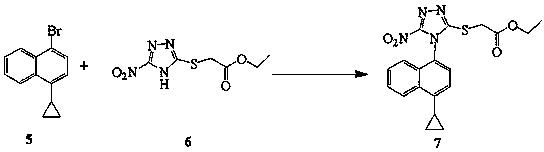
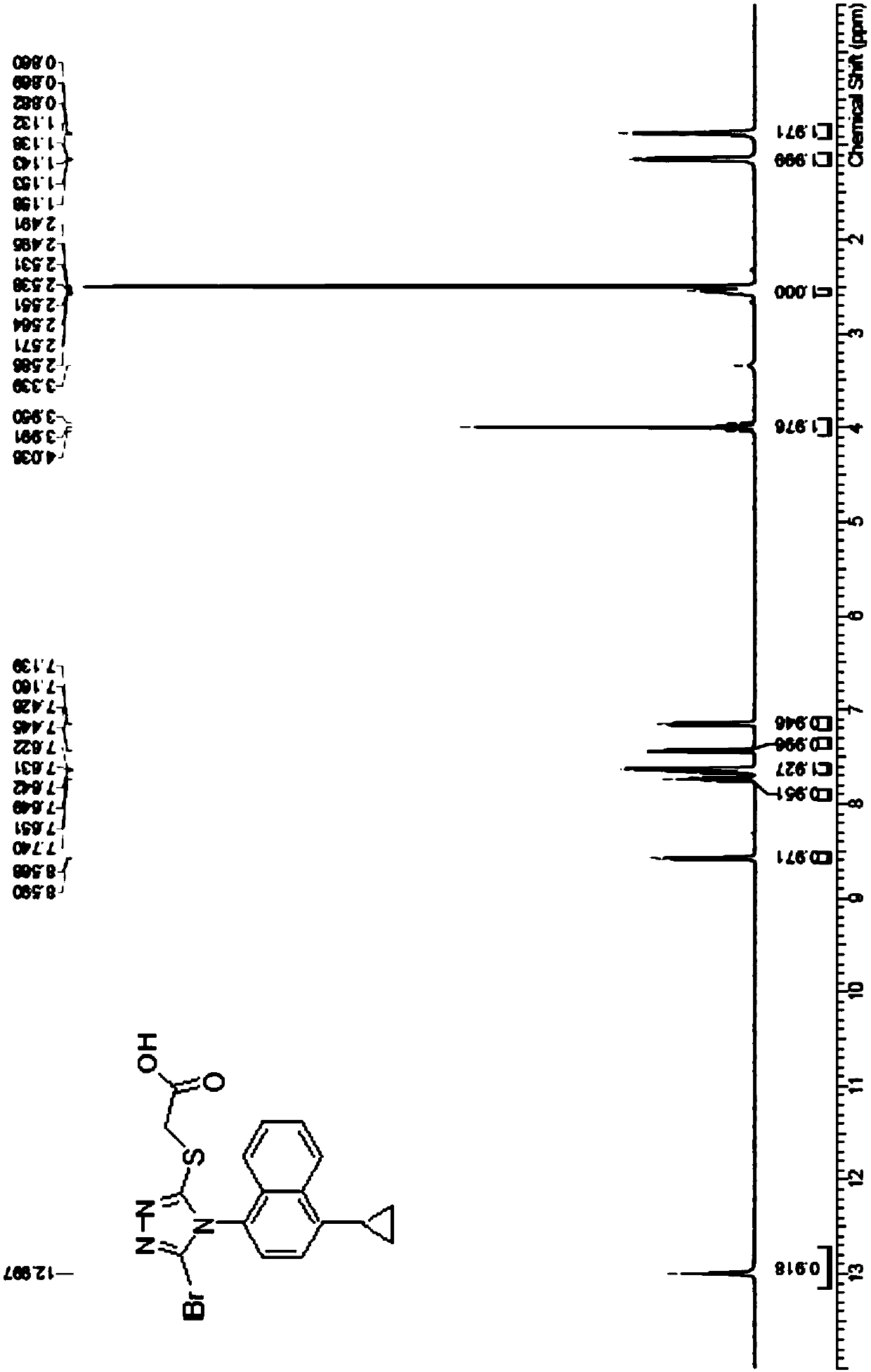
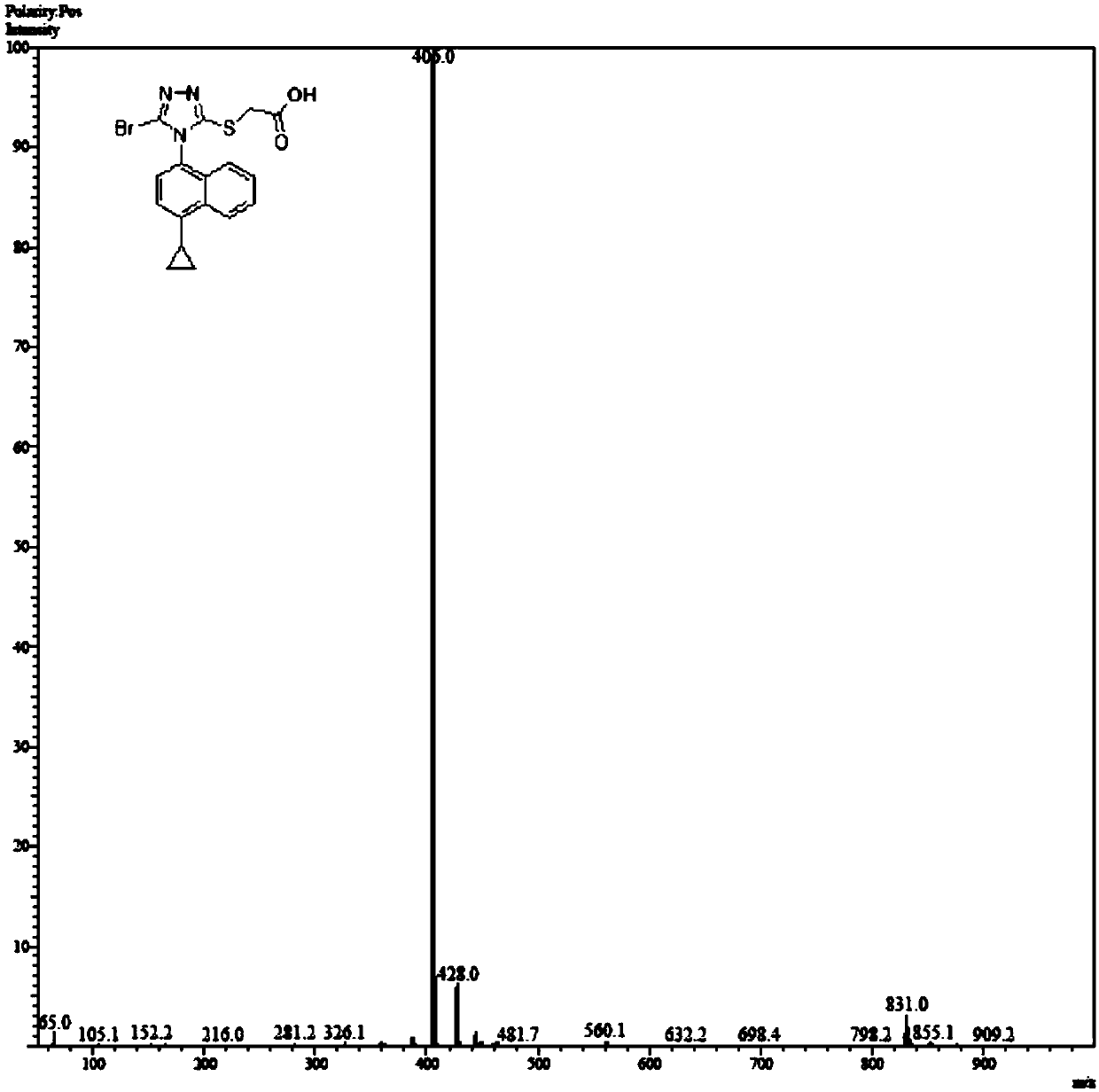
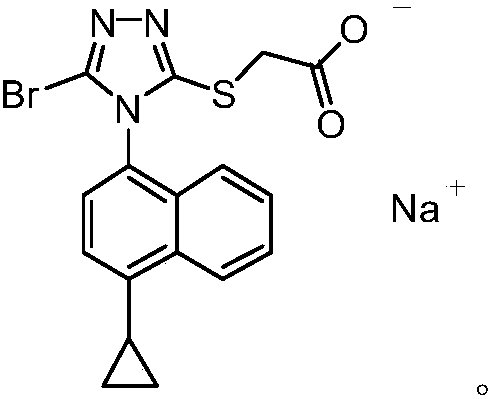
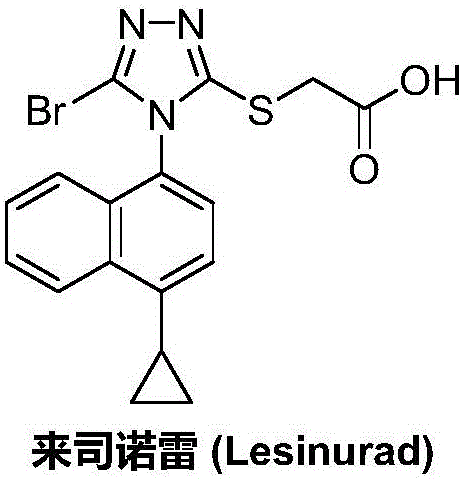
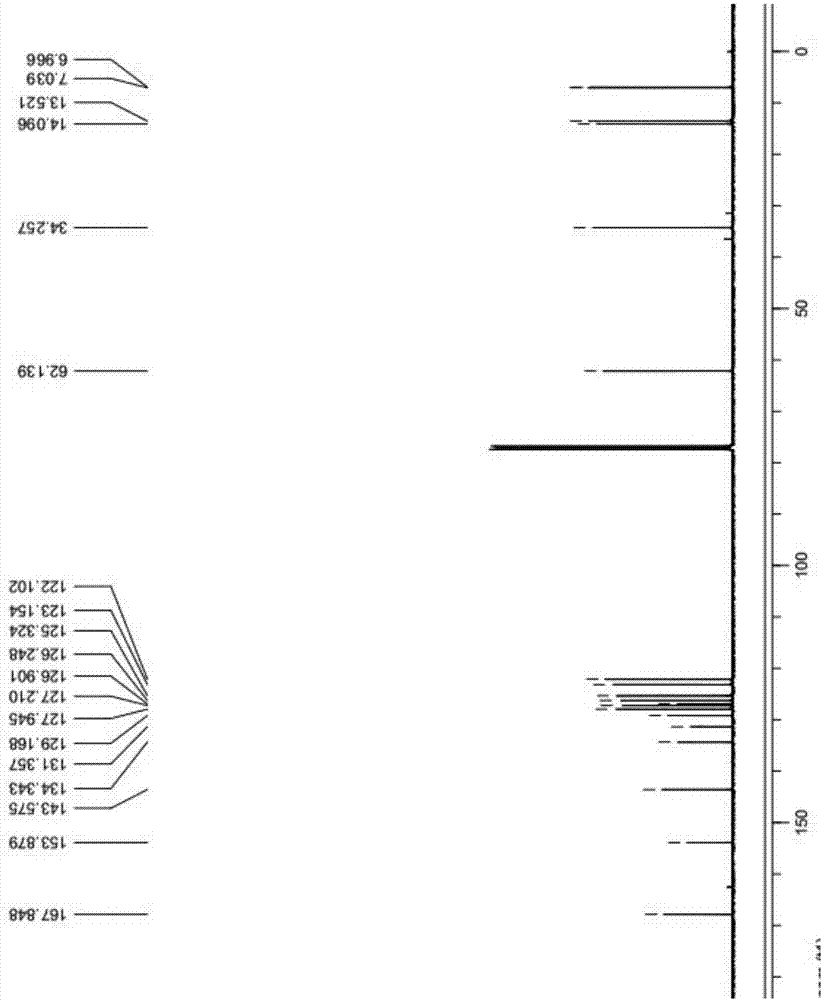
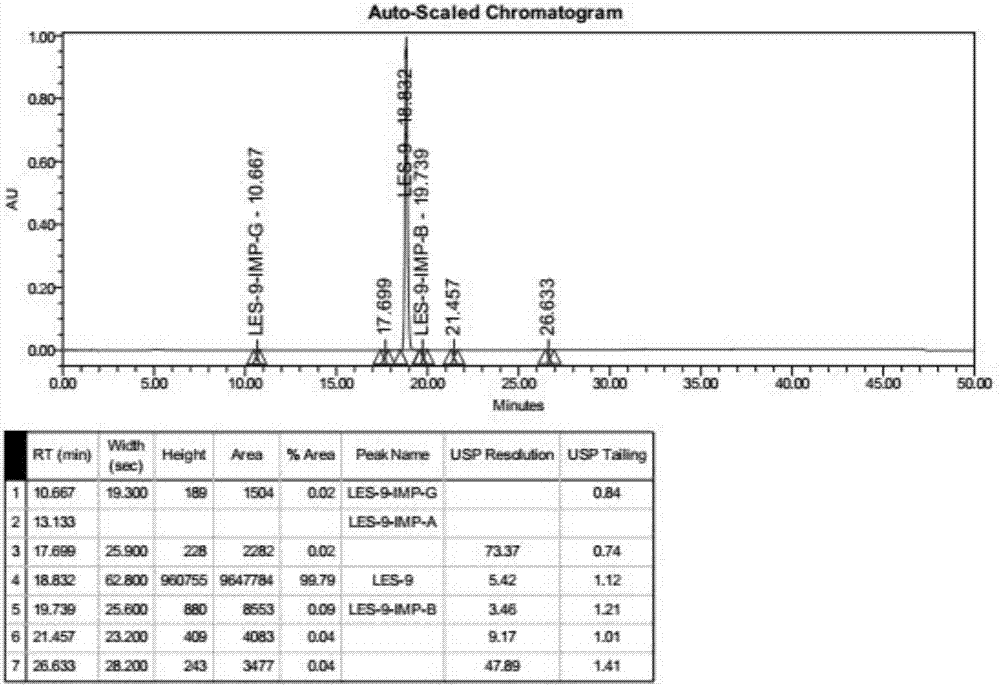
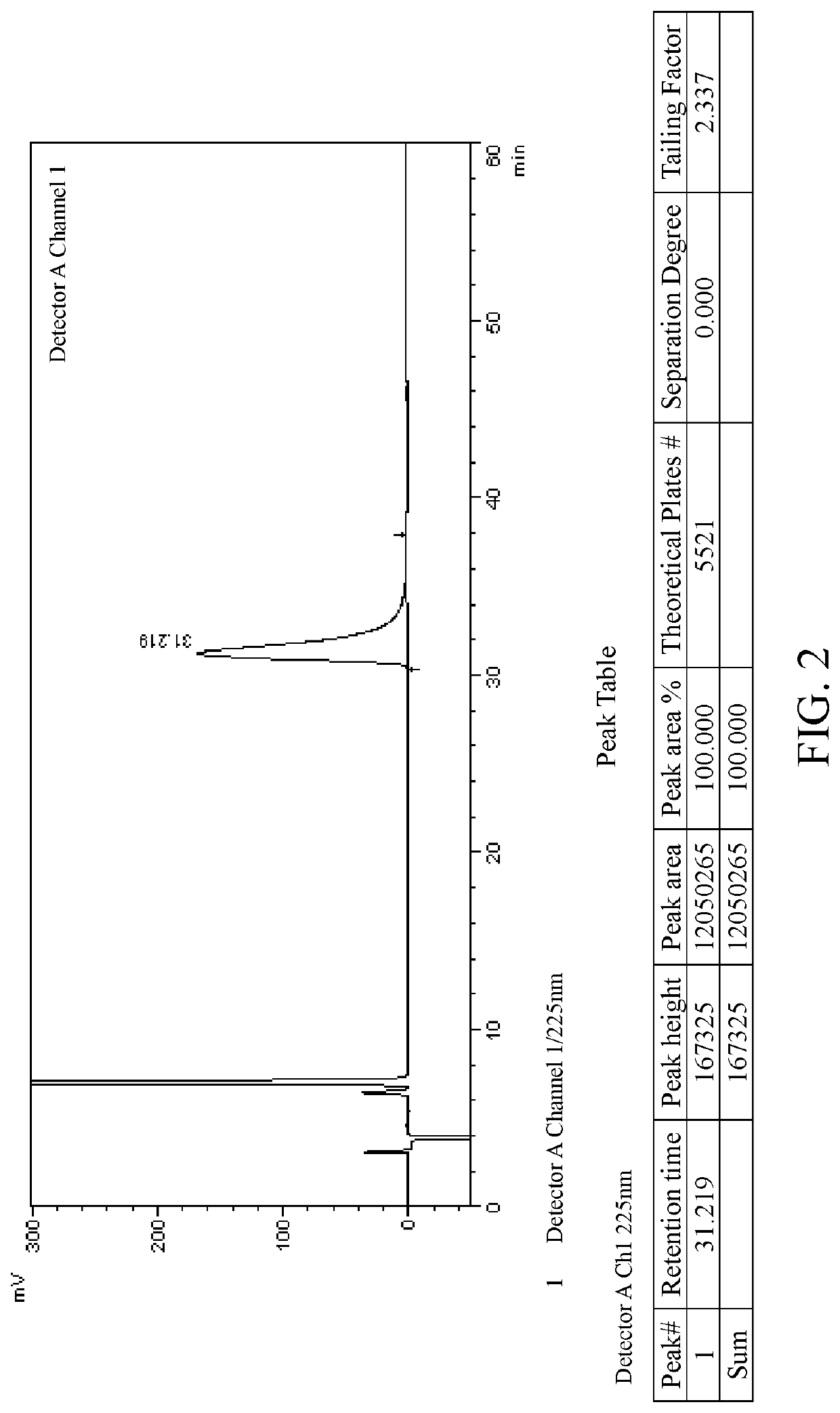
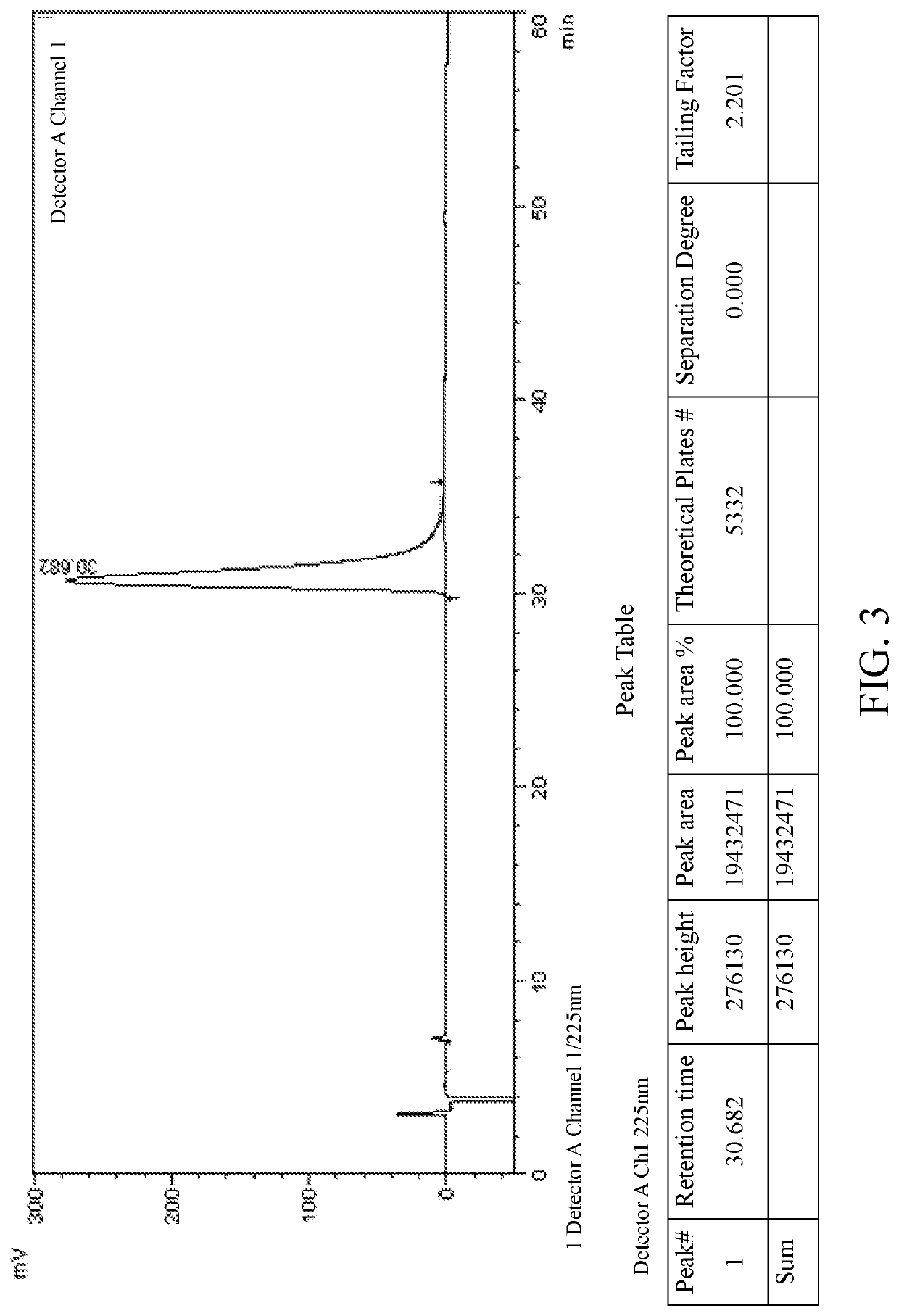
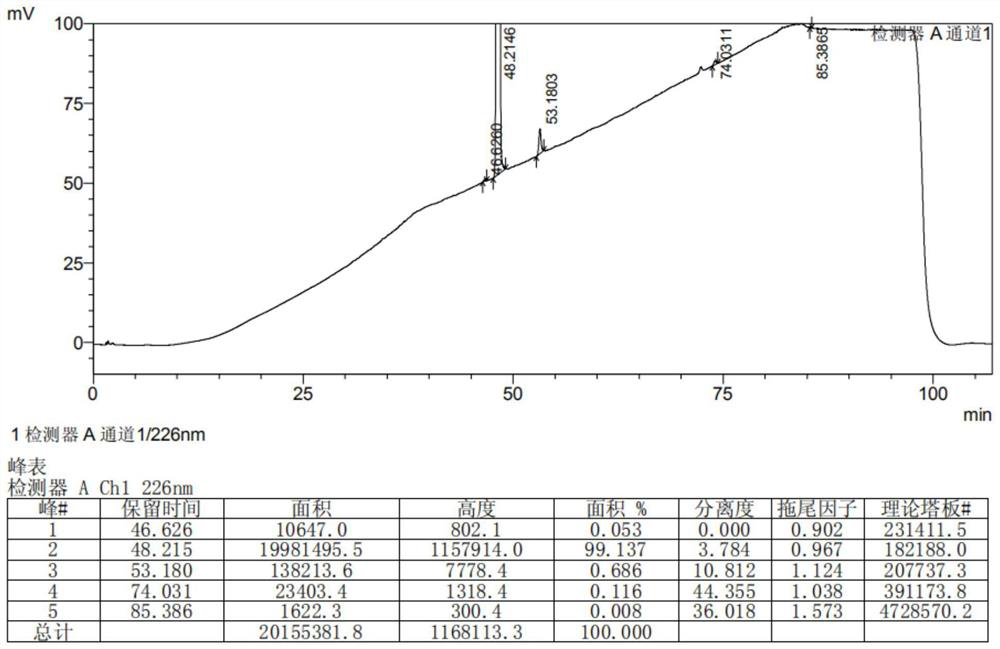
