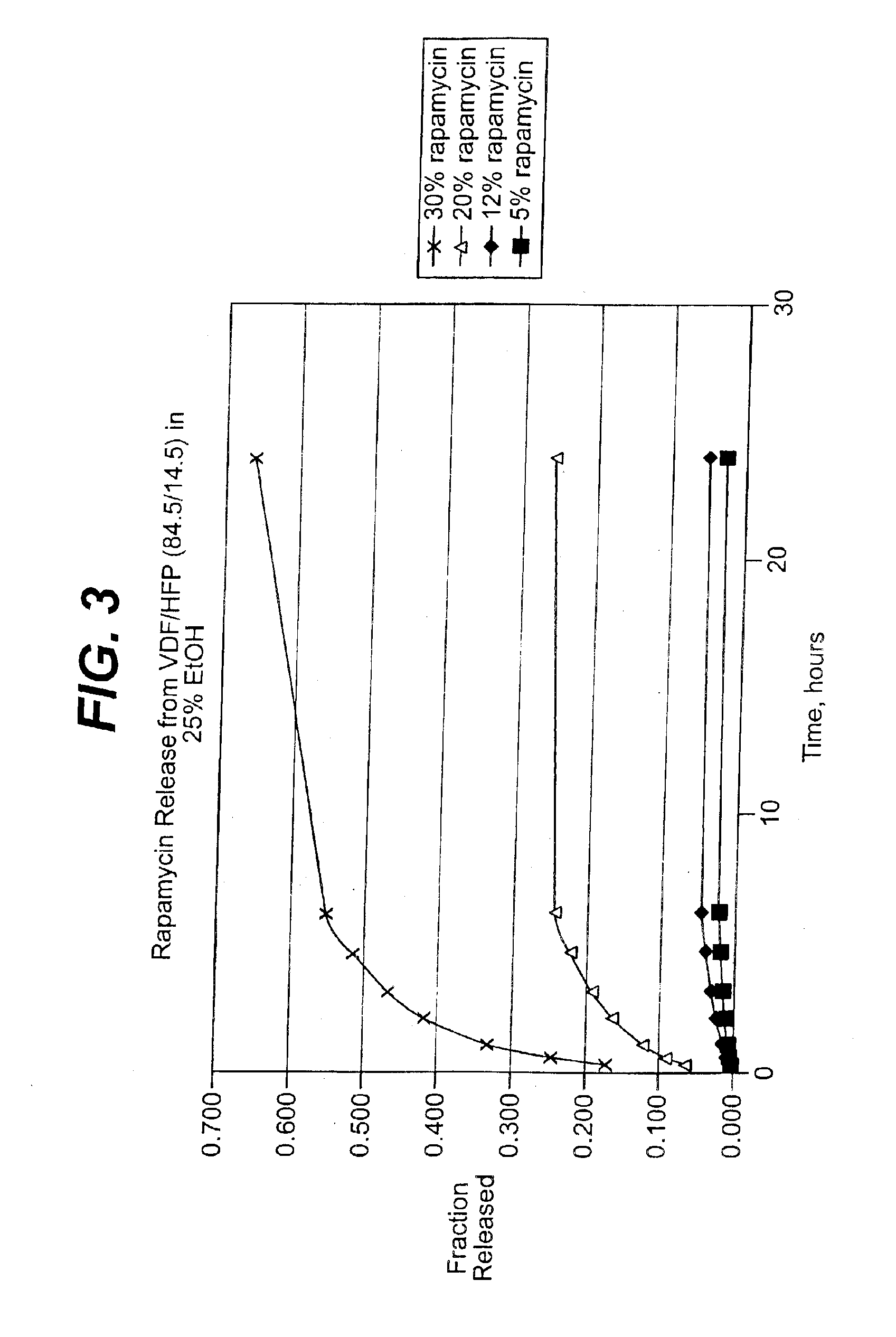
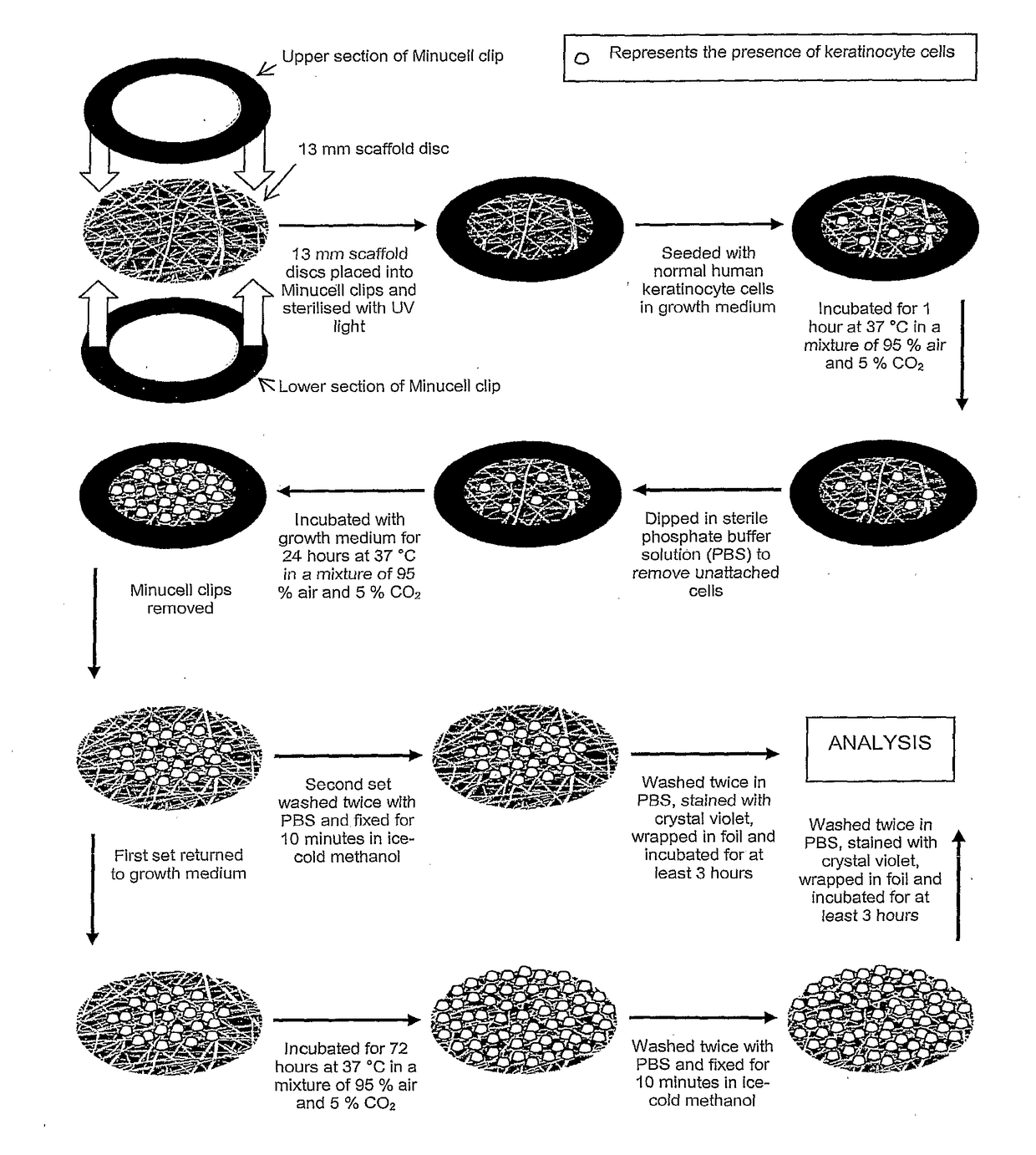
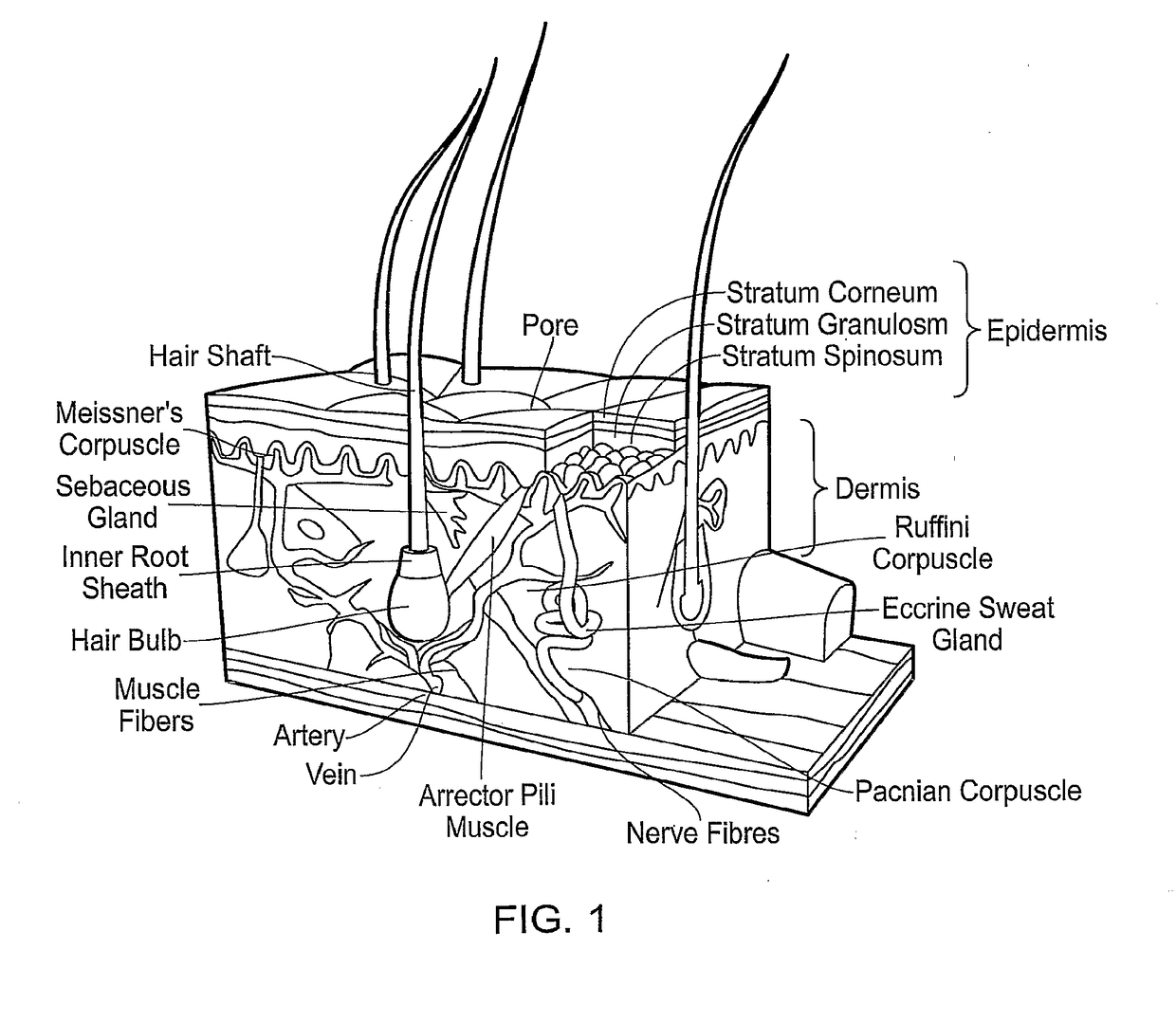
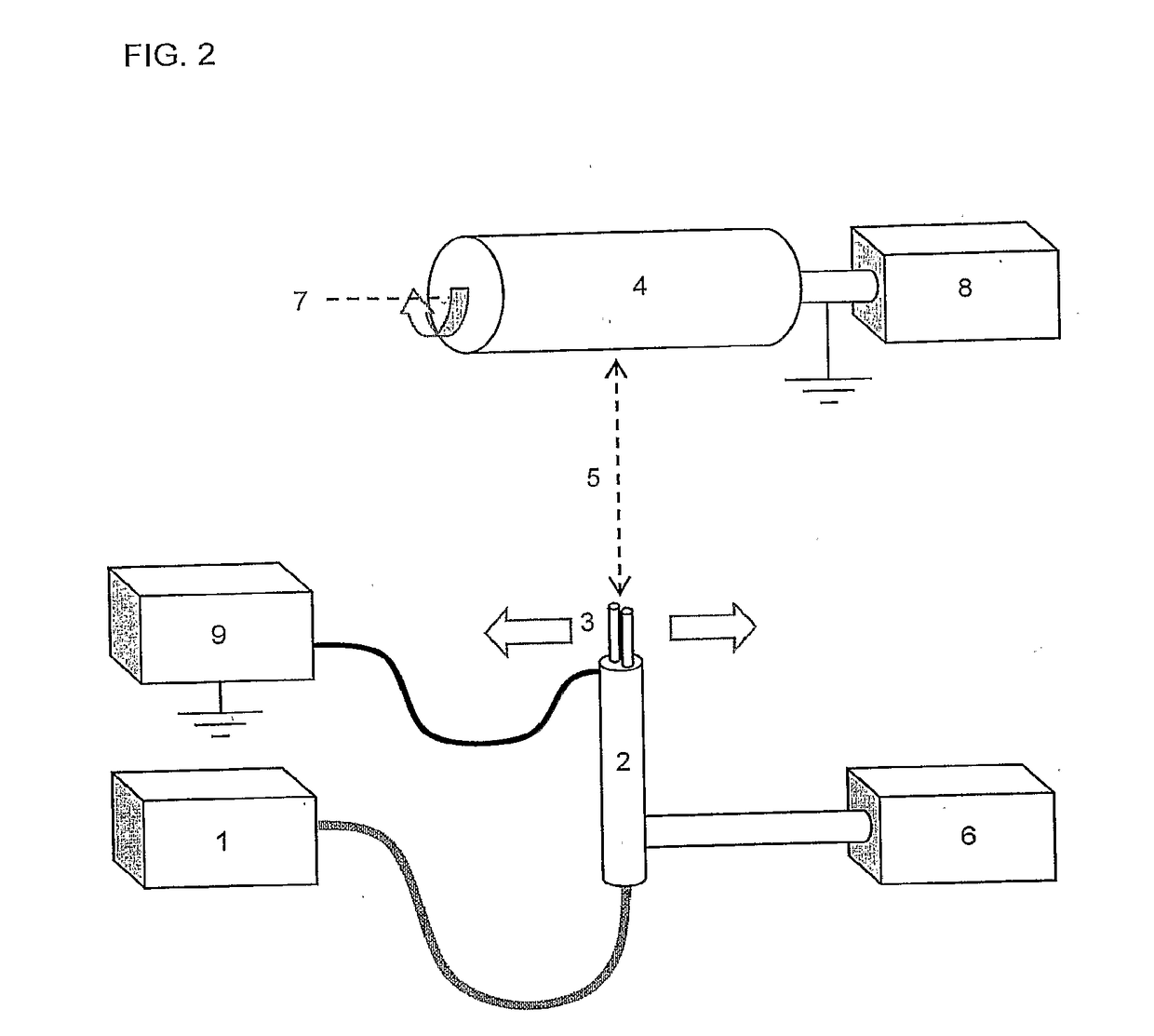
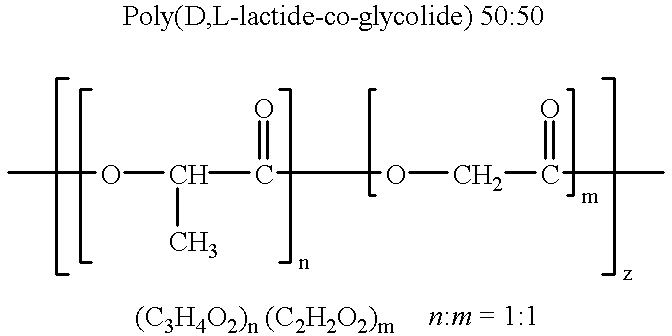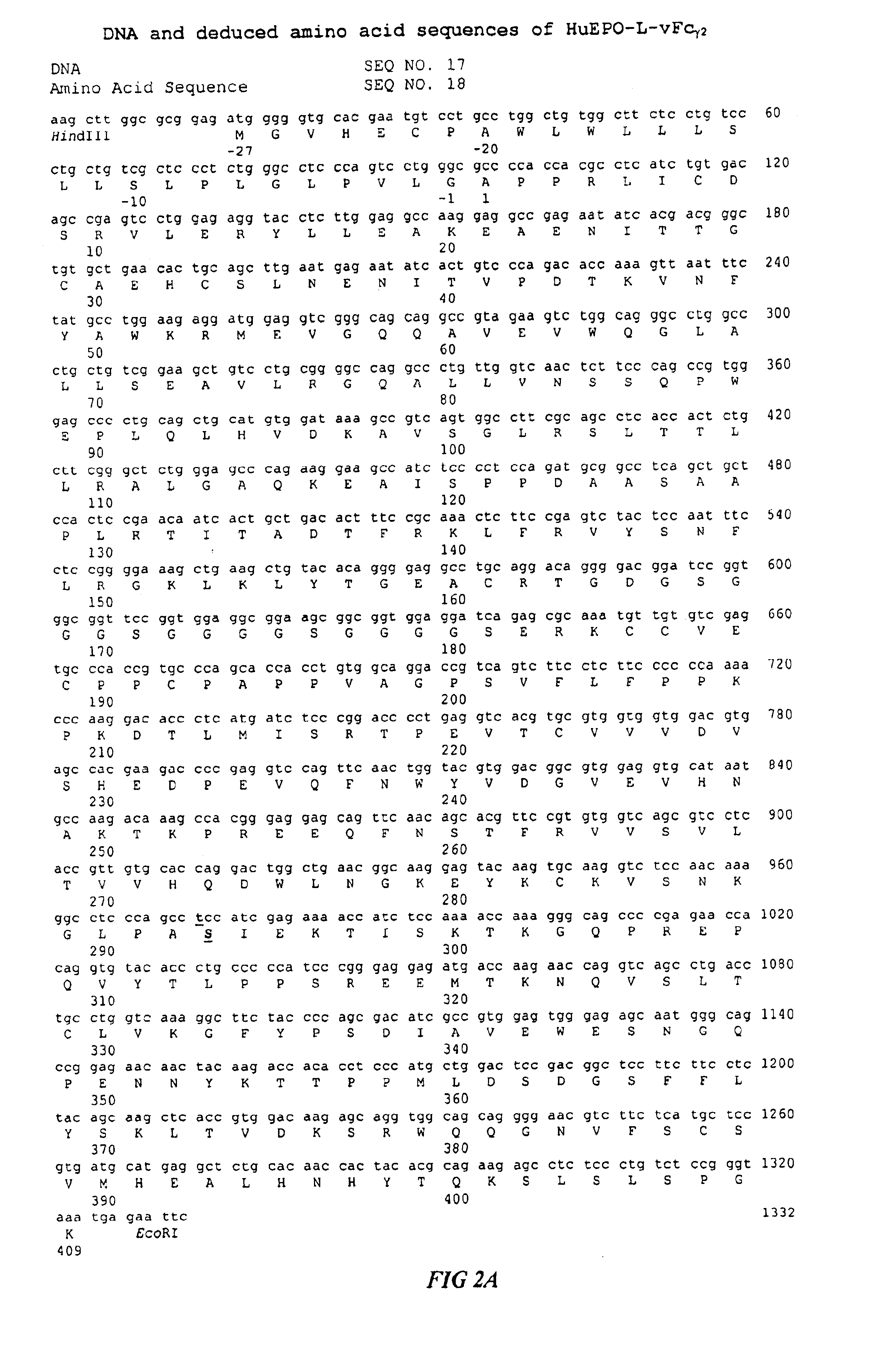Patents
Literature
69755 results about "Biological organism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Humans As Biological Organisms. Organism can be defined as an assembly of molecules functioning as a stable whole that exhibit the properties of life. The word organism originates from the Greek organon meaning organ of sense. Organisms have the tendency of responding to stimuli, homeostasis, reproduction, growth, and development.
