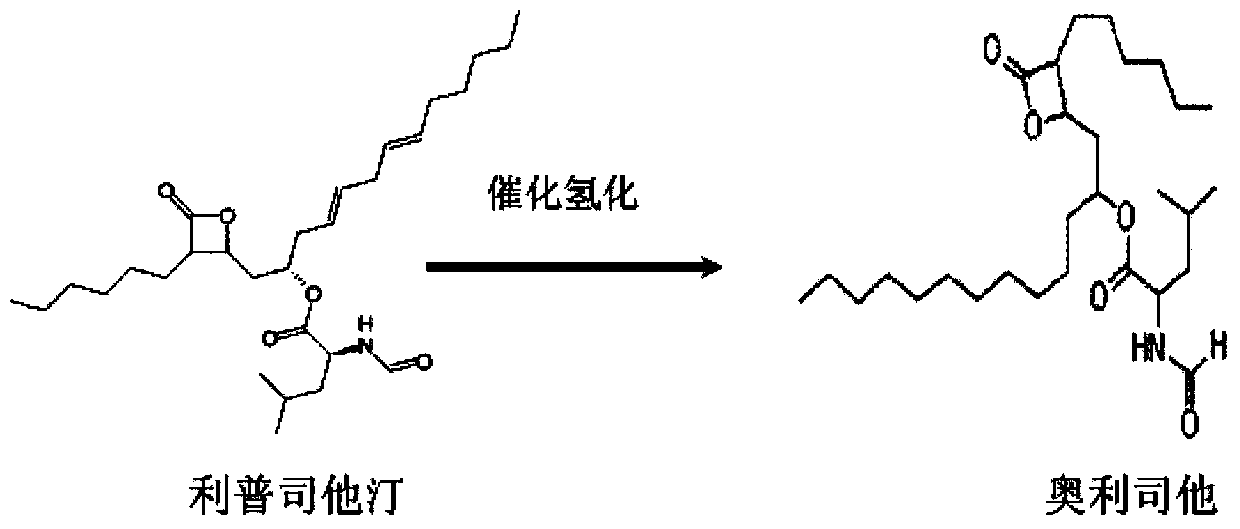
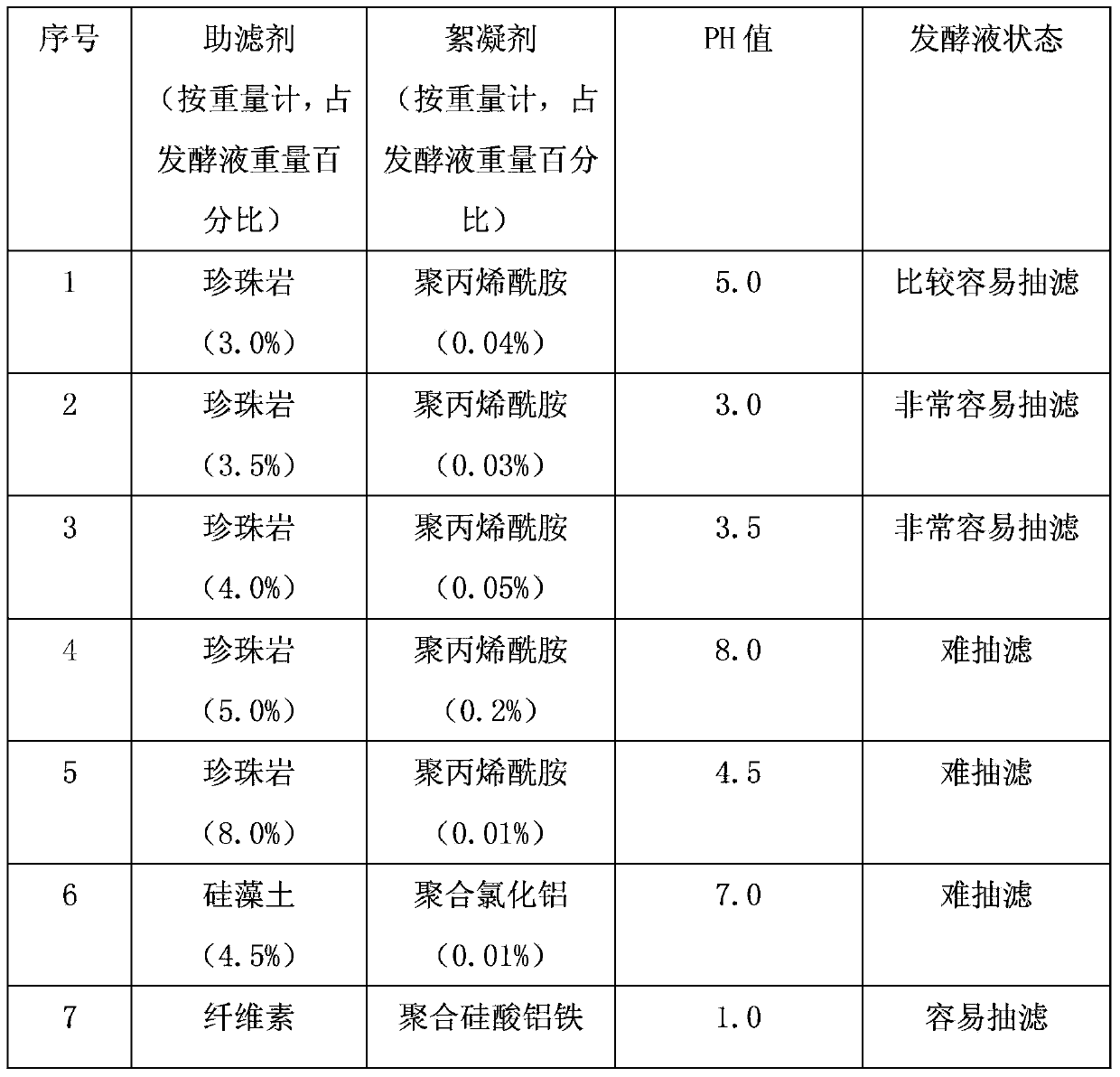
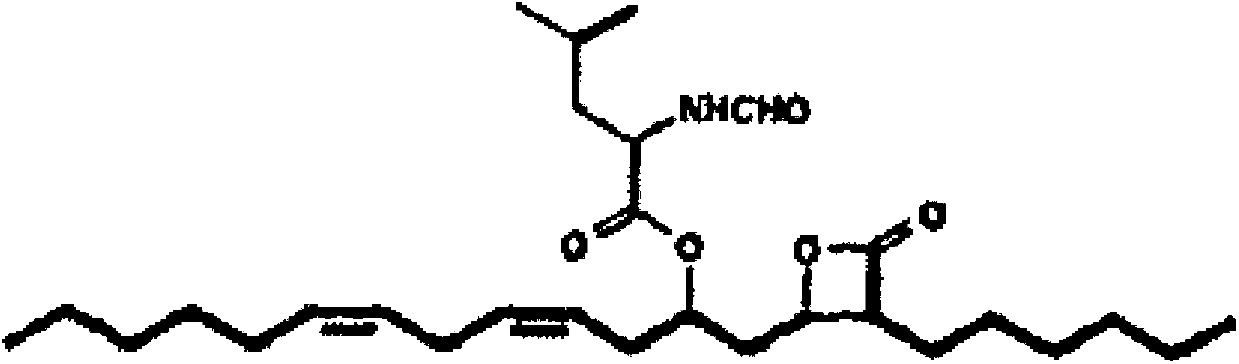
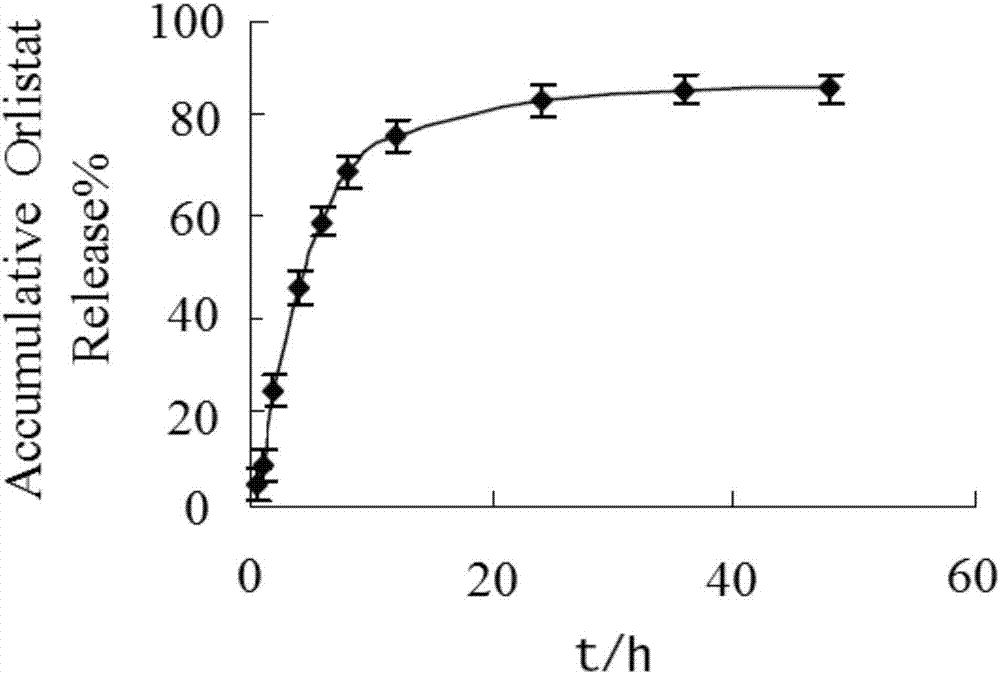
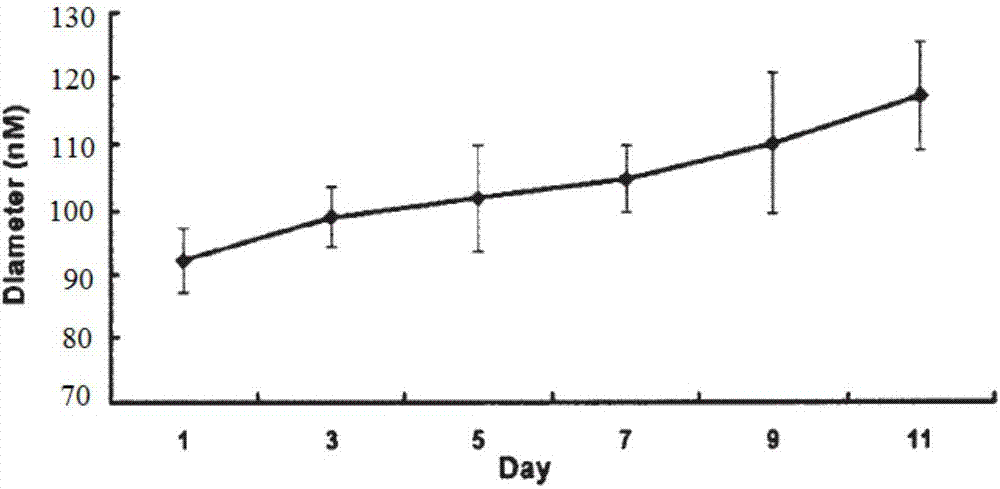
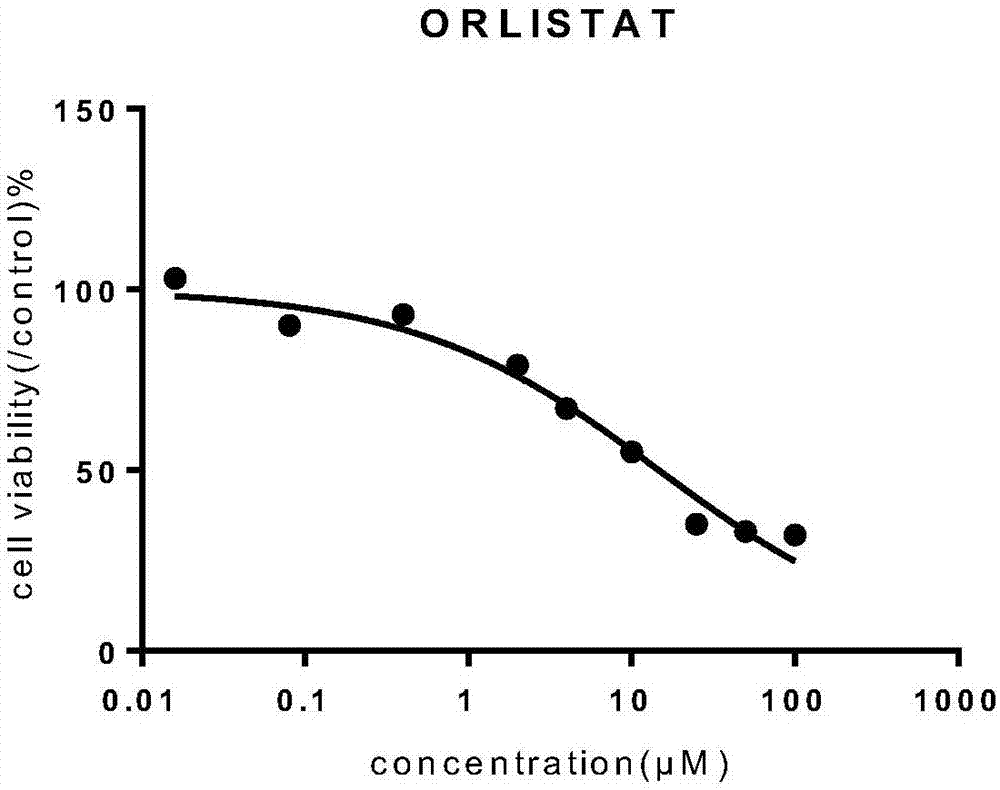
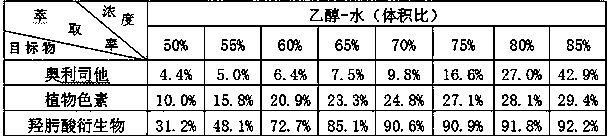
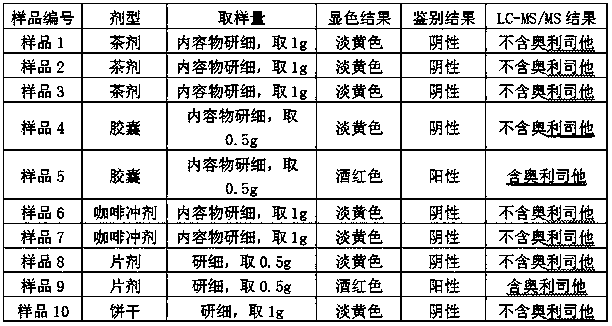
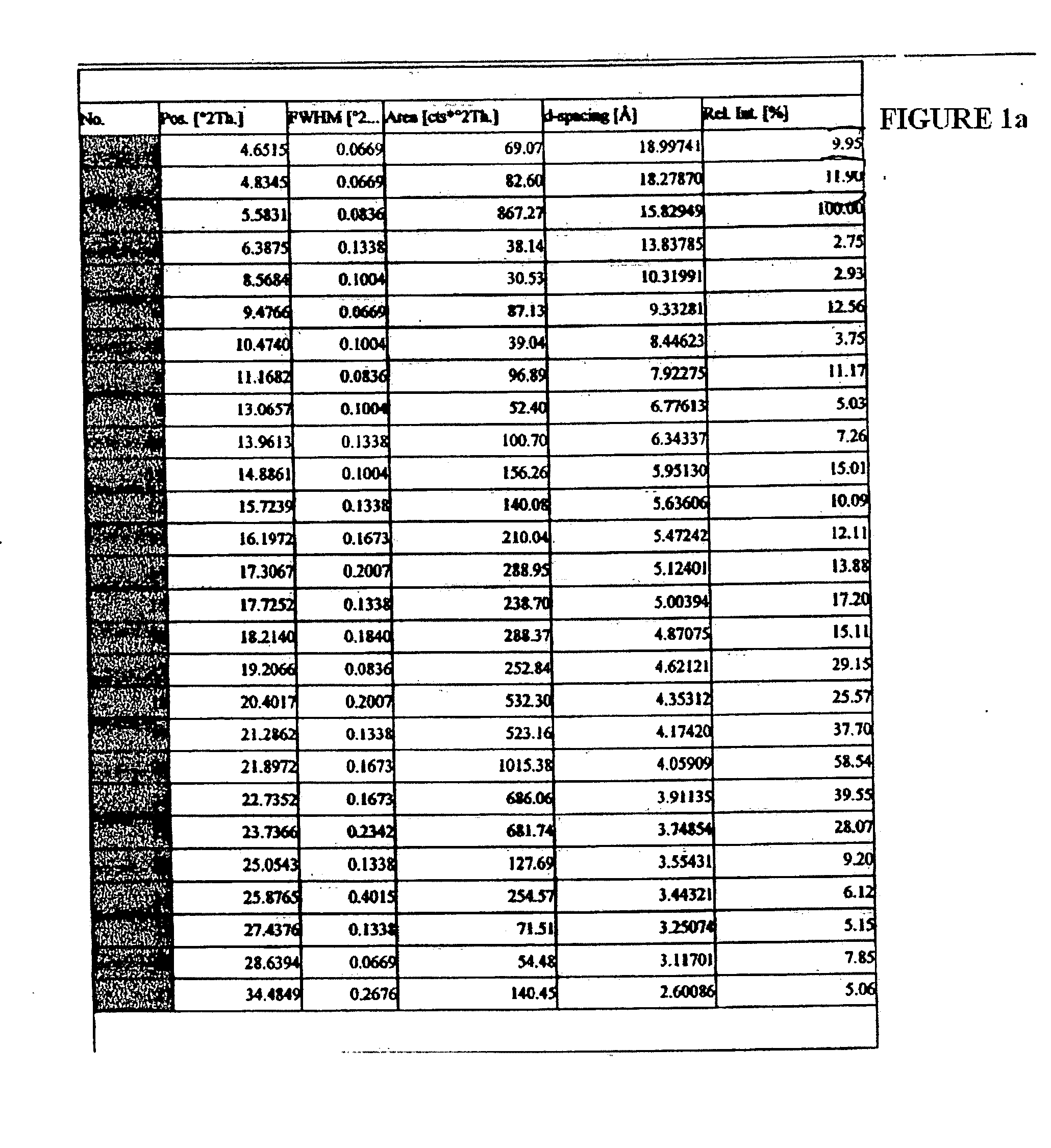
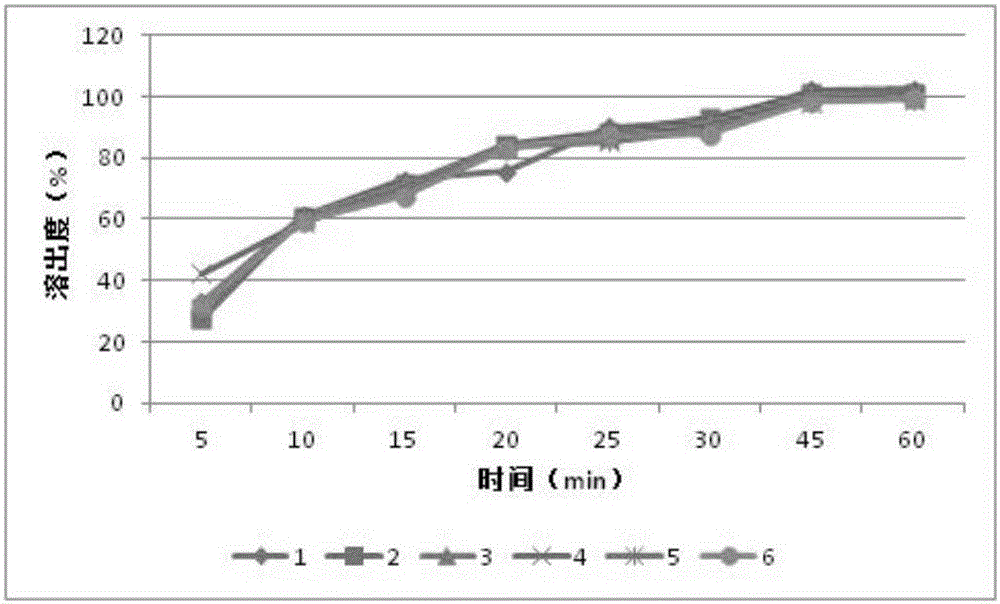
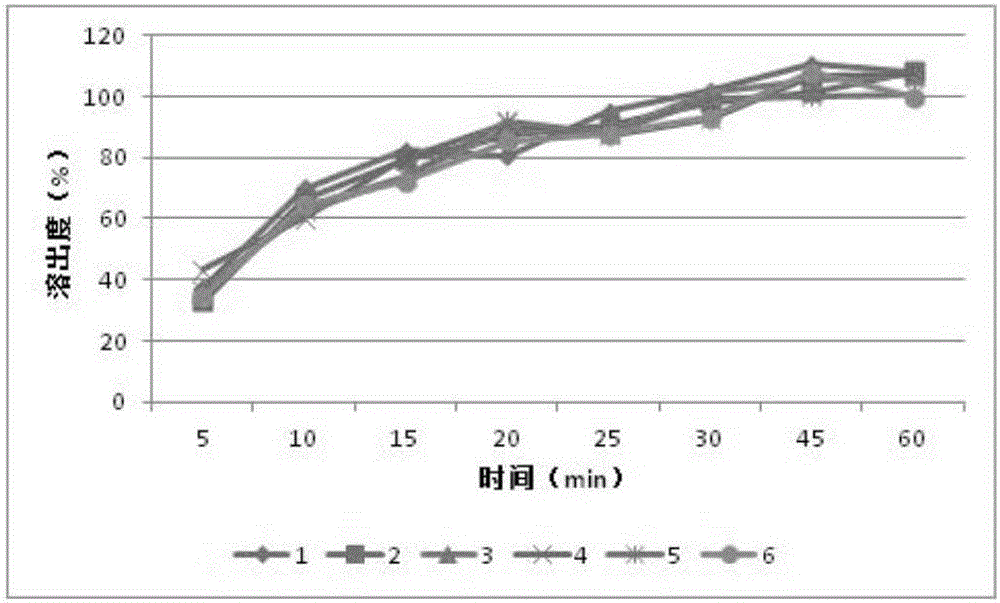
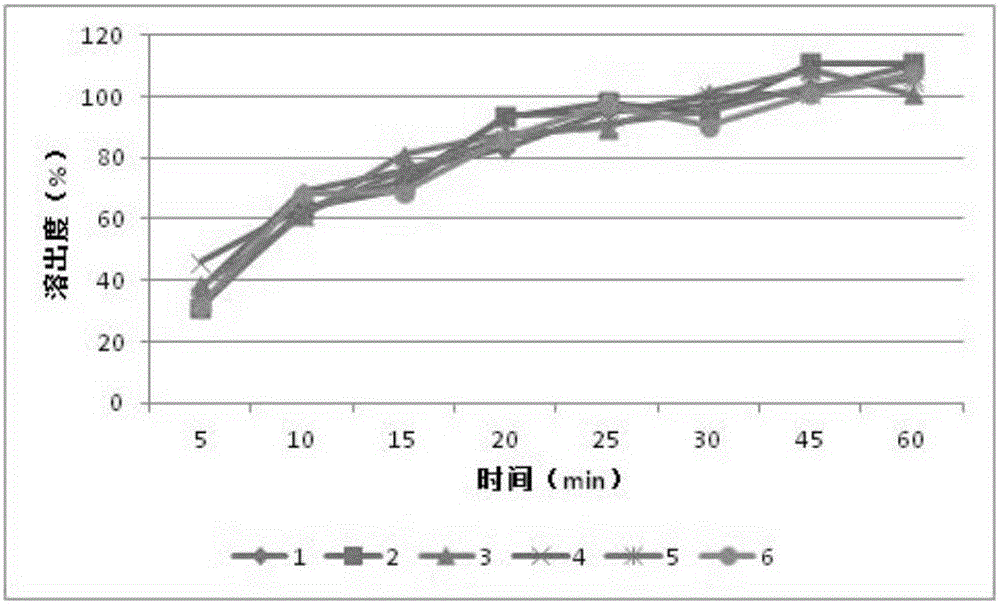
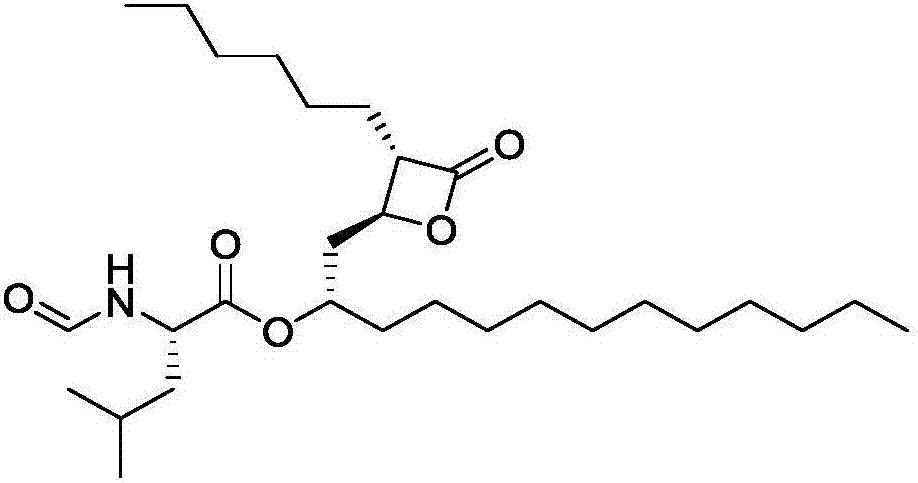
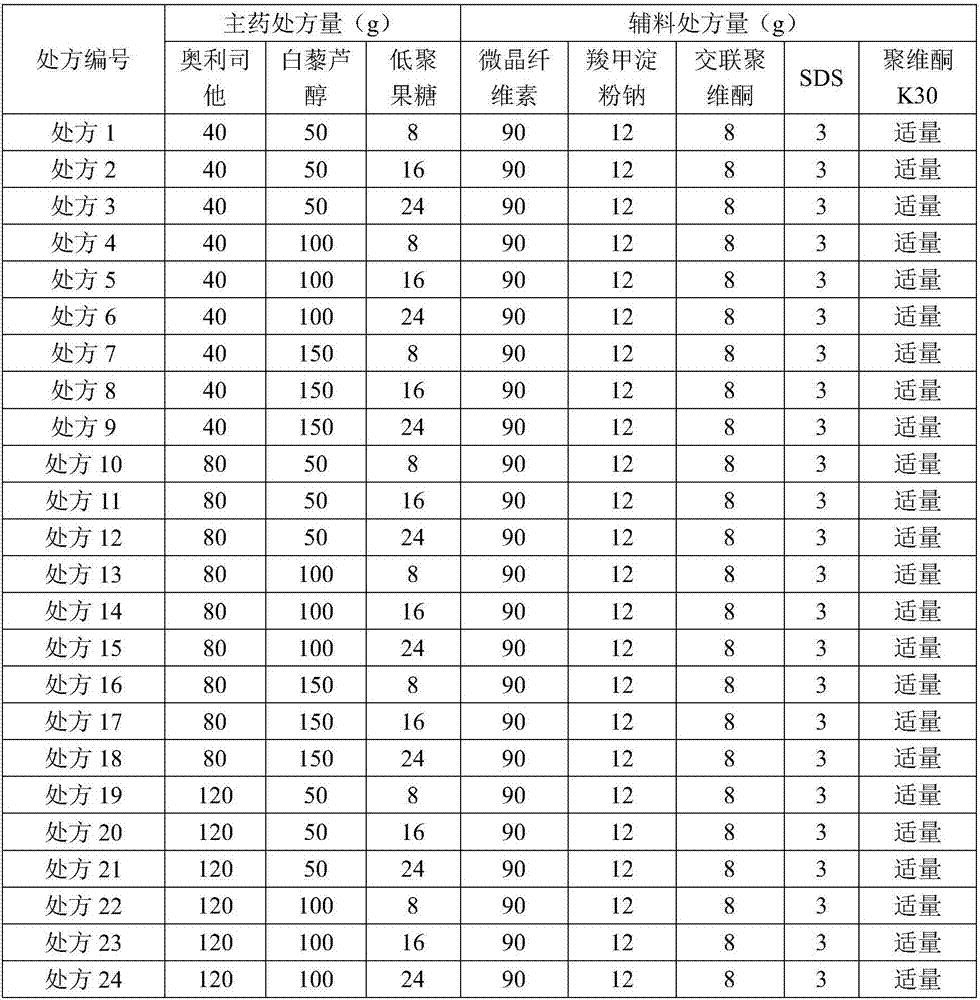
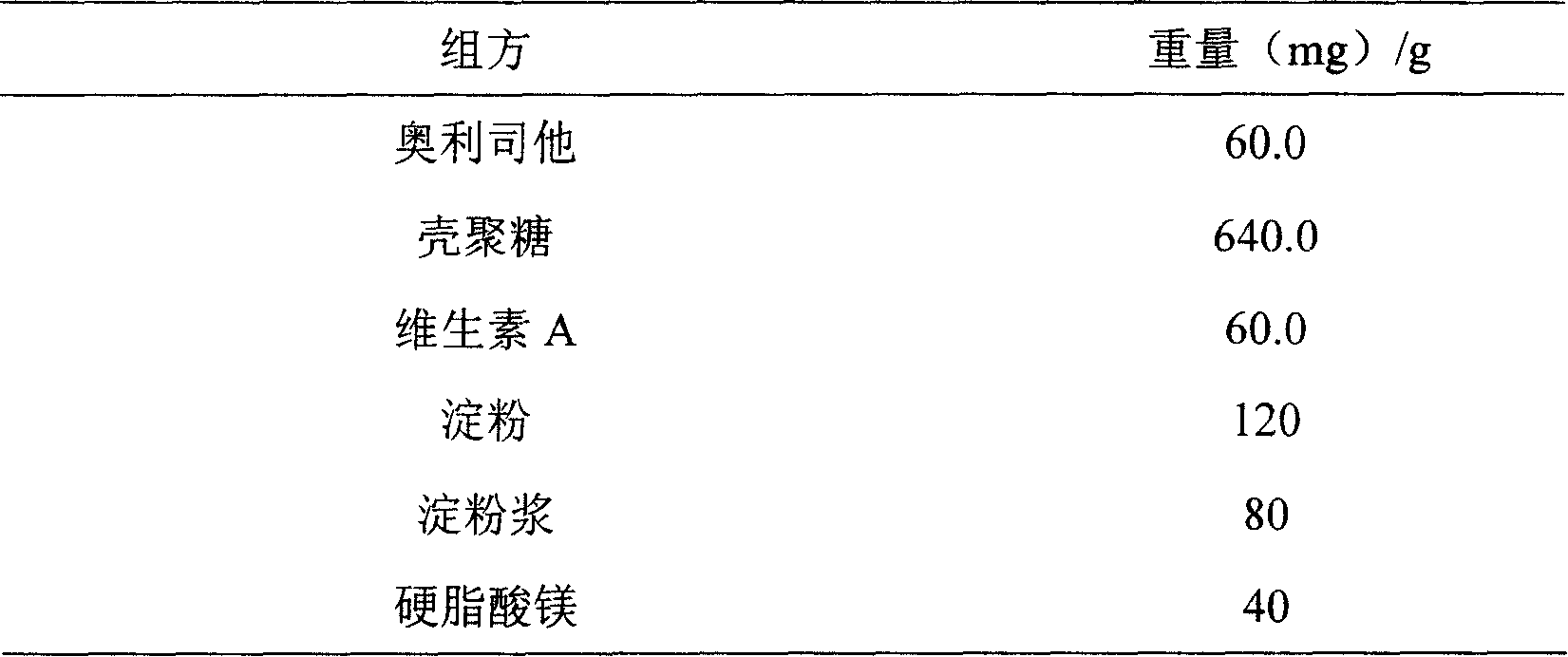
Patents
Literature
139 results about "Orlistat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used with a doctor-approved exercise, behavior change, and reduced-calorie diet program to help you lose weight. It is used by certain overweight people, such as those who are obese or have weight-related medical problems.
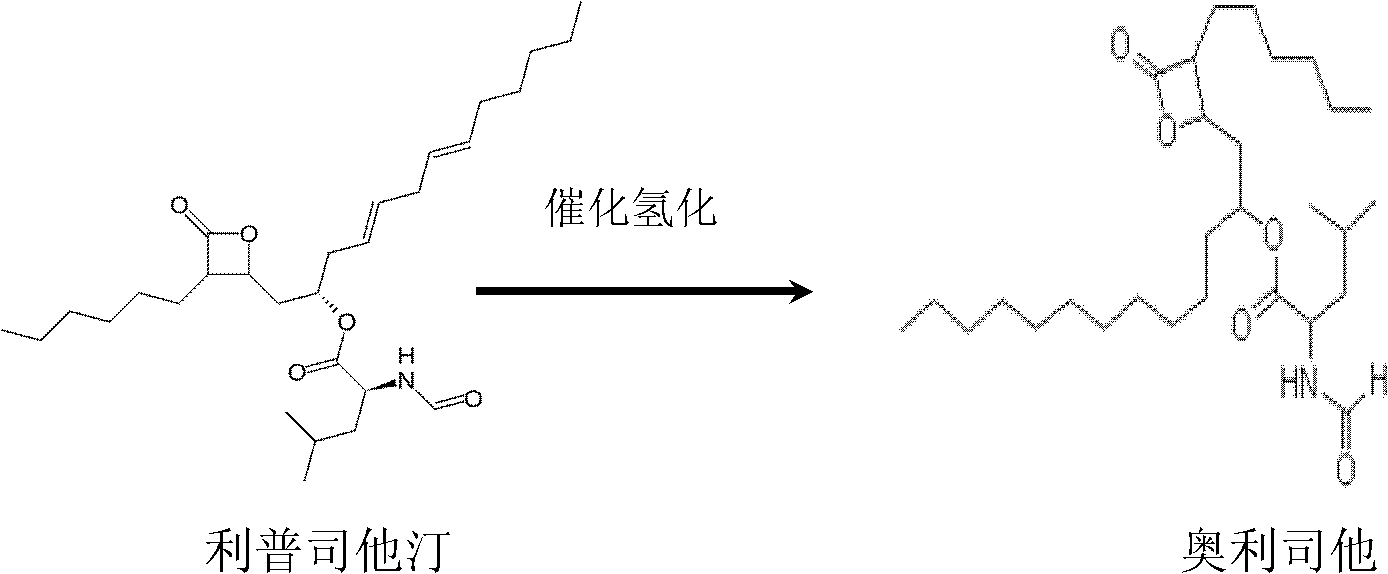
Method for preparing orlistat
The invention belongs to the technical field of medicaments, and particularly relates to a novel method for preparing weight-losing medicament orlistat by a fermentation method. The method for preparing the orlistat comprises the following steps of: extraction and filtering of fermentation solution, chromatographic impurity removal by macroporous absorption resin, silica column chromatography of orlistat after hydrogenation and the like. By method, the effects of better removing impurities and purifying products are achieved.
Owner:鲁南新时代生物技术有限公司
Medicinal composition for treating or preventing obesity and metabolic syndromes
InactiveCN102872062APrevent obesityPrevent Metabolic SyndromeOrganic active ingredientsMetabolism disorderOrlistatActive component
The invention discloses a medicinal composition for treating or preventing the obesity and metabolic syndromes, and belongs to the medicine field. The medicinal composition treats orlistat and acarbose as medicinal active components, so the application amount of orlistat in the medicinal composition is substantially