Patents
Literature
261 results about "Opioid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, reversing opioid overdose, suppressing cough, as well as for executions in the United States. Extremely potent opioids such as carfentanil are only approved for veterinary use. Opioids are also frequently used non-medically for their euphoric effects or to prevent withdrawal.
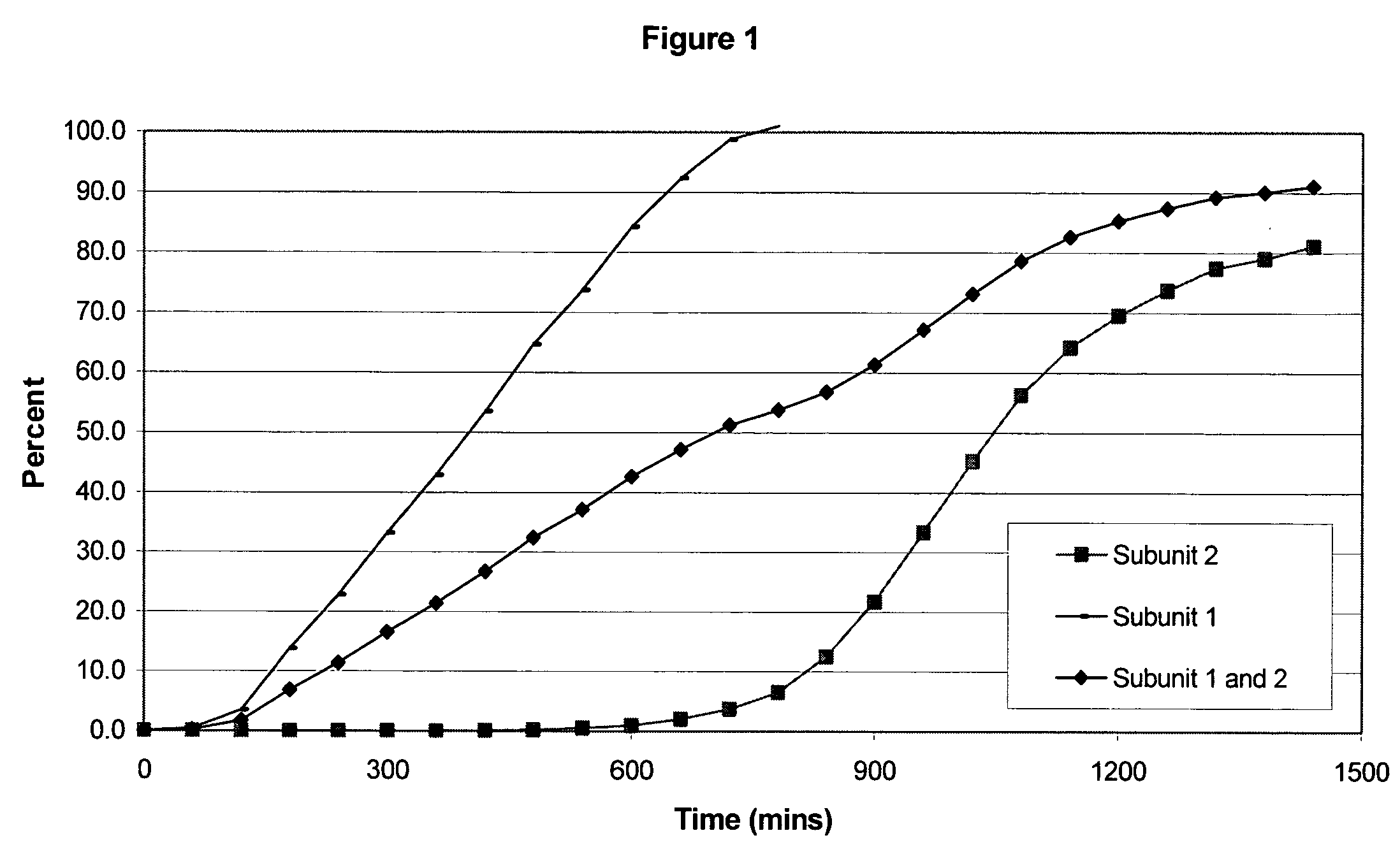
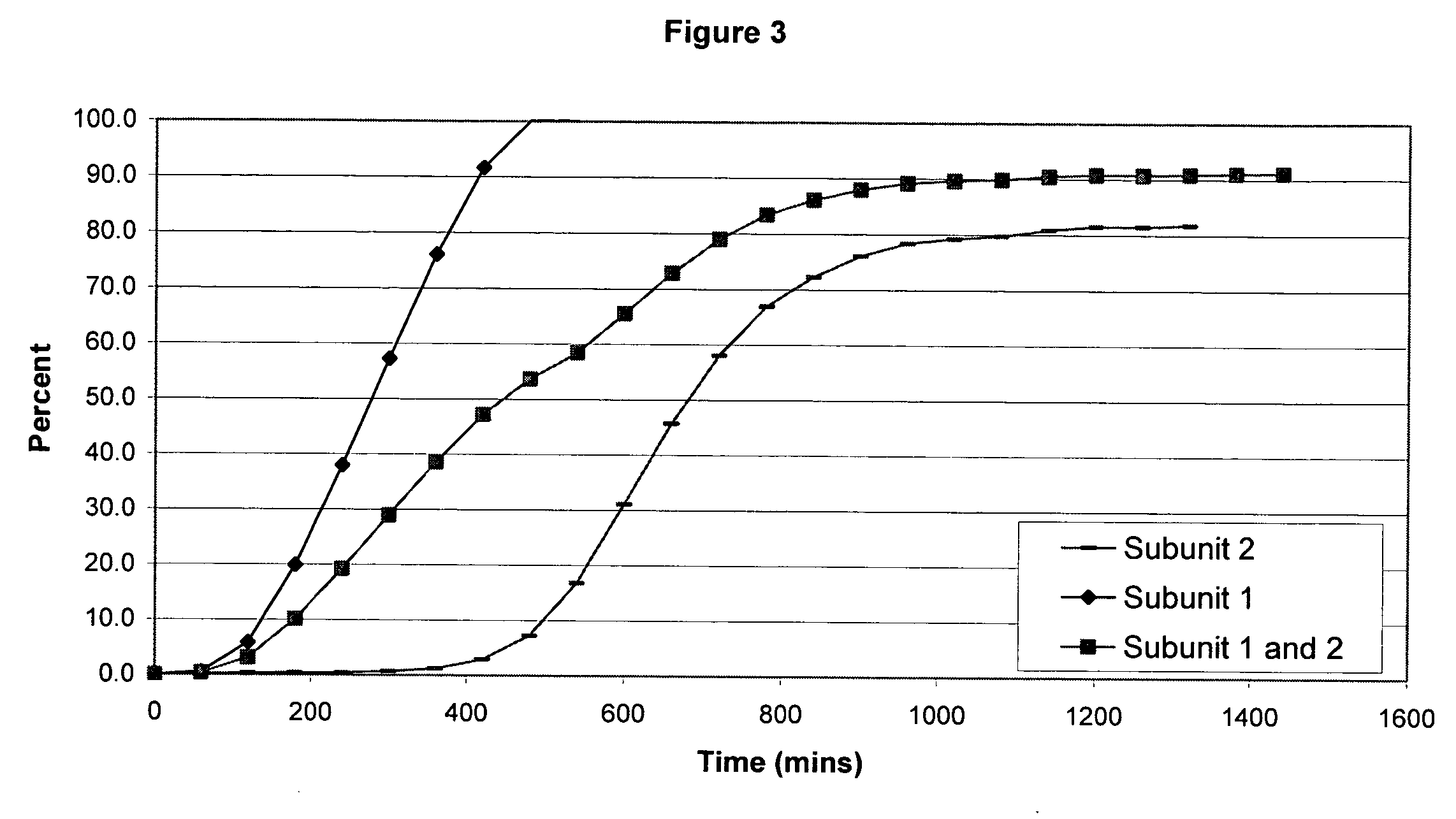
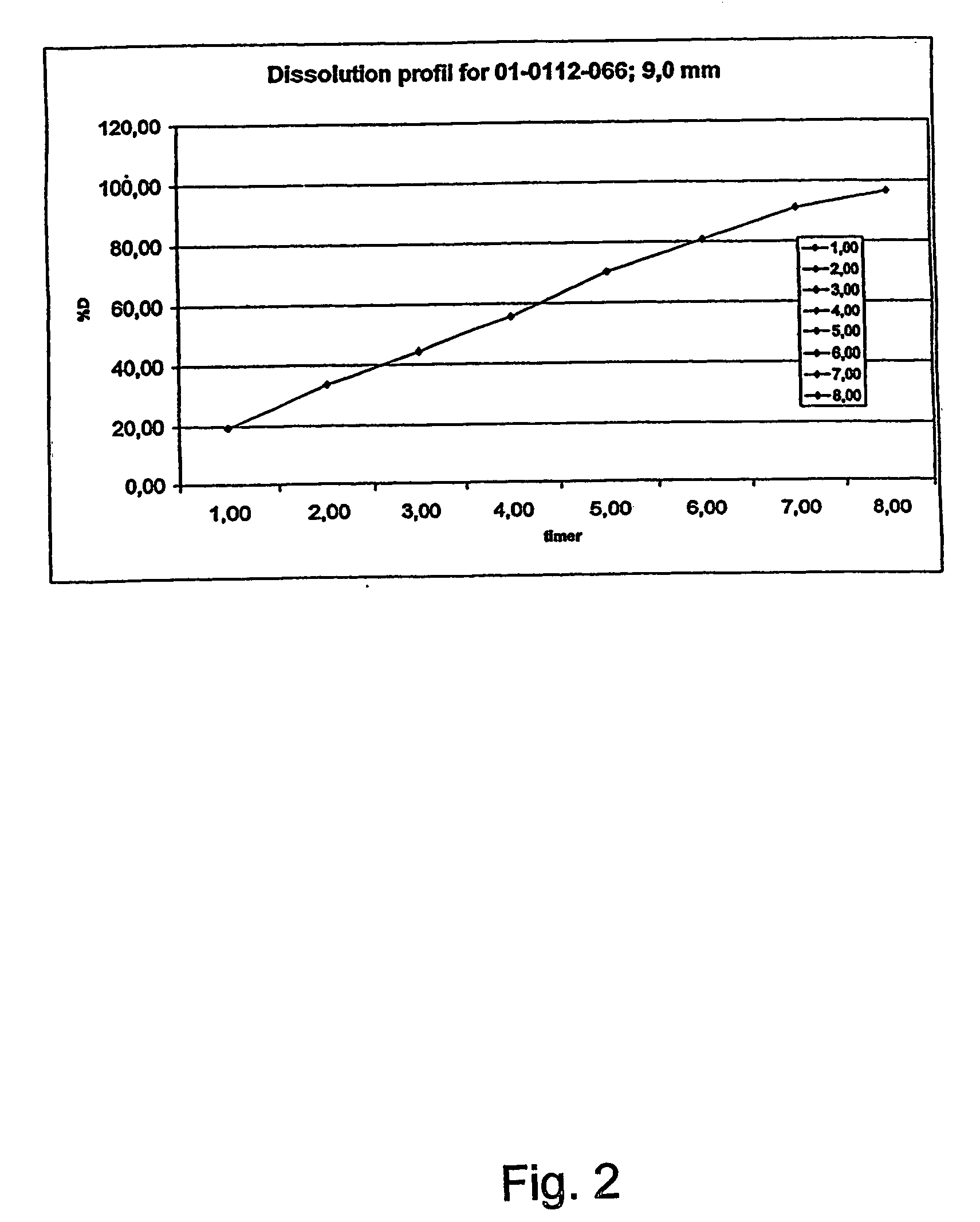
Sustained release opioid formulations and method of use
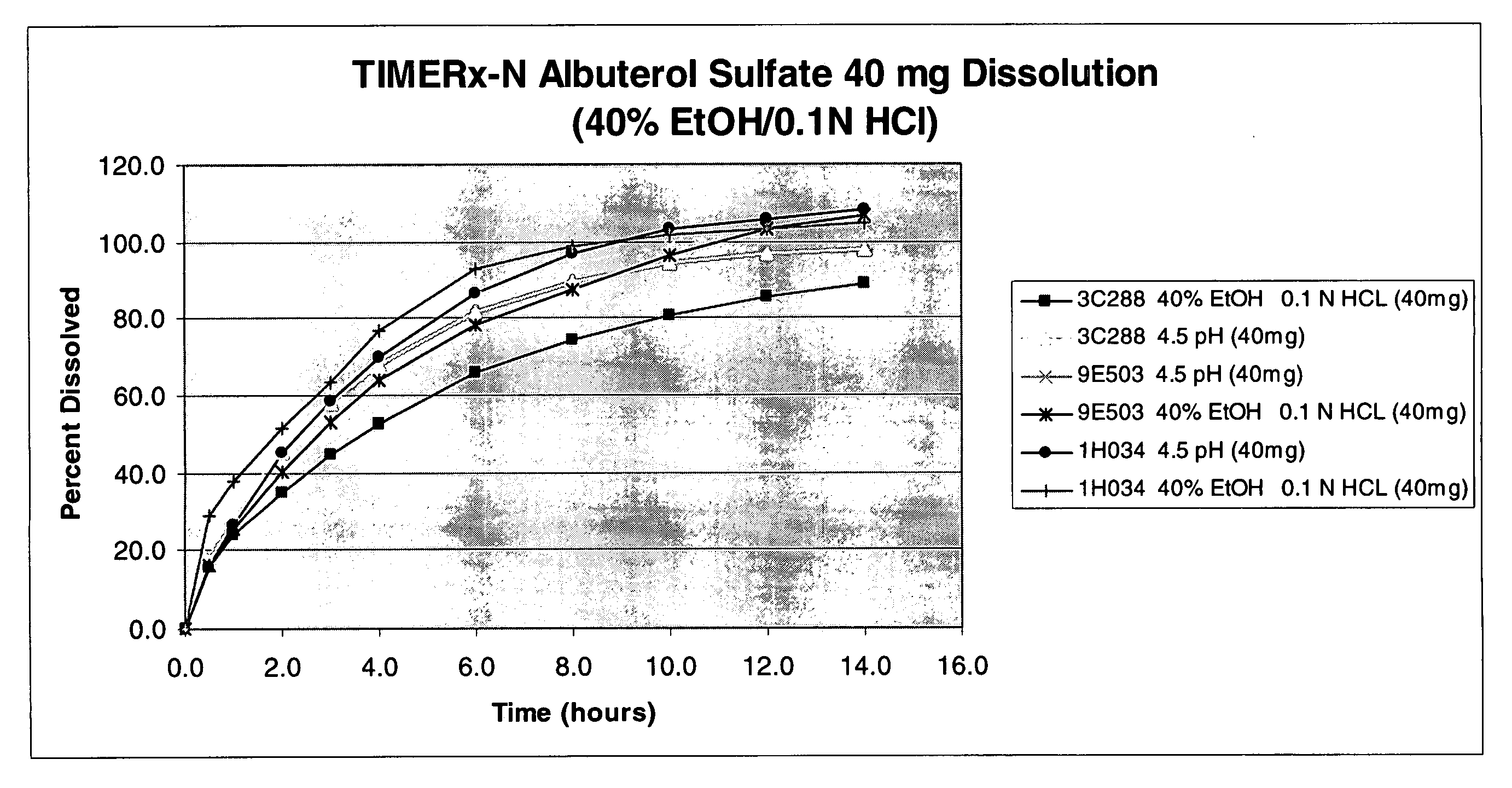
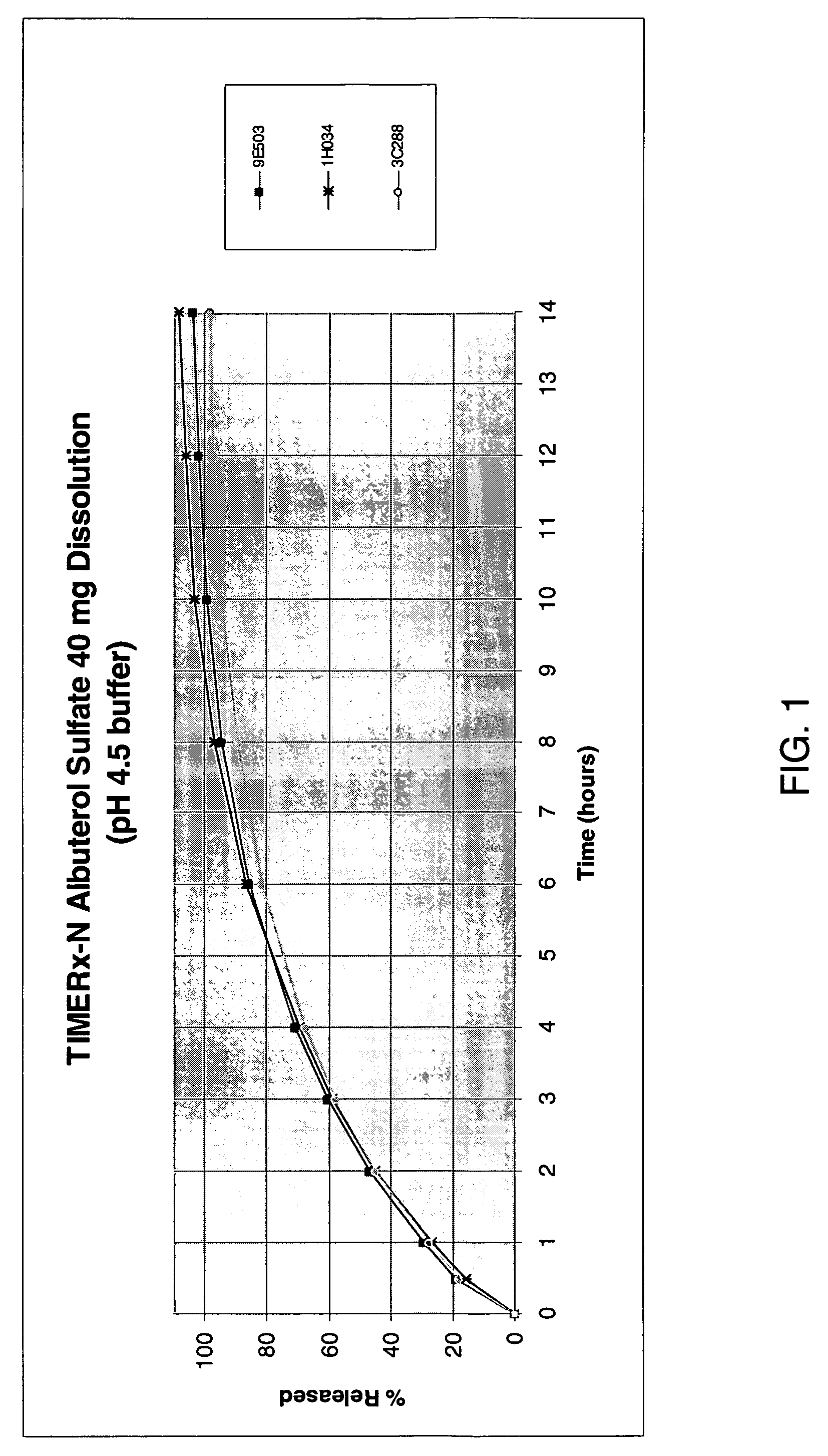
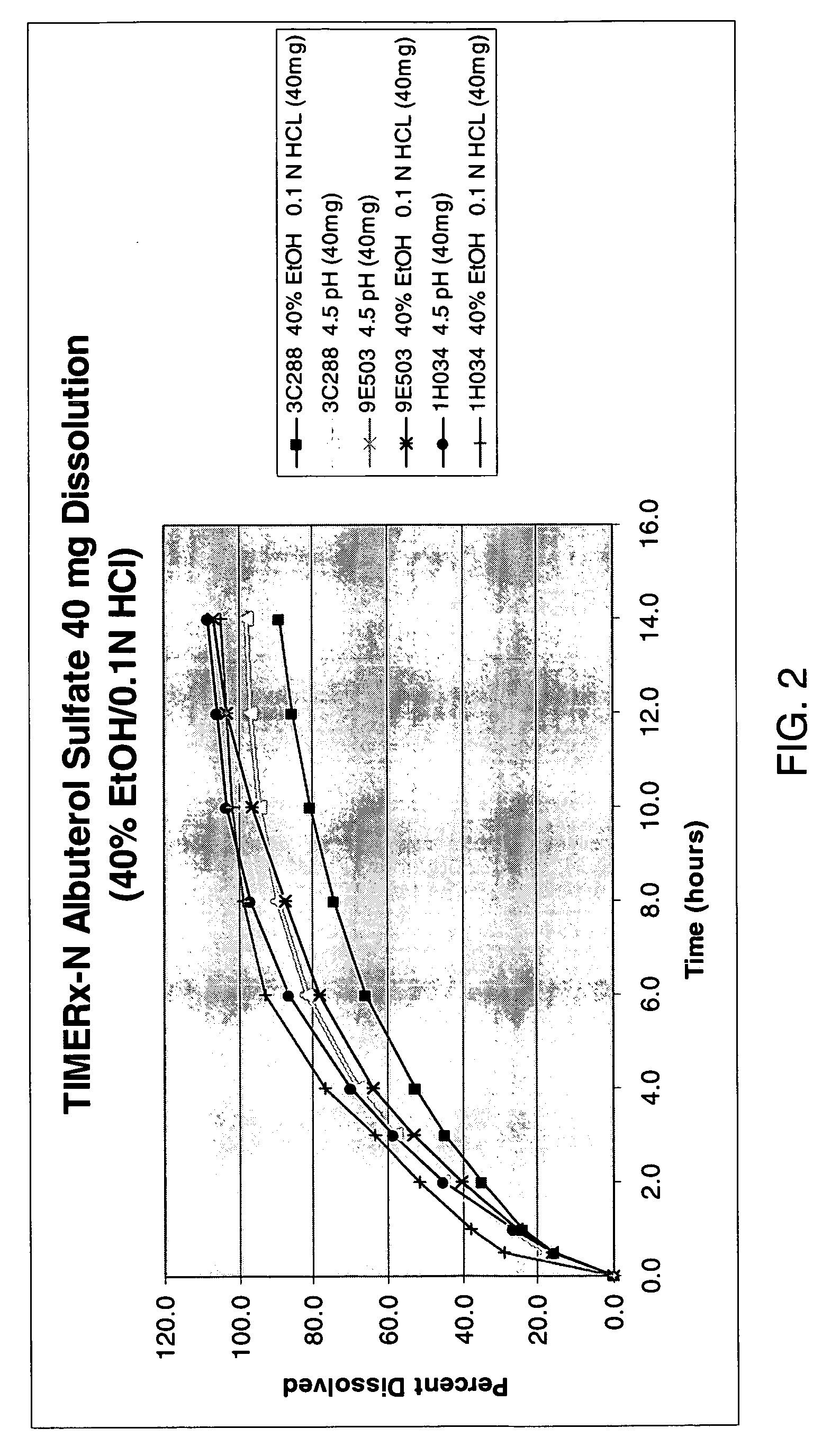
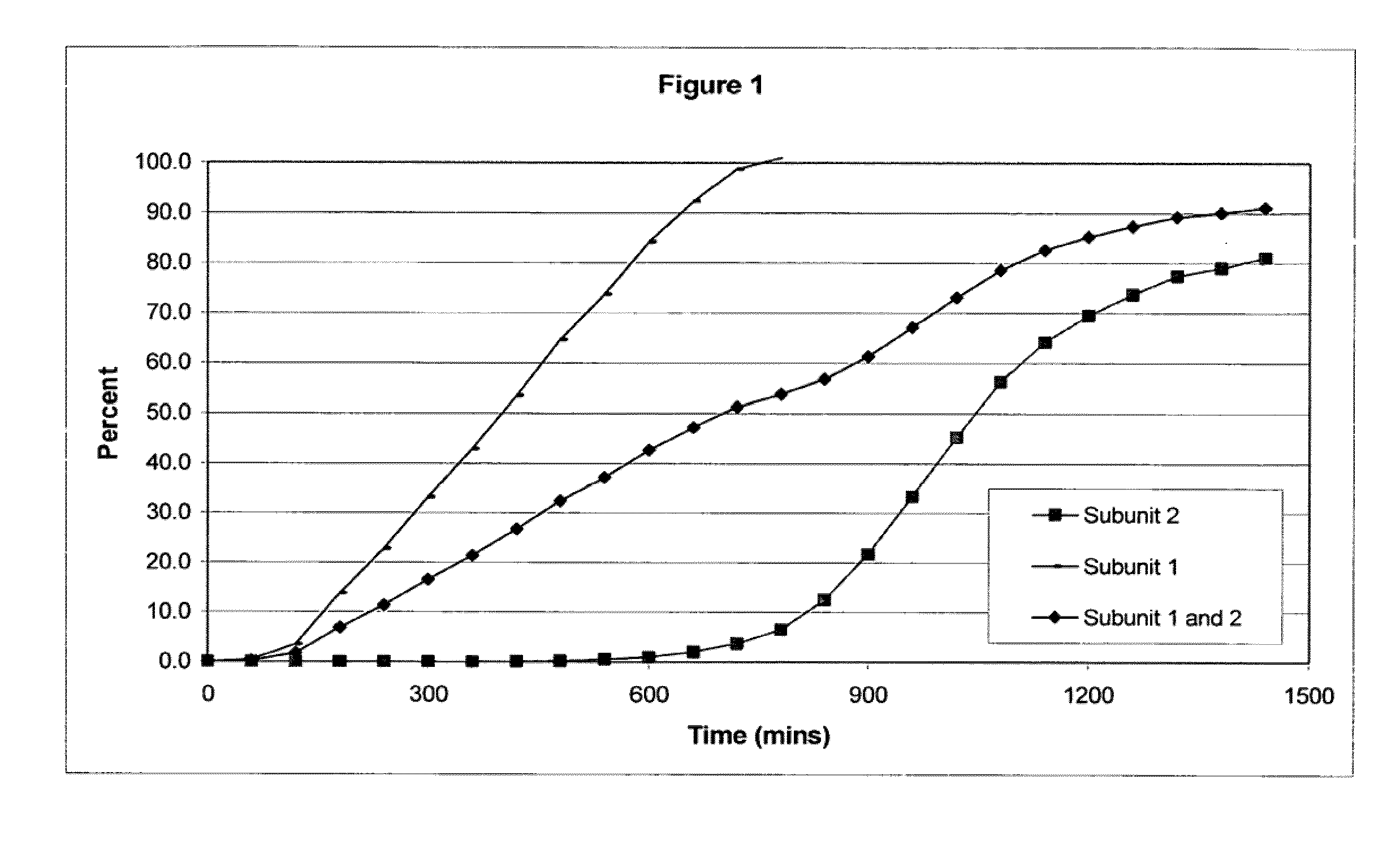
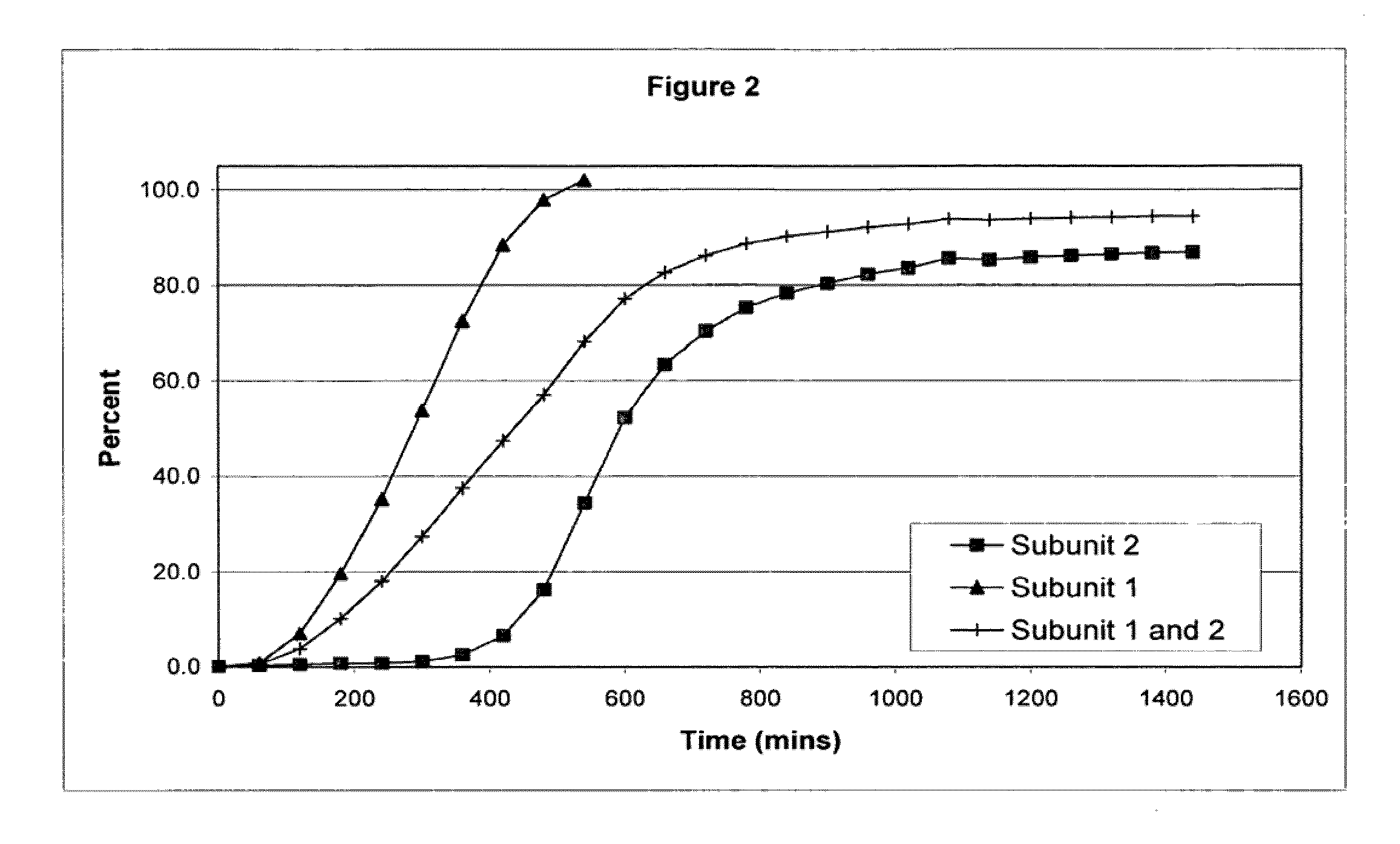
