Patents
Literature
5816 results about "Quinoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C₉H₇N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.
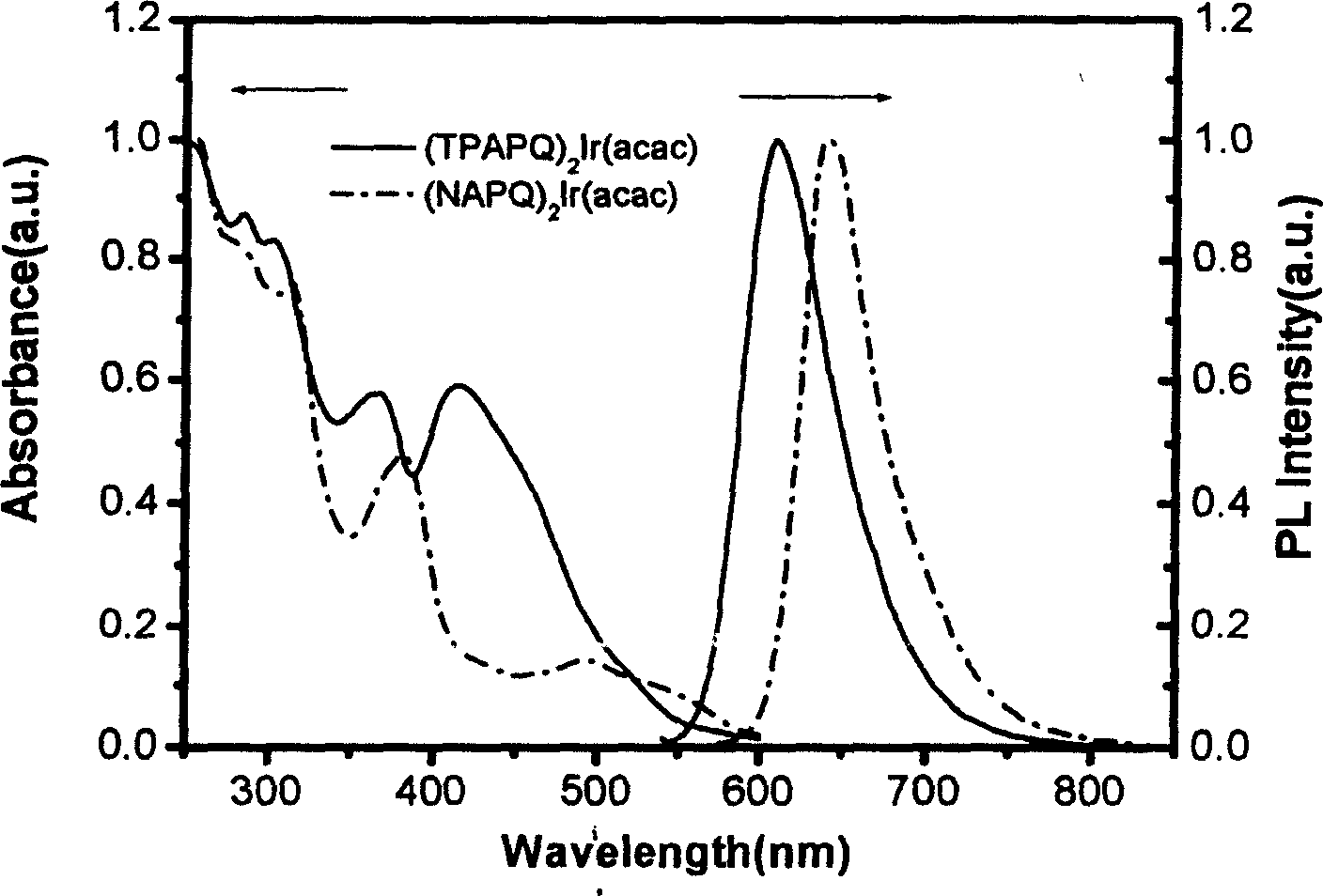
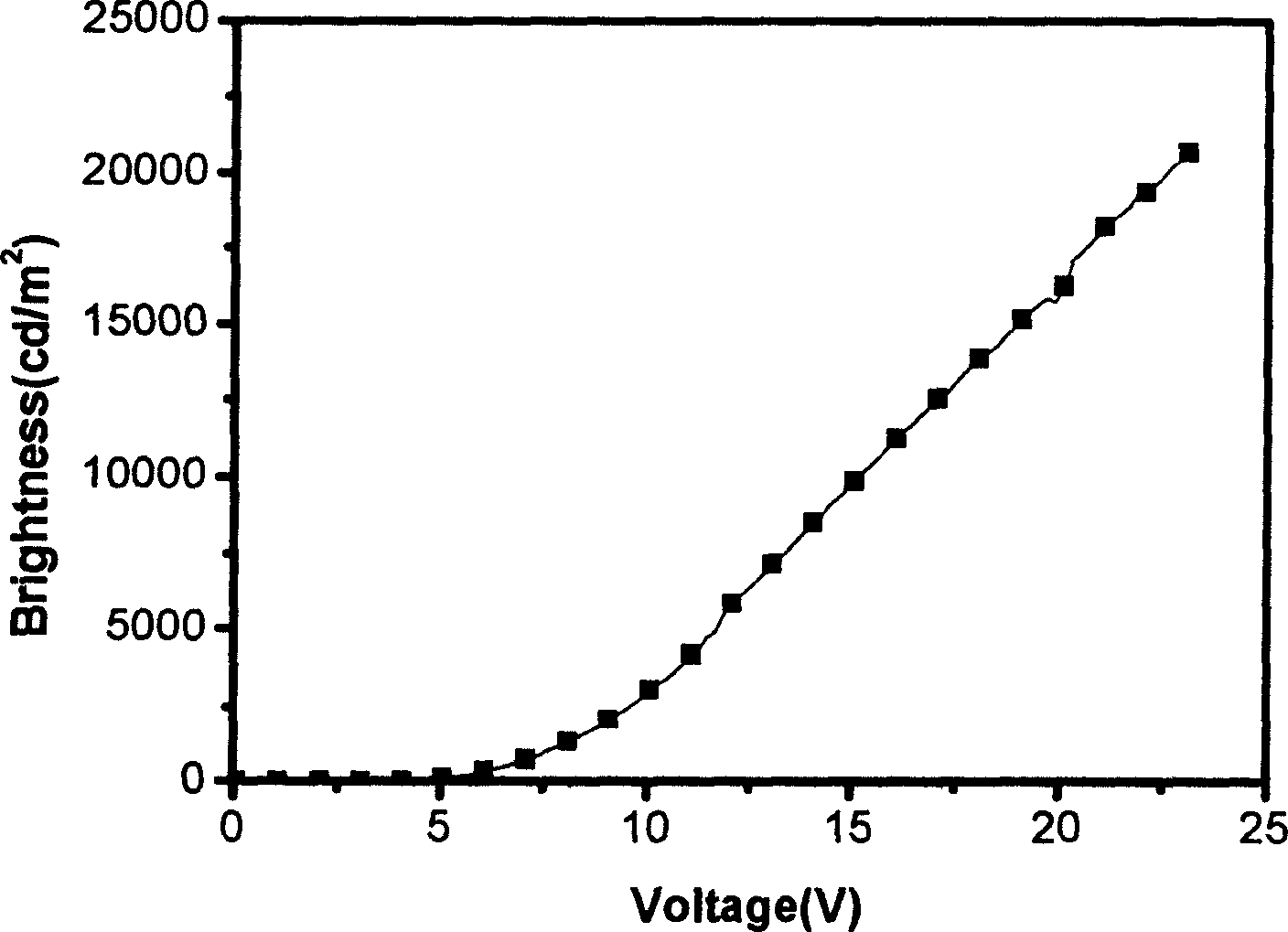
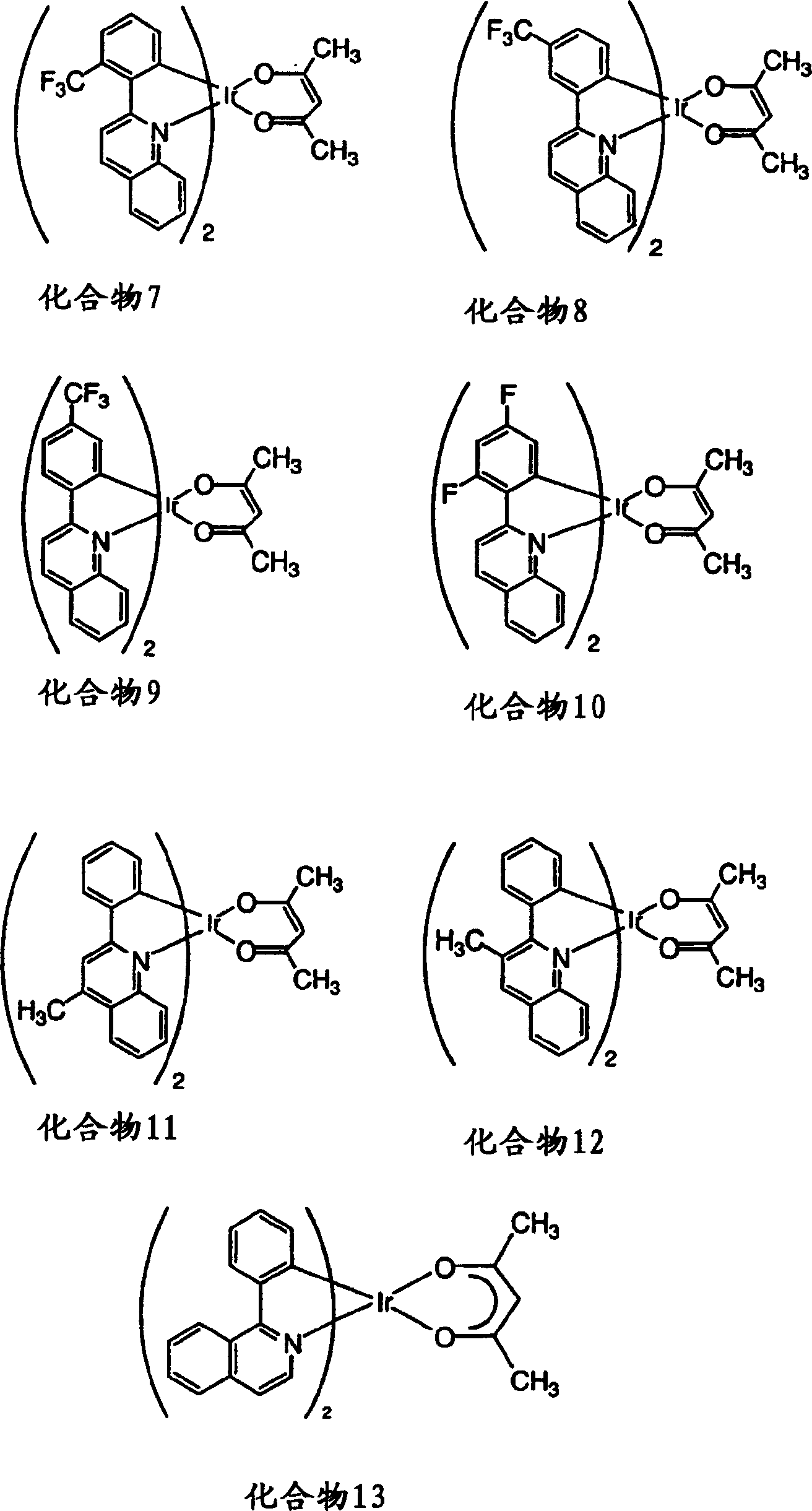
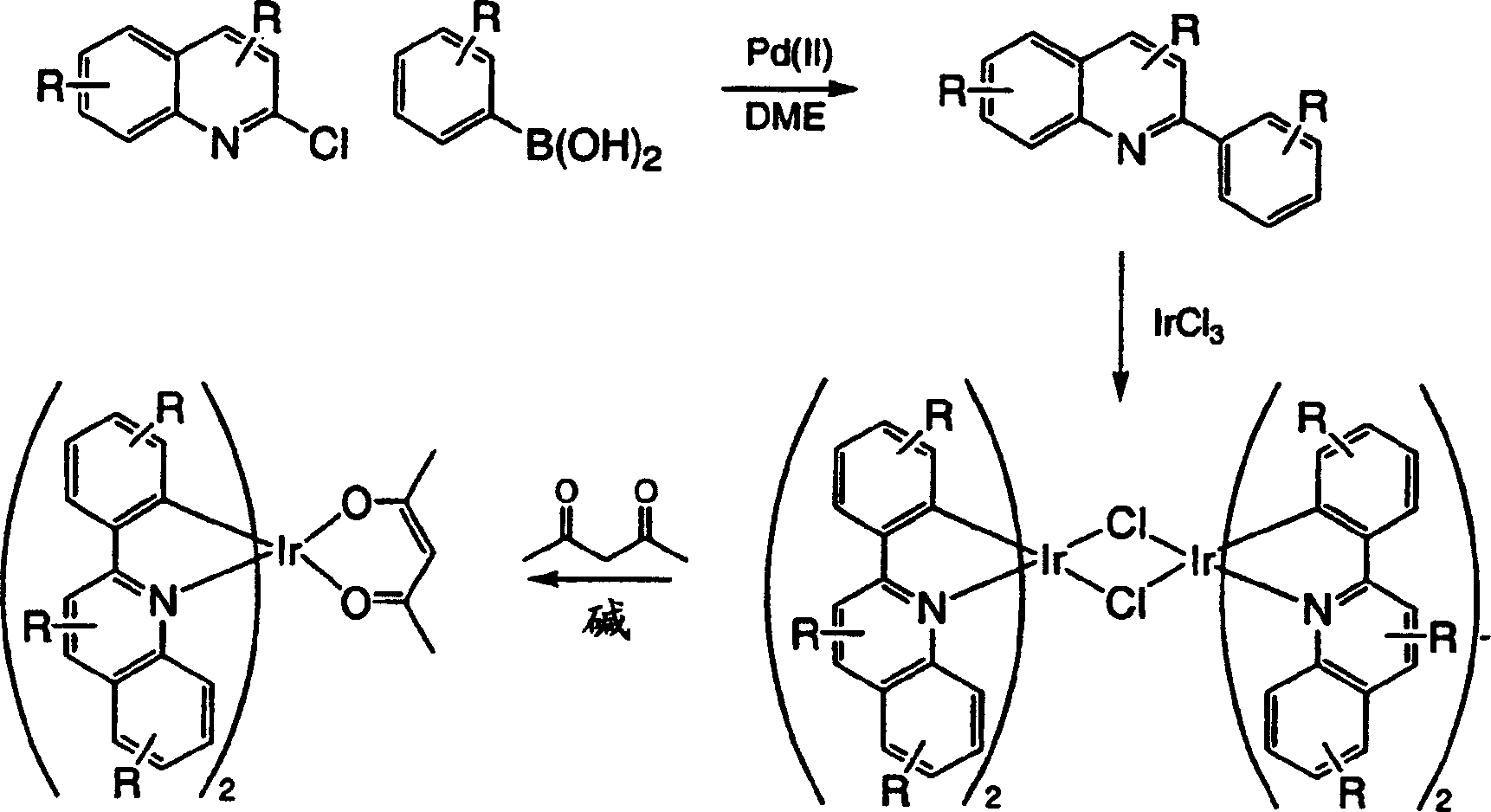
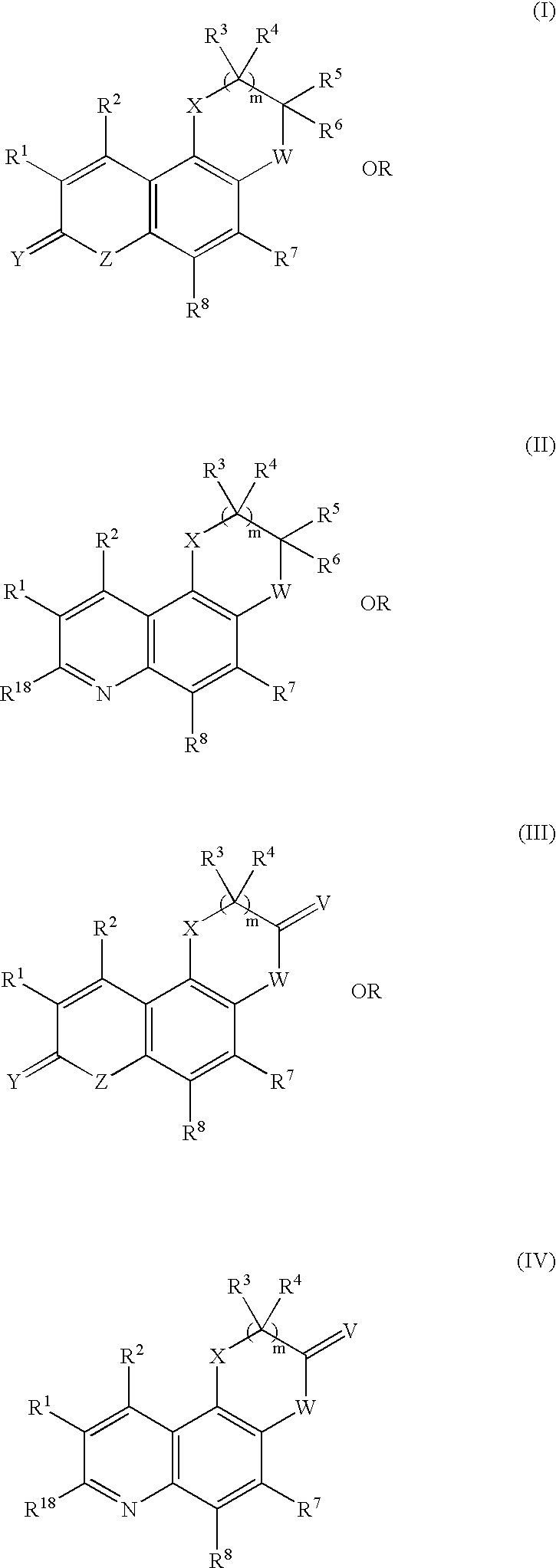
Complexes of red light iridium by using nitrogen heterocycles in quinoline as ligand, and application
ActiveCN1696137AShort lifeImprove efficiencyElectrical apparatusGroup 8/9/10/18 element organic compoundsIridiumNitrogen
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
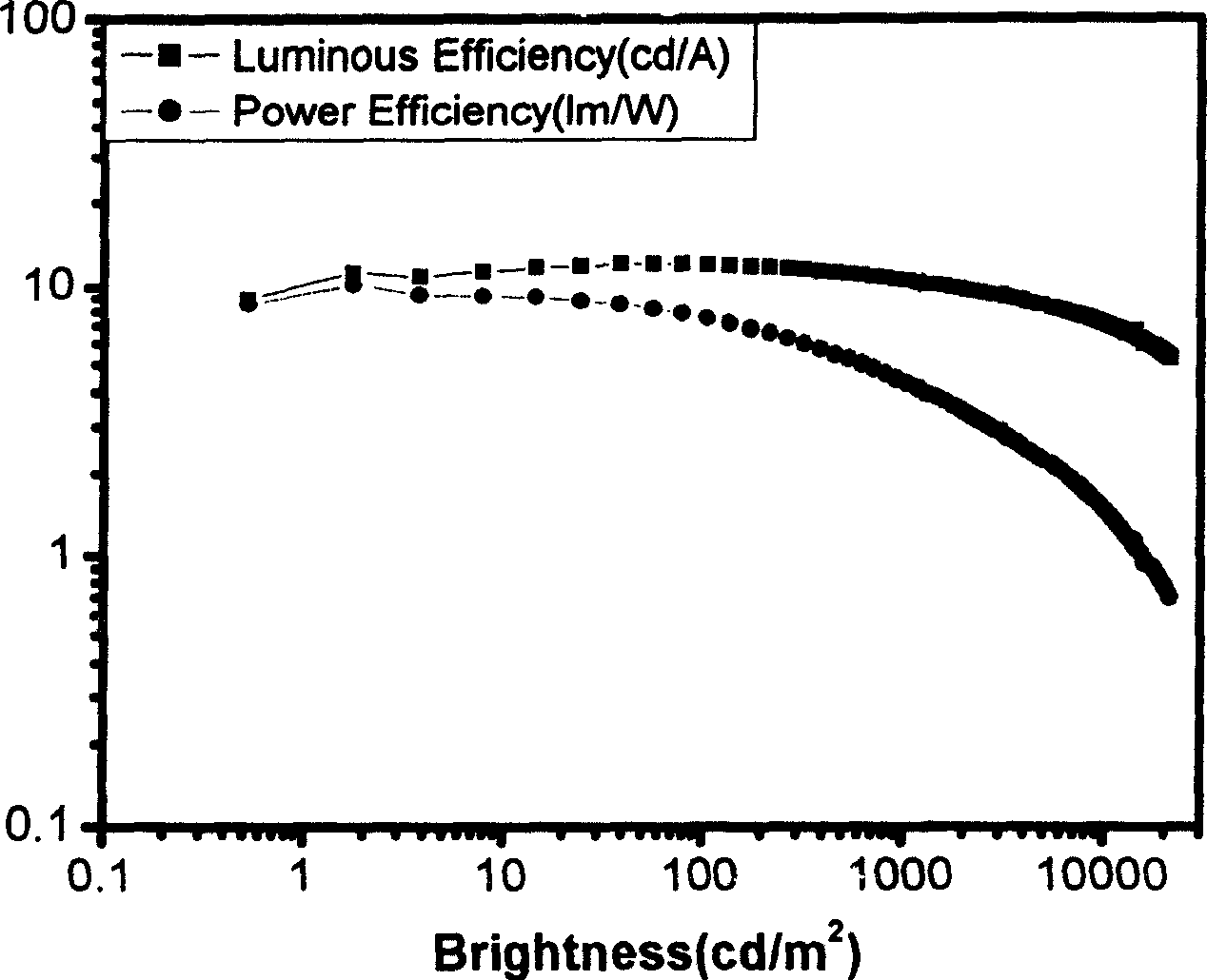
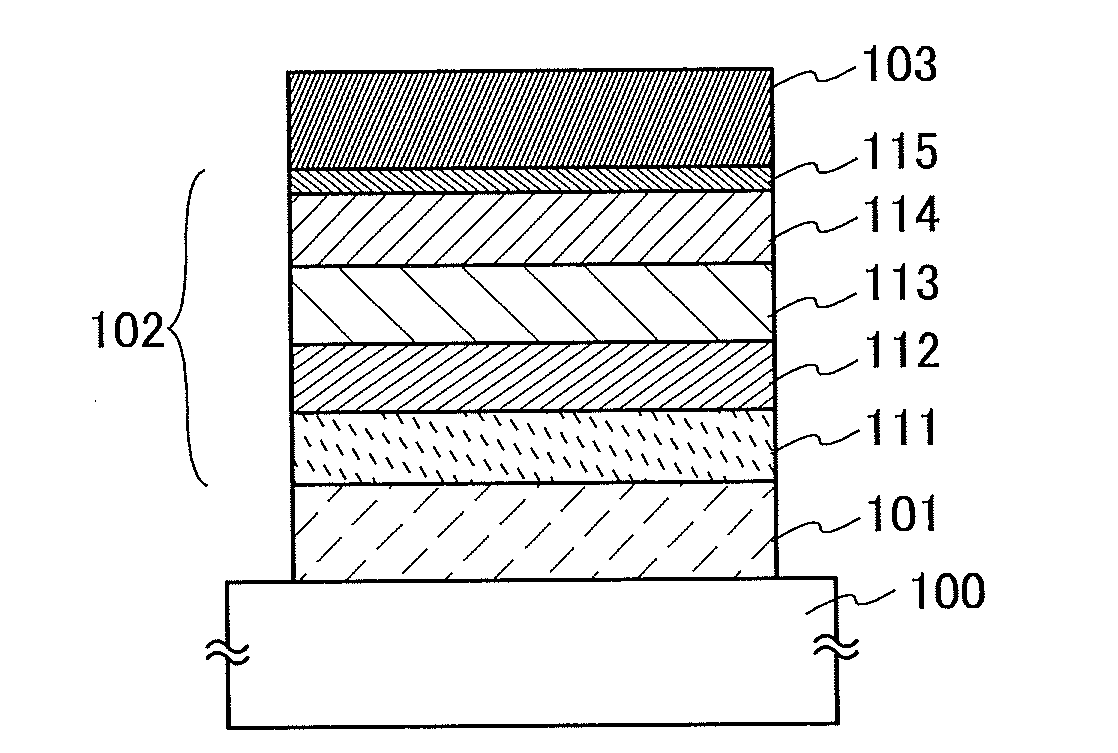
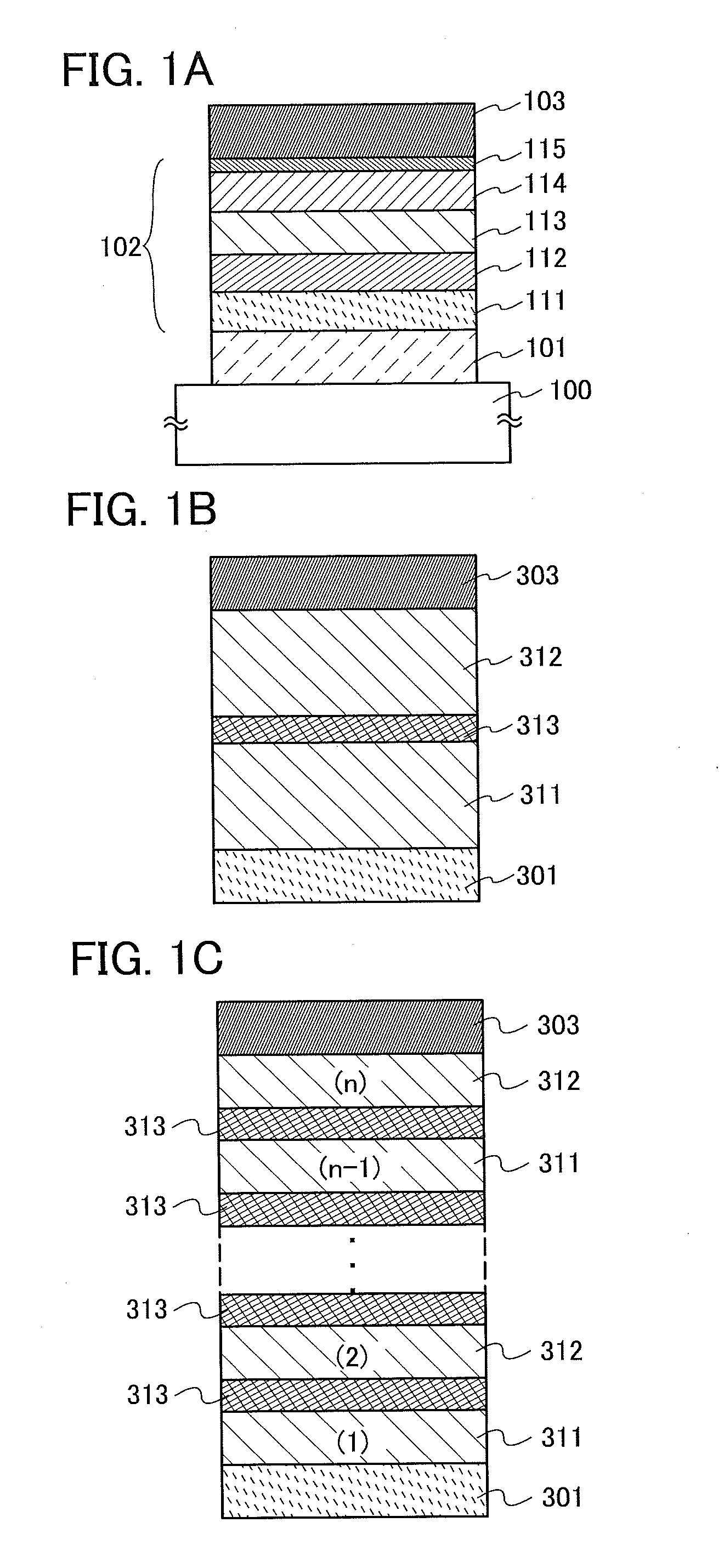
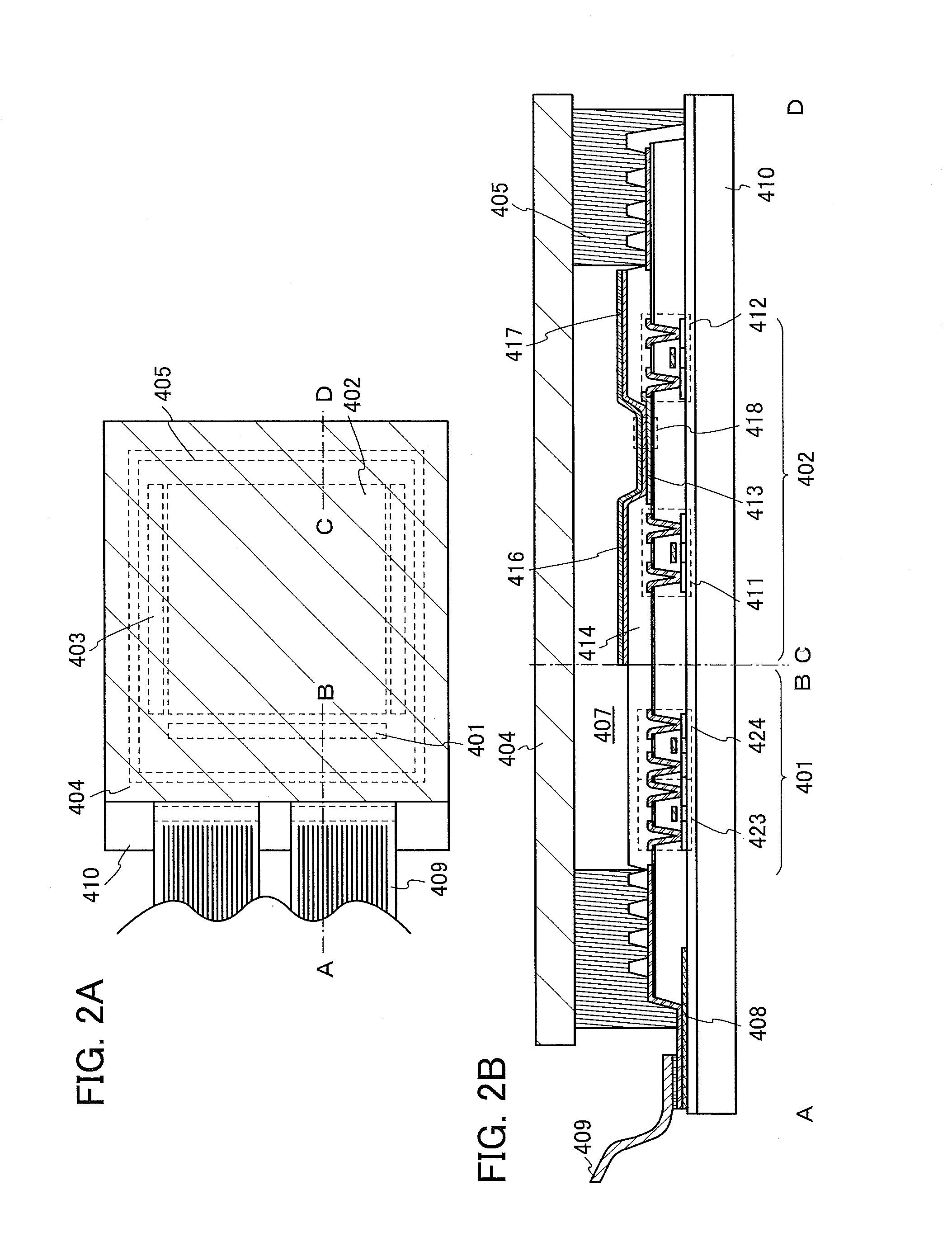
Light-Emitting Element, Light-Emitting Device, Electronic Appliance, and Lighting Device
InactiveUS20140183503A1Reduce the driving voltageImprove current efficiencyOrganic chemistrySolid-state devicesLow voltageQuinoline
