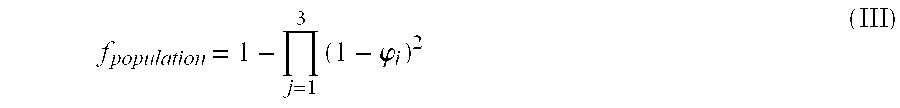Patents
Literature
42 results about "Helper epitope" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthetic vaccine agents
InactiveUS7097837B2Promote absorptionImprove responsePeptide/protein ingredientsAntibody mimetics/scaffoldsCtl epitopeHelper epitope
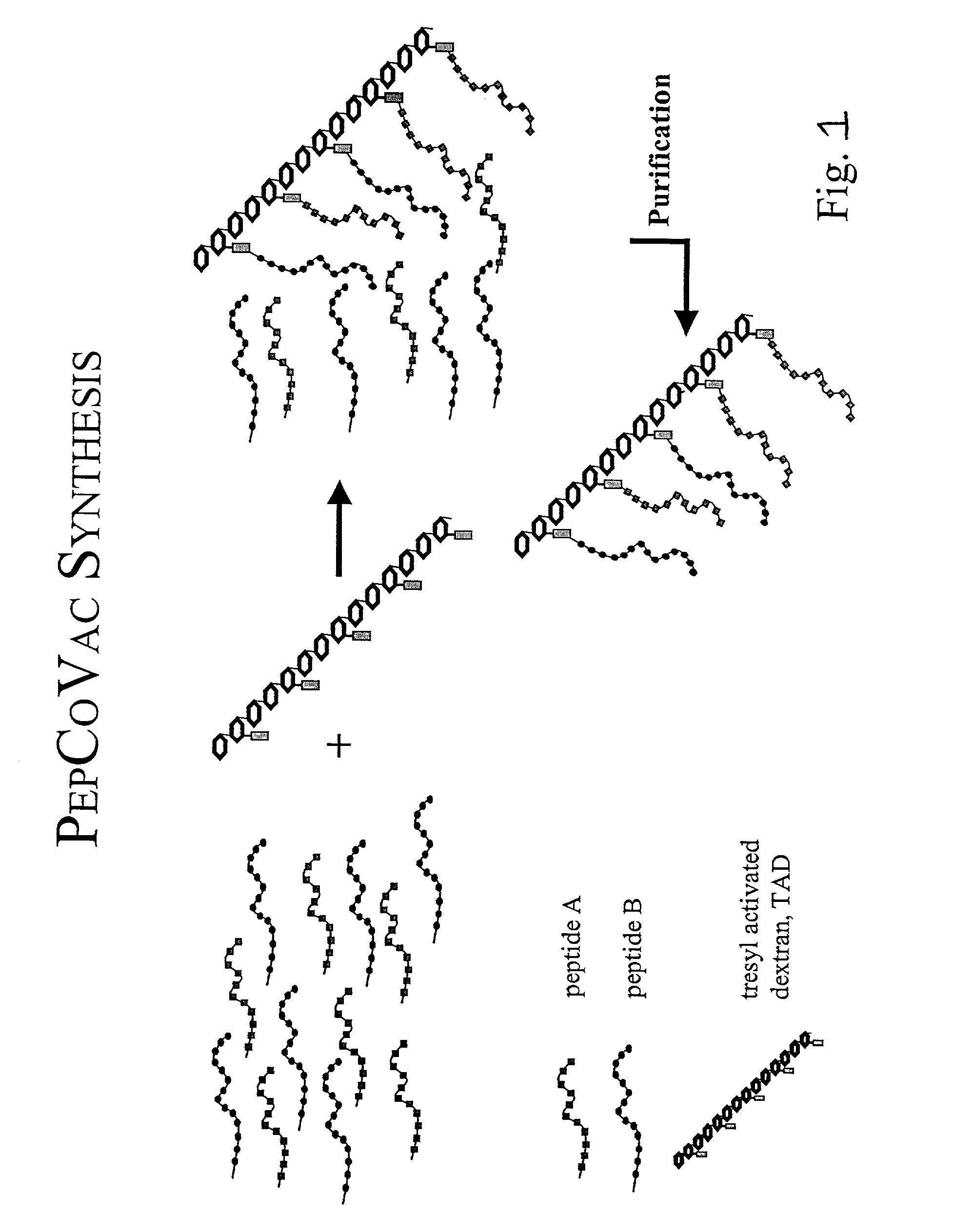
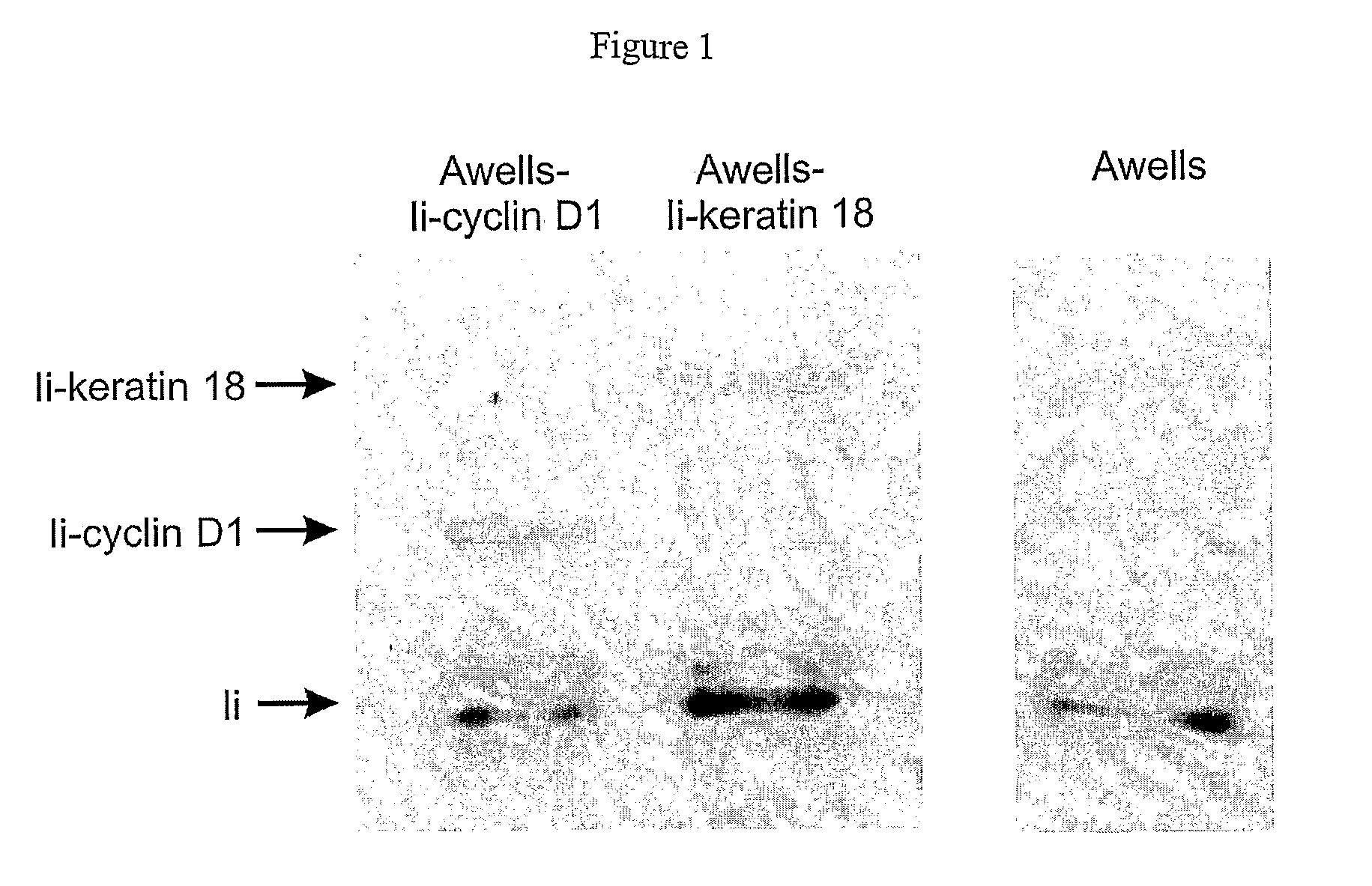
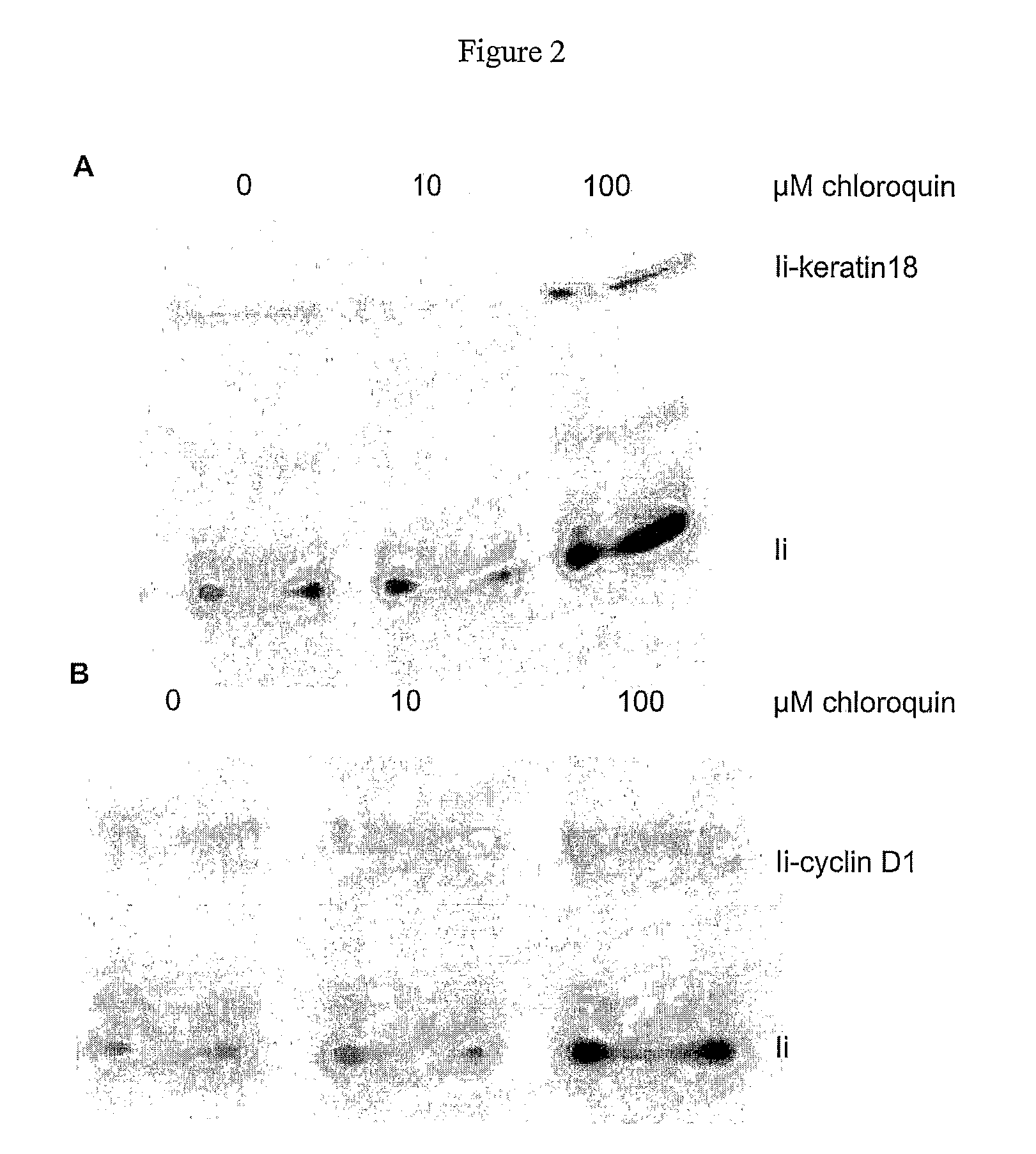
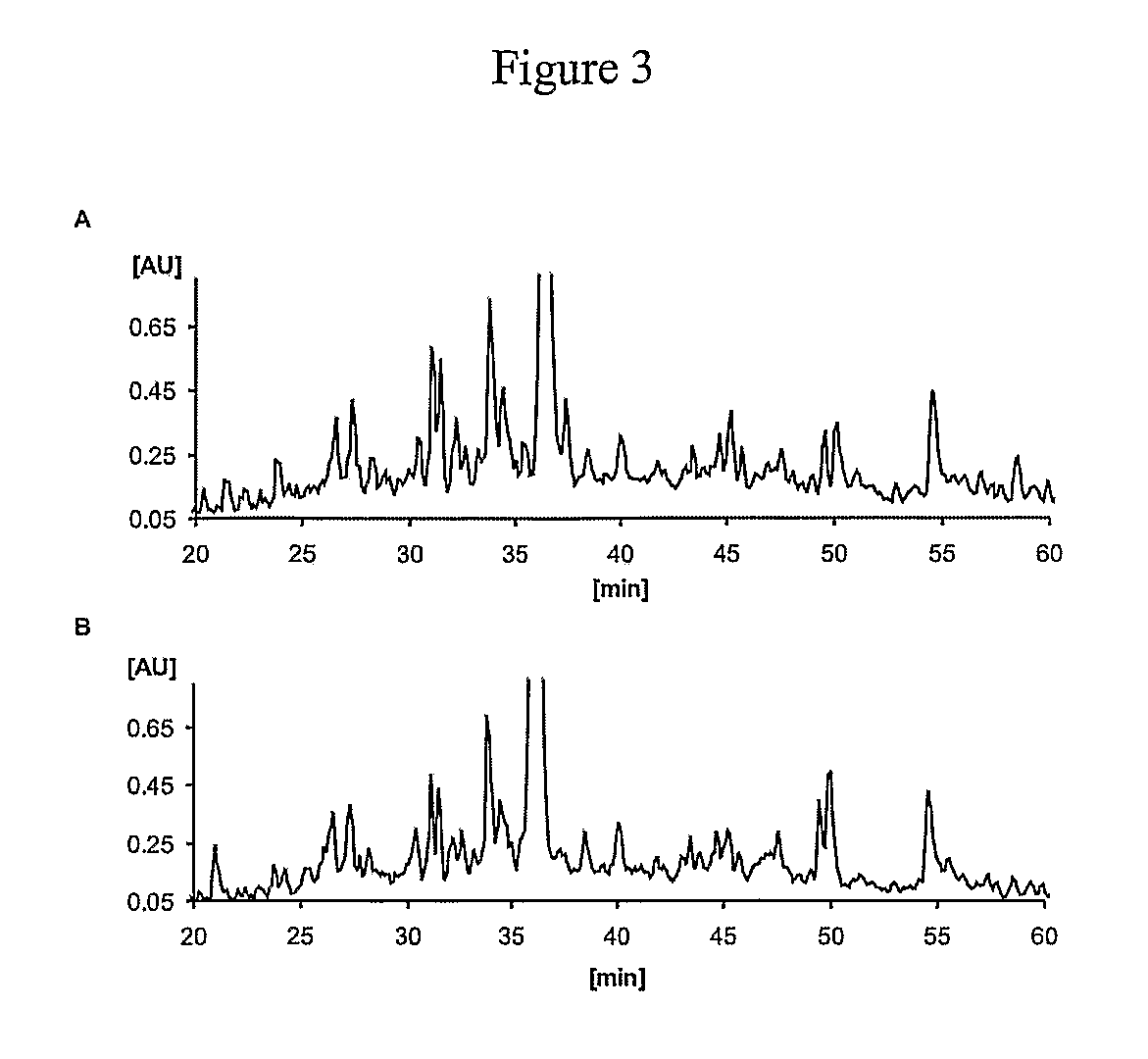
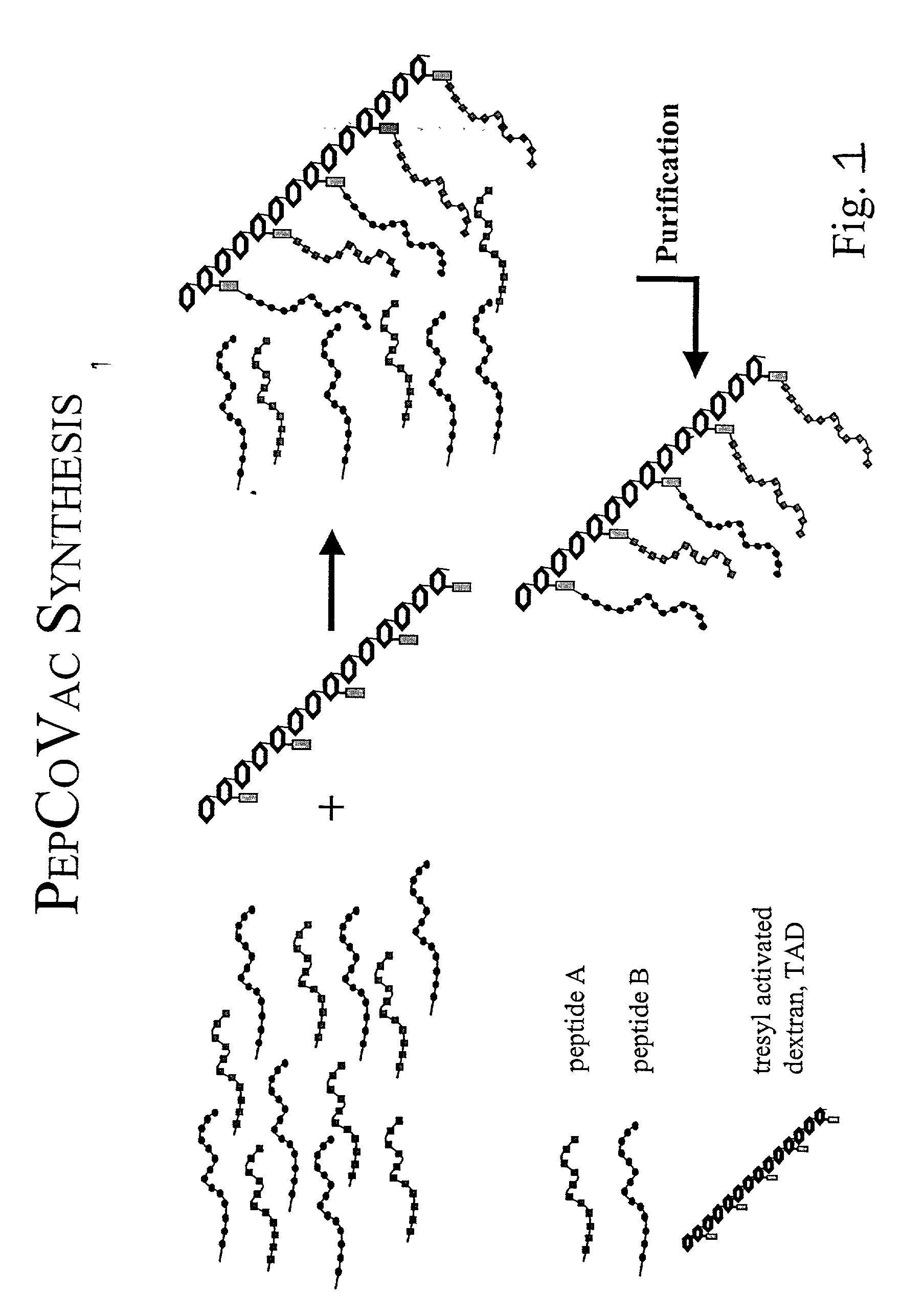
The present invention provides for novel immungens that are comprised of an activated polyhydroxypolymer backbone to which is attached 2 separate antigenic determinants. The 1st antigenic determinant includes a B-cell or CTL epitope and the 2nd antigenic determinant includes a T-helper epitope. In preferred embodiments, the antigenic determinants are derived from different molecules and species. Exemplary immunogens of the invention are constituted of a linear tresyl-activated dextran backbone to which is coupled B-cell or CTL epitopes of an antigen and to which is also coupled universal T-helper epitopes. Also disclosed are immunogenic compositions comprising the immunogens, methods of immunization and a method for identification of suitable immunogens of the invention.