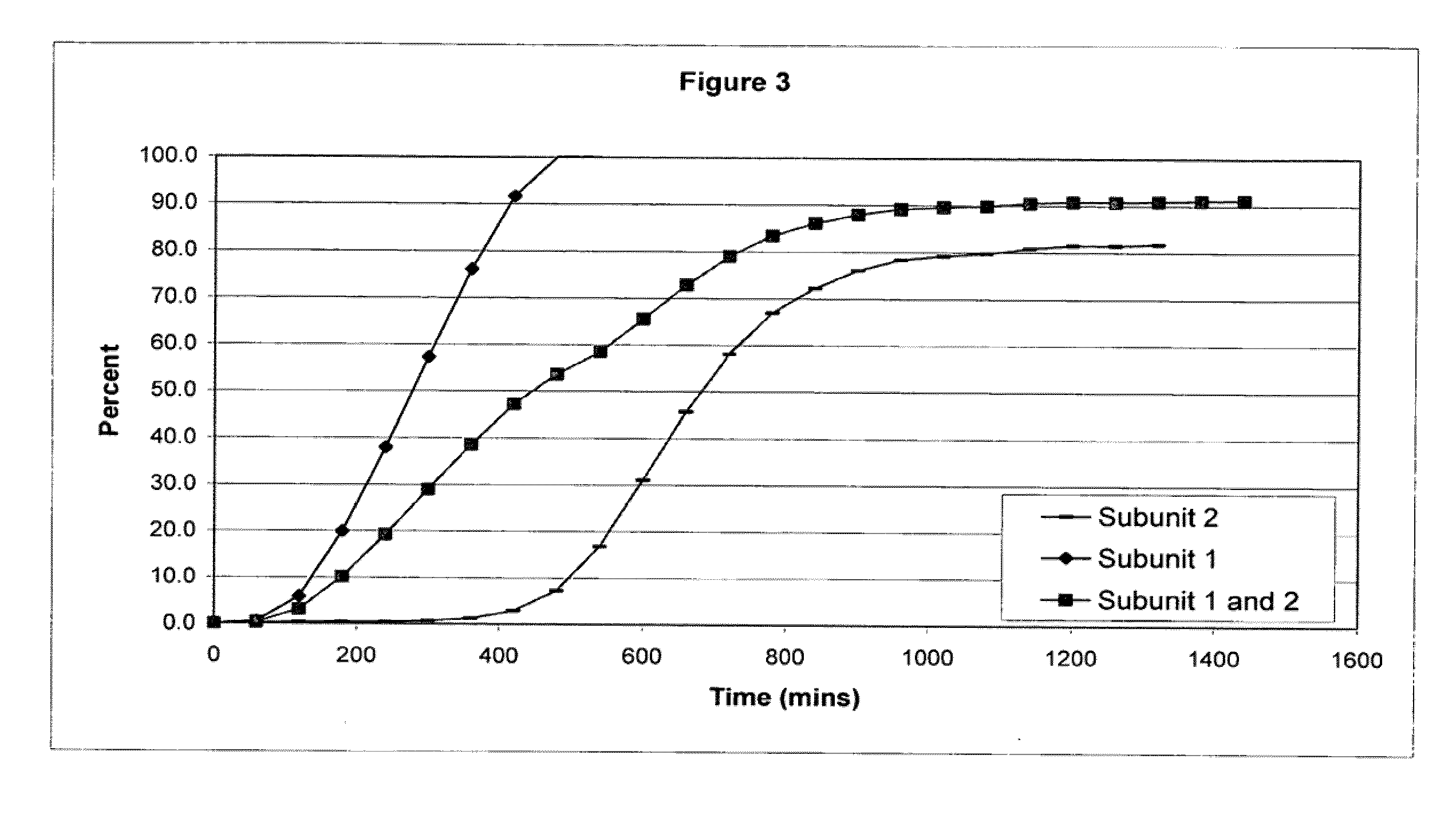
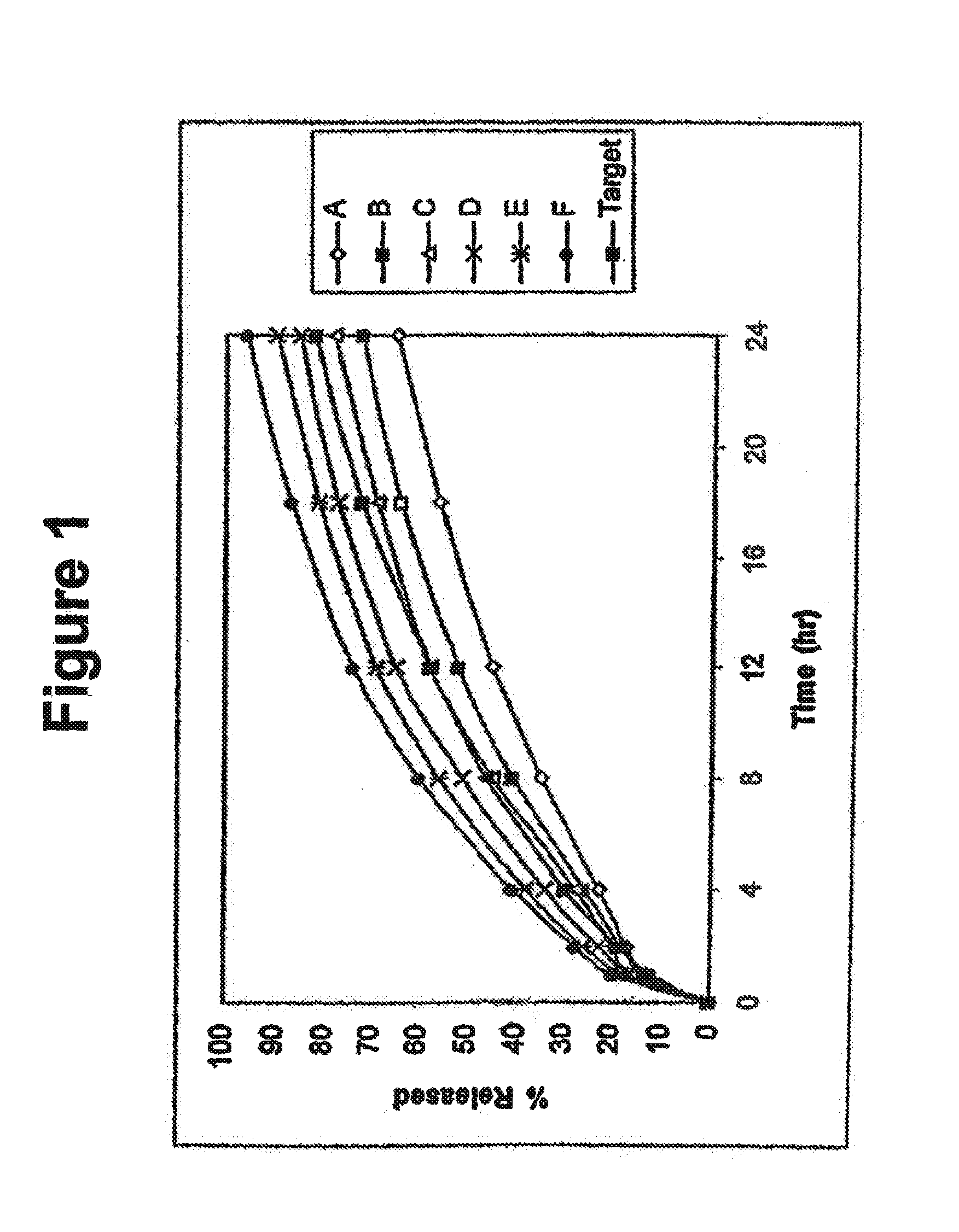
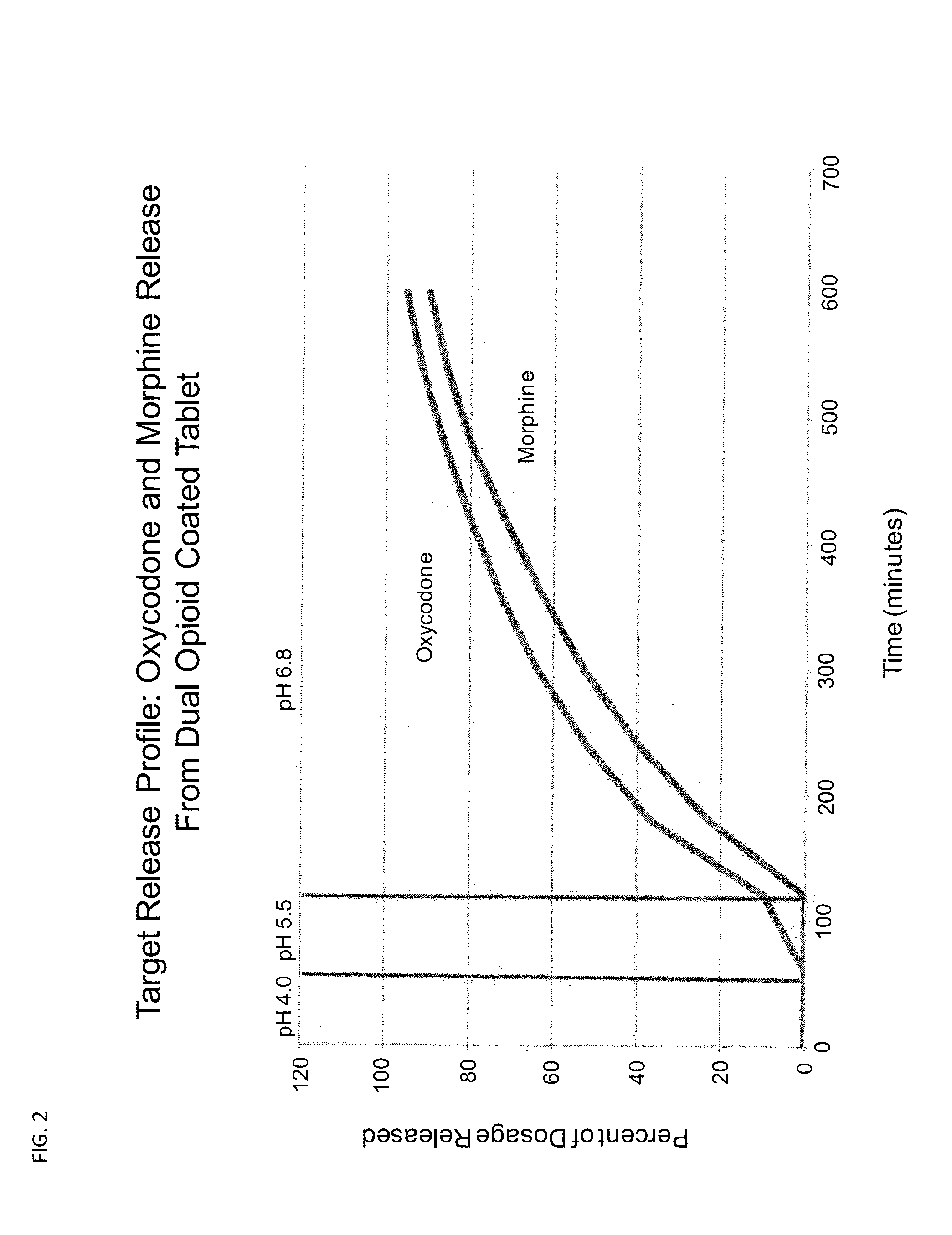
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; less than about 10% within about 10 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
Abuse-resistant sustained-release opioid formulation
InactiveUS20050163856A1Prevent extractionDissuades abuseBiocidePowder deliveryCompound (substance)Solvent
A method for reducing the abuse potential of an oral dosage form of an opioid extractable by commonly available household solvents said method comprising combining a therapeutically effective amount of the opioid compound, or a salt thereof, a matrix-forming polymer and an ionic exchange resin.