reduced, thereby the risk of the damage of orlistat to the liver of a body is reduced. The medicinal composition has a substantial synergistic effect when the medicinal composition is used for treating or preventing the obesity and the metabolic syndromes, can reduces the diabetes and angiocardiopathy suffering risks of obese patients, and has a wide medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing high-purity Orlistat
The invention belongs to the technical field of medicine, particularly relates to a method for preparing high-purity Orlistat, and more particularly relates to a method for preparing high-purity Orlistat by utilizing mixed silica gel medium-pressure chromatography and crystallization as core technologies. The specific scheme of the method comprises filtration, lixiviation, extraction, chromatography, decoloring, hydrogenation, crystallization and drying processes of fermentation broth. According to the invention, the method provided by the invention is adopted to prepare Orlistat, the total yield of Orlistat is larger than 30%, and the productivity of Orlistat can reach 1.5-3 ton / month; and by detection of HPLC (high performance liquid chromatography), the purity of Orlistat is above 99.5%, the single impurity content in Orlistat is less than 0.1% and is higher than a medicinal standard. In addition, equipment used in the method provided by the invention is common chemical equipment, thus investment is less, and used solvents can be recovered and reused, thereby greatly reducing production cost and environmental pollution and achieving clean production.
Owner:鲁南新时代生物技术有限公司
Combinations of statins and anti-obesity agent and glitazones
Co-therapy of an anti-obesity agent, a statin, and a glitazone is disclosed along with fixed combinations thereof. Atorvastatin, rosiglitazone, and orlistat are preferred as the various components. Non-glitazone antidiabetic agents may be optionally added to the therapy and / or to the fixed combination product.
Owner:PALEPU NAGESWARA R
Method for purifying orlistat
