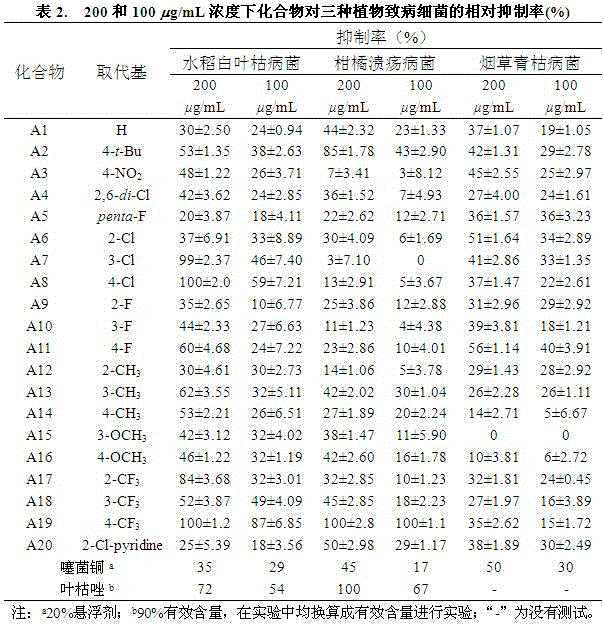
Quinazolinone compound containing 1, 2, 4-triazole thioether and synthesizing method and application of quinazolinone compound
A technology of triazole sulfide and quinazolinone, which is applied in the field of chemistry, can solve the problems of high toxicity of organic fungicides, does not conform to the development of the times, environmental pollution, etc., and achieves good effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
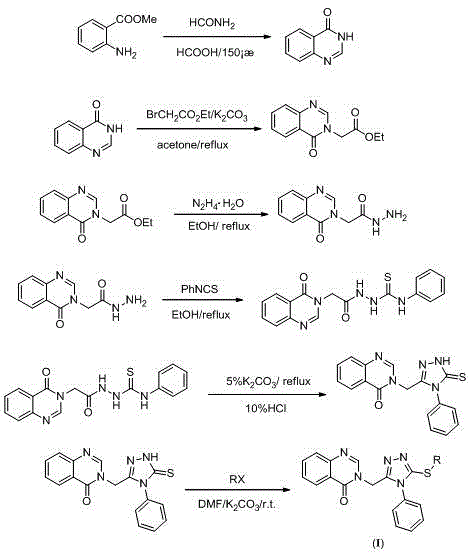
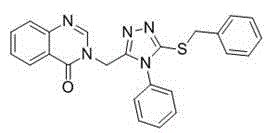
[0045] Example 1: Compound 3-((5-(benzylthio)-4-phenyl-4H-1,2,4-triazol-3-yl)methyl)quinazolin-4(3H)-one Synthesis:
[0046] (1) Preparation of quinazolin-4-one
[0047] Add 9.0 g (59.54 mmol) methyl anthranilate, 13 mL (327.3 mmol) formamide, and 3 mL (79.52 mmol) formic acid to a 100 mL three-necked flask in sequence, and heat up to 130-140 °C for reflux reaction; stop after 6 h After the reaction, the reaction liquid was cooled and poured into an appropriate amount of cold water, a large amount of white solids precipitated, stirred for 0.5 h, filtered with water, washed with water, dried, and recrystallized with absolute ethanol to obtain 4.10 g of white flocs, with a yield of 47.1% .
[0048] (2) Preparation of 2-(4-oxoquinazolin-3-(4H)-yl) ethyl acetate
[0049] Add 0.20 g (1.37 mmol) quinazolin-4-one, 5 mL acetone, 0.28 g (2.04 mmol) potassium carbonate to a 25 mL single-necked bottle, slowly drop 0.34 g (2.05 mmol) ethyl bromoacetate at room temperature, and heat up ...
Embodiment 2
[0060] Example 2: Compound 3-((5-((4-(tert-butyl)benzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)methyl) Synthesis of quinazolin-4-(3H)-one:
[0061] (1) Preparation of intermediate 3-((4-phenyl-5-mercapto-4H-1,2,4-triazol-3-yl)methyl)quinazolin-4-(3H)-one: Synthetic steps and processing conditions are with embodiment one (1~5);
[0062] (2) Target product 3-((5-((4-(tert-butyl)benzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)methyl) The preparation of quinazolin-4-(3H)-one (compound number A2):
[0063]
[0064] The synthesis steps and process conditions are the same as in Example 1 (6), the difference being that p-tert-butylbenzyl bromide is used as a raw material, and white flocs are obtained after recrystallization from ethanol-dichloromethane (10:1, v / v), and the yield is : 58.0%; m.p. 175~178 ℃.
[0065] IR (KBr, cm -1 ) v : 2962, 1685, 1610, 1172; 1 H NMR (CDCl 3 , 500 MHz) δ : 1.27 (s, 9H), 4.38 (s, 2H), 5.18 (s, 2H), 7.04 (d, J = 8.05 Hz, 2H), 7.19 (d, J = 8.00...
Embodiment 3
[0066] Example 3: Compound 3-((5-((4-nitrobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)methyl)quinazoline Synthesis of -4(3H)-one:
[0067] (1) Preparation of intermediate 3-((4-phenyl-5-mercapto-4H-1,2,4-triazol-3-yl)methyl)quinazolin-4-(3H)-one: Synthetic steps and processing conditions are with embodiment one (1~5);
[0068] (2) Target product 3-((5-((4-nitrobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)methyl)quinazoline - Preparation of 4(3H)-one (compound number A3):
[0069]
[0070] The synthesis steps and process conditions are the same as in Example 1 (6), except that p-nitrobenzyl chloride is used as a raw material, and red granular crystals are obtained after recrystallization from ethanol-dichloromethane (10:1, v / v). The yield is: 50.0%; m.p. 188~190℃.
[0071] IR (KBr, cm -1 ) v : 3076, 1672, 1610, 1165; 1 H NMR (CDCl 3 , 500 MHz) δ : 4.46 (s, 2H), 5.14 (s, 2H), 7.18 (d, J = 7.45 Hz, 2H), 7.50~7.45 (m, 4H), 7.53 (d, J = 8.60 Hz, 2H), 7.70 (d, J = 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com