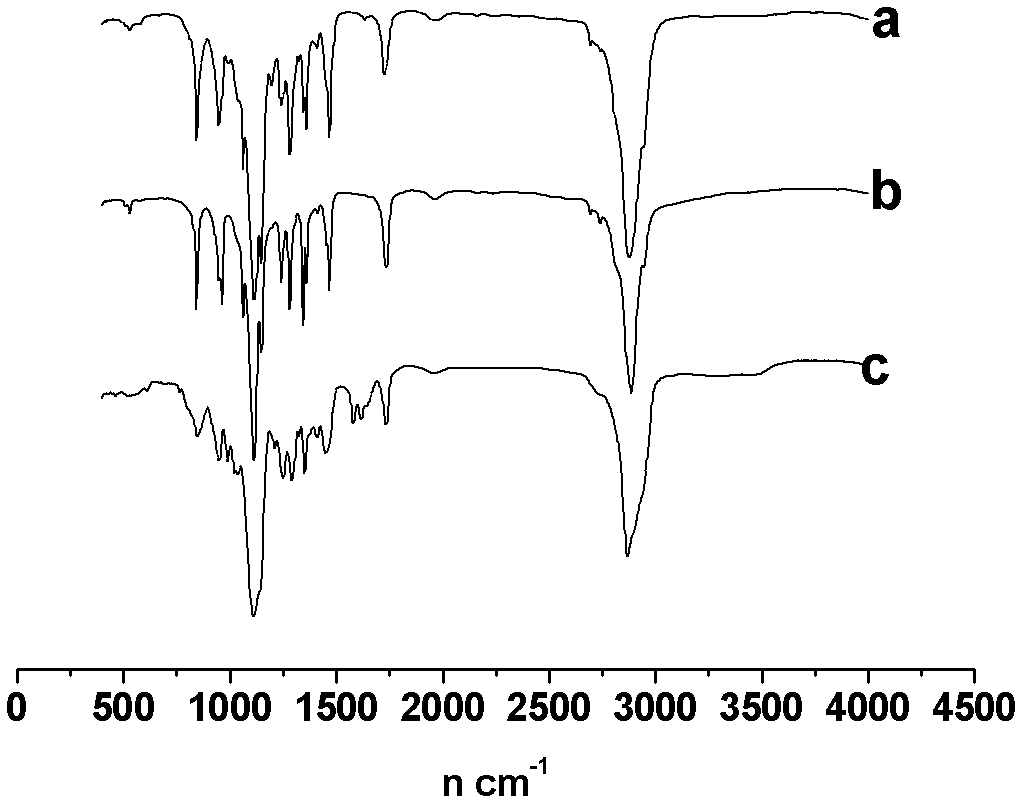
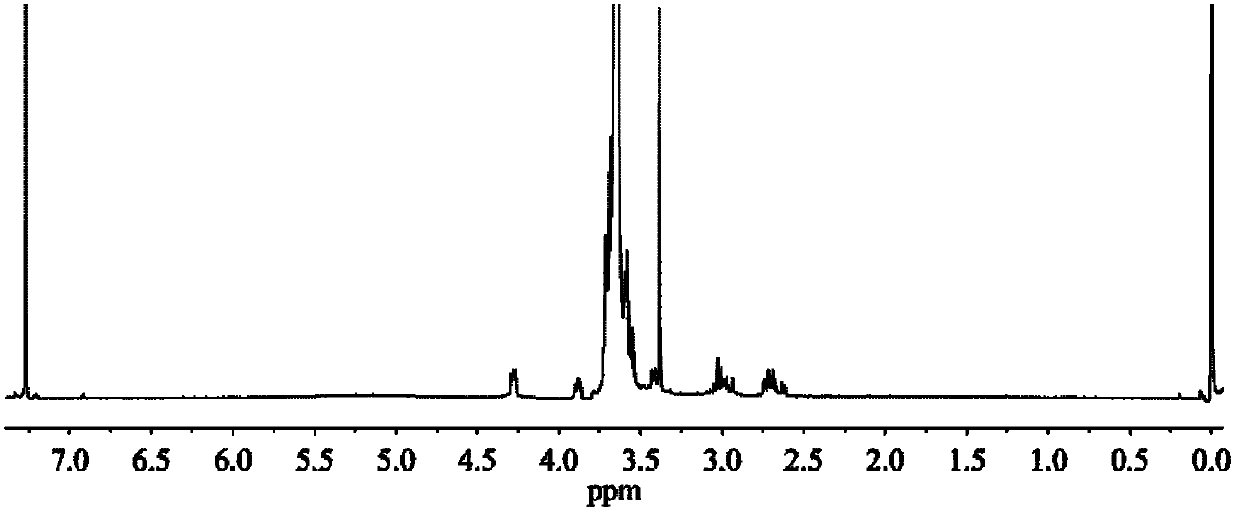
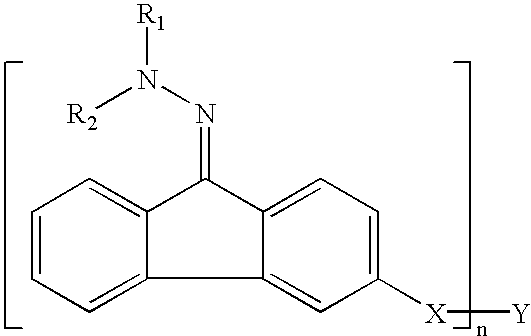
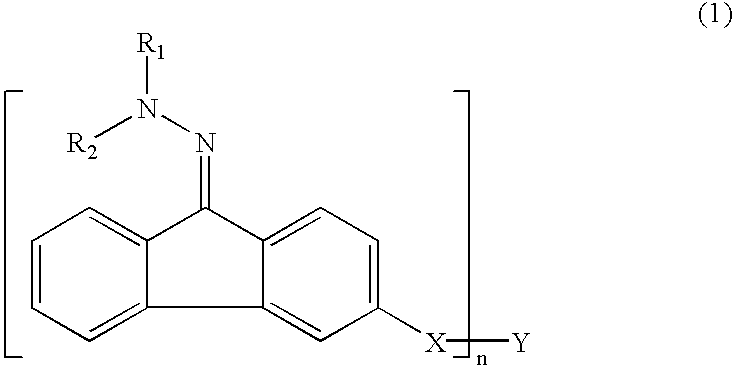
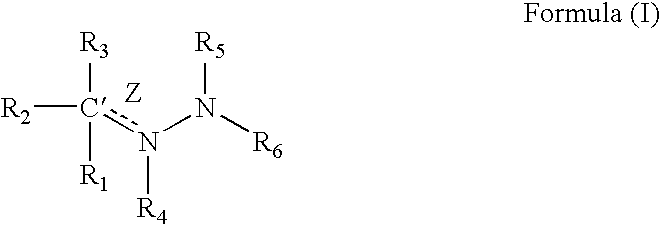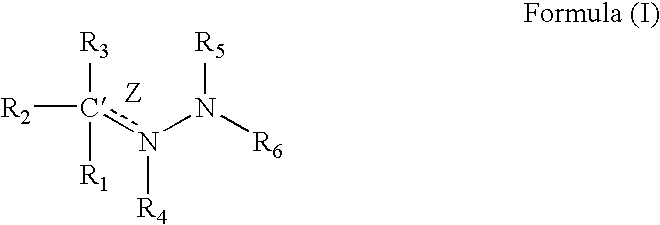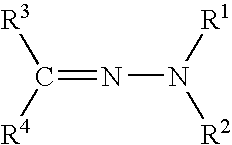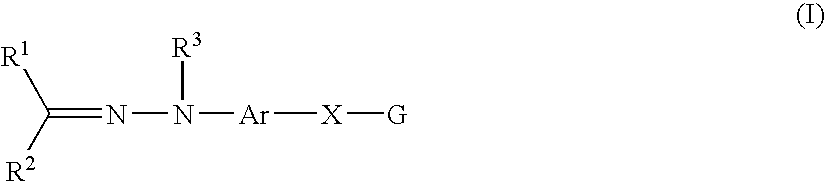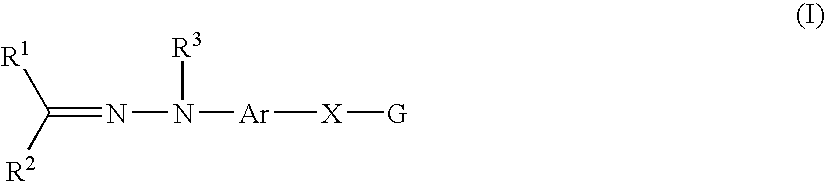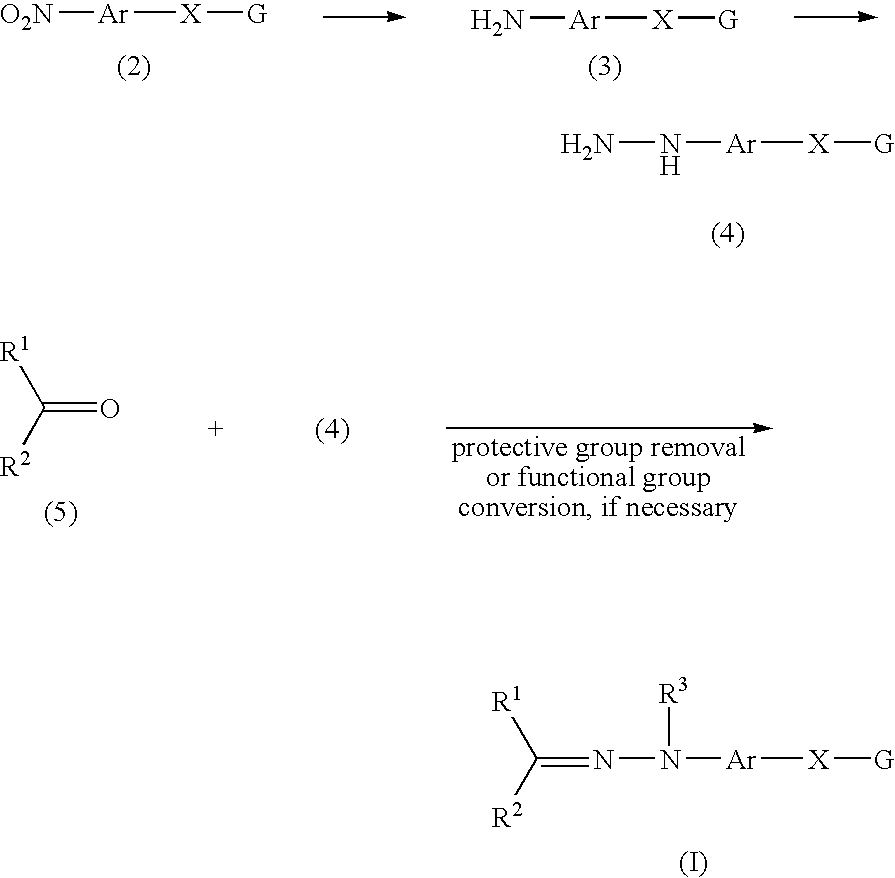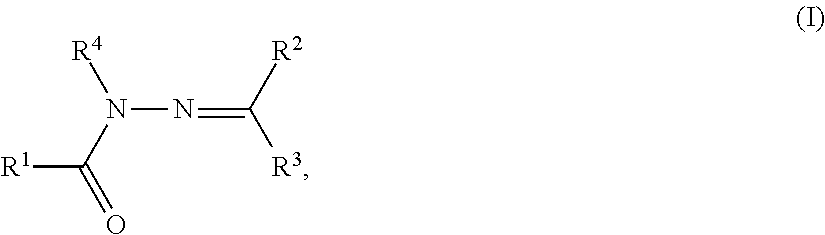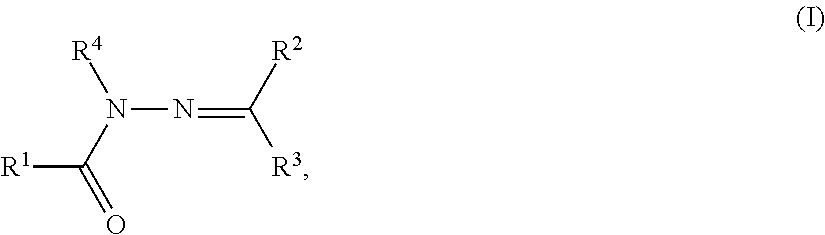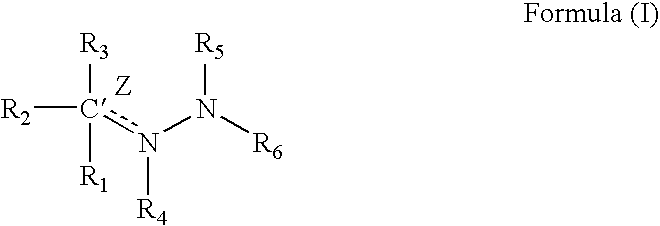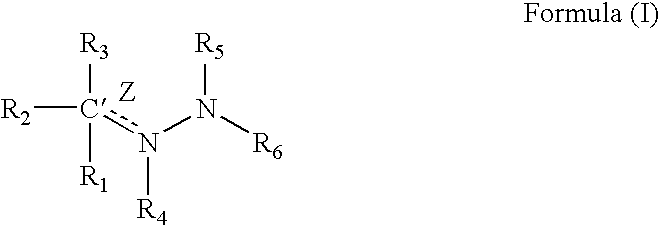Patents
Literature
1142 results about "Hydrazone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
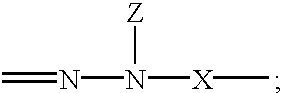
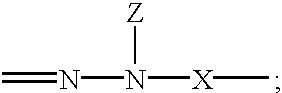
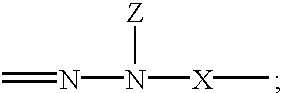
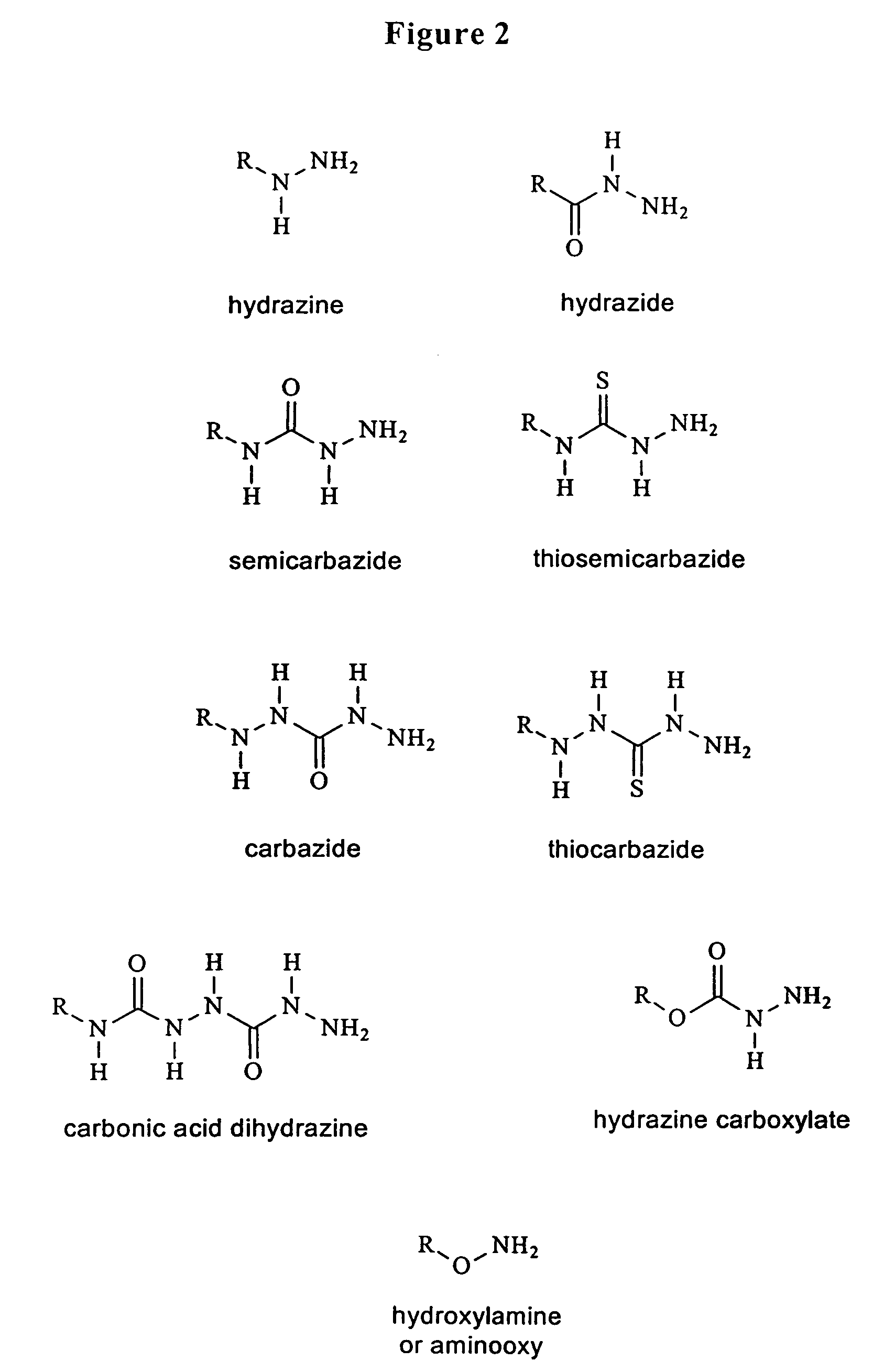
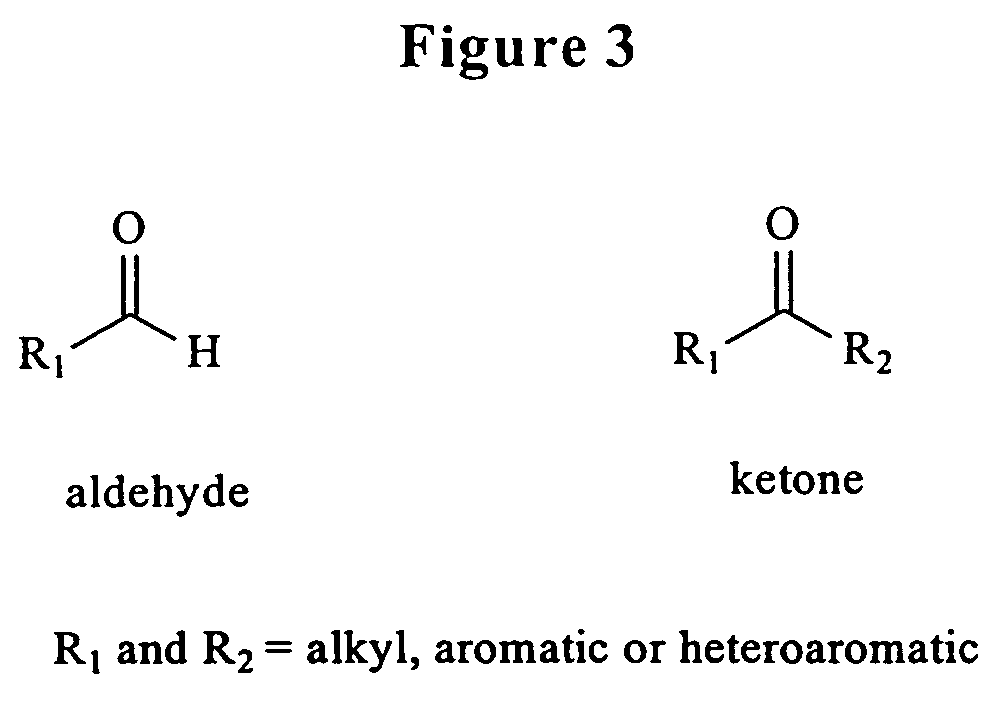
Hydrazones are a class of organic compounds with the structure R₁R₂C=NNH₂. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH₂ functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.
Non-radical photochemical crosslinked hydrogel material preparation method, product and application
ActiveCN105131315APrecise and controllable time and spaceEasy to operateOrganic active ingredientsCosmetic preparationsPolymer scienceHydroxylamine
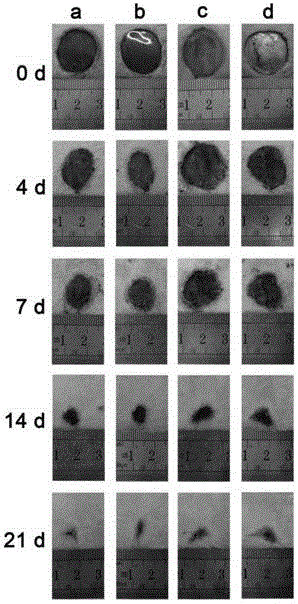
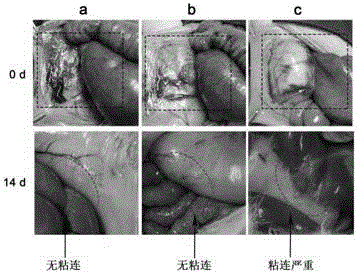
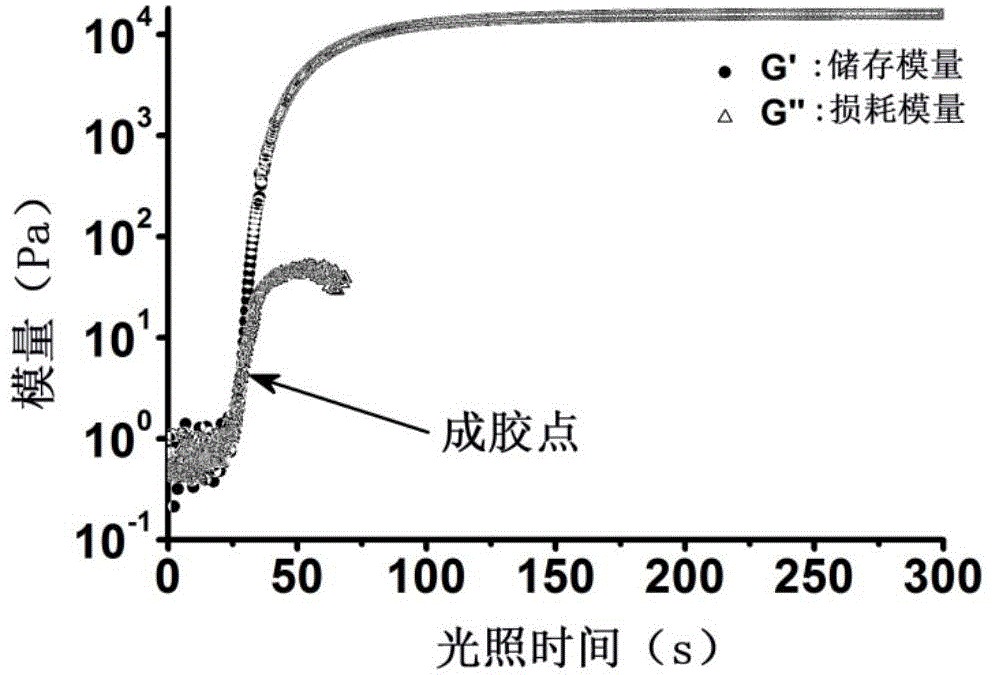
The present invention provides a non-radical photo-crosslinked hydrogel preparation method, comprising the following steps: a component A is dissolved in a biocompatible medium to obtain a solution A, component B-hydrazide, hydroxylamine or primary amine high molecular derivative is dissolved in a biocompatible medium to obtain a solution B; the solution A and the solution B are evenly mixed to obtain a hydrogel precursor solution; under illumination, aldehyde group produced by light excitation of o-nitrobenzyl in the component A of the hydrogel precursor solution is crosslinked with hydrazone, hydroxylamine or primary amine group in the component B in the form