Disclosed is a light-emitting element having high emission efficiency, capable of driving at low voltage, and showing a long lifetime. The light-emitting element contains a compound between a pair of electrodes, and the compound is configured to give a first peak of m / z around 202 and a second peak of m / z around 227 in a mass spectrum. The first and second peaks are product ions of the compound and possess compositions of C16H9 and C17H10N, respectively, which are derived from a dibenzo[f,h]quinoline unit.
Owner:SEMICON ENERGY LAB CO LTD
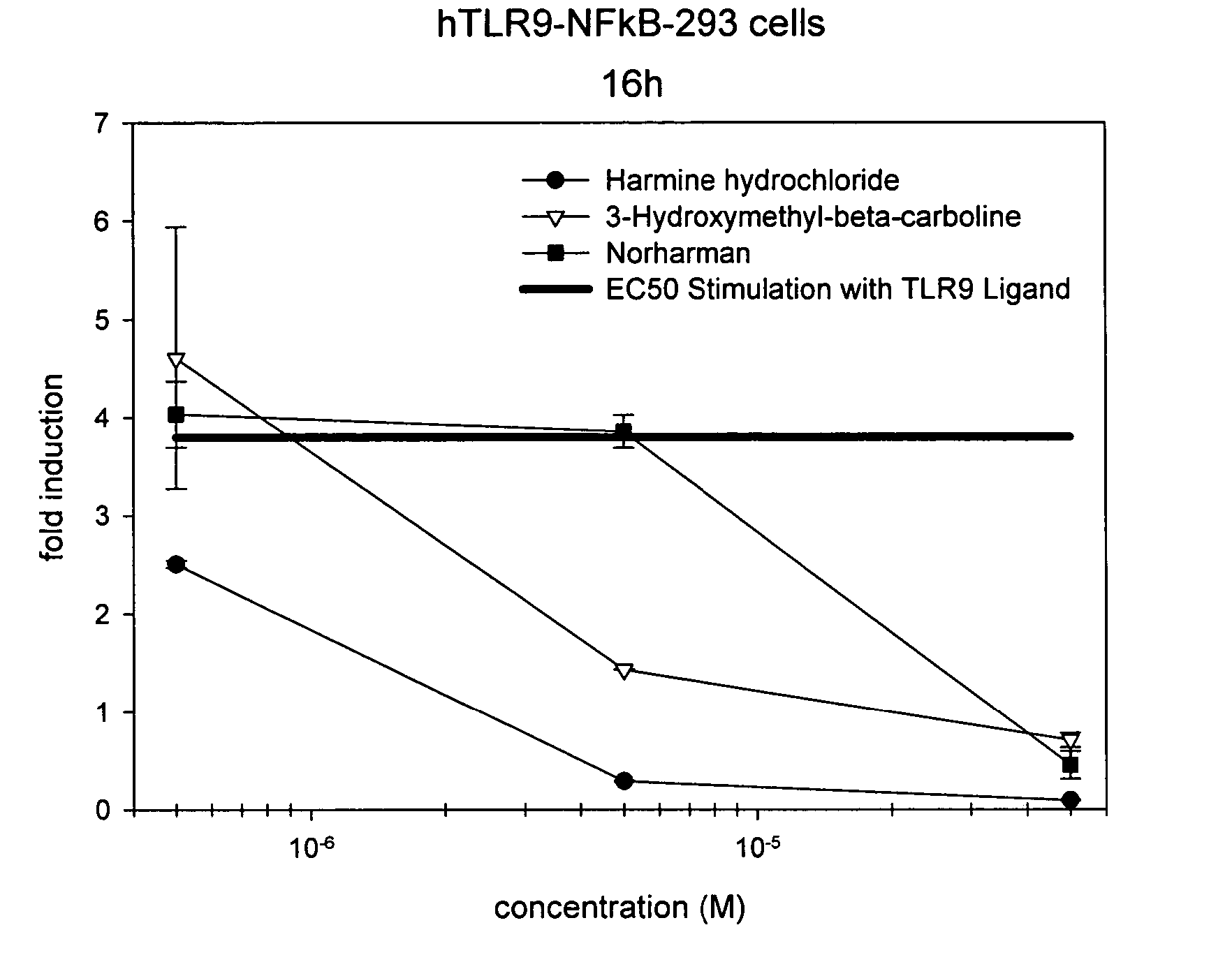
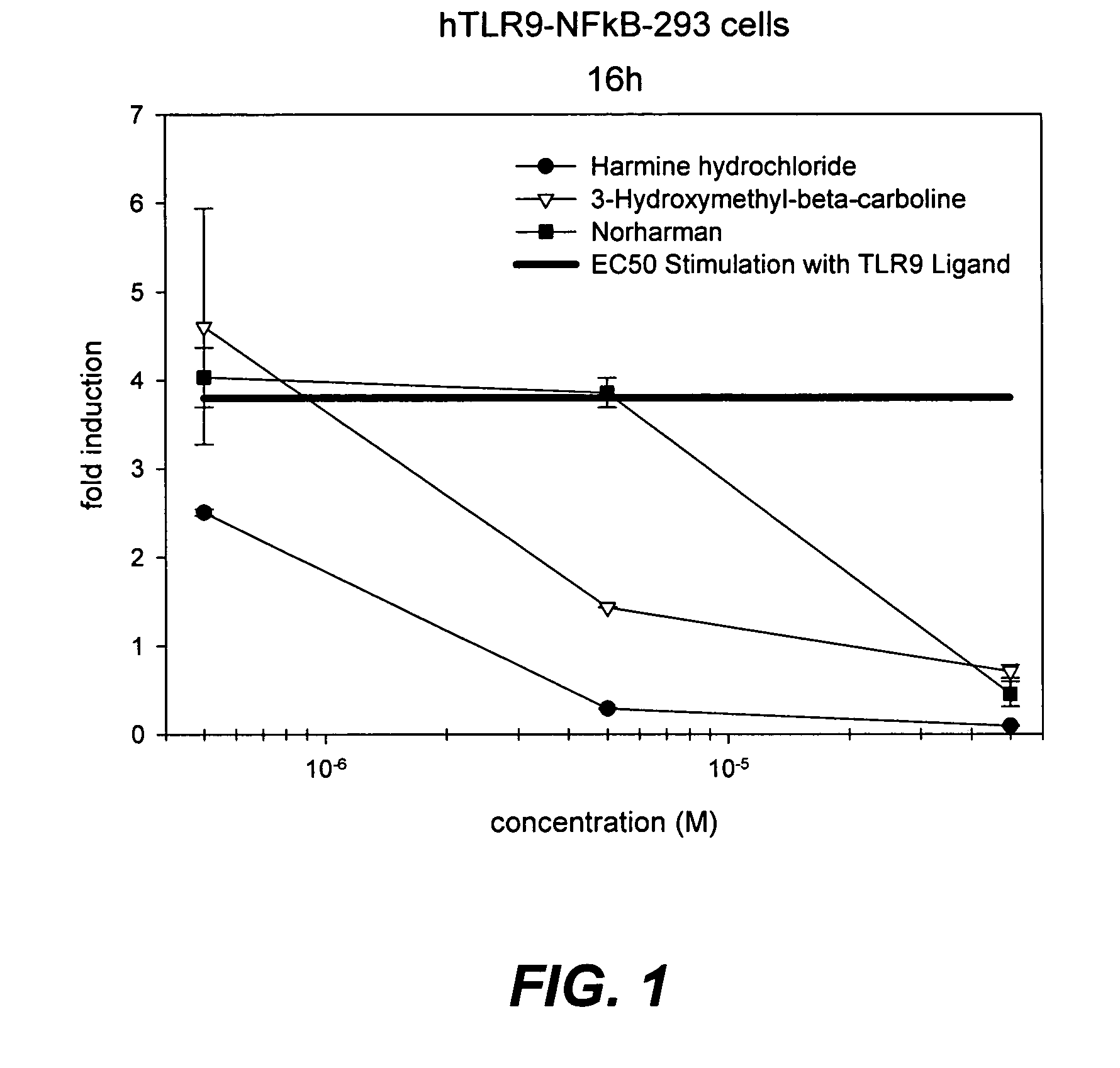
Small molecule toll-like receptor (TLR) antagonists
The invention provides methods and compositions useful for modulating signaling through Toll-like receptors. The methods involve contacting a TLR-expressing cell with a small molecule having a core structure including at least two rings. Certain of the compounds are 4-primary amino quinolines. Many of the compounds and methods are useful specifically for inhibiting immune stimulation involving at least one of TLR9, TLR8, TLR7, and TLR3. The methods may have use in the treatment of autoimmunity, inflammation, allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer, and immunodeficiency.
