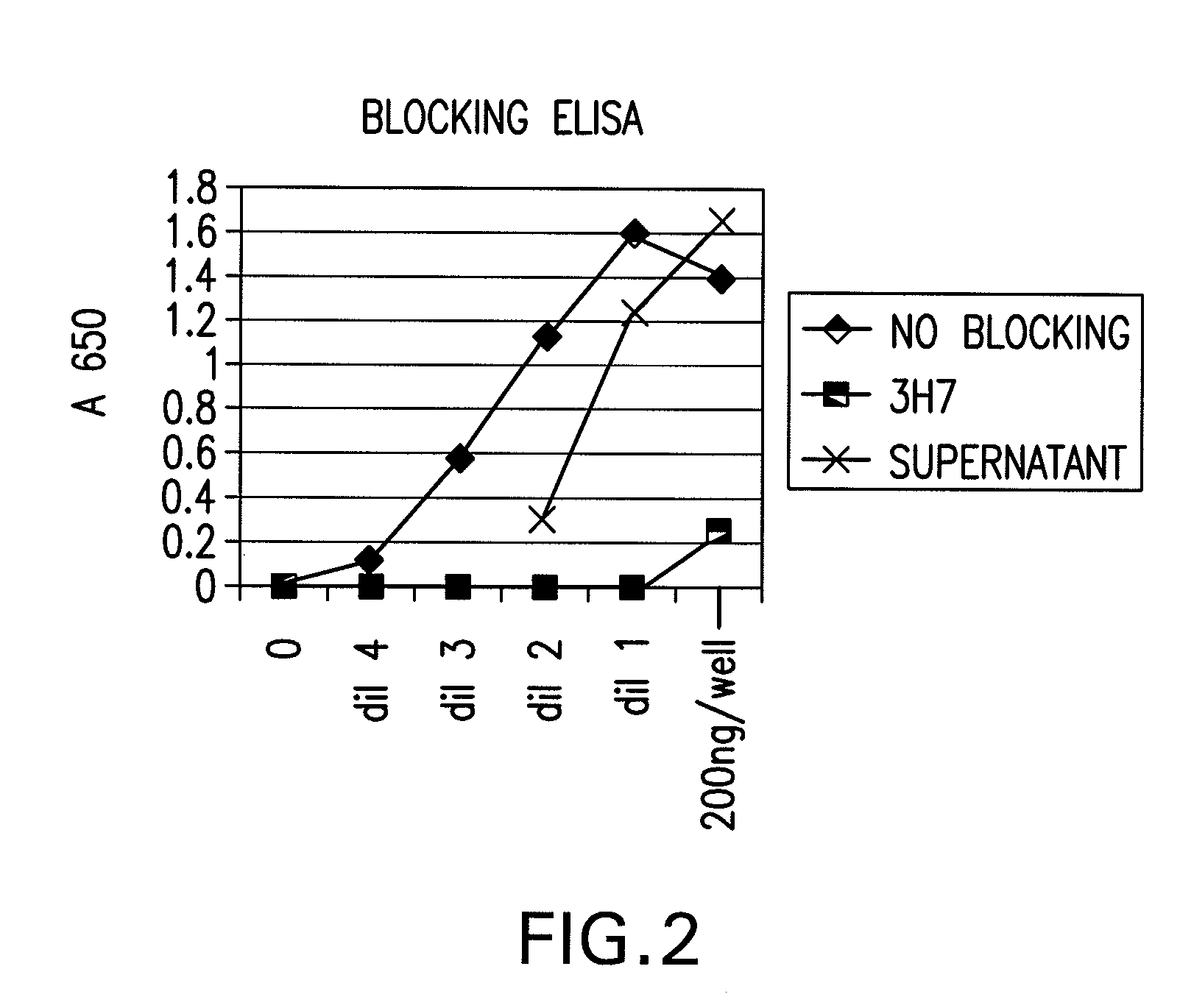
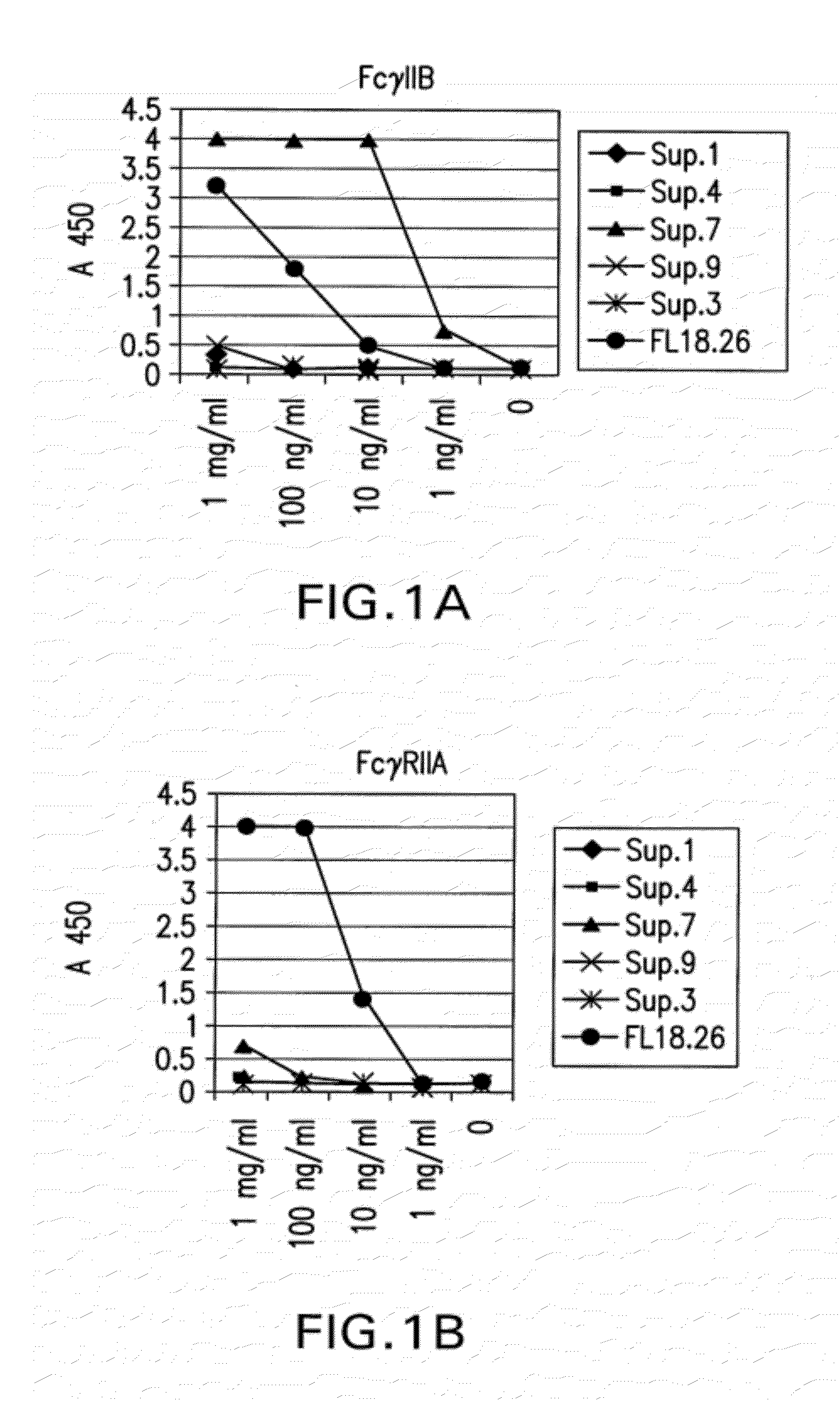
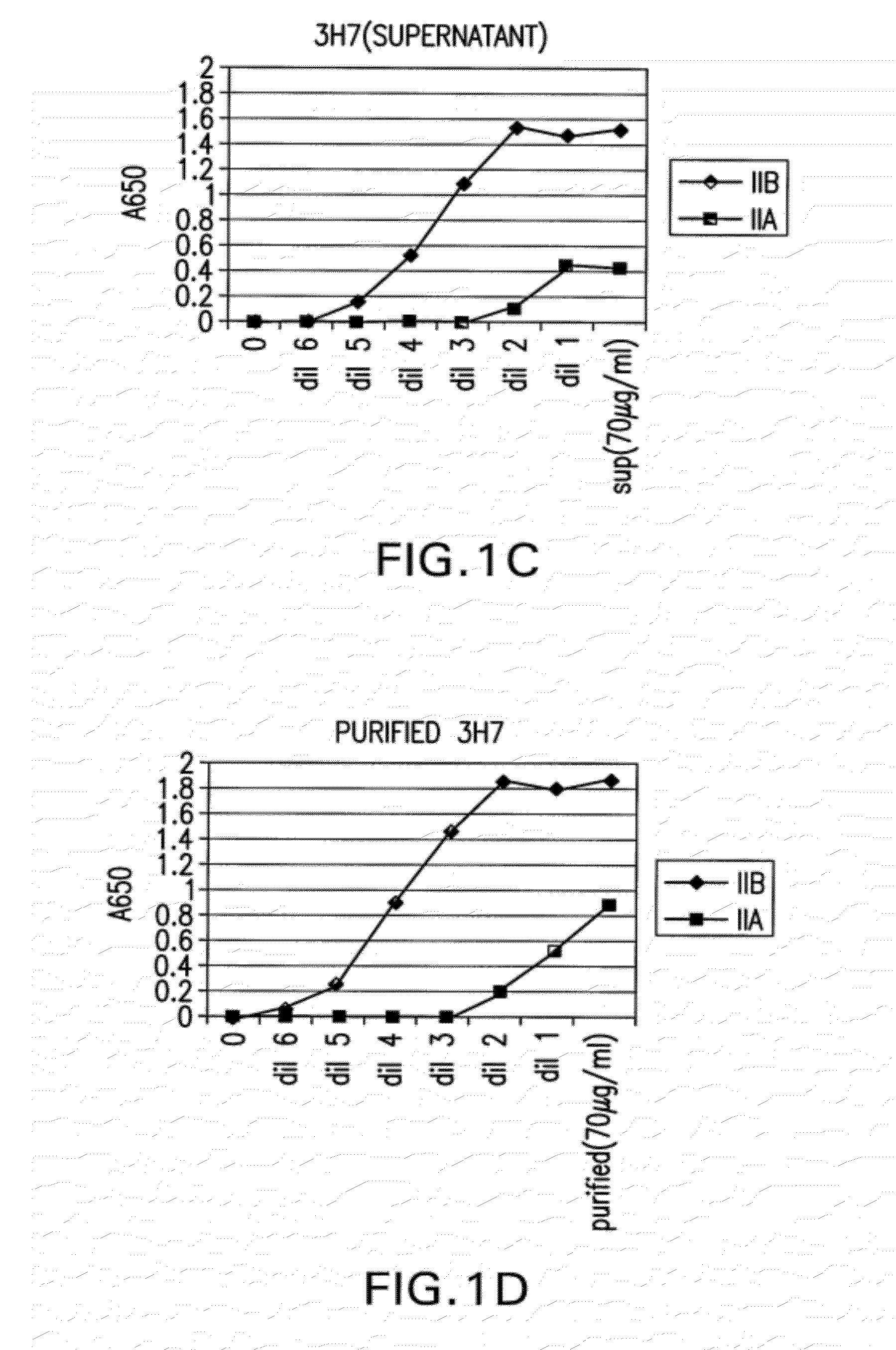
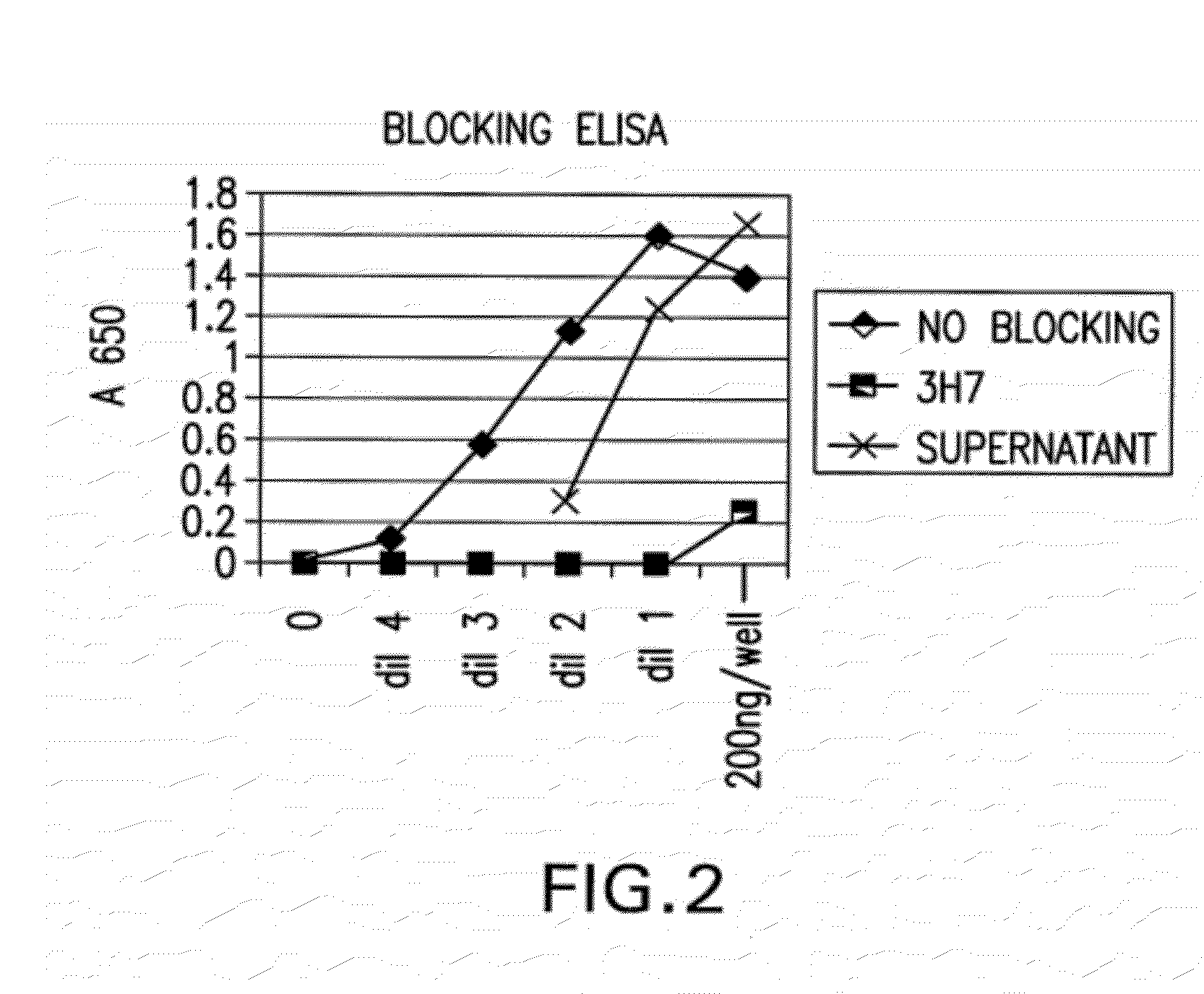
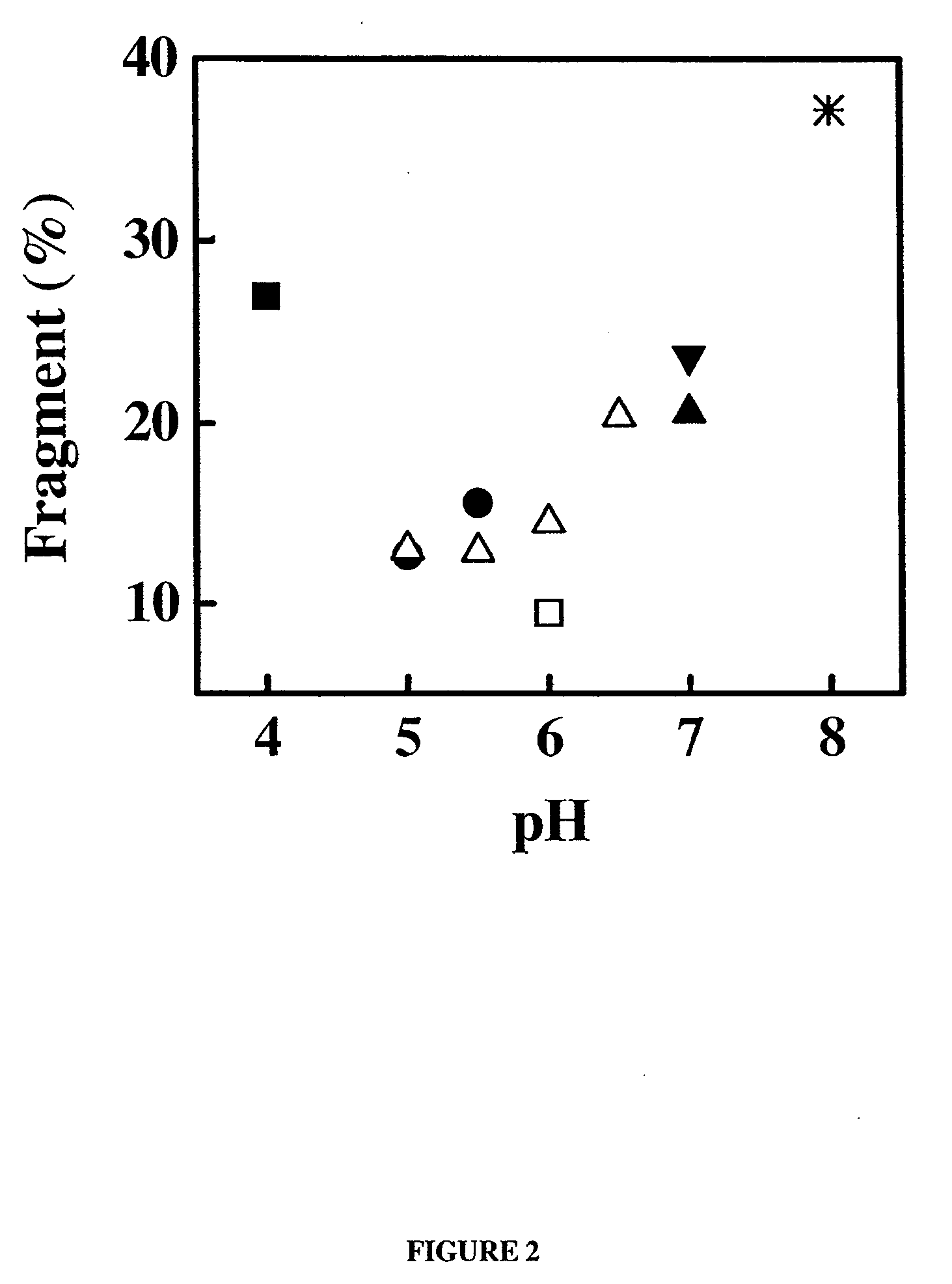
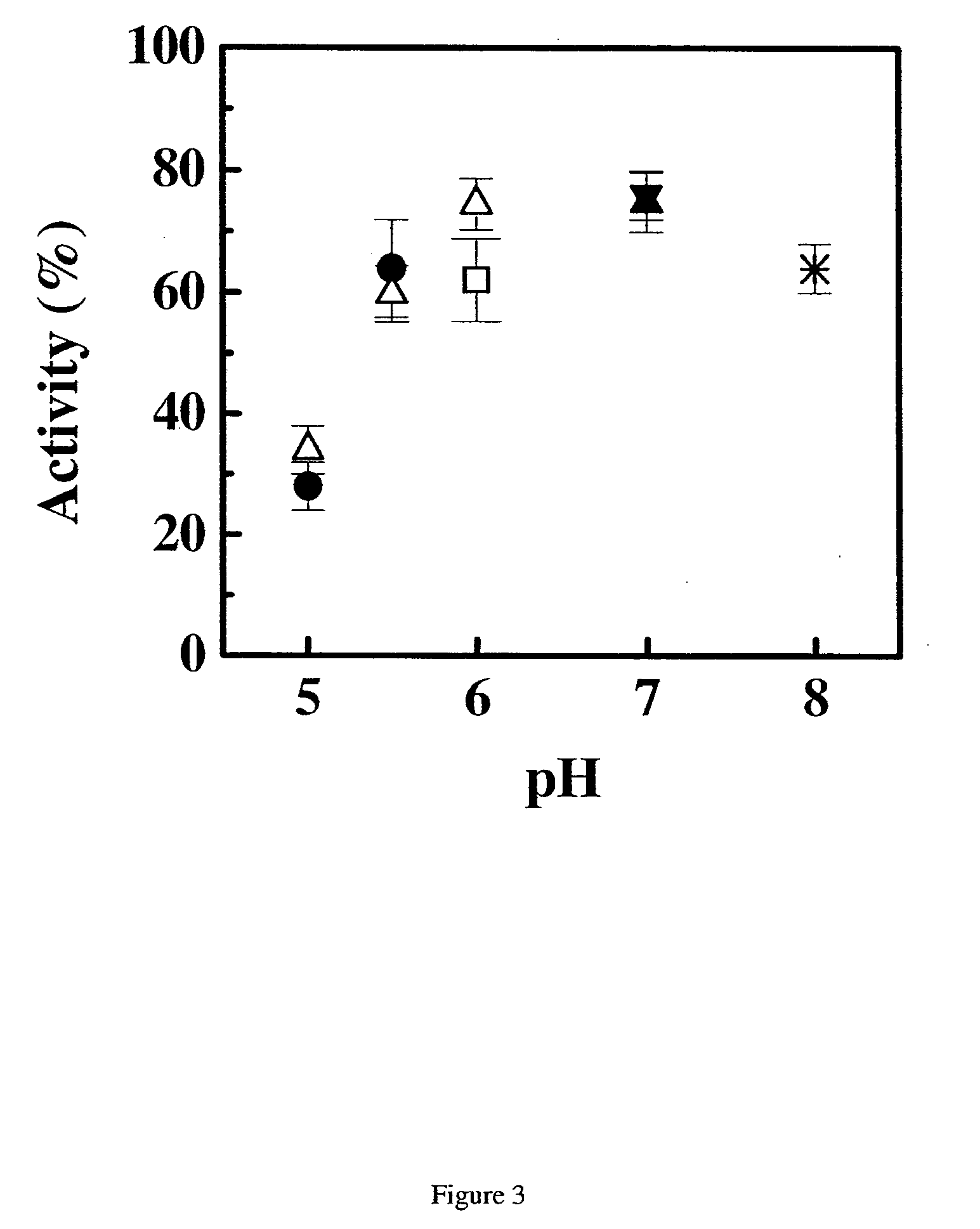
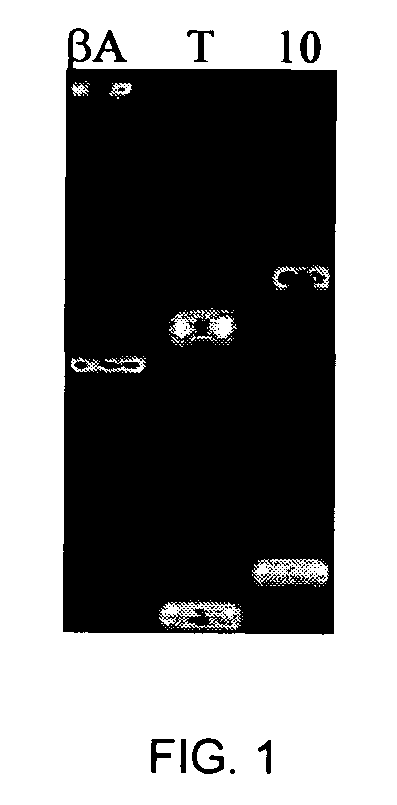
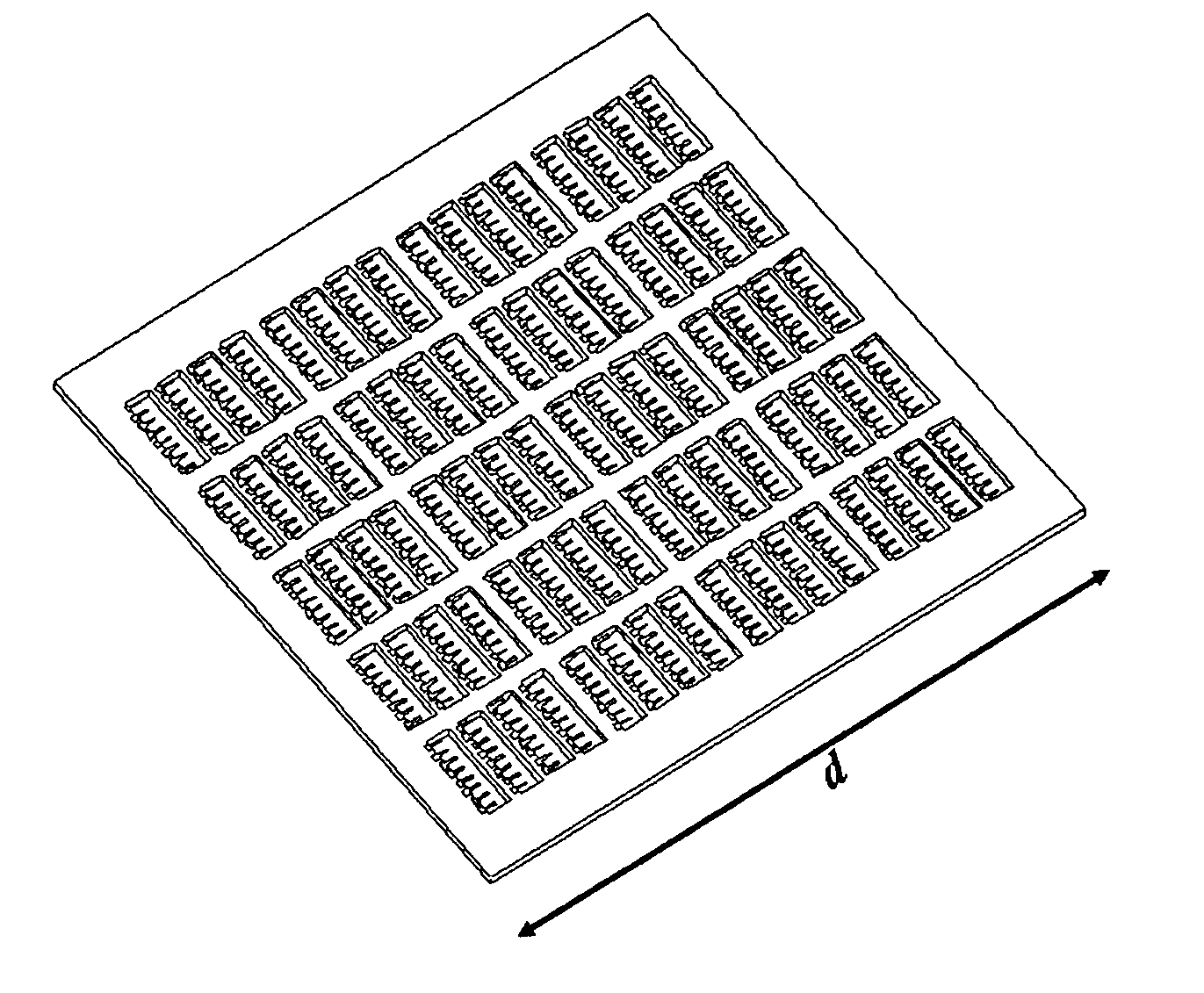
Patents
Literature
104 results about "Ige mediated" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
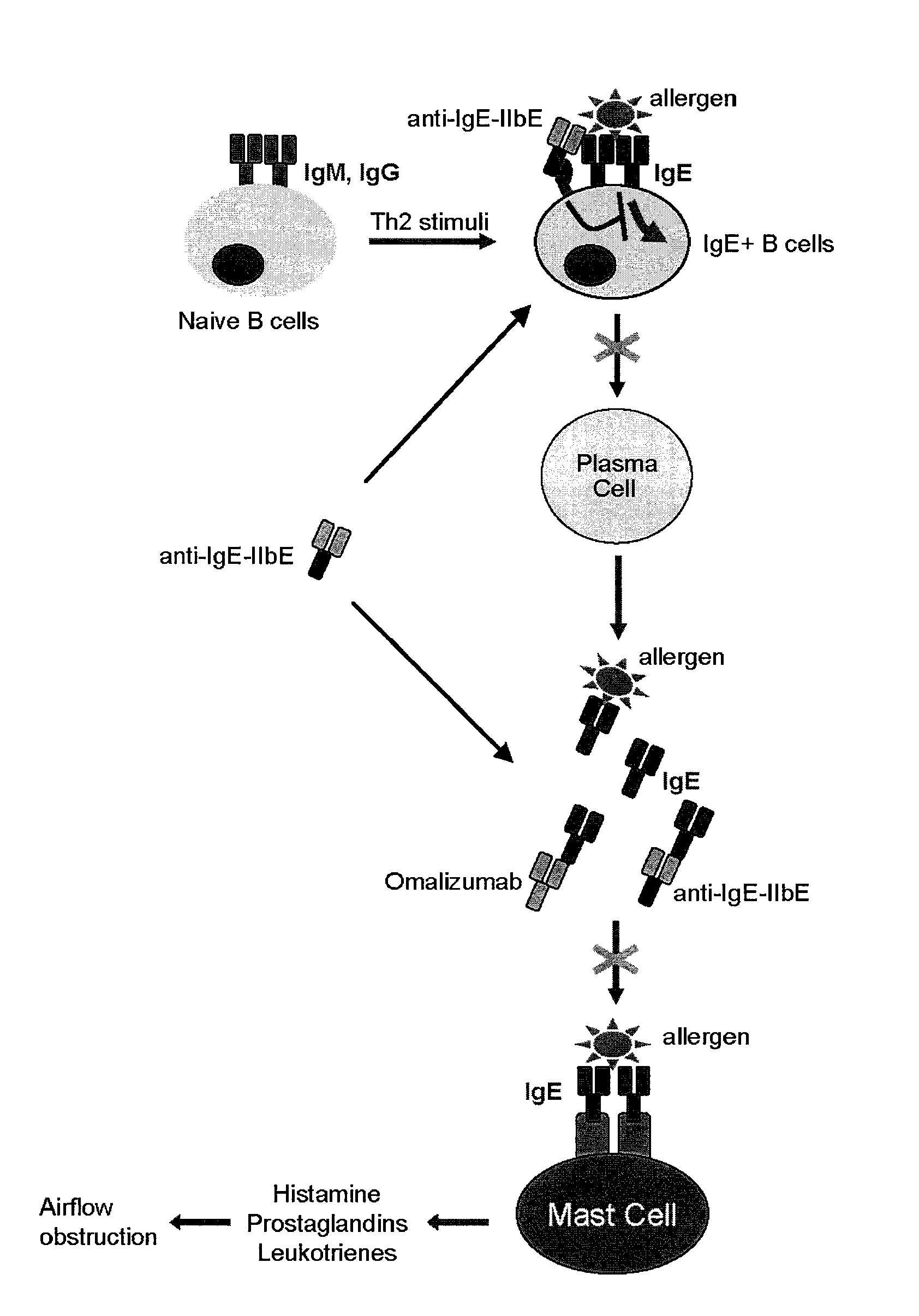
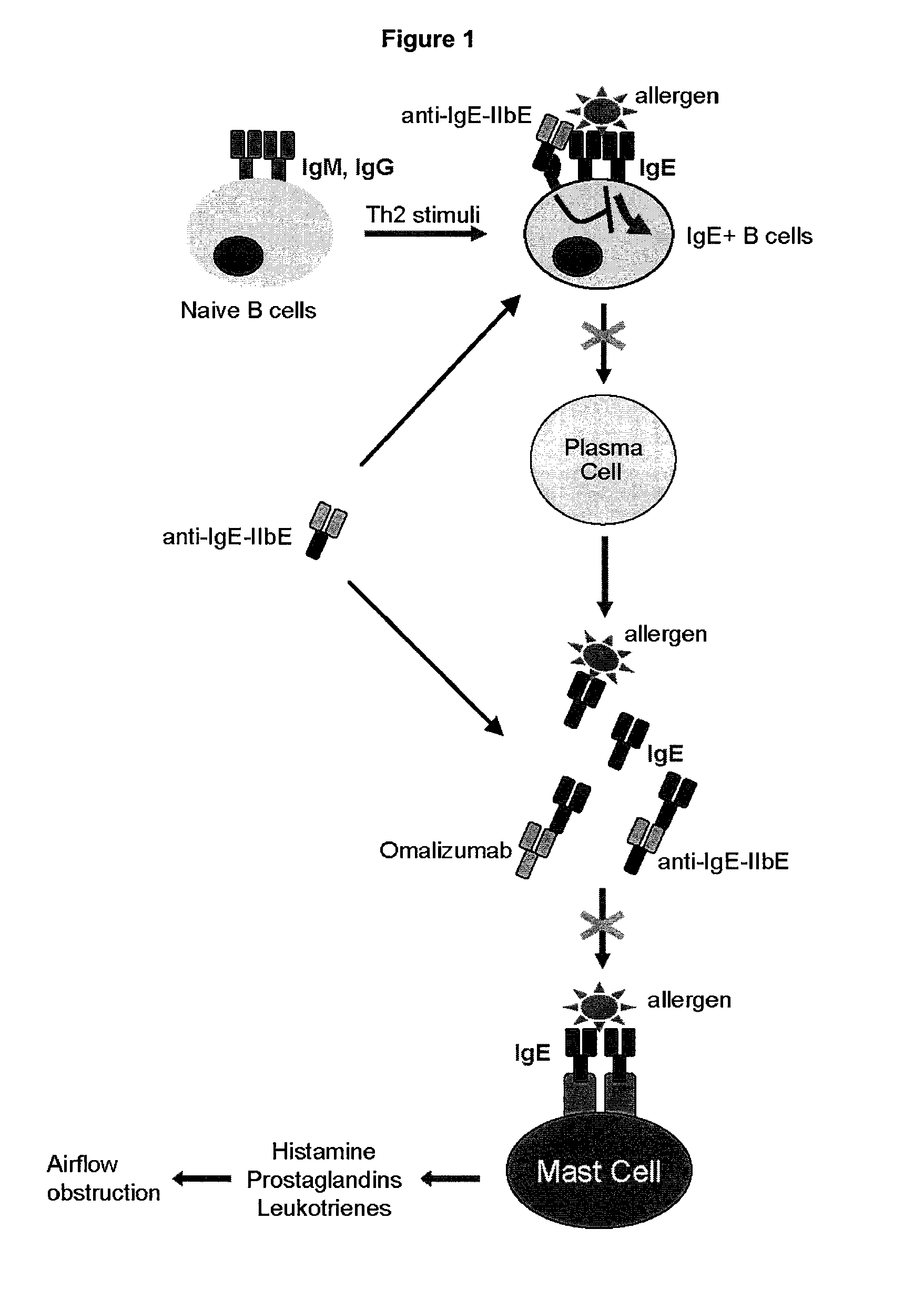
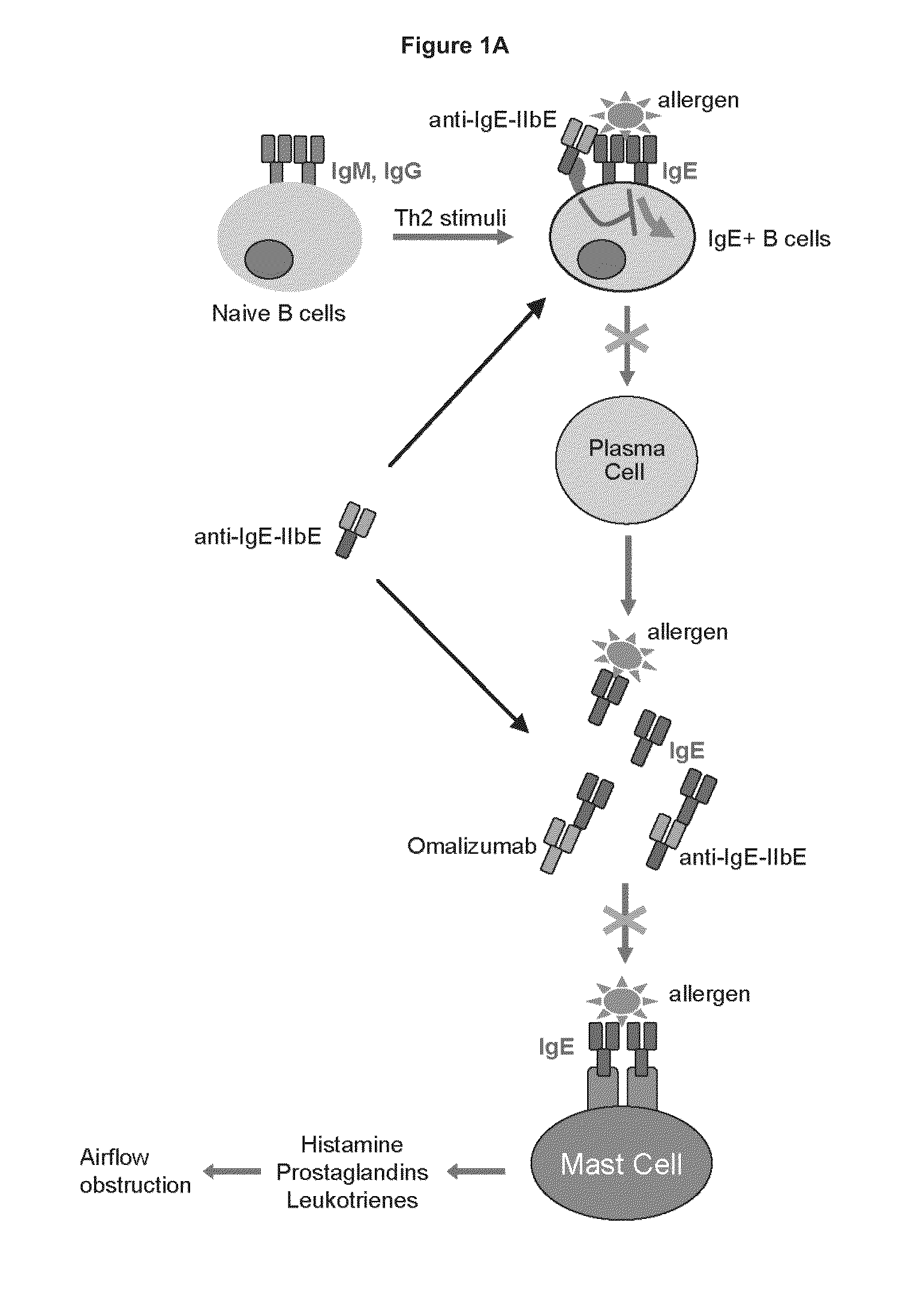
IgE mediated asthma, also known as allergic asthma, is the most common form of asthma. In allergic asthma, the body initiates an immune response against an allergen which results in the production of IgE antibodies.
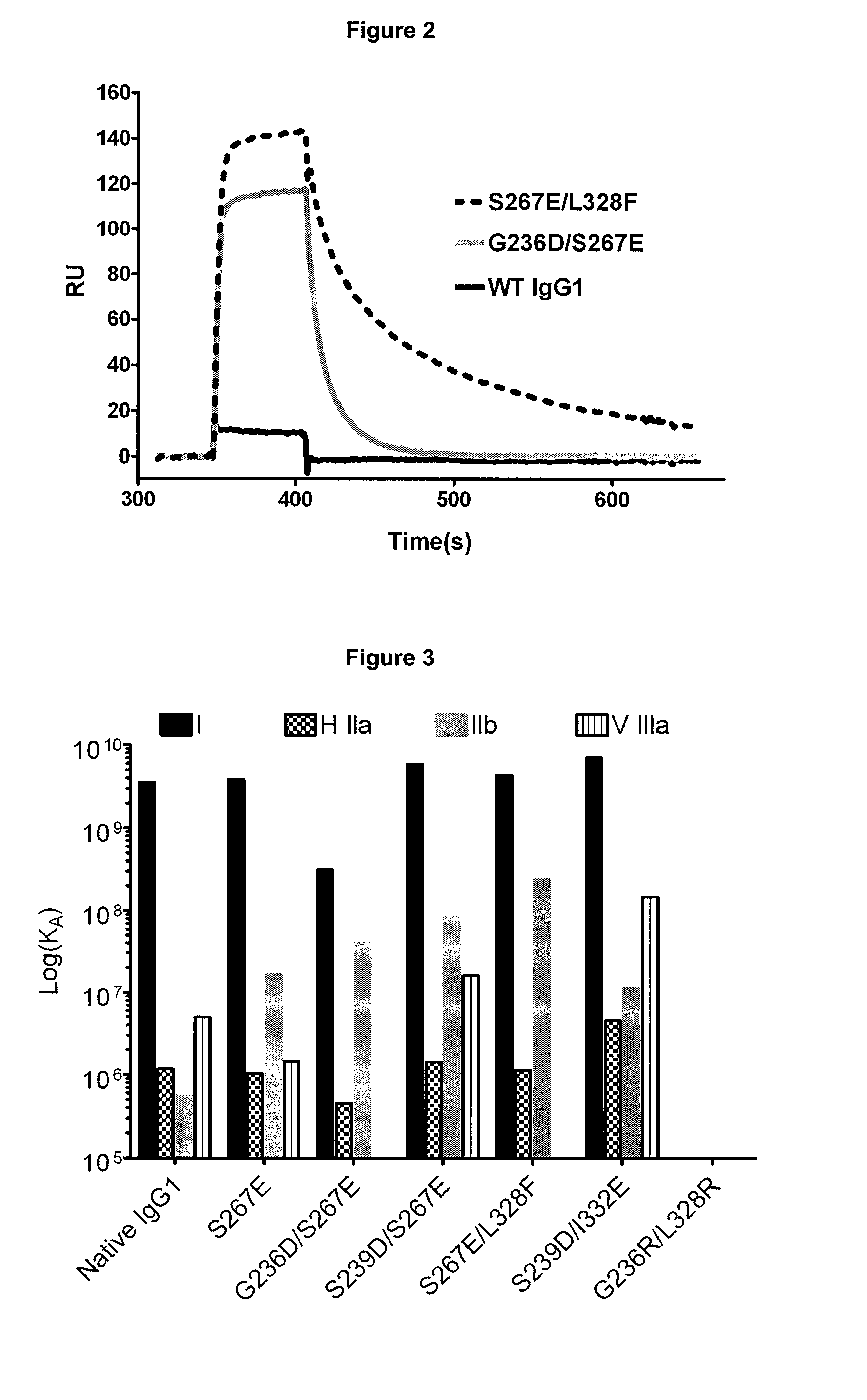
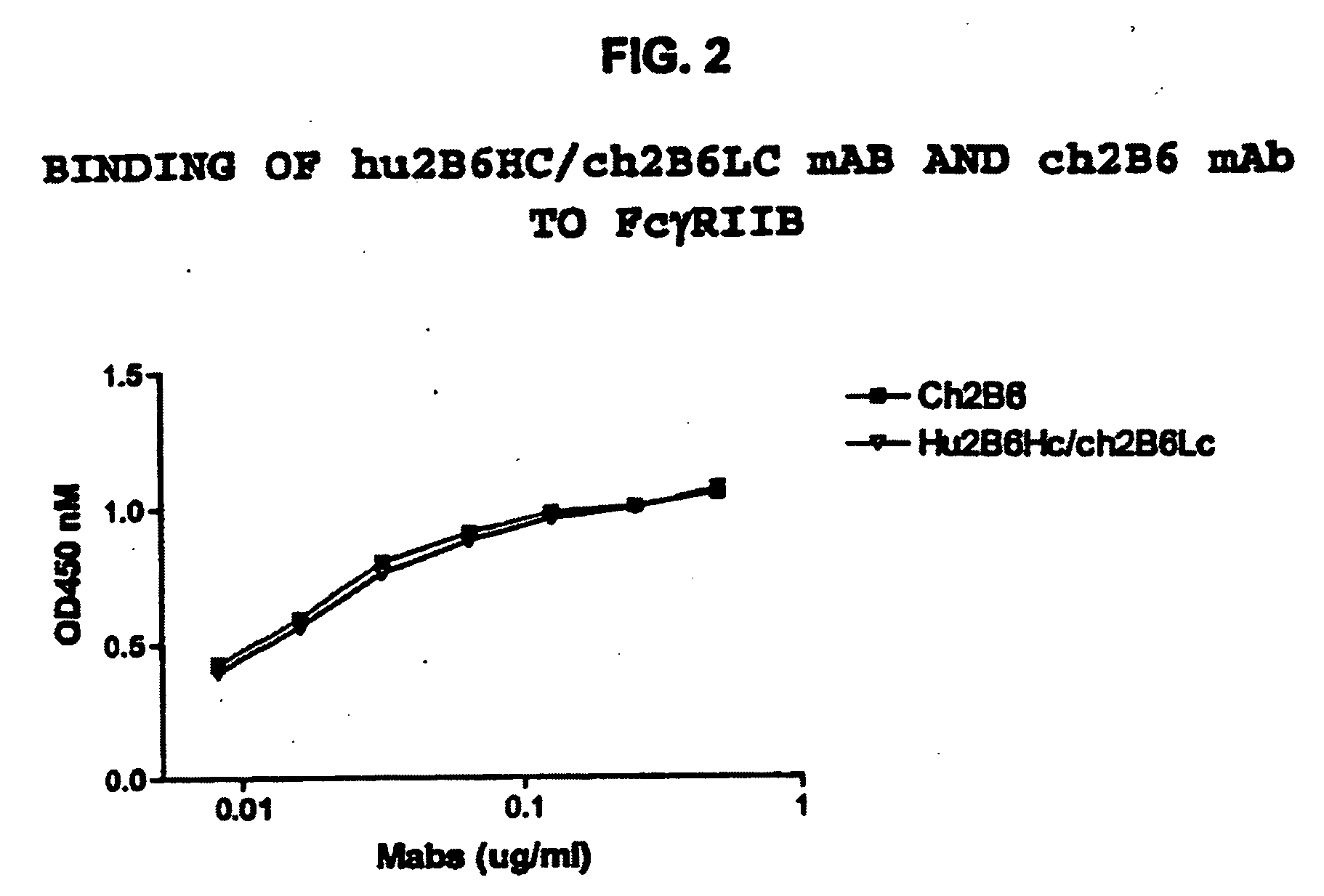
Fcgamma-RIIB-specific antibodies and methods of use thereof
InactiveUS20050260213A1Good curative effectAvoid managementHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen Binding FragmentTherapeutic effect
