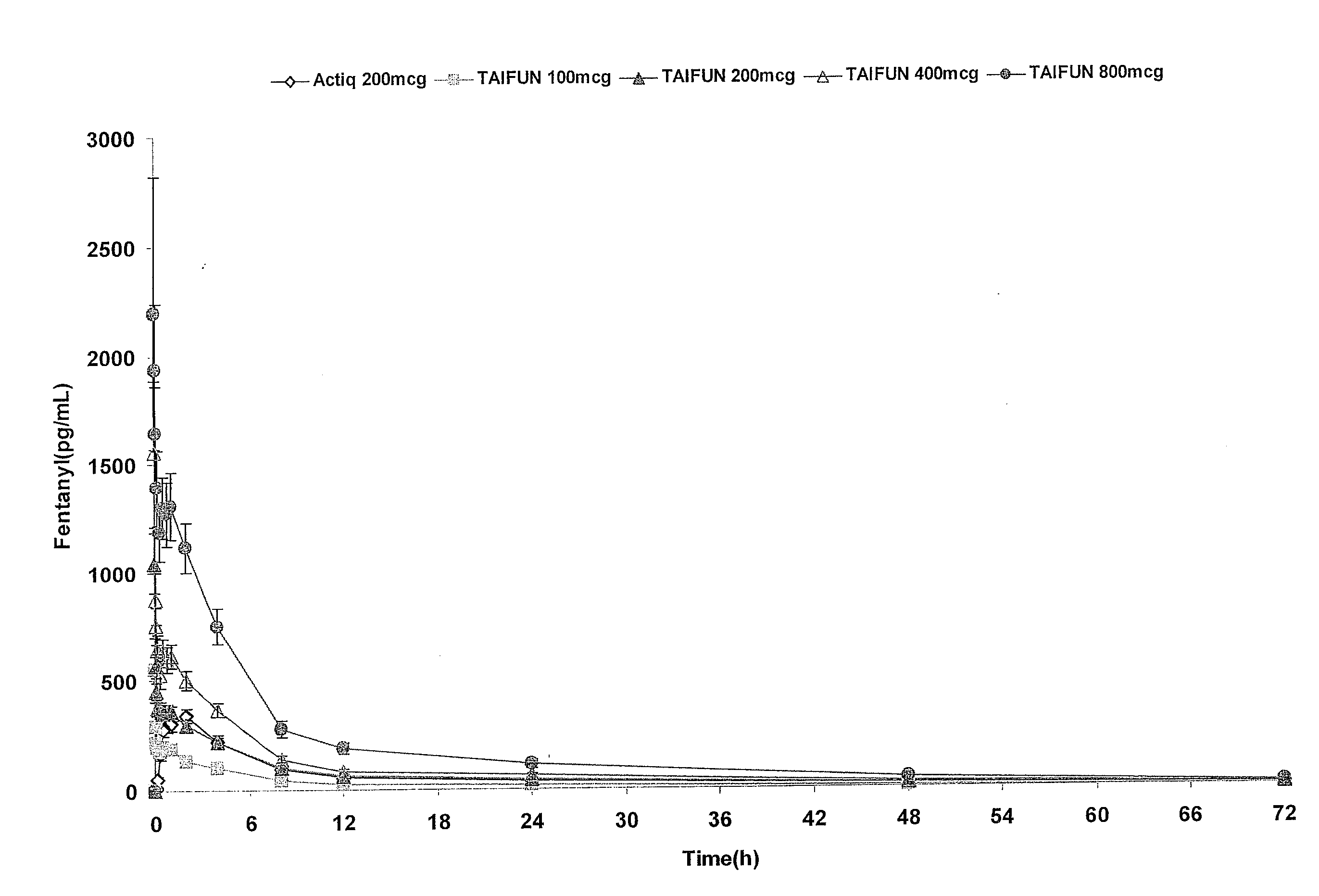
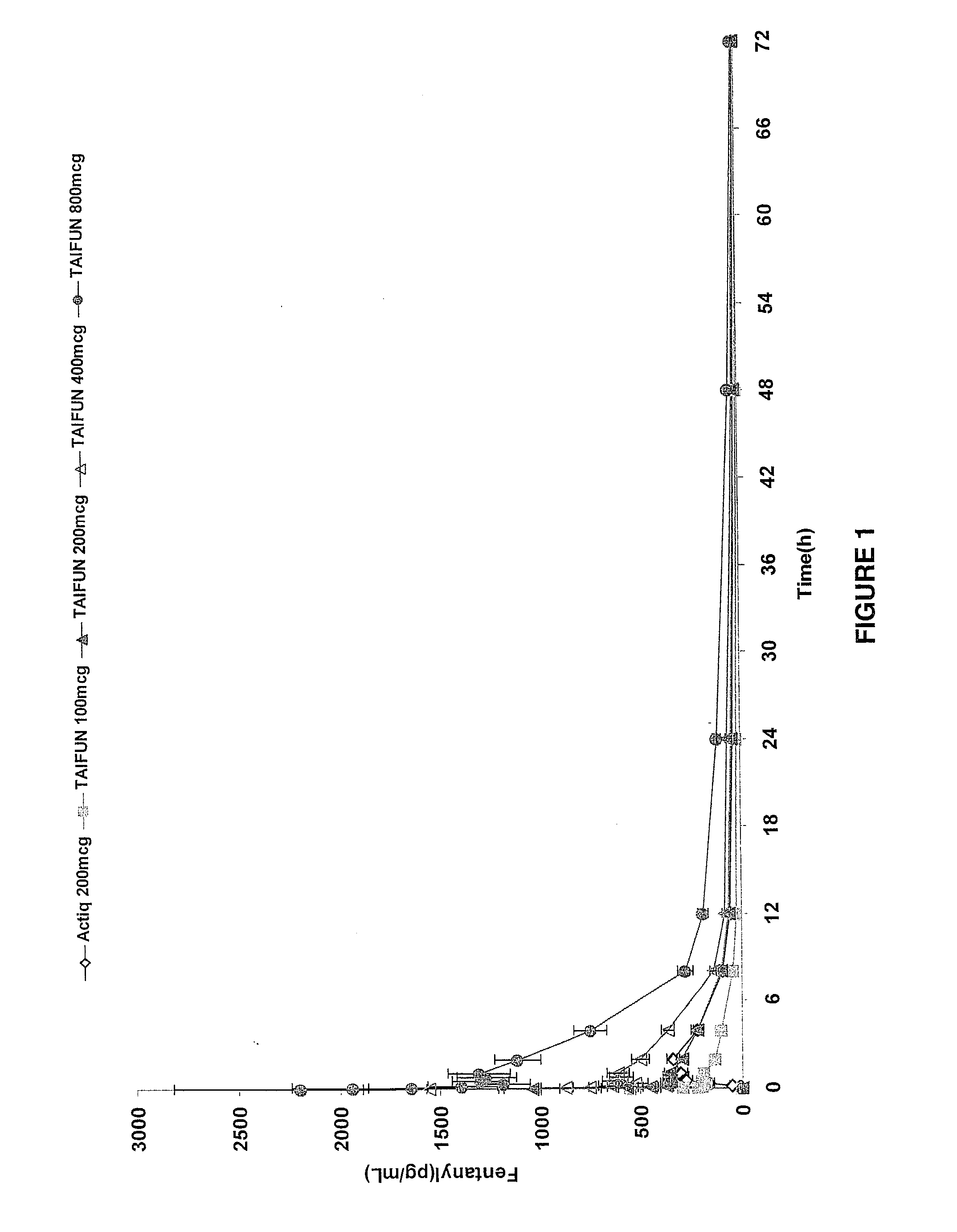
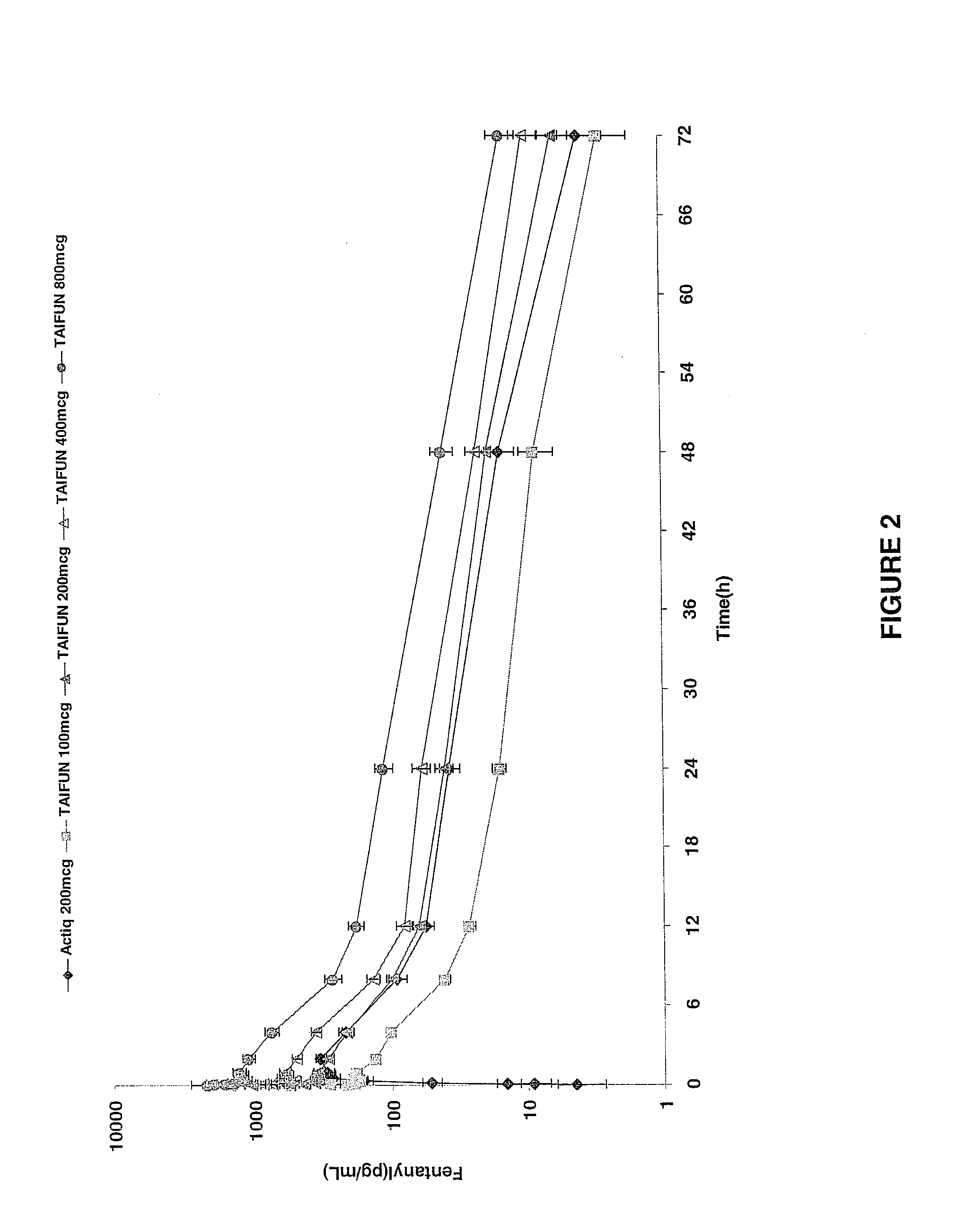
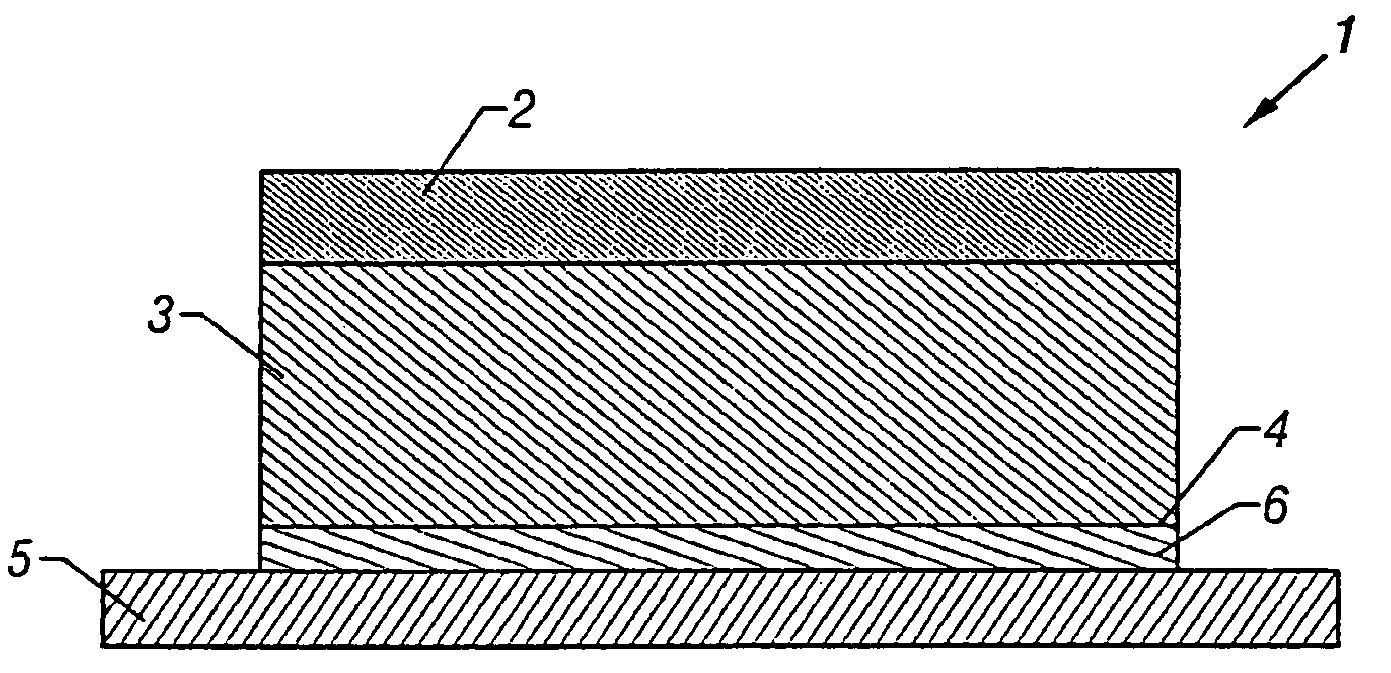
Patents
Literature
213 results about "Fentanyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to help relieve severe ongoing pain (such as due to cancer).
