Toxicity Management for Anti-Tumor Activity of CARs
a technology of toxic management and car, which is applied in the field of toxic management of car antitumor activity, can solve the problems of insufficient investigation of car t cells on all blasts, more immature leukemia with a more rapid progression, and insufficient investigation of cytokine secretion and disorders associated with in vivo chimeric antigen recept t-cell expansion, so as to reduce or avoid an adverse effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
[0160]The invention is further described in detail by reference to the following experimental examples. These examples are provided for purposes of illustration only, and are not intended to be limiting unless otherwise specified. Thus, the invention should in no way be construed as being limited to the following examples, but rather, should be construed to encompass any and all variations which become evident as a result of the teaching provided herein.
[0161]Without further description, it is believed that one of ordinary skill in the art can, using the preceding description and the following illustrative examples, make and utilize the compounds of the present invention and practice the claimed methods. The following working examples therefore, specifically point out the preferred embodiments of the present invention, and are not to be construed as limiting in any way the remainder of the disclosure.
example 1
Cytokine Therapy in Combination with CAR T Cell Infusion
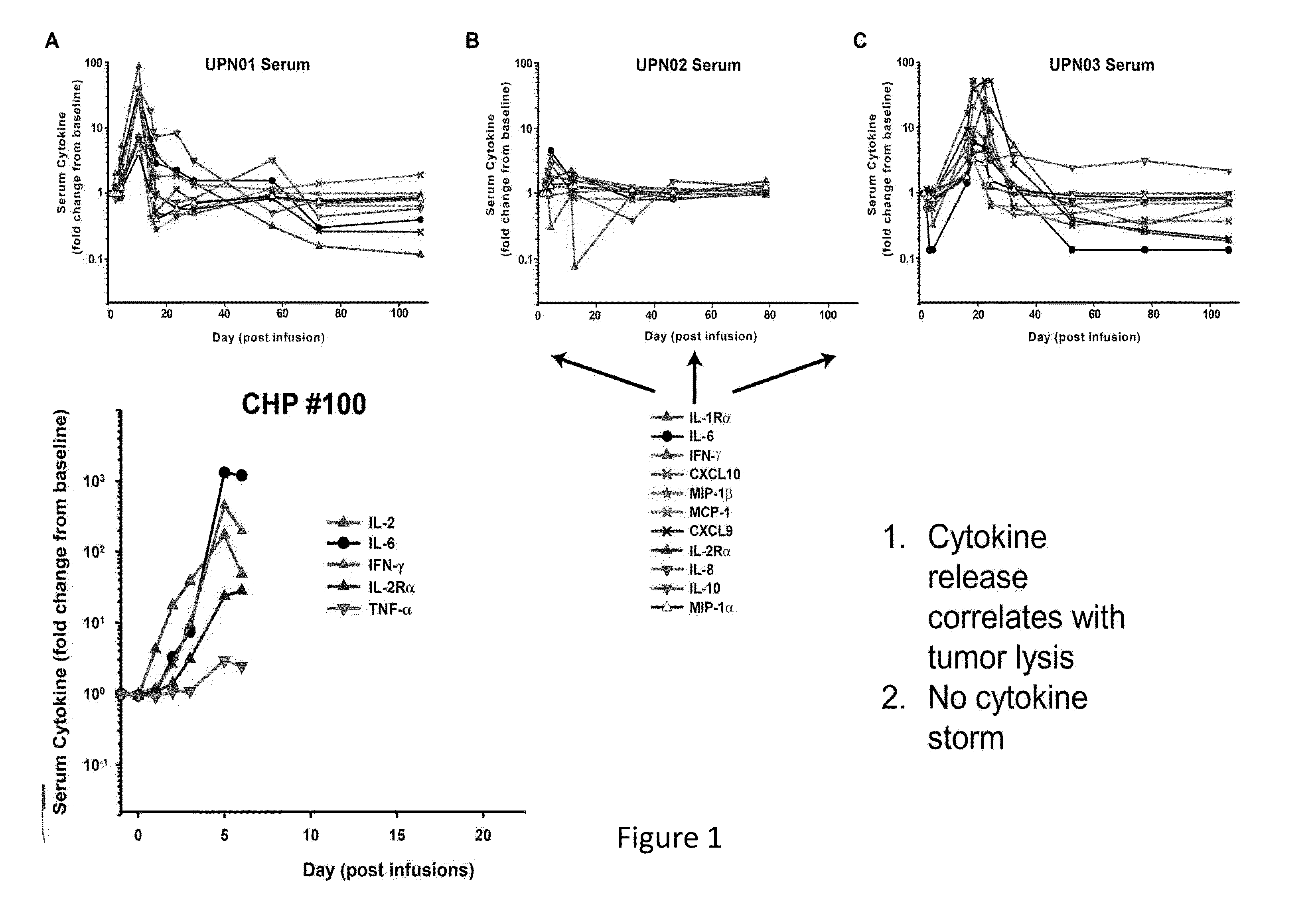
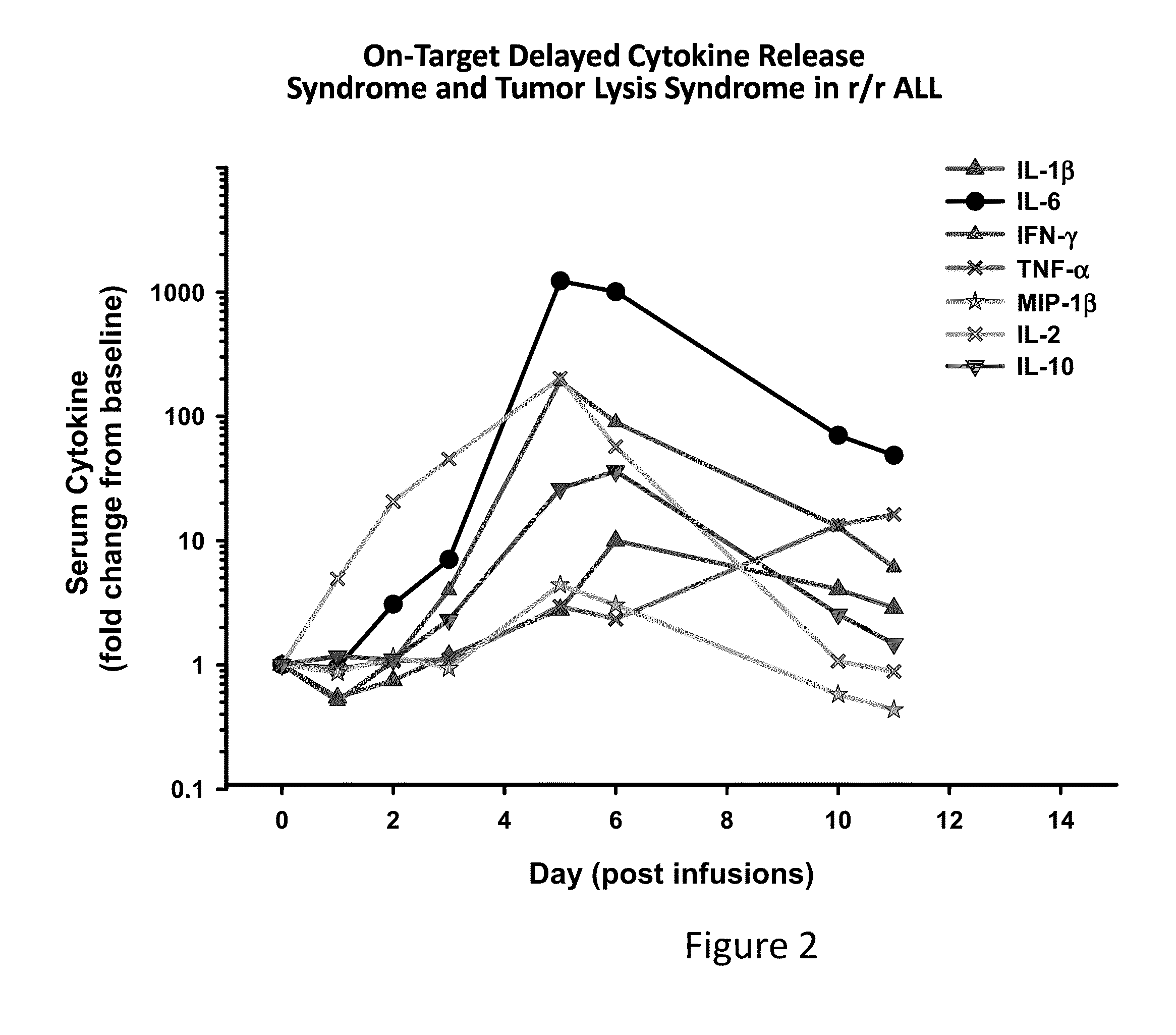
[0162]The results presented herein demonstrate that patients following infusion of CART cells exhibit differential expression levels of various cytokines. In some instances, the elevated levels of some cytokines are a result of the toxicity of the infused CAR T cells (FIG. 1). It was observed that tocilizumab (anti-IL6) can ameliorate the toxicity of CARs and seemingly preserve antitumor effects in 2 of 2 patients (FIG. 2). Without wishing to be bound by any particular theory, it is believed that anakinra and other reagents that block IL-1 may also be useful in this regard. The data presented herein also demonstrates that IL-1 is elevated in patients, and this may lead to the later rise in IL-6. Anakinra is an IL-1Ra recombinant protein which binds to the IL1 receptors and blocks both IL-1 alpha and beta signaling. Anakinra has a short ½ life. There is an advantage to use Anakinra to start treating patients since both IL-1 alph...
