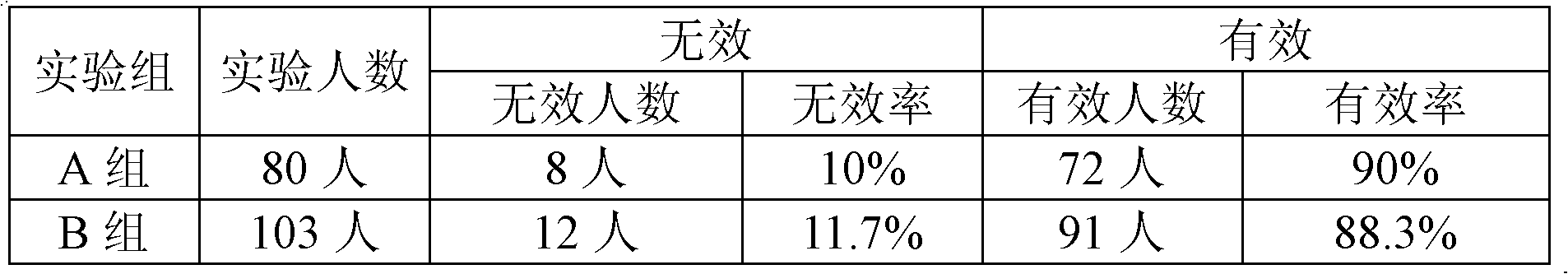
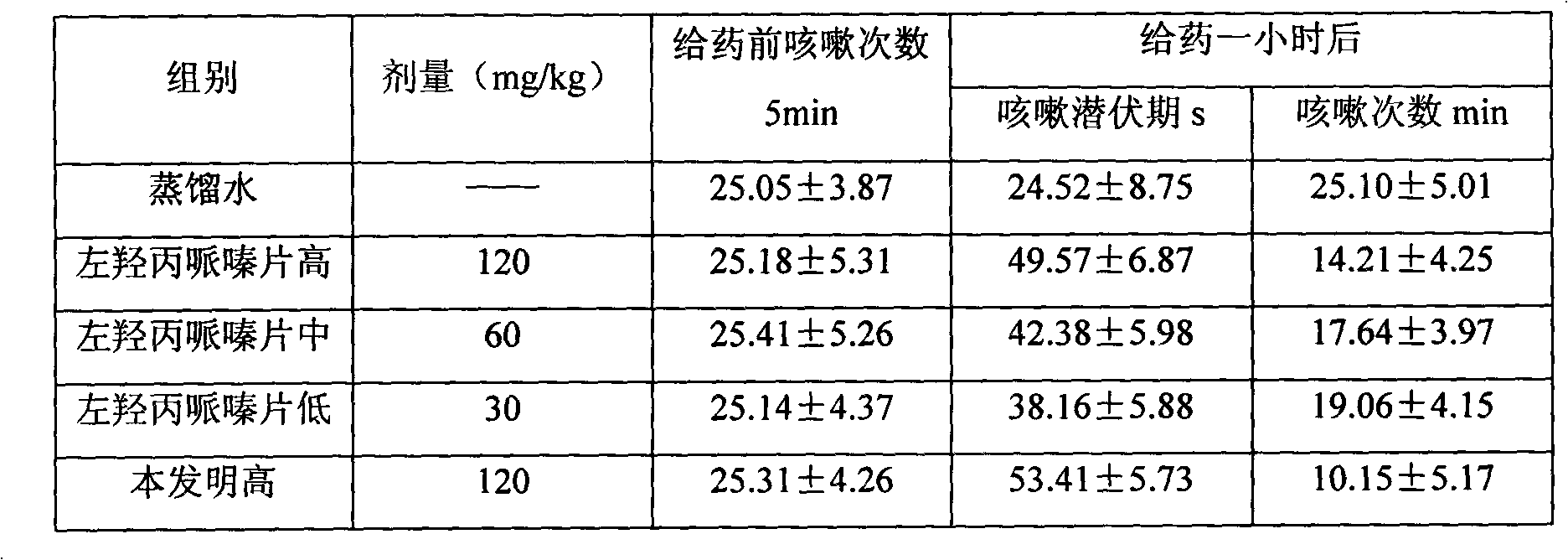
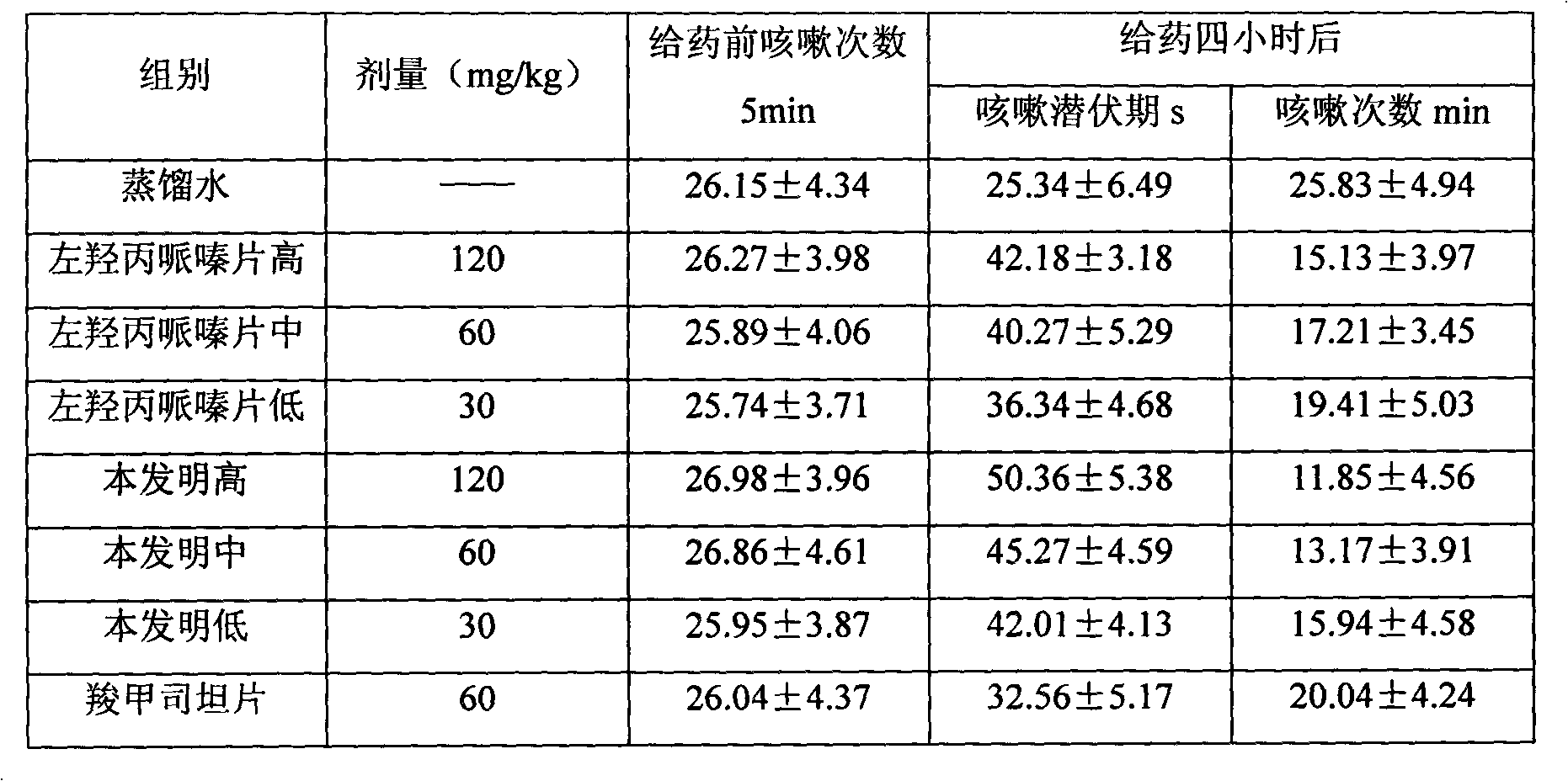
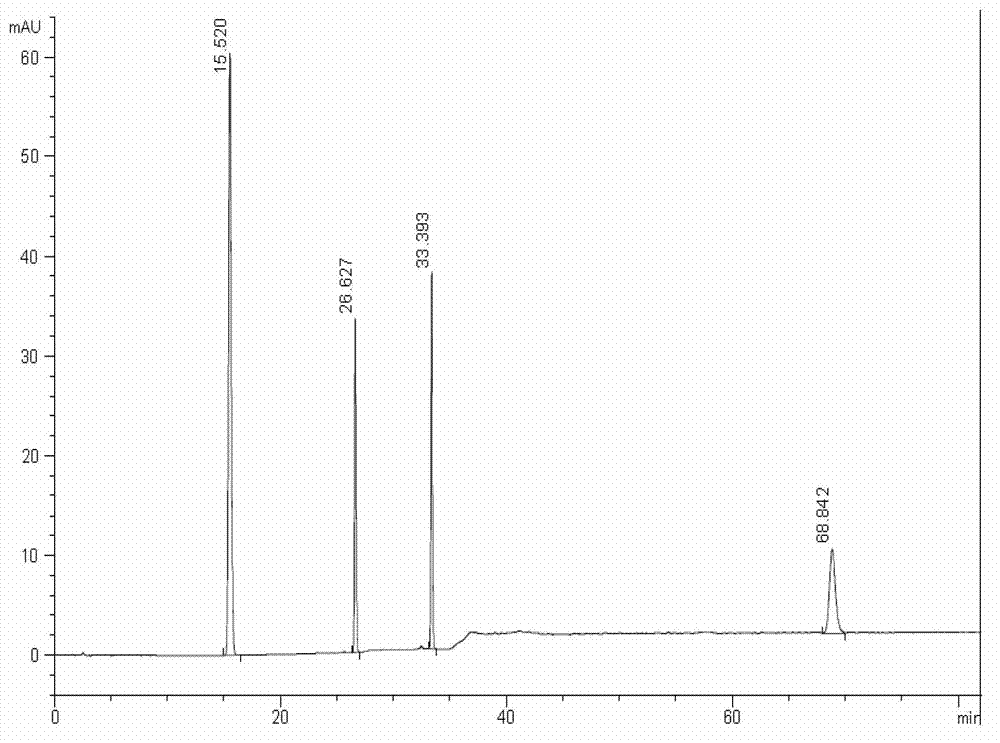
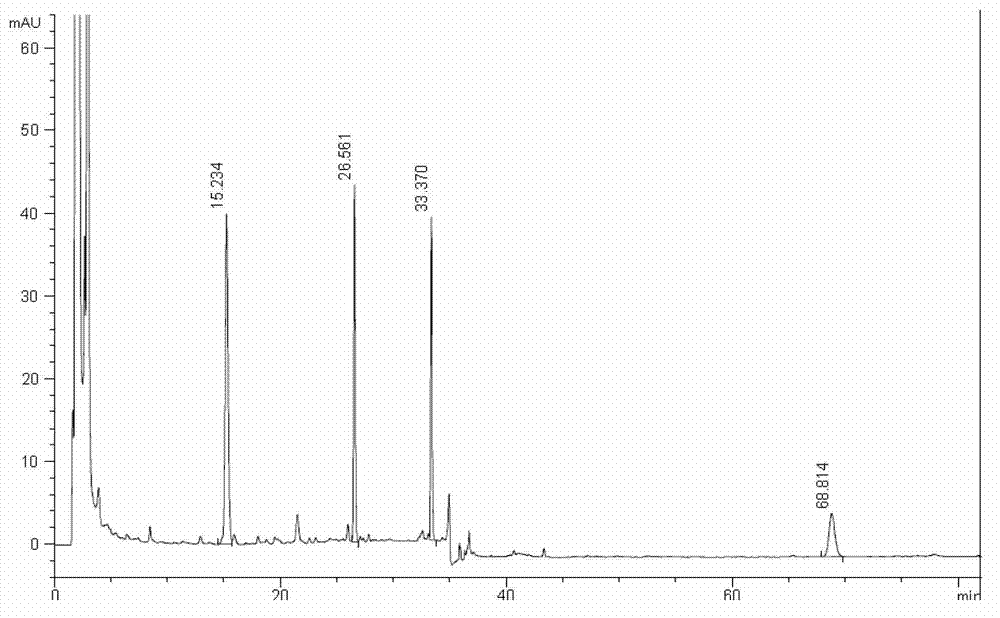
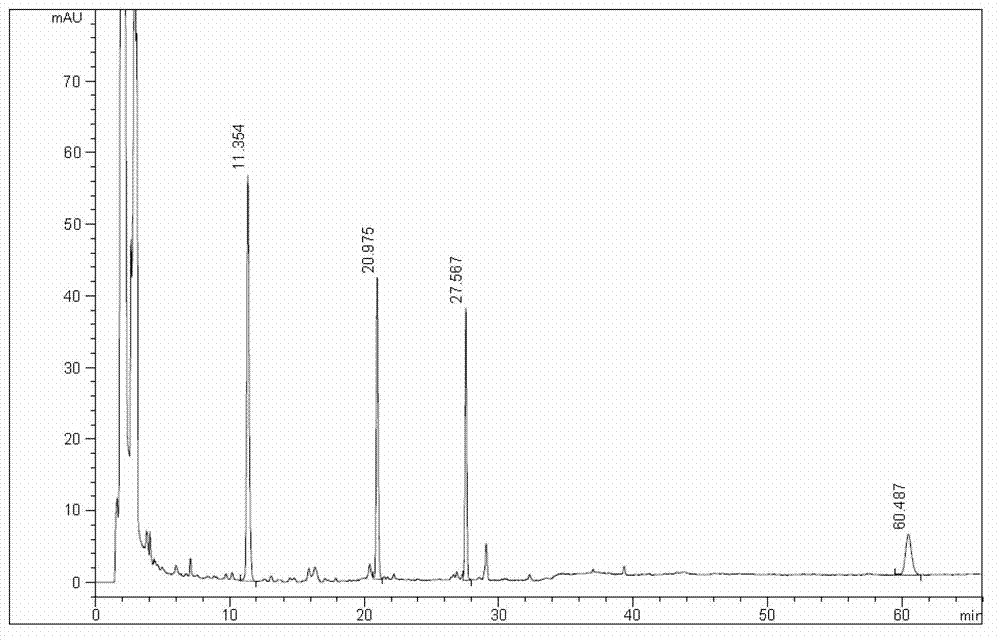
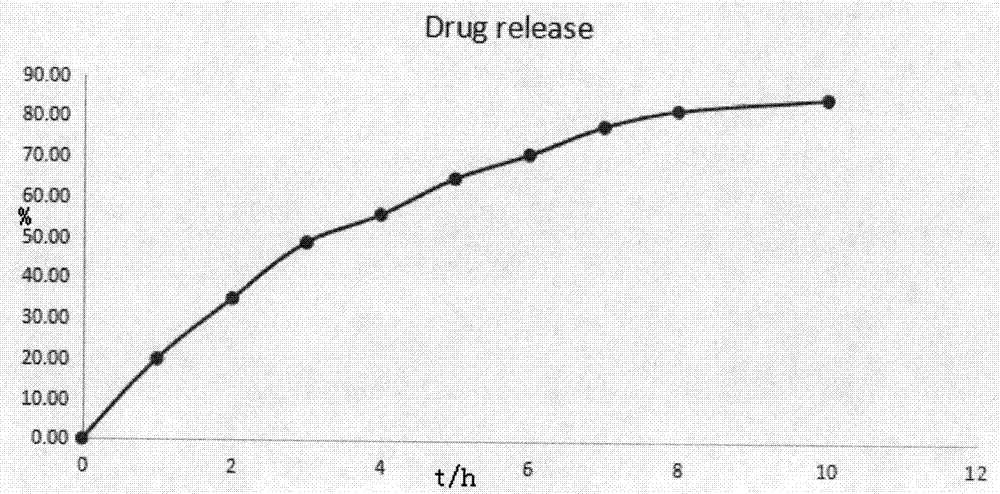
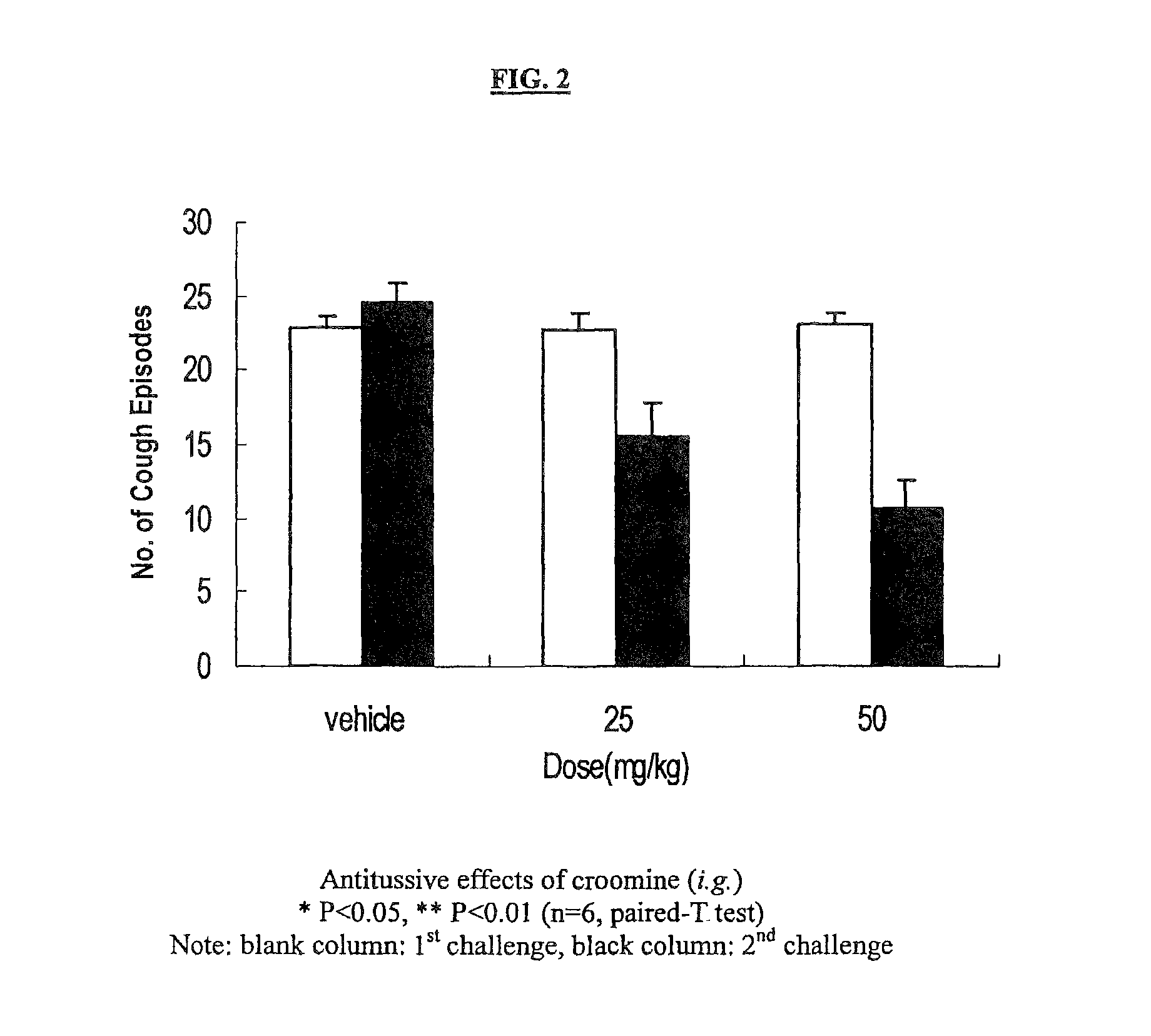
