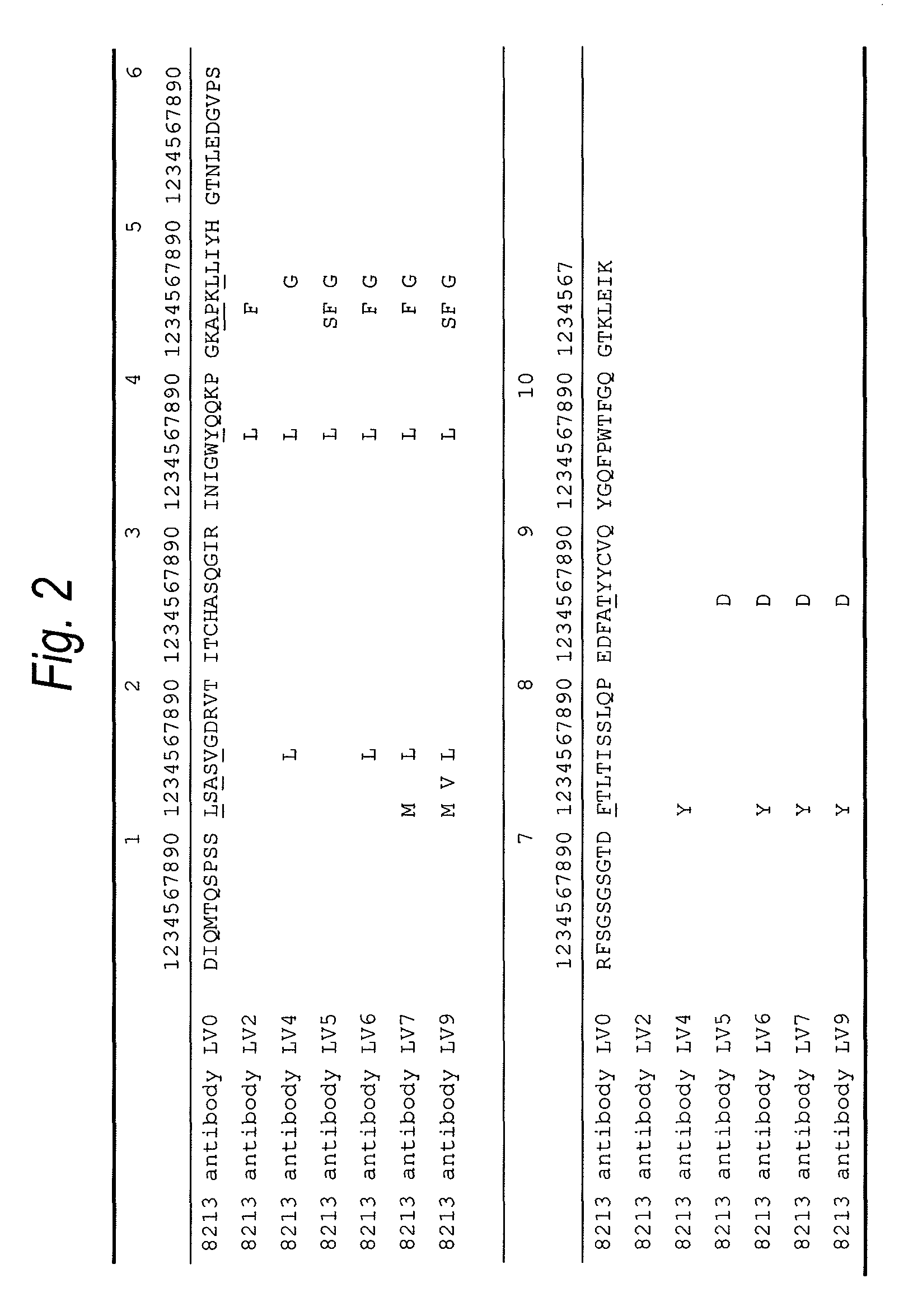
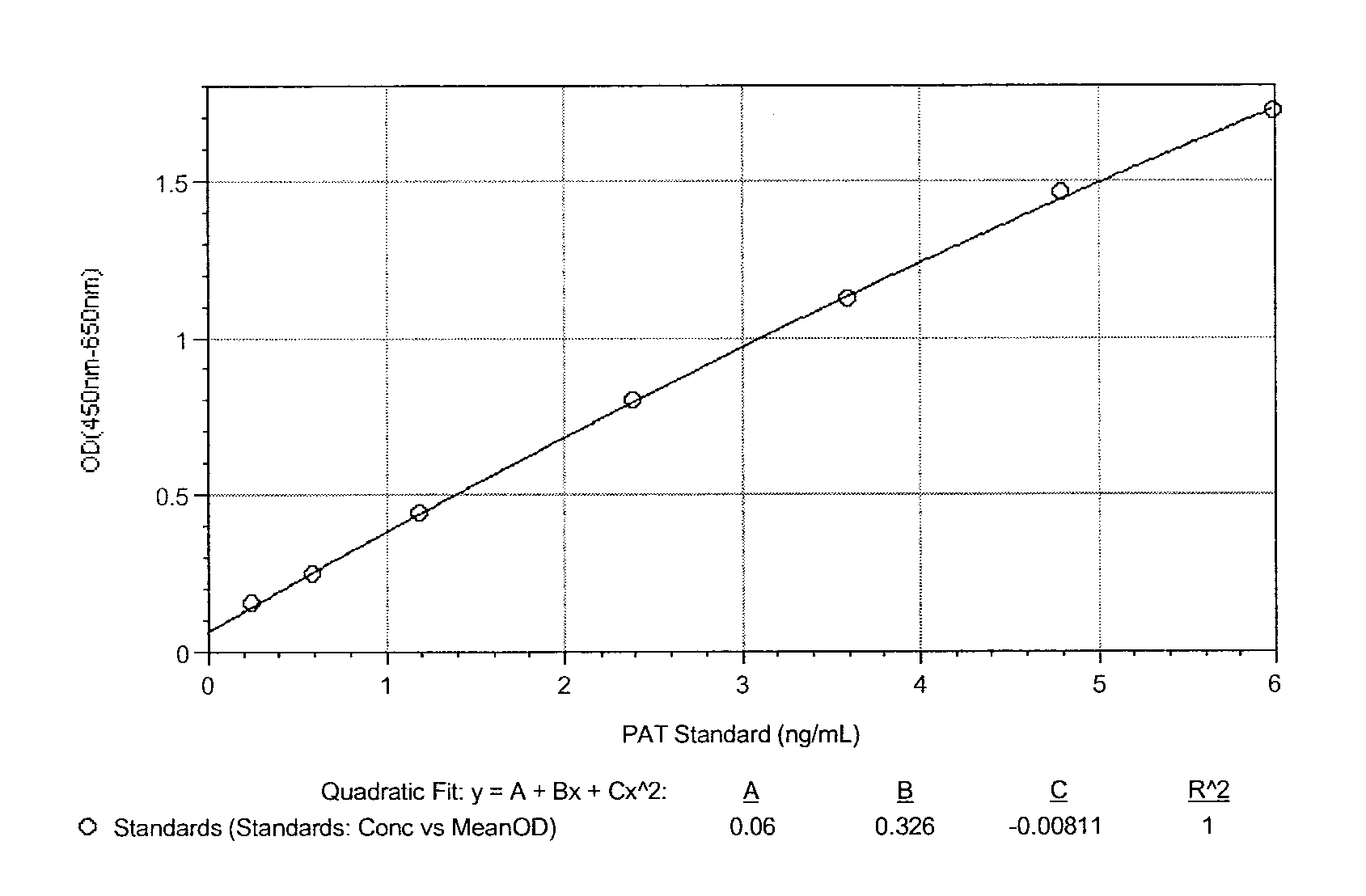
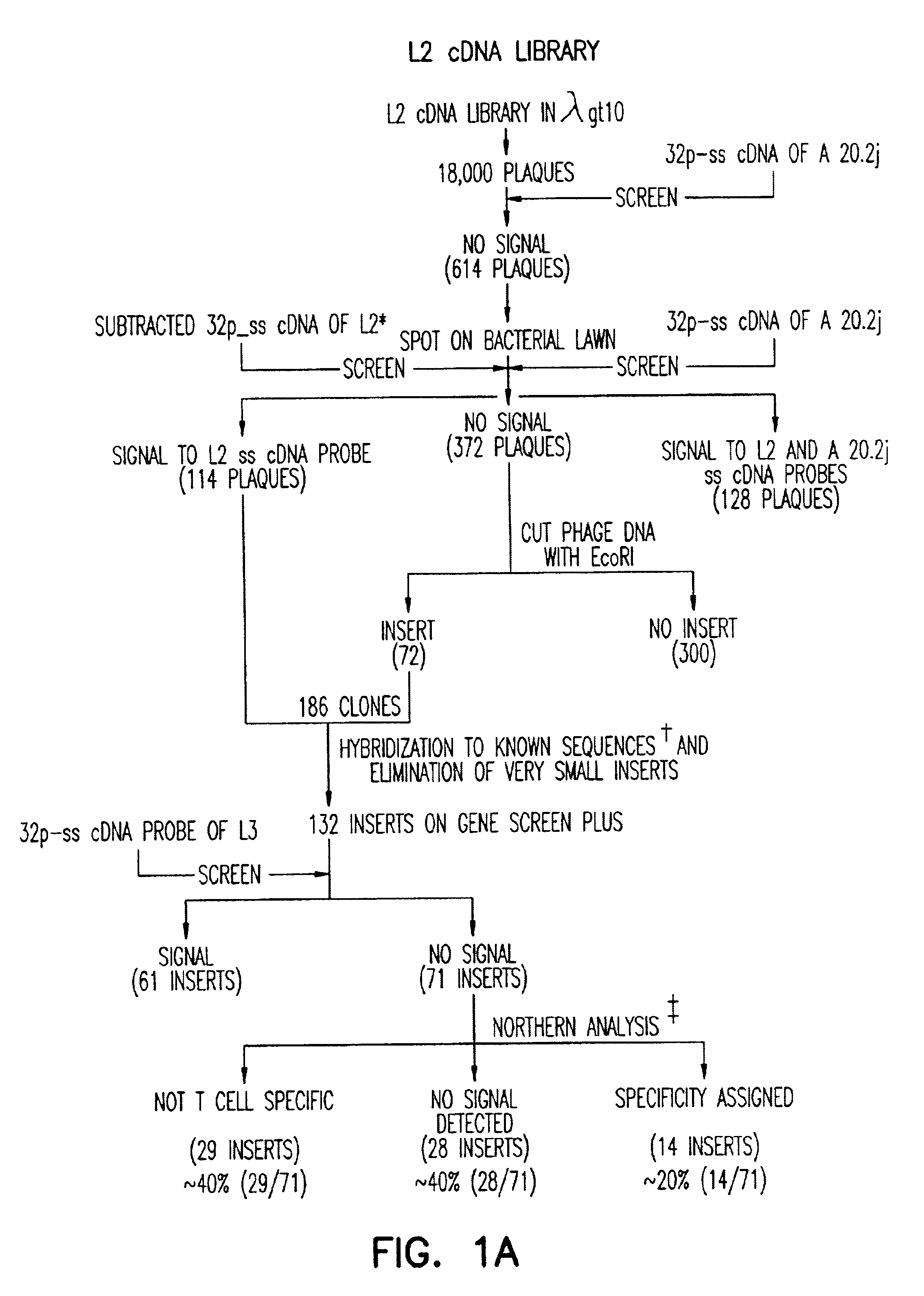
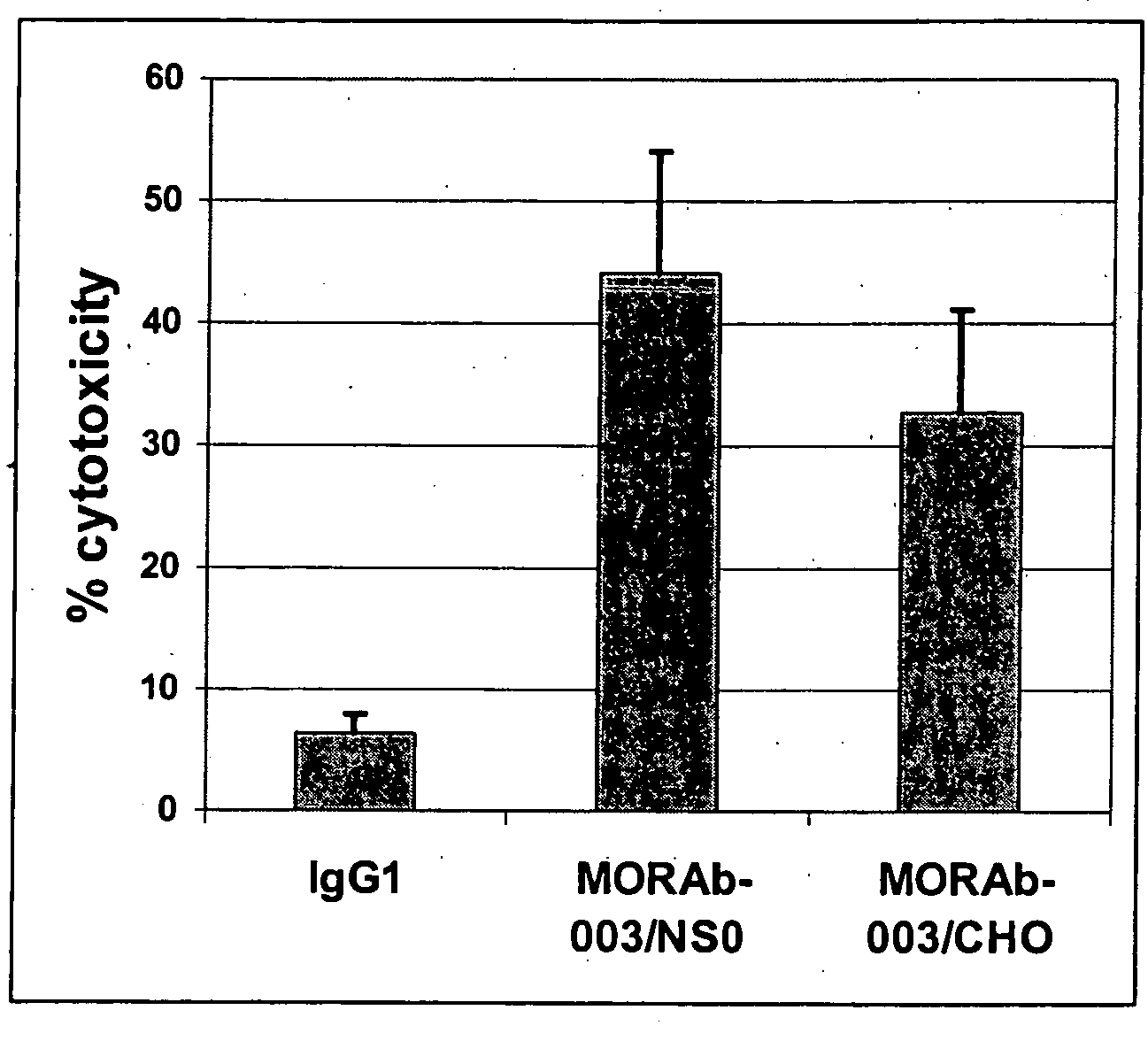
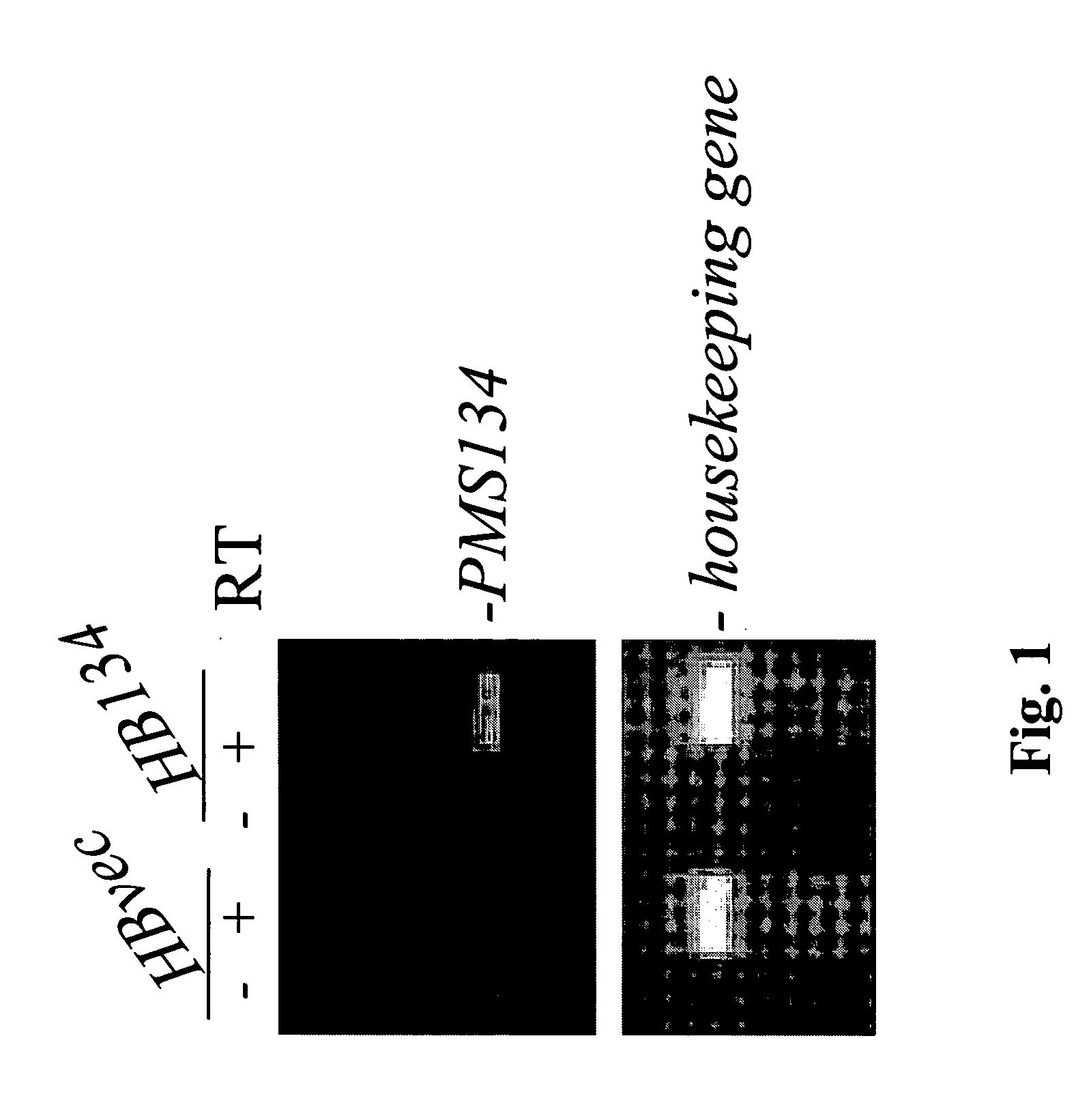
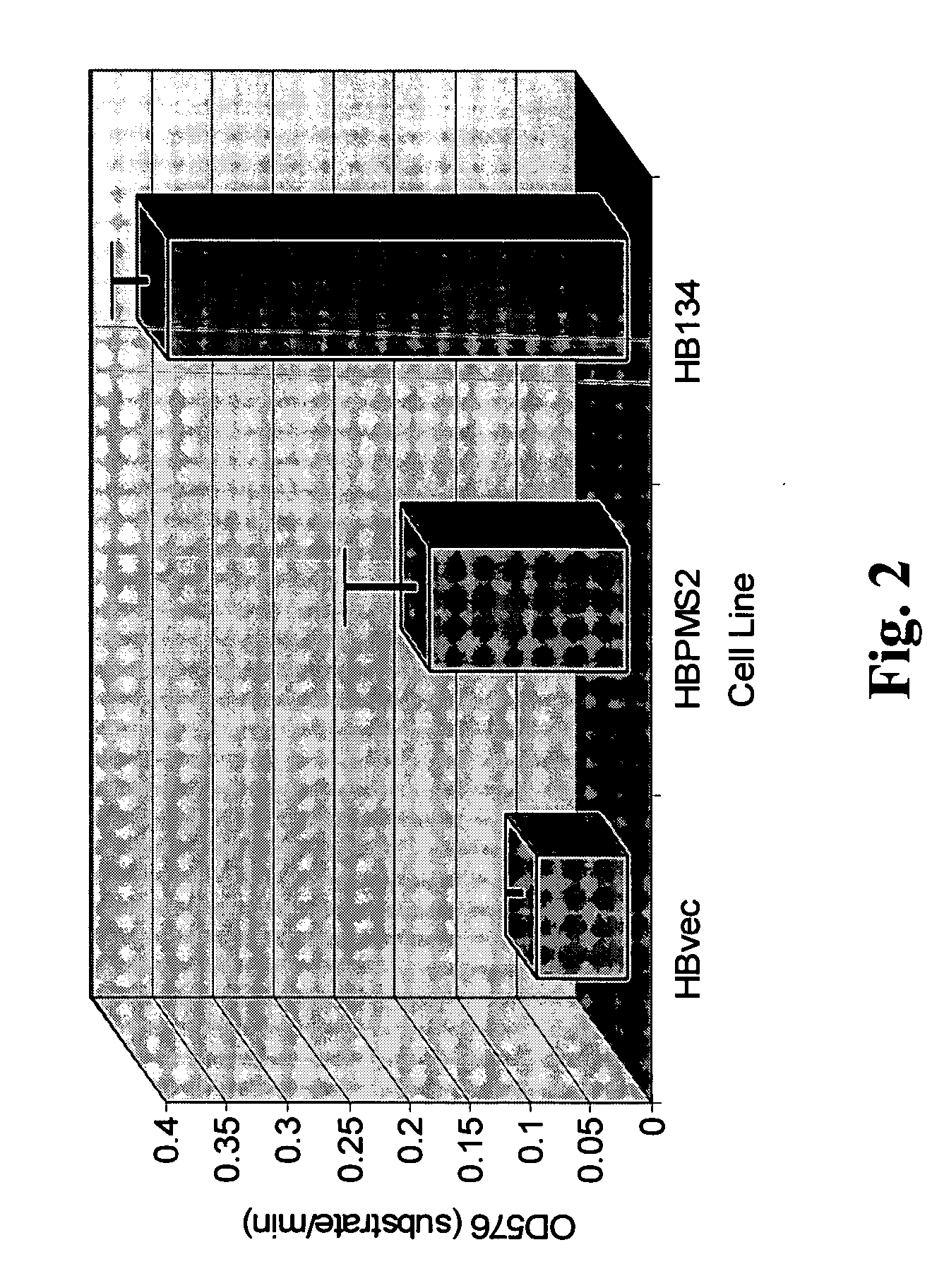
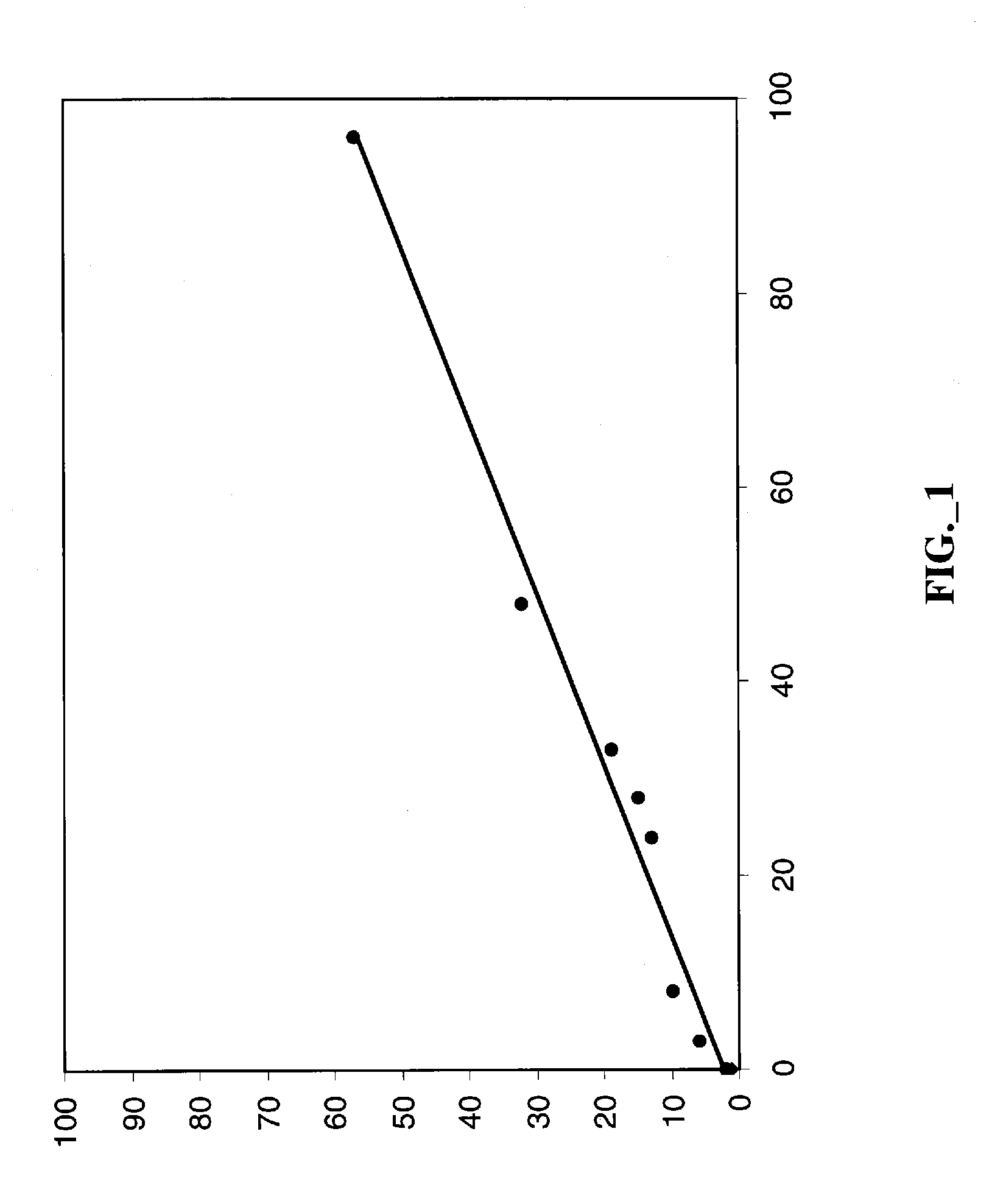
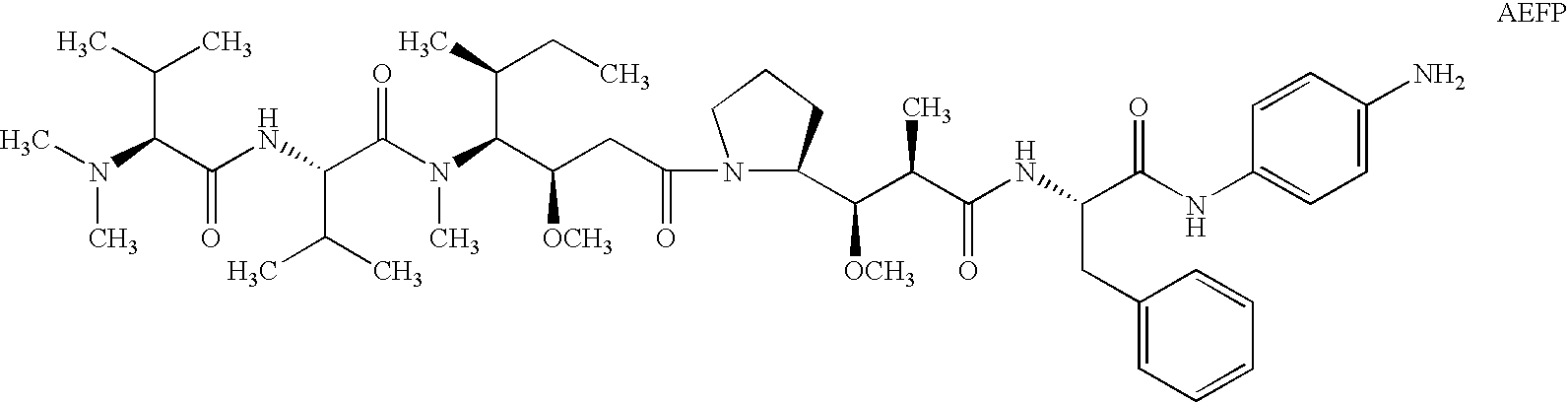
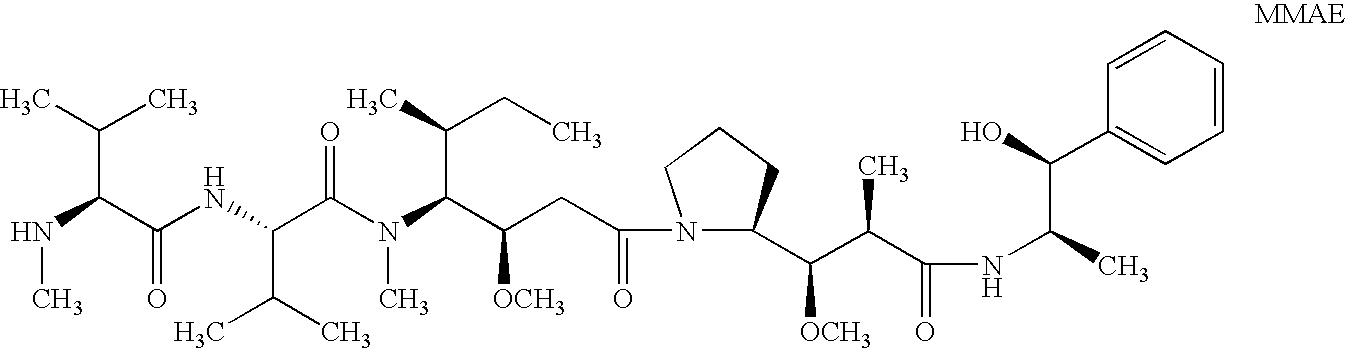
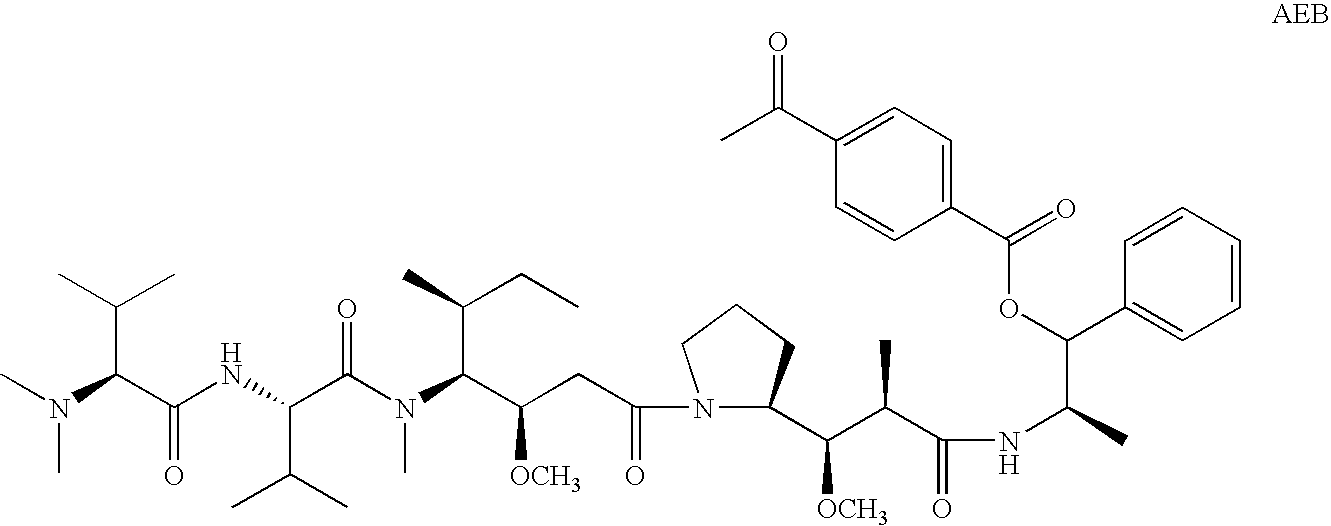
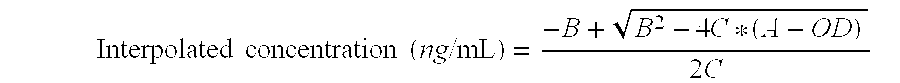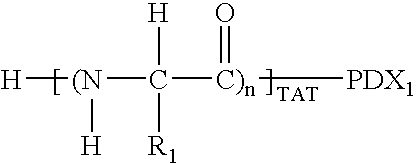Patents
Literature
5118 results about "Monoclonal antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monoclonal antibodies (mAb or moAb) are antibodies that are made by identical immune cells that are all clones of a unique parent cell. Monoclonal antibodies can have monovalent affinity, in that they bind to the same epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different plasma cell (antibody secreting immune cell) lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one single monoclonal antibody to two epitopes.
CE7-specific redirected immune cells
Genetically engineered, CE7-specific redirected immune cells expressing a cell surface protein having an extracellular domain comprising a receptor which is specific for CE7, an intracellular signaling domain, and a transmembrane domain, and methods of use for such cells for cellular immunotherapy of CE7+ neuroblastoma are disclosed. In one embodiment, the immune cell is a T cell and the cell surface protein is a single chain FvFc:ζ receptor where Fv designates the VH and VL chains of a single chain monoclonal antibody to CE7 linked by peptide, Fc represents a hinge —CH2—CH3 region of a human IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. DNA constructs encoding a chimeric T-cell receptor and a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor are also disclosed.
Owner:CITY OF HOPE
Modification assisted profiling (MAP) methodology
Methods of utilizing a biosensor platform for the purpose of studying macromolecule interactions is provided. Also provided are methods of sorting monoclonal antibodies directed against a particular antigen into functional groups wherein each group exhibits a characteristic binding profile to the antigen.
Owner:REGENERON PHARM INC
Antibody formulations
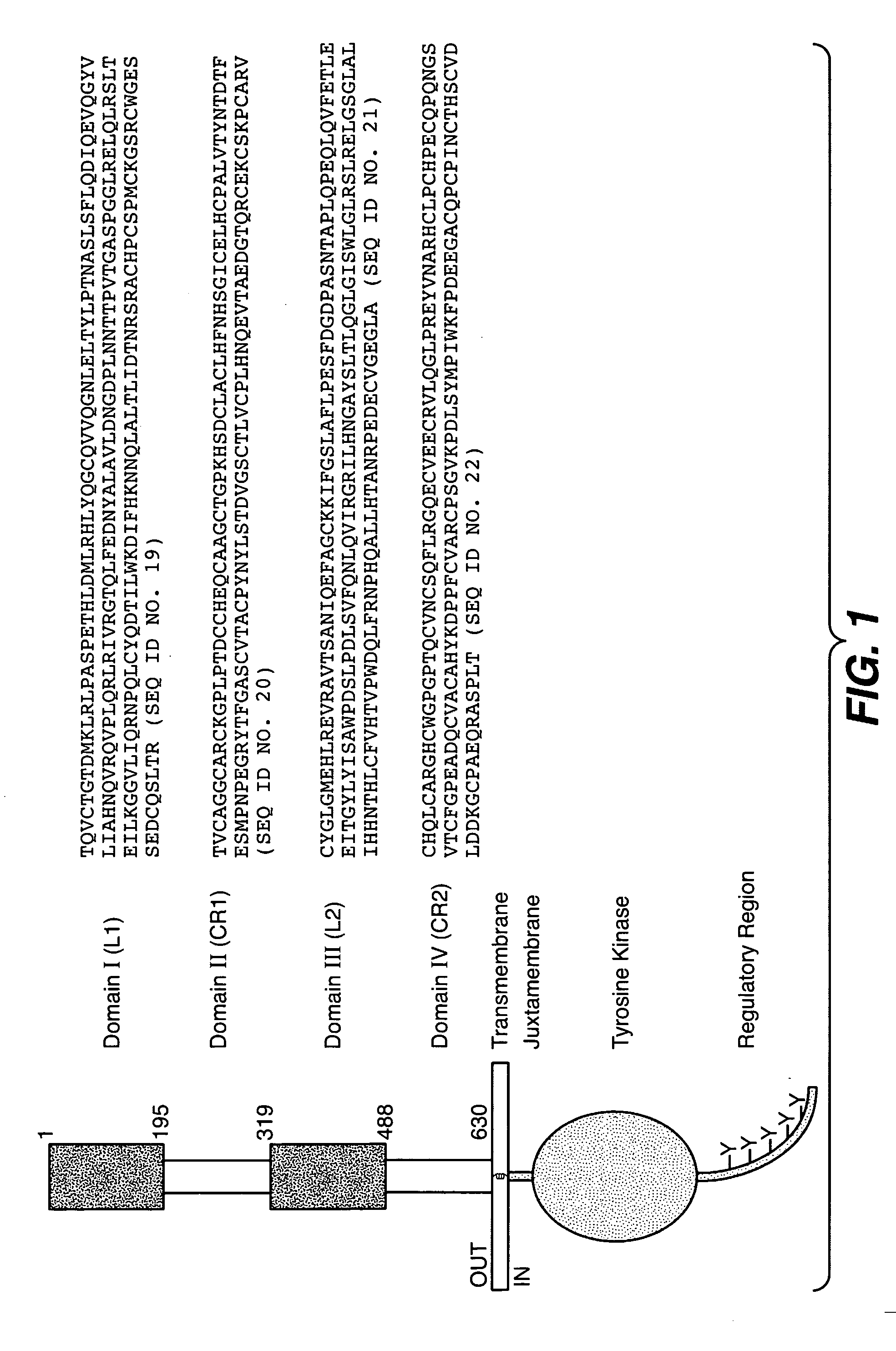
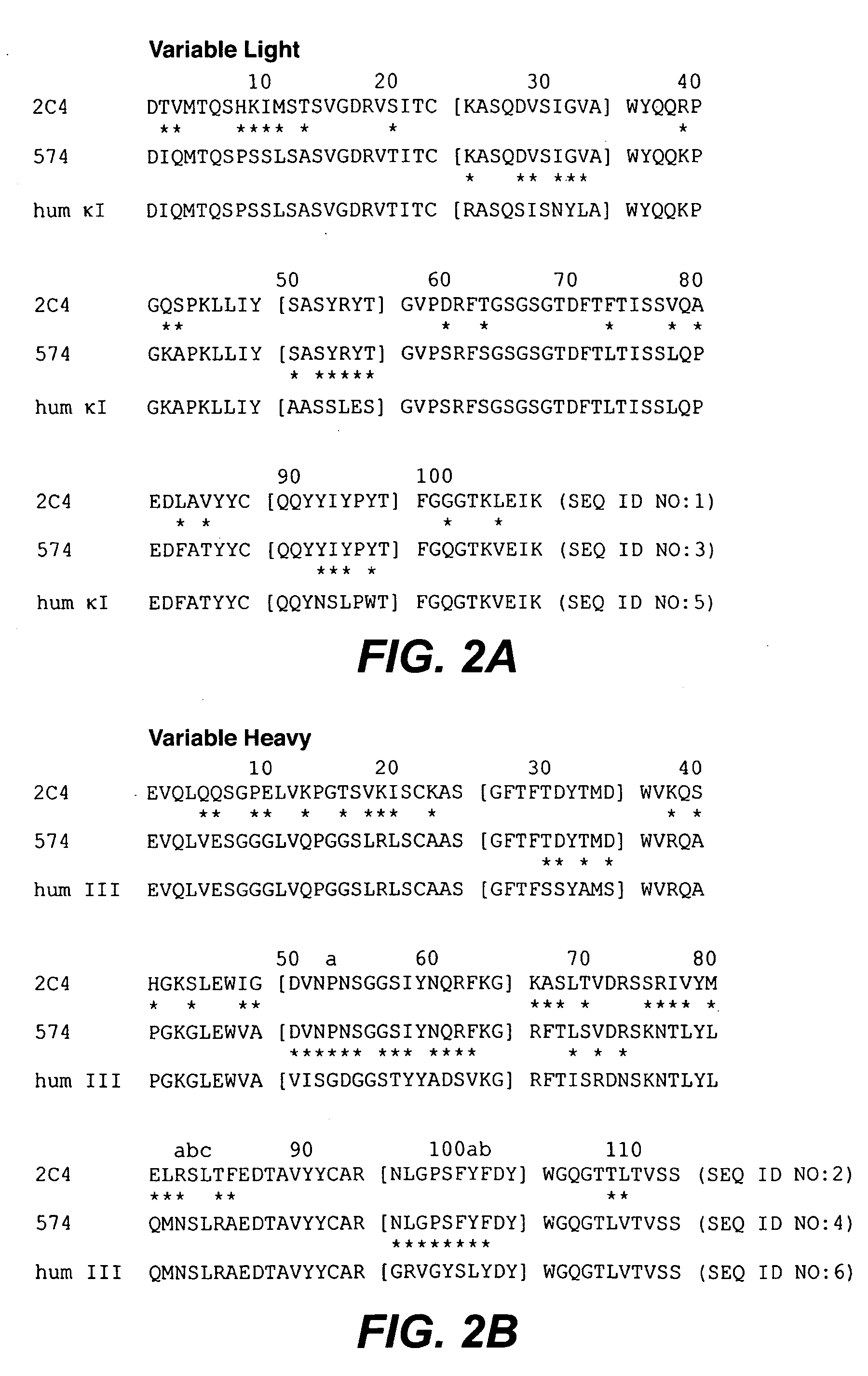
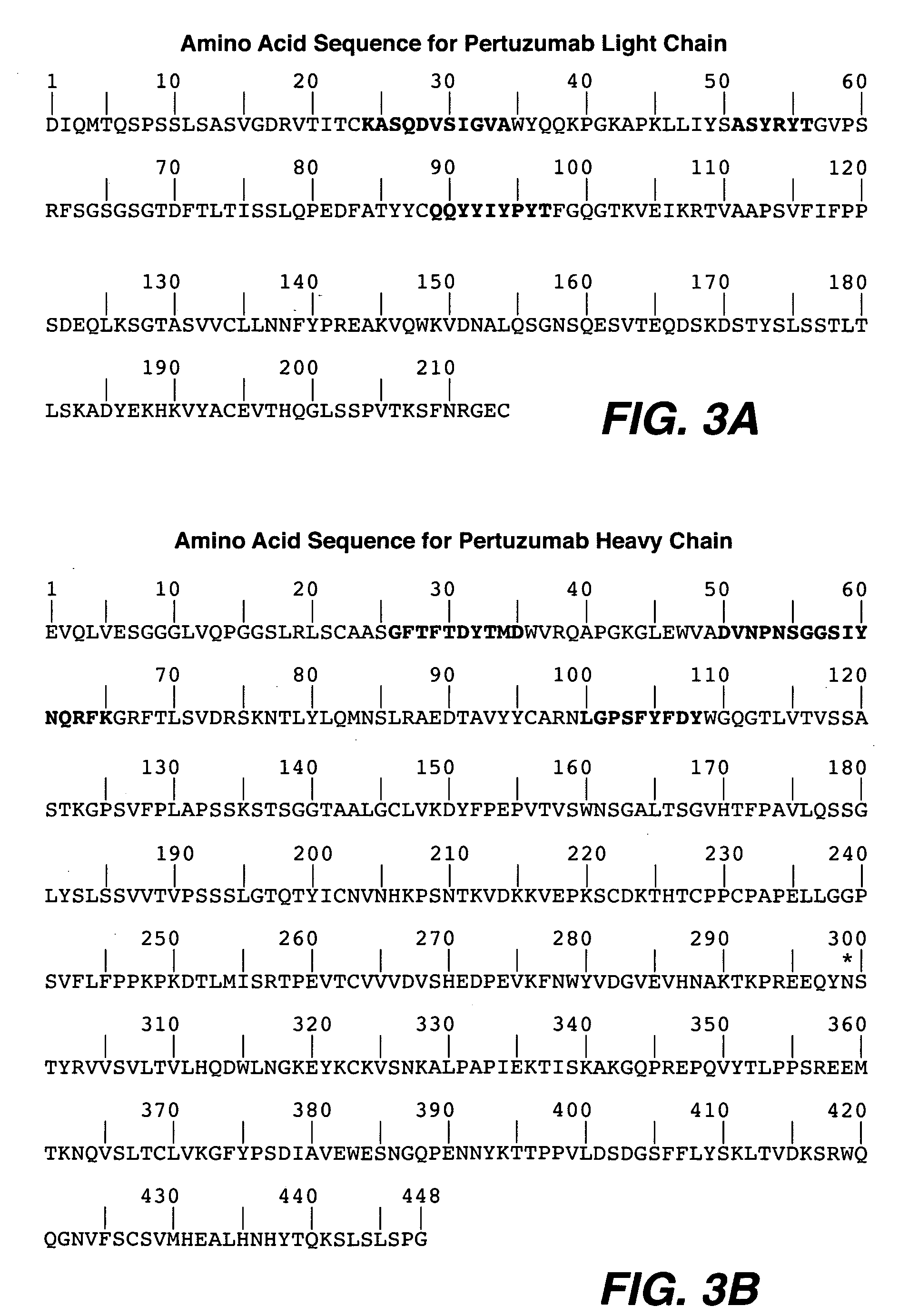
The present application describes antibody formulations, including monoclonal antibodies formulated in histidine-acetate buffer, as well as a formulation comprising an antibody that binds to domain II of HER2 (for example, Pertuzumab), and a formulation comprising an antibody that binds to DR5 (for example, Apomab).
