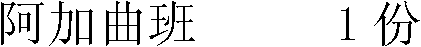Patents
Literature
120results about How to "Reduce drug risk" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
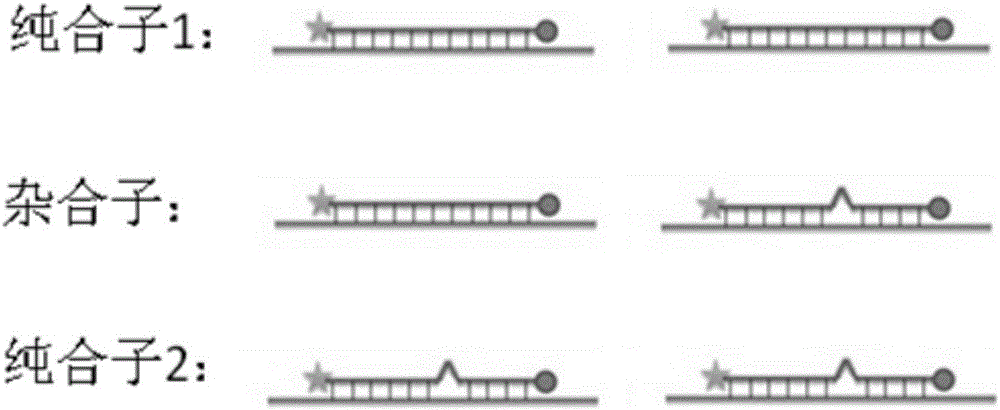
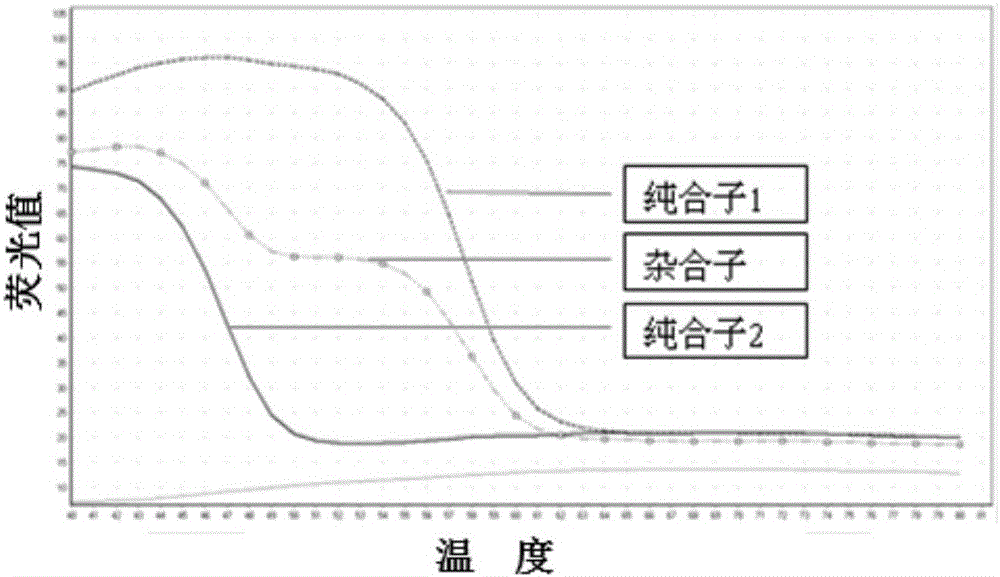
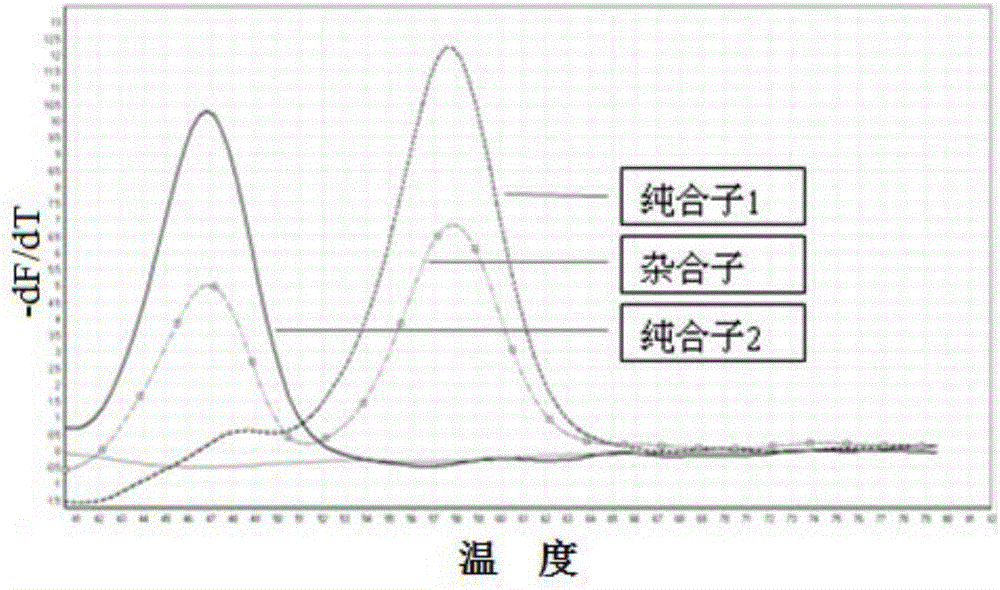
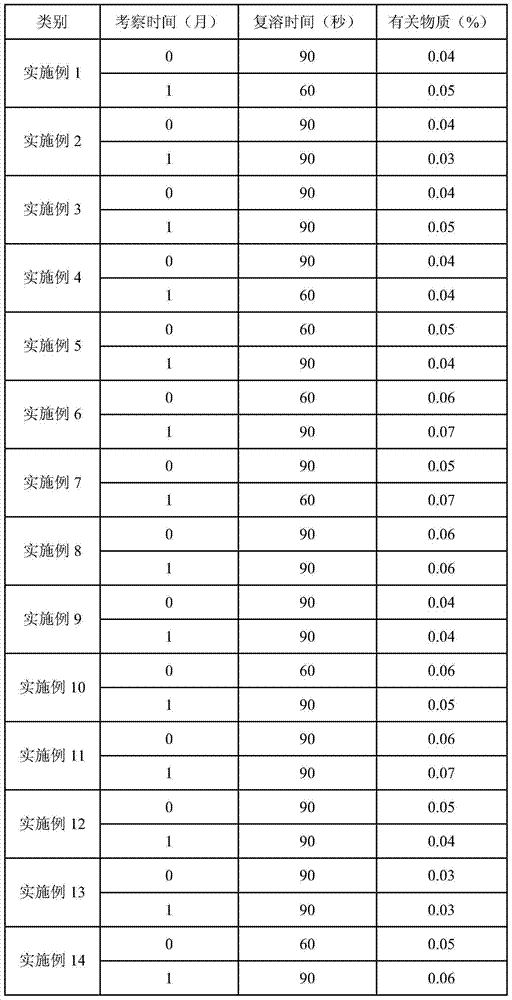
SNP (single nucleotide polymorphism) combination, detection method and kit for detecting liver damage susceptible genotype of antitubercular drug
ActiveCN106119363AReduce drug riskImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationGeneticsAntituberculous drugs
The present invention relates to an SNP (single nucleotide polymorphism) combination, detection method and kit for detecting liver damage susceptible genotype of an antitubercular drug and belongs to the technical field of medical molecular biological diagnosis; the SNP combination includes 7 SNP sites, and nucleotide sequences of the 7 SNP sites are shown sequentially as in SEQ ID NO. 1-7; the present invention also relates to an SNP detection method, comprising PCR (polymerase chain reaction) amplification and double-labeled probe melting curve analytical reaction, and primer pairs and double-labeled probe sequences for detection of the 7 SNP sites are shown as in SEQ ID NO. 8-20. The SNP site combination, detection method and kit provided herein enables quick, accurate, simple and high-throughput detection for a patient's genotype and prediction for the liver damage risk due to the patient using the antitubercular drug.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY +1
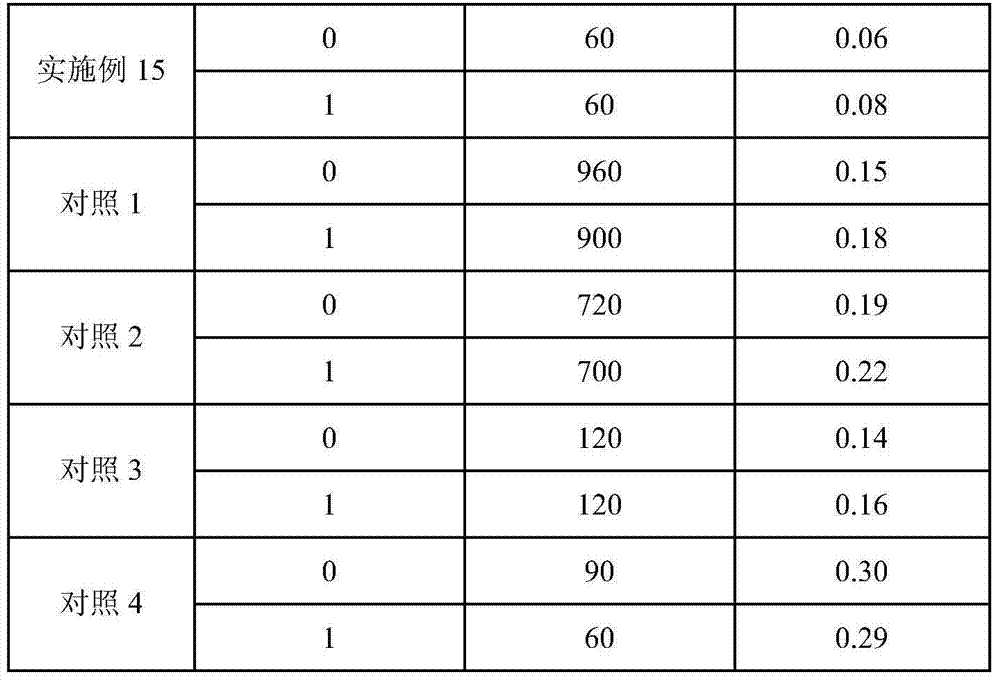
Medicinal composition for treating or preventing obesity and metabolic syndromes
InactiveCN102872062APrevent obesityPrevent Metabolic SyndromeOrganic active ingredientsMetabolism disorderOrlistatActive component
The invention discloses a medicinal composition for treating or preventing the obesity and metabolic syndromes, and belongs to the medicine field. The medicinal composition treats orlistat and acarbose as medicinal active components, so the application amount of orlistat in the medicinal composition is substantially reduced, thereby the risk of the damage of orlistat to the liver of a body is reduced. The medicinal composition has a substantial synergistic effect when the medicinal composition is used for treating or preventing the obesity and the metabolic syndromes, can reduces the diabetes and angiocardiopathy suffering risks of obese patients, and has a wide medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Sodium fusidate freezing-dried powder injection
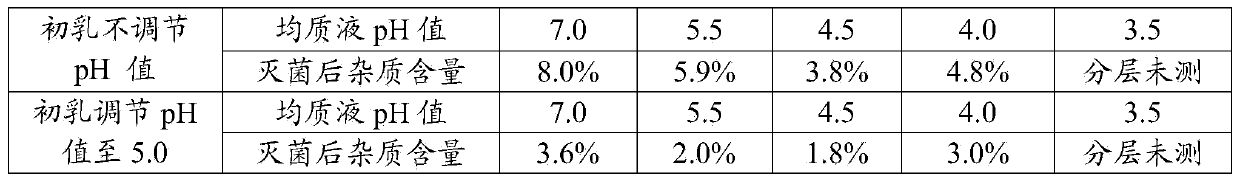
ActiveCN101143133AImprove securityReduce drug riskAntibacterial agentsPowder deliveryMedication riskBiomedical engineering
The invention discloses a sodium fusidate freeze-dried powder injection, including the following components and contents (weight portions) of 450 to 550 of sodium fusidate, 30 to 500 of glycin and 40 to 600 of arginine. The sodium fusidate freeze-dried powder injection has good long-period stability and improves the medication safety for the patient and reduces the medication risk, because certain quantity of stabilizer is added into the sodium fusidate freeze-dried powder injection.