Owner:BOEHRINGER INGELHEIM ROXANE
Morphine controlled release system
InactiveUS20070003617A1Low administration frequencyAffecting extent of drug bioavailabilityBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid-when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed pead concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
Abuse resistant melt extruded formulation having reduced alcohol interaction
InactiveUS20090317355A1Reduced and limited dose-dumping effectReduce interactionBiocideNervous disorderVerapamilOral medication
The present invention relates to compositions for oral administration. The invention preferably comprises at least one abuse-resistant drug delivery composition for delivering a drug having potential for dose dumping in alcohol, related methods of preparing these dosage forms, and methods of treating a patient in need thereof comprising administering the inventive compositions to the patient. Most preferably, the dosage form includes verapamil. These formulations have reduced potential for abuse. In another formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Ethanol-resistant sustained release formulations
InactiveUS20070212414A1High drug safetyLower potentialBiocideNervous disorderSustained Release FormulationsDelivery system
The invention provides formulations that resist dose dumping in the presence of ethanol and methods of use thereof. The formulations can be used to prevent dose dumping, to increase safety of drugs, and to reduce abuse of drugs prone to such abuse. The formulations comprise at least one drug and a sustained release delivery system. In one embodiment, the drug is an opioid.
Owner:ENDO PHARMA INC
Abuse resistant melt extruded formulation having reduced alcohol interaction
InactiveUS20100172989A1Reduced and limited dose-dumping effectReduce interactionPowder deliveryBiocideVerapamilOral medication
The present invention relates to compositions for oral administration. The invention preferably comprises at least one abuse-resistant drug delivery composition for delivering a drug having potential for dose dumping in alcohol, related methods of preparing these dosage forms, and methods of treating a patient in need thereof comprising administering the inventive compositions to the patient. Most preferably, the dosage form includes verapamil. These formulations have reduced potential for abuse. In another formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG +1
Robust sustained release formulations
InactiveUS20080085304A1Avoid dose dumpingHigh drug safetyPowder deliveryPill deliverySolid Dose FormOxymorphone
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Opioid Dosage Forms Having Dose Proportional Steady State Cave and Auc and Less Than Dose Proportional Single Dose Cmax
