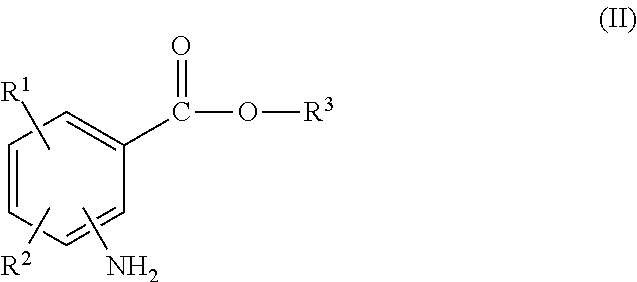Patents
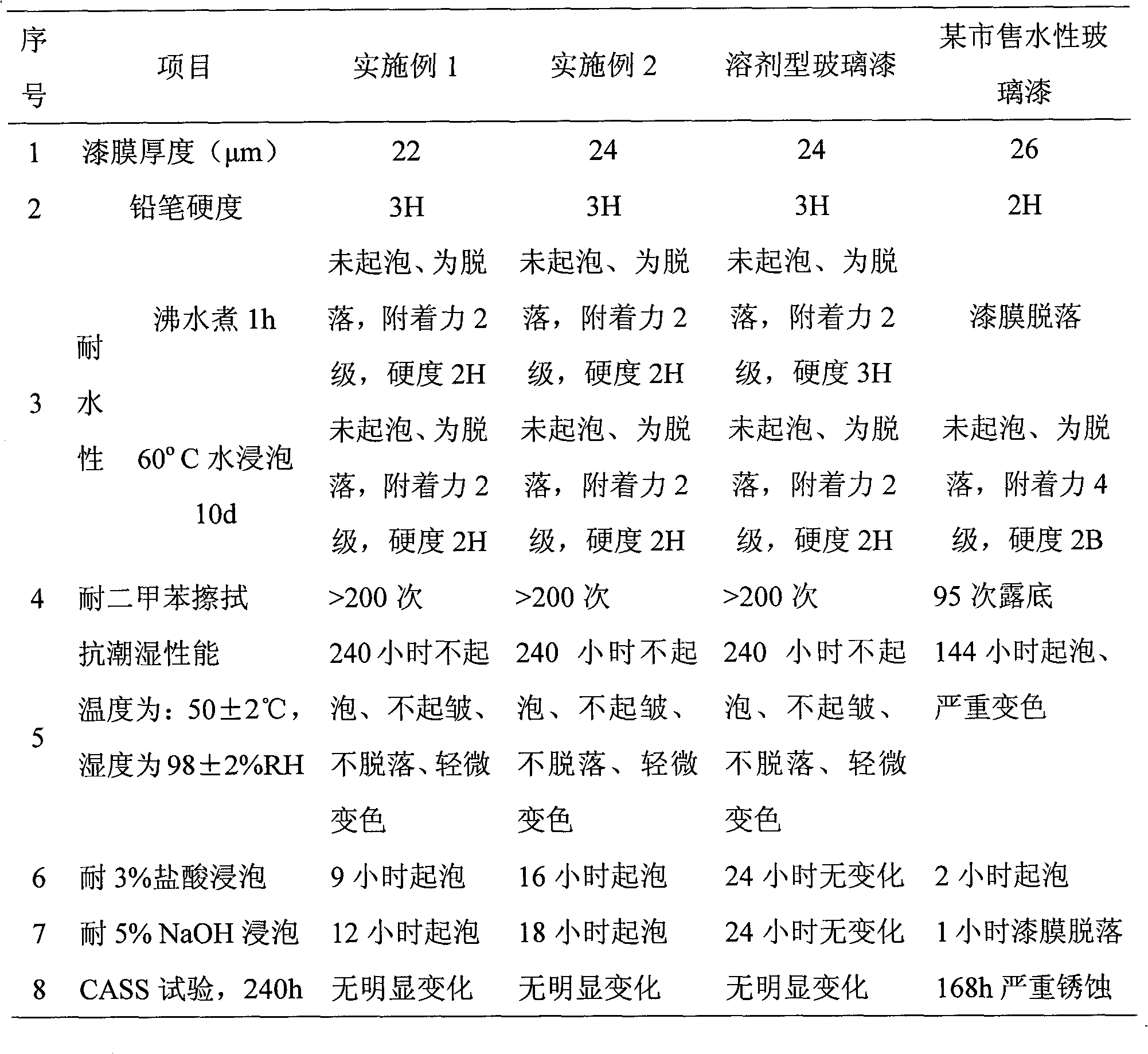
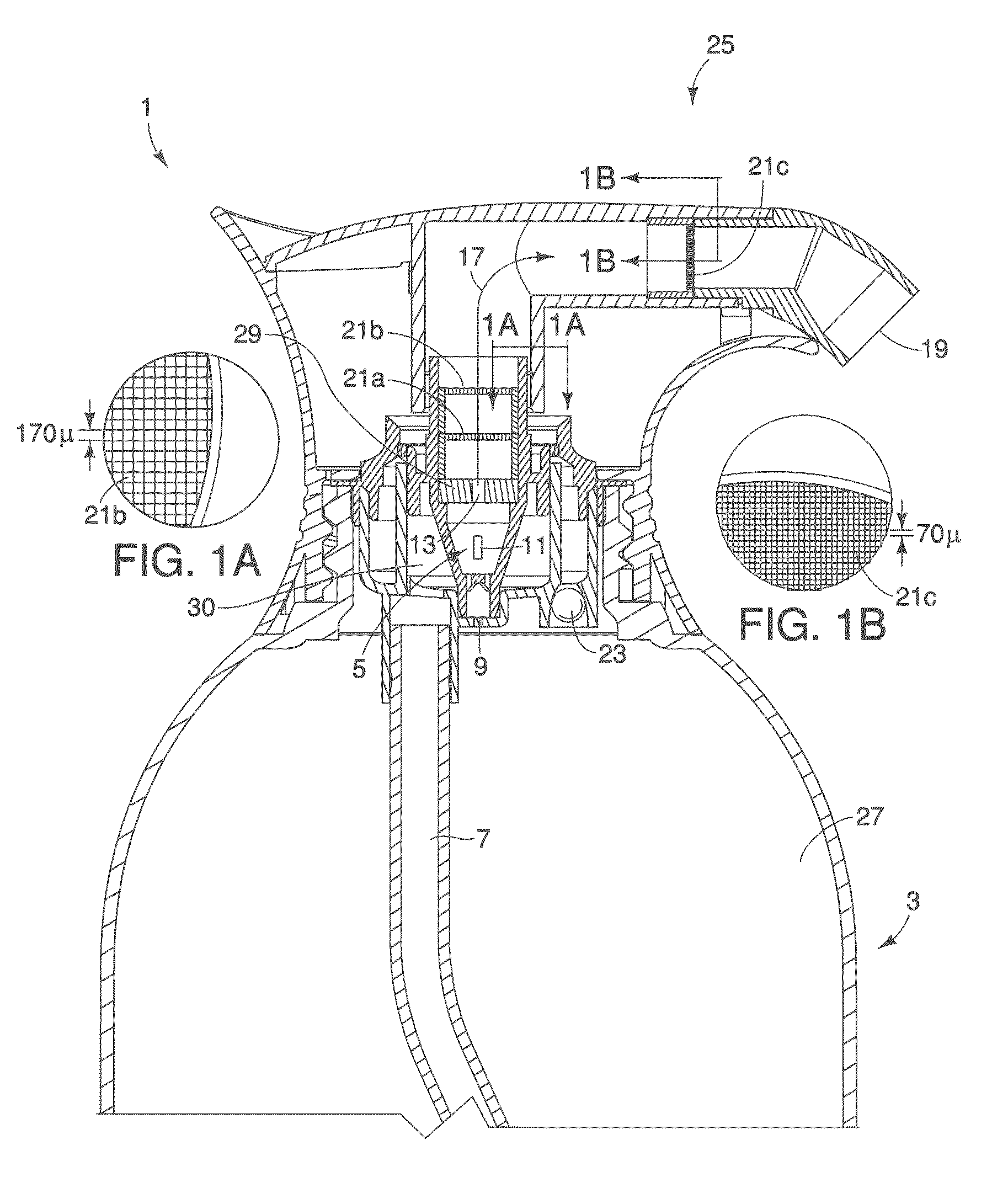
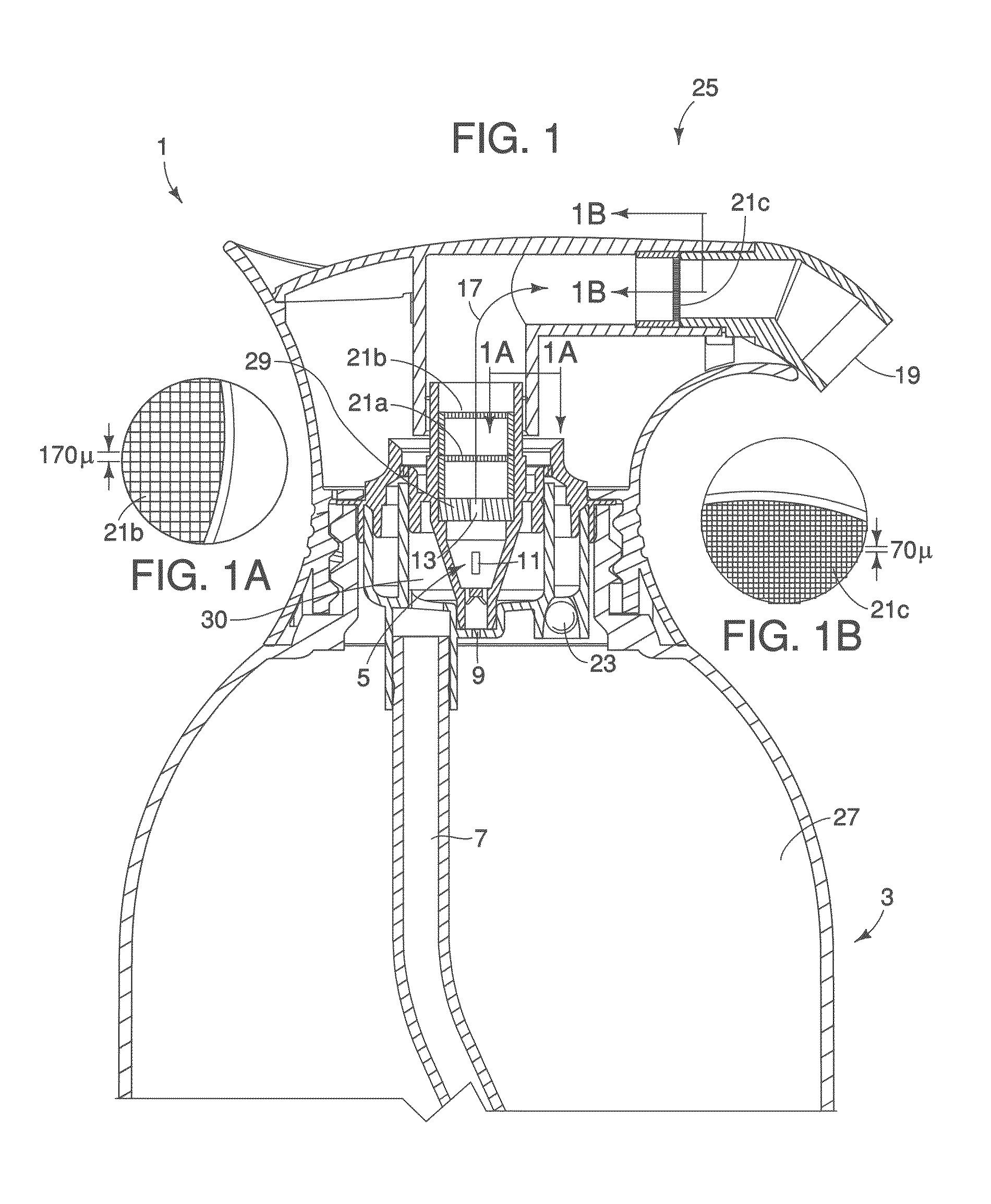
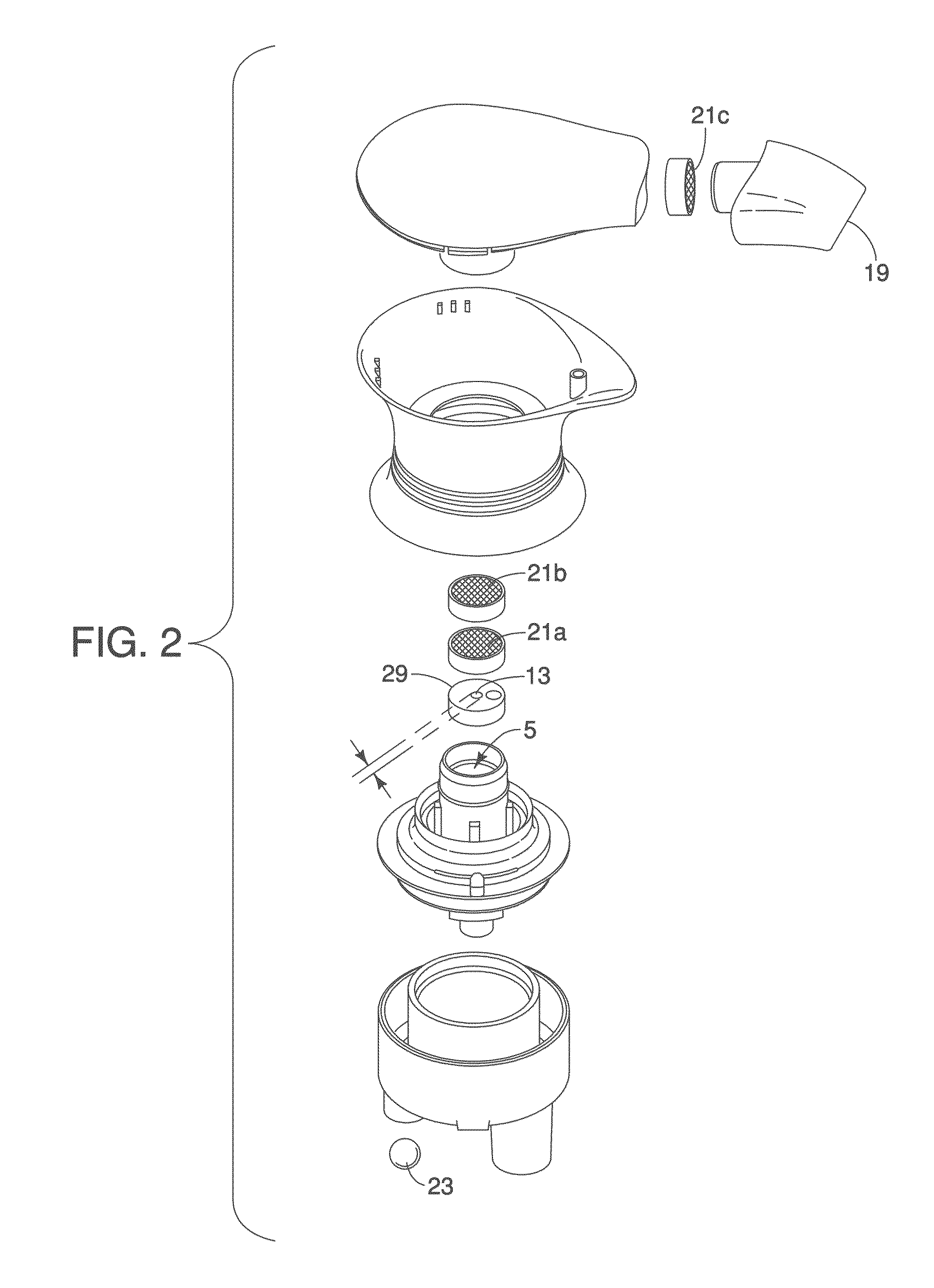
Literature
756 results about "Free base" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Free base (freebase, free-base) is the conjugate base (deprotonated) form of an amine, as opposed to its conjugate acid (protonated) form. The amine is often an alkaloid, such as nicotine, cocaine, morphine, and ephedrine, or derivatives thereof. Freebasing is a more efficient method of self-administering alkaloids via the smoking route.
Triclosan-containing medical devices
InactiveUS6106505ALower levelAvoiding undesirably high releaseWrappers shrinkageShrinkage connectionsTriclosanAntiinfective agent
PCT No. PCT / US96 / 20932 Sec. 371 Date Jun. 30, 1998 Sec. 102(e) Date Jun. 30, 1998 PCT Filed Dec. 23, 1996 PCT Pub. No. WO97 / 25085 PCT Pub. Date Jul. 17, 1997The present invention relates to polymeric medical articles comprising the antiinfective agents chlorhexidine and triclosan. It is based, at least in part, on the discovery that the synergistic relationship between these compounds permits the use of relatively low levels of both agents, and on the discovery that effective antimicrobial activity may be achieved when these compounds are comprised in either hydrophilic or hydrophobic polymers. It is also based on the discovery that chlorhexidine free base and triclosan, used together, are incorporated into polymeric medical articles more efficiently. Medical articles prepared according to the invention offer the advantage of preventing or inhibiting infection while avoiding undesirably high release of antiinfective agent, for example into the bloodstream of a subject.
Owner:COLUMBIA UNIV OF THE CITY OF NEW YORK TRUSTEES OF THE
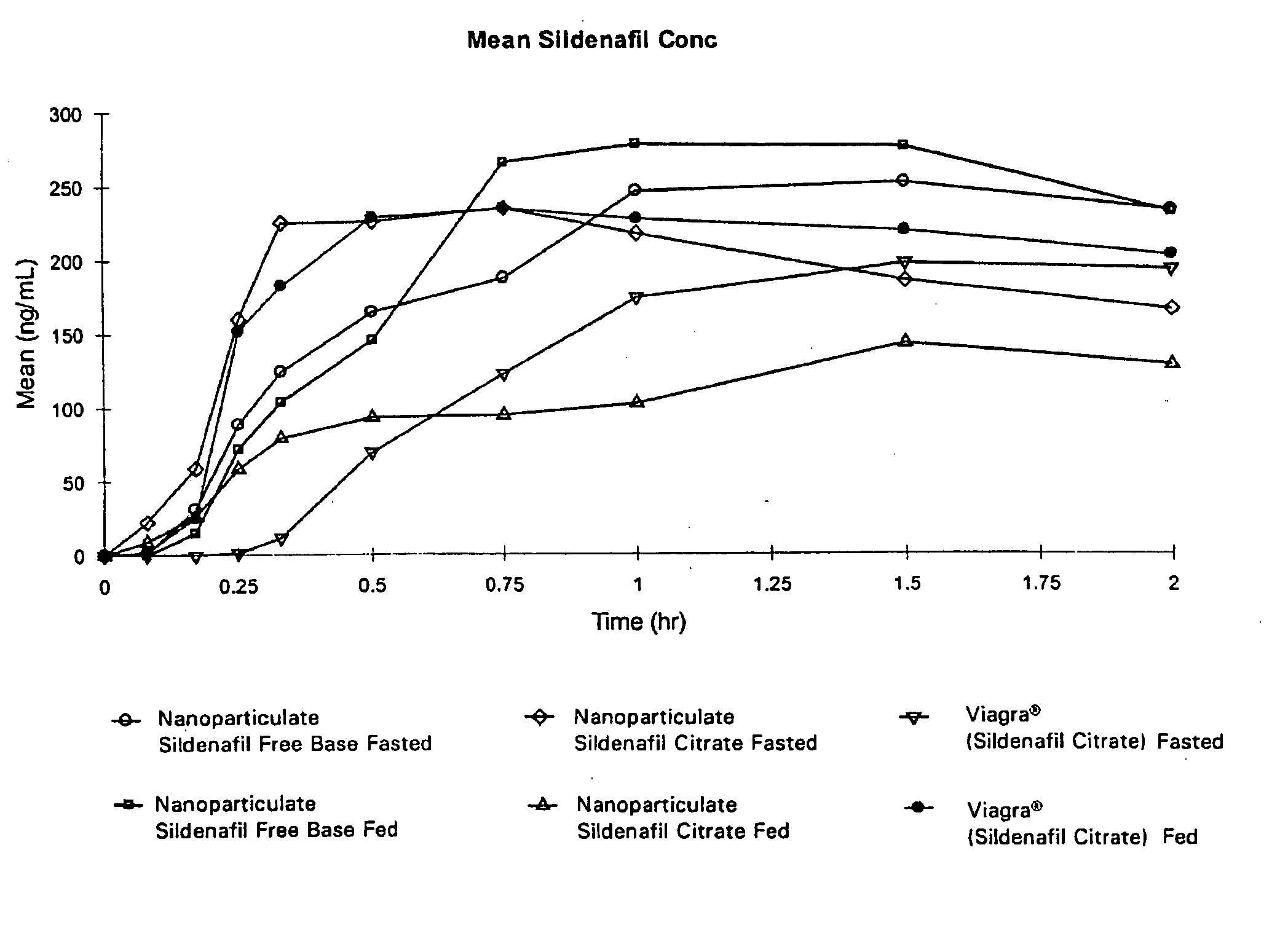
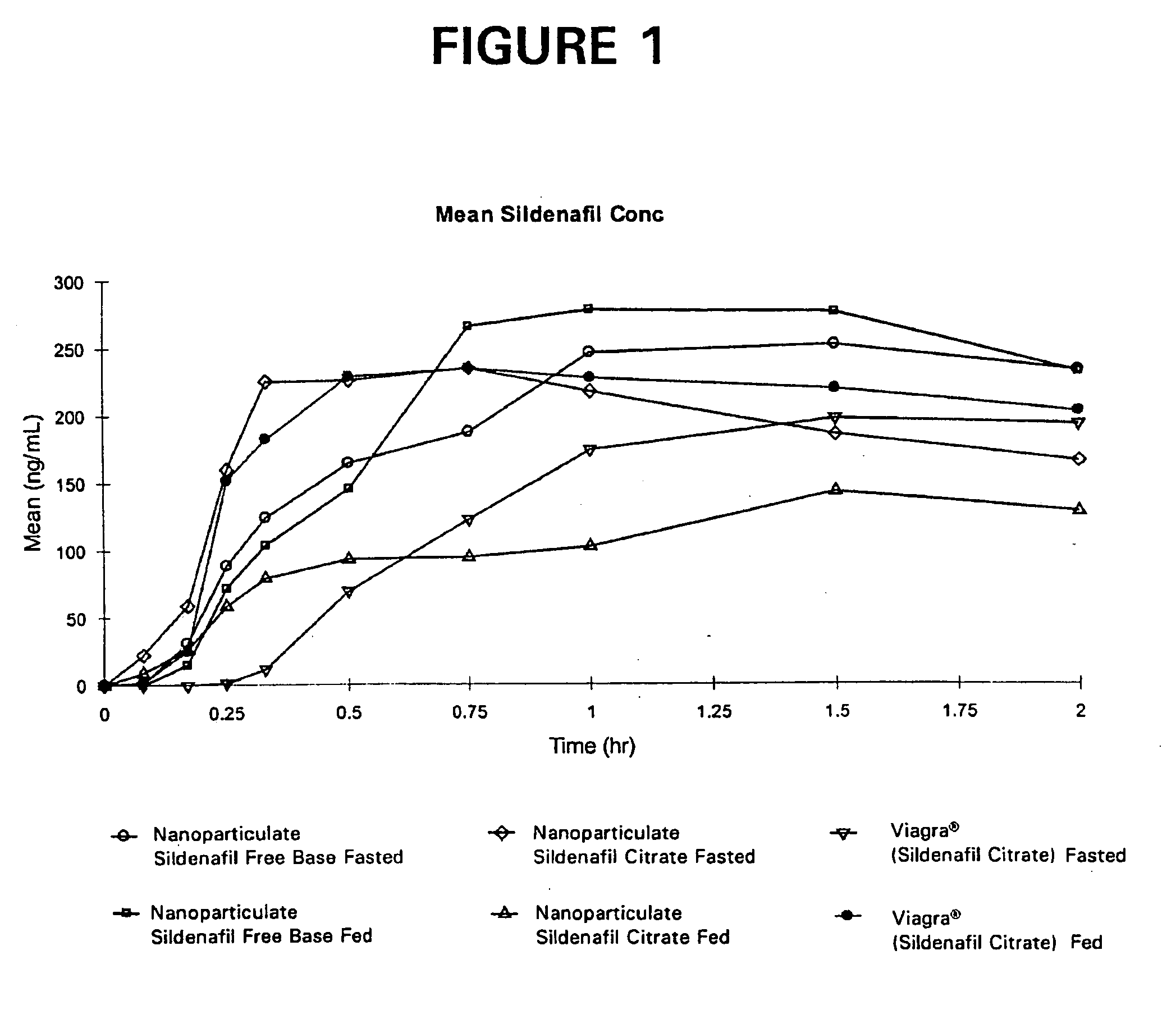
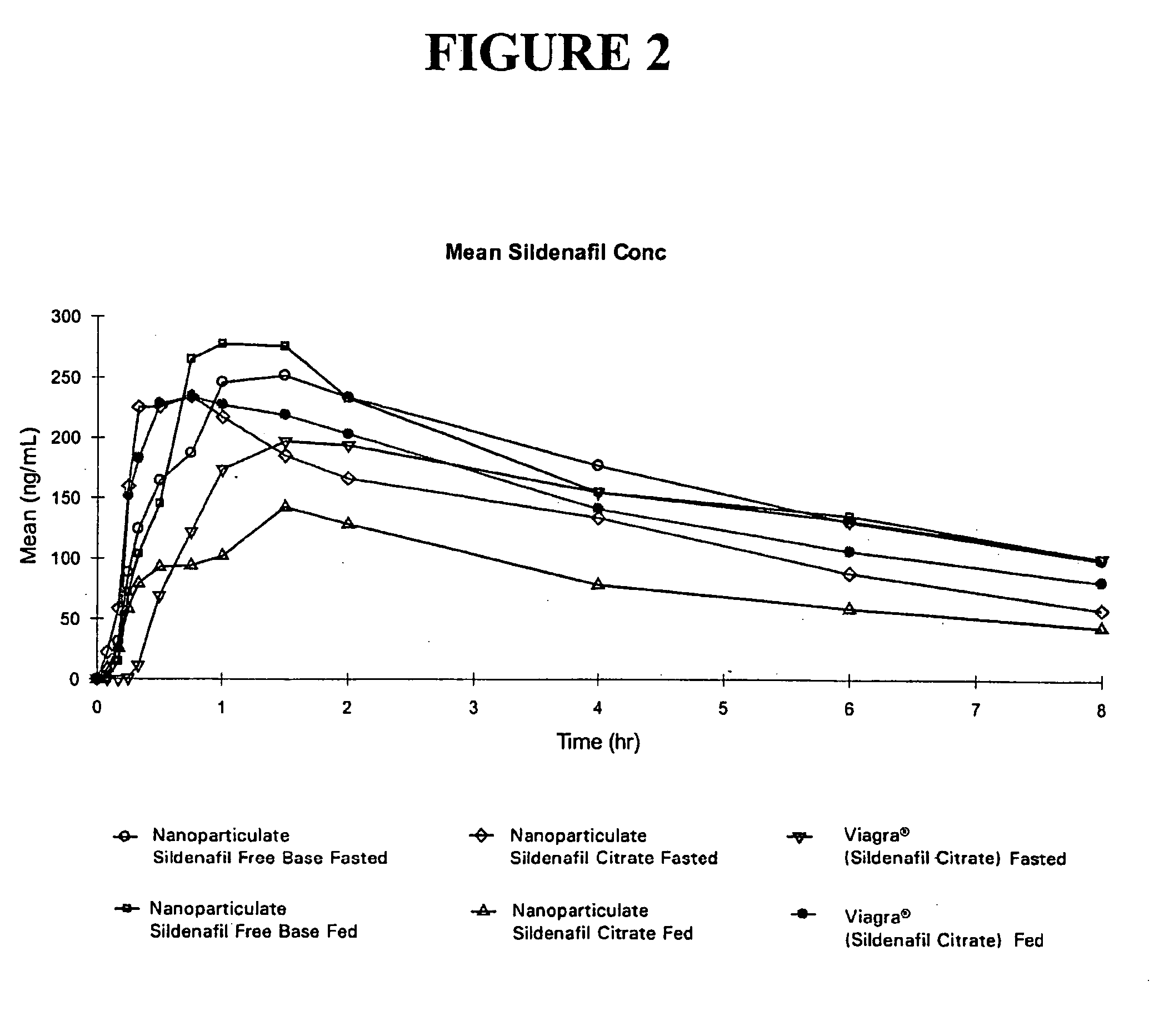
Novel compositions of sildenafil free base
InactiveUS20050042177A1Eliminate the effects ofOrganic active ingredientsPowder deliveryMicroparticleMedicinal chemistry
The present invention is directed to nanoparticulate compositions comprising sildenafil free base. The sildenafil free base particles of the composition have an effective average particle size of less than about 2000 nm.
