Solid dispersions containing an apoptosis-promoting agent
A technology of solid dispersion and solid matrix, which can be used in medical preparations containing active ingredients, organic active ingredients, oil/fat/wax non-active ingredients, etc., and can solve problems such as impracticality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0217] Embodiment 1: Preparation of the solid dispersion of ABT-263 diHCl
[0218] ABT-263 diHCl crystalline salt was mixed with surfactant and water-soluble polymer in the following weight ratio:
[0219] 10.8% ABT-263 salt (10% free base equivalent); 10% surfactant; 79.2% polymer
[0220] 21.5% ABT-263 salt (20% free base equivalent); 10% surfactant; 68.5% polymer
[0221] 32.3% ABT-263 salt (30% free base equivalent); 10% surfactant; 57.7% polymer
[0222] 43% ABT-263 salt (40% free base equivalent); 10% surfactant; 47% polymer
[0223] Surfactants in different series are TPGS, Span™ 20 or Tween™ 20. The polymers in different series are copovidone (Kollidon® VA 64), povidone K-30 or HPMC-AS.
[0224] The ingredient mixture was dissolved in methanol in each case. Methanol was removed in vacuo at 65°C using a Genevac® system, and the resulting solid dispersion was allowed to cool to ambient temperature.
[0225] The solid dispersion in each case was sieved through a 4...
Embodiment 2
[0228] Embodiment 2: Preparation of solid dispersion of ABT-263 free base
[0229] ABT-263 diHCl crystalline salt was dissolved in acetone, and NaOH was added to convert ABT-263 diHCl to free base. The NaCl by-product was precipitated and removed by filtration.
[0230] In the acetone solution of obtained ABT-263 free base, add surfactant and water-soluble polymer of following weight ratio:
[0231] 10% ABT-263 free base; 10% surfactant; 80% polymer
[0232] 20% ABT-263 free base; 10% surfactant; 70% polymer
[0233] 30% ABT-263 free base; 10% surfactant; 60% polymer
[0234] 40% ABT-263 free base; 10% surfactant; 50% polymer
[0235] Surfactants in different series are TPGS, Span™ 20 or Tween™ 20. Polymers in different series are copovidone (Kollidon® VA 64) or HPMC-AS.
[0236] Acetone was removed in vacuo at 65°C using a Genevac® system, and the resulting solid dispersion was allowed to cool to ambient temperature.
[0237] The solid dispersion in each case was sie...
Embodiment 3
[0240] Example 3: Dissolution Profile of Solid Dispersions
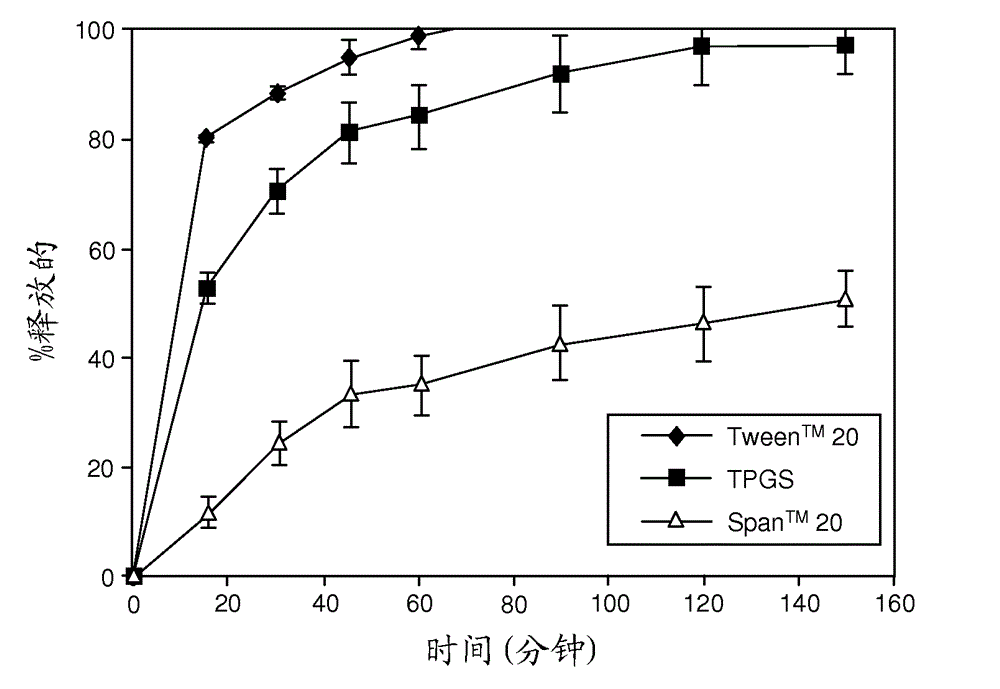
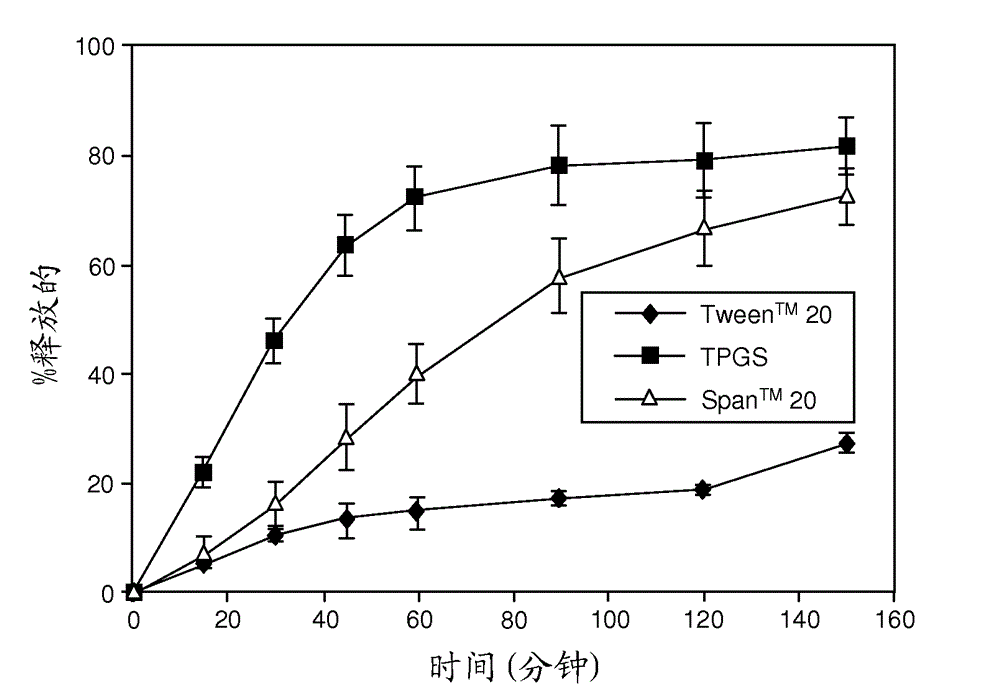
[0241]Representative dissolution (drug release) profiles in pH 6.5 buffered media containing 7.6 mM Tween® 80 are shown in figure 1 (ABT-263 diHCl) and figure 2 (ABT-263 free base).
[0242] like figure 1 As shown in , at 20% drug loading level, ABT-263 diHCl solid dispersion with 68.5% copovidone and 10% TPGS showed a moderate rate of drug release, which reached a plateau at about 80% release. The release from similar dispersions with Span™ 20 or especially Tween™ 20 as surfactant was much slower.
[0243] In contrast, if figure 2 ABT-263 free base solid dispersion with 70% copovidone and 10% Tween® 20 or TPGS showed fast drug release at the same 20% drug loading level as shown in . In the case of the free base dispersion, only Span™ 20 surfactant resulted in a much slower release.
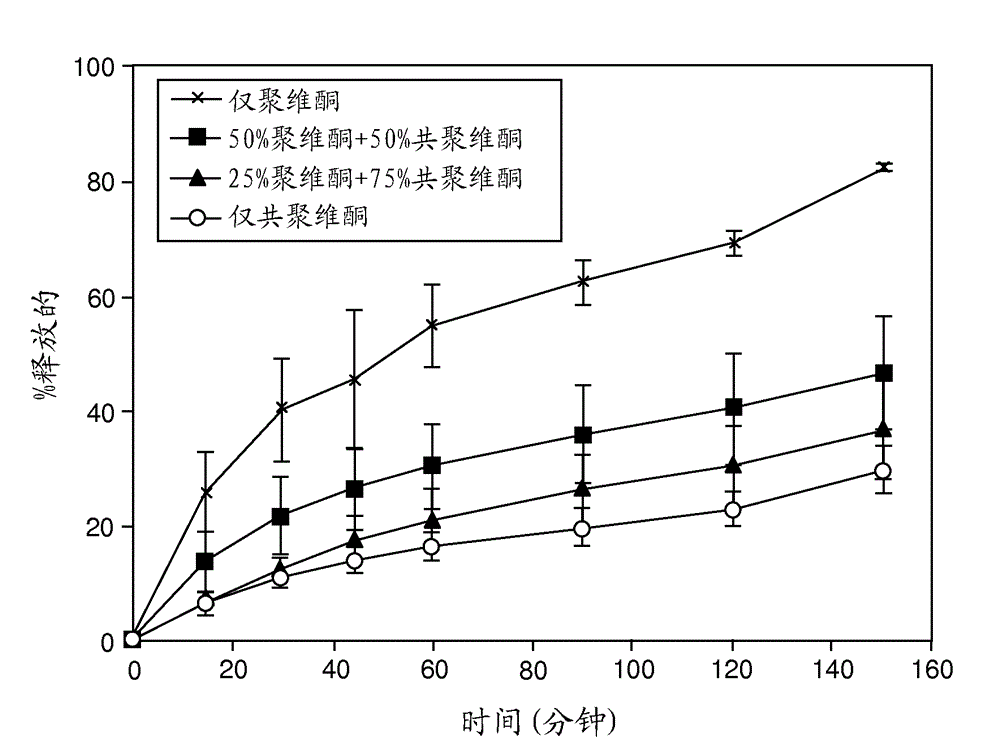
[0244] The release rate was drug load dependent for both ABT-263 diHCl and free base dispersion formulations, with the 20%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com