Owner:COLEY PHARMA GMBH +1
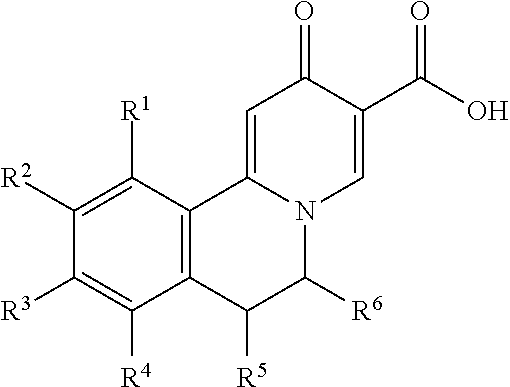
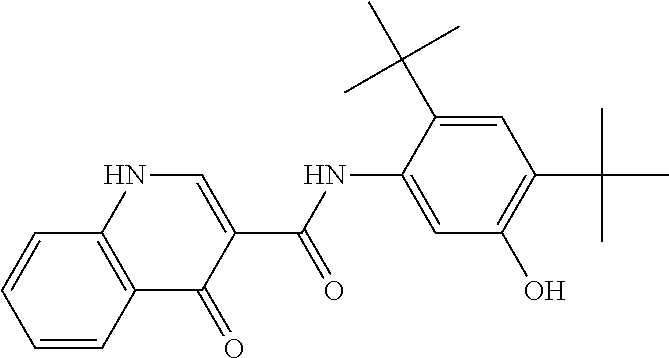
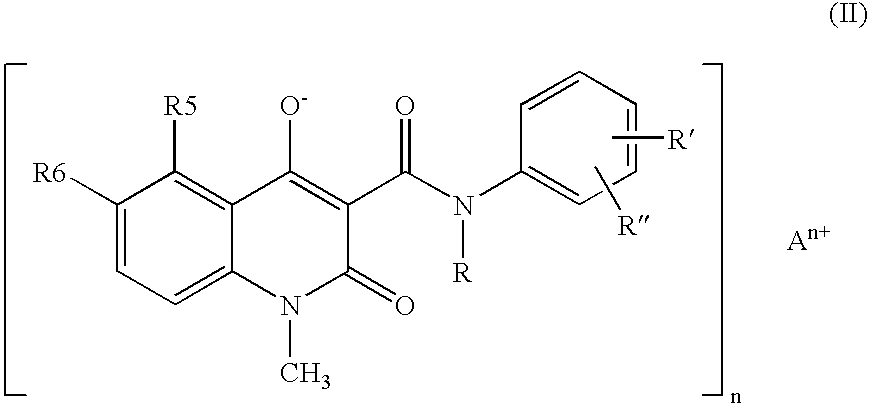
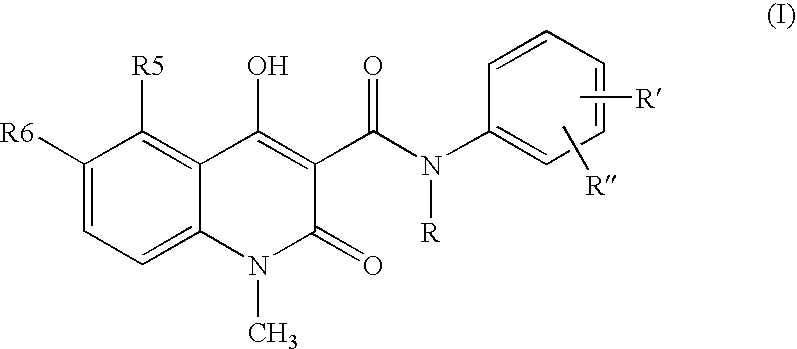
Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide
Pharmaceutical compositions including N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide (Compound 1) and methods of using such compositions are described herein.
Owner:VERTEX PHARMA INC
Metal coordination compound, luminescence device and display apparatus
InactiveUS20030054198A1Group 5/15 element organic compoundsSolid-state devicesQuinolinePerylene derivatives
An organic EL device includes a luminescence layer containing, as a luminescent material allowing a high-luminescence and high-efficiency luminescence for a long period of time, a metal coordination compound represented by the following formula (1): LmML'n, wherein M denotes Ir, Pt, Ph or Pd; L denotes a bidentate ligand; L' denotes a bidentate ligand different from L; m is an integer of 1, 2 or 3; and n is an integer of 0, 1 or 2 with the proviso that the sum of m and n is 2 or 3. The partial structure MLm is represented by a formula (2) or a formula (3) shown below, and the partial structure ML'n is represented by a formula (4) or a formula (5) shown below: wherein CyN1, CyN2 and CyN3 independently denote a substituted or unsubstituted cyclic group containing a nitrogen atom connected to M; CyN4 denotes a cyclic group containing 8-quinoline or its derivative having a nitrogen atom connected to M; CyC1, CyC2 and CyC3 independently denote a substituted or unsubstituted cyclic group containing a carbon atom connected to M, with the proviso that the metal coordination compound is represented by the formula (2) when n is 0.
Owner:CANON KK
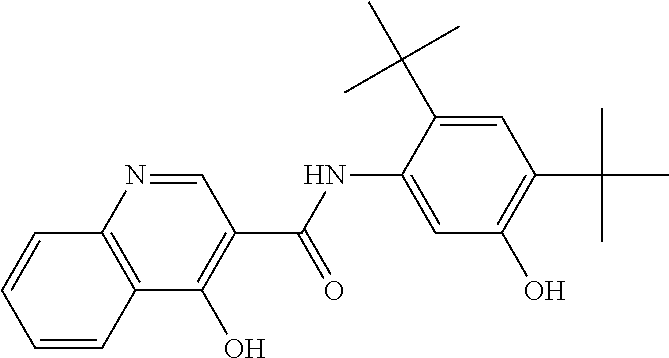
Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide
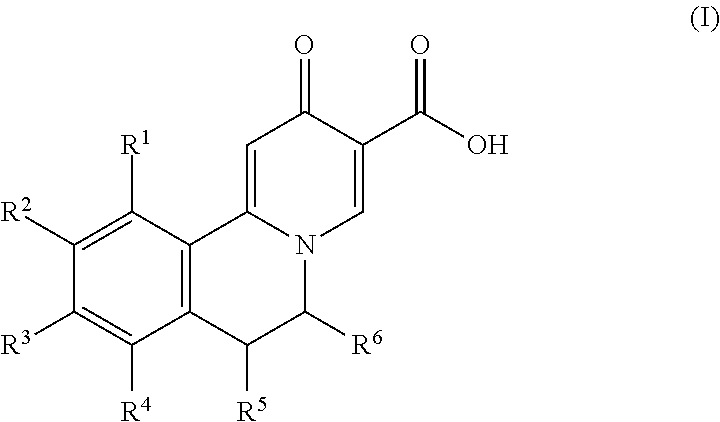
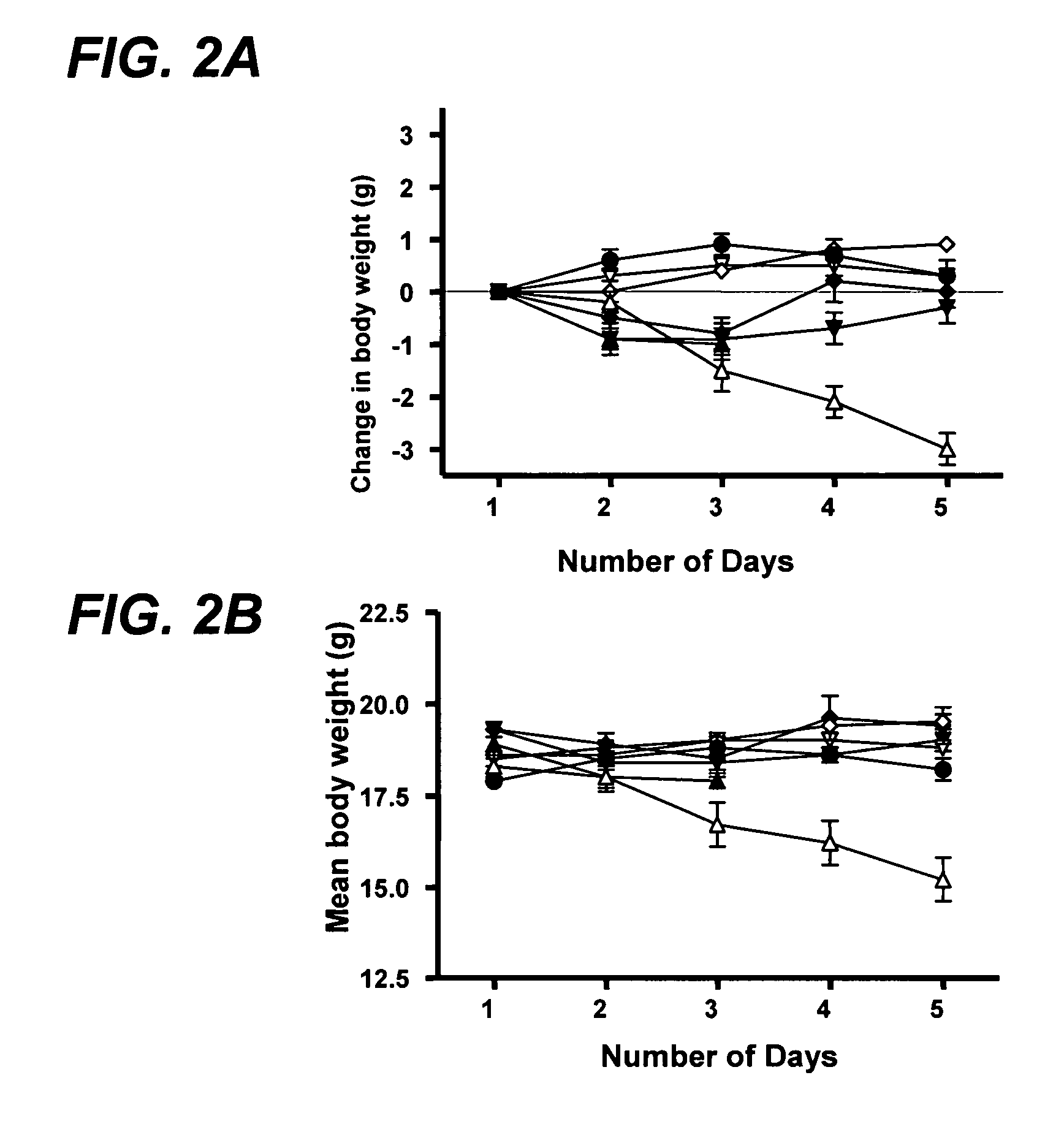
InactiveUS20110064811A1Improve solubilityGood physical and chemical stabilityBiocidePowder deliveryFormamideOxoquinolines
The present invention relates to solid state forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide (Compound 1), pharmaceutical compositions thereof and methods therewith.
Owner:VERTEX PHARMA INC
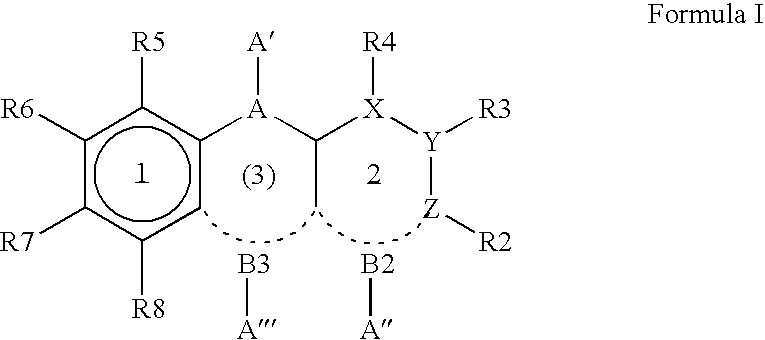
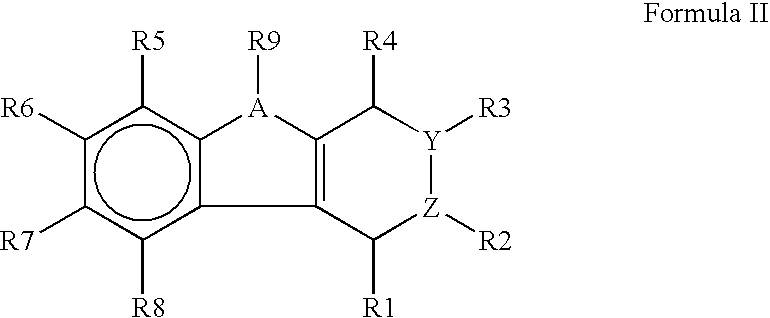
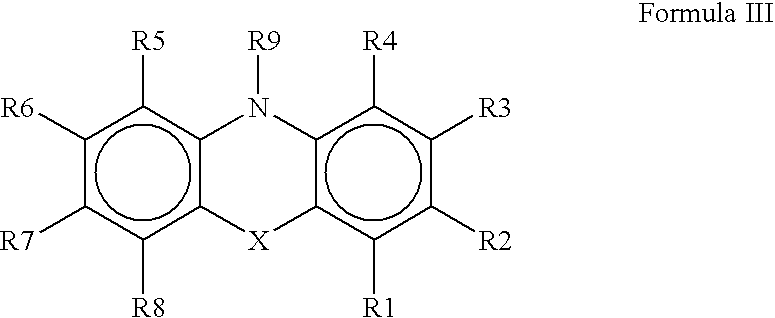
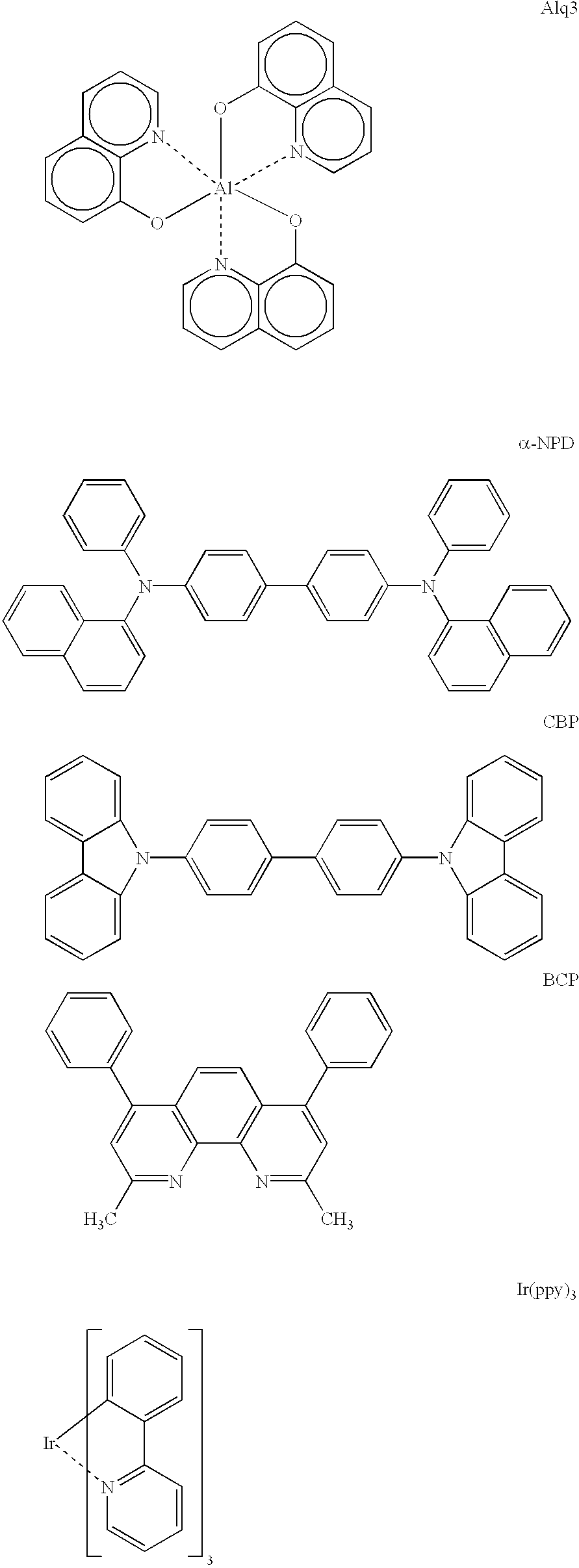
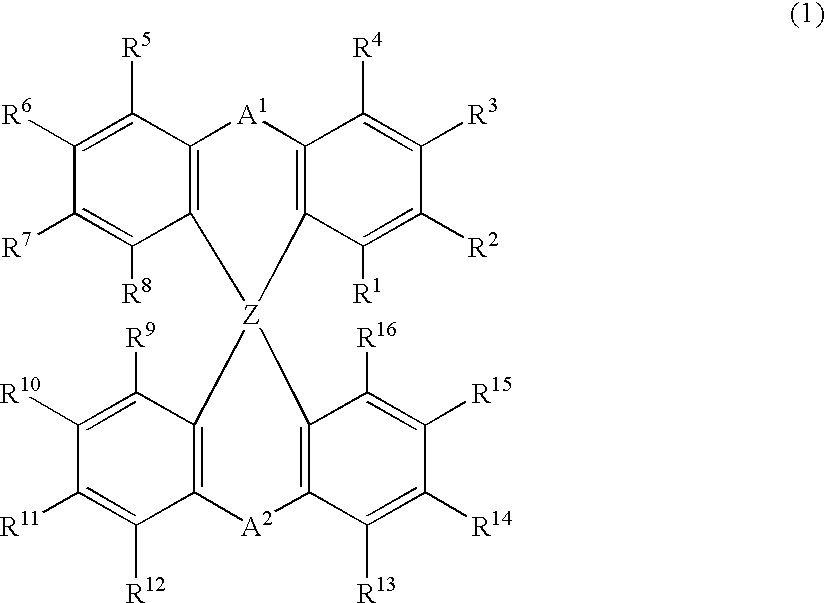
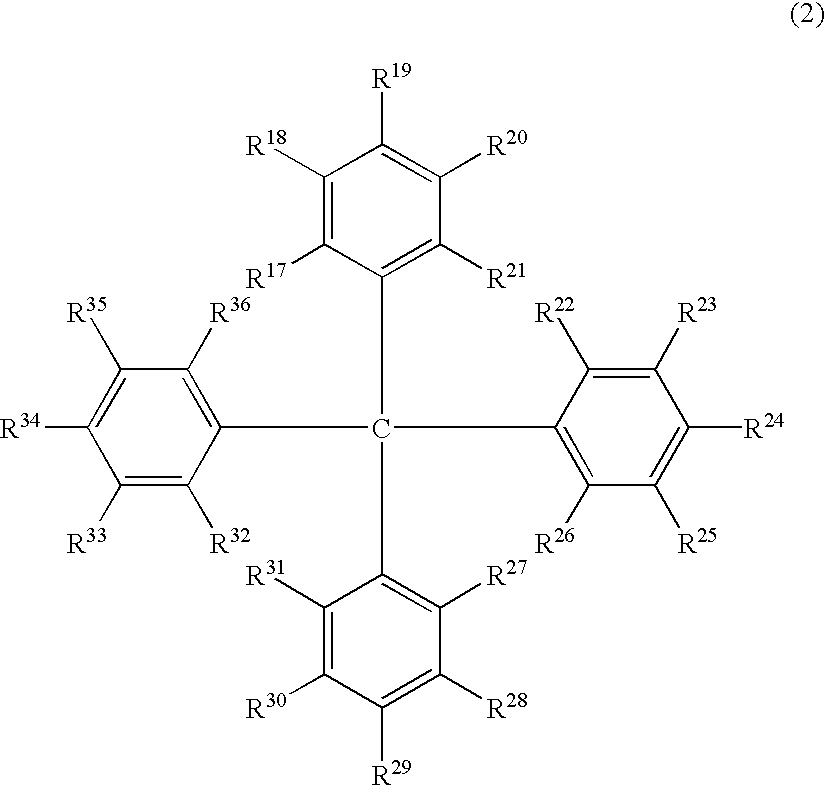
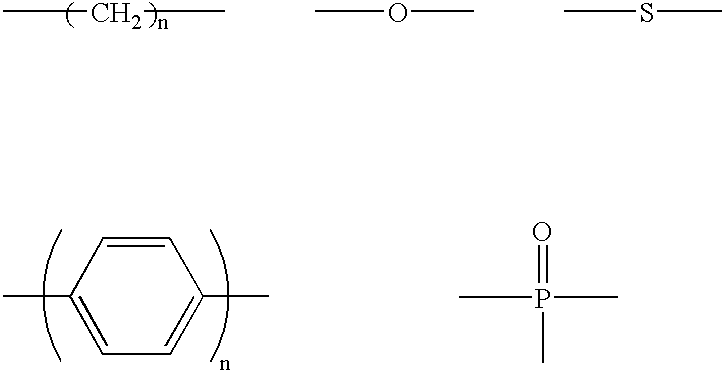
Luminescent element material and luminescent element comprising the same
The light emitting device of the present invention relates to a light emitting device which is characterized in that it is a device with an emissive substance present between an anode and cathode, and which emits light by means of electrical energy, and said device has a least one type of compound denoted by (a) to (d) below. (a) A compound having a plurality of 1,7-phenanthroline skeletal structures (b) A benzoquinoline derivative (c) A spiro compound represented by general formula (1) A1 and A2 are each selected from single bonds, substituted or unsubstituted alkyl chains, ether chains, thioether chains, ketone chains and substituted or unsubstituted amino chains. However, A1<> A2. Z represents carbon or silicon. R1 to R16 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. (d) A tetraphenylmethane derivative represented by general formula (2) R17 to R36 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. However, at least one of R17 to R36 is selected from substituents represented by general formula (3). -X-Ar (3) X is a single bond or is selected from the following, and Ar denotes a condensed aromatic ring or heteroaromatic ring. In the case where X is phosphorus oxide, then Ar represents an aromatic hydrocarbon or heteroaromatic ring. n is an natural number.