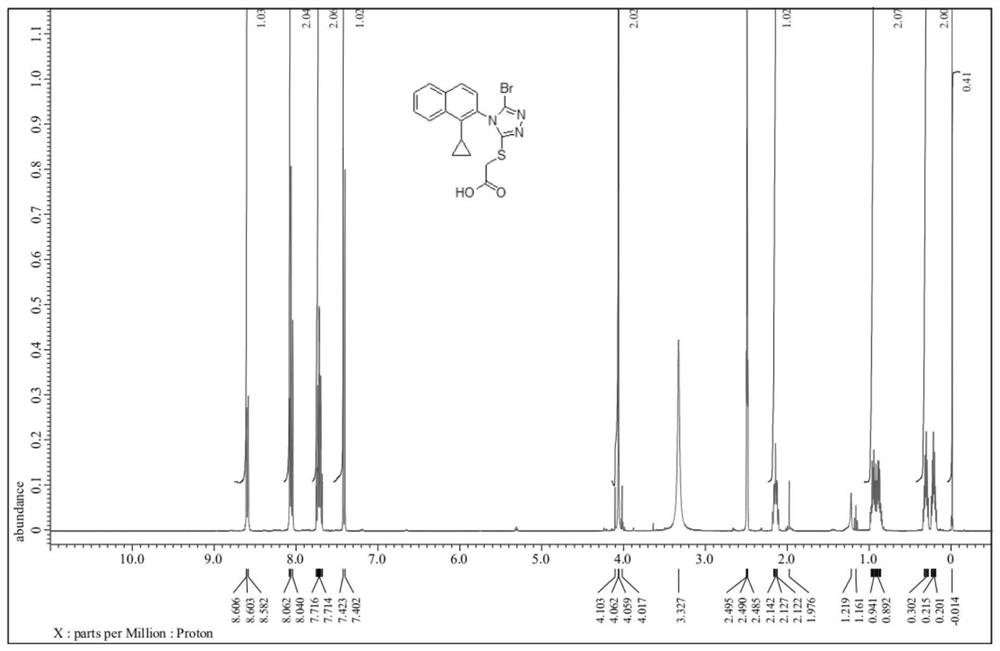
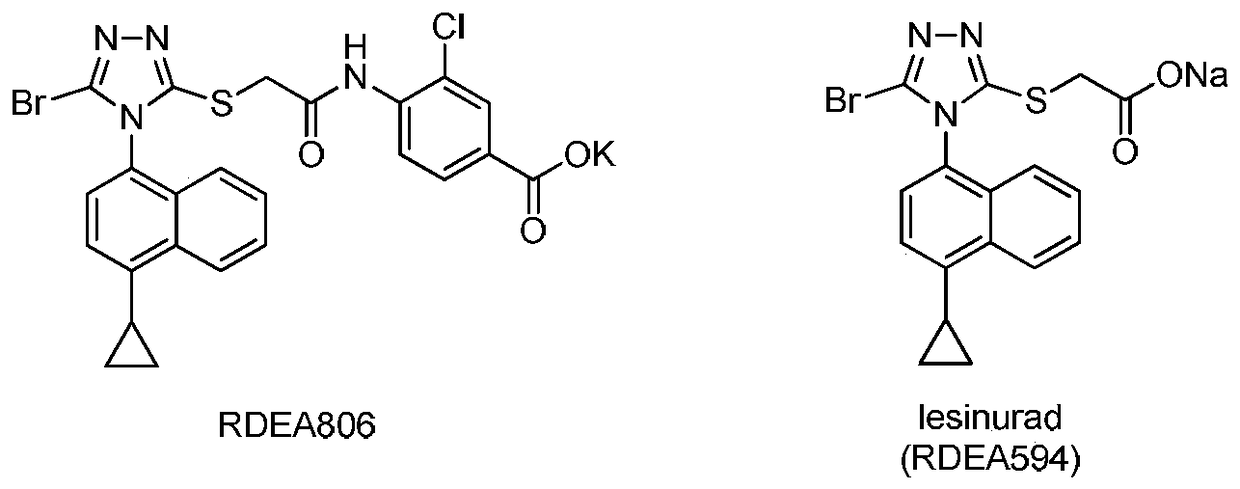
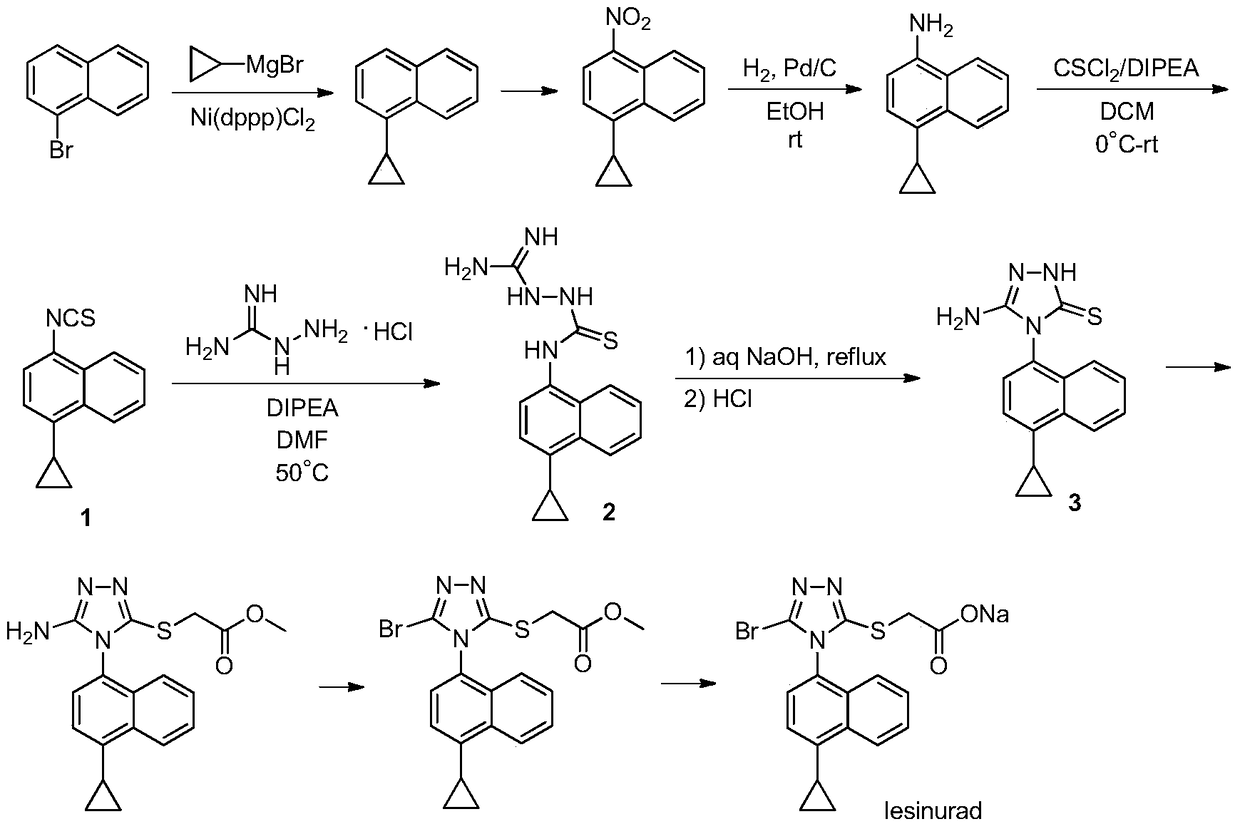
Patents
Literature
50 results about "Lesinurad" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
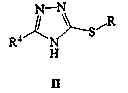
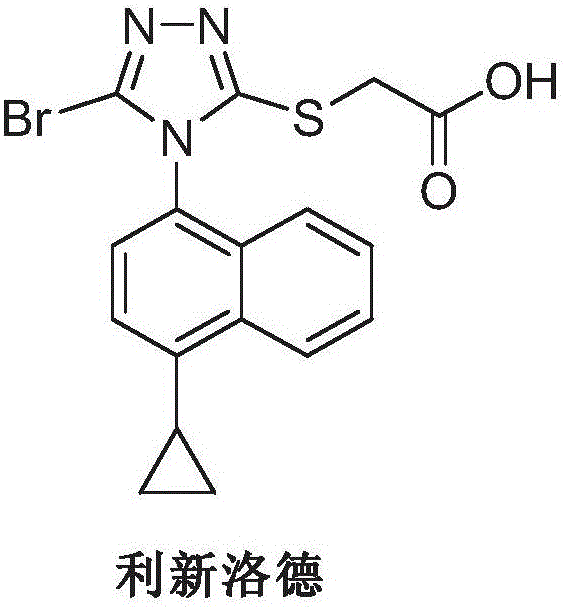
Lesinurad is used with another medication (such as allopurinol, febuxostat) to lower uric acid levels in people with gout.
