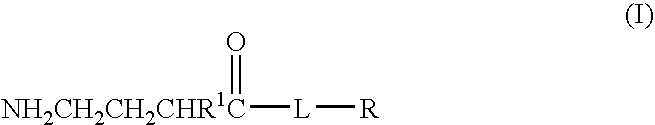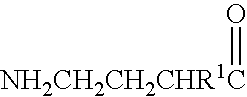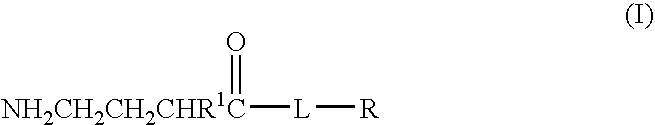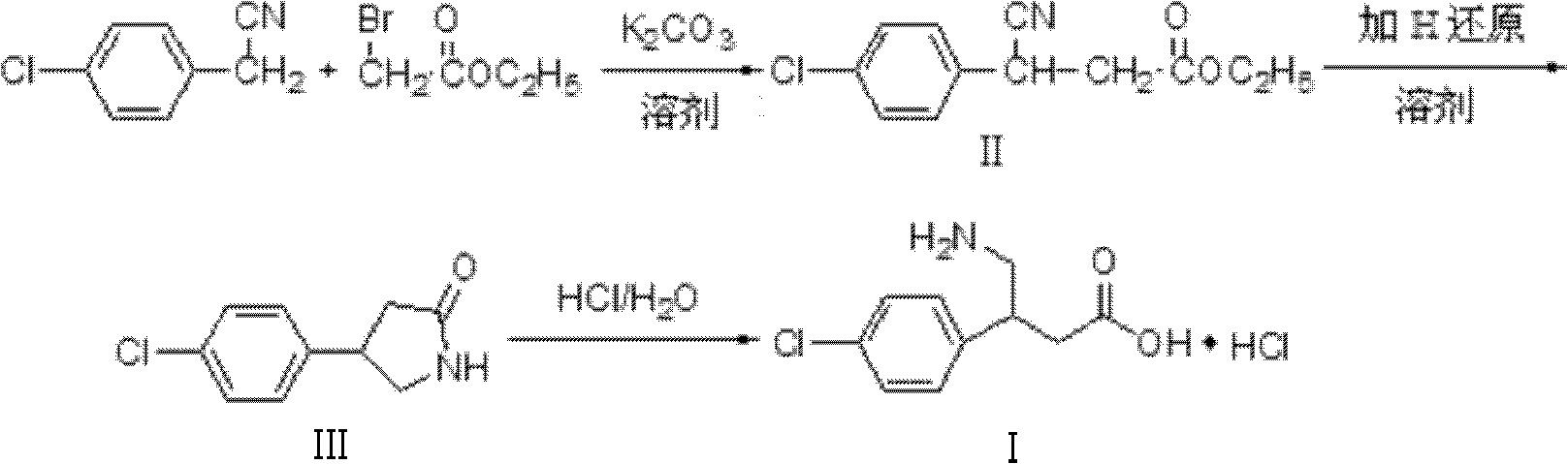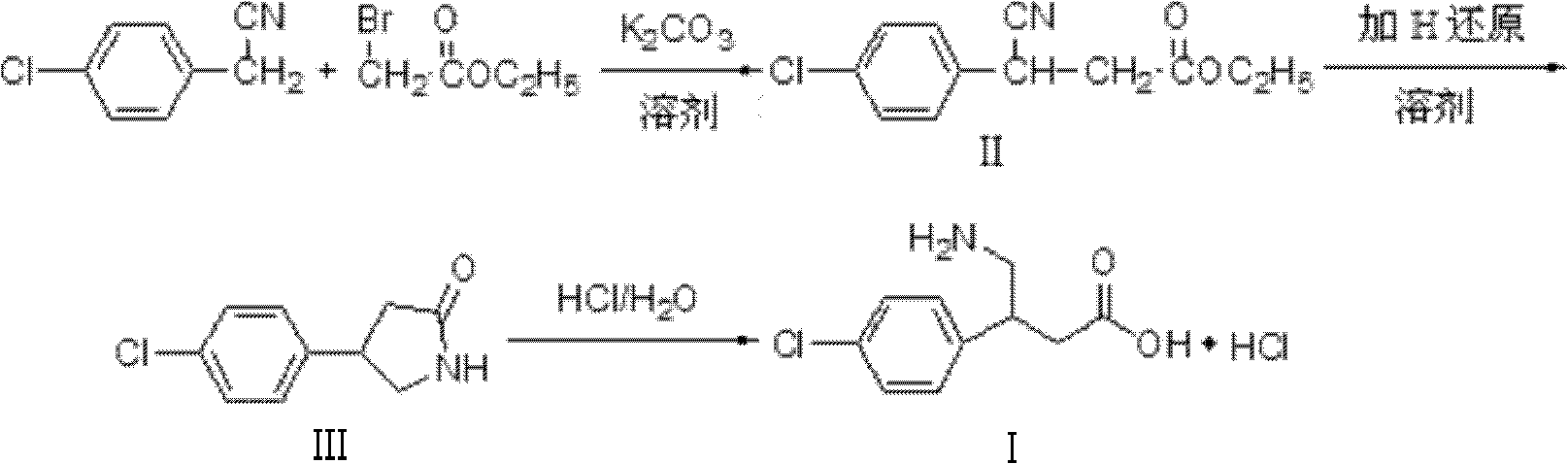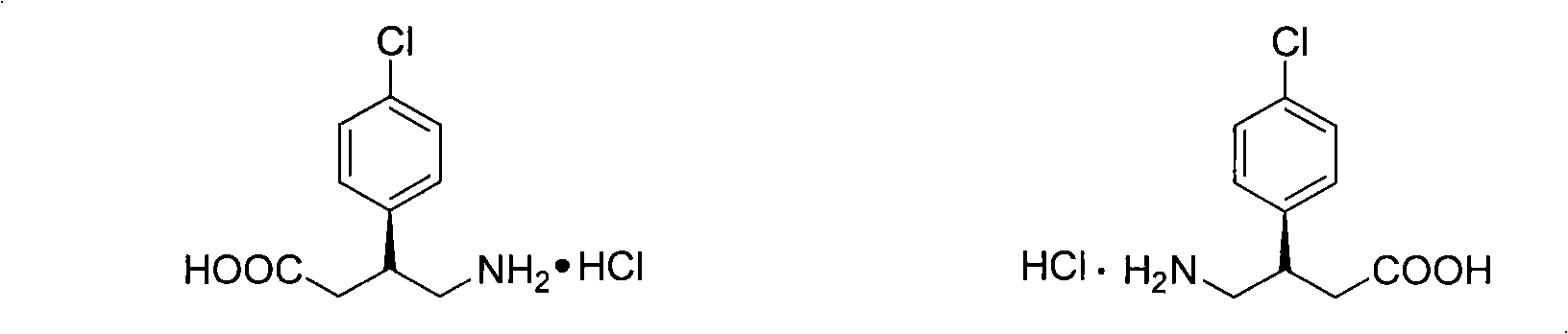Patents
Literature
86 results about "Baclofen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
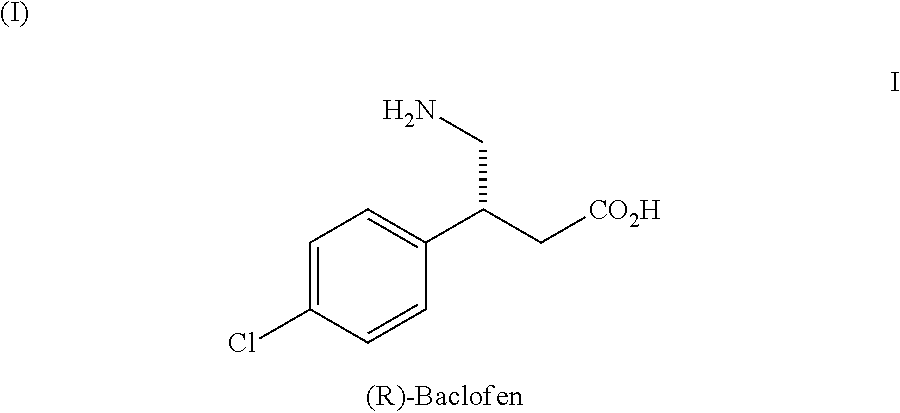
Baclofen is used to treat muscle spasms caused by certain conditions (such as multiple sclerosis, spinal cord injury/disease).
Compositions and methods for enhancing cognitive function and synaptic plasticity
InactiveUS20060089335A1Improve cognitive functionEnhance synaptic plasticityBiocidePeptide/protein ingredientsNR1 NMDA receptorAction potential firing
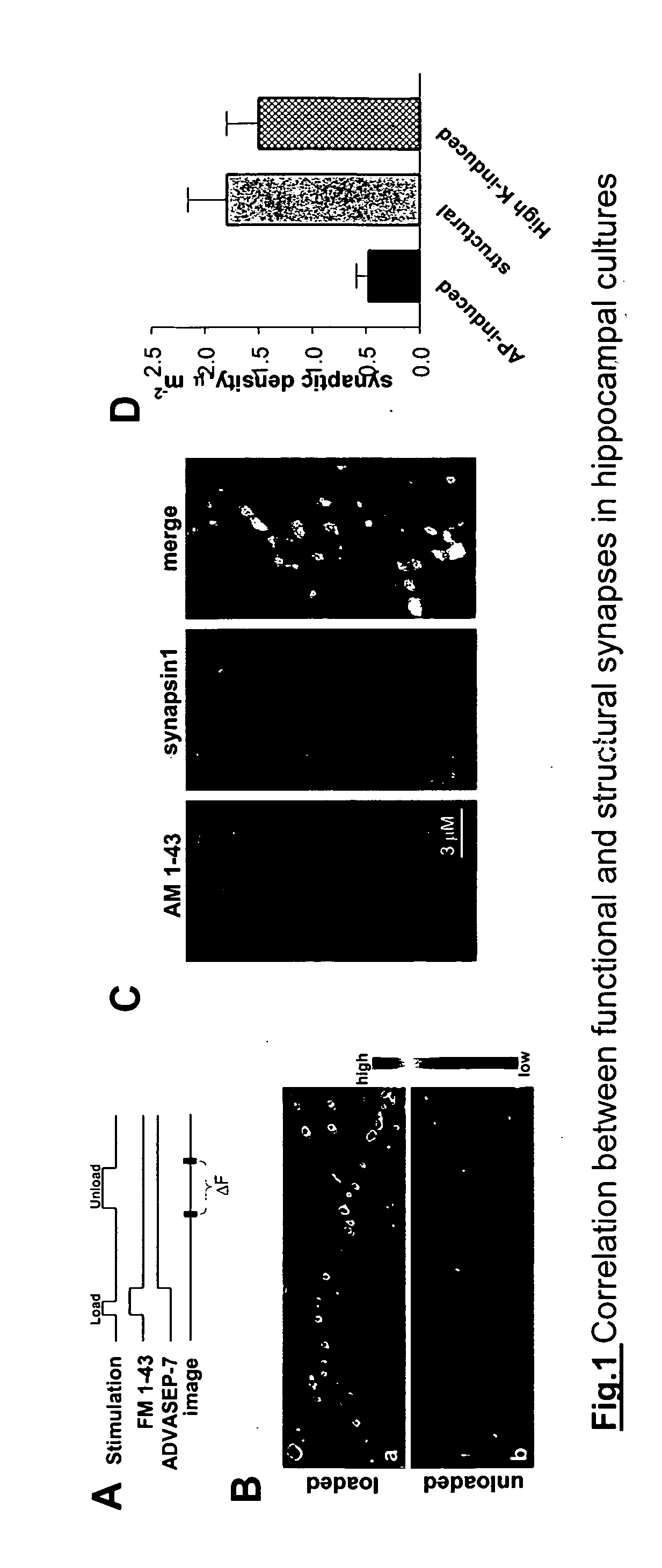
The present invention provides compositions and methods for enhancing cognitive function and synaptic plasticity. According to the method, Ca++ influx into excitatory neurons (nerve cells) is decreased by treatment with a number of different agents including divalent cations (e.g., Mg++), GABAB agonists, GABAA agonists, calcium channel blockers, and / or compounds that decrease action potential firing such as sodium channel blockers. Decreasing Ca++ influx results in increased synaptic plasticity and enhanced cognitive function. In particular, decreasing Ca++ influx associated with uncorrelated neural activity results in long-lasting increases in synaptic plasticity and cognitive function. This is achieved by administration of agents that cause a voltage-dependent block of NMDA receptors (e.g., divalent cations such as Mg++) or by administration of GABAB agonists such as baclofen. The invention further provides screening methods useful in identifying compounds that enhance synaptic plasticity and cognitive function.
Owner:MASSACHUSETTS INST OF TECH
Pharmaceutical Compositions and Related Methods of Treatment
InactiveUS20080021074A1Decrease and prevent sleepinessDecrease and prevent and lethargyBiocideNervous disorderAdrenergic receptor agonistsClumsiness
Pharmaceutical compositions comprising at least one alpha2-adrenergic agonist or baclofen and at least one alpha1-adrenergic agonist are disclosed. Pharmaceutical compositions comprising tizanidine and modafinil are disclosed. Methods for reducing somnolence, sleepiness, lethargy, dizziness, drowsiness, somnolence, tiredness, lightheadedness, increased weakness, confusion, unsteadiness, clumsiness, or a combination of the symptoms thereof in a human patient; treating pain; and attenuating muscle spasticity, using pharmaceutical compositions comprising at least one alpha2-adrenergic agonist or baclofen and at least one alpha1-adrenergic agonist are disclosed.