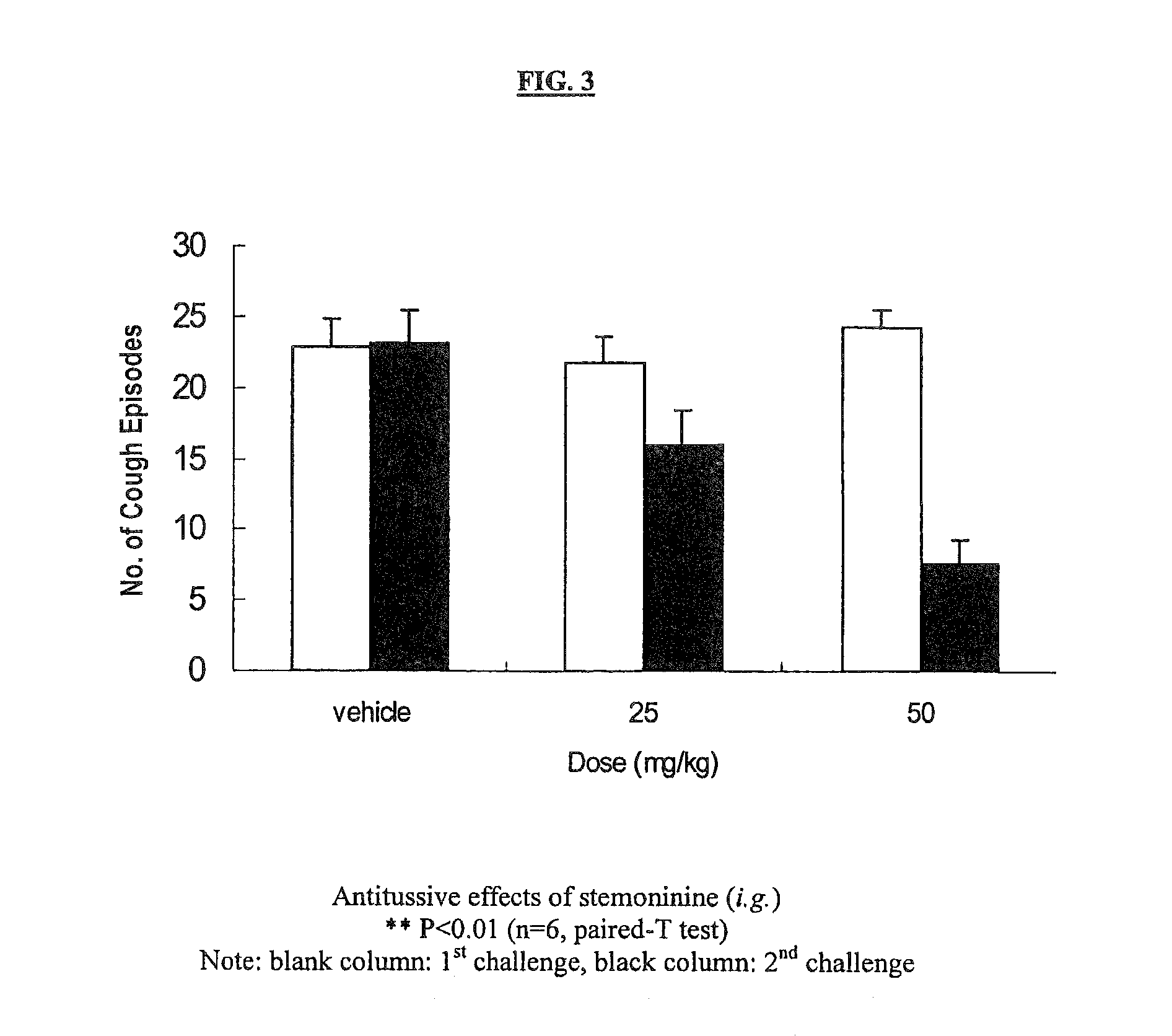
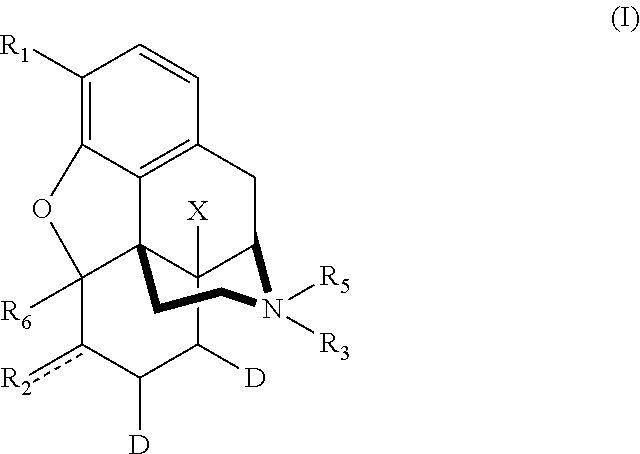
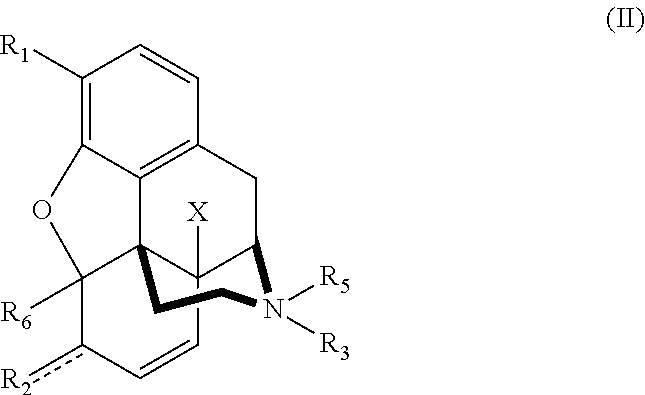
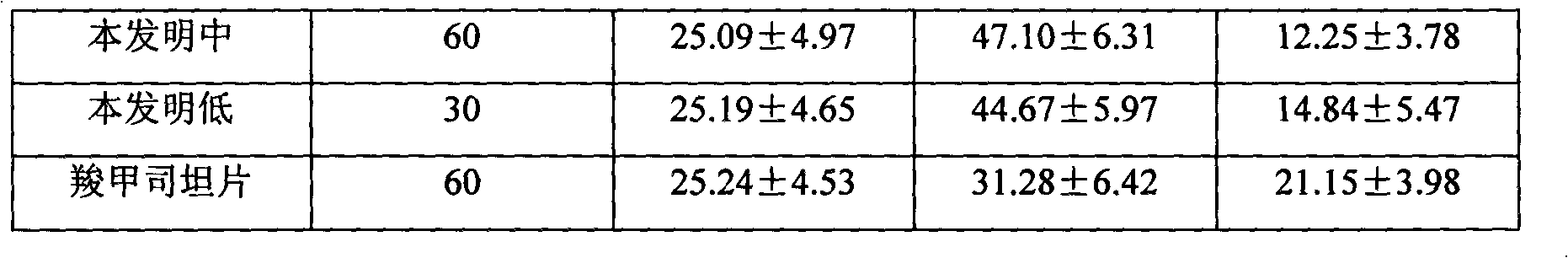Patents
Literature
57 results about "Antitussive Agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any substance that is capable of relieving or suppressing coughing.
