Patents
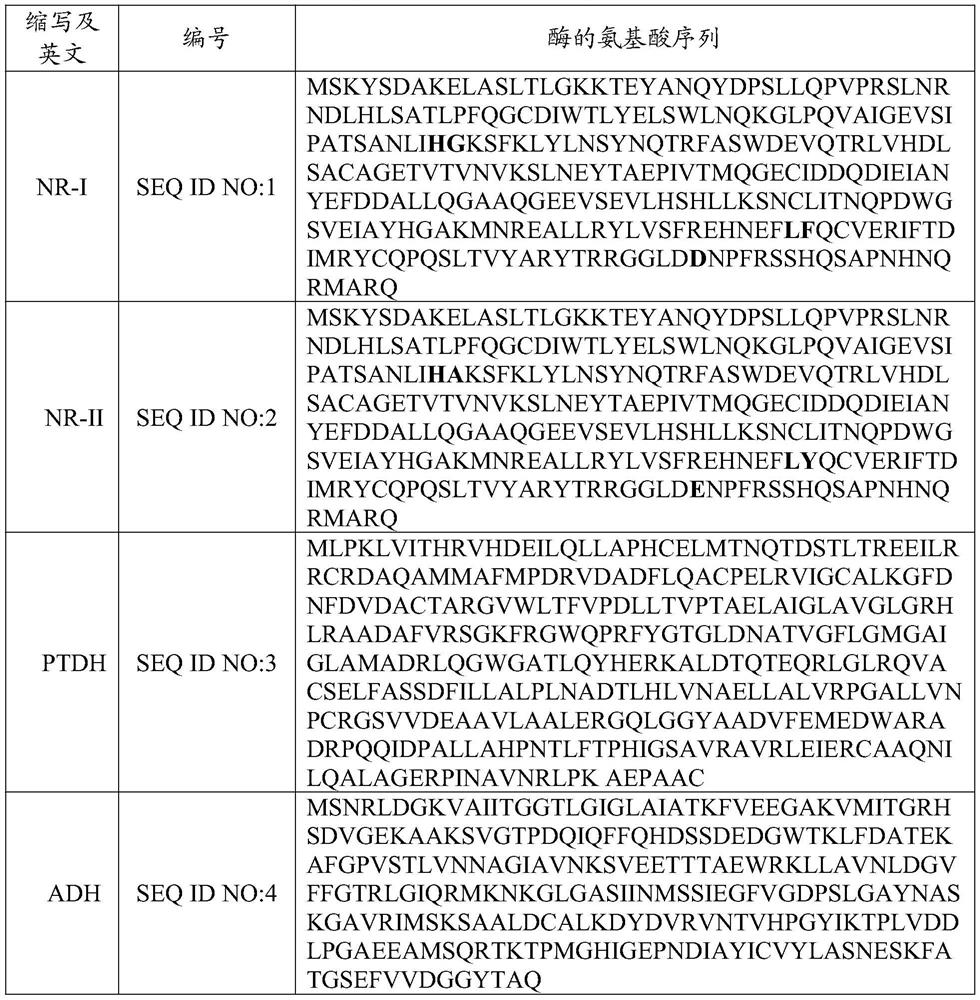
Literature
131 results about "Ethyl bromoacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ethyl bromoacetate is the chemical compound with the formula CH₂BrCO₂C₂H₅. It is the ethyl ester of bromoacetic acid and is prepared in two steps from acetic acid. It is a lachrymator and has a fruity, pungent odor. It is also a highly toxic alkylating agent and may be fatal if inhaled.
Sofalcone preparation method
InactiveCN1733682AFew synthetic stepsFew reaction stepsOrganic compound preparationCarboxylic compound preparationKetone2-Butene
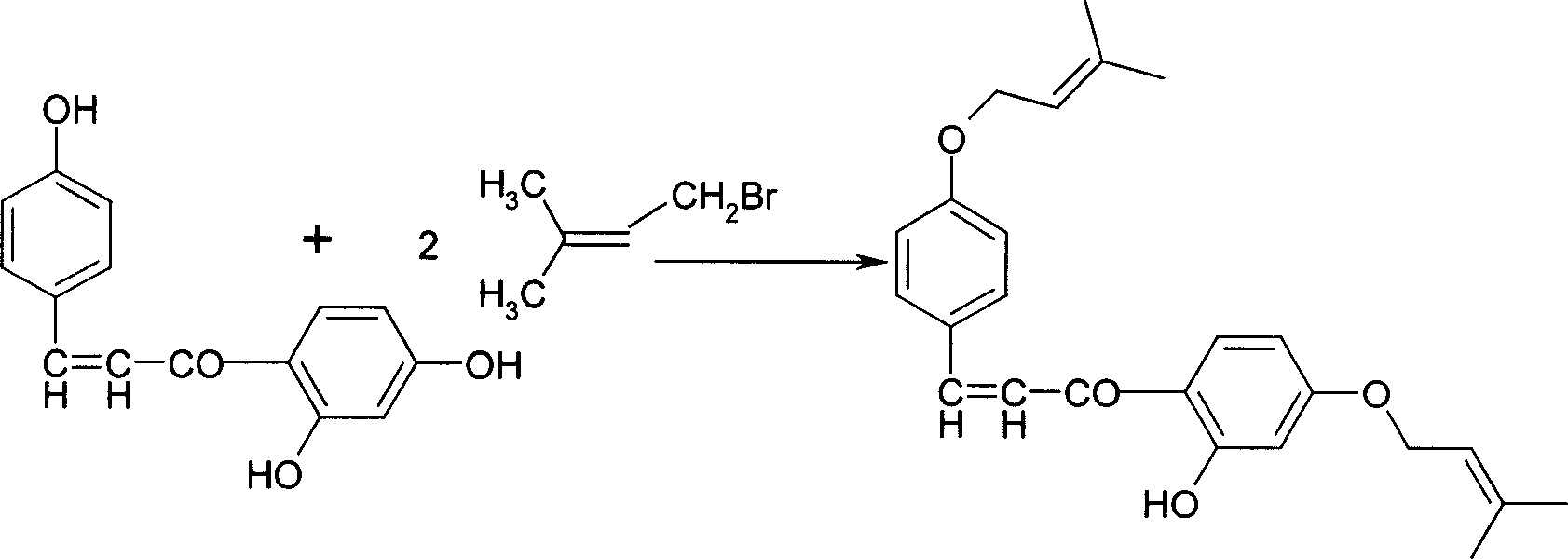
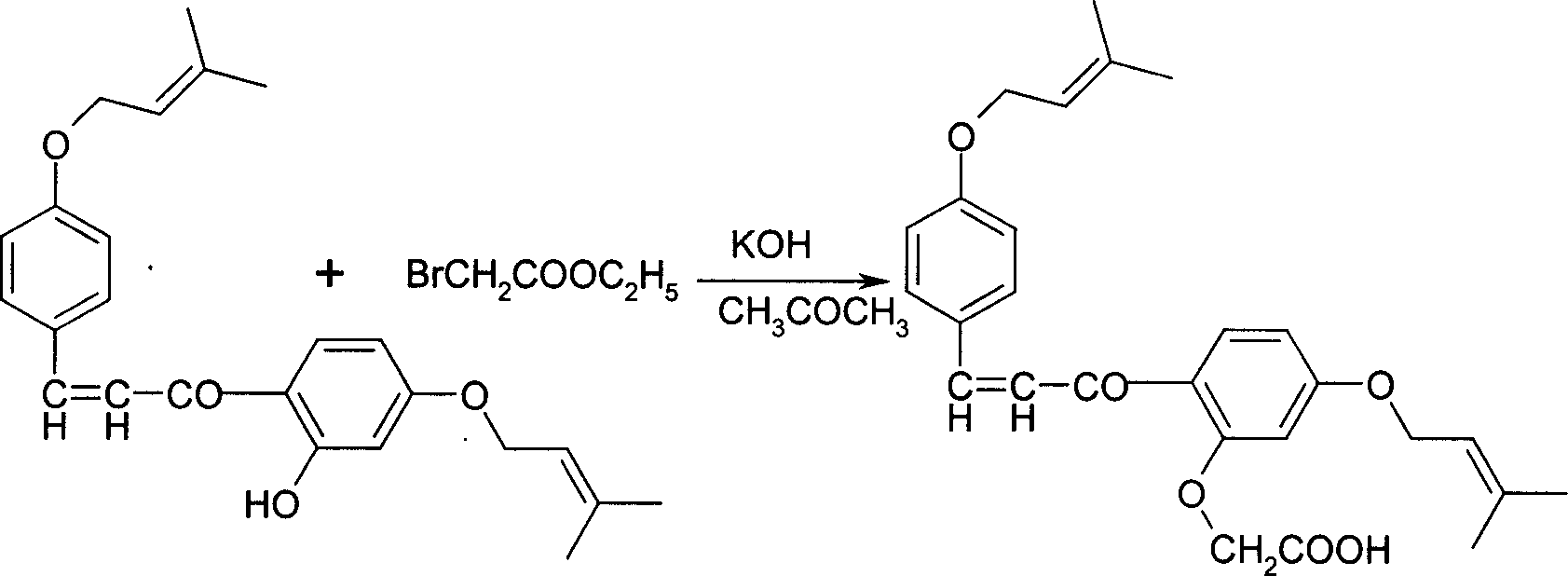
Disclosed is a Sofalcone preparation method which mainly comprises, (1) subjecting p-hydroxybenzaldehyde and 2,4-ihydroxyacetophenone to condensation reaction under alkaline condition, obtaining 2,4,4-Trihydro-xychalcone, (2) subjecting 2,4,4-trihydro-xychalcone and 1-bromo-3-methyl-2-butene to condensation reaction, obtaining 2-hydroxyl-4,4-bis (3-methyl-2-butenyloxy) chalcone, (3) subjecting the 2-hydroxyl-4,4-bis (3-methyl-2-butenyloxy) chalcone and ethyl bromoacetate to condensation under the action of potasium carbonate, thus obtaining sofalcone. The mol ratio of p-hydroxybenzaldehyde, 2.4-dihydroxyacetophenone, 1-bromo-3-methyl-2-butene, and ethyl bromoacetate can be 1 : (0.90-1.50) : (1.60-2.55) : (0.60-1.25).
Owner:阮华君
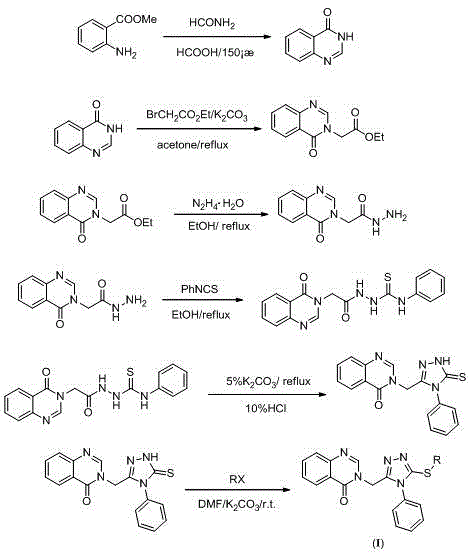
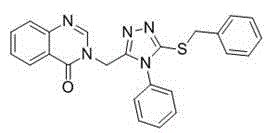
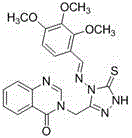
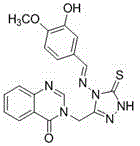
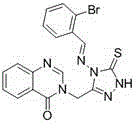
Quinazolinone compound containing 1, 2, 4-triazole thioether and synthesizing method and application of quinazolinone compound
Owner:北京传奇优声文化传媒有限公司
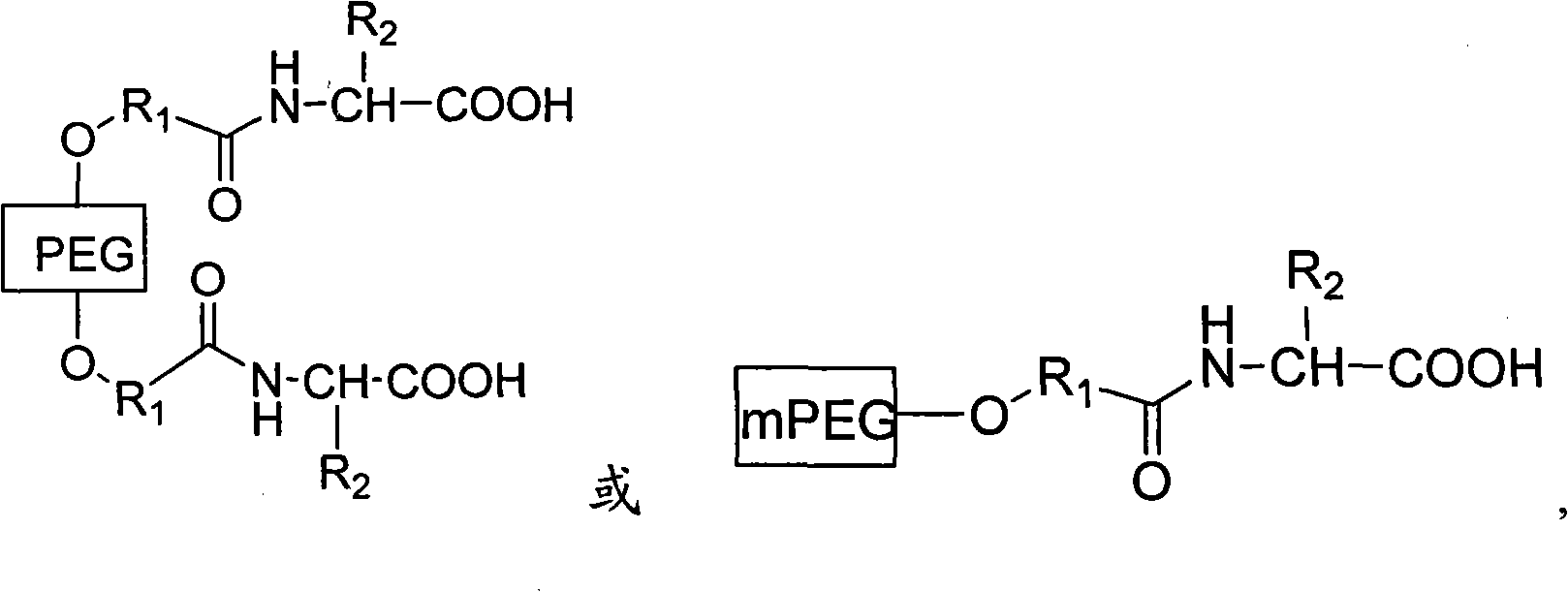
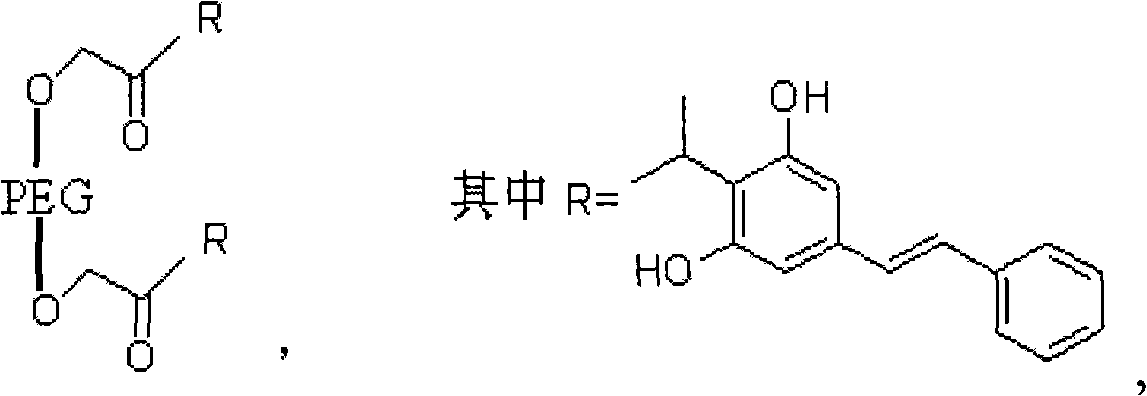
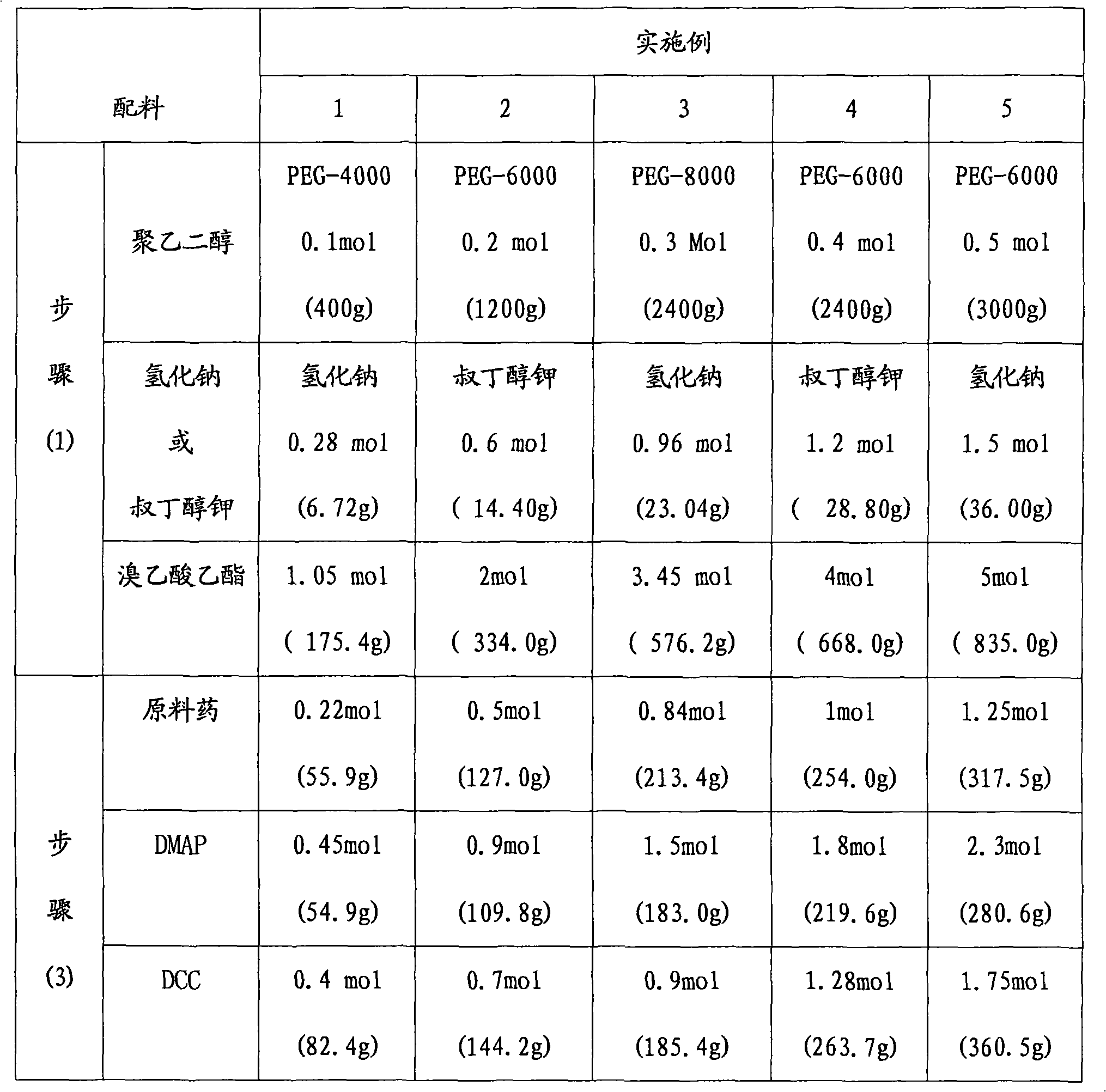
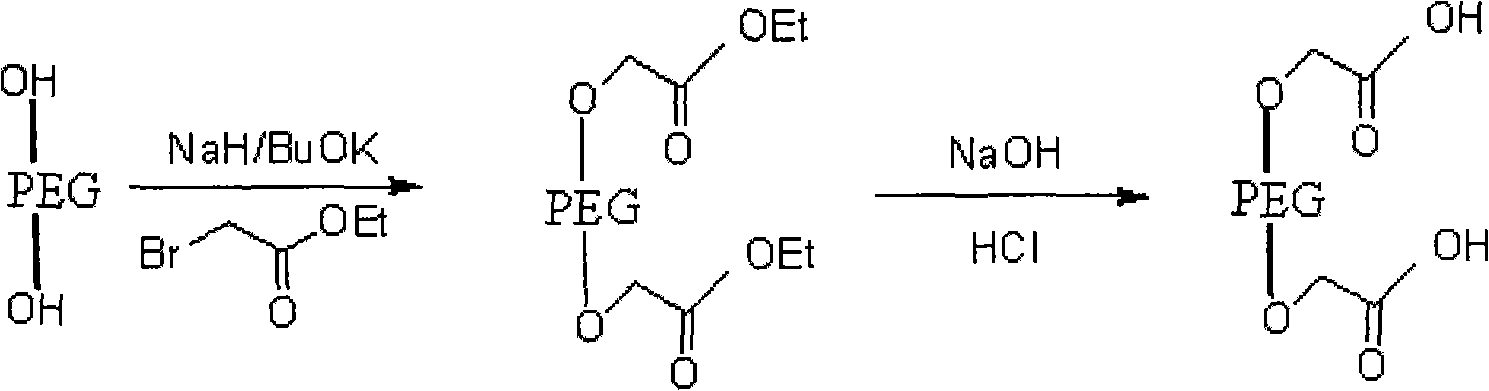
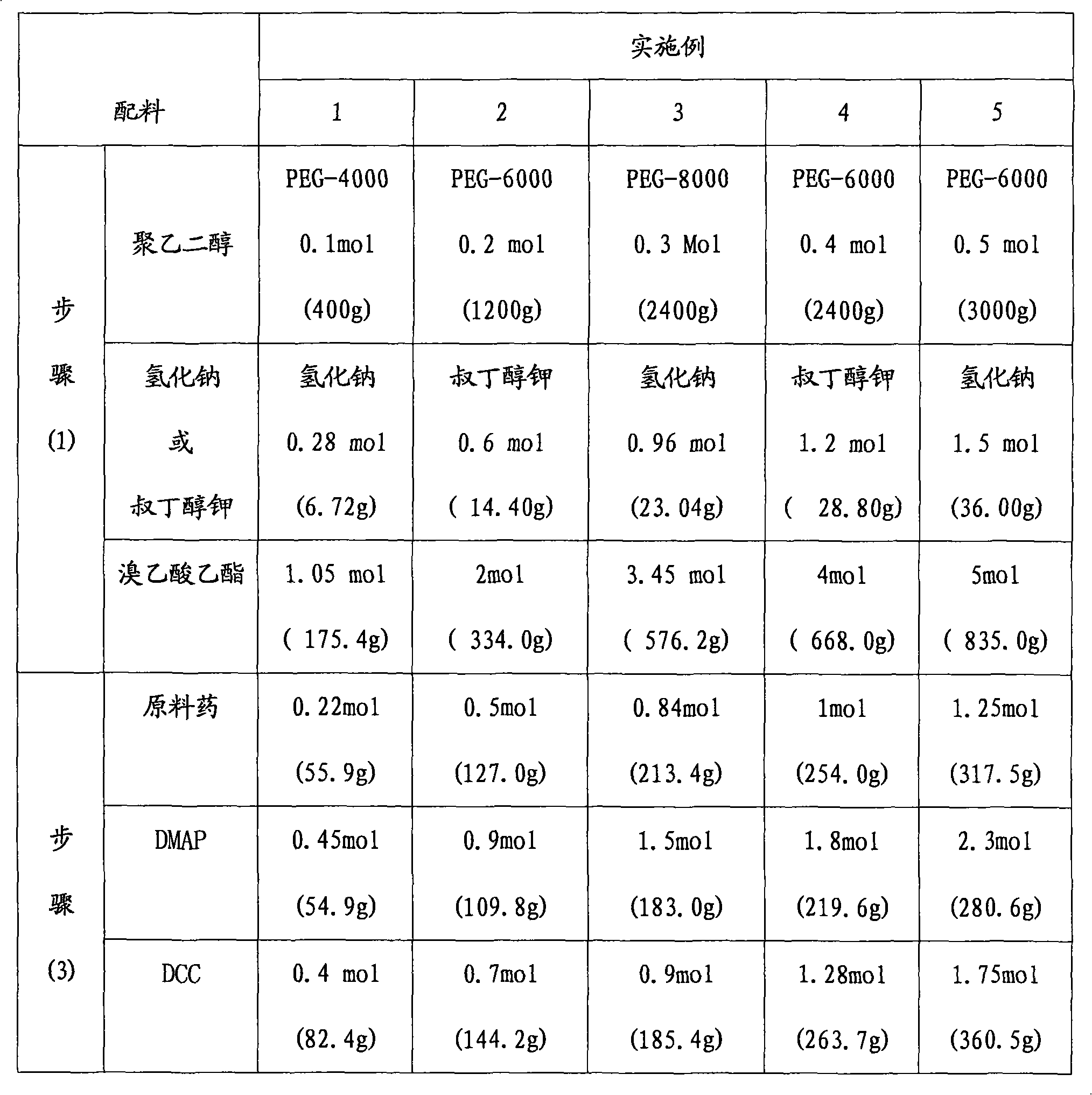
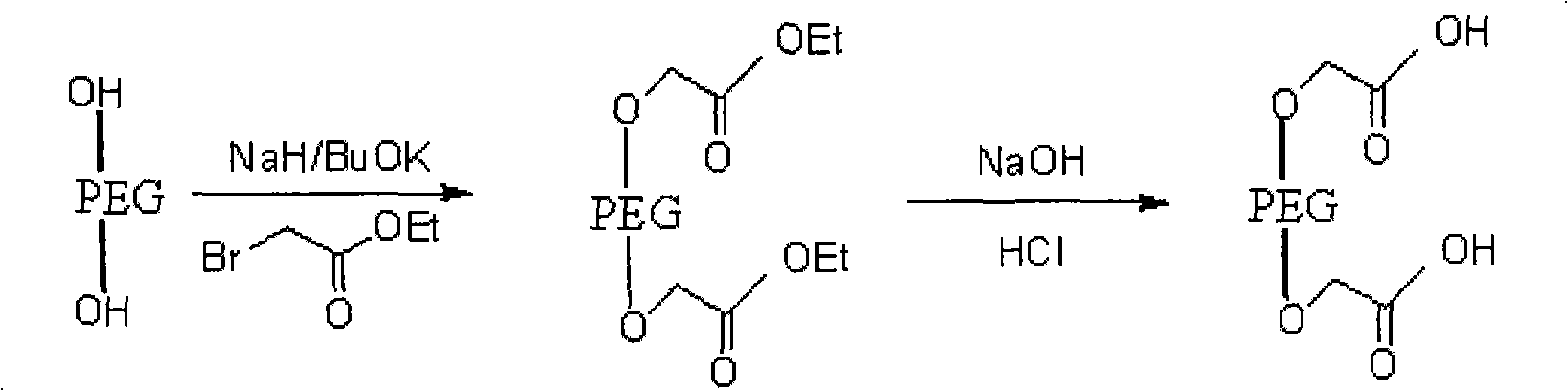
PEG (Polyethylene Glycol), mPEG (Methoxy Polyethylene Glycol) chemical modifier and method thereof for preparing water-soluble resveratrol prodrug
The invention discloses a PEG (Polyethylene Glycol) / mPEG (Methoxy Polyethylene Glycol) chemical modifier and a method thereof for preparing a water-soluble resveratrol prodrug. In the PEG / mPEG chemical modifier, PEG or mPEG is used as a vector, and the chemical modifier is prepared through reacting succinic anhydride, bromoacetate or isobutyl bromoacetate with amino acid. The preparation conditions and steps of the chemical modifier are simple. The chemical modifier is used for modifying the resveratrol to prepare the resveratrol prodrug, can change the water solubility and the stability of the resveratrol, is convenient to use and improves the bioactivity. When the prodrug is decomposed in vivo, amino acid required by the human body is also released while the resveratrol is released, thus the application scope of the prodrug is wider than that of the resveratrol.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Anti-morphine monoclonal antibody, cell strain for generating anti-morphine monoclonal antibody, morphine detection kit and manufacturing method thereof