Owner:QUESTCOR PHARMA
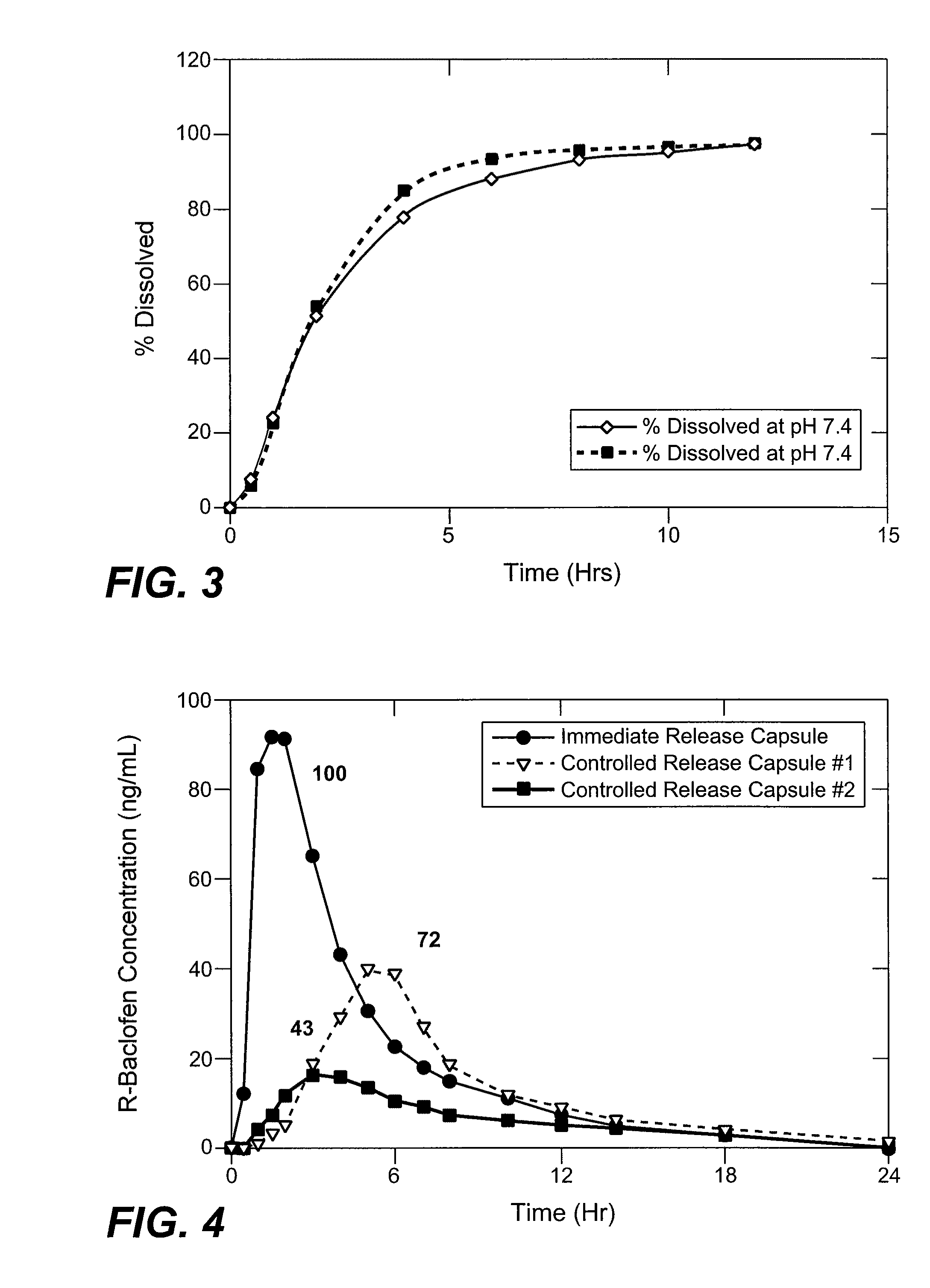
Baclofen and r-baclofen gastroretentive drug delivery systems
InactiveUS20110091542A1Reduce concentrationStrong therapeutic activityOrganic active ingredientsBiocideControlled releaseSide effect
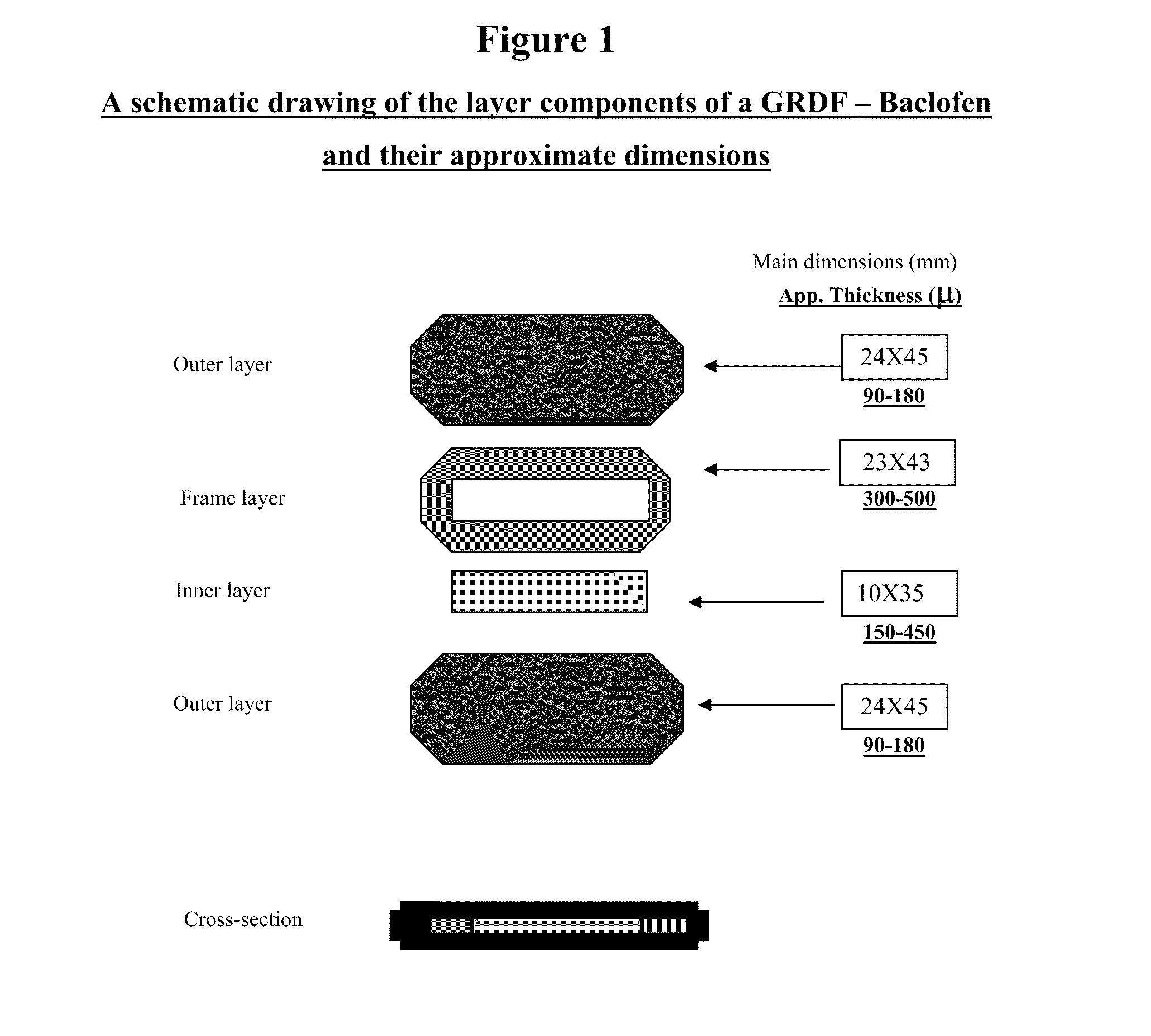
A biodegradable, multi-layered controlled release gastroretentive baclofen or R-baclofen dosage form which is optionally divided into a first dosage of baclofen or R-baclofen for immediate release and a second dosage of baclofen or R-baclofen for controlled release in the stomach and gastrointestinal tract of a patient, folded into a capsule which disintegrates upon contact with gastric juice and the dosage form unfolds rapidly upon contact with gastric juice. The biodegradable, multi-layered gastroretentive dosage forms of the invention provide fast onset of baclofen or R-baclofen activity with prolonged absorption and minimal undesirable side effects.
Owner:INTEC PHARMA
Method for reducing pain
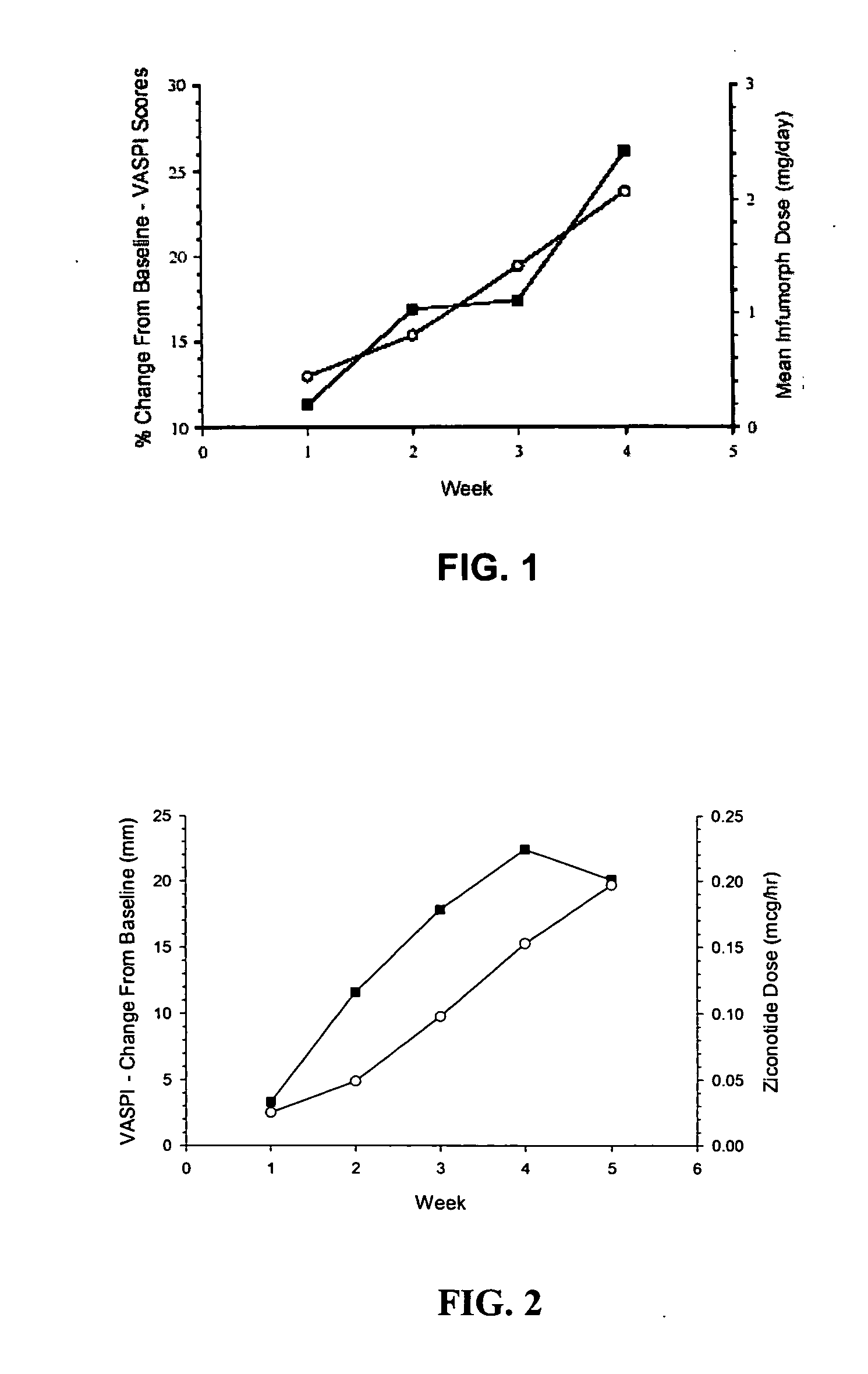
ActiveUS20050192218A1Relieve painRetain potencyBiocideNervous disorderIntrathecal usePharmaceutical formulation
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
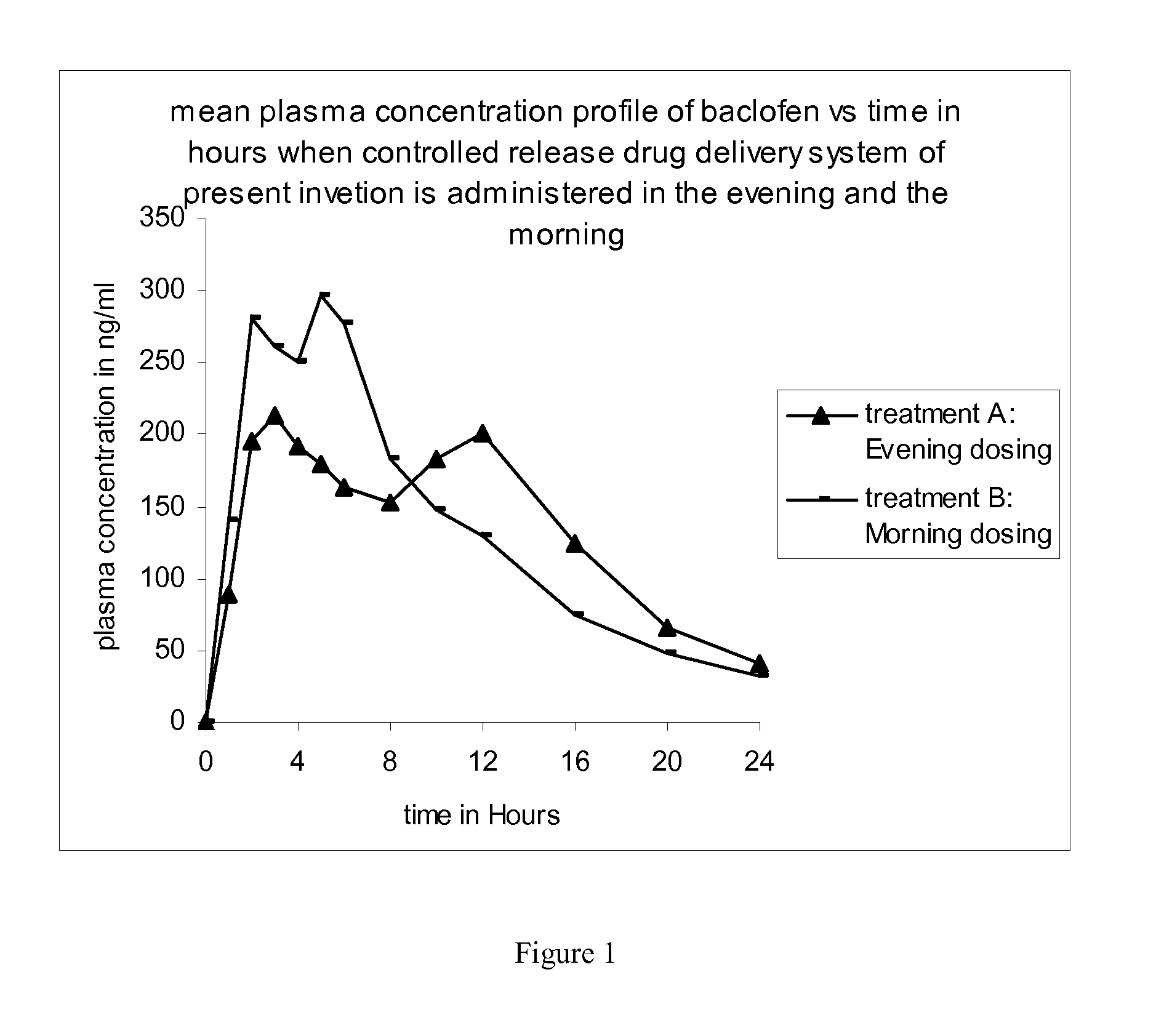
Method of treating a disease condition susceptible to baclofen therapy
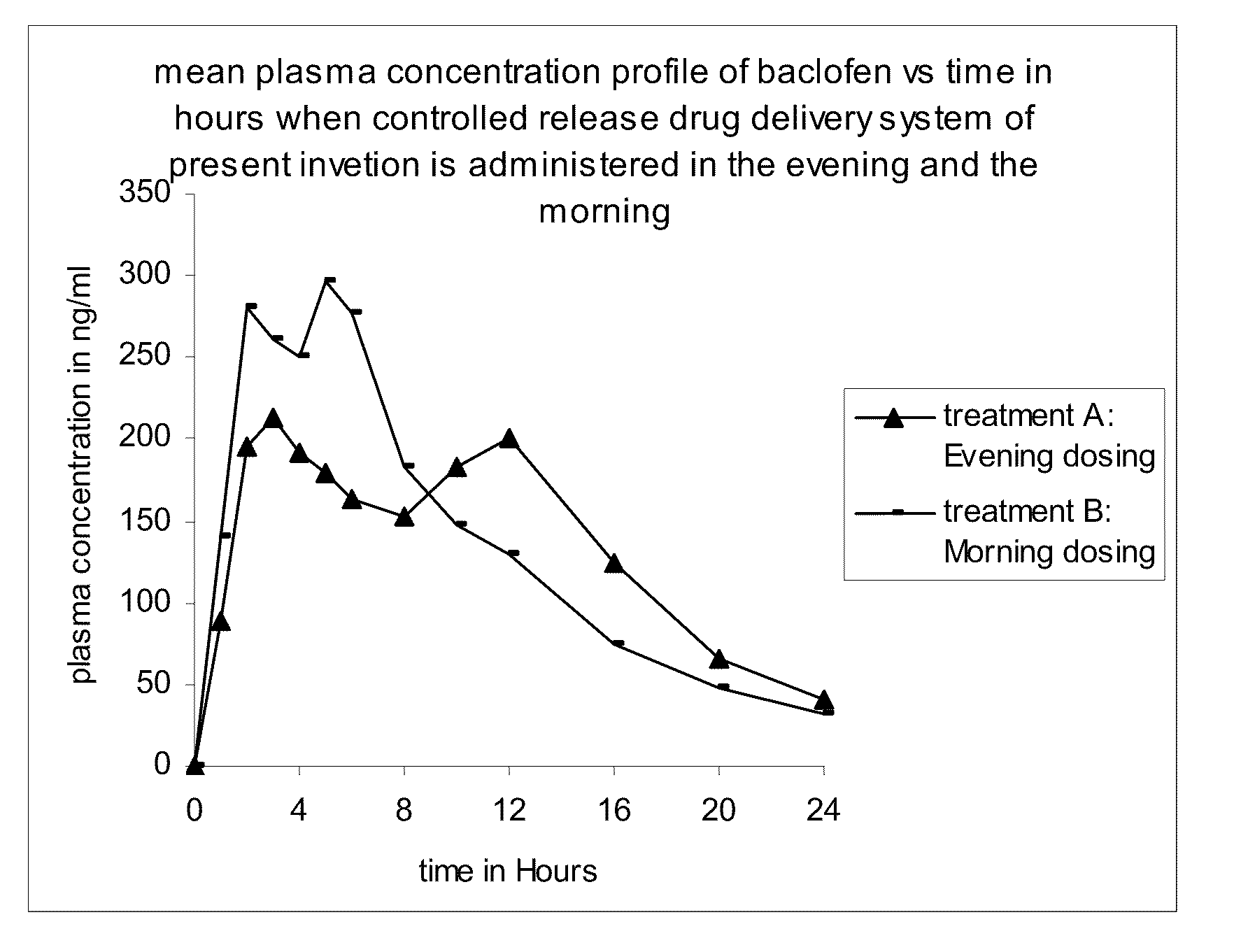
InactiveUS20110200671A1High plasma levelExtended durationOrganic active ingredientsBiocideDiseaseMedicine
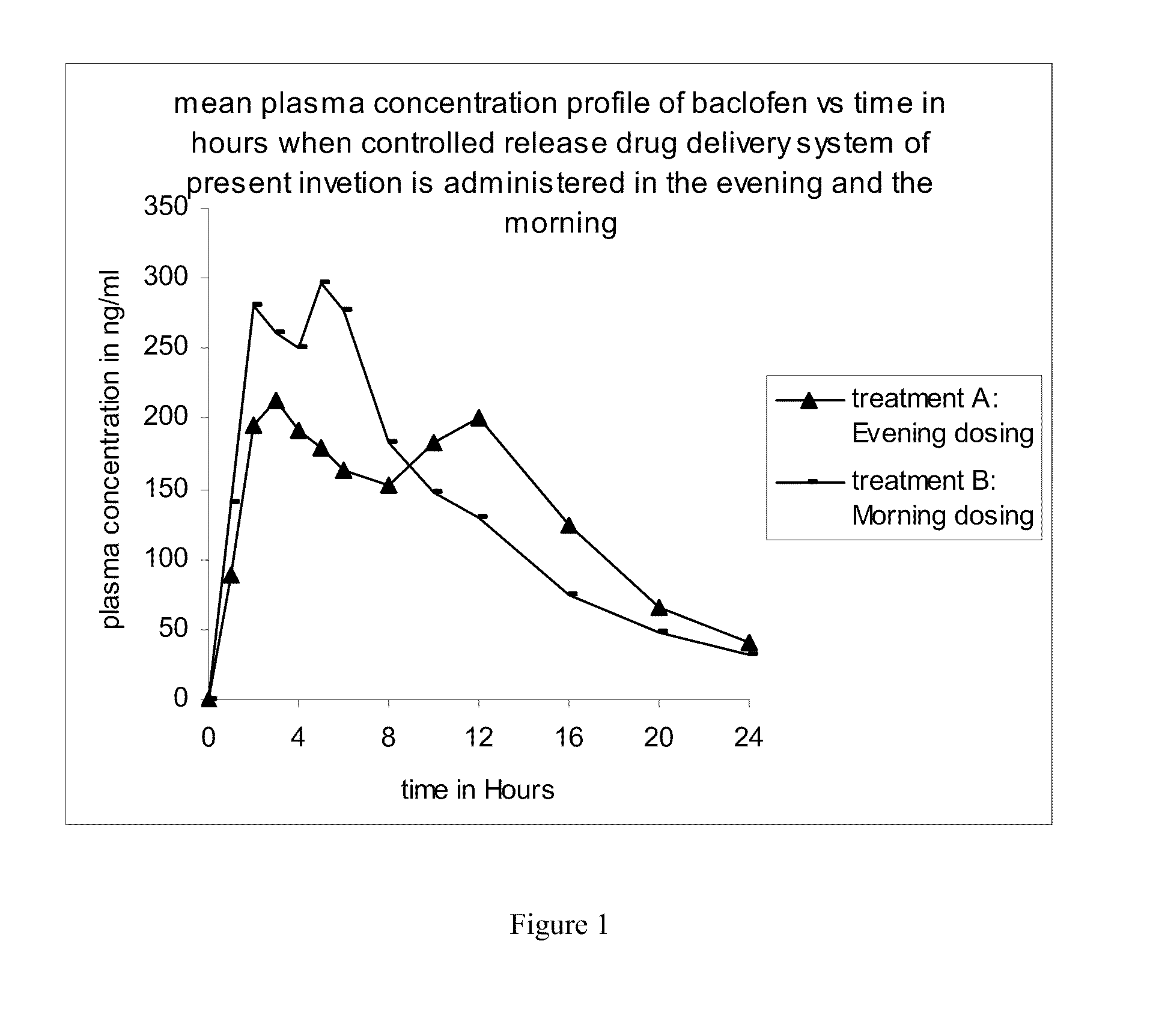
The present invention discloses a method of treating a disease condition susceptible to baclofen therapy, said method comprising orally administering once-a-day in the evening a controlled release drug delivery system comprising baclofen or its pharmaceutically acceptable salt or its derivatives and pharmaceutically acceptable excipients.
Owner:SUN PHARMA INDS
Baclofen and acamprosate based therapy of neurological disorders
The present invention relates to combinations and methods for the treatment of neurological disorders related to glutamate excitotoxicity and Amyloid β toxicity. More specifically, the present invention relates to novel combinatorial therapies of Multiple Sclerosis, Alzheimer's disease, Alzheimer's disease related disorder, Amyotrophic Lateral Sclerosis, Parkinson's disease, Huntington's disease, neuropathic pain, alcoholic neuropathy, alcoholism or alcohol withdrawal, or spinal cord injury, based on Baclofen and Acamprosate combination.
Owner:PHARNEXT
Nutrigenomics methods and compositions