Owner:ALKERMES PHARMA IRELAND LTD
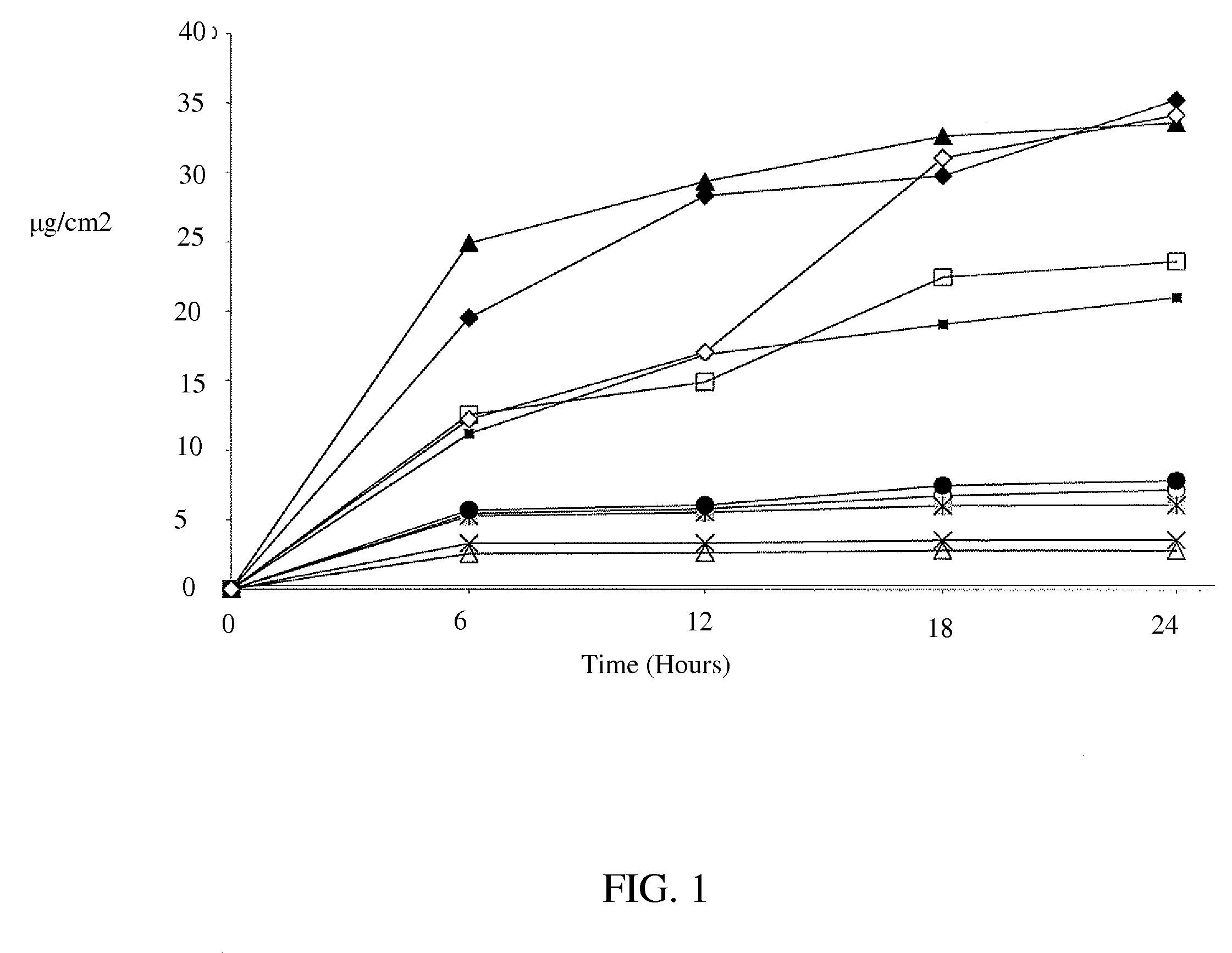
Transdermal dosage form comprising an active agent and a salt and a free-base form of an adverse agent
InactiveUS20080020028A1Sufficient amountInhibition effectBiocideNervous disorderOpioid antagonistPreventing pain
This invention relates to a tamper-resistant transdermal dosage form comprising an active agent, such as an opioid, or a pharmaceutically acceptable salt thereof, a free base of an adverse agent, such as an opioid antagonist, and a pharmaceutically acceptable salt of an adverse agent, such as an opioid antagonist. The transdermal dosage form allows an effective amount of the active agent, or a pharmaceutically acceptable salt thereof, to be transdermally administered to an animal. The invention further relates to methods for treating or preventing pain in an animal comprising contacting the skin of an animal in need thereof with the transdermal dosage form of the invention for an amount of time sufficient to treat or prevent pain.
Owner:PURDUE PHARMA LP
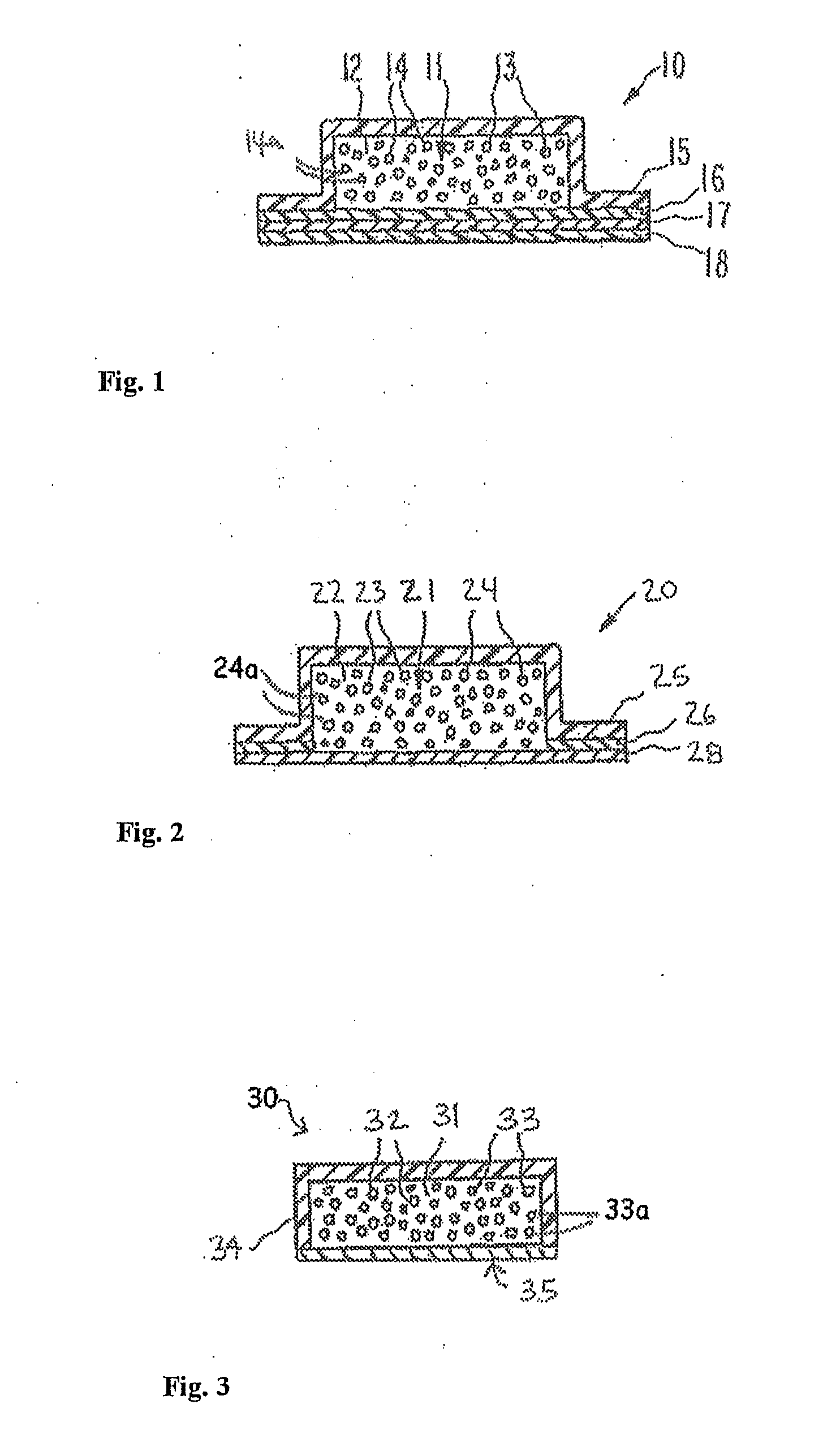
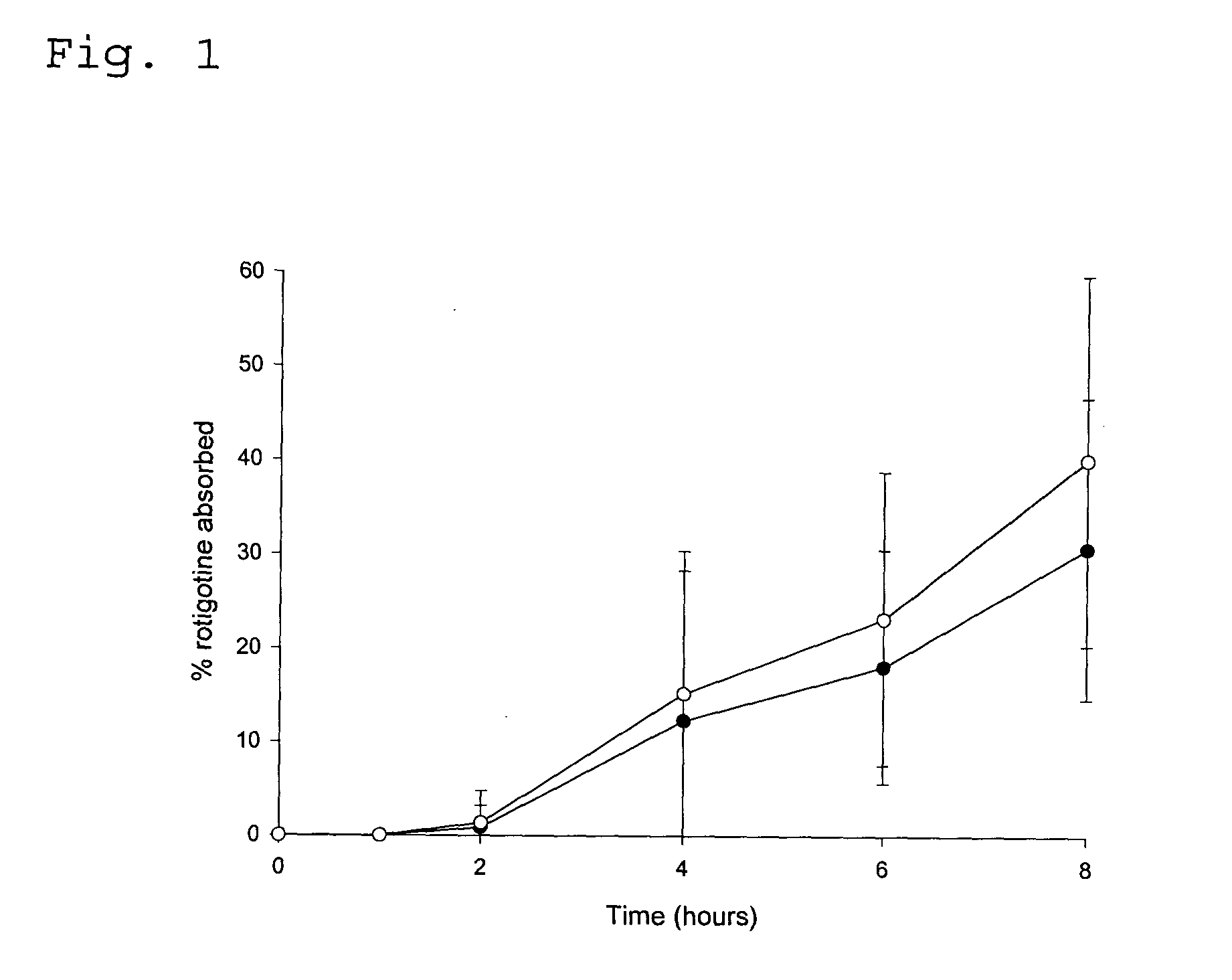
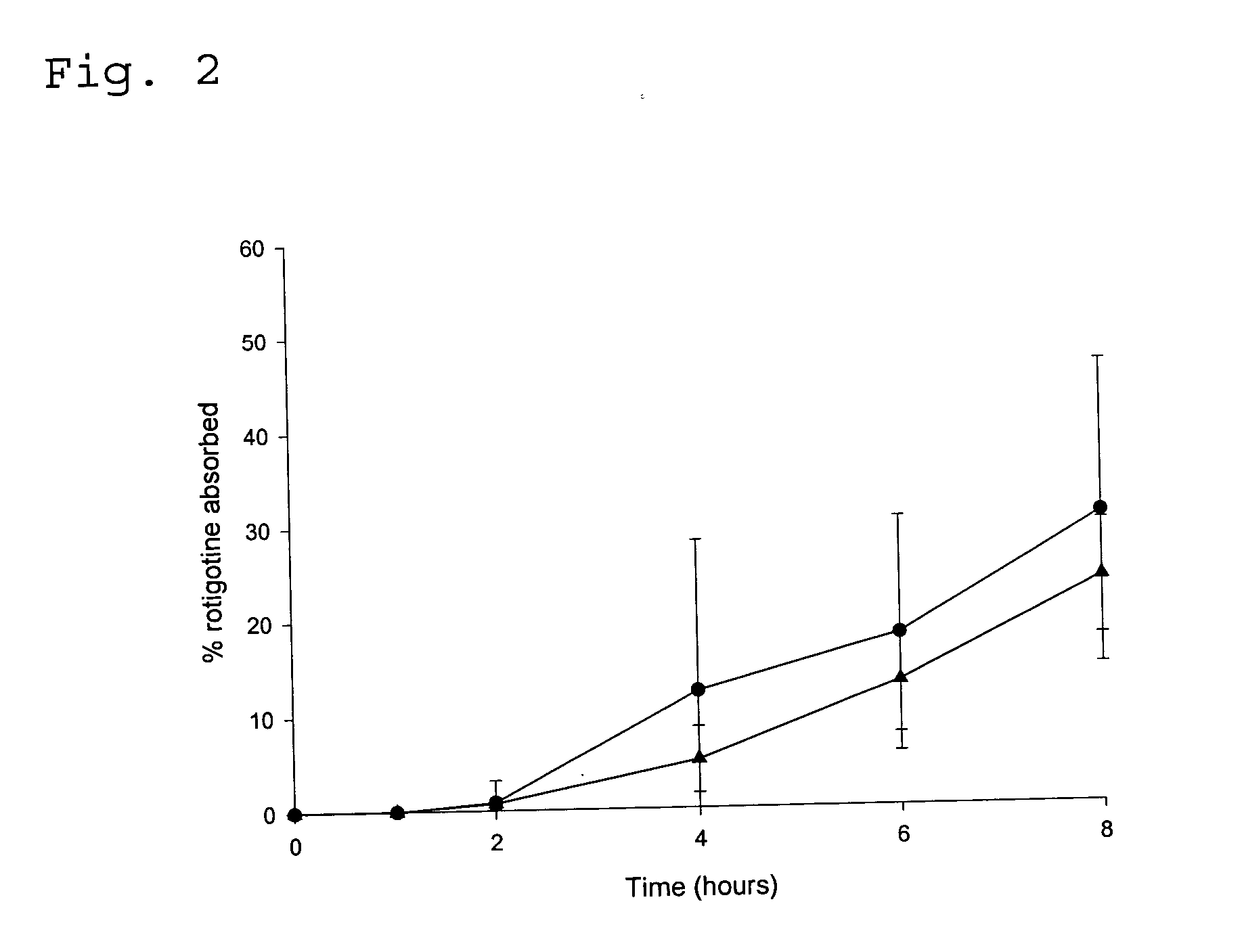
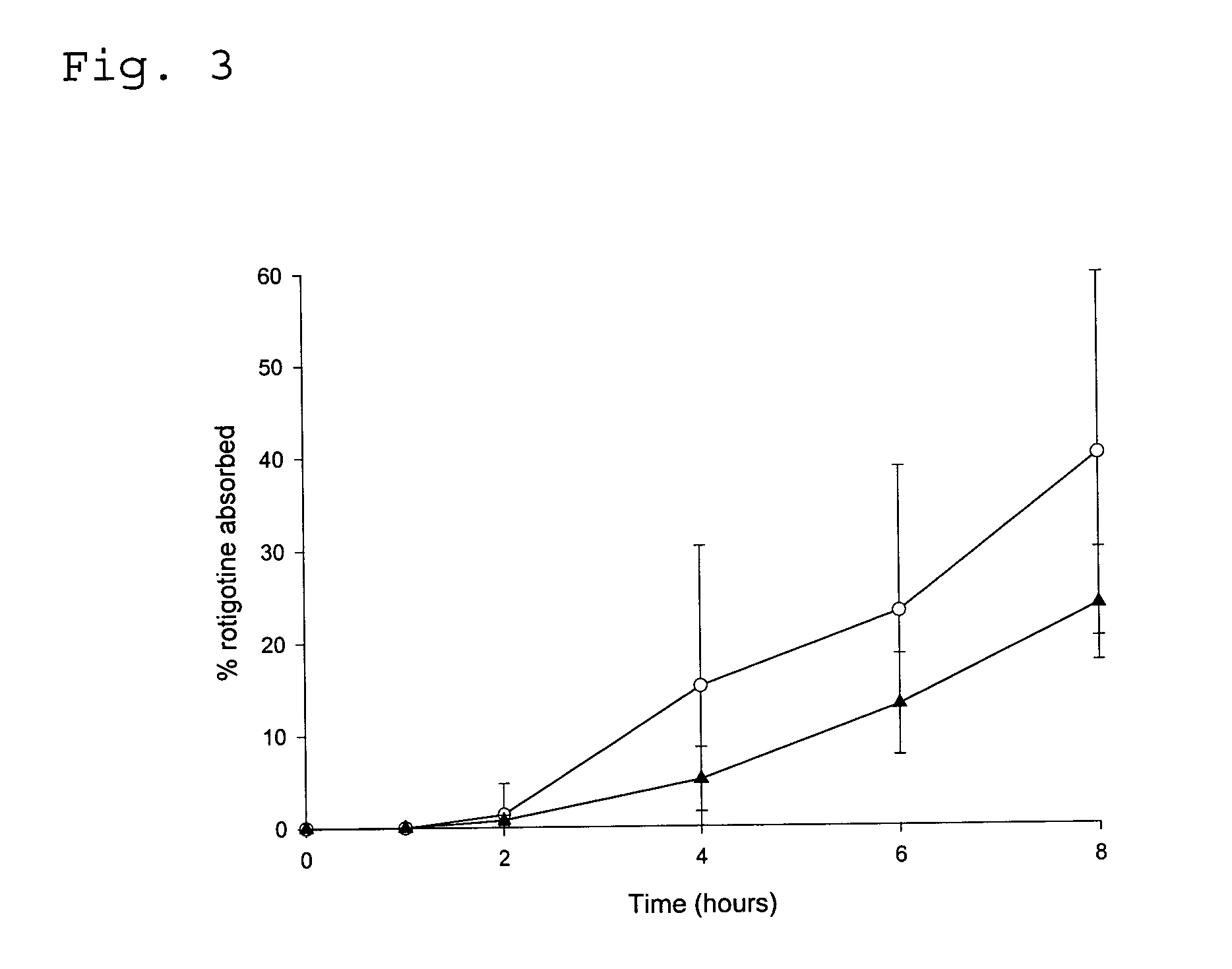
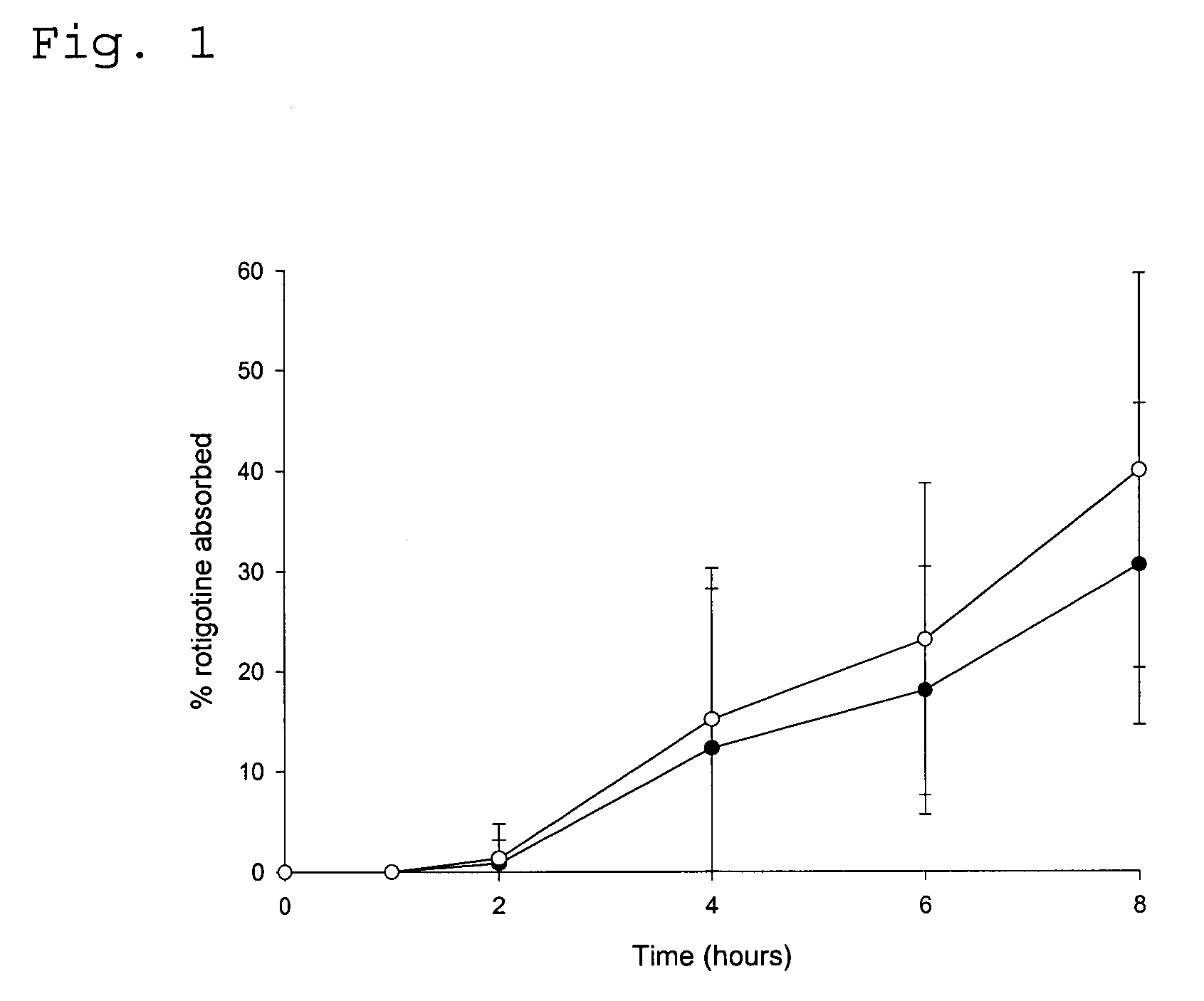
Transdermal delivery system for the administration of rotigotine
An improved Transdermal Delivery System (TDS) comprising a backing layer inert to the components of the matrix, a self-adhesive matrix containing rotigotine and a protective foil or sheet to be removed prior to use, characterized in that the self-adhesive matrix consists of a solid or semi-solid semi-permeable polymer (1) wherein rotigotine in its free base form has been incorporated, (2) which is saturated with rotigotine and contains said rotigotine as a multitude of microreservoirs within the matrix, (3) which is highly permeable for the free base of rotigotine, (4) which is impermeable for the protonated form of rotigotine, (5) wherein the maximum diameter of the microreservoirs is less than the thickness of the matrix. is provided. Said TDS provides for enhanced flux of rotigotine across the TDS / skin interface.