Owner:BEALE STREET 143 INVEST APS
Synthetic vaccine agents
InactiveUS20040191264A1Promote absorptionImprove responseBacterial antigen ingredientsSnake antigen ingredientsCtl epitopeHelper epitope
The present invention provides for novel immungens that are comprised of an activated polyhydroxypolymer backbone to which is attached 2 separate antigenic determinants. The 1st antigenic determinant includes a B-cell or CTL epitope and the 2nd antigenic determinant includes a T-helper epitope. In preferred embodiments, the antigenic determinants are derived from different molecules and species. Exemplary immunogens of the invention are constituted of a linear tresyl-activated dextran backbone to which is coupled B-cell or CTL epitopes of an antigen and to which is also coupled universal T-helper epitopes. Also disclosed are immunogenic compositions comprising the immunogens, methods of immunisation and a method for identification of suitable immunogens of the invention.
Owner:PHARMEXA
Wt1 antigenic polypeptide, and Anti-tumor agent containing said polypeptide
[Problem] To provide a polypeptide which enables stimulation of numerous T cells of different derivation, and to induce strong immunological response against numerous types of cancers.[Solution] Provided is a fused polypeptide that includes a helper epitope derived from the WT1 protein, and a killer epitope derived from the WT1 protein, wherein the polypeptide includes a total of 3 to 6 of the helper epitopes and the killer epitopes per molecule of the fused polypeptide.
Owner:BIOIMMULANCE CO LTD +1
Immunogenic CEA
InactiveUS20050063952A1Effective immune responseBiocideGenetic material ingredientsVaccinationCarcinoembryonic antigen
The present invention provides for methods for immunizing actively against autologous carcinoembryonic antigen (CEA). The method encompasses that the immune system is engaged with variant CEA which is either administered as a protein vaccine, or is effected expressed by nucleic acid vaccination or live-viral vaccination. Preferred embodiments include immunization with variants that include at least one foreign T-helper epitope introduced in the CEA sequence. Also disclosed is variant proteins, DNA, vectors, and host cells useful for practising the method of the invention.
Owner:PHARMEXA
Immunogenic lipopeptides comprising T-helper and B-cell epitopes
InactiveUS7569225B2Increase profitEasy to specifyAntibacterial agentsBiocideSynthetic ImmunogensVaccination
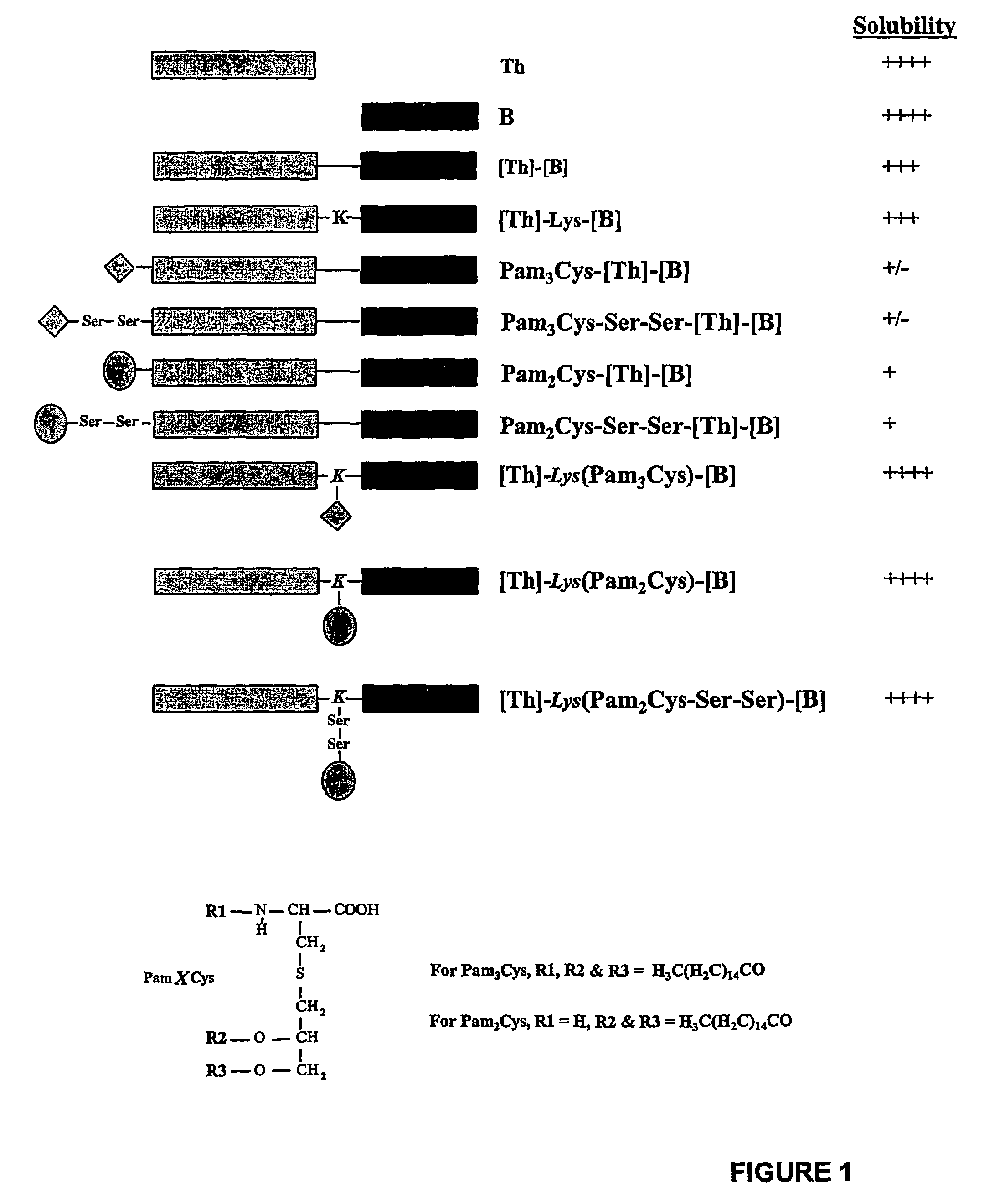
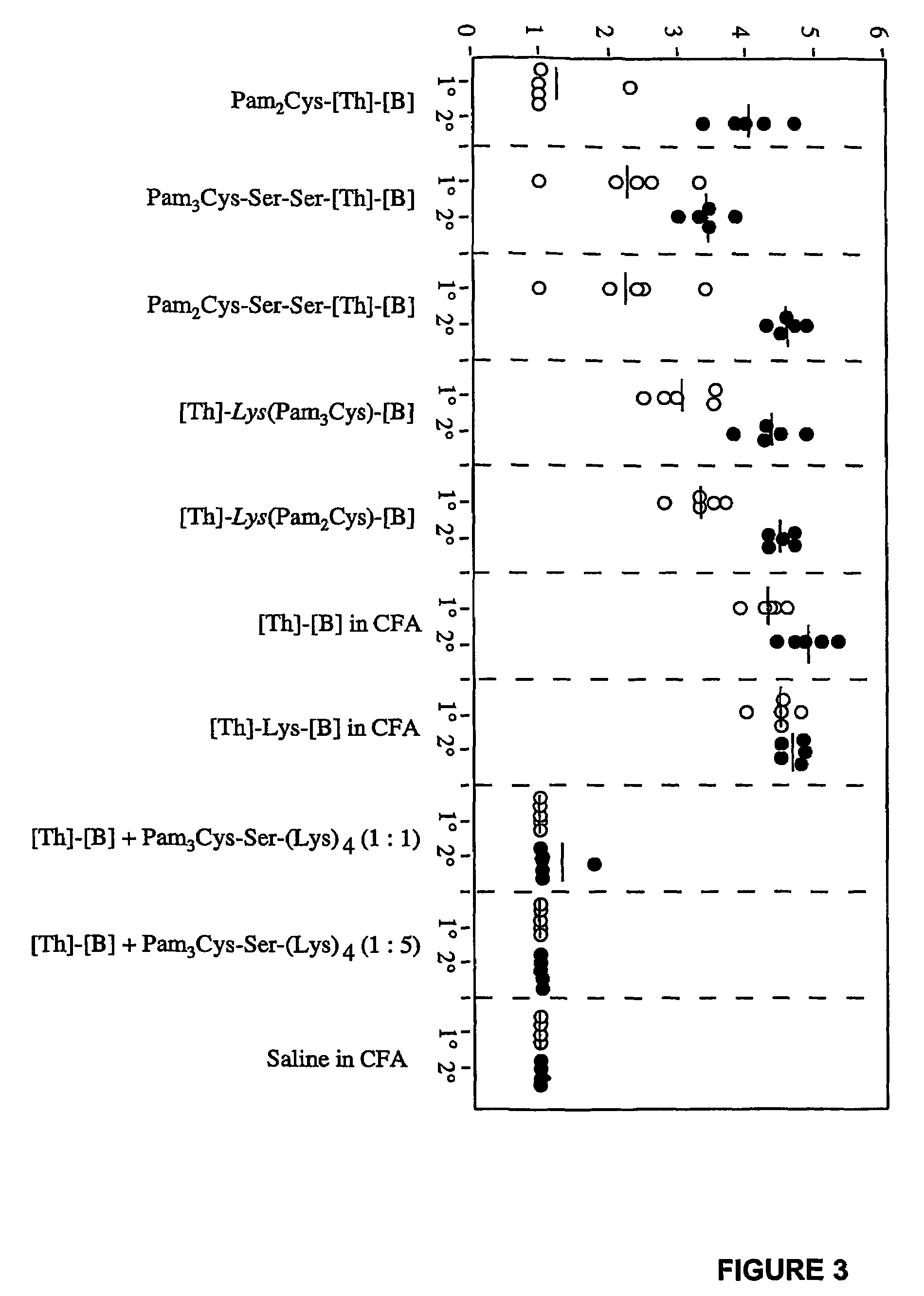
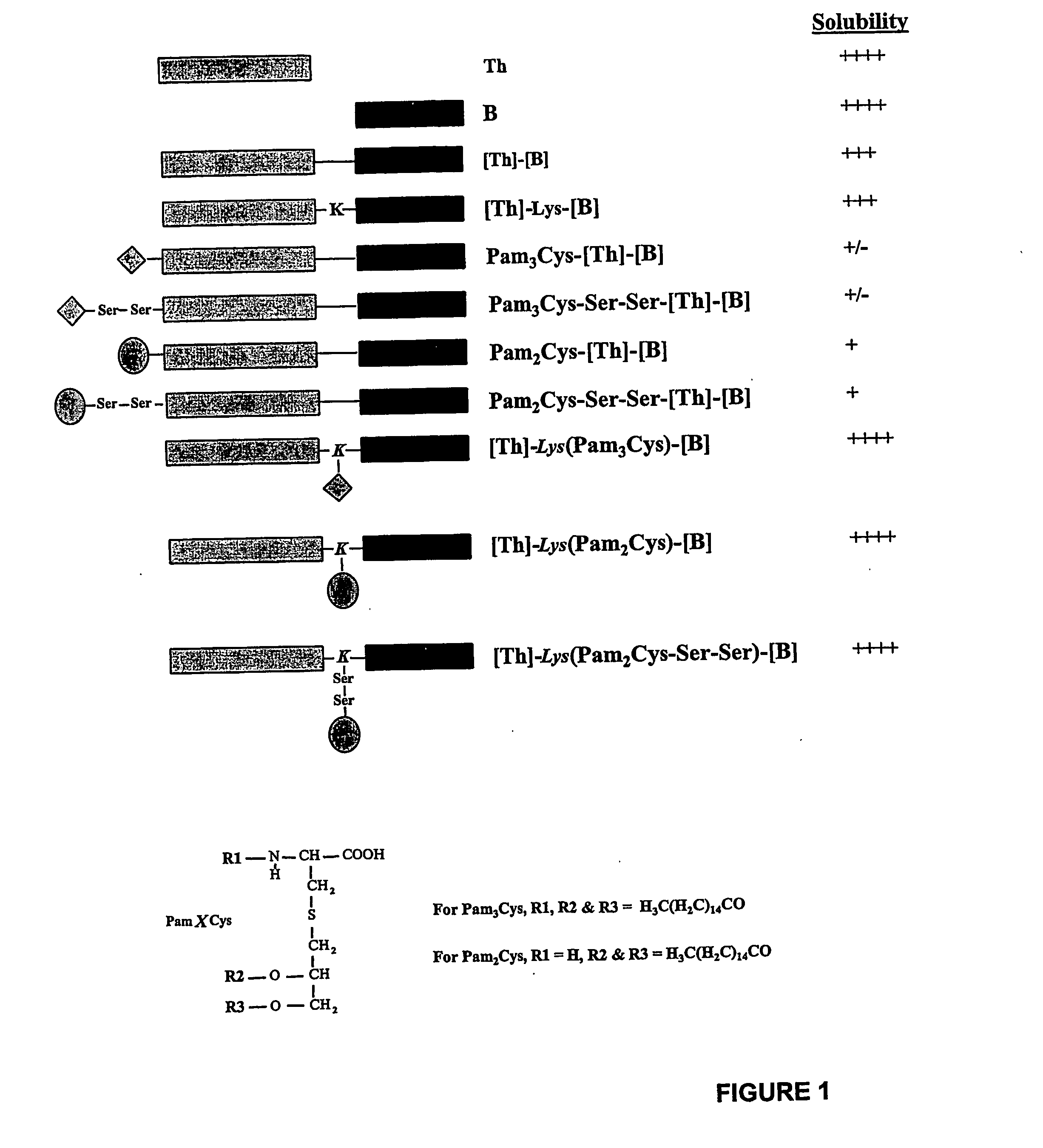
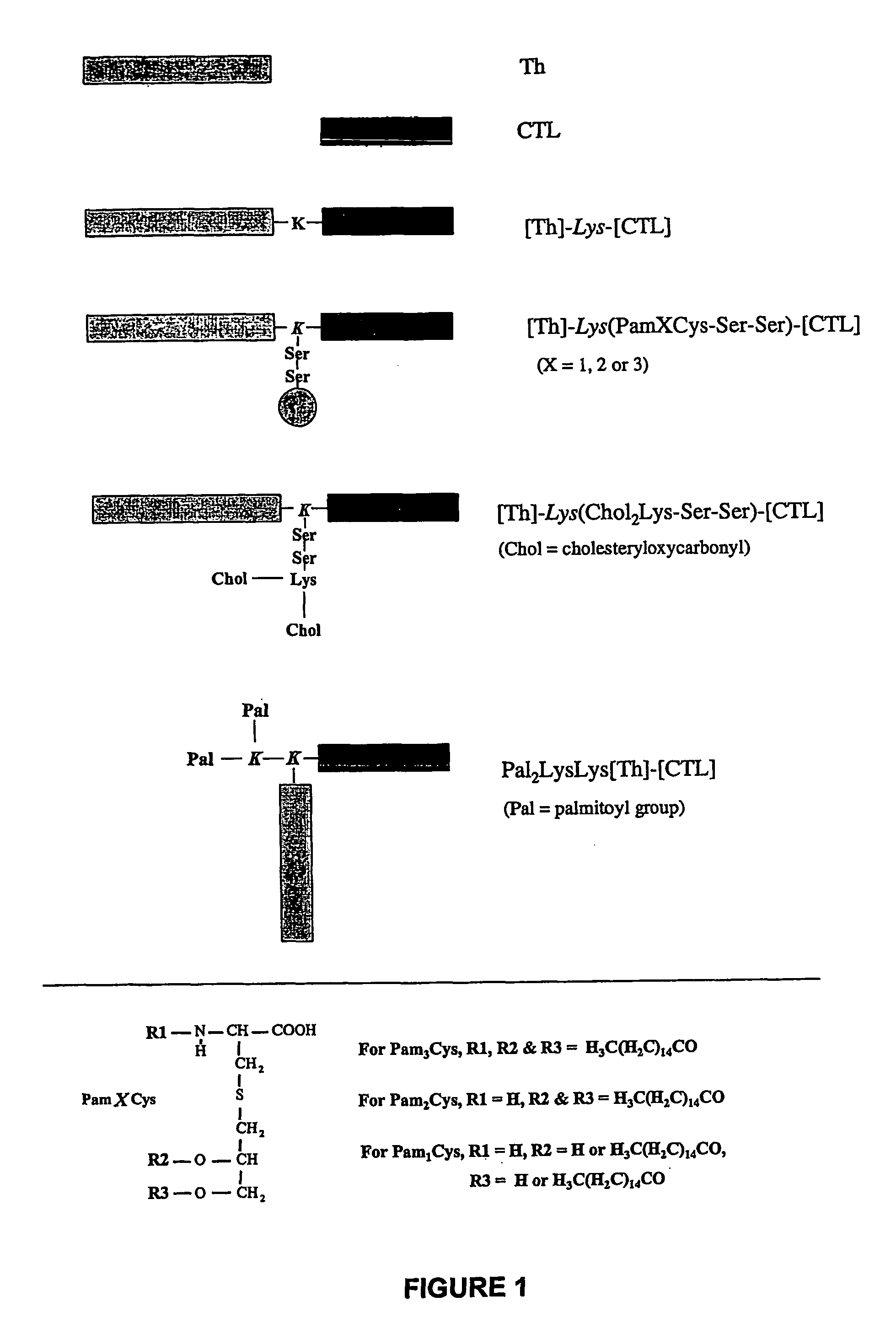
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and B cell epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular antigens. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the B cell epitope or within the T-helper epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Cancer Vaccines Against Mucosal Antigens and Methods of Making and Using the Same
Nucleic acid molecules comprising a nucleotide sequence that encodes a chimeric protein are disclosed. The chimeric proteins comprise at least one epitope of a mucosally restricted antigen, at least one CD4+ helper epitope, and, optionally, a secretion sequence. Chimeric proteins that comprise at least one epitope of a mucosally restricted antigen, at least one CD4+helper epitope and, optionally a secretion sequence are also disclosed. Compositions including pharmaceutical compositions and injectables comprising nucleic acid molecule and proteins are disclosed. Methods of treating individuals diagnosed with cancer of a mucosal tissue and methods of preventing cancer of a mucosal tissue are disclosed.
Owner:THOMAS JEFFERSON UNIV
Novel immunogenic lipopeptides comprising t-helper and b-cell epitopes