Owner:TORAY IND INC
Pyridine, pyrimidine, quinoline, quinazoline, and naphthalene urotensin-II receptor antagonists
The present invention relates to urotensin II receptor antagonists, pharmaceutical compositions containing them and their use.
Owner:ENCYSIVE PHARMA INC
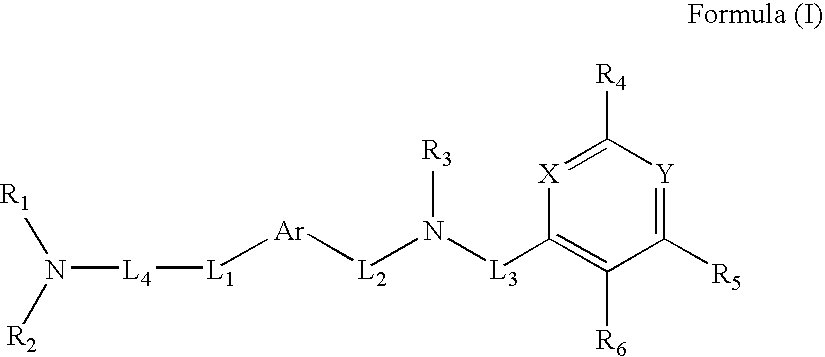
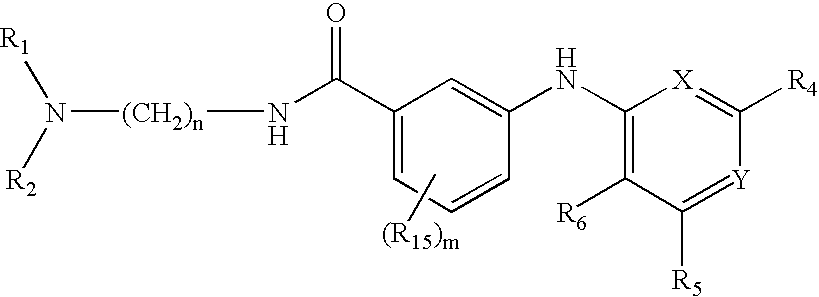
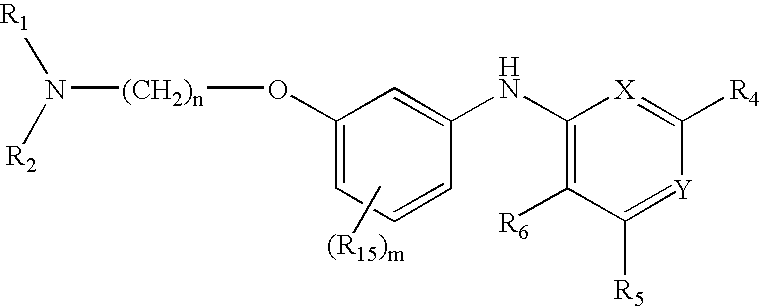
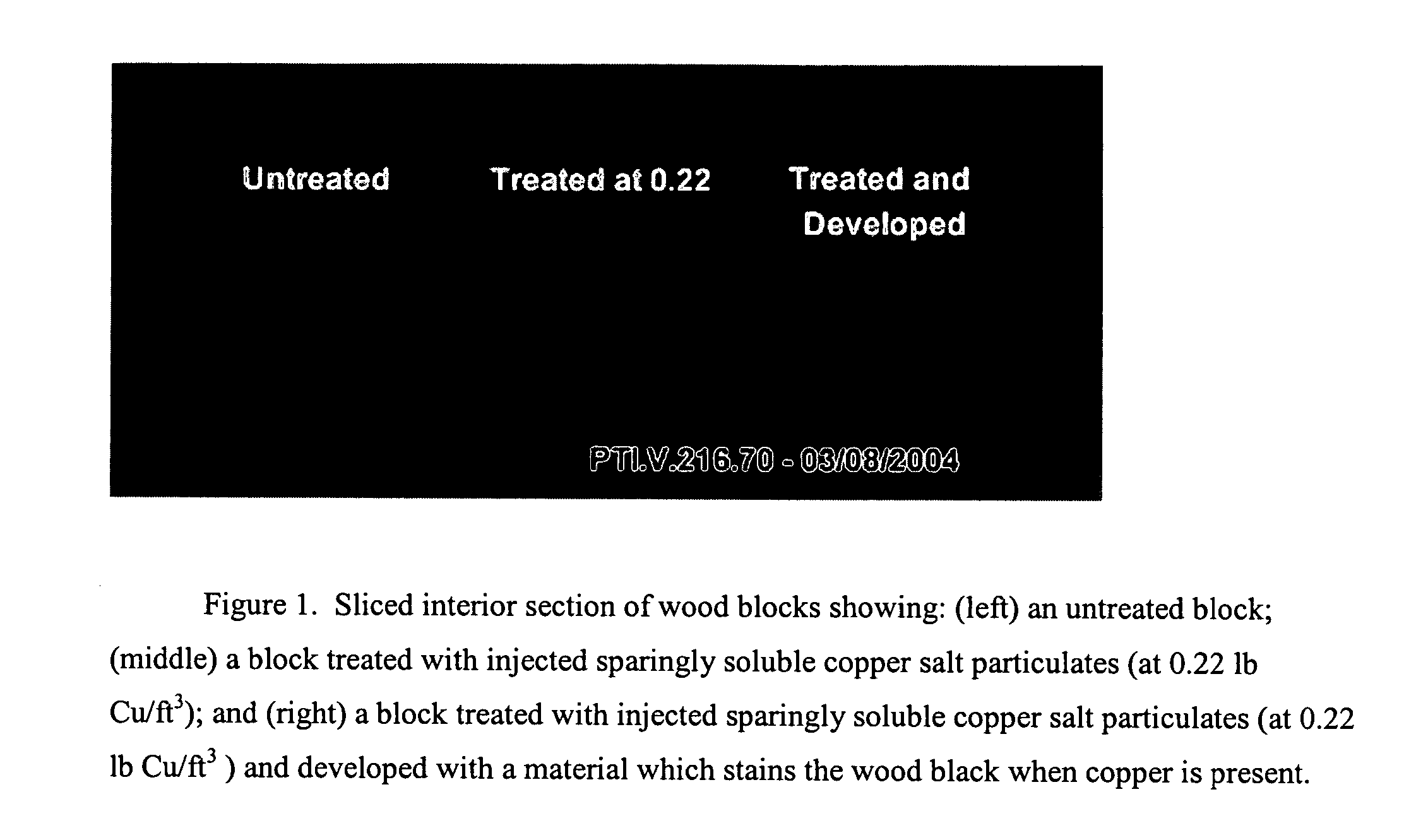
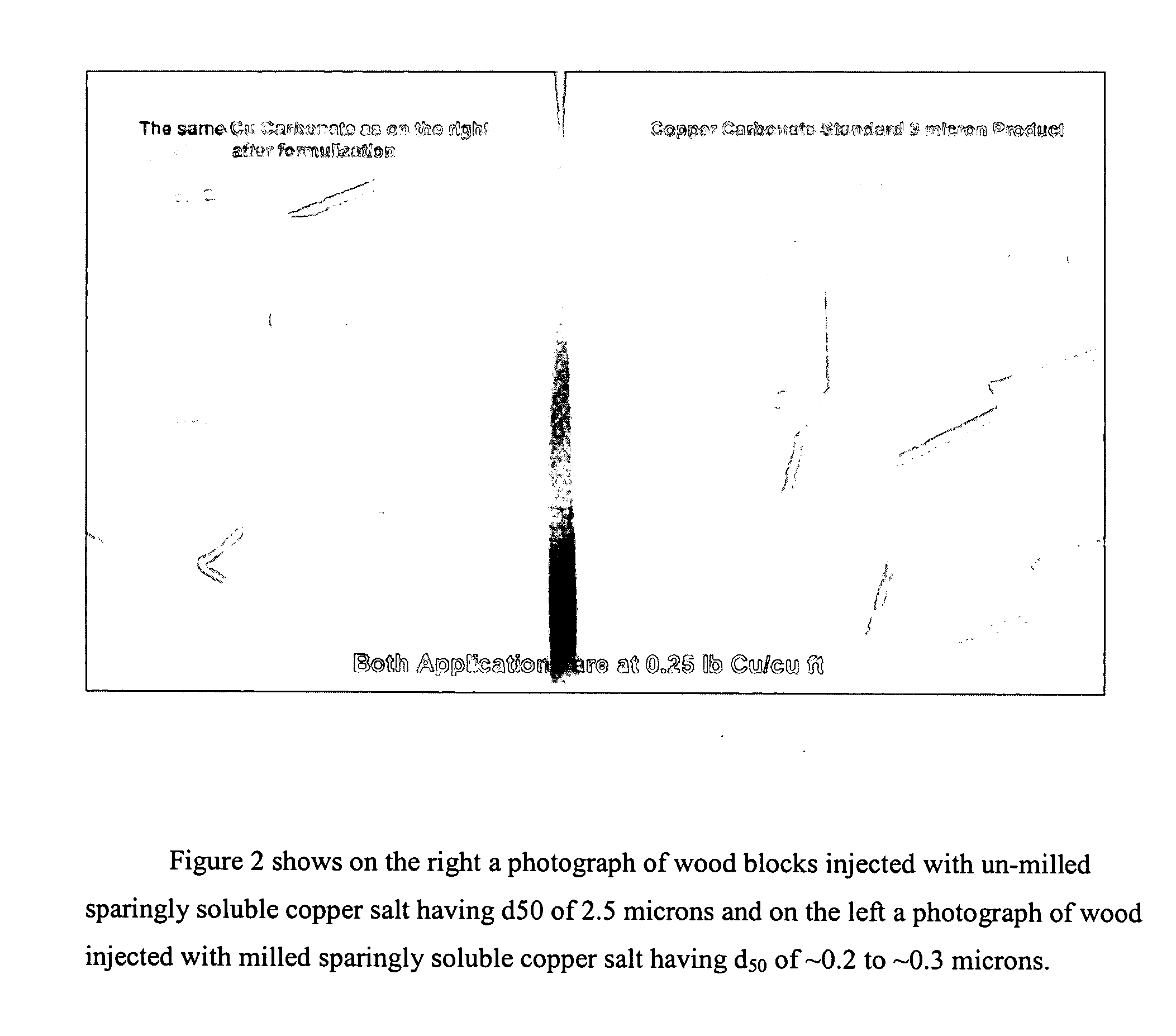
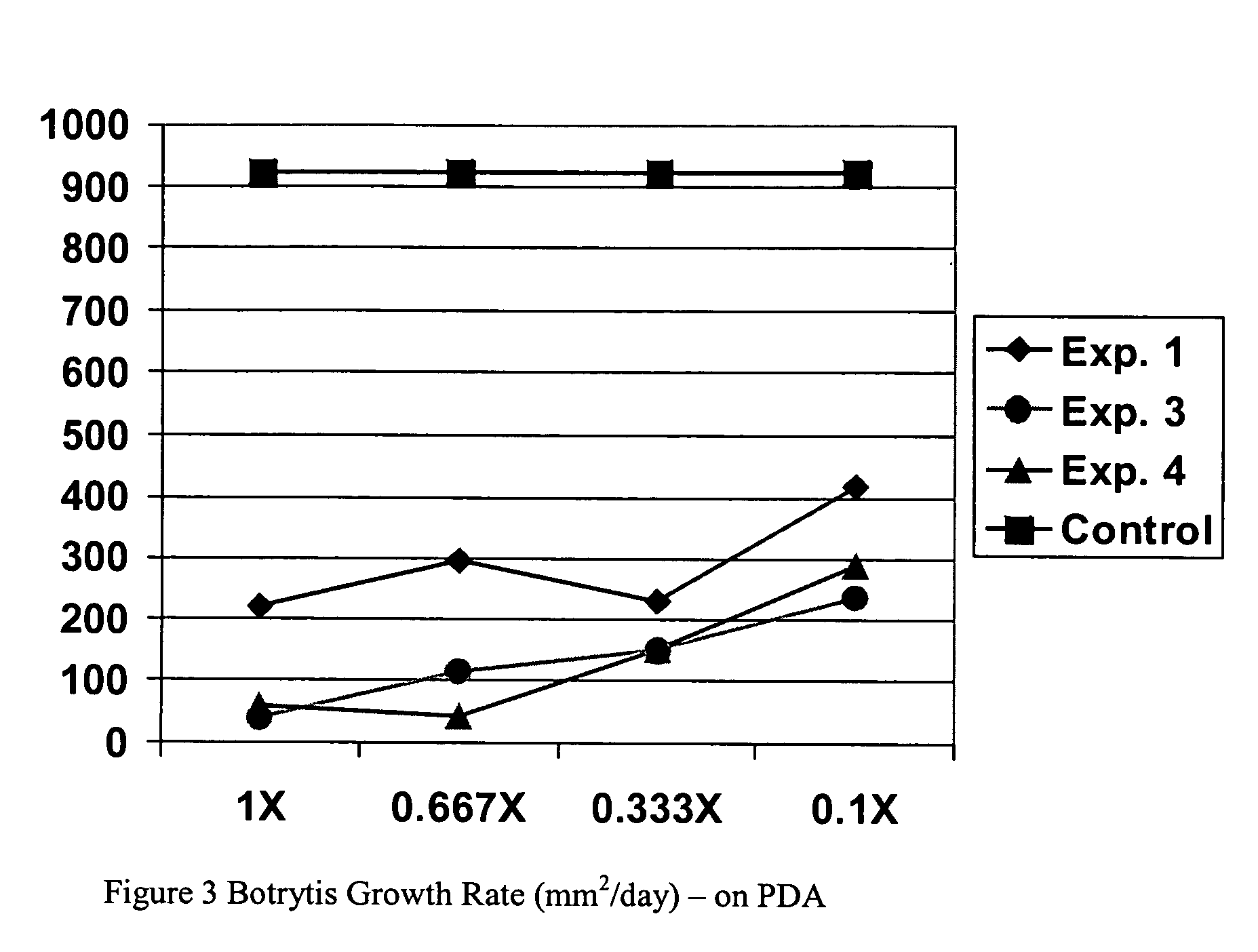
Composition, method of making, and treatment of wood with an injectable wood preservative slurry having biocidal particles
A method of preserving wood includes injecting into the wood an effective amount of a aqueous wood-injectable biocidal slurry, said a wood-injectable biocidal slurry containing dispersants and sub-micron biocidal particles selected from at least one of the following classes: 1) a plurality of particles containing at least 25% by weight of a solid phase of sparingly soluble salts selected from copper salts, nickel salts, tin salts, and / or zinc salts; 2) a plurality of particles containing at least 25% by weight of a solid phase of sparingly soluble metal hydroxides selected from copper hydroxide, nickel hydroxide, tin hydroxide, and / or zinc hydroxide; 3) a plurality of particles containing at least 25% by weight of a solid phase comprising a substantially-insoluble organic biocide selected from triazoles, chlorothalonil, iodo-propynyl butyl carbamate, copper-8-quinolate, fipronil, imidacloprid, bifenthrin, carbaryl, strobulurins, and indoxacarb; 4) a plurality of particles containing on the outer surface thereof a substantially-insoluble organic biocide; 5) a plurality of particles containing a solid phase of a biocidal, partially or fully glassified composition comprising at least one of Zn, B, Cu, and P. The particles may advantageously contain metallic copper, a leachability barrier, pigments, dyes, or other adjuvants disposed on the outer surface thereof.
Owner:OSMOSE
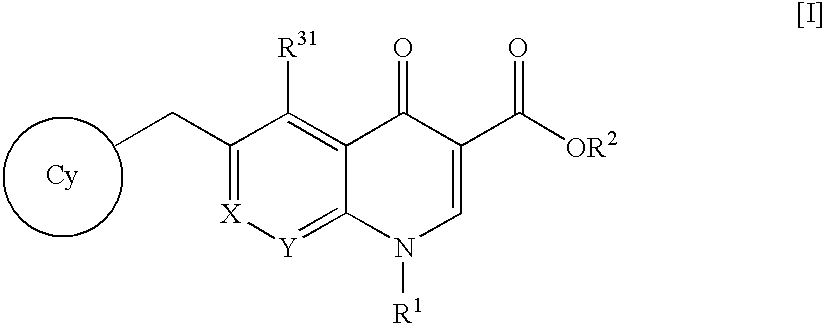
Quinoline and quinoxaline compounds
Quinoline and quinoxaline compounds, pharmaceutical compositions containing such compounds and the use of such compounds to elevate certain plasma lipid levels, including high density lipoprotein-cholesterol and to lower certain other plasma lipid levels, such as LDL-cholesterol and triglycerides and accordingly to treat diseases which are exacerbated by low levels of HDL cholesterol and / or high levels of LDL-cholesterol and triglycerides, such as atherosclerosis and cardiovascular diseases in some mammals, including humans.
