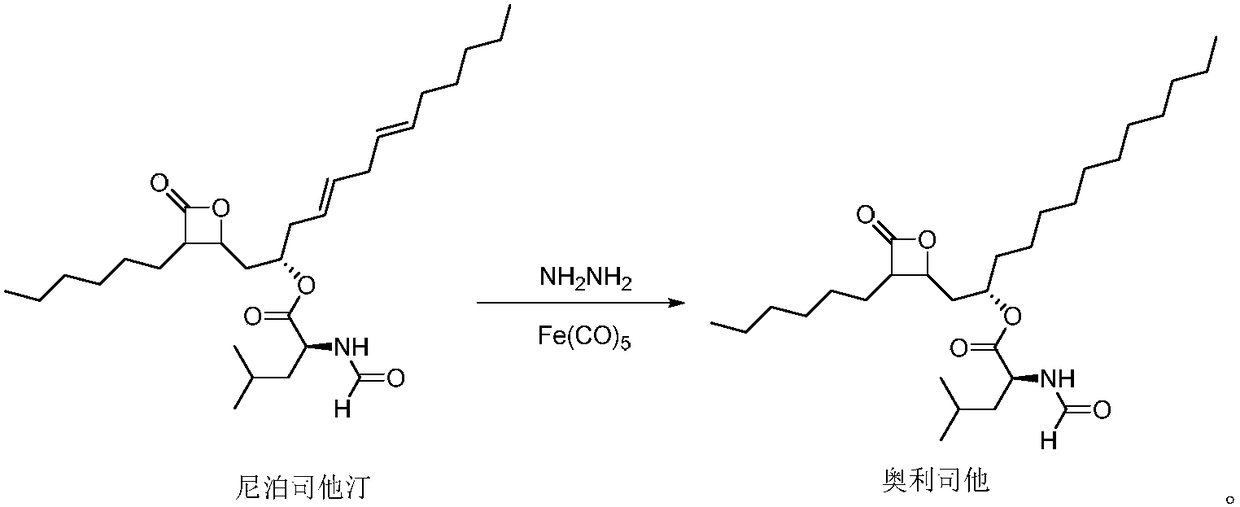
Patents
Literature
34 results about "Lipstatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
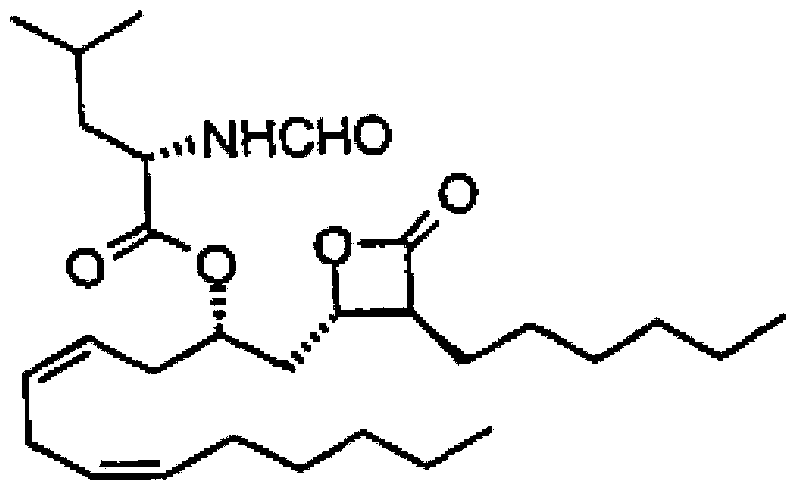
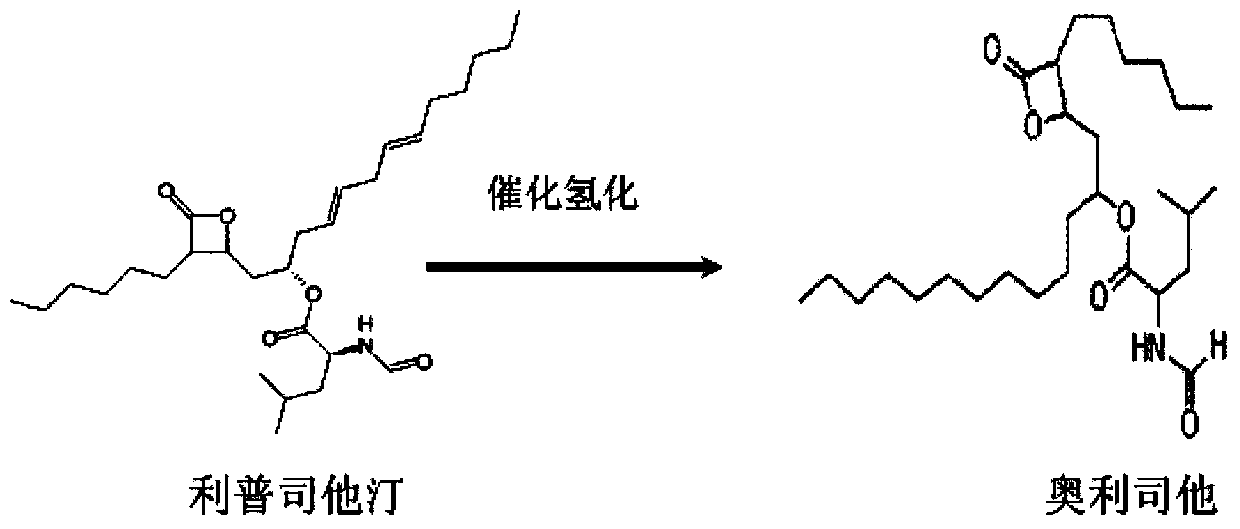
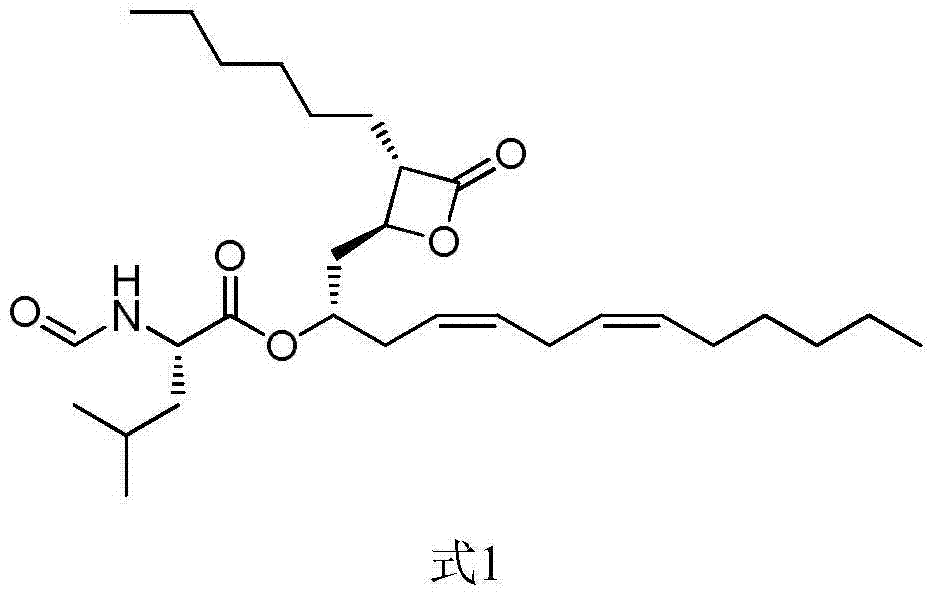
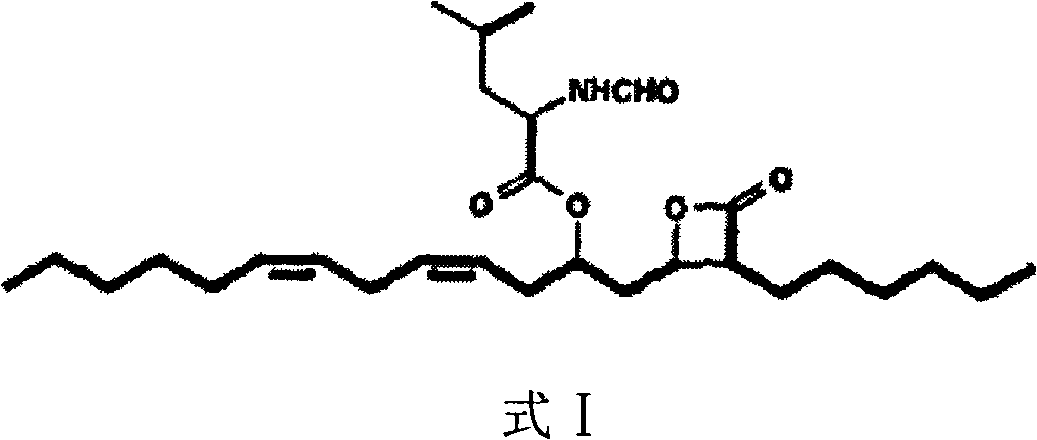
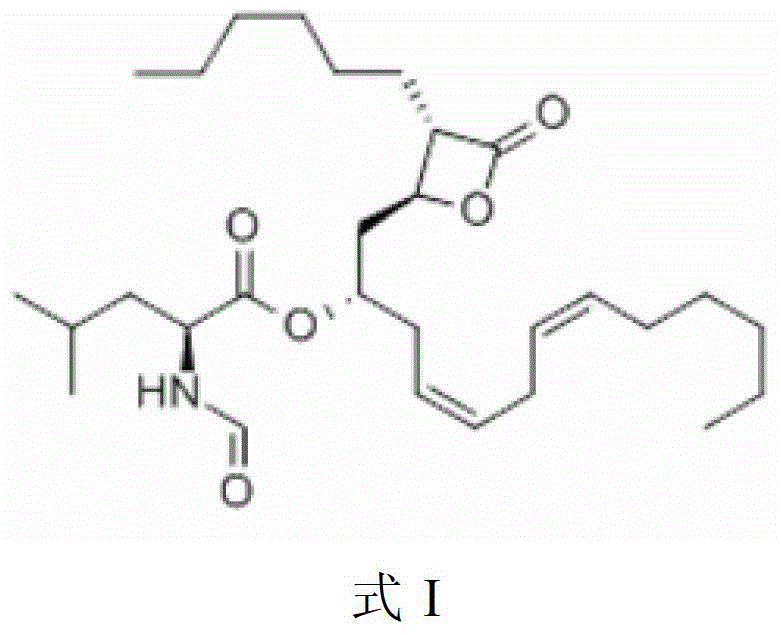
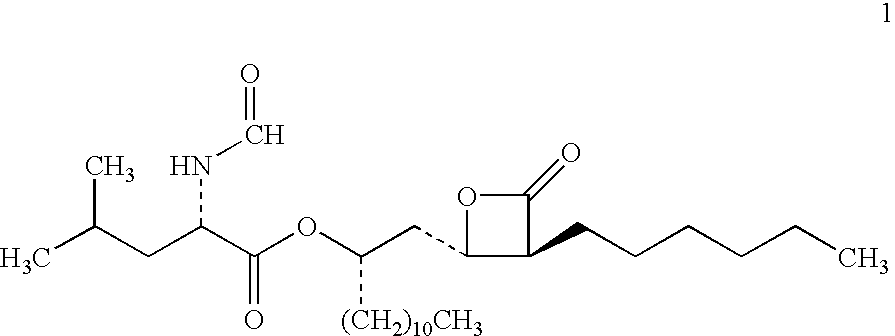
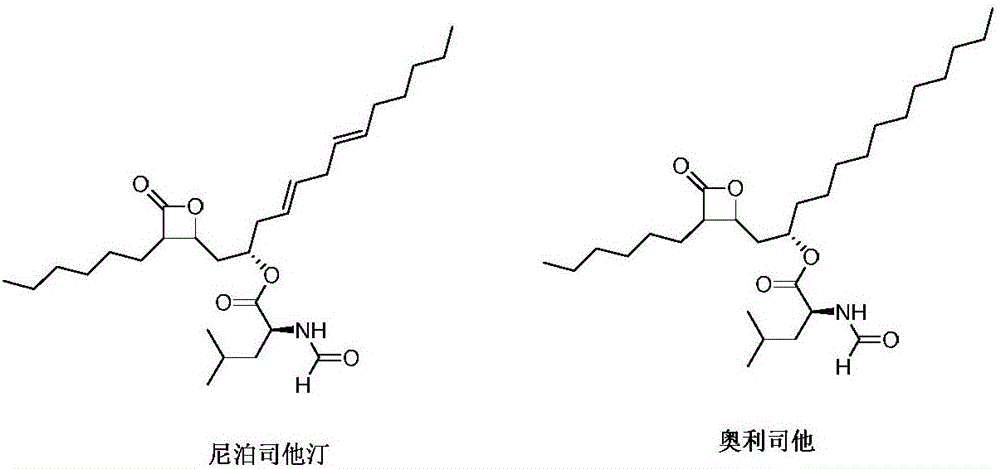
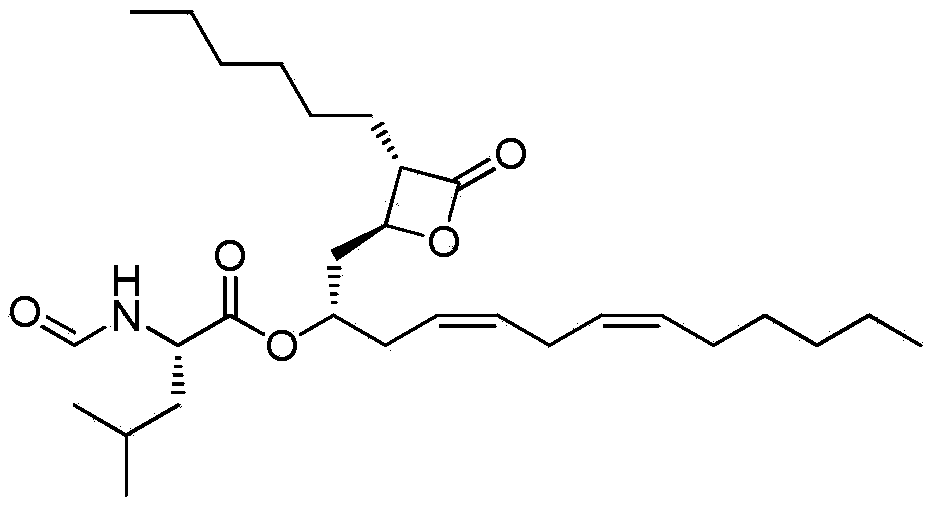
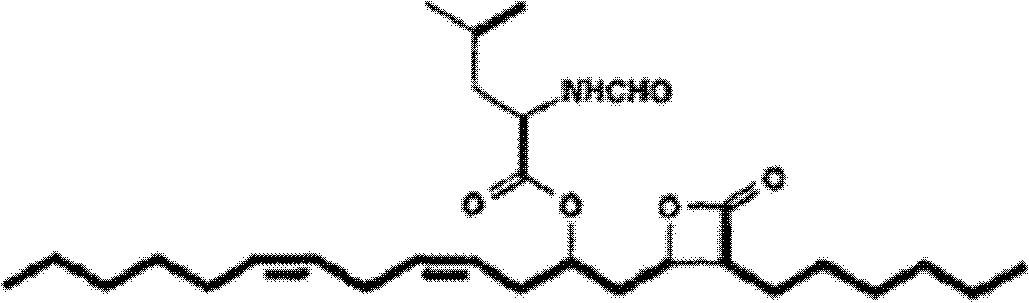