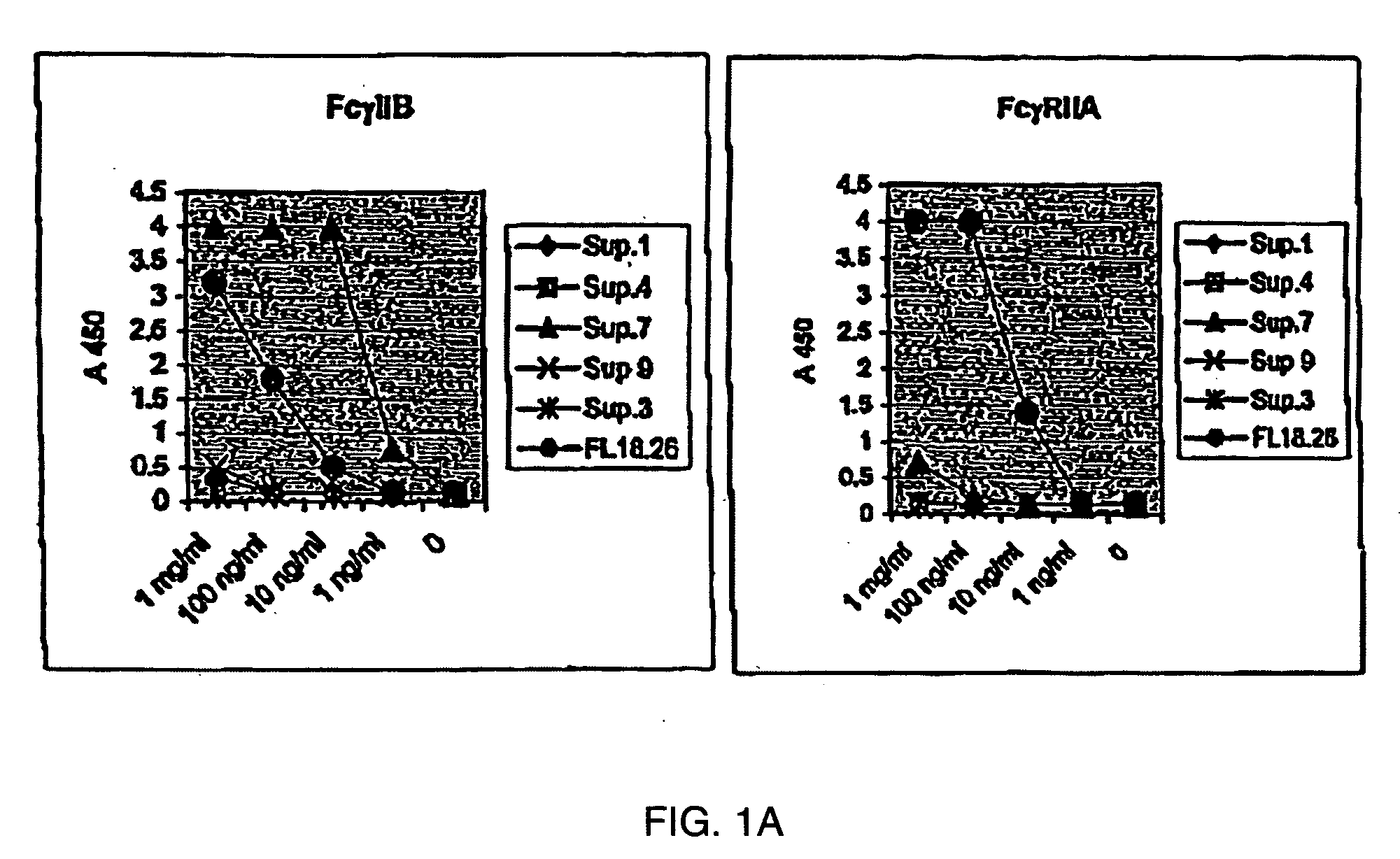
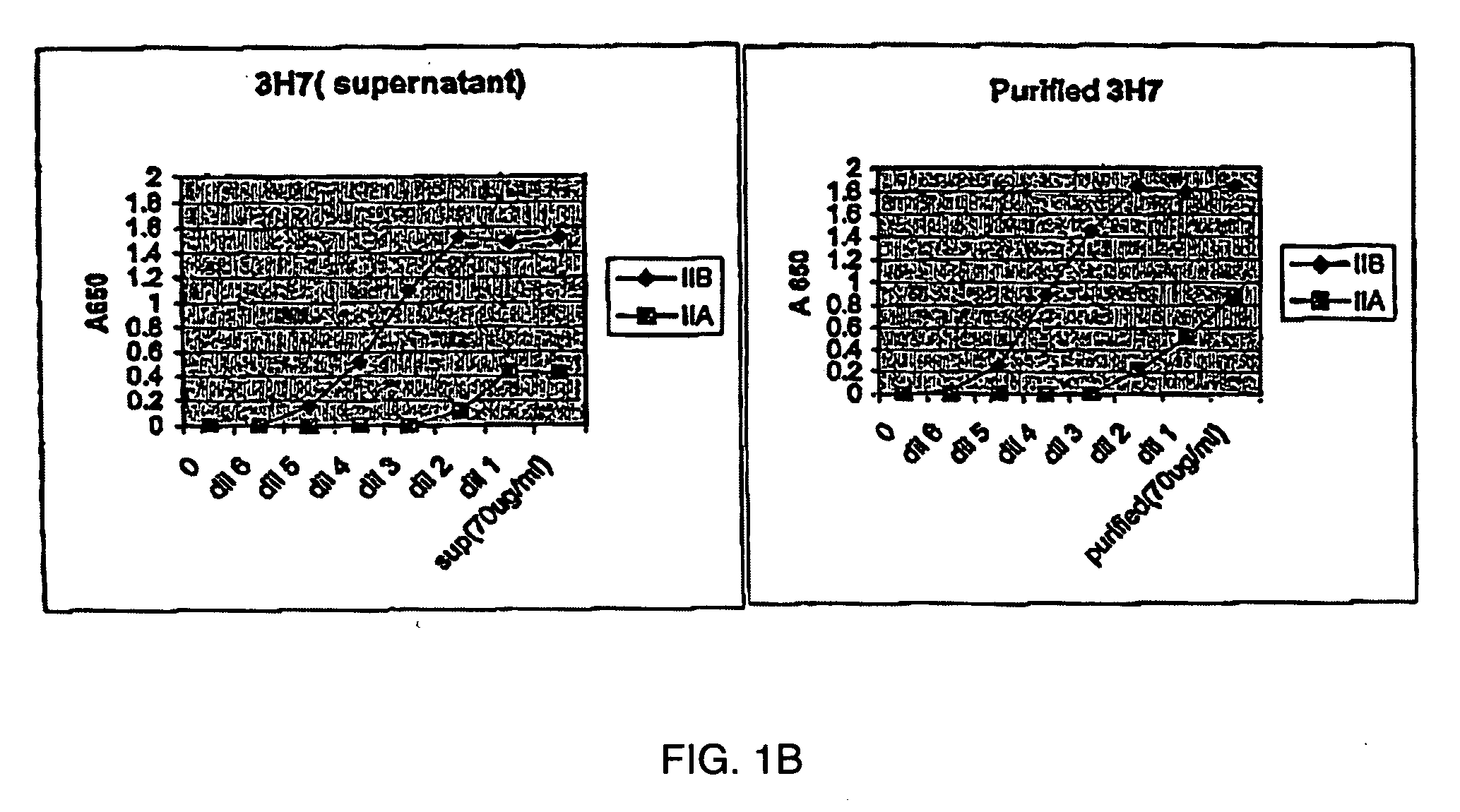
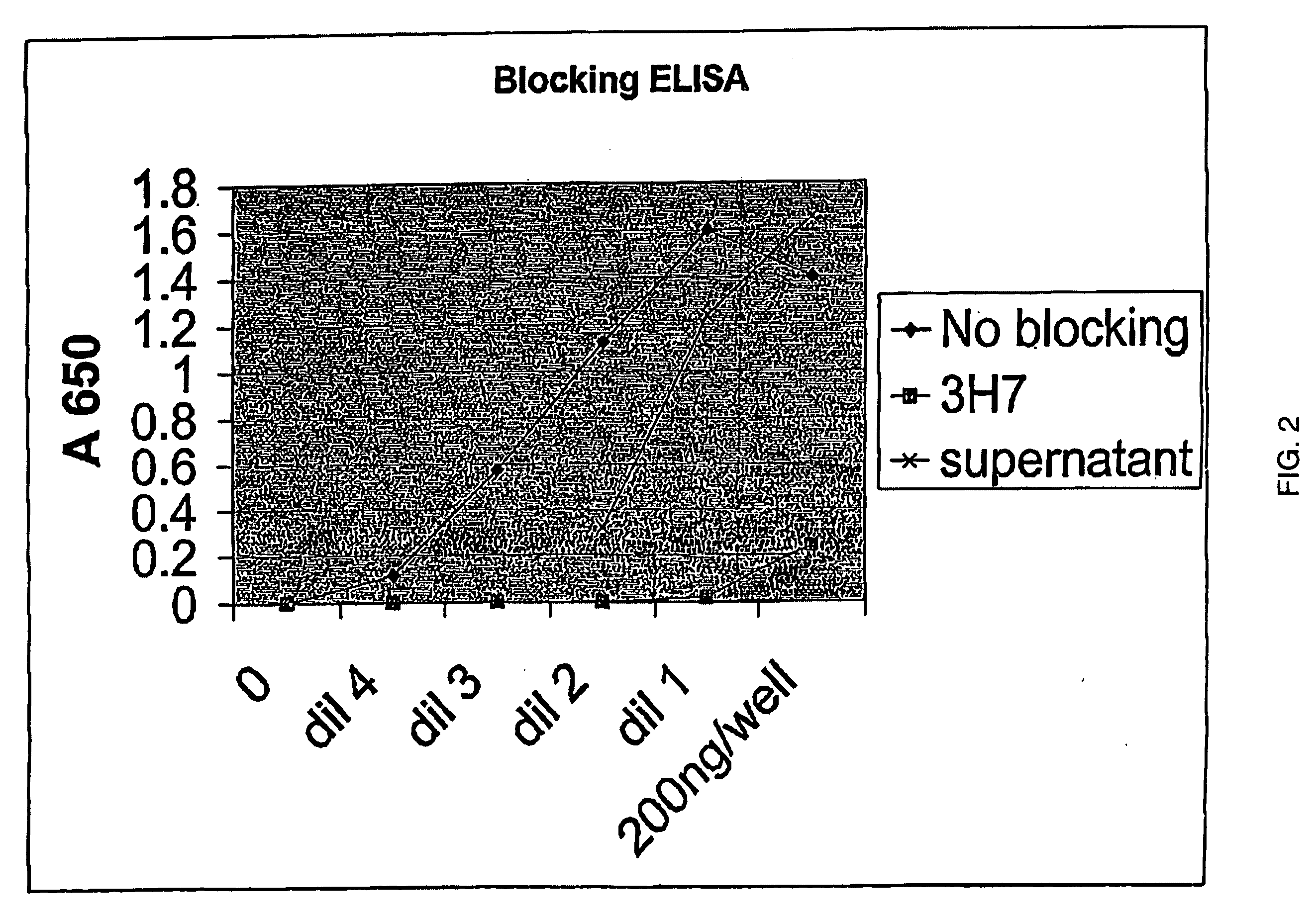
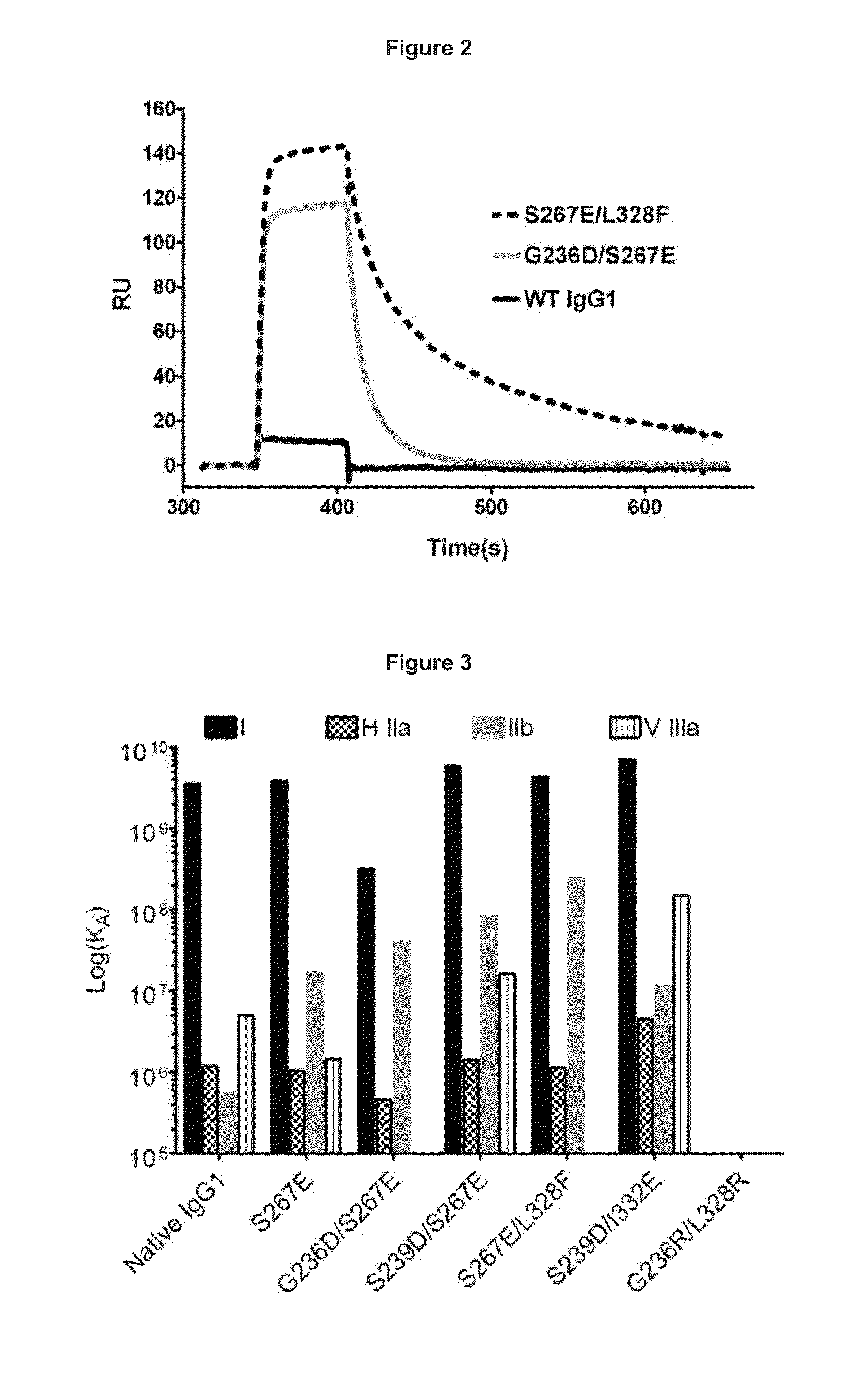
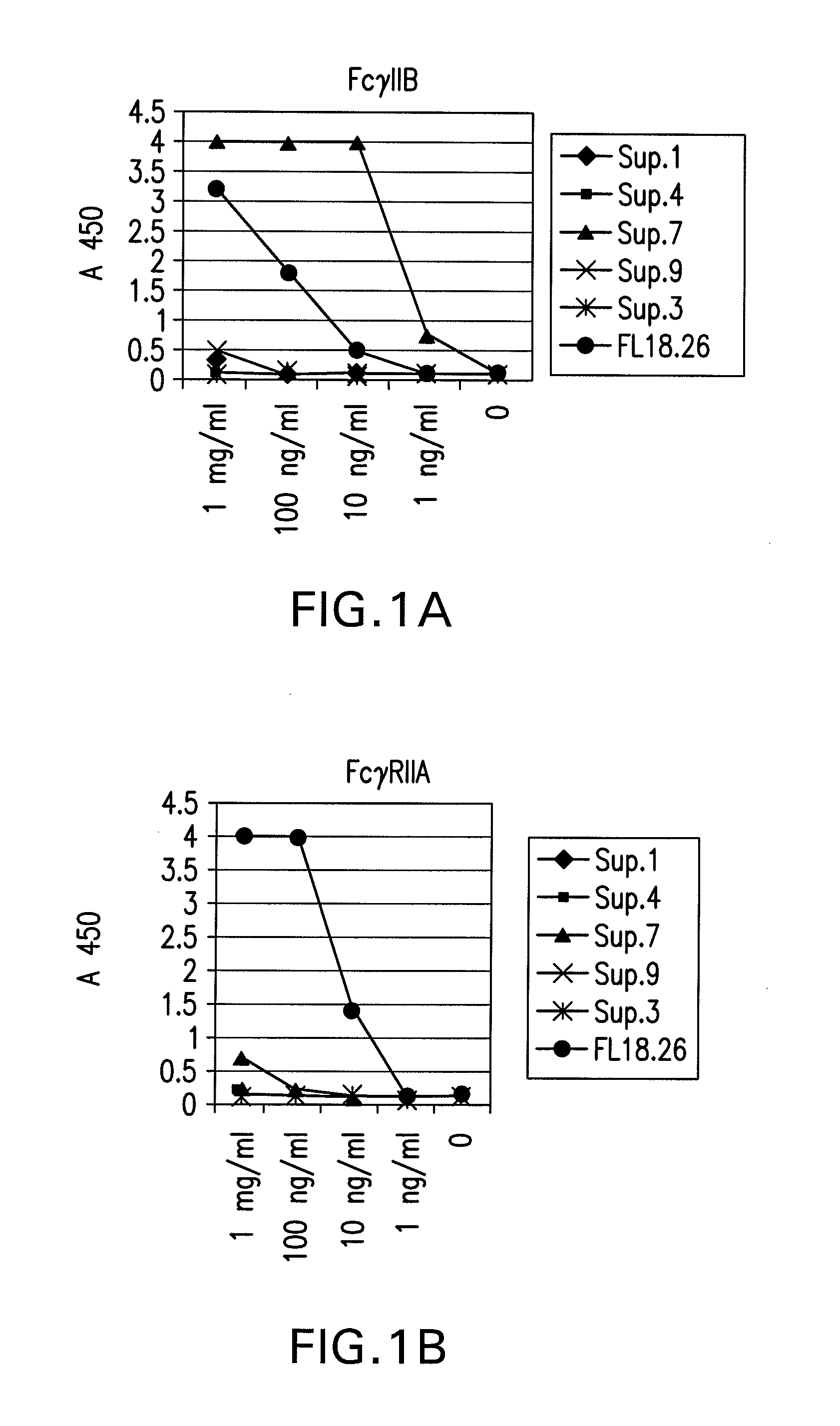
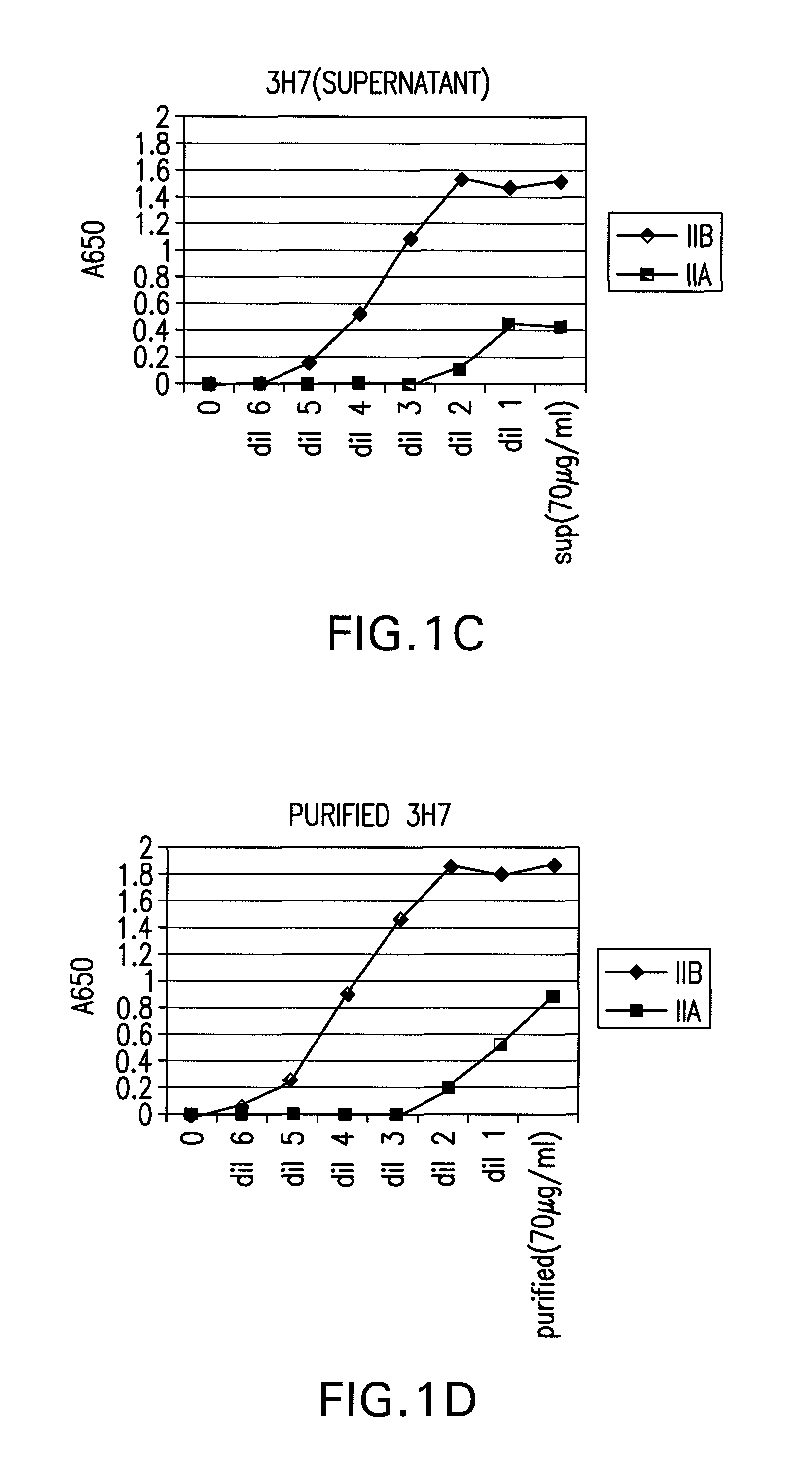
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
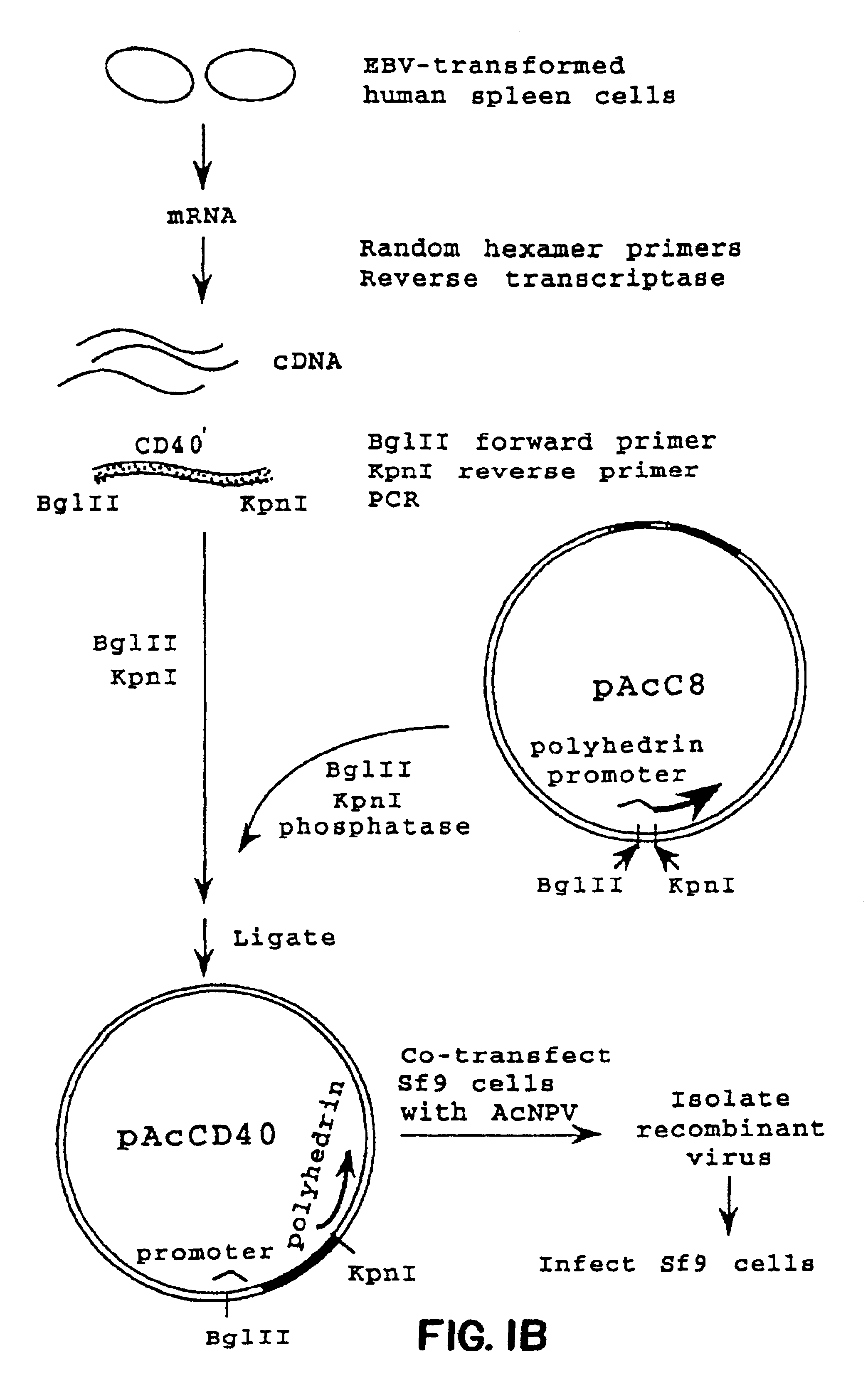
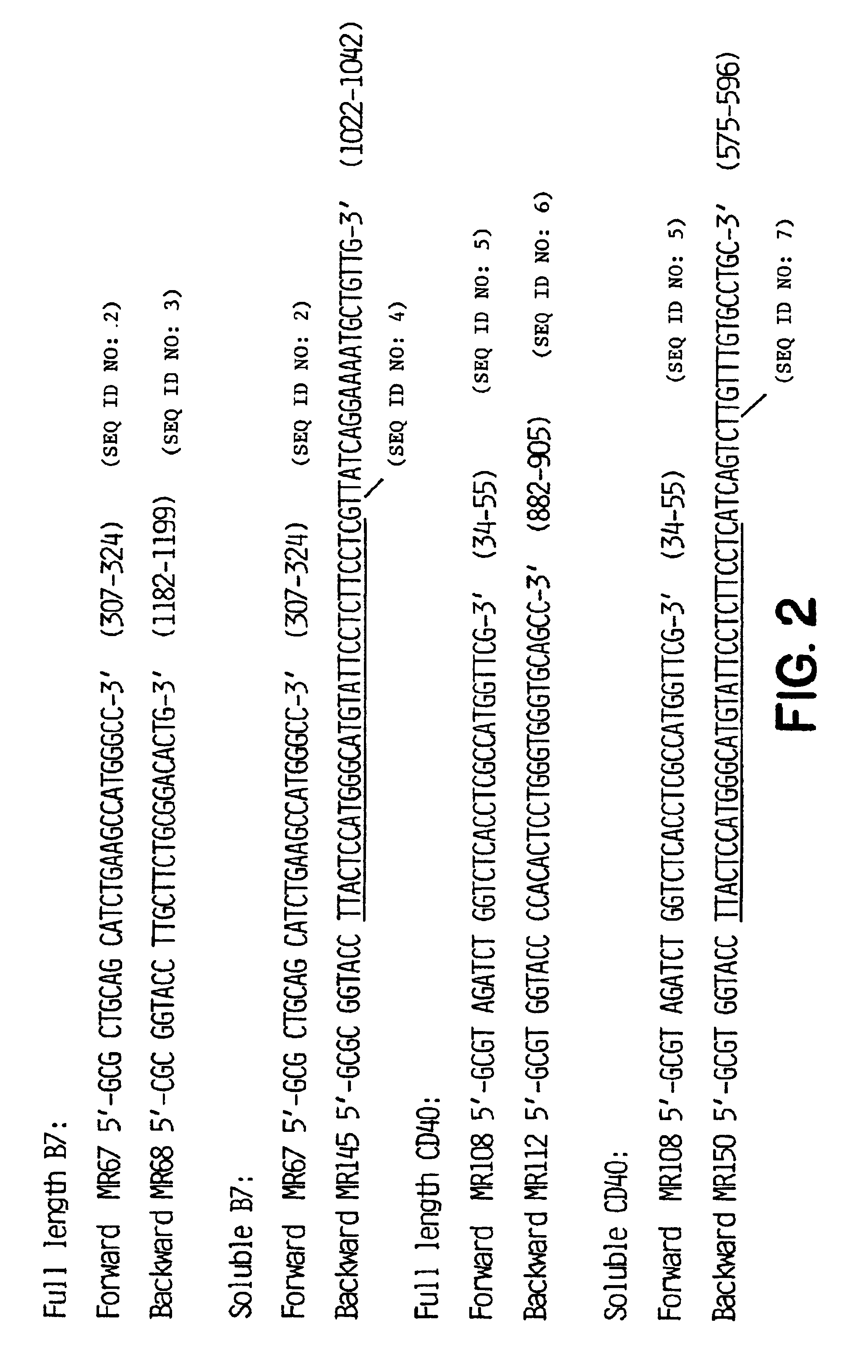
Method for treating an IgE-mediated disease in a patient using anti-CD40 monoclonal antibodies
InactiveUS6899879B2Inhibition of differentiationInhibit growthOrganic active ingredientsVirusesDiseaseEpitope
Methods for preventing or treating an IgE-mediated allergic disease in a patient are presented, the methods comprising administration of a monoclonal antibody capable of binding to a human CD40 antigen located on the surface of a human B cell, wherein binding of the antibody to the CD40 antigen prevents the growth or differentiation of the B cell. Monoclonal antibodies useful in these methods, and epitopes immunoreactive with such monoclonal antibodies are also presented.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Real time electronic cell sensing systems and applications for cell-based assays