The present invention provides a proprietary compositions and systems to modulate genetic and metabolomic contributing factors affecting disease diagnosis, stratification, and prognosis, as well as the metabolism, efficacy and / or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients, and other ingredients for the purposes of customizing a subject's nutritional supplement formulation to optimize specific health outcomes. Specific to this invention the utilization of certain known polymorphic genes associated with Substance Use Disorder (SUD) are analyzed to target certain genetic anomalies that lead to a high risk and predisposition to SUD. The genotypic patterns are then utilized to provide certain nutritional customized solutions especially related to the attenuation of aberrant abuse of physician prescribed narcotic pain medication across all pain conditions. A priority GENOPROFILE is measured and directs the customization of a subsequent nutraceutical to act as a therapeutic modality. Specifically the treatment includes slow attenuation of the pain medication by incorporating orals (shakes, liquid beverages, pills, tablets, troche, ointments etc.), Intramuscular, Intravenous, intra-rectal and any form necessary to deliver a sufficient amount of an anti-craving and anti-stress nutraceutical. Moreover, the invention includes examples of novel analgesic ointments coupling Synaptamine and such analgesic and other anesthetic compounds including but not limited to Gabapentin, Ketamine, Baclofen, Ketoprofen, Amitriptyline, Lidocaine, Cyclobenzapine, Diclofenac, Menthol, Camphor and Capsaicin. The GENOPROFILE will be used to determine pain sensitivity Intolerance.
Owner:BLUM KENNETH +3
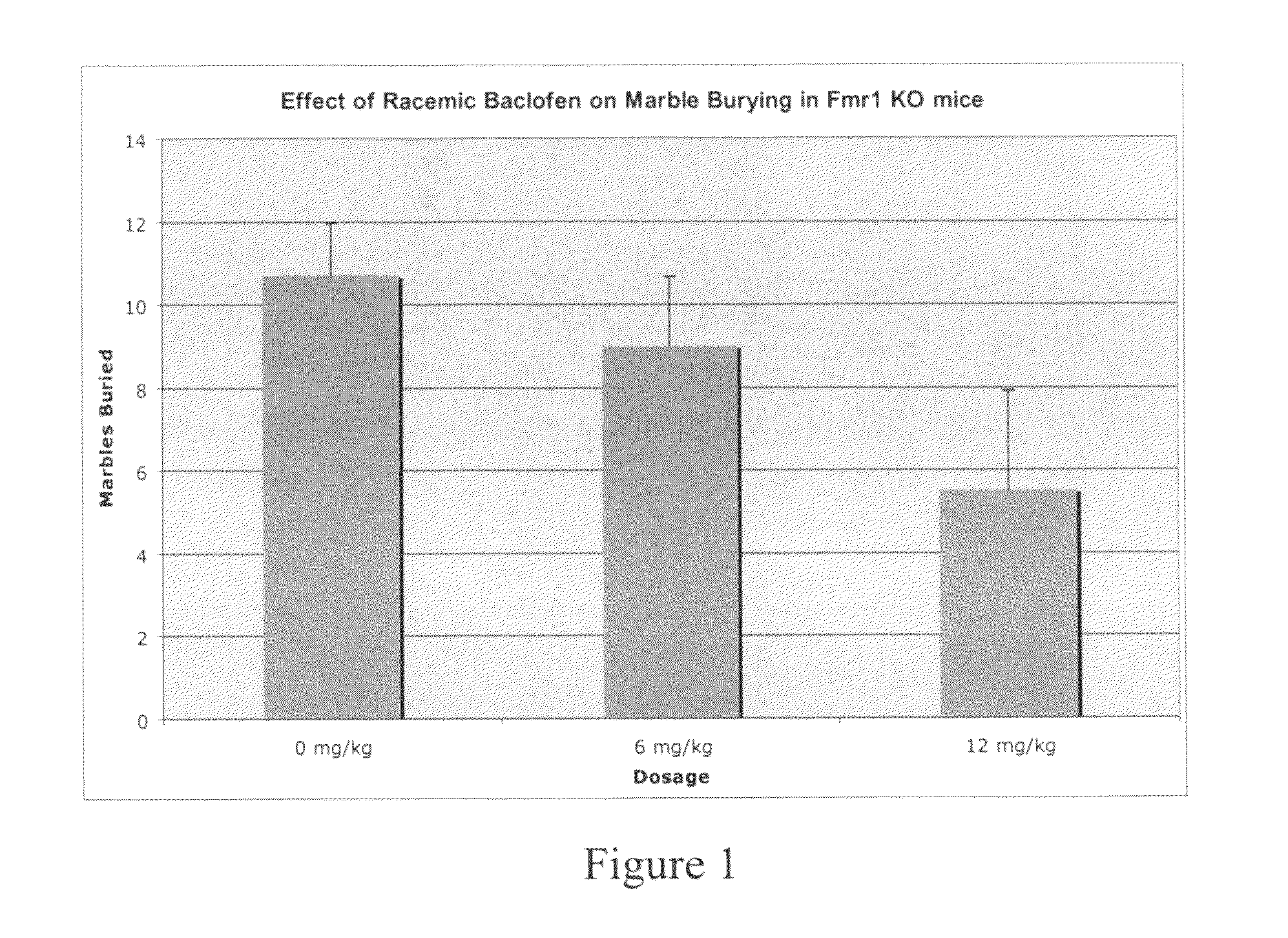
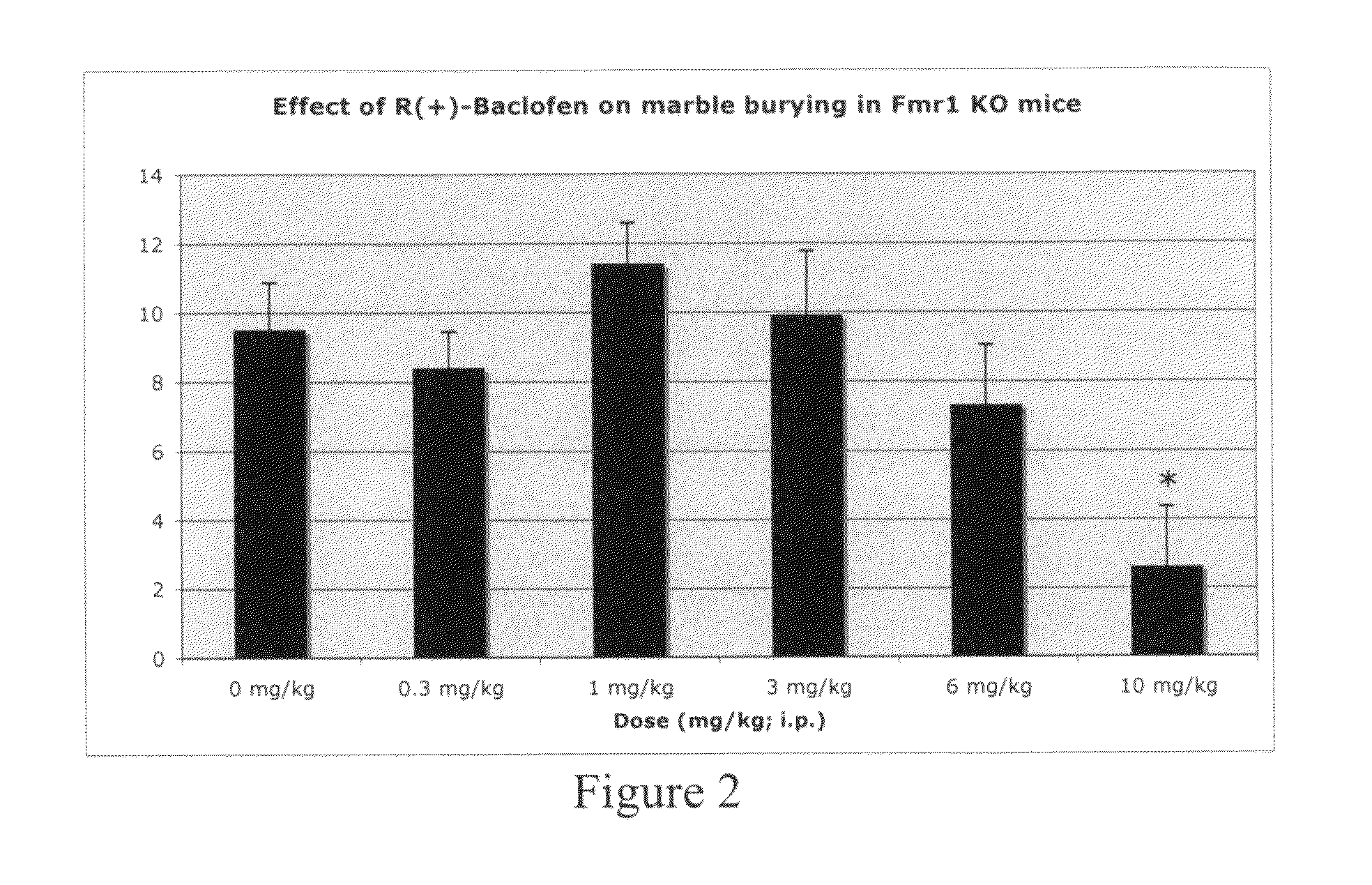
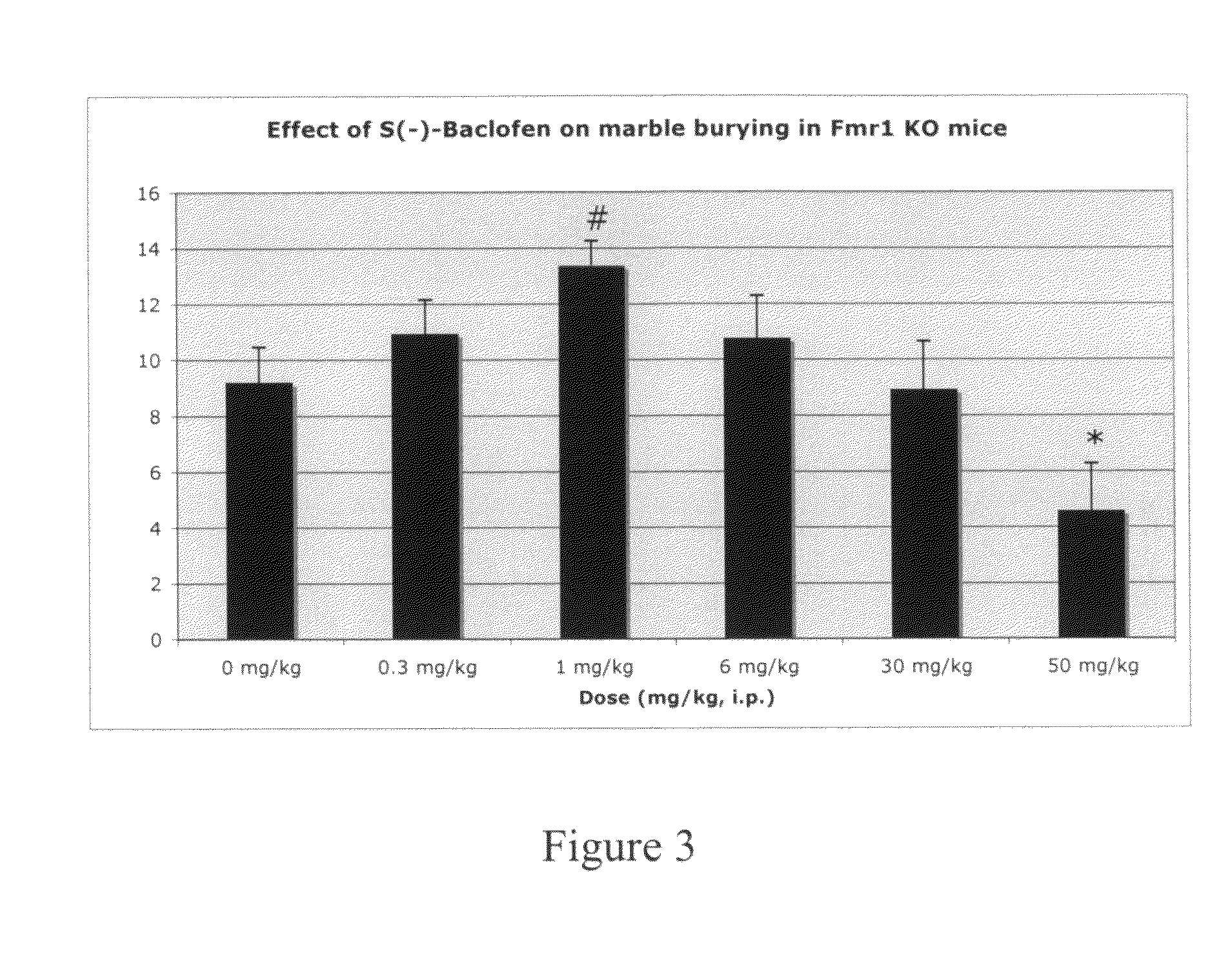
Methods of treating mental retardation, down's syndrome, fragile X syndrome and autism
ActiveUS20100029770A1Symptoms improvedReduce restlessnessOrganic active ingredientsBiocideFragile X chromosomeGamma-Aminobutyric acid
Subjects having at least one condition selected from the group consisting of mental retardation, Down's syndrome, fragile X syndrome and autism are treated with a composition that includes gamma-aminobutyric acid agonists and / or M1 muscarinic receptor antagonists. The gamma-aminobutyric acid agonist (GABA) can be a GABA(B) agonist, such as baclofen. GABA(B) agonists can be used in combination with Group I mGluR antagonists and M1 muscarinic receptor antagonists in methods of treating humans.