Owner:PFIZER INC +1
4-oxoquinoline compound and use thereof as pharmaceutical agent
Owner:JAPAN TOBACCO INC
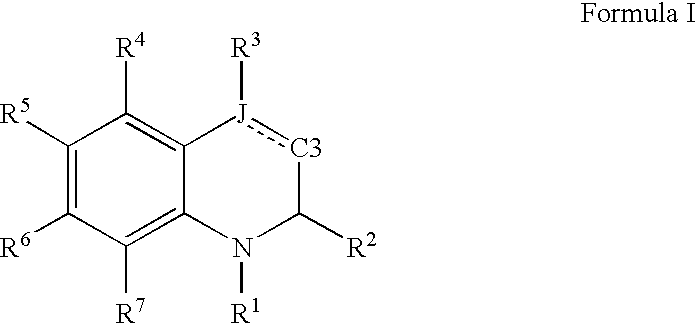
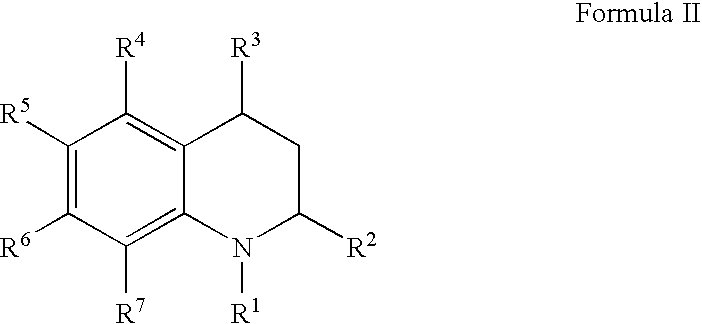
Dihydro- and tetrahydro-quinoline compounds
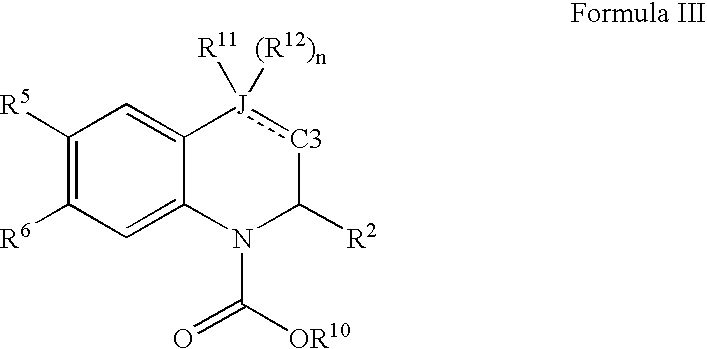
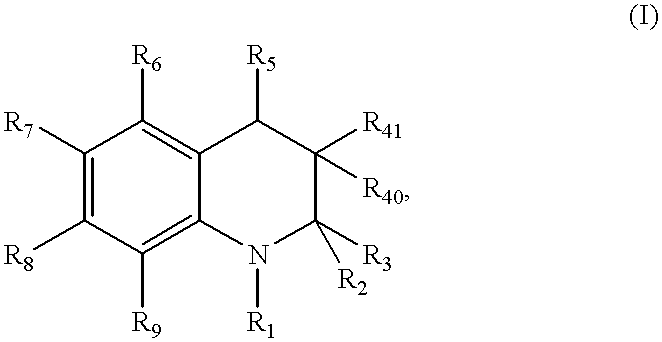
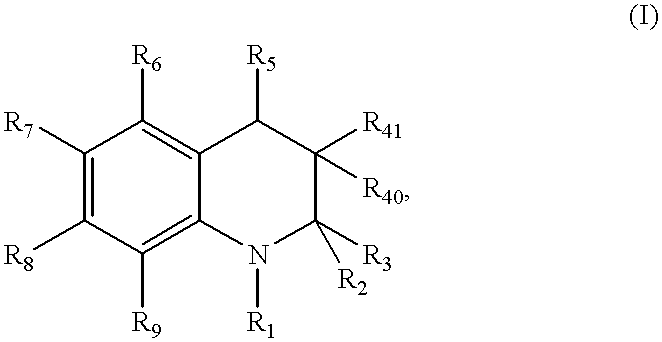
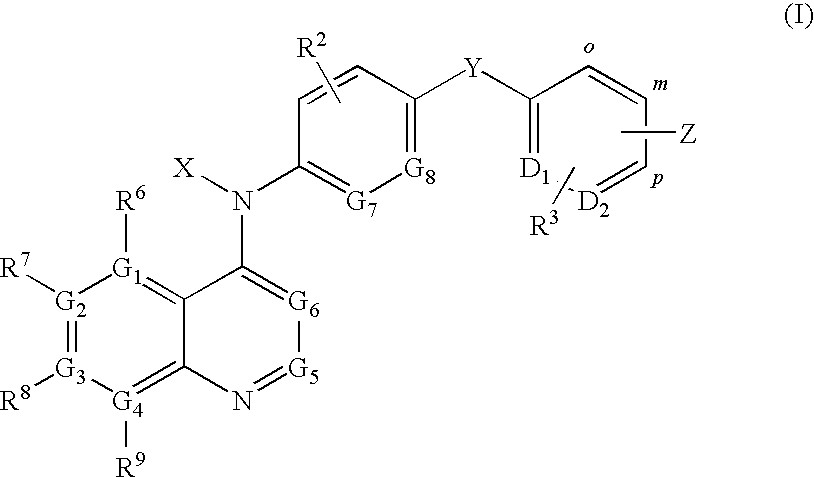
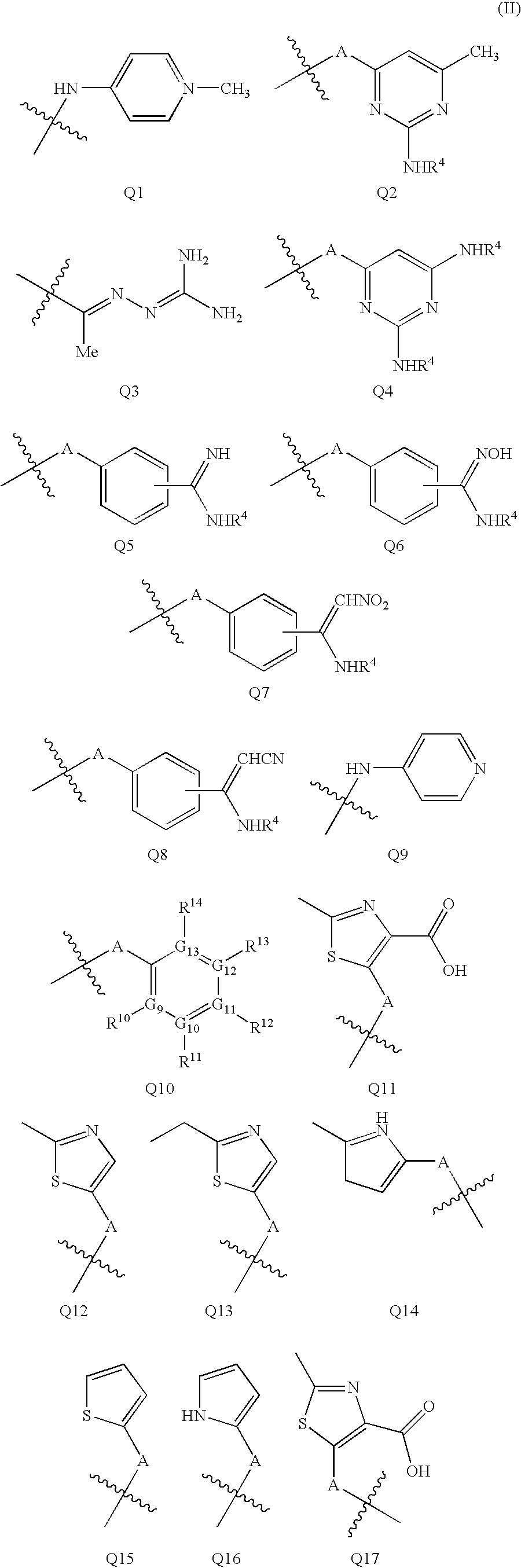
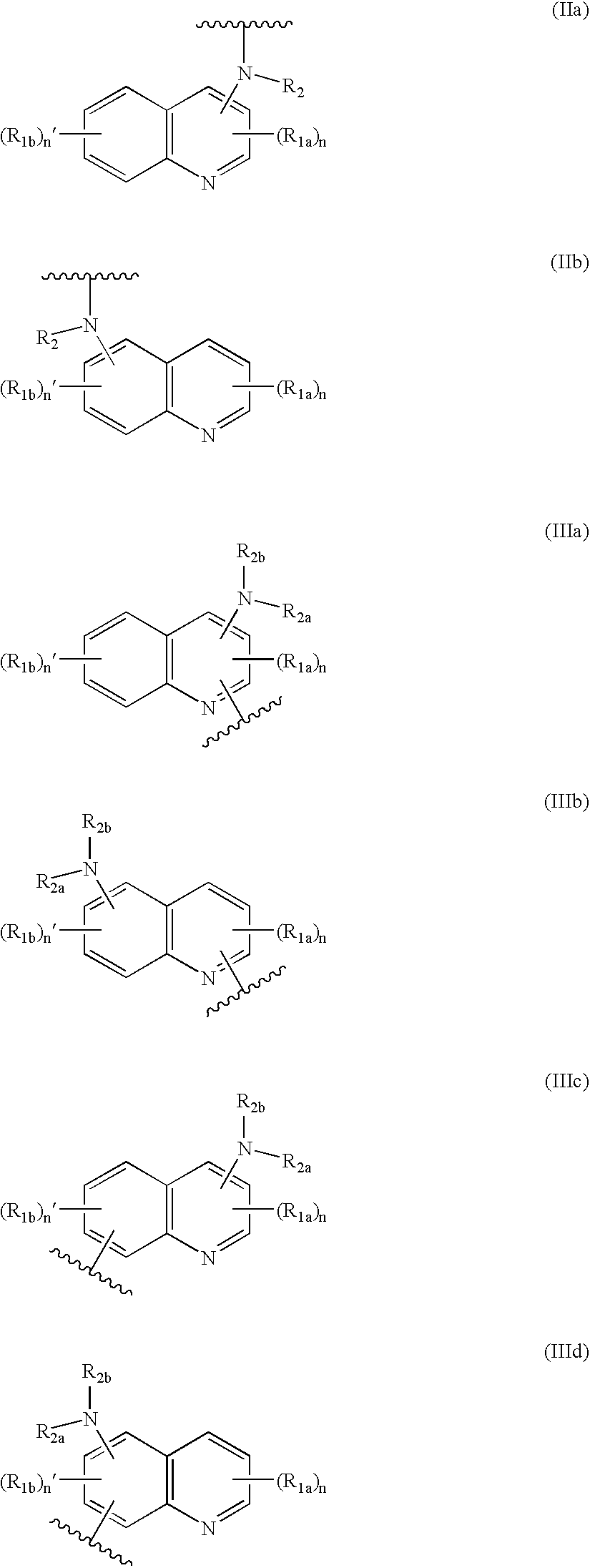
Compound of formula (I):wherein:R1 represents hydrogen orwherein A is as defined in the description,R2 and R3 each independently represents alkyl, cycloalkyl, heterocycloalkyl, optionally substituted aryl, optionally substituted heteroaryl, cycloalkylalkyl, heterocycloalkylalkyl, optionally substituted arylalkyl, optionally substituted heteroarylalkyl,or optionally substituted aminoalkyl or R2 and R3, together with the carbon atom carrying them, form cycloalkyl or monocyclic heterocyclic group, substituted or unsubstituted,R40 represents hydrogen or a group selected from optionally substituted alkyl, optionally substituted alkenyl and optionally substituted alkynyl or Q or -V-Q wherein V represents alkylene, alkenylene or alkynylene and Q represents optionally substituted cycloalkyl, optionally substituted aryl, optionally substituted heterocycloalkyl,or optionally substituted heteroaryl,R41 and R5 together form a bond or each represents hydrogen,R6, R7, R8 and R9 each independently represents hydrogen, halogen, alkyl, (C3-C8)cycloalkyl,or -OW wherein W is as defined in the description, and medicinal products containing the same which are useful as antioxidative agents.
Owner:LES LAB SERVIER
Pharmaceutical composition and administrations thereof
The present invention relates to pharmaceutical compositions comprising a solid dispersion of N-[2,4-Bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide, methods of manufacturing pharmaceutical compositions of the present invention, and methods of administering pharmaceutical compositions of the present invention.
Owner:VERTEX PHARMA INC
Small molecule toll-like receptor (TLR) antagonists
The invention provides methods and compositions useful for modulating signaling through Toll-like receptors. The methods involve contacting a TLR-expressing cell with a small molecule having a core structure including at least two rings. Certain of the compounds are 4-primary amino quinolines. Many of the compounds and methods are useful specifically for inhibiting immune stimulation involving at least one of TLR9, TLR8, TLR7, and TLR3. The methods may have use in the treatment of autoimmunity, inflammation, allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer, and immunodeficiency.
Owner:COLEY PHARMA GMBH +1
Biosensor
InactiveUS20050175509A1Eliminate measurement errorsHigh precisionMicrobiological testing/measurementBiological material analysisCompound (substance)Organic compound
The present invention relates to a biosensor which comprises an electrode system including at least one pair of electrodes, at least one insulating base plate for supporting the electrode system, a first reaction layer provided at least on a working electrode of the electrode system, including an organic compound having a functional group capable of bonding or being adsorbed to an electrode and a hydrophobic hydrocarbon group, a second reaction layer provided on the first reaction layer, including an amphiphilic lipid capable of bonding or being adsorbed to a hydrophobic portion of the first reaction layer, and a reagent system carried in a two-component membrane composed of the first and second reaction layers, including at least membrane-binding type pyrroquinoline quinone-dependent glucose dehydrogenase and an electron mediator.
Owner:PANASONIC CORP
Compositions containing quinoline compounds
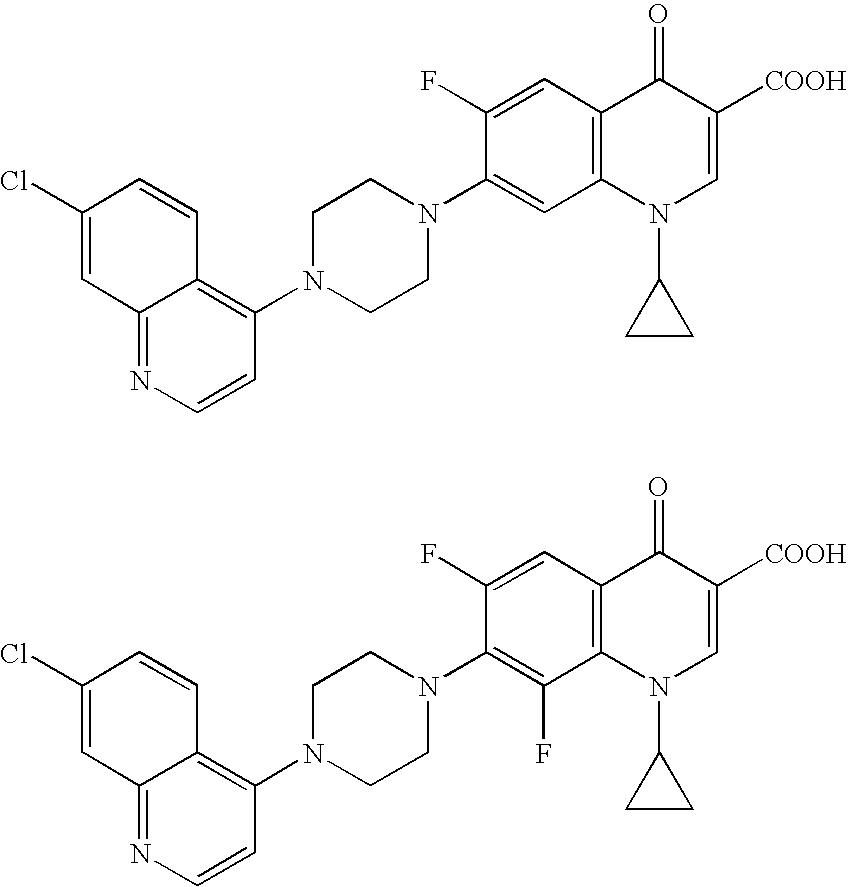
A stable solid pharmaceutical composition consisting essentially of an effective amount of a salt of formula (II)together with an alkaline-reacting component maintaining the pH preferably above 8, or a salt with a divalent metal cation; andat least one pharmaceutical excipient;said salt of formula (II) being essentially stable during storage at room temperature for a period of at least 3 years. A process for stabilizing the salt of formula (II).A crystalline salt of formula (II) and a process for preparing said salt.
Owner:ACTIVE BIOTECH AB
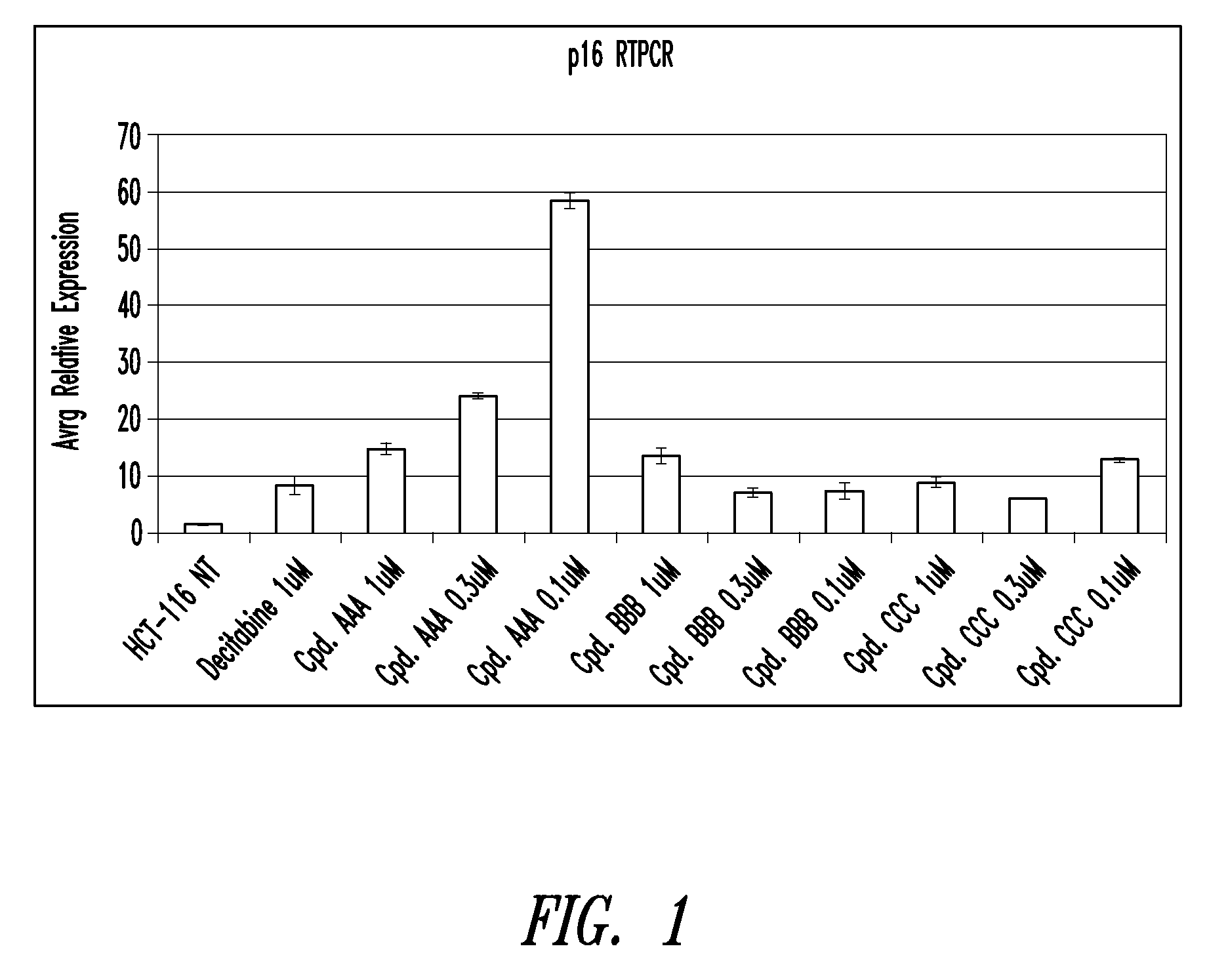
Quinoline derivatives for modulating DNA methylation
InactiveUS20090285772A1Reduction in function/activityReduced activityBiocideOrganic chemistryCytosineDNA methylation
Quinoline derivatives, particularly 4-anilinoquinoline derivatives, are provided. Such quinoline derivatives can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position, for example via selective inhibition of DNA methyltransferase DNMT1. Methods for synthesizing numerous 4-anilinoquinoline derivatives and for modulating DNA methylation are provided. Also provided are methods for formulating and administering these compounds or compositions to treat conditions such as cancer and hematological disorders.
