Weight-reducing medicine orlistat synthesis method
A technology of orlistat and a synthesis method, applied in the field of drug synthesis, can solve the problems of long hydrogenation reaction time, low yield and the like, and achieve the effects of easy product purification, few side reactions and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
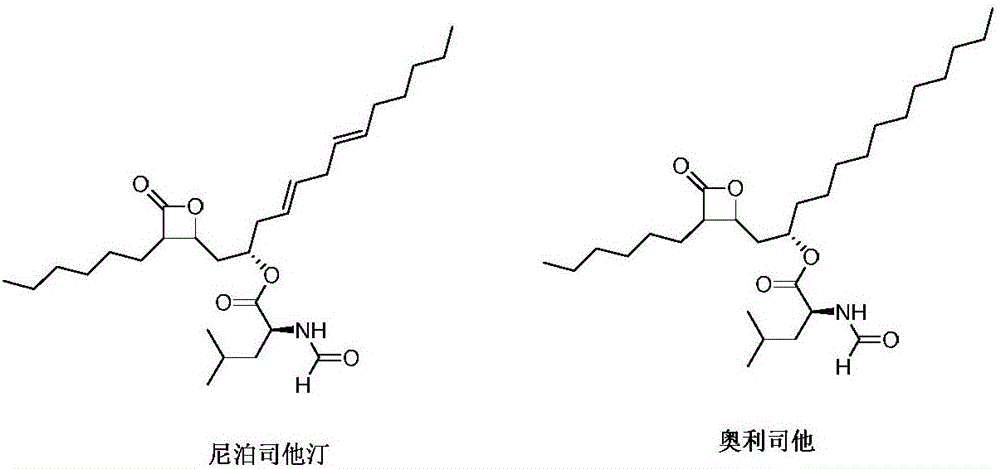
[0019] Synthesis of orlistat
[0020] Under nitrogen protection, 9.8g of niborestatin, (R,R)-(-)-N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexyldi Add 0.7g of cobaltamine, 0.6g of cuprous chloride and 30ml of acetonitrile into the reaction flask, start stirring, adjust the temperature to 20°C, then add 2.3g of sodium borohydride in three batches and stir for 2 to 4 hours. After the reaction is complete, the The reaction solution was poured into ice water to quench the reaction, extracted with dichloromethane, washed with water, and the organic phase was concentrated under reduced pressure to obtain orlistat. Dissolve the obtained orlistat in dichloromethane / petroleum ether mixed solvent (volume ratio 1:20), then heat to 80°C and stir for 10-20 minutes, filter while hot, cool the filtrate naturally, suction filter, and vacuum-dry to obtain refined 9.3 g of orlistat, the yield was 94.3%, and the HPLC purity was 99.56%.
Embodiment 2
[0022] Synthesis of orlistat
[0023] Under nitrogen protection, 9.8g of niborestatin, (R,R)-(-)-N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexyldi Add 0.6g of cobaltamine, 0.6g of cuprous chloride and 30ml of acetonitrile into the reaction flask, start stirring, adjust the temperature to 15°C, then add 3g of sodium borohydride in three batches and stir for 2 to 4 hours. After the reaction is complete, the reaction The solution was poured into ice water to quench the reaction, extracted with dichloromethane, washed with water, and the organic phase was concentrated under reduced pressure to obtain orlistat. The obtained orlistat was dissolved in dichloromethane / petroleum ether mixed solvent (volume ratio 1:20), then heated to 70°C and stirred for 10-20 minutes, filtered while hot, the filtrate was naturally cooled, suction filtered, and vacuum-dried to obtain refined 9.4 g of orlistat, the yield was 95.2%, and the HPLC purity was 99.66%.
Embodiment 3
[0025] Synthesis of orlistat
[0026] Under nitrogen protection, 9.8g of niborestatin, (R,R)-(-)-N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexyldi Add 0.9g of cobaltamine, 0.4g of cuprous chloride and 30ml of acetonitrile into the reaction flask, start stirring, adjust the temperature to 10°C, then add 3.8g of sodium borohydride in three batches and stir for 2 to 4 hours. After the reaction is complete, the The reaction solution was poured into ice water to quench the reaction, extracted with dichloromethane, washed with water, and the organic phase was concentrated under reduced pressure to obtain orlistat. Dissolve the obtained orlistat in dichloromethane / petroleum ether mixed solvent (volume ratio 1:20), then heat to 80°C and stir for 10-20 minutes, filter while hot, cool the filtrate naturally, suction filter, and vacuum-dry to obtain refined 9.4 g of orlistat, the yield was 94.5%, and the HPLC purity was 99.76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com