Patents
Literature
5482results about How to "Reduce degradation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
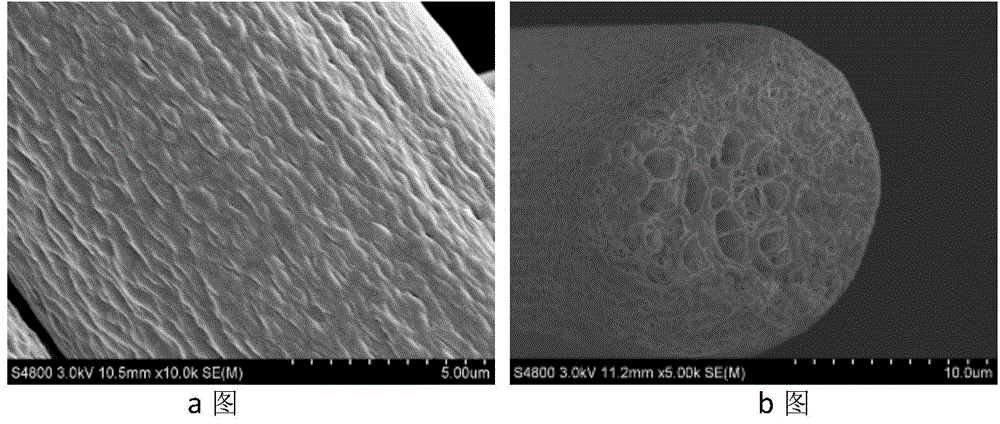
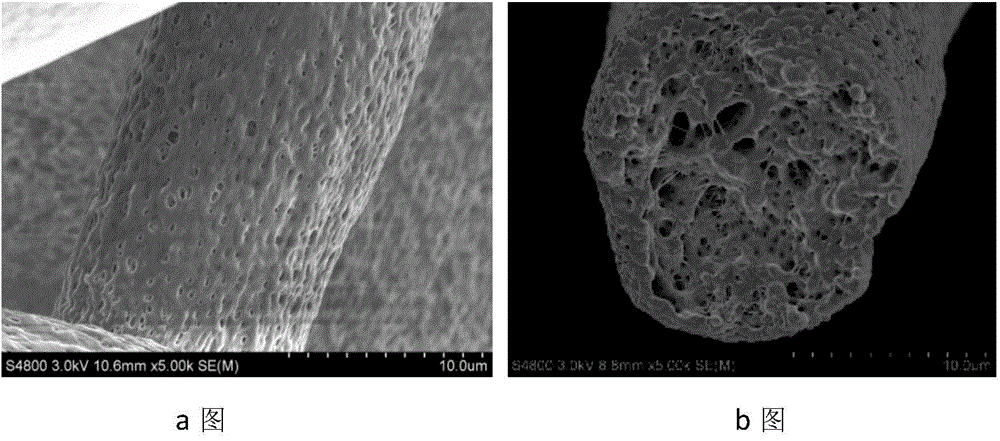
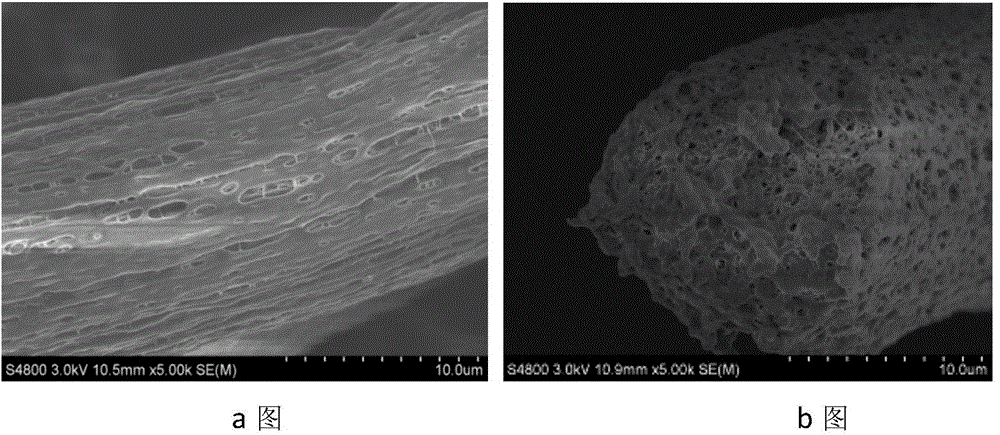
Preparation method of porous fiber non-woven fabric
ActiveCN103981635AReduce melt viscosityReduce degradationFilament forming substance formingMelt spinning methodsDiluentNonwoven fabric
The invention relates to a preparation method of a porous fiber non-woven fabric. The aim of the preparation method is to improve the product performance of the conventional non-woven fabric, so that the non-woven fabric meets the requirements on high-precision and high-performance filter. The technical scheme is that the preparation method of the porous fiber non-woven fabric comprises the following steps in sequence: (1) uniformly mixing a polymer and a diluent to obtain a blend with 10 to 60 percent of polymer; (2) melting and extruding the blend in the step (1) by adopting a screw extruder granulator, and directly cooling and granulating in air; (3) producing master batches in the step (2) by melt-down equipment to obtain a primary non-woven fabric; (4) extracting to remove the diluent from the primary non-woven fabric in the step (3), performing pore-forming on fibers in the non-woven fabric, and drying to obtain the porous fiber non-wave fabric; (5) recovering mixed waste liquid of the diluent and an extraction agent for reuse.
Owner:浙江省轻工业品质量检验研究院
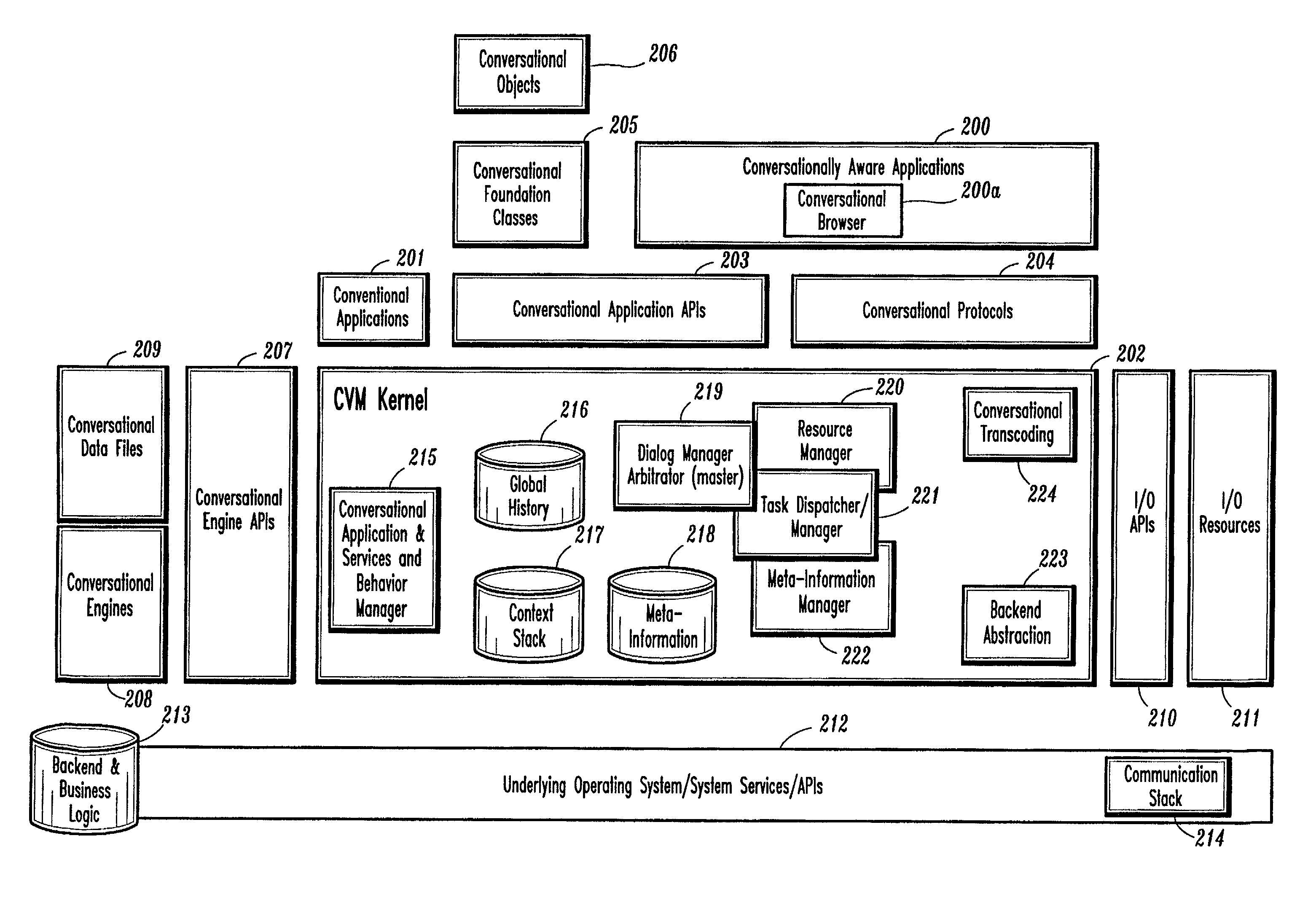
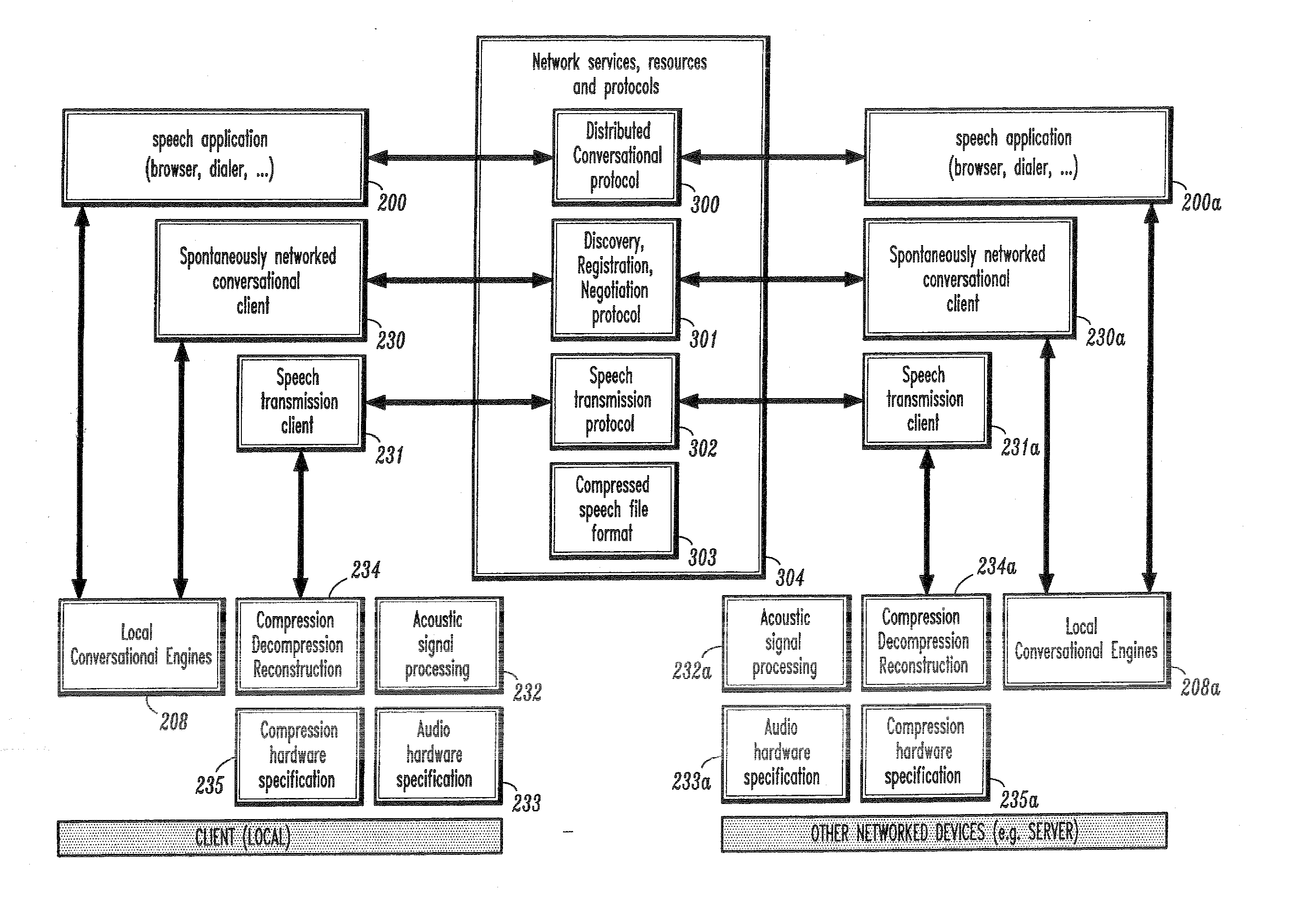
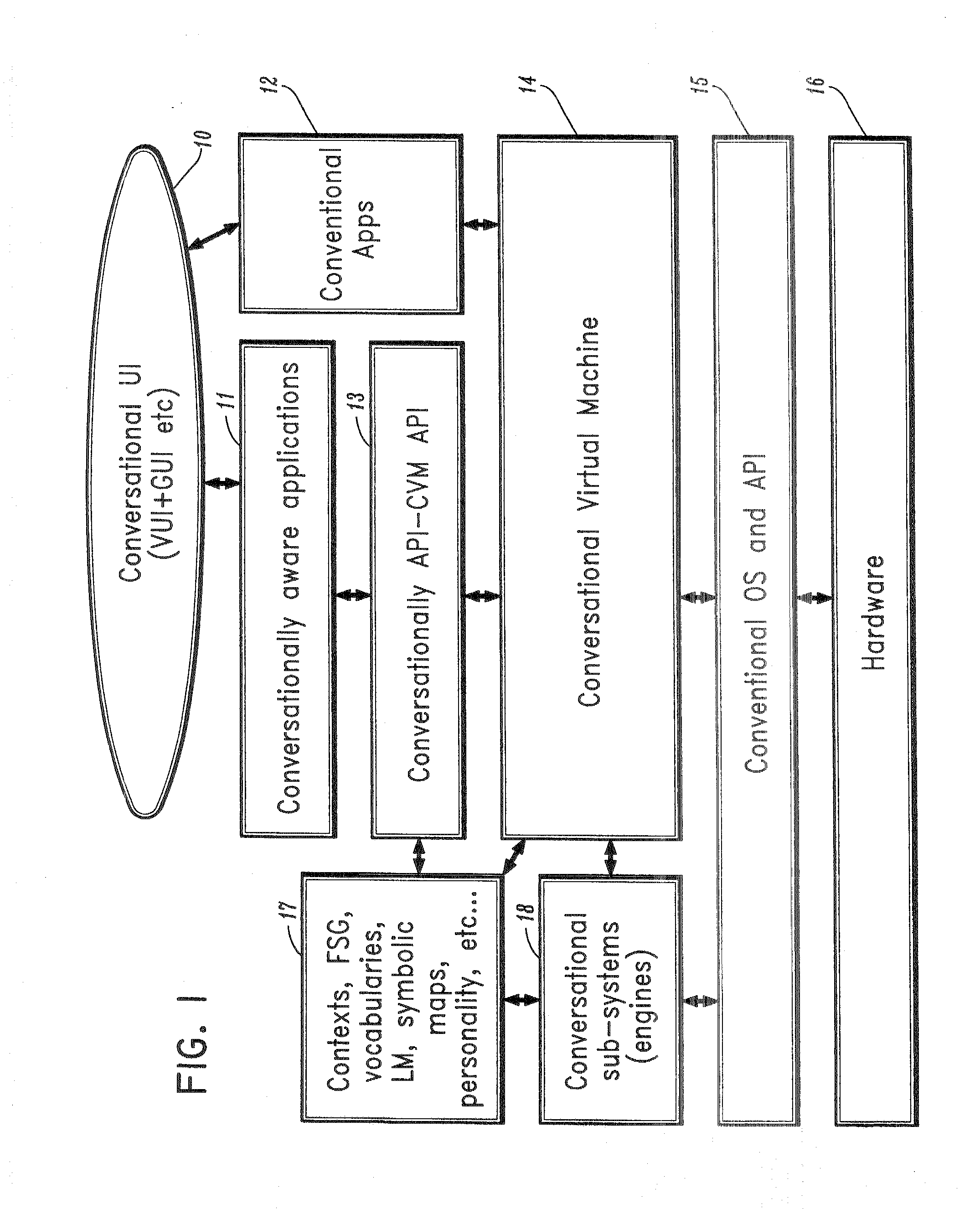
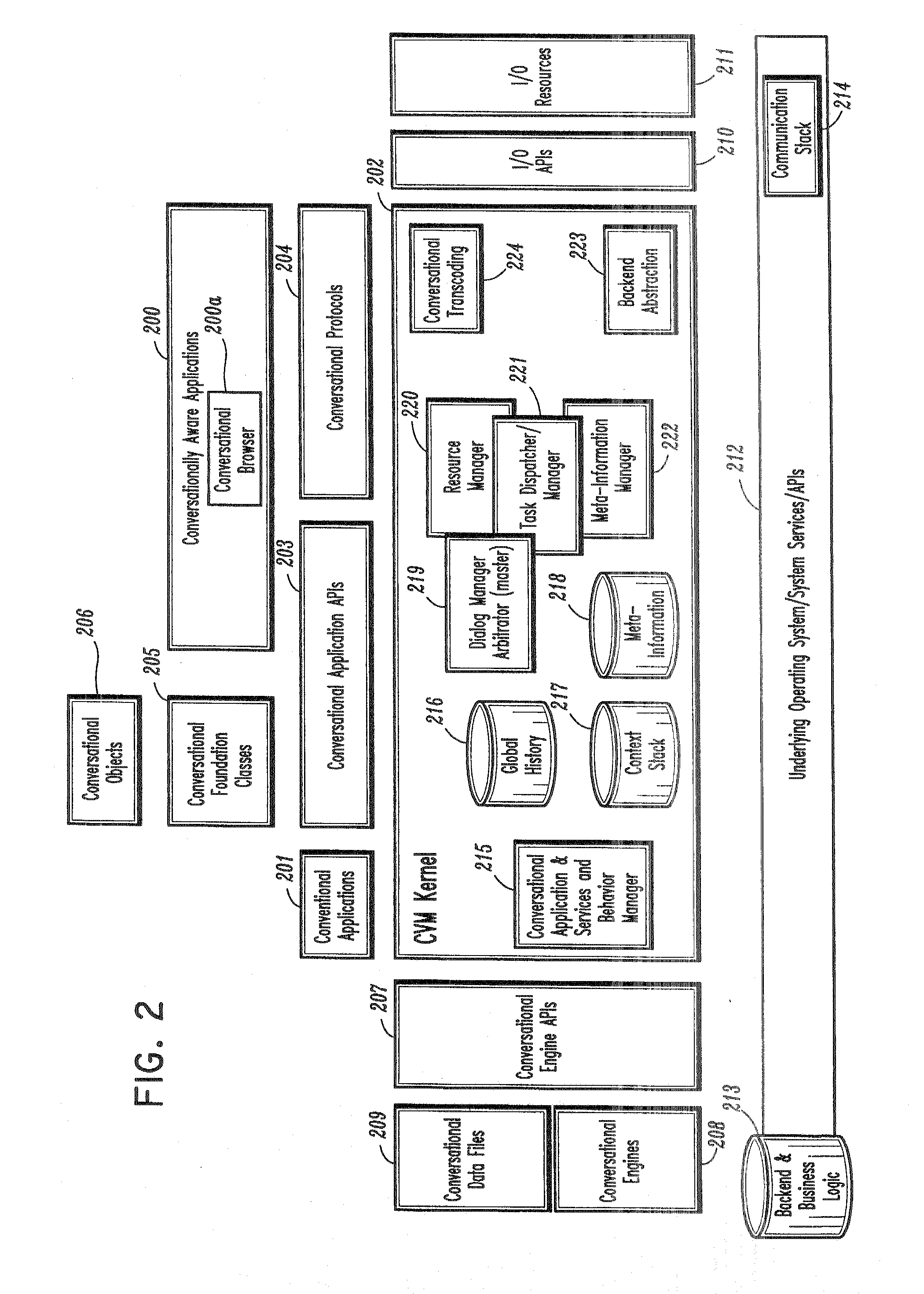
Conversational computing via conversational virtual machine
InactiveUS7137126B1Limitation for transferReduce degradationInterconnection arrangementsResource allocationConversational speechApplication software
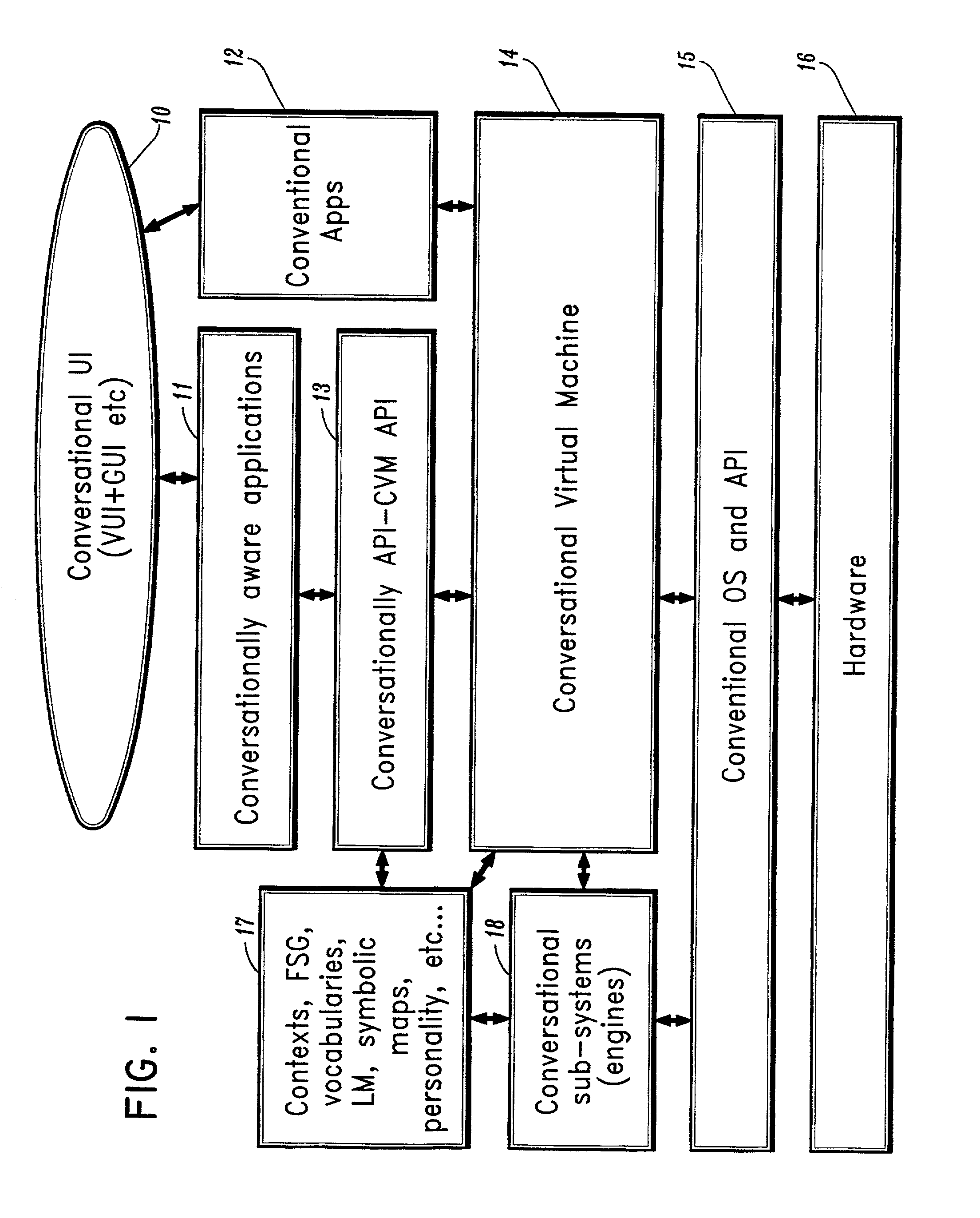
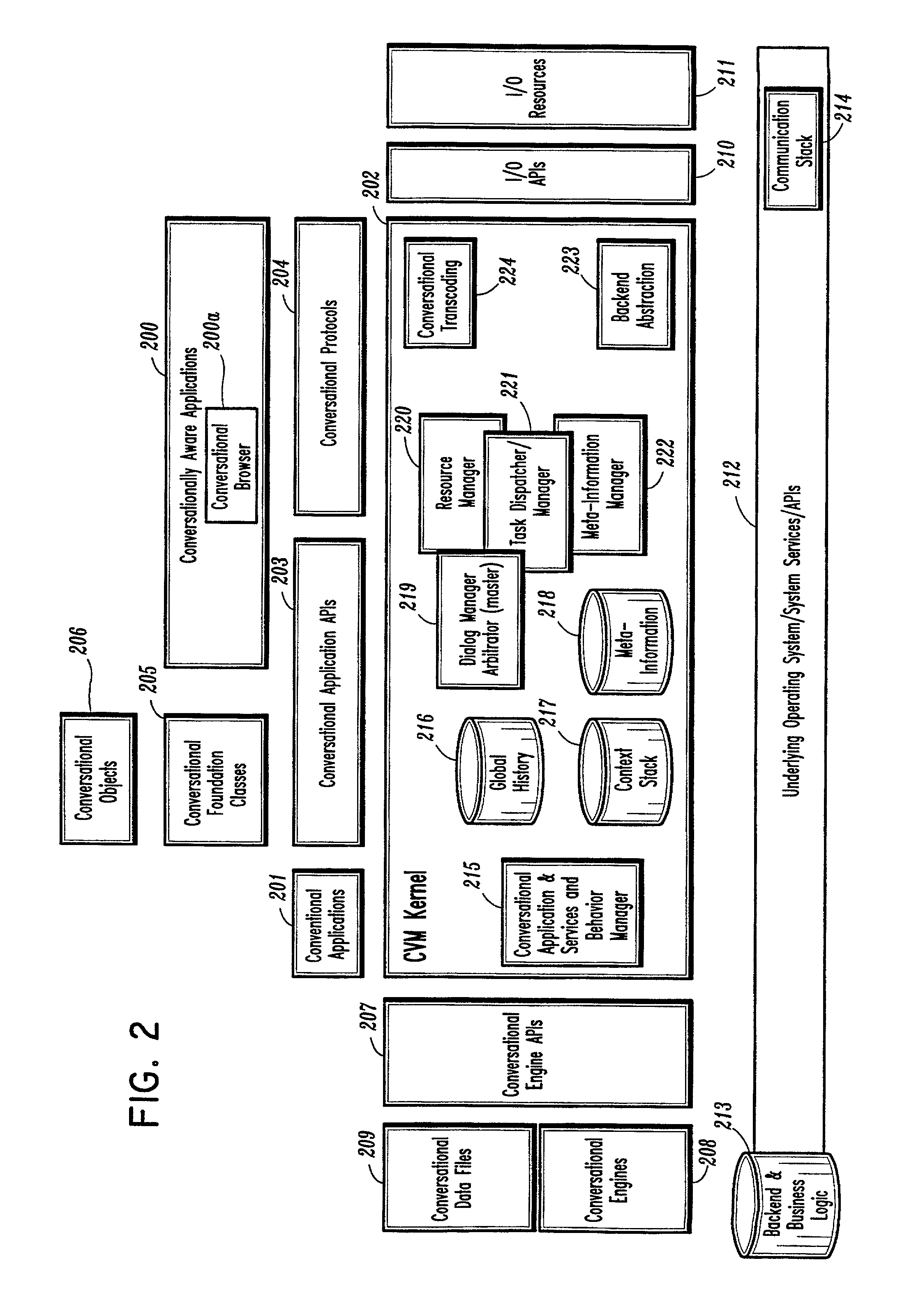
A conversational computing system that provides a universal coordinated multi-modal conversational user interface (CUI) (10) across a plurality of conversationally aware applications (11) (i.e., applications that “speak” conversational protocols) and conventional applications (12). The conversationally aware maps, applications (11) communicate with a conversational kernel (14) via conversational application APIs (13). The conversational kernel (14) controls the dialog across applications and devices (local and networked) on the basis of their registered conversational capabilities and requirements and provides a unified conversational user interface and conversational services and behaviors. The conversational computing system may be built on top of a conventional operating system and APIs (15) and conventional device hardware (16). The conversational kernel (14) handles all I / O processing and controls conversational engines (18). The conversational kernel (14) converts voice requests into queries and converts outputs and results into spoken messages using conversational engines (18) and conversational arguments (17). The conversational application API (13) conveys all the information for the conversational kernel (14) to transform queries into application calls and conversely convert output into speech, appropriately sorted before being provided to the user.
