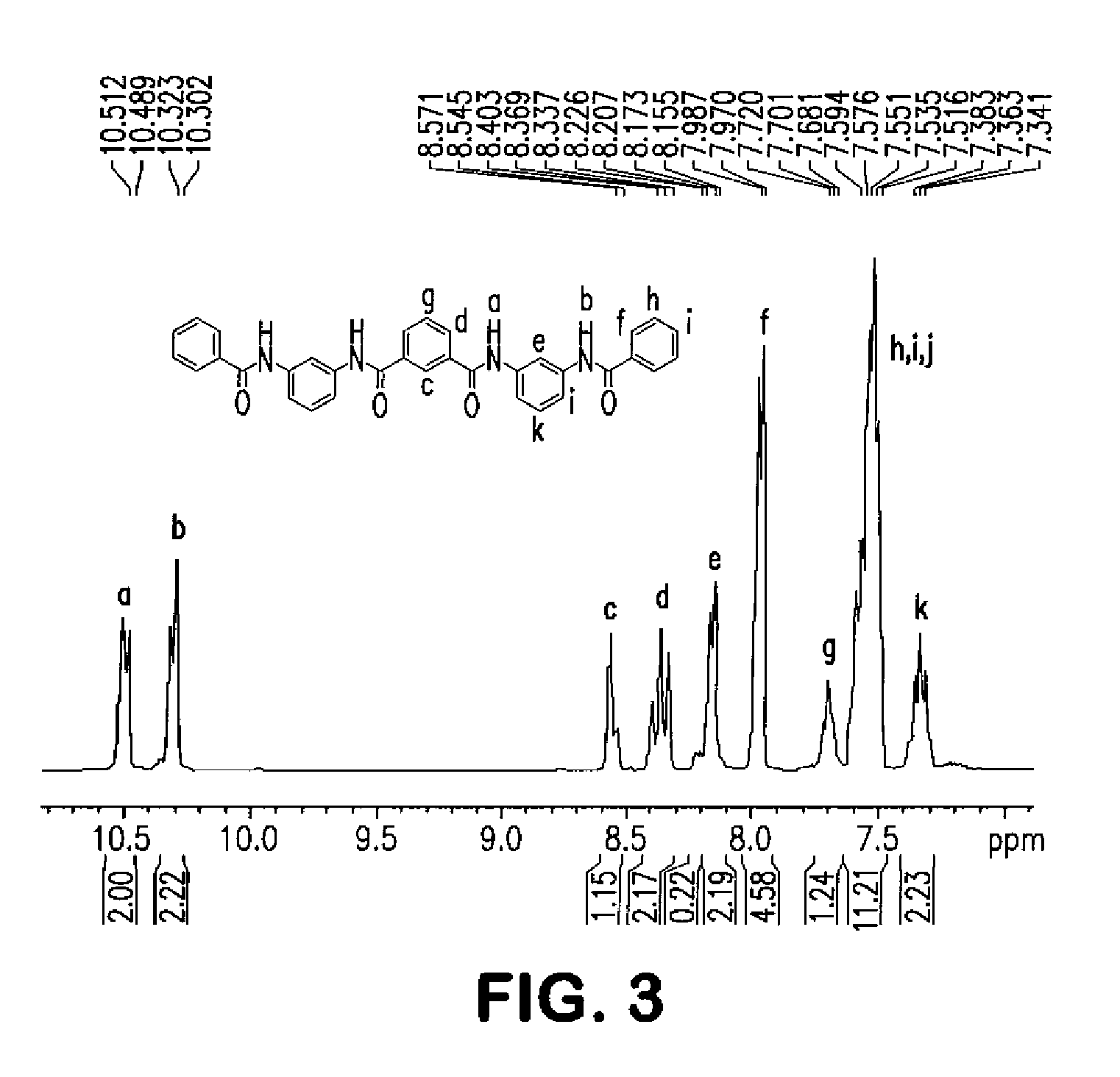
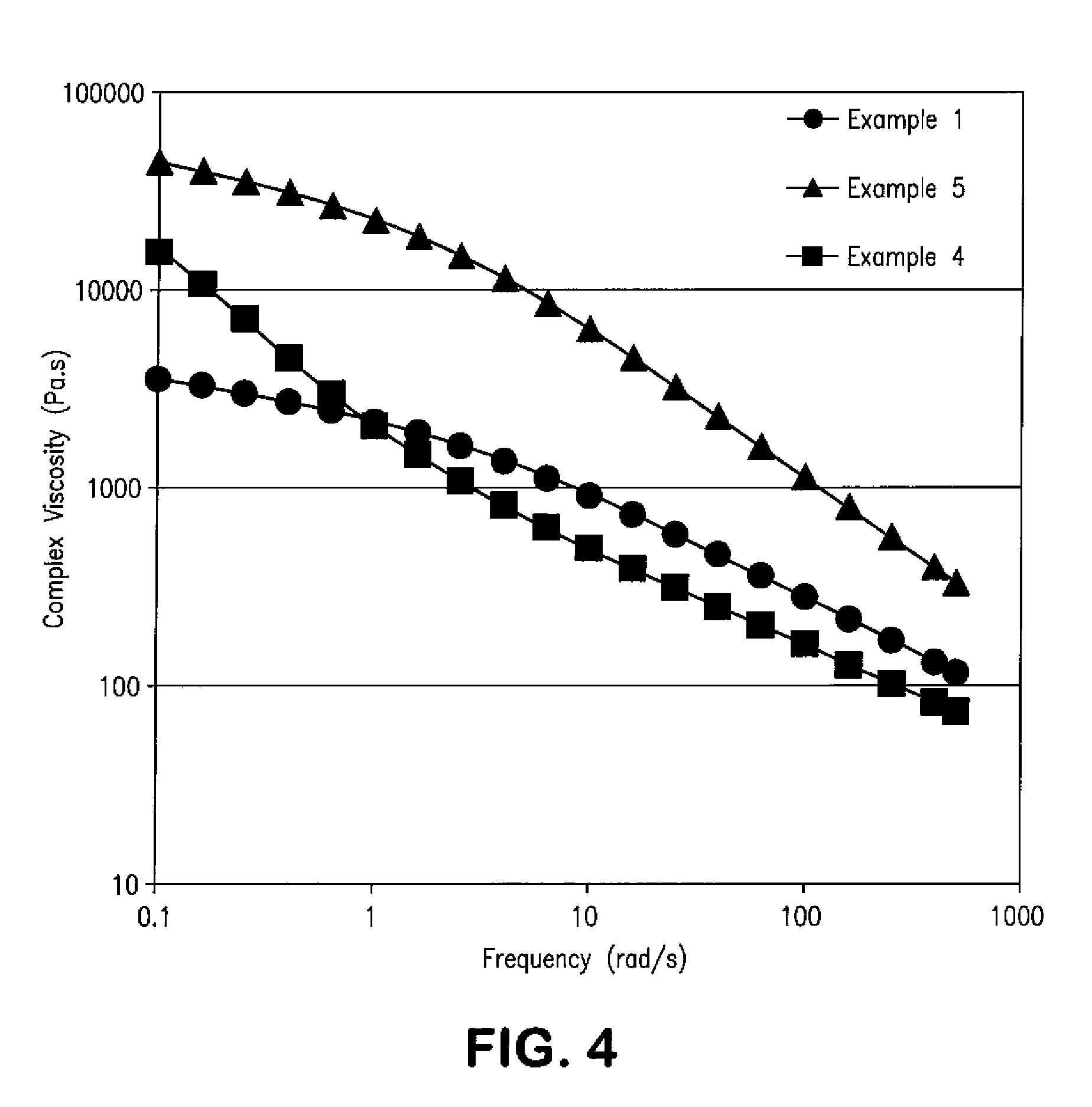
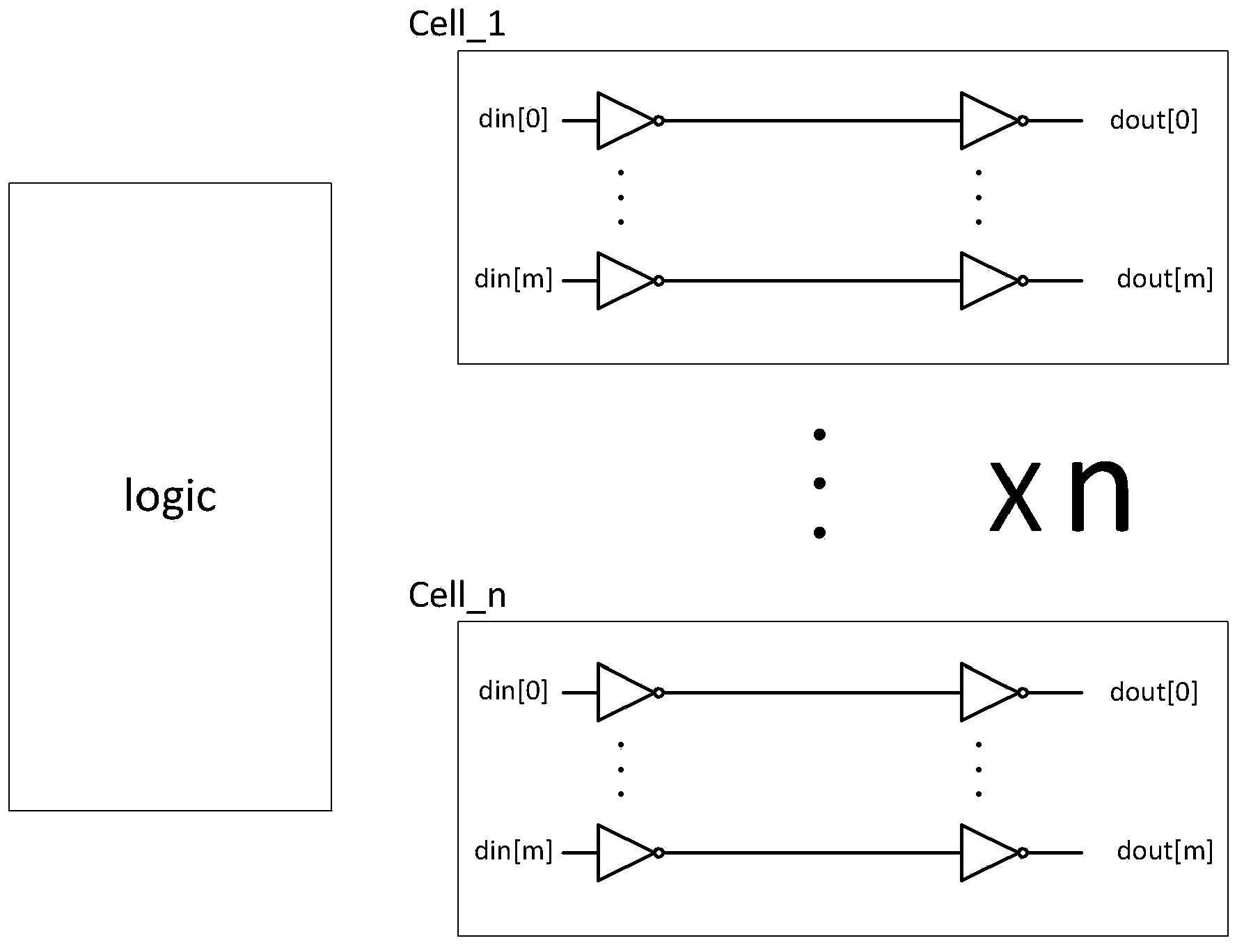
Patents
Literature
73 results about "Physical integrity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
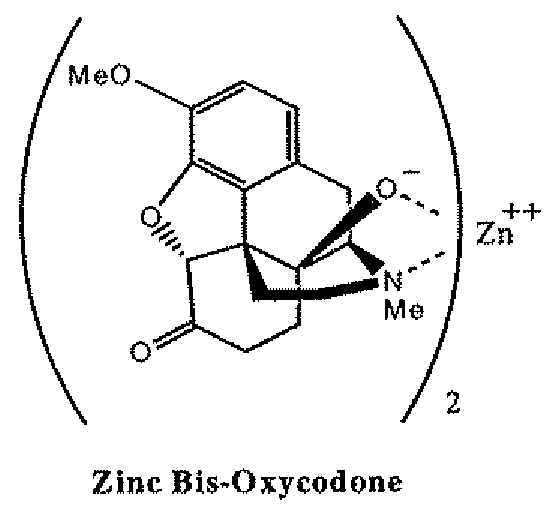
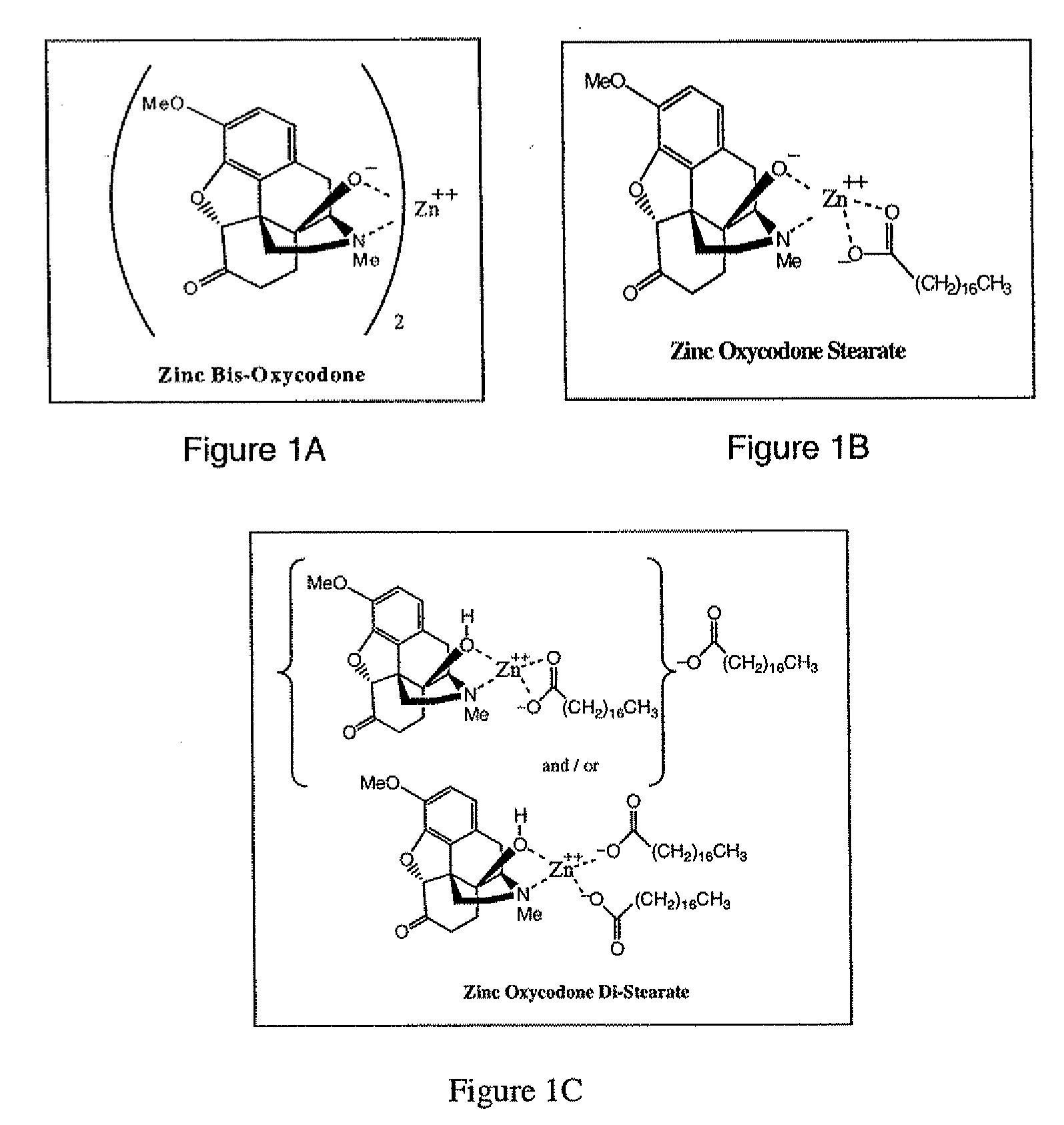
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
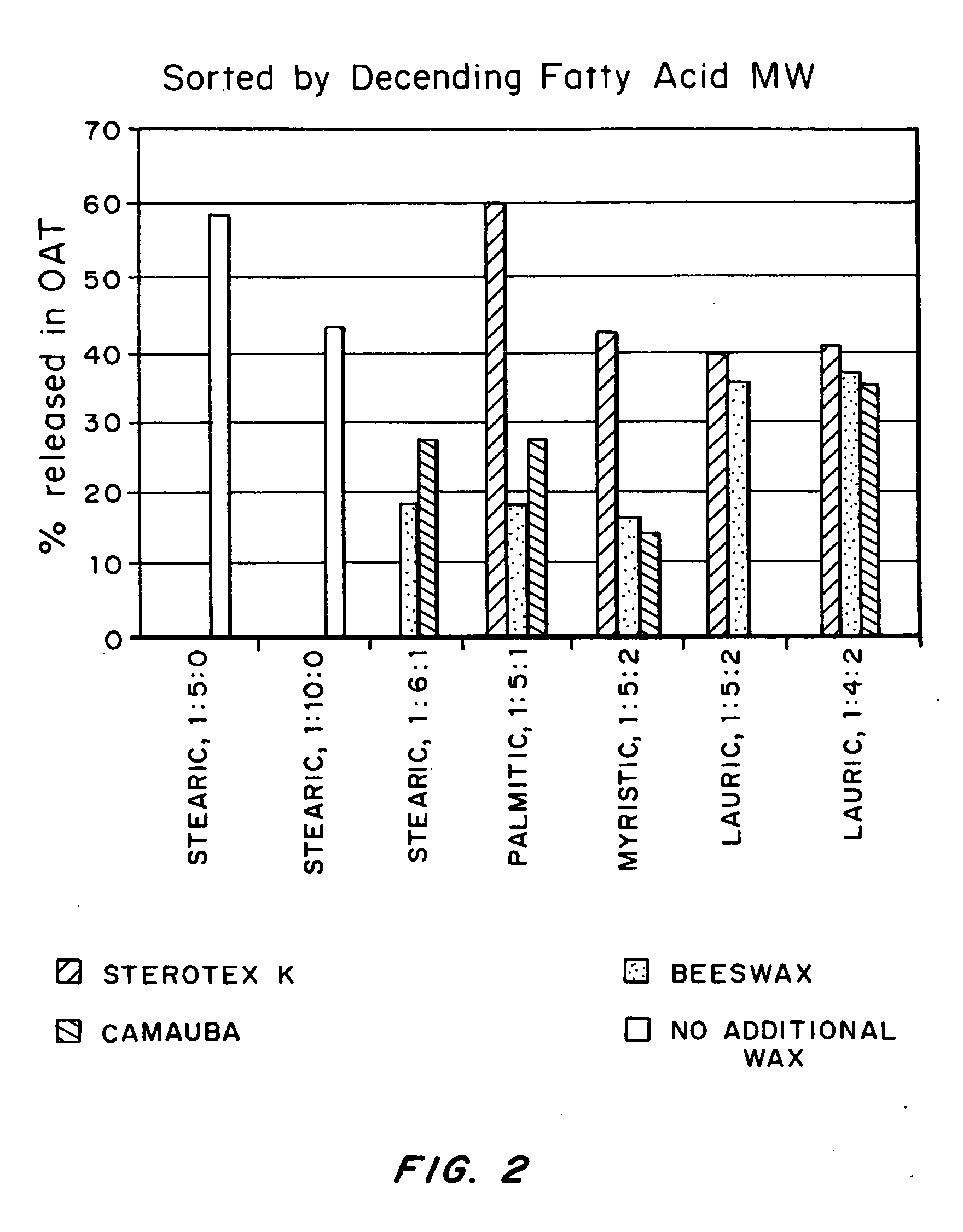
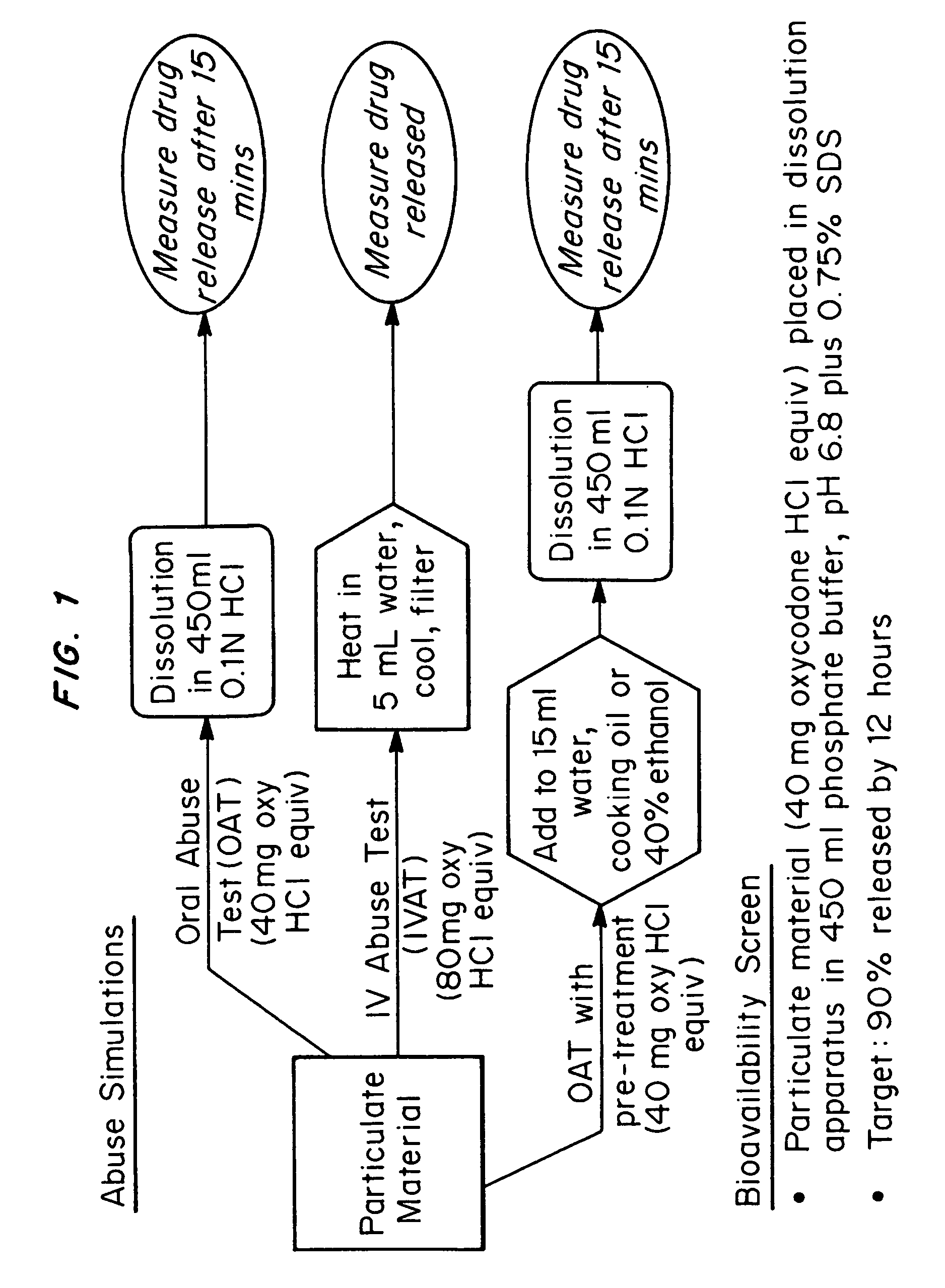
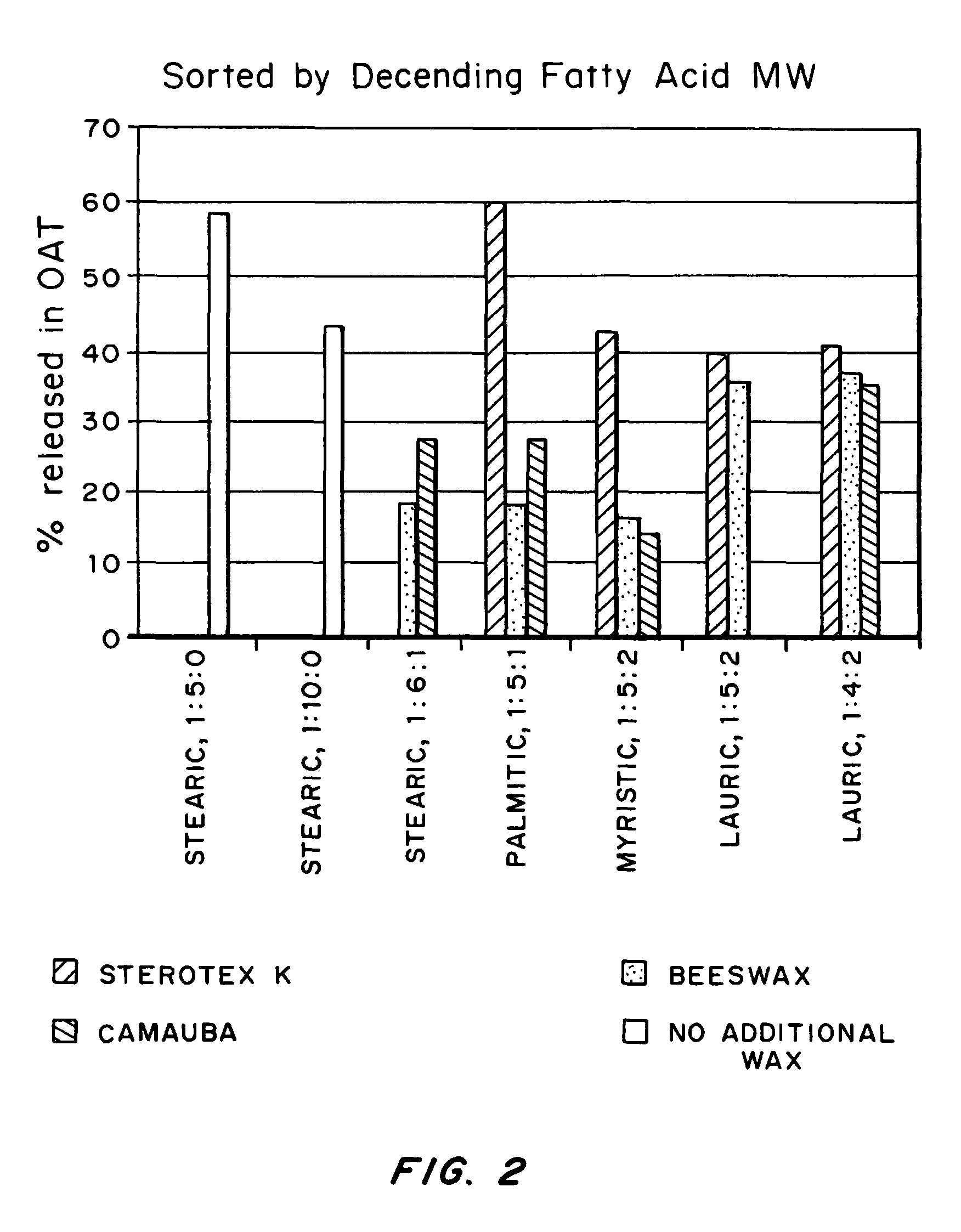
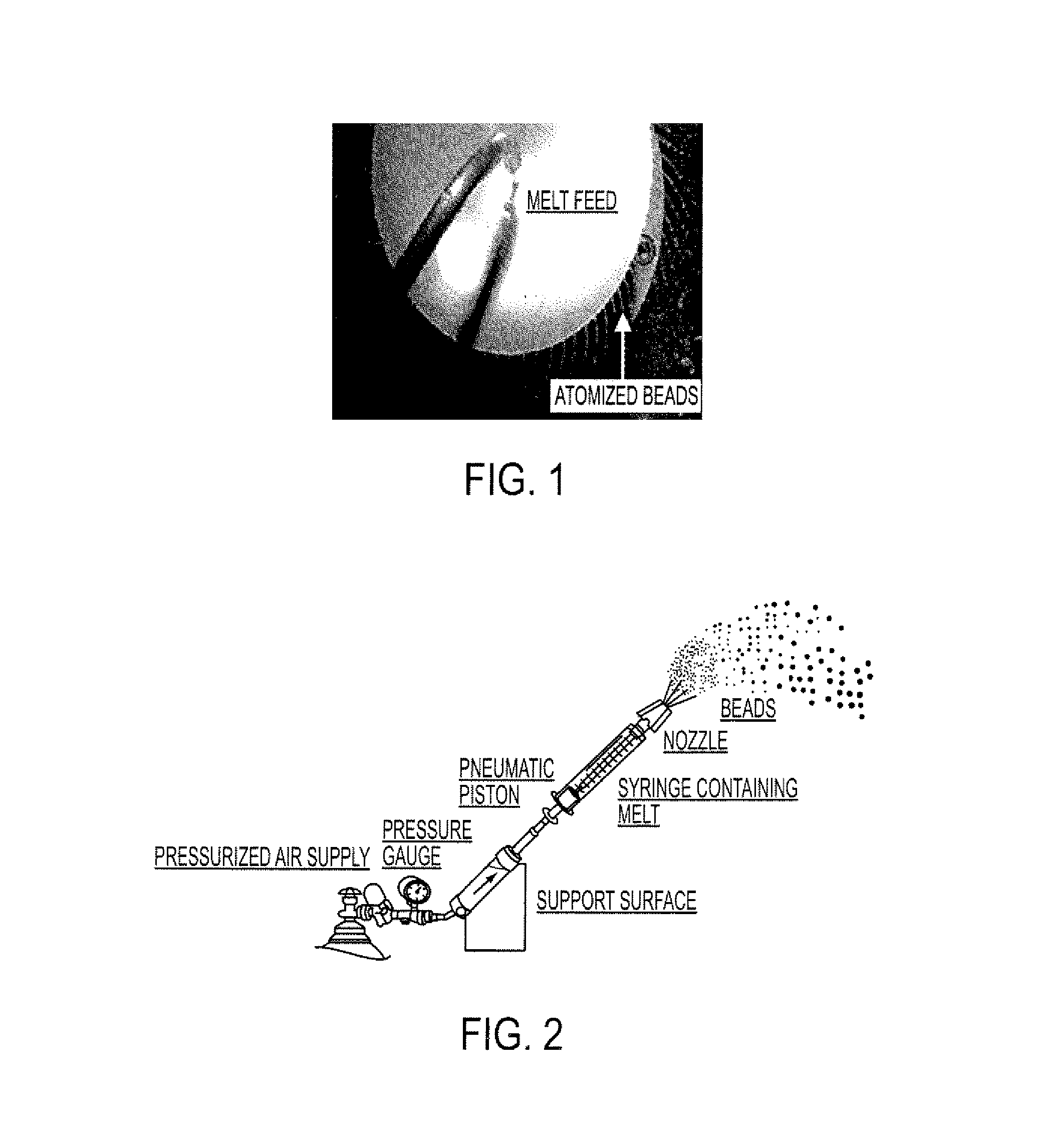
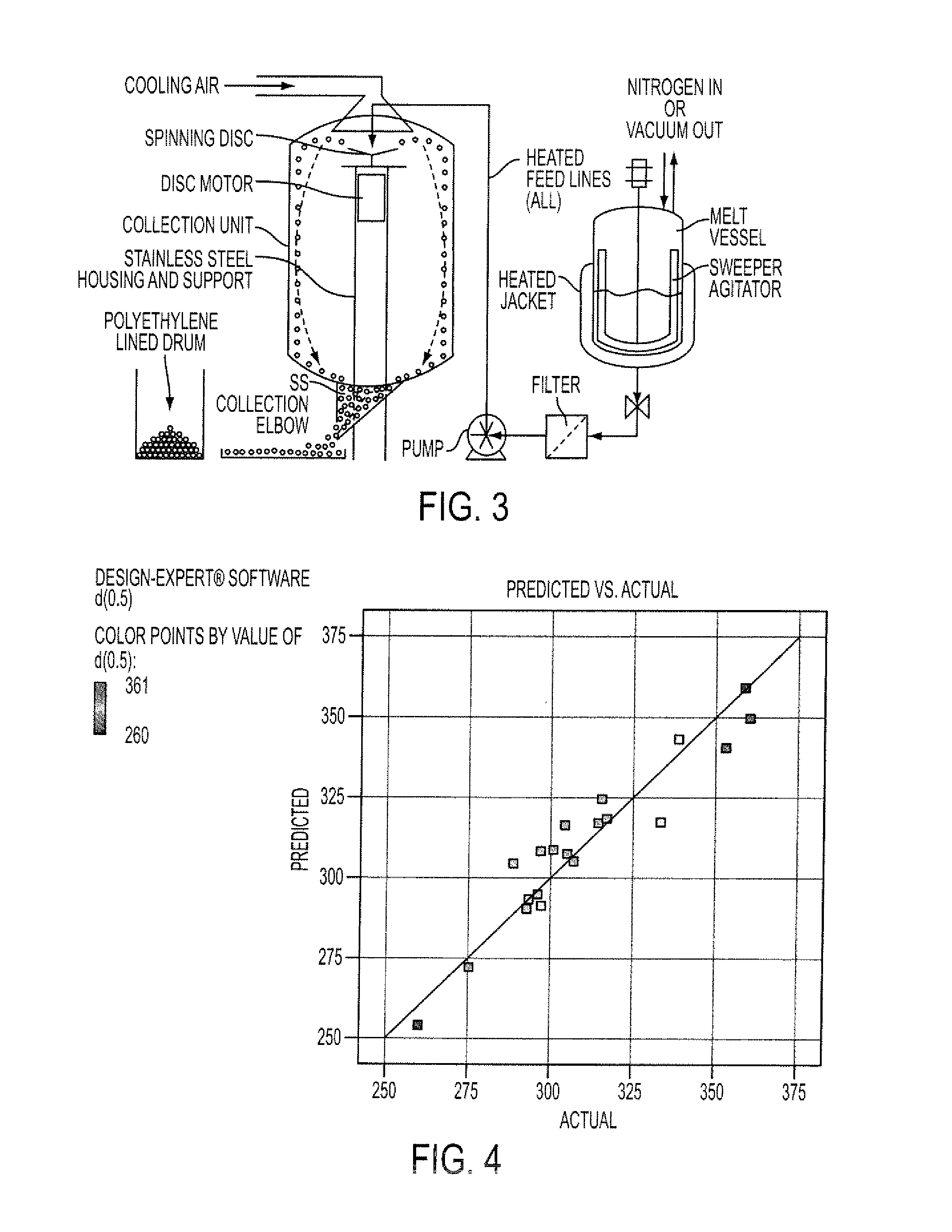
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
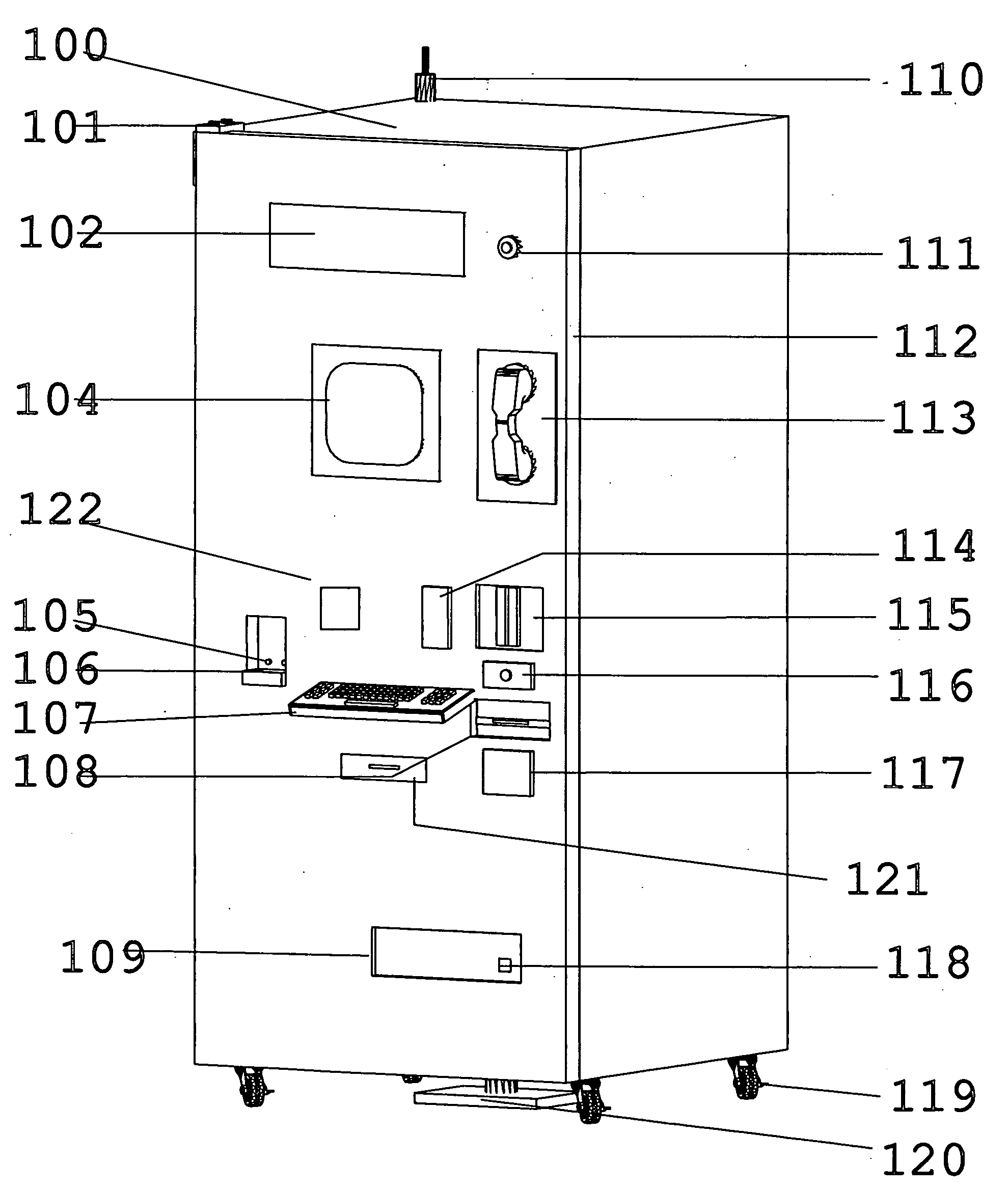
Inventory control and prescription dispensing system
ActiveUS20070043469A1Ensure integrityEnsure safetyDrug and medicationsCoin-freed apparatus detailsEngineeringPatient registration
An inventory control and prescription management and dispensing system including a dispensing vault for storing and dispensing prescriptions, the dispensing vault in communication with a central computer system that, in turn, communicates with prescription providers, insurance companies, and other third parties; the dispensing vault including robotic means for randomly accessing pre-filled prescriptions within the vault, with the vault further including RFID, bar code, and other means for verifying the content and internal location of pre-filled prescriptions; a customer interface that uses customer biometrics to ID a customer to ensure that prescriptions are only dispensed to the correct person; a patient registration system in communication with the central computer system for collecting insurance, doctor, biometric, and other information to facilitate transactions; a labeling system for