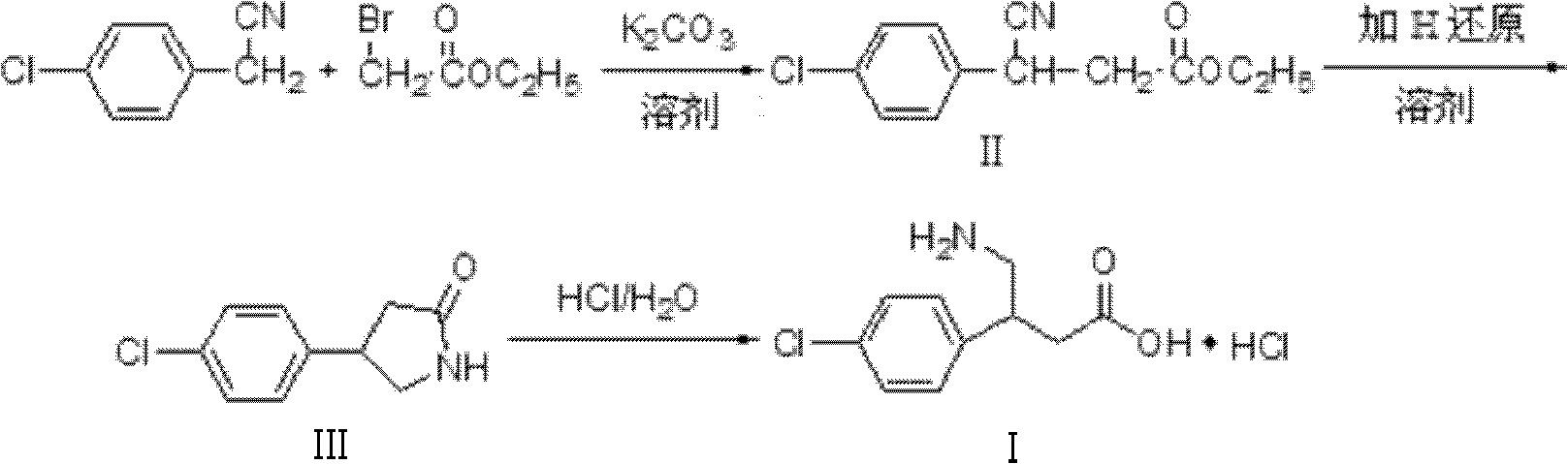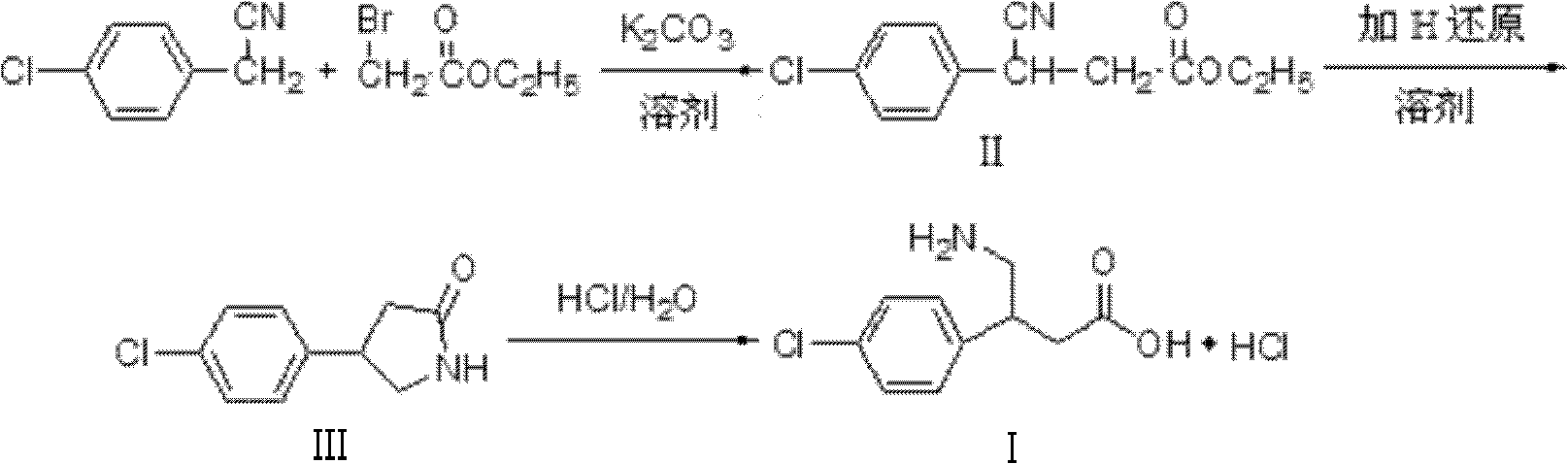Method for synthesizing hydrochloric acid baclofen
A technology of baclofen hydrochloride and synthetic method, which is applied in the field of drug synthesis, can solve the problems of expensive phase transfer catalyst, high toxicity of solvent toluene, and long synthetic process route, achieve good industrial prospects, simple reaction process, and avoid low temperature reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
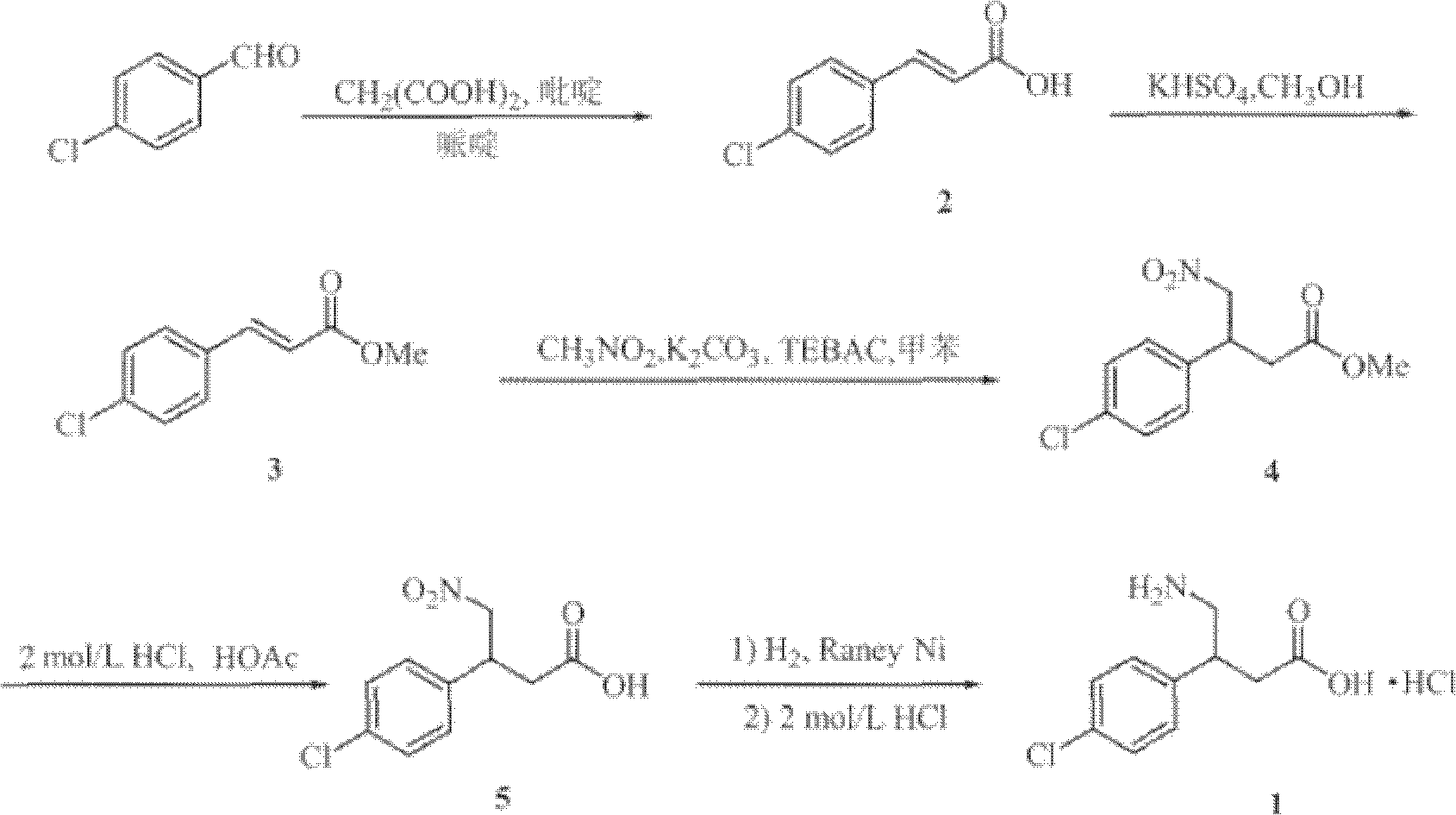
[0029] First prepare the used ultrafine potassium carbonate of the present invention, 100g potassium carbonate, 500g dehydrated alcohol are added grinder, seal all grinder openings, grind 5h under normal temperature, sampling, the average particle diameter of testing potassium carbonate by particle size distribution is 500nm , pour out spare. Wherein, the grinding time length, and the input amount of potassium carbonate, the input amount of dehydrated alcohol etc. all can affect the particle diameter of the ultrafine potassium carbonate prepared at last, the required potassium carbonate particle diameter of following embodiment is at 100~1000nm All have better performance.