Owner:UNILOC 2017 LLC
Bottom up fill in high aspect ratio trenches
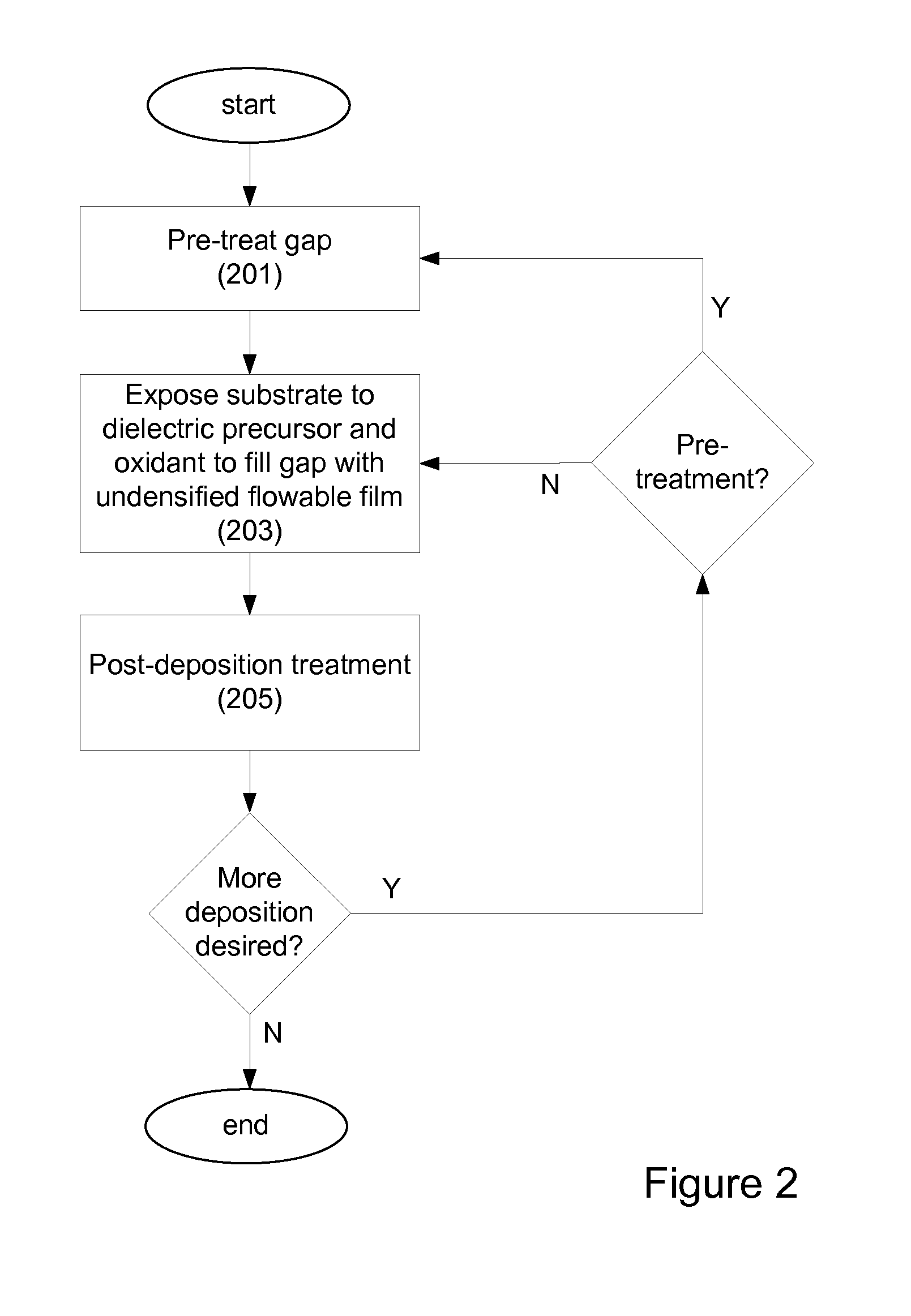
InactiveUS20120149213A1Improve gap fillingReduce nucleation delayLiquid surface applicatorsSemiconductor/solid-state device manufacturingHydrogenNitrogen
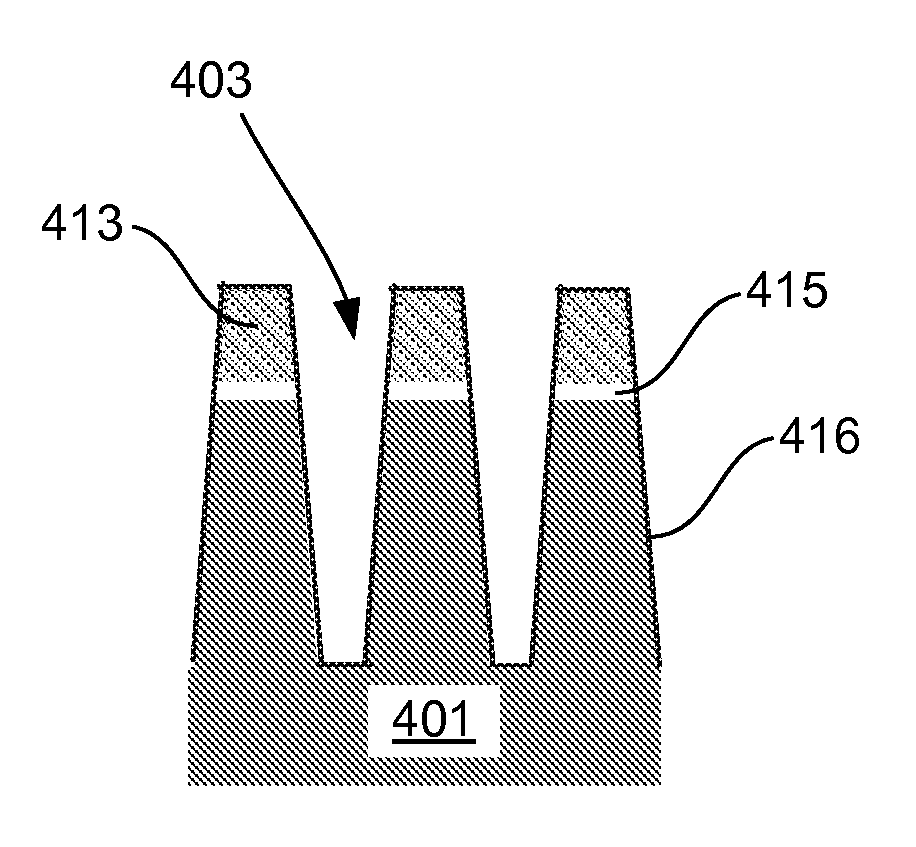
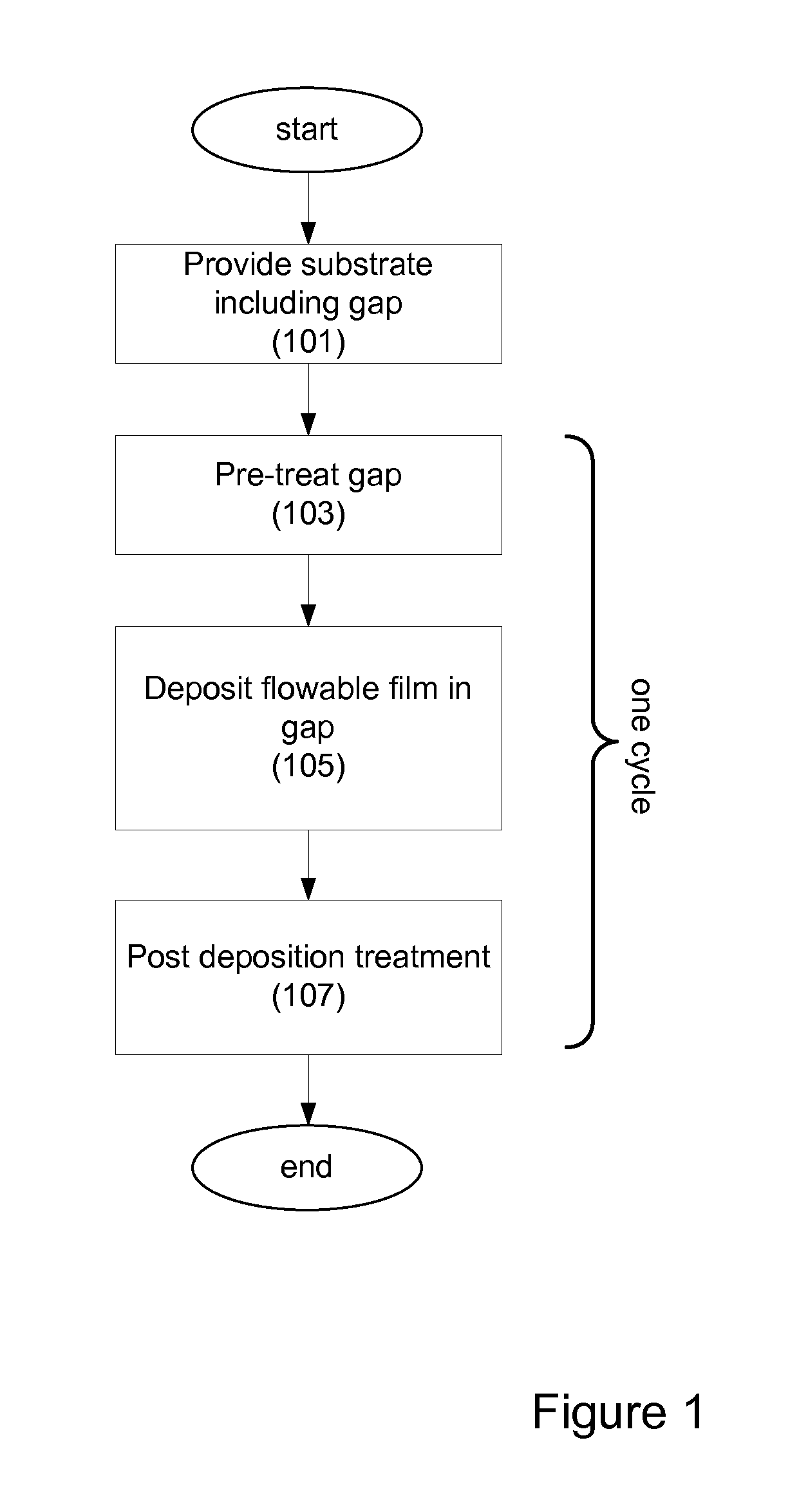
Provided are novel methods of filling gaps with a flowable dielectric material. According to various embodiments, the methods involve performing a surface treatment on the gap to enhance subsequent bottom up fill of the gap. In certain embodiments, the treatment involves exposing the surface to activated species, such as activated species of one or more of nitrogen, oxygen, and hydrogen. In certain embodiments, the treatment involves exposing the surface to a plasma generated from a mixture of nitrogen and oxygen. The treatment may enable uniform nucleation of the flowable dielectric film, reduce nucleation delay, increase deposition rate and enhance feature-to-feature fill height uniformity.
Owner:NOVELLUS SYSTEMS
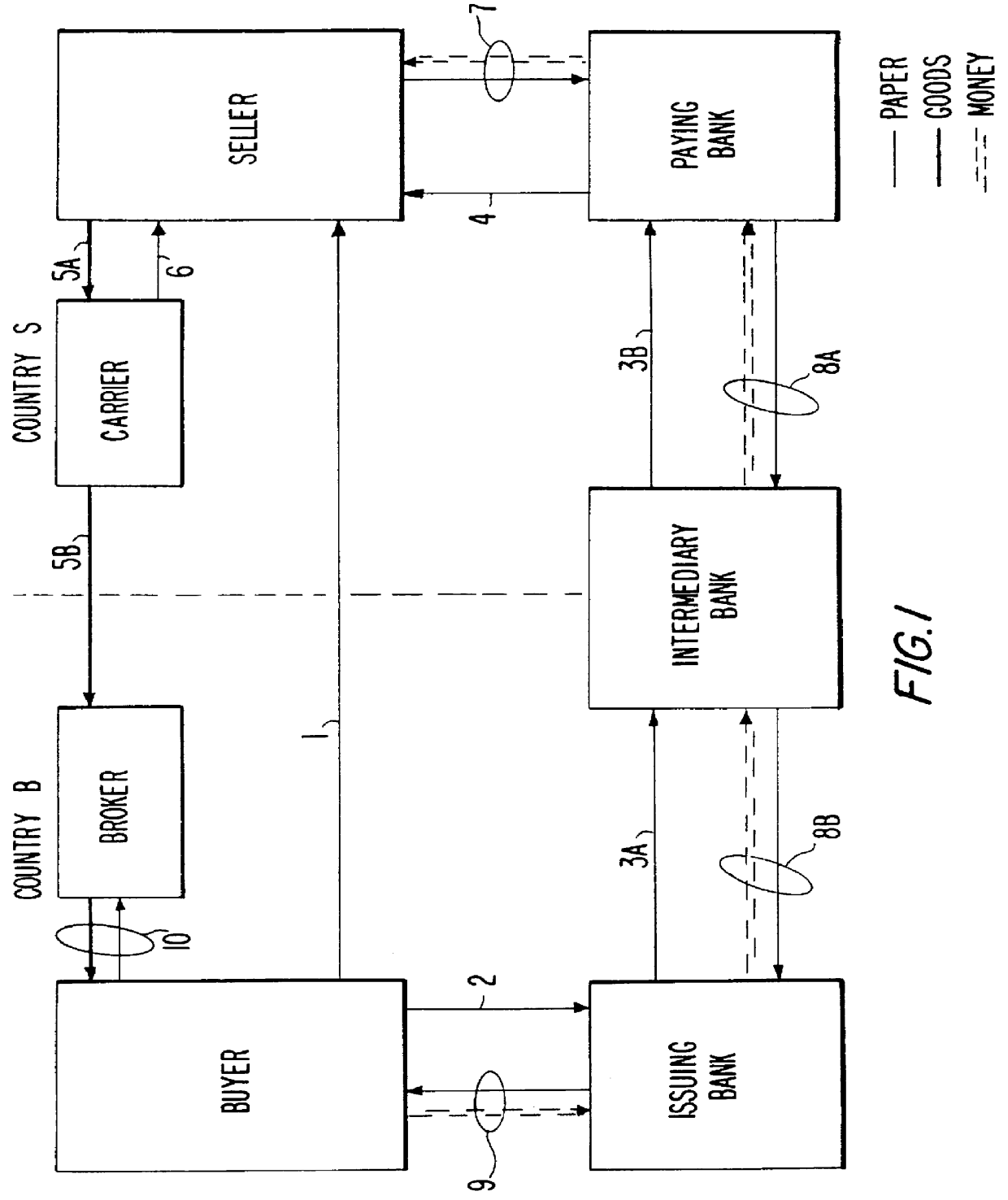
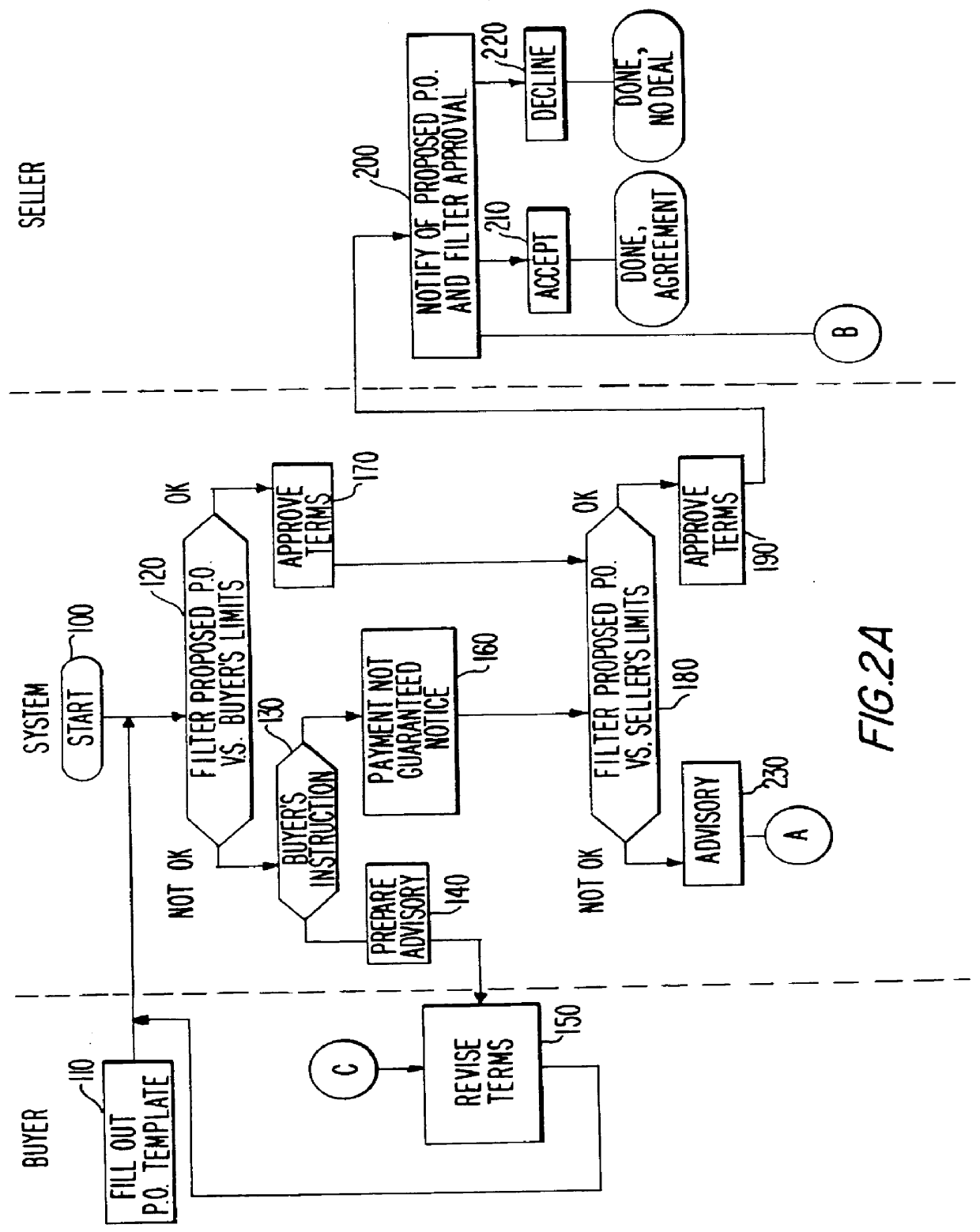
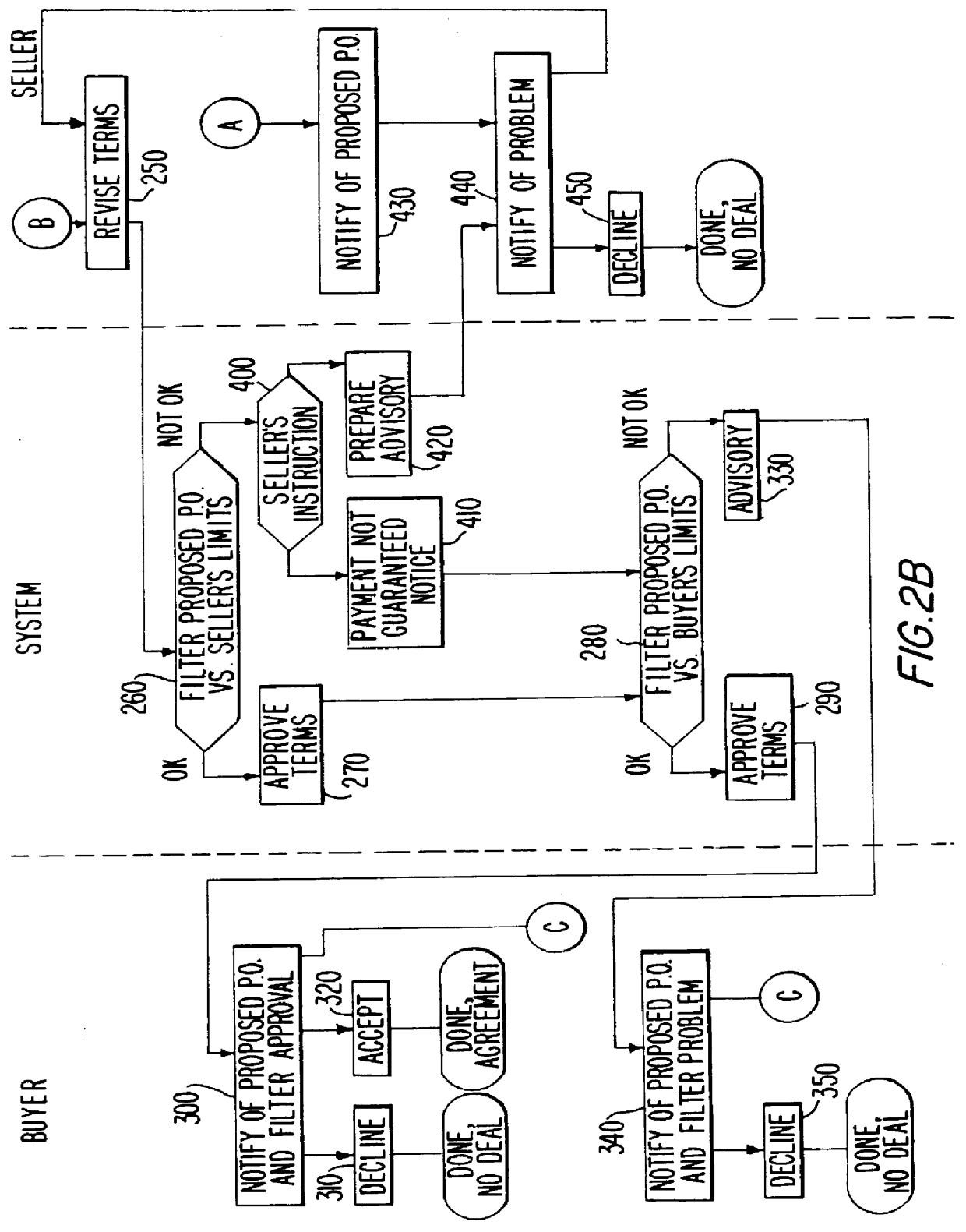
Full service trade system
InactiveUS6151588AAvoid disadvantagesReduce degradationFinancePayment architecturePaymentComputer science
A system stores criteria specified by a funder relating to trade transactions for buyers and sellers. The system compares the criteria with a proposed purchase order to determine whether the system can generate a payment guarantee on behalf of the funder for the buyer to the seller. The system also compares subsequent documents relating to an original purchase order with the original purchase order to ensure that the terms of the purchase order are properly fulfilled. When the appropriate conditions for payment are met, the system issues a funds transfer instruction to transfer payment from the buyer to the seller.