Owner:HAISCO PHARMA GRP INC
Irinotecan hydrochloride liquor type injection and preparation method thereof
ActiveCN101953781AReduce drug riskImprove product qualityOrganic active ingredientsPharmaceutical delivery mechanismStabilizing AgentsChemistry
The invention discloses an irinotecan hydrochloride liquor type injection and a preparation method thereof, which is characterized in that the injection contains buffer salt, no stabilizing agent, i.e. sorbierite. In addition, the invention also discloses a preparation method of the irinotecan hydrochloride injection. The irinotecan hydrochloride injection obtained by utilizing the method has stable quality, pharmacy safety and low production cost.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Temozolomide lyophilized powder preparation and preparation method thereof
ActiveCN104721155AReduce adverse reactionsLow toxicityOrganic active ingredientsPowder deliveryDissolutionBULK ACTIVE INGREDIENT
The invention belongs to the technical field of pharmaceutical preparations and specifically relates to a temozolomide lyophilized powder preparation. The temozolomide lyophilized powder preparation comprises an active ingredient of temozolomide or a pharmaceutically acceptable salt thereof, and a solution before lyophilization further contains an excipient, a wetting agent, a buffer agent, an osmotic pressure regulating agent, a pH regulating agent, water for injection and an organic solvent, wherein the organic solvent is selected from one or any combination of ethanol, acetone, isopropanol, n-propanol, butanone, sec-butyl alcohol and methanol, and is preferably ethanol. The temozolomide lyophilized powder preparation provided by the invention has the advantages of stable quality, high re-dissolution speed and a small residual amount of the organic solvent. The invention further provides a method for preparing the preparation. The process provided by the invention is simple and convenient in preparation process and easy to control production links, the organic solvent accounts for a relatively small part of total volume of material liquid, the pollution to production equipment and environment caused by the organic solvent is reduced, and thus the method is suitable for large-scale production.
Owner:QILU PHARMA HAINAN
Planting method for improving quality of corn
InactiveCN107409674AImprove survival rateReduce the occurrence of pests and diseasesCalcareous fertilisersMagnesium fertilisersSite managementSowing
The invention discloses a planting method for improving the quality of corn, and relates to the technical field of corn planting. The planting method comprises the following steps of land selection and preparation, seed selection, seed treatment, sowing, film mulching, field management and harvesting. By means of the planting method, the survive rate of the corn can be improved, plant diseases and insect pests can be reduced, and the overall quality is improved.
Owner:黄勇
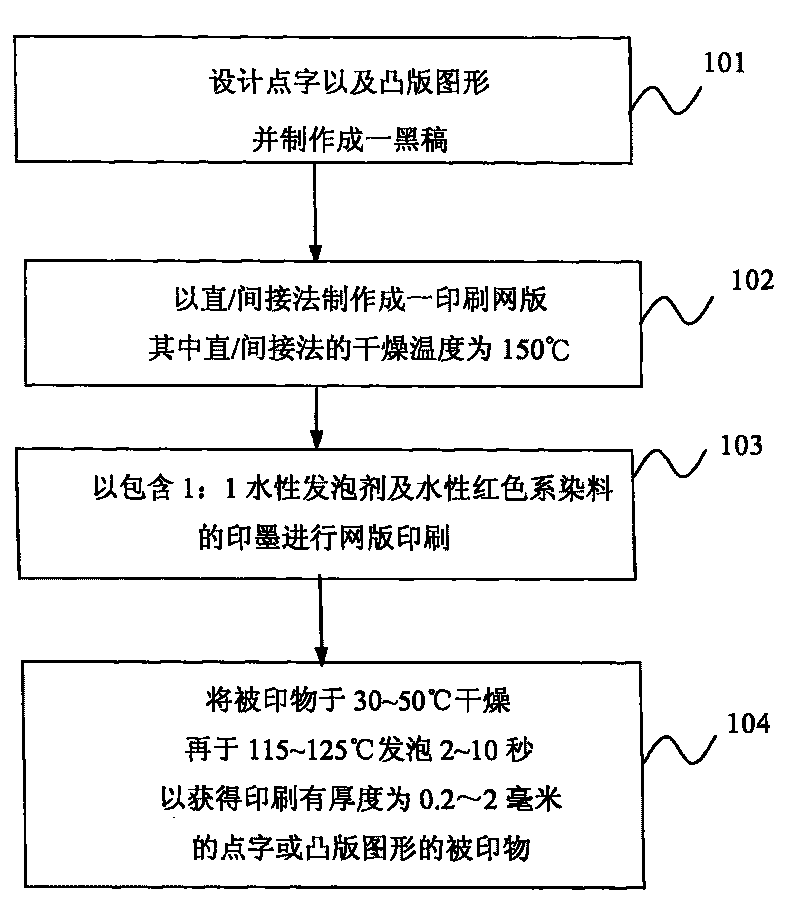
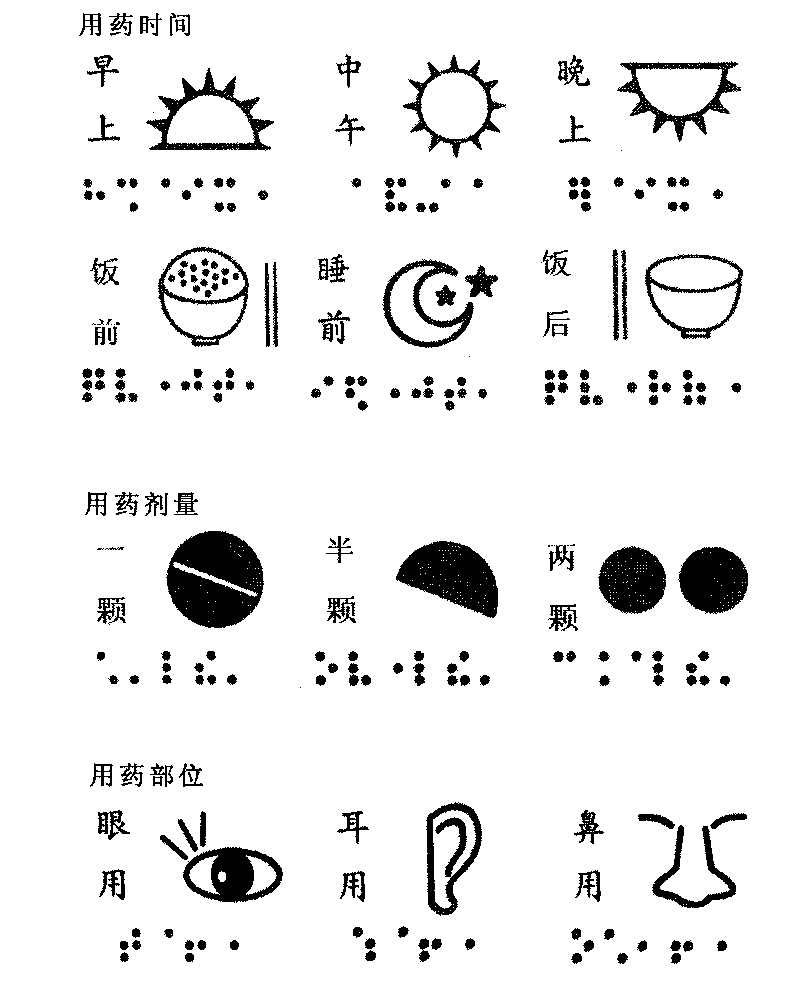
Method for manufacturing medicament application identification
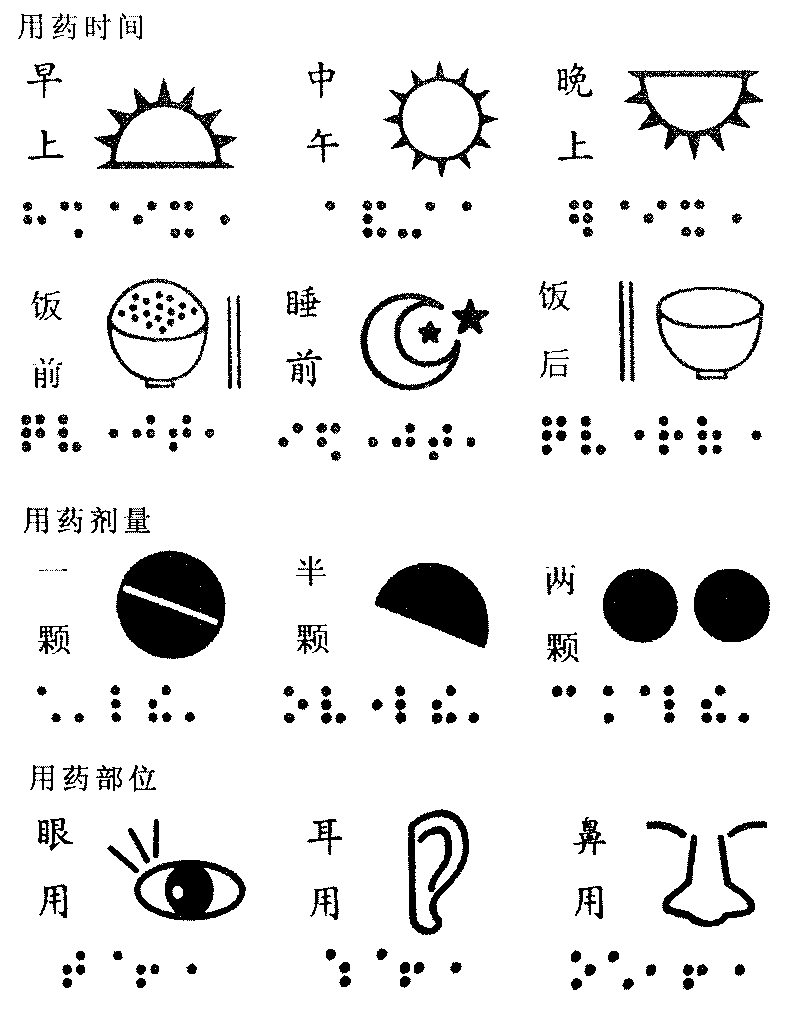
InactiveCN101722750AImprove the effect of medicationImprove ease of useStampsOther printing matterScreen printingImpaired visual acuity
The invention relates to a method for manufacturing medicament application identification. The medicament application identification comprises a braille capable of providing medicament application information and a typography graph capable of providing medicament application information. The method comprises the following steps: (a) designing the braille and the typography graph, photographing the braille and the typography graph and drawing a black draft; (b) manufacturing a printing plate by using the black draft, a negative plate with thickness of 35 microns and a screen printing plate through a direct / indirect method which uses the drying temperature of 150 DEG C; (c) adding printing ink into the printing plate to print a printed object, wherein the printing ink contains aqueous foaming agent and aqueous red dye in a ratio of 1 to 1; and (d) drying the printed object at the temperature of between 30 and 50 DEG C, and foaming the printed object for 2 to 10 seconds at the temperature of between 115 and 125 DEG C to obtain the printed object printed with the braille or the graph with the thickness of 0.2 to 2 millimeters. The method of the invention can manufacture the medicament application identification enabling visual impairment people, illiterates and seniors to accurately learn the medicament application information by touching or looking at the graph.
Owner:CHANGHUA CHRISTIAN HOSPITAL
Argatroban injection and preparation method thereof
ActiveCN103070822AReduce solubilityReduce drug riskPeptide/protein ingredientsPharmaceutical delivery mechanismSolventActive carbon
The invention relates to an argatroban injection and a preparation method thereof. The injection is composed of 1 part of argatroban, 80-150 parts of a solubilizer, a proper amount of a pH regulation agent, and injection water. The addition amount of the pH regulation agent satisfies that the pH value of a full amount of the solution is regulated to 4.2-7.0. During medicine solution preparation, the medicine solution with argatroban added is not subjected to an active carbon absorption treatment. The argatroban injection provided by the invention has the advantages of no organic solvent, simple components, stable quality, and convenient application.
