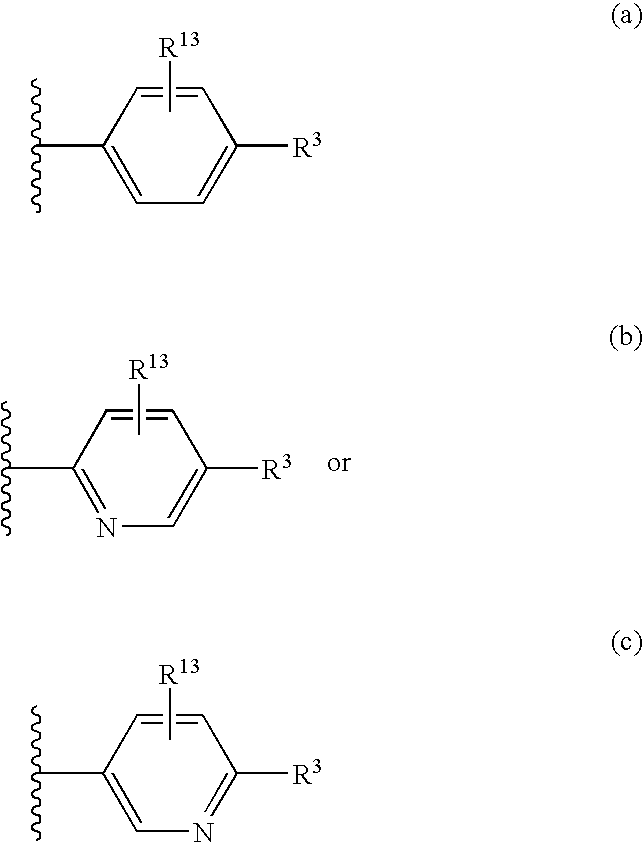
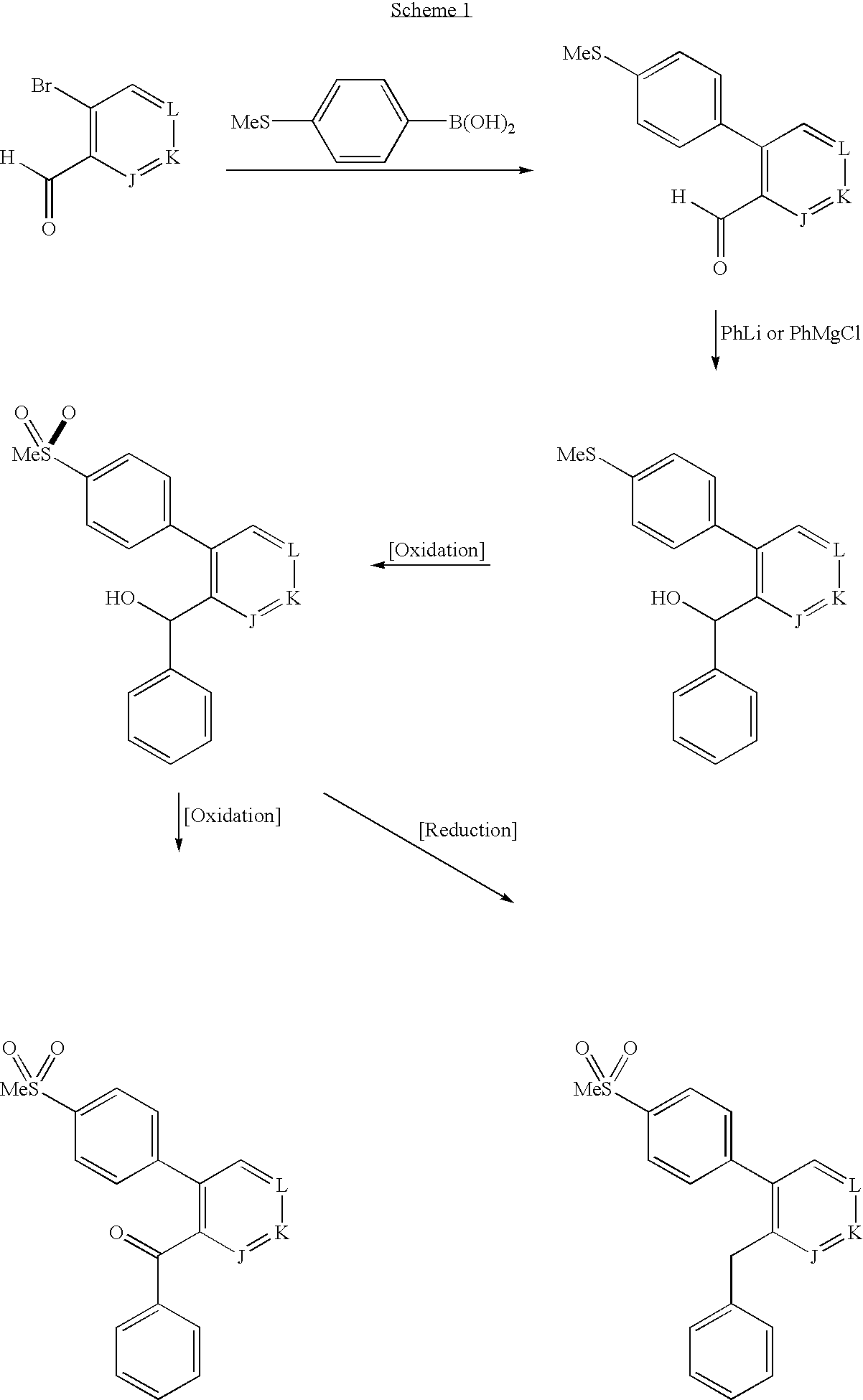
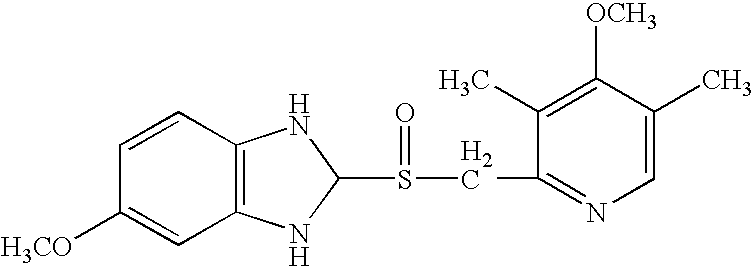
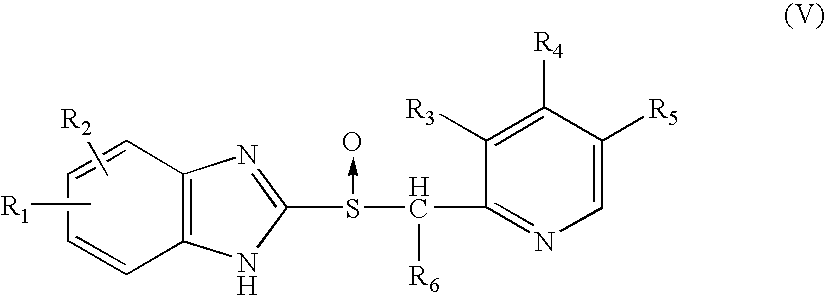
Patents
Literature
77 results about "Decongestant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A decongestant, or nasal decongestant, is a type of pharmaceutical drug that is used to relieve nasal congestion in the upper respiratory tract. The active ingredient in most decongestants is either pseudoephedrine or phenylephrine (the latter of which has disputed effectiveness). Intranasal corticosteroids can also be used as decongestants and antihistamines can be used to alleviate runny nose, nasal itch, and sneezing.
Dosage form containing multiple drugs
A pharmaceutical dosage form comprising a first drug and a second drug, both of which are selected from decongestants, antitussives, expectorants, analgesics and antihistamines. The dosage form provides a plasma concentration within a therapeutic range of the second drug over a period which is coextensive with at least about 70% of a period over which the dosage form provides a plasma concentration within a therapeutic range of the first drug. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Ophthalmic fluid delivery device and method of operation
An ophthalmic fluid atomizer configured to safely deliver an ophthalmic fluid, the ophthalmic fluid atomizer including a body having a proximal end and a distal end and a reservoir connected to the body, wherein the reservoir contains an ophthalmic fluid disposed therein, wherein the ophthalmic fluid is selected from the group consisting of a decongestant and a tear substitute. The atomizer further includes a discharge plate disposed at the distal end, wherein the discharge plate includes a plurality of openings extending therethrough. The atomizer further includes a propulsion means for transmitting the ophthalmic fluid from the reservoir to the discharge plate, wherein transmission of the ophthalmic fluid across the discharge plate generates a plume of ophthalmic fluid along a direction directly toward the eye, wherein the plume of ophthalmic fluid travels unassisted from the discharge plate to the eye and at the eye has a momentum that has a magnitude that is insufficient to trigger at least one of an ocular blink reflex and a lacrimation reflex of the eye.
Owner:OPTIMYST SYST
Decongestant and expectorant tablets
The present invention relates to a sustained release oral pharmaceutical tablet formulation containing an expectorant and a decongestant.
Owner:ANDRX LABS
Solution forms of cyclodextrins for nasal or throat delivery of essential oils
This invention further relates to a method for preventing or treating diseases or conditions of the oral cavity, throat or nose of warm-blooded animals including humans. More particularly, the invention pertains to a composition and method for spraying essential oils to the oral cavity, throat or nasal mucosa as cyclodextrin inclusion complexes. The spray composition includes a cyclodextrin in an amount of from about 0.1% w / v to about 20% w / v; at least one essential oil in an amount of from about 0.001% w / v to about 5.0% w / v; an effective amount of an antimicrobial preservative composition; and water. The composition may further comprise an alcohol co-solvent, a thickening agent, a sweetener, an antitussive, an anticholinergic, a decongestant, an antihistamine, an astringent, an anti-inflammatory steroid composition, a vitamin, a respiratory stimulant, a mucolytic agent, a bronchodilator, a beta-antagonist, an antidiarrheal agent, or combinations thereof.
