Patents
Literature
69902 results about "Additive ingredient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An artificial ingredient usually refers to an ingredient which is artificial or man-made, such as: Artificial flavor. Food additive. Food coloring. Preservative. Sugar substitute, artificial sweetener.
Breed-specific canine food formulations
InactiveUS6156355AUnique shapeManaged fat levelMilk preparationAnimal feeding stuffFood formulationAdditive ingredient
Breed-specific dog food formulations that comprise chicken meat as the major ingredient, rice as the predominant (or sole) grain source, fruit and / or vegetable fiber as the primary or sole fiber source, unique fat and antioxidant blend, vitamins, herbs and spices, carotenoids, and no corn or artificial colors, preservatives, flavors or sugars are provided.
Owner:BIG HEART PET INC
Systems and methods for a vaporization device and product usage control and documentation
Owner:CANOPY GROWTH CORP
Skin Firming Anti-Aging Cosmetic Mask Compositions
InactiveUS20040161435A1Promote excess fat reductionPromote cellulite controlBiocideCosmetic preparationsAdditive ingredientPhase mask
I have discovered cosmetic mask compositions suitable for face, neck, chin or body applications. These compositions synergistically combine at least one skin beneficial cosmetic or drug composition with at least one composition to promote excess fat reduction, cellulite control, or muscle toning benefits. The mask composition also contains at least one binder composition that binds with other beneficial ingredients by electrostatic, atomic, or ionic charges to synergistically enhance their topical site-specific benefits. These mask compositions are suitable for a variety of delivery system methods that include peel-off mask, leave-in mask, moisturizing mask, exfoliating mask, prosthetic mask, soaking mask, depilatory mask, foaming mask, rinse-off mask, sloughing mask, rub-off mask, two-phase mask, dual-chamber mask, and self-heating (heat releasing) mask.
Owner:GUPTA SHYAM K
Food preparation system
InactiveUS20050193901A1Eliminate all packaging materialGood for foodFeeding apparatusRoasters/grillsAdditive ingredientControl system
An automated food preparation system is described. It allows precise, automated control of the food preparation process, and has the ability to perform an automated cleanup. It comprises at least one manipulator to process and move ingredients, a control system, an autonomously accessible ingredient storage system, and at least one cooking receptacle.
Owner:BUEHLER DAVID BENJAMIN
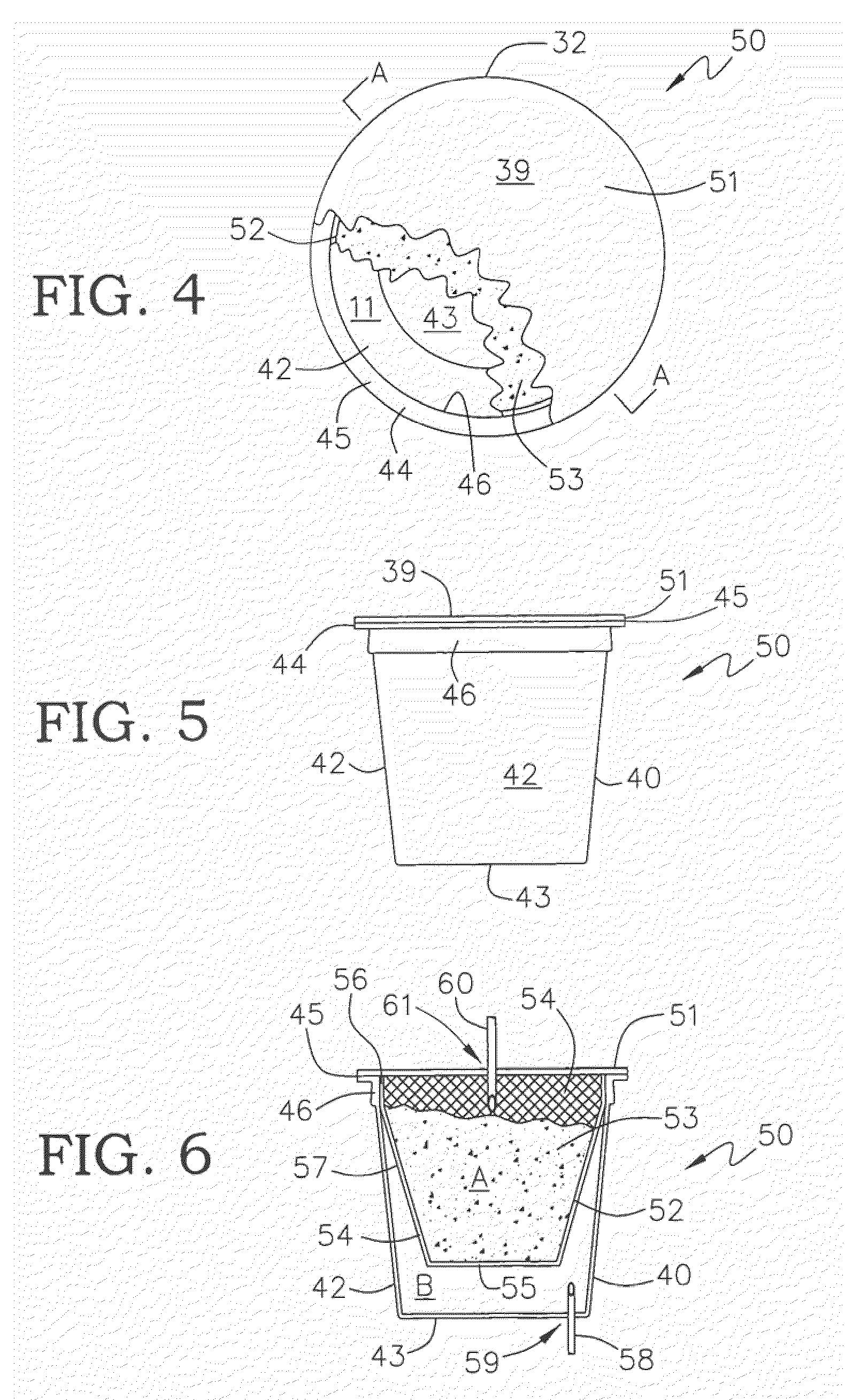
Mineral Composite Beverage Brewing Cup and Cartridge
A beverage filter cartridge having a cup, a filter, a lid and at least one beverage ingredient is provided where the cup and lid are sealed together to form an interior chamber which is separated into a first and second compartments by the filter, which is preferably basket or cone shaped, and a beverage ingredient such as coffee is contained inside first compartment, and at least one of the cup, lid and filter contains calcium carbonate in an amount effective to either improve the ability of beverage makers to pierce the cup or to provide a means for the cartridge to absorb CO2 emitted by the ingredient thereby reducing the hold time to packing and reducing the number of defective cartridges.
Owner:BEMIS COMPANY INC
Solid solution perforator for drug delivery and other applications
InactiveUS6945952B2Fast biodegradationBarrier property can be diminished and controlledSurgeryMicroneedlesDrug reservoirDrugs solution
A solid drug solution perforator (SSP) system and an associated drug reservoir are provided for delivering therapeutic, prophylactic and / or cosmetic compounds, for nutrient delivery and for drug targeting. For drug delivery, the SSP system includes an active drug ingredient and a matrix of perforator material that biodegrades or dissolves quickly upon contact with a patient's body. The SSP system provides a skin barrier perforator and a controller for prompt initiation and cut-off drug delivery. In a preferred method of transdermal drug delivery, an SSP system containing a selected drug penetrates into an epidermis or dermis, and the drug is promptly released from the (dissolving) SSP system perforator. An additional drug is optionally delivered from a patch reservoir through skin pores created by insertion of the perforator. Formulation and fabrication procedures for the SSP and associated reservoir are also provided. An SSP system can be fabricated with variety of shapes and dimensions.
Owner:THERAJECT INC.