InactiveUS20070066534A1Promote maturityEnhance antigen presentationAntibacterial agentsBiocideSynthetic ImmunogensDiamino acid
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and B cell epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular antigens. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the B cell epitope or within the T-helper epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Novel immunogenic mimetics of multimer proteins
InactiveUS20030185845A1High yieldLow toxicityCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsInterleukin 5ADAMTS Proteins
The present invention relatas to novel immunogenic variants of multimeric proteins such as immunogenic variants of interleukin 5 (IL5) and tumour necrosis factor alpha (TNF, TNFalpha). The variants are, besides from being immunogenic in the autologous host, also highly similar to the native 3D structure of the proteins from which they are derived. Certain variants are monomeric mimics of the multimers, where peptide linkers (inert or T helper epitope containing) ensure a spatial organisation of the monomomer units that facilitate correct folding. A subset of variants are monomer TNFalpha variants that exhibit a superior capability of assembling into multimers with a high structural similarity to the native protein. Also disclosed are methods of treatment and production of the variants as well as DNA fragments, vectors, and host cells.
Owner:PHARMEXA
Immunogenic lipopeptides comprising T-helper and cytotoxic T lymphocyte (CTL) epitopes
InactiveUS7833532B2Easy to synthesizeIncrease profitSsRNA viruses negative-senseBiocideSynthetic ImmunogensCtl epitope
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and CTL epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular CTL epitopes. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the CTL epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Immunogenic T-Helper Epitopes From Human Tumour Antigens and Immunotherapeutic Methods Using Said Epitopes
InactiveUS20090317428A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer, in particular renal cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 338 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
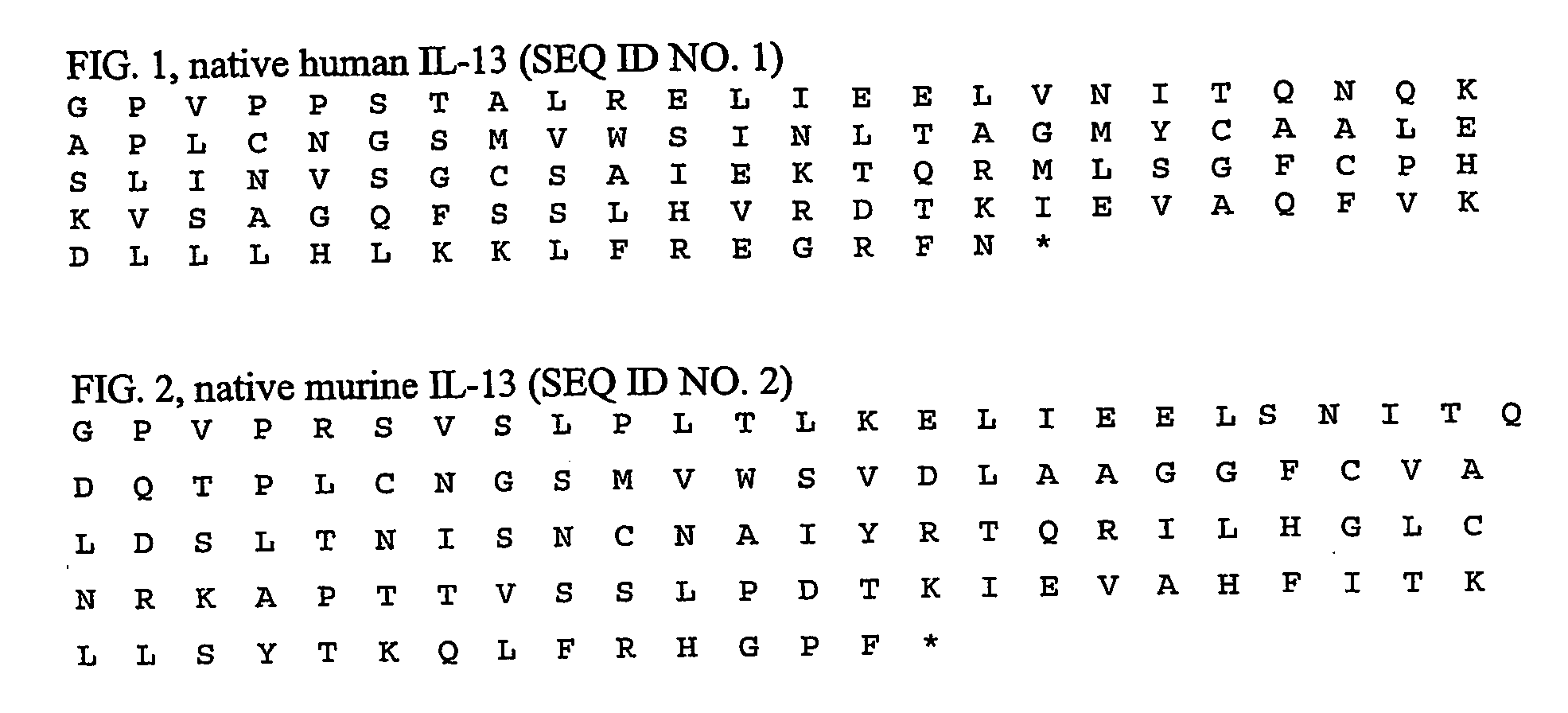
Immunogenic composition comprising an il-13 element and t cell epitopes, and its therapeutic use
InactiveUS20060147417A1Improve assessmentEnhanced antibodyBacterial antigen ingredientsPeptide/protein ingredientsVaccinationContact allergy
Owner:GLAXO GROUP LTD
Synthetic peptide-based marker vaccine and diagnostic system for effective control of porcine reproductive and respiratory syndrome (PRRS)
ActiveUS20140335118A1Enhance their respective immunogenicityRisk minimizationAntibacterial agentsSsRNA viruses positive-senseHelper epitopeMonitoring and control
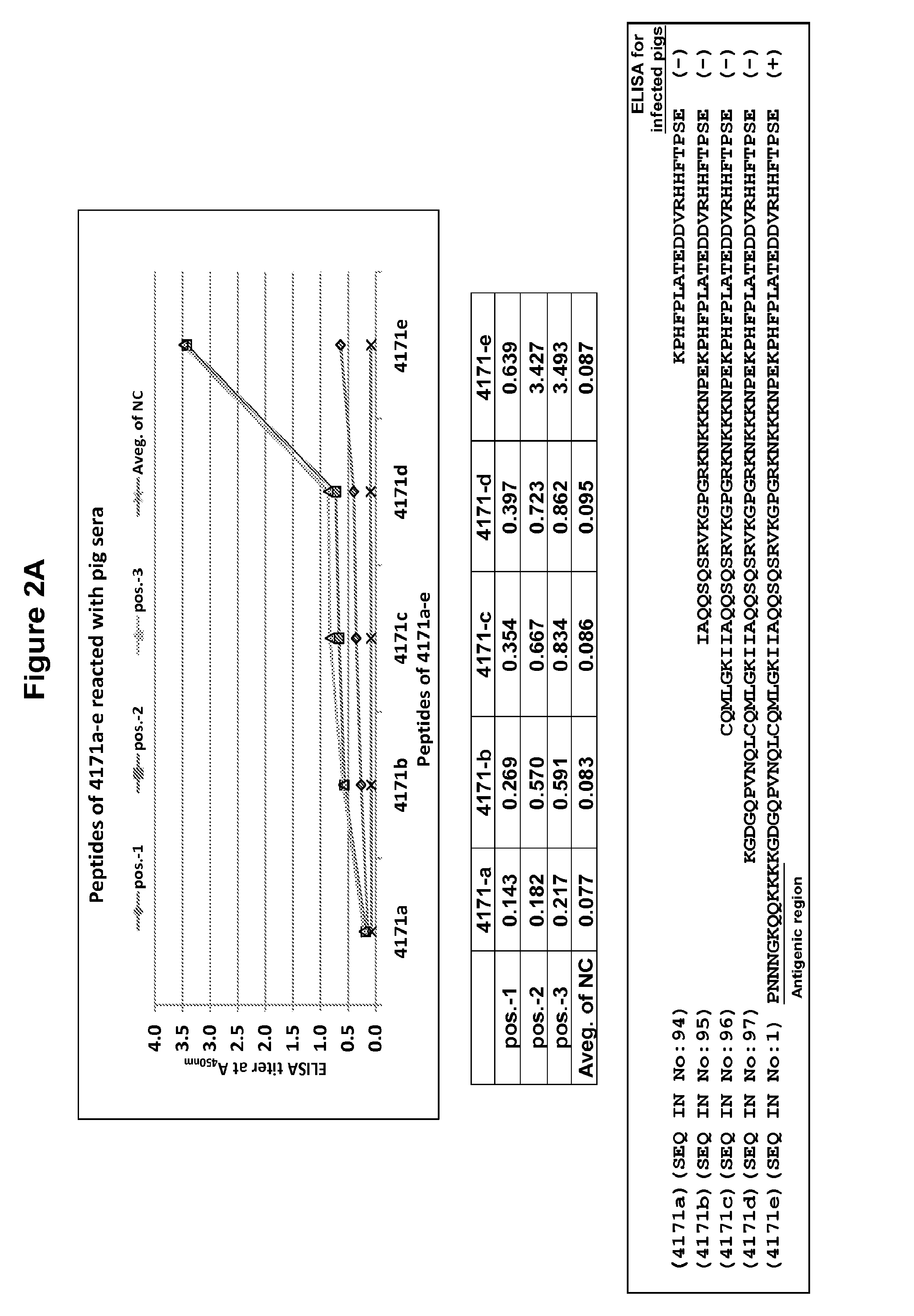
A peptide-based marker vaccine against Porcine Reproductive and Respiratory Syndrome (PRRS) and a set of immunodiagnostic tests for the prevention, monitoring and control of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) are disclosed. Vaccine formulations according to various embodiments of the invention contain a mixture of peptides derived from PRRSV GP2, GP3, GP4, or GP5 proteins; each peptide individually contains a B cell PRRSV neutralizing / receptor binding epitope which is individually linked to an artificial T helper epitope for enhancement of the respective peptide's immunogenicity; and which can be supplemented with a mixture of peptides representing the T helper epitopes derived from the PRRSV GP4, GP5, M and Nucleocapsid proteins to provide cell mediated immunity. Such viral peptide compositions are prepared in an acceptable delivery system as vaccine formulations and can provide cross protection of PRRSV antibody free pigs from infection upon PRRSV challenge.