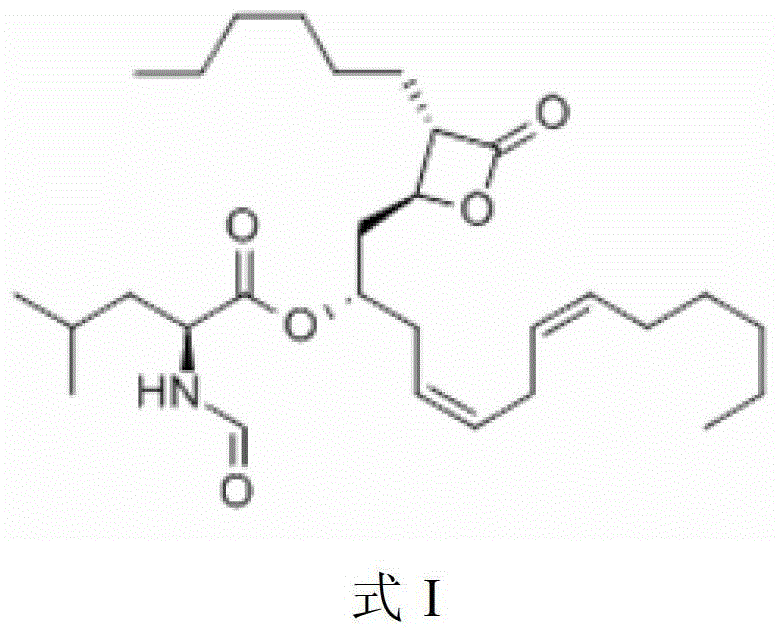
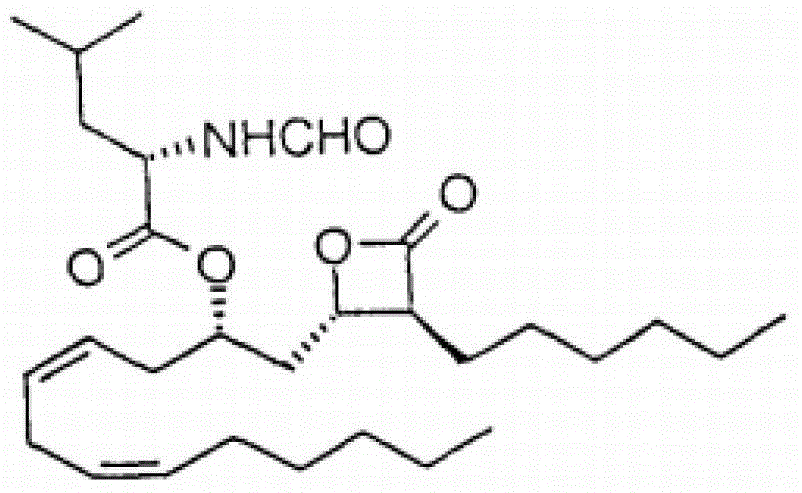
Lipstatin is a potent, irreversible inhibitor of pancreatic lipase. It is a natural product that was first isolated from the Actinobacterium Streptomyces toxytricini. The popular antiobesity drug orlistat (trade names Xenical and alli) is a saturated derivative of lipstatin.
New process for separating and extracting lipstatin from stretomyces toxytricini fermentation liquor
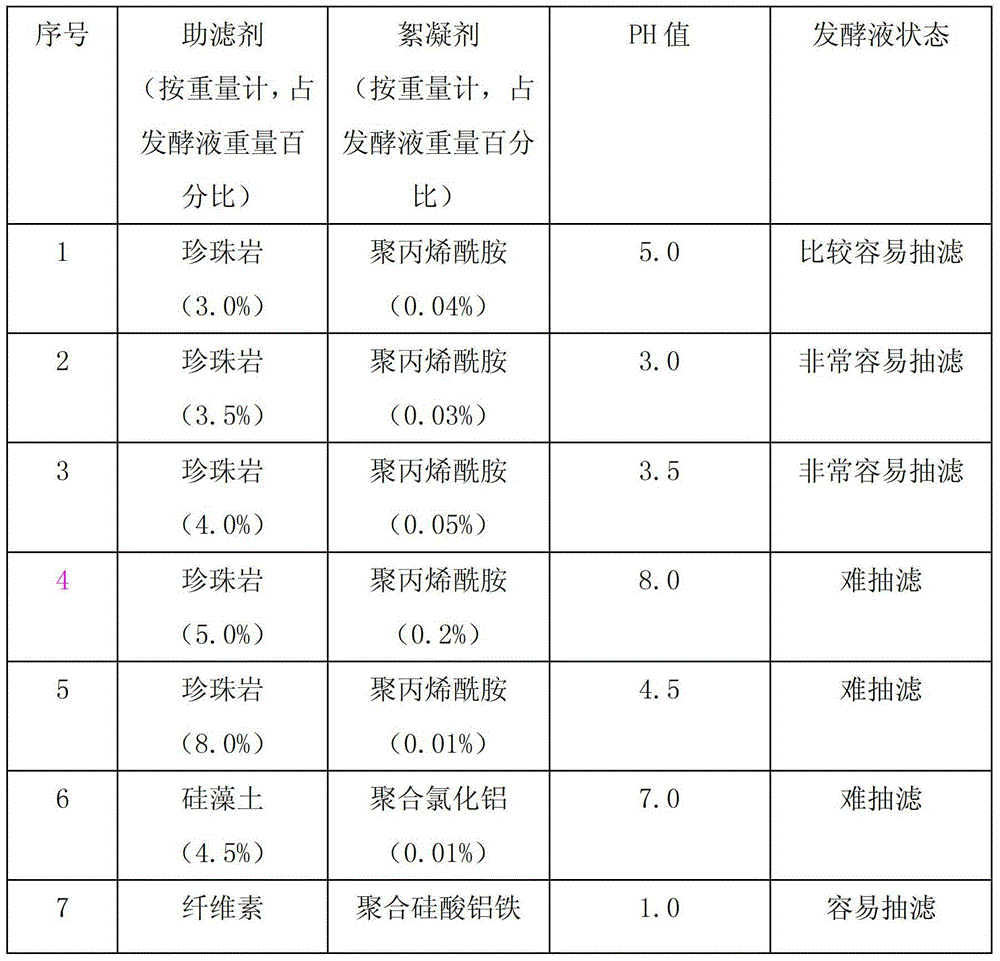
The invention discloses a new process for separating and extracting lipstatin from stretomyces toxytricini fermentation liquor. The process mainly comprises the following steps of: pretreating fermentation liquor, pre-concentrating the fermentation liquor, drying, extracting fungi residue with a solvent and performing solid-liquid separation on the fungi residue, concentrating the extract, and the like. The overall yield (based on the total lipstatin content of the fermentation liquor) of the lipstatin is up to more than 80 percent.