High yield method of producing pure rebaudioside A
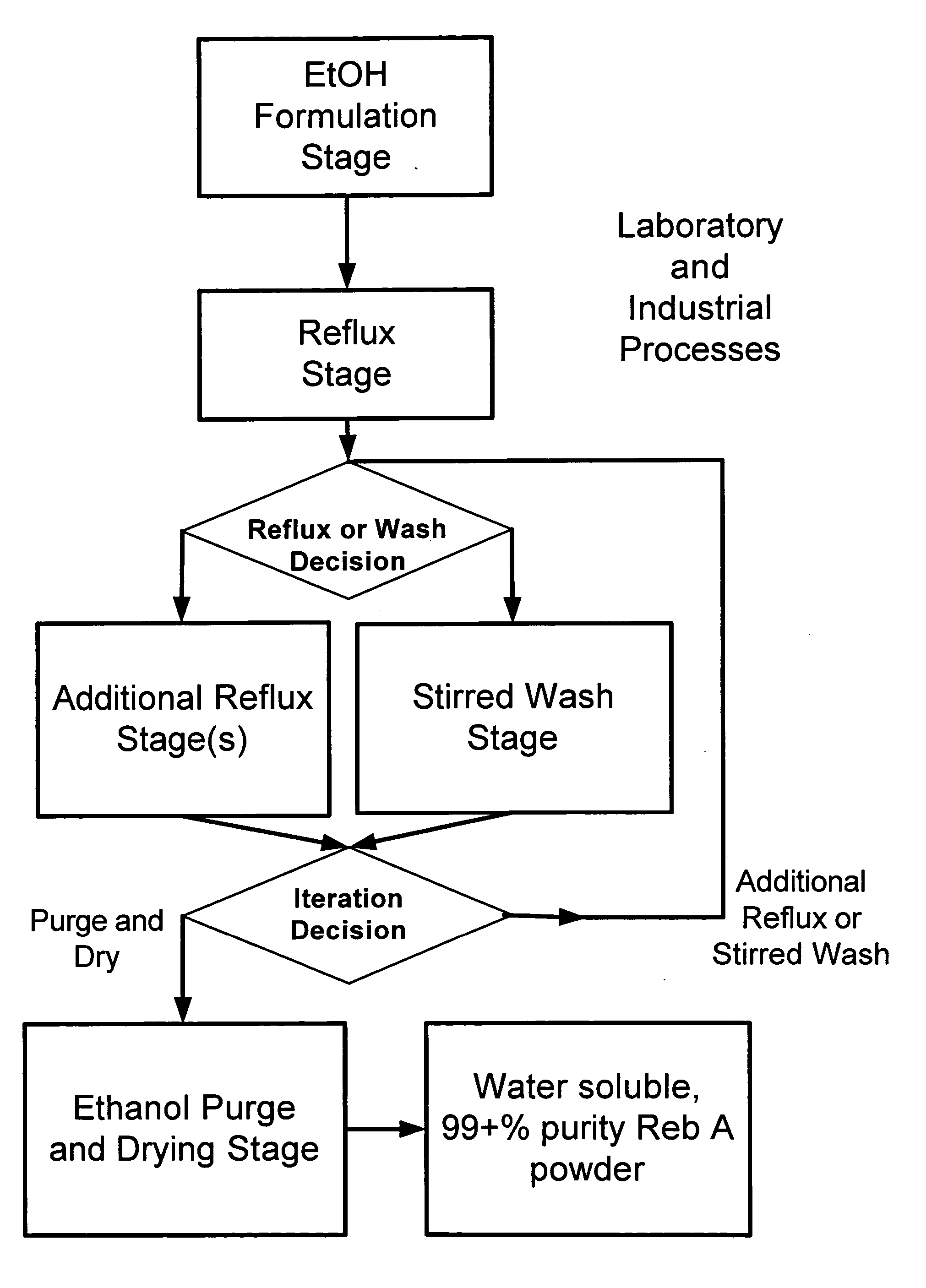
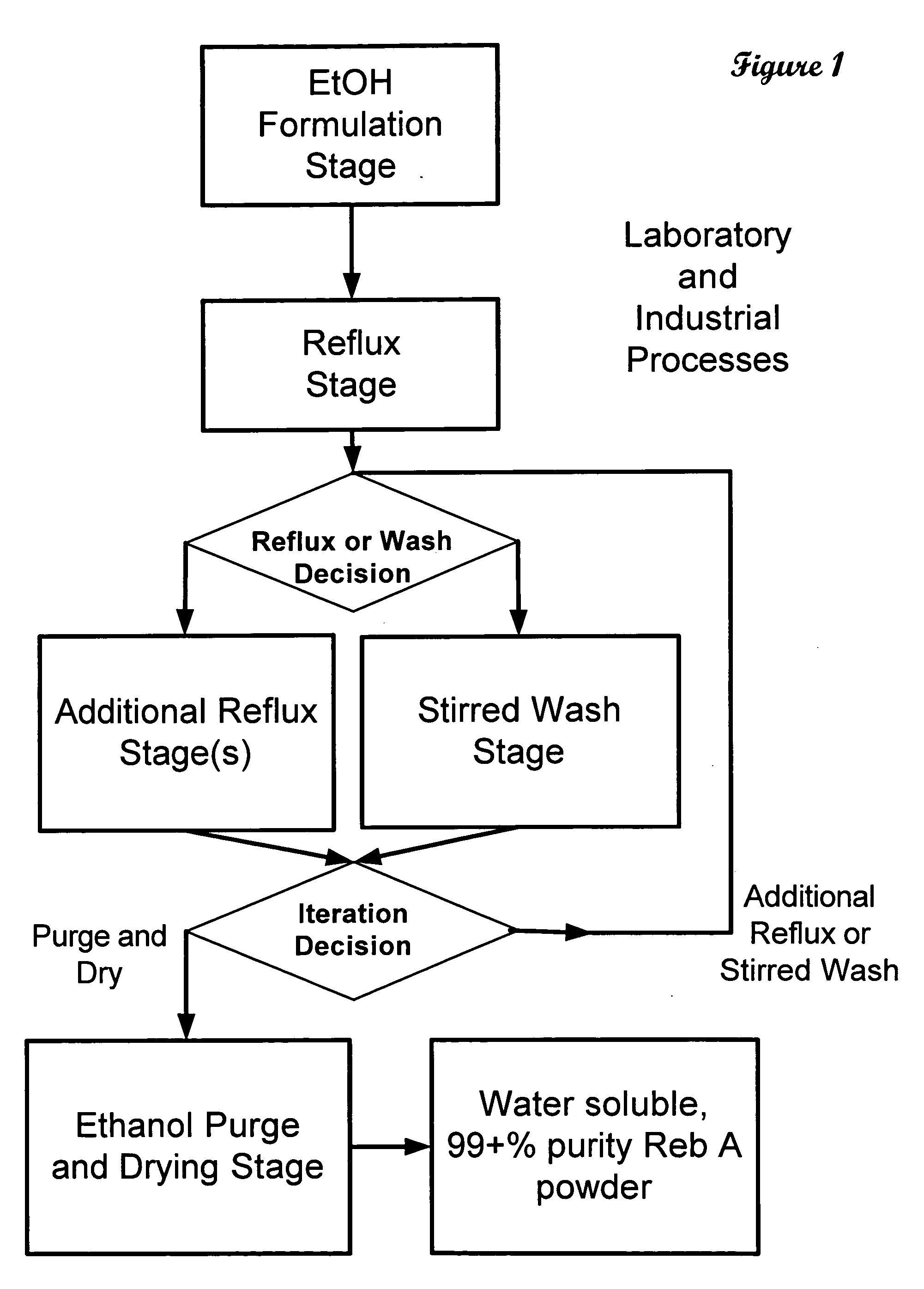
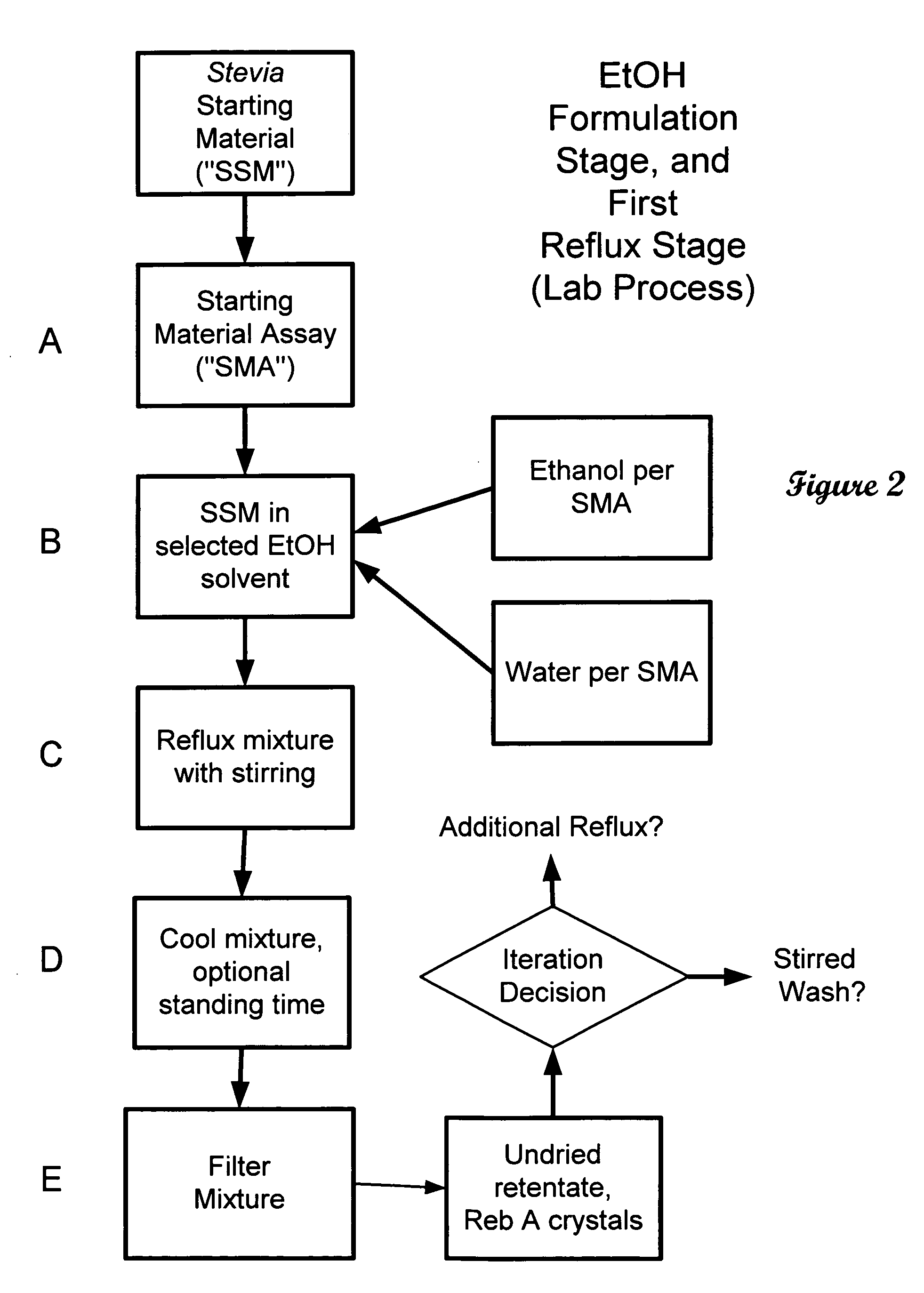
ActiveUS20060083838A1Reduction in yieldQuality improvementSugar derivativesMetabolism disorderSolubilityAdditive ingredient
The invention provides a high throughput, high purity, high yield system and method of isolating and purifying rebaudioside A (“Reb A”), with acceptable water solubility for all commercial uses, from commercially available Stevia rebaudiana starting material. The invention also provides a means of maximizing yields of 99+% purity Reb A based on the attributes of a given batch of Stevia starting material. The Reb A produced by the invention is water soluble, devoid of bitterness heretofore associated with rebaudioside sweeteners, non-caloric, and suitable for use as a reagent and as an ingredient in orally consumed products, e.g., as a sweetener, flavor enhancer, and flavor modifier.
Owner:SWEET GREEN FIELDS INT CO LTD
Topical Nutraceutical Compositions with Selective Body Slimming and Tone Firming Antiaging Benefits
InactiveUS20040146539A1Cut skinReduction in signCosmetic preparationsToilet preparationsPimpleWrinkle skin
I have discovered cosmetic or topical pharmaceutical compositions for external body part or organ slimming, firming, cellulite reduction, fat-reduction, and obesity control benefits that are in synergistic combination with benefits for the treatment of skin aging, skin wrinkles reduction, skin exfoliating, treatment of acne, treatment of rosacea, age-spots reduction, skin surface whitening, skin surface brightening striae distensae (stretch marks) reduction, treatment of pimples, treatment of skin infections and lesions, spider veins reduction, blood microcirculation (venous insufficiency) improvement, UVA / UVB protection of skin, and skin redness reduction. These compositions thus provide multiple combinations of skin and external body part or organ enhancement benefits that can be selective and specific for external body parts and organs such as face, chin, cheeks, arms, "love handles" in abdomen area, eye lids and eye zone, neck, breasts, thighs, and hips. These compositions include a body beneficial composition selected from certain nutraceutical, cosmetic, and pharmaceutical ingredients, a composition to promote collagen and elastin synthesis in the skin, and a cosmetically or pharmaceutically acceptable delivery system.