ActiveCN103820394AMaintain structural specificityPromote productionTissue cultureImmunoglobulinsAntigenCross-link
Owner:HANGZHOU CLONGENE BIOTECH
Alpha-glycosidase inhibitor preparation method and purpose
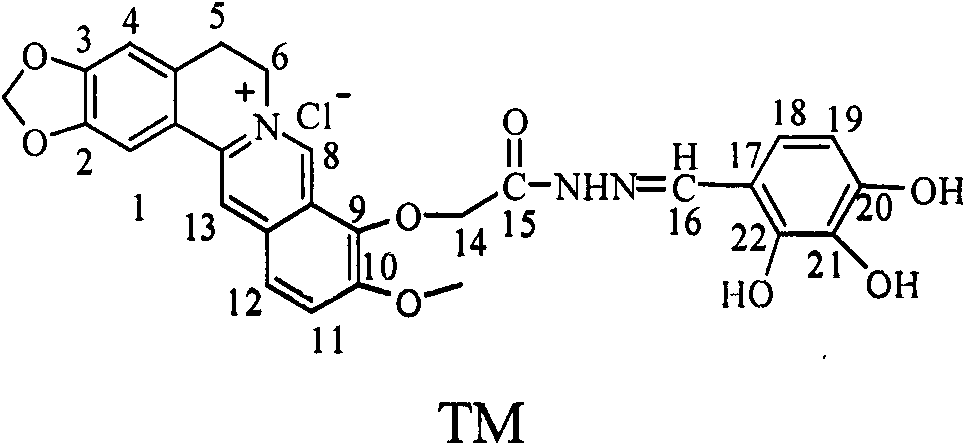
InactiveCN103159755AStrong inhibitory activityOrganic active ingredientsOrganic chemistryBenzaldehydeStructural formula
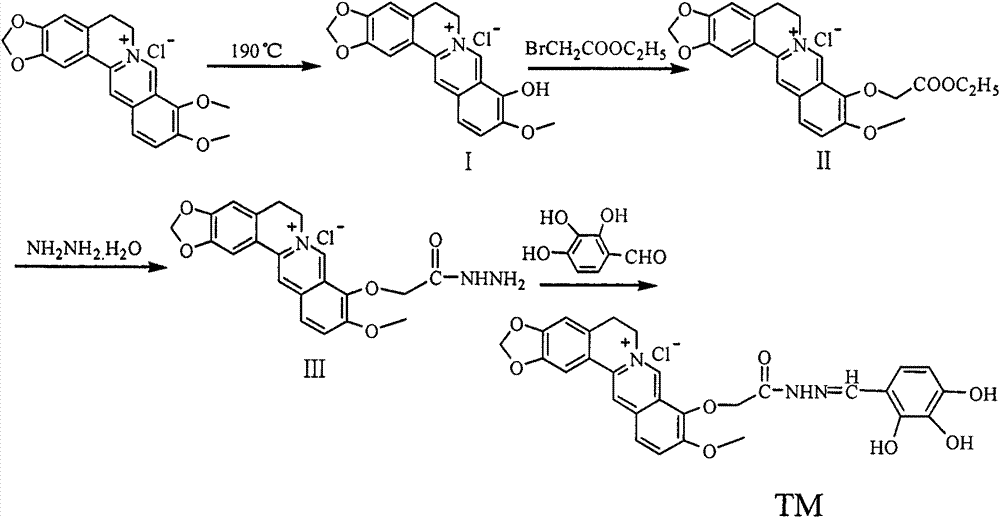
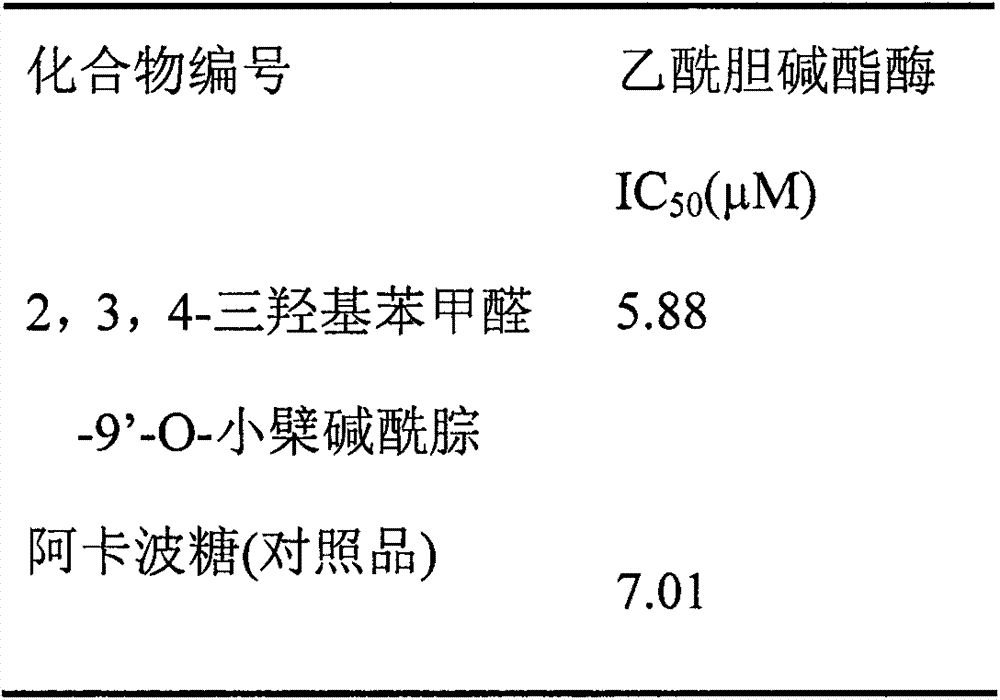
The invention discloses a preparation method of an alpha-glycosidase inhibitor 2,3,4-trihydroxy benzaldehyde-9'-O-berberine acylhydrazone, and can be used for preparing the medicines to treat diabetes. A structural formula of a compound is shown in the description. The preparation method comprises the following steps: 1) taking berberine hydrochloride as an initial raw material, demethylating under pressure reduction 190 DEG C condition, wherein the products can not be separated to obtain berberrubine (I), bridging the obtained compound I and alpha-ethyl bromoacetate through a nucleophilic substitution reaction to form a berberine ethyl acetate derivative (II); 2) performing hydrazinolysis of the compound II and hydrazine hydrate to obtain berberine hydrazides (III); and 3) performing nucleophilic addition-dehydration reaction on the compound III and the 2,3,4-trihydroxy benzaldehyde to obtain the 2,3,4-trihydroxy benzaldehyde-9'-O-berberine acylhydrazone (TM), and purifying the products through a recrystallization method. The 2,3,4-trihydroxy benzaldehyde-9'-O-berberine acylhydrazone has strong alpha-glucosidase inhibition activity through alpha-glucosidase inhibition experiment, and its inhibition performance is higher than that of a comparison product acarbose by 1.2 times.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Preparation and use of hydroxyl oximated calix[6]arene efficient uranium extractant
InactiveCN103242193AEasy extractionHigh selectivityOrganic chemistryHydroxylaminePotassium hydroxide
The invention discloses a synthetic method of hydroxyl oximated calix[6]arene suitable for efficient uranium extraction. The synthetic method is completed by two steps: firstly, performing reaction between calix[6]arene and ethyl bromoacetate under the catalytic effect of anhydrous potassium carbonate to obtain a calix[6]arene ethyl acetate derivative; and secondly, performing reaction between the calix[6]arene ethyl acetate derivative and hydroxylamine hydrochloride under the catalytic effect of potassium hydroxide to obtain hydroxyl oximated calix[6]arene. The method is simple and convenient in synthetic line, mild in reaction condition and easy to control and realize; and synthesized hydroxyl oximated calix[6]arene has efficient extraction performance and selective complexation performance to uranium and has wide application prospect in uranium separation, uranium-bearing wastewater treatment and uranium recovery.
