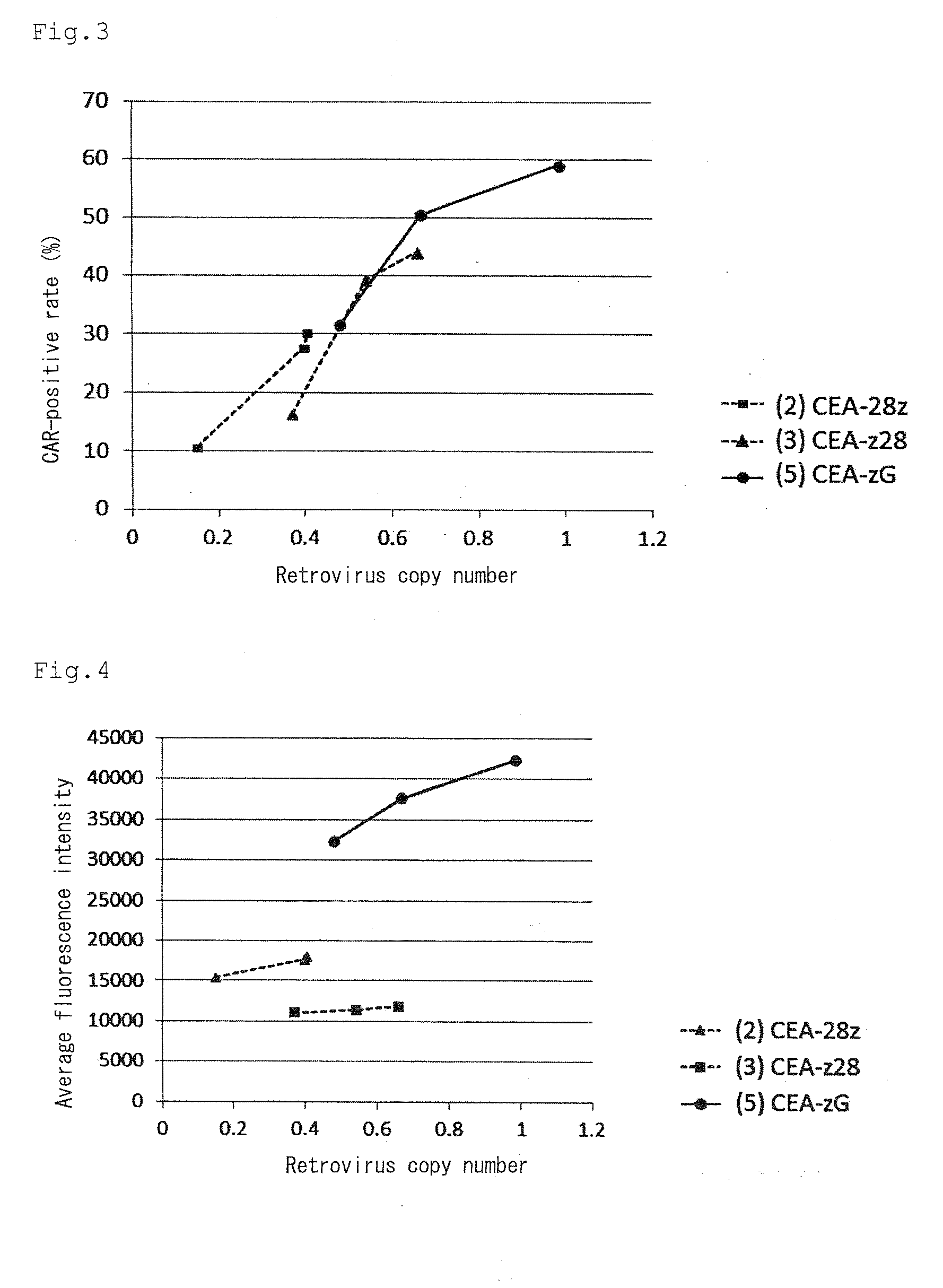
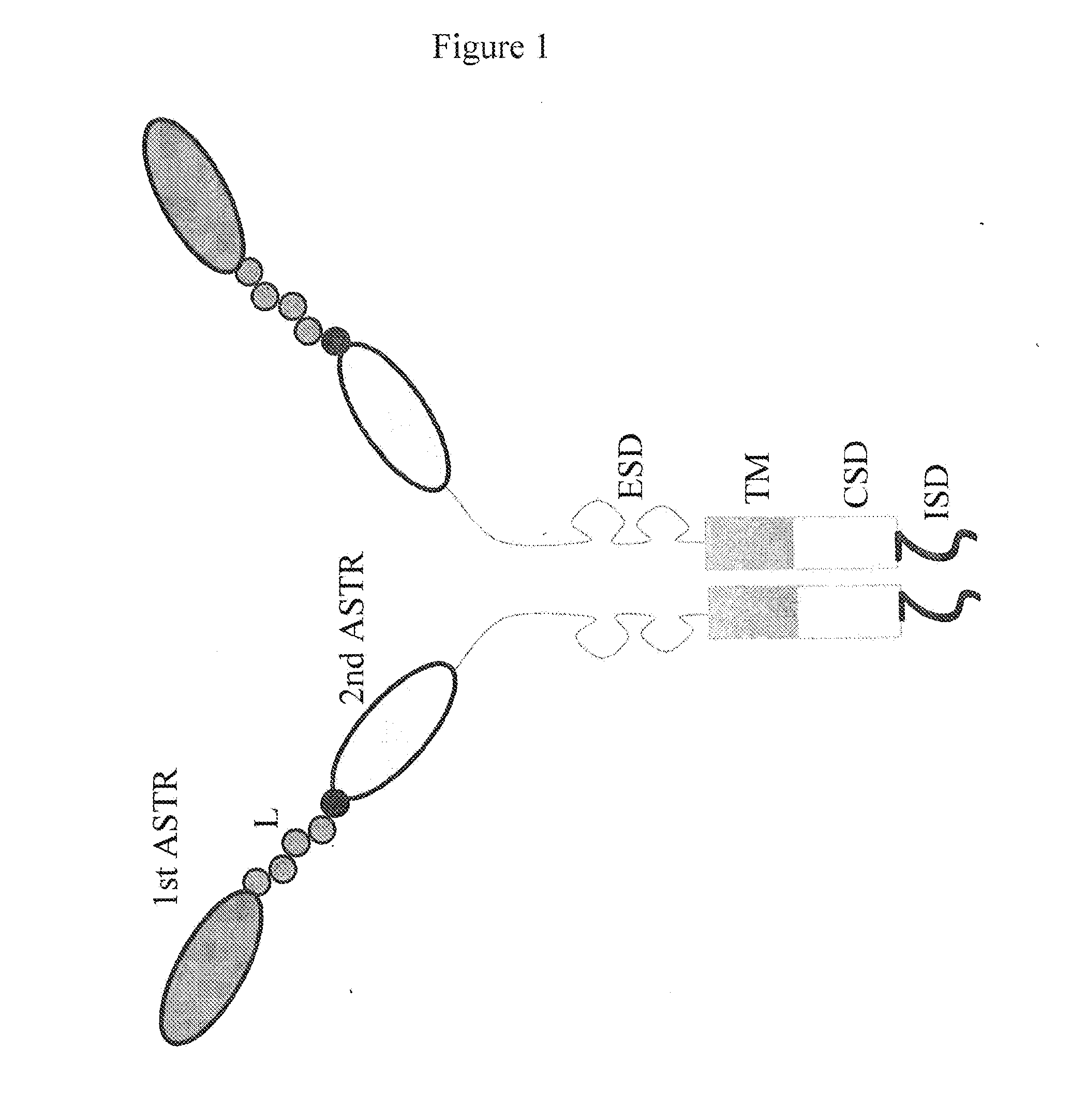
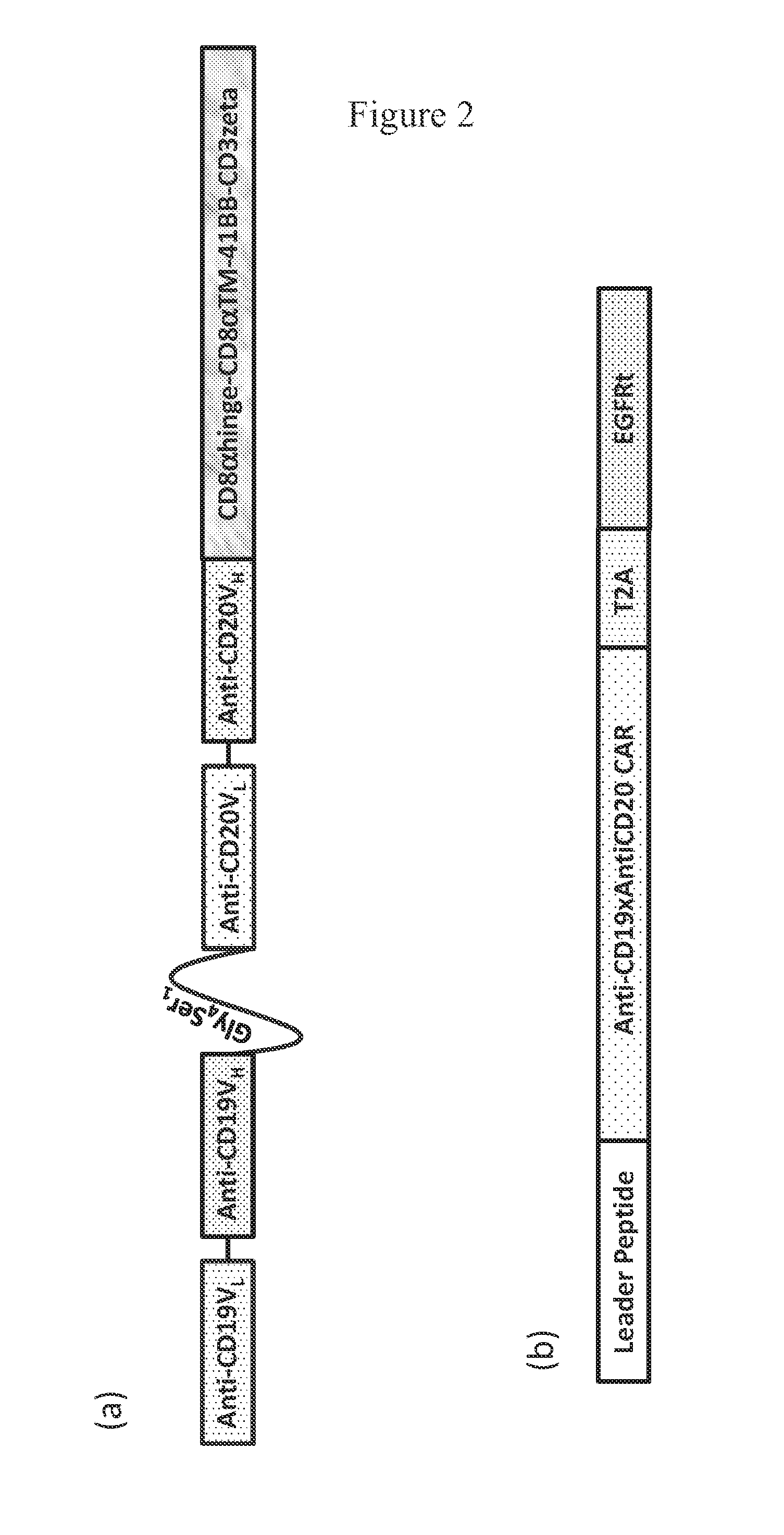
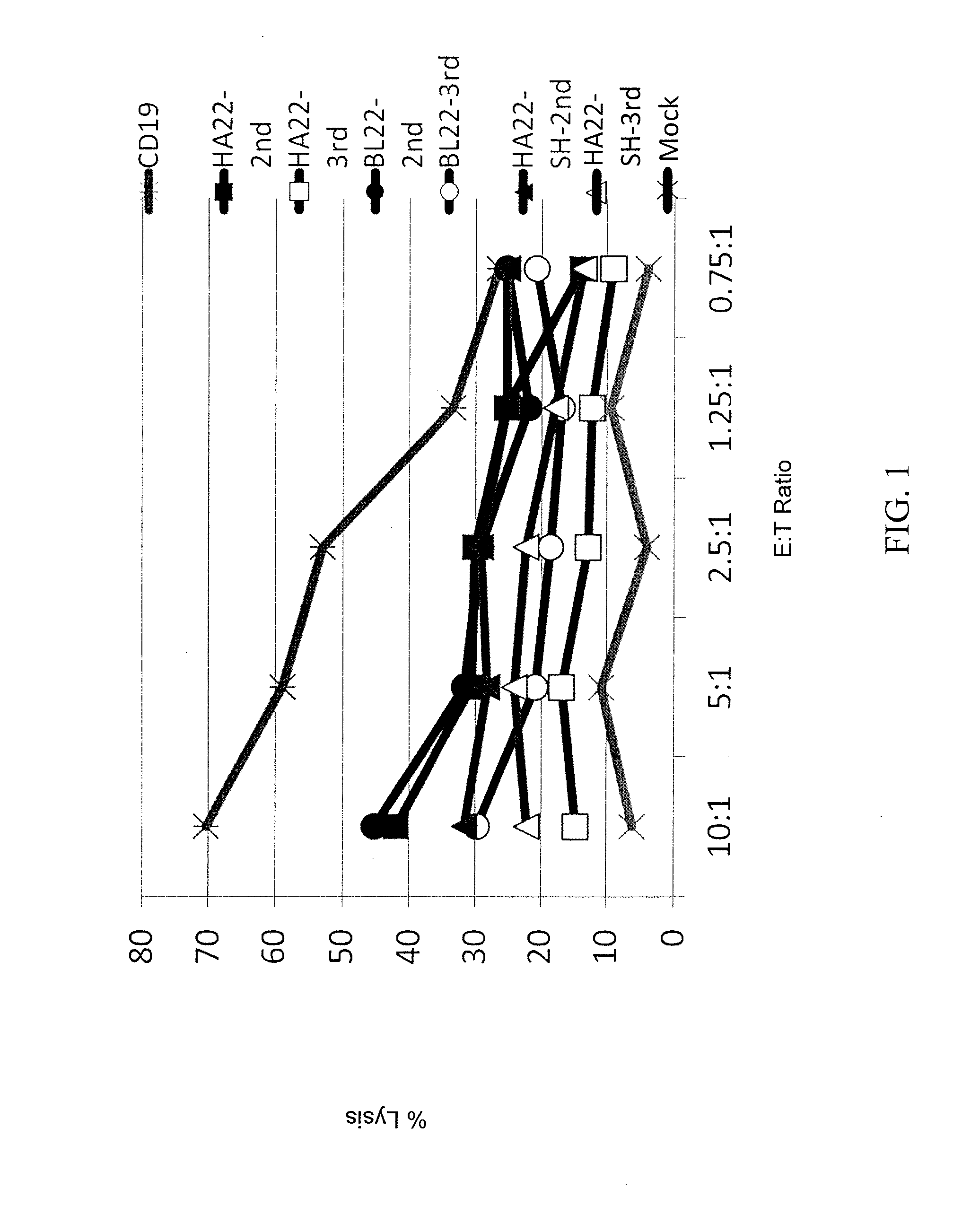
Patents
Literature
685 results about "Transmembrane domain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
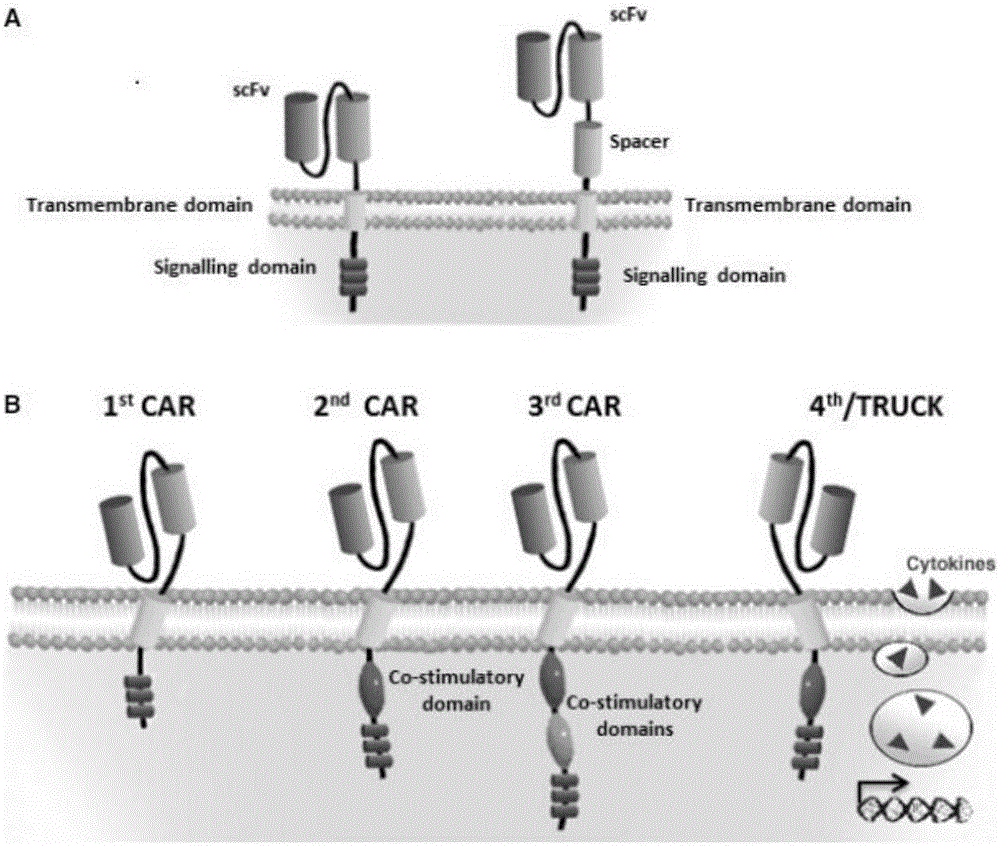
Transmembrane domain usually denotes a transmembrane segment of single alpha helix of a transmembrane protein. More broadly, a transmembrane domain is any membrane-spanning protein domain.