Owner:ARGUS PHARMA
Method for purifying orlistat intermediate
The invention belongs to the technical field of medicine and particularly relates to a method for purifying the important intermediate lipstatin of orlistat serving as a weight-reducing aid. The method provided by the invention comprises fermentation liquor pretreatment, extracting, phase inversion and concentration. The product obtained by using the method is high in purity, and the process provided has the advantages of high yield, short period, simplicity and easiness in control, less equipment menstruum investment and low production cost, and is very suitable for industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Purification method for lipstatin
ActiveCN102993134AOmit the preprocessing stepHigh purityOrganic chemistryPurification methodsOrganic solvent
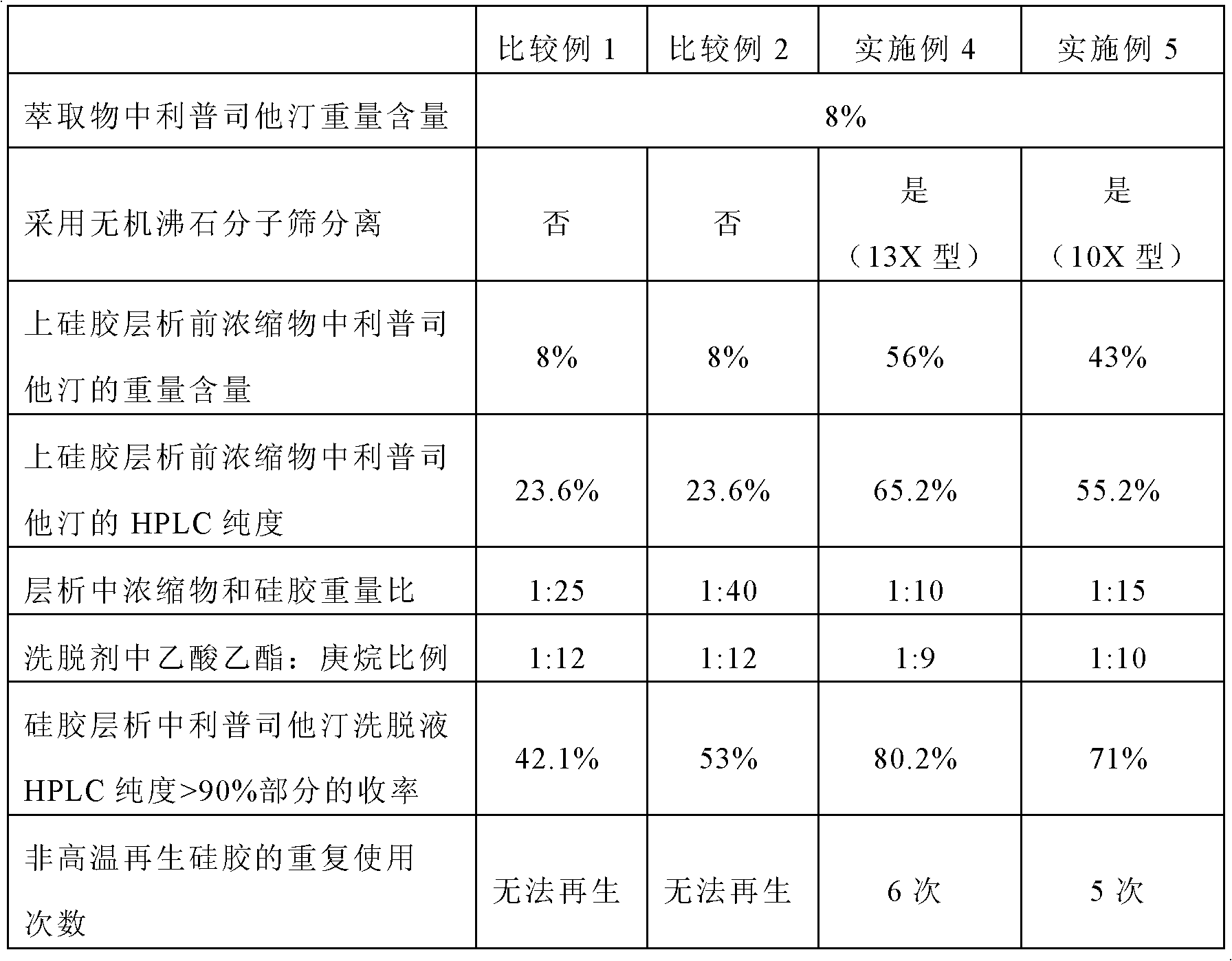
The invention belongs to the technical field of medicines and particularly relates to a purification method for lipstatin. The technical scheme is that the purification method mainly comprises the steps of fermentation liquid direct extraction concentration, organic solvent dissolving filtering, silica-gel column chromatography and crystallization and the like, lipstatin with the weight content of 75%-85% and the high performance liquid chromatography (HPLC) detection purity of no smaller than 97% is obtained, and the total yield is 72%. The purification method is simple in steps, short in period, high in product yield and good in quality, all solvents can be recycled, the production cost is greatly reduced, and pollution to the environment is reduced.
Owner:鲁南新时代生物技术有限公司
Method for fermentation production of lipstatin
ActiveCN103937847ANutrient balanceReduce fermentation costsMicroorganism based processesFermentationStearic acidOleic Acid Triglyceride
The present invention relates to a method for fermentation production of lipstatin, particularly to application of a mixed fatty acid to replace linoleic acid supplemented in a fed-batch manner during a fermentation culture process, wherein the mixed fatty acid comprises, by weight, 2.0-7.0% of stearic acid, 14.0-30% of oleic acid, 61-78% of linoleic acid, and 5.0-7.0% of palmitic acid. According to the present invention, on the basis of the existing invention CN102268466, the mixed fatty acid is adopted to replace the linoleic acid supplemented in the fed-batch manner during the fermentation culture process, such that the fermentation cost is reduced, and the HPLC detection results show that the fermentation broth does not contain the methionine analog lipstatin; and with the variable speed material supplementing method, nutrient balance is always maintained in the culture medium, the sufficient and non-excess nutrients are provided at the lipstatin synthesis stage, and the fermentation titer is improved by 6-16%.
Owner:鲁南新时代生物技术有限公司
Method for purifying lipstatin
InactiveCN102558104AEasy to saveHigh boiling pointOrganic chemistryBulk chemical productionLipstatinChemistry
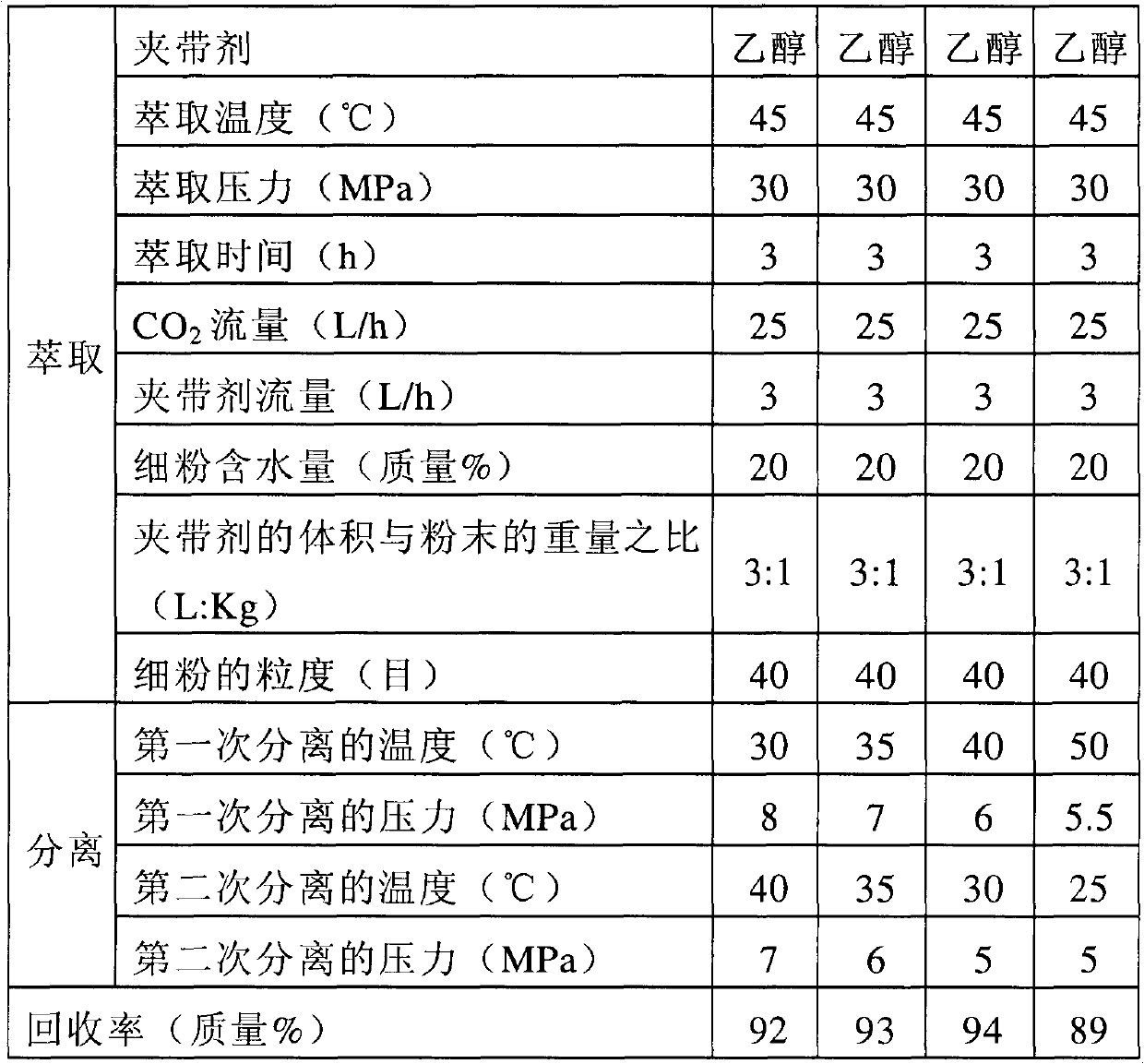
The invention provides a method for purifying lipstatin. The method comprises the following steps of: carrying out extraction on a solid mixture containing the lipstatin by using supercritical CO2 fluid, and then, purifying an obtained lipstatin extract to obtain the lipstatin. The method provided by the invention has the characteristics of high efficiency, environment-friendliness, simple process, high purity of finished products, high yield and the like.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Production process for preparing lipstatin
InactiveCN103131739AReduce manufacturing costImprove fermentation titerMicroorganism based processesFermentationPhosphateGlycerol
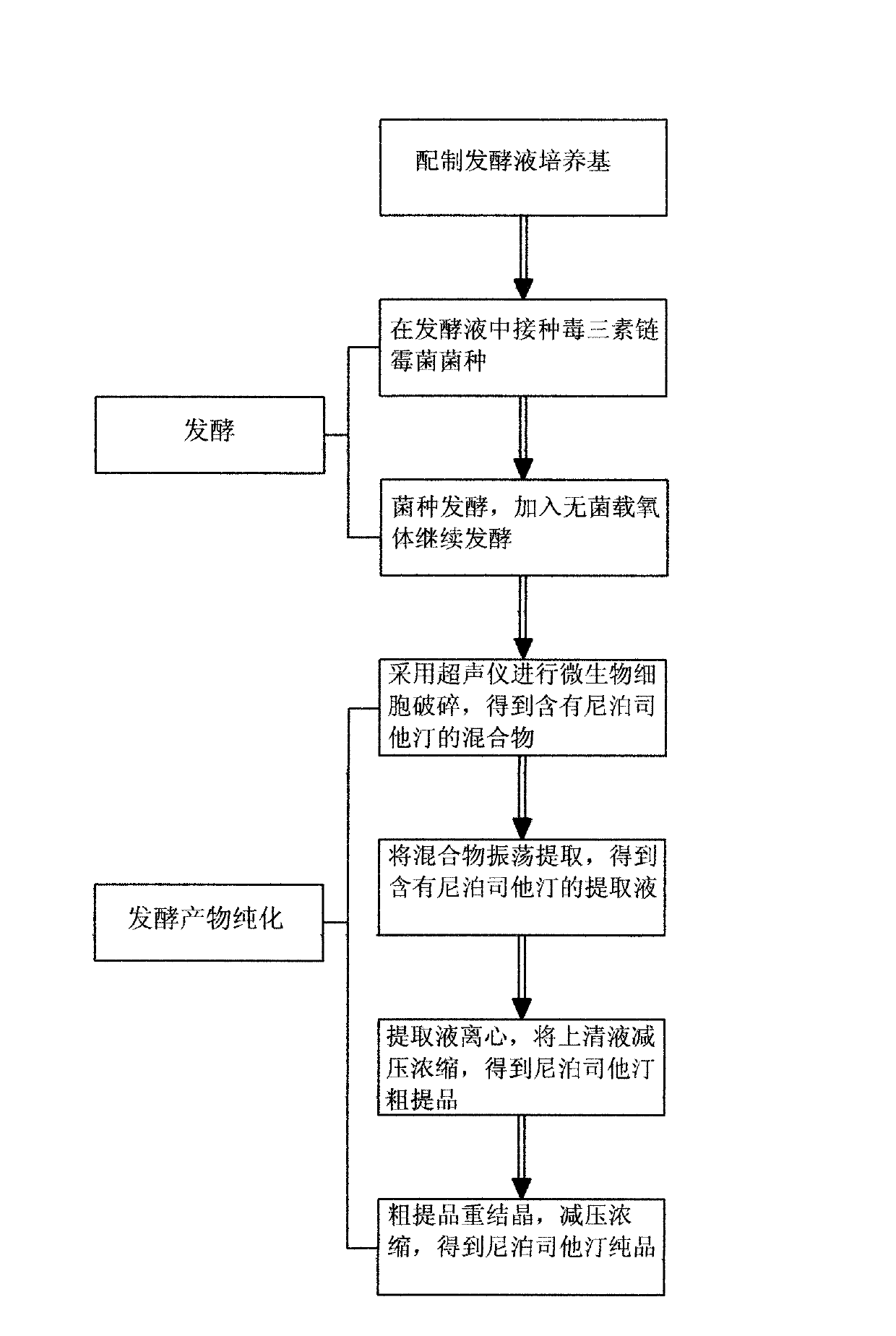