Owner:UNITED BIOMEDICAL INC
Immunogenic mimetics of multimer proteins with promiscuous T cell epitope inserts
InactiveUS20040258660A1High expressionImprove purification effectPeptide/protein ingredientsAntibody mimetics/scaffoldsInterleukin 5ADAMTS Proteins
The present invention relatas to novel immunogenic variants of multimeric proteins such as immunogenic variants of interleukin 5 (IL5) and tumour necrosis factor alpha (TNF, TNFalpha). The variants are, besides from being immunogenic in the autologous host, also highly similar to the native 3D structure of the proteins from which they are derived. Certain variants are monomeric mimics of the multimers, where peptide linkers (inert or T helper epitope containing) ensure a spatial organisation of the monomomer units that facilitate correct folding. A subset of variants are monomer TNFalpha variants that exhibit a superior capability of assembling into multimers with a high structural similarity to the native protein. Also disclosed are methods of treatment and production of the variants as well as DNA fragments, vectors, and host cells.
Owner:PHARMEXA
Synthetic immunogen useful for generating long lasting immunity and protection against pathogens
InactiveUS20130183377A1Induced proliferationGenerating long lasting protective immunityAntibacterial agentsPowder deliverySynthetic ImmunogensTrypanosomiasis
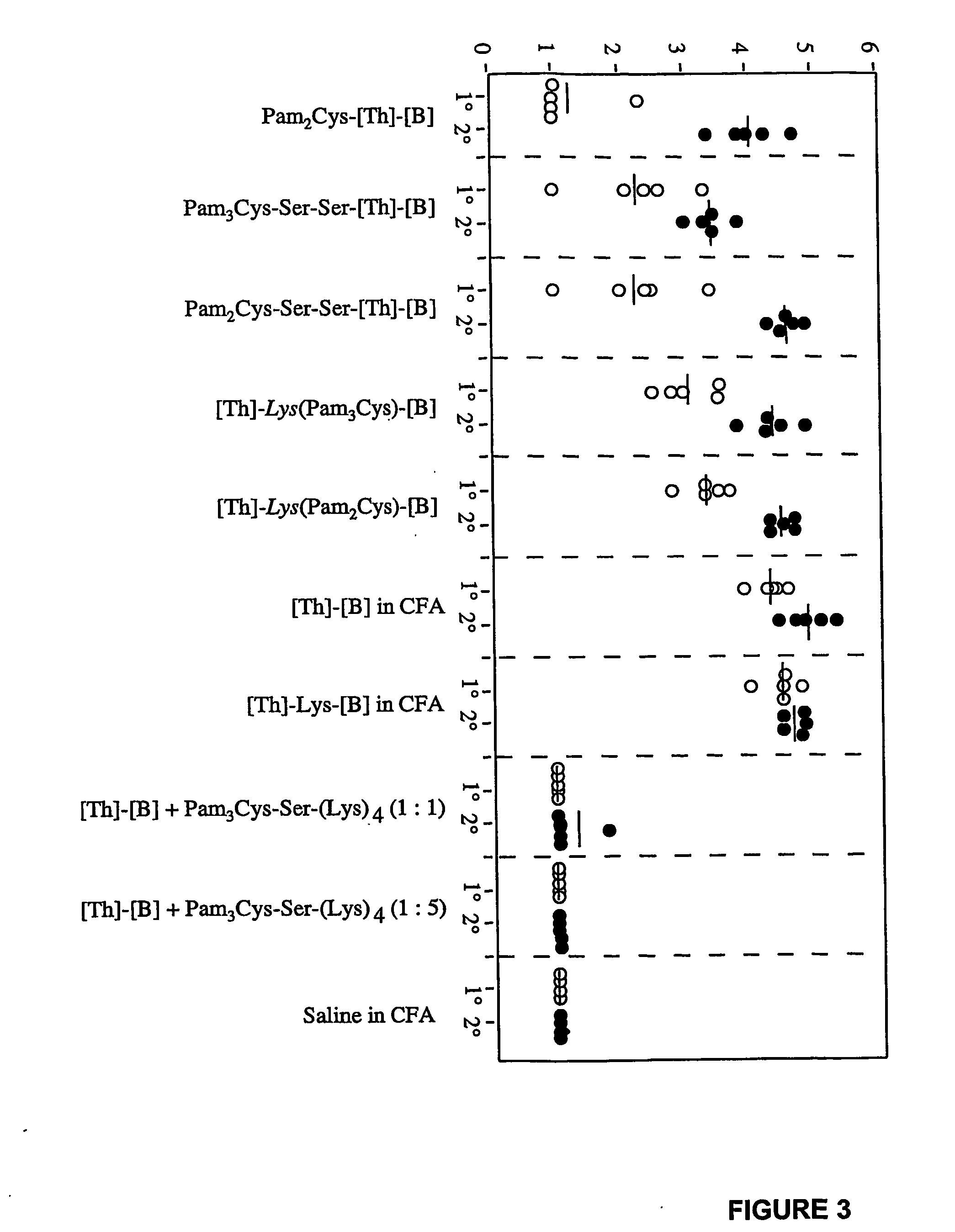
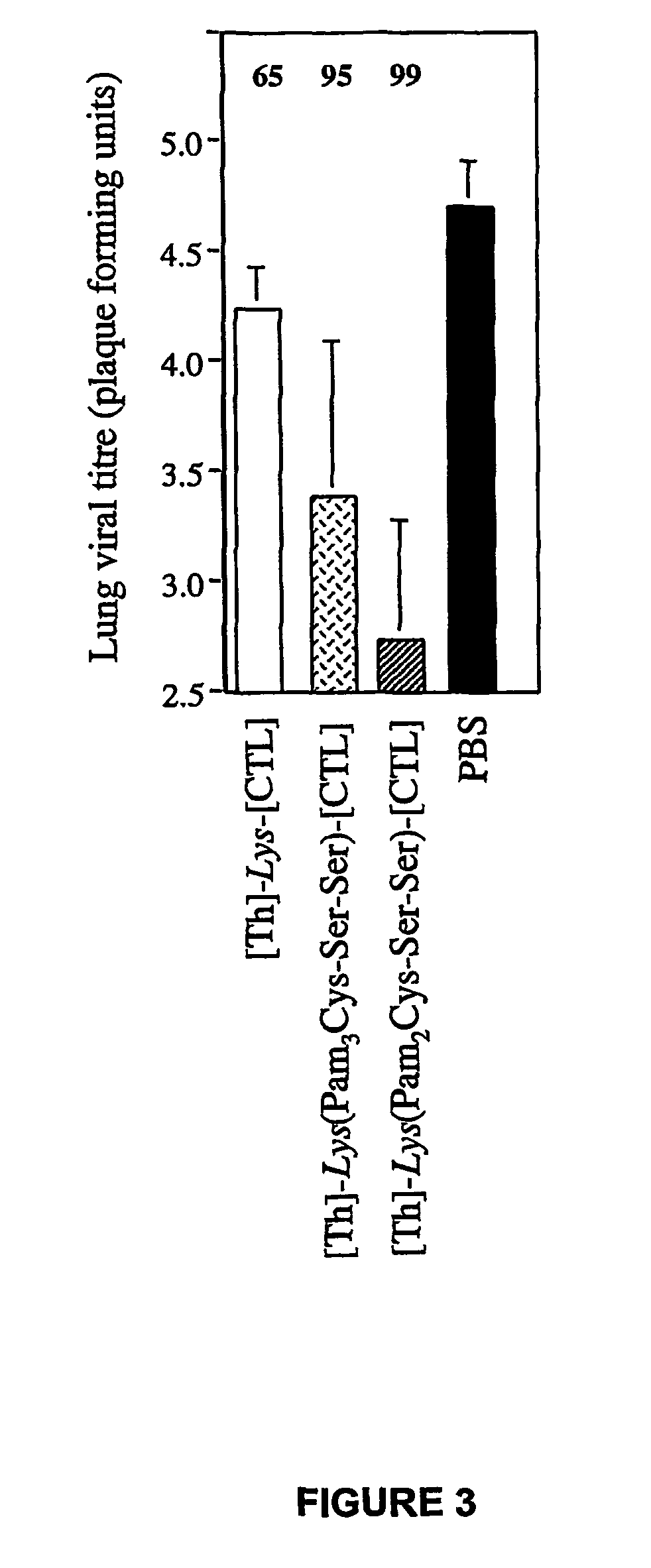
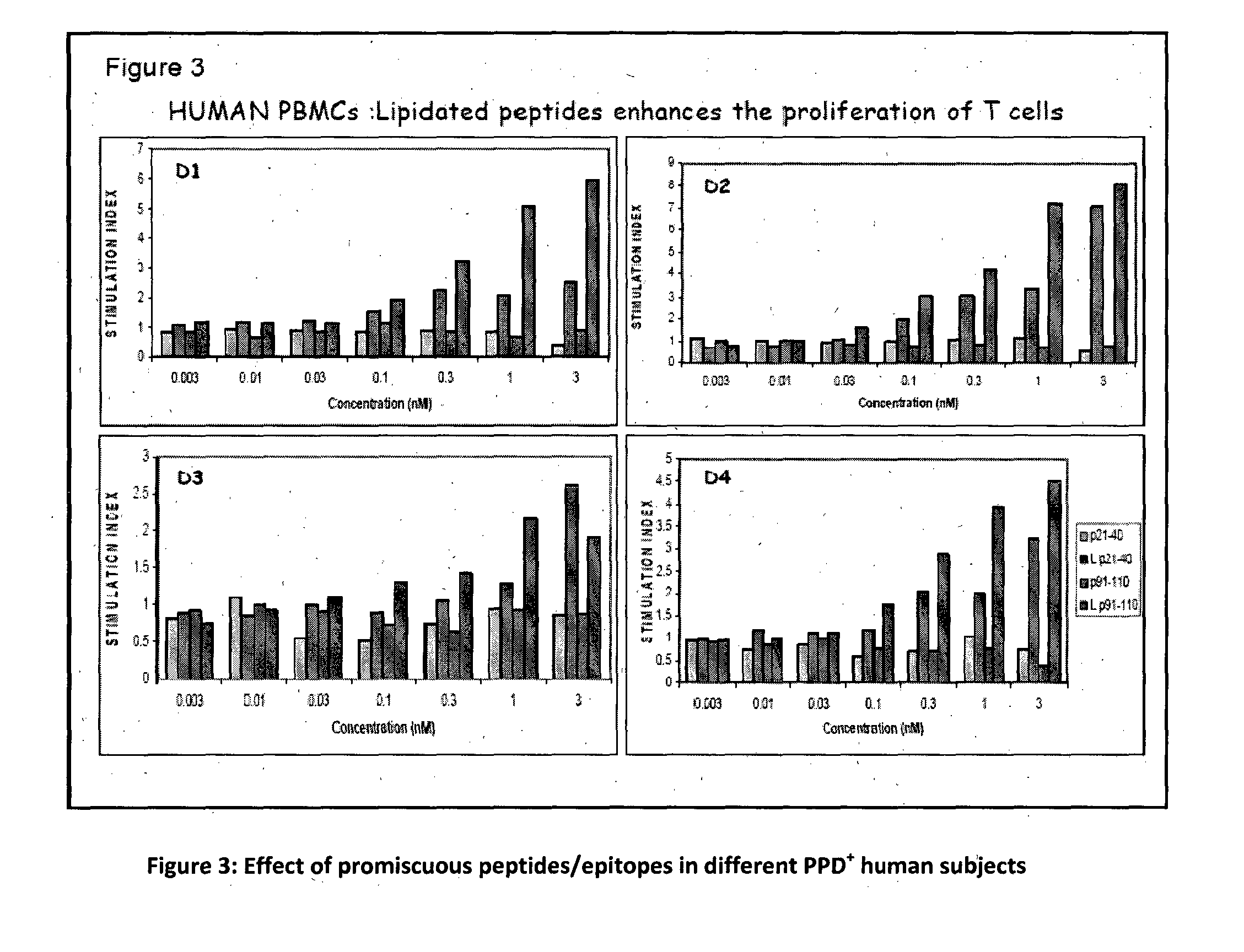
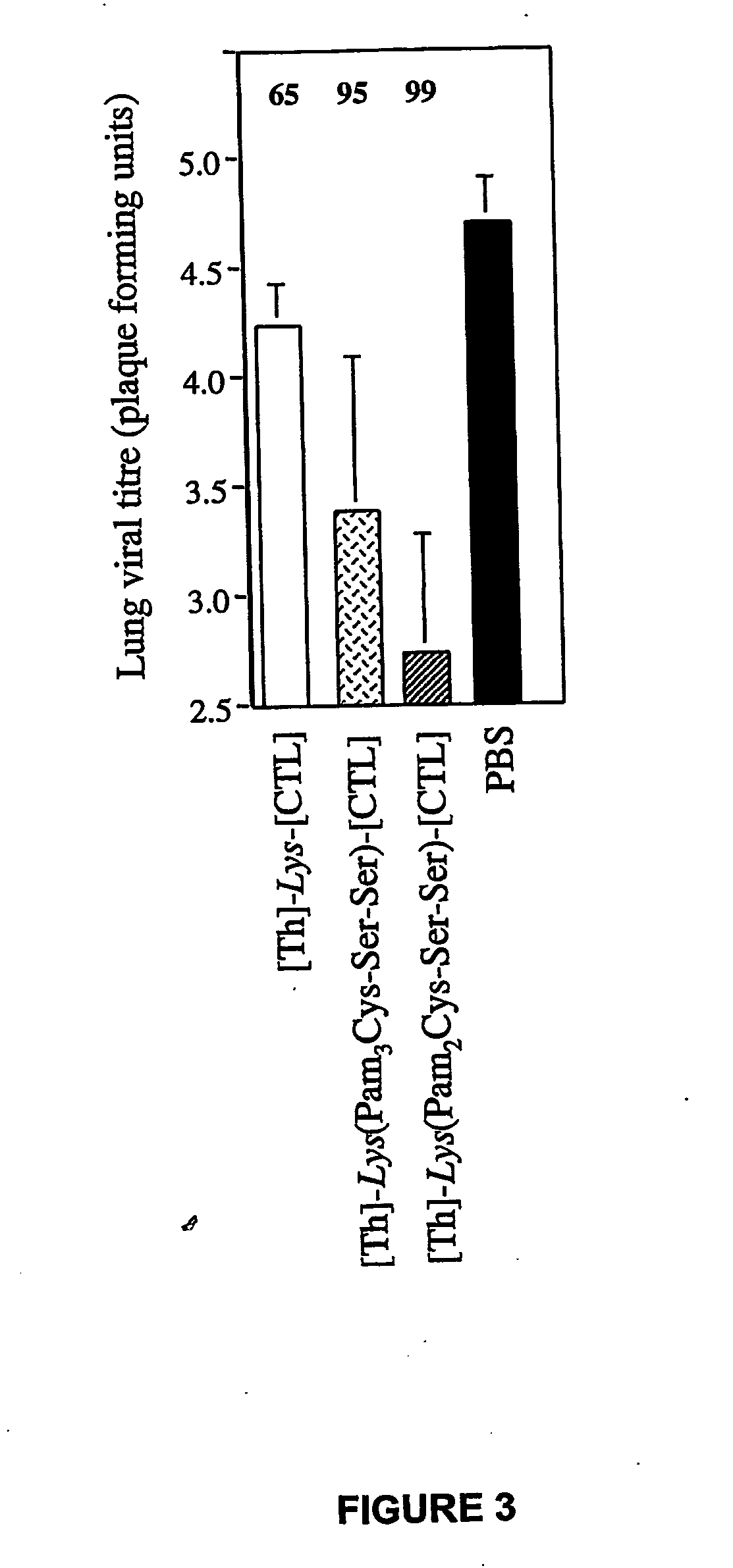
The present invention relates to a synthetic immunogen represented by the general formula 1, useful for generating long lasting protective immunity against various intracellular pathogens which are the causative agents of tuberculosis, leishmaniasis, AIDS, trypanosomiasis, malaria and also allergy, cancer and a process for the preparation thereof. The developed immunogen is able to circumvent HLA restriction in humans and livestock. The invention further relates to a vaccine comprising the said immunogen for generating enduring protective immunity against various diseases. The said vaccine is targeted against intracellular pathogens, more particularly the pathogen M. tuberculosis in this case. In the present invention, promiscuous peptides of M. tuberculosis are conjugated to TLR ligands especially; Pam2Cys to target them mainly to dendritic cells and therefore elicit long-lasting protective immunity. (The formula (I) should be inserted here) General formula (I) wherein, X1=a promiscuous CD4 T helper epitope selected from SEQ ID No. 1 to 98 OR nil; X2=a promiscuous CD8 T cytotoxic epitope selected from SEQ ID No. 99 to 103 OR nil; when X1=nil; X2=SEQ ID No. 99 to 103 and when X2=nil; X1=SEQ ID No. 1 to 98; Y=Lysine; and S=Serine.
