Post-transition metal catalyst system for oligomerization of ethylene
A post-transition metal and ethylene oligomerization technology, which is applied in the direction of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problems of complex catalyst preparation and achieve convenient large-scale Mass production and storage, good water and oxygen resistance, good stability, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
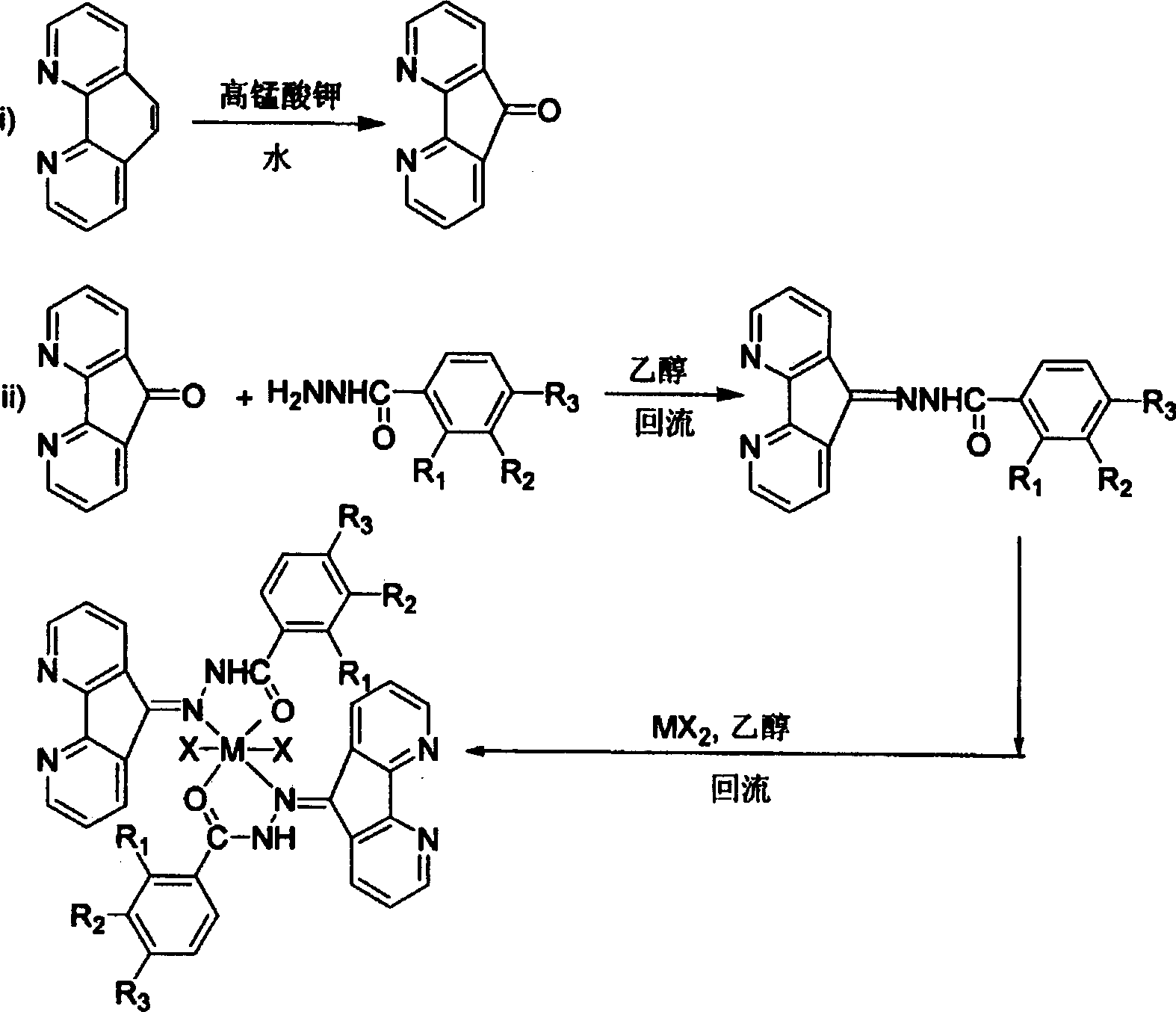
[0023] 1. Synthesis of ligand 4,5-diazafluorene-9-ketobenzoylhydrazone
[0024] Under the protection of nitrogen, dissolve 4,5-diazafluoren-9-one (0.911g, 5mmol) in absolute ethanol (25ml), add a catalytic amount of p-toluenesulfonic acid, heat slightly to reflux, and stir for 10 Add dropwise a solution of benzoyl hydrazide (0.681g, 5mmol) in absolute ethanol (15mml) within minutes, continue to reflux in absolute ethanol after dripping, stop the reaction after 8h, cool to precipitate a large amount of precipitate, filter with suction, and wash with ethanol. It was dried in vacuum to obtain 1.36 g of yellow solid, yield: 90%. EI-MS(m / z): 300(M + , 61.0%), 197 (15.7%), 181 (16.9%), 169 (13.7%), 154 (7.9%), 139 (3.6%), 122 (10.1%), 105 (100%), 94 (5.6 %), 77 (33.7%).