Owner:GUPTA SHYAM K
Method of treating inflammatory intestinal diseases containing as the ingredient IL-6 receptors antibodies
A preventive or therapeutic agent for treating bowel disease, including Crohn's disease and ulcerative colitis, where the agent has as an active ingredient an antibody directed against IL-6 receptor which is an interleukin-6 antagonist.
Owner:CHUGAI PHARMA CO LTD +1
Shaving aid material
A shaving aid material is provided that includes a water-soluble lubricious shaving aid in combination with a water-insoluble erodable medium. In some embodiments, the shaving aid material further includes a water-soluble thermoplastic polymer, and may also include a plasticizer. Optional additional ingredients include emulsifiers, surfactants, skin conditioners, fragrances, depilatory agents, cleaning agents, medicinal agents, etc.
Owner:EDGEWELL PERSONAL CARE BRANDS LLC
Pro-fragrances
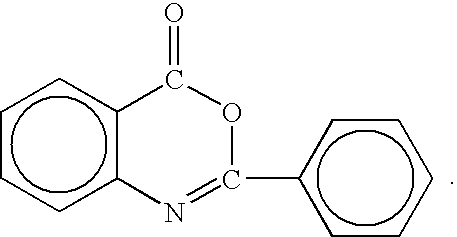
InactiveUS6861402B1Enhanced perfume longevityCosmetic preparationsOrganic chemistryFlavorAdditive ingredient
The present invention relates to fragrance delivery systems which comprise: A) from about 0.01% by weight of a pro-fragrance component which comprises pro-fragrances or pro-accords selected from at least two of the following: i) aldehyde and ketone releasing pro-fragrances, preferably an oxazolidine pro-fragrance; ii) β-amino pro-fragrances; and iii) orthoester pro-accords; and B) the balance carries and others adjunct ingredients.
Owner:THE PROCTER & GAMBLE COMPANY
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
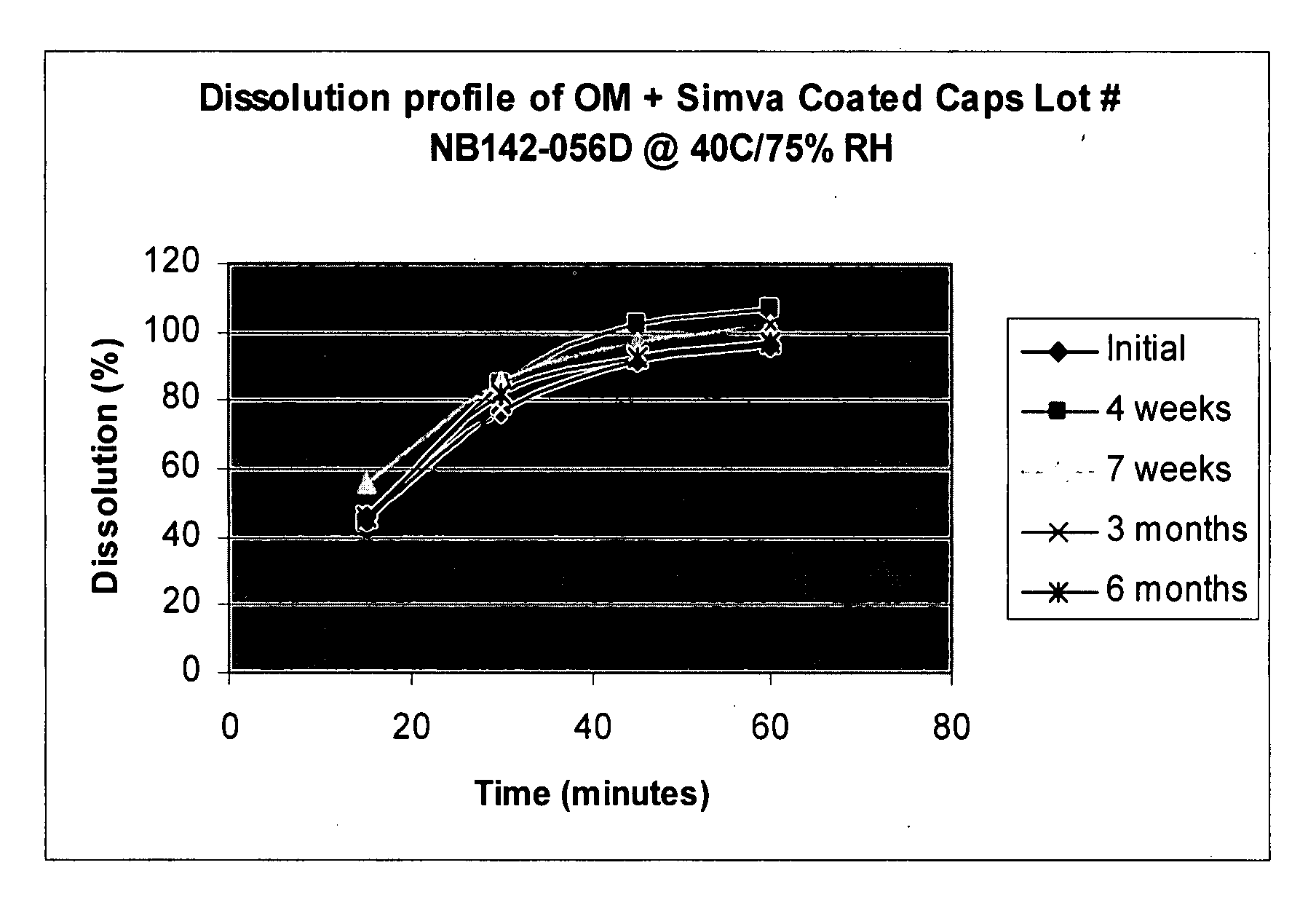
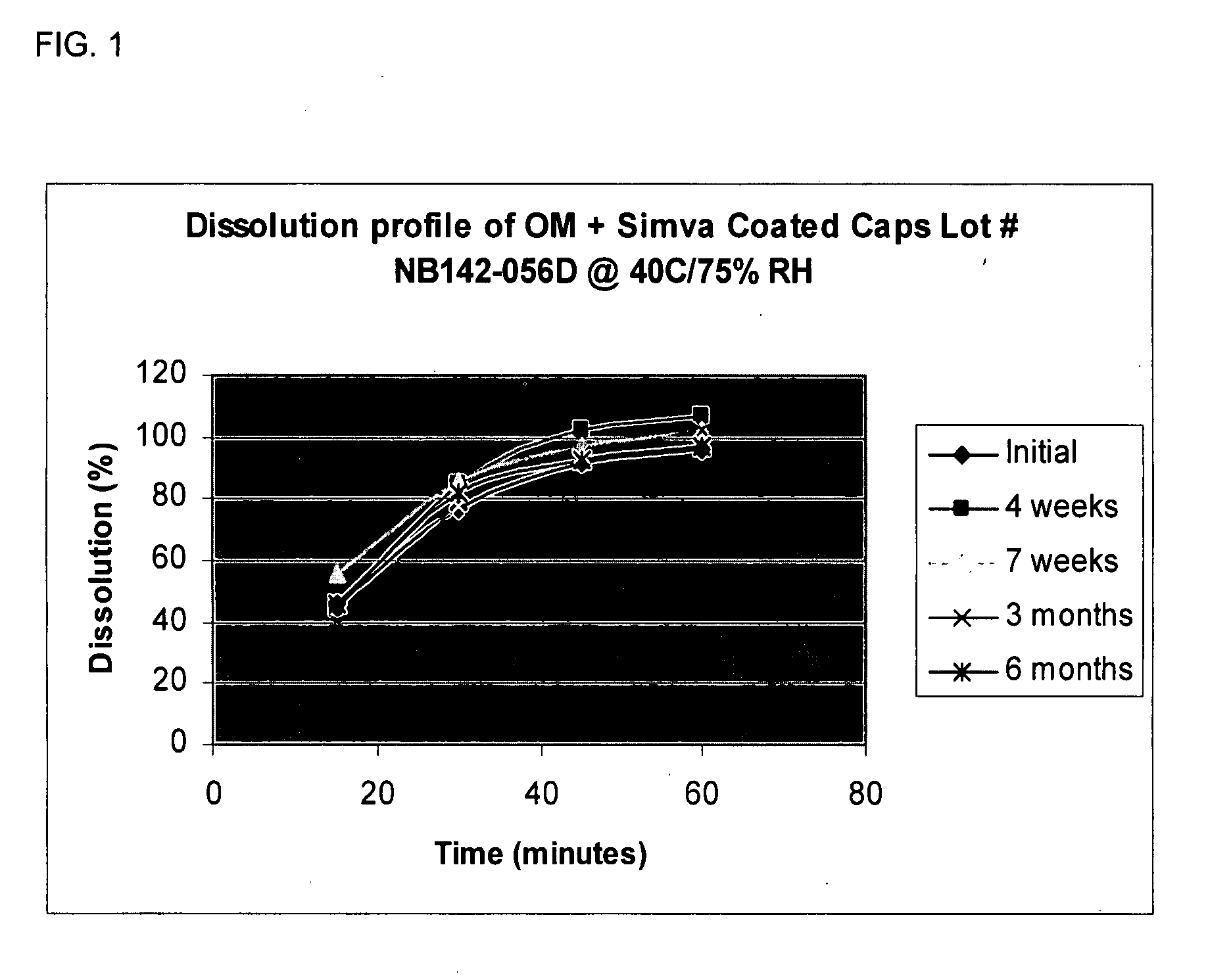
Coating capsules with active pharmaceutical ingredients
Pharmaceutical compositions in unit dose form comprising capsules containing one or more first active pharmaceutical ingredient in a pharmaceutically acceptable vehicle, coated with one or more second active pharmaceutical ingredients, wherein the unit dose form is a pharmaceutical grade finished dosage form, and methods of making and using the same.