The invention discloses a production process for preparing lipstatin. The production process comprises the following process flow: preparing a culture medium of a fermentation solution, fermenting and purifying a fermentation product. The culture medium of the fermentation solution contains 3.5%-7.0% of glycerol, 4.5%-8.0% of soybean flour, 1.0%-3.5% of lecithin, 5%-12% of soybean oil, 0.05-0.2% of magnesium sulfate, 0.05-0.2% of calcium carbonate and 0.05-0.2% of potassium dihydrogen phosphate. According to the production process disclosed by the invention, the soybean flour, the soybean oil, the glycerol and the like are taken as a main nitrogen source and a carbon source of the fermentation culture medium, ultrasonic disruption is performed on cells, oscillating extraction is performed to get a lipstatin crude product, and then recrystallization is performed on the crude product to get a lipstatin pure product. The production process disclosed by the invention has the characteristics of simple process, short production cycle, high extraction rate and low production cost.
Owner:GUANGZHOU MEDCAN PHARMATECH
Method for purifying lipstatin
The invention provides a method for purifying lipstatin, which comprises the following steps: 1)providing an organic solvent extract of lipstatin; 2)concentrating the organic solvent extract of lipstatin; 3)mixing an concentrate obtained in the step 2) with C5-C20 alkane and a liquid state organic salt, filtering to obtain a filtrate; and 4)cooling the filtrate obtained in the step 3) and crystallizing to obtain the lipstatin. Compared with the prior art, the method has the following advantages and active effect that the simple processes of extraction, concentration and crystallization can realize extraction of lipstatin, the HPLC purity of lipstatin can reach as high as 86% (for instance); and absolute content can reach as high as 74% (for instance). According to the method, defects of high equipment cost, large energy consumption, complex operation and little one-time treatment capacity in prior art can be overcome, production cost is reduced, production efficiency is increased, and the method is suitable for industrial large-production.
Owner:NEW FOUNDER HLDG DEV LLC +2
Fat-binding polymers
InactiveUS7048917B1Excellent fat binding propertyLow toxicityBiocideDrug compositionsSide effectLipase inhibitors
The present invention relates to a method for treating obesity, a method for reducing the absorption of dietary fat, and a method for treating hypertriglyceridemia in a patient and to particular polymers for use in the methods or in a manufacture of a medicament. The methods comprise the step of orally administering to a mammal, such as a human, a therapeutically effective amount of one or more fat-binding polymers. The administration of the fat-binding polymer of the invention facilitates the removal of fat from the body prior to digestion, with minimal side effects and low toxicity. In a preferred embodiment, the one or more fat-binding polymers are administered in combination with one or more lipase inhibitors, for example, lipstatin and tetrahydrolipstatin.
Owner:GENZYME CORP
Fermentation process for lipstatin and method of extracting lipstatin from a fermentation broth
InactiveUS20030138919A1Regulate the fermentation broth viscosityImprove bioavailabilityOrganic chemistryFermentationBiotechnologyMicroorganism
The present invention provides a fermentation process for producing lipstatin comprising the steps of: a) preparing a fermentation medium containing a lipstatin-producing microorganism comprising an oil and an assimilable carbon source, wherein the wt / wt ratio of oil and assimilable carbon source is regulated to achieve an optimal lipstatin biosynthesis by the microorganism; and b) feeding the fermentation medium with an emulsifier, wherein the emulsifier provides an optimal viscosity for the fermentation medium and optimal pH during the fermentation to permit fermentation for lipstatin production. The disclosed process also provides a process for extracting a lipstatin from a fermentation broth.