Owner:SHANDONG NEWTIME PHARMA
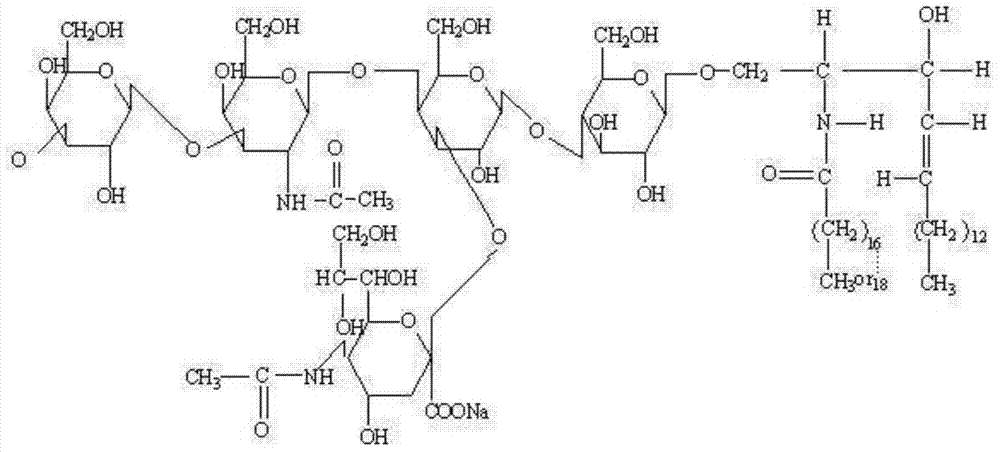
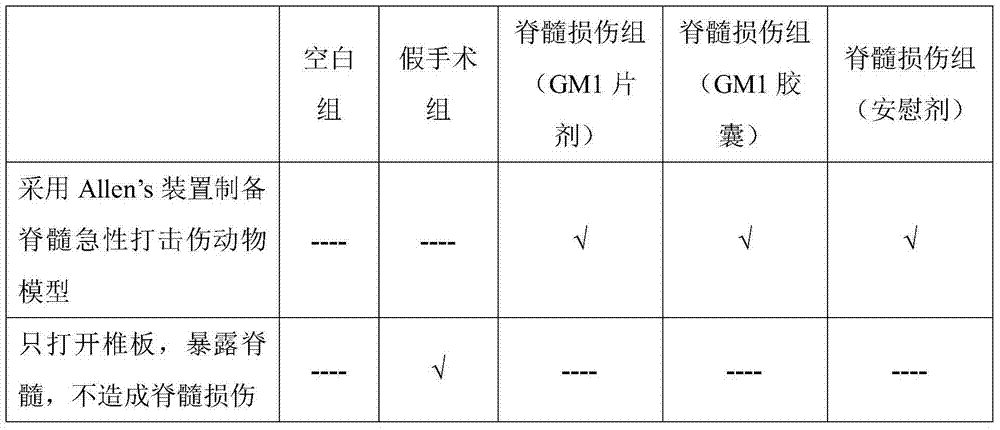
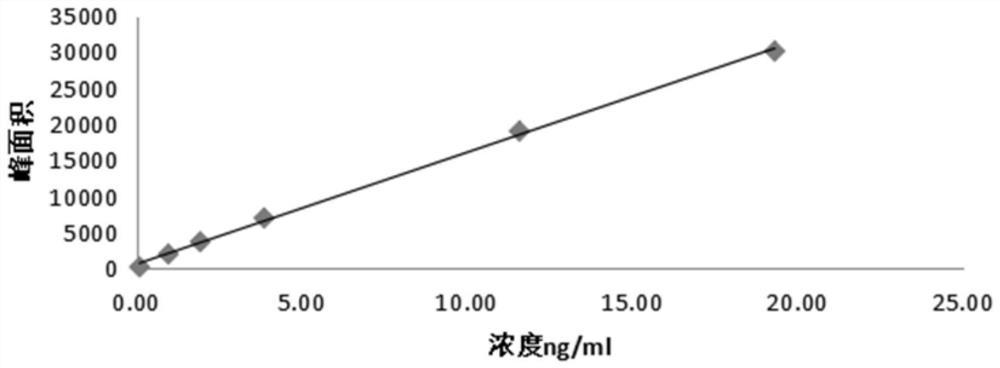
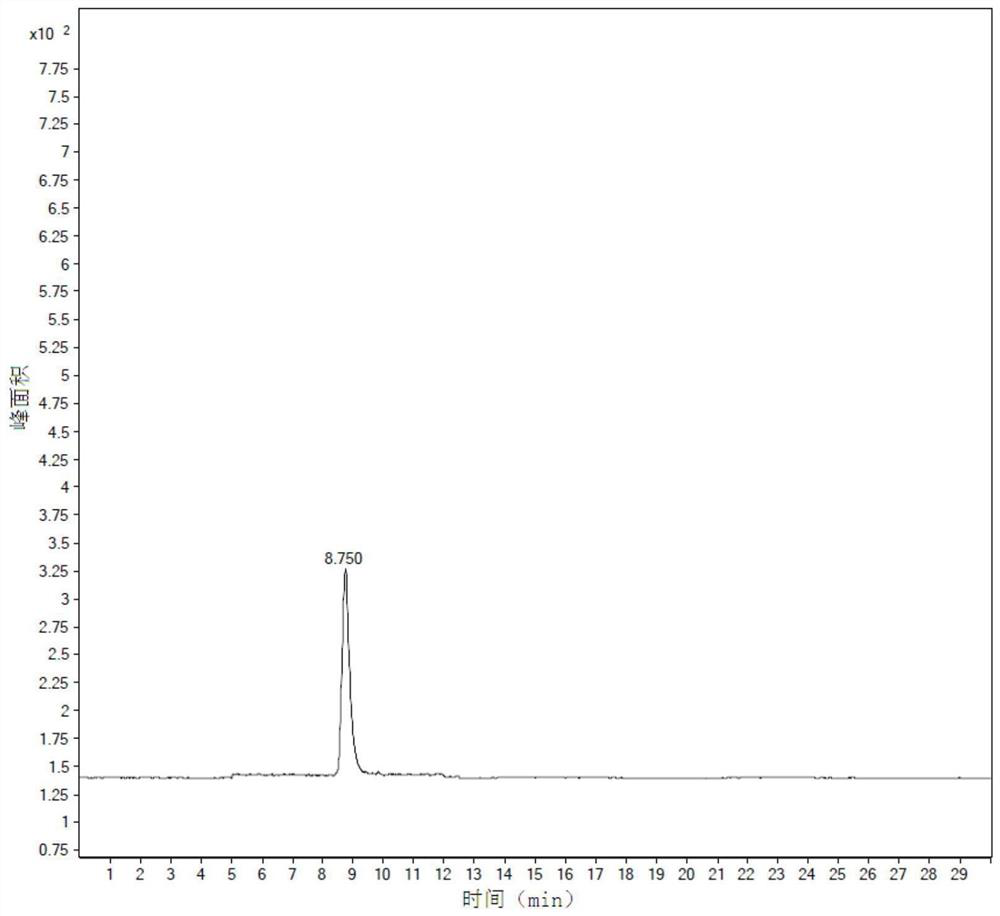
Oral preparation of monosialotetrahexosyl ganglioside sodium
InactiveCN104490837AEffectively exert biological activityImprove efficacyOrganic active ingredientsNervous disorderHard CapsuleAdhesive
The invention discloses an oral preparation of monosialotetrahexosyl ganglioside sodium, belongs to the field of medicines, and aims at solving the problems that a monosialotetrahexosyl ganglioside sodium injection hurts a human body and is high in cost. A monosialotetrahexosyl ganglioside sodium tablet is prepared from monosialotetrahexosyl ganglioside sodium, a filler, a drying adhesive, a disintegrating agent and a lubricant; and a hard capsule of monosialotetrahexosyl ganglioside sodium is prepared from monosialotetrahexosyl ganglioside sodium, a diluent, a lubricant, a glidant, a disintegrating agent and a wetting agent. The oral preparation has the advantages of relatively low cost, small medication pain and high medicine effect.
Owner:哈尔滨医科大学科技开发总公司
Linezolid injection and preparation method thereof
ActiveCN111686072AImprove quality and safetyImprove medication safetyAntibacterial agentsOrganic active ingredientsUse medicationMorpholine
The invention provides a linezolid injection and a preparation method thereof. The preparation method of the linezolid injection comprises the steps of weighing, preparation, filtration, encapsulationand sterilization, and nitrogen filling protection is carried out on liquid medicine in the steps of preparation and / or encapsulation. The content of 3-fluoro-4-(4-morpholinyl) aniline in the linezolid injection product can be obviously reduced by performing nitrogen charging protection on the liquid medicine in the steps of preparation and / or encapsulation, so that the product quality and the medication safety of the linezolid injection are favorably improved, and the medication risk of the variety is also obviously reduced by reducing the content of genotoxic impurities.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Curcumin nano micelle eye drops as well as preparation method and application
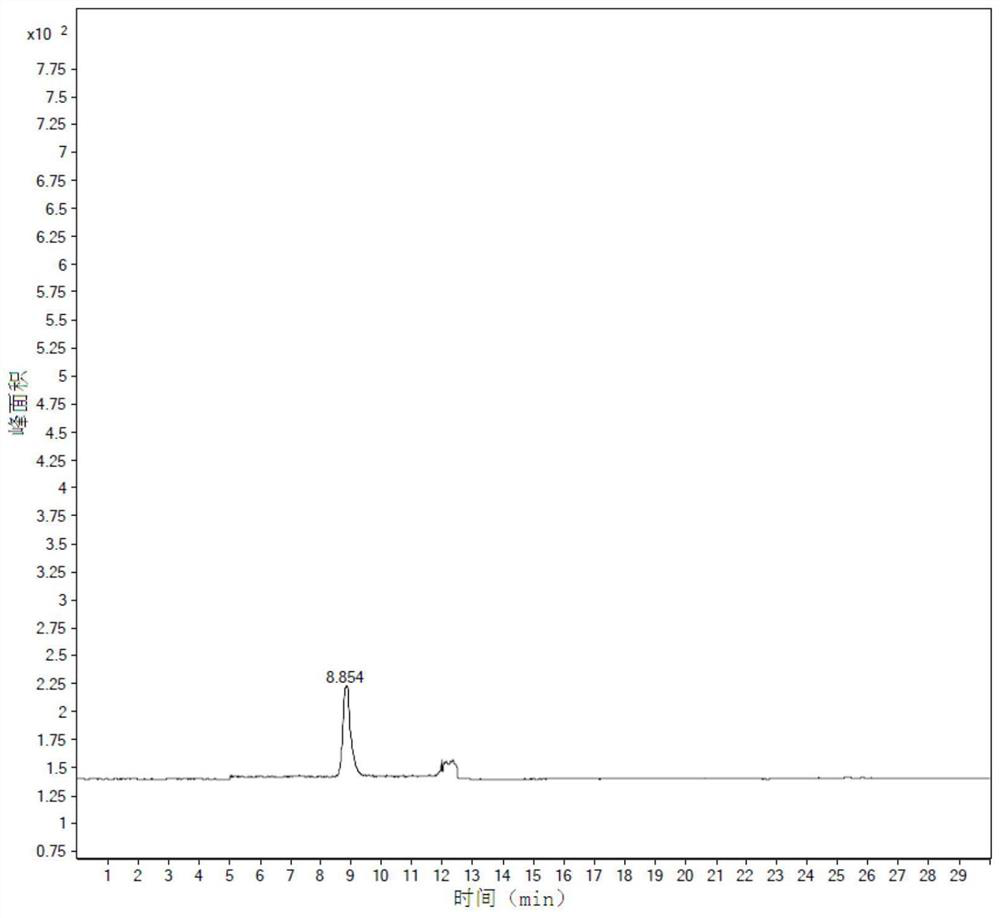
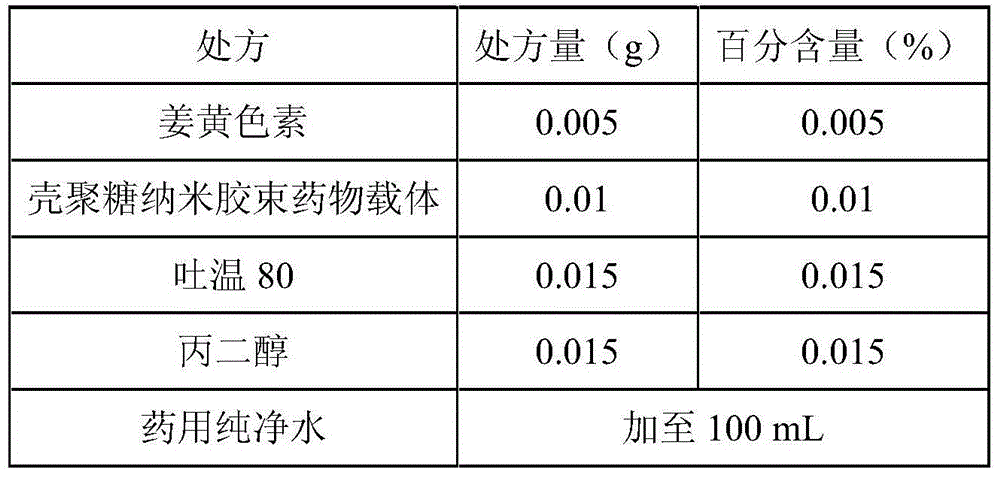
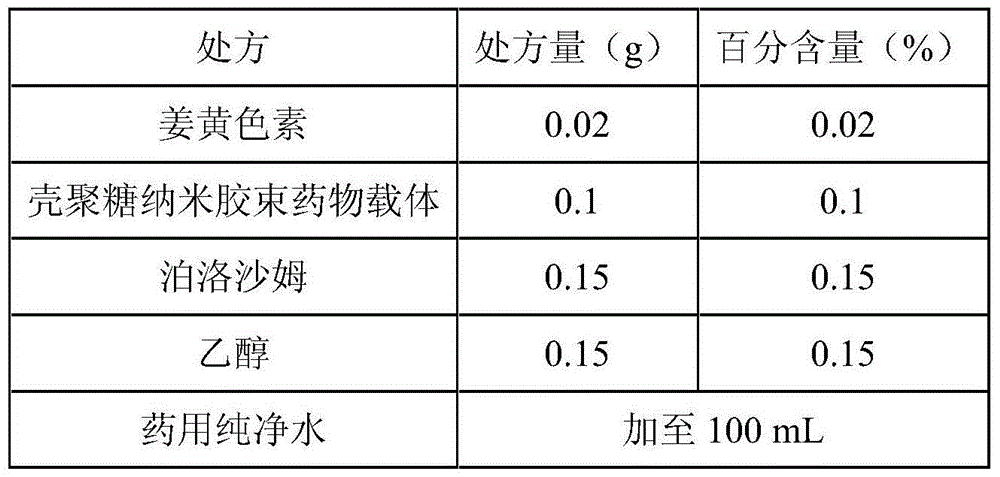