Owner:UCB SA
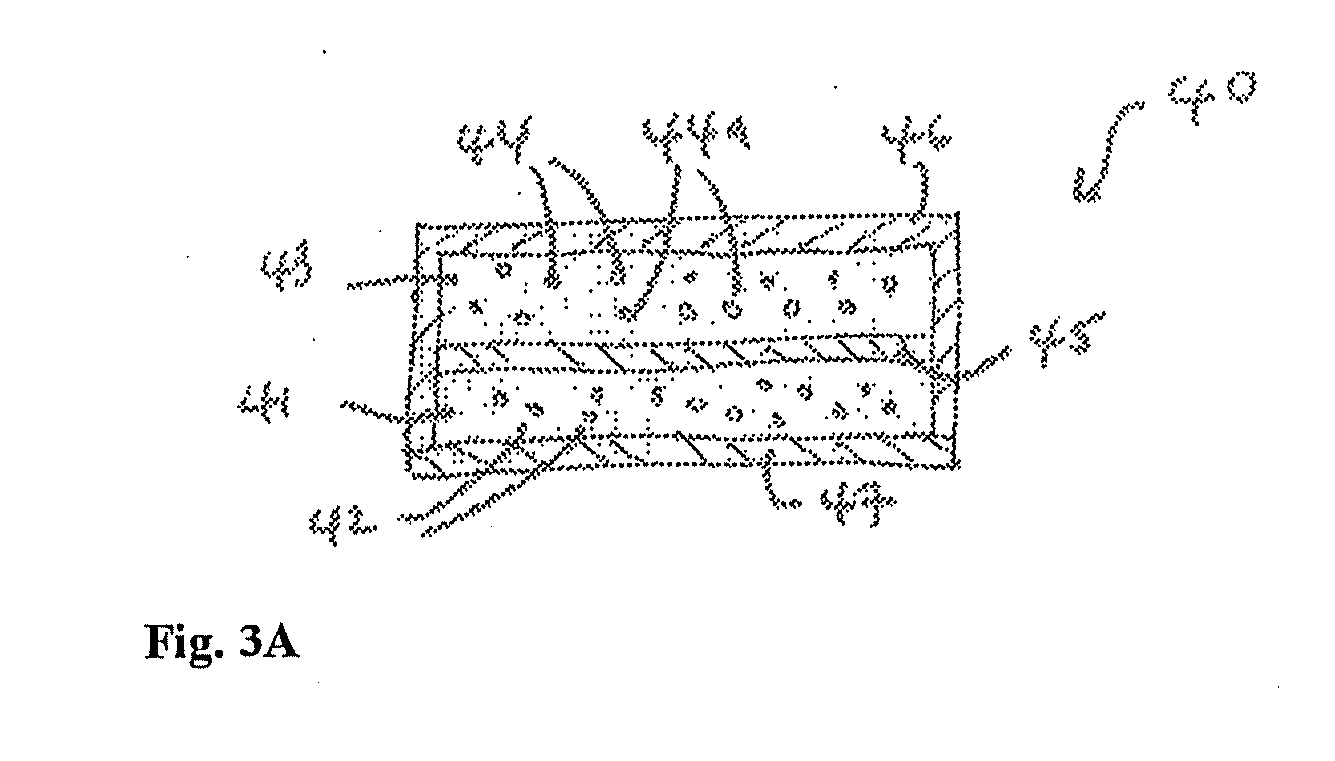
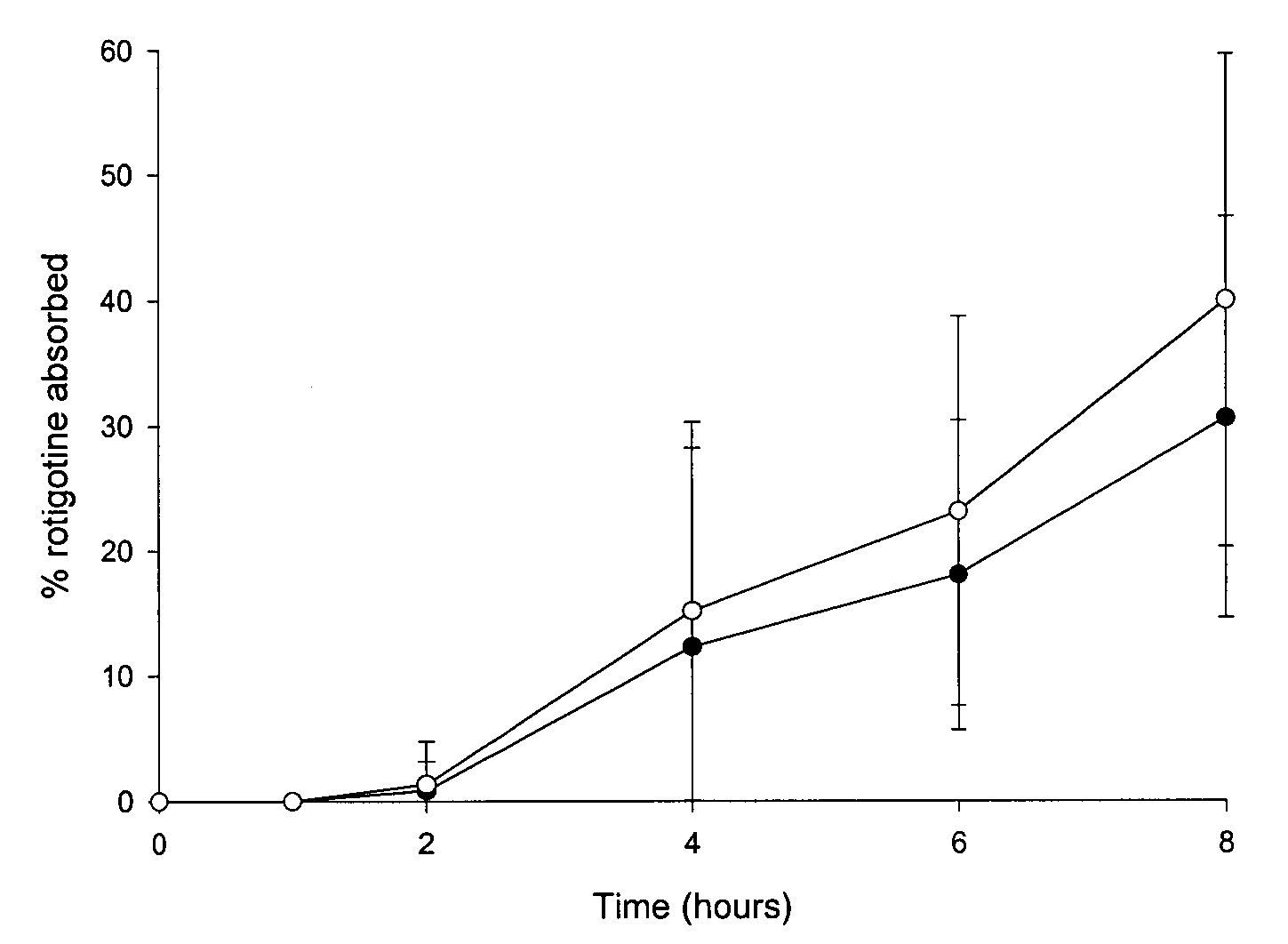
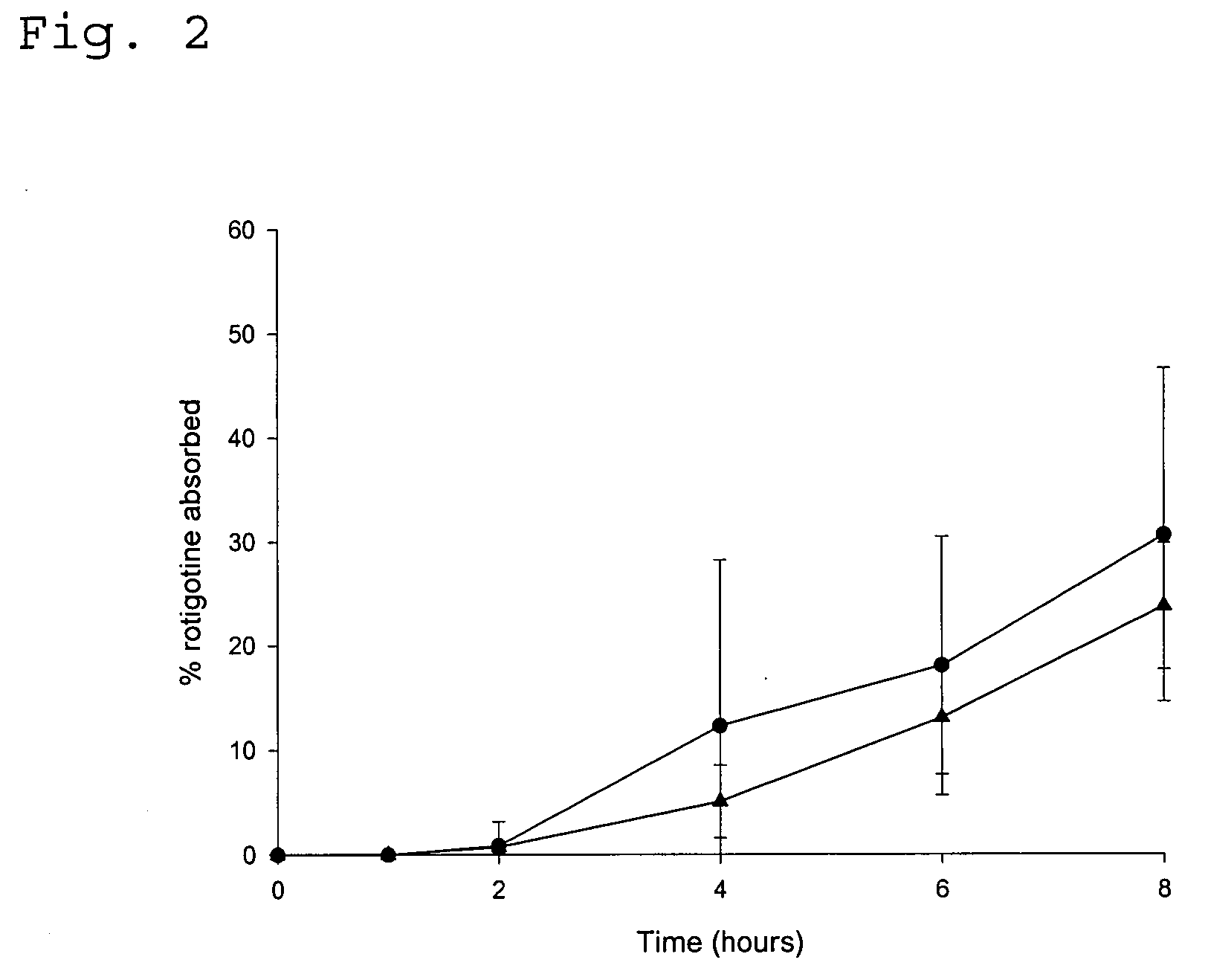
Transdermal delivery system
ActiveUS20040081683A1Facilitated releaseGood release effectPowder deliveryBiocideProtonationMedicine
An improved Transdermal Delivery System (TDS) comprising a backing layer inert to the components of the matrix, a selfadhesive matrix containing an amine-functional drug and a protective foil or sheet to be removed prior to use, characterized in that the self-adhesive matrix consists of a solid or semi-solid semi-permeable polymer (1) wherein an amine functional drug in its free base form has been incorporated, (2) which is saturated with the amine functional drug and contains said drug as a multitude of microreservoirs within the matrix, (3) which is highly permeable for the free base of the amine functional drug, (4) which is impermeable for the protonated form of the amine functional drug, (5) wherein the maximum diameter of the microreservoirs is less than the thickness of the matrix. is provided. Said TDS provides for enhanced flux of the amine functional drug across the TDS / skin interface.
Owner:UCB SA
Formulation for oral administration of apoptosis promoter
InactiveUS20100297194A1Improve oral bioavailabilityConvenient route of administrationOrganic active ingredientsBiocideDiseaseAntioxidant
An orally deliverable pharmaceutical composition comprises as a sole or first active ingredient a compound of Formula I defined herein or a pharmaceutically acceptable salt thereof, for example ABT-263 free base or ABT-263 bis-HCl salt, dispersed, in a free base equivalent amount of at least about 2.5% by weight of the composition, in a pharmaceutically acceptable carrier; wherein said active ingredient is in solid-state form and / or the composition further comprises, dispersed in the carrier, a pharmaceutically acceptable heavier-chalcogen antioxidant in an amount effective to inhibit oxidation of the active ingredient at a thioether linkage thereof. The composition is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBOTT LAB INC +1
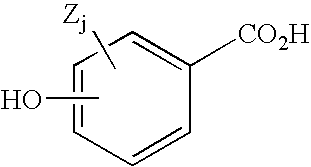
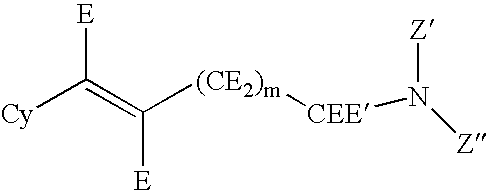
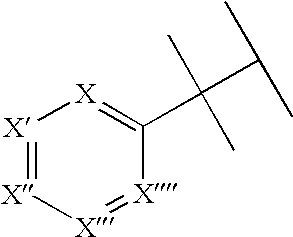
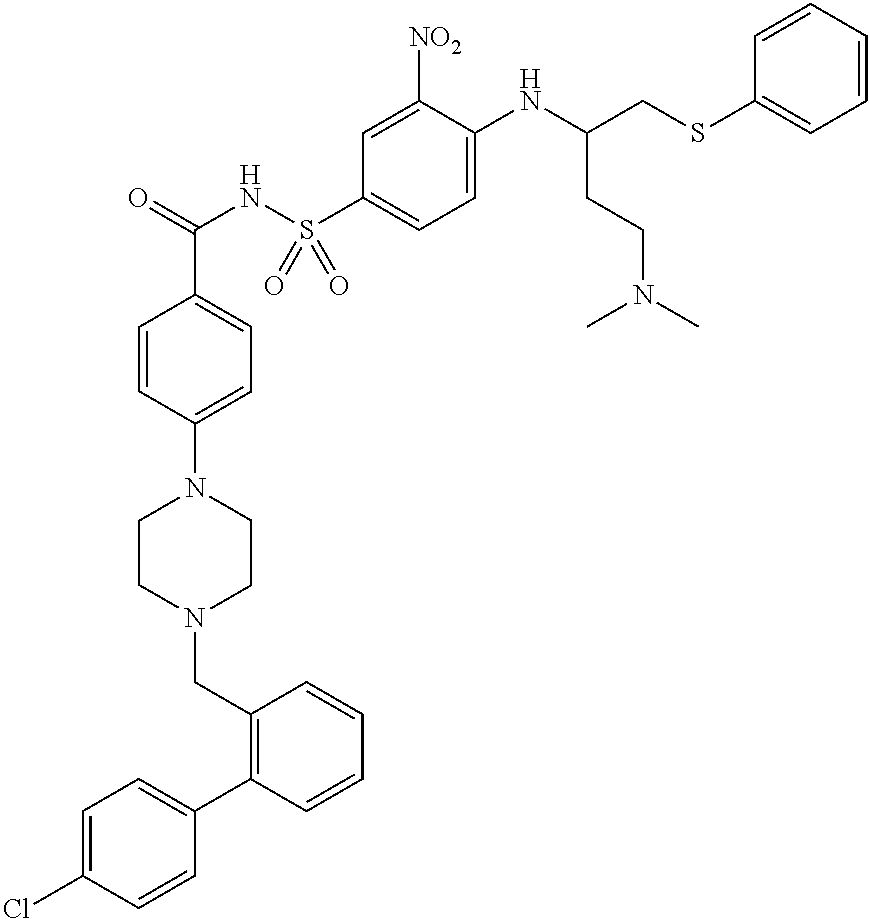
Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity
ActiveUS9708322B2High activityPromote disseminationOrganic active ingredientsOrganic chemistryQuinoxalineDisease
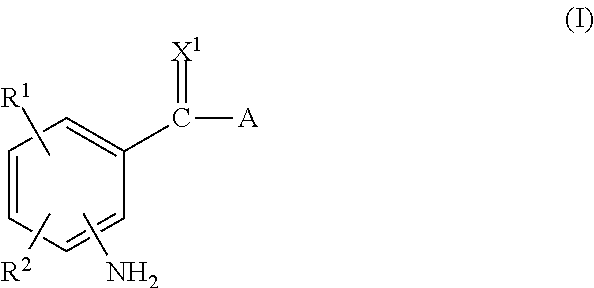
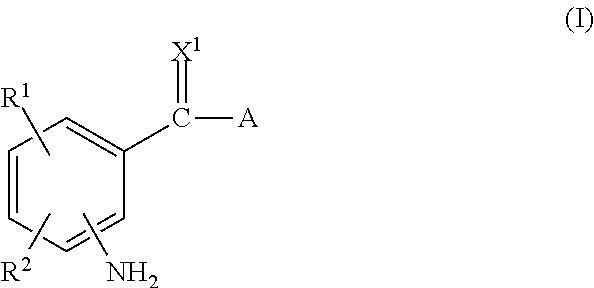
The invention relates to substituted heterocycle fused gamma-carbolines of the Formula Q as described herein, in free base or pharmaceutically acceptable salt form, and / or pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving the 5-HT2A receptor pathway, the serotonin transporter (SERT) pathway and / or the dopamine D2 receptor pathway signaling systems.
