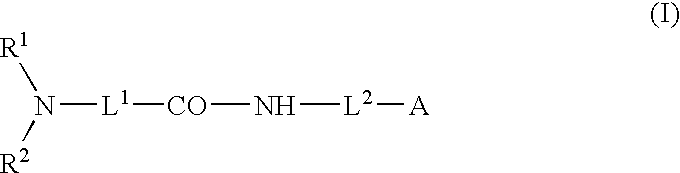Patents
Literature
1672 results about "Vaccination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccination is the administration of a vaccine to help the immune system develop protection from a disease. Vaccines contain a microorganism or virus in a weakened or killed state, or proteins or toxins from the organism. In stimulating the body's adaptive immunity, they help prevent sickness from an infectious disease. When a sufficiently large percentage of a population has been vaccinated, herd immunity results. The effectiveness of vaccination has been widely studied and verified. Vaccination is the most effective method of preventing infectious diseases; widespread immunity due to vaccination is largely responsible for the worldwide eradication of smallpox and the elimination of diseases such as polio and tetanus from much of the world.
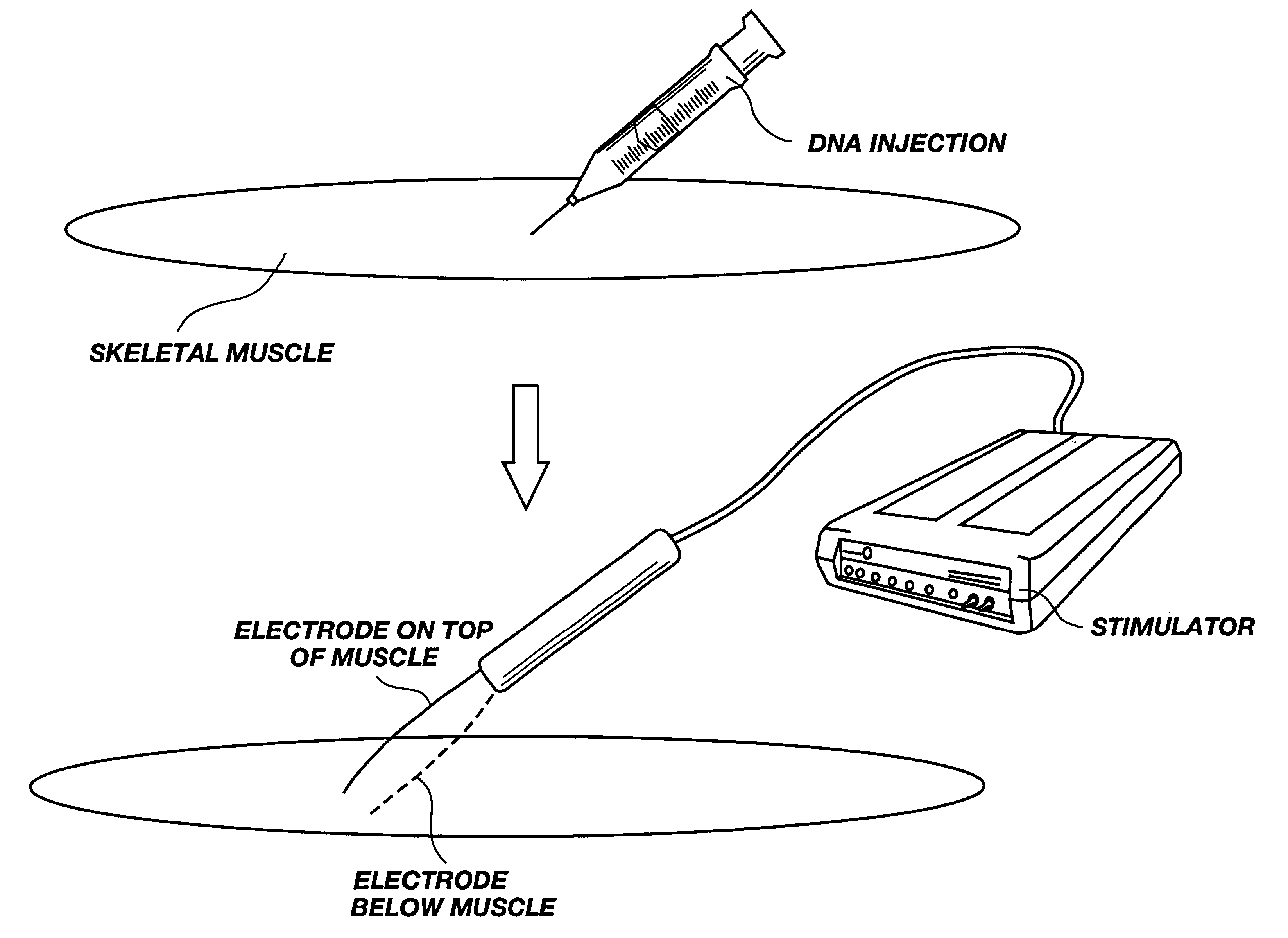
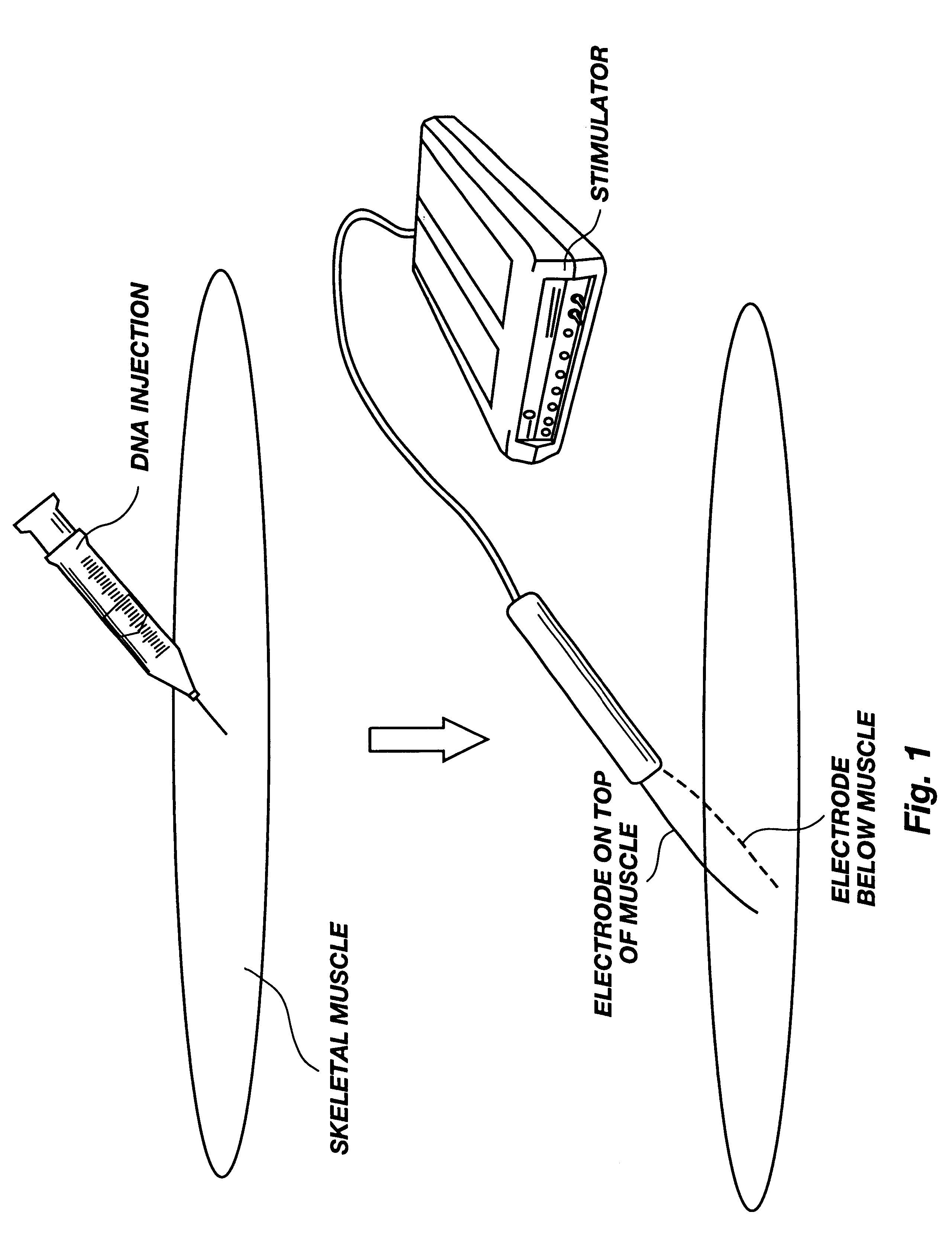
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
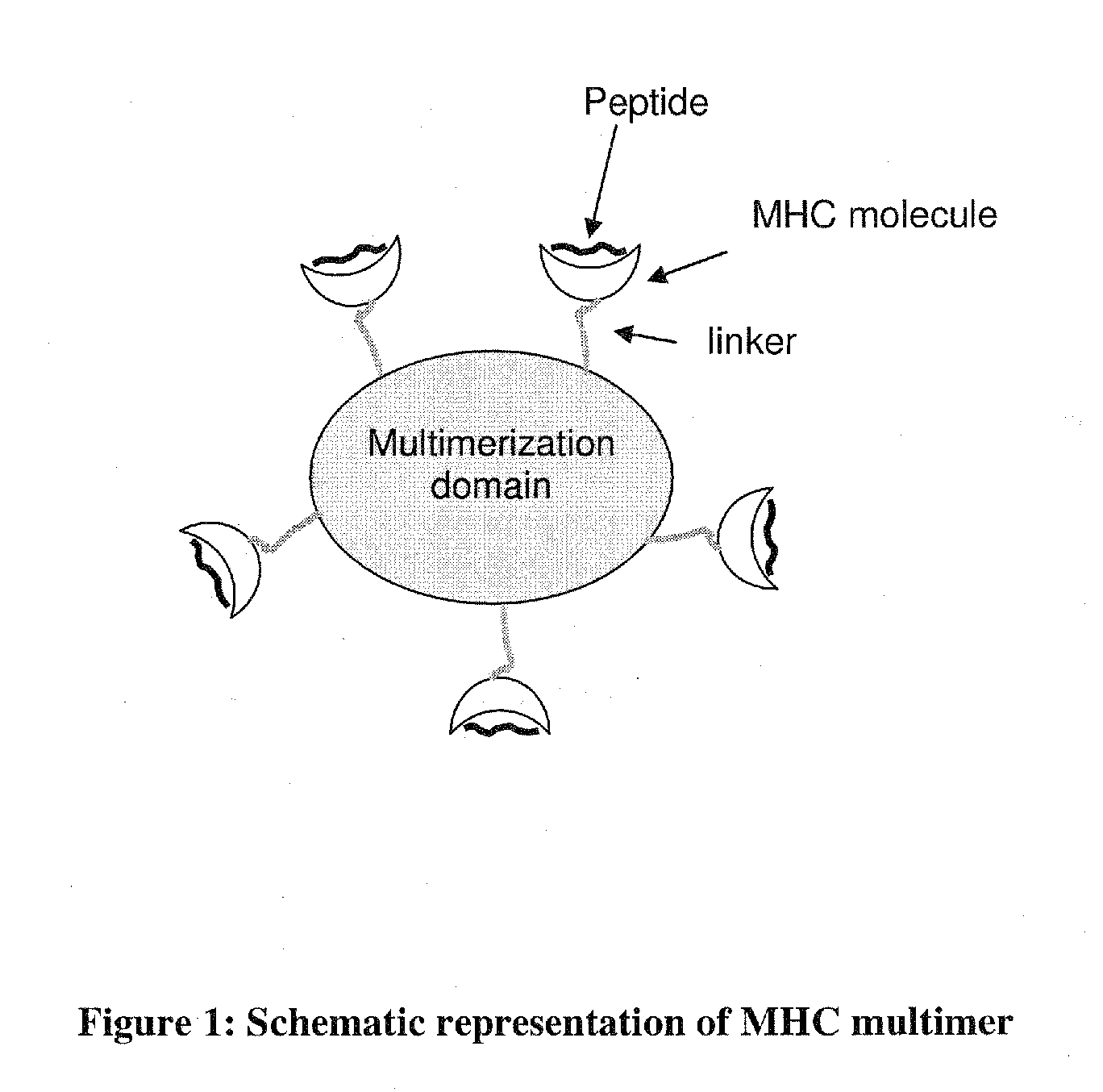
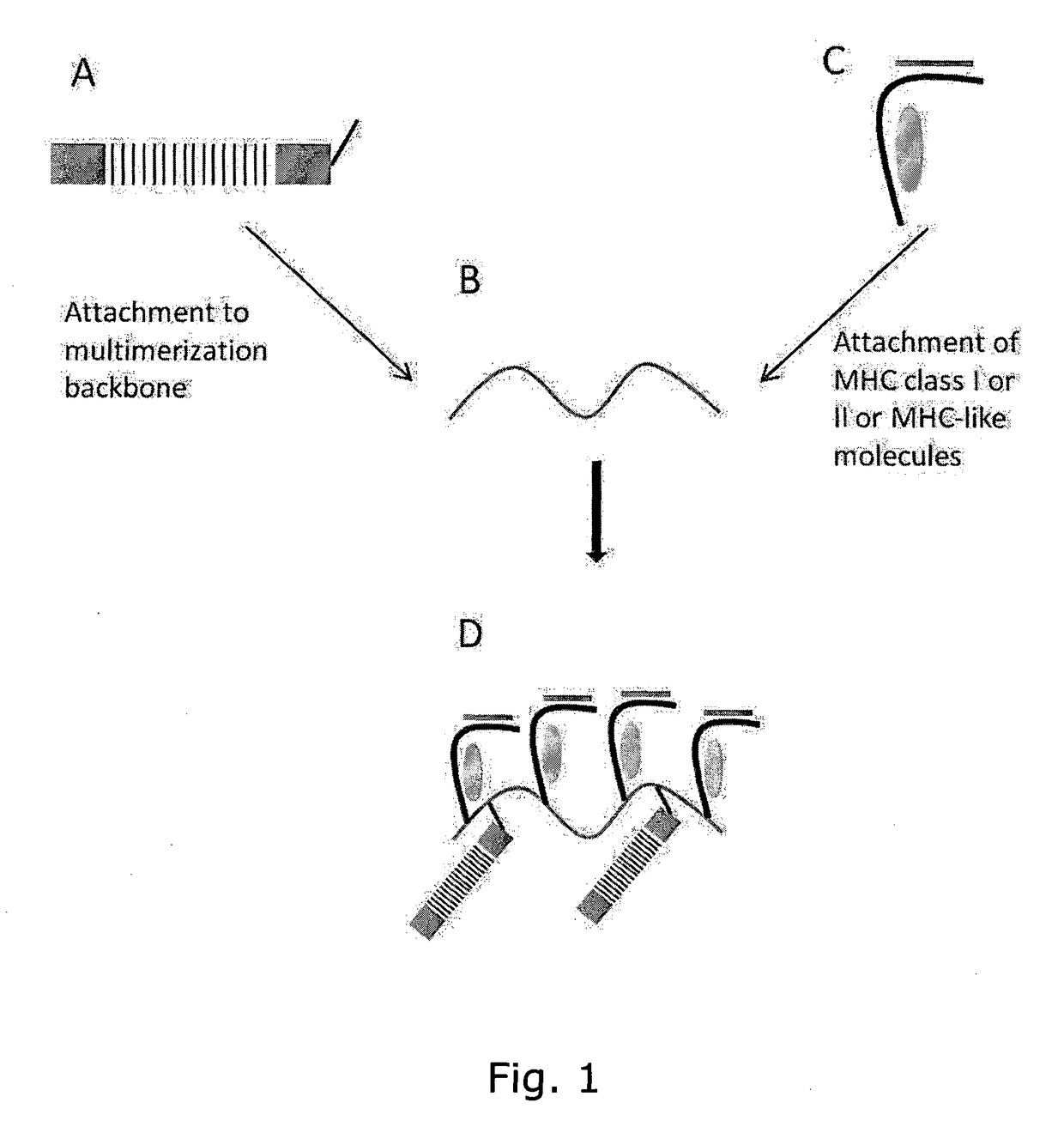
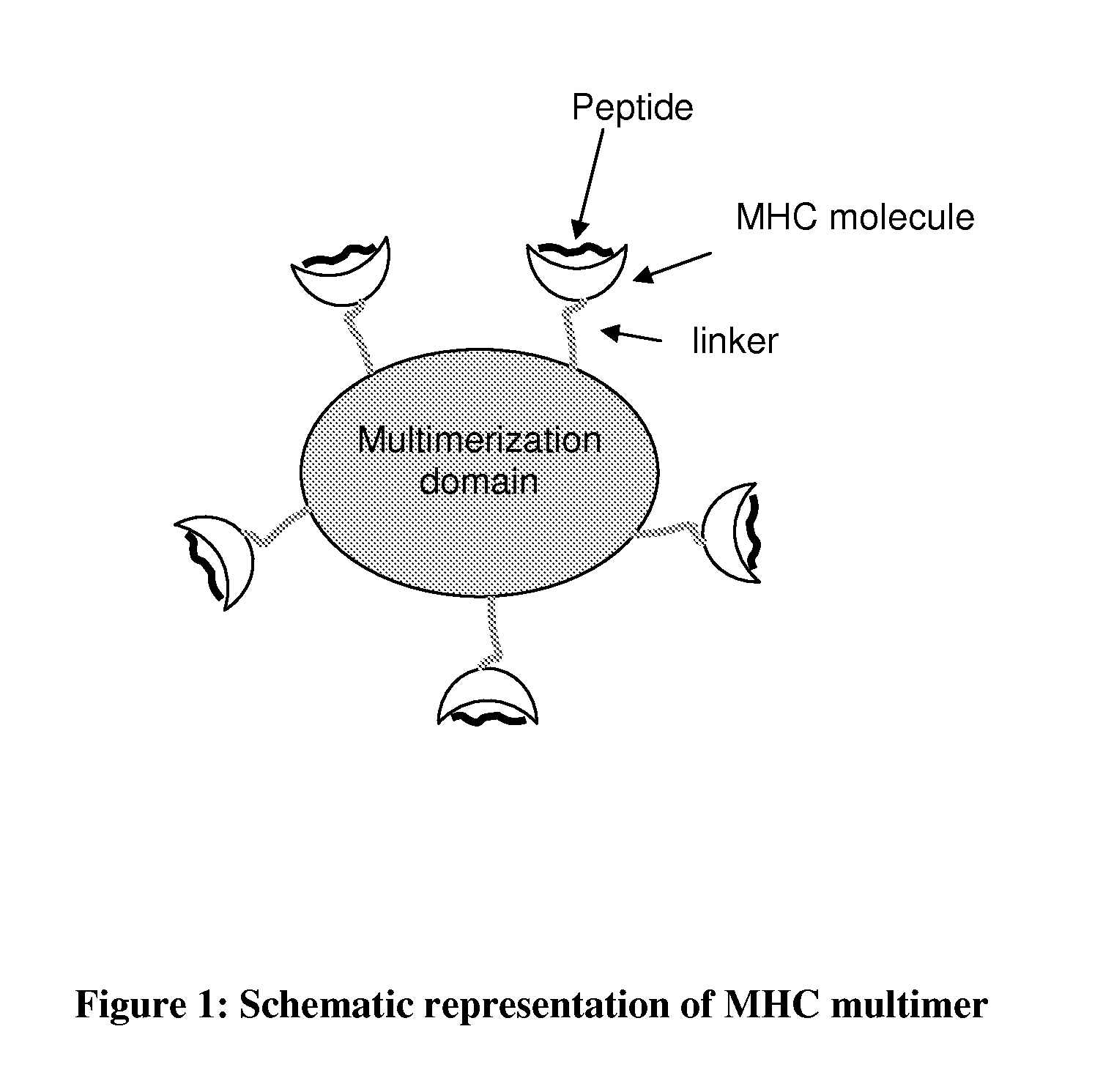
MHC Multimers in Cancer Vaccines and Immune Monitoring
InactiveUS20110318380A1Reduces infectious titerImprove efficacyPeptide/protein ingredientsImmunoglobulinsAntigenDisease
The present invention relates to MHC-peptide complexes and uses thereof in the diagnosis of, treatment of or vaccination against a disease in an individual. More specifically the invention discloses MHC complexes comprising cancer antigenic peptides and uses there of.