Owner:GENENTECH INC
Anti-TIM-3 antibody
The present invention provides an anti-human TIM-3 antibody having high ADCC activity or antibody fragment thereof by screening a monoclonal antibody or antibody fragment thereof which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure and exhibits ADCC activity; a hybridoma which produces the antibody; a DNA encoding the antibody; a vector comprising the DNA; a transformant which is obtainable by introducing the vector; a method for producing the antibody or the antibody fragment thereof which comprises using the hybridoma or the transformant; a therapeutic agent and a diagnostic agent comprising the antibody or the antibody fragment thereof as an active ingredient.
Owner:KYUSHU UNIV +1
Monoclonal antibodies detection methods for enzymes that confer resistance to 2,4-dichlorophenoxyacetic acid in plants
ActiveUS8460891B2Animal cellsImmunoglobulins against cytokines/lymphokines/interferonsMonoclonal antibodyDioxygenase
Owner:CORTEVA AGRISCIENCE LLC
Monoclonal antibodies and detection methods for phosphinothricin-N-acetyl-transferase enzyme
ActiveUS9371394B2Immunoglobulins against bacteriaBiological material analysisN-acetyltransferaseAcetyltransferase
Described herein are monoclonal antibodies and methods useful for determining and quantitating the presence of a phosphinothricin-N-acetyl-transferase enzyme. The claimed antibodies and methods are particularly useful for identifying and quantitating the presence of phosphinothricin-N-acetyl-transferase expressed in trangenic plants.
Owner:CORTEVA AGRISCIENCE LLC
Antibody for 4-1BB
The present invention includes the receptor protein 4-1BB and the cDNA gene encoding for receptor protein 4-1BB. The nucleotide sequence of the isolated cDNA is disclosed herein along with the deduced amino acid sequence. The 4-1BB protein and fragments and derivatives can be used: 1) as a probe to isolate ligands to receptor protein 4-1BB, 2) to stimulate proliferation of B-cell's expressing 4-1BB, or 3) to block 4-1BB ligand binding. A monoclonal antibody against 4-1BB was developed which specifically recognizes an epitope on the extracellular domain of receptor protein 4-1BB. The monoclonal antibody can be used enhance T-cell proliferation and activation by treating T-cells that have expressed receptor protein 4-1BB with the monoclonal antibody. The effectiveness of the treatment was enhanced when conducted in the presence of protein tyrosinase kinase. A fusion protein for detecting cell membrane ligands to receptor protein 4-1BB was developed. It comprises the extracellular portion of the receptor protein 4-1BB and a detection protein bound to the portion of the receptor protein 4-1BB.