Owner:QPHARMA
Sustained-release drug delivery compositions and methods
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
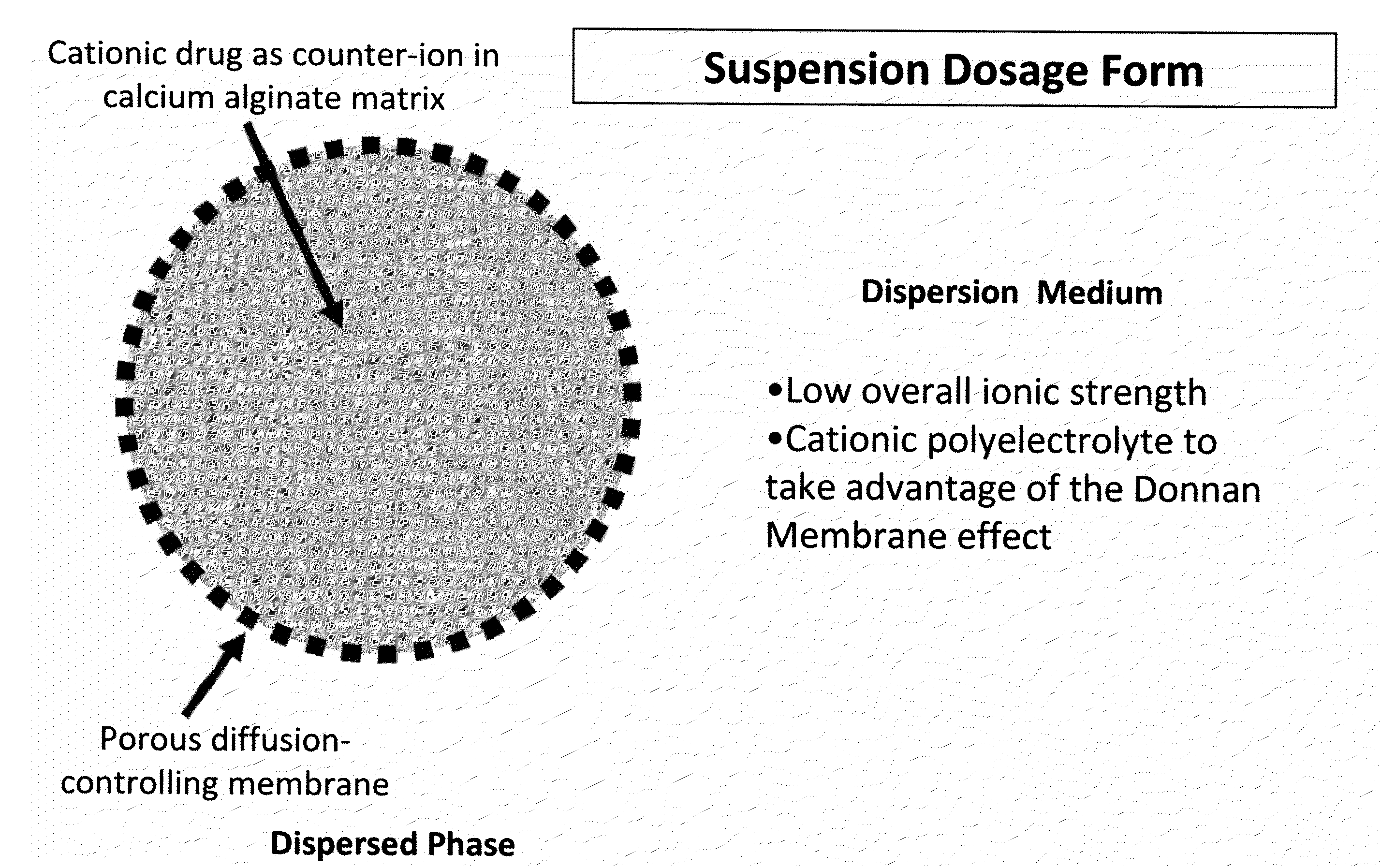
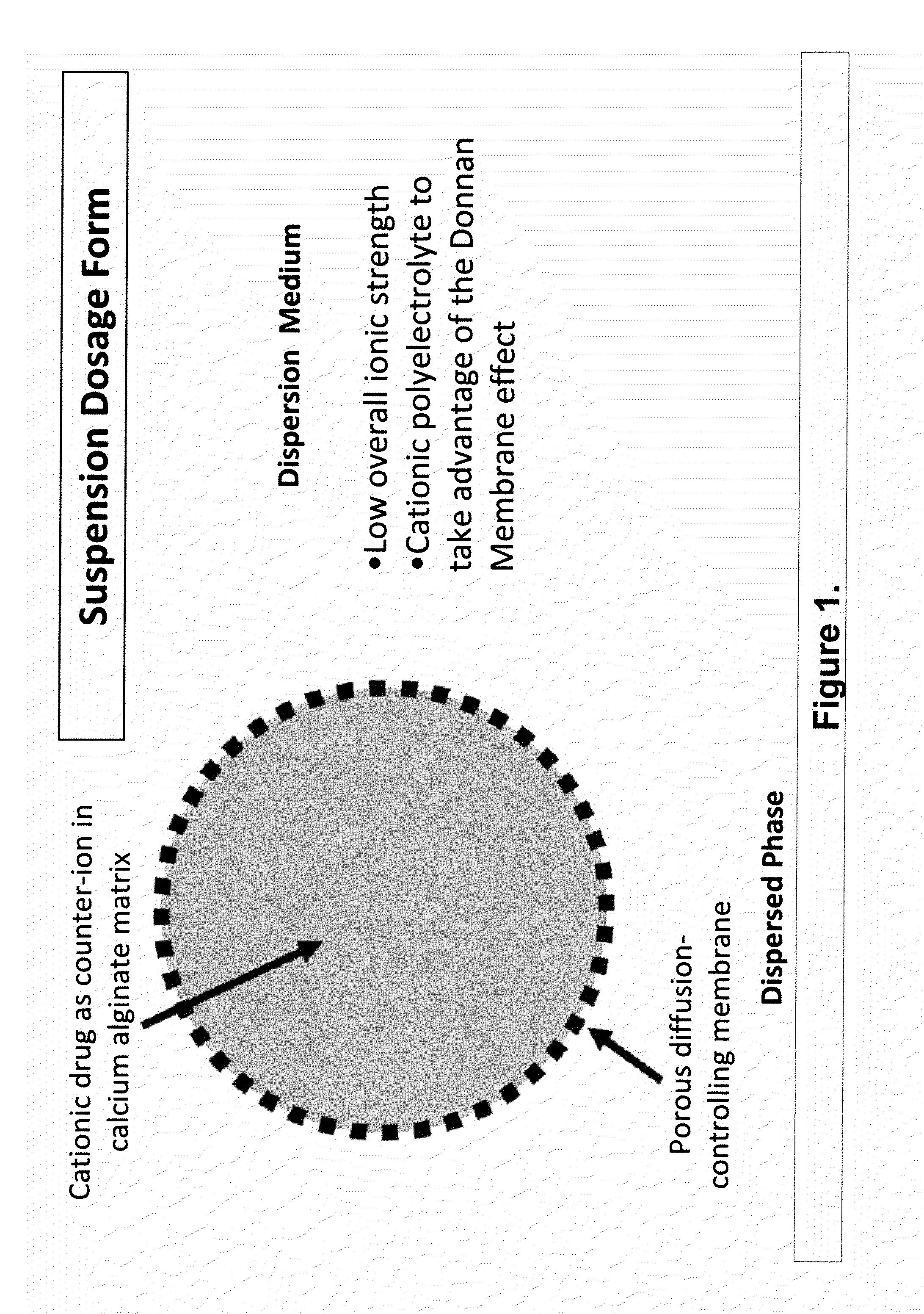
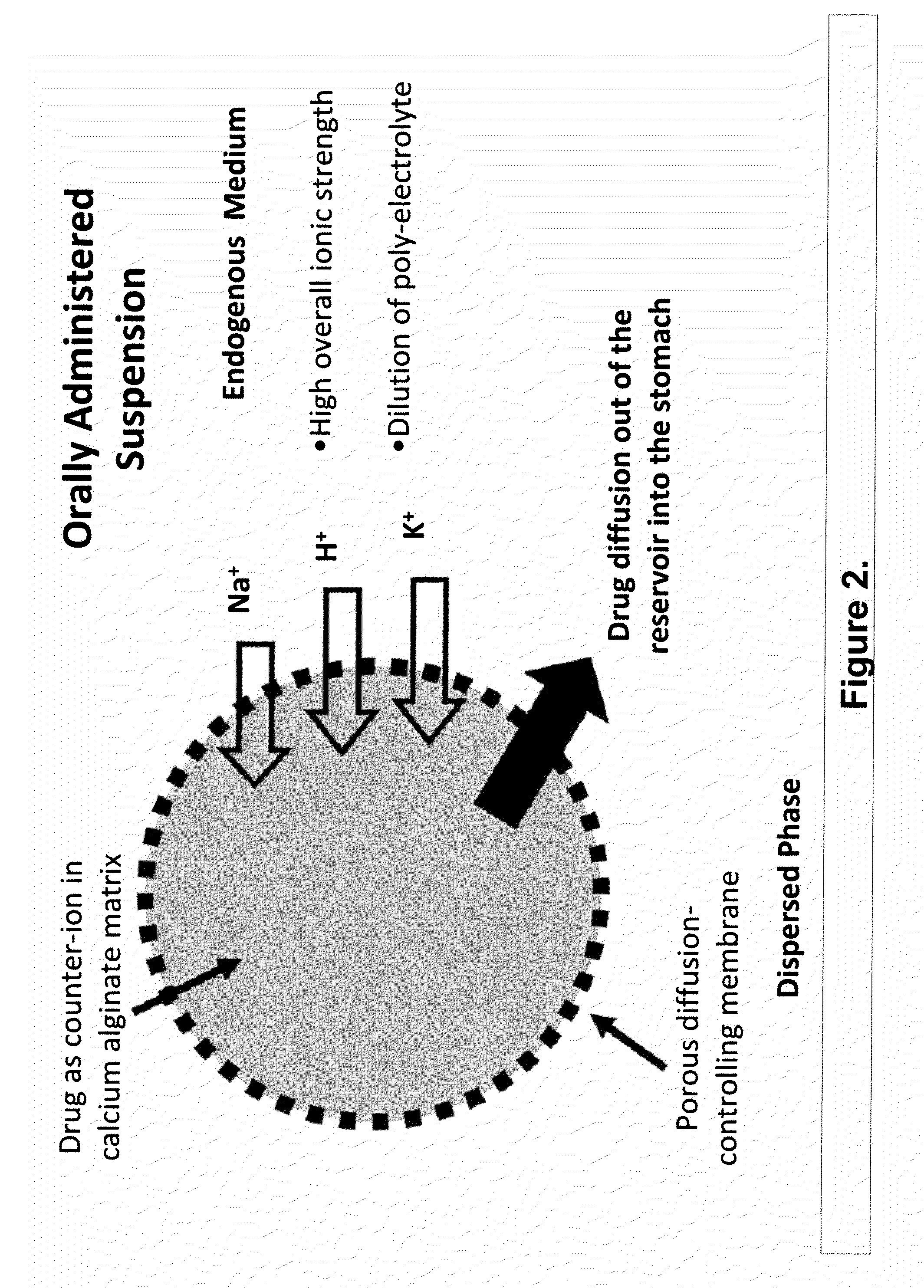
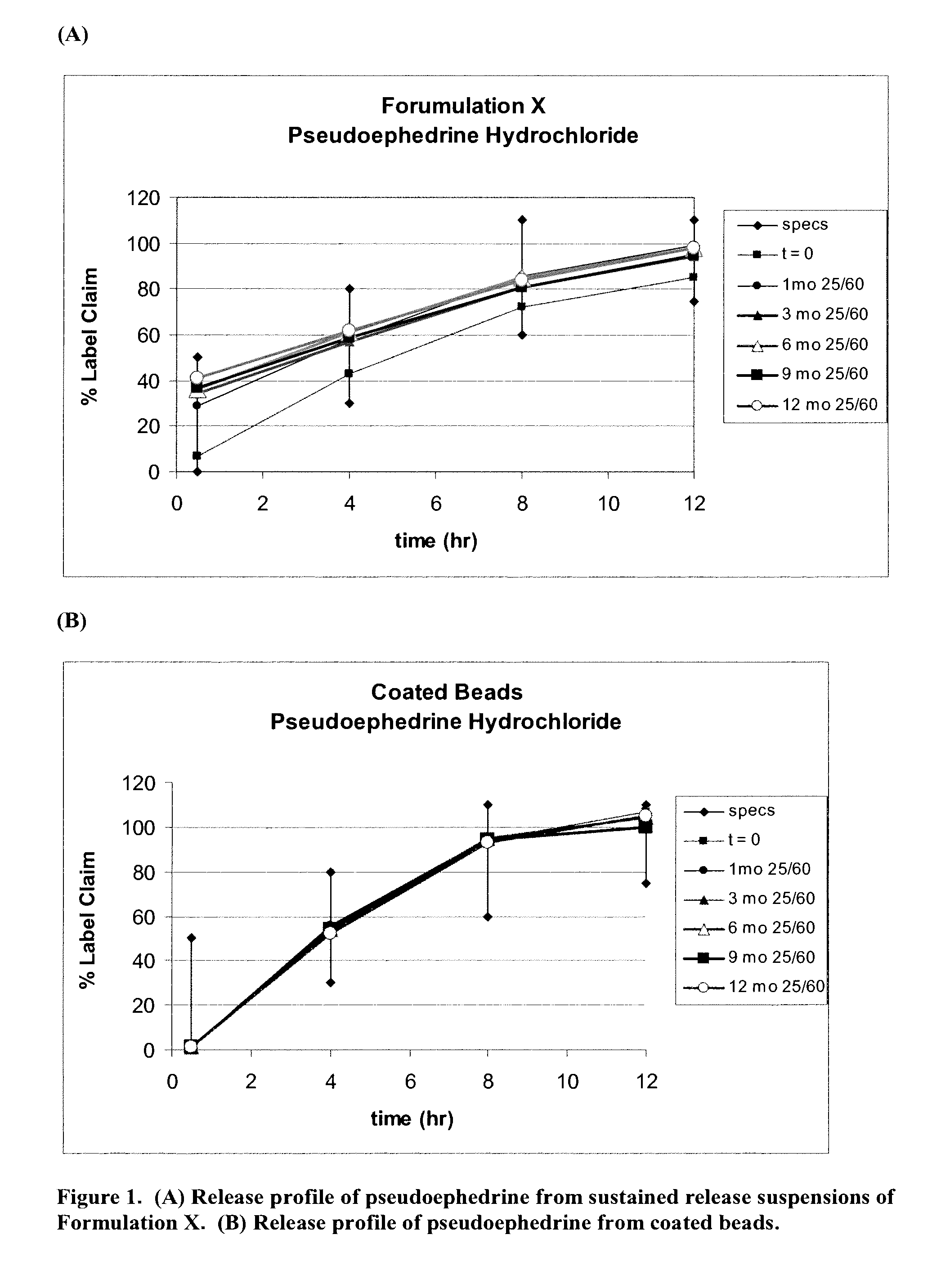
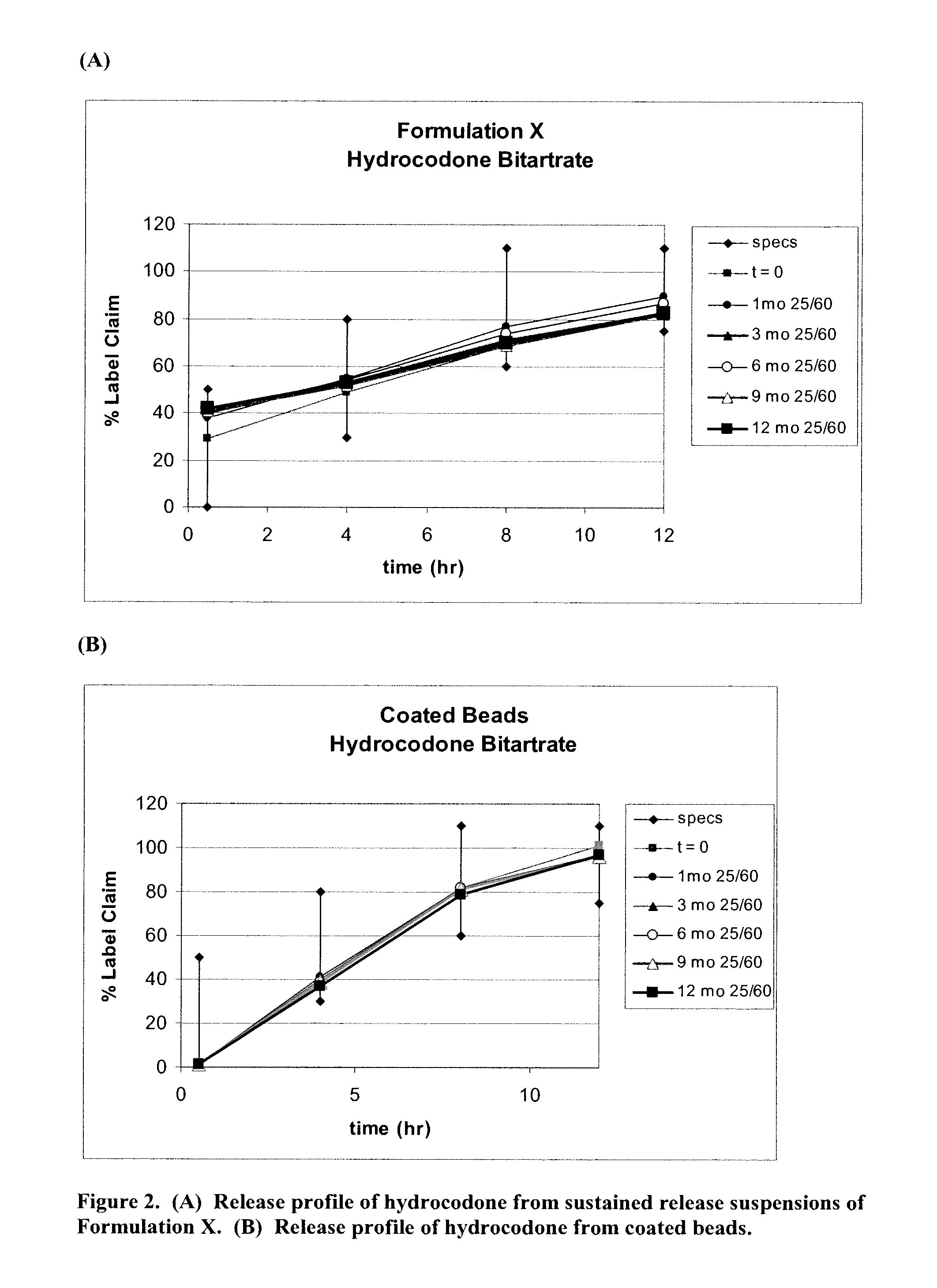
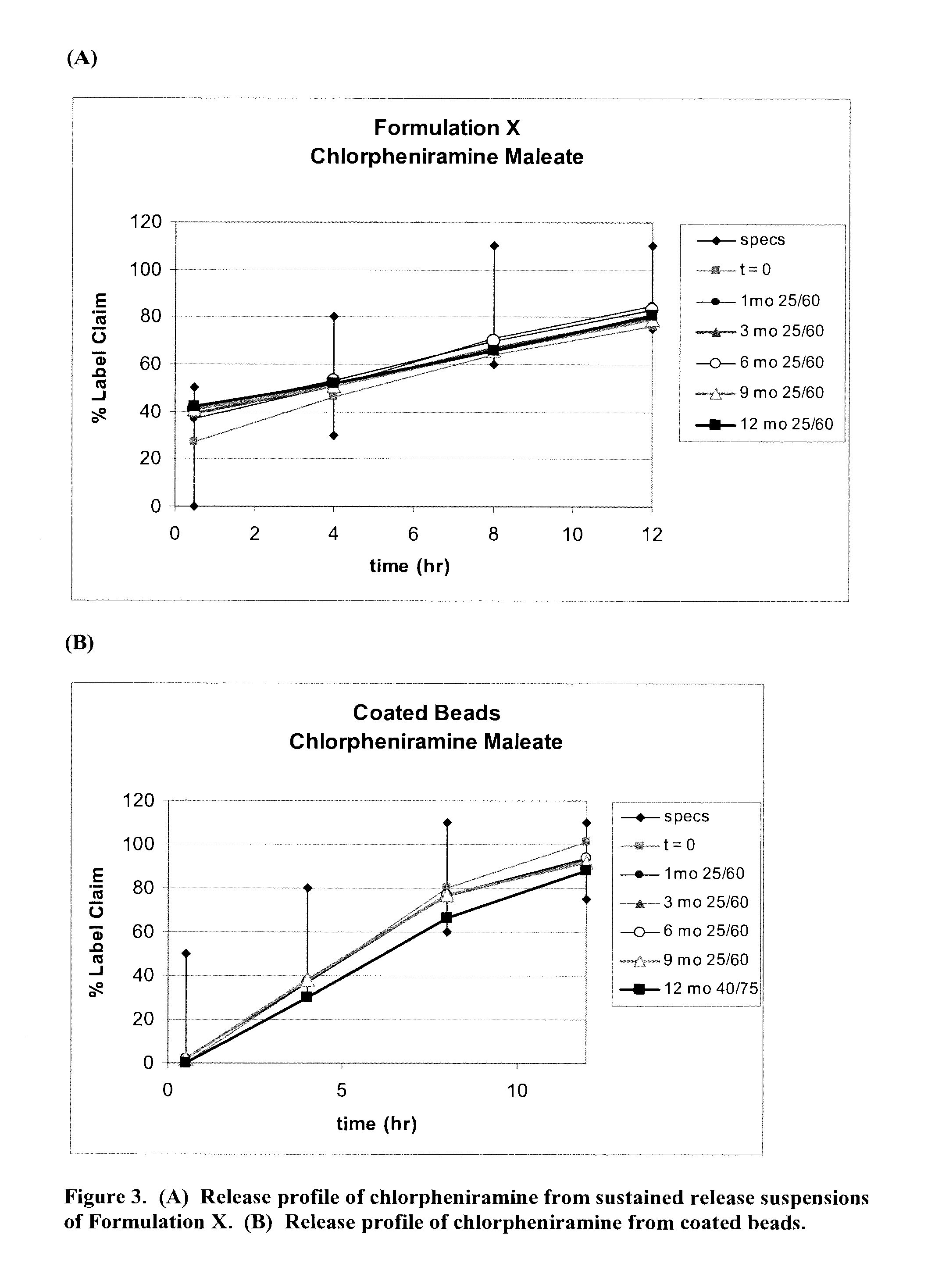
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
Thin film delivery systems for volatile decongestants
A volatile decongestant delivery vehicle composition includes (i) a flowable water-soluble film-forming matrix; and (ii) a particulate volatile decongestant agent uniformly stationed therein. A useful volatile decongestant agent includes menthol, for example menthol crystals. The composition may further include a decongesting volatile oil, such as, but not limited to eucalyptus oil, menthol oil, pine oil, terpine hydrate oil, and combinations thereof. The volatile decongestant agent may be present in amounts of up to about 0.1% to about 60% by weight of the total composition.
Owner:MONOSOL RX
Dynamic variable release
InactiveUS20050152967A1Improve efficiencyReduce in quantityBiocideOrganic active ingredientsDiseaseCommon cold
The present invention relates to novel mixed release pharmaceutical formulations that include a expectorant available for immediate release and a decongestant for extended release that provide for the symptomatic relief of cough associated with respiratory tract conditions such as the common cold, bronchial asthma, acute and chronic bronchitis.
Owner:NEOS THERAPEUTICS LP
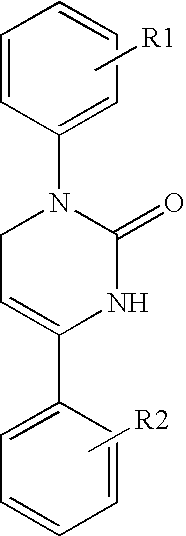
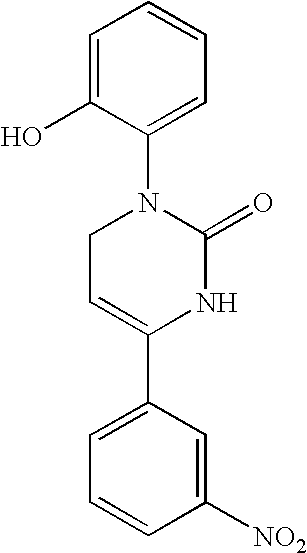
Therapeutic 1,2,3,6-tetrahydropyrimidine-2-one compositions and methods therewith
Owner:CRAGMONT PHARMA
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions that comprise an expectorant, an extended release antitussive, and an extended release decongestant. Specifically, the compositions comprise guaifenesin, phenylephrine tannate, and dextromethorphan tannate. The present invention also includes methods for using these compositions for treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions comprising an antitussive, a decongestant and an expectorant, and in a specific embodiment comprising hydrocodone, phenylephrine hydrochloride and guaifenesin, wherein the composition may be substantially free of added sugar and added alcohol, and methods for using these compositions for the treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Solid pharmaceutical dosage unit for alleviating symptoms of rhinorrhea