labeling pre-filled prescriptions with customer specific information upon dispensing; transport container that integrate into the dispensing vault and provide secure transportation from a pharmaceutical manufacturer or repackager to the dispensing vault, security provided through RFID tags which communicate with the central computer to verify that the transport container contains the correct formulary and that the integrity of the container (temperature range, time in transit, tampering) has not been compromised; a payment system integral to the dispensing vault that is in communication with third party banks and pharmacy billing management systems, credit agencies and the central computer; a verification system for ensuring that pre-filled bottles received from the manufacturer (before they are placed into the storage locations inside the vault) have not had their integrity compromised, with this system evaluating each container's weight, size, moisture content, shape, velocity change, color, pattern, and physical integrity (all comparisons made against standards stored in the central computer).
Owner:DRAPER LONNIE
Abuse-deterrent drug formulations
ActiveUS20050281748A1Reduce the possibilityImprove lipophilicityTelevision system detailsPowder deliveryImmediate releaseActive agent
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, the drug is modified to increase its lipophilicity by forming a salt between the drug and one or more fatty acids wherein the concentration of the one or more fatty acids is one to 15 times the molar amount of the active agent, preferably two to ten times the molar amount of the active agent. In one embodiment the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble. The abuse-deterrent composition prevents the immediate release of a substantial portion of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