Owner:LOGICBLOX
Packet switching network, packet switching equipment and network management equipment
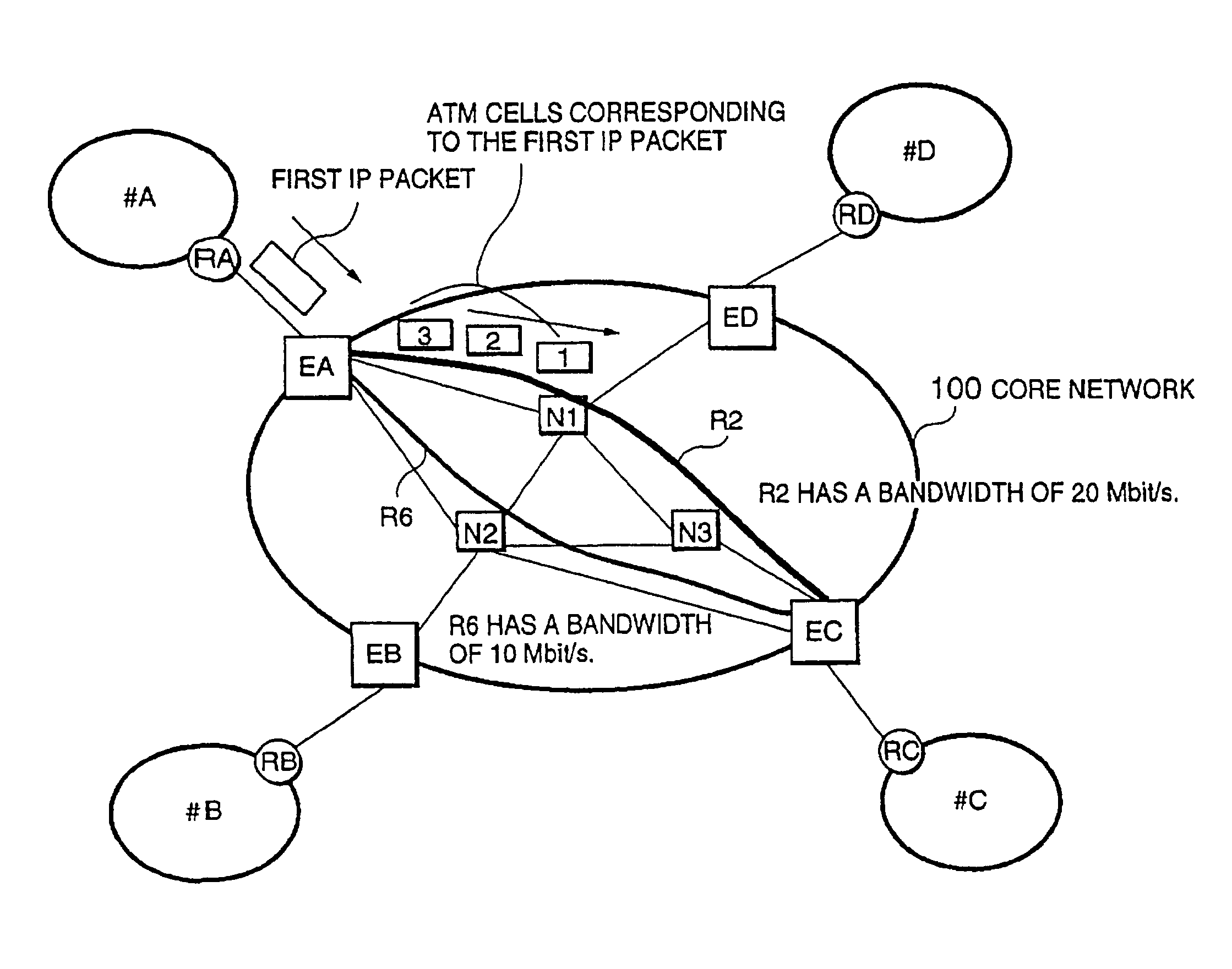
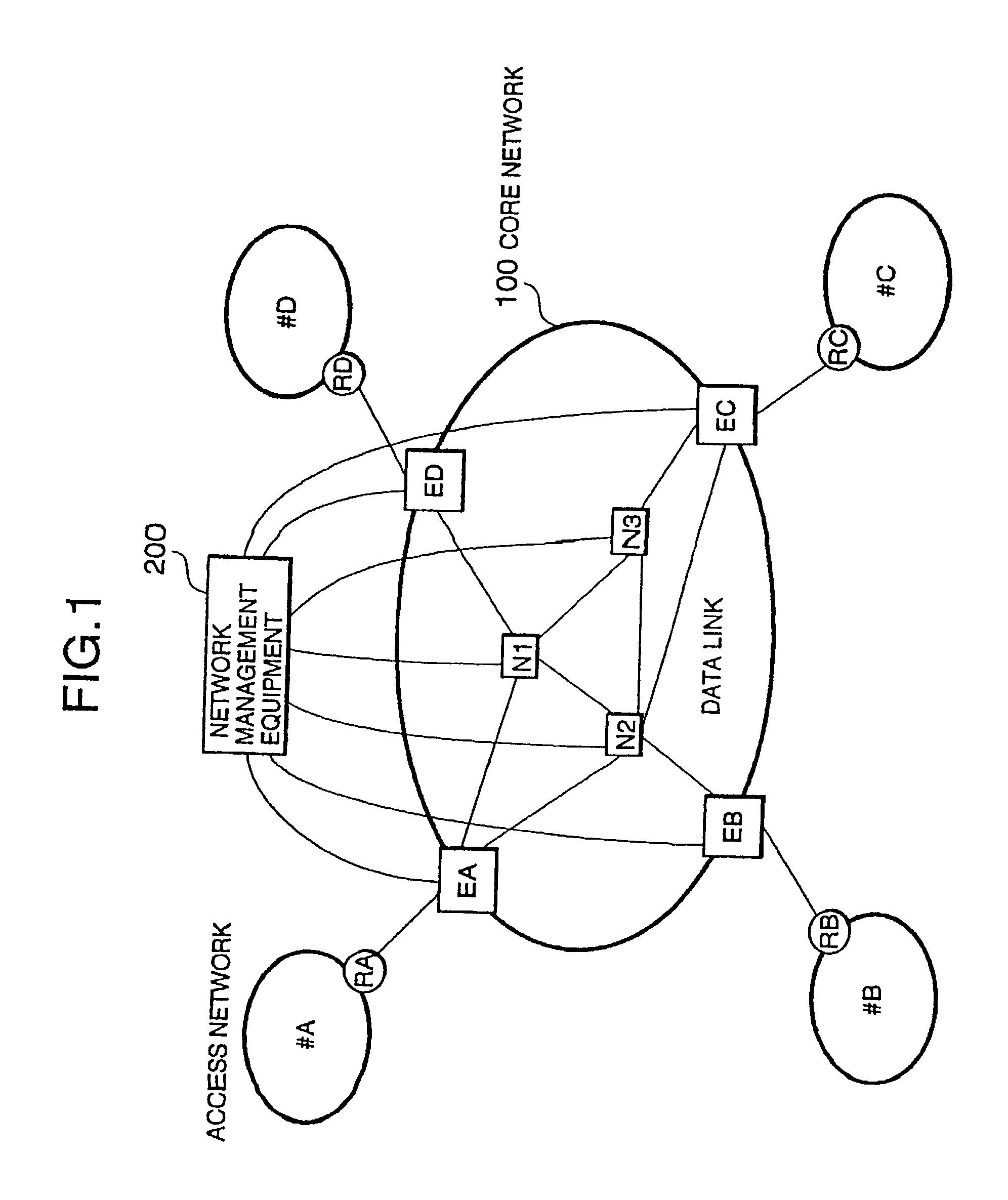
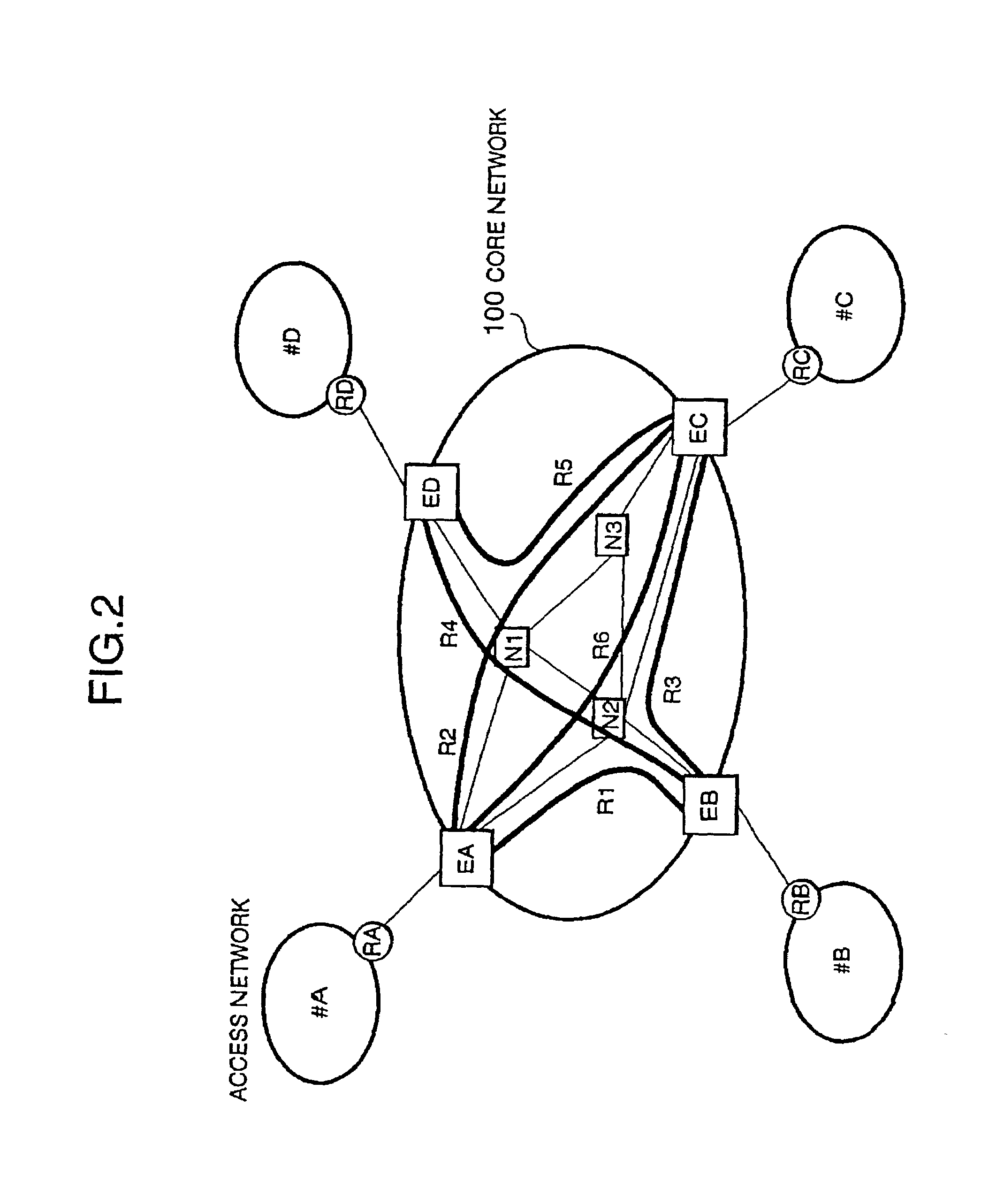
InactiveUS7046630B2Reduces delay variationReduce in quantityError preventionTransmission systemsAccess networkDelayed time
This invention provides a network management equipment and a packet switching equipment which eliminate a connection setup delay time, reduce a delay and a delay variation involved in data transfer, and effectively perform connectionless data flow processing in a large data network. The network is divided into a connection-oriented core network and a plurality of connectionless access networks connected to the core network where a plurality of connections (called permanent virtual route (PVR)) are set up among a plurality of edge nodes. The network management equipment selects one route from a plurality of PVRs for connectionless data flow received from one of the access networks and transfers data along the PVR.
Owner:HITACHI LTD
Compositions, devices, and methods for nicotine aerosol delivery
ActiveUS20140345635A1Reduce degradationConstant efficiencyTobacco treatmentTobacco devicesSolventElectron
The present disclosure generally relates to compositions, and related devices and methods, useful in vaporizing devices such as electronic cigarettes. The composition may comprise nicotine, at least one solvent, and at least one ion pairing agent, and may be vaporized to form a condensation aerosol, wherein inhalation of the aerosol allows for deposition of nicotine with the respiratory system, including deep lung deposition. The vaporizing device may comprise a vaporization unit, a battery, and an integrated circuit coupled to the battery, wherein the integrated circuit is configured to control the battery for rapid initial vaporization without overheating, producing thermal degradation products, or draining battery energy. The battery may operate with pulse width modulation for at least a portion of the time the vaporizing device is being used.
Owner:NJOY LLC
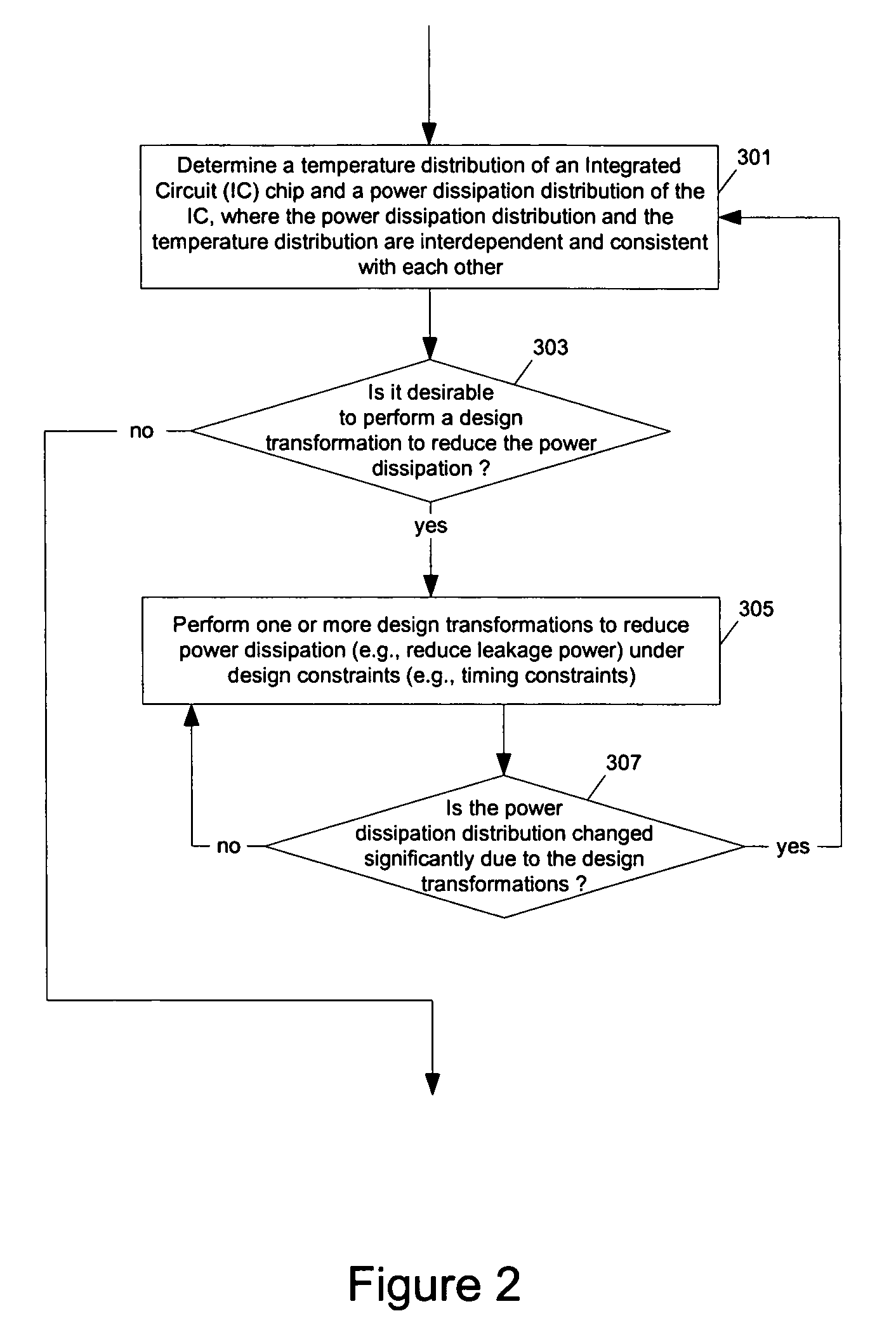
Methods and apparatuses for thermal analysis based circuit design
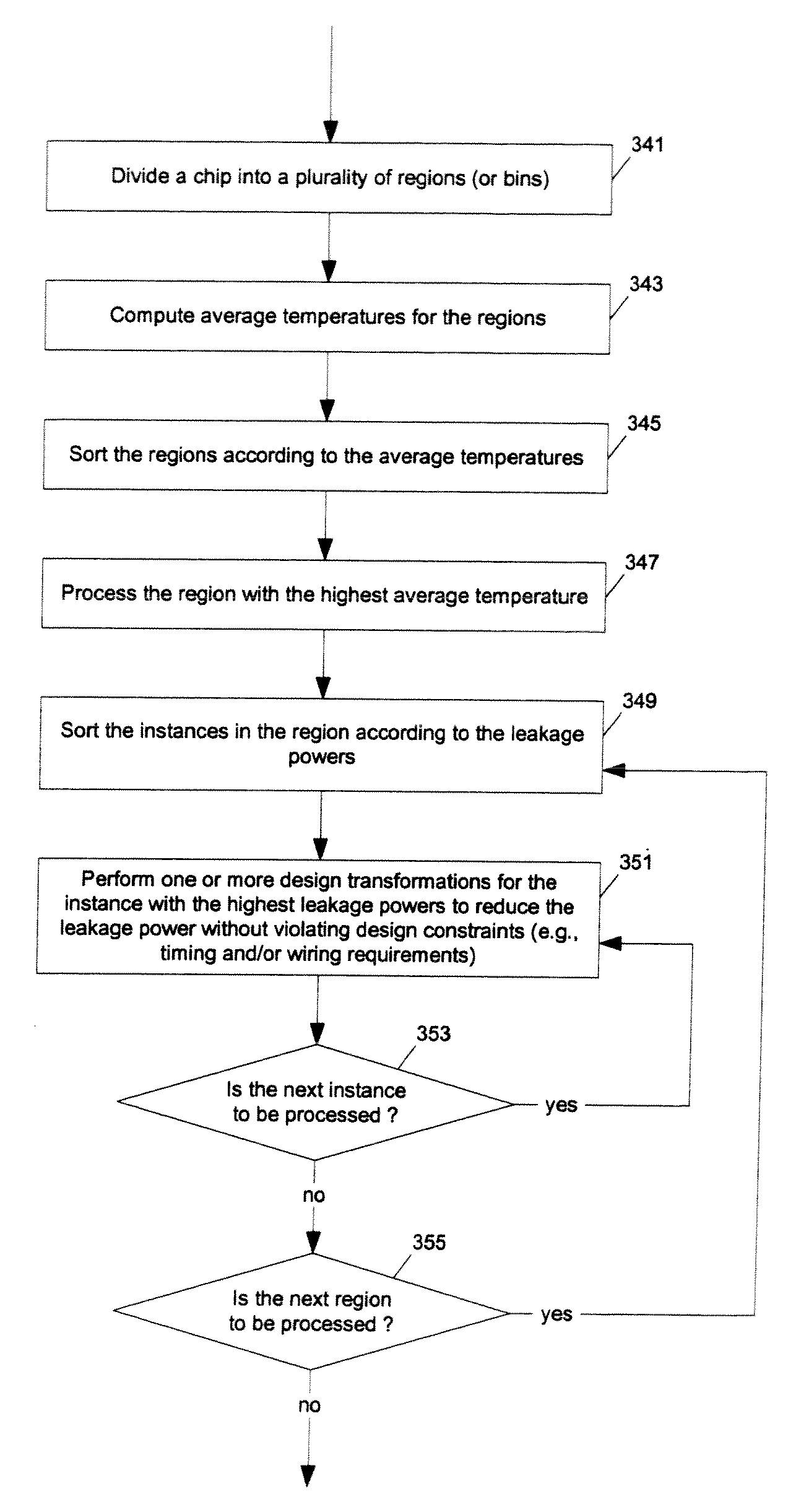
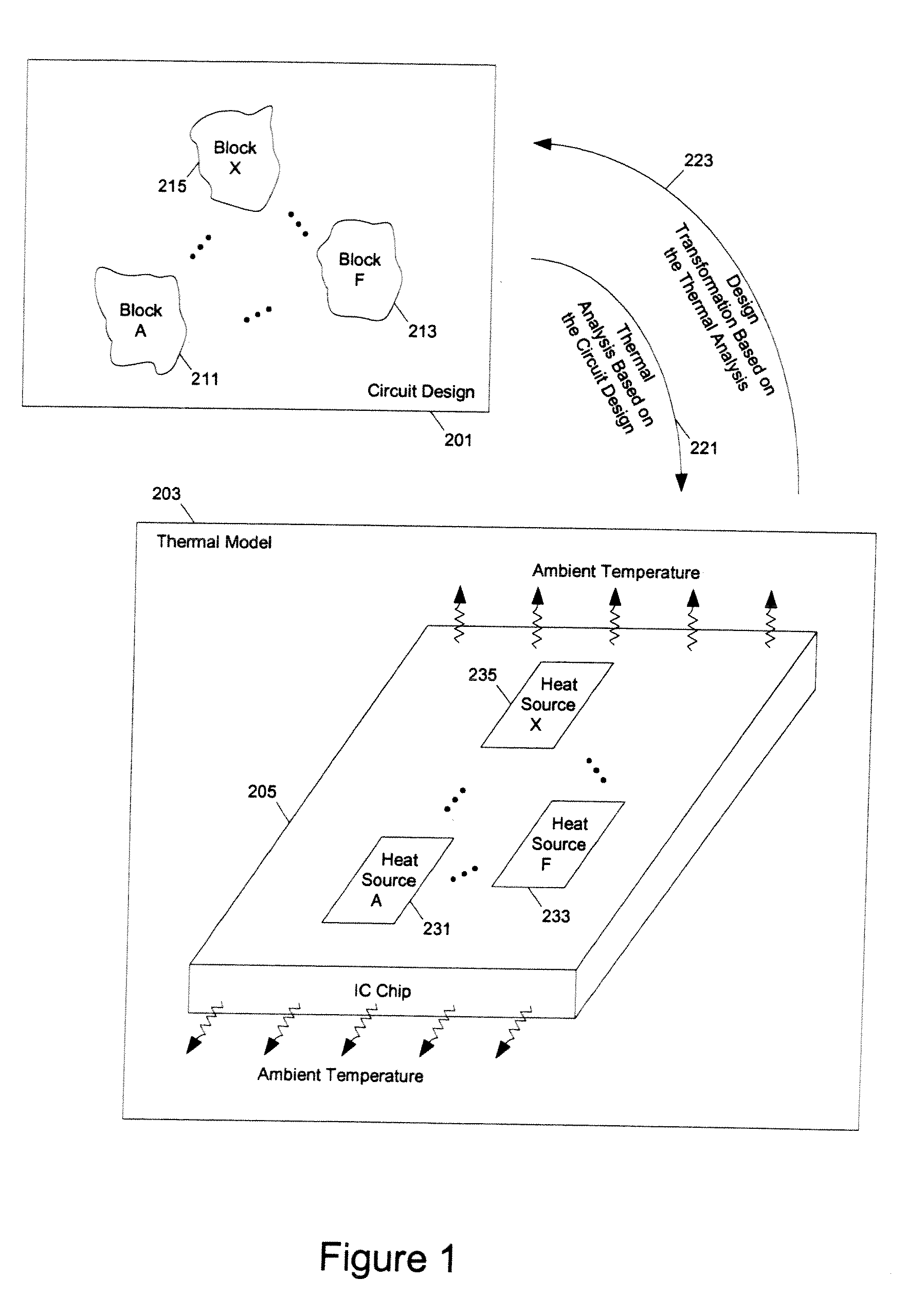
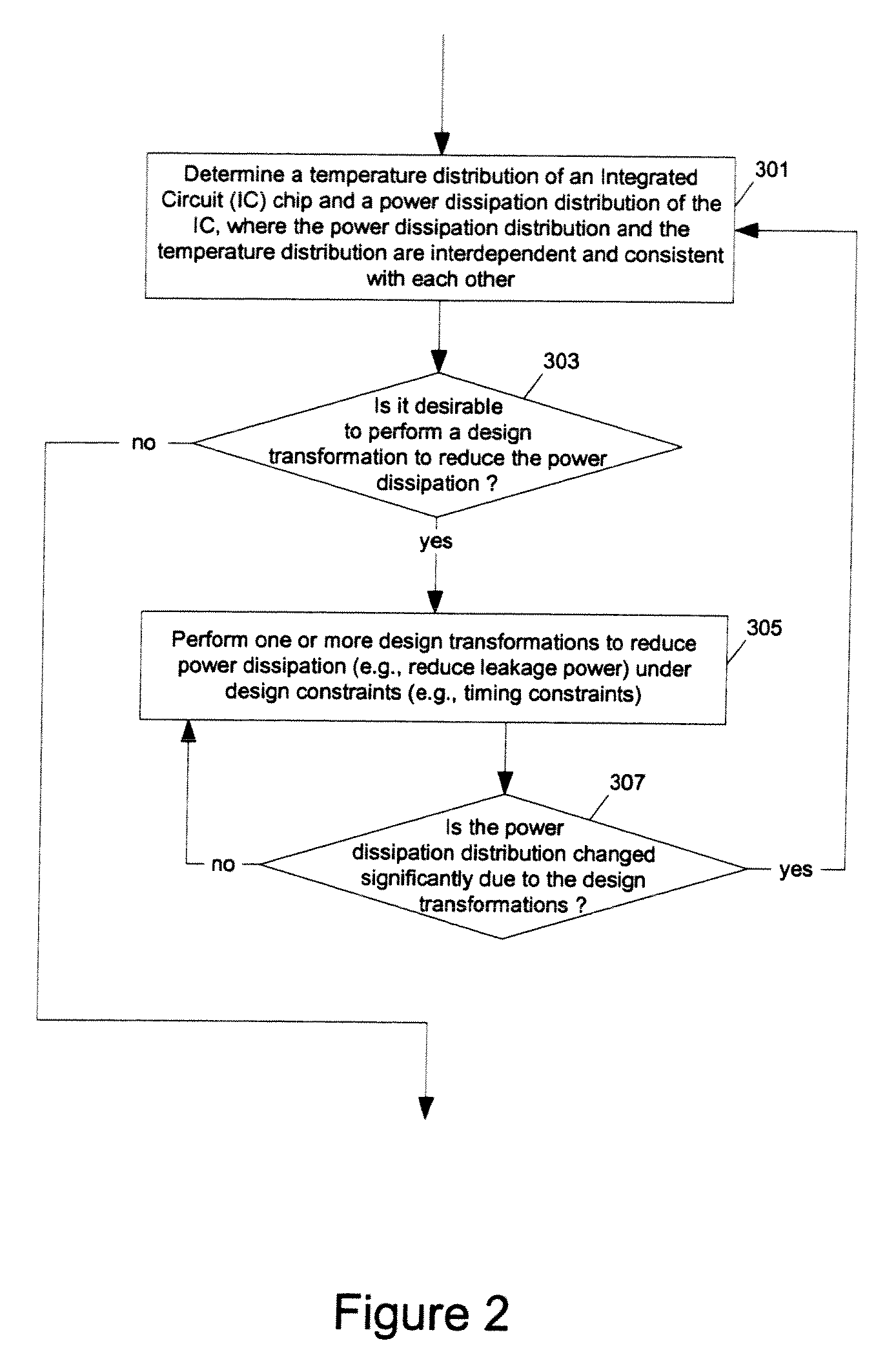
ActiveUS7366997B1Reduce power consumptionExtension of timeSoftware simulation/interpretation/emulationSpecial data processing applicationsPower usageTransition time
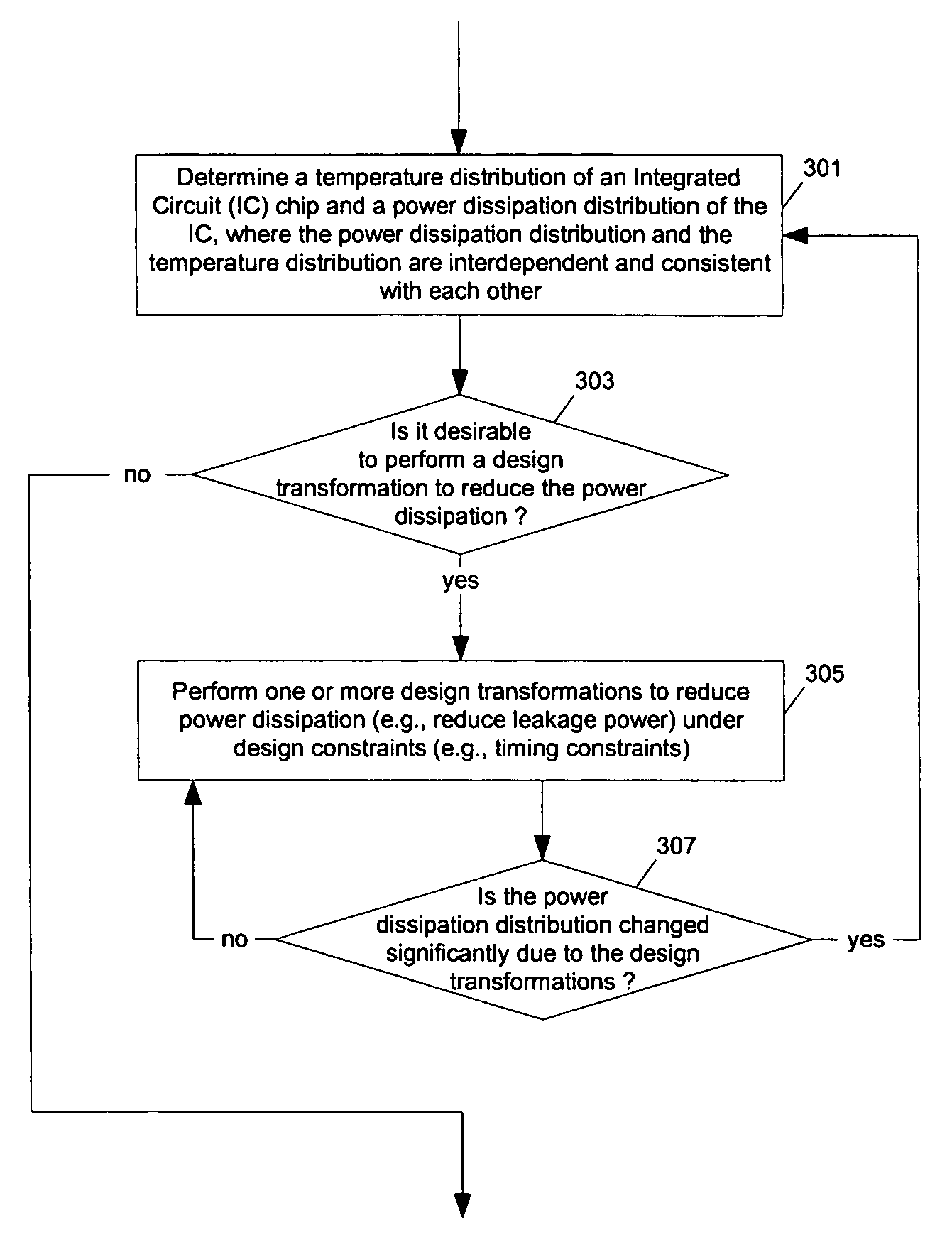
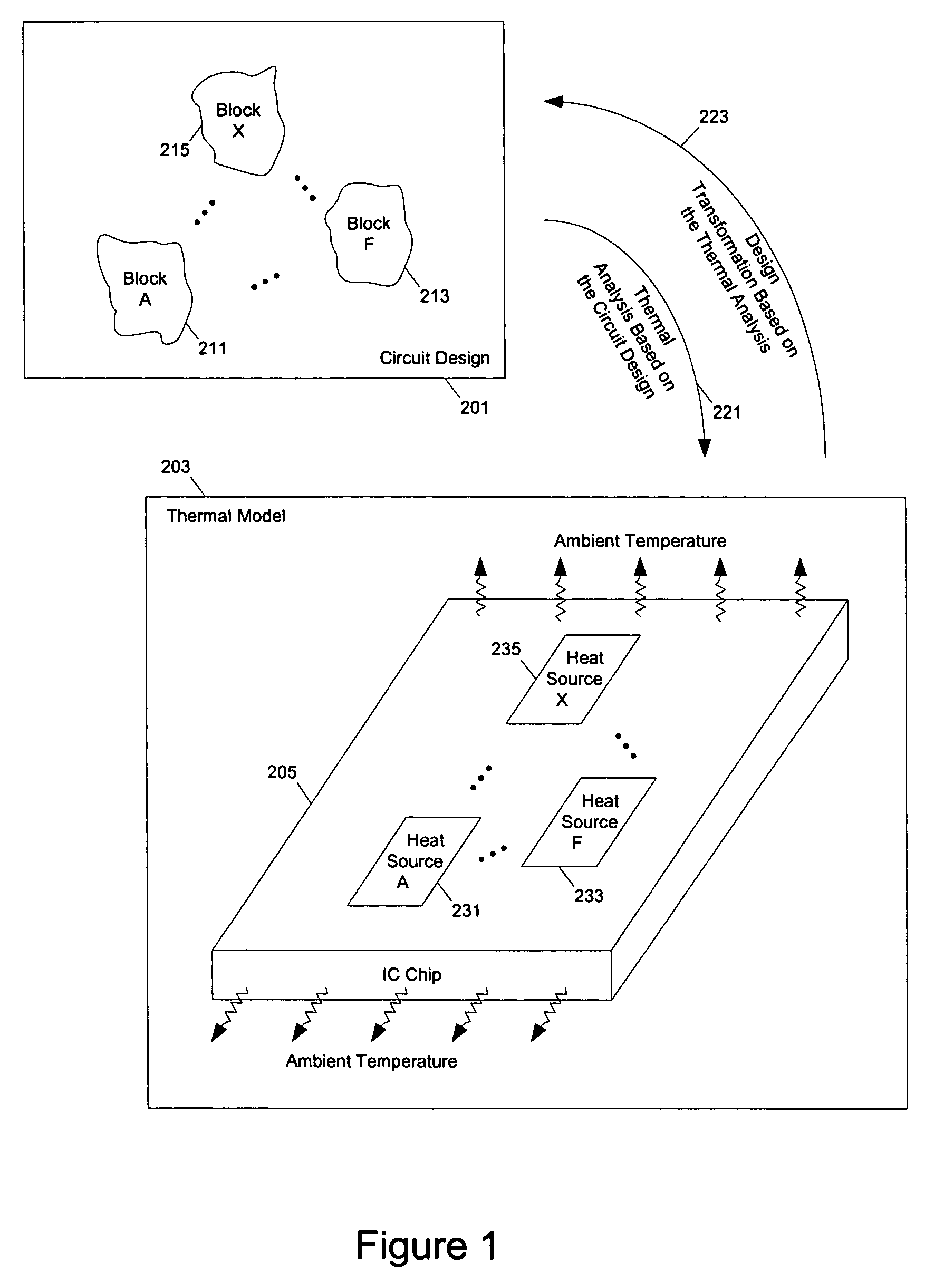
Methods and apparatuses for circuit design to reduce power usage, such as reducing temperature dependent power usage, and / or to improve timing, such as reducing temperature dependent delay or transition time. At least one embodiment of the present invention reduces the power dissipation and improves the timing of an integrated circuit to optimize the design. A thermal analysis is used to determine the temperature dependent power dissipation of a circuit and the temperature distribution of the circuit resulting from dissipating the heat created by the temperature dependent power dissipation. Then, the components of the design are selectively transformed to reduce the power dissipation and to improve timing based on the temperature solution. The transformation may include placement changes and netlist changes, such as the change of transistor threshold voltages for cells or for blocks of the circuit chip.