Owner:INTRA CELLULAR THERAPIES INC
Use
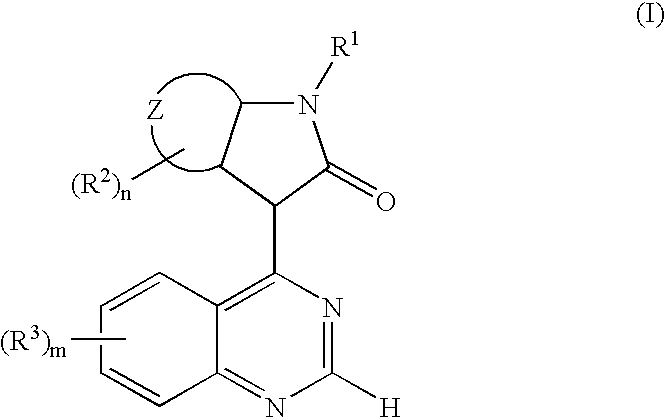
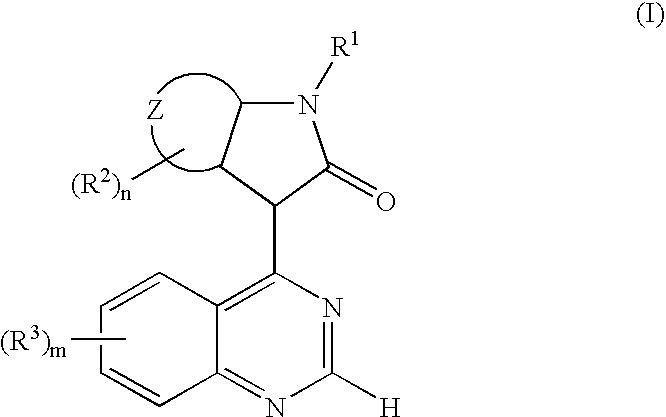
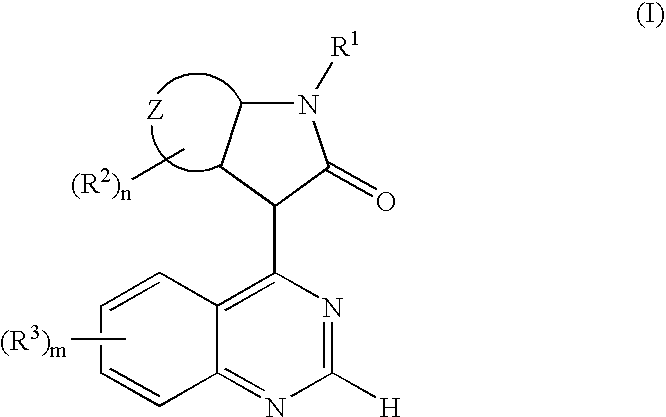
The present invention relates to a new use of oxindole derivatives of formula I, as a free base or pharmaceutically acceptable salts thereof, in the manufacture of a medicament for the prevention and / or treatment of dementia related diseases, Alzheimer's Disease and conditions associated with glycogen synthase kinase-3. Formula (I) wherein R1, R2, R3, ring Z, m and n are as defined as in claim 1. The present invention further relates to a method of prevention and / or treatment of dementia related diseases, Alzheimer's Disease and conditions associated with glycogen synthase kinase-3, as well as a pharmaceutical composition for said use.
Owner:ASTRAZENECA AB
Synthesis, split and racemization of chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
ActiveCN101130504AReduce dosageLow costOrganic compound preparationCarboxylic acid amides optical isomer preparationButyramidePyrrolidine
The invention discloses a new synthesizing technique of chiral drug (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetamide ( left Piracetam) intermediate (S)-(+)-2-aminobutanamide hydrochlorate, which comprises the following steps: adopting 2-brobutyrate as initial raw material; aminating; esterifying; ammonolyzing; detaching; looping; obtaining the object compound; making mixed rotary free alkaline (+-)-2-aminobutanamide; adopting half-quantum resolution method to connect chemical detaching salt to evolve salt; removing the detaching agent through alkalization; obtaining the (S)-(+)-2-aminobutanamide hydrochlorate with optical activity; using the mother liquor to make the product. The invention improves the receiving rate and saves the cost of raw material with simply technical operation and low cost, which resolves the resolved mother liquor after racemic action again to reduce the pollution of environment, therefore fitting for industrialized manufacturing.
Owner:ABA CHEM CORP
Wet granulation process
InactiveUS20060057073A1Improve solubilityImprove efficiencyOrganic active ingredientsAerosol deliveryBULK ACTIVE INGREDIENTActive ingredient
The invention is in the field of pharmaceutical dosage forms, and more particularly in the field of pharmaceutical granules and processes for making granules. The invention provides a process for preparing pharmaceutical granules which contain an active ingredient in the form of a salt, said process comprising the steps of (a) providing a powder containing the active ingredient as a free base or acid, and (b) agglomerating the powder by adding a granulation liquid to form granules; wherein step (b) is conducted in the presence of a neutralization agent capable of neutralizing the active ingredient, and for a sufficient amount of time to allow the active ingredient to become at least partially converted into a salt. The invention also provides pharmaceutical granules obtainable by said process and pharmaceutical compositions comprising said granules. The invention further provides the use of pharmaceutical granules for the pulmonary delivery of an active ingredient.
Owner:PARI PHARMA GMBH
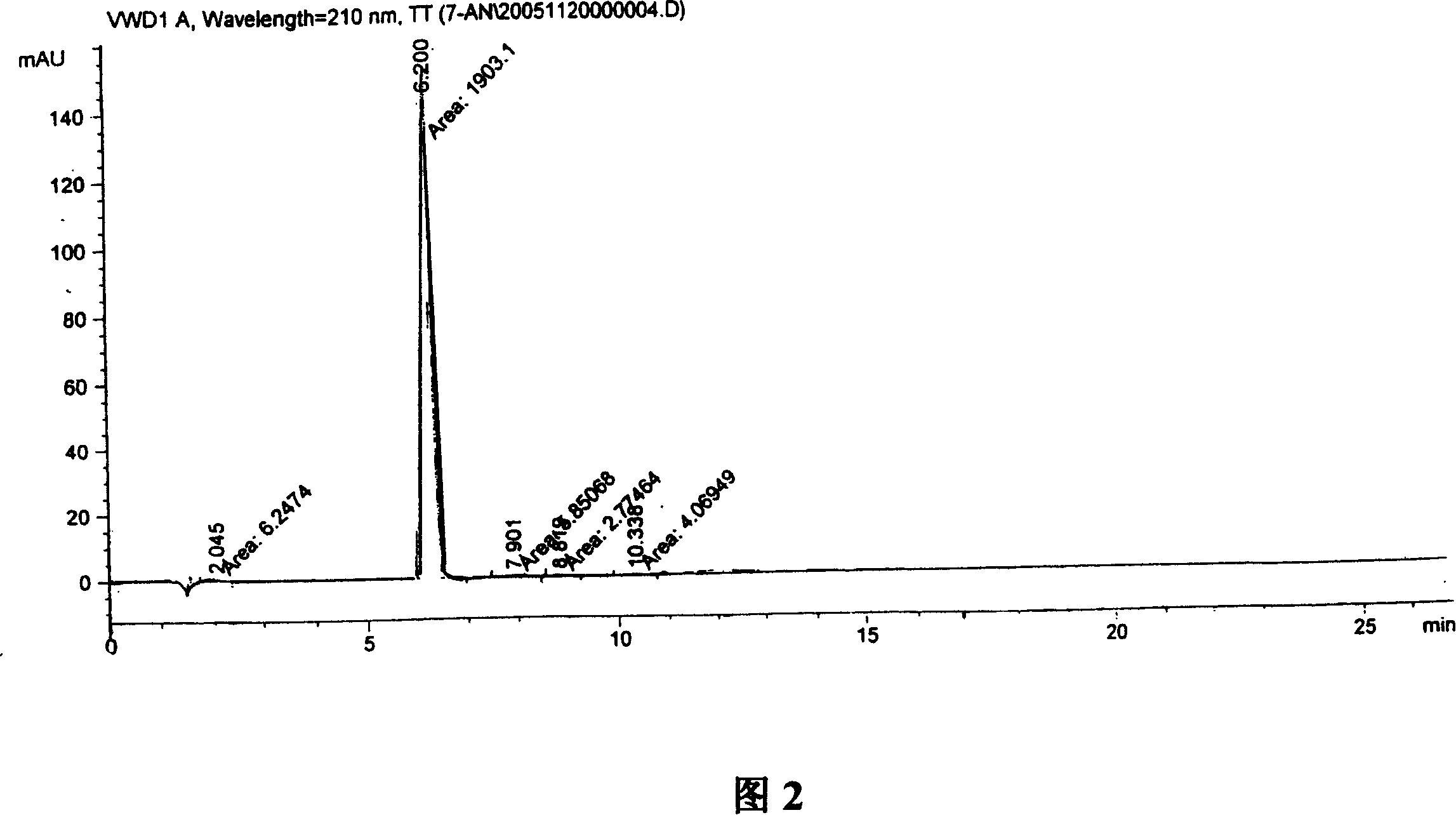
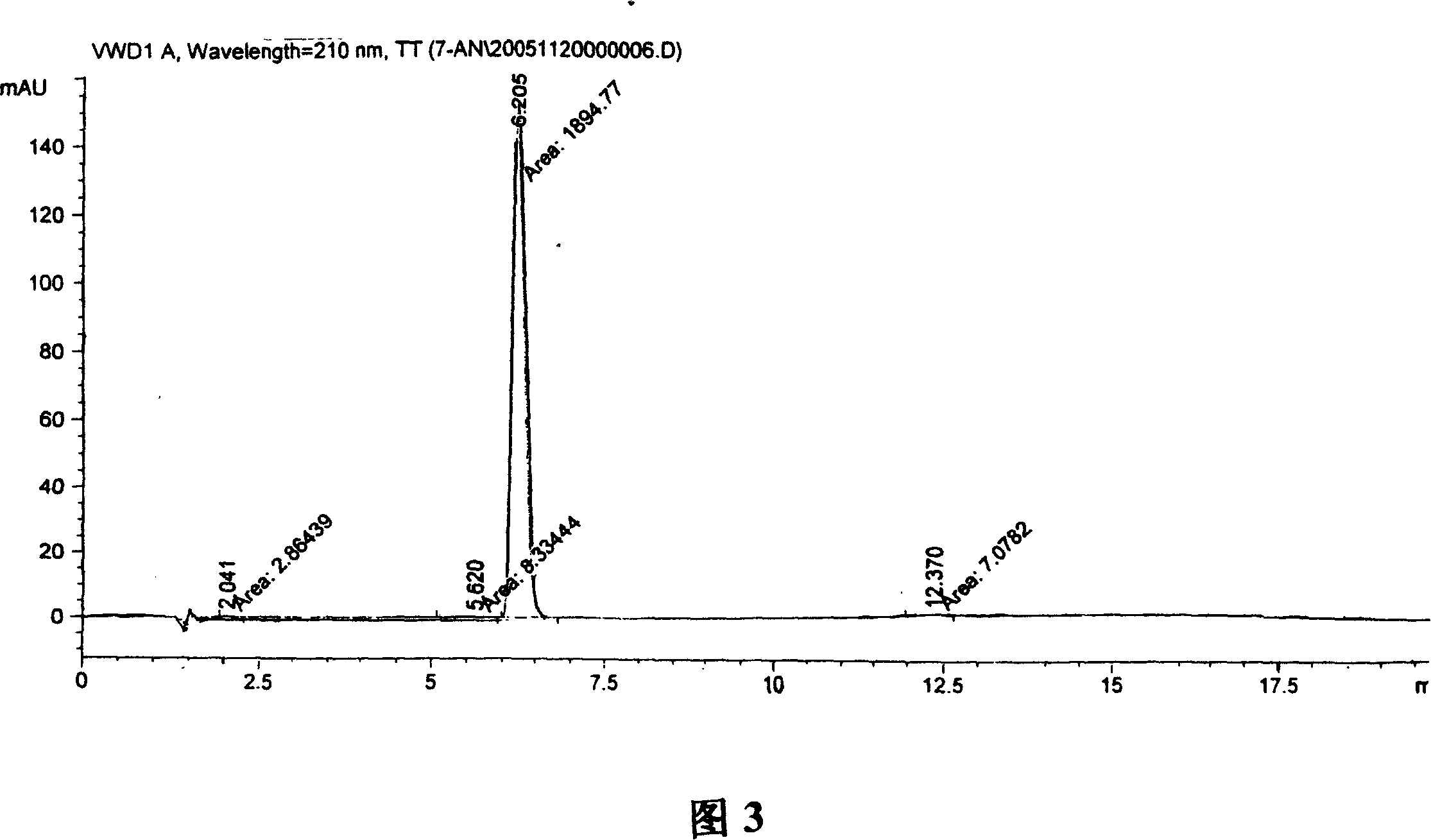