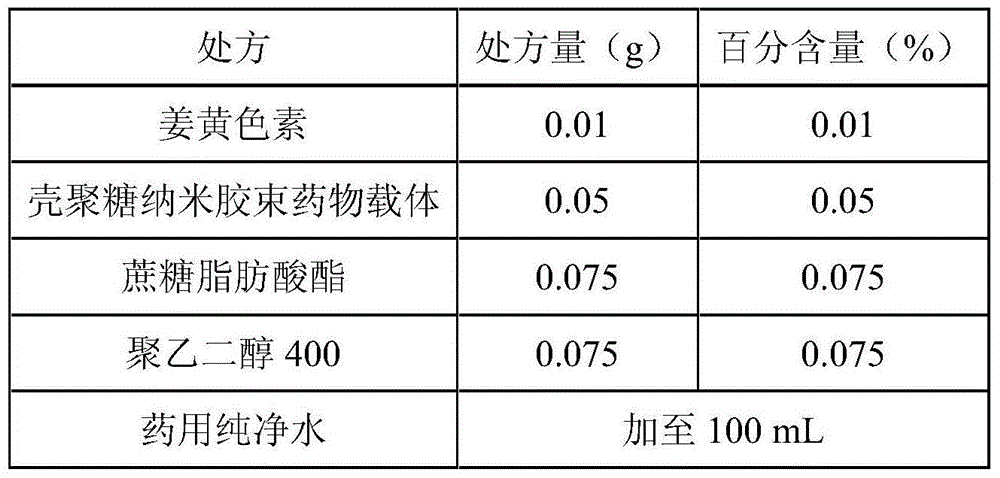
InactiveCN104644550AImprove stabilityReduce drug riskSenses disorderPharmaceutical delivery mechanismMass-Volume PercentageDrug carrier
The invention provides curcumin nano micelle eye drops. The curcumin nano micelle eye drops comprise curcuminoid, a chitosan nano micelle drug carrier, a surfactant, a co-surfactant and medical pure water, wherein the mass volume percentage of curcuminoid is 0.005-0.02% and the mass volume percentage of the chitosan nano micelle drug carrier is 0.01-0.1%. The curcumin nano micelle eye drops provided by the invention are good in stability, high in bioavailability and low dosage.
Owner:GUANGZHOU BAIEN BIOTECH CO LTD
Wheat planting method
InactiveCN106613066AEnhance drought and lodging resistanceIncrease root layer and root countBiocideSeed and root treatmentCommon wheatSowing
Owner:朱玉生
Processing device of toxic traditional Chinese medicine
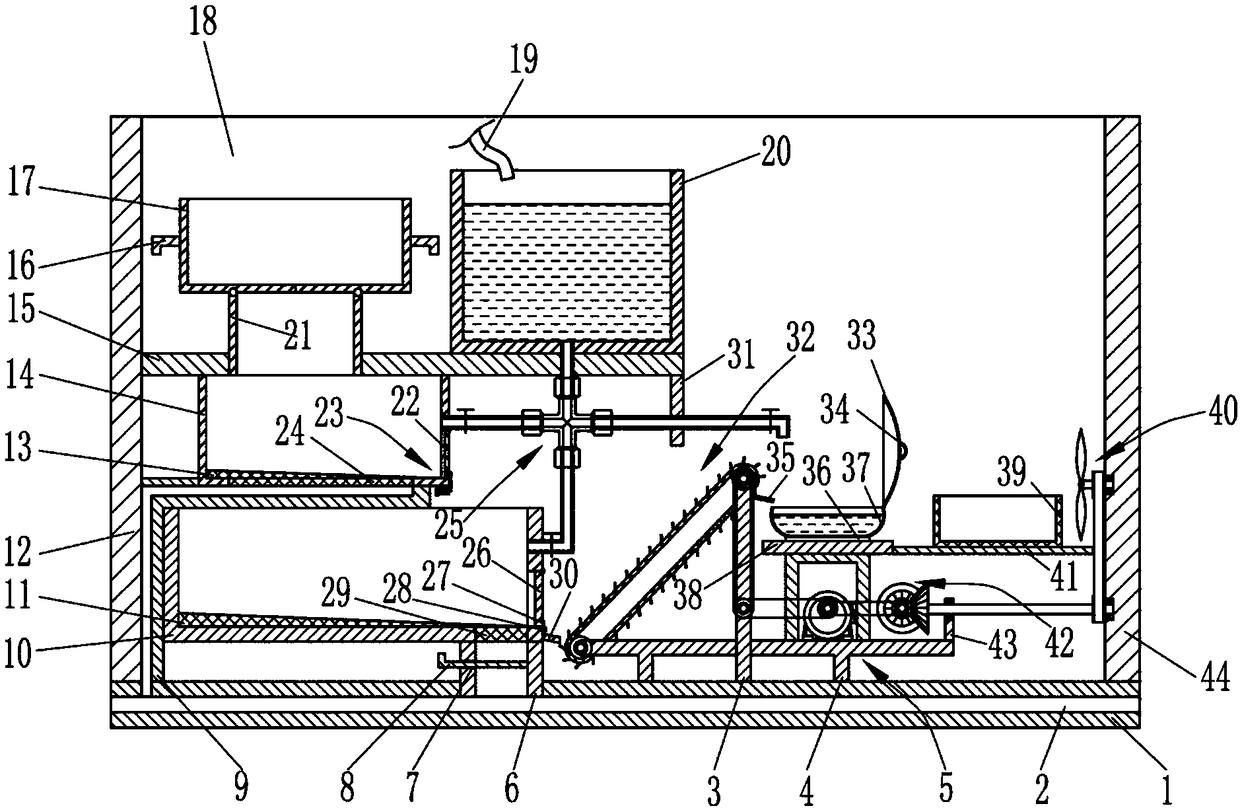
ActiveCN109010051AReduce toxic ingredientsLow toxicityPharmaceutical product form changeChemical/physical/physico-chemical processesFixed frameMedicine
The invention relates to a traditional Chinese medicine processing device, in particular to a processing device of toxic traditional Chinese medicines. The technical problem to be solved by the invention is to provide device for carrying out hydrolysis treatment on highly toxic traditional Chinese medicines, and effectively reducing toxic components in the toxic traditional Chinese medicines. Theprocessing device comprises a mounting bottom plate, a toxic water discharge pipe, a supporting rod, a fixing frame, a driving mechanism, a first drainage pipe, a first baffle, a second drainage pipe,a soaking frame, a first inclined mesh plate, a first mounting plate, a second inclined mesh plate, a cleaning frame, a second mounting plate, a first lifting rod, a material selecting frame, a mounting wall plate, a water conveying pipe, a water storage tank, a discharging pipe, a first cover plate, a fixing mechanism, a first net plate, a water conveying mechanism, a second cover plate and a first fixing plate. The processing device disclosed by the invention has the beneficial effects that the toxic traditional Chinese medicines can be subjected to hydrolytic treatment, and toxic components in the toxic traditional Chinese medicines are effectively reduced.