The present invention discloses orlistat purifying method, which includes dissolving coarse orlistate product in medium polarity solvent, filtering, eliminating impurity, crystallization, dissolving in non-polar solvent, recrystallization, and repeating some steps until reaching the medicinal purity standard. The said method is suitable for purifying coarse orlistate product with purity of 50-85 %, and has the advantages of high yield, short purification period, simple purification process and being suitable for industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Preparation method of orlistat
ActiveCN102070568AReduce consumptionShorten the production cycleOrganic chemistryOrlistatPolymer resin
The invention discloses a preparation method of orlistat, which comprises the following steps: (1) using reverse phase silica gel or reverse phase polymer resin as a filler of a DAC (dynamic axial compression) preparative column, and uniformly mixing an organic solvent and the filler to prepare a slurry; (2) filling the filler slurry into a cylinder of the DAC preparative column, and starting a column packing machine to carry out axial compression; (3) pumping a mobile phase into the DAC preparative column by using a preparative pump, thereby carrying out displacement washing on the organic solvent in the column and balancing the preparative column; (4) injecting a crude orlistat product solution into the DAC preparative column by using a high-pressure preparative pump to carry out column adsorption; (5) after the column adsorption, analyzing the orlistat solution by using a mobile phase, and collecting the analytic liquid in multiple sections; and (6) merging samples with qualified purity, concentrating, crystallizing and drying to obtain the finished orlistat product. By using the purification and preparation method of orlistat researched and developed by the invention, the purification and preparation amount of orlistat per batch is up to several kilograms and even myriagrams, and the purification production cycle is shortened to about 1-2 hours from tens of hours in the past.
