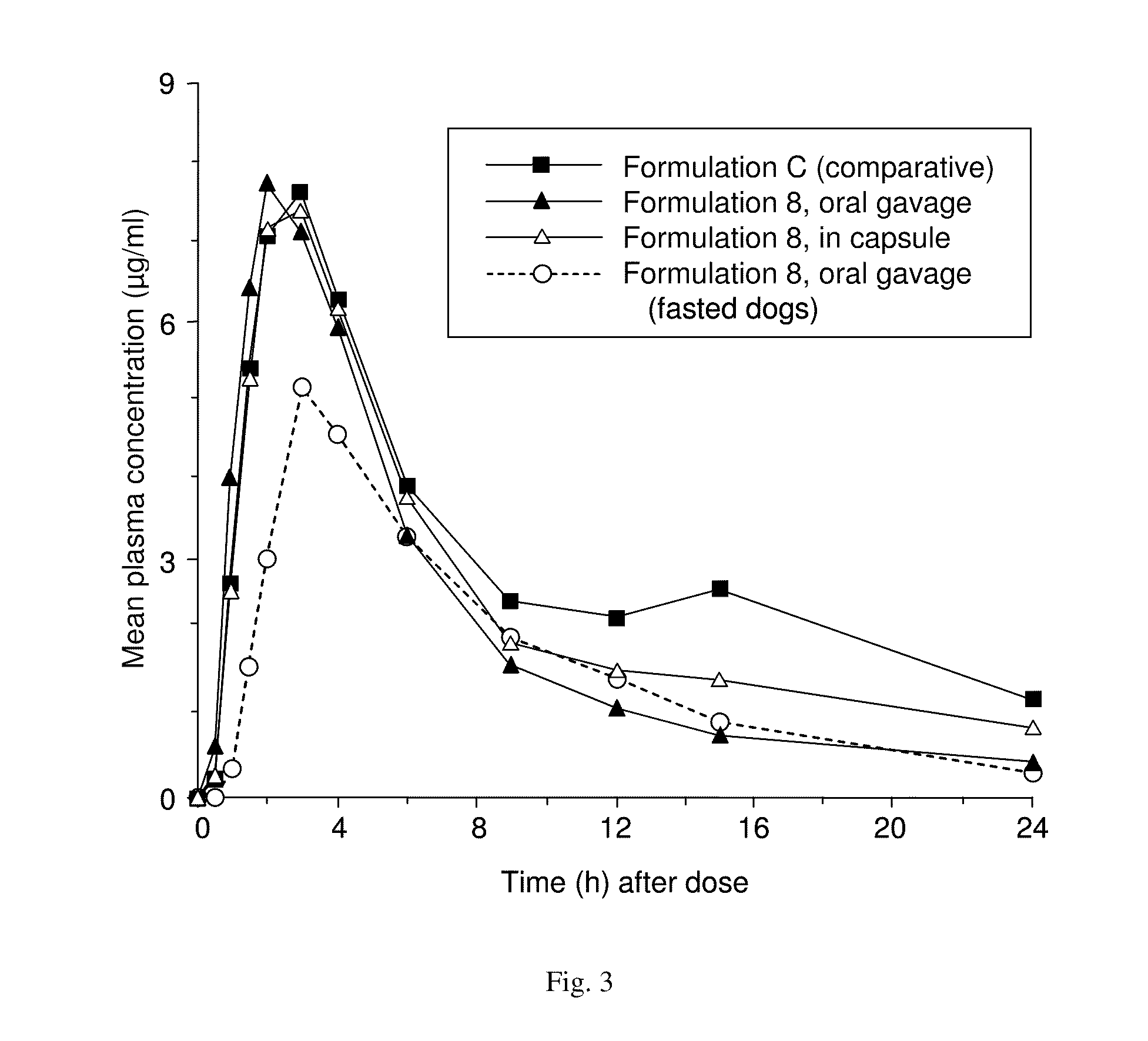
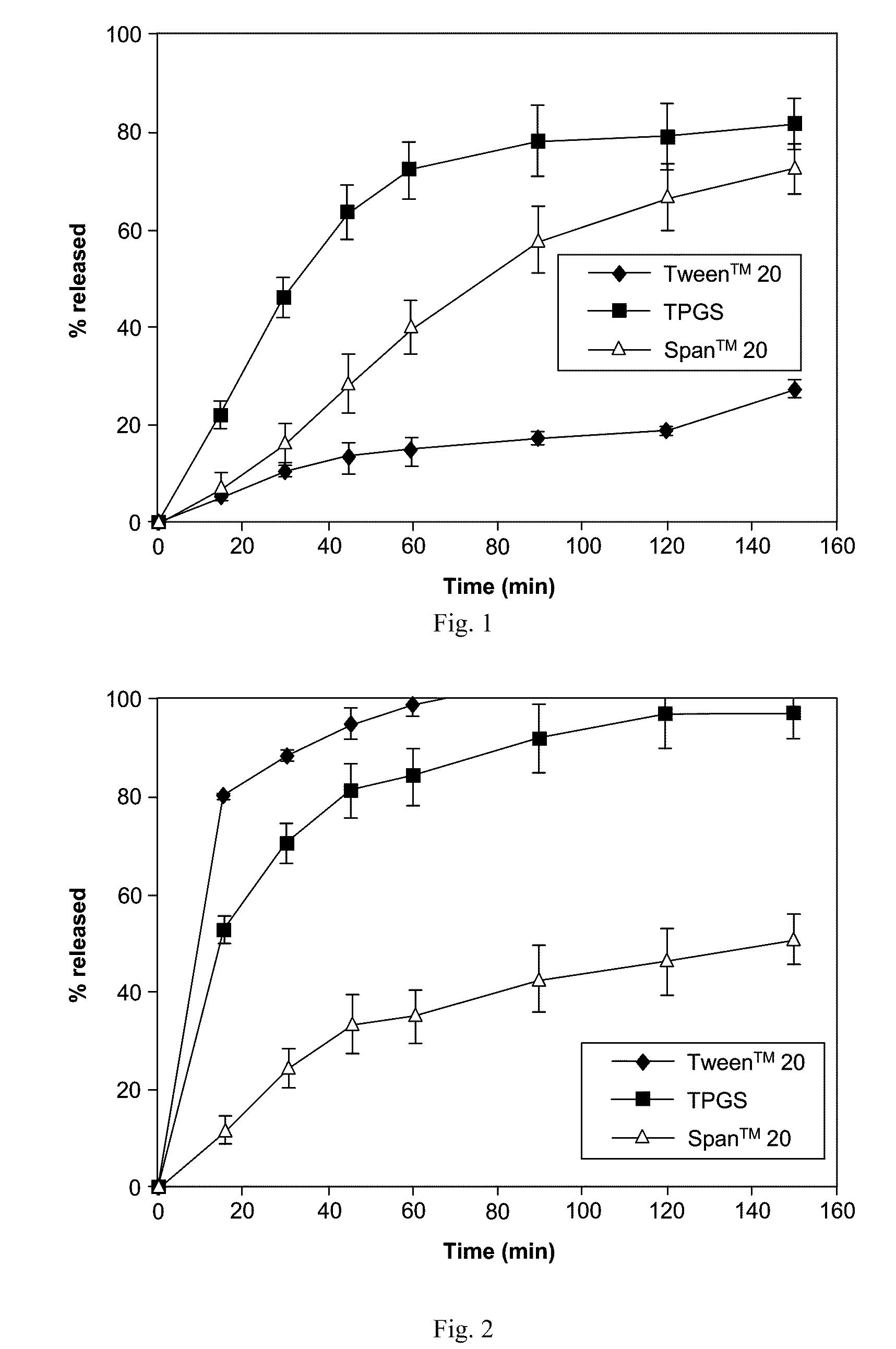
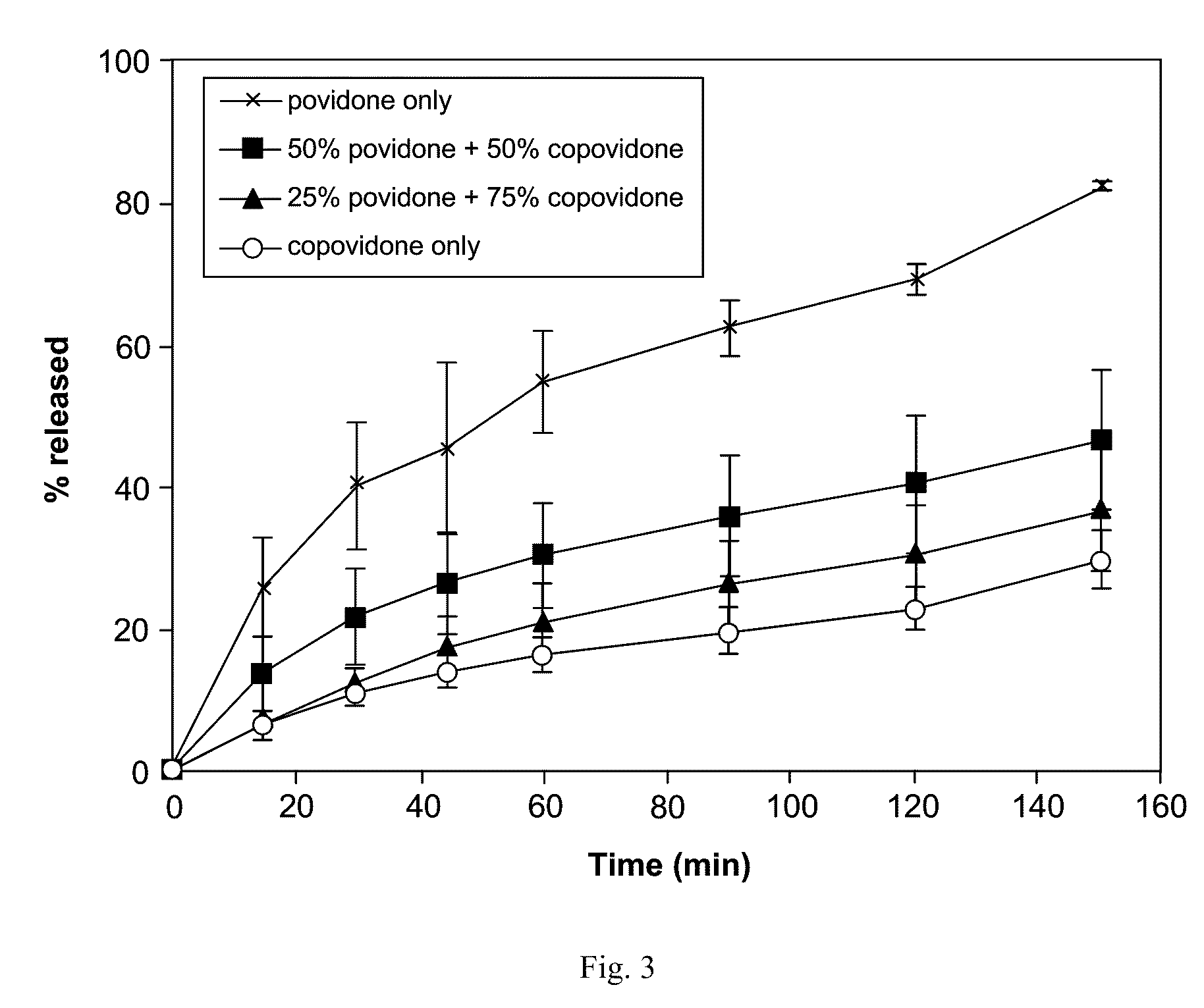
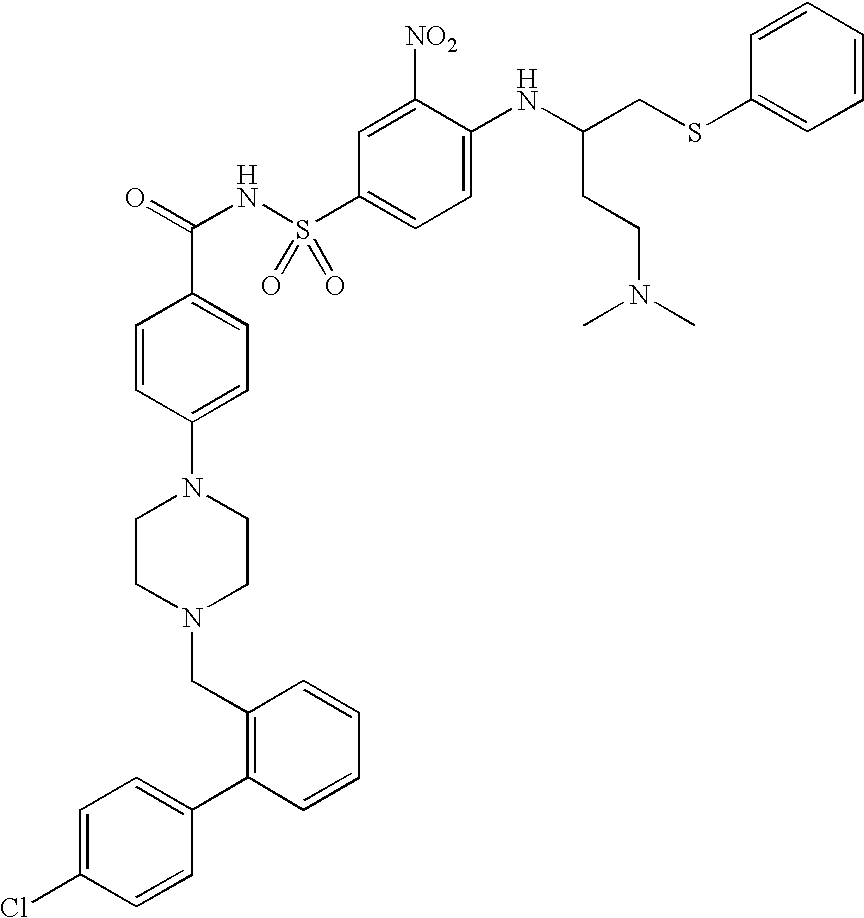
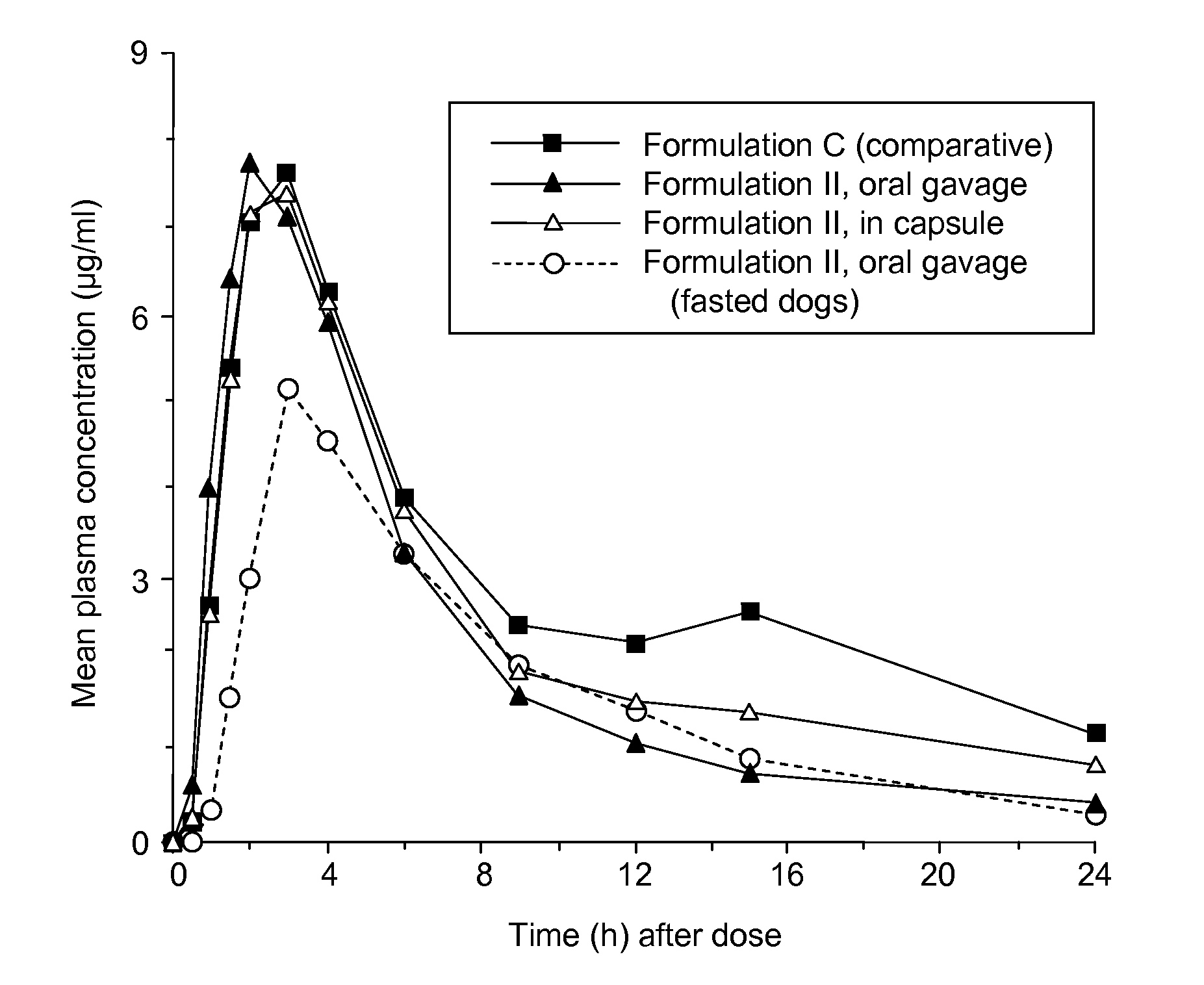
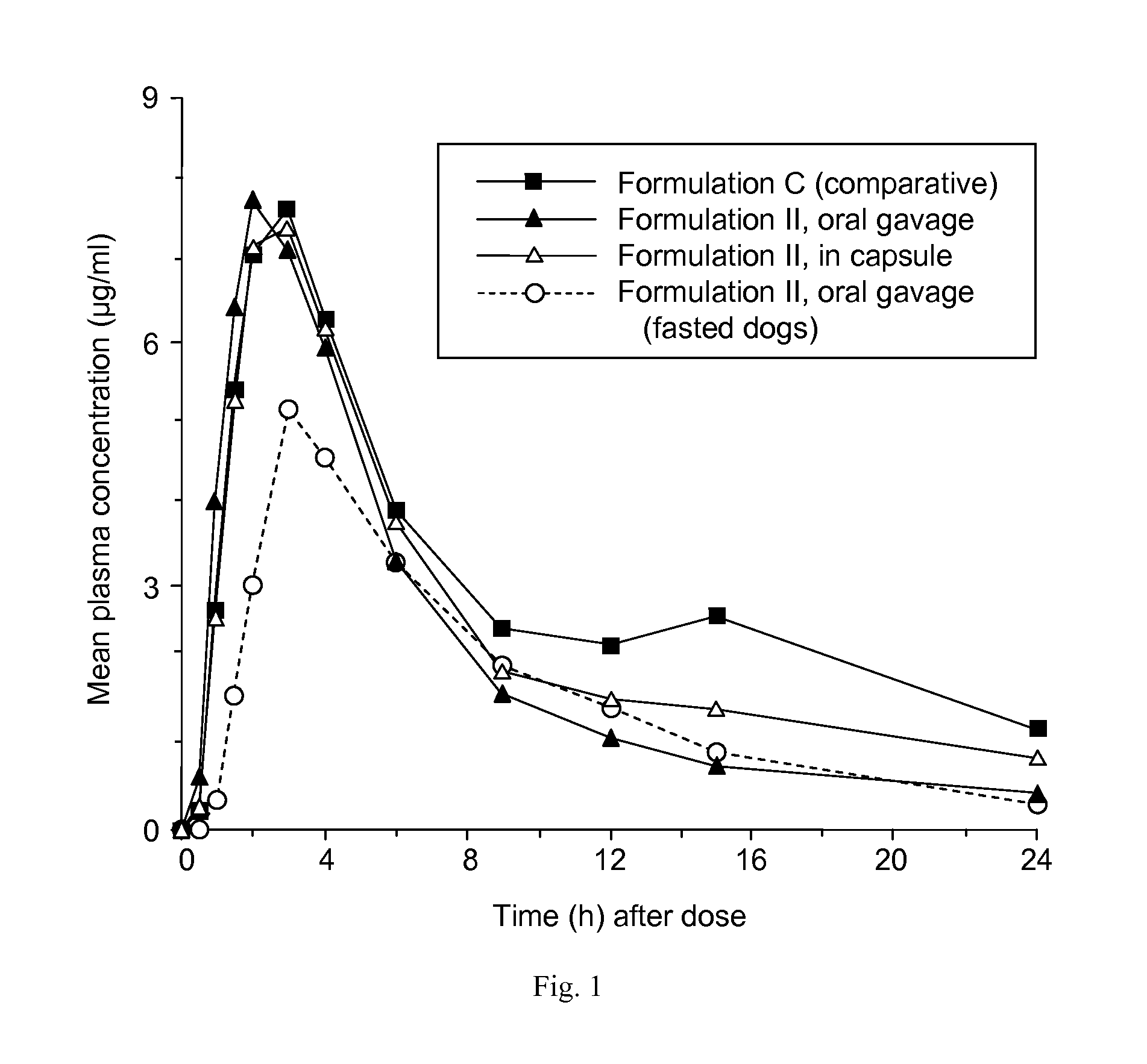
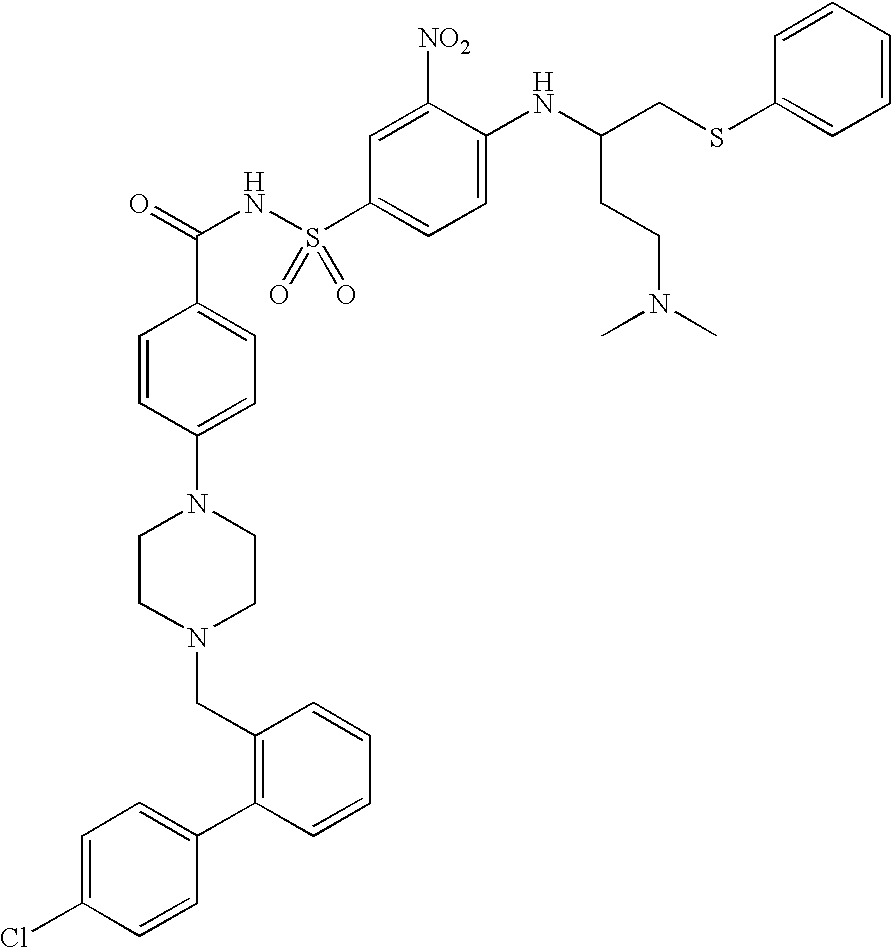
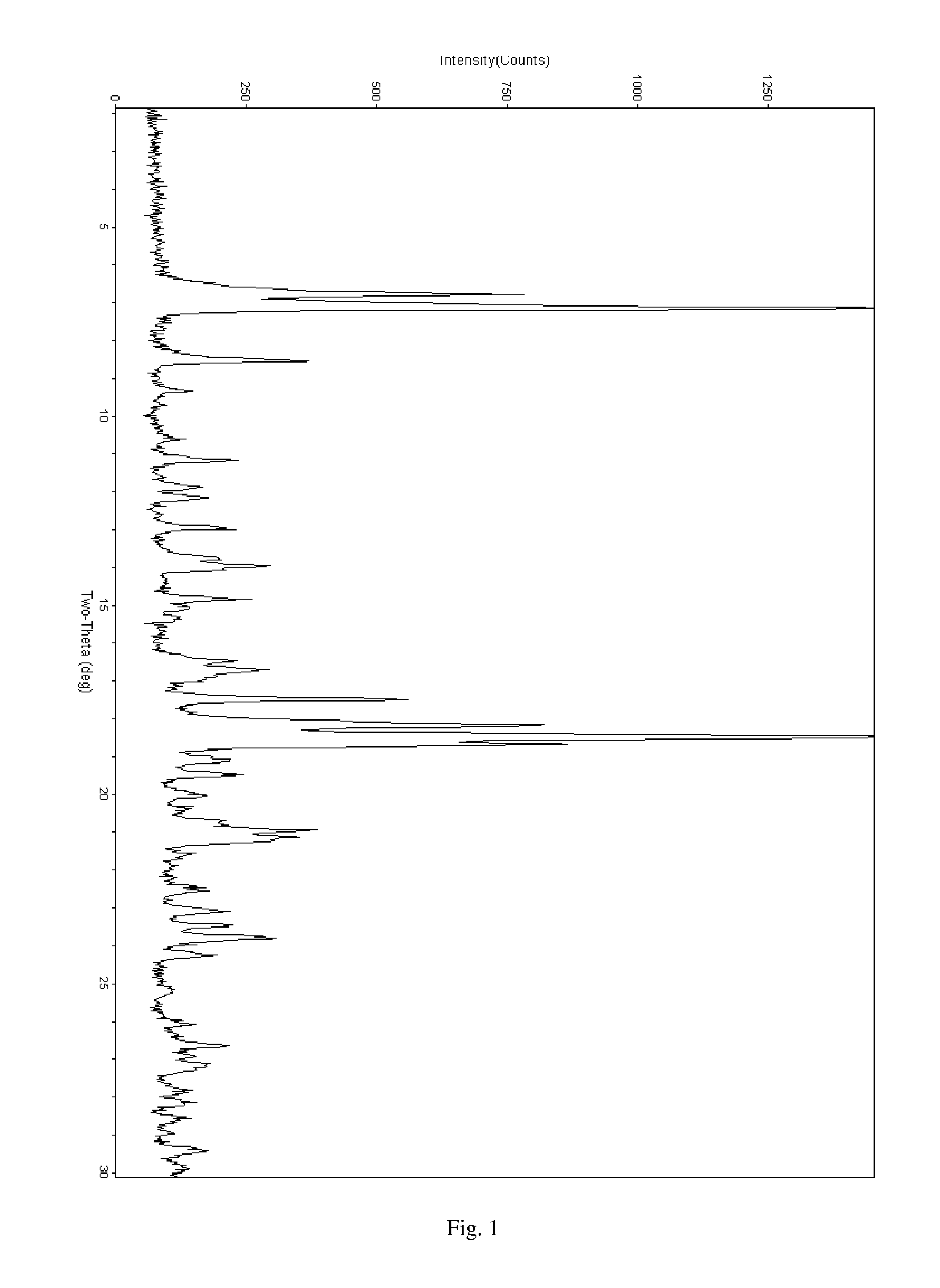
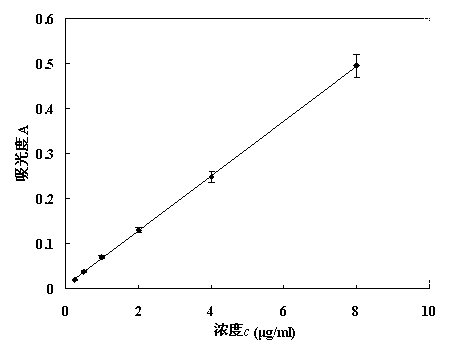
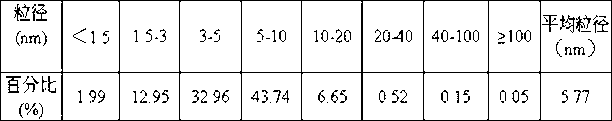
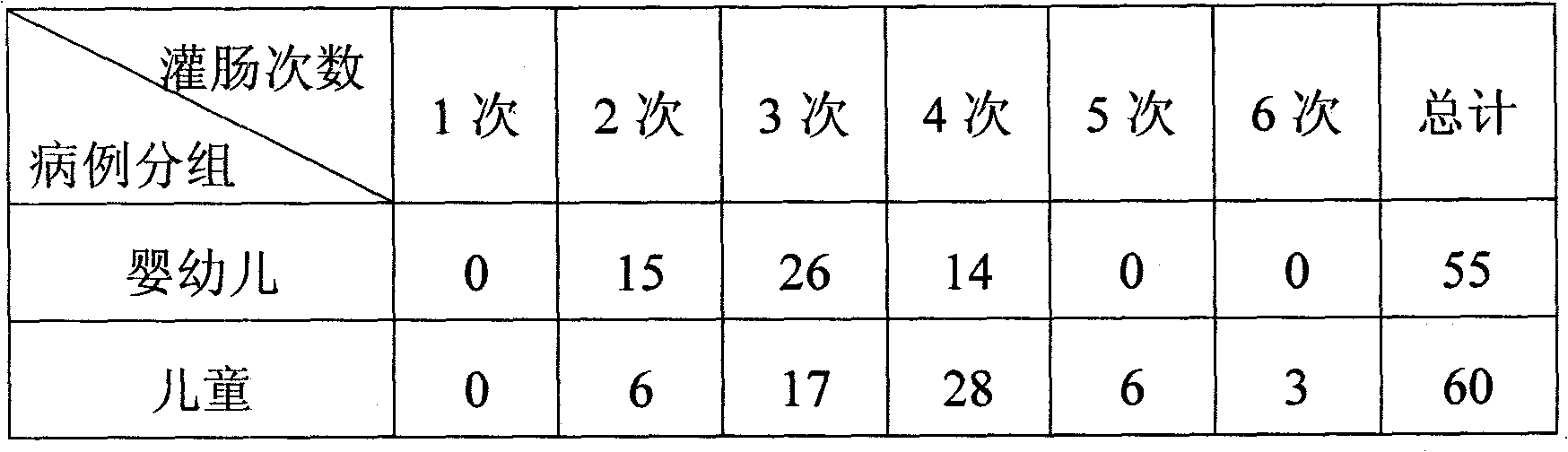
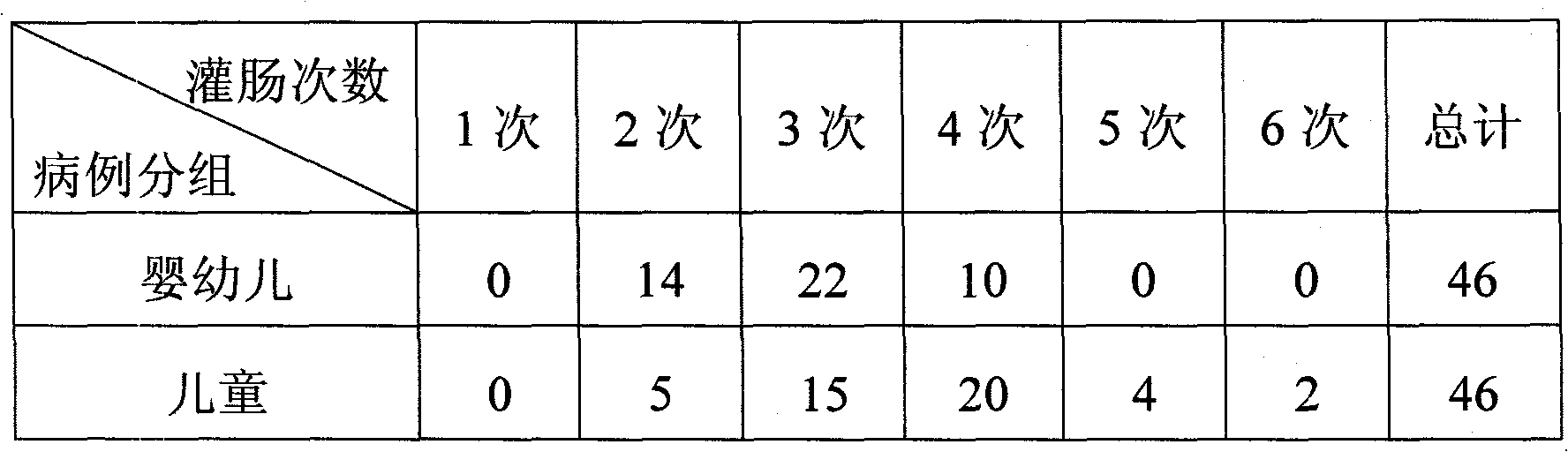
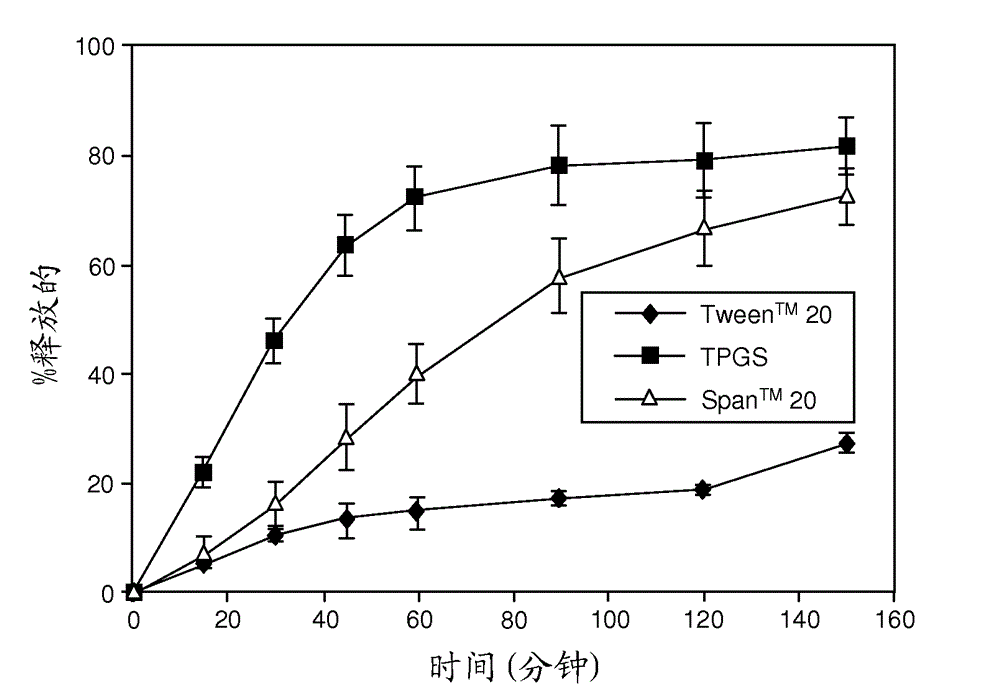
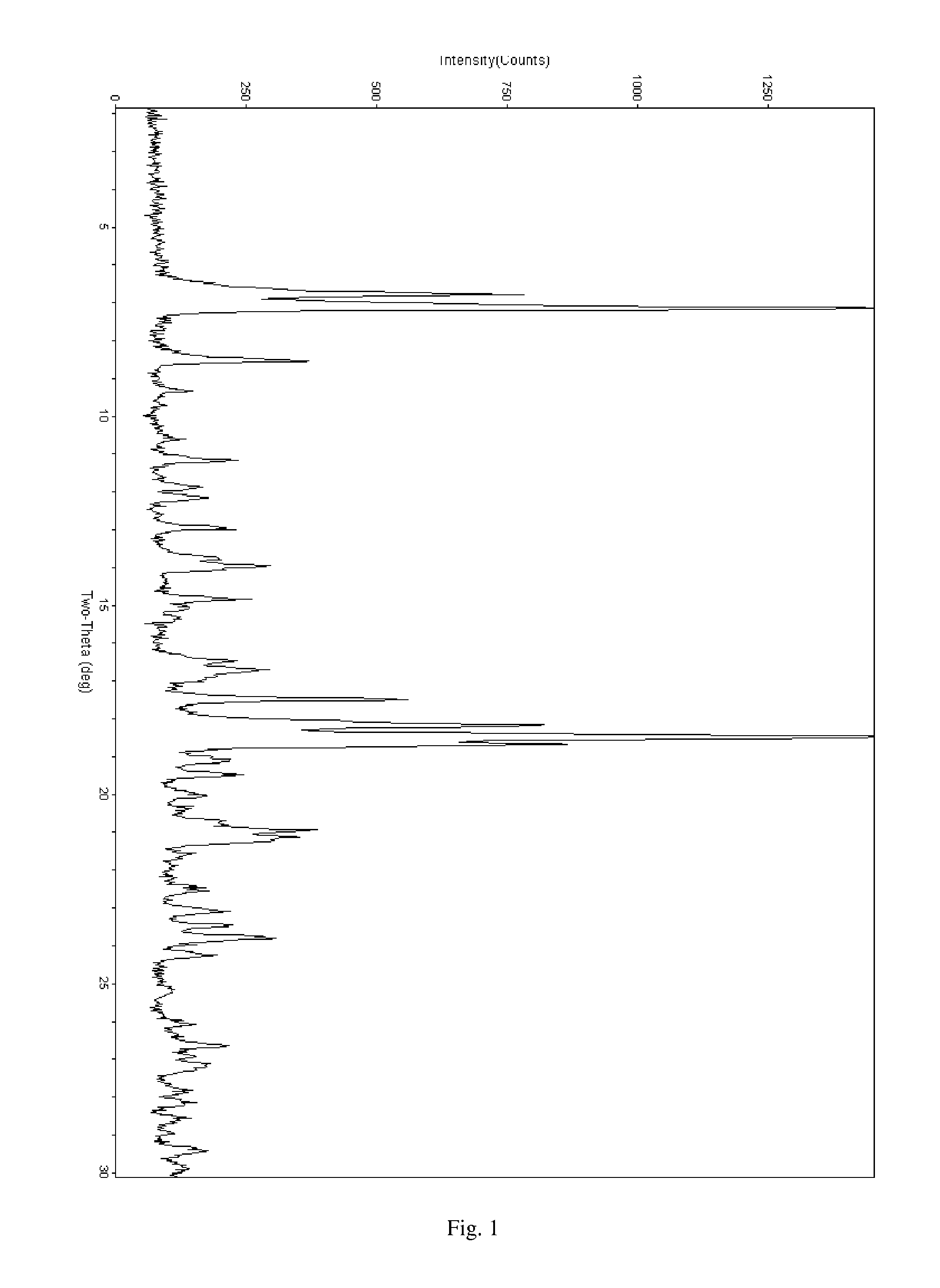
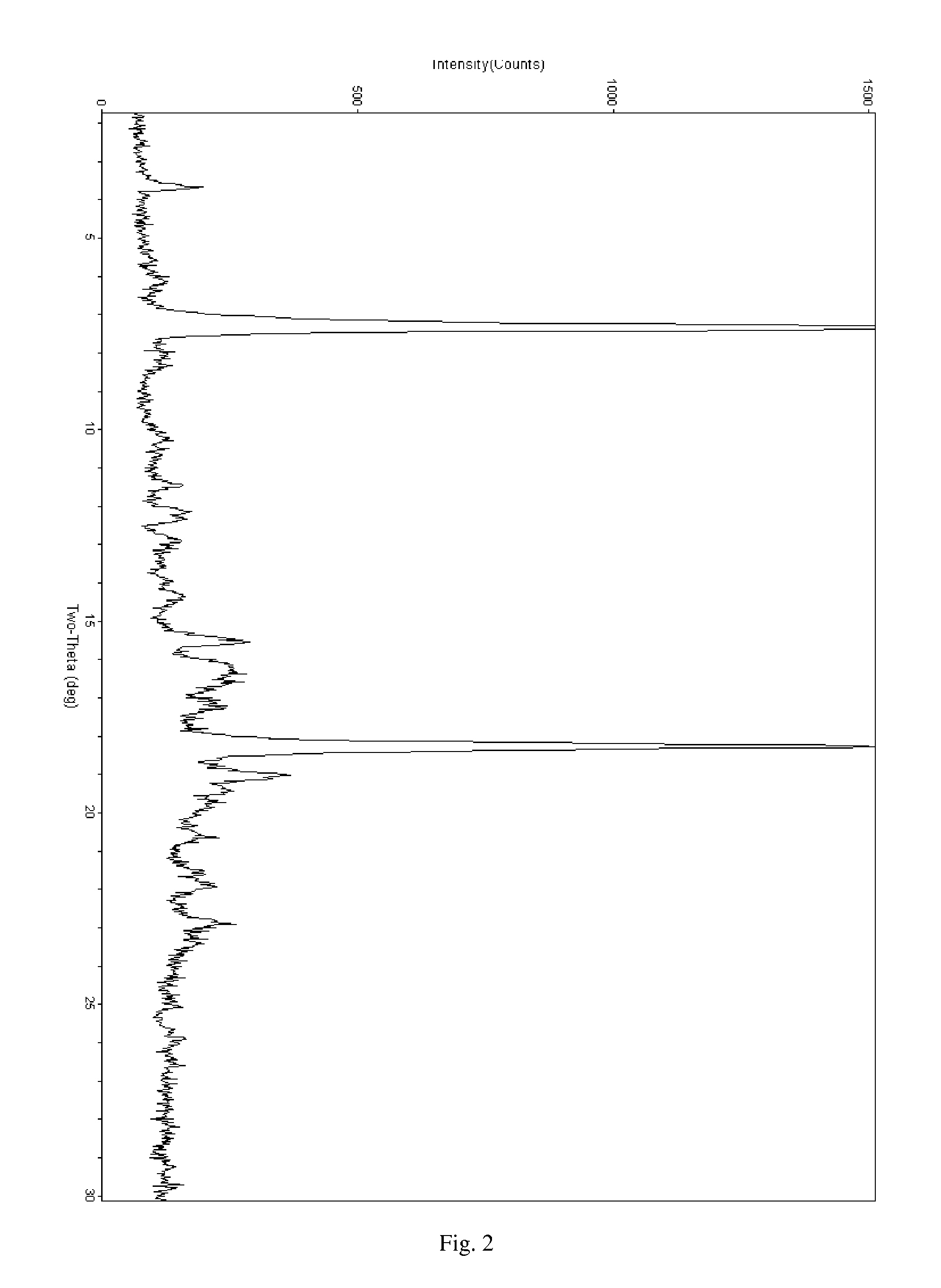
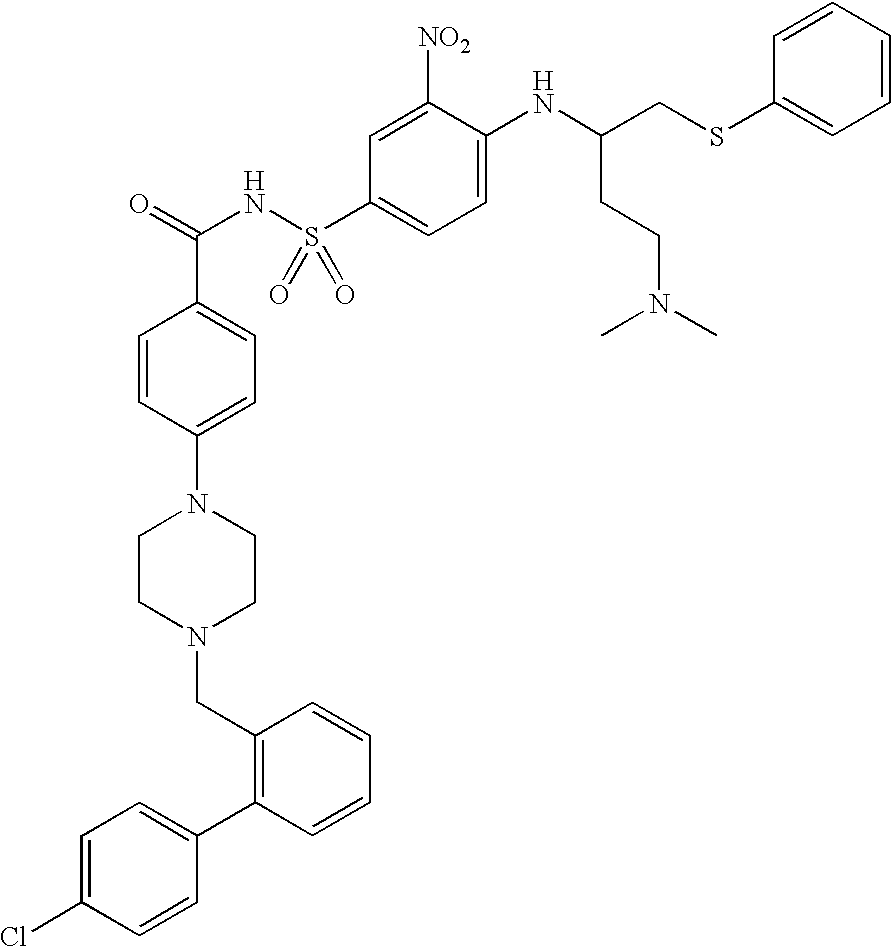
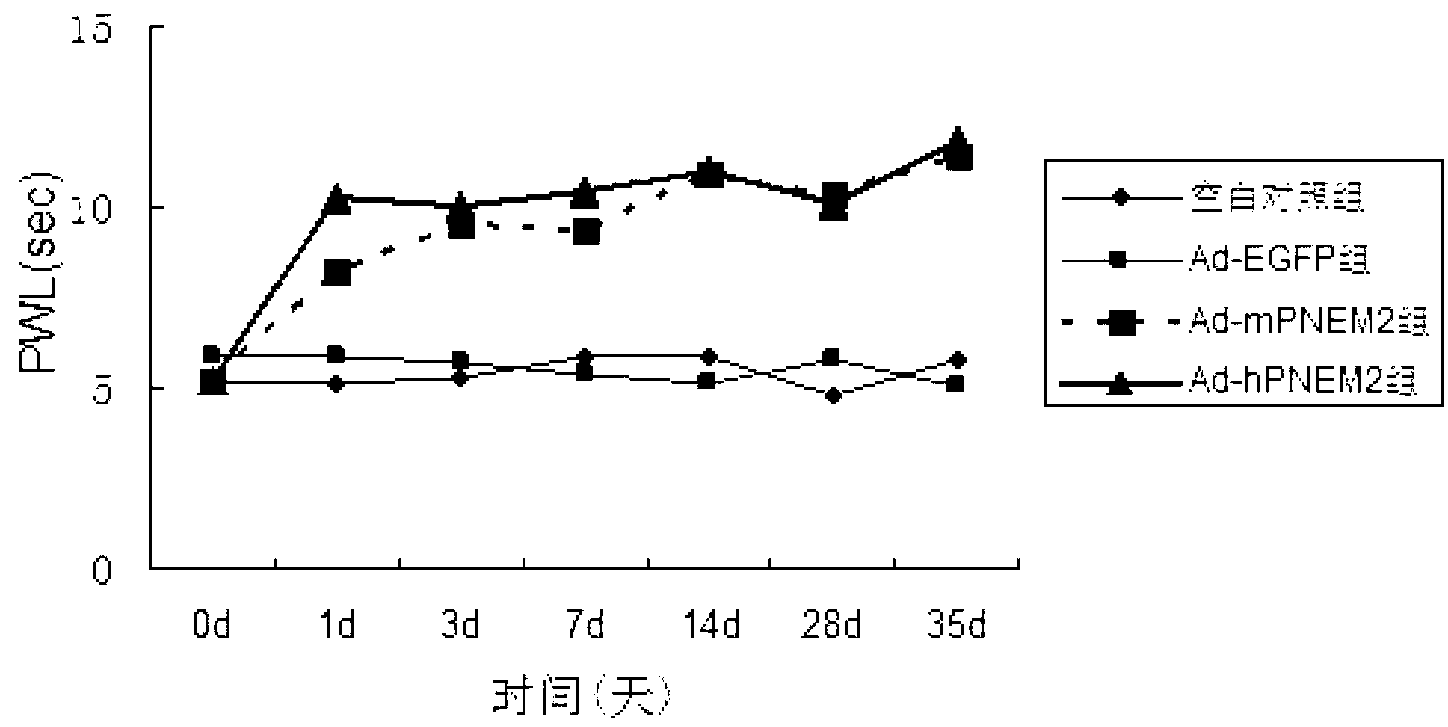
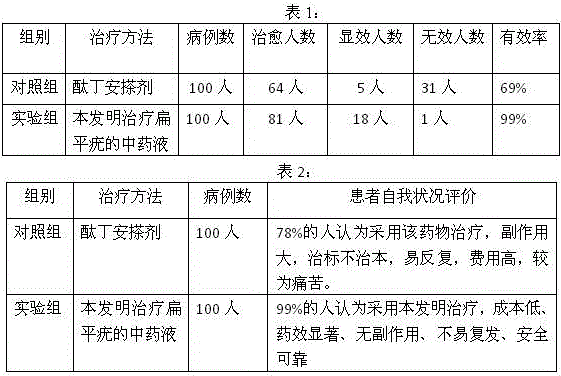
Patents
Literature
59results about How to "Convenient route of administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
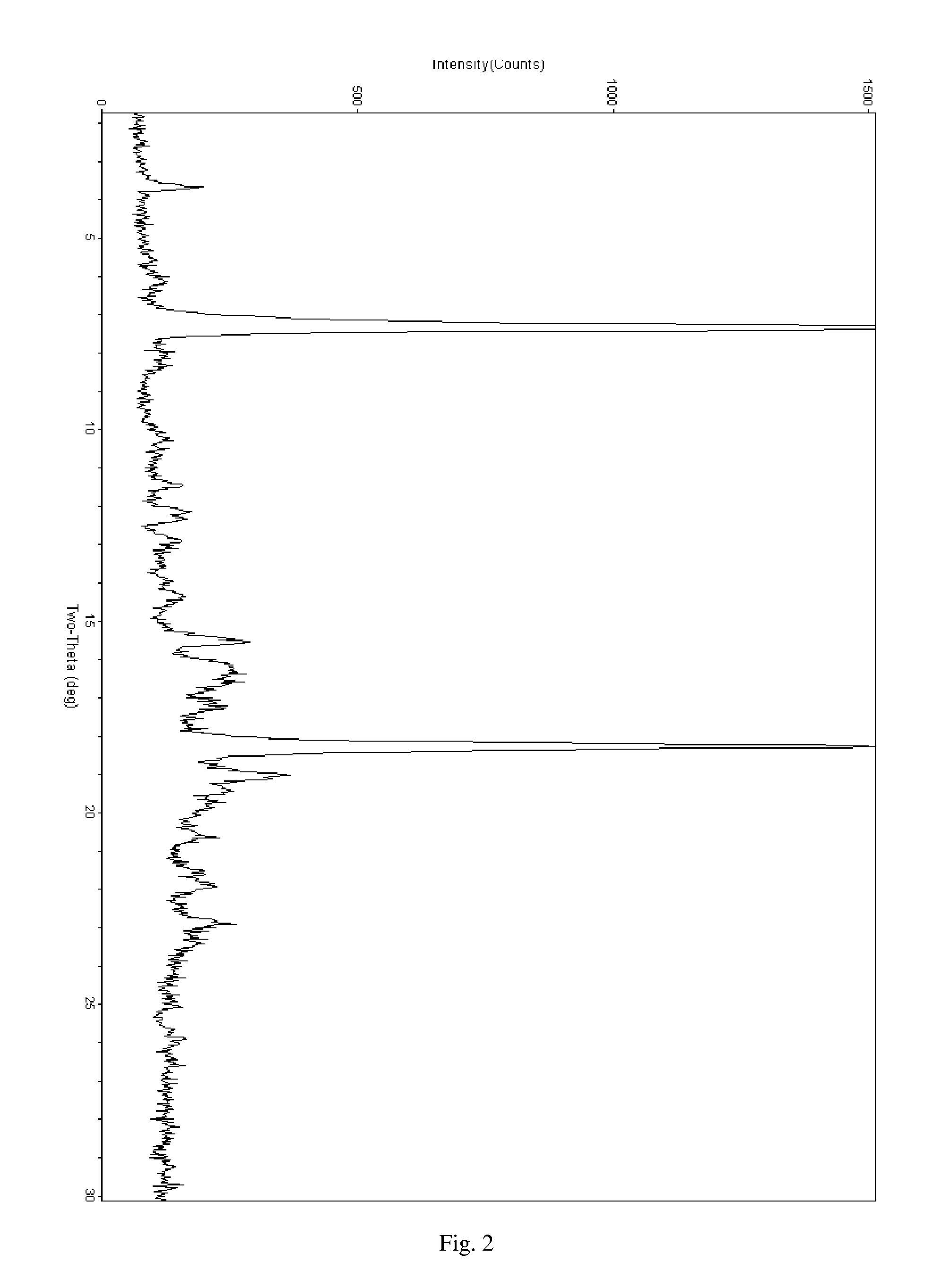
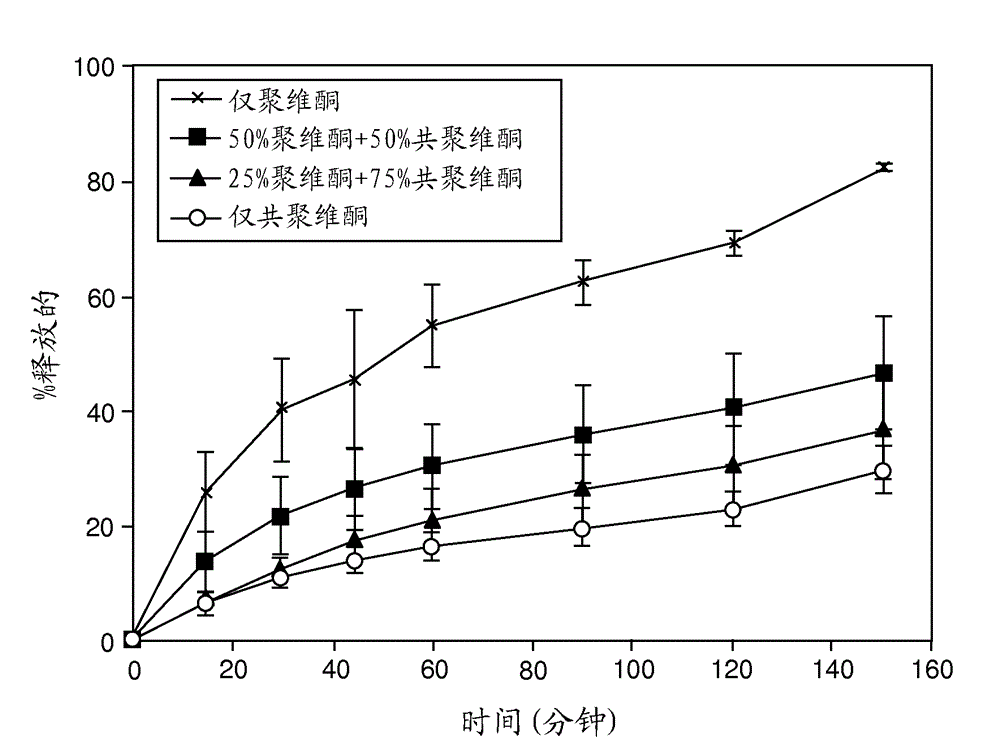
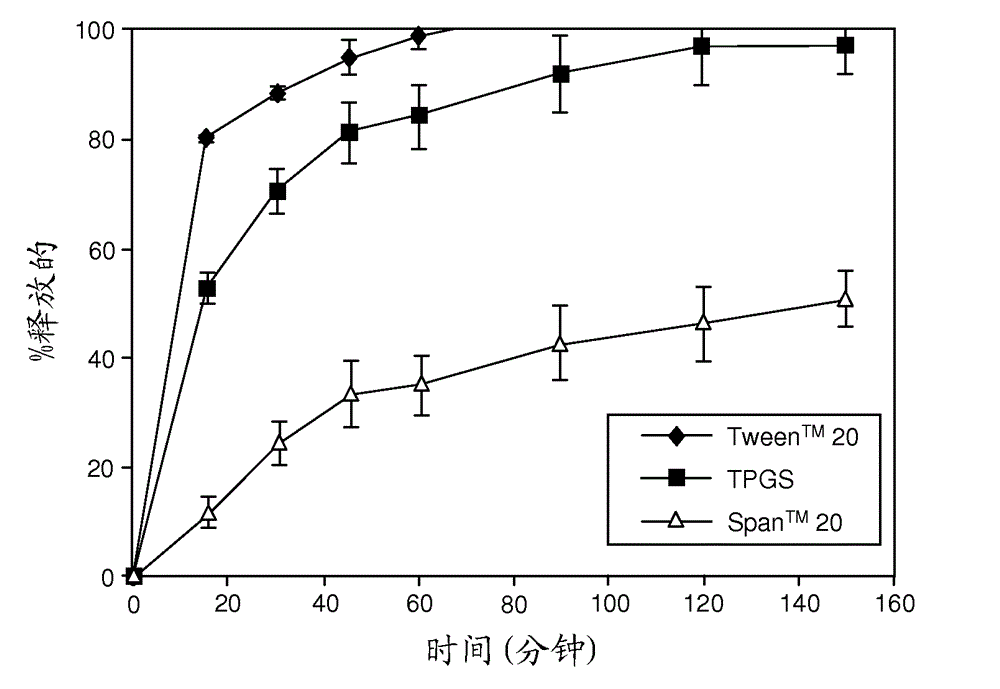
Solid dispersions containing an apoptosis-inducing agent
InactiveUS20120277210A1Improve clinical utilityStay focusedPowder deliveryBiocidePolymer scienceOral medication
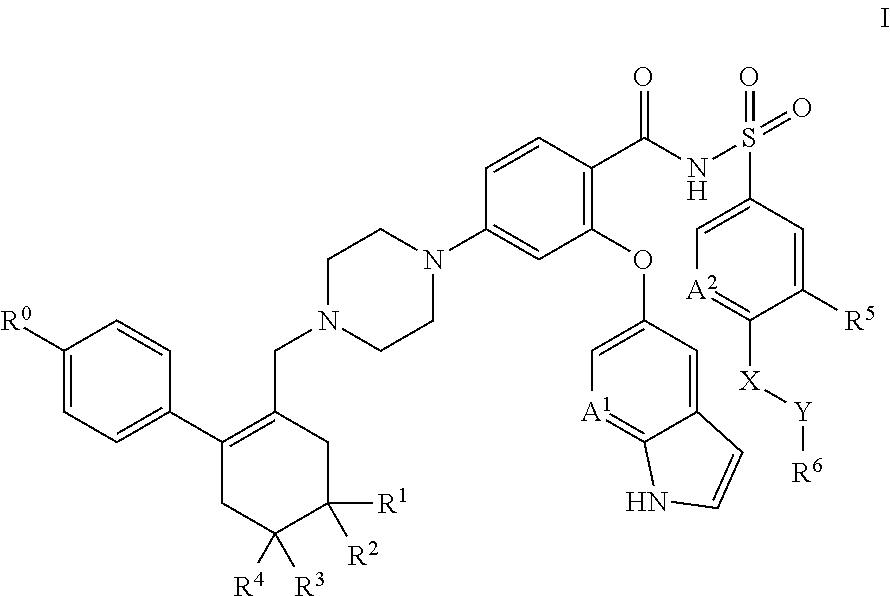
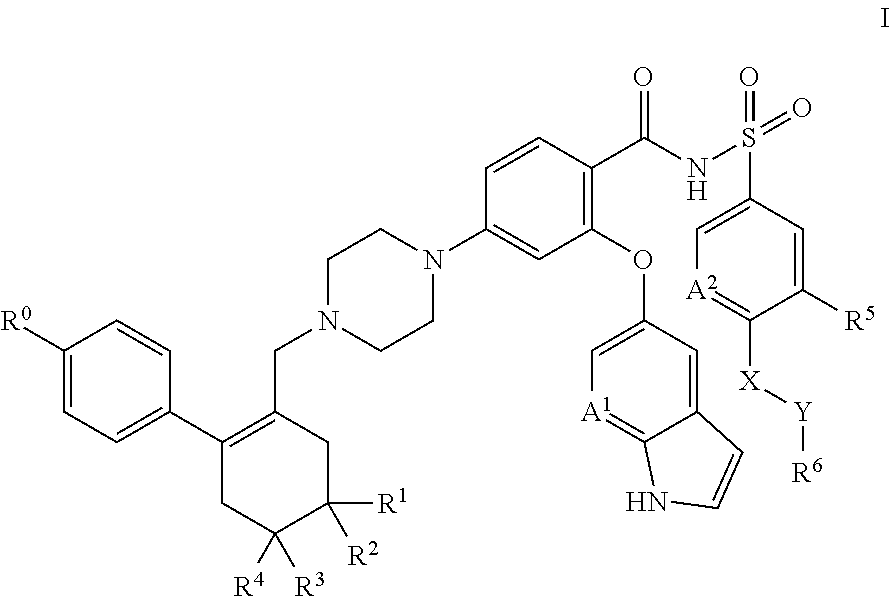
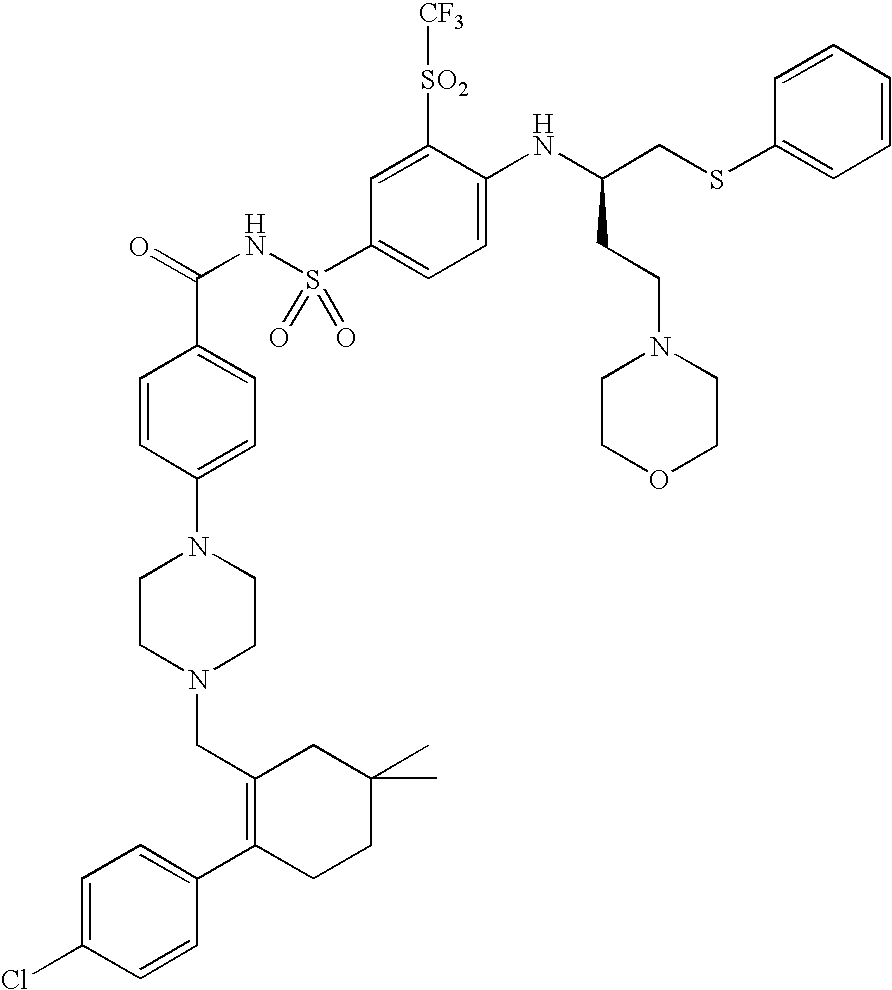
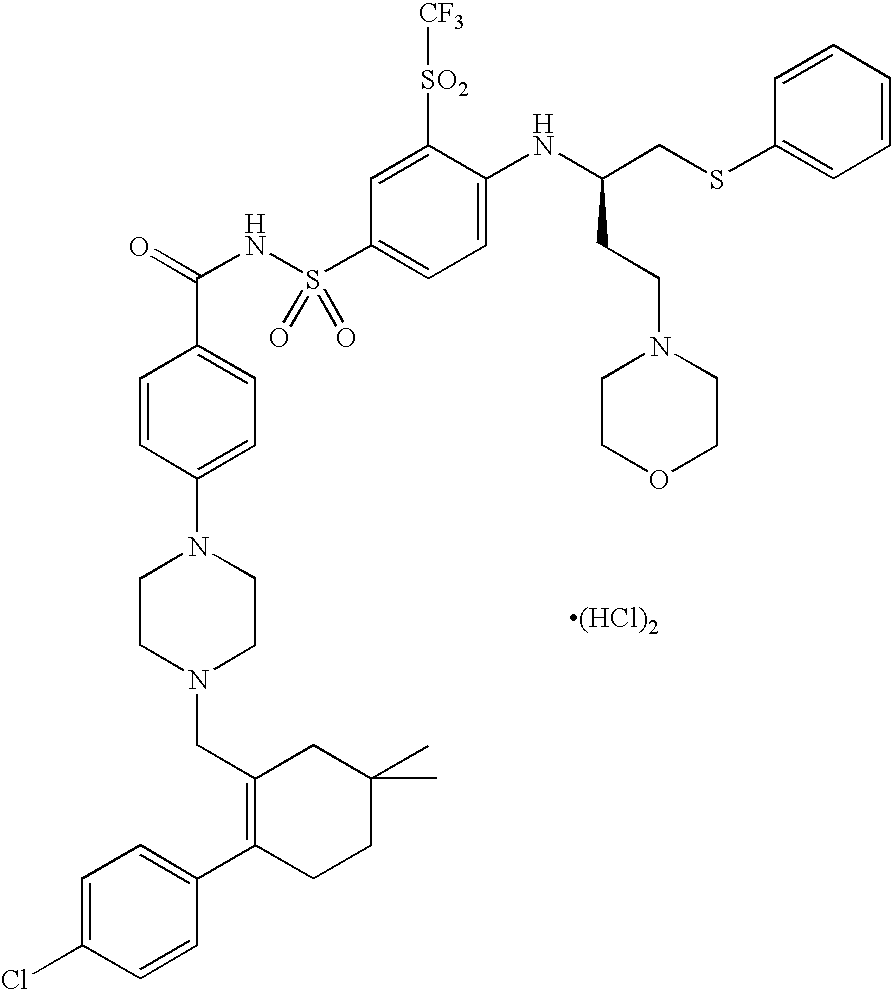
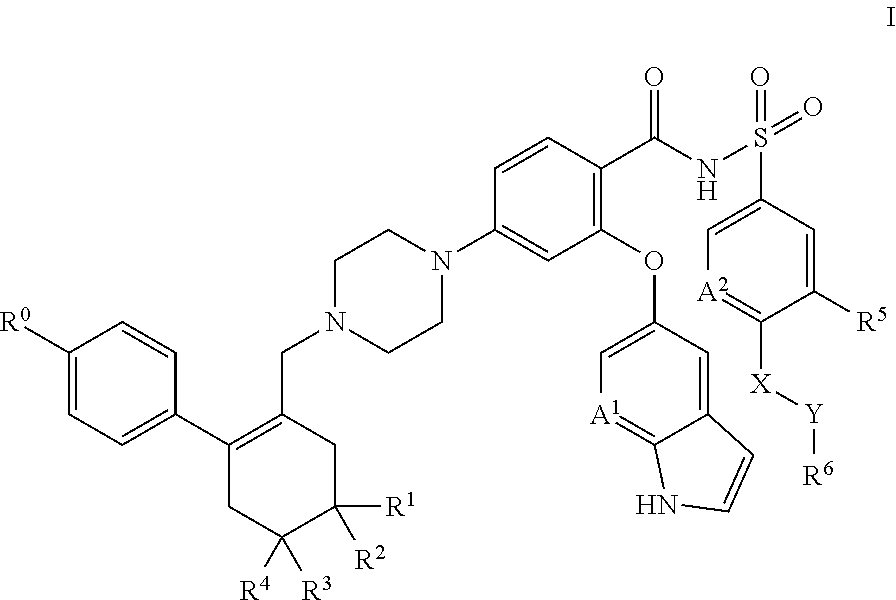