Owner:安徽精诚本草中药饮片有限公司
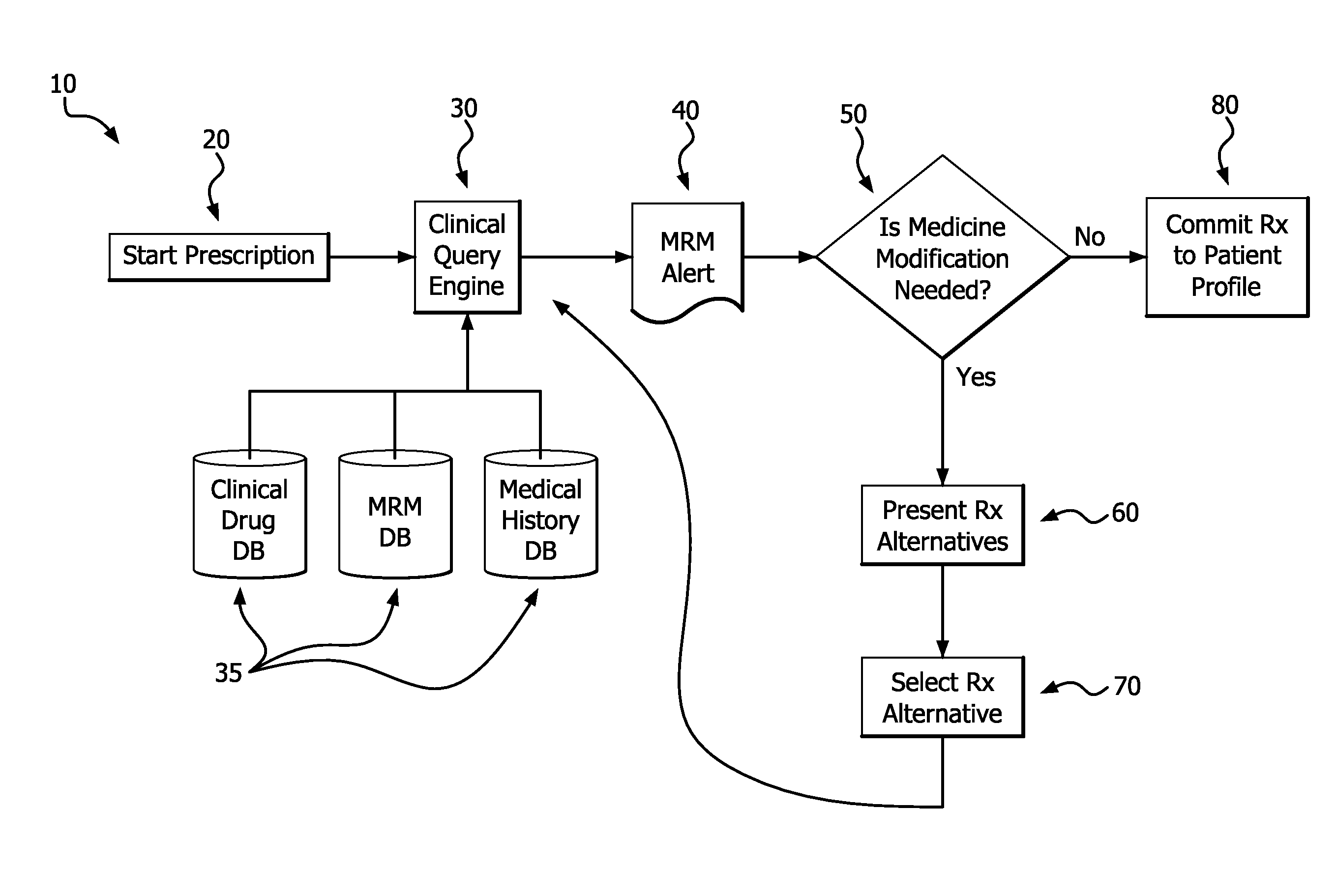
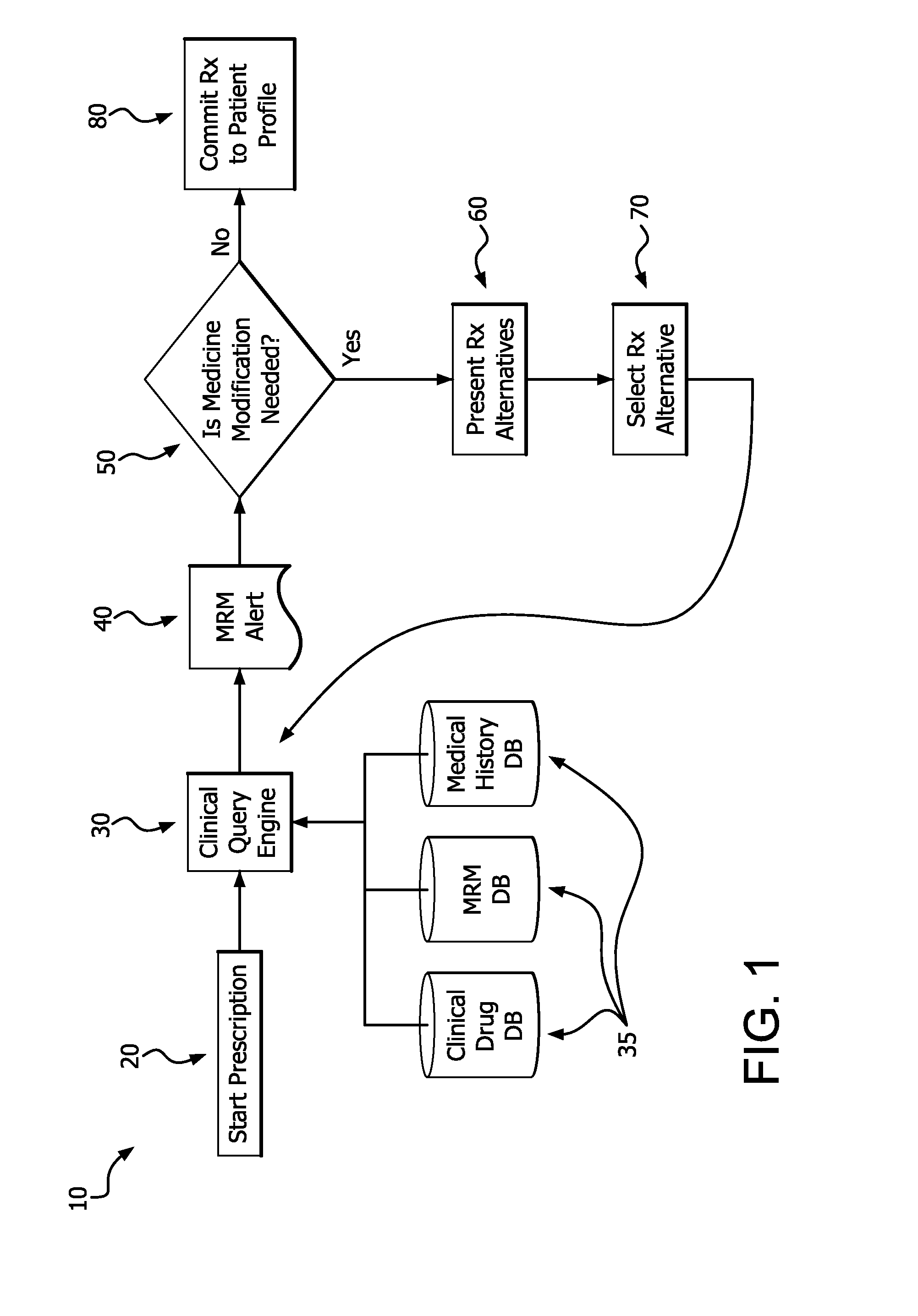
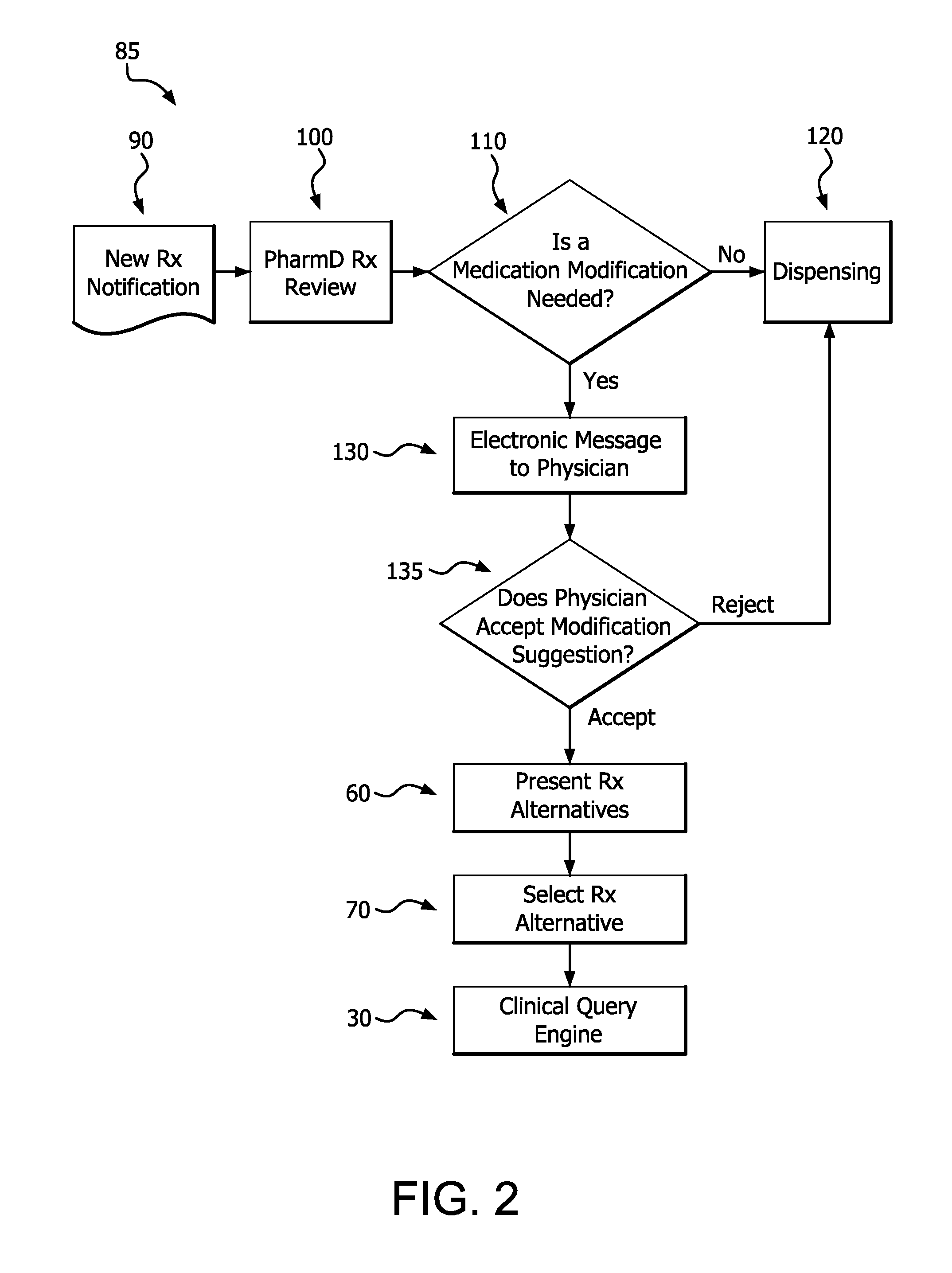
Medication risk mitigation system and method
ActiveUS20150178465A1Reduce drug riskData processing applicationsHealth-index calculationIntervention measuresMedication risk
A medication risk mitigation method utilizing three interventions: a prospective intervention performed by a prescriber, a concurrent intervention performed by a pharmacist and a retroactive intervention performed by a pharmacist. At each intervention, the system of the instant invention utilizes a computer program to compare each prescribed medication to a series of intrinsic and extrinsic data sources in order to identify potential contraindications and, if necessary, modify a prescription. The system also permits secure messaging between prescribers and pharmacists, each with access to the computer program, so as to facilitate communication and reduce medication risks. The system of this invention also permits modeling for hypothetical medication modifications based on the same intrinsic and extrinsic data sources.
Owner:CAREKINESIS
Oral pharmaceutical composition for treating or preventing obesity-related hypertension and its application
InactiveCN105343056AHighlight substantiveSignificant progressOrganic active ingredientsMetabolism disorderVascular diseaseOrlistat
The invention discloses an oral pharmaceutical composition for treating or preventing obesity-related hypertension and its application and belongs to the field of medicine. The pharmaceutical composition comprises orlistat and lol-suffixed pharmaceutical active ingredients. The usage of orlistat and lol-suffixed pharmaceuticals in the pharmaceutical composition is greatly reduced, thus the risk of pharmaceuticals injuring the human body is reduced. The oral pharmaceutical composition has significant synergistic effect in treating or preventing obesity-related hypertension, can reduce the weight of a patient and the risk of hypertension being complicated by cardiovascular diseases and has higher social value.