InactiveUS20080292699A1Reducing and eliminating over-dryingBiocidePill deliveryAnticholinergic agentsDecongestant
A solid pharmaceutical dosage unit for alleviating the symptoms of rhinorrhea. The dosage unit comprises an anticholinergic agent and an antihistamine and, optionally, a decongestant and, when placed in a basket in 500 ml of 0.01 N HCl of 37° C. which is stirred at 100 rpm, releases at least about 75% of the at least one anticholinergic agent within 45 minutes and releases the at least one antihistamine at a rate of from about 20% to about 60% after 2 hours, from about 45% to about 80% after 4 hours and at least about 75% after 8 hours. This Abstract is not intended to define the invention disclosed in the specification, nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
Owner:EVERETT LAB
System and method for suppressing a cough
A system and method for suppressing coughs, congestion and related symptoms is provided. The composition sprayed into the oral cavity may contain a cough suppressant and other ingredients such as decongestants, flavorings, sweeteners, stablizers, and / or preservatives. The composition is sprayed into the oral cavity utilizing a spray pattern having a spray pattern ratio from about 1.0 to about 1.19. The method of application comprises multiple applications of the composition every four to six hours according to condition severity and a user's age characteristics.
Owner:MATRIXX INITIATIVES
Compositions to reduce congestion and methods for application thereof to the nasal membrane
A gelled composition formulated to maintain an active ingredient in association with the nasal membrane for an extended period of time is provided. The gelled composition may be formulated as a decongestant or a sinus discomfort relieving agent. The invention further includes a system and method for applying the gelled composition to the nasal membrane.
Owner:CHURCH & DWIGHT CO INC
Kits for Prevention and Treatment of Rhinitis