Owner:SYNOPSYS INC
MEMS Packaging Including Integrated Circuit Dies
InactiveUS20090194829A1Highly integratedReduced package footprintSemiconductor/solid-state device detailsSolid-state devicesOn boardElectrical connection
MEMS packaging schemes having a system-on-package (SOP) configuration and a system-on-board (SOB) configuration are provided. The MEMS package comprises one or more MEMS dies, a cap section having one or more integrated circuit (IC) dies, and a packaging substrate or a printed circuit board (PCB) arranged in a stacking manner. Vertical connectors, such as through-silicon-vias (TSVs), are formed to provide short electrical connections between the various components. The MEMS packaging schemes enable higher integration density, reduced MEMS package footprints, reduced RC delays and power consumption.
Owner:TAIWAN SEMICON MFG CO LTD
Delivery of Nanoparticles and/or Agents to Cells
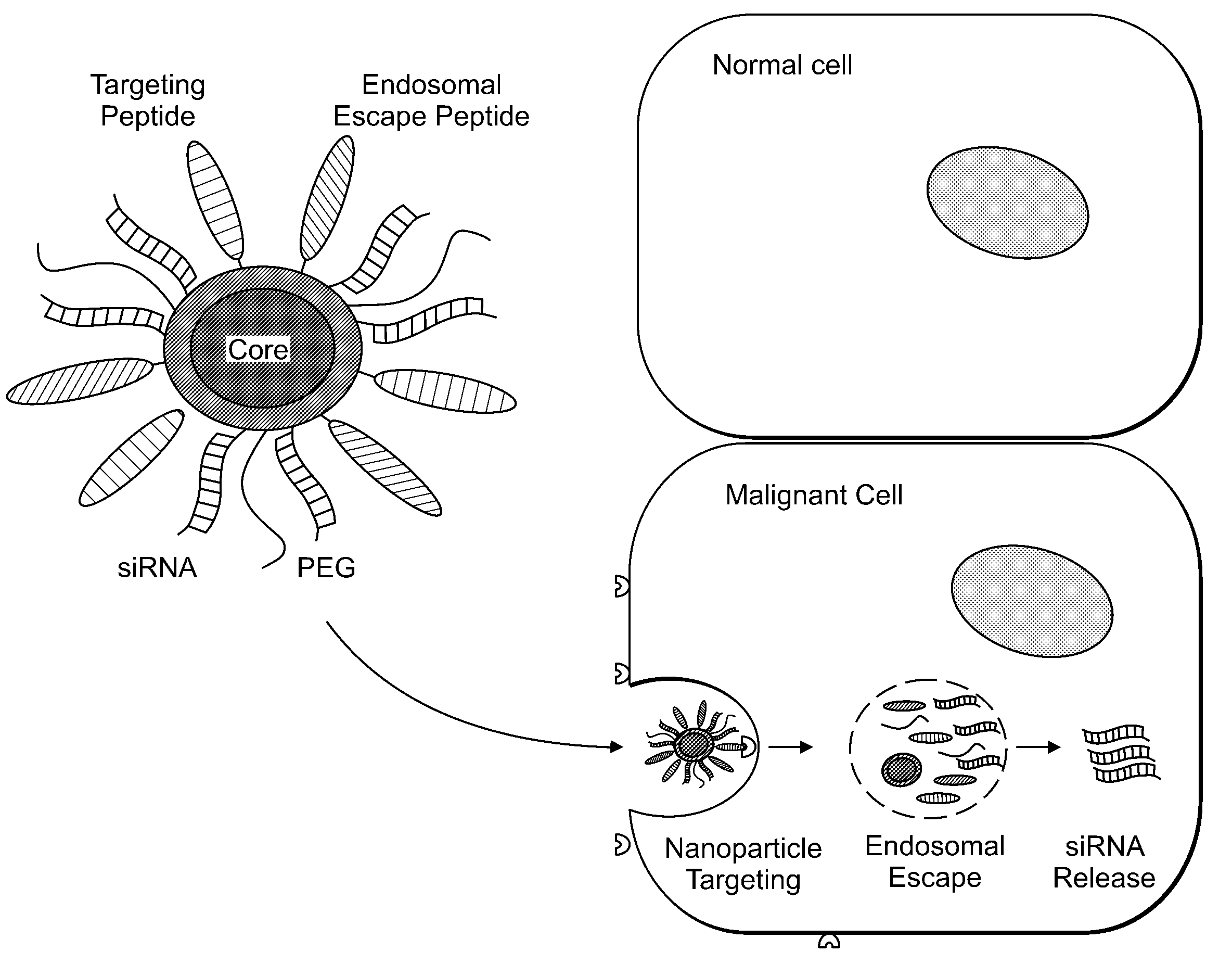
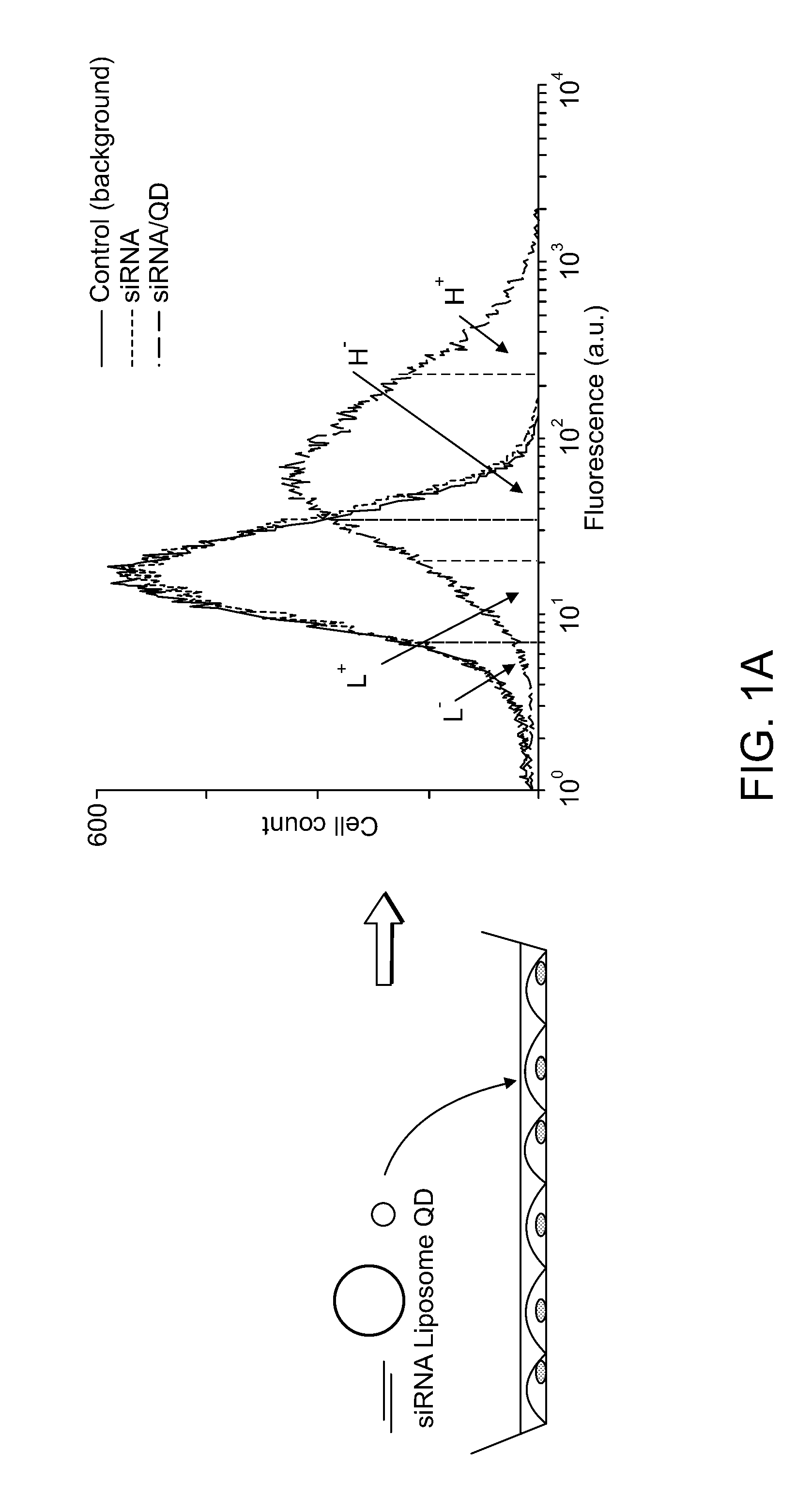
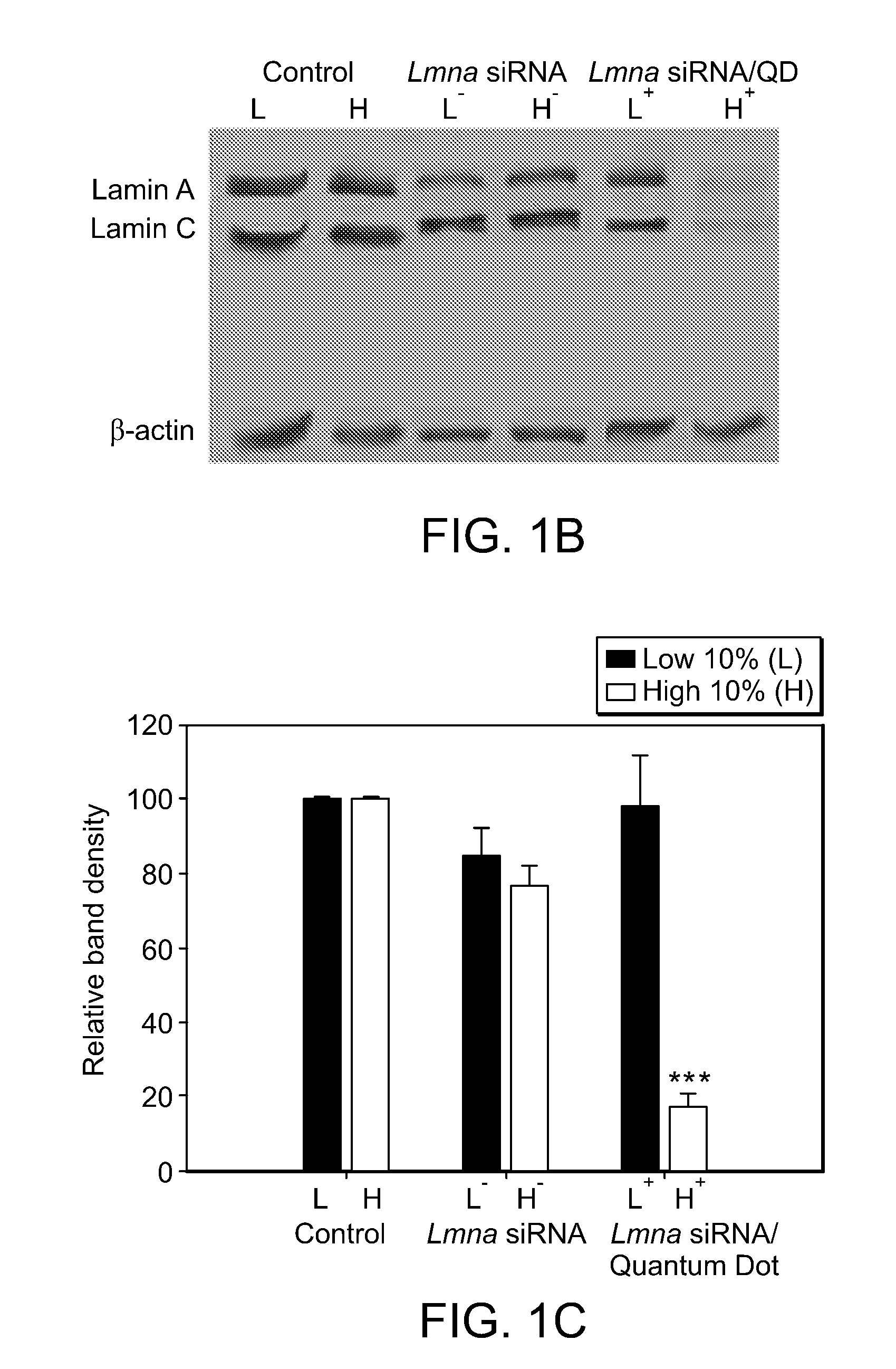
InactiveUS20080213377A1Extended circulation timeReduce degradationPowder deliverySugar derivativesDiagnostic agentNanoparticle
The present invention provides systems, methods, and compositions for targeted delivery of nanoparticles and / or agents to tissues, cells, and / or subcellular locales. In general, compositions comprise a nanoparticle (e.g. quantum dot, polymeric particle, etc.), at least one modulating entity (such as a targeting moiety, transfection reagent, protective entity, etc.), and at least one agent to be delivered (e.g. therapeutic, prophylactic, and / or diagnostic agent). The present invention provides methods of making and using nanoparticle entities in accordance with the present invention.
Owner:MASSACHUSETTS INST OF TECH
Use of matrix metalloproteinase inhibitors in skin care
InactiveUS20090068255A1Preventing and reducing of and sun damageImprove skin appearanceBiocideCosmetic preparationsWrinkle skinDisease
The application of matrix metalloproteinase (MMP) inhibitors to the skin inhibits the degradation of proteins found in the skin including collagen, elastin, and other basement membrane and extracellular matrix protein. MMP inhibitors may be used in both cosmetic compositions and pharmaceutical compositions for application to skin. MMP inhibitors are formulated with a cosmetically suitable vehicle or pharmaceutically acceptable excipient for application to the skin as creams, lotions, ointments, solutions, face masks, etc. As cosmetics, the inventive MMP inhibitor compositions are applied to the skin to prevent or reduce the appearance of wrinkles, pigmentation changes, loss of elasticity, or other effects associated with aging or sun damage. As pharmaceuticals, the inventive MMP inhibitor compositions may also be applied to the skin to treat or prevent a skin disease (e.g., proliferative disease, inflammatory disease).
Owner:LIVING PROOF INC
Heparin barrier coating for controlled drug release
ActiveUS20050004663A1Reduce frictional forceReduce forceSuture equipmentsStentsCompound (substance)Antioxidant
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations as well as other therapeutic agents may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:WYETH
Lighting device and method of making
ActiveUS20070274063A1Reduced optical degradationGood mechanical integrityOptical articlesSemiconductor/solid-state device manufacturingEffect lightEngineering
A lighting device comprises a solid state light emitter, first and second electrodes connected to the emitter, an encapsulant region comprising a silicone compound and a supporting region. The encapsulant region extends to an external surface of the lighting device. At least a portion of the first electrode is surrounded by the supporting region. The encapsulant region and the supporting region together define an outer surface which substantially encompasses the emitter. A method of making a lighting device, comprises electrically connecting first and second electrodes to an emitter; inserting the emitter into mold cavity; inserting an encapsulant composition comprising a one silicone compound; and then inserting a second composition to substantially surround at least a portion of the first electrode.
Owner:CREELED INC
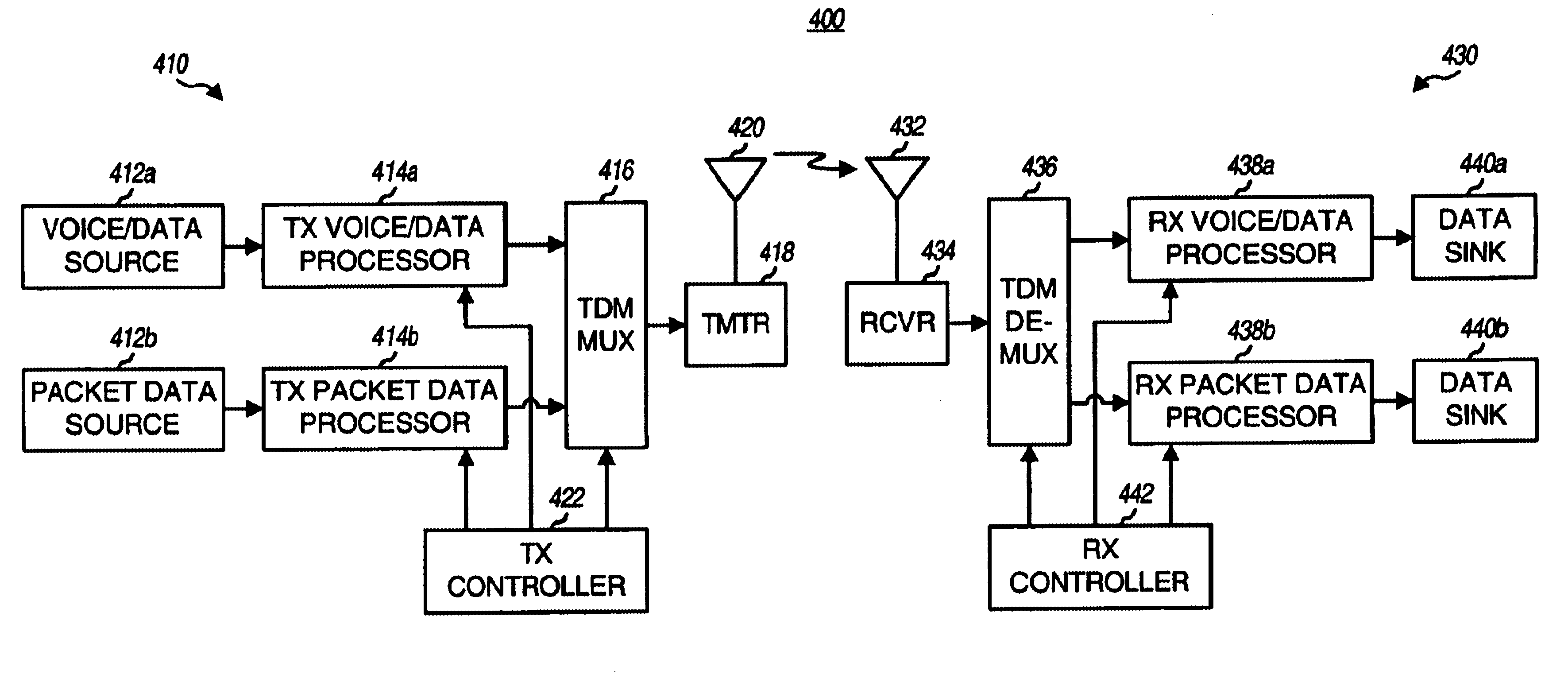
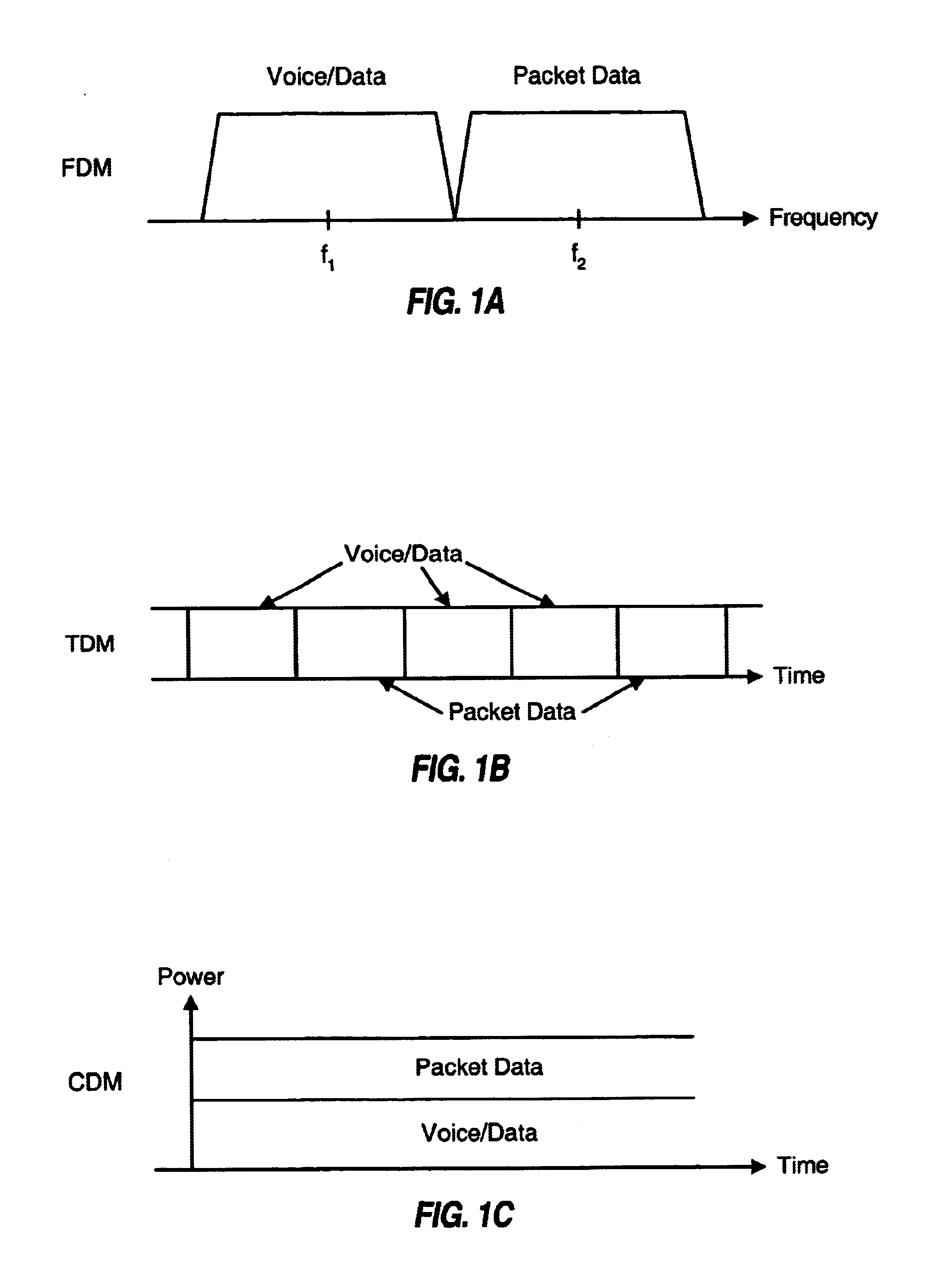
Method and apparatus for multiplexing high-speed packet data transmission with voice/data transmission
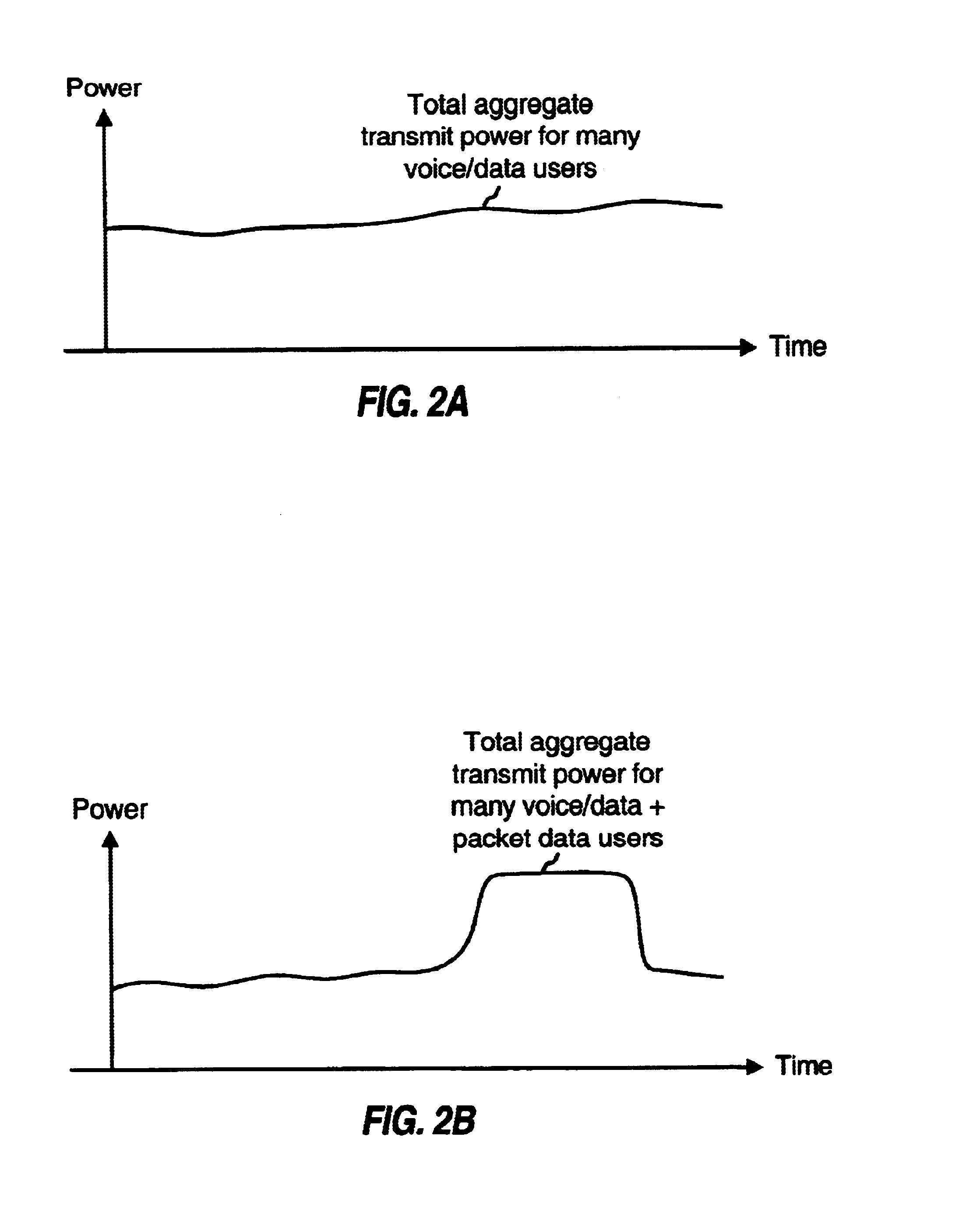
InactiveUS6775254B1Minimize impactLarge fluctuationsPower managementNetwork traffic/resource managementData transmissionTransmitted power
Techniques for transmitting voice / data and packet data services such that packet data transmissions have less impact on voice / data transmissions. In one aspect, voice / data and packet data can be multiplexed within a transmission interval such that the available resources are efficiently utilized. In another aspect, the amount of variation in the total transmit power from a base station is controlled to reduce degradation to transmissions from this and other base stations. In a specific method for concurrently transmitting a number of types of data, a first data type (e.g., voice, overhead, and some data) and a second data type are respectively processed in accordance with first and second signal processing schemes to generate first and second payloads, respectively. First and second partitions are then defined in a transmission interval. The first and second payloads are time multiplexed into the first and second partitions, respectively, and the multiplexed payloads are transmitted.