Owner:QINGDAO YUNTIAN BIOTECH
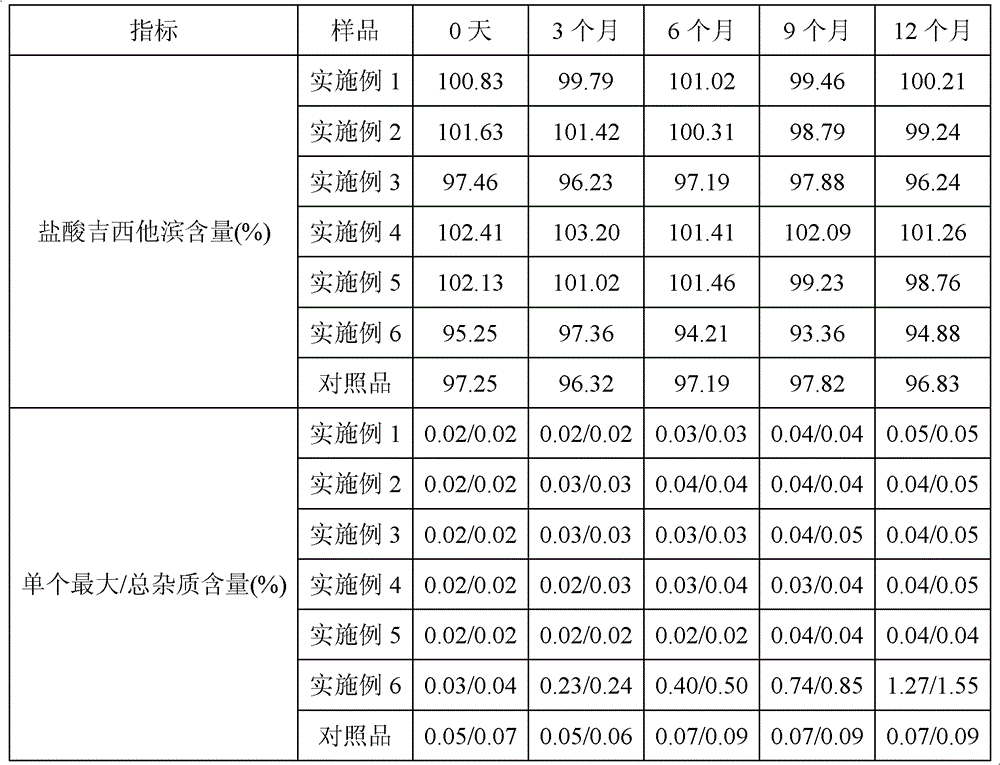
Gemcitabine hydrochloride lyophilized preparation
ActiveCN102302462BFix stability issuesGuarantee product qualityPowder deliveryLyophilised deliveryGemcitabine HydrochlorideAntioxidant
The present invention discloses a gemcitabine hydrochloride lyophilized preparation. The lyophilized preparation comprises, by weight, 20-70 parts of gemcitabine hydrochloride, 15-60 parts of a lyoprotectant and 0.005-0.5 parts of an antioxidant. Compared to the prior art, with the gemcitabine hydrochloride lyophilized preparation provided by the present invention, the problem of the stability ofthe gemcitabine hydrochloride is solved; the product quality is ensured; the risk of drug using is reduced; the preparation process is simple, the cost is low; and the preparation is suitable for theindustrial production, and has practical value.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Composition containing paclitaxel and preparation method thereof
ActiveCN103736096AAvoid hemolytic reactionsReduce drug riskOrganic active ingredientsPowder deliveryPatient complianceMedication risk
The invention relates to the technical field of antitumor drugs, and discloses a composition containing paclitaxel and a preparation method thereof. The composition containing paclitaxel comprises 1 part by weight of paclitaxel and 1.2-15 parts by weight of egg yolk lecithin; the pH value range of the composition is 4.0-6.0. According to the composition containing paclitaxel of the invention, egg yolk lecithin is adopted as an emulsifier; the pH value of the composition is adjusted to be within a proper range; no polyoxyethylated castor oil with large toxicity is adopted; no ethanol is contained; the medication risk of the composition containing paclitaxel is reduced; and the patient compliance is improved.
Owner:李宏 +1
Pharmaceutical composition for treatment or prevention of obesity-related hypertension and usage of pharmaceutical composition for treatment or prevention of obesity-related hypertension
InactiveCN105233288AHighlight substantiveSignificant progressDipeptide ingredientsMetabolism disorderCvd riskBULK ACTIVE INGREDIENT
The invention belongs to the field of medicines and discloses a pharmaceutical composition for treatment or prevention of obesity-related hypertension. The pharmaceutical composition comprises active ingredients of orlistat and angiotensin converting enzyme inhibitors, and risks of physical damages are reduced since consumption of the orlistat and angiotensin converting enzyme inhibitors in the pharmaceutical composition is greatly reduced. The pharmaceutical composition has a remarkable synergistic effect in treatment or prevention of obesity-related hypertension, is capable of reducing weights of obese patients and risks of hypertension and cardiavascular diseases and has a promising medical application prospect.
Owner:QINGDAO YUNTIAN BIOTECH
Medical composition for losing weight or treating metabolic syndromes
ActiveCN101756993ALose weightLower blood pressureOrganic active ingredientsMetabolism disorderTG - TriglycerideLost Weight
The invention discloses a medical composition for treating metabolic syndromes, belonging to the field of medicines, and in particular relates to a medical composition containing orlistat or cetilistat and actos, wherein the composition is solid preparations such as compressed tablets, dispersible tablets, sustained release tablets, capsules, granules, and the like. In experiments, the medical composition containing the orlistat or the cetilistat and the actos is accidentally found to have obvious synergistic effect on the aspects of reducing the blood pressure, the serum total cholesterol, the serum triglyceride and the low density lipoprotein cholesterin and enhancing the carbohydrate tolerance.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of argatroban injection and preparation method thereof
ActiveCN103070822BReduce solubilityReduce drug riskPeptide/protein ingredientsPharmaceutical delivery mechanismOrganic solventArgatroban Injection
The invention relates to an argatroban injection and a preparation method thereof. The injection is composed of 1 part of argatroban, 80-150 parts of a solubilizer, a proper amount of a pH regulation agent, and injection water. The addition amount of the pH regulation agent satisfies that the pH value of a full amount of the solution is regulated to 4.2-7.0. During medicine solution preparation, the medicine solution with argatroban added is not subjected to an active carbon absorption treatment. The argatroban injection provided by the invention has the advantages of no organic solvent, simple components, stable quality, and convenient application.