Owner:GLAXO SMITHKLINE LLC
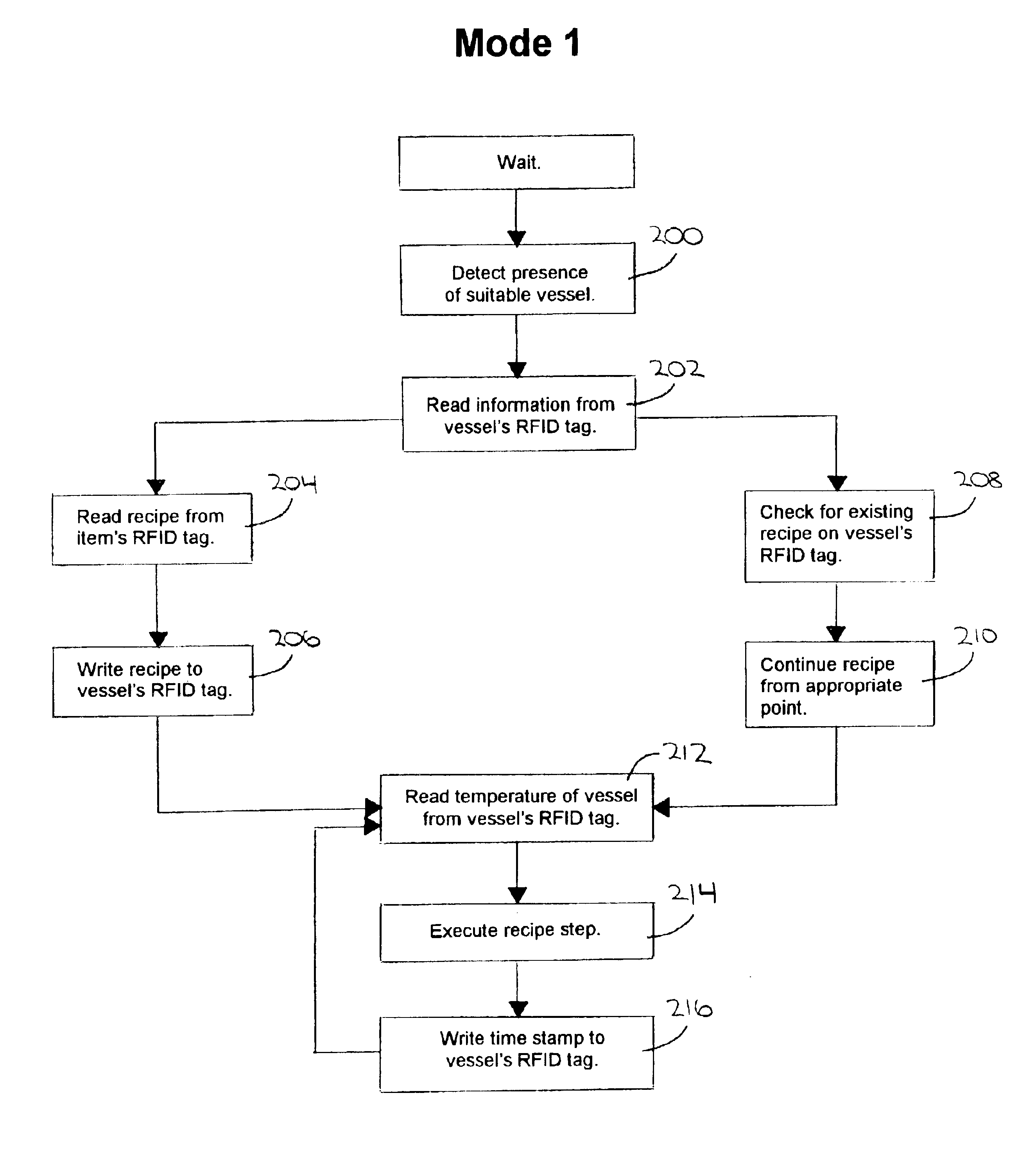
RFID-controlled smart range and method of cooking and heating
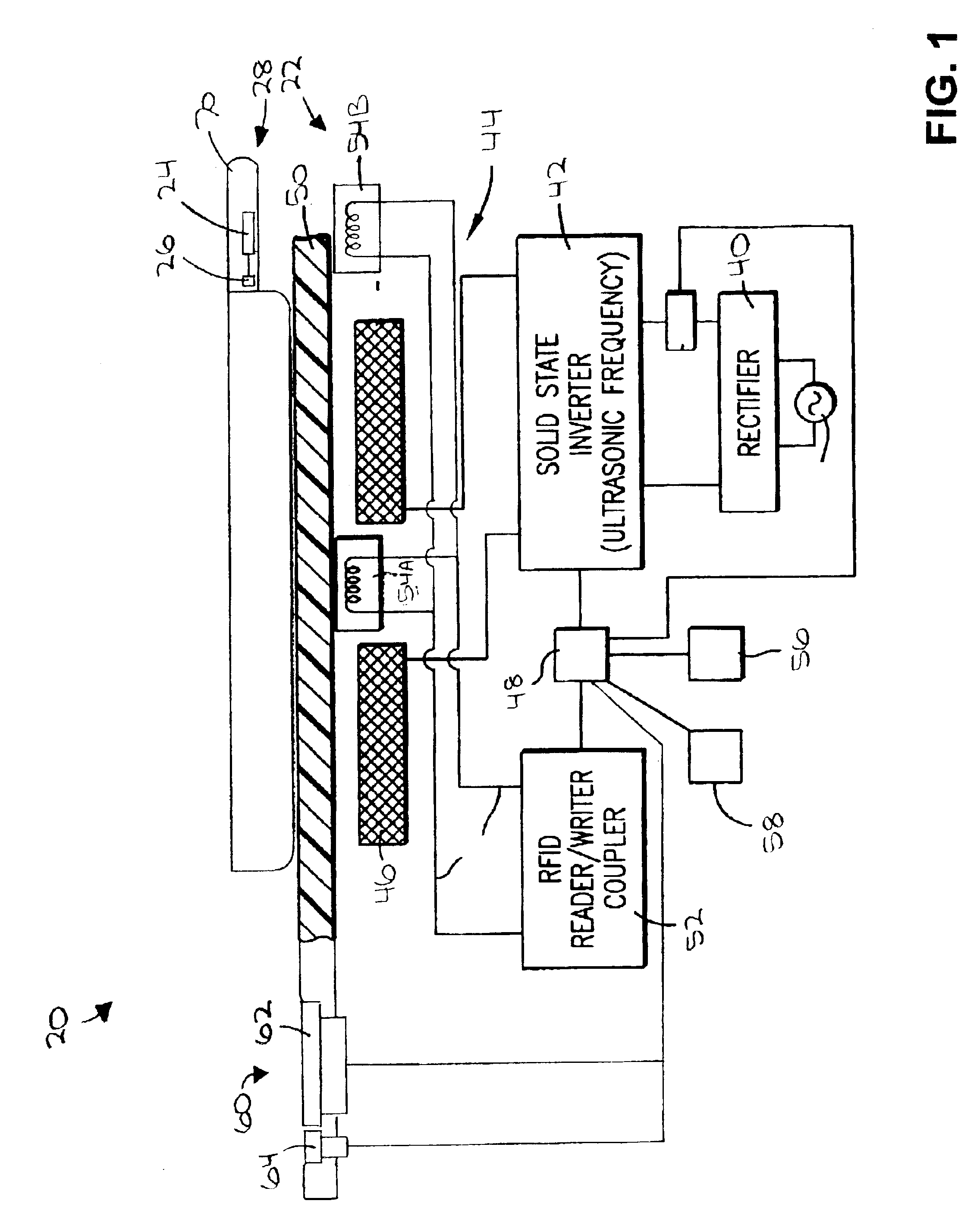
InactiveUS6953919B2Facilitate communicationFacilitates informationCooking vesselsDeep fat fryersAdditive ingredientEngineering
A system and method for providing multiple cooking modes and an ability to automatically heat cooking vessels and other objects using RFID technology, and an ability to read and write heating instructions and to interactively assist in their execution. An induction heating range is provided with two antennas per hob, and includes a user interface display and input mechanism. The vessel includes an RFID tag and a temperature sensor. In a first cooking mode, a recipe is read by the range and the range assists a user in executing the recipe by automatically heating the vessel to specified temperatures and by prompting the user to add ingredients. The recipe is written to the RFID tag so that if the vessel is moved to another hob, into which the recipe has not been read, the new hob can read the recipe from the RFID tag and continue in its execution.