Owner:ZHUHAI UNITED LAB
Orlistat tablets and preparation method thereof
ActiveCN101791296AGood chemical stabilityImprove physical stabilityOrganic active ingredientsMetabolism disorderOrlistatPharmacy
The invention belongs to the technical field of the medicament and in particular relates to tablets comprising orlistat and a preparation method thereof. Orlistat and cyclodextrin which are subjected to enclosing and auxiliary materials acceptable for pharmacy are pressed into the tablets. The tablets solve the sticking problem of carrying out pressing on the orlistat by the conventional process, obviously improve the chemical and physical stability of the orlistat, cover the unpleasant taste of the orlistat, improve the administration compliance of the dysphagia patients, have good dissolution and improve the curative effect of the orlistat.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for purifying orlistat intermediate
The invention belongs to the technical field of medicine and particularly relates to a method for purifying the important intermediate lipstatin of orlistat serving as a weight-reducing aid. The method provided by the invention comprises fermentation liquor pretreatment, extracting, phase inversion and concentration. The product obtained by using the method is high in purity, and the process provided has the advantages of high yield, short period, simplicity and easiness in control, less equipment menstruum investment and low production cost, and is very suitable for industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Method for purifying orlistat
ActiveCN102993135ASimple processGood effect of removing impuritiesOrganic chemistryOrlistatPurification methods
The invention belongs to the field of pharmaceutical synthesis and discloses a method for purifying orlistat. Two-step refining is performed by crystallization of a polar solvent and a non-polar solvent, according to the high performance liquid chromatography (HPLC) measurement, the content of the orlistat is above 99%, the maximum single impurity of the orlistat is within 0.1%, and the orlistat accords with United States pharmacopoeia (USP) 35 standards. The method for purifying the orlistat has the advantages of being simple, small in equipment investment, low in production costs, high in product quality, stable in process and suitable for industrialized production.
Owner:SHANDONG NEWTIME PHARMA
Orlistat nano-microsphere as well as preparation method and application thereof in anti-tumor drugs
ActiveCN107412196AChange hydrophobic propertiesHigh clinical valueOrganic active ingredientsInorganic non-active ingredientsOrlistatDisease
The invention belongs to the technical field of bio-medicine, and in particular relates to an orlistat nano-microsphere as well as a preparation method and an application thereof in anti-tumor drugs. The orlistat nano-microsphere provided by the invention is prepared by mixing orlistat and a drug-carrying material, wherein the drug-carrying material is polyethylene glycol-polycaprolactone (mPEG-PCL); the mass ratio of the orlistat to the mPEG-PCL is at (1-2) to (3-10); and the molecular weight ratio of mPEG to PCL is at 2000 to 10000 or 2000 to 20000. The polymer nano-microsphere provided by the invention is prepared by loading the orlistat on the nano-material, so that a hydrophobic property of the nano-microsphere is changed; and through the sustained-release property of the microsphere, the microsphere can be used for preparing drugs for resisting tumor diseases; therefore, the nano-microsphere is huge in potential clinical value.
Owner:ZEIN BIOTECHNOLOGY CO LTD
Orlistat rapid detection method
ActiveCN105510312ASimple and fast operationReduce analysis costsMaterial analysis by observing effect on chemical indicatorOrlistatTest sample
The present invention discloses an orlistat rapid detection method, a to-be-tested sample is extracted, treated with a washing solution, reacted with a hydroxylamine hydrochloride solution and a strong alkali solution for extraction, and then acidified for coloration, and whether orlistat is contained can be judged by color. The detection method is simple and low in cost for analysis, the to-be-tested sample is pretreated, and then reacted with a detection agent, so that the interference of relevant factors can be eliminated, observation of the color of the solution is facilitated, and whether the orlistat is contained in the sample can be judged more accurately. The detection method is strong in specificity, interfering substances can be eliminated by extraction, washing, extraction and separation, the sensitivity of the coloration reaction is increased, test result correct rate is high, the coloration reaction is rapid, the phenomenon is obviously, and the method is suitable for on-site rapid detection.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Orlistat industrial liquid chromatogram preparation method
The invention discloses an orlistat preparation method, the orlistat synthesis crude product is employed as a raw material, and is performed through a HPLC optimization condition to obtain an appropriate mobile phase and column separating condition, and is amplified to an industrial preparation scale liquid chromatogram separating system for further to obtain the orlistat with high purity. The orlistat industrial liquid chromatogram preparation method has the advantages that industrial preparation scale liquid chromatogram separating system is directely used on the crude product after synthesis to obtain the required substance, so that good separating and purification effects can be reached, the orlistat industrial liquid chromatogram preparation method has the advantages of simple operation, short period and high efficiency, the purity can reach more than 99.5%, and the individual impurity can be controlled below 0.3%.
Owner:江苏汉邦科技股份有限公司
Method for purifying orlistat
ActiveCN102010387AIncrease productivitySimple processOrganic chemistryMetabolism disorderOrlistatHuman use