The present invention relates to a plurality of dosage forms comprising a first dosage form and second dosage form each comprising a therapeutic agent, such as an opioid; wherein the dosage strength of the second dosage form is greater than that of the first dosage form; and wherein the steady state Cave and the steady state AUC of the first and second dosage forms are dose proportional and the single dose Cmax of the second dosage form is less than the minimum level for dose proportionality with respect to the first dosage form. The present invention also relates to methods of administering such dosage forms to a patient, as well as to kits comprising such dosage forms and instructions for administration of the dosage forms to a patient. The inventors believe that the dosage forms and methods of the present invention will lead to improved safety and patient acceptance.
Owner:PURDUE PHARMA LP
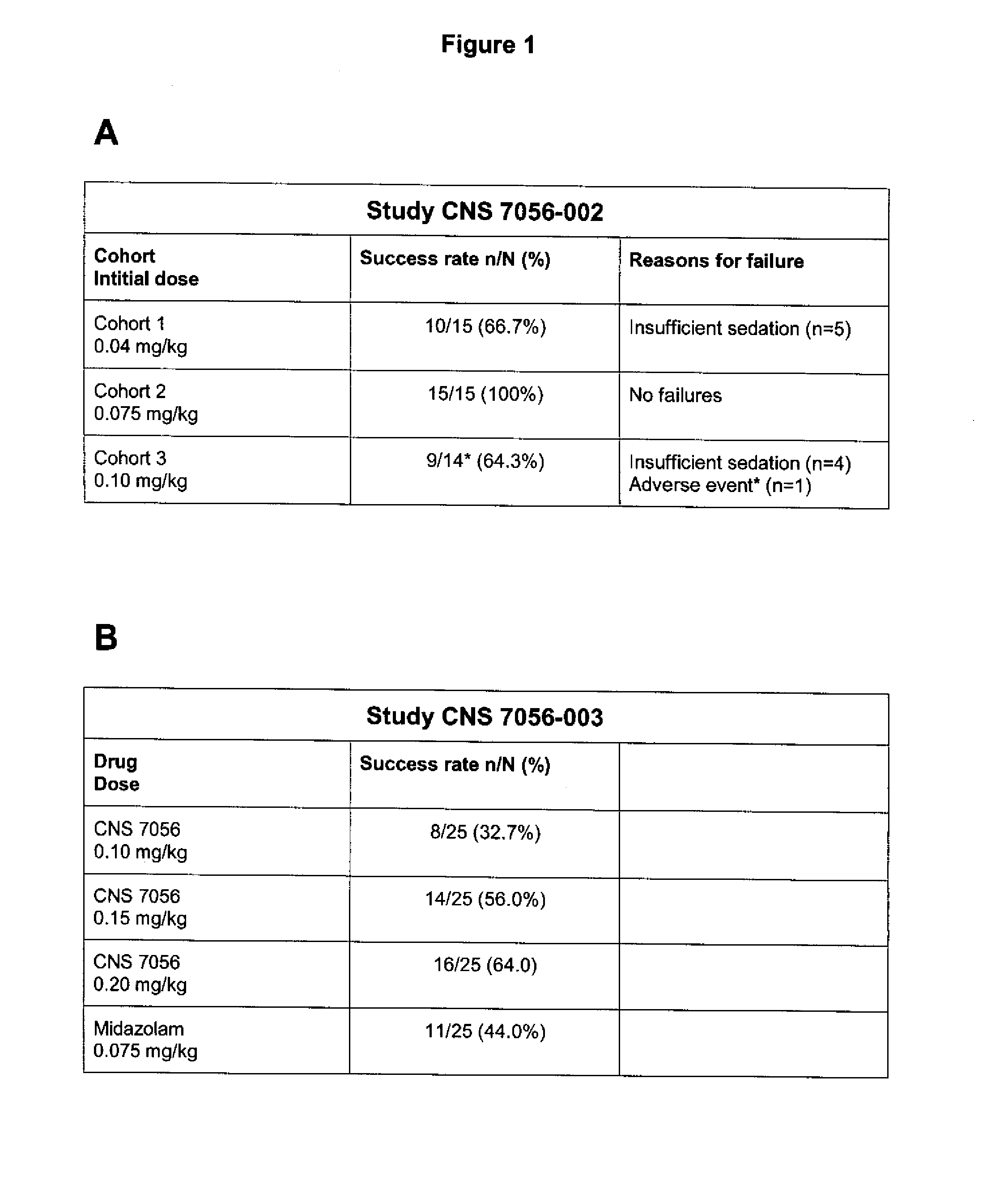
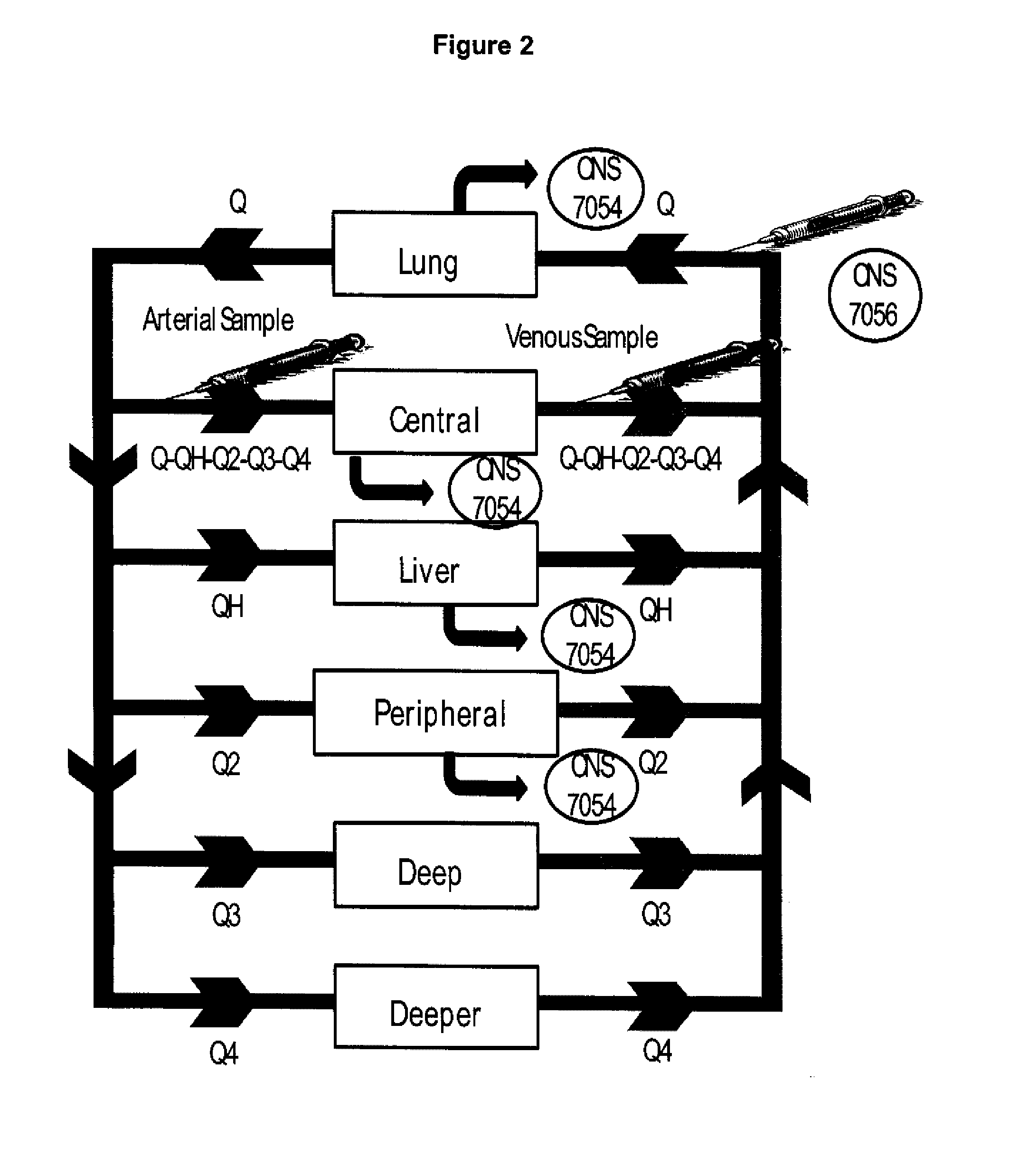
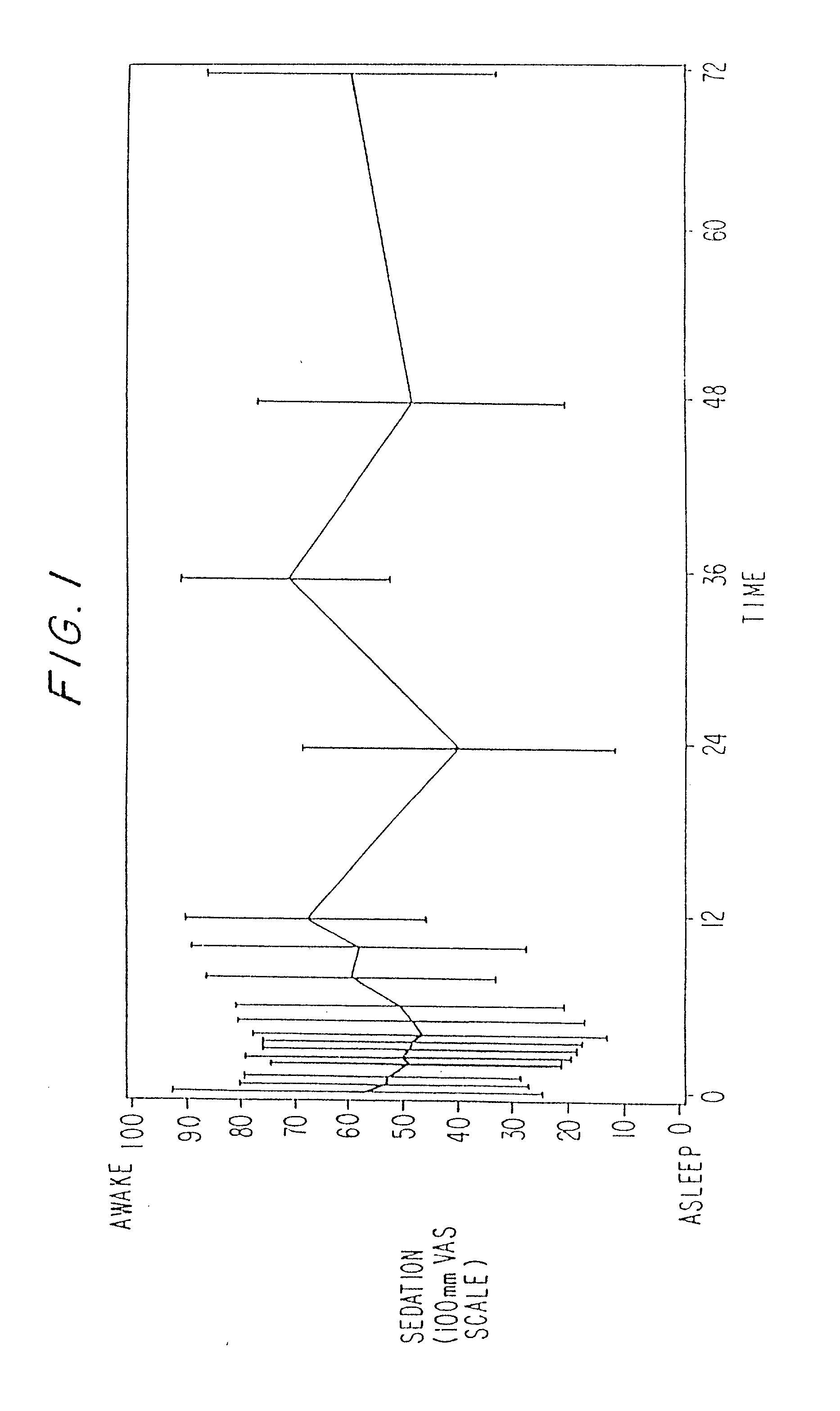
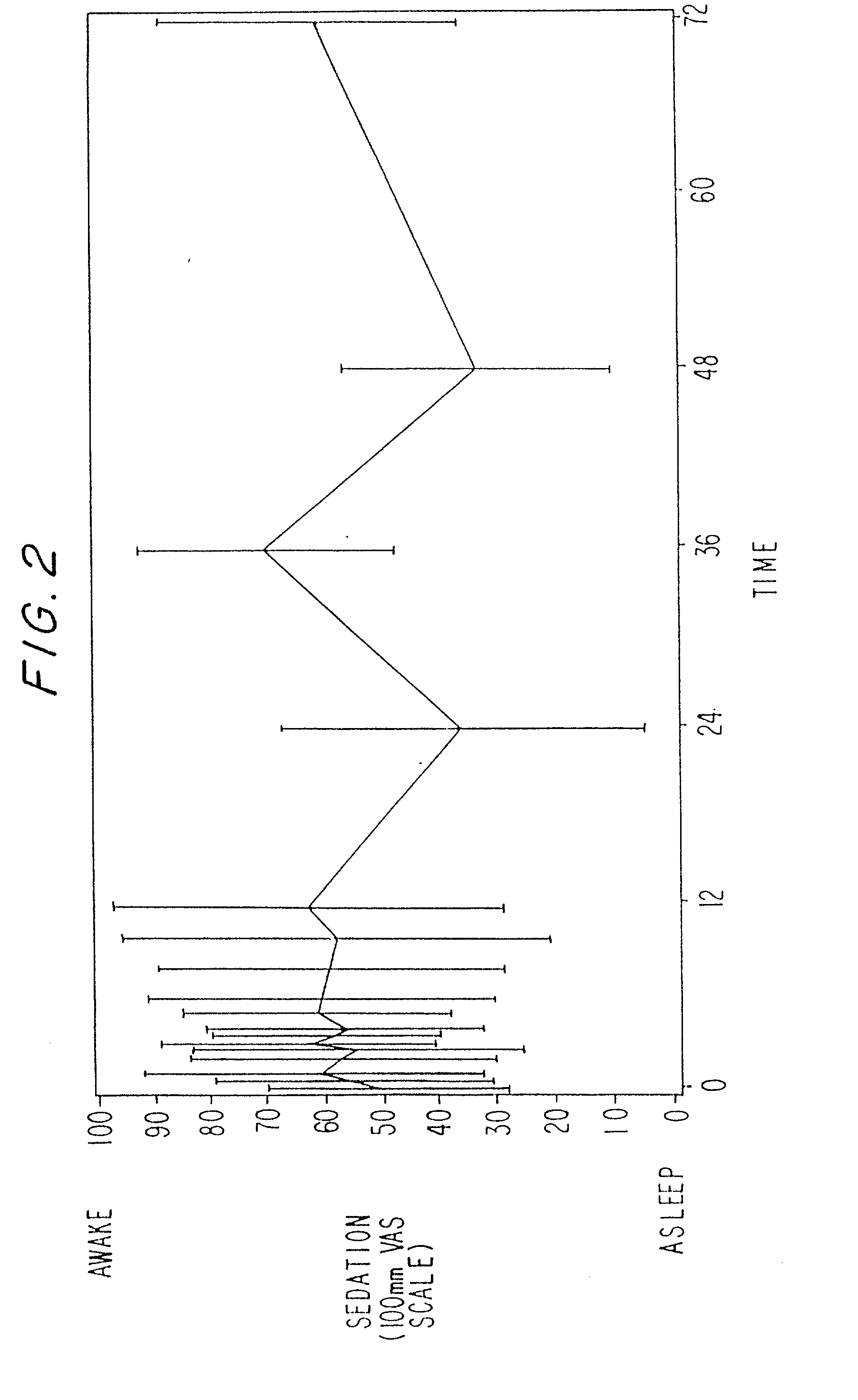
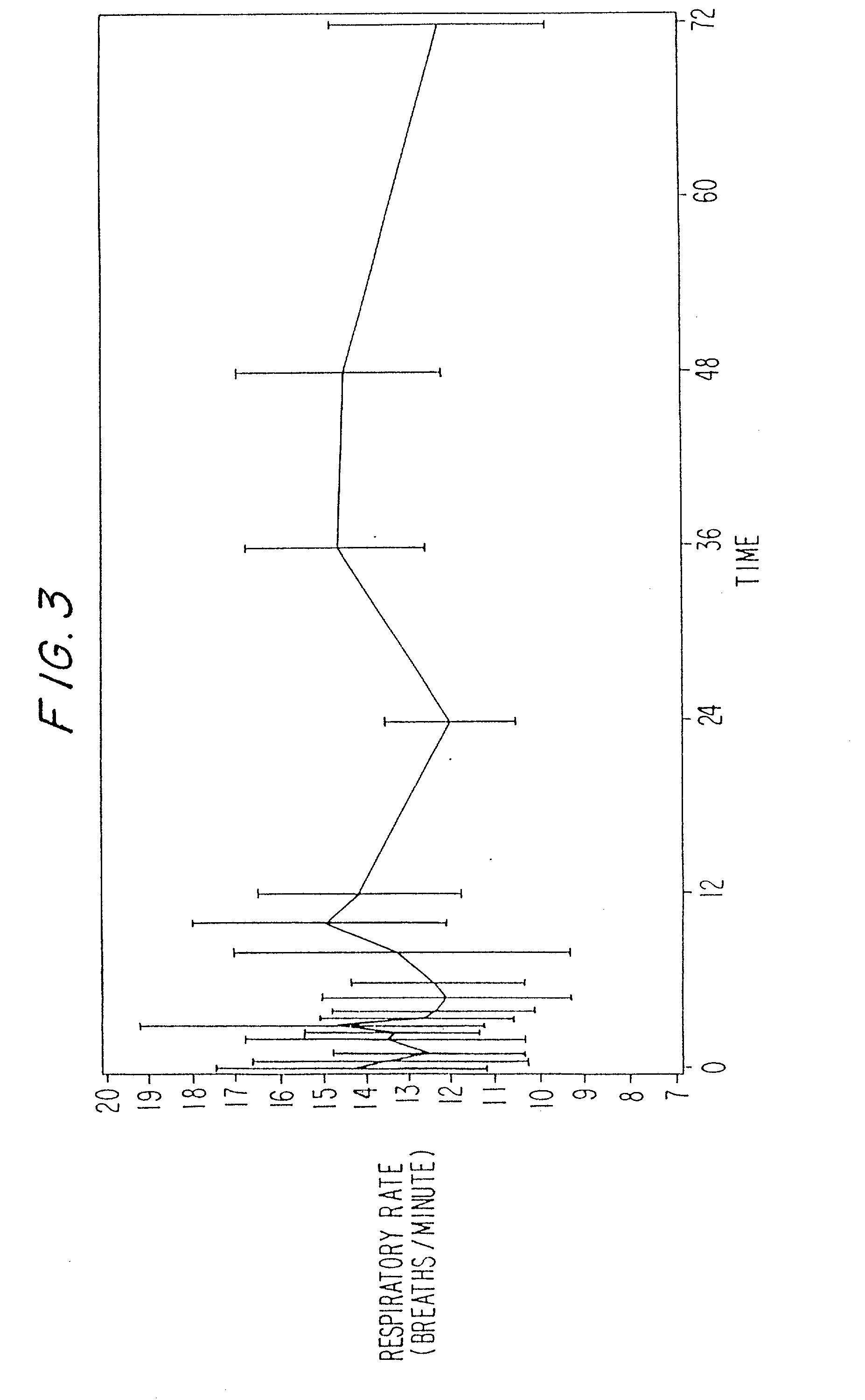
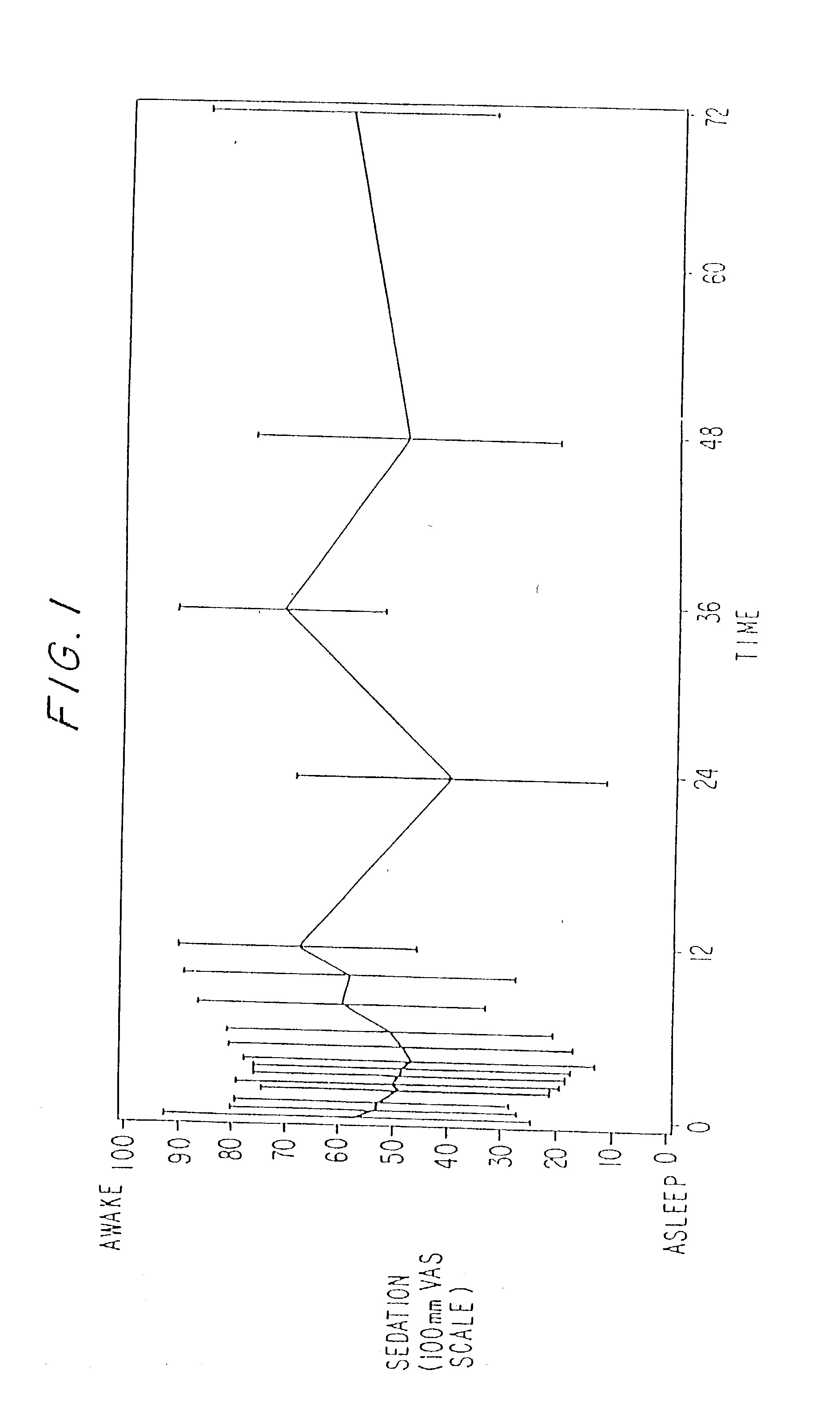
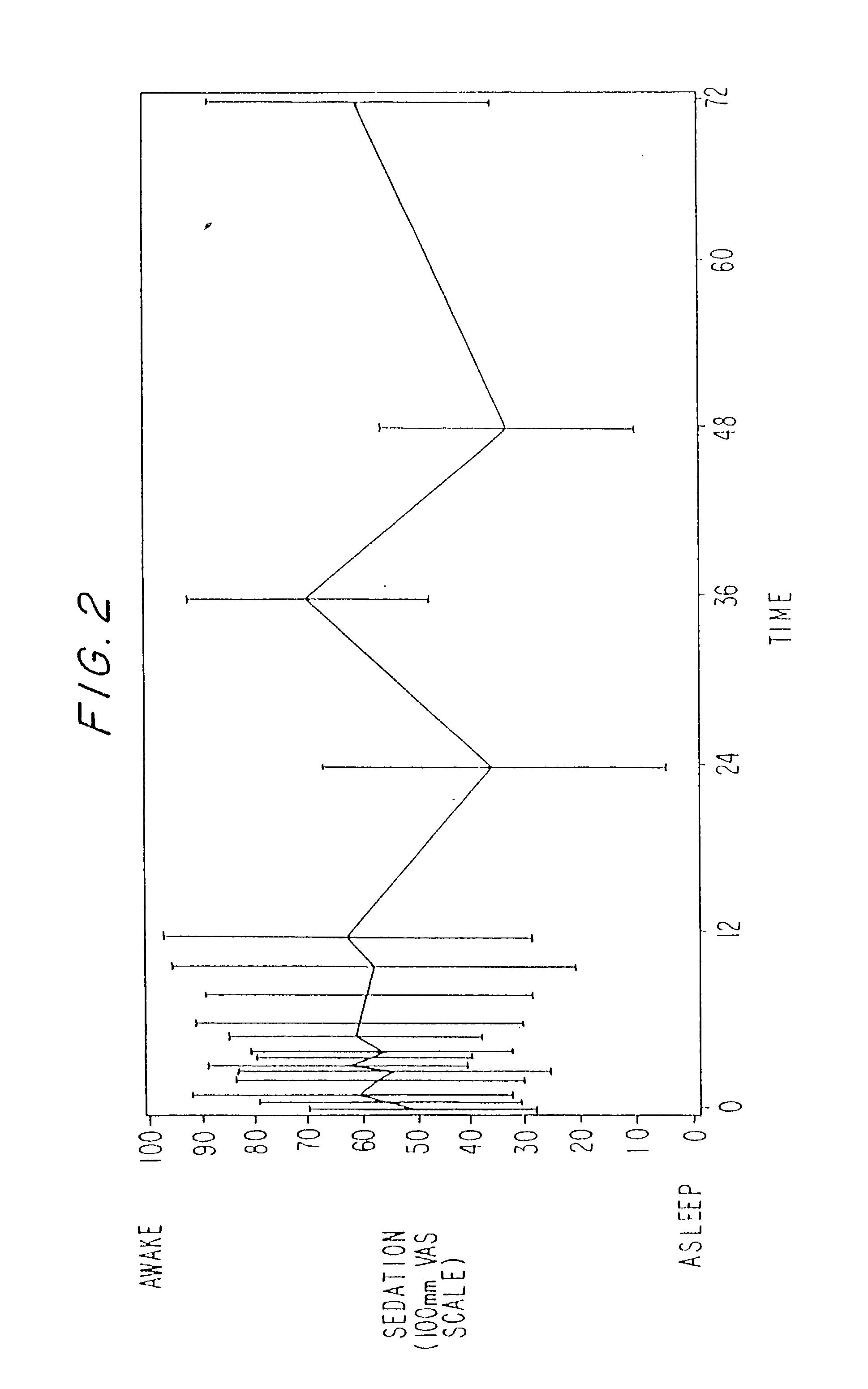
Dosing regimen for sedation with CNS 7056 (remimazolam)
ActiveUS20140080815A1Good sedative effectSafe and convenientBiocideNervous disorderDosing regimenRegimen
The invention relates to a dosing regimen for sedation with the fast-acting benzodiazepine CNS 7056 in combination with an opioid, in particular fentanyl, whereas CNS 7056 is given in a dose of 2 to 10 mg, preferably between 4 and 9 mg and most preferably between 5 and 8 mg.
Owner:PAION UK
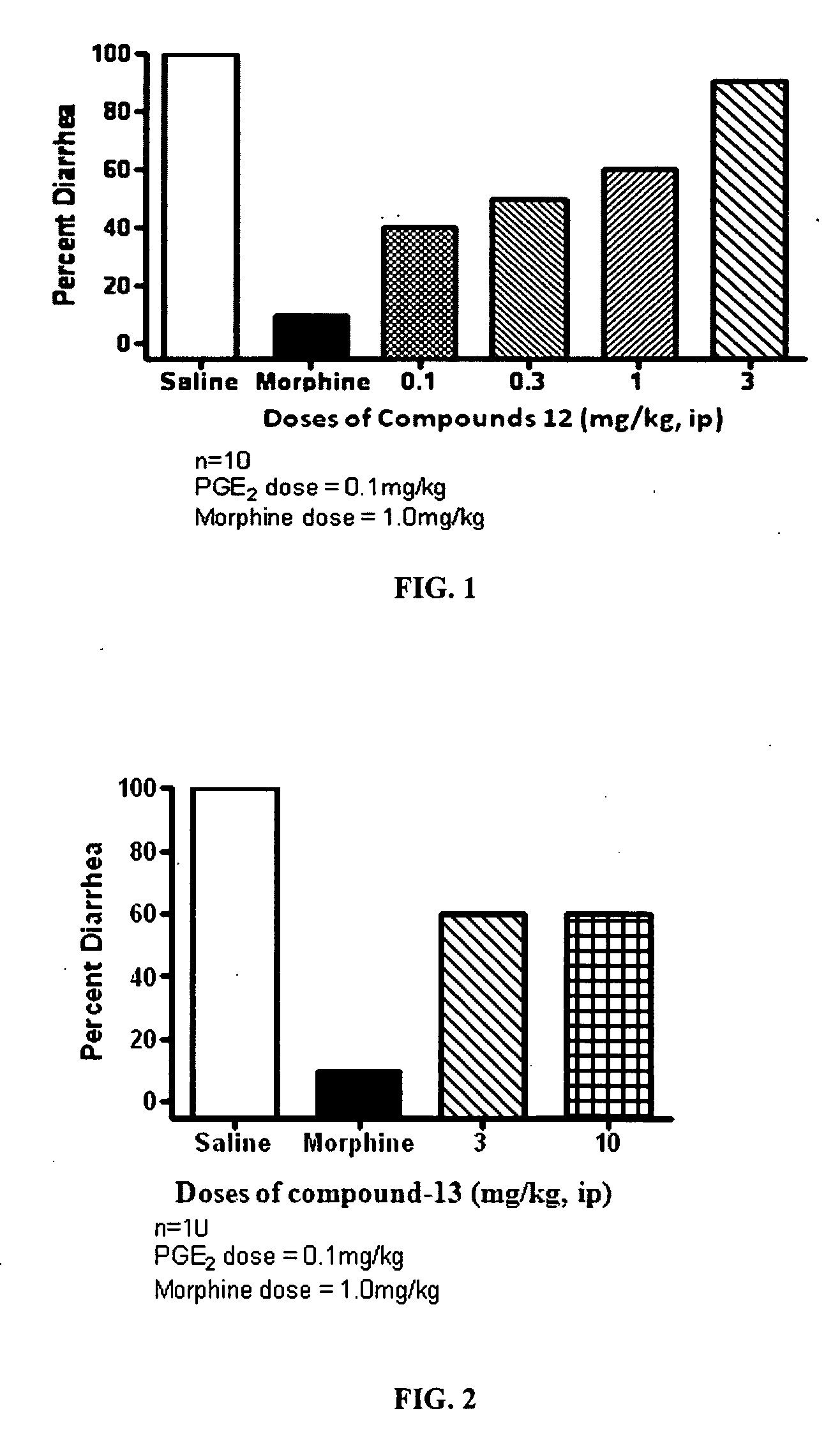
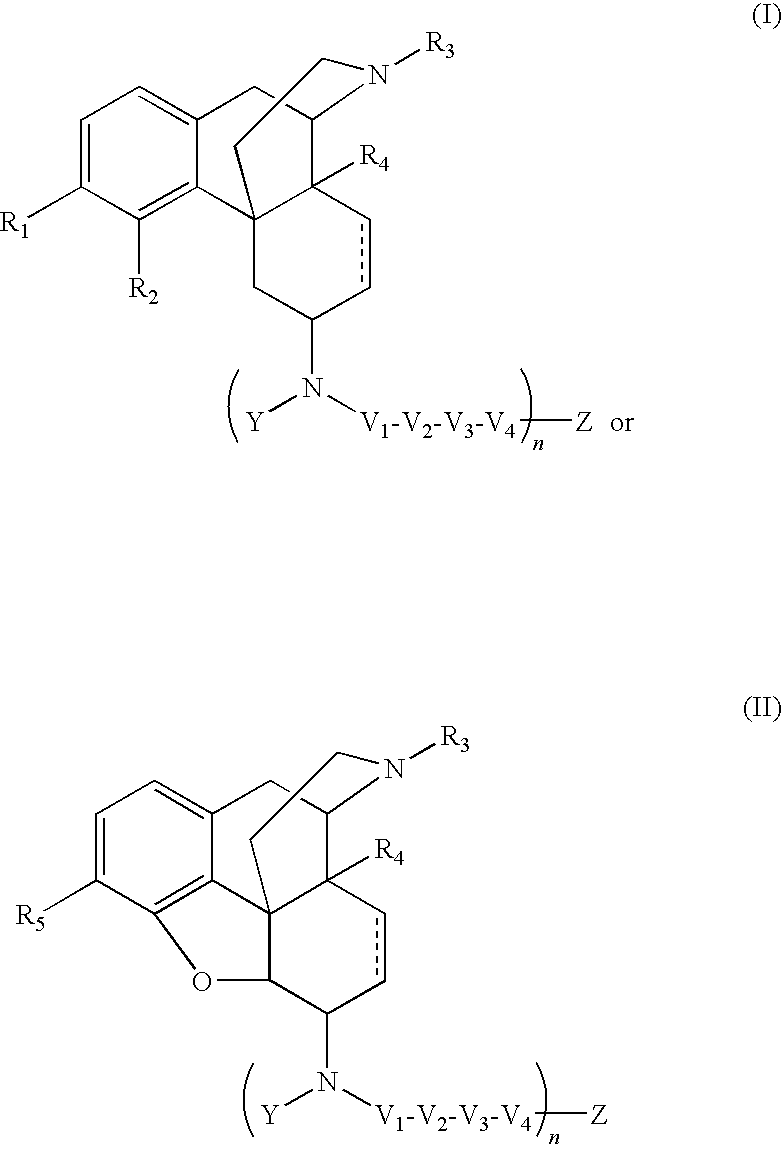
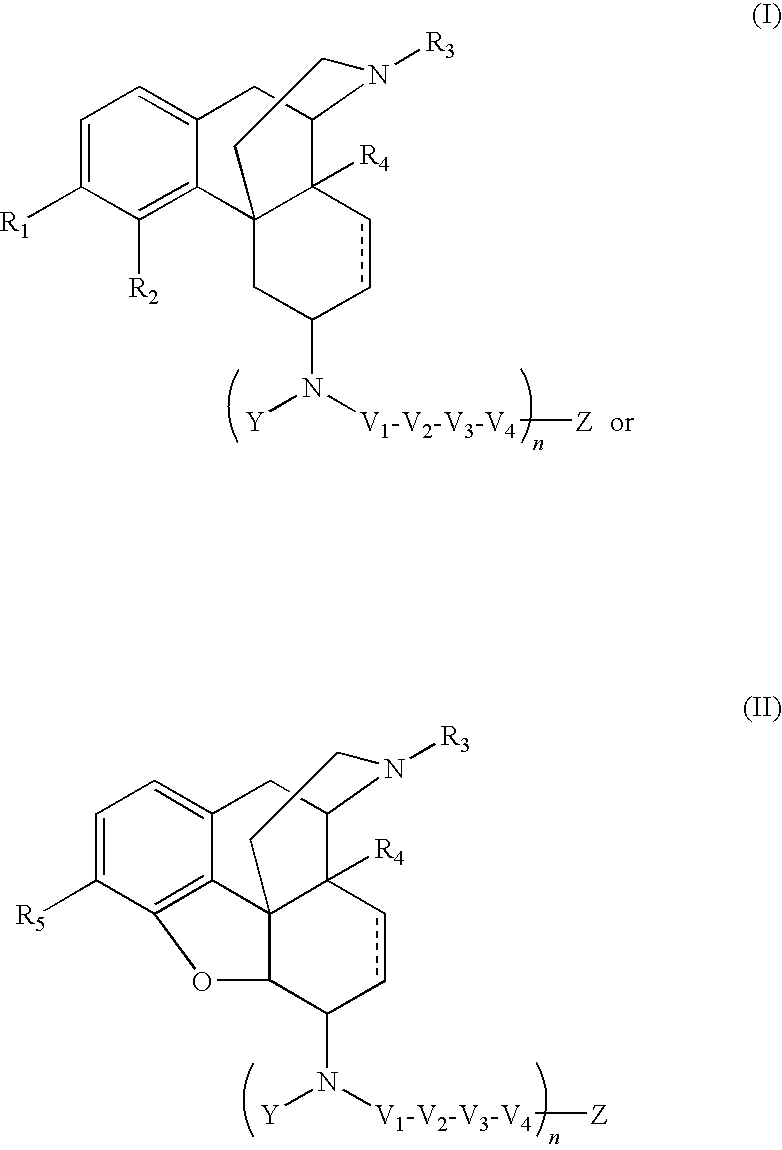
Selective opioid compounds
ActiveUS20090209569A1Reducing lipid permeability of drugReduce penetrationAntibacterial agentsBiocideDiseaseInterstitial cystitis
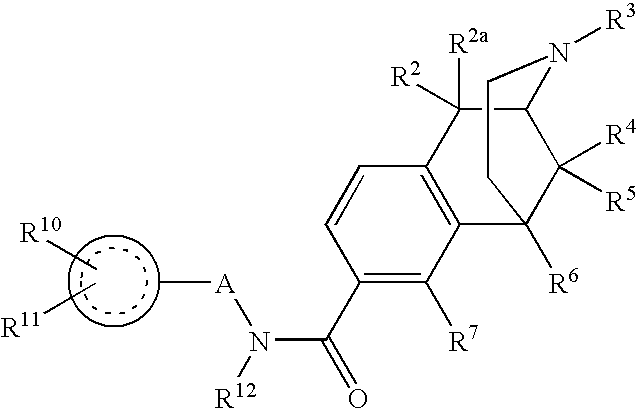
The present invention relates to compounds of Formula I or II, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which relates to morphinan compounds useful as μ, δ, and / or κ receptor opioid compounds and pharmaceuticals containing same that may be useful for mediating analgesia, combating drug addiction, alcohol addiction, drug overdose, mental illness, bladder dysfunctions, neurogenic bladder, interstitial cystitis, urinary incontinence, premature ejaculation, inflammatory pain, peripherally mediated and neuropathic pain, cough, lung edema, diarrhea, cardiac disorders, cardioprotection, depression, and cognitive, respiratory, diarrhea, irritable bowel syndrome and gastro-intestinal disorders, immunomodulation, and anti-tumor agents.
