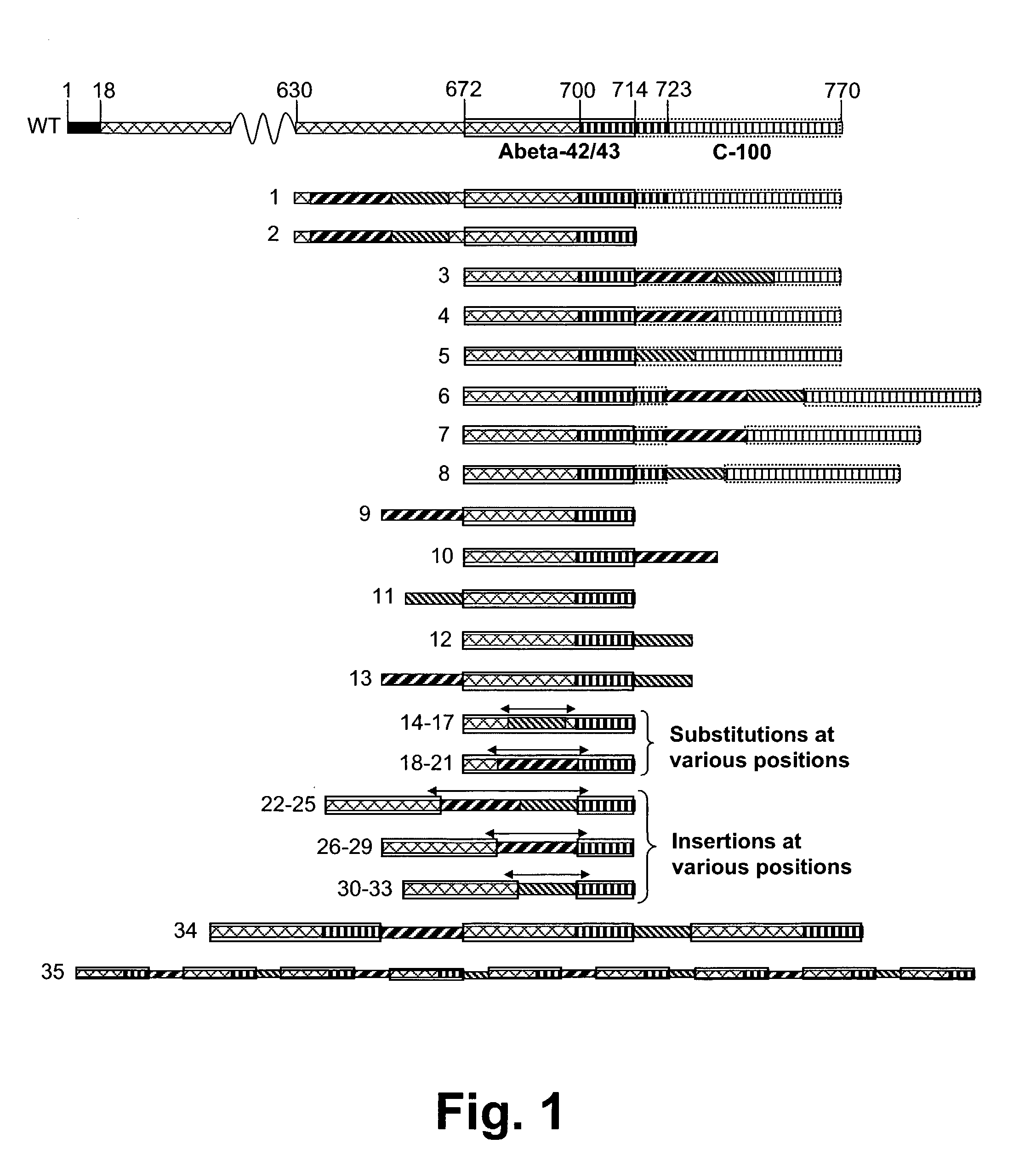
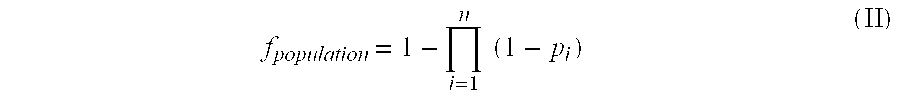Novel method for down-regulation of amyloid
a technology of amyloid and amyloid ligand, which is applied in the field of amyloid downregulation, can solve the problems of losing all reasoning ability, forgetting even simple tasks, and usually fatal diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0271] The Auto Vaccination Approach for Immunizing Against AD
[0272] The fact that A.beta. protein knock out mice does not show any abnormalities or adverse side effects, suggest that removal or lowering the amounts of AP will be safe, Zheng H. (1996).
[0273] Published experiments where transgenic animals are immunized against the transgenic human A.beta. protein suggest that if it was possible to break the self tolerance, down-regulation of AP could be obtained by auto-reactive antibodies. These experiments further suggest that such down regulation of A.beta. potentially would both prevent the formation of plaques, and even clear already formed A.beta. plaques from the brain, cf. Schenk et al. (1999). But, traditionally it is not possible to raise antibodies against self-proteins.
[0274] The published data does thus not provide the means for breaking true self-tolerance towards true self-proteins. Nor does the data provide information on how to ensure that the immune reaction is dire...
example 2
[0297] Immunisation of Transgenic Mice with A.beta. and Modified Proteins According to the Invention
[0298] Construction of the hAB43+-34 encoding DNA. The hAB43+-34 gene was constructed in several steps. First a PCR fragment was generated with primers ME#801 (SEQ ID NO: 10) and ME#802 (SEQ ID NO: 11) using primer ME#800 (SEQ ID NO: 9) as template. ME#800 encodes the human abeta-43 fragment with E. coli optimised codons. ME#801 and 802 adds appropriate restriction sites to the fragment.
