Complexes of red light iridium by using nitrogen heterocycles in quinoline as ligand, and application
A technology of iridium complex and nitrogen heterocycle is applied to red light iridium complex with quinoline nitrogen heterocycle as ligand and its application field, which can solve the problem that it is not easy to synthesize in large quantities, the internal quantum efficiency cannot exceed 25%, and the synthetic The method is complicated and other problems, to achieve the effect of easy synthesis and purification, short life, high efficiency red light emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] For the examples given, use the complex (1-NAPQ) 2 Ir(acac) is doped in the CBP host material to make organic EL devices. First, 50nm of N,N'-bis(1-naphthyl)-N,N'-diphenyl-1,1'-diphenyl-4,4'- is deposited on the surface coated with ITO glass. Diamine (NPB) serves as the hole transport layer. Then, CBP was deposited on the hole transport layer to form a 30nm light-emitting layer, which was doped with 3% (1-NAPQ) 2 Ir(acac). Finally, deposit a hole blocking layer (BCP: 10mm), an electron transport layer (Alq 3 : 40nm), interface layer (LiF: 1nm) and cathode (Al: 100nm).
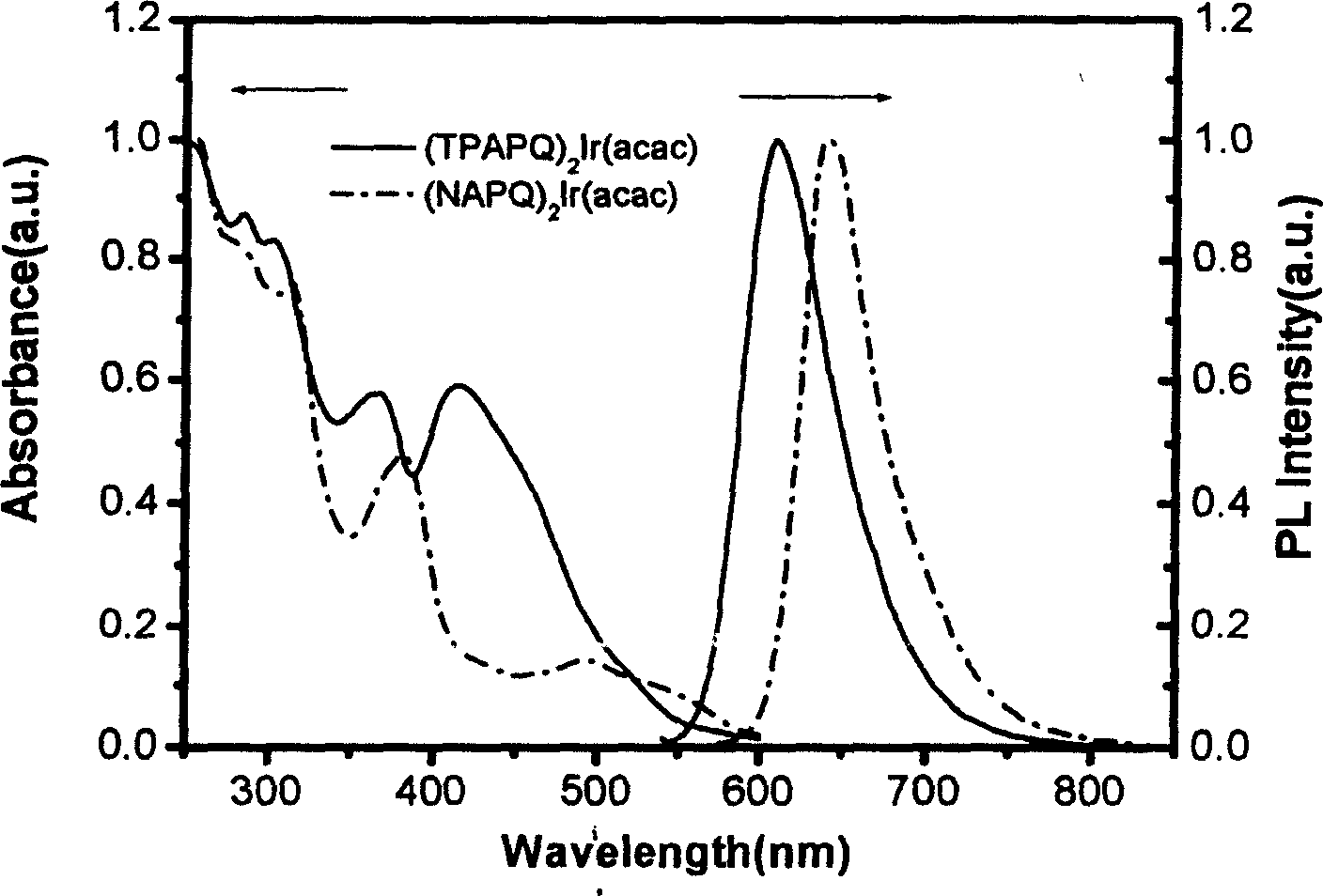
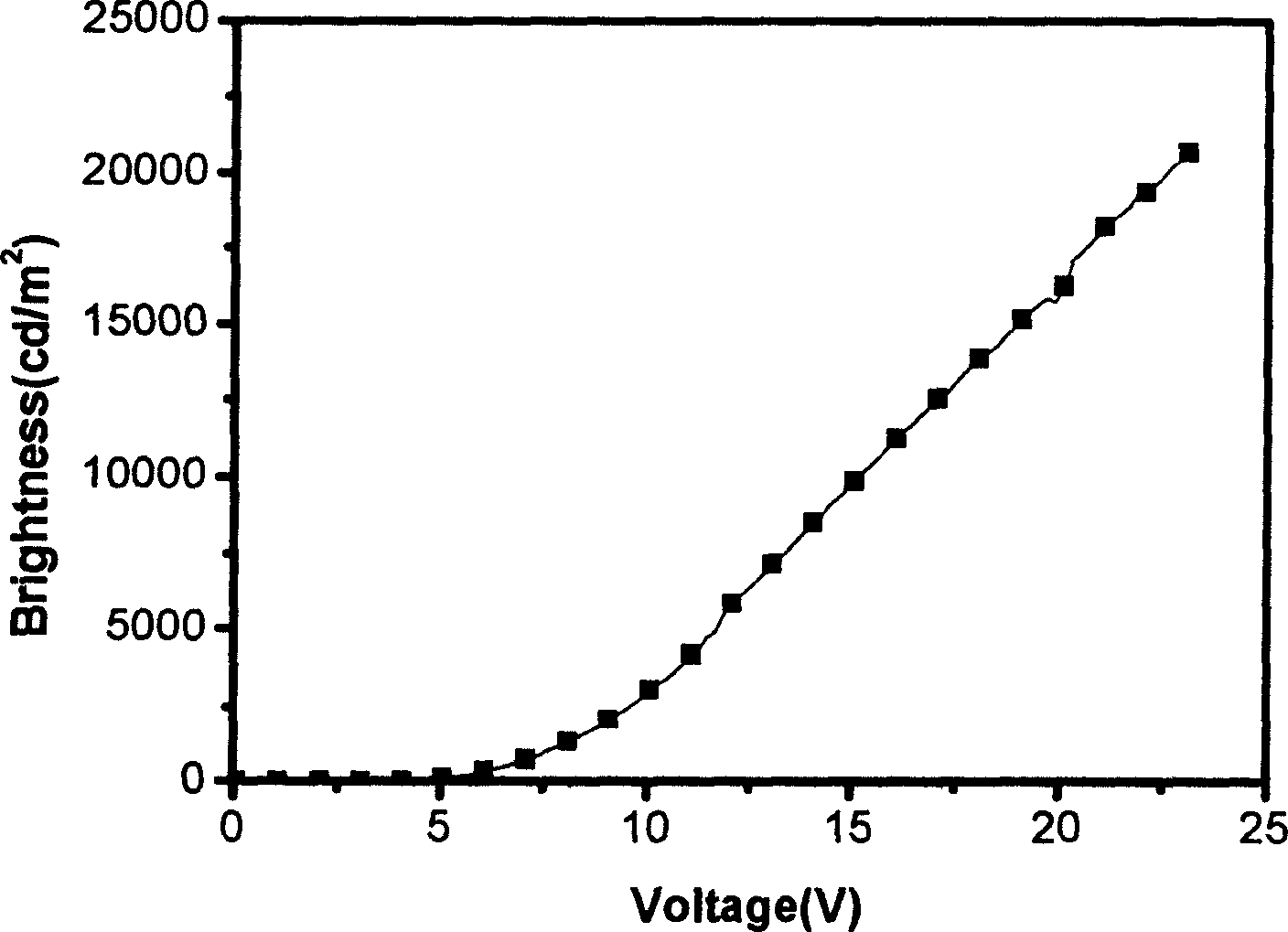
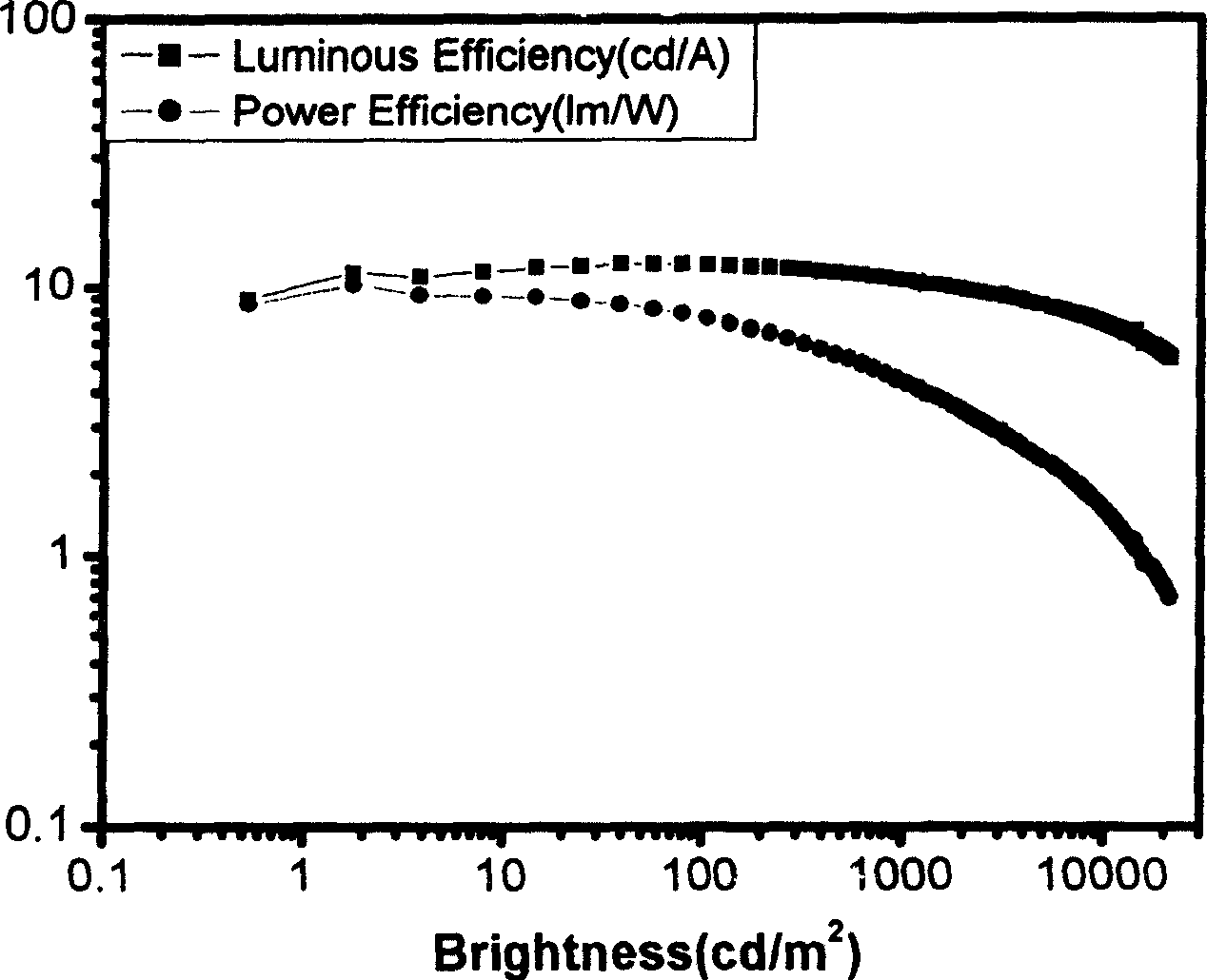
[0063] The resulting EL device is at 100cd / m 2 The luminous efficiency is 2.2cd / A, the external quantum efficiency is 3.0%, the emission peak is 642nm, the half-value width is 35nm, the color coordinate CIE value x=0.71, y=0.29.
Embodiment 2
[0065] For this example, a complex (TPAPQ) is used 2 Ir(acac) is doped in the CBP host material to make organic EL devices. First, 50nm of N,N'-bis(1-naphthyl)-N,N'-diphenyl-1,1'-diphenyl-4,4'- is deposited on the surface coated with ITO glass. Diamine (NPB) serves as the hole transport layer. Then, CBP was deposited on the hole transport layer to form a 30nm light-emitting layer, which was doped with 7% (TPAPQ) 2 Ir(acac). Finally, a hole blocking layer (BCP: 10nm), an electron transport layer (Alq 3 : 40nim), interface layer (LiF: 1nm) and cathode (Al: 100nm).
[0066] The resulting EL device is at 100cd / m 2 The luminous efficiency is 12.2cd / A, the external quantum efficiency is 9.0%, the emission peak is 616nm, the half-value width is 48nm, the color coordinate CIE value x=0.67, y=0.32.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| full width at half maximum | aaaaa | aaaaa |
| full width at half maximum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com