Owner:CLINICAL RES ASSOC
Baclofen and acamprosate based therapy of neurological disorders
The present invention relates to combinations and methods for the treatment of neurological disorders related to glutamate excitotoxicity and Amyloid β toxicity. More specifically, the present invention relates to novel combinatorial therapies of Multiple Sclerosis, Alzheimer's disease, Alzheimer's disease related disorder, Amyotrophic Lateral Sclerosis, Parkinson's disease, Huntington's disease, neuropathic pain, alcoholic neuropathy, alcoholism or alcohol withdrawal, or spinal cord injury, based on Baclofen and Acamprosate combination.
Owner:PHARNEXT
Compounded transdermal pain management
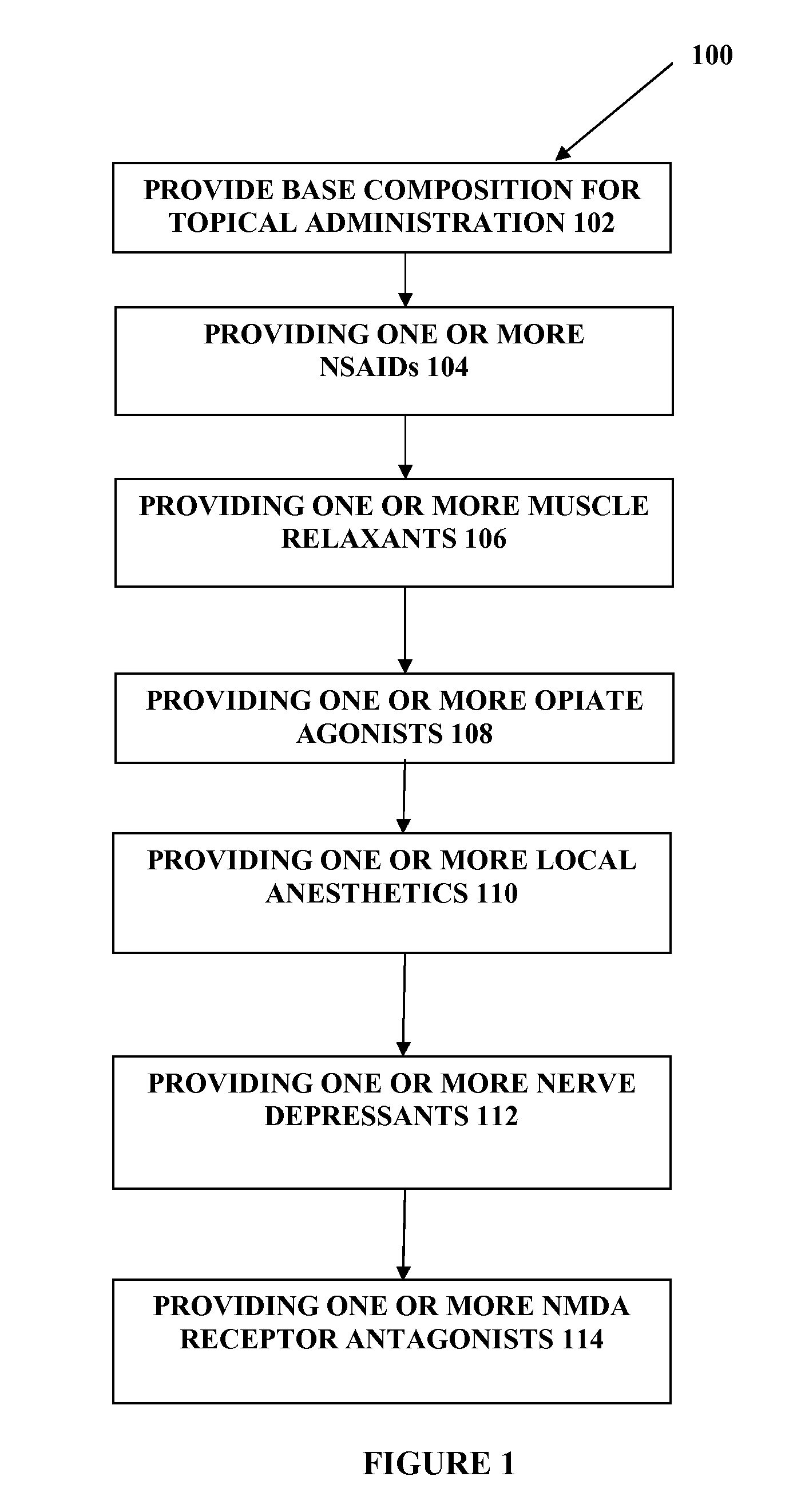
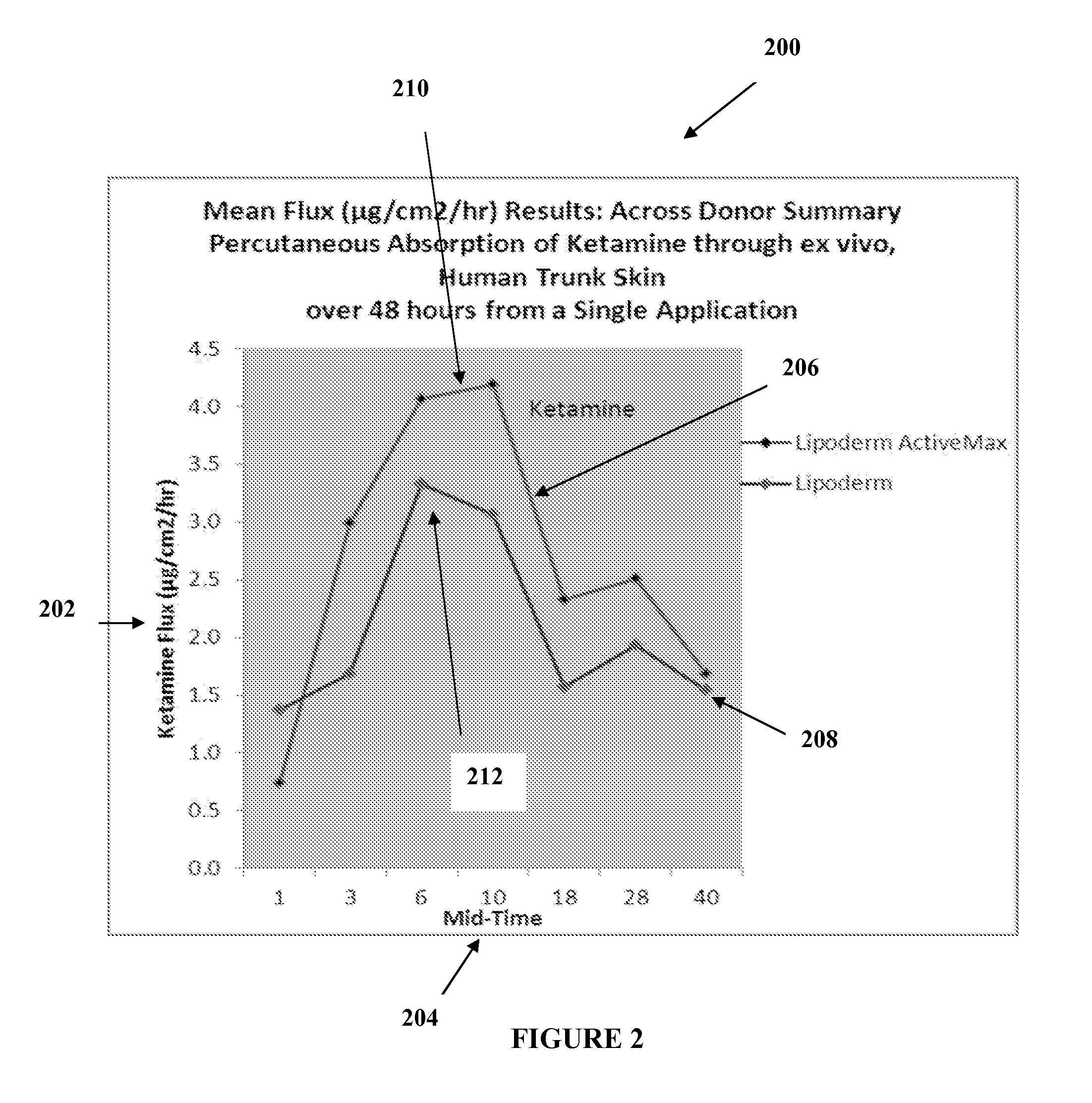
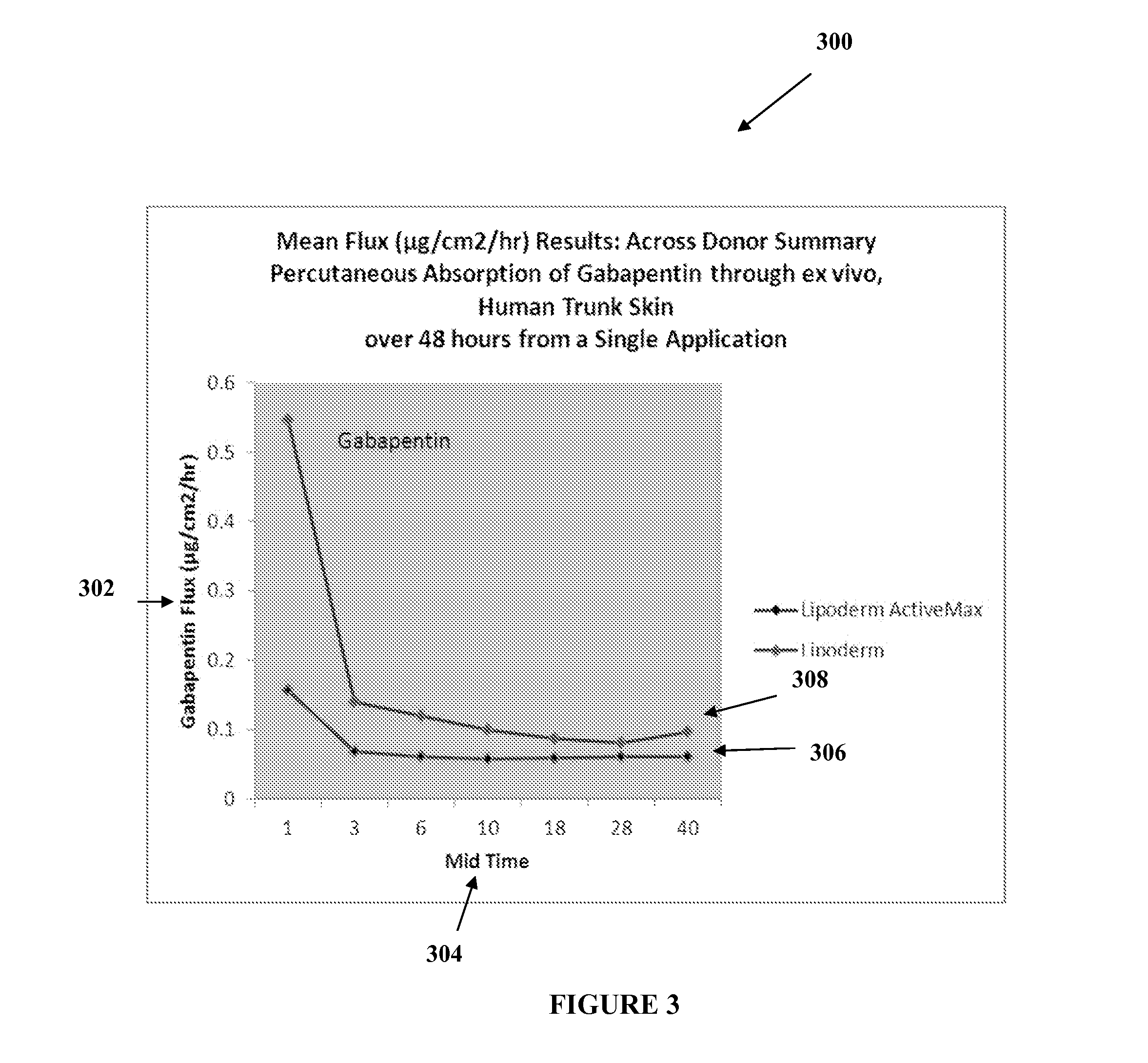
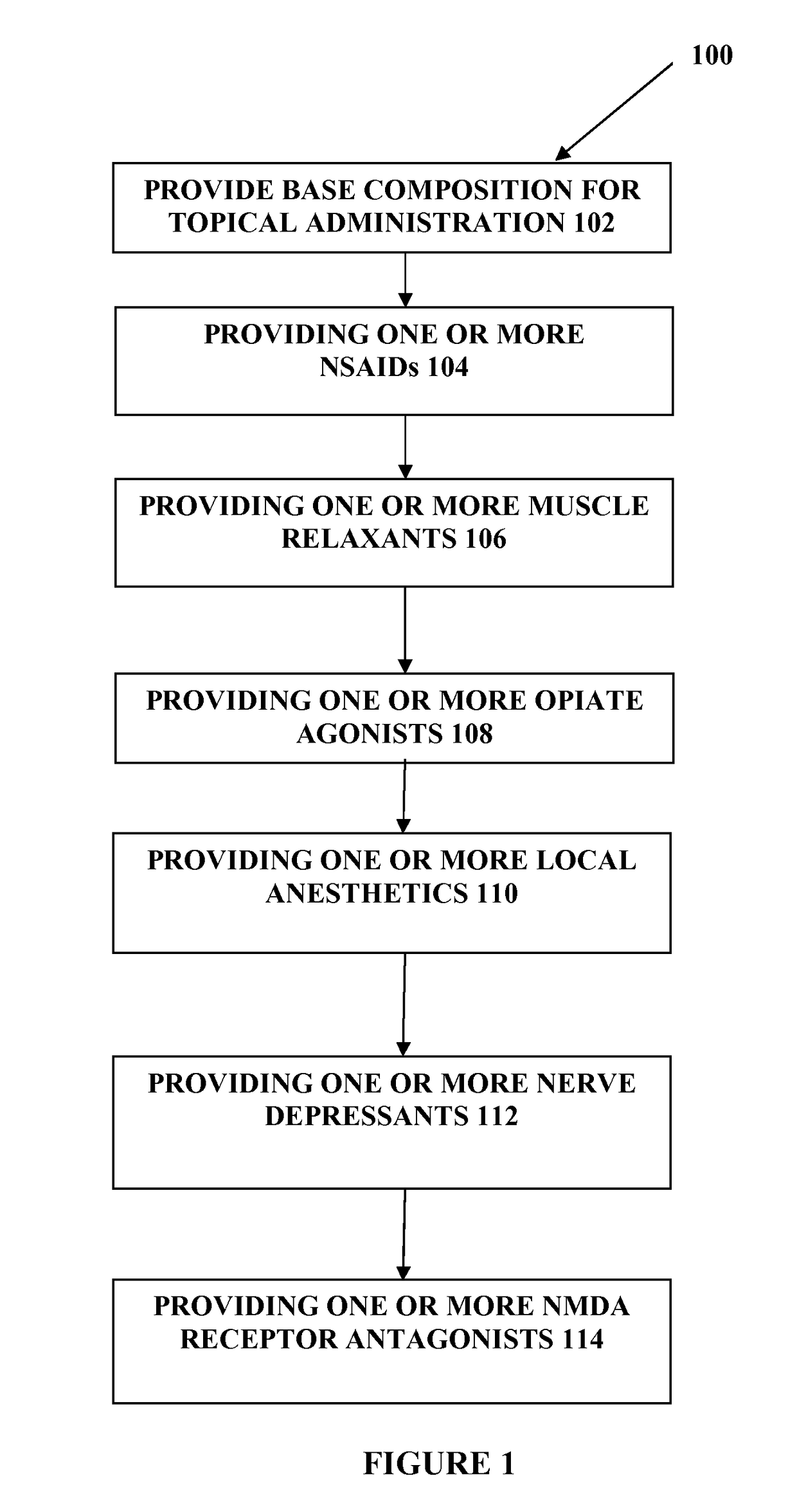
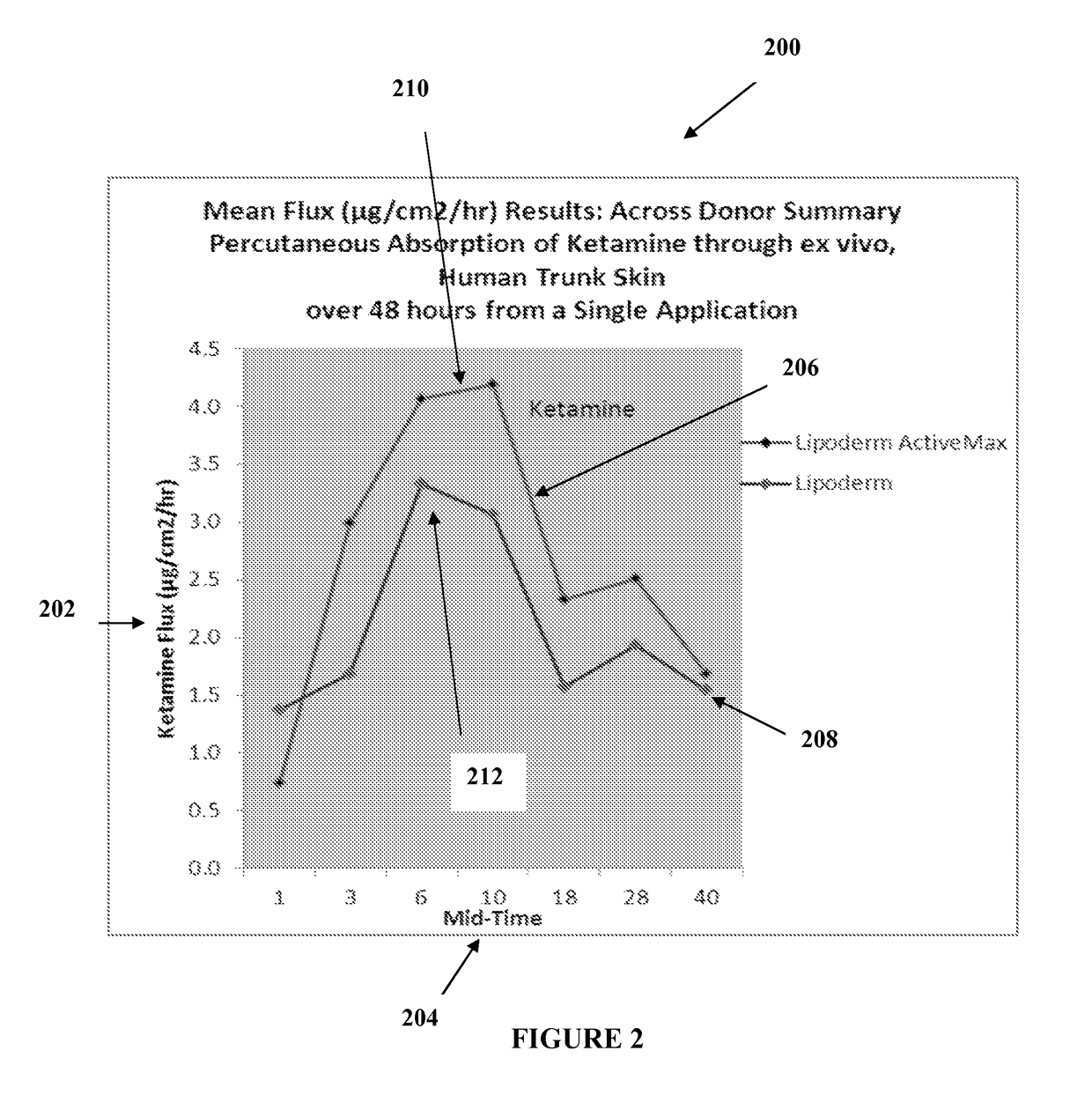
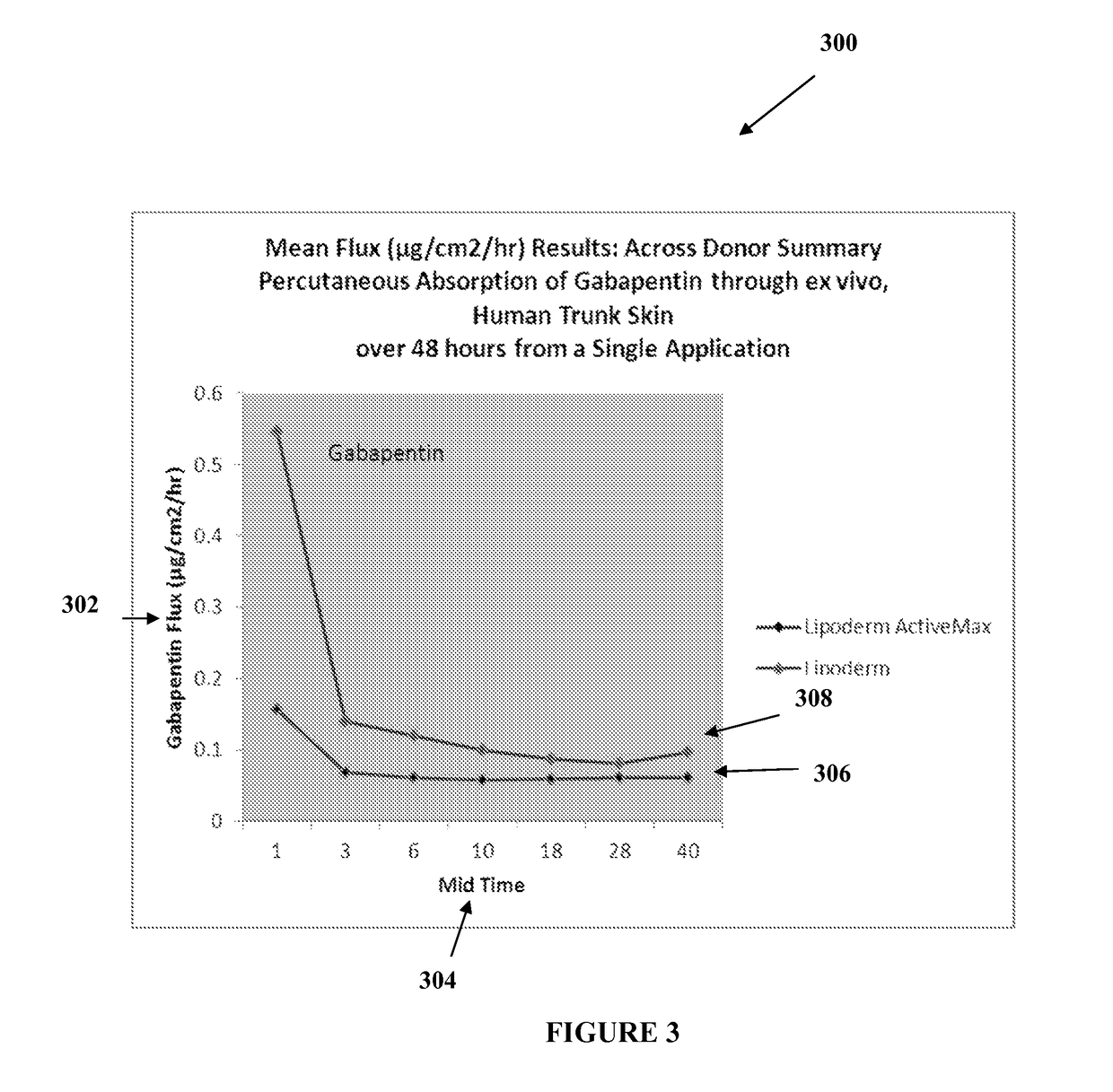
The present embodiments relate to topically delivered medication (compounded) for treatment of pain, inflammation, muscle fatigue, spasms, and / or other ailments. A transdermal cream may provide the effective topical administration of multiple medications simultaneously. The transdermal cream may include a salt load of approximately 30% or greater. The transdermal cream may include a unique base composition such that the transdermal cream may be able to remain stable and avoid degradation for six months or more and capable of effective delivery of active ingredient concentrations exceeding approximately 40% or more of the total formulation weight. The active ingredients may include a nerve depressant, NSAID, muscle relaxant, opiate agonist, local anesthetic, NMDA receptor antagonist, and a tricyclic antidepressant. In one embodiment, the transdermal cream may comprise ketamine HCL, gabapentin, clonidine HCL and baclofen. The transdermal cream may deliver an enhanced topical delivery flux of ketamine via a single transdermal application.
Owner:CMPD LICENSING
Sustained Release Particulate Oral Dosage Forms of (R)-Baclofen and Methods of Treatment
InactiveUS20100137442A2Enhanced bioavailability profileImproved profileBiocideOrganic active ingredientsDisease causeBaclofen
Owner:XENOPORT
Method for reducing pain
ActiveUS7268109B2Nervous disorderPeptide/protein ingredientsPharmaceutical formulationAnalgesic agents
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
Method for alleviating signs and symptoms of spasticity
ActiveUS8426470B2Lower Level RequirementsAlleviation of signBiocideOrganic active ingredientsSedative EffectsImmediate release
A method of alleviating signs and symptoms of spasticity in human patient comprising orally administering to said human patients once in a day a controlled drug delivery system comprising an effective daily dose of baclofen or its pharmaceutically acceptable salt. The controlled drug delivery system is operable to produce a level of sedation lower than a sedation produced by three times a day immediate release tablets. A total daily dosage of the controlled release tablets and a total daily dosage of the three times a day immediate release tablets remain same.