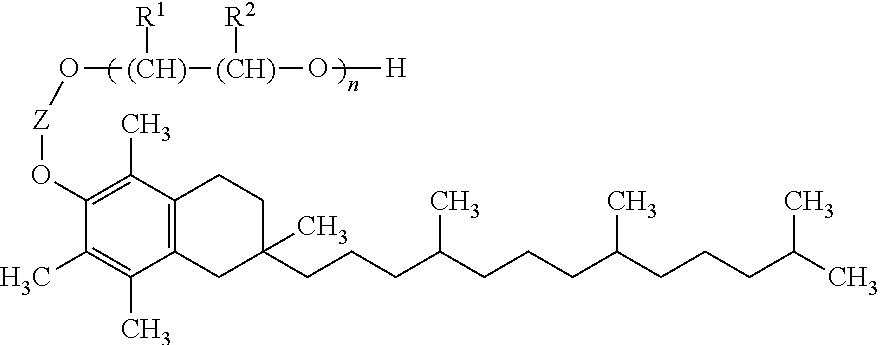
A pro-apoptotic solid dispersion comprises, in essentially non-crystalline form, a Bcl-2 family protein inhibitory compound of Formula I as defined herein, dispersed in a solid matrix that comprises (a) a pharmaceutically acceptable water-soluble polymeric carrier and (b) a pharmaceutically acceptable surfactant. A process for preparing such a solid dispersion comprises dissolving the compound, the polymeric carrier and the surfactant in a suitable solvent, and removing the solvent to provide a solid matrix comprising the polymeric carrier and the surfactant and having the compound dispersed in essentially non-crystalline form therein. The solid dispersion is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
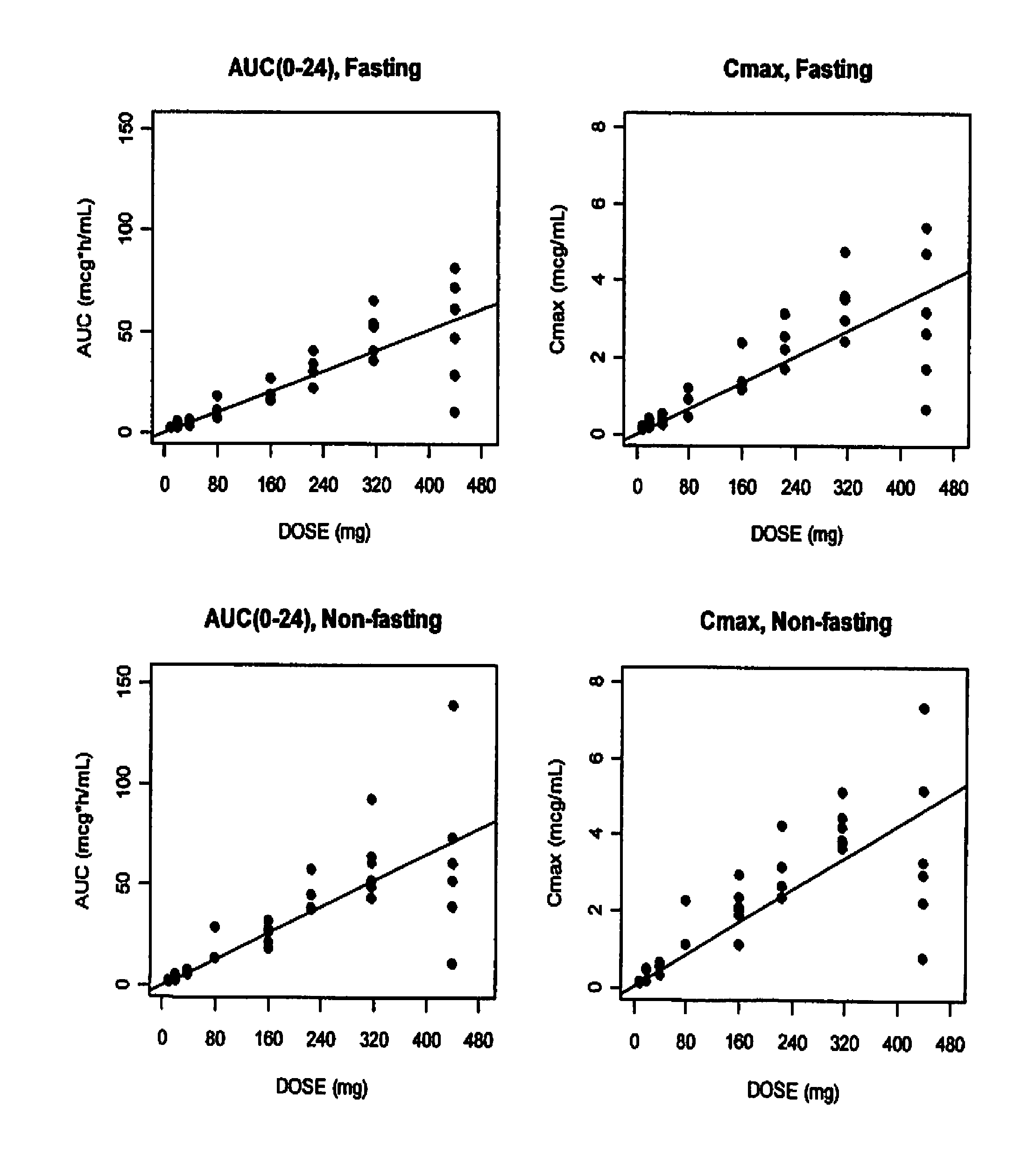
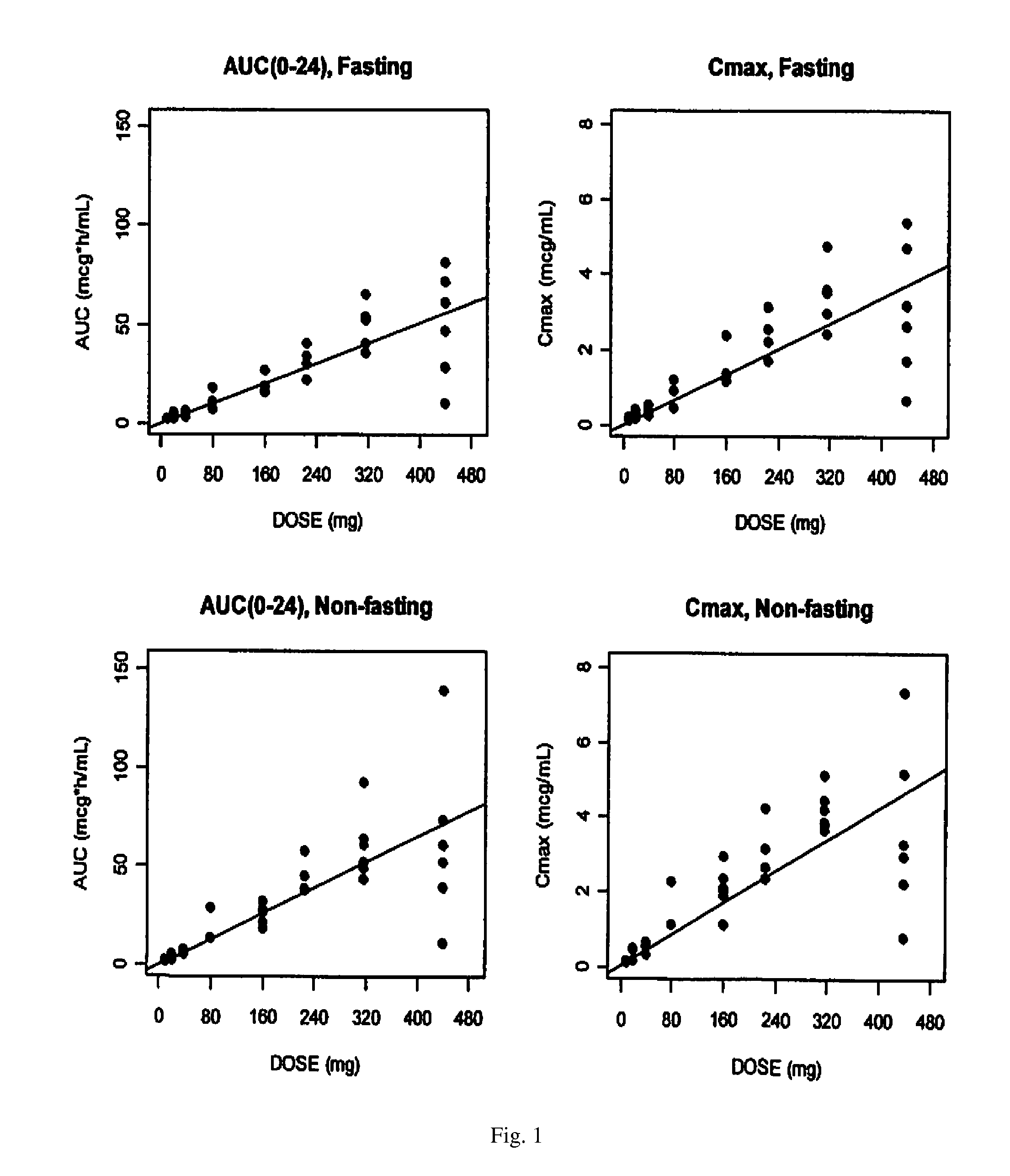
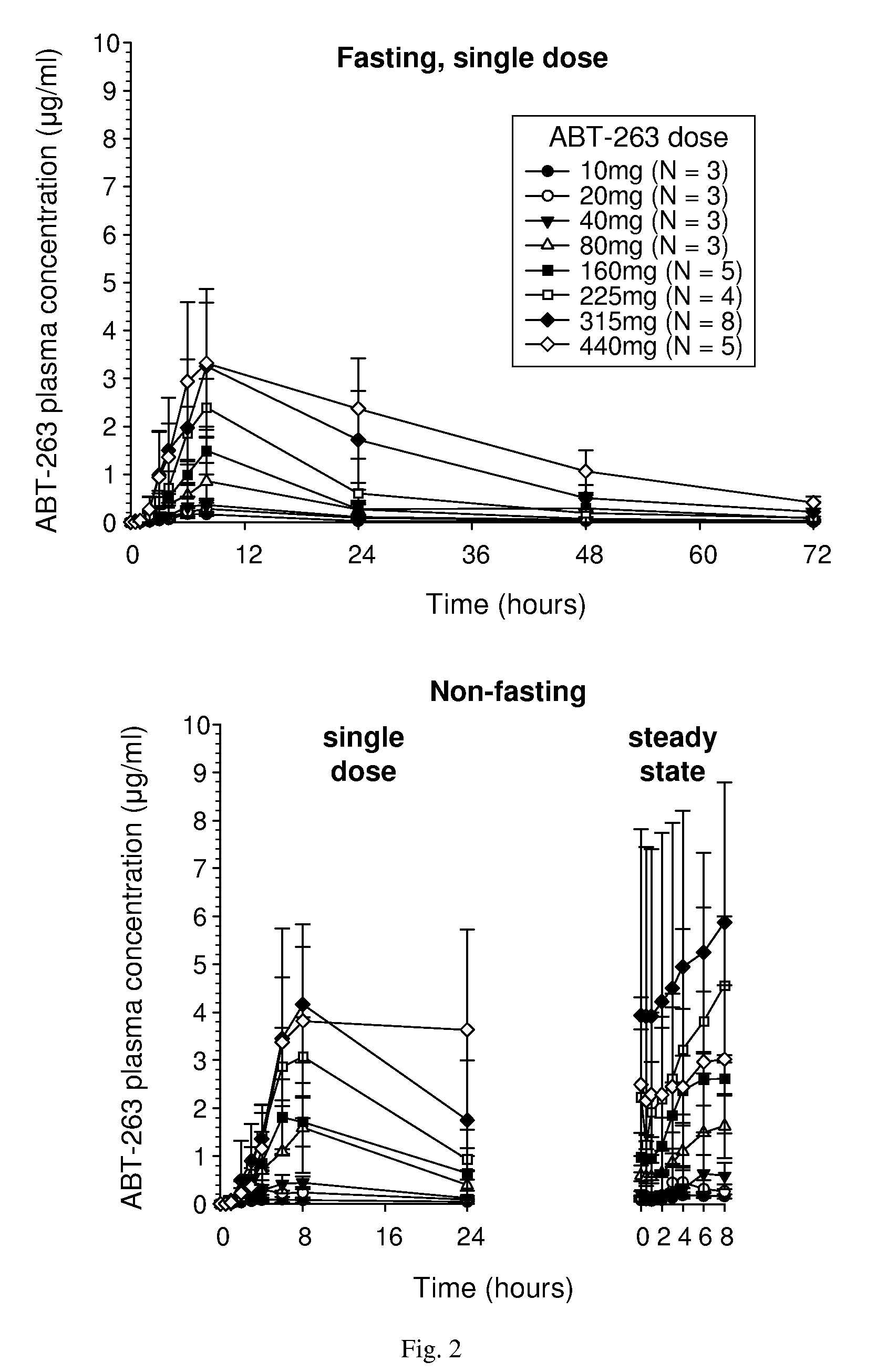
Solid oral formulation of abt-263
InactiveUS20100278921A1Convenient route of administrationEasy to doOrganic active ingredientsPowder deliveryDiseaseOral medication
An orally deliverable pharmaceutical composition comprises (a) a pharmaceutically acceptable acid addition salt of ABT-263 in solid particulate form, and (b) a plurality of pharmaceutically acceptable excipients including at least a solid diluent and a solid disintegrant; wherein the salt is formed from more than one equivalent of acid per equivalent of ABT-263. The composition is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBOTT LAB INC