Owner:INDIANA UNIV RES & TECH CORP
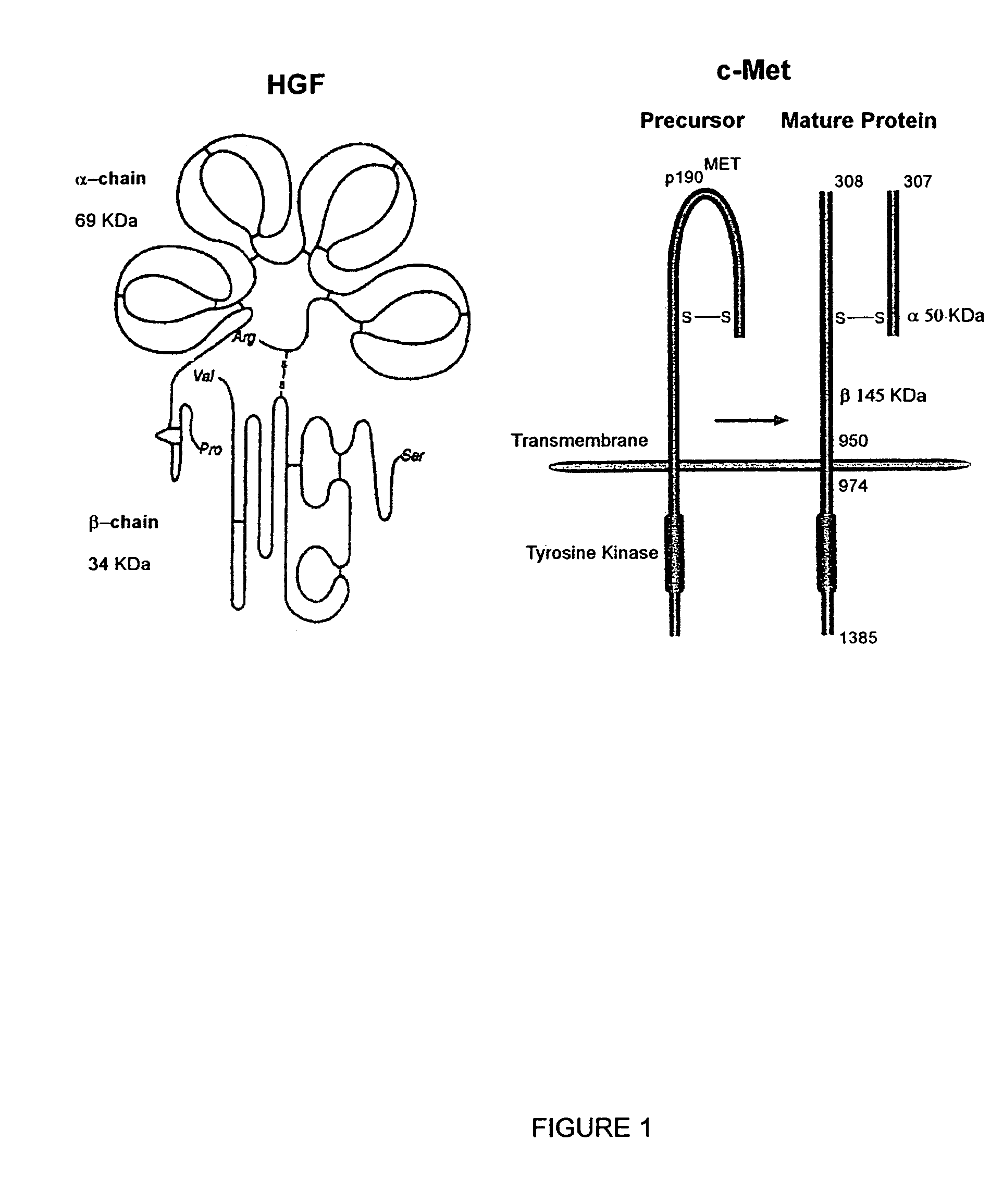
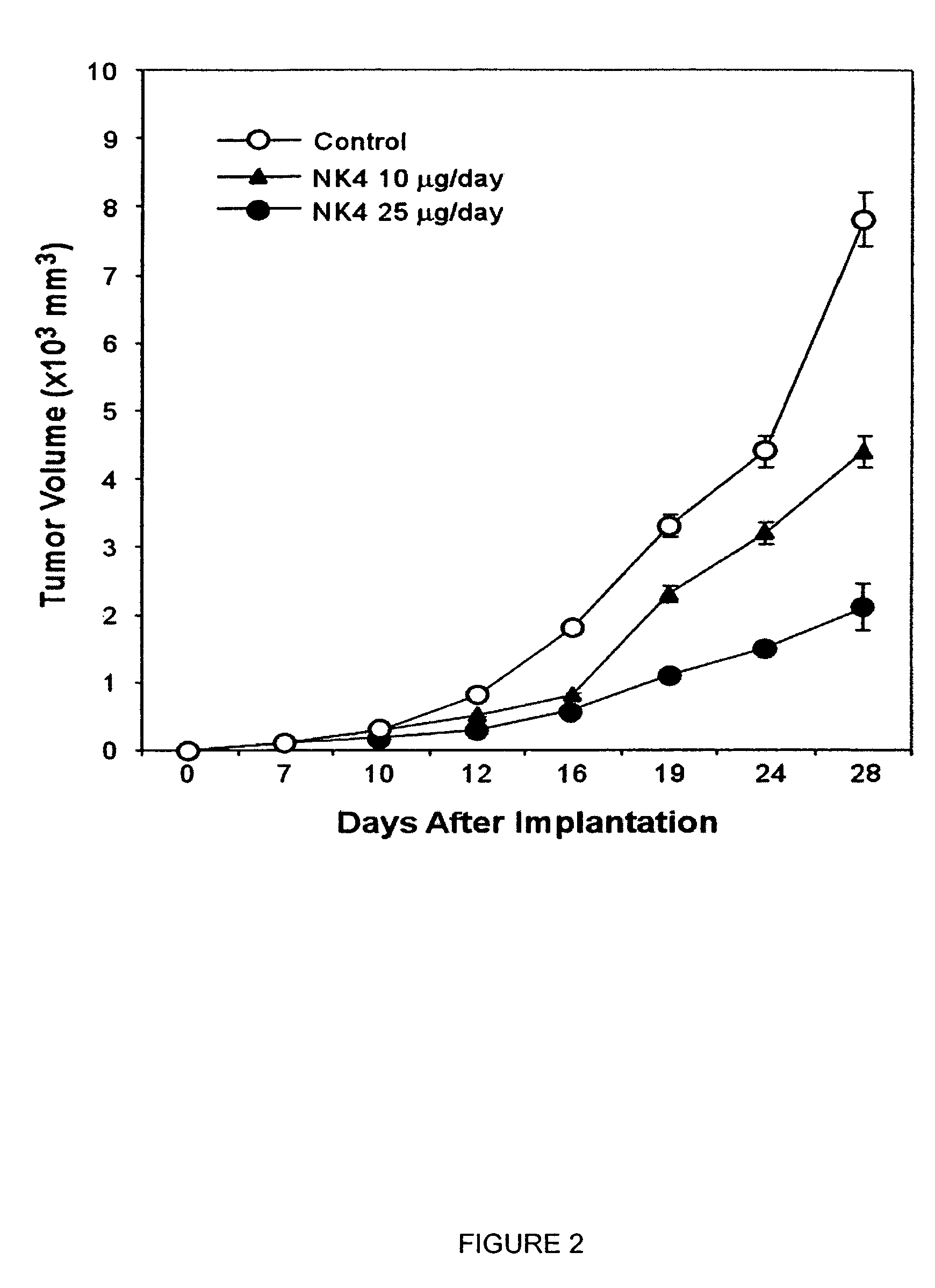
Monoclonal antibodies to hepatocyte growth factor
InactiveUS7220410B2Animal cellsBiological material analysisMonoclonal antibodyHepatocyte growth factor
The present invention is directed toward a neutralizing monoclonal antibody to hepatocyte growth factor, a pharmaceutical composition comprising same, and methods of treatment comprising administering such a pharmaceutical composition to a patient.
Owner:GALAXY BIOTECH LLC
Murine expression of a human IgA lambda locus
InactiveUS6998514B2High expressionTissue cultureImmunoglobulinsImmunoglobulin light chainMonoclonal antibody
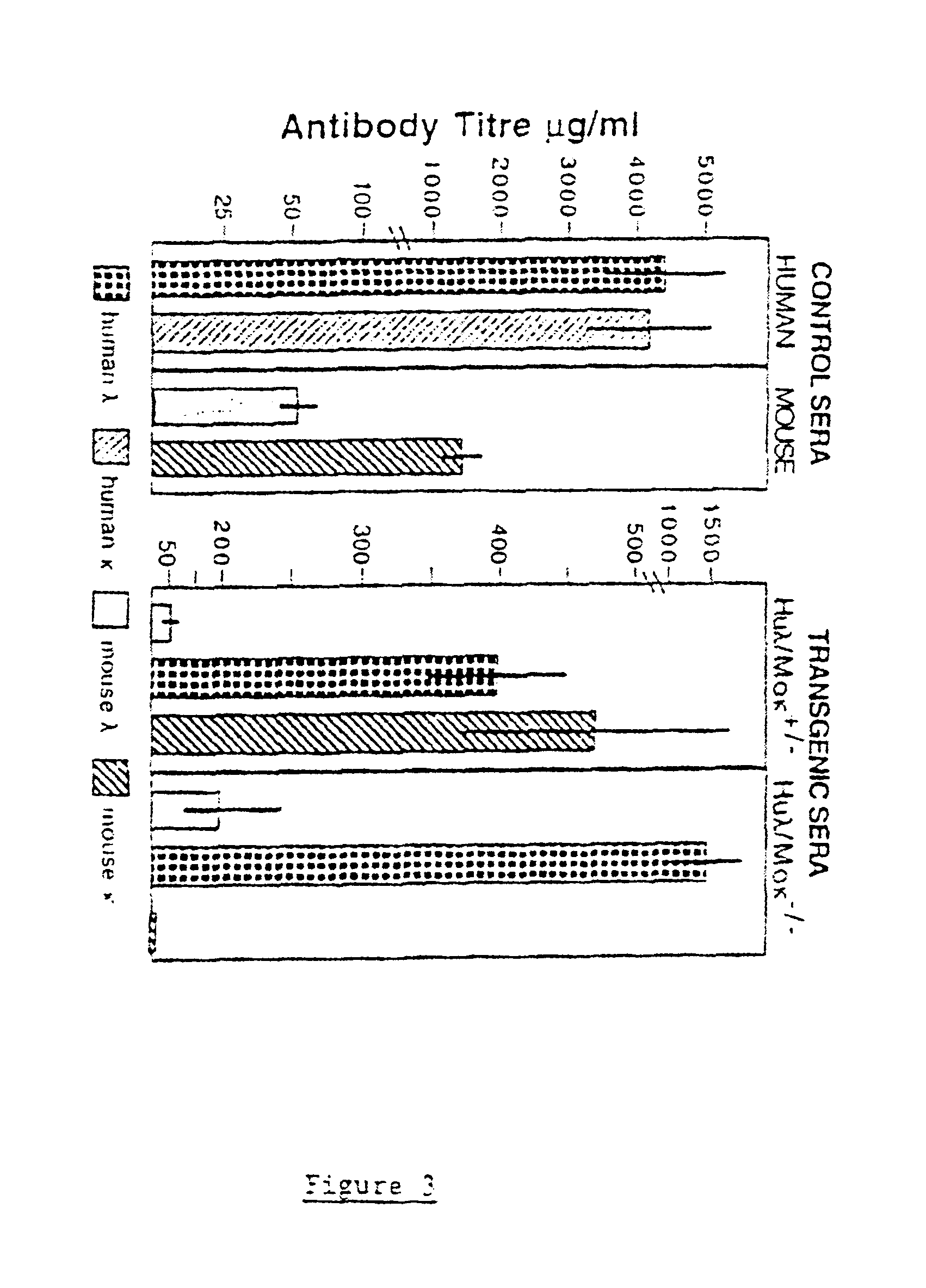
In humans, approximately 60% of expressed immunoglobulin light chains are of the Kappa type and 40% of the Lambda type. In mice, there is almost no expression from the Lambda locus and over 95% of light chains are of Kappa type. The present invention discloses, among other things, transgenic mice carrying most of the human Ig Lambda light chain locus in their genome. The resulting mice express light chains with Kappa / Lambda ratio similar to the human ratio. Breeding of HuIg Lamda mice to Kappa-deficient mice also is described, as well as the generation of human monoclonal antibodies from transgenic mice with human Ig Lambda locus.
Owner:BABRAHAM INST
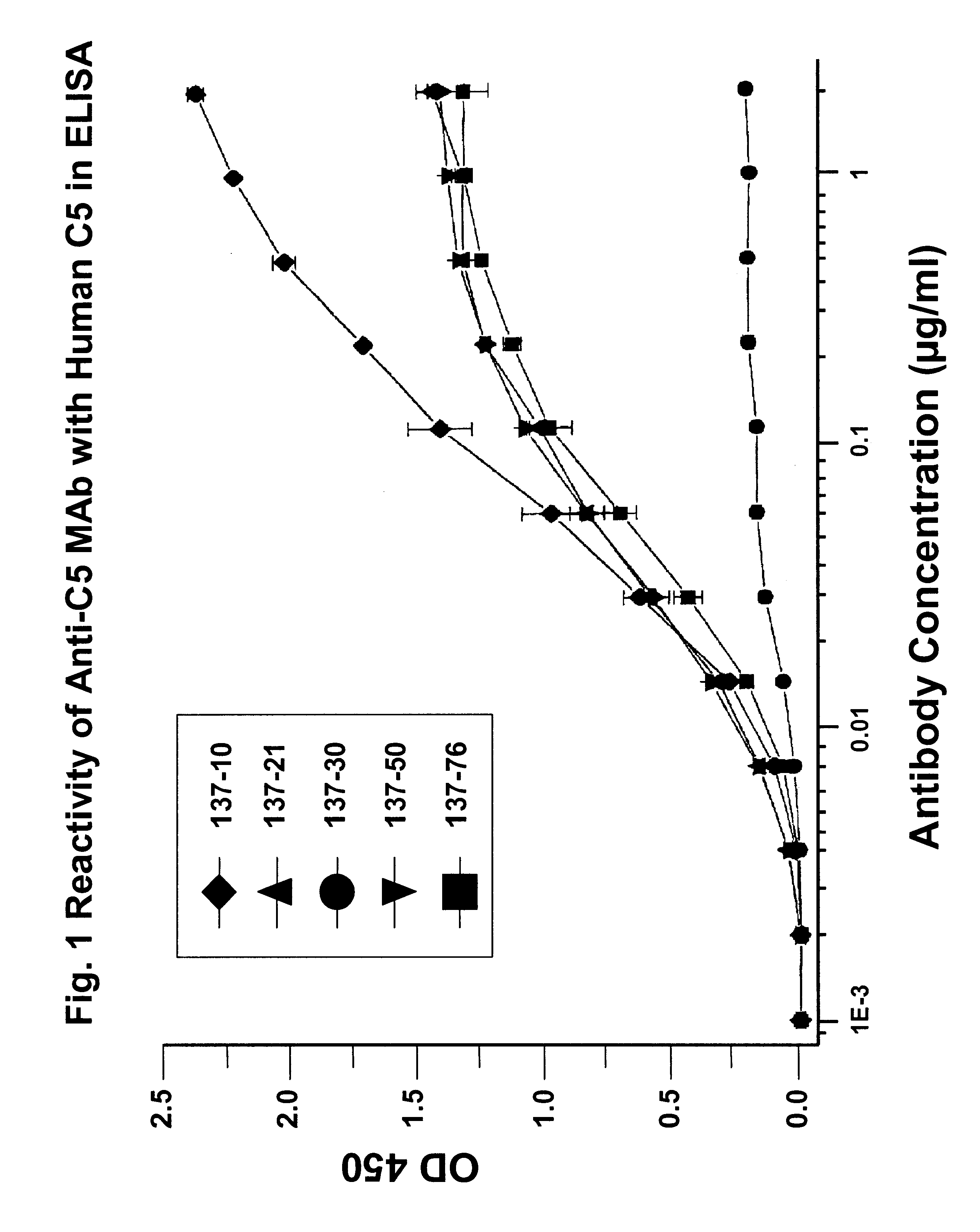
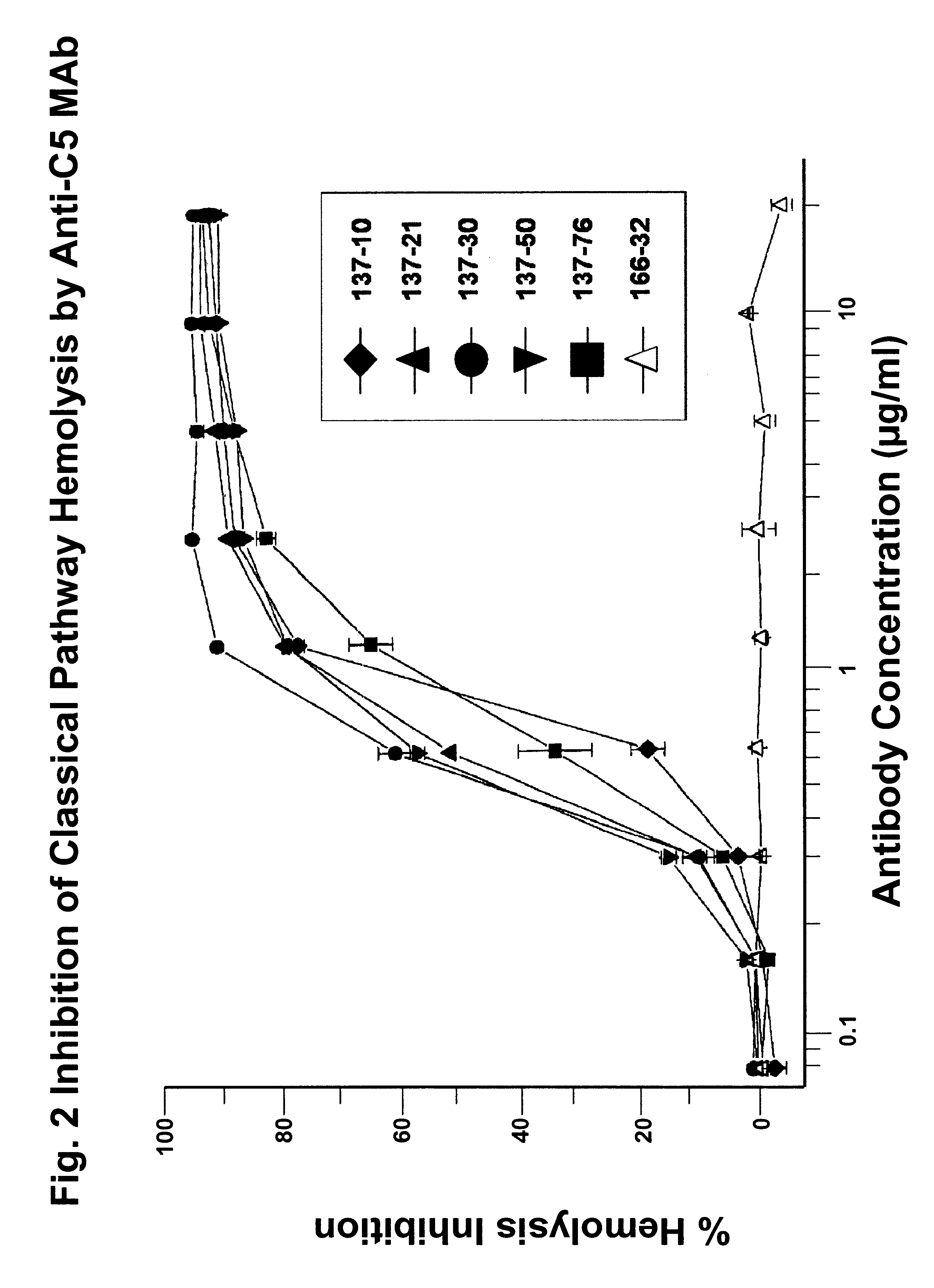
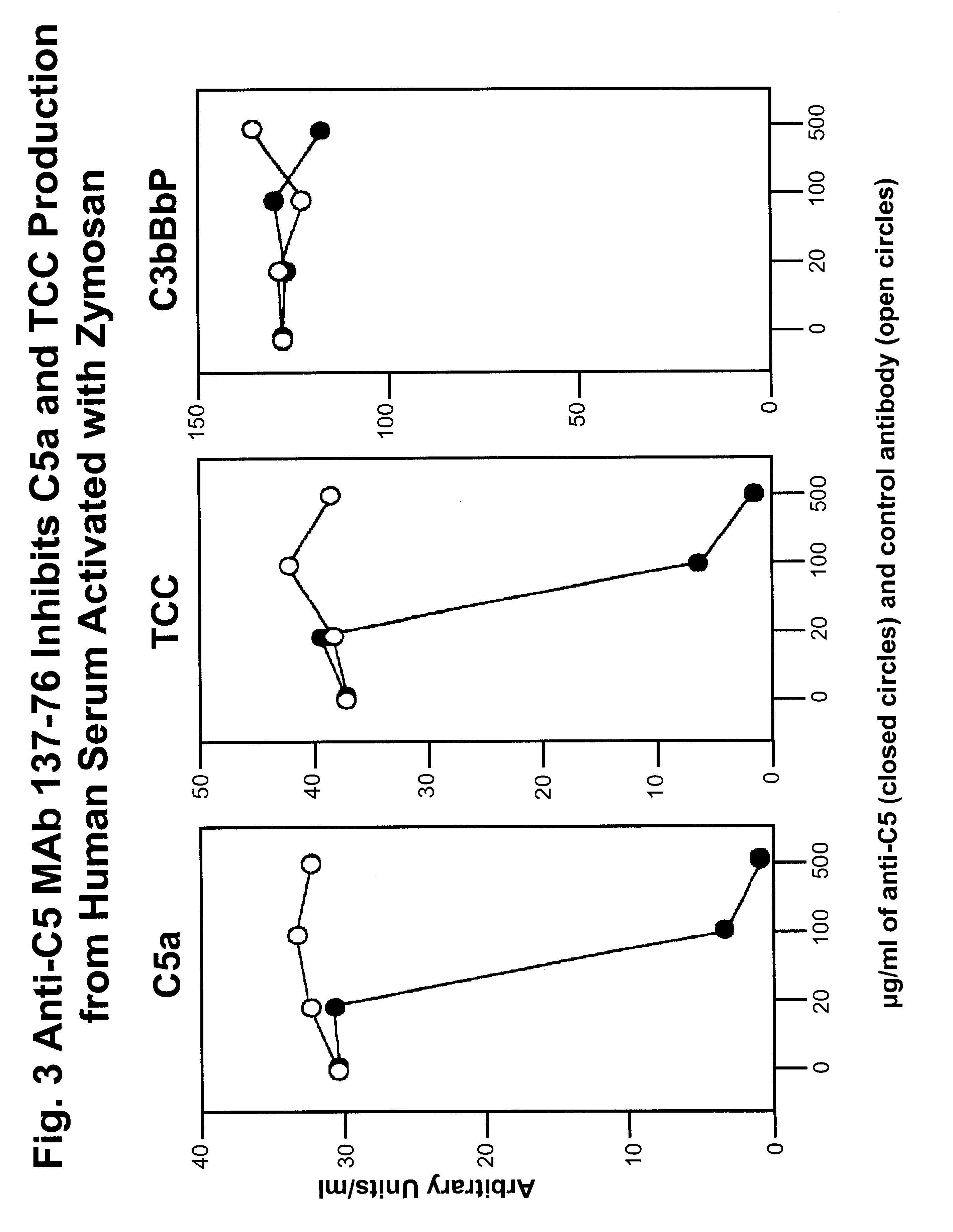
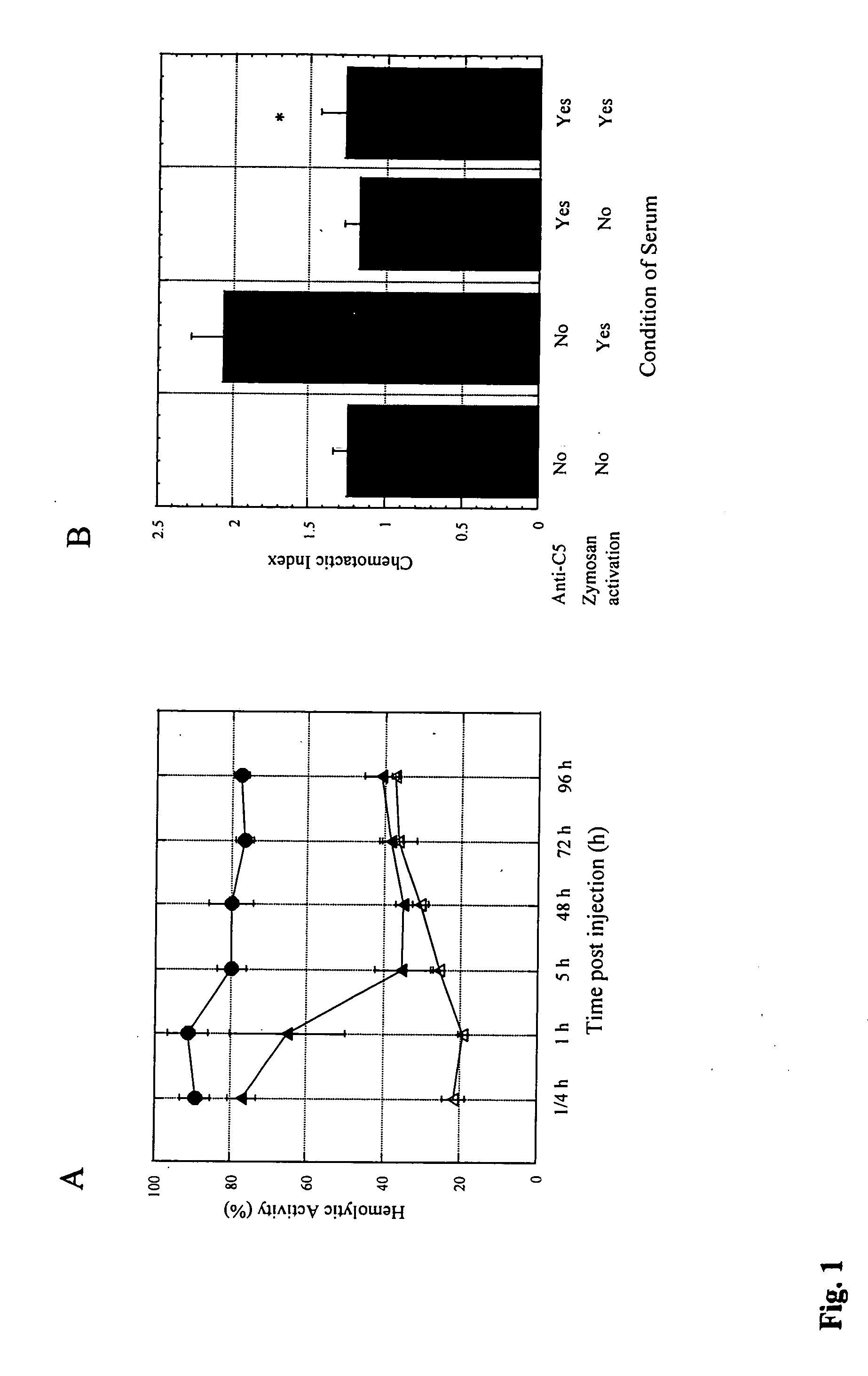
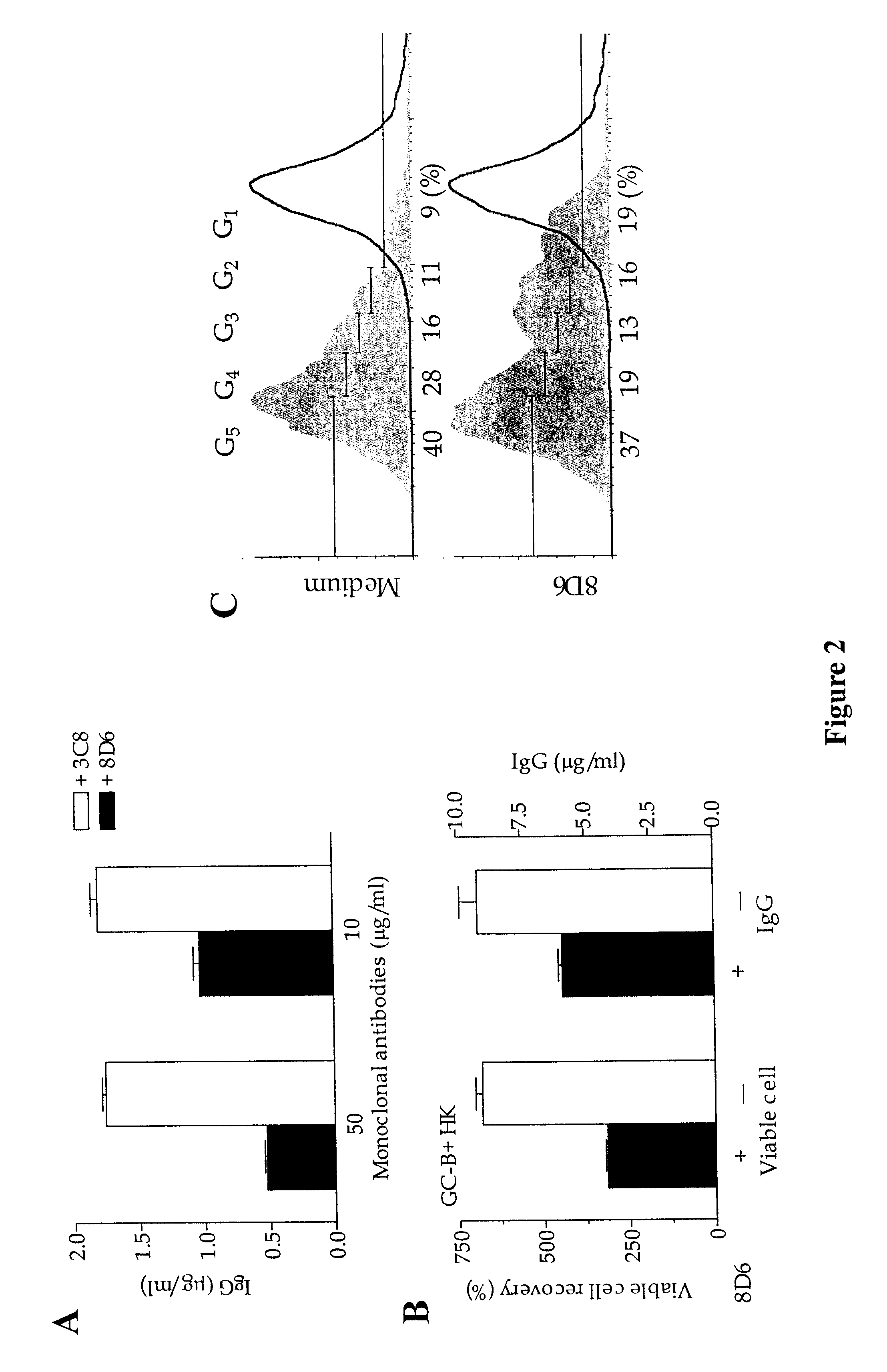
Anti-C5 monoclonal antibodies
InactiveUS6534058B2High affinityMaximizing characteristicImmunoglobulins against blood coagulation factorsAnimal cellsAcute vascular rejectionOligonucleotide
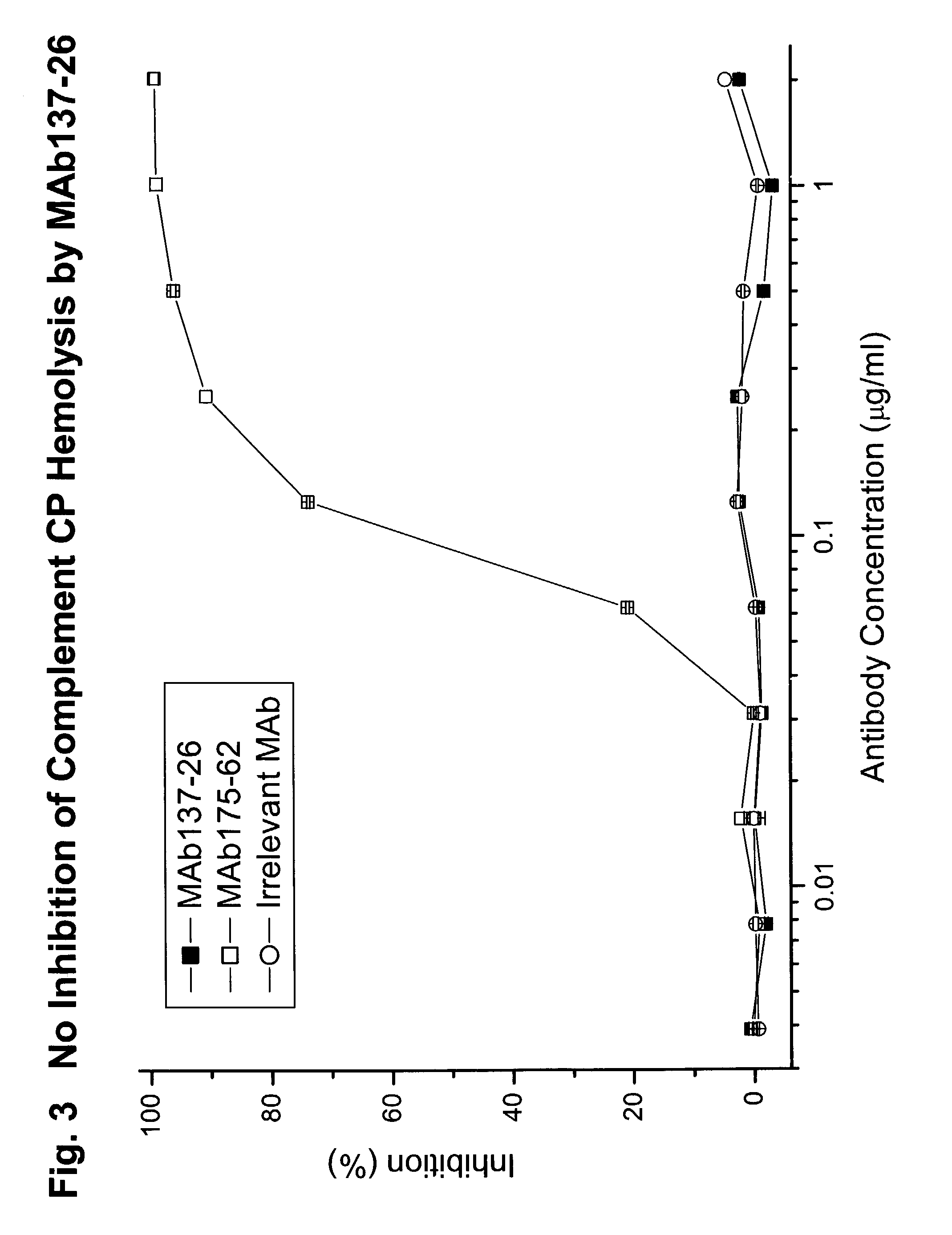
The invention relates to C5 inhibitors, which inhibit type II endothelial cell activation, wherein the inhibition is manifested by the suppression of E-selectin. These inhibitors are useful in treatment of delayed xenograft rejection or acute vascular rejection. The inhibitors include antibody molecules, as well as homologues, analogues and modified or derived forms thereof, including immunoglobulin fragments like Fab, F(ab')2 and Fv, small molecules, including peptides, oligonucleotides, peptidomimetics and organic compounds. Examples of monoclonal antibodies, which bind to and inhibit C5, were generated and are designated MAb 137-76 and MAb 137-30.