InactiveUS7192752B2Reducing cell-impedance response of cellImmobilised enzymesBioreactor/fermenter combinationsAntigenCytotoxicity
The present invention includes devices, systems, and methods for assaying cells using cell-substrate impedance monitoring. In one aspect, the invention provides cell-substrate impedance monitoring devices that comprise electrode arrays on a nonconducting substrate, in which each of the arrays has an approximately uniform electrode resistance across the entire array. In another aspect, the invention provides cell-substrate monitoring systems comprising one or more cell-substrate monitoring devices comprising multiple wells each having an electrode array, an impedance analyzer, a device station that connects arrays of individual wells to the impedance analyzer, and software for controlling the device station and impedance analyzer. In another aspect, the invention provides cellular assays that use impedance monitoring to detect changes in cell behavior or state. In some preferred aspects, the assays are designed to investigate the affects of compounds on cells, such as cytotoxicity assays. In other preferred aspects, the assays are designed to investigate the compounds that effect IgE-mediated responses of cells to antigens.
Owner:AGILENT TECH INC
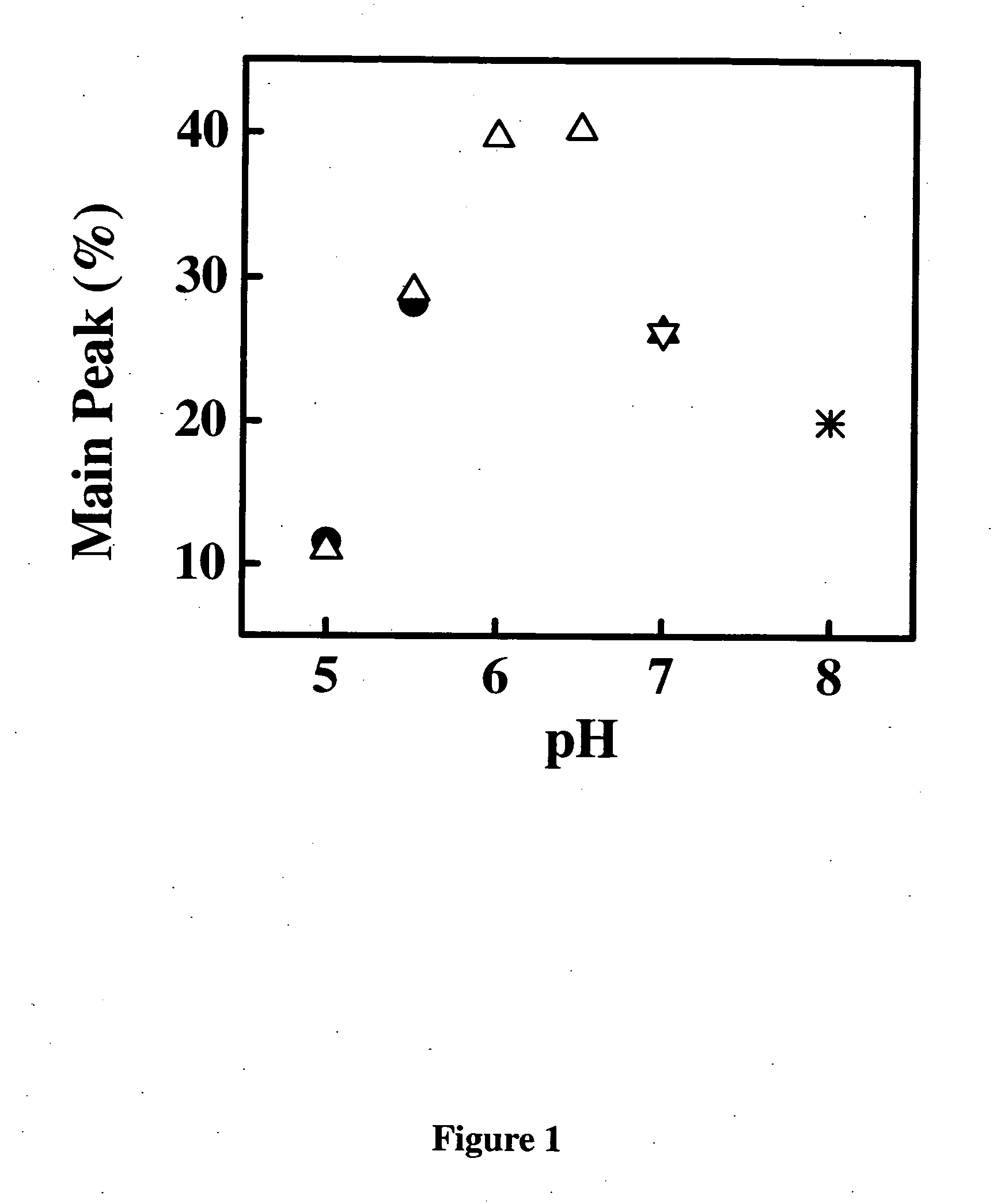
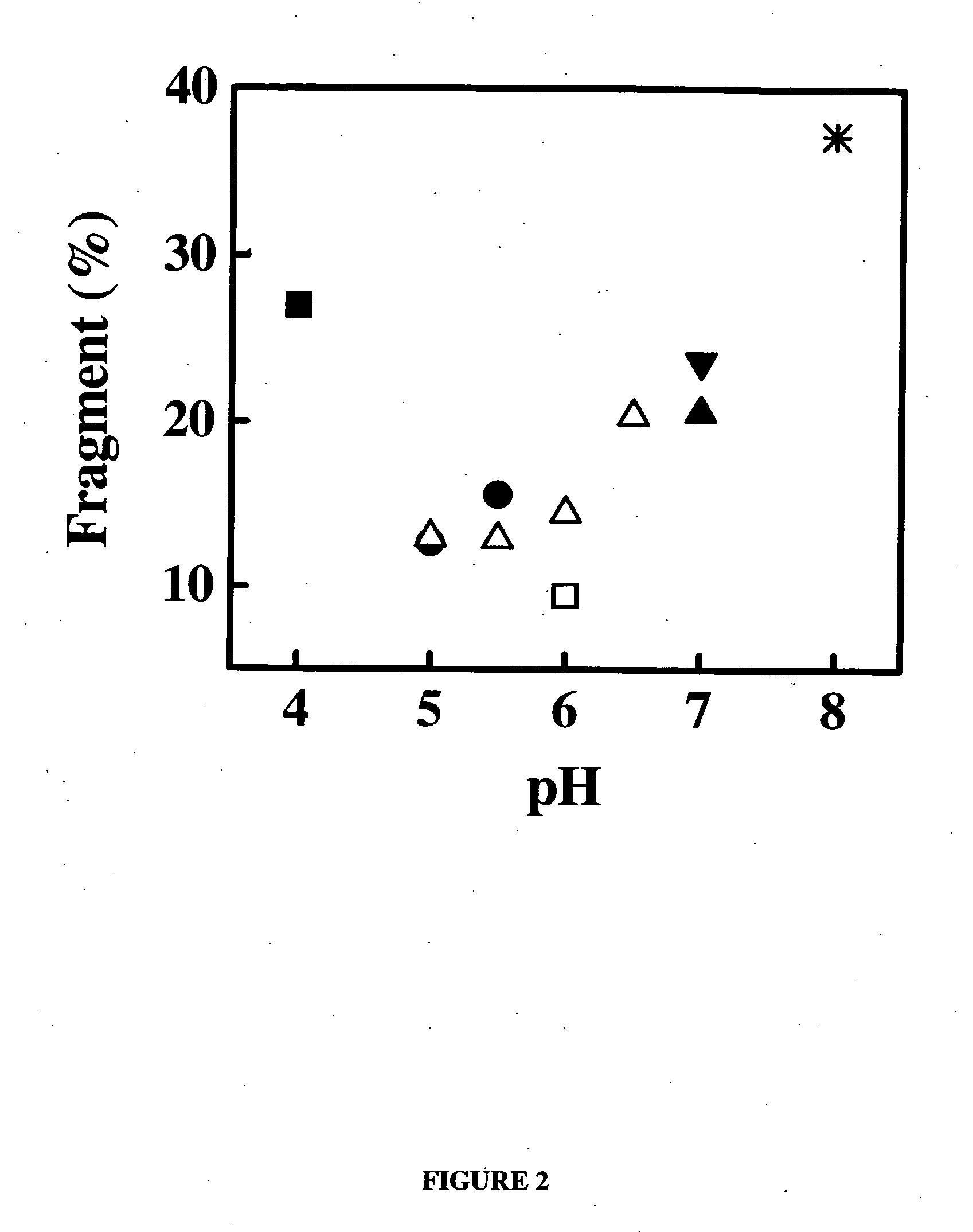
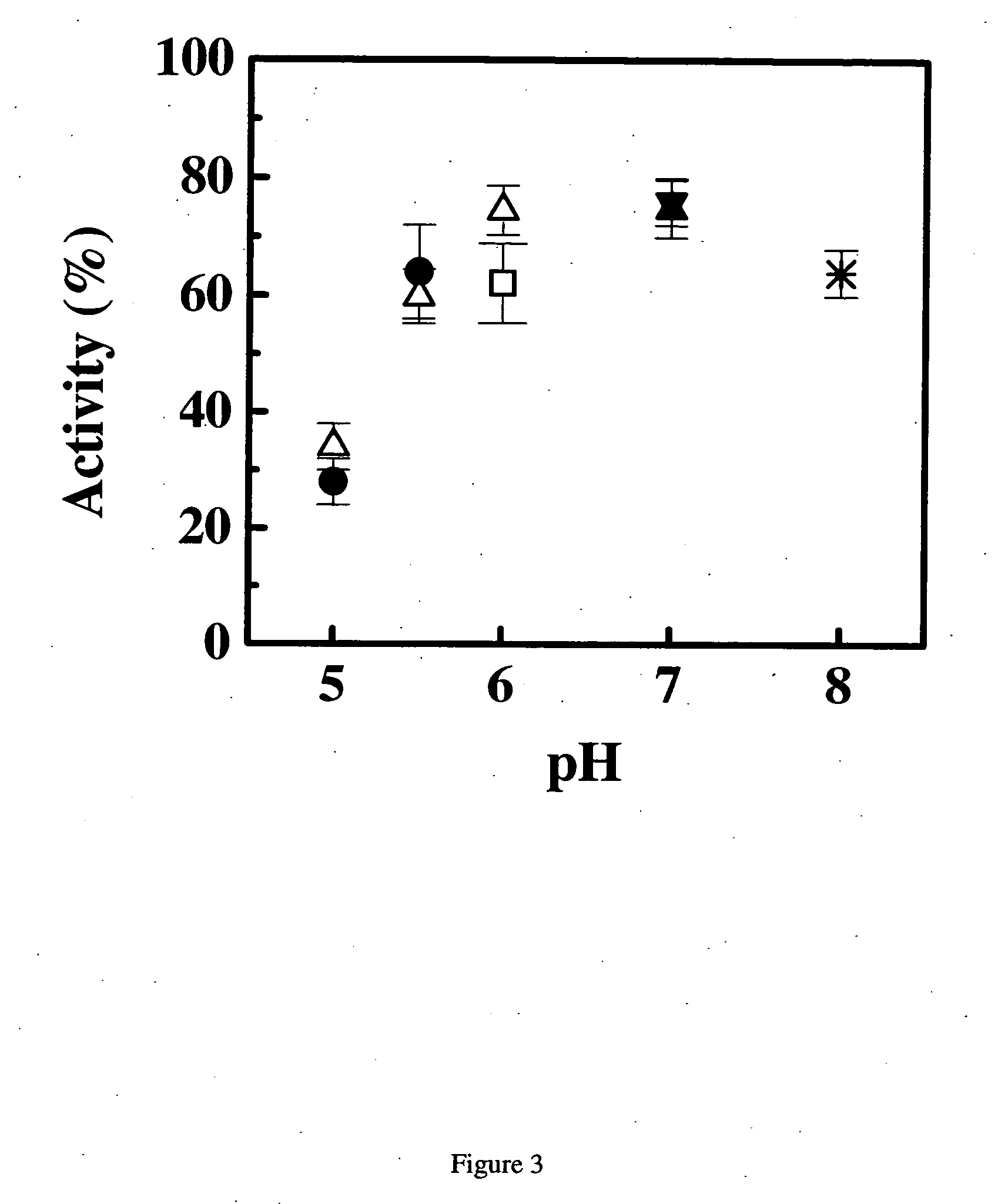
Methods of treating IgE-mediated disorders comprising the administration of high concentration anti-IgE antibody formulations
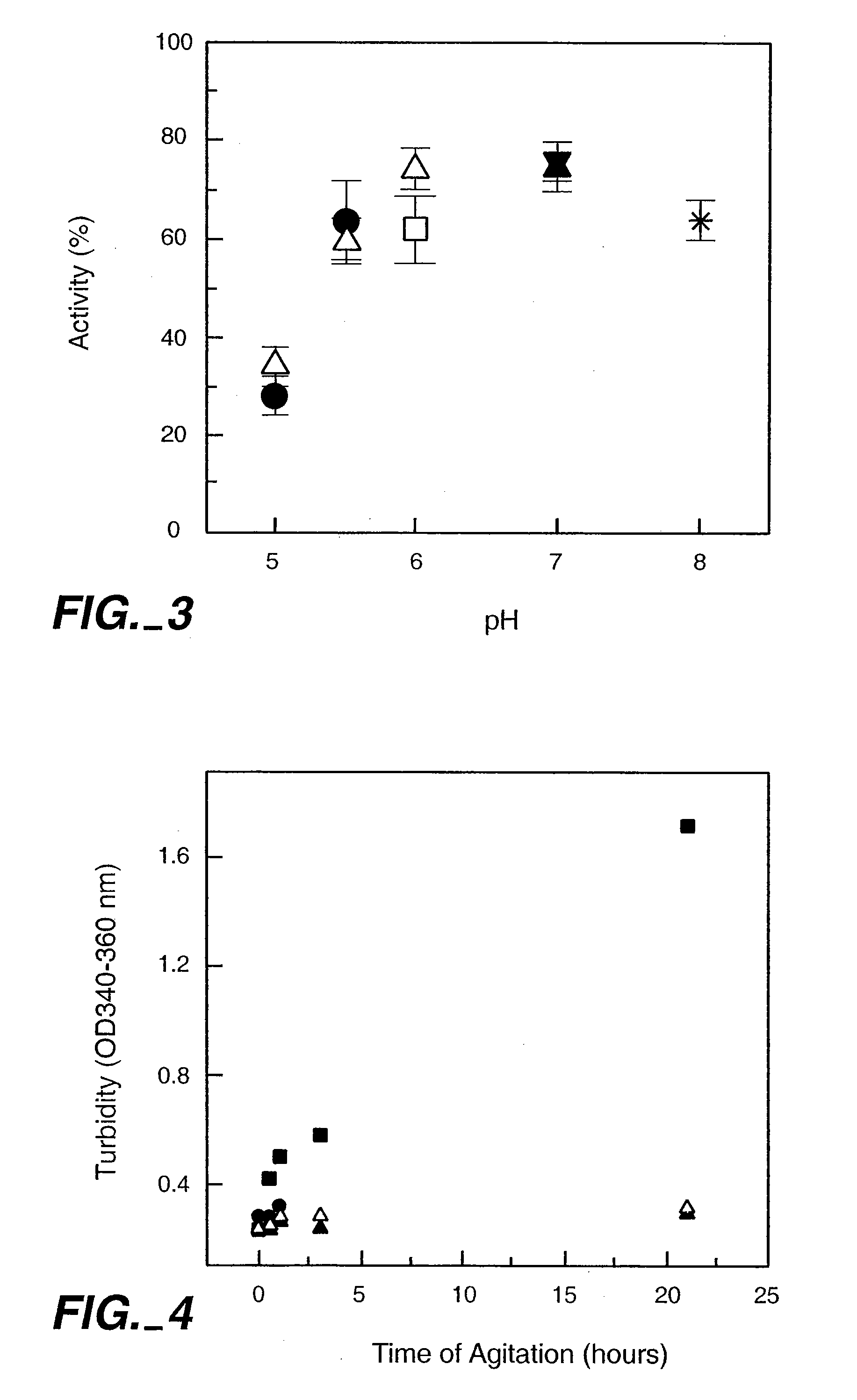
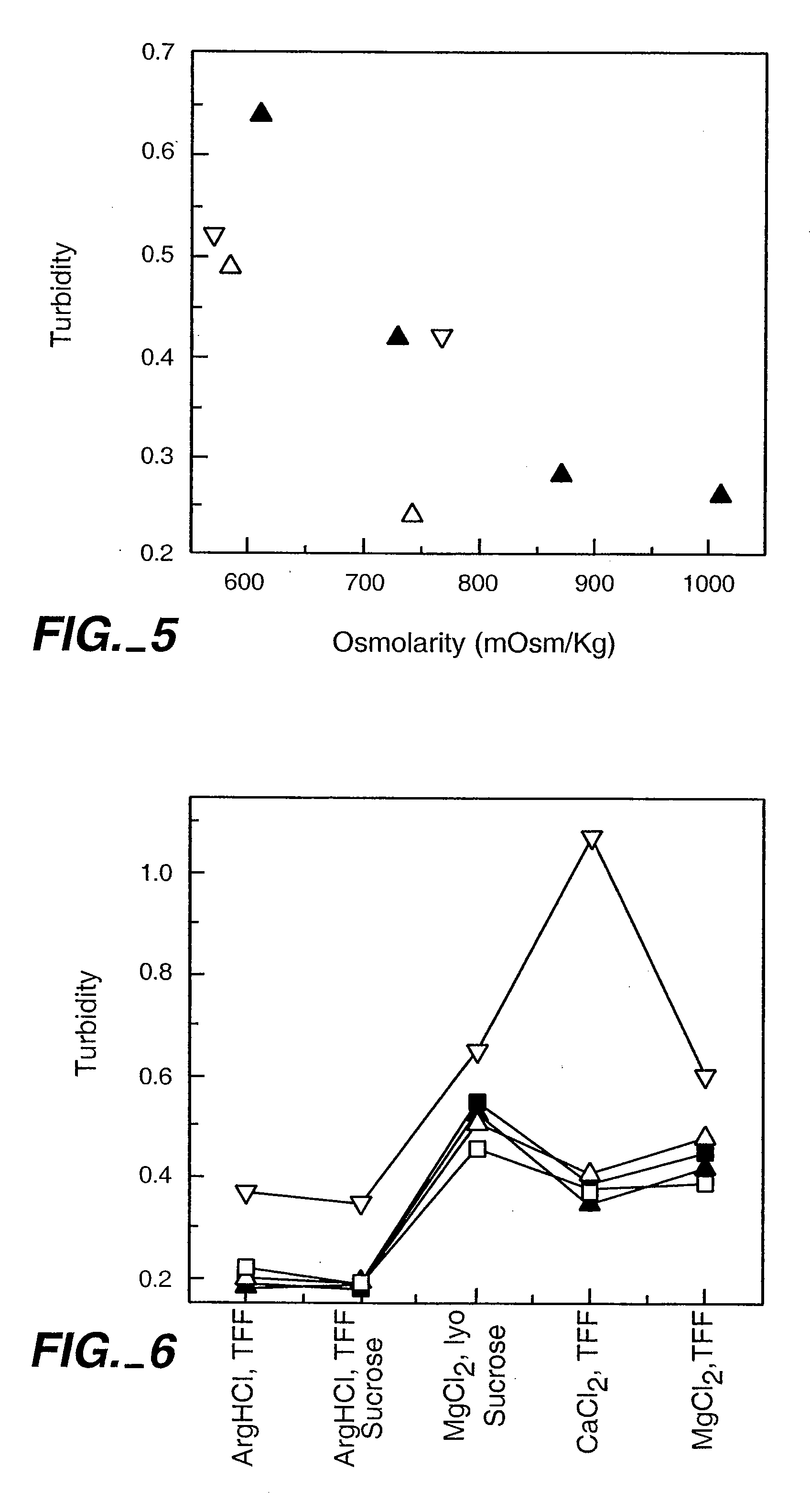
InactiveUS20050158303A1Speed up concentrationReduce turbidityOrganic active ingredientsBiocideIge mediatedAnti-IgE Antibody
The present application relates to methods of treating IgE-mediated disorders comprising the administration of highly concentrated anti-IgE antibody formulations with reduced viscosity that are stable, relatively isotonic and are of low turbidity. The formulations are particularly suitable for subcutaneous administration.