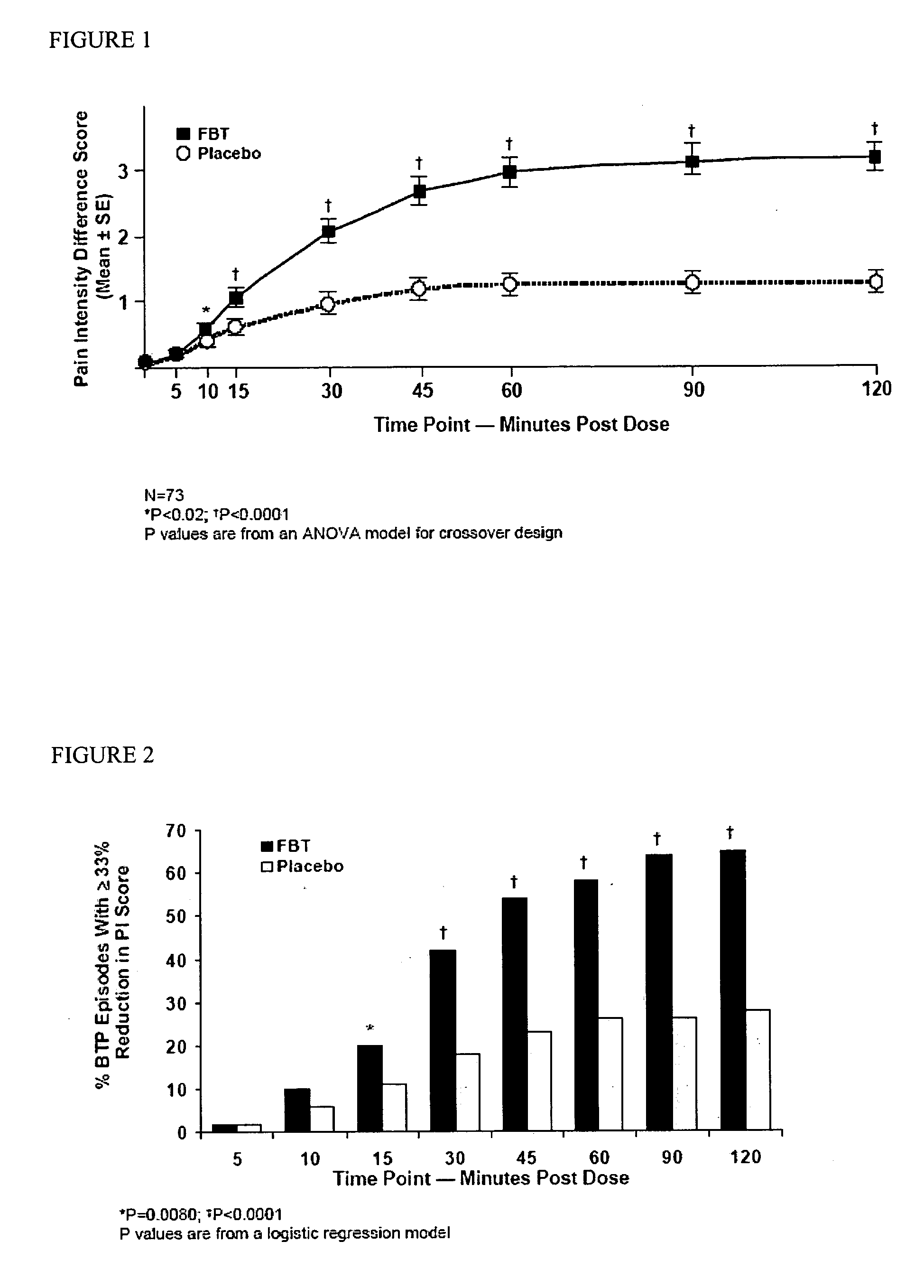
Fentanyl composition for the treatment of acute pain
A pharmaceutical composition for the treatment of acute pain by sublingual administration is described. The composition comprises an essentially water-free, ordered mixture of fentanyl or a pharmaceutically acceptable salt thereof in the form of microparticles which are adhered to the surface of carrier particles which are substantially larger than the particles of fentanyl, and are essentially water-soluble. In a preferred embodiment, the composition also contains a bioadhesion and / or mucoadhesion promoting agent. The invention also relates to the preparation of the composition, and to the use of the composition for the treatment of acute pain.
Owner:DIABAKT AB
Generally linear effervescent oral fentanyl dosage form and methods of administering
Fentanyl containing dosage forms and methods using same are described. These dosage forms include substantially less fentanyl by weight than know oral formulation and have advantages in terms of cost and side effects. These dosage forms are intended for oral administration of fentanyl across the oral mucosa.
Owner:CIMA LABS
Generally linear effervescent oral fentanyl dosage form and methods of administering
Fentanyl containing dosage forms and methods using same are described. These dosage forms include substantially less fentanyl by weight than know oral formulation and have advantages in terms of cost and side effects. These dosage forms are intended for oral administration of fentanyl across the oral mucosa.
Owner:CEPHALON INC
Effervescent oral fentanyl dosage form and methods of administering fentanyl
Fentanyl-containing dosage forms and methods using same are described. These dosage forms include substantially less fentanyl by weight than known oral formulation and have advantages in terms of reduced cost and reduced side effects. These dosage forms are intended for oral administration of fentanyl across the oral mucosa.
Owner:CEPHALON INC
