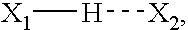Patents
Literature
1052 results about "Bile fluid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bile or gall is a dark green to yellowish brown fluid, produced by the liver of most vertebrates, that aids the digestion of lipids in the small intestine.
Devices and methods for pyloric anchoring
ActiveUS20050055039A1Avoiding erosion and ulcerationSuture equipmentsElectrotherapyPylorusPatient characteristics
A device for performing one or more functions in a gastrointestinal tract of a patient includes an anchoring member and at least one actuator, sensor, or combination of both coupled with the anchoring device. The anchoring device is adapted to maintain at least part of the device within a pyloric portion of the patient's stomach and to intermittently engage, without directly attaching to, stomach tissue. Actuators perform any suitable function, such as transmitting energy to tissue, acting as a sleeve to reduce nutrient absorption, occupying space in the stomach, eluting a drug and / or the like. Sensors may be adapted to sense any suitable patient characteristic within the patient's gastrointestinal tract, such as pH, temperature, bile content, nutrient content, fats, sugars, alcohol, opiates, drugs, analytes, electrolytes and / or hemoglobin.
Owner:BARONOVA
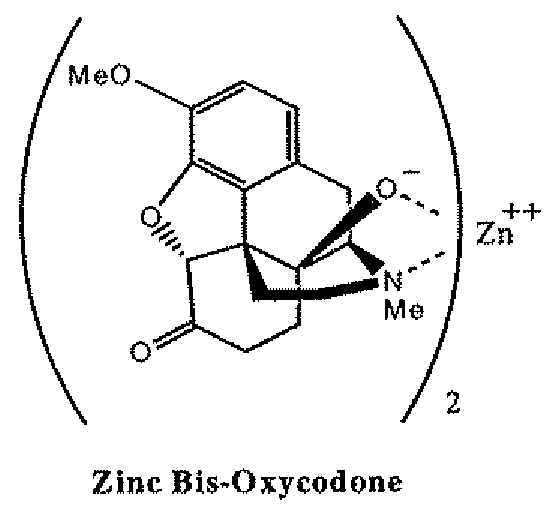
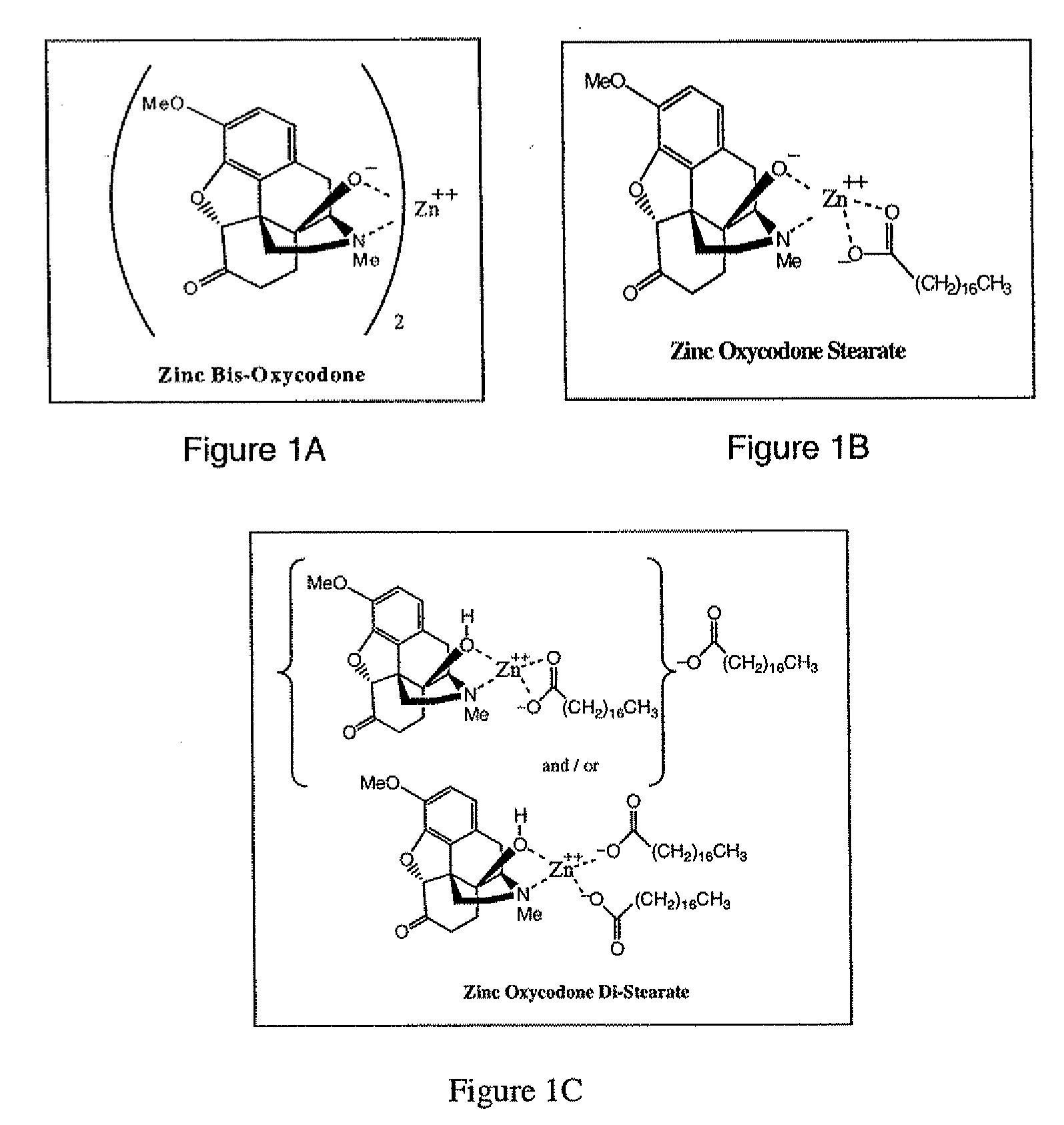
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
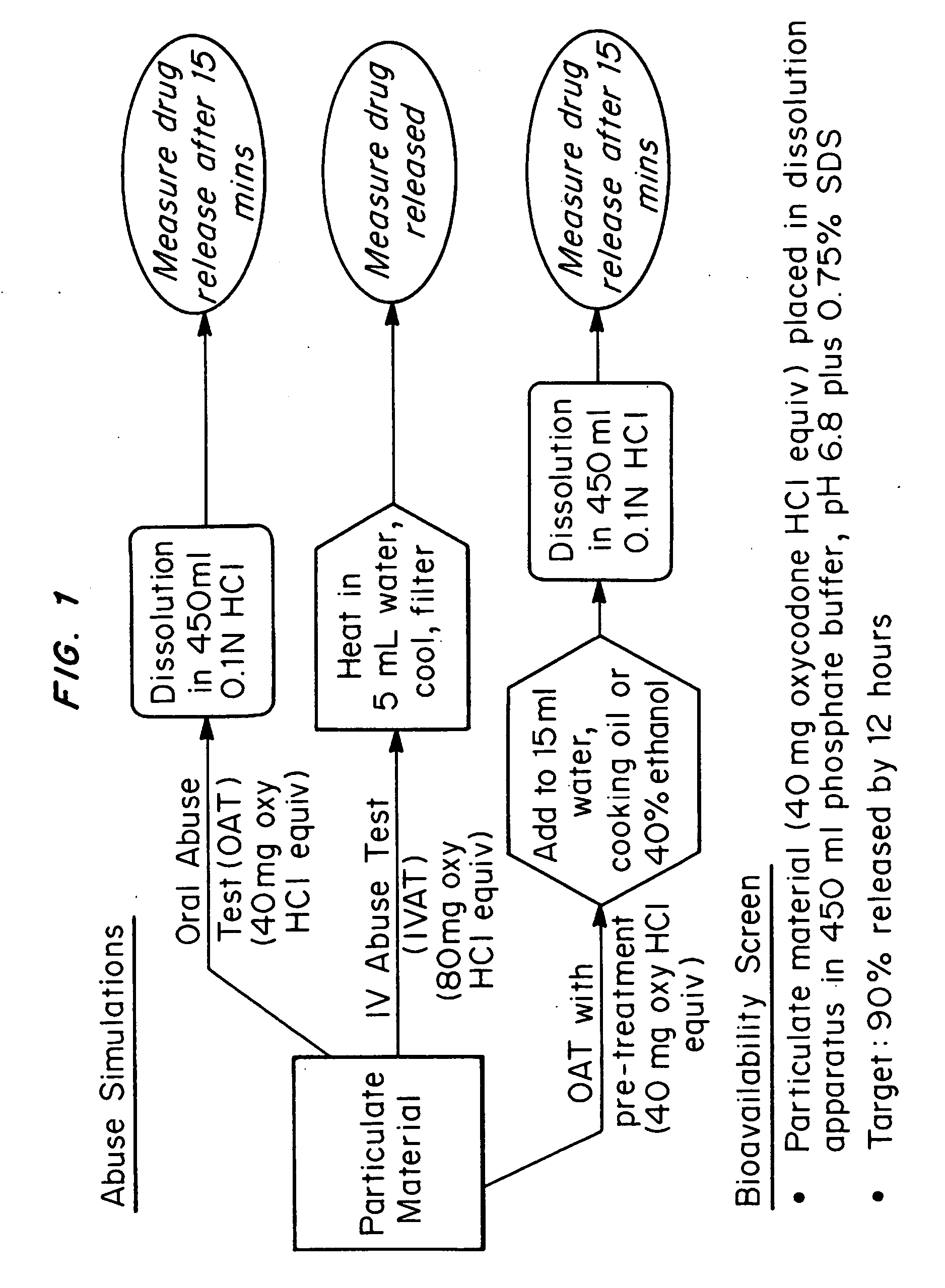
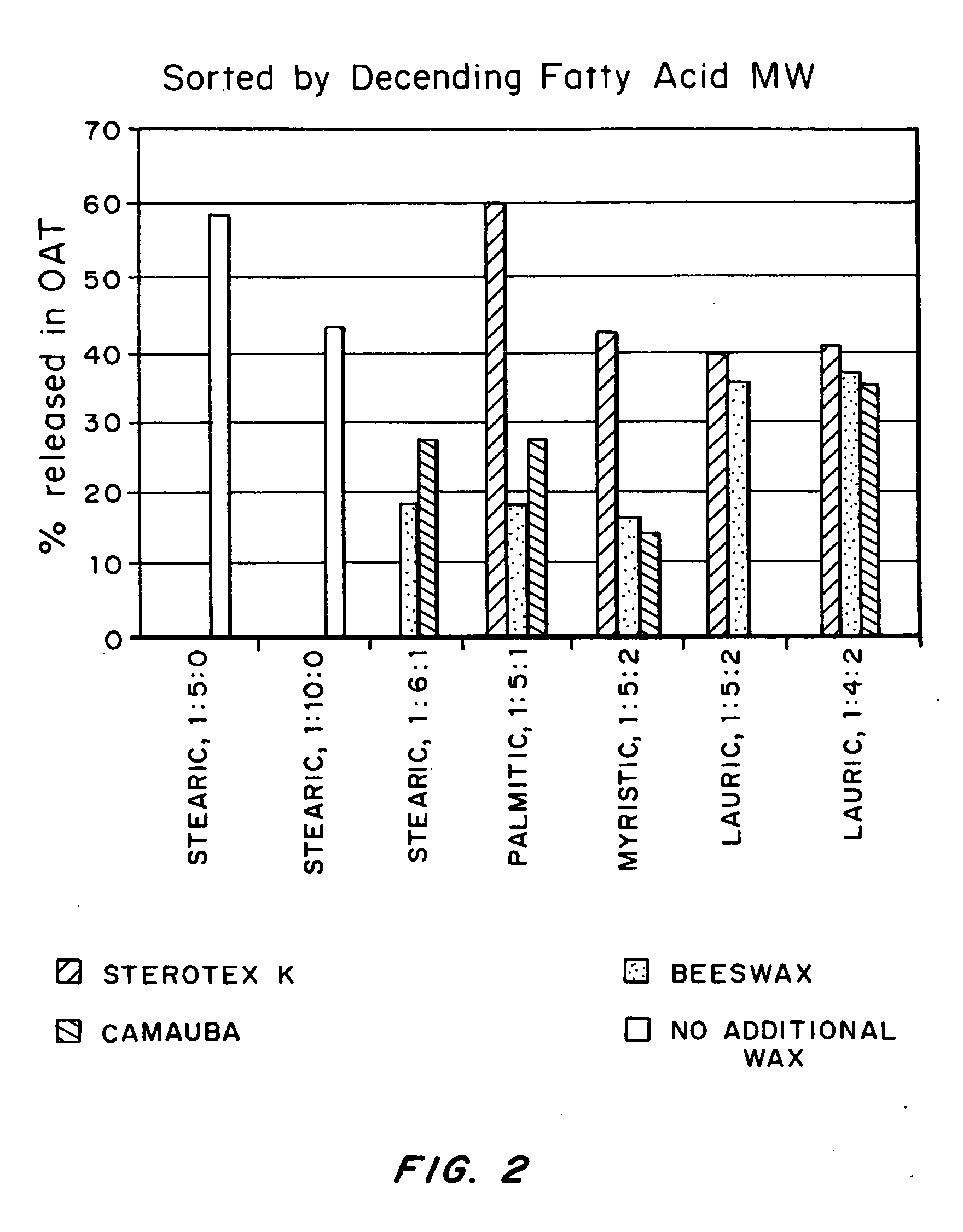