of respective formation of oxime and Schiff base to produce the hydrogel. The present invention also provides a kit for the hydrogel preparation, and application of the hydrogel in tissue repair, beauty and as a cell, protein or drug carrier. The tissue surface light-situ gel can be achieved by the hydrogel, in particular, wound surface in-situ thin glue formation can be achieved, and the hydrogel is especially suitable for clinical wound surface tissue repair and isolation.
Owner:上海戴云化工科技有限公司 +2
Medicinal uses of hydrazones
InactiveUS6660737B2Increasing endogenous EPO and vascularizationIncreased vascularizationBiocideNervous disorderGynecologyHydrazone
Compounds having a structure according to Formula (I):are effective in a method of increasing erythropoietin and vascularization of tissue in a subject in need thereof.
Owner:THE PROCTER & GAMBLE COMPANY
Flat panel display
ActiveUS20050116240A1Avoid pinholesImprove picture qualityDischarge tube luminescnet screensElectroluminescent light sourcesOrganic layerDisplay device
The present invention discloses an organic light emitting device for preventing element defects and improving picture quality by reducing a taper angle of a substrate surface. The flat panel display of the present invention comprises, an insulating substrate, a lower layer formed on the insulating substrate and having a first step and a first taper angle with respect to the substrate surface, and an upper layer formed on the insulating substrate and for reducing the taper angle of the lower layer. The upper layer has a second taper angle smaller than the first taper angle of the lower layer. The upper layer is a conductive layer that may be applied by a wet coating method, has a charge transporting capability, and is selected from at least one of a small-molecule organic layer including a carbazole-based, arylamine-based, hydrazone-based, stilbene-based, oxadiazole-based, starburst-based derivatives, and a polymer organic layer including PEDOT, PANI, carbazole-based, arylamine-based, perylene-based, pyrrole-based, oxadiazole-based derivatives.