Owner:AGILENT TECH INC
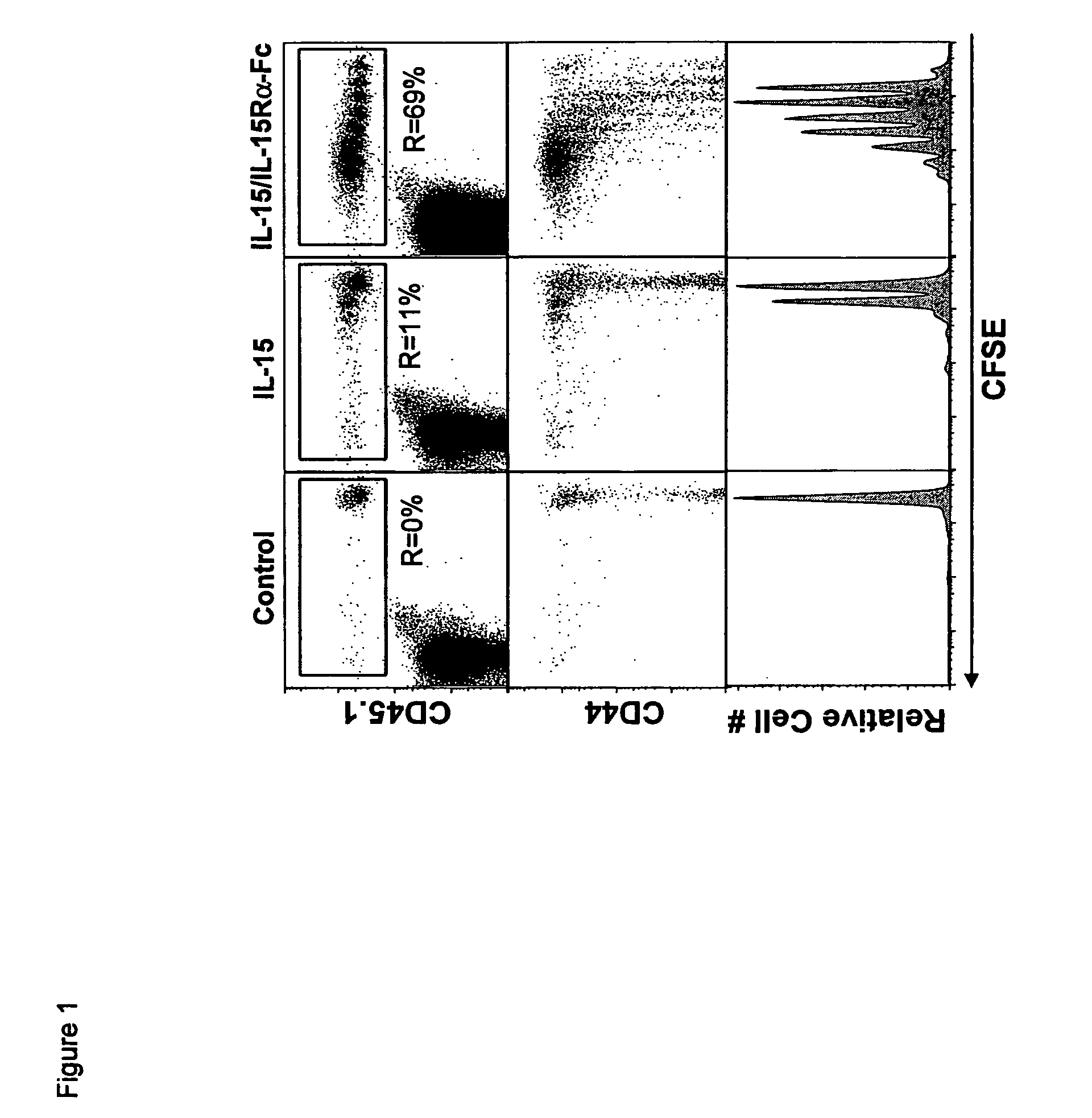
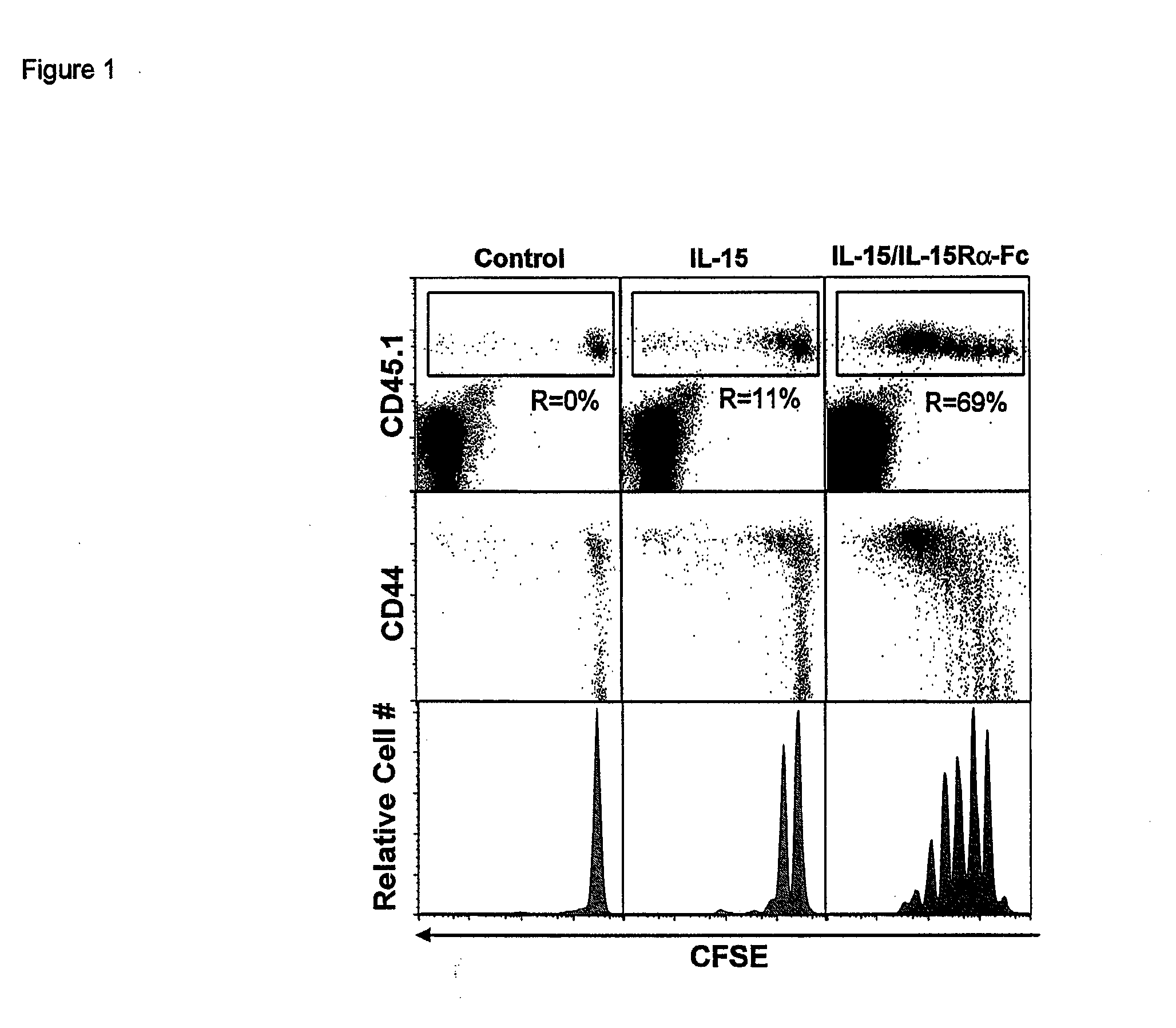
Compositions and methods for immunomodulation in an organism using IL-15 and soluble IL-15Ra
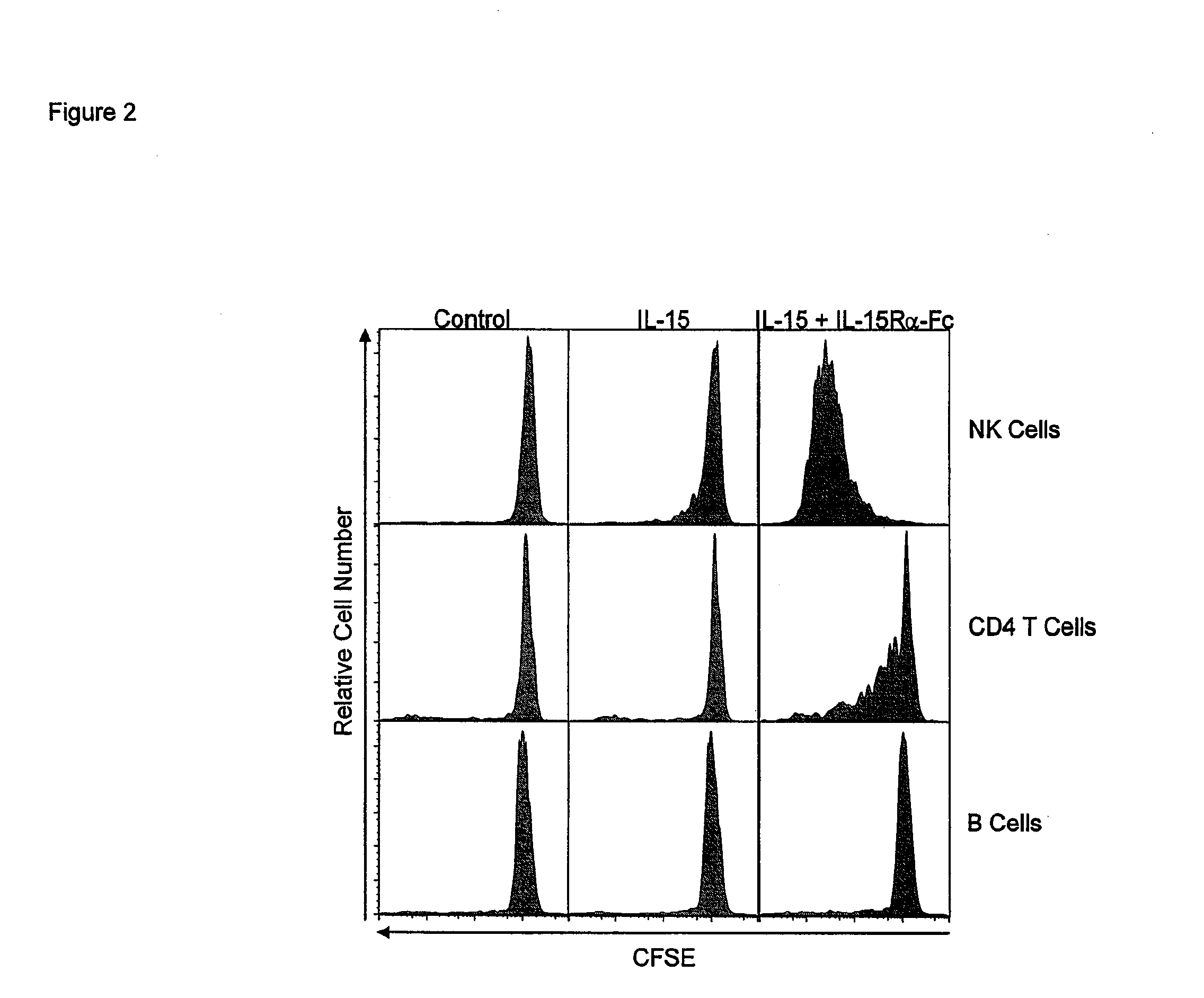
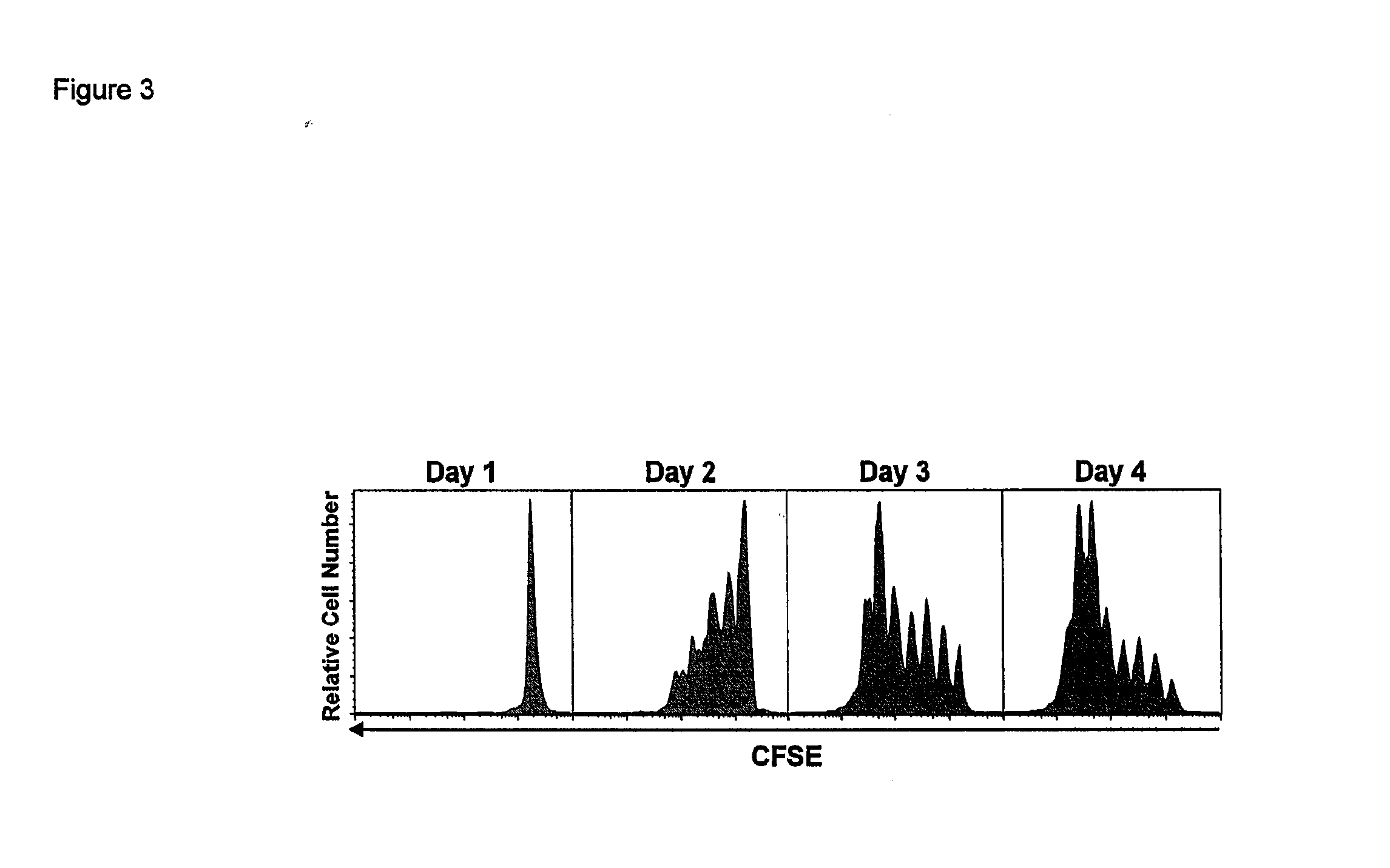
ActiveUS8124084B2Extended half-lifeImprove bioavailabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiological bodyVaccination
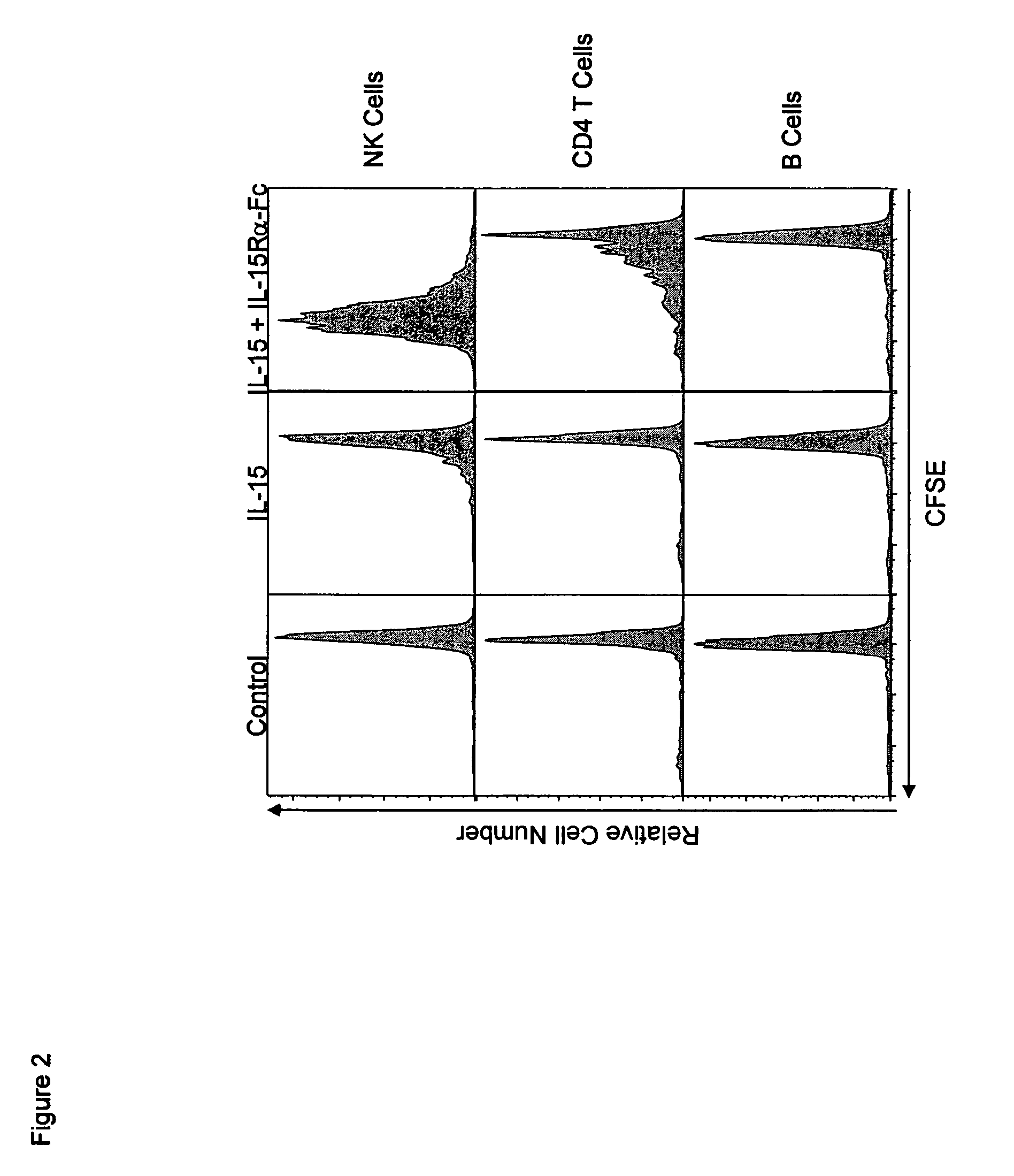
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
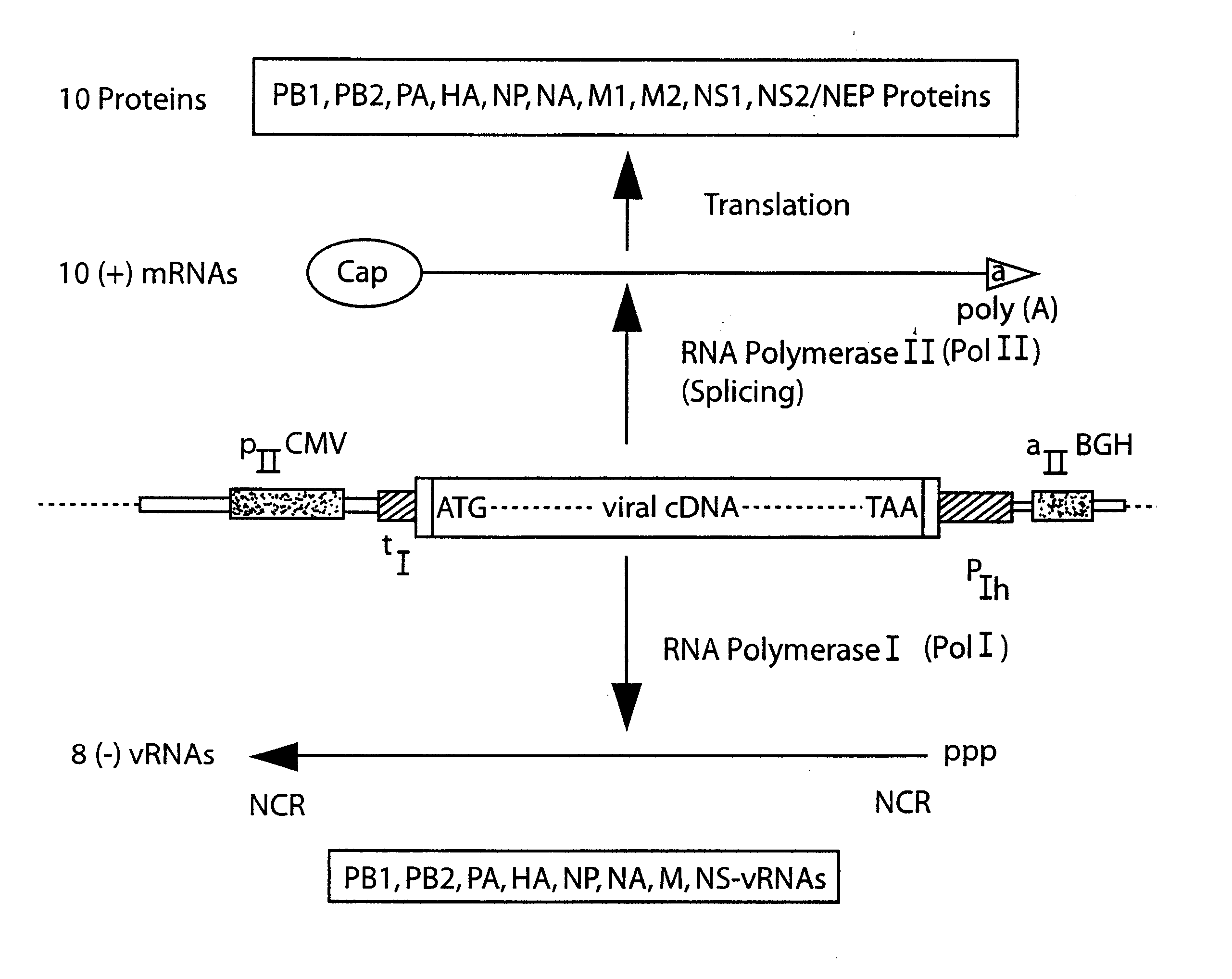
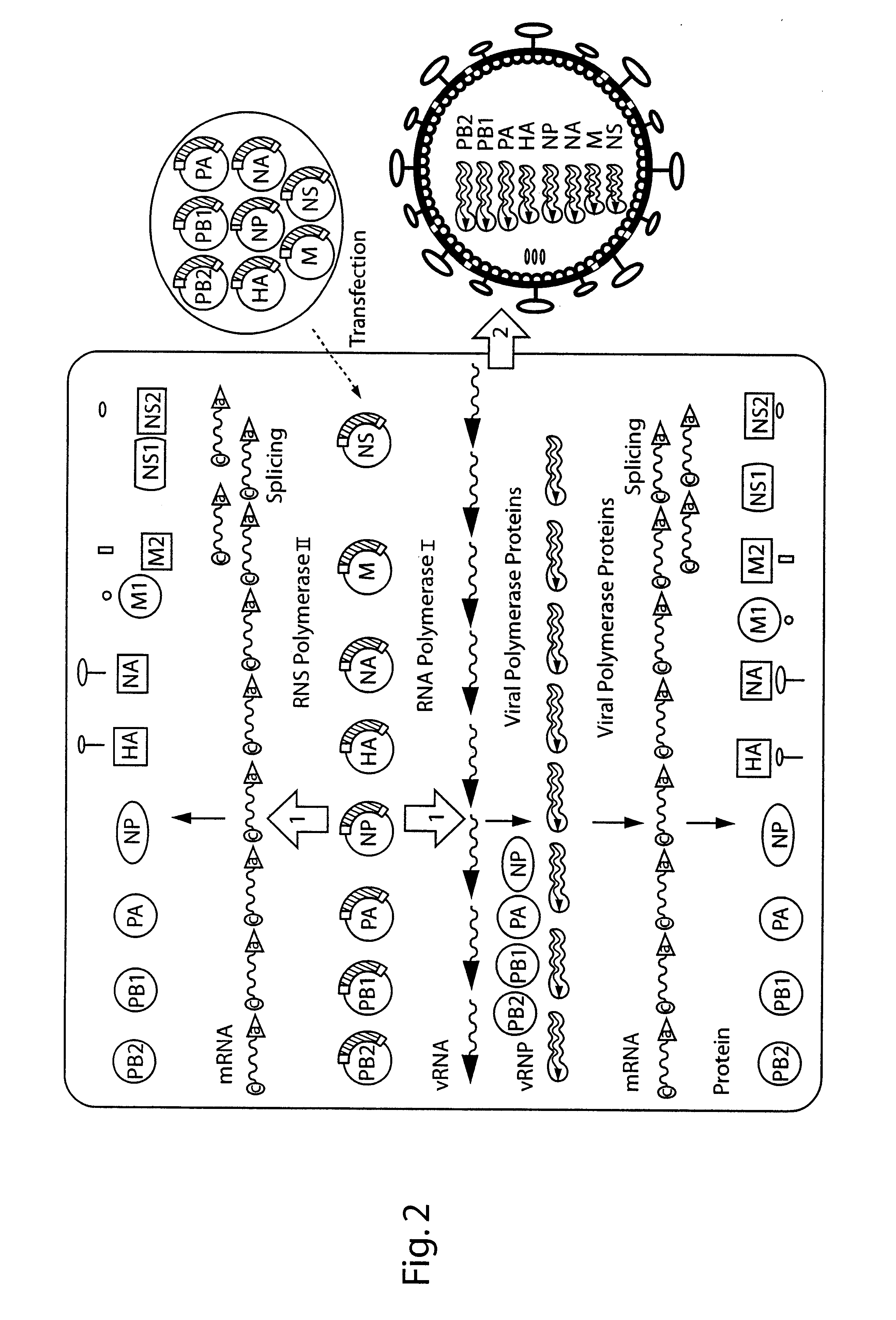
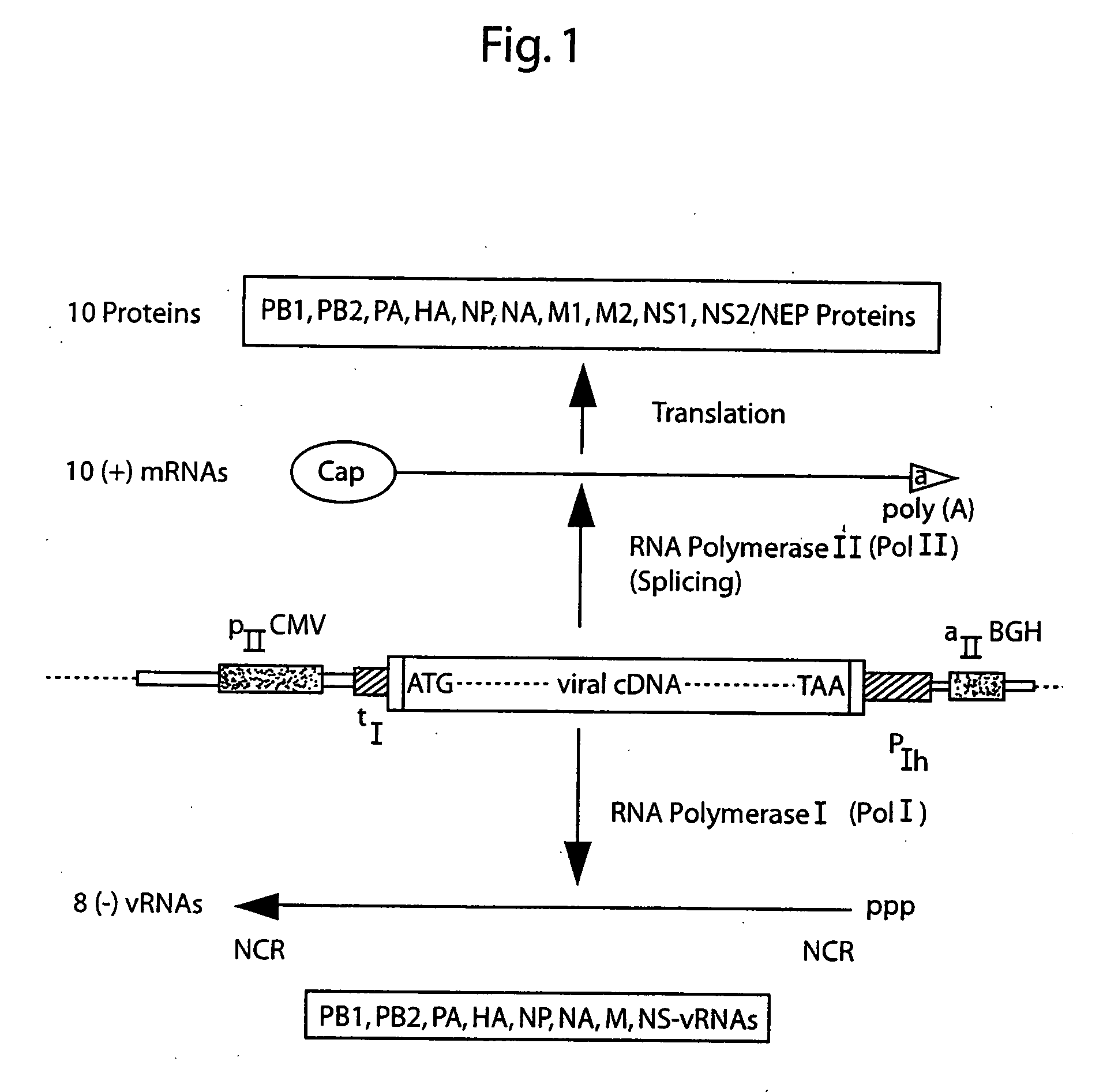
DNA transfection system for the generation of infectious influenza virus
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuramimidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
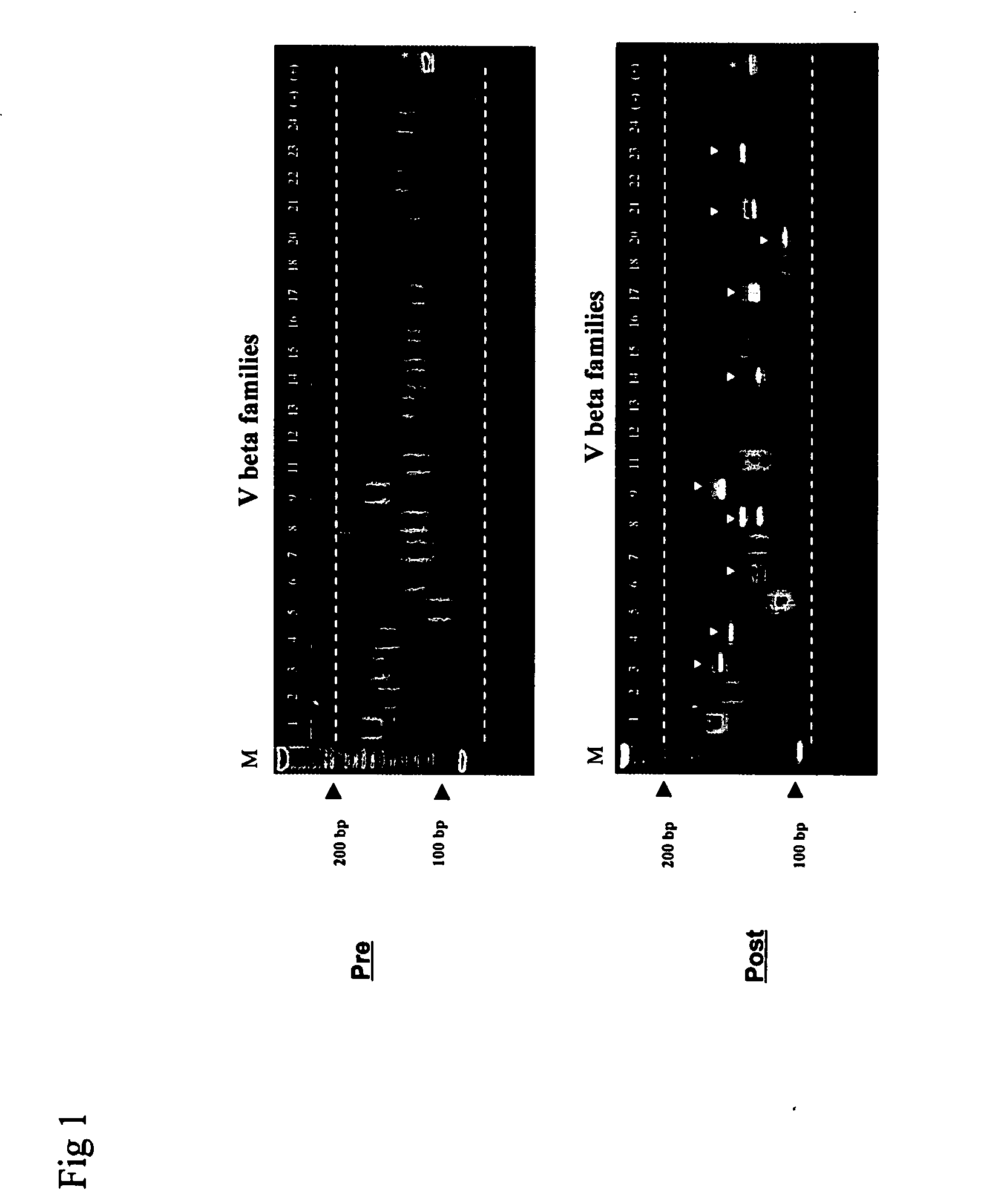
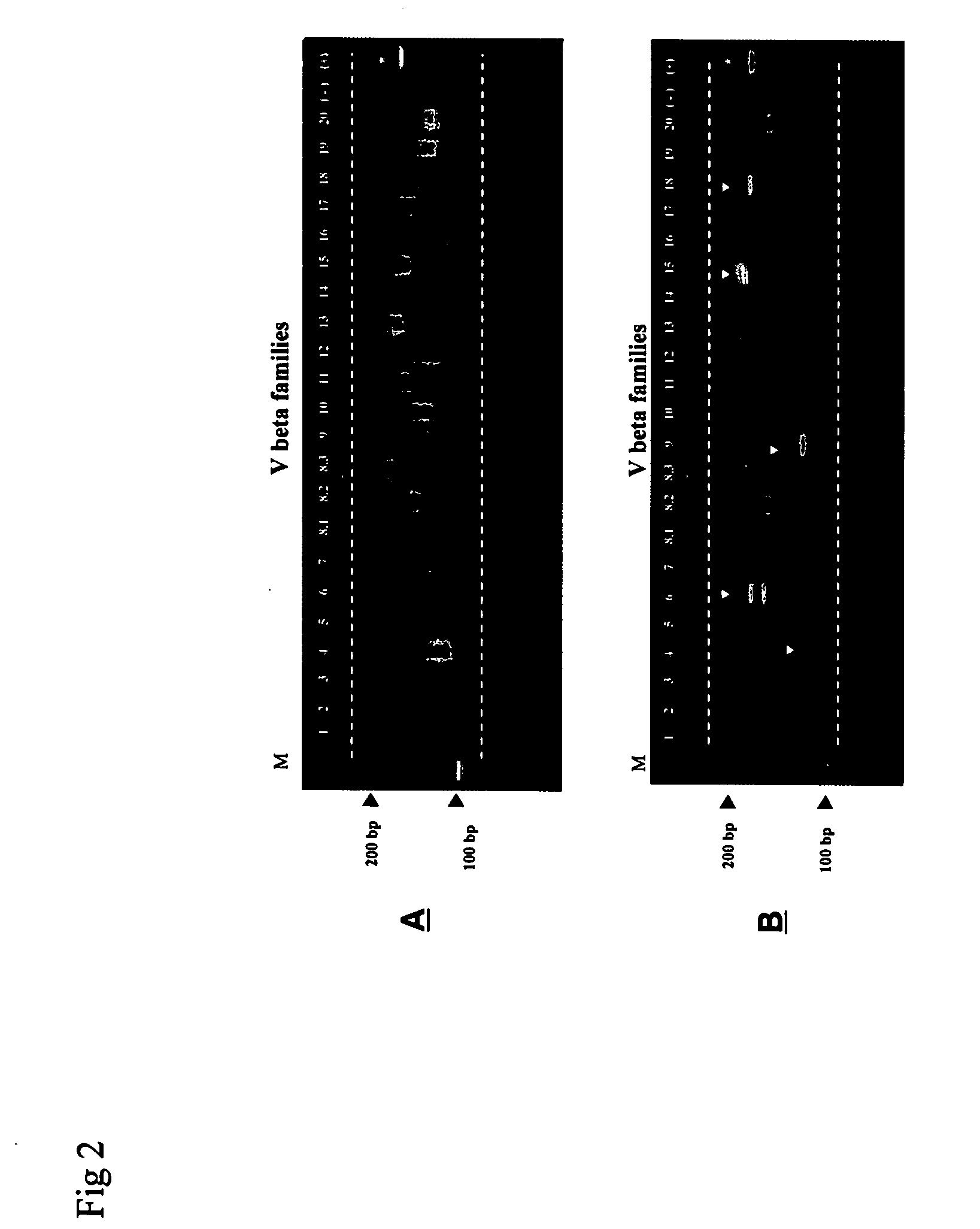
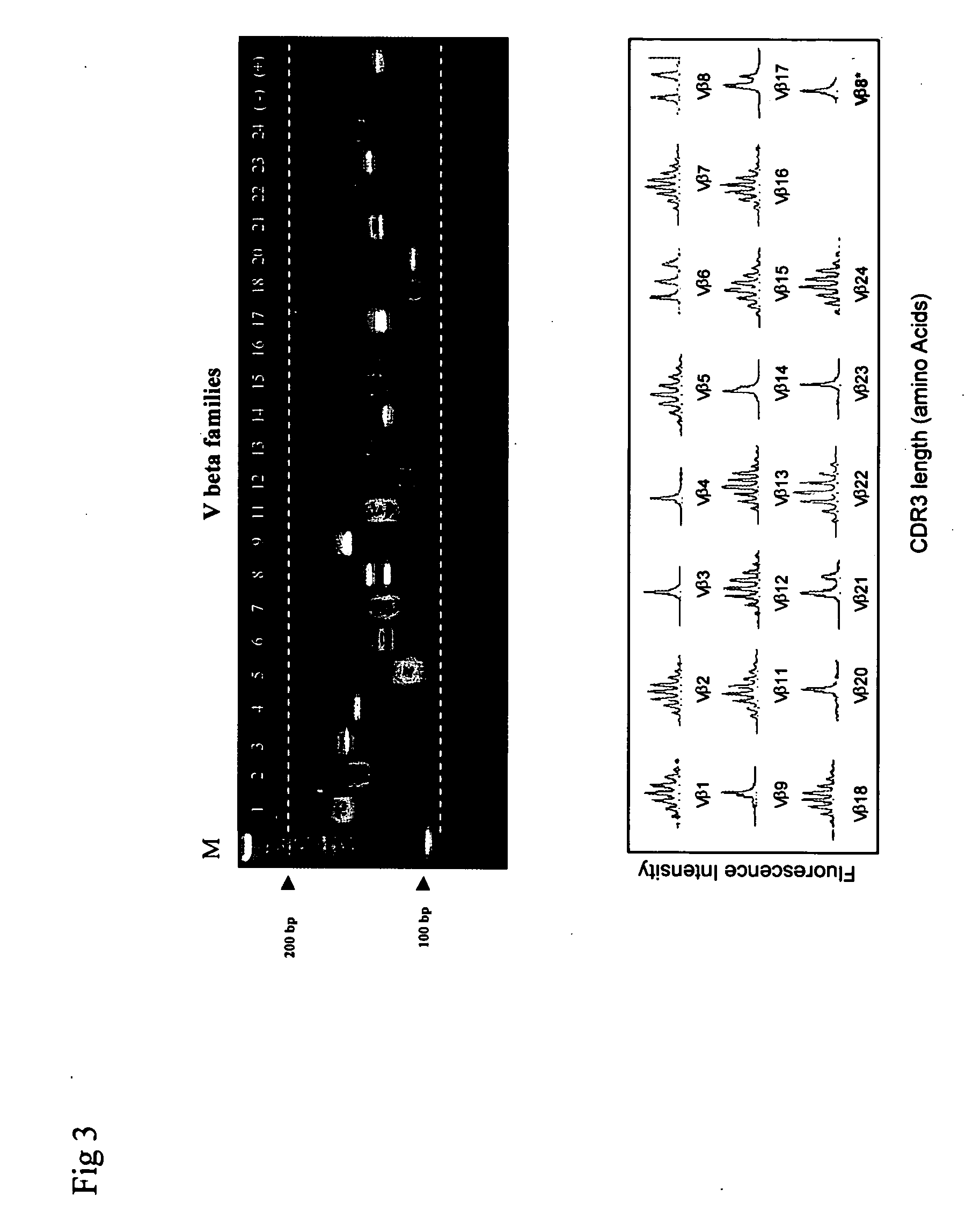
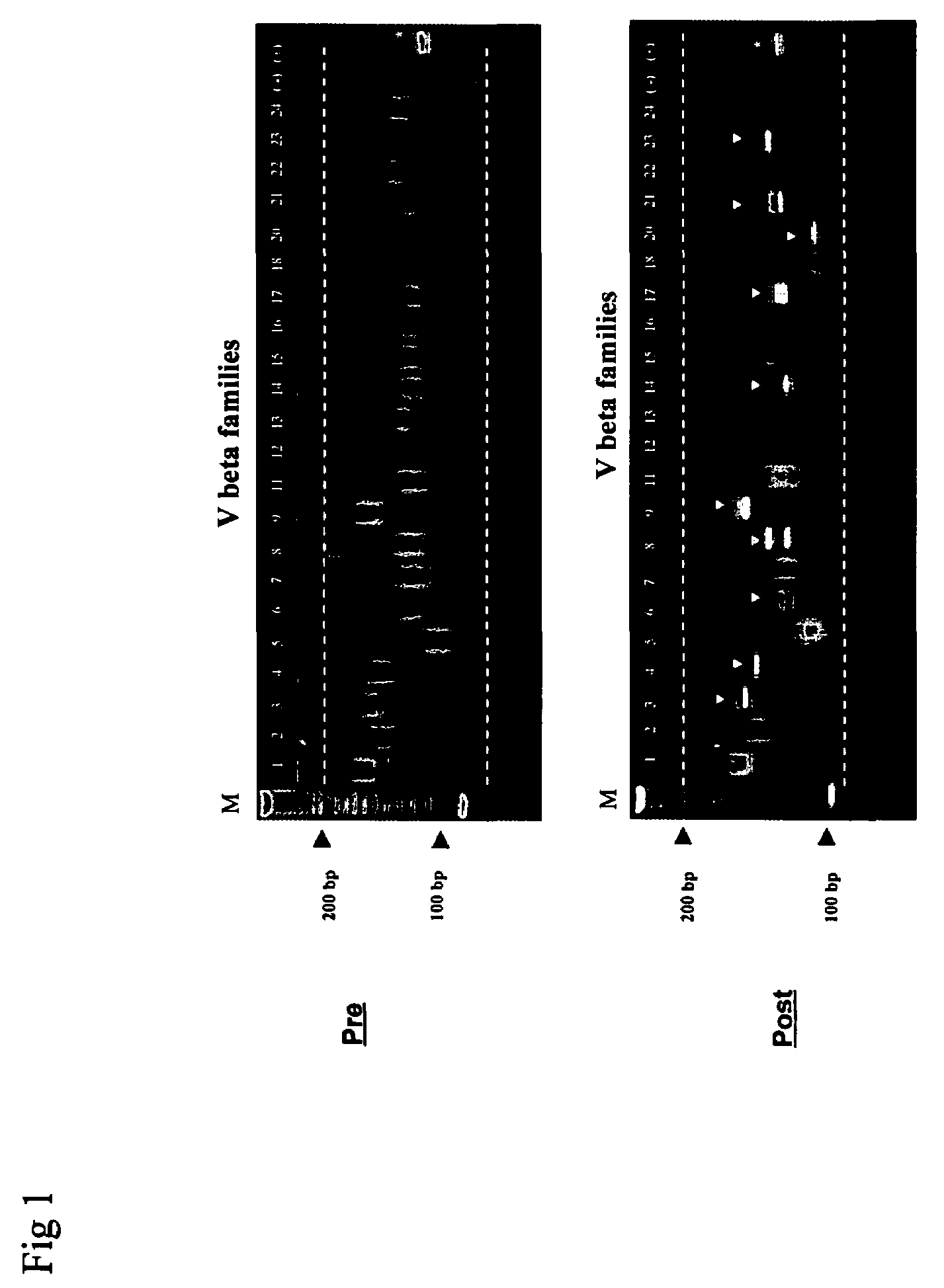
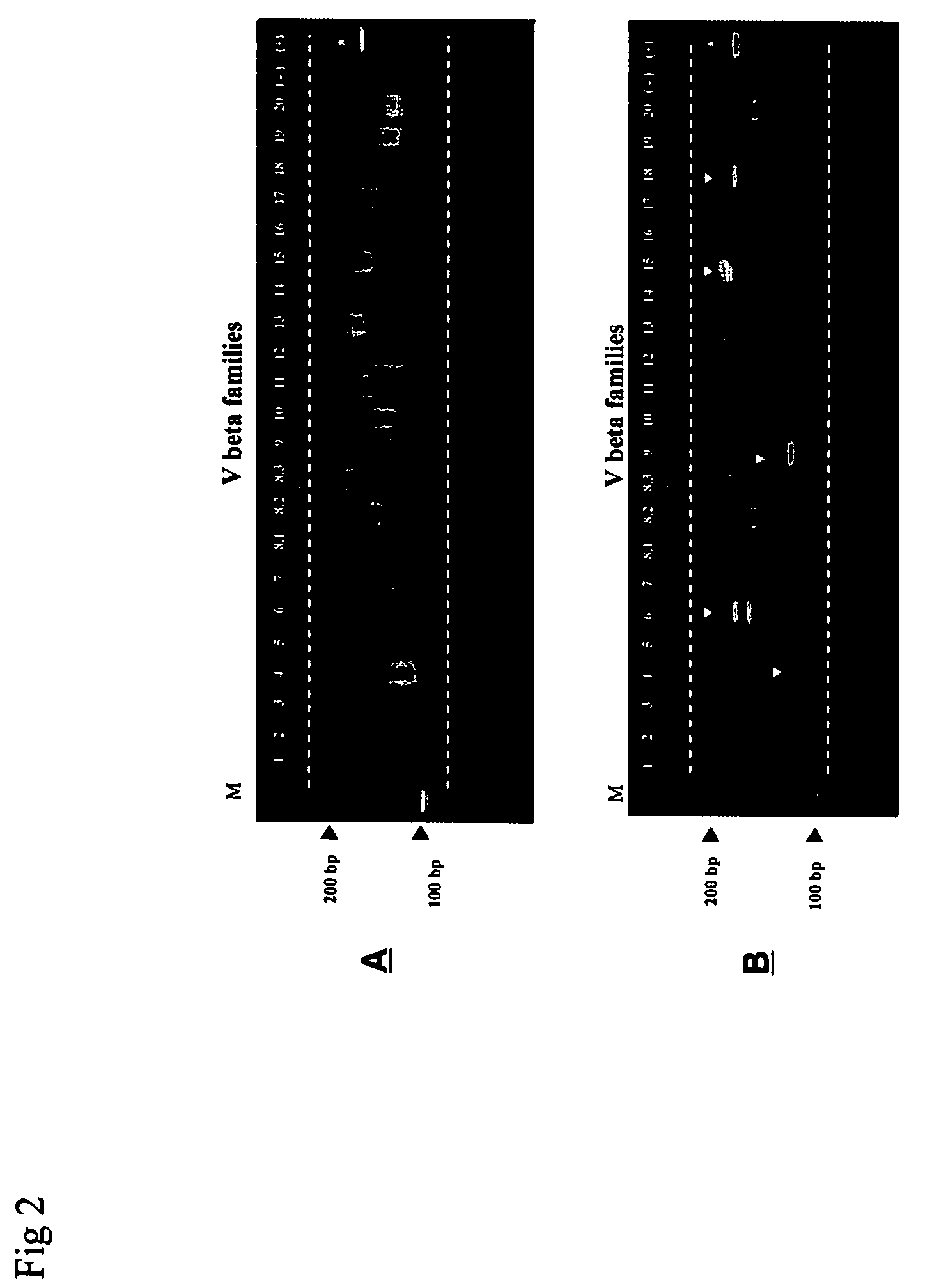
Method for detection and quantification of T-cell receptor Vbeta repertoire
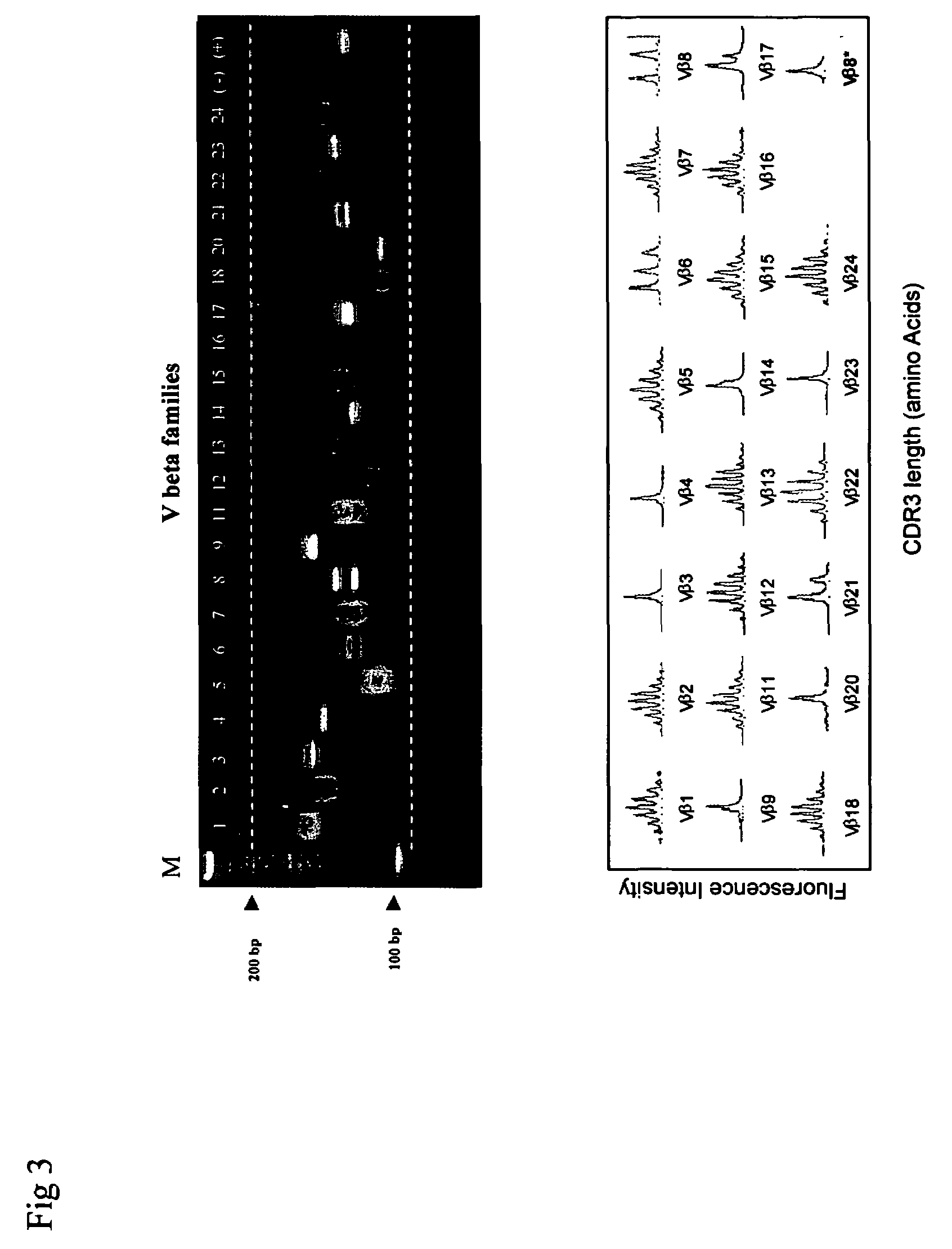
ActiveUS20070117134A1Enhances PCR reaction sensitivityRapid determinationAnalysis using chemical indicatorsSugar derivativesVaccinationAntigen stimulation