Kits providing a combination of one or more pharmaceutical information comprising one or more agent(s) for the treatment or alleviation of symptoms commonly associated with a cold and an immunonutritional composition comprising immunonutritional agent and methods of using these kits are described . The kits provide both the pharmaceutical agent(s) and the immunonutritional agent in a convenient form for administration. The kit typically includes instruction for coordinating the administration of the pharmaceutical formulation with the administration of the immunonutritional composition. The preferred immunonutritional agents are compounds that contain a pharmaceutically acceptable form of zinc, such as zinc acetate, zinc gluconate, zinc gluconate glycine, and zinc sulfate. Preferably the kit contains multiple dosage forms containing the immunonutritional composition. In the most preferred embodiment, the immunonutritional composition is in the form of a lozenge. Suitable pharmaceutical agents include but are not limited to antihistamines, decongestants, anticholinergies, antitussives, analgestics, mucolytics, expectorants, and combinations thereof. The pharmaceutical formulations may be in any suitable dosage form, including forms which provide controlled release of the pharmaceutical agent, including immediate, sustained, modified, delayed or pulsed release pharmacokinetic mechanism or a combination thereof. The combined treatment requires administration of both the pharmaceutical formulation(s) for the treatment of symptoms commonly associated with a cold and the administration of the immunonutritional composition, which supplies nutritional support for the patient's innate immune response to the presence of infectious organisms.
Owner:AURIGA LAB
Methods and compositions using optically pure (-) cetirizine in combination with leukotriene inhibitors or decongestants
InactiveUS6790849B2Potent antihistaminic activityAvoid it happening againRespiratory disorderOptically-active compound separationDecongestantCetirizine
Methods and pharmaceutical compositions employing (+) cetirizine, (-) cetirizine, or racemic cetirizine, or a pharmaceutically acceptable salt thereof, and a leukotriene inhibitor, or a pharmaceutically acceptable salt thereof, or decongestant for the treatment, management, and / or prevention of inflammation, asthma or symptoms thereof, allergic disorders such as allergic rhinitis, and dermatitis.