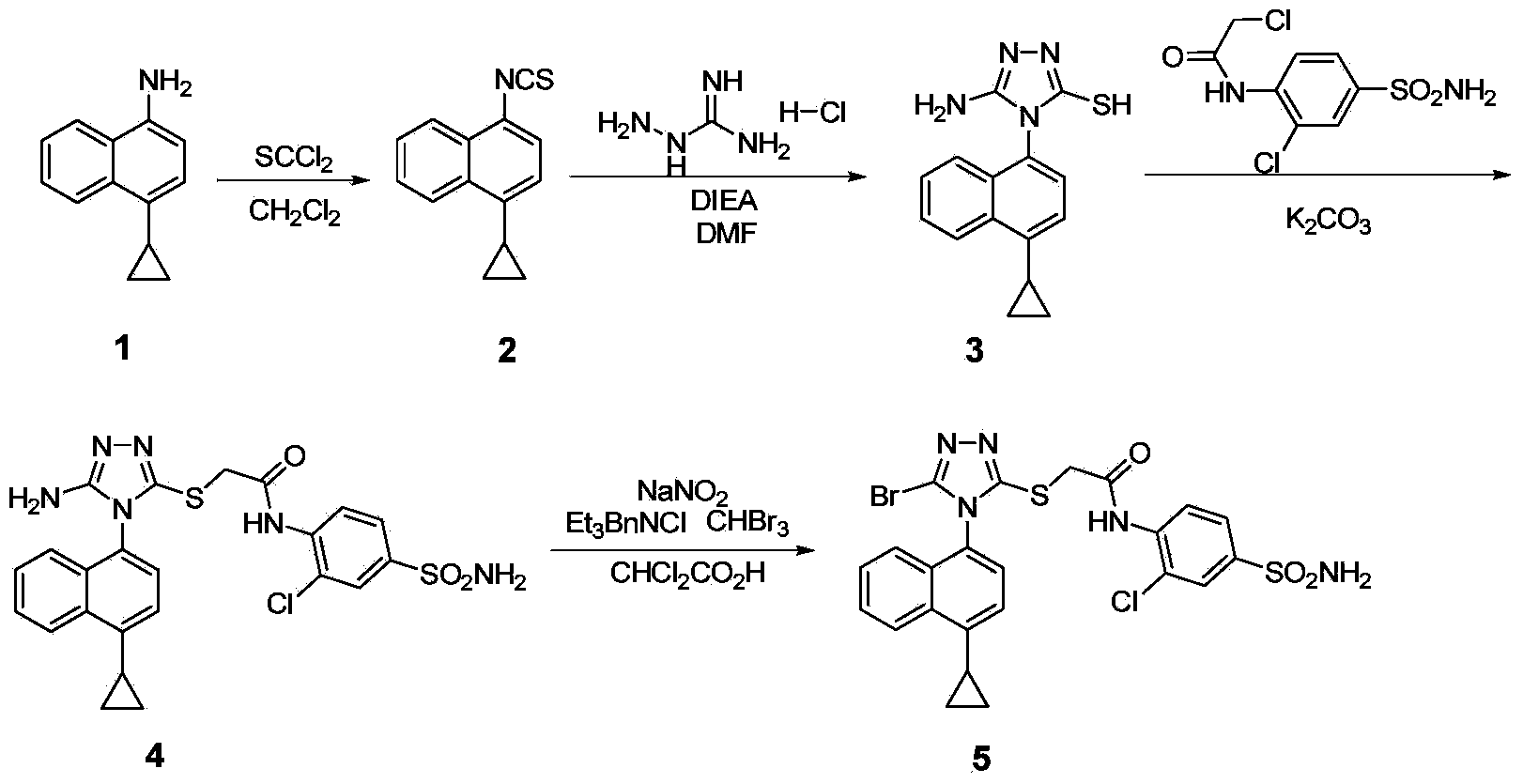
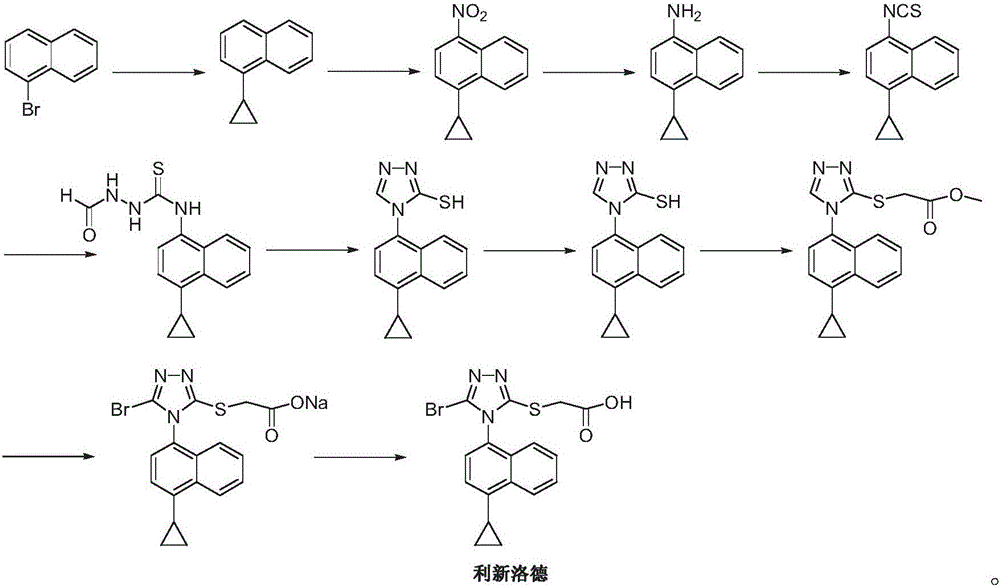
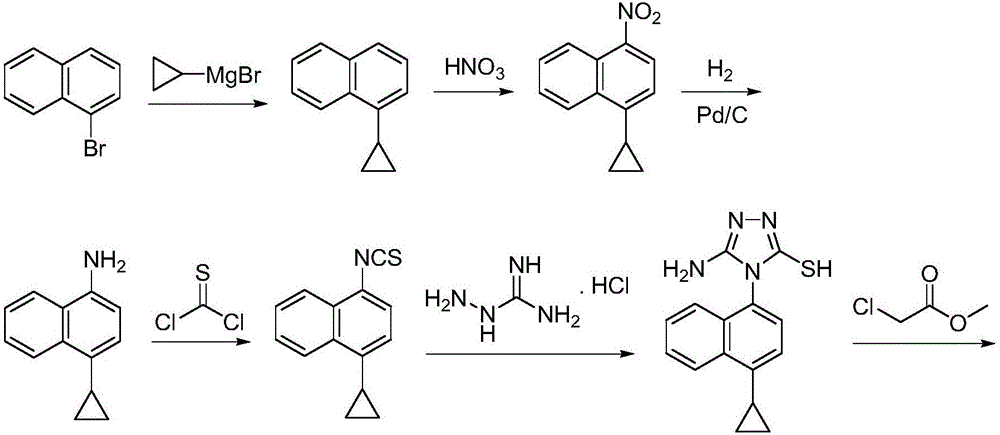
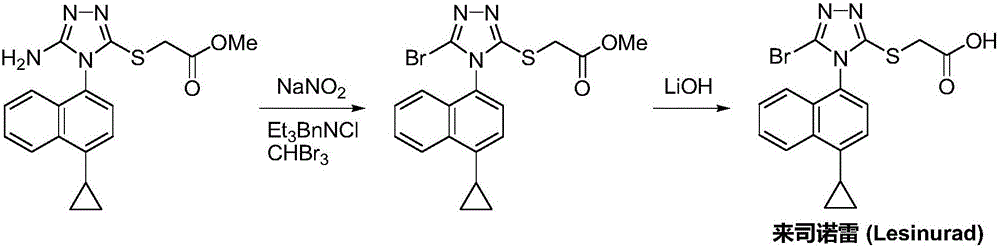
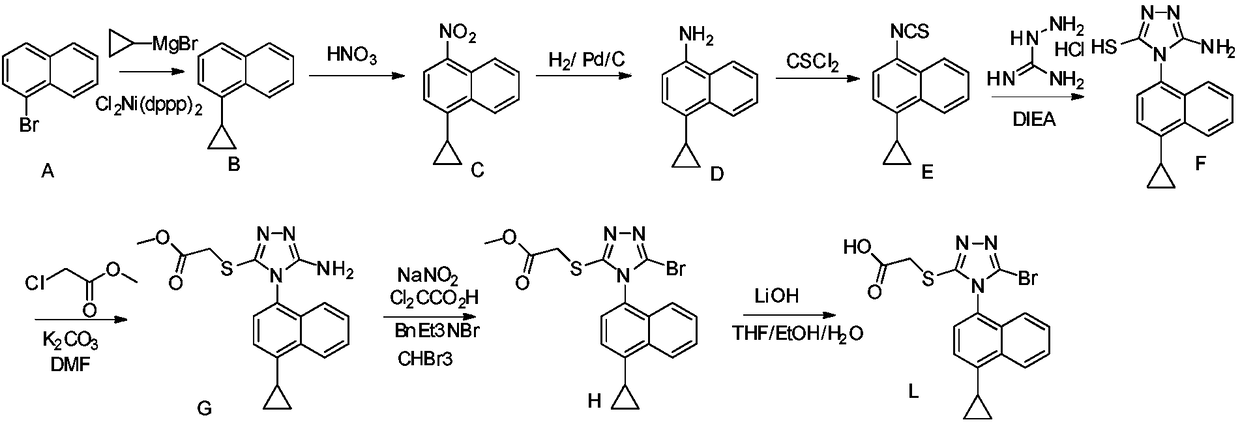
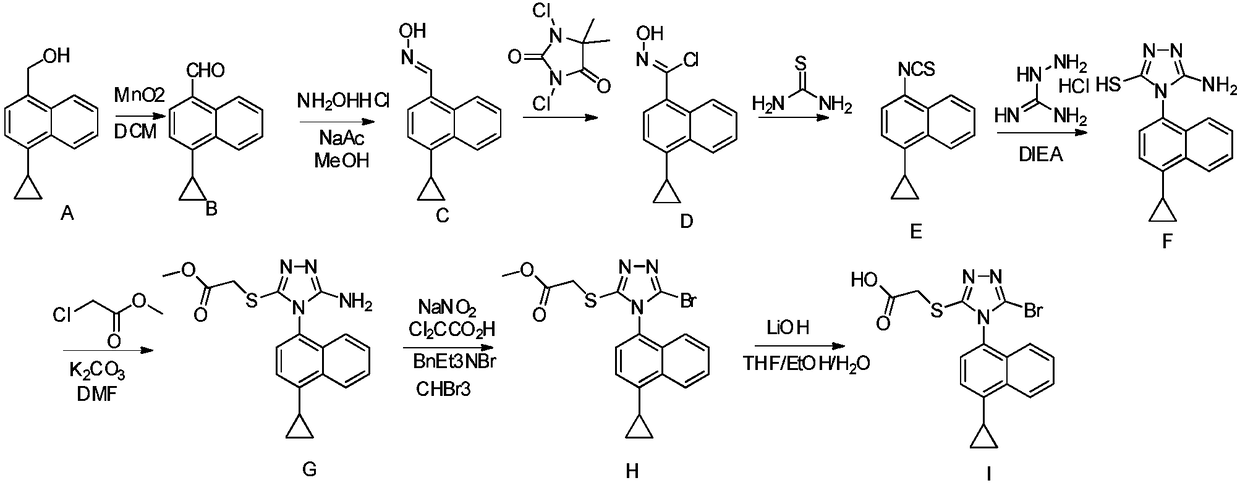
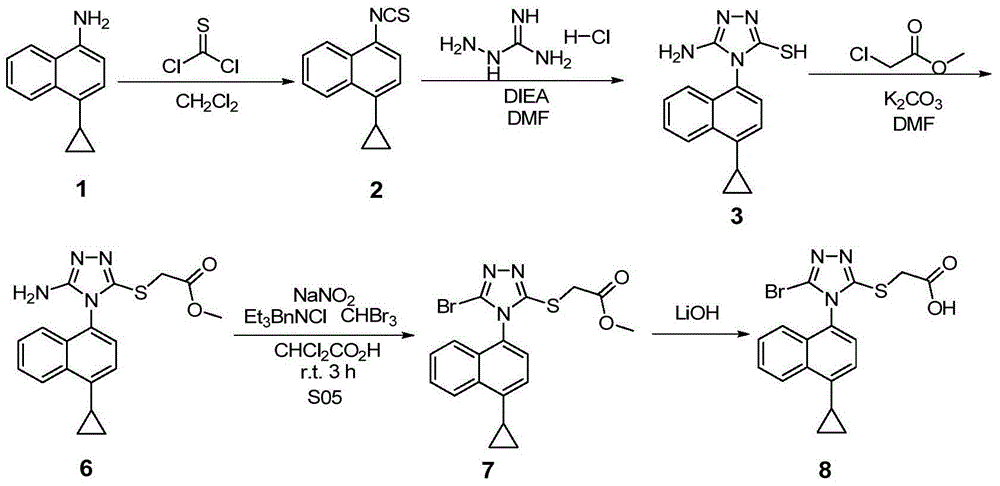
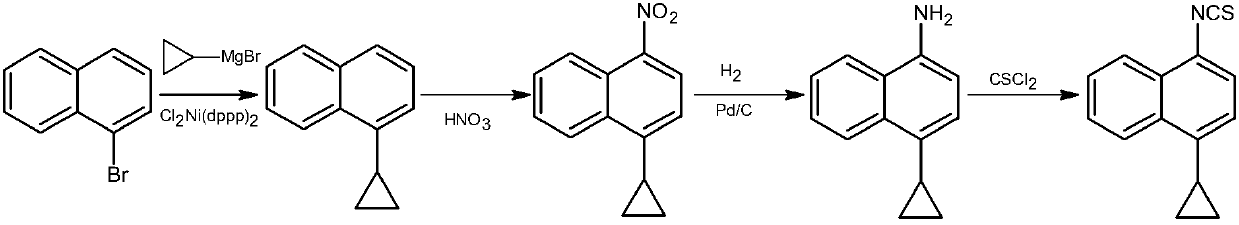
Preparation method of gout curative medicine Lesinurad and midbody of Lesinurad
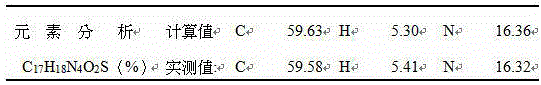
The invention provides a novel Lesinurad midbody and provides a synthesis process of Lesinurad, which is economic, efficient, safe, environment-friendly and applicable to large-scale industrial production. The invention further provides a method for preparing the conventional Lesinurad midbody and Lesinurad by using the novel Lesinurad midbody. When synthesized from the novel Lesinurad midbody provided by the invention, the Lesinurad has advantages that the price of necessary raw materials is low, the raw materials are easy to obtain, heavy metal and solvents which are harmful to the environment are not used, the midbody and a product can be easily separated and purified, the operation is easy, application of thiophosgene which is high in toxicity and not easy to operate is avoided, and the total reaction yield is approximate to or higher than that in the prior art.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
Resolving method of medicine lesinurad axial chiral enantiomer
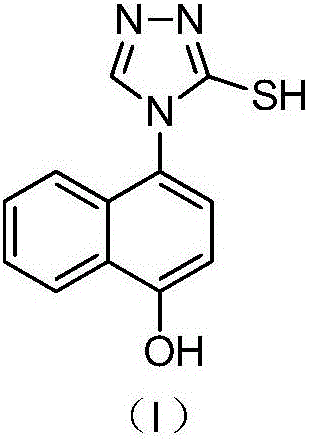
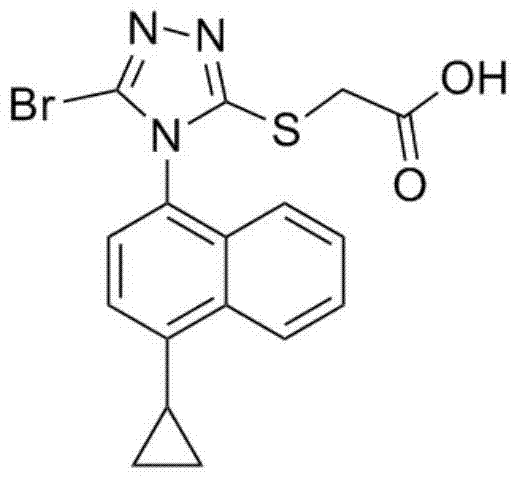
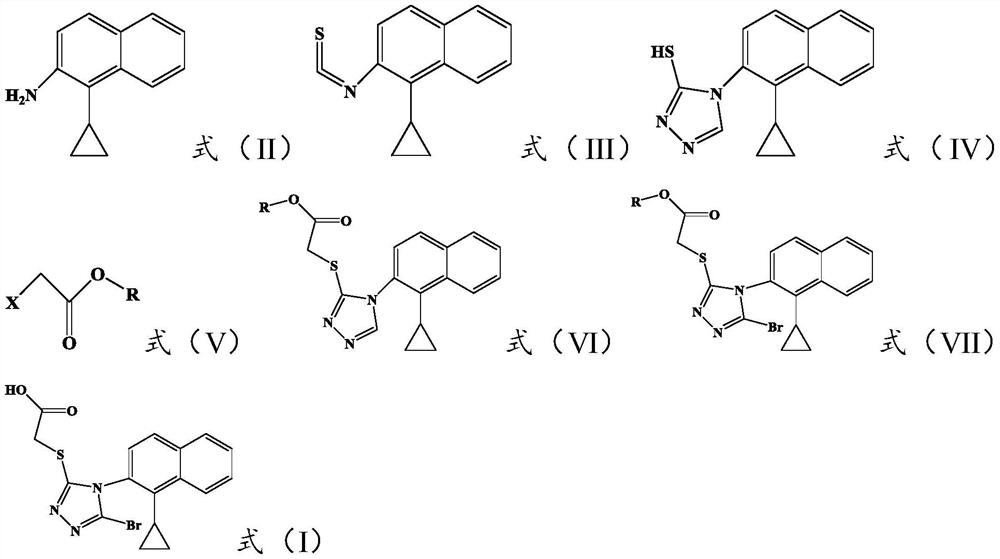
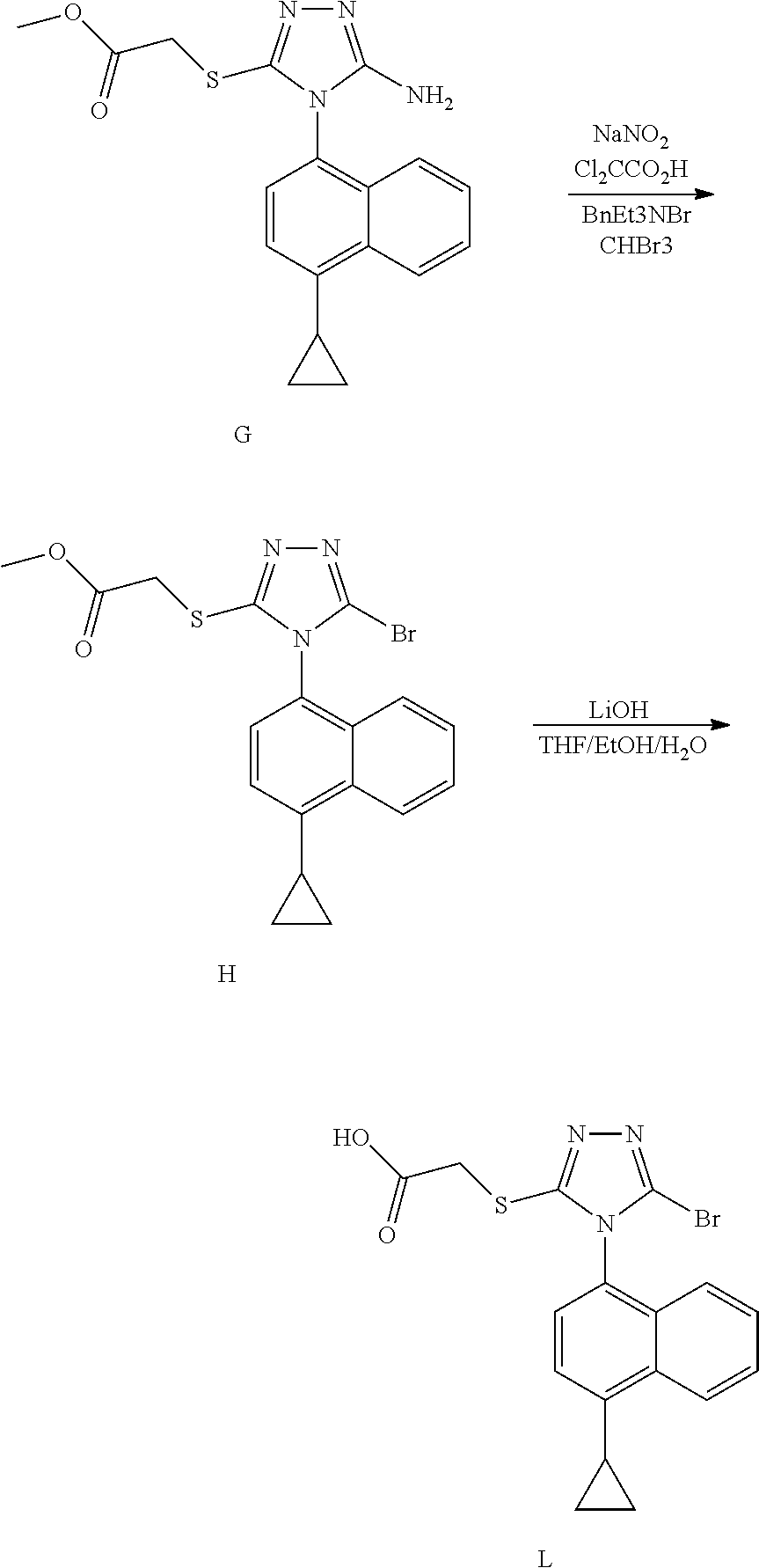
The invention discloses a resolving method of a medicine lesinurad axial chiral enantiomer. An alkamine derivative with optical activity is used as a resolving agent and reacts with a lesinurad racemic body in an organic solvent to form salts; the salts are dissociated to obtain (R)- or (S)-2-(5-bromo-4-(4-cyclopropylnaphthalene-1-yl-4H-1,2,4-triazole-3-sulfenyl)acetic acid with optical activity is obtained. By the method, an S configuration axial chiral enantiomer and an R configuration axial chiral enantiomer with the optical activity ee reaching 93 percent or higher can be obtained.
Owner:ZHEJIANG JINGXIN PHARMA +1
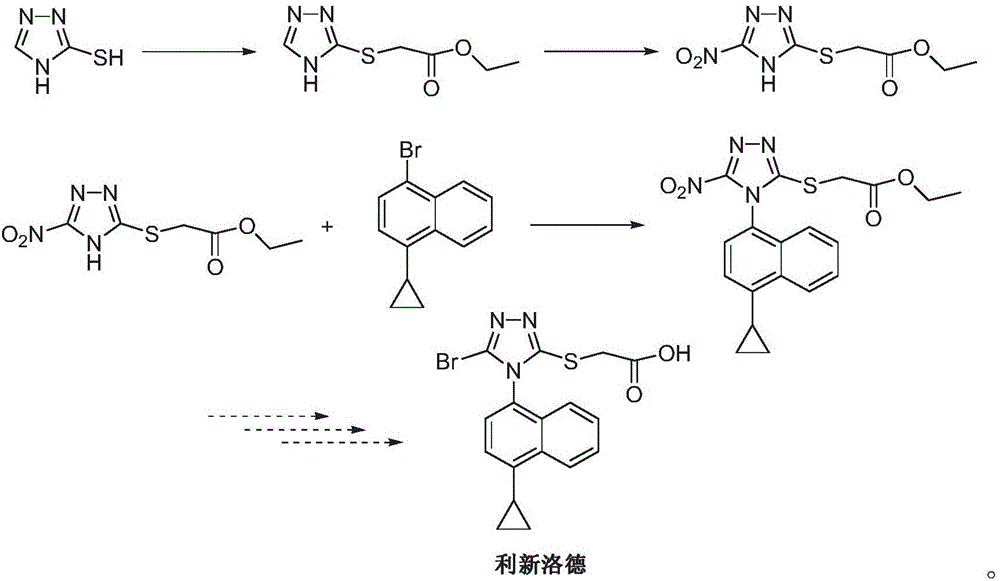
Preparing method for (4-(4-cyclopropyl-naphthalene-1-yl)-5-nitr-4H-(1, 2, 4) triazole-3-ylsulfanyl)-ethyl acetate and intermediate (5-nitr-4H-(1, 2, 4) triazole-3-sulfenyl)-ethyl acetate thereof
The invention provides a preparing method for (4-(4-cyclopropyl-naphthalene-1-yl)-5-nitr-4H-(1, 2, 4) triazole-3-ylsulfanyl)-ethyl acetate and an intermediate (5-nitr-4H-(1, 2, 4) triazole-3-ylsulfenyl)-ethyl acetate thereof. The intermediate is used for synthesizing an anti-gout drug Lesinurad, and has the advantages of being economical, environmentally friendly, efficient, high in yield and the like.