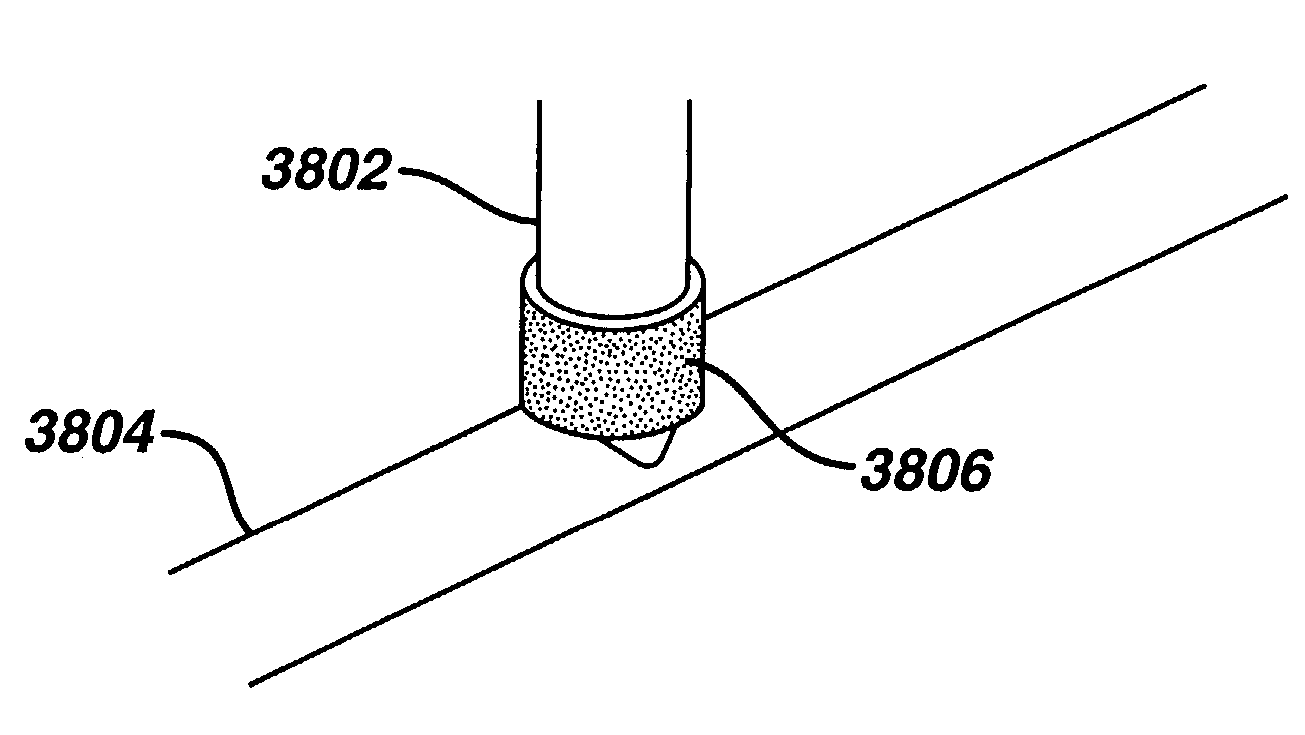
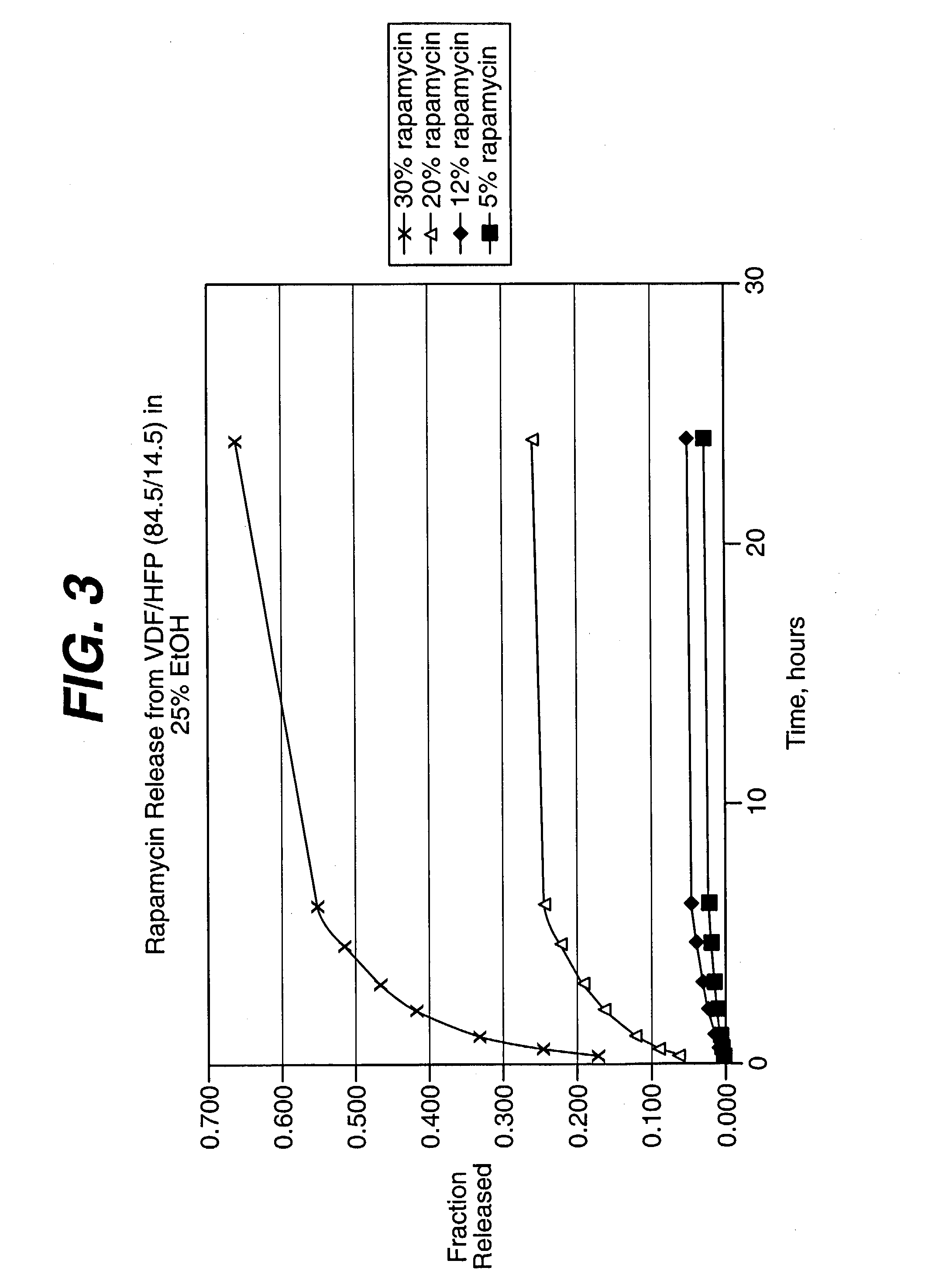
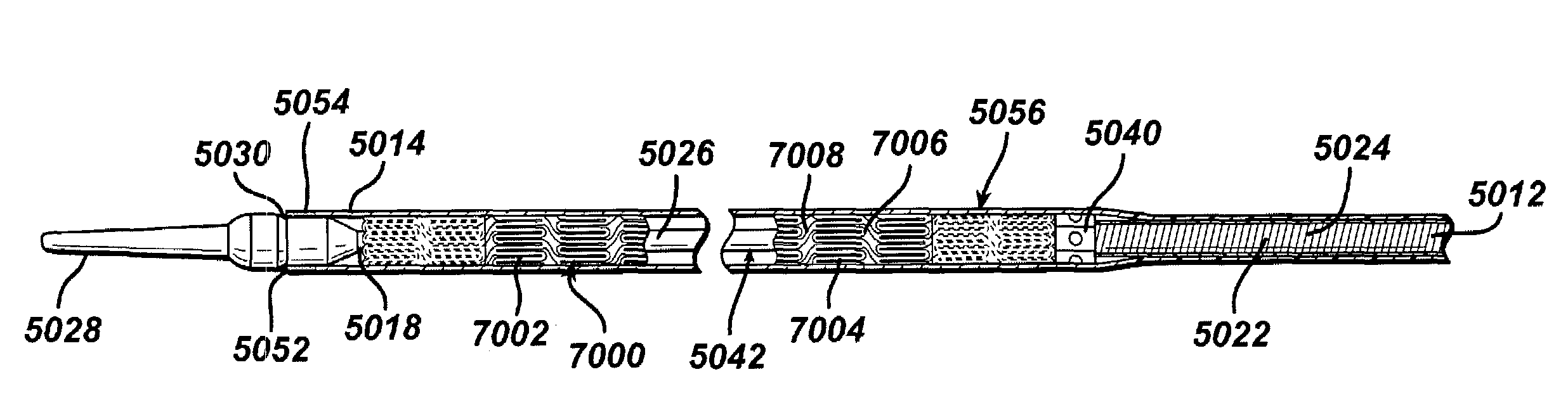
Drug releasing anastomosis devices and methods for treating anastomotic sites
ActiveUS7108701B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH
Self-supporting, shaped, three-dimensional biopolymeric materials and methods
Self-supporting, shaped, three-dimensional cross-linked proteinaceous biopolymeric materials that may be implanted in vivo, and methods of making such materials are disclosed. The biopolymeric materials most preferably include reinforcing media, such as biocompatible fibrous or particulate materials. In use, the preformed, shaped biopolymeric materials may be applied to tissue in need of repair and then sealed around its edges with a liquid bioadhesive. In such a manner, repaired tissue which is capable of withstanding physiological pressures may be provided.
Owner:CRYOLIFE
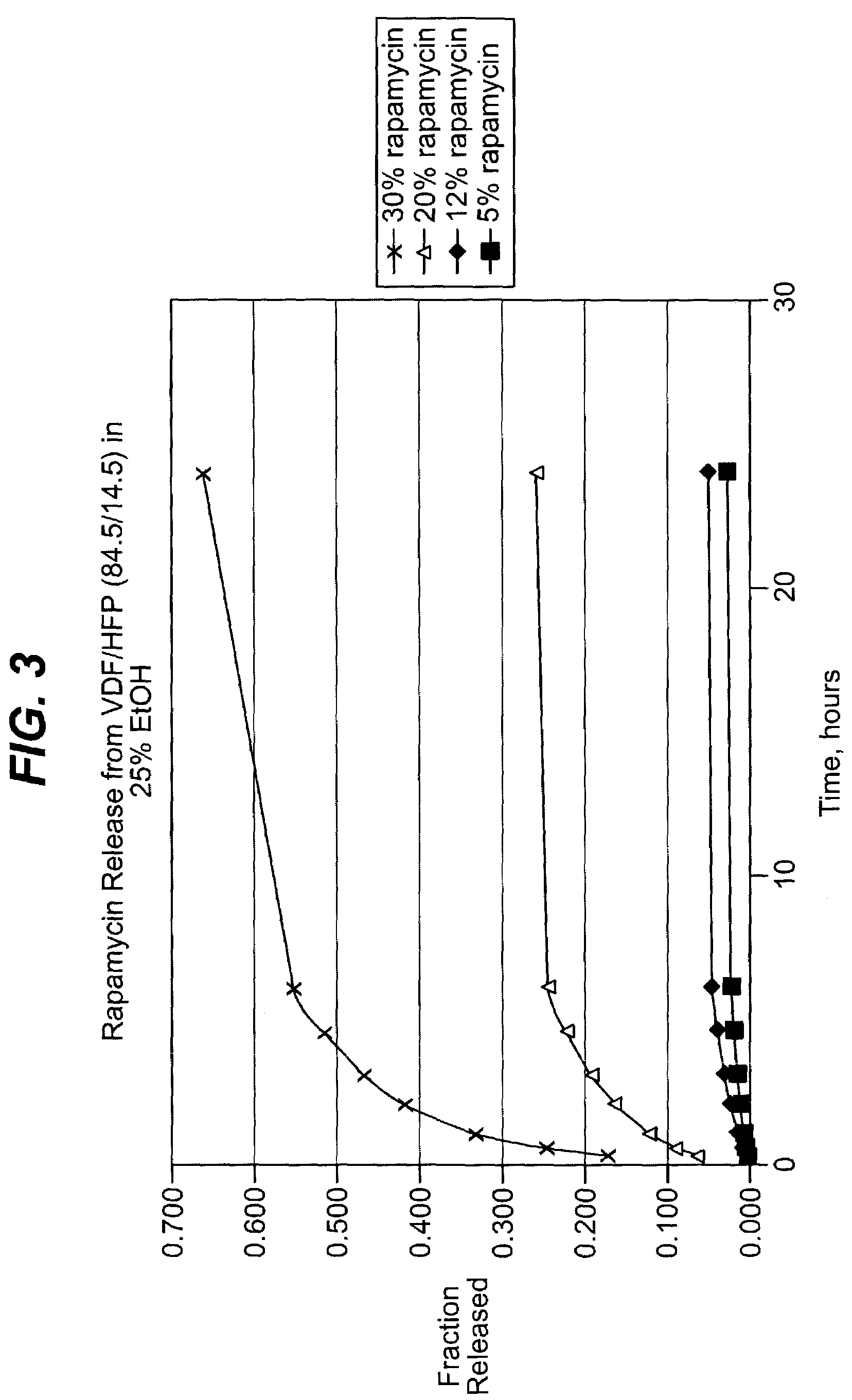
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Multilayer scaffold
InactiveUS20170182211A1Avoid disease riskAvoids the potential ethical and religious barriers to the useSkin implantsTissue regenerationMedicinePlla scaffold
Owner:SMITH & NEPHEW PLC
Modified delivery device for coated medical devices
InactiveUS7527632B2Minimize potential risk of damageReduce frictionStentsEar treatmentBiological bodyMedical device
Medical devices, and in particular implantable medical devices, including self-expanding stents, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
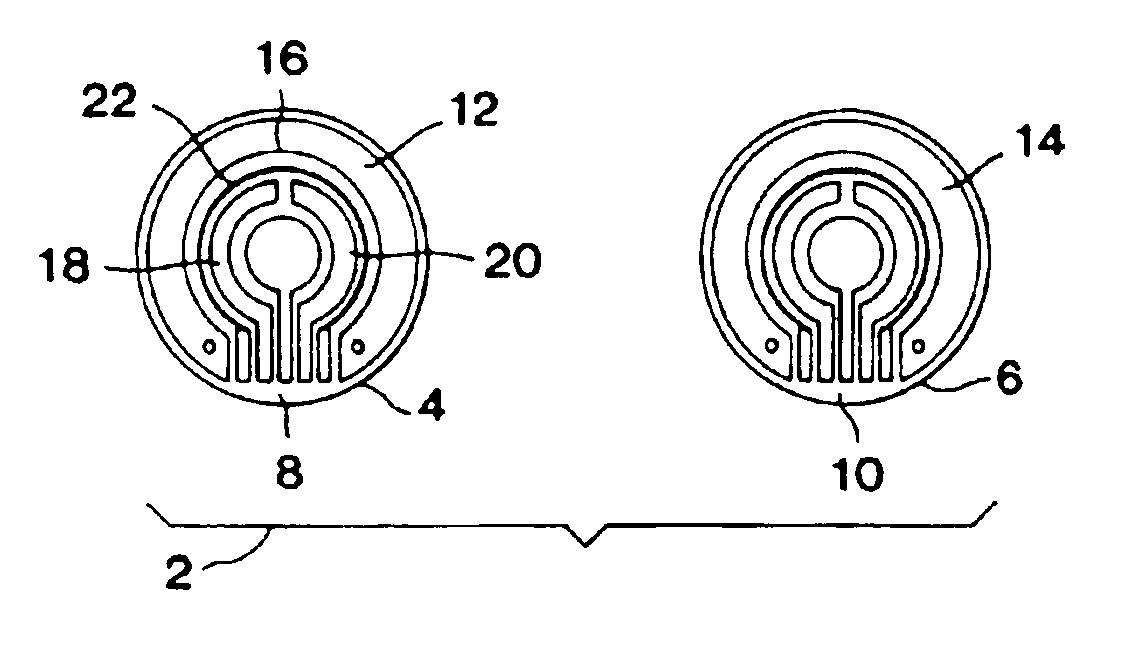
Monitoring of physiological analytes
InactiveUS6850790B2Minimize impactReduce presenceElectrotherapyMicrobiological testing/measurementAnalyteEngineering
Methods and devices are provided for measuring the concentration of target chemical analytes present in a biological system. Device configuration and / or measurement techniques are employed in order to reduce the effect of interfering species on sensor sensitivity. One important application of the invention involves a method and device for monitoring blood glucose values.
Owner:LIFESCAN IP HLDG LLC +1
Means to achieve sustained release of synergistic drugs by conjugation
A codrug composition of at least two drug compounds covalently linked to one another via a labile bond to form a single codrug composition, or ionically linked to one another to form a single workings composition, and methods of use of the codrug for the treatment of various medical conditions. The codrug may be administered by itself or in the form of a bioerodible or nonbioerodible substance.
Owner:UNIVERSITY OF KENTUCKY +1
In vivo biosensor apparatus and method of use
InactiveUS6673596B1Less can be administeredCost-effective administration of drugBioreactor/fermenter combinationsBiological substance pretreatmentsIn vivoGenetically engineered
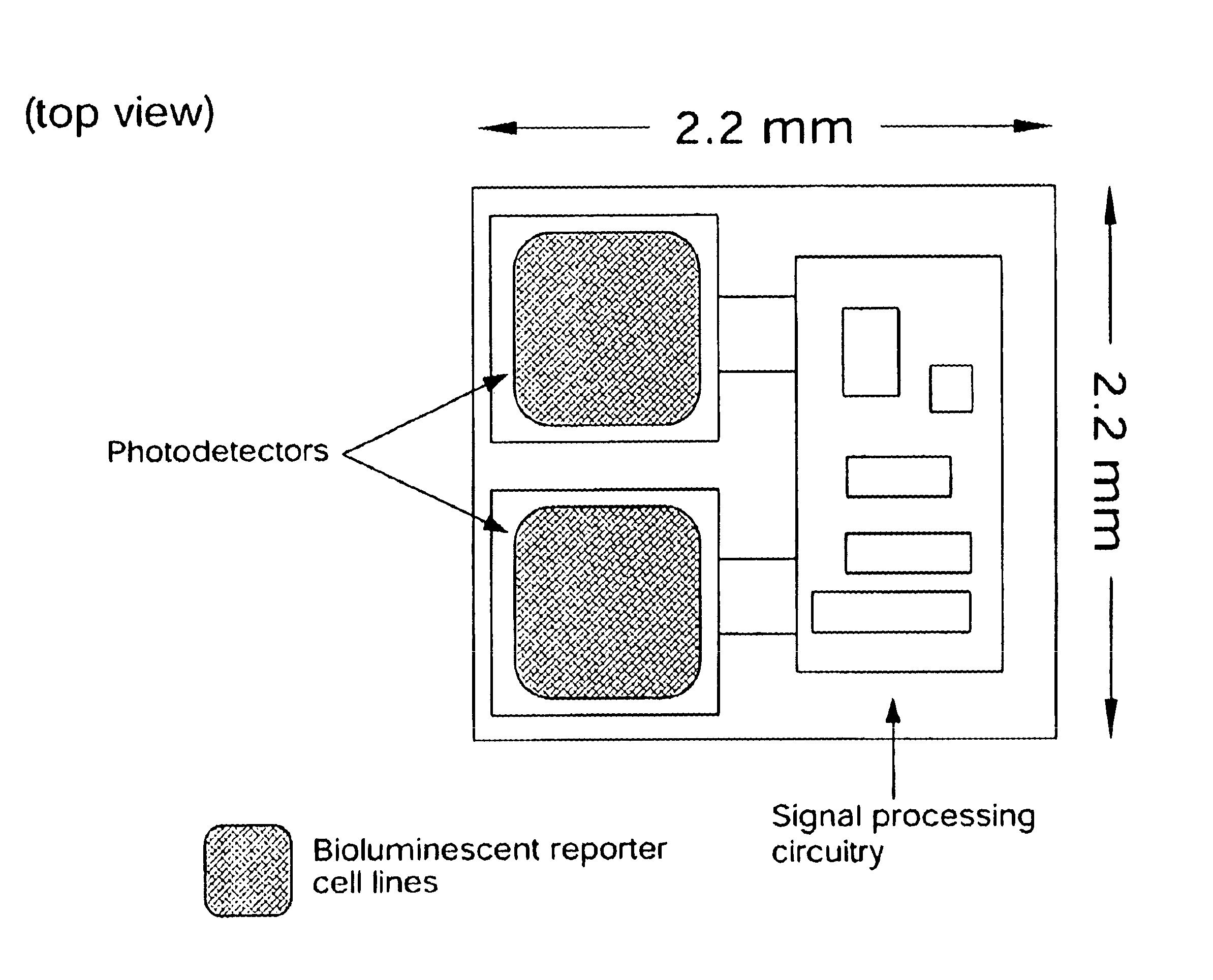
Disclosed are bioluminescent bioreporter integrated circuit devices that detect selected analytes in fluids when implanted in the body of an animal. The device comprises a bioreporter that has been genetically engineered to contain a nucleic acid segment that comprises a cis-activating response element that is responsive to the selected substance operably linked to a gene encoding a bioluminescent reporter polypeptide. In preferred embodiments, the target analyte is glucose, glucagons, or insulin. Exposure of the bioreporter to the target substance causes the response element to up-regulate the nucleic acid sequence encoding the reporter polypeptide to produce a luminescent response that is detected and quantitated. In illustrative embodiments, the bioreporter device is encapsulated on an integrated circuit that is capable of detecting the emitted light, processing the resultant signal, and then remotely reporting the results. Also disclosed are controlled drug delivery systems capable of being directly or indirectly controlled by the detection device that provide drugs such as insulin to the animal in reponse to the amount of target analyte present in the body fluids.