Owner:SUPERGEN
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
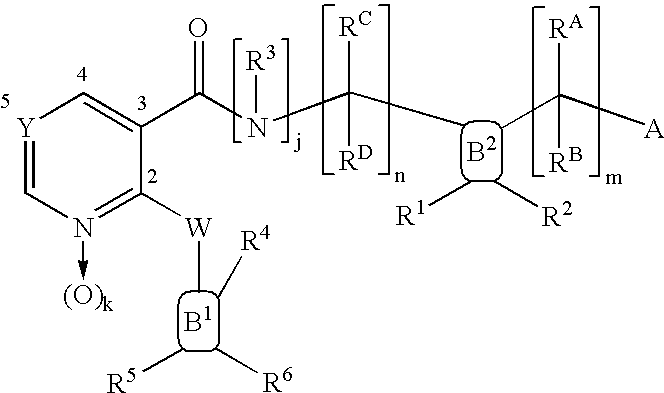
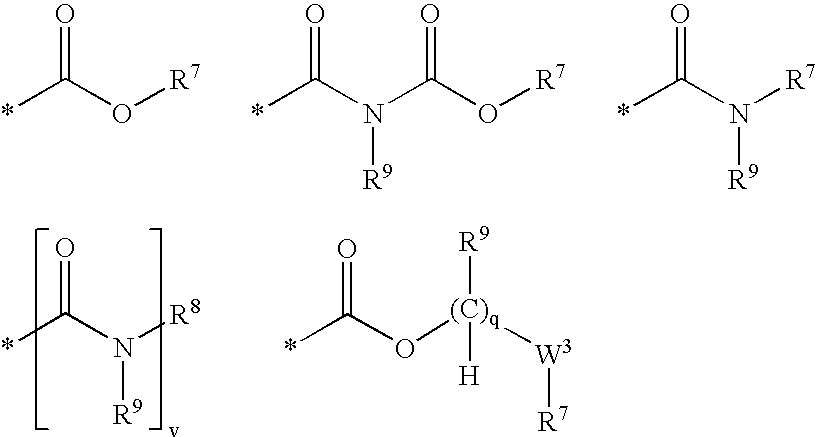
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
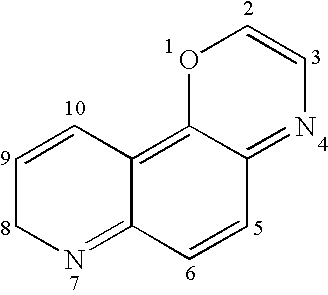
8-Hydroxy quinoline derivatives
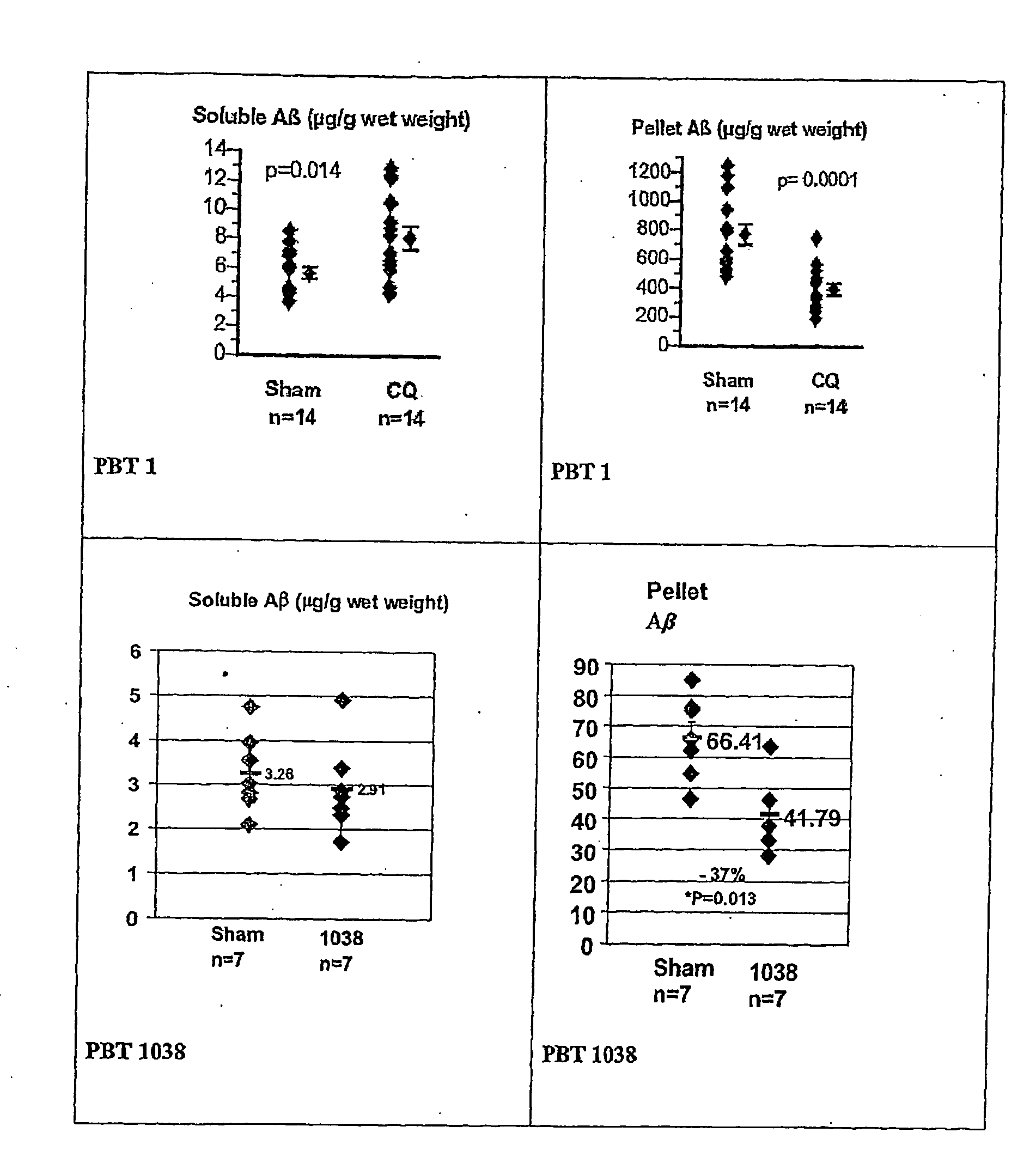
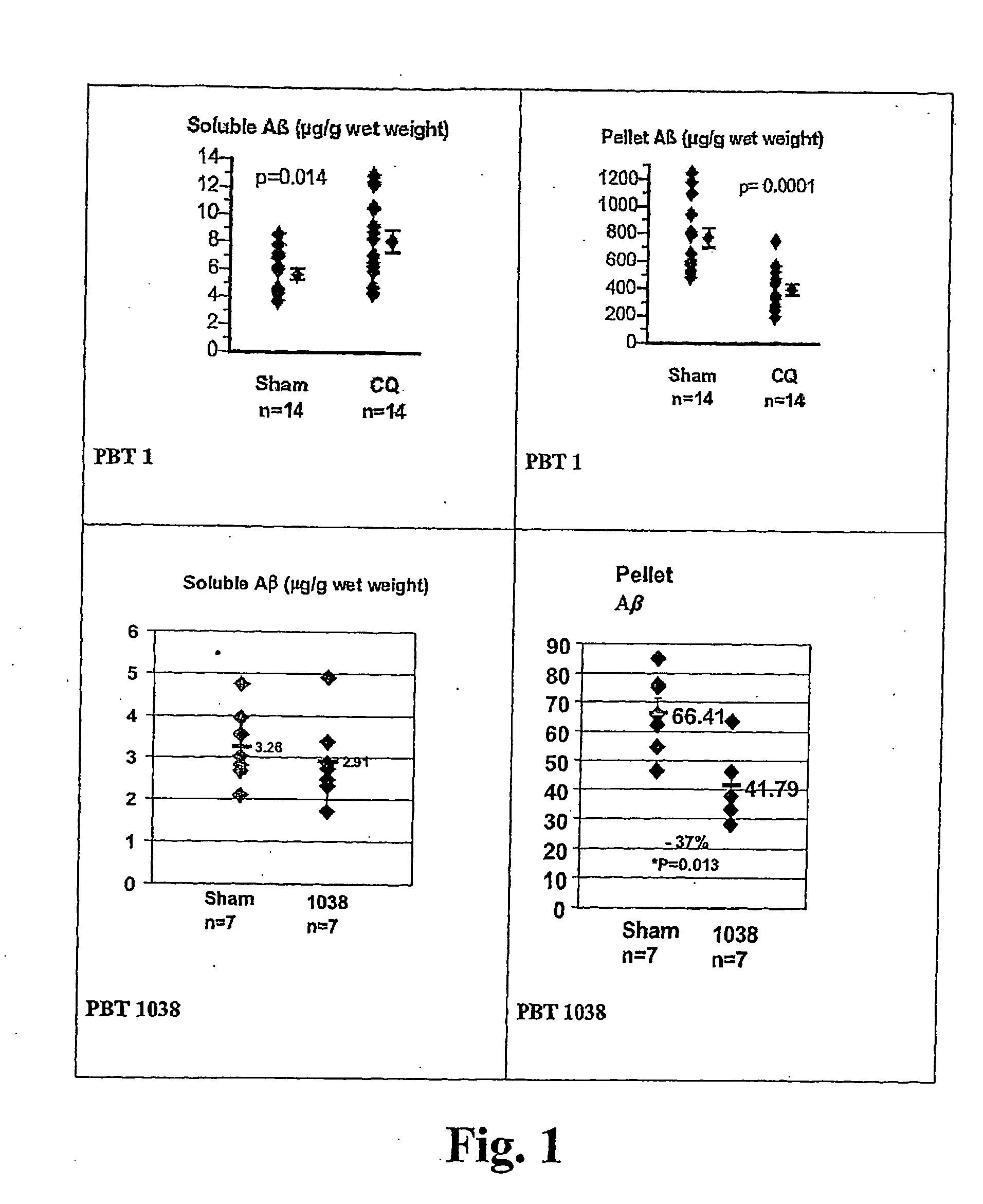
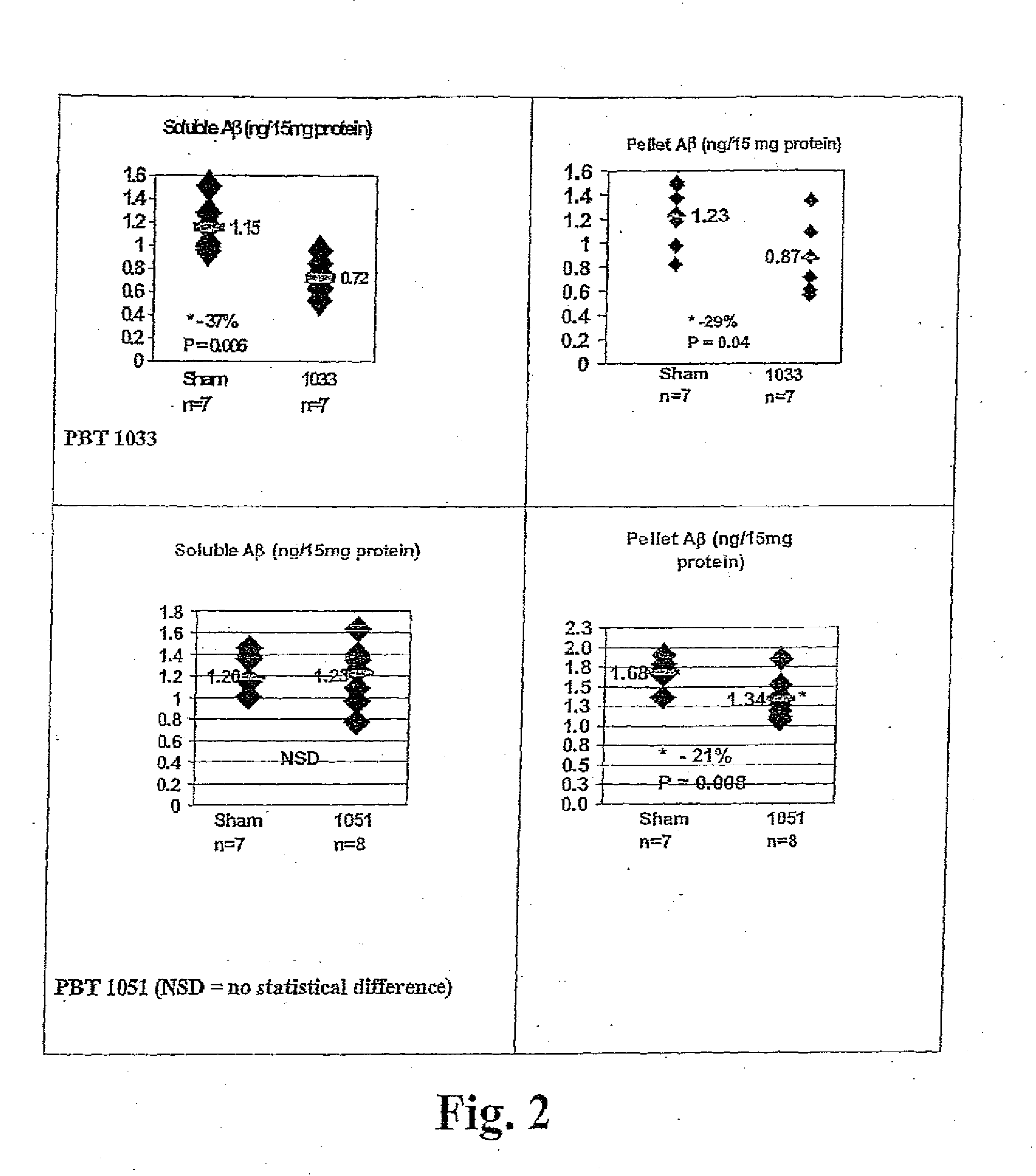
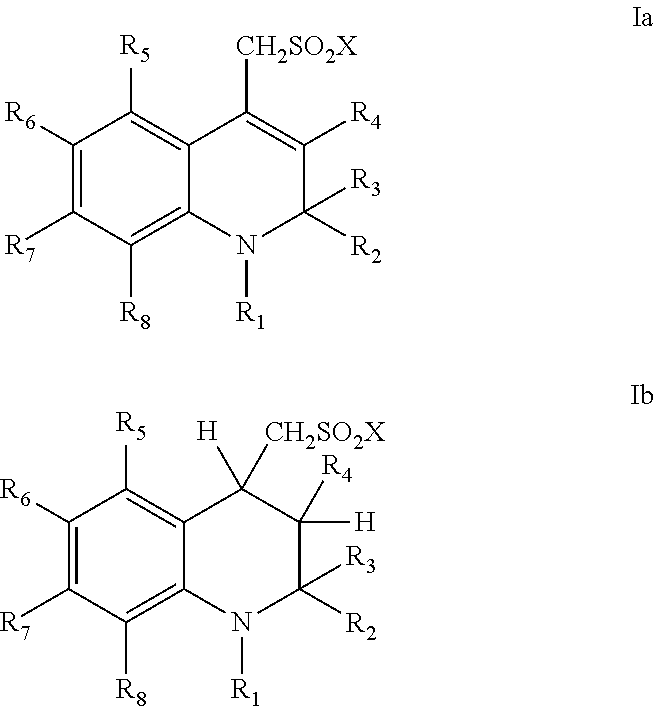
ActiveUS20060089380A1Improve solubilityMaintaining membrane permeabilityBiocideSenses disorderQuinolineNeuro-degenerative disease
Owner:PRANA BIOTECHNOLOGY LTD
Hybrid molecules QA where Q is an aminoquinoline and A is an antibiotic residue, the synthesis and uses thereof as antibacterial agents
The invention concerns an aminoquinoline-antibiotic hybrid compound of general formula (I): Q-(Y1)p-(U)p-(Y2)p-A: wherein Q represents an aminoquinoline, (Y1)p(U)p-(Y2)p'' is an optional spacer and A is an antibiotic residue. The invention enables the antibiotic residue activity to be unexpectedly enhanced.
Owner:PALUMED +1
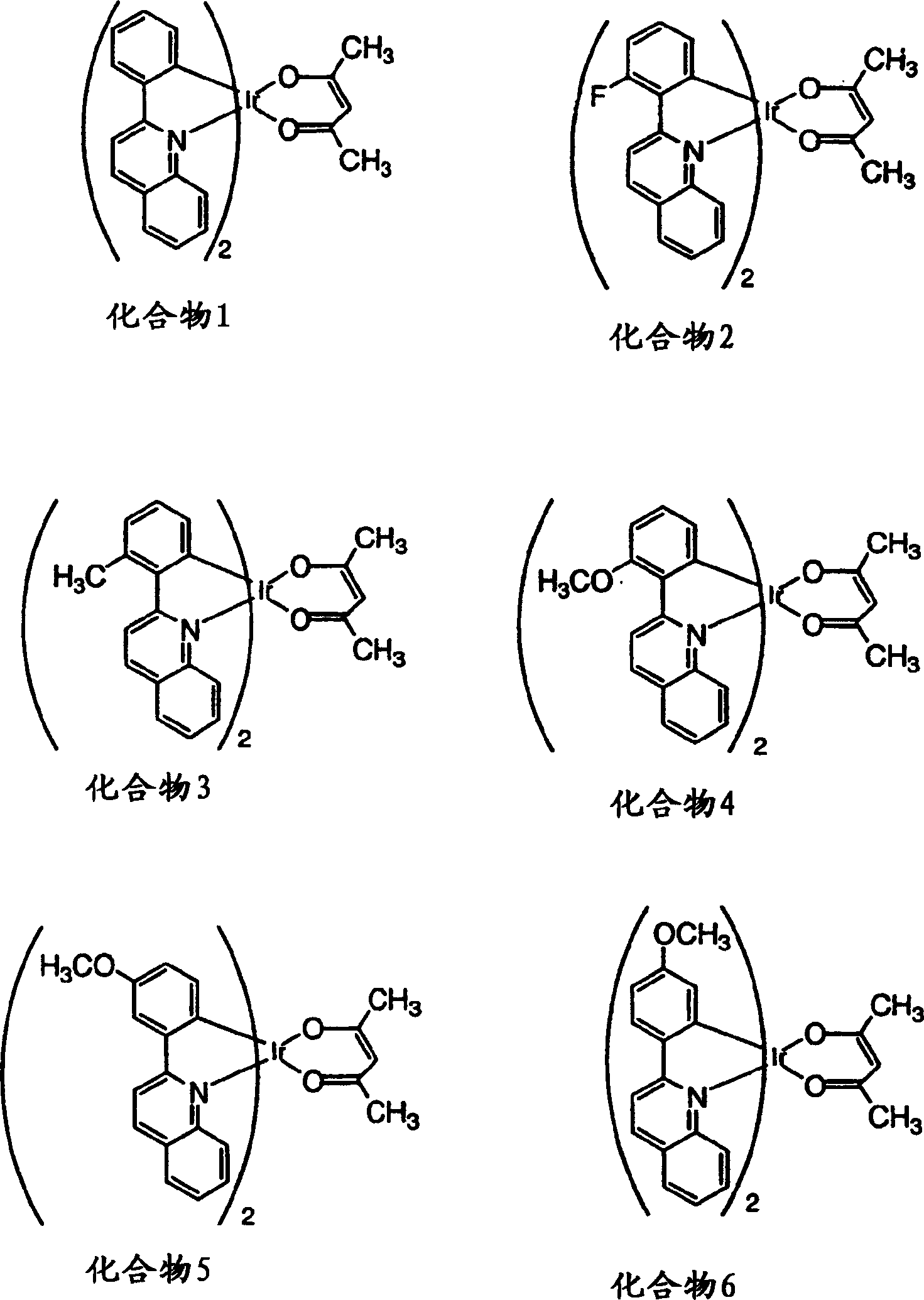
Phosphorescent compounds and devices comprising the same
InactiveCN1589307ARuthenium organic compoundsIndium organic compoundsOrganic light emitting devicePhosphorescence
Owner:UNIVERSAL DISPLAY +1
Tricyclic quinolinone and tricyclic quinoline androgen receptor modulator compounds and methods
Owner:LIGAND PHARMA INC
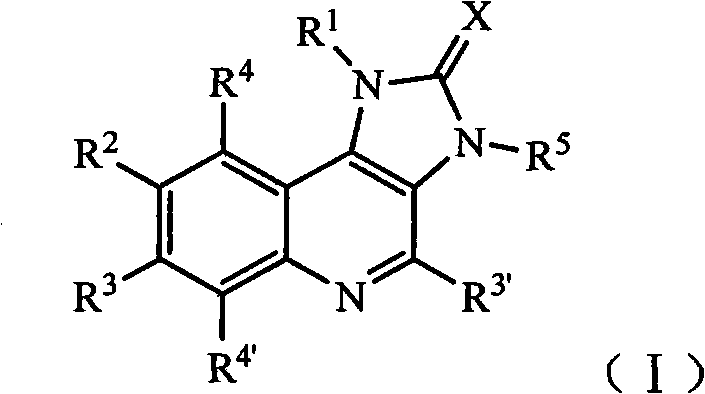
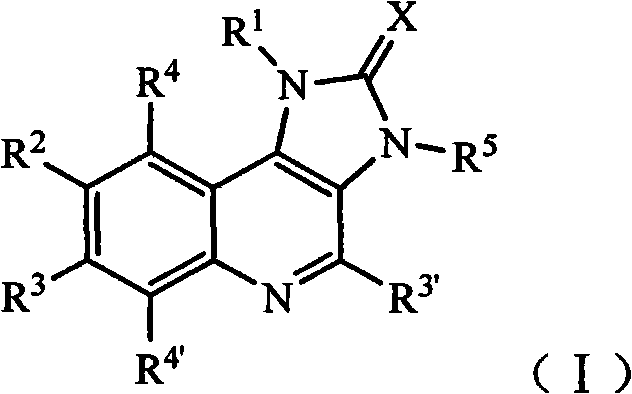
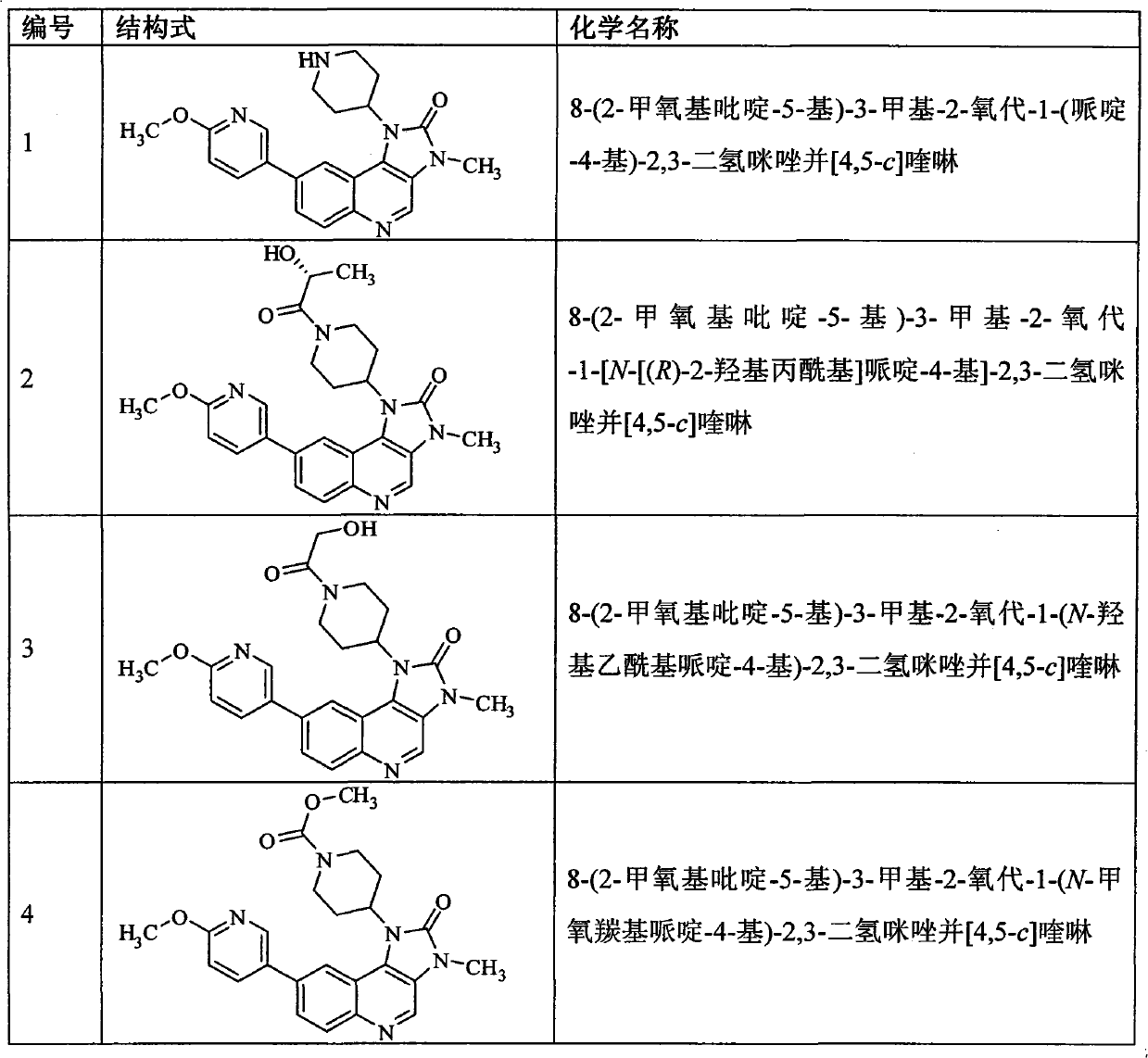
Imidazo quinoline PI3K and mTOR (mammalian target of rapamycin) dual inhibitor
ActiveCN102372711ASignificant progressEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryQuinolineDual inhibitor
The invention belongs to the technical field of medicines, and particularly relates to an imidazo quinoline PI3K and mTOR (mammalian target of rapamycin) dual inhibitor disclosed in a general formula (I) and clinically-acceptable salt or stereoisomer thereof, wherein R1, R2, R3, R3', R4, R4', R5 and X are defined as in a specification. The invention also relates to a preparation method of the compounds, a medicine preparation containing the compounds and the application of the compounds in preparing medicines capable of treating and / or preventing proliferative diseases.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
Absorbing liquid for eliminating sulfide from gas mixture
InactiveCN1421264AReduce processing stepsEconomical desulfurization methodDispersed particle separationSulfur preparation/purificationSulfolaneMorpholine
The present invention belongs to gas producing technology. The absorbing liquid for eliminating sulfide from gas mixture includes main absorbent comprising steric hindrance amine and N-methyl diethanolamine; cosolvent of one or several of sulfolane, N-methyl pyrrolidine, polyglycol dialkyl ether, morpholine and its derivative; catalyst of one or several of C2-C12 alkanolamine, piperazine and its derivative, quinoline, urea and metal phthalocyanine complex. Using the absorbing liquid can eliminate H2S, COS, mercaptan, thioether and other sulfide in less steps.