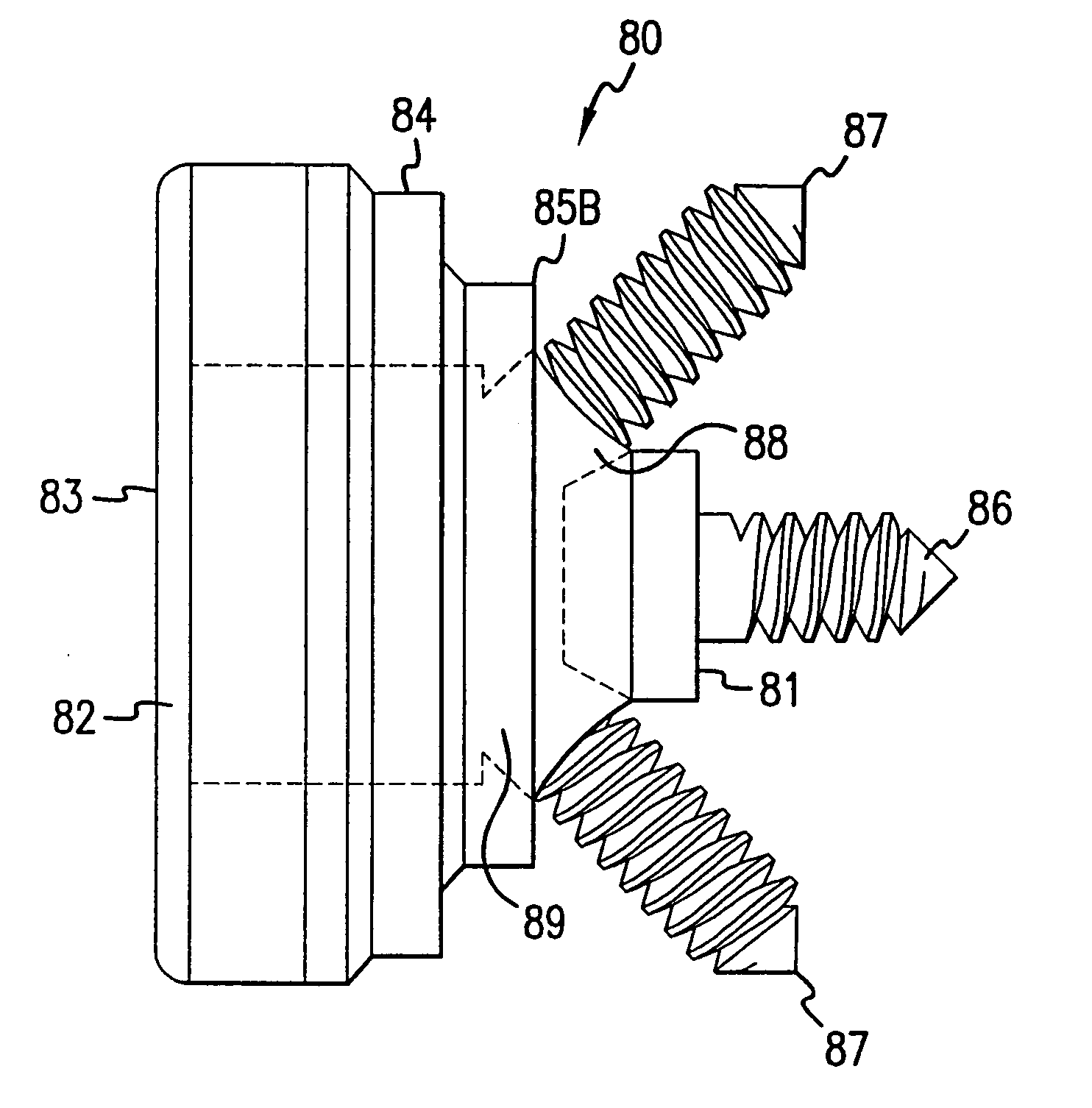
Transosseous core approach and instrumentation for joint replacement and repair
InactiveUS6984248B2Convenient treatmentWithout integrityFinger jointsWrist jointsArticular surfacesArticular surface
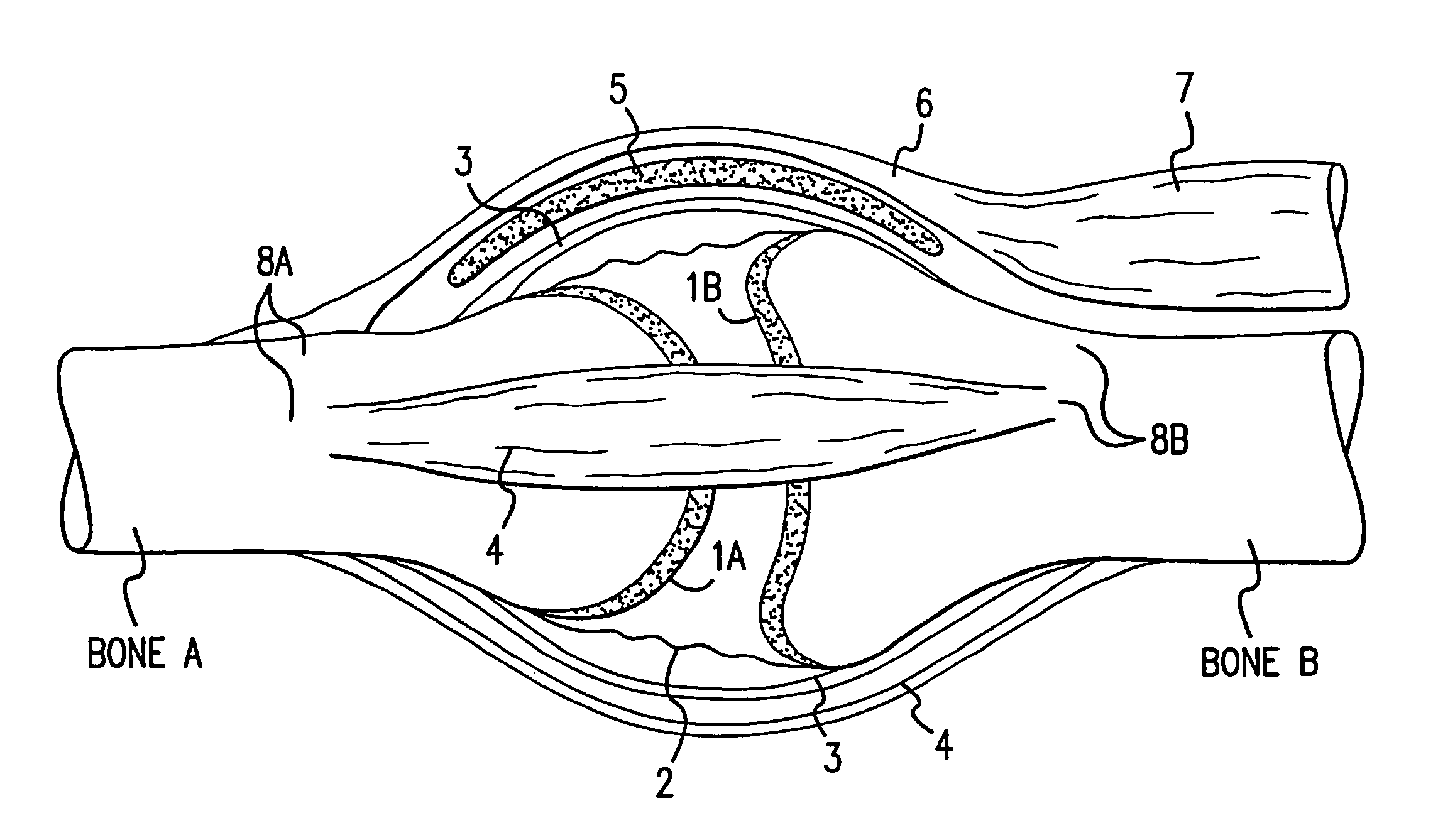
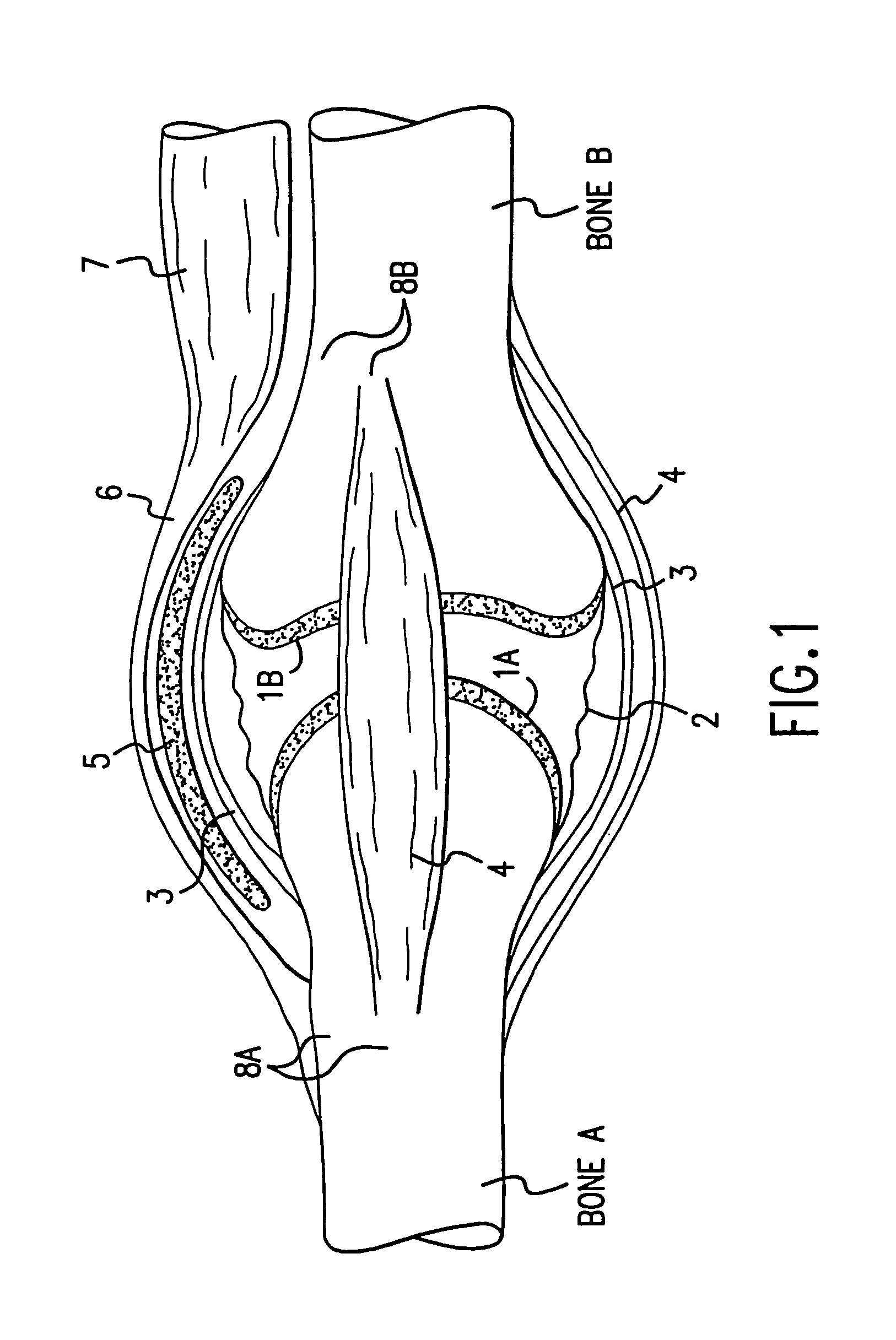
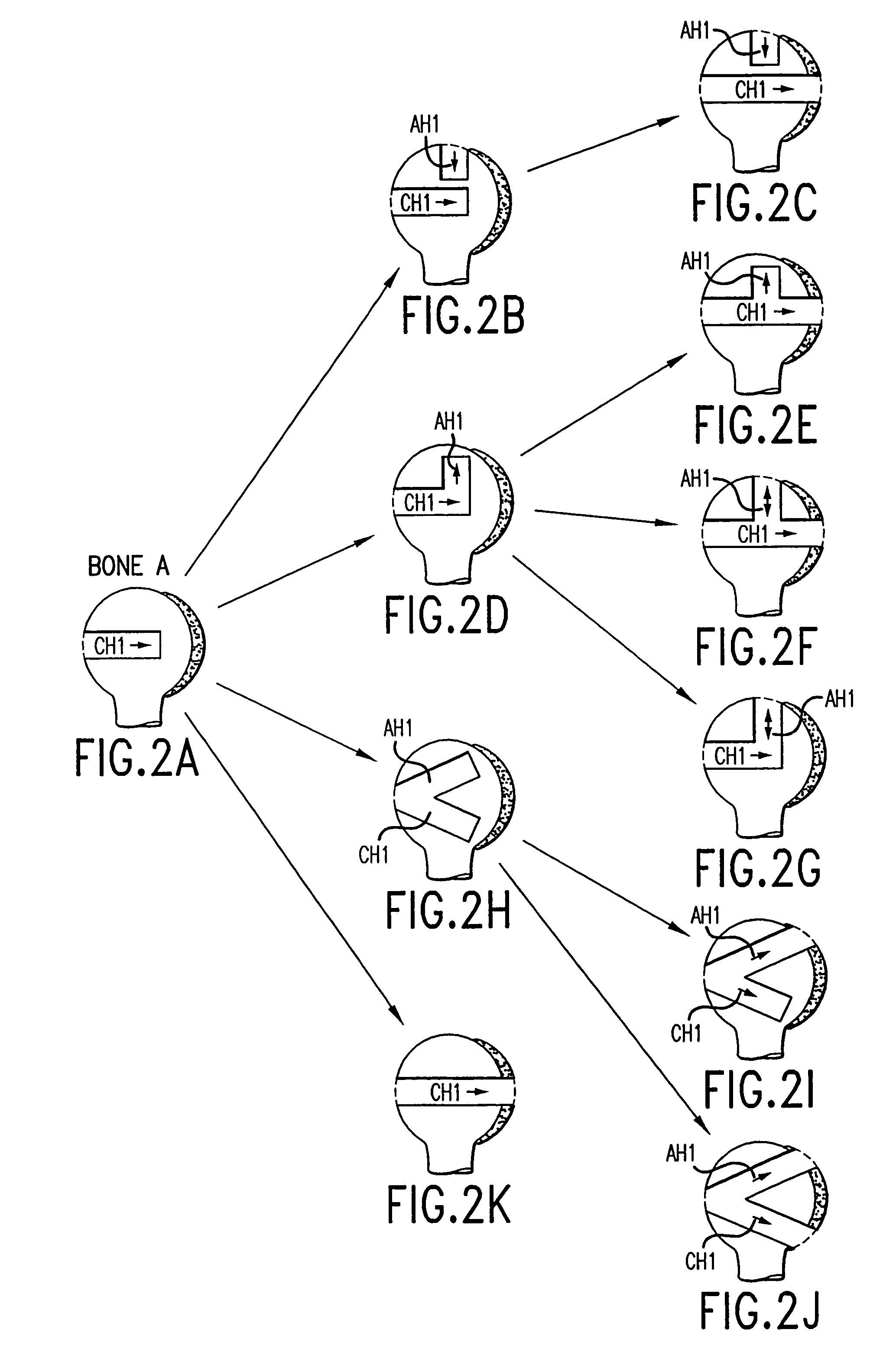
A method and instrumentation is disclosed for gaining access to areas in and around joints for treatment and to provide new implants and instrumentation adapted for the new method. In a transosseous core approach, the joint is entered through a pathway provided in a portion of a joint bone. Such pathway is preferably made by removing a bone core from the bone in or adjacent to the joint, wherever possible without substantially compromising physical integrity and physiological viability of the joint. A route for the transosseous core approach traverses through a more-accessible bone of the joint which can be aligned with a less-accessible bone in order to facilitate treatment of articular surfaces and / or other structures in the joint. Implant modules are provided which can be inserted into the joint through the transosseous pathway and assembled in situ inside the joint to form an implant assembly.
Owner:HYDE JR EDWARD R
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS20090297617A1Reduce the possibilityImprove lipophilicityBiocidePowder deliveryAs DirectedOrganic solvent
