Method for preparing recombinant human IgE receptor protein and application of recombinant human IgE receptor protein
A technology of recombinant protein and receptor protein, applied in the field of application, can solve the problems of high cost, increase of IgE concentration, aggravated allergic reaction, etc., and achieve the effect of reducing production cost, ensuring safety, and alleviating the disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
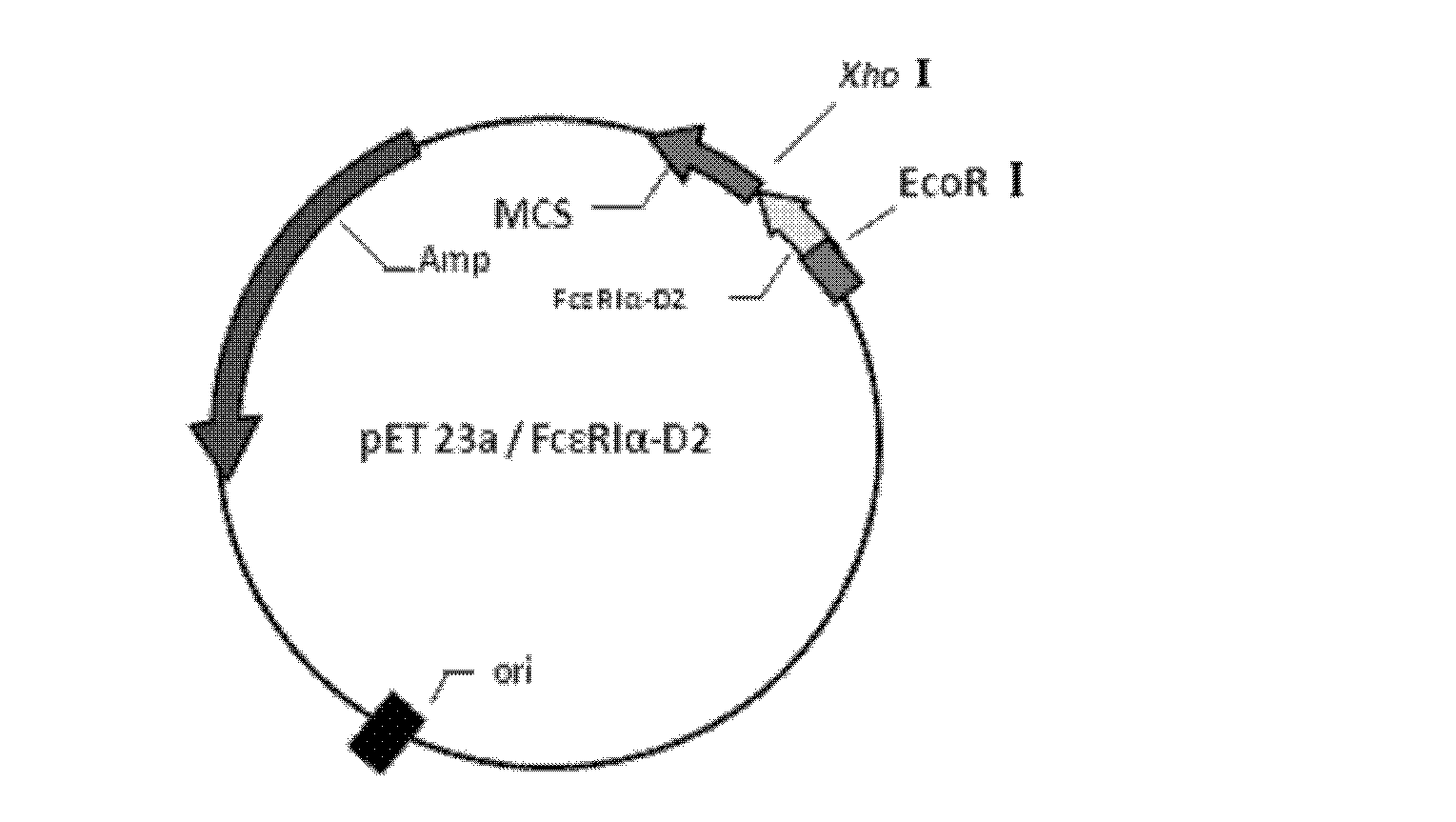
[0028] Example 1: Construction and Identification of pET23a / FcεR I α-D2 Recombinant Plasmid
[0029] (1) PCR amplification of the target gene fragment
[0030] Search the National Center for Biological Information (NCBI) in the United States to obtain the complete gene sequence of human FcεRI (NCBI Reference Sequence: NM 002001.3), and select the gene sequence of the D2 domain (87-173 amino acids of FcεRIα) with IgE affinity properties of human FcεRIα . EcoR I and Xho I were used as upstream and downstream restriction sites respectively, and a 6×His tag was added to the C-terminus. Shanghai Xuguan Biotechnology Development Co., Ltd. was commissioned to synthesize the gene sequence (SEQ ID NO: 1) and the target gene Linked to pET 28a expression vector, named pET 28a / FcεRIα-D2.