Owner:GENENTECH INC
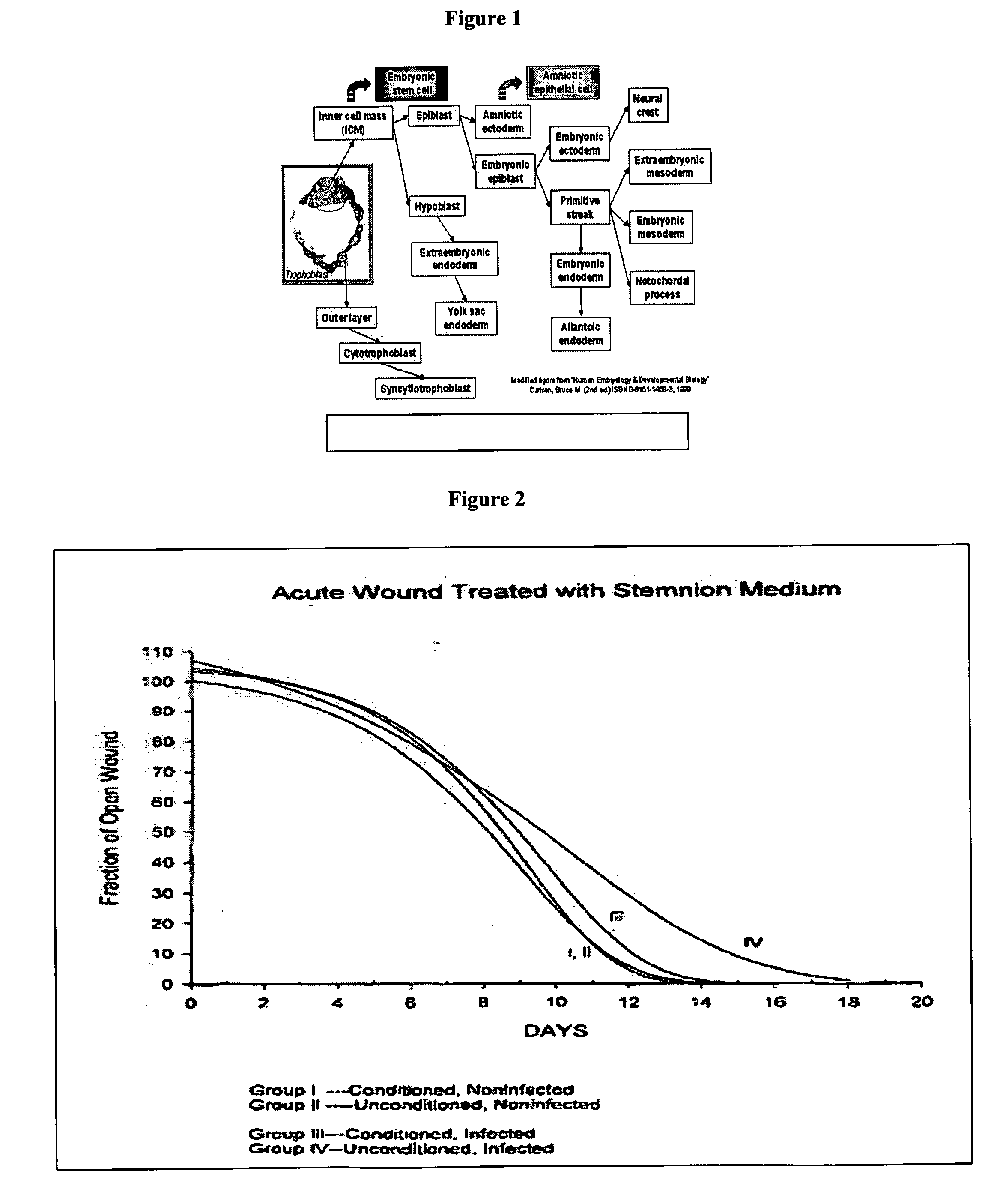
Amnion-derived cell compositions, methods of making and uses thereof
The invention is directed to substantially purified amnion-derived cell populations, compositions comprising the substantially purified amnion-derived cell populations, and to methods of creating such substantially purified amnion-derived cell populations, as well as methods of use. The invention is further directed to antibodies, in particular, monoclonal antibodies, that bind to amnion-derived cells or, alternatively, to one or more amnion-derived cell surface protein markers. The invention is further directed to methods for producing the antibodies, methods for using the antibodies, and kits comprising the antibodies.
Owner:STEMNION
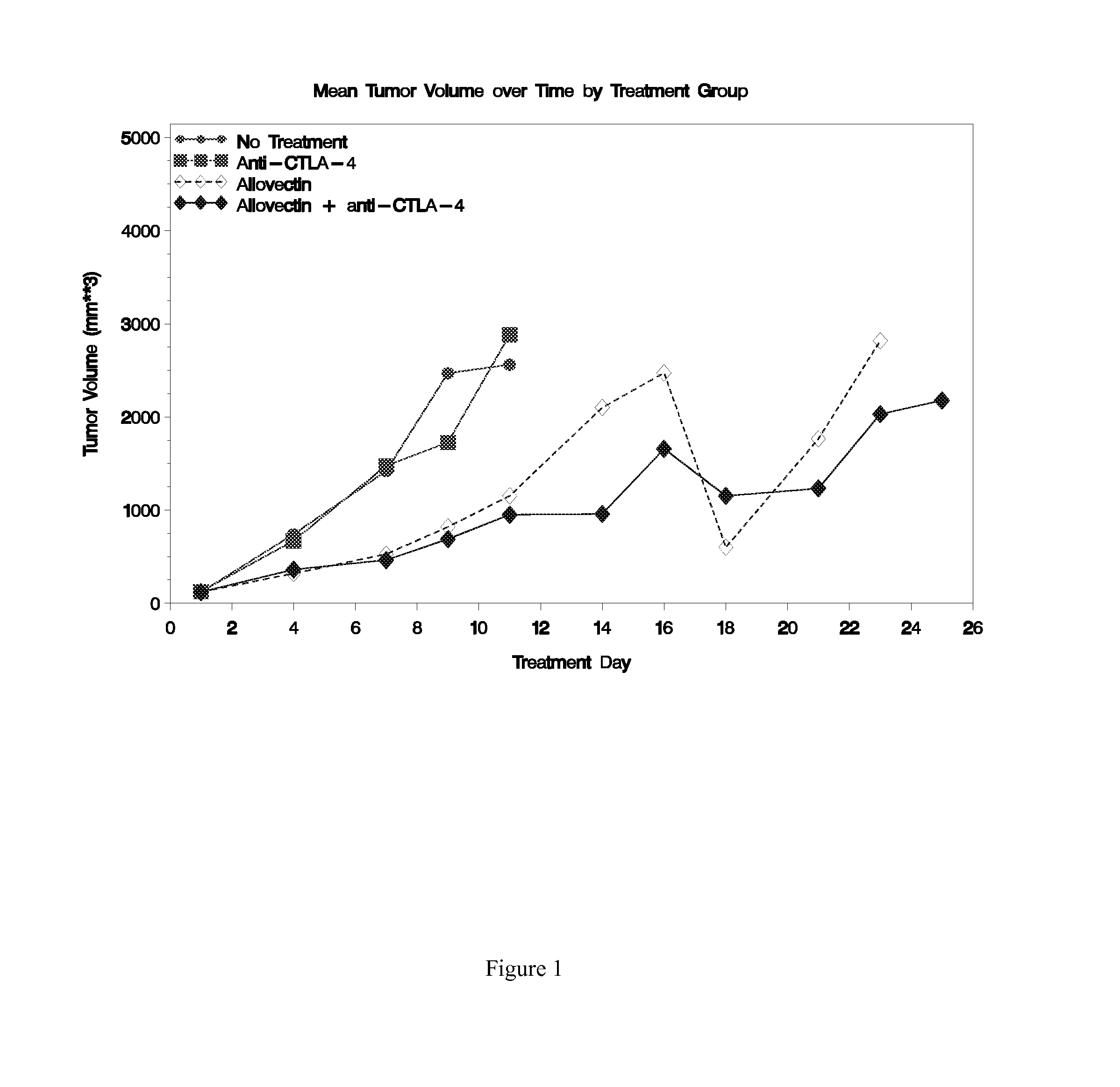
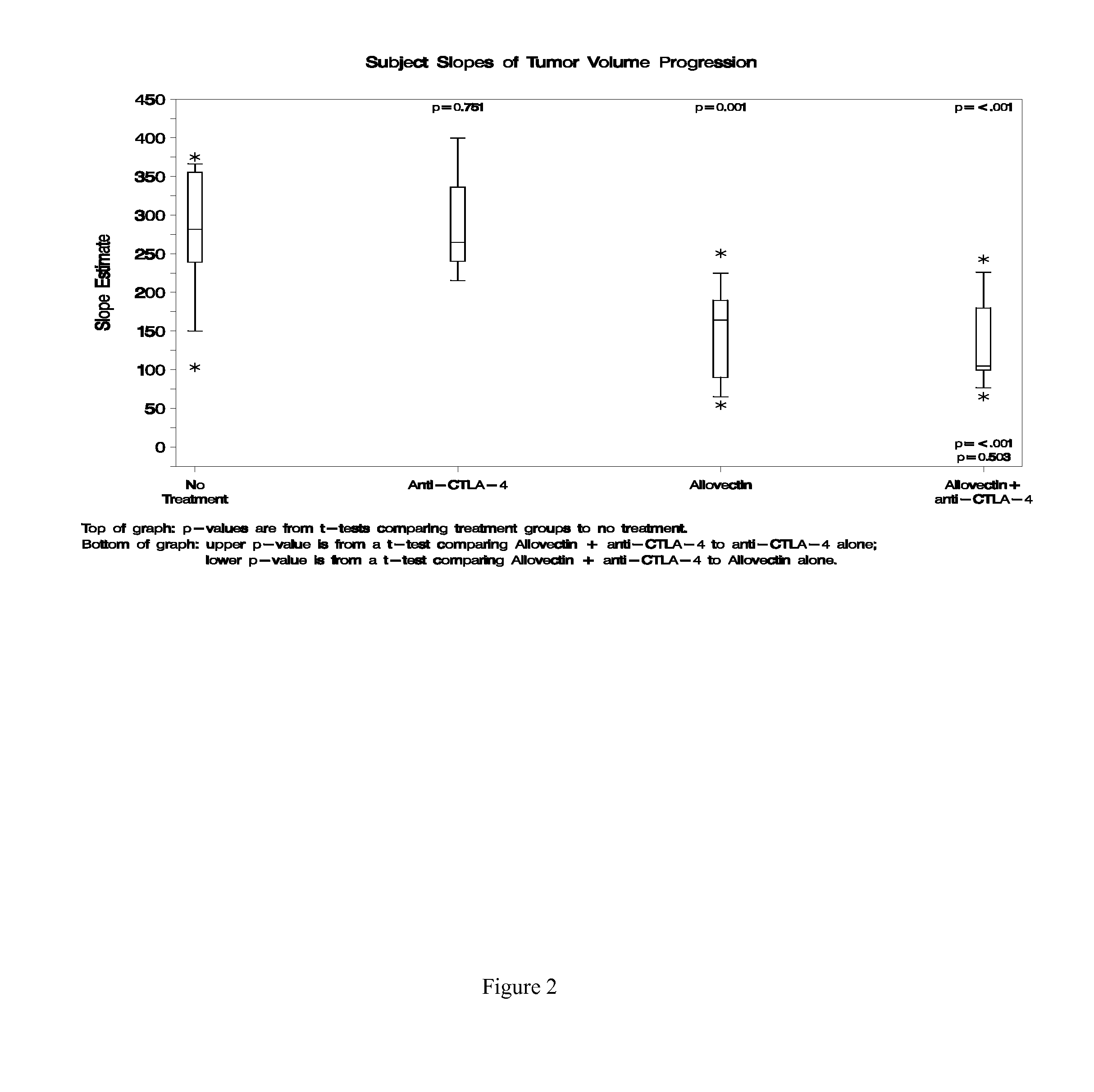
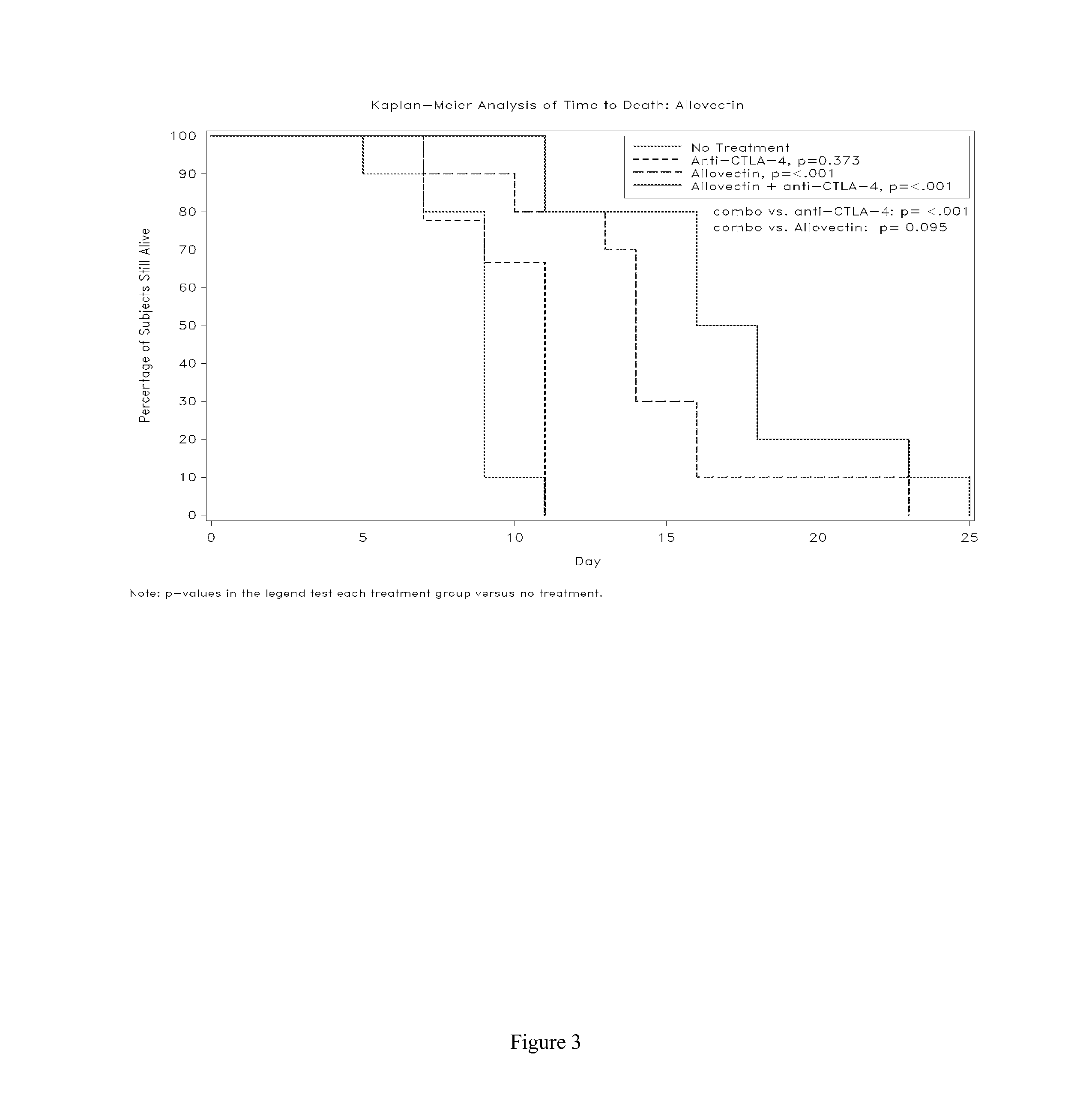
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130071403A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentIrritation
The present disclosure provides combinations of immunotherapeutics and methods for treating medical conditions that are characterized by the lack of an effective immune response, for example as would result following a down-regulation of MHC class I, such as in cancer. The immunotherapeutic compositions of the invention, which can be used to treat the medical conditions, include one or more immunostimulatory antibodies or molecules having specificity for CTLA-4, PD-1, PD-L1, PD-L2, CD40, OX40, CD137, GITR, ILT2, or ILT3, or ligands for these molecules (e.g., an isolated fully-human monoclonal antibody) in association with one or more alloantigens, such as, vector(s) capable of expressing protein(s) or peptide(s) that stimulate T-cell immunity against tissues or cells, formulated in a pharmaceutically acceptable carrier. The proteins or peptides may comprise class I major histocompatibility complex (MHC) antigens, β2-microglobulins, or cytokines. The MHC antigen may be foreign to the subject. The MHC antigen may be HLA-B7.