Owner:UNIV OF TENNESSEE RES FOUND +1
Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
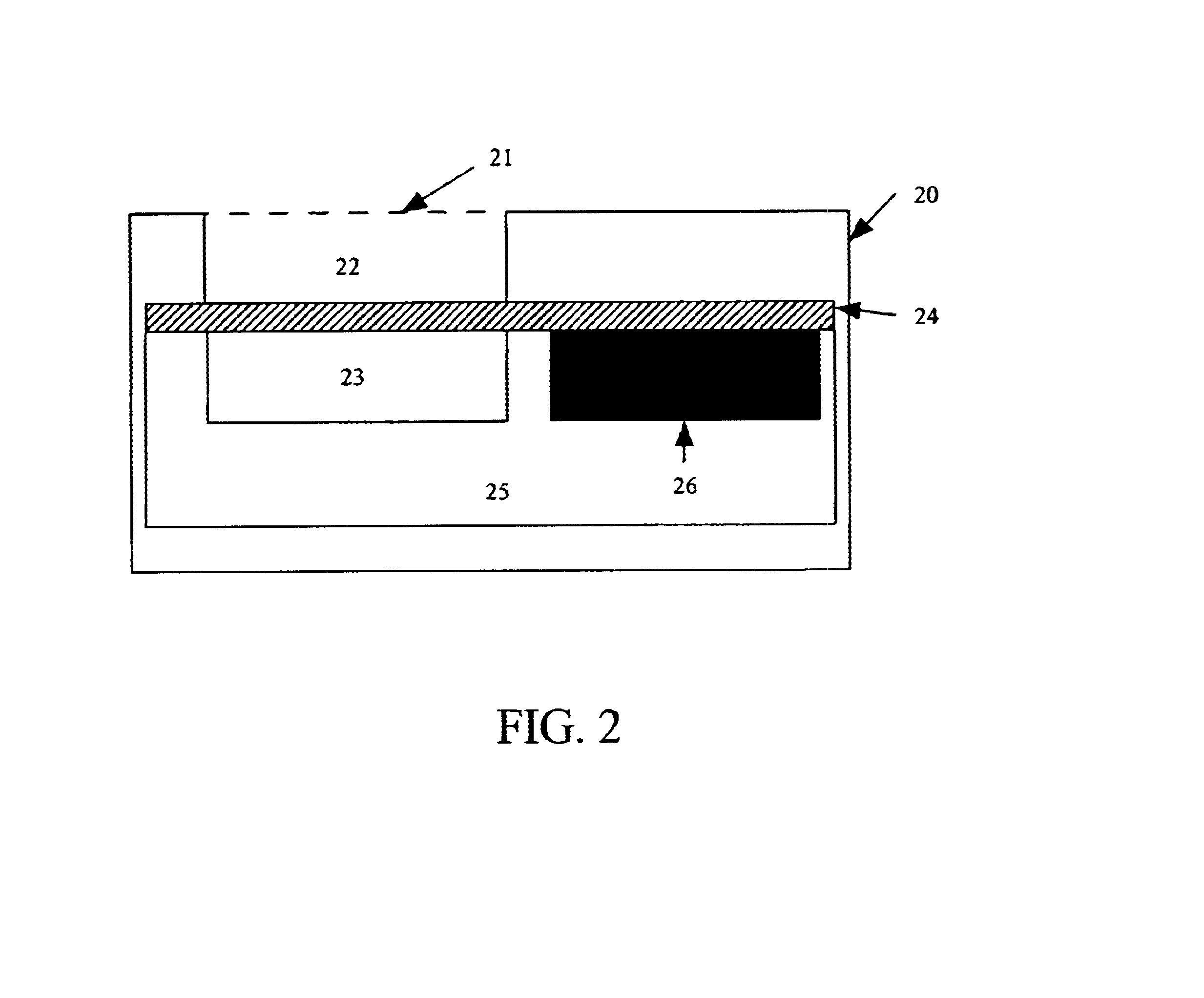
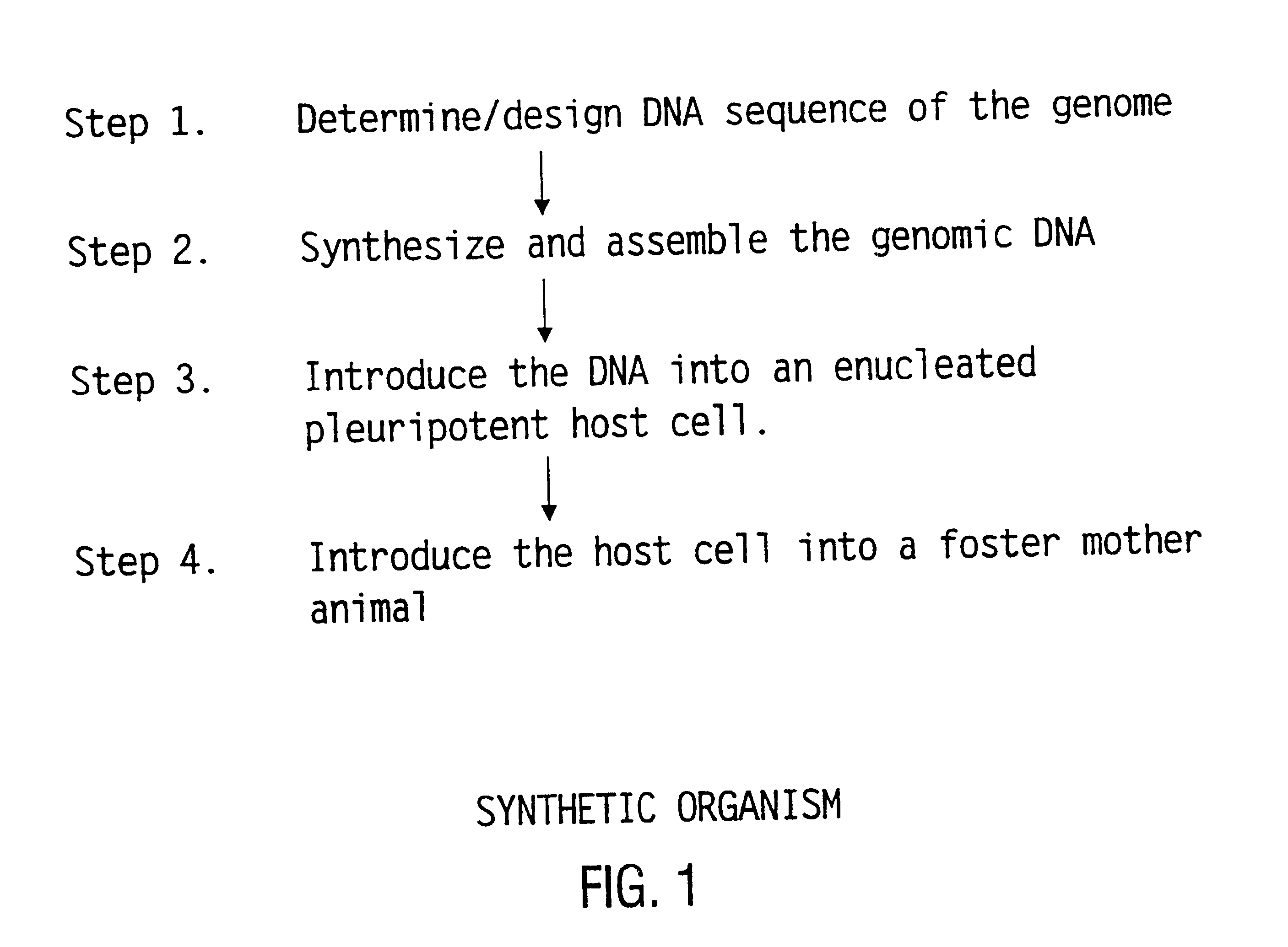
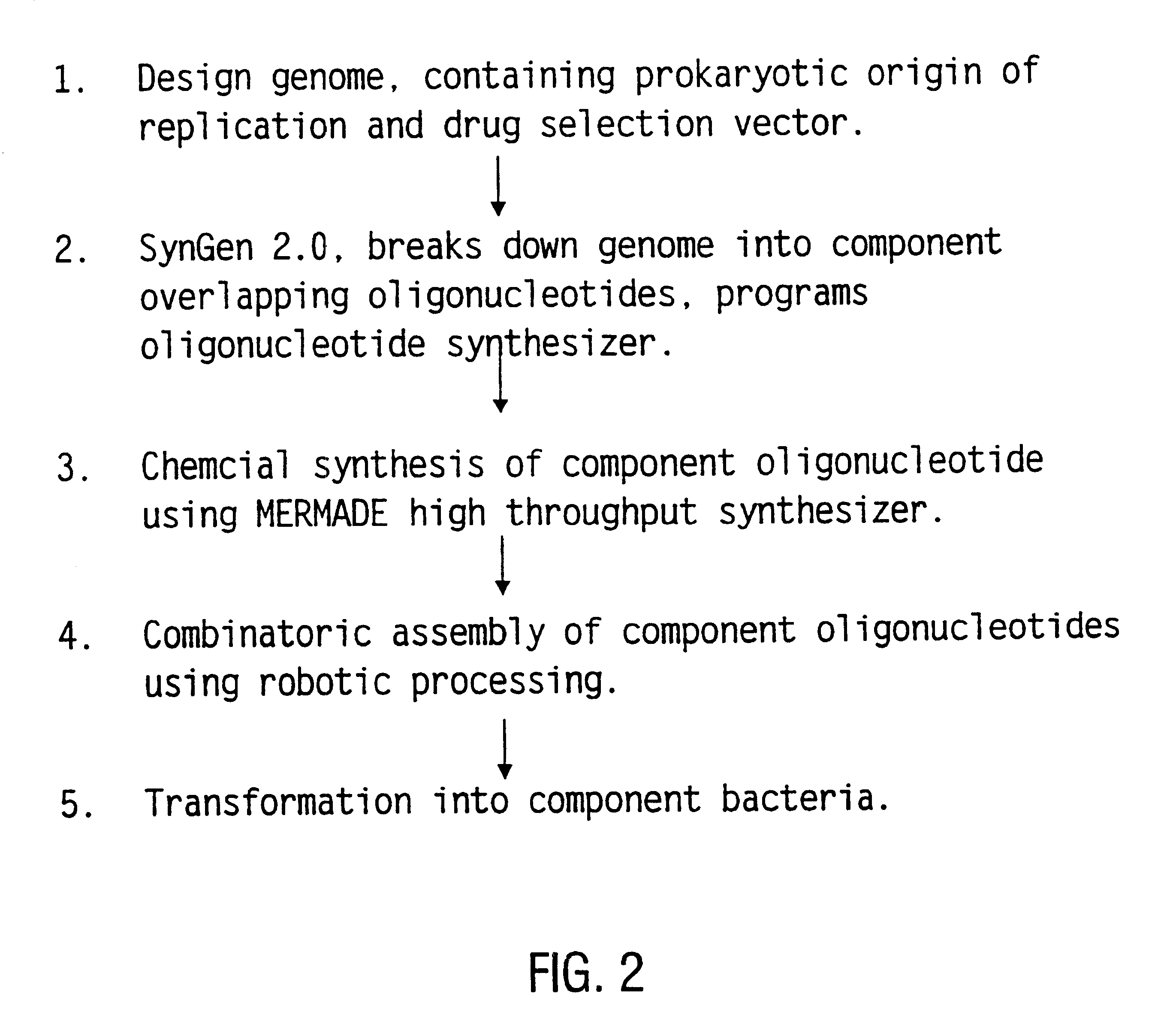
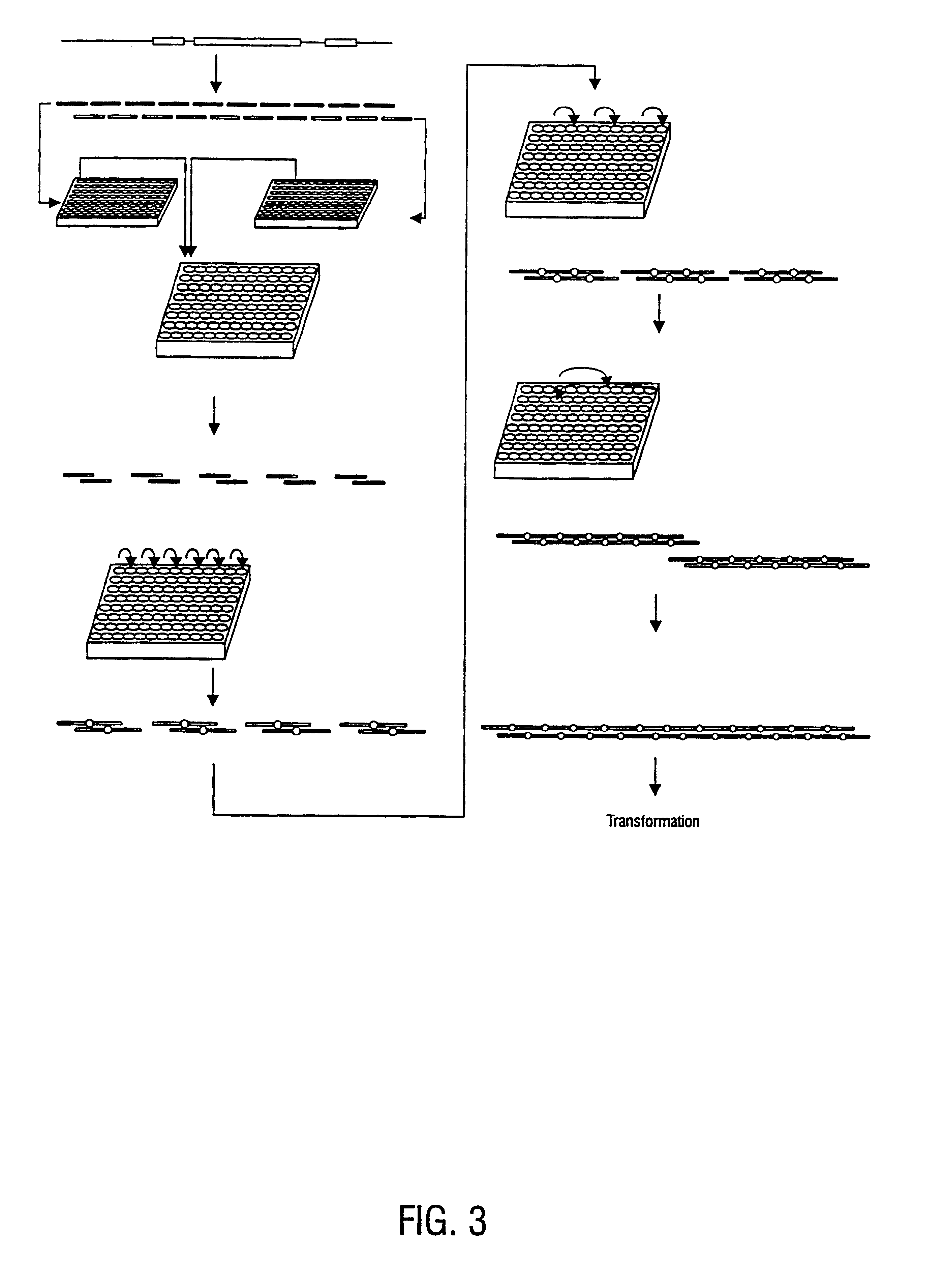
Method for the complete chemical synthesis and assembly of genes and genomes
InactiveUS6521427B1BiocideSequential/parallel process reactionsChemical synthesisHuman genome database
The present invention relates generally to the fields of oligonucleotide synthesis. More particularly, it concerns the assembly of genes and genomes of completely synthetic artificial organisms. Thus, the present invention outlines a novel approach to utilizing the results of genomic sequence information by computer directed gene synthesis based on computing on the human genome database. Specifically, the present invention contemplates and describes the chemical synthesis and resynthesis of genes defined by the genome sequence in a host vector and transfer and expression of these sequences into suitable hosts.
Owner:JOHNSON & JOHNSON INC (US) +3
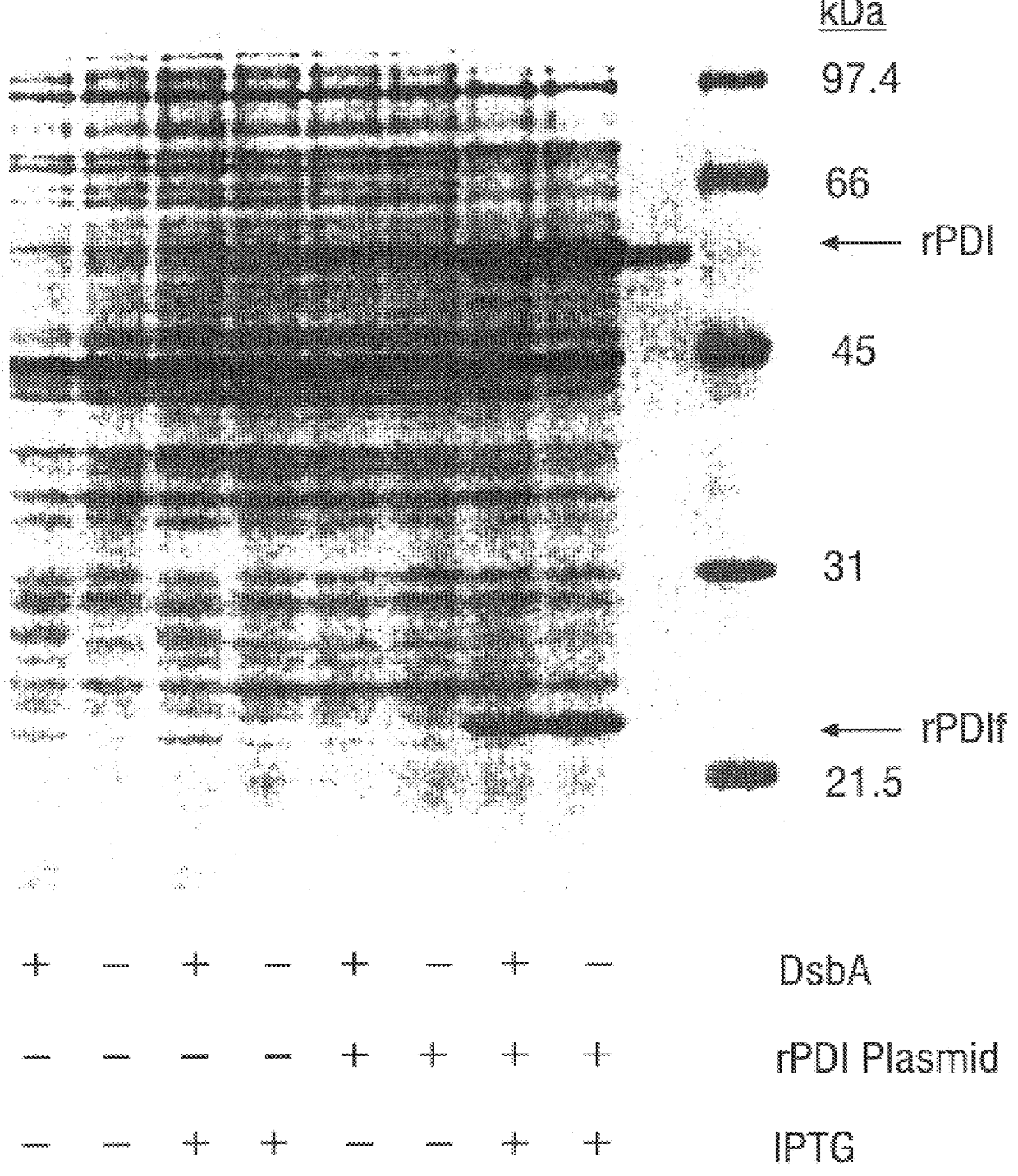
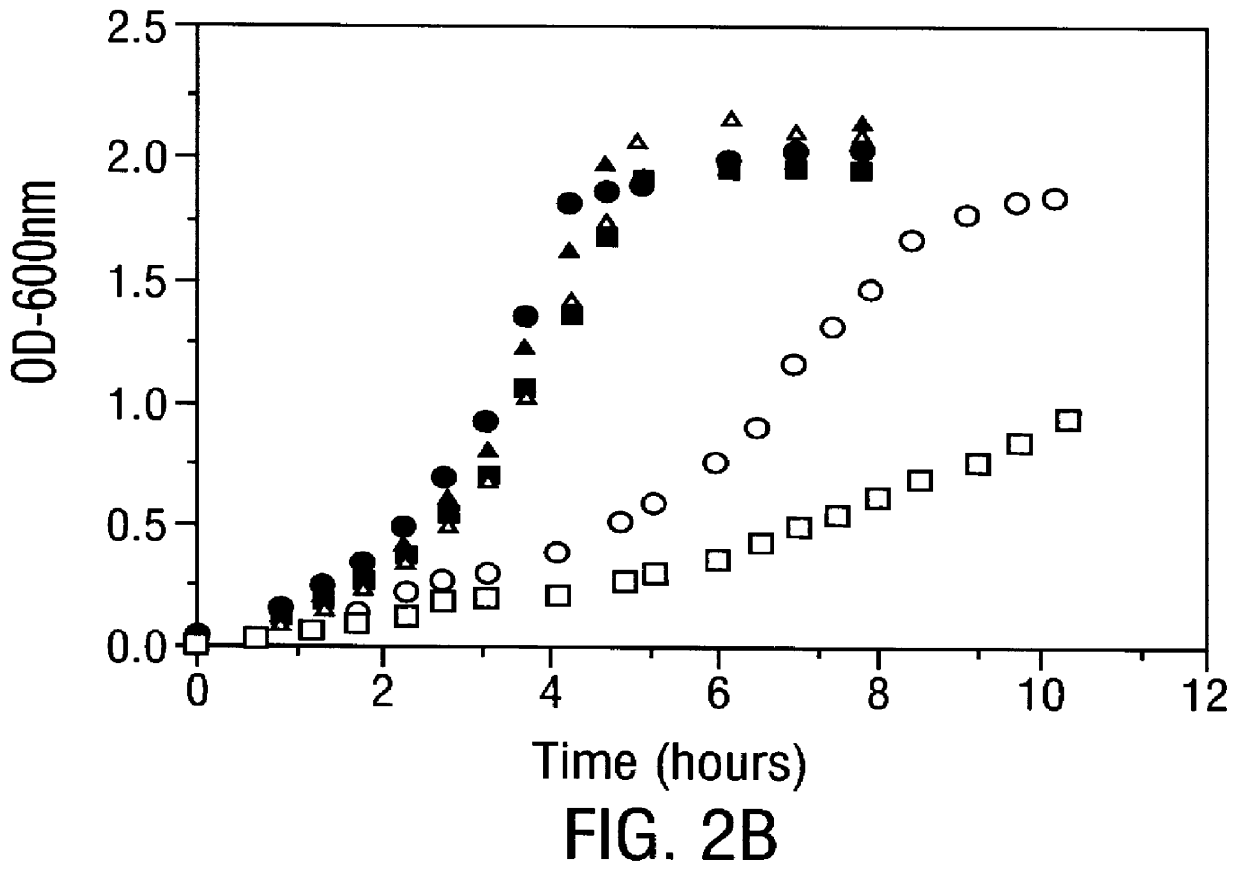
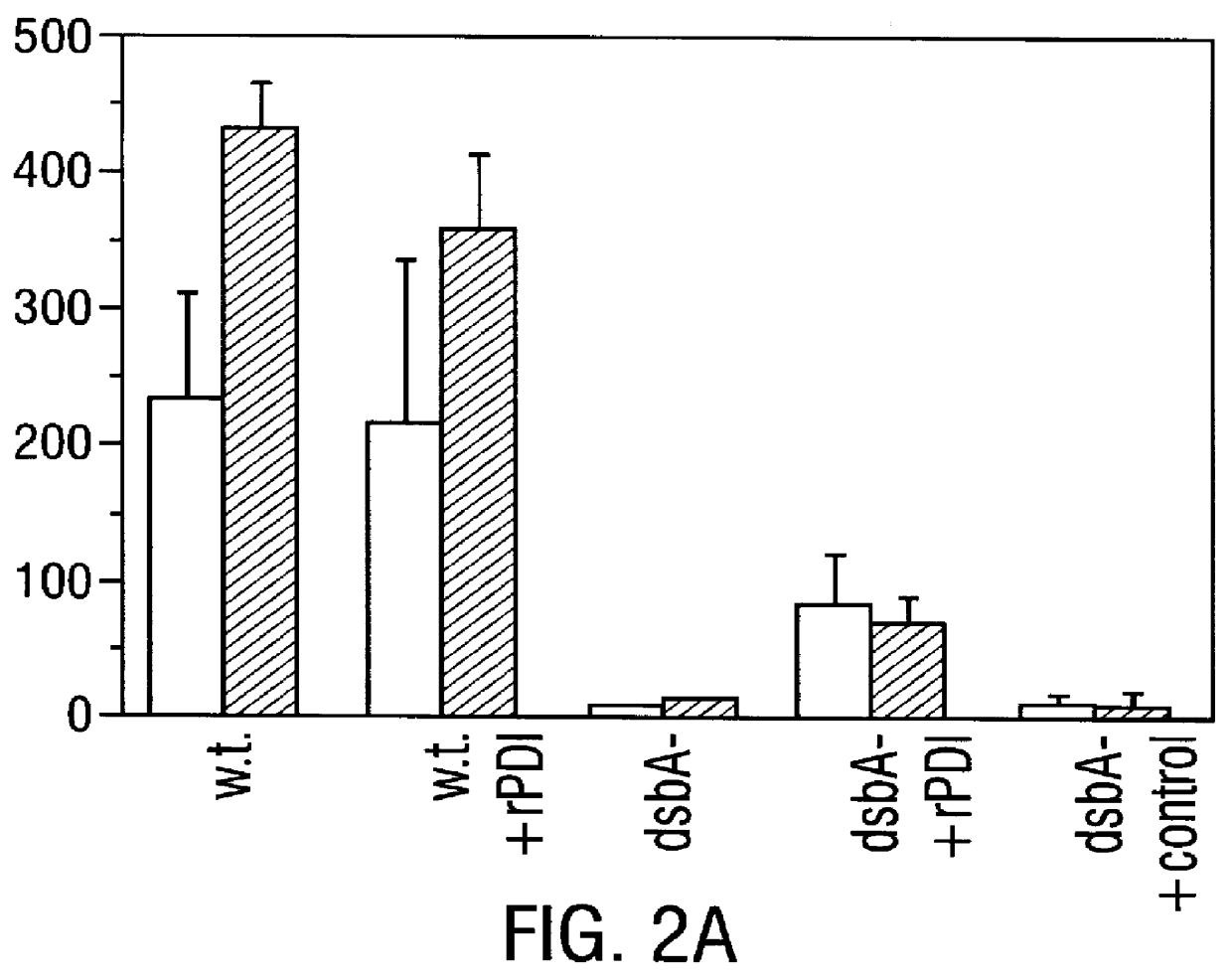
Methods for producing soluble, biologically-active disulfide-bond containing eukaryotic proteins in bacterial cells
InactiveUS6027888AEfficient productionFold preciselyPeptide/protein ingredientsMicroorganismsDisulfide bondingZymogen
Disclosed are methods of producing eukaryotic disulfide bond-containing polypeptides in bacterial hosts, and compositions resulting therefrom. Co-expression of a eukaryotic foldase and a disulfide bond-containing polypeptide in a bacterial host cell is demonstrated. In particular embodiments, the methods have been used to produce mammalian pancreatic trypsin inhibitor and tissue plasminogen activator (tPA) in soluble, biologically-active forms, which are isolatable from the bacterial periplasm. Also disclosed are expression systems, recombinant vectors, and transformed host cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
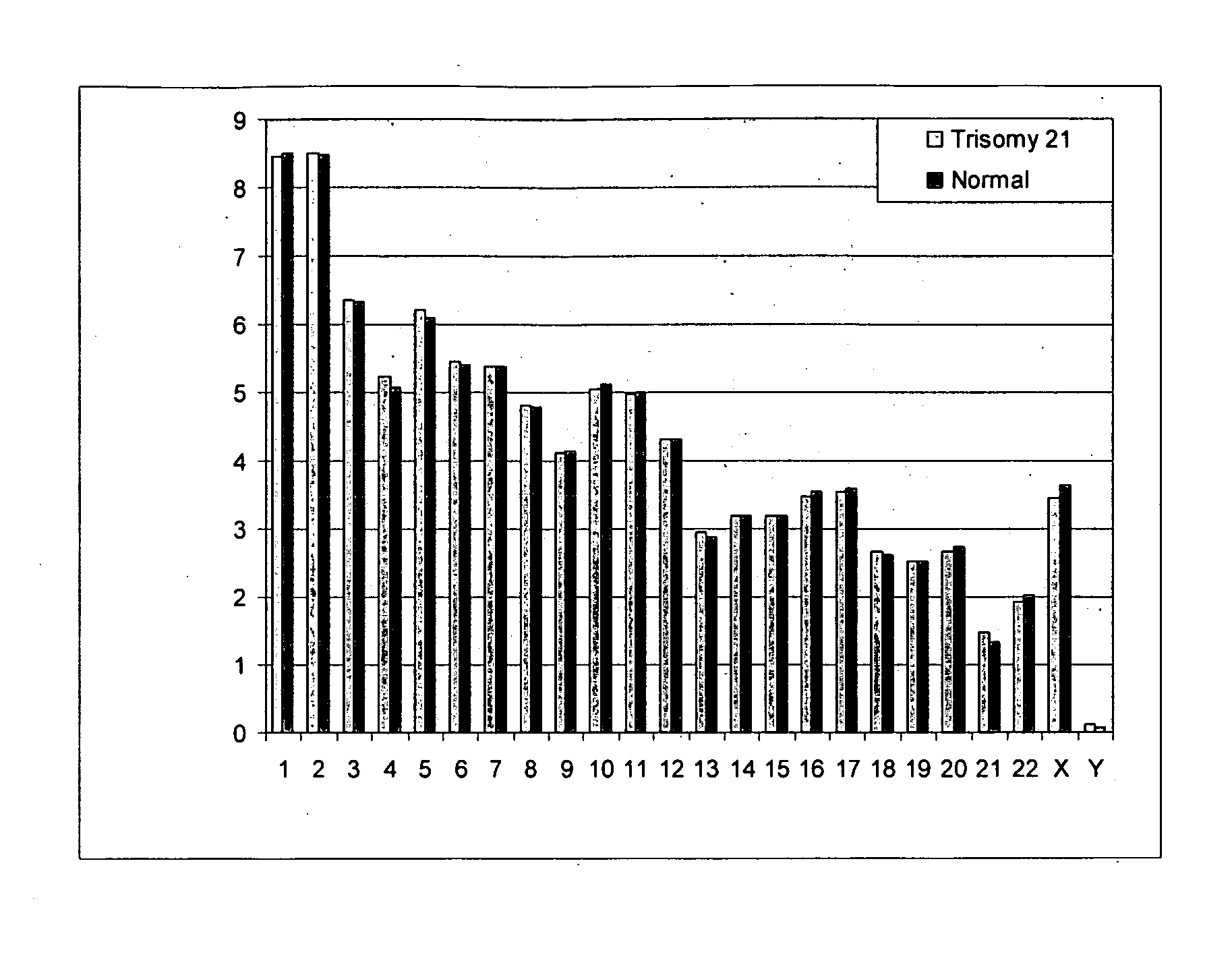
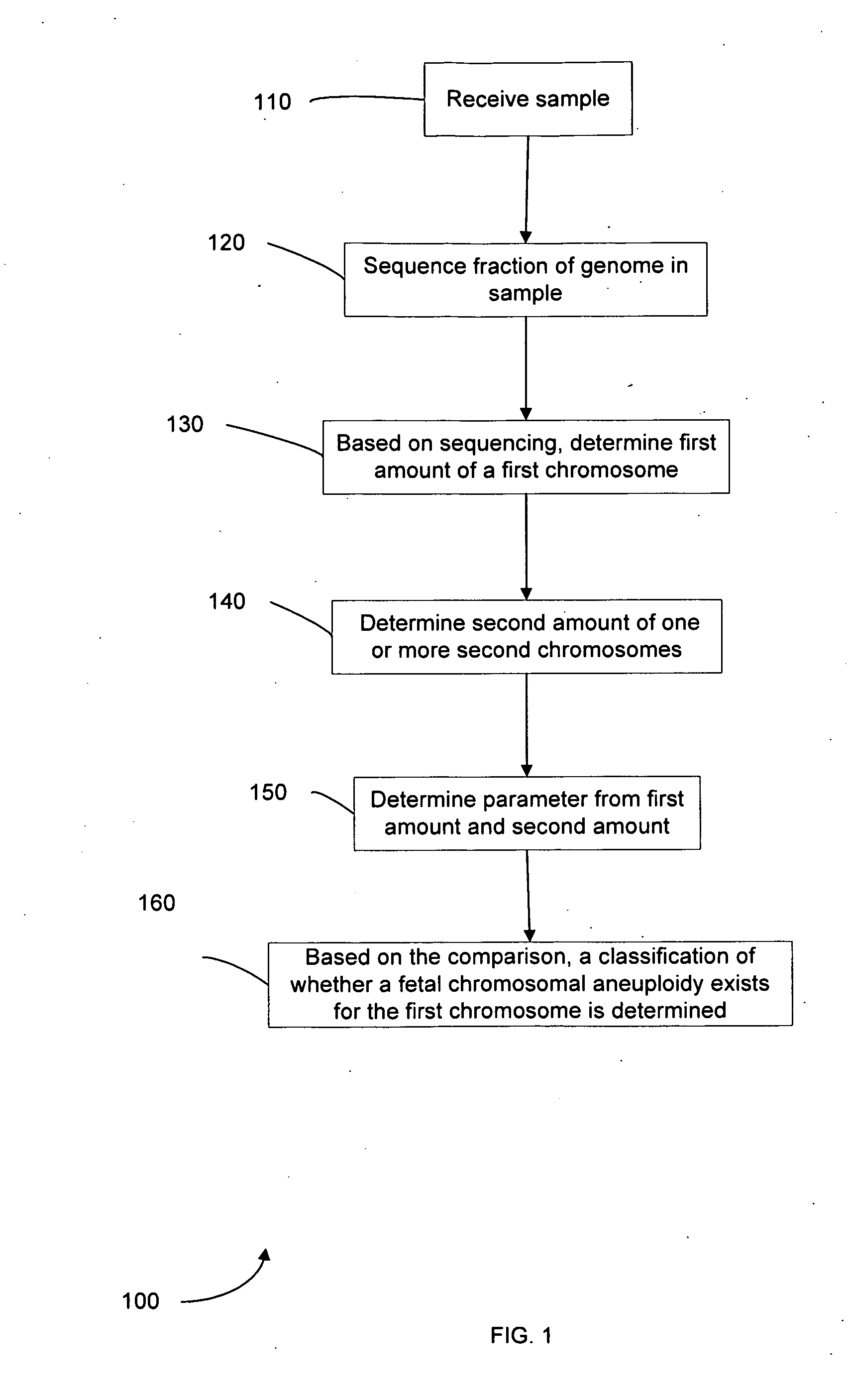
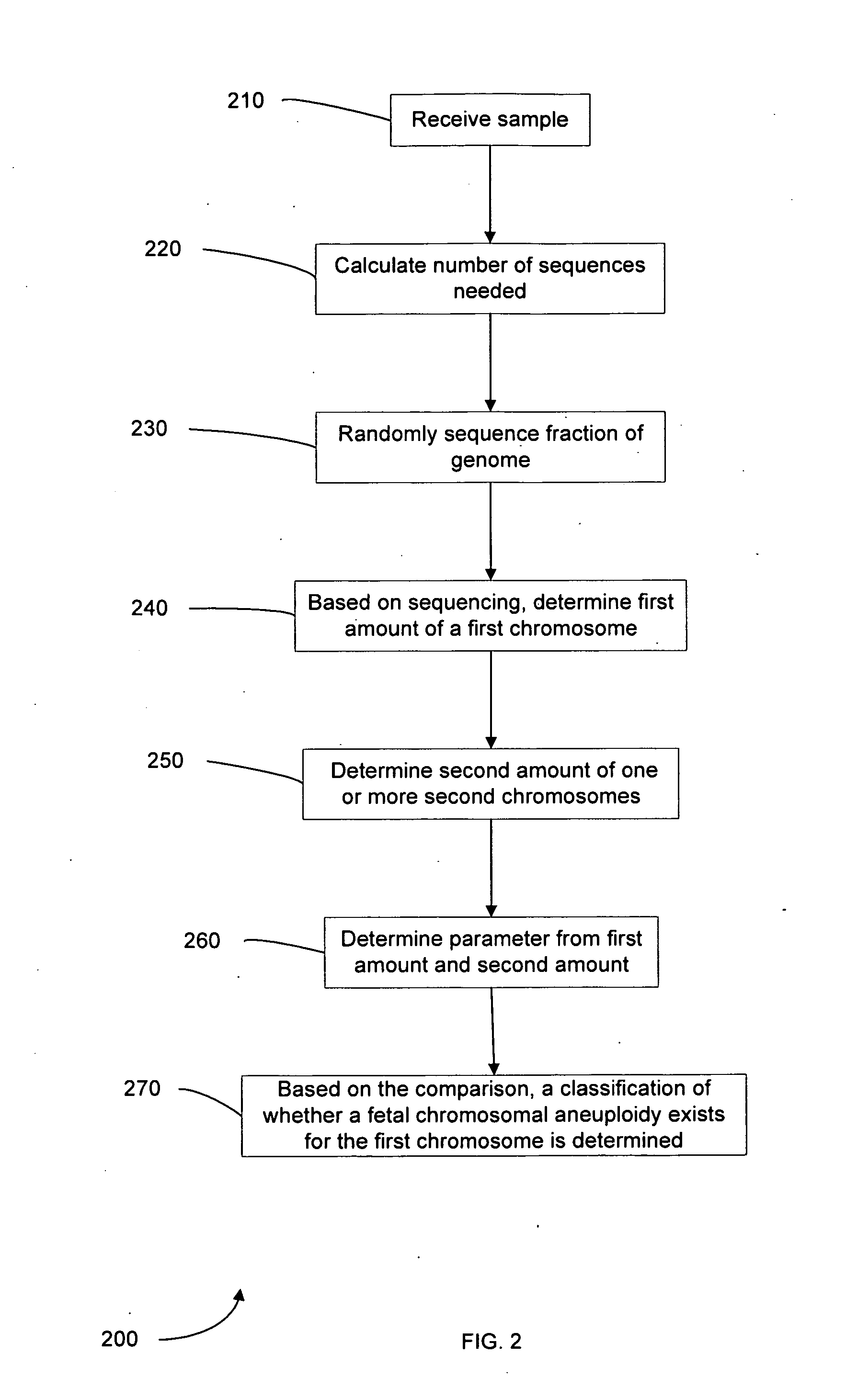
Diagnosing fetal chromosomal aneuploidy using massively parallel genomic sequencing
PendingUS20090029377A1Quantity maximizationSufficient amountMicrobiological testing/measurementDisease diagnosisGenomic sequencingGenome
Embodiments of this invention provide methods, systems, and apparatus for determining whether a fetal chromosomal aneuploidy exists from a biological sample obtained from a pregnant female. Nucleic acid molecules of the biological sample are sequenced, such that a fraction of the genome is sequenced. Respective amounts of a clinically-relevant chromosome and of background chromosomes are determined from results of the sequencing. A parameter derived from these amounts (e.g. a ratio) is compared to one or more cutoff values, thereby determining a classification of whether a fetal chromosomal aneuploidy exists.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