Formulation for oral administration of apoptosis promoter
InactiveUS20100297194A1Improve oral bioavailabilityConvenient route of administrationOrganic active ingredientsBiocideDiseaseAntioxidant
An orally deliverable pharmaceutical composition comprises as a sole or first active ingredient a compound of Formula I defined herein or a pharmaceutically acceptable salt thereof, for example ABT-263 free base or ABT-263 bis-HCl salt, dispersed, in a free base equivalent amount of at least about 2.5% by weight of the composition, in a pharmaceutically acceptable carrier; wherein said active ingredient is in solid-state form and / or the composition further comprises, dispersed in the carrier, a pharmaceutically acceptable heavier-chalcogen antioxidant in an amount effective to inhibit oxidation of the active ingredient at a thioether linkage thereof. The composition is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBOTT LAB INC +1
Solid dispersions containing an apoptosis-promoting agent
InactiveUS20100311751A1Attenuate induction of apoptosisStrong cytotoxicityPowder deliveryOrganic active ingredientsDiseaseOral medication
A pro-apoptotic solid dispersion comprises, in essentially non-crystalline form, a Bcl-2 family protein inhibitory compound, e.g., ABT-263, dispersed in a solid matrix that comprises (a) a pharmaceutically acceptable water-soluble polymeric carrier and (b) a pharmaceutically acceptable surfactant. A process for preparing such a solid dispersion comprises dissolving the compound, the polymeric carrier and the surfactant in a suitable solvent, and removing the solvent to provide a solid matrix comprising the polymeric carrier and the surfactant and having the compound dispersed in essentially non-crystalline form therein. The solid dispersion is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
Stable nanoparticulate drug suspension
InactiveUS20100323020A1Convenient route of administrationEasy to doOrganic active ingredientsPowder deliverySodium bicarbonateDisease
A liquid pharmaceutical composition comprises an aqueous medium having suspended therein a solid particulate Bc1-2 family protein inhibitory compound such as ABT-263, having a D90 particle size not greater than about 3 μm; wherein the aqueous medium further comprises at least one pharmaceutically acceptable surfactant and at least one pharmaceutically acceptable basifying agent such as sodium bicarbonate in amounts that are effective together to inhibit particle size increase. The composition is suitable for oral or parenteral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bc1-2 family proteins, for example cancer.