Owner:ANHUI WANBANG MEDICAL TECH
Preparation method of Lesinurad
InactiveCN106220577AEase of industrial productionEco-friendly economyOrganic chemistryLesinuradAcetic acid
The invention discloses a preparation method of a novel drug Lesinurad for gout treatment. The preparation method comprises the preparation step that Lesinurad is prepared by taking 1-bromo-4-cyclopropyl naphthalene and 2-[(3-bromo-4H-1,2,4-triazole-5-yl)sulfo]acetic acid as raw materials through a condensation reaction in one step. According to the preparation method of Lesinurad, the raw materials are easy to obtain, the process is simple, the economical property and environmental friendliness are achieved, and the method is suitable for industrialized production.
Owner:SUZHOU MIRACPHARMA TECH
Refining method of lesinurad
The invention discloses a synthetic refining method of lesinurad. The method includes: taking 4-(4-cyclopropyinaphthalene-1-yl)-1H-1, 2, 4-triazole-5(4H)-mercaptan as the starting raw material, carrying out alkylation reaction with ethyl bromoacetate, bromination reaction with N-bromosuccinimide, sodium hydroxide hydrolysis, hydrobromic acid neutralization, refining and drying to successfully synthesize a high purity lesinurad bulk drug finished product, and purifying the intermediates of all steps and the product, thus obtaining the high purity final product meeting the requirements. The method provided by the invention is easy for product collection, and is suitable for industrial production.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
Preparation method of lesinurad
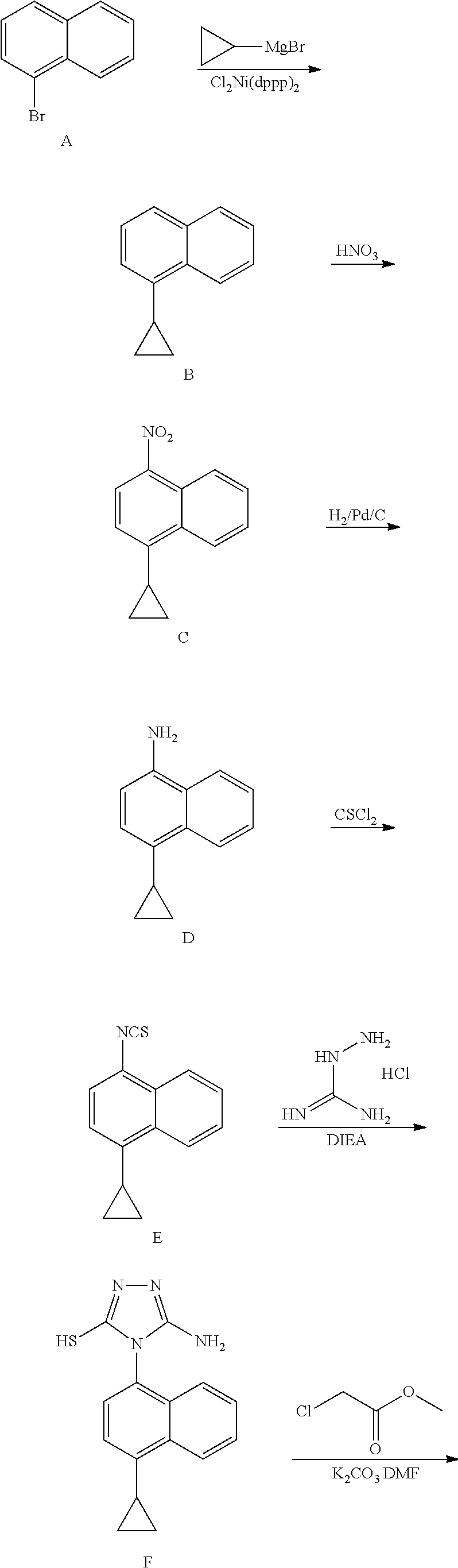
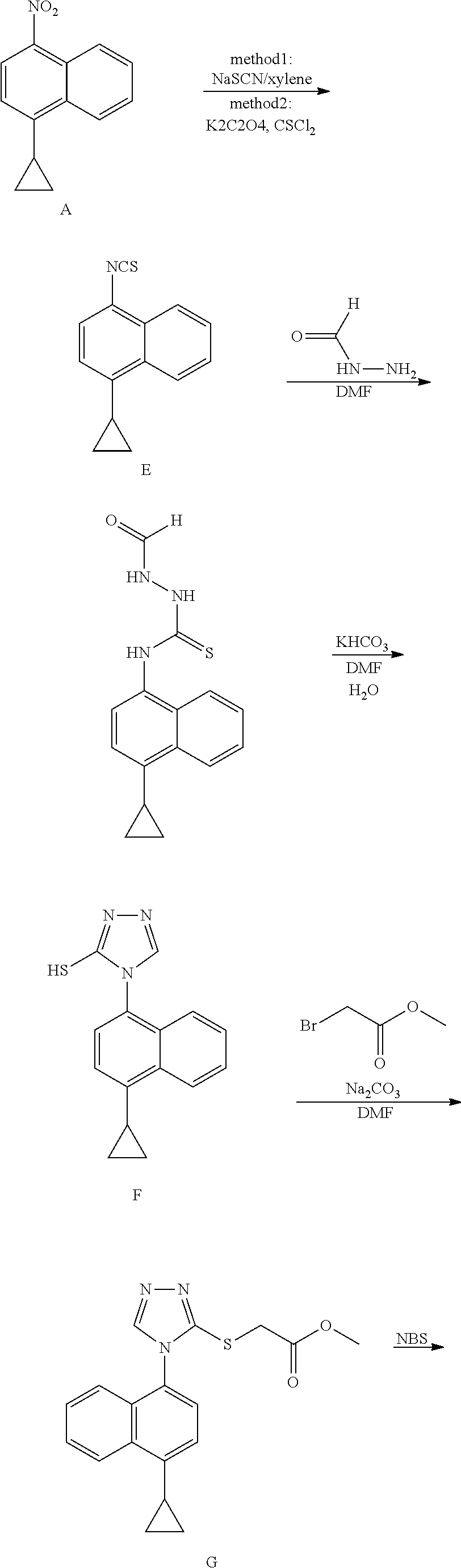
The invention discloses a preparation method of lesinurad. The method comprises: allowing 1-cyclopropylnaphthalene-4-yl isorhodanate and acethydrazide to undergo an addition reaction, allowing the obtained 4-acetyl-1-(4-cyclopropylnaphthalene-1-yl) thiosemicarbazide to undergo a cyclization reaction in the function of an alkali reagent, allowing the obtained 4-(4-cyclopropylnaphthalene-1-yl)-5-methyl-4H-1,2,3-triazole-3-mercaptan and methyl chloroacetate (or methyl bromoacetate) to undergo a condensation reaction, allowing the obtained 2-{[4-(4-cyclopropylnaphthalene-1-yl)-5-methyl-4H-1,2,4-triazole-3-yl]sulfo} methyl acetate to undergo an oxidation reaction, allowing the obtained 5-[(methoxycarbonyl)methylthio]-4-(4-cyclopropylnaphthalene-1-yl)-4H-1,2,4-triazole-3-carboxylic acid undergo a bromination reaction, and carrying out an esterolysis reaction to obtain lesinurad. The method is reasonable and simple in technical route, simplified in operation and low in cost. The method is green and eco-friendly and suitable for industrial production.
Owner:湖南欧亚药业有限公司
Method for preparing lesinurad intermediate
The invention discloses a method for preparing a lesinurad intermediate shown in the formula (I). By means of the method, triazole functional groups can be directly introduced, the reaction yield is as high as 96.30%, and use of thiophosgene and other toxic reagents is avoided. Besides, a cyclization step is not needed in the synthetic route, the final product can be obtained through six-step reaction, preparation is easy, cost is low, and the method is suitable for industrial production. Please see the structural formula in the description.
Owner:CHENGDU BAIYU PHARMA CO LTD
Hyperuricemia medicine composition and purpose thereof
ActiveCN111714485ASmall toxicityGood for lowering uric acidHydroxy compound active ingredientsAntipyreticLesinuradEster prodrug
The invention belongs to the field of chemical medicine, and particularly relates to a hyperuricemia medicine composition and a purpose thereof, including the purpose of a carbene compound in a structure shown as the formula (I) and pharmaceutically acceptable salts, ester, prodrug, a solvate, a polymorphic substance, a hydrate or a derivative of the carbene compound combined with hyperuricemia medicine to preparation of combined medicine for treating hyperuricemia. The invention also provides a hyperuricemia medicine composition. The hyperuricemia medicine composition comprises the compound,and either benzbromarone or lesinurad. The hyperuricemia medicine composition can achieve the equivalent or even better urate lowering effects than the hyperuricemia medicine in the prior art, but thetoxic and side effects of the hyperuricemia medicine can be obviously reduced; the safety is improved; and the hyperuricemia medicine composition can be used for treating hyperuricemia, and gout or gout complications caused by hyperuricemia.
Owner:CATCH BIO SCI & TECH
New compound for gout and preparation method thereof, and application and pharmaceutical preparation of new compound
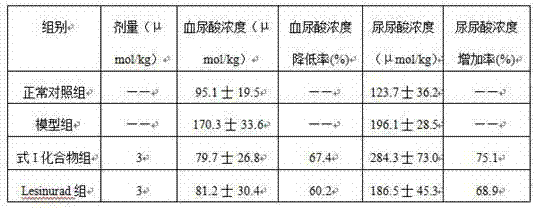
InactiveCN104710374AEasy to prepareLower blood uric acid concentrationOrganic active ingredientsOrganic chemistryLesinuradHydroxylamine
The invention relates to a new compound lesinurad hydroxamate disclosed as Formula I and a preparation method thereof, a pharmaceutical composition and preparation containing the new compound, and application of the new compound in drug preparation. The preparation method is implemented by reacting lesinurad methyl ester under the action of hydroxylamine hydrochloride. The method has the advantages of accessible raw materials, mild reaction conditions, high product yield and high product purity, and is simple to operate. The new compound has specific therapeutic actions on hyperuricemia, gout, arthritis and gouty arthritis, has the characteristics of high efficiency, low toxicity and high metabolism stability, and has wide application prospects.
Owner:ANHUI YIXINMING PHARMA TECH
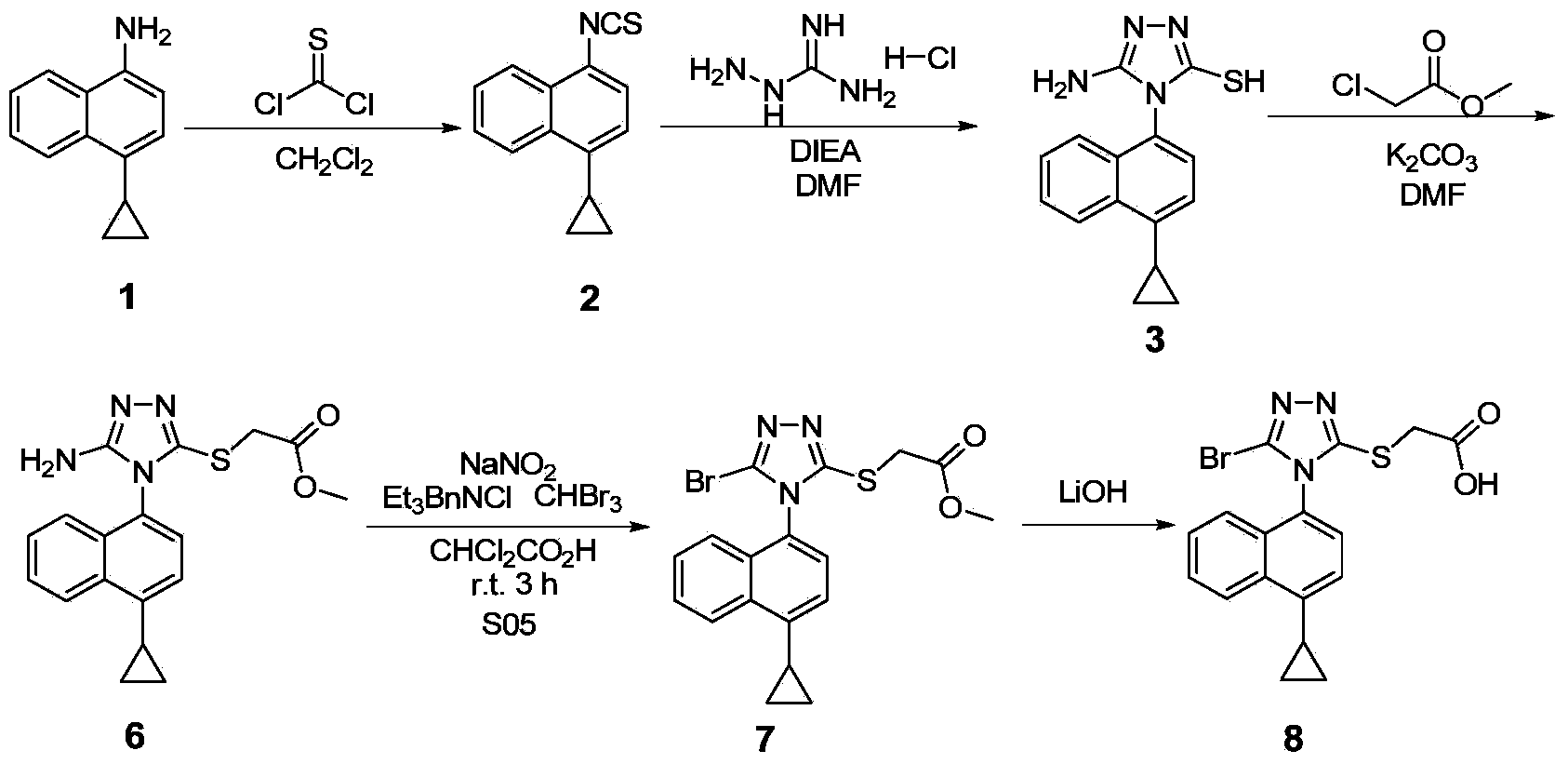
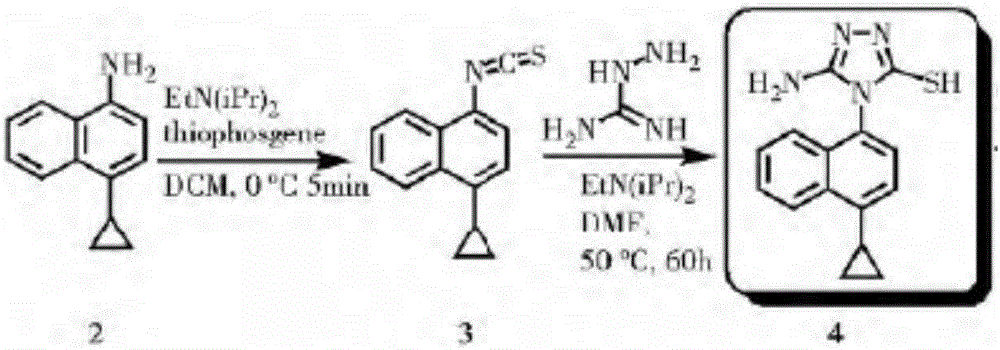
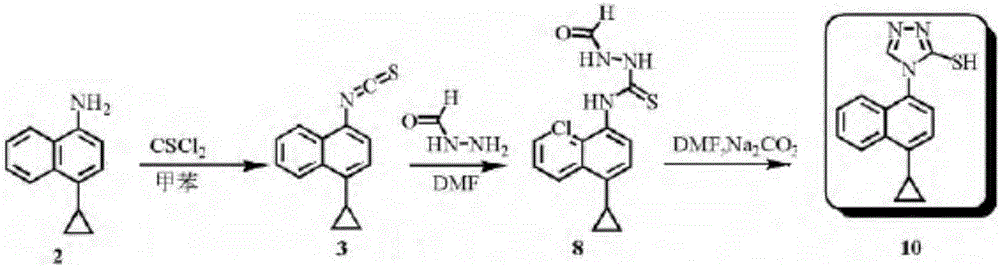
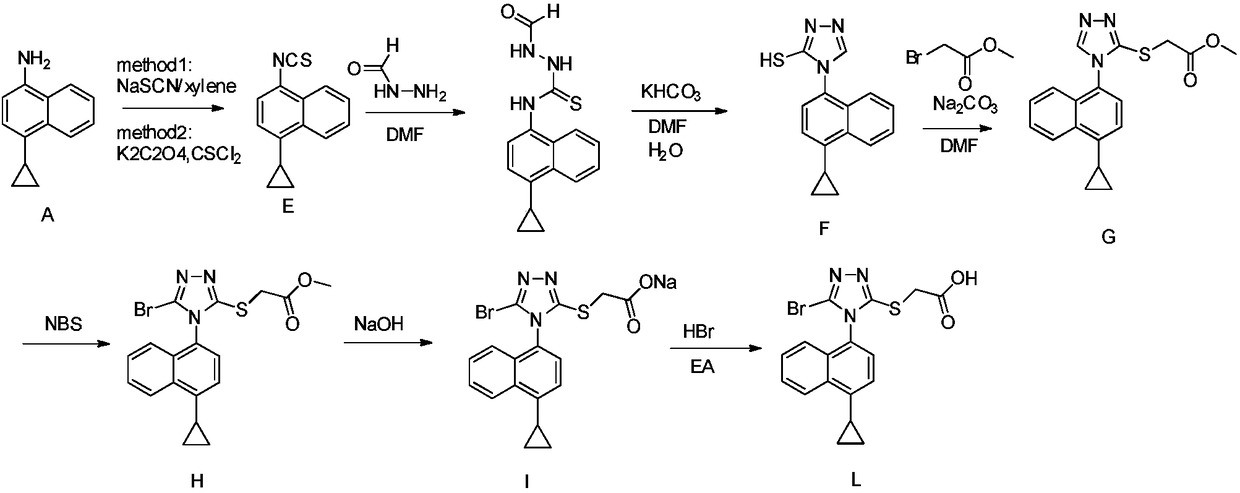
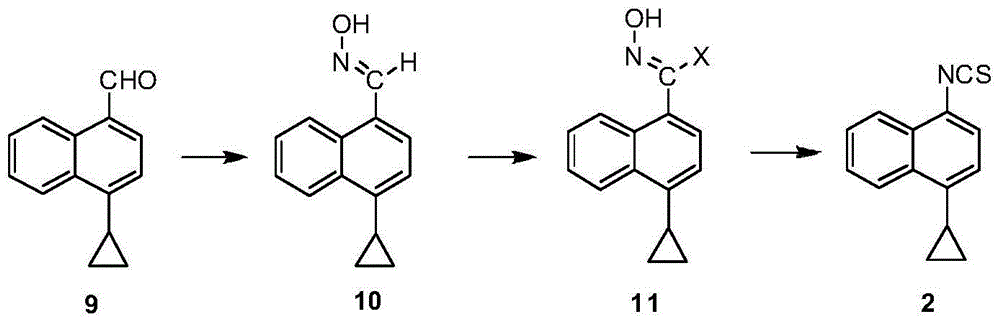
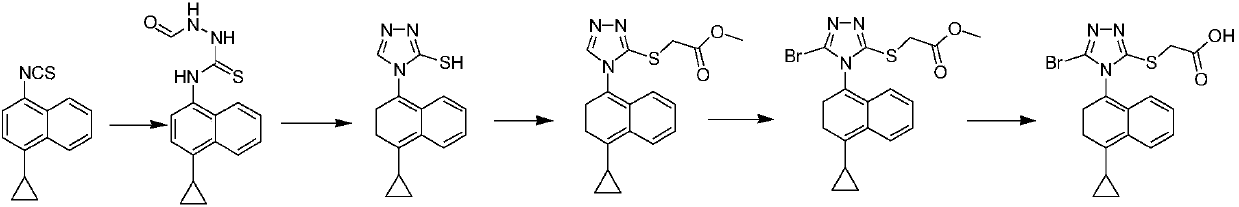
Preparation method of lesinurad intermediate namely 1-naphthyltriazole thioketone
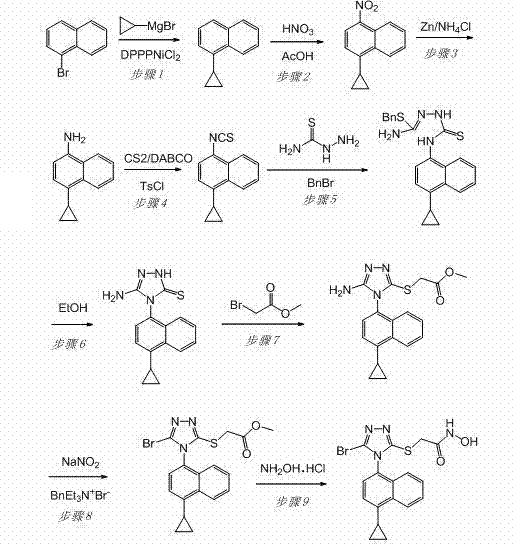
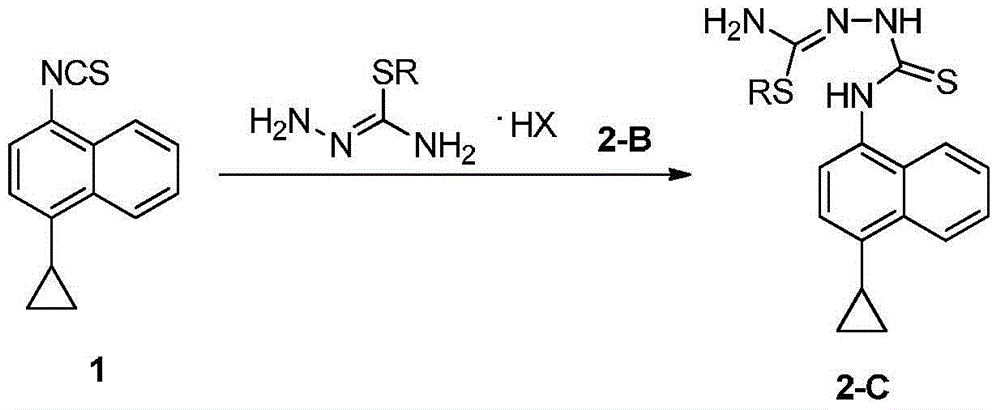
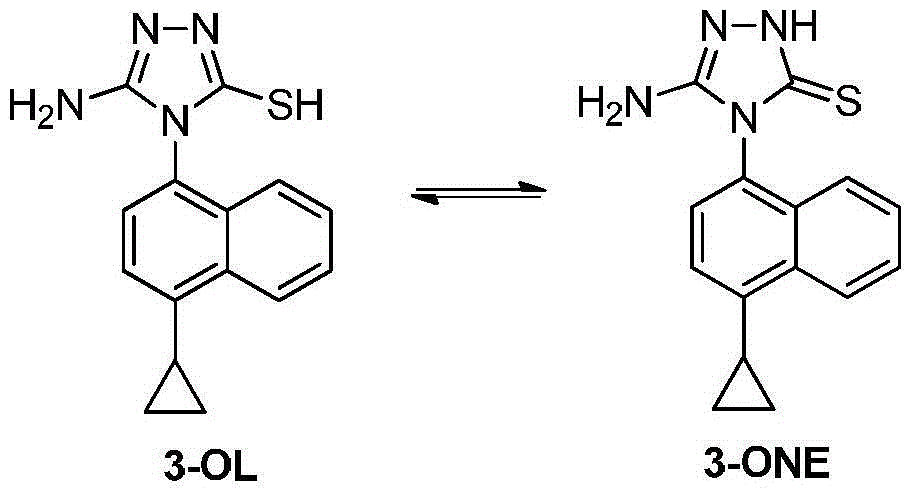
The invention discloses a preparation method of an intermediate, which can be used to prepare the drug lesinurad for treating gout. The intermediate is 3-amino-4-(4-cyclopropylnaphthalene-1-yl)-1H-1,2,4-triazole-5(4H)-thioketone(3). The preparation method has the advantages of short reaction time, high yield, and no need of column chromatography purification.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Lesinurad-containing compound composition and preparation method thereof
InactiveCN104814957ASolve common problemsImprove liquidityPowder deliveryOrganic active ingredientsLesinuradOrganic chemistry
The invention relates to a Lesinurad-containing compound composition. The Lesinurad-containing compound composition comprises Lesinurad and Febuxostat. The invention also relates to a preparation method of the Lesinurad-containing compound composition. The preparation method comprises the following steps of 1, Lesinurad-Febuxostat solid composition preparation and 2, solid agent preparation. The preparation method is simple and fast and is suitable for industrial production.