Owner:VICAL INC
Method for preparing monoclonal antibody
ActiveUS20060059575A1Improve productivityImprove expression efficiencyNucleic acid vectorImmunoglobulinsImmunoglobulin heavy chainMonoclonal antibody
A significantly increased amount of a monoclonal antibody is obtained from the culture medium of recombinant hybridoma prepared by introducing genes encoding a protein identical to the immunoglobulin heavy chain polypeptide of the specific monoclonal antibody into an immortalized B cell (hybridoma) producing the monoclonal antibody.
Owner:JAPAN TOBACCO INC +1
Antibody formulations
The present application describes antibody formulations, including monoclonal antibodies formulated in histidine-acetate buffer, as well as a formulation comprising an antibody that binds to domain II of HER2 (for example, Pertuzumab), and a formulation comprising an antibody that binds to DR5 (for example, Apomab).
Owner:GENENTECH INC
Polyclonal antibody composition for treating allergy
InactiveUS6849259B2Efficient removalPotential clinical advantageImmunoglobulins against animals/humansImmunoglobulins against plantsMicrosphereBULK ACTIVE INGREDIENT
A pharmaceutical composition for treating allergy is described. The composition comprises as an active ingredient a recombinant polyclonal antibody or a mixture of different monoclonal antibodies capable of reacting with or binding to an allergen together with one or more pharmaceutically acceptable excipients. The composition may be used topically as a solution, dispersion, powder, or in the form of microspheres. The polyclonal antibody is preferably a recombinant polyclonal antibody produced by phage display technology. The pairing of specific immunoglobulin variable region light chain and heavy chain maintained from the original polyclonal immune response or selected by panning using the allergen in question is preferably maintained by bulk transfer of the pairs into an expression vector.
Owner:SYMPHOGEN AS
Tumor-associated marker
This invention provides monoclonal antibody-producing hybridomas designated 27.F7 and 27.B1. The invention also provides methods for detecting TIP-2 antigen-bearing cancer cells in a sample, detecting the presence of TIP-2 antigen, optionally on the surface of cancer cells, immunohistochemical screening of a tissue section for the presence of TIP-2 antigen bearing cancer cells, diagnosing cancer in a subject, monitoring progression of cancer wherein the cancer cells are TIP-2 antigen-bearing cells, delivering exogenous material to TIP-2 antigen-bearing cancer cells of a human subject, and treating cancer in a human subject. This invention further provides a kit for detecting the presence of TIP-2 antigen-bearing cancer cells. This invention also provides isolated peptides having the amino acid sequences Lys Leu Leu Gly Gly Gln Ile Gly Leu (SEQ ID No:3) and Ser Leu Leu Gly Cys Arg His Tyr Glu Val (SEQ ID NO:4).
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Monoclonal antibodies with enhanced ADCC function
InactiveUS20050249722A1Preventing alloimmunizationAntibacterial agentsImmunoglobulins against blood group antigensMonoclonal antibodyAnti-d antibody
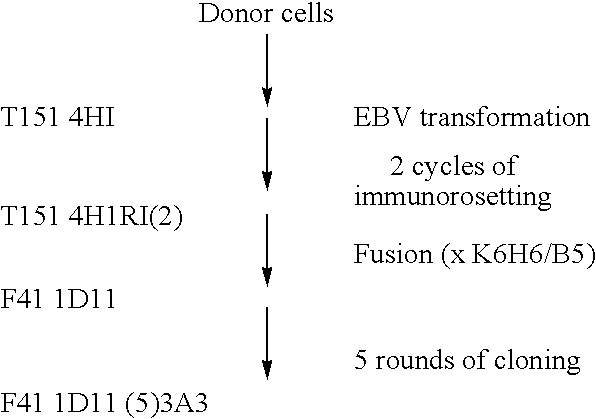
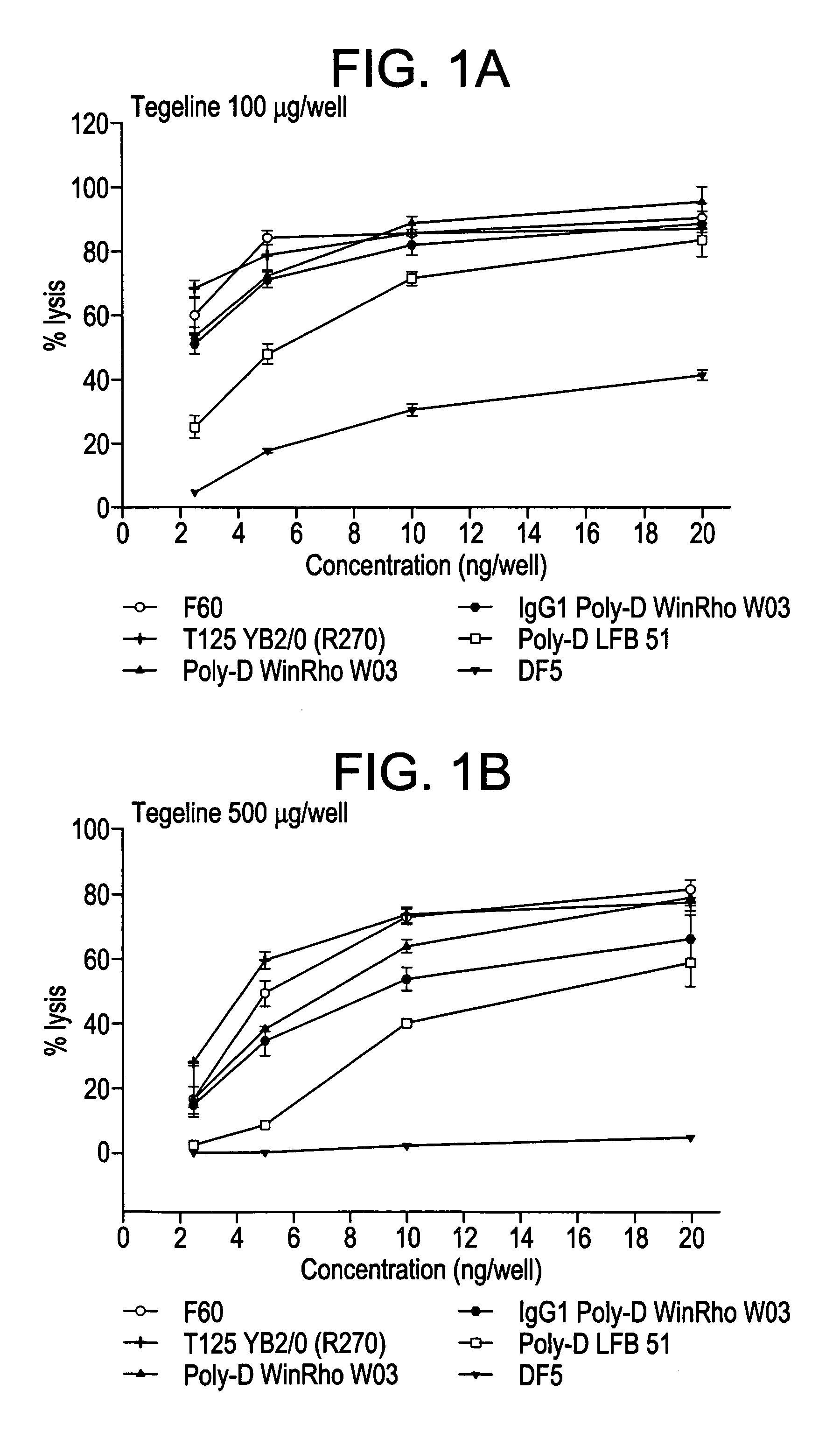
The invention concerns a method for obtaining and selecting monoclonal antibodies by an ADDC-type test, said antibodies capable of activating type III Fcy receptors and having a particular glycan structure. The inventive anti-D antibodies can be used for preventing Rhesus isoimmunisation in Rh negative persons, in particular for haemolytic disease in a new-born baby of for uses such as idiopathic thrombocytopenic pupura 9ITP)
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Anti-tim-3 antibody
ActiveUS20140044728A1High activityPeptide/protein ingredientsAntipyreticExtracellularDiagnostic agent
Disclosed are an anti-human TIM-3 antibody having high ADCC activity or antibody fragment thereof by screening a monoclonal antibody or antibody fragment thereof which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure and exhibits ADCC activity; a hybridoma which produces the antibody; a DNA encoding the antibody; a vector comprising the DNA; a transformant which is obtainable by introducing the vector; a method for producing the antibody or the antibody fragment thereof which comprises using the hybridoma or the transformant; and a therapeutic agent and a diagnostic agent comprising the antibody or the antibody fragment thereof as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
Antibodies and methods for generating genetically altered antibodies with enhanced effector function
InactiveUS20050054048A1Increase variabilityBetter pharmacokinetic profileAnimal cellsSugar derivativesGenetic diversityMonoclonal antibody
Dominant negative alleles of human mismatch repair genes can be used to generate hypermutable cells and organisms. By introducing these genes into cells and transgenic animals, new cell lines and animal varieties with novel and useful properties can be prepared more efficiently than by relying on the natural rate of mutation. These methods are useful for generating genetic diversity within immunoglobulin genes directed against an antigen of interest to produce altered antibodies with enhanced biochemical activity. Moreover, these methods are useful for generating antibody-producing cells with increased level of antibody production. The invention also provides methods for increasing the effector function of monoclonal antibodies and monoclonal antibodies with increased effector function.
Owner:EISAI INC
Anthracycline derivative conjugates, process for their preparation and their use as antitumor compounds
The present invention relates to conjugates of therapeutically useful anthracyclines with carriers such as polyclonal and monoclonal antibodies, proteins or peptides of natural or synthetic origin; methods for their preparation, pharmaceutical composition containing them and use thereof in treating certain mammalian tumors.
Owner:GENENTECH INC +1
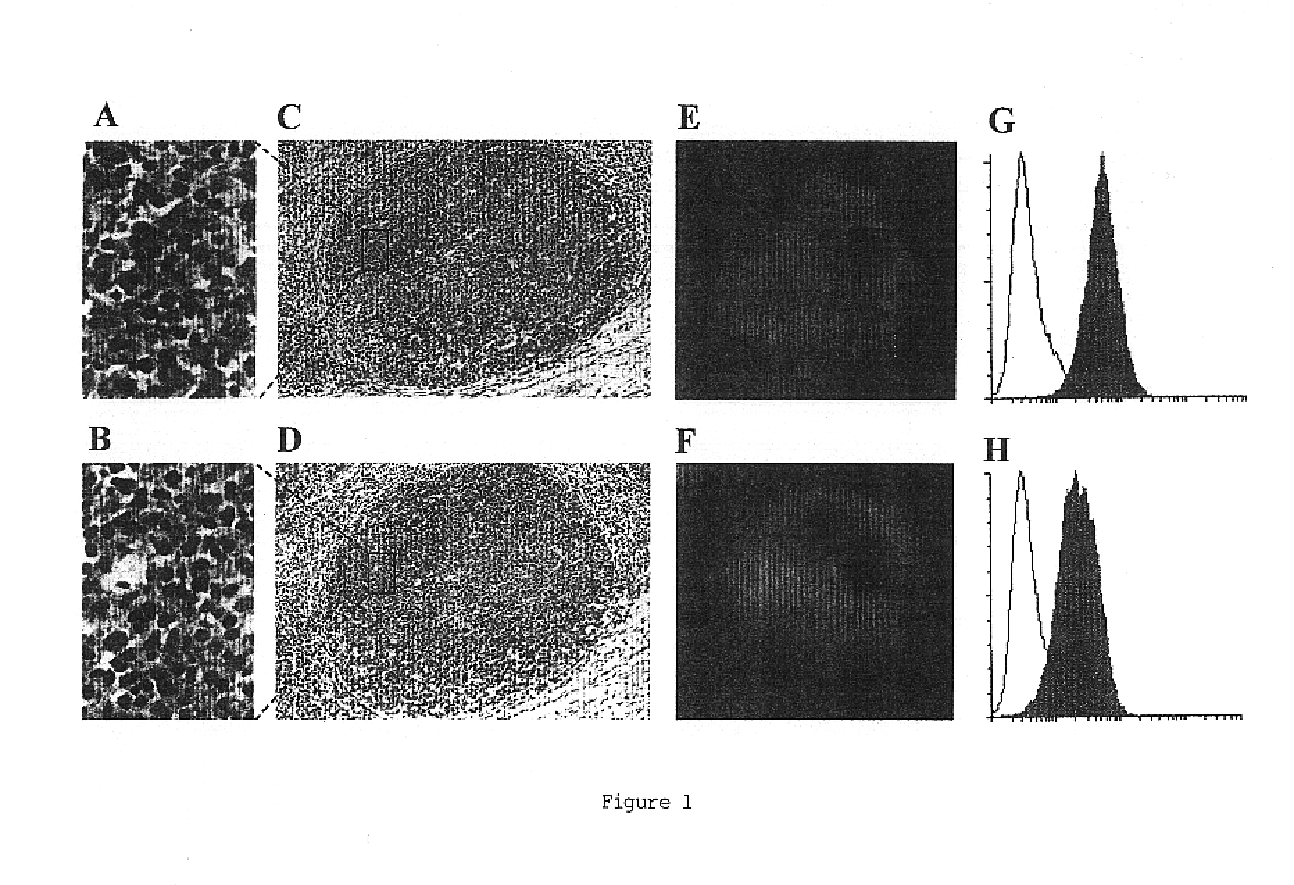
Fusion partner for production of monoclonal rabbit antibodies
The invention provides a rabbit-derived immortal B-lymphocyte capable of fusion with a rabbit splenocyte to produce a hybrid cell that produces an antibody. The immortal B-lymphocyte does not detectably express endogenous immunoglobulin heavy chain and may contain, in certain embodiments, an altered immunoglobulin heavy chain-encoding gene. A hybridoma resulting from fusion between the subject immortal B-lymphocyte and a rabbit antibody-producing cell is provided, as is a method of using that hybridoma to produce an antibody. The subject invention finds use in a variety of different diagnostic, therapeutic and research applications.
Owner:EPITOMICS INC
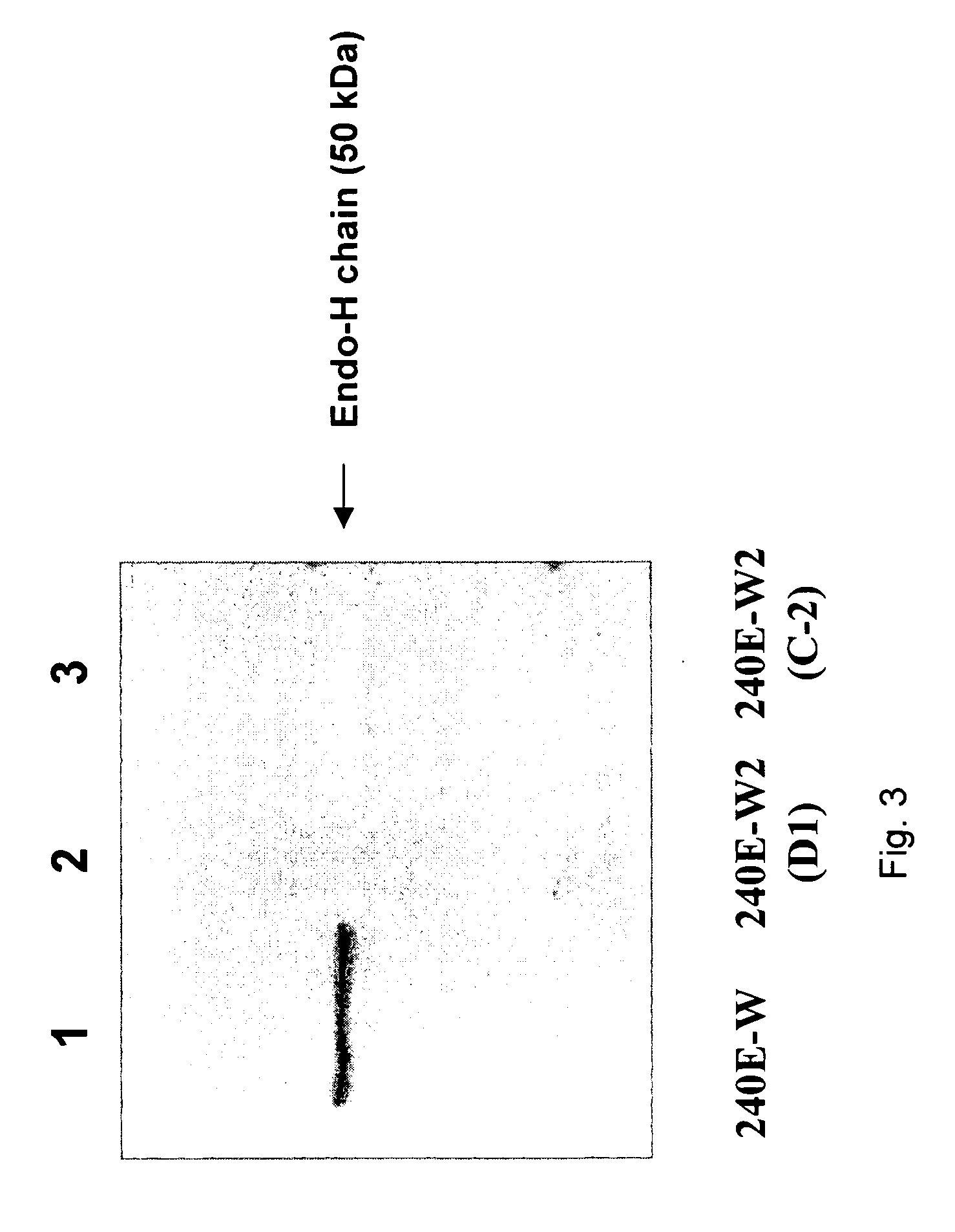
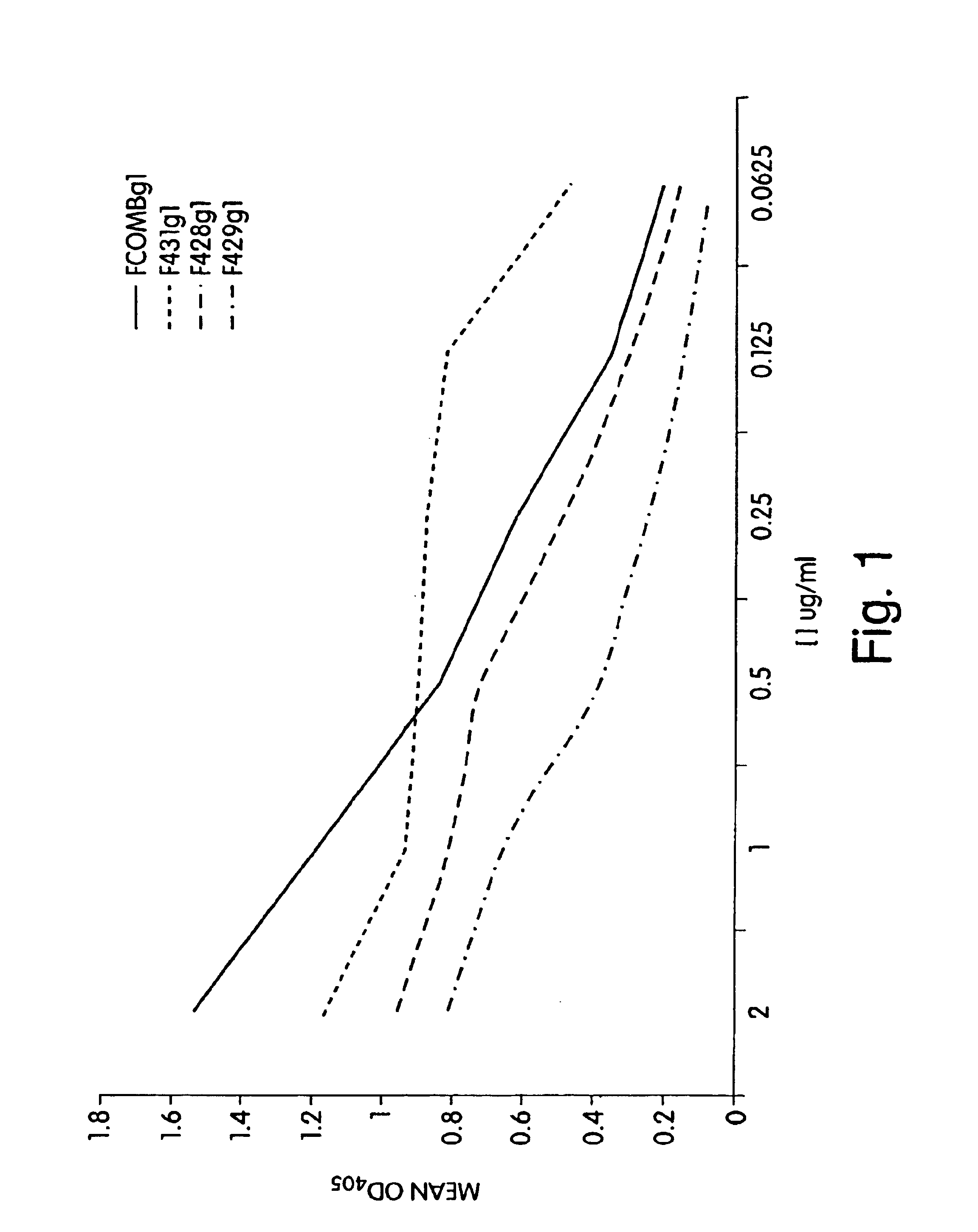
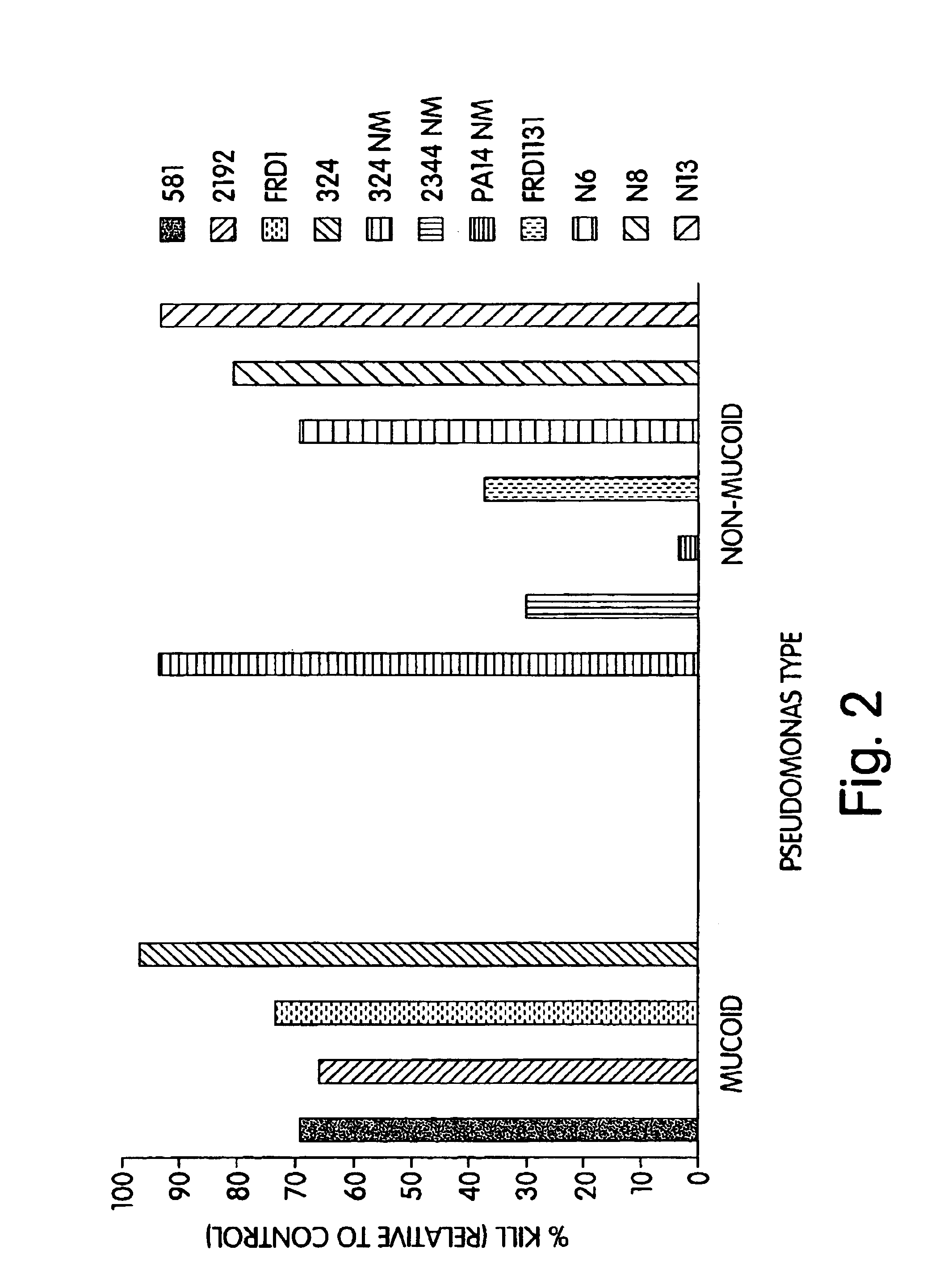
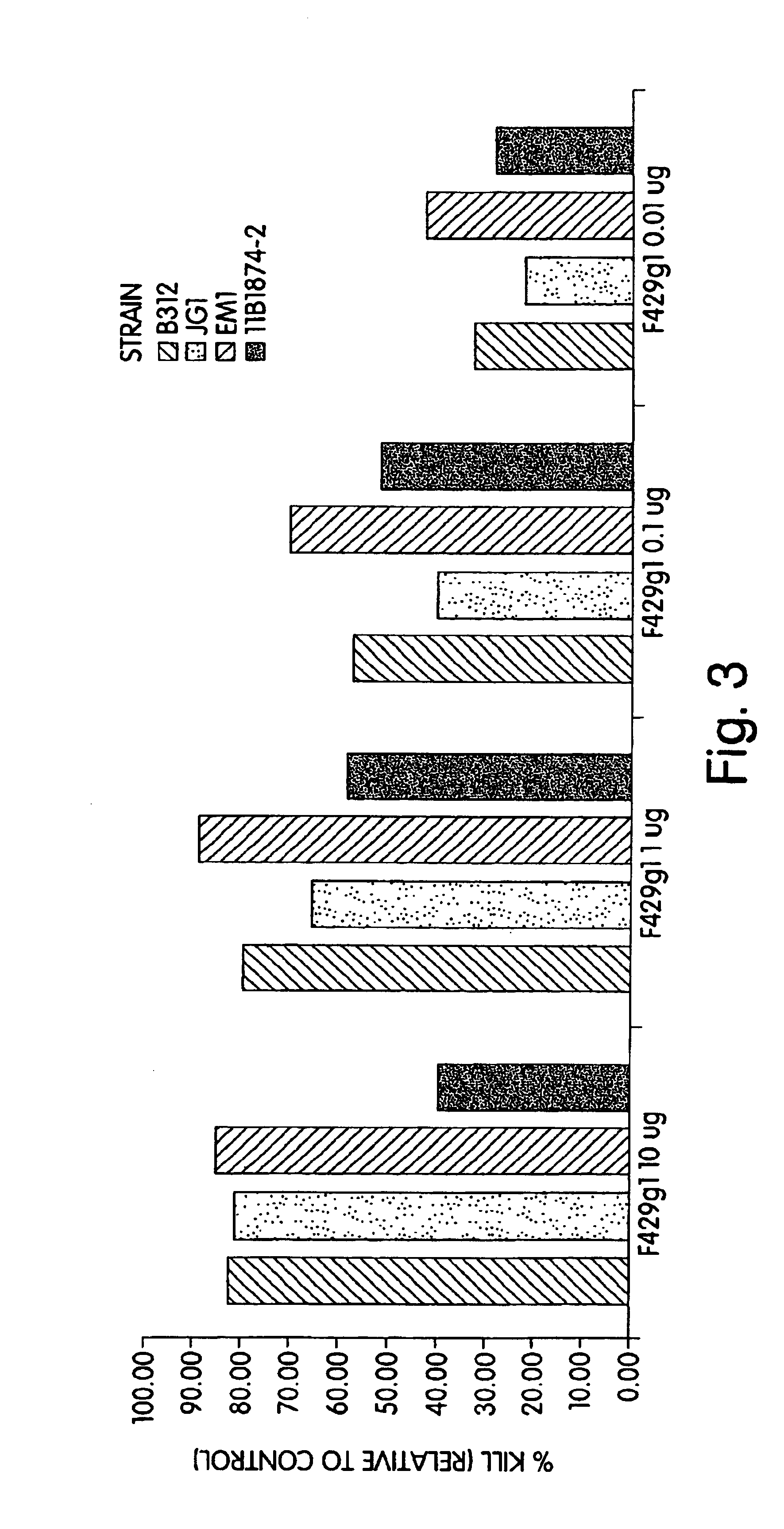
P. aeruginosa mucoid exopolysaccharide specific binding peptides
InactiveUS6962813B2Enhance opsonization and phagocytosisEnhance cytocidal effectAnimal cellsImmunoglobulins against bacteriaDiseaseMonoclonal antibody
The present invention relates to peptides, particularly human monoclonal antibodies, that bind specifically to P. aeruginosa mucoid exopolysaccharide. The invention further provides methods for using these peptides in the diagnosis, prophylaxis and therapy of P. aeruginosa infection and related disorders (e.g., cystic fibrosis). Some antibodies of the invention enhance opsonophagocytic killing of multiple mucoid strains of P. aeruginosa. Compositions of these peptides, including pharmaceutical compositions, are also provided, as are functionally equivalent variants of such peptides.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Treatment of immunological disorders using anti-dc30 antibodies
InactiveUS20050123536A1Enhancing cytotoxicEnhancing cytostatic effectOrganic active ingredientsSenses disorderDiseaseAntibody conjugate
The present invention relates to methods for the treatment of immunological disorders other than cancer, comprising administering proteins characterized by their ability to bind to CD30 and exert a cytostatic or cytotoxic effect on an activated lymphocyte. Such proteins include monoclonal antibodies AC10 and IleFi1. AC10 and HeFi-1 derivatives, and antibodies that compete with AC10 and HeFi-1 for binding to CD30. Other such proteins include multivalent anti-CD30 antibodies and anti-CD30 antibodies conjugated to cytotoxic agents. Treatment modalities with antibodies of the invention are also provided.