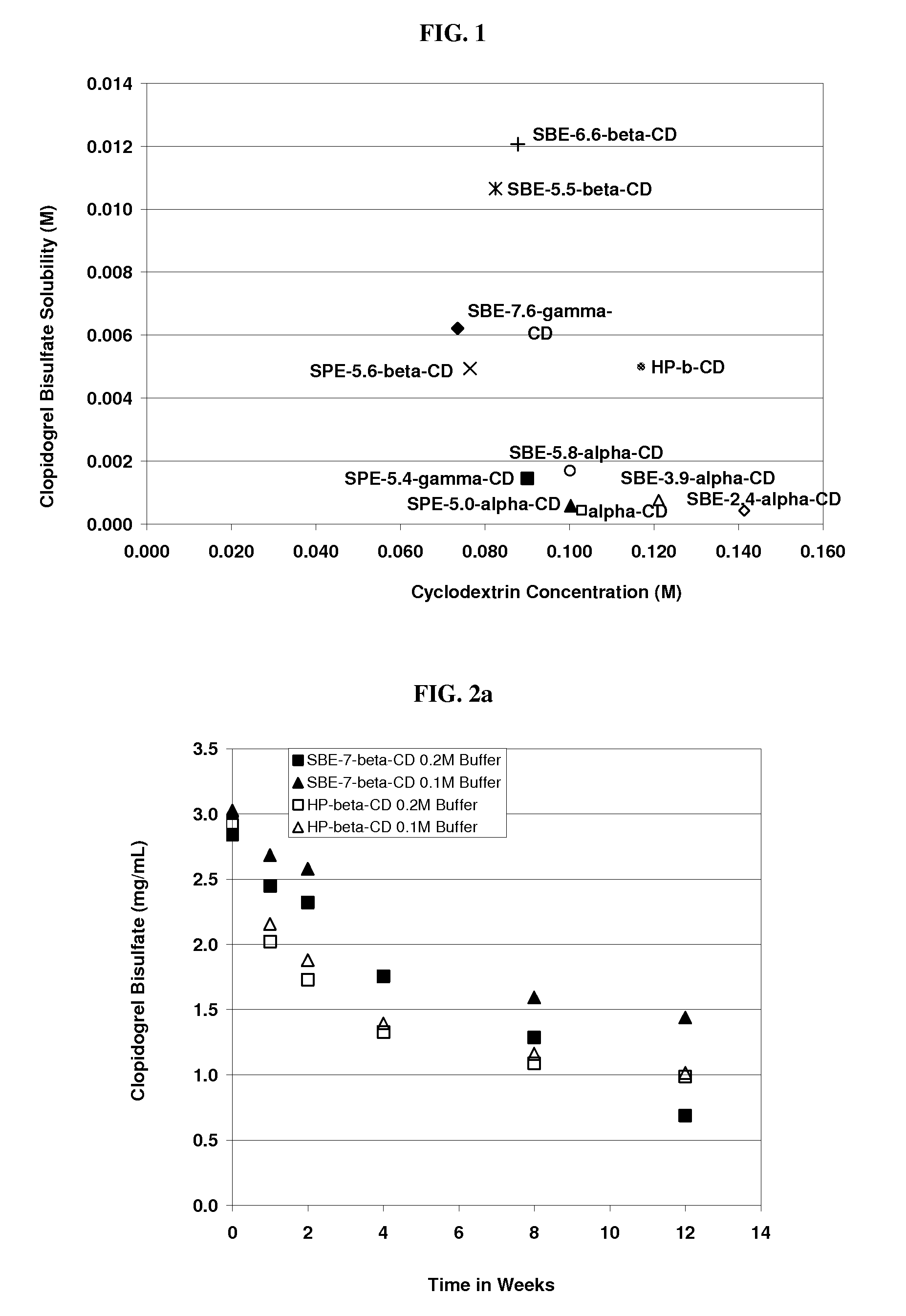
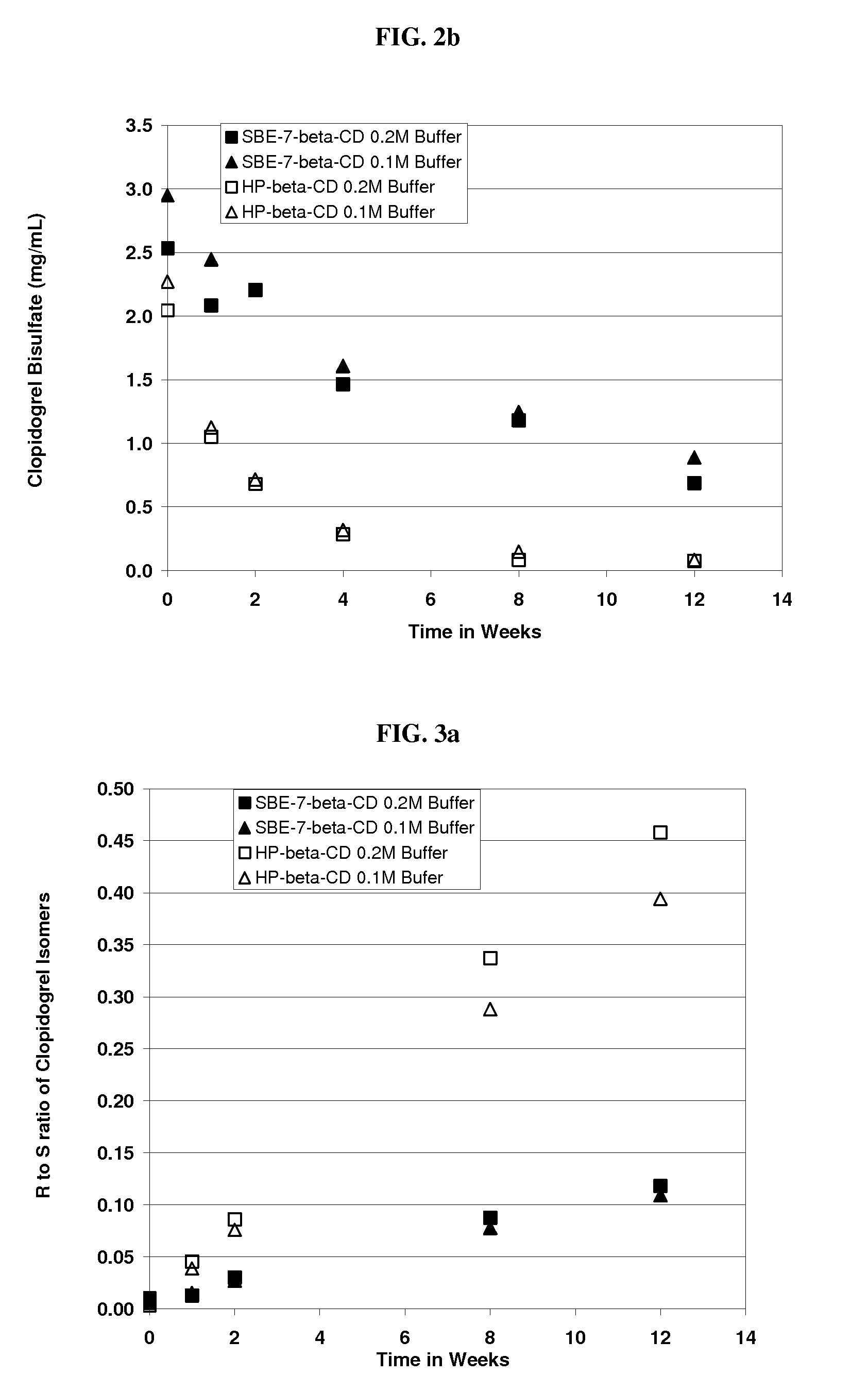
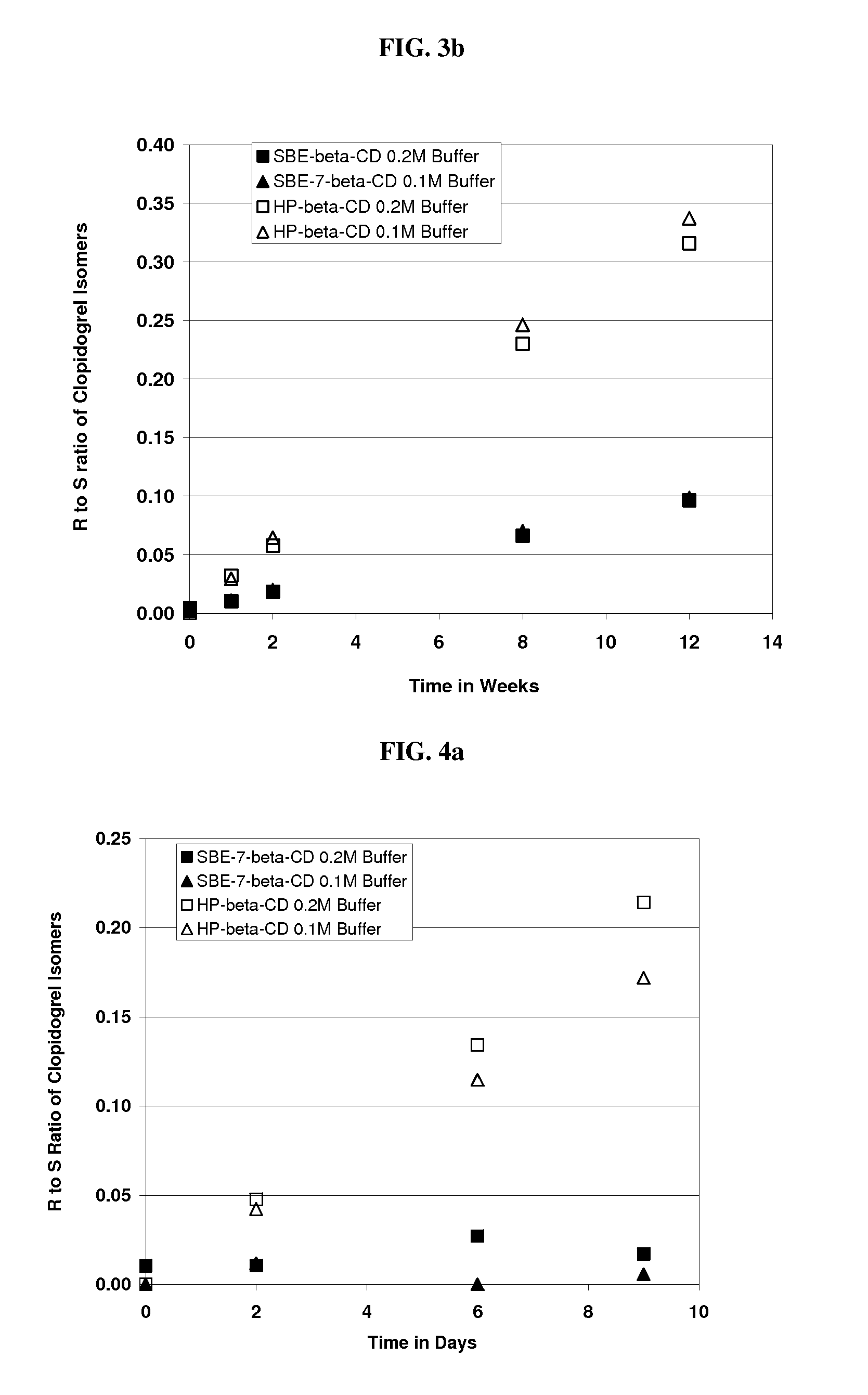
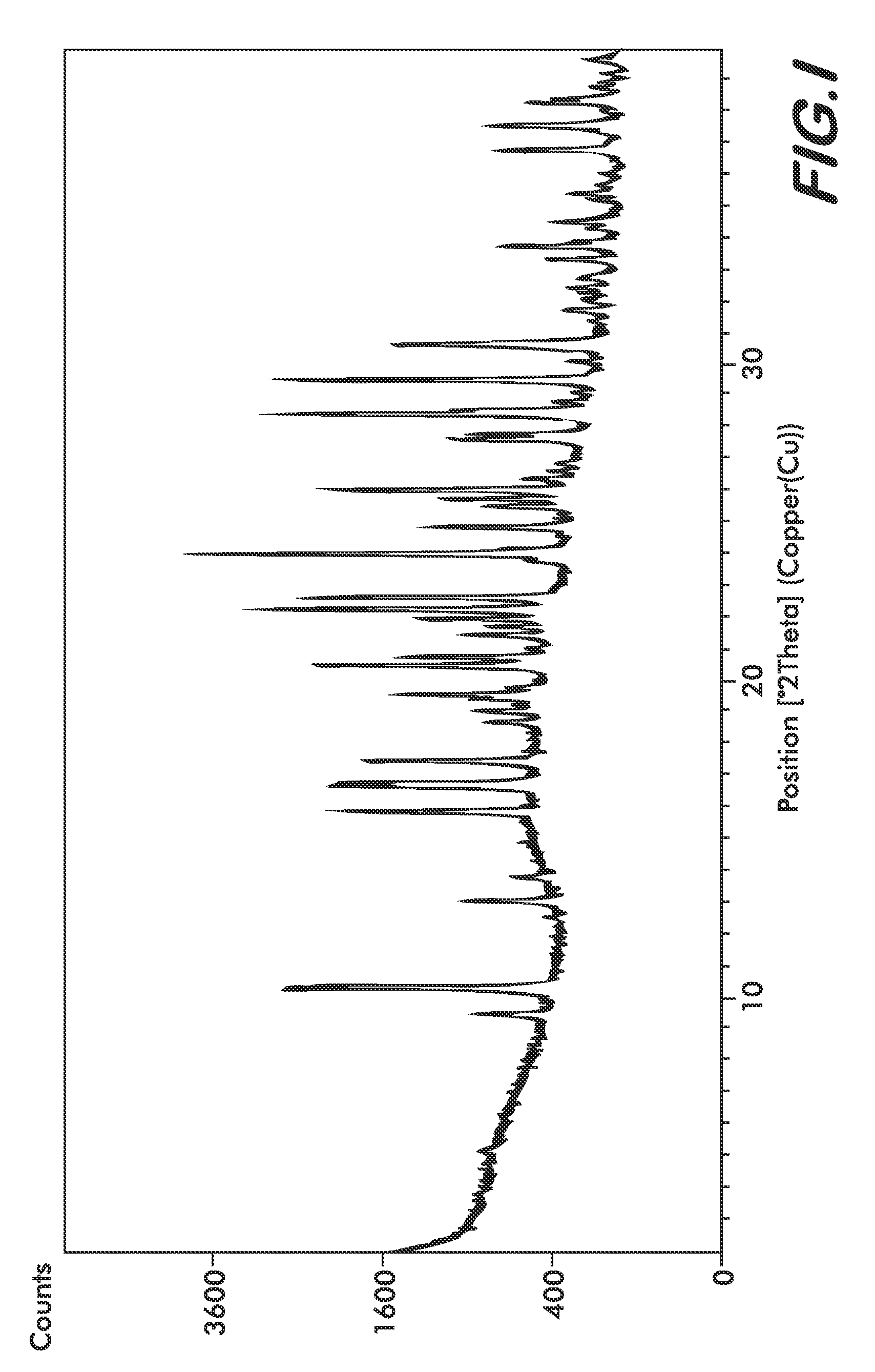
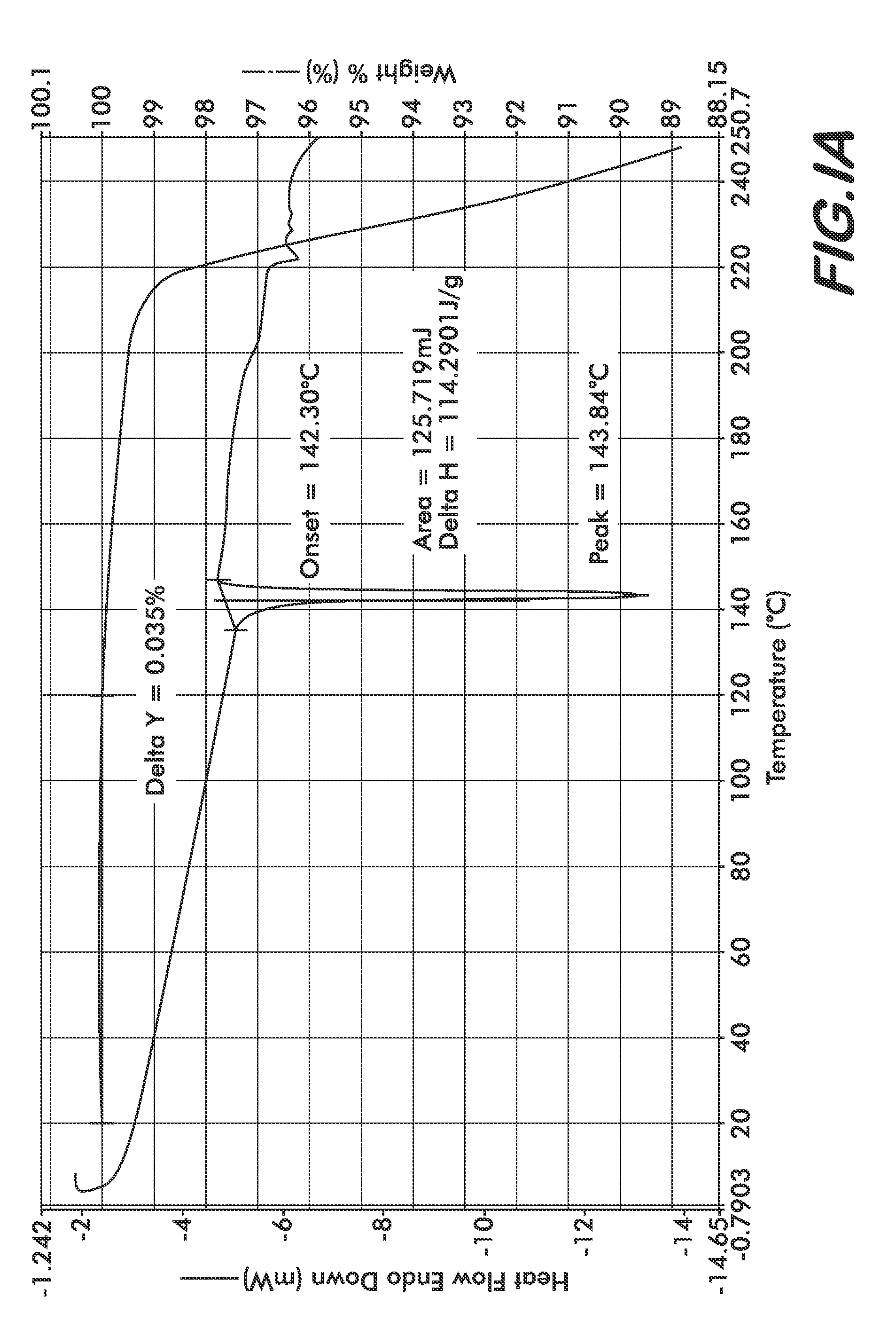
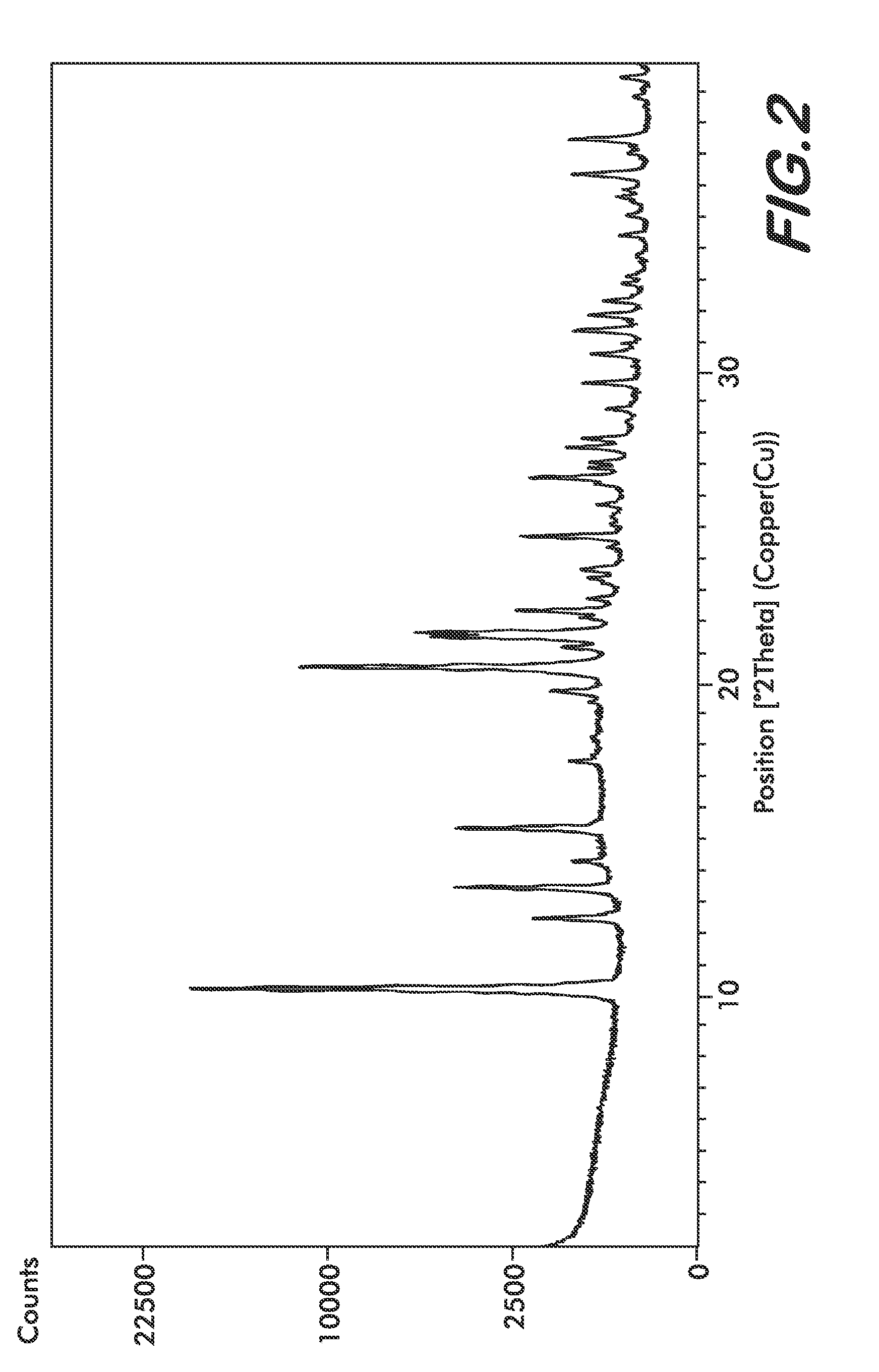
Formulations Containing Clopidogrel and Sulfoalkyl Ether Cyclodextrin and Methods of Use
ActiveUS20100292268A1Reduce chemical degradationReduce probabilityBiocideAntipyreticEtherCyclodextrin derivative
The present invention provides compositions containing clopidogrel, present as a free base or a pharmaceutically acceptable salt thereof, and sulfoalkyl ether cyclodextrin (SAE-CD). The compositions can be liquid, suspension or solid compositions. They can be adapted for oral, peroral or parenteral administration. The SAE-CD serves to aid in dissolution and stabilization of the clopidogrel in aqueous media. The stability of clopidogrel against hydrolytic degradation, thermal degradation, and photolytic degradation are improved. SAE-CD provides improved results over other cyclodextrin derivatives. The SAE-CD-containing composition of clopidogrel can be provided in liquid form, solid form or as a reconstitutable powder. Both ready-to-use and concentrated liquid compositions can be prepared. The liquid composition is optionally available as a clear solution. The compositions herein can be administered perorally or parenterally and provide substantial pharmacokinetic, pharmacodynamic and / or therapeutic advantages over a tablet composition administered perorally and excluding SAE-CD.
Owner:CYDEX PHARMACEUTICALS INC
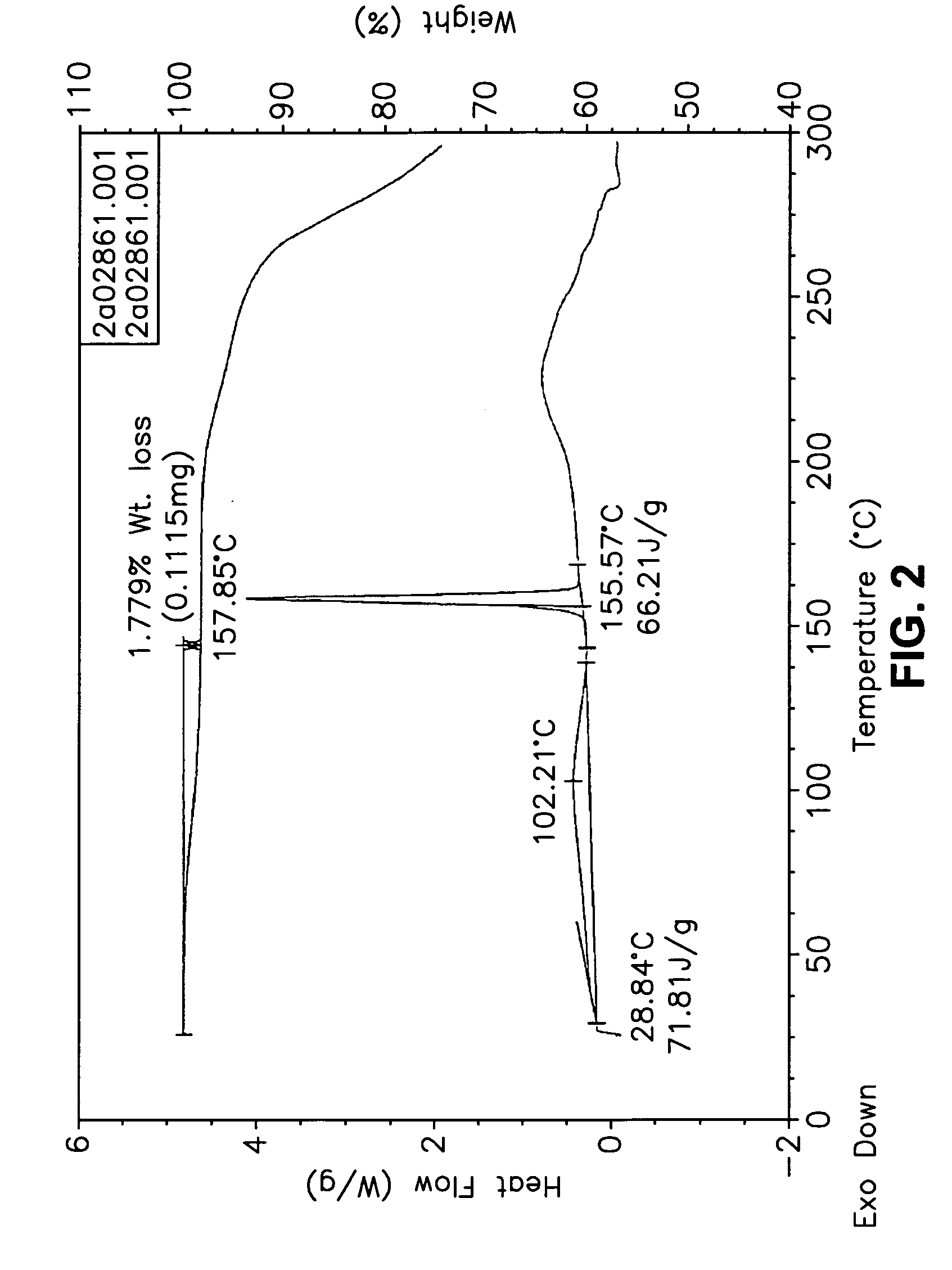
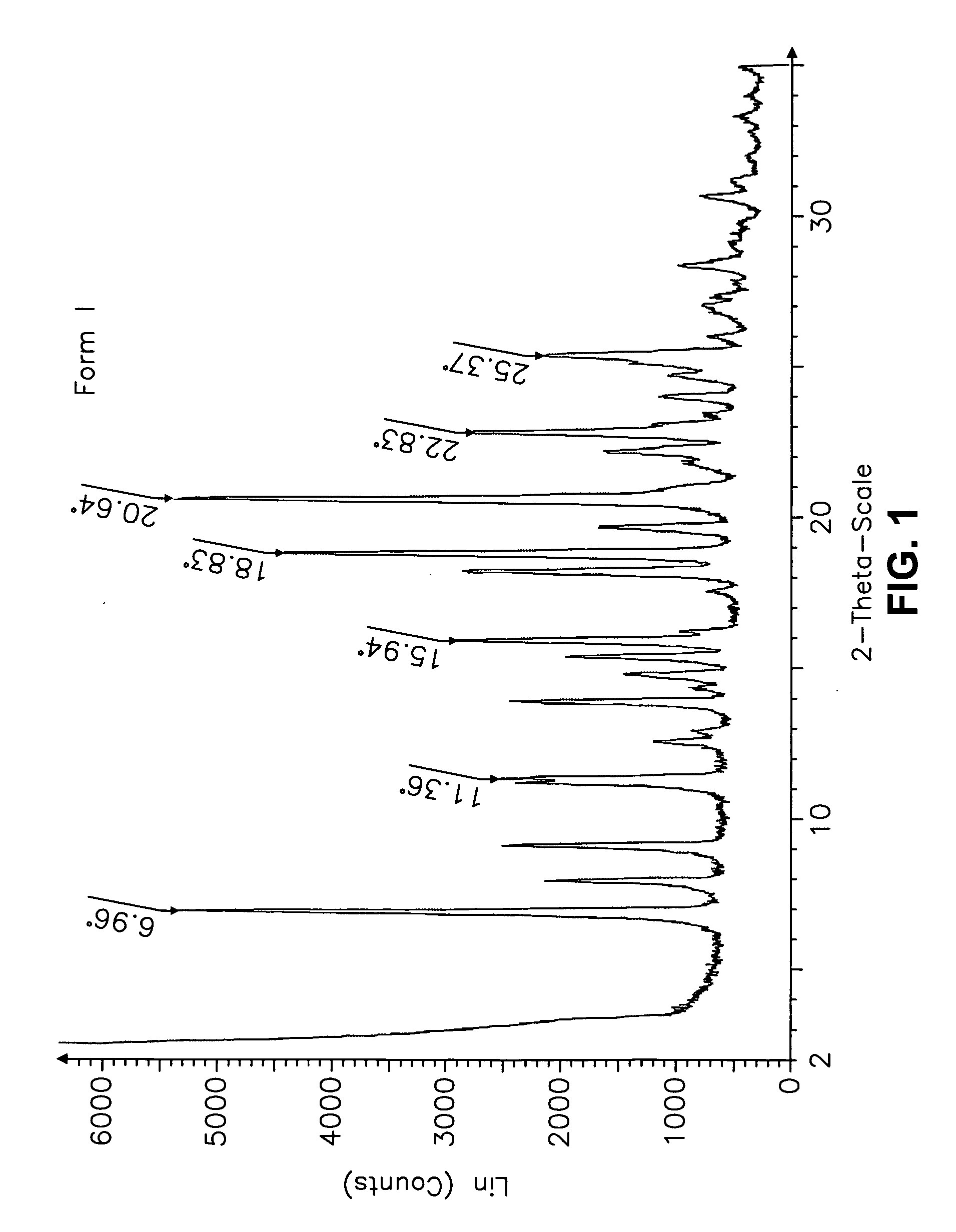
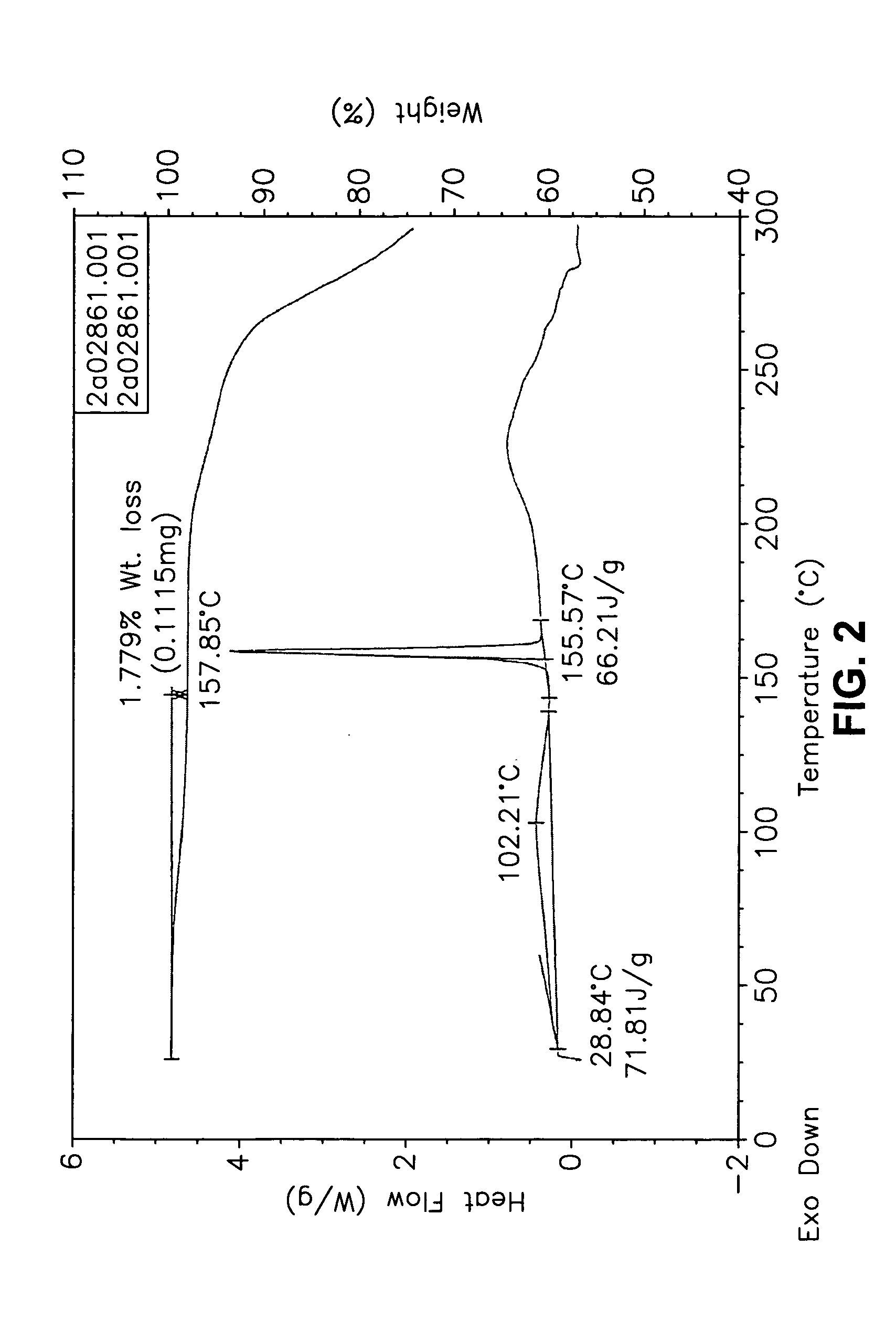
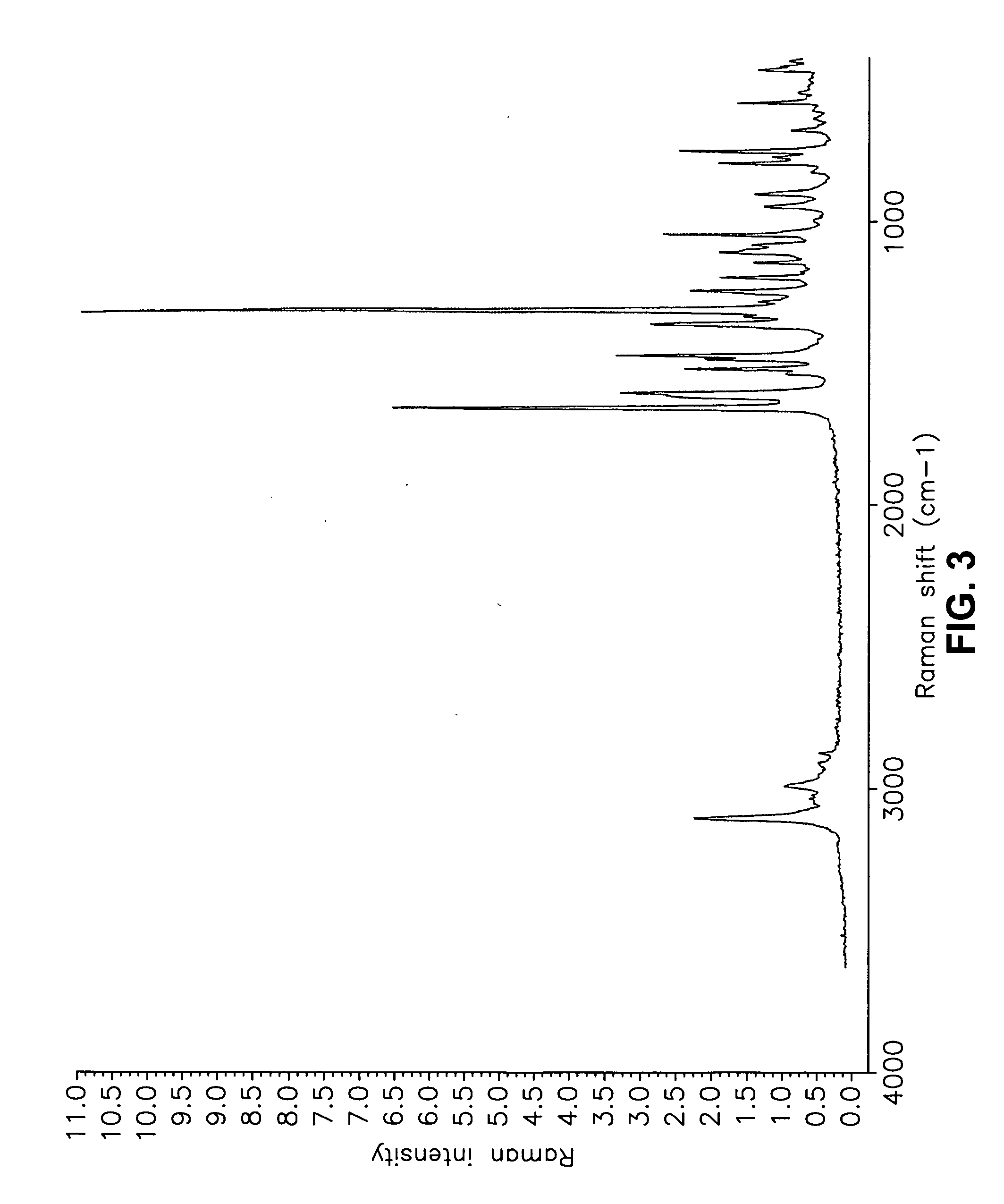
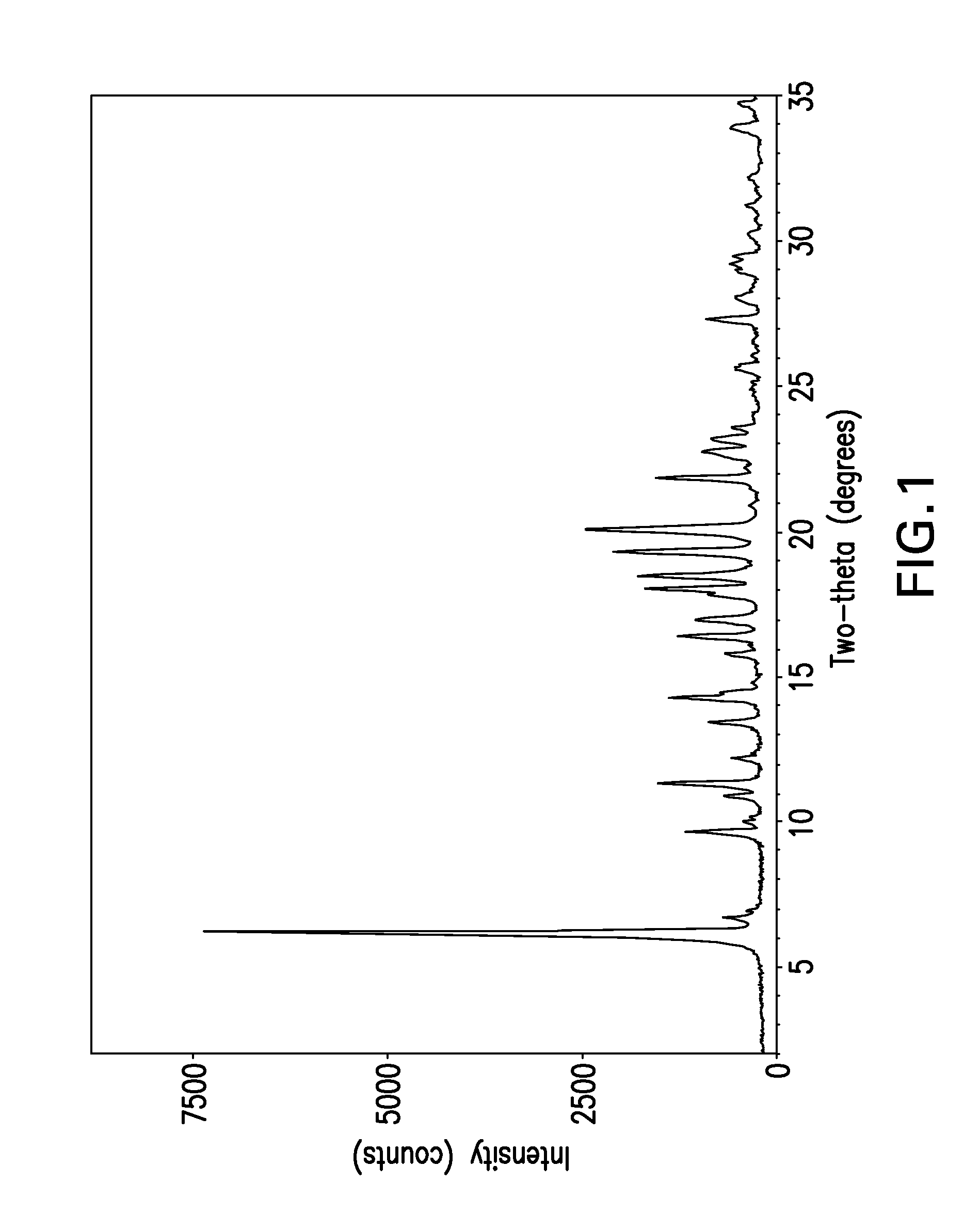
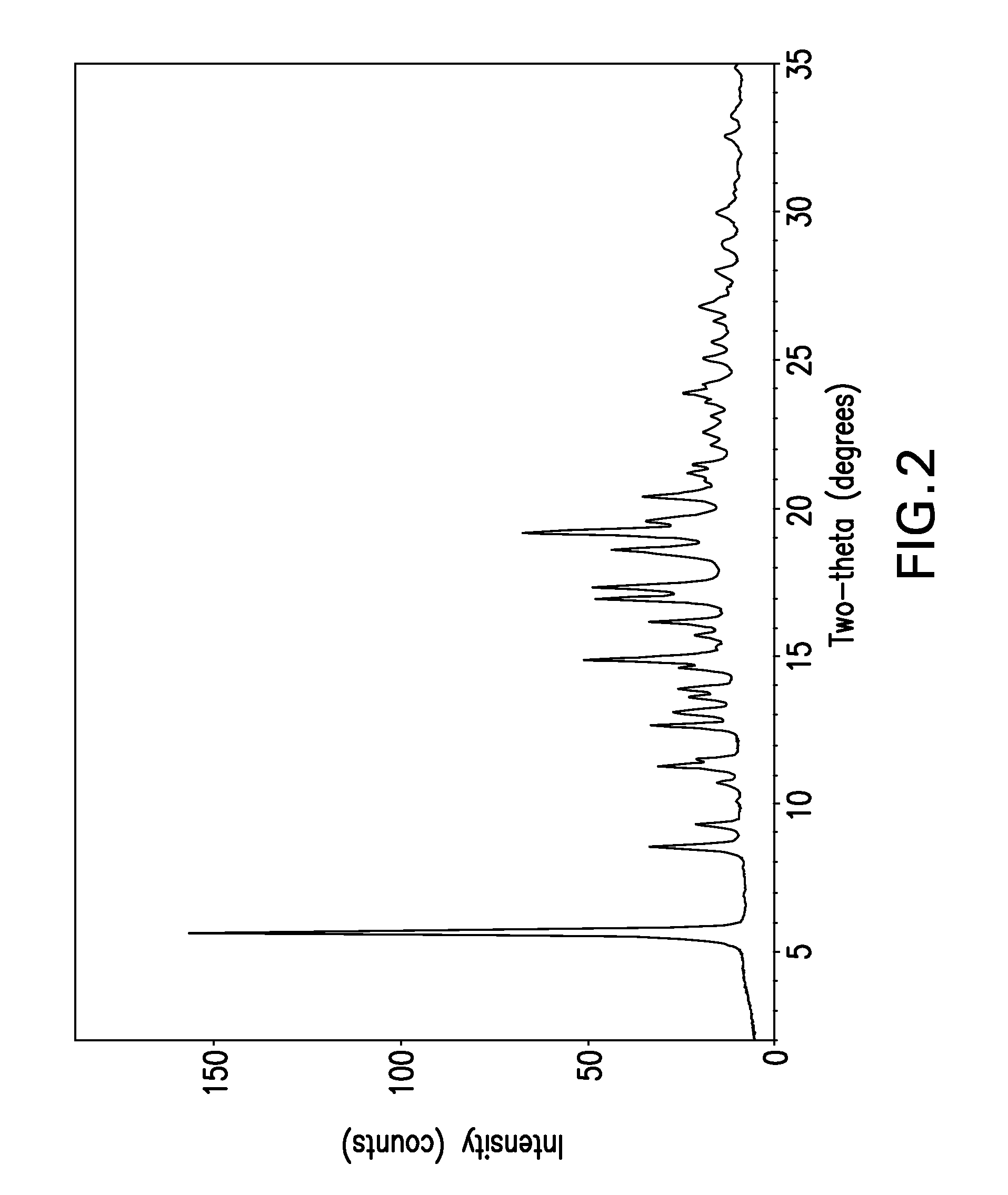
Forms of bendamustine free base
Novel polymorphic forms of bendamustine free base are described, including amorphous bendamustine free base, six anhydrous crystalline forms, four hydrate forms, and five solvate forms, with methods of their preparation and use also being described.
Owner:CEPHALON INC
Antimicrobial medical devices containing chlorhexidine free base and salt
InactiveUS7329412B2Avoid adverse reactionsImprove antibacterial propertiesSuture equipmentsOrganic active ingredientsChloraPrepDentistry
The present disclosure invention relates to medical devices treated with a solution comprising one or more solvents and a combination of chlorhexidine free base and a water-soluble chlorhexidine salt in a weight / weight ratio of between about 1:1 to about 1:5, preferably about 1:1.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Anthranilic Esters as Additives in Lubricants
This invention relates to anthranilate esters and their use in lubricants, such as engine oils. The invention particularly relates to compositions that deliver an ash-free base to a lubricant in the form of a basic amine additive, without adversely impacting seal compatibility and / or degradation, and methods thereof.
Owner:THE LUBRIZOL CORP
Carvedilol free base, salts, anhydrous forms or solvates thereof, corresponding pharmaceutical compositions, controlled release formulations, and treatment or delivery methods