Owner:GENENTECH INC +1
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090053218A1Good curative effectAvoid managementAntibody ingredientsImmunoglobulinsTreatment effectAutoimmune responses
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition.
Owner:MACROGENICS INC
Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
ActiveUS20080044429A1Good curative effectEnhanced effector functionSugar derivativesPeptide/protein ingredientsTreatment effectAntigen Binding Fragment
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090074771A1Strong therapeutic activityEnhancing antibody-mediated effector functionAntibody ingredientsImmunoglobulinsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
FcγRIIB specific antibodies and methods of use thereof
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition.
Owner:MACROGENICS INC
NOVEL COMPOSITIONS AND METHODS FOR TREATING IgE-MEDIATED DISORDERS
ActiveUS20100080814A1Reducing IgE secretionInhibition of maturationAntibody ingredientsImmunoglobulinsDiseaseMembrane anchor
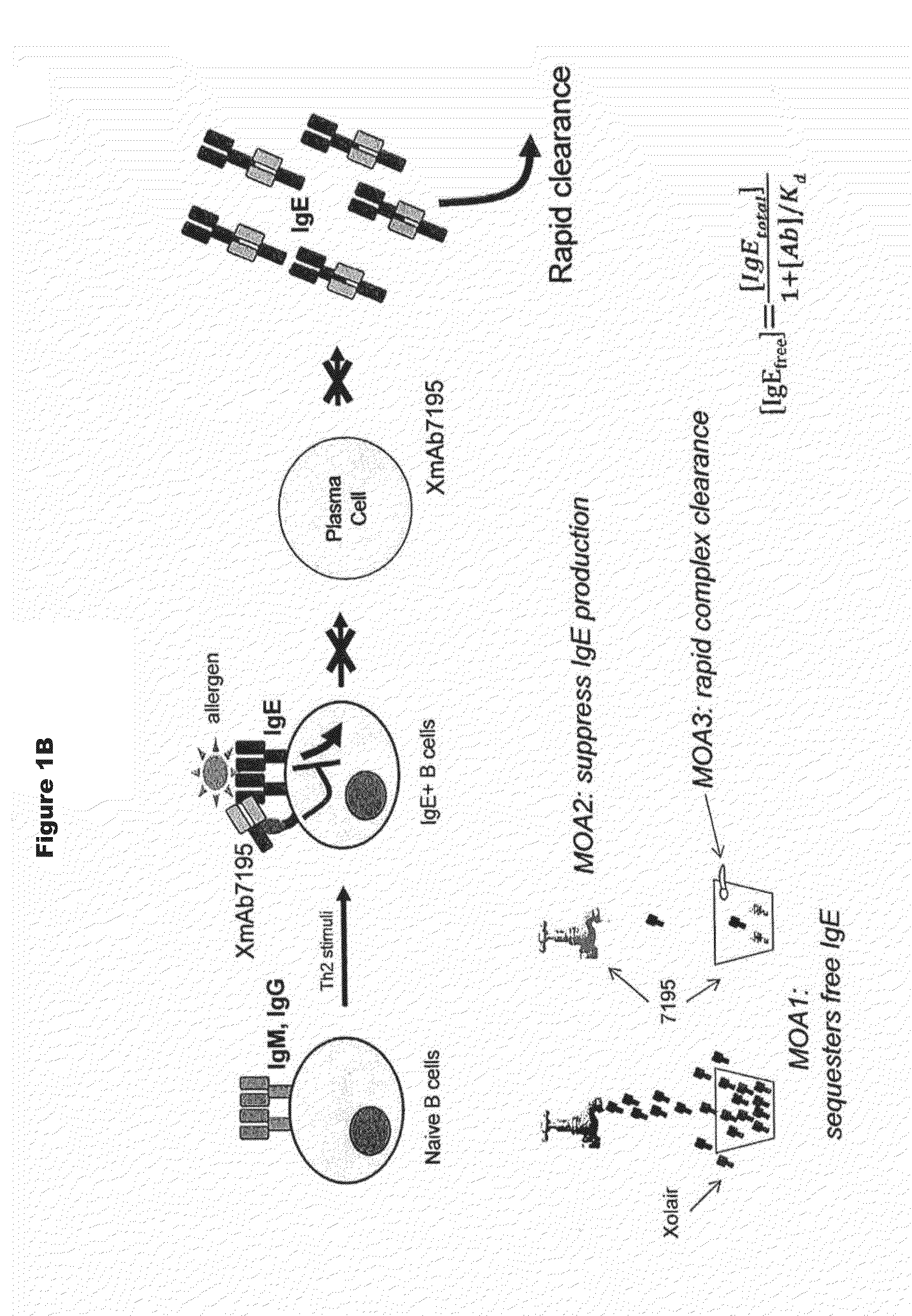
The present invention relates to immunoglobulins that bind IgE and FcγRIIb with high affinity, said compositions being capable of inhibiting cells that express membrane-anchored IgE. Such compositions are useful for treating IgE-mediated disorders, including allergies and asthma.
Owner:XENCOR
Rapid clearance of antigen complexes using novel antibodies
InactiveUS20140212435A1Reduce serum concentrationHigh affinityAntibody ingredientsImmunoglobulinsAntigenDisease
The present invention relates to immunoglobulins that bind IgE and FcγRIIb with high affinity, said compositions being capable of inhibiting cells that express membrane-anchored IgE. Such compositions are useful for treating IgE-mediated disorders, including allergies and asthma.
Owner:XENCOR
FcγRIIB specific antibodies and methods of use thereof
InactiveUS8946387B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090076251A1Strong therapeutic activityEnhancing antibody-mediated effector functionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityAutoimmune responses
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090202537A1Balanced functionImmune responseSenses disorderNervous disorderFc(alpha) receptorImmunologic disorders
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer (preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma), autoimmune disease, inflammatory disease or IgE-mediated allergic disorder. The present invention also encompasses the use of a humanized FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention with a vaccine composition.
Owner:MACROGENICS INC
Methods of treating IgE-mediated disorders comprising the administration of high concentration anti-IgE antibody formulations
The present application relates to methods of treating IgE-mediated disorders comprising the administration of highly concentrated anti-IgE antibody formulations with reduced viscosity that are stable, relatively isotonic and are of low turbidity. The formulations are particularly suitable for subcutaneous administration.
Owner:GENENTECH INC
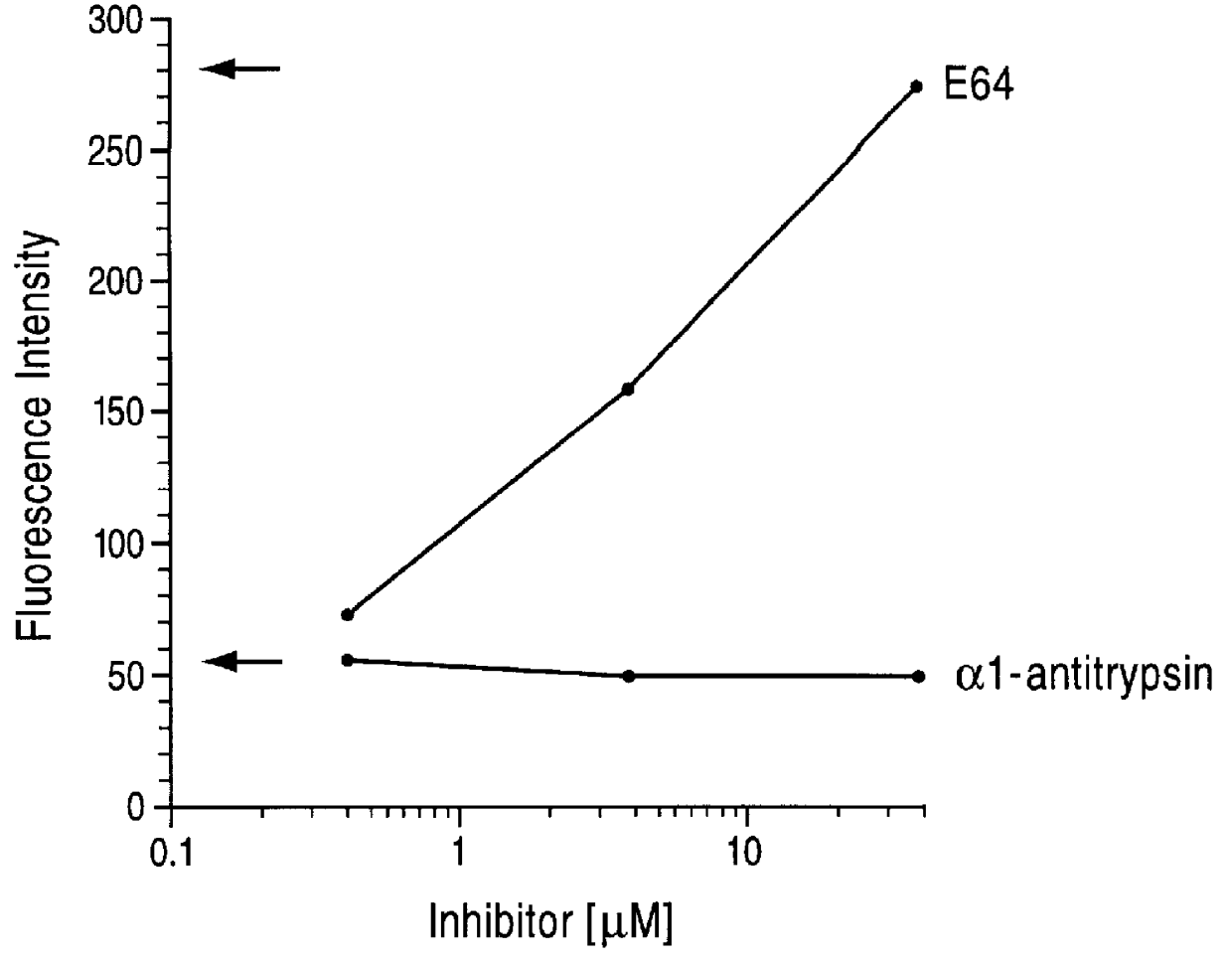
Cysteine protease inhibitors for use in treatment of IGE mediated allergic diseases
InactiveUS6034066AOrganic active ingredientsGroup 5/15 element organic compoundsOccupational allergensCysteine Proteinase Inhibitors
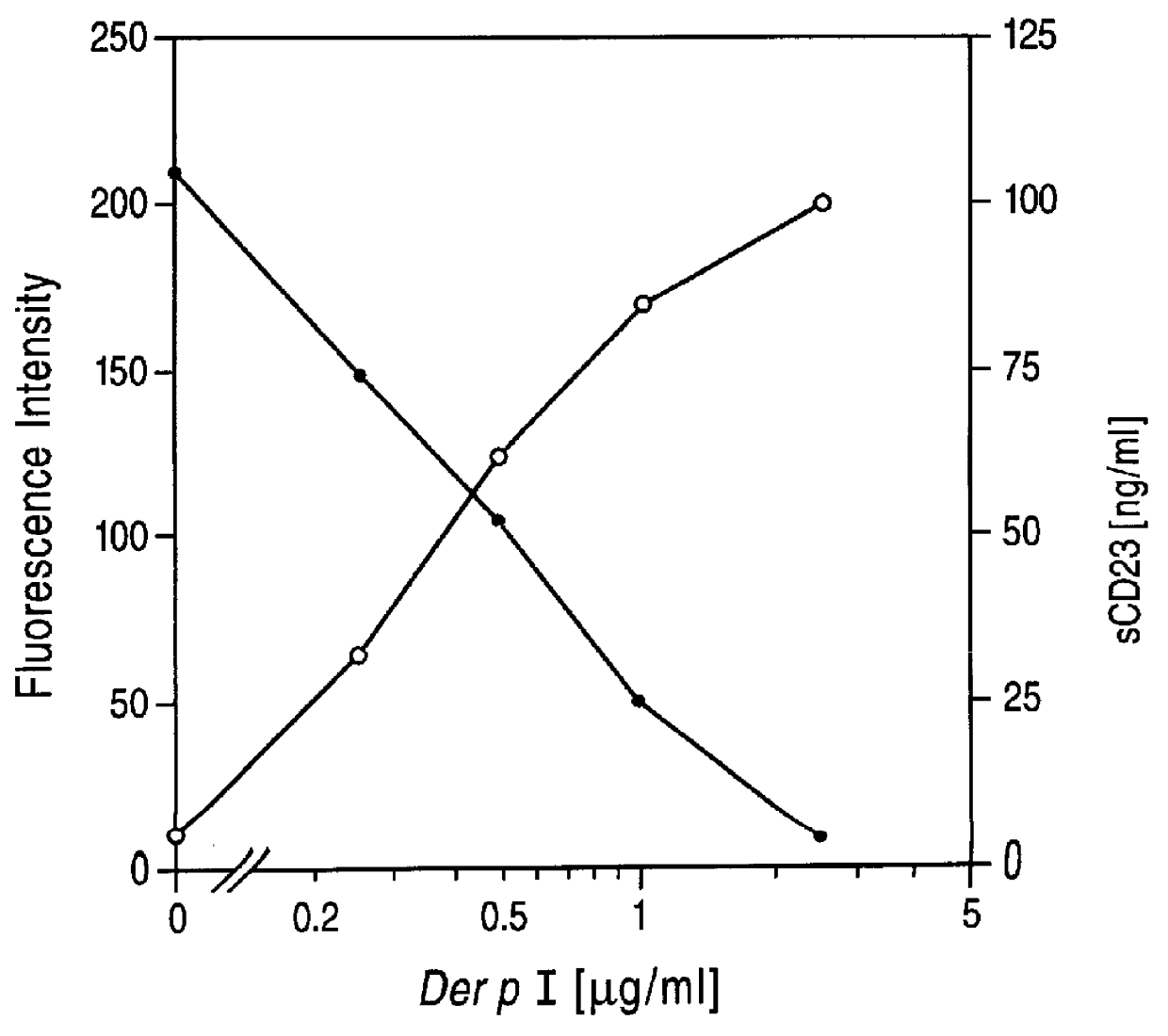
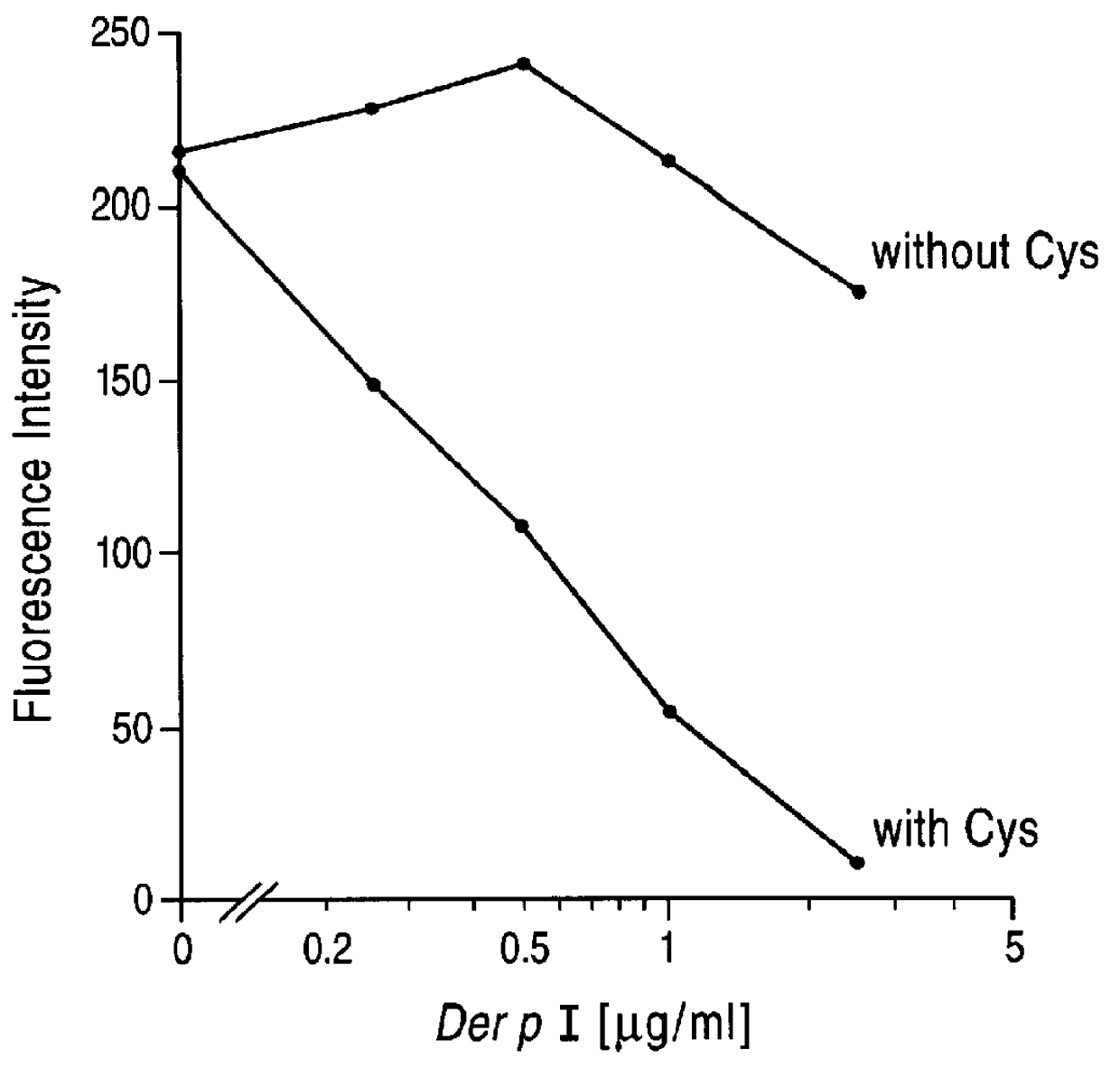
PCT No. PCT / GB96 / 01707 Sec. 371 Date Feb. 26, 1998 Sec. 102(e) Date Feb. 26, 1998 PCT Filed Jul. 17, 1996 PCT Pub. No. WO97 / 04004 PCT Pub. Date Feb. 6, 1997The invention provides compounds for use in the treatment of allergic diseases including juvenile asthma and eczema. The compounds can inhibit IgE mediated reaction to major environmental and occupational allergens and can also have a prophylactic effect against allergic disease by preventing allergic sensitization to environmental and occupational allergens when administered to at-risk individuals (e.g., those at genetic risk of asthma and those exposed to occupational allergens in the workplace). The compounds are also useful for inactivation or attenuation of the allergenicity of allergens in situ. The invention provides compounds and ligands per se, pharmaceutical compositions containing the compounds, processes for producing the compounds and pharmaceutical compositions, and methods for using the compounds and compositions in treatment or prophylaxis of IgE mediated allergic diseases and in inactivation or attenuation of allergens in situ. The invention also enables the reduction or destruction of the viability of allergy-causing organisms.