Owner:QUALCOMM INC
System and method of providing intent predictions for an utterance prior to a system detection of an end of the utterance
ActiveUS20160148610A1Unnecessary delay in identifying (and generating) one orReduce degradationSpeech recognitionSpeech identificationNatural language
In certain implementations, intent prediction is provided for a natural language utterance based on a portion of the natural language utterance prior to a system detection of an end of the natural language utterance. In some implementations, a first portion of a natural language utterance of a user may be received. Speech recognition may be performed on the first portion of the natural language utterance to recognize one or more words of the first portion of the natural language utterance. Context information for the natural language utterance may be obtained. Prior to a detection of an end of the natural language utterance, a first intent may be predicted based on the one or more words of the first portion and the context information. One or more user requests may be determined based on the first predicted intent.
Owner:VOICEBOX TECH INC
Photoresist composition for deep UV radiation containing an additive
InactiveUS6723488B2Reduce impactPrevent degradationPhotosensitive materialsElectric discharge tubesUltravioletAqueous solution
Owner:AZ ELECTRONICS MATERIALS USA CORP
Thin film transistor and method of forming the same
ActiveUS20080197350A1Reduce layeringReduce degradationSolid-state devicesSemiconductor/solid-state device manufacturingEngineeringSemiconductor
A thin film transistor (TFT) may include a channel layer, a source electrode, a drain electrode, a protective layer, a gate electrode, and / or a gate insulating layer. The channel layer may include an oxide semiconductor material. The source electrode and the drain electrode may face each other on the channel layer. The protective layer may be under the source electrode and the drain electrode and / or may cover the channel layer. The gate electrode may be configured to apply an electric field to the channel layer. The gate insulating layer may be interposed between the gate electrode and the channel layer.
Owner:SAMSUNG ELECTRONICS CO LTD
Apparatus comprising a broadcast receiver circuit and provided with an antenna
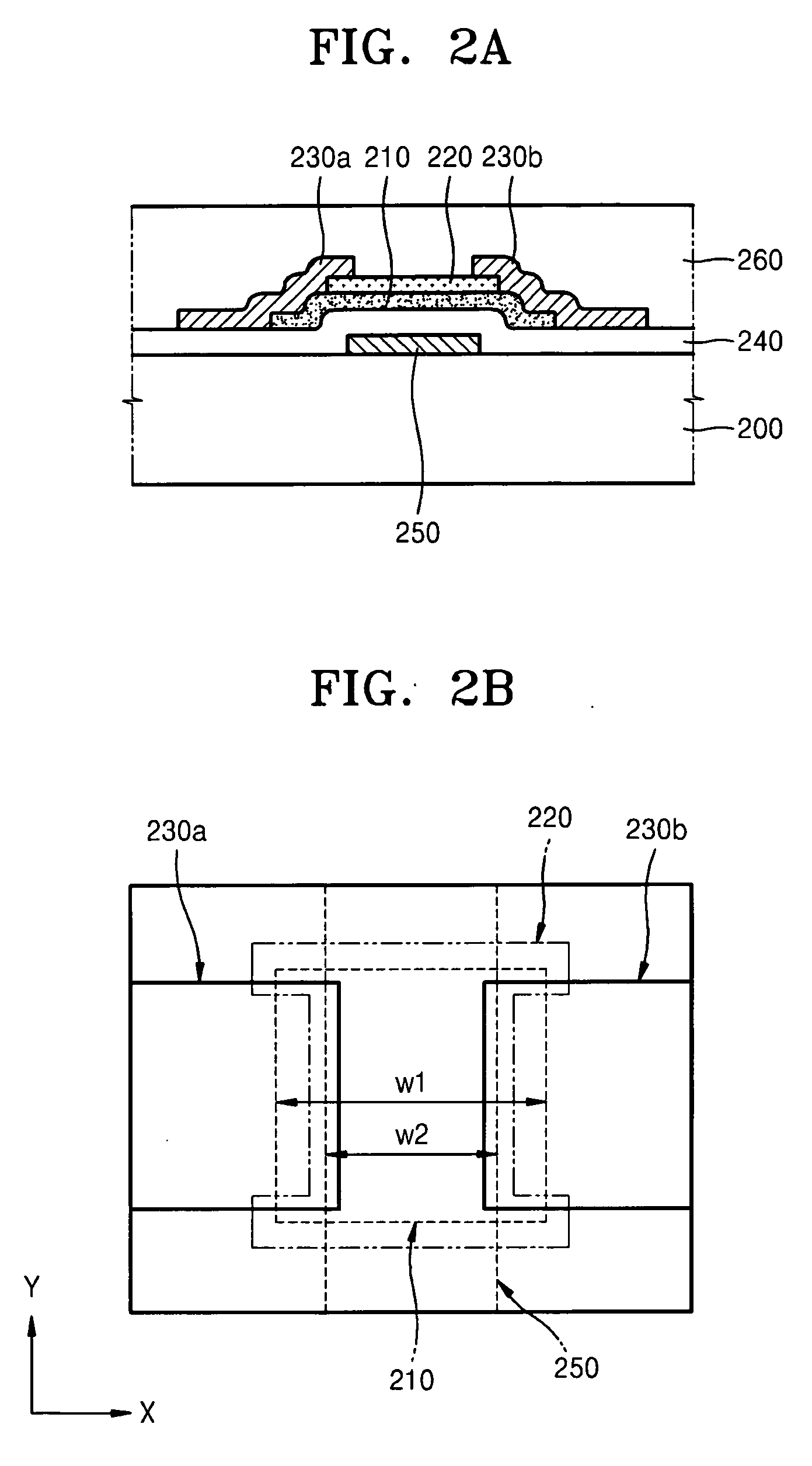
ActiveUS20110028114A1Meet the requirementsMore powerTransmissionBroadcast domainAudio power amplifier
The invention relates to an apparatus 1 comprising a broadcast receiver circuit, an embedded antenna for receiving broadcast signals and a tuning circuit coupled between the antenna and the receiver circuit, which tuning circuit comprises a filter circuit coupled to ground, wherein the tuning circuit is designed to have a first resonance at a first frequency below a broadcast band of interest, and a second resonance at a second frequency above the broadcast band and wherein the tuning circuit comprises an amplifier with an output to the receiver circuit and with an input to the filter circuit, and wherein the tuning circuit is provided with a carrier to noise ratio (CNR) which is substantially fiat across the broadcast band.
Owner:NXP BV
Conversational computing via conversational virtual machine
InactiveUS20070043574A1Limitation for transferReduce degradationInterconnection arrangementsInterprogram communicationConversational speechApplication software
A conversational computing system that provides a universal coordinated multi-modal conversational user interface (CUI) 10 across a plurality of conversationally aware applications (11) (i.e., applications that “speak” conversational protocols) and conventional applications (12). The conversationally aware applications (11) communicate with a conversational kernel (14) via conversational application APIs (13). The conversational kernel 14 controls the dialog across applications and devices (local and networked) on the basis of their registered conversational capabilities and requirements and provides a unified conversational user interface and conversational services and behaviors. The conversational computing system may be built on top of a conventional operating system and APIs (15) and conventional device hardware (16). The conversational kernel (14) handles all I / O processing and controls conversational engines (18). The conversational kernel (14) converts voice requests into queries and converts outputs and results into spoken messages using conversational engines (18) and conversational arguments (17). The conversational application API (13) conveys all the information for the conversational kernel (14) to transform queries into application calls and conversely convert output into speech, appropriately sorted before being provided to the user.
Owner:NUANCE COMM INC
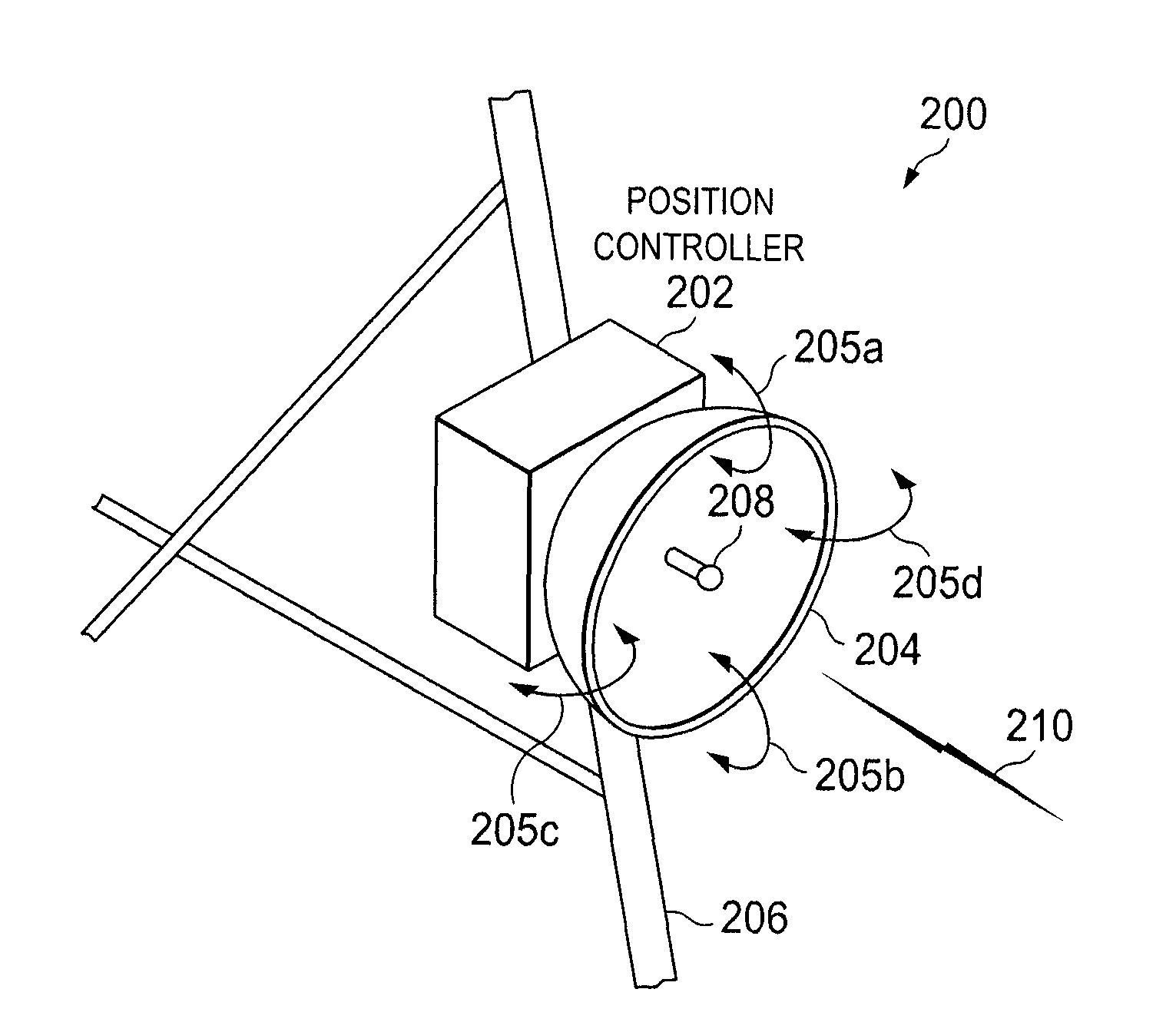
System and method for re-aligning antennas
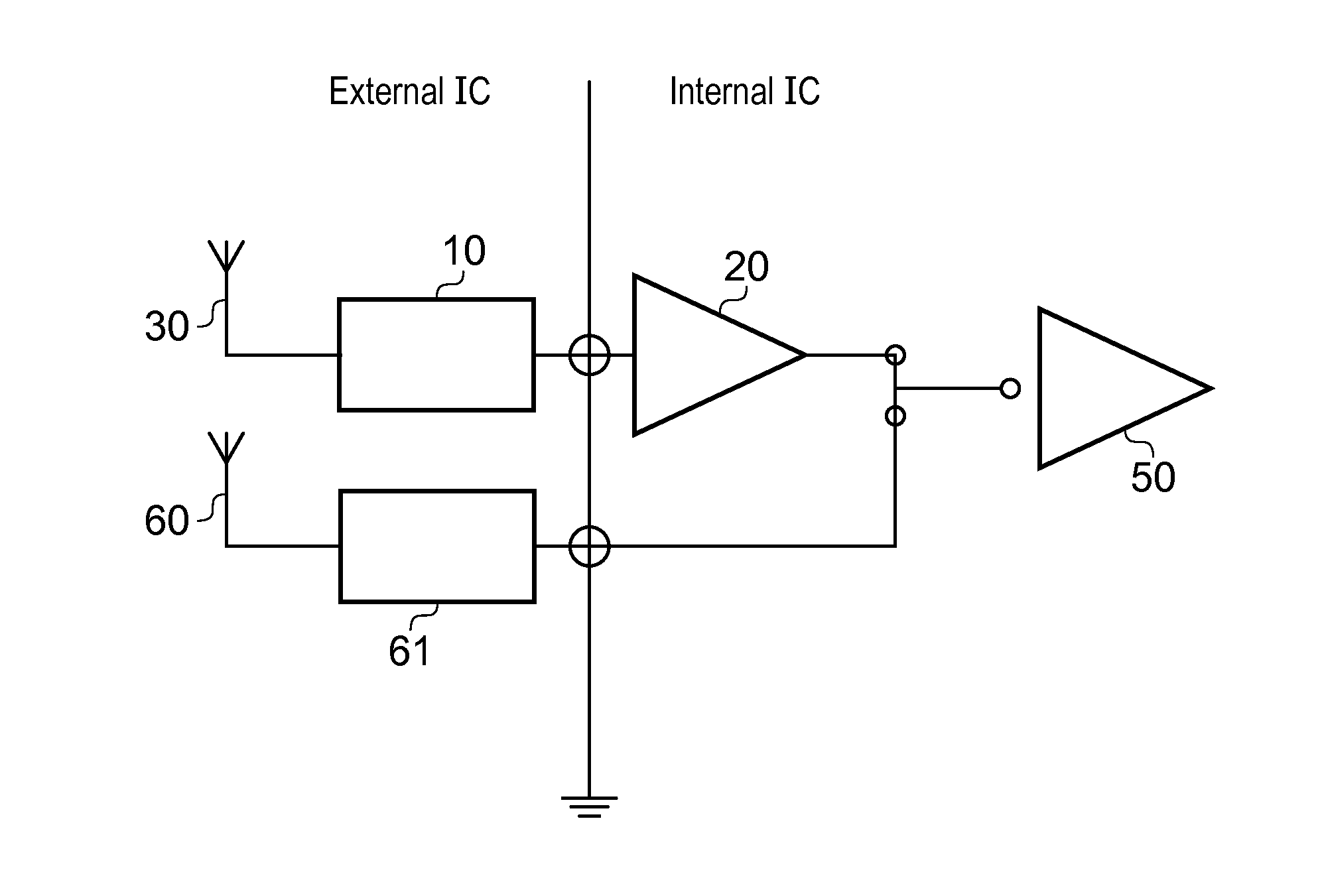
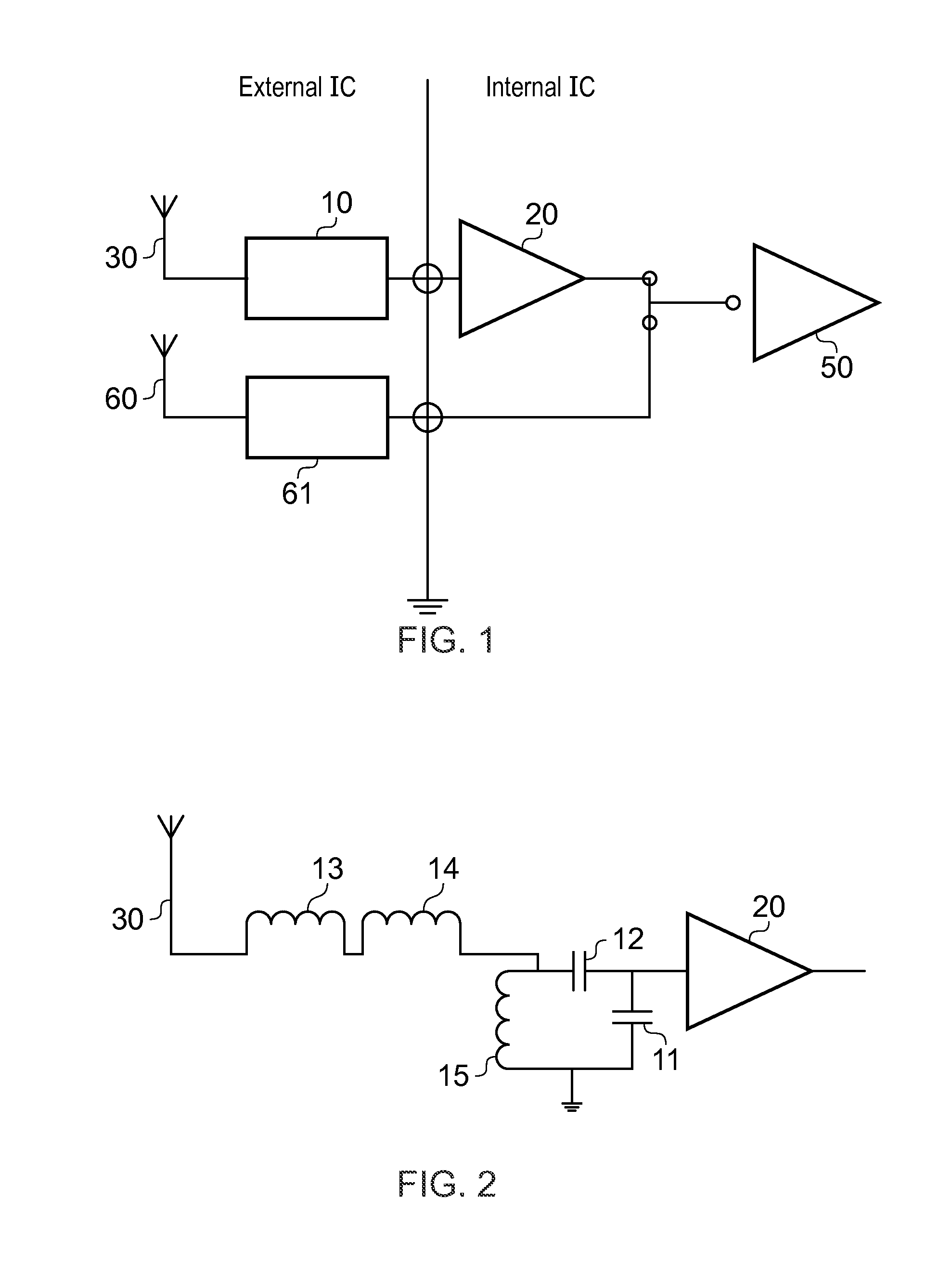
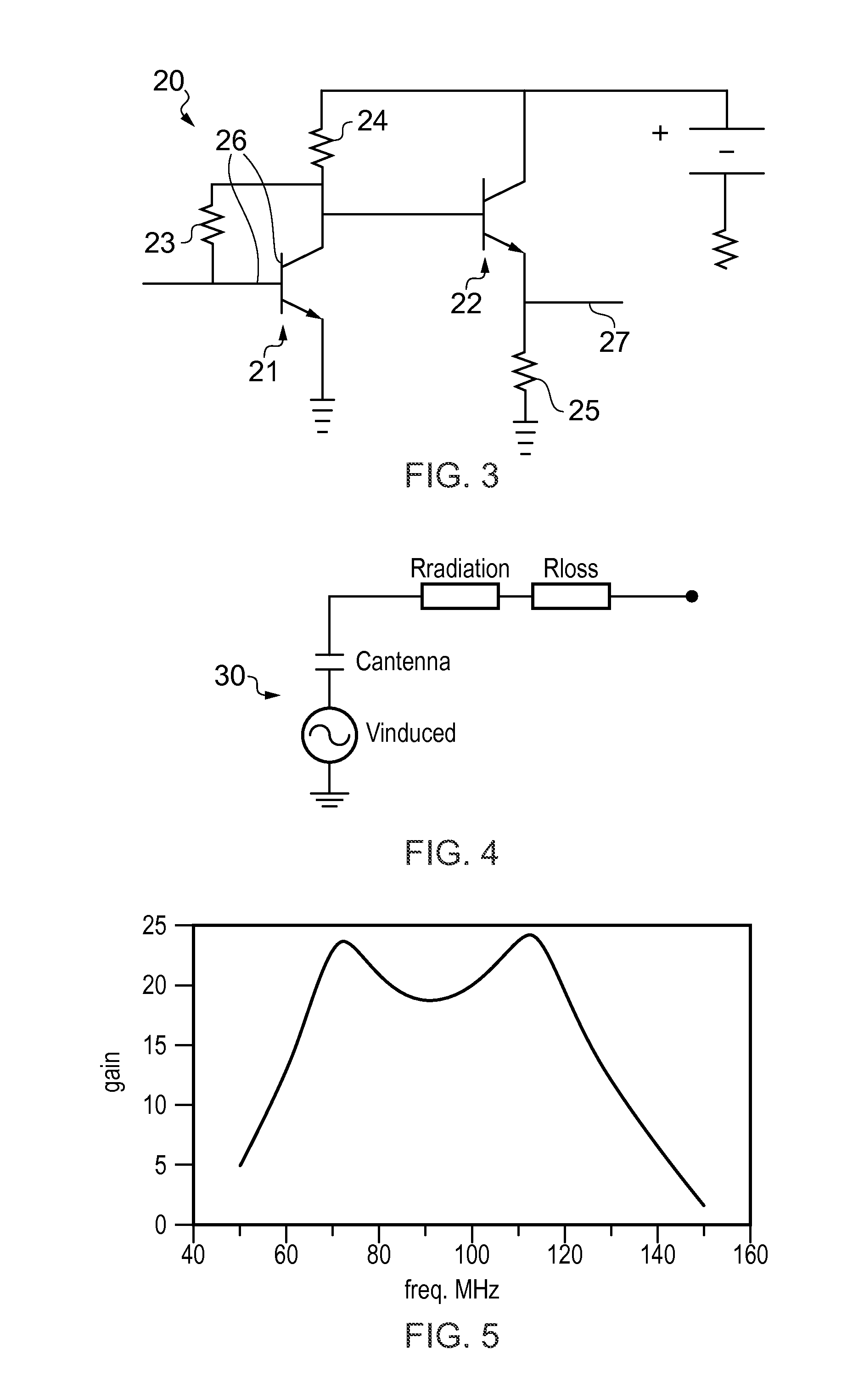
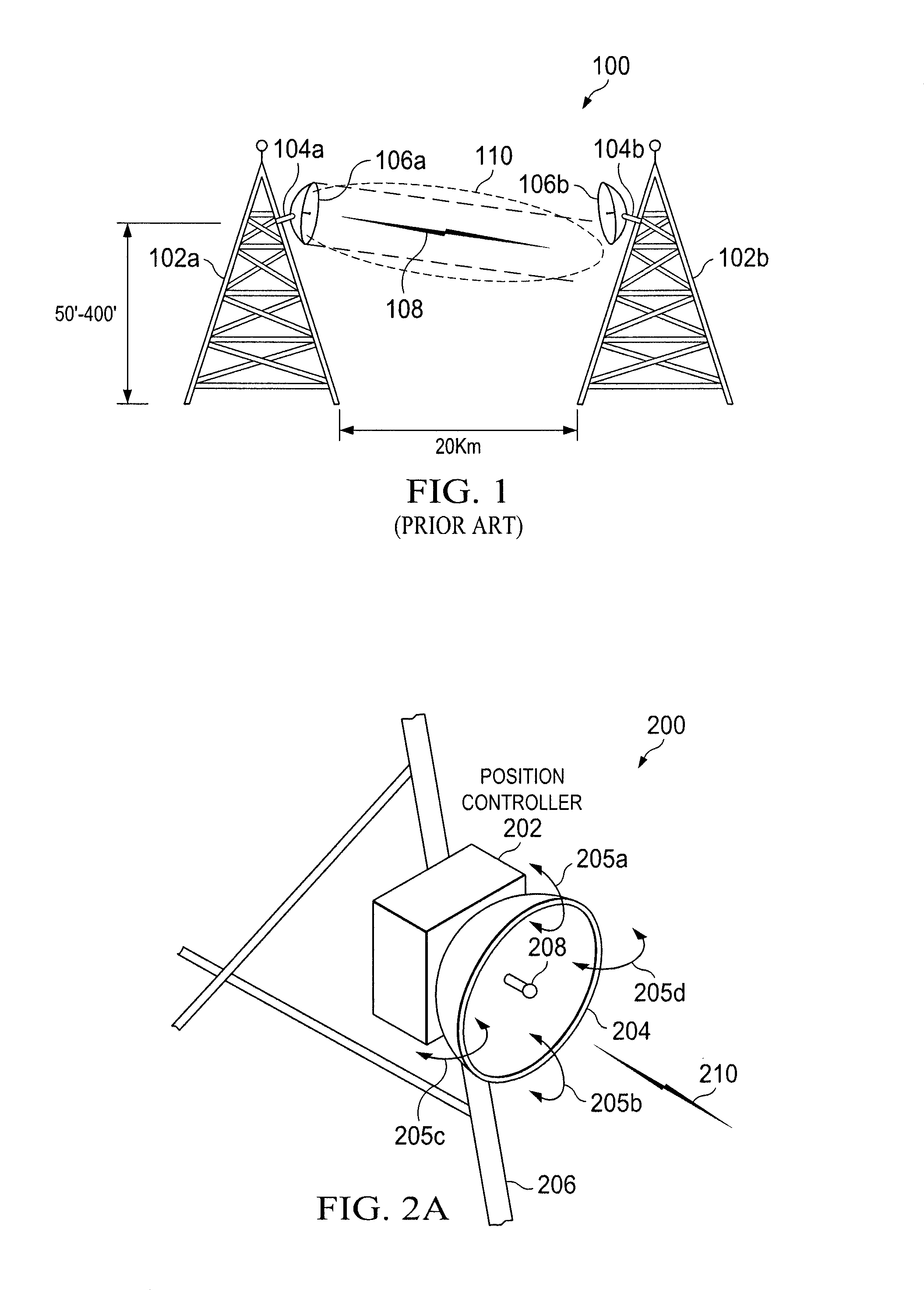
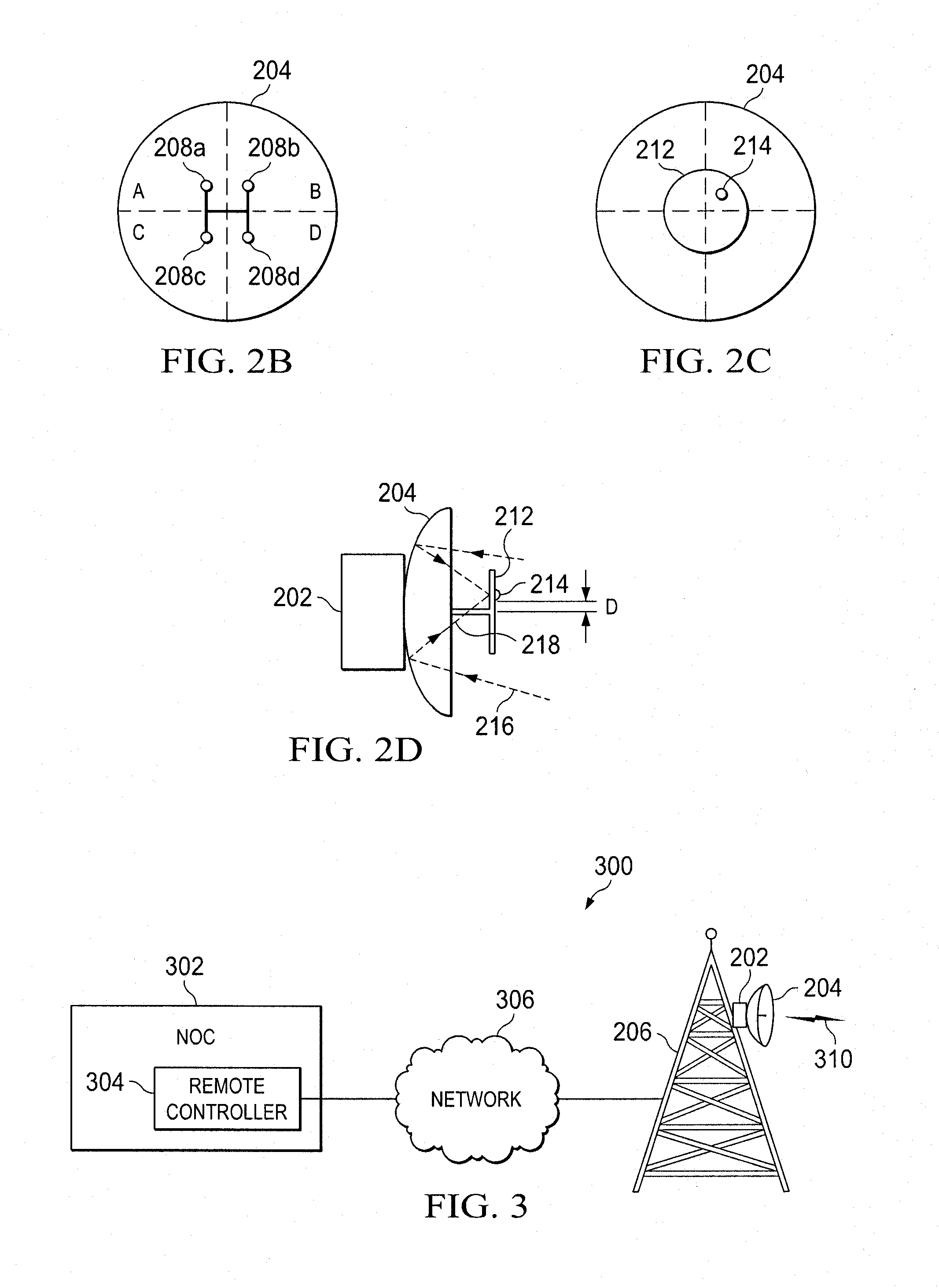
ActiveUS8022885B2Low costReduce degradationAntenna detailsEngineeringElectrical and Electronics engineering
A system for re-aligning an antenna communicating signals point-to-point. The system may include a first antenna, a second antenna configured to communicate a communications signal with the first antenna using point-to-point communications, and a position controller coupled to the first antenna and configured to re-align the first antenna with respect to the second antenna in response to determining a misalignment of the antenna.