[0030] Add 4-chlorophenylacetonitrile, ethyl bromoacetate, and superfine potassium carbonate into the reactor at a molar ratio of 1.00:1.05:1.3, and at the same time add 5 times the mass of absolute ethanol to superfine potassium carbonate, and react at 30°C for 10 hours , filtered, and the filtrate was c...
Embodiment 2
[0034] Add 200g of potassium carbonate and 1200g of absolute ethanol into the grinder, close all the openings of the grinder, grind for 6 hours at room temperature, take a sample, test the average particle size of potassium carbonate by particle size distribution to be 200nm, and pour it out for later use.
[0035] Add 4-chlorophenylacetonitrile, ethyl bromoacetate, and superfine potassium carbonate into the reactor at a molar ratio of 1.00:1.20:2.0, and at the same time add methanol 10 times the mass of superfine potassium carbonate, react at 70°C for 4 hours, and filter , the filtrate was concentrated to give white solid 3-(4-chlorophenyl)-3-cyanopropionic acid ethyl ester, yield 95%, mp56~57°C.
[0036] The white solid 3-(4-chlorophenyl)-3-cyanopropionic acid ethyl ester and Raney nickel obtained in the above reaction were mixed in 3-(4-chlorophenyl)- Put the methanol of ethyl cyanopropionate into the dry hydrogenation kettle in turn, start stirring after sealing, first rep...
Embodiment 3
[0039] Add 300g of potassium carbonate and 1500g of absolute ethanol into the grinder, close all the openings of the grinder, grind for 8 hours at room temperature, take a sample, and test the average particle size of potassium carbonate by particle size distribution to be 230nm, pour it out for later use.
[0040] Add 4-chlorophenylacetonitrile, ethyl bromoacetate, and superfine potassium carbonate into the reactor at a molar ratio of 1.00:1.20:1.7, and at the same time add methanol 10 times the mass of superfine potassium carbonate, react at 60°C for 6 hours, and filter , and the filtrate was concentrated to obtain ethyl 3-(4-chlorophenyl)-3-cyanopropionate (II) as a white solid, yield 96%, mp 56-57°C.
[0041]Add 20 g of 3-(4-chlorophenyl)-3-cyanopropionic acid ethyl ester (II) and 100 ml of water obtained by the above reaction into a reactor equipped with a reflux device, stir carefully in batches at room temperature Add 12g of sodium borohydride (NaBH 4 ), continue to st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com