The invention is a method for detecting and measuring T-cell receptor (TCR) repertoires from mammalian lymphocytes. The method is based on the use of the multiple sets of unique primers to amplify 22 regions of the TCR Vβ region and thereby detect clonal expansions related to antigen stimulation of the immune system. Kits containing sets of primers and specialized analytical statistical software for use in determining clonal expansion in humans and mice are disclosed. The reliability, efficiency and short assay time in using the method is well suited to monitoring immune response to vaccination and therapeutic treatments for immune disorders.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Continuous Feed Hypodermic Syringe with Self Contained Cartridge Dispenser
InactiveUS20080058732A1Provide impact resistancePrevented being caughtAmpoule syringesAutomatic syringesPatient needSterile environment
A hypodermic syringe and a plurality of single use cartridges able to be successively loaded into said syringe for providing rapid dispensing of a medicant to numerous users without possibility of contamination. The cartridges are continuously fed through the syringe for the quick and efficient inoculation of patients. An operator inserts a needle into a patient by pushing in a trigger. Prior to the needle being inserted into the patient, a disinfectant is dispersed from the cartridge to maintain sterility. An operator then pushes in a plunger forcing the medication through the needle into the patient. The plunger is pulled back into its original location after use. The needle is pulled back with the plunger out of the patient's skin and back into the cartridge. A new cartridge on a clip is then advanced into the syringe for use in inoculating the next patient needing medication. The syringe and cartridge dispenser further maintains a sterile environment during successive vaccinations reducing the transmission of any disease from patient to patient.
Owner:HARRIS ARTHUR
Compositions and Methods for Immunomodulation in an Organism
ActiveUS20120177598A1Long half-lifeGood treatment effectPolypeptide with localisation/targeting motifPeptide/protein ingredientsVaccinationHalf-life
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
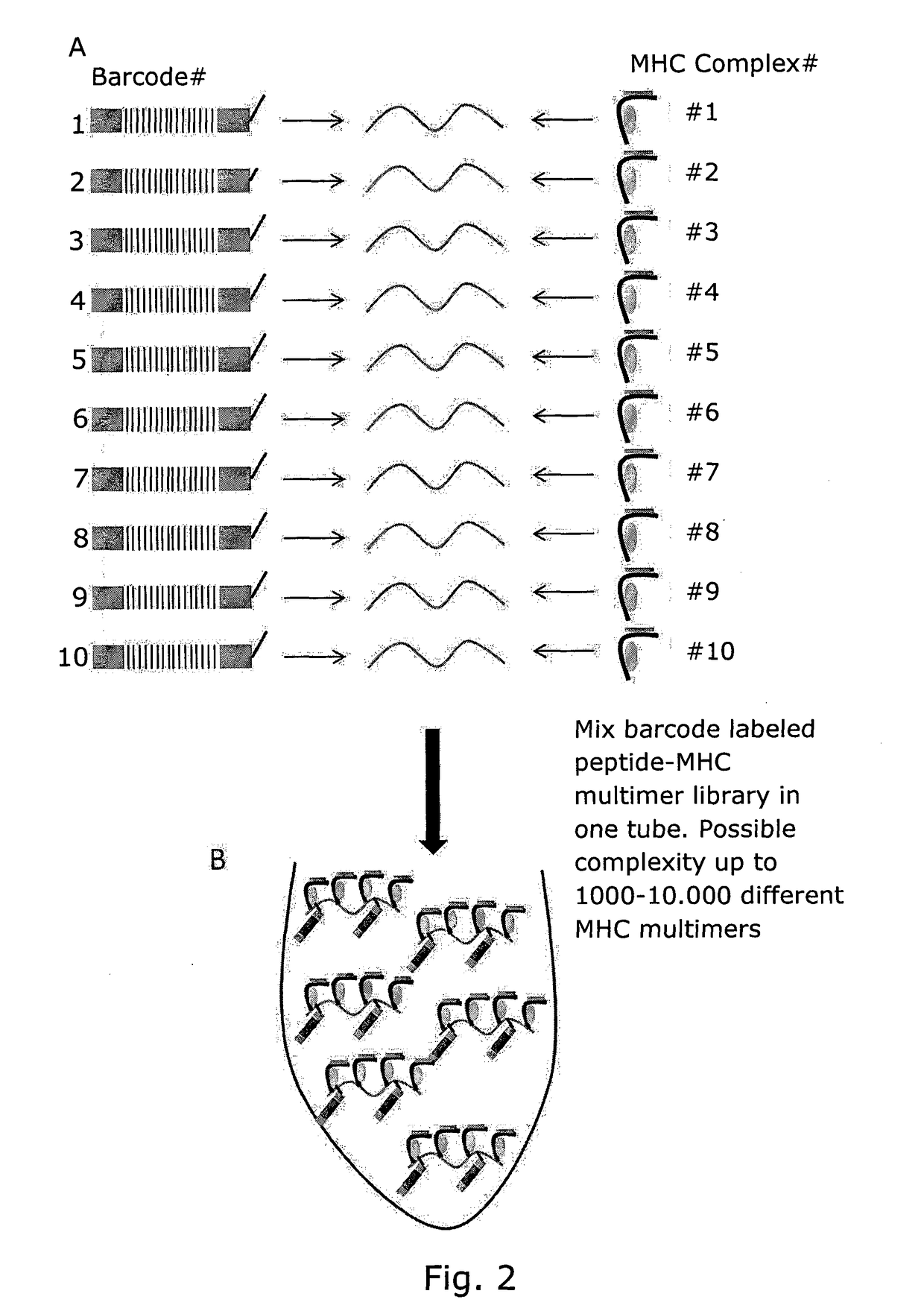
Determining Antigen Recognition through Barcoding of MHC Multimers
PendingUS20170343545A1Improve understandingMicrobiological testing/measurementBiological material analysisSingle sampleVaccination
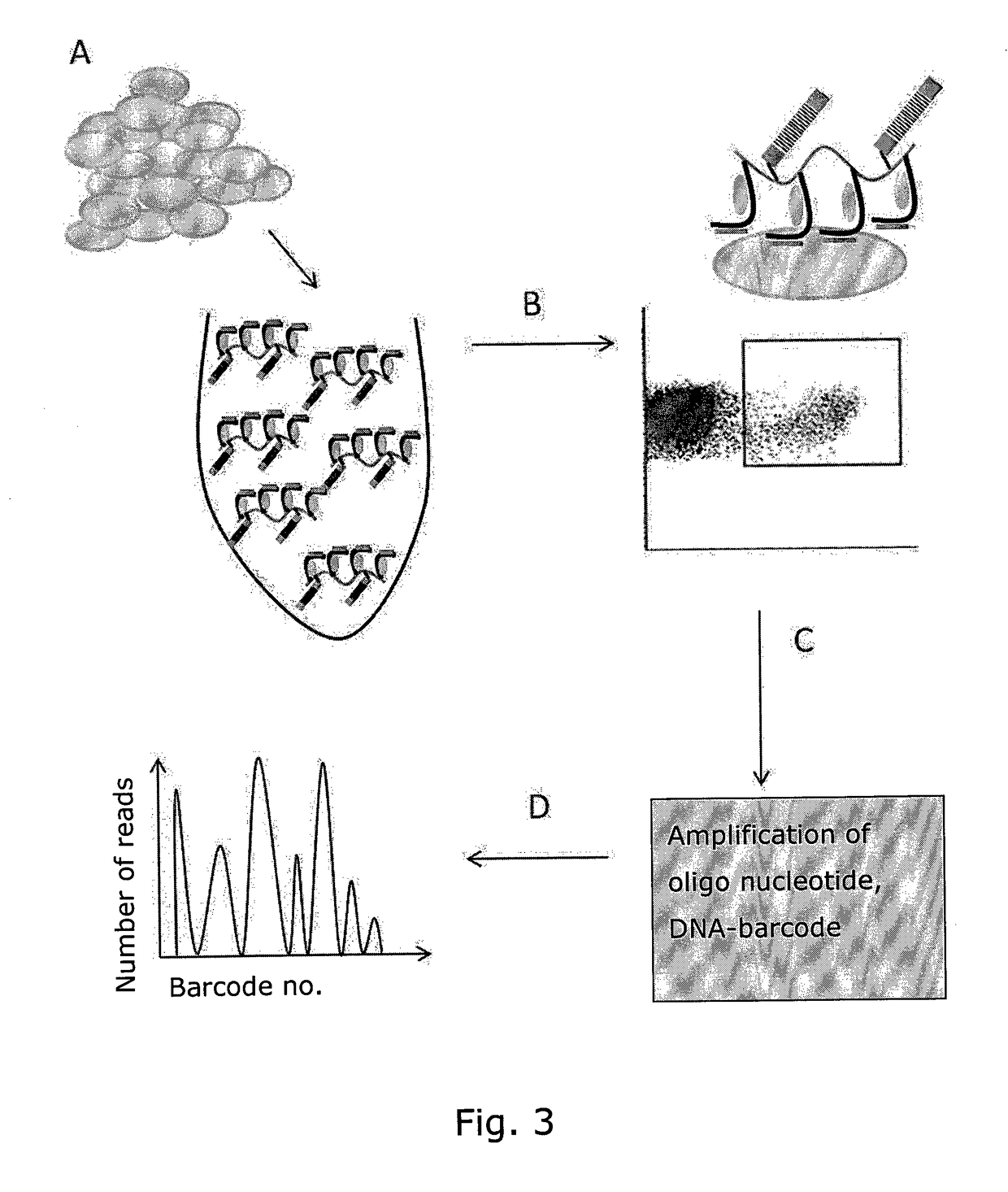
The present invention describes the use of nucleic acid barcodes as specific labels for MHC multimers to determine the antigen responsiveness in biological samples. After cellular selection the barcode sequence will be revealed by sequencing. This technology allows for detection of multiple (potentially >1000) different antigen-specific cells in a single sample. The technology can be used for T-cell epitope mapping, immune-recognition discovery, diagnostics tests and measuring immune reactivity after vaccination or immune-related therapies.