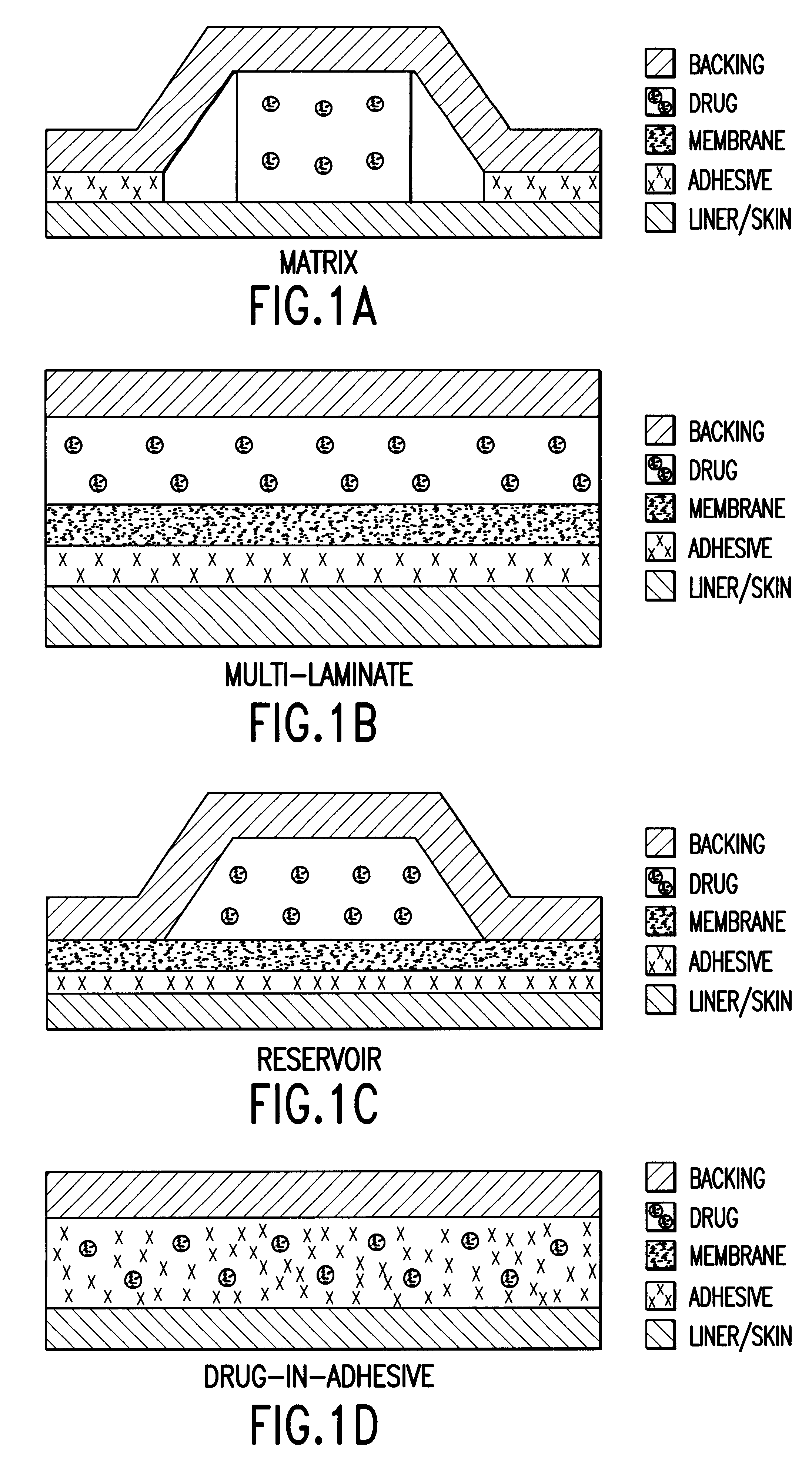
Dosage form containing multiple drugs
A pharmaceutical dosage form comprising a first drug and a second drug, both of which are selected from decongestants, antitussives, expectorants, analgesics and antihistamines. The dosage form provides a plasma concentration within a therapeutic range of the second drug over a period which is coextensive with at least about 70% of a period over which the dosage form provides a plasma concentration within a therapeutic range of the first drug. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Fast dissolving orally consumable films containing an antitussive and a mucosa coating agent
A consumable film adapted to adhere to and dissolve in the oral cavity of a consumer comprising at least one water soluble polymer, at least one antitussive agent and a mucosa-coating effective amount of a mucosa-coating agent.
Owner:MCNEIL PPC INC
Sustained-release drug delivery compositions and methods
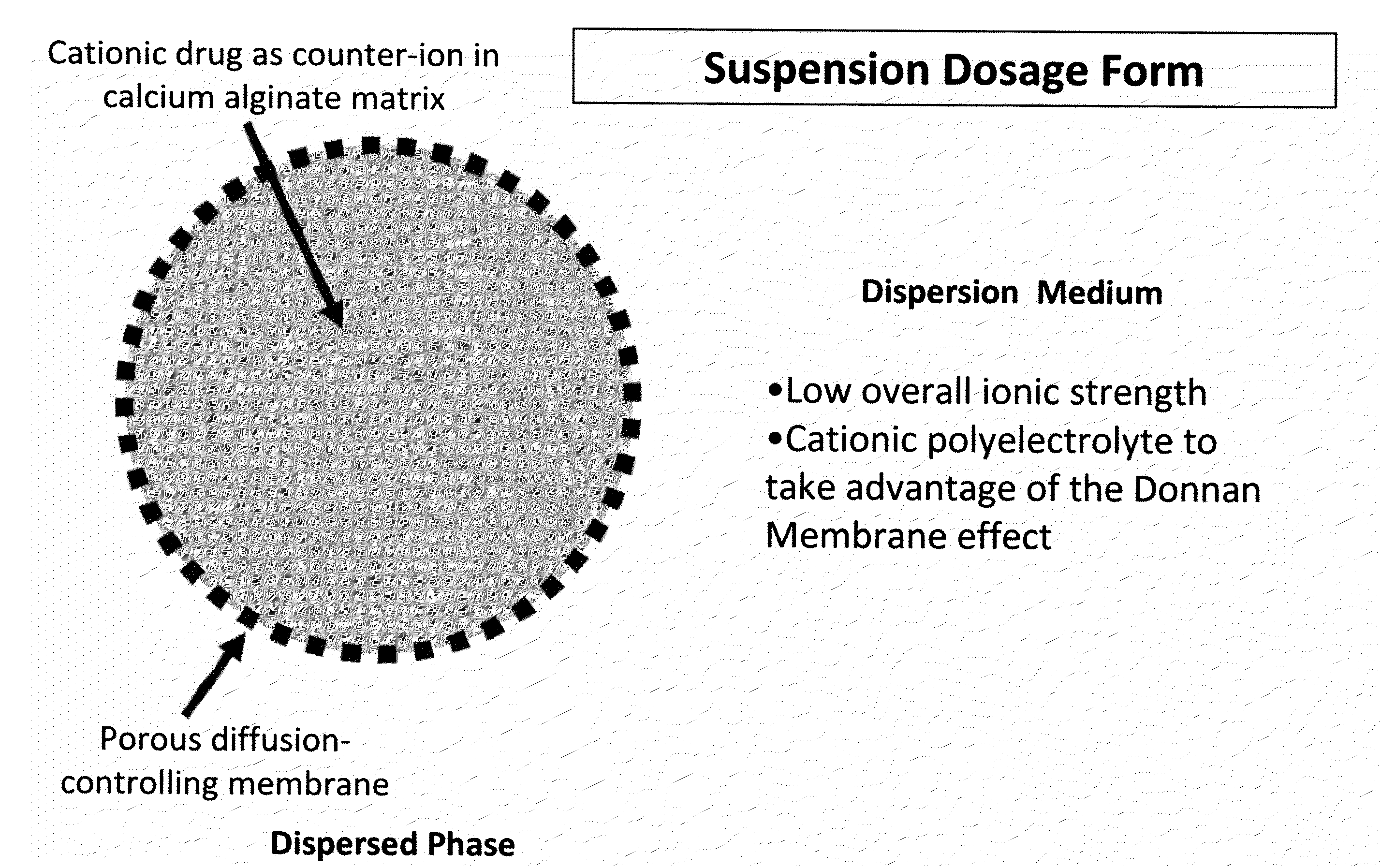
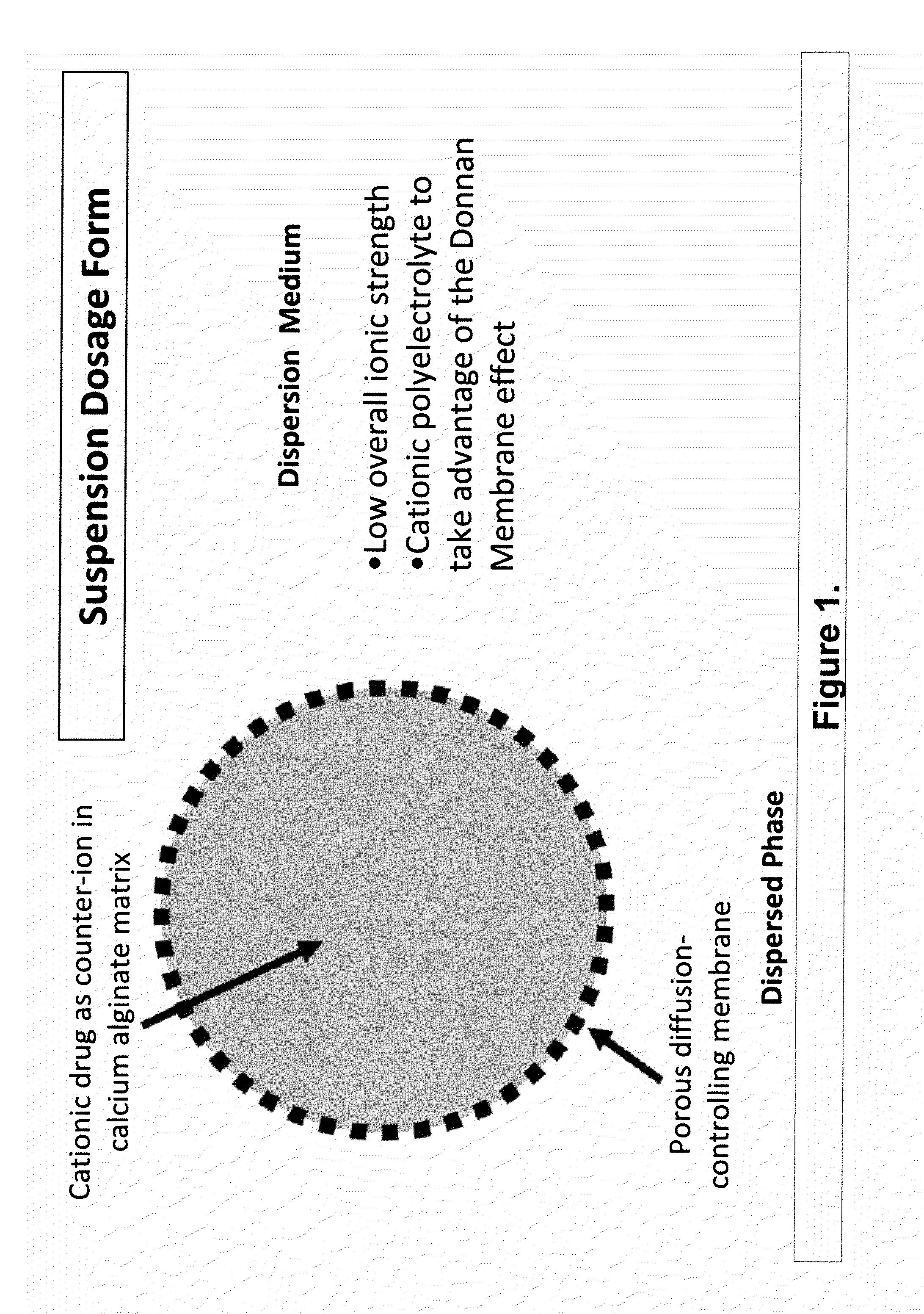
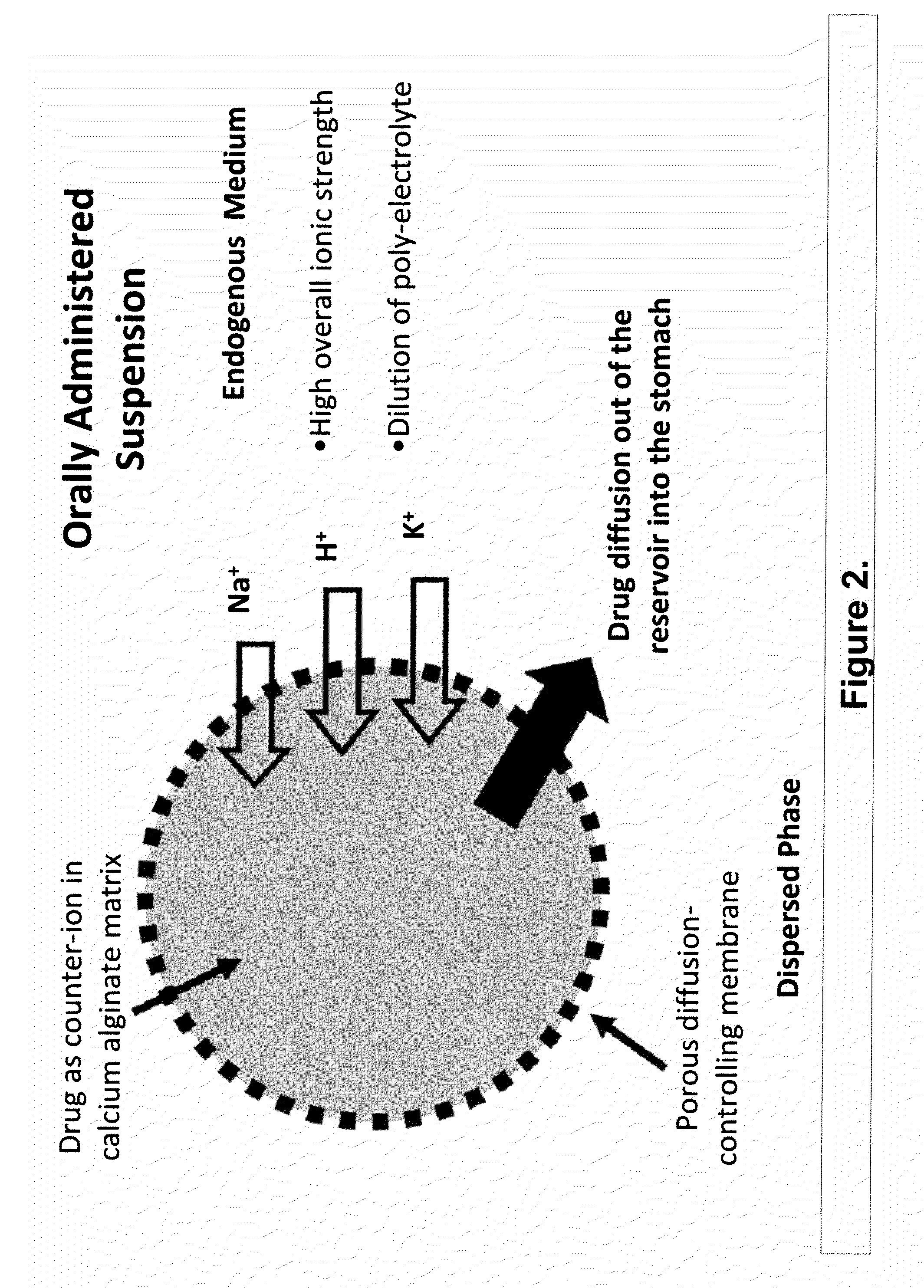
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
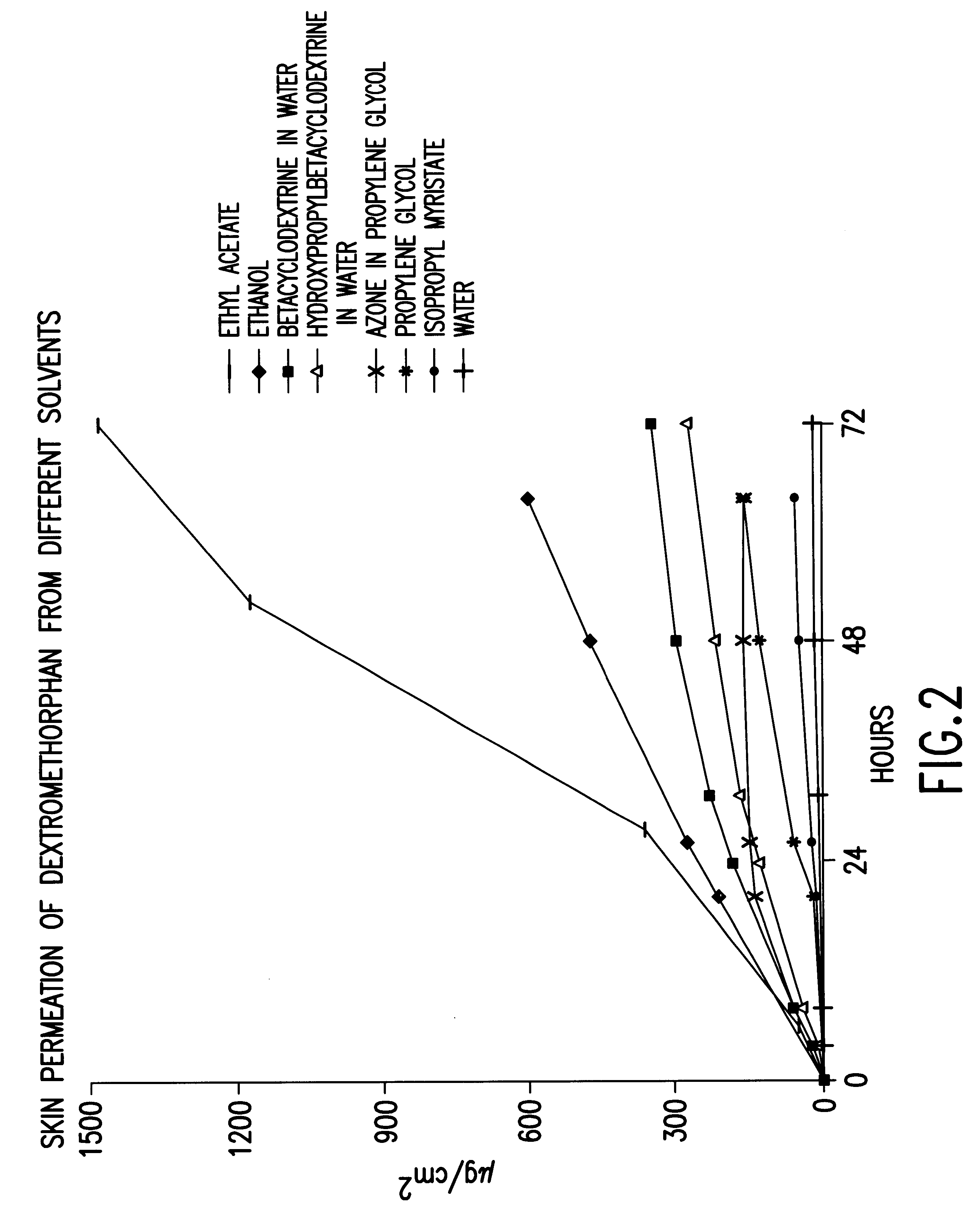
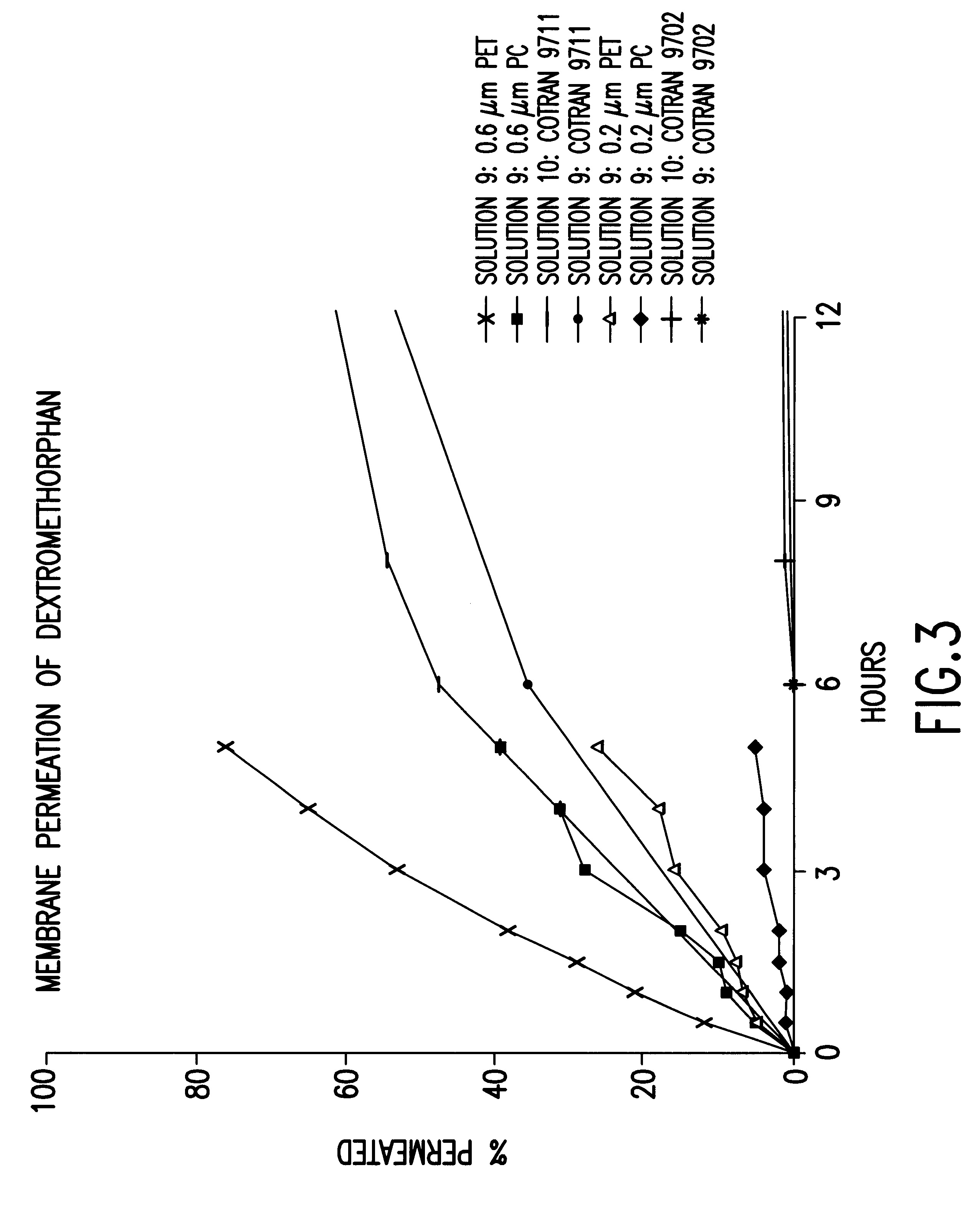
Transdermally administered dextromethorphan as antitussive agent
The present invention is drawn to a device for the transdermal administration of dextromethorphan, (+)-3-methoxy-17-methyl-9a,13a,14a-morphanin, and salts, prodrugs and metabolites thereof, together with a pharmaceutically acceptable carrier, to a human being or animal in need thereof, to achieve an antitussive effect. The present invention is further drawn to a method of achieving an antitussive effect in a human being or animal which comprises transdermally administering dextromethorphan, (+)-3-methoxy-17-methyl-9a,13a,14a-morphanin, and salts, prodrugs and metabolites thereof, together with a pharmaceutically acceptable carrier.
Owner:MCNEIL AB +1
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions that comprise an expectorant, an extended release antitussive, and an extended release decongestant. Specifically, the compositions comprise guaifenesin, phenylephrine tannate, and dextromethorphan tannate. The present invention also includes methods for using these compositions for treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions comprising an antitussive, a decongestant and an expectorant, and in a specific embodiment comprising hydrocodone, phenylephrine hydrochloride and guaifenesin, wherein the composition may be substantially free of added sugar and added alcohol, and methods for using these compositions for the treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
Owner:EVERETT LAB
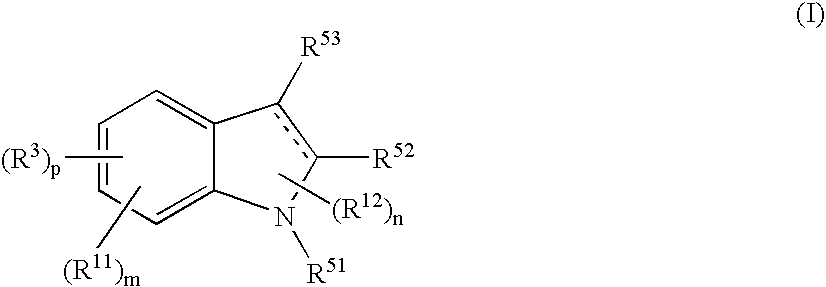
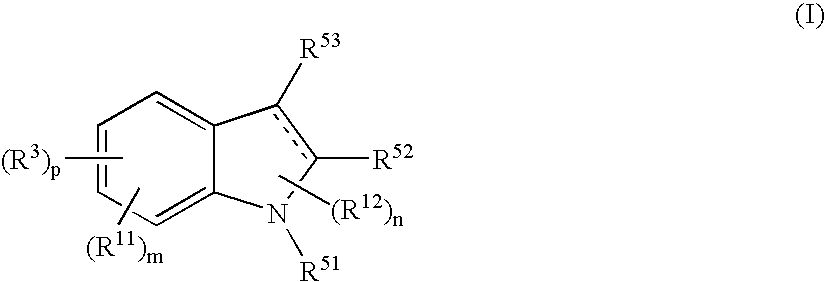
Indole Compound and Use Thereof
InactiveUS20080188532A1Increased airway hyperreactivityImprove respiratory functionBiocideSenses disorderDiseaseBronchial epithelium
The present invention relates to a compound represented by the formula (I),wherein all symbols are as defined in the description,a salt thereof, a solvate thereof, or a prodrug thereof, which has a leukotriene receptor antagonistic activity which is expected to be more effective than those of the leukotriene receptor antagonists currently used in clinical trials. Therefore, it is useful as an agent for the prevention and / or treatment of a leukotriene-mediated disease such as a respiratory diseases such as bronchial asthma, chronic obstructive pulmonary disease, pulmonary emphysema, chronic bronchitis, pneumonia (e.g. interstitial pneumonia etc.), severe acute respiratory syndrome (SARS), acute respiratory distress syndrome (ARDS), allergic rhinitis, sinusitis (e.g. acute sinusitis, chronic sinusitis, etc.), or the like, or as an expectorant or an antiitussive.
Owner:ONO PHARMA CO LTD
Combination cough treatment compounds and method of treating common coughs
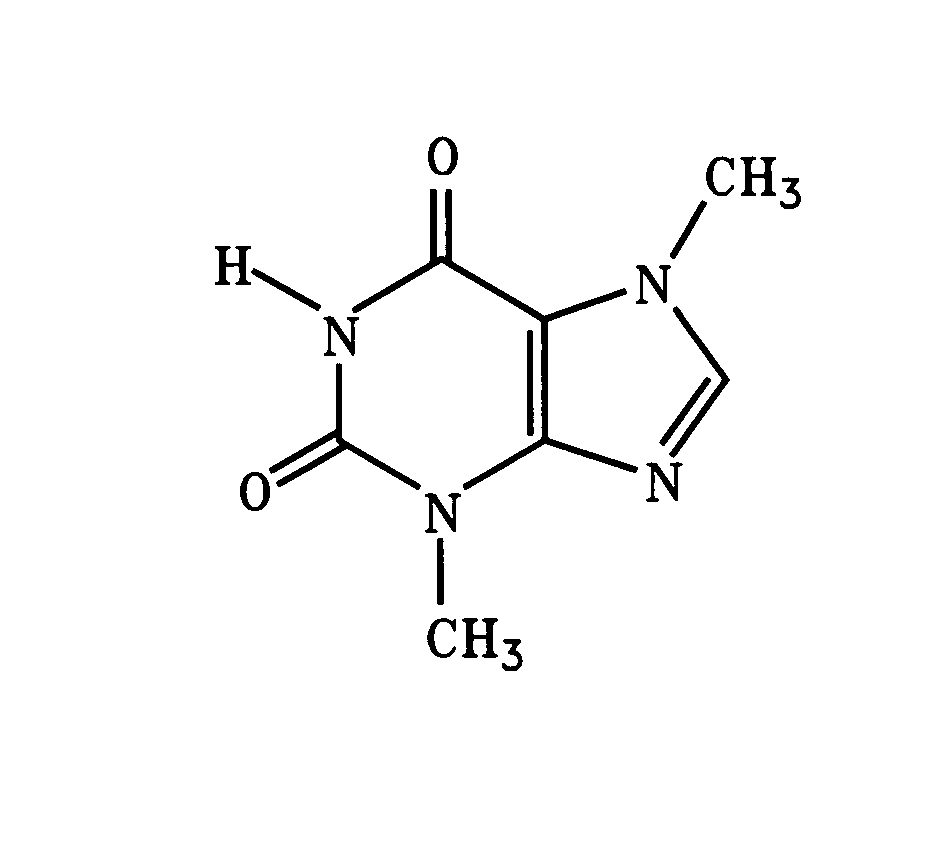
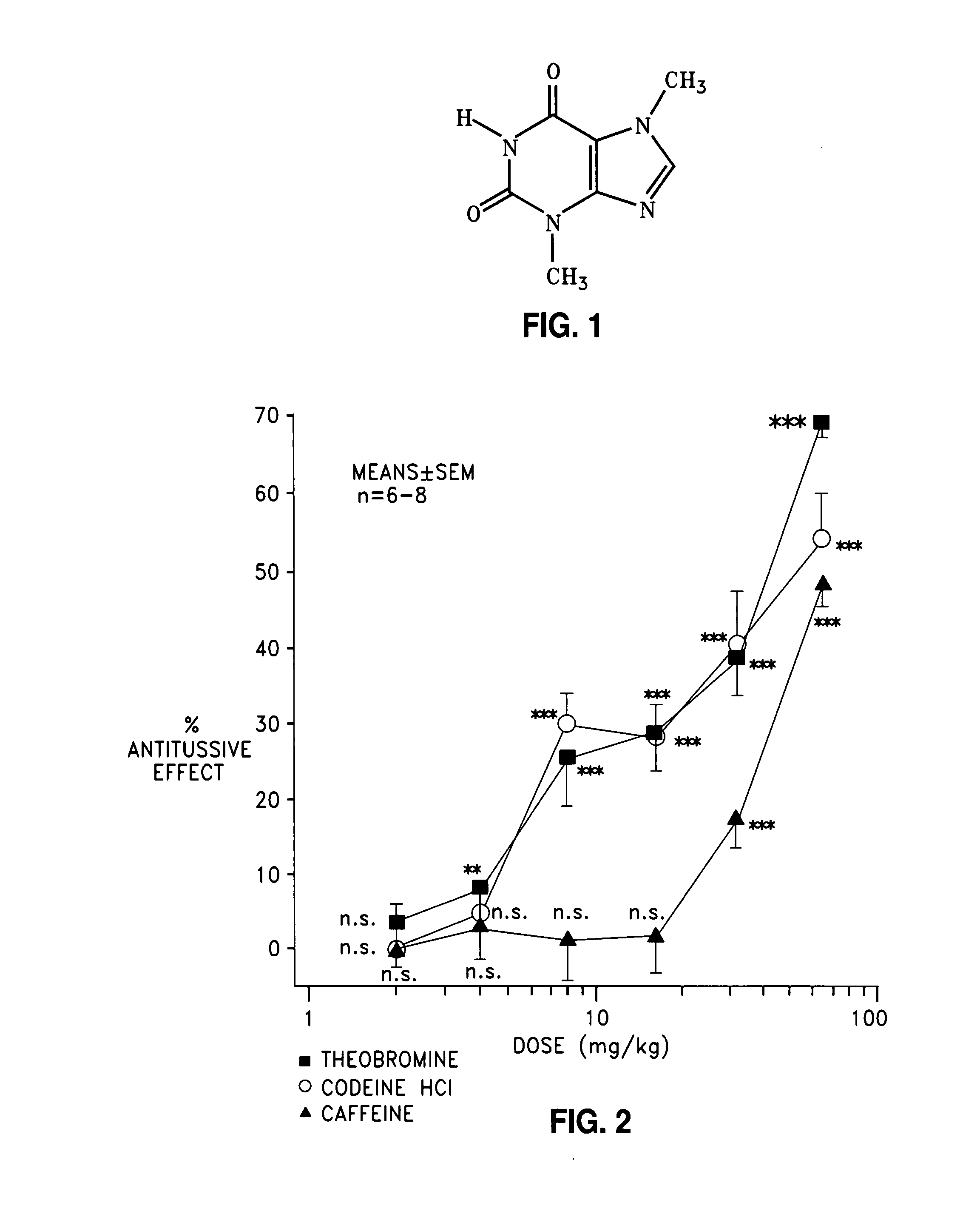
A novel composition of three recognized antitussive agents, when used in combination, work in an additive fashion to suppress cough. Each drug has a desirable effect of suppressing cough in a unique fashion. However, undesirable side effects can occur in humans at concentrations at which the drug has its maximal antitussive effect. Pharmaceutical compositions of theobromine, dextromethorphan, and an antihistamine with central nervous system effect, such as dexbrompheniramine, maximize cough suppression while decreasing the likelihood of side effects when used in combination.