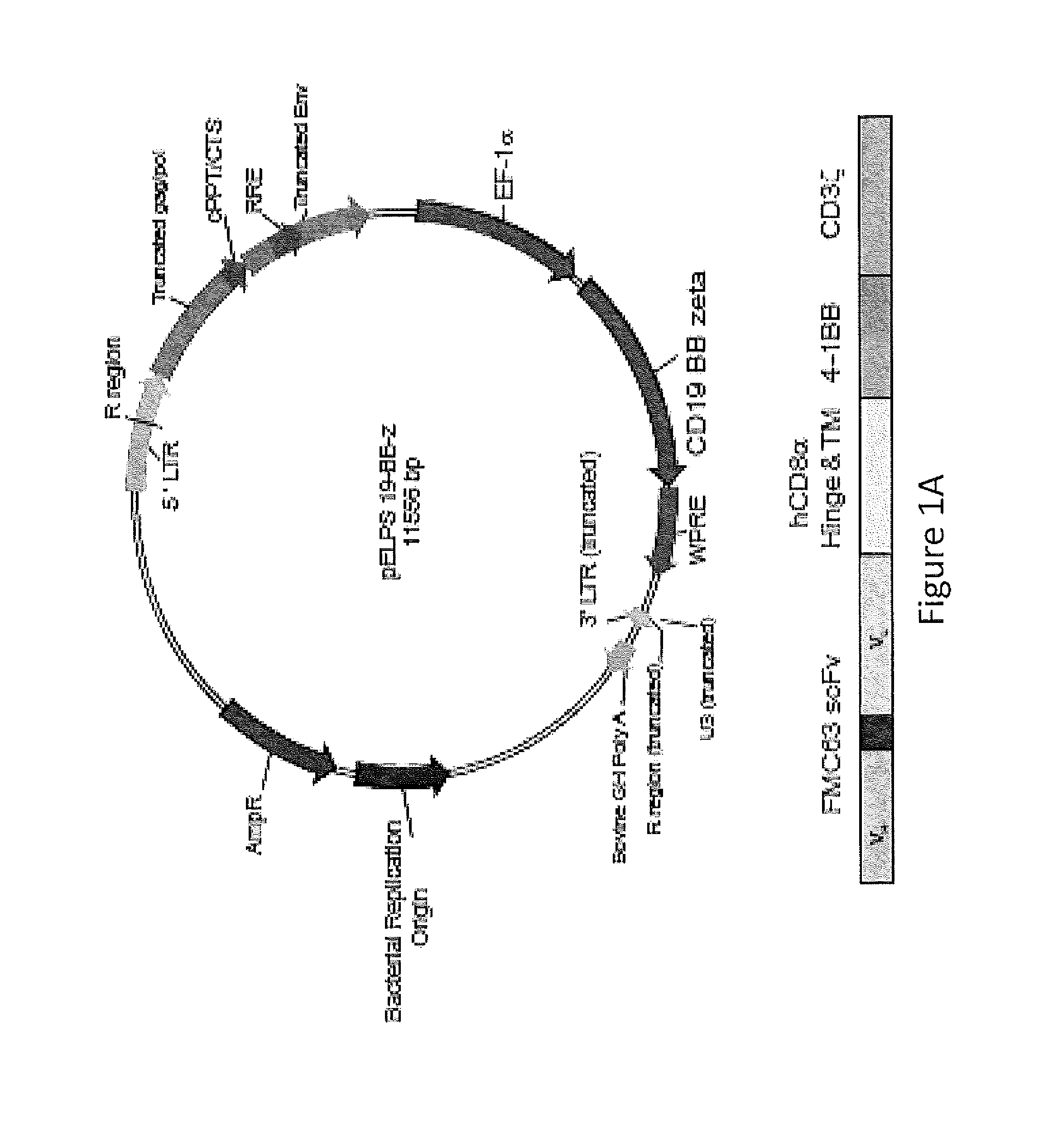
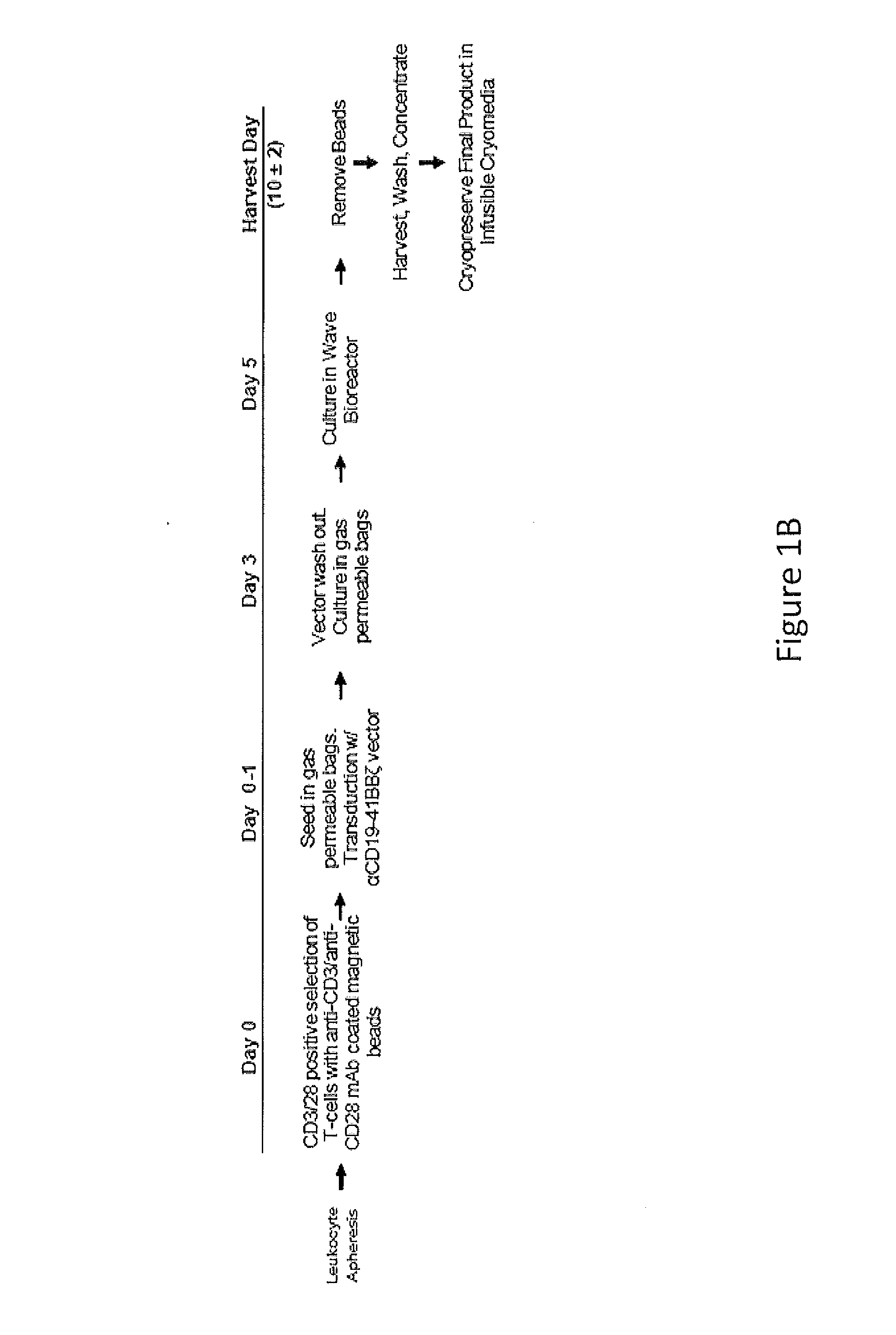
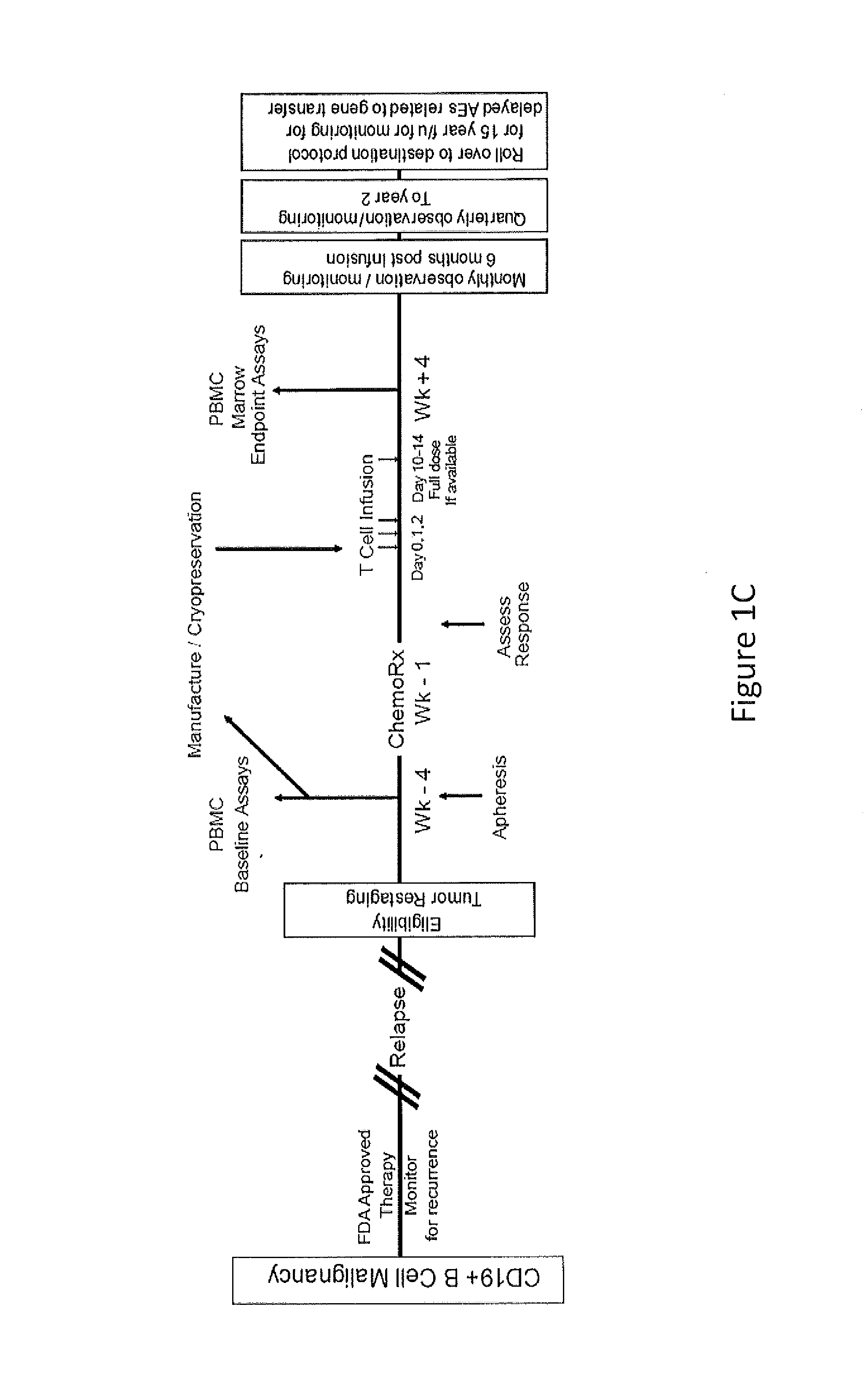
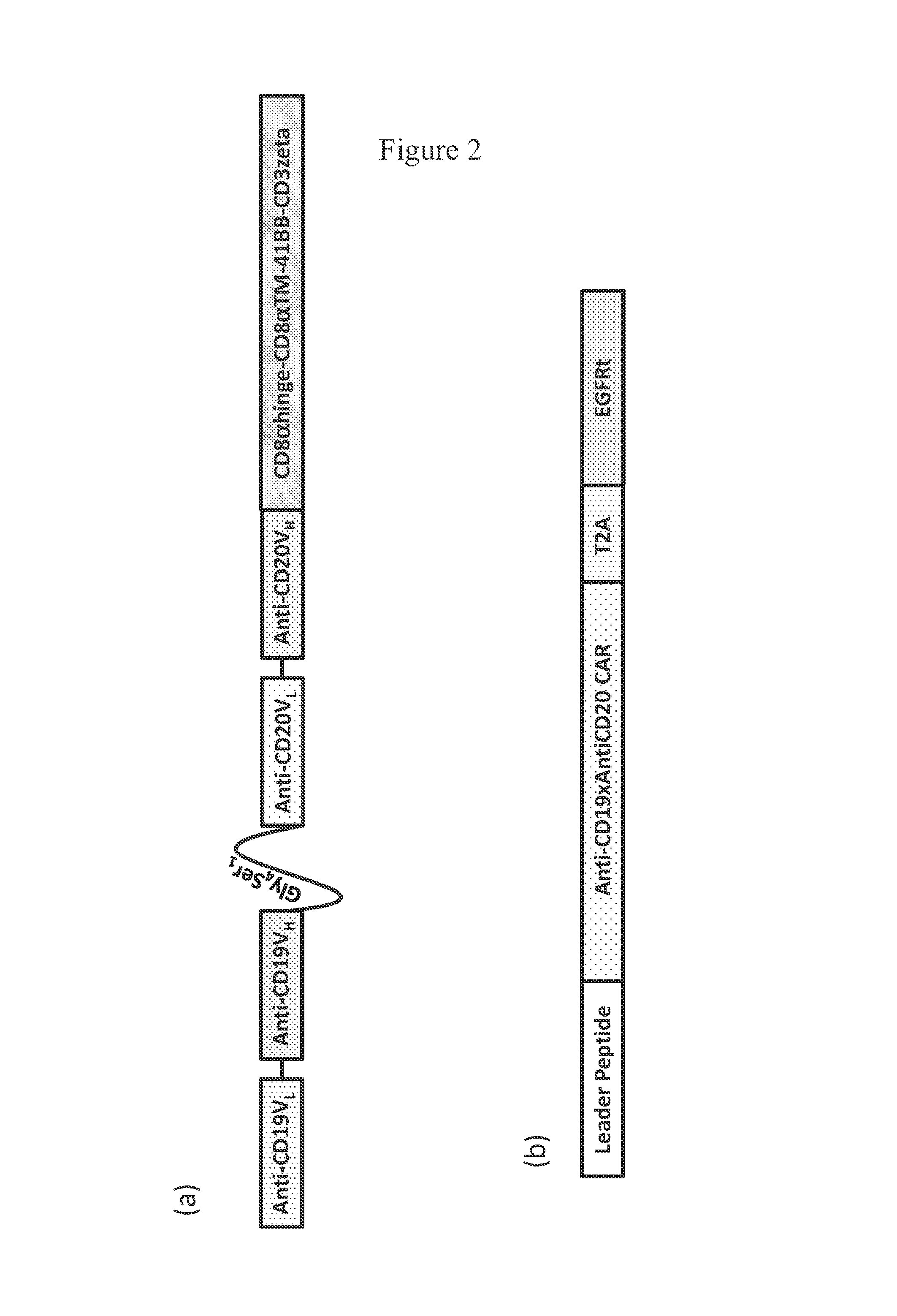
Use of Chimeric Antigen Receptor-Modified T-Cells to Treat Cancer
ActiveUS20130287748A1Stimulate immune responseVirusesPeptide/protein ingredientsBinding domainAntigen binding
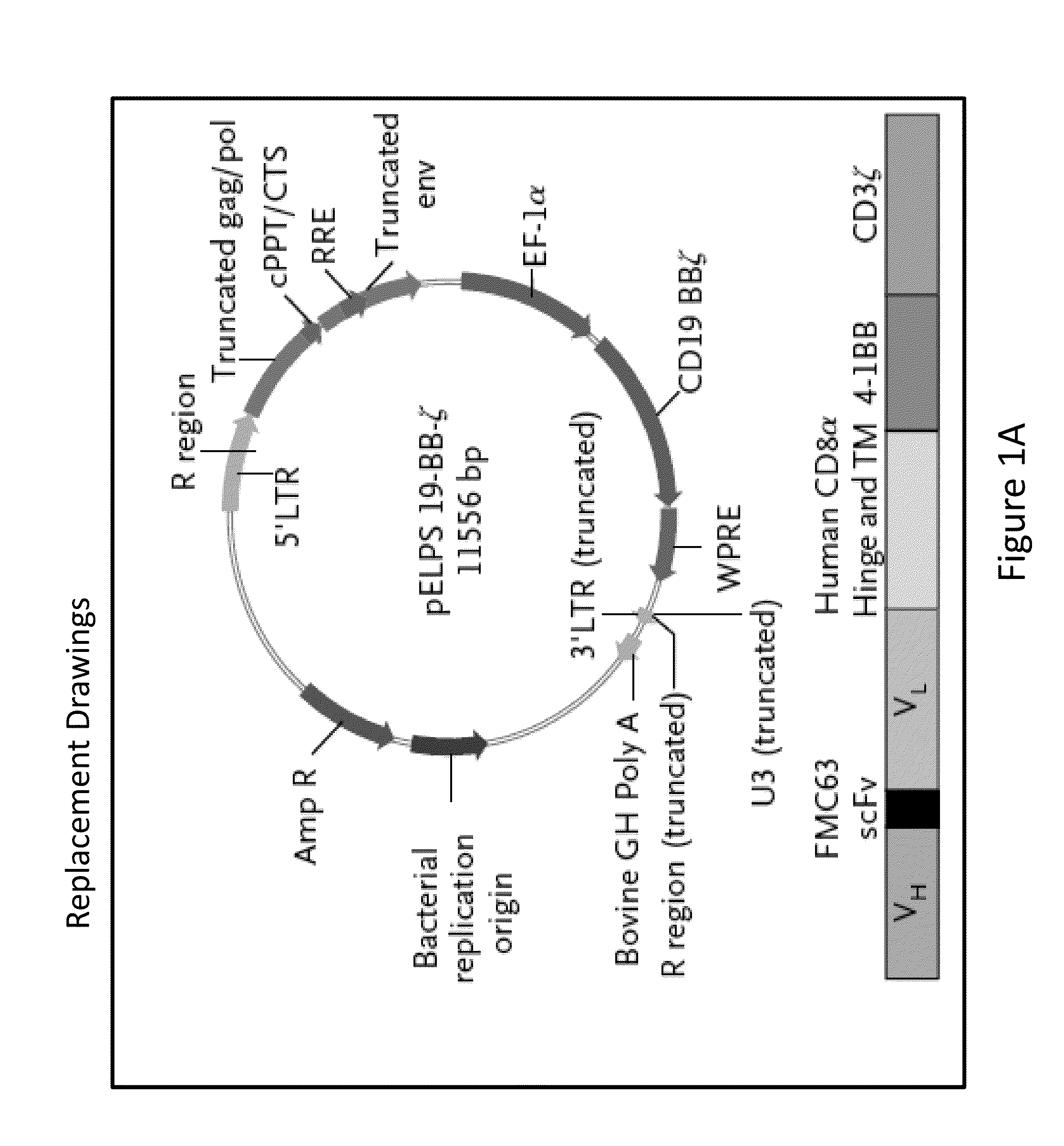
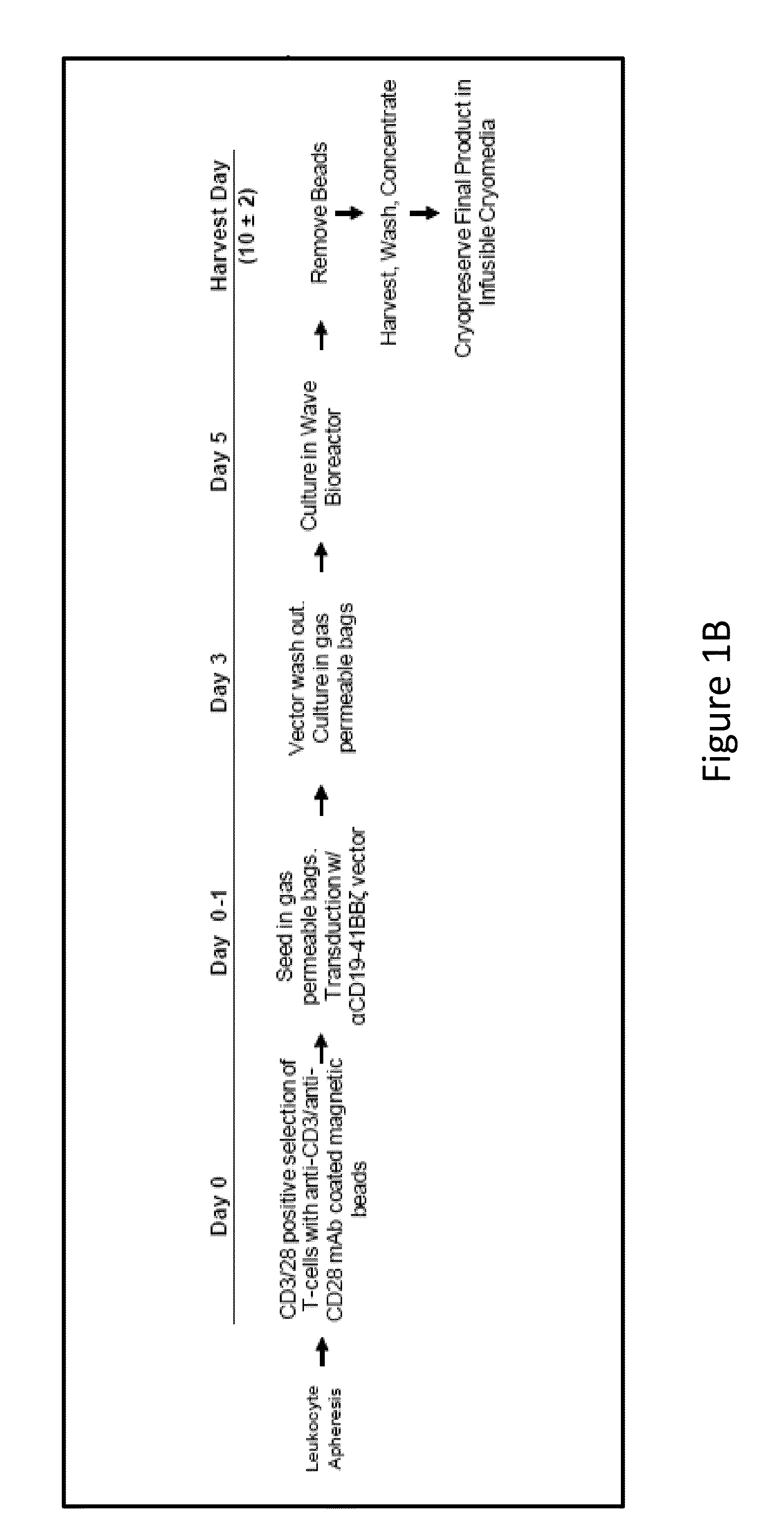
The present invention provides compositions and methods for treating cancer in a human. The invention includes relates to administering a genetically modified T cell to express a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
CE7-specific redirected immune cells
Genetically engineered, CE7-specific redirected immune cells expressing a cell surface protein having an extracellular domain comprising a receptor which is specific for CE7, an intracellular signaling domain, and a transmembrane domain, and methods of use for such cells for cellular immunotherapy of CE7+ neuroblastoma are disclosed. In one embodiment, the immune cell is a T cell and the cell surface protein is a single chain FvFc:ζ receptor where Fv designates the VH and VL chains of a single chain monoclonal antibody to CE7 linked by peptide, Fc represents a hinge —CH2—CH3 region of a human IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. DNA constructs encoding a chimeric T-cell receptor and a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor are also disclosed.
Owner:CITY OF HOPE
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
Chimeric immunoreceptor useful in treating human gliomas
InactiveUS7514537B2Negligible toxicityPotent and selectiveBiocideAntibody mimetics/scaffoldsIntracellular signallingHuman glioma
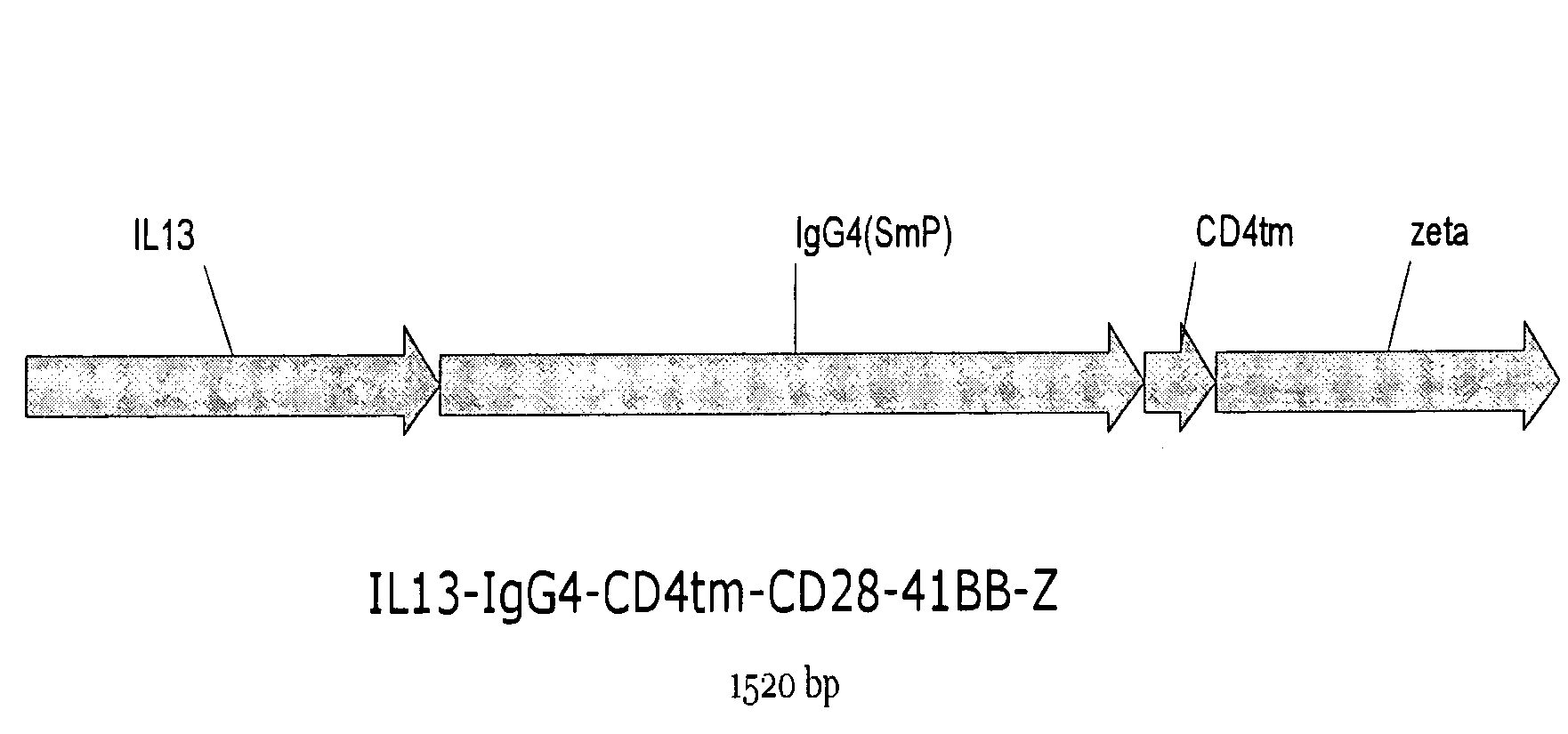
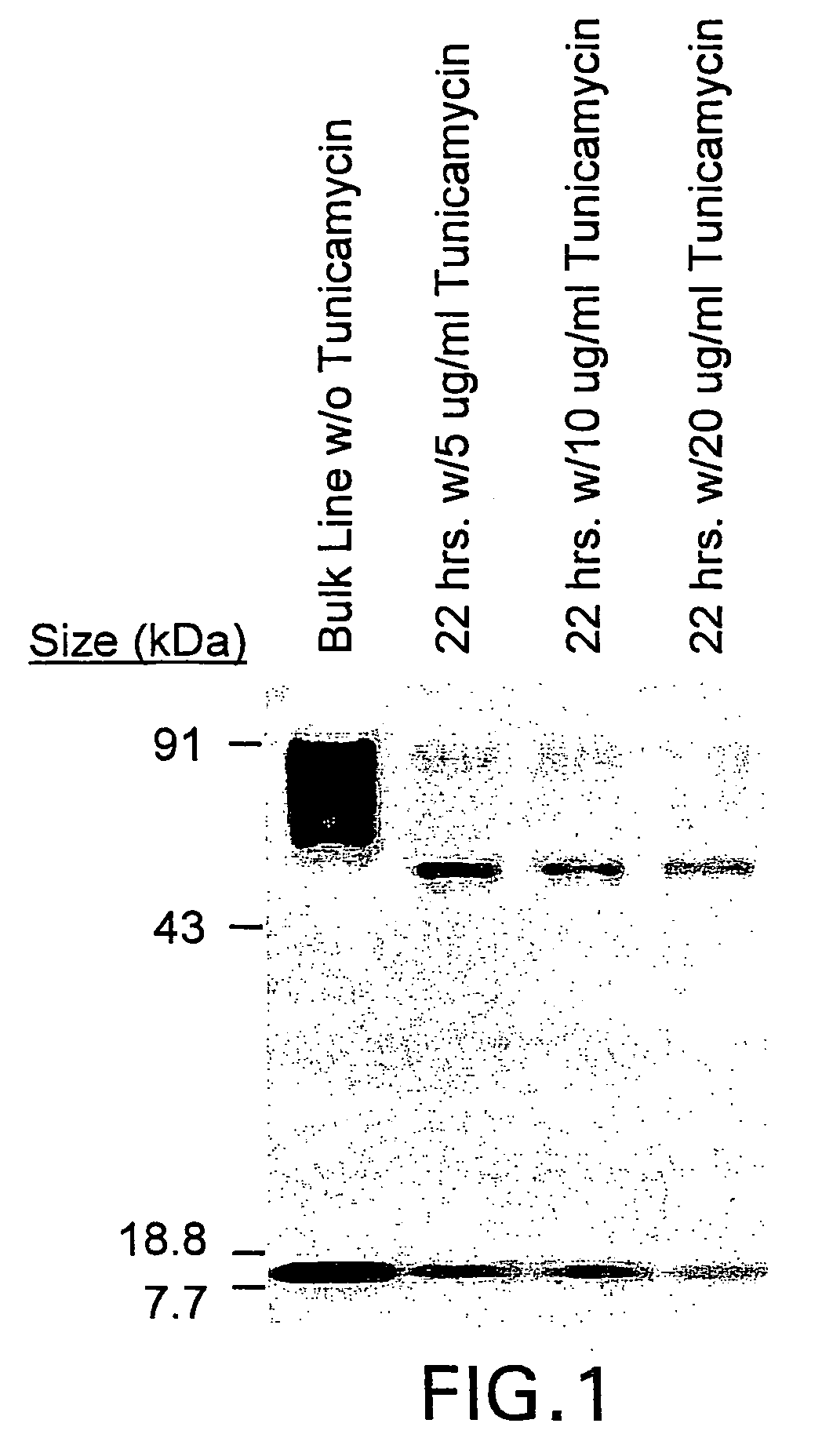
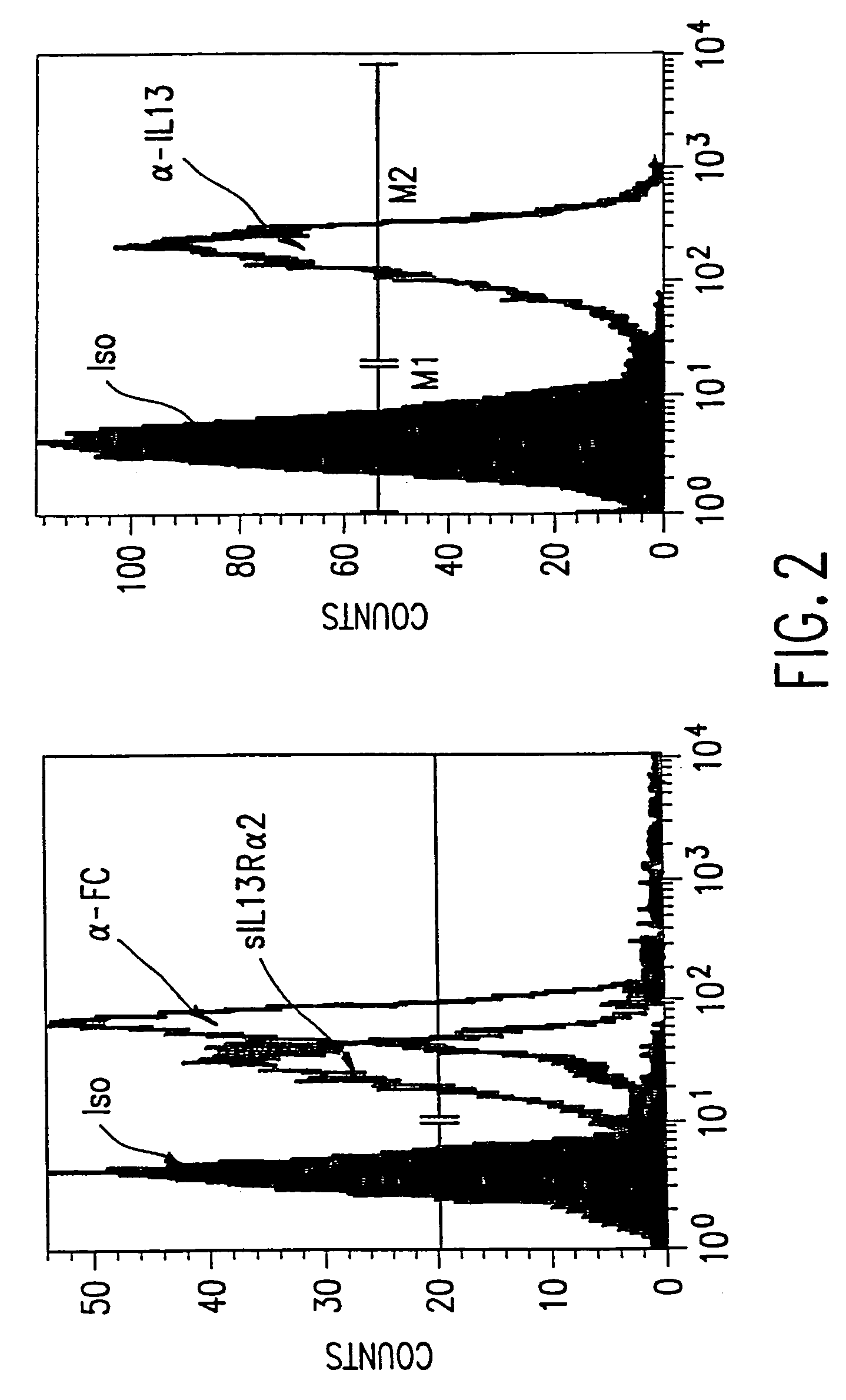
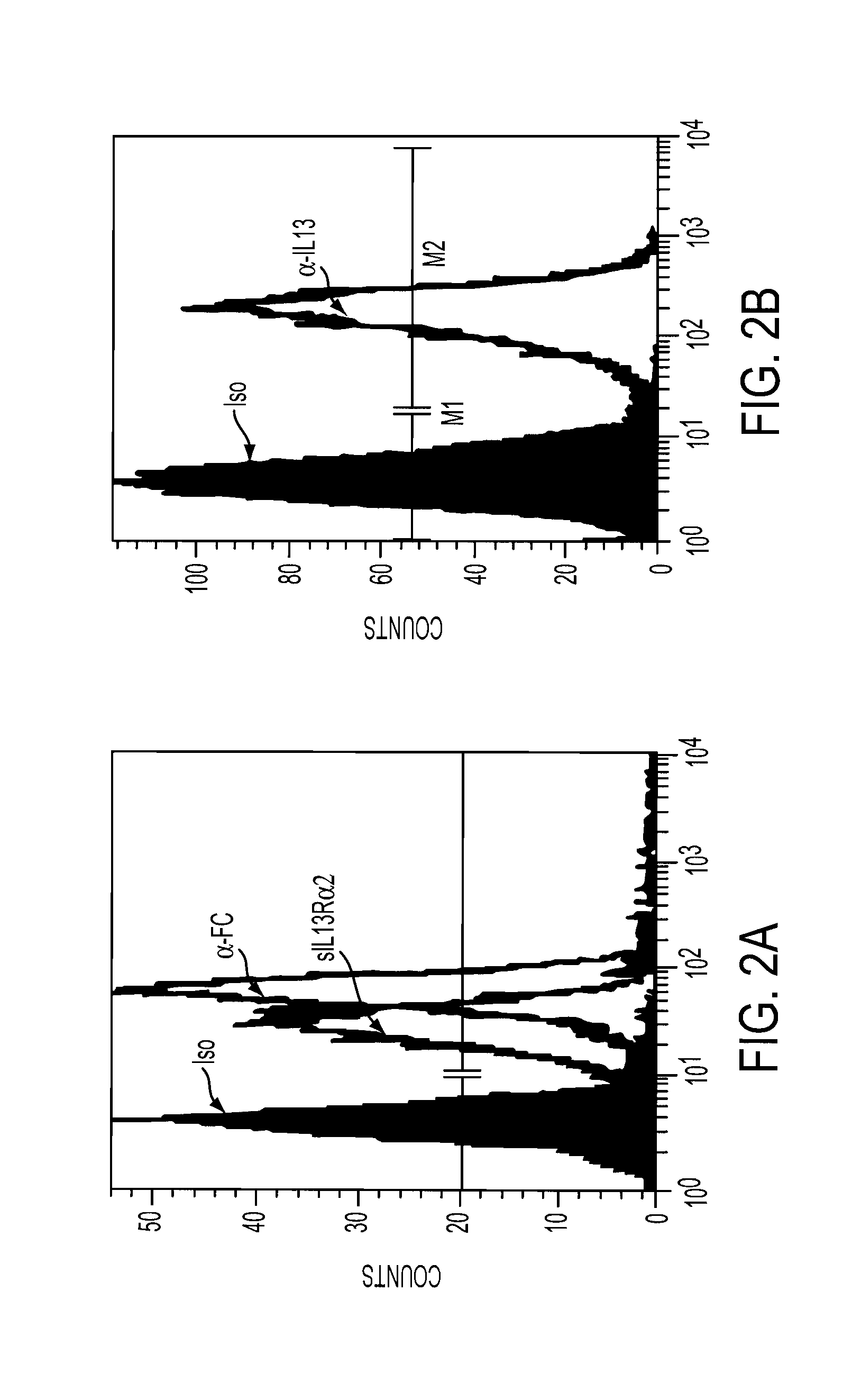
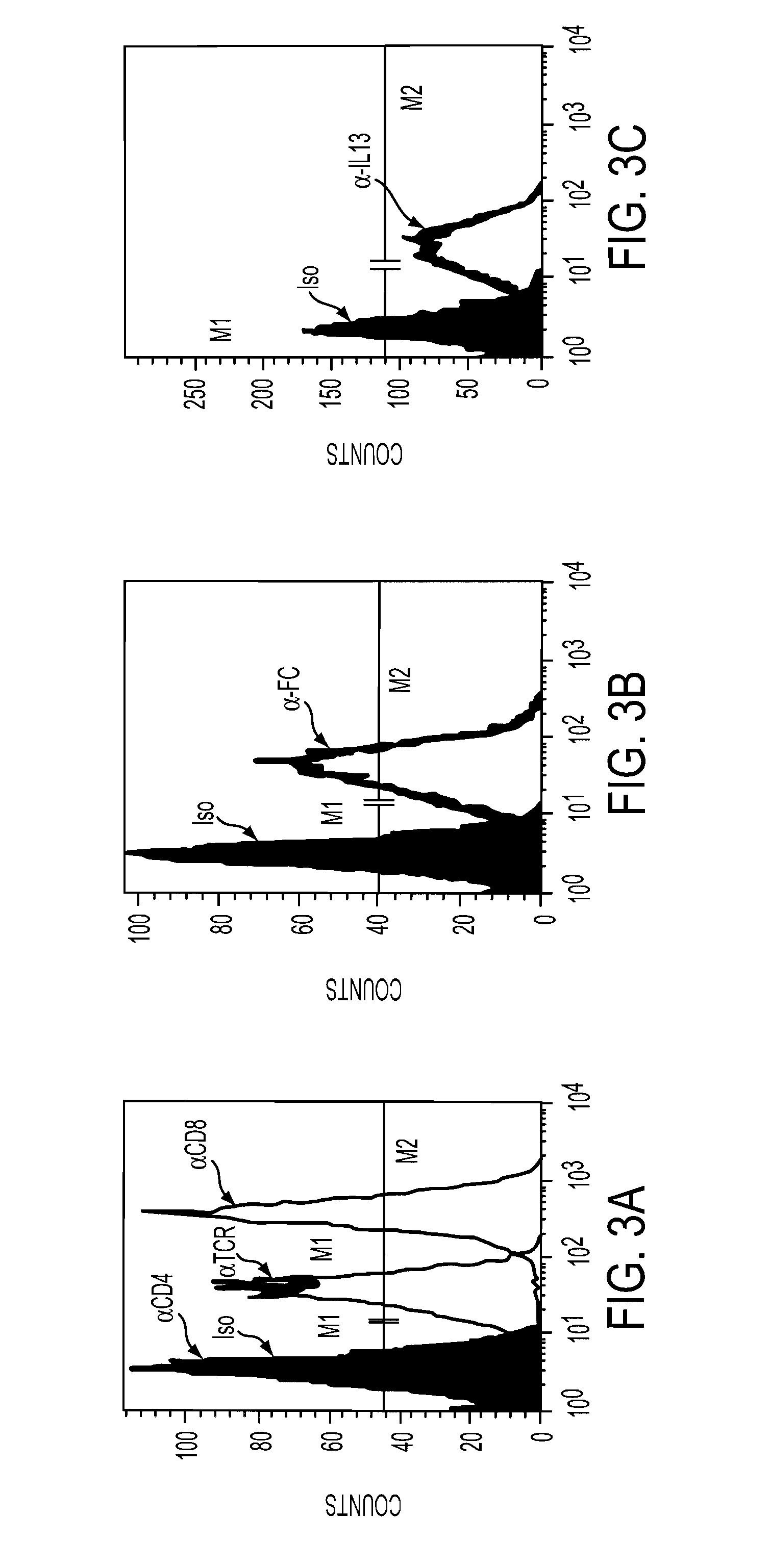
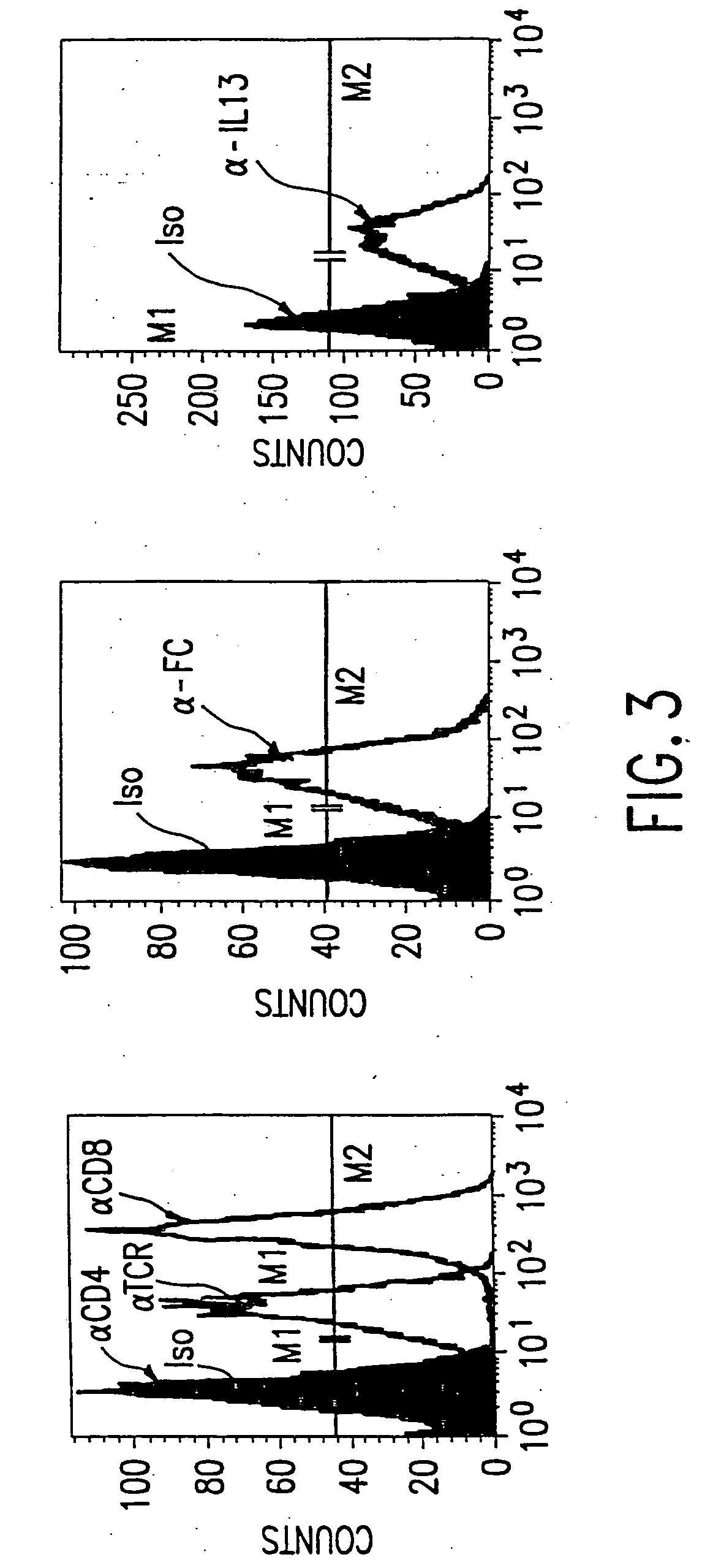
