Method for producing endomorphin 2 via high-efficiency expression, and application for same
A high-efficiency expression technology of endomorphin, applied in the direction of biochemical equipment and methods, application, botany equipment and methods, etc., to achieve good effect and convenient administration route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Acquisition of Fusion Gene and Construction of Adenovirus
[0031] 1. Acquisition and identification of target genes
[0032] The target gene was amplified from the plasmid pTCNE by PCR method, and the primers used for the amplification were sequenced as follows:
[0033] Forward sequence: 5'-ACGCGAATTCACCATGTCCCAT-3' (SEQ ID NO: 4);
[0034] Reverse sequence: 5'-CAAGAGCAAGCGCTACCCCTTCTTCTAAGGATCCATAT-3' (SEQ ID NO: 5);
[0035] PCR system:
[0036]
[0037] Melting at 94°C for 5 minutes; 32 cycles of 94°C for 50s, 56°C for 50s, and 72°C for 90s; elongation at 72°C for 10 minutes. Take 5 μl and do 1% agarose gel electrophoresis (containing EB 0.5 μg / ml; voltage: 80V) for identification; the rest is used for recovery and purification of target fragments.
[0038] The target gene and pUC19 plasmid were digested by EcoRI and BamHI respectively, then ligated into pUC19-NEM2 with T4 DNA ligase, and transformed into E.coli.DH5α competent cells (purchased from ...
Embodiment 2
[0047] Example 2: Expression of Endomorphin 2
[0048] The adenovirus Ad-hPNEM2 constructed in Example 1 was transfected into host cells: HEK293 cells (purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences), and the culture condition of HEK293 cells was DMEM with 10% fetal bovine serum (purchased from GIBICO Company) , 37°C, 5% CO 2 .
[0049] On the 7th day after virus infection, the cells were broken by high-speed centrifugation to remove cell fragments, and centrifuged again to collect the cell supernatant culture medium, which contained a large amount of EM-2 protein, and the concentration of endomorphin 2 was detected by EIA method to reach 1873.56± 225.17ng / ml.
[0050] The expression product endomorphin 2 was collected, and endomorphin 2 was purified by silica gel column chromatography, followed by gradient elution with dichloromethane, dichloromethane:methanol=19:1 to obtain a yellow oil, which was dried in vacuum A yellow blocky solid was o...
Embodiment 3