Owner:LEVINE BRIAN M +1
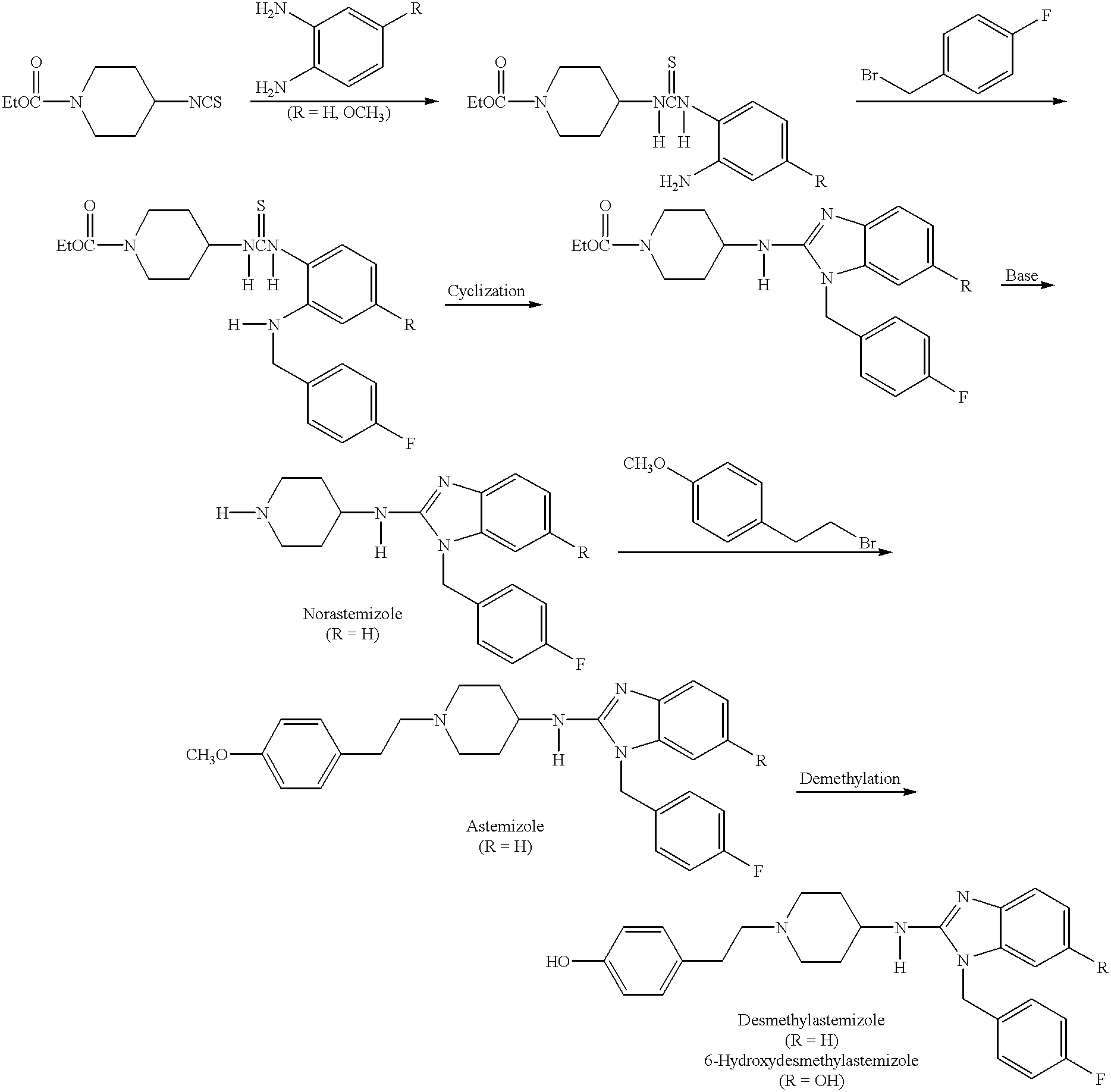
Compositions for treating allergic and other disorders using norastemizole in combination with other active ingredients
InactiveUS6303632B1Reduce adverse effectsUseful in treatmentBiocideOrganic chemistryMotion sicknessWhole body
Methods and compositions are disclosed utilizing metabolic derivatives of astemizole for the treatment of allergic disorders while avoiding the concomitant liability of adverse effects associated with the astemizole. The metabolic derivatives of astemizole are also useful for the treatment of retinopathy and other small vessel disorders associated with diabetes mellitus and such other conditions as may be related to the antihistamine activity of astemizole. For example, the metabolic derivatives of astemizole are useful for the treatment of asthma, motion sickness, and vertigo, without the concomitant liability of adverse effects associated with astemizole. Furthermore, the metabolic derivatives of astemizole, in combination with non-steroidal anti-inflammatory agents or other non-narcotic analgesics, or in combination with a decongestant, cough suppressant / antitussive or expectorant, are useful for the treatment of cough, cold, cold-like, and / or flu symptoms and the discomfort, headache, pain, fever, and general malaise associated therewith, without the concomitant liability of adverse effects associated with astemizole.
Owner:SEPACOR INC
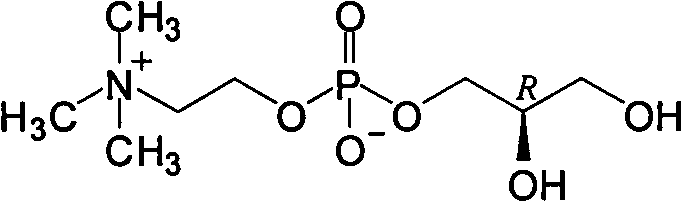
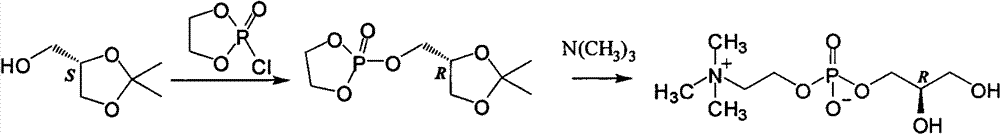
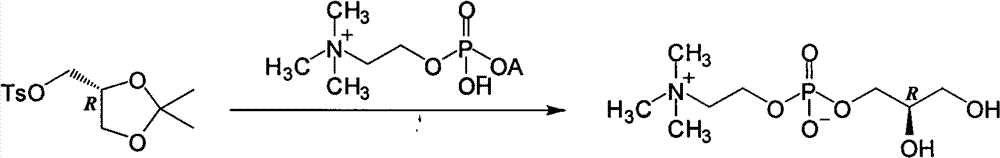
L-alpha-choline glycerophosphate synthesis method
InactiveCN103087091AOvercome stabilityPhosphatide foodstuff compositionsSynthesis methodsCholine Phosphate
The present invention relates to a method, which comprises that (R)-(-)-3-chloro-1,2-propanediol and a phosphocholine tetramethyl ammonium salt are subjected to a substitution reaction, and ion exchange resin purification is performed to obtain a L-alpha-choline glycerophosphate pure product. According to the present invention, the used chiral intermediate (R)-(-)-3-chloro-1,2-propanediol is further a key chiral intermediate of an antitussive agent levodropropizine, has characteristics of stable chemical property and convenient and easy obtaining, and is especially for L-alpha-choline glycerophosphate industrial production.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
Methods and compositions for treating allergic disorders and other disorders using metabolic derivatives of astemizole
InactiveUS6130233AReduce adverse effectsUseful in treatmentBiocideOrganic chemistryMotion sicknessDecongestant
Owner:SEPACOR INC
Method of making antitussive medicine and relieving cough
InactiveUS20070060564A1Good treatment effectFew side-effectsBiocideAnimal repellantsMedicinal herbsChemical composition
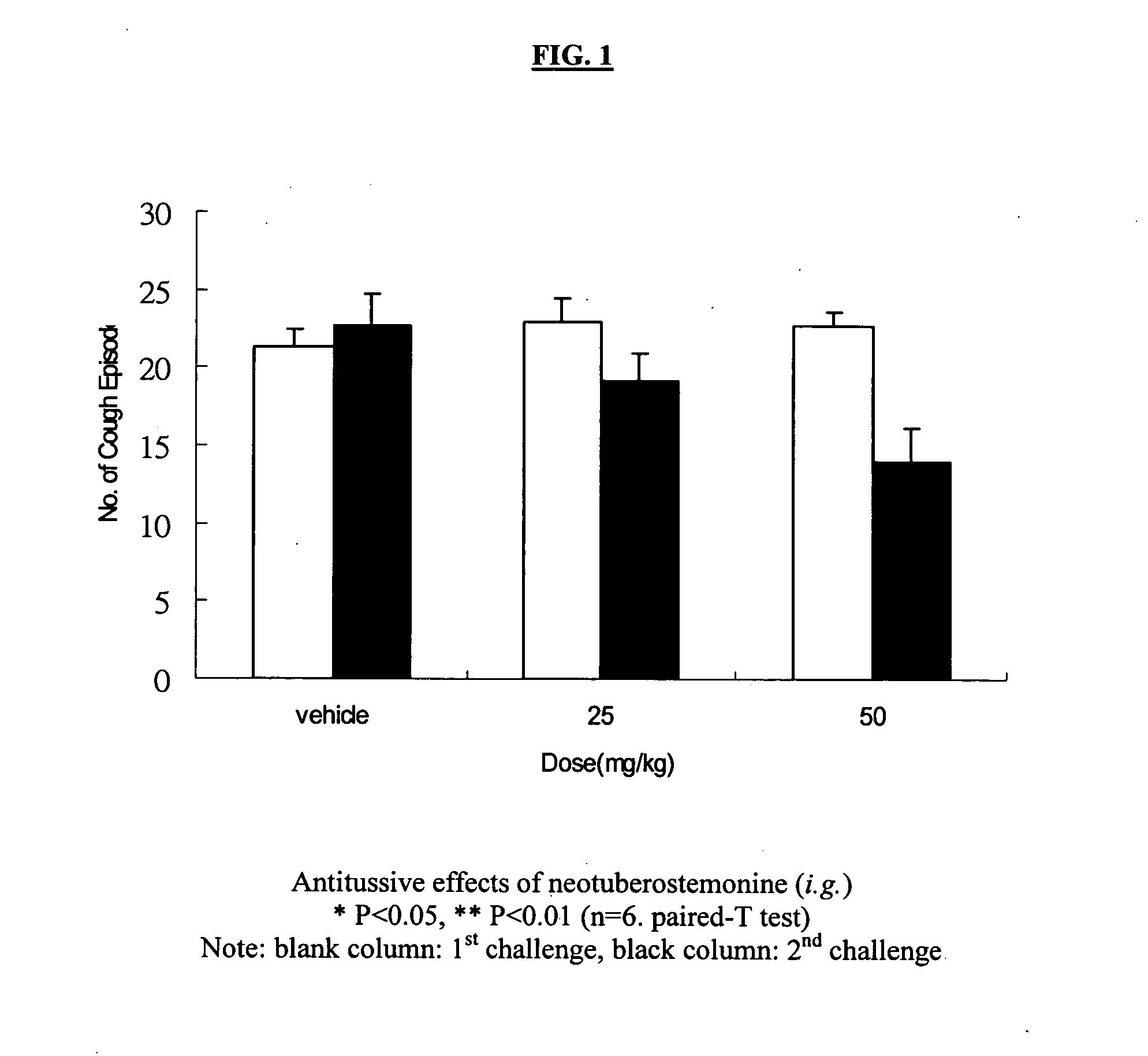
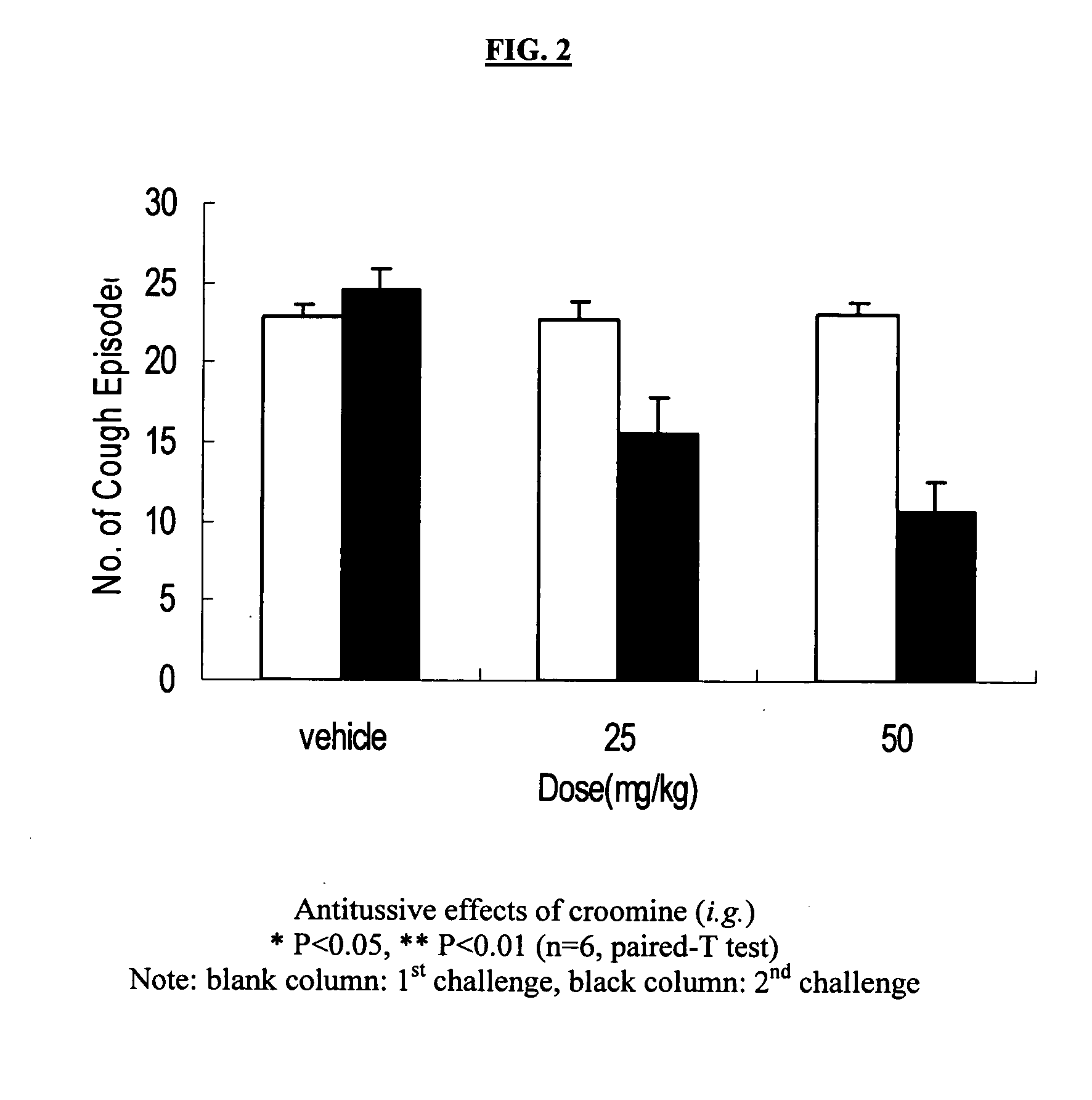
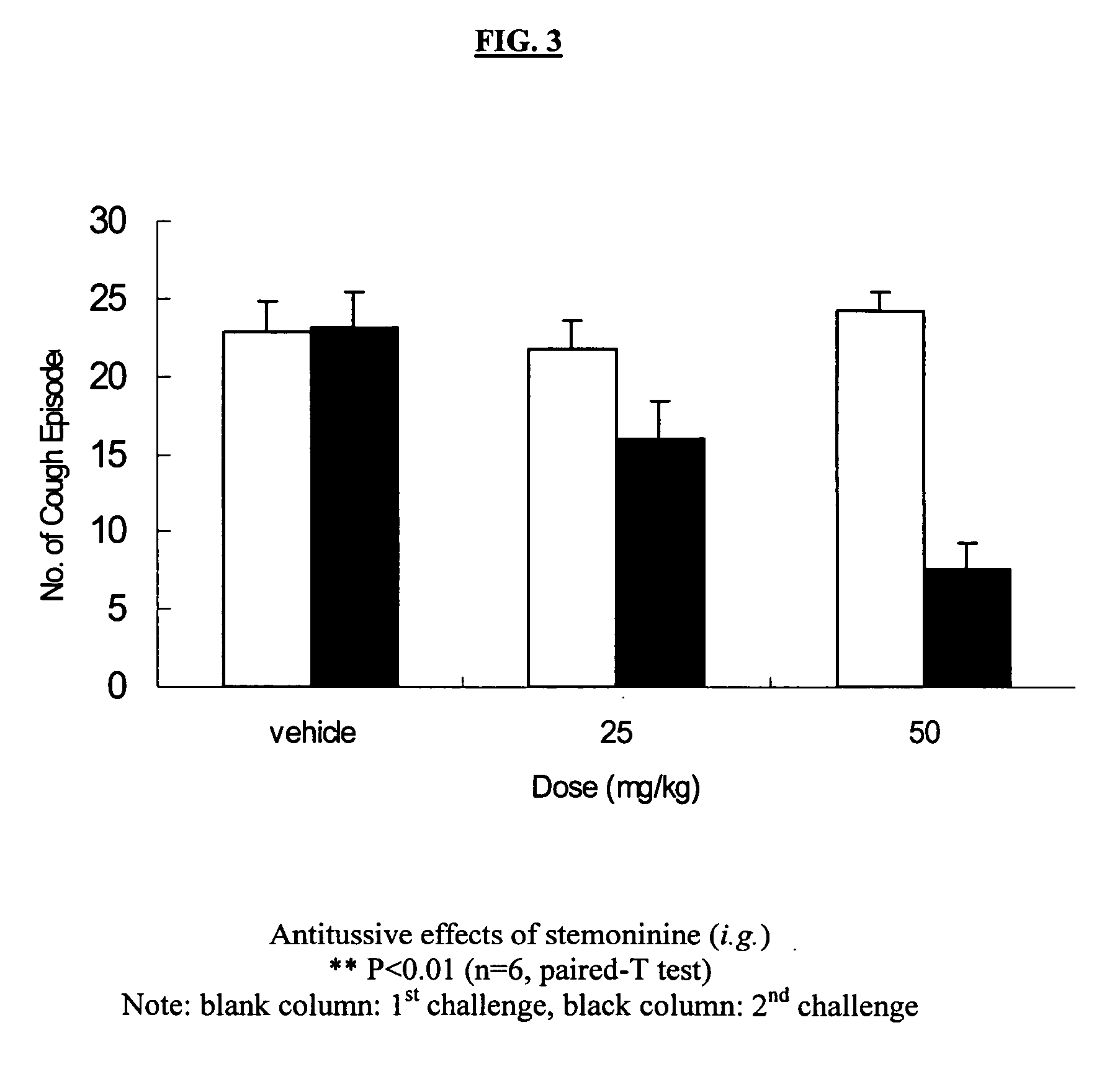
Method for relieving cough using an effective antitussive chemical compound belonging to stemoamide or tuberostemospironine and method for manufacturing pharmaceutical compositions for treating or relieving cough in human and animal subjects which including a stemoamide or tuberostemospironine compound. In addition, the antitussive property of the compounds provides a method for assessing the quality of herbs traditionally used in treating cough by analyzing the content of the effective chemical ingredient, i.e., compounds of stemoamide type and / or tuterostemospironine type. It further provides a method for identifying medicinal herbs which may be used for relieving cough by phytochemical determination of the existence of compounds that are of stemoamide or tuterostemospironine type.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Methods for treating allergic disorders using norastemizole