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signaling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human maligancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targeting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Chimeric receptors and uses thereof in immune therapy
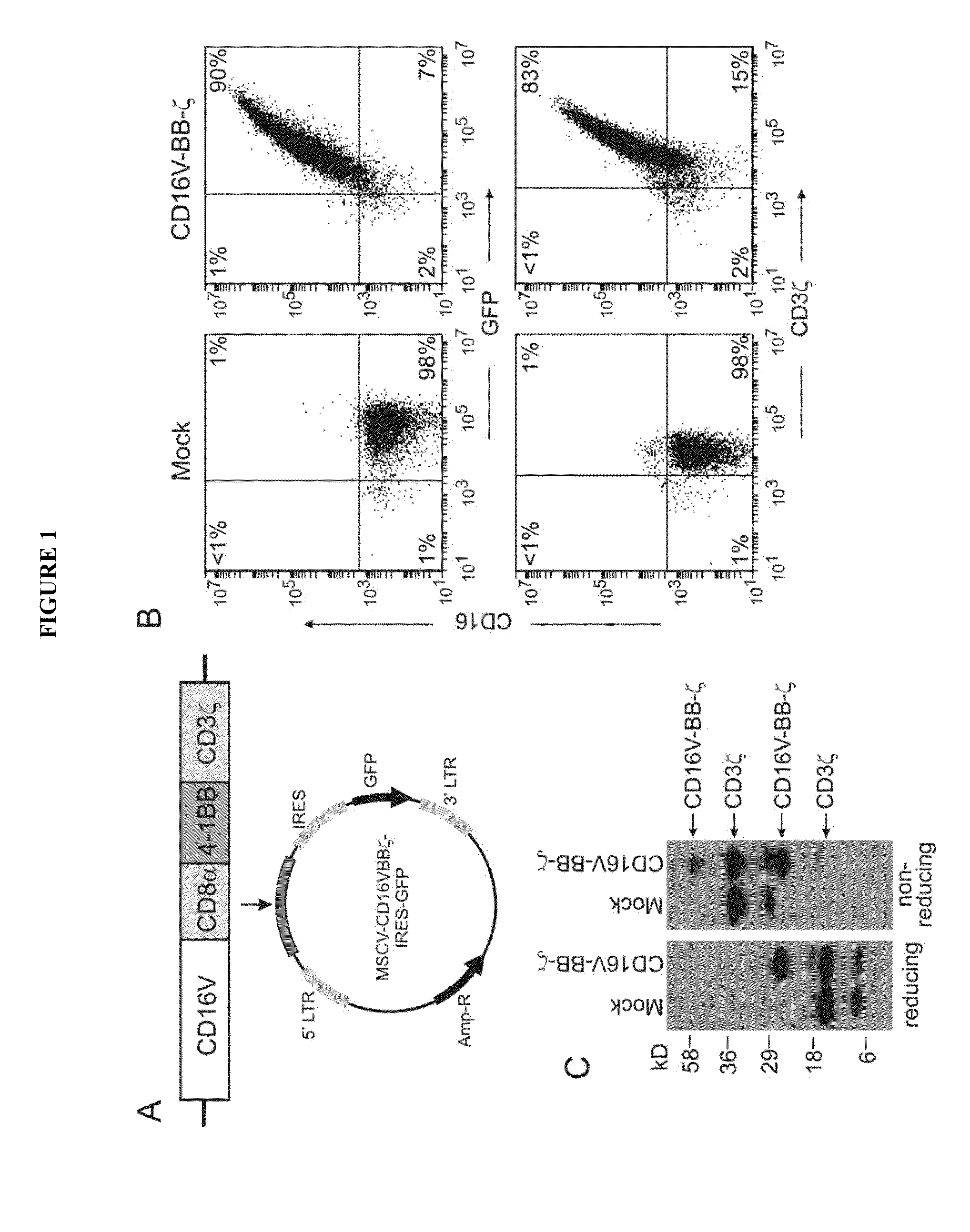
ActiveUS20150139943A1Good curative effectEnhanced ADCC activityVirusesPeptide/protein ingredientsCytotoxicityCD8
Owner:COGENT BIOSCIENCES INC +2
Chimeric antigen receptor
ActiveUS20140242701A1High expressionHigh cytotoxic activityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAntigen receptor
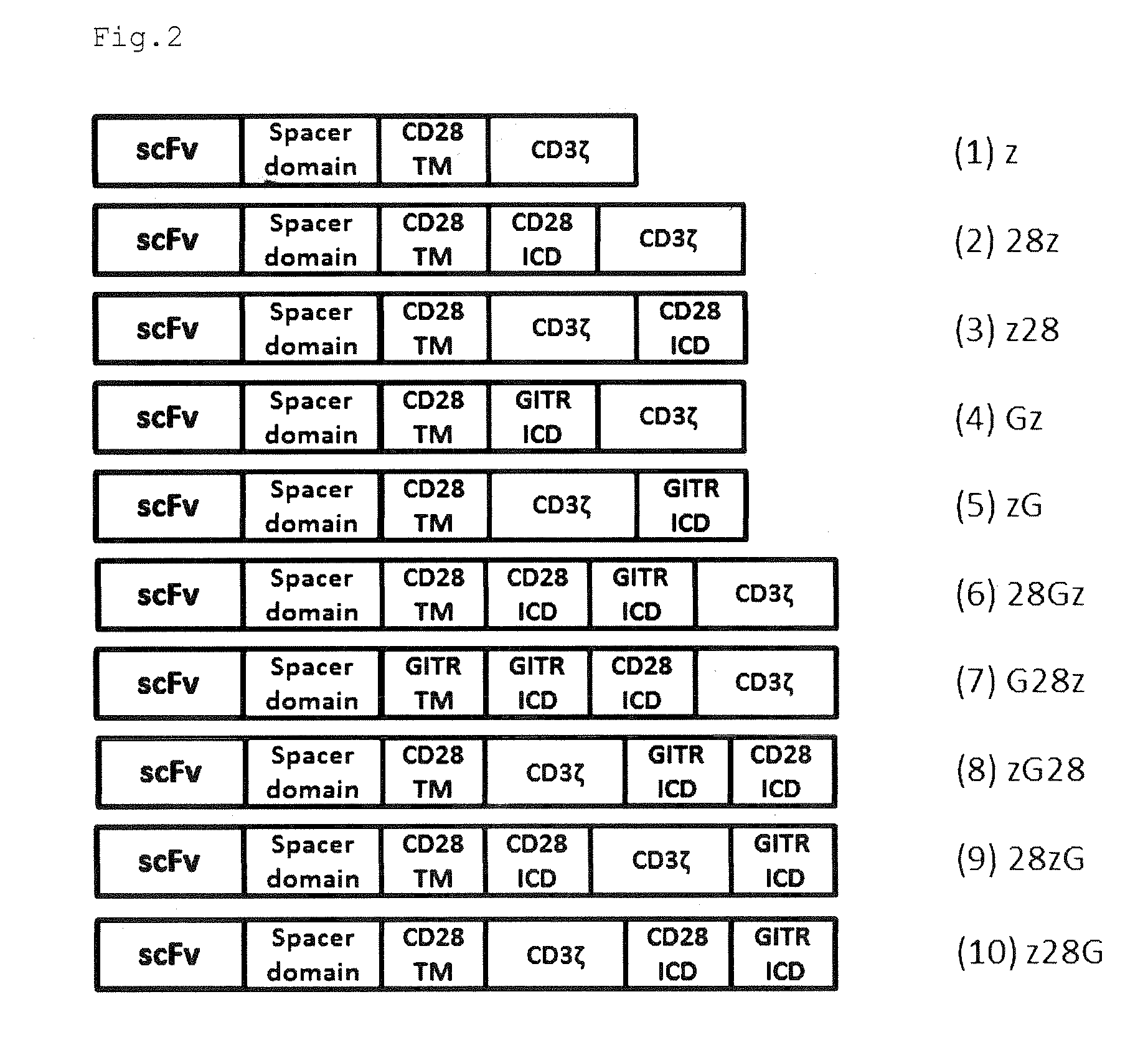
Provided are a chimeric antigen receptor comprising an extracellular domain capable of binding to an antigen, a transmembrane domain and at least one intracellular domain, the chimeric antigen receptor being characterized in that an intracellular domain of a glucocorticoid-induced tumor necrosis factor receptor (GITR) is contained as the intracellular domain; a nucleic acid encoding the chimeric antigen receptor; a cell expressing the chimeric antigen receptor; and a method for producing the cell.
Owner:MIE UNIVERSITY
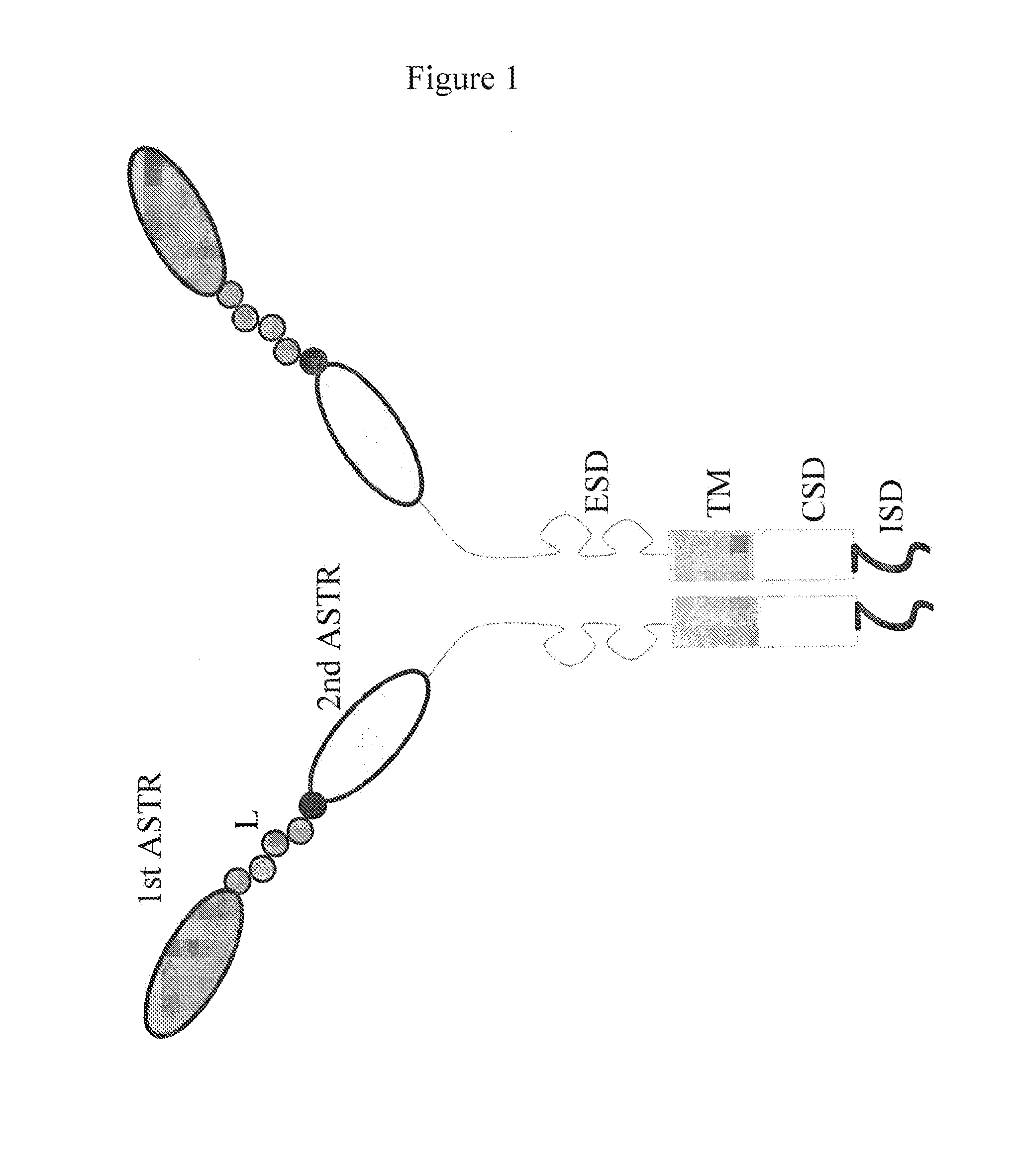
Bispecific chimeric antigen receptors and therapeutic uses thereof
ActiveUS20150038684A1Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingAntigen receptors
The invention is directed to a bispecific chimeric antigen receptor, comprising: (a) at least two antigen-specific targeting regions; (b) an extracellular spacer domain; (c) a transmembrane domain; (d) at least one co-stimulatory domain; and (e) an intracellular signaling domain, wherein each antigen-specific targeting region comprises an antigen-specific single chain Fv (scFv) fragment, and binds a different antigen, and wherein the bispecific chimeric antigen receptor is co-expressed with a therapeutic control. The invention also provides methods and uses of the bispecific chimeric antigen receptors.