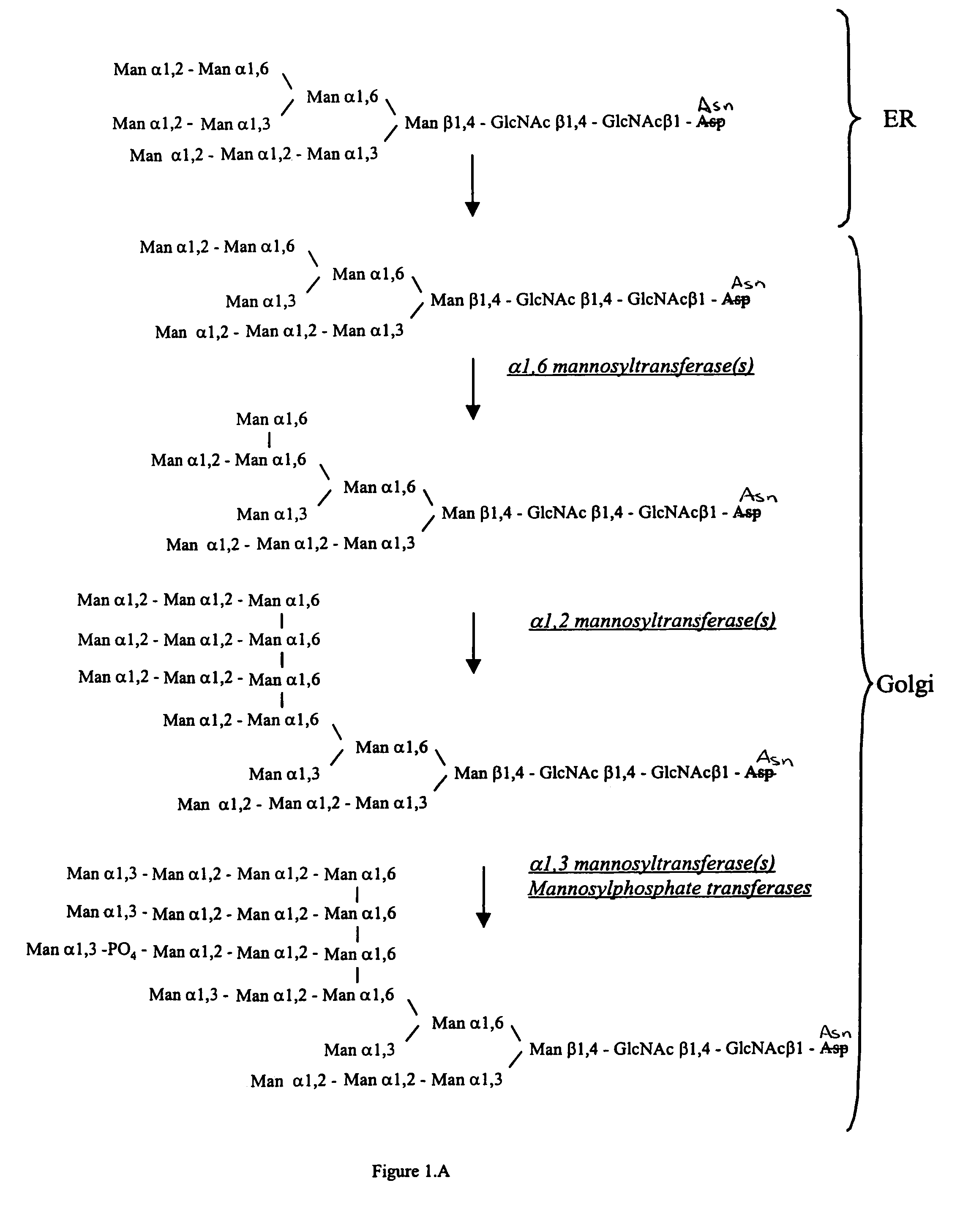
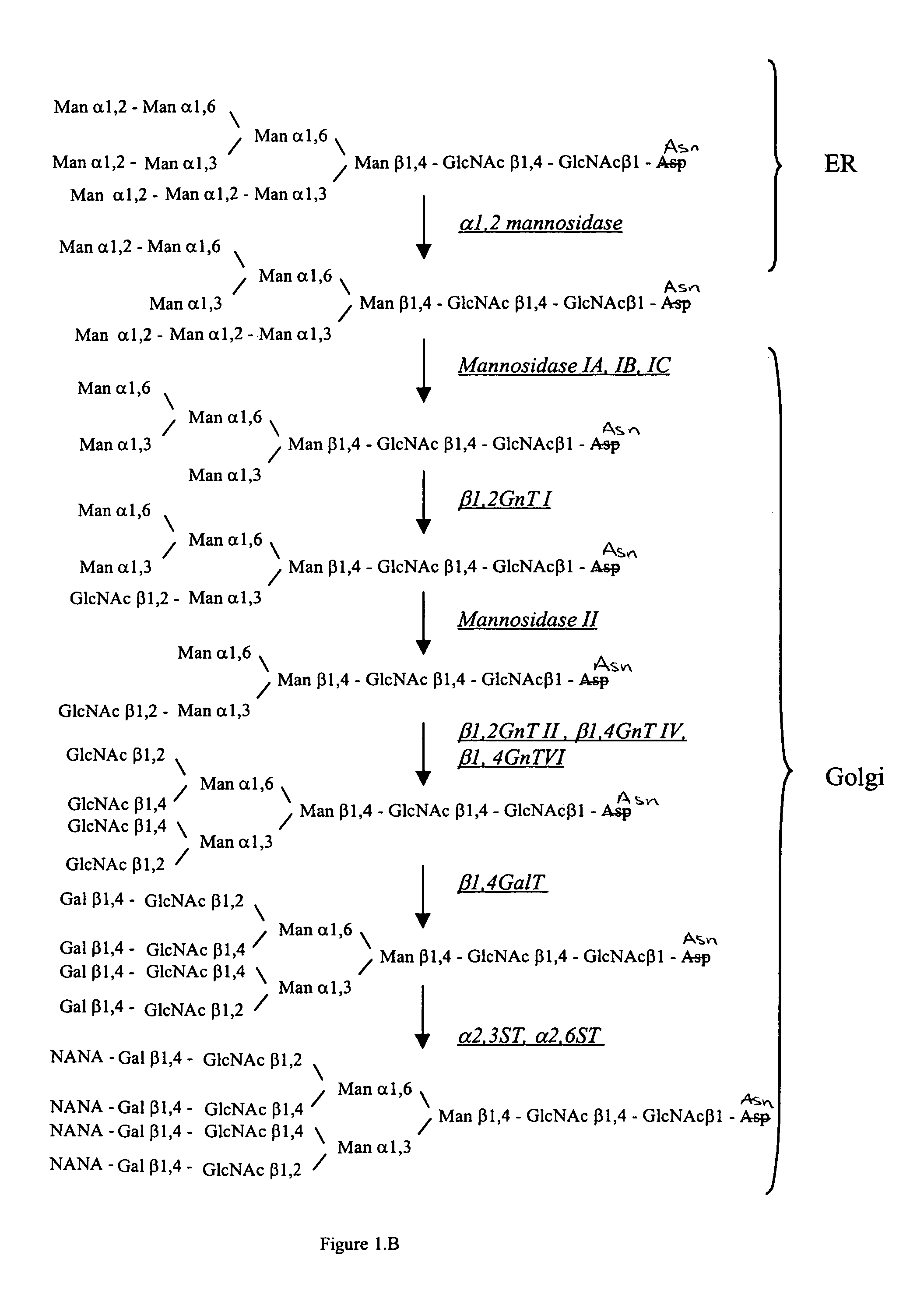
Methods for producing modified glycoproteins
Cell lines having genetically modified glycosylation pathways that allow them to carry out a sequence of enzymatic reactions, which mimic the processing of glycoproteins in humans, have been developed. Recombinant proteins expressed in these engineered hosts yield glycoproteins more similar, if not substantially identical, to their human counterparts. Thelower eukaryotes, which ordinarily produce high-mannose containing N-glycans, including unicellular and multicellular fungi are modified to produce N-glycans such as Man5GlcNAc2 or other structures along human glycosylation pathways. This is achieved using a combination of engineering and / or selection of strains which: do not express certain enzymes which create the undesirable complex structures characteristic of the fungal glycoproteins, which express exogenous enzymes selected either to have optimal activity under the conditions present in the fungi where activity is desired, or which are targeted to an organelle where optimal activity is achieved, and combinations thereof wherein the genetically engineered eukaryote expresses multiple exogenous enzymes required to produce “human-like” glycoproteins.
Owner:GLYCOFI
Apparatus and methods for analyte measurement and immuno assay
ActiveUS20030170881A1Avoid disadvantagesBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careOrganism
The present invention relates to an apparatus for conducting a variety of assays for the determination of analytes in liquid samples, and relates to the methods for such assays. In particular, the invention relates to a single-use cartridge designed to be adaptable to a variety of real-time assay protocols, preferably assays for the determination of analytes in biological samples using immunosensors or other ligand / ligand receptor-based biosensor embodiments. The cartridge provides novel features for processing a metered portion of a sample, for precise and flexible control of the movement of a sample or second fluid within the cartridge, for the amending of solutions with additional compounds during an assay, and for the construction of immunosensors capable of adaptation to diverse analyte measurements. The disclosed device and methods of use enjoy substantial benefits over the prior art, including simplicity of use by an operator, rapid in situ determinations of one or more analytes, and single-use methodology that minimizes the risk of contamination of both operator and patient. The disclosed invention is adaptable to the point-of-care clinical diagnostic field, including use in accident sites, emergency rooms, surgery, nursing homes, intensive care units, and non-medical environments.
