Functional reactive dye for zinc ion probe, and preparation method and application thereof
A reactive dye and functional technology, applied in reactive dyes, dyeing methods, azo dyes, etc., can solve the problems of not indicating the ion detection range, etc., and achieve the effects of low cost, good selectivity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Synthesis of rhodamine B hydrazide
[0042] Take a 100mL three-neck flask, weigh 1.200g (2.5mmol) Rhodamine B and dissolve it in 30mL absolute ethanol, stir vigorously at room temperature and slowly add 1mL (19.8mmol excess) 98% hydrazine hydrate dropwise. Heat to reflux at 78°C for 2 hours until the solution turns from dark purple to clear yellow. After the reaction is completed, cool to room temperature, and use a rotary evaporator to evaporate the solvent and excess hydrazine hydrate to obtain a light yellow crude product of rhodamine B hydrazide. Take 50mL of newly prepared 1M HCl and add it to the crude product, and the product dissolves into a pink solution. Slowly add 1M NaOH to the solution under stirring to adjust the pH value to between 9-10. When NaOH was added dropwise to the mixed solution, when the pH of the solution was adjusted to about 6, pale pink flocculent precipitates began to precipitate out. Filter and wash the precipitate with 15ml deioniz...
Embodiment 2
[0049] Synthesis of Functional Reactive Dyes as Zinc Ion Probes
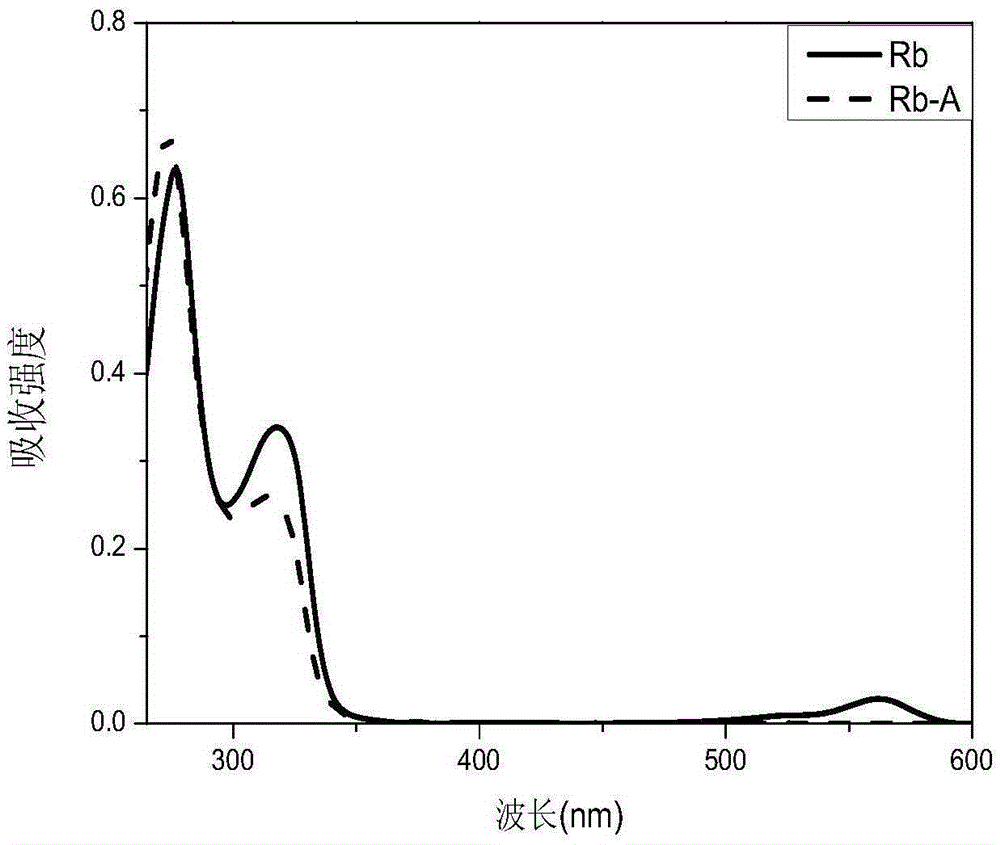
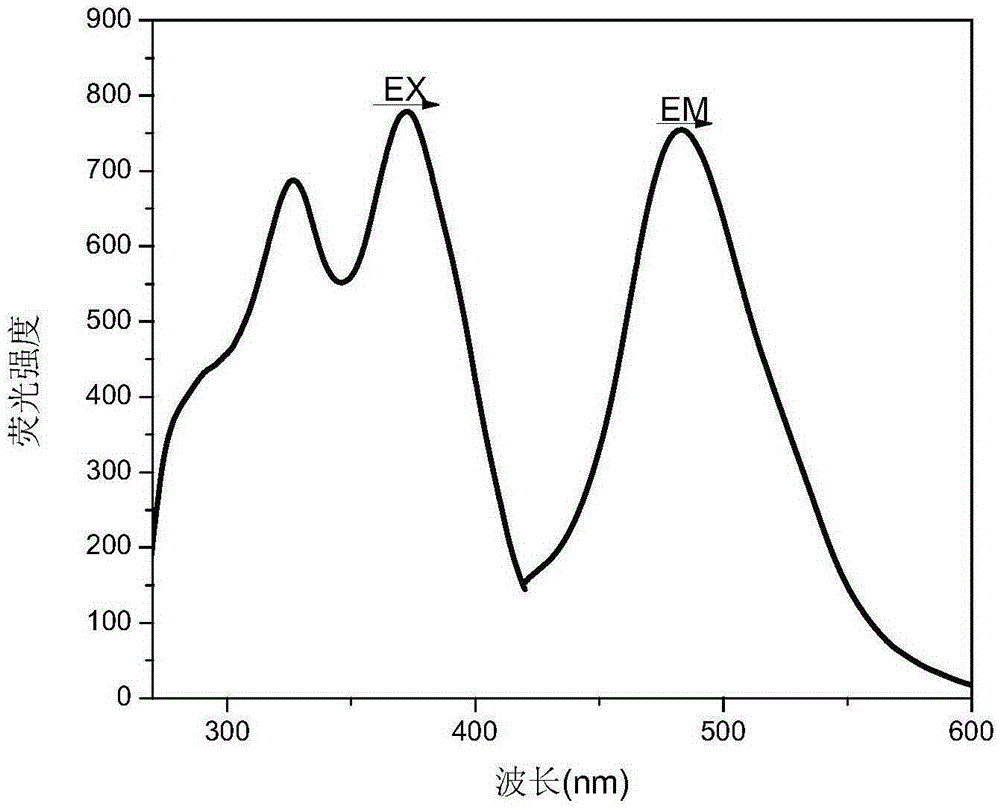