The invention relates to a method for purifying an organic compound, in particular to a method for preparing high-quality orlistat and a process thereof. By adopting the process disclosed by the invention for separating and preparing the high-quality orlistat with a dynamic axial compression preparation column, the chromatography content of the orlistat can reach more than 99.0% when the content of the orlistat is tested by adopting a high performance liquid chromatography, the content of known impurities is less than or equal to 0.15%, the content of unknown impurities is less than 0.10%, and the invention is completely in line with high quality requirements on ICH (The International Conference on Harmonization of Technical Requirements for Registration of pharmaceuticals for Human use).
Owner:HISUN PHARMACEUTICAL (HANGZHOU) CO LTD
Method for separating and purifying Orlistat
ActiveCN102558103AReduce consumptionSimple processIon-exchange process apparatusOrganic chemistryOrlistatQuality control
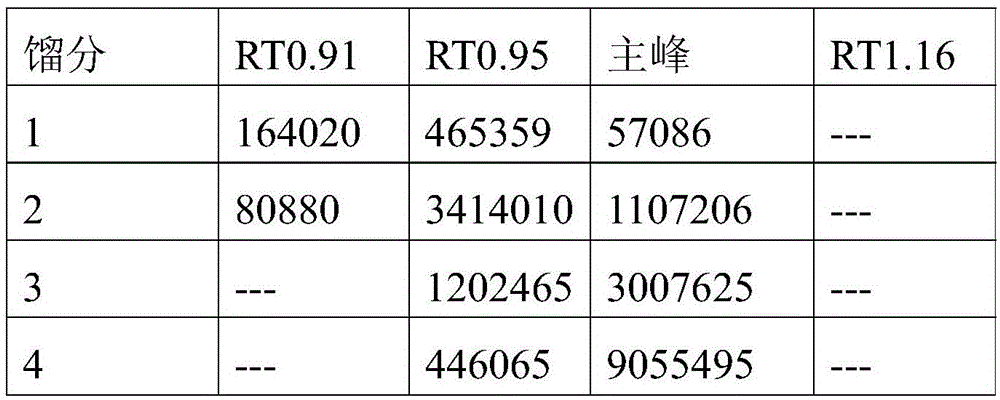
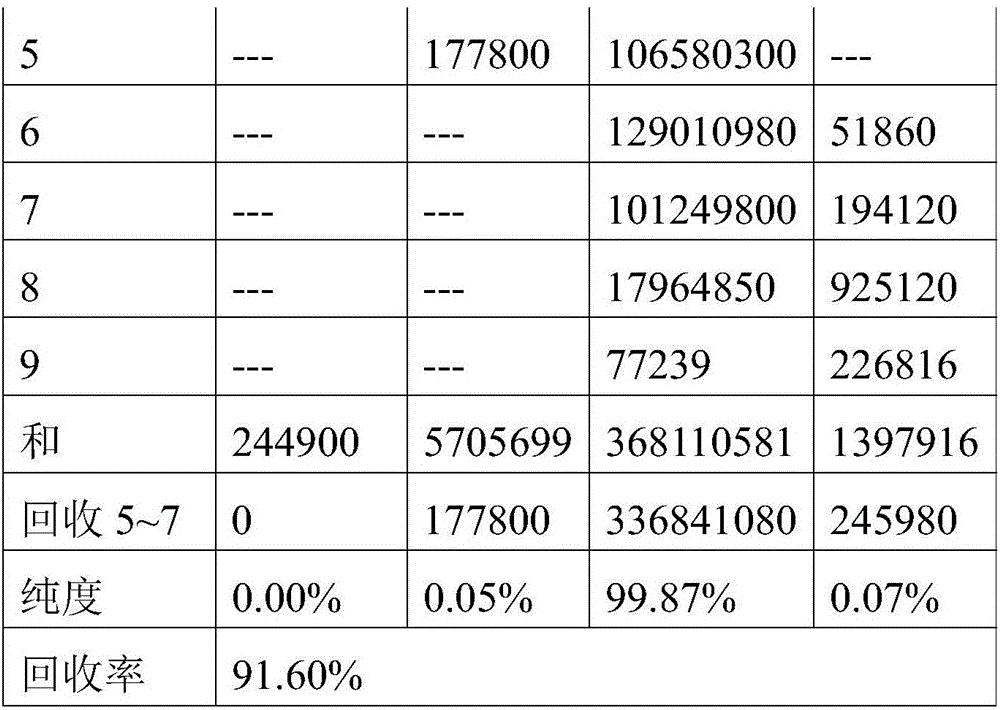
The invention belongs to the field of separation and purification and relates to a method for separating and purifying Orlistat by reverse-phase high-performance preparative liquid chromatography. The Orlistat prepared by using the method has purity of more than 99.6%, recovery rate of above 90% and the largest single impurity content of not more than 0.1%. The method has the advantages of simplicity in process operation, easiness in quality control and suitability for wide industrial scale application.
Owner:SHANDONG NEWTIME PHARMA
Orlistat soft capsule and preparation method thereof
ActiveCN108785272ALittle side effectsImprove efficacyOrganic active ingredientsMetabolism disorderOrlistatSide effect
The invention belongs to the technical field of medicines and in particular relates to an orlistat soft capsule and a preparation method thereof. The orlistat soft capsule consists of a content and capsule skin, wherein the content consists of orlistat, polysorbate 80 and vitamin E polyethylene glycol succinate; the capsule skin consists of fish gelatin, glycerinum and purified water. The orlistatsoft capsule provided by the invention has the advantages of being high in dissolution rate, low in related substance content, high in stability and low side effect, the medicinal effect of orlistatcan be effectively improved, meanwhile, the orlistat soft capsule has no problem of adhesion, pasting or re-accumulation in a production process, is easy to control and is beneficial to large-scale production.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Streptomyces toxytricini and applications of streptomyces toxytricini in preparation of lipstatin
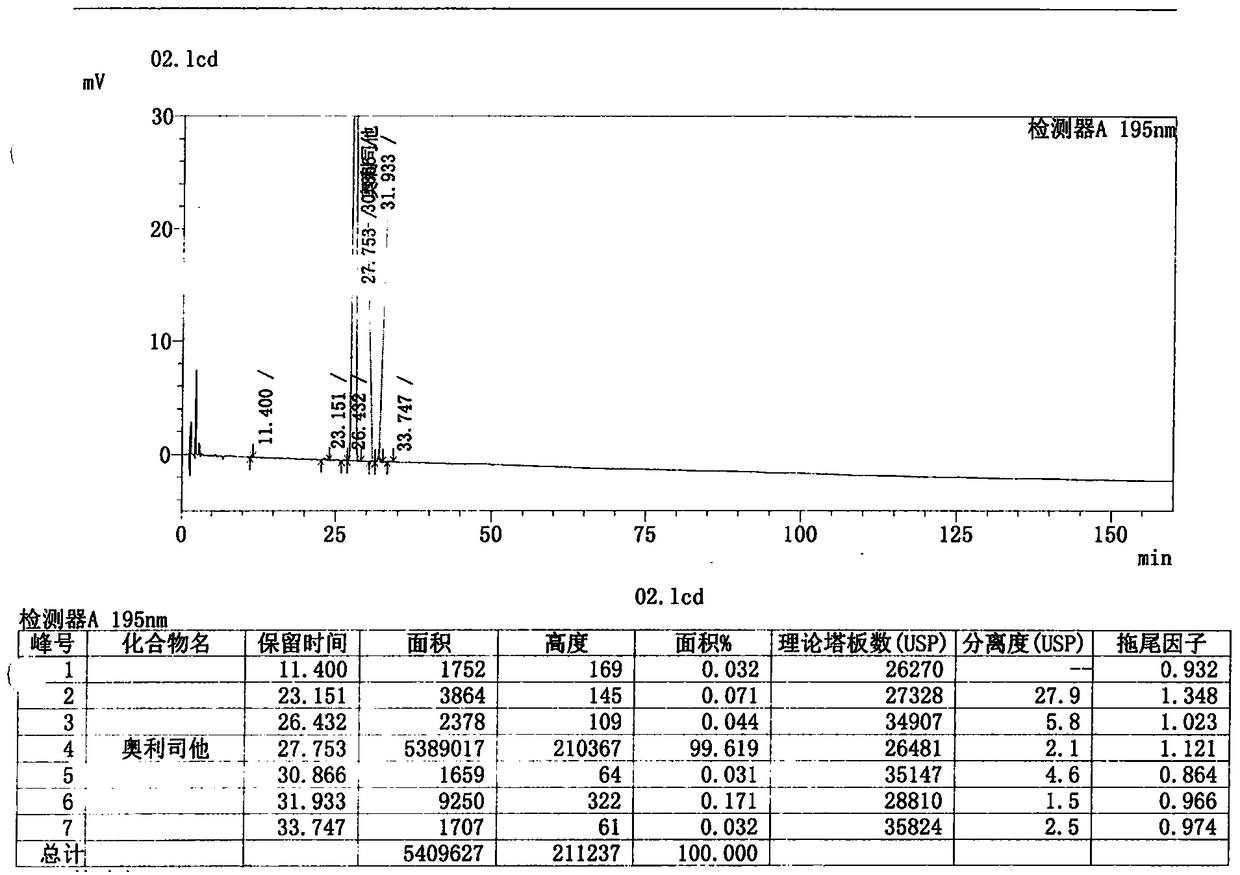
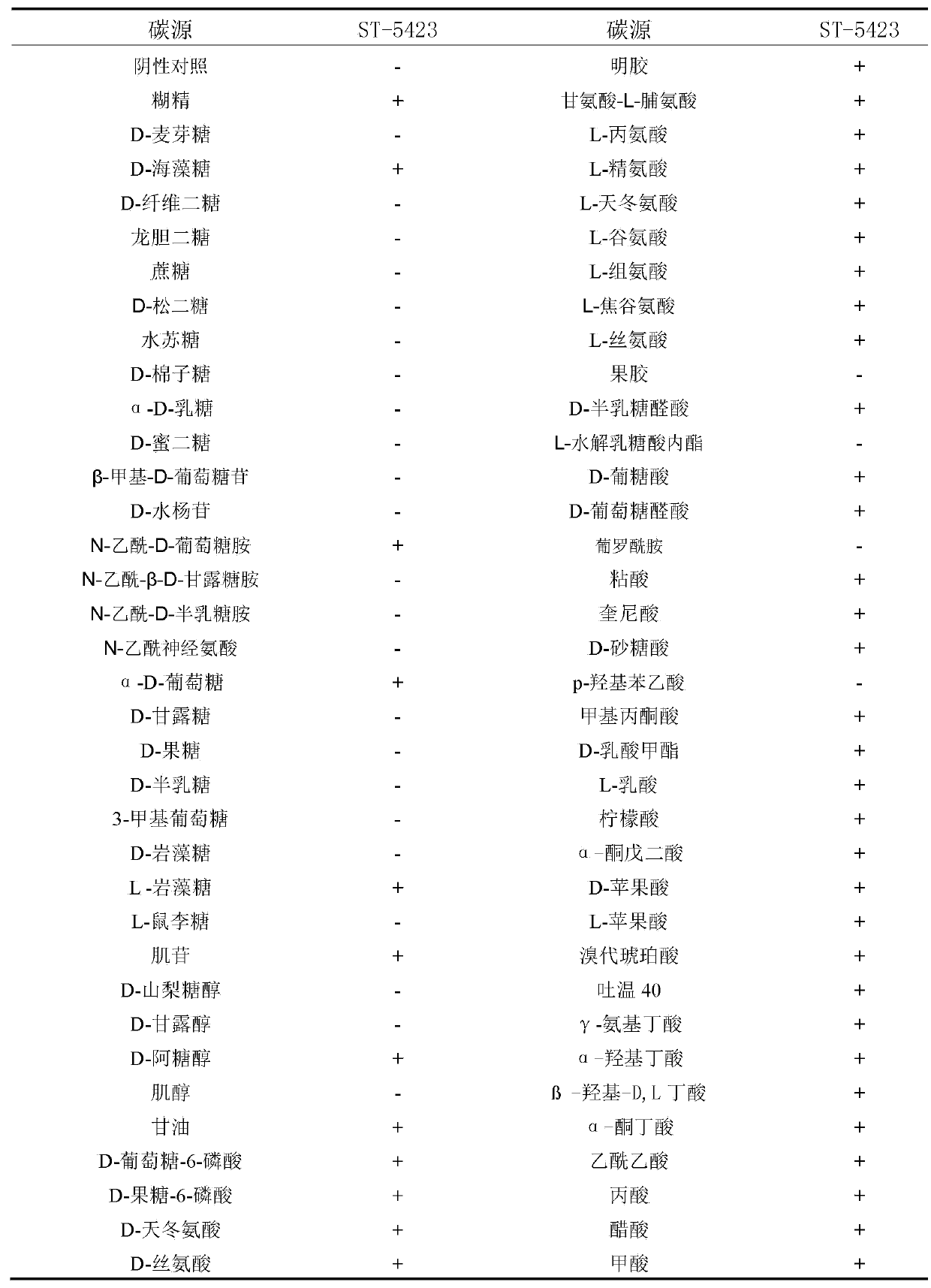
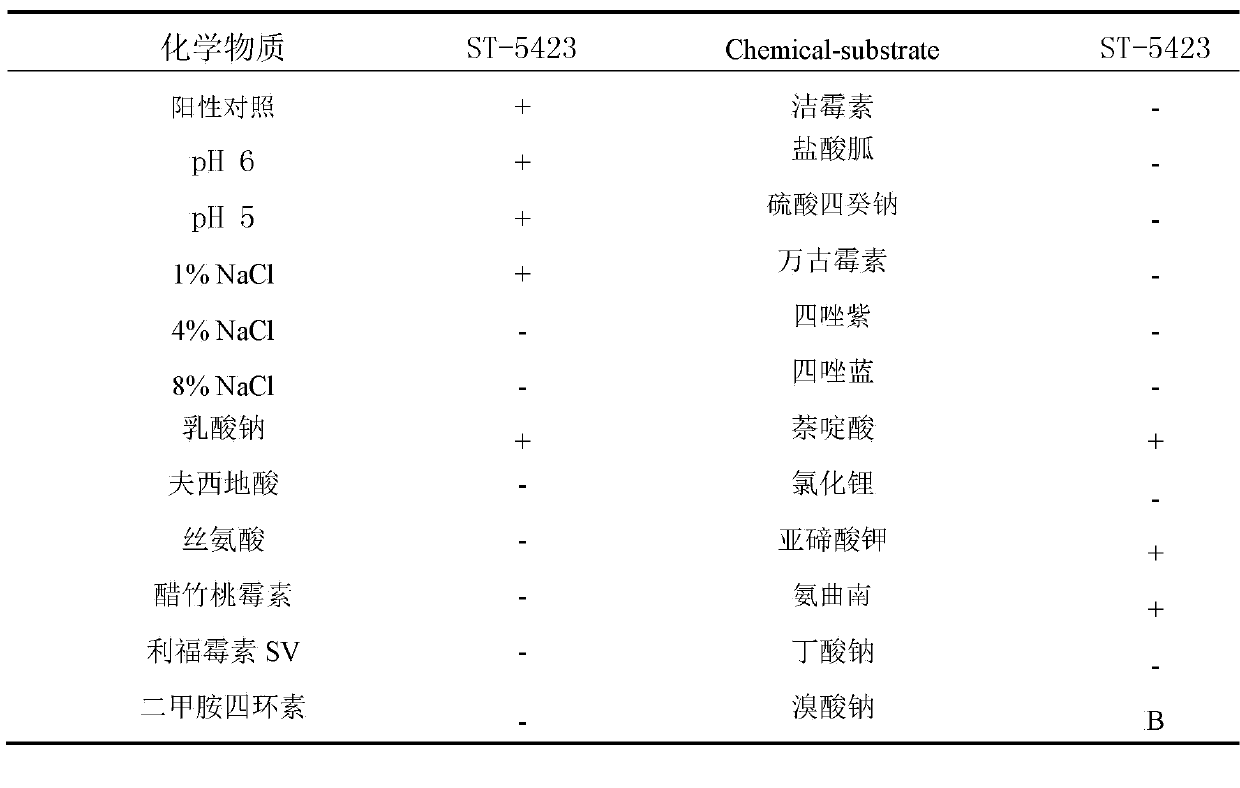
The invention discloses Streptomyces toxytricini ST-5423 and applications of Streptomyces toxytricini ST-5423. The bacterial strain is preserved in China Center for Type Culture Collection, the preservation number is CCTCC M2013312, and the preservation date is July, 4th, 2013. The invention also discloses a preparation method of an orlistat intermediate by fermentation using the bacterial strain. It is confirmed by production practices, in which 10t and 30t fermentation tanks are used, that production capacity of fermentation technology disclosed in the invention is stable, fermentation titer unit is high, fermentation by-products are relatively less, the difficulty of post-extraction is reduced greatly, the fermentation technology is suitable for industrialized large-scaled production, and quality of the obtained orlistat products is high.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
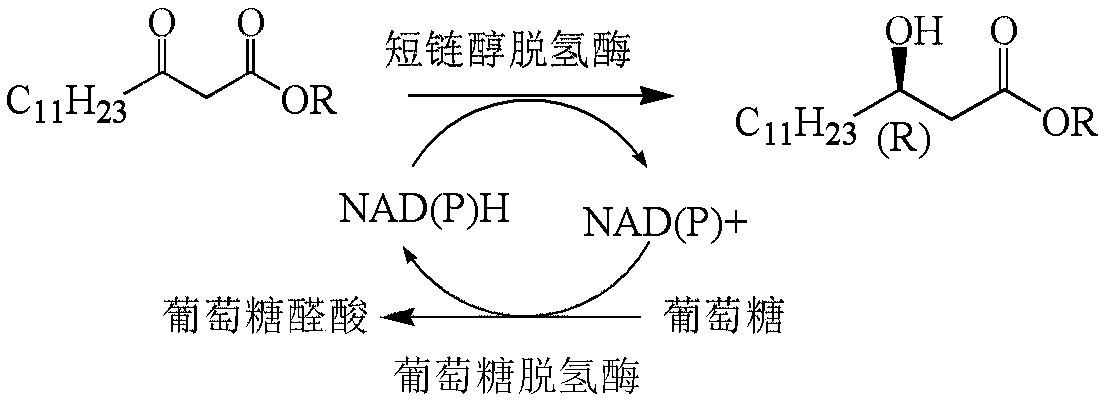
Method for preparing orlistat intermediate by enzymatic technology
The invention relates to the technical field of medicines, and discloses a method for preparing an orlistat intermediate by an enzymatic technology. The method comprises the following steps: synthesisof a short-chain alcohol dehydrogenase original gene, expression of a short-chain alcohol dehydrogenase, cell breaking and enzymatic catalysis. The production of the high-chiral ee value (ee > 99%) orlistat intermediate methyl (R)-3-hydroxytetradecanoate by a short-chain alcohol dehydrogenase catalysis process is sued to substitute a traditional chemical synthesis method, so the production cost and the environmental pollution are reduced. The method for synthesizing the orlistat intermediate has the advantages of mild reaction conditions, fast reaction speed, high reaction yield and chiral eevalue, low production cost, and suitableness for industrial expansion production.
Owner:ZHEJIANG OCEAN UNIV
Compound orlistat nano-emulsion oral liquid and preparation method thereof
InactiveCN102552409AIncrease fat solubilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderActive agentBrown adipose tissue
The invention discloses a compound orlistat nano-emulsion oral liquid which contains the following components by mass percentage: 35%-45% of surfactant, 0-1% of cosurfactant, 0-1% of oil phase, 0.5%-4% of orlistat, 0.5%-3% of fructus cannabis oil, 0.5%-2% of evening primrose oil, 0.1%-0.5% of beta-carotene, 0.0001%-0.002% of vitamin D, 0.1%-0.5% of vitamin E, 0.0001-0.002% of vitamin K, and the balance of deionized water, and the sum of the mass percentage of the components is 100 percent. Aiming at the features of obese patients, the compound orlistat nano-emulsion oral liquid has multiple effects of reducing absorption of fat and heat in food, accelerating consumption of stubborn brown adipose tissues accumulated in the body, replenishing micronutrient matters lost during weight loss, reducing blood fat and cholesterol and the like, and does not have the side effects of insomnia, strength lack, palpitation and the like.