Owner:NANHUA UNIV
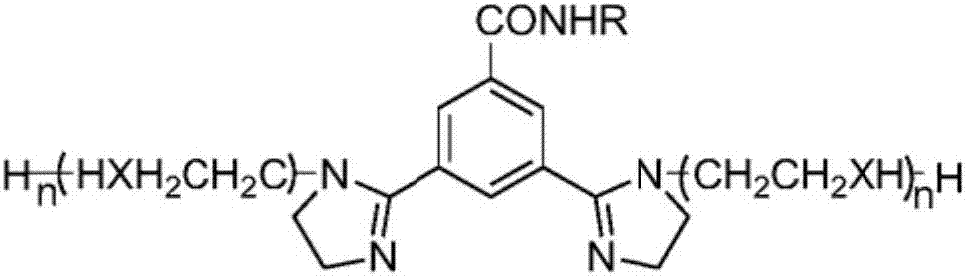
Benzotriazole imidazoline derivative, and preparation method and application thereof
InactiveCN107253945AHigh yieldReaction conditions are easy to controlOrganic chemistryAdditivesSodium chloroacetateDiethylenetriamine
The invention discloses an imidazoline group-containing benzotriazole derivative, and a synthesis process and application thereof. The synthesis process comprises the following steps: taking benzotriazole as a raw material, and reacting with sodium monochloroacetate / ethyl chloroacetate / ethyl bromoacetate (wherein the reacting yield of the sodium monochloroacetate is highest) to form benzotriazole acetic acid, and performing dehydration and cyclization reaction with diethylenetriamine / triethylenetetramine / tetraethylenepentamine to generate the benzotriazole imidazoline derivative. The reaction conditions are easy to control; and the yield is high. The benzotriazole imidazoline derivative has a good corrosion inhibiting effect on YG8 hard alloy and Q235 low-carbon steel in an acidic water environment.
Owner:EAST CHINA JIAOTONG UNIVERSITY
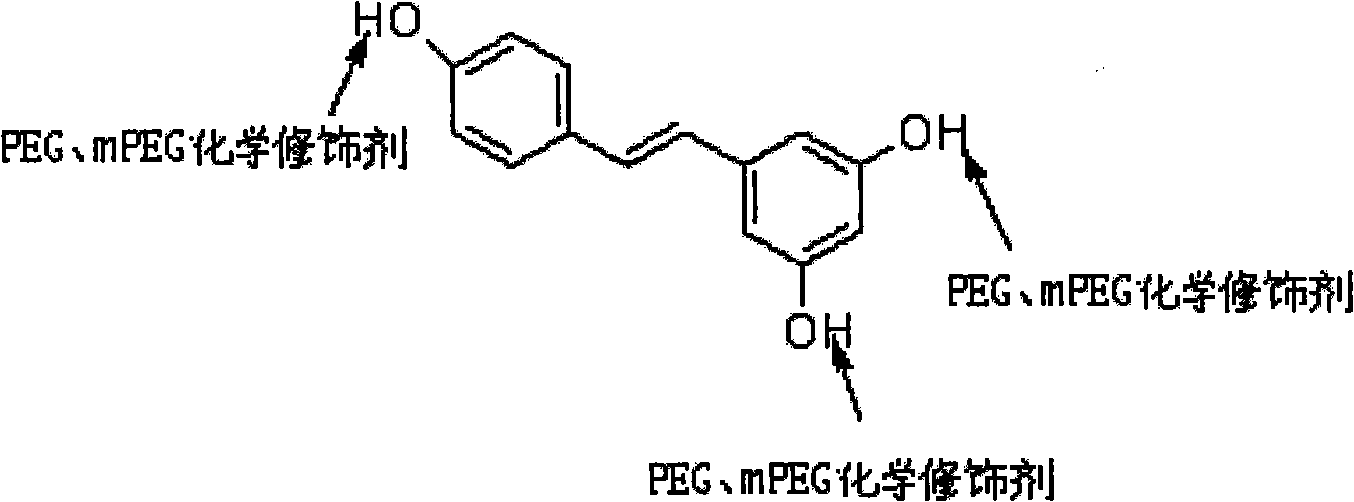
3, 5-dihydroxy-4-isopropyl diphenyl ethylene-ethyl bromoacetate-polyoxyethylene compound and synthetic method thereof
InactiveCN101564537AGood water solubilityGood biocompatibilityAntimycoticsHydroxy compound active ingredientsSolubilityPolyethylene glycol
The invention discloses a 3, 5-dihydroxy-4-isopropyl diphenyl ethylene-ethyl bromoacetate-polyoxyethylene compound, namely polyoxyethylene-di(acetic acid-4-isopropyl-5-hydroxy diphenyl ethylene-3-phenolic ester). The original medicine is 3, 5-dihydroxy-4-isopropyl diphenyl ethylene (tolylene maud); and the modifier is polyoxyethylene and the linker arm is ethyl bromoacetate. The synthetic method of the compound includes three reactions, namely esterification reaction, hydrolysis reaction and esterification reaction. Compared with the original medicine, the compound has enhanced water-solubility and stability; the internal medicine distribution is changed; the bioavailability is enhanced; the synthetic method is simple; the reaction condition is mild; and the cost is low, without numerous unpleasant experimental procedures. In the method, the 3, 5-dihydroxy-4-isopropyl diphenyl ethylene-ethyl bromoacetate-polyoxyethylene compound can be easily made.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Preparation method of organic/layered double hydroxide (LDH) complex
InactiveCN102225917AHigh crystallinityHigh affinityMaterial nanotechnologyOrganic chemistryCrystallinitySolvent
The invention provides a preparation method of an organic / layered double hydroxide (LDH) complex. The preparation method comprises the following steps of a) dissolving 1,10-dioxo-4,7,13, 16-tetranitro-18-crown-6 in a CH2Cl2 solvent, adding anhydrous potassium carbonate into the solution and mixing them, heating and refluxing the mixed solution, then adding ethyl bromoacetate into the mixed solution and refluxing it, then filtering and evaporating the refluxed solution to obtain an oil-like substance, adding hydrochloric acid solution into the oil-like substance, heating, refluxing and filtering the mixed solution, and evaporating the filtrate to make crystals be separated out to obtain 4,7,13,16-tetracarboxymethyl-1,10-dioxo-4,7,13, 16-tetraazaoctadecane (TECA), and b) mixing TECA, NaOH and methanamide, adding MgAl-NO3-LDH into the mixture, standing for 10 minutes to 16 hours, and then centrifuging, washing and drying the mixture to obtain a TECA / LDH complex, wherein a mass ratio of TECA to MgAl-NO3-LDH is 1. Through the preparation method, a TECA / LDH complex can be prepared in a short reaction time thus a production period of the TECA / LDH complex is saved; corrosive effects of methanamide on LDH laminates are reduced and compositions of the laminates are maintained well; and a crystallinity and a yield of complex products are improved.
Owner:BEIJING NORMAL UNIVERSITY +1
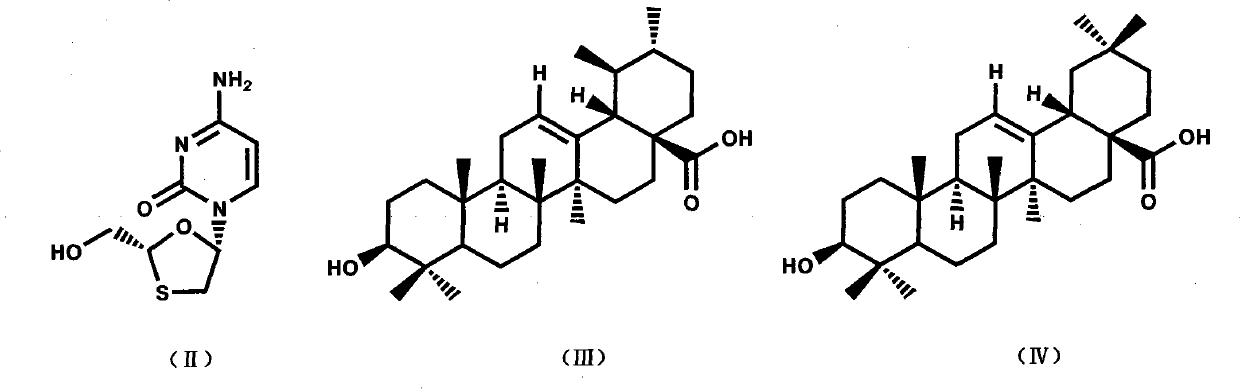
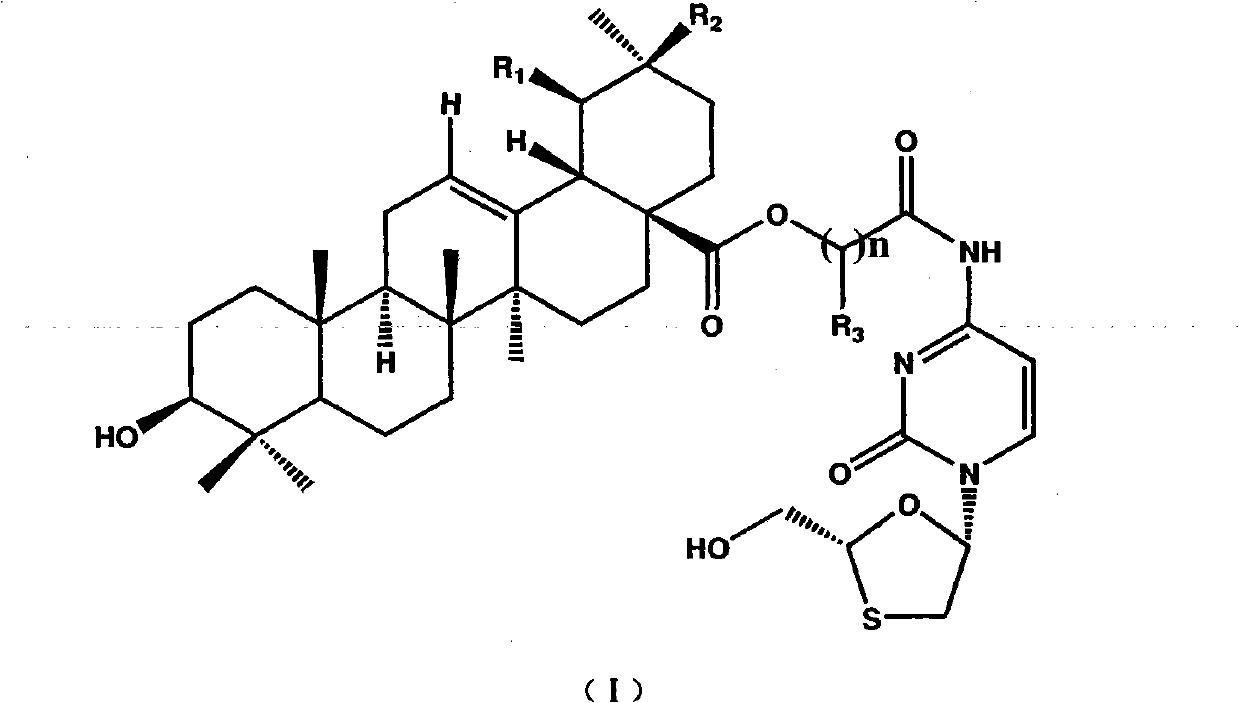
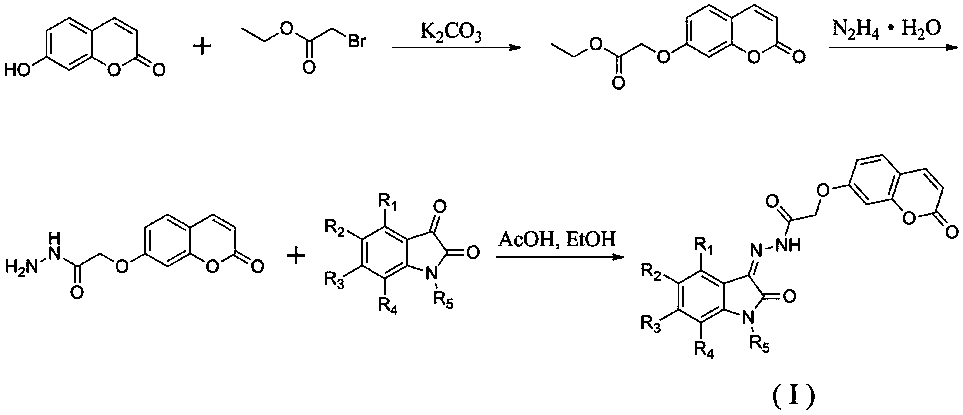
Preparation method and applications of lamivudine twin drug
InactiveCN101766632AHas antiviral effectImprove liver functionOrganic active ingredientsDigestive systemHepatic inflammationPropanoic acid
The invention provides a preparation method and applications of a lamivudine twin drug. Lamivudine, and ursolic acid or oleanolic acid are condensed into the lamivudine-ursolic acid or lamivudine-oleanolic acid twin drug at a low temperature by using ethyl chloroacetate (ethyl bromoacetate) or ethyl chloropropionate (ethyl bromopropionate) as the linking group. The twin drug possibly has a dual-action mechanism; and the twin drug has the action of antivirus, and also has the actions of resisting inflammations, protecting the liver cell membranes, improving the liver function and resisting fibrosis. Thus, the invention organically combines the antivirus therapy and the liver protection treatment and provides a new concept for the research and development of drugs for treating hepatitis.