[0050] Take a 250ml three-necked flask, dissolve 603mg (1.00mmol) R1 in 20mL of anhydrous THF, stir in the three-necked flask and place it in an oil bath, N 2 Under protection, the temperature was maintained at 42°C. Weigh 0.16gNaHCO 3 Add to the mixed solution as an acid-binding agent to control the pH value of the system. Take 268 mg (1.00 mmol) of 4-aminosalicylaldehyde benzoylhydrazone P, dissolve it in 40 ml of THF, and slowly drop it into a three-necked flask while stirring. The reaction was carried out under reflux and stirring at 50°C for six hours, followed by TLC running until the reaction of the raw materials was complete. The filtrate was rotary evaporated to remove the solvent, the solid product was washed with a small amount of distilled water several times, and dried in a vacuum drying oven to constant weight to obtain the final product of functional reactive dye of zinc ion probe. figure 2 D...
Embodiment 3
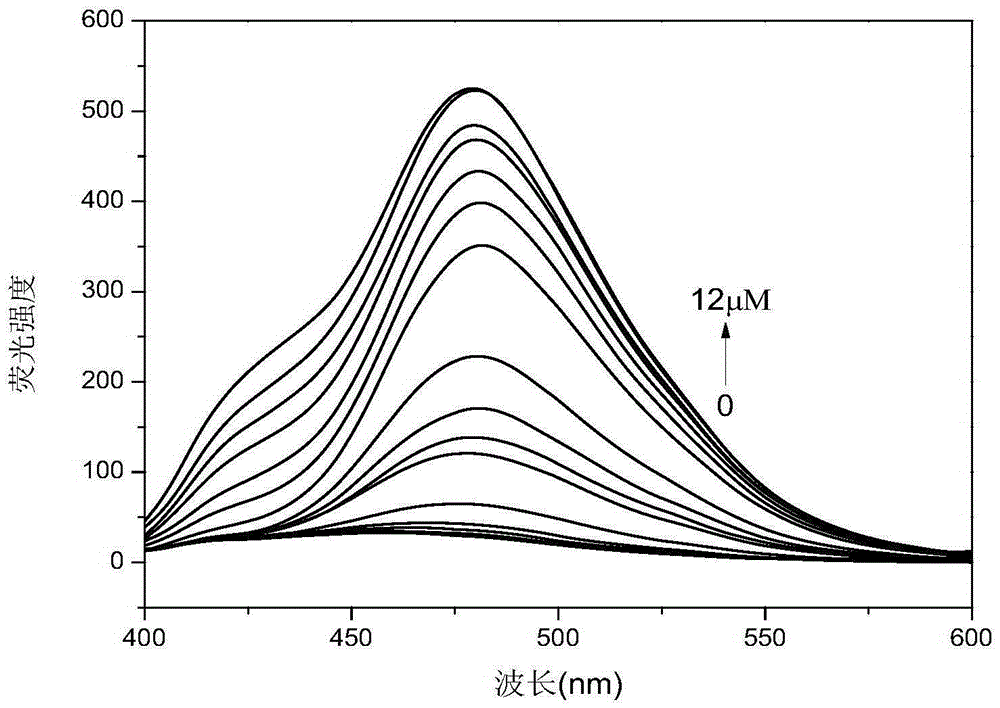
[0055] Add different concentrations of zinc ions to the DMF solution, such as image 3 As indicated, the fluorescence intensity of the liganded fluorescent probe (10 μM) was measured at an excitation wavelength of 372 nm. When adding only 10nM concentration of zinc ions to the fluorescent probe (10μM) solution, the fluorescent probe solution presents a fluorescence emission peak at around 483nm, with the Zn 2+ The intensity of the fluorescence emission peak at 483nm was also significantly enhanced with the increase of ion concentration. When Zn 2+When the ion concentration reaches 10μM, the fluorescence intensity of the ligand fluorescent probe hardly changes with the increase of ion concentration. In the DMF solution of fluorescent probe R2, different metal ions (Zn 2+ 、Cd 2+ 、Sr + 、Cr 3+ , Fe 3+ 、Co 2+ 、Ni 2+ 、Cu 2+ 、Na + 、Ba 2+ , Mn 2+ , Ca 2+ , Pb 2+ , Sn 2+ 、 Bi + , Hg 2+ ) for selective interference detection. Figure 4 It can be seen from the results ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com