Owner:SUN PHARMA INDS
Method of treating a disease condition susceptible to baclofen therapy
The present invention discloses a method of treating a disease condition susceptible to baclofen therapy, said method comprising orally administering once-a-day in the evening a controlled release drug delivery system comprising baclofen or its pharmaceutically acceptable salt or its derivatives and pharmaceutically acceptable excipients.
Owner:SUN PHARMA INDS
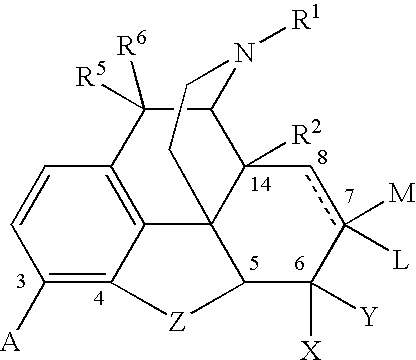
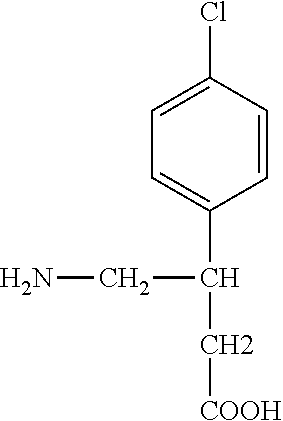
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formulaNH2CH2CH2CHR1C(O)N—R (I)where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Methods of treating fragile X syndrome and autism
ActiveUS8143311B2Symptoms improvedReduce restlessnessBiocideOrganic active ingredientsFragile X chromosomeM1 muscarinic receptor
Subjects having autism are treated with a composition that includes gamma-aminobutyric acid agonists. Subjects having fragile X syndrome are treated with M1 muscarinic receptor antagonists. The gamma-aminobutyric acid agonist (GABA) can be a GABA(B) agonist, such as baclofen. GABA(B) agonists can be used in combination with Group I mGluR antagonists and M1 muscarinic receptor antagonists in methods of treating humans.
Owner:CLINICAL RES ASSOC
Zwitterion solution for low-volume therapeutic delivery
A formulation is provided that includes a volume of an aqueous multivalent physiological ion solution compatible with cerebrospinal fluid containing at least one divalent cation of magnesium or calcium, and at least one anion of carbonate or phosphate, and having a pH between 6.5 and 8.0. A zwitterionic therapeutic agent other than baclofen is dissolved the solution to achieve higher concentration or ease of solution and / or storage relative to therapeutic saline solutions of the same agent. A process of delivering a zwitterionic therapeutic agent into a subject is provided that includes dissolving a therapeutic amount of the zwitterionic therapeutic agent in a volume of artificial cerebrospinal fluid to form a stable formulation. The solution is then administered to the subject using an intrathecal pump.
Owner:WAYNE STATE UNIV
Baclofen solution for low-volume therapeutic delivery
A high concentration baclofen solution is provided suitable for therapeutic use in a medical setting. A high concentration solution of baclofen in multivalent physiological ion solution such as artificial cerebrospinal fluid is provided with concentrations of baclofen of 10 mg / ml. Artificial cerebrospinal fluid is particularly advantageous as a baclofen solvent. A medical package is also provided for baclofen delivery to patients suffering from spasticity.