The present invention also relates to carvedilol free base, salts, anhydrous forms, or solvates thereof, corresponding pharmaceutical compositions or controlled release formulations, and methods delivery of carvedilol forms to the lower gastrointestingal tract or methods to treat cardiovascular diseases, which may include, but are not limited to hypertension, congestive heart failure, and angina. The present invention relates to control release formulations, which comprise various cavedilol forms, which may include, but are not limited to carvedilol free base and corresponding carvedilol salts, anhydrous forms or solvates thereof.
Owner:BURKE MATTHEW D +7
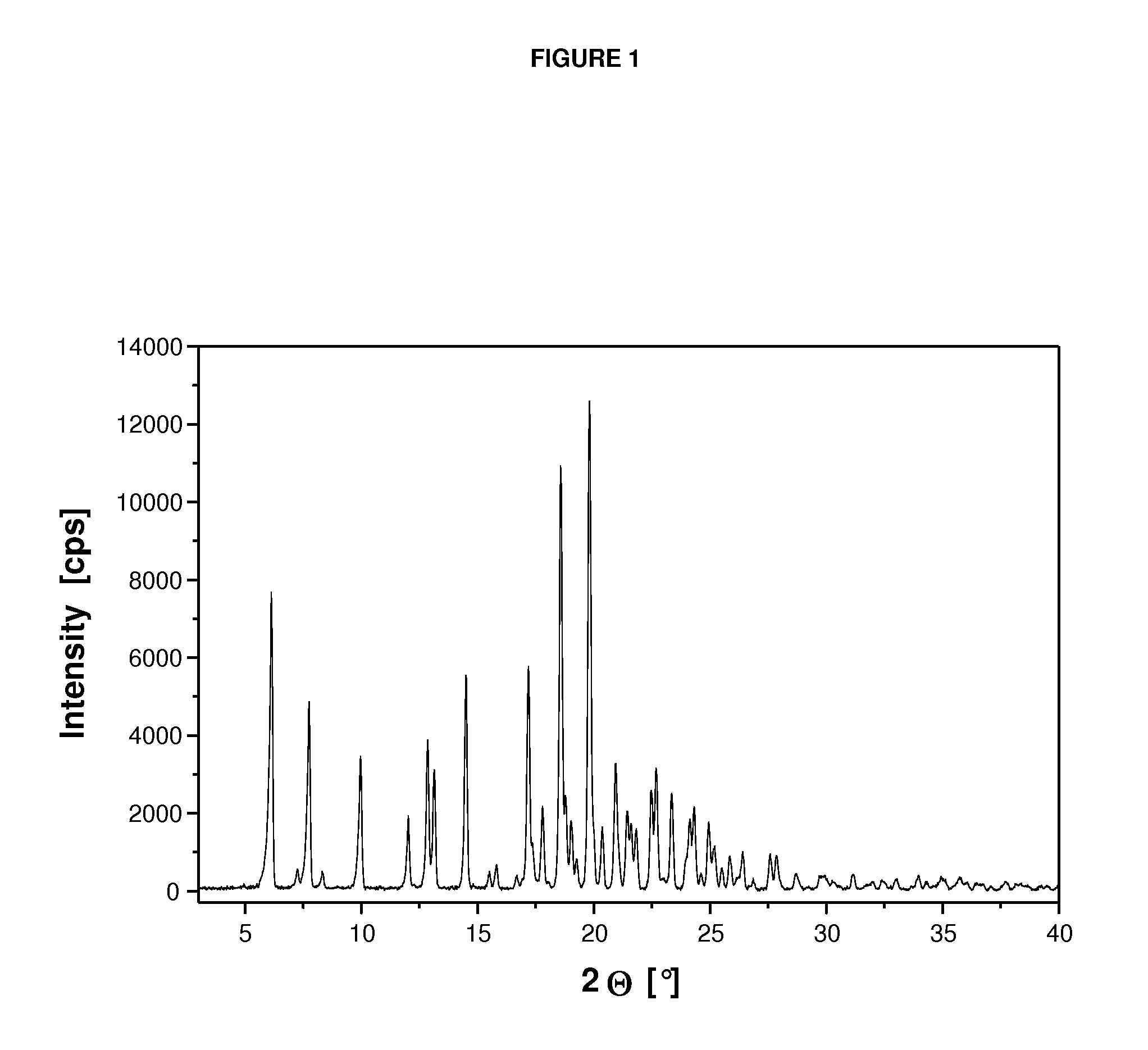
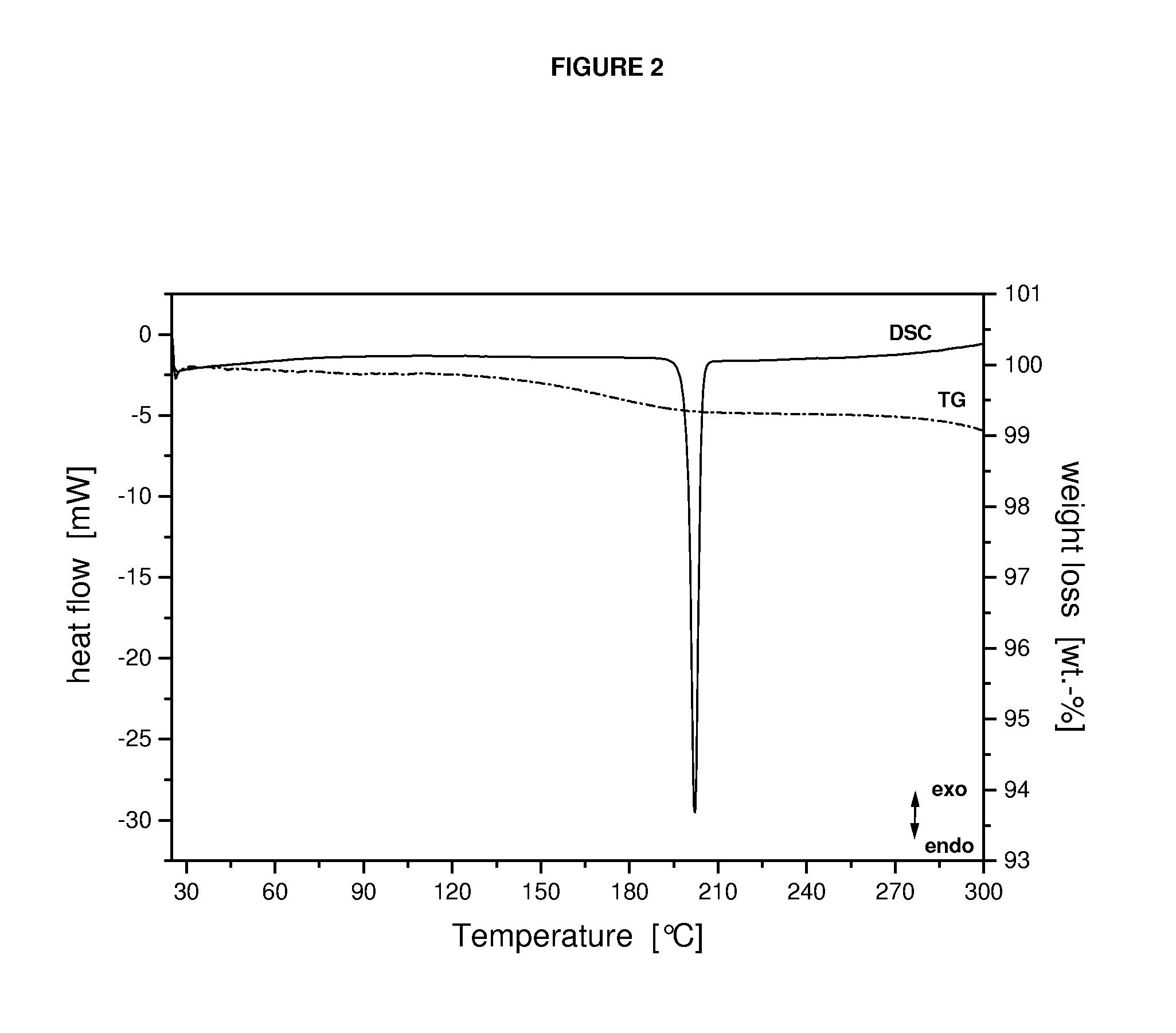
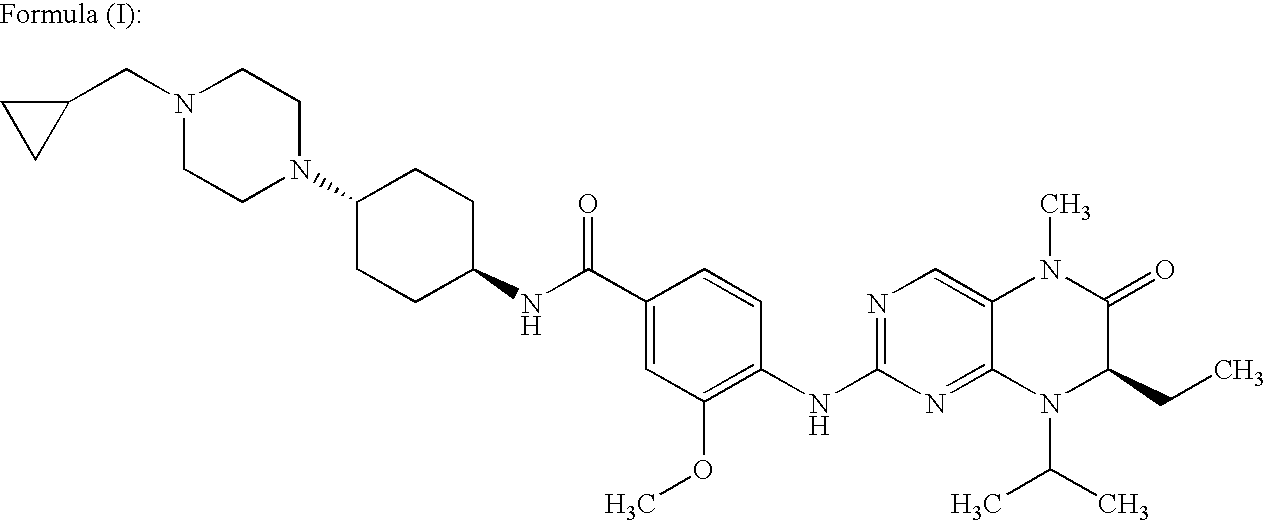
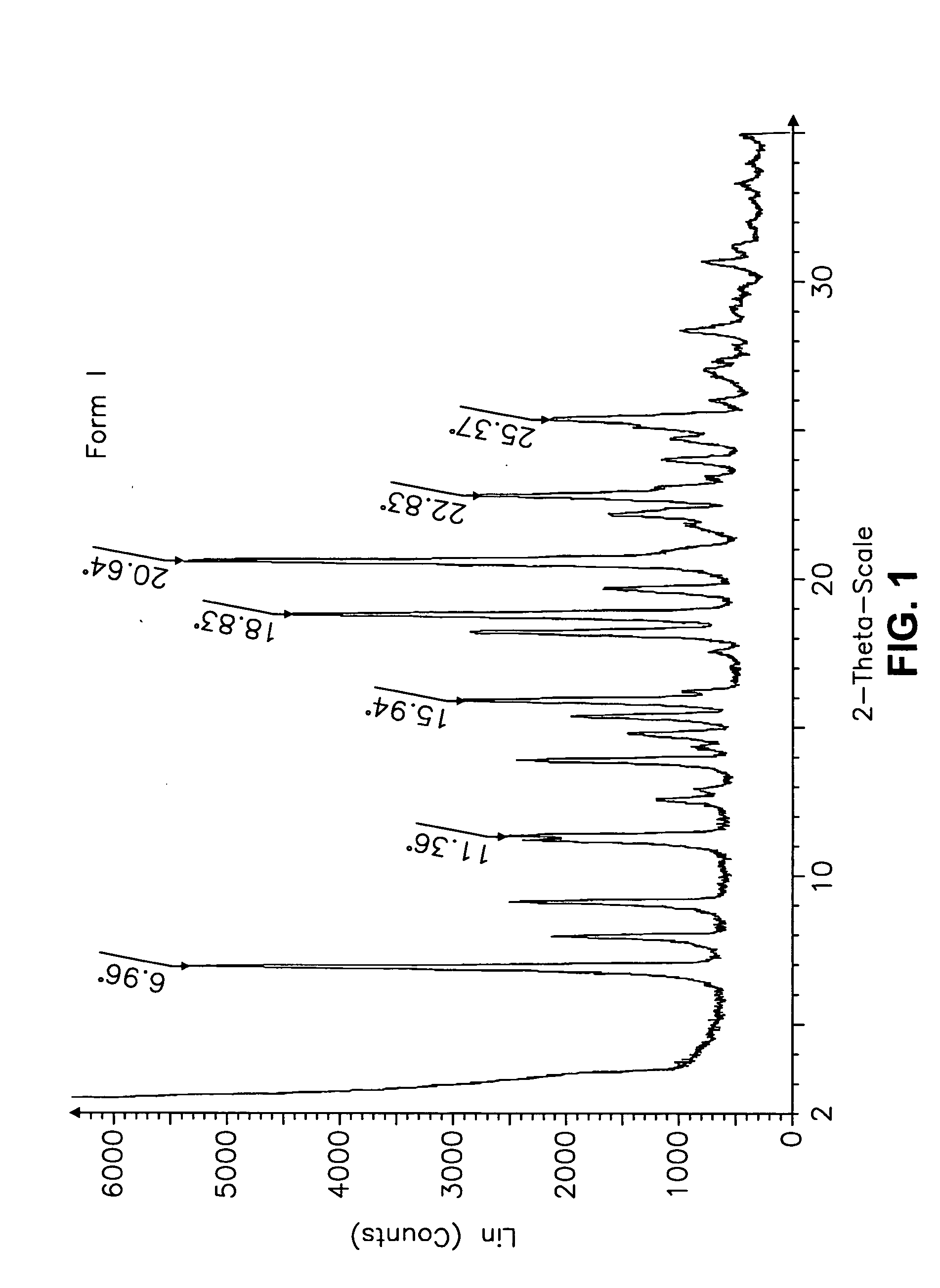
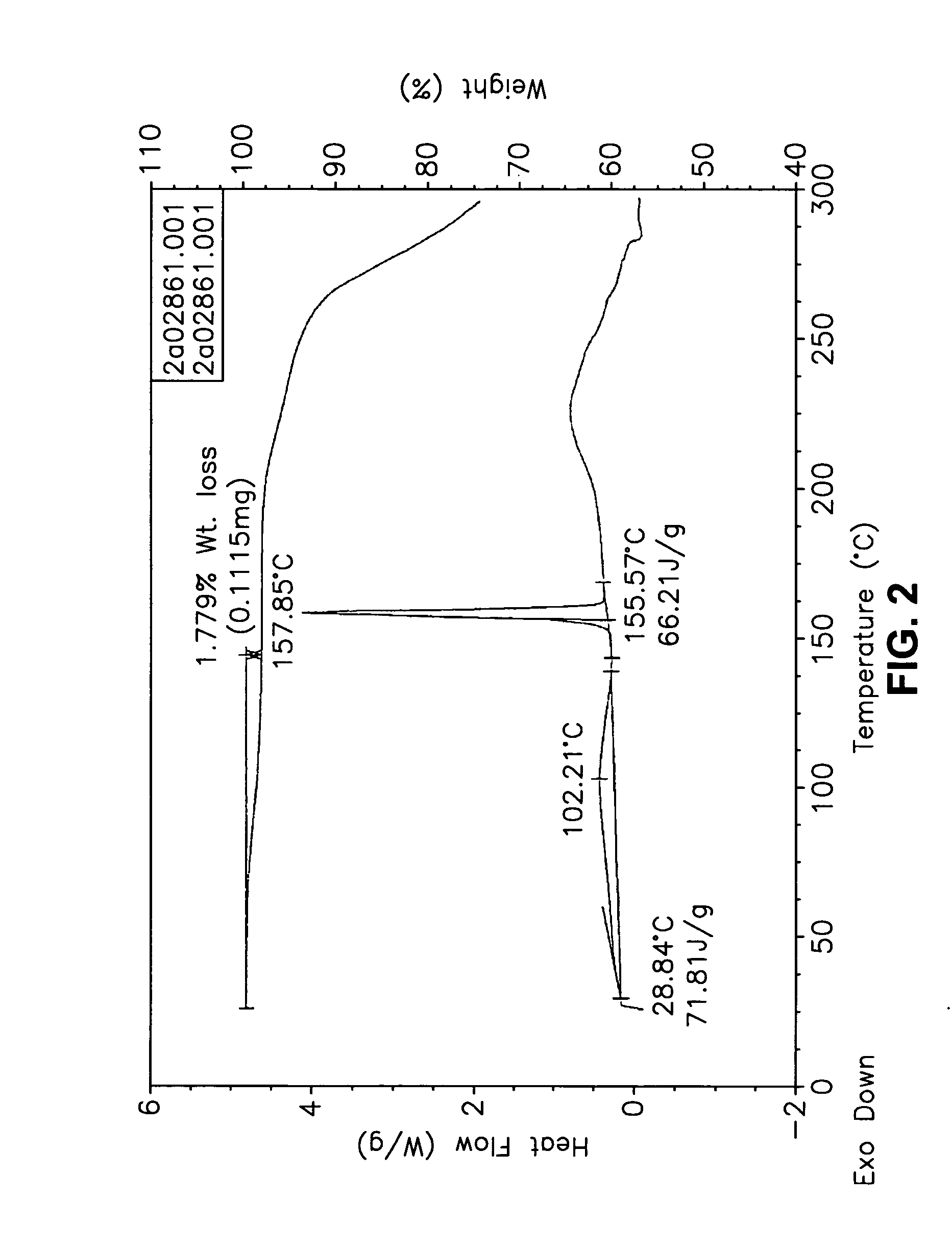
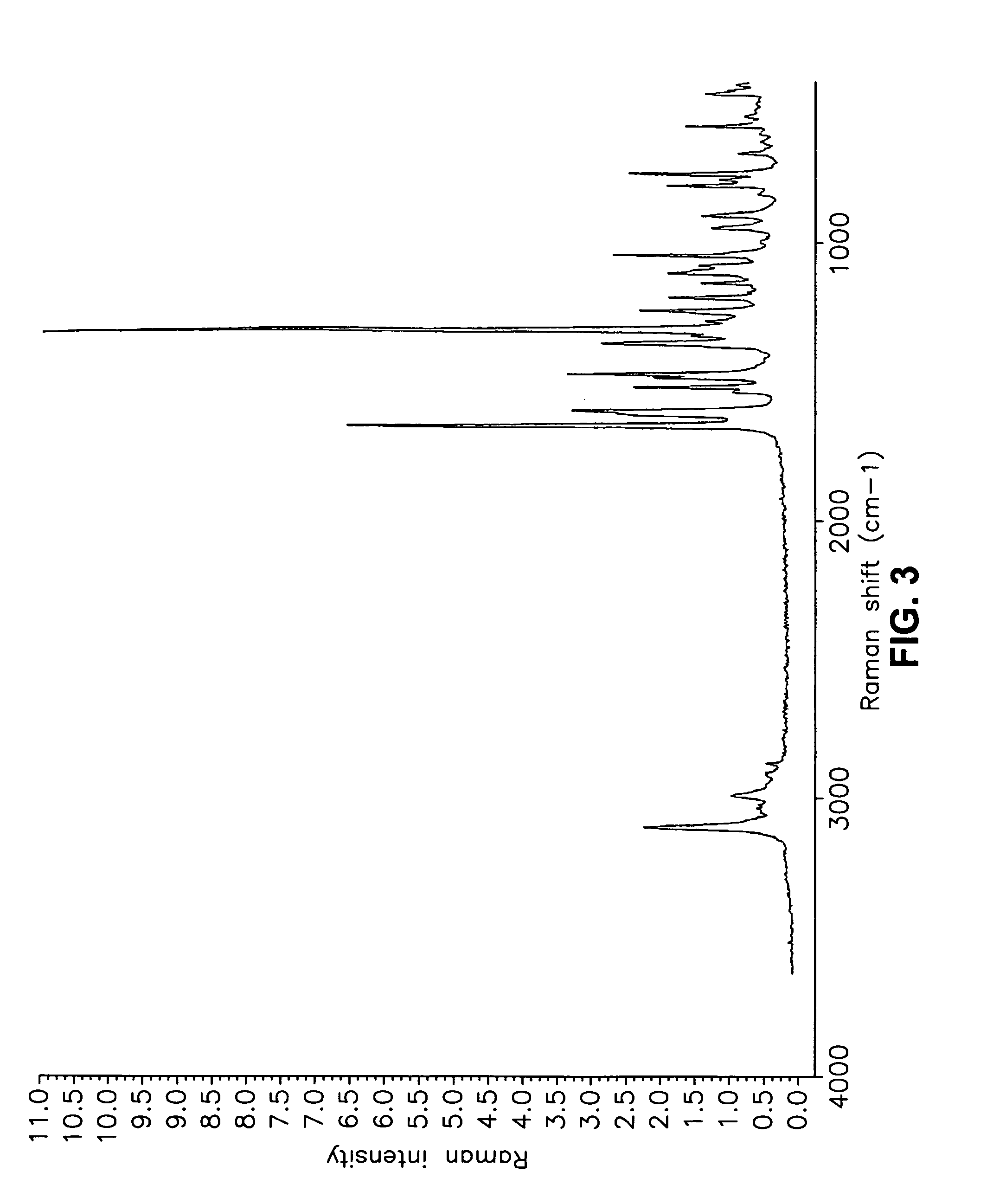
Crystalline form of a dihydropteridione derivative
The present invention relates to a crystalline form a dihydropteridione derivative, namely a crystalline form of the free base N-[trans-4-[4-(cyclopropylmethyl)-1-piperazinyl]cyclohexyl]-4-[[(7R)-7-ethyl-5,6,7,8-tetrahydro-5-methyl-8-(1-methylethyl)-6-oxo-2-pteridinyl]amino]-3-methoxy-benzamide, to a process for the manufacture thereof, and to the use thereof in a pharmaceutical composition.
Owner:BOEHRINGER INGELHEIM INT GMBH
Carvedilol free base, salts, anhydrous forms or solvates thereof, corresponding pharmaceutical compositions, controlled release formulations, and treatment or delivery methods
The present invention also relates to carvedilol free base, carvedilol salts, anhydrous forms, or solvates thereof, corresponding pharmaceutical compositions or controlled release formulations, and delivery or dosing methods of carvedilol forms to the lower gastrointestingal tract or methods to treat cardiovascular diseases, which may include, but are not limited to hypertension, congestive heart failure, atherosclerosis, and angina. The present invention relates to controlled release formulations, which comprise various carvedilol forms, which may include, but are not limited to a carvedilol free base or corresponding carvedilol salts, anhydrous forms or solvates thereof.
Owner:FLAMEL TECHNOLOGIES +1
Carvedilol free base, salts, anhydrous forms or solvates thereof, corresponding pharmaceutical compositions, controlled release formulations, and treatment or delivery methods
The present invention also relates to carvedilol free base, salts, anhydrous forms, or solvates thereof, corresponding pharmaceutical compositions or controlled release formulations, and methods delivery of carvedilol forms to the lower gastrointestingal tract or methods to treat cardiovascular diseases, which may include, but are not limited to hypertension, congestive heart failure, and angina. The present invention relates to control release formulations, which comprise various cavedilol forms, which may include, but are not limited to carvedilol free base and corresponding carvedilol salts, anhydrous forms or solvates thereof.
Owner:SMITHKLINE BEECHAM (CORK) LTD
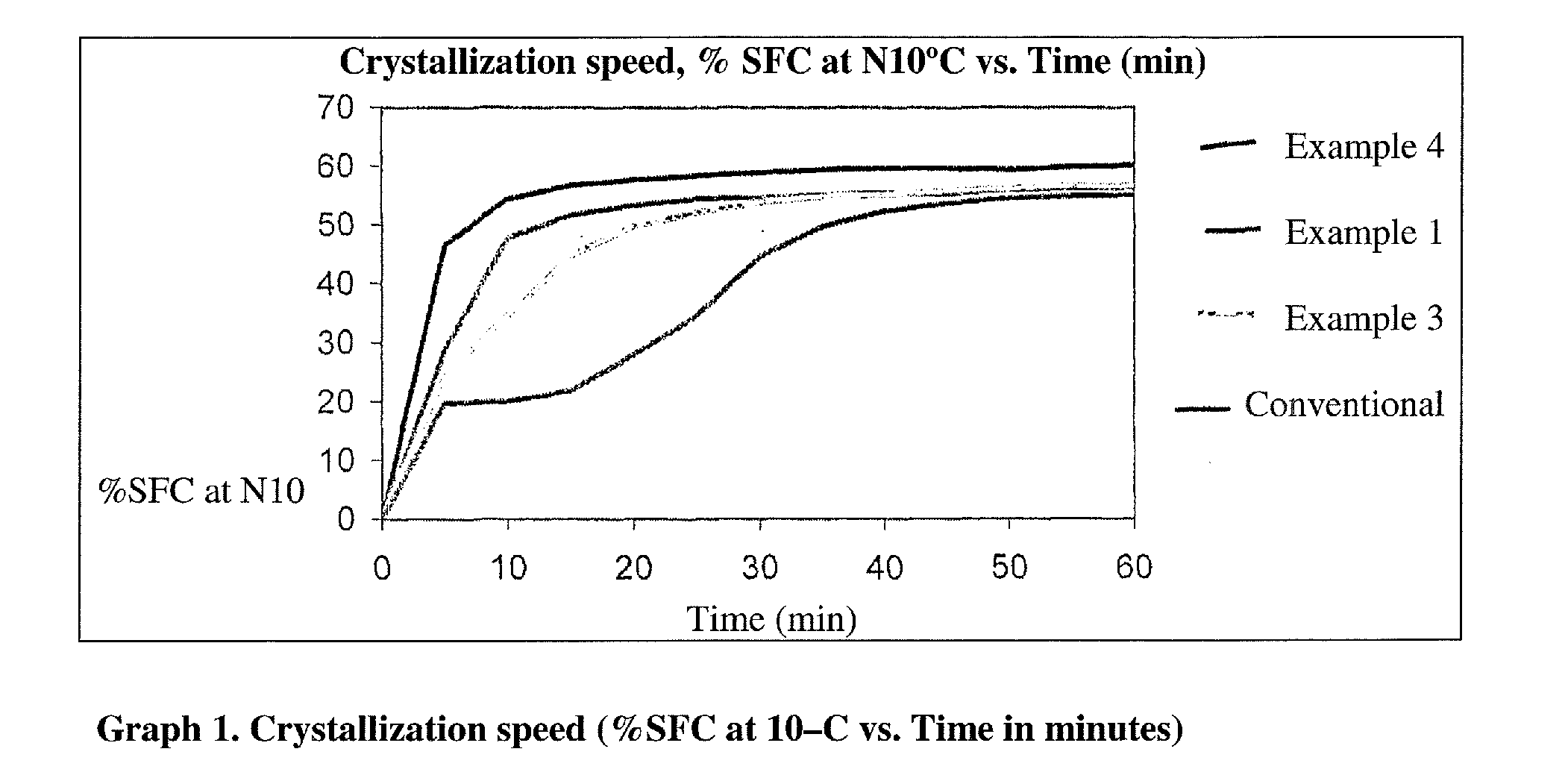
Trans-free fat base for application in filling creams
InactiveUS20110177227A1Great tasteAvoid layeringFatty acid esterificationFatty-oils/fats separationStearic acidOleic Acid Triglyceride