Owner:HR TECH
Fire-resistant panel and method of manufacture
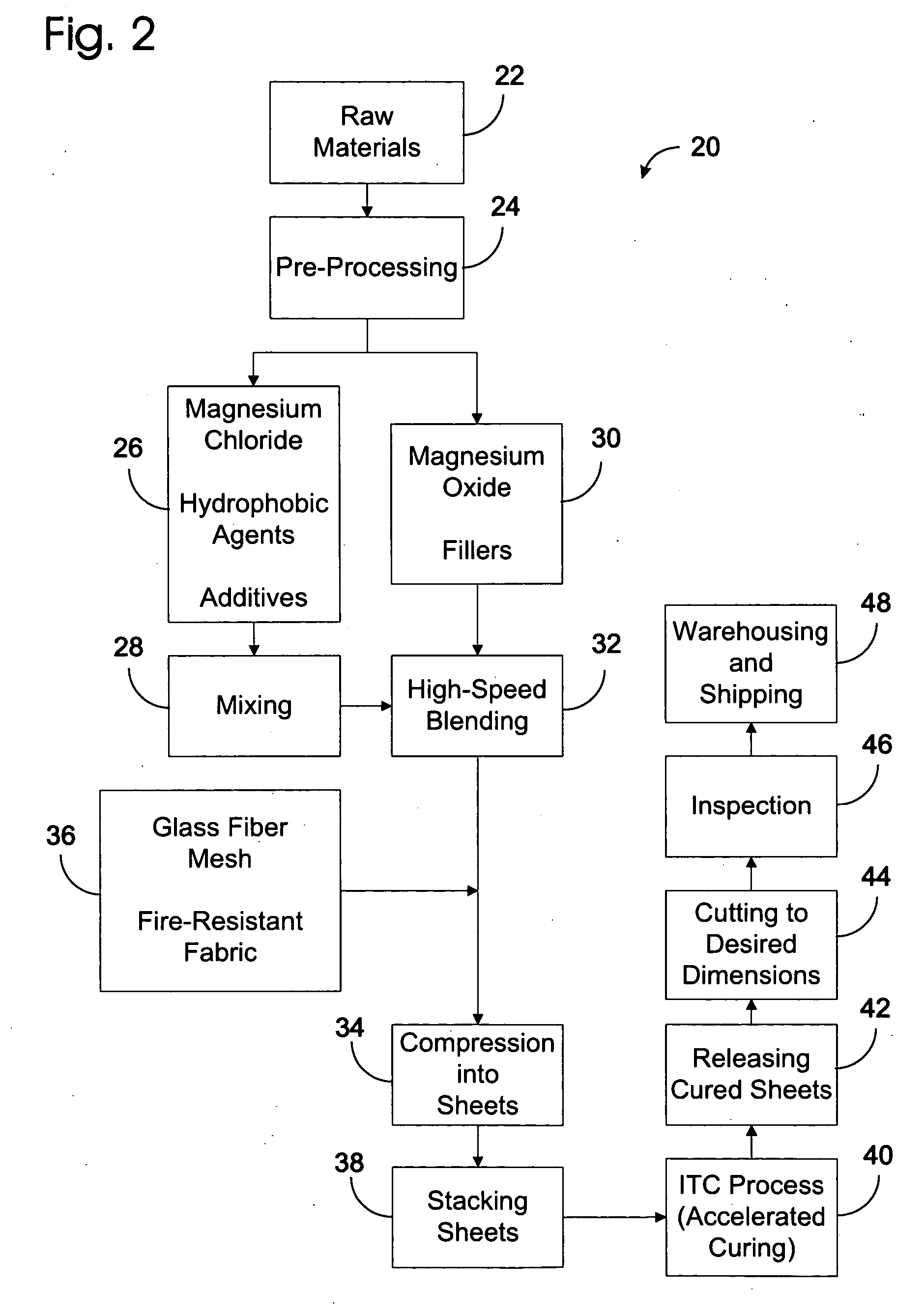
A highly fire-resistant and environmentally-friendly panel of 2 mm to 28 mm may be manufactured by a blending of magnesium compounds, sodium silicate, kaolin, fillers, and additives to form the core materials, reinforced by 4 layers of fire-resistant glass fiber meshes and fabrics. Using a proprietary ITC process that accelerates the chemical reactions of the ingredients to generate sufficient heat without external supply of energy, the panels may be completely cured within 24 hours instead of 10 days. The use of waste materials, energy-saving curing system and no gas emission manufacturing process combined to make this panel an eco-friendly product which offers the world's highest-rated fire resistance of 5 hours, high flexural strength, low density, durability and effective water-resistance.
Owner:REP TECH
Food Compositions of Microalgal Biomass
InactiveUS20100239712A1Cheaply and efficiently scaleReduce the amount requiredMilk preparationDough treatmentDry weightAdditive ingredient
The invention provides algal biomass, algal oil, food compositions comprising microalgal biomass, whole microalgal cells, and / or microalgal oil in combination with one or more other edible ingredients, and methods of making such compositions by combining algal biomass or algal oil with other edible ingredients. In preferred embodiments, the microalgal components are derived from microalgal cultures grown and propagated heterotrophically in which the algal cells comprise at least 10% algal oil by dry weight.
Owner:TERRAVIA HLDG INC
Rapid prototyping and fabrication method for 3-D food objects
InactiveUS6280785B1Versatile and Realistic Rapid PrototypingRapid productionLayered productsConfectioneryFree formControl signal
A freeform fabrication method for making a three-dimensional food object from a design created on a computer, including: (a) providing a support member by which the object is supported while being constructed; (b) operating a material dispensing head for dispensing a continuous or intermittent strand of food composition in a fluent state; this food composition including a liquid ingredient and a primary body-building food material and the dispensed food composition having a rigidity and strength sufficient for permitting the food composition to be built up layer by layer into a three-dimensional shape in a non-solid state; and (c) operating control devices for generating control signals in response to coordinates of the object design and controlling the position of the dispensing head relative to the support member in response to the control signals to control dispensing of the food composition to construct a 3-D shape of this object. The method optionally includes an additional step of applying a heat treatment to the 3-D shape after this 3-D shape is constructed. This method can be used to form an intricate shape of a cake mix, which is then baked in an oven. It can also be used to form a custom-designed decorative shape on the top surface of a pre-made cake.
Owner:NANOTEK INSTR GRP LLC
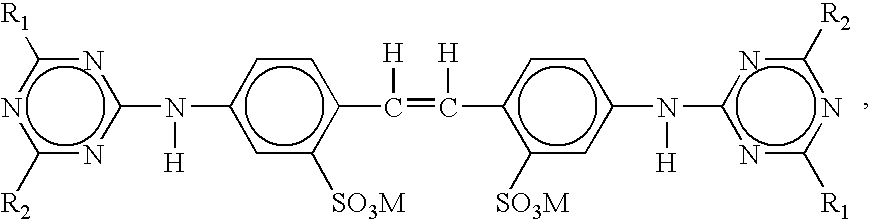
High-potency sweetener for hydration and sweetened hydration composition
InactiveUS20070116823A1Improve flavor profileImproving temporal profile profileMetabolism disorderFood preparationAdditive ingredientSweetness
The present invention relates generally to functional sweetener compositions comprising non-caloric or low-caloric natural and / or synthetic high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different functional sweetener compositions comprising at least one non-caloric or low-caloric natural and / or synthetic high potency sweetener, at least one sweet taste improving composition, and at least one functional ingredient, such as a hydration product. The present invention also relates to functional sweetener compositions and methods that can improve the tastes of non-caloric or low-caloric high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the functional sweetener compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
Multi-phase, multi-compartment capsular delivery apparatus and methods for using same
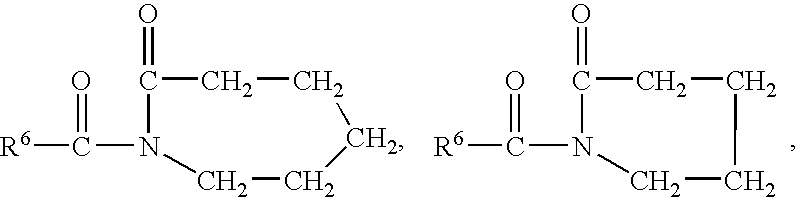