Owner:SUNOVION PHARMA INC
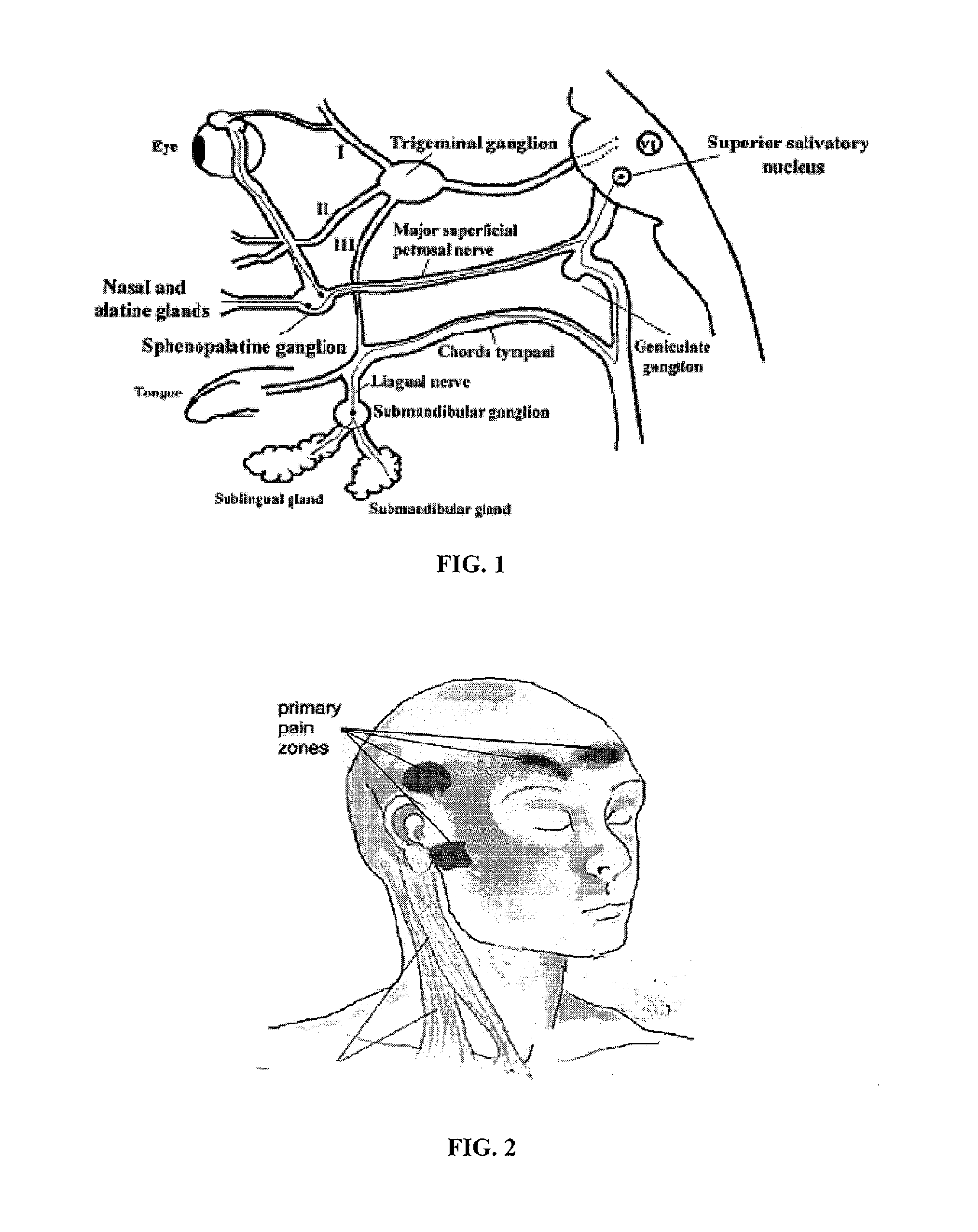
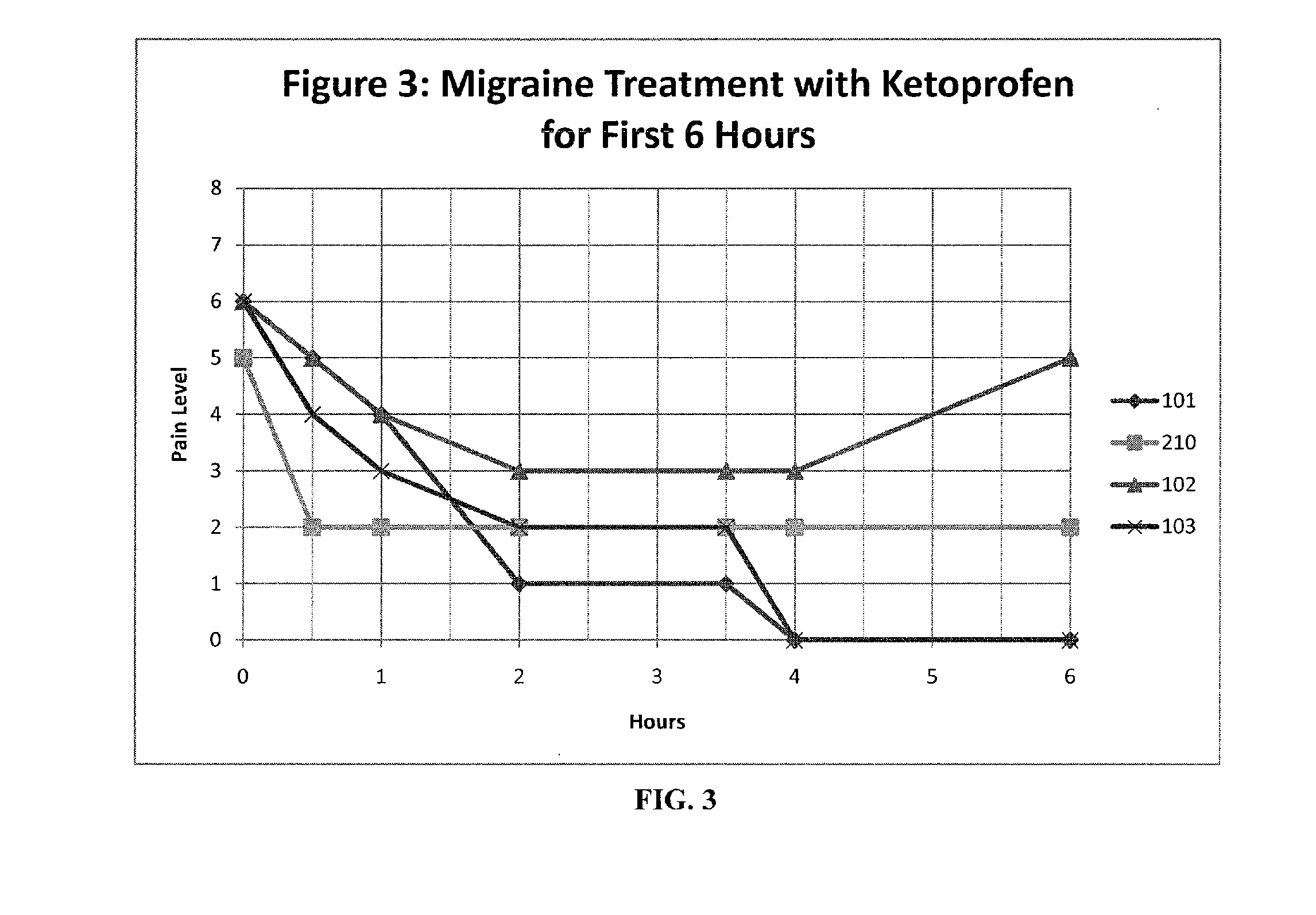
Methods and compositions for treating and preventing trigeminal autonomic cephalgias, migraine, and vascular conditions
The present invention relates to, among other things, methods for treating trigeminal cephalgias such as migraine and migraine like headaches and other cerebrovascular conditions associated with pain and or inflammation. When non-steroidal anti inflammatory drugs (NSAIDs), such as ketoprofen, are applied locally using specific topical formulations immediate relief of pain is obtained. Intense pain is typically reduced to mild pain or no pain within 30 minutes of application of the topical formulation. The NSAID may be given in combination with other pharmacological agents, such as vasoconstrictors, opioids, decongestants and / or non-opioid migraine drugs, such as triptans and ergots and agents that affect serotonin receptors as agonists, antagonists or partial agonists.
Owner:ACHELIOS THERAPEUTICS
Nitrosated and nitrosylated cyclooxygenase-2 inhibitors, compositions and methods of use
InactiveUS7166618B2Improving gastrointestinal property of COX-Promote wound healingBiocideSenses disorderSedating AntihistaminesHydrolase inhibitor
The present invention describes novel nitrosated and / or nitrosylated cyclooxygenase 2 (COX-2) inhibitors and novel compositions comprising at least one nitrosated and / or nitrosylated cyclooxygenase 2 (COX-2) inhibitor, and, optionally, at least one compound that donates, transfers or releases nitric oxide, stimulates endogenous synthesis of nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor or is a substrate for nitric oxide synthase, and / or optionally, at least one therapeutic agent, such as, steroids, nonsteroidal antiinflammatory compounds (NSAID), 5-lipoxygenase (5-LO) inhibitors, leukotriene B4 (LTB4) receptor antagonists, leukotriene A4 (LTA4) hydrolase inhibitors, 5-HT agonists, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors, H2antagonists, antineoplastic agents, antiplatelet agents, decongestants, diuretics, sedating or non-sedating anti-histamines, inducible nitric oxide synthase inhibitors, opioids, analgesics, Helicobacter pylori inhibitors, proton pump inhibitors, isoprostane inhibitors, and mixtures thereof. The present invention also provides novel compositions comprising at least one parent COX-2 inhibitor and at least one nitric oxide donor, and, optionally, at least one therapeutic agent. The present invention also provides kits and methods for treating inflammation, pain and fever; for treating and / or improving the gastrointestinal properties of COX-2 inhibitors; for facilitating wound healing; for treating and / or preventing renal toxicity; and for treating and / or preventing other disorders resulting from elevated levels of cyclooxygenase-2.