Owner:ALKERMES INC
Method for qualitatively screening 242 kinds of compounds by liquid phase chromatography-mass spectra at the same times
ActiveCN101398414AFast wayStrong detection specificityComponent separationMaterial analysis by electric/magnetic meansBenzodiazepineCarbamate
The invention discloses a method which can carry out qualitative screening to 242 compounds (drugs or toxicants) simultaneously. A mode of liquid chromatogram-mass spectrometry (LC-MS / MS) multi-reaction monitoring (MRM) is adopted to carry out determinedness to objects by two pairs of parent ion-daughter ion pair and retention time. The 242 drugs or toxicants comprise toxic products such as opioids, amphetamine and cocaines and the like, bromazepam such as benzodiazepines and barbiturates, and common drugs, alkaloid, pesticide (including organophosphates, carbamates, pyrethroid pesticide residues and organochlorine pesticide), weed killer, raticide and the like.
Owner:上海市公安局刑事侦查总队
Drug treatment for restless leg syndrome
A method for the treatment of Restless Leg Syndrome (RLS), which comprises administering an alpha2-agonist and a second agent selected from the group consisting of the dopamine agonists, opioids, benzodiazepines and the combination of L-DOPA plus a decarboxylase inhibitor.
Owner:BRECHT HANS MICHAEL
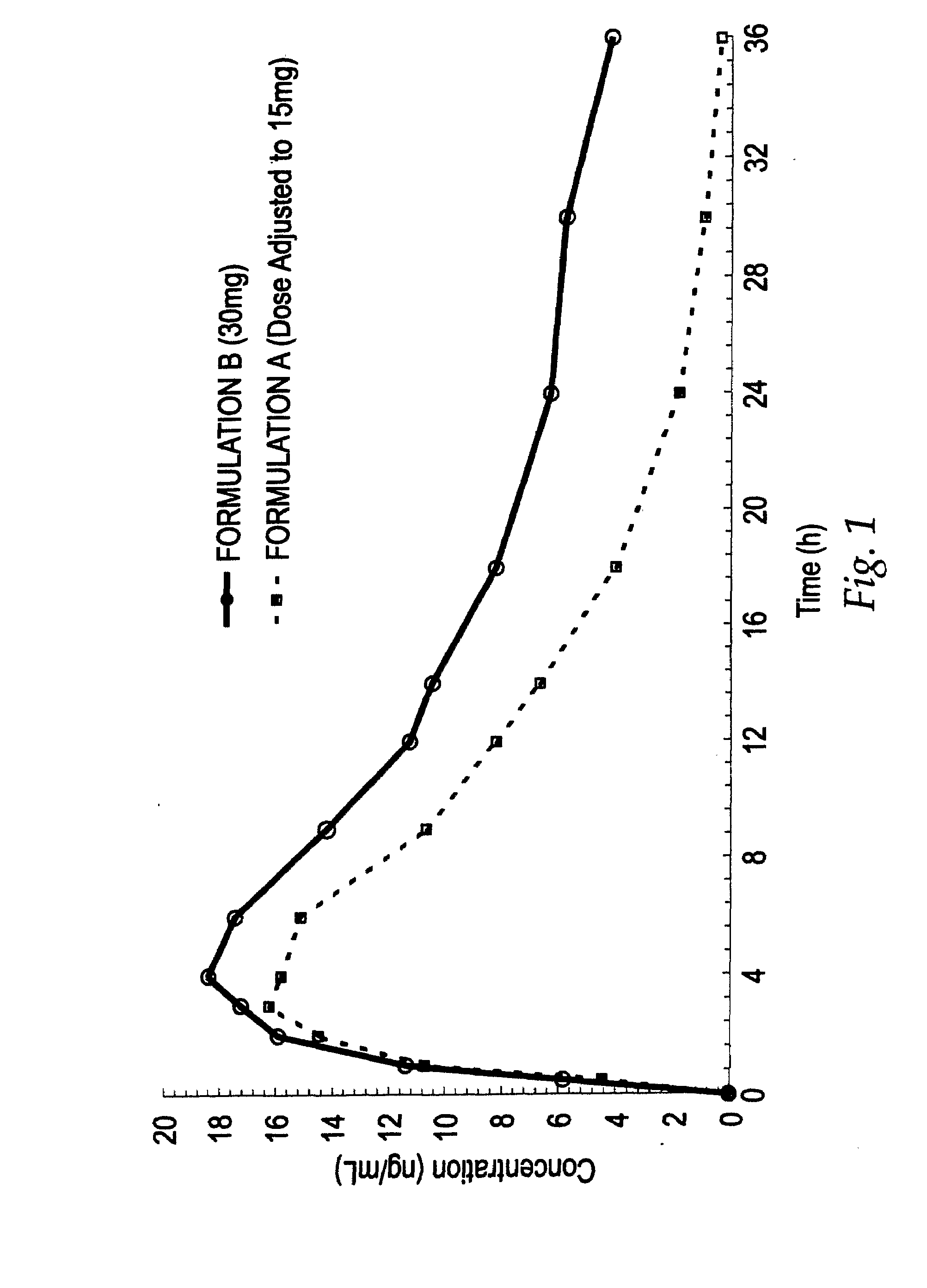
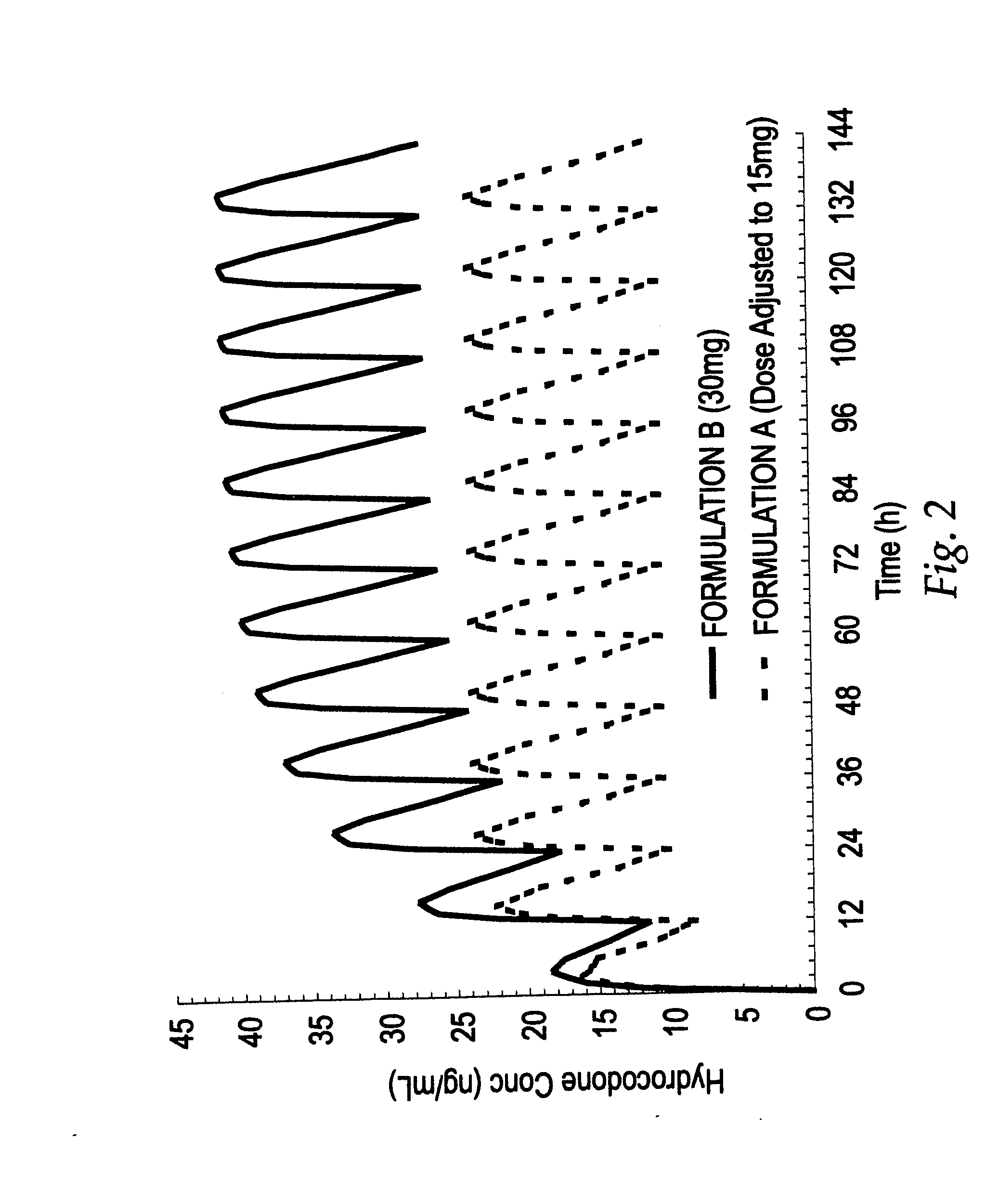
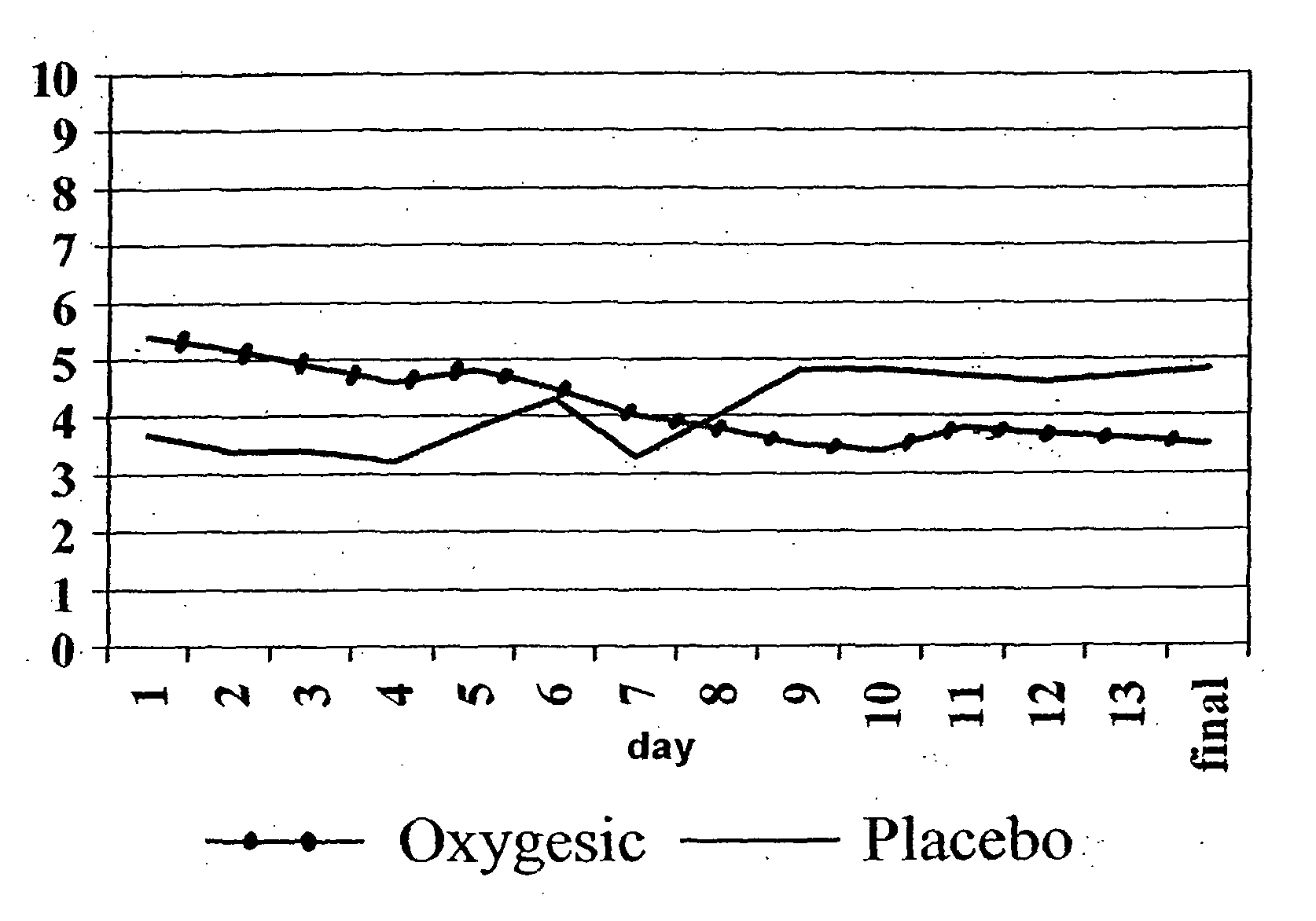
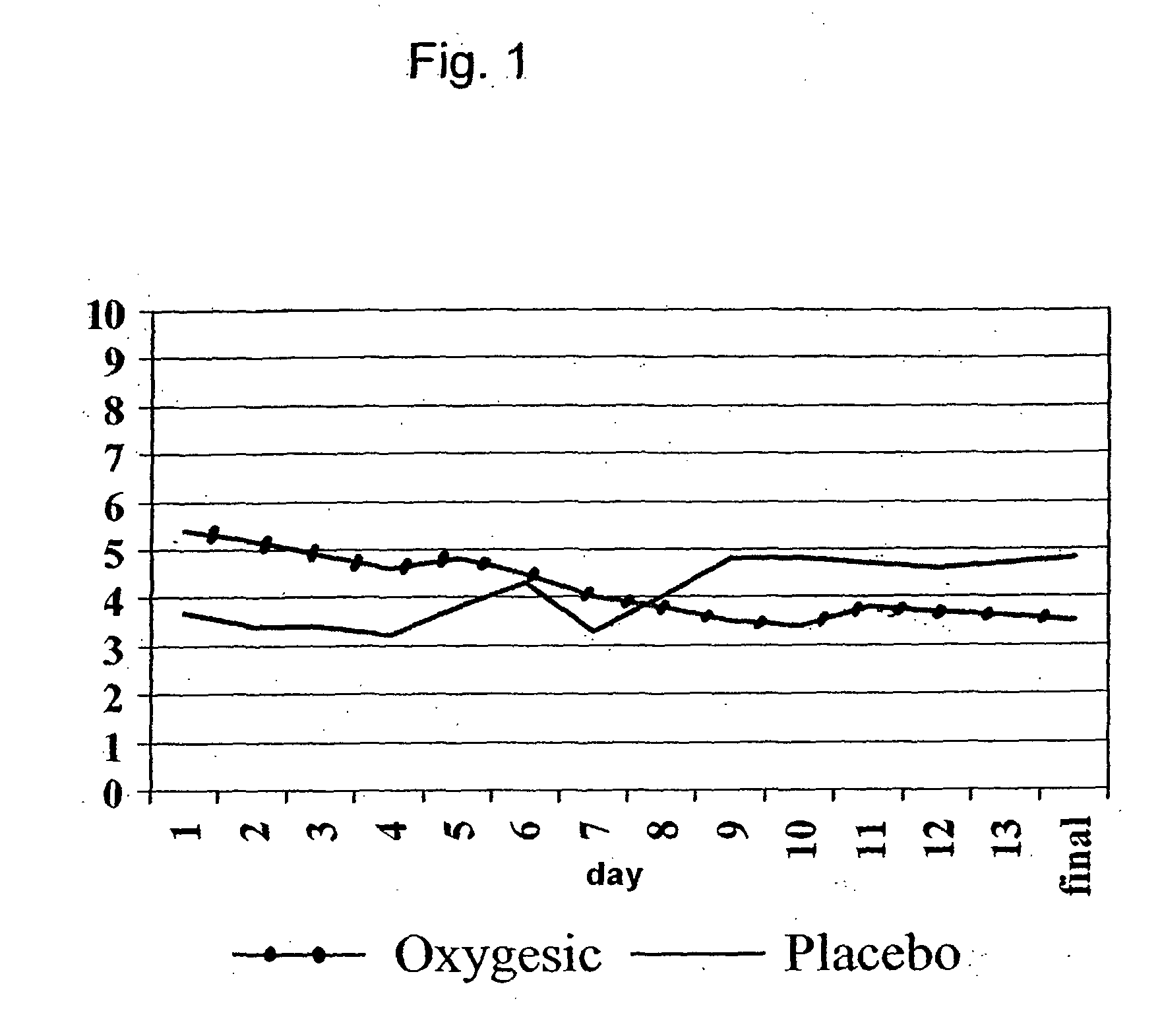
Treating pain by administering 24 hours opioid formulations exhibiting rapid rise of drug level
InactiveUS20020058050A1Great analgesic efficacyQuick releaseOrganic active ingredientsCosmetic preparationsAbsorption Half-LifeOral medication
Patients are treated with 24-hour oral sustained release opioid formulations which, upon administration, provide an initially rapid opioid absorption such that the minimum effective analgesic concentration of the opioid is more quickly achieved. These sustained release opioid formulations include an effective amount of at least one retardant material to cause said opioid analgesic to be released at a such a rate as to provide an analgesic effect after oral administration to a human patient for at least about 24 hours, and are characterized by providing an absorption half-life from 1 to about 8 hours. A method of titrating a human patient utilizing these sustained release opioid formulations is also disclosed.
Owner:PURDUE PHARMA LP
Method of treating pain by administering 24 hour oral opioid formulations exhibiting rapid rate of initial rise of plasma drug level
InactiveUS20030035837A1Good analgesic effectQuick releaseBiocidePowder deliveryAbsorption Half-LifeOral medication
Patients are treated with 24-hour oral sustained release opioid formulations which, upon administration, provide an initially rapid opioid absorption such that the minimum effective analgesic concentration of the opioid is more quickly achieved. These sustained release opioid formulations include an effective amount of at least one retardant material to cause said opioid analgesic to be released at a such a rate as to provide an analgesic effect after oral administration to a human patient for at least about 24 hours, and are characterized by providing an absorption half-life from 1 to about 8 hours. A method of titrating a human patient utilizing these sustained release opioid formulations is also disclosed.