InactiveUS6124320AReduce adverse effectsUseful in treatmentBiocideOrganic chemistryMotion sicknessDecongestant
Methods and compositions are disclosed utilizing metabolic derivatives of astemizole for the treatment of allergic disorders while avoiding the concomitant liability of adverse effects associated with the astemizole. The metabolic derivatives of astemizole are also useful for the treatment of retinopathy and other small vessel disorders associated with diabetes mellitus and such other conditions as may be related to the antihistamine activity of astemizole. For example, the metabolic derivatives of astemizole are useful for the treatment of asthma, motion sickness, and vertigo, without the concomitant liability of adverse effects associated with astemizole. Furthermore, the metabolic derivatives of astemizole, in combination with non-steroidal anti-inflammatory agents or other non-narcotic analgesics, or in combination with a decongestant, cough suppressant / antitussive or expectorant, are useful for the treatment of cough, cold, cold-like, and / or flu symptoms and the discomfort, headache, pain, fever, and general malaise associated therewith, without the concomitant liability of adverse effects associated with astemizole.
Owner:SEPACOR INC
Lozenge for delivery of dextromethorphan
InactiveUS20050238695A1Powder deliveryOrganic active ingredientsDextromethorphan+diphenhydramineEthylmorphine
The present invention provides an organoleptically pleasing lozenge containing an antitussive selected from the group consisting of dextromethorphan, diphenhydramine, caramiphen, carbapentane, ethylmorphine, noscapine, codeine, and mixtures thereof, complexed with an ion exchange resin wherein the particle size of the resin is 38 μm or less in diameter. Also provided is a process for producing the lozenge and methods of administering the lozenge.
Owner:MCNEIL PPC INC +1
A kind of cough medicine and preparation method thereof
The invention discloses an antitussive medicine and a preparation method thereof. The antitussive medicine of the present invention is made of the following raw materials in the weight ratio: 40-60 parts of Houttuynia cordata, 15-30 parts of chrysanthemum root, 20-40 parts of Radix isatidis, 20-40 parts of fat sea, 30-50 parts of jade butterfly, 20-40 parts of lily, Tangerine peel 5~15, licorice 8~18, Atractylodes macrocephala 10~25, lotus leaf 30~50, mulberry leaf 20~45, mint leaf 5~15, loquat leaf 40~60, Ophiopogon japonicus 10~25, Bletilla striata 10~28, Habitat 15-30. The antitussive drug of the present invention is refined from a variety of natural raw materials. The raw material compatibility is scientific and reasonable. The effect of blood moistening dryness has a good therapeutic effect on coughs caused by various diseases such as upper respiratory tract infection, acute pharyngitis, and bronchitis.
Owner:徐弋舒
Compound pharmaceutical chemical acting on respiratory diseases and preparation process and application thereof
The invention provides pharmaceutical composition for respiratory diseases and a preparation process thereof, in particular to pharmaceutical composition and a preparation process and application thereof. The pharmaceutical composition comprises active ingredients, namely levodropropizine and carbocysteine, and pharmaceutically acceptable accessories. Compared with commonly used existing antitussive drugs or expectorants in the market, the pharmaceutical composition has more evident effect of cough relieving and fewer adverse reactions and has certain social benefit and economic benefit.