Owner:CHINA PHARM UNIV
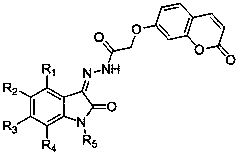
Coumarin-isatin type compounds, and preparation method and purpose thereof
The invention discloses a kind of coumarin-isatin type compounds, and a preparation method and a purpose thereof. The compounds possess a structure shown as a formula (I). The preparation method comprises taking 7-hydroxycoumarin and ethyl bromoacetate as raw materials to obtain [(2-oxo-2H-chromen-7-yl)oxy]acetic acid, and then performing hydrazinolysis reaction to obtain [(2-oxo-2H-chromen-7-yl)oxy]acetohydrazide, and finally reacting with various substituted isatins to obtain the corresponding target compounds. The compounds can be used as a raw material of an anti-diabetes medicament, and the preparation method is simple and easily-available in raw material and convenient to operate.
Owner:贺州市八步区市场监督管理局
Synthesis method of 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid
InactiveCN103965191AReaction raw materials are readily availableReasonable priceOrganic chemistry2-amino-5-bromopyridineOrganic synthesis
The invention belongs to the field of organic synthesis, and particularly relates to a synthesis method of 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid. The method comprises the following steps: reacting N,N-dimethylformamidodimethyl acetal with 2-amino-5-bromopyridine at 40-100 DEG C to obtain an intermediate, and reacting the intermediate with ethyl bromoacetate at 60-160 DEG C for 3-15 hours; after the reaction finishes, cooling to room temperature, and concentrating by rotary evaporation to obtain an ethyl 6-bromoimidazo[1,2-alpha]pyridyl-3-formate crude product; and under the action of an alkali, carrying out hydrolysis reaction on the ethyl 6-bromoimidazo[1,2-alpha]pyridyl-3-formate in a certain solvent for 1-5 hours, neutralizing with hydrochloric acid, filtering, washing with water, and drying to directly obtain the 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid pure product. The method has the advantages of accessible reaction raw materials, reasonable price, mild reaction conditions and simple after-treatment, and is easy to operate and control; and the product has the advantages of stable quality and high purity.
Owner:SHANDONG YOUBANG BIOCHEM TECH
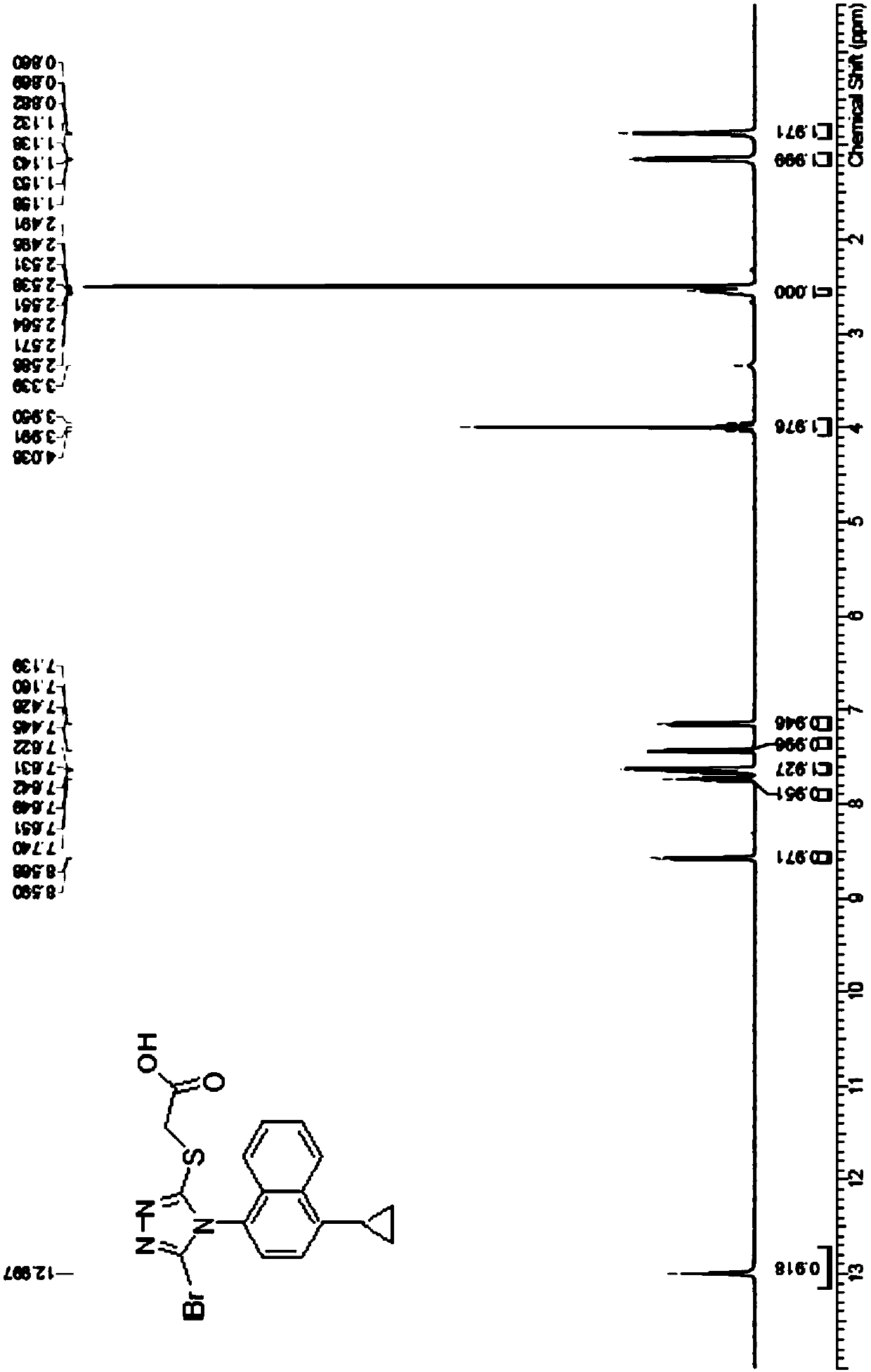
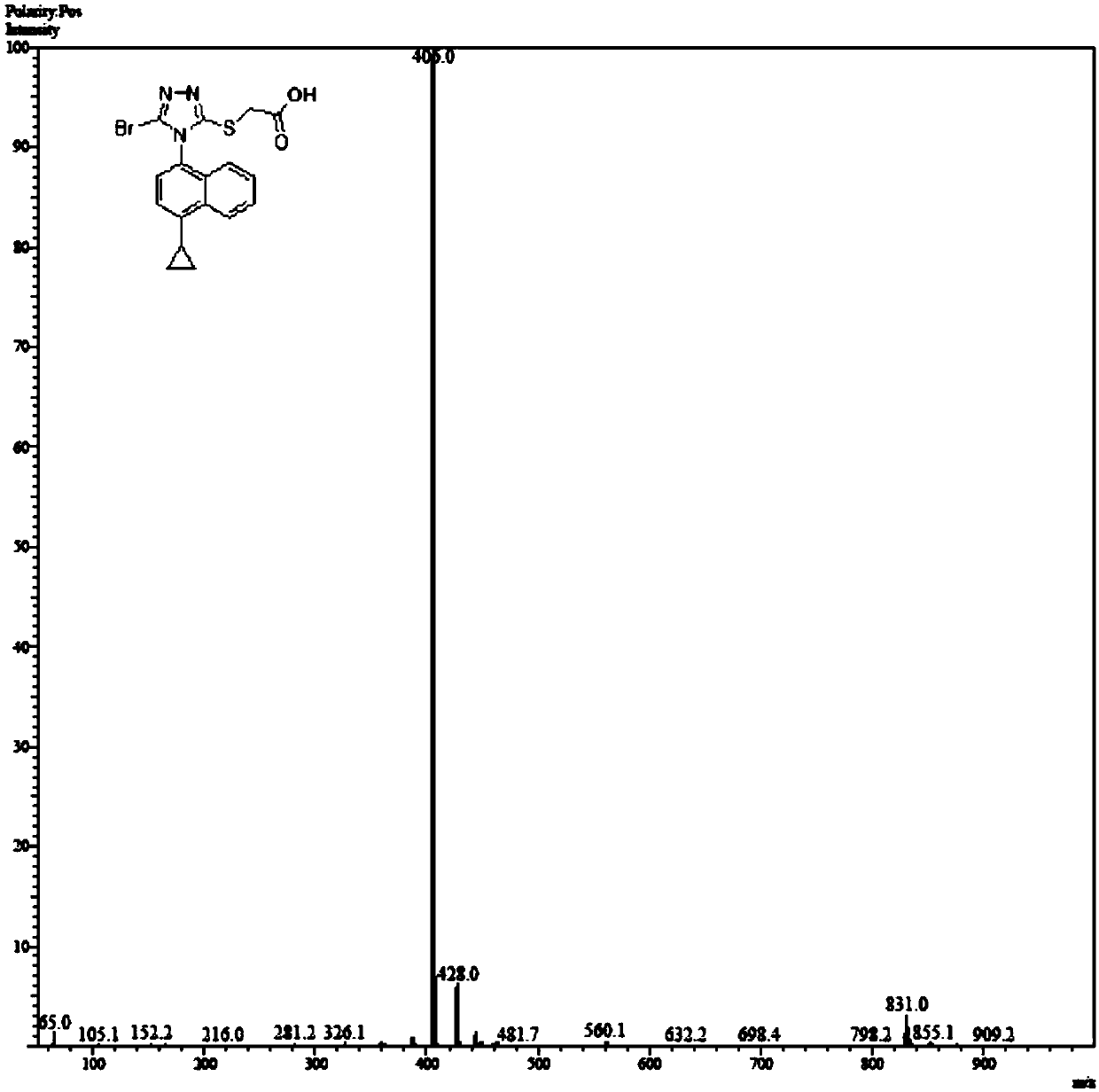
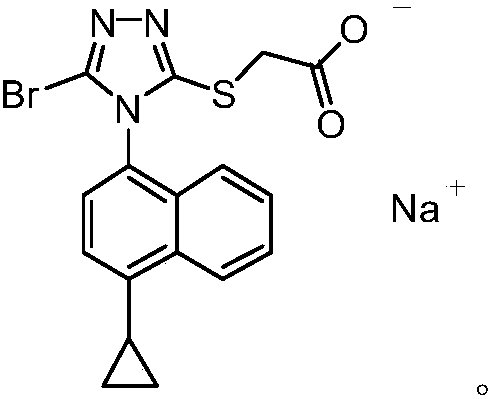
Refining method of lesinurad
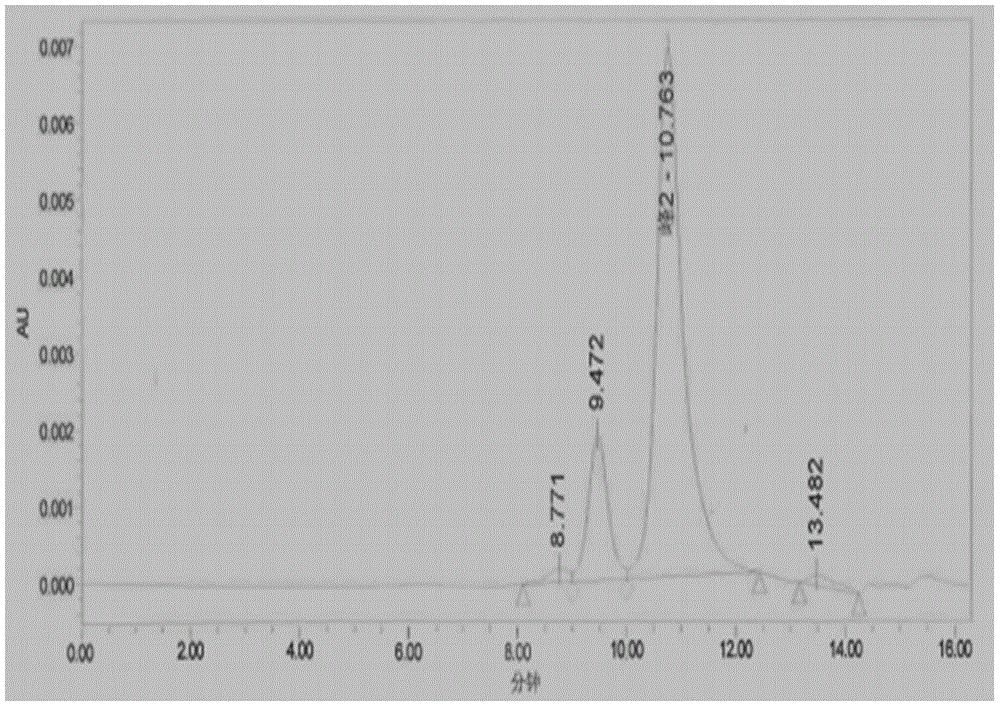
The invention discloses a synthetic refining method of lesinurad. The method includes: taking 4-(4-cyclopropyinaphthalene-1-yl)-1H-1, 2, 4-triazole-5(4H)-mercaptan as the starting raw material, carrying out alkylation reaction with ethyl bromoacetate, bromination reaction with N-bromosuccinimide, sodium hydroxide hydrolysis, hydrobromic acid neutralization, refining and drying to successfully synthesize a high purity lesinurad bulk drug finished product, and purifying the intermediates of all steps and the product, thus obtaining the high purity final product meeting the requirements. The method provided by the invention is easy for product collection, and is suitable for industrial production.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
Green synthesis method of ethyl bromodifluoroacetate
ActiveCN107400053ASolving Recycling ProblemsHigh yieldPreparation from carboxylic acid halidesPreparation by hydrogen halide split-offSynthesis methodsHigh pressure
The invention provides a green synthesis method of ethyl bromodifluoroacetate. According to the method, 1,1-difluoro-1,2-dichloroethane is used as a starting material; elimination reaction is performed to obtain 1,1-difluoro-2-chloroethylene; the 1,1-difluoro-2-chloroethylene and bromine are subjected to addition to obtain 1,1-difluoro-1,2-dibromo-2-chloroethane; then, the elimination reaction is performed to obtain a 1,1-difluoro-2-bromine-2-chloroethylene; then, the 1,1-difluoro-2-bromine-2-chloroethylene and the bromine are subjected to addition to obtain 1,1-difluoro-1,2,2-tribromo-2-chloroethane; then, sulfur trioxide is used for oxidizing the 1,1-difluoro-1,2,2-tribromo-2-chloroethane; 1,1-difluoro-1-bromoacetyl chloride is obtained; finally, the 1,1-difluoro-1-bromoacetyl chloride and ethyl alcohol are esterified to obtain 2,2-difluoro-2-ethyl bromoacetate. The synthesis method has the advantages that the problems of recovery and utilization of waste materials of 1,1-difluoro-1,2-dichloroethane (R132b) are solved; no organic solvents are used in the reaction process; the oxidation step uses SO3 for oxidation; high temperature and high pressure or concentrated sulfuric acid is not needed; the safety is enhanced; the discharging of waste acid is reduced; the green production requirement is met; the product yield is relatively high; the green synthesis method is suitable for industrial production.