Owner:TEVA PHARM USA INC
Fermentation process for lipstatin and method of extracting lipstatin from a fermentation broth
Owner:TEVA PHARM USA INC
Sterilizing agent capable of inhibiting Klebsiella pneumonia
InactiveCN109548786AInhibition of growth and reproductionBiocideDisinfectantsK pneumoniaePublic place
The invention discloses a sterilizing agent capable of remarkably inhibiting growth and propagation of Klebsiella pneumonia. The main component of the sterilizing agent is lipstatin. The sterilizing agent comprises the following components in percentage by mass: 5-13% of lipstatin, 0.5-2% of an ethylenediaminetetraacetic acid (EDTA) disodium salt and 0.5-2% of NaOH. The invention provides the sterilizing agent for inhibiting the growth and propagation of the Klebsiella pneumoniae, can be used for indoor disinfection, particularly can be used for public places such as hospitals and the like where the Klebsiella pneumoniae is spread easily, and has a good effect on inhibiting the growth and propagation of the Klebsiella pneumoniae.
Owner:PINGDU PEOPLES HOSPITAL
Application of Lipstatin for preventing untoward reaction after CT enhance examination
InactiveCN107137396AMitigation and relief of adverse reactionsSignificant adverse reactionsOrganic active ingredientsAntinoxious agentsAdditive ingredientMedicine
The invention discloses an application of liplastatin in preventing adverse reactions after CT-enhanced examination, and at the same time discloses a medicine for alleviating adverse reactions after CT-enhanced examination. The effective content of statins is 0.1‑20%. The present invention discovers and proves for the first time that liprestatin can treat adverse reactions caused by CT, especially hypersensitivity patients who are highly sensitive to contrast agents, and can reduce and relieve their adverse reactions during enhanced scanning. It has been clinically verified that liprestatin has a very significant effect on adverse reactions after CT examination.
Owner:付国田
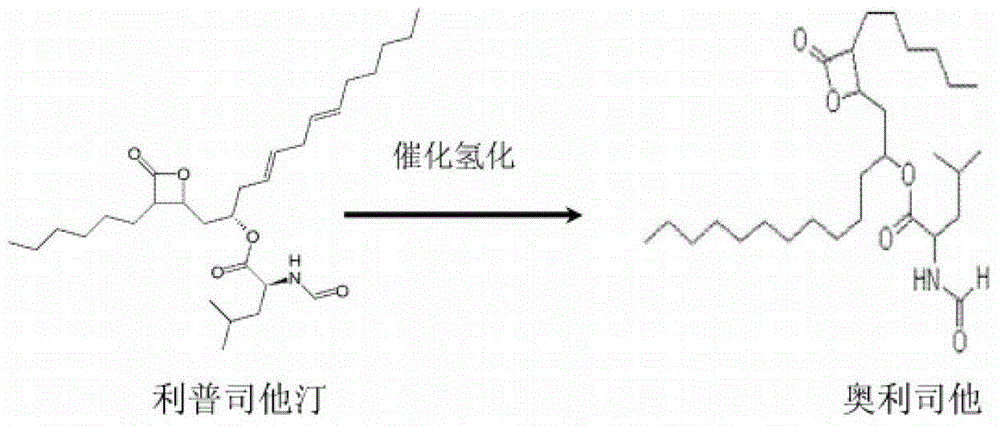
Weight-reducing medicine orlistat synthesis method
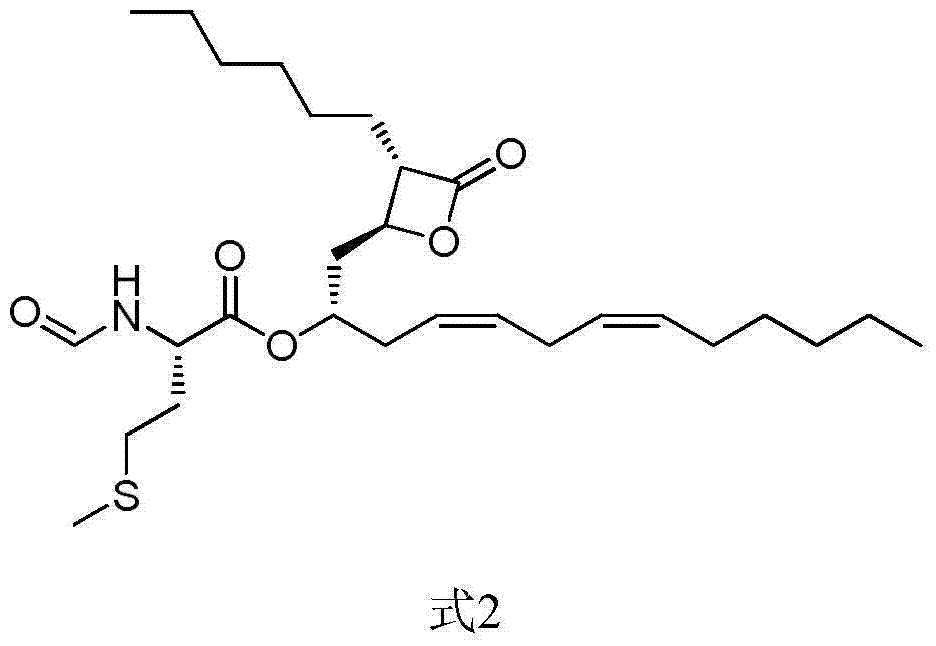
The invention discloses a weight-reducing medicine orlistat synthesis method. The synthesis method includes that in existence of (R,R)-(-)-N,N'-bis(3,5-ditert-butyl-salicylidene)-1,2-cyclohexyl diamine cobalt, lipstatin and sodium borohydride are subjected to reduction reaction under catalysis of cuprous chloride to obtain orlistat. The orlistat synthesis method is quick in reaction, and reaction yield is increased effectively. By adoption of (R,R)-(-)-N,N'-bis(3,5-ditert-butyl-salicylidene)-1,2-cyclohexyl diamine cobalt as a stabilizer of a reducing agent, low side reaction quantity, easiness in product purification and suitableness for industrial expanded production are realized.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Method for purifying lipstatin
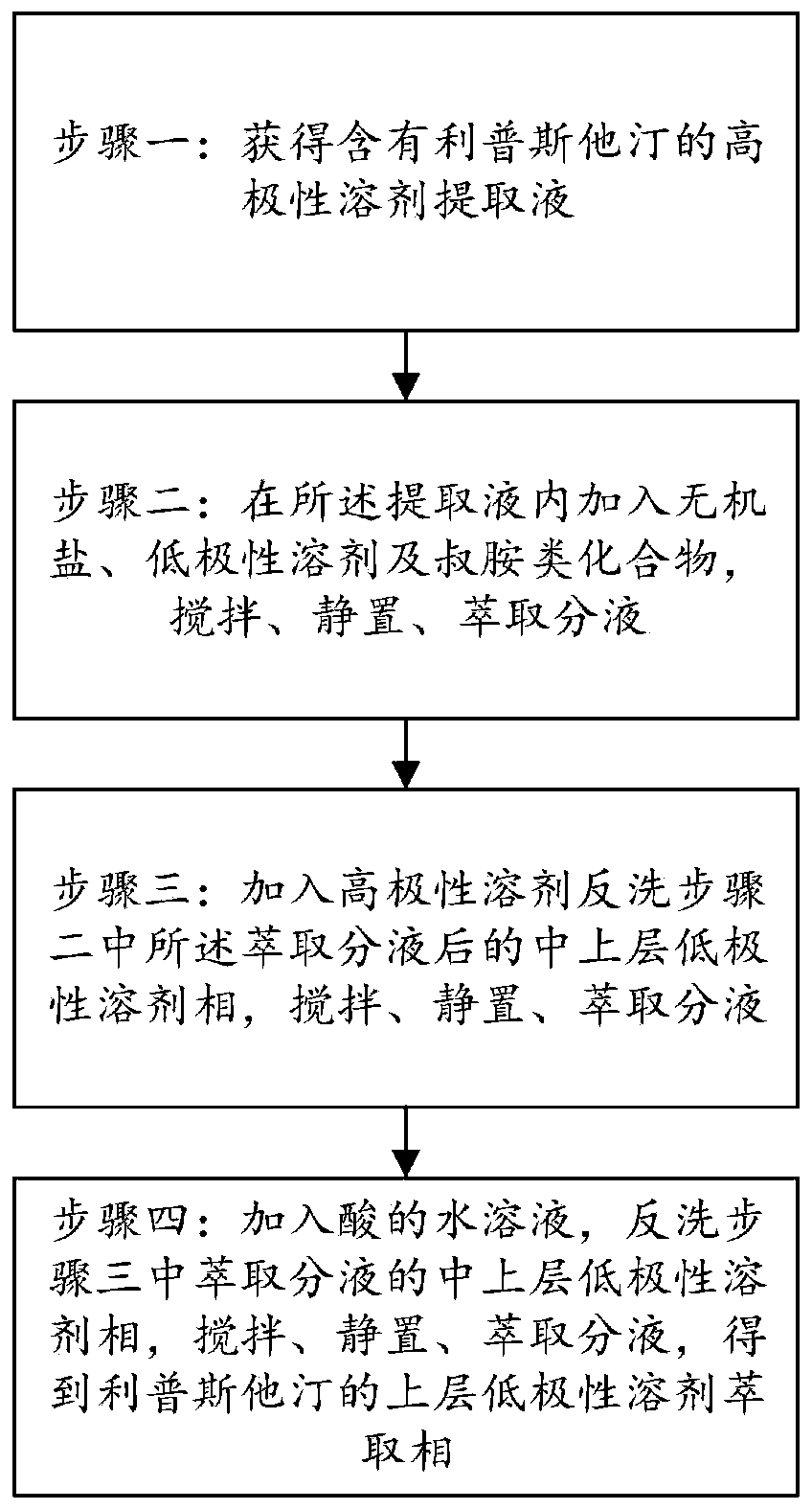
ActiveCN110066260AEasy to separate and removeFacilitate automated continuous productionOrganic chemistryInorganic saltsPurification methods
The invention provides a method for purifying lipstatin. The method comprises the steps that step one, a high-polarity solvent extraction solution containing the lipstatin is obtained; step two, inorganic salt, a low-polarity solvent and tertiary amine compounds are added into the extraction solution, and stirring, standing and extraction and solution separation are conducted; step three, a high-polarity solvent is added for backflushing of a low-polarity solvent phase of the upper middle layer after the extraction and solution separation in the step two, and stirring, standing and extractionand solution separation are conducted; step four, an acid aqueous solution is added for backflushing of the low-polarity solvent phase of the upper middle layer after the extraction and solution separation in the step three, stirring, standing and extraction and solution separation are conducted, and a low-polarity solvent extraction phase of the upper layer of the lipstatin is obtained. Accordingto the method for purifying the lipstatin, impurities can be easily separated and removed through solution separation of the high-polarity solution and the low-polarity solution, the product is not easily damaged, and the yield is high; the operation is simple, the requirements for equipment are low, and the convenience is provided for industrial automatic continuous production; the production period can be greatly shortened, and the production cost is reduced.