[0051] Example 3: Analysis of the secretion and expression of endomorphin-2 in vitro
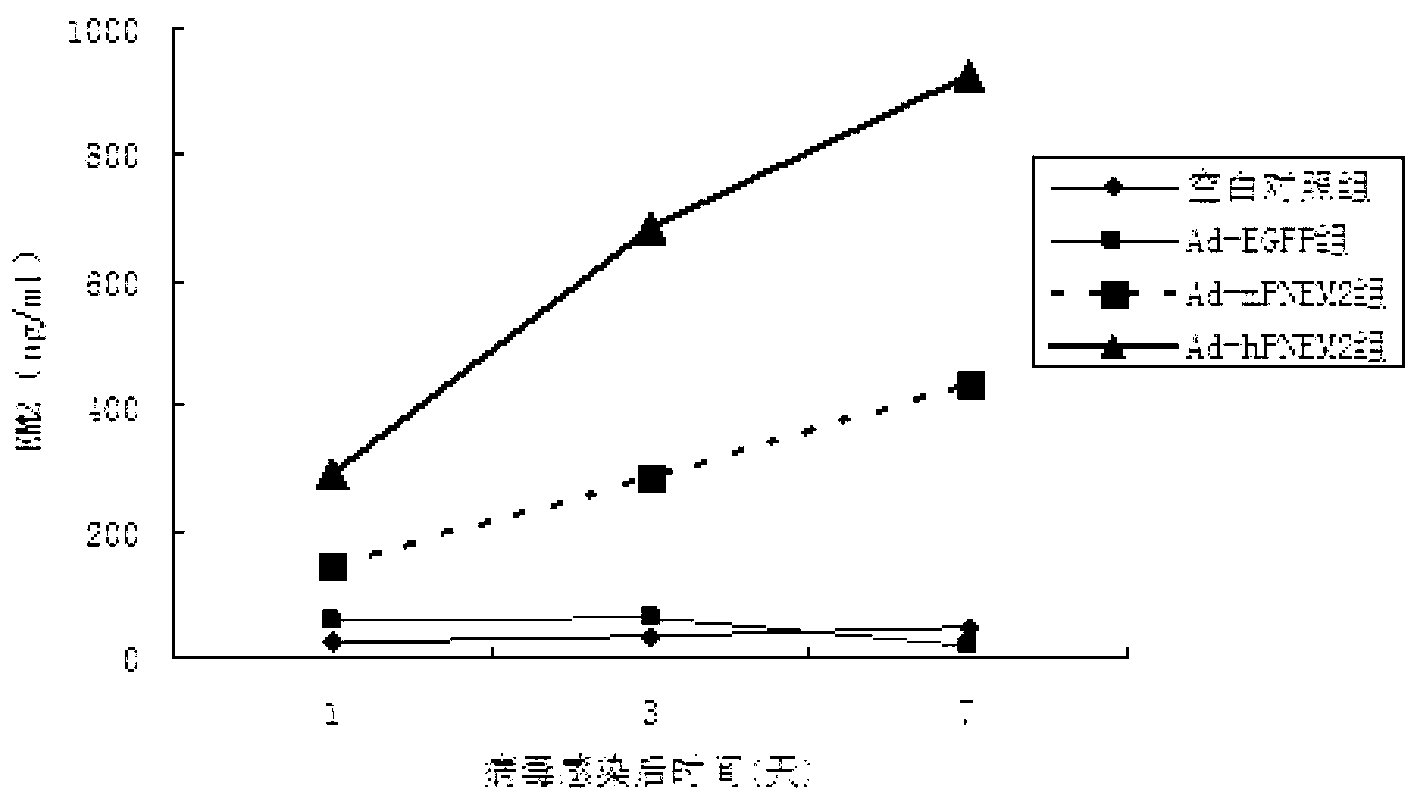
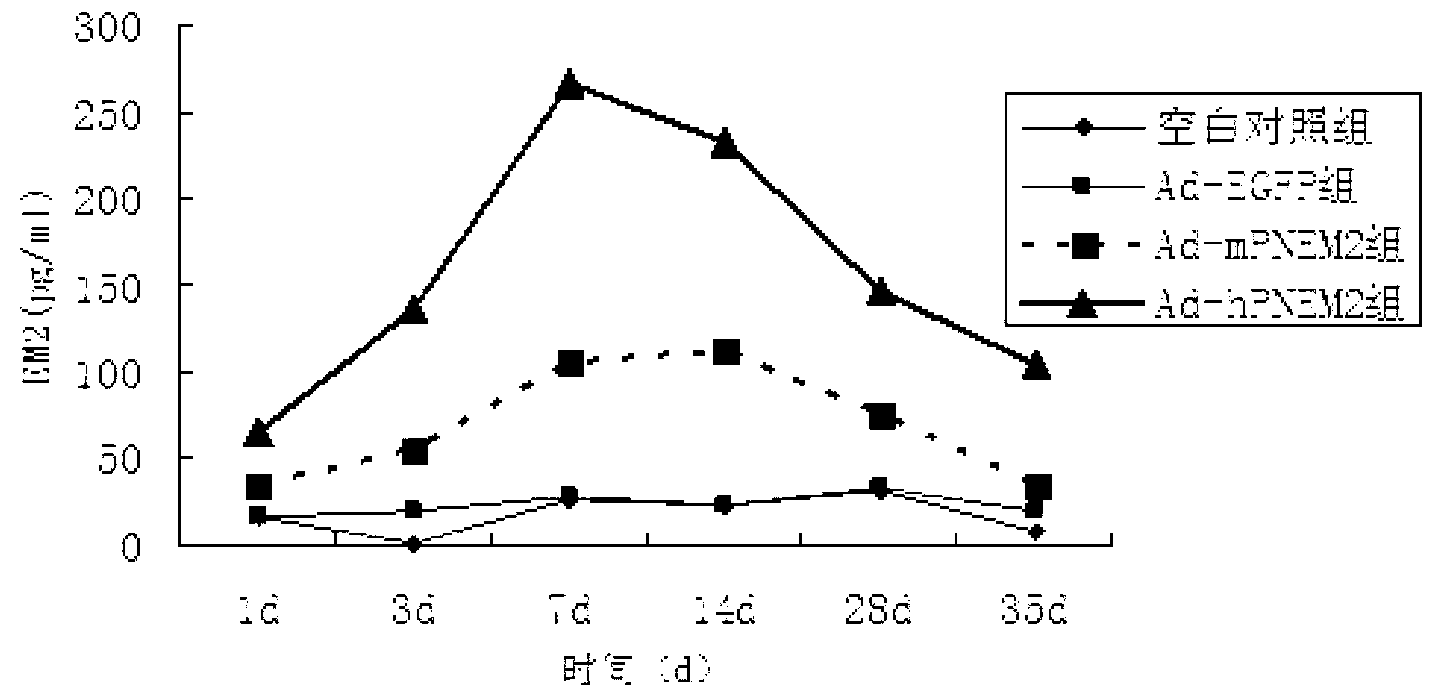
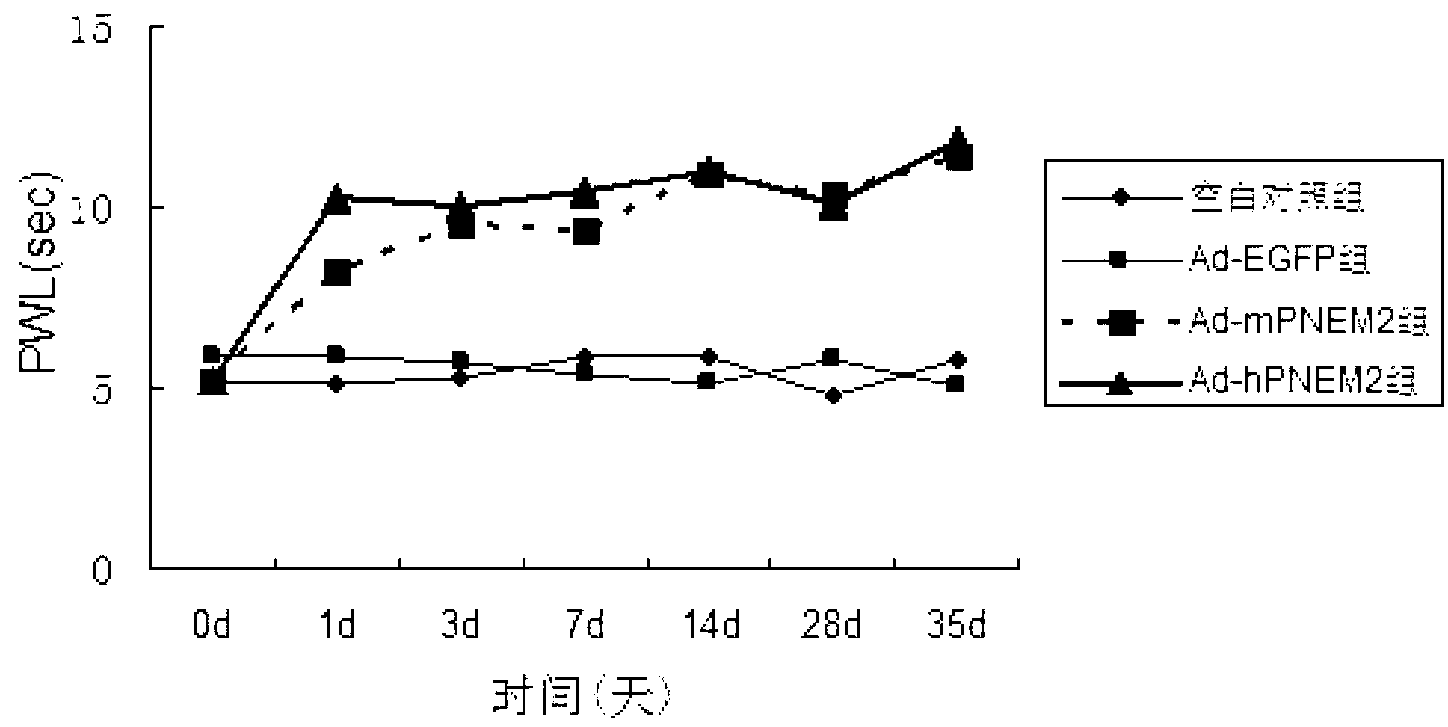
[0052] The six-well plate NIH3T3 cell adenovirus Ad-hPNEM2 infection test was carried out. The control groups were the Ad-EGFP group (constructed in the previous experiment), the blank group without virus infection, and Ad-rPNEM2 with mouse-derived nerve growth factor as the leader peptide. Control group (preliminary experimental construction). On the 7th day after the virus infection, collect the cell culture fluid on the 1st, 3rd, 7th, and 10th day after the infection, centrifuge to remove the cells, and measure the concentration of EM-2 protein.
[0053] The results found that the expression of endomorphin-2 in Ad-hPNEM2 group and Ad-rPNEM2 group was significantly increased, and the content of endomorphin-2 in Ad-hPNEM2 group was significantly higher than that of Ad-rPNEM2 group (Pfigure 1 ). The results show that the DNA sequence inserted into the viral genome can correctly translate th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com