Owner:SAMSUNG DISPLAY CO LTD
Method fro determining content of volatile carbonyl compound in main stream smoke of cigarette
InactiveCN101701941AEasy to separateSeparation reachedComponent separationHydrazoneChromatographic column
The invention discloses a method for determining content of volatile carbonyl compound in main stream smoke of cigarette. 2, 4-dinitrophenylhydrazine is utilized to gather volatile carbonyl compound in main stream smoke of cigarette, so as to form hydrazone compound, and the content of the hydrazone compound is determined by a high performance liquid chromatograph by adopting external standard method; and the high performance liquid chromatograph adopts chromatographic column. The determination method of the invention adopts chromatographic column, has excellent separating effect on nitro substituted arene derivative, especially on hydrazone compound formed after reaction of 2, 4-dinitrophenylhydrazine and volatile carbonyl compound, and meanwhile chromatographic analysis condition is optimized, thus achieving complete separation of volatile carbonyl compound and interfering component in main stream smoke of cigarette and improving accuracy of determination.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
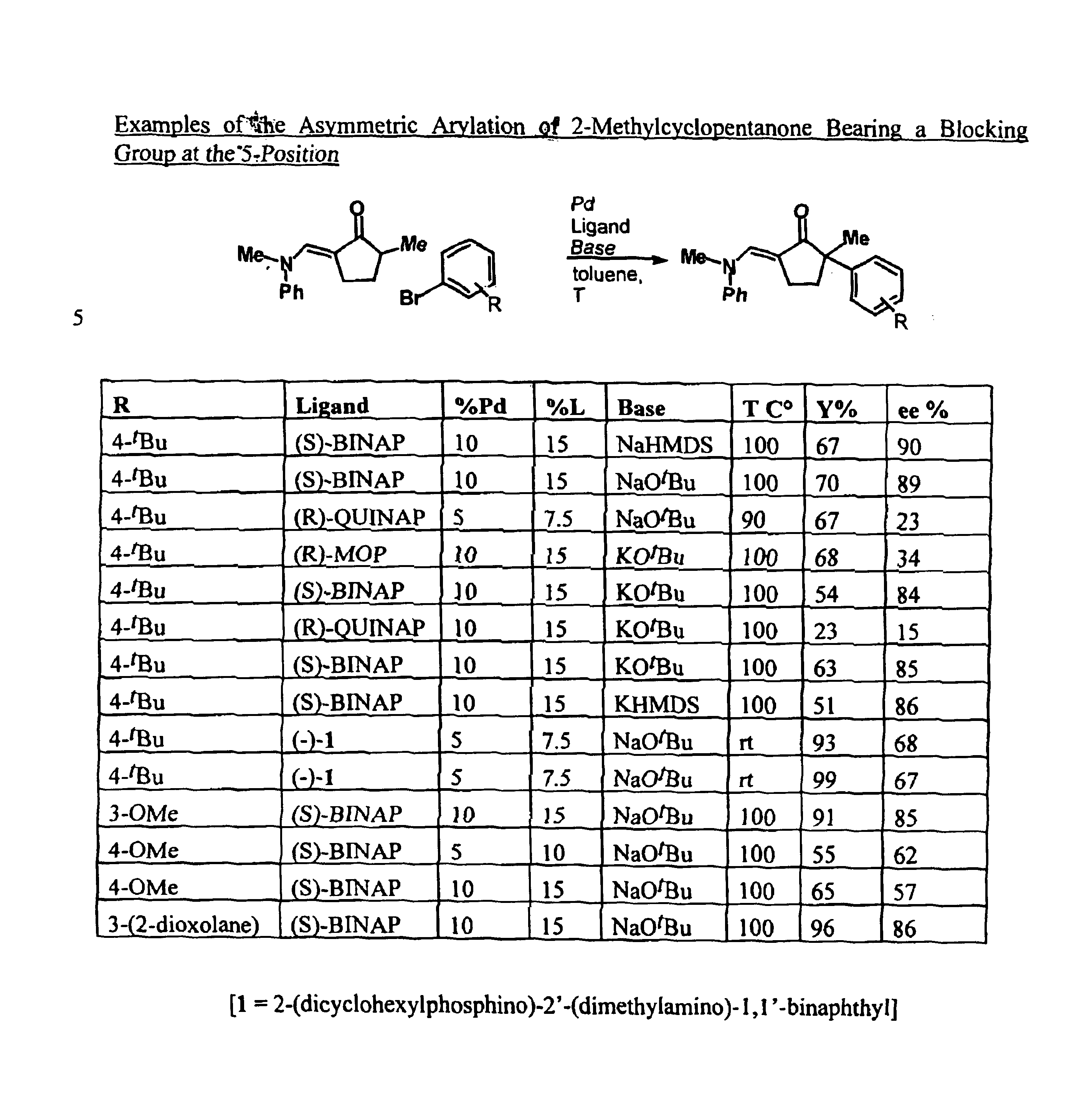
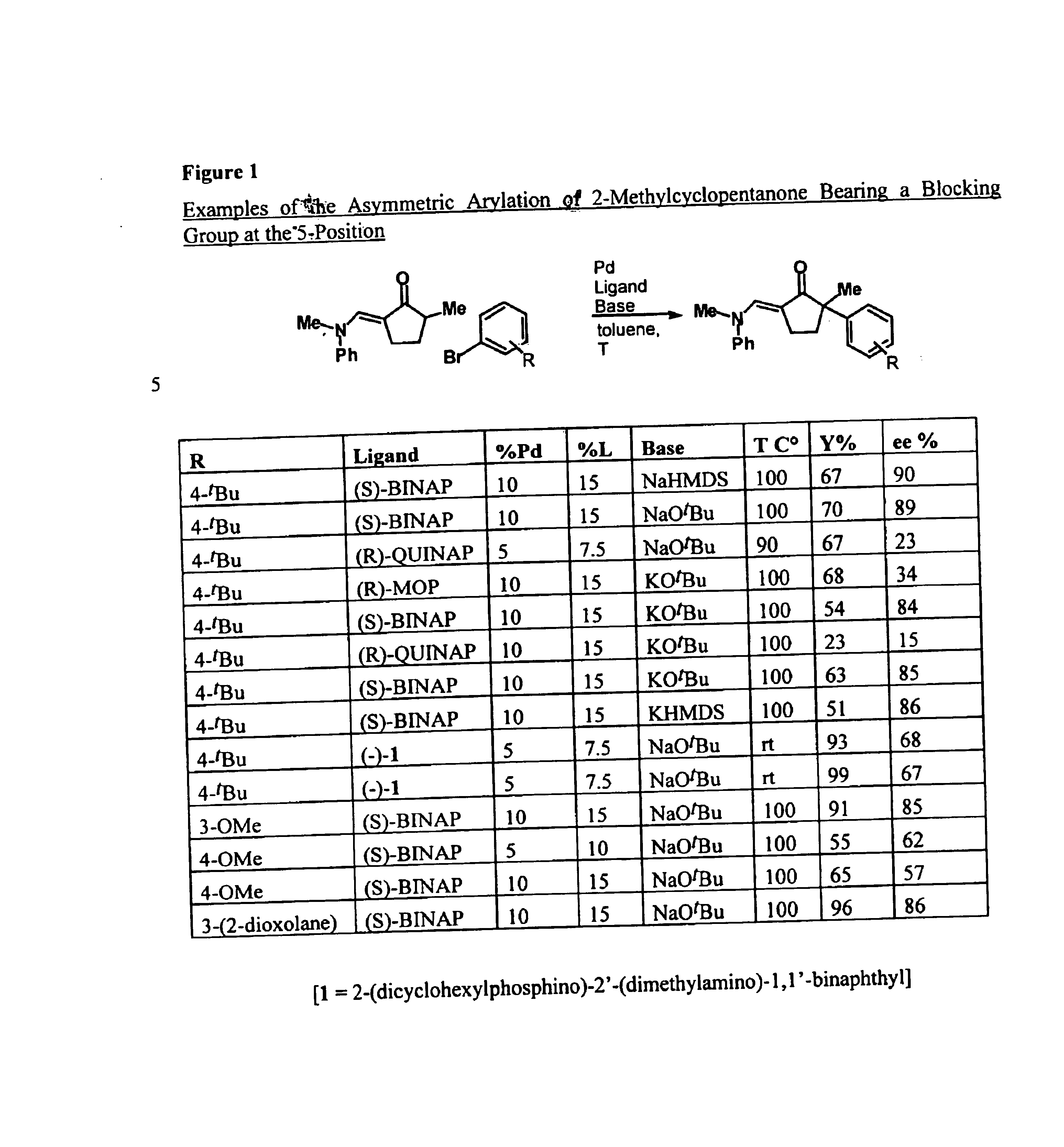
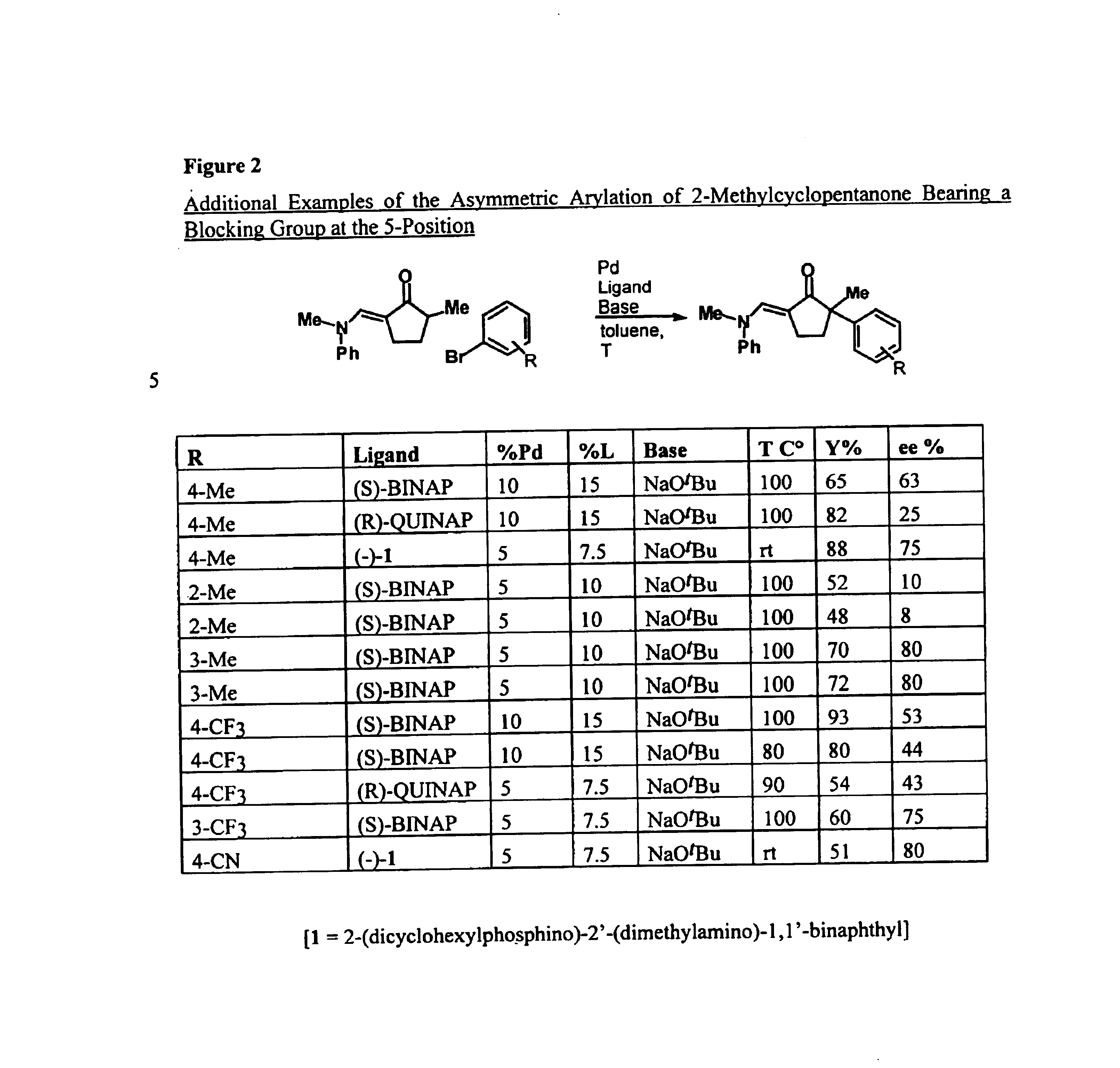
Arylation and vinylation of activated carbons
InactiveUS6867310B1Carboxylic acid nitrile preparationGroup 5/15 element organic compoundsActivated carbonHydrazone
The present invention provides transition-metal-catalyst-based methods for the arylation and vinylation of activated methyl, methylene, and methine carbons with aryl halides, vinyl halides, and the like. The methods of the invention provide several improvements over existing methods, including the ability to synthesize efficiently and under mild conditions α-aryl and α-vinyl products from a wide range of starting materials, including ketones, esters, hydrazones, and imines. Furthermore, the methods of the invention may be used in an asymmetric sense, i.e. to produce enantiomerically-enriched chiral α-aryl and α-vinyl products.