Owner:ABBVIE INC
Salt of abt-263 and solid-state forms thereof
InactiveUS20100305125A1Convenient route of administrationEasy to doOrganic active ingredientsOrganic chemistryDiseaseMedicine
Owner:ABBVIE INC
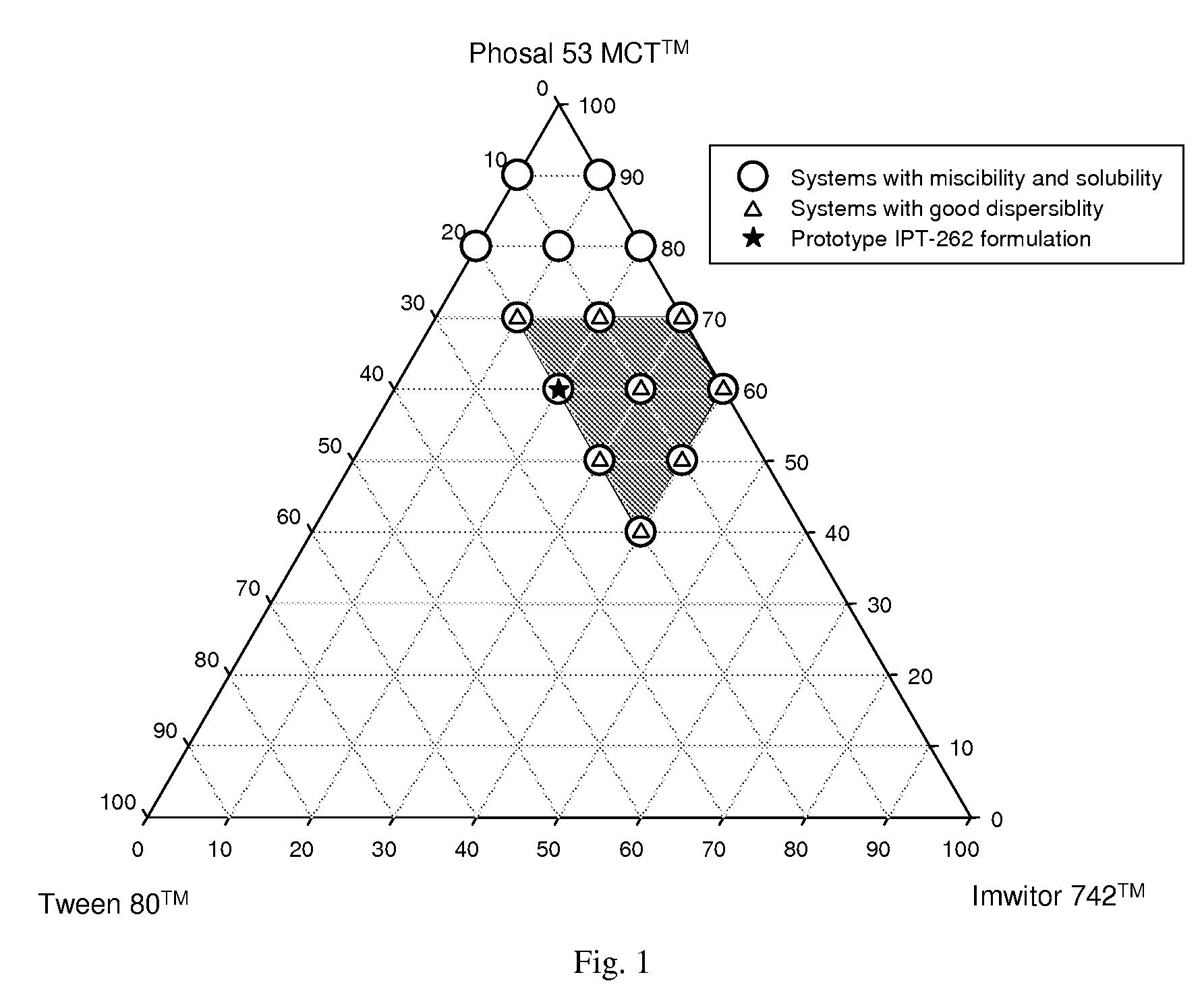
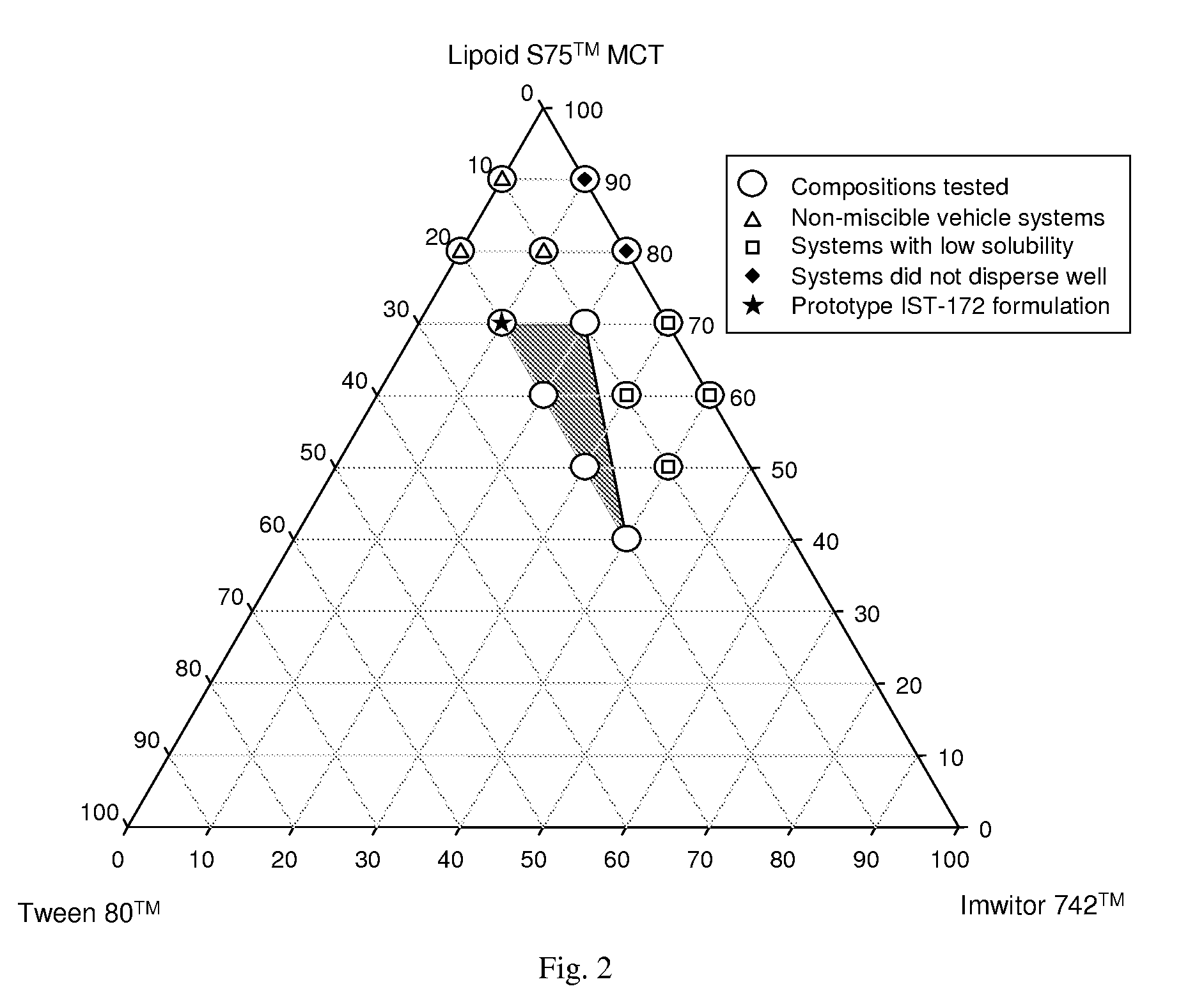
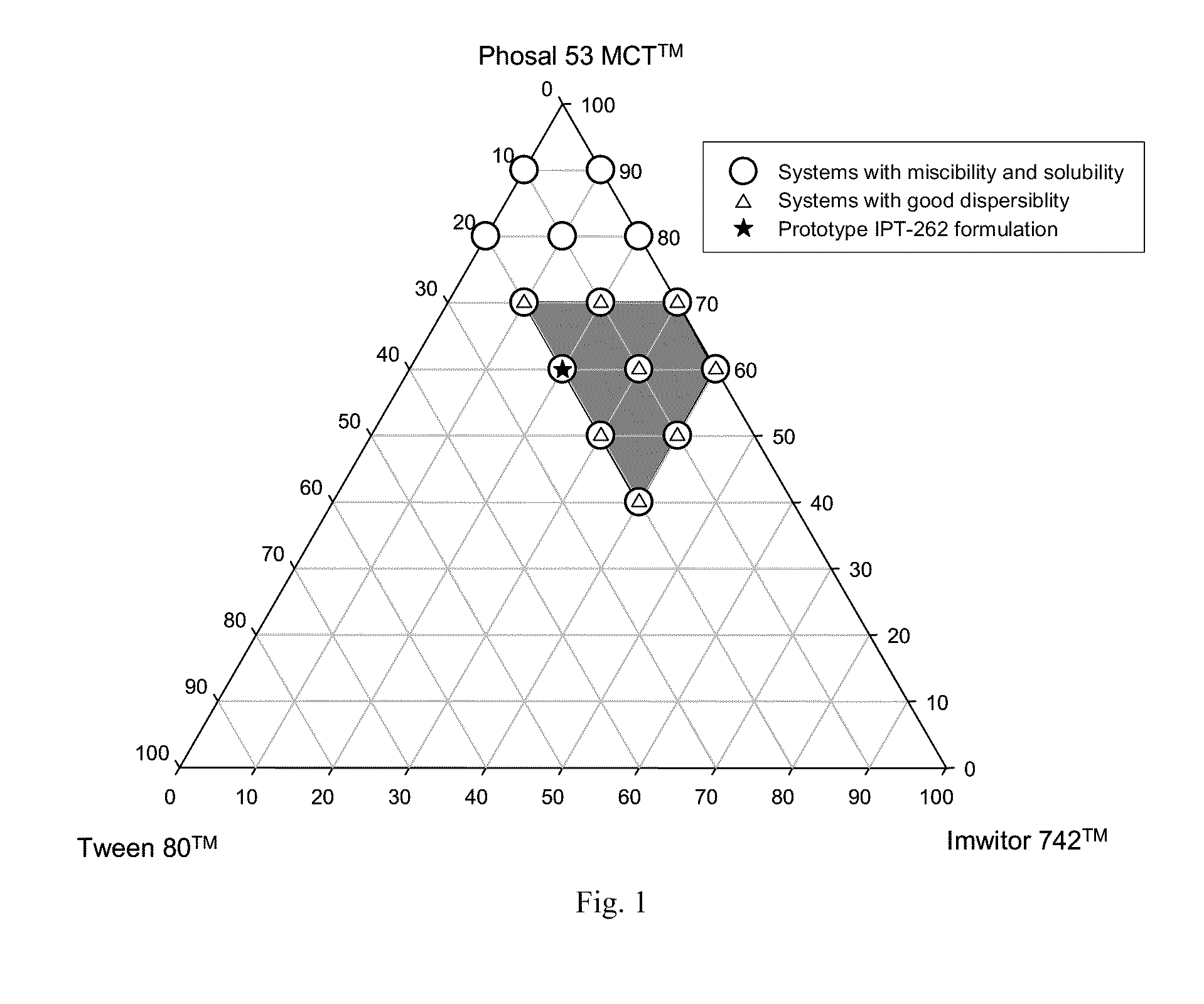
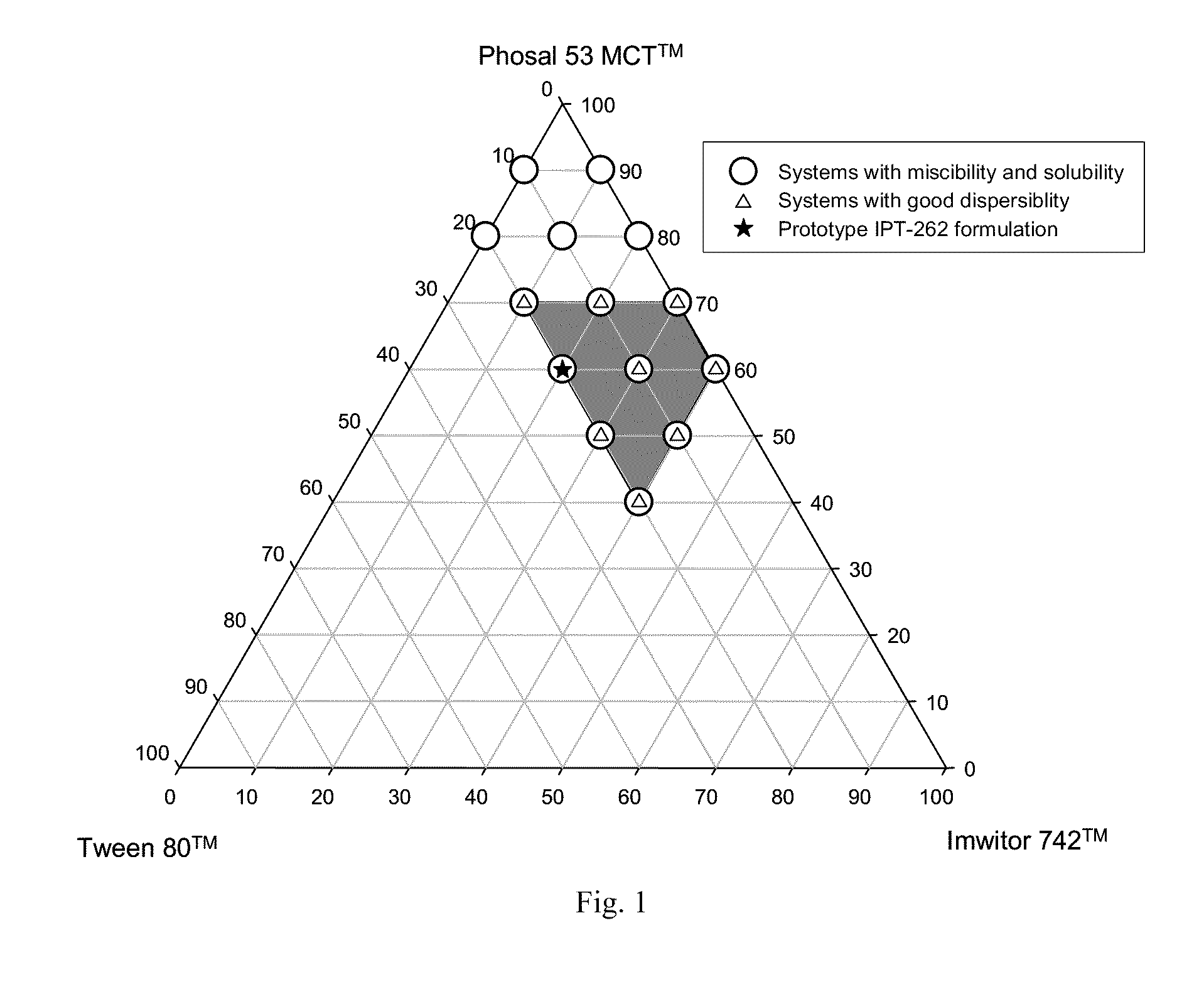
Stabilized lipid formulation of apoptosis promoter
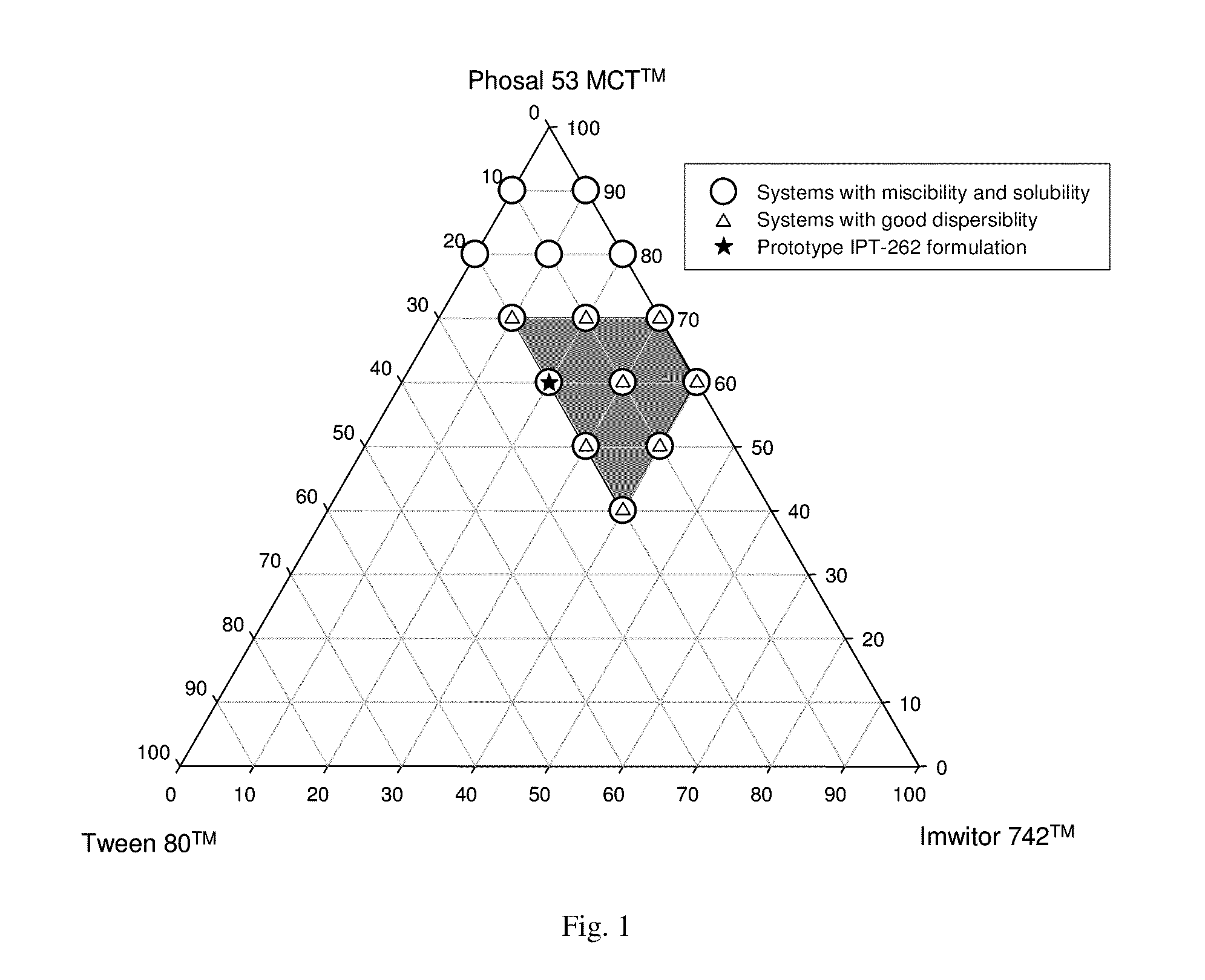
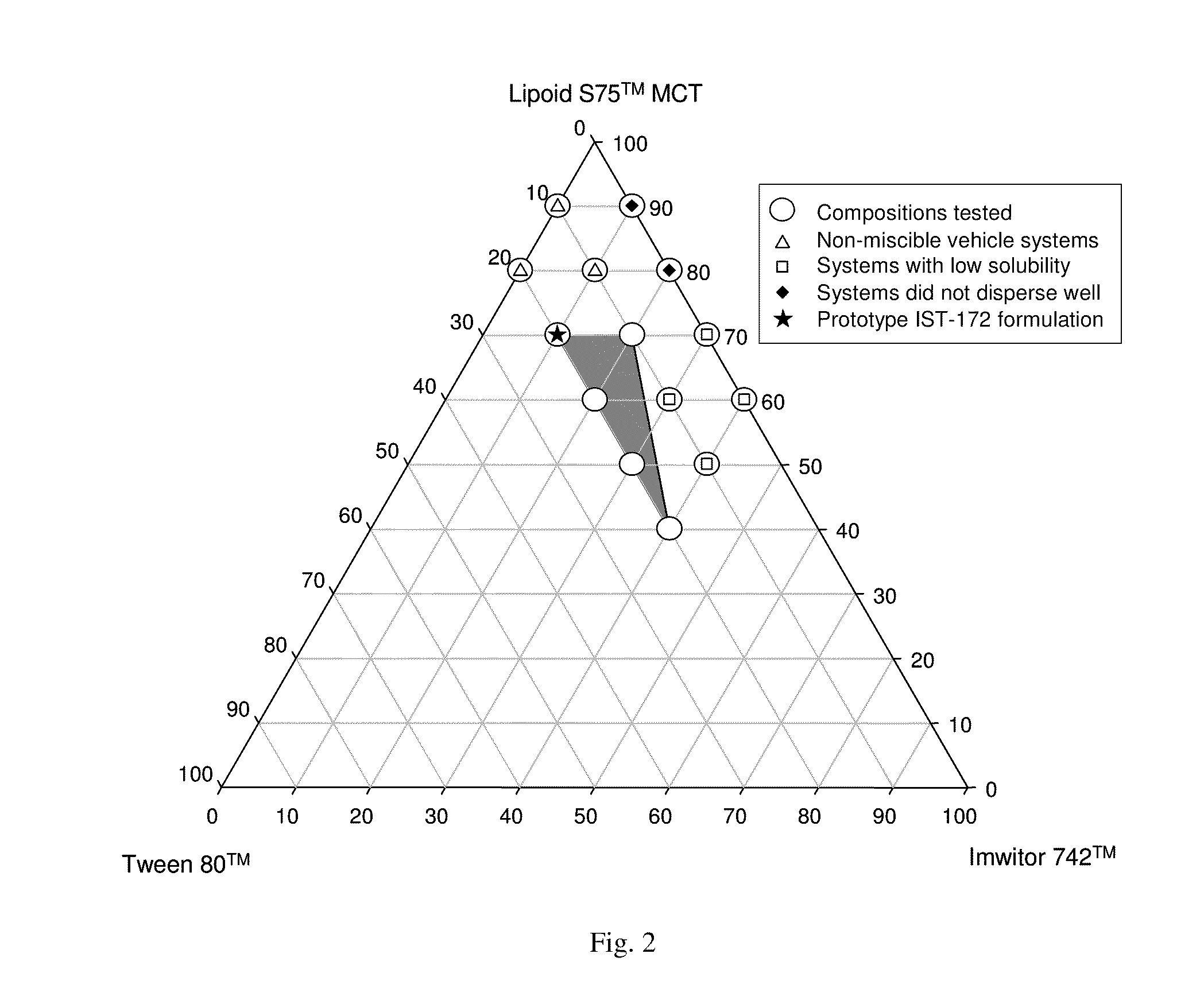
InactiveUS20100278905A1Convenient route of administrationEasy to doOrganic active ingredientsDispersion deliveryLipid formationDisease
An orally deliverable pharmaceutical composition comprises a Bcl-2 family protein inhibitory compound, e.g., ABT-263, a heavier-chalcogen antioxidant and a substantially non-aqueous lipid carrier, wherein said compound and said antioxidant are in solution in the carrier. The composition is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
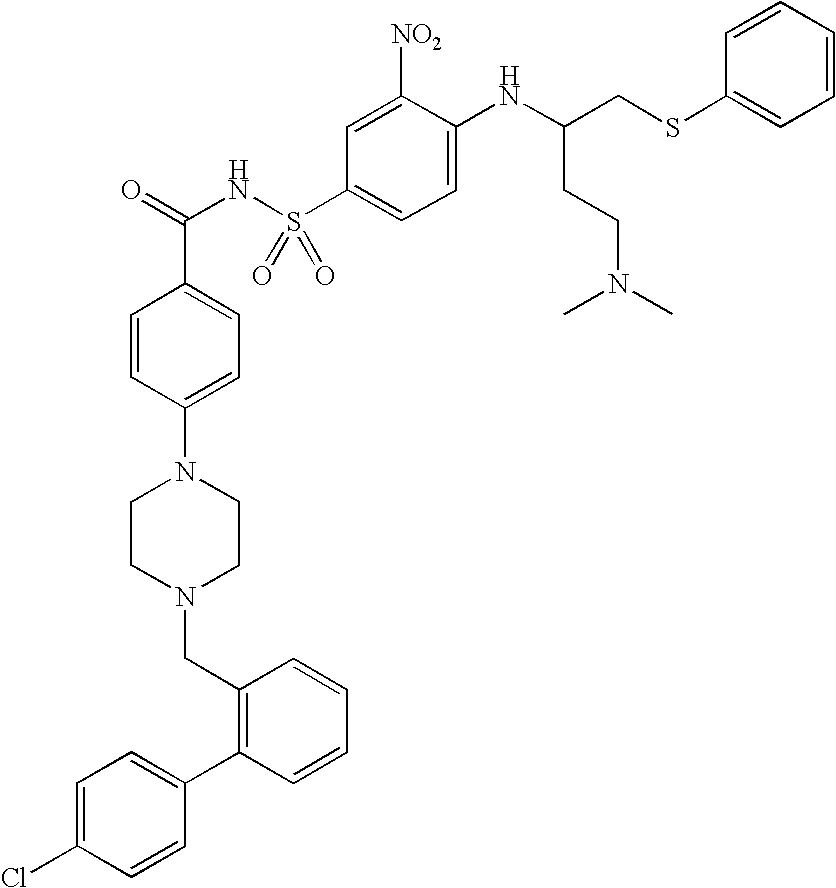
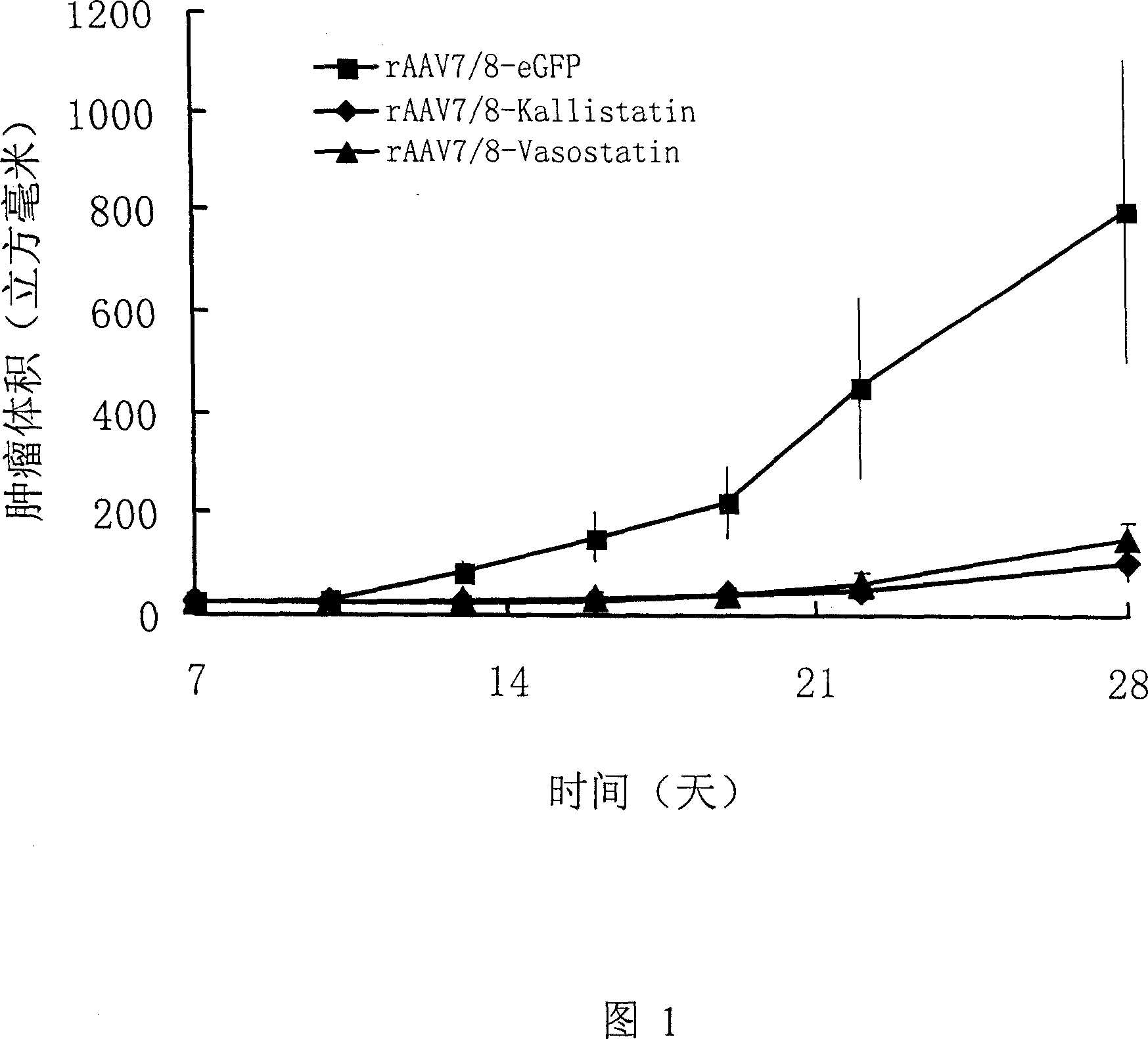
Genetic medicine for preventing and treating cancer of colon and rectum, preparation process and use thereof
InactiveCN1966082AEfficient transfectionConvenient route of administrationGenetic material ingredientsAntineoplastic agentsKallistatinOncology