Owner:CENTURYLINK INTPROP
Apparatus and method for adjusting beam pattern in communication system supporting beam division multipile access scheme
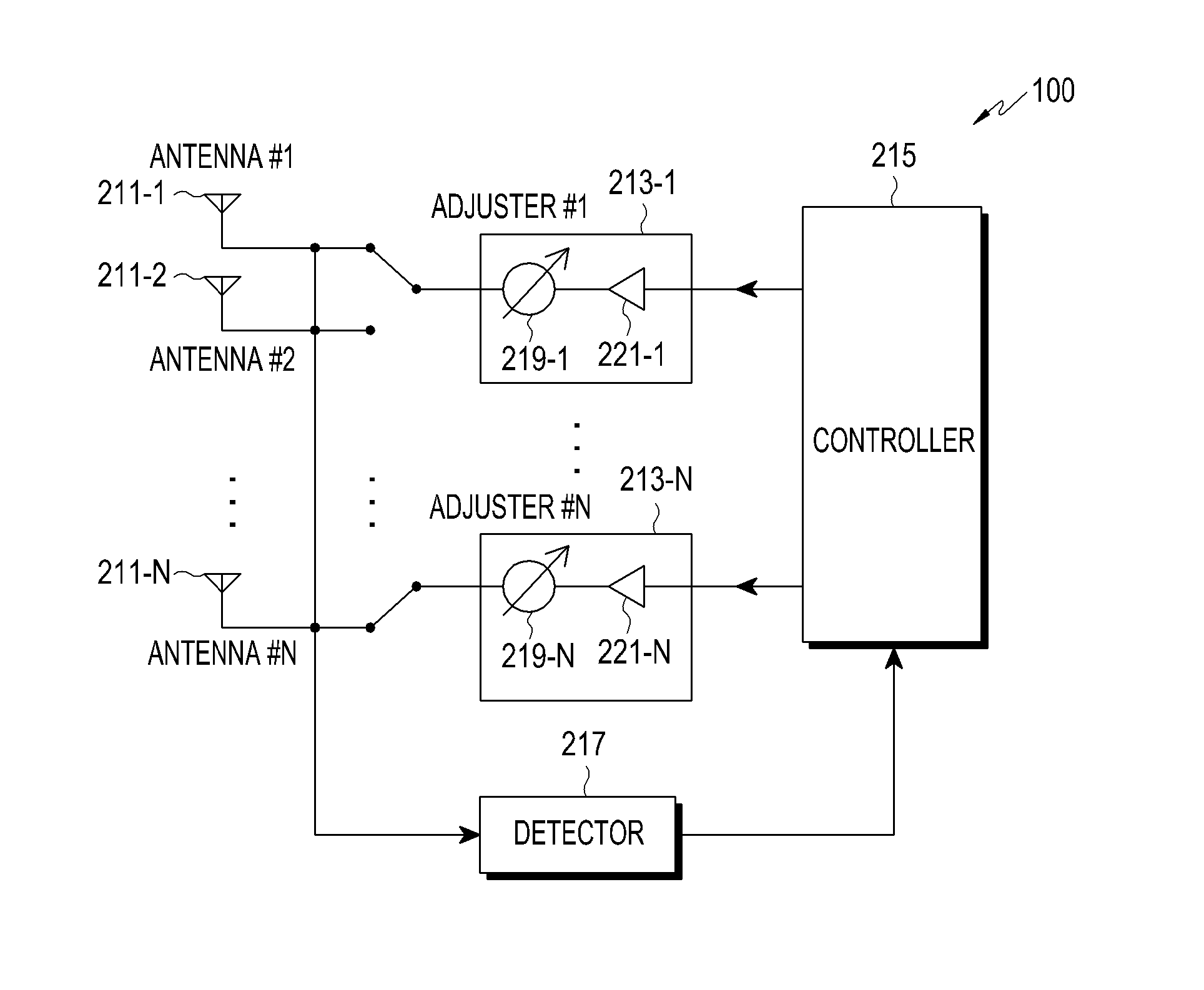
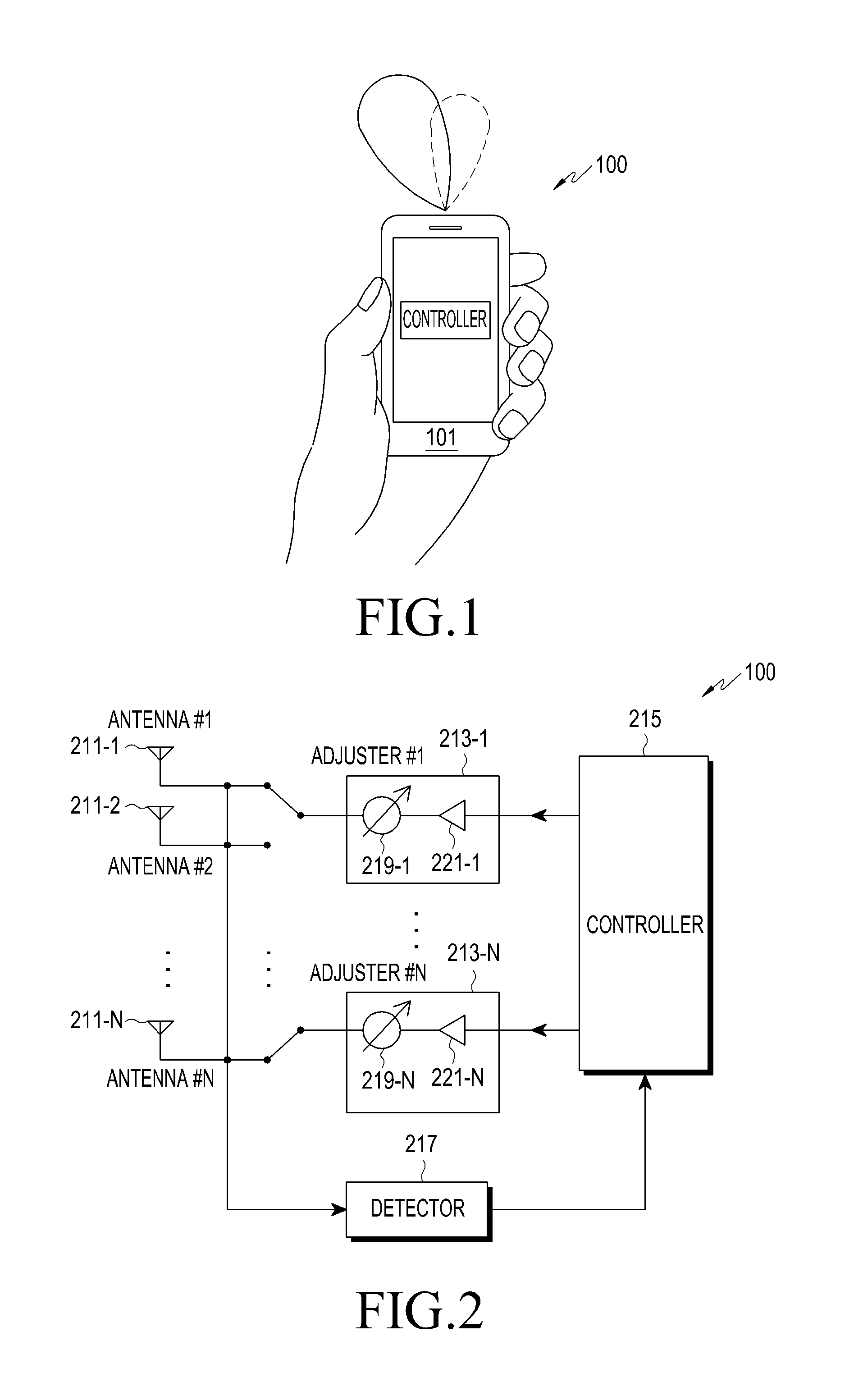
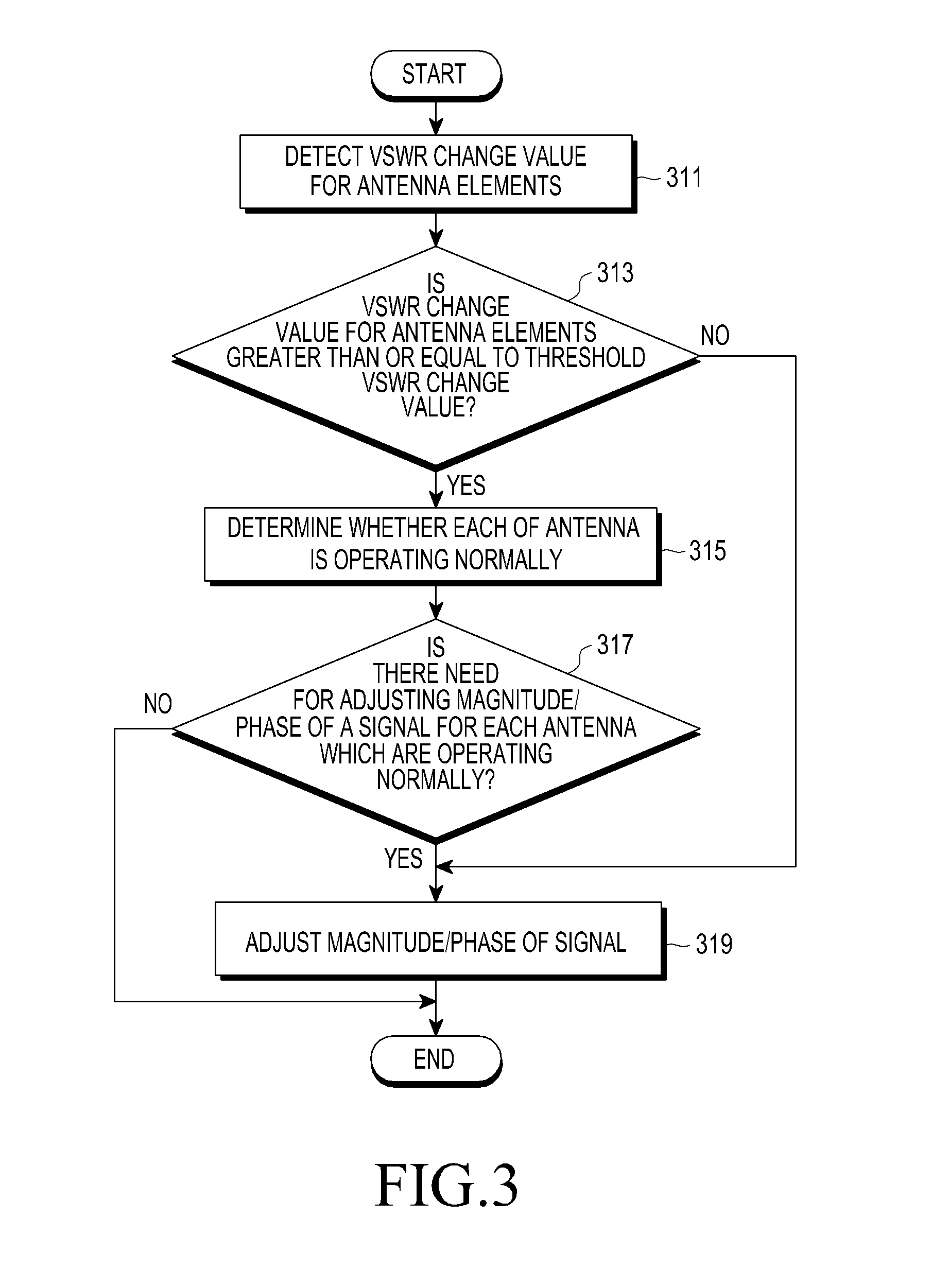
ActiveUS20150318610A1Reduce degradationImprove communication system performanceTransmitters monitoringSpatial transmit diversityCommunications systemBeam pattern
A method for adjusting a beam pattern in a beam pattern adjusting apparatus in a communication system supporting a Beam Division Multiple Access (BDMA) scheme is provided. The method includes determining whether a Voltage Standing Wave Ratio (VSWR) value for each antenna included in an antenna array included in the beam pattern adjusting apparatus is greater than or equal to a threshold VSWR value, if it is determined that an antenna of the antenna array has a VSWR value that is greater than or equal to the threshold VSWR, detecting whether each of the antenna elements is operable, and if it is determined that at least one of the antennas is inoperable, adjusting a beam pattern of at least one of the antennas that is operable.
Owner:SAMSUNG ELECTRONICS CO LTD +1
Methods and apparatuses for thermal analysis based circuit design
ActiveUS20080168406A1Reduce power consumptionExtension of timeSoftware simulation/interpretation/emulationSpecial data processing applicationsEngineeringPower usage
Methods and apparatuses for circuit design to reduce power usage, such as reducing temperature dependent power usage, and / or to improve timing, such as reducing temperature dependent delay or transition time. At least one embodiment of the present invention reduces the power dissipation and improves the timing of an integrated circuit to optimize the design. A thermal analysis is used to determine the temperature dependent power dissipation of a circuit and the temperature distribution of the circuit resulting from dissipating the heat created by the temperature dependent power dissipation. Then, the components of the design are selectively transformed to reduce the power dissipation and to improve timing based on the temperature solution. The transformation may include placement changes and netlist changes, such as the change of transistor threshold voltages for cells or for blocks of the circuit chip.
Owner:SYNOPSYS INC
Method and apparatus for patch panel patch cord documentation and revision
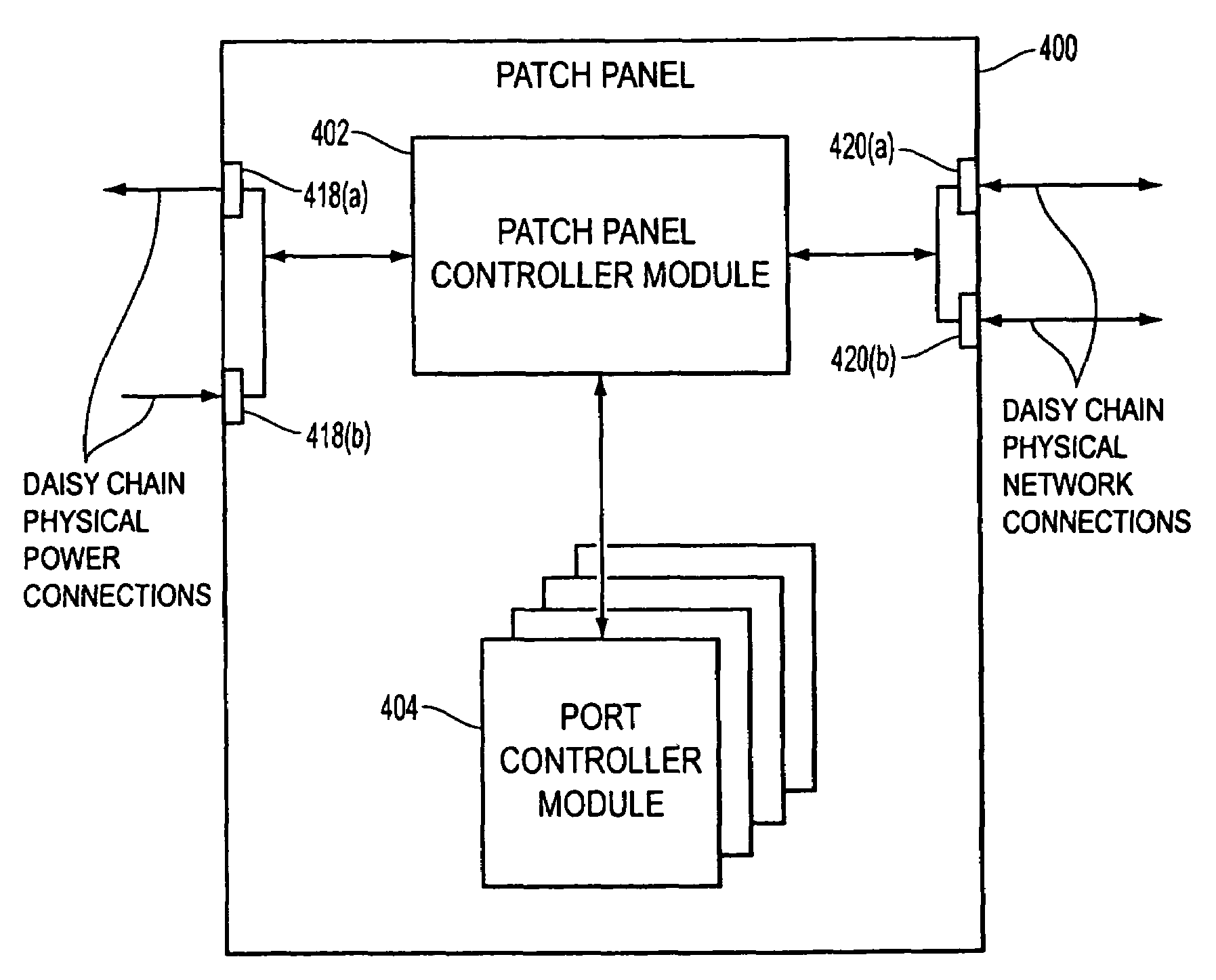
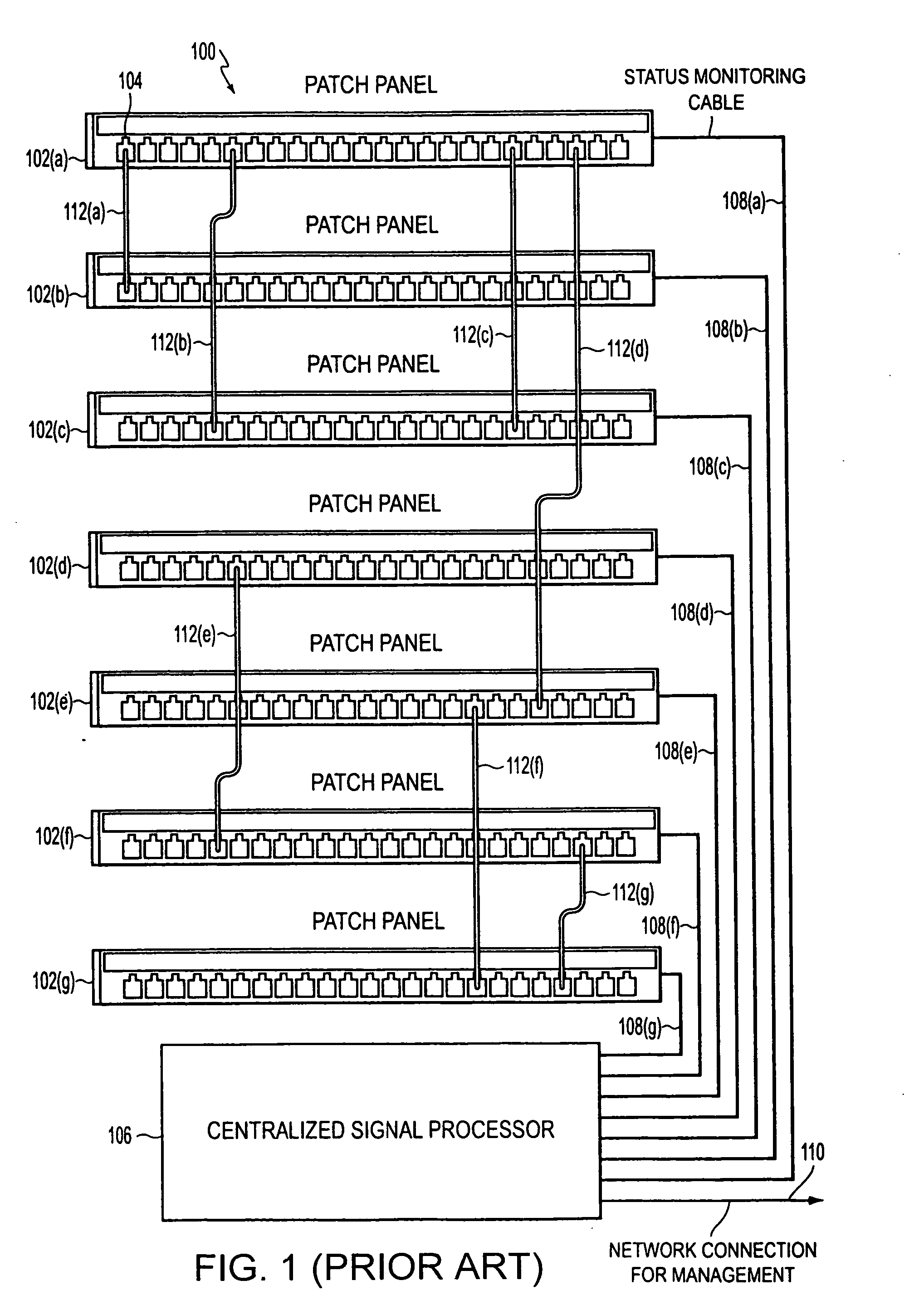
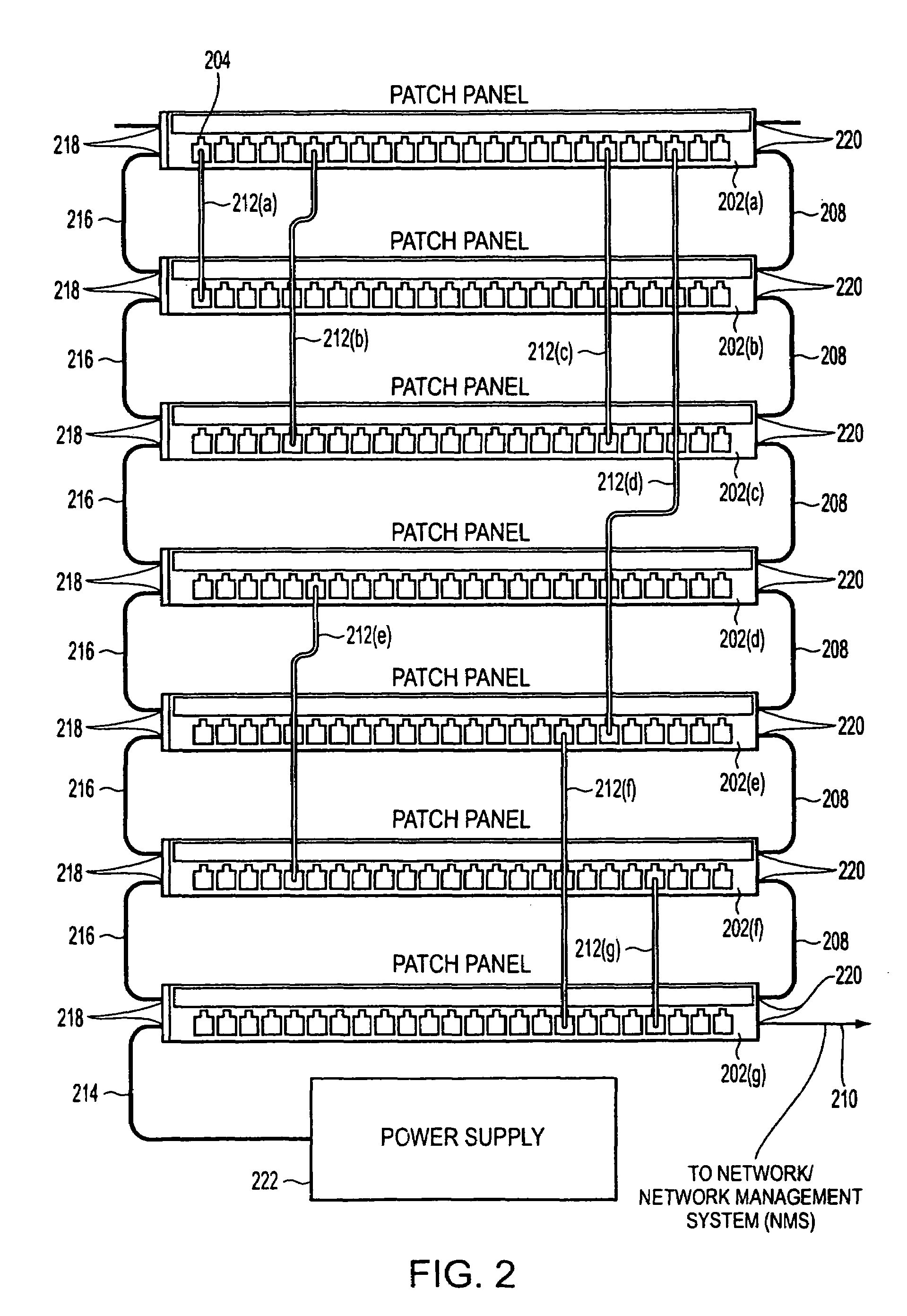
ActiveUS7297018B2Improved real-time reportingReduce degradationElectrically conductive connectionsCoupling device detailsPatch panelDocumentation procedure
A method and apparatus are provided for monitoring and reporting cable connectivity such as patch panel port-level connectivity on a real-time basis. For patch panel systems, the approach is based upon a distributed architecture that may be modularly scalable and may reduce, if not eliminate, the need for a centralized signal processor and complex cabling between patch panels and the centralized signal processor. Each patch panel may determine port level connectivity independently. Polling delays and polling-related overhead processing may be reduced or eliminated by supporting real-time monitoring of port connectivity at the port level. The approach provides improved real-time reporting of patch panel connectivity with reduced cabling complexity, increased reliability, and decreased maintenance costs. In addition, the approach is compatible with (i.e., may communicate with and be controlled by) a multipurpose network management system (NMS). In addition, a compatible revision system is provided.
Owner:PANDUIT
Methods, compositions and systems for local delivery of drugs
ActiveUS20110195123A1Reduce degradationImprove permeabilityBiocidePowder deliveryCell-Extracellular MatrixBrachytherapy
Implantable medical device eluting drug locally and in prolonged period is provided, including several types of such a device, the treatment modes of implementation and methods of implantation. The device comprising of polymeric substrate, such as a matrix for example, that is used as the device body, and drugs, and in some cases additional scaffolding materials, such as metals or additional polymers, and materials to enhance visibility and imaging. The selection of drug is based on the advantageous of releasing drug locally and in prolonged period, where drug is released directly to the extracellular matrix (ECM) of the diseased area such as tumor, inflammation, degeneration or for symptomatic objectives, or to injured smooth muscle cells, or for prevention. One kind of drug is the gene silencing drugs based on RNA interference (RNAi), including but not limited to si RNA, sh RNA, or antisense RNA / DNA, ribozyme and nucleoside analogs. The modes of implantation in some embodiments are existing implantation procedures that are developed and used today for other treatments, including brachytherapy and needle biopsy. In such cases the dimensions of the new implant described in this invention are similar to the original implant. Typically a few devices are implanted during the same treatment procedure.