Owner:MEDIVIR UK
Anti-IgE antibodies
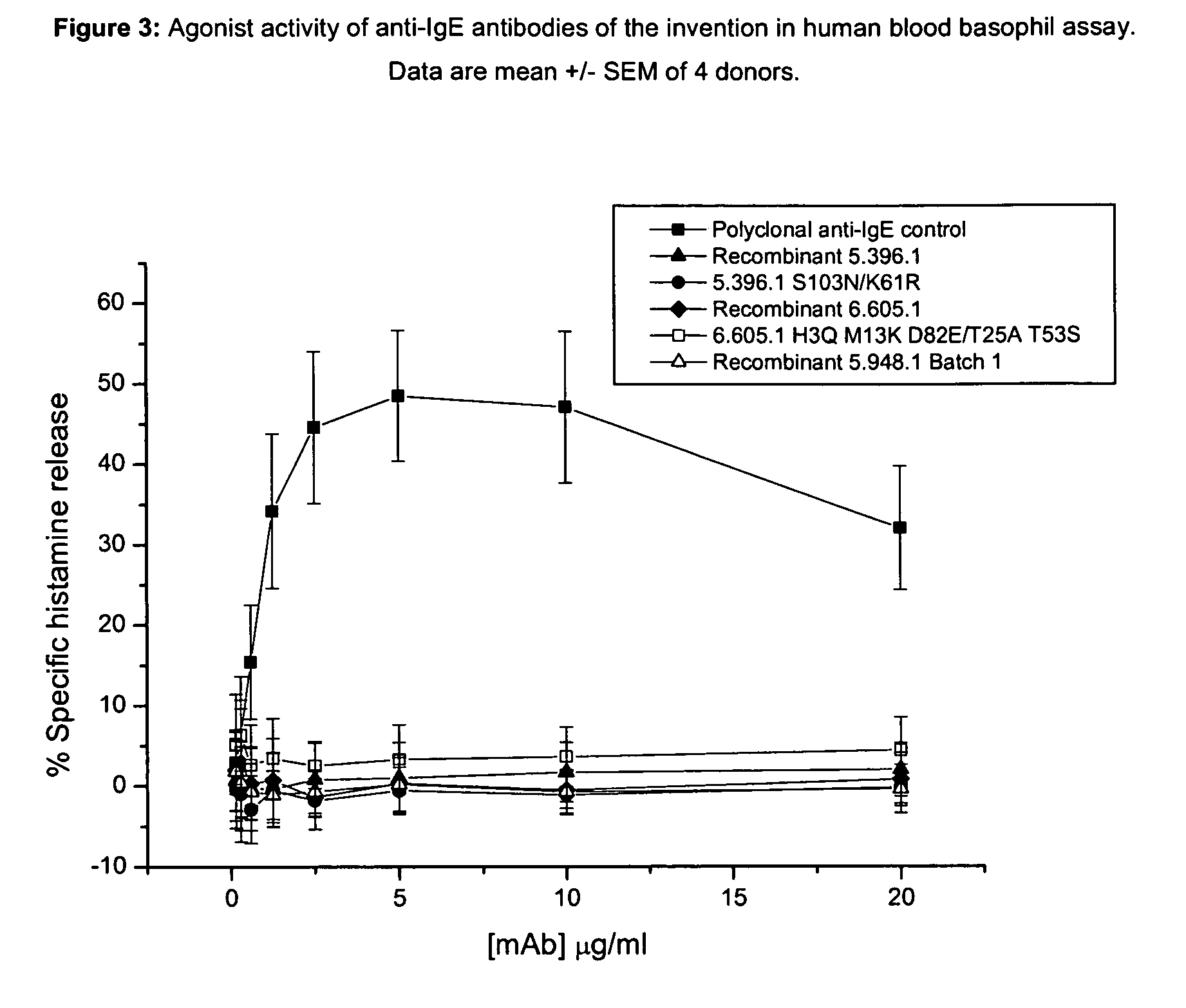
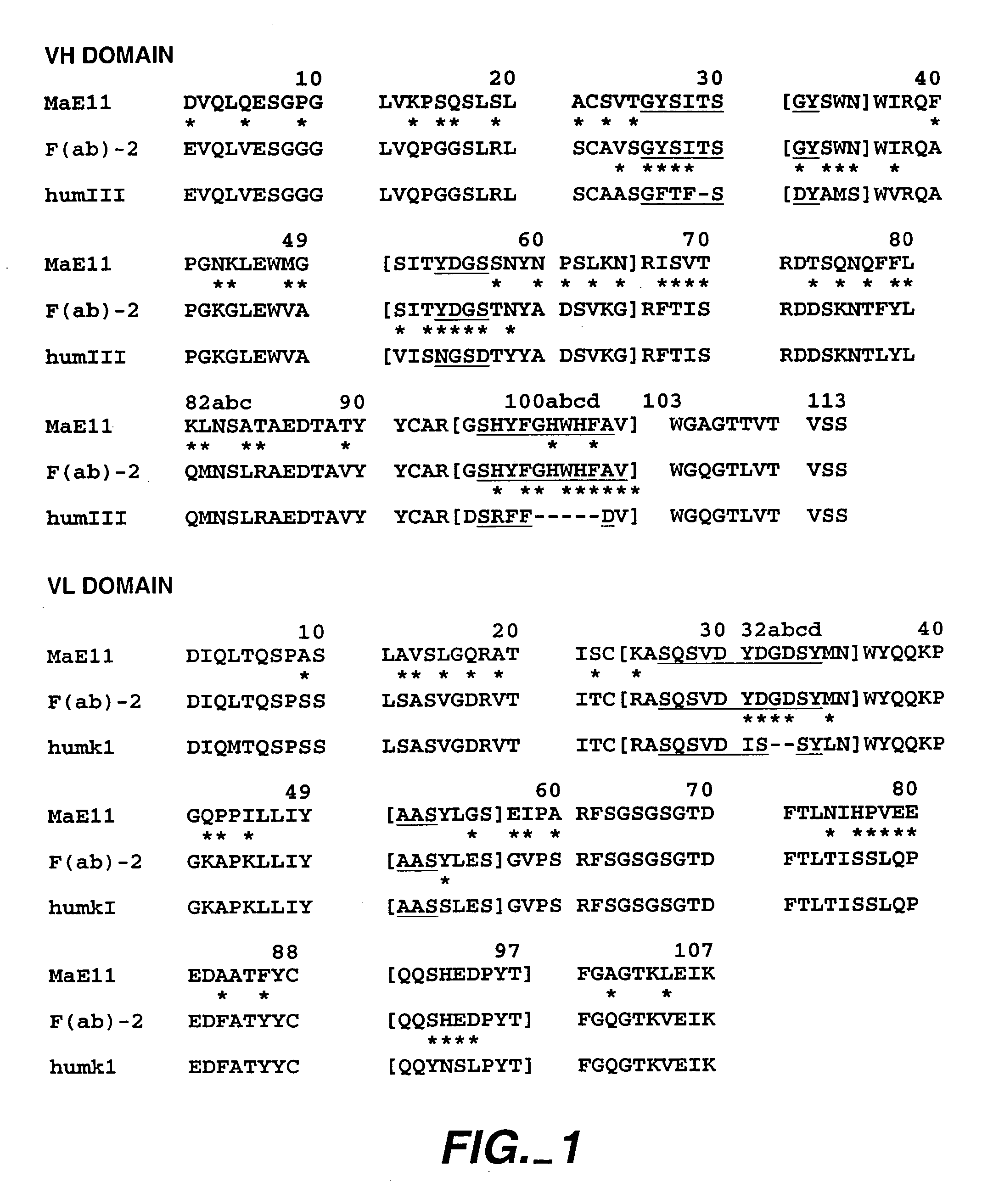
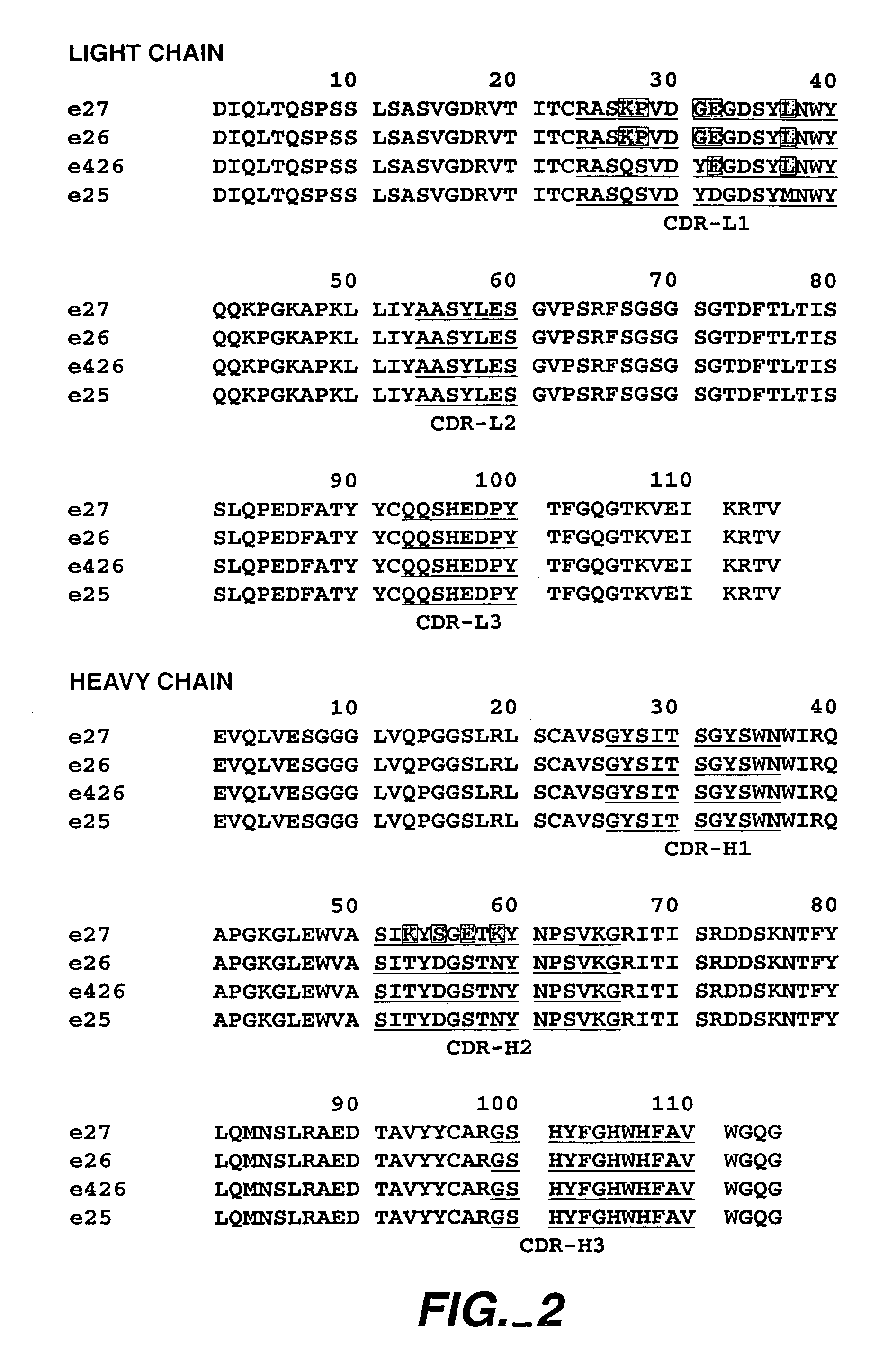
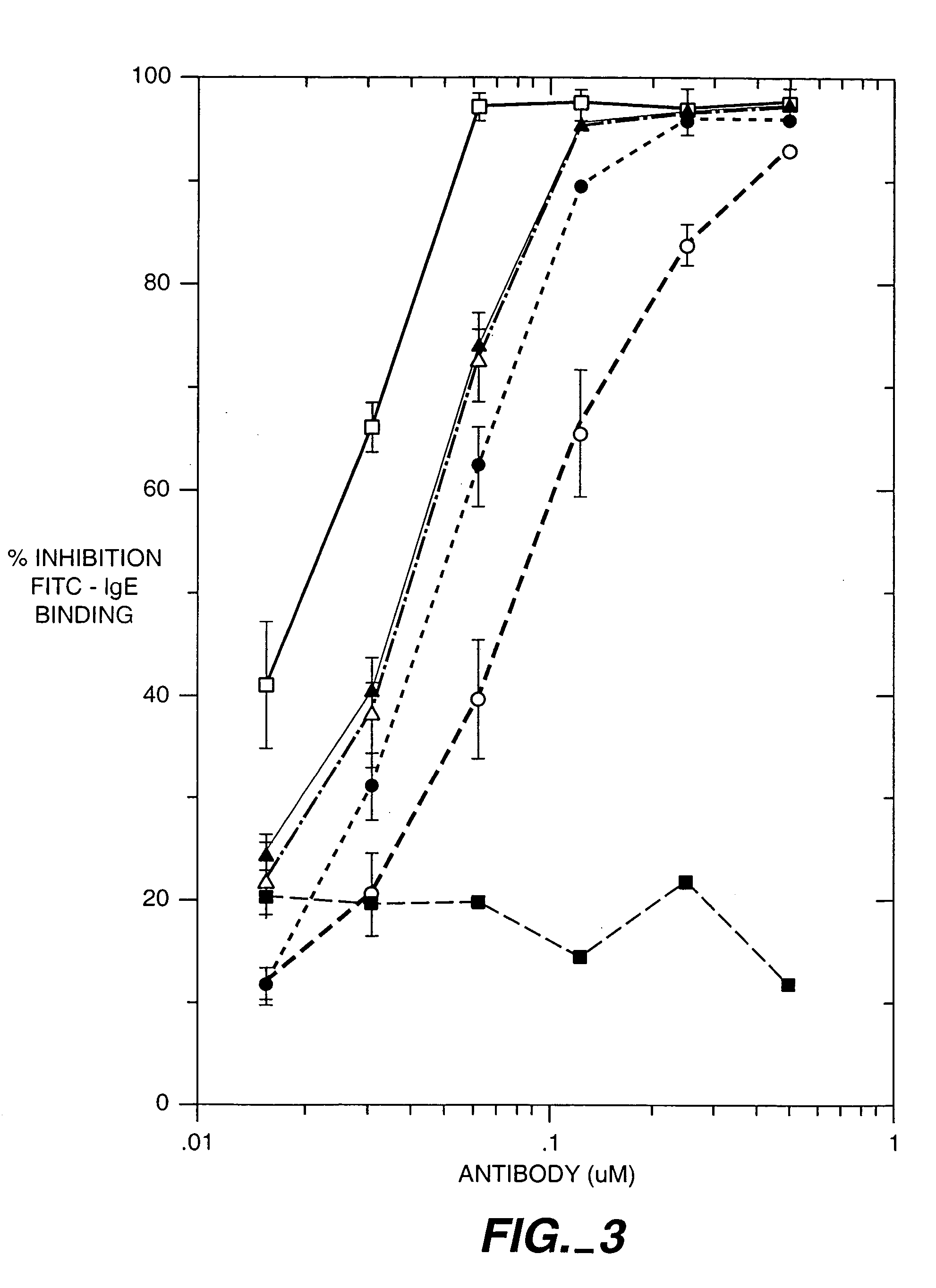
The present invention describes IgE antagonists (including variant anti-IgE antibodies) and their use in diagnosis, therapy or prophylaxis of allergic and other IgE-mediated disorders, including asthma, food allergies, hypersensitivity and anaphylactic reactions.
Owner:GENENTECH INC
METHODS OF TREATING IgE-MEDIATED DISORDERS COMPRISING THE ADMINISTRATION OF HIGH CONCENTRATION ANTI-IgE ANTIBODY FORMULATIONS
The present application relates to methods of treating IgE-mediated disorders comprising the administration of highly concentrated anti-IgE antibody formulations with reduced viscosity that are stable, relatively isotonic and are of low turbidity. The formulations are particularly suitable for subcutaneous administration.
Owner:GENENTECH INC +1
Use of parasitic biological agents for prevention and control of allergic and other IgE-mediated disorders
InactiveUS20070087020A1Reduce and eliminate and ameliorate inappropriate immune responseStimulate immune responseProtozoa antigen ingredientsLeech/worm material medical ingredientsDiseaseInterleukin 10
The present invention describes using, on a repetitive basis, a non-human colonizing helminth compound, in an amount sufficient to establish a transitory parasitic helminth infection and or to simulate in a parasitic helminth infection, thereby having immunosuppressive effect against benign antigens and or stimulating a regulatory immune response characterized by the production of T helper cells 2 (Th2), T regulatory helper cells (TREG) and certain cytokines, including, but not limited to interleukin 10 (IL-10), as a therapy or prophylaxis of allergy and other IgE-mediated disorders, which are marked by an inappropriate IgE immune response including, but not limited to an aberrant and or enhanced IgE antibody production to benign antigens. The invention presents using helminth compound by administering it in a frequency and amount sufficient to eliminate or ameliorate the inappropriate immune response in an asthmatic and or allergic individual.
Owner:MILESTONE RES
Fusion molecules and treatment of IgE-mediated allergic diseases
InactiveUS20030082190A1Negatively expressionFind utility in the treatmentSenses disorderFungiLate phaseVaccination
The invention concerns bifunctional fusion molecules for the treatment of IgE-mediated allergic conditions and FcepsiRI-mediated autoimmune conditions. The invention provides a new therapeutic approach for the treatment of both acute and late-phase allergic responses due to ingestion, inhalation, cutaneous and parenteral exposure to allergens, responses including asthma, allergic rhinitis, atopic dermatitis, severe food allergies, chronic urticaria and angioedema, as well as anaphylactic reactions due to exposures such as bee stings or penicillin allergy. In addition, the invention provides for a novel, safer and more efficacious form of allergy vaccination.
Owner:RGT UNIV +1
Large scale parallel immuno-based allergy test and device for evanescent field excitation of fluorescence
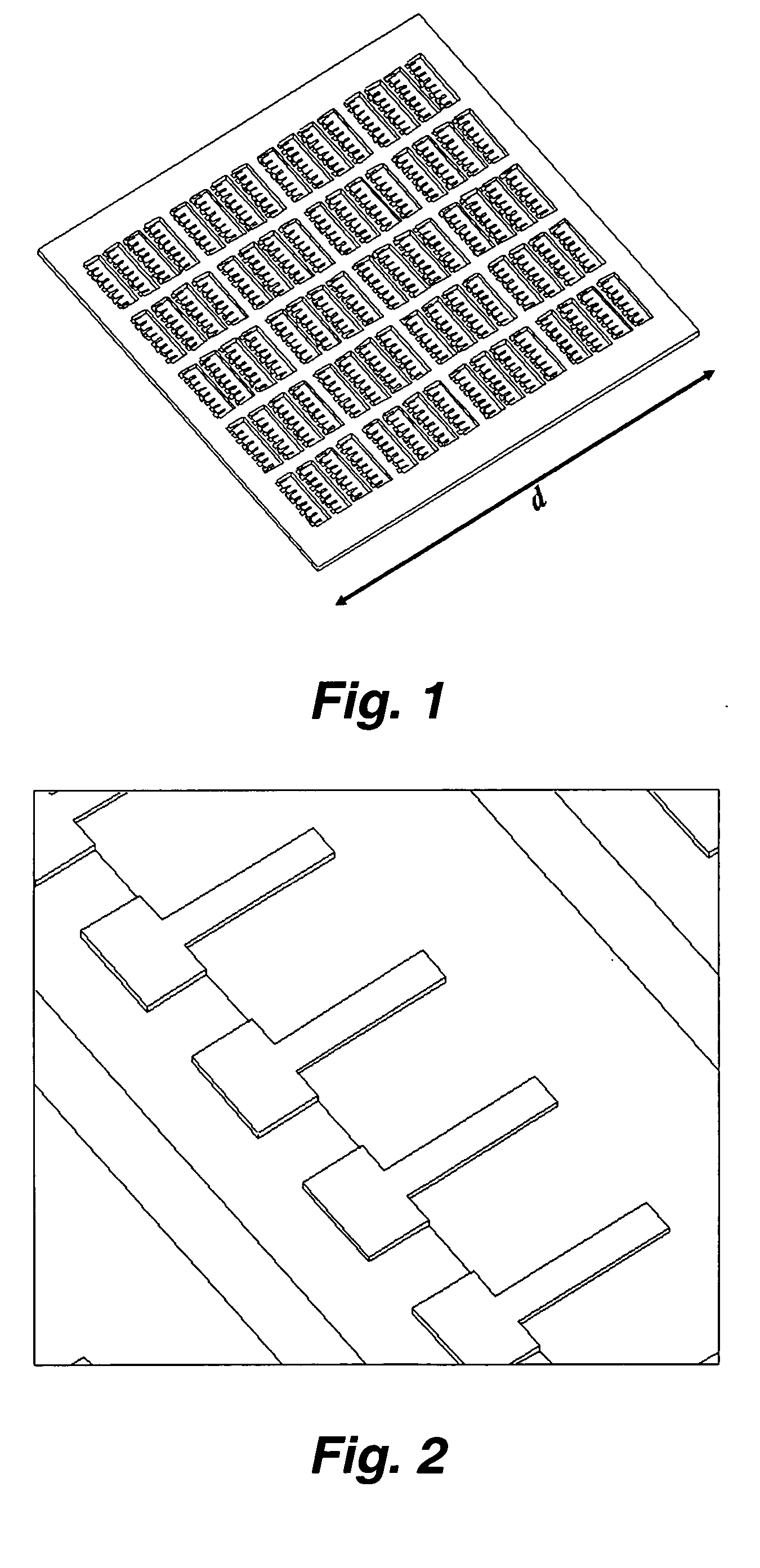
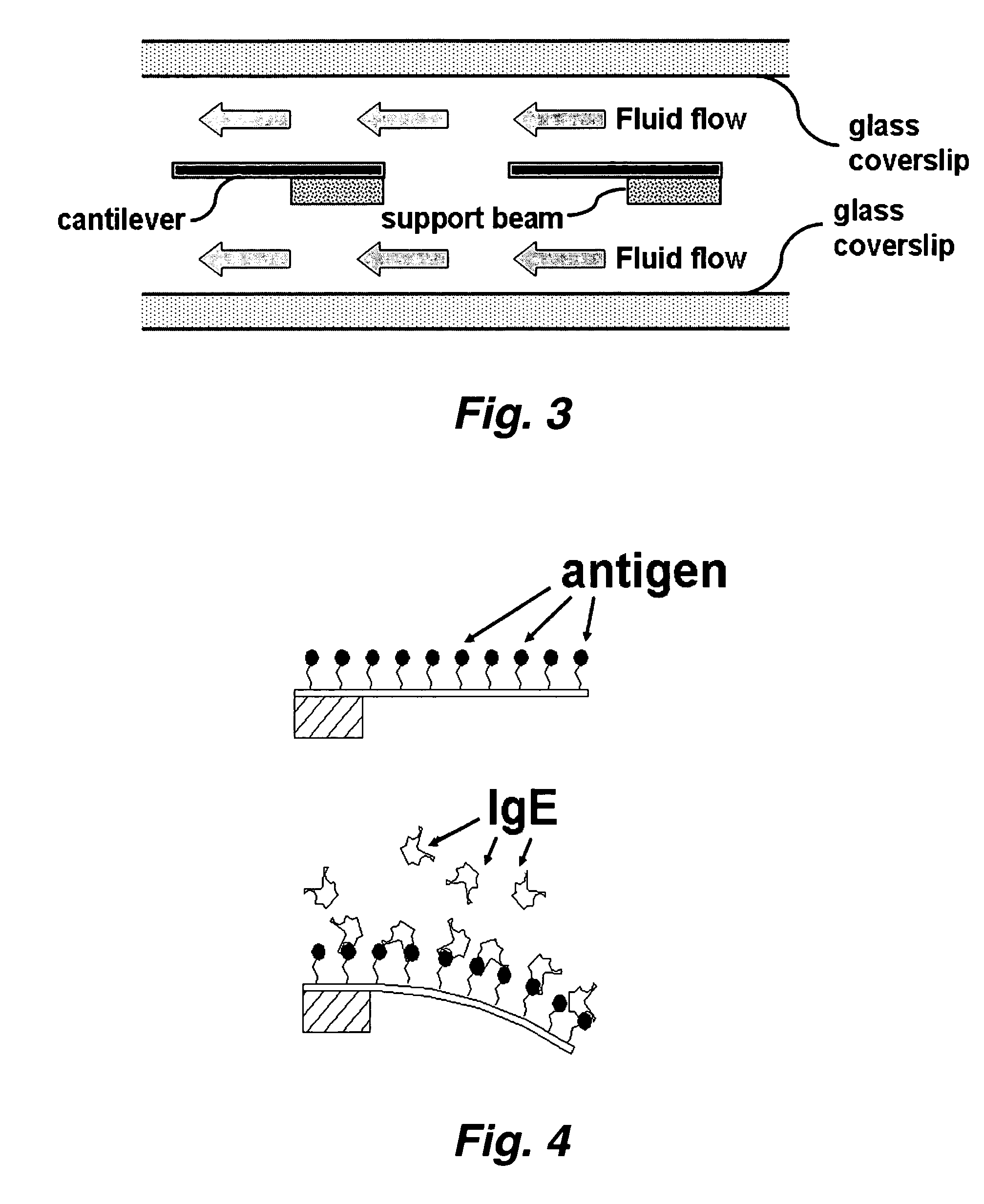
InactiveUS20070117217A1Quick checkRapid diagnosisMaterial analysis by optical meansDisease diagnosisAntigenMassively parallel
This invention provides a device and methods for the rapid detection and / or diagnosis and / or characterization of one or more allergies (e.g., causes IgE mediated allergic reaction (immediate hypersensitvity) in a mammal (e.g., a human or a non-human mammal). In certain embodiments, the device comprises a microcantilever array where different cantilevers comprising the array bear different antigens. Binding of IgE to the antigen on a cantilever causes bending of the cantilever which can be readily detected.