Owner:FUKANGREN BIO PHARMA
New preparation method of anti-gout medicine Lesinurad and its key intermediate
ActiveCN108947919AAvoid damageImprove conversion rateOrganic chemistryBulk chemical productionLesinuradTriflic acid
The invention provides a new preparation method of an anti-gout medicine Lesinurad and its key intermediate. By using the process provided by the invention, a compound IV can be directly converted into a product III without separation, the reaction yield is greatly increased, and the operation step can be simplified. In addition, the synthesis of the novel intermediate provided by the invention does not require the high-toxicity thiophosgene and carbon disulfide, which greatly improves the safety and environmental protection of the process. The novel synthesis preparation process of Lesinuradis highly efficient, economical, safe, environmentally friendly and suitable for industrial production. In the specification, wherein R is cyclopropane, halogen, trifluoro mesylate, mesylate, and p-toluenesulfonate, and preferably R is cyclopropane; R3 represents COCH3, or R3 represents benzyl or CH2R4, wherein R4 represents an ester group, CN, CH2OH or a phenyl group substituted with one or moreselected from C1-C6 alkyl group and halogen; and X is a halogen.
Owner:SHANGHAI AOBO PHARMTECH INC LTD +1
Preparation method of gout treatment drug Lesinurad and Lesinurad intermediate
Provided are a novel Lesinurad intermediate, providing a synthetic process more economical, more efficient, safer, more environmentally friendly, and suitable for large-scale industrial production for the preparation of Lesinurad. Also provided is a method for preparing the known Lesinurad intermediate and Lesinurad via the provided novel Lesinurad intermediate. Using the novel Lesinurad intermediate of the present invention to synthesize Lesinurad has the following advantages of using cheap and easily available required raw materials, avoiding the use of heavy metals and solvents harmful to the environment, easily separating and purifying the intermediate and the product with a simple operation, avoiding the use of the thiophosgene having high toxicity and difficulty of operation, and enabling the total yield of the reaction to reach a level equal to or higher than the prior art.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
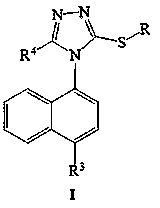
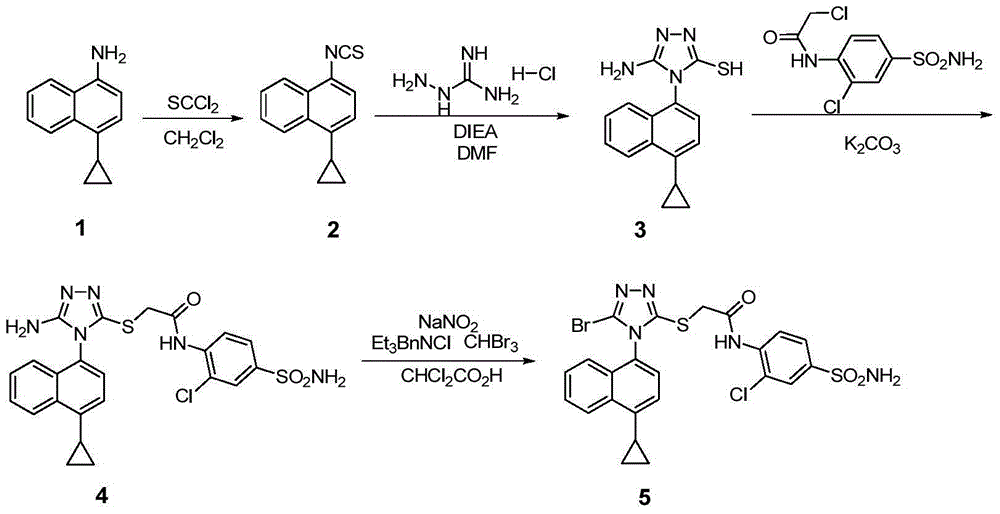
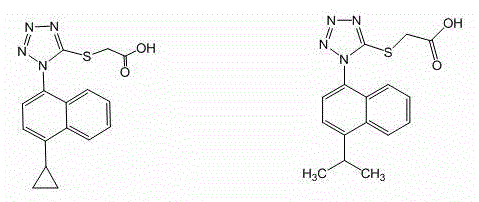
Lesinurad analog and preparation method and medical application thereof
ActiveCN104817509ASmall toxicityImprove securityOrganic chemistrySkeletal disorderLesinuradCombinatorial chemistry
The invention relates to a lesinurad analog as shown in the general formula (I), its preparation method, a pharmaceutical composition containing the derivative and an application of the pharmaceutical composition used as a therapeutic agent, especially as a medicine for treating hyperuricemia and gout, wherein definition of each substituent group in the general formula (I) is as the same as definition in the specification.
Owner:ANHUI HEALSTAR PHARM CO LTD
Preparation method of Lesinurad
ActiveCN107955029AShort routeHigh yieldGroup 3/13 element organic compoundsBulk chemical productionHigh selectivityDrug product
The invention discloses a preparation method of Lesinurad, and belongs to the technical field of chemical drug synthesis. A compound of the formula Les-03 in the description is prepared from compoundsshown in formulas Les-01 and Les-02 as raw materials, a compound of the formula Les-04 is added, and a compound of the formula Les-05 is prepared. The compound of the Les-05 has high selectivity during coupling, so that the purity of a reaction product is high, post-treatment is facilitated, and quality of an obtained final product is controllable; the compound in Les-07 is prepared from the compound in Les-05 and the compound in Les-06 by Suzuki coupling reaction, the Suzuki coupling reaction has high reliability and good repeatability, and finally Lesinurad is obtained through protecting group removal. The preparation method has the advantages of short process route, high yield and low cost; adopted reagents are non-toxic or low-toxic conventional reagents, and are basically harmless tooperators and basically pollution-free to the environment; the whole process is simple and convenient to operate, the process stability is good, the quality of the obtained final product is controllable and stable, and the method is suitable for commercial production.
Owner:成都美域高制药有限公司
Synthetic method of Lesinurad
ActiveCN106866560AHigh purityLess side effectsOrganic chemistryBulk chemical productionLesinuradHydrolysis
The invention relates to a synthetic method of Lesinurad. The method comprises the following steps: carrying out a nucleophilic addition reaction on an initial raw material 1-cyclopropylnaphthalene-4-yl-isorhodanate (compound 7) and methyl hydrazinocarboxylate to generate a compound 6, cyclizing the compound 6 under the action of sodium hydroxide to generate a compound 5, carrying out a condensation reaction on the compound 5 and a compound 4 to remove one molecule of halogen hydride in order to generate a compound 3, brominating the compound 3 to generate a compound 2, and hydrolyzing the compound 2 under the action of an alkali to obtain the target product Lesinurad. The preparation method is simple and easy; the compound 2 is prepared through carrying out the nucleophilic addition reaction on the hydroxyl group of the compound 3 by an introduced bromine atom, so generation of an alpha-bromocarboxylic acid impurity in the target product is avoided; the purity of the reaction product is high and reaches 99.5% or above; side reactions are few, so the yield of the target product is high and reaches 96%; and most raw materials used in the whole preparation process are nontoxic, so industrial production of the target product is conveniently realized.
Owner:ZHEJIANG MENOVO PHARMA
Naphthyl thiocarbonyl compound as well as preparation method and application thereof
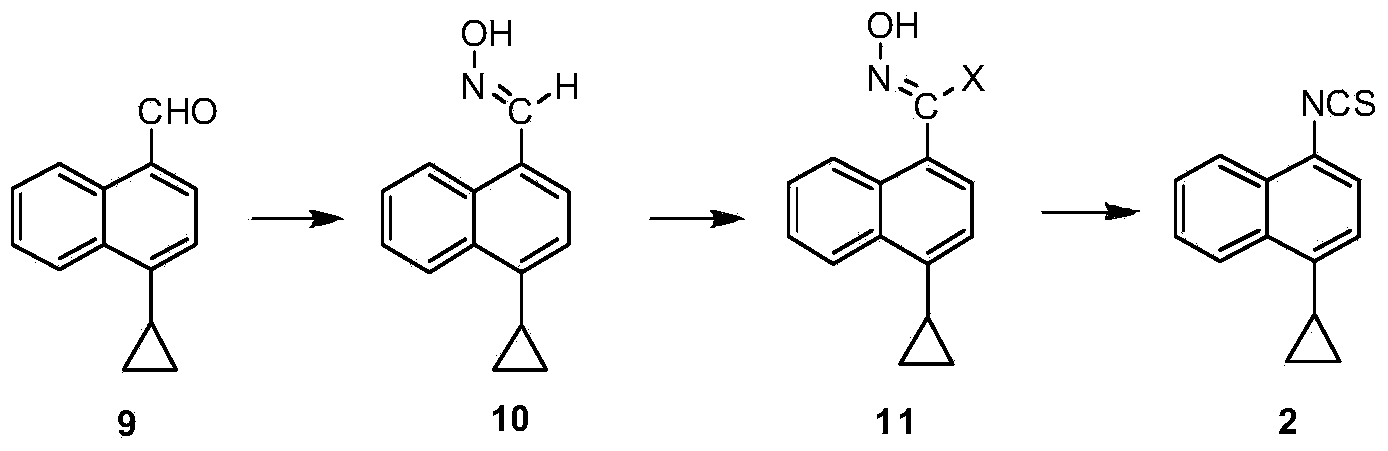
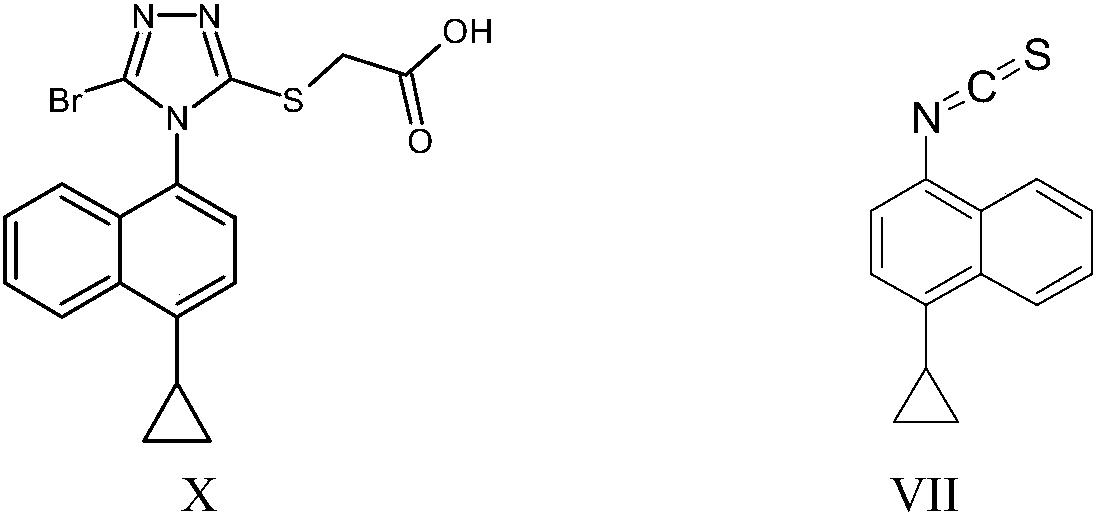
InactiveCN109516935ALow toxicityReduce pollutionOrganic compound preparationHydrocarbon from halogen organic compoundsLesinuradThio-
The invention provides a naphthyl thiocarbonyl compound of a structural formula I. The invention further provides a preparation method of the compound and application of the compound in preparation ofa lesinurad key intermediate 1-cyclopropylnaphthalene-4-benzyl isothiocyanate of a structural formula VII. The structural formula is as shown in the specification. In the formula, R represents hydroxyl or thio.
Owner:JIANGXI SYNERGY PHARMA
Medicinal composition containing febuxostat
The invention provides a medicinal composition containing febuxostat. Febuxostat and lesinurad as active components and proper auxiliaries are adopted to prepare the stable preparation through a preparation process. The preparation has excellent in vitro dissolution, and quickly acts in a body.