Owner:ABBOTT POINT CARE
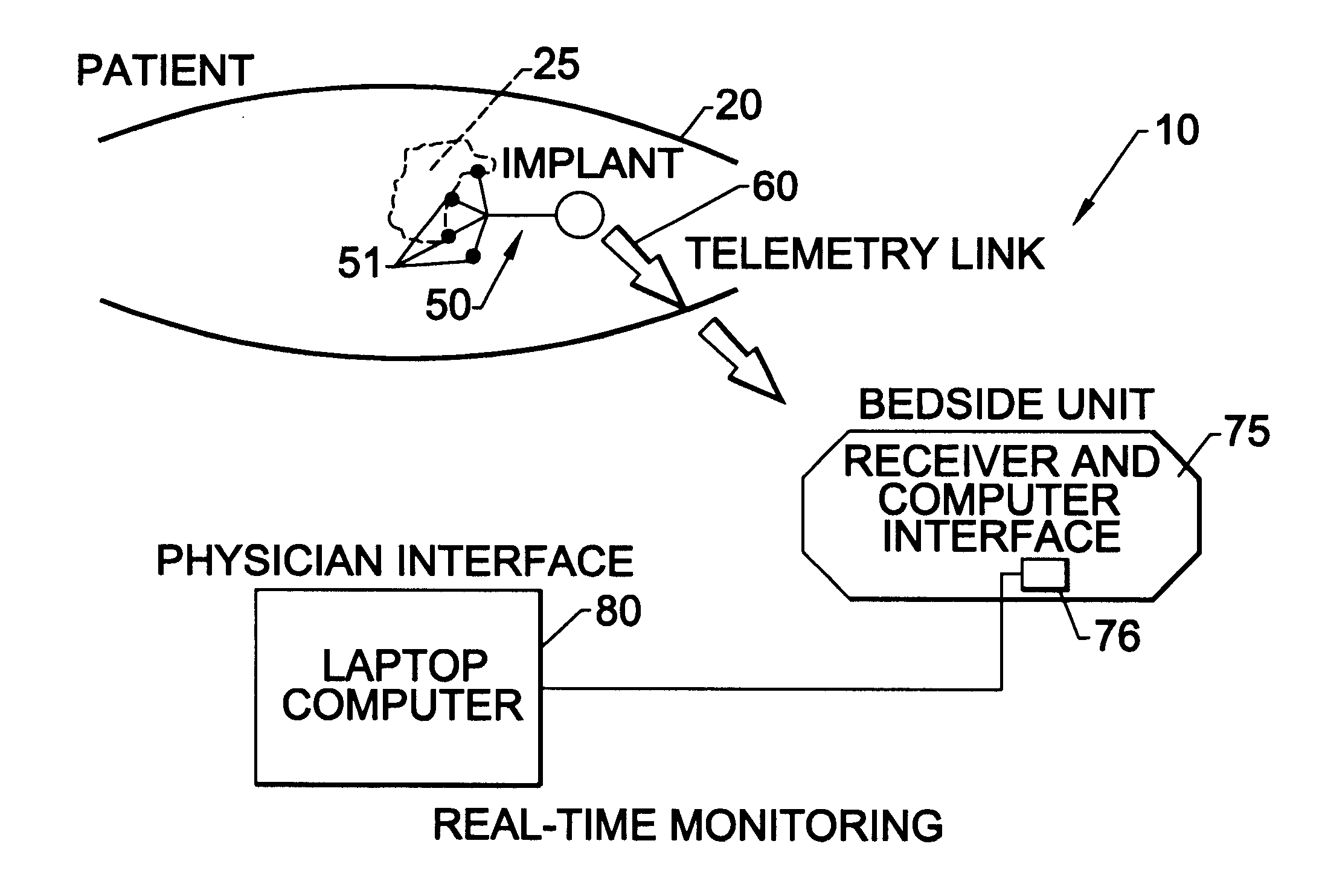
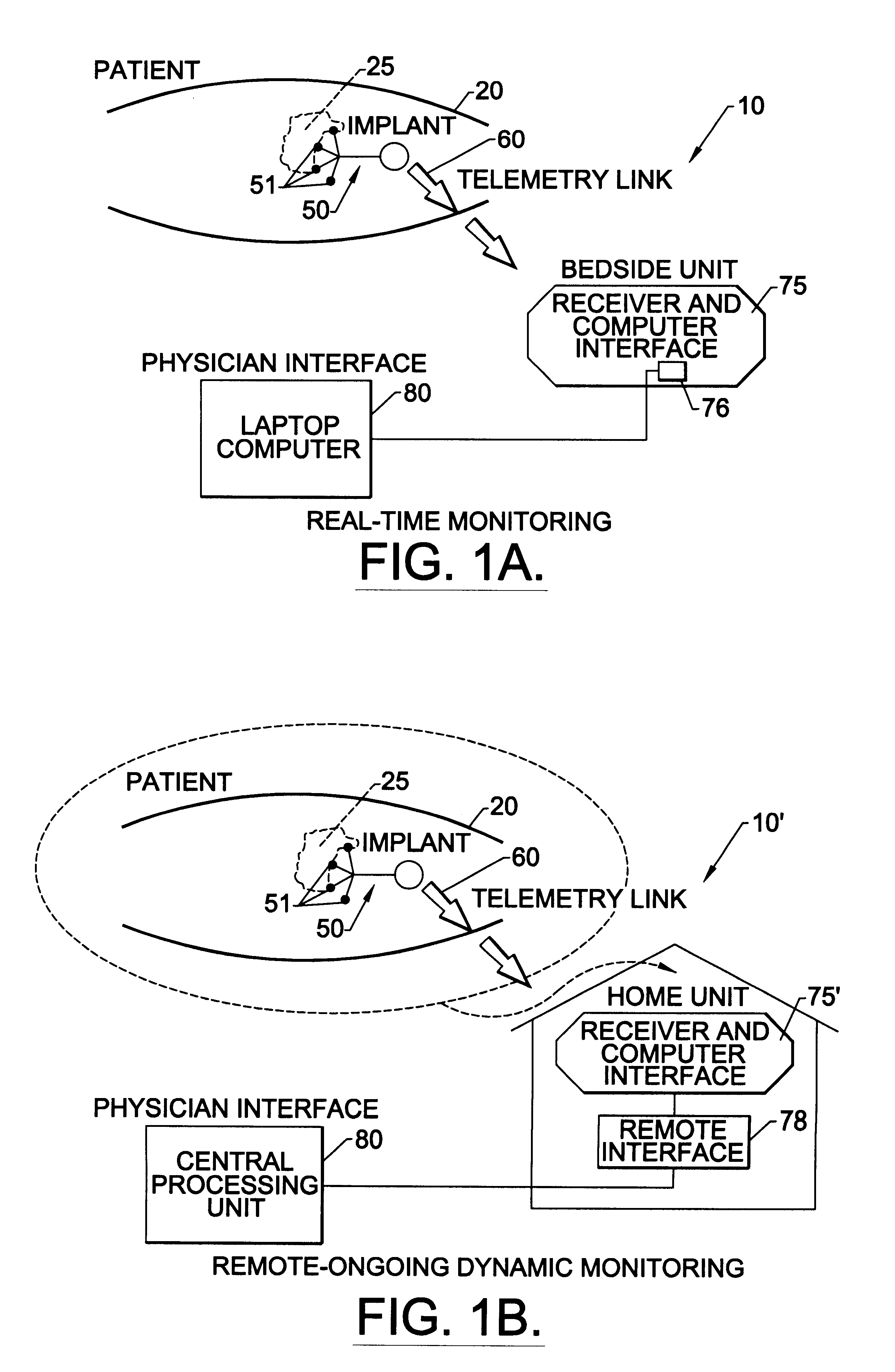
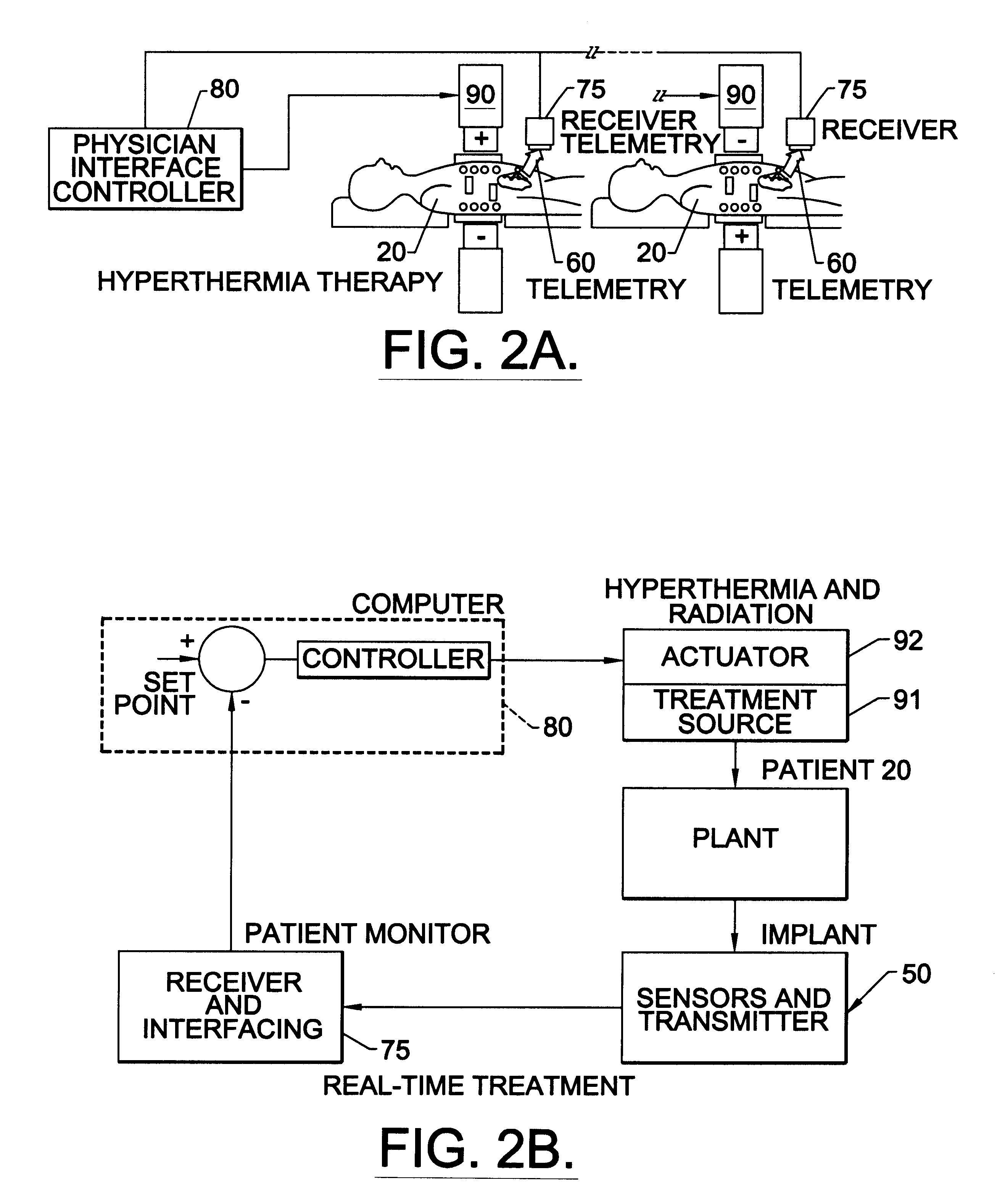
Methods, systems, and associated implantable devices for dynamic monitoring of physiological and biological properties of tumors
InactiveUS6402689B1Enhanced and favorable treatmentMinimize couplingMechanical/radiation/invasive therapiesSurgeryDynamic monitoringEngineering
Methods of monitoring and evaluating the status of a tumor undergoing treatment includes monitoring in vivo at least one physiological parameter associated with a tumor in a subject undergoing treatment, transmitting data from an in situ located sensor to a receiver external of the subject, analyzing the transmitted data, repeating the monitoring and transmitting steps at sequential points in time and evaluating a treatment strategy. The method provides dynamic tracking of the monitored parameters over time. The method can also include identifying in a substantially real time manner when conditions are favorable for treatment and when conditions are unfavorable for treatment and can verify or quantify how much of a known drug dose or radiation dose was actually received at the tumor. The method can include remote transmission from a non-clinical site to allow oversight of the tumor's condition even during non-active treatment periods (in between active treatments). The disclosure also includes monitoring systems with in situ in vivo biocompatible sensors and telemetry based operations and related computer program products.
Owner:NORTH CAROLINA STATE UNIV +1
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Apparatus and method for bioelectric stimulation, healing acceleration, pain relief, or pathogen devitalization
ActiveUS7117034B2Minimal stressPromote healingElectrotherapyArtificial respirationEngineeringAnimal body
An method and method for generating an electrical signal for use in biomedical applications, including two timing-interval generators, each optionally driving a multistep sequencer; analog, digital or hybrid means for combining the resulting timed signals into a complex electrical signal; optional filtering means for blocking direct current, removing selected frequency components from the resulting signal, and / or providing voltage step-up if needed; and conductive means for coupling the resulting signal to a human or animal body, food, beverage or other liquid, cell or tissue culture, or pharmaceutical material, in order to relieve pain, stimulate healing or growth, enhance the production of specific biochemicals, or devitalize selected types of organisms.
Owner:HEALTHONICS INC
Seed specificity highly effective promoter and its application
InactiveCN101063139AReduce adverse effectsFermentationVector-based foreign material introductionHeterologousNucleotide
The invention discloses a special promoter separated from millet, expressing carrier with nucleic acid sequence of SEQ ID No. 1 host with the expressing carrier and appliance of the promoter, which is characterized by the following: utilizing Tail-PCR (colored body step moving method); getting the special promoter from gene group DNA; possessing nucleic acid sequence of SEQ ID No. 1; ;linking downstream of the promoter to non-homologous or homologous gene; constructing plant expressing carrier; transferring host plant; driving the downstream gene to high effective and special express goal protein in the seed; realizing genetic modification of plant; or using as effective tool for studying plant and biological reactor.
Owner:CHINA AGRI UNIV
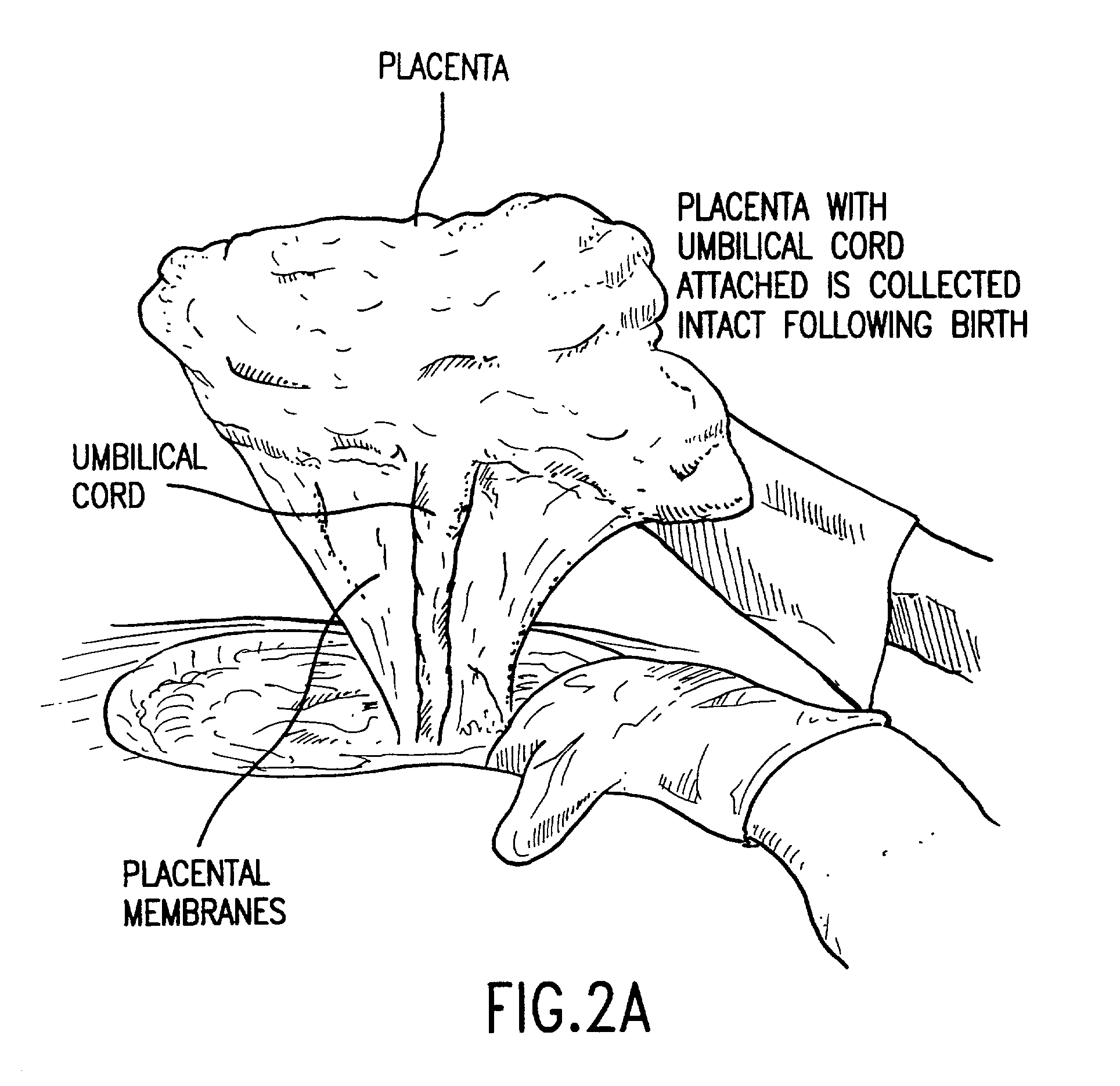
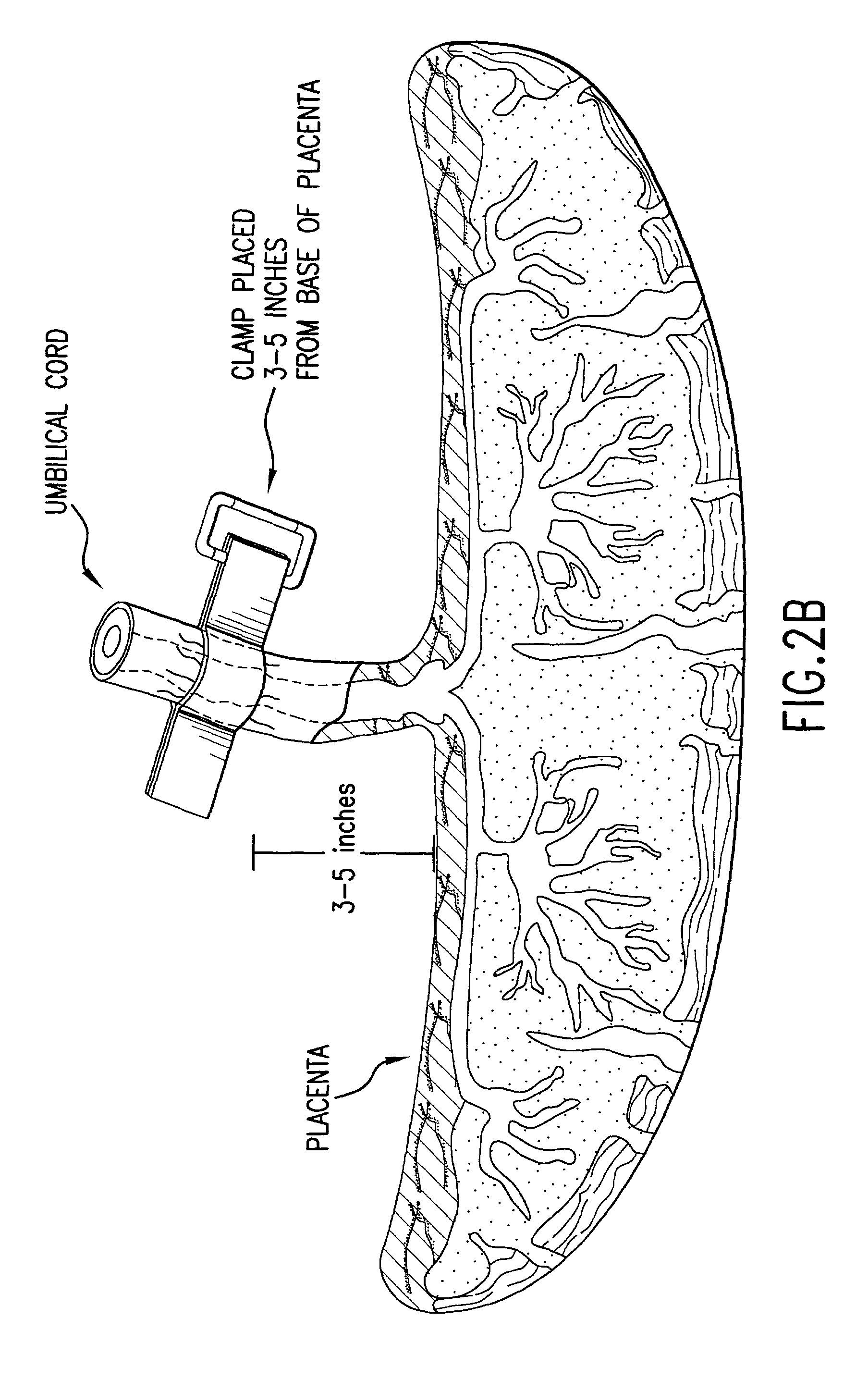
Post-partum mammalian placenta, its use and placental stem cells therefrom
InactiveUS20030032179A1Enhance exsanguinationEnhance sterile conditionSenses disorderAntipyreticAnticoagulant AgentEmbryo
The present invention provides a method of extracting and recovering embryonic-like stem cells, including, but not limited to pluripotent or multipotent stem cells, from an exsanguinated human placenta. A placenta is treated to remove residual umbilical cord blood by perfusing an exsanguinated placenta, preferably with an anticoagulant solution, to flush out residual cells. The residual cells and perfusion liquid from the exsanguinated placenta are collected, and the embryonic-like stem cells are separated from the residual cells and perfusion liquid. The invention also provides a method of utilizing the isolated and perfused placenta as a bioreactor in which to propagate endogenous cells, including, but not limited to, embryonic-like stem cells. The invention also provides methods for propagation of exogenous cells in a placental bioreactor and collecting the propagated exogenous cells and bioactive molecules therefrom.
Owner:CELULARITY INC
Methods and systems for annotating biomolecular sequences
A method of annotating biomolecular sequences. The method comprises (a) computationally clustering the biomolecular sequences according to a progressive homology range, to thereby generate a plurality of clusters each being of a predetermined homology of the homology range; and (b) assigning at least one ontology to each cluster of the plurality of clusters, the at least one ontology being: (i) derived from an annotation preassociated with at least one biomolecular sequence of each cluster; and / or (ii) generated from analysis of the at least one biomolecular sequence of each cluster thereby annotating biomolecular sequences.