Owner:山东飞源新材料有限公司
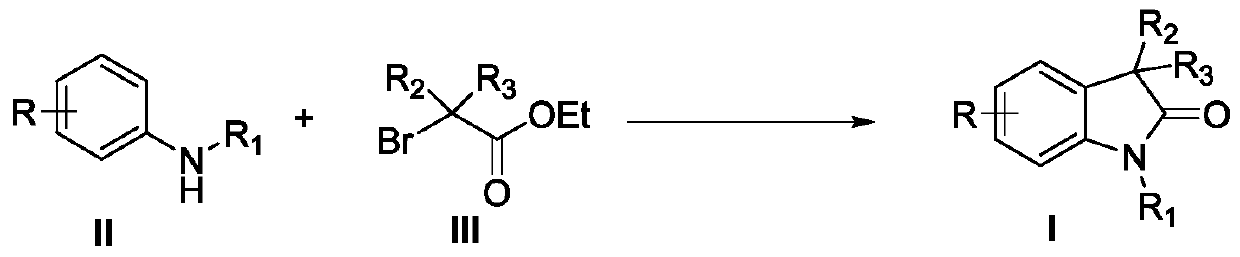
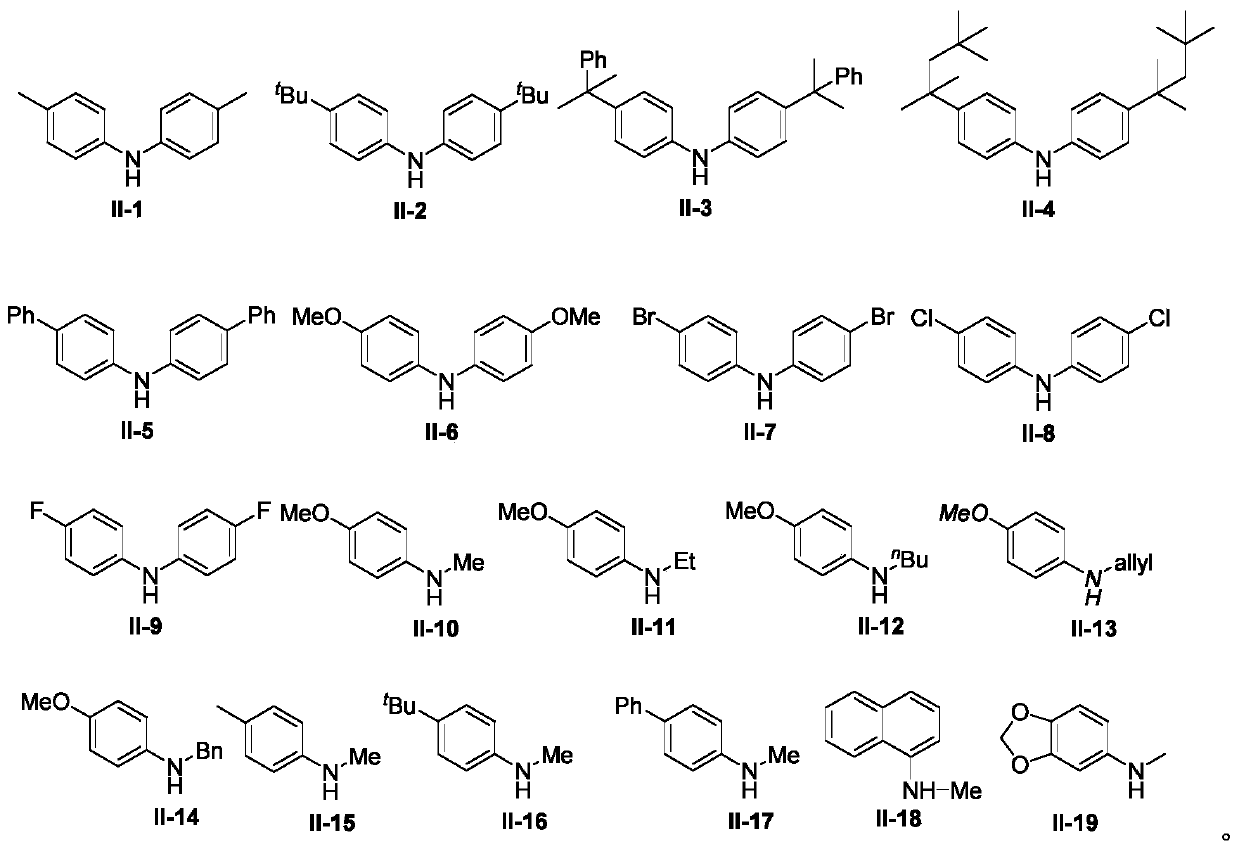
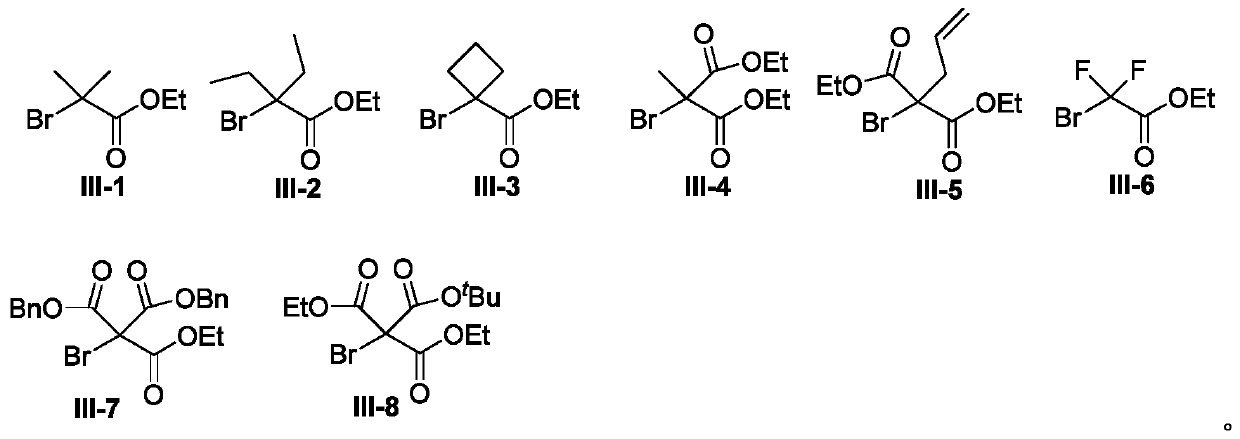
Synthesis method of 3,3'-disubstituted-2-indolone compound
The invention discloses a synthesis method of a 3,3'-disubstituted-2-indolone compound. The synthesis method comprises the following steps: with an aniline compound as a raw material, directly carrying out a reaction with ethyl bromoacetate compound for obtaining the 3,3'-disubstituted-2-indolone compound. The method has the advantages of economy, convenience, easily available raw material sources, wide reaction substrate application range and high target product yield.
Owner:NANCHANG HANGKONG UNIVERSITY
Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate
InactiveCN109503624AReasonable reaction process designMethod route shortOrganic chemistryNonaneSolvent
The invention relates to a method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate, and mainly solves a technical problem of absence of a method suitable for industrialsynthesis at present. The method provided by the invention comprises the following three steps: step one, firstly enabling a compound 1, paraformaldehyde and tetrabutylammonium fluoride added into asolvent N, N-dimethylformamide to be in reaction to obtain a compound 2; step two, enabling the compound 2, ethyl bromoacetate and cesium carbonate to be in reaction in acetone to obtain a compound 3;and step three, enabling the compound 3 and iron and ammonium chloride to be in reaction in ethanol and water to obtain a final compound 4, wherein a reaction formula is as shown in the Specification.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
Synthetic method and application of quinazolinone compounds containing 1,2,4-triazolethione Schiff base
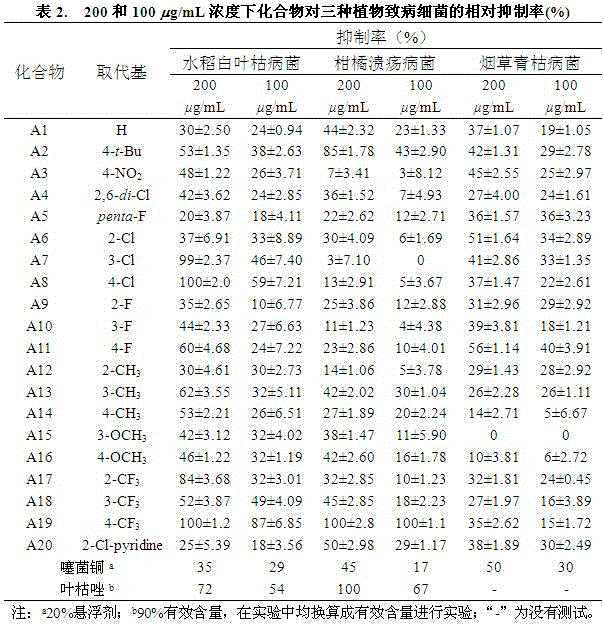
The invention discloses a preparation method and a bacteriostatic activity of plant pathogen prevention and treatment compounds quinazolinone compounds containing a 1,2,4-triazolethione Schiff base, and concretely relates to compounds represented by general formula (I), and a preparation method thereof. The quinazolinone target compounds containing the 1,2,4-triazolethione Schiff base are synthesized from methyl ortho-aminobenzoate, methanamide, ethyl bromoacetate, hydrazine hydrate, carbon disulfide, potassium hydroxide and aromatic aldehyde through the steps of ring closure, alkylation, hydrazinolysis, salt formation, ring closure and a Schiff base reaction. Compounds F11 and F19 have better Xanthomonas oryzae and Xanthomonas axonopodis pv. citri inhibition activity than a contrast medicate bismerthiazol under 200mg / mL or 100mg / mL; and the synthesized compounds have medium to excellent Sclerotinia scleotiorum inhibition activity, and compounds F4, F5, F6, F8, F9, F17 and F25 have broad-spectrum inhibition activity on six test fungi.
Owner:GUIZHOU UNIV
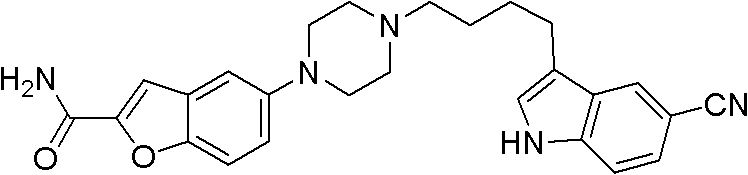
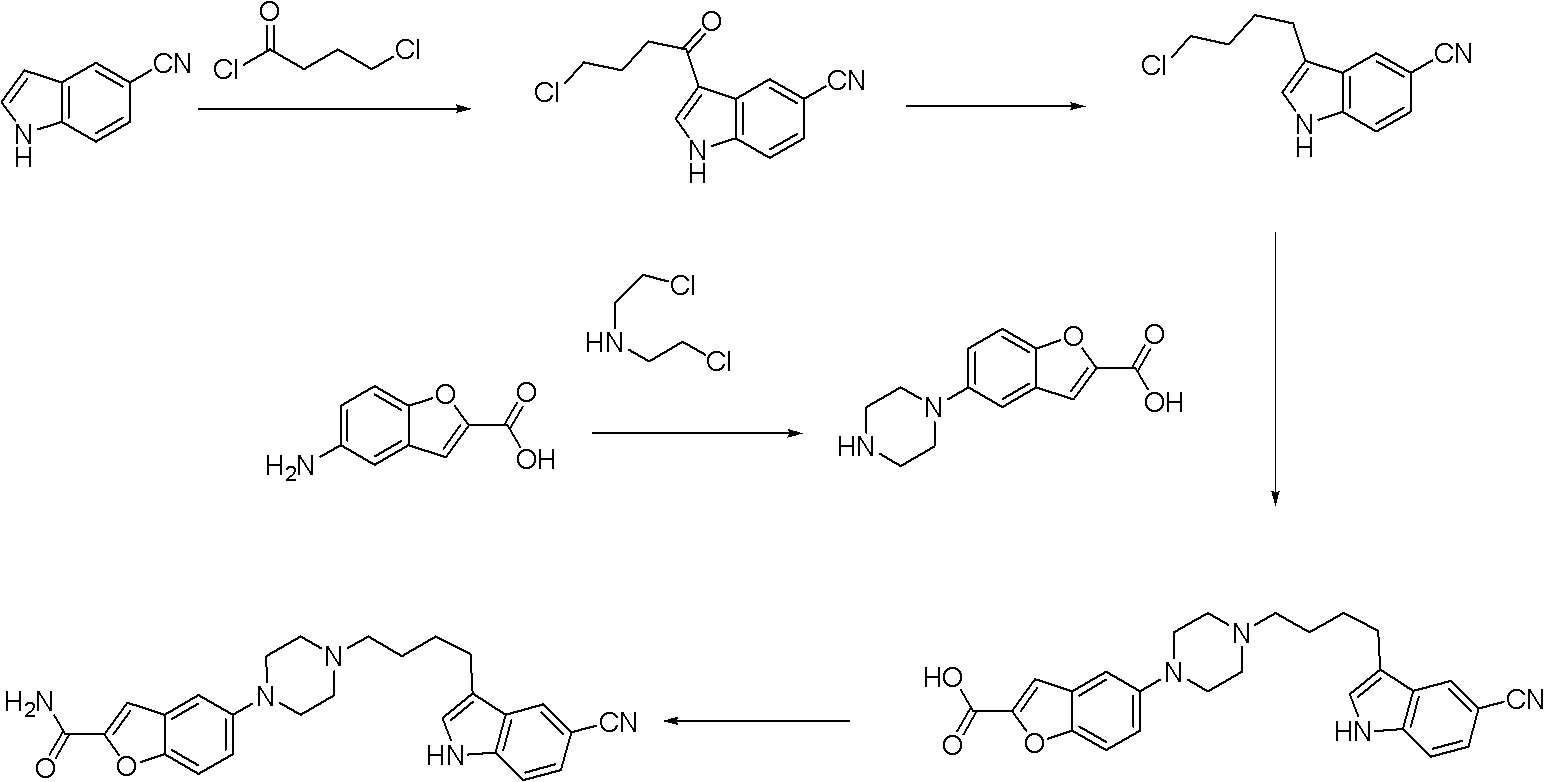
Synthesis method for antidepressant drug vilazodone
InactiveCN103159749AOvercome the disadvantage of low yieldOrganic chemistrySynthesis methodsCarboxylic acid
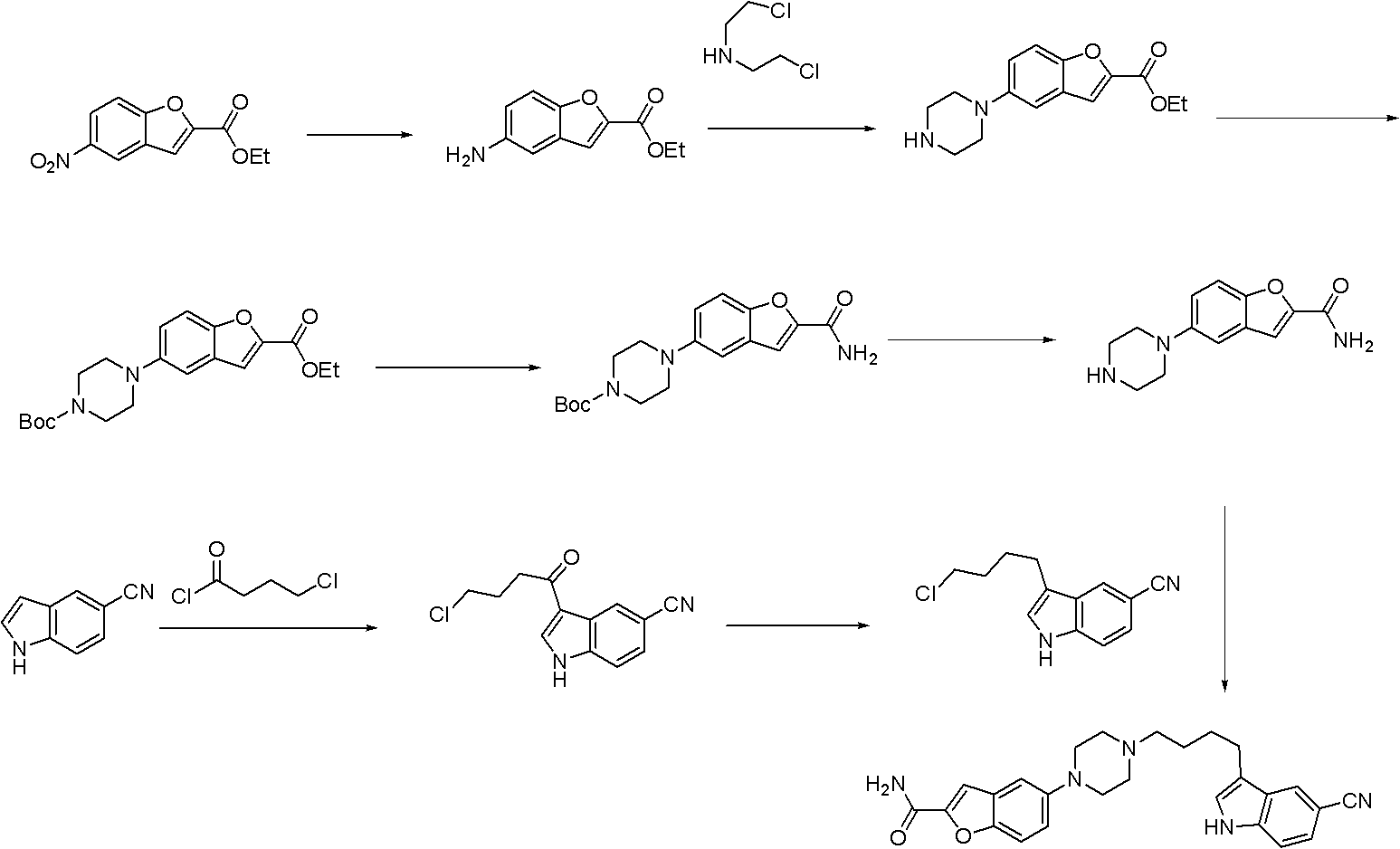
The present invention provides a synthesis method for an antidepressant drug vilazodone, belonging to the technical field of drug synthesis, and the method comprises the steps of: reacting 5-nitrosalicylaldehyde as a raw material with ethyl bromoacetate under the action of potassium carbonate by heating, to obtain a compound 5-nitrobenzofuran-2-carboxylic acid via hydrolysis; adding in aqueous ammonia after the reaction between 5-nitro-benzofuran-2-carboxylic acid and isobutyl chloroformate is completed, to give 5-nitrobenzofuran-2-carboxamide; reducing the 5-nitrobenzofuran-2-carboxamide by using sodium hydrosulfite to give an intermediate 5-aminobenzofuran-2-carboxamide; cyclizing the 5-aminobenzofuran-2-carboxamide with bis(2-chloroethyl)amine in the action of an alkaline to give 5-piperazinyl-benzofuran-2-carboxamide; and subjecting the 5-piperazinyl-benzofuran-2-carboxamide and 3-(4-chloro-butyl)-5-cyano indole to a substitution reaction to obtain the vilazodone. According to the synthesis method of the invention, the raw materials are cheap, and the reaction process is simple.