Owner:SEATTLE GENETICS INC
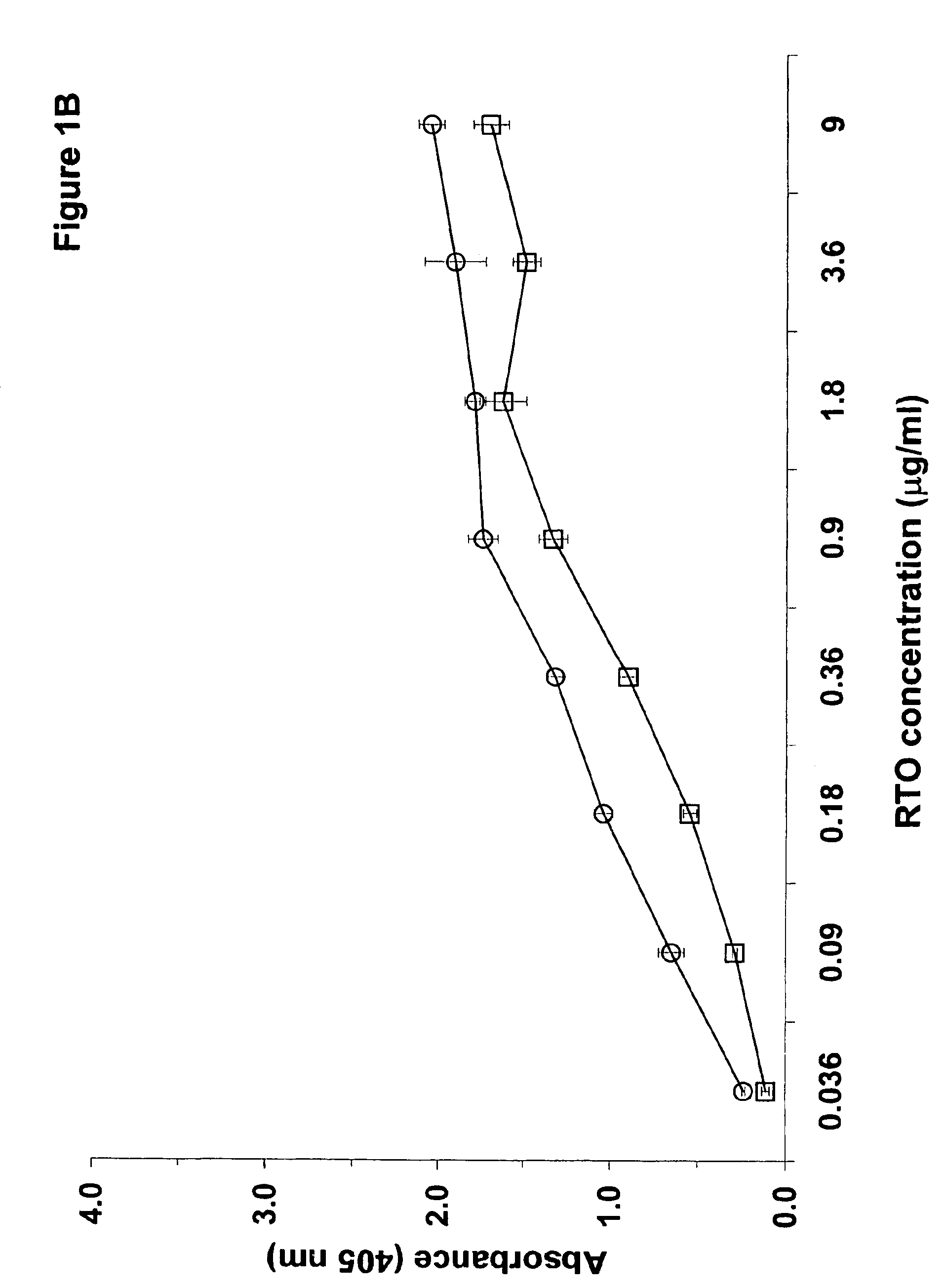
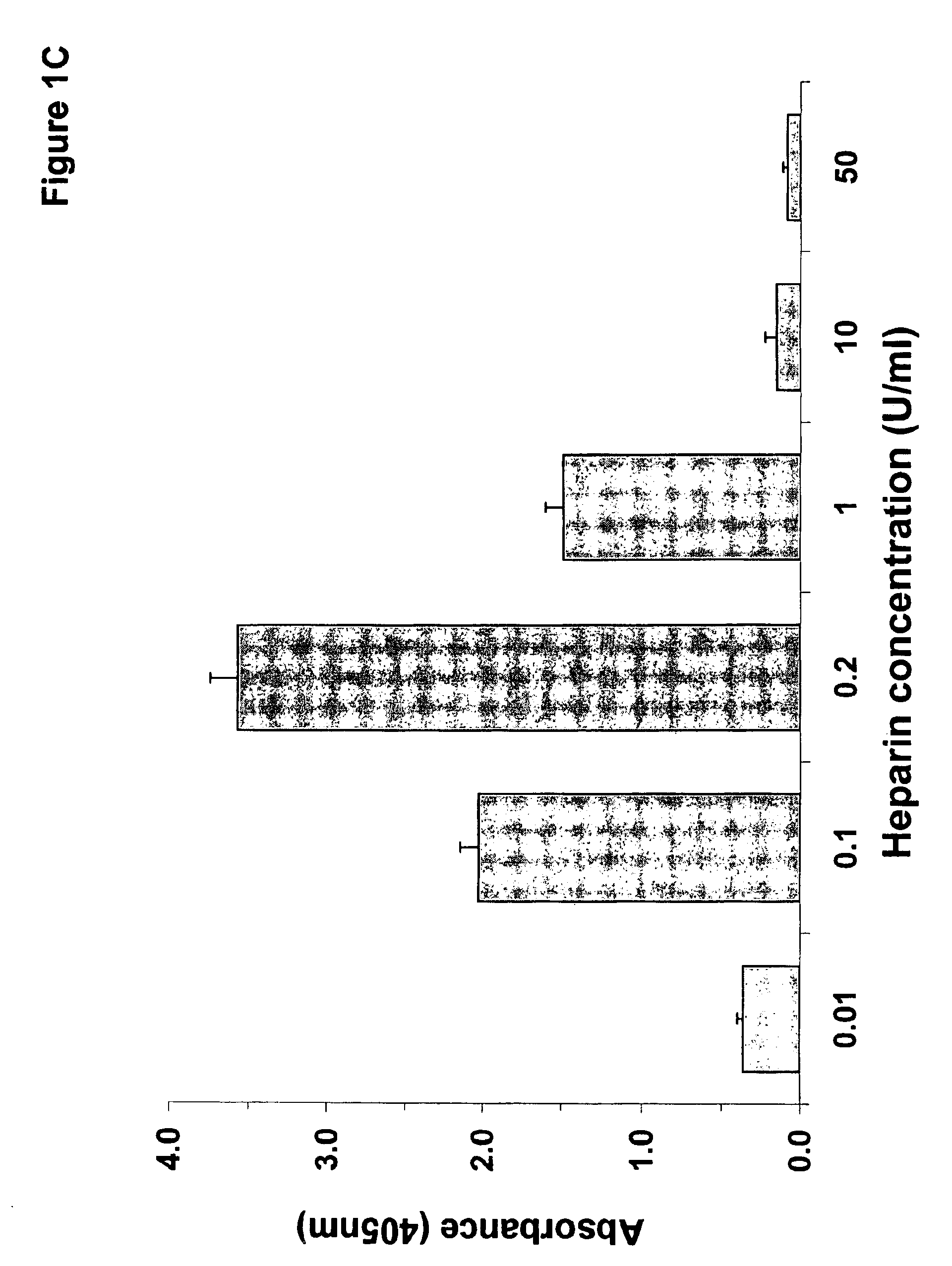
Compositions and methods useful for the diagnosis and treatment of heparin induced thrombocytopenia/thrombosis
InactiveUS6964854B1Animal cellsImmunoglobulins against cytokines/lymphokines/interferonsHeparin antibodyMammal
The invention includes compositions, kits and methods comprising a monoclonal antibody which shares key functional properties with the polyclonal antibodies which participate in the pathogenesis of heparin induced thrombocytopenia / thrombosis (HIT / HITT) in a mammal. The monoclonal antibody of the invention preferentially binds with a PF4 / heparin complex relative to the binding of the antibody with PF4 or heparin alone. The monoclonal antibody of the invention also binds specifically with PF4 in a complex with other glycosaminoglycans besides heparin, and also activates platelets. The monoclonal antibody of the invention is useful in methods for diagnosing and treating HIT / HITT in a mammal. A humanized version of the monoclonal antibody of the invention is also included, along with a process for humanizing the monoclonal antibody of the invention.
Owner:STC UNM
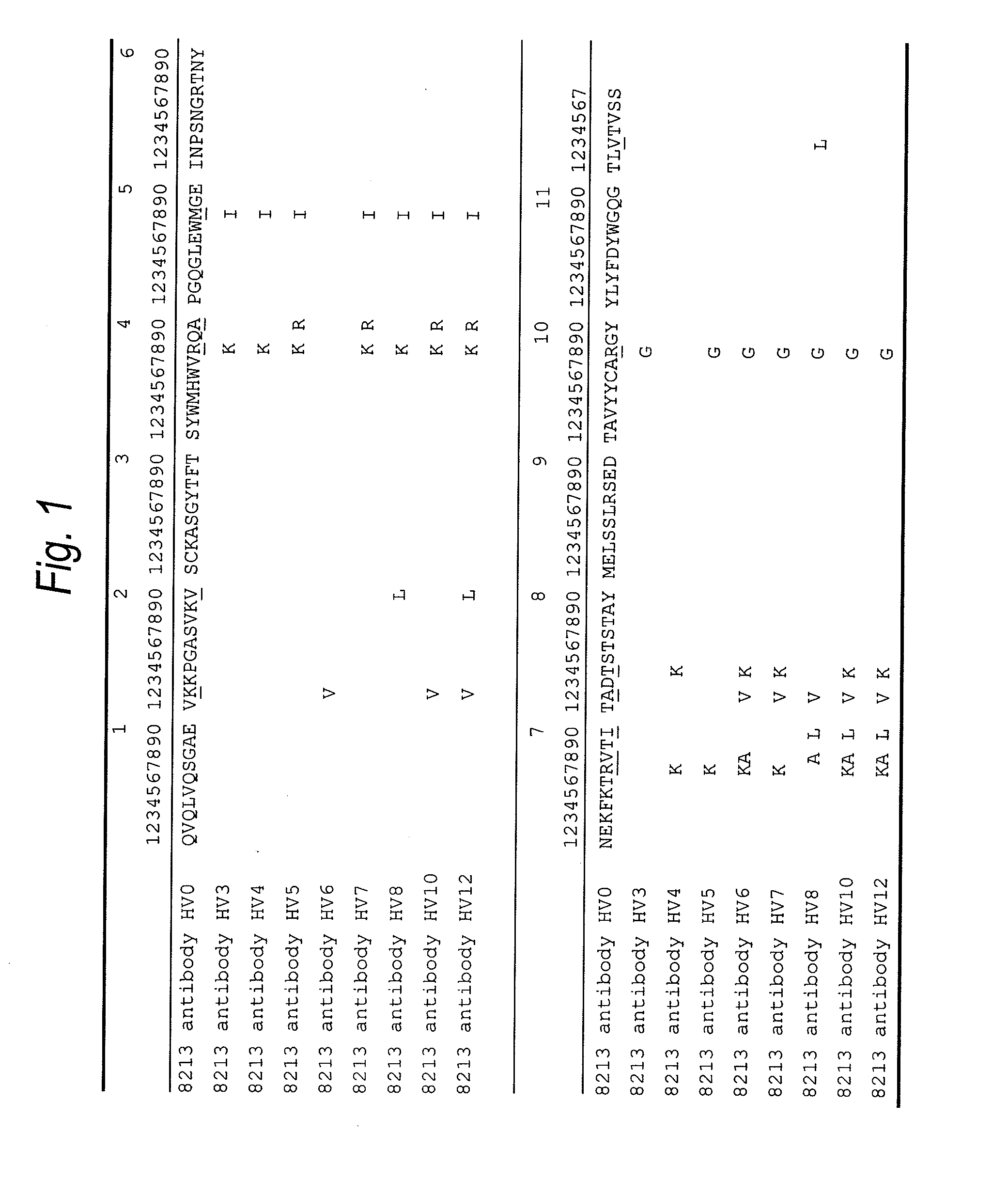
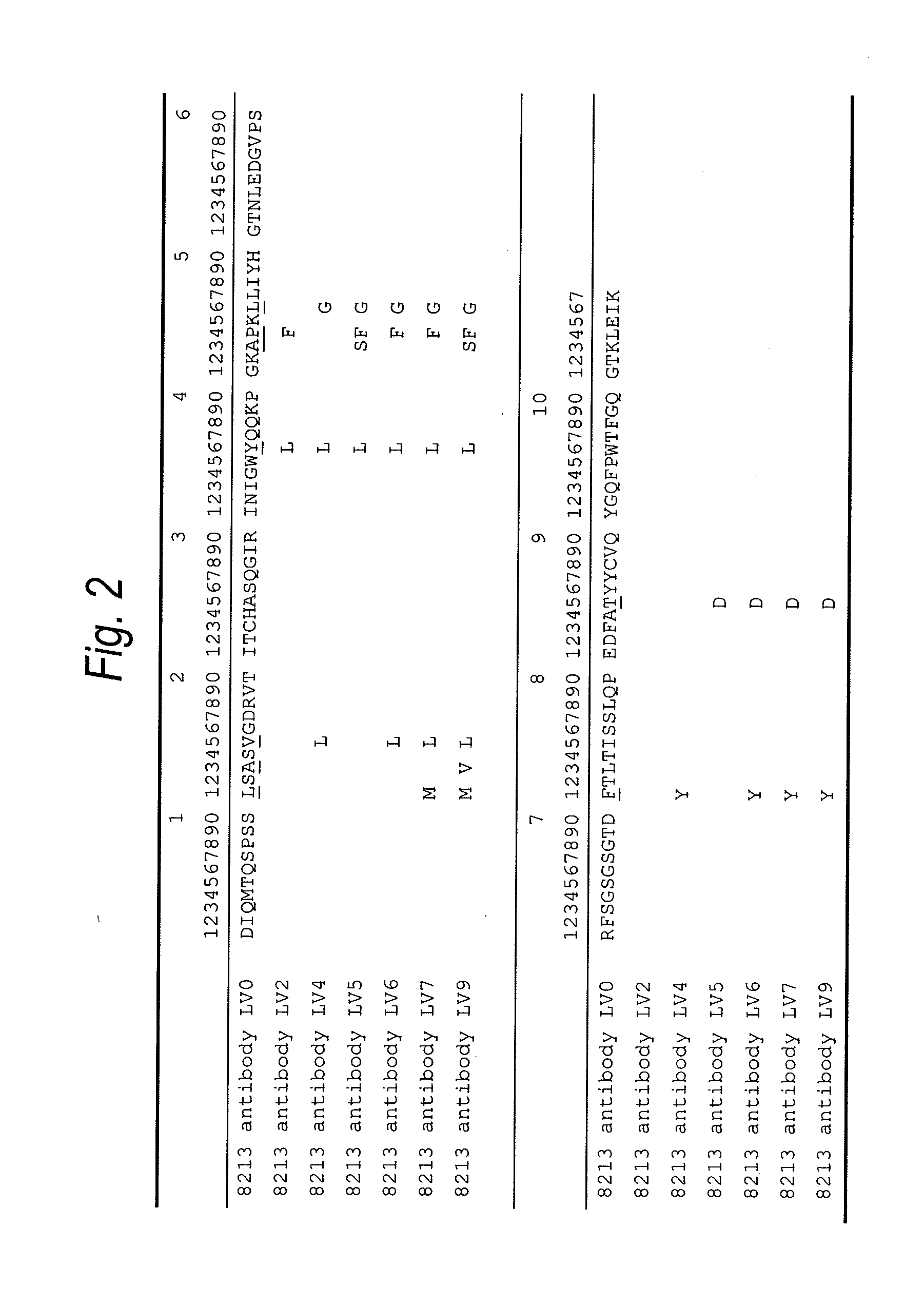
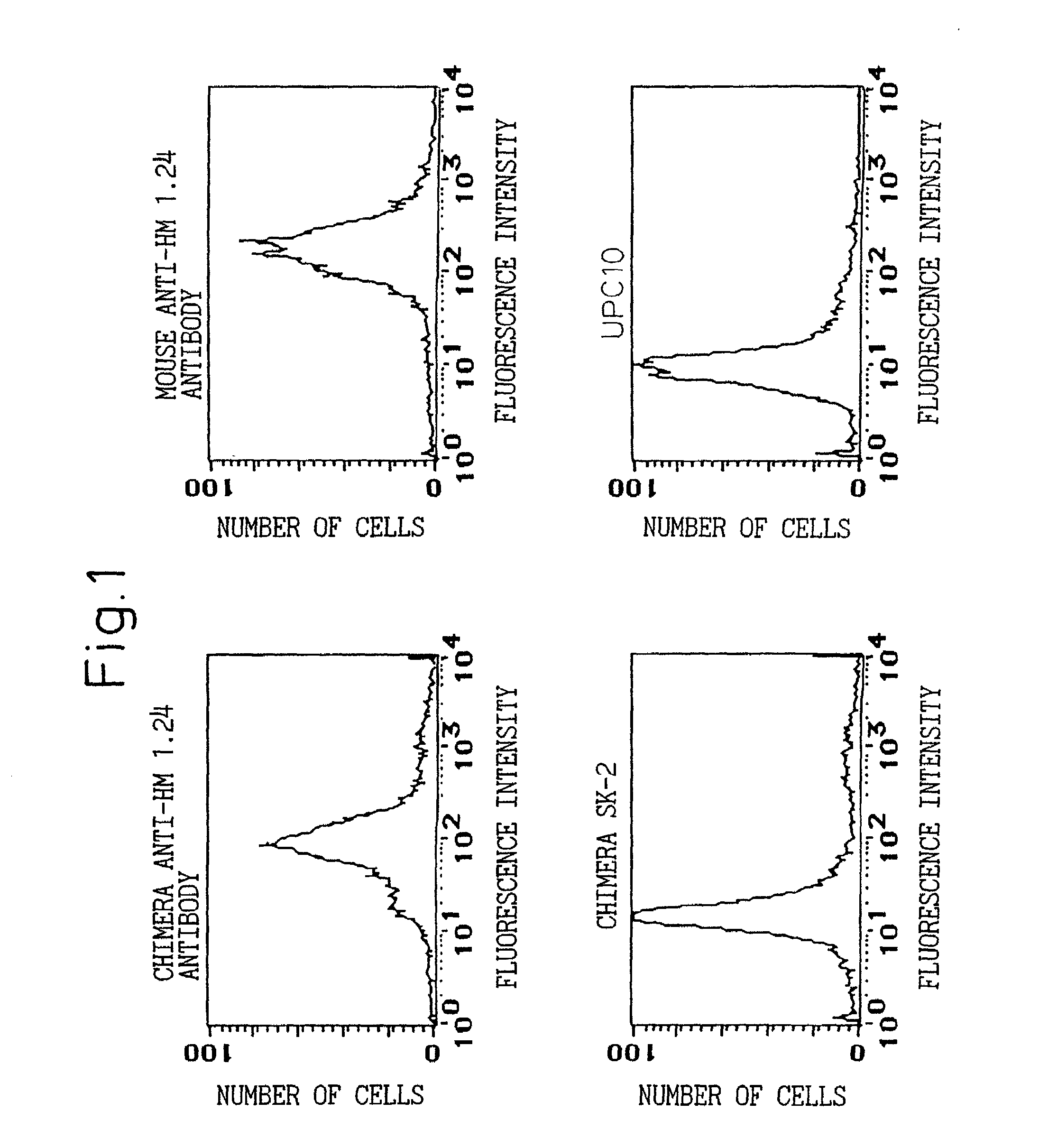
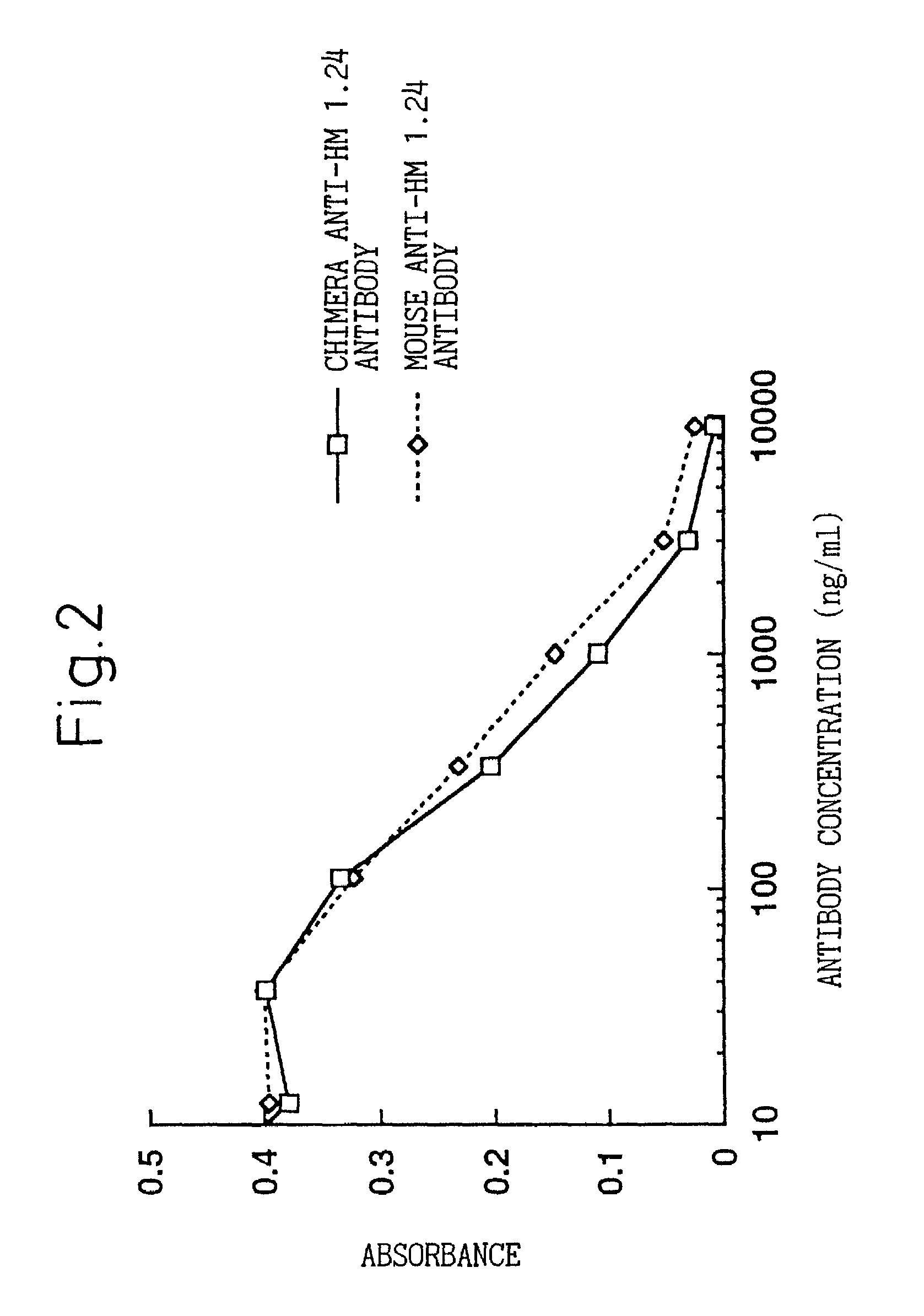
Natural human antibody
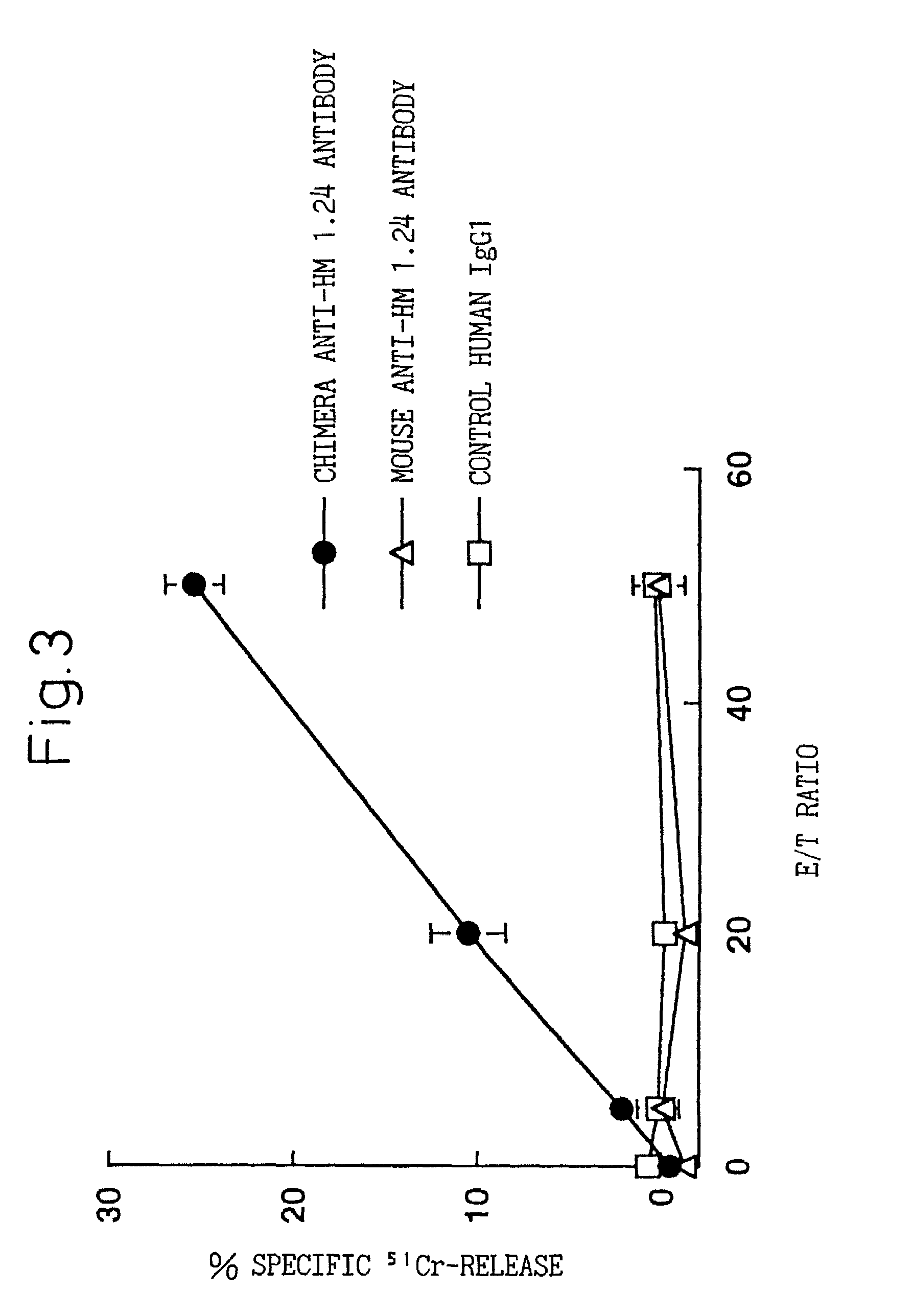