Owner:SHANDONG NEWTIME PHARMA
Method for applying pesticide on corn plants
ActiveCN103181312AImprove lodging resistanceDisease controlBiocidePlant growth regulatorsToxicologyTebuconazole
The invention provides a method for applying a pesticide on corn plants. The method comprises steps of selecting a corn lodging-resistant agent, a bactericide and an insecticide which have the consistent medicinal pH values; respectively weighing 7.5-12 grams of the corn lodging-resistant agent, 8-18 grams of the bactericide and 7.5-13.5 grams of the insecticide according to the dosages of the corn lodging-resistant agent, the bactericide and the insecticide, then adding water for 25-30 kilograms, and mixing so as to obtain a spray dosage of compound liquid medicine for each mu of corns, wherein the corn lodging-resistant agent consists of one of or a mixture of Jindele and Zhuangfengan, the bactericide consists of one of or a mixture of triadimefon and tebuconazole, and the insecticide is carbosulfan; when the leaves of the corns grow into 8-12 blades, adopting the compound liquid medicine to spray onto the corns. The method for applying the pesticide on corn plants has the advantages of labor saving, time saving, small hazard, good control effect and reduced cost.
Owner:河南金苑种业股份有限公司
Feed for milking sows
InactiveCN104304828AFeed value improvementImprove internal environmentAnimal feeding stuffNutrientMango leaf extract
The invention provides feed for milking sows and belongs to the field of pig feed. The feed for the milking sows solves the problems that the sows have low immunity, and drug residue is caused because chemical medicine is injected into the sows. The feed for the milking sows comprises, by weight, 550-600 parts of corn flour, 50-100 parts of wheat bran, 20-50 parts of fish meal, 20-50 parts of silkworm chrysalis, 50-100 parts of fermented oil tea cakes, 5-10 parts of mango leaf extracts, 30-80 parts of whey powder, 13-16 parts of calcium hydrophosphate, 10-50 parts of limestone powder, 0.05-0.1 part of papain, 0.1-0.3 part of lysine, 0.1-0.3 part of methionine, 0.12-0.16 part of iron, 0.001-0.005 part of manganese, 0.05-0.15 part of zinc, 0.003-0.008 part of copper and 0.5-1.5 parts of decavitamin. According to the feed for the milking sows, nutrient substances in the feed are reasonably matched due to variety selection and content selection of materials, galactopoiesis of the milking sows is facilitated, the immunity of the milking sows is improved, and the drug use risk of the sows is reduced.
Owner:成都理想财富投资咨询有限公司
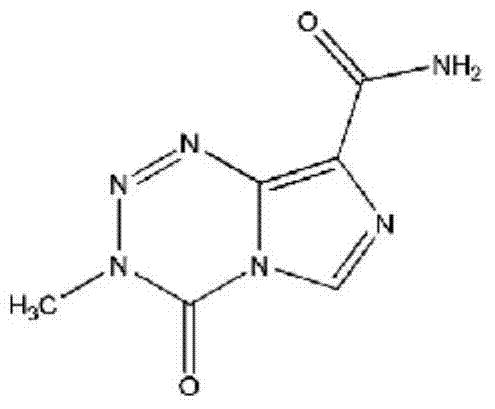
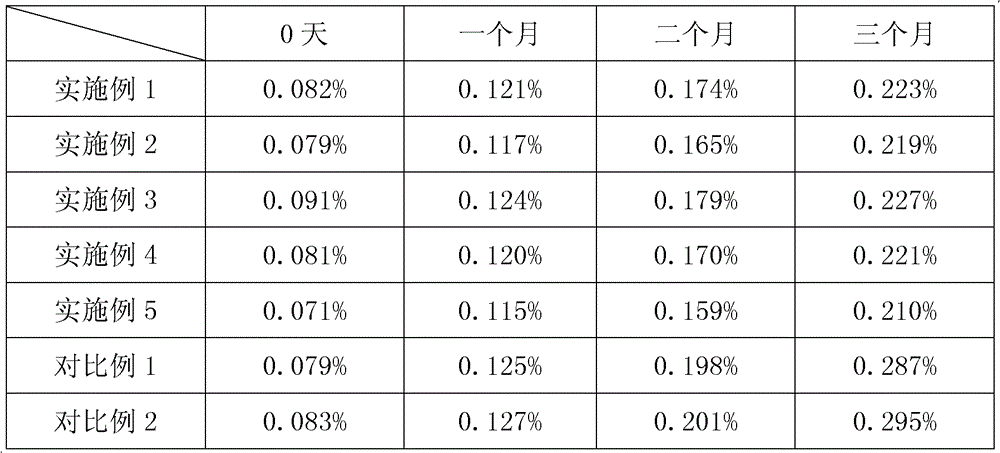
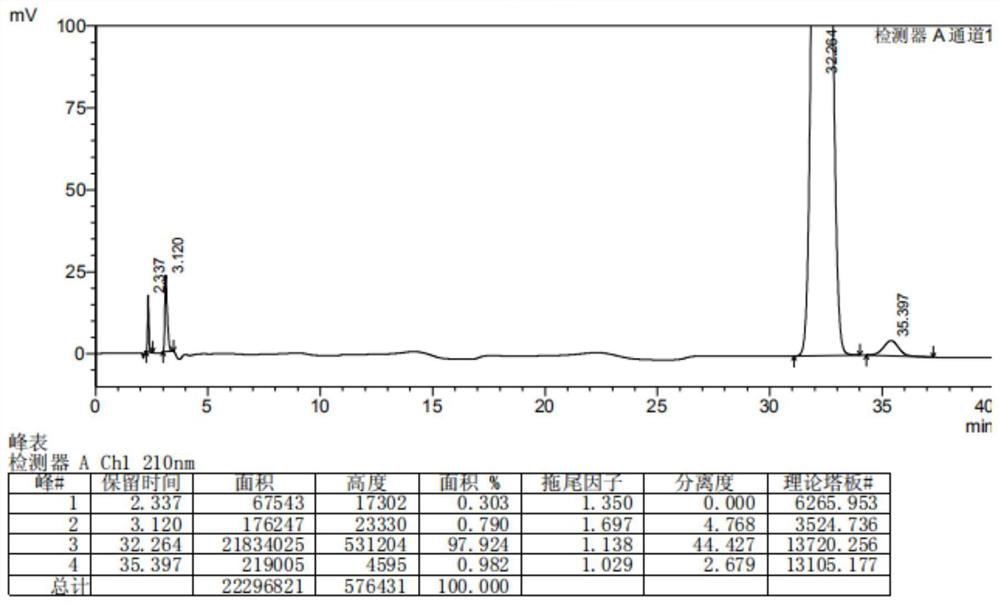
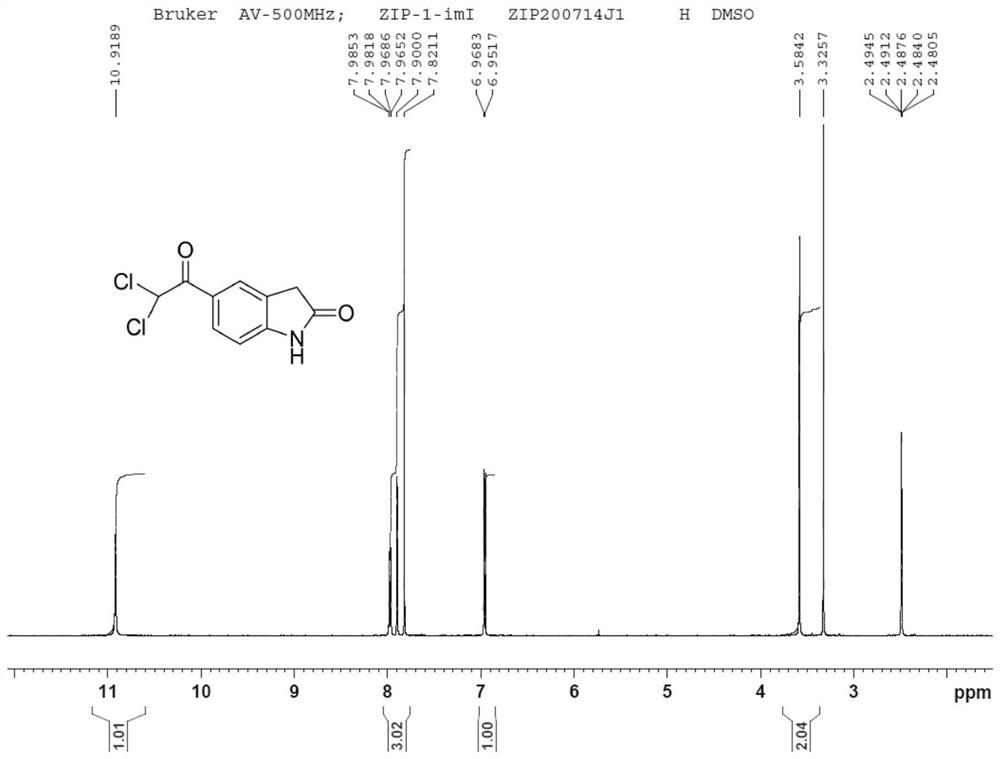
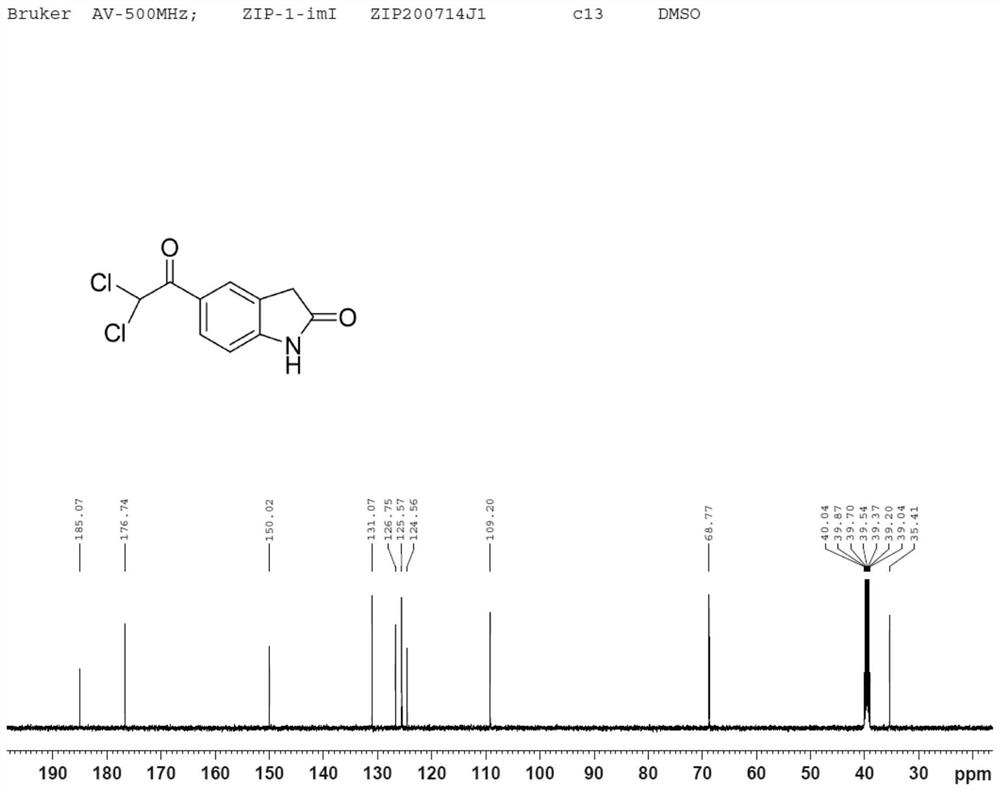
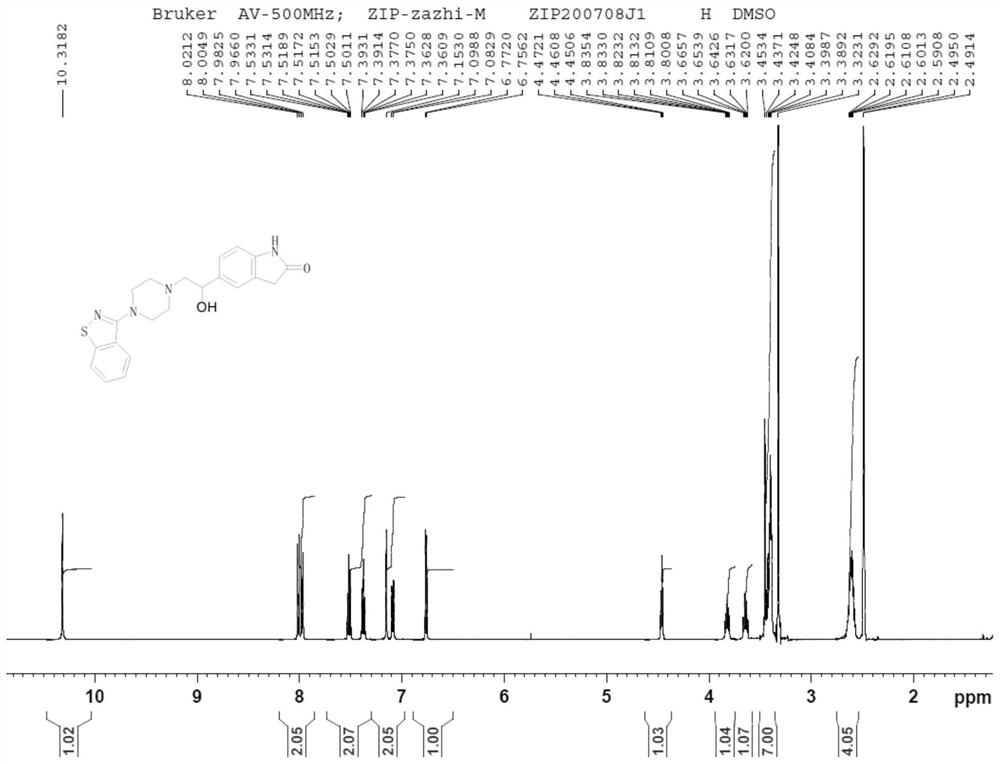
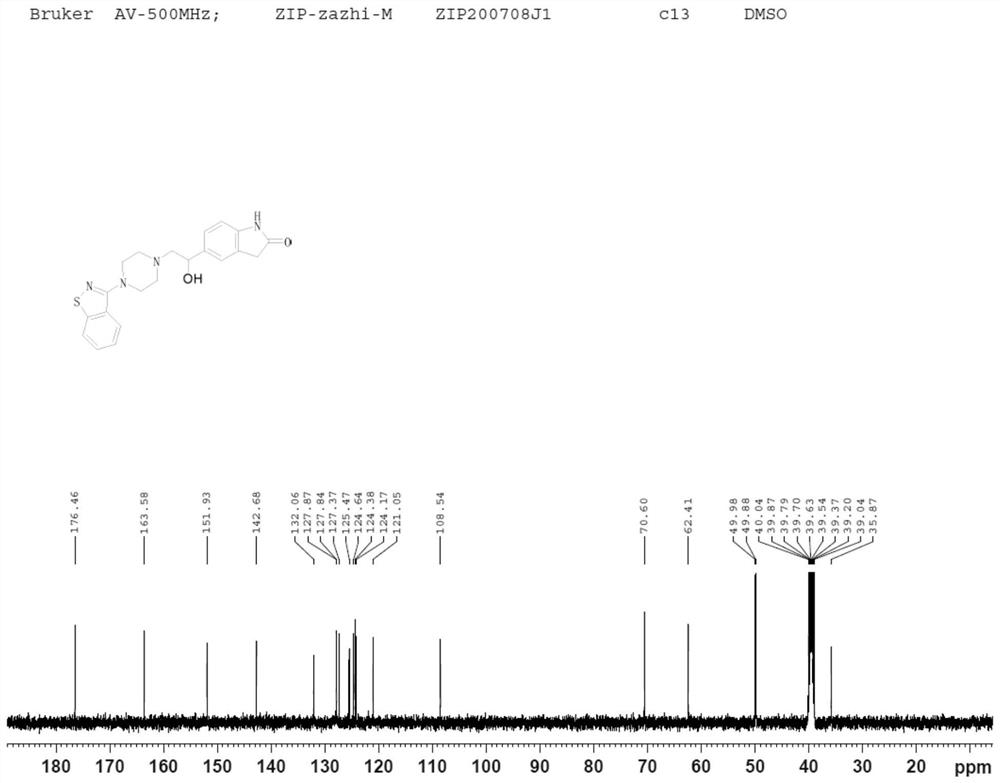
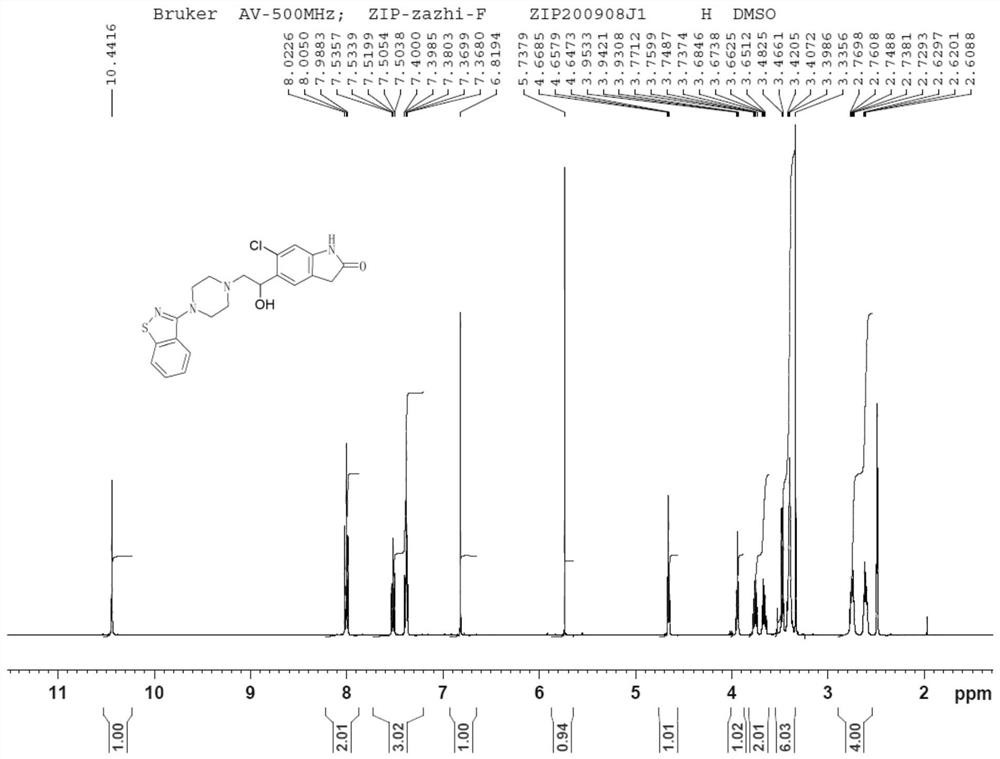
Dihalide impurity in ziprasidone hydrochloride intermediate and preparation method of dihalide impurity
ActiveCN112724066AQuality improvementReduce drug riskOrganic chemistryCombinatorial chemistryMedicinal chemistry
The invention provides a dihalide impurity in a ziprasidone hydrochloride intermediate. The dihalide impurity has a structure shown as a formula 1. On the basis that the ziprasidone hydrochloride intermediate can bring in the dechlorination impurity or the preparation process contains the dechlorination step, the dechlorination impurity with a specific structure is obtained, and the preparation steps of the corresponding impurity are provided, so a corresponding technical support is provided for preparation of ziprasidone hydrochloride. The synthesis method provided by the invention has the advantages of simple process, strong controllability and mild conditions, can be used for quality standard establishment and quality control links of ziprasidone hydrochloride process research and development, production and the like, and provides technical support for ziprasidone hydrochloride medication safety. The method can be used for quality research such as qualitative and quantitative analysis of impurities in ziprasidone hydrochloride synthesis, so that the quality of ziprasidone hydrochloride can be improved, and important guiding significance is provided for reducing the medication risk of ziprasidone hydrochloride.