Owner:MASSACHUSETTS INST OF TECH
Electrophotographic organophotoreceptors with novel charge transport compounds
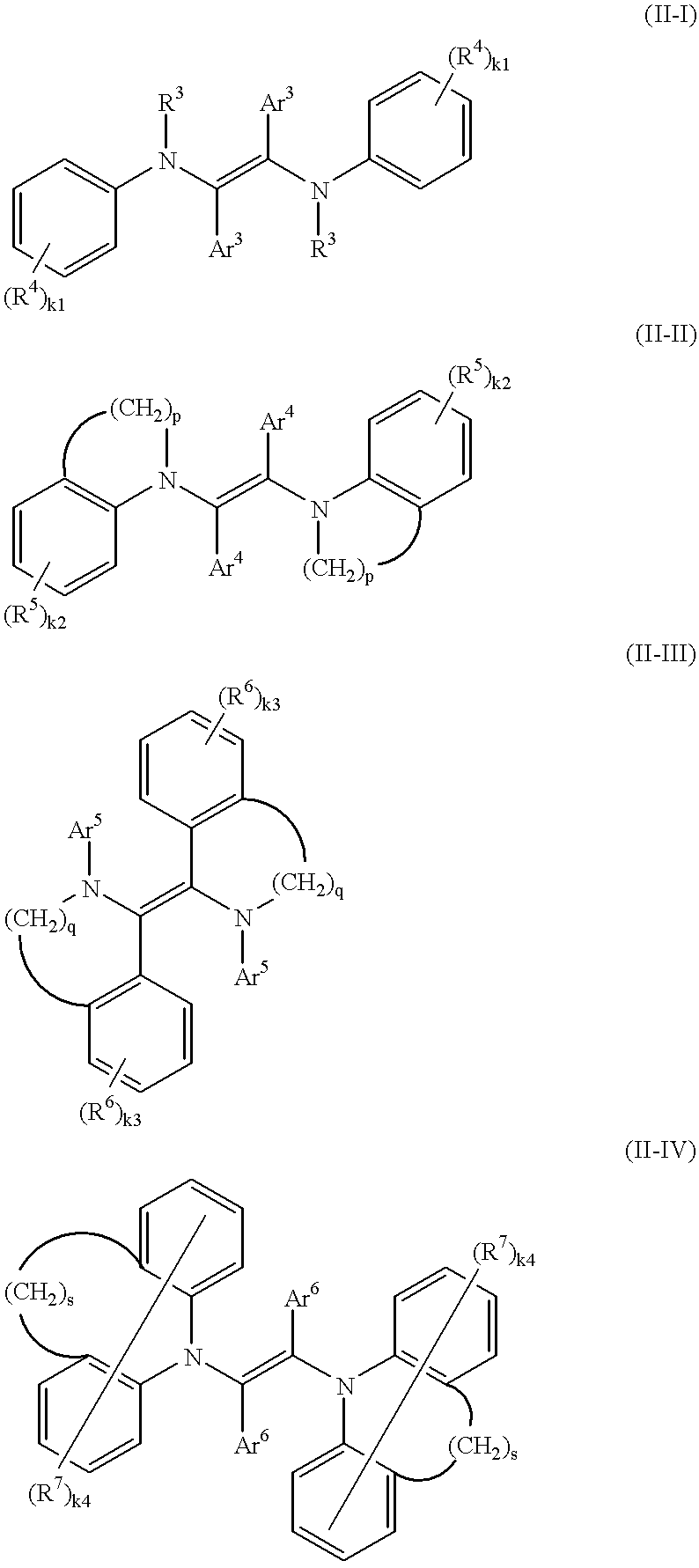
Charge transport compounds are described that have a multiple number of hydrazone-bridged heterocyclic groups (especially julolidine, carbazole and / or triarylmethane groups) connected by a central bridging group. An example of charge transport compounds are those having the following generic formula:(R-Q)n-Y Formula Iwherein R is selected from the group consisting of julolidine ring groups, carbazole ring groups, and triarylmethane ring groups; Q comprises an aromatic hydrazone linking group, such as Y comprises a bridging group between R-Q- groups, such as a bond, carbon atom, nitrogen atom, oxygen atom, sulfur atom, a methylene group; Z is an aryl group, preferably a phenyl group or naphthyl group; X is a linking group, preferably amethylene group, and for example having the formula -(CH2)m- (branched or linear), where m is an integer between 0 and 20, inclusive, and one or more of the methylene groups is optionally replaced by an oxygen atom, a carbonyl group, urethane, urea, an ester group, a -NR6 group, a CHR7 group, or a CR8R9 group where R6, R7, R8, and R9 are, independently, H, an alkyl group, or aryl group; and n is an integer between 2 and 6, inclusive. An organic photoreceptor includes that compound and (b) a charge generating compound; and (c) an electrically conductive substrate.
Owner:S PRINTING SOLUTION CO LTD
Functionalized polymer
A functionalized polymer includes a directly bonded moiety, which can be located at a terminus of the polymer, defined by the formula —NH—NR1R2 where R1 and R2 independently are substituted or unsubstituted alkyl, alkenyl, cycloalkyl, cycloalkenyl, aryl, allyl, aralkyl, alkaryl, or alkynyl groups, or together form a substituted or unsubstituted alkylene, alkenylene, cycloalkylene, cycloalkenylene, or arylene group. The functionalized polymer can be provided by reacting a living polymer with a hydrazone. Such polymers can be used in the production of compositions that include particulate fillers.
Owner:BRIDGESTONE CORP
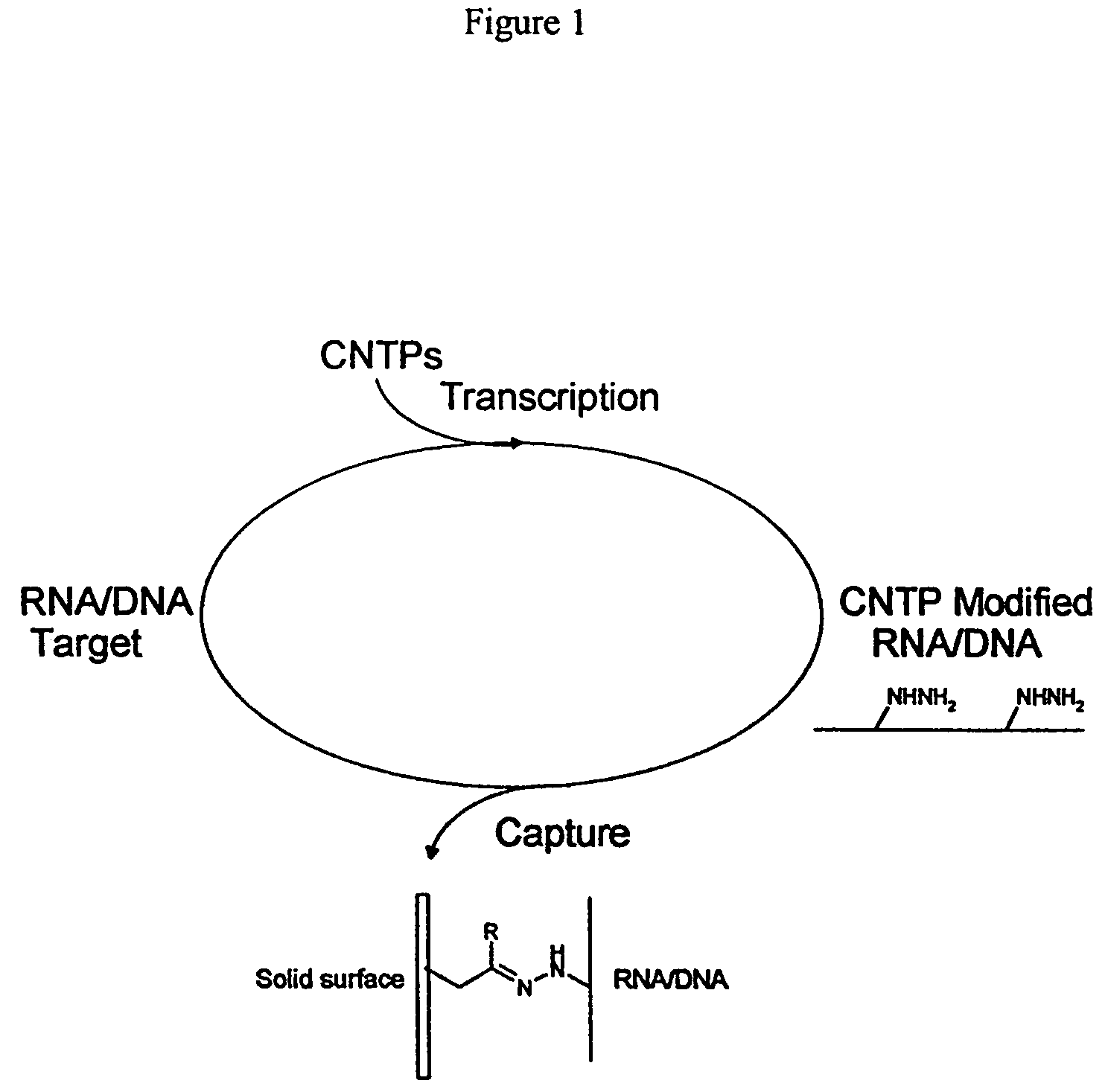
Triphosphate oligonucleotide modification reagents and uses thereof
InactiveUS7173125B2Accurate measurementImprove the immunitySugar derivativesCarboxylic acid nitrile preparationEnzymatic synthesisHydrazone
Hydrazino, oxyamino and carbonyl-based monomers and methods for incorporation into oligonucleotides during enzymatic synthesis are provided. Modified oligonucleotides are provided that incorporate the monomers provided herein. Immobilized oligonucleotides and oligonucleotide conjugates that contain covalent hydrazone or oxime linkages are provided. Methods for preparation of surface bound oligonucleotides are provided. Methods for the preparation of oligonucleotide conjugates are also provided.
Owner:VECTOR LAB +1
Preparation and application of dual-sensitivity amphiphilic polysaccharide-doxorubicin conjugate and pharmaceutical composition thereof
ActiveCN105727309APayloadImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsHigh concentrationTherapeutic effect
The invention relates to a dual-sensitivity amphiphilic polysaccharide-doxorubicin conjugate.According to the conjugate, hydrophobic antitumor drug doxorubicin is introduced in a polysaccharide framework through a connecting arm containing a disulfide bond and a hydrazone bond, so that the polysaccharide-doxorubicin conjugate has an amphiphilic property, can be self-assembled into a nano-micelle in water and directly used for tumor treatment, and can also be physically loaded with an antitumor drug to be used for antitumor treatment.The conjugate is mainly characterized in that after the nano-micelle reaches a focus, the disulfide bond in the connecting arm can be degraded specifically by high-concentration reduction substances in focus cells, and meanwhile the hydrazone bond in the connecting arm can be degraded in a special pH environment of the focus, so that the micelle is degraded, the drug is quickly released, and the treatment effect is improved; the antitumor drug is loaded in two modes of chemical conjugation and physical package, and therefore the joint treatment effect is achieved.The polysaccharide conjugate and a pharmaceutical composition can be used for injection or oral administration or external administration, can remarkably improve antitumor activity, and provides a new thought for development of the antitumor drug.
Owner:CHINA PHARM UNIV
Hydrazone derivatives and uses thereof
Owner:REDPOINT BIO CORP
Electrophotographic photosensitive member, image forming device using same, and electrophotographic photosensitive member cartridge
ActiveUS20090232551A1Improve wear resistanceDevelopersElectrographic process apparatusHydrazonePolyester resin
To realize an electrophotographic photoreceptor excellent in abrasion resistance, the photosensitive layer of the photoreceptor comprises a polyester resin containing a repeating structural unit represented by the formula (1) and a hydrazone compound.(In the formula (1), Ar1 to Ar4 each represents, independently of each other, an arylene group which may have a substituent. X1 represents a bivalent group (including a single bond) and X2 represents a bivalent group (including a single bond) with 3 or less atoms.)