Owner:COUNCIL OF SCI & IND RES +1
Immunogenic T-helper epitopes from human tumour antigens and immunotherapeutic methods using said epitopes
InactiveUS8067529B2Tumor rejection antigen precursorsPeptide/protein ingredientsHla class iiHelper epitope
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer, in particular renal cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 338 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Synthetic vaccine agents
InactiveUS20020119162A1Promote absorptionImprove responsePeptide/protein ingredientsAntibody mimetics/scaffoldsCtl epitopeHelper epitope
The present invention provides for novel immungens that are comprised of an activated polyhydroxypolymer backbone to which is attached 2 separate antigenic determinants. The 1st antigenic determinant includes a B-cell or CTL epitope and the 2nd antigenic determinant includes a T-helper epitope. In preferred embodiments, the antigenic determinants are derived from different molecules and species. Exemplary immunogens of the invention are constituted of a linear tresyl-activated dextran backbone to which is coupled B-cell or CTL epitopes of an antigen and to which is also coupled universal T-helper epitopes. Also disclosed are immunogenic compositions comprising the immunogens, methods of immunization and a method for identification of suitable immunogens of the invention.
Owner:BEALE STREET 143 INVEST APS
Novel immunogenic lipopeptides comprising t-helper and cytotoxic t lymphocyte (ctl) epitopes
InactiveUS20070160631A1Conveniently synthesizedEasy to synthesizeSsRNA viruses negative-senseBiocideSynthetic ImmunogensCtl epitope
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and CTL epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular CTL epitopes. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the CTL epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
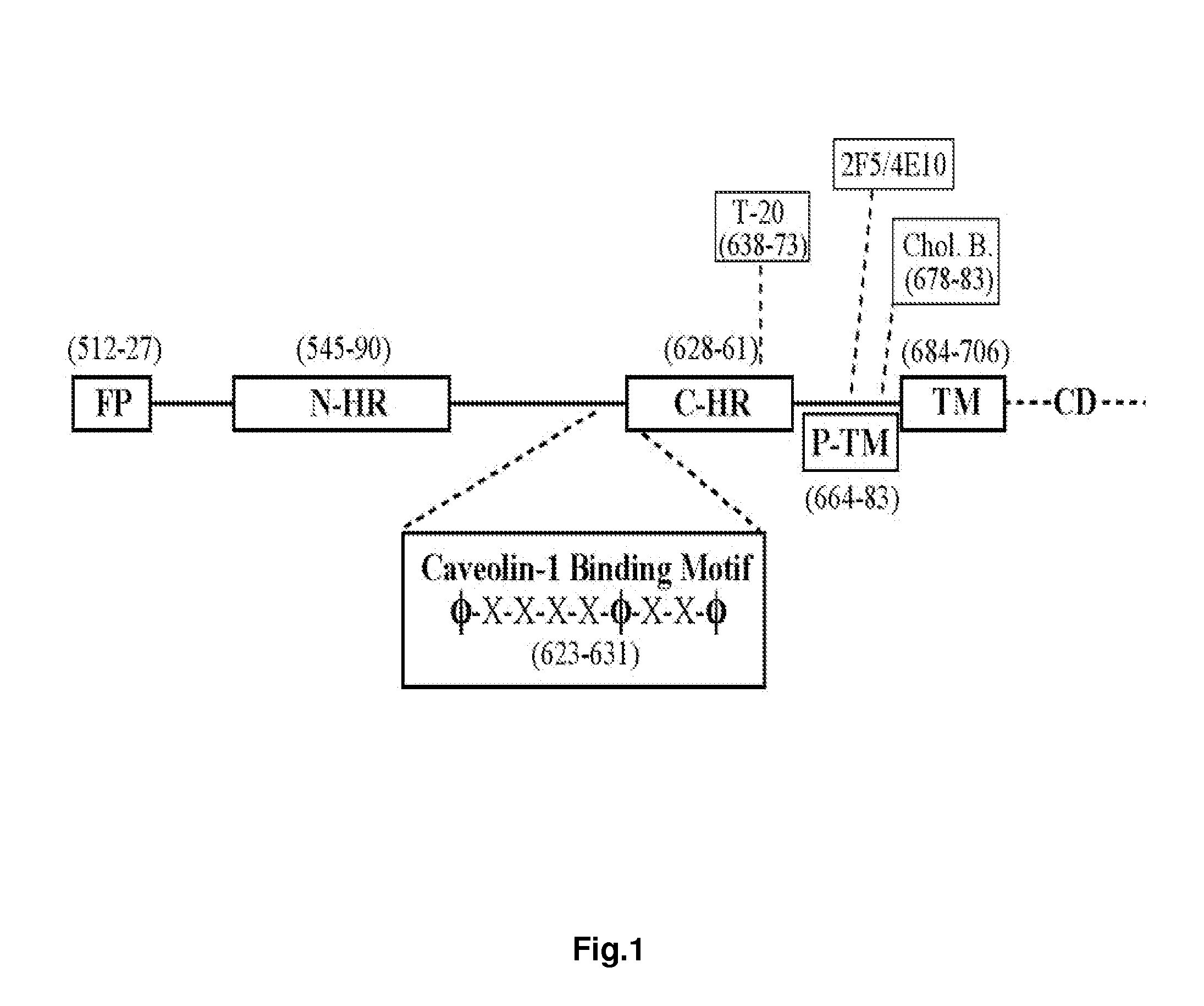
Synthetic peptides corresponding to overlapping neutralizing determinants in the cbd1 epitope induce broadly neutralizing antibodies
InactiveUS20110217307A1Peptide/protein ingredientsViral antigen ingredientsNeutralizing antibodyHiv 1 gp41
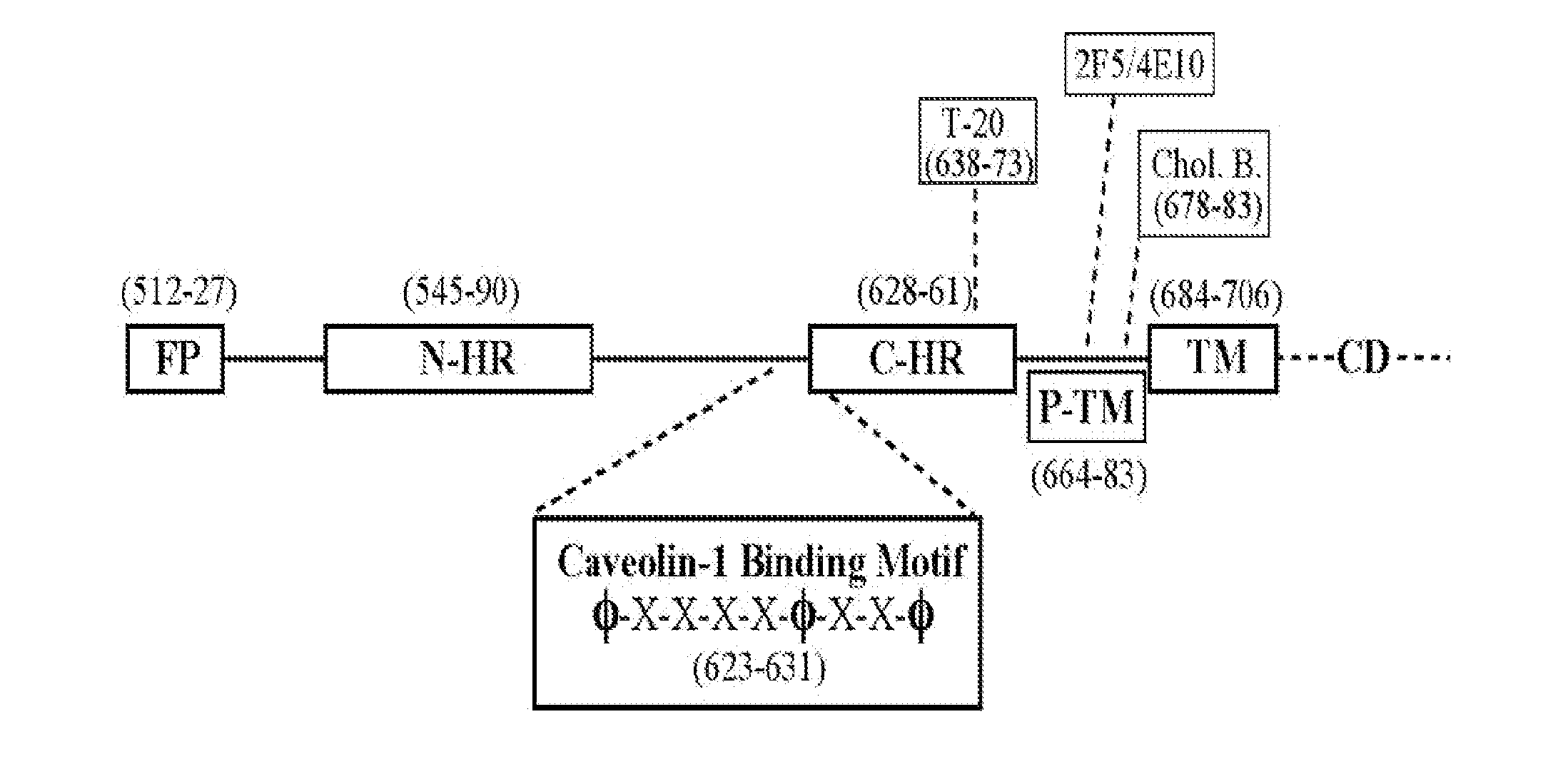
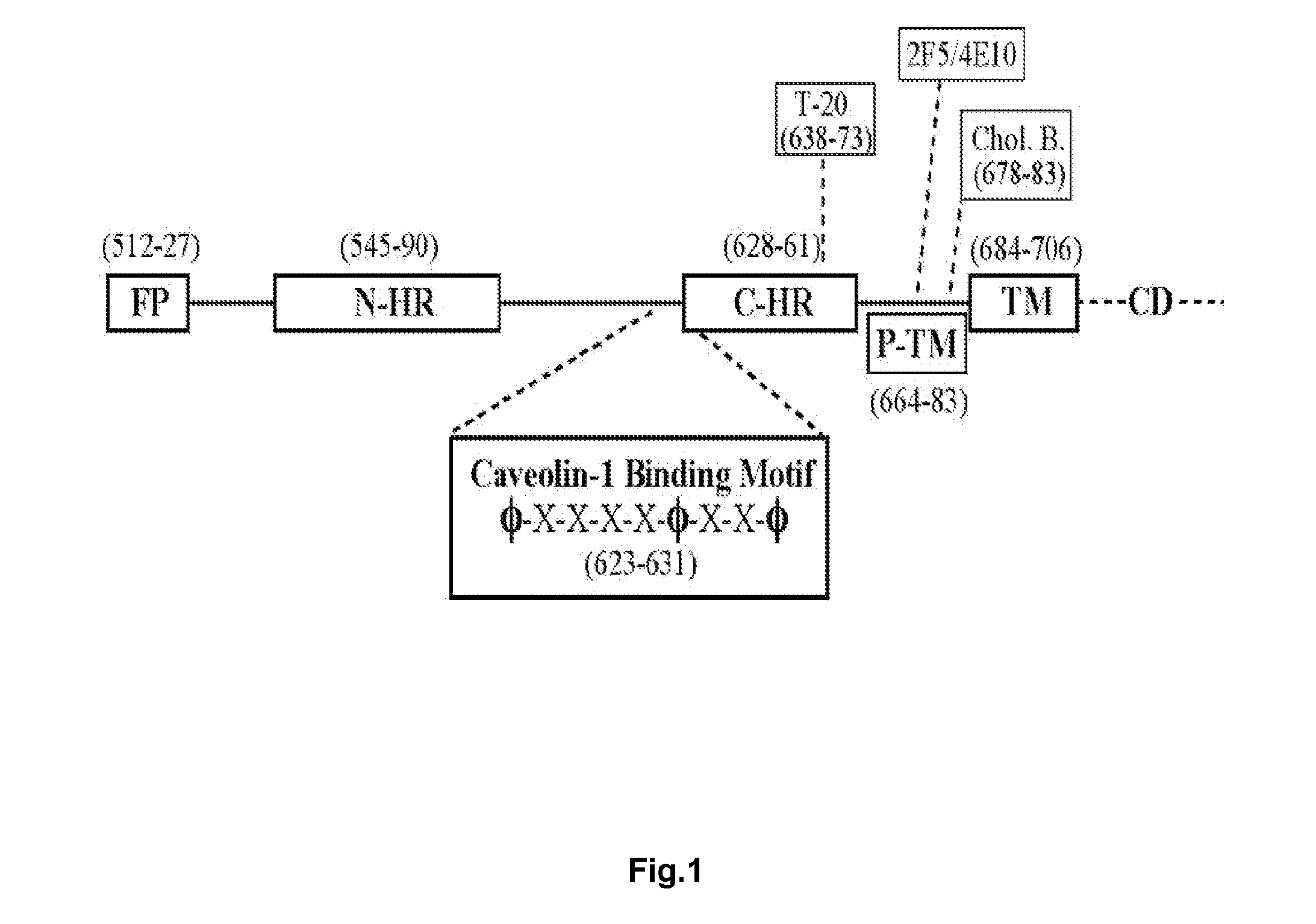
The present invention relates to chimeric peptides having a caveolin-1 binding domain of an HIV-1 gp41 (CBD1) peptide or a variant of said CBD1, fused to a T helper epitope. In one aspect, the T epitope is from a peptide selected from the group consisting of a tetanus toxin, an HIV-1 Gag p24 and an HIV-1 Env-gp120. Compositions containing these chimeric peptides and pharmaceutical and immunogenic compositions as well as vaccines comprising these chimeric peptides also are part of the present invention. Methods to induce neutralizing antibodies against HIV-1 activity and uses of the chimeric peptides to treat or to prevent HIV-1 infection are also disclosed.
Owner:CENT NAT DE LA RECHERCHE SCI
Designer peptide-based pcv2 vaccine
ActiveUS20130236487A1Improving immunogenicityImprove quality controlSsRNA viruses positive-sensePeptide/protein ingredientsPeptide antigenHelper epitope
Porcine circovirus (PCV2) vaccine compositions comprising a peptide antigen derived from a PCV2 capsid protein are described. In various embodiments, the peptide antigen contains amino acids of the capsid protein from about amino acid 47 to about amino acid 202. In some embodiments, the peptide antigen is optionally linked to an artificial T helper epitope and / or mixed with T helper epitopes derived from the ORF1 and ORF3 proteins of PCV2. Methods of using PCV2 vaccine compositions are also described. In various embodiments, a vaccine composition is used in animals for the prevention of PCV2 infection. In other embodiments, a PCV2 vaccine composition is used as an antigen for diagnosing PCV2 infection.