Owner:SEATTLE CHILDRENS HOSPITAL
Anti-cd22 chimeric antigen receptors
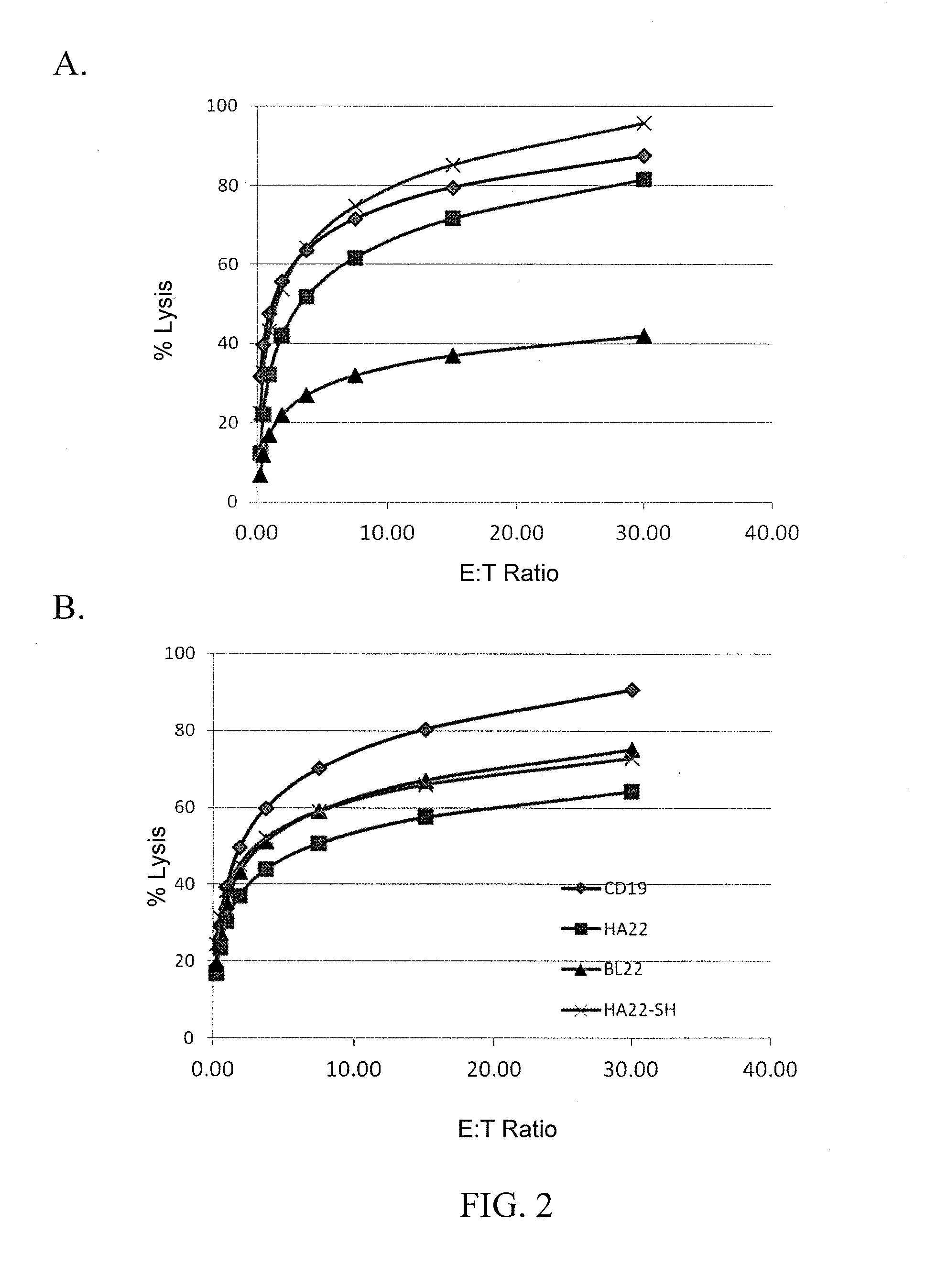
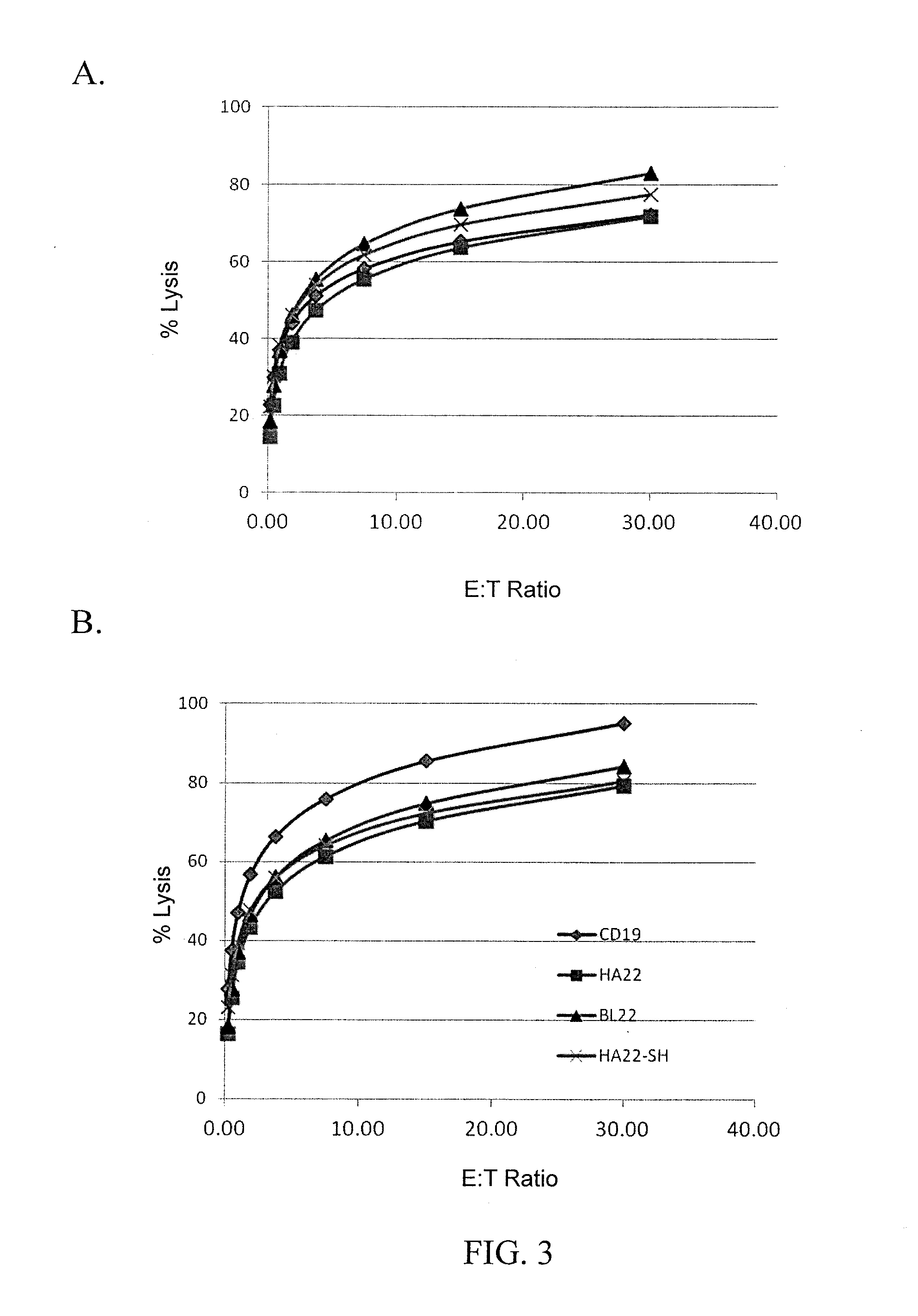
The disclosure provides a chimeric antigen receptor (CAR) comprising a) an antigen binding domain of HA22, a transmembrane domain, and an intracellular T cell signaling domain; or b) an antigen binding domain of BL22, a transmembrane domain, and an intracellular T cell signaling domain comprising CD28 and / or CD137. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a mammal and methods of treating or preventing cancer in a mammal are also disclosed.
Owner:UNITED STATES OF AMERICA
Methods for Treatment of Cancer
The present invention provides compositions and methods for treating cancer in a human. The invention includes relates to administering a genetically modified T cell to express a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions for Treatment of Cancer
The present invention provides compositions and methods for treating cancer in a human. The invention includes relates to administering a genetically modified T cell to express a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Use of icos-based cars to enhance antitumor activity and car persistence
ActiveUS20150017141A1Stimulate immune responseUnlikely can be integratedBiocideAntibody mimetics/scaffoldsBinding domainAntigen binding
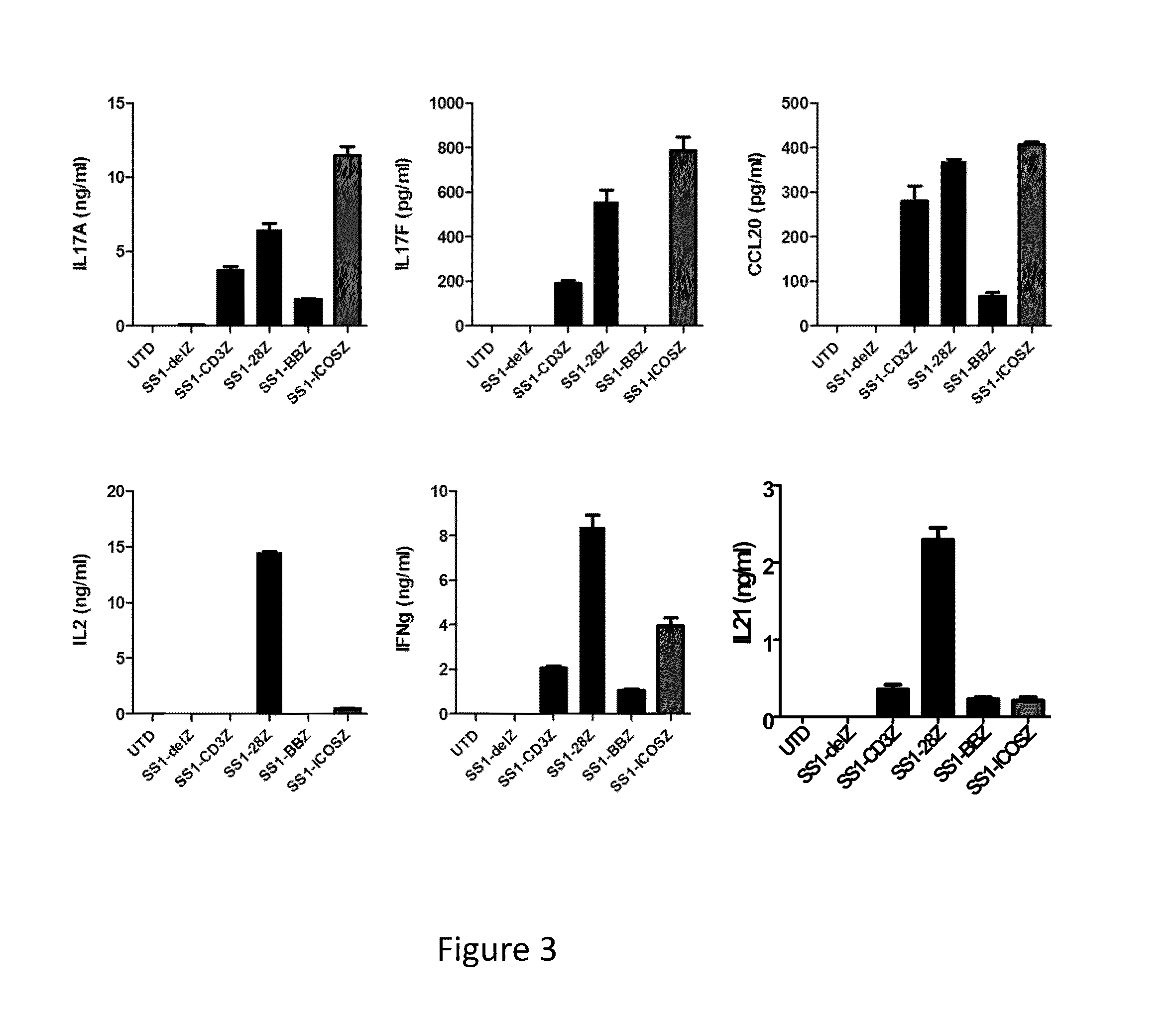
The present invention provides compositions and methods for treating cancer in a human. The invention includes administering a genetically modified Th17 cell to express a CAR having an antigen binding domain, a transmembrane domain, and an ICOS intracellular signaling domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
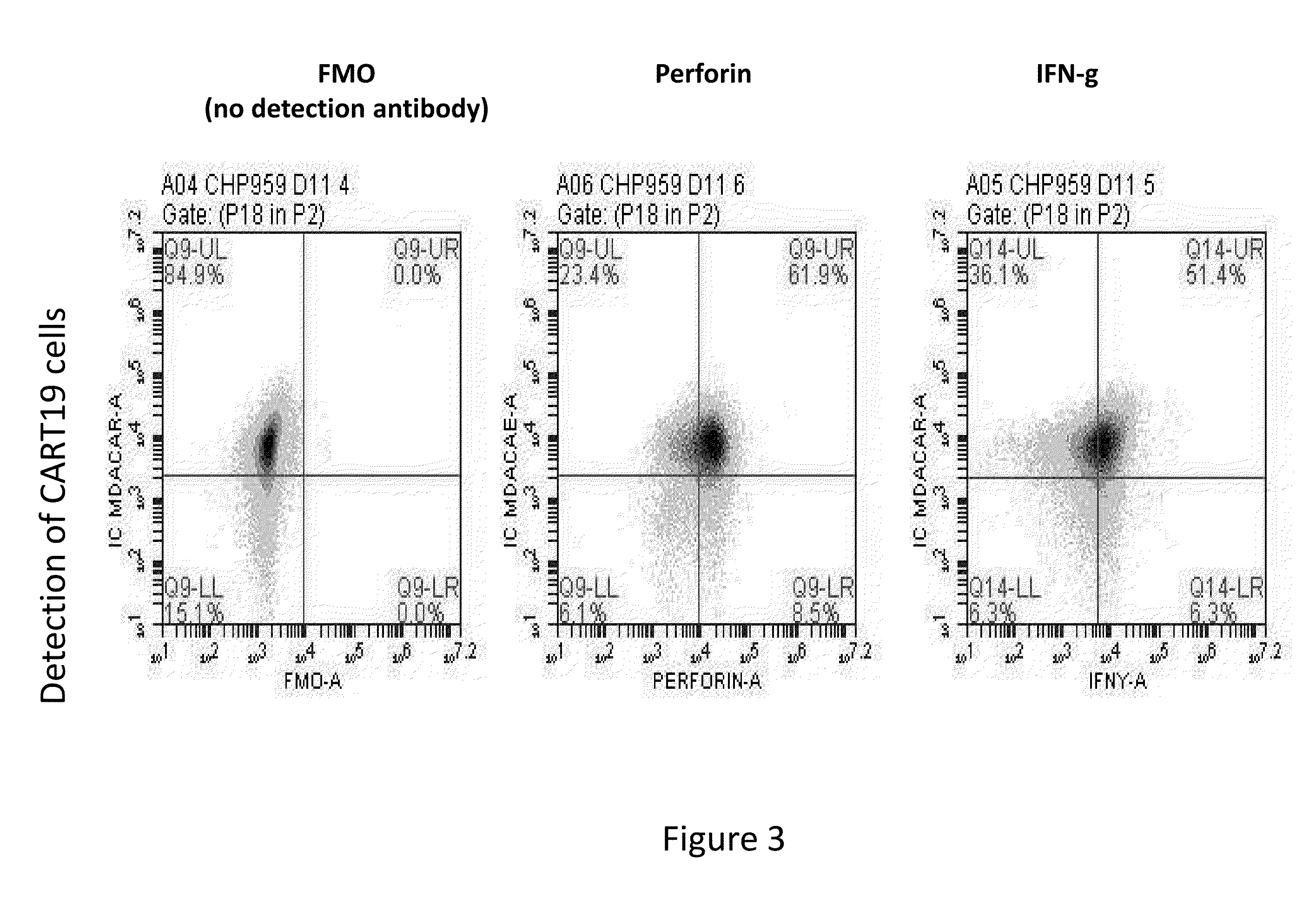
Use of cart19 to deplete normal b cells to induce tolerance
InactiveUS20150290244A1Peptide/protein ingredientsSnake antigen ingredientsBinding domainAntigen binding
The present invention provides compositions and methods for inducing tolerance in a human. The invention includes administering a genetically modified T cell expressing a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain.
Owner:LG CHEM LTD +1
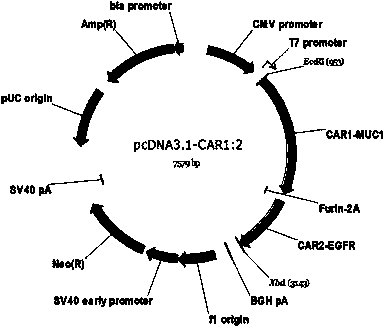
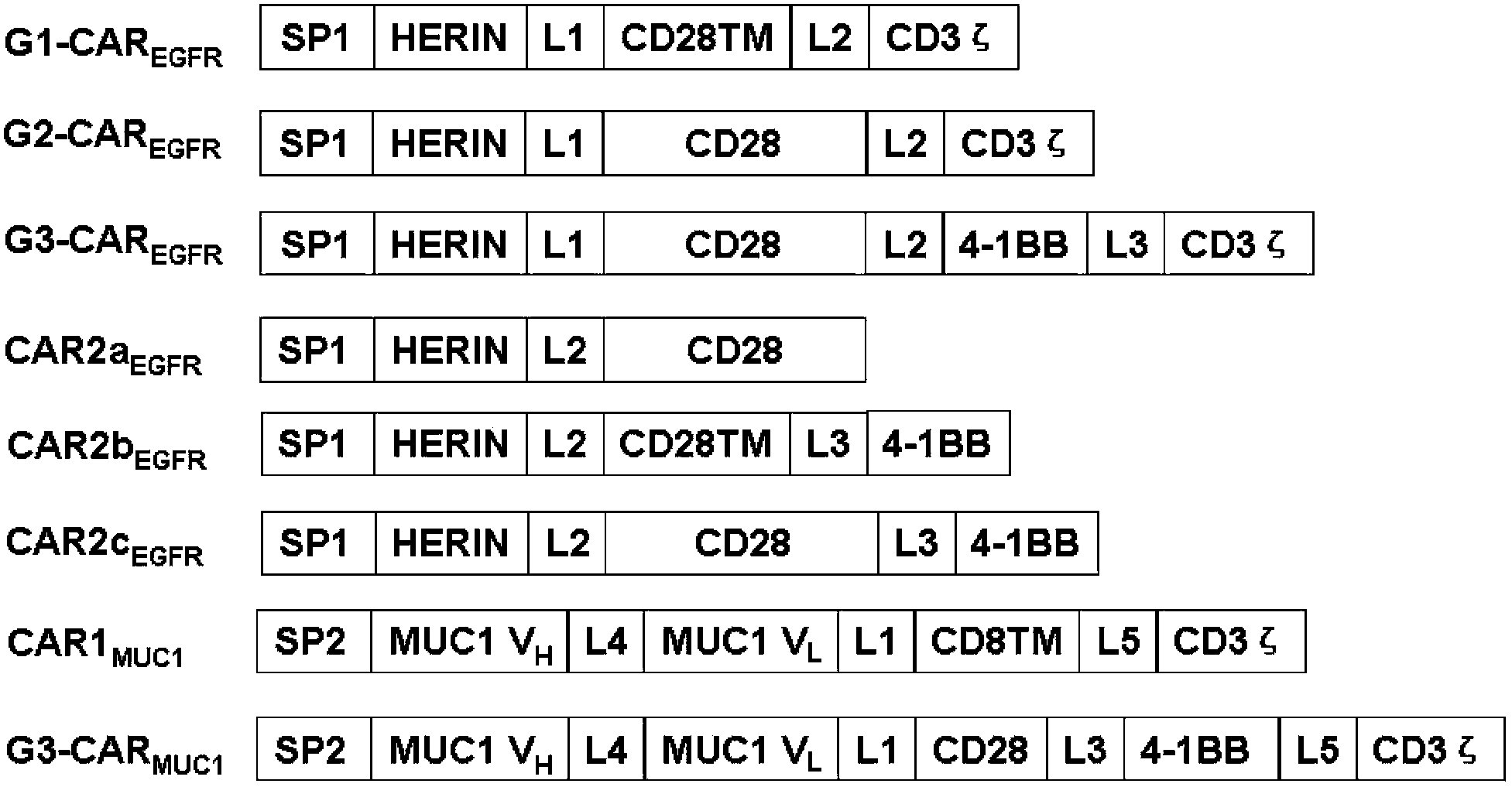
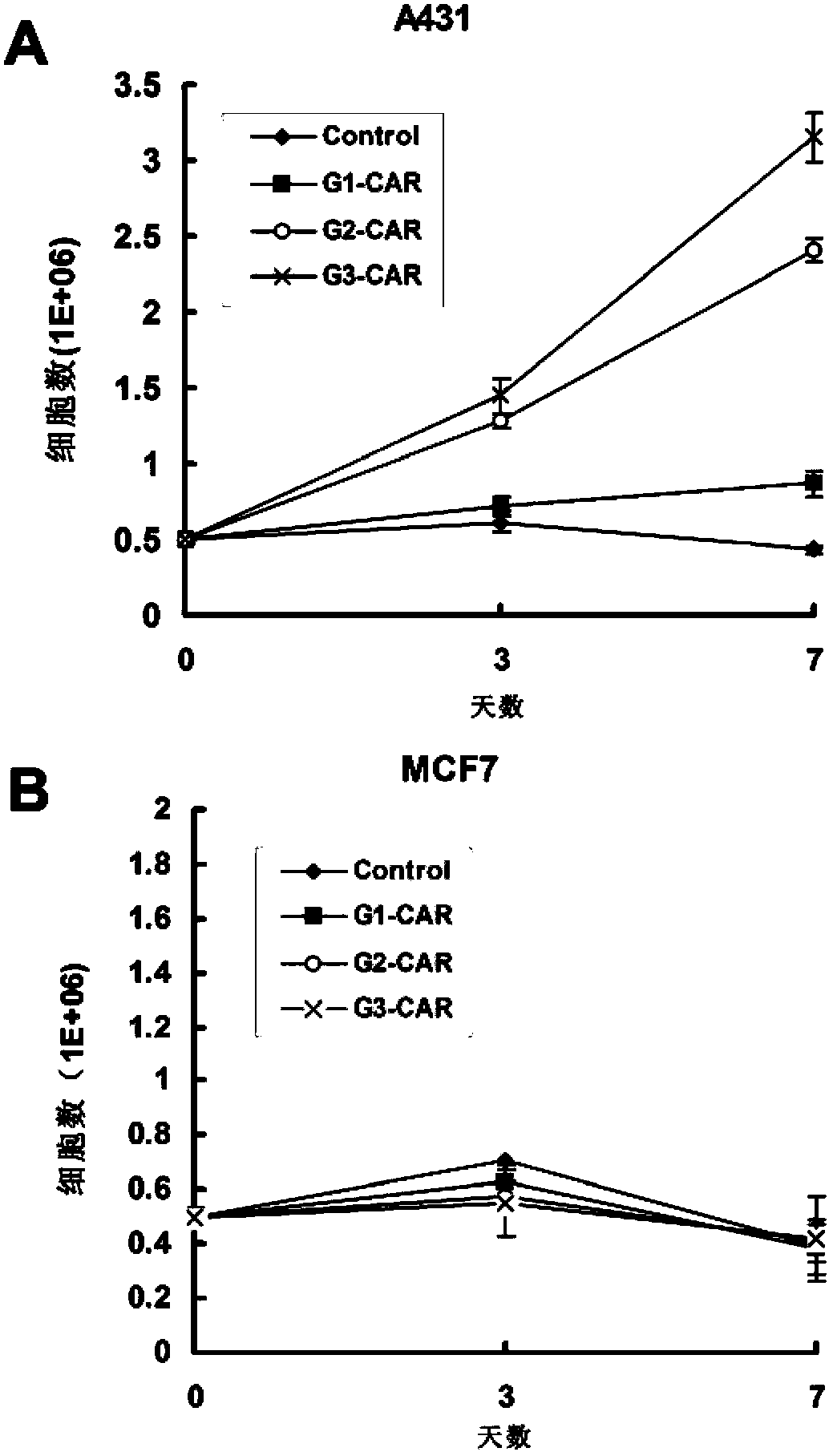
Chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and composition and uses thereof
ActiveCN103483453AFix security issuesImprove proliferative abilityPeptide/protein ingredientsGenetic material ingredientsEpidermal Growth Factor Receptor KinaseAntigen
The present invention belongs to the fields of molecular biology and immunology and relates to a chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and a composition and uses thereof The present invention particularly relates to the chimeric antigen receptor comprising polypeptides efficiently combining the EGFR family proteins and transmembrane domains, wherein the polypeptides efficiently combining EGFR family proteins comprise HERIN. The chimeric antigen receptor can promote the proliferation ability and killing effect of T cell at the premise of maintaining the killing specificity of the T cell.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
Bispecific chimeric antigen receptors and encoding polynucleotides thereof
ActiveUS9447194B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellularAntigen receptors
The invention is directed to a bispecific chimeric antigen receptor, comprising: (a) at least two antigen-specific targeting regions; (b) an extracellular spacer domain; (c) a transmembrane domain; (d) at least one co-stimulatory domain; and (e) an intracellular signaling domain, wherein each antigen-specific targeting region comprises an antigen-specific single chain Fv (scFv) fragment, and binds a different antigen, and wherein the bispecific chimeric antigen receptor is co-expressed with a therapeutic control. The invention also provides methods and uses of the bispecific chimeric antigen receptors.