Owner:MITSUBISHI CHEM CORP
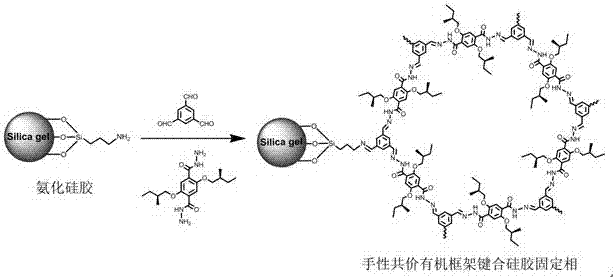
Hydrazone bond-connected chiral covalent organic framework bonded silica gel stationary phase and application thereof
ActiveCN107362785AClear structureEasy to prepareOther chemical processesSolid sorbent liquid separationSilica gelHplc mass spectrometry
The invention relates to a hydrazone bond-connected chiral covalent organic framework bonded silica gel stationary phase and an application thereof. The stationary phase is prepared by a method comprising the following steps: uniformly mixing a hydrazide chiral precursor, 1,3,5-benzenetricarboxaldehyde and ammoniated silica gel in an organic solvent in an inert atmosphere, carrying out a reaction under the catalysis of acetic acid, and filtering, washing and drying the obtained reaction product to obtain the chiral covalent organic framework bonded silica stationary phase. The structure of the hydrazide chiral precursor is represented by formula (I) shown in the description, a mass ratio of the ammoniated silica gel to the hydrazide chiral precursor is (2-12):1, and molar ratio of the hydrazide chiral precursor to the 1,3,5-benzenetricarboxaldehyde is 1:(0.5-2). The chiral covalent organic framework-bonded silica gel stationary phase is uniform in particle size; and the stationary phase has the advantages of high column efficiency, moderate column pressure and good separation effect on cis-trans isomers and position isomers when used in high performance liquid chromatography, and also has the advantages of definite structure, simple preparation method and good batch reproducibility.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Bifunctional polyethylene glycol and adriamycin conjugate and preparation method thereof
InactiveCN103357022ASmall toxicityGood water solubilityOrganic active ingredientsPharmaceutical non-active ingredientsCancer cellKetone
The invention relates to a bifunctional polyethylene glycol and adriamycin conjugate and a preparation method thereof. The bifunctional polyethylene glycol and adriamycin conjugate is prepared in the following steps: modifying methoxy polyethylene glycol which serves as a raw material through hydroformylation, carrying out a reductive amination reaction on the modified methoxy polyethylene glycol and disulfide-bond containing dihydrazide to generate terminal-hydrazide polyethylene glycol, and carrying out a reaction on the terminal hydrazide of the terminal-hydrazide polyethylene glycol and the ketone carbonyl of the adriamycin to generate a hydrazone bond to obtain the bifunctional polyethylene glycol and adriamycin conjugate. The bifunctional polyethylene glycol and adriamycin conjugate provided by the invention can adapt to the reductive acidic environment in a cancer cell to release drugs rapidly to improve the cancer treatment effect and reduce the drug resisting possibility of the cancer cell, and in addition, the bifunctional polyethylene glycol and adriamycin conjugate can be self-assembled into a nano particle in a water phase to be able to circulate for a long time in blood to improve the pharmacokinetics of the doxorubicin. The method for preparing the bifunctional polyethylene glycol and adriamycin conjugate has the advantages of simple process, mild reaction conditions, high drug carrying rate and low cost, and the raw materials are easy to obtain. The bifunctional polyethylene glycol and adriamycin conjugate has potential application values in the aspects of targeted delivery of drugs, controlled release of drugs and improvement of clinical cancer resisting and treating effects.
Owner:XI AN JIAOTONG UNIV
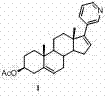
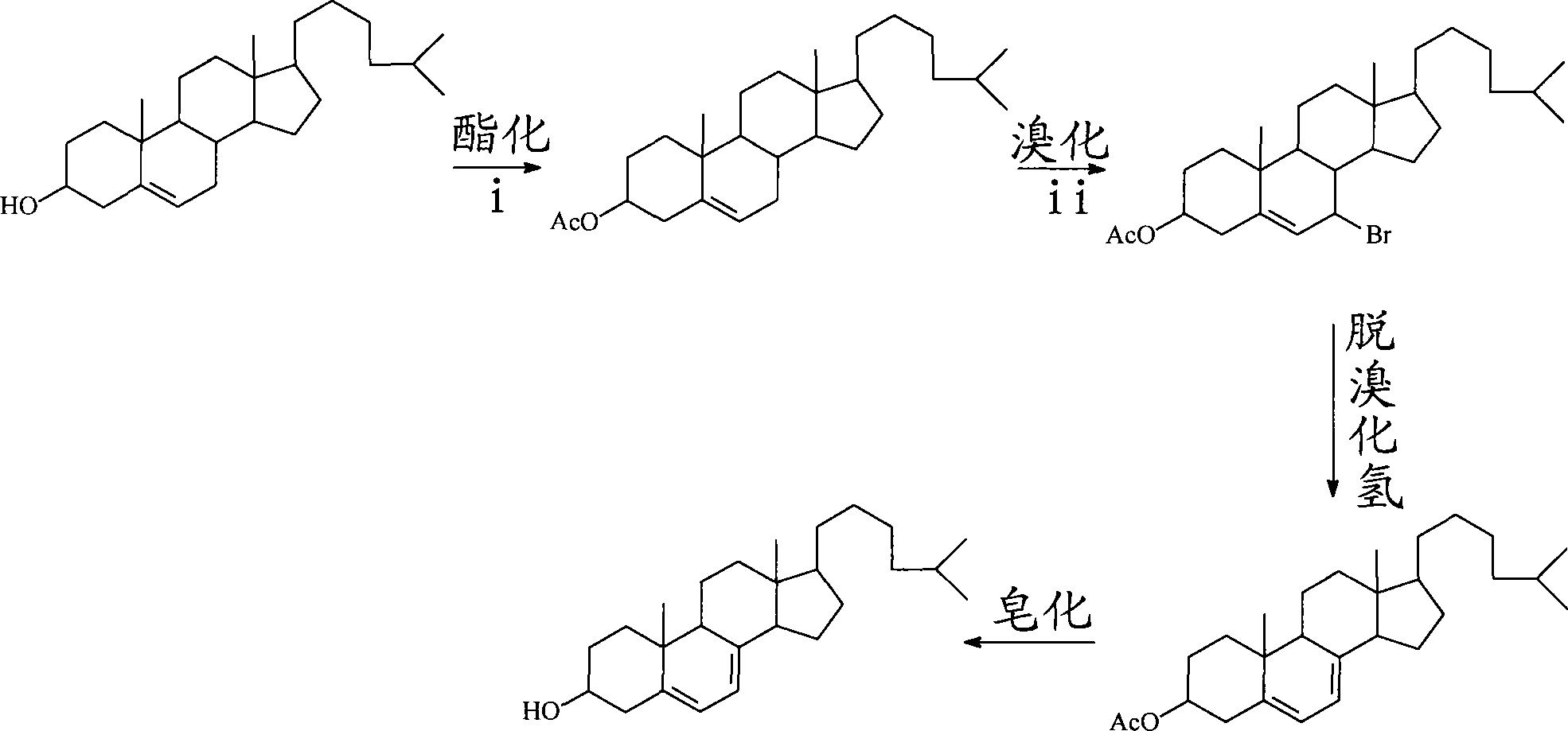
Method for preparing abiraterone acetate
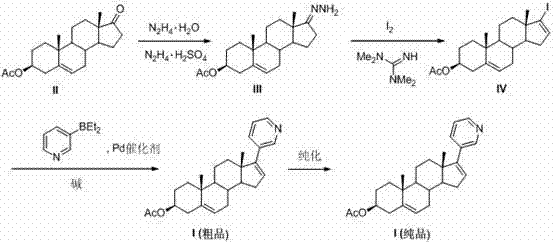
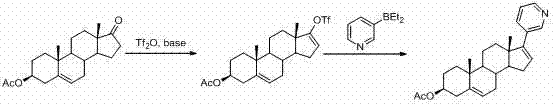
The invention discloses a convenient, rapid and economical method for massively preparing abiraterone acetate. According to the method, dehydroepiandrosterone acetate is used as a starting material, a keto carbonyl group is converted to hydrazone, and the abiraterone acetate is directly synthesized through iodination and Suzuki coupling reaction. According to the method, the operation is simple and convenient, the total yield of the three-step reaction is 34.7%, the product purity is 98.5%, the column chromatography separation purification is not required in the postprocessing, and the method is suitable for industrial production.
Owner:SUN YAT SEN UNIV
Hydrazone derivative
A compound represented by the following formula (I): wherein R1 represents hydrogen, aryl which may have a substituent, a saturated or unsaturated 5- to 7-membered heterocyclic group which may have a substituent, etc.; R2 represents hydrogen, aryl which may have a substituent, a saturated or unsaturated 5- to 7-membered heterocyclic group which may have a substituent, etc.; R3 represents hydrogen, etc.; Ar represents a divalent group derived from aromatic hydrocarbon, etc.; X represents a single bond, linear or branched alkylene having from 1 to 3 carbon atoms which may have a substituent, etc.; and G represents halogen, a saturated or unsaturated 5- or 6-membered cyclic hydrocarbon group which may have a substituent, a saturated or unsaturated 5- to 7-membered heterocyclic group which may have a substituent, etc., a salt thereof or a solvate thereof; and an agent for inhibiting aggregation and / or deposition of an amyloid protein or an amyloid-like protein, which comprises the compound, a salt thereof or a solvate thereof.
Owner:DAIICHI PHARMA CO LTD
Film-transferring printing method
InactiveCN101148127AEffective peelingEfficient transferDecorative surface effectsPattern printingEngineeringPrinting ink
The film transfer printing process includes the following steps: 1. painting polyethylene terephthalate (PET) film with strippable layer of silica and methyl hydrazone acetaldehyde and in pH 7-8, solid content of 45+ / -2 % and proper viscosity; 2. printing graphic context on the PET film with intaglio printing or flexible plate printing ink; 3. painting water soluble glue of vinyl acetate-ethylene emulsion and in pH 8-10, solid content of 52+ / -2 % and proper viscosity; and 4. compounding the PET film to paper material and stripping to transfer the printed graphic context onto the paper material. The present invention can strip and transfer the printed ink layer effectively to obtain printed matter with strong metal texture in less influence of the paper quality on the printed matter quality.
Owner:SHENZHEN JINJIA GRP
Aqueous cross-linking compositions and methods
ActiveUS20120142847A1Improve mechanical propertiesGood physical propertiesInksEster polymer adhesivesCross-linkHydrazone
Water-borne cross-linking polymeric compositions and related embodiments, such as methods of making and using the compositions, as well as products formed with said compositions are described. For example, the water-borne composition may comprise polymers incorporating cross-linking functionality such as, but not limited to, carbonyl or epoxy functionality, and a blocked cross-linking agent, for example, a hydrazone. The cross-linking functionality does not react with the blocked cross-linking agent; however, the blocked cross-linking agent is capable of reacting with the alternative form cross-linking agent such as, but not limited to, a hydrazide, to yield a cross-linked polymer. The alternative form cross-linking agent may be formed in an equilibrium reaction including the blocked cross-linking agent.
Owner:BENJAMIN MOORE & CO
Acyl hydrazones as bleach-boosting active substances
InactiveUS20140323381A1Organic detergent compounding agentsNon-surface-active detergent compositionsHydrazoneBleach
The aim of the invention is to improve the cleaning performance of washing and cleaning agents against stains from polysaccharide-containing food residue. This is substantially achieved by incorporating a combination of peroxide bleaching agents with specific acyl hydrazones.