A fat base is described comprising a mixture containing from 4 to 20% C12:0 lauric acid, preferentially from 5 to 17%, from 30 to 50% C16:0 palmitic acid, preferentially from 34 to 45%, and 4 to 10% C18:0 stearic acid, preferentially from 4.5 to 7.5%, and from 20 to 40% C18:1 oleic acid, preferentially from 25 to 35%, the balance being a mixture of other C4:0 to C22:0 fatty acids, having application, for example, as filling for wafer biscuits.
Owner:TEAM FOODS COLOMBIA
Hydroxybenzoate salts of metanicotine compounds
InactiveUS20060122237A1Prevent and suppress symptomsIncrease the number ofBiocideNervous disorderDouble bondMedicinal chemistry
Patients susceptible to or suffering from conditions and disorders, such as central nervous system disorders, are treated by administering to a patient in need thereof compositions that are hydroxybenzoate salts of E-metanicotine-type compounds. The formation of hydroxybenzoate salts of the E-metanicotine compounds is also useful in purifying the E-metanicotine compounds, as the hydroxybenzoate salts tend to crystallize out, leaving impurities such as Z-metanicotine compounds, and compounds where the double bond has migrated, in solution. If desired, the hydroxybenzoate salts can be converted to either the free base (the E-metanicotine) or to another pharmaceutically acceptable salt form.
Owner:TARGACEPT INC
Epoxy modified silicon-contained waterborne acrylic resin and coating thereof
The invention provides an epoxy modified silicon-contained waterborne acrylic resin which is prepared by the following steps of: carrying out addition reaction on epoxy resin and acrylic acid to produce epoxy monoacrylate resin; and then carrying out free-base co-polymerization by taking the epoxy monoacrylate resin as a monomer and matching an acrylic acid monomer and an amio silicone monomer to obtain a product. The method overcomes the defects of overlarge viscosity, inconvenient operation, and the like during reaction caused by the fact that the epoxy resin is directly inoculated with the acrylic acid in the past; the resin is matched with soluble amino resin to form a film forming matter, then the resin is matched with relevant pigment and assistants to obtain a waterborne coating which can form a film after solidified at 70-80 DEG C for 20 min when used for the surface of glass; and epoxy amino can obtain excellent adhesive force through solidification and has excellent acid-alkali resistance. The amio silicone monomer has the performance of being baked and solidified at low temperature and achieves fine flexibility, and still has higher hardness and better adhesive force after being water-tolerant, thereby overcoming the defect of being scratched by hard matters in damp and hot environments.
Owner:常州市弘可利办公用品有限公司 +1
Foam Oxidative Hair Colorant Composition with the Free-Base of 1,4-Diamino-2-Methoxymethyl Benzene
ActiveUS20130081647A1Increase shear viscosityReduce shearCosmetic preparationsHair cosmeticsHair ColorantsMethoxymethylbenzene
An oxidative hair colorant composition to be dispensed from a manually-actuable, non-aerosol dispenser as a foam. The oxidative hair colorant composition contains the free-base of 1,4-diamino-2-methoxymethyl benzene to achieve for efficient dye precursor levels in formulation and to achieve a desire rheology profile of the oxidative hair colorant composition.