Owner:NORTHWEST A & F UNIV
Stable solutions of orlistat for pharmaceutical dosage forms
InactiveUS20100087520A1Increase loadPatient compliance is goodBiocideCapsule deliveryTG - TriglycerideMedium-chain triglyceride
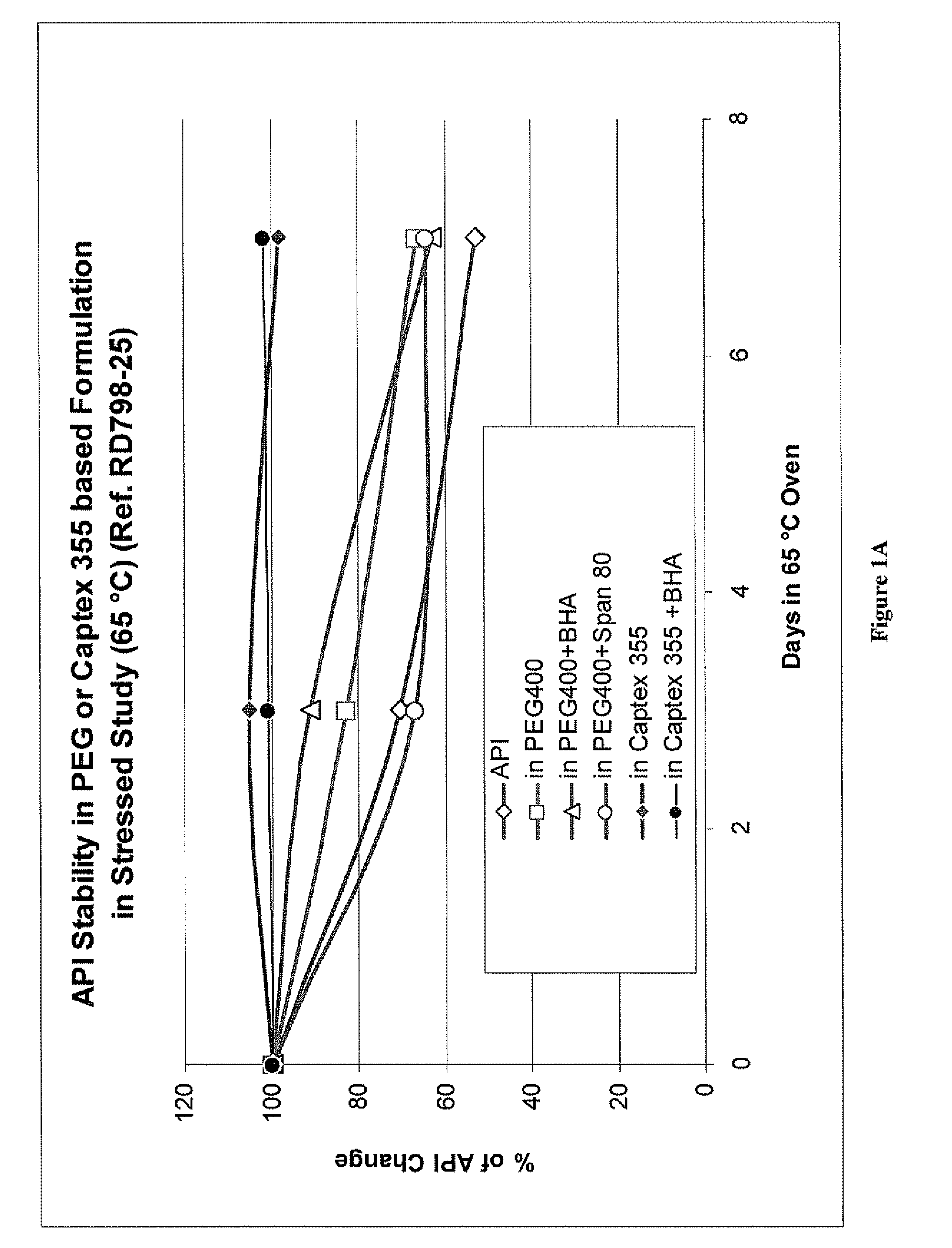
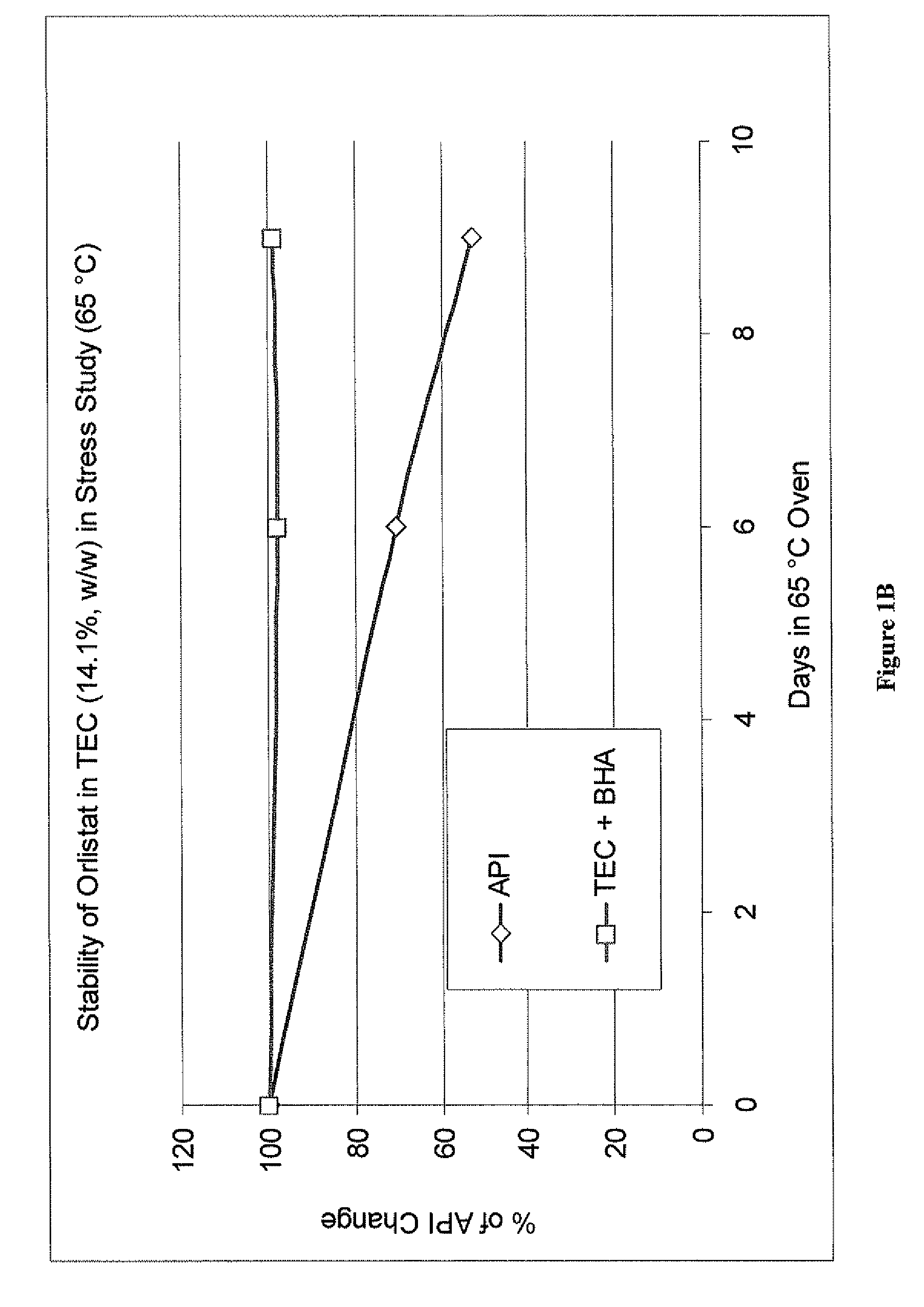
Liquid orlistat-containing fill materials suitable for encapsulating in hard or soft capsules are described herein. The fill material contains orlistat dissolved in one or more medium chain triglycerides or medium chain partial triglycerides, one or more citrate esters, and combinations thereof. The fill material can also contain one or more pharmaceutically acceptable excipients. In one embodiment, the fill material is substantially free of surfactants. The fill material can be encapsulated in hard or soft, gelatin or non-gelatin capsules. The capsules may be coated to modify release of orlistat from the capsule. Alternatively, the fill material can be encapsulated in an enteric capsule, wherein the enteric polymer is a component of the capsule shell, rather than a coating over the capsule shell. The fill materials are stable at elevated temperatures over an extended period of time and allow for high loadings of orlistat (e.g., 20% w / w or higher).
Owner:BIONPHARMA INC
Preparation method of orlistat
The invention belongs to the technical field of medicines, and in particular relates to a preparation method of orlistat, which specifically comprises the steps of pre-processing a fermentation solution, extracting through a filter cake, concentrating, extracting through a non-polar solvent, concentrating, extracting through a polar solvent, freezing to remove impurity, concentrating, crystallizing through a non-polar solvent, hydrogenating, backwashing, crystallizing and re-crystallizing. The content of orlistat prepared by adopting the preparation method disclosed by the invention is above 99%; the maximum individual impurity is within 0.1% and is higher than medicine standard; the preparation method disclosed by the invention is short in route, good in quality, convenient to operate, low in equipment investment and low in solvent use amount; furthermore, all solvents can be recycled and reused; and the production cost and the environmental pollution of orlistat are greatly reduced.
Owner:鲁南新时代生物技术有限公司
Stable pharmaceutical compositions of orlistat
The present invention relates to stable pharmaceutical compositions of orlistat for treatment or prevention of obesity and hyperlipidemia. The pharmaceutical compositions contain Orlistat form I, which does not convert to form II at the temperatures encountered during manufacturing of an orlistat dosage form.
Owner:RANBAXY LAB LTD
Orlistat-containing pharmaceutical composition for reducing weight
ActiveCN106822097AExcellent dissolution behaviorOrganic active ingredientsMetabolism disorderOrlistatObesity
The invention relates to an orlistat-containing pharmaceutical composition for reducing weight. The pharmaceutical composition contains orlistat and oligosaccharide, wherein the weight ratio of the orlistat to the oligosaccharide is 1:(0.1-0.9). The orlistat-containing pharmaceutical composition has the advantages that the weight-reducing effect of the pharmaceutical composition on a rat with nutritional obesity is equivalent to that of the orlistat, and the animal oil discharge degree, caused by the pharmaceutical composition, of an experiment animal is evidently lower than the animal oil discharge degree, caused by the orlistat, of the experiment animal; surprisingly, in-vitro dissolution research results show that the dissolution behavior of the orlistat-containing pharmaceutical composition is better than that of orlistat capsules on the market; the orlistat-containing pharmaceutical composition can keep the weight reducing effect of current orlistat products on the market while the gastrointestinal tract untoward effects caused by the orlistat products can be reduced.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Medicine composition having weight reducing function and containing orlistat
ActiveCN106924270AReduce gastrointestinal adverse reactionsLose weightHydroxy compound active ingredientsMetabolism disorderOrlistatSide effect
The invention relates to a medicine composition having a weight reducing function and containing orlistat. The medicine composition contains orlistat, resveratrol and oligosaccharide, wherein a weight ratio of the orlistat to the resveratrol to the oligosaccharide is 1 to (0.42 to 3.75) to (0.1 to 0.6). The low dose of orlistat (one third of a conventional dose) and high dose of resveratrol are combined with any dose (high, medium, low) of oligosaccharide to use, a significant weight reducing effect can be produced for nutritional obesity mice, the weight reducing effect is better than that of the conventional dose of orlistat, and the adverse reaction morbidity of gastrointestinal tract is lower than that of the conventional dose of orlistat. The medicine composition is a health weight reducing drug with low side effect and meets the requirement of modern people on the weight reducing drugs.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Oral pharmaceutical composition for treating or preventing obesity-related hypertension and its application
InactiveCN105343056AHighlight substantiveSignificant progressOrganic active ingredientsMetabolism disorderVascular diseaseOrlistat
The invention discloses an oral pharmaceutical composition for treating or preventing obesity-related hypertension and its application and belongs to the field of medicine. The pharmaceutical composition comprises orlistat and lol-suffixed pharmaceutical active ingredients. The usage of orlistat and lol-suffixed pharmaceuticals in the pharmaceutical composition is greatly reduced, thus the risk of pharmaceuticals injuring the human body is reduced. The oral pharmaceutical composition has significant synergistic effect in treating or preventing obesity-related hypertension, can reduce the weight of a patient and the risk of hypertension being complicated by cardiovascular diseases and has higher social value.
Owner:QINGDAO YUNTIAN BIOTECH
Compound preparation containing orlistat
InactiveCN101167721AReduce food intakeObvious weight loss effectHydroxy compound active ingredientsMetabolism disorderVitamin K2Amylase
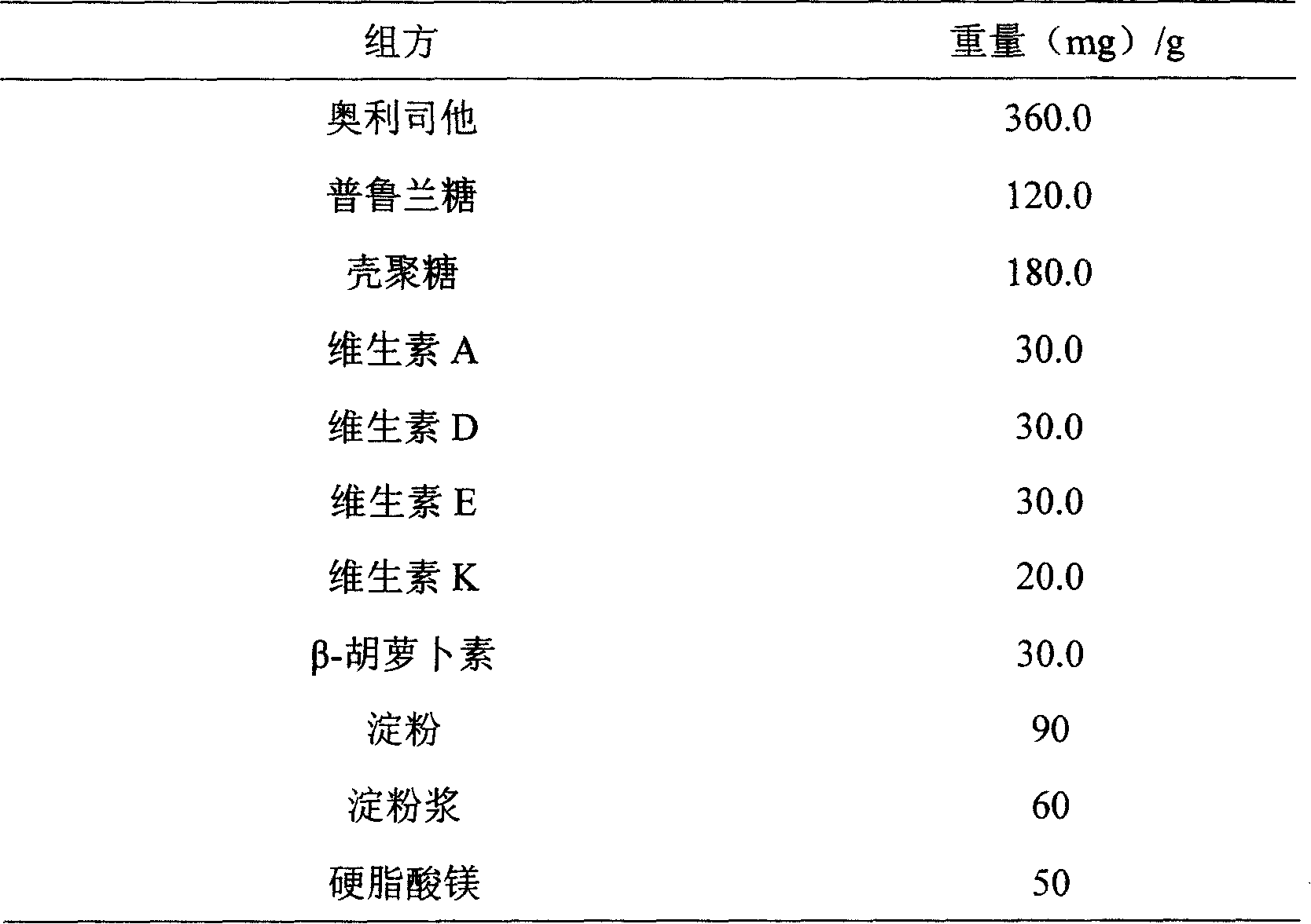
The invention belongs to the field of medical technique and relates to a compound fat-reducing containing orlistat. The invention basically contains orlistat and synergistic agent, wherein the synergistic agent is one or several ingredients chosen from pulullan saccharide, chitin, vitamin A, vitamin D, vitamin E, vitamin K and / or beta-carotene. Any solid preparation for oral administration can be made by charging other active component or finding component on the basic components. The invention is characterized in that quick fat reducing can be achieved by inhibiting the absorption of exogenous fat, meanwhile the adsorption action of pulullan saccharide, chitin and other amylase is brought in to effect, the ipidized loose bowel movement, unctuous stool, anal fistula and other side effects of the patient of adiposis are reduced, and fatsoluble vitamin reduced by absorption is complemented.