Owner:HENKEL KGAA
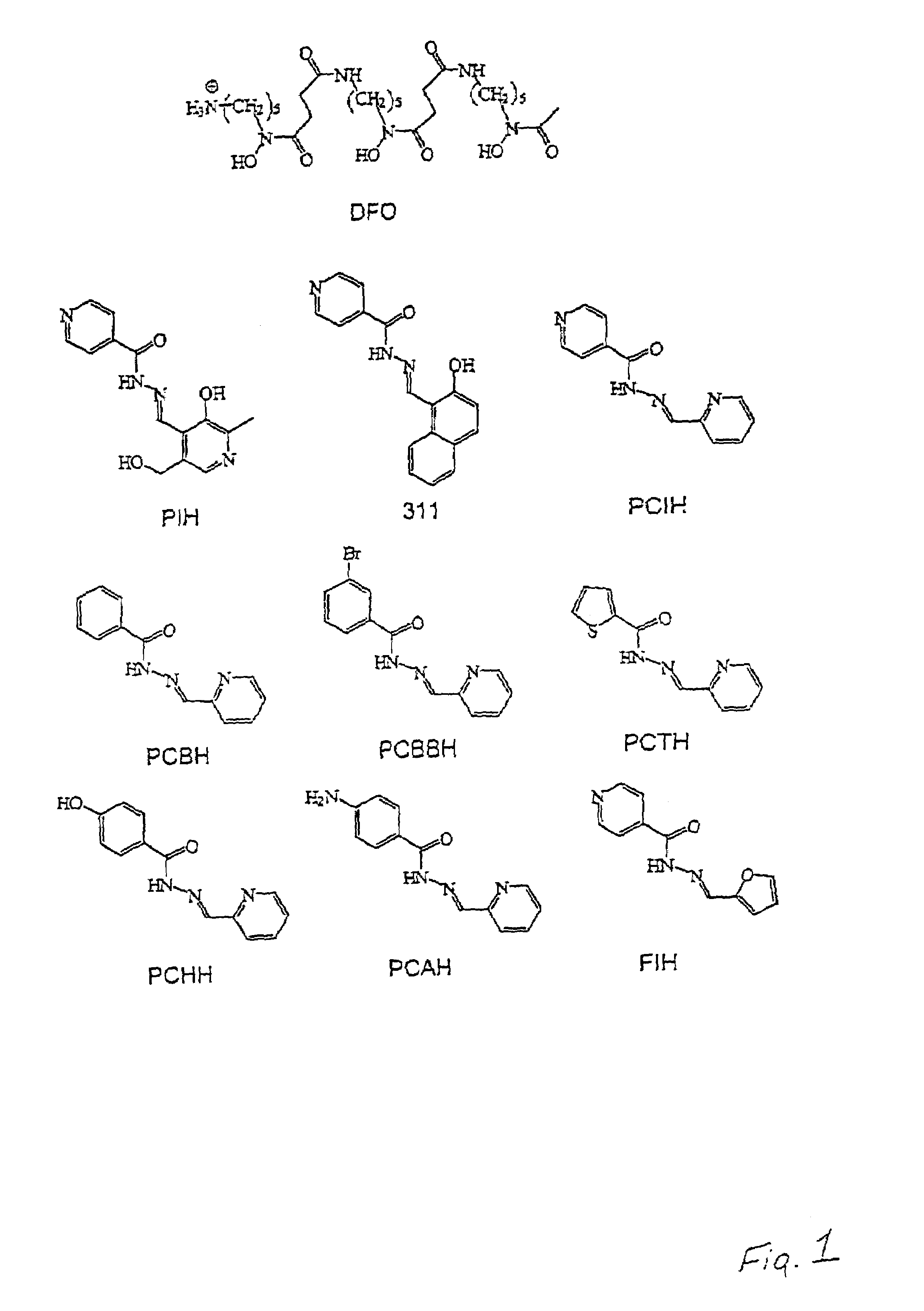
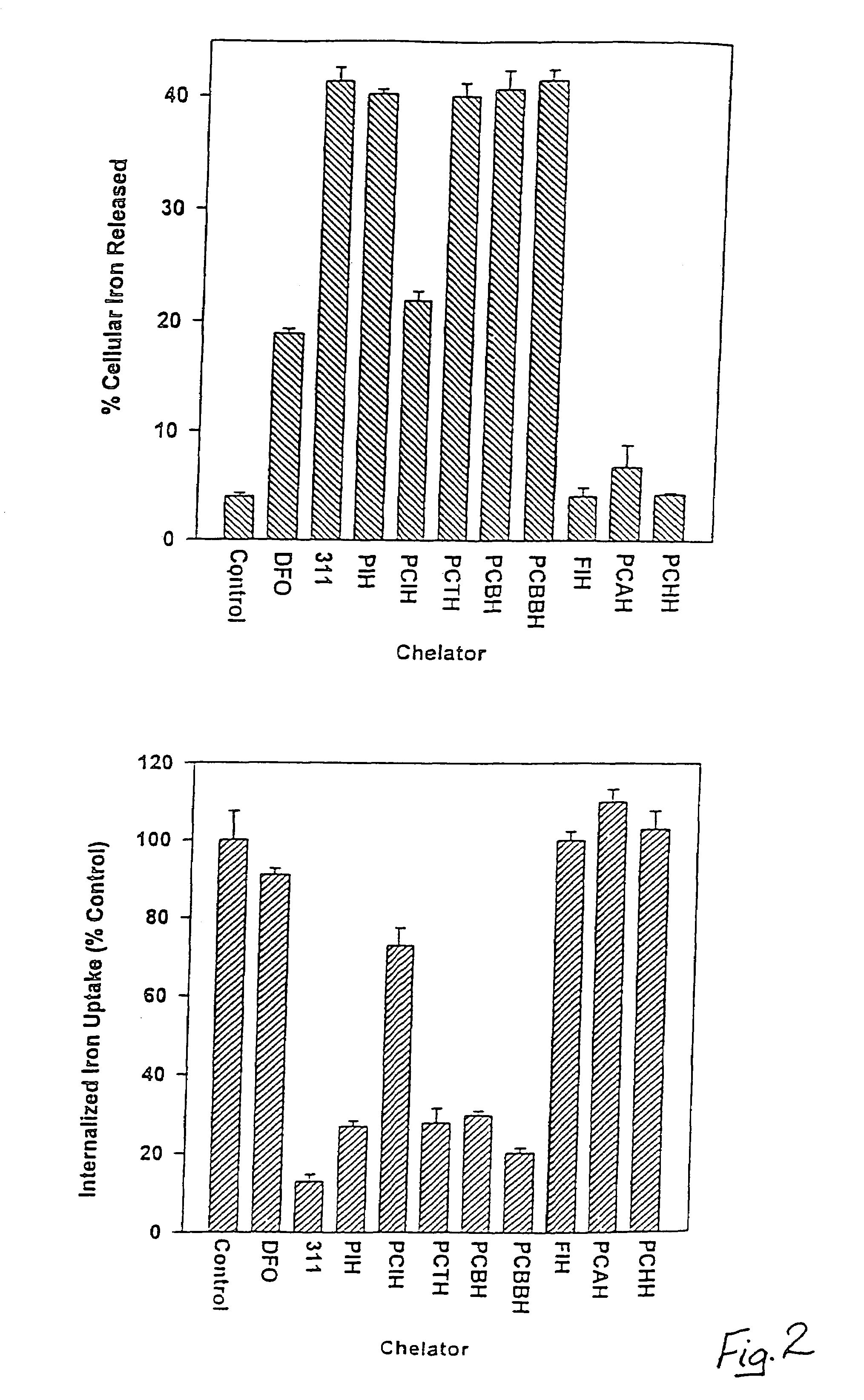
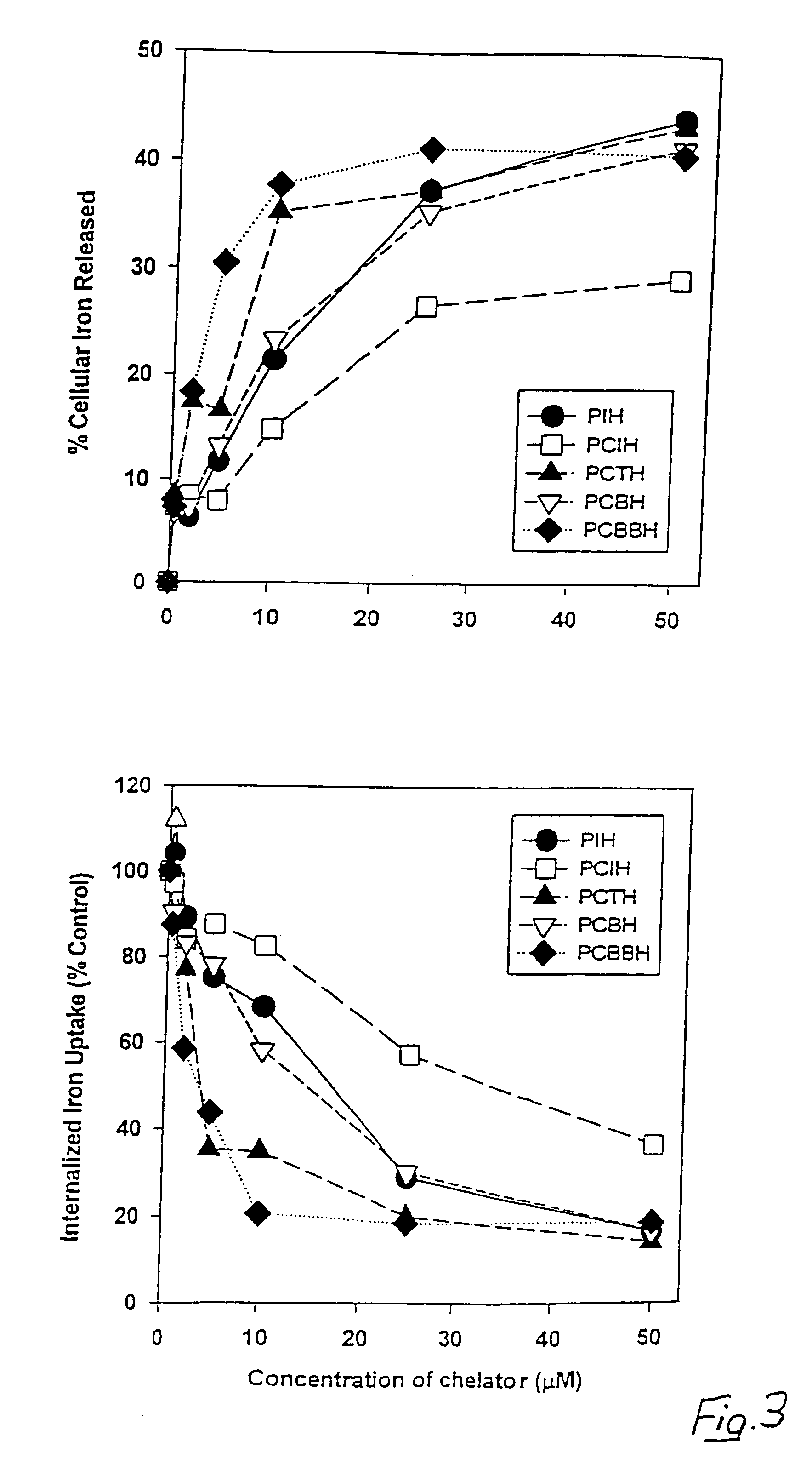
Iron chelators and uses thereof
The present invention provides 2-pyridylcarboxaldehyde isonicotinoyl hydrazone (PCIH) analogues suitable for use as an in vivo iron chelators, the PCIH analogue having Formula 1: wherein R1 is an aromatic or heterocyclic group and R2 is either H or OH; isomers thereof or salts thereof; pharmaceutical compositions containing the analogues; and uses of the analogues in the treatment of iron-overload diseases.
Owner:THE UNIV OF QUEENSLAND +1
Crystalline oxotitanylphthalocyanine and electrophotographic photoreceptor using the same
An object of the invention is to improve the photosensitivity characteristics, characteristics on repeated use and stability of a photoreceptor. A photosensitive layer formed on a conductive support of a photoreceptor contains crystalline oxotitanylphthalocyanine having major peaks in an X-ray diffraction spectrum at Bragg angles (2theta±0.2°) of 7.3°, 9.4°, 9.6°, 11.6°, 13.3°, 17,9°, 24.1° and 27.2°, wherein a peak bundle formed by overlapping the peaks at 9.4° and 9.6° is the largest peak, and the peak at 27.2° is the secondary largest peak as a charge generating substance. Furthermore, it contains a bisamine compound, an N,N'-bisenamine compound, a styryl compound, an amine-hydrazone compound, a benzofuran-bishydrazone compound, a bisenamine compound, a benzofuran-bishydrazone compound or a benzofuran-bis-cyclic hydrazone compound represented by the particular structural formulae as a charge transporting substance.