Owner:SEATTLE CHILDRENS HOSPITAL
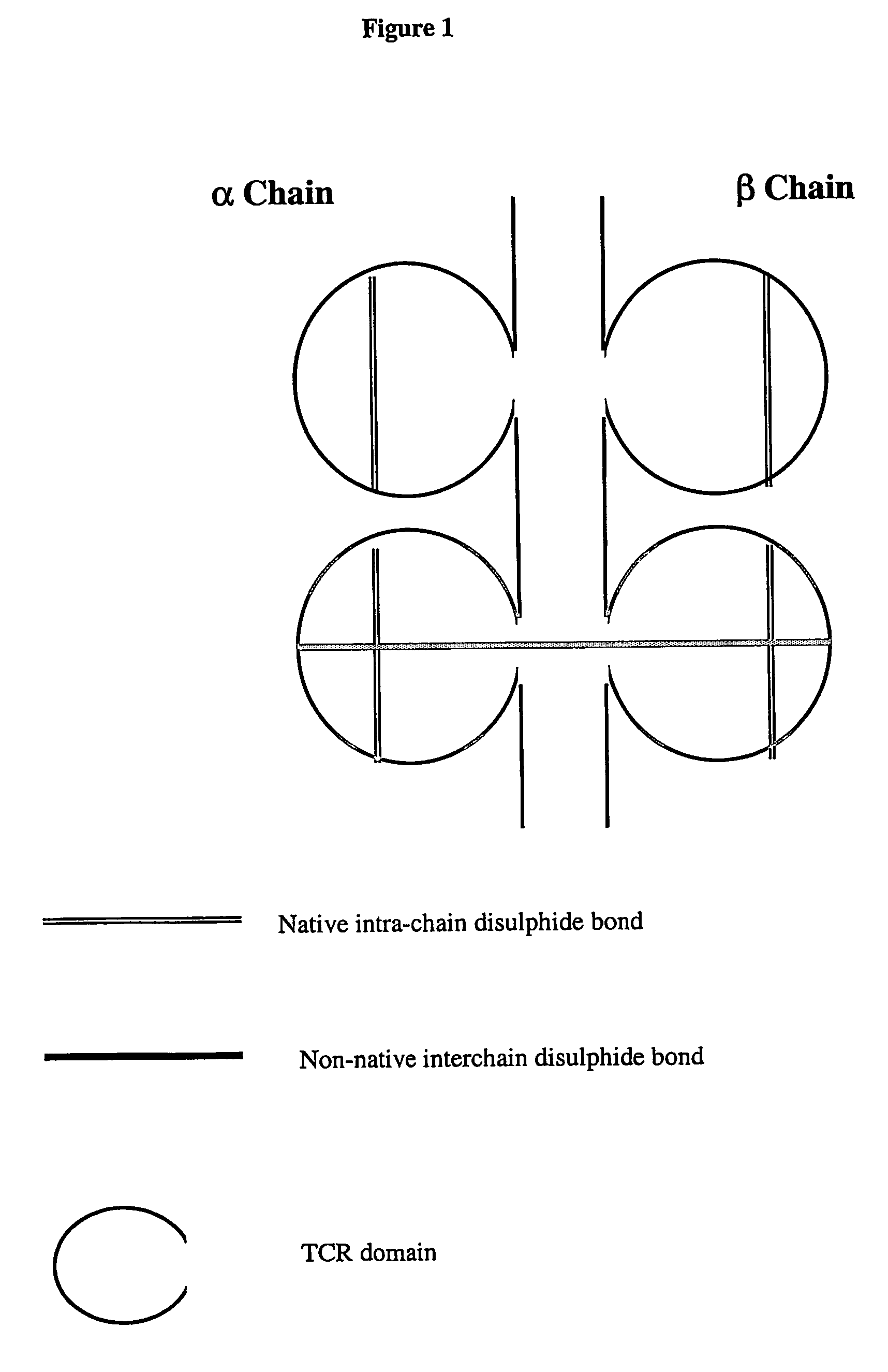
Modified soluble T cell receptor
The present invention provides a soluble T cell receptor (sTCR), which comprises (i) all or part of a TCR $g(a) chain, except the transmembrane domain thereof, and (ii) all or part of a TCR $g(b) chain, except the transmembrane domain thereof. (i) and (ii) each comprise a functional variable domain and at least a part of the constant domain of the TCR chain, and are linked by a disulphide bond between constant domain residues which is not present in native TCR, characterized in that the sTCR recognizes a CD1-antigen complex, a bacterial superantigen or a peptide-MHC / superantigen complex.
Owner:IMMUNOCORE LTD +1
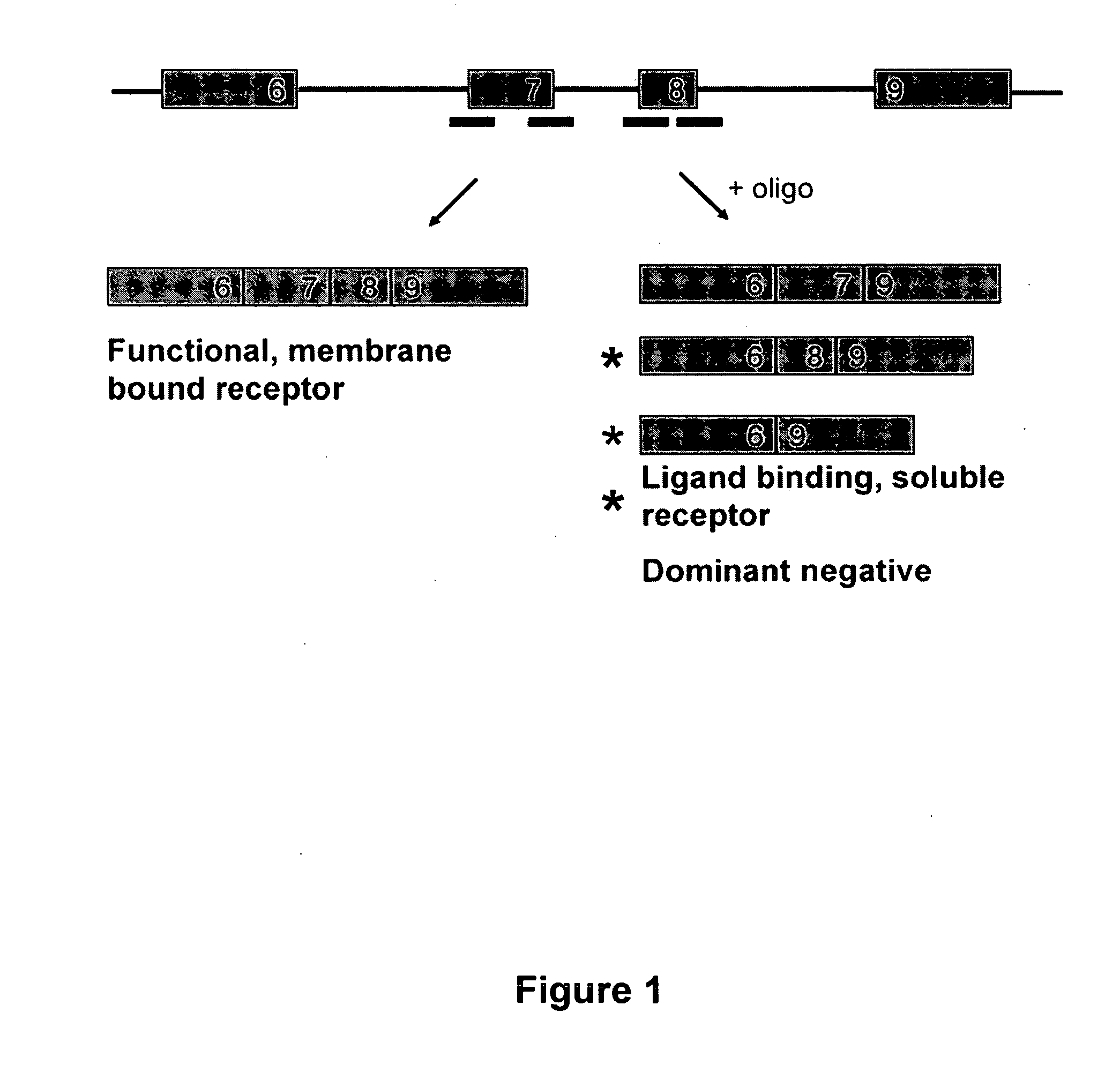
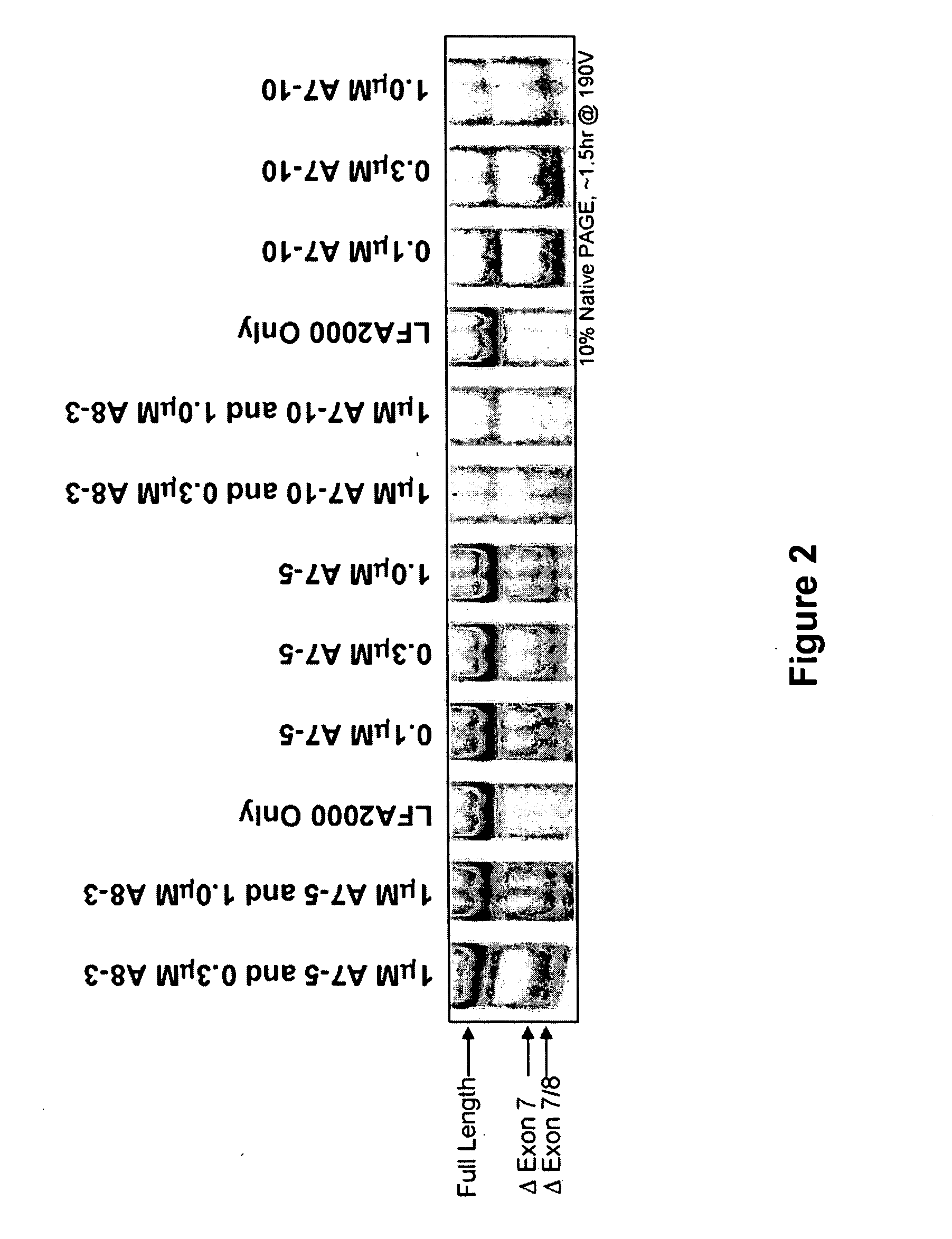
Splice switch oligomers for TNF superfamily receptors and their use in treatment of disease
InactiveUS20070105807A1High expressionReduce expressionAntibacterial agentsOrganic active ingredientsSplice switchingPrecursor mRNA
Methods and compositions are disclosed for controlling expression of TNF receptors (TNFR1 and TNFR2) and of other receptors in the TNFR superfamily using compounds that modulate splicing of pre-mRNA encoding these receptors. More specifically these compounds cause the removal of the transmembrane domains of these receptors and produce soluble forms of the receptor which act as an antagonist to reduce TNF-α activity or activity of the relevant ligand. Reducing TNF-α activity provides a method of treating or ameliorating inflammatory diseases or conditions associated with TNF-α activity. Similarly, diseases associated with other ligands can be treated in like manner. In particular, the compounds of the invention are splice-splice switching oligomers (SSOs) which are small molecules that are stable in vivo, hybridize to the RNA in a sequence specific manner and, in conjunction with their target, are not degraded by RNAse H.
Owner:SANTARIS PHARMA AS +3
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20090257994A1Negligible toxicityPotent and selectiveBiocidePeptide/protein ingredientsIntracellular signallingMalignancy
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human malignancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Novel chimeric antigen receptor and applications thereof
PendingCN108276493AMild release responseHigh ability to target and recognize tumor antigensPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsAntigen binding
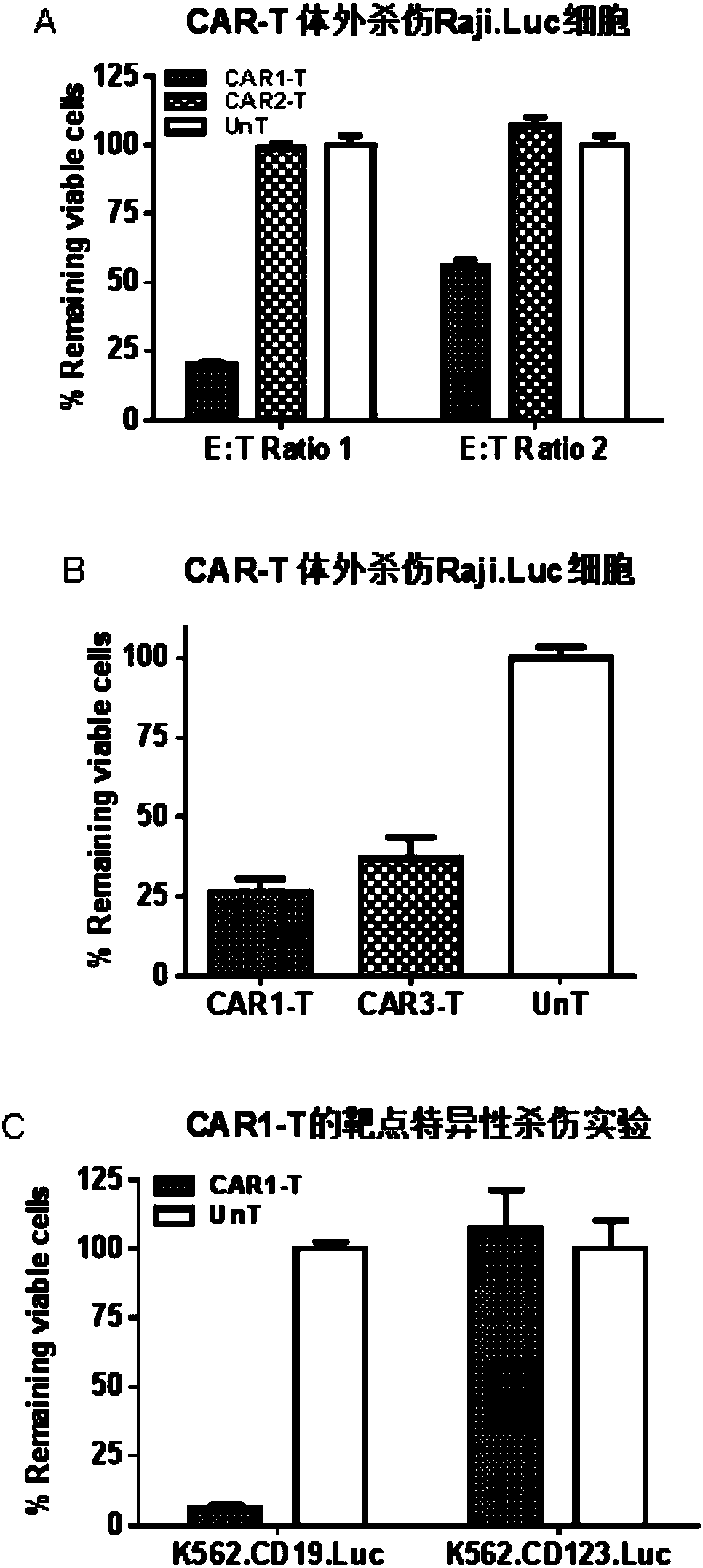
The present invention discloses a novel chimeric antigen receptor and applications thereof, wherein the novel chimeric antigen receptor comprises a signal peptide, an antigen binding domain, a transmembrane domain and an intracellular signal domain, and comprises a 4-1BB signal peptide and / or a 4-1BB molecular transmembrane domain. According to the present invention, a variety of chimeric antigenreceptor nucleic acid sequences are separated and purified, the chimeric antigen receptor specifically for CD19 malignant tumor antigens and the CAR-T cells are provided, and the blood cell line malignant tumor killing test results show that the tumor-cell-targeting ability of immune cells is significantly enhanced, and the tumor cell killing activity is enhanced.