Owner:南京正济医药销售有限公司
Anti-cocaine monoclonal antibody, cell line capable of secreting same and preparation method
InactiveCN107988168AMaintain structural specificityPromote productionTissue cultureImmunoglobulinsAntigenMicrobiology
The invention provides a hybridoma cell line COC 2B1 which is capable of producing an anti-cocaine monoclonal antibody and preserved in CCTCC (China Center for Type Culture Collection) with the preservation number being NO.C201727. The invention further provides the anti-cocaine monoclonal antibody secreted by the hybridoma cell line COC 2B1, a kit for detecting cocaine in urine as well as a detection method and a production method of the cocaine detection kit. The invention has the following beneficial effects: ethyl bromoacetate as a crosslinking agent is used for activating cocaine, and anobtained cocaine artificial antigen keeps structural specificity of cocaine. A continuous immunization method is adopted, antigen dosage is saved effectively, immunization time is shortened, secretionyield of the hybridoma cell line COC 2B1 is high, and the cocaine monoclonal antibody secreted by the hybridoma cell line COC 2B1 has the characteristics of high affinity, high specificity and high sensitivity. The detection product has more advantages in aspects of sensitivity, specificity, detection limit and the like.
Owner:HANGZHOU CLONGENE BIOTECH
Method for synthesizing hydrochloric acid baclofen
InactiveCN102351726AGuaranteed sourceInhibit side effectsOrganic compound preparationAmino-carboxyl compound preparationChlorobenzeneBACLOFEN HYDROCHLORIDE
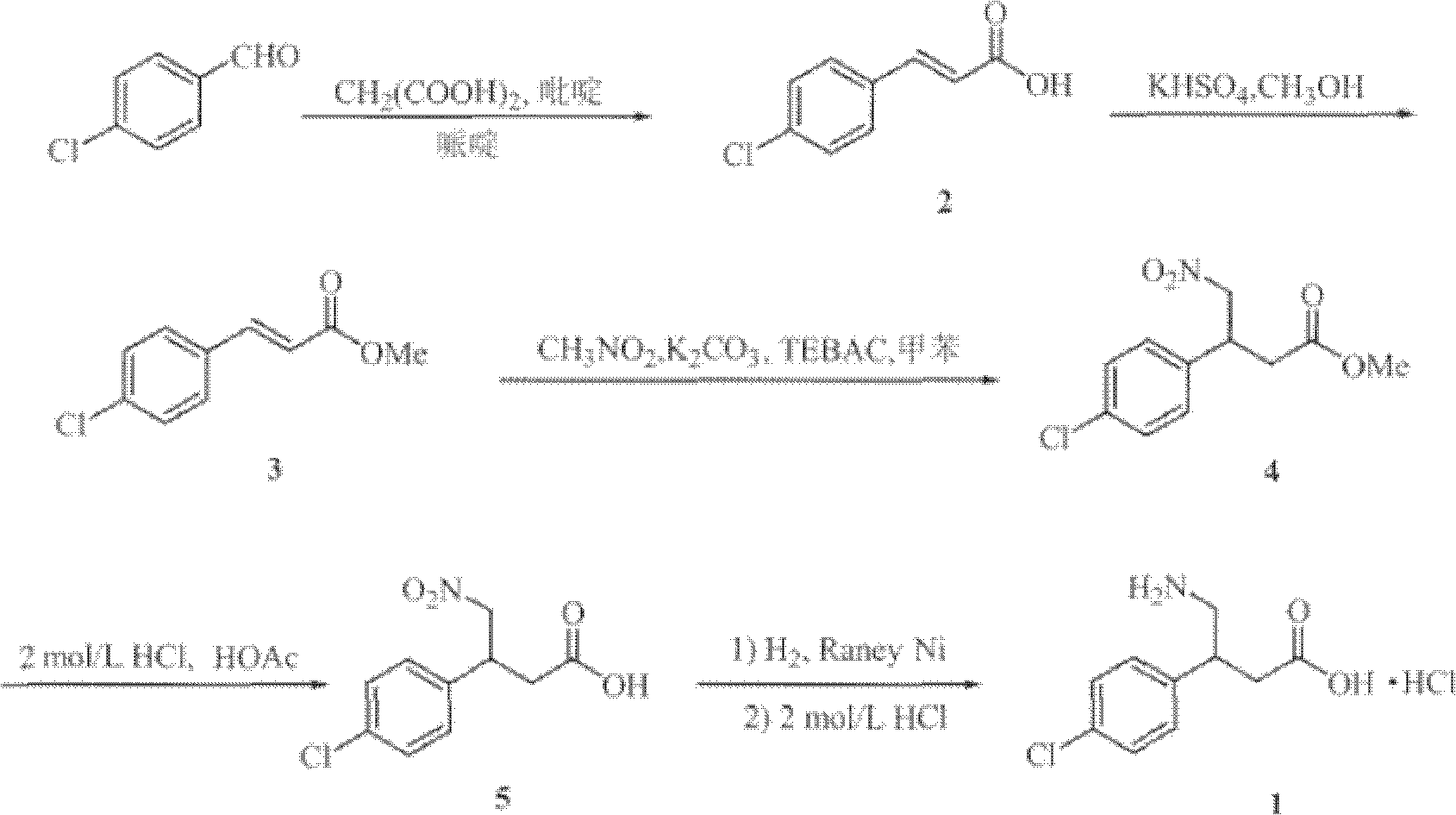
The invention relates to a novel method for synthesizing medicament hydrochloric acid baclofen. The method comprises the following steps of: adding 4-chlorobenzene acetonitrile, bromoacetate and ultrafine potassium carbonate into C1-C4 low carbon alcohol, reacting at the temperature of 30-70 DEG C, filtering and removing a potassium salt, and concentrating the filtrate to obtain a white solid, i.e., 3-(4-chlorphenyl)-3-cyan ethyl propionate (II) of which the melting point is 56-57 DEG C; undergoing a hydrogenation reduction reaction on the white solid (II) to obtain a white solid, i.e., 4-(4-chlorphenyl)-2-pyrrolidone (III) of which the melting point is 109-111 DEG C; and refluxing the white solid (III) in a hydrochloric acid aqueous solution for 8-30 hours, concentrating under reduced pressure to obtain hydrochloric acid baclofen when finishing the reaction , and recrystallizing by using isopropanol to obtain refined hydrochloric acid baclofen of which the melting point is 178-179 DEG C. Raw materials used in the method are readily available, the process is easy and reliable, and the total yield of the hydrochloric acid baclofen is up to 59 percent; and the method has a good industrial prospect.
Owner:HEBEI UNIV OF TECH +1
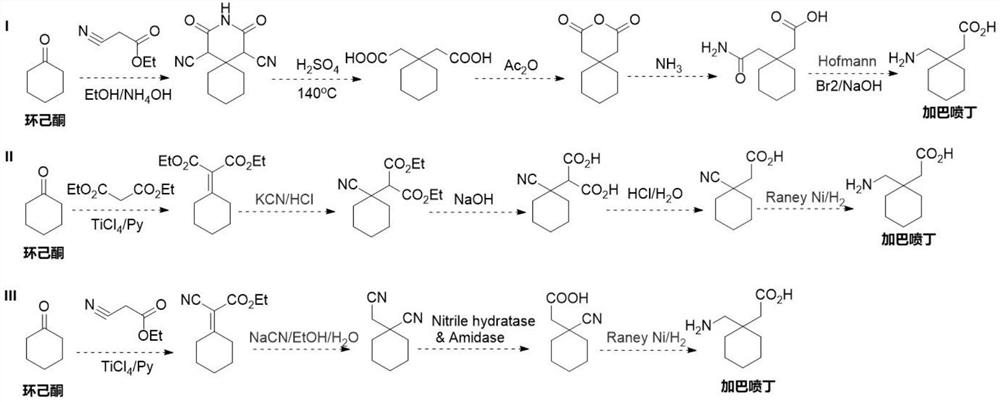
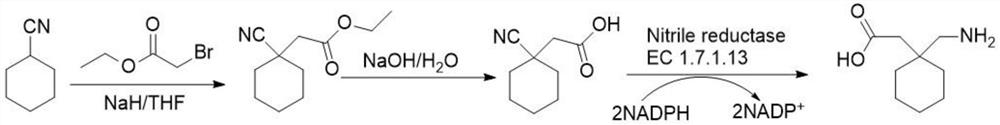
Preparation method of cyano reductase and gabapentin
The invention relates to the technical field of medicine synthesis, in particular to a preparation method of cyano reductase and gabapentin. The method comprises the following steps: by taking sodium hydride as a catalyst, carrying out condensation reaction on cyclohexyl methyl cyanide and 2-ethyl bromoacetate to generate 2-cyano-2-cyclohexyl ethyl acetate; under an alkaline condition, carrying out hydrolysis reaction on 2-cyano-2-cyclohexyl ethyl acetate to obtain 2-cyano-2-cyclohexyl acetic acid; and converting the 2-cyano-2-cyclohexyl acetic acid into gabapentin under the action of the cyano reductase. Aiming at the defects (the problem of traditional chemical preparation of gabapentin) of a literature scheme, cyano reductase is introduced, and a route is systematically optimized, so that reaction steps are reduced, and meanwhile, reaction conditions are milder and more environment-friendly. According to the route, the overall yield of gabapentin can be remarkably increased, and meanwhile, the gabapentin better meets the requirements of current green production.
Owner:SHENZHEN READLINE BIOTECH CO LTD
Preparation method of artificial antigen of ketamine
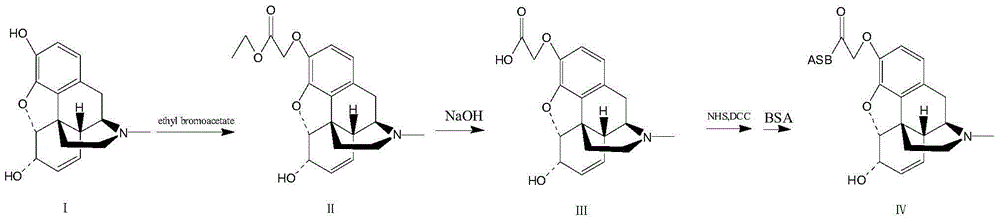
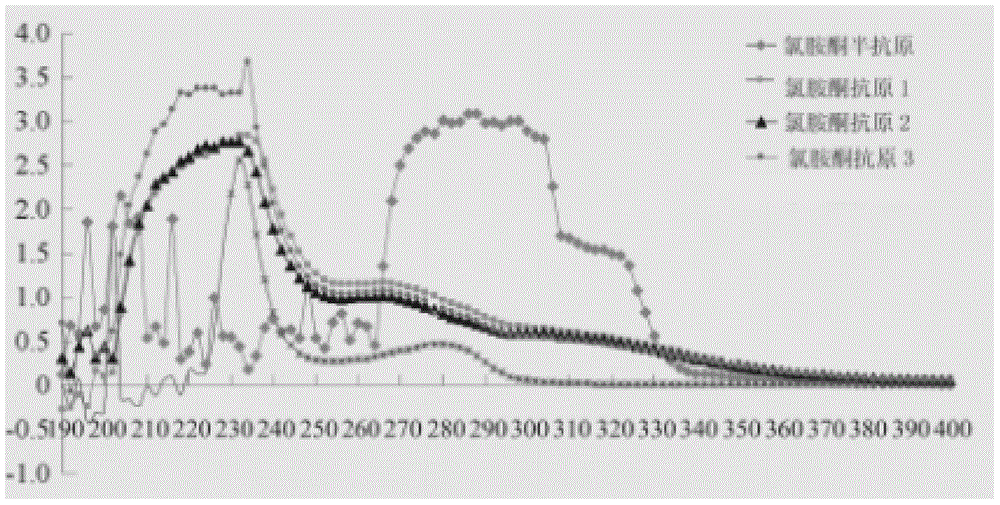
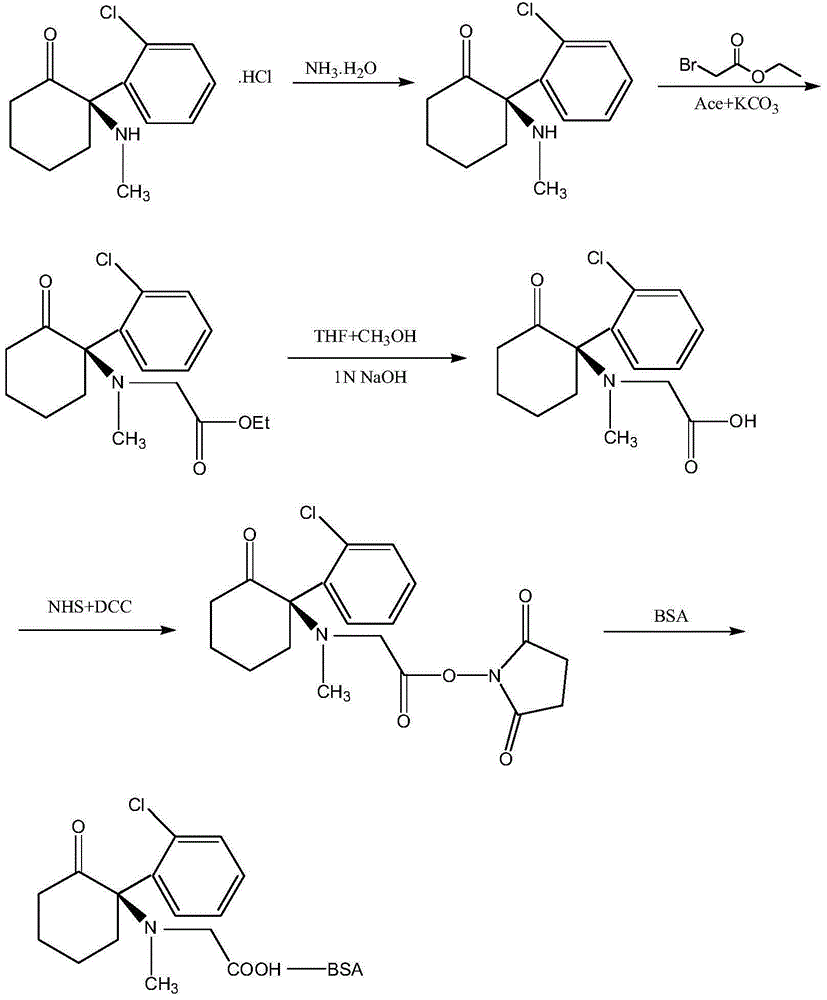
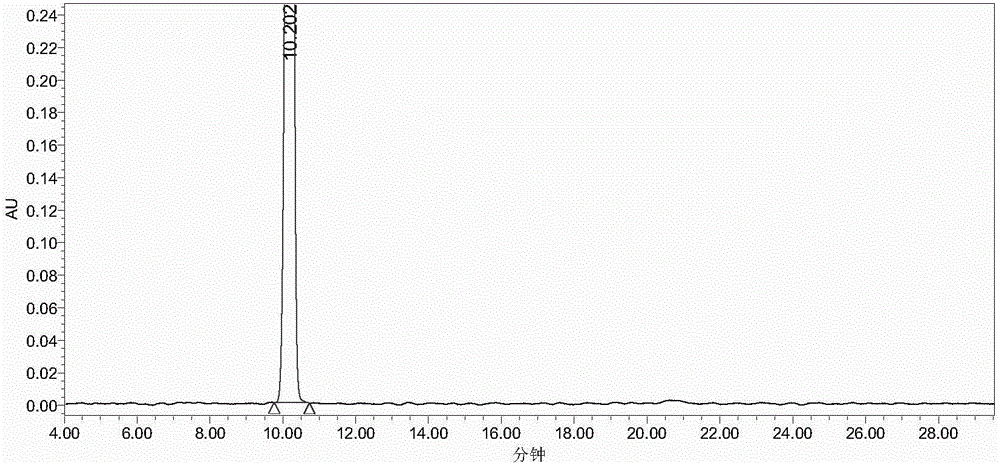
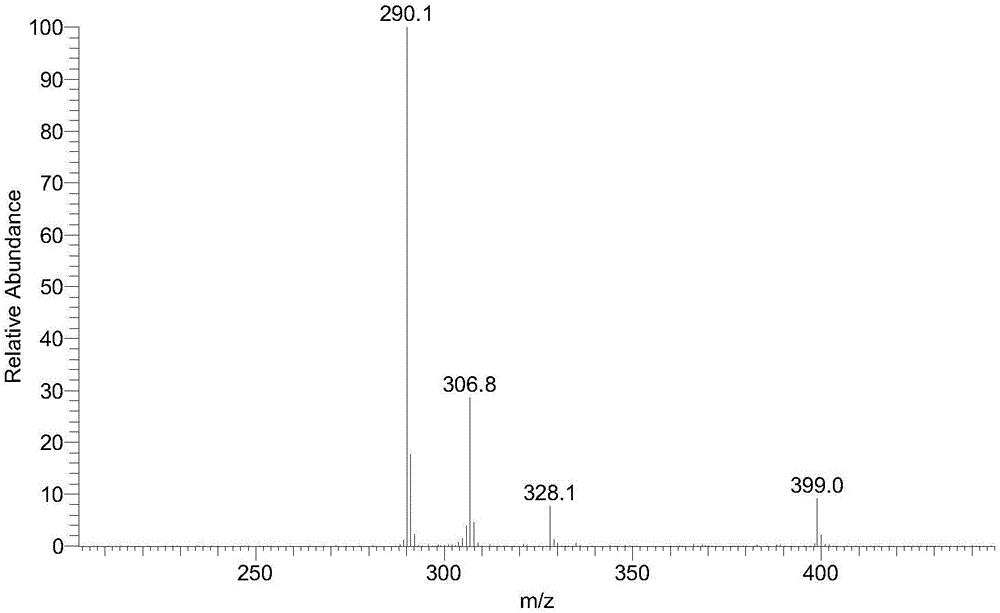
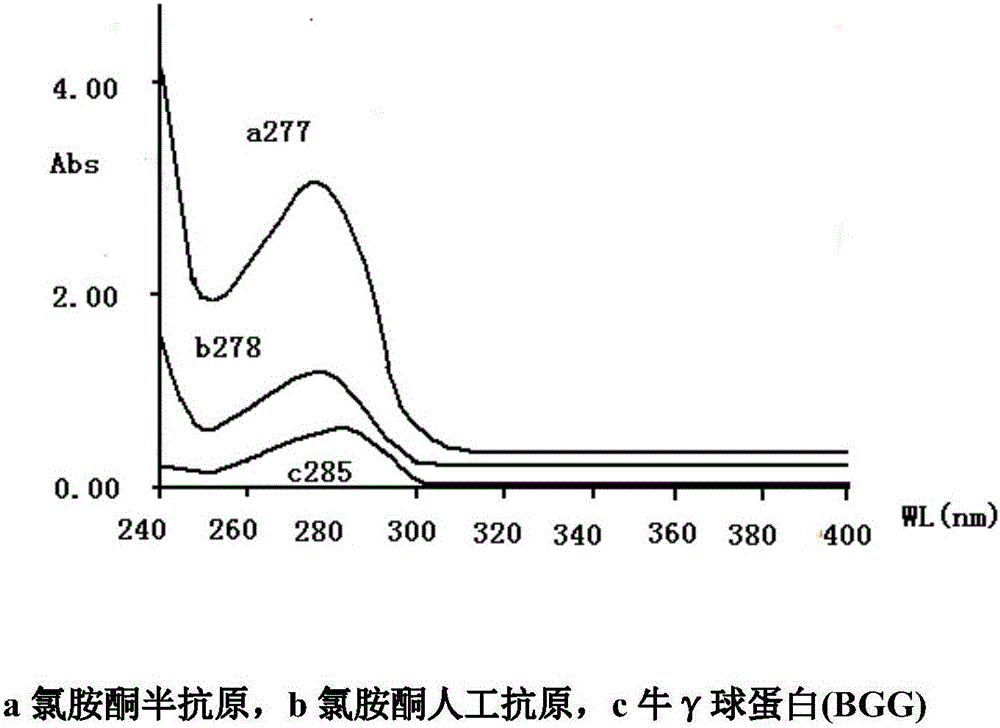
InactiveCN104558140AHigh yieldHigh purityPeptide preparation methodsHybrid peptidesAntigenHydrolysis
The invention provides a preparation method of an artificial antigen of ketamine. The preparation method comprises the following steps: (1) preparing and detecting a hapten, namely reacting the hapten containing carboxyl from ketamine hydrochloride and ethyl bromoacetate by virtue of dissociation, arm linkage and hydrolysis; (2) preparing and detecting the artificial antigen, namely combining the hapten with bovine gamma globulin (BGG) by virtue of a carbodiimide method so as to prepare the artificial antigen of the ketamine, namely ketamine-bovine gamma globulin. The prepared artificial antigen of the ketamine can be utilized for preparing corresponding ketamine antibodies by virtue of animal immunization and can be applied for researches of various ketamine immunoassay methods, and a convenient, rapid and accurate way is provided for the detection of the ketamine.
Owner:ABON BIOPHARM HANGZHOU
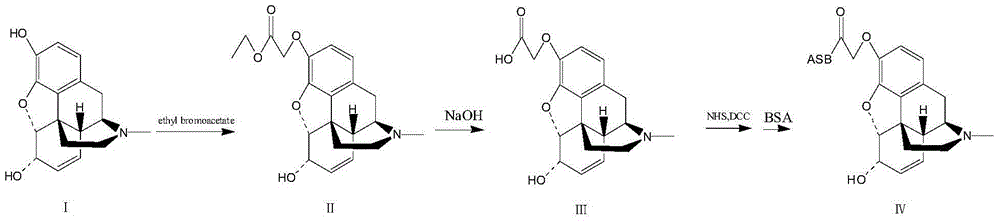
Preparation method for ketamine artificial antigen
InactiveCN105968184AAdvanced synthesis technologyStrong specificityOrganic compound preparationHybrid peptidesAcetic acidAntigen