Owner:RGT UNIV OF CALIFORNIA
Anti-IgE antibodies
The present invention relates to novel human antibodies specifically directed against human immunoglobulin E (anti-IgE). The present invention also relates to pharmaceutical compositions and methods for treating asthma, in particular allergic asthma, as well as other IgE-mediated disorders including allergic rhinitis and food allergies.
Owner:PFIZER INC +1
Fusion molecules and treatment of IgE-mediated allergic diseases
The invention concerns bifunctional fusion molecules for the treatment of IgE-mediated allergic conditions and FcεRI-mediated autoimmune conditions. The invention provides a new therapeutic approach for the treatment of both acute and late-phase allergic responses due to ingestion, inhalation, cutaneous and parenteral exposure to allergens, responses including asthma, allergic rhinitis, atopic dermatitis, severe food allergies, chronic urticaria and angioedema, as well as anaphylactic reactions due to exposures such as bee stings or penicillin allergy. In addition, the invention provides for a novel, safer and more efficacious form of allergy vaccination.
Owner:RGT UNIV OF MINNESOTA +1
Method for treating IgE-mediated disorders
InactiveUS7157085B2Reducing and inhibiting IgE-mediated productionSugar derivativesMicrobiological testing/measurementDiseaseIsomerization
The present invention relates to a method for adjusting the affinity of a polypeptide to a target molecule by a combination of steps, including: (1) the identification of aspartyl residues which are prone to isomerization; (2) the substitution of alternative residues and screening the resulting mutants for affinity against the target molecule. In a preferred embodiment, the method of subtituting residues is affinity maturation with phage display (AMPD). In a further preferred embodiment the polypeptide is an antibody and the target molecule is an antigen. In a further preferred embodiment, the antibody is anti-IgE and the target molecule is IgE. In another embodiment, the invention relates to an anti-IgE antibody having improved affinity to IgE.
Owner:GENENTECH INC
IgE CH3 Peptide Vaccine
InactiveUS20110300163A1Reducing treatmentPreventing and alleviatingPeptide/protein ingredientsVertebrate antigen ingredientsPeptide vaccineImmunogenicity
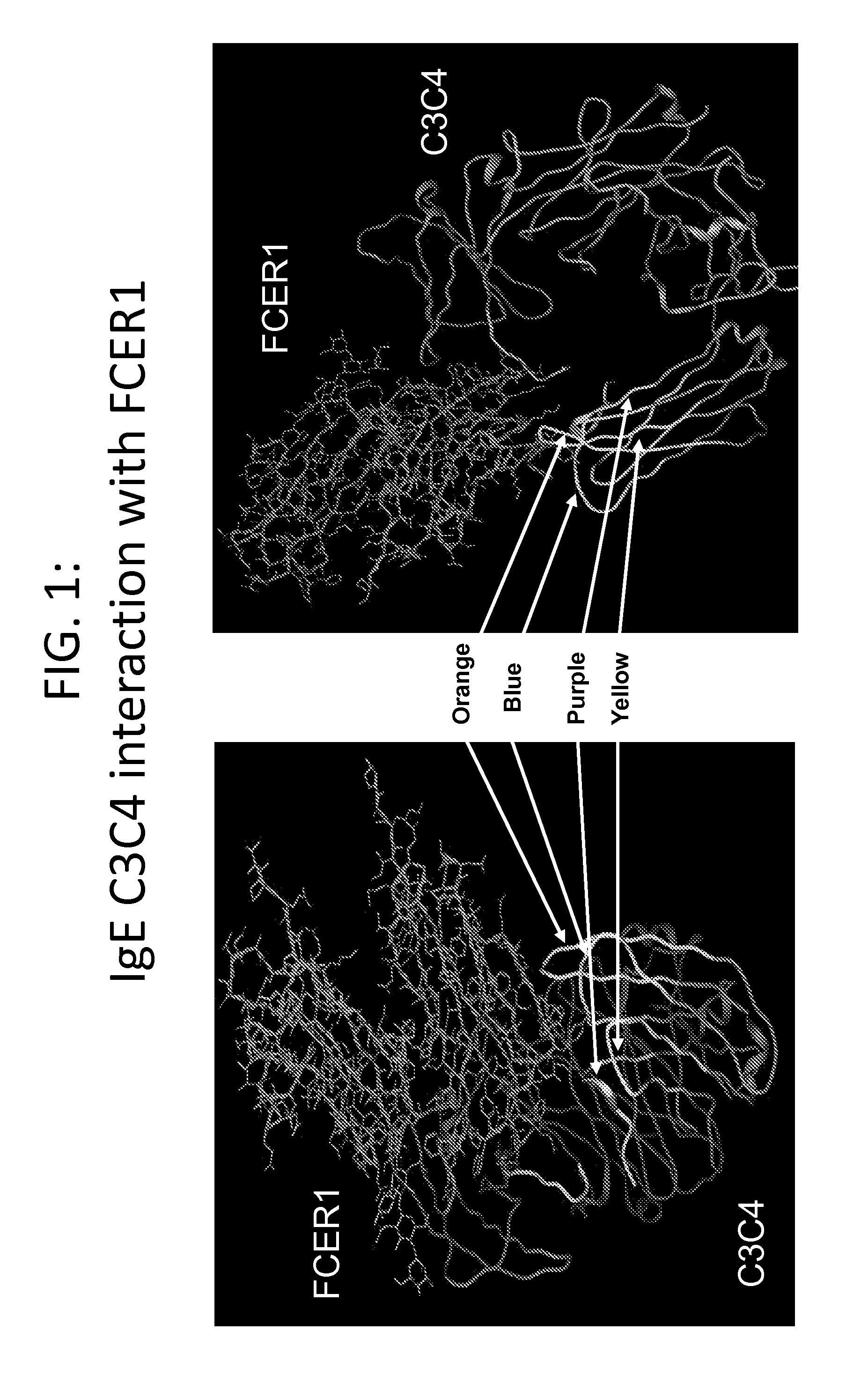
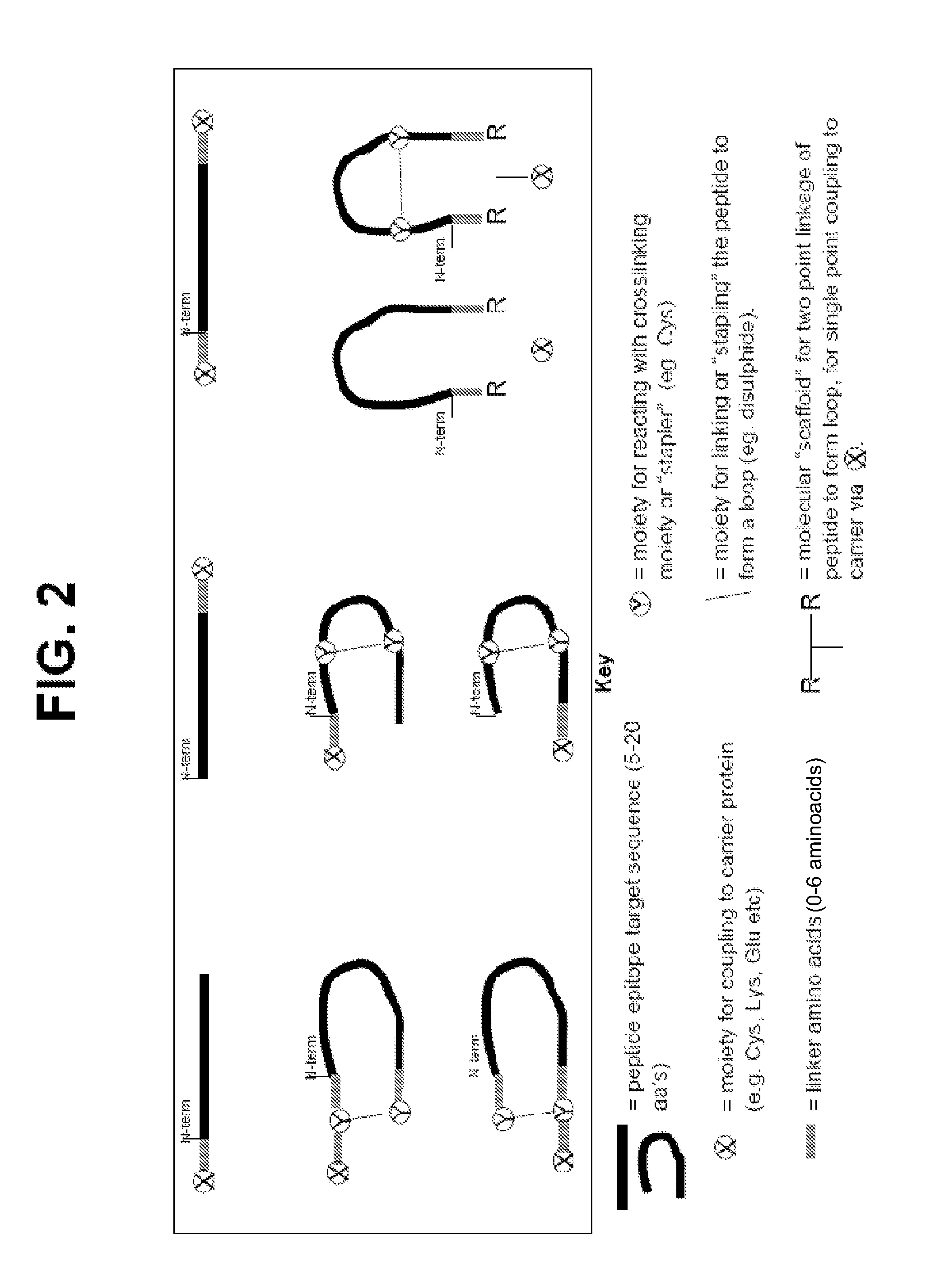
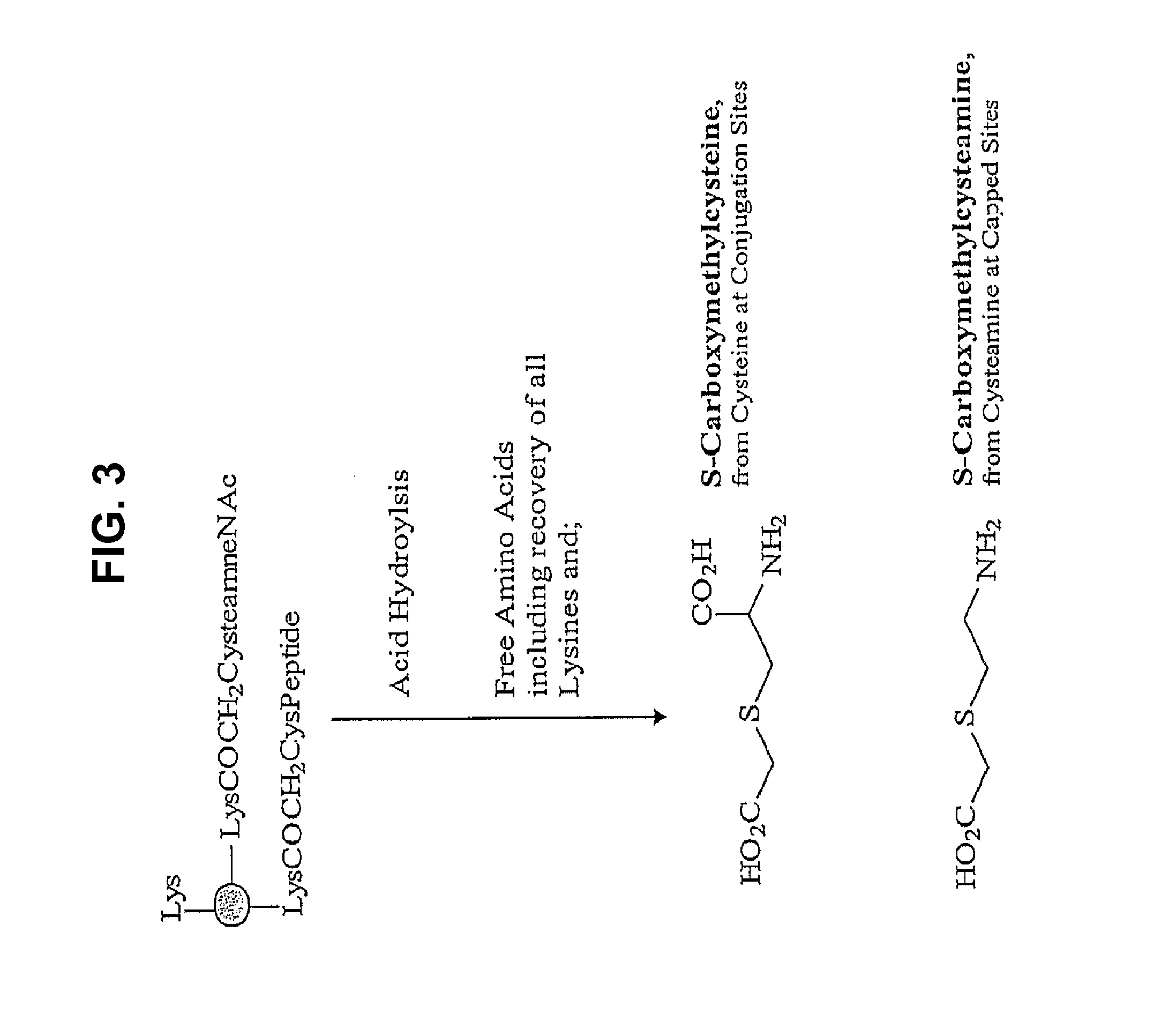
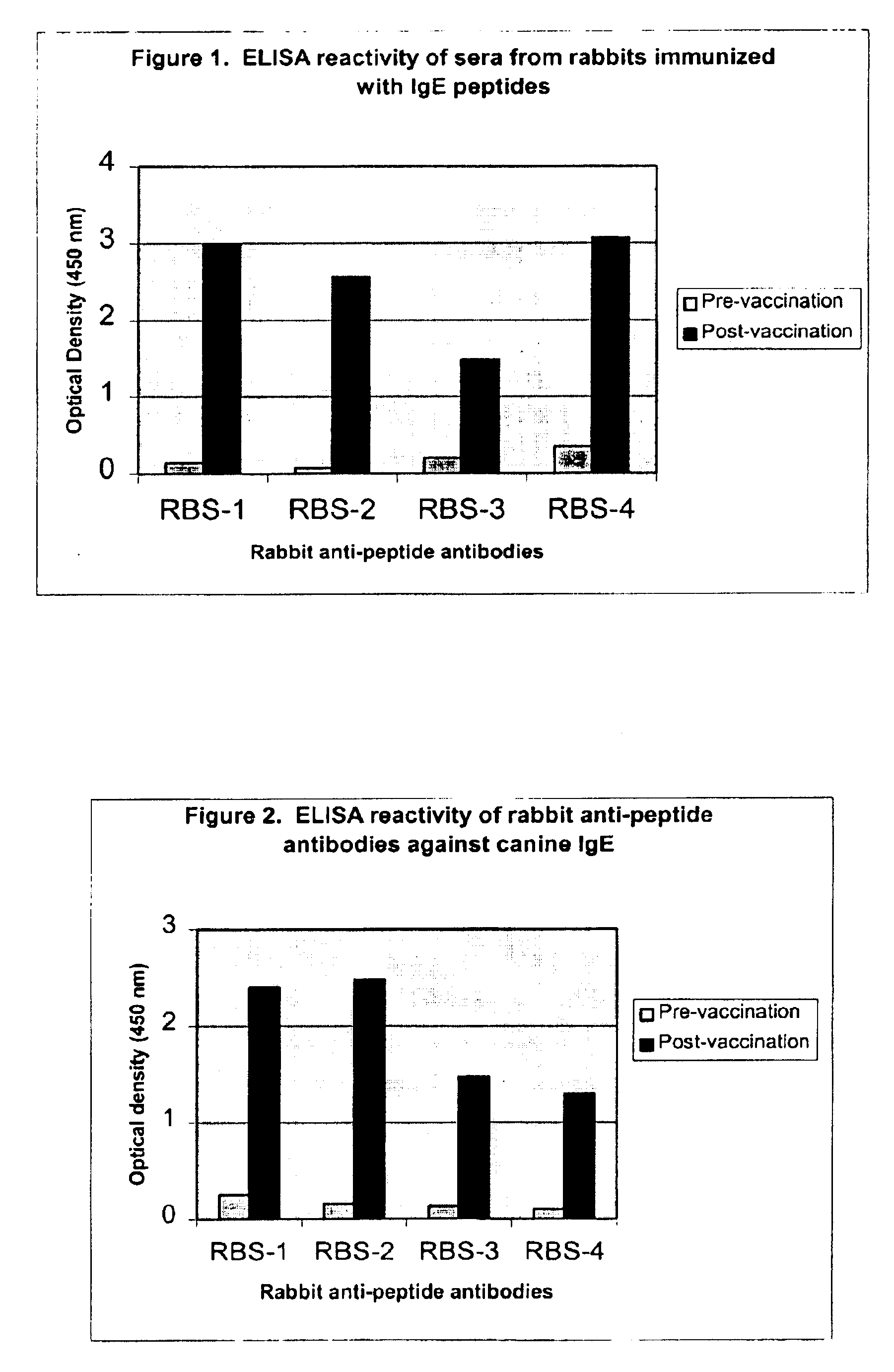
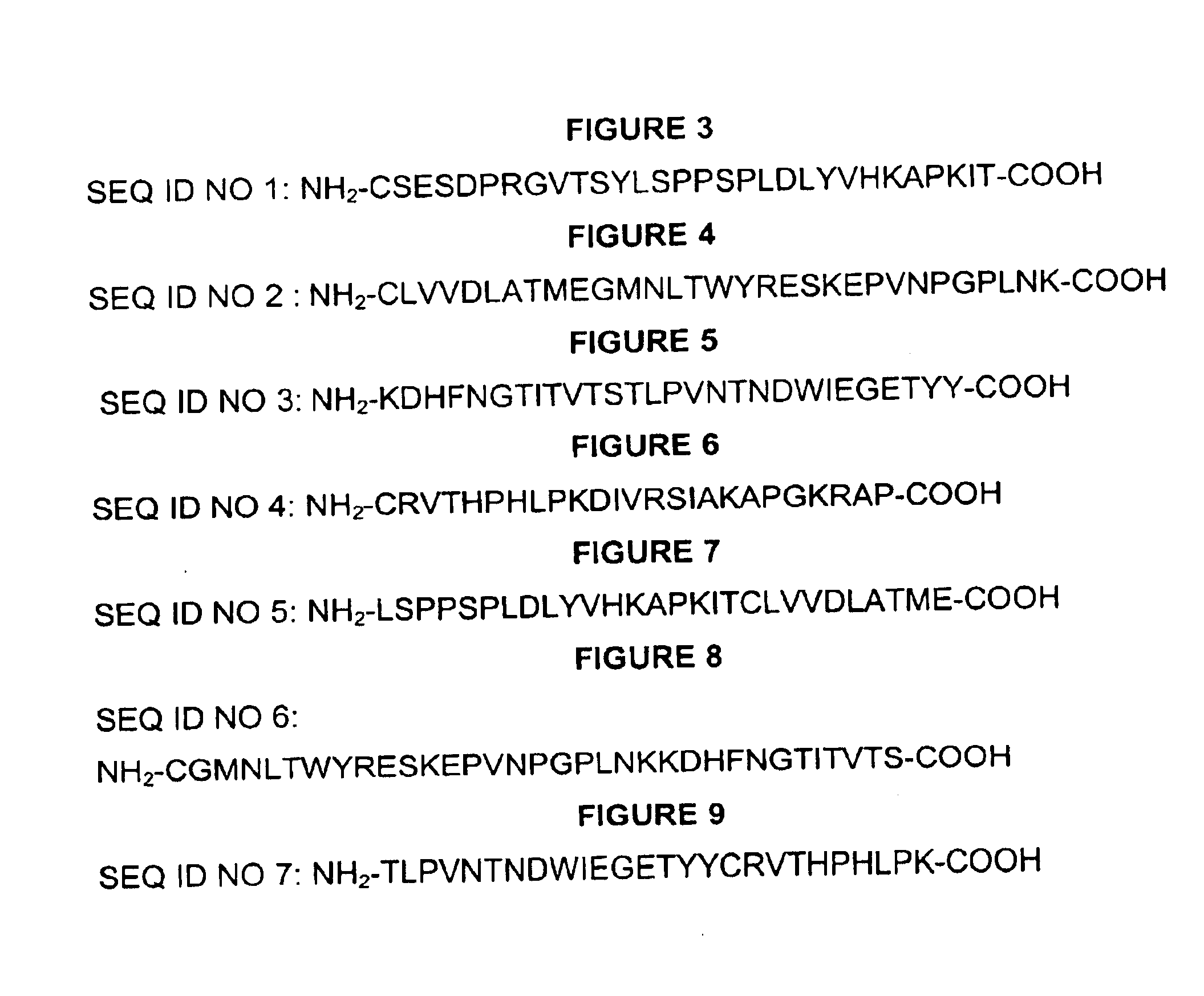
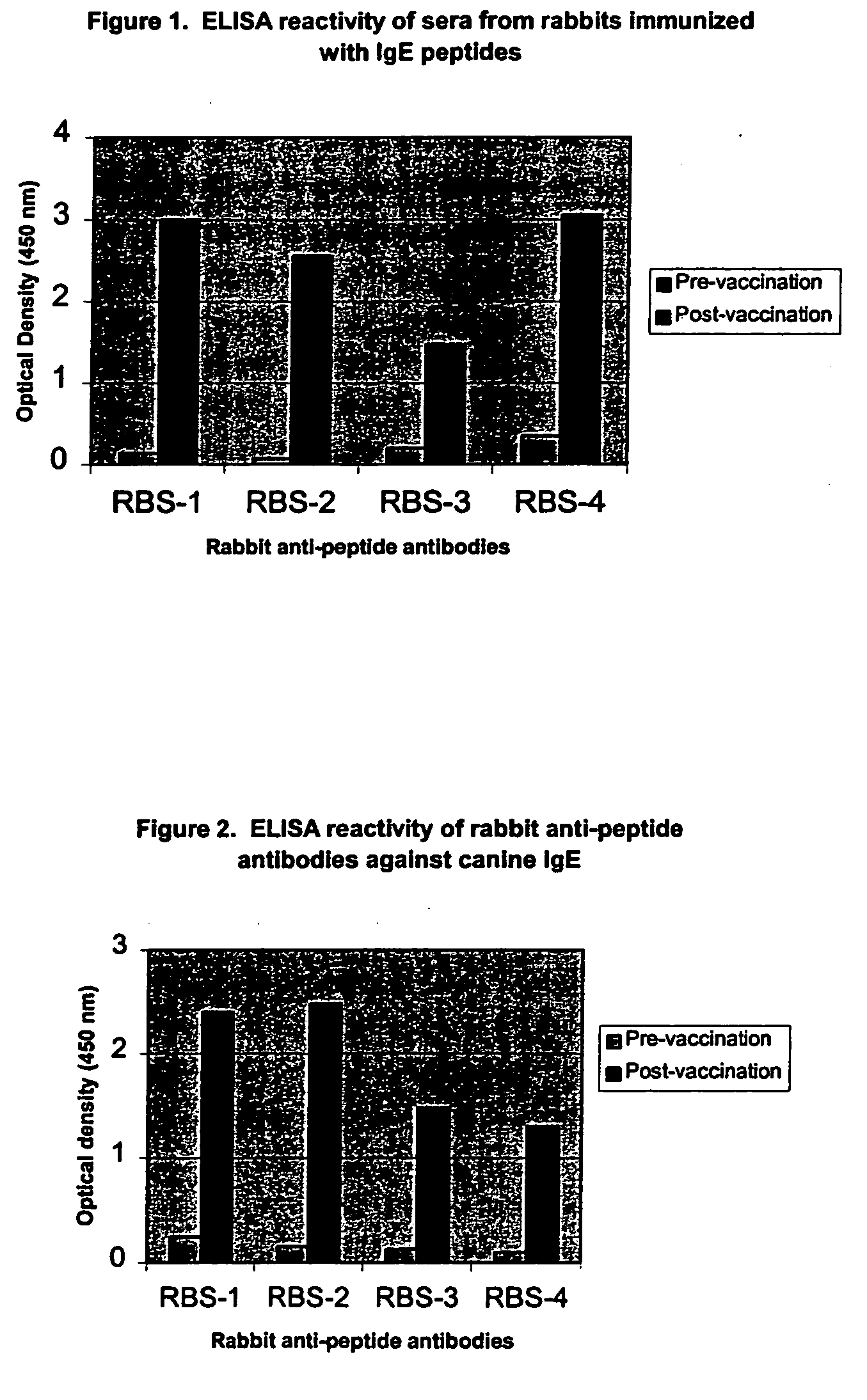
The present invention relates to the provision of novel immunogens comprising an antigenic IgE peptide preferably linked to an immunogenic carrier, compositions comprising the immunogens, and methods for the prevention, treatment or alleviation of IgE-mediated disorders. The invention further relates to methods for production of these medicaments, immunogenic compositions and pharmaceutical compositing thereof and their use in medicine.