Owner:NANJING LEGEND BIOTECH CO LTD
Method and compositions for cellular immunotherapy
ActiveUS20150306141A1Enhanced cytokine productionIncreased proliferationPeptide/protein ingredientsAntibody mimetics/scaffoldsIn vivoTransmembrane domain
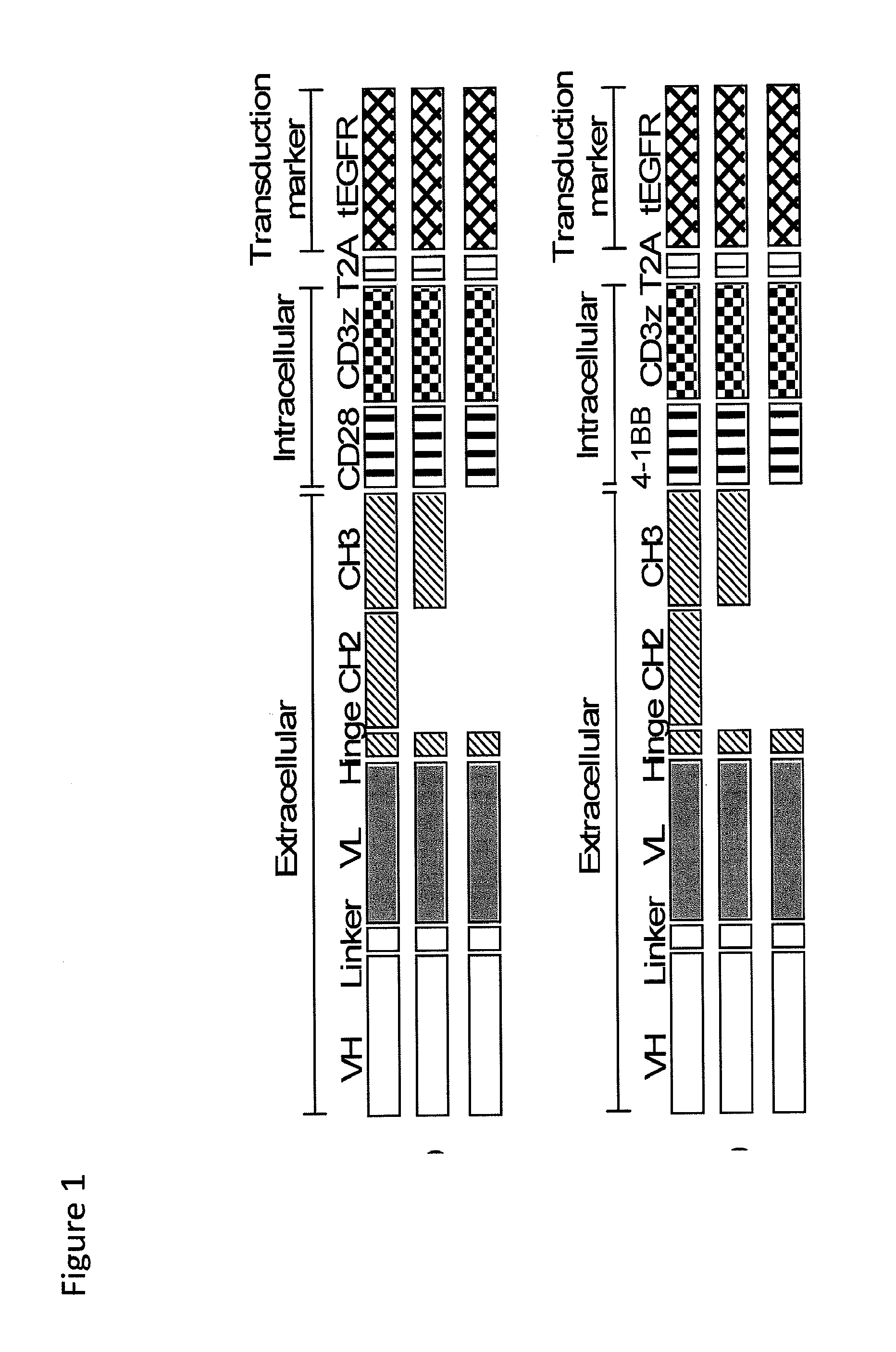
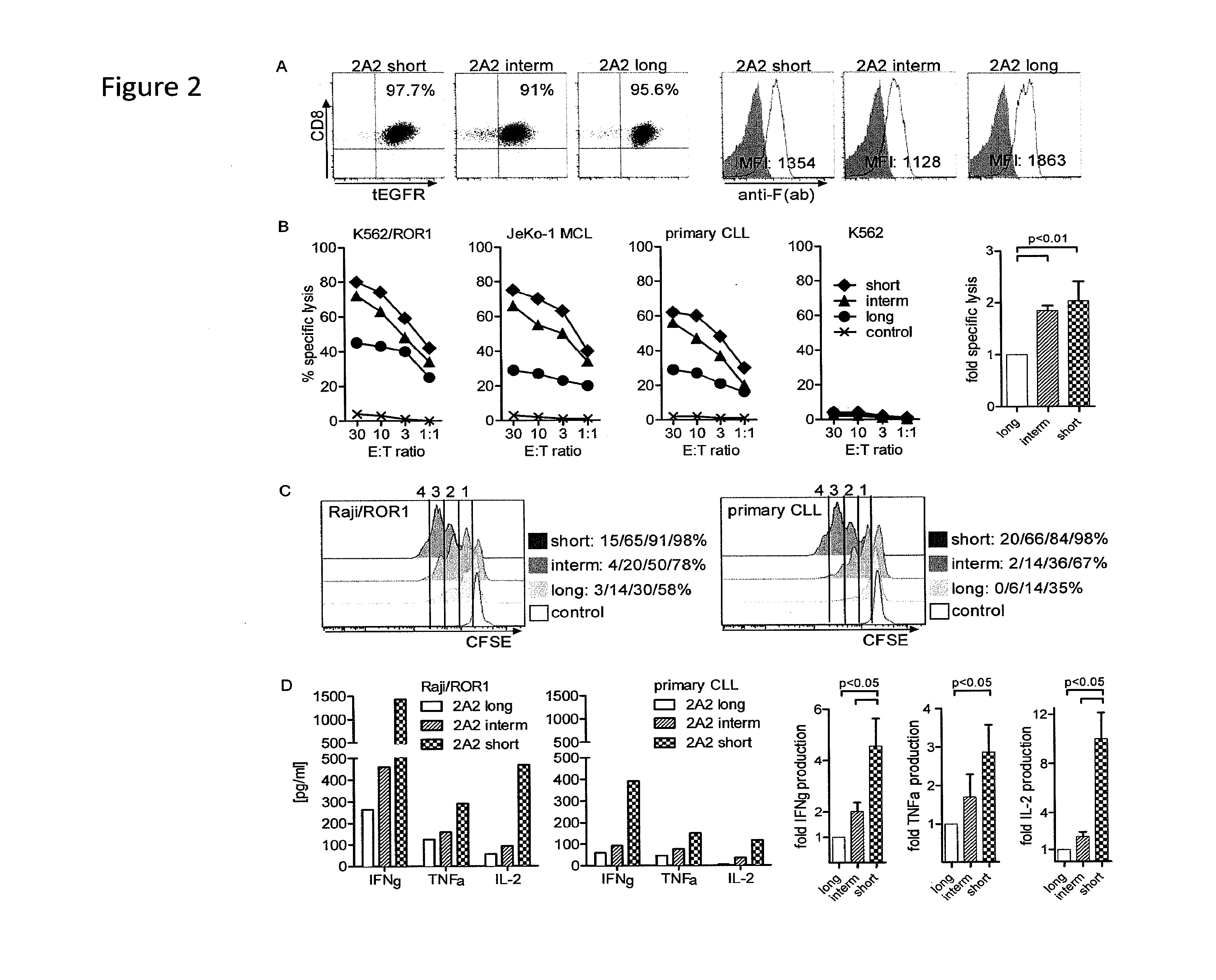
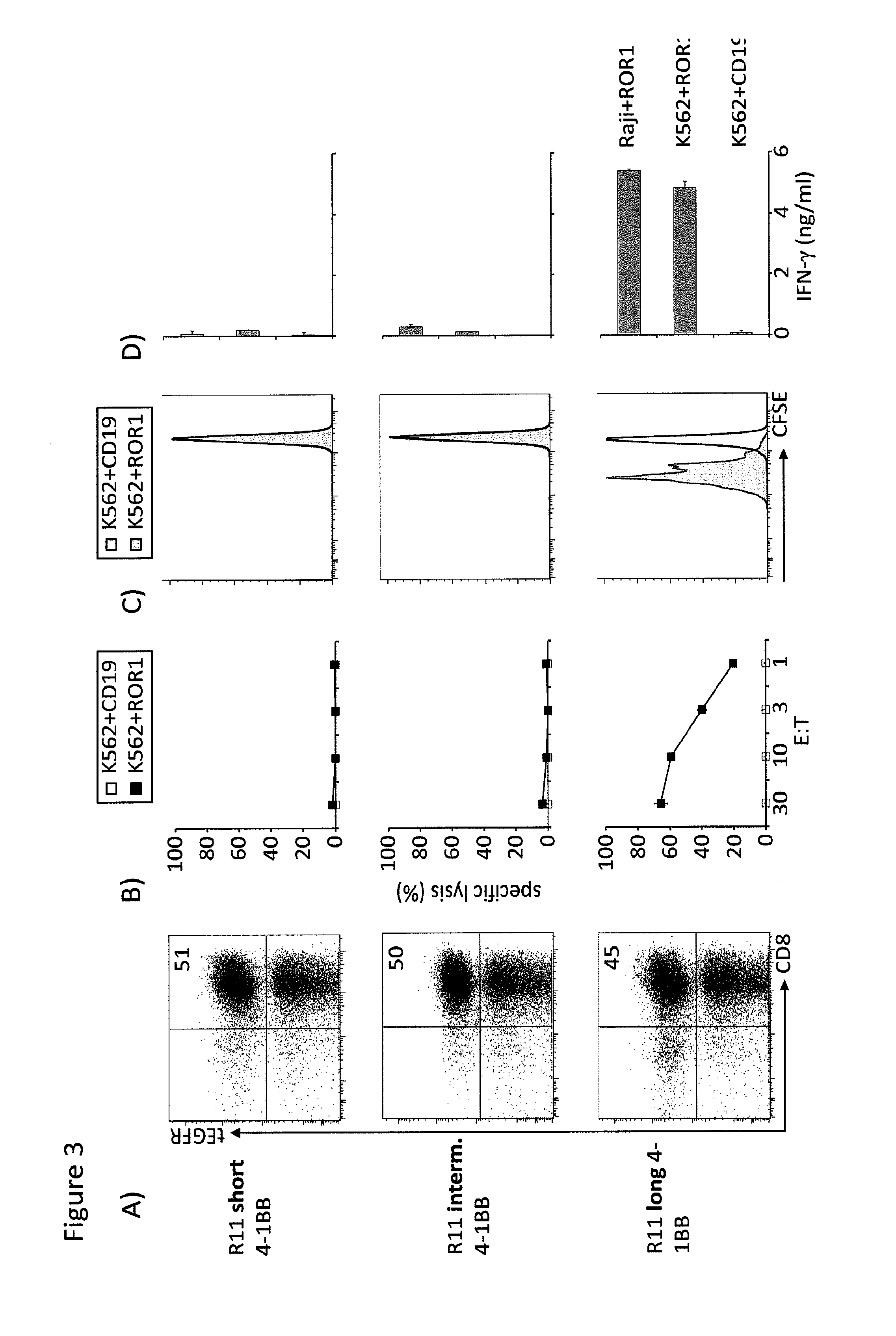
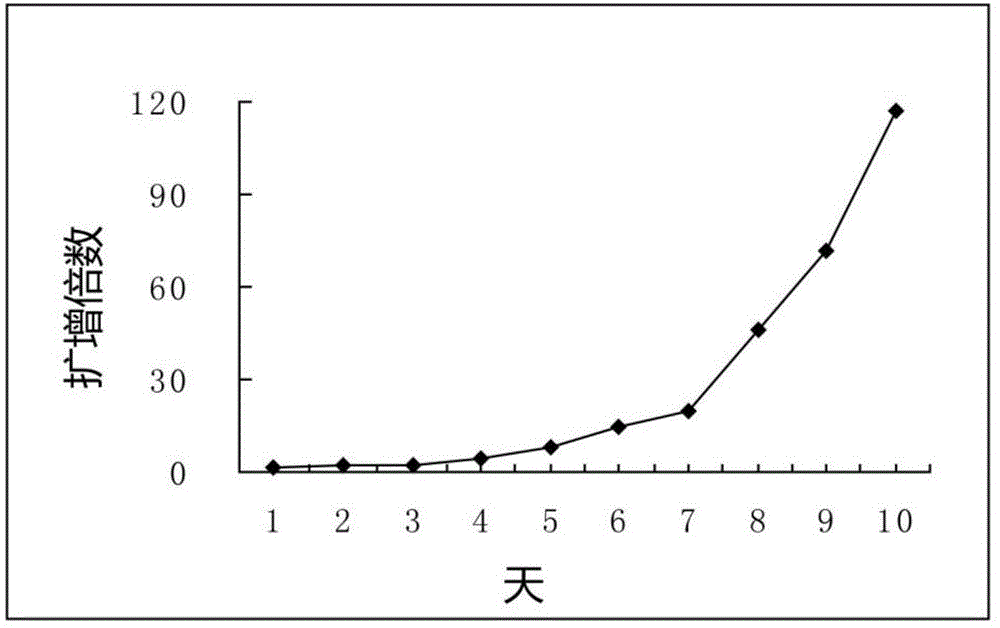
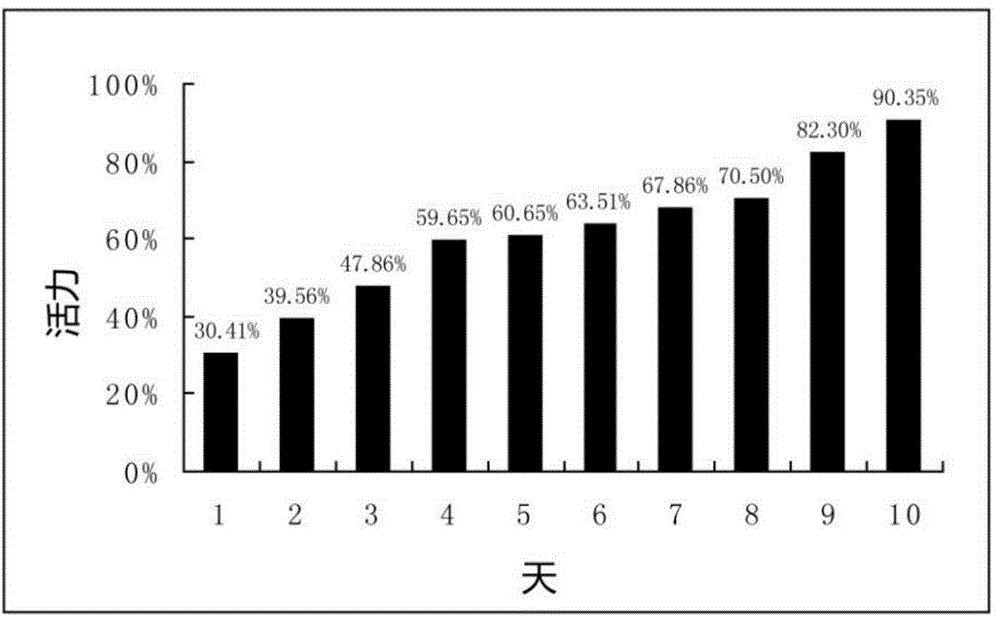
The present invention provides nucleic acids, vectors, host cells, methods and compositions to confer and / or augment immune responses mediated by cellular immunotherapy, such as by adoptively transferring CD8+ central memory T cells or combinations of central memory T cells with CD4+ T cells that are genetically modified to express a chimeric receptor. In embodiments the genetically modified host cell comprises a nucleic acid comprising a polynucleotide coding for a ligand binding domain, a polynucleotide comprising a customized spacer region, a polynucleotide comprising a transmembrane domain, and a polynucleotide comprising an intracellular signaling domain. It has been surprisingly found that the length of the spacer region can affects the ability of chimeric receptor modified T cells to recognize target cells in vitro and affects in vivo efficacy of the chimeric receptor modified T cells. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:SEATTLE CHILDRENS HOSPITAL +1
Chimeric antigen receptor with stable antigen binding units, method for preparing chimeric antigen receptor and application thereof
ActiveCN104829733APeptide/protein ingredientsPharmaceutical non-active ingredientsTumor targetAntigen receptors
The invention relates to a chimeric antigen receptor with stable antigen binding units. The chimeric antigen receptor comprises the extracellular antigen binding units, trans-membrane domains and intracellular co-stimulation signal domains. The antigen binding units can be stably expressed as compared with specific tumor targets and comprise heavy chains and light chains which are connected with one another by hinges of SEQ ID NO.2 (sequence identity number.2) amino acid sequence codes. The invention further relates to cells for expressing the receptor and application of the receptor.
Owner:GUANGZHOU BIO GENE TECH CO LTD
Compositions that include hemagglutinin, methods of making and methods of use thereof
InactiveUS20070253982A1Stimulate immune responseCost effectiveSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininToll-like receptor
Owner:VAXINNATE