Owner:IMMUDEX APS +1
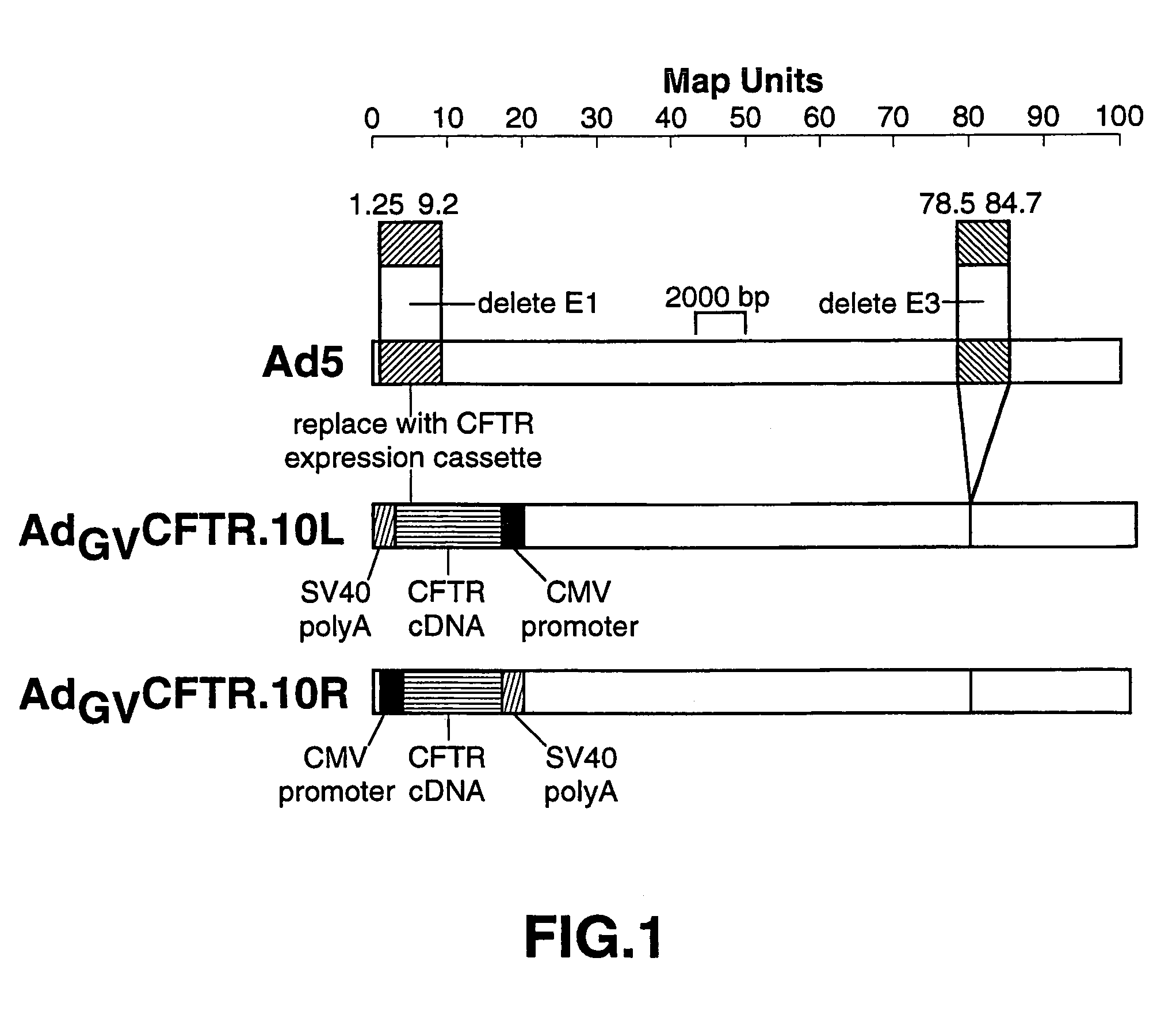
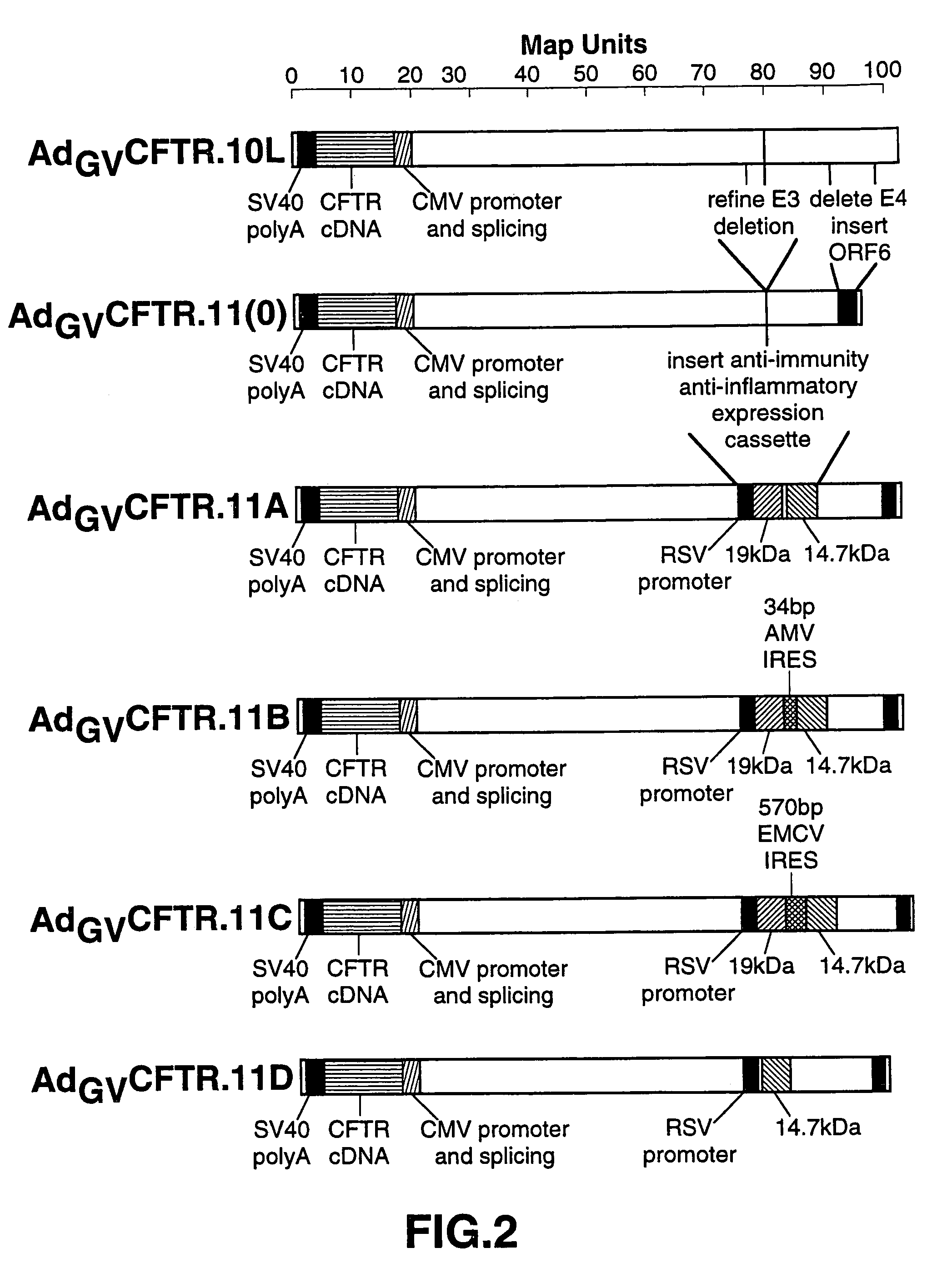
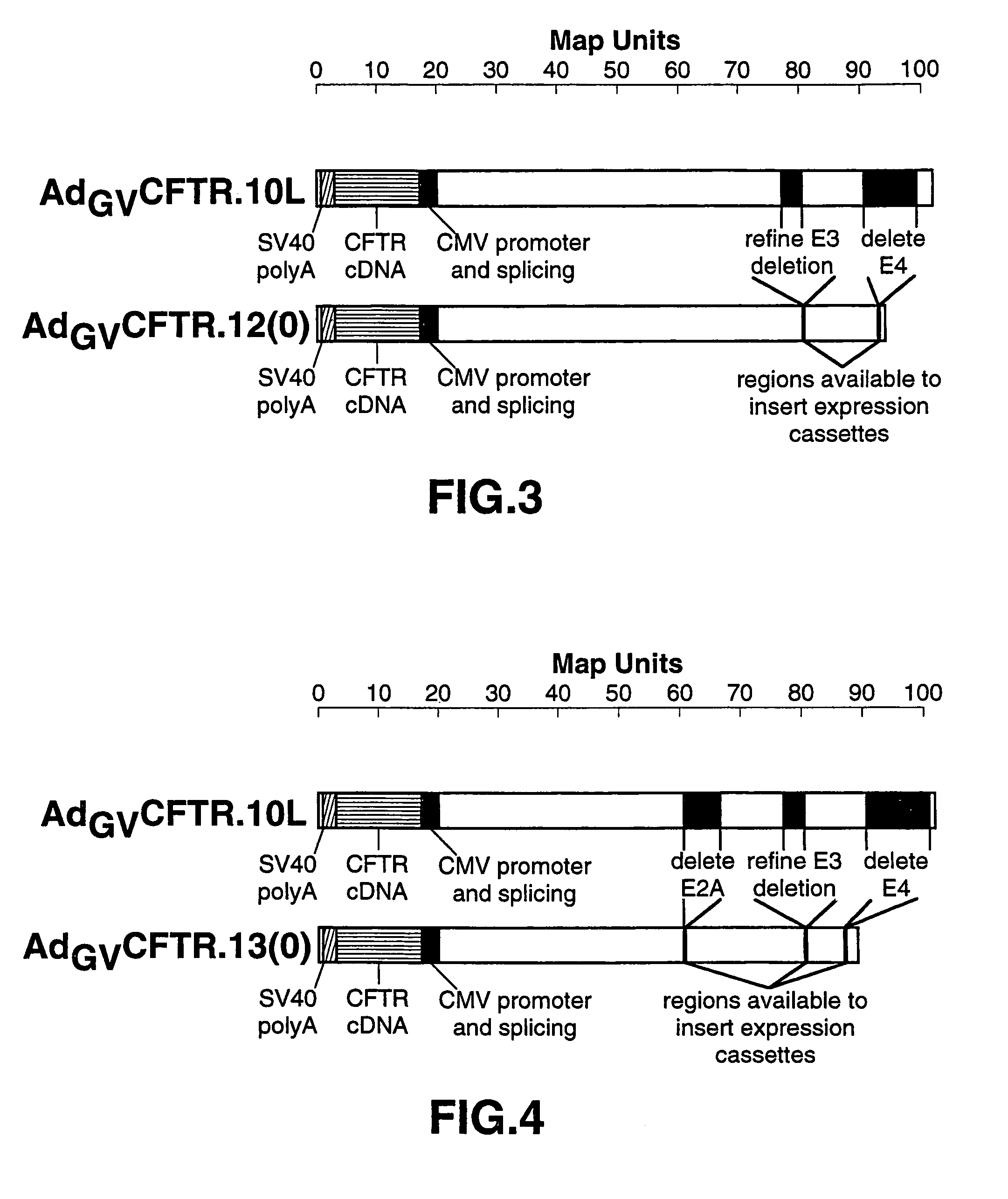
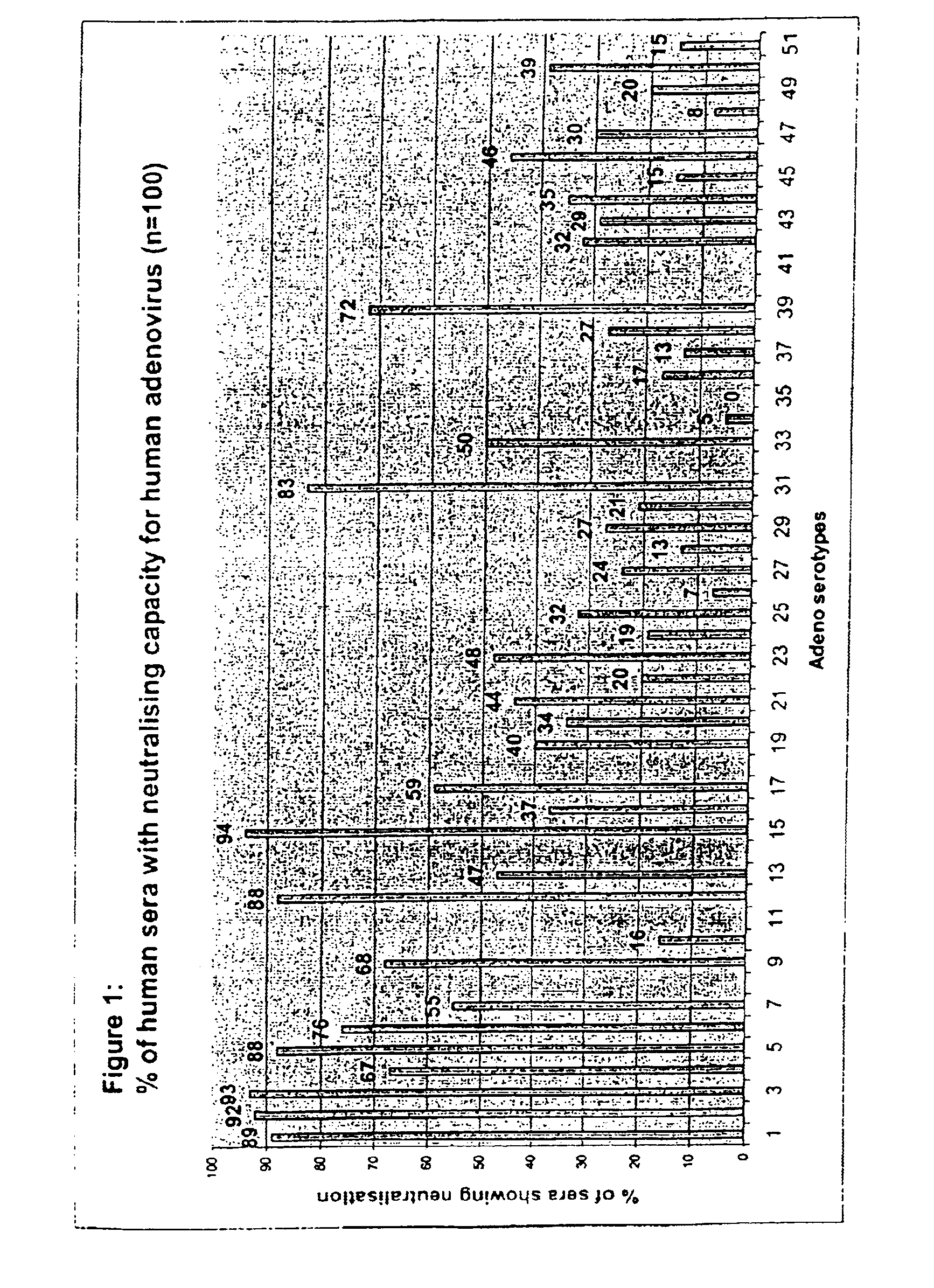
Complementary adenoviral vector systems and cell lines
The present invention provides multiply deficient adenoviral vectors and complementing cell lines. Also provided are recombinants of the multiply deficient adenoviral vectors and a therapeutic method, particularly relating to gene therapy, vaccination, and the like, involving the use of such recombinants.
Owner:GEN VEC INC
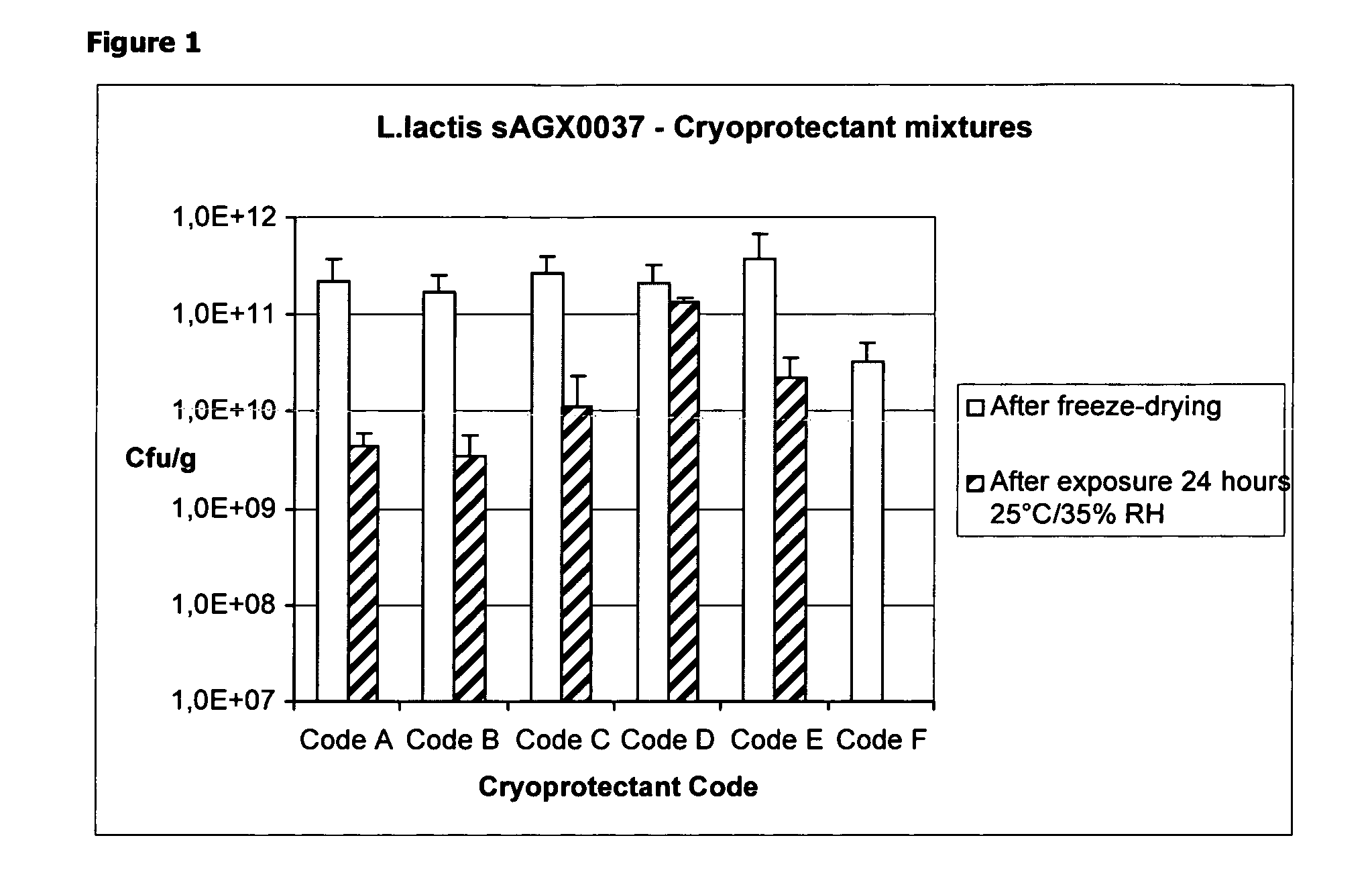
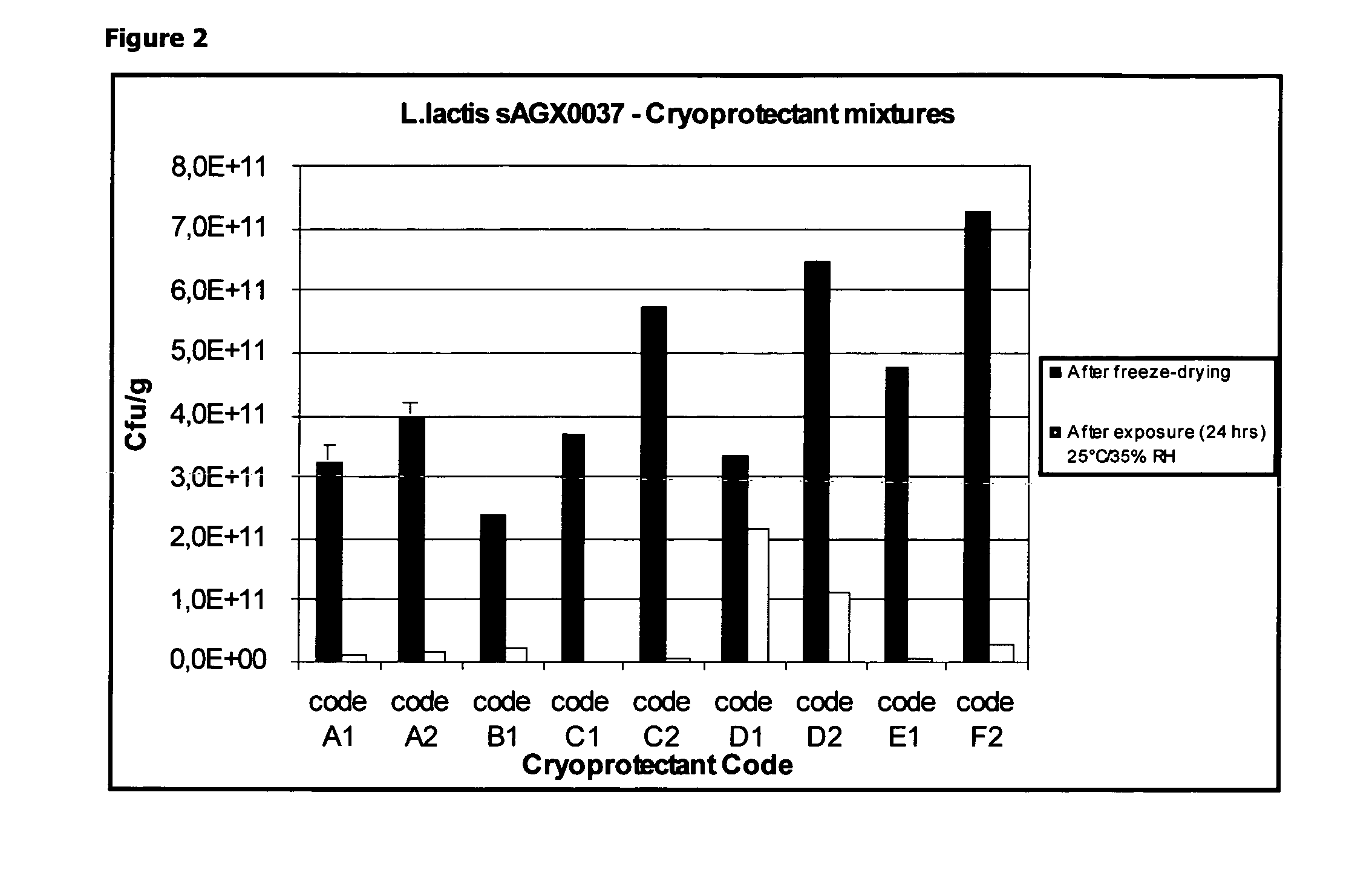
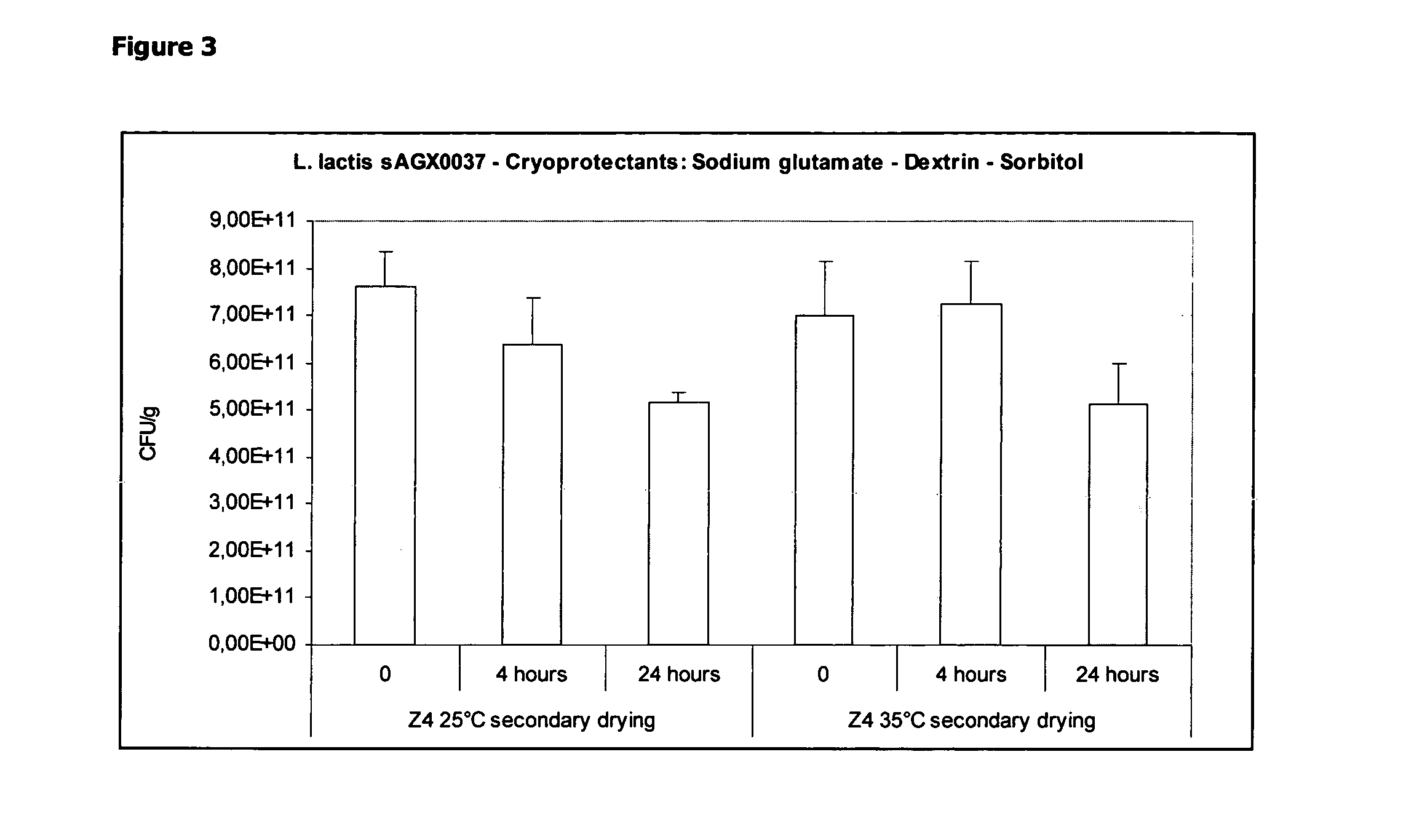
Cryoprotectants for freeze drying of lactic acid bacteria
ActiveUS20120039853A1Improve survivabilityImprove textureBiocideMilk preparationBacteroidesVaccination
The present invention comprises the discovery and development of an effective cryoprotectant composition, without containing skim milk or any other animal-derived compounds, to achieve long-term stability of freeze-dried lactic acid bacteria (LAB), at different temperatures, whereby the retention of viability of the freeze-dried LAB after 6 months of storage, preferably after 9 months of storage, more preferably after 12 months of storage is more than 50%. The invention is in the field of producing freeze dried bacteria, in particular Lactic acid bacteria. More in particular, the invention relates to the use of a novel combination of cryoprotectants for increasing the viability of bacteria after freeze drying, improving the texture of the lyofilized cake for easy grinding and improving the long term stability of the freeze dried bacteria at different temperature conditions. The invention further relates to such freeze dried bacteria for use in food industry or in human or animal health applications. More in particular, the invention relates to the increased viability and long-term storage of recombinant bacteria capable of expressing heterologous proteins or peptides and administered to humans or animals for therapeutic or vaccination purposes.