Owner:UNITED BIOMEDICAL INC
Novel Promiscuous HPV16-Derived T Helper Epitopes for Immunotherapy
The present invention relates to novel amino acid sequences of peptides derived from HPV16 that are able to bind to MHC complexes of class II, and elicit an immune response. The present invention further relates to pharmaceutical products, such as vaccines and T-cells, based on said epitopes.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
Synthetic peptides corresponding to overlapping neutralizing determinants in the CBD1 epitope induce broadly neutralizing antibodies
The present invention relates to chimeric peptides having a caveolin-1 binding domain of an HIV-1 gp41 (CBD1) peptide or a variant of said CBD1, fused to a T helper epitope. In one aspect, the T epitope is from a peptide selected from the group consisting of a tetanus toxin, an HIV-1 Gag p24 and an HIV-1 Env-gp120. Compositions containing these chimeric peptides and pharmaceutical and immunogenic compositions as well as vaccines comprising these chimeric peptides also are part of the present invention. Methods to induce neutralizing antibodies against HIV-1 activity and uses of the chimeric peptides to treat or to prevent HIV-1 infection are also disclosed.
Owner:CENT NAT DE LA RECHERCHE SCI
Construction and application of fusion protein vaccine platform
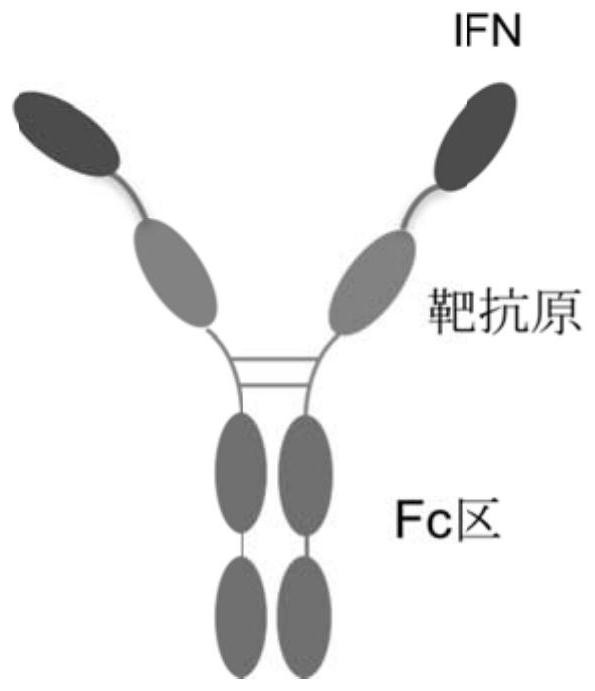
PendingCN113876938AIncrease flexibilityReduce deliveryAntibacterial agentsSsRNA viruses positive-senseHelper epitopeTGE VACCINE
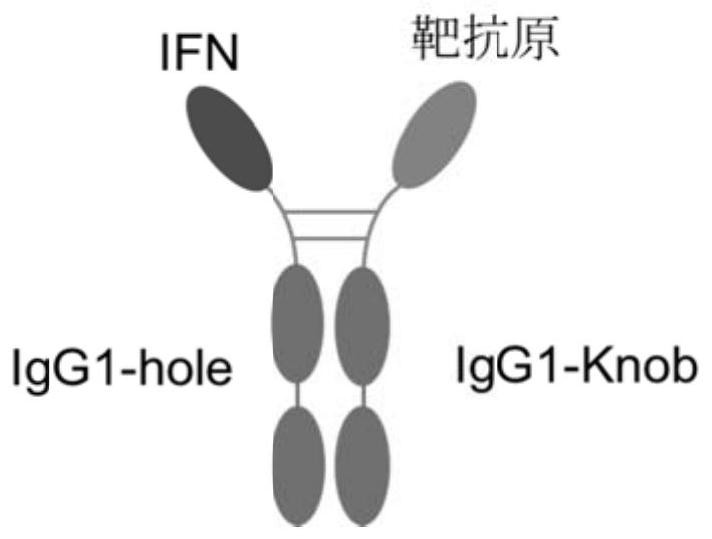
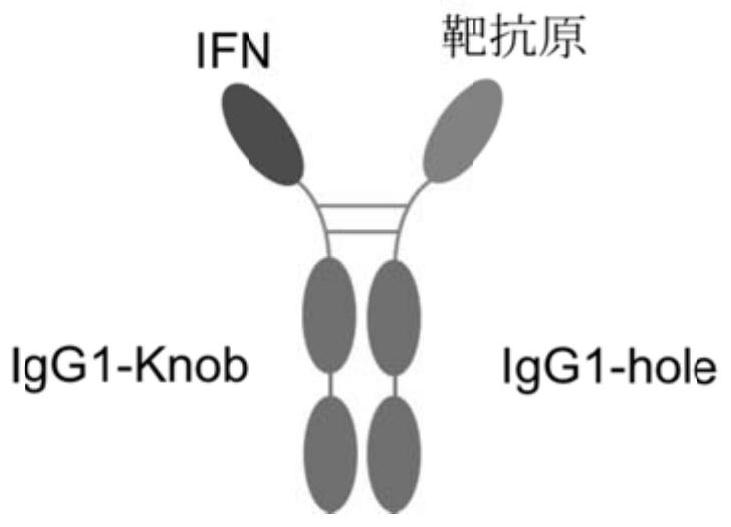
The invention relates to construction and application of a fusion protein vaccine platform. The present invention provides a vaccine, which comprises a fusion protein containing an interferon-target antigen-immunoglobulin Fc region (or antibody) and a Th cell helper epitope. The invention also relates to the use of a fusion protein containing an interferon-target antigen-immunoglobulin Fc (or antibody) region and a Th cell helper epitope for the preparation of a prophylactic or therapeutic composition. The vaccine provided by the invention can be generated through an eukaryotic cell expression system, the vaccine can be inoculated through subcutaneous / muscle or nasal cavity immune ways and the like, and can cause strong immune response of a body. The vaccine of the present invention can be used as a prophylactic or therapeutic vaccine.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
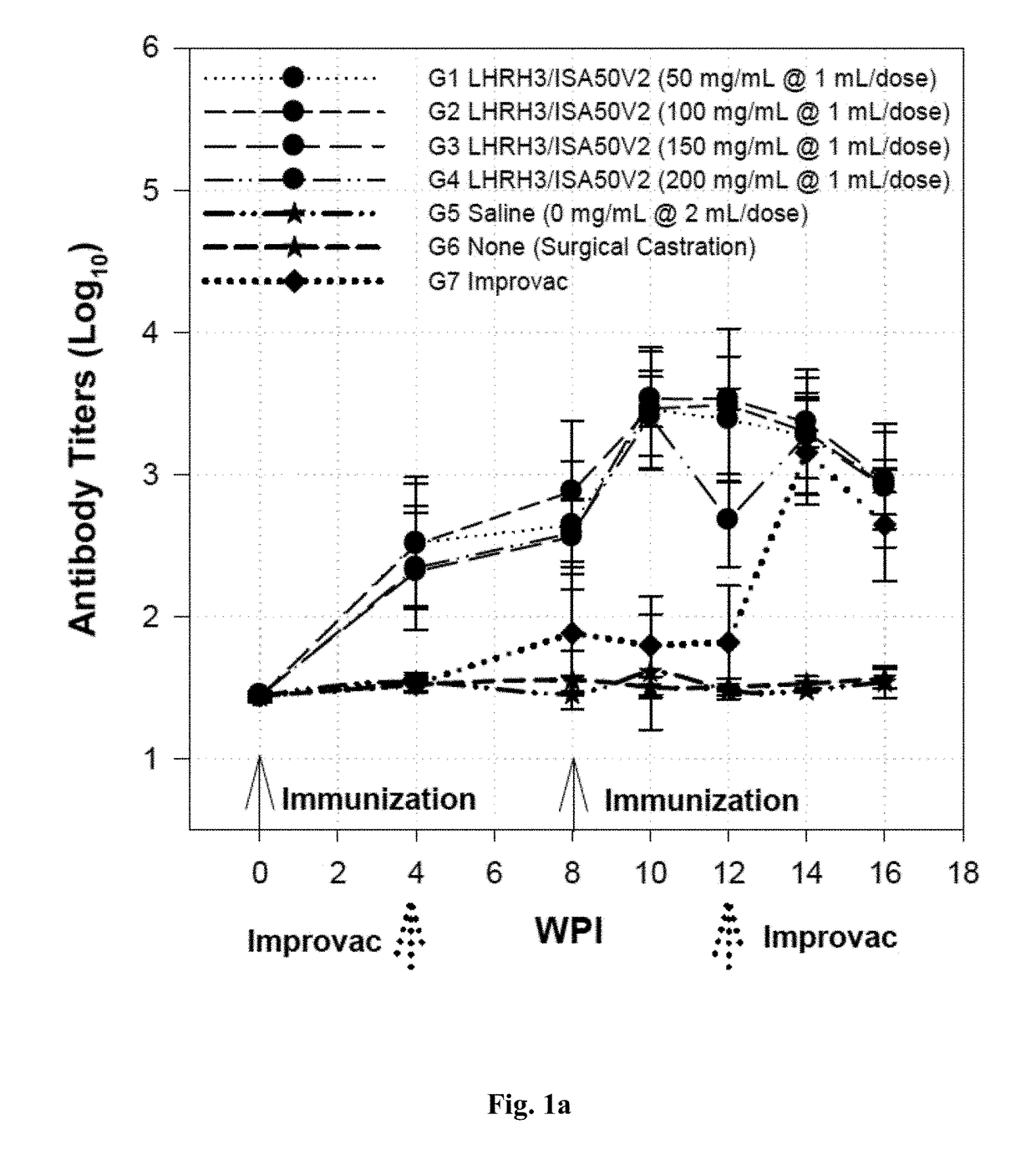
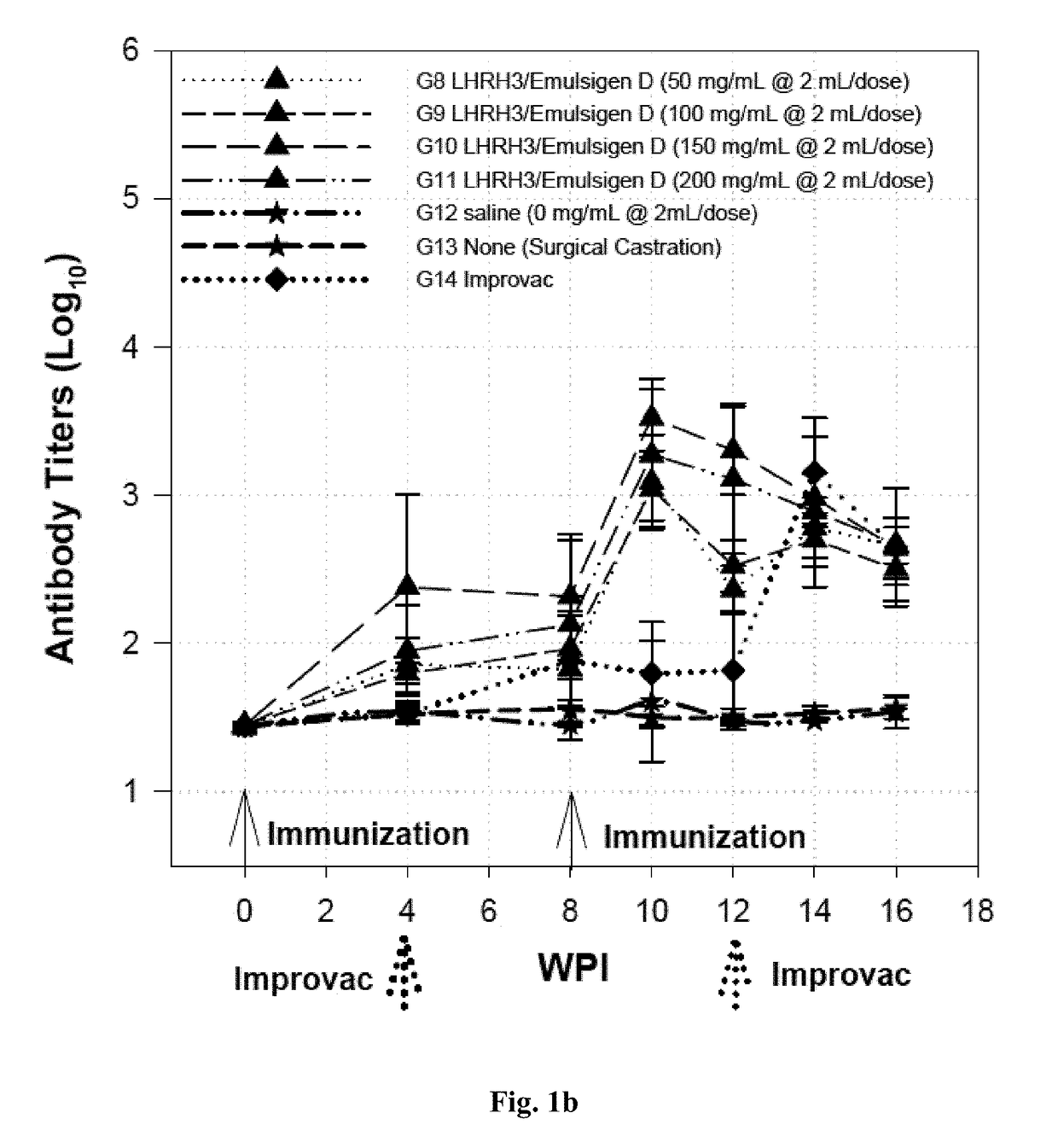
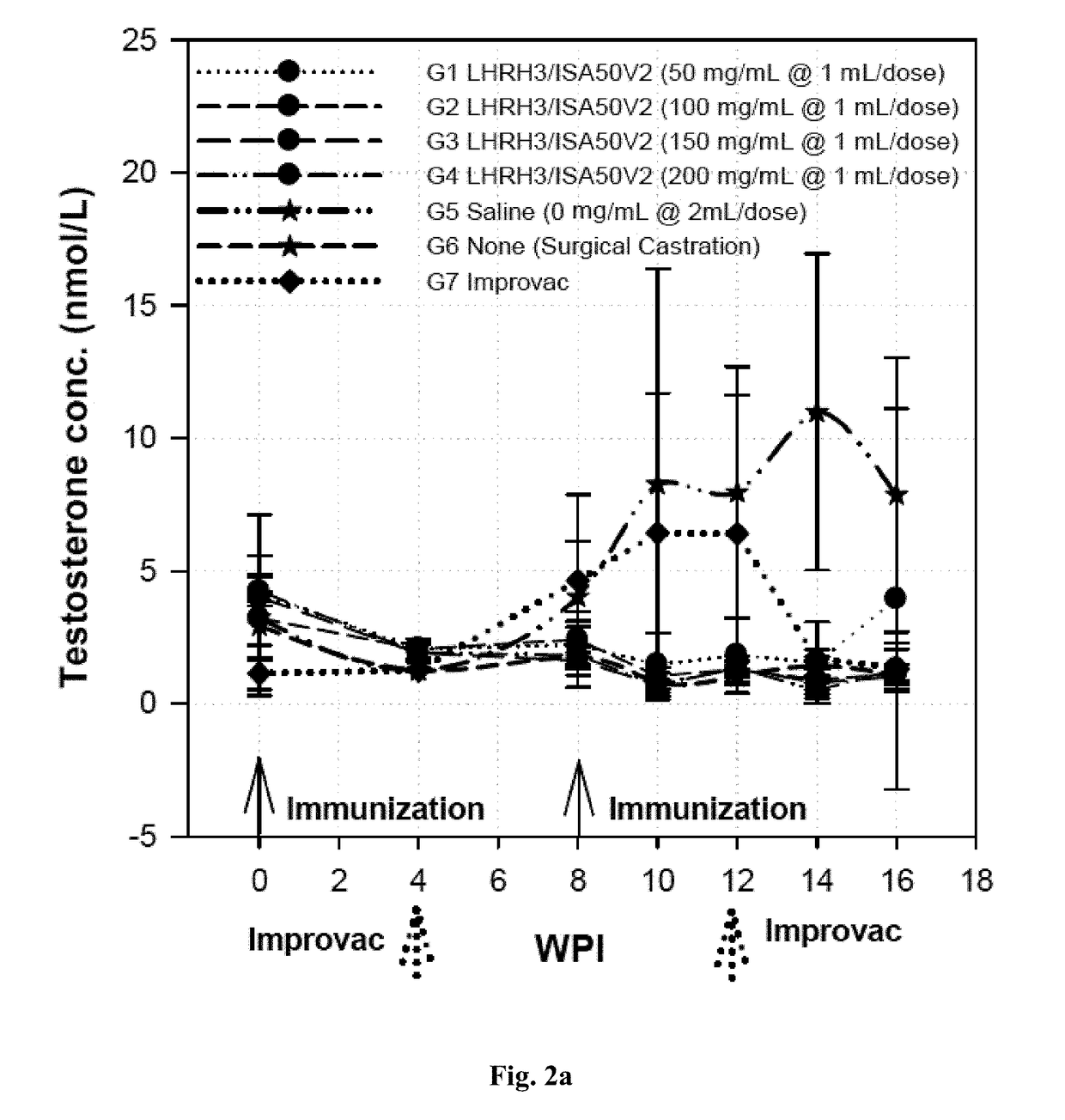
Immunogenic lhrh composition and use thereof in pigs
InactiveUS20170216418A1Effective immunocastrationImprove scalabilityLuteinising hormone-releasing hormoneContraceptive vaccin ingredientsAdjuvantBoar taint
A vaccine composition for castrating pigs, comprising a peptide immunogen and a veterinarily acceptable delivery vehicle or adjuvant, wherein the peptide immunogen comprises (a) a LHRH peptide of SEQ ID NO: 1, and (b) at least one T helper epitope selected from a group consisting of SEQ ID NOs: 2, 3, 4, and 5, and, optionally, an immunostimulatory peptide of SEQ IN NO: 6, wherein the LHRH peptide is covalently linked through its N-terminus residue to the T helper epitope or immunostimulatory peptide. A method for castrating or inhibiting characteristics, including boar taint, induced by the sexual maturation of pigs using the vaccine composition is also disclosed.