The invention relates to a gene drug based on genes as kallistatin and vasostatin, to treat carcinoma of colon, wherein it also provides relative application to improve treatmene effect. The carrier of genes is adenovirus (AAV), while the best one is AAV7 / 8. The inventive drug can be made into injection or dried powder, and it can be injected into muscle.
Owner:许瑞安 +2
Solid Dispersions Containing Kinase Inhibitors
InactiveUS20110306632A1Attenuate induction of apoptosisStrong cytotoxicityPowder deliveryBiocideWater solubleSolvent
A solid dispersion comprises, in essentially non-crystalline form, a kinase inhibitory compound, e.g., N-(4-{4-amino-7-[1-(2-hydroxyethyl)-1H-pyrazol-4-yl]thieno[3,2-c]pyridin-3-yl}phenyl)-N′-(3-fluorophenyl)urea, dispersed in a solid matrix that comprises (a) a pharmaceutically acceptable water-soluble polymeric carrier and (b) a pharmaceutically acceptable surfactant. A process for preparing such a solid dispersion comprises dissolving the compound, the polymeric carrier and the surfactant in a suitable solvent, and removing the solvent to provide a solid matrix comprising the polymeric carrier and the surfactant and having the compound dispersed in essentially non-crystalline form therein. The solid dispersion is suitable for oral administration to a subject in need thereof for treatment of a cancer.