Owner:江苏蓝电环保股份有限公司
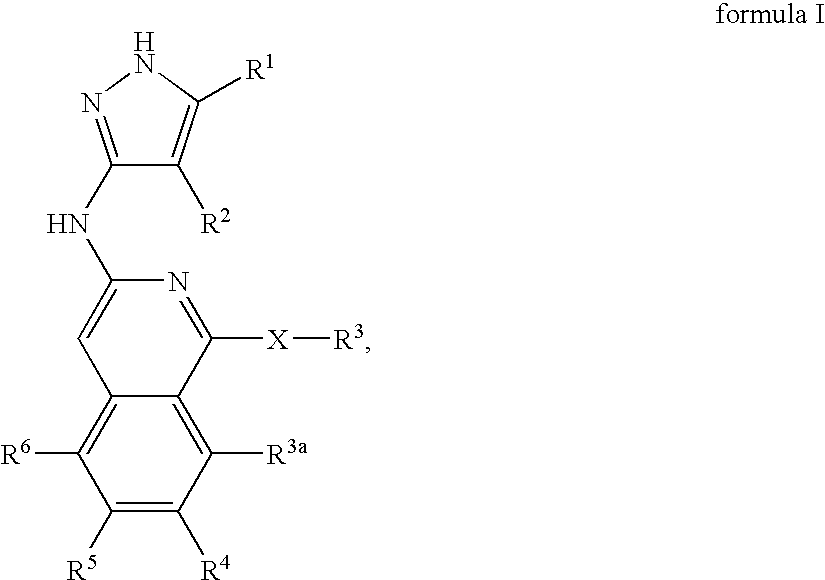
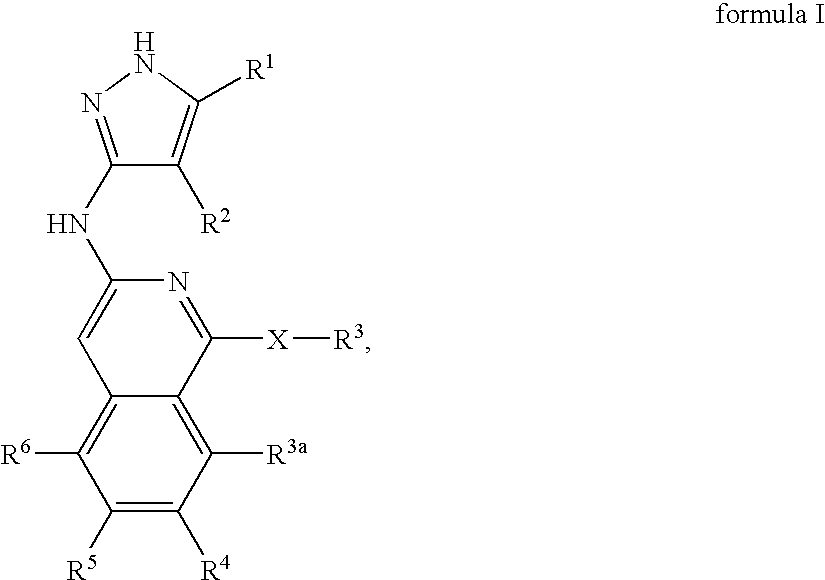
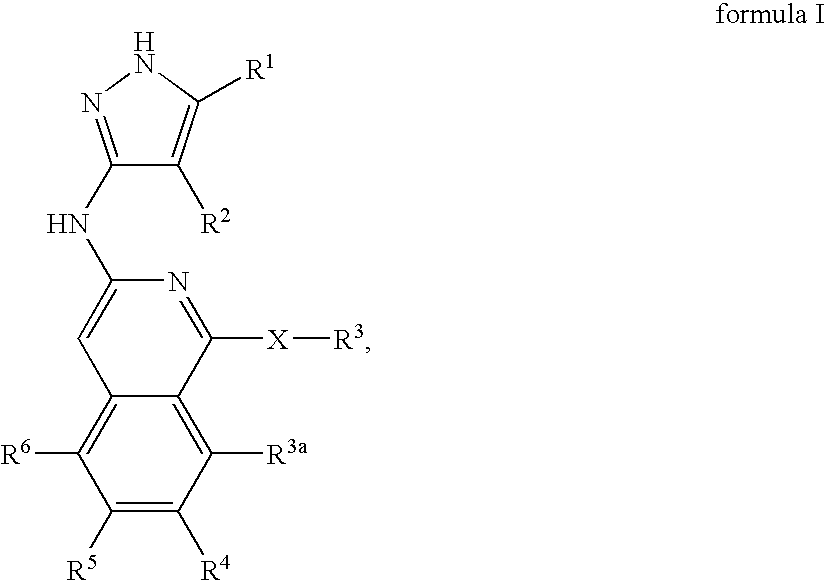
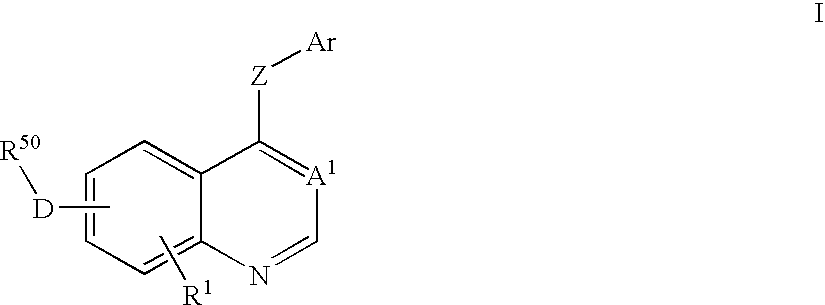
Isoquinoline aminopyrazole derivatives
The present invention relates to the compounds of formula I: their pharmaceutically acceptable salts or esters, enantiomeric forms, diastereoisomers and racemates, the preparation of such compounds, pharmaceutical compositions containing them and their manufacture, as well as the use of such compounds in the control or prevention of illnesses such as cancer.
Owner:F HOFFMANN LA ROCHE & CO AG
c-Met modulators and methods of use
The present invention provides compounds for modulating protein kinase enzymatic activity for modulating cellular activities such as proliferation, differentiation, programmed cell death, migration and chemoinvasion. More specifically, the invention provides quinazolines and quinolines which inhibit, regulate and / or modulate kinase receptor, particularly c-Met, KDR, c-Kit, flt-3 and flt-4, signal transduction pathways related to the changes in cellular activities as mentioned above, compositions which contain these compounds, and methods of using them to treat kinase-dependent diseases and conditions. The present invention also provides methods for making compounds as mentioned above, and compositions which contain these compounds.
Owner:EXELIXIS INC
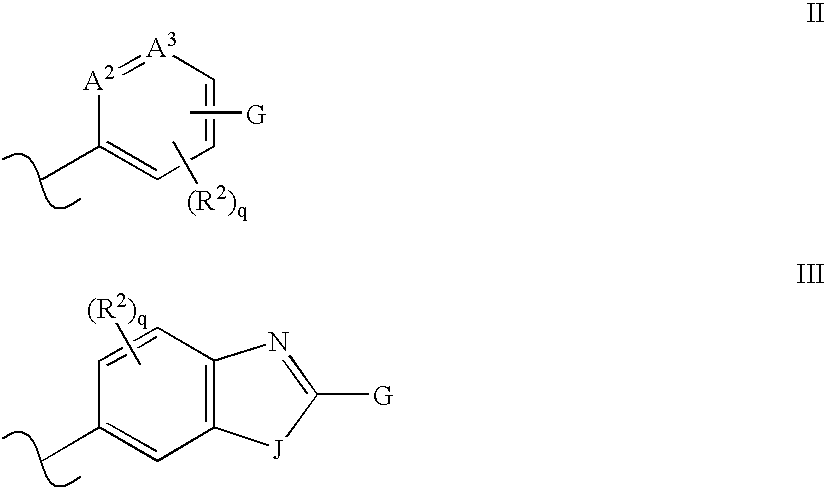
Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide
Owner:VERTEX PHARMA INC
8-Hydroxy quinoline derivatives
ActiveUS20080161353A1Improve solubilityMaintain permeabilityBiocideSenses disorderQuinoline8-Hydroxyquinoline
Owner:PRANA BIOTECHNOLOGY LTD
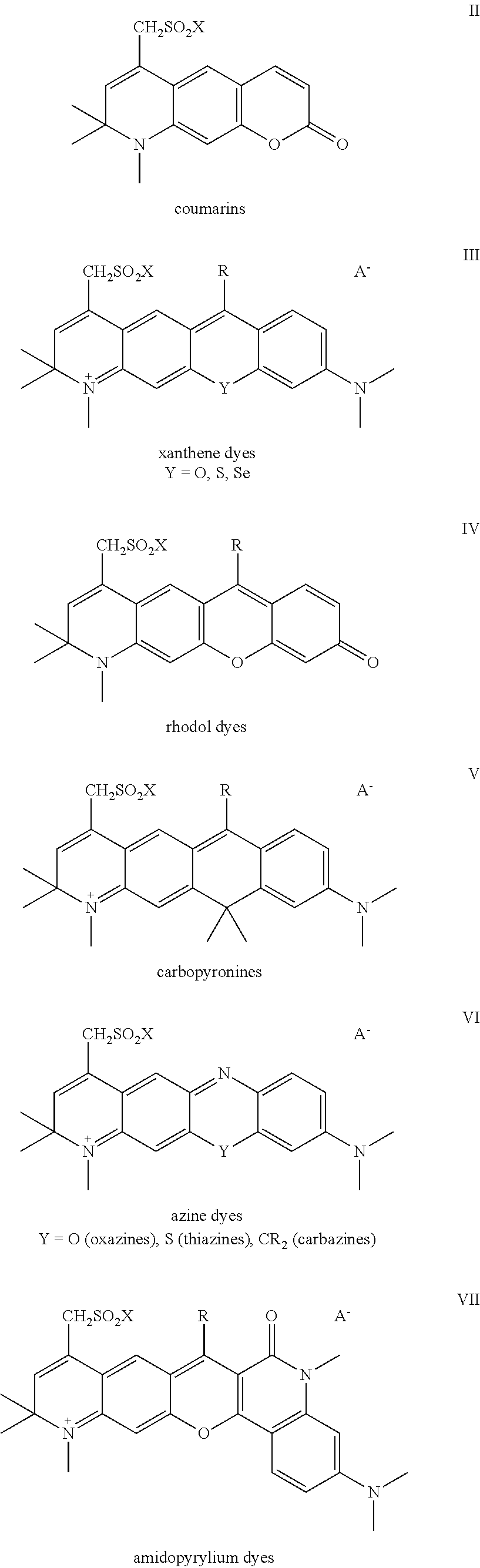
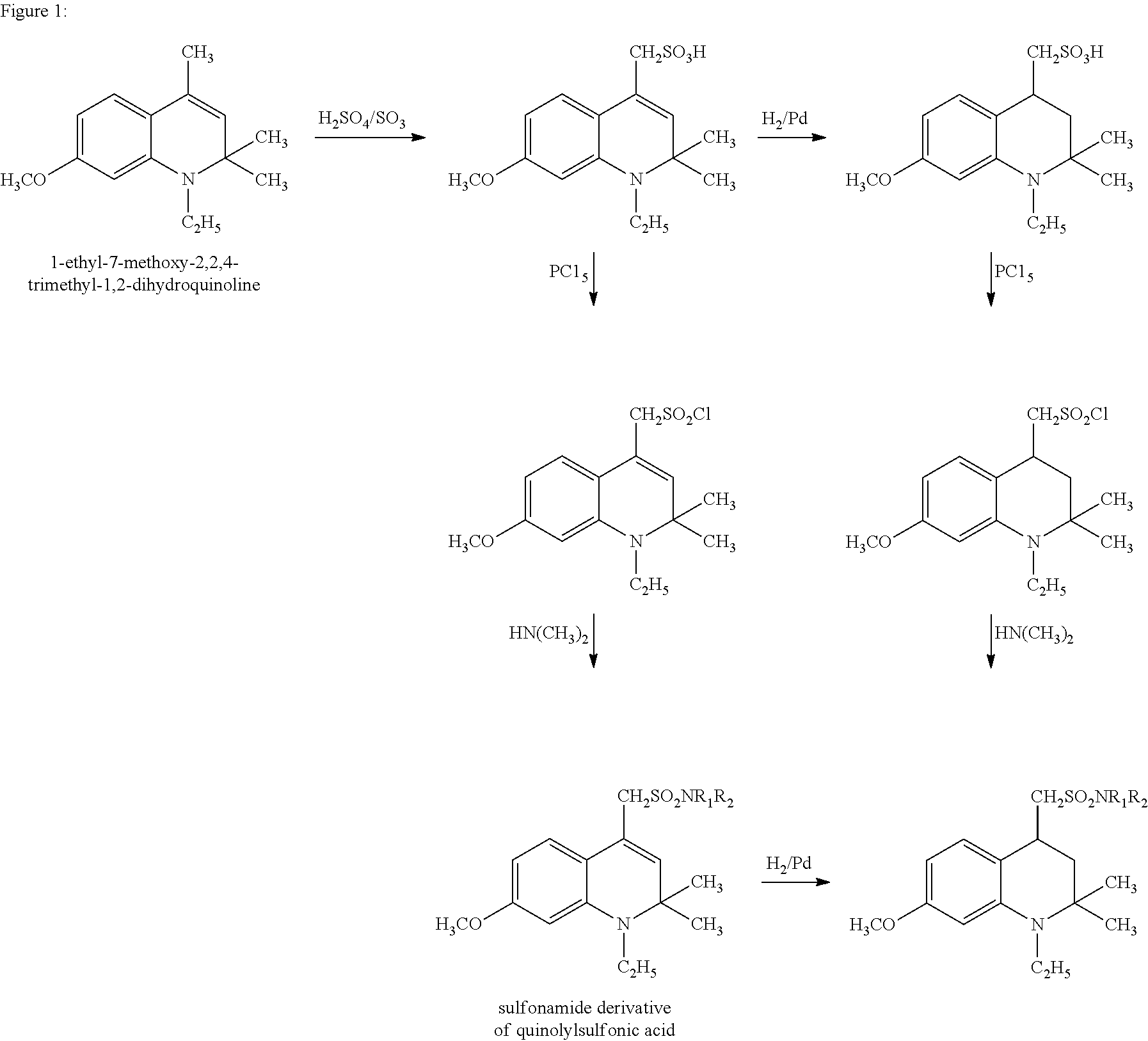
Sulfonamide derivatives of polycyclic dyes used for analytical applications
ActiveUS20110190486A1Simple waySimple and cost-effectiveSugar derivativesPyronine/xanthon/thioxanthon/selenoxanthan/telluroxanthan dyesQuinolinePhotochemistry
The invention concerns the production of quinoline compounds containing sulfonic acid groups, the said quinoline compounds and their conversion into dyes containing sulfonic acid groups. The dyes according to the invention are used especially to label analytes, for example to label biomolecules.