[0031] Using the pET 28a / FcεRIα-D2 genomic DNA as a template, design primers and add enzyme cutting sites. The sequence shown in the upstream primer sequence SEQ ID NO: 2, the sequence shown in the d...
Embodiment 2
[0058] Embodiment 2: the shaking flask culture of recombinant bacterium
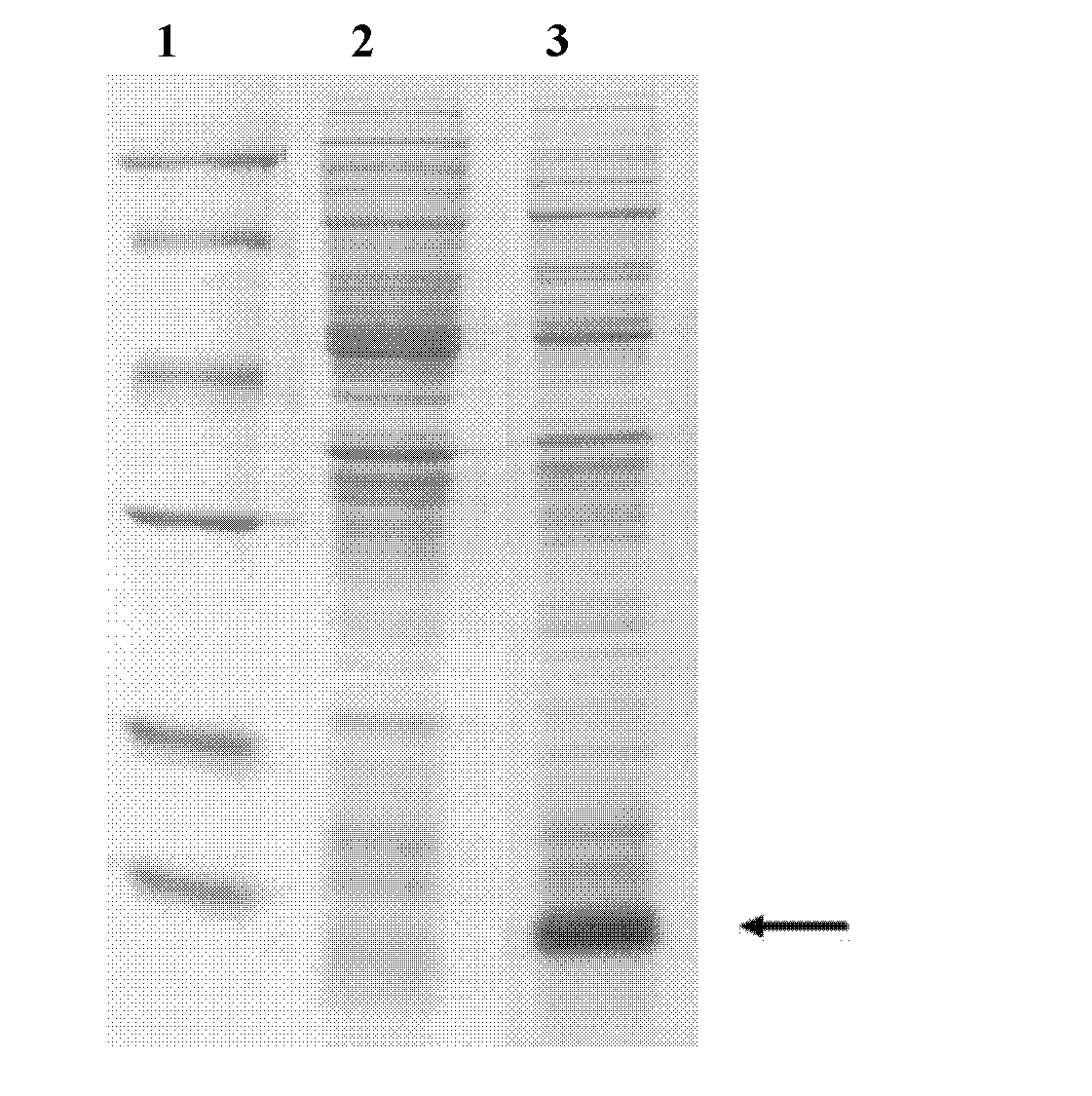
[0059] Pick a single colony of E.coli BL21 (DE3) containing the recombinant plasmid pET23a / FcεRIα-D2 on the LB solid medium plate in Example 1, inoculate it in 20 mL of LB liquid medium containing 1 mg / mL ampicillin, and shake it at a constant temperature Cultivate overnight in bed at 37°C, 170rpm. Inoculate the activated bacteria into 100mL LB liquid medium containing 1mg / mL ampicillin according to the inoculum amount of 1%, cultivate at 37°C and 170rpm for 3-4 hours, and wait until the OD of the bacteria 600nm When reaching 0.5-1, add IPTG to a final concentration of 0.1 mM, and continue culturing for 5 hours. SDS-PAGE electrophoresis detection, the results are attached figure 2 As shown in the middle lane 3, the recombinant bacteria expressed FcεRIα-D2 at about 13kDa, which was consistent with the theoretical molecular weight of 12950.19Da. The soluble detection of the target protein is carried ou...