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opioids. In a preferred embodiment, a drug is modified to increase its lipophilicity. In some embodiments the modified drug is homogeneously dispersed within spherical microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and / or organic solvent insoluble. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is passes through the GI tract.
Owner:COLLEGIUM PHARMA INC
Abuse-deterrent drug formulations
ActiveUS7771707B2Reduce the possibilityImprove lipophilicityPowder deliveryTelevision system detailsActive agentWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, the drug is modified to increase its lipophilicity by forming a salt between the drug and one or more fatty acids wherein the concentration of the one or more fatty acids is one to 15 times the molar amount of the active agent, preferably two to ten times the molar amount of the active agent. In one embodiment the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble. The abuse-deterrent composition prevents the immediate release of a substantial portion of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
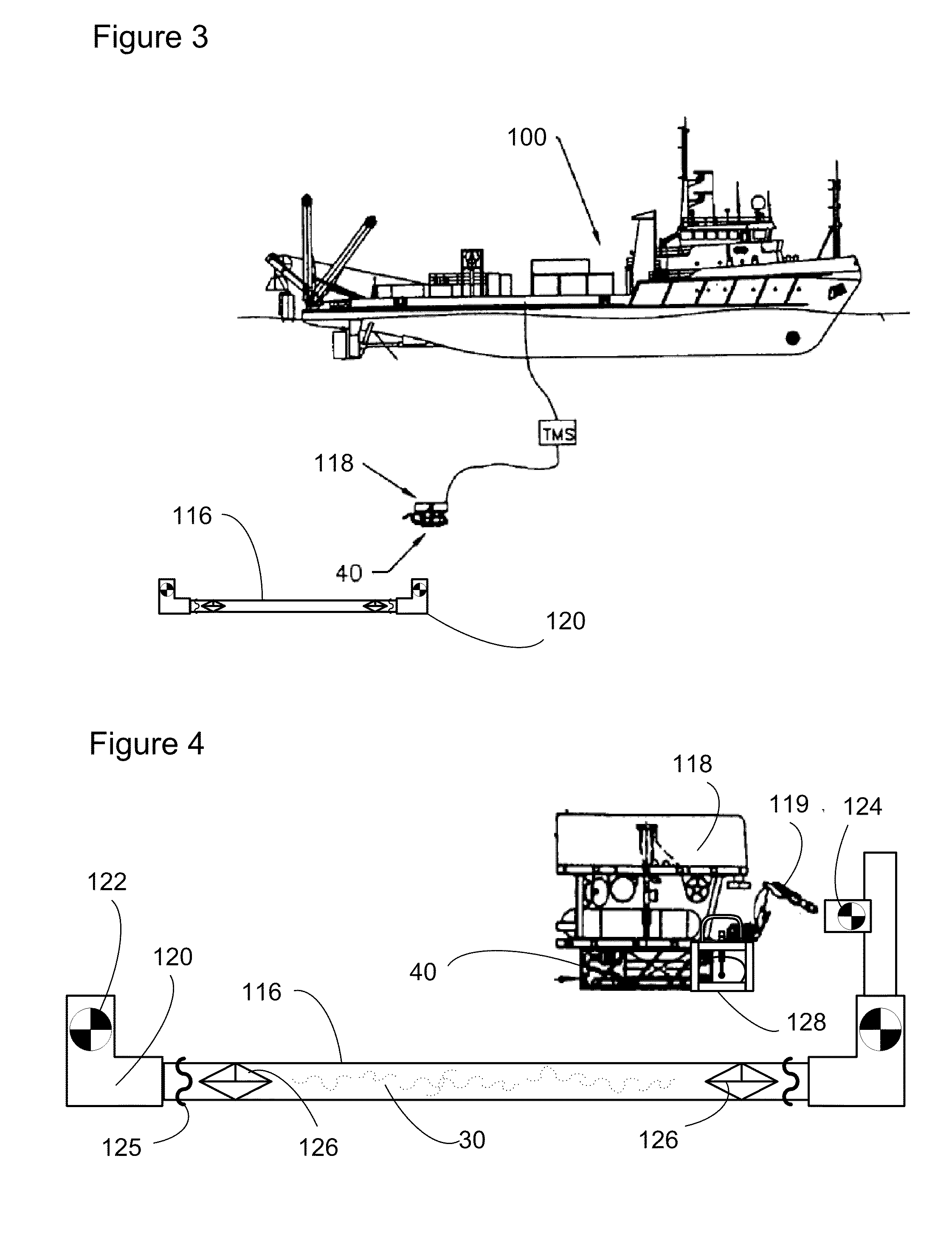
Subsea pipeline service skid
InactiveUS20100089126A1Sufficient quantityEasy to operateDetection of fluid at leakage pointArtificial islandsOcean bottomMarine engineering
Apparatus and methods are described for subsea pipeline servicing, including line-pack testing, physical integrity testing, recovery of damaged sections of pipelines, and product removal from subsea structures. In one embodiment of the invention, a subsea pipeline service skid is provided including at least one sample collection bladder affixed to the skid and in fluid communication with a skid mounted pump dimensioned to pull a sample from the subsea pipeline. In another embodiment, a product removal bladder is provided for removal of the hydrocarbons from a subsea structure.