[0299] The PCR fragment was purified, digested with NcoI and HindIII, purified again and cloned into NcoI-HindIII digested and purified pET28b+E. coli expression vector. The resulting plasmid encoding wildtype human A.beta.-43 is named pAB1.
[0300] In the next step the T-helper epitope, P2, is added to the C-terminus of the molecule. Primer ME#806 (SEQ ID NO: 12) contains the sequence encoding the P2 epitope, thus generating a fusion of P2 and Abeta-43 by the PCR reaction.
[0301] The cloning was performe...
example 3
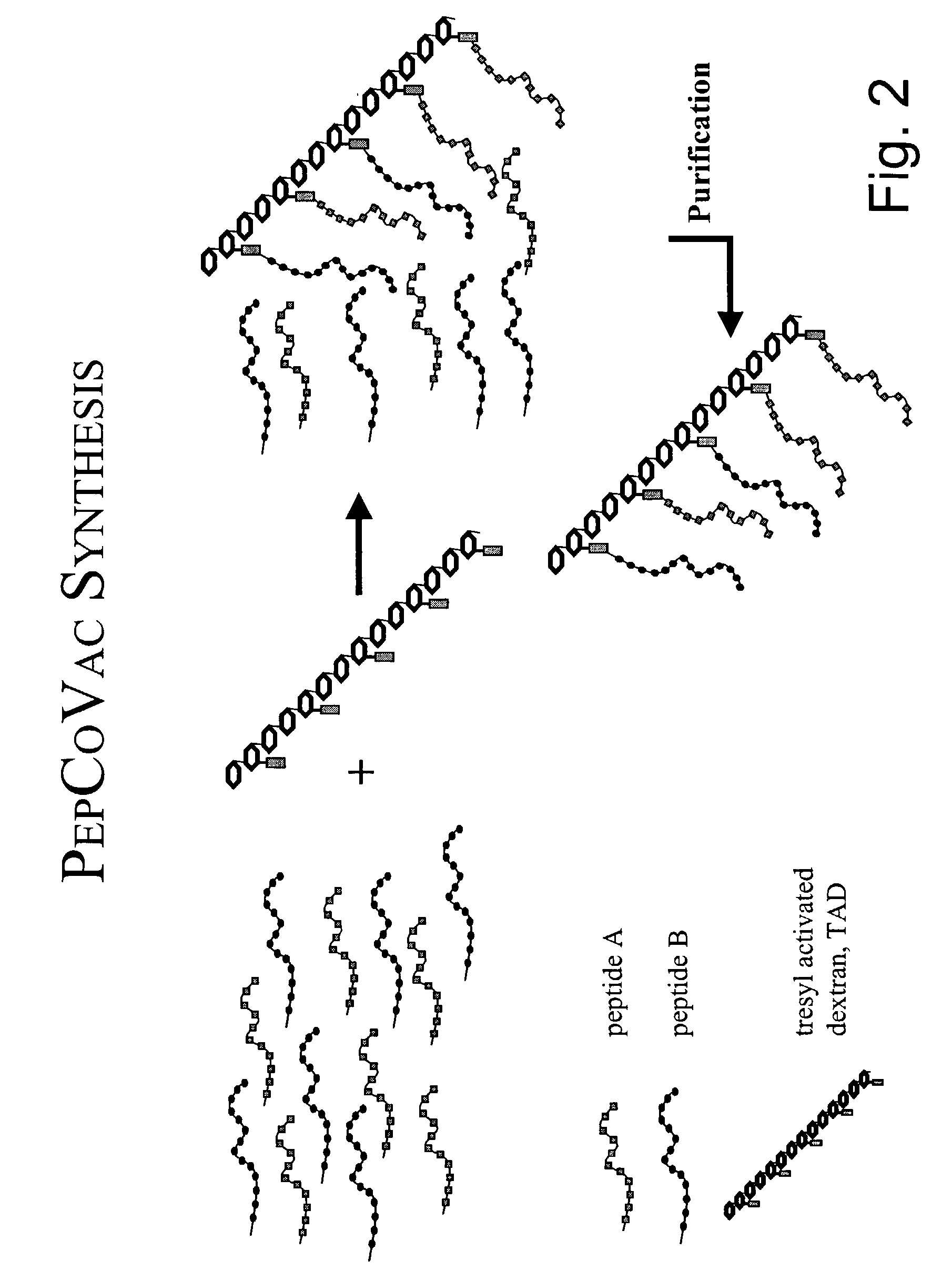
[0314] Synthesis of an A.beta. Peptide Copolymer Vaccine Using Activated Poly-Hydroxypolymer as the Cross-Linking Agent.
[0315] Introduction. A traditional conjugate vaccine consists of a (poly)peptide coupled covalently to a carrier protein. The peptide contains the B-cell epitope(s) and the carrier protein provides T-helper epitopes. However, most of the carrier protein will normally be irrelevant as a source for T-helper epitopes, since only aminor part of the total sequence contains the relevant T-helper epitopes. Such epitopes can be defined and synthesized as peptides of e.g. 12-15 amino acids. If these peptides are linked covalently to peptides containing the B-cell epitopes, e.g. via a multivalent activated poly-hydroxypolymer, a vaccine molecule that only contains the relevant parts can be obtained. It is further possible to provide a vaccine conjugate that contains an optimized ratio between B-cell and T-cell epitopes.
[0316] Synthesis of the activated poly-hydroxypolymer. P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com