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Systems and methods for administration of small volume sufentanil drug dosage forms to the sublingual mucosa of a subject using a device are disclosed. The dispensing device includes a lock-out feature and a means to retard or prevent saliva and / or moisture ingress such that the drug dosage forms in the device remain dry prior to administration.
Owner:ACEIRX PHARM INC
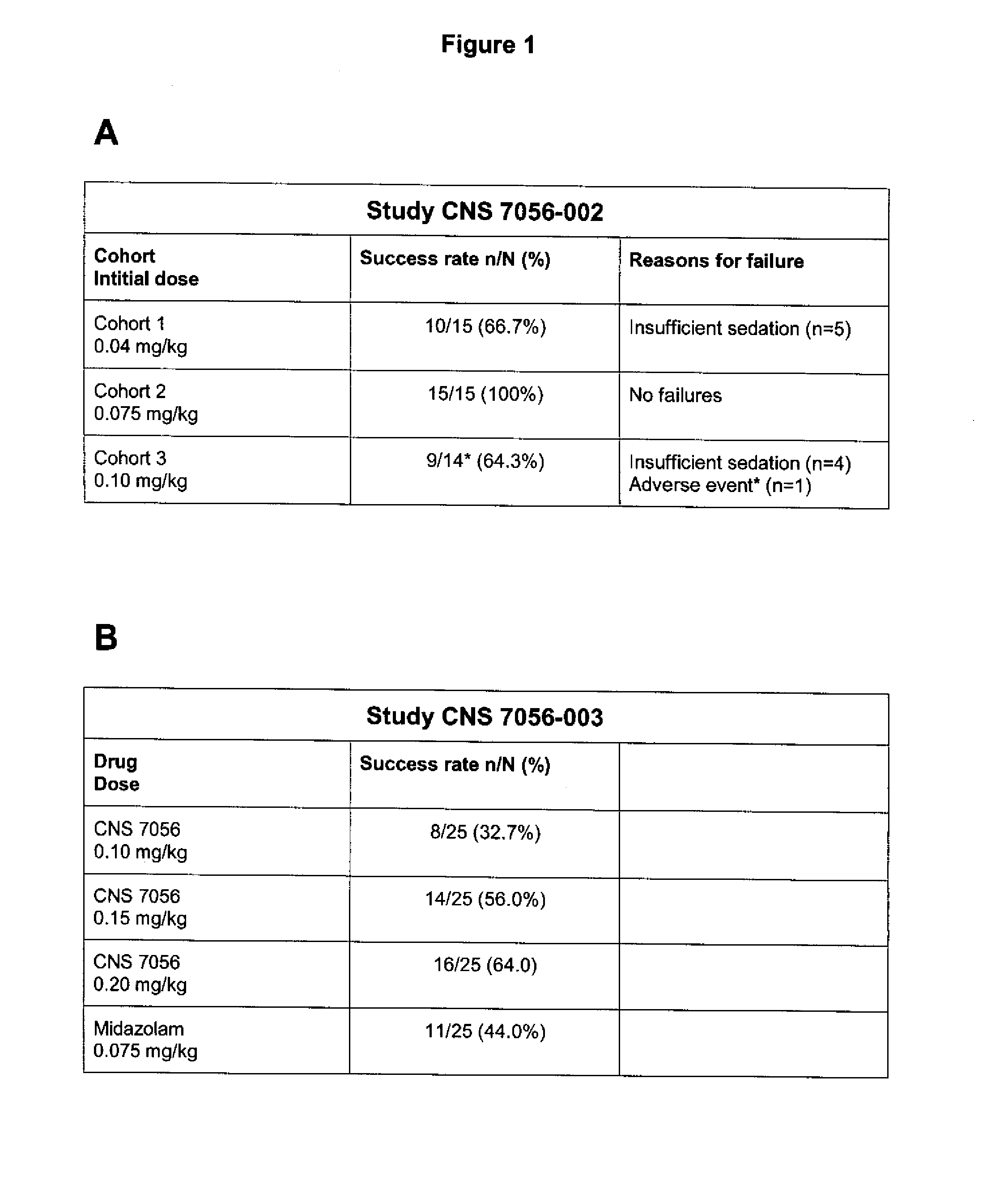
Dosing regimen for sedation with CNS 7056 (remimazolam)
ActiveUS20140080815A1Good sedative effectSafe and convenientBiocideNervous disorderDosing regimenRegimen
The invention relates to a dosing regimen for sedation with the fast-acting benzodiazepine CNS 7056 in combination with an opioid, in particular fentanyl, whereas CNS 7056 is given in a dose of 2 to 10 mg, preferably between 4 and 9 mg and most preferably between 5 and 8 mg.
Owner:PAION UK
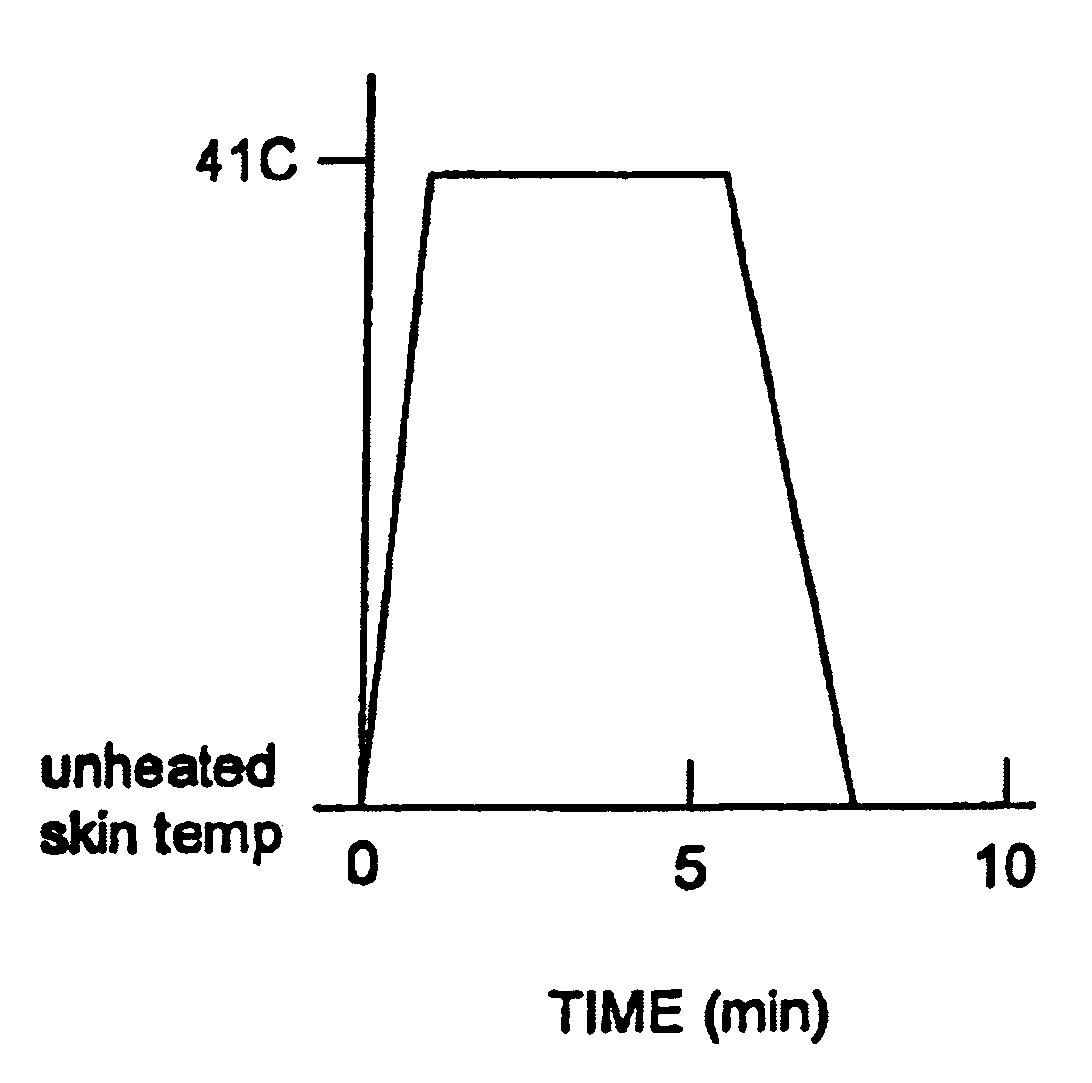
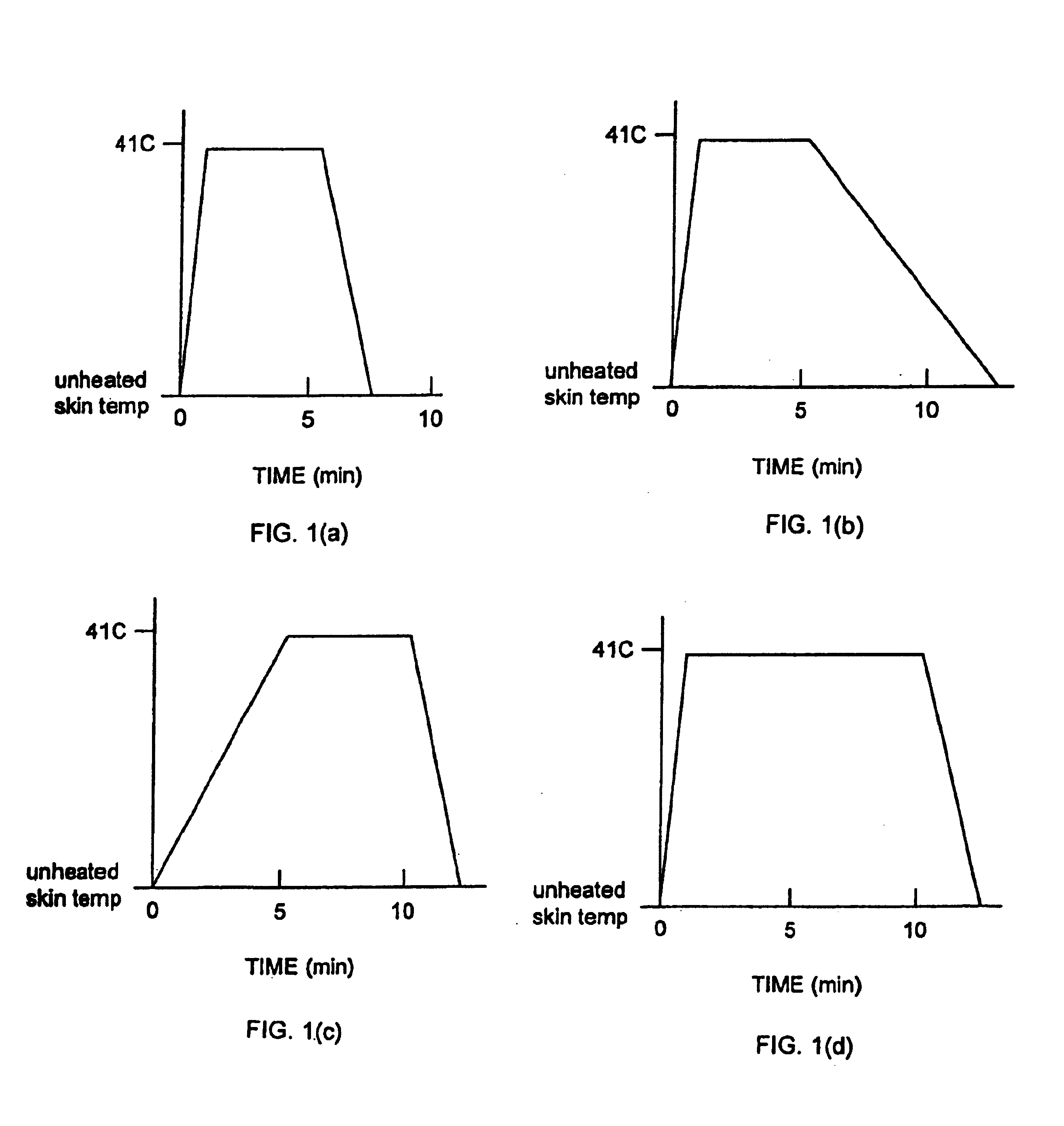
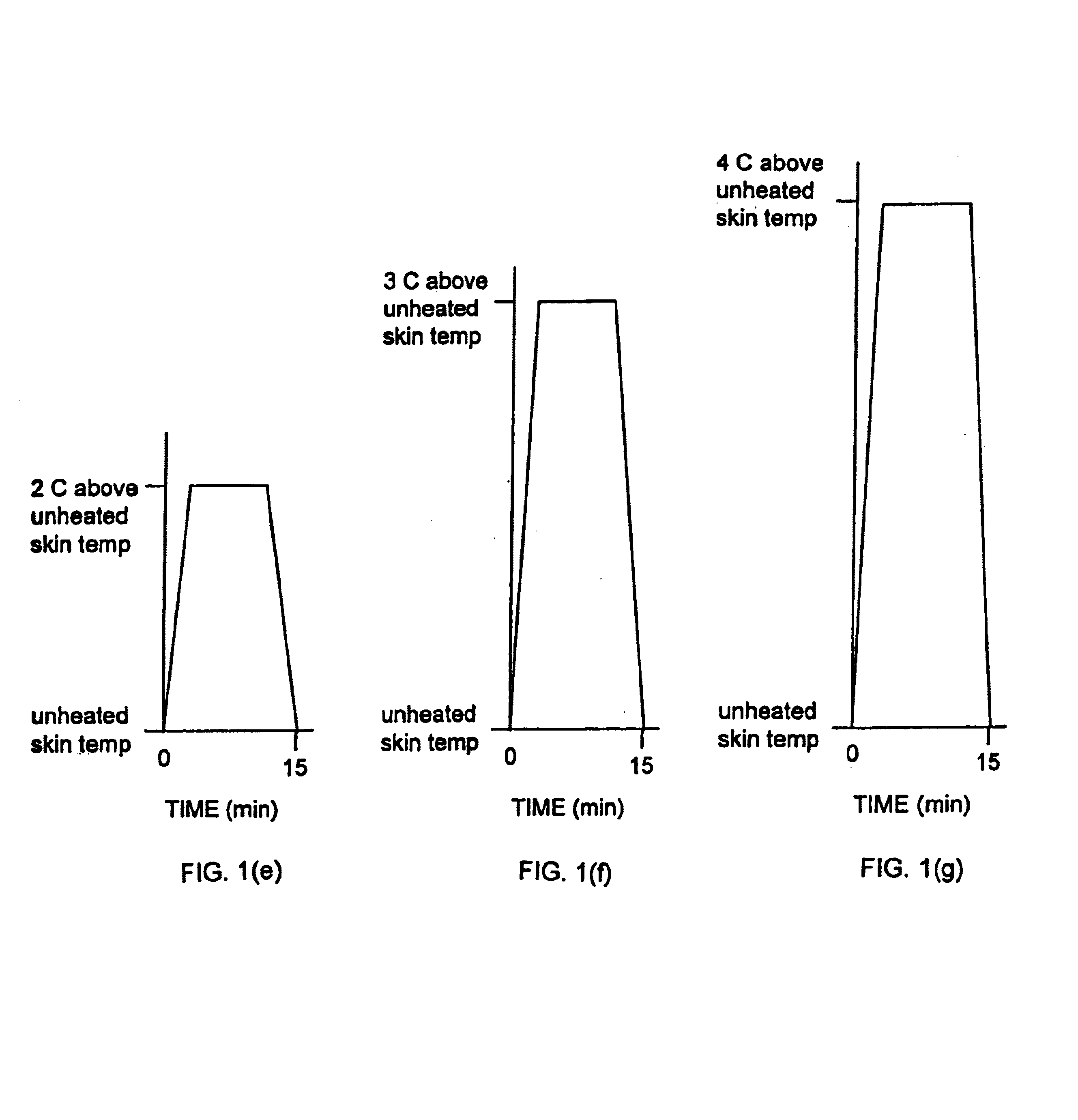
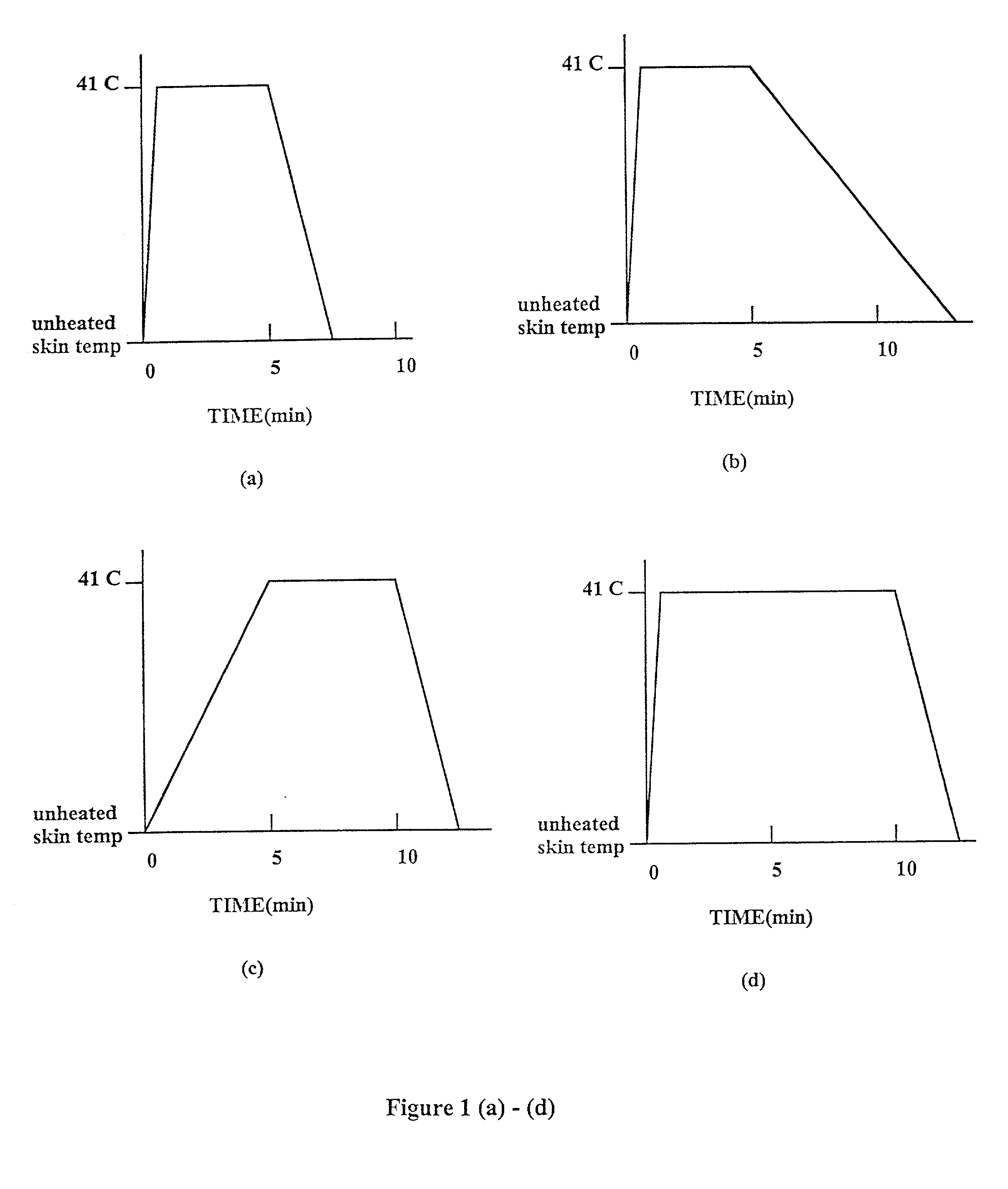
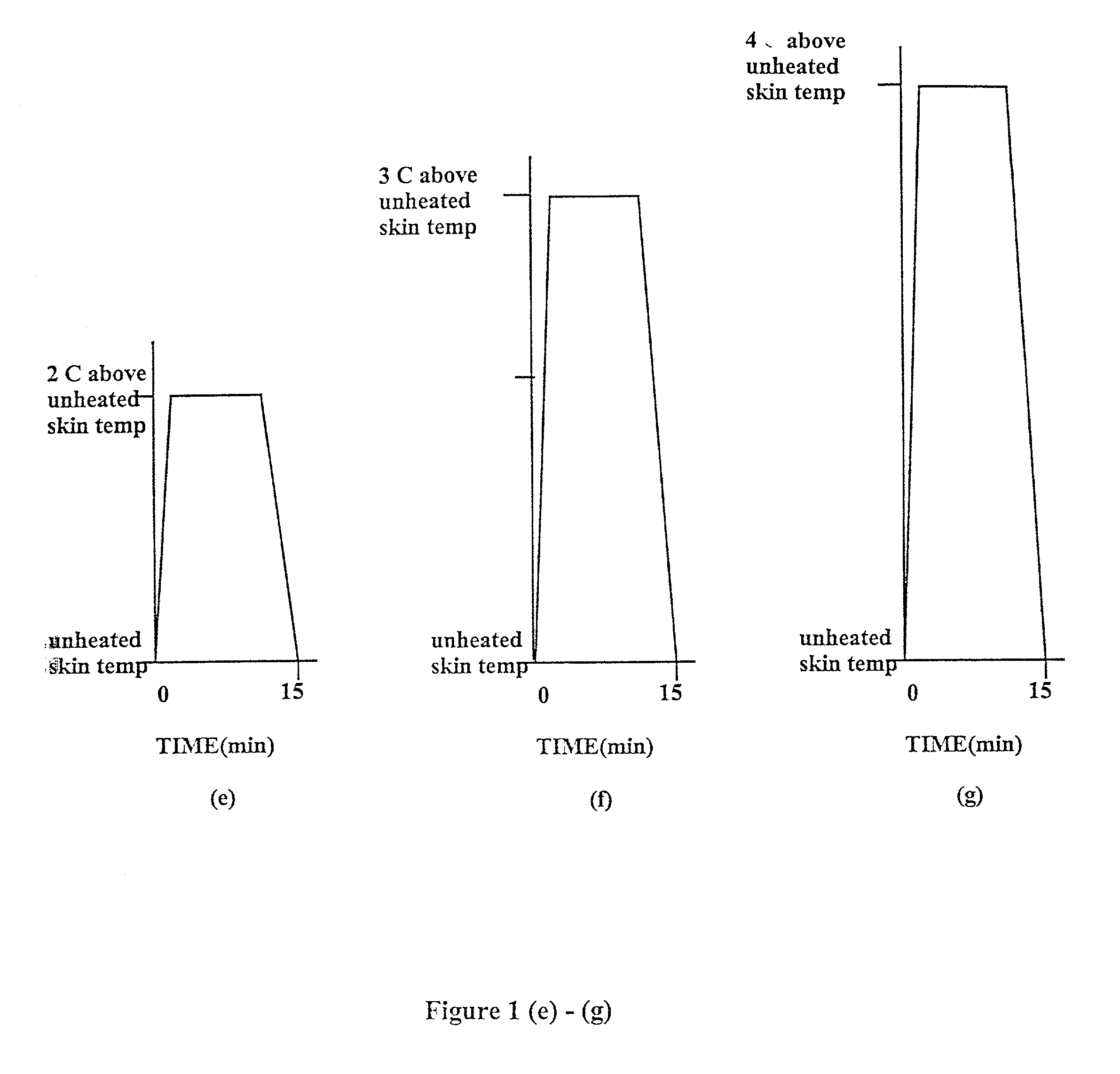
Methods and apparatus for using controlled heat to regulate transdermal and controlled release delivery of fentanyl, other analgesics, and other medical substances
InactiveUS6955819B2Increase blood flowDrive fastCosmetic preparationsElectrotherapyControlled releaseMedicine
The present invention is directed toward an apparatus to rapidly deliver a drug to a patient The invention comprises a drug beneath a patient's skin in a drug depot site. A heating component is placed near the drug depot site and generates heat in and near the drug depot site. A control component connected to the heating component is used to control the magnitude and duration of heat generated by the heating component.
Owner:CRESCITA THERAPEUTICS INC
Generally linear effervescent oral fentanyl dosage form and methods of administering
Fentanyl containing dosage forms and methods using same are described. These dosage forms include substantially less fentanyl by weight than know oral formulation and have advantages in terms of cost and side effects. These dosage forms are intended for oral administration of fentanyl across the oral mucosa.