Owner:COMPUGEN
Multivalent immunoglobulin-based bioactive assemblies
ActiveUS7527787B2Efficacious for arrestingInhibition formationPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDiagnostic agent
Owner:IBC PHARMACEUTICALS INC
Methods and compositions for generating bioactive assemblies of increased complexity and uses
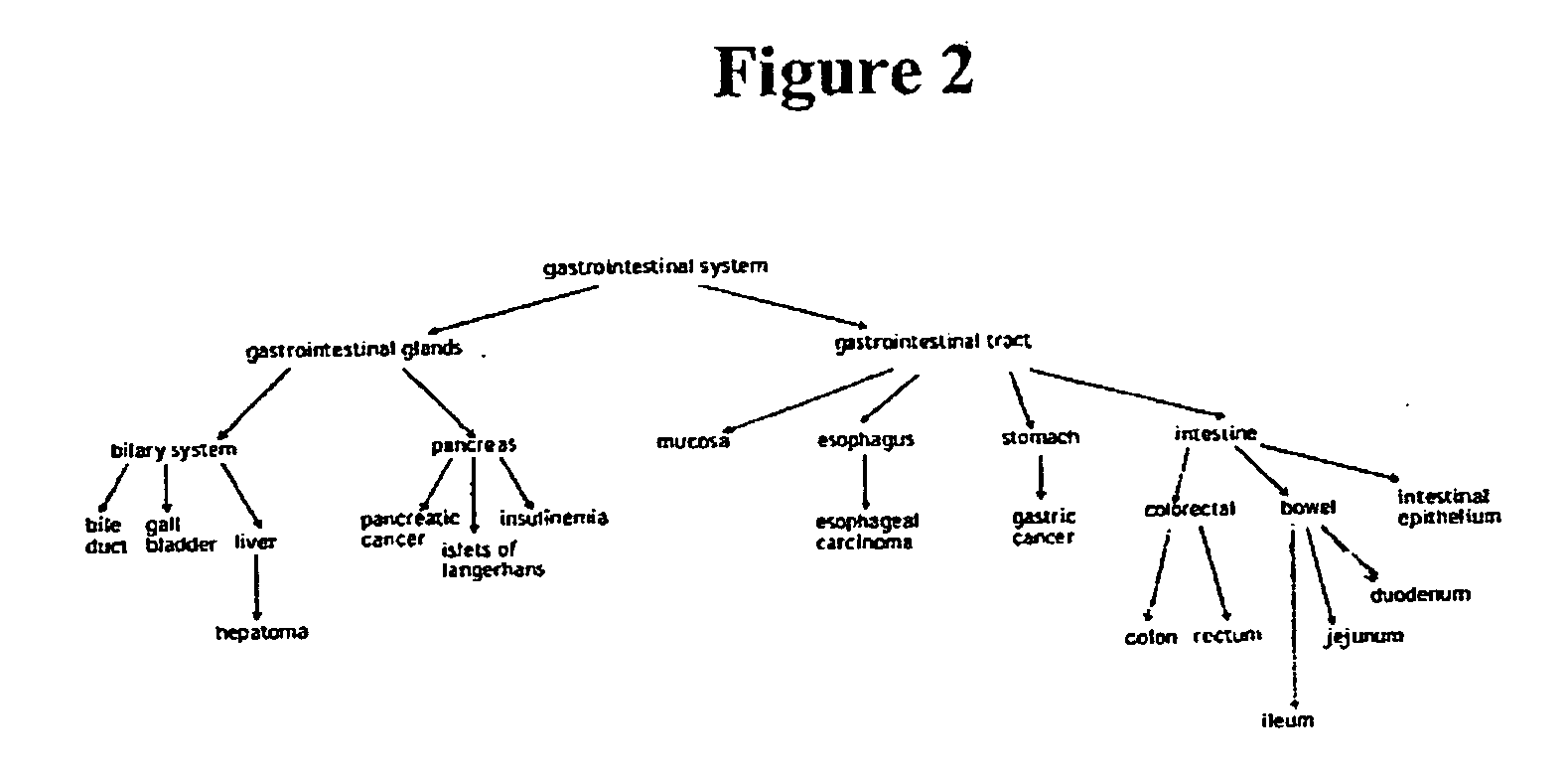
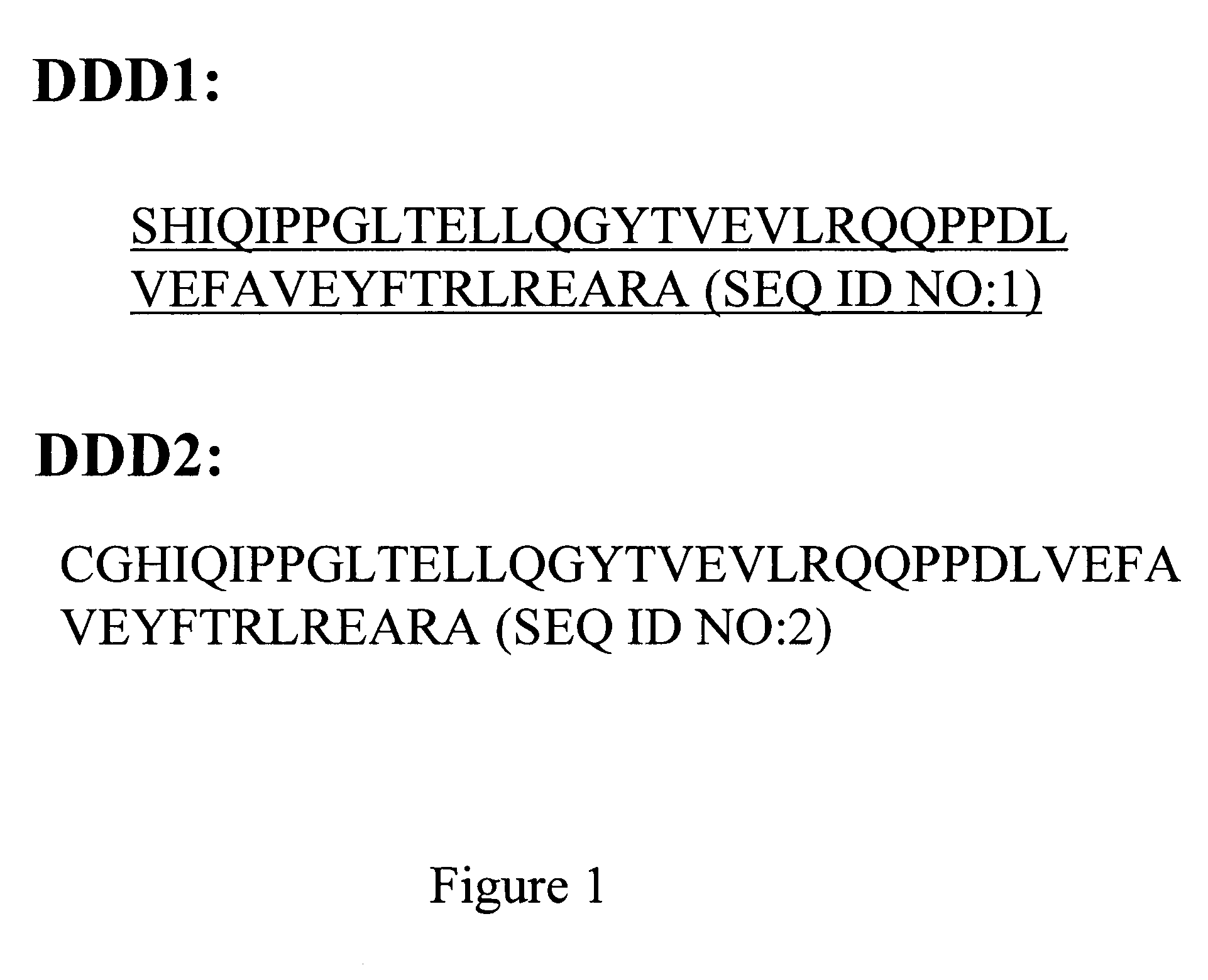
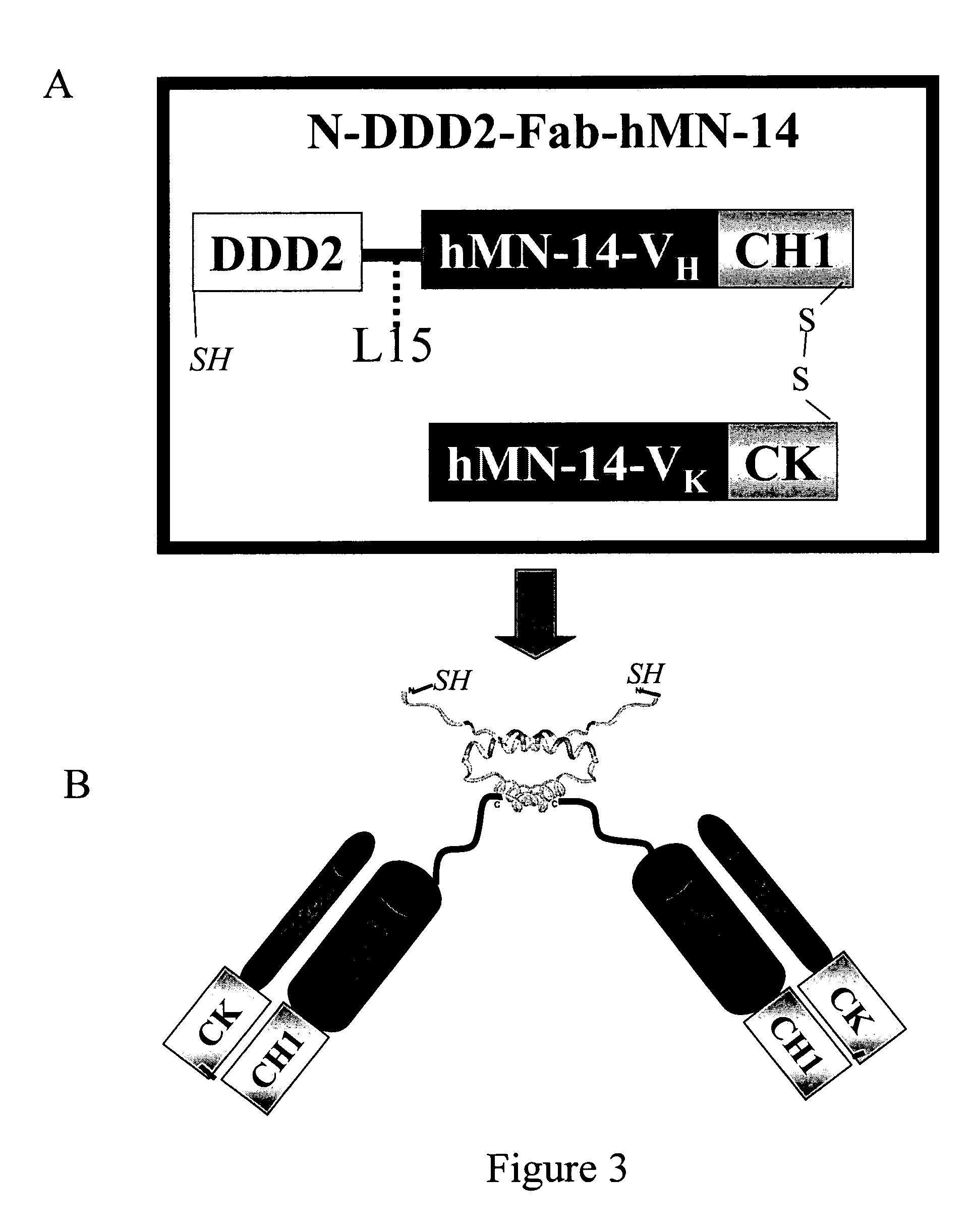
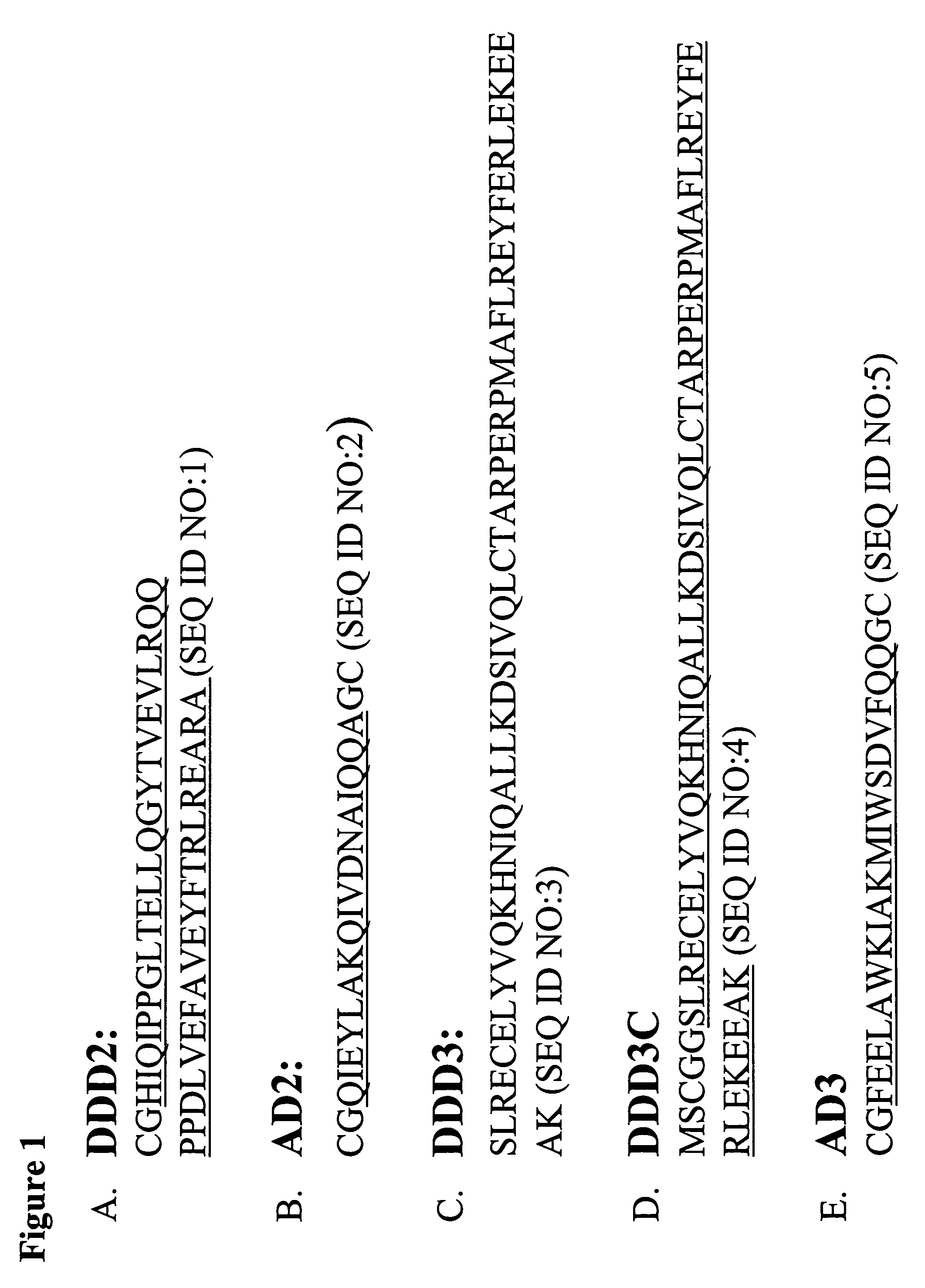
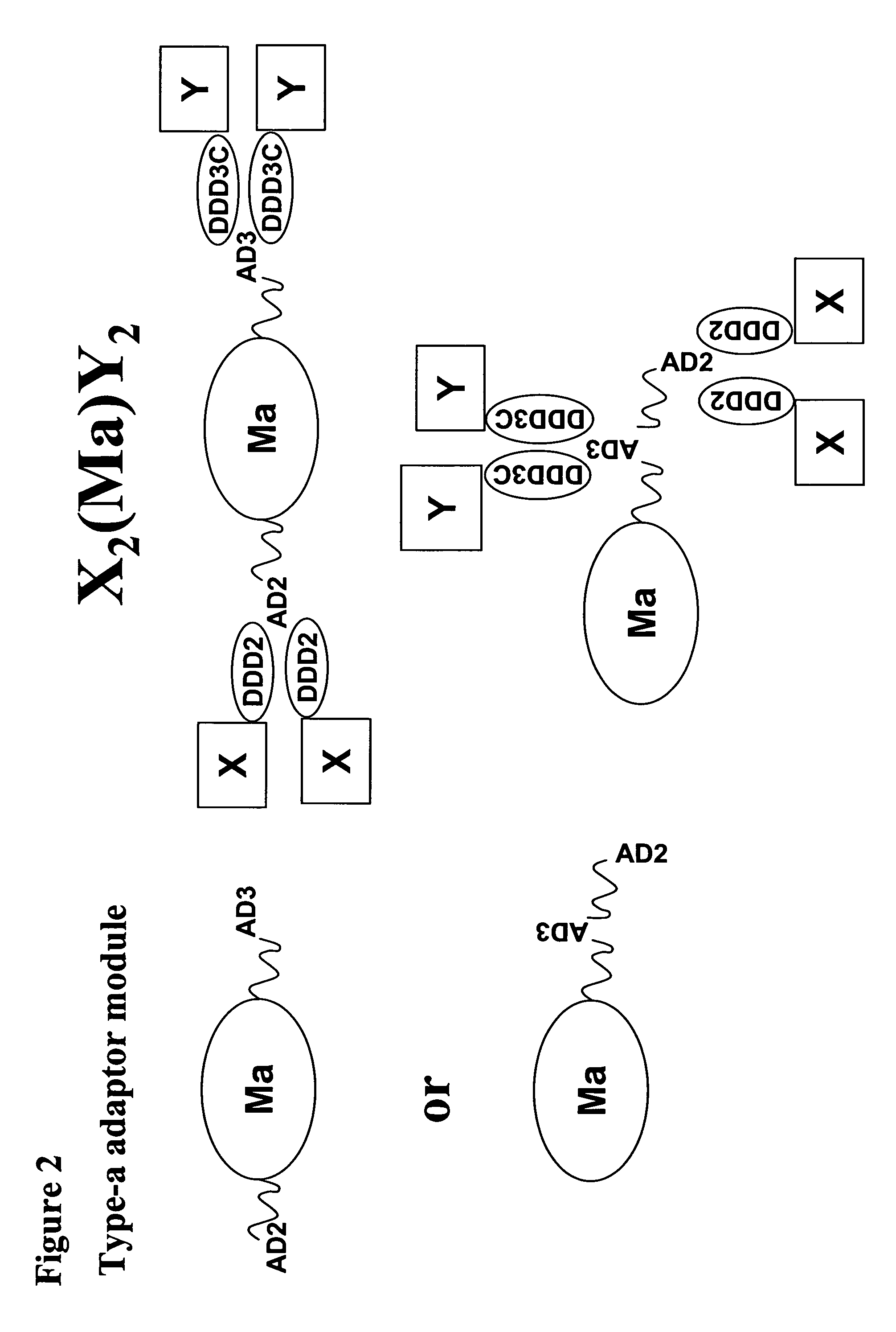
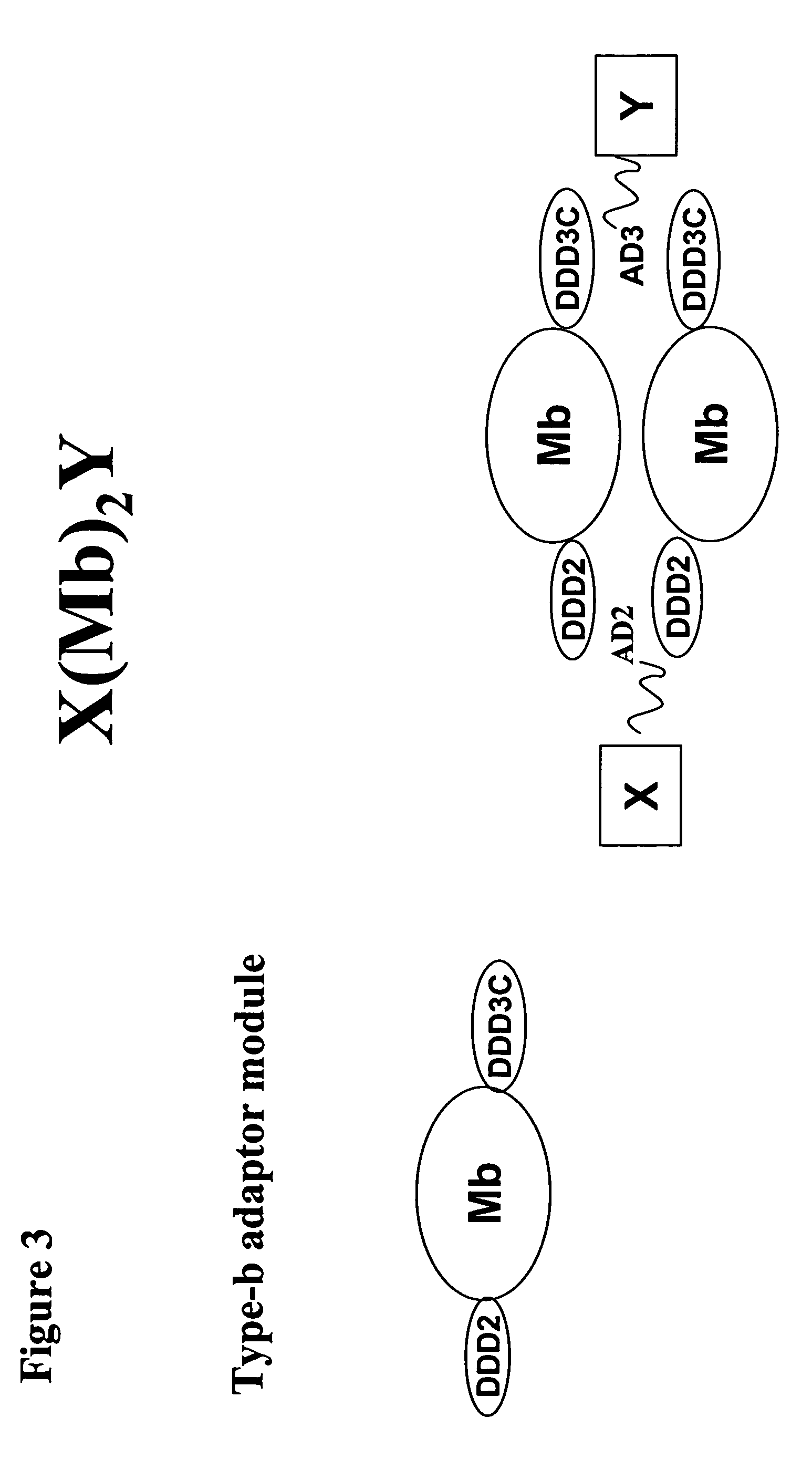
The present invention concerns methods and compositions for making and using bioactive assemblies of defined compositions, which may have multiple functionalities and / or binding specificities. In particular embodiments, the bioactive assembly is formed using dock-and-lock (DNL) methodology, which takes advantage of the specific binding interaction between dimerization and docking domains (DDD) and anchoring domains (AD) to form the assembly. In various embodiments, one or more effectors may be attached to a DDD or AD sequence. Complementary AD or DDD sequences may be attached to an adaptor module that forms the core of the bioactive assembly, allowing formation of the assembly through the specific DDD / AD binding interactions. Such assemblies may be attached to a wide variety of effector moieties for treatment, detection and / or diagnosis of a disease, pathogen infection or other medical or veterinary condition.