Owner:ARGUS PHARMA
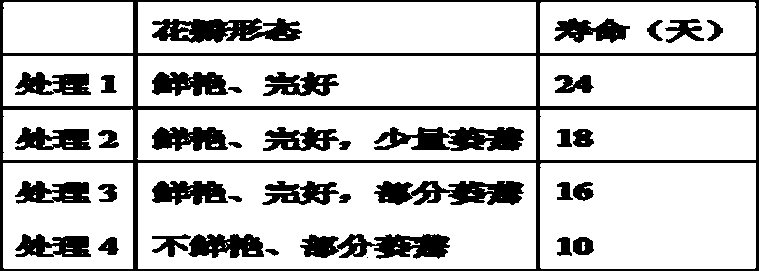
Canxnation vase solution
The invention discloses a canxnation vase solution. The canxnation vase solution is prepared from, by weight, 3 % of saccharose, 10-30 mg / L of penicillin, 30-90 mg / L of inositol, 100 mg / L of pyruvic acid and 30-90 mg / L of lipstatin. The invention provides a new use of lipstatin, and the lipstatin is conductive to prolonging the refreshing time of flowers. The canxnation vase solution has the advantages of being long in refreshing lifetime, having non-toxic and non-irritating smell, being used for the canxnation vase solution, lasting long flower color, being strong in petals and good freshnessin flowers, and keeping the lifetime of the canxnation vase up to 23-24 days.
Owner:WEIFANG UNIVERSITY
Bactericidal agent for inhibiting growth and propagation of Legionella pneumophila
InactiveCN109453163AInhibition of growth and reproductionAntibacterial agentsOrganic active ingredientsAdditive ingredientLegionella pneumophila
The invention discloses a bactericidal agent for inhibiting growth and propagation of Legionella pneumophila. Lipstatin serves as a main ingredient. The invention further discloses the bactericidal agent for inhibiting the growth and propagation of the Legionella pneumophila. The bactericidal agent comprises the following ingredients: Lipstatin, EDTA disodium salt and pure water, wherein the masspercent of the Lipstatin is 10-15%, and the mass percent of the EDTA disodium salt is 3-10%. The bactericidal agent for inhibiting the growth and propagation of the Legionella pneumophila, provided bythe invention, has a very good effect on inhibiting the growth and propagation of the Legionella pneumophila.
Owner:PINGDU PEOPLES HOSPITAL
Composition for fermentation process of lipstatin
The invention belongs to the technical field of medicine chemical industry, and relates to a composition used in the fermentation process of lipstatin and preparation method of the composition. Specifically, the composition is obtained by saponifying sunflower seed oil into salt, separating the salt, acidizing and washing; the composition contains linoleic acid, and the cost is only a half of the linoleic acid on market, the linoleic acid in the fermentation process is replaced by the composition, and the fermentation valence is improved by 12-18%.
Owner:鲁南新时代生物技术有限公司
Method for purifying lipstatin
InactiveCN103030613AIncrease weight contentReduce usageOrganic chemistryMolecular sieveOrganic solvent
The invention provides a method for purifying lipstatin. The method comprises the following steps: 1) providing a solid mixture containing the lipstatin; 2) extracting the solid mixture by utilizing a low-polarity first organic solvent or a non-polarity organic solvent, so as to obtain an extract liquor containing the lipstatin; 3) adsorbing the extract liquor by a molecular sieve; eluting the extract liquor by a first eluent so as to obtain the first extract liquor containing the lipstatin; and 4) purifying the first eluent containing the lipstatin obtained in the step 3) through chromatography. The method provided by the invention has the following beneficial effects: the solvent containing the lipstatin is selectively adsorbed by adopting the organic molecular sieve; the method is low in cost and more stable at high temperature; the use amount of silica gel is reduced, the utilization ratio of the silica gel is improved, and the separation effect is improved, so that the production running cost is greatly reduced.
Owner:NEW FOUNDER HLDG DEV LLC +2
Preparation method of orlistat
InactiveCN111499599ANovel preparation routeSimple and fast operationOrganic chemistryLipstatinMedicinal chemistry
The invention discloses a preparation method of orlistat. According to the invention, Lipstatin is used as an initial raw material, and cuprous chloride catalyzes reduction reaction of Lipstatin and sodium borohydride to obtain orlistat. The preparation method disclosed by the invention has the advantages of cheap and easily available raw materials, mild process conditions, simple post-treatment,high product yield and few byproducts, and the whole process is very suitable for industrialization.