Owner:CIMA LABS
Methods and apparatus for using controlled heat to regulate transdermal and controlled release delivery of fentanyl, other analgesics, and other medical substances
InactiveUS20010037104A1Increase blood flowDrive fastCosmetic preparationsToilet preparationsControlled releaseMedicine
The present invention is directed toward an apparatus to rapidly deliver a drug to a patient The invention comprises a drug beneath a patient's skin in a drug depot site. A heating component is placed near the drug depot site and generates heat in and near the drug depot site. A control component connected to the heating component is used to control the magnitude and duration of heat generated by the heating component.
Owner:CRESCITA THERAPEUTICS INC
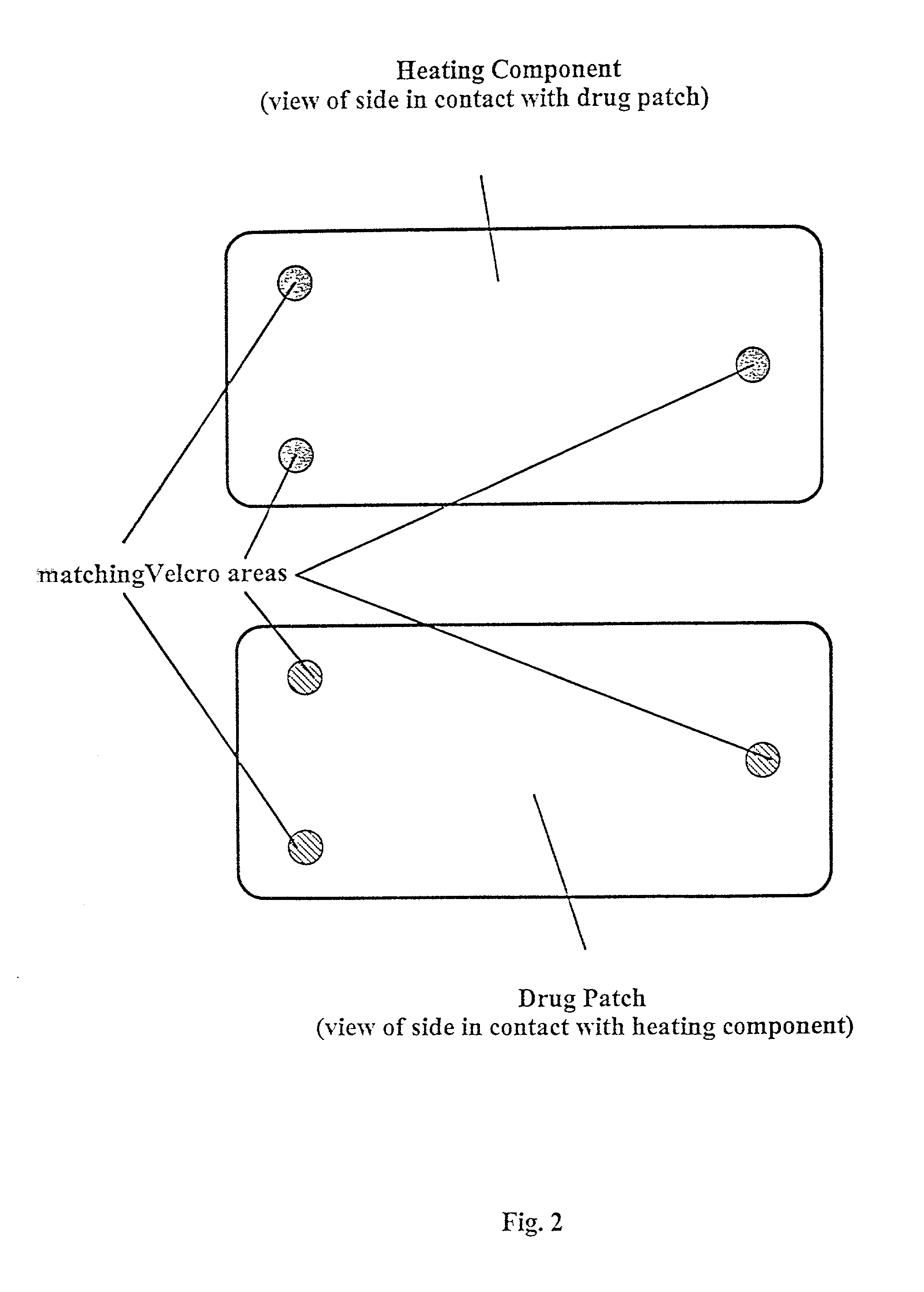
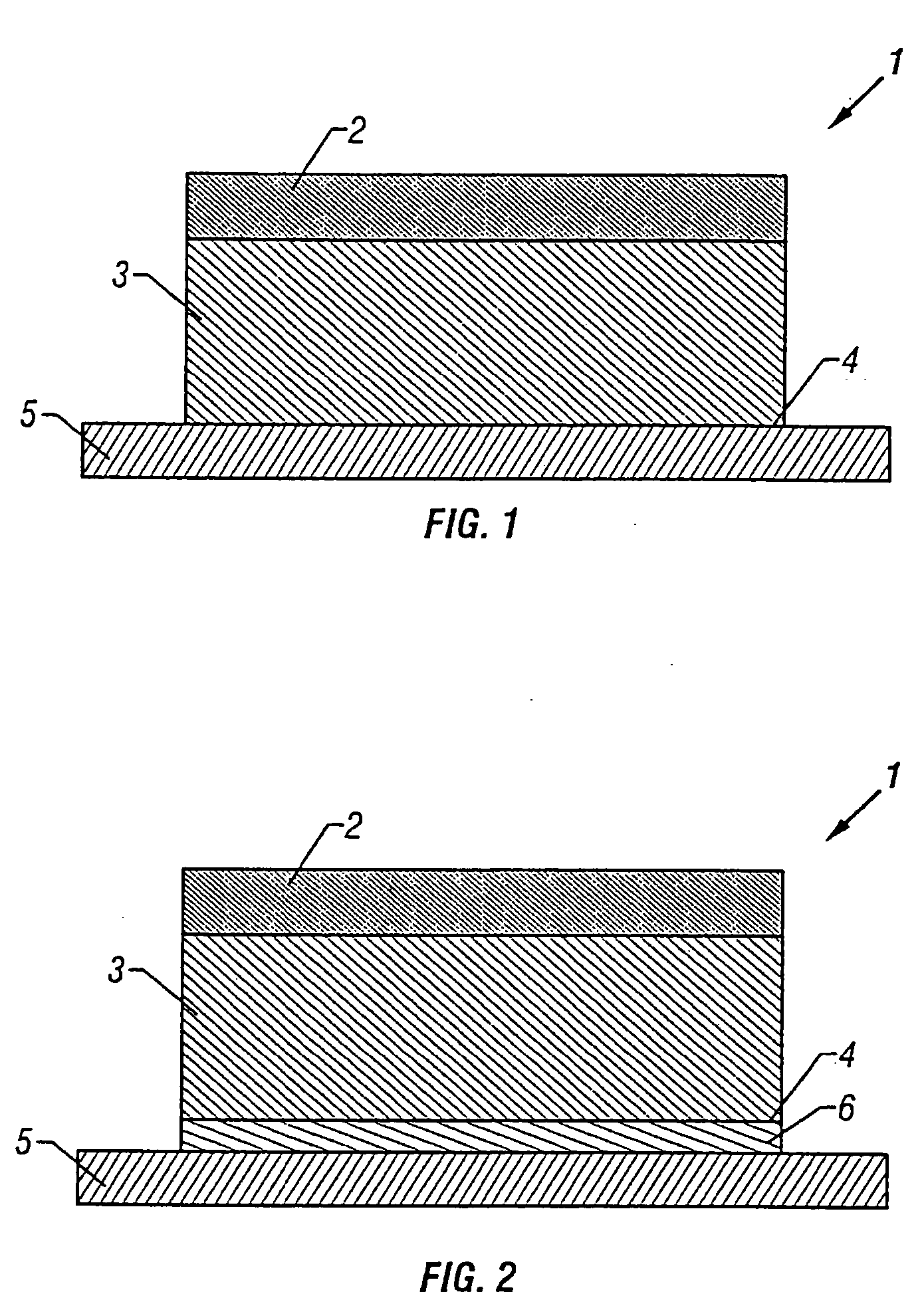
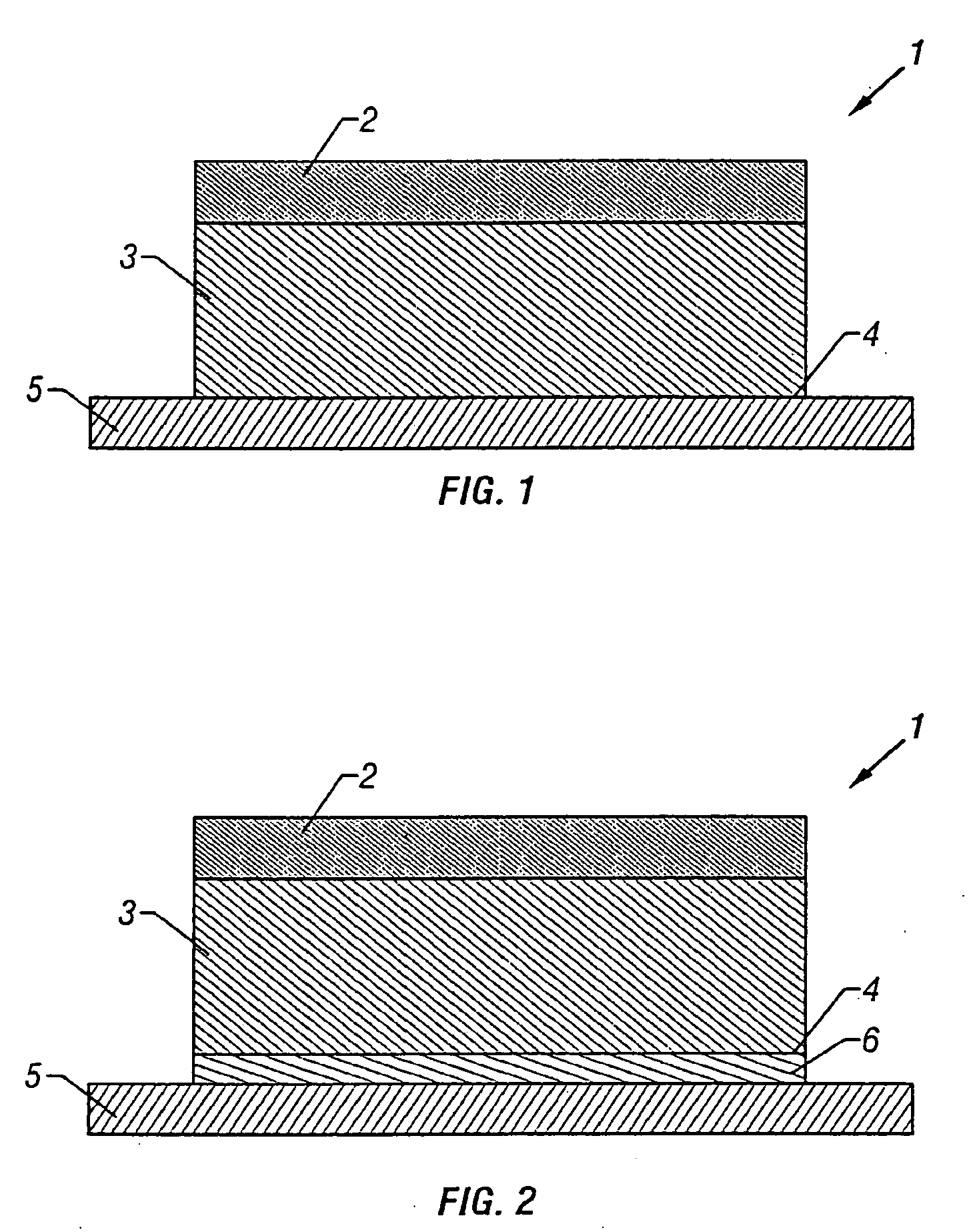
Transdermal drug patch
InactiveUS20010033858A1Sufficient transdermal permeabilityConstant concentrationNervous disorderSheet deliverySolubilityTransdermal patch
The present invention is directed toward a formulation for supplying additional drug for delivery in a transdermal drug delivery device. The invention comprises a drug, such as fentanyl that is capable of transdermal delivery, and a solution having a pre-designed solubility for the drug. The solution dissolves only a portion of said drug and allows a significant portion of the drug to remain undissolved in solution, thus providing extra drug to be delivered at a consistent, controlled delivery rate. The invention may used in conjunction with controlled heat.
Owner:ZARS INC
Sublingual fentanyl spray
ActiveUS20070261695A1Reducing patient to patient variabilityLow variabilityBiocideNervous disorderPharmaceutical medicinePerylene derivatives
The present invention is directed to sublingual formulations containing fentanyl, a pharmaceutically acceptable salt thereof, or derivative thereof, suitable for administration to a patient, and methods for treatment with the formulations.
Owner:BTCP PHARMA LLC
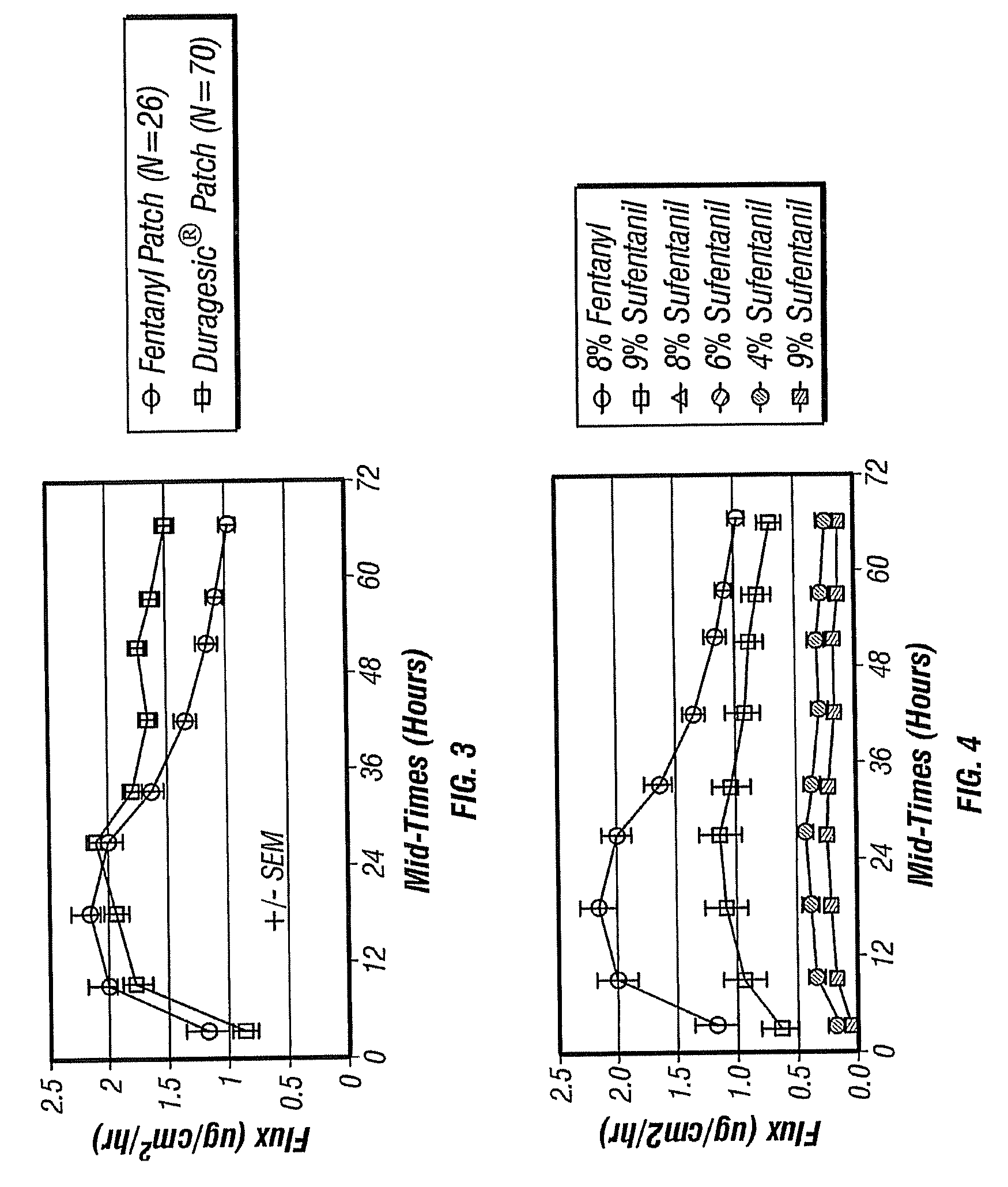
Small Volume Oral Transmucosal Dosage Forms Containing Sufentanil for Treatment of Pain
Compositions, methods and systems for administration of small volume sufentanil-containing drug dosage forms to the oral mucosa of a subject are disclosed.
