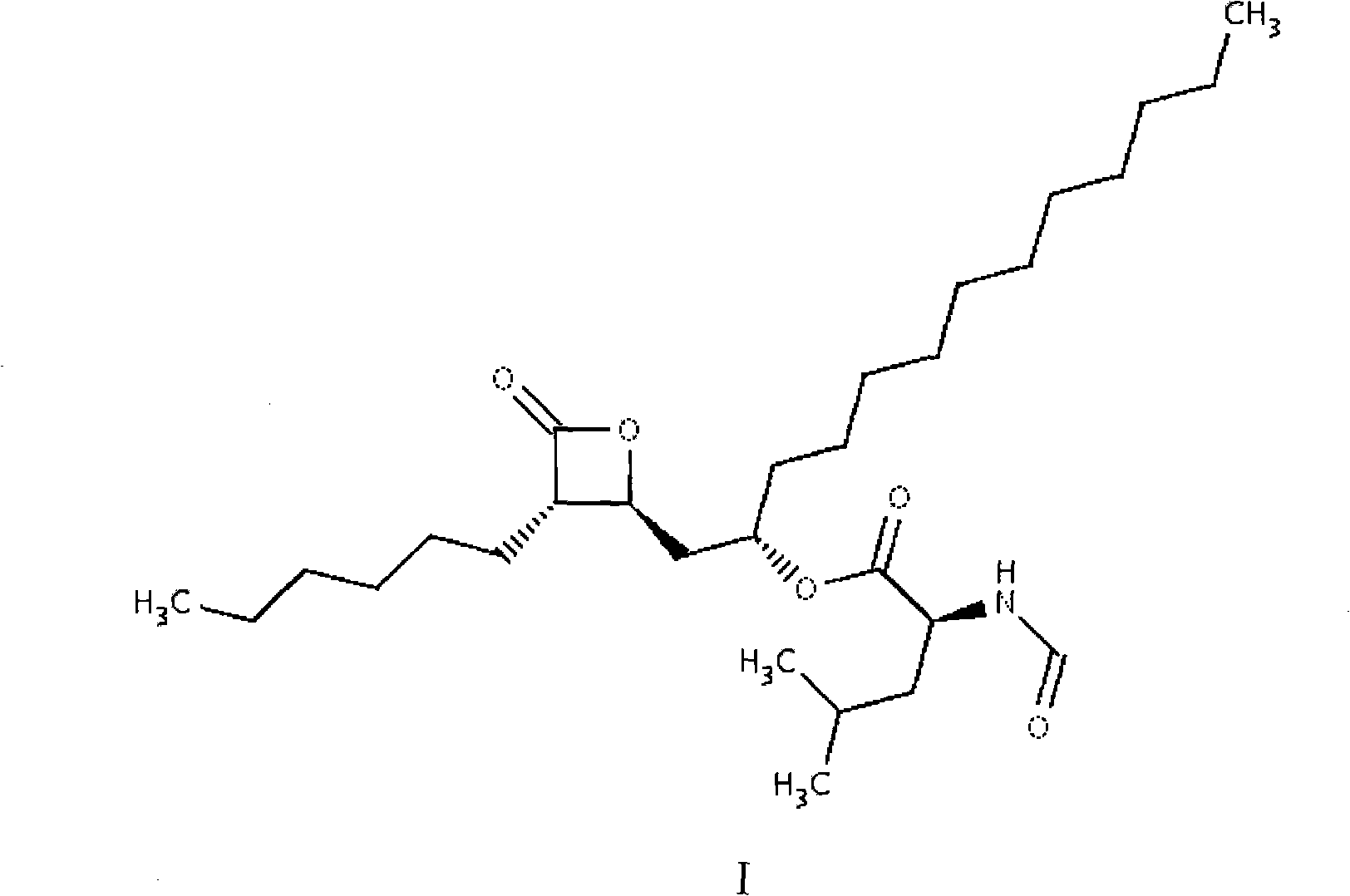
Orlistat tablets and preparation method thereof
A technology of orlistat and tablets, which is applied in the field of medicine, can solve the problems of sticky punching, hot punching, etc., and achieve the effects of simple preparation process, improved inhibition, and avoiding unfavorable factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
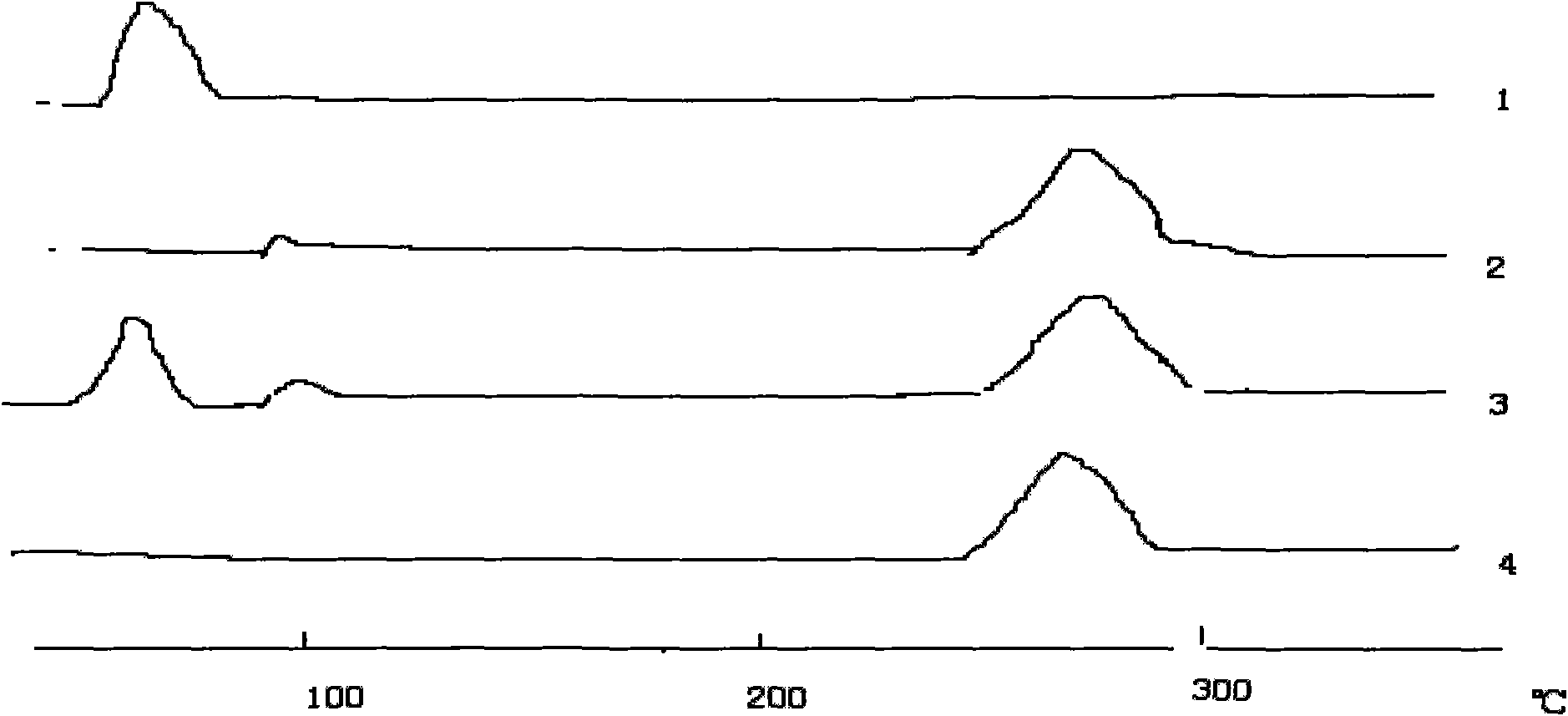
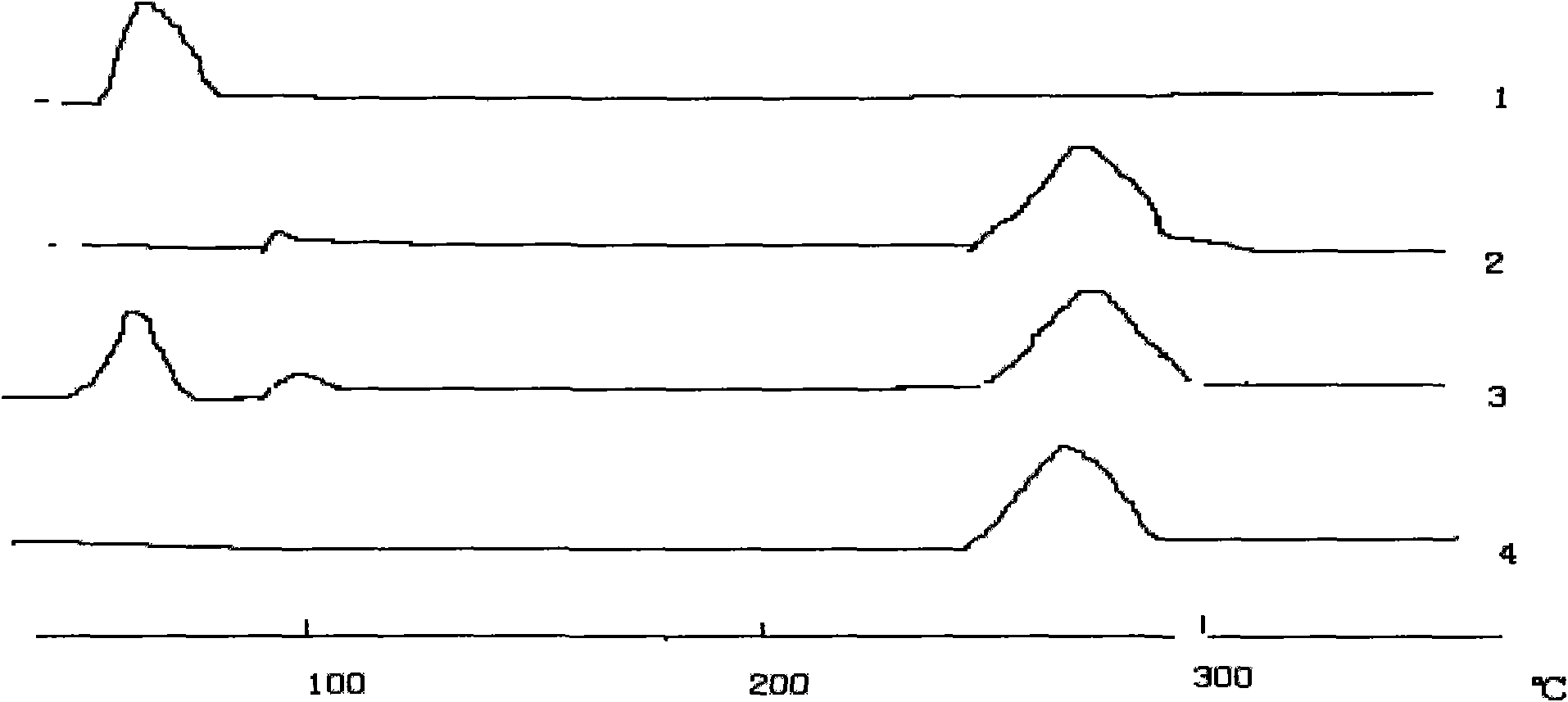
Image
Examples
Embodiment 1
[0045] Embodiment 1 orlistat tablet
[0046] 1. Orlistat tablet prescription
[0047] 1.1 Inclusion compound formula
[0048] Orlistat 12.4g (0.025mol)
[0049] α-cyclodextrin 24.325g (0.025mol)
[0050] 50% ethanol solution 1500ml
[0051] 1.2 Tablet formulation
[0052] 10 parts of clathrate
[0053] Microcrystalline cellulose 87 parts
[0054] Sodium carboxymethyl starch 2 parts
[0055] 1 part magnesium stearate
[0056] 2. Preparation process
[0057] (1) Dissolve the prescribed amount of α-cyclodextrin in the prescribed amount of 50% ethanol, add the prescribed amount of orlistat, and then heat the mixture. When the orlistat becomes oily, continue heating to 40°C , and then use an ultrasonic homogenizer with a frequency of 20kHz, 60W, and heat preservation and ultrasonication for 46min to prepare a uniformly mixed solution.