Owner:四川奇格曼药业有限公司
Lipstatin fermentation method and fermentation medium
ActiveCN112852898AReduce consumption costEasy to controlBacteriaMicroorganism based processesBiotechnologyEngineering
In order to overcome the problems of low fermentation unit, high cost of fermentation raw materials, complex and difficult control of process, unsuitability for large-scale industrial production and the like in the existing fermentation method of the lipstatin, the invention provides a simple, convenient and efficient fermentation method of the lipstatin and a fermentation culture medium. The method comprises the following steps: plate separation and slant culture, strain slant shake flask fermentation screening, shake flask seed liquid preparation, primary seed tank culture, secondary seed tank culture and fermentation tank culture. The fermentation method and the fermentation culture medium of the lipstatin, which are obtained through combined optimization strategies such as shake flask fermentation screening of high-yield strains, optimization of culture media at all levels and improvement of a fermentation process technology, have the advantages of high fermentation unit, low fermentation raw material cost, simplicity and convenience in fermentation process control, no need of adding exogenous toxic precursors and the like; and the method has very important significance for improving the fermentation yield and the product quality of the lipstatin and reducing the production cost.
Owner:ARGUS PHARMA
Culture medium for producing lipstatin and use method of culture medium
PendingCN111979277AImprove certaintyImprove fermentation titerBacteriaMicroorganism based processesBiotechnologyScreening cultures
The invention provides a culture medium for producing lipstatin. The culture medium is a combined culture medium comprising a solid screening culture medium, a slant solid culture medium, a seed solution culture medium and a fermentation culture medium. The solid screening culture medium and the slant solid culture medium both contain caprylyl-coenzyme and leucine; and the fermentation culture medium comprises a fermentation basal culture medium and a fermentation fed-batch culture medium. The idea that people depend on experience more than theory for a long time is broken through; a reactionmechanism is carefully researched and analyzed; and the mode of combining the solid screening culture medium, the slant solid culture medium, the seed solution culture medium and the fermentation culture medium is adopted, so that the certainty of the lipstatin culture process is improved, the process is more targeted, the fermentation titer of the lipstatin is obviously improved, and the averagefermentation titer of the lipstatin can reach 12.5 g / L or above and can reach 13.3 g / L to the maximum.
Owner:HEBEI SHENGXUE DACHENG PHARMA
Application of ribosome sigma factor, mutant thereof and protein obtained by encoding in increasing yield of lipstatin
ActiveCN112342203AImprove abilitiesIncreased ability to produce riprestatinBacteriaTransferasesNucleotideSigma factor
The invention discloses application of a ribosome sigma factor, a mutant thereof and protein obtained by encoding in increasing the yield of lipstatin. The ribosome sigma factor is a ribosome sigma factor A or a ribosome sigma factor B, the nucleotide sequence of the ribosome sigma factor A is shown as SEQ ID NO.5, and the nucleotide sequence of the ribosome sigma factor B is as shown in SEQ ID NO. 7; and the mutants of the ribosome sigma factors A and B are further comprised, the nucleotide sequence of the mutant of the ribosome sigma factor A is shown as SEQ ID NO. 1, and the nucleotide sequence of the mutant of the ribosome sigma factor B is shown as SEQ ID NO. 3. According to the application, the overexpression of the ribosome sigma factors A and B obtained by screening and the mutantsof the ribosome sigma factors A and B can improve the yield of lipstatin.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
Purification method of liprestatin
The invention provides a method for purifying lipstatin, which comprises the following steps: 1)providing an organic solvent extract of lipstatin; 2)concentrating the organic solvent extract of lipstatin; 3)mixing an concentrate obtained in the step 2) with C5-C20 alkane and a liquid state organic salt, filtering to obtain a filtrate; and 4)cooling the filtrate obtained in the step 3) and crystallizing to obtain the lipstatin. Compared with the prior art, the method has the following advantages and active effect that the simple processes of extraction, concentration and crystallization can realize extraction of lipstatin, the HPLC purity of lipstatin can reach as high as 86% (for instance); and absolute content can reach as high as 74% (for instance). According to the method, defects of high equipment cost, large energy consumption, complex operation and little one-time treatment capacity in prior art can be overcome, production cost is reduced, production efficiency is increased, and the method is suitable for industrial large-production.
Owner:NEW FOUNDER HLDG DEV LLC +2
A kind of method of purifying orlistat intermediate
The invention belongs to the technical field of medicine and particularly relates to a method for purifying the important intermediate lipstatin of orlistat serving as a weight-reducing aid. The method provided by the invention comprises fermentation liquor pretreatment, extracting, phase inversion and concentration. The product obtained by using the method is high in purity, and the process provided has the advantages of high yield, short period, simplicity and easiness in control, less equipment menstruum investment and low production cost, and is very suitable for industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
A kind of method for the production of liprestatin by multi-stage fermentation
ActiveCN106497995BHigh potencyLow costMicroorganism based processesFermentationBiotechnologyStreptomyces
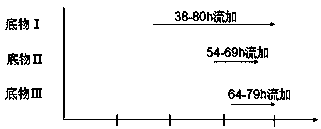
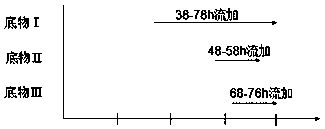
The invention discloses a method for producing Lipstatin through multi-stage fermentation, comprising the stages of strain activation, seed culture and fermentation culture. The first 10-40 hours of the fermentation culture stage is a cell growth stage, with dissolved oxygen level controlled at 50-100%. The Lipstatin production stage occurs after 40 hours. When the wet weight of bacteria reaches 10-12 g / L, the bacteria are transferred into a micro-oxygen fermentation stage, with the dissolved oxygen level controlled at 20-30%. When glucose concentration is less than 1 g / L, flow feeding with a substrate I is started, and fermentation is conducted for 60-80 hours with the glucose concentration in a fermentation tank controlled in the range of 1.0-1.5 g / L. 10-20 hours after feeding with the substrate I, flow feeding with a substrate II is begun, and flow feeding with a substrate III is begun 20-30 hours after feeding with the substrate I. The fermentation adopts multi-stage dissolved oxygen control and feeding fermentation in batches, and makes more than 80% of the product secreted outside the mycelium by multi-stage fermentation production of Lipstatin and simultaneously controls the permeability of Streptomyces mycelium. The method has the advantages of low cost, simple operation, short fermentation time, high yield of Lipstatin and easy separation of the product, and is beneficial to industrial production.
Owner:SOUTHEAST UNIV CHENGXIAN COLLEGE
A kind of purification method of liprestatin
ActiveCN102993134BOmit the preprocessing stepHigh purityOrganic chemistryOrganic solventPurification methods
The invention belongs to the technical field of medicines and particularly relates to a purification method for lipstatin. The technical scheme is that the purification method mainly comprises the steps of fermentation liquid direct extraction concentration, organic solvent dissolving filtering, silica-gel column chromatography and crystallization and the like, lipstatin with the weight content of 75%-85% and the high performance liquid chromatography (HPLC) detection purity of no smaller than 97% is obtained, and the total yield is 72%. The purification method is simple in steps, short in period, high in product yield and good in quality, all solvents can be recycled, the production cost is greatly reduced, and pollution to the environment is reduced.
Owner:鲁南新时代生物技术有限公司
A kind of Lipistatin fermentation method and fermentation medium
ActiveCN112852898BReduce consumption costEasy to controlBacteriaMicroorganism based processesBiotechnologyEngineering
In order to overcome the problems of low fermentation unit, high cost of fermentation raw materials, complex and difficult process, and unsuitability for large-scale industrial production in the existing lipastatin fermentation methods, the present invention provides a simple and efficient fermentation method for lipastatin. and fermentation medium, including the following steps: plate separation and slant culture, bacterial strain slant shake flask fermentation screening, shake flask seed liquid preparation, primary seed tank culture, secondary seed tank culture and fermenter culture. In the present invention, the lipistatin fermentation method and fermentation medium obtained by combining optimization strategies such as screening of high-yielding strains by shaking flask fermentation, optimization of medium at all levels, and improvement of fermentation process technology have the advantages of high fermentation unit, low cost of fermentation raw materials, and control of fermentation process. The advantages of simplicity and convenience, no need to add exogenous toxic precursors, etc., are of great significance for improving lipstatin fermentation yield and product quality, and reducing production costs.
Owner:ARGUS PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com