Owner:FUKANGREN BIO PHARMA
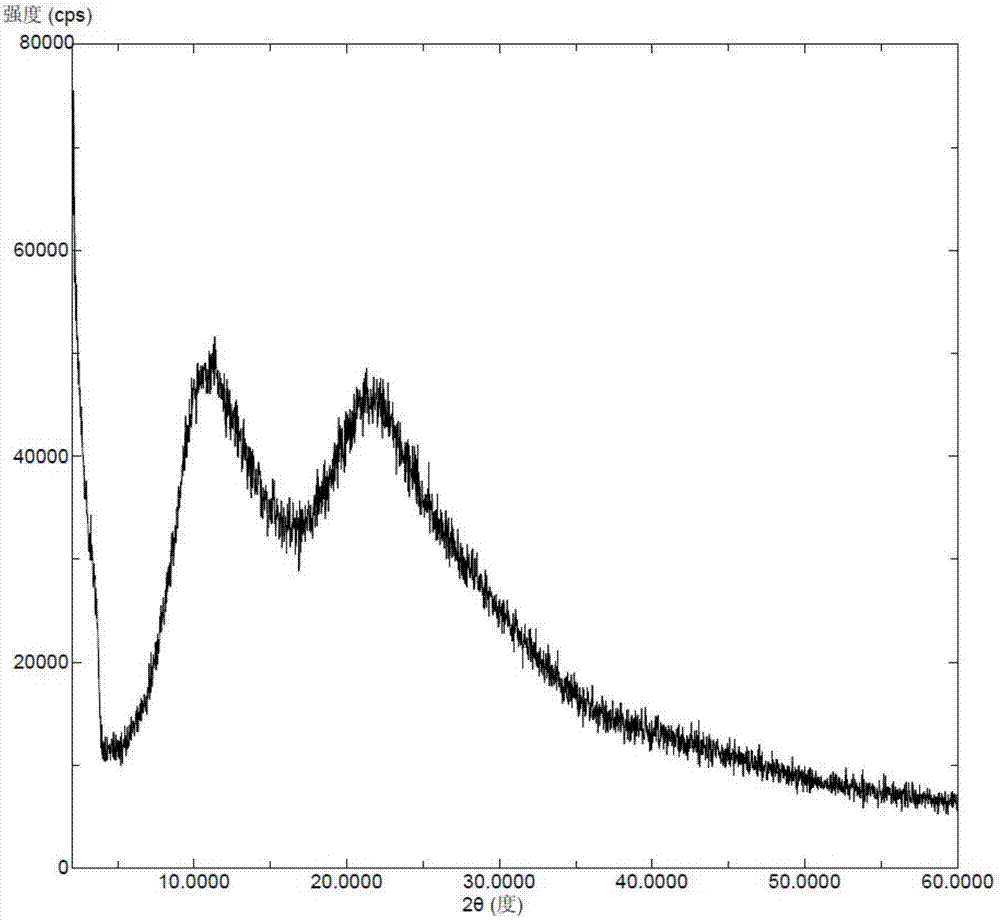
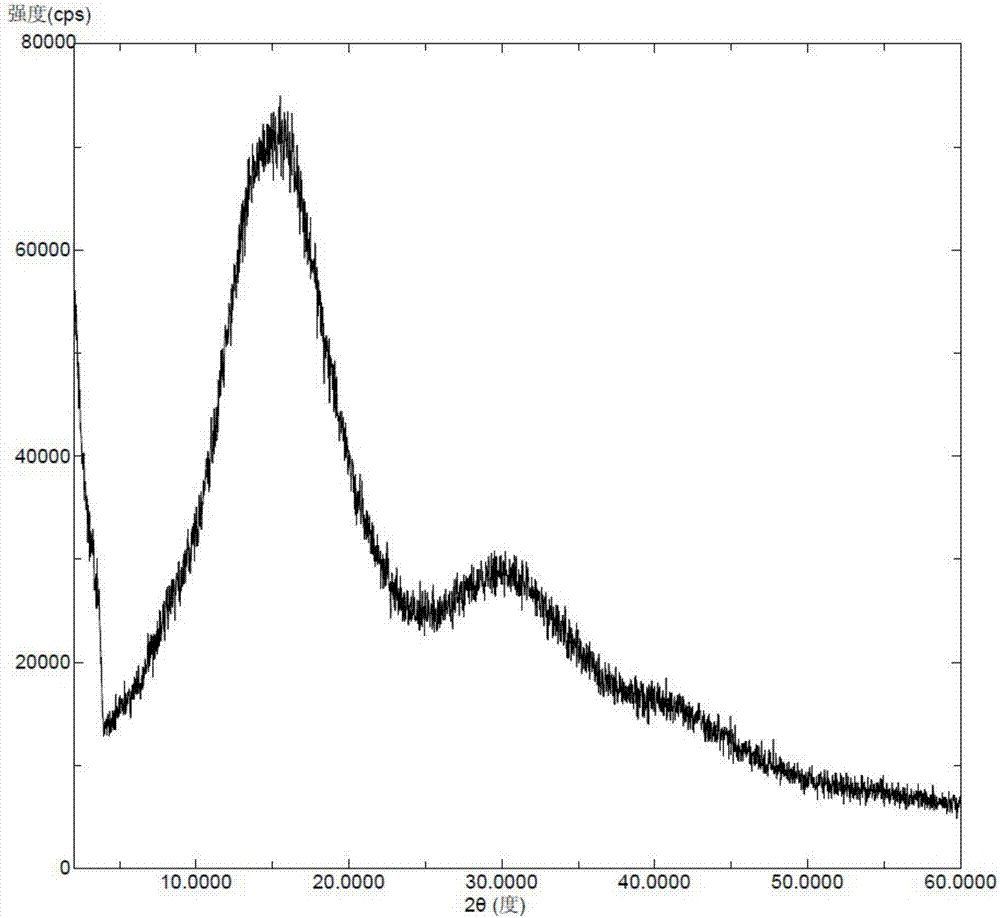
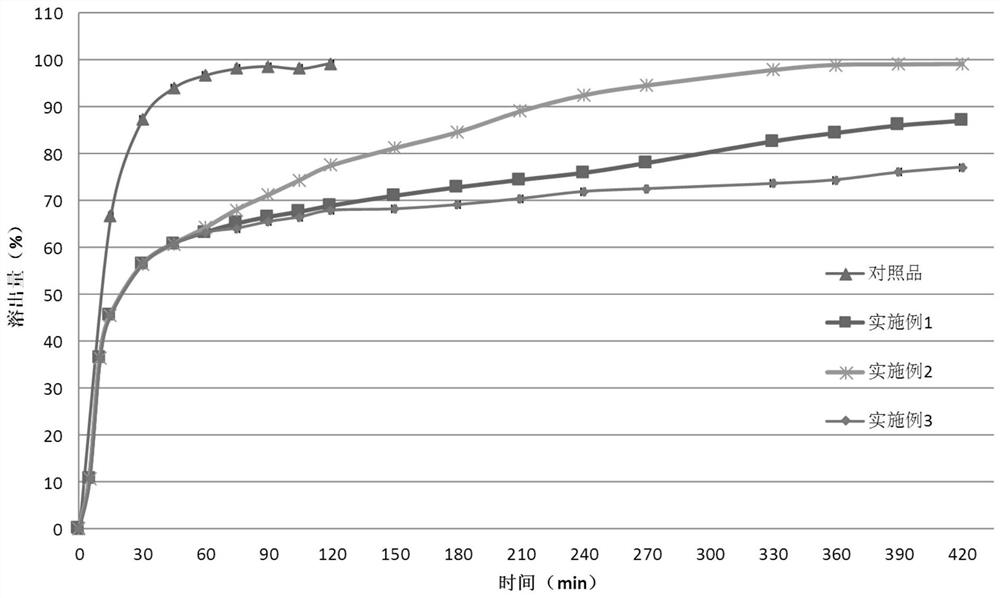
Solid dispersoid of lesinurad and pharmaceutic adjuvant and preparation method of solid dispersoid
InactiveCN107281108AImprove stabilityLow pricePowder deliveryOrganic active ingredientsLesinuradDispersity
The invention discloses solid dispersoid of amorphous lesinurad and a pharmaceutic adjuvant and a preparation method of the solid dispersoid. The solid dispersoid is prepared from the lesinurad and the pharmaceutic adjuvant; the weight ratio of the lesinurad to the pharmaceutic adjuvant is 1 to (0.1 to 100), wherein the lesinurad is in an amorphous state; the X-ray powder diffraction spectrum of the solid dispersoid has no the characteristic peak of a crystal of the lesinurad after the background peak of the pharmaceutic adjuvant is deducted. The solid dispersoid of the amorphous lesinurad and the pharmaceutic adjuvant, which is provided by the invention, is favorable in stability and dispersity, is used for increasing the dissolution rate of the lesinurad, is used for more beneficially improving the bioavailability of a medicinal preparation and the absorption of an organism to a medicine, and in an accelerated test condition, can be used for maintaining favorable physical stability and chemical stability. The preparation method of the amorphous solid dispersoid, which is provided by the invention, is simple to operate, low in cost, good in repeatability and easy to realize, and is suitable for industrialized production.
Owner:CHANGZHOU FANGNAN MEDICINE TECH CO LTD
Preparation method of lesinurad oxidation impurity
PendingCN112778223AHigh purityThe synthesis method is simpleOrganic chemistryLesinuradOrganic solvent
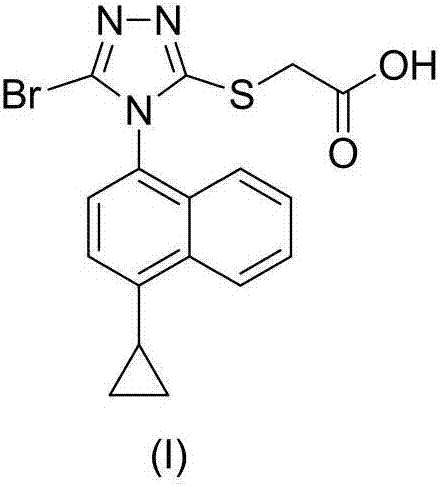
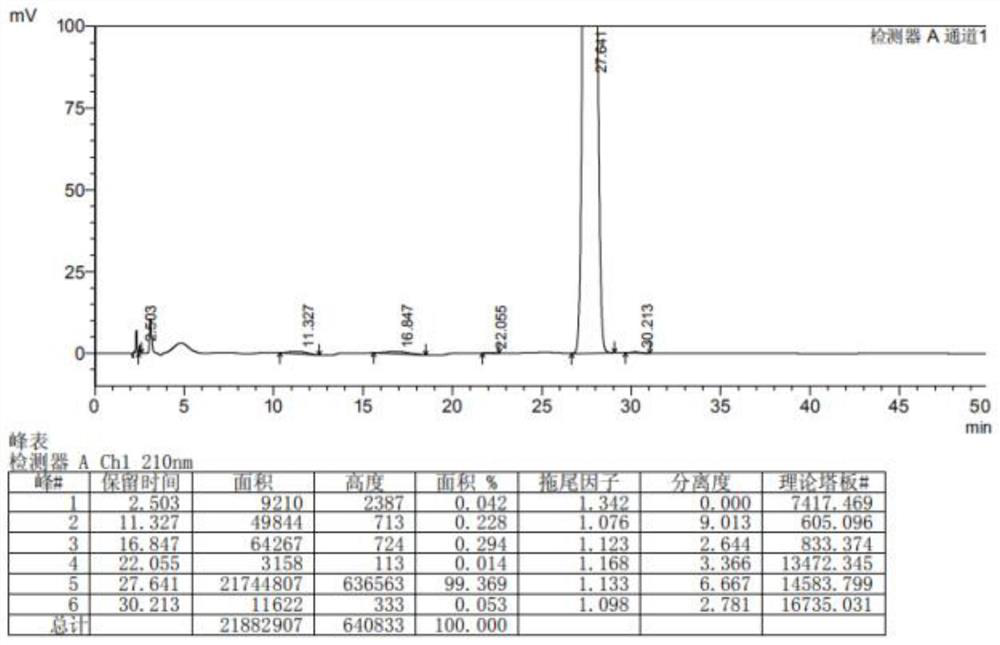
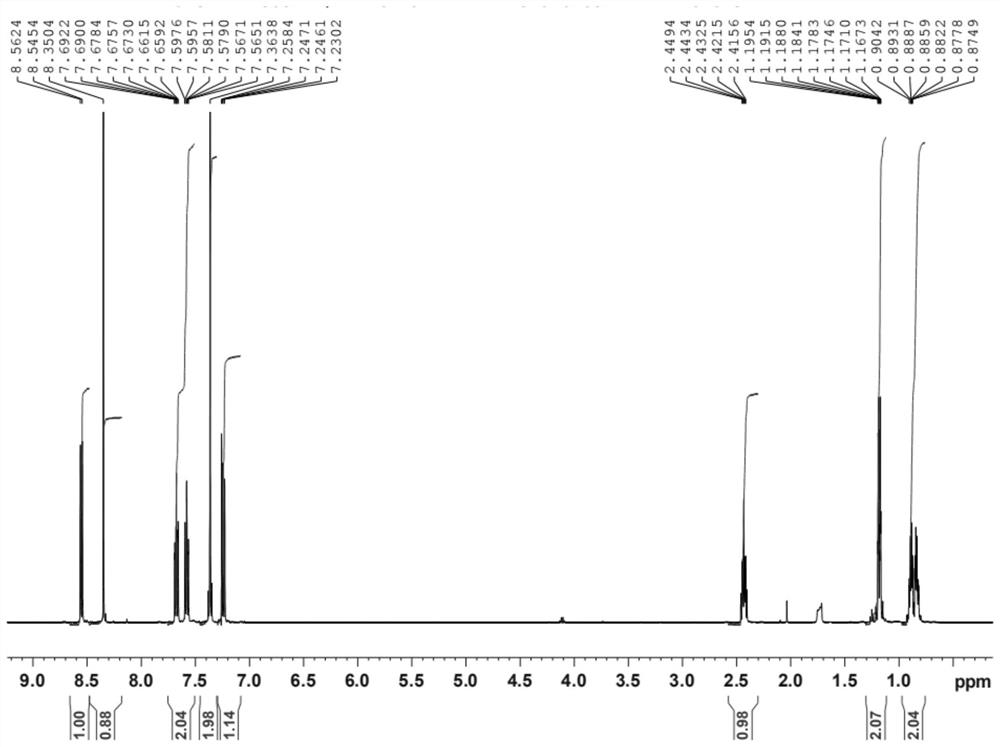
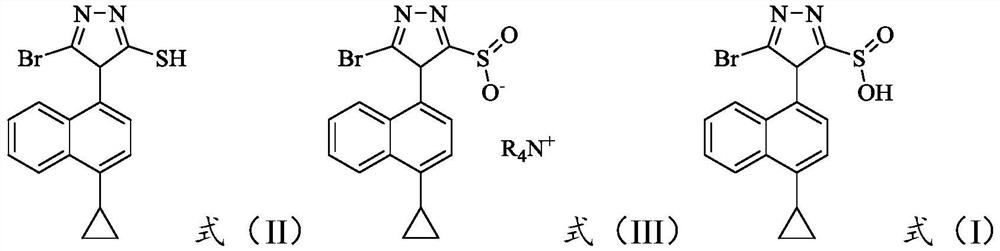
The invention provides a preparation method of a lesinurad oxidation impurity, which comprises the following steps: S1) oxidizing a compound as shown in a formula (II) under an alkaline condition, then adjusting the pH value of a reaction solution to be acidic, and conducting reacting with a quaternary ammonium salt to obtain a compound as shown in a formula (III); and S2) dissolving the compound as shown in the formula (III) in a mixed solvent of water and a first organic solvent, adjusting the pH value to be acidic, and then separating an organic phase to remove the solvent, thereby obtaining the lesinurad oxidation impurity as shown in a formula (I). Compared with the prior art, the synthetic method disclosed by the invention is simple, the obtained sample is high in purity, the method can be used for the links of process research and development, production, quality standard establishment and quality control of the lesinurad, and a technical support is provided for the medication safety of the lesinurad.
Owner:HAINAN XINOPEN SOURCE MEDICAL TECH CO LTD
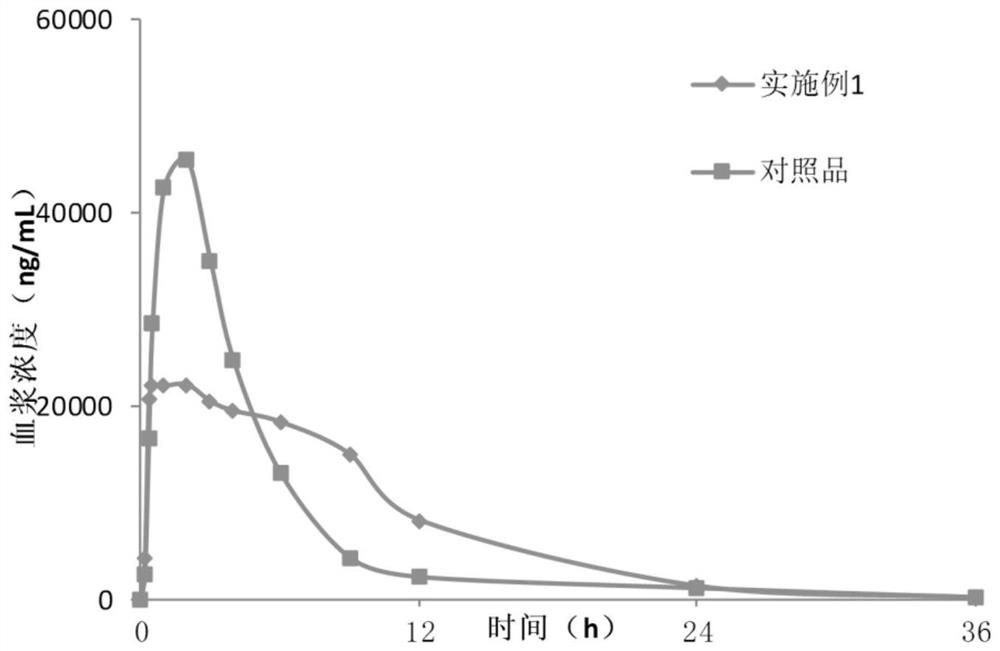
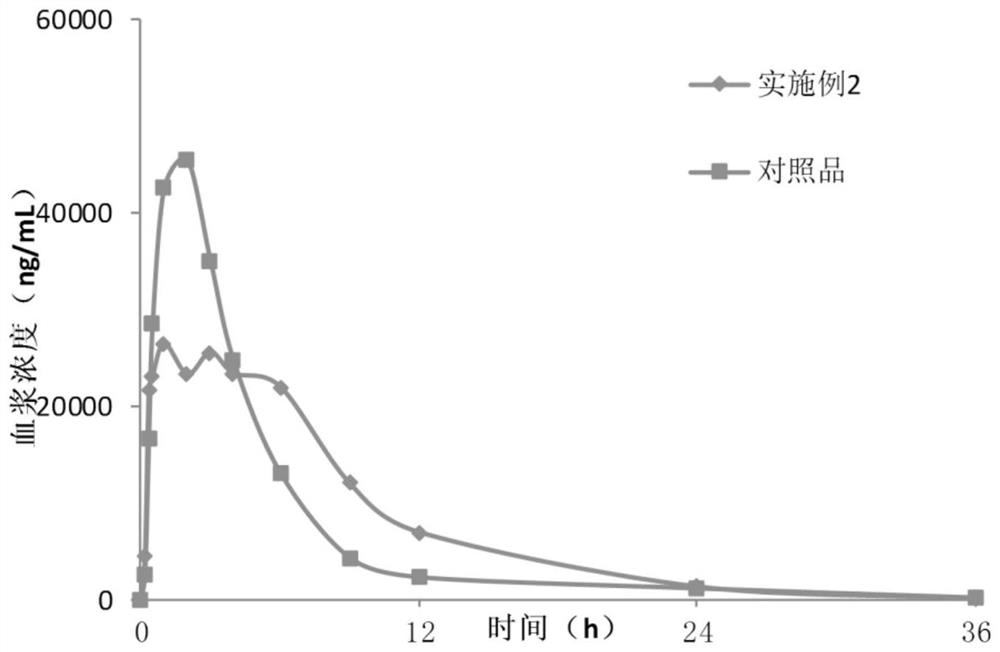
Lesinurad controlled-release pharmaceutical composition
ActiveCN112057429AReduce drug riskIncrease release speedOrganic active ingredientsSkeletal disorderLesinuradImmediate release
The invention discloses a lesinurad controlled-release pharmaceutical composition. The lesinurad controlled-release pharmaceutical composition has the following characteristics: (i), within 12 hours after oral administration of the pharmaceutical composition, the plasma concentration of lesinurad is kept above 6 mu g / mL; and (ii), after oral administration of the pharmaceutical composition, the maximum plasma concentration of lesinurad is 45%-60% of the equal dose of lesinurad administered orally alone. The lesinurad controlled-release pharmaceutical composition in the invention has improved release and dissolution rates; and, compared with common quick-release tablets, the lesinurad controlled-release pharmaceutical composition has lower toxic and side effects, enhances the drug effect, can obviously reduce the medication risk of lesinurad, and meanwhile, enhances the drug administration compliance.