ActiveUS20050008690A1Extended shelf lifeFacilitate desirable propertyPowder deliveryCapsule deliveryDietary supplementAdditive ingredient
A multi-compartment capsule, comprising, a first receiving chamber comprising at least one ingredient having a first physical state, wherein said ingredient is selected from the group consisting of a nutraceutical, a vitamin, a dietary supplement and a mineral; and a second receiving chamber comprising at least one ingredient having a second physical state, wherein said ingredient is selected from the group consisting of a nutraccutical, a vitamin, a dietary supplement and a mineral; wherein said first physical state of said ingredient of said first receiving chamber being different from said second physical state of said ingredient of said second receiving chamber; and said ingredient of said first receiving chamber being different from said ingredient of said second receiving chamber.
Owner:INNERCAP TECH
Vaccine composition containing synthetic adjuvant
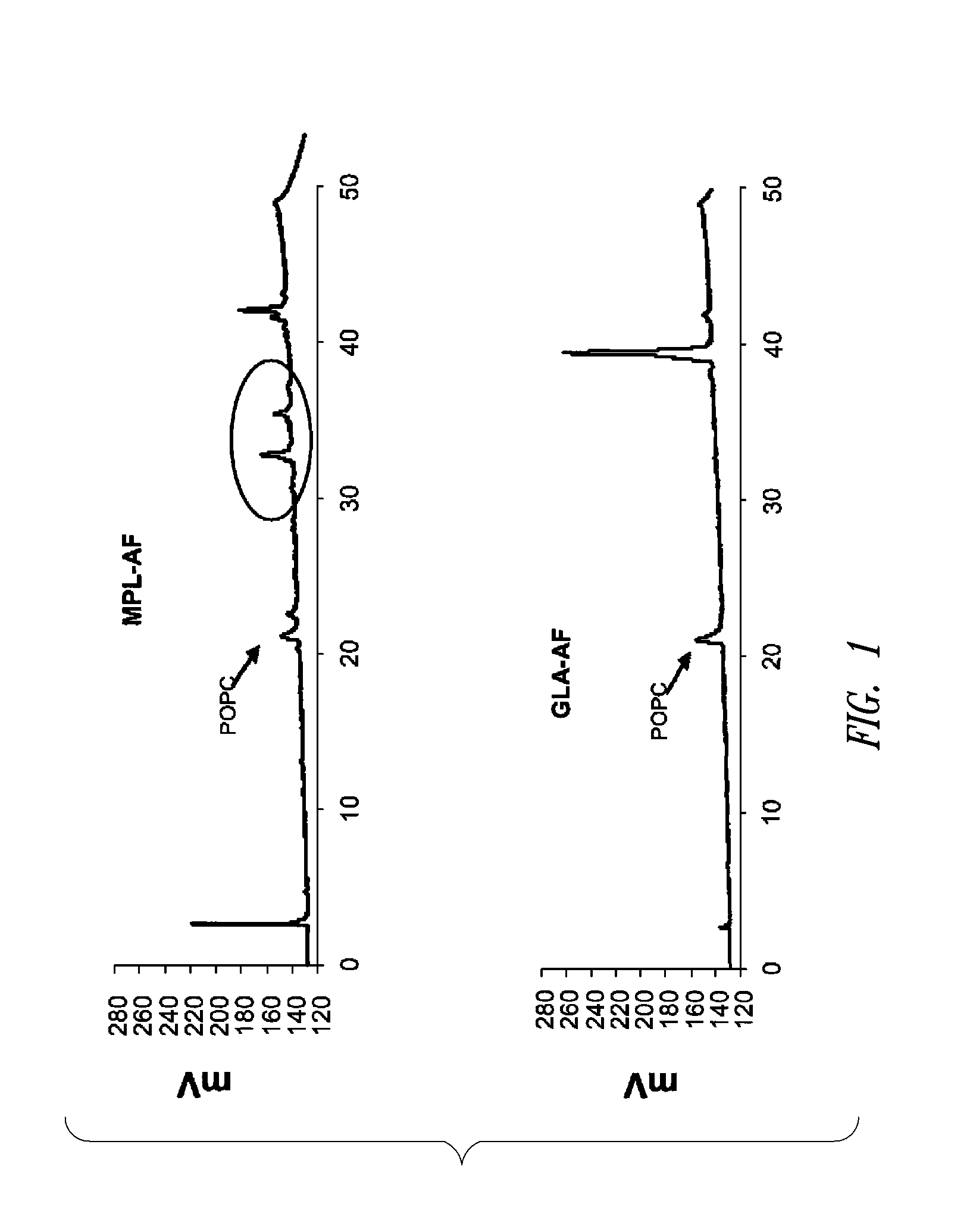
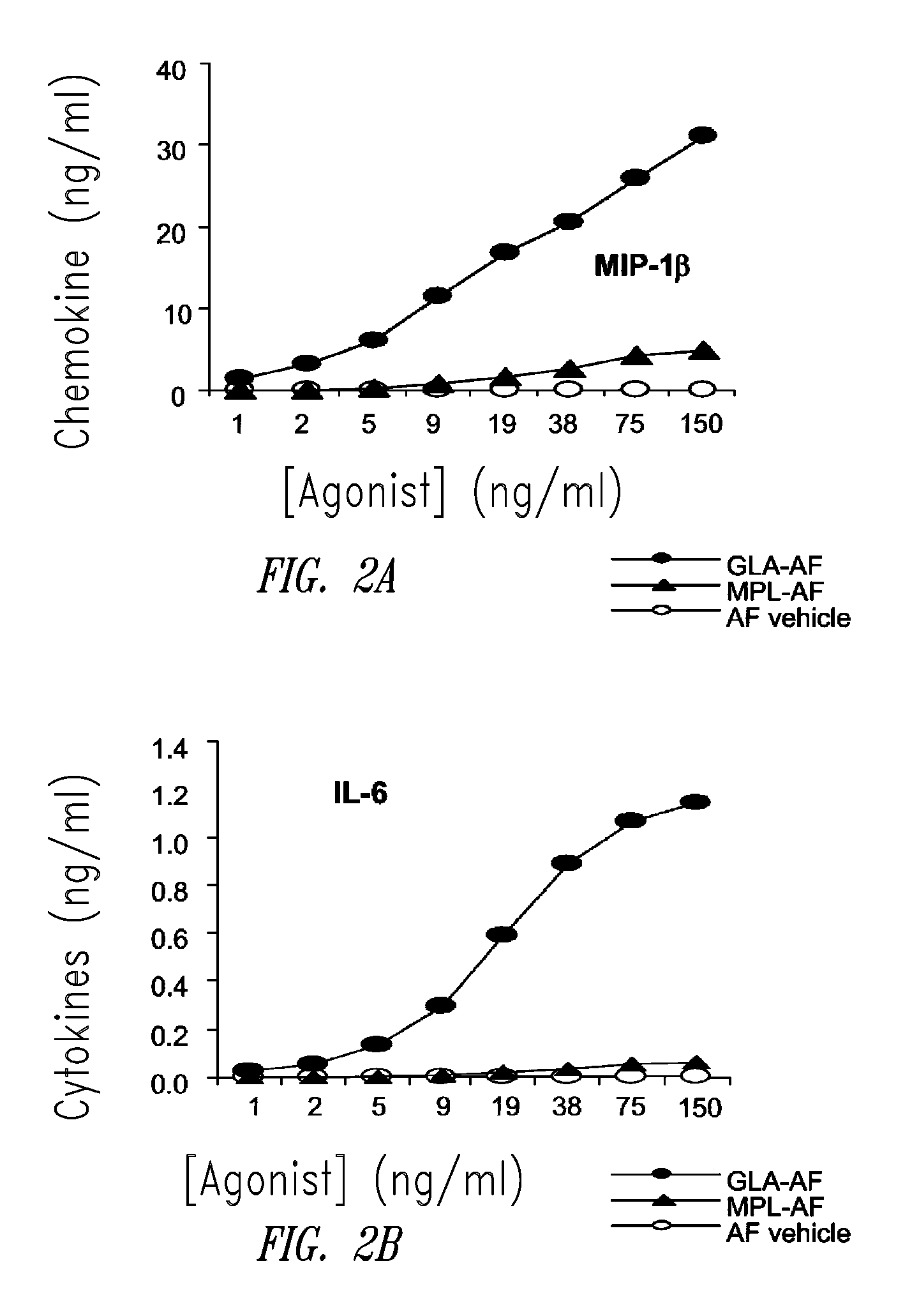
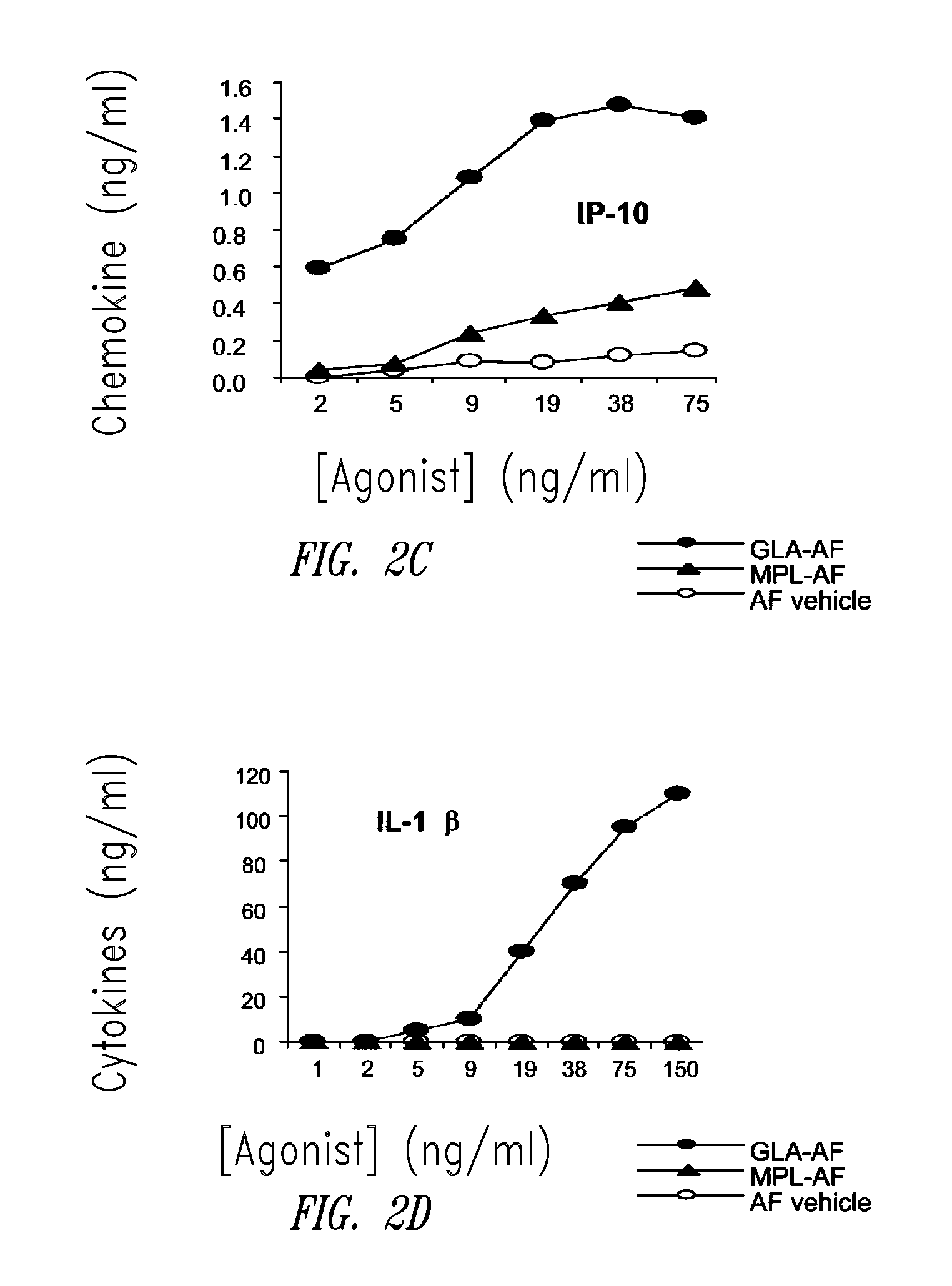
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
Topically Bioavailable Acne and Rosacea Treatment Compositions
InactiveUS20040156873A1Reduce stimulationSynergistic superior anti-acneBiocideCosmetic preparationsAdditive ingredientIrritation
The present invention relates to acne and rosacea compositions by a six-prong synergistic combination treatment strategy that includes (1) control of excess sebum production, (2) control of undesirable bacteria or mites, (3) control of inflammation, (4) enhanced desquamation of follicular infundibulum cells, (5) reduction of irritation from anti-acne or rosacea compositions themselves, and (6) enhancement of the topical bioavailability of anti-acne and rosacea compositions. This is achieved by a synergistic combination of commonly utilized topical anti-acne and rosacea ingredients with a topical bioavailability enhancement composition, which results in enhanced anti-acne and rosacea action from such ingredients. Moreover, additional inclusion of an anti-inflammatory composition, and also a vascular micro-circulation enhancement composition, further results in synergistic superior anti-acne and rosacea benefits from such compositions. The present invention discloses additional surprising synergistic combinations for the control of acne and rosacea that are suitable for a variety of delivery systems and packaging forms.
Owner:GUPTA SHYAM K
Spontaneous emulsions containing cyclosporine
A pharmaceutical composition contains cyclosporine as the active ingredient. More specifically, the composition is an orally administered pharmaceutical formulation in the form of a spontaneous emulsion comprising cyclosporine, ethanol ethyl oleate and polyoxyethylene glycerol trioleate. A method for preparing an orally administered pharmaceutical composition involves first dissolving cyclosporine in ethanol. Polyoxyethylene glycerol trioleate and an oil component are then added, mixed and diluted in an aqueous media to form a spontaneous emulsion.
Owner:WOCKHARDT EU OPERATIONS SWISS
Method and Device for Ophthalmic Administration of Active Pharmaceutical Ingredients
InactiveUS20080233053A1Efficient transferImprove bioavailabilityOrganic active ingredientsPeptide/protein ingredientsAdditive ingredientBioavailability
Disclosed is the use of a mist of a pharmaceutical composition for ophthalmic delivery of a protein or peptide active pharmaceutical ingredient, a related method of treatment and a device useful in implementing the use and method. Disclosed is also the use of a mist for ophthalmic delivery of a pharmaceutical composition including a highly irritating penetration enhancer and an ophthalmically acceptable carrier, a related method of treatment and a device useful in implementing the use and method. Disclosed is also a device for ophthalmic administration configured to direct a mist of a pharmaceutical composition to the eye only when the eye is open. Disclosed is also a self-sterilizing device for ophthalmic administration. Disclosed is also a device and a method for increasing the bioavailability of an ophthalmically administered API in a pharmaceutical composition.