Owner:ABBVIE INC
Solid dispersions containing kinase inhibitors
InactiveUS8557995B2Convenient route of administrationEasy to doBiocidePowder deliverySolventKinase inhibition
Owner:ABBVIE INC
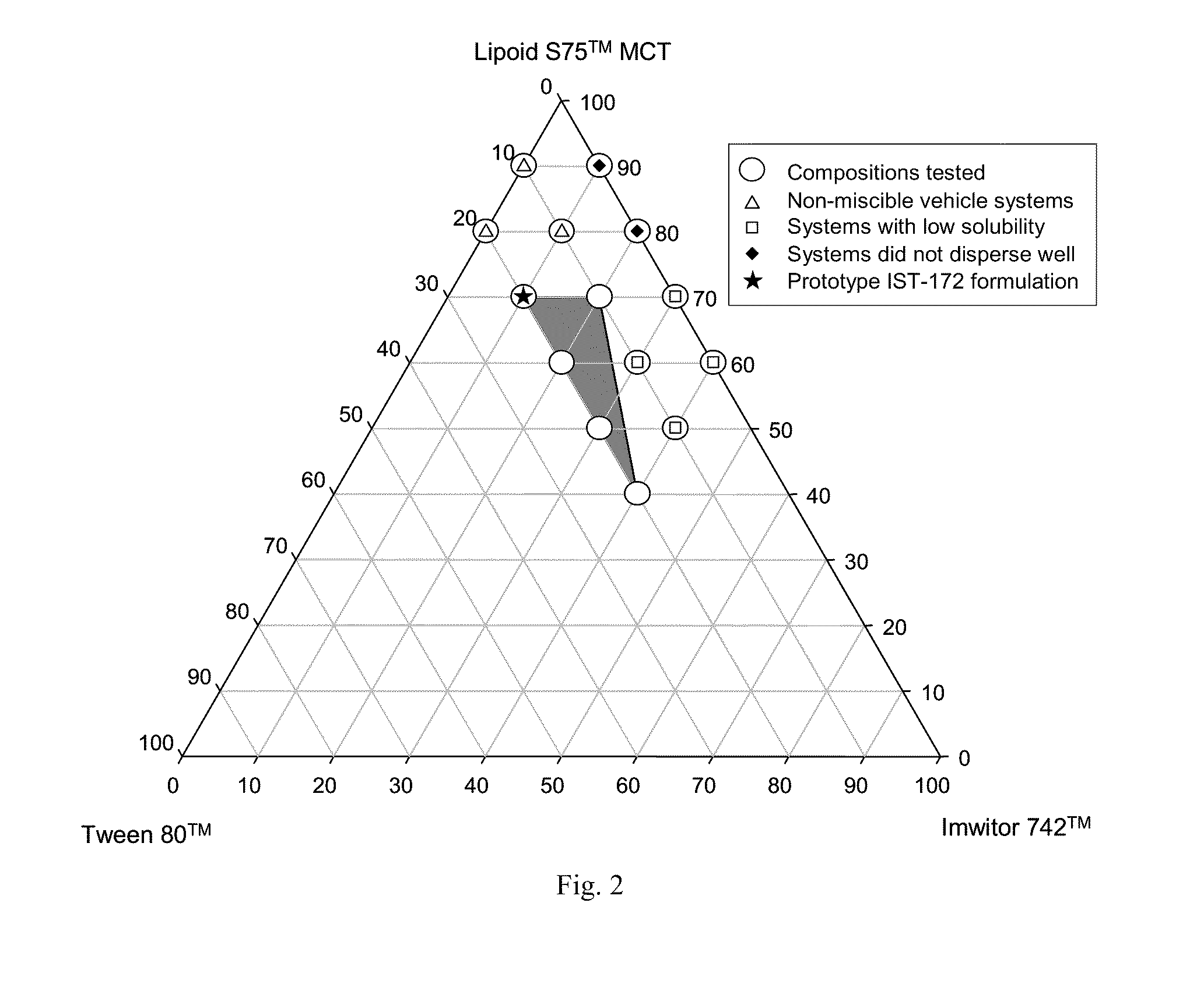
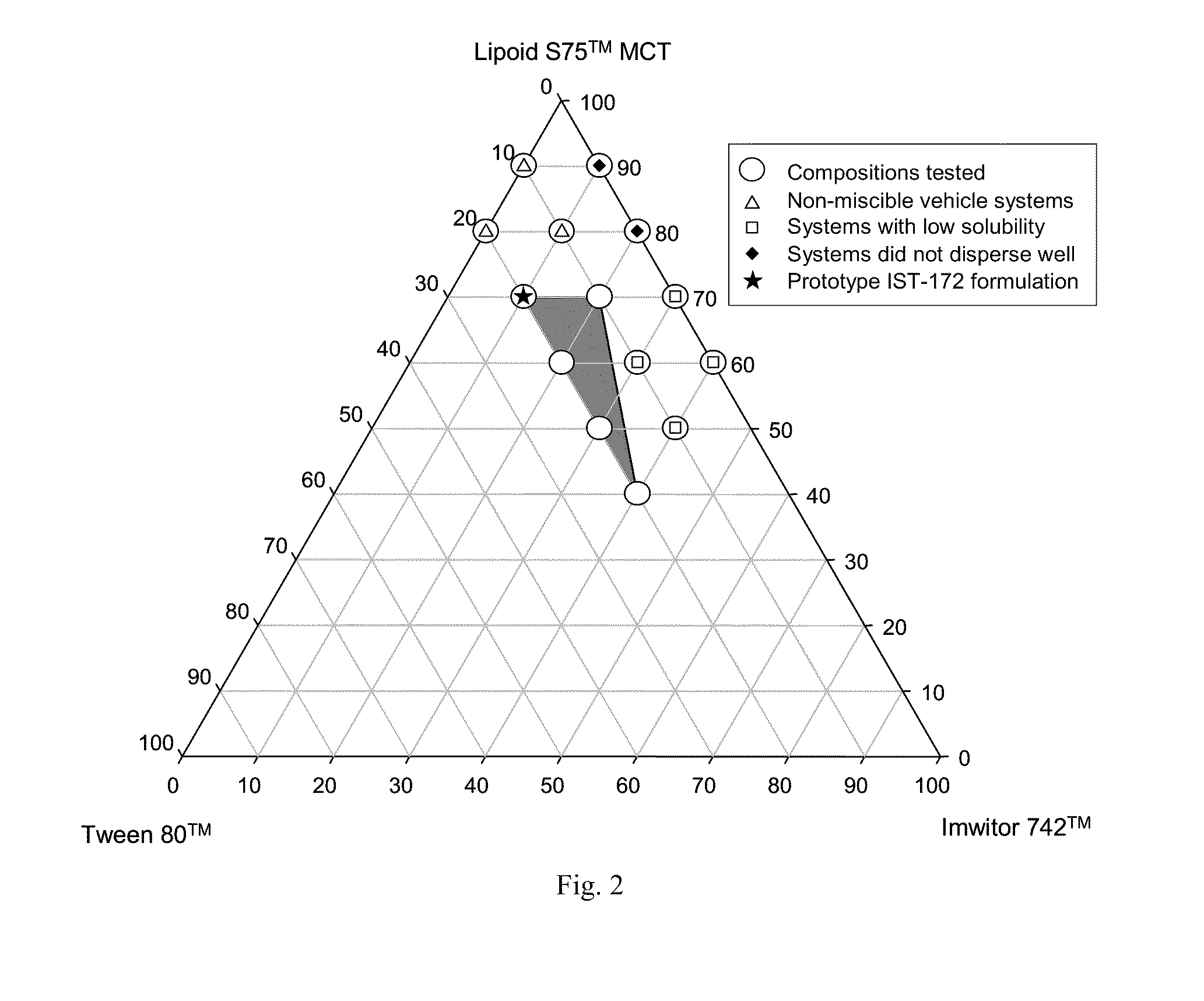
Lipid formulation of apoptosis promoter
InactiveUS20100280031A1Improve oral bioavailabilityConvenient route of administrationOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseOral medication
An orally deliverable pharmaceutical composition comprises a drug-carrier system having a Bcl-2 family protein inhibitory compound, e.g., ABT-263, in solution in a substantially non-aqueous carrier that comprises at least one phospholipid and a pharmaceutically acceptable solubilizing agent. The composition is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBOTT LAB INC
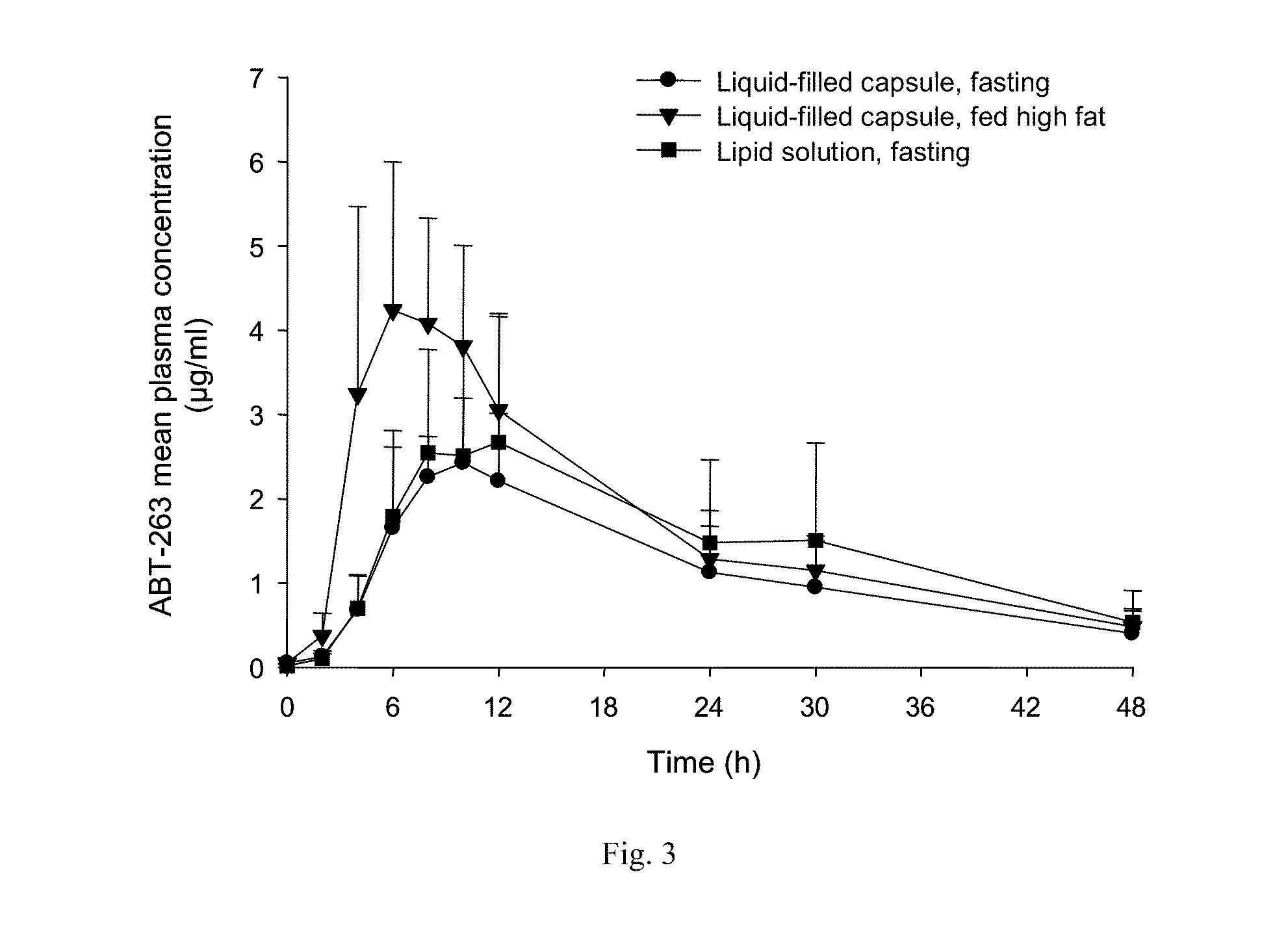
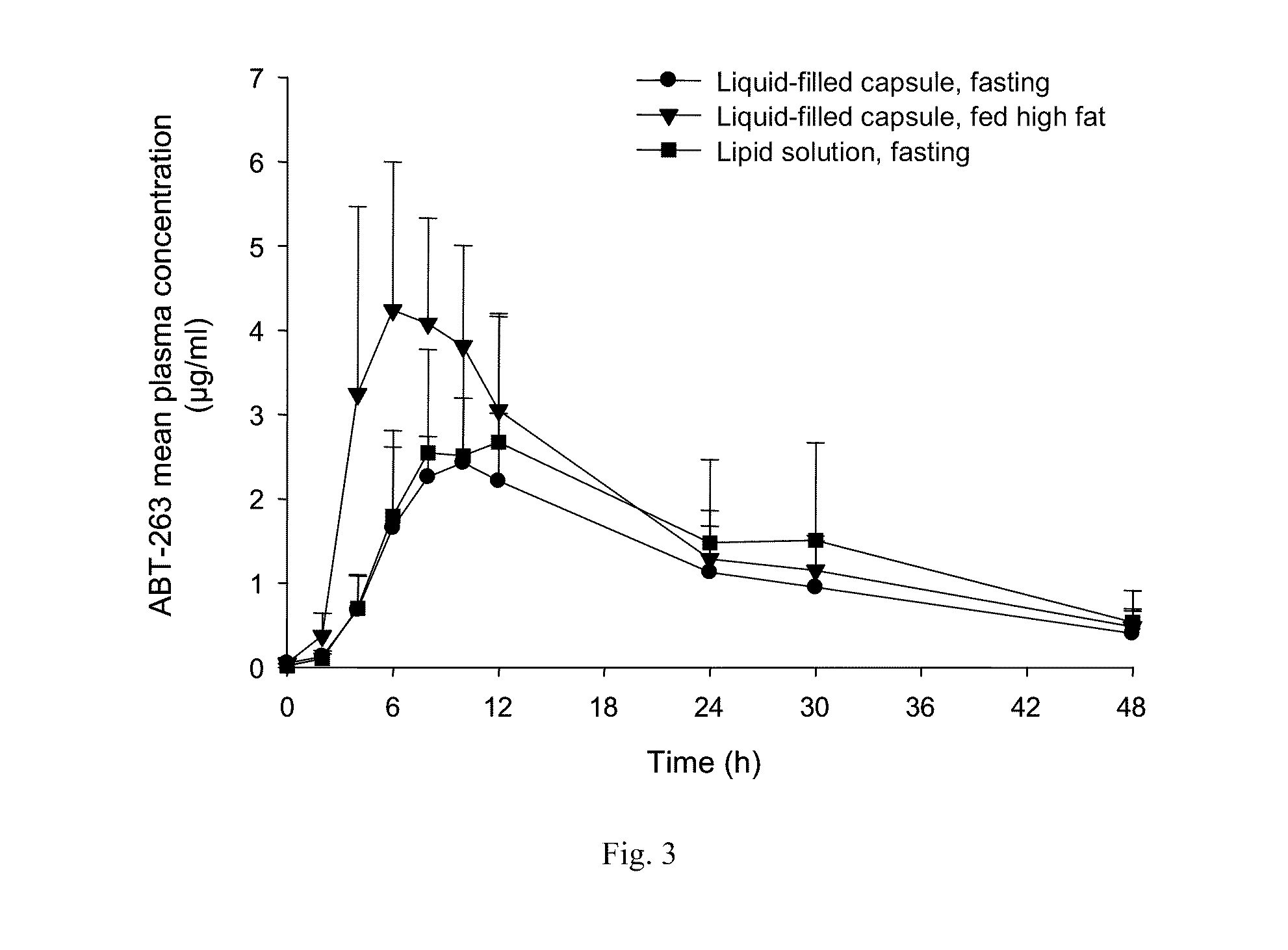
Abt-263 capsule
InactiveUS20110159085A1Reduce formationReduce rateOrganic active ingredientsPharmaceutical product form changeSolventDrug
A pharmaceutical capsule comprises a shell having encapsulated therewithin a liquid solution of ABT-263 or a pharmaceutically acceptable salt thereof in a substantially non-ethanolic carrier that comprises as pharmaceutically acceptable excipients (a) at least one phospholipid, (b) at least one solubilizing agent for the at least one phospholipid, selected from the group consisting of glycols, glycerides and mixtures thereof, (c) at least one non-phospholipid surfactant and (d) at least one sulfur-containing antioxidant. The capsule is useful in treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
Recombinant expression vector for expressing LL-37 polypeptide, recombinant lactococcus lactis, antiviral drug, construction method and application
ActiveCN111500615AAvoid cumbersomenessAvoid efficiencyAntibacterial agentsPolypeptide with localisation/targeting motifStaphylococcus lactisNucleotide
The invention provides a recombinant expression vector for expressing LL-37 polypeptide, recombinant lactococcus lactis, an antiviral drug, a construction method and application, and belongs to the technical field of gene engineering. A skeleton plasmid for constructing the recombinant expression vector is PNZ8149; a fusion gene is inserted into the recombinant expression vector; the nucleotide sequence of the fusion gene is shown as SEQ ID NO: 1. The recombinant expression vector disclosed by the invention can be efficiently expressed in lactococcus lactis, and the LL-37 obtained by expression can effectively inhibit SARS coronavirus and SARS-CoV2 coronavirus. The invention also provides recombinant lactococcus lactis containing the recombinant expression vector in the scheme. According to the recombinant lactococcus lactis disclosed by the invention, an antiviral polypeptide drug LL-37 can be input into a human body in an oral form, the antiviral effect is good, and the production cost is low.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Lipofectin nanometer silver gel and preparation method thereof
ActiveCN103006561AImprove stabilityRelease stabilityInorganic active ingredientsAntisepticsCholesterolGlycerol
The invention relates to a lipofectin nanometer silver gel and a preparation method of the lipofectin nanometer silver gel. The lipofectin nanometer silver gel is prepared from the following raw materials in parts by weight: 1.5 to 4.5 parts of soya bean lecithin and cholesterol, 1 to 9 parts of chitosan, 1 to 20 parts of glycerol, 0.1 to 2 parts of oleum menthae, 30 to 70 parts of acetum, 20 to 60 parts of nanometer silver solution, and the balance of deionized water. According to the preparation method, the nanometer silver is prepared into lipofectin, most of the nanometer silvers are encapsulated in a bilayer formed by the lipofectin, and therefore the stability of the nanometer silver can be improved; and the proper macromolecular optimum formula is adopted to prepare the gel, thus the convenience is provided for paving medicine on the skin. The prepared preparation has the advantages of being stable in medicine release rate, convenience in medicine paving, low in frequency of using the medicine, free from toxicity and stimulation and high in compliance of a user, and can be used for preventing and treating skin intolerance caused by various pathogenic microorganisms.
Owner:ZHEJIANG SANHE NANOMETER SCI & TECH
Enema liquid for treating hyperpyrexia and cough and asthma
InactiveCN101766787AEffective treatmentPrevention of cough and asthmaAnthropod material medical ingredientsAntipyreticDiseaseSide effect
The invention discloses an enema liquid for treating hyperpyrexia and cough and asthma, which is prepared by using stiff silkworm, curcuma longa, cicada slough, wine-processed rhubarb, raw ephedra herb, cassia twig, gypsum, almond, earthworm, red paeony root, tangerine peel, mix-fried tatarian aster root and mix-fried liquoric root as the raw material through the following steps: firstly adding water into gypsum to decoct; after decocting, adding the decocted gypsum in other raw material medicaments which are marinate in advance to decoct; and after decocting the raw material medicaments, filtering out a medicinal liquid, i.e. the enema liquid for treating hyperpyrexia and cough and asthma. The product of the invention mainly has the effects of expelling wind and cold pathogens externally, hot and suffocating of upper, middle and lower warmer internally, clearing and descending lung qi, purging fu-organs and relieving flatulence, relieving cough and asthma and relieving dizziness and convulsion, and is suitable to treat high fever in children, cough and dyspnea and tachypnea or the diseases with symptoms of aversion to cold, lassitude, syncope with convulsion, clonic convulsion and the like. The medicament formula of the product of the invention directly guides and is applied clinically according to the principles of Chinese medicine. The clinical curative effect shows that the product of the invention has the advantages of quick response, recurrence prevention, no any toxic or side effects, obvious treating effect and valuable popularization and application.