Owner:SACKLER RICHARD S +2
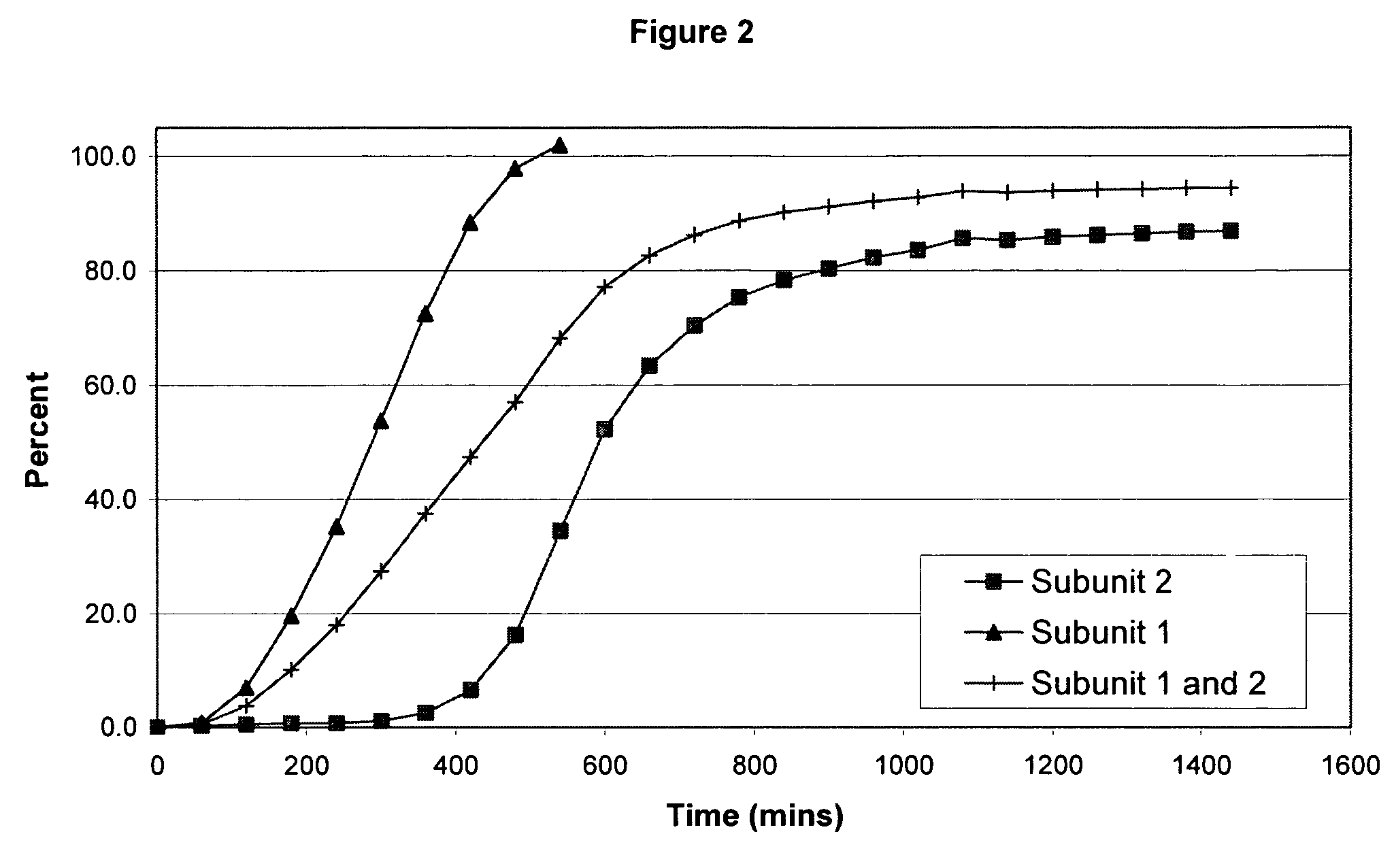
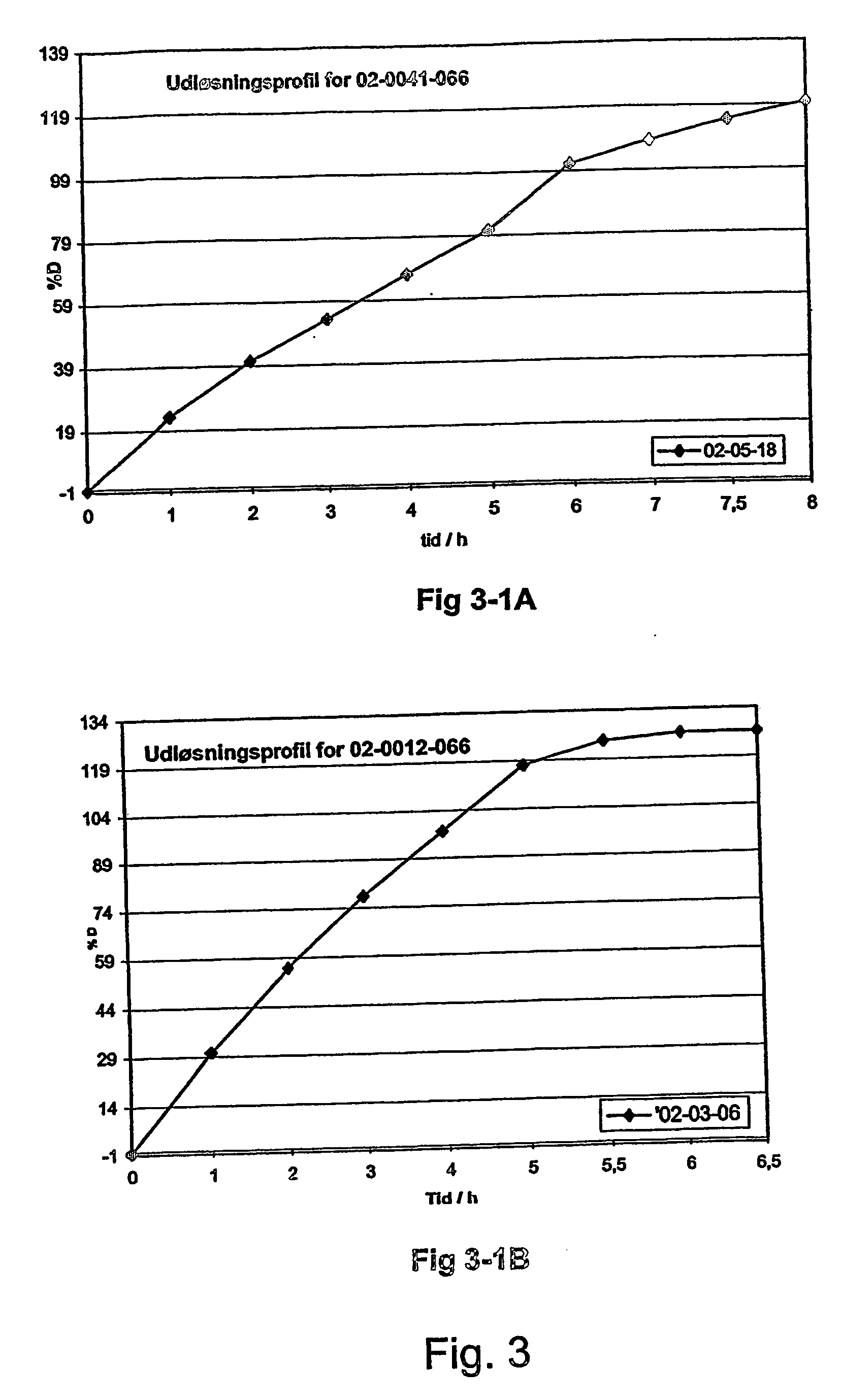
Sustained release opioid formulations and methods of use
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
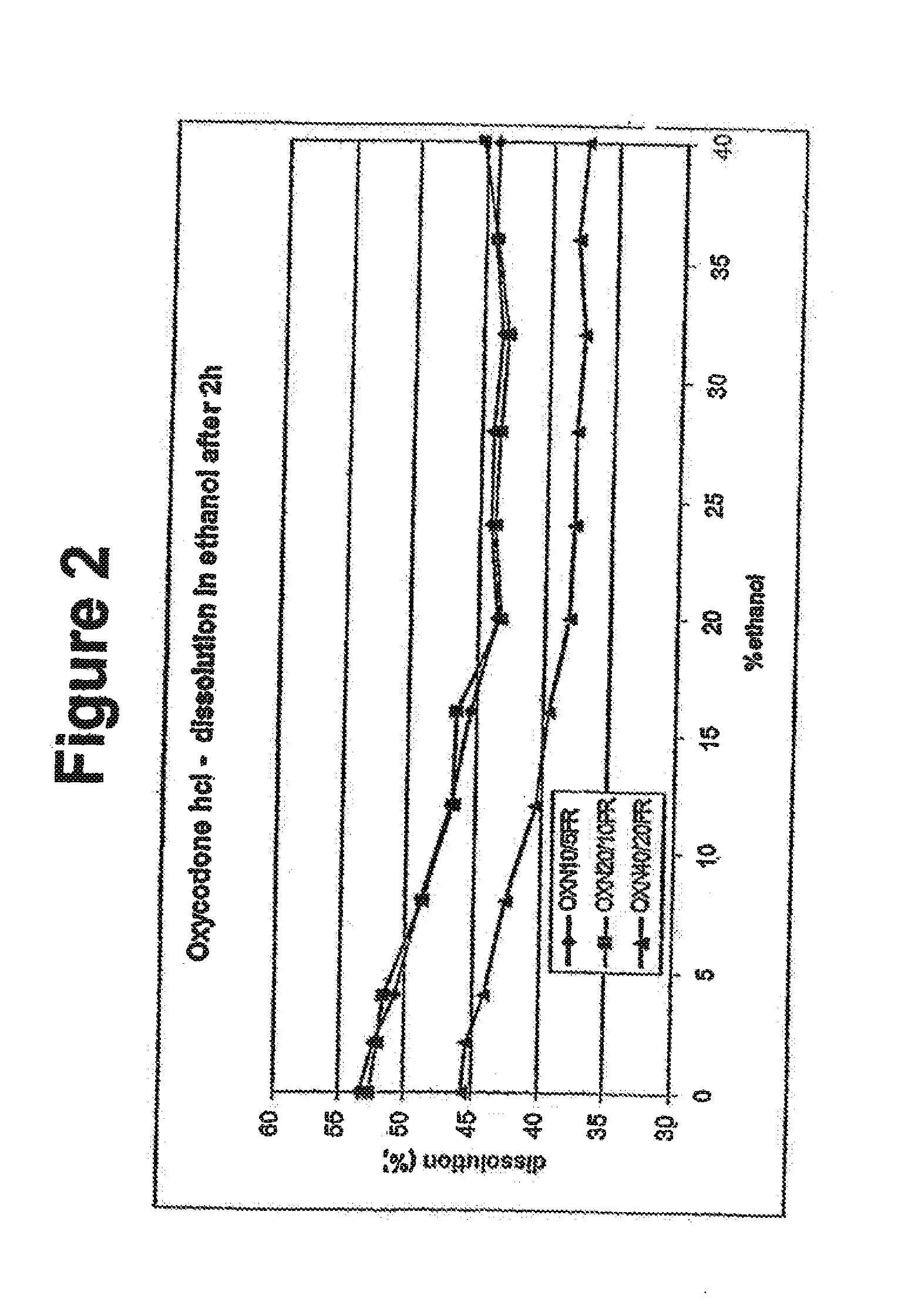
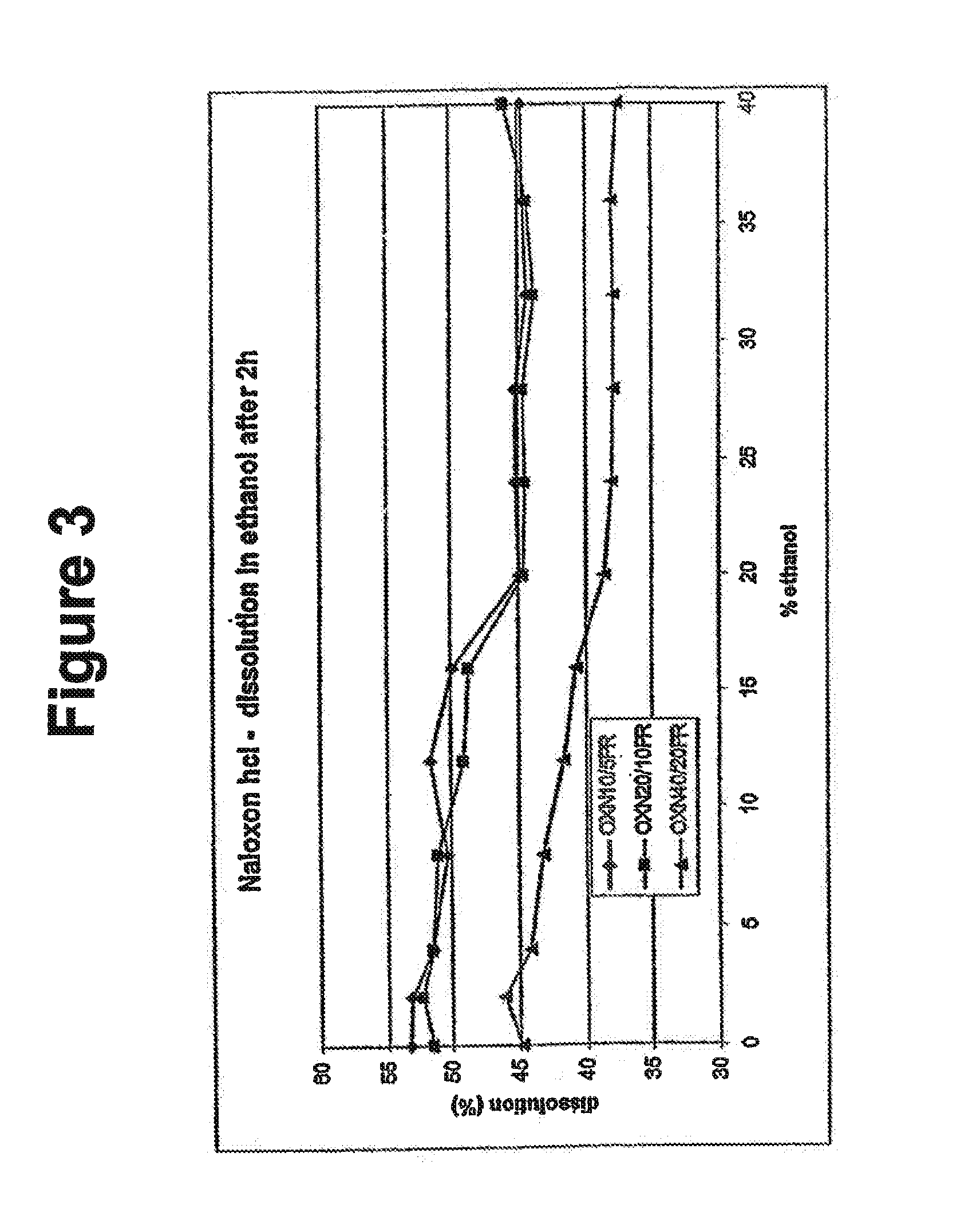
Alcohol resistant dosage forms
Owner:PURDUE PHARMA LP
A2a adenosine receptor antagonists
The present invention relates to novel compounds that are A2A adenosine receptor antagonists, and to their use in treating mammals for various disease states, such as obesity, CNS disorders, including the “movement disorders” (Parkinson's disease, Huntington's Chorea, and catelepsy), and cerebral ischemia, excitotoxicity, cognitive and physiological disorders, depression, ADHD, and drug addiction (alcohol, amphetamine, cannabinoids, cocaine, nicotine, and opioids) and to their use in the enhancement of immune response. The invention also relates to methods for the preparation of such compounds, and to pharmaceutical compositions containing them.
Owner:GILEAD SCI INC
Therapy for enteric infections
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
Topical anesthetic formulation
The topical medicament gel formulation of the present invention includes an anesthetic, an anti-microbial, an oxidant, a nutrient, a diuretic, an opioid, an anti-emetic, an anti-seizure drug, and a non-steroidal anti-inflammatory drug (NSAID), USP in a molecular, as opposed to a salt form, as the active ingredient. Additional constituents illustratively include a skin penetration enhancer and a gelling agent. This invention deals with problems commonly associated with topical application of local medicaments such as: slow onset of action; need for occlusion; and rapid loss of effect due to rapid systemic dispersion. The invention permits enhanced penetration of the medicament and thereby allows for a lesser total dosage of pharmaceutically active ingredient. The use of a lesser total dosage also decreases systemic toxicity.
Owner:WEPFER SCOTT
Oral dosage form safeguarded against abuse
The present invention relates to an abuse-proofed, oral dosage form with controlled opioid-release for once daily administration, characterised in that it comprises at least one opioid with potential for abuse (A), at least one synthetic or natural polymer (C), optionally delayed-release matrix auxiliary substances, physiologically acceptable auxiliary substances (B), optionally a wax (D) and optionally at least one delayed-release coating, component (C) or (D) in each case exhibiting a breaking strength of at least 500 N, preferably of at least 1000 N.