Owner:VERTICAL PHARMA LLC
Apparatus and method for transdermal delivery of fentanyl-based agents
An apparatus and method for transdermally delivering a biologically active agent comprising a delivery system having a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers. In one embodiment, the fentanyl-based agent is contained in a biocompatible coating that is applied to the microprojection member. In a further embodiment, the delivery system includes a gel pack having a fentanyl-based agent-containing hydrogel formulation that is disposed on the microprojection member after application to the skin of a patient. In an alternative embodiment, the fentanyl-based agent is contained in both the coating and the hydrogel formulation.
Owner:ALZA CORP
Method for reducing pain
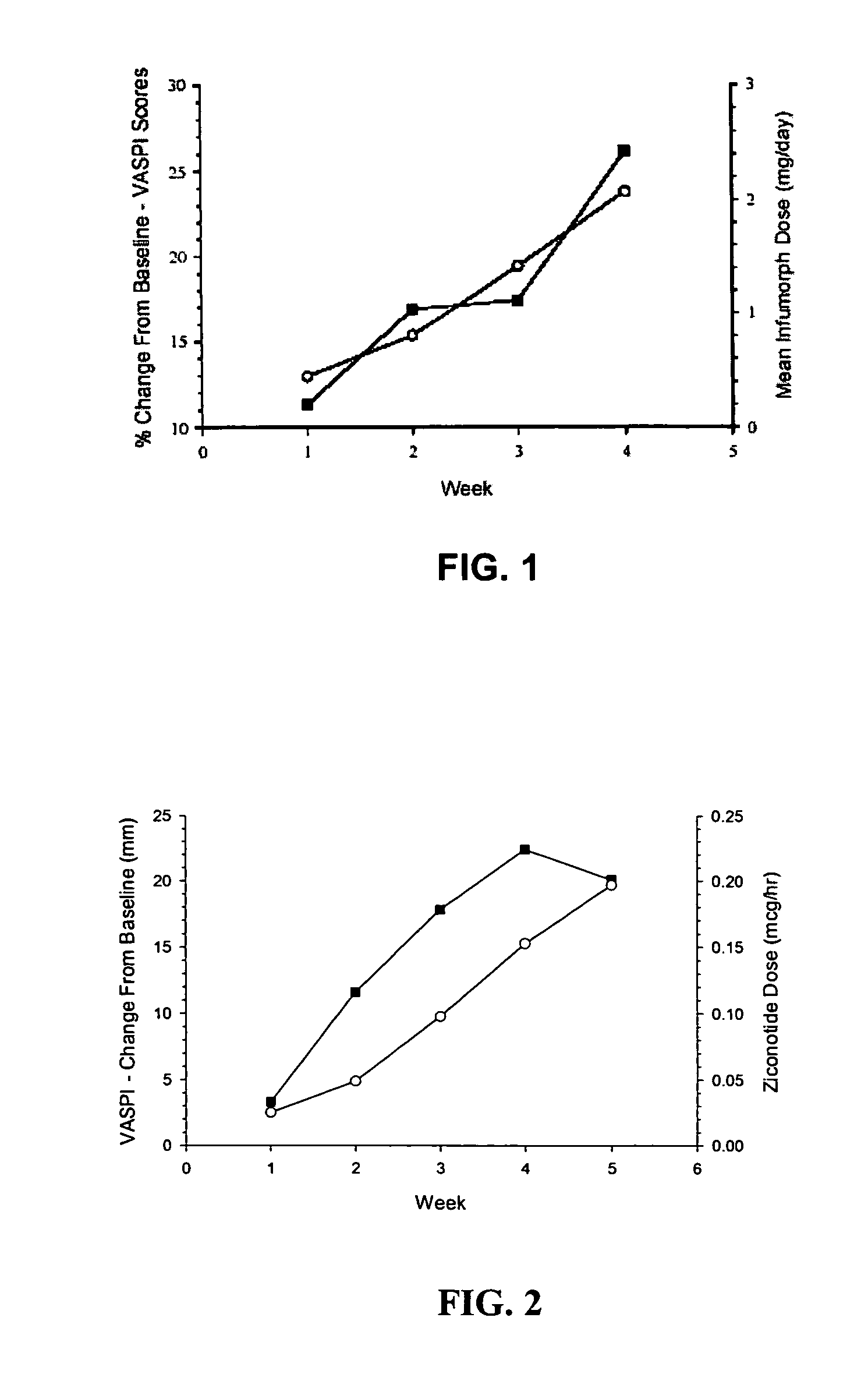
ActiveUS20050192218A1Relieve painRetain potencyBiocideNervous disorderIntrathecal usePharmaceutical formulation
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
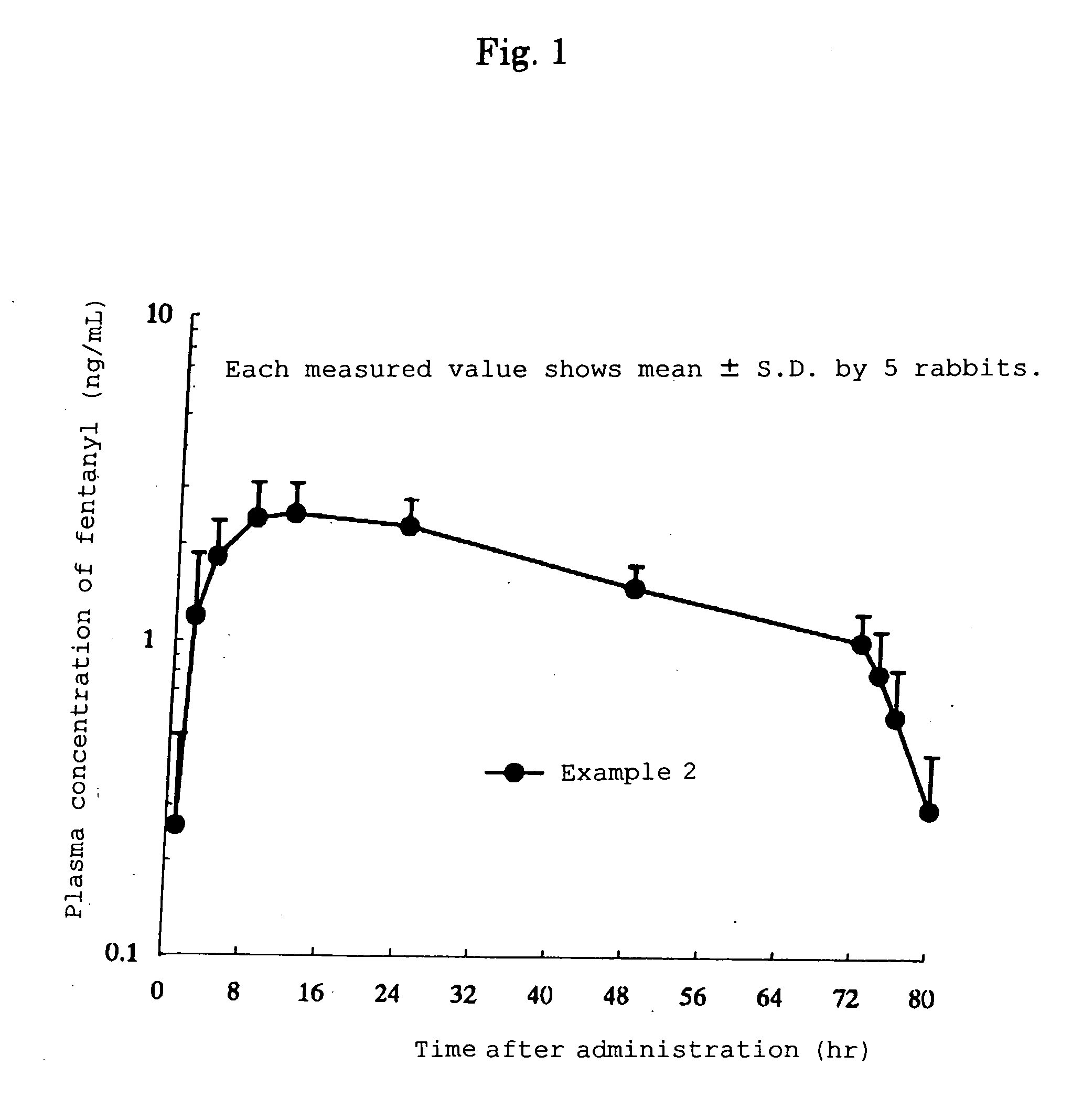
Transdermal patch for external use comprising fentanyl
ActiveUS20060013865A1Efficient methodReduce the burden onBiocideAntipyreticTransdermal patchTolerability
A transdermal patch for external use having a backing layer and a pressure-sensitive adhesive layer formed on one surface of the backing layer, which contains polyisobutylene, a mineral oil and fentanyl employed as the active ingredient in the pressure-sensitive adhesive layer and in which the contents of polyisobutylene and fentanyl in the pressure-sensitive adhesive layer respectively range from 75.2 to 94.2% by mass and 1 to 6% by mass while the content of the mineral oil is from 0.25 to 0.05 parts by mass based on polyisobutylene. This patch can be easily produced, has a long-lasting effect and is excellent in adhesion to the skin and tolerability against movement to the body parts.
Owner:HISAMITSU PHARM CO INC
Pharmaceutical composition
A pharmaceutical composition comprising an analgesic or analgesic combination and a stool softener is disclosed. The analgesic is selected from morphine, meperidine, fentanyl, hydromorphone, oxymorphone, oxycodone, hydrocodone, methadone, propoxyphene, pentazocine, levorphanol, codeine, acetaminophen and combinations of these analgesics. The composition is formulated for oral administration as a liquid or solid dosage form for immediate, slow, delayed or sustained-release characteristics.
Owner:BRANDED PRODS FOR THE FUTURE
Transdermal system with fentanyl
InactiveUS20040001882A1Improve stabilityImprove mechanical stabilityBiocideUnknown materialsAdhesiveAdditive ingredient
This invention relates to a transdermal system containing fentanyl as the active ingredient and consisting of or comprising a substrate, a mixture of the following ingredients applied to the substrate: the active ingredient, an oil-based aloe vera extract, a resin, and an adhesive, as well as a layer laminated to the mixture applied to the substrate.
Owner:HEXAL AG
Sublingual coated tablet of fentanyl
The present invention relates to a fentanyl coated tablet and to the method for the preparation thereof.
Owner:ETHYPHARM SA
Refining method of 4-phenylaminopiperidine analgesic
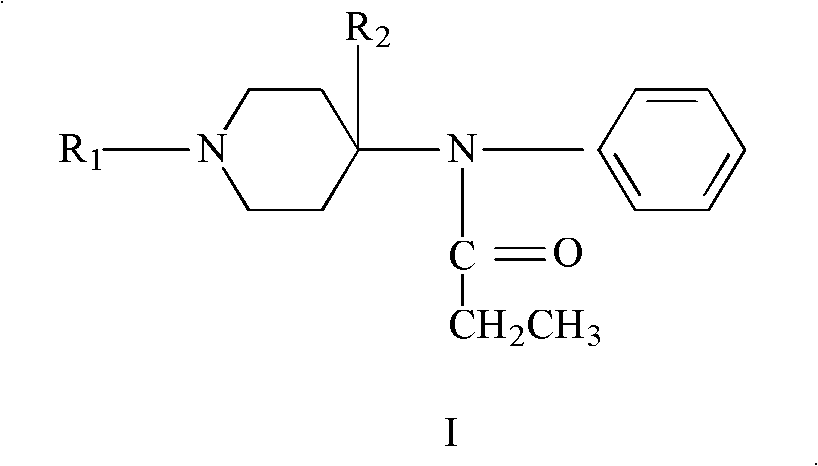
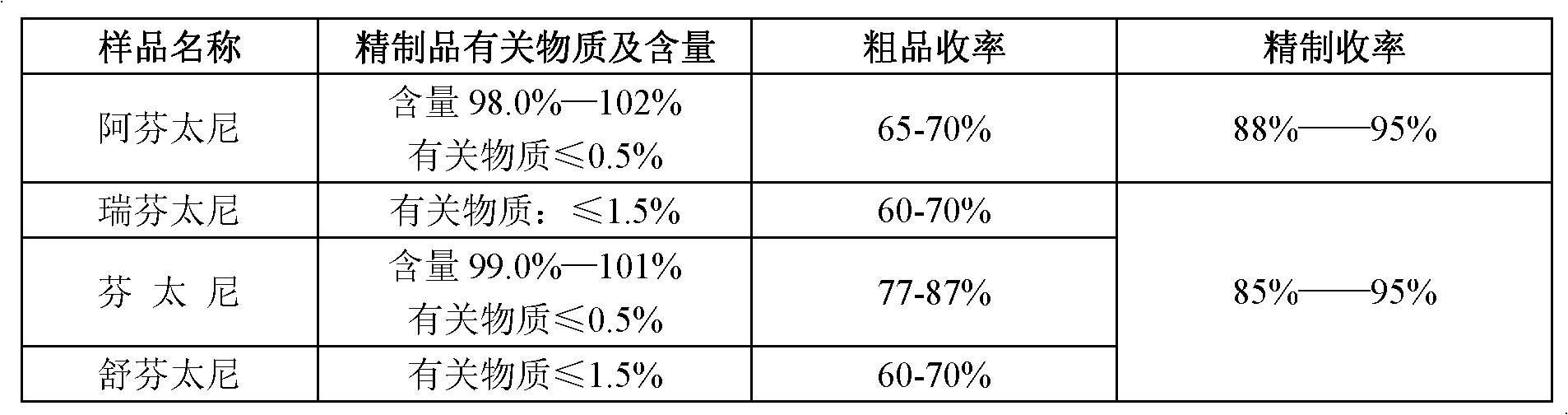
The invention discloses a refining method of 4-phenylaminopiperidine analgesic, comprising the following steps of firstly preparing an acid salt from the 4-phenylaminopiperidine analgesic, purifying the acid salt after recrystallization, alkalizing the salt again to obtain free basic groups, adding another acid to the mixture to obtain another acid salt, re-crystallizing and refining the obtainedanother acid salt by a second solvent to obtain the acid salt. According to the invention, the process is simple, the used agents are simply and easily obtained, and only twice salifying operation and recrystallization operation are needed; and by utilizing the refining method, the content of impurities is remarkably reduced, the purity reaches the weight standard associated with the pharmacopoeia, the purity and the yield of fentanyl compound acid salts prepared from fentanyl compound free basic groups are remarkably improved, the production cost is reduced, and the production efficiency is remarkably improved.