Owner:MEYTHALER JAY M +1
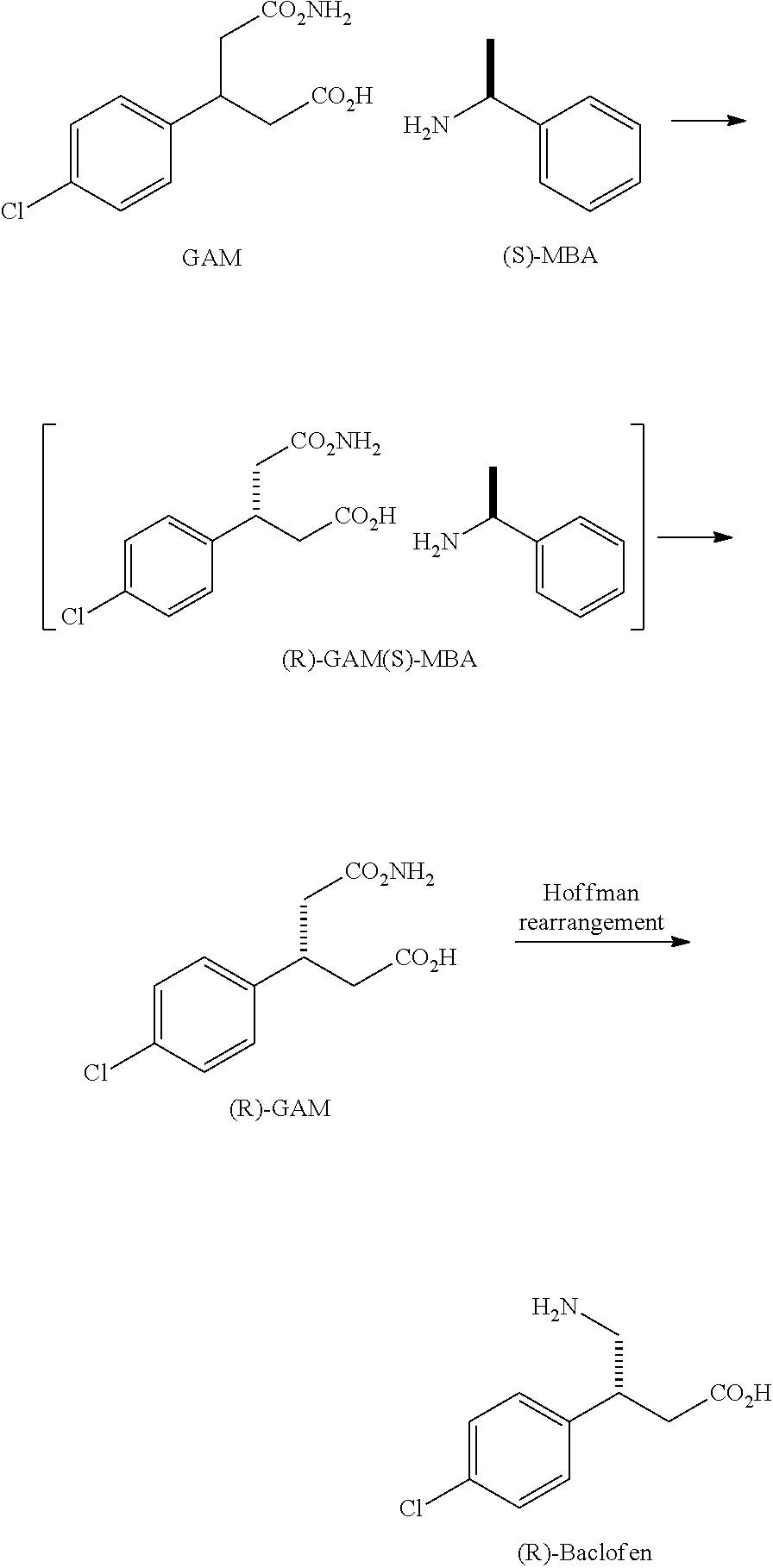
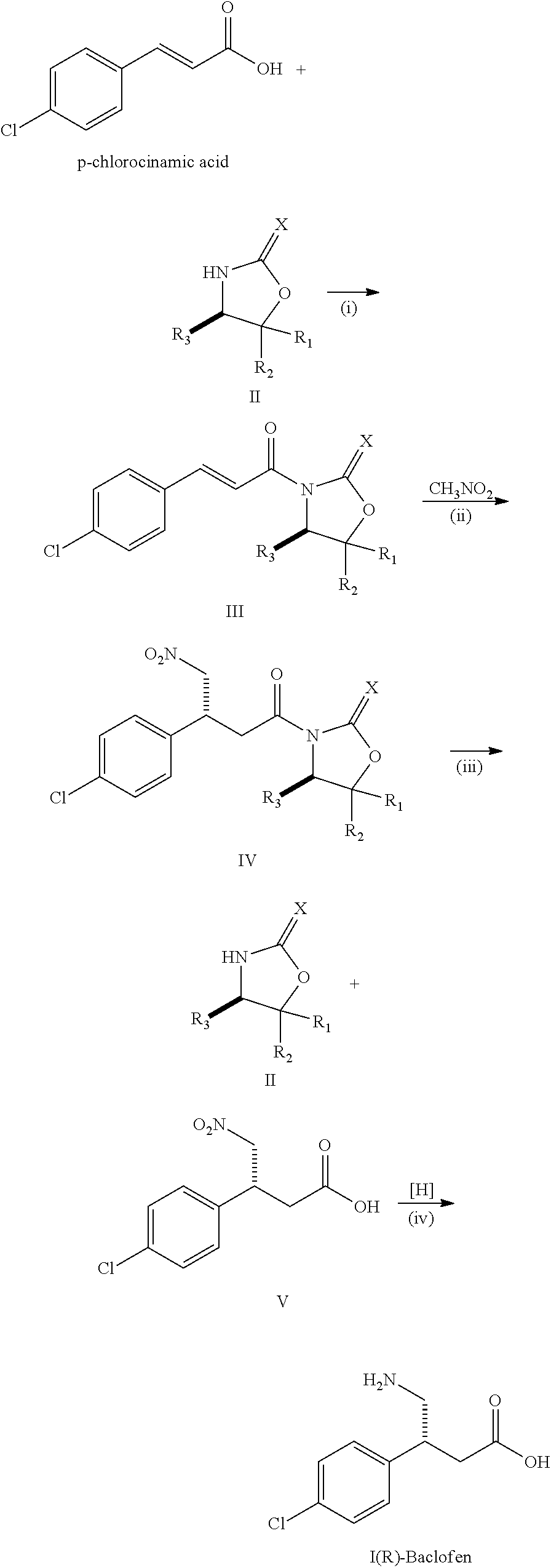
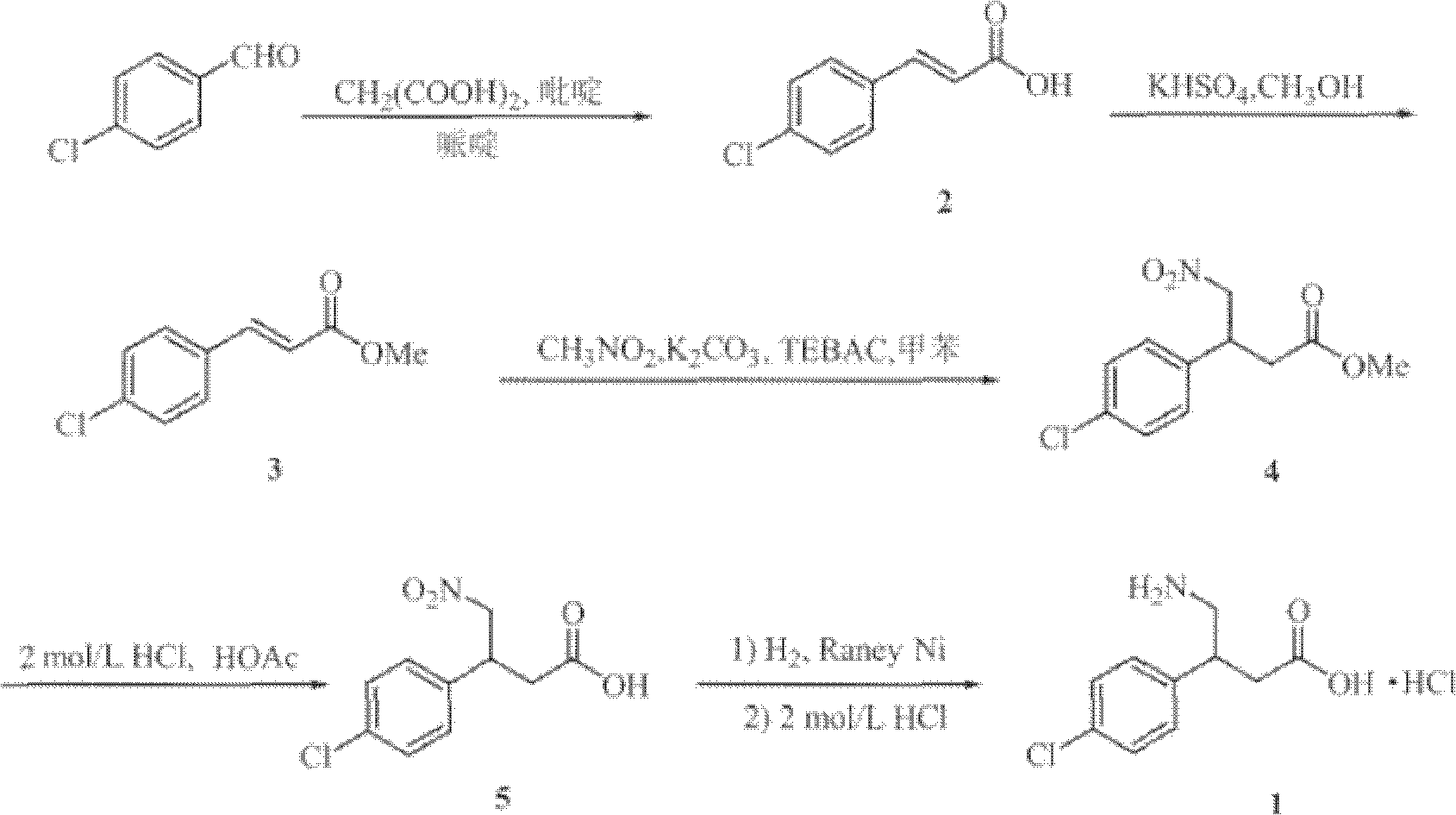
Method for preparing chiral baclofen
The present invention provides a novel method for preparing chiral Baclofen with higher yield, higher e.e. value, and lower cost via chiral Michael addition.
Owner:SCI PHARMTECH
Methods of Treating alcoholism and alcohol related disorders using combination drug therapy and swellable polymers
The current invention provides methods of treating alcohol related disorders by providing a sustained release oral drug dosage form comprising a plurality of solid state drugs i.e., baclofen and naltrexone, dispersed in a solid state unitary matrix formed from a combination of swellable polymers. The combination of swellable polymers in a single oral drug dosage form is beneficial in terms of release rate control for combination therapies.
Owner:ALKERMES INC
Methods of administering drugs in an implantable multi-chamber pump
InactiveUS20140296830A1Relieve painReducing severe and chronic painMedical devicesPressure infusionNeuropathic painZiconotide
One embodiment of the present invention is a method for reducing pain using a multi chamber pump to separately administer multiple drugs. For example, one chamber may contain an omega conopeptide, such as ziconotide, and the other chamber or chambers may contain one or more other drugs, which may include of morphine, hydromorphone, fentanyl, sufentanil, bupivacaine, baclofen, clonidine, and buprenorphine, or their pharmaceutically acceptable salts thereof. Other applications of the present invention include methods for treating severe chronic pain due to cancer, failed back syndrome, CRPS, neuropathic pain, mixed neuropathic and nociceptive pain.
Owner:JAZZ PHARMA
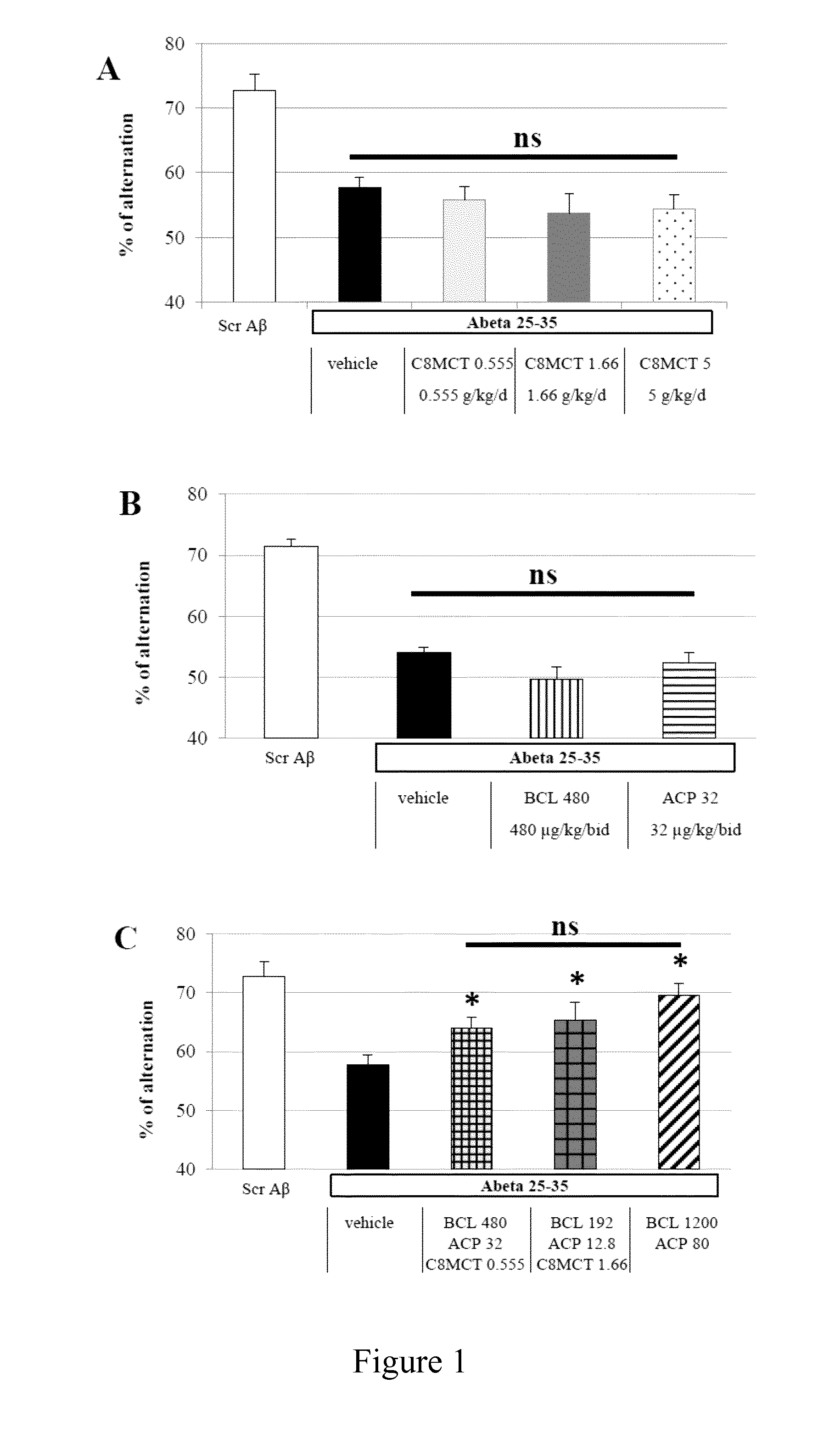
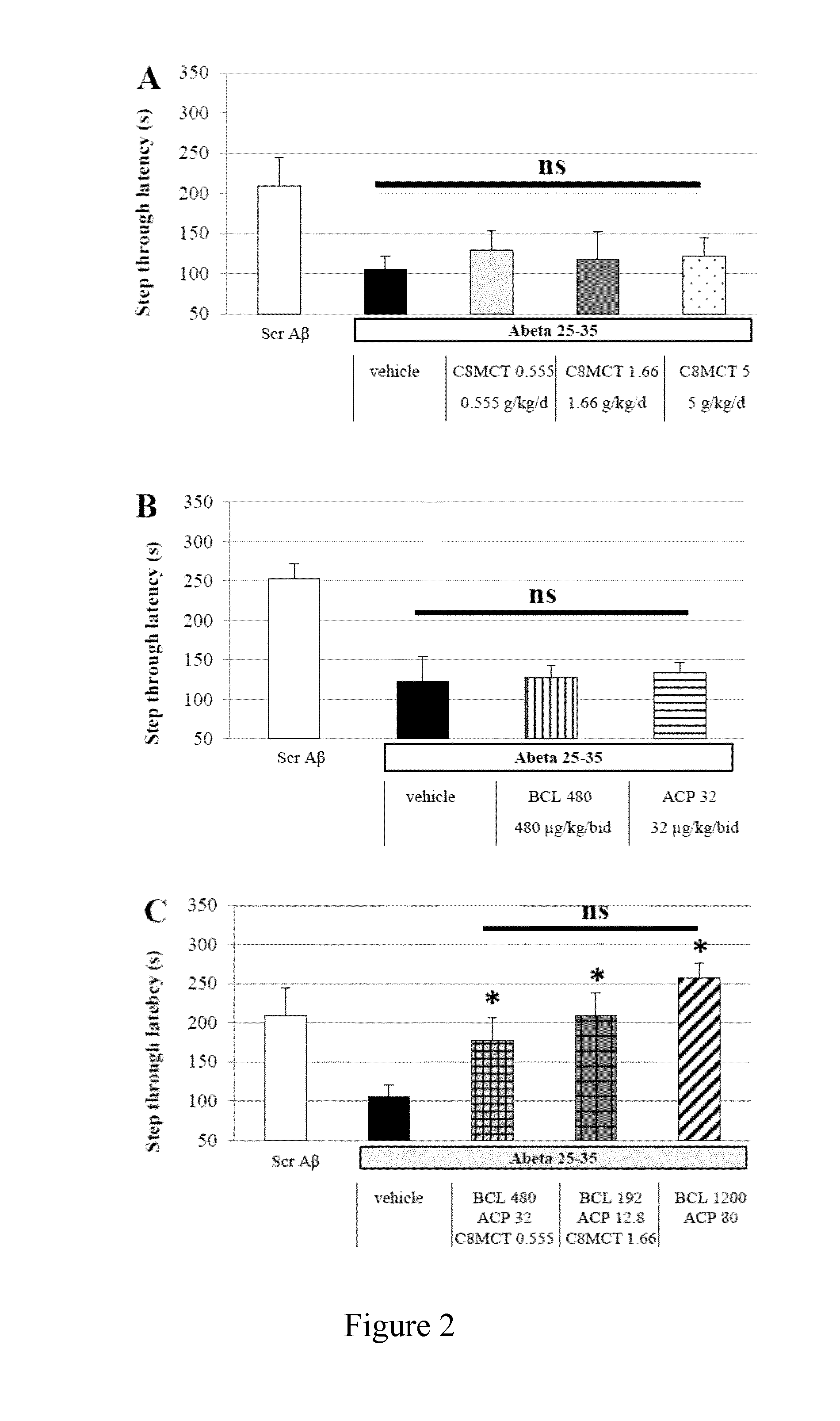
Combination of baclofen, acamprosate and medium chain triglycerides for the treatment of neurological disorders
ActiveUS20160354335A1Improve cognitive functionProtected growthNervous disorderMuscular disorderAlcoholismsHuntingtons chorea
The present invention relates to combinations and methods for the treatment of neurological disorders related Amyloid beta toxicity and / or neuronal death and / or glucose impaired neuronal metabolism. More specifically, the present invention relates to novel combinatorial therapies of Alzheimer's disease, Alzheimer's disease related disorders, frontotemporal dementia, Parkinson's disease, Lewy body dementia, Huntington's disease, peripheral neuropathies, alcoholism or alcohol withdrawal, neurological manifestations of drug abuse or drug abuse withdrawal, amyotrophic lateral sclerosis, multiple sclerosis, spinal cord injury, epilepsy, traumatic brain injury or brain ischemic events based on baclofen, acamprosate and at least one medium chain triglyceride.