Owner:GRUNENTHAL GMBH
Medicinal uses of mu-opioid receptor agonists
ActiveUS7498297B2Reduce intensityControl rateAntipyreticAnalgesicsDiseaseMu-Opioid Receptor Agonists
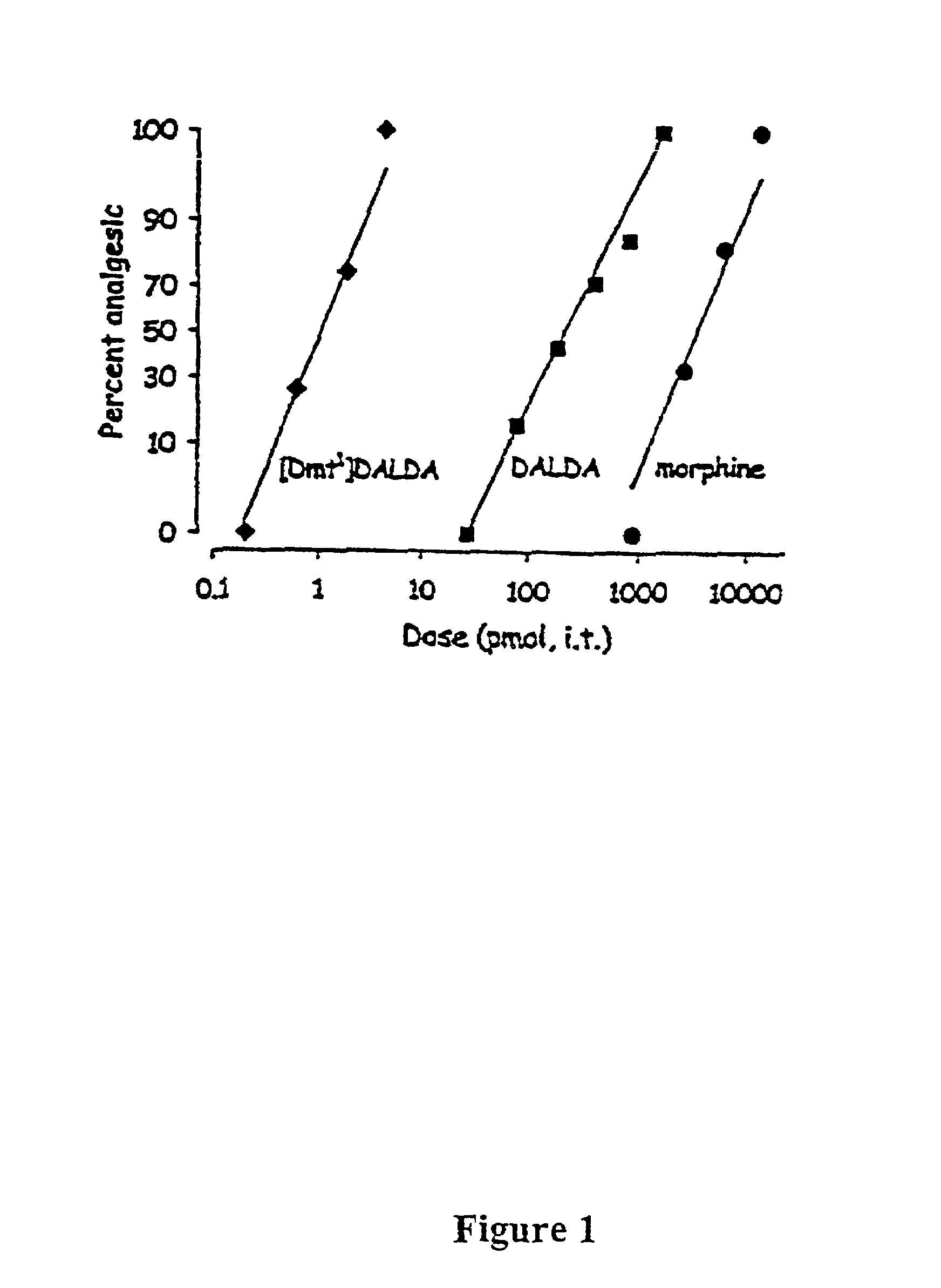
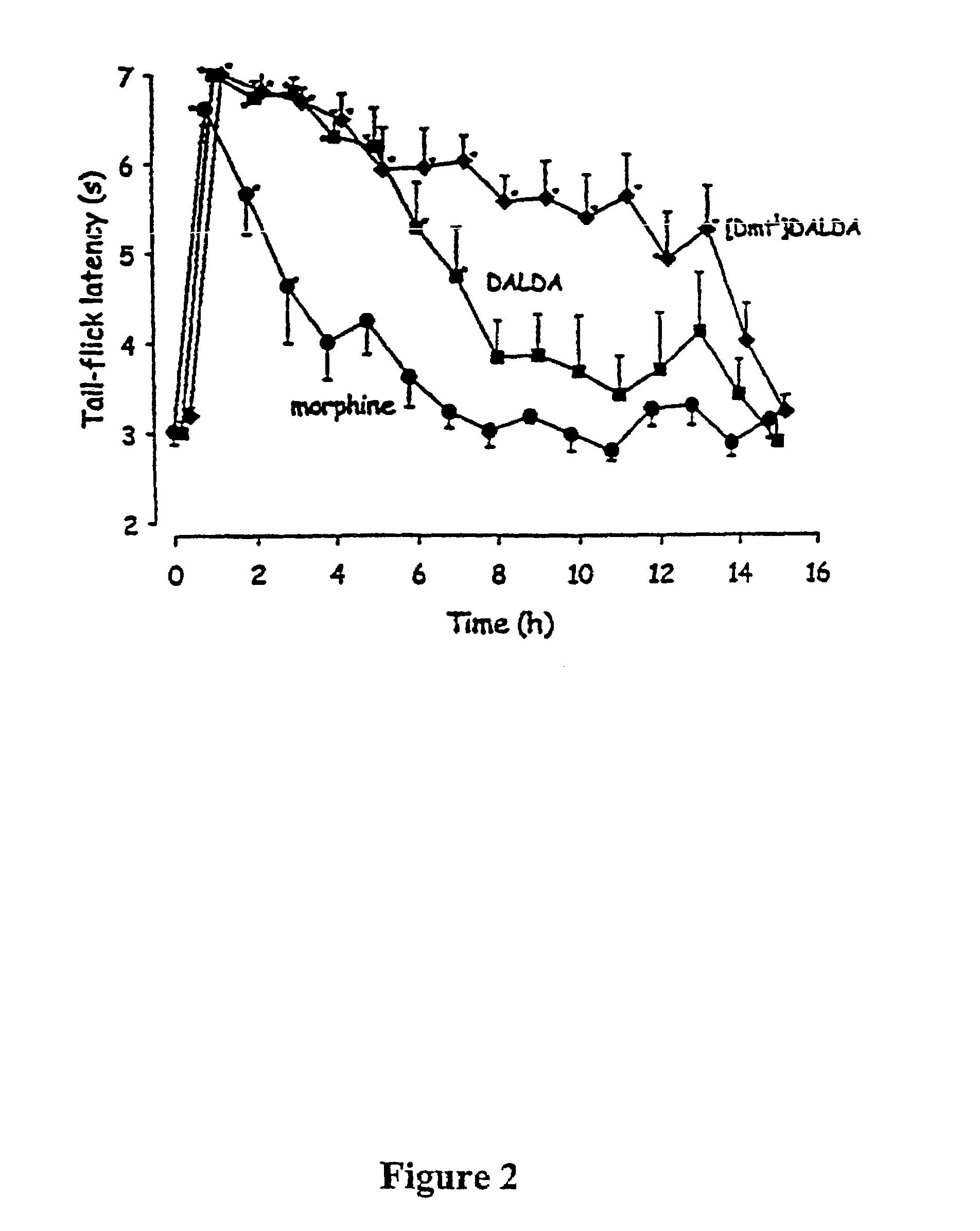
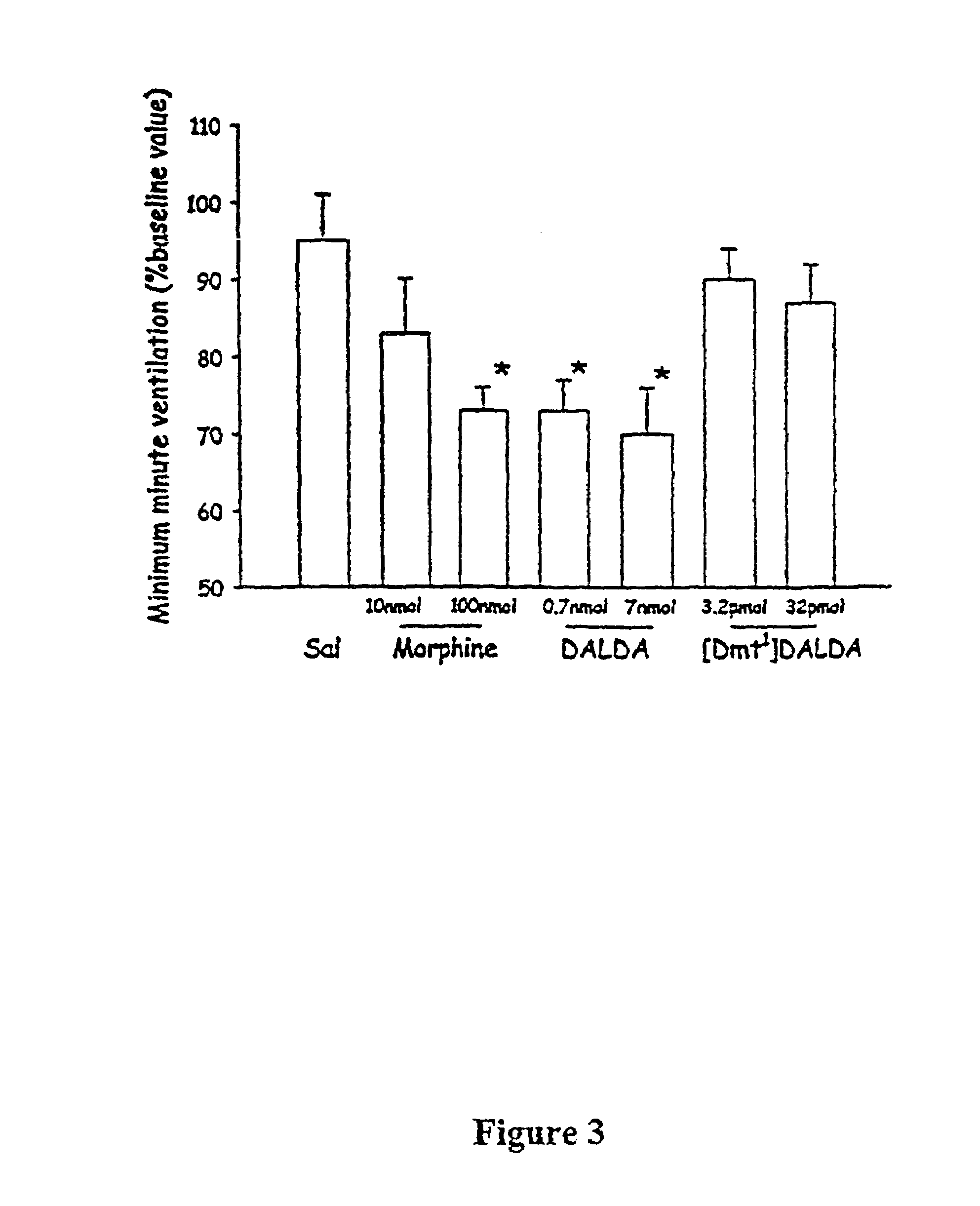
The present invention provides methods for stimulating a mu-opioid receptor agonist peptide in a mammal in need thereof. The methods comprise administering to the mammal an effective amount of a selective mu-opioid receptor agonist peptide that comprises at least two α-amino acid residues. At least one of the amino acid residues has a positive charge. The amino acid residue in the first position is a tyrosine or tyrosine derivative. The amino acid in the second position is a D-α-amino acid. The present invention also provides methods of treating a mammal suffering from conditions or diseases by administering to the mammal an effective amount of the peptides.
Owner:CORNELL RES FOUNDATION INC +1
Large Substituent, Non-Phenolic Opioids
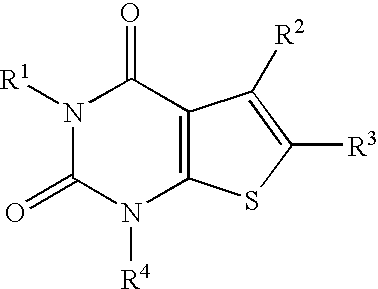
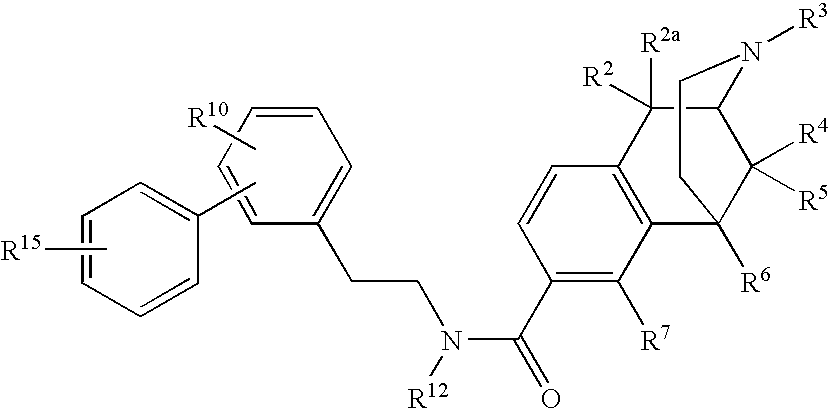
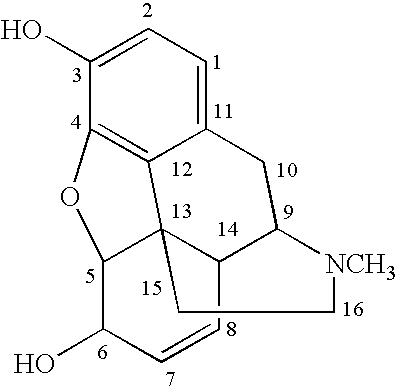
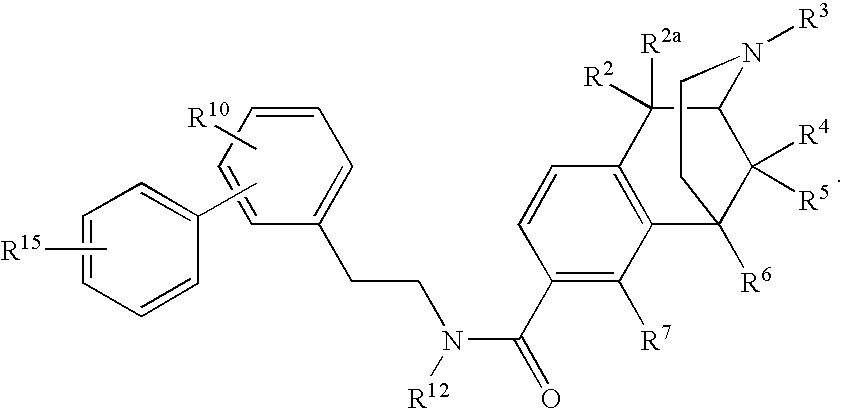
ActiveUS20070021457A1Excellent opioid bindingGood metabolic stabilityBiocideNervous disorderBenzazocineAnalgesic agents
8-Substituted-2,6-methano-3-benzazocines of general structure are useful as analgesics, anti-diarrheal agents, anticonvulsants, antitussives and anti-addiction medications. One embodiment is the subgenus of biphenylethyl compounds:
Owner:RENESSELAER POLYTECHNIC INST
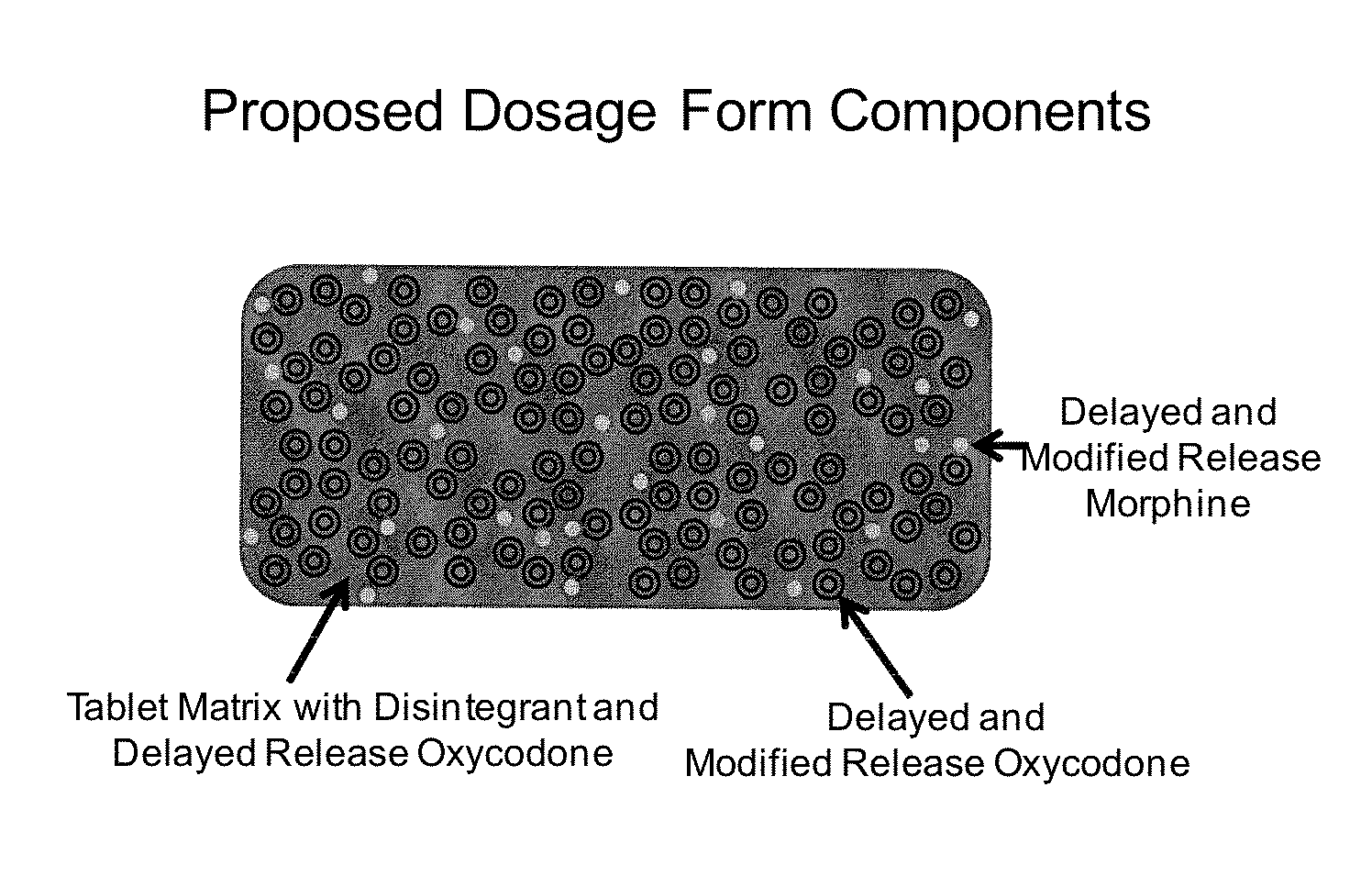
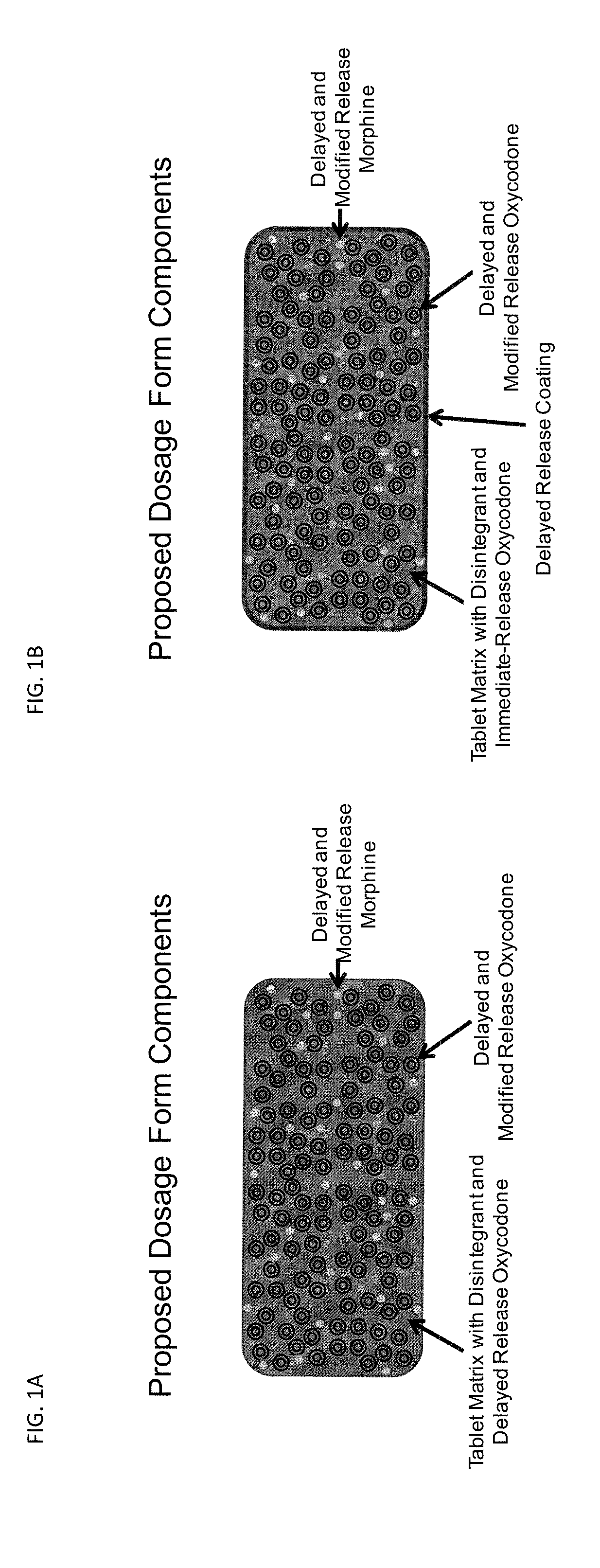
Controlled Release Formulations of Opioids
InactiveUS20130022646A1BiocideNervous disorderPharmaceutical formulationControlled-Release Formulations
Pharmaceutical formulations containing opioid components that each has a release profile. The components may provide immediate or controlled release of the opioid. The invention is also directed to methods of controlling release of one or more opioid compounds and methods of treating pain.
Owner:QRXPHARMA
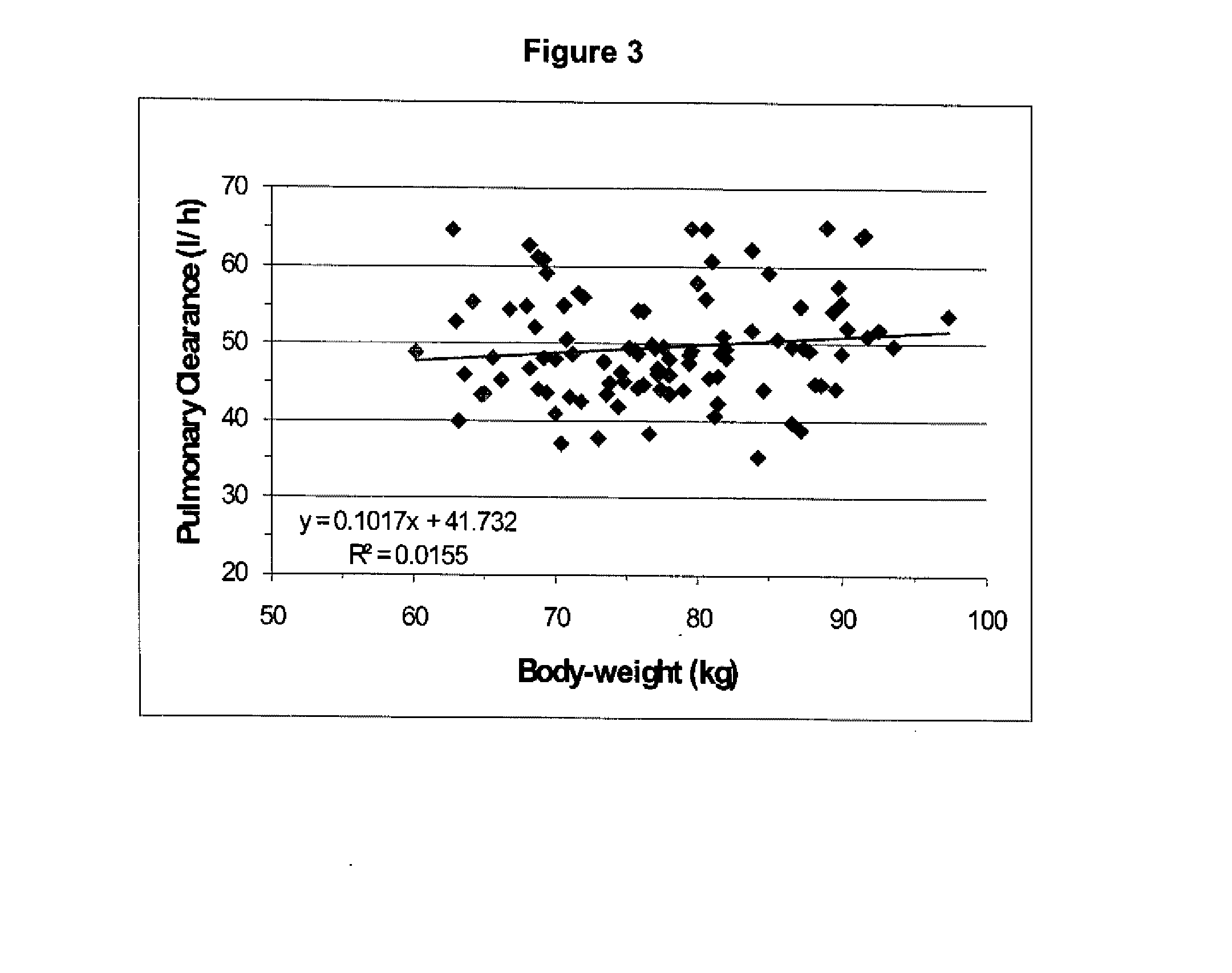
Opioids for the treatment of the chronic obstructive pulmonary disease (copd)
ActiveUS20070185146A1Less side effectsBiocideRespiratory disorderControl releaseObstructive Pulmonary Diseases
The present invention relates to an opioid controlled release oral dosage form comprising at least one opioid for the manufacture of a medicament to treat patients with Chronic Obstructive Pulmonary Disease (COPD).