A reshaped human anti-HM1.24 antibody comprising:(A) L chains each comprising (1) a constant region of a human L chain, and (2) FRs of a human L chain, and CDRs of L chain of mouse anti-HM1.24 monoclonal antibody; and(B) H chains each comprising (2) a constant region of a human H chain, and (2) FRs of a human H chain, and CDRs of H chain of mouse anti-HM1.24 monoclonal antibody. Since the majority of the reshaped human antibody is derived from human antibody and the CDR has a low antigenicity, the reshaped human antibody of the present invention has low antigenicity and therefore is very promising in medical and therapeutic applications.
Owner:CHUGAI PHARMA CO LTD
Methods for treating neuropathy by agonist anti-trk-C monoclonal antibodies
InactiveUS7615383B2Effective prevention and treatmentMaintain good propertiesNervous disorderGenetic material ingredientsBiologic DMARDCell system
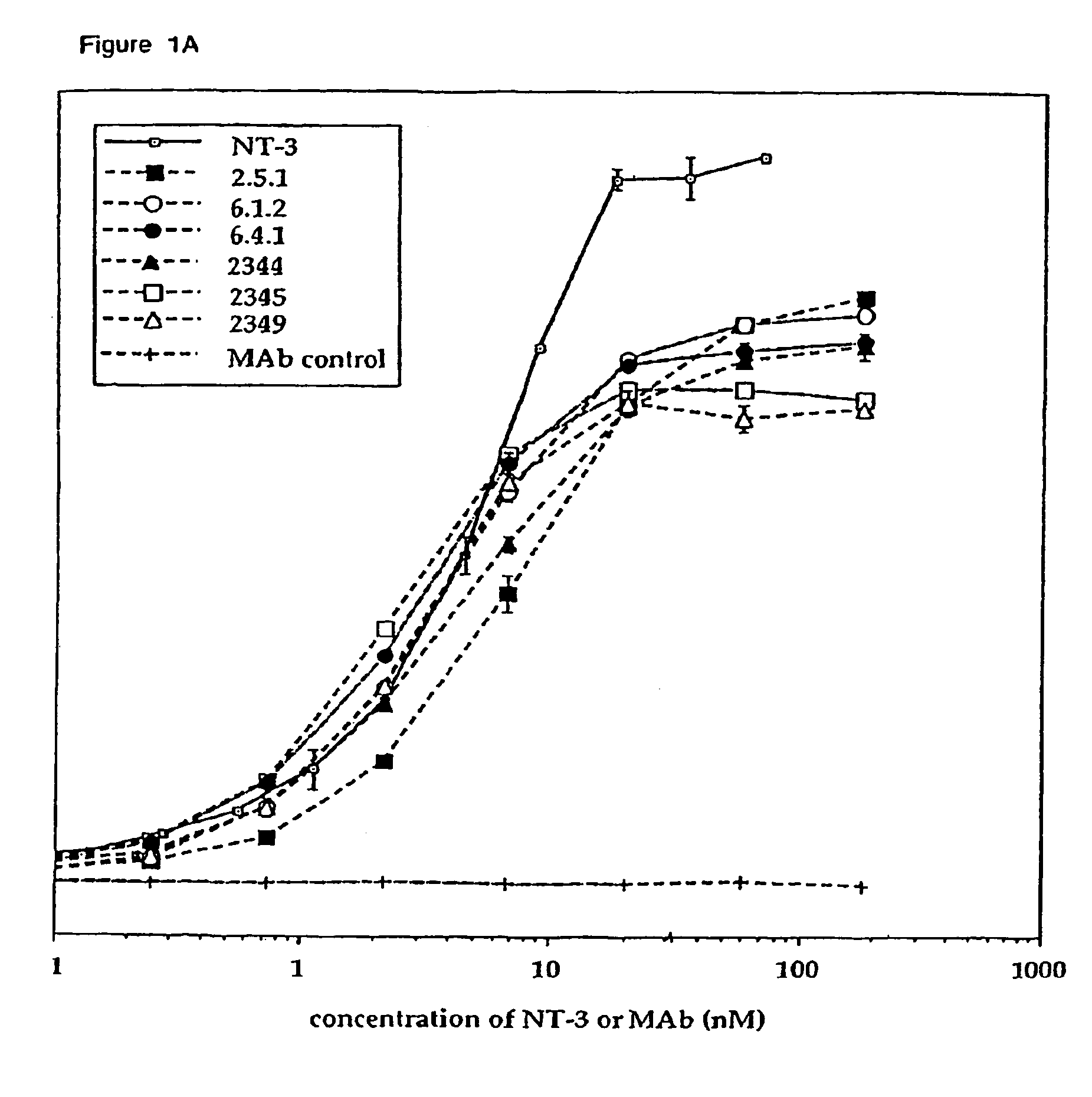
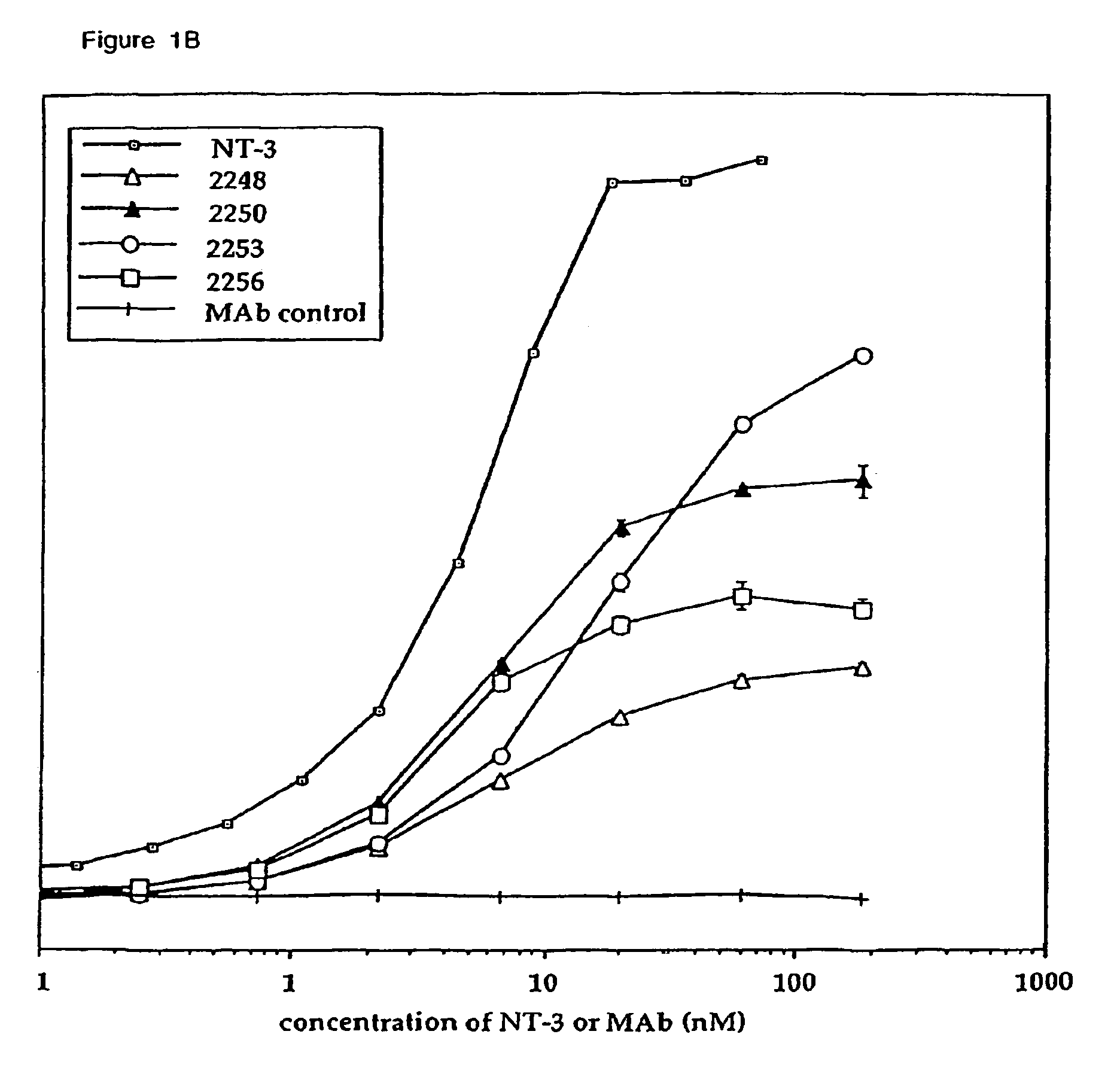
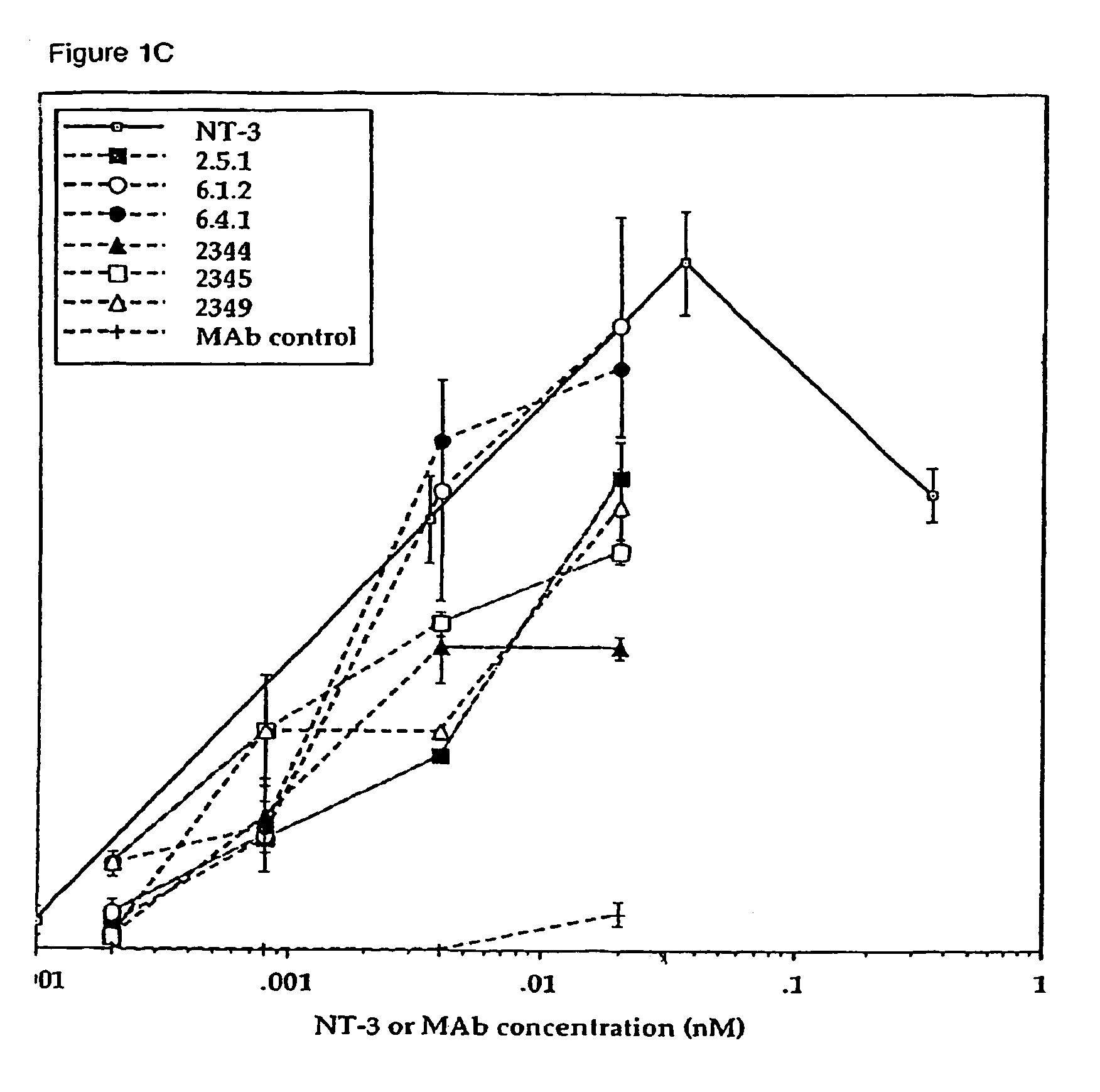
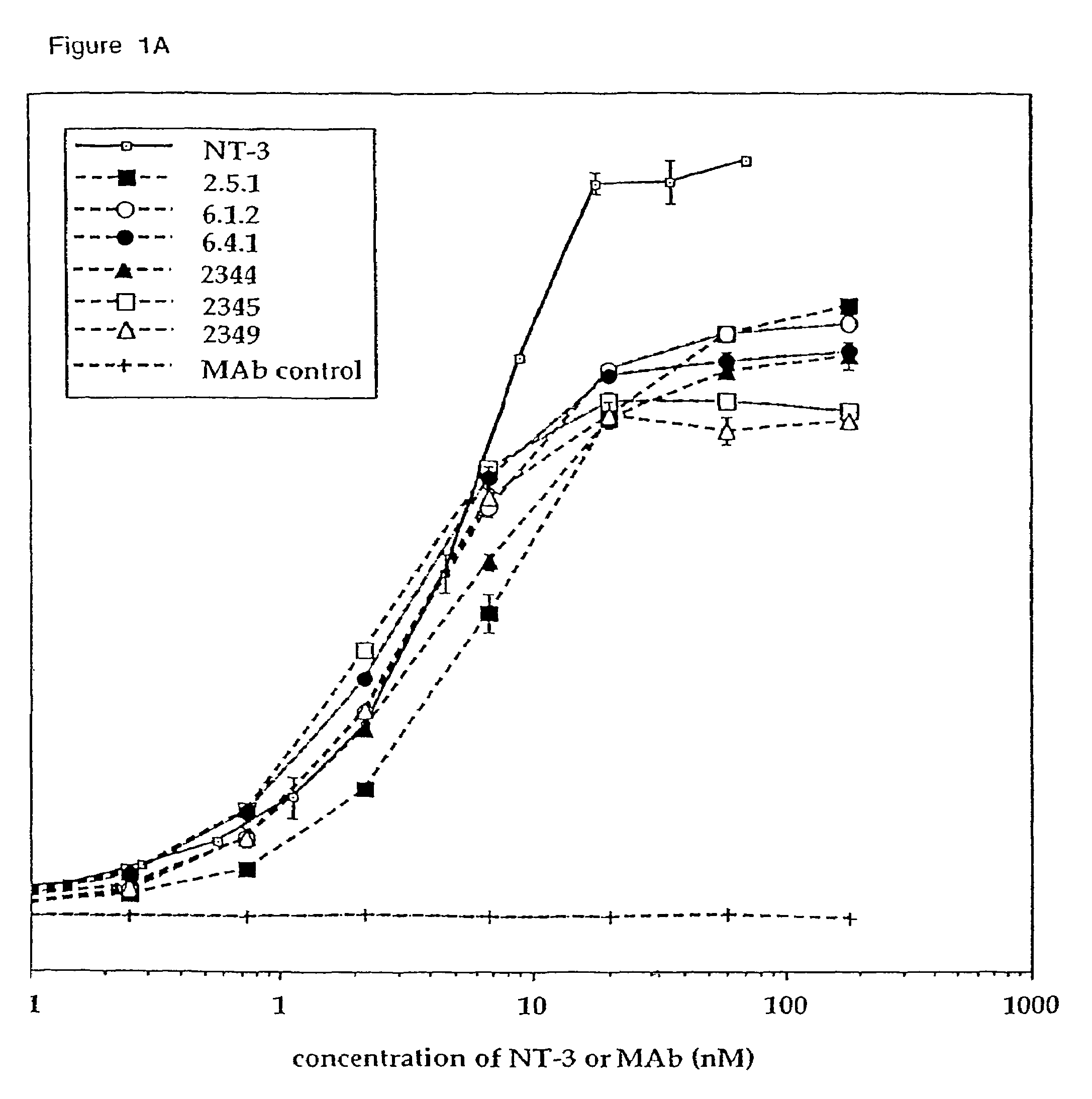
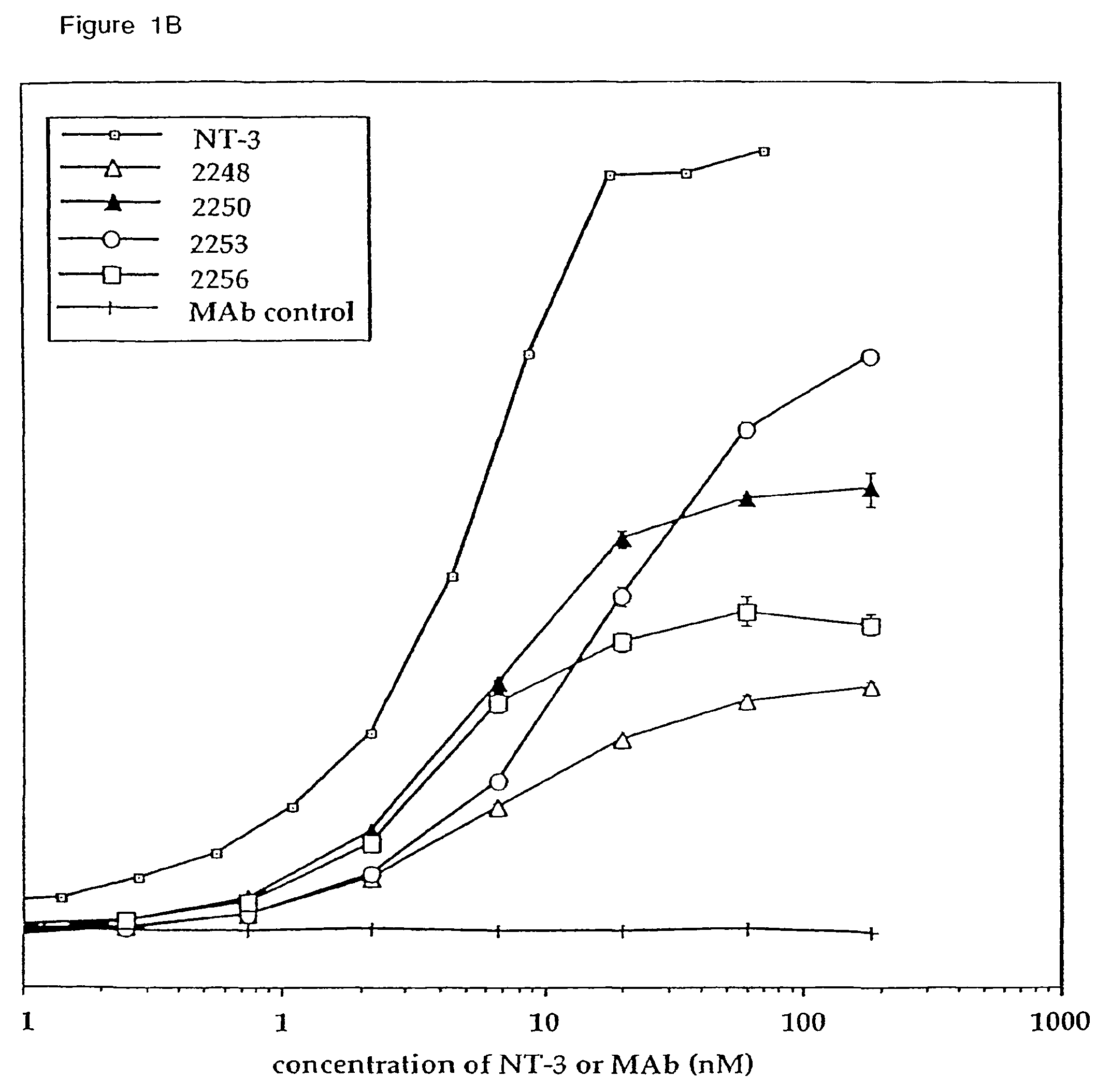
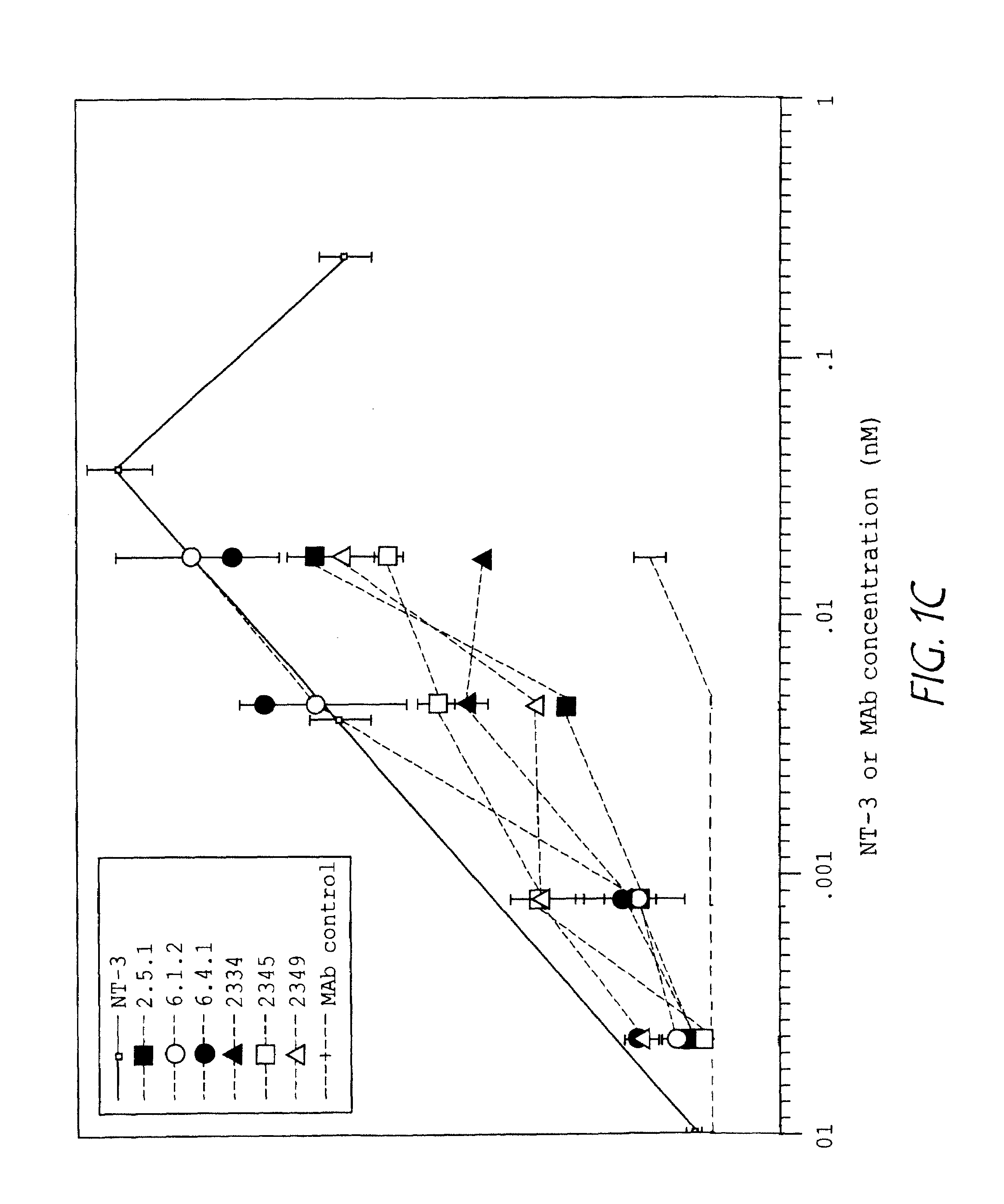
The invention concerns agonist anti-trkC monoclonal antibodies which mimic certain biological activities of NT-3, the native ligand of trkC. The invention further concerns the use of such antibodies in the prevention and / or treatment of cellular degeneration, including nerve cell damage associated with acute nervous cell system injury and chronic neurodegenerative diseases, including peripheral neuropathy.
Owner:GENENTECH INC
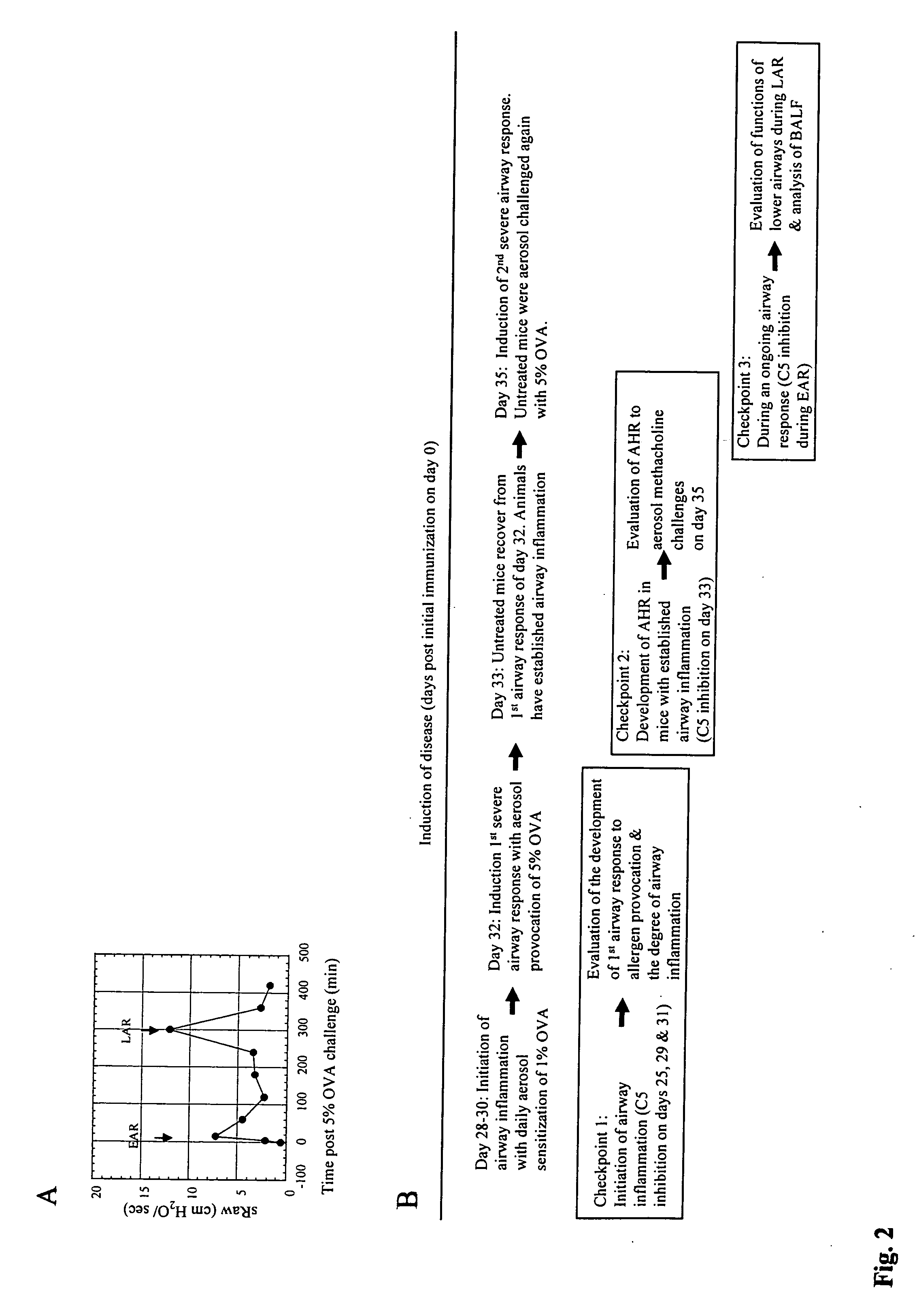
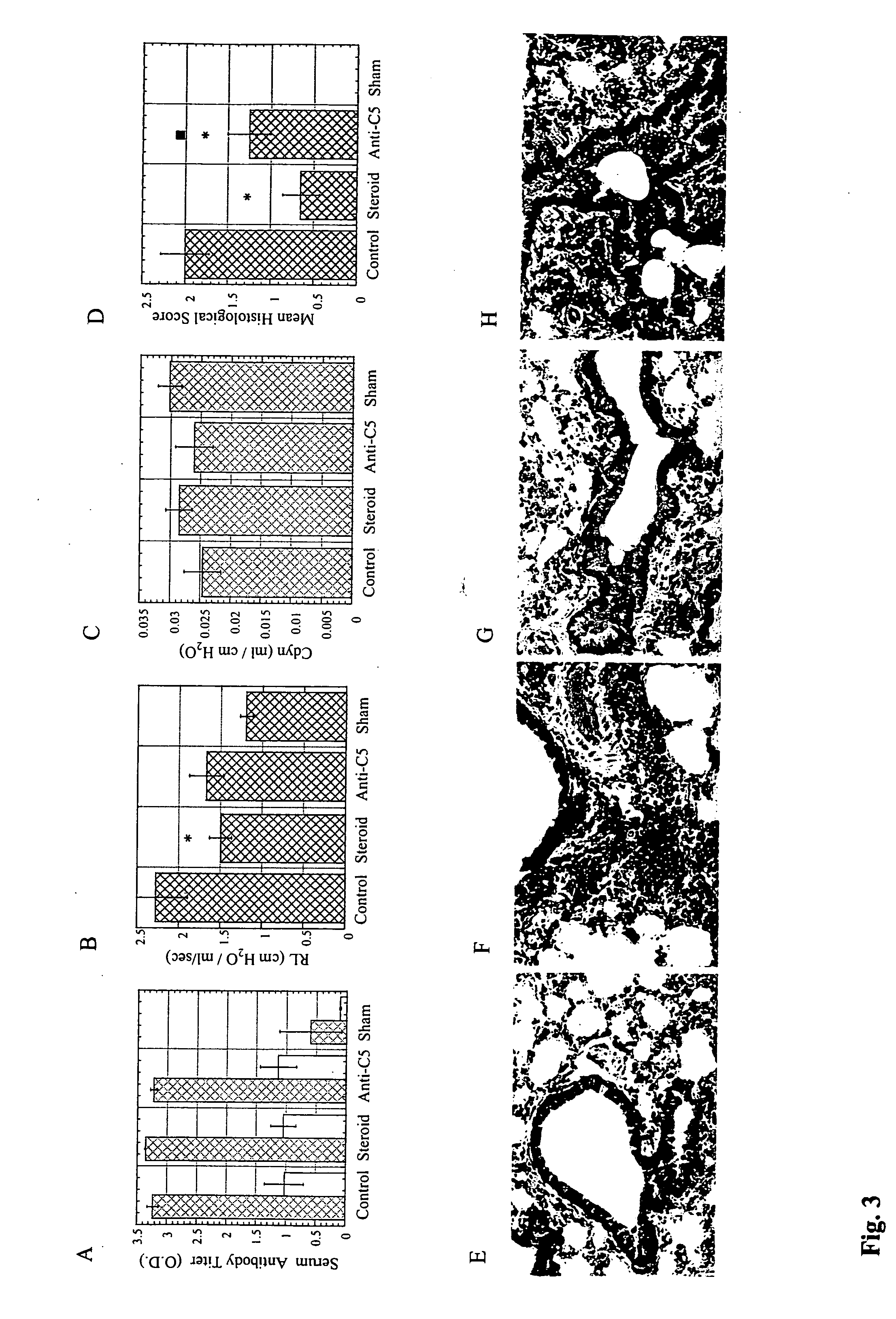