The invention provides a preparation method for a ketamine artificial antigen. The preparation method comprises the first step of hapten preparation and detection, wherein an ester group is introduced through reaction with ethyl bromoacetate, hydrolysis is carried out under the alkaline condition, and ketamine hapten containing carboxy groups is obtained; the second step of artificial antigen preparation and detection, wherein the ketamine hapten and bovine gamma globulin (BGG) are combined through a carbodiimide method to prepare the ketamine artificial antigen, namely, ketamine-BGG. The prepared ketamine artificial antigen can be used for animal immunization, a corresponding ketamine antibody is obtained, the preparation method can be used for studies of various kinds of ketamine immunoassays, and a more convenient, quicker and more accurate approach is provided for ketamine detection.
Owner:HANGZHOU LAIHE BIOTECH CO LTD
3, 5-dihydroxy-4-isopropyl diphenyl ethylene-ethyl bromoacetate-polyoxyethylene compound and synthetic method thereof
InactiveCN101564537BGood water solubilityGood biocompatibilityAntimycoticsHydroxy compound active ingredientsSolubilityPolyethylene glycol
The invention discloses a 3, 5-dihydroxy-4-isopropyl diphenyl ethylene-ethyl bromoacetate-polyoxyethylene compound, namely polyoxyethylene-di(acetic acid-4-isopropyl-5-hydroxy diphenyl ethylene-3-phenolic ester). The original medicine is 3, 5-dihydroxy-4-isopropyl diphenyl ethylene (tolylene maud); and the modifier is polyoxyethylene and the linker arm is ethyl bromoacetate, the molecular weight of polyoxyethylene is 4000 to 8000. The synthetic method of the compound includes three reactions, namely esterification reaction, hydrolysis reaction and esterification reaction. Compared with the original medicine, the compound has enhanced water-solubility and stability; the internal medicine distribution is changed; the bioavailability is enhanced; the synthetic method is simple; the reaction condition is mild; and the cost is low, without numerous unpleasant experimental procedures. In the method, the 3, 5-dihydroxy-4-isopropyl diphenyl ethylene-ethyl bromoacetate-polyoxyethylene compound can be easily made.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
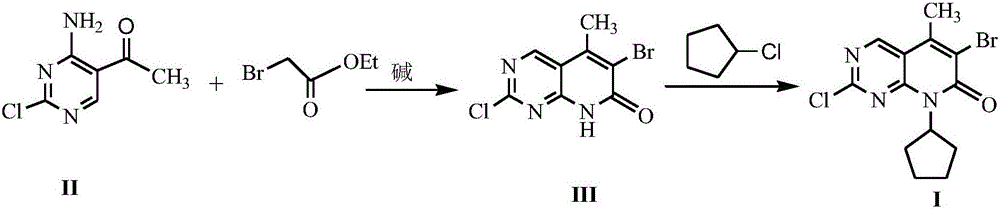
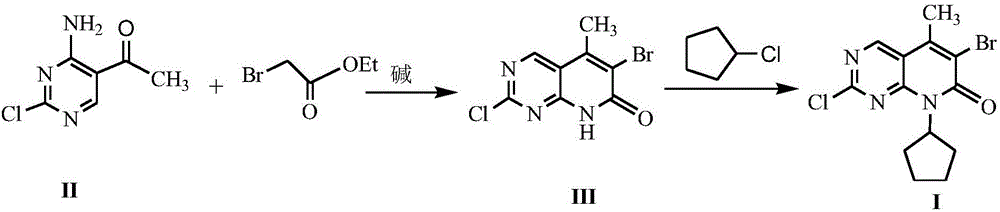
Method for synthesizing palbociclib intermediate
The invention discloses a method for synthesizing a palbociclib intermediate 2-chloro-6-bromo-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one. The method comprises the following steps of 1) preparing 2-chloro-6-bromo-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one by means of performing cyclization on 4-amino-2-chloro-5-pyrimidine ethanone and ethyl bromoacetate under the effect of alkali; 2) preparing a target product by means of performing amine alkylation reaction on the 2-chloro-6-bromo-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one and chlorinated cyclopentane. The method for synthesizing an important palbociclib intermediate 2-chloro-6-bromo-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one provided by the invention has the advantages that the steps are few, the reaction condition is mild, the operation is easy and convenient, the synthesis efficiency is high, impurities are few, the method is suitable for industrialized production, and a new approach is provided for the preparation of palbociclib and the palbociclib intermediate. The formula is shown in the description.
Owner:HUAIHAI INST OF TECH
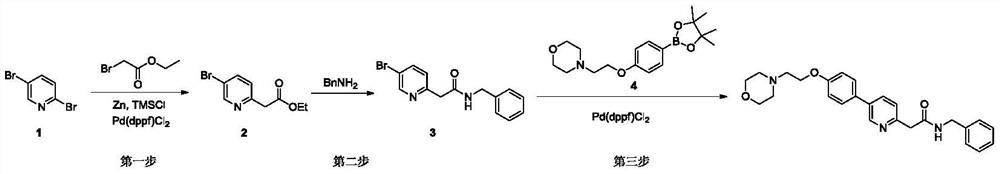
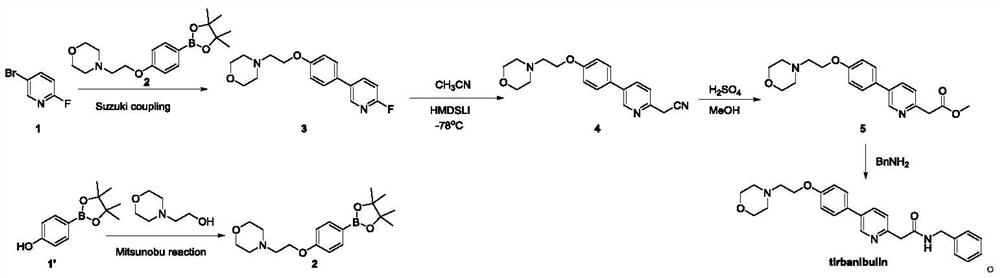
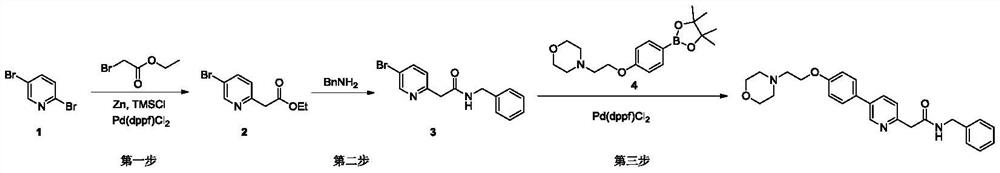
Synthesis method of tirbanibulin
ActiveCN113354575ARaw materials are cheap and easy to getMild reaction conditionsOrganic chemistryCombinatorial chemistryEthyl acetate
The invention belongs to the technical field of synthesis of medical compounds, which particularly discloses a synthesis method of tirbanibulin. The preparation method comprises the following steps of taking 2, 5-dibromopyridine as a raw material, and reacting with ethyl bromoacetate to obtain a compound 2, then carrying out ammonolysis reaction on the compound 2 and benzylamine to obtain a compound 3, and enabling the compound 3 and the compound 4 to be mixed and react to obtain the final product tirbanibulin. The method has the advantages of cheap and easily available raw materials, convenience in production and easiness in purification, and can be developed into an industrial production method.
Owner:HENAN VOCATIONAL COLLEGE OF APPLIED TECH
Synthesis of 2-(1-benzylpiperidine)-2, 8-diazaspiro[4, 5]decane
The invention discloses synthesis of 2-(1-benzylpiperidine)-2, 8-diazaspiro[4, 5]decane. Directed at the shortages of expensiveness, low reaction yield, and difficult purification in previous synthesis methods of 2-(1-benzylpiperidine)-2, 8-diazaspiro[4, 5]decane, the invention provides the following synthesis route of: taking piperidine-4 carboxylic acid ethyl ester as the raw material and conducting protection on it, then bringing the protected raw material into butt joint with ethyl bromoacetate, and carrying out ring closing with 1-benzylpiperidine-4-ammonia, performing deprotection, and implementing reduction with tetrahydro lithium aluminum, thus obtaining the 2-(1-benzylpiperidine)-2, 8-diazaspiro[4, 5]decane. According to the invention, the whole process is easy to operate, the product is easy to purify, and the raw material cost is greatly reduced.