[0058] (2) Turn on the freeze dryer to pre-freeze the product, reduce the temperature of the product to about -40°C at 2°C / min, and keep ...
Embodiment 2
[0060] Embodiment 2 orlistat tablet
[0061] 1. Orlistat tablet prescription
[0062] 1.1 Inclusion compound formula
[0063] Orlistat 12.4g (0.025mol)
[0064] γ-cyclodextrin 32.425g (0.025mol)
[0065] water 400ml
[0066] 1.2 Tablet formulation
[0067] 20 parts of clathrate
[0068] Microcrystalline cellulose 55 parts
[0069] Hydroxypropyl cellulose 20 parts
[0070] 3 parts of 2% povidone ethanol solution
[0071] 2 parts magnesium stearate
[0072] 2. Preparation process
[0073] (1) Dissolve the prescribed amount of γ-cyclodextrin in the prescribed amount of water, add the prescribed amount of orlistat, and then heat the mixture. When the orlistat becomes oily, continue heating to 60°C, and then use Stir with an electric mixer for 30 minutes;
[0074] (2) Turn on the freeze dryer to pre-freeze the product, reduce the temperature of the product to about -40°C at 2°C / min, and keep it warm for about 4 hours. After the product is completely frozen, turn on the c...
Embodiment 3
[0076] Embodiment 3 orlistat tablet
[0077] 1. Orlistat tablet prescription
[0078] 1.1 Inclusion compound formula
[0079] Orlistat 12.4g (0.025mol)
[0080] β-cyclodextrin 28.375g (0.025mol)
[0081] water 500ml
[0082] 1.2 Tablet formulation
[0083] 10 parts of clathrate
[0084] Lactose 75 parts
[0085] 10 parts of hydroxypropyl cellulose
[0086] Anhydrous ethanol amount
[0087] Magnesium stearate 5 parts
[0088] 2. Preparation process
[0089] (1) Dissolve the prescribed amount of β-cyclodextrin in the prescribed amount of water, add the prescribed amount of orlistat, and then heat the mixture. When the orlistat becomes oily, continue heating to 50°C, and then use Stir with an electric mixer for 30 min.
[0090] (2) Dry the mixed solution obtained in step (1) by spray drying, wherein the spray drying parameters are: inlet temperature 55°C, outlet temperature 50°C, spray speed 6ml / min, spray pressure 2.5bar. Finally, the dried orlistat cyclodextrin incl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com