Owner:HUNAN JIUDIAN PHARMA
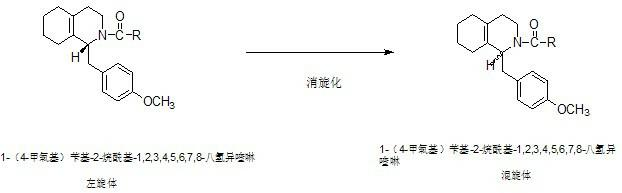
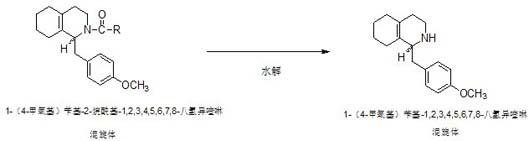
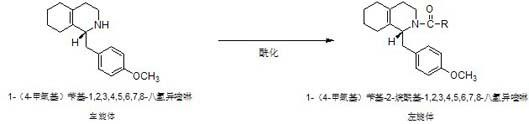
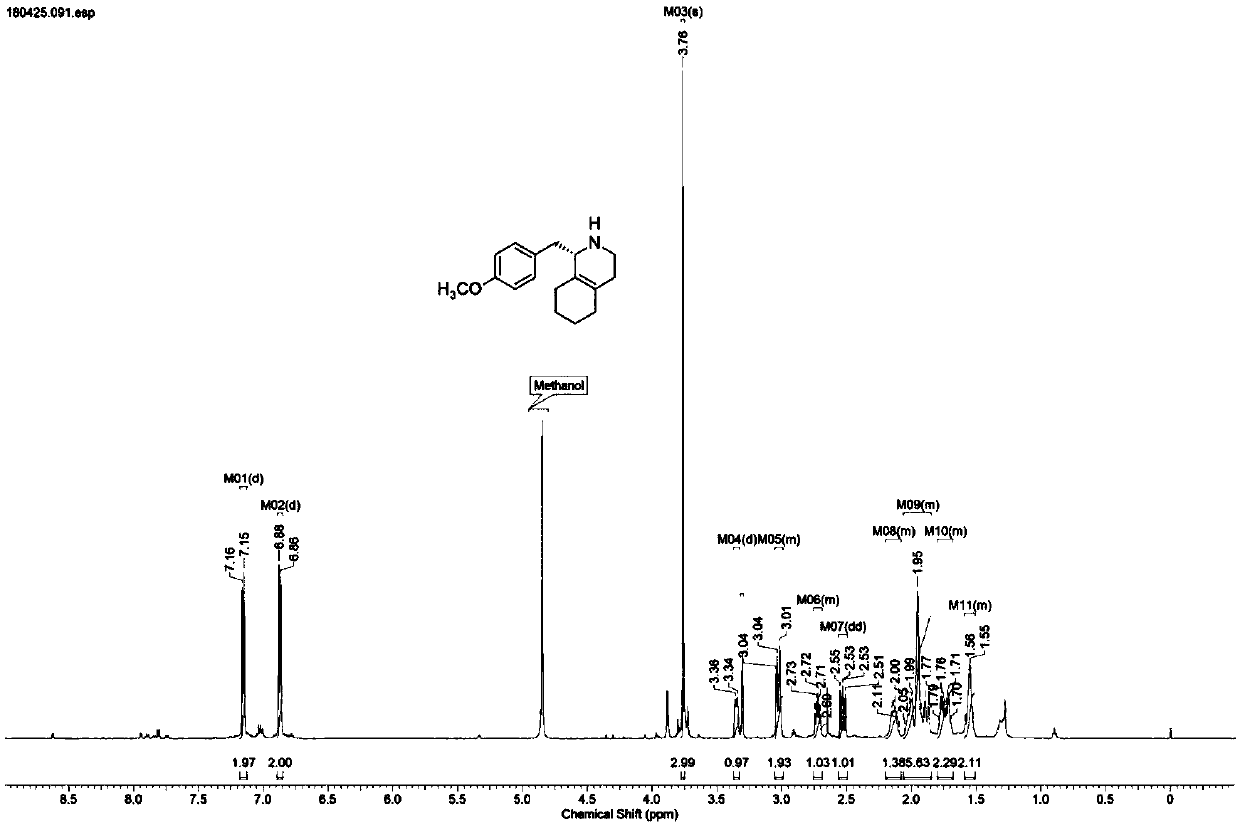
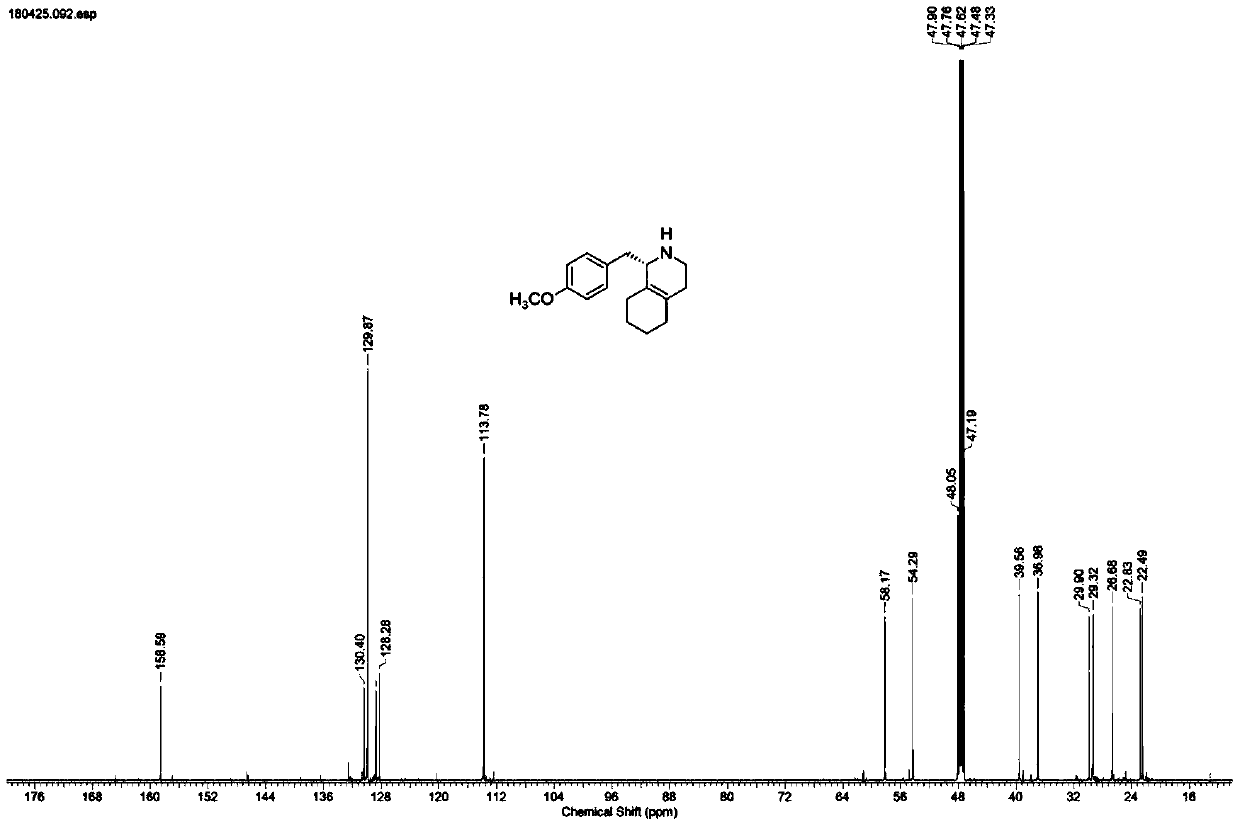
Preparation process for key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as cough relieving medicine
ActiveCN102219737ALow costSimple and safe operationOrganic chemistryIsoquinolineDextromethorphan Hydrobromide
The invention discloses a preparation process for a key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as a cough relieving medicine, which comprises the following steps of: performing acylation reaction to obtain 1-(4-methoxyl)benzyl-2-alkylacyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (laevo isomer); and racemizing under the alkaline condition to obtain 1-(4-methoxyl)benzyl-2-alkylacyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer); and hydrolyzing under the alkaline condition to obtain the 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer). The process aims to overcome the defect of the synthetic process and reduce cost, so that the process is simply and safely operated, the industrial production is qualified, and the yield of products is between 55 and 69 percent.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Slow release levodropropizine pharmaceutical composition
InactiveCN1520820AEffective plasma concentrationLower peak plasma concentrationOrganic active ingredientsPharmaceutical delivery mechanismSide effectLevodropropizine
The present invention provides one kind of delayed releasing levohydroxypropyl piperazine medicine composition and the medicine composition consists of levohydroxypropyl piperazine 10-80 weight portions, supplementary material for delayed releasing 0.5-60 weight portions and other supplementary material 0.5-90 weight portions. The delayed releasing levohydroxypropyl piperazine medicine composition is cough relieving medicine and the supplementary material for delayed releasing results in the effective blood medicine concentration maintained for relatively long period, relatively low blood medicine concentration, raised curative effect, reduced toxic side effect and less medicine taking times.
Owner:王志刚
Compositions of non-steroidal anti-inflammatory drugs decongestants and anti-histamines
The present invention is directed to a pharmaceutical composition and a method for the treatment of rhinitis and cold-like symptoms which includes a non-steroidal anti-inflammatory drug (NSAID), a decongestant, and an antihistamine. It has been found that the NSAID enhances the activity of a decongestant and an anti-histamine, thus permitting a reduction in either or both in administration of separate dosage forms. The same enhancement can also occur with an anti-tussitive. Thus, the effective amount of the decongestant or the antihistamine or both is less than about 75 % of an amount presentin an approved dose of the decongestant or the antihistamine, or both, relative to an amount of the NSAID corresponding to about 100% of the amount present in a normal strength dosage form of the NSAID.
Owner:PF CONSUMER HEALTHCARE 1 LLC
Method for preparing safe and effective antibechic dimimorfan phosphate
The invention relates to a method for preparing a safe and effective antibechic dimimorfan phosphate. The method comprises steps of reacting dextrorphane with trifluoro-methylsulfonyl chloride in triethylamine so as to generate dextrorphane trifluoro-methyl sulfone chloride ester; reacting the dextrorphane trifluoro-methyl sulfone chloride ester with tetramethyltin in methylbenzene so as to generate (9s, 13s, 14s)-3, 17-dimethyl morphinan (or adding the dextrorphane trifluoro-methyl sulfone chloride ester into mixed solvent of THF and N-methyl-2-pyrrolidone, adding catalyst ferric acetylacetonate and methyl magnesium bromide, stirring and heating reflux for 12 hours, so as to prepare (9s, 13s, 14s)-3, 17-dimethyl morphinan), furthermore, reacting with phosphoric acid so as to salify to prepare target product (9s, 13s, 14s)-3, 17-dimethyl morphine phosphate-dimimorfan phosphate. The method takes commercially available dextrorphane as the raw material, and prepares the target product through three steps of esterification, methylation and salification. The method has advantages of easily obtained raw materials, few synthesis steps, simple technology, low cost, high yield, high purity of the product and strong economic practicability, and is applicable to industrial production.
Owner:WUHAN YAOGU BIOLOGICAL ENG
Novel method for synthesizing key intermediate of dextromethorphan through enzyme catalysis asymmetric synthesis
ActiveCN110628841ASimple process routeMild reaction conditionsFermentationAntitussive AgentDextromethorphan
The invention discloses imine reductase AtIR(WP_027931121.1) derived from Amycolatopsis thermoflava, imine reductase StIR(WP_023587323.1) derived from Streptomyces thermolilacinus, imine reductase PmIR(WP_091804541.1) derived from Prauserella marina, imine reductase SmIR(WP_020496004.1) derived from Sciscionella marina, imine reductase AaIR(WP_020635634.1) derived from Amycolatopsis alba or iminereductase ShIR(SHE96216.1) derived from Streptoalloteichus hindustanus, and the imine reductase is adopted as a biocatalyst for preparing a key intermediate (S)-1-(4-methoxy benzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline of a central antitussive, namely dextromethorphan. A 5-50g / L substrate can be catalyzed by using corresponding imine reductase, the conversion rate is greater than 99%, and the method has remarkable characteristics of being high in yield, good in stereoselectivity, mild in reaction condition, and the like.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Ibuprofen-Hydrocodone-Antihistamine Composition
InactiveUS20080305159A1Relieve the coldRelieving cough symptomBiocideDrug compositionsDecongestantCoughing symptom
A pharmaceutical composition for use in relieving cold and cough symptoms in a mammal includes ibuprofen, hydrocodone bitartrate and an antihistamine (or a decongestant). The ibuprofen is in a therapeutically effective amount sufficient to act as an analgesic and an anti-inflammatory. The hydrocodone bitartrate is in a therapeutically effective amount sufficient to act as an antitussive and an analgesic. The antihistamine is in a therapeutically effective amount to act as an antihistamine. A fatty excipient may be added to the active ingredients to facilitate encapsulation. Multiple layers of the fatty excipient may be employed in varying release formulations and to facilitate identification of the composition.
Owner:MIKART
Method for simultaneously detecting contents of four effective ingredients in antitussive tablet
ActiveCN103048409AImprove quality controlQuality improvementComponent separationSilanesAdditive ingredient
The invention provides a method for simultaneously detecting contents of four effective ingredients (including schisandrin, praeruptorin A, praeruptorin and shionone) in an officinal antitussive tablet. The method comprises the steps of applying a modern high performance liquid chromatography technology, and taking octodecyl silane bonded silica gel as a filling agent; taking acetonitrile as a mobile phase A, and water as a mobile phase B; and calculating the number of theoretical plates to be not less than 5000 according to a schisandrin peak. In an elution process, gradient elution that allows a concentration proportion of the mobile phase A to the mobile phase B to change gradually is combined with isocratic elution that allows a proportion of the mobile phase A to the mobile phase B to be constant, so that a specific gradient elution procedure is formed; two or three gradient elutions are conducted; and the change detection is conducted on wavelength in a gradient elution process. The method can simultaneously detect the contents of schisandrin, praeruptorin A, praeruptorin and shionone in the antitussive tablet qualitatively and quantitatively, is simple, convenient, quick and high in specificity, and can raise the quality control level of the antitussive tablet effectively.
Owner:TEYI PHARMACEUTICAL GROUP CO LTD
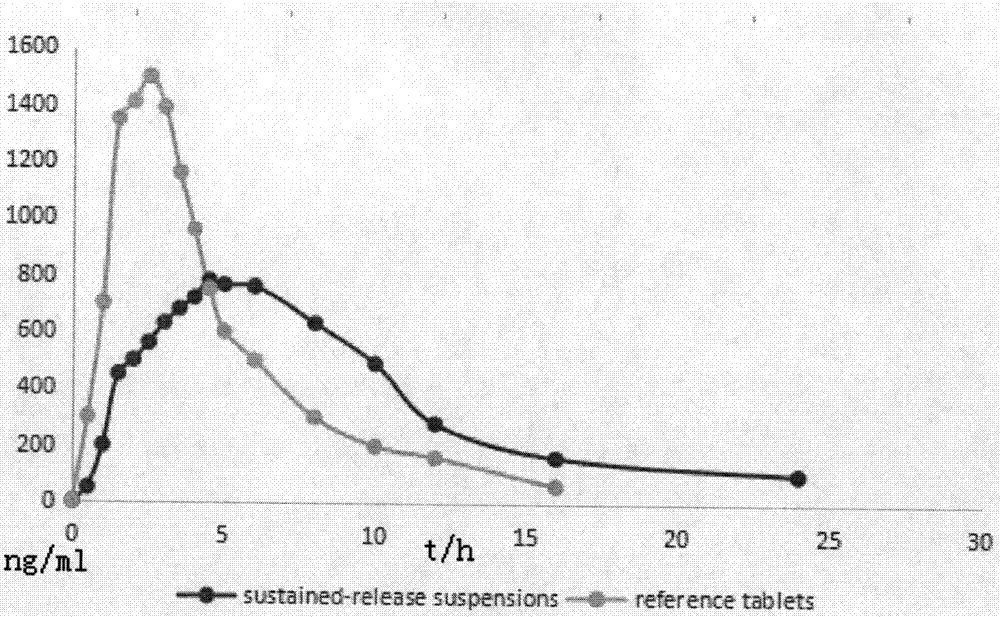
Dextromethorphan hydrobromide slow-release dry suspension and preparation method thereof
The invention discloses a slow-release dry suspension of dextromethorphan hydrobromide which is slowly released in the gastrointestinal tract environment. The formulation contains dextromethorphan hydrobromide and a pharmaceutically acceptable polymer. According to weight percentage, the preparation contains 10-90% of dextromethorphan hydrobromide and 10-90% of auxiliary materials. The supplementary materials for sustained release are one or more of cation exchange resin, methyl cellulose, ethyl cellulose, acrylic resin and hydroxypropyl methyl cellulose. Compared with the immediate-release preparation, the controlled-release preparation of the present invention can maintain the effective blood drug concentration within 24 hours, improve the curative effect, have less toxic and side effects, be convenient to take and carry, and reduce the number of times of taking. The sustained-release preparation of the invention can maintain a more stable blood drug concentration within 24 hours, improve curative effect, and have less toxic and side effects. This preparation only needs to be administered once a day. The controlled-release preparation of the present invention will be clinically used as an antitussive.