Owner:UNITED BIOMEDICAL INC
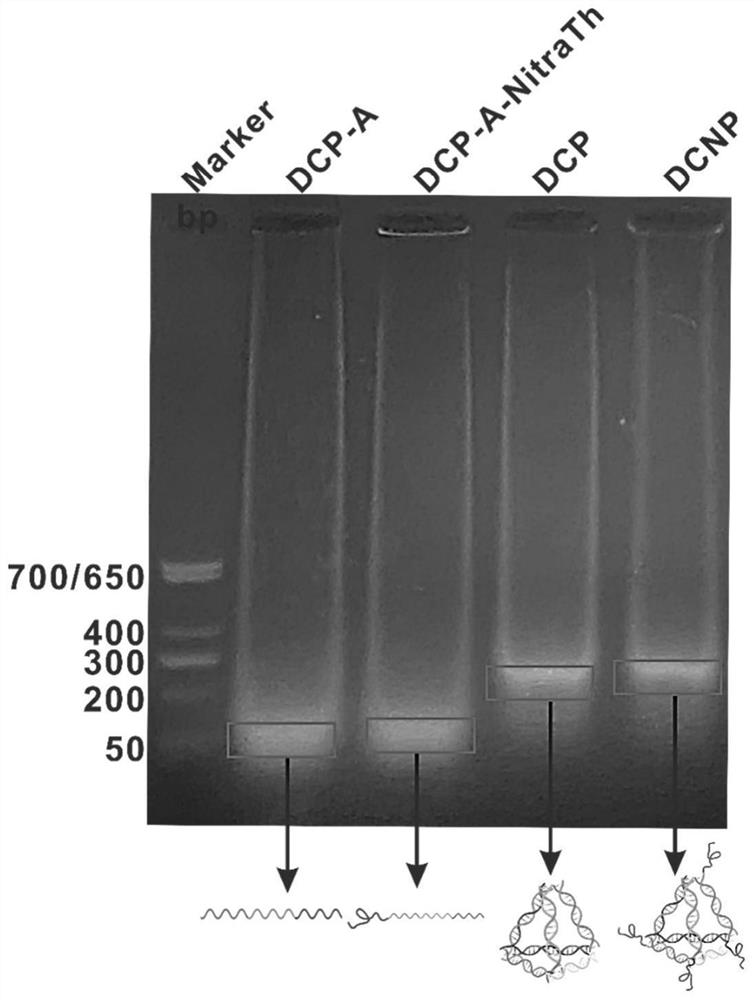
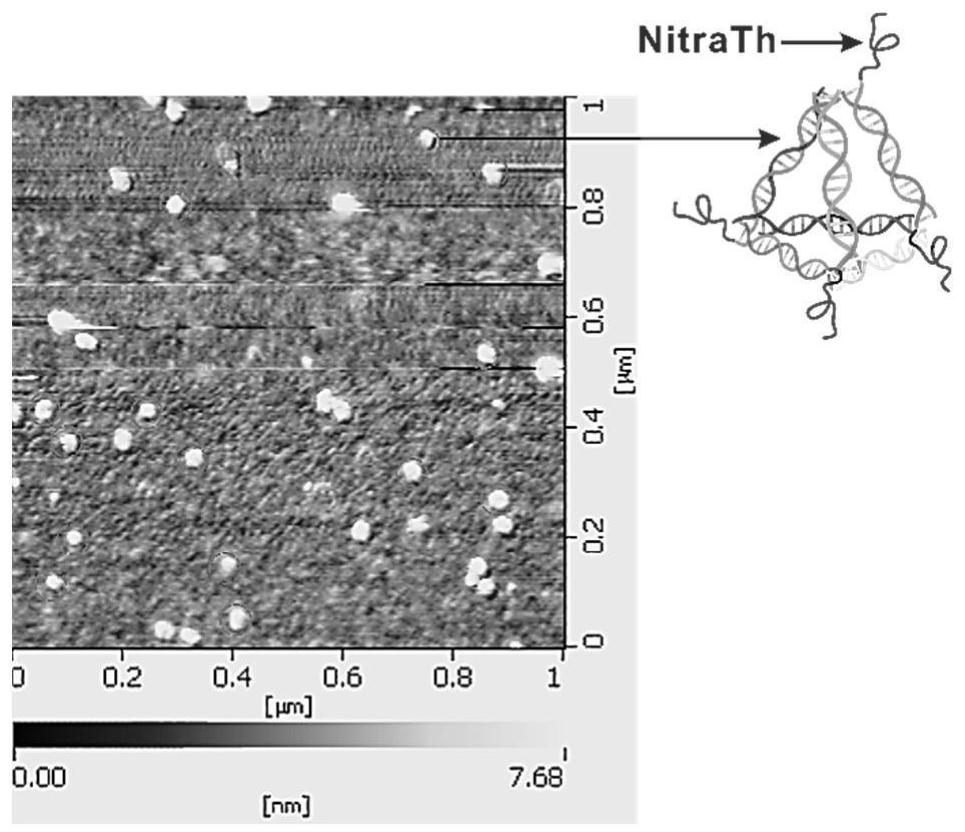
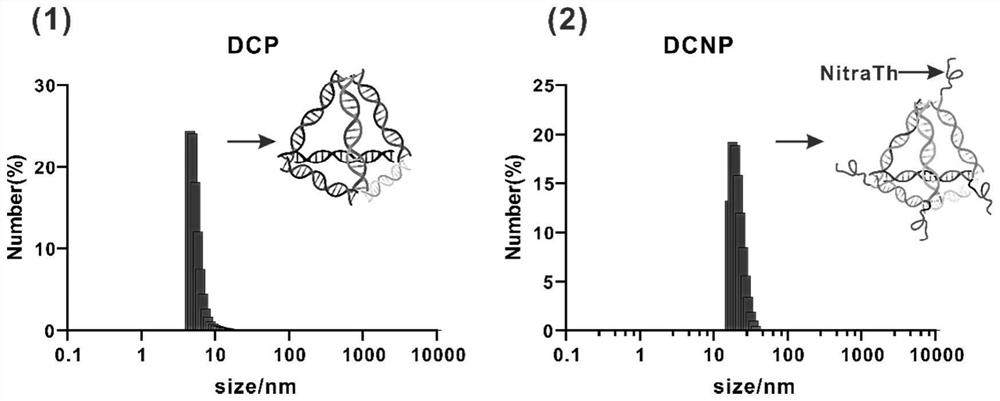
Self-assembled DNA tetrahedron and peptide vaccine delivery system
PendingCN114181935APromote recruitmentGood biocompatibilityCancer antigen ingredientsPharmaceutical non-active ingredientsAntigenHelper epitope
The invention relates to a self-assembled DNA tetrahedron and a peptide vaccine delivery system. And the self-assembled DNA tetrahedron is composed of four single-stranded DNAs. The recombinant self-assembled DNA tetrahedron is obtained by recombining a self-assembled DNA tetrahedron. According to the peptide vaccine delivery system, a recombinant self-assembly DNA tetrahedron serves as a main body, and the 5'end or the 3 'end of each single-stranded DNA of the main body is coupled with helper epitope peptide or peptide vaccine.By means of the delivery system, the vaccines can efficiently reside in lymph nodes and are taken and presented by DC cells, so that antigen-specific T cells are effectively activated, and tumor growth is inhibited.
Owner:CHINA PHARM UNIV +1
Novel immunogenic mimetics of multimer proteins
InactiveCN1615316AAntibody mimetics/scaffoldsGenetic material ingredientsInterleukin 5White blood cell
The present invention relates to novel immunogenic variants of multi-subunit proteins such as interleukin 5 (IL5) and tumor necrosis factor alpha (TNF, TNF alpha). In addition to being immunogenic in the autologous host, the variants also highly resemble the native 3D structure of the protein from which they were derived. Certain variants are monomeric mimics of multimers in which a peptide linker (containing an inert or T helper epitope) ensures spatial organization of the monomeric unit that facilitates correct folding. One subtype of variant is the monomeric TNFα variant, which displays a superior ability to assemble into multimers with a high structural similarity to the native protein. Methods of processing and producing variants as well as DNA fragments, vectors and host cells are also disclosed.
Owner:PHARMEXA
Pig O/Mya98 and O/PanAsia type foot-and-mouth disease gene engineering inactivated vaccine
The invention relates to a fusion protein for preventing O-type foot-and-mouth disease virus, and a preparation method and application of the fusion protein. In particular, the present invention relates to a fusion protein, and the fusion protein comprises a molecular adjuvant IL-2 polypeptide, a T cell helper epitope polypeptide, a polypeptide with seven segments of epitopes related to O / Mya98 and O / PanAsia type foot-and-mouth disease virus strain main structural protein VP1, VP2 and VP3, and a killer T cell epitope polypeptide. The invention also relates to the preparation method and application of the fusion protein. The vaccine production preparation process is stable and is suitable for large-scale production. Tests show that the vaccine is safe to use and can effectively prevent infection of different O-type foot-and-mouth disease epidemic strains.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
Novel Scaffolded HIV-1 Vaccine Immunogens
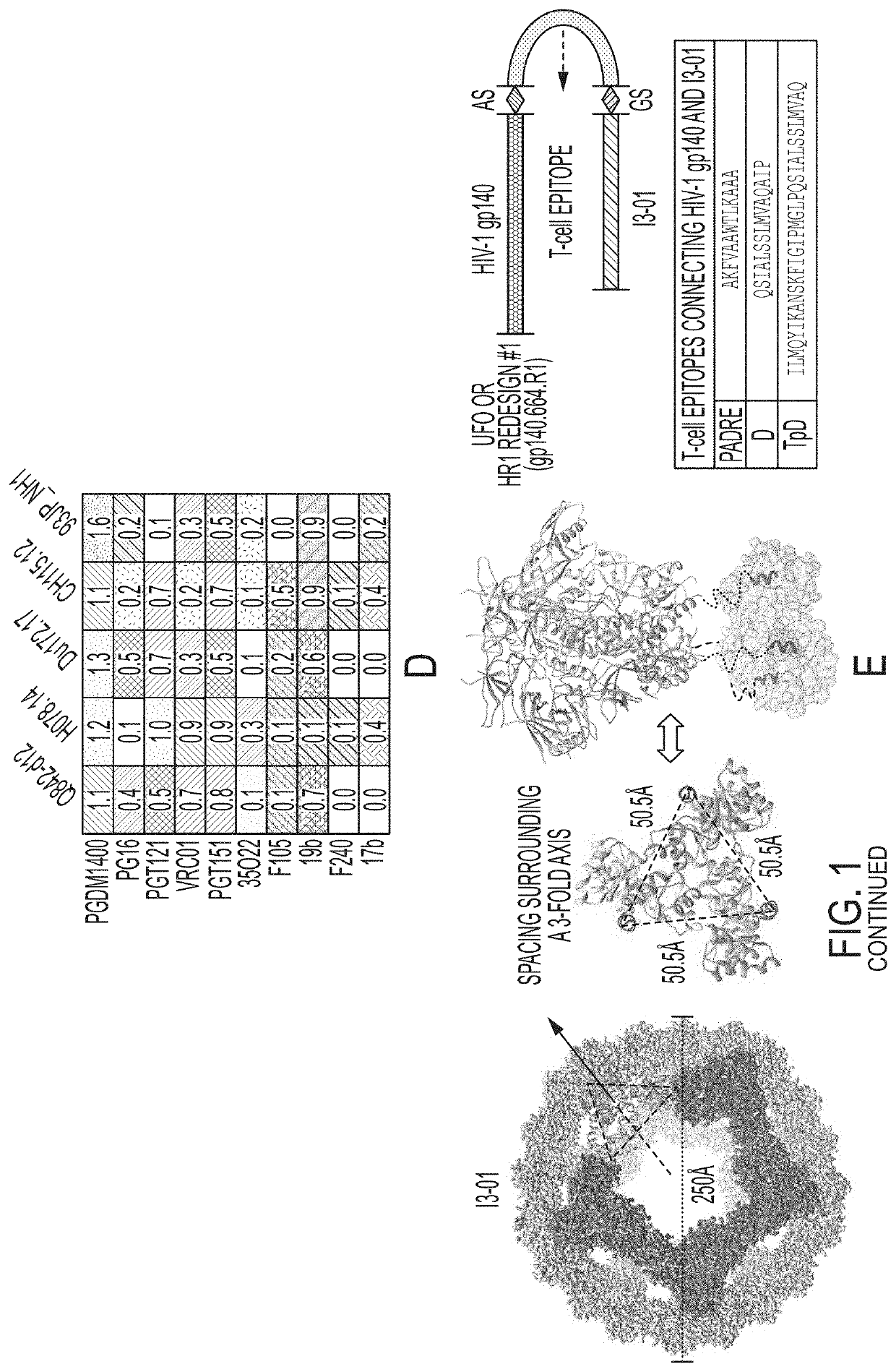
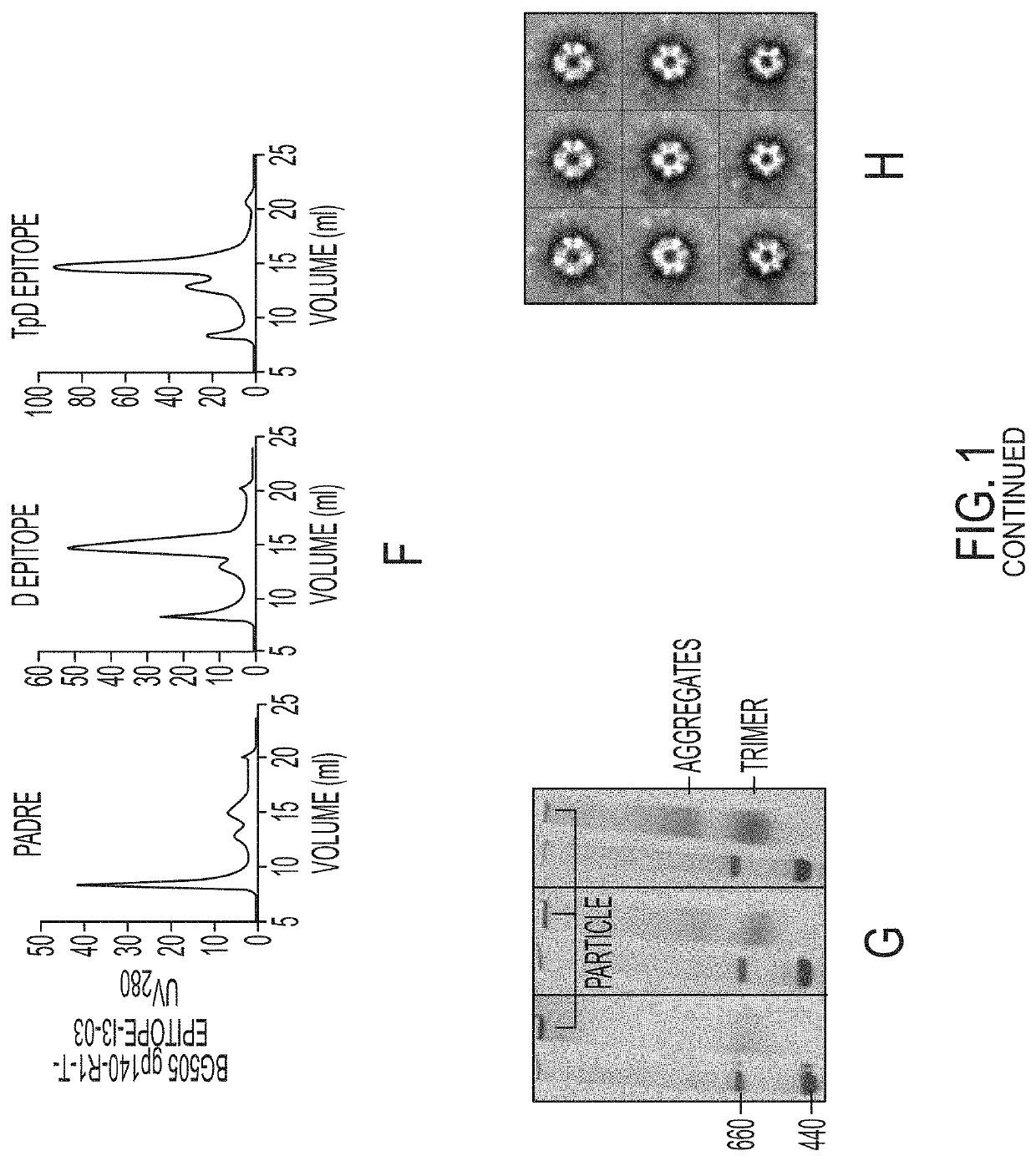
The present invention provides novel scaffolded HIV-1 vaccine immunogens. Some of the scaffolded immunogens contain a soluble gp140 trimer linked to the N-terminus of the nanoparticle subunit and a T-helper epitope that is fused via a short peptide spacer to the C-terminus of the nanoparticle subunit. Some other immunogens of the invention contain a soluble gp140 trimer protein that is linked to a stable nanoparticle via a short peptide spacer that is a T-helper epitope. Some of the scaffolded immunogens contain a gp140 trimer immunogen presented on a nanoparticle platform formed with I3-01 protein, E2p, or variants of protein 1VLW. Also provided in the invention are nucleic acids that encode the various vaccine immunogens described herein, and expression vectors and host cells harboring the nucleic acids. The invention further provides methods of using the scaffolded HIV-1 vaccine immunogens for preventing or treating HIV infections.