Owner:SILENSEED LTD
Online search system, method and computer program
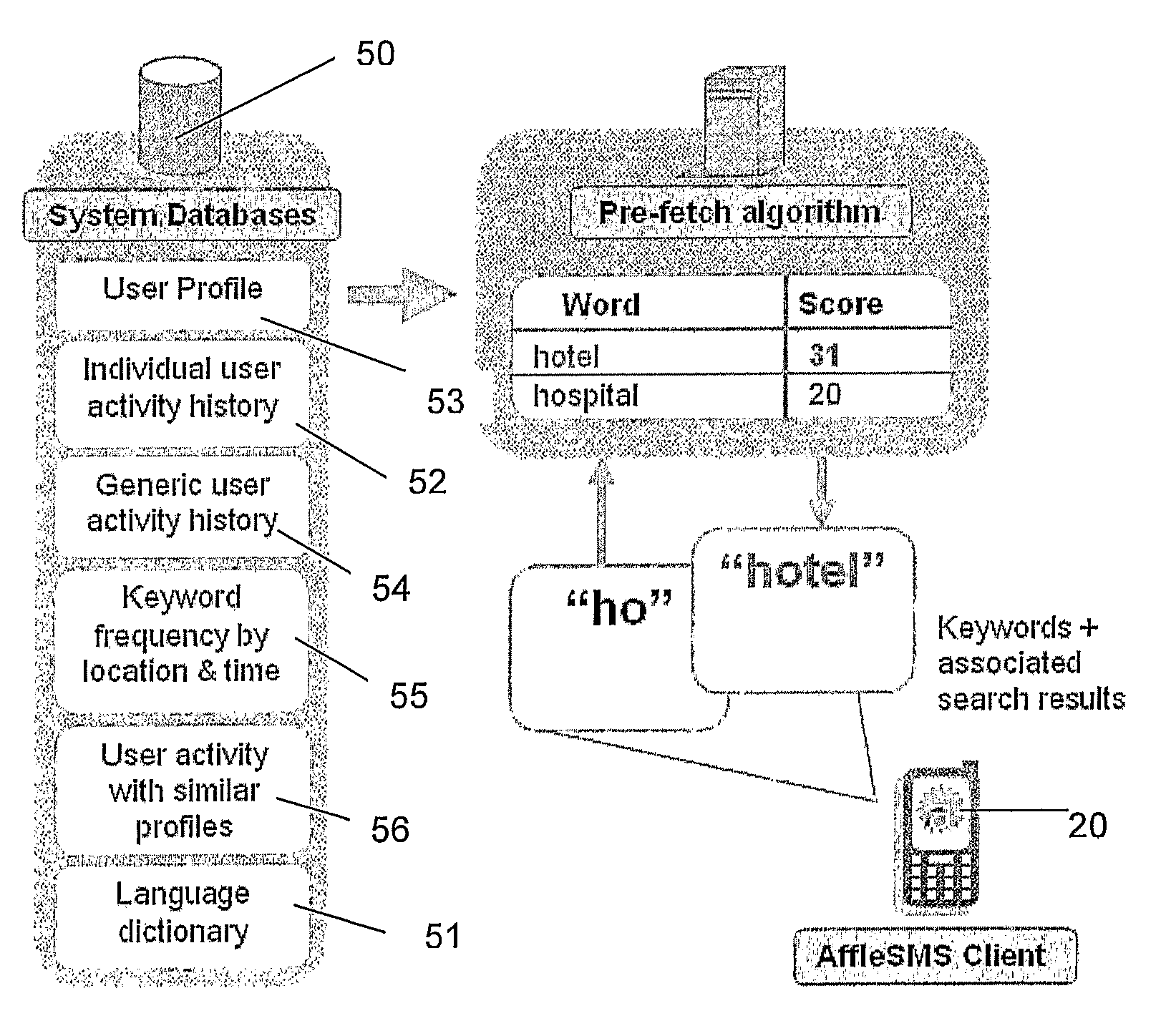
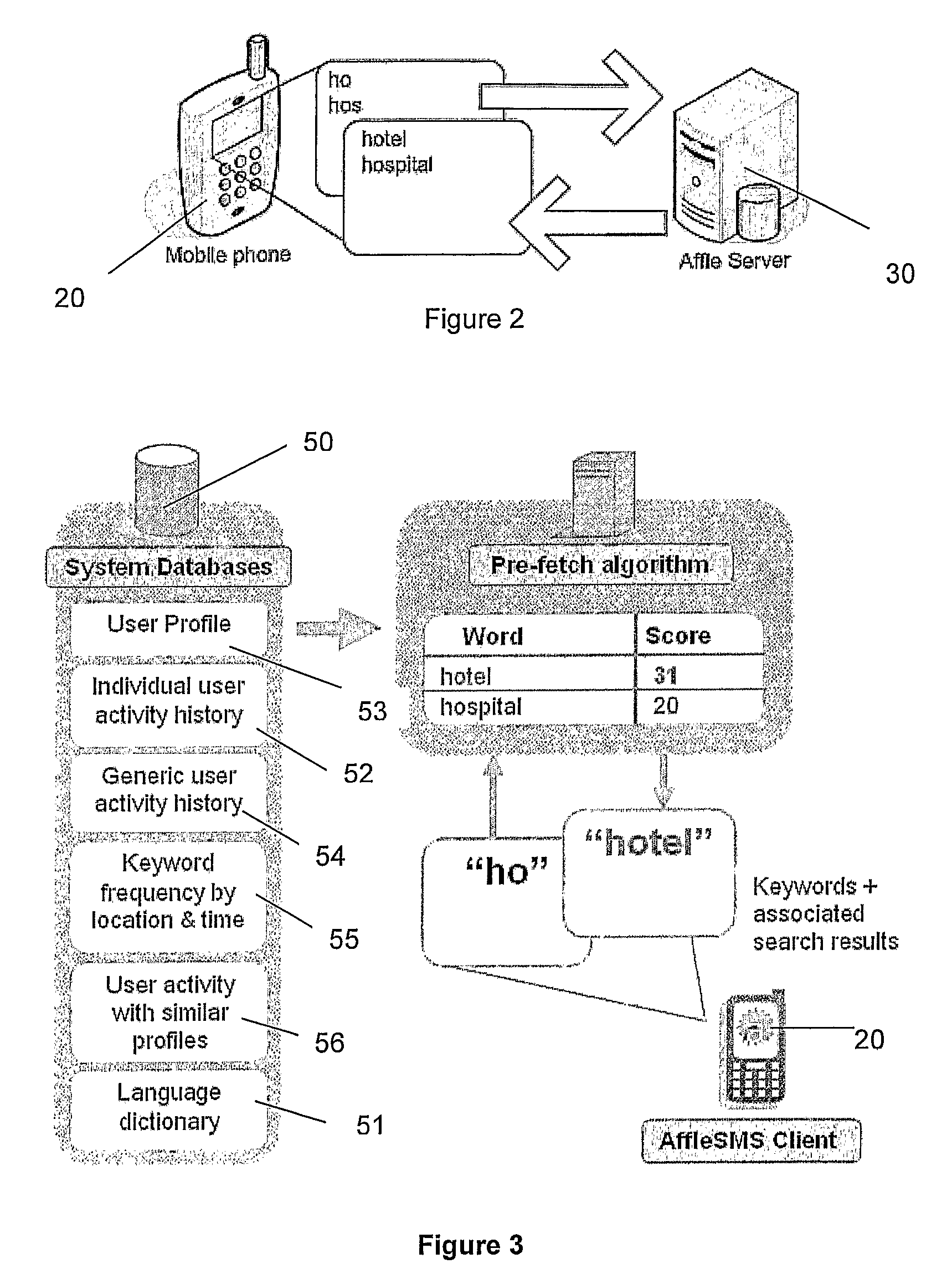
ActiveUS8452745B2Lower latencyReduce degradationWeb data indexingDigital data processing detailsClient-sideSearch terms
A search system, method and computer program are disclosed in which characters of a search term are captured as they are entered into a client system (20) and used to predict search terms. Search results are obtained for a predetermined number of the predicted search terms and cached at the client system (20). Upon determining the complete search term has been entered, search results corresponding to the complete search term are displayed.
Owner:AFFLE INT PTE LTD
High resolution 2D-3D switchable autostereoscopic display apparatus
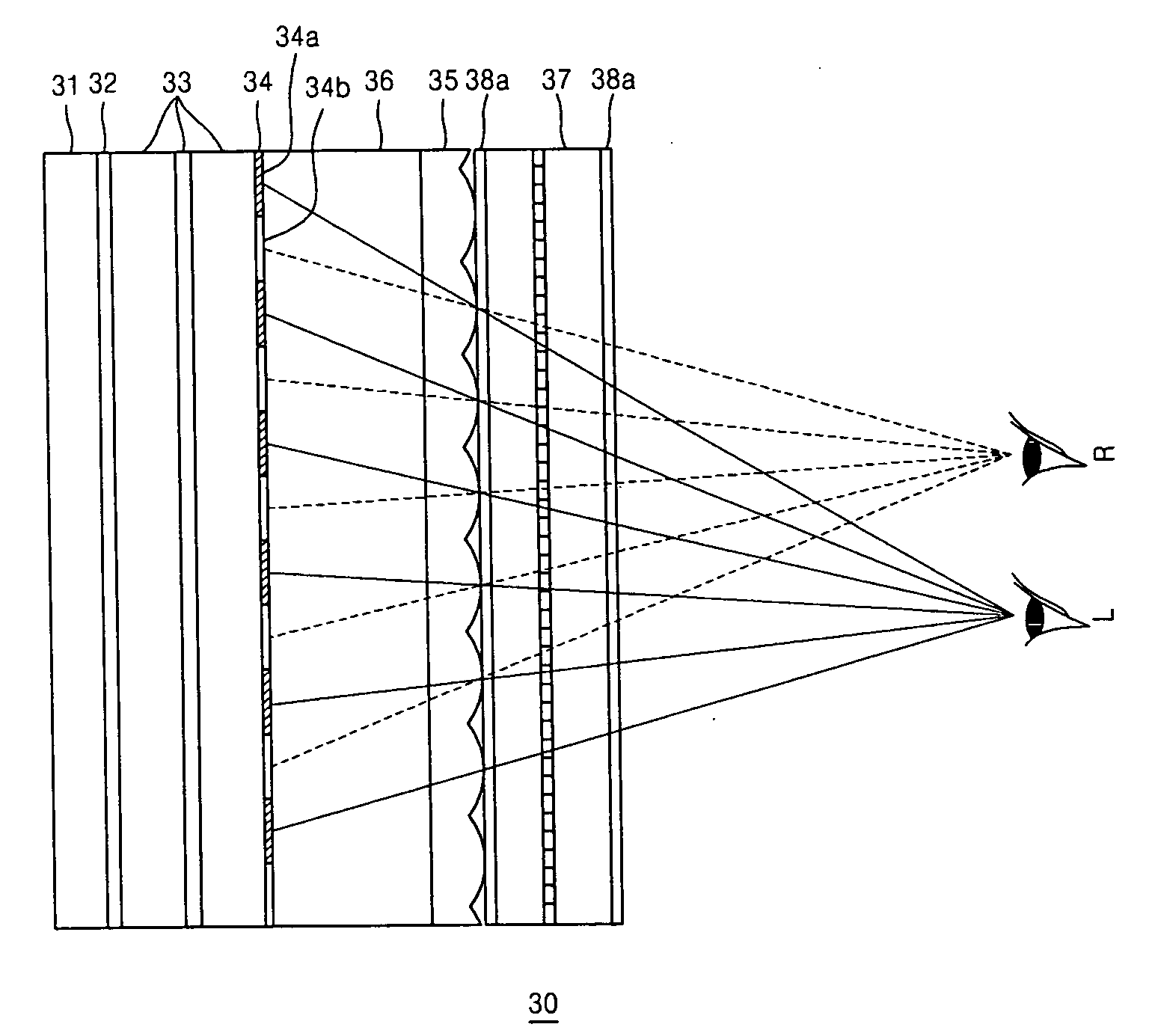
ActiveUS20070008406A1Reduce degradationReduce crosstalkTelevision system detailsColor television detailsPhysicsPolarizer
A high resolution 2D-3D switchable autostereoscopic display apparatus includes: a backlight unit emitting light; a polarizer sheet changing the light emitted from the backlight unit so that the light has only a specific polarization direction; a polarization switch converting the direction of the polarization of incident light; a birefringent element array comprising a plurality of alternating first and second birefringent elements and changing the polarization direction of incident light so that the polarization of light transmitted by the first birefringent elements is perpendicular to the polarization of light transmitted by the second birefringent elements; a lenticular lens sheet separating and emitting incident light to a first eye viewing zone and a second eye viewing zone; and a display panel displaying an image.
Owner:SAMSUNG ELECTRONICS CO LTD
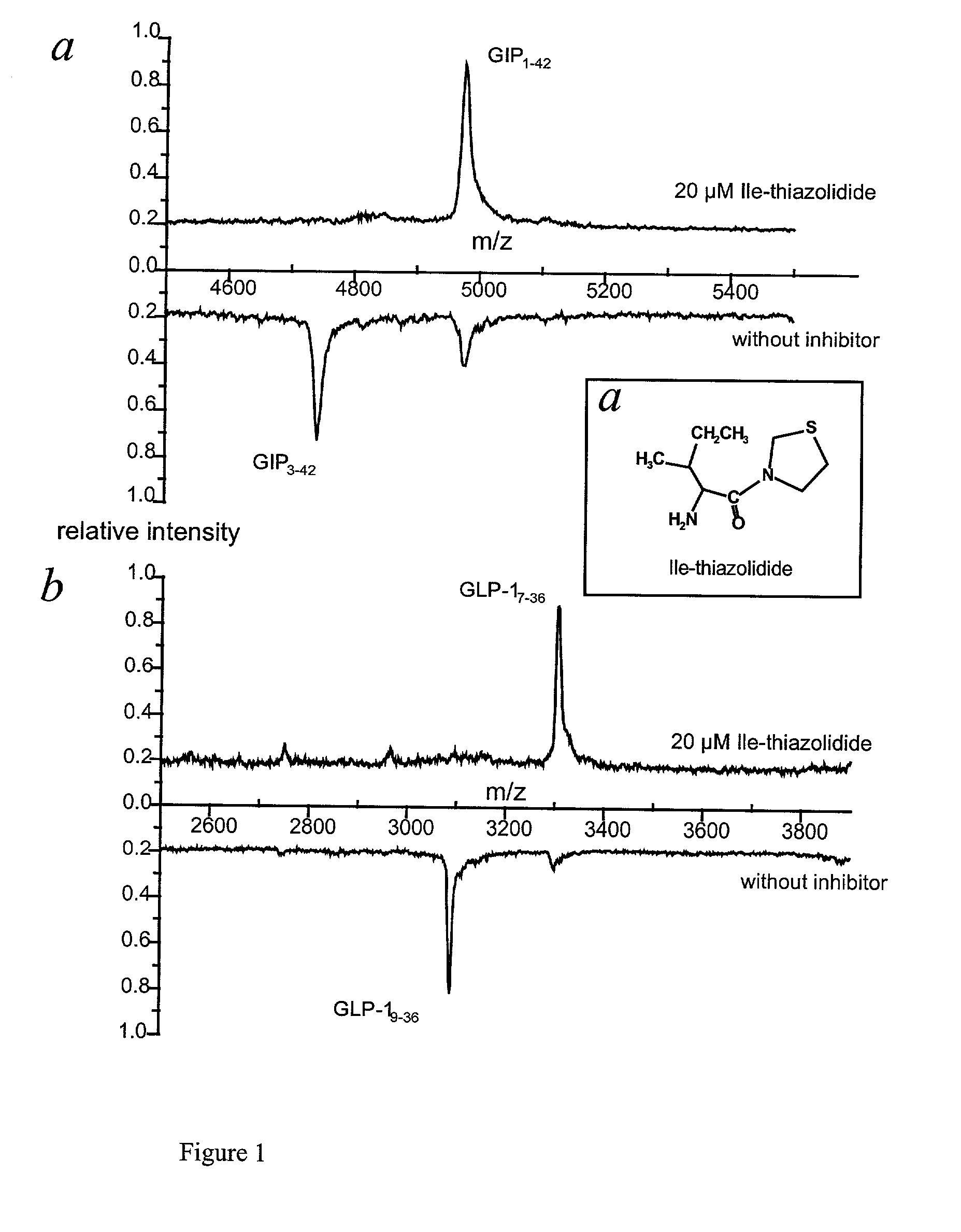
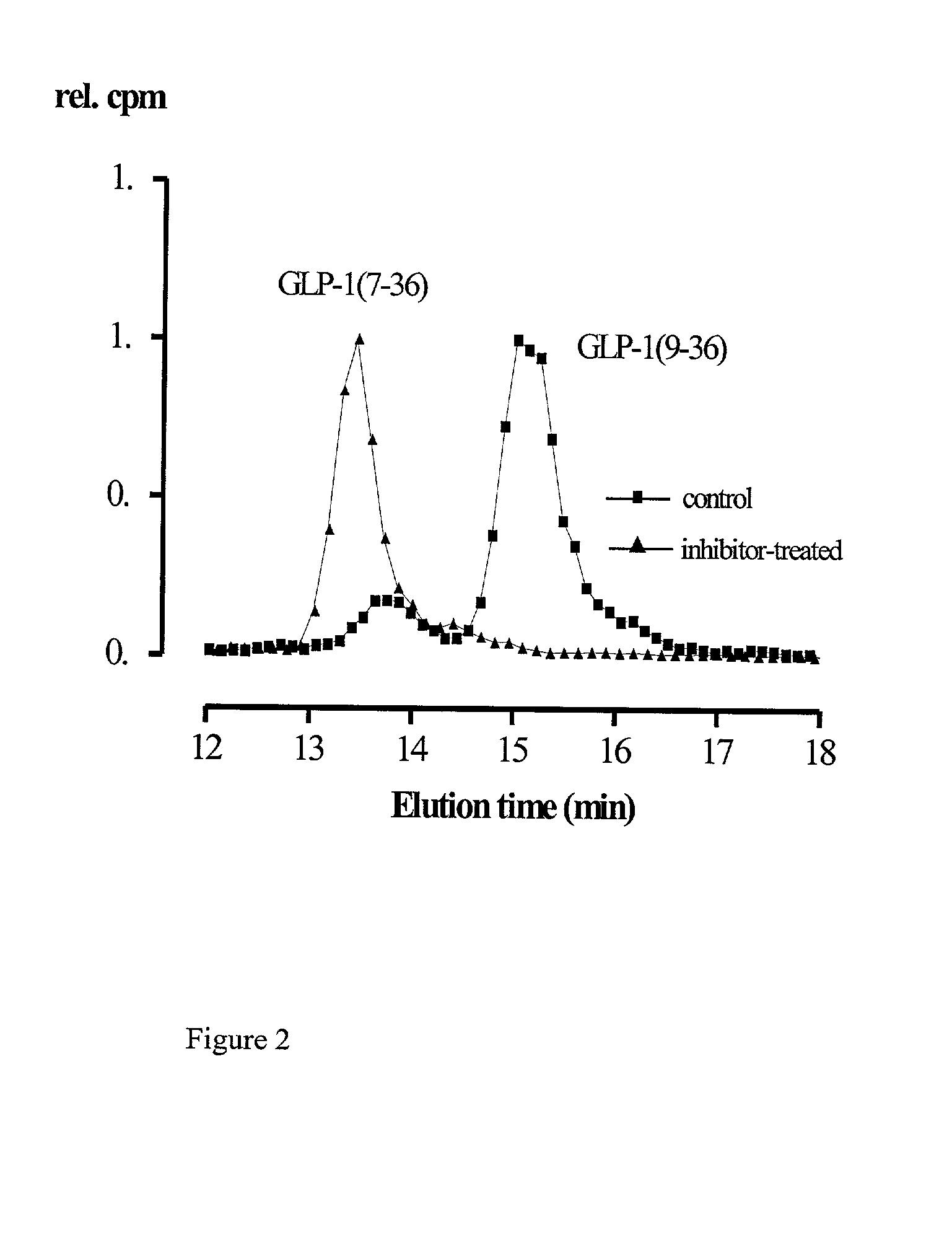
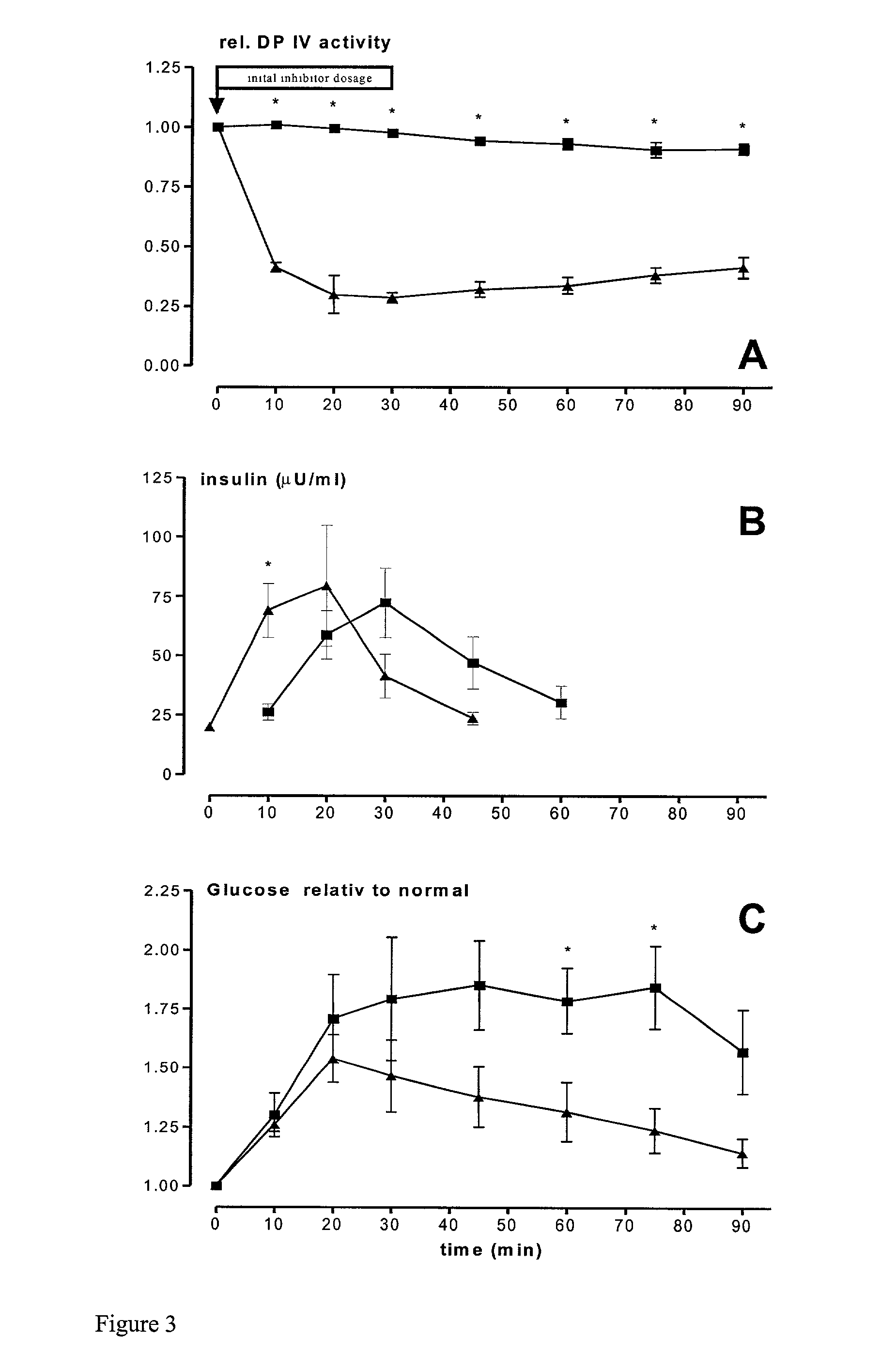
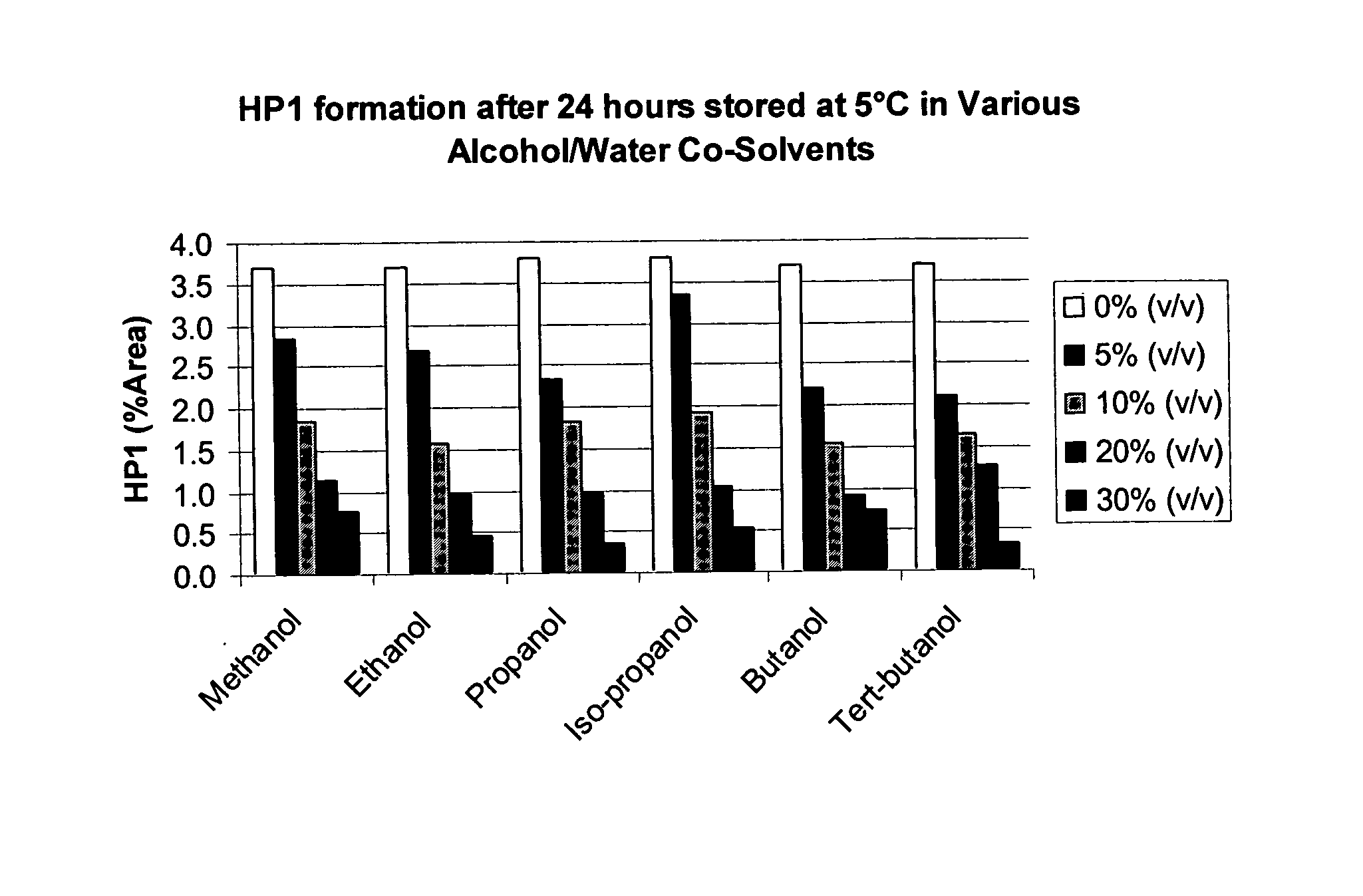
Use of dipeptidyl peptidase IV effectors for lowering blood pressure in mammals
InactiveUS20020006899A1Reduce degradationReduce concentrationBiocideOrganic chemistryBiological bodyDipeptidyl peptidase
The invention comprises the use of activity-reducing effectors of dipeptidyl peptidase (DP IV) and DP IV-analogous enzyme activity in the blood of a mammal to lower the blood sugar level and the blood pressure in mammalian organisms.