Owner:刘宏飞
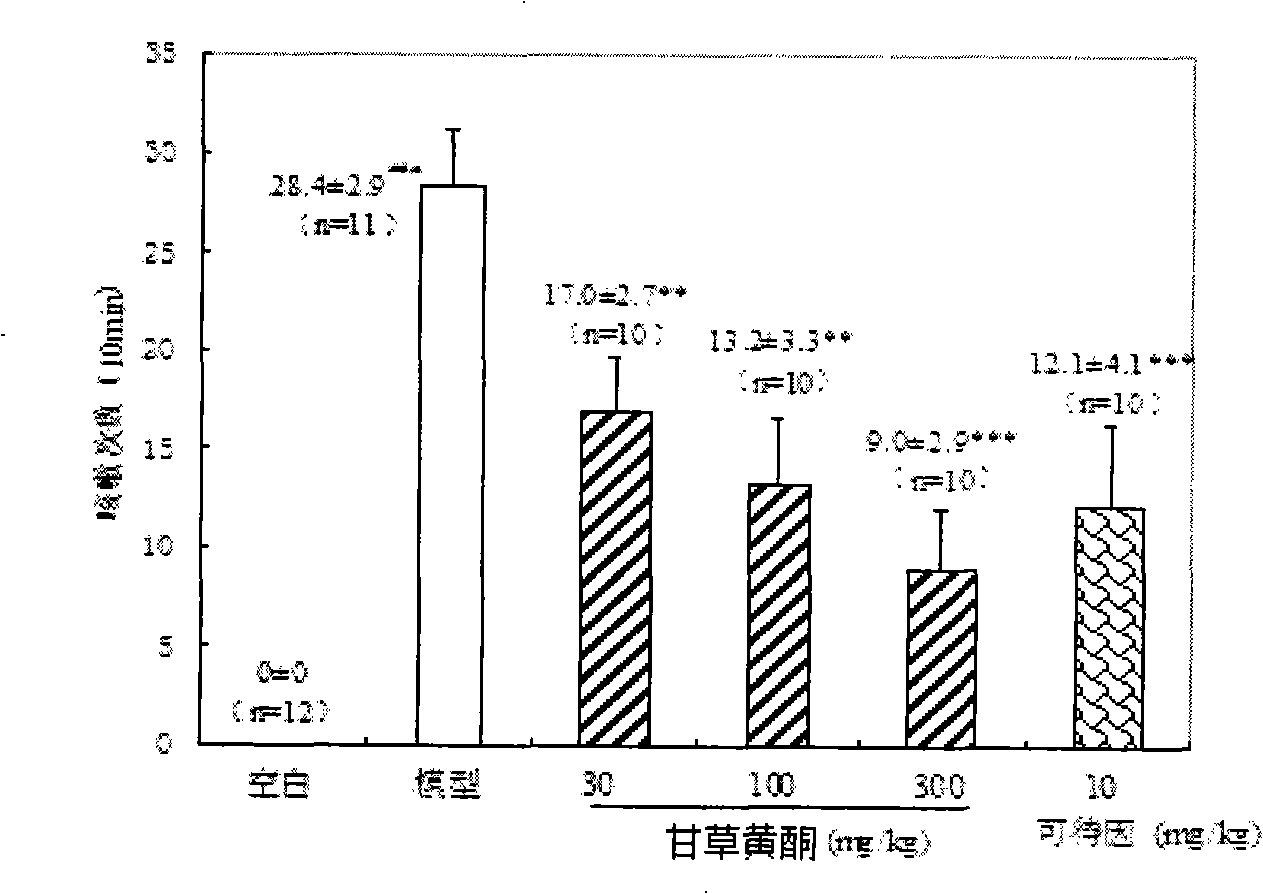
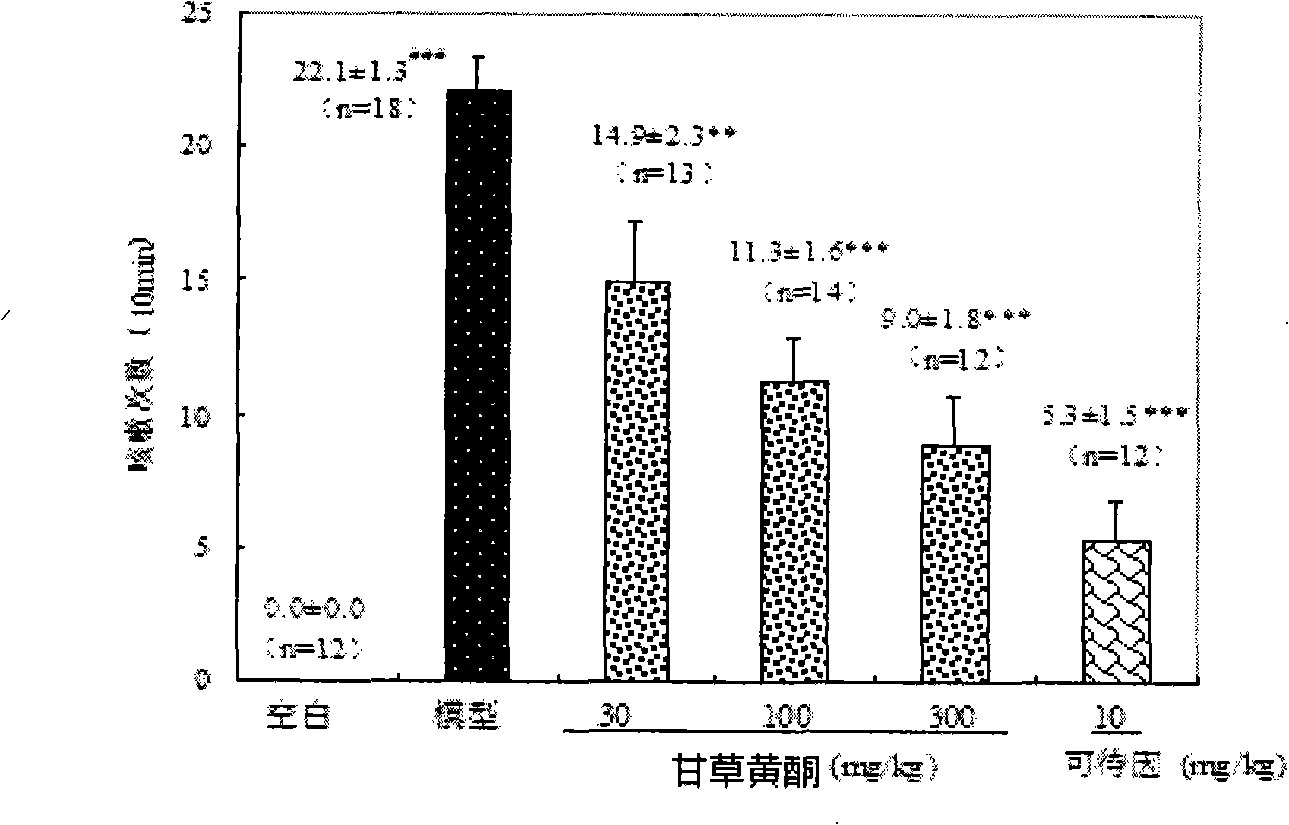
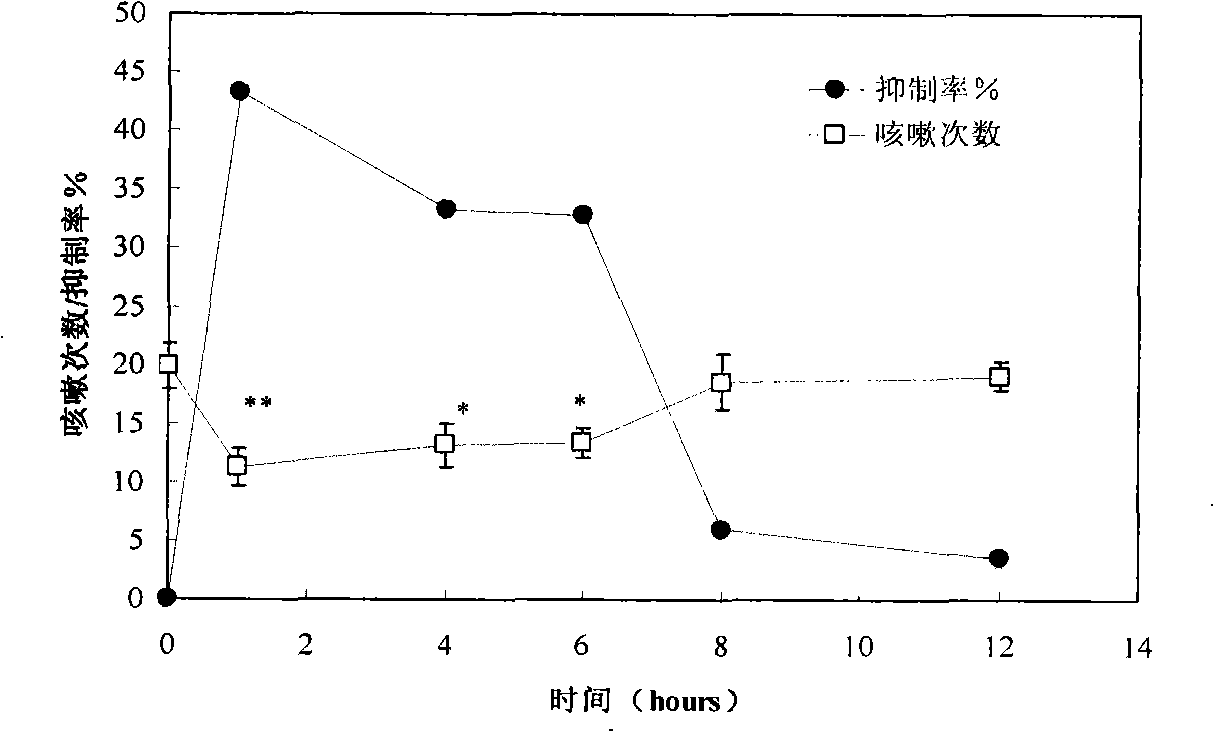
Licorice flavonoids antitussive and application thereof
InactiveCN101524397ASignificant antitussive effectAddressing the lack of strong non-narcotic cough suppressantsRespiratory disorderFood preparationDiseaseChronic cough
The invention provides an application of licorice flavonoids in preparing antitussives for treating acute and chronic cough caused by a plurality of reasons. The related acute and chronic cough comprises cough symptoms caused by various diseases such as acute and chronic tracheitis, asthma, chronic obstructive disease of lung, idiopathic pulmonary fibrosis, tuberculosis, bacterial pneumonia, bronchiectasis and the like. The licorice flavonoids extracted from liquorice proves to have very strong effect in preventing cough according to pharmacological tests; drug potency thereof is similar to the drug potency of codein in narcotic antitussive; the function mechanism analyses indicate that the licorice flavonoids plays the role of preventing cough through the non-narcotic nerve centre. In addition, the licorice flavonoids extracted from natural plants can also be prepared into health food. The invention solves the problem that the non-narcotic potent antitussives are badly needed, provides a non-narcotic potent antitussives which has fine social and economical benefits.
Owner:ZHEJIANG UNIV
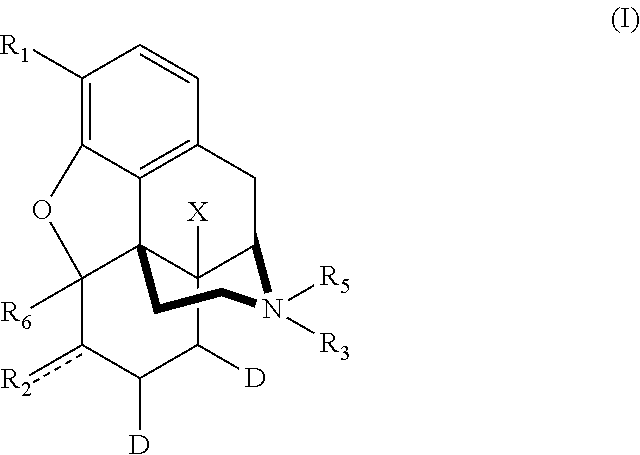
Deuterated morphine derivatives
ActiveUS20160052931A1Broad utilityReduce riskHeavy metal active ingredientsBiocideNormorphineΜ-opioid receptor
The invention relates to new morphine derivatives deuterated at the 7,8-position of the morphine ring, furthermore to a process for the preparation thereof, and to pharmaceutical compositions comprising them. The new deuterated morphine derivatives show high and selective μ-opioid receptor binding activity leading to the benefit of higher analgesic activity at lower dosages inducing thereby reduced adverse effects compared to the hydrogenated derivatives. The compounds of the invention are useful for example in the treatment of pain or can be used as antitussive agents with a reduced risk of the possibility of drug abuse.
Owner:SZEGEDI TUDOMANYEGYETEM
Antitussive Agent
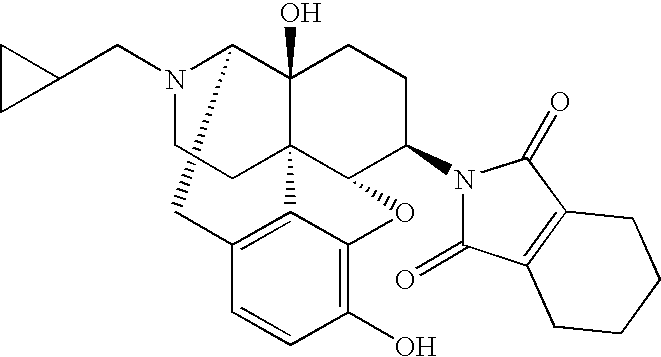
InactiveUS20090176818A1Good effectLittle side effectsBiocideOrganic chemistrySide effectAdditive ingredient
An antitussive, which can be used for therapy or prophylaxis of coughing, is disclosed. The antitussive comprises as an effective ingredient a morphinan derivative having a nitrogen-containing cyclic substituent or a pharmaceutically acceptable acid addition salt thereof, having a specific structure, such as the compound below [N-(17-cyclopeopylmethyl-4,5α-epoxy-3,14-dihydroxy-morphinan-6β-yl)-3,4,5,6-tetrahydrophthalimide]. The antitussive has an excellent therapeutic or prophylactic effect against coughing and the side effects thereof are small.
Owner:TORAY IND INC
Method of making antitussive medicine and relieving cough
InactiveUS7867998B2Good treatment effectFew side-effectsBiocideRespiratory disorderMedicinal herbsChemical composition
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Deuterated morphine derivatives
ActiveUS9447108B2Wide range of usesHigh and selective μ-opioid receptor activityOrganic active ingredientsNervous disorderΜ-opioid receptorSubstance abuse
The invention relates to new morphine derivatives deuterated at the 7,8-position of the morphine ring, furthermore to a process for the preparation thereof, and to pharmaceutical compositions comprising them. The new deuterated morphine derivatives show high and selective μ-opioid receptor binding activity leading to the benefit of higher analgesic activity at lower dosages inducing thereby reduced adverse effects compared to the hydrogenated derivatives. The compounds of the invention are useful for example in the treatment of pain or can be used as antitussive agents with a reduced risk of the possibility of drug abuse.
Owner:SZEGEDI TUDOMANYEGYETEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com