Owner:武月萍
Medicine for treating hypomenorrhea due to deficiency of kidney and preparation method thereof
InactiveCN104721582AImprove performanceTo promote metabolismAnthropod material medical ingredientsMammal material medical ingredientsPenisFresh water organism
The invention discloses a medicine for treating hypomenorrhea due to the deficiency of the kidney and a preparation method thereof. The medicine is prepared from the following medicinal raw materials: dried human placenta, eucommia bark, flastem milkvetch seeds, tokay, hairy antlers, sea horses, ursine seal testes and penes, prepared rhizome of adhesive rehmannias, dried longan pulp, cochinchinese asparagus roots, noble dendrobium stems, manyflower solomonseal rhizomes, barbary wolfberry fruits, glossy privet fruit, tortoise shells, rose flowers, gaulis aristolochiae, Danshen roots, twotooth achyranthes roots, suberect spatholobus stems, goat or sheep testes, dragonflies, Elaphurus Davidianus Milne-Edwards, greywhitehair raspberry fruits, air bladders of yellow croakers, freshwater sponge, wild groundnut seeds, cushaw stems, dahurian rose fruits and ford metalleaf roots. The medicine has the functions of nourishing the kidney, strengthening the essence, nourishing blood and regulating menstruation and is mainly used for treating hypomenorrhea due to the deficiency of the kidney. The medicine is prepared by integrating the concept of wholism with the treatment based on syndrome differentiation, can be used for improving the overall function, state and immunity of the human body, regulating qi and blood and improving the metabolism of blood, is targeted at hypomenorrhea due to the deficiency of the kidney, can take effect on the diseased part directly, is short in course of treatment, causes less adverse reactions, is safe and efficient, can be used selectively according to different clinical symptoms, can be applied conveniently and can be easily accepted by patients, and patients can not become resistant to the medicine.
Owner:李佃场
Anti-cancer anti-disease buccal tablet
InactiveCN102441000AAvoid lossImprove absorption and utilizationOrganic active ingredientsMetabolism disorderDiseaseMedicine
The invention discloses an anti-cancer anti-disease buccal tablet which is prepared from the following components in parts by weight: 25-32 parts of yellow fungus polysaccharide, 25-32 parts of Grifola frondosa polysaccharide, 23-29 parts of aweto polysaccharide, 7-12 parts of microcrystalline cellulose, 0.6-2 parts of low-substituted hydroxypropyl cellulose, 2-4 parts of xylitol, 4-7 parts of magnesium stearate and 0.2-0.5 part of essence sodium. The anti-cancer anti-disease buccal tablet is convenient to take and carry and easy to store, has obviously higher bioavailability than common preparations, and provides a convenient administration route for adapting to the present fast-paced living demands and increasingly enhanced living taste.
Owner:SHANDONG UNIV
Chinese herbal compound functional food for adjuvant therapy of diabetes and preparation method thereof
InactiveCN107969698AExtend the life cycleImprove the quality of lifeMetabolism disorderNatural extract food ingredientsVitamin CLife quality
The invention discloses a Chinese herbal compound functional food for adjuvant therapy of diabetes and a preparation method thereof. The Chinese herbal compound functional food consists of the following raw materials: a burdock root extract, a pueraria extract, a rhizoma polygonati extract, vitamin C, xylooligosaccharide, xylitol, an arabian jasmine flower extract and magnesium stearate. Accordingto the Chinese herbal compound functional food for adjuvant therapy of diabetes, the postprandial blood sugar and fasting blood-glucose can be reduced effectively, the food can be used for adjuvant therapy of diabetics, the food is reasonable in formula and high in safety and has prevention and treatment effects for diabetic complications, the kidney injury degree of diabetics can be improved, the life cycle of diabetics is prolonged, and the life quality of diabetics is improved; the Chinese herbal compound functional food for adjuvant therapy of diabetes disclosed by the invention is rich in hypoglycemic activity substances such as fructo-oligosaccharides, polysaccharides, anthraquinone compounds and various amino acids and vitamins, is sour and sweet and unique in flavor, and has the functions of health care and nutrition.
Owner:博德生物技术(德州)有限公司
Recombinant adenovirus, preparation method and application
InactiveCN109679929AImprove securityNo cancer riskPeptide/protein ingredientsDigestive systemSingle injectionShuttle plasmid
The invention discloses recombinant adenovirus, a preparation method and application. The preparation method comprises the following steps: cloning a TMEM88 gene into a cloning region of adenovirus shuttle plasmids and constructing recombinant shuttle plasmids for expressing TMEM88; co-transforming the recombinant shuttle plasmids and adenovirus framework plasmids into host cells; carrying out homologous recombination to generate recombinant adenovirus plasmids; packaging through packaging cells to obtain adenovirus particles with infection activity and amplifying in the packaging cells; aftercollecting and cracking the packaging cells, purifying to obtain the recombinant adenovirus. According to the recombinant adenovirus, adenovirus is used as a carrier and is safe and reliable; the adenovirus is not combined with host chromosome DNA (Deoxyribonucleic Acid) and the risk of canceration is not caused; the recombinant adenovirus has a remarkable effect on hepatic fibrosis and the reversing effect can be observed with a single injection. The recombinant adenovirus is convenient for drug administration due to the drug administration manner; the recombinant adenovirus is introduced into rats by adopting a tail vein injection manner. The best time for reversing the hepatic fibrosis is obtained.
Owner:ANHUI MEDICAL UNIV
Solid dispersions containing an apoptosis-inducing agent
ActiveUS20150157639A1Convenient route of administrationEasy to doOrganic active ingredientsPowder deliveryDiseaseOral medication
A pro-apoptotic solid dispersion comprises, in essentially non-crystalline form, a Bcl-2 family protein inhibitory compound of Formula I as defined herein, dispersed in a solid matrix that comprises (a) a pharmaceutically acceptable water-soluble polymeric carrier and (b) a pharmaceutically acceptable surfactant. A process for preparing such a solid dispersion comprises dissolving the compound, the polymeric carrier and the surfactant in a suitable solvent, and removing the solvent to provide a solid matrix comprising the polymeric carrier and the surfactant and having the compound dispersed in essentially non-crystalline form therein. The solid dispersion is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
Solid dispersions containing an apoptosis-promoting agent
InactiveCN102802607AConvenient route of administrationPowder deliveryOrganic active ingredientsDiseaseOral medication
A pro-apoptotic solid dispersion comprises, in essentially non-crystalline form, a Bcl-2 family protein inhibitory compound, e.g., ABT-263, dispersed in a solid matrix that comprises (a) a pharmaceutically acceptable water-soluble polymeric carrier and (b) a pharmaceutically acceptable surfactant. A process for preparing such a solid dispersion comprises dissolving the compound, the polymeric carrier and the surfactant in a suitable solvent, and removing the solvent to provide a solid matrix comprising the polymeric carrier and the surfactant and having the compound dispersed in essentially non-crystalline form therein. The solid dispersion is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
Salt of ABT-263 and solid-state forms thereof
InactiveUS8362013B2Convenient route of administrationEasy to doOrganic active ingredientsOrganic chemistryDiseaseMedicine
Owner:ABBVIE INC
ABT-263 capsule
InactiveUS8927009B2Convenient route of administrationEasy to doOrganic active ingredientsPharmaceutical product form changeDiseaseActive agent
A pharmaceutical capsule comprises a shell having encapsulated therewithin a liquid solution of ABT-263 or a pharmaceutically acceptable salt thereof in a substantially non-ethanolic carrier that comprises as pharmaceutically acceptable excipients (a) at least one phospholipid, (b) at least one solubilizing agent for the at least one phospholipid, selected from the group consisting of glycols, glycerides and mixtures thereof, (c) at least one non-phospholipid surfactant and (d) at least one sulfur-containing antioxidant. The capsule is useful in treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
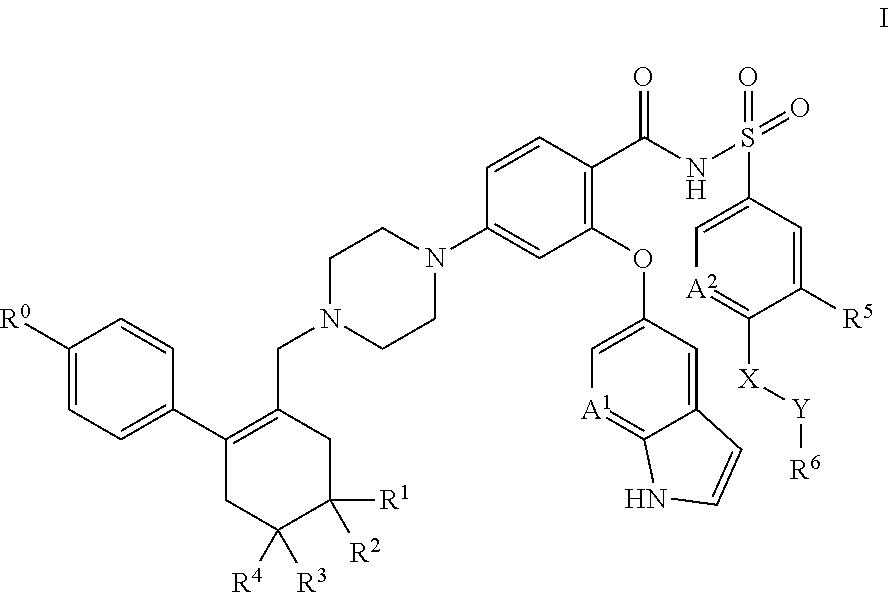
Application of compound in treatment of autoimmune skin diseases caused by inflammation
PendingCN111840287AEffective treatmentImprove the quality of lifeOrganic active ingredientsOrganic chemistryPharmaceutical medicinePharmacology
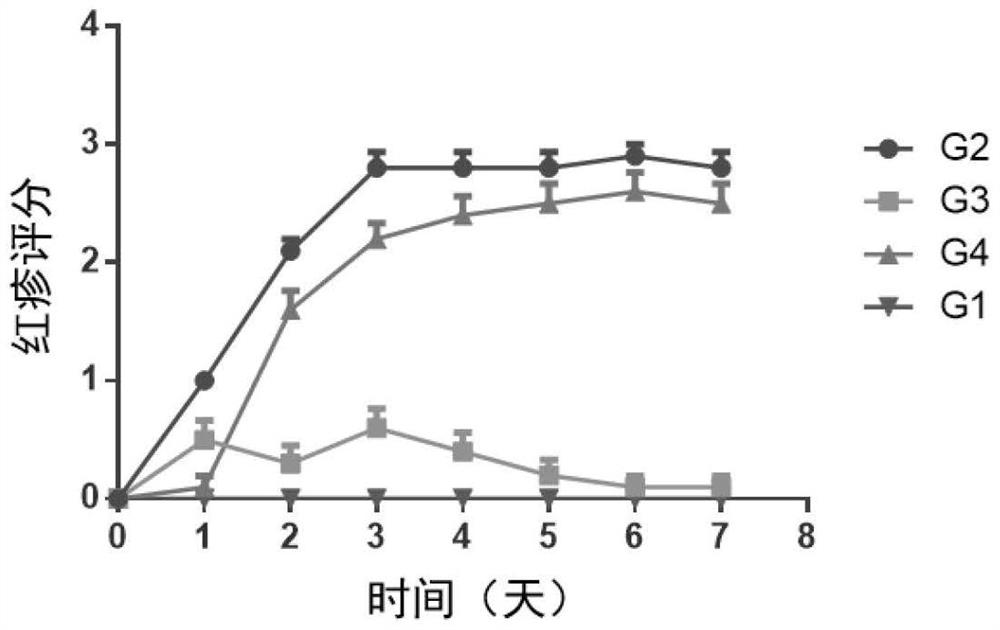
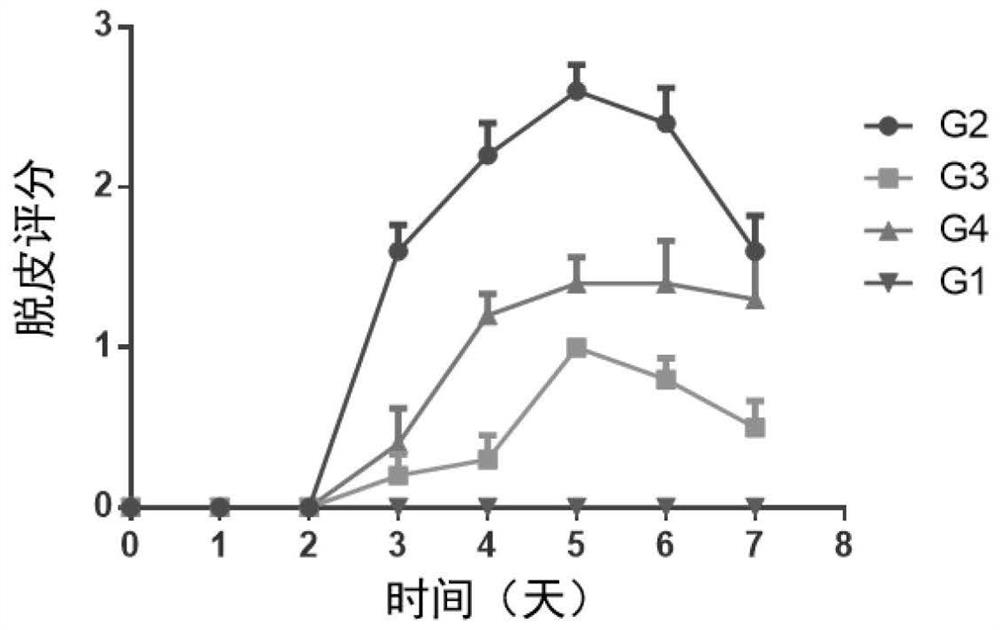
The invention discloses application of a compound Nib1, a pharmaceutically acceptable salt, a solvate, a solvate of the pharmaceutically acceptable salt or a crystal form thereof in preparation of a medicine for treating autoimmune skin diseases caused by inflammation, improving skin erythema, improving skin decrustation, reducing skin thickening, reducing epidermal hyperplasia, improving angiectasis and / or reducing immune cell infiltration. The chemical formula of the Nib1 is C28H29F2N3O. The compound provided by the invention is a new treatment mechanism aiming at autoimmune skin diseases (such as psoriasis diseases) caused by inflammation, is small in side effect and convenient in administration route, can achieve the balance of safety and effectiveness, can be used for a long time, andimproves the life quality of patients. The compound can significantly improve clinical symptoms of autoimmune skin diseases caused by inflammation, such as erythema cutanea, skin peeling, skin thickening, epidermal hyperplasia, improve angiectasis and reduce immune cell infiltration and the like.
Owner:GENEROS BIOPHARMA LTD
Method for producing endomorphin 2 via high-efficiency expression, and application for same
InactiveCN103320472ATo achieve the purpose of long-term pain treatmentConvenient route of administrationNervous disorderTetrapeptide ingredientsSequence signalEndogenous Opiates
The invention belongs to the technical field of biological genes, wherein the related literature reports of realizing high-efficiency secretion for endomorphin 2 by virtue of a human-original nerve growth factor leader peptide are inexistent at present. The invention provides a method for producing endomorphin 2 via high-efficiency expression. The method comprises the following steps of: designing a fused gene including a human-original nerve growth factor signal peptide sequence and an endomorphin 2 expression sequence; then inserting the fused gene in an expression carrier; then converting host cells, screening and authenticating positive recombinants, performing induced culture, and externally secreting expression protein; and finally collecting the product via purification. The expression product, namely, endomorphin 2, prepared by the method, is applied to preparation for medicines treating chronic pain and cancer pain. The product is an endogenous opiate peptide, thus being free form the reactions of habituation, tolerance and the like, as well as capable of being expressed for a long term in a body, so as to achieve the purpose of treating pain for a long time.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Cyclocarya paliurus granule tea bag and preparation method thereof
InactiveCN106615376AHigh health valueGood hypoglycemic activityPre-extraction tea treatmentBlack teaGlucose polymers
The invention belongs to the technical field of healthy drinks, and specifically relates to a cyclocarya paliurus granule tea bag and a preparation method thereof. The cyclocarya paliurus granule tea bag is prepared from the following components in percentage by weight: 50% to 85% of cyclocarya paliurus old leaf black tea powder and 15% to 50% of a filler. The cyclocarya paliurus granule tea bag maintains the color, fragrance and taste of natural tea, and also abandons the defect that traditional powder-like tea bags are likely to pollute the environment in the production process and are likely to go bad in the storage process; meanwhile, the quality, perception and blood glucose reduction effects of tea are improved, and a convenient administration route is also provided for adapting to the requirement of modern fast pace life and continuously increasing life taste; and the cyclocarya paliurus granule tea bag is simple in technology, low in production cost and great in health care value.
Lipid-lowering buccal tablets
InactiveCN101837062APromote decompositionPromote absorptionMetabolism disorderPill deliveryAdditive ingredientIsomalt
Owner:SHANDONG UNIV
Liquid traditional Chinese medicine for treating verruca plana and preparation method thereof
InactiveCN105878581AConvenient route of administrationQuick effectAnthropod material medical ingredientsPharmaceutical delivery mechanismPeppermintsOldenlandia diffusa
The invention discloses a liquid traditional Chinese medicine for treating verruca plana and a preparation method thereof. The liquid traditional Chinese medicine is prepared from the following traditional Chinese medicinal raw materials in parts by weight: 15-25 parts of oldenlandia diffusa, 15-25 parts of opuntia stricta, 10-20 parts of herba portulacae, 10-20 parts of salvia miltiorrhiza, 10-20 parts of clerodendrum bungei, 5-15 parts of sedum lineare, 5-15 parts of radix semiaquilegiae, 5-15 parts of flos sophorae, 4-12 parts of frankincense, 2-8 parts of periostracum cicada, 2-8 parts of peppermint and 0.02-0.06 part of cantharides. The liquid traditional Chinese medicine is prepared by adopting common traditional Chinese medicinal materials as raw materials, is convenient in administration route, quick in response and low in cost, does not have irritation, toxic or side effect, has remarkable curative effect, prevents scar or relapse, has the effects of clearing heat, removing toxicity, nourishing blood, dispelling wind, regulating liver, strengthening spleen, softening hard mass and removing verruca, and can be used for quickly and effectively treating verruca plana.
Owner:孙明香
Hydrogenated castor oil nano-lipophilic medicine preparation and preparation method thereof
InactiveCN101143220AReduce releaseGood dispersionPowder deliveryOrganic active ingredientsPolyvinyl alcoholDrugs preparations
The invention provides a nanometer-lipophilic drug preparation of hydrogenated castor oil and the preparation method of the nanometer-lipophilic drug preparation. The nanometer drug preparation consists of a lipophilic drug, the hydrogenated castor oil and little polyvinyl alcohol. The nanometer-lipophilic drug preparation of hydrogenated castor oil of the invention has the efficacy of slowly releasing and at the same time is capable of improving the dispersion of the drug, the utilization degree of the drug and the drug effect, and that the storage, the transportation and the administration route of the drug are more convenient.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com