Owner:PHARMALIGHT
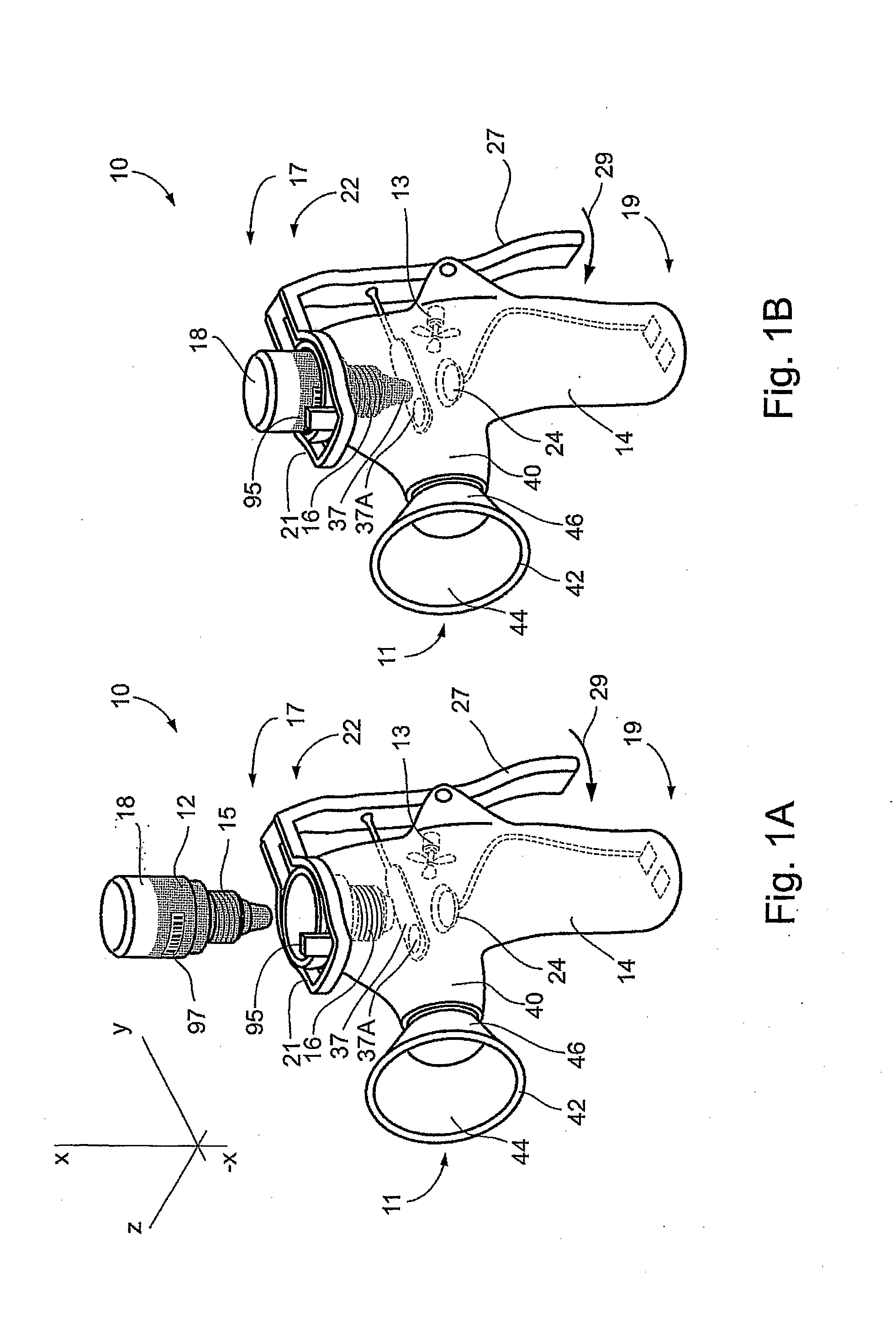
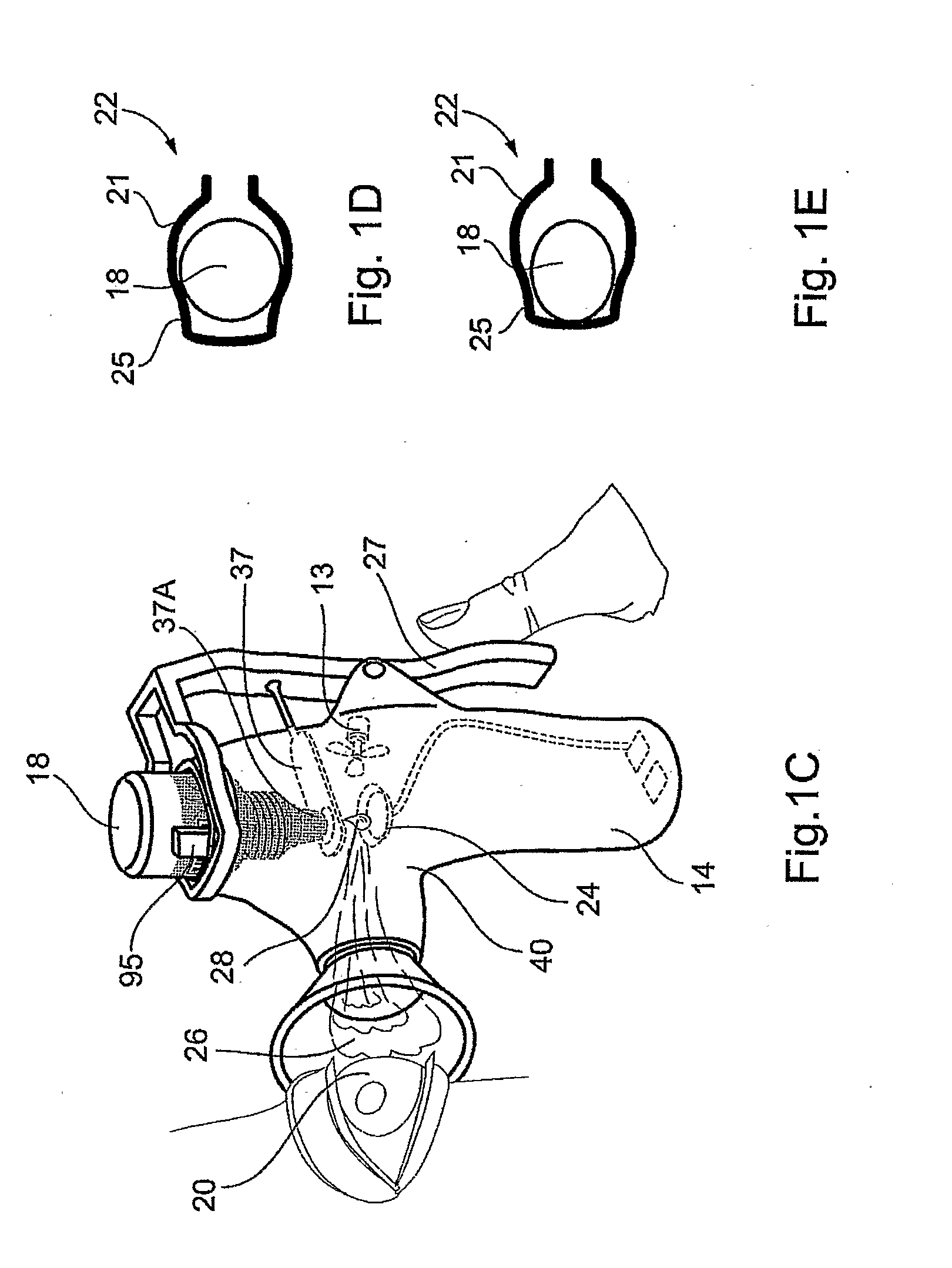
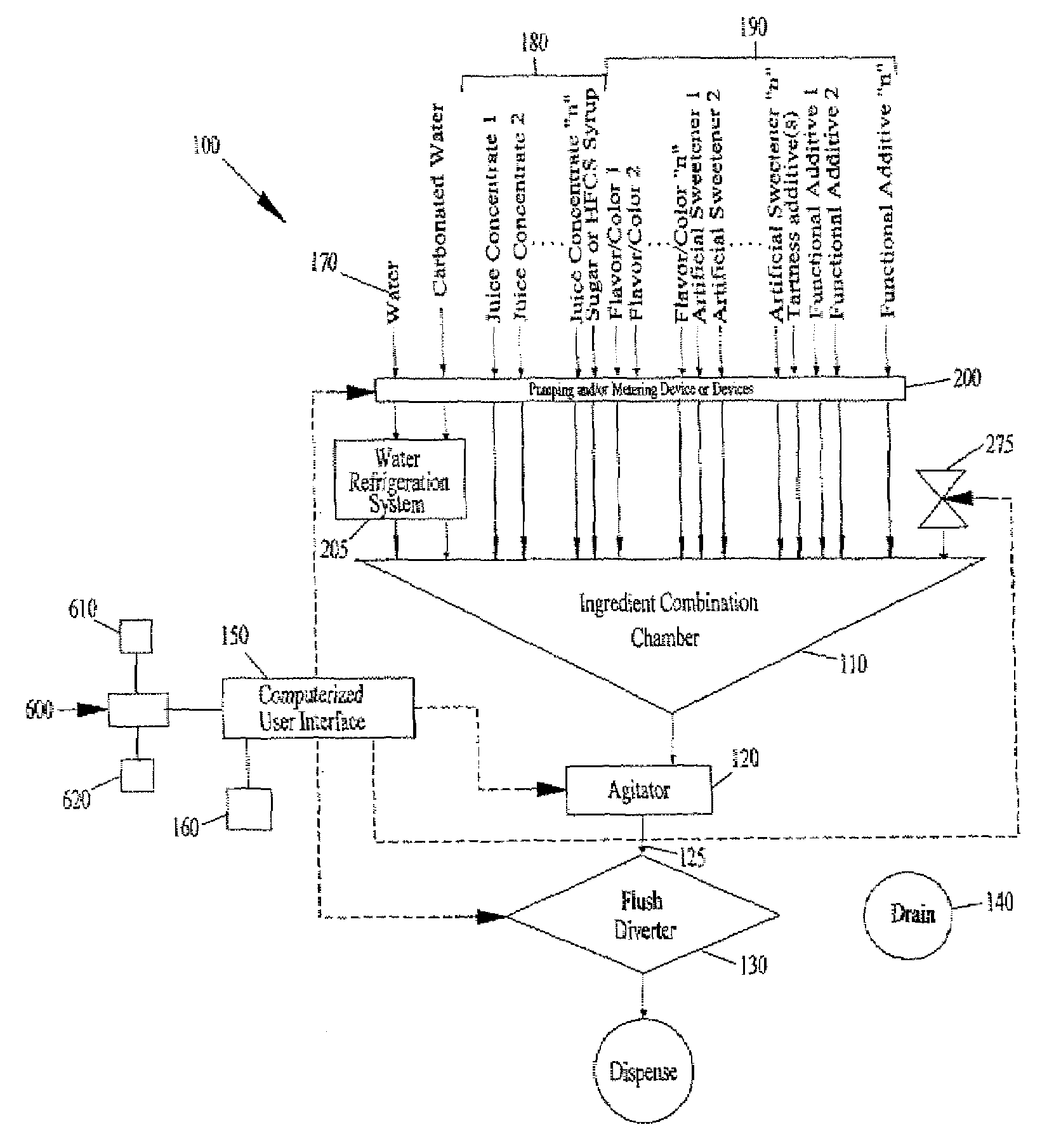
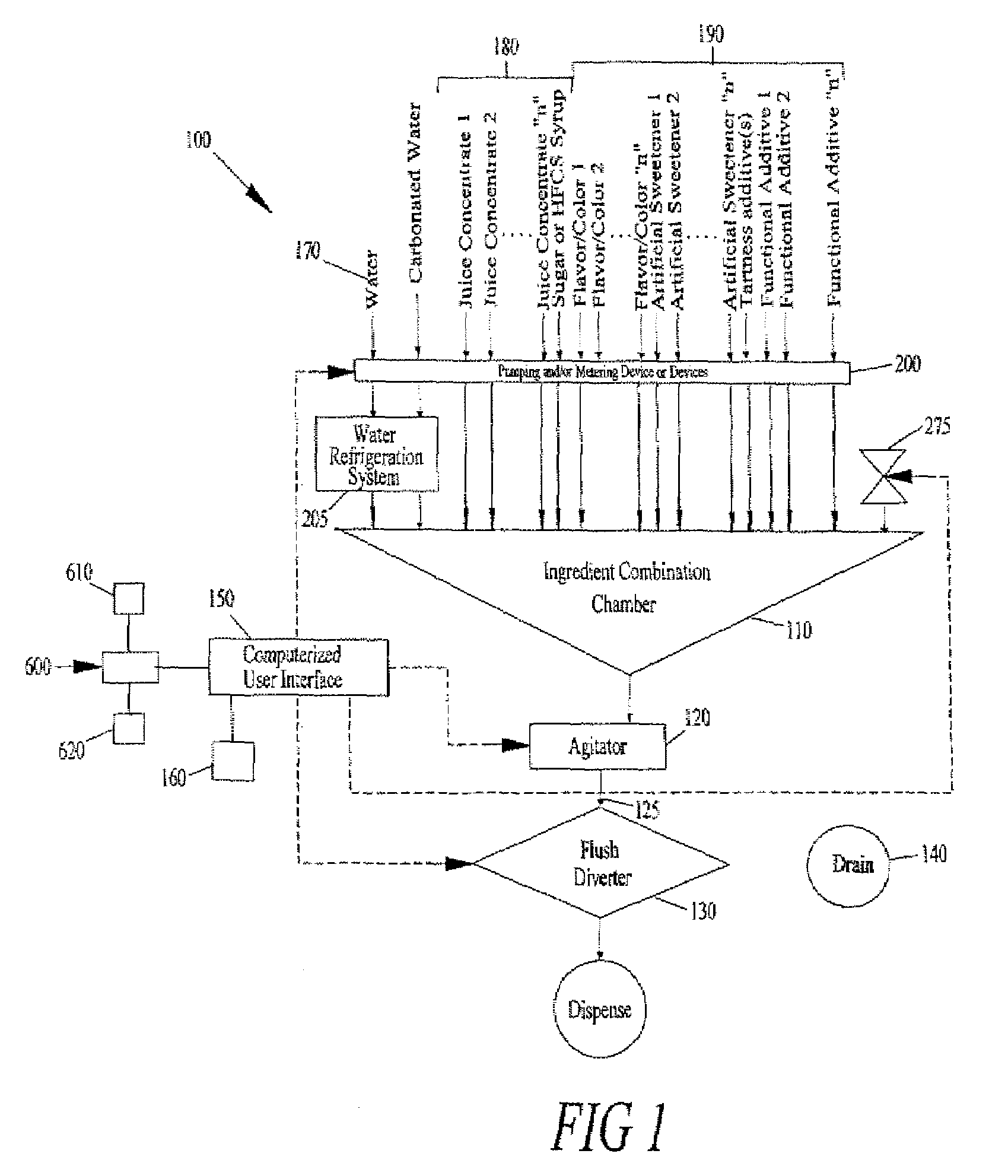
Juice Dispensing System
ActiveUS20070205220A1Liquid flow controllersLiquid transferring devicesFruit juiceAdditive ingredient
The present application describes a product mixing device. The product mixing device includes an ingredient combination chamber and means for agitation positioned about the ingredient combination chamber. The ingredient combination chamber includes a diluent inlet, a number of macro-ingredient inlets, a number of micro-ingredient inlets, and an outlet.
Owner:THE COCA-COLA CO
Method for preparing a beverage or food liquid and system using brewing centrifugal force
A capsule, device, system and method for preparing a liquid food or beverage. The capsule comprises an enclosure containing one or more extractable or infusible ingredients and configured and dimensioned to have a perimeter and be radially symmetrical about a central axis to facilitate rotation; and an opening arrangement that allows liquid food to leave the capsule after passing through the ingredient(s), wherein the opening arrangement is configured and positioned near or upon the perimeter of the enclosure to allow the liquid food to leave the capsule radially due to the application of the centrifugal forces. The method and device introduce liquid into the capsule to form the liquid food while applying centrifugal forces to the capsule to urge the liquid to pass through the ingredient(s).
Owner:SOC DES PROD NESTLE SA
Effervescent green tea extract formulation
InactiveUS6299925B1Fast absorptionMaintain good propertiesAntibacterial agentsPre-extraction tea treatmentNatural productAdditive ingredient
A solid state water soluble formulation in granular or tablet form is provided. The formulation is a natural products formulation containing a green tea plant extract in combination with other ingredients which create an effervescent liquid composition upon dispensing the formulation in a liquid. The liquid form of administration, as well as the effervescent properties of the dissolved formulation increase bioavailability of the advantageous components of the green tea plants such as Polyphenols, by increasing absorption speed and amount in the human body. The formulation may include additional components such as, other plant extracts, vitamins, ionic minerals, and other substances purported to be of a health benefit.
Owner:XEL HERBACEUTICALS INC
Oral transmucosal delivery of drugs or any other ingredients via the inner buccal cavity
InactiveUS6210699B1Avoid irritationAvoid the tasteAdhesive dressingsPill deliverySide effectAdditive ingredient