Owner:凌沛学
Pharmaceutical composition for treatment or prevention of obesity-related hypertension and usage of pharmaceutical composition for treatment or prevention of obesity-related hypertension
InactiveCN105233288AHighlight substantiveSignificant progressDipeptide ingredientsMetabolism disorderCvd riskBULK ACTIVE INGREDIENT
The invention belongs to the field of medicines and discloses a pharmaceutical composition for treatment or prevention of obesity-related hypertension. The pharmaceutical composition comprises active ingredients of orlistat and angiotensin converting enzyme inhibitors, and risks of physical damages are reduced since consumption of the orlistat and angiotensin converting enzyme inhibitors in the pharmaceutical composition is greatly reduced. The pharmaceutical composition has a remarkable synergistic effect in treatment or prevention of obesity-related hypertension, is capable of reducing weights of obese patients and risks of hypertension and cardiavascular diseases and has a promising medical application prospect.
Owner:QINGDAO YUNTIAN BIOTECH
Orlistat purification process
ActiveCN106543109ALower purchase priceReduce lossOrganic chemistryChromatographic separationOrlistat
The invention provides an orlistat purification process. The purification process is characterized by comprising the steps of filtering, centrifuging, carrying out liquid-phase preparation chromatographic separation, concentrating and drying. The liquid-phase chromatographic separation adopts spherical silica gel filling as a separation medium, and an orlistat crude product is subjected to isocratic elution by utilizing an alcohol type water liquid under the balance pressure of 2Mpa-5Mpa, wherein the alcohol type water liquid is a mixed liquid containing fatty alcohol with 1-3 carbon atoms and water in the volume ratio ranging from (80 to 20)-(92 to 8); and the grain diameter of spherical silica gel is 15 microns-30 microns and the pore diameter is 80A-200A.
Owner:ARGUS PHARMA +1
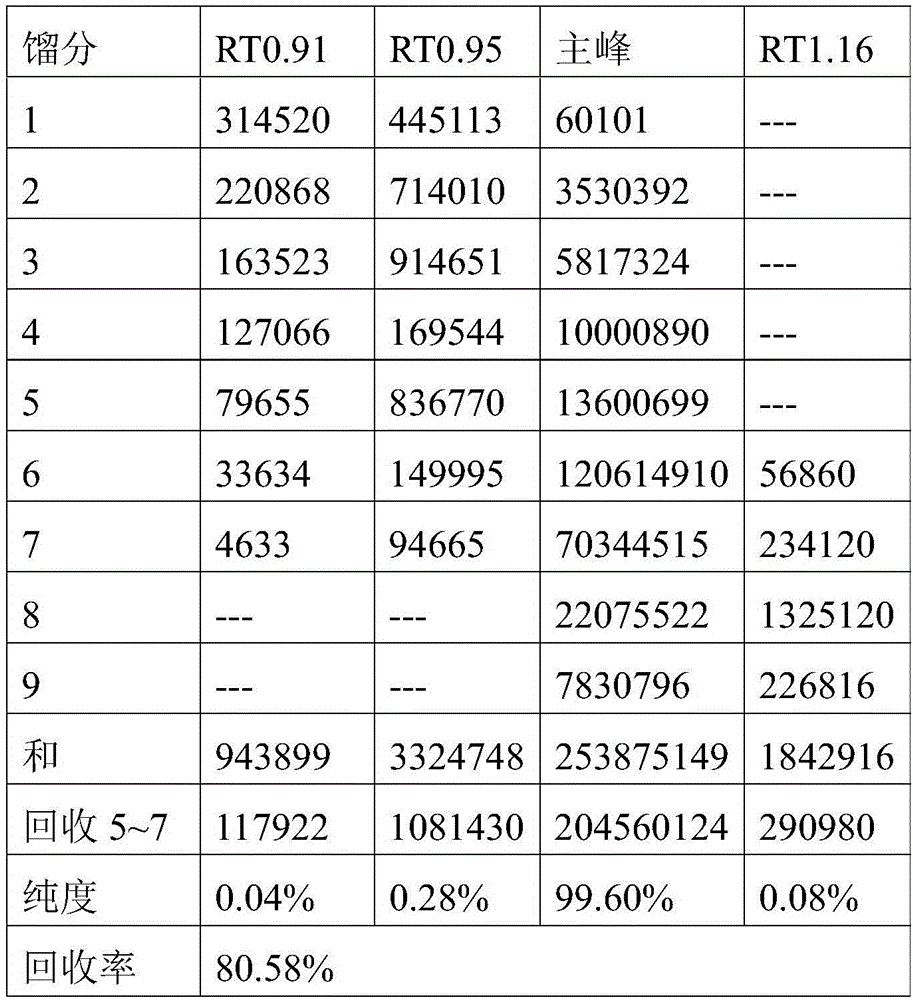
The preparation method of Nordrestat
ActiveCN104892509BEase of industrial productionRaw materials are easy to getOrganic chemistryOrlistatDehydrogenation
The invention discloses a preparation method of Roxadustat. The preparation steps are as follows: the Roxadustat is prepared from tyrosine through esterification, etherification, cyclization, dehydrogenation, oxidative rearrangement and acylation reaction. The preparation method has the advantages that the raw materials are easily obtained; the process is simple; and the preparation method is economic and environmental-friendly, and is suitable for industrialized production.
Owner:南通华宇化工科技有限公司
Compositions and methods for prevention and treatment of obesity and obesity related metabolic syndrome
ActiveUS20120121753A1Superior risk-benefit profileLimit caloric intakeMilk preparationBacteriaOrlistatBody weight
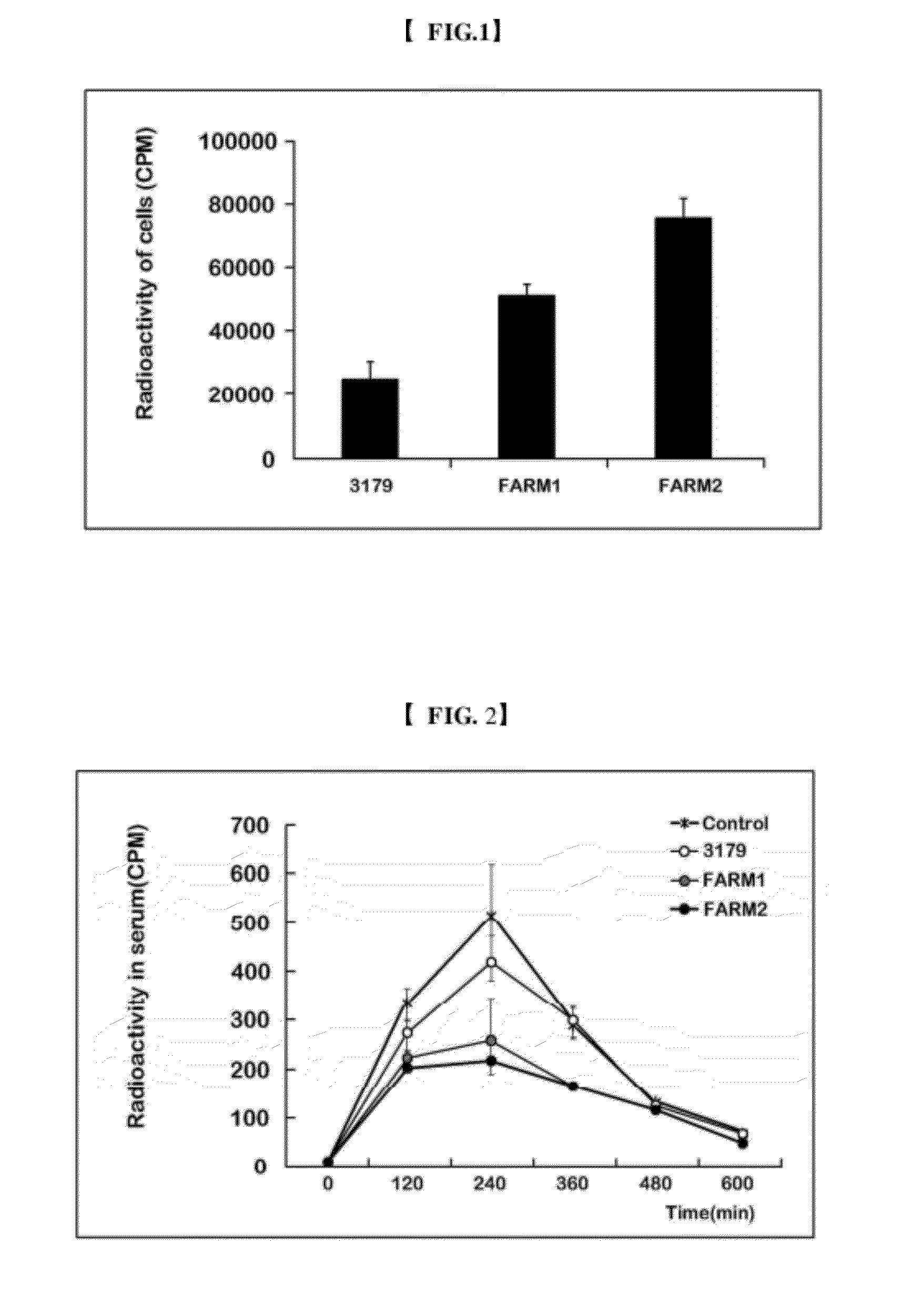
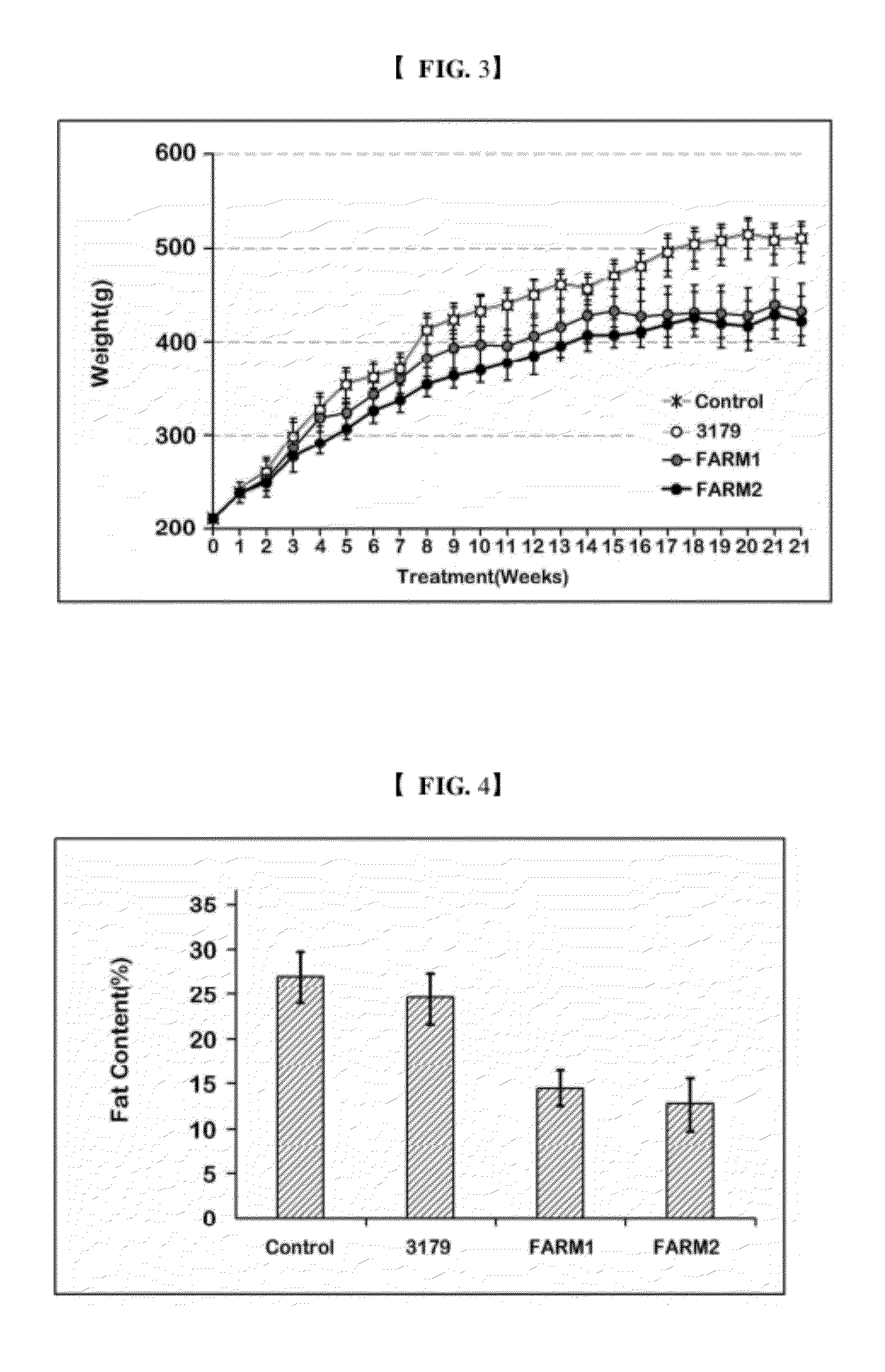
The present invention relates to prevention and treatment of obesity and obesity related metabolic syndrome, particularly to prevention and treatment of obesity by change of intestinal flora. In the present invention, it was ascertained that the characteristics of intestinal bacteria are transformed by administration of a microorganism preparation which improves free fatty acid absorption by the bacteria, and free fatty acid absorption in the gastrointestinal tract is thereby decreased by introduction thereof. The present invention provides a method for preventing and treating obesity and obesity related metabolic syndrome, a pharmaceutical composition and diet supplement for prevention and treatment thereof, and a modified probiotic strains usable for such purposes on the basis of these experimental results. The present invention shows a weight loss effect equal to that of orlistat which is most widely used as an anti-obesity therapeutic agent. The present invention shows that the absorption of fatty acids in the gastrointestinal tract is blocked by improving the characteristics of intestinal bacteria and transplanting them, thereby enabling the treatment of obesity.