Owner:PFIZER VACCINES
Method for preparing recombinant human IgE receptor protein and application of recombinant human IgE receptor protein
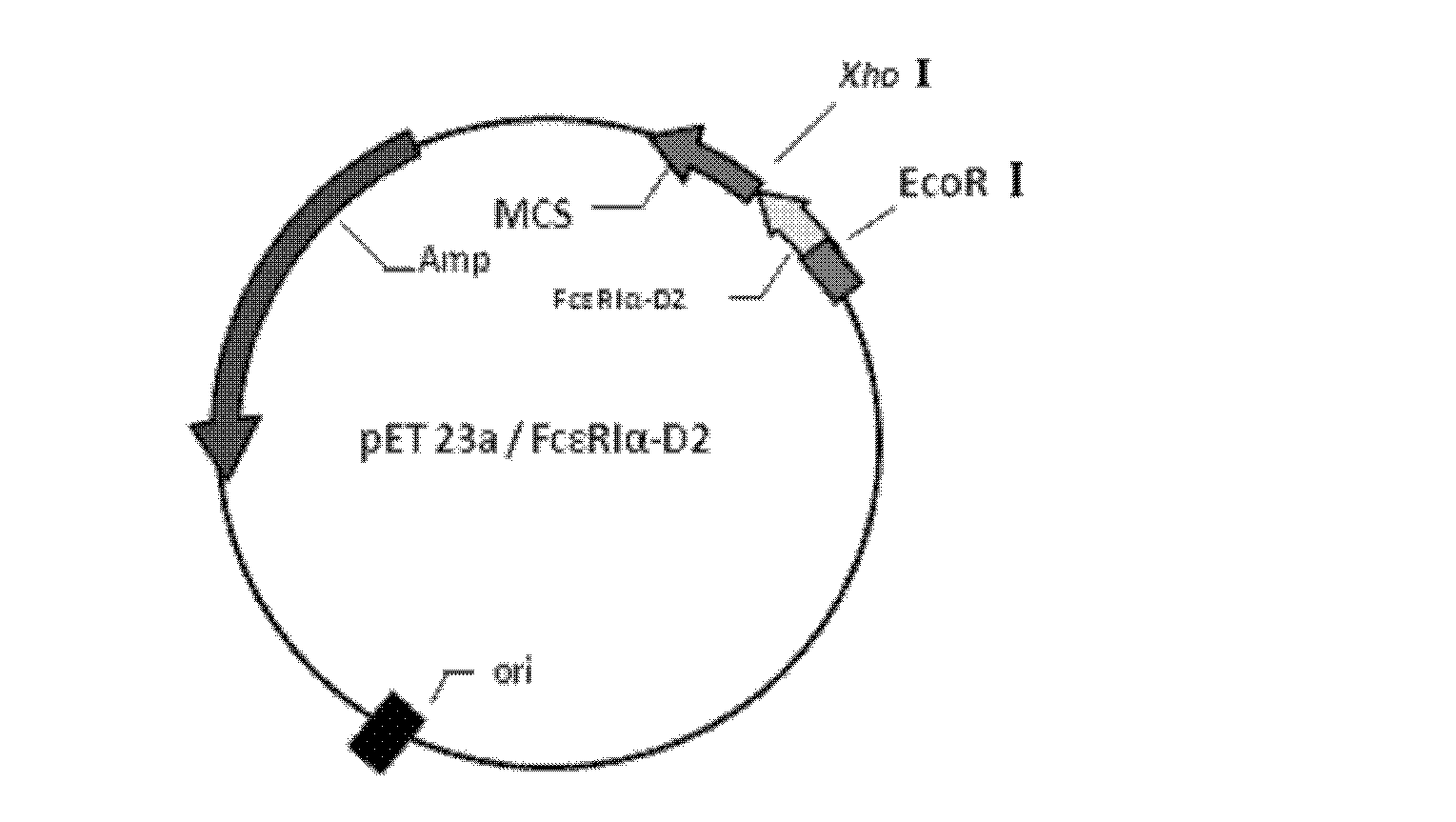
ActiveCN102660569AImprove bindingGuaranteed removal effectBacteriaPeptide/protein ingredientsSorbentHypersensitive response
The invention discloses a method for preparing recombinant human IgE receptor protein and application of the recombinant human IgE receptor protein, and belongs to the field of biological engineering and blood purification technology. Fc epsilon RI alpha-D2 with high binding force with IgE is highly expressed by means of gene recombination technology, recombinant protein is obtained by means of purification and used as a genin of an adsorbent, and the prepared adsorbent fixed onto a solid phase can be used for binding the IgE in a high-affinity manner. The method solves the problems of low safety and high cost of the prior art in the field of IgE removal by means of the blood purification technology. The method has an excellent application prospect in terms of removing excessive IgE in blood of an allergic reaction patient by means of the blood purification technology, treating allergic reaction mediated by the IgE, purifying the IgE and the like.
Owner:DALIAN UNIV OF TECH
Anti-IgE vaccines
The present invention provides compositions and methods for the use of antigenic peptides derived from the Fc portion of the epsilon heavy chain of an IgE molecule as vaccines for the treatment and prevention of IgE-mediated allergic disorders. In particular, the invention provides compositions, methods for the treatment and prevention of IgE-mediated allergic disorders comprising an immunogenic amount of one or more antigenic peptides derived from the CH3 domain or junction of Ch-3 / CH4 domain of an IgE molecule and methods for the evaluation of IgE mediated allergies in dogs.
Owner:ZOETIS SERVICE LLC
Compositions and methods for treating IgE-mediated disorders
Owner:XENCOR INC
MINIMALLY INVASIVE ASSESSMENT OF IgE MEDIATED ALLERGY
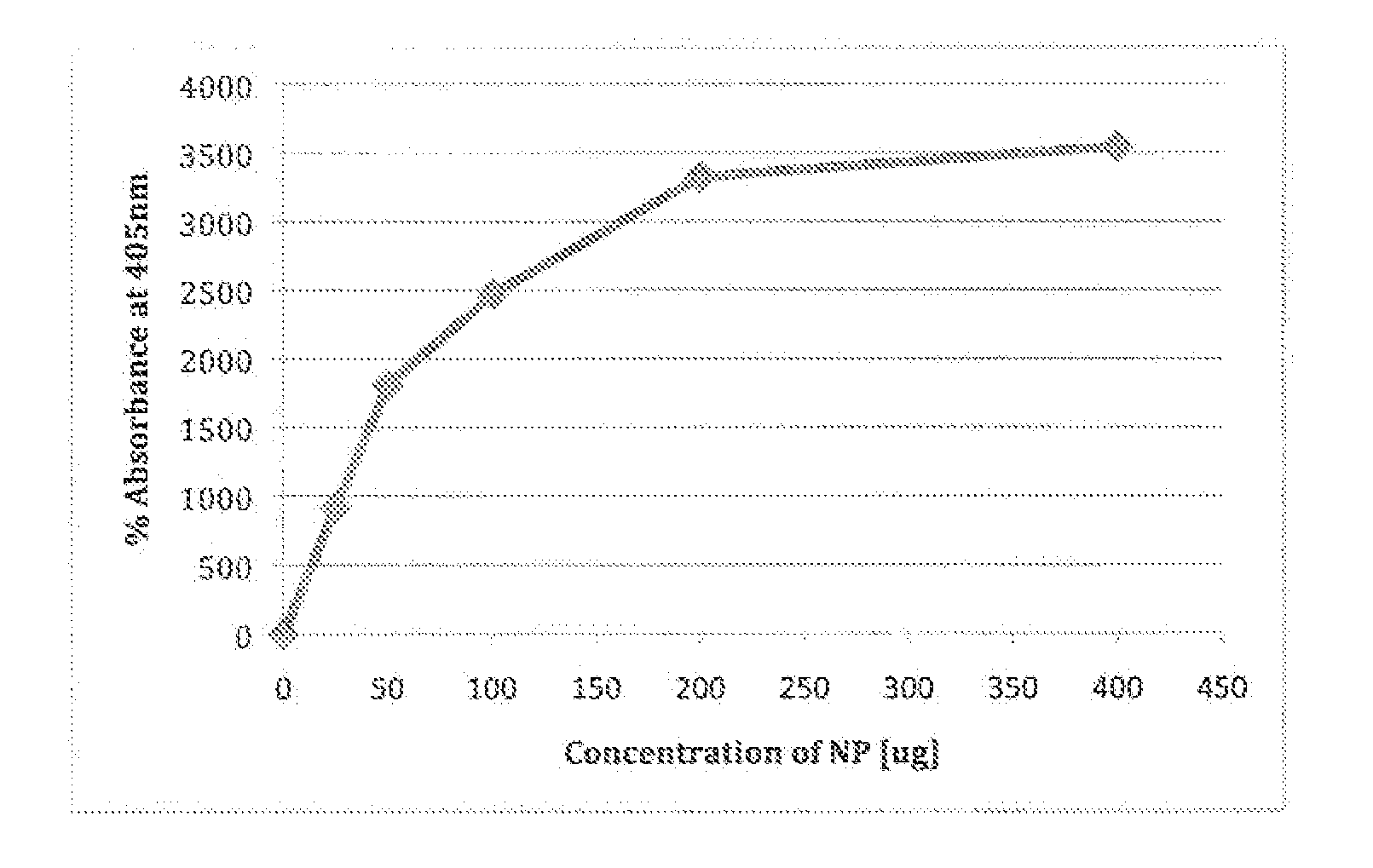
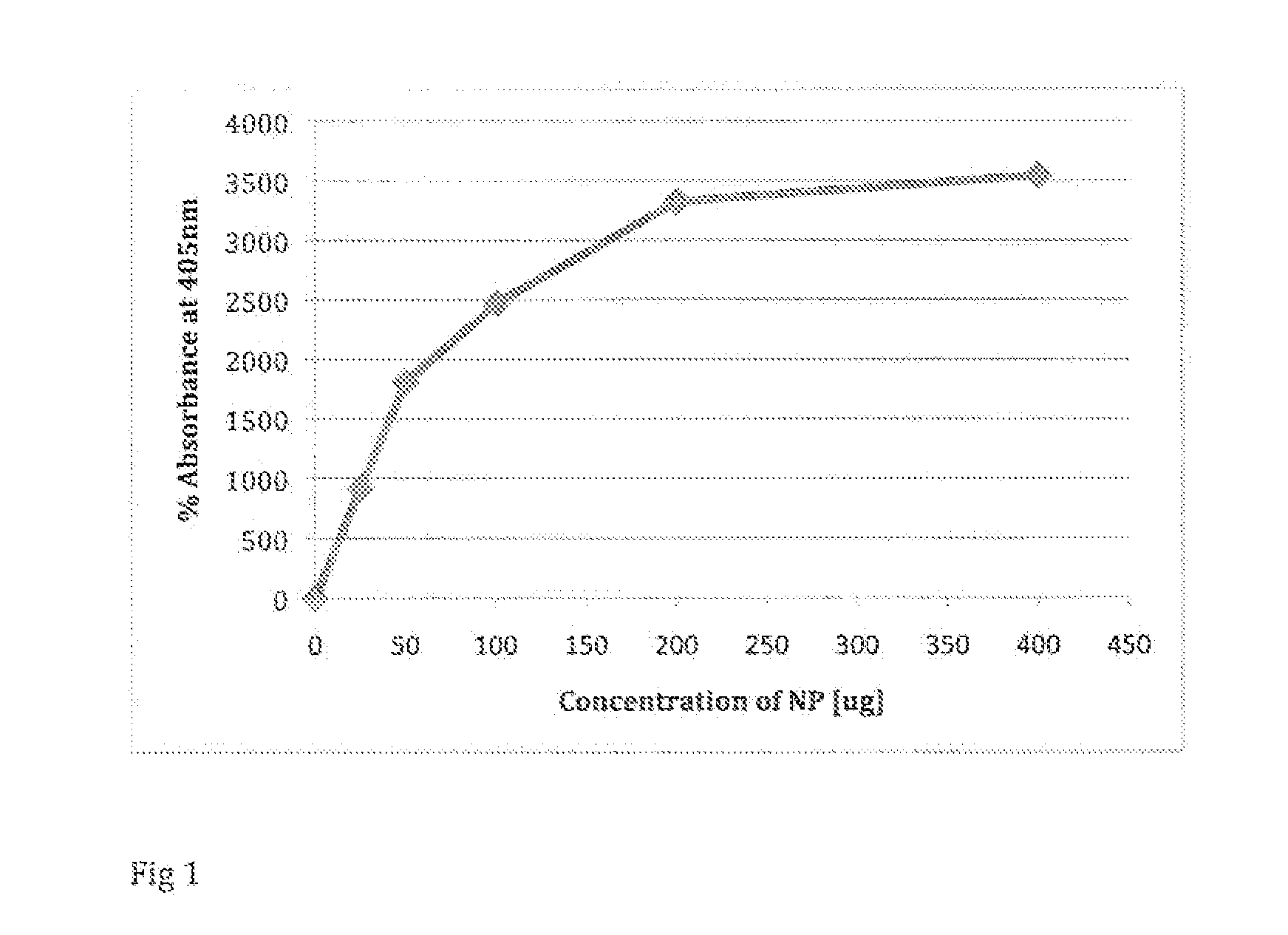
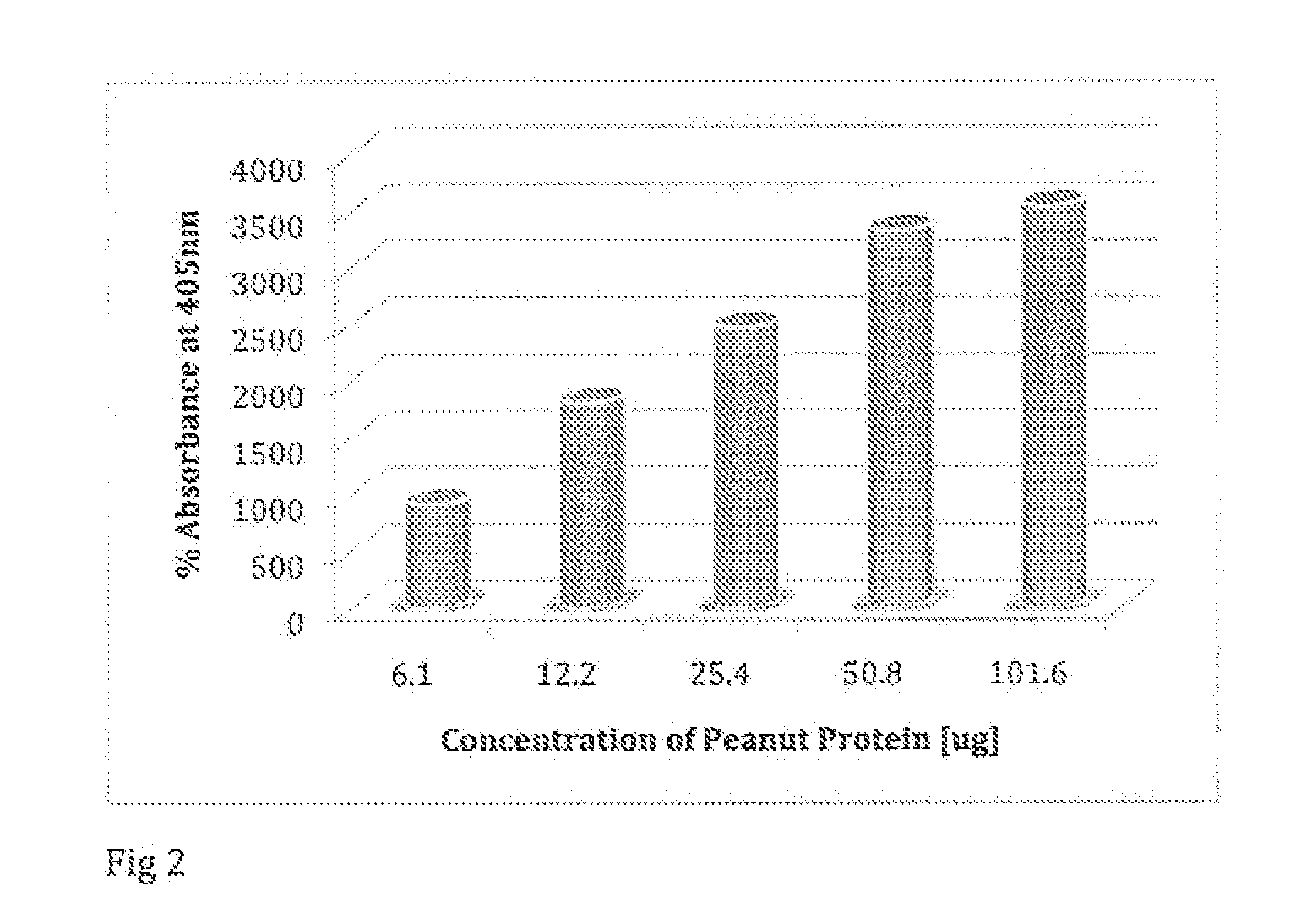
A system and method for determining the presence and level of allergy indicators in a human fluid sample such as, but not limited to, blood serum and saliva, is disclosed. In another embodiment, the method may assess a level of allergens in a consumable product. The system and method may make use of functionalized magnetic nanoparticles that have modified surfaces suitable for attracting allergy indicators from human fluid sample and allergens from consumable products. The system and method may provide a minimally invasive assessment of allergy indicators to determine whether one is allergic to a substance.
Owner:UNITED ARAB EMIRATES UNIVERSITY +1
Anti-IgE vaccines
Owner:ZOETIS SERVICE LLC
Synergistic compositions containing aromatic compounds and terpenoids present in Alpinia galanga
InactiveUS7252845B2Suppress hypersensitivity reactionCosmetic preparationsBiocideImmunologic disordersUlcerative colitis
Novel compositions of matter containing aromatic compounds and terpenoids which are present in and may preferably be derived from the plant Alpinia galanga (Zingiberaceae) show synergistic effects with respect to immunomodulation, and they significantly suppress hypersensitivity reactions. Thus they are used for preparing medicaments for these purposes and, more specifically, for the treatment or prevention of IgE mediated allergic reactions and conditions, such as asthma, allergic rhinitis, atopic eczema or anaphylaxis, and autoimmune disorders, such as Crohn's disease, ulcerative colitis, rheumatoid arthritis or psoriasis, as well as for the alleviation of pain. They can for example be formulated into pharmaceuticals, cosmetics or dietary supplements. A method of preparing such compositions from Alpinia galanga is also described.
Owner:AS FERROSAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com