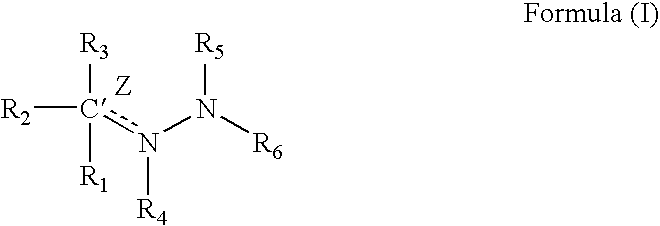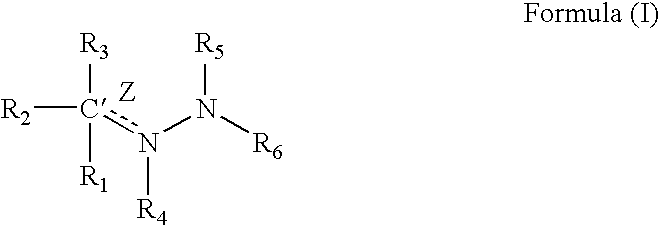Medicinal uses of hydrazones
a technology of hydrazone and hydrazone, which is applied in the field of medicinal uses of hydrazone, can solve the problems of lack of oxygen supply, tissue damage, health problems affecting hundreds of millions of people, etc., and achieve the effects of increasing the transcription of hif-1 target genes, and increasing the biological activity of hif-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-32
[0112] The following chart shows the structure of compounds made according to the procedures described in Examples 1-32.
1TABLE I 9 Example R1 R3 R2 / R4 R5 R6 Z (bond) EC.sub.50.sup.1 1 2-pyridyl H Nil H 2-pyridyl Double 1.6 2 2-pyridyl CH.sub.2 Nil H 2-pyridyl Double 0.65 3 2-pyridyl H Nil CH.sub.3 2-pyridyl Double 0.75 4 2-pyridyl H H / H H 2-pyridyl Single 5.7 5 2-pyridyl CH.sub.3 Nil CH.sub.3 2-pyridyl Double 26.2 6 2-pyridyl H Nil H 2-benzothiazole Double 1.5 7 2-pyridyl H Nil H 2-quinoline Double 3.15 8 2-pyridyl H Nil H 2-(5,7-bis- Double 1.57 trifluoromethyl- [1,8]-naphthyridyl) 9 2-pyridyl H Nil H 3-chloro-6-Double 6.7 pyridazine 10 2-pyridyl H Nil H 3-chloro-6-Double 1.56 trifluoromethyl-2- pyridyl 11 2-pyridyl CH.sub.3 Nil H 3-chloro-6- Double 15 trifluoromethyl-2- pyridyl 12 2-pyridyl H Nil CH.sub.3 4,6-dimethyl-2- Double 4.78 pyrimidine 13 2-pyridyl H Nil CH.sub.3 4-trifluoromethyl- Double 4.3 phenyl 14 2-pyridyl H Nil H 9H-1,3,4,9- Double 2.3 Tetraaza-2-fluorene 15 2-pyrid...
example 1
[0114] N-Pyridin-2-yl-N'-2-ylmethylene-hydrazine (1c): 10
[0115] To a solution of 2-pyridinecarboxaldehyde 1a (10 mmol, 1 equiv.) in ethanol (20 mL) is added 2-hydrazinopyridine 1b (10 mmol, 1 equiv.) to form a 0.5 M solution. The mixture is heated at reflux (60.degree. C.) for 6 hours or until the TLC (66% ethyl acetate / hexanes) shows disappearance of starting material. Upon forming or cooling, the desired product N-pyridin-2-yl-N'-2-ylmethylene-hydrazine 1c, precipitates and is filtered and washed with diethylether. The pale yellow solid is dried under vacuum for 15 hours. Generally, only the first crop is collected, characterized and tested.
[0116] Utililizing substantially the method of Example 1 and appropriate hydrazine, aldehyde, or ketone, the following subject compounds of Examples 2-108 are obtained. Modifications are described below.
example 2
[0117] N-Pyridin-2-yl-N'-(1-pyridin-2-yl-ethylidene)hydrazine: 11
[0118] In a procedure analogous to Example 1, 2-acetylpyridine is combined with 2-hydrazinopyridine to form N-Pyridin-2-yl-N'-(1-pyridin-2-yl-ethyli-dene)hydrazine. N-Pyridin-2-yl-N'-(1-pyridin-2-yl-ethylidene)hydrazine is precipitated out of ethanol with water. The solid is filtered and dried to afford the product as a dihydrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com