Owner:WELLA OPERATIONS US LLC
Therapy for the treatment of disease
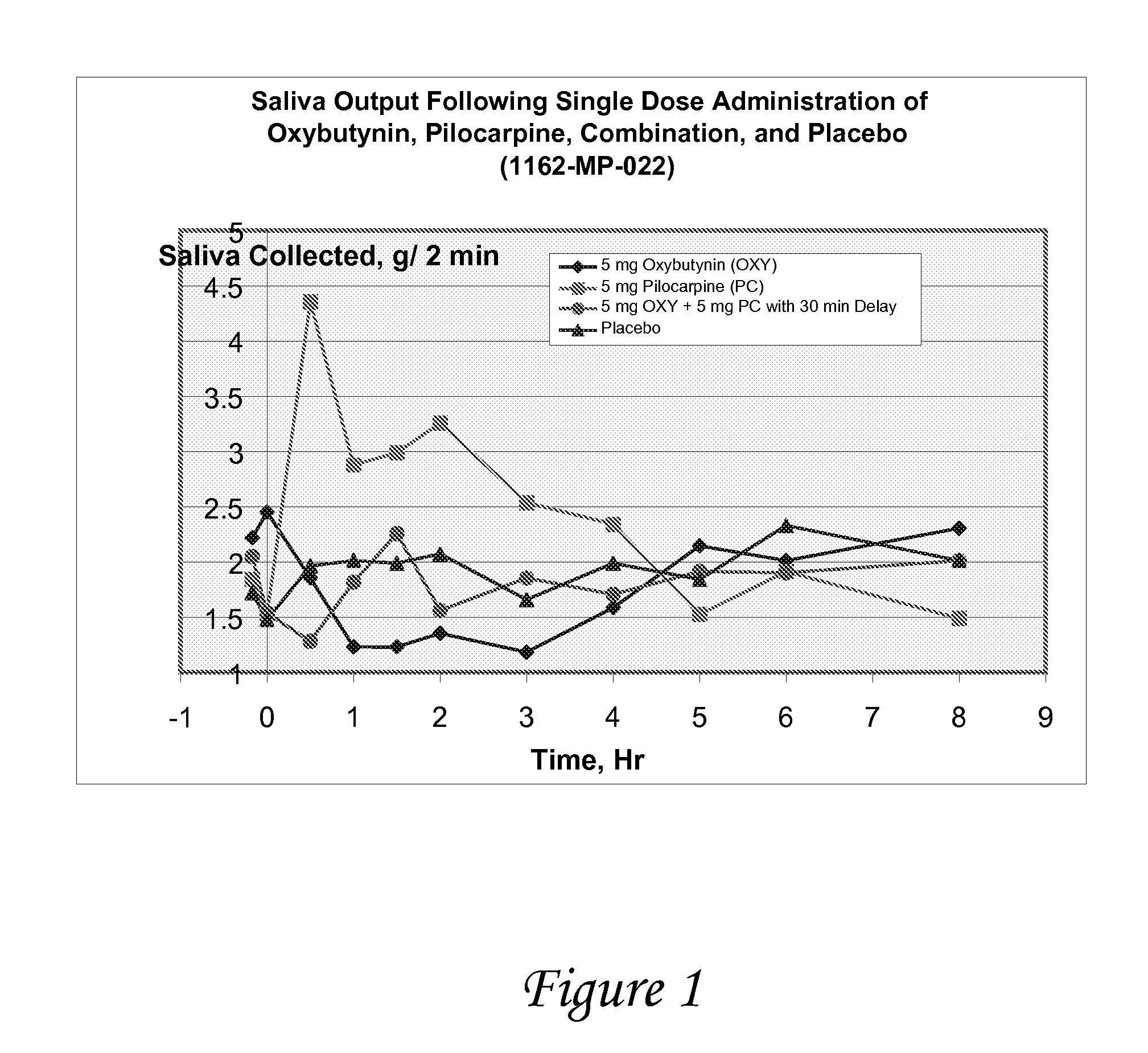
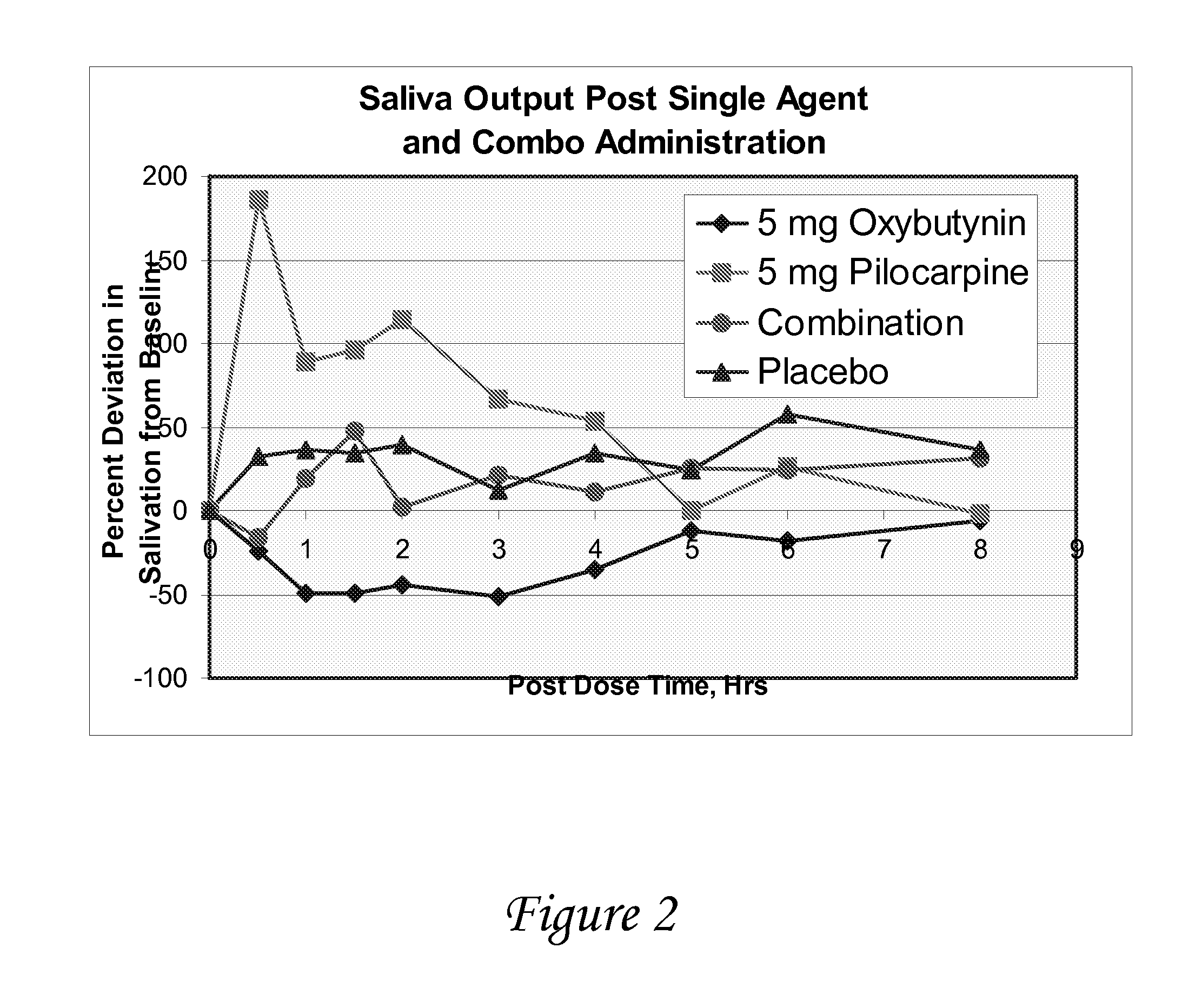
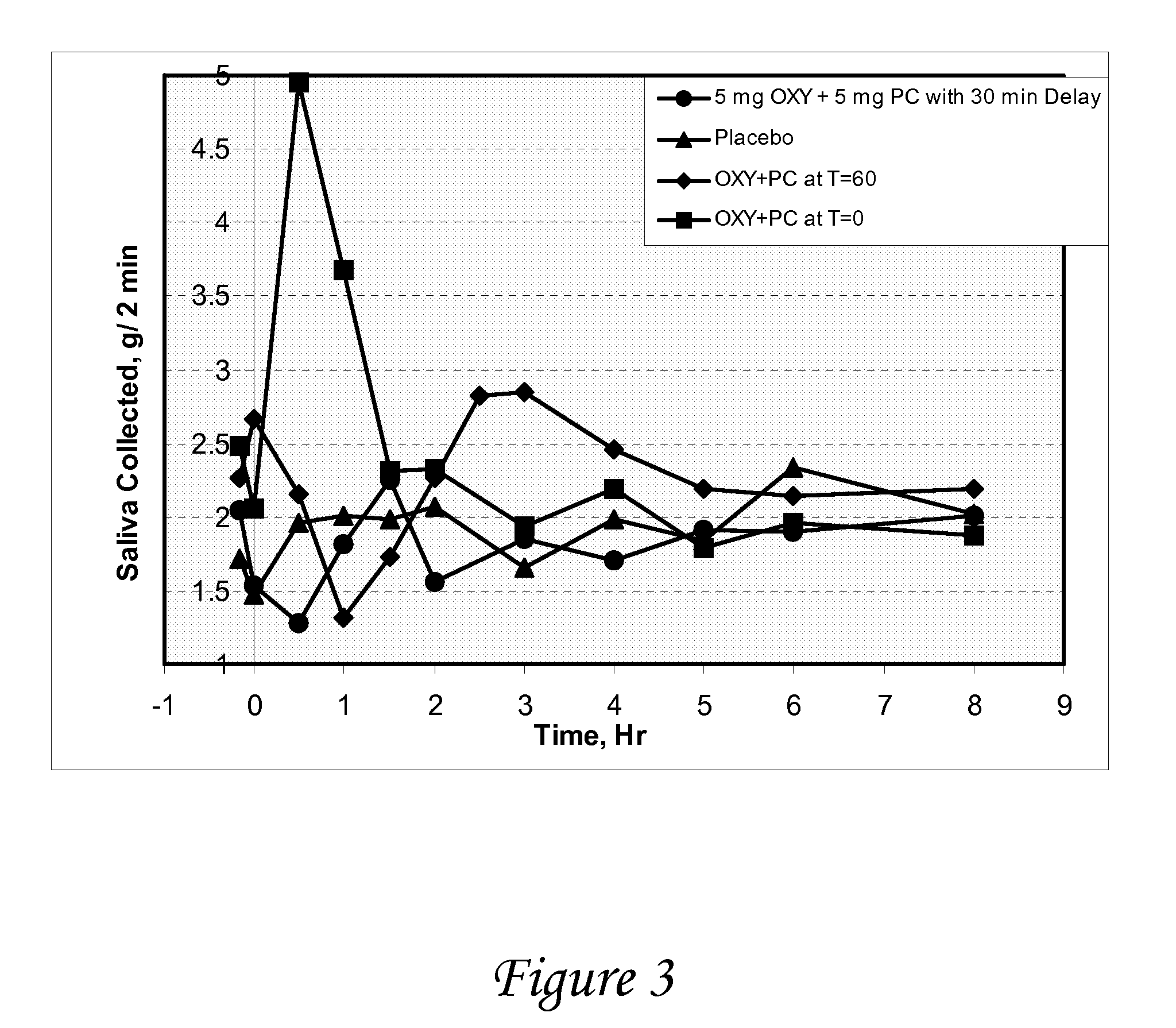
Disclosed herein are pharmaceutical compositions comprising oxybutynin, or a free base thereof or a pharmaceutically acceptable salt thereof, and pilocarpine, or a free base thereof or a pharmaceutically acceptable salt thereof. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS7955616B2Lower potentialMinimally inhibit osteogenesis and subsequent bone healingSurgical adhesivesSkeletal disorderPyrrolidinonesCarboxylic acid
The present application is directed to compositions and methods for mechanically controlling the bleeding of bone. The compositions comprise in intimate admixture the following Components 1, 2 and 3: (1) a finely powdered, carboxylic acid salt comprising a carboxylate anion and a metallic cation, (2) a composition comprising pyrrolidone or an N-alkyl pyrrolidone wherein alkyl is a C1-C12 alkyl radical and an optional, biocompatible liquefying agent if the composition is in solid form at room temperature, and (3) an optional analgesic, wherein the analgesic is present in a free base and salt form.
Owner:ABYRX
High concentration local anesthetic formulations
InactiveUS20090048296A1Ameliorate and inhibit painAmeliorate and eliminate painBiocideNervous disorderAnesthetic AgentHigh concentration
A transdermal topical anesthetic formulation, which can be used to ameliorate or inhibit neuropathic pain, has been developed. In the preferred embodiment, the topical anesthetic is a local anesthetic such as lidocaine, most preferably lidocaine free-base in a gel, and the dosage of the local anesthetic is effective in the painful area or immediately adjacent areas, to ameliorate or eliminate the pain. High concentration of local anesthetic in solution in the carrier is used to drive rapid release and uptake of the drug. Relief is typically obtained for a period of several hours.
Owner:CENTXION I INC CENTXION
Abt-263 crystalline forms
ActiveUS20110071151A1Purification less economicalPurification difficult and expensiveOrganic active ingredientsOrganic chemistry methodsDiseaseProtein C
Owner:ABBVIE INC
Antimicrobial medical devices
InactiveUS20020122876A1Improve antibacterial propertiesFacilitated releaseSuture equipmentsOrganic active ingredientsMedicineChlorhexidine
The present disclosure invention relates to medical devices treated with a solution comprising one or more solvents and a combination of chlorhexidine free base and a water-soluble chlorhexidine salt in a weight / weight ratio of between about 1:1 to about 1:5, preferably about 1:1.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Local anesthetic phospholipid-mixed solvent-oil sustained-release drug delivery system and preparation method thereof
ActiveCN108743952AExtended release timeLess irritatingAntipyreticAnalgesicsVitamin E AcetatePhospholipid
The invention relates to a local anesthetic sustained-release preparation with phospholipid-mixed solvent-oil as a carrier, and a preparation method thereof. The sustained-release preparation is prepared from local anesthetic as an active ingredient and a mixture of phospholipid-mixed solvent-oil as a sustained-release drug delivery system, and selectively is prepared from an antioxidant, whereinthe local anesthetic is selected from one of bupivacaine or ropivacaine free base or a mixture thereof; the mixed solvent is a mixture of benzyl benzoate and one or two of benzyl alcohol, ethanol; theantioxidant is vitamin E acetate, lipoic acid and the like; in the phospholipid-mixed solvent-oil mixture, the content of phospholipid does not exceed 50%, the content of benzyl benzoate does not exceed 10%, and the rest is oil.
Owner:XIAN LIBANG PHARMA TECH
Acid Salt Forms of Polymer-Drug Conjugates and Alkoxylation Methods
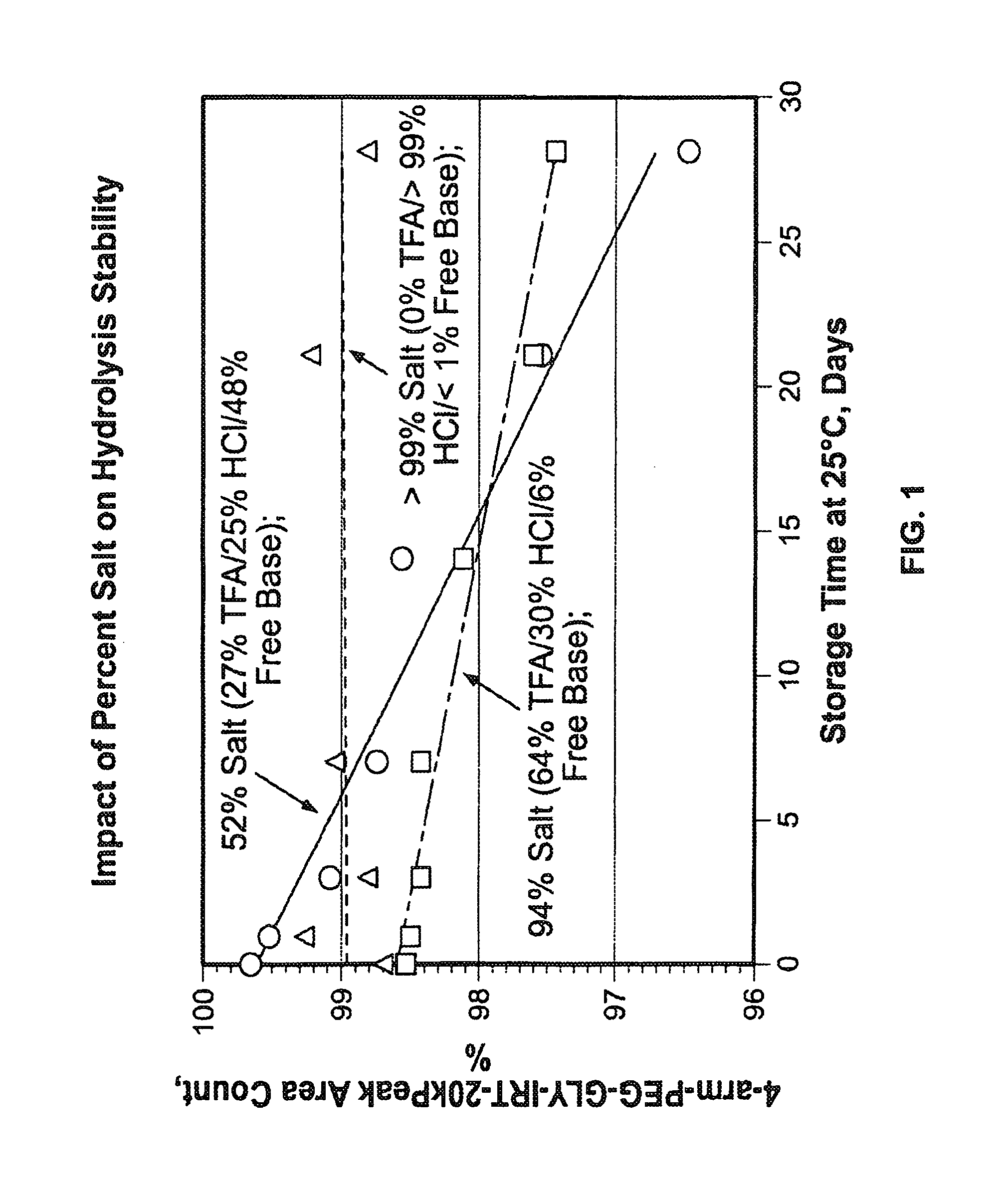
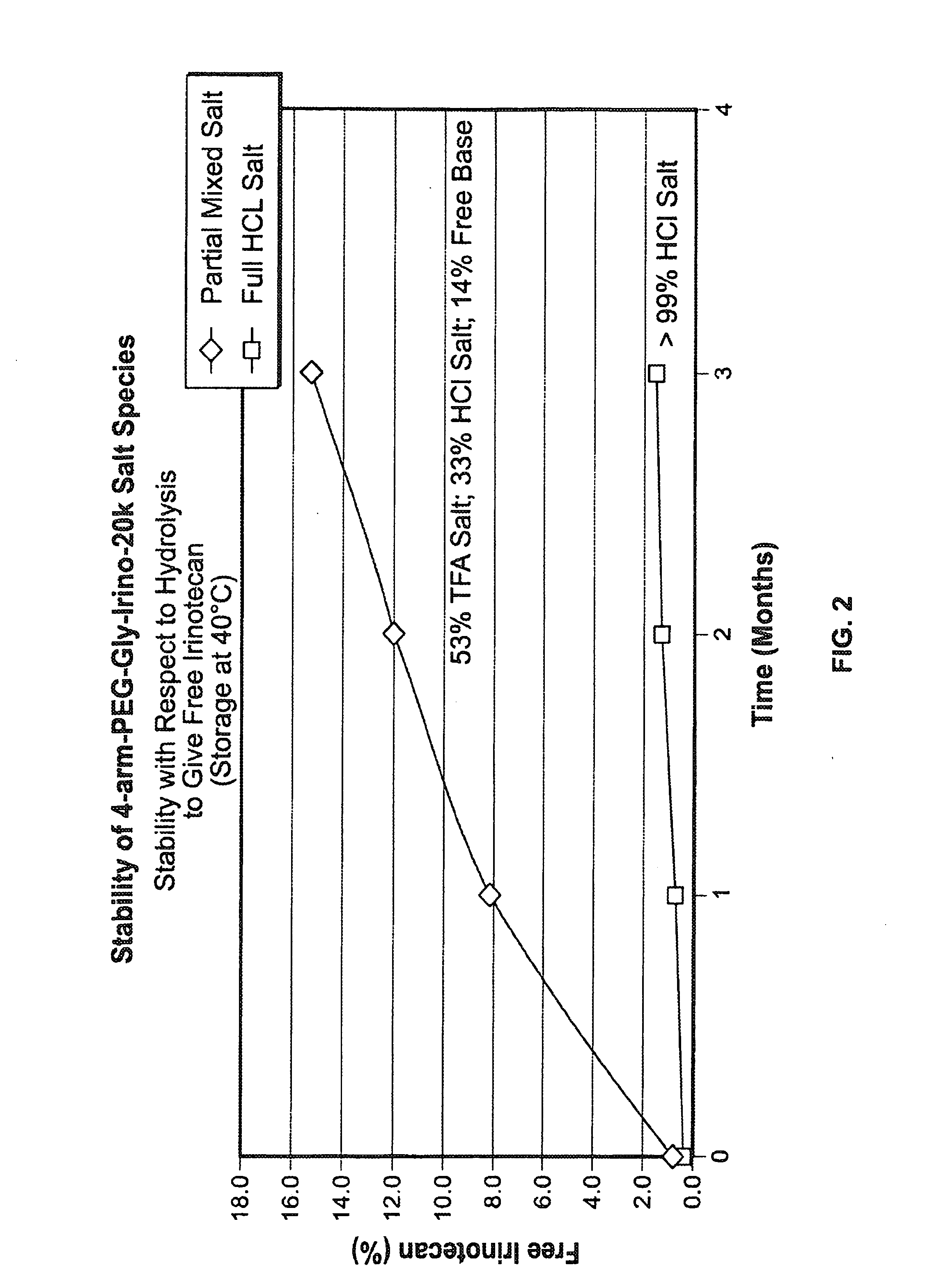
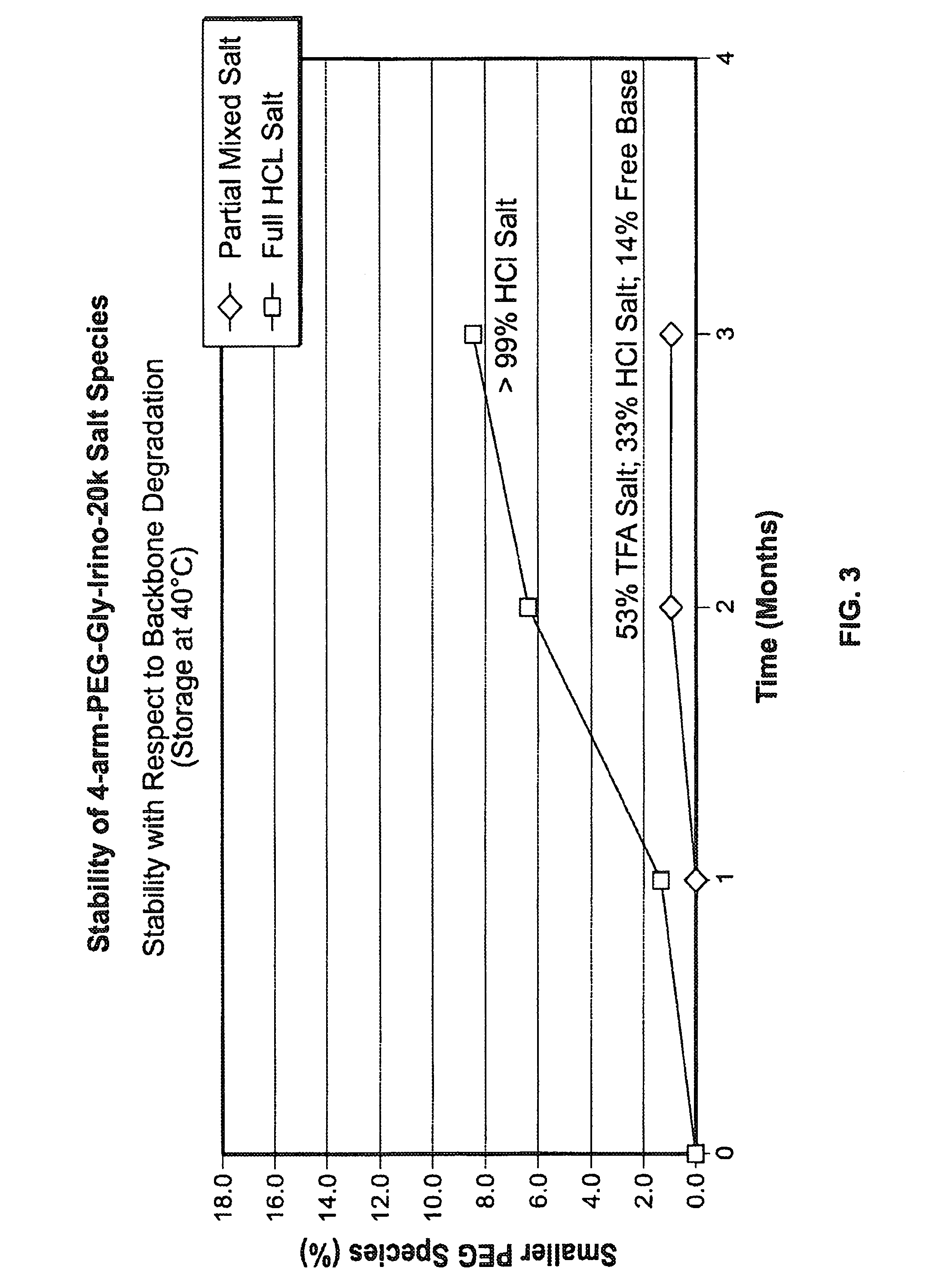
Among other aspects, provided herein is a mixed-acid salt of a water-soluble polymer-drug conjugate, along with related methods of making and using the same. The mixed-salt acid salt is stably formed, and appears to be more resistant to hydrolytic degradation than the corresponding predominantly pure acid salt or free base forms of the polymer-drug conjugate. The mixed acid salt is reproducibly prepared and recovered, and provides surprising advantages over non-mixed acid salt forms of the water-soluble polymer drug conjugate.
Owner:NEKTAR THERAPEUTICS INC
Process for preparing a dipeptidyl peptidase IV inhibitor and intermediates employed therein
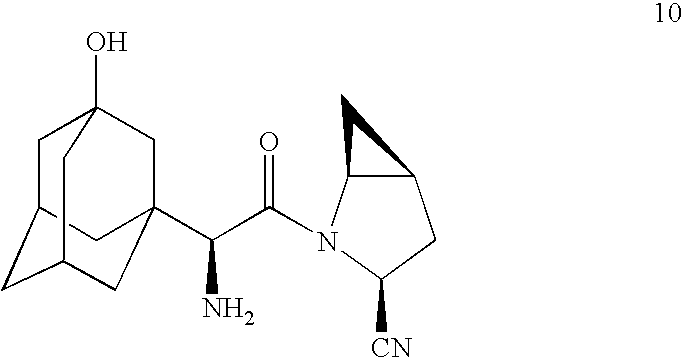
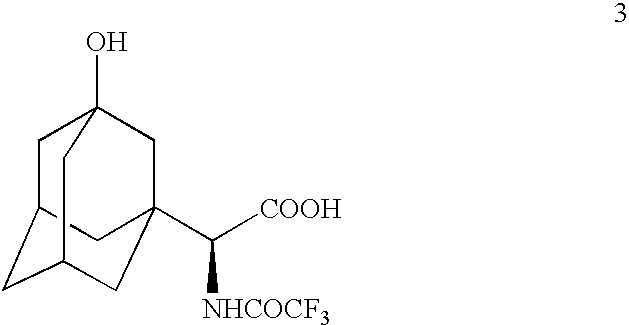
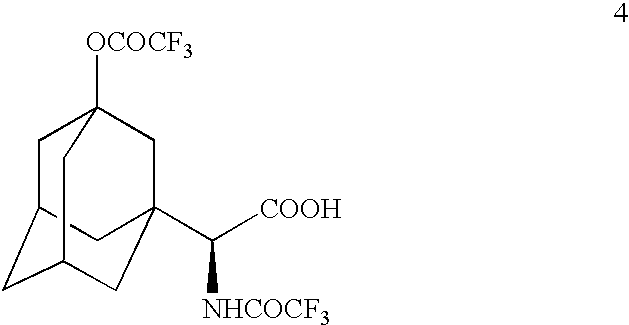
A process is provided for preparing a dipeptidyl peptidase IV inhibitor of the structurewhereinis treated with TFAA in isopropyl acetate to protect the tertiary hydroxyl group as a trifluoroacetate group to form 4(which is a novel intermediate)which is converted to acid chloride compound 5(which is a novel compound)using Vilsmeier reagent or other chloro reagent and coupled with compound 6 in a heterogeneous mixture of ethyl acetate and aqueous bicarbonate to give compound 7The N,O-bis(trifluoroacetyl) groups of compound 7 are deprotected to give free base compound 10.
Owner:ASTRAZENECA AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity](https://images-eureka.patsnap.com/patent_img/98a75e2d-75e1-48ce-aec0-985d9e07e9a0/US09708322-20170718-C00001.png)
![Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity](https://images-eureka.patsnap.com/patent_img/98a75e2d-75e1-48ce-aec0-985d9e07e9a0/US09708322-20170718-C00002.png)
![Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity](https://images-eureka.patsnap.com/patent_img/98a75e2d-75e1-48ce-aec0-985d9e07e9a0/US09708322-20170718-C00003.png)