Owner:YICHANG HUMANWELL PHARMA
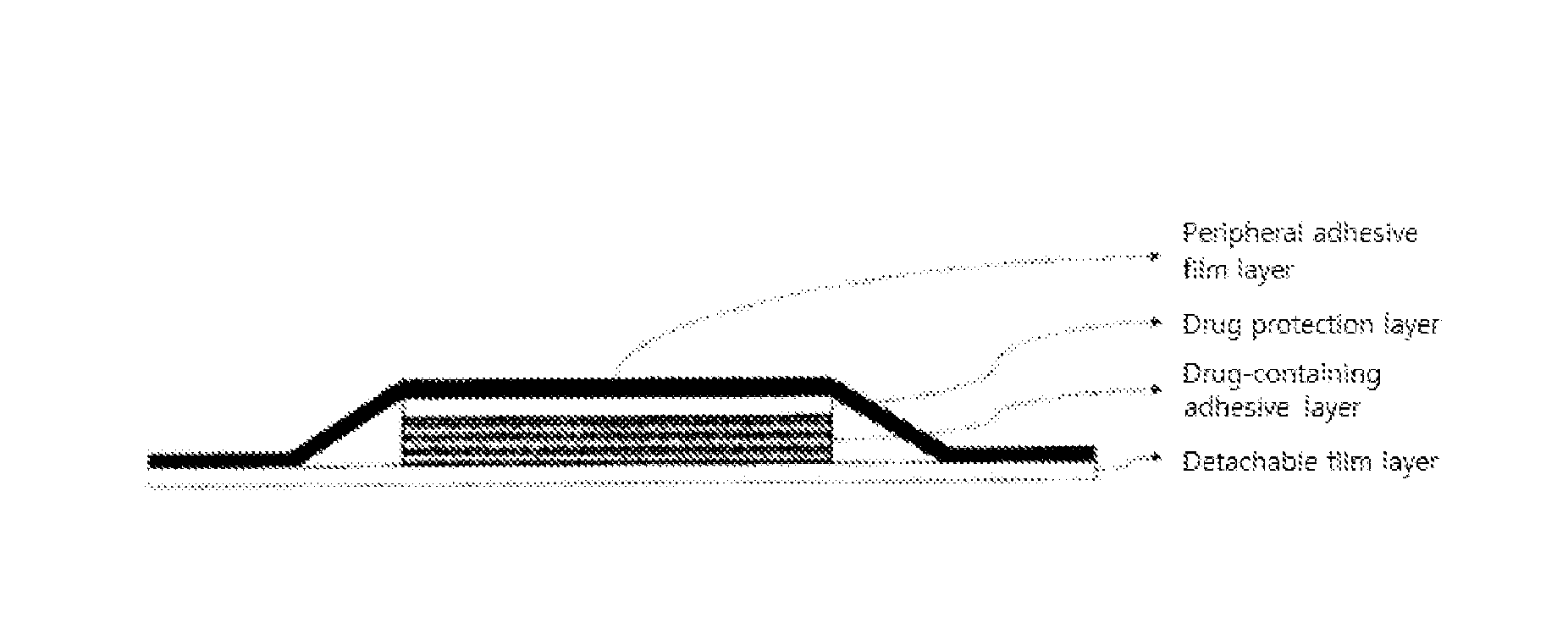
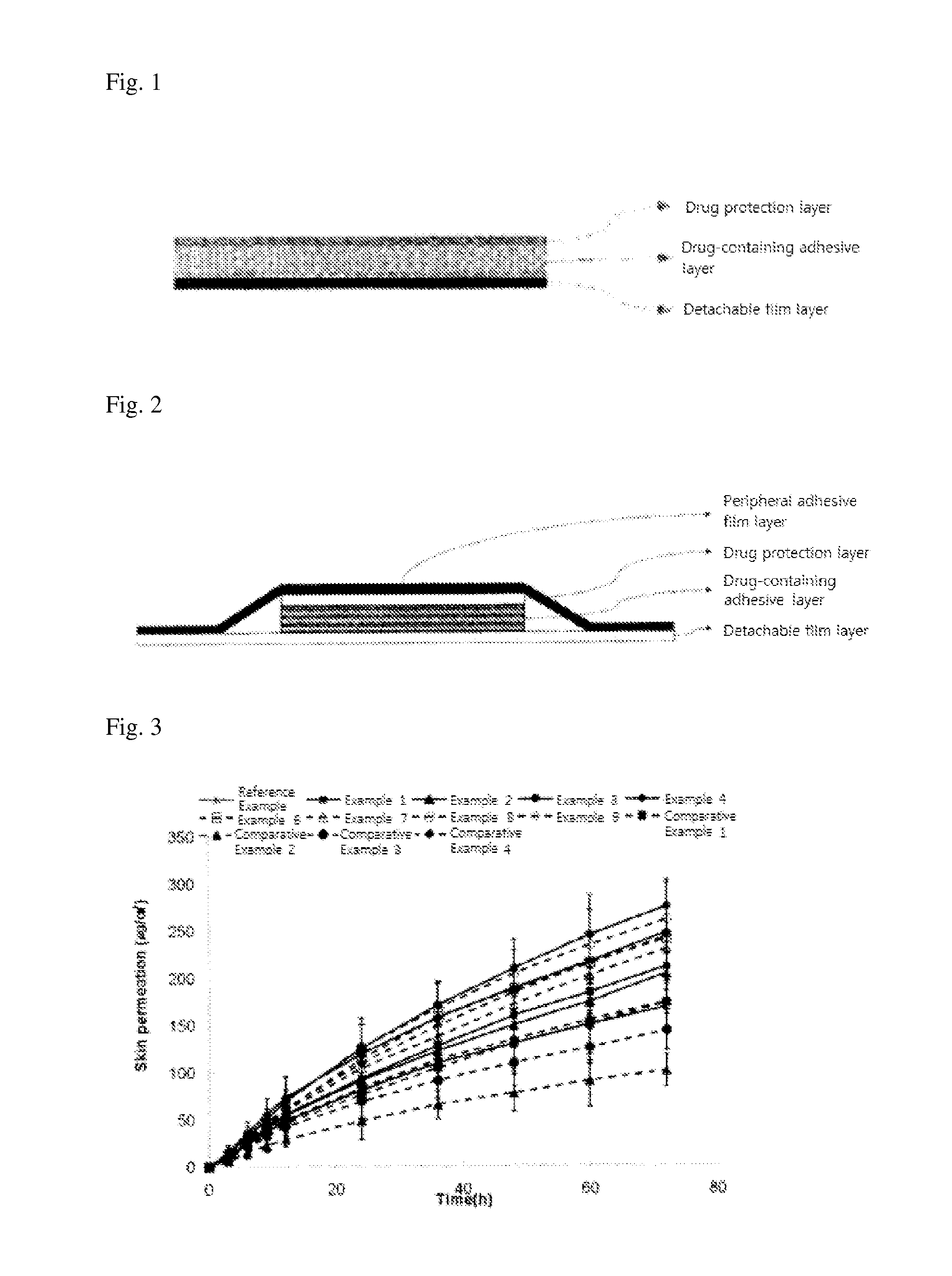
Adhesive preparation containing fentanyl
ActiveUS20060034900A1Sufficient skin permeabilityControlling skin permeabilityBiocideNervous disorderSkin permeabilityOrganic liquids
The present invention provides a Fentanyl-containing percutaneously absorbable adhesive preparation, which is obtained from economic starting materials, has a constitution simpler than that of conventional ones, has sufficient skin permeability, and which permits control of skin permeability by changing the mixing ratio of two kinds of polyisobutylene having different molecular weights, a tackifier and an organic liquid. Specifically, the present invention provides a percutaneously absorbable adhesive preparation comprising a support and an adhesive layer laminated on one surface thereof, wherein the adhesive layer comprises Fentanyl, two kinds of polyisobutylene having different molecular weights, a tackifier and an organic liquid compatible with the aforementioned two kinds of polyisobutylene and the aforementioned tackifier.
Owner:NITTO DENKO CORP
Method for reducing pain
ActiveUS7268109B2Nervous disorderPeptide/protein ingredientsPharmaceutical formulationAnalgesic agents
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
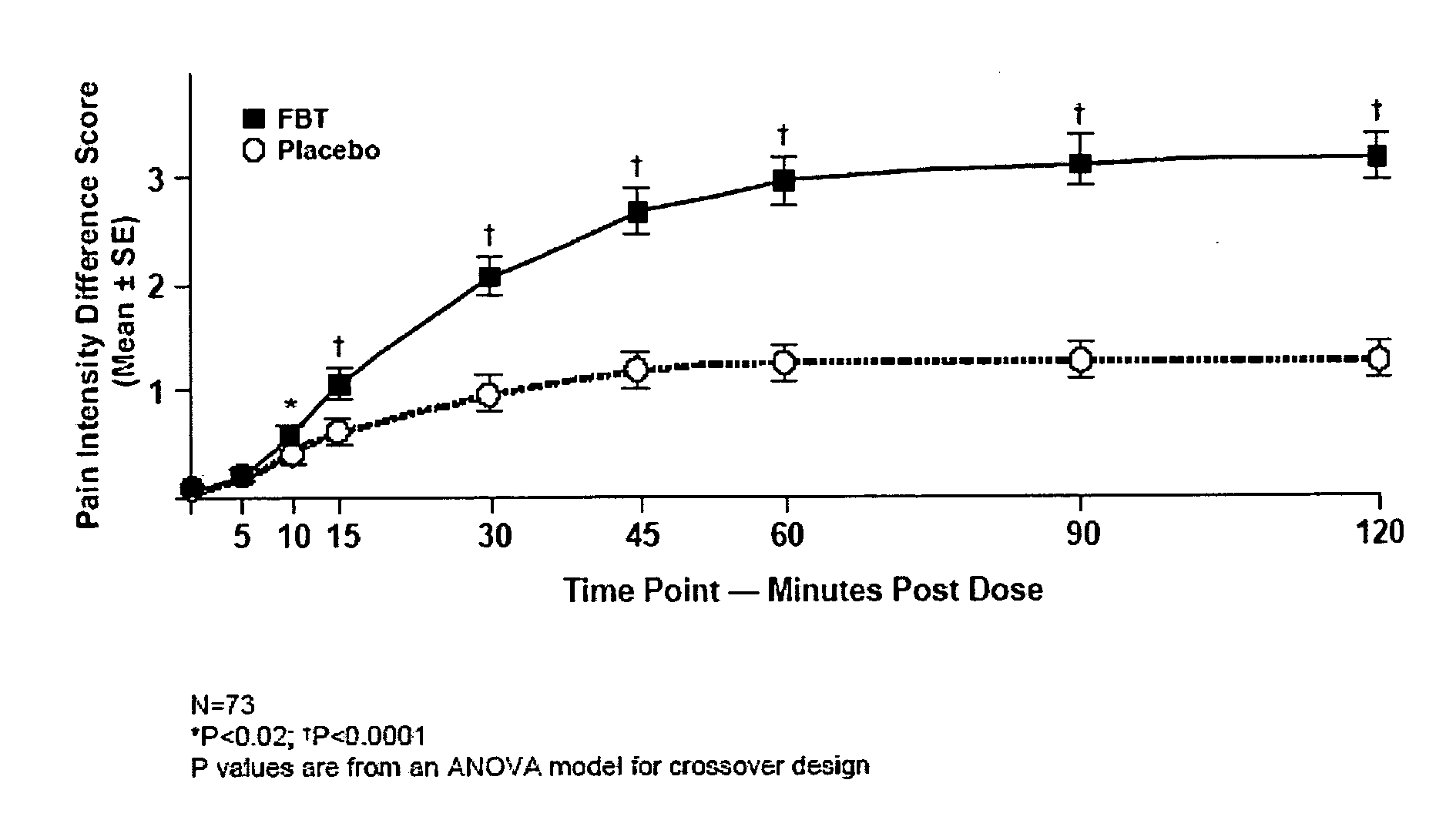
Breakthrough pain management
InactiveUS20090011030A1Treatment and alleviation of painAct quicklyBiocidePowder deliveryPulmonary inhalationFentanyl
The present invention is directed to a powdered formulation comprising an analgesic, preferably fentanyl, for use in pulmonary inhalation administration for the rapid analgesic titration of pain, in particular breakthrough pain. Upon administration, the powdered formulation is able to provide a narrower titration range in patients suffering from pain, as well as effective analgesic amounts of fentanyl in a shorter time and at lower dose levels of administered fentanyl when compared to fentanyl administered by an oral transmucosal route.
Owner:LAB INT
Adhesive patch
ActiveUS20070009588A1Easy to processAdjust pressure-sensitive adhesivenessBiocideNervous disorderMedicineBULK ACTIVE INGREDIENT
An adhesive patch for percutaneous fentanyl administration which is easily produced, has long-term continuity, and is excellent in adhesion and conformability to the skin. The adhesive patch comprises a backing layer and a pressure-sensitive adhesive layer formed on one side thereof, wherein the pressure-sensitive adhesive layer comprises fentanyl as an active ingredient, a pressure-sensitive adhesive base, and a tackifier resin, the pressure-sensitive adhesive base comprising polyisobutylene and a styrene / isoprene / styrene block copolymer, the proportion of the polyisobutylene in the adhesive base being, 8 to 15 wt. %, and the ratio of the concentration of the polyisobutylene to that of the styrene / isoprene / styrene block copolymer being from 2 / 3 to 3 / 2.