Owner:SHANGHAI JINGXIN BIOLOGICAL MEDICAL +1
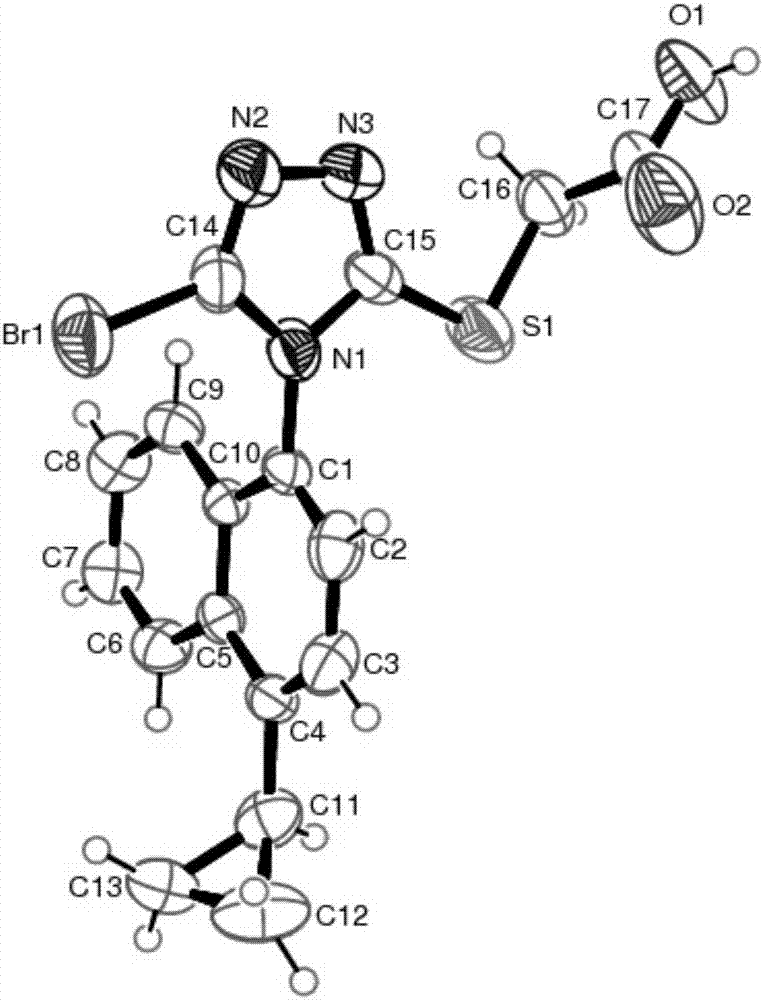
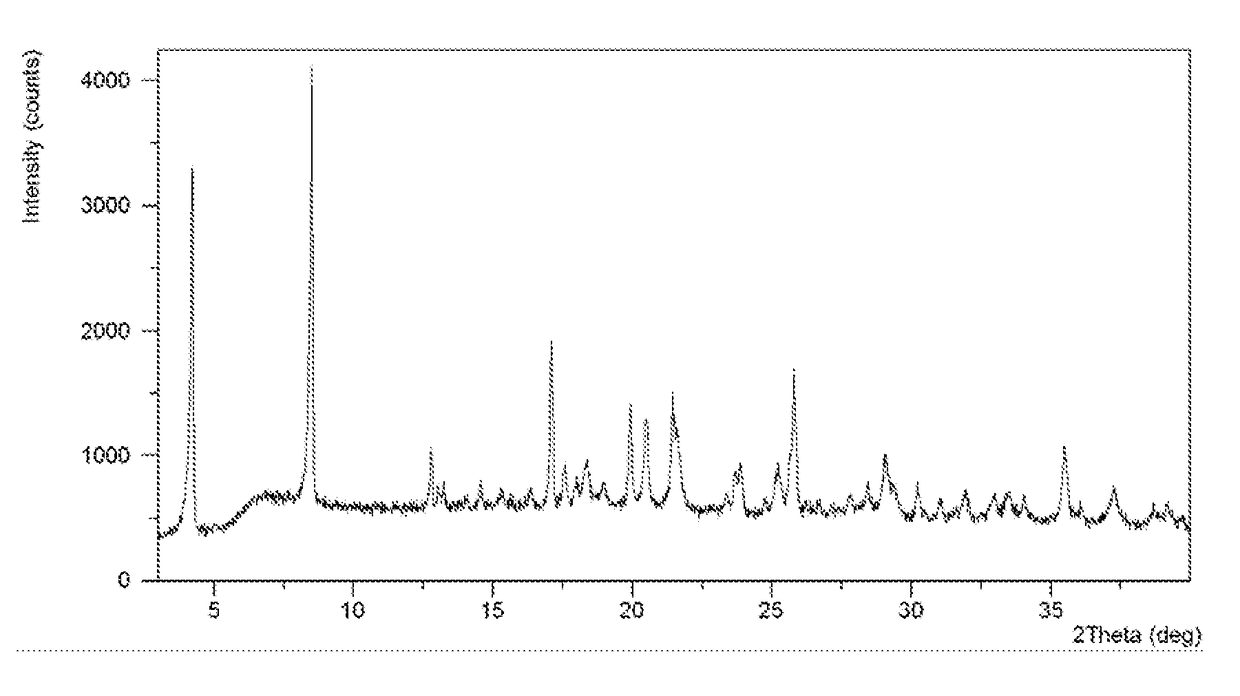
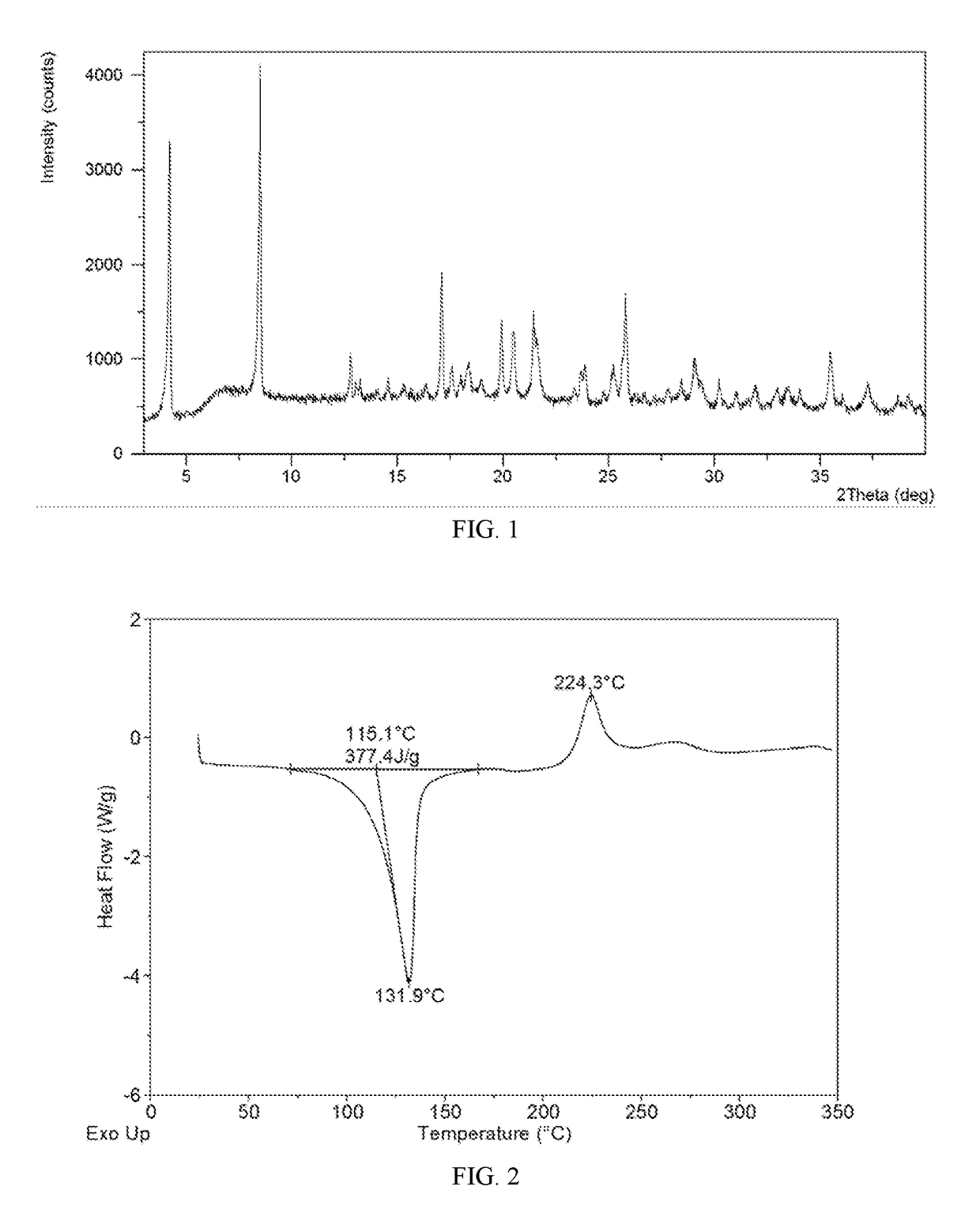
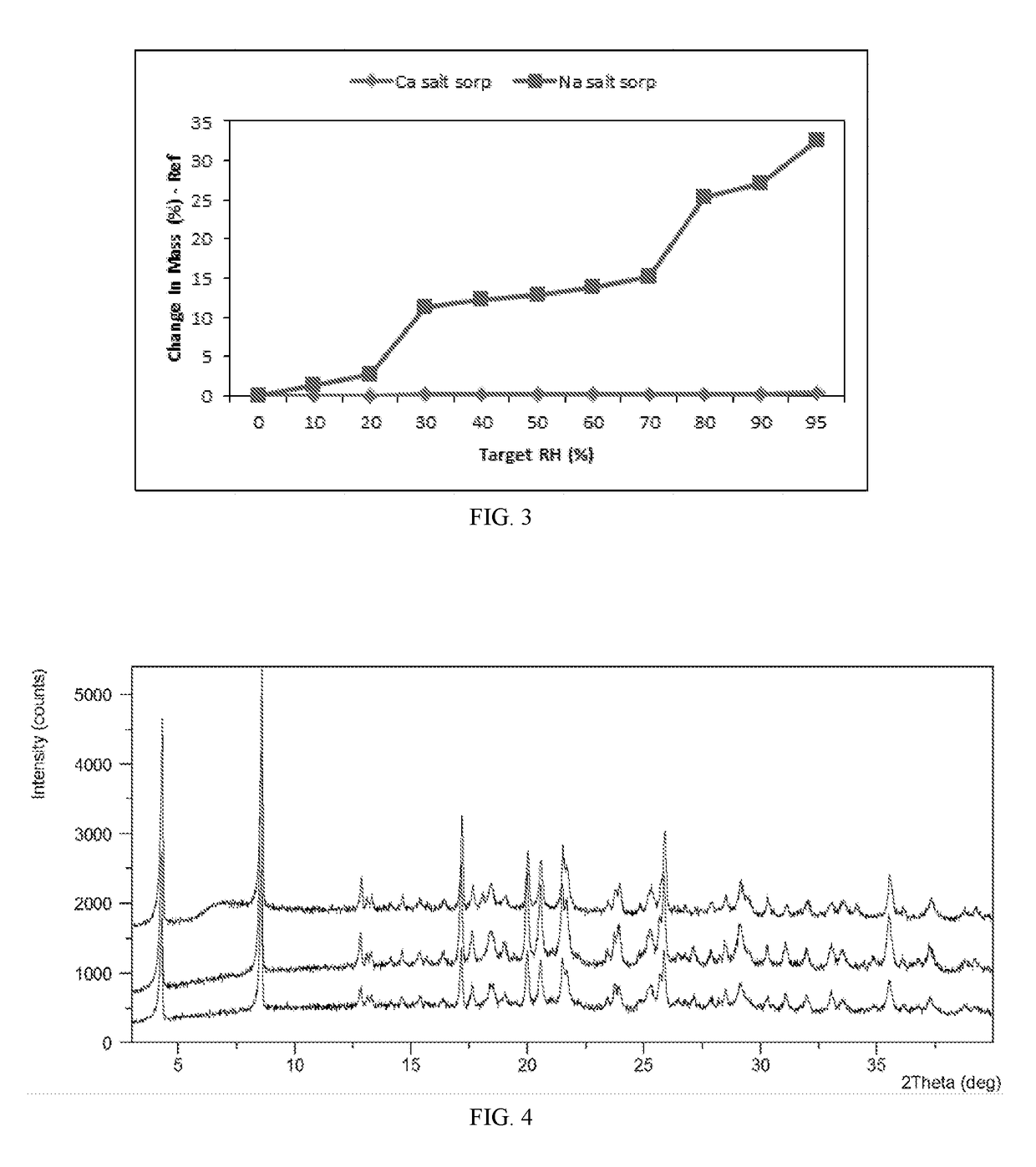
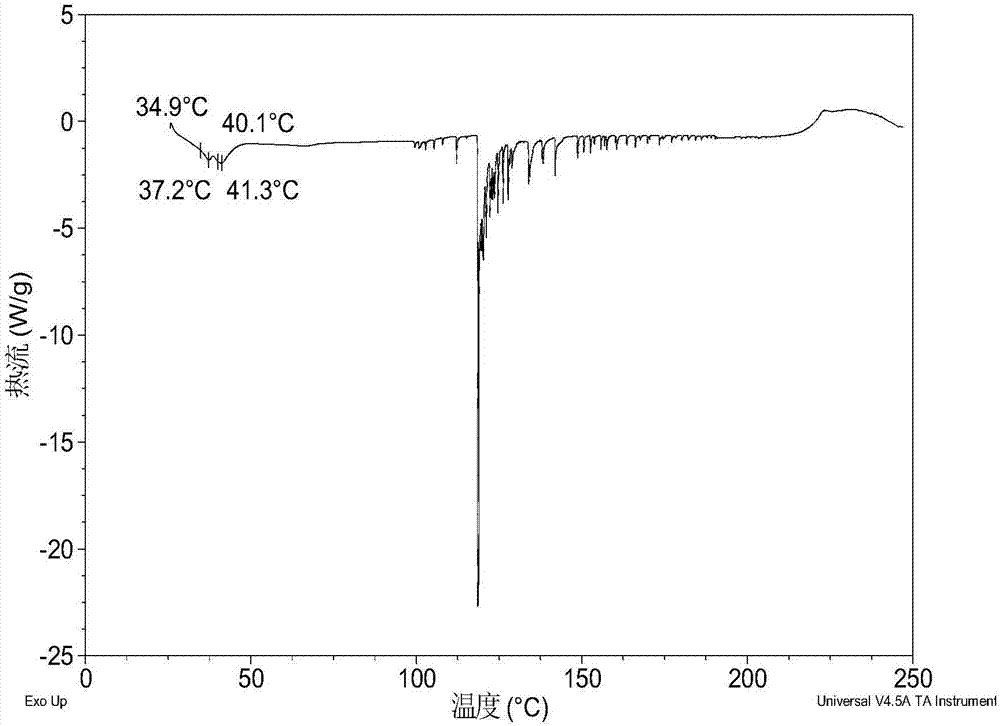
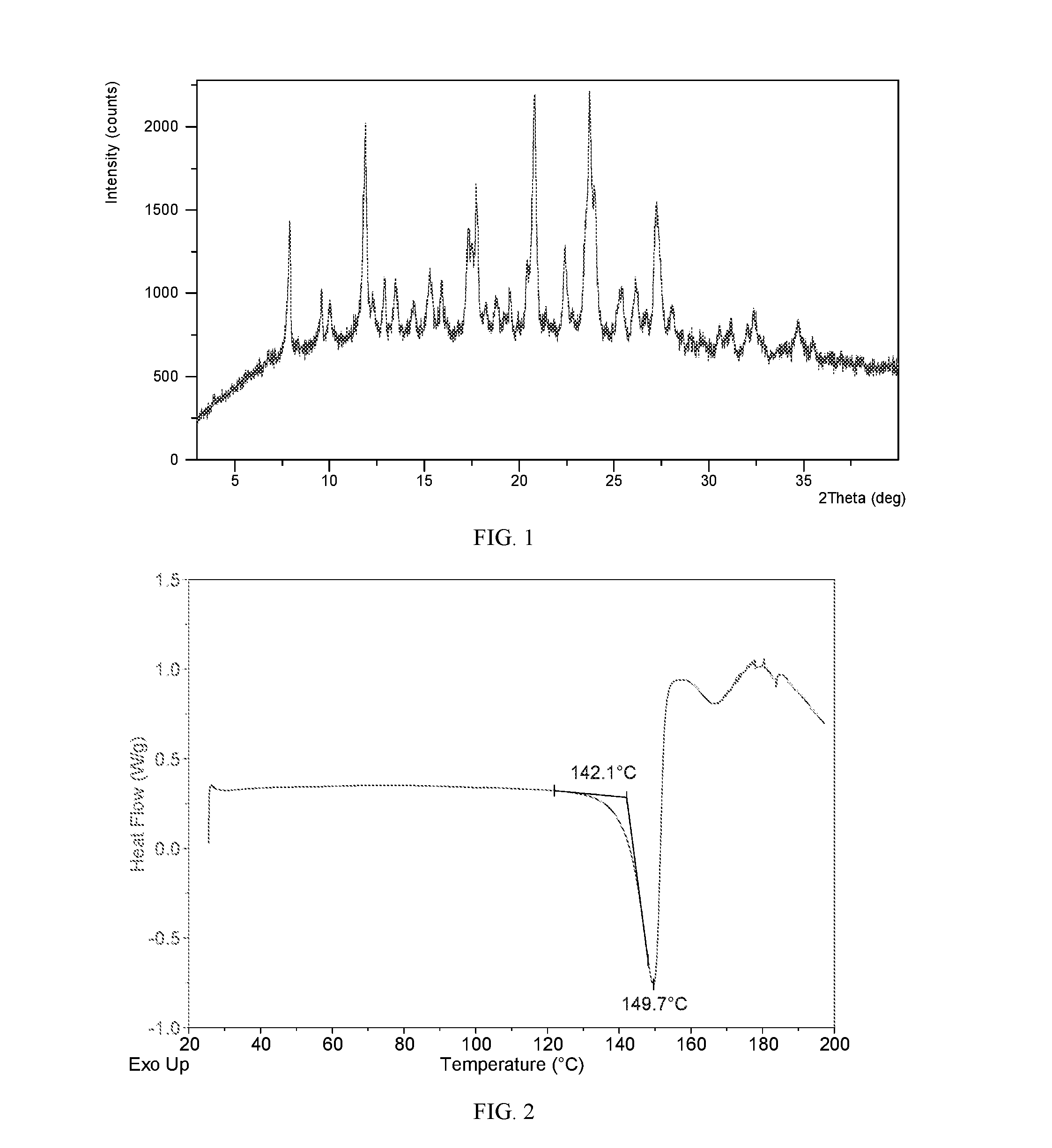
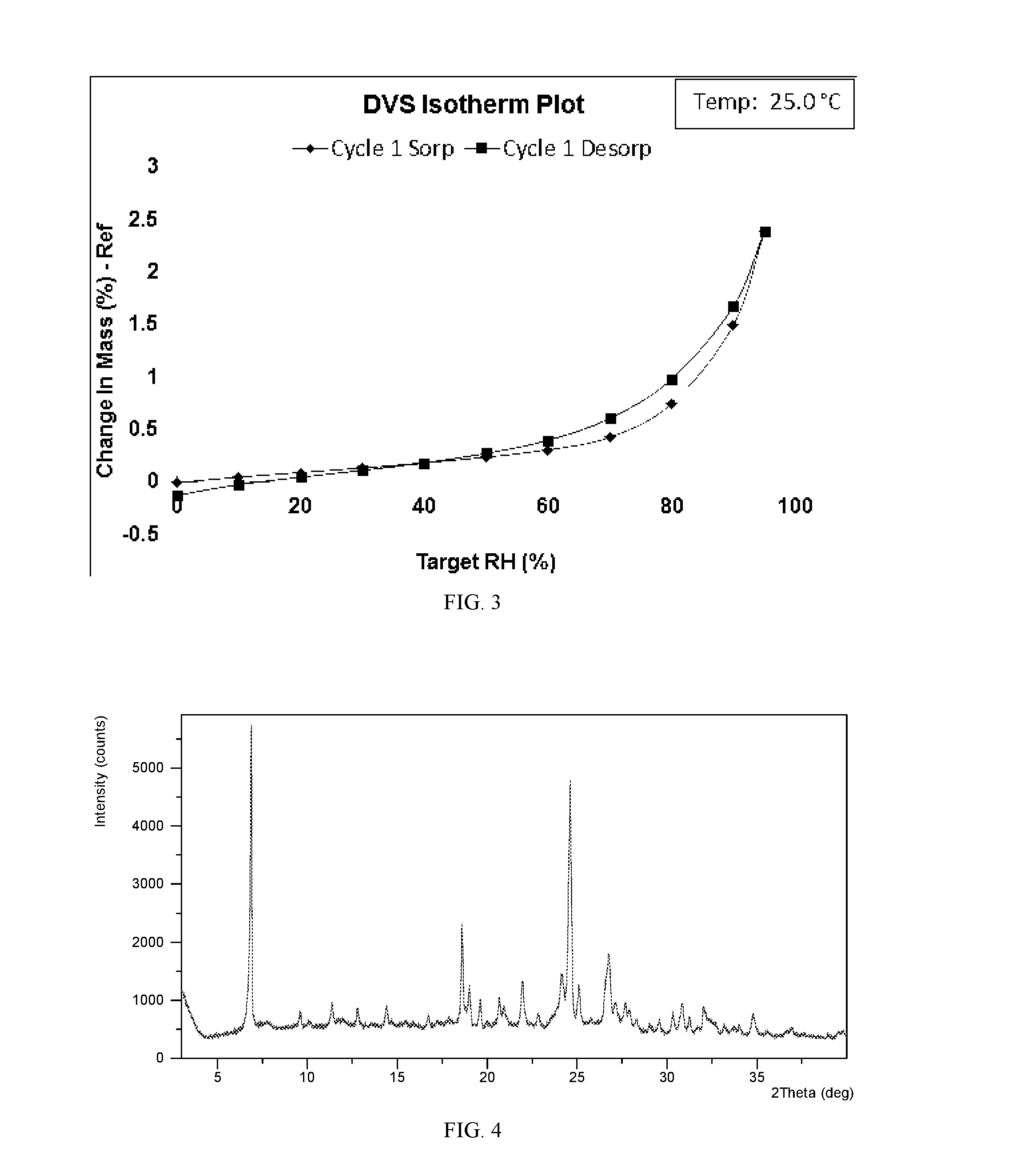
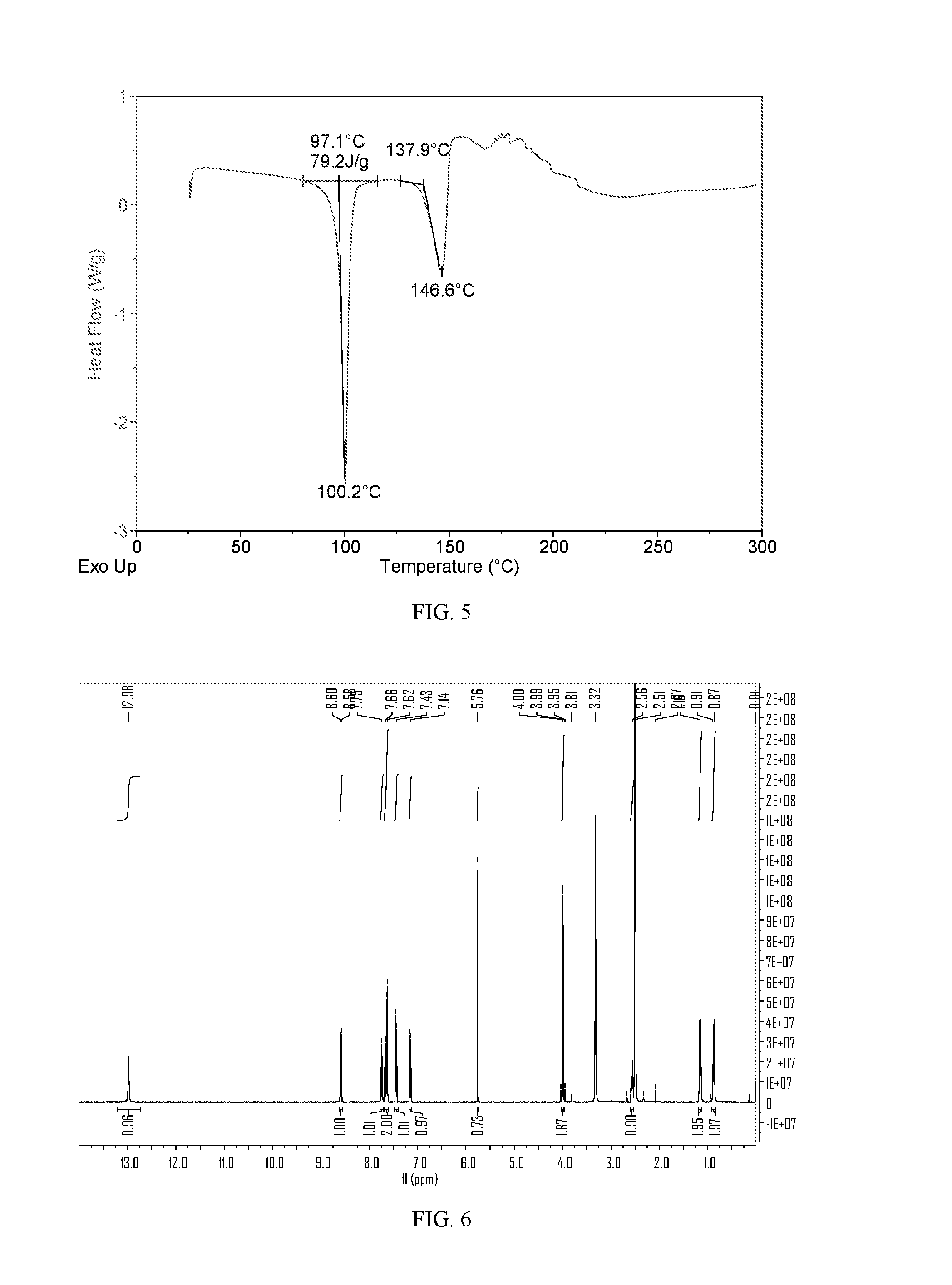
Salts and co-crystals of lesinurad
Novel salts and cocrystals of lesinurad, processes for their preparation, pharmaceutical compositions comprising these new salt forms and co-crystals, and use of them for treating or delaying progression or onset of diseases or disorders related to activity of uric acid transport 1 (URAT1) proteins are disclosed. These novel forms were characterized by X-ray powder diffraction, differential scanning calorimetry, and other techniques. They can be readily prepared and are suitable for preparation of solid dosage forms owing to their ease of handling and superior pharmacological properties.
Owner:CRYSTAL PHARMATECH CO LTD +1
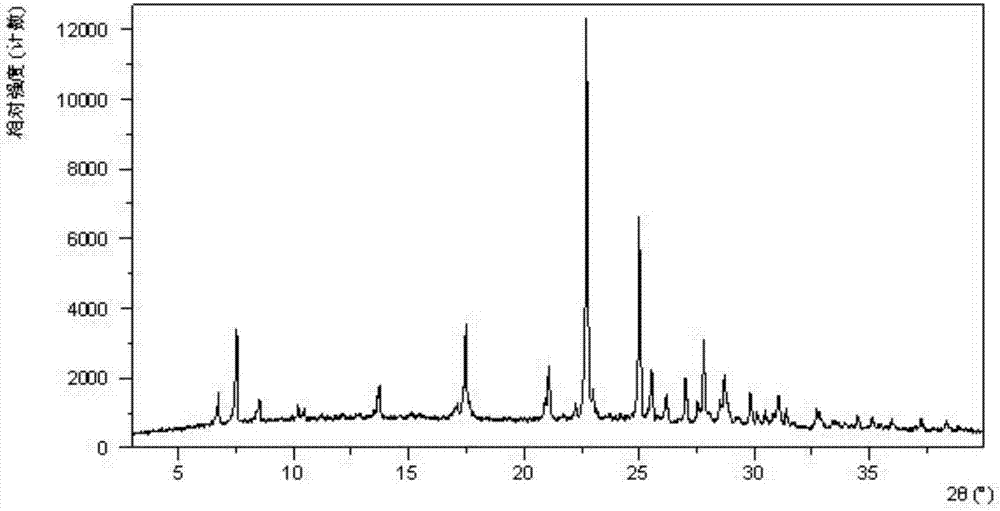
Crystal form of sodium salt of lesinurad and preparation method thereof
InactiveCN106883190AGood for long-term storageImprove stabilityOrganic active ingredientsOrganic chemistry methodsLesinuradCost Controls
The invention relates to a crystal form of a sodium salt of lesinarad and a preparation method thereof. The crystal form provided by the invention is good in stability and facilitates the long-term storage of chemicals. Moreover, the preparation method is simple in operation, good in repeatability, capable of facilitating the cost control in the industrialized production and extremely high in economic value.
Owner:CRYSTAL PHARMATECH CO LTD +1
Method for producing lesinurad intermediate 4-cyclopropyl-1-naphthylamine
InactiveCN105622427AHigh purityImprove securityOrganic compound preparationAmino compound preparationLesinuradPtru catalyst
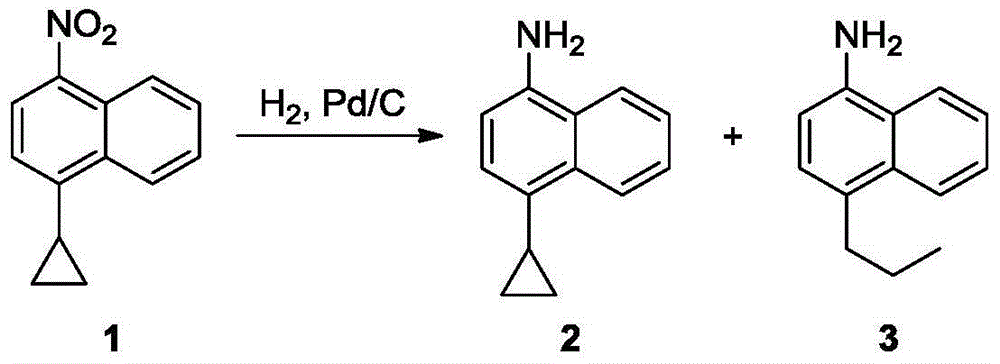
The invention discloses a synthetic method which can be applied to produce gout treating drug lesinurad intermediate 4-cyclopropyl-1-naphthylamine 2. The method adopts metal or low-price salt as a reducing agent, to provide a production technique which can avoid the application of hydrogen and precious metal catalysts without producing a by-product 3. The present technique has the characteristics of high purity products, good security and low cost.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Crystalline forms of lesinurad and its sodium salt
Novel crystalline forms of lesinurad and its sodium salt, processes for their preparation, pharmaceutical compositions comprising these new forms, and use of them for treating or delaying progression or onset of diseases or disorders related to activity of uric acid transporter 1 (URAT1) proteins are disclosed. These novel forms were characterized by X-ray powder diffraction, differential scanning calorimetry, and other techniques. They can be readily prepared and are suitable for preparation of solid dosage forms owing to their ease of handling and superior pharmacological properties.
Owner:CRYSTAL PHARMATECH CO LTD +1
Resolution method for axis chiral enantiomers of lesinurad
ActiveUS20210053928A1Easy to separateConveniently recovered and reusedOrganic active ingredientsSkeletal disorderLesinuradEnantiomer
A resolution method of axial chiral enantiomers of lesinurad (2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid) adopts inexpensive and readily available quinoline natural products and derivatives thereof, such as quinine, cinchonine, quinidine or cinconidine as resolving agents to react with lesinurad racemate in an organic solvent to form a salt, and the salt is dissociated by acidification so as to obtain optically pure (R)- or (S)-2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid. The method can give axial chiral enantiomer of lesinurad in R configuration with a chiral purity ee of up to 100% and a total yield of 90% or more. The obtained axial chiral enantiomer of lesinurad in S configuration can reach a chiral purity ee of up to 99.9% and a total yield of 80% or more.
Owner:CHINA RESOURCES SAIKE PHARMA
Preparation method of lesinurad related impurities