Owner:INTREXON ACTOBIOTICS NV
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
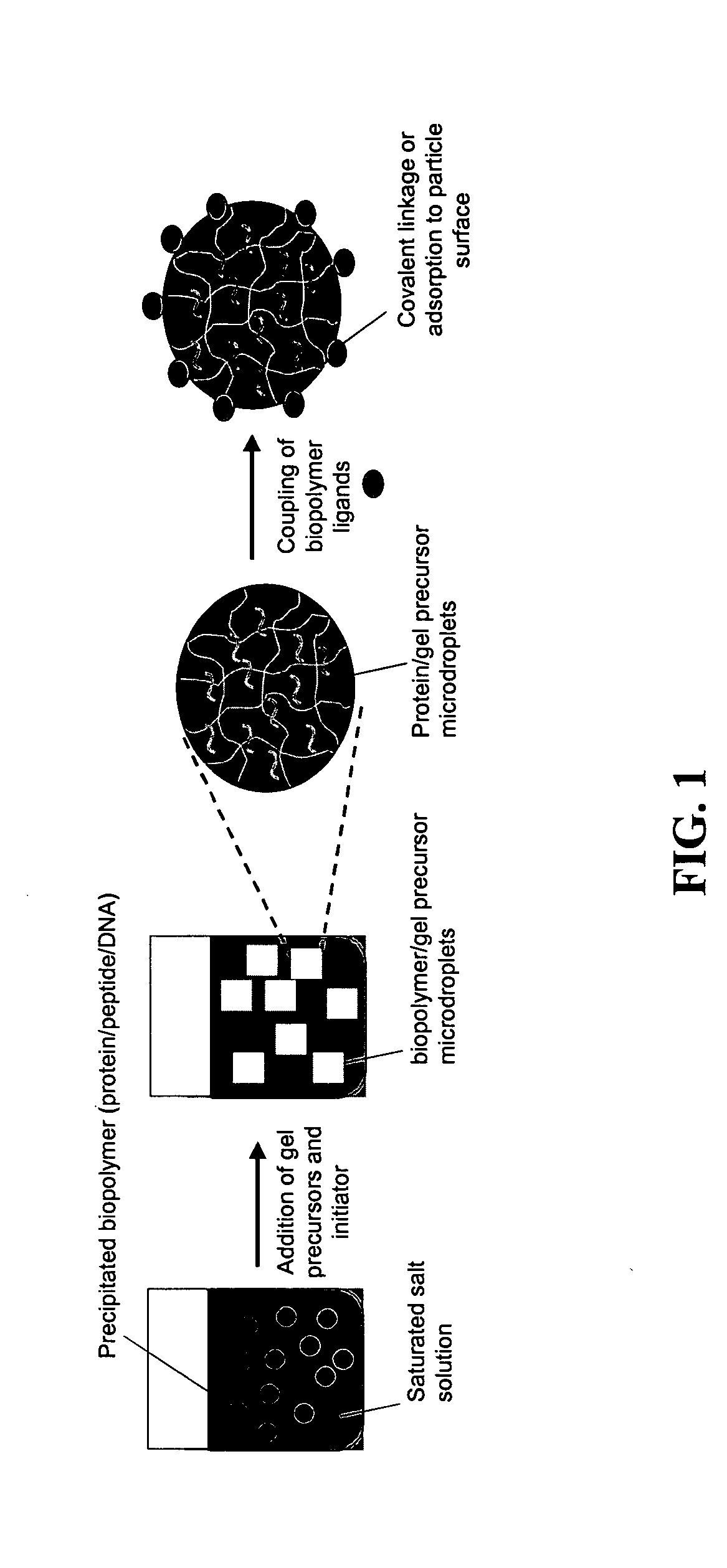
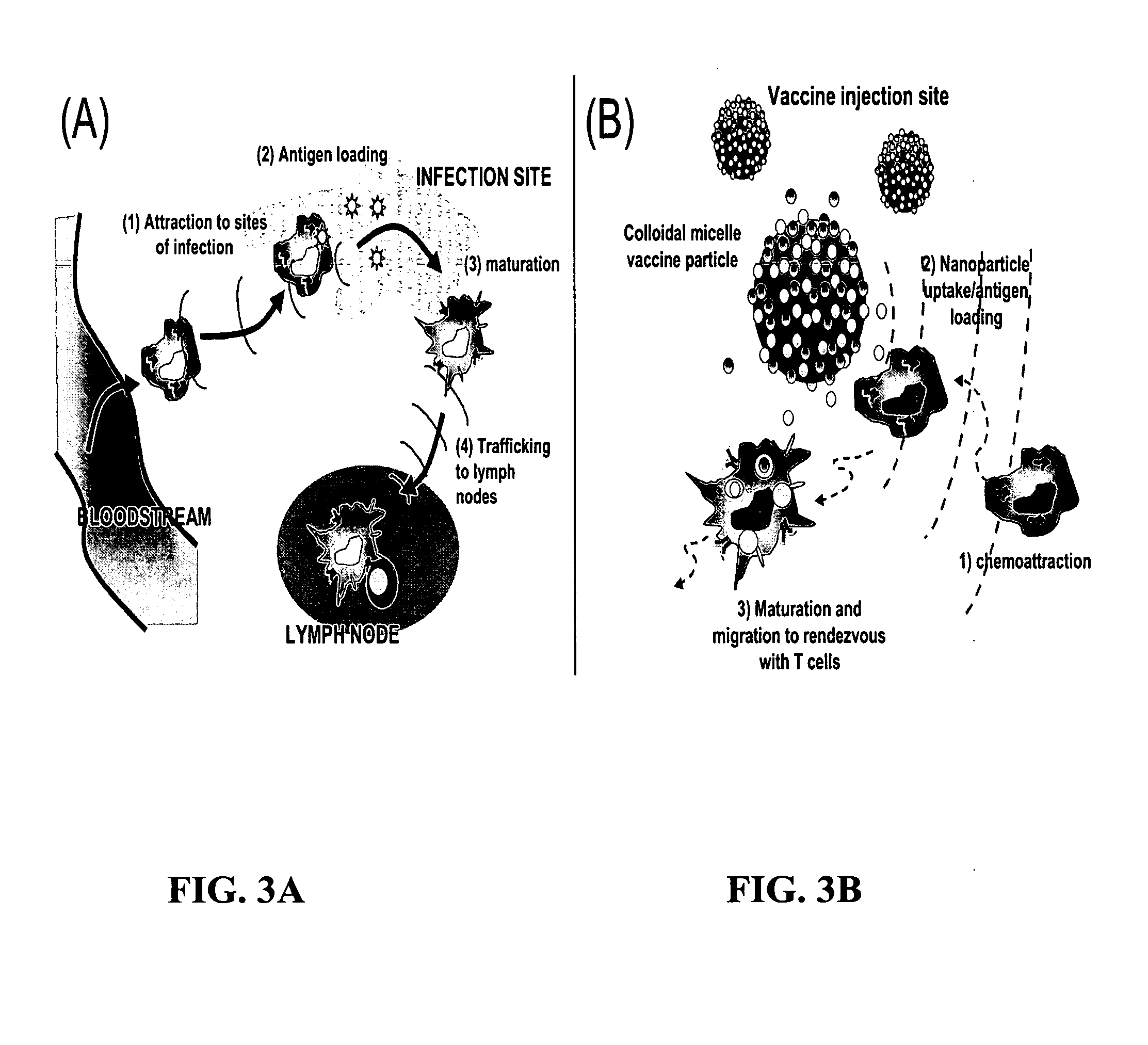
Programmed immune responses using a vaccination node
The present invention provides compositions and methods for modulating immune responses to antigens. One aspect of the present invention relates to a particle-based antigen delivery system (vaccination node) that comprises a hydrogel particle capable of both antigen presentation and DC activation. The VN may further comprise a chemoattractant-loaded microsphere capable of attracting DCs to the site of administration. Another aspect of the present invention relates to the use of the VN to modulate antigen presenting cells activation for the prevention and / treatment of various diseases, such as infectious diseases, cancers and autoimmune diseases.
Owner:VAXDESIGN
DNA transfection system for the generation of infectious influenza virus
InactiveUS20050186563A1Improve effectivenessElicit protective immunitySsRNA viruses negative-senseFungiDual promoterSingle-Stranded RNA
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuraminidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
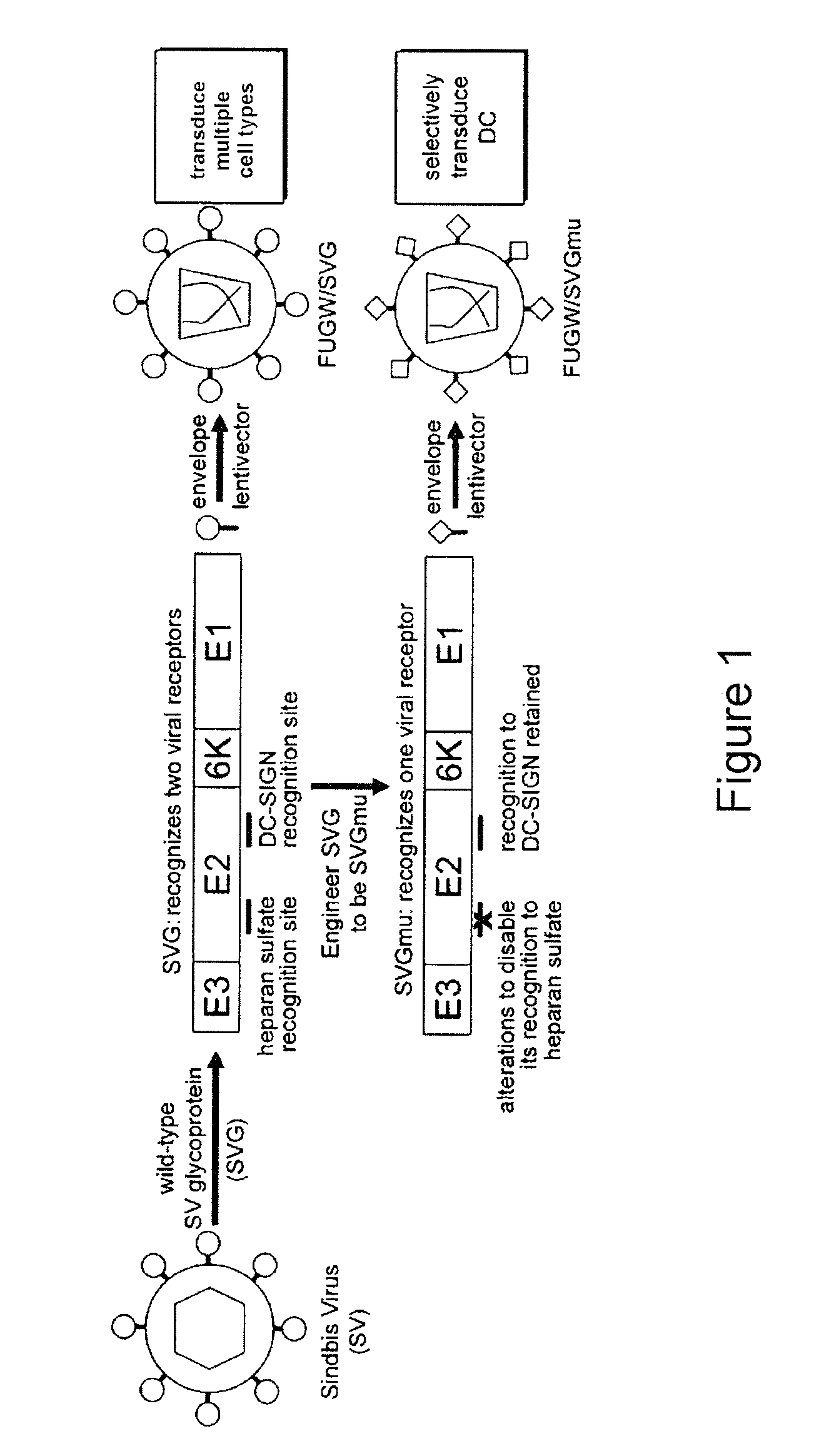
Targeted gene delivery for dendritic cell vaccination
Methods and compositions are provided for delivery of a polynucleotide encoding a gene of interest, typically an antigen, to a dendritic cell (DC). The virus envelope comprises a DC-SIGN specific targeting molecule. The methods and related compositions can be used to treat patients suffering from a wide range of conditions, including infection, such as HIV / AIDS, and various types of cancers.
Owner:CALIFORNIA INST OF TECH
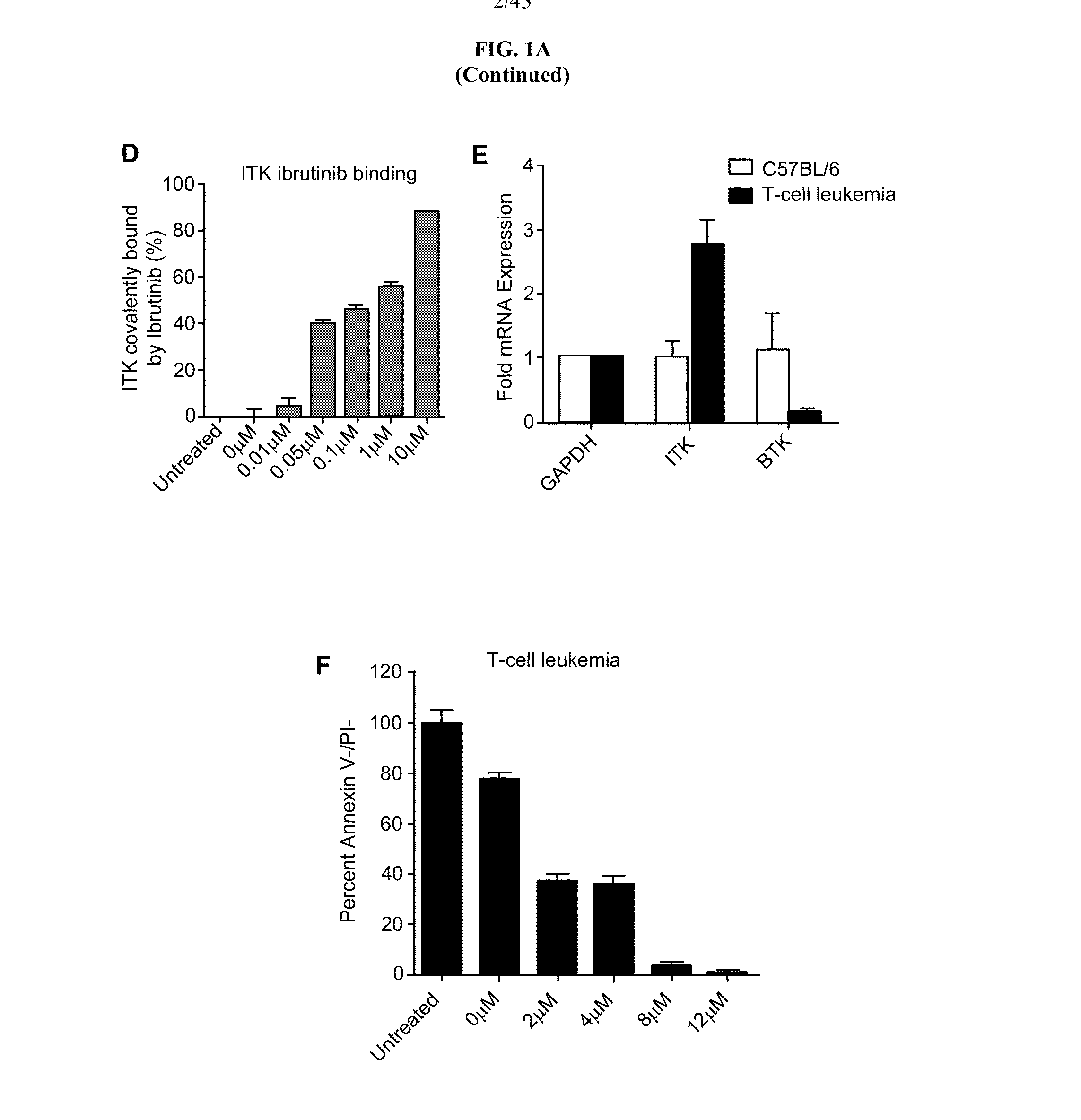
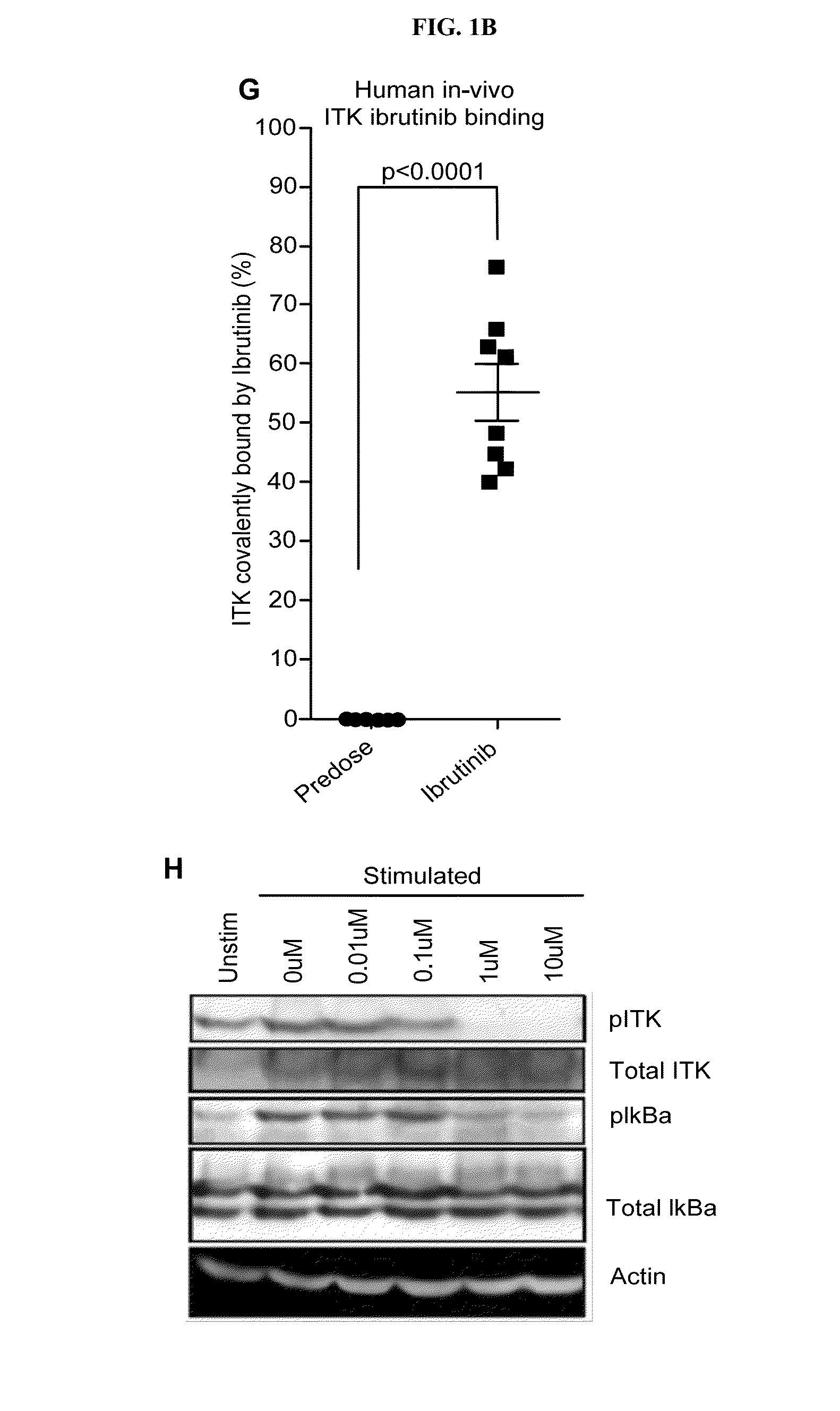
Tec family kinase inhibitor adjuvant therapy
Described herein are methods and compositions comprising a covalent TEC family kinase inhibitor for use in adjuvant therapy, including adjuvant cancer therapy, vaccination and treatment of immune disorders and pathogenic infections.