Owner:PHARNEXT
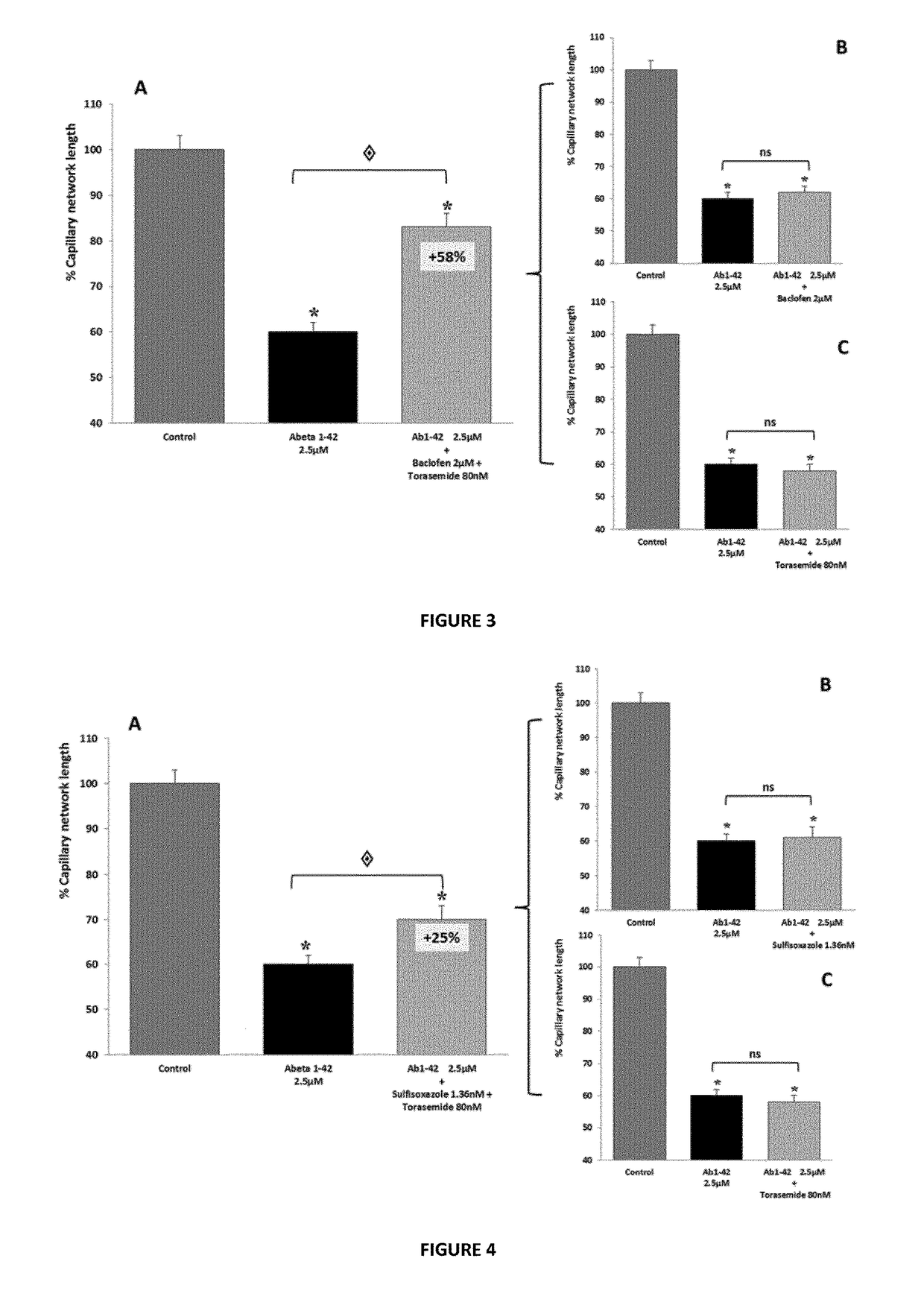
Composition comprising torasemide and baclofen for treating neurological disorders
ActiveUS9931326B2Sulfonylurea active ingredientsAnhydride/acid/halide active ingredientsAlcoholismsHuntingtons chorea
The present invention relates to compositions and methods for the treatment of neurological disorders related to glutamate excitotoxicity and Amyloid β toxicity. More specifically, the present invention relates to novel combinatorial therapies of multiple sclerosis, Alzheimer's disease, Alzheimer's disease-related disorders, amyotrophic lateral sclerosis, Parkinson's disease, Huntington's disease, neuropathic pain, alcoholic neuropathy, alcoholism or alcohol withdrawal, or spinal cord injury.
Owner:PHARNEXT
Intravenous baclofen formulations and treatment methods
ActiveUS20160213631A1Rapid attainmentAccurate and precise dose titrationOrganic active ingredientsNervous disorderIV injectionAnesthesia
An intravenous baclofen solution is disclosed, along with methods of dosing and treatment therewith.
Owner:RGT UNIV OF MINNESOTA +1
Compounded transdermal pain management
ActiveUS9724315B2Efficient deliveryPrevent degradationBiocideNervous disorderNR1 NMDA receptorGabapentin
The present embodiments relate to topically delivered medication (compounded) for treatment of pain, inflammation, muscle fatigue, spasms, and / or other ailments. A transdermal cream may provide the effective topical administration of multiple medications simultaneously. The transdermal cream may include a salt load of approximately 30% or greater. The transdermal cream may include a unique base composition such that the transdermal cream may be able to remain stable and avoid degradation for six months or more and capable of effective delivery of active ingredient concentrations exceeding approximately 40% or more of the total formulation weight. The active ingredients may include a nerve depressant, NSAID, muscle relaxant, opiate agonist, local anesthetic, NMDA receptor antagonist, and a tricyclic antidepressant. In one embodiment, the transdermal cream may comprise ketamine HCL, gabapentin, clonidine HCL and baclofen. The transdermal cream may deliver an enhanced topical delivery flux of ketamine via a single transdermal application.
Owner:CMPD LICENSING
Method for synthesizing hydrochloric acid baclofen
InactiveCN102351726AGuaranteed sourceInhibit side effectsOrganic compound preparationAmino-carboxyl compound preparationChlorobenzeneBACLOFEN HYDROCHLORIDE
The invention relates to a novel method for synthesizing medicament hydrochloric acid baclofen. The method comprises the following steps of: adding 4-chlorobenzene acetonitrile, bromoacetate and ultrafine potassium carbonate into C1-C4 low carbon alcohol, reacting at the temperature of 30-70 DEG C, filtering and removing a potassium salt, and concentrating the filtrate to obtain a white solid, i.e., 3-(4-chlorphenyl)-3-cyan ethyl propionate (II) of which the melting point is 56-57 DEG C; undergoing a hydrogenation reduction reaction on the white solid (II) to obtain a white solid, i.e., 4-(4-chlorphenyl)-2-pyrrolidone (III) of which the melting point is 109-111 DEG C; and refluxing the white solid (III) in a hydrochloric acid aqueous solution for 8-30 hours, concentrating under reduced pressure to obtain hydrochloric acid baclofen when finishing the reaction , and recrystallizing by using isopropanol to obtain refined hydrochloric acid baclofen of which the melting point is 178-179 DEG C. Raw materials used in the method are readily available, the process is easy and reliable, and the total yield of the hydrochloric acid baclofen is up to 59 percent; and the method has a good industrial prospect.
Owner:HEBEI UNIV OF TECH +1
Method for preparing chiral baclofen
ActiveCN101514167AHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsGlutaric anhydrideGlutaric acid
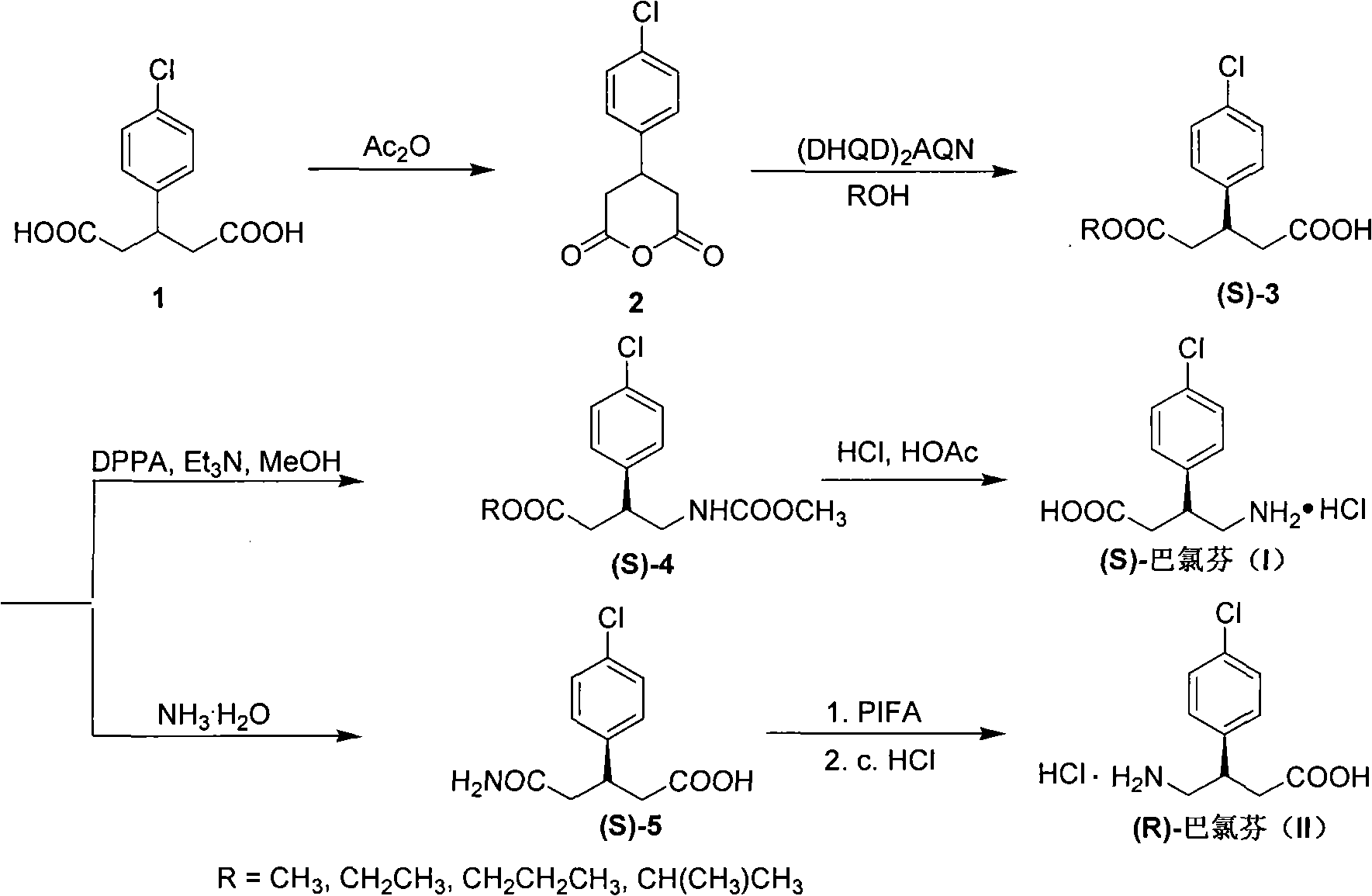
The invention relates to a method for preparing chiral baclofen, belonging to the synthesis field of chiral compounds. The synthetic route of the method is as follows: 3-(4-chlorphenyl) glutarate used as a starting material is condensed to prepare 3-(4-chlorphenyl) glutaric anhydride; a key intermediate (S)-3-(4-chlorphenyl) monoester glutarate is prepared by the 3-(4-chlorphenyl) glutaric anhydride under the action of chiral catalysts and the chiral baclofen is prepared by Curtius (or Hofmann) rearrangement reaction. The method of the invention has short reaction steps, convenient operation, high ee value of product, low cost and high yield.