[0025] 2. Synthesis of 4,5-diazafluorene-9-ketobenzoylhydrazone nickel (II) complex catalyst precursor
[0026] Under the protection of nitrogen, dissolve 4,5-diazafluoren-9-one benzoyl hydrazone (0.060g, 0.2mmol) in a...
Embodiment 2
[0030] 1. Synthesis of ligand 4,5-diazafluoren-9-one-4-nitrobenzoylhydrazone:
[0031] Under the protection of nitrogen, dissolve 4,5-diazafluoren-9-one (0.911g, 5mmol) in absolute ethanol (25ml), add a catalytic amount of p-toluenesulfonic acid, heat slightly to reflux, and stir for 10 minutes Add 4-nitrobenzoyl hydrazide (0.906g, 5mmol) in anhydrous ethanol (25mml) solution dropwise, continue to reflux in anhydrous ethanol after dripping, stop the reaction after 8h, cool to precipitate a large amount of precipitate, filter with suction, It was washed with ethanol and dried in vacuum to obtain 1.59 g of yellow solid. Yield: 92%. IR(KBr): 3437, 3251, 3177, 3064, 3003, 1644(vs), 1592(s), 1566(m), 1517(s), 1400(s), 1337(vs), 1288, 1175, 1143 , 1097, 1009, 948, 861, 821, 756(m), 715(m); 1HNMR (DMSO): δ 7.50~7.65 (2H, m), 8.25 (4H, d), 8.4 (2H, d), 8.63 (1H, s), 8.80 (2H, d); FAB-MS (m / z): 344(M + -1); Anal.Calcd.for C 18 H 11 N 5 O 3 : C, 62.61%; H, 3.21%; N, 20.28%; O, 13.90%. Foun...
Embodiment 3
[0037] 1. Synthesis of ligand 4,5-diazafluorene-9-one-2-hydroxybenzoylhydrazone
[0038] Under the protection of nitrogen, dissolve 4,5-diazafluoren-9-one (0.911g, 5mmol) in absolute ethanol (25ml), add a catalytic amount of p-toluenesulfonic acid, heat slightly to reflux, and stir for 10 minutes Add 2-Hydroxybenzoic acid hydrazide (0.761g, 5mmol) in absolute ethanol (20mml) solution dropwise, continue to reflux in absolute ethanol after dripping, stop the reaction after 8h, cool to precipitate a large amount of precipitation, suction filter, ethanol Washing, vacuum drying, to obtain 1.34 g of light yellow solid, yield: 85%. IR(KBr): 3430(m), 3320(s), 3081(m), 2702, 2564, 1937, 1775, 1685(vs), 1604(s), 1565(s), 1519(vs), 1489( s), 1453(s), 1405(vs), 1365, 1282(s), 1234(m), 1201, 1162(m), 1132(s), 1107(s), 1060(m), 960, 894 , 753(vs); MALDI-TOF(m / z): 317(M + +1).
[0039] 2. Synthesis of catalyst precursor of 4,5-diazafluoren-9-one-2-hydroxybenzoylhydrazone nickel (II) complex:
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com