Owner:VALKYRIE COMMISSIONING SERVICES
Monolithic zeolite coated structures and a method of manufacture
InactiveUS6936561B2Tight controlHigh degreeMolecular sieve catalystsDispersed particle separationHoneycombHoneycomb like
Structured zeolite coated structures comprising thick porous inorganic zeolite coatings disposed on monolithic support structures, which can be honeycomb shaped, are disclosed. The zeolite coatings have open interconnected pores of controlled pore size and are characterized by improved durability, physical integrity, and adherence sufficient to enable use as supports for catalysts in liquid phase applications under harsh reaction conditions. Methods for making zeolite coated structures are also disclosed.
Owner:CORNING INC
Tamper-resistant pharmaceutical compositions of opiods and other drugs
InactiveUS20110142943A1Reduce the possibilityImprove lipophilicityBiocidePowder deliveryAs DirectedOpioid
Tamper-resistant pharmaceutical compositions have been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opioids. The tamper-resistant compositions retard the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is passes through the GI tract.
Owner:COLLEGIUM PHARMA INC
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
InactiveUS20080199530A1Reduce the possibilityImprove lipophilicityPowder deliveryNervous disorderAdditive ingredientWater insoluble
Owner:COLLEGIUM PHARMA INC
Subsea pipeline service skid
InactiveUS8381578B2Reduce pressureDetection of fluid at leakage pointPipe elementsMarine engineeringSubmarine pipeline
Owner:VALKYRIE COMMISSIONING SERVICES
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS8557291B2Reduce the possibilityImprove lipophilicityPowder deliveryBiocideOrganic solventAs Directed
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opioids. In a preferred embodiment, a drug is modified to increase its lipophilicity. In some embodiments the modified drug is homogeneously dispersed within spherical microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and / or organic solvent insoluble. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is passes through the GI tract.
Owner:COLLEGIUM PHARMA INC
Device For The Leak-Tight Sealing Of Packaging Containers For Sensitive Products
ActiveUS20070272646A1Eliminate the problemReduce disadvantagesCapsClosure using stoppersEngineeringAmbient air
The invention relates to a device for the leak-tight sealing of openings in containers and for the purification of the ambient air located therein as a result of opening and closing cycles, said containers being intended for the packaging of products that are sensitive to gaseous pollutants present in the ambient air. The inventive device is particularly suitable for the packaging of solid-state medicaments in the form of pellets, tablets or capsules, having a reactivity and physical integrity that must be fully conserved.
Owner:AIRNOV INC
Transosseous core approach and instrumentation for joint replacement and repair
InactiveUS20060142865A1Without integrityWithout viabilityFinger jointsWrist jointsArticular surfacesArticular surface
A method and instrumentation is disclosed for gaining access to areas in and around joints for treatment and to provide new implants and instrumentation adapted for the new method. In a transosseous core approach, the joint is entered through a pathway provided in a portion of a joint bone. Such pathway is preferably made by removing a bone core from the bone in or adjacent to the joint, wherever possible without substantially compromising physical integrity and physiological viability of the joint. A route for the transosseous core approach traverses through a more-accessible bone of the joint which can be aligned with a less-accessible bone in order to facilitate treatment of articular surfaces and / or other structures in the joint. Implant modules are provided which can be inserted into the joint through the transosseous pathway and assembled in situ inside the joint to form an implant assembly.
Owner:HYDE EDWARD R JR
Tamper-resistant pharmaceutical compositions of opioids and other drugs
Tamper-resistant pharmaceutical compositions have been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opioids. The tamper-resistant compositions retard the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is passes through the GI tract.
Owner:COLLEGIUM PHARMA INC
Gastric retention controlled drug delivery system
ActiveUS7776345B2Fast flotationMaintain physical integrityOrganic active ingredientsNervous disorderControlled releaseExcipient
The present invention provides a gastric retention controlled drug delivery system comprising: (a) a controlled release core comprising a drug, a highly swellable polymer and a gas generating agent, said core being capable of swelling and achieving floatation rapidly while maintaining its physical integrity in gastrointestinal fluids for prolonged periods, and (b) a rapidly releasing coat composition comprising the same drug as in the core and pharmaceutically acceptable excipients, wherein the coating composition surrounds the core such that the system provides a biphasic release of the drug in gastrointestinal fluids.
Owner:SUN PHARMA INDS
Tamper-resistant pharmaceutical compositions of opioids and other drugs
InactiveUS20150005332A1Reduced likelihoodImprove lipophilicityBiocidePowder deliveryPharmaceutical SubstancesMedicinal chemistry
Tamper-resistant pharmaceutical compositions have been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opioids. The tamper-resistant compositions retard the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is passes through the GI tract.
Owner:COLLEGIUM PHARMA INC
Wound care device having fluid transfer and adhesive properties
This disclosure relates to a wound care device which contains capillary force one-way pumps that are capable of transporting fluid, such as wound exudate, away from a wound site to the opposite side of the wound care device, which functions as a segregated fluid reservoir. This fluid transport mechanism generally aids in reducing wound maceration by removing excess wound fluid and the protease enzymes and infectious bacteria contained within the wound fluid. The wound care device performs this function, often times for multiple days, without the loss of the physical integrity of the wound care device. In addition to providing a uni-directional fluid transport mechanism, the wound care device contains a perforated adhesive layer.
Owner:MILLIKEN & CO
Microcrystalline cellulose cushioning granules
InactiveUS6858725B1Improve cushioning propertiesImprove buffering effectOrganic active ingredientsPowder deliveryControlled releaseOrganic solvent
Granulation of microcrystalline cellulose with a granulating fluid consisting of water and a water-miscible, volatile, polar organic solvent yields porous granules which are comprised of particles that are larger than the ungranulated microcrystalline cellulose. This granulated microcrystalline cellulose is capable of cushioning controlled release particles and barrier coated particles from the compression forces used in tableting, thereby maintaining the physical integrity of the components of the tablet.
Owner:R P SCHERER TECH INC
Slow release fertilizer spike
InactiveUS6120574AHigh physical integritySignificant attritionBiocideAlkali orthophosphate fertiliserParticulatesMagnesium phosphate
An attrition resistant fertilizer spike composition, exhibiting a mechanical strength which allows the spike to be hammered unsupported into the soil without suffering damage, to provide an effective source of slow releasing plant nutrients to the soil. The composition comprises between 93 and 98 percent of particulate plant nutrient compounds containing one phosphate ion chemically combined with one divalent cation and one monovalent cation, including the compounds magnesium ammonium phosphate and magnesium potassium phosphate. The composition includes a coating of between 2 and 7 percent of thermoplastic adhesive with a softening temperature between 65 and 160 DEG C. on the particles. The coated particles are formed into an attrition and shatter resistant spike shape, suitable for hammering into the soil, by pressing into a die at a temperature higher than the softening temperature of the thermoplastic adhesive and then cooling to a temperature lower than the softening temperature. A method of preparing these improved spikes is provided. In the method the thermoplastic adhesive is applied as an aqueous dispersion or emulsion along with a die lubricant prior to pressing the coated particles into a spike exhibiting a high degree of physical integrity.
Owner:AGRINUTRIENTS TECH GRP INC
Tamper-resistant pharmaceutical compositions of opioids and other drugs
ActiveUS20150004244A1Reduced likelihoodImprove lipophilicityPowder deliveryBiocidePharmaceutical SubstancesMedicinal chemistry
Tamper-resistance pharmaceutical compositions have been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opioids. The tamper-resistant compositions retard the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is passes through the GI tract.
Owner:COLLEGIUM PHARMA INC
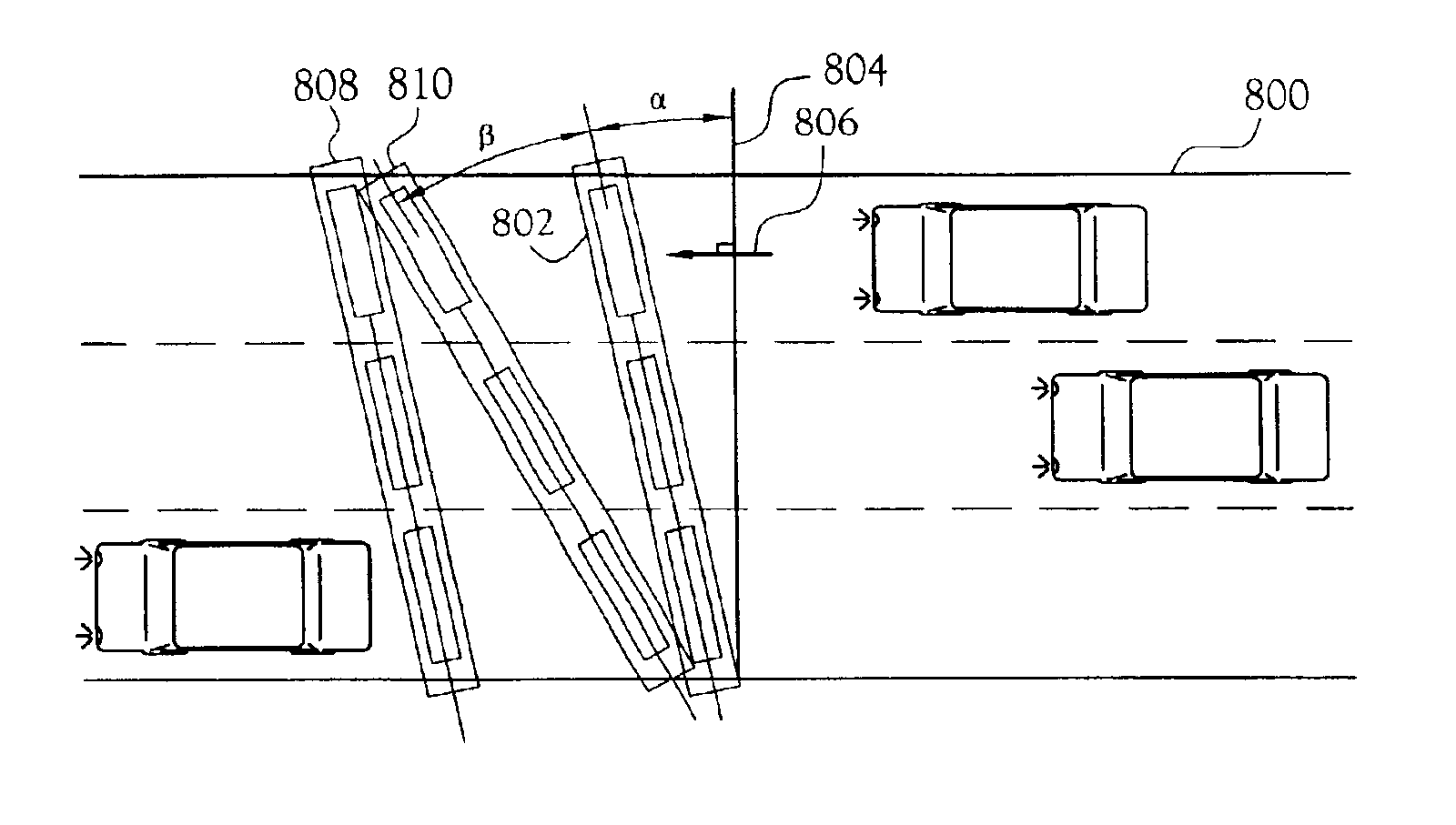
Surface-mount traffic sensors
InactiveUS6917308B2Easy to deployOptimizes useful informationSurveyDetection of traffic movementInfrastructure planningEngineering
Surface-mounted traffic monitoring sensors that do not require substantial disruption to traffic flow to install or maintain, and that do not substantially degrade the physical integrity of the road. Pneumatic road-tube wedges and surface-mount inductive blades detect wheel-spikes and / or inductive signatures in both fixed and portable installations, single or multi-lane roadways, and provides accurate vehicle speed, volume, occupancy, turning movement counts, weaving sections, classification, re-identification, travel-time, origin and destination, lane-keeping variation, speed-variation, angle-of-attack, and vehicle weight and load distribution. This data is useful to infrastructure planners, traffic-flow modelers, to enhance the safety of work-zone crews, law enforcement, and for real-time traffic operations, etc.
Owner:INDUCTIVE SIGNATURE TECH
Thermotropic Liquid Crystalline Polymer with Improved Low Shear Viscosity
A thermotropic liquid crystalline polymer melt polymerized in the presence of a viscosity modifier that can help increase the “low shear” viscosity of the resulting composition is provided. The increased “low shear” viscosity can minimize drooling of the polymer composition during processing and also allow it to possess a greater melt strength, which facilitates its ability to be processed in a wide variety of applications without losing its physical integrity. Despite having a relatively high “low shear” viscosity, the present inventors have discovered that the viscosity modifier does not substantially increase the “high shear” melt viscosity of the polymer composition. In this regard, the ratio of the “low shear” viscosity to the melt viscosity is generally very high, such as within a range of from about 50 to about 1000.