Owner:ATTO TEC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/7fc68549-2d37-4f83-89dd-f36e605466b3/US07553855-20090630-D00001.png)
![Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/7fc68549-2d37-4f83-89dd-f36e605466b3/US07553855-20090630-D00002.png)
![Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Compositions of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/7fc68549-2d37-4f83-89dd-f36e605466b3/US07553855-20090630-D00003.png)
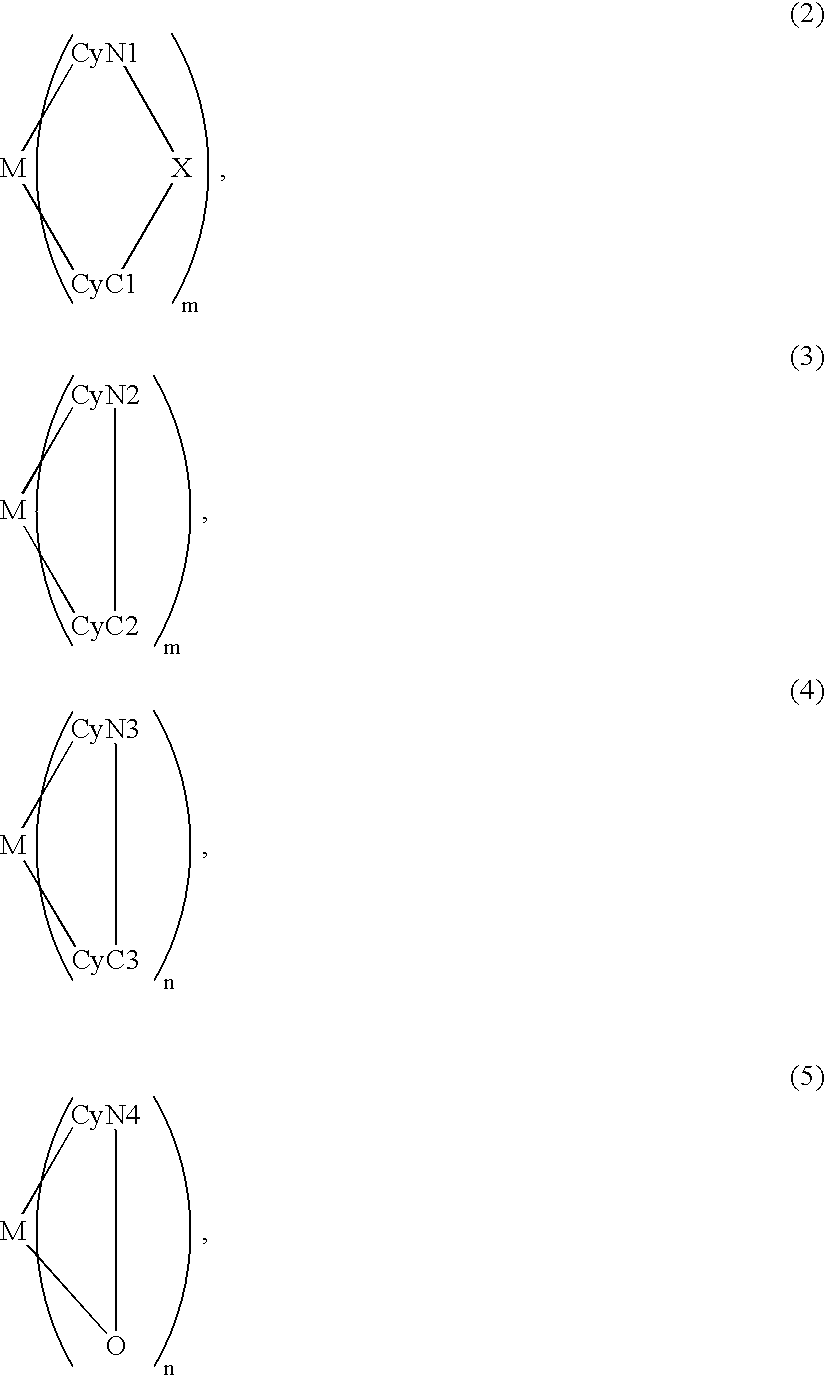
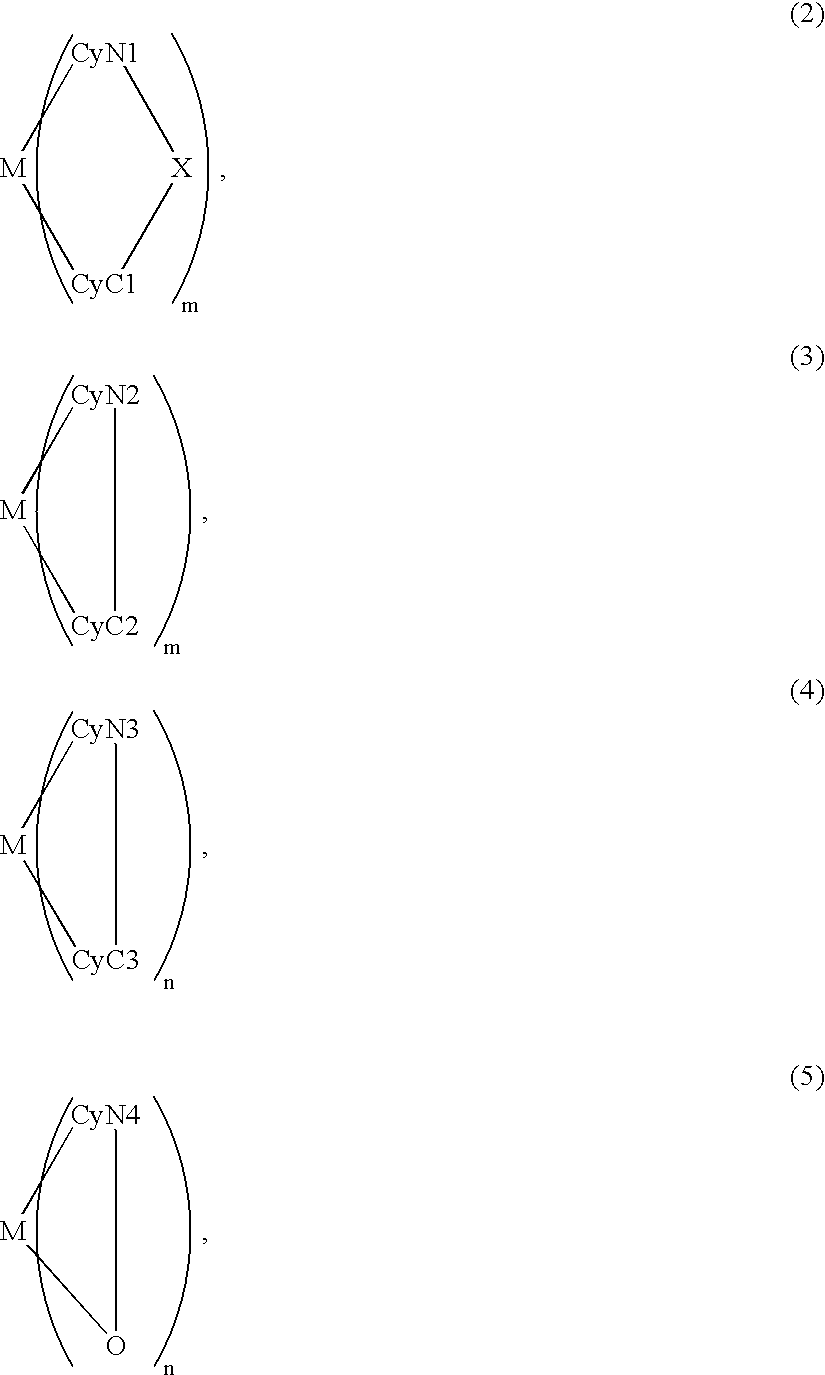
![Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/a113b4d7-c198-49c0-a9ea-68b5e5fb4c63/US20110064811A1-20110317-D00000.png)
![Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/a113b4d7-c198-49c0-a9ea-68b5e5fb4c63/US20110064811A1-20110317-D00001.png)
![Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/a113b4d7-c198-49c0-a9ea-68b5e5fb4c63/US20110064811A1-20110317-D00002.png)
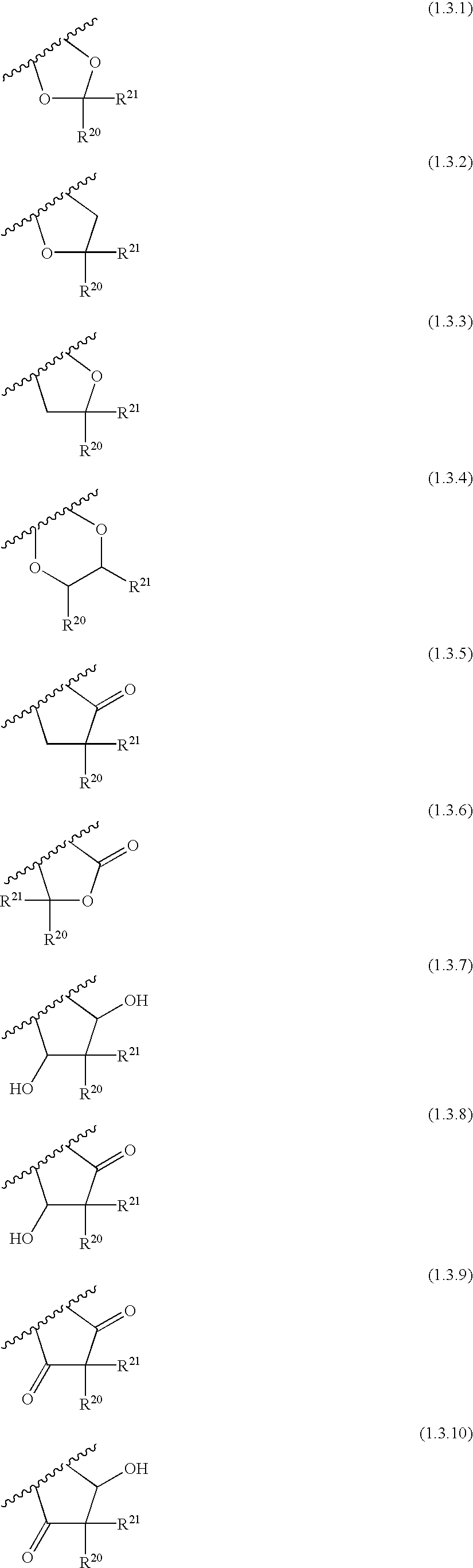
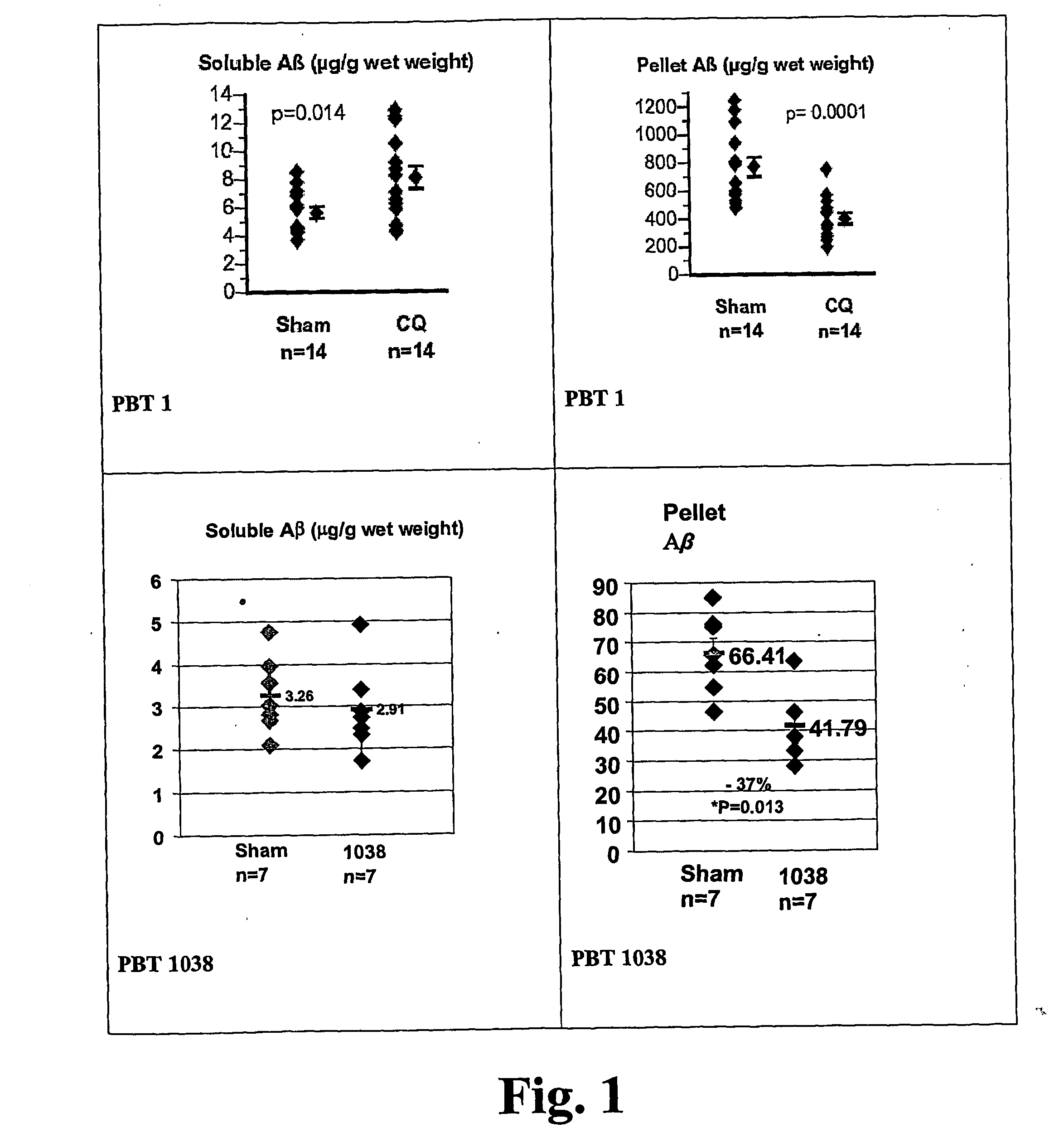
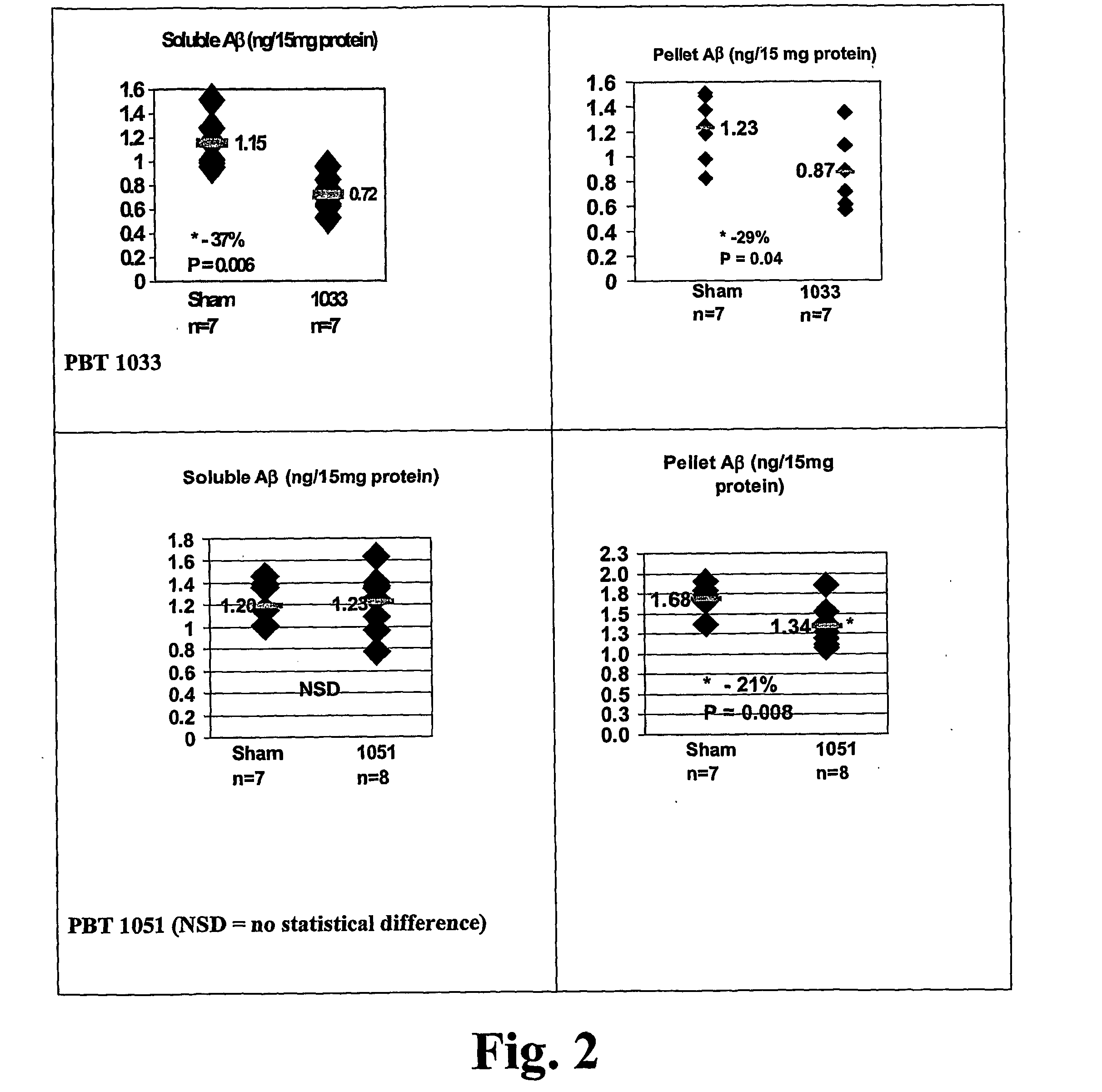
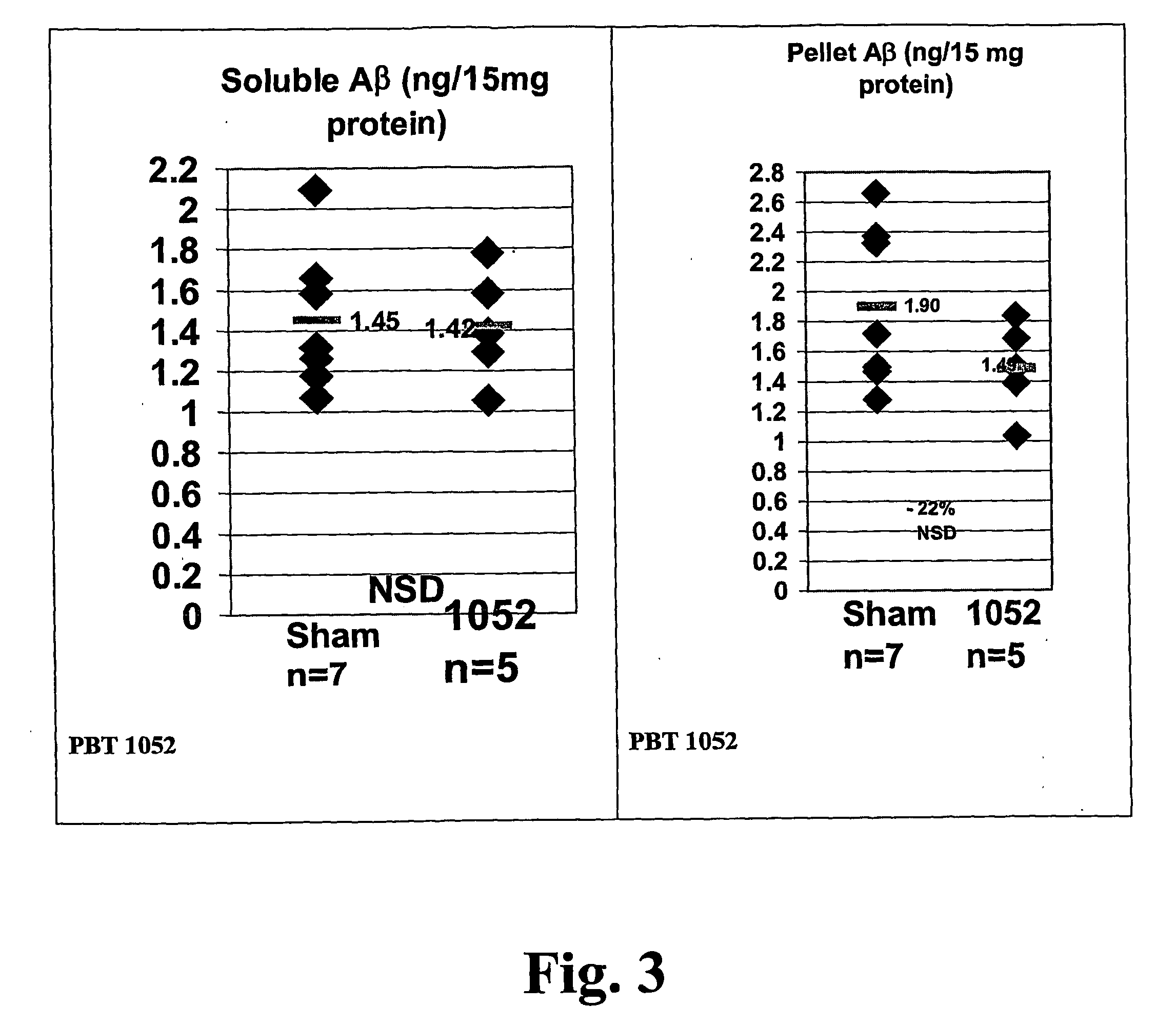
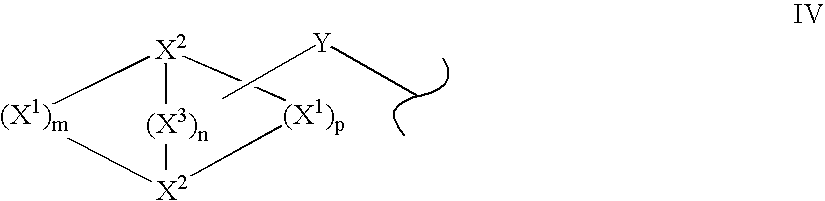
![Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/7e454e27-3fb8-45f3-af3c-0febeb707734/US08674108-20140318-D00001.png)
![Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/7e454e27-3fb8-45f3-af3c-0febeb707734/US08674108-20140318-D00002.png)
![Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/7e454e27-3fb8-45f3-af3c-0febeb707734/US08674108-20140318-D00003.png)