Owner:HISAMITSU PHARM CO INC
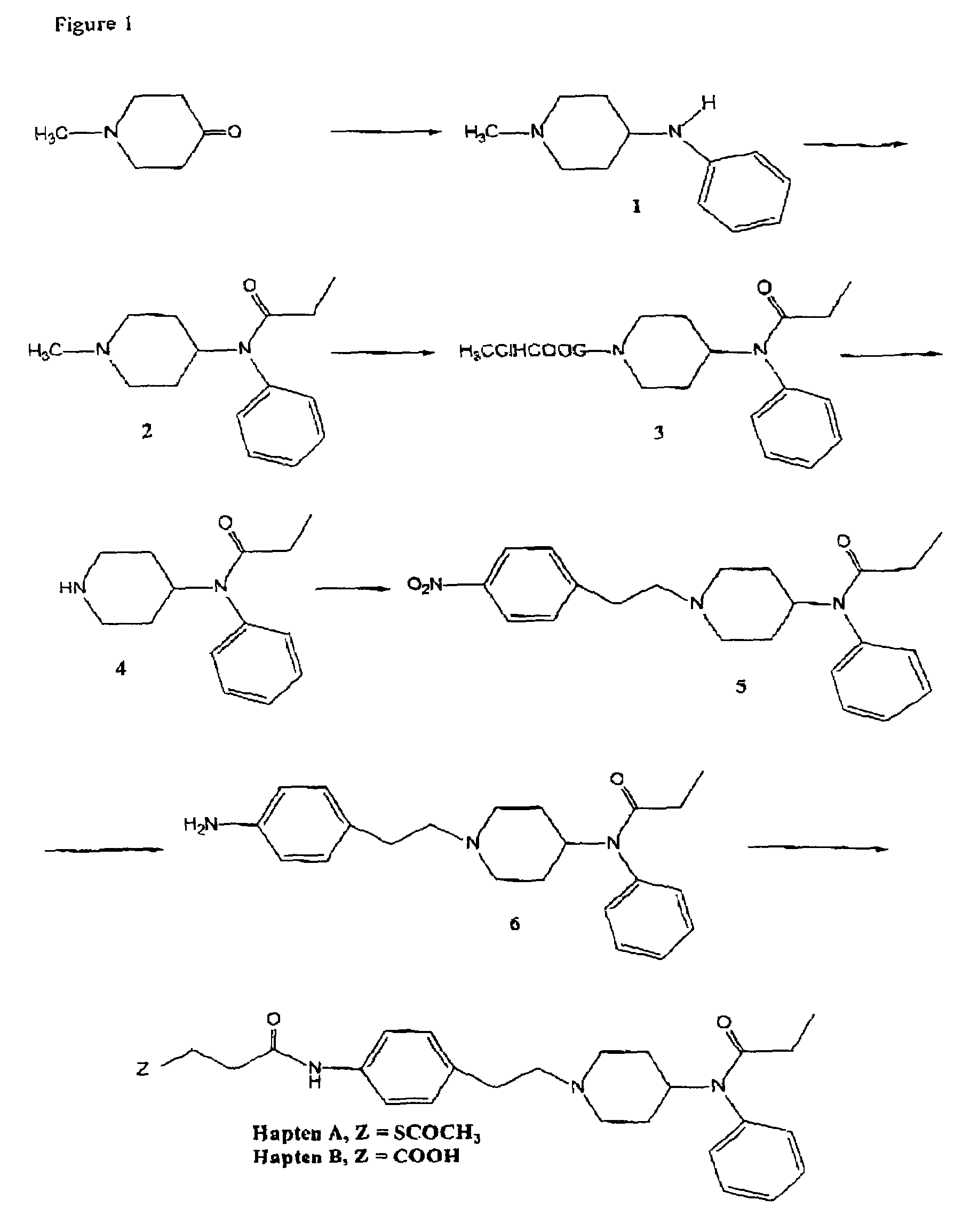
Method and kit for detecting, or determining the quantity of, metabolites of fentanyl and metabolites of fentanyl analogs
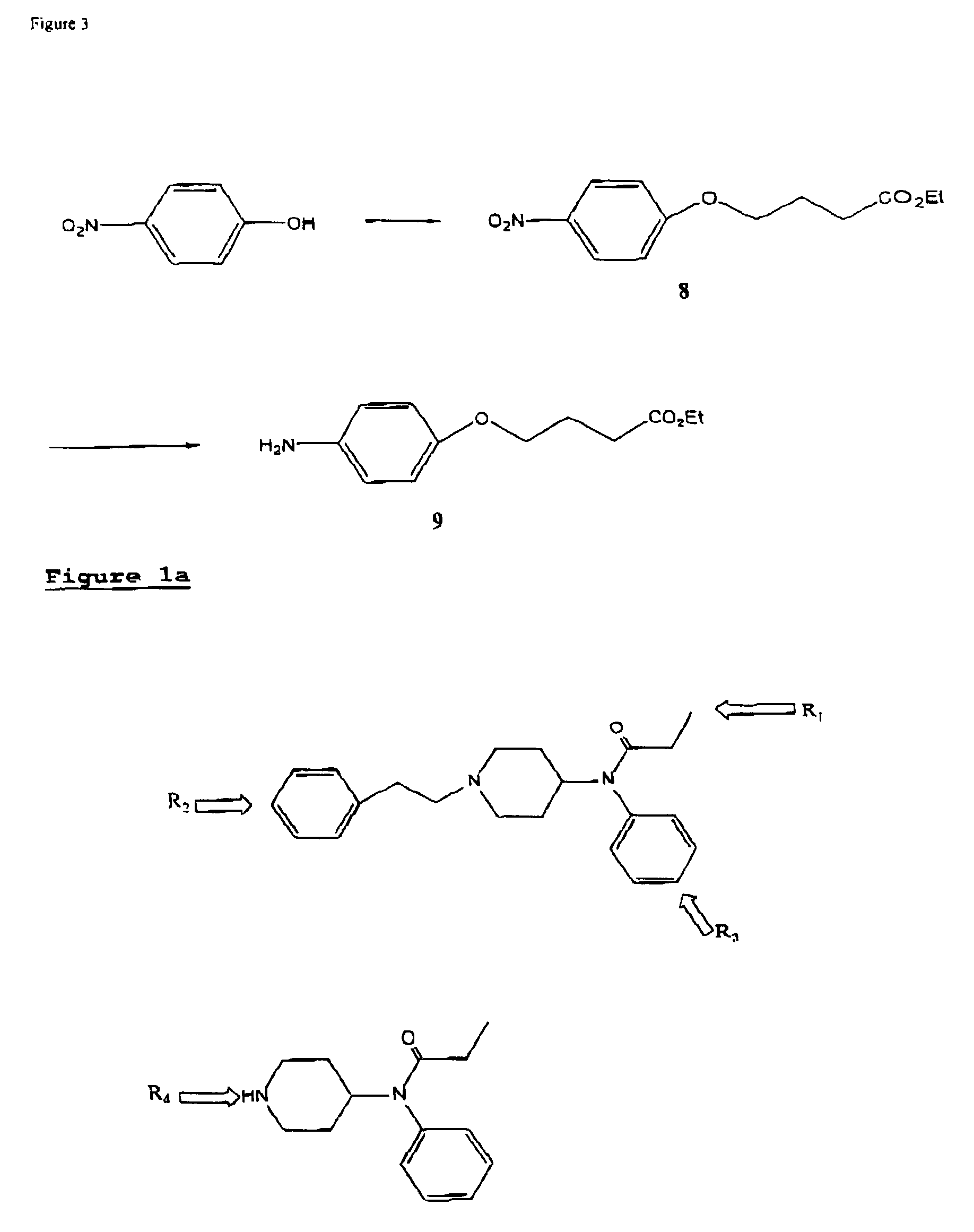
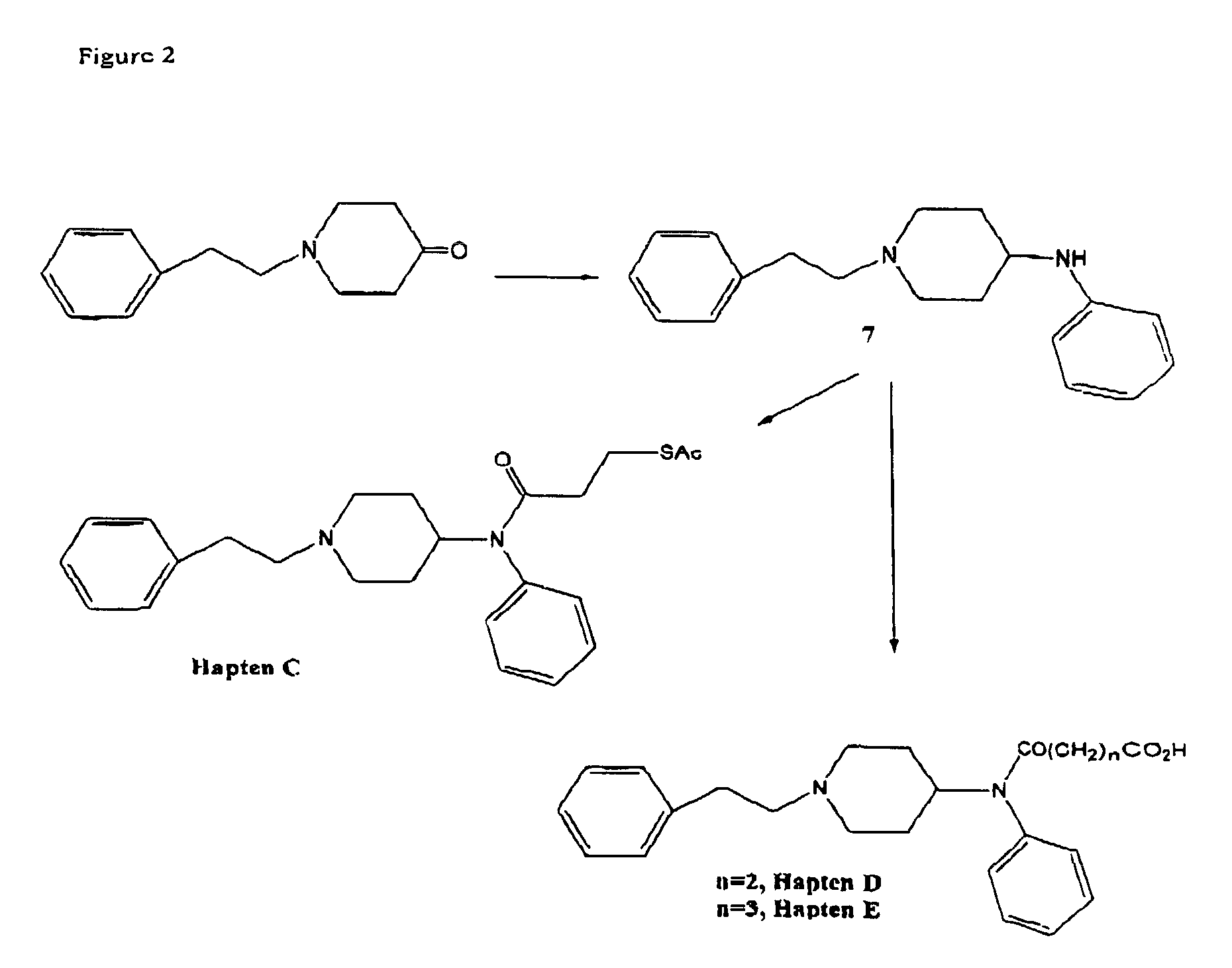
The invention provides an immunogen comprising a hapten coupled to an antigenicity-conferring carrier material, a conjugate comprising the aforementioned hapten coupled to a labelling agent, as well as, antibodies raised against the aforementioned immunogen and capable of binding with at least one structural epitope of metabolites of fentanyl and of metabolites of fentanyl analogs.
Owner:RANDOX LAB LTD
Fentanyl transdermal patch
ActiveUS20140005617A1Maintain constant fentanyl skin permeabilityPrevent disengagementBiocideAdhesive dressingsSkin permeabilityAcrylic rubber
Provided is a fentanyl transdermal patch comprising an acrylic-rubber hybrid as a drug-adhesive layer. The fentanyl transdermal patch can maintain constant fentanyl skin permeability for three days by maintaining close contact with the skin such that desorption, release by moisture and sweat, and skin stimulation are all improved.
Owner:ICURE
Transdermal patch for external use comprising fentanyl
A transdermal patch for external use having a backing layer and a pressure-sensitive adhesive layer formed on one surface of the backing layer, which contains polyisobutylene, a mineral oil and fentanyl employed as the active ingredient in the pressure-sensitive adhesive layer and in which the contents of polyisobutylene and fentanyl in the pressure-sensitive adhesive layer respectively range from 75.2 to 94.2% by mass and 1 to 6% by mass while the content of the mineral oil is from 0.25 to 0.05 parts by mass based on polyisobutylene. This patch can be easily produced, has a long-lasting effect and is excellent in adhesion to the skin and tolerability against movement to the body parts.