Owner:TICONA LLC
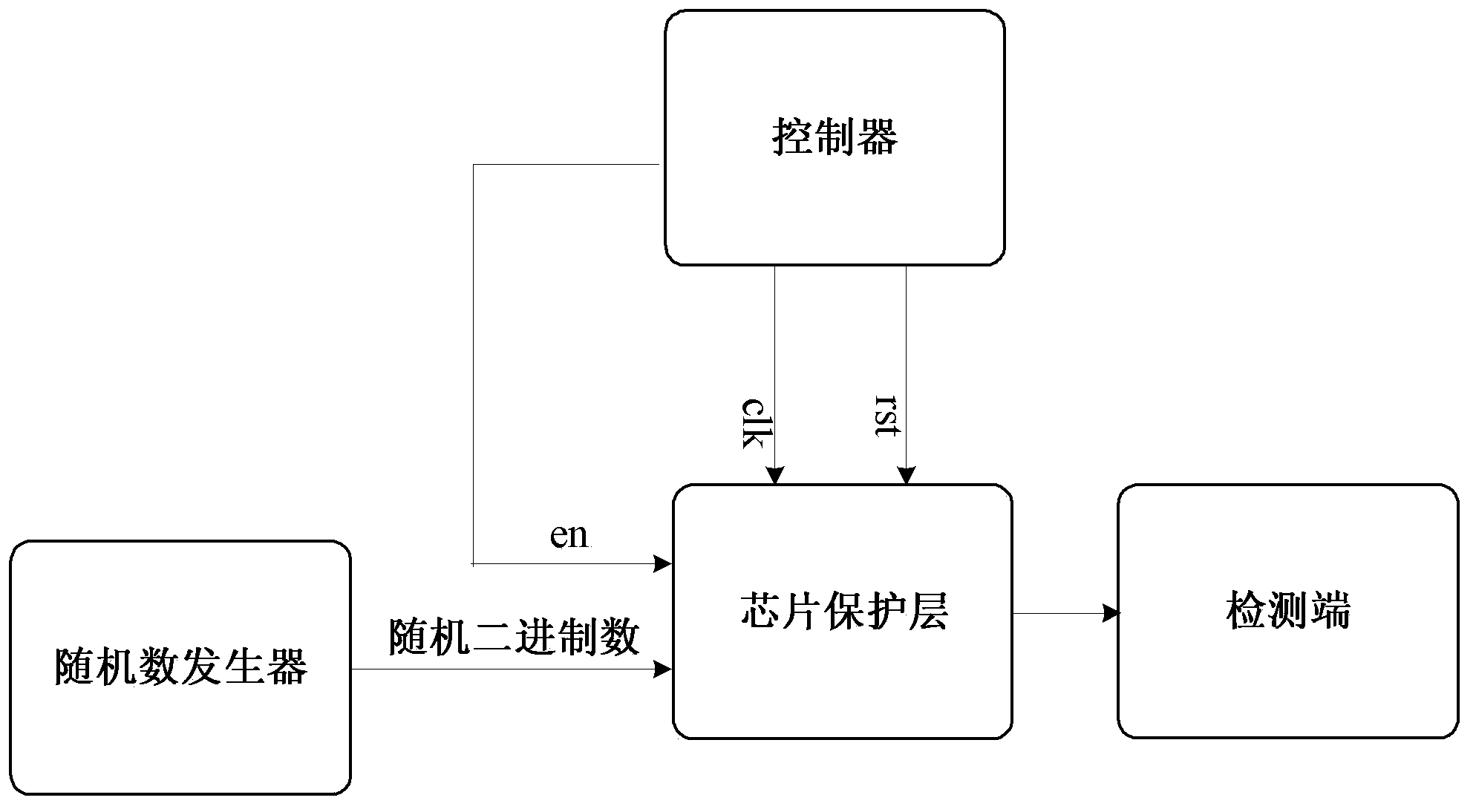
Method and device for protecting chip top-layer covering integrity
ActiveCN103413106AFix security issuesSolve the technical defects of high power consumptionInternal/peripheral component protectionPhysical layerEngineering
The invention discloses a method and a device for protecting chip top-layer covering integrity, and relates to the field of chip physical integrity protection. The method is applied to a physical layer protecting circuit, the physical layer protecting circuit is divided into n groups, and each group has M metal wires. In each detection cycle, the method includes A, generating random binary numbers with at least M bits; B, inputting the random binary numbers into the input ends of the M metal wires in each group; C, detecting output signals of the M metal wires, and if the output signals are not identical to the random binary numbers inputted into the M metal wires, determining that a chip is attacked. The device comprises a random number generator, a controller and a detection side. Chip top-layer metal covering integrity protection of the method and the device is based on a random number comparison method, and compared with the prior art, the method and the device have the advantages that the technical defects of low safety and high power consumption are overcome.
Owner:DATANG MICROELECTRONICS TECH CO LTD
Wound care device having fluid transfer and adhesive properties
This disclosure relates to a wound care device which contains capillary force one-way pumps that are capable of transporting fluid, such as wound exudate, away from a wound site to the opposite side of the wound care device, which functions as a segregated fluid reservoir. This fluid transport mechanism generally aids in reducing wound maceration by removing excess wound fluid and the protease enzymes and infectious bacteria contained within the wound fluid. The wound care device performs this function, often times for multiple days, without the loss of the physical integrity of the wound care device. In addition to providing a uni-directional fluid transport mechanism, the wound care device contains a perforated adhesive layer.
Owner:MILLIKEN & CO
Fluidizing gravity conveyor with high temperature multi-layered fluid distributor member
ActiveUS20090003942A1Reduce temperature gradientWithstand high temperatureContainer filling methodsFertiliser distributersRigid gas permeable lensPorous medium