Owner:HAINAN XINOPEN SOURCE MEDICAL TECH CO LTD
Drug combination for treating or preventing fatty hyperlipidemia
InactiveCN105232554AFunction and effect enhancementLose weightOrganic active ingredientsMetabolism disorderLow density lipoprotein cholesterolObesity prevention
The invention relates to a drug combination for treating or preventing fatty hyperlipidemia, in particular to a drug combination containing orlistat or cetilistat and acipimox, and belongs to the field of pharmaceuticals. The drug combination can be solid preparations such as conventional tablets, dispersible tablets, sustained-release tablets, capsules and granules. Experiments accidentally show that a drug combination containing orlistat or cetilistat and rosuvastatin calcium has an obvious synergistic effect in the aspects of lowering serum total cholesterol, serum triglyceride and low-density lipoprotein cholesterol. A preparation method is simple, convenient to operate and suitable for industrialized production.
Owner:QINGDAO YUNTIAN BIOTECH
Triazole derivative and application thereof
ActiveCN113004247APromote growthPrevent elongationBiocidePlant growth regulatorsGrowth plantTriazole derivatives
The invention belongs to the field of chemistry, and particularly relates to a triazole derivative and application of the derivative in plant growth regulation and bactericide. The triazole derivative is a compound as shown in a general formula I defined in the description, a diastereoisomer, a chiral isomer or a salt thereof. The definition of each substituent group in the formula is shown in the description. The compound provided by the invention can be applied to plant growth regulation and bactericides.
Owner:SHENYANG RES INST OF CHEM IND
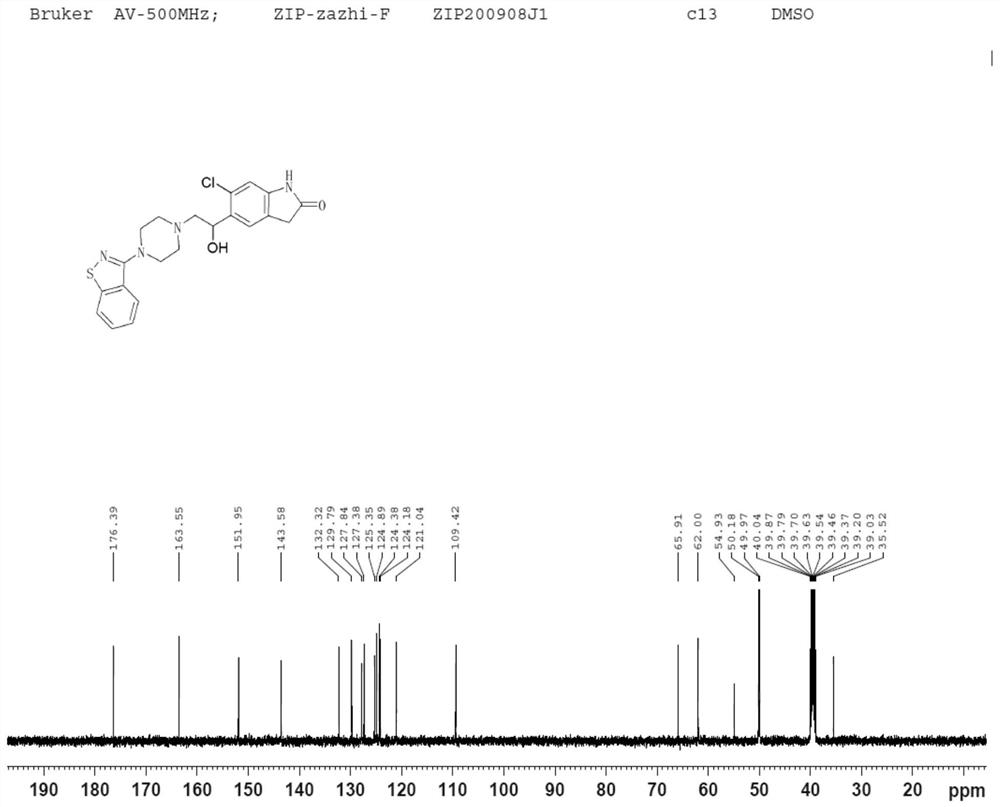
Impurity in ziprasidone hydrochloride and preparation method thereof
The invention provides an impurity in ziprasidone hydrochloride. The impurity has a structure as shown in formula 1. The impurity with a specific structure is obtained on the basis that chlorine impurities are doped in the ziprasidone hydrochloride preparation process, or impurities are brought in and transferred into a final product in the dechlorination step in the acylation reaction process, and the corresponding impurity preparation steps are provided, so that corresponding technical support is provided for preparation of ziprasidone hydrochloride. The synthesis method provided by the invention is simple in process, strong in controllability and mild in condition, can be used for quality standard establishment and quality control links in ziprasidone hydrochloride process research and development, production and the like, and provides technical support for the medication safety of ziprasidone hydrochloride. The method can be used for quality research such as qualitative and quantitative analysis of impurities in ziprasidone hydrochloride synthesis, so that improvement of the quality of ziprasidone hydrochloride is facilitated, and important guiding significance is provided for reducing the medication risk of ziprasidone hydrochloride.
Owner:HAINAN XINOPEN SOURCE MEDICAL TECH CO LTD
Compound vitamin injection medicine composition and preparation method
ActiveCN104523714AStable pHImprove stabilityMetabolism disorderPharmaceutical non-active ingredientsVitamin injectionBULK ACTIVE INGREDIENT
The invention discloses a compound vitamin injection medicine composition and a preparation method, and belongs to the field of medicine manufacturing. According to the compound vitamin injection medicine composition, the active ingredients per dosage unit comprise 110 mg of vitamin B, 5 mg of riboflavin in riboflavin sodium phosphate and 200 mg of vitamin C. The compound vitamin injection medicine composition and the preparation method have the advantages that the PH is stable, the PH value is small in change, the content of main drugs is stable, related matter is less, the process is simplified, the liquid dosing time is shortened, and the operation difficulty is lowered.
Owner:北京柏雅联合药物研究所有限公司
Novel guaiacol bromine composition and preparation method thereof
InactiveCN111135156AImprove stabilitySmall increaseEther/acetal active ingredientsPharmaceutical delivery mechanismEdetic AcidBromine
The invention relates to a novel guaiacol bromine composition and a preparation method thereof. According to the invention, edetic acid (edetate) is found to be able to well control growth of a bromhexine hydrochloride impurity E and impurity B, so the stability of bromhexine hydrochloride is remarkably improved. Compared with a conventional product with a similar kind, the novel guaiacol brominecomposition prepared by using the preparation method provided by the invention has the advantage that the high-temperature, accelerated and long-term stability results are obviously improved. The preparation method provided by the invention can be used for industrial large-scale production and has good prospect.
Owner:北京柏雅联合药物研究所有限公司
Material for transfusion hose and application of material
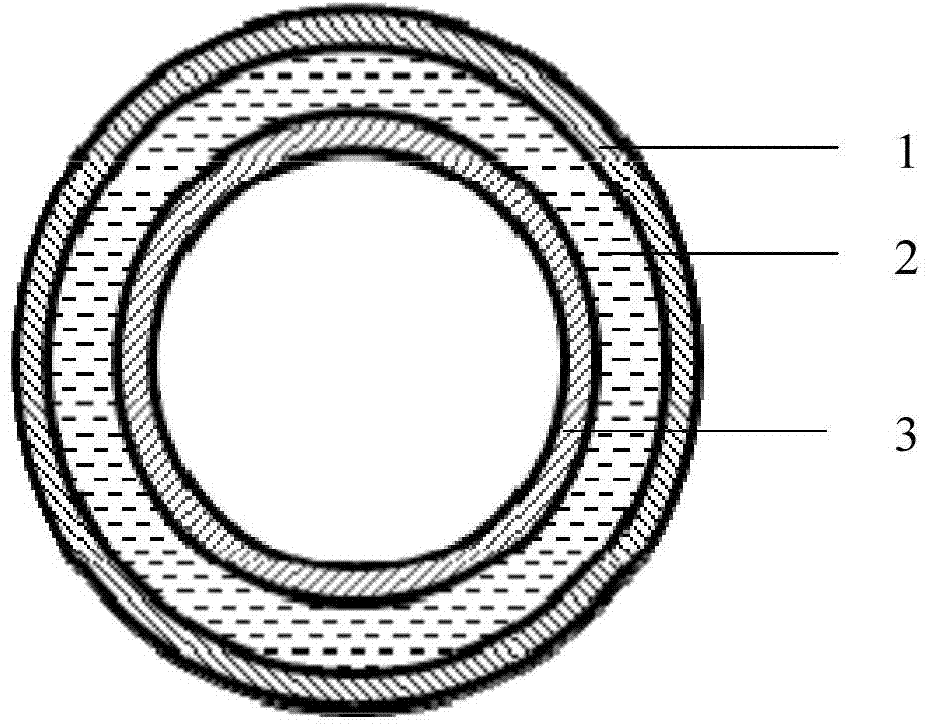
ActiveCN104772934AGood welding performanceReduce leak rateSynthetic resin layered productsCatheterComposite materialWeldability
The invention provides a material for a transfusion hose and an application of the material. The material is obtained by coextruding at least one outer layer material, a middle layer material and an inner layer material, wherein the outer layer material is prepared from the following components in percentage by weight: 50-99 percent of PP and 1-50 percent of SEBS, totally 100 percent; the middle layer material is prepared from the following components in percentage by weight: 30-70 percent of PP and 30-70 percent of SEBS, totally 100 percent; the inner layer material is prepared from the following components in percentage by weight: 10-45 percent of PP and 55-90 percent of SEBS, totally 100 percent. The material is high in weldability, soft, high in transparency, high in elasticity and suitable for coextrusion.
Owner:LANGHUO MEDICAL MATERIAL BEIJING
Impurity in ziprasidone hydrochloride and preparation method of impurity
ActiveCN112778298AQuality improvementReduce drug riskOrganic chemistryBulk chemical productionMedication riskCombinatorial chemistry
The invention provides an impurity in ziprasidone hydrochloride. The impurity has a structure as shown in formula 1. According to the present invention, the impurity with the specific structure is obtained based on incomplete reaction during the ziprasidone hydrochloride preparation process, and the impurity is introduced and transferred to a final product, and the corresponding impurity preparation steps are provided so as to provide the corresponding technical support for the ziprasidone hydrochloride preparation. The synthesis method provided by the invention is simple in process, high in controllability and mild in condition, can be used for quality standard establishment and quality control links such as ziprasidone hydrochloride process research and development, production and the like, and provides technical support for ziprasidone hydrochloride medication safety. The method can be used for quality research such as qualitative and quantitative analysis of impurities in ziprasidone hydrochloride synthesis, so that improvement of the quality of ziprasidone hydrochloride is facilitated, and great guiding significance is provided for reducing the medication risk of ziprasidone hydrochloride.
Owner:HAINAN XINOPEN SOURCE MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com