Owner:IBC PHARMACEUTICALS INC
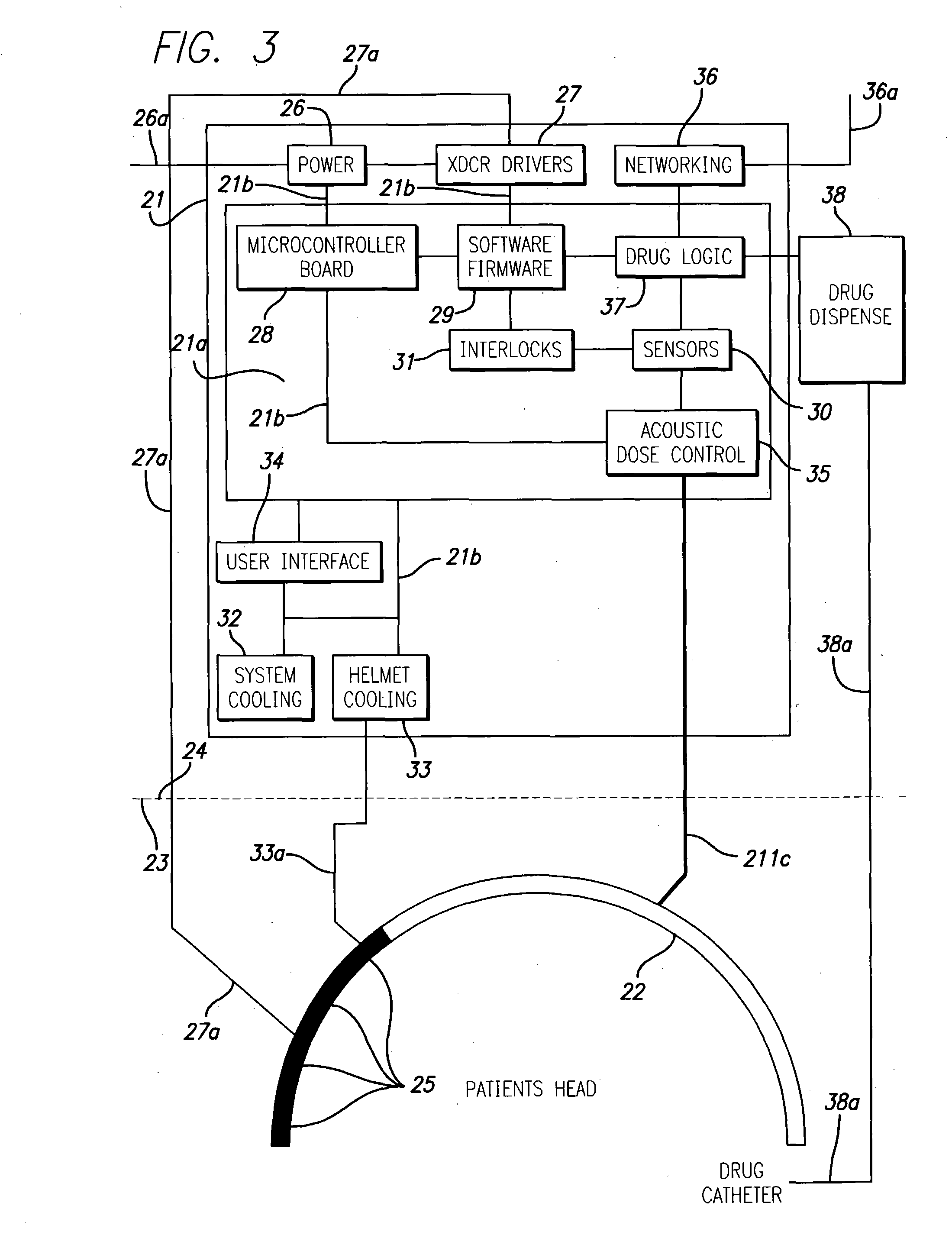
System and methods for treatment of alzheimer's and other deposition-related disorders of the brain
InactiveUS20040049134A1Slowing, stopping or avoiding a patient's cognitive lossesMinimal adverse side effectUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDiseaseSide effect
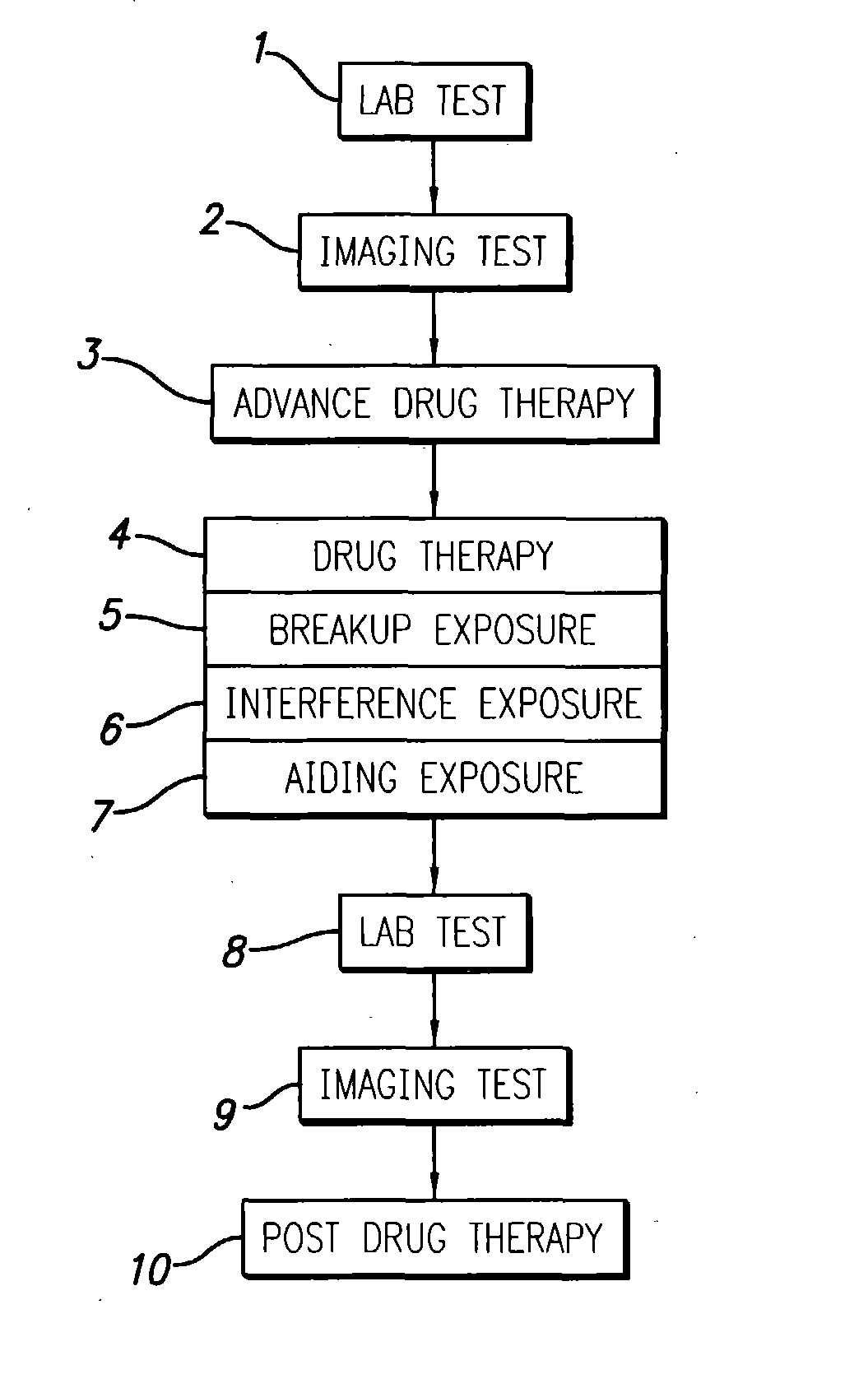
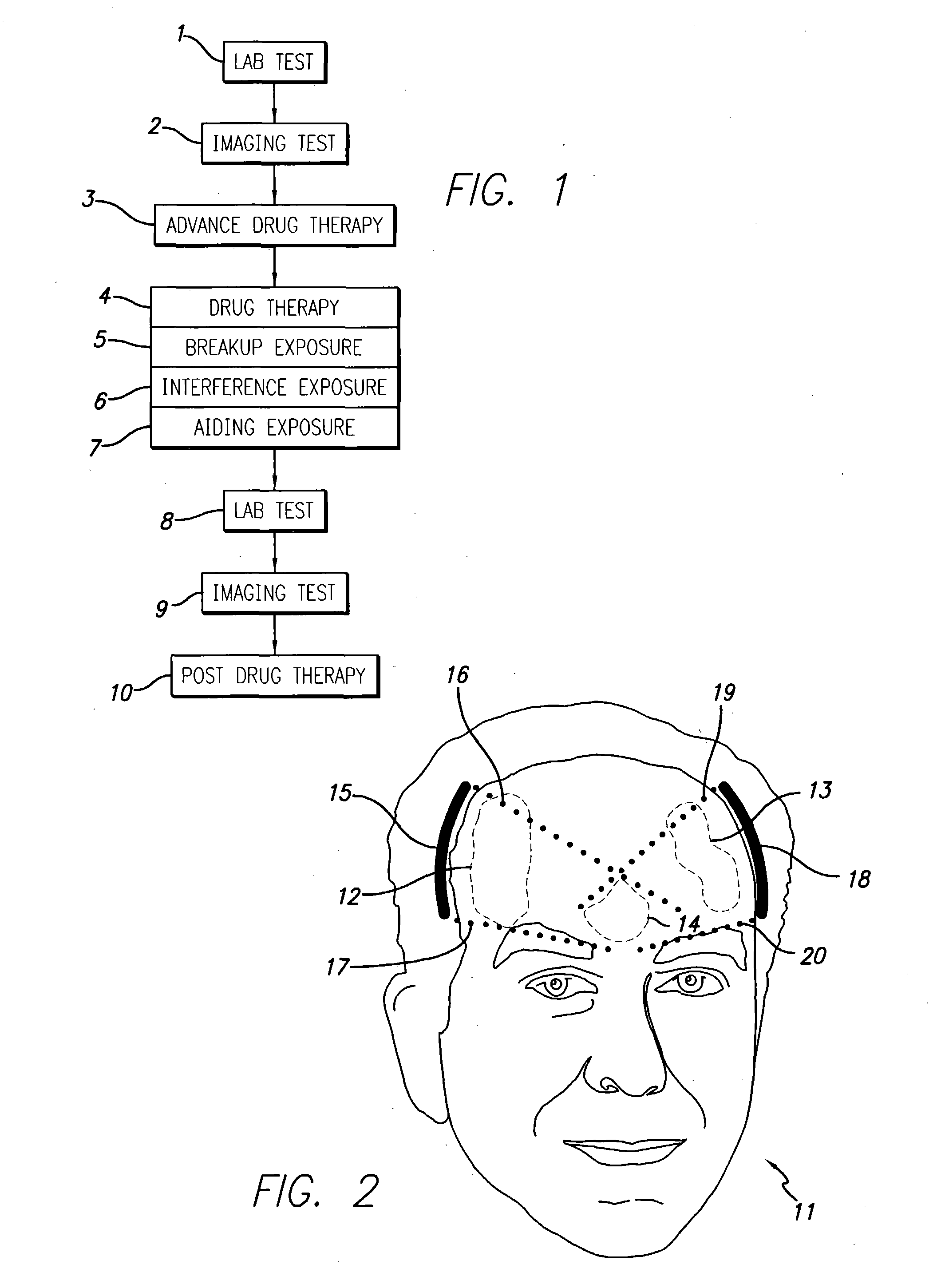
A system and methods are provided for the therapeutic treatment of brain-plaques, fibrils, abnormal-protein related or aggregation-prone protein related deposition-diseases. The system employs acoustic exposure therapy means for delivering therapeutic energy to at least one brain region. The therapy supports at least one of the following processes: (i) physical breakup, erosion, disentanglement, de-aggregation, dissolution, de-agglomeration, de-amalgamation or permeation of the deposits, (ii) interference in at least one deposit formation process, deposition related chemical reaction or biological or genetic pathway contributing to the deposits or deposition-related processes, and (iii) aiding the recovery, growth, regrowth or improved functionality of brain-related cells or functional pathways negatively impacted by, stressed by or disposed to the deposits, deposition-processes or deposition disease state, or supporting the growth of newly transplanted cells anywhere in the brain-related anatomy. The system and methods treat Alzheimer's and other deposition-related disorders of the brain, with minimal adverse side effects to the patient and may be used in cooperation with a drug.
Owner:TOSAYA CAROL A +1
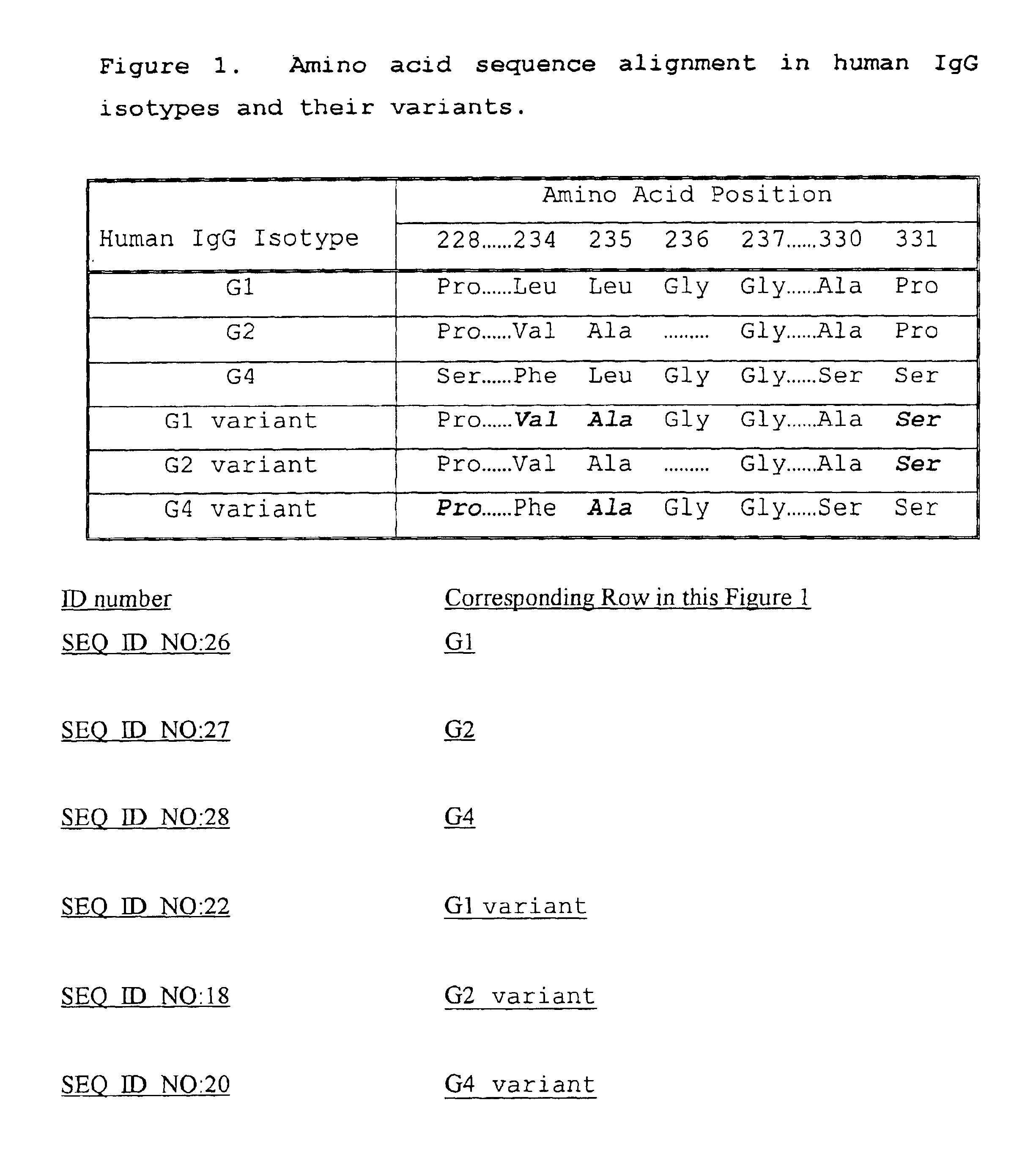
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
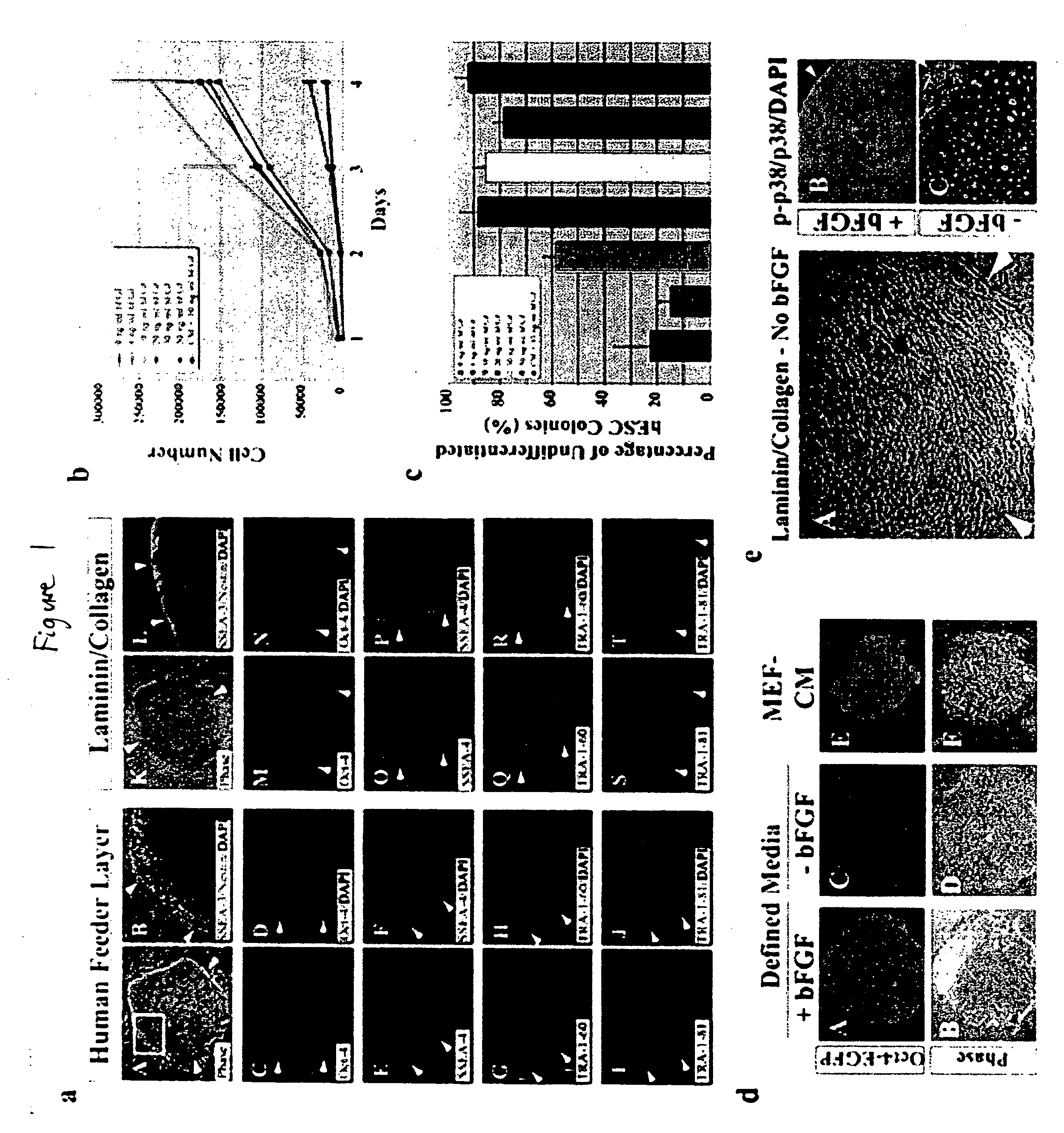
Defined media for stem cell culture
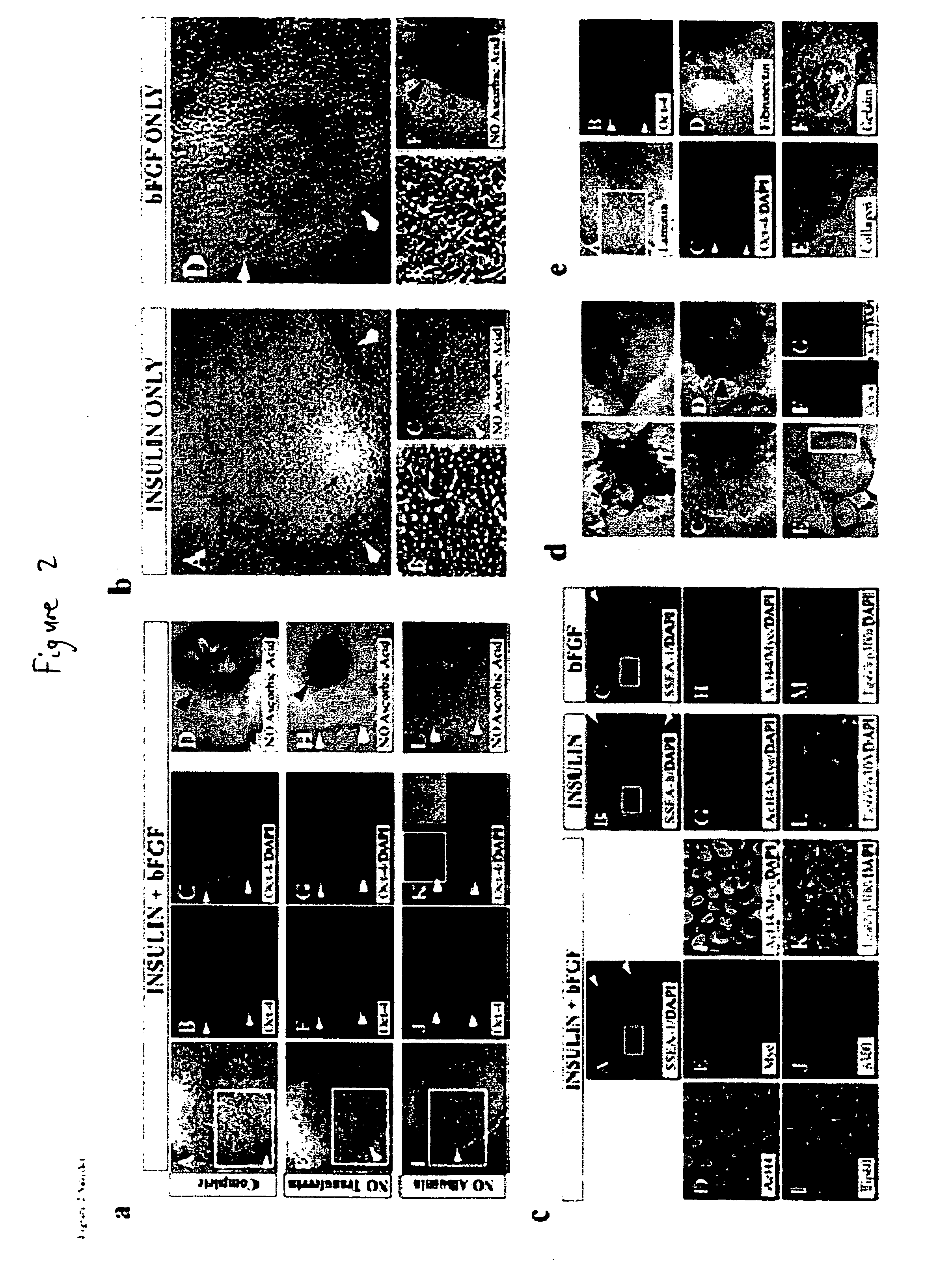
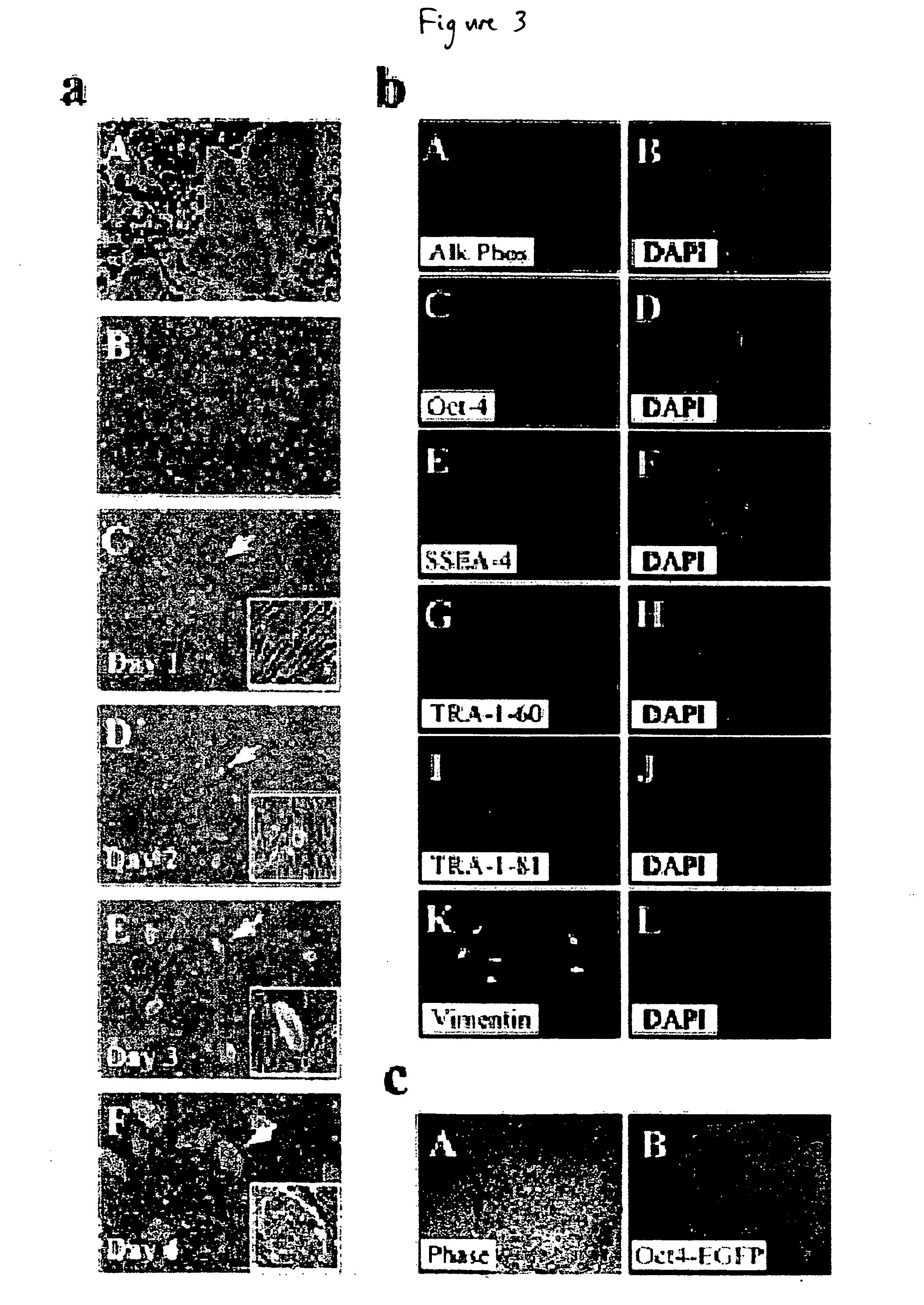
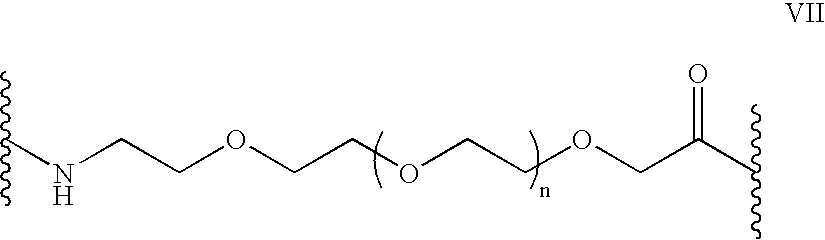
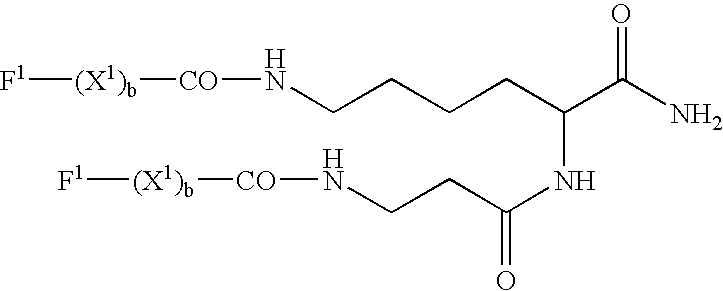
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
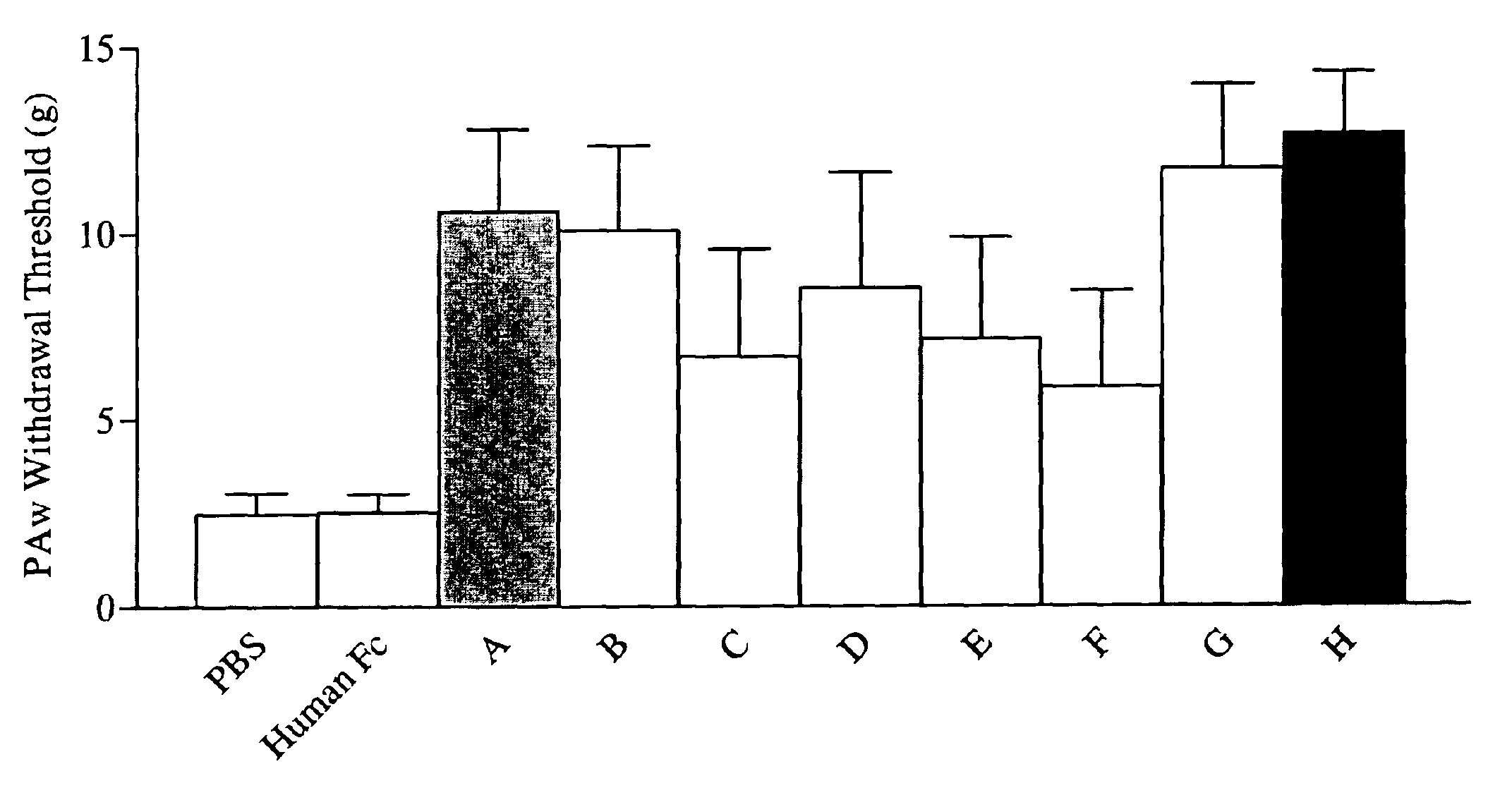
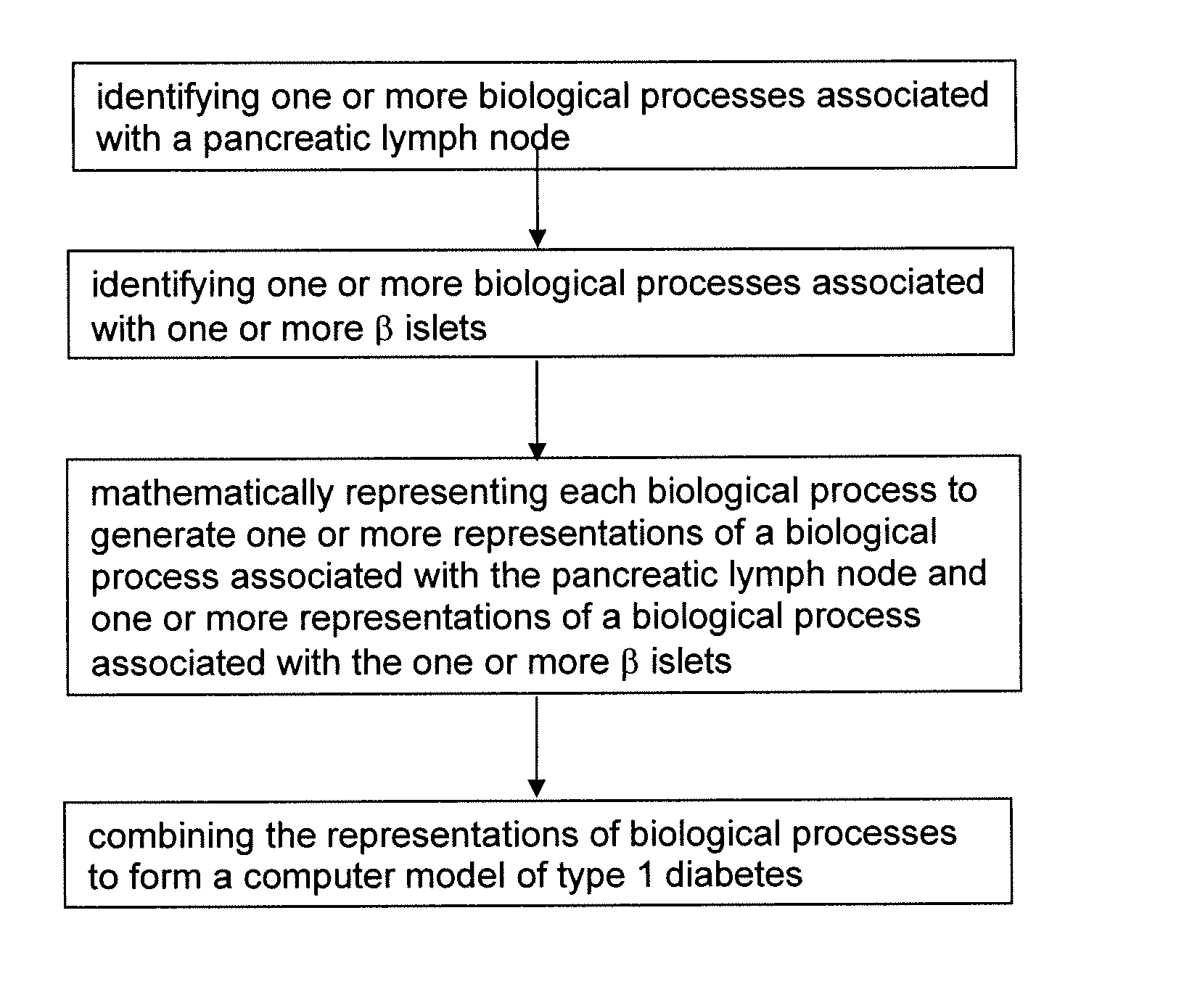
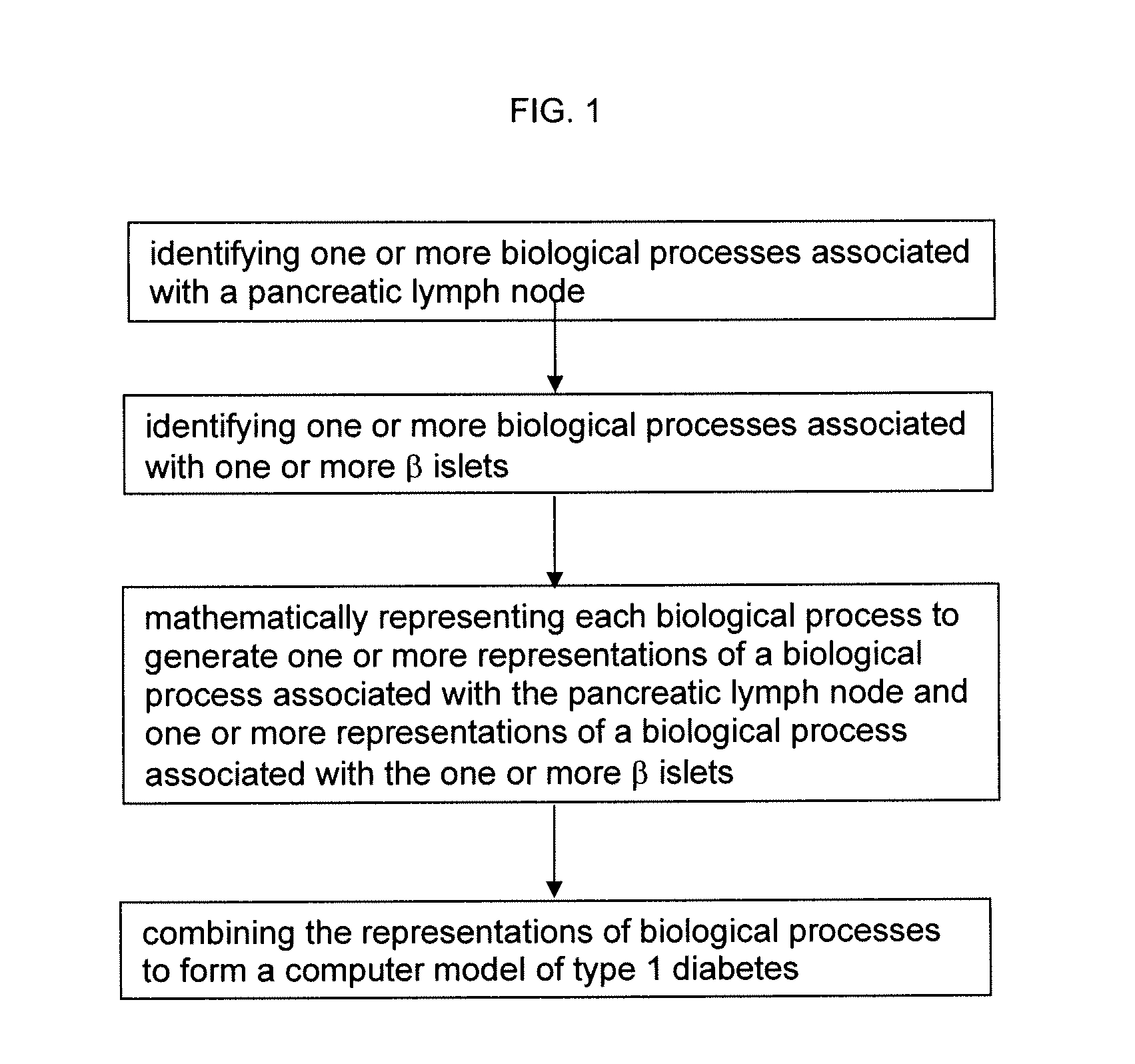
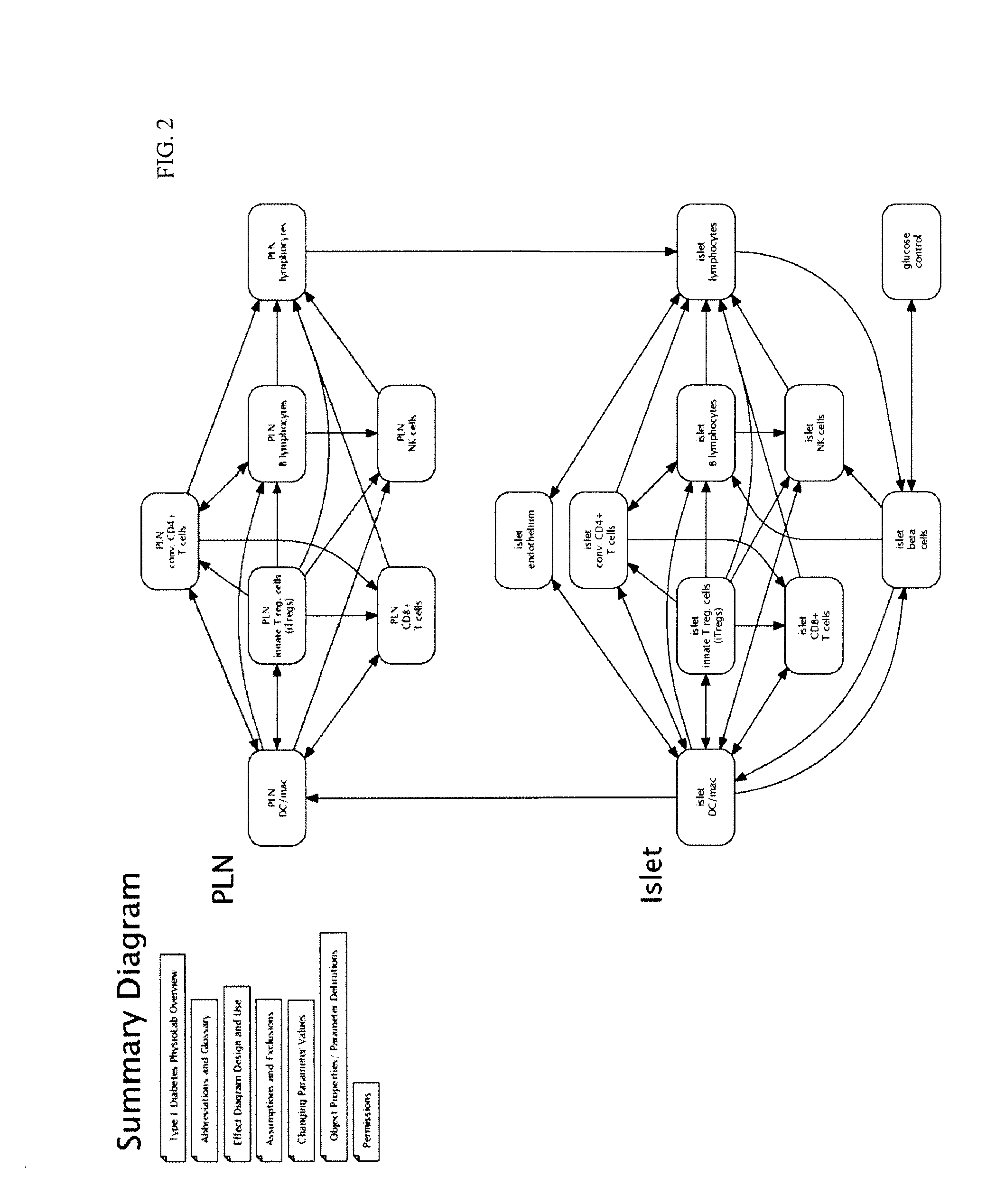
Apparatus and method for computer modeling type 1 diabetes
The invention encompasses novel methods for developing a computer model of type 1 diabetes in a mammal. In particular, the models can include representations of biological processes associated with a pancreatic lymph node and one or more pancreatic islets. Alternatively, the models can include representations of biological processes associated with at least two conditions selected from the group consisting of autoreactive T cell production, autoreactive T cell priming, insulitis and hyperglycemia. The invention also provides methods for developing a computer model of a non-insulin replacement treatment of type 1 diabetes. The invention also encompasses computer models of type 1 diabetes, methods of simulating type 1 diabetes and computer systems for simulating type 1 diabetes and the uses thereof.
Owner:ENTELOS INC
Microfluidic devices comprising biochannels
The present invention is directed to a variety of microfluidic devices with configurations including the use of biochannels or microchannels comprising arrays of capture binding ligands to capture target analytes in samples. The invention provides microfluidic cassettes or devices that can be used to effect a number of manipulations on a sample to ultimately result in target analyte detection or quantification.
Owner:MOTOROLA INC
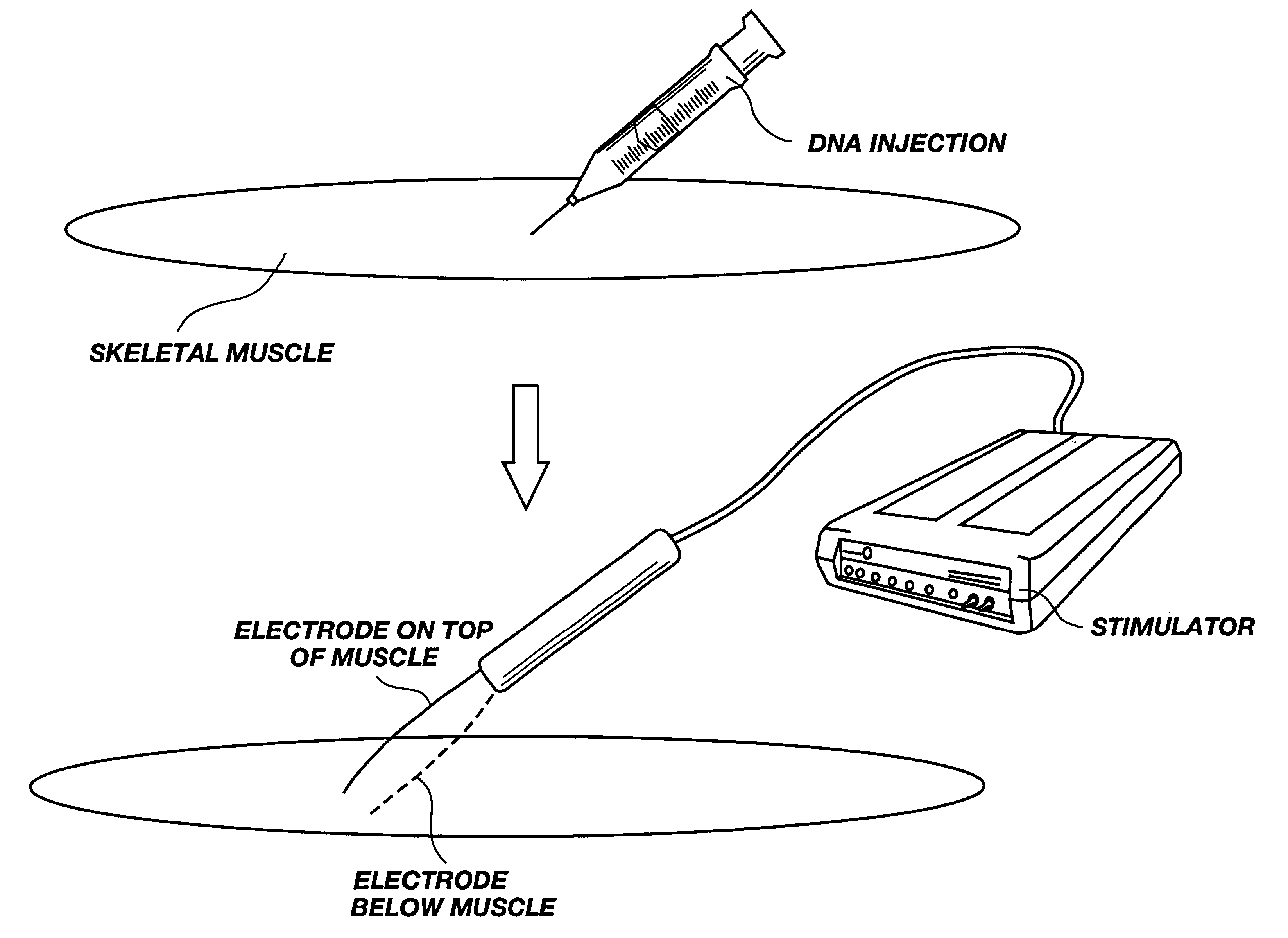
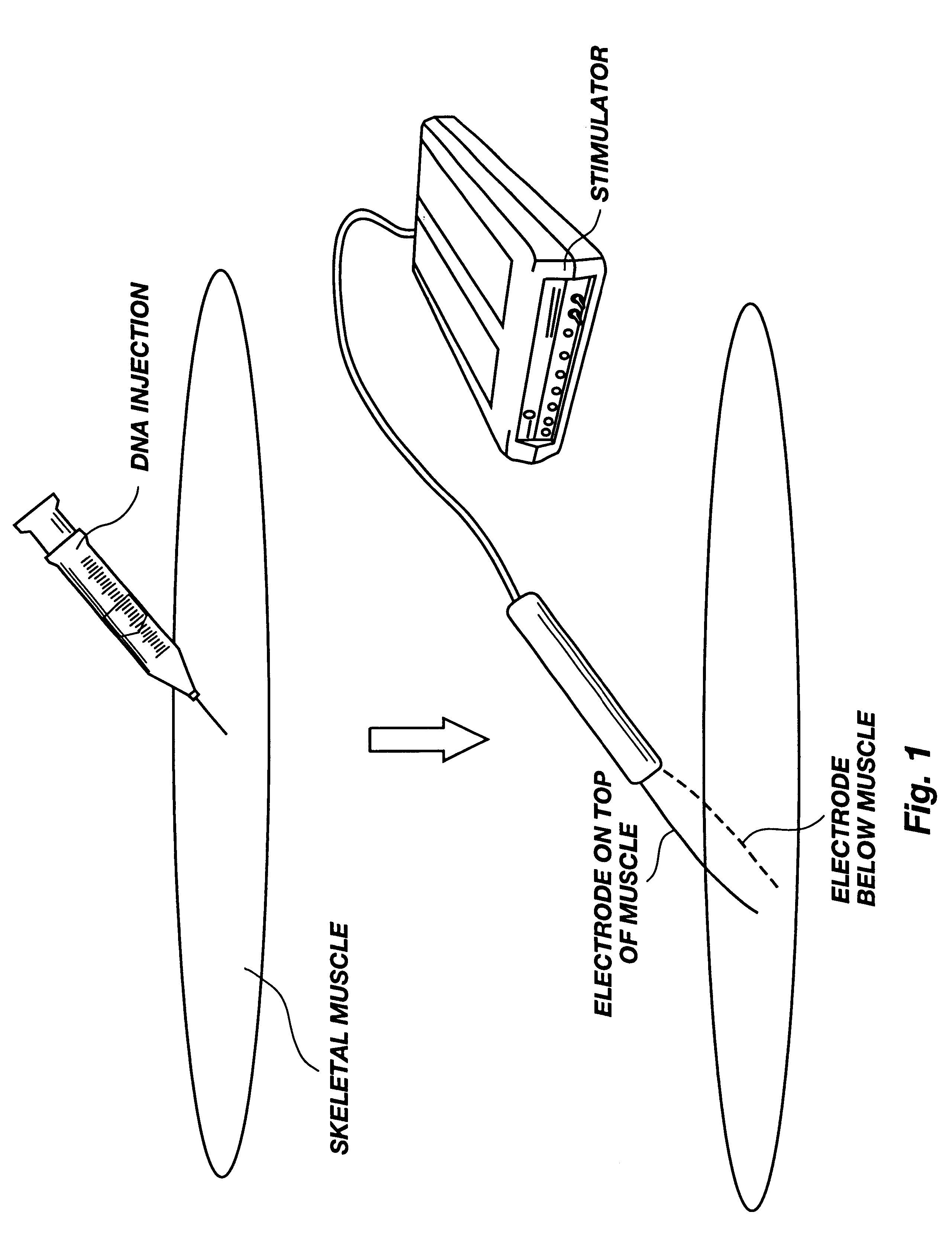
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com