A device and method for the oral transmucosal delivery of active substances to the oral cavity utilizing an unplasticized polyvinyl pyrrolidone polymer (PVP) as the primary mucoadhesive. The device is applied and adheres to the mucosa of the oral cavity without causing side effects or leaving an unpleasant taste. Preferably the device is a bilayer tablet having a mucoadhesive layer and an overlying active substance containing layer. The mucoadhesive layer may contain PVP as the only adhesive or may be combined with other hydrophilic polymeric substances. The active layer also contains a hydrophilic polymer carrier. The layers in the device dissolve and release the active substance to the oral cavity and is particularly adapted for the delivery of substances active in the oral cavity such as breath fresheners and substances to combat dry mouth. It is also useful for the delivery of ionic drugs such as peptides.
Owner:WATSON PHARMA INC +1
Method for preparing glycolysis Chinese herbal medicine preparations for feed
InactiveCN101116473AGood effectQuick balanceFood processingClimate change adaptationAdditive ingredientChinese traditional
A preparation method of a ferment Chinese Traditional Herbs for feeding belongs to the production field of the feed additives. The technical proposal provided by the present invention is that one or various intestinal probiotics is inoculated on a cultivation matrix containing extracts of Chinese Traditional Herbs, and undergoes ferment cultivation in a proper condition. The probiotics include Lactobacillus acidophilus, Bacillus subtilis, and candida utilis, etc, and the selected Chinese Traditional Herbs include astragalus, atractlylis lancea formalyrata, codonopsis pilosula, pericarpium citri reticulatae, and atractylis atractylis ovata, etc. The ferment Chinese Traditional Herbs for feeding provided by the present invention used to breed the livestock and the poultry is capable of preventing and treating epidemic diseases and improving the growth of the animal. The biological ferment of the water extract of Chinese Traditional Herbs by the probiotics is utilized to produce the elements with higher activities, thereby improving the absorption, transportation, metabolism, activation and medical efficacy of the Chinese Traditional Herbs in human body.
Owner:济南亿民动物药业有限公司
Beverage system, including bubble beverage, instant beverage, beverage with dissolved gas, and beverage with ingredient
InactiveUS20140234488A1Modest expenseTea substituesAlcoholic beverage preparationAdditive ingredientLoment
A beverage system is created having beverage, with at least one liquid. The beverage is created in a variety, including tea drink, coffee drink, milk drink, yogurt drink, malted drink, roasted cereal grain beverage, roasted nut and seed beverage, roasted bean beverage, distilled water, mineral water, sports drink, fruit juice, vegetable juice, fruit drink, vegetable drink, fruit skin drink, vegetable skin drink, plant drink, soft drink, alcoholic drink, and soup drink. The beverage is also created with or without addition of ingredient, including flavoring ingredient, nutritional ingredient, health ingredient, and other ingredient. The beverage can be served as hot drink, cold drink, cold drink with ice, and warm drink. The beverage can be caffeinated, or non caffeinated; and diet or non diet. The liquid includes water, oil, and alcohol. The beverage system is created in various form, including bubble beverage, instant beverage, and beverage with dissolved gas.
Owner:CHANG ALICE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com