An apparatus for conveying fluidized high temperature finely divided dry material by gravity utilizes a multi-layered, gas permeable gas distributor member comprising (i) an upper layer a flat, rigid gas permeable, porous medium capable of withstanding temperatures up to about 2900° F. through which gas to fluidize the material will pass; (ii) a middle layer comprised of a gas permeable insulation material that maintains its physical integrity when exposed to temperature differentials ranging from about 500° F. to about 2900° F.; and (iii) a lower layer that supports the top and middle layers comprising a substantially flat plate having a plurality of openings therethrough through which fluidizing gas can pass.
Owner:FLSMIDTH AS
Method and device or system to monitor the state of tires, and detection of snow chains or spikes use, on a vehicle
InactiveUS20080001728A1Overcome effectEasy to detectTyre measurementsVehicle tyre testingHarmonicRotation velocity
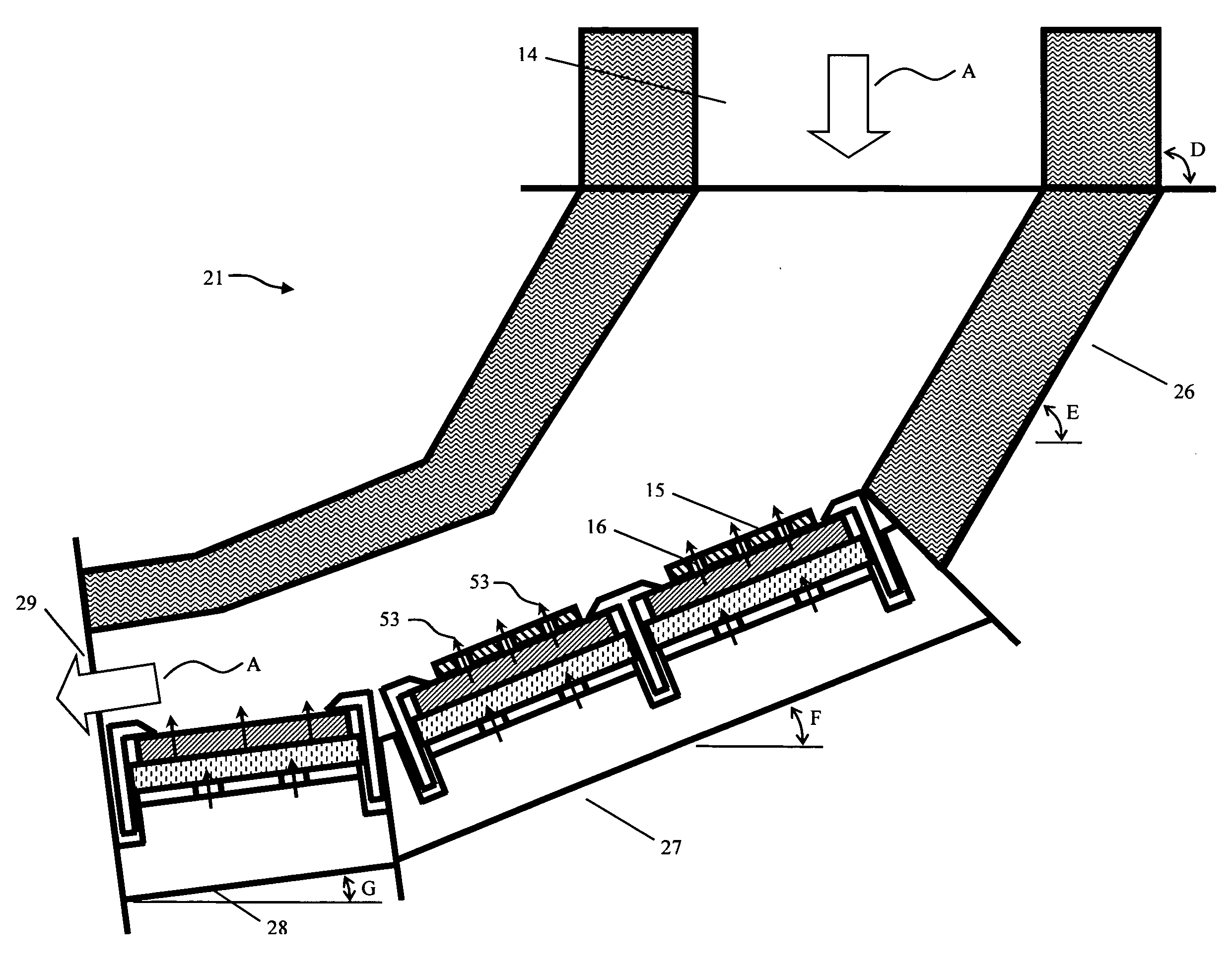
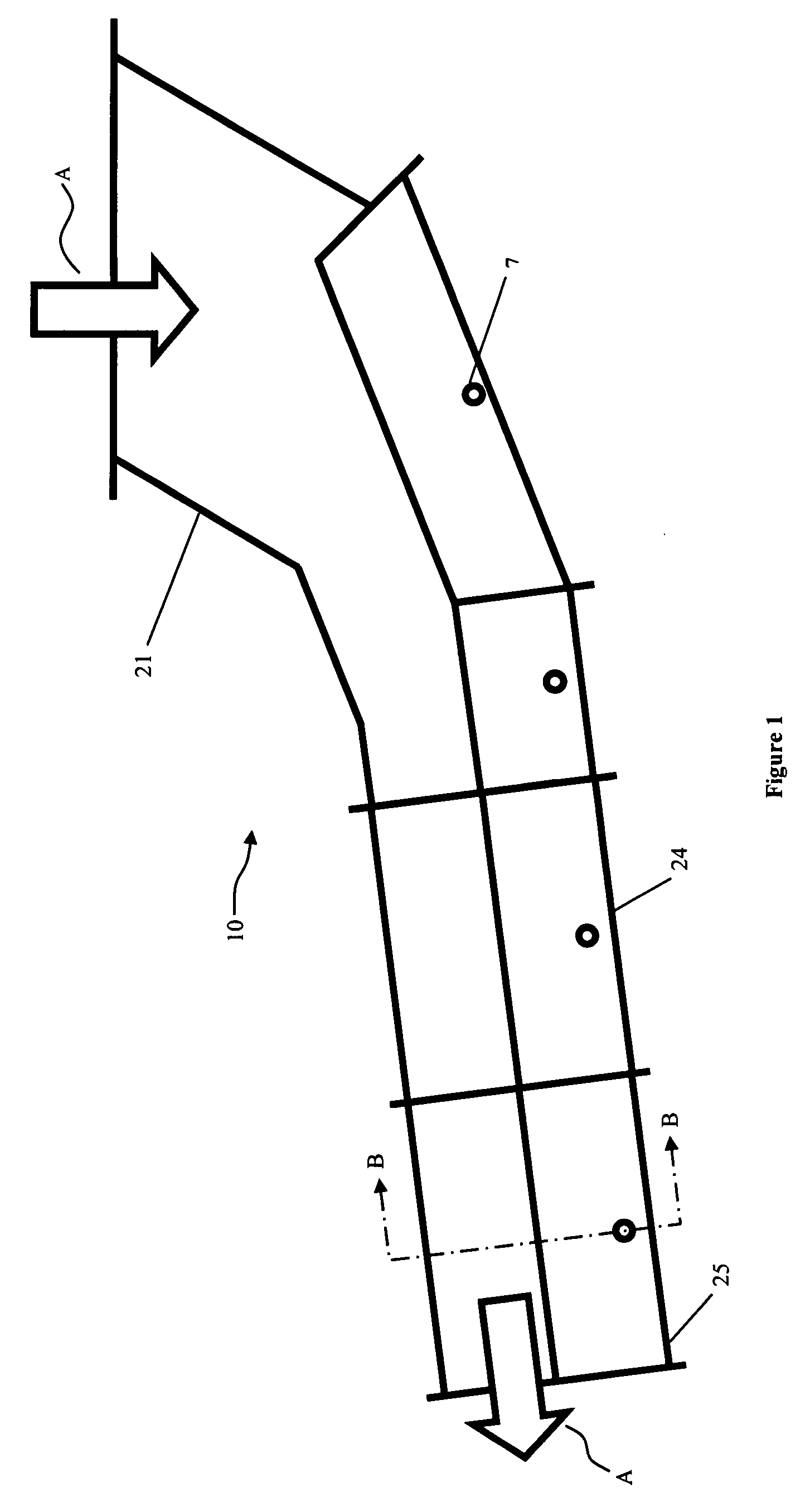
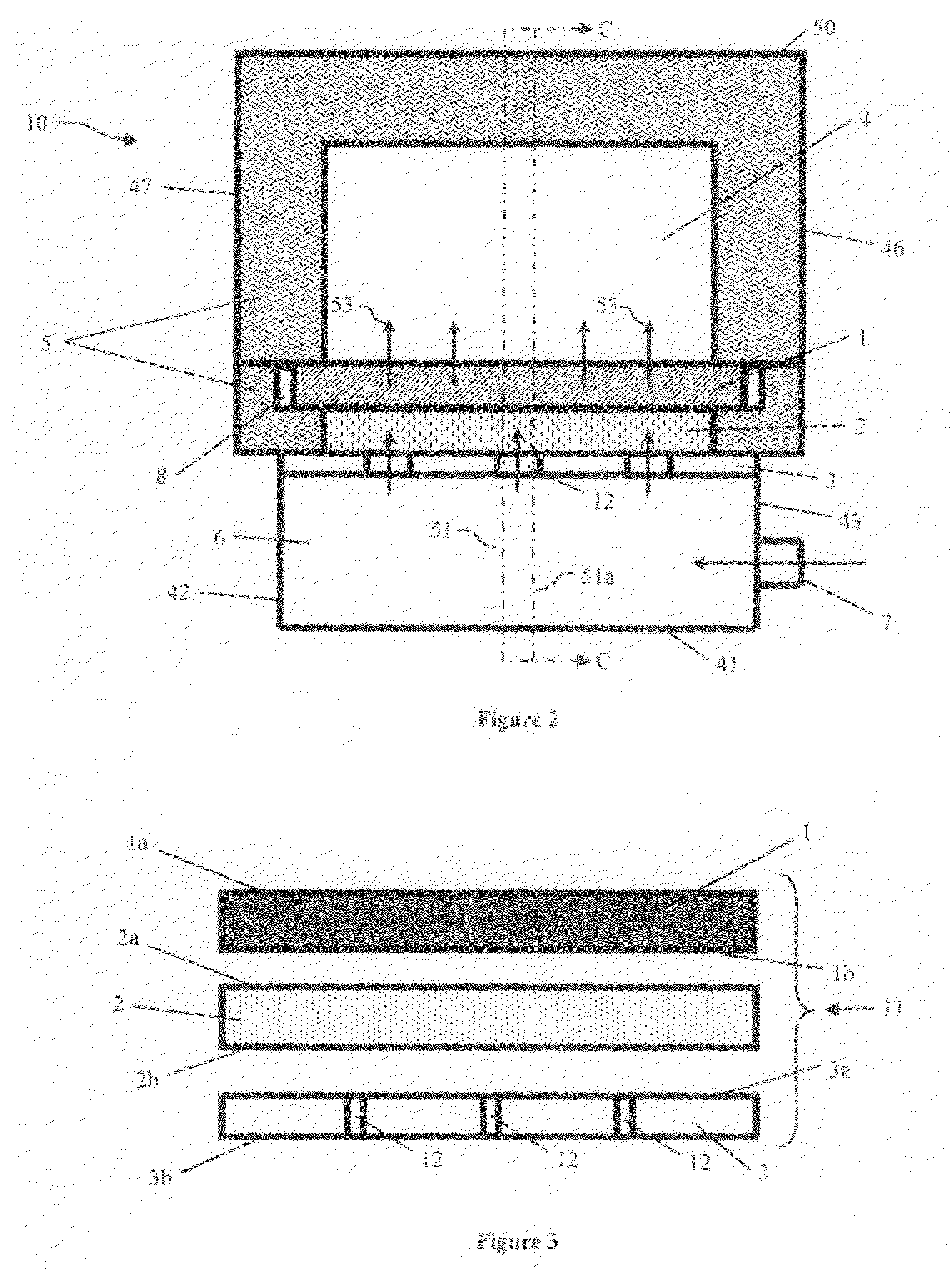
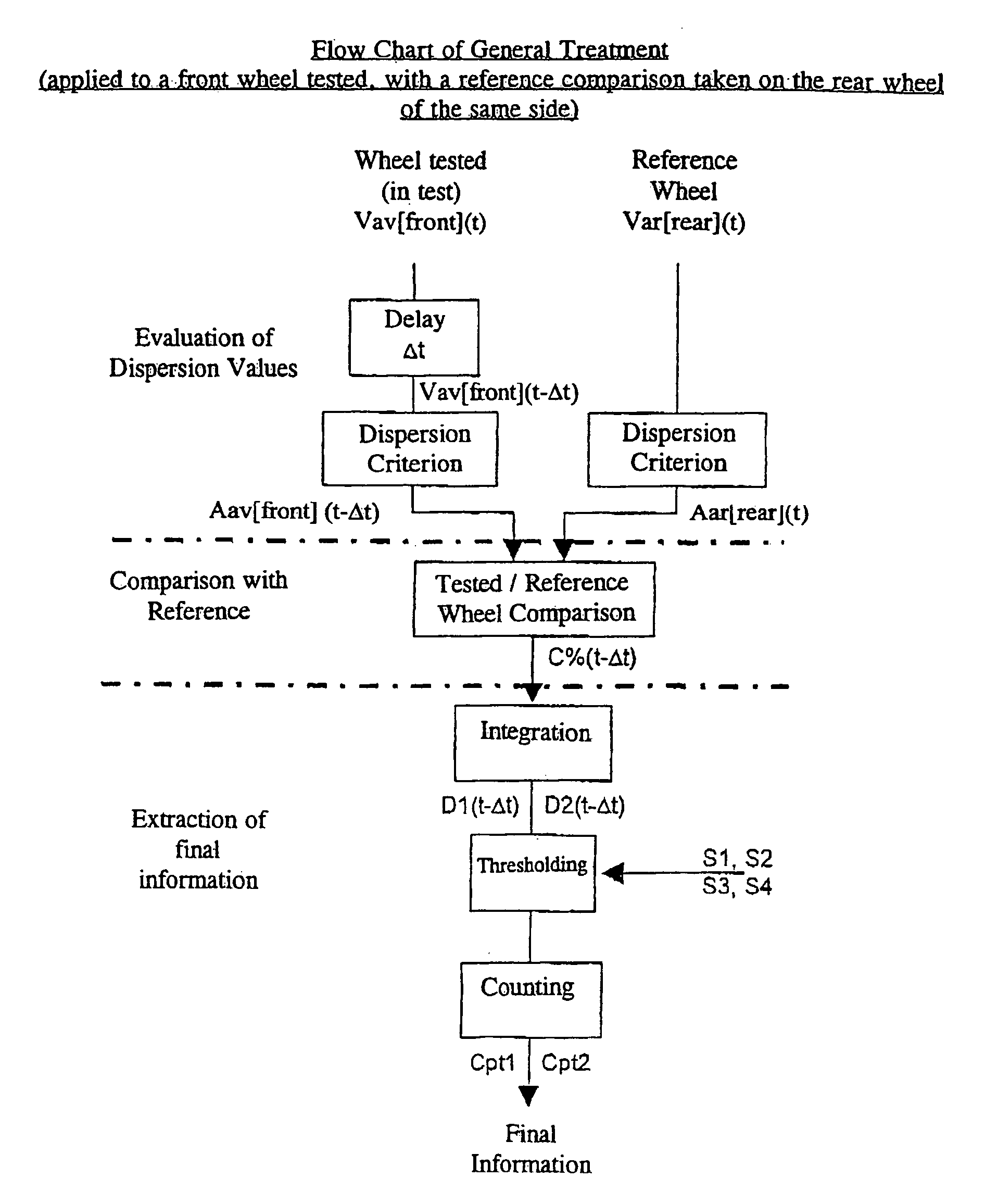
A system and method are provided for monitoring the physical integrity of a tire. The system and method include measuring a signal proportional to the speed of rotation of the wheel and calculating a harmonic signal of the round of wheel (e.g., equal to the energies of the harmonics of this signal), then comparing the result with a threshold and activating an alarm when over-passing this threshold.
Owner:DUFOURNIER TECH
Securized and portable electric vehicle charger
InactiveUS20170361719A1Reduce usageEasy to disassembleCharging stationsElectrical testingElectrical conductorElectric power
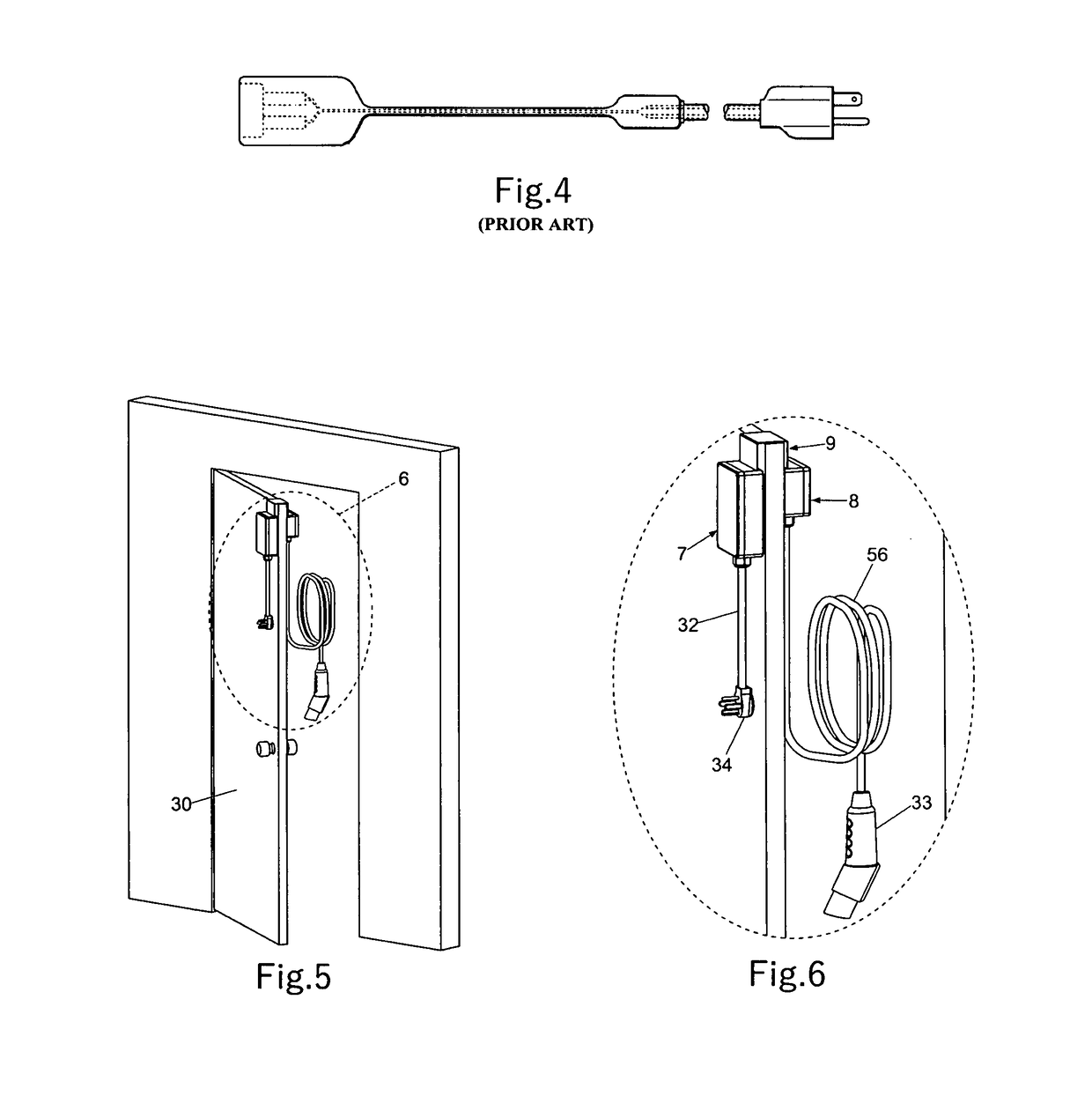
An electric vehicle supply equipment (EVSE) having ability to charge electric vehicle from indoor located 208-240V outlets. The EVSE comprises a flattened armored cable to securely transmit electrical power to outdoor location from indoor located electrical source. In the preferred embodiment, the flattened armored section acts as EVSE anchoring system which providing ease of installation when temporary electric vehicle recharging is required. The EVSE has ability to disconnect electrical power passing through the flat armored cable in case of non proper conditions or if any electrical hazard occurs. A further embodiment comprises a flexible version of flat cable. To enhance its electrical safety, flat wire conductor comprising damage sensor is used to improve the electrical safety. The EVSE may validate flat wire physical integrity before applying electrical power to the flattened flexible cable.