Owner:NICOX SA
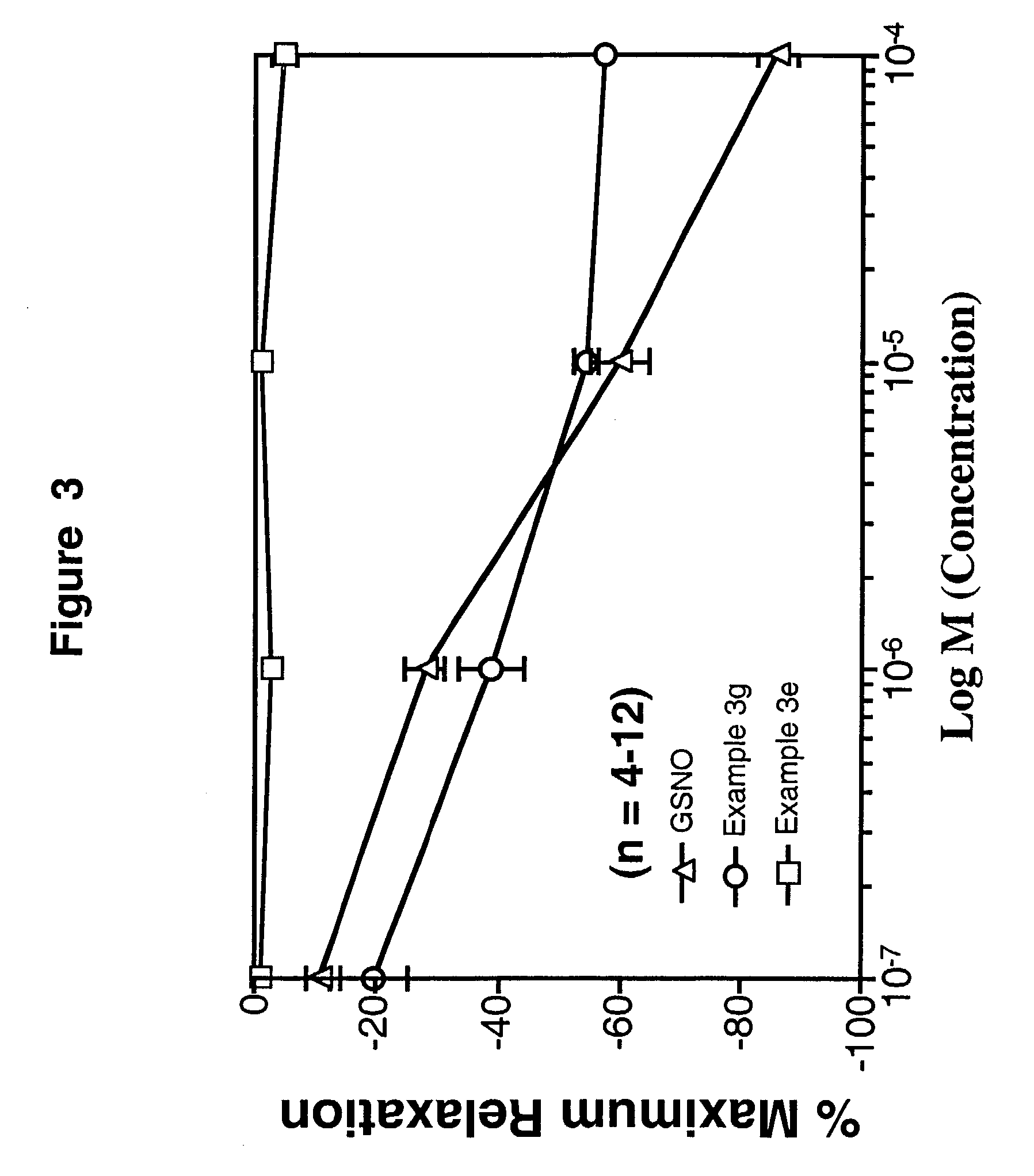
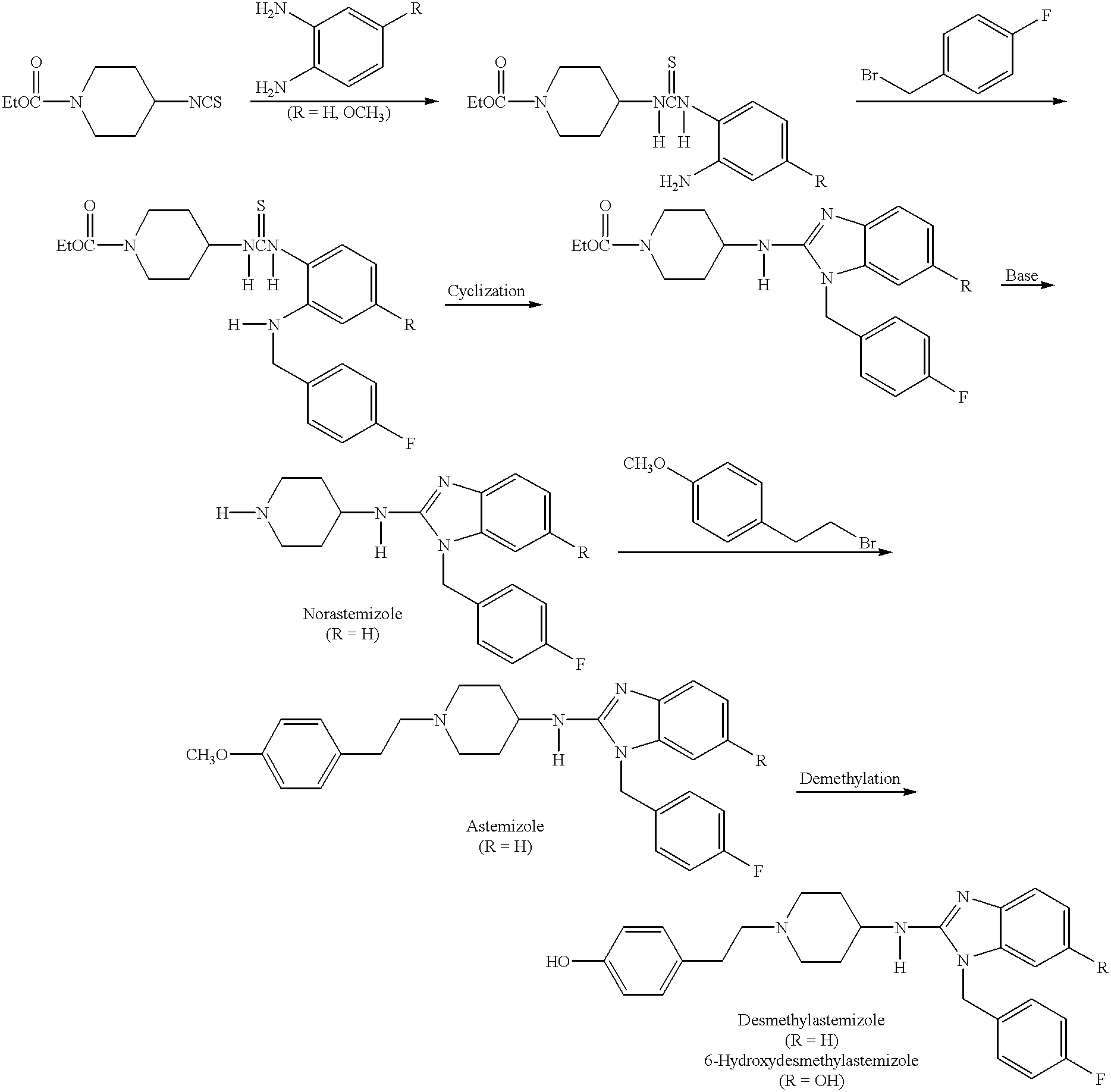
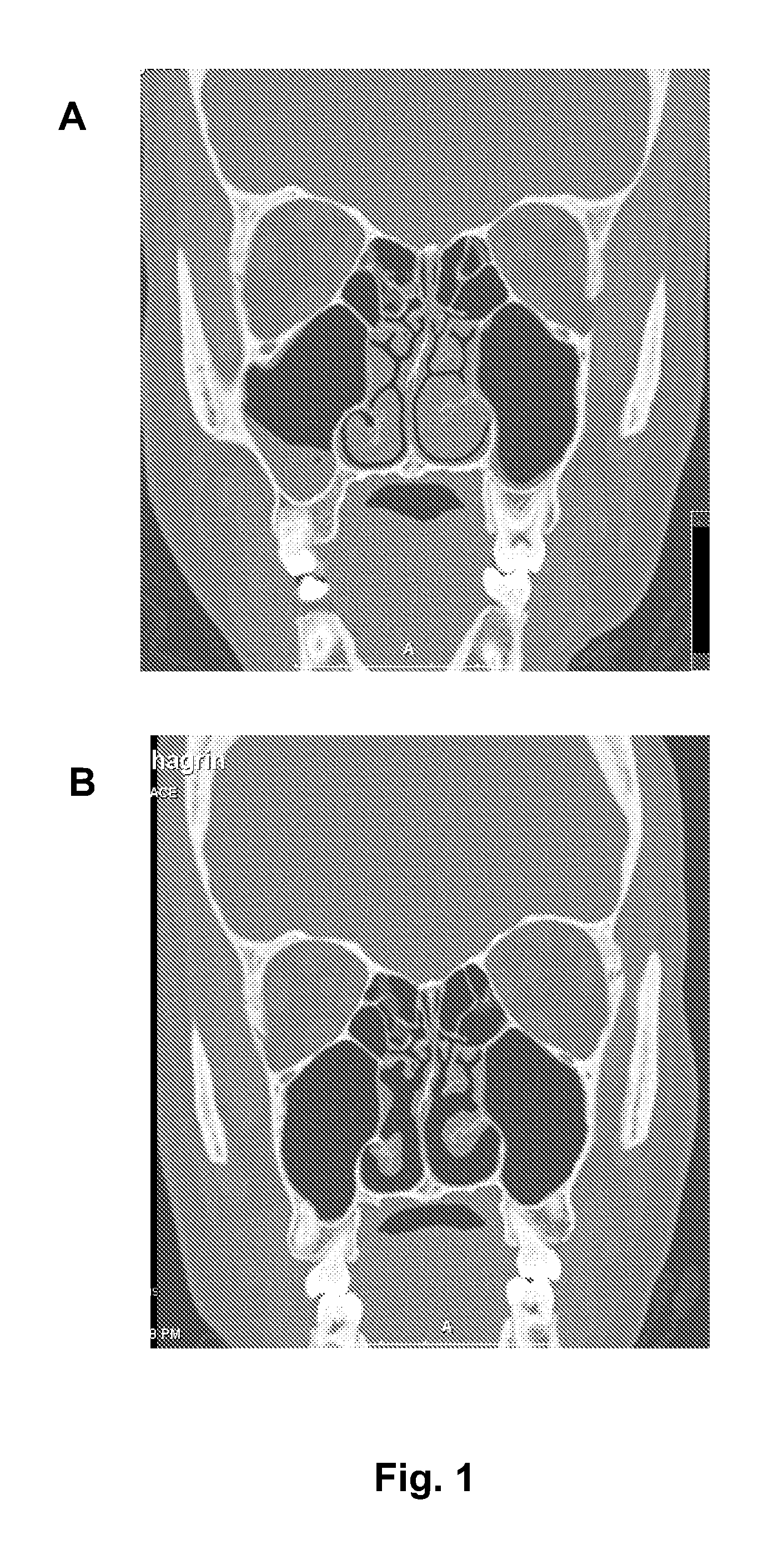
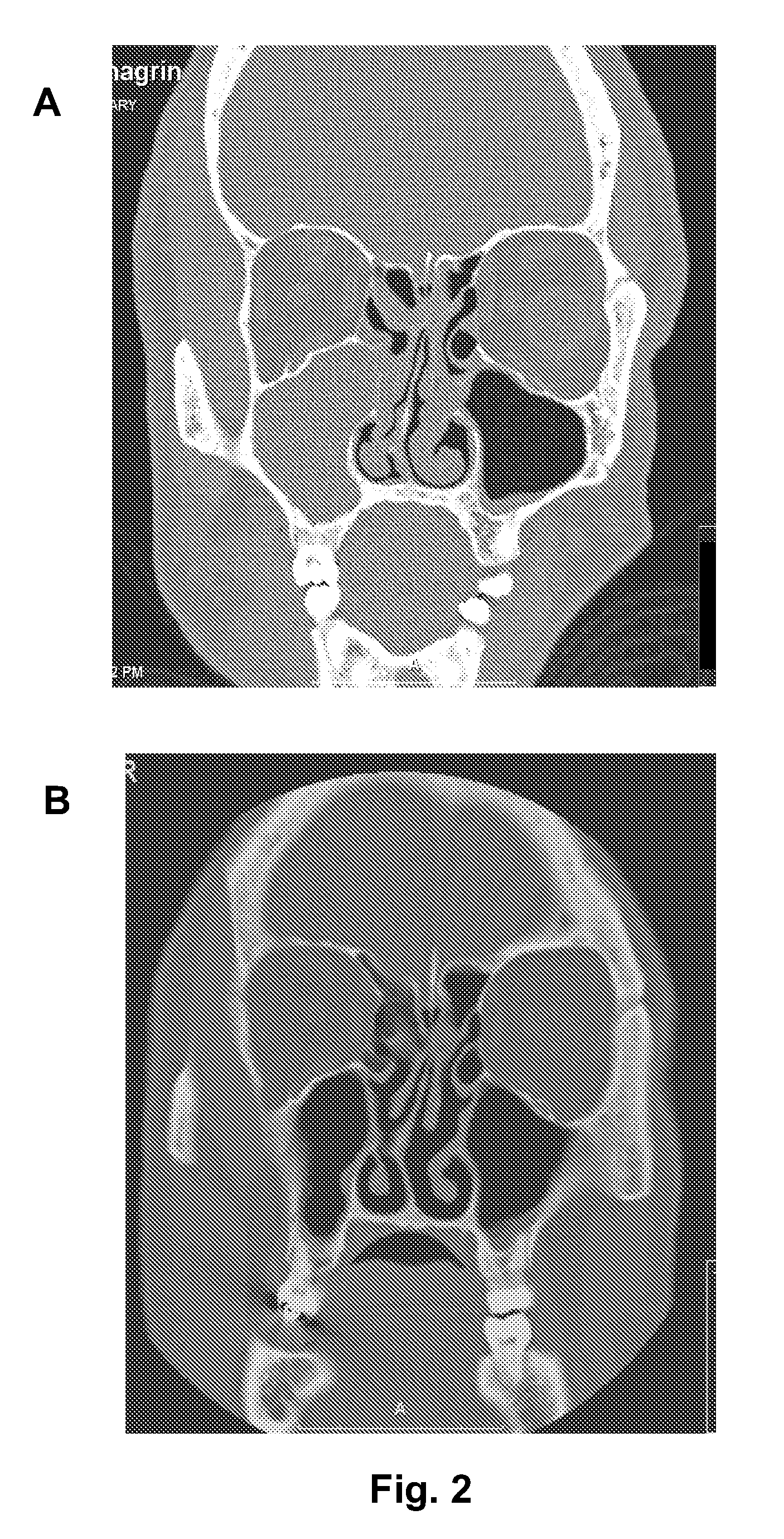
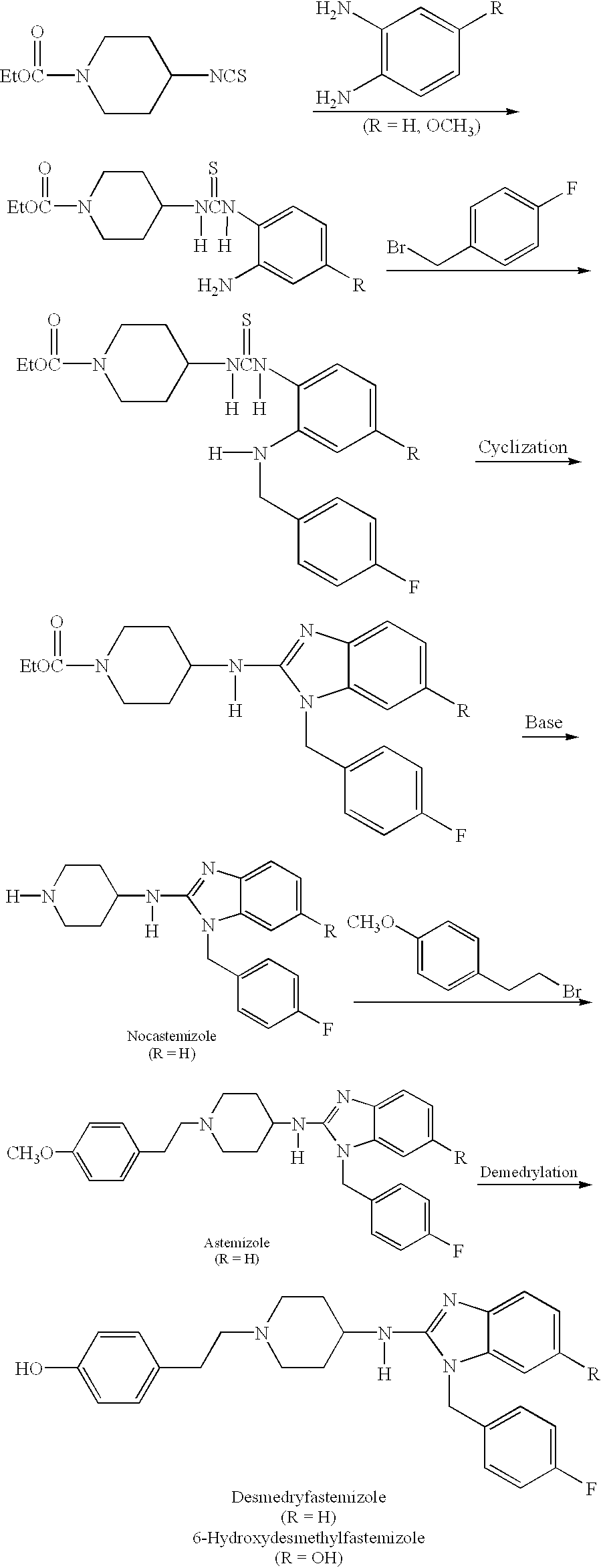
Compositions for treating allergic and other disorders using norastemizole in combination with other active ingredients
InactiveUS6303632B1Reduce adverse effectsUseful in treatmentBiocideOrganic chemistryMotion sicknessWhole body
Methods and compositions are disclosed utilizing metabolic derivatives of astemizole for the treatment of allergic disorders while avoiding the concomitant liability of adverse effects associated with the astemizole. The metabolic derivatives of astemizole are also useful for the treatment of retinopathy and other small vessel disorders associated with diabetes mellitus and such other conditions as may be related to the antihistamine activity of astemizole. For example, the metabolic derivatives of astemizole are useful for the treatment of asthma, motion sickness, and vertigo, without the concomitant liability of adverse effects associated with astemizole. Furthermore, the metabolic derivatives of astemizole, in combination with non-steroidal anti-inflammatory agents or other non-narcotic analgesics, or in combination with a decongestant, cough suppressant / antitussive or expectorant, are useful for the treatment of cough, cold, cold-like, and / or flu symptoms and the discomfort, headache, pain, fever, and general malaise associated therewith, without the concomitant liability of adverse effects associated with astemizole.