Toxicity Management for Anti-Tumor Activity of CARs
InactiveUS20150202286A1Reducing and avoiding adverse effectReducing and avoiding adverseOrganic active ingredientsBiocideAbnormal tissue growthAntigen binding
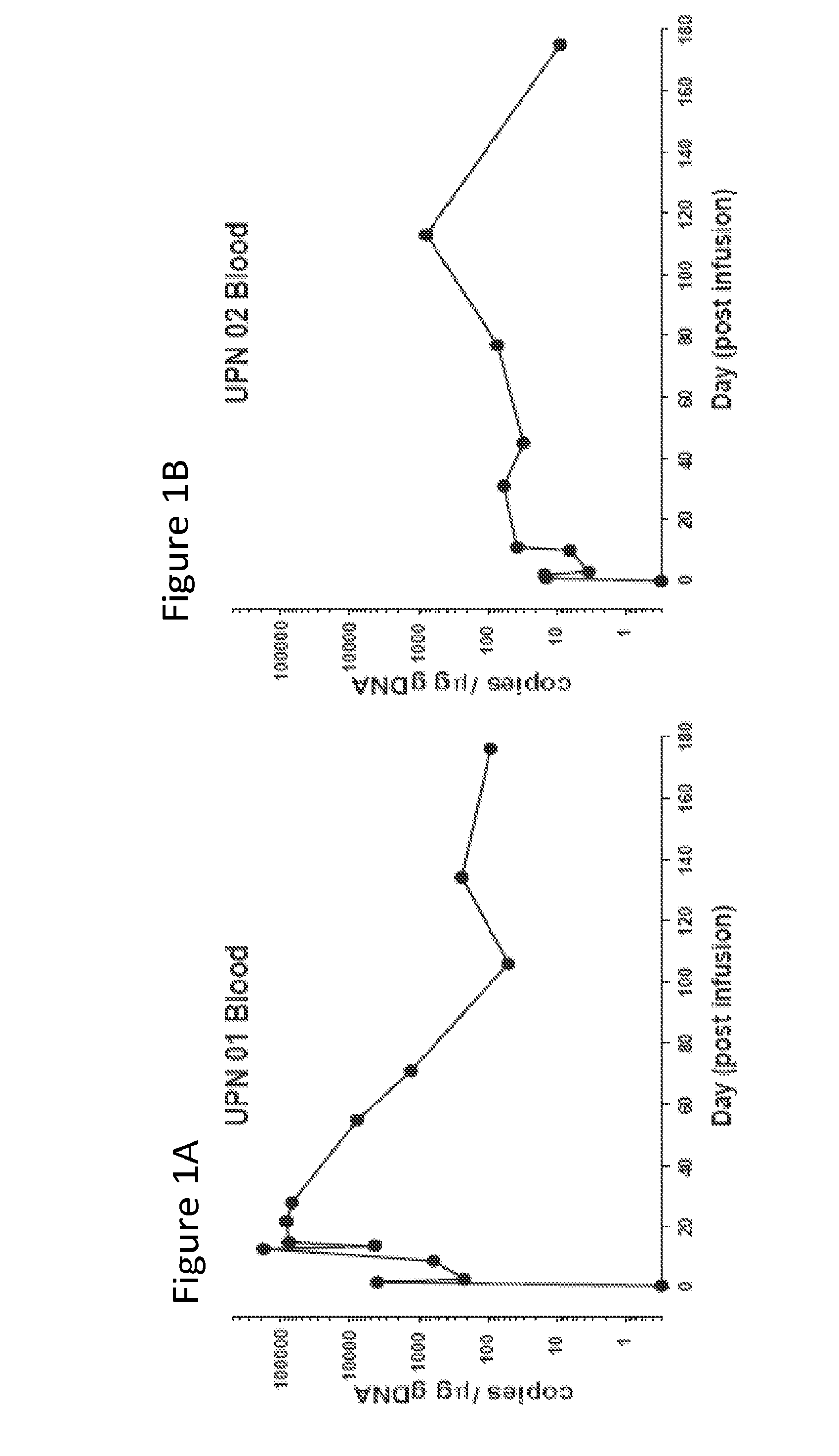
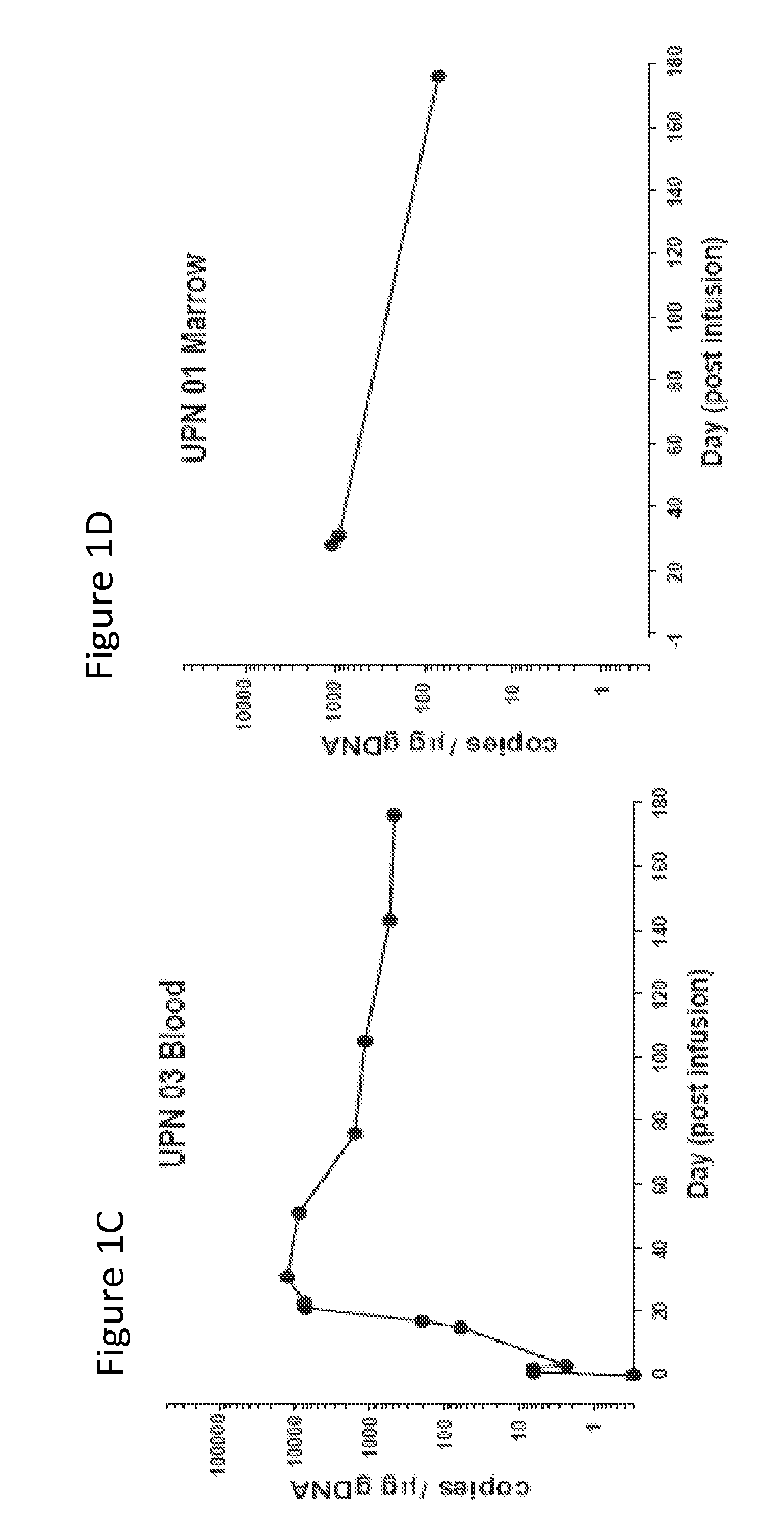
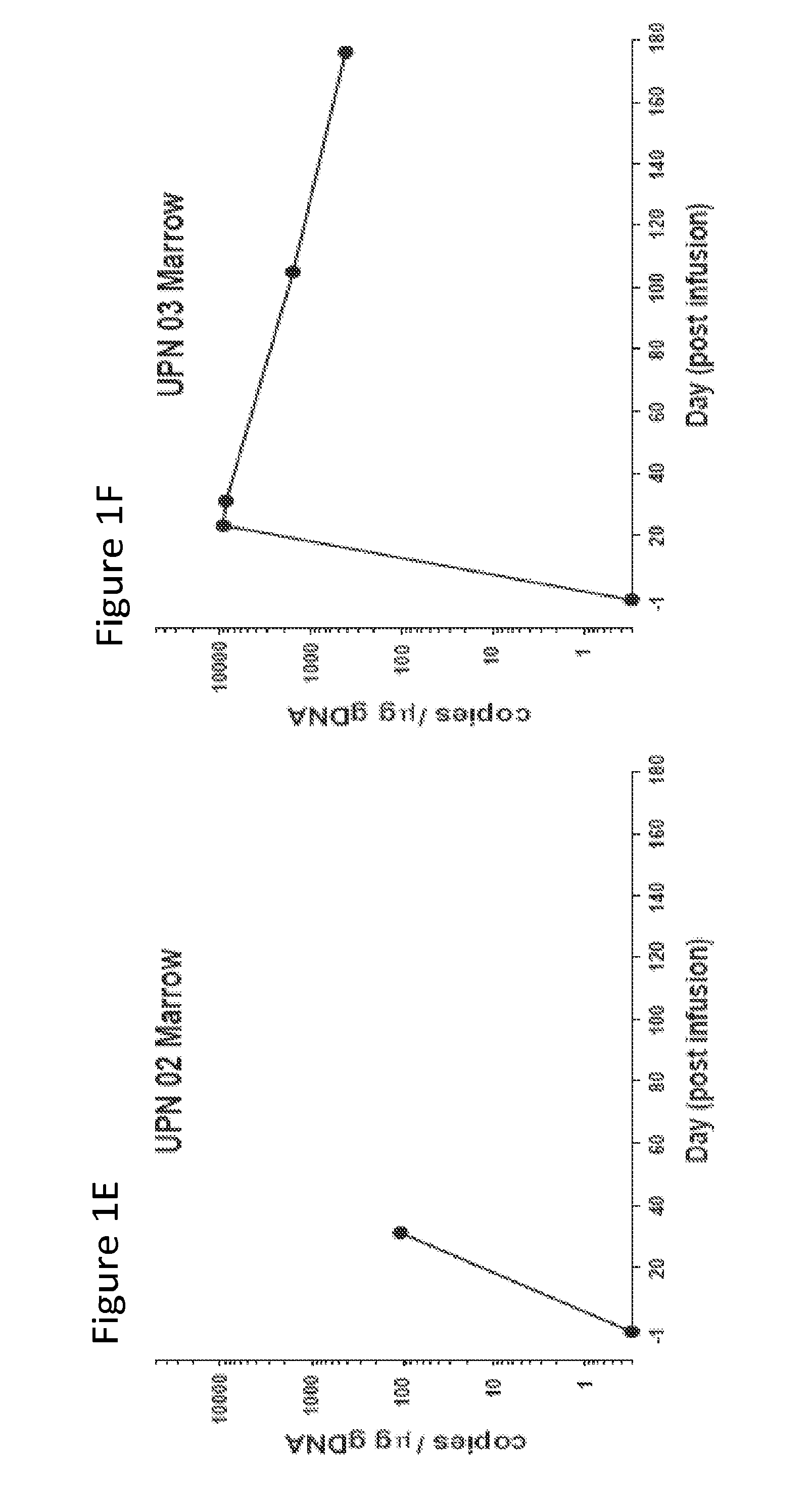
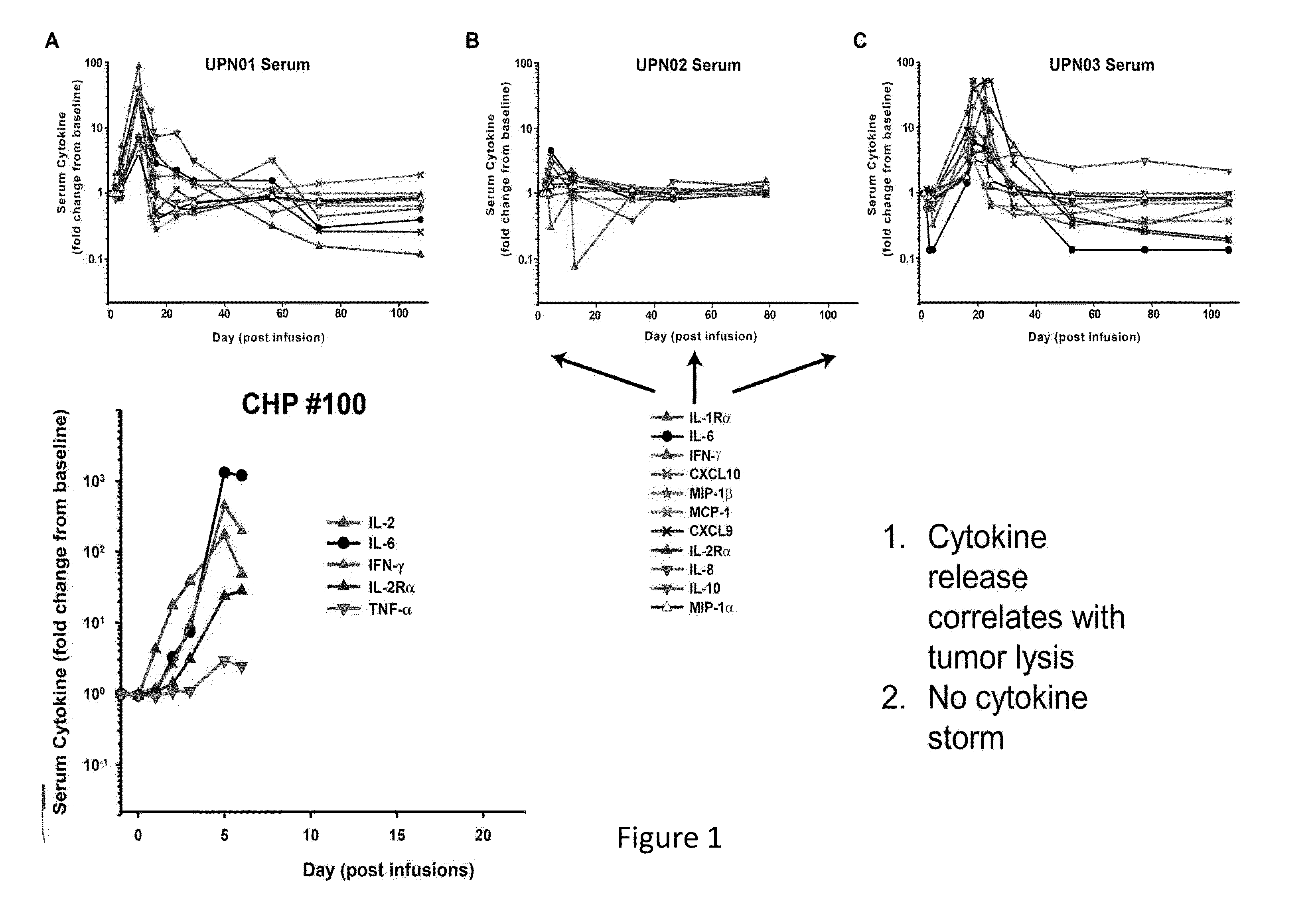
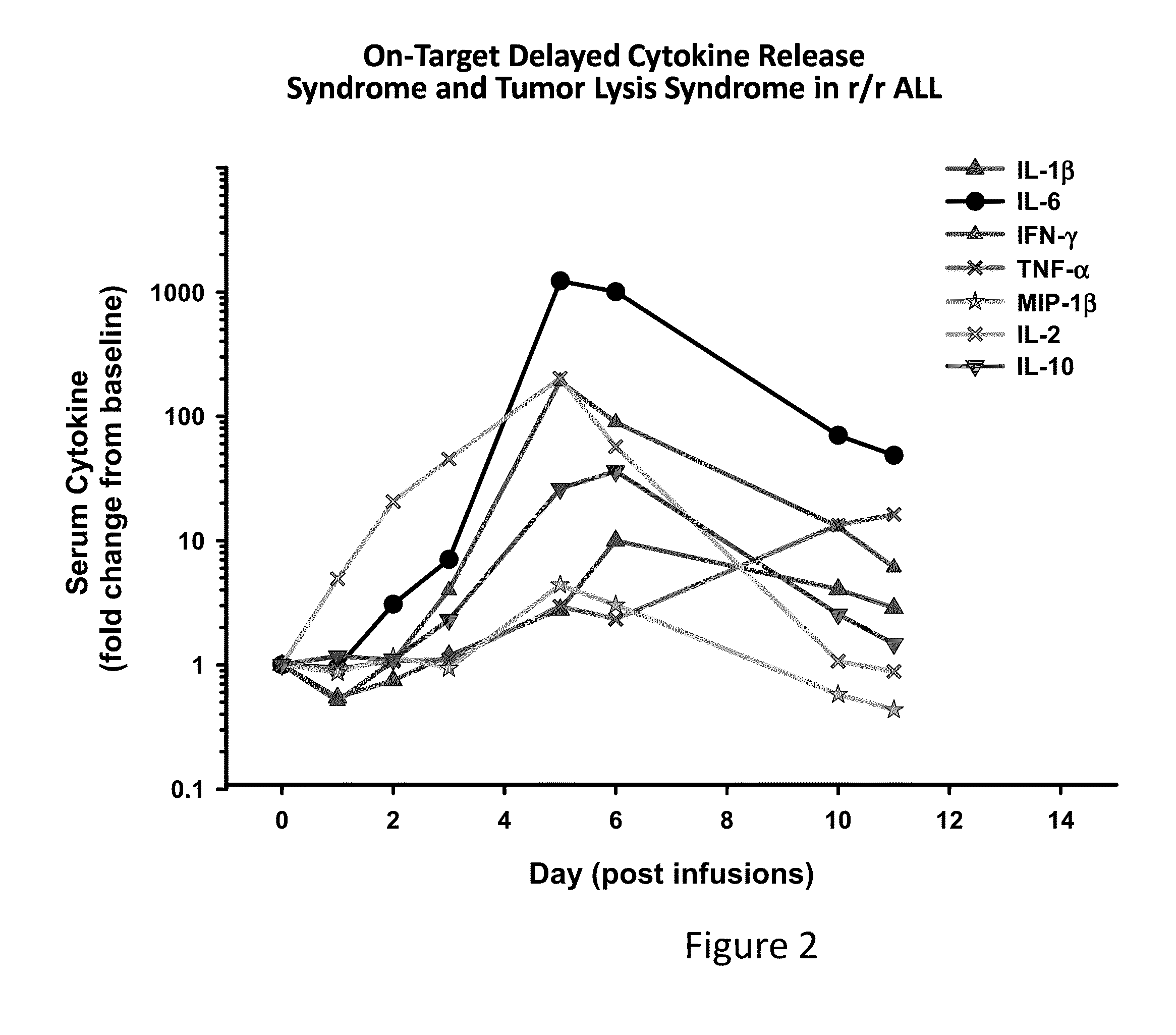
The present invention provides compositions and methods for treating cancer in a patient. In one embodiment, the method comprises a first-line therapy comprising administering to a patient in need thereof a genetically modified T cell expressing a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain and monitoring the levels of cytokines in the patient post T cell infusion to determine the type of second-line of therapy appropriate for treating the patient as a consequence of the presence of the CAR T cell in the patient.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA +1
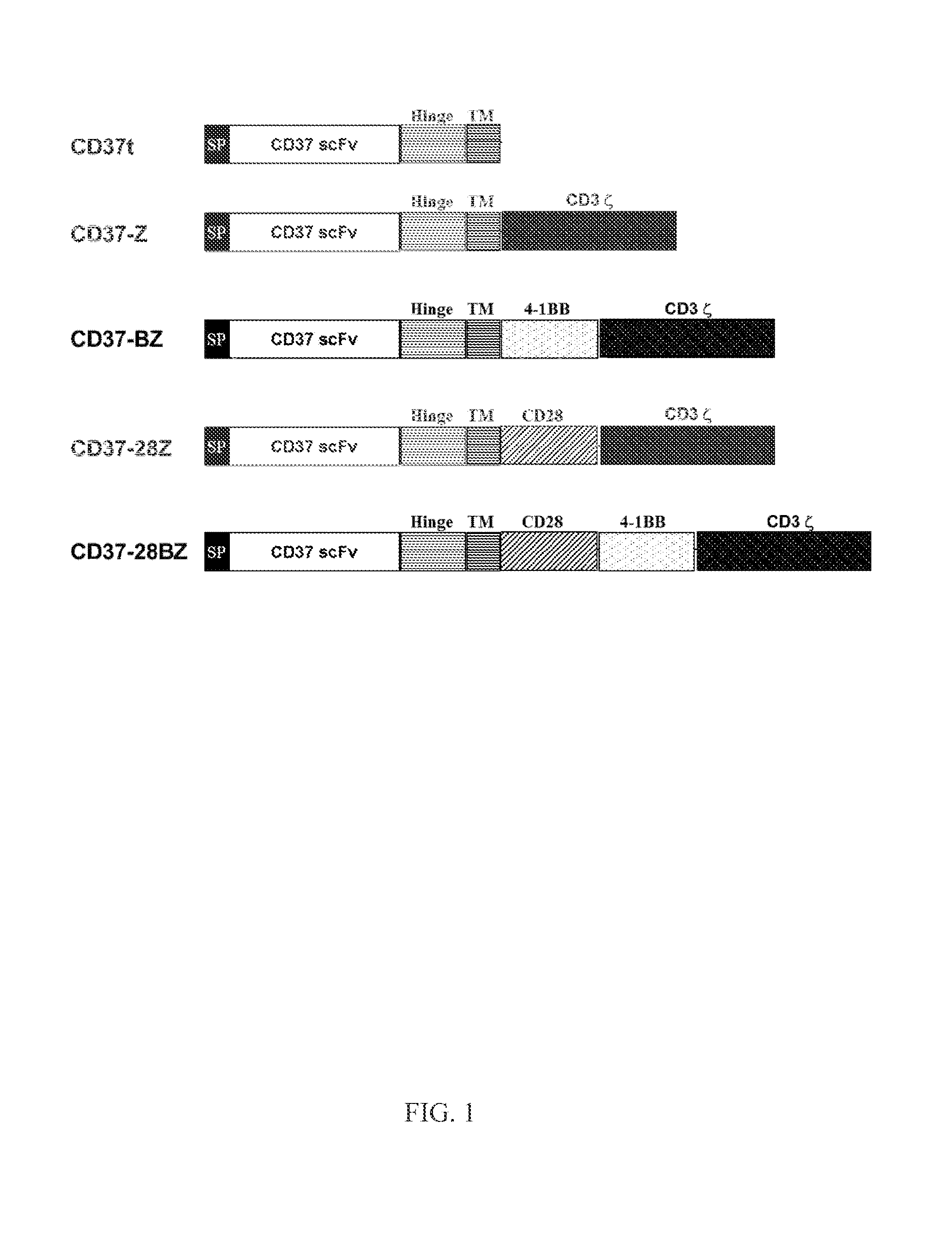
Chimeric antigen receptors and immune cells targeting b cell malignancies
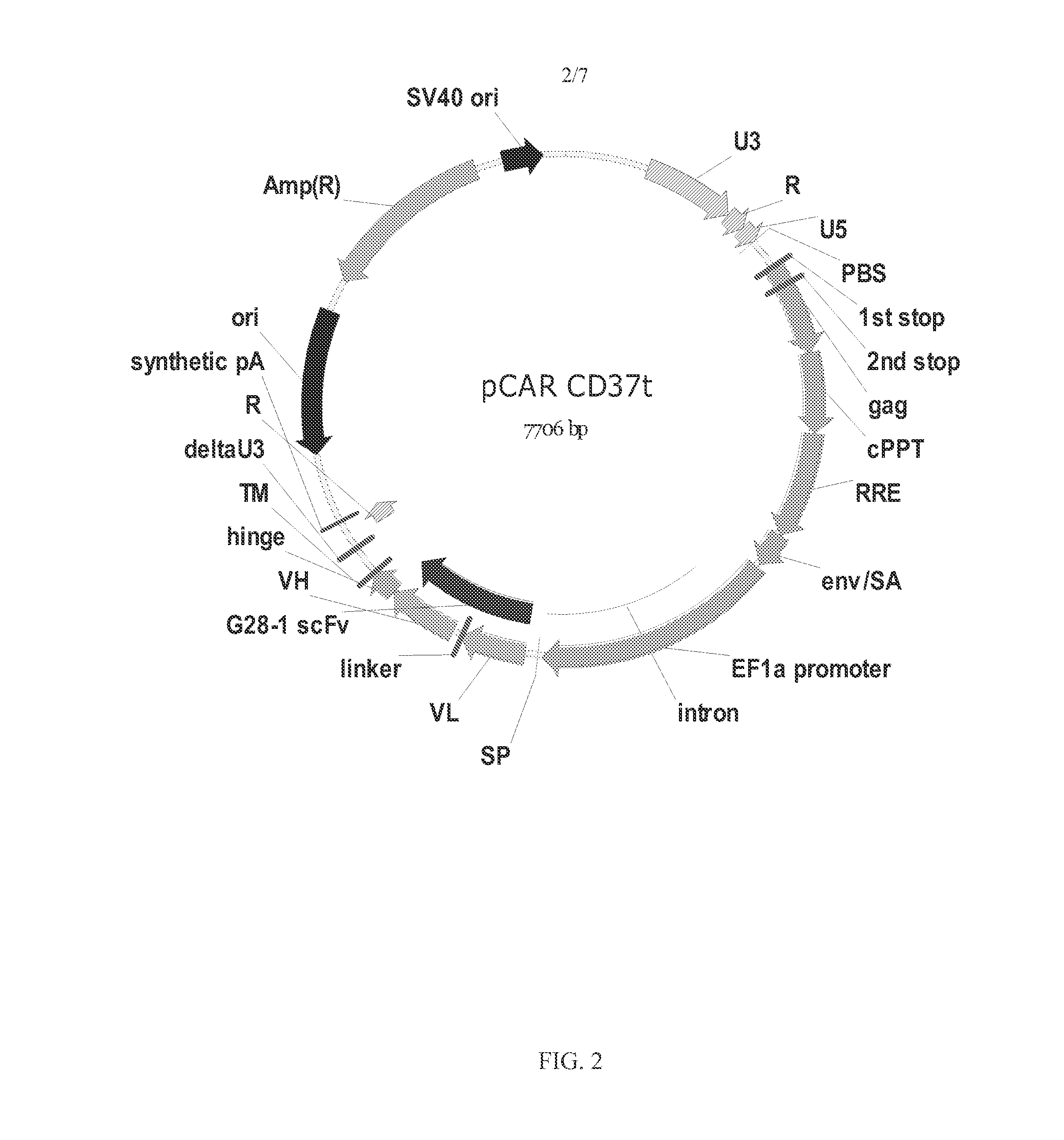
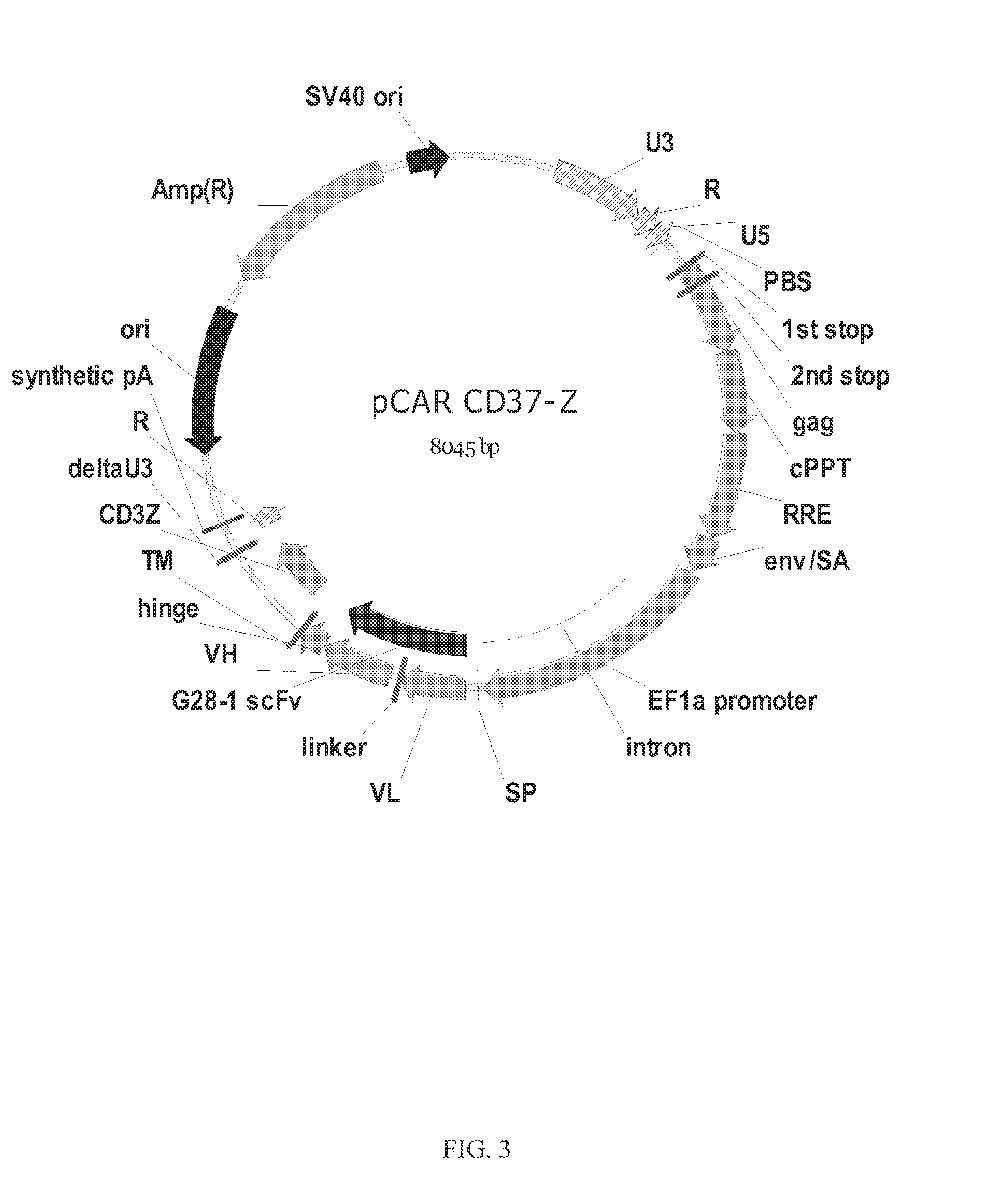
The disclosure describes genetically engineered CD37 specific redirected immune effector cells expressing a chimeric antigen receptor (CAR) protein comprising an antigen binding domain derived from an antibody, a single chain antibody or portion thereof that binds CD37; a hinge region; a transmembrane domain and an intracellular signaling domain derived from human CD3ζ or FcRγ; and optionally one or more co-stimulatory intracellular signaling domains The invention includes nucleic acids, vectors and immune effector cells associated with the production of the CAR protein, as well as methods of treating B cell malignancies in humans by cellular immunotherapy.
Owner:BLUEBIRD BIO INC
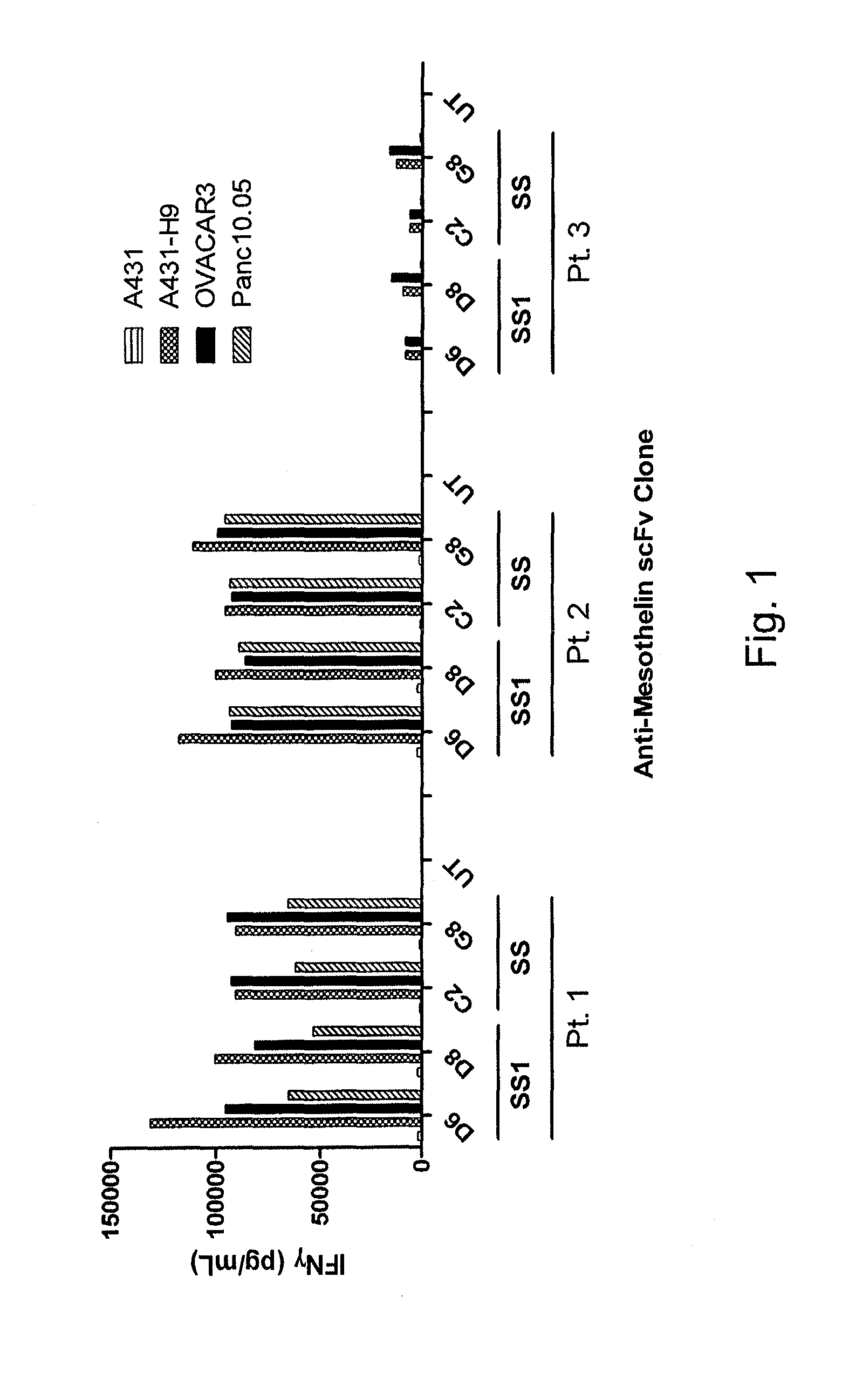
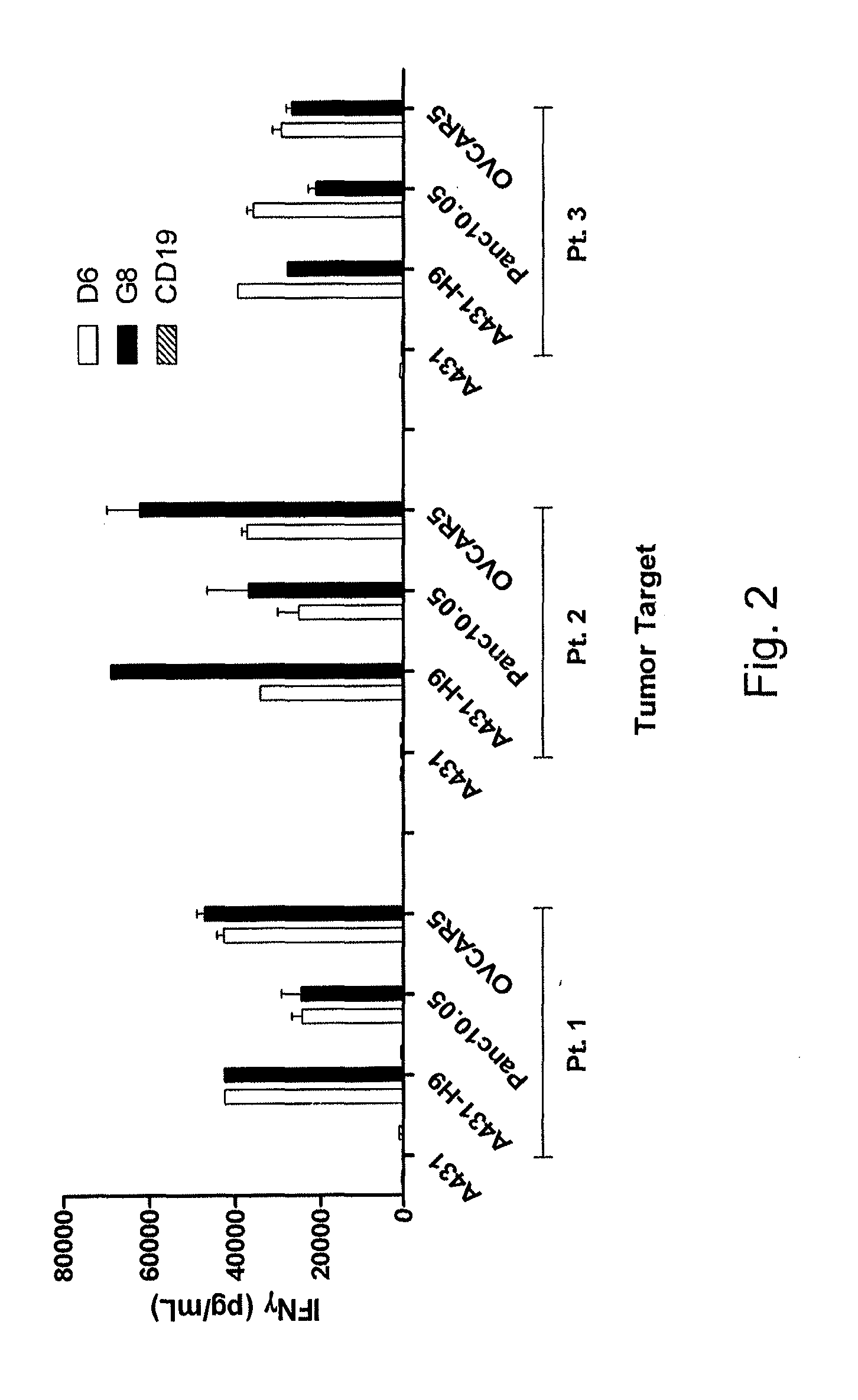
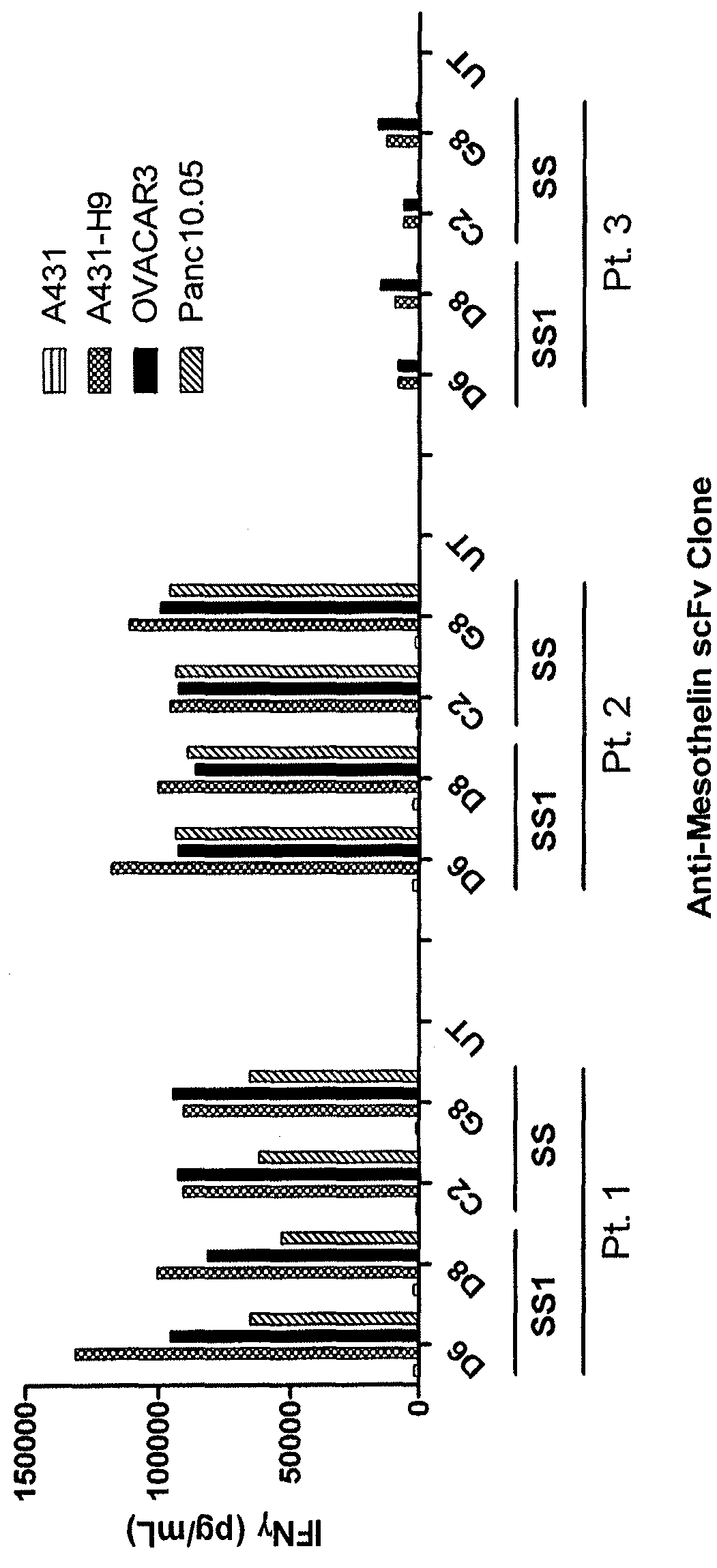
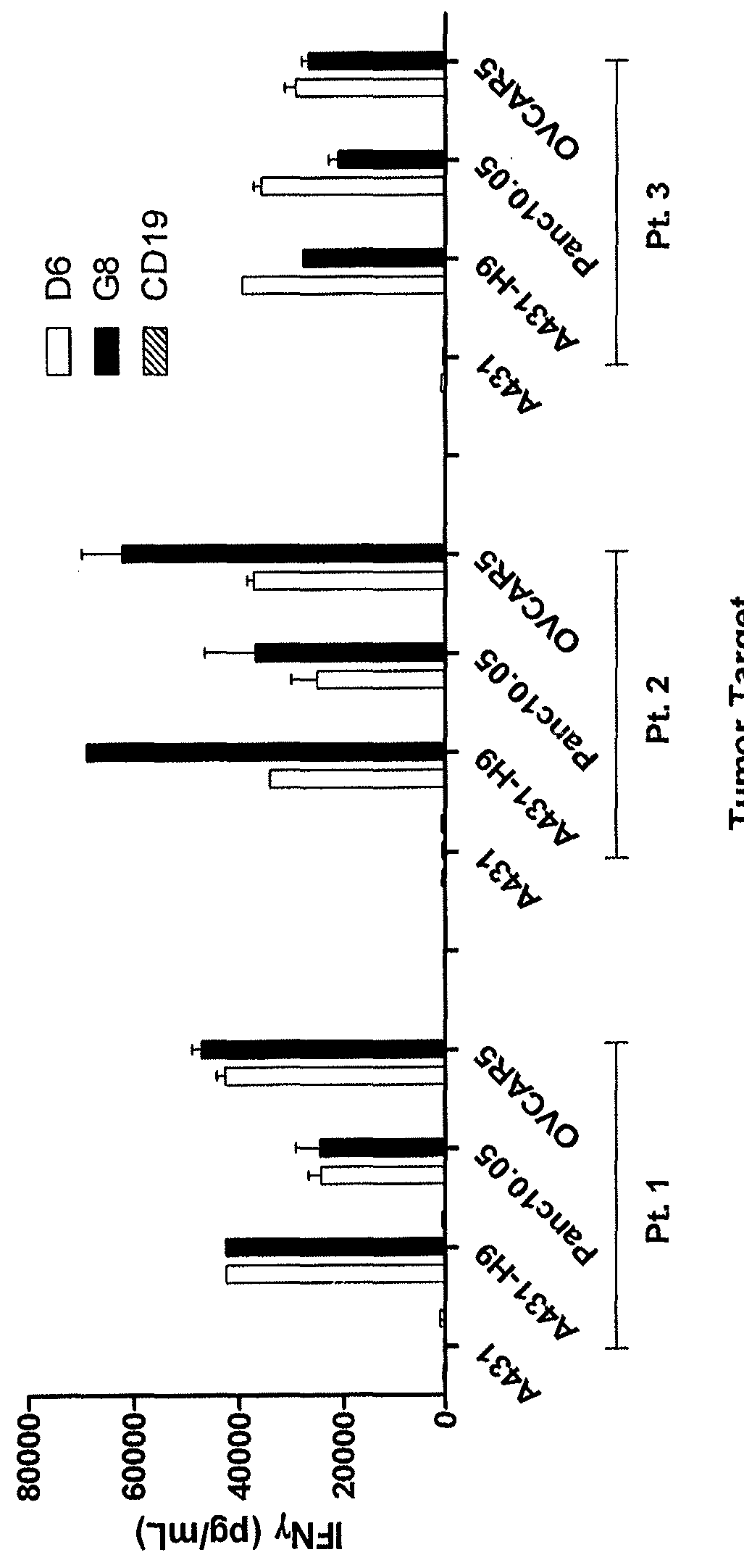
Anti-mesothelin chimeric antigen receptors
The invention provides a chimeric antigen receptor (CAR) (a) an antigen binding domain of HN1 or SS, a transmembrane domain, and an intracellular T cell signaling domain, or (b) an antigen binding domain of SS1, a transmembrane domain, an intracellular T cell signaling domain, and a granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor 2 leader. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a mammal and methods of treating or preventing cancer in a mammal are also disclosed.