Owner:HISAMITSU PHARM CO INC
Phentanyl-containing adhesive patch for application to oral-cavity mucosa
ActiveUS20050232982A1Sufficient adhesivityEasy to handleBiocideNervous disorderAdhesiveBULK ACTIVE INGREDIENT
To provide a patch containing fentanyl for mucous membrane of the oral cavity (oral transmucosal fentanyl), which rapidly increases the serum concentration of the drug, is easy in handling and is superior in safety. A patch containing fentanyl for mucous membrane of the oral cavity, which can be prepared by laminating on one side of a drug layer which contains fentanyl or its salt as an active ingredient, methyl vinyl ether-maleic anhydride copolymer as an adhesive, and at least one substance selected from the group consisting of hydroxypropylcellulose, hydroxypropylmethylcellulose and hydroxyethylcellulose as a thickener, a support layer hardly soluble or insoluble in water, and a backing in their order.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Transdermal therapeutic system (TTS) with fentanyl as a active ingredient
ActiveUS7390500B2Improve securityOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTActive ingredient
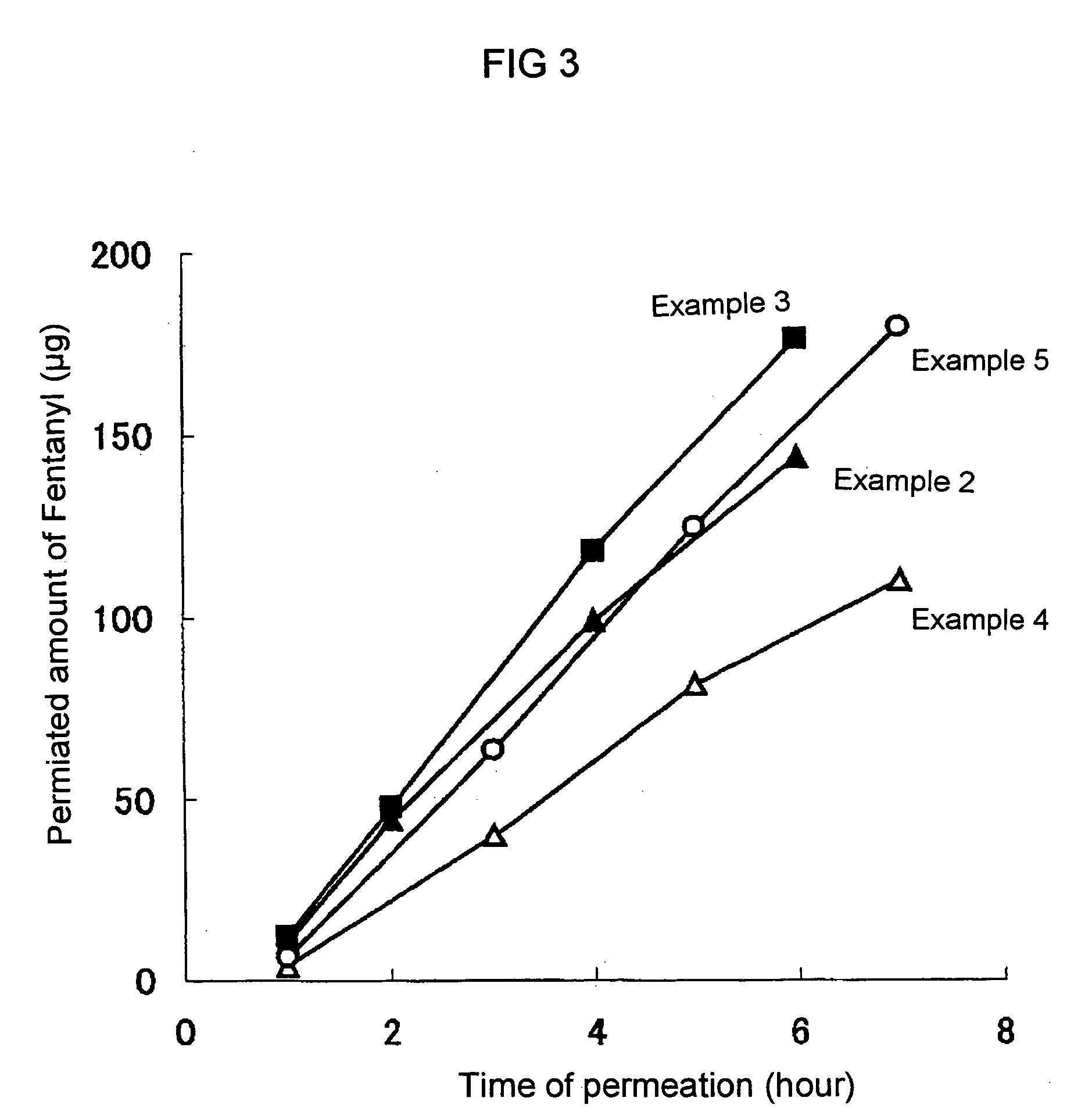
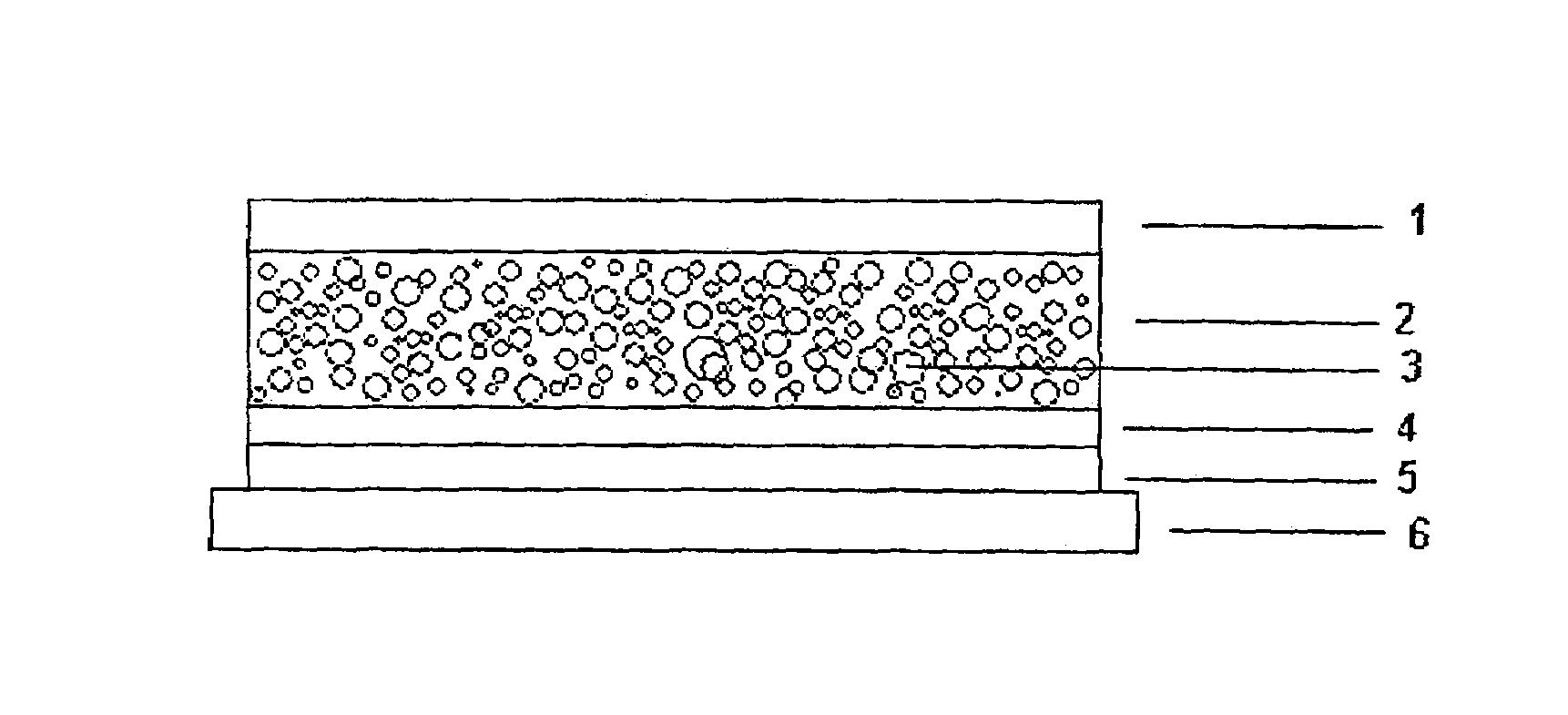
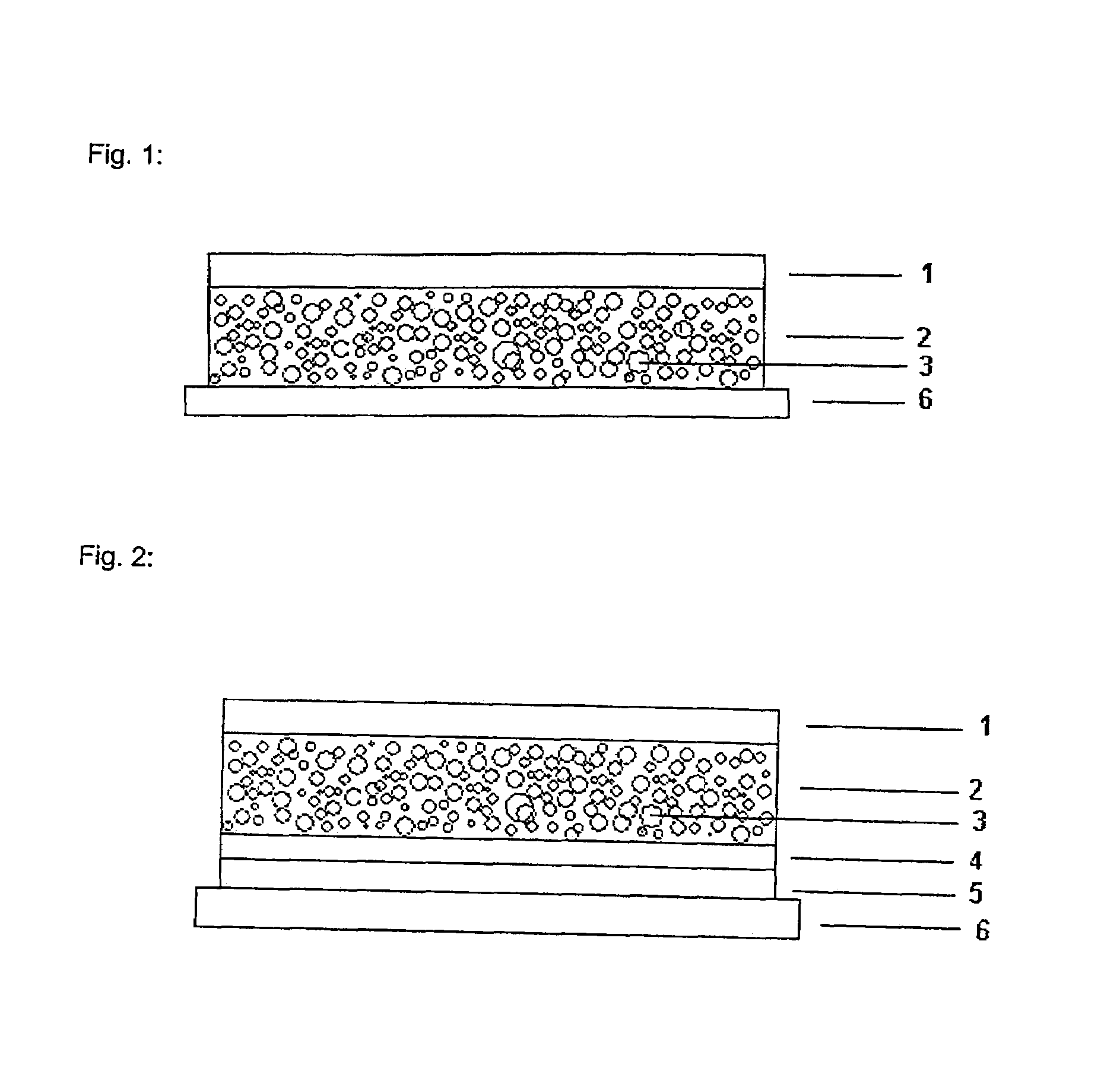
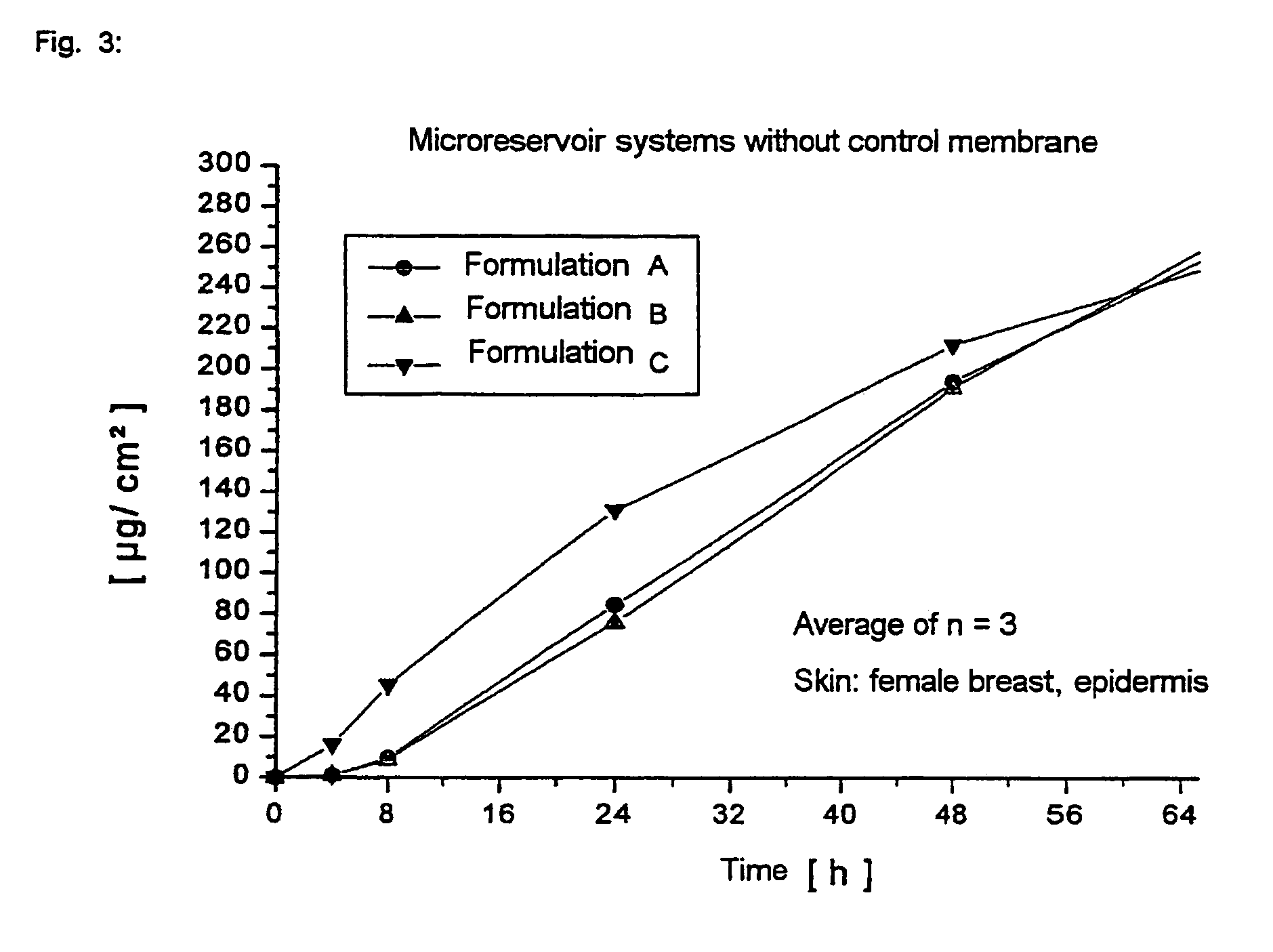
The invention relates to a transdermal therapeutic systems with fentanyl or an analogous fentanyl derivative as active ingredient. In order to prevent inadvertent overdosage by uncontrolled release of active ingredient as a result of damage, the active ingredient is contained in fluid-filled micro-reservoirs in the layer containing the active ingredient. The layer containing the active ingredient can optionally be provided with a membrane.
Owner:LTS LOHMANN THERAPIE-SYST AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com