Owner:PHARMACYCLICS
Method for detection and quantification of T-cell receptor Vbeta repertoire
ActiveUS7375211B2Rapid determinationEasily detecting clonalityAnalysis using chemical indicatorsSugar derivativesVaccinationAntigen stimulation
The invention is a method for detecting and measuring T-cell receptor (TCR) repertoires from mammalian lymphocytes. The method is based on the use of the multiple sets of unique primers to amplify 22 regions of the TCR Vβ region and thereby detect clonal expansions related to antigen stimulation of the immune system. Kits containing sets of primers and specialized analytical statistical software for use in determining clonal expansion in humans and mice are disclosed. The reliability, efficiency and short assay time in using the method is well suited to monitoring immune response to vaccination and therapeutic treatments for immune disorders.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
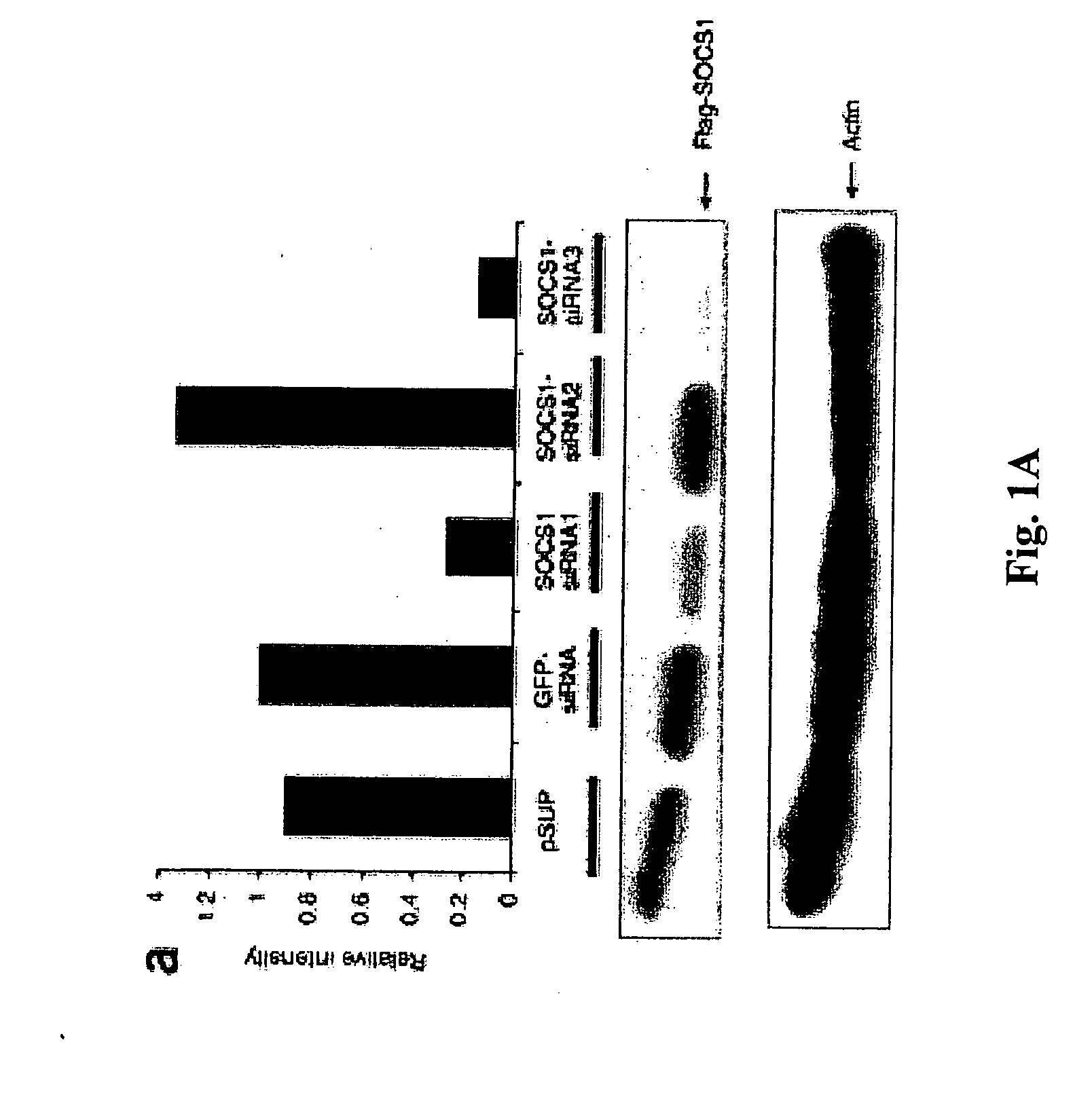
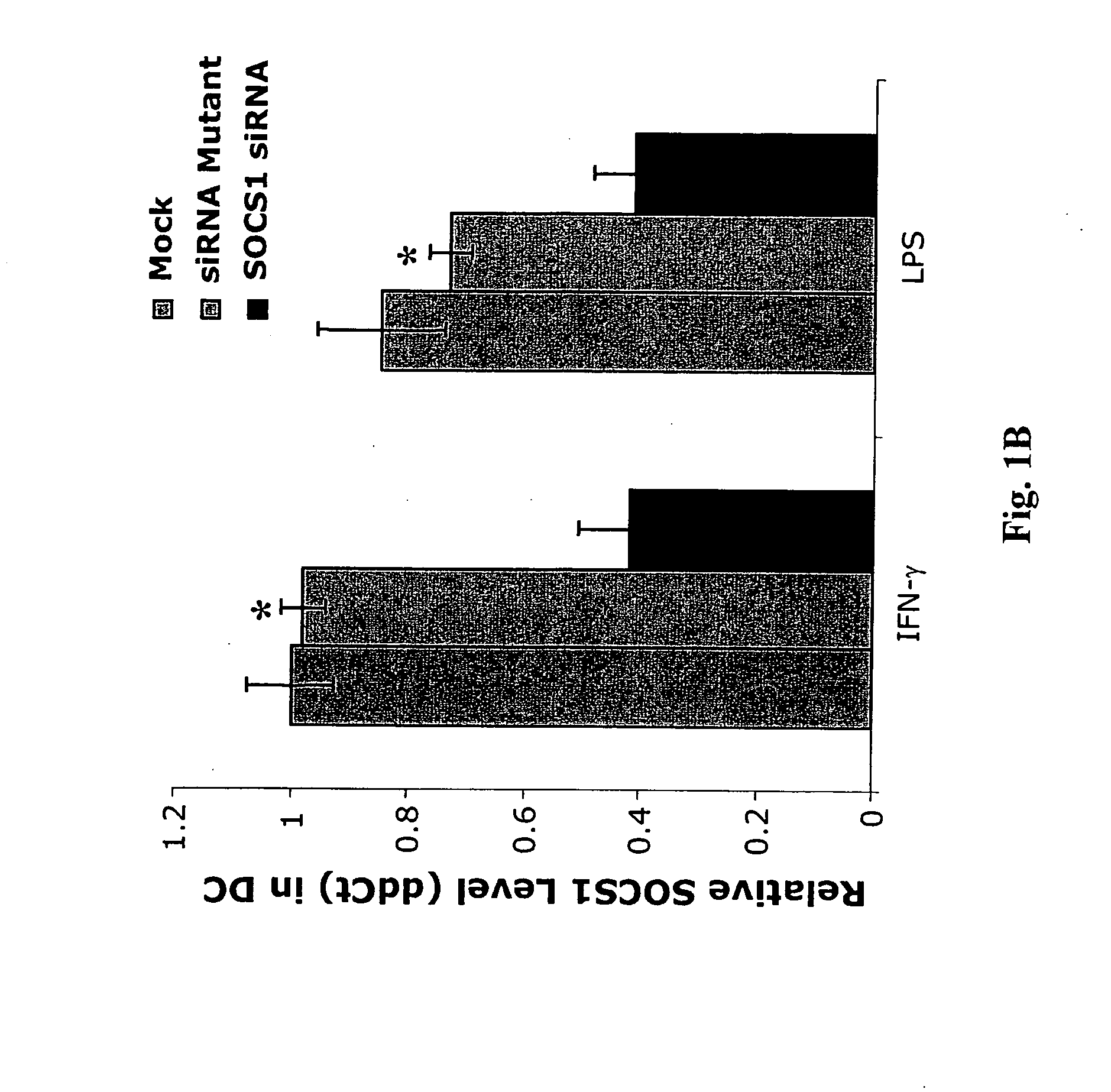
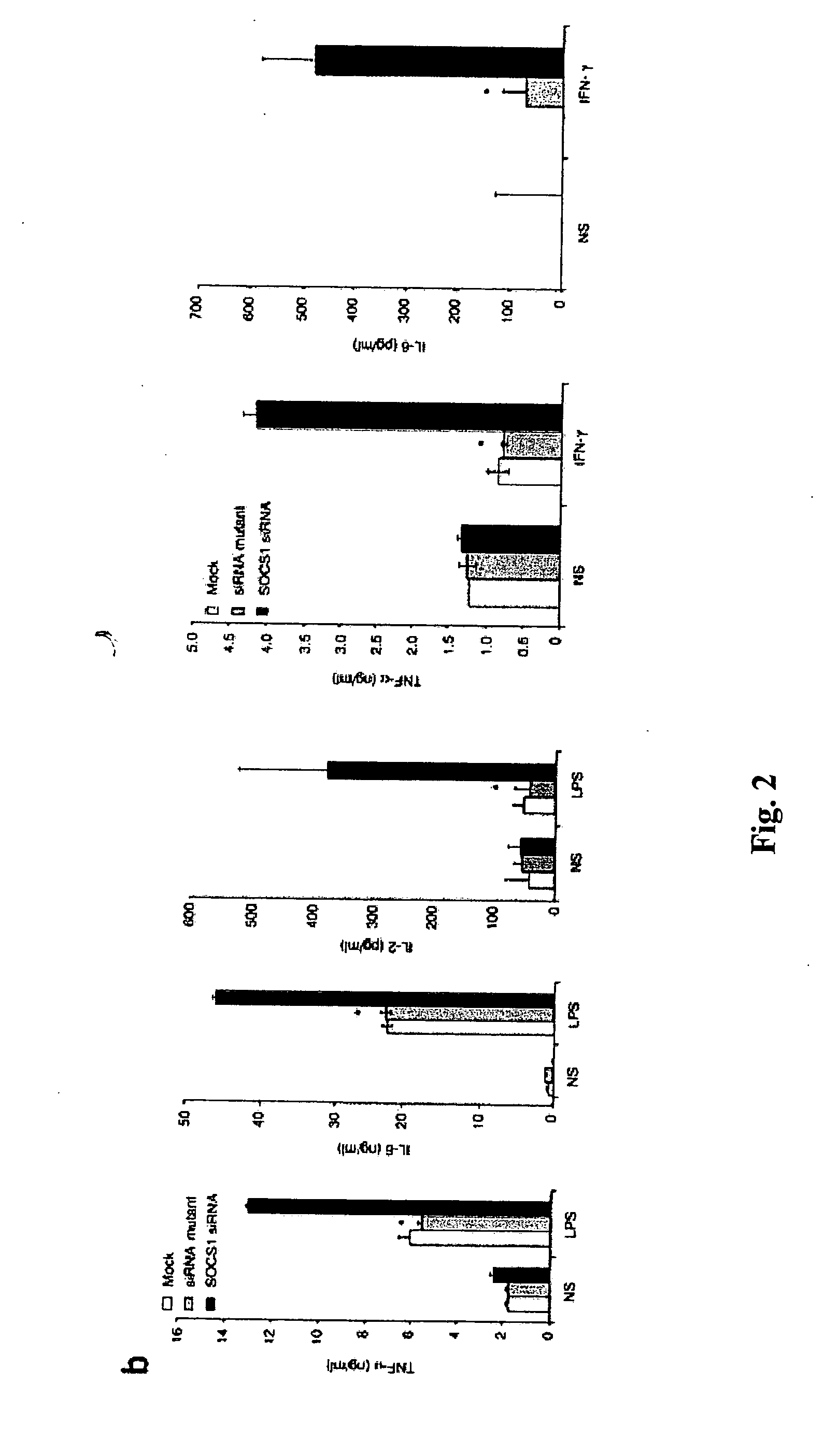
Modulation of negative immune regulators and applications for immunotherapy
ActiveUS20060292119A1Easy to integrateBiocideSsRNA viruses positive-senseVaccinationImmunocompetence
The invention includes compositions and methods for enhancing immunopotency of an immune cell by way of inhibiting a negative immune regulator in the cell. The present invention provides vaccines and therapies in which antigen presentation is enhanced through inhibition of negative immune regulators. The present invention also provides a mechanism to break self tolerance in tumor vaccination methods that rely on presentation of self tumor antigens.
Owner:BAYLOR COLLEGE OF MEDICINE
Methods and compositions for increased priming of t-cells through cross-presentation of exogenous antigens
InactiveUS20080171059A1Easy to demonstrateEffective vaccineTissue cultureCancer antigen ingredientsDiseaseVaccination
Methods for eliciting in an animal in need thereof a cell-mediated immune response specific to an antigen, the method comprising providing an antigen preparation comprising particles on the surface of which the antigen is attached, and administering the antigen preparation to the animal, wherein the particles are taken up by antigen presenting cells (APC) of the animal via phagocytosis, forming a phagosome inside the APC, wherein the antigen is attached to the surface of the particle in such a way that the antigen is released in the phagosome before the phagosome fuses with a late endosome or a lysosome, and wherein the antigen is cross-presented on a Class I MHC molecule. Also provided are particulate antigen preparations or particulate vaccines that can be delivered to an animal in need thereof for vaccination against, for preventing or treating, a disease related to the antigen, such as cancer and a viral infection.
Owner:LUDWIG INST FOR CANCER RES +1
Vaccination by topical application of recombinant vectors
InactiveUS20030045492A1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseGenetic material ingredientsGene deliveryVaccination
The present invention relates to techniques of skin-targeted non-invasive gene delivery to elicit immune responses and uses thereof. The invention further relates to methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal following topical application of vectors, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal as well as such a method further including disposing the vector in and / or on the delivery device. The vector can be gram negative bacteria, preferably Salmonella and most preferably Salmonella typhimurium.
Owner:UAB RES FOUND
Recombinant vaccine against botulinum neurotoxin
InactiveUS7081529B2Fast and efficient purificationBacterial antigen ingredientsBacteriaVaccinationRecombinant vaccines
This invention is directed to preparation and expression of synthetic genes encoding polypeptides containing protective epitopes of botulinum neurotoxin (BoNT). The invention is also directed to production of immunogenic peptides encoded by the synthetic genes, as weel as recovery and purification of the immunogenic peptides from recombinant organisms. The invention is also directed to methods of vaccination against botulism using the expressed peptides.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Methods and compositions for the targeting of a systemic immune response to specific organs or tissues
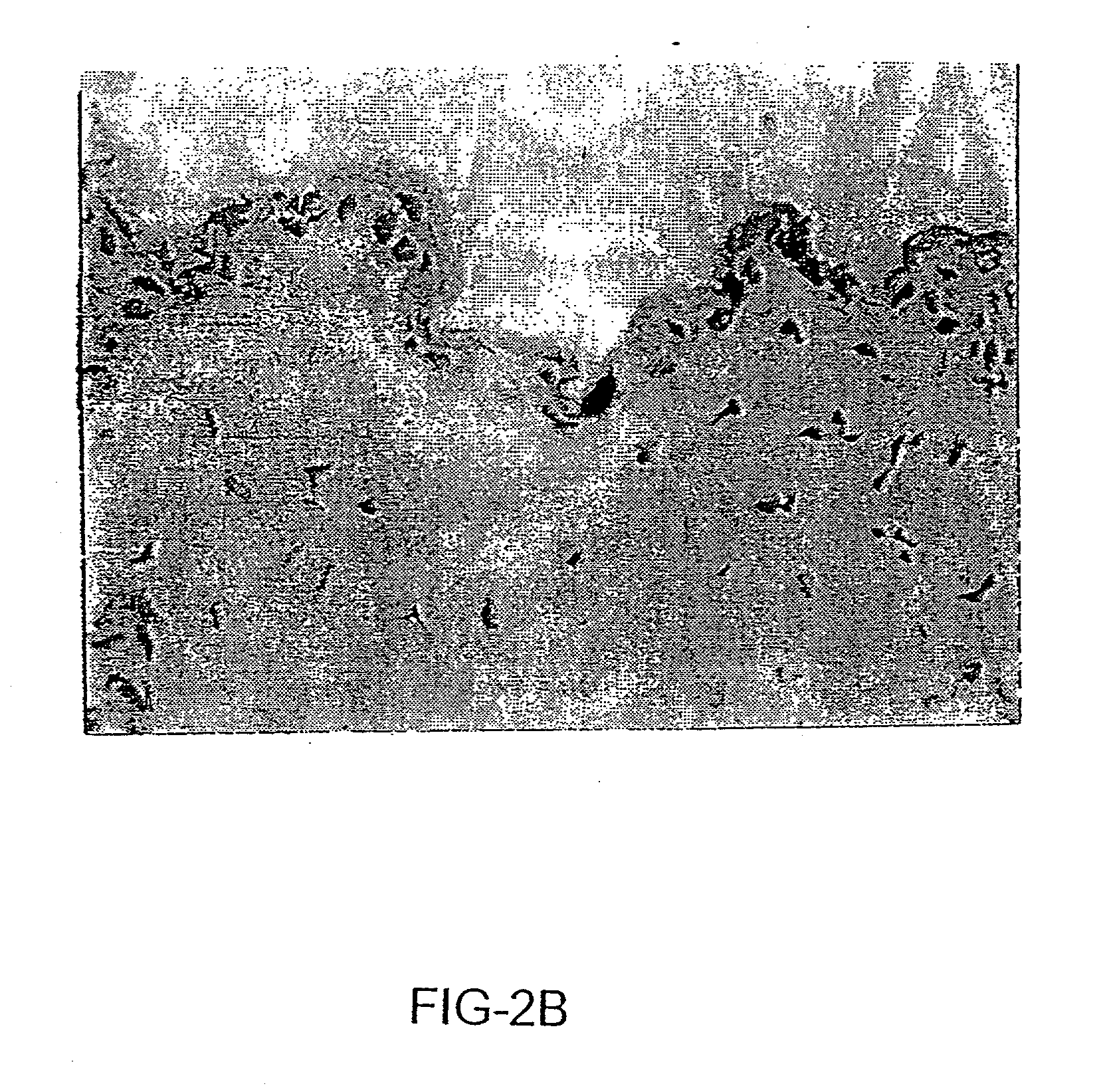
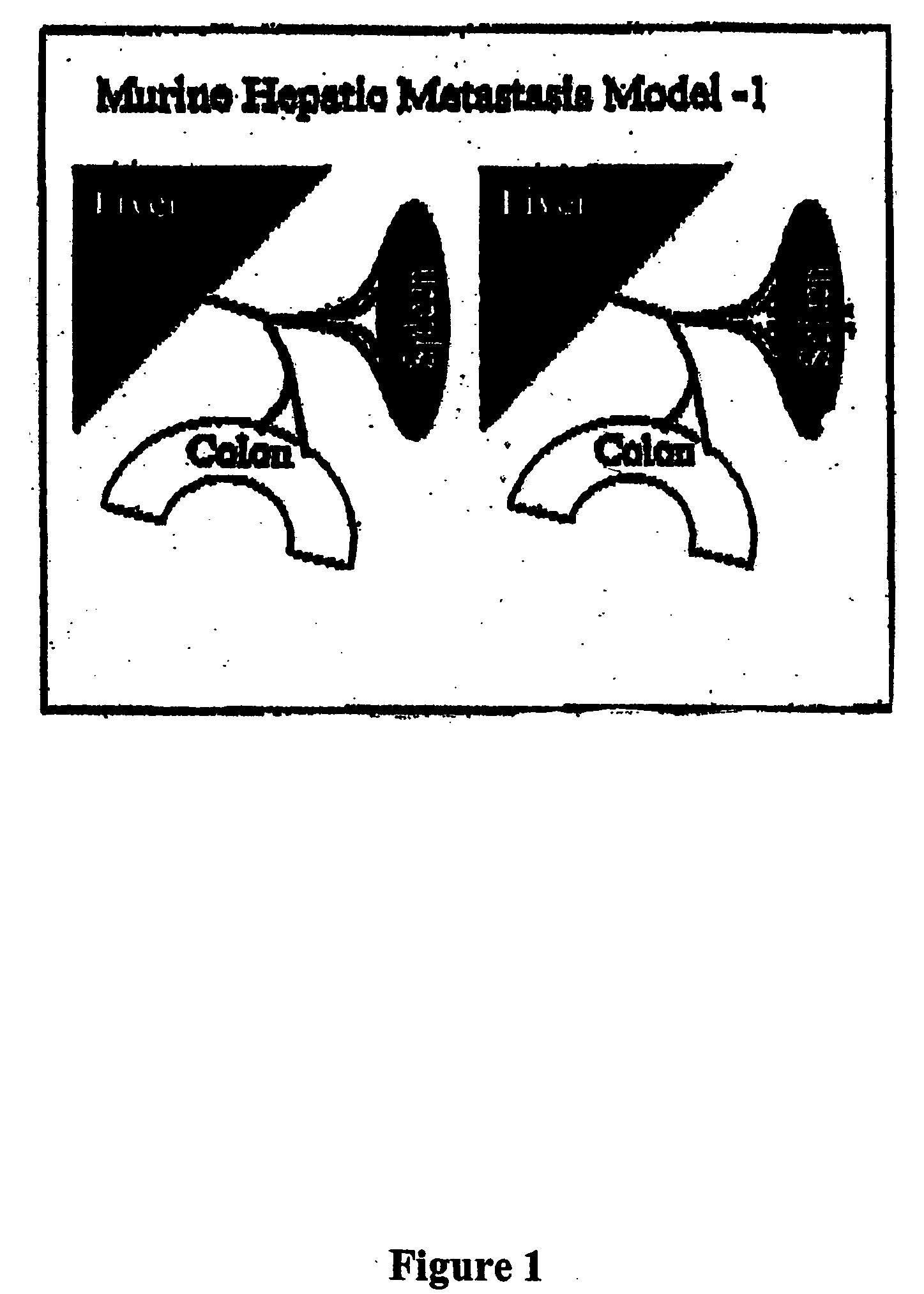
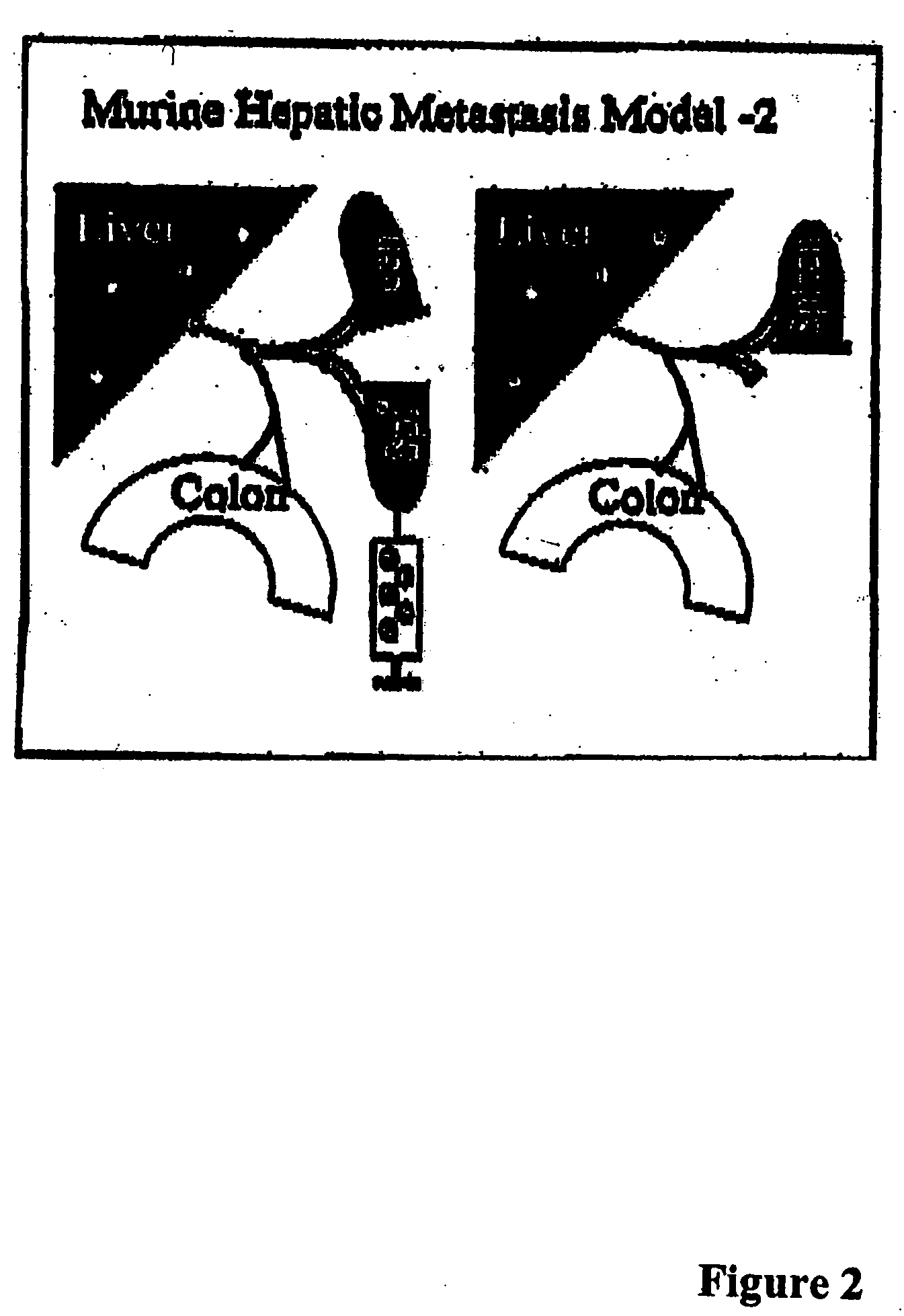
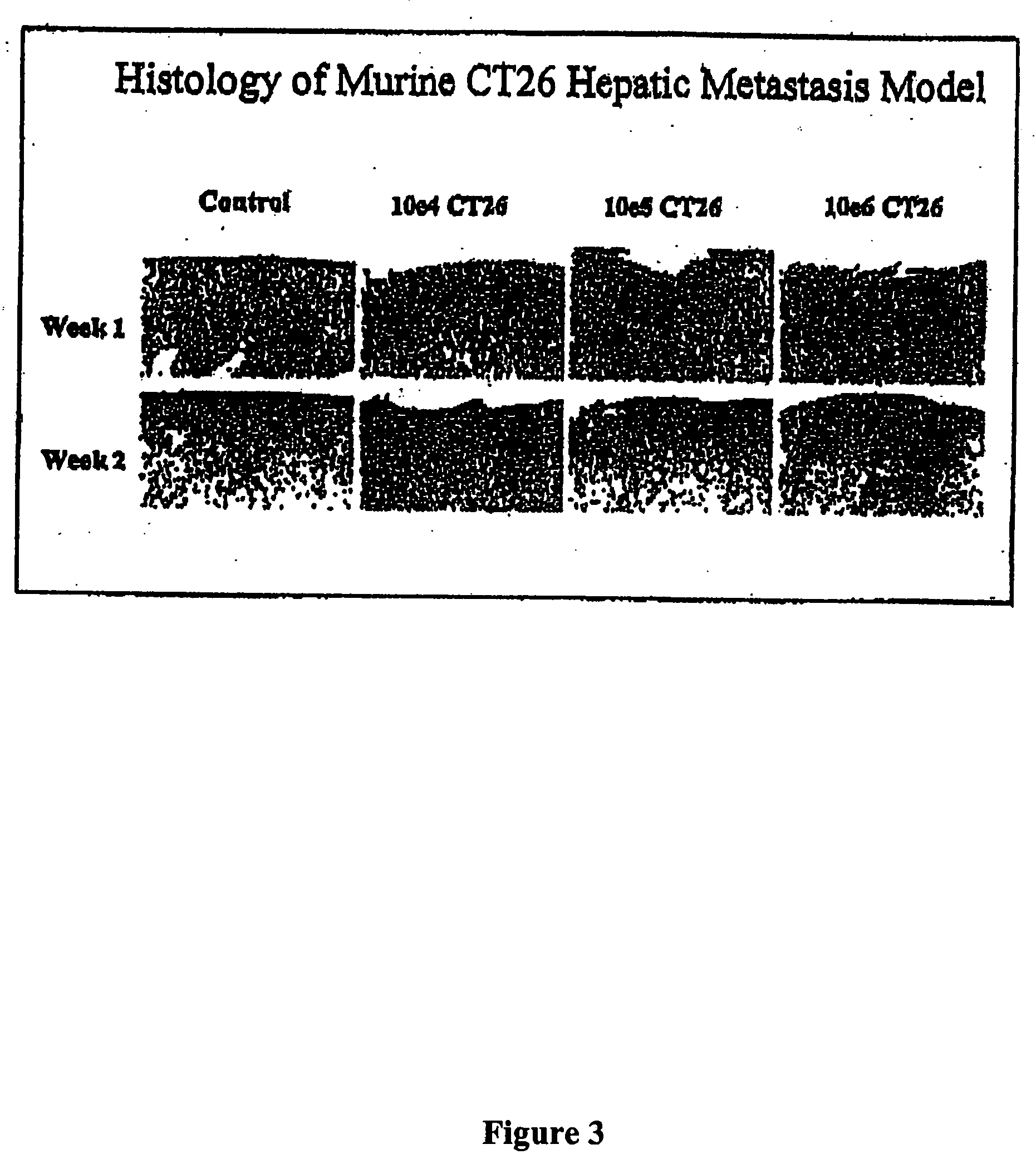
The invention provides methods and compositions for targeting a separately generated immune response to a specific organ or tissue, e.g. one affected by cancer, using one or more agents with a tropism for the organ or tissue or that can be specifically localized to the desired organ or tissue. For example, the invention provides methods ands compositions for treating liver metastases from colorectal cancer using a combination of a granulocyte / macrophage colony stimulating factor (GM-CSF) augmented tumor cell vaccination and Listeria monocytogenes (LM) infection.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Special microorganism composite bacterial agent for directly decomposing and fermenting crops straws to generate marsh gas and application method thereof
InactiveCN101775359AGood degradation activityEasy to storeFungiBacteriaBacillus licheniformisVaccination
The invention discloses a special microorganism composite bacterial agent for directly decomposing and fermenting crops straws to generate marsh gas and application method thereof. The special microorganism composite bacterial agent is liquid or solid, and is formed by fermenting, culturing, combining and compositing one or a plurality of bacillus cereus, bacillus subtilis, bacillus licheniformis, bacillus megaterium, bacillus mucilaginosus, bacillus circulans, bacillus sphaerieus, clostridium sporogenes, clostridium acetobutylicum, clostridium barati, clostridium beijerinckii, clostridium thermocellum, trichoderma viride, trichodermareesei and aspergillus niger. Simultaneously, by taking the special microorganism composite bacterial agent as a vaccination bacterial agent, the invention provides an application method for decomposing the crops straws and accelerating the ferment to generate marsh gas; and the microorganism composite bacterial agent-treated crops straws have fast ferment speed, large marsh gas generation quantity, low treating cost and no secondary pollution, can be fermented to generate the marsh gas under the condition of no faeces of poultry, and have very wide application range.