example 2
CD19-Redirected Chimeric Antigen Receptor T (CART19) Cells Induce a Cytokine Release Syndrome (CRS) and Induction of Treatable Macrophage Activation Syndrome (MAS) that can be Managed by the IL-6 Antagonist Tocilizumab (Toc)
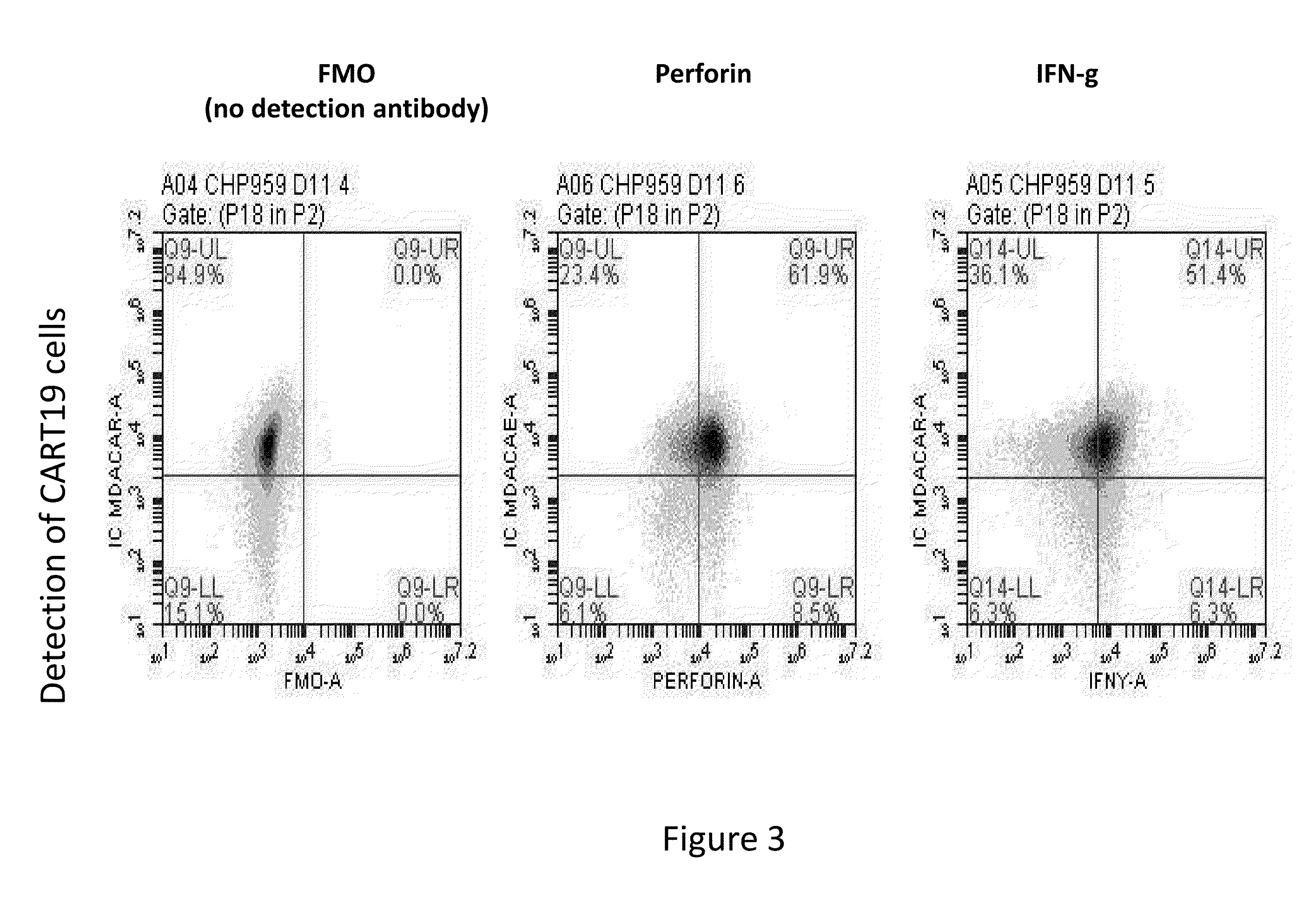
[0164]Infusion of CART19 cells results in 100 to 100,000× in vivo proliferation, tumor lysis syndrome followed by durable antitumor activity, and prolonged persistence in patients with B cell tumors. The results presented herein demonstrate that in vivo proliferation of CART19 cells and potent anti-tumor activity therefrom is associated with CRS, leading to hemophagocytic lymphohistiocytosis (HLH), also termed MAS. Without wishing to be bound by any particular theory, it is believed that MAS / HLH is a unique biomarker that is associated with and may be required for potent anti-tumor activity.
[0165]Autologous T cells were lentivirally transduced with a CAR composed of anti-CD19 scFv / 4-1BB / CD3-zeta, activated / expanded ex-vivo with anti-CD3 / anti-CD28 beads, and then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com