Owner:CGENETECH (SUZHOU CHINA) CO LTD
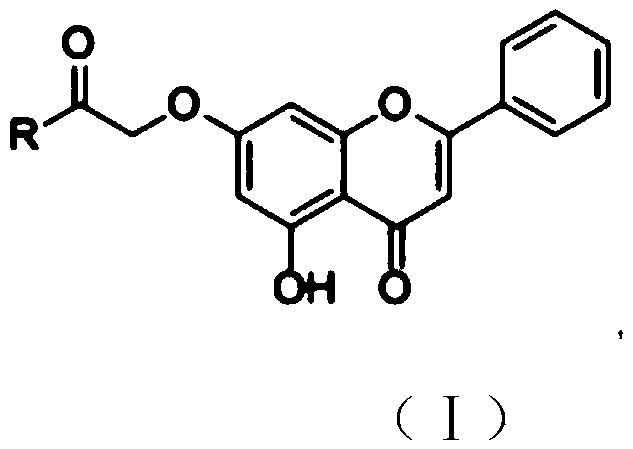
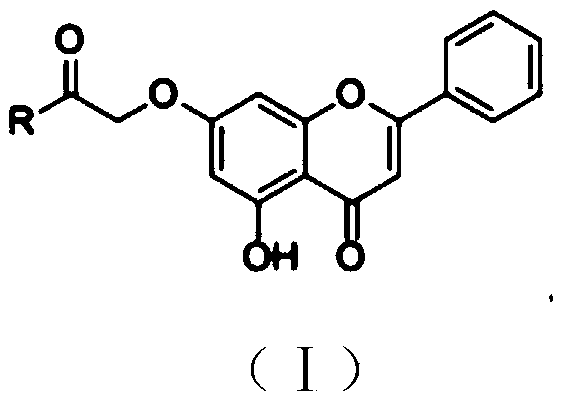
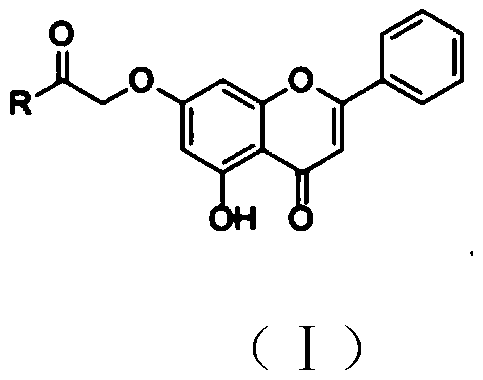
Chrysin amide derivative as well as preparation method and medical application thereof
PendingCN109761944AGood water solubilityImprove stabilityGroup 5/15 element organic compoundsSkeletal disorderSolubilityEthyl acetate
The invention relates to the technical field of pharmaceutical chemistry and in particular relates to a chrysin amide derivative as well as a preparation method and medical application of the derivative. A structural formula of the chrysin amide derivative provided by the invention is shown as a formula I; a substituent group R in the compound is a series of acylamino. The chrysin amide derivativeprovided by the invention has the characteristics of good water solubility, stability and pharmacological activity; the preparation method of each chrysin amide derivative is the same and comprises the following steps: connecting 7th-site hydroxyl of chrysin with ethyl bromoacetate and then hydrolyzing; finally, carrying out amide condensation reaction on a series of amide compounds respectively,so as to obtain a series of chrysin amide derivatives. A pharmacodynamic screening experiment result shows that compared with the chrysin, the compound has better water solubility and better uric acid lowering and gout and inflammation resisting effects and is a compound which has a prospect of being developed into a novel anti-gout drug.
Owner:武汉翼博济生生物科技有限公司
Synthesis method of imidazo[1,2-alpha]pyridyl-3-formic acid
InactiveCN103965190AReaction raw materials are readily availableReasonable priceOrganic chemistrySynthesis methodsOrganic synthesis
Owner:SHANDONG YOUBANG BIOCHEM TECH
Novel preparation method for butyrolactone derivative
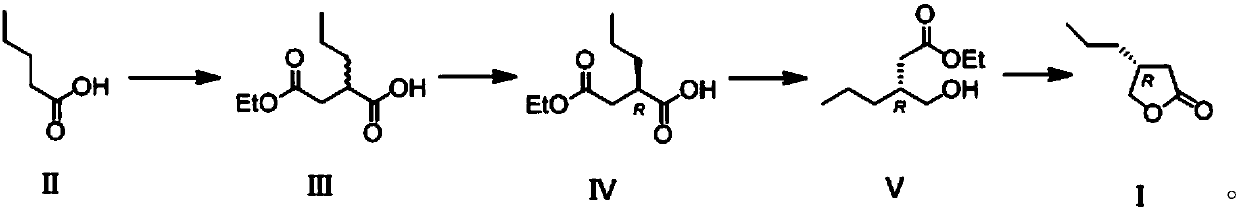
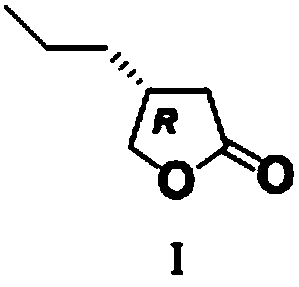
The invention discloses a novel preparation method for a butyrolactone derivative. The method includes the steps of: (1) reacting pentanoic acid shown as formula (II) with ethyl bromoacetate under theaction of lithium diisopropylamide to obtain a compound shown as formula (III); (2) splitting the compound shown as formula (III) with chiral phenylethylamine to obtain a compound shown as formula (IV); (3) subjecting the compound shown as formula (IV) to reduction of carboxyl by borane so as to obtain a compound shown as formula (V); and (4) carrying out cyclization reaction on the compound shown as formula (V) to obtain a butyrolactone derivative shown as formula (I). The brand new butyrolactone derivative synthesis method provided by the invention has the advantages of low cost of synthetic raw material pentanoic acid, need of just 4-step reaction, and good stereoselectivity, and can obviously reduce the production cost. And the synthesis route is shown as the specification.
Owner:安徽华胜医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
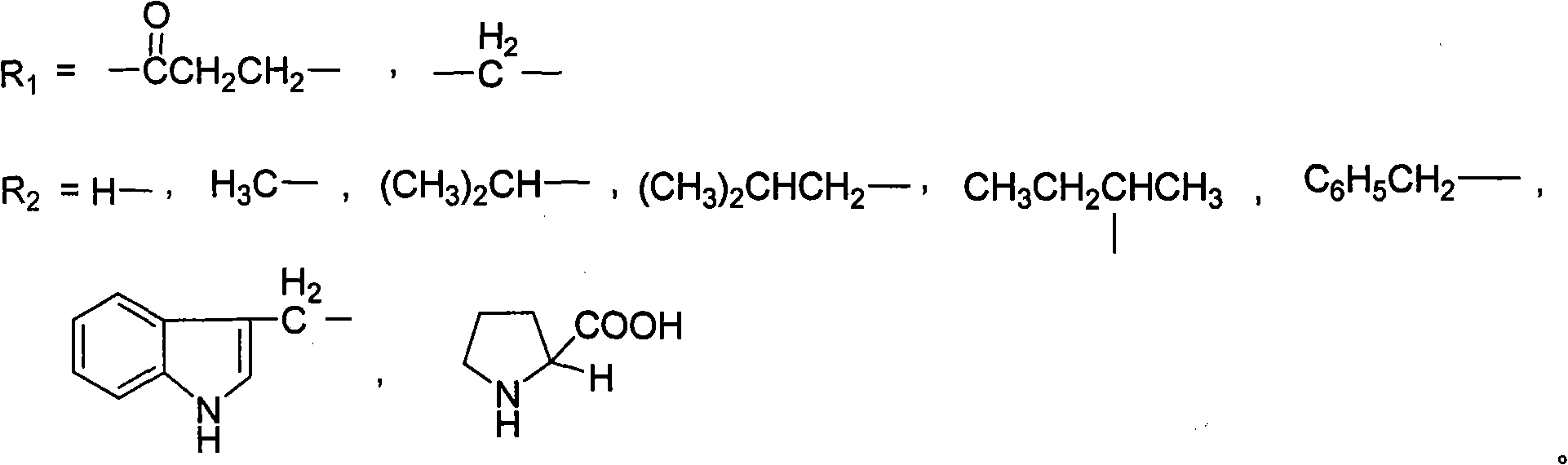
![Preparation and use of hydroxyl oximated calix[6]arene efficient uranium extractant Preparation and use of hydroxyl oximated calix[6]arene efficient uranium extractant](https://images-eureka.patsnap.com/patent_img/5b052ffd-9068-4ed7-aaf1-73b6d2f7f9a2/130517205948.PNG)
![Preparation and use of hydroxyl oximated calix[6]arene efficient uranium extractant Preparation and use of hydroxyl oximated calix[6]arene efficient uranium extractant](https://images-eureka.patsnap.com/patent_img/5b052ffd-9068-4ed7-aaf1-73b6d2f7f9a2/130517205956.PNG)
![Preparation and use of hydroxyl oximated calix[6]arene efficient uranium extractant Preparation and use of hydroxyl oximated calix[6]arene efficient uranium extractant](https://images-eureka.patsnap.com/patent_img/5b052ffd-9068-4ed7-aaf1-73b6d2f7f9a2/236159DEST_PATH_IMAGE004.PNG)
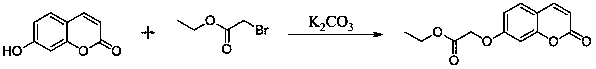
![Synthesis method of 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid Synthesis method of 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid](https://images-eureka.patsnap.com/patent_img/e1ecd4ae-f3a6-4447-886a-cb494a9c350b/201410212550X100002DEST_PATH_IMAGE001.PNG)
![Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate](https://images-eureka.patsnap.com/patent_img/c0d300b0-9773-430c-84c7-853737052f0a/DEST_PATH_IMAGE001.png)
![Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate Method for synthesizing tert-butyl 6-oxo-8-oxa-2, 5-diazaspiro[3.5]nonane-2-carboxylate](https://images-eureka.patsnap.com/patent_img/c0d300b0-9773-430c-84c7-853737052f0a/DEST_PATH_IMAGE003.png)

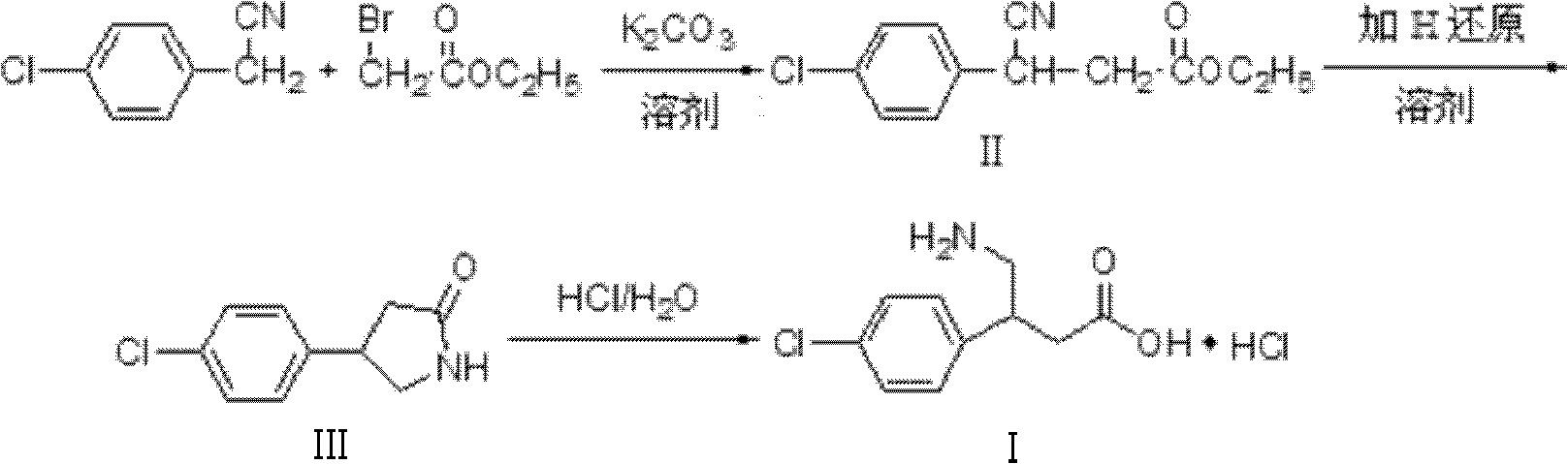
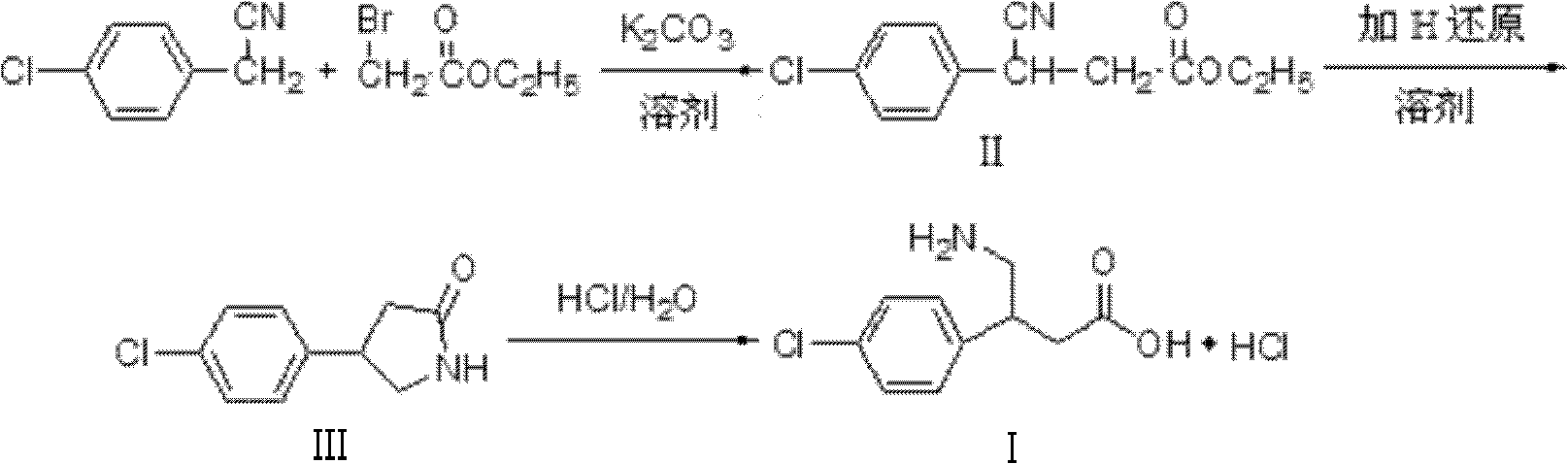
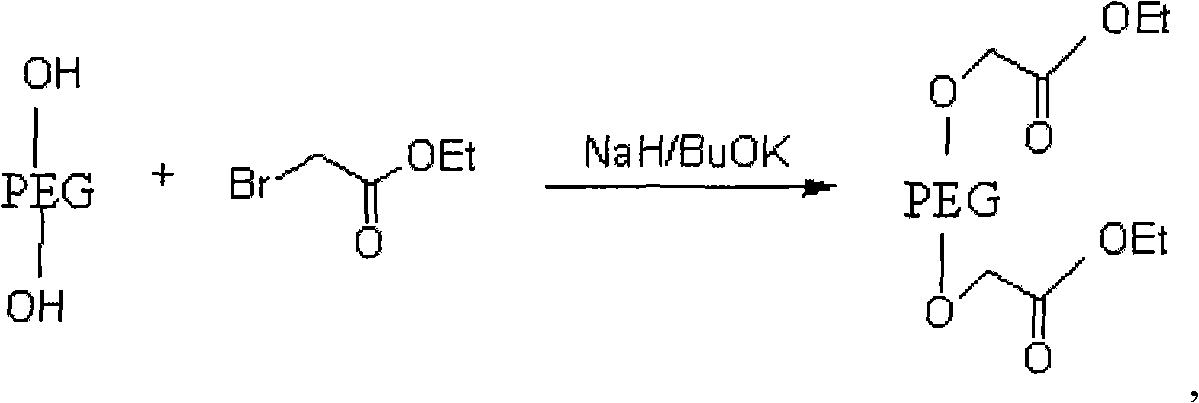
![Synthesis of 2-(1-benzylpiperidine)-2, 8-diazaspiro[4, 5]decane Synthesis of 2-(1-benzylpiperidine)-2, 8-diazaspiro[4, 5]decane](https://images-eureka.patsnap.com/patent_img/5a829d05-c874-4b22-8df9-130345de382d/255932DEST_PATH_IMAGE002.PNG)
![Synthesis of 2-(1-benzylpiperidine)-2, 8-diazaspiro[4, 5]decane Synthesis of 2-(1-benzylpiperidine)-2, 8-diazaspiro[4, 5]decane](https://images-eureka.patsnap.com/patent_img/5a829d05-c874-4b22-8df9-130345de382d/418645DEST_PATH_IMAGE001.PNG)
![Synthesis method of imidazo[1,2-alpha]pyridyl-3-formic acid Synthesis method of imidazo[1,2-alpha]pyridyl-3-formic acid](https://images-eureka.patsnap.com/patent_img/6c5326a8-765a-4e51-9c45-6ba25beb8c9e/201410212487X100002DEST_PATH_IMAGE001.PNG)