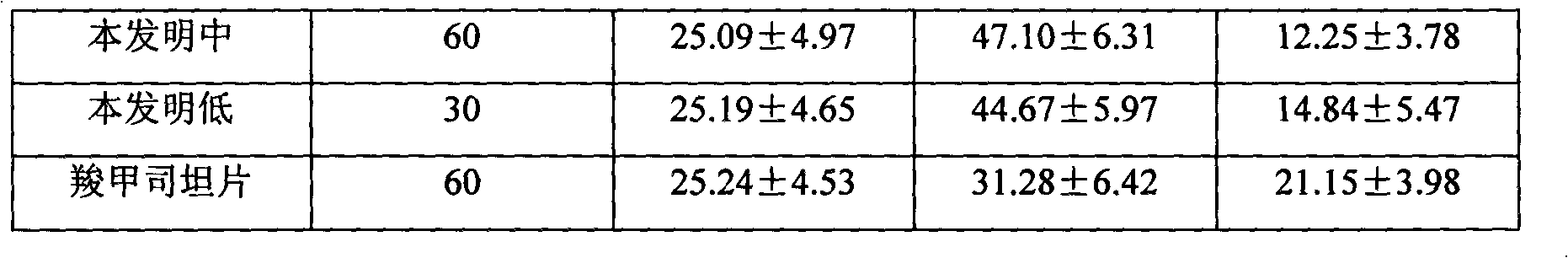Compound pharmaceutical chemical acting on respiratory diseases and preparation process and application thereof
A technology for respiratory diseases and pharmaceutical preparations, which is applied in the field of pharmaceutical compositions using levopromazine and carbocisteine as active ingredients, and can solve problems such as reducing the sensitivity of sensory nerves in the lungs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
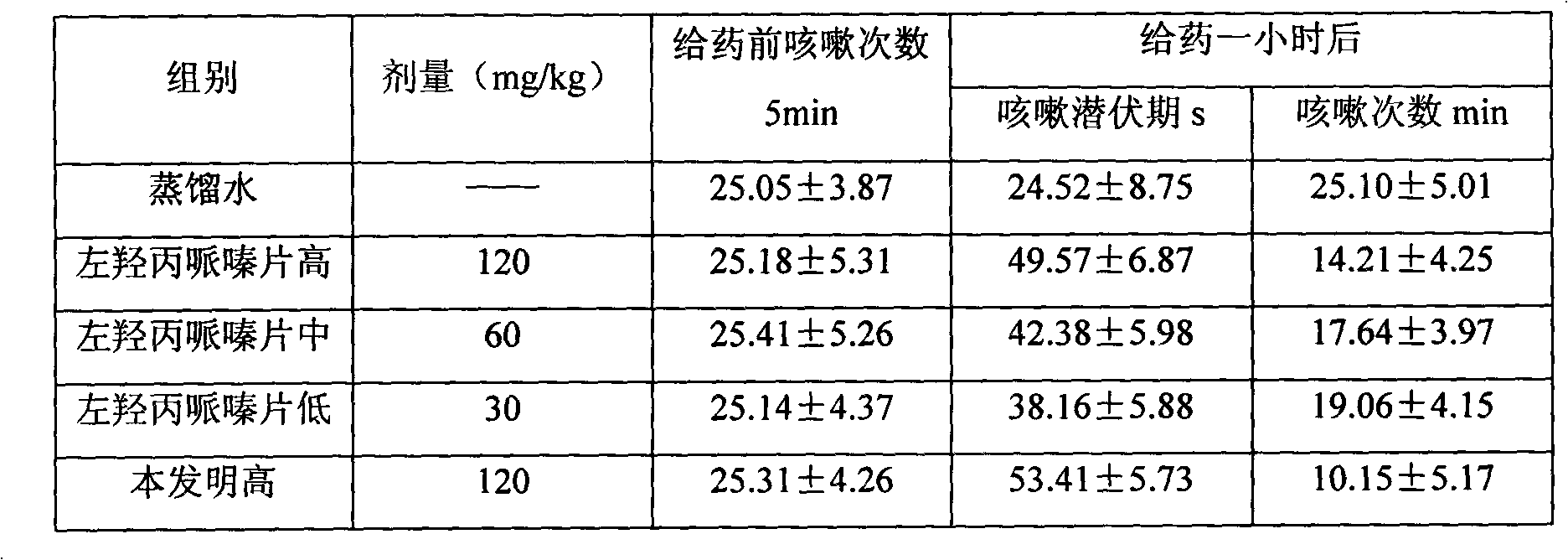
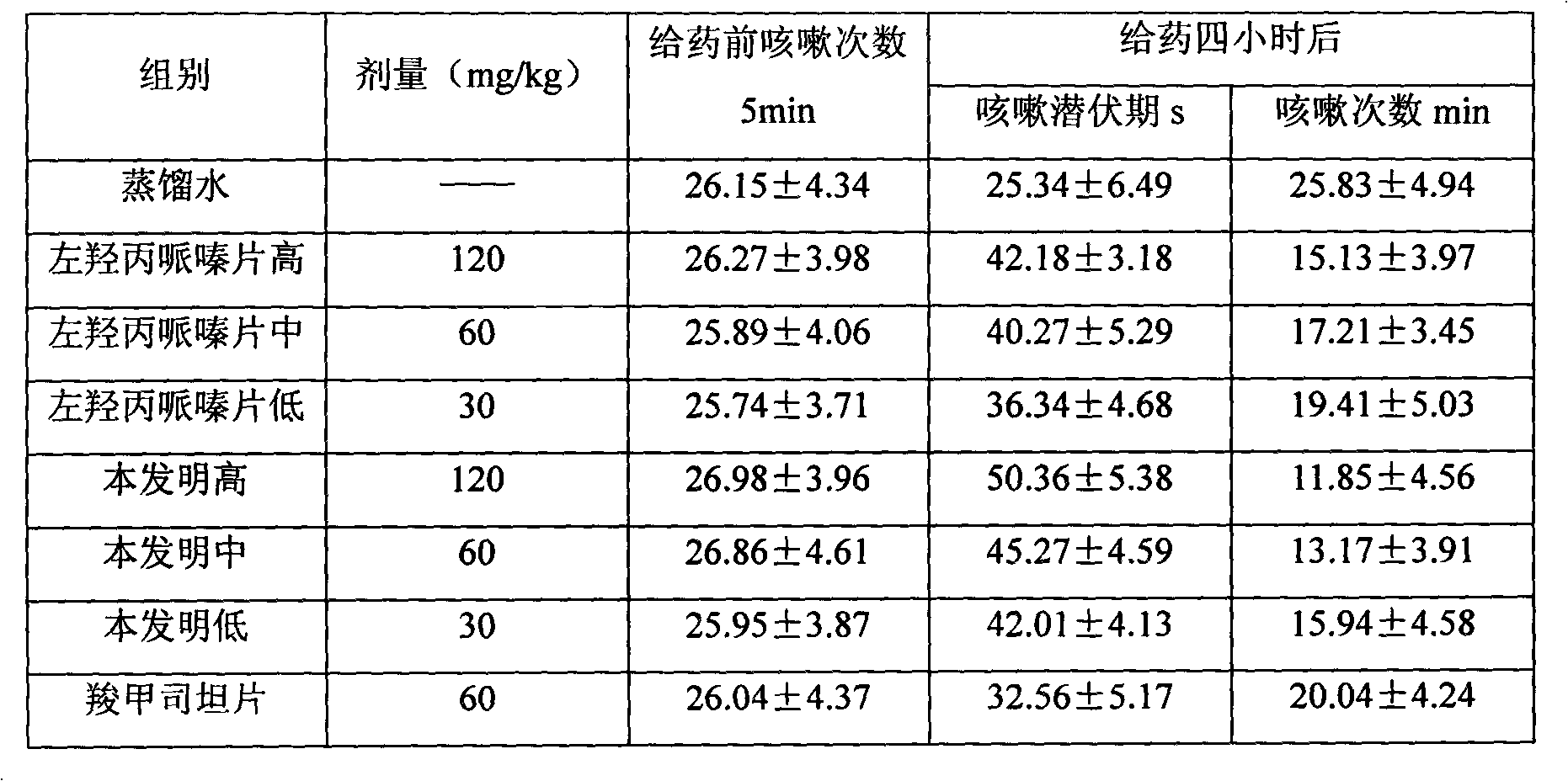
[0043] Take by weighing levodropropizine 60g, carbocisteine 250g, be prepared into common oral tablet as follows:
[0044] Weigh 400g of starch, 20g of microcrystalline cellulose, 10g of polyvinylpyrrolidone, 5g of talcum powder and 20g of aspartame, pulverize and sieve the raw and auxiliary materials respectively, and set aside. Dissolve the starch in water and add levodropropizine and carbocisteine to grind into a paste, dry, crush, and sieve; then add other excipient fine powder and mix well, add an appropriate amount of gelatin slurry, granulate, and bake , granulated, compressed into tablets, and prepared into 1000 tablets of the present invention.
Embodiment 2
[0046] Take levodropropizine 100g, carbocisteine 150g, prepare oral liquid as follows:
[0047] Grind levodropropizine and carbocisteine respectively, sieve and mix evenly, add ethanol to the mixture, stir and dissolve, dissolve in about 500ml purified water, filter, adjust its pH to 8~9, add appropriate amount of sucrose, sorbic acid and methylparaben again, and add water to 1000ml, obtain the pharmaceutical composition oral liquid 1000ml of the present invention.
Embodiment 3
[0049]Take by weighing levodropropizine 10g, carbocisteine 200g appropriate amount, be prepared into capsules as follows:
[0050] Weigh 50g of starch, 10g of micropowder silica gel, 5g of magnesium stearate, 10g of pregelatinized starch and 10g of low-substituted hydroxymethylcellulose, pulverize levodropropizine and carbocisteine respectively, sieve and mix evenly, Dissolve starch in water and add levodropropizine and carbocisteine to grind into a paste, dry, crush, and sieve; then add other excipient fine powder and mix well, add appropriate amount of gelatin slurry, granulate, and bake , whole grain, and filling gelatin capsules to obtain 1000 capsules of the pharmaceutical composition of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com