The invention provides a preparation method of lesinurad related impurities. Compared with the prior art, the preparation method has the advantages that intermediate related impurities and position isomer impurities of the lesinurad can be obtained, the synthesis method is simple, the obtained target product is high in purity, technological research and quality standard formulation of the lesinurad are facilitated, the research and development cost is effectively reduced, and the preparation method is of great significance to drug development.
Owner:HAINAN XINOPEN SOURCE MEDICAL TECH CO LTD
Novel preparation method for Anti-gout drug lesinurad, and key intermediate thereof
InactiveUS20200062720A1SuitableProcess economyOrganic chemistryBulk chemical productionLesinuradEthyl acetate
A novel preparation method for the anti-gout drug Lesinurad, and a key intermediate thereof. The method comprises the following reaction steps: 1) the compound of formula II undergoing a substitution reaction with R3—SH in the presence of a first solvent and a first alkali to generate a mixture containing the compound of formula III and the compound of formula IV; 2) adding a second alkali and R3X to the resulting mixture for a reaction to obtain the compound of formula III, wherein: R represents a cyclopropane group, a halogen, a triflate group, a mesylate group or a tosylate group, preferably a cyclopropane group; R3 represents —COCH3, a benzyl group or —CH2R4, wherein R4 represents a methyl acetate group, an ethyl acetate group, —C(O)OC2H5, —C(O)OCH3, —CN, —CH2OH or a phenyl group substituted with one or more of a C1-C6 alkyl group and a halogen; X represents a halogen. The process of the present invention directly converts the compound of formula IV into the product compound of formula III without separation, significantly increasing the reaction yield and simplifying the operation steps. In addition, the synthesis of the new intermediate of the present invention does not require the use of highly toxic thiophosgene and carbon disulphide, significantly improving the safety and environmental friendliness of the process.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Pharmaceutical composition for treating hyperuricemia
ActiveCN111714487ASmall toxicityGood for lowering uric acidOrganic active ingredientsSkeletal disorderLesinuradCo medication
The invention belongs to the field of chemical medicines, and particularly relates to a pharmaceutical composition for treating hyperuricemia. The invention relates to application of a sesquiterpene lactone compound with a structure shown as a formula (I) and pharmaceutically acceptable salts, esters, prodrugs, solvates, polymorphs, hydrates or derivatives of the sesquiterpene lactone compound topreparation of combined drugs for treating the hyperuricemia in combination with hyperuricemia drugs, and further provides the pharmaceutical composition for treating the hyperuricemia. The pharmaceutical composition comprises the compound and one of benzbromarone or lesinurad. The pharmaceutical composition for treating the hyperuricemia can achieve the uric acid reducing effect equivalent to oreven better than that of the drugs for treating the hyperuricemia in the prior art, can obviously reduce the toxic and side effects of the drugs for treating the hyperuricemia in the prior art and improve the safety, and can be used for treating the hyperuricemia and gout or gout complications caused by the hyperuricemia.
Owner:CATCH BIO SCI & TECH
A process for refining the lesinurad intermediate 1-naphthyltriazolethione that can be industrialized
The invention discloses a synthesis process and refining process for a lesinurad intermediate 3-amino-4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione which can be used for preparation of gout treatment drugs. The process comprises the following steps: adding a sodium carbonate solution into a mixture prepared from a compound 1, a compound 2-B1, ethanol and water drop by drop; supplementing a proper amount of water after completion of reaction; and treating a crude product 3 of 3-M containing impurities with alkali in a solvent, hydrolyzing 3-M and subjecting the obtained crude solid of 3 to ethanol beating so as to obtain a high-purity product 3. The process has the advantages of suitability for large-scale industrial operation and capacity of producing the high-purity product.
Owner:天津天诚新药评价有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com