Nebulization of monoclonal antibodies for treating pulmonary diseases
The present application relates to methods and compositions employing an antibody that inhibits activation of the complement system and can be used to prevent or treat a pulmonary disease or condition.
Owner:ALEXION PHARMA INC
Monoclonal antibodies that suppress B cell growth and/or differentiation
The present invention provides monoclonal antibodies which interfere with the interactions between FDCs and B cells, thereby suppressing the proliferation and / or differentiation of B cells in lymphoid follicles. The monoclonal antibodies of the present invention are useful for treating follicular lymphomas, multiple myeloma as well as autoimmune diseases.
Owner:OCHSNER CLINIC FOUND
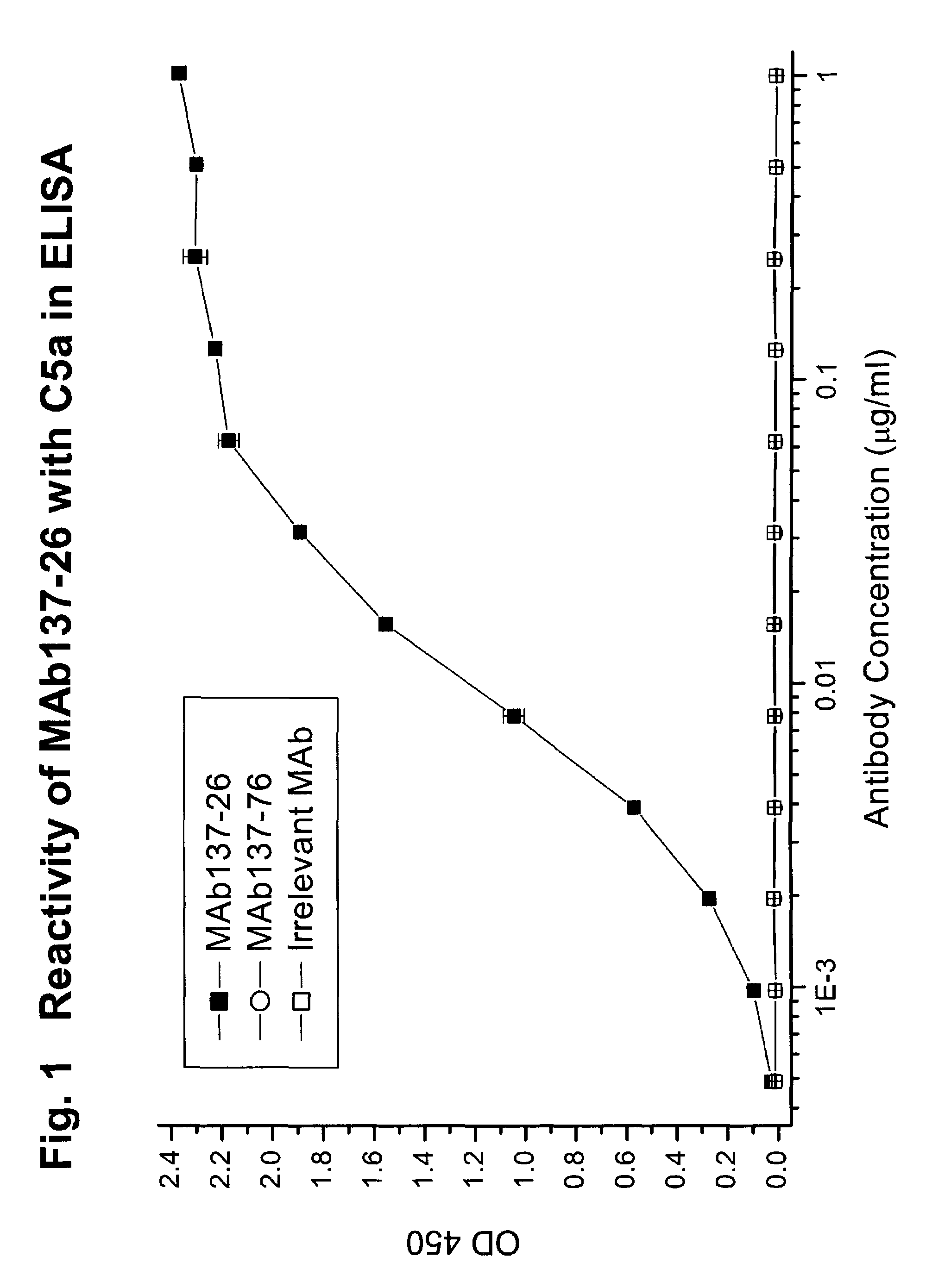
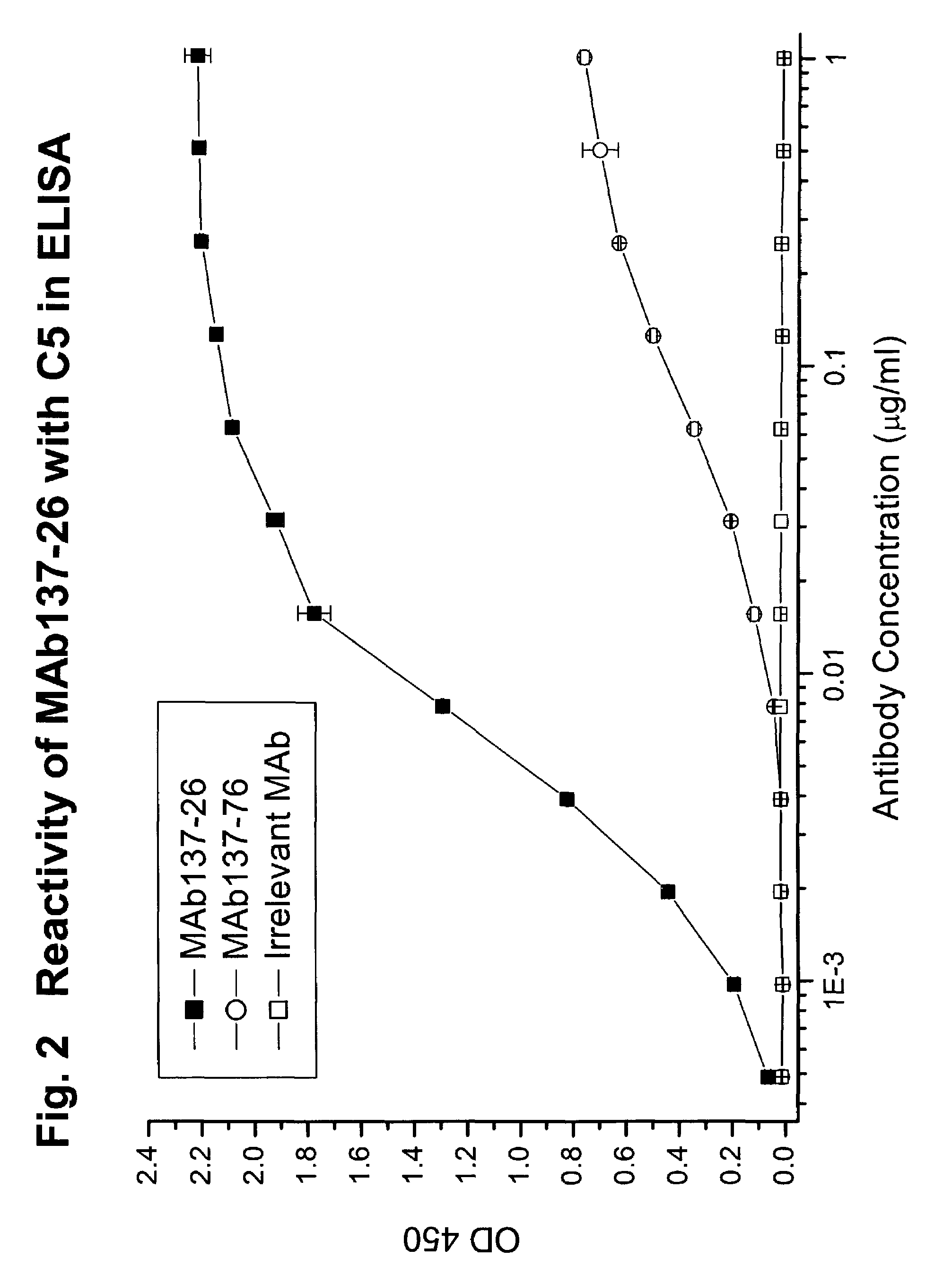
Complement pathway inhibitors binding to C5 and C5a without preventing formation of C5b
The invention relates to inhibitors that bind to C5 and C5a, but which do not prevent the activation of C5 and do not prevent formation of or inhibit the activity of C5b. One example of such an inhibitor molecule is the monoclonal antibody designated MAb137-26, which binds to a shared epitope of human C5 and C5a. These inhibitors may be used to inhibit the activity of C5a in treating diseases and conditions mediated by excessive or uncontrolled production of C5a. The inhibitor molecules are also useful for diagnostic detection of the presence / absence or amount of C5 or C5a.
Owner:GENENTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com