Owner:PROSIDION LIMITED
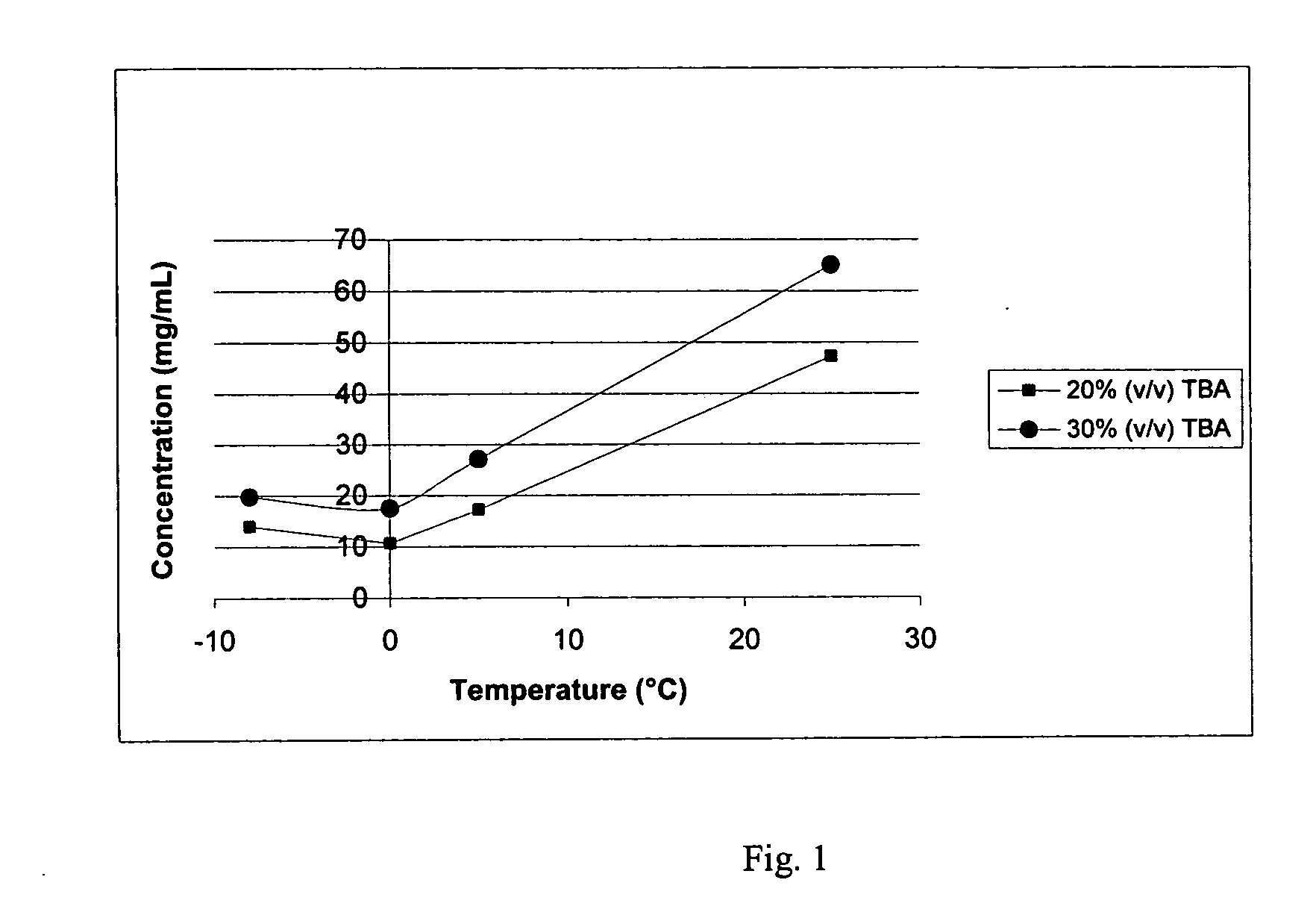
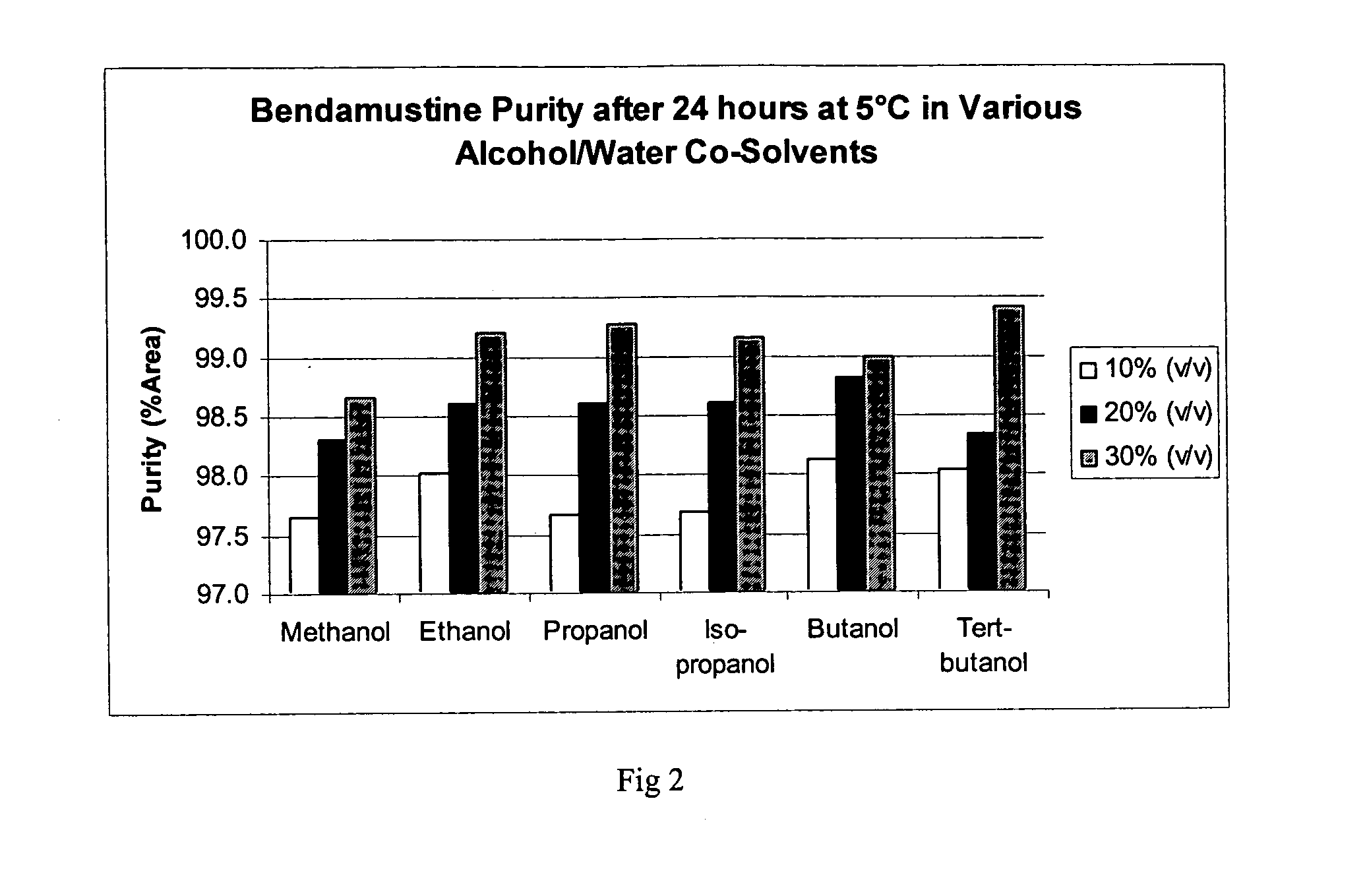
Bendamustine pharmaceutical compositions
The present invention provides pharmaceutical formulations of lyophilized bendamustine suitable for pharmaceutical use. The present invention further provides methods of producing lyophilized bendamustine. The pharmaceutical formulations can be used for any disease that is sensitive to treatment with bendamustine, such as neoplastic diseases.
Owner:CEPHALON LLC
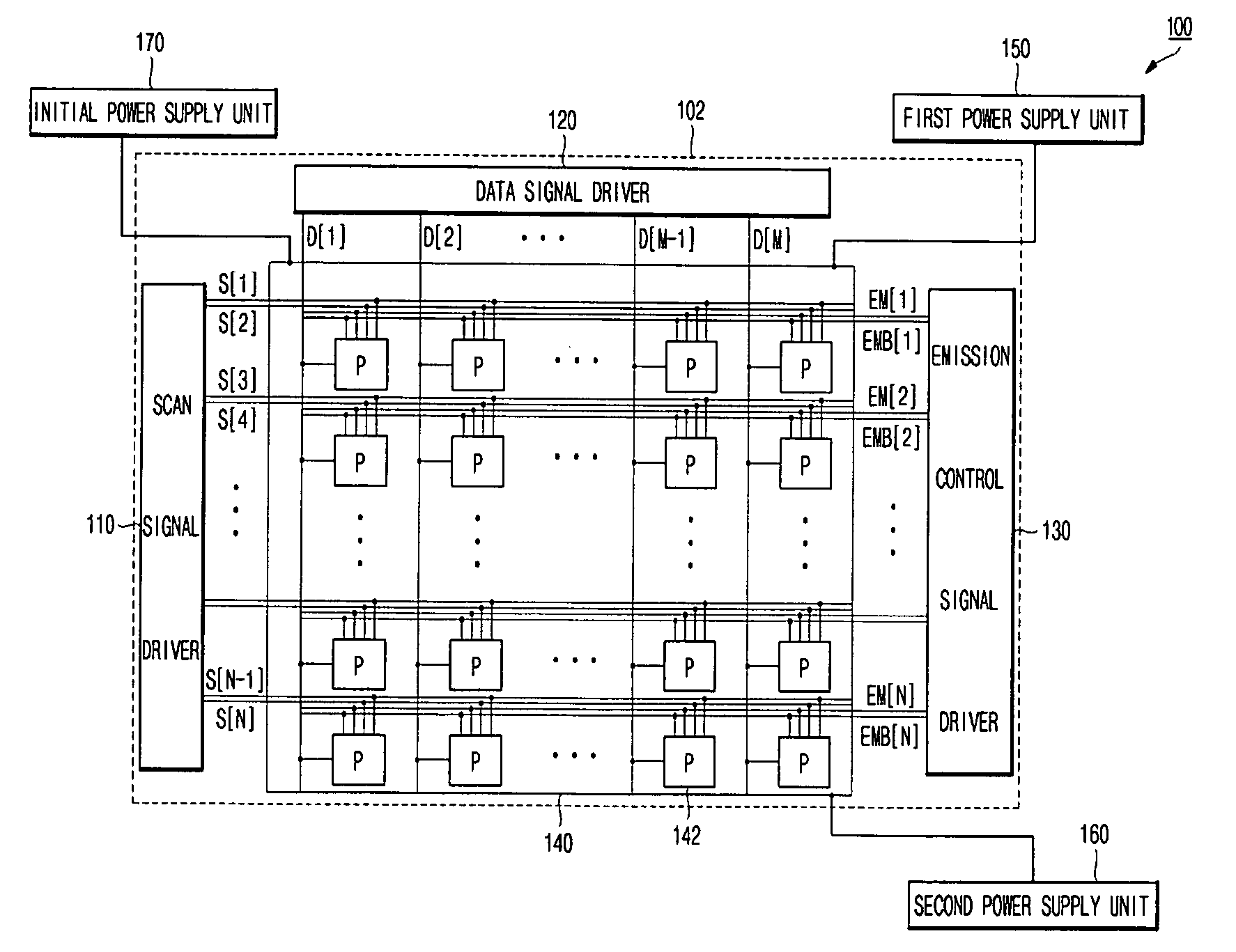
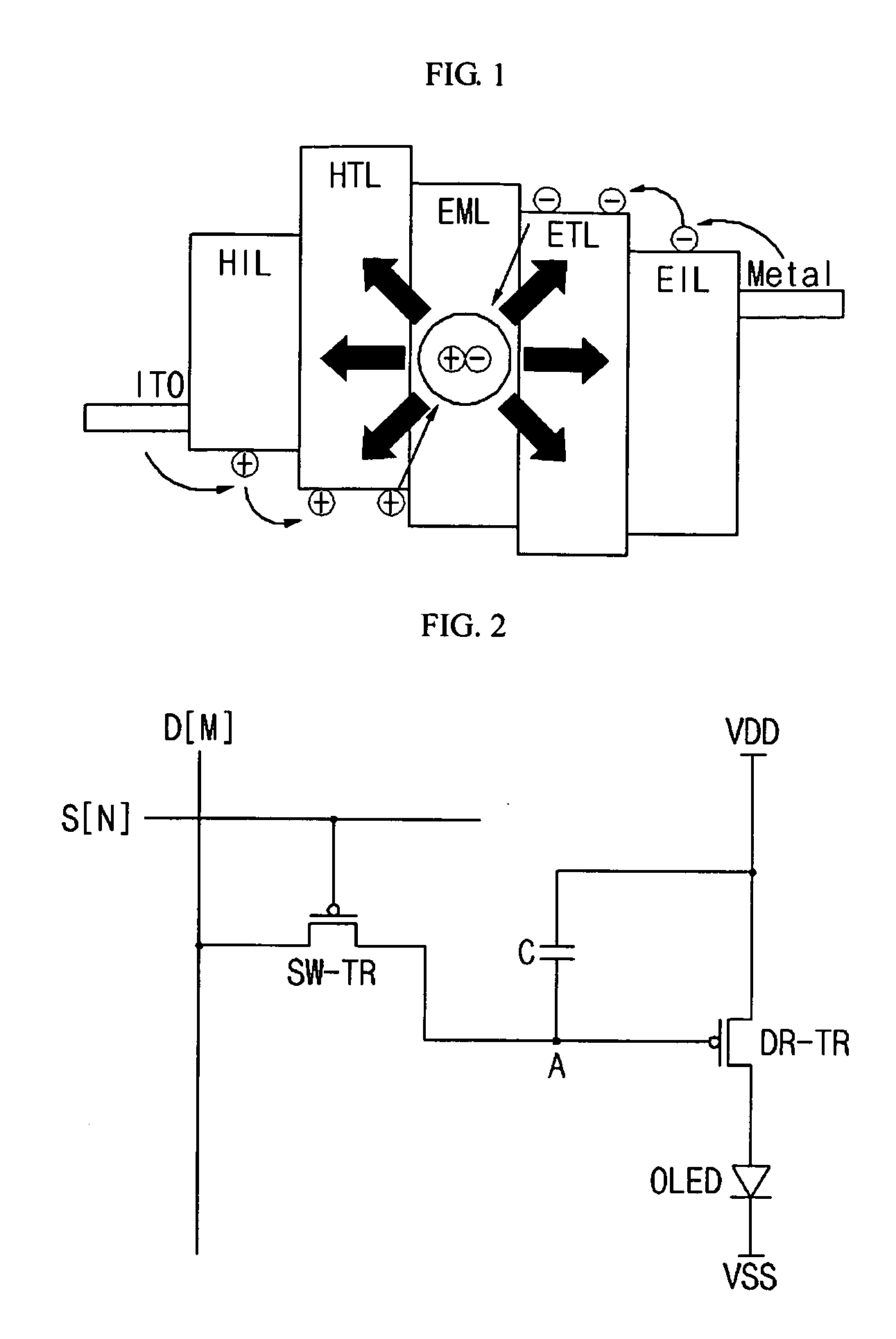
Organic light emitting display and driving method thereof
ActiveUS20080150846A1Operation margin can be ensuredAvoid voltage dropStatic indicating devicesDisplay deviceLight-emitting diode
An organic light emitting display including a scan signal line forwarding a scan signal, a data line sending a data signal and a pixel coupled to the scan signal line and the data line, the organic light emitting diode display, wherein the pixel includes a first switching transistor transmitting a data signal from the data line in response to the scan signal of the scan signal line, a driving transistor, coupled to the first switching transistor, controlling driving current from a first power source line, a storage capacitor coupled between the driving transistor and the first power source line, an organic light emitting diode, coupled between the driving transistor and a second power source line, displaying an image with the driving current controlled by the driving transistor, an initial switching transistor, coupled between the storage capacitor and an initial power source line, initializing the storage capacitor, and a switching transistor for applying a reverse bias, coupled between the second power source line and the initial power source line, applying a reverse bias voltage to the organic light emitting diode.
Owner:SAMSUNG DISPLAY CO LTD
Flip chip assembly structure for semiconductor device and method of assembling therefor
InactiveUS6798072B2Improve productivityImprove reliabilitySemiconductor/solid-state device detailsSolid-state devicesDevice materialMetallic materials
A semiconductor device includes a semiconductor chip and a printed circuit board. Metal electrodes of the semiconductor chip and the internal connection terminals of the printed circuit board are electrically connected through the metallic joining via precious metal bumps. A melting point of a metal material constituting each of the metallic joining parts is equal to or higher than 275 degrees, and a space defined between the chip and the board is filled with resin (under fill) containing 50 vol % or more inorganic fillers.
Owner:HITACHI LTD
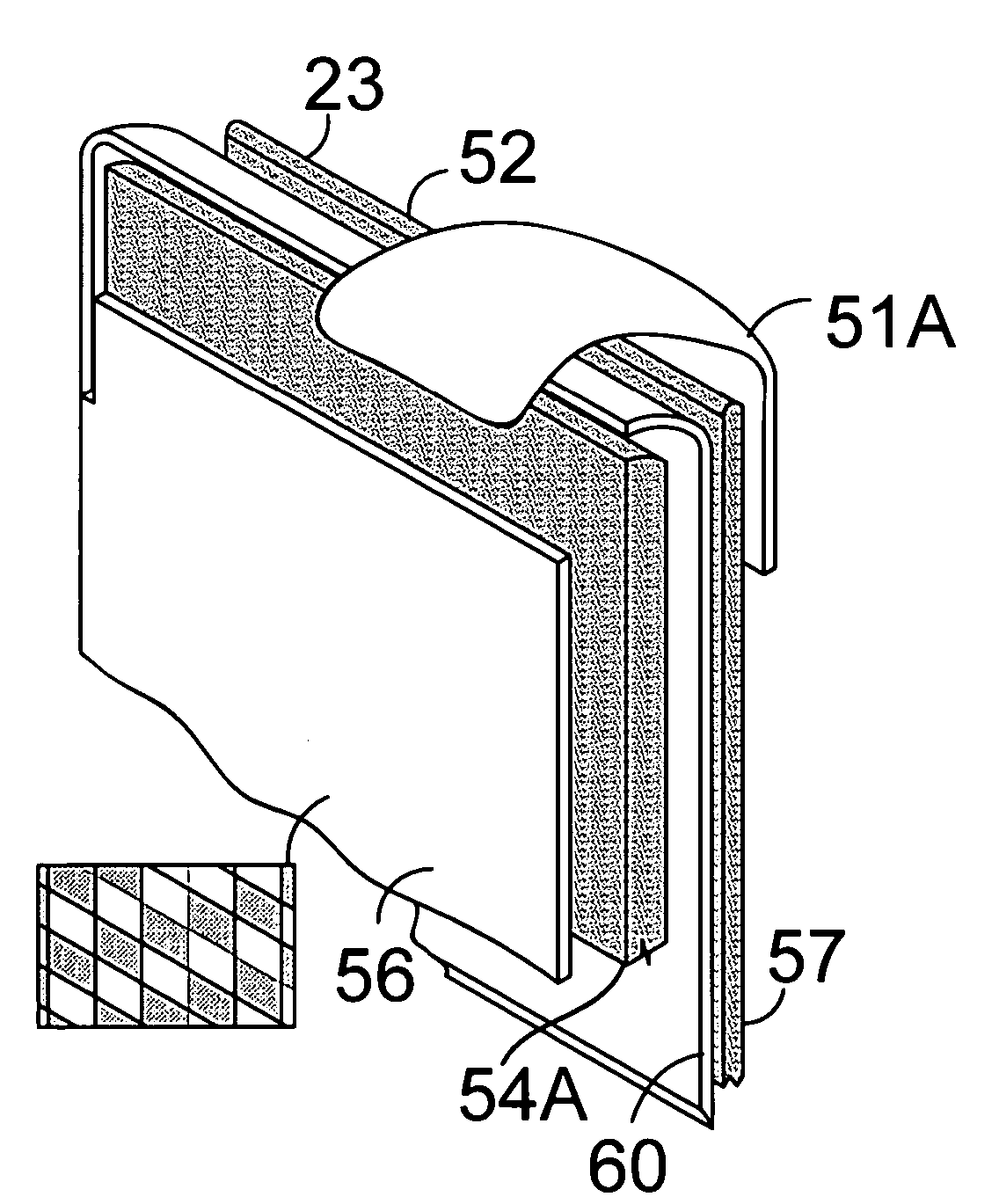
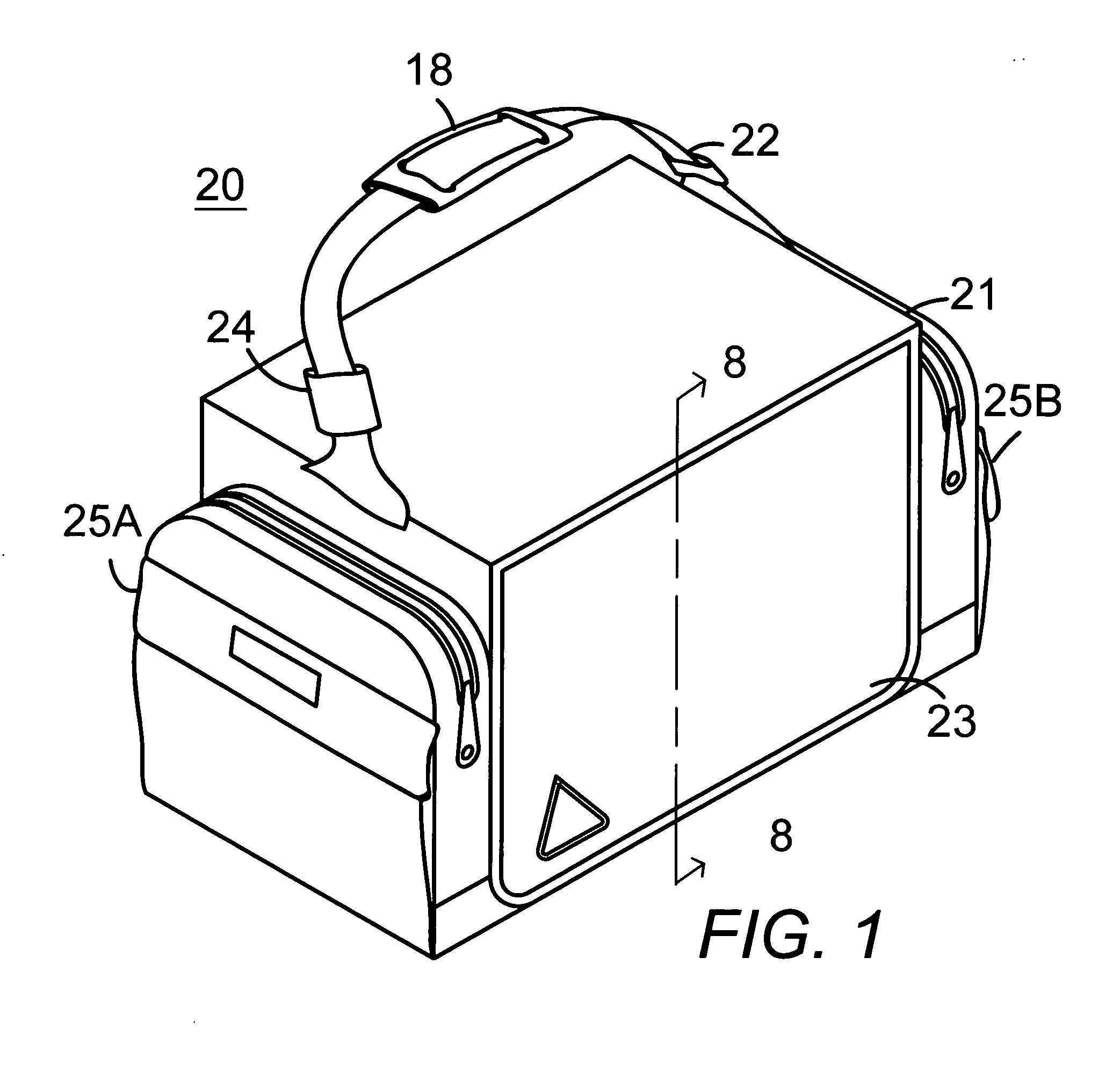
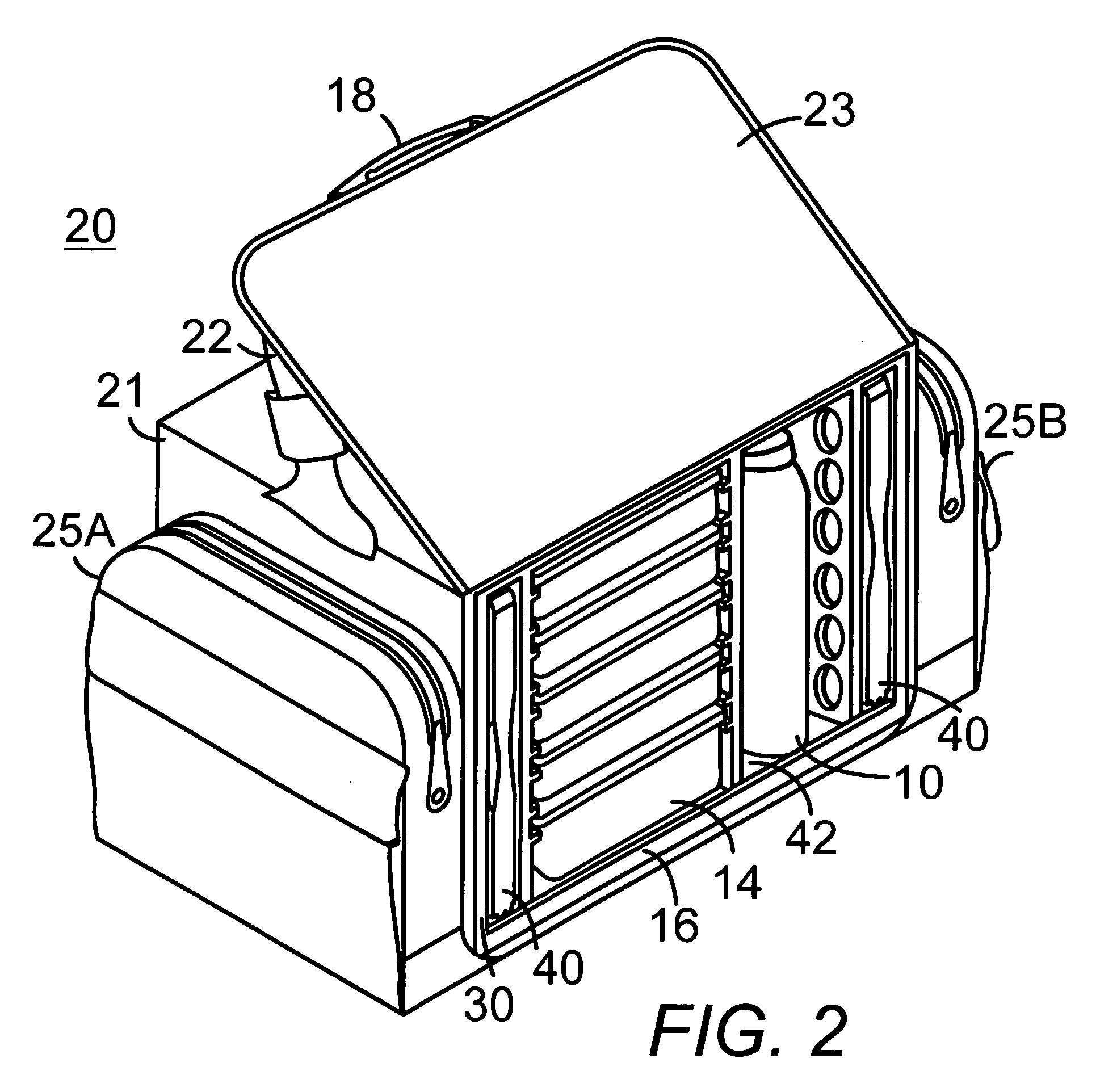
Portable soft shell cooler with compartmented rack for individual meal and beverage containers
InactiveUS7313927B2Easy to manageImprove cooling effectLighting and heating apparatusOther accessoriesWater circulationEngineering
A soft thermally insulated padded shell houses a rigid rack with a series of vertical walls forming compartments. One pair of walls has a series of mating horizontal tracks to receive slide-in meal containers in a spaced vertical array. Narrow side compartments house coolant containers. Another compartment houses beverage containers. Upper holes in the walls admit air flow between compartments. A vertical lip on the open front edge of the bottom of the rack forms a condensation basin beneath the elevated meal containers. An elevated floor section insertable in the beverage compartment serves as a beverage compartment condensation basin. Holes through the bottoms of the walls allow condensation water circulation between compartments.
Owner:BARKER GAREN S
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com