Embodiment 3
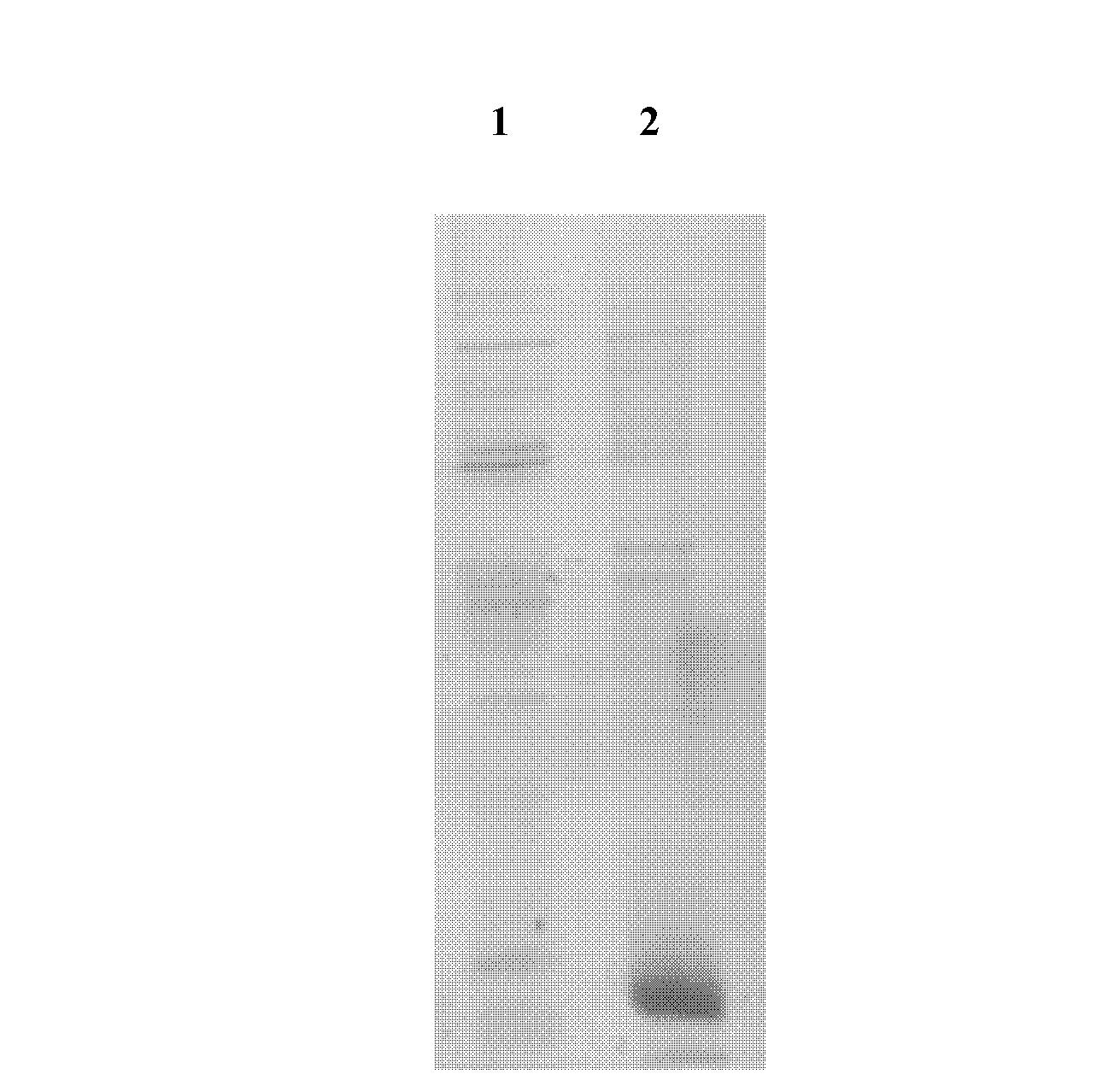
[0060] Example 3: Renaturation of recombinant protein FcεRIα-D2 inclusion body and separation and purification of protein
[0061] (1) Extraction and preliminary purification of inclusion bodies (cloning, prokaryotic expression and purification of human FcγR I receptor extracellular region gene [J]. Journal of Cell and Molecular Immunology. 2010, (002): 178-180.): Will The obtained inclusion bodies were washed 5 times with a washing buffer, and then washed once with a resuspension buffer to obtain preliminarily purified inclusion bodies containing the recombinant protein FcεRIα-D2. The pH of the washing buffer is 8.0, containing 0.01M Tris-HCl, 0.1M NaCl, 0.01M EDTA, 2mM DTT and 10% Triton-100; the pH of the resuspension buffer is 8.0, containing 0.01M Tris-HCl, 0.1M NaCl, 0.01M EDTA and 2mM DTT.
[0062] (2) Renaturation of the recombinant protein FcεRIα-D2: Denaturation and renaturation of the initially purified inclusion bodies containing the recombinant protein FcεRIα-D2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com