Owner:MICROBIOTICA GMBH
Application of nanoparticle containing orlistat in preparing anti-hepatitis B virus medicine
ActiveCN108524472AHigh plasma drug concentrationGood sustained release effectOrganic active ingredientsDigestive system2-methacryloyloxyethyl phosphorylcholineMicrosphere
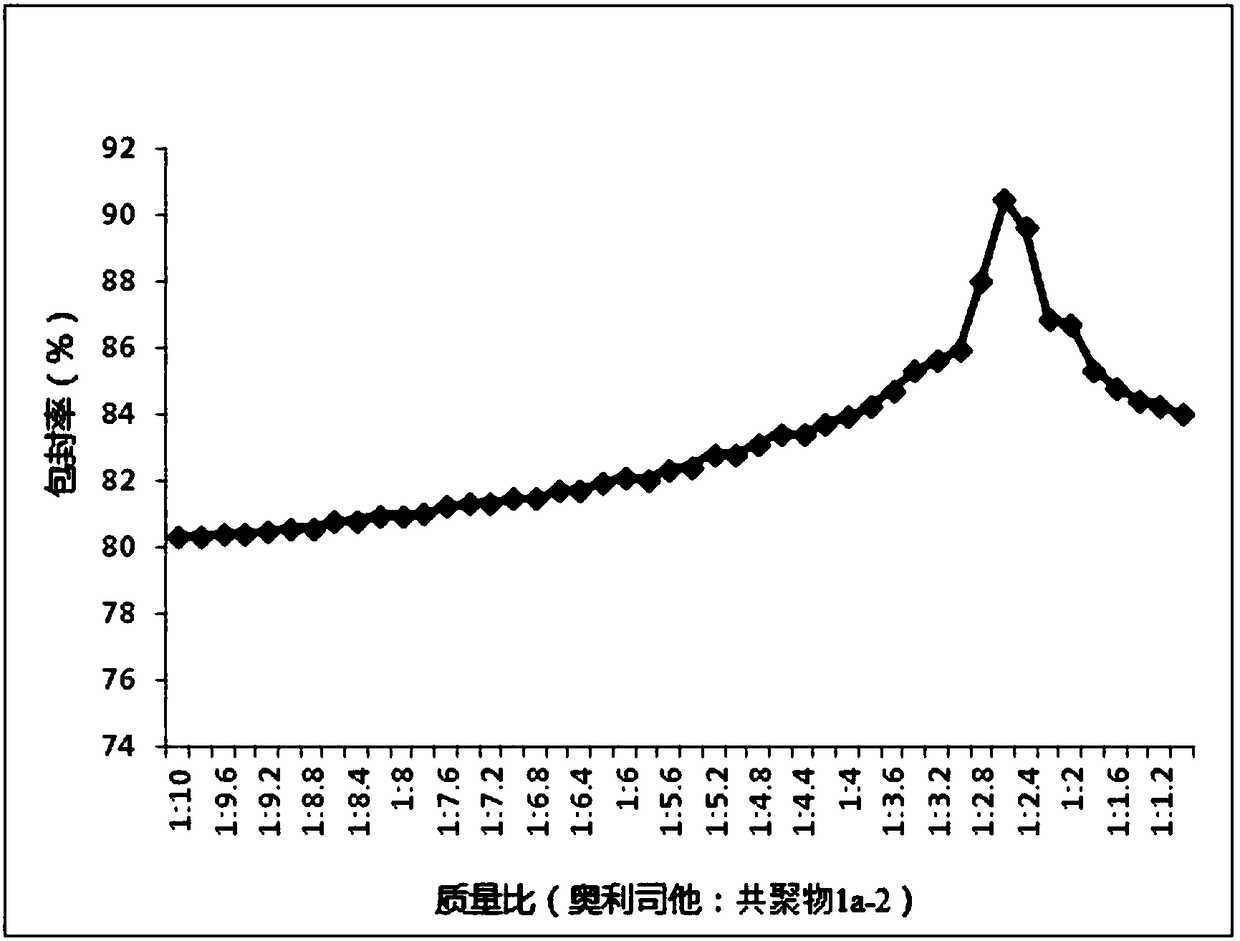
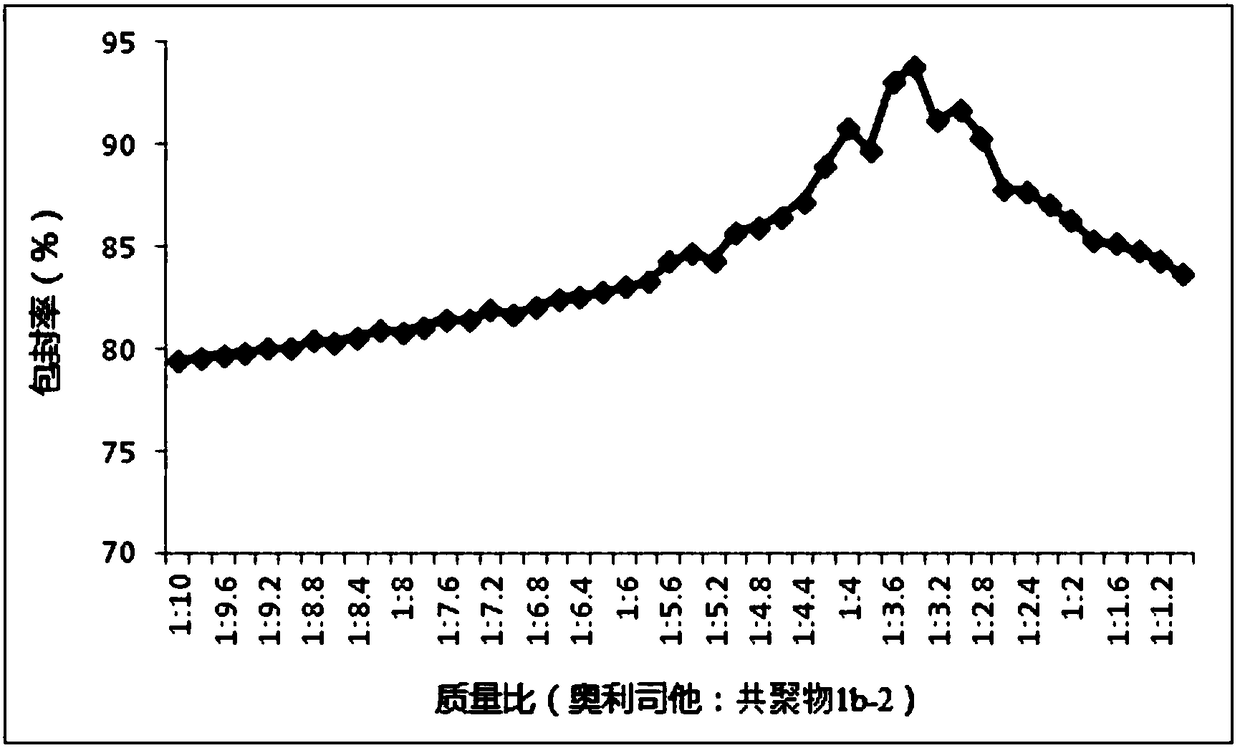
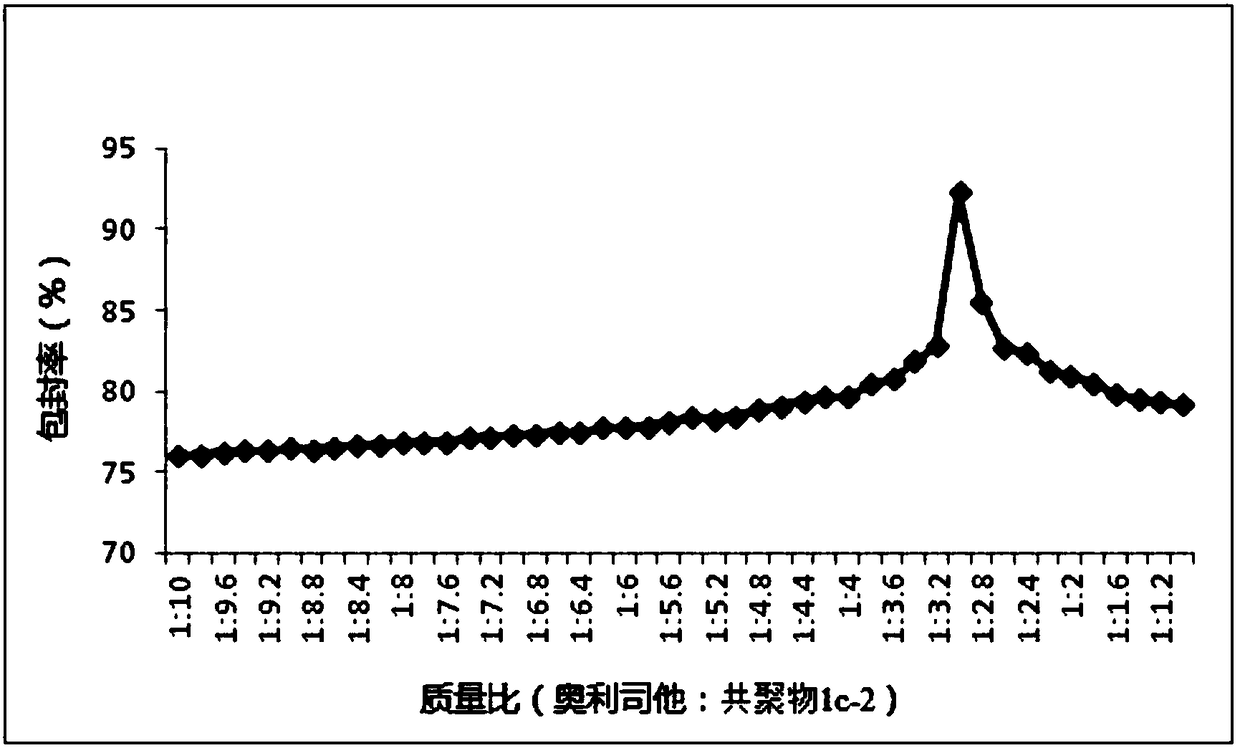
The invention relates to the technical field of medicines, in particular to application of a nanoparticle containing orlistat in preparing an anti-hepatitis B virus (HBV) medicine. The nanoparticle isprepared from the orlistat and a copolymer chosen from a vitamin A methacrylate-2-methacryloyloxyethyl phosphorylcholine copolymer or a vitamin E methacrylate-2-methacryloyloxyethyl phosphorylcholinecopolymer or a vitamin D2 methacrylate-2-methacryloyloxyethyl phosphorylcholine copolymer. The encapsulation efficiency of the nanoparticle is 82.67-93.83%; animal test shows that after oral administration of the nanoparticle, the blood drug concentration is higher, and the sustained-release effect is good; an in-vitro test result shows that the inhibition ratio of extracellular HBV DNA replication is used as an index, and the activity of the nanoparticle for resisting HBV infection is significantly higher than those of the orlistat and a contrast nanoparticle prepared by adopting PMB30W as acarrier.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com