Owner:PURDUE PHARMA LP
Tamper-resistant pharmaceutical compositions of opiods and other drugs
InactiveUS20110142943A1Reduce the possibilityImprove lipophilicityBiocidePowder deliveryAs DirectedOpioid
Tamper-resistant pharmaceutical compositions have been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opioids. The tamper-resistant compositions retard the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is passes through the GI tract.
Owner:COLLEGIUM PHARMA INC
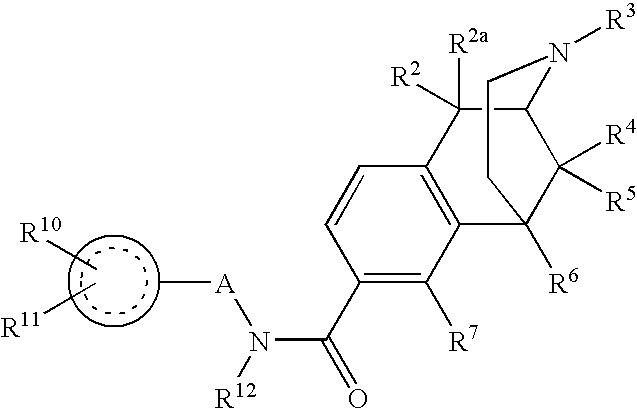
Quaternary opioid carboxamides
ActiveUS20090197905A1Good to excellent peripheral opioid antagonism activityLess susceptibleBiocideNervous disorderSide effectMedicine
Owner:RENESSELAER POLYTECHNIC INST
Phosphate derivatives of pharmaceutical products
InactiveUS20070042999A1Quick conversionReduce solubilityBiocideNervous disorderAnesthetic AgentPhosphate
According to the invention, there is provided a complex of a pharmaceutical compound selected from the group consisting of opioids, hormones, anaethetics and chemotherapeutic agents comprising the reaction product of: (a) one or more phosphate derivatives of one or more opioids, steroid hormones, thyroid hormones, anaesthetics or chemotherapeutic agents having a phenolic, primary alcohol, secondary alcohol or tertiary hydroxyl group; and (b) a complexing agent selected from the group comprising amphoteric surfactants, cationic surfactants, amino acids having nitrogen functional groups and proteins rich in these amino acids.
Owner:VITAL HEALTH SCIENCES PTY LTD
Method of Improving Treatments in Rheumatic and Arthritic Diseases
Improved treatments of joint diseases, such as, e.g. osteoarthritis and rheumatoid arthritis, and pain, wherein a strontium-containing compound is administered alone or in combination with one or more second therapeutically and / or prophylactically active substances, selected from the group consisting of bisphosphonates, glucosamine, pallitative agents, analgesic agents, disease modifying anti-rheumatic compounds (DMARDs), selective estrogen receptor modulators (SERMs), aromatase inhibitors, non-steroidal anti-inflammatory agents (NSAIDs), COX-2 inhibitors, COX-3 inhibitors, opioids, inhibitors / antagonists of IL-1, inhibitors / antagonists of TNF-alpha, inhibitors of matrix metallo-proteinases (MMPs), cathepsin K inhibitors, inhibitors / antagonists of RANK-ligand, statins, glucocorticoids, chondroitin sulphate, NMDA receptor antagonists, inhibitors of interleukin-I converting enzyme, Calcitonin gene related peptide antagonists, glycine antagonists, vanilloid receptor antagonists, inhibitors of inducible nitric oxide synthetase (iNOS), N-acetylcholine receptor agonists, neurokinin antagonists, neuroleptic agents, PAR2 receptor antagonists and anabolic growth factors acting on joint tissue components. Pharmaceutical compositions comprising a strontium-containing compound and a second therapeutically and / or prophylactically active substance as defined above.
Owner:OSTEOLOGIX AS
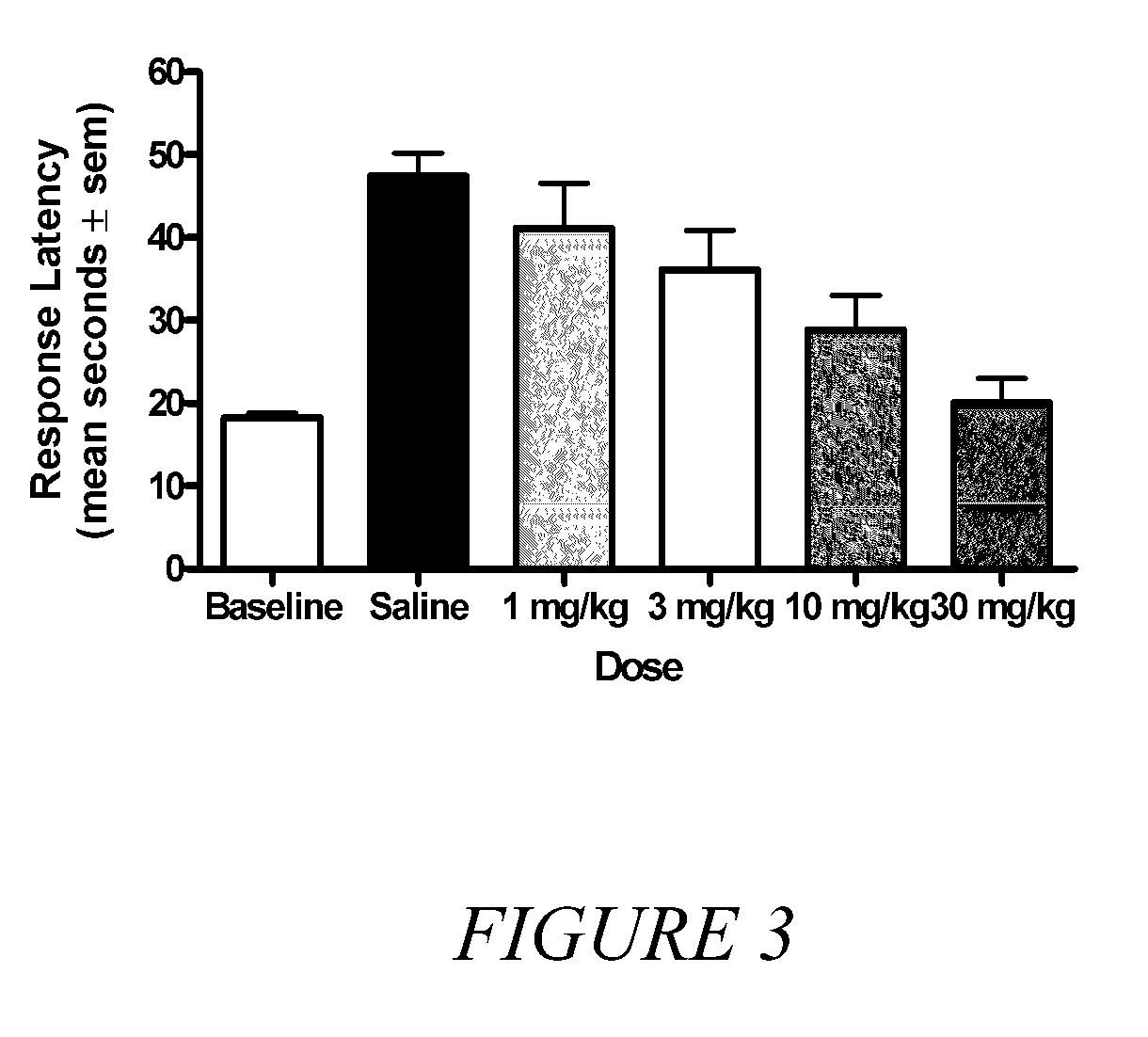
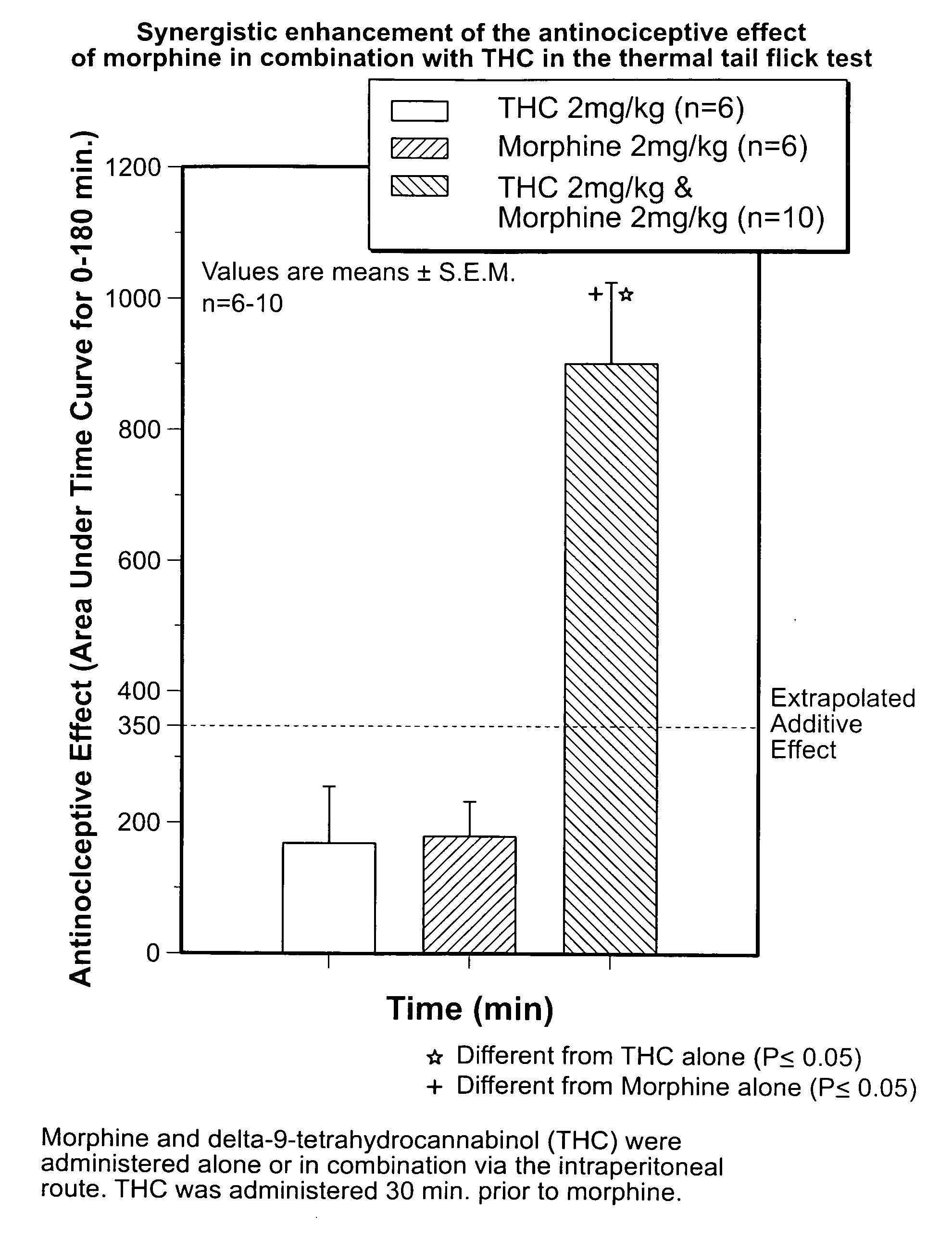
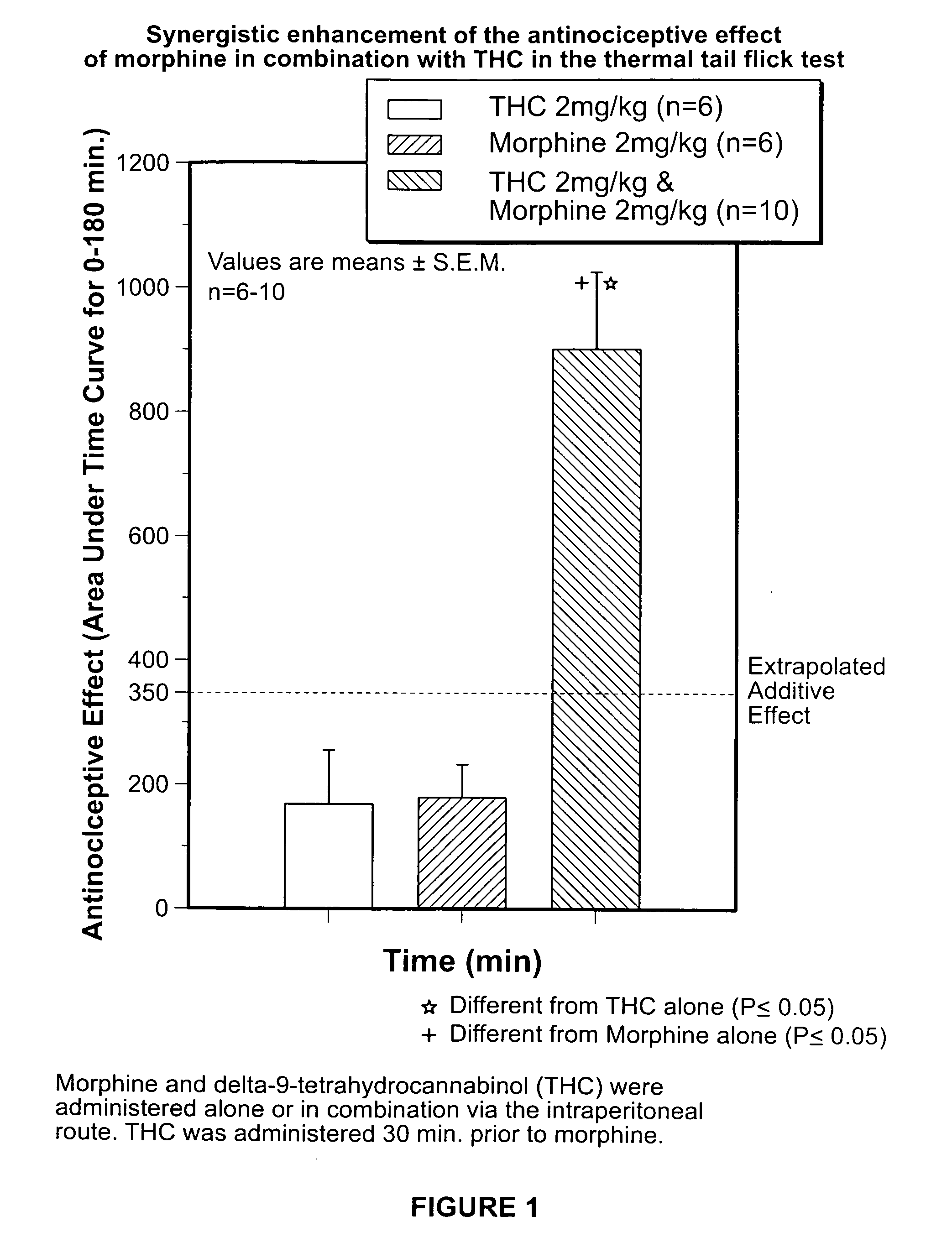
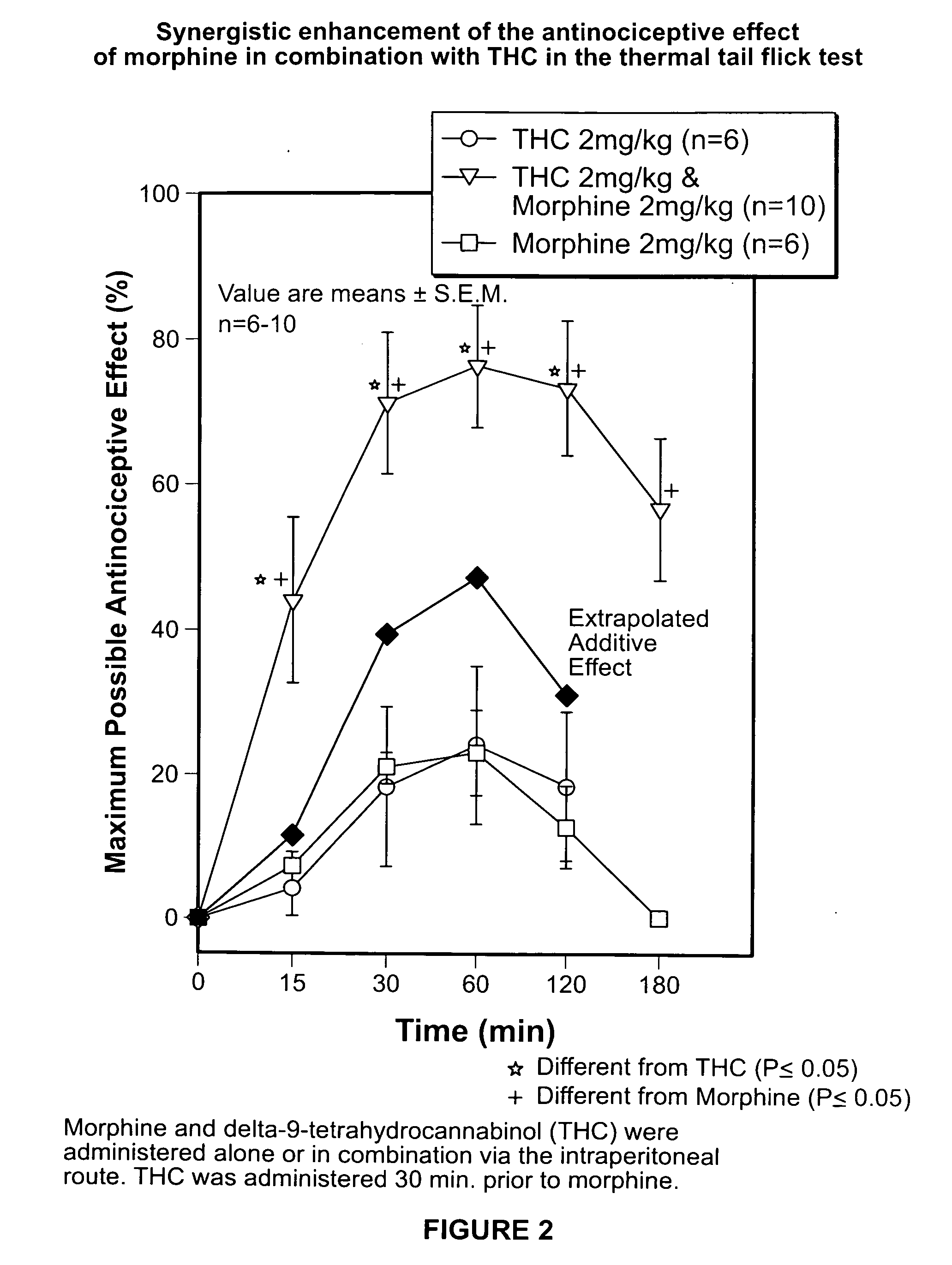
Novel synergistic opioid-cannabinoid codrug for pain management
InactiveUS20080176885A1Slow of dependenceReduce clinical side effectsBiocideOrganic chemistryCannabinoidPain management
Compounds including an opioid, and a cannabinoid covalently bound by a linker; pharmaceutical formulations including codrugs; methods of manufacture as well as methods of treatment are disclosed.
Owner:INSYS THERAPEUTICS
Large substituent, non-phenolic opioids
ActiveUS7557119B2Excellent opioid bindingGood metabolic stabilityBiocideNervous disorderBenzazocineAnalgesic agents
8-Substituted-2,6-methano-3-benzazocines of general structureare useful as analgesics, anti-diarrheal agents, anticonvulsants, antitussives and anti-addiction medications. One embodiment is the subgenus of biphenylethyl compounds:
Owner:RENESSELAER POLYTECHNIC INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com