Owner:SHARP KK
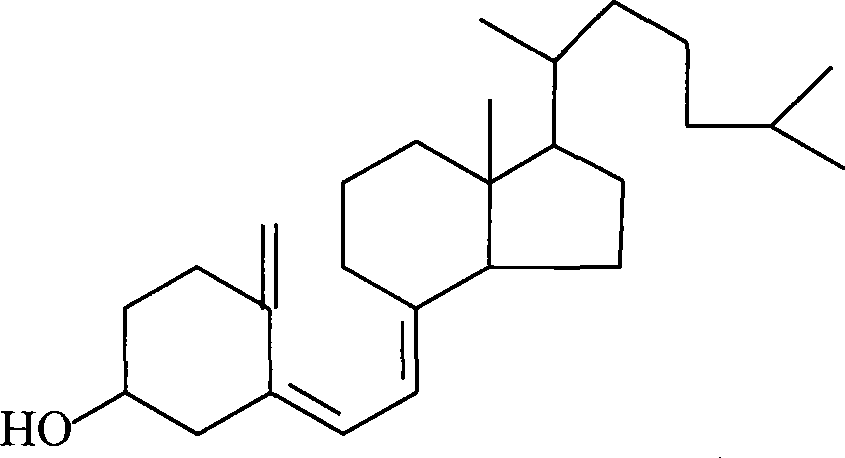
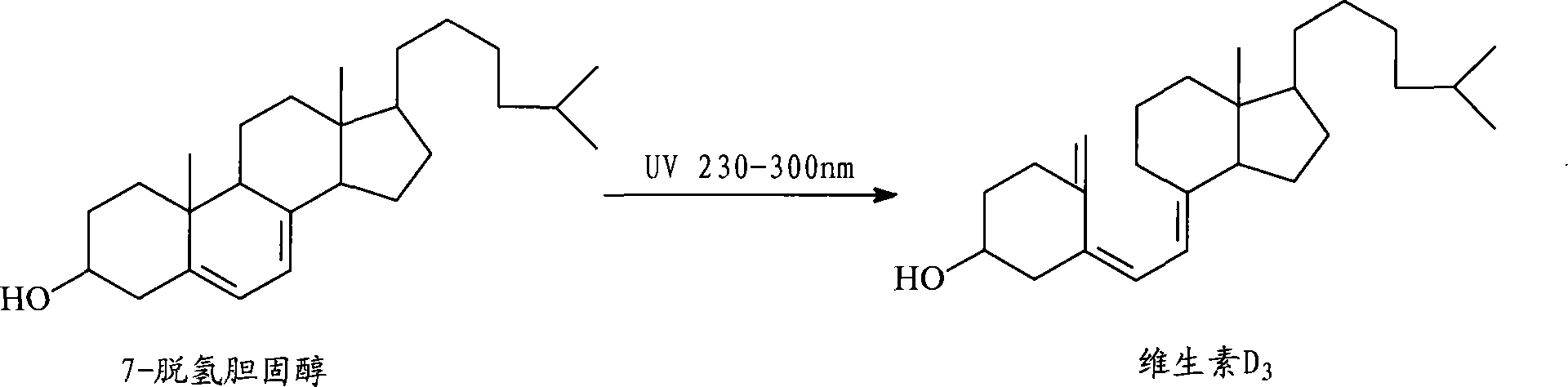
Preparation method for 7-dehydrochol esterol
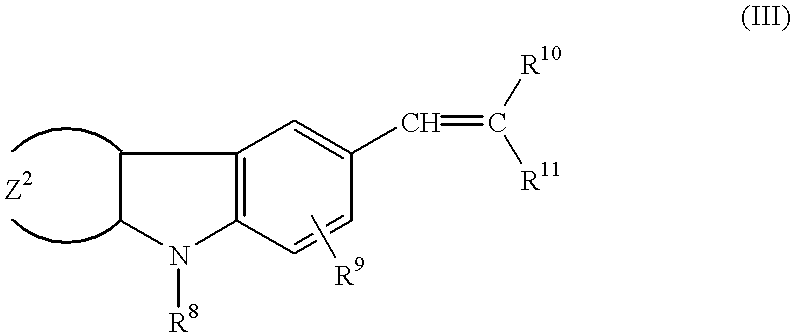
The invention discloses a preparation method of a 7-dehydrocholesterol, which comprises the following steps: a) protecting a hydroxyl on the 3 position of a cholesterol; b) oxidizing a carbon on the 7 position of the product made in step a) into a carbonyl; c) carrying out hydrazone reaction to the product made in step b) with hydrazine derivatives, the structure of the hydrazine derivatives being at most one H to be substituted by the following hydrazine; d) reacting the product made in step c) with a strong basic reagent having stronger alkaline than a sodium hydroxide; e) reacting the product made in step d) with water or an acid. In the preparation method, no halogen group elements such as Br element are participated, the side effects are less and the yield is high, and the prepared product is safe and innocuous.
Owner:BEIJING UNIV OF CHEM TECH
Fire ant bait agent and method of use thereof
The invention relates to a medicine for killing solenopsis invicta buren and the use method thereof. The medicine comprises 0.0025%-5.0000% of insecticidal active constituent according to mass percent, which is selected from one of or the mixture of the following component: fipronil, fluorine insects amines, spinosad, Arab-Israeli (Iraq) ivermectin, vants hydrazone, imidacloprid, rotenone, matrine, Yanggakdo twisting glycosides, cicuta alkali, boric acid; 15%-25% of glucide, 10%-25% of thickening agent, 0.005%-6% of menstruum, 0.01%-0.03% of preservative and the rest of water. The use method of the solenopsis invicta buren baits is that: pouring suitable quantity baits agent into a container with nozzle, and diluting the baits agent with water, then agitating the anthill lightly, and sprinkling the water-based baits agent on the body surface of the ergates and the soil above the anthill surface after a mass of ergates come out of the anthill. The manufacturing process of the medicine is simple, the cost is lower, and the use method is suitable for large-scale spraying.
Owner:惠州市南天生物科技有限公司
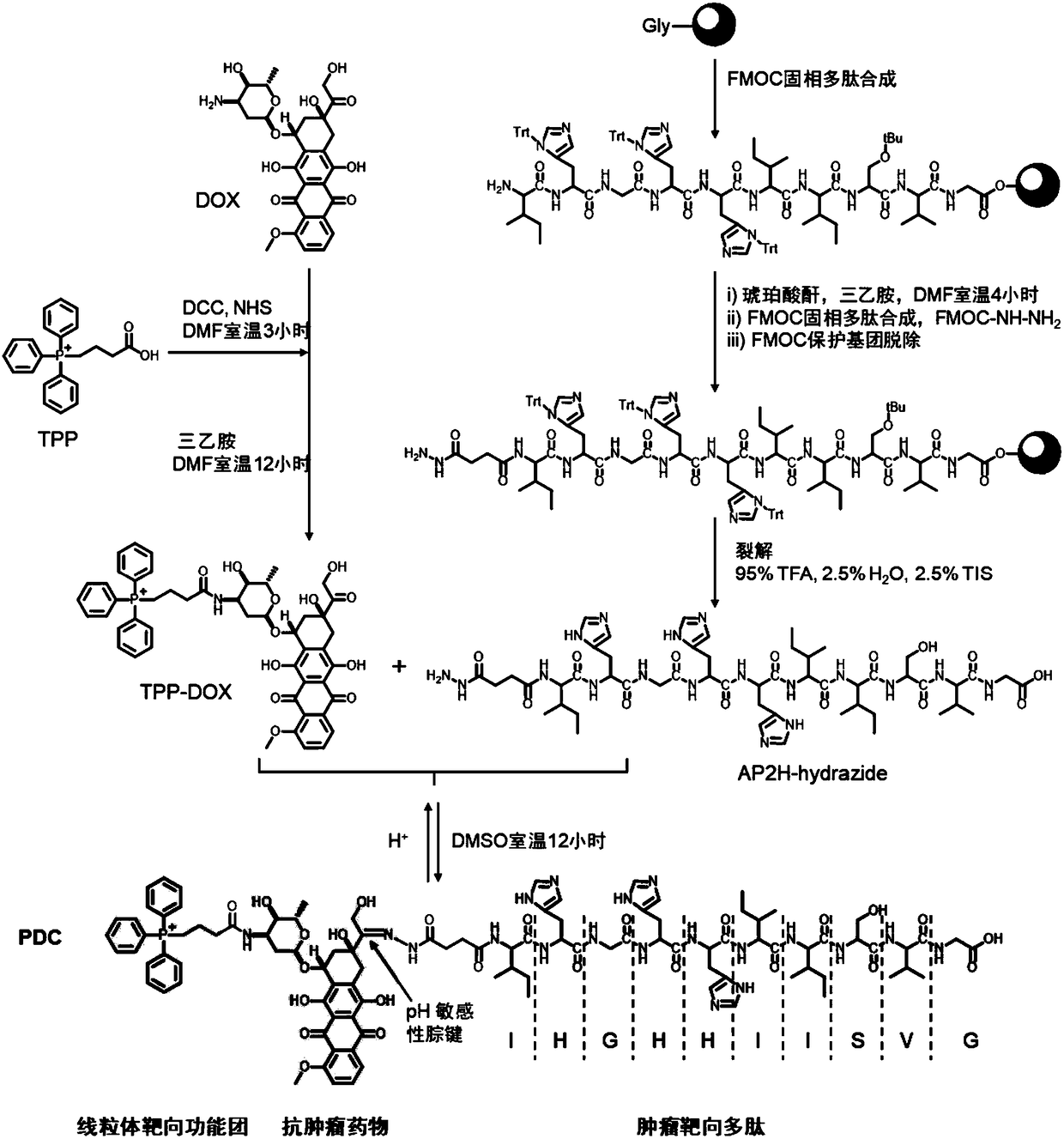
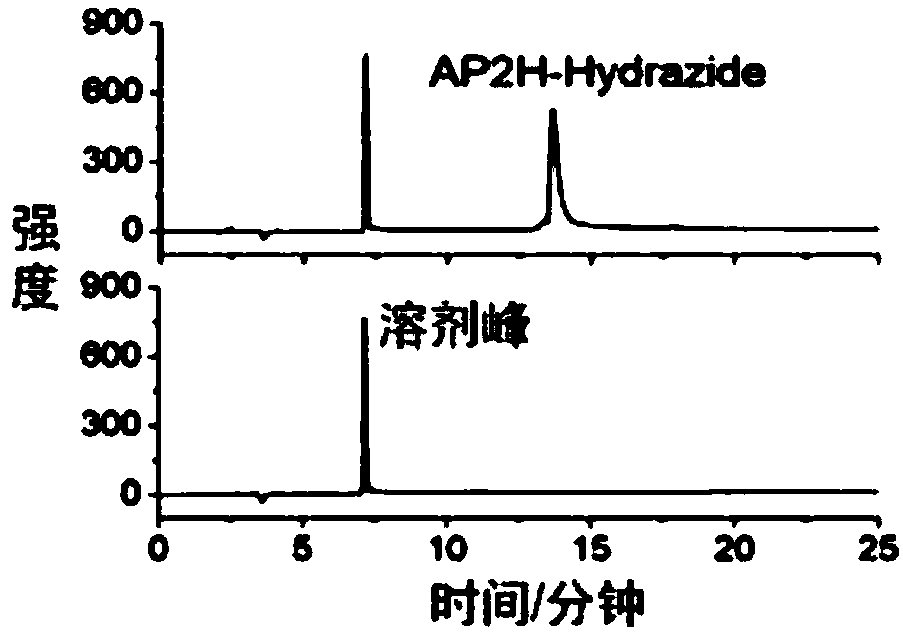
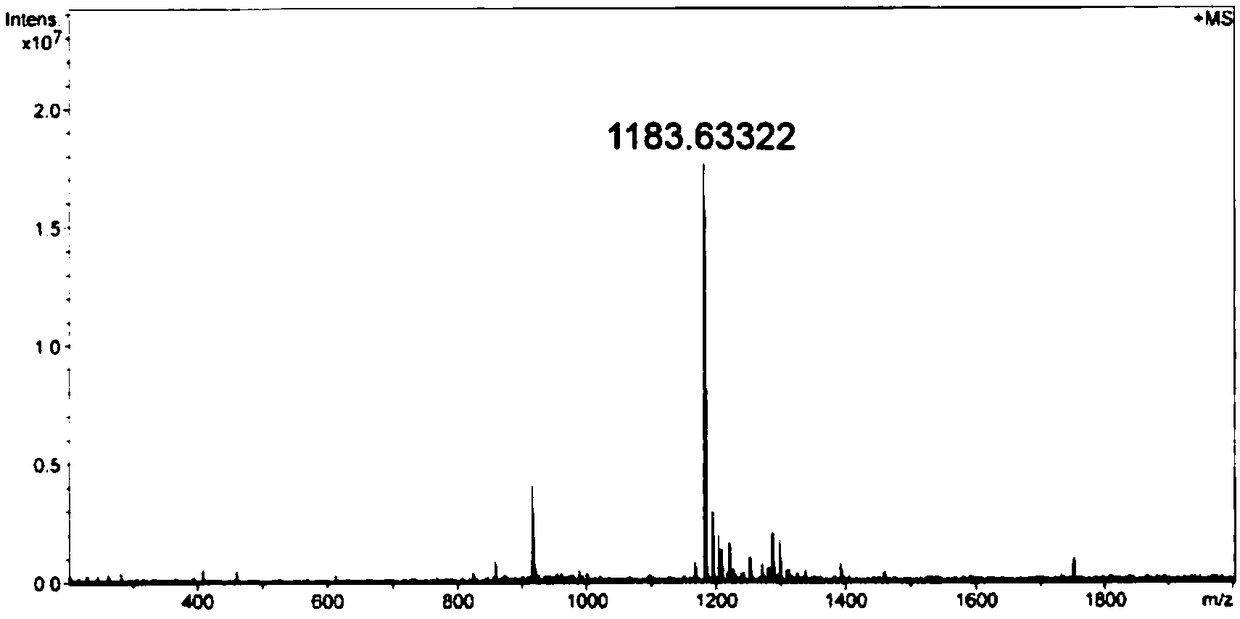
Double-targeting polypeptide-antibody-drug conjugate, and prepared method and antineoplastic application thereof
InactiveCN108578708AIncreased selective uptakeOvercome toxicityOrganic active ingredientsPharmaceutical non-active ingredientsChemical LinkageMitophagy
The invention discloses a double-targeting polypeptide-antibody-drug conjugate, and a prepared method and antineoplastic application thereof. The double-targeting polypeptide-antibody-drug conjugate structurally includes four components of (A), tumor specificity targeting polypeptide; (B), an antineoplastic drug; (C), a mitochondria target functional group; and (D), hydrazone bonds used for connecting the polypeptide and the antineoplastic drug. The tumor specificity targeting polypeptide is connected with the antineoplastic drug by a connecting arm comprising the hydrazone bonds; the antineoplastic drug is connected with the mitochondria target functional group by chemical bonds; and the tumor specificity targeting polypeptide is suitable for targeting the conjugate to a tumour cell and marker protein LAPTM4B carried on the surface of the tumour cell is used as a specificity target. A molecular target is combined with an organelle target, selective uptake of the drug in the tumour cell can be increased, an acting site of a DOX drug can be transferred form cell nucleus to mitochondria, and therefore the condition that the tumour cell is killed by drug resistance is avoided.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Antitumor prodrug and preparation method thereof
ActiveCN102276826AGood biocompatibilityPromote degradationSugar derivativesSugar derivatives preparationSolubilitySide effect
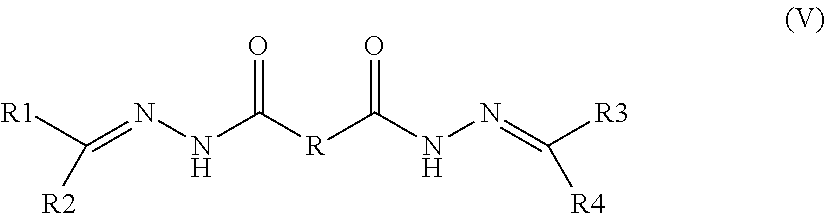