Owner:SEPACOR INC
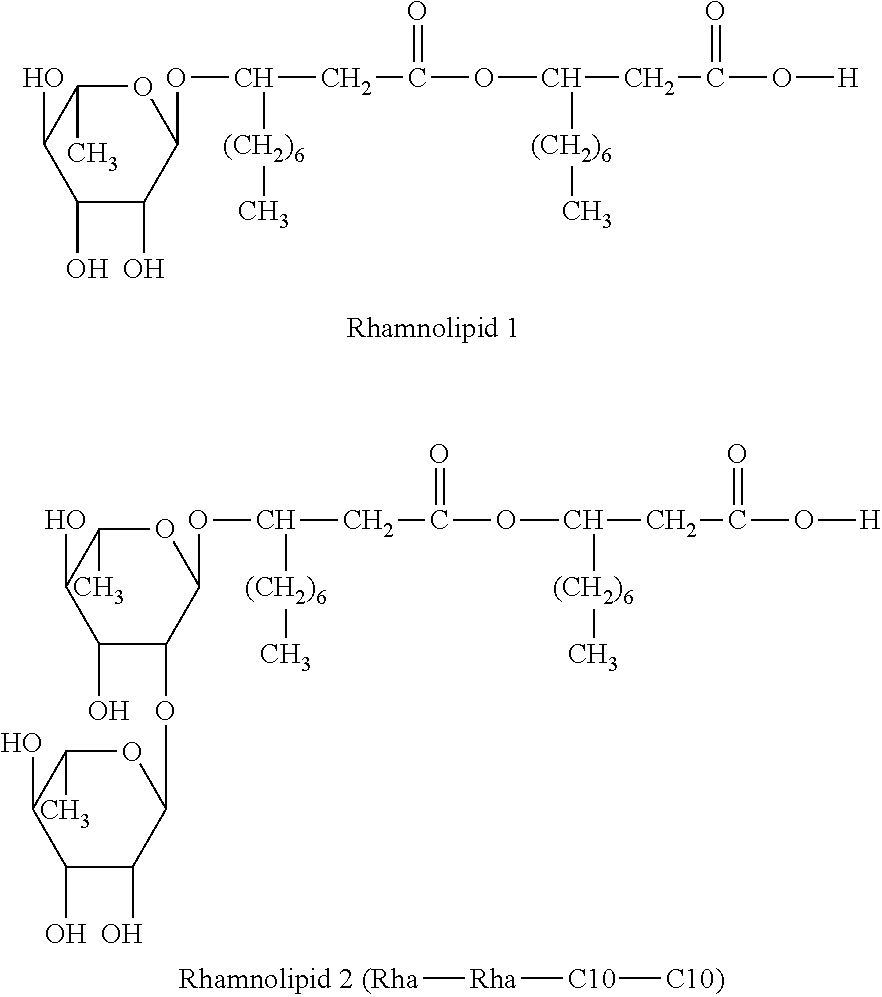
Method for treating rhinitis and sinusitis by rhamnolipids
The present invention is directed to methods for treating rhinitis or sinusitis in a subject. In one embodiment, the method comprises the steps of: identifying a subject in need thereof, and administering intranasally to the subject a formulation comprising an only active ingredient of an effective amount of rhamnolipid. In another embodiment, the method comprises the steps of: identifying a subject in need thereof, and administering intranasally to the subject a first active ingredient of an effective amount of a rhamnolipid and a second active ingredient of an effective amount of a corticosteroid, an antihistamine, a leukotriene antagonist, cromylin, an antibiotic, a sphingolipid, or a decongestant.
Owner:LEIGHTON ANTON
Methods and compositions for treating allergic disorders and other disorders using metabolic derivatives of astemizole
InactiveUS6130233AReduce adverse effectsUseful in treatmentBiocideOrganic chemistryMotion sicknessDecongestant
Owner:SEPACOR INC
Nasal spray composition and method for treating rhinitis, sinusitis or both
A nasal spray composition for treating mucosal inflammation associated with rhinitis, sinusitis, or both can include a decongestant and at least one therapeutic agent. The therapeutic agent is selected from the group consisting of an anti-inflammatory agent and an anti-histamine agent. The nasal spray composition is non-habituating and is administered intranasally to a subject in need thereof.
Owner:KNAUER KENT A
Methods for treating allergic disorders using norastemizole
InactiveUS6124320AReduce adverse effectsUseful in treatmentBiocideOrganic chemistryMotion sicknessDecongestant
Methods and compositions are disclosed utilizing metabolic derivatives of astemizole for the treatment of allergic disorders while avoiding the concomitant liability of adverse effects associated with the astemizole. The metabolic derivatives of astemizole are also useful for the treatment of retinopathy and other small vessel disorders associated with diabetes mellitus and such other conditions as may be related to the antihistamine activity of astemizole. For example, the metabolic derivatives of astemizole are useful for the treatment of asthma, motion sickness, and vertigo, without the concomitant liability of adverse effects associated with astemizole. Furthermore, the metabolic derivatives of astemizole, in combination with non-steroidal anti-inflammatory agents or other non-narcotic analgesics, or in combination with a decongestant, cough suppressant / antitussive or expectorant, are useful for the treatment of cough, cold, cold-like, and / or flu symptoms and the discomfort, headache, pain, fever, and general malaise associated therewith, without the concomitant liability of adverse effects associated with astemizole.
Owner:SEPACOR INC
Methods for treating disorders using norastemizole in combination with other active ingredients
InactiveUS6458809B1Reduce adverse effectsUseful in treatmentBiocideAnimal repellantsDiseaseAntiinflammatory drug
Methods and compositions are disclosed utilizing metabolic derivatives of astemizole for the treatment of allergic disorders while avoiding the concomitant liability of adverse effects associated with the astemizole. The metabolic derivatives of astemizole are also useful for the treatment of retinopathy and other small vessel disorders associated with diabetes mellitus and such other conditions as may be related to the antihistamine activity of astemizole. For example, the metabolic derivatives of astemizole are useful for the treatment of asthma, motion sickness, and vertigo, without the concomitant liability of adverse effects associated with astemizole. Furthermore, the metabolic derivatives of astemizole, in combination with non-steroidal anti-inflammatory agents or other non-narcotic analgesics, or in combination with a decongestant, cough suppressant / antitussive or expectorant, are useful for the treatment of cough, cold, cold-like, and / or flu symptoms and the discomfort, headache, pain, fever, and general malaise associated therewith, without the concomitant liability of adverse effects associated with astemizole.
Owner:SEPACOR INC
Substituted aryl compounds as novel cyclooxygenase-2 selective inhibitors, compositions and methods of use
InactiveUS20050059665A1Unexpected potential for facilitating wound healingHave antiinflammatory propertiesBiocideSenses disorderHydrolase inhibitorThromboxanes
The invention describes novel substituted aryl compounds that are cyclooxygenase 2 (COX-2) selective inhibitors and novel compositions comprising at least one cyclooxygenase 2 (COX-2) selective inhibitor, and, optionally, at least one compound that donates, transfers or releases nitric oxide, stimulates endogenous synthesis of nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor or is a substrate for nitric oxide synthase, and / or, optionally, at least one therapeutic agent, such as, steroids, nonsterodal anti-inflammatory compounds (NSAID), 5-lipoxygenase (5-LO) inhibitors, leukotriene B4 (LTB4) receptor antagonists, leukotriene A4 (LTA4) hydrolase inhibitors, 5-HT agonists, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors, H2 antagonists, antineoplastic agents, antiplatelet agents, thrombin inhibitors, thromboxane inhibitors, decongestants, diuretics, sedating or non-sedating anti-histamines, inducible nitric oxide synthase inhibitors, opioids, analgesics, Helicobacter pylori inhibitors, proton-pump-inhibitors, isoprostane inhibitors, and mixtures thereof. The invention also provides novel kits comprising at least one COX-2 selective inhibitor, and, optionally, at least one nitric oxide donor, and / or, optionally, at least one therapeutic agent. The novel cyclooxygenase 2 selective inhibitors of the invention can be optionally nitrosated and / or nitrosylated. The invention also provides methods for treating inflammation, pain and fever; for treating and / or improving the gastrointestinal properties of COX-2 selective inhibitors; for facilitating wound healing; for treating and / or preventing renal toxicity or other toxicities; for treating and / or preventing other disorders resulting from elevated levels of cyclooxygenase-2; and for improving the cardiovascular profile of COX-2 selective inhibitors.
Owner:NICOX SA
Method of treating snoring and other obstructive breathing disorders
InactiveUS20040258621A1Reduce eliminateRelieve symptomsBiocideImpression capsNasal cavityGastrointestinal reflux
A method of pharmaceutically managing snoring and impaired breathing is provided. This invention relates to treating snoring, sleep apnea, and other forms of sleep-disordered breathing in those with or without symptoms of or the diagnosis of gastro-intestinal reflux disease (GERD). It comprises administration of a therapeutically effective dose of Prevacid (Lansoprazole) or any other medication that can be used to treat symptoms of hyper-acidity or gastrointestinal reflux disease (GERD). The therapeutic medication may be used alone or in combination with other pharmacologic agents or mechanical modalities including but not limited to decongestants, antihistamines or mechanical nasal toilet. It also is of benefit in improving breathing disorders that are present while awake.
Owner:SOHNSTEARNS & STERN
Tannate dry powder formulations
Dry powder tannate compositions containing bioactive agents, tannic acid, dispersants, and viscosity modifying agents are disclosed. Specifically, the bioactive agents are antihistamines, decongestants, antitussives, and anticholinergics. The dry powder formulations can further include pharmaceutically acceptable excipients. The dry powder formulations exhibit increased stability for extended shelf life. Bioactive agent tannate salts remain suspended for at least two weeks following formation of the suspension in a pharmaceutically acceptable aqueous liquid.
Owner:ACELLA PHARMA
Compositions comprising an antihistamine, antitussive and decongestant in extended release formulations
The invention provides oral formulations for the treatment of cold and allergy symptoms. Each formulation combines an antihistamine, an antitussive, and / or a decongestant into one extended release composition. The invention further provides for methods of making and using such formulations, as well as for methods for preventing abuse or extraction of a single drug present in an oral extended release composition comprising two or more of an antihistamine, antitussive, and / or decongestant.
Owner:ATTKISSON ELIZABETH E
Kits for prevention and treatment of rhinitis
Owner:HALL MISCHELLE +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com