Owner:孟坤
Method for Resolution of Baclofen Salts
ActiveUS20190345098A1Low compositionSubstantial discriminationOrganic compound preparationAmino-carboxyl compound preparationButyrateEnantiomer
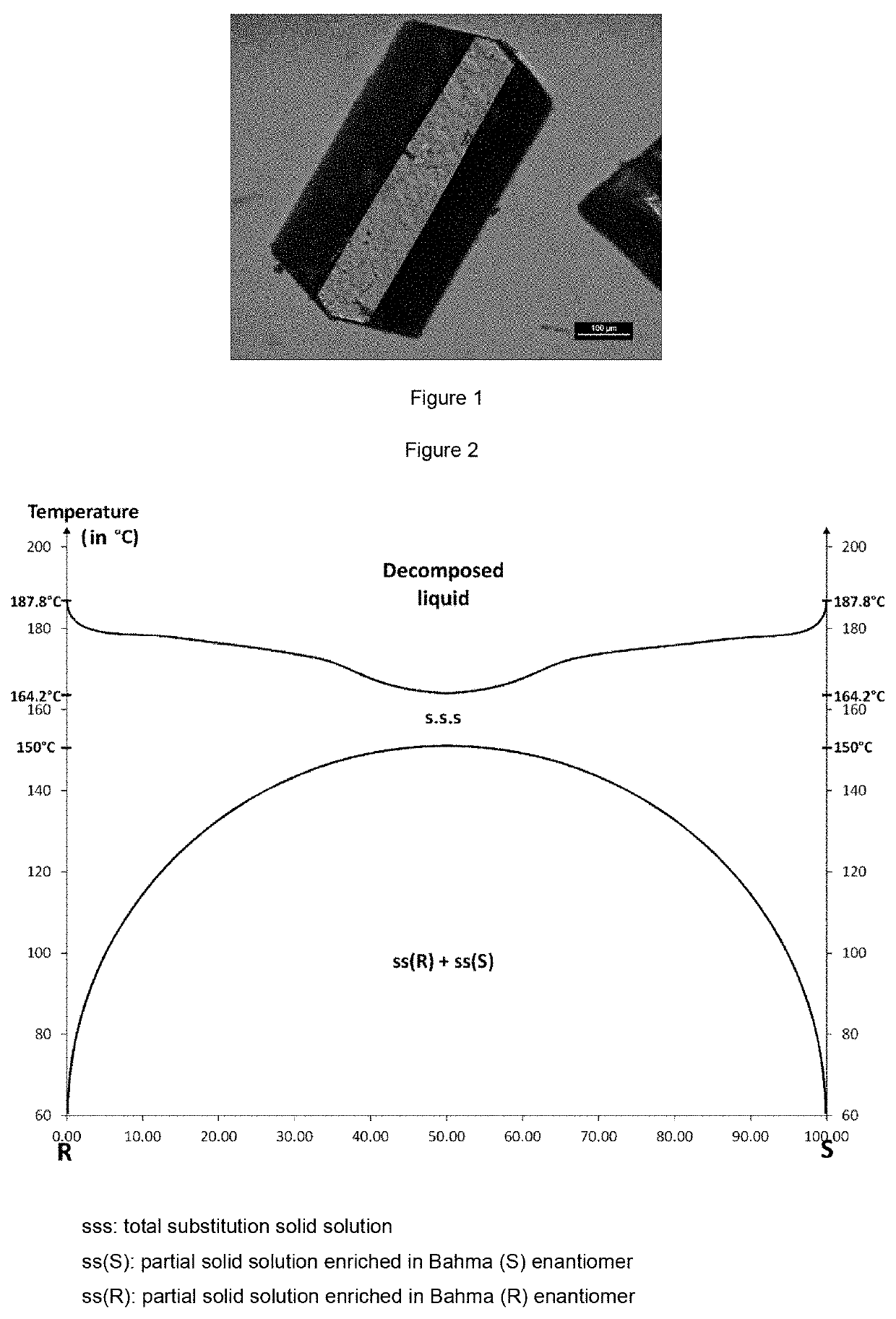
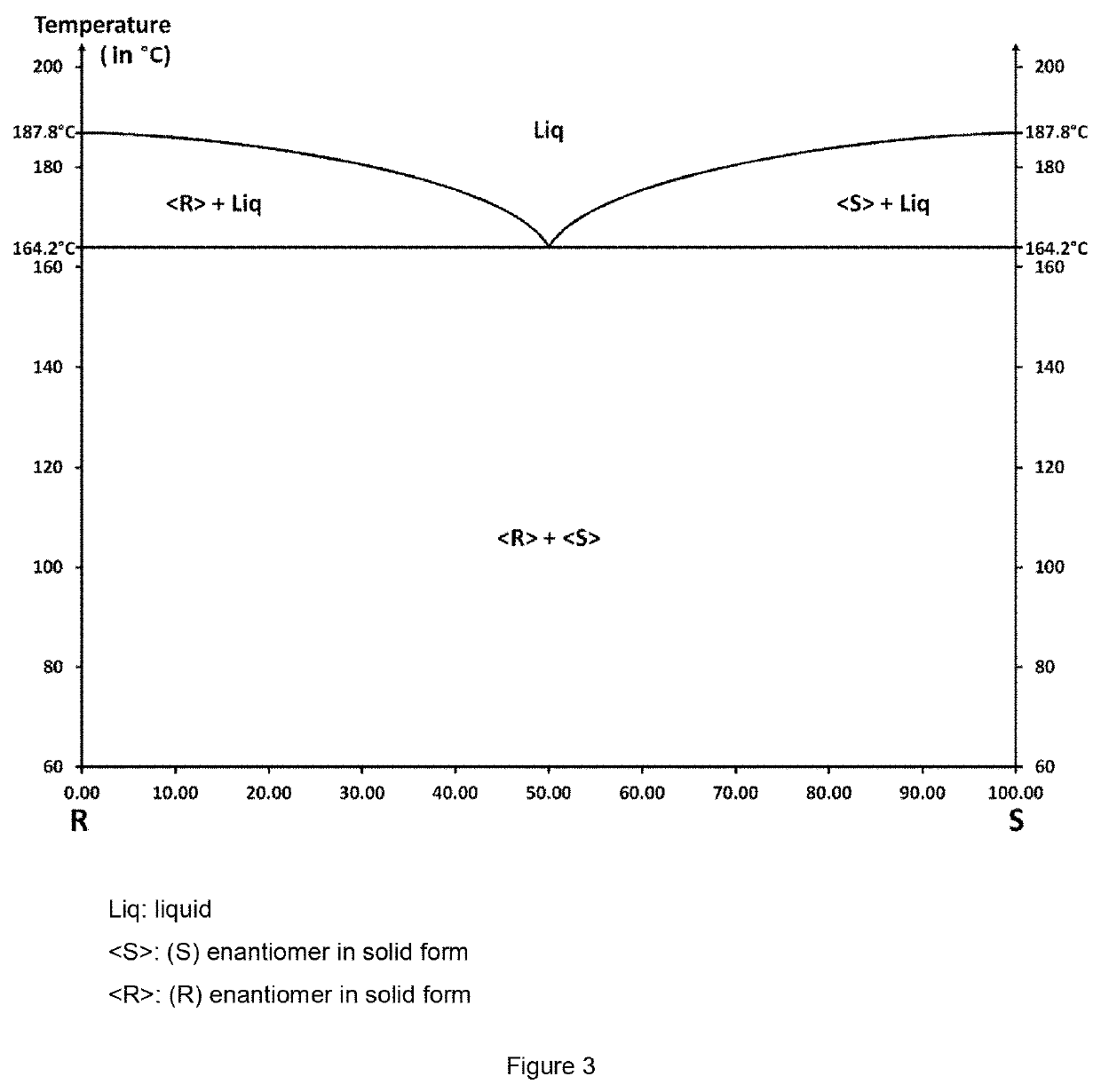
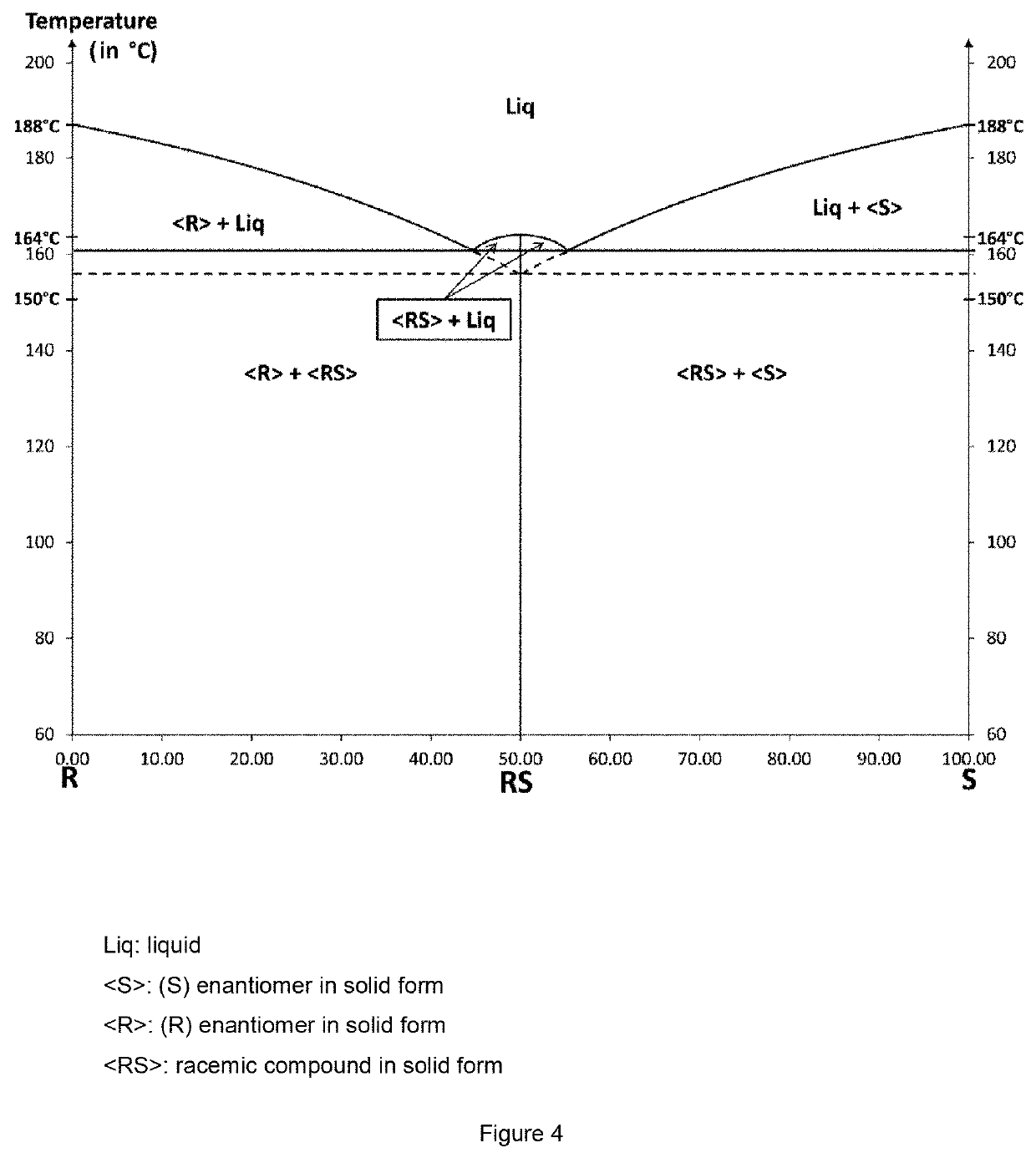
The invention relates to the field of resolution of chiral compounds existing in the form of two optical antipodes (enantiomers), such as Baclofen. More particularly, the invention relates to the production of the pure enantiomer (R)(−) Baclofen, of chemical nomenclature (R)-4-amino-3-(4-chlorophenyl)-butanoic acid, and the hydrogen maleate salt thereof. More specifically, the invention relates to the resolution of hydrogen maleate salts of racemic Baclofen by preferential crystallisation and particularly by the AS3PC method (auto-seeded and programmed polythermal preferential crystallisation).
Owner:UNIV DE ROUEN (FR)
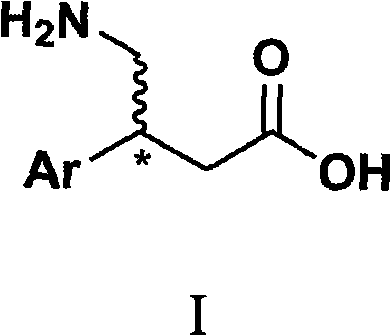
Novel method for synthesizing chiral beta-aryl-gamma-aminobutyric acid compounds
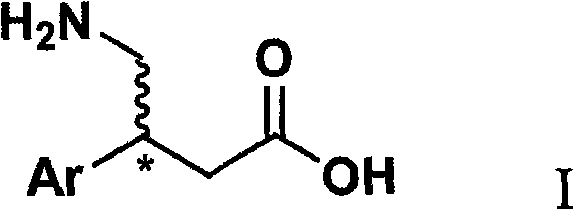
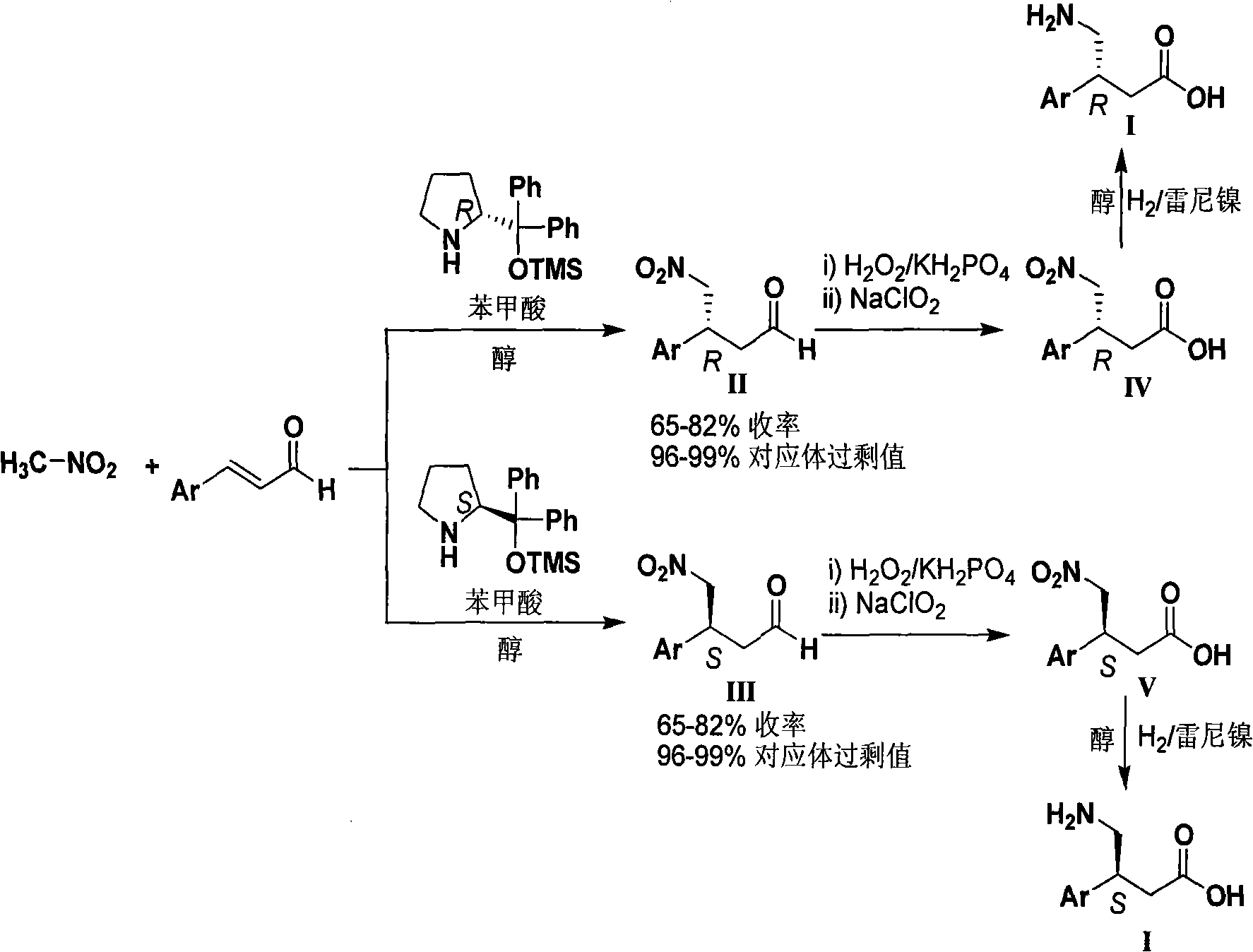
InactiveCN101333168ALower synthesis costFew reaction stepsOrganic chemistryOrganic compound preparationNitromethaneStructural formula
The invention relates to a novel and simple synthetic methodsynthesis method for chiral beta-aryl-gamma-aminobutyric acid compounds as shown in structural formula I. The method takes chiral diphenyl pyrrolidine trimethylsilyl ether as a catalyst and takes the cheap nitromethane and 3-aryl-acrolein as raw materials; the catalyst and the raw materials are reacted in alcohol solvent for 5 to 40 hours to prepare the chiral beta-aryl-gamma-nitryl-butyraldehyde with high yield (65-82%) and high counterpart selectivity (96-99%); then the chiral beta-aryl-gamma-nitryl-butyraldehyde is taken as the key intermediate to go through aldehyde oxidation and nitro reduction to get the chiral beta-aryl-gamma-aminobutyric acid (baclofen compounds). Compared with the existing synthetic route for baclofen compounds, the reaction steps of the invention are significantly shortened from the usual 6-8 steps to 3 steps, and the reaction conditions are mild and controllable, thereby greatly reducing the synthetic costs of baclofen drugs.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com