Owner:THE SCRIPPS RES INST
Synthetic immunogen useful for generating long lasting immunity and protection against pathogens
InactiveUS9340622B2Generating long lasting protective immunityInduced proliferationAntibacterial agentsPowder deliverySynthetic ImmunogensDisease
The present invention relates to a synthetic immunogen represented by the general formula 1, useful for generating long lasting protective immunity against various intracellular pathogens which are the causative agents of tuberculosis, leishmaniasis, AIDS, trypanosomiasis, malaria and also allergy, cancer and a process for the preparation thereof. The developed immunogen is able to circumvent HLA restriction in humans and livestock. The invention further relates to a vaccine comprising the said immunogen for generating enduring protective immunity against various diseases. The said vaccine is targeted against intracellular pathogens, more particularly the pathogen M. tuberculosis in this case. In the present invention, promiscuous peptides of M. tuberculosis are conjugated to TLR ligands especially; Pam2Cys to target them mainly to dendritic cells and therefore elicit long-lasting protective immunity.wherein, X1=a promiscuous CD4 T helper epitope selected from SEQ ID No. 1 to 98 OR nil;X2=a promiscuous CD8 T cytotoxic epitope selected from SEQ ID No. 99 to 103 OR nil;when X1=nil; X2=SEQ ID No. 99 to 103 and when X2=nil; X1=SEQ ID No. 1 to 98;Y=Lysine; andS=Serine.
Owner:COUNCIL OF SCI & IND RES +1
Vaccines for suppressing ige-mediated allergic disease and methods for using the same
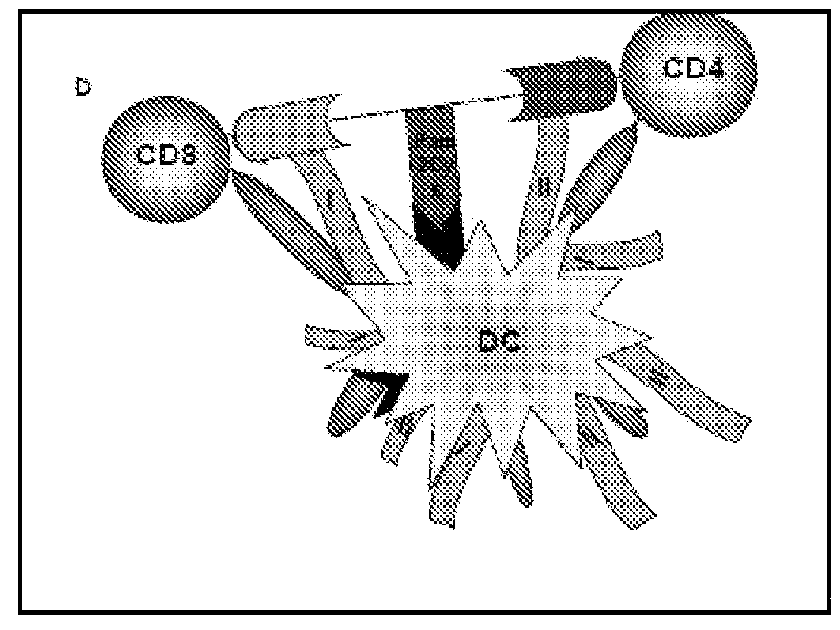
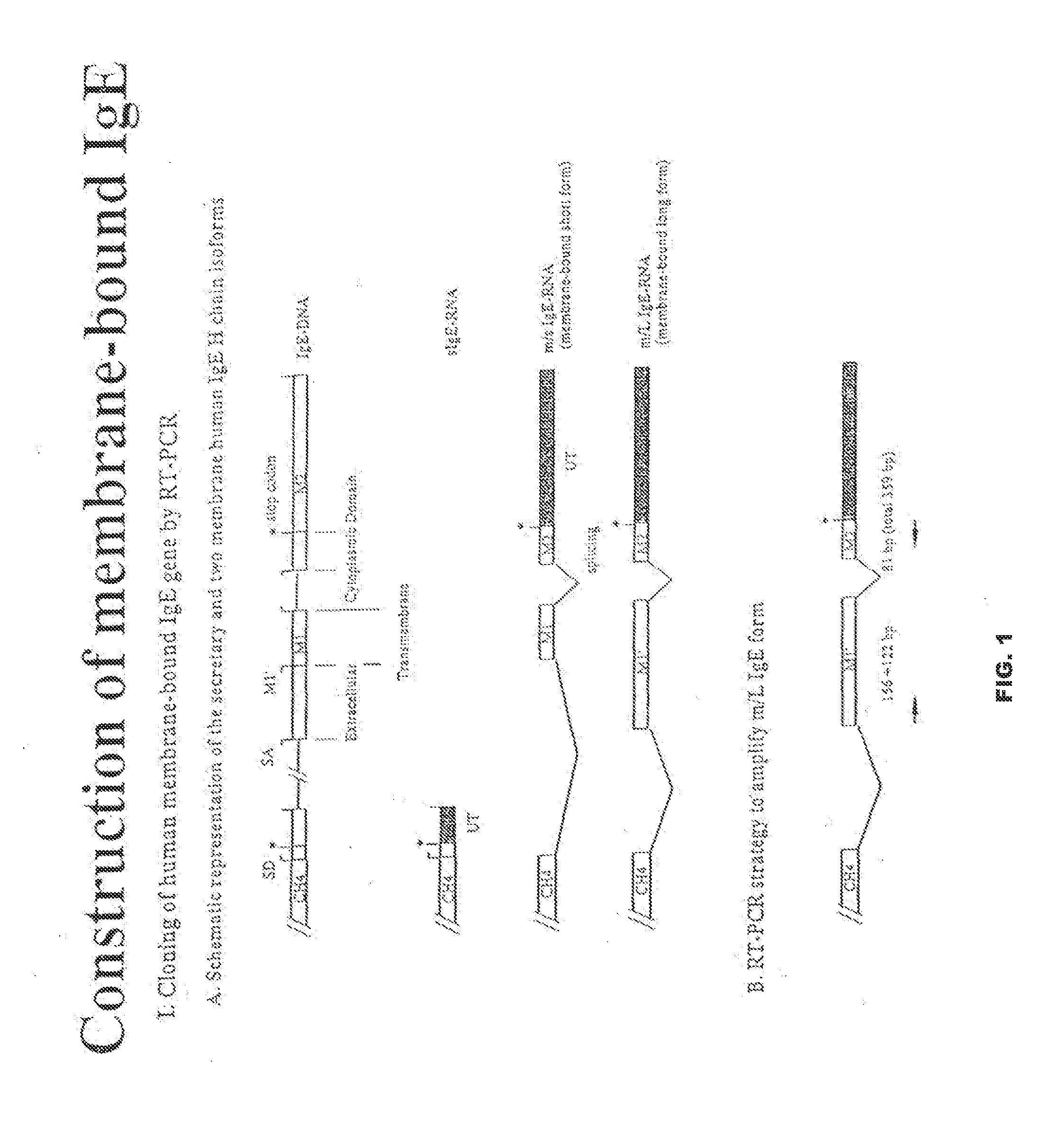
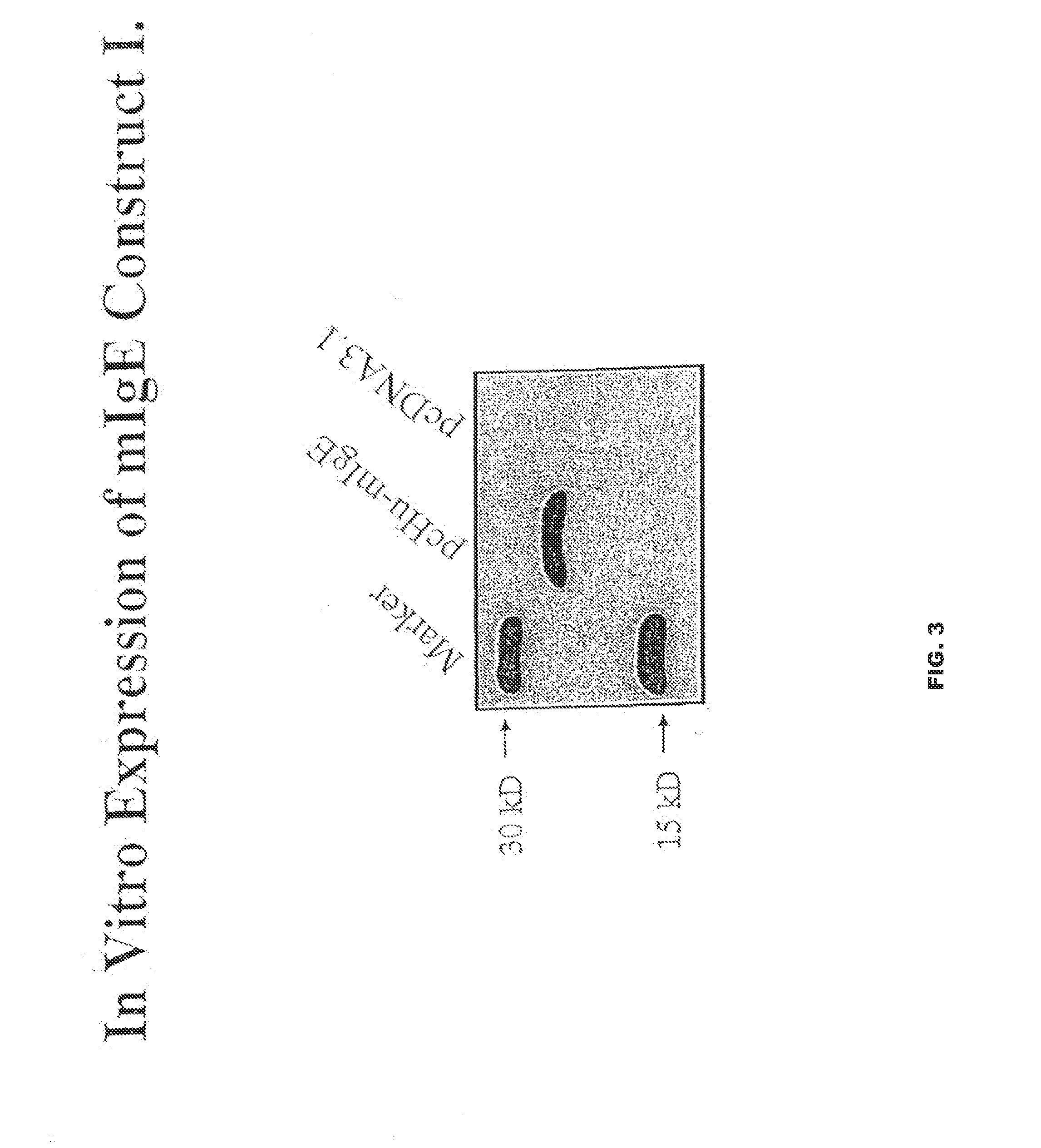
Nucleic acid molecules that encode a protein comprising at least one epitope of membrane IgE free of epitopes present on the serum IgE, including proteins that further comprise non-IgE T cell helper epitope are disclosed. Vaccines, vectors and host cells that comprise such nucleic acid molecules are disclosed. Isolated proteins, including haptenized proteins, comprising at least one epitope of membrane IgE free of epitopes present on the serum IgE, including proteins that further comprise non-IgE T cell helper epitope are disclosed. Vaccines that comprise and methods of making such proteins and antibodies that specifically bind to such proteins are disclosed. Vaccines that comprise killed or inactivated cells or particles are disclosed. Methods of treating and preventing IgE mediated allergic disease or condition are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Cancer vaccines against mucosal antigens and methods of making and using the same
Nucleic acid molecules comprising a nucleotide sequence that encodes a chimeric protein are disclosed. The chimeric proteins comprise at least one epitope of a mucosally restricted antigen, at least one CD4+ helper epitope, and, optionally, a secretion sequence. Chimeric proteins that comprise at least one epitope of a mucosally restricted antigen, at least one CD4+ helper epitope and, optionally a secretion sequence are also disclosed. Compositions including pharmaceutical compositions and injectables comprising nucleic acid molecule and proteins are disclosed. Methods of treating individuals diagnosed with cancer of a mucosal tissue and methods of preventing cancer of a mucosal tissue are disclosed.
Owner:THOMAS JEFFERSON UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com