Owner:UNITED STATES OF AMERICA
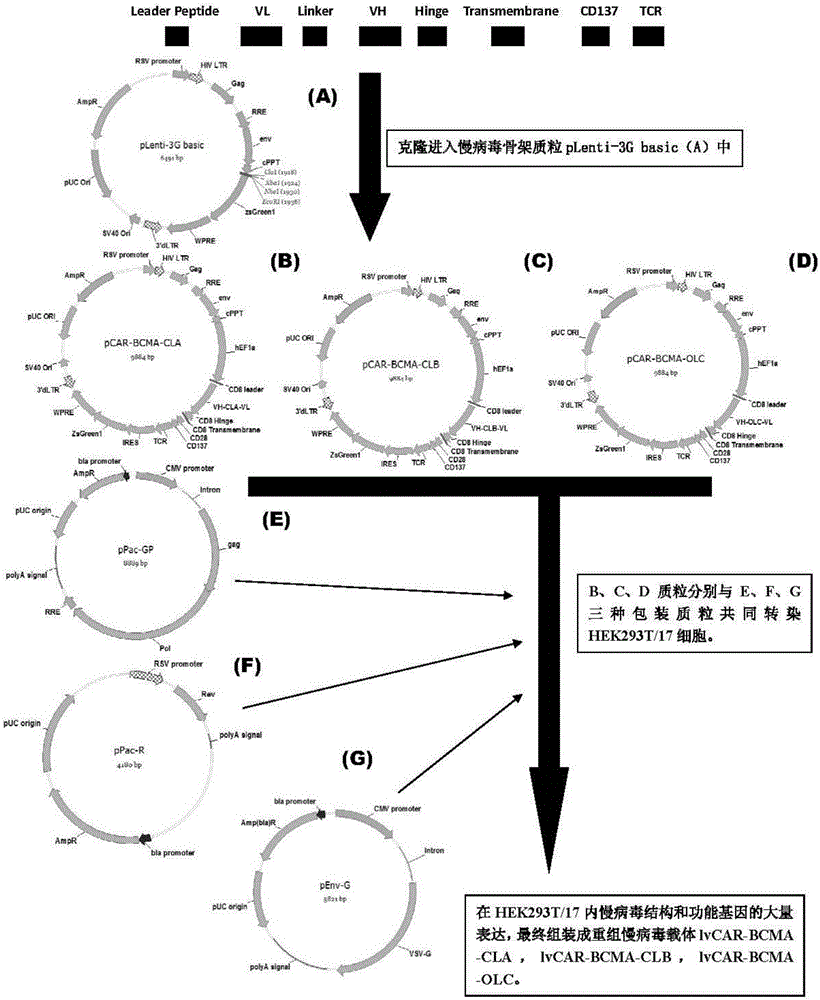
Anti-BCMA chimeric antigen receptor, encoding gene, recombinant expression vector and establishing method and application of anti-BCMA chimeric antigen receptor, encoding gene and recombinant expression vector
ActiveCN105777911AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSequence signalSingle-Chain Antibodies
The invention discloses an anti-BCMA chimeric antigen receptor, an encoding gene, a recombinant expression vector and an establishing method and application of the anti-BCMA chimeric antigen receptor, the encoding gene and the recombinant expression vector. The receptor comprises a CD8 leader chimeric receptor signal peptide, a BCMA single-chain antibody heavy chain VH, an Optimal Linker C, a BCMA single-chain antibody light chain VL, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulatory factor and a TCR chimeric receptor T cell activating domain which are sequentially connected in series. In addition, the invention further discloses the encoding gene and the recombinant expression vector of the anti-BCMA chimeric antigen receptor and the establishing method and application of the encoding gene and the recombinant expression vector. The secretion of cell factors and the cytotoxicity in vitro of CAR-T cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Peptides derived from STEAP1
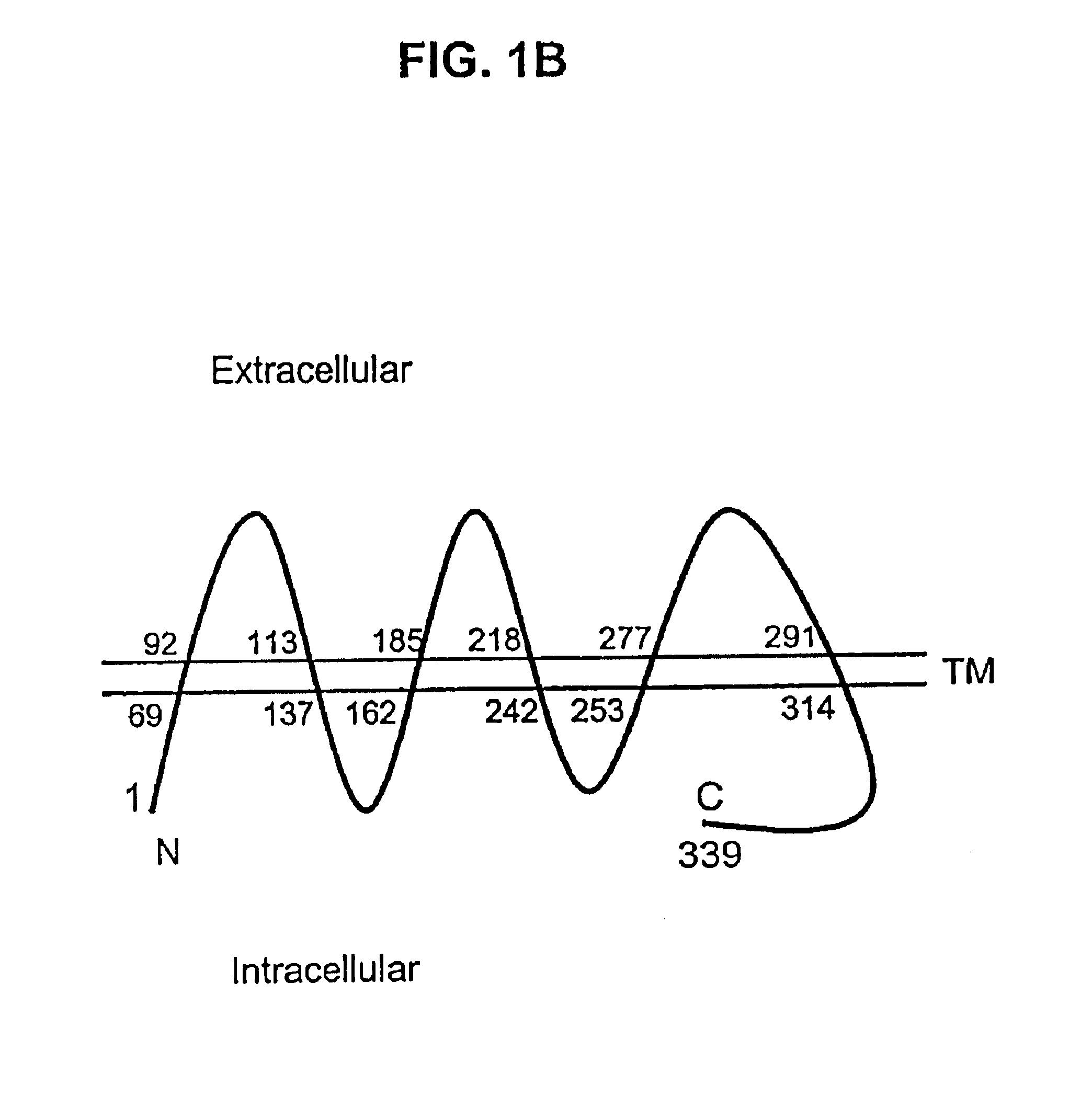
Described is a novel family of cell surface serpentine transmembrane antigens. Two of the proteins in this family are exclusively or predominantly expressed in the prostate, as well as in prostate cancer, and thus members of this family have been termed “STEAP” (serpentine transmembrane antigens of the prostate). Four particular human STEAPs are described and characterized herein. The human STEAPs exhibit a high degree of structural conservation among them but show no significant structural homology to any known human proteins. The prototype member of the STEAP family, STEAP-1, appears to be a type IIIa membrane protein expressed predominantly in prostate cells in normal human tissues. Structurally, STEAP-1 is a 339 amino acid protein characterized by a molecular topology of six transmembrane domains and intracellular N- and C-termini, suggesting that it folds in a “serpentine” manner into three extracellular and two intracellular loops. STEAP-1 protein expression is maintained at high levels across various stages of prostate cancer. Moreover, STEAP-1 is highly over-expressed in certain other human cancers.
Owner:AGENSYS
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20060067920A1Negligible toxicityPotent and selectiveBiocideAntibody mimetics/scaffoldsIntracellular signallingAutocrine paracrine
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human maligancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Anti-mesothelin chimeric antigen receptors
ActiveUS9359447B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyCancer preventionAntiendomysial antibodies
Owner:UNITED STATES OF AMERICA
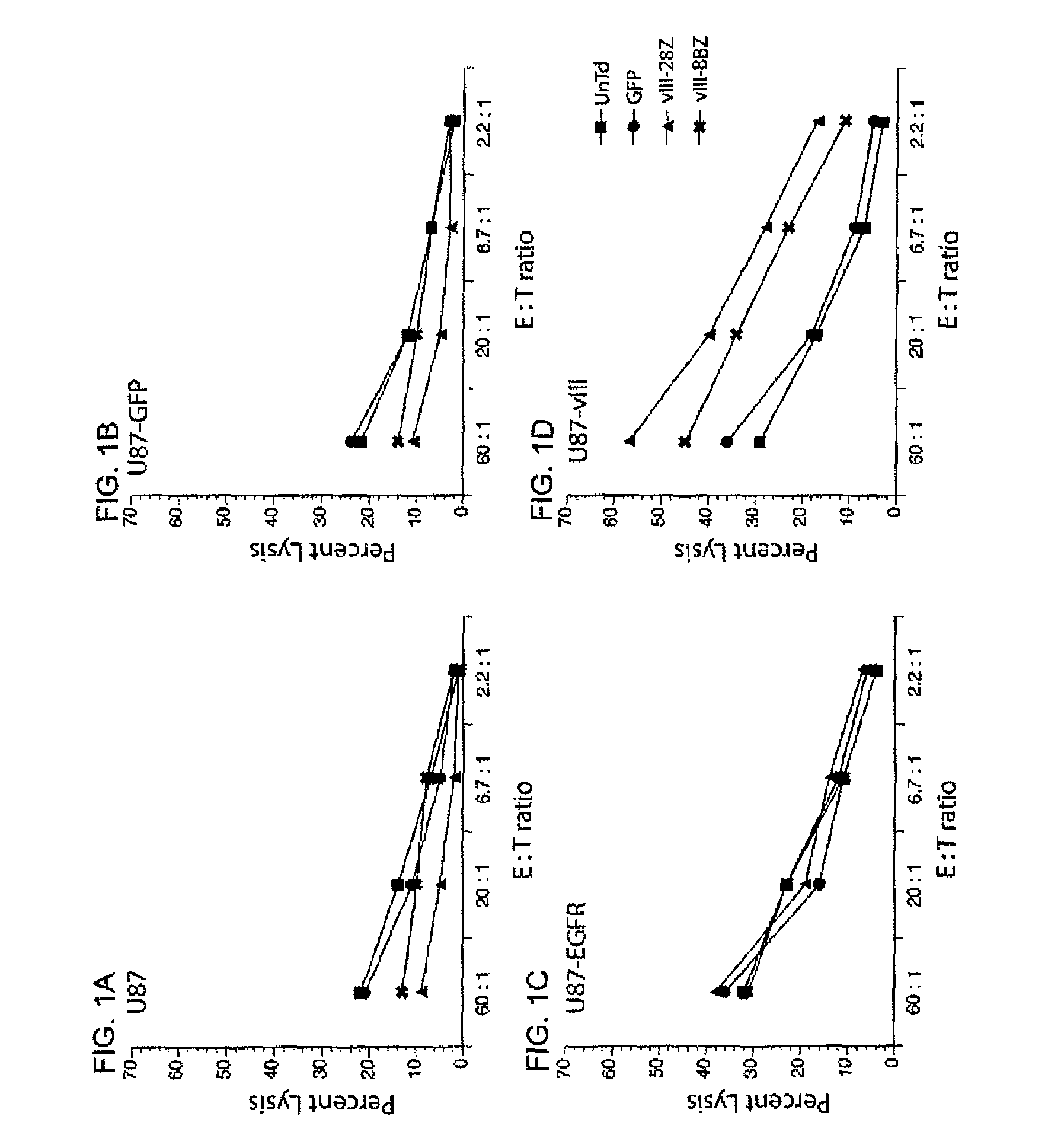
Anti-epidermal growth factor receptor variant III chimeric antigen receptors and use of same for the treatment of cancer
ActiveUS9266960B2Peptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsAntigen binding
The disclosure provides chimeric antigen receptors (CARs) comprising an antigen binding domain of human antibody 139, an extracellular hinge domain, a transmembrane domain, and an intracellular domain T cell receptor signaling domain. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a host and methods of treating or preventing cancer in a host are also disclosed.
Owner:UNITED STATES OF AMERICA
Humanized monoclonal antibody targeting to human CD19 antigen
ActiveCN107312091AEfficient killingNo side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsSingle-Chain AntibodiesTreatment effect
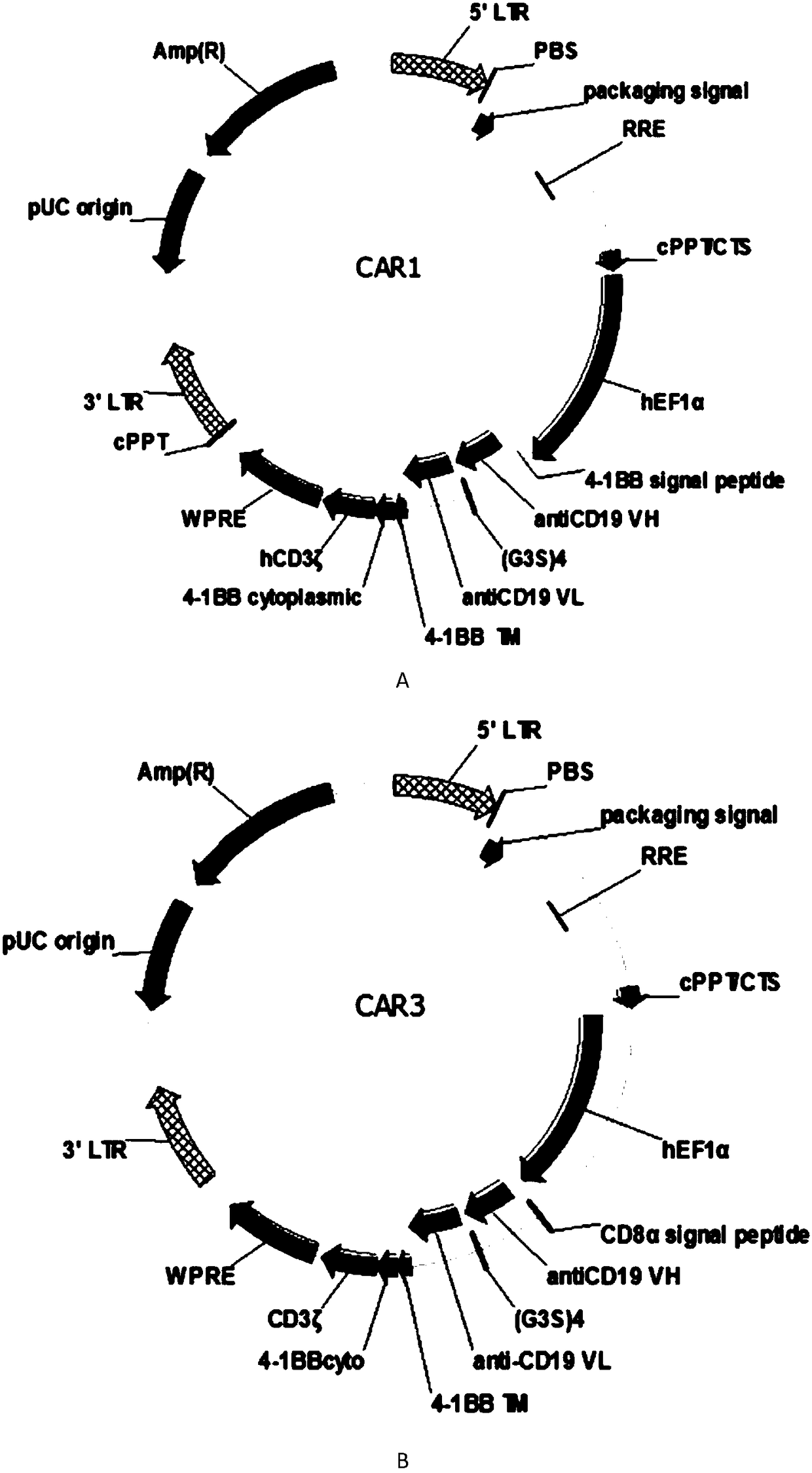
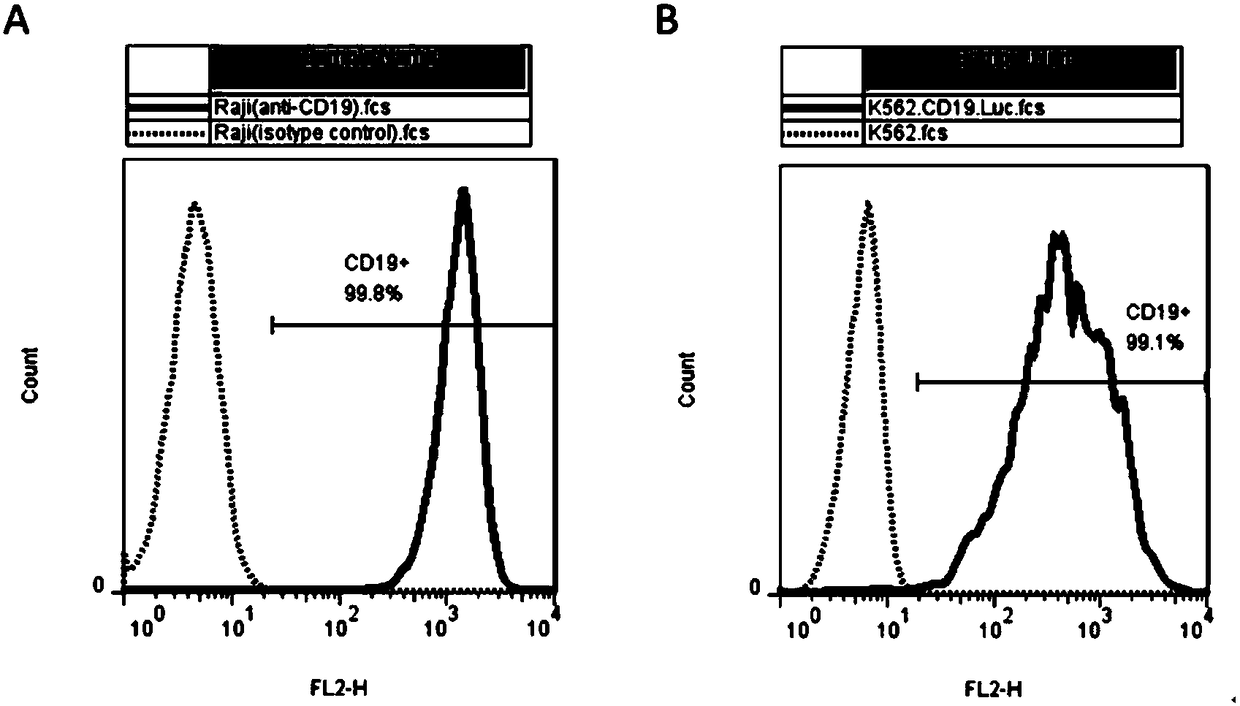
The invention discloses a humanized monoclonal antibody targeting to human CD19 antigen. The humanized monoclonal antibody is applied to construction and preparation of chimeric antigen receptor T cells; and subsequent in-vitro experiments verify the tumor treatment effect of the chimeric antigen receptor T cells. A chimeric antigen receptor targeting to the human CD19 antigen is composed of a leader peptide, a single-chain antibody targeting to the human CD19 antigen, a hinge area for connection with ScFv, a transmembrane domain and an immune receptor tyrosine activation motif; a lentiviral expression vector is constructed from the chimeric antigen receptor and then used for infection of immune cells, so the chimeric antigen receptor targeting to the human CD19 antigen can be expressed in the immune cells; and tumors expressing the CD19 antigen are killed by recognizing the CD19 antigen on the surfaces of tumor cells, so the purpose of tumor treatment is achieved. The invention provides a novel tool for targeting therapy of tumors.
Owner:CHONGQING PRECISION BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com