Owner:DOUCET SERGE +1
Systems, devices, and methods for self-preservation of robotic apparatus
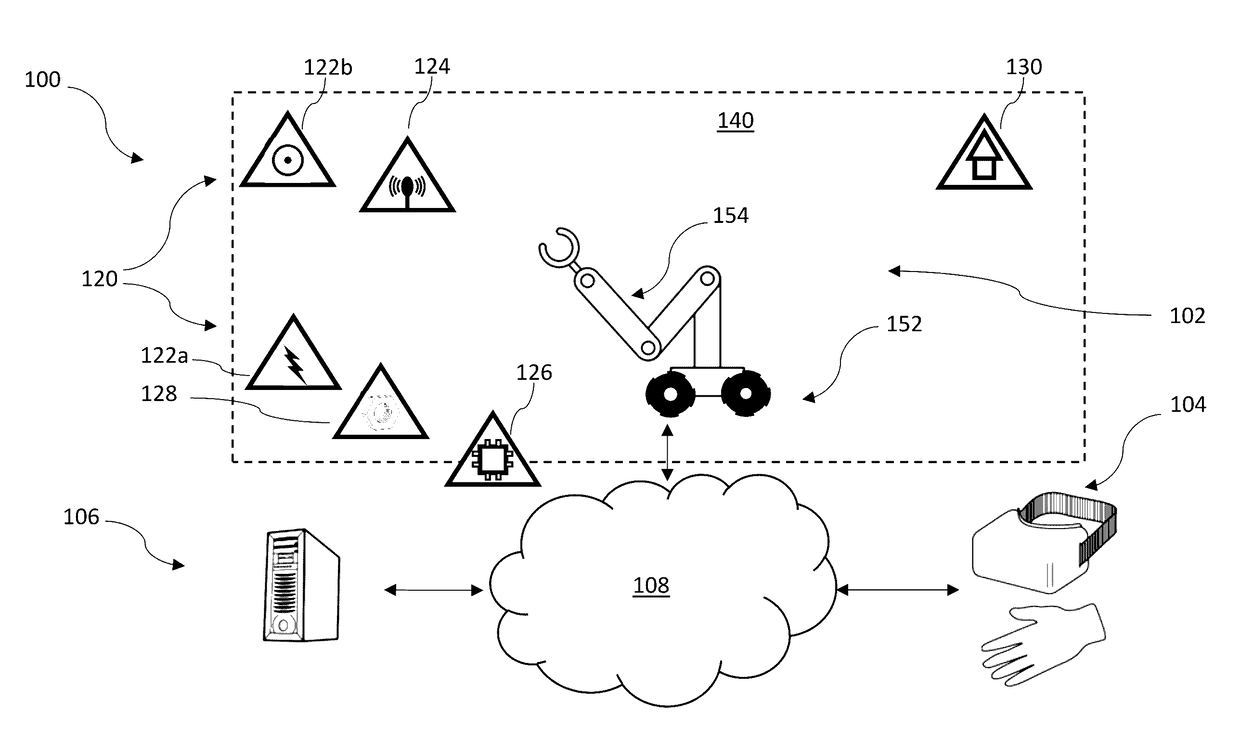
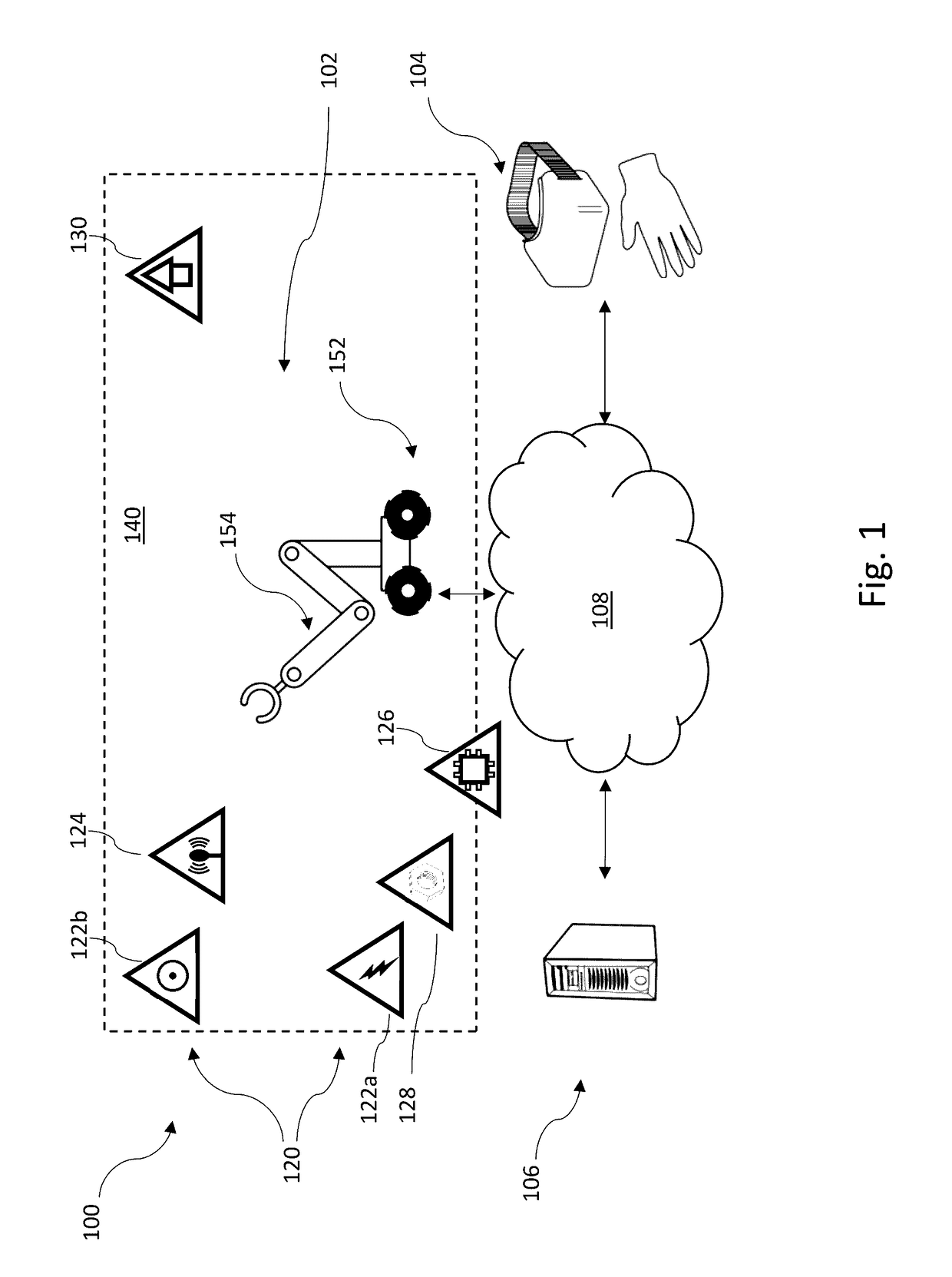
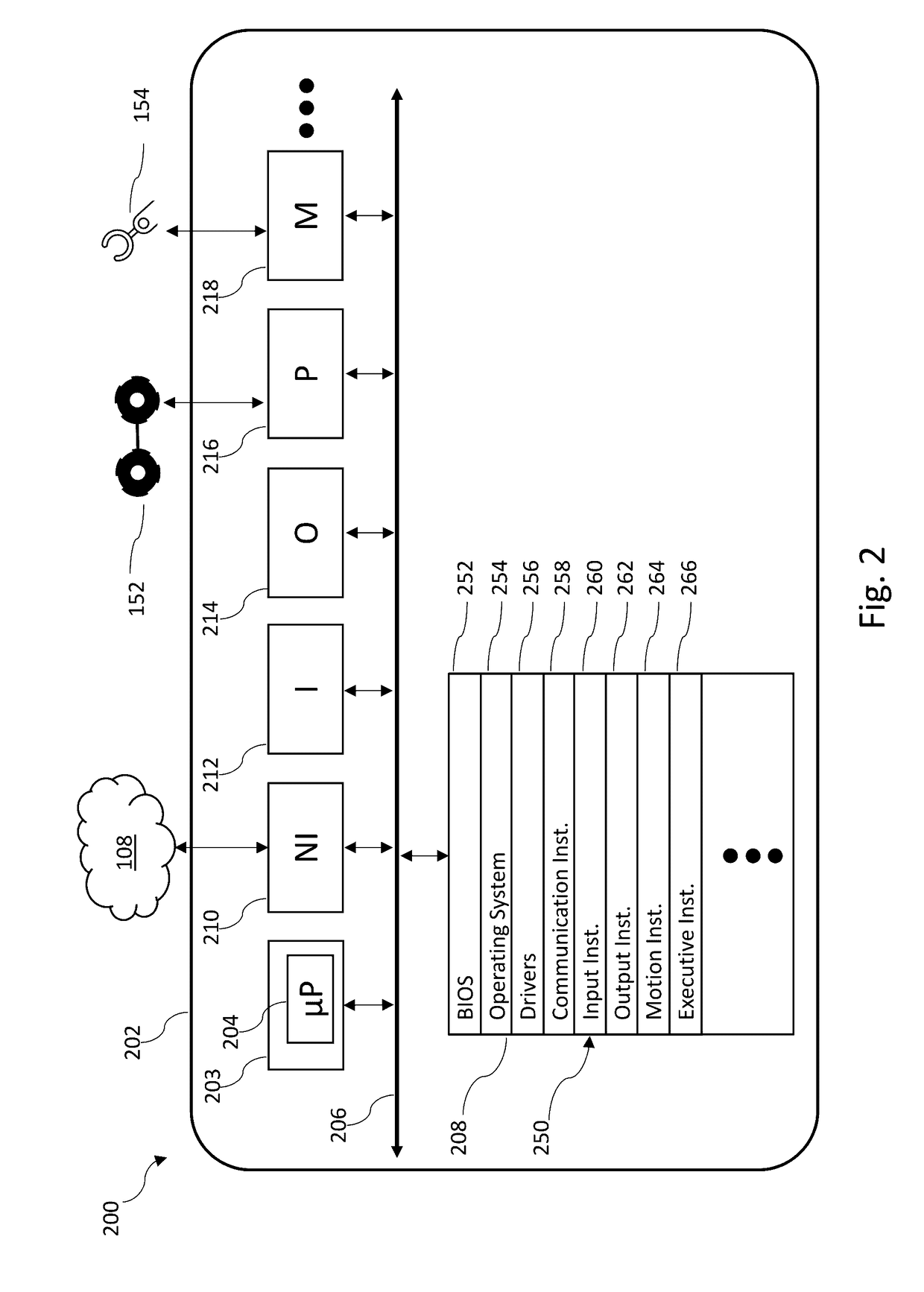
Robotic apparatus employ a large variety of resources to operate. A robotic apparatus seeks out sources of energy, computational capacity, shelter, communications, and / or other resources to preserve or renew its energy stores, computational resources, or physical integrity and / or to receive further guidance or direction or to report collected or sensed data or information. A robotic apparatus can determine the existence of a resource deficiency or projected resource deficiency, assess a ranking of such, identify one or more remedial actions, and execute the remedial action(s). A robotic apparatus can assess a ranking of a resource deficiency or projected resource deficiency based on a value of the resource, a severity of need or urgency for the resource, and ability to obtain or replenish the source.
Owner:OCADO INNOVATION
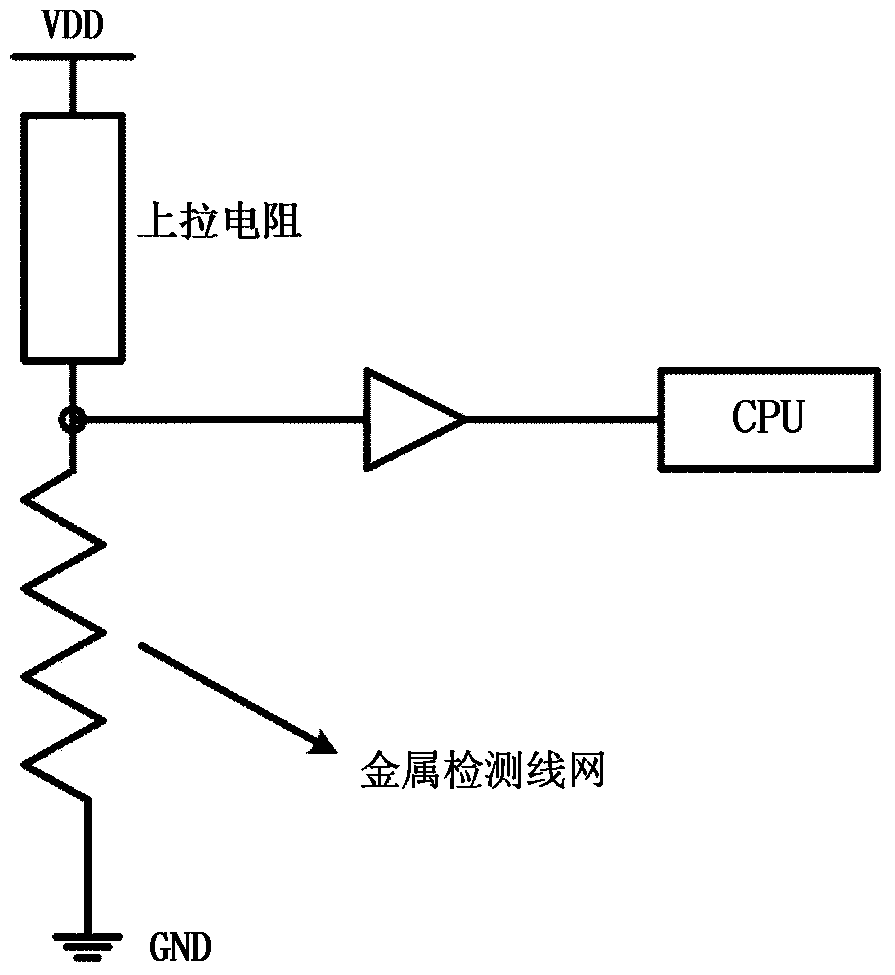
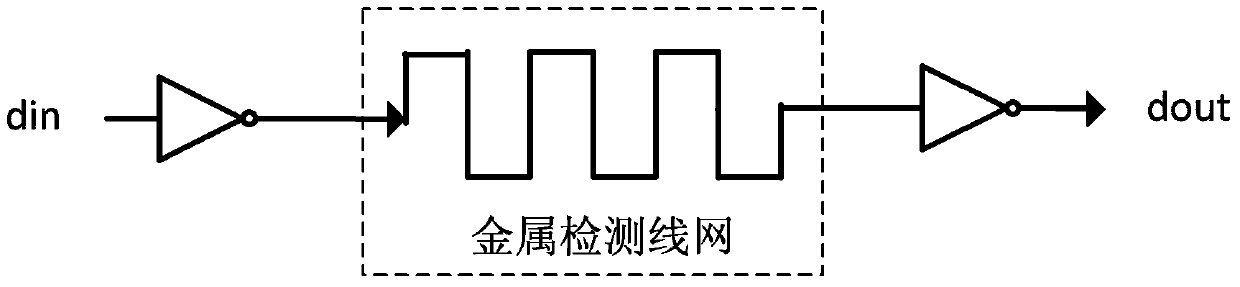
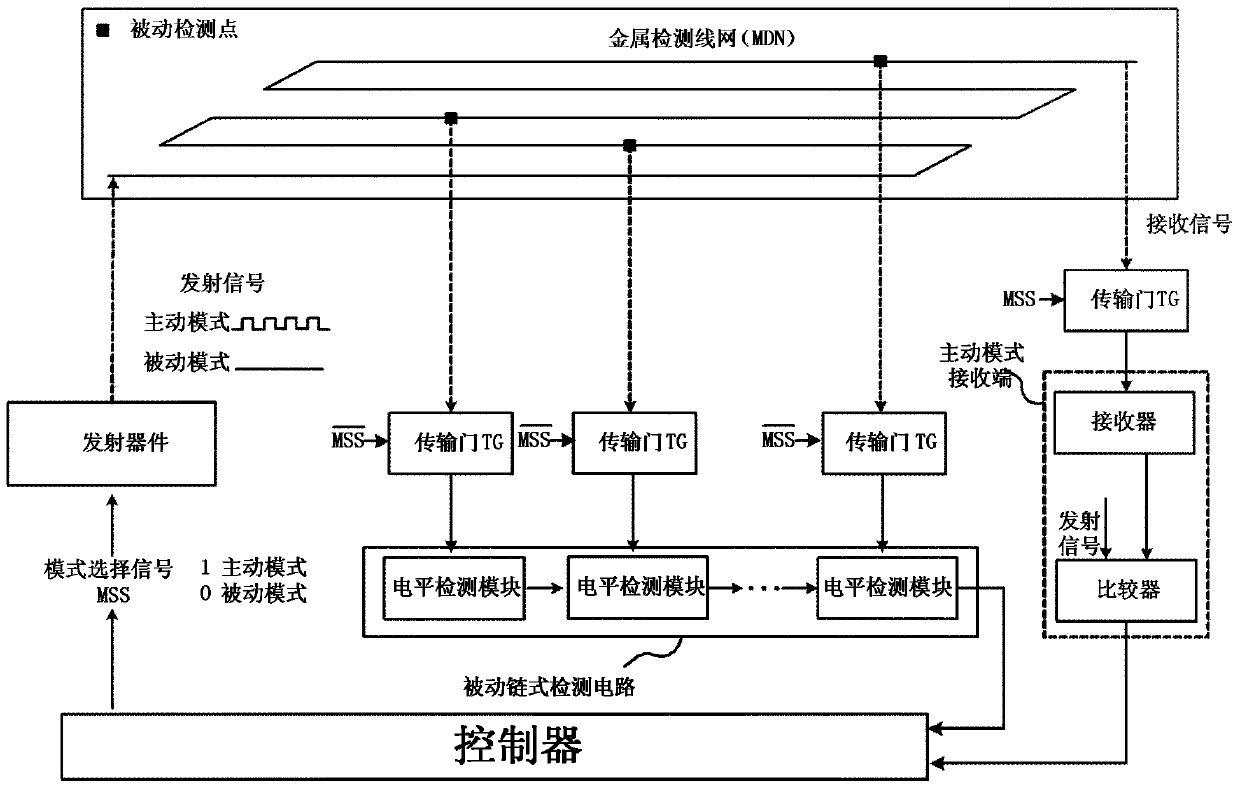
Chip physical integrity detecting device
ActiveCN103440452AReduce areaExtending the Coverage of Detectable VandalismPlatform integrity maintainanceActive detectionMode selection
The invention discloses a chip physical integrity detecting device and belongs to the field of chip physical integrity detection. The chip physical integrity detecting device comprises a controller, a transmitter, a metal detection net and a detector, wherein the controller is used for selecting detection modes and emitting mode selection signals according to the working state of a chip to be detected, the controller is also used for judging whether the chip to be detected is abnormal according to potential information fed back by the detector, the transmitter is used for transmitting signals to the metal detection net according to the mode selection signals, and the detector is used for detecting potential information of a signal end of the metal detection net connected with the detector according to the mode selection signals and sending the detected potential information to the controller. According to the chip physical integrity detecting device, due to the fact that work in carried out by means of switching between two detection modes, the detachable destroy behavior coverage can be enlarged; compared with a device only using an active detection mode always, the chip physical integrity detecting device has the advantage of being capable of reducing power consumption remarkably.
Owner:DATANG MICROELECTRONICS TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com