The invention provides antineoplastic prodrugs with structures represented by formulas (I), (II), (V), (VI), (VII), or (VIII), and a preparation method thereof. According to the invention, polyethylene glycol or polyethylene glycol monomethyl ether is adopted as a carrier, and anthracycline antineoplastic drugs are bonded on the carriers through ester bonds, amide bonds or hydrazone bonds, such that the antineoplastic prodrugs are obtained. According to the antineoplastic prodrugs provided by the invention, polyethylene glycol or polyethylene glycol monomethyl ether is adopted as a carrier, such that the drug-loading of the anthracycline antineoplastic drugs is improved, toxic and side-effects are reduced, and the bioavailability is improved. Polyethylene glycol or polyethylene glycol monomethyl ether has good biocompatibility and biodegradation property. When polyethylene glycol or polyethylene glycol monomethyl ether is used as an antineoplastic prodrug carrier, no harm is brought to human bodies. With polyethylene glycol or polyethylene glycol monomethyl ether, anthracycline antineoplastic drug dissolvability and circulation period in human bodies can be improved. Therefore, the method assists in improving the performances of anthracycline antineoplastic drugs.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Electrophotographic organophotoreceptors with novel charge transport materials
InactiveUS6905804B2High quality imagingQuality improvementOrganic chemistryOrganic compound preparationArylHydrazone
Owner:S PRINTING SOLUTION CO LTD
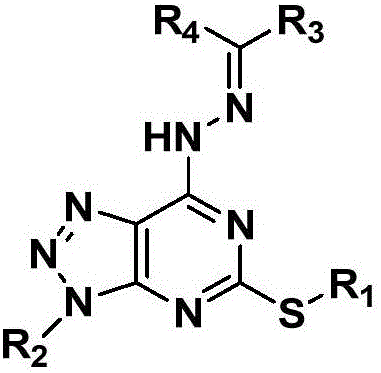
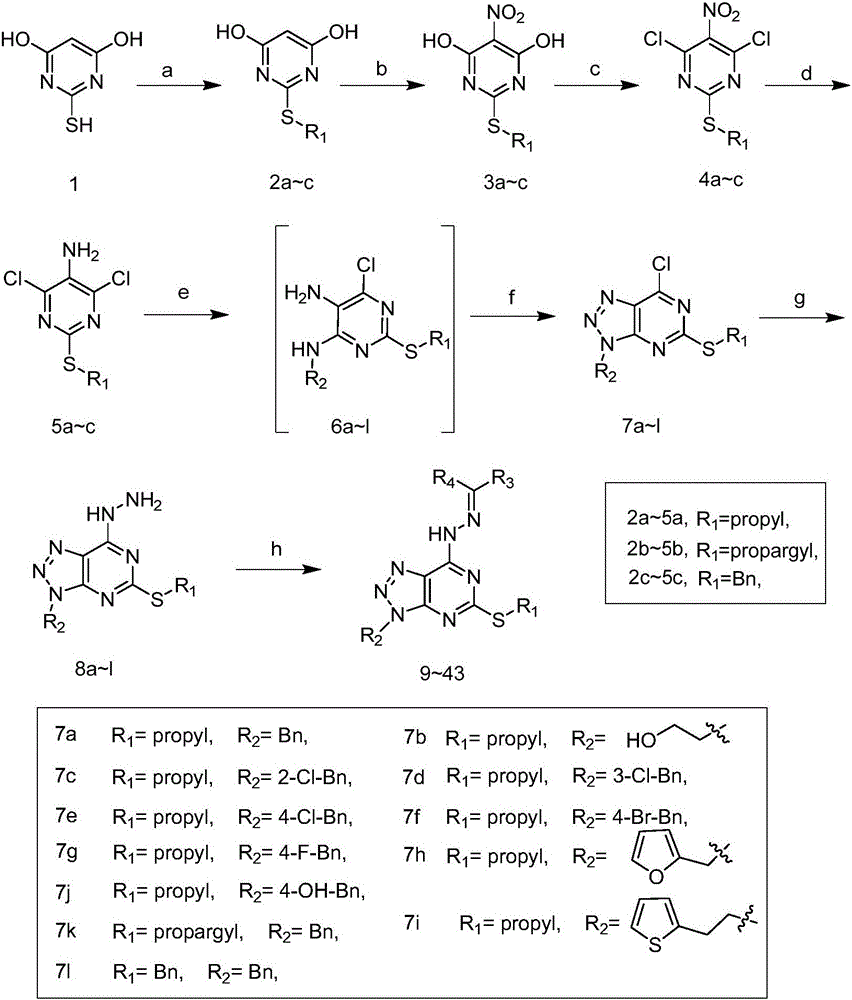
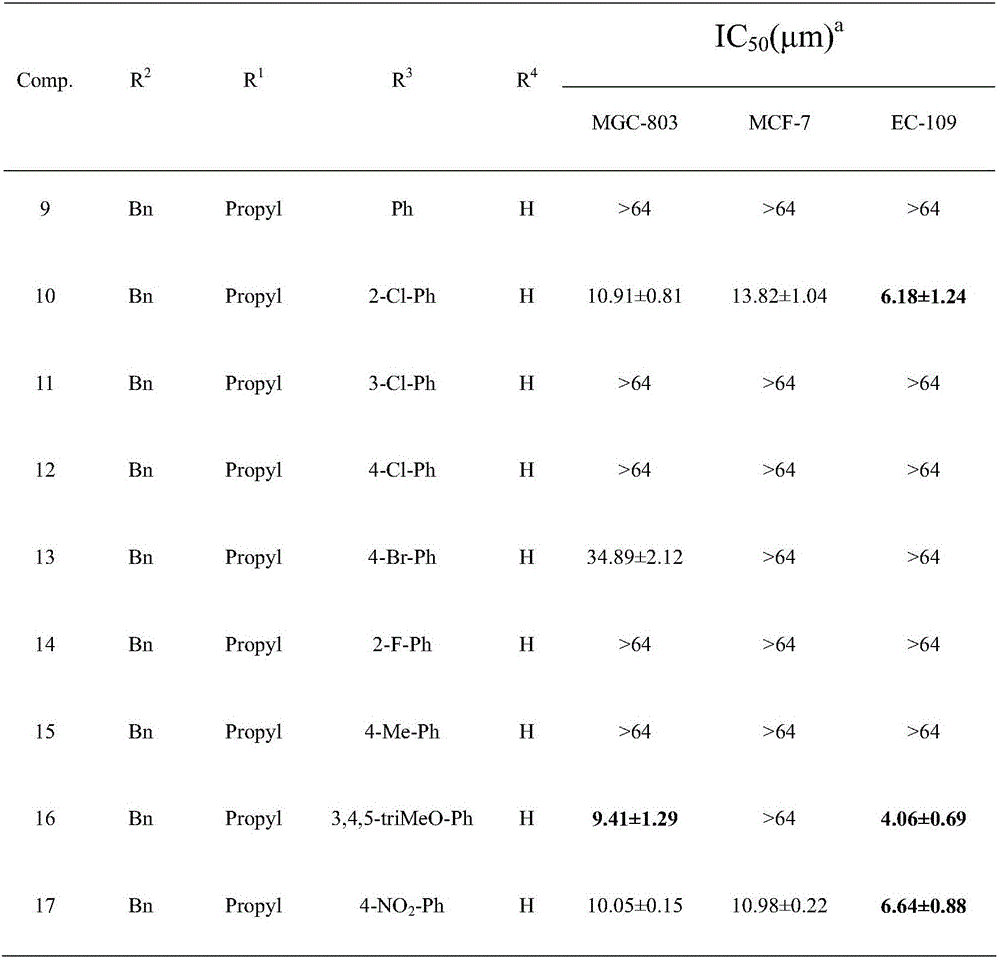
Pyrimidotriazole compounds containing hydrazone bonds as well as preparation method and application of pyrimidotriazole compounds
InactiveCN106432247AEnhanced inhibitory effectSignificant in vitro antitumor activityOrganic chemistryAntineoplastic agentsHydrazoneDrugs preparations
The invention belongs to the field of medicinal chemistry and discloses pyrimidotriazole compounds containing hydrazine bonds as well as a preparation method and an application of the pyrimidotriazole compounds in drug preparation. The general formula I of the compounds is shown in the specification. The compounds have remarkable inhibition and killing functions on multiple tumor cells such as MGC-803, MCF-7 and EC-109, can serve as candidate or lead compounds for further development and are applied to preparation of an anti-tumor drug.
Owner:ZHENGZHOU UNIV
Functional biopolymer modification reagents and uses thereof
InactiveUS7102024B1Accurate measurementImprove the immunityGroup 4/14 element organic compoundsSugar derivativesHydrazoneBiopolymer
Hydrazino, oxyamino and carbonyl-based reagents and methods for incorporation into oligonucleotides during their solid phase synthesis are provided. Modified oligonucleotides are provided that incorporate the reagents provided here in. Immobilized oligonucleotides and oligonucleotide conjugates that contain covalent hydrazone or oxime linkages are provided. Methods for preparation of surface bound oligonucleotides are provided. Methods for the preparation of oligonucleotide conjugates are also provided.
Owner:VECTOR LAB +1
Medicinal uses of hydrazones
InactiveUS20040053977A1Increasing endogenous EPO and vascularizationIncreased vascularizationBiocideNervous disorderGynecologyHydrazone
Owner:ALMSTEAD JI IN KIM +3
Functional reactive dye for zinc ion probe, and preparation method and application thereof
InactiveCN105400233AWith zinc ion probe functionHas functional reactive dye propertiesReactive dyesDyeing processStructural formulaSolvent
The invention relates to a functional reactive dye for a zinc ion probe, and a preparation method and application thereof. The dye has a structural formula as described in the specification. The preparation method comprises the following steps: subjecting rhodamine B and hydrazine hydrate to a heating reflux reaction so as to obtain rhodamine B hydrazide; subjecting methyl p-aminobenzoate and hydrazine hydrate to a heating reflux reaction, dissolving a product in a solvent, adding salicylaldehyde drop by drop and then carrying out a heating reflux reaction so as to obtain salicylaldehyde-4-aminobenzoyl hydrazone; and dissolving cyanuric chloride in a solvent, adding an acid binding agent, adding rhodamine B hydrazide drop by drop at a temperature in a range of -5 to 5 DEG C, carrying out a reaction under stirring so as to obtain a concentration product, dissolving the concentration product in a solvent, adding the acid binding agent, adding salicylaldehyde-4-aminobenzoyl hydrazone drop by drop and carrying out a reflux reaction with temperature controlled so as to obtain the functional reactive dye. The functional reactive dye has good selectivity on zinc ions, is convenient to use in sewage treatment and exerts good usage effect.
Owner:DONGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com