Owner:刘相梅 +1
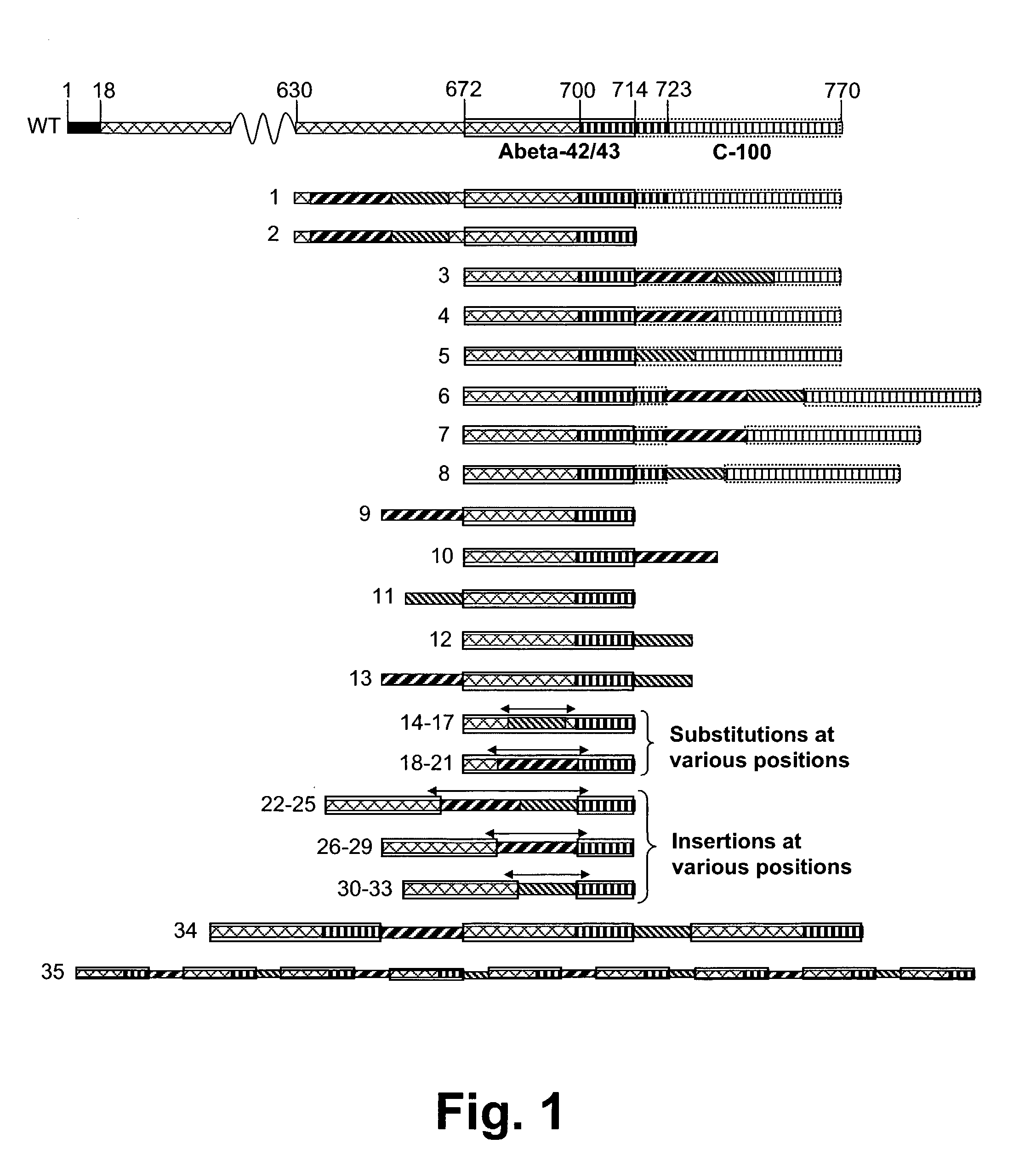
Novel method for down-regulation of amyloid
Disclosed are novel methods for combatting diseases characterized by deposition of amyloid. The methods generally rely on immunization against amyloid precursor protien (APP) or beta amyloid (Abeta). Immunization is preferably effected by administration of analogues of autologous APP or Abeta, said analogues being capable of inducing antibody production against the autologous amyloidogenic polypeptides. Especially preferred as an immunogen is autologous Abeta which has been modified by introduction of one single or a few foreign, immunodominant and promiscuous T-cell epitopes. Also disclosed are nucleic acid vaccination against APP or Abeta and vaccination using live vaccines as well as methods and means useful for the vaccination. Such methods and means include methods for the preparation of analogues and pharmaceutical formulations, as well as nucleic acid fragments, vectors, transformed cells, polypeptides and pharmaceutical formulations.
Owner:H LUNDBECK AS
Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens and Uses Thereof
ActiveUS20150202309A1Excellent characteristicsHigh yieldAntibacterial agentsMedical devicesStreptococcus pneumoniaeVaccination
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates) and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR®, SYNFLORIX® and / or PREVNAR 13®. The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
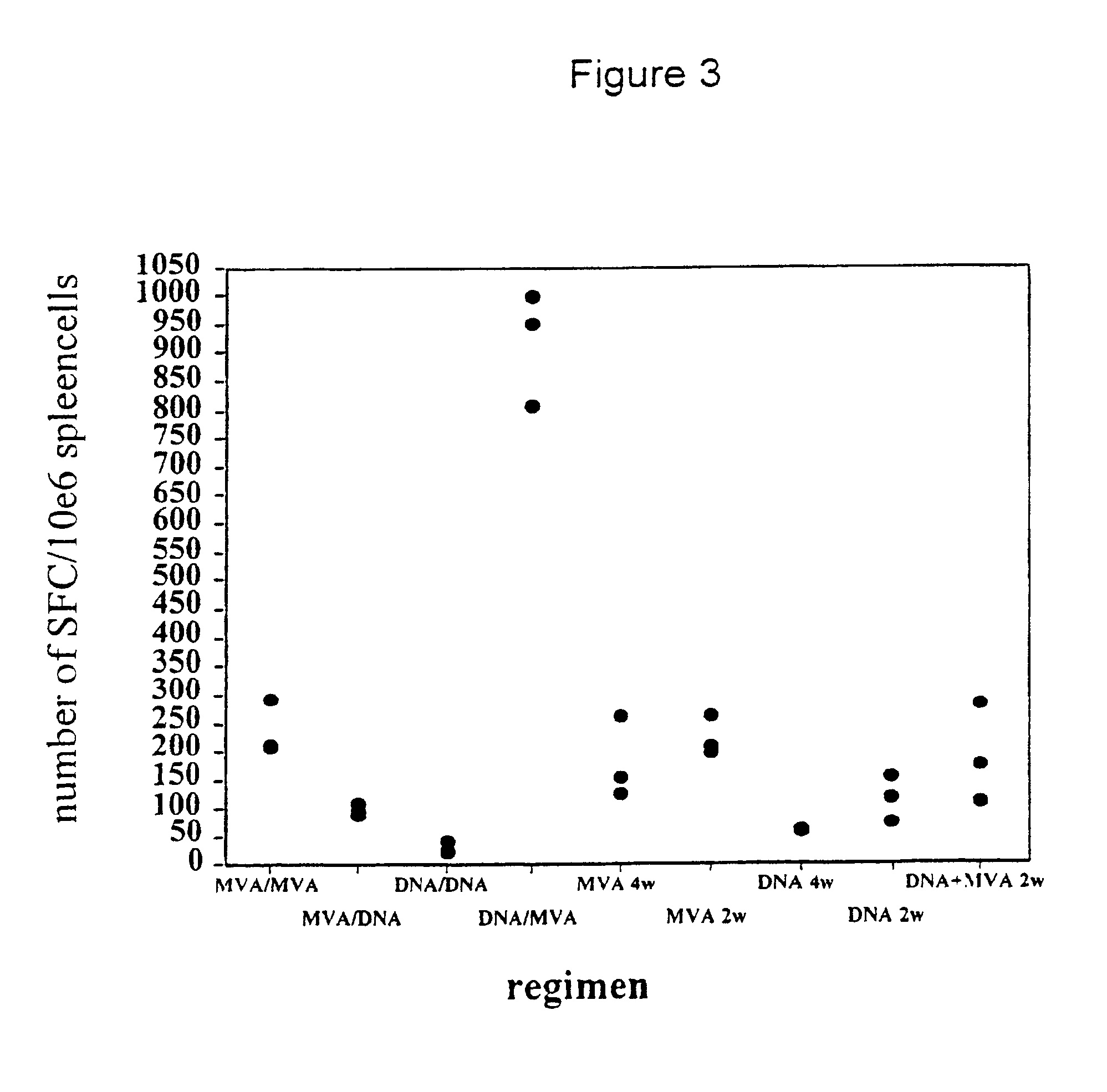
Methods and reagents for vaccination which generate a CD8 T cell immune response
InactiveUS6663871B1Increase boost effectGood effectVirusesPeptide/protein ingredientsAntigenVaccination
New methods and reagents for vaccination are described which generate a CD8 T cell immune response against malarial and other antigens such as viral and tumour antigens. Novel vaccination regimes are described which employ a priming composition and a boosting composition, the boosting composition comprising a non-replicating or replication-impaired pox virus vector carrying at least one CD8 T cell epitope which is also present in the priming composition.
Owner:OXXON THERAPEUTICS LTD
MHC Multimers in Tuberculosis Diagnostics, Vaccine and Therapeutics
The present invention relates to MHC-peptide complexes and uses thereof in the diagnosis of, treatment of or vaccination against a disease in an individual. More specifically the invention discloses MHC complexes comprising Mycobacterium tuberculosis antigenic peptides and uses there of.
Owner:AGILENT TECH INC
Novel strategies for delivery of active agents using micelles and particles
InactiveUS20100062968A1Reduce chanceRapid responseAntibacterial agentsOrganic active ingredientsVaccinationActive agent
The present invention provides biodegradable particles (e.g., three-dimensional particles) and micelles which can be used to encapsulate active agents for delivering to a subject. The present invention further provides methods for producing and delivering such particles and micelles. Additionally, the invention provides vaccination strategies that encompass the use of the novel particles and micelles.
Owner:EMORY UNIVERSITY +2
Treating viral infection at smallpox vaccination site
InactiveUS7288265B1Useful in treatmentAvoid infectionAdhesive dressingsAbsorbent padsVaccinationAdhesive
An adhesive patch is provided wherein the patch includes a porous backing having a front side and a back side. The patch also includes a therapeutic formulation located on the front side of the backing. The backing includes a flexible sheet of water insoluble porous material. The therapeutic formulation includes a combination of a antiviral agent useful for treating a viral infection in a mammal (e.g., human), a medicament that relieves topical discomfort, an adhesive, and a solvent. The solvent can preferably include a fragrance.
Owner:LECTEC CORP
Low molecular weight compounds administered together with anti-cancer agents to prevent or treat cancer and pharmaceutical compositions thereof
InactiveUS6762174B1Remarkable effectLow toxicityBiocidePeptide/protein ingredientsVaccinationAnticarcinogen
The present invention relates to peptide-like compounds, e.g. aminocarboxylic acid amide derivatives, and to methods of using same to stimulate cells of the immune system, bone marrow and other organs. The present compounds can be used to enhance vaccination, increase synthesis of and enhance function of blood cell components and enhance anti-neoplastic effects of various agents. The compounds of the invention can be used to produce a variety of further pharmacologic effects.
Owner:DOVETAIL TECH
Inactivated Zika virus vaccine
ActiveCN105749268AEase the epidemicReduce the burden onSsRNA viruses positive-senseViral antigen ingredientsZika virusSide effect
The invention provides an inactivated Zika virus vaccine. The inactivated Zika virus vaccine is obtained by: performing ultrafiltration and concentration on Zika virus liquid after inactivation, centrifuging the concentrated virus liquid by adopting a sucrose density zone, performing ion exchange and concentration sterilization on a centrifugal product to obtain a Zika virus vaccine stock solution, diluting the vaccine stock solution until the total protein content is not more than 20mu g / ml, and adding an adjuvant to obtain a vaccine semi-finished product. The method for preparing the vaccine provided by the invention is simple, convenient and easy to operate, the cost is saved, the produced vaccine is suitable for Asian people, a unit dose of the Zika virus liquid is high in immunogenicity, the content of hybrid protein is low, the side effect after injection is small, and the safety is high, so that the vaccine is suitable for vaccination of fertile women before pregnancy, can avoid newborn Brazil microcephaly caused by infection of Zika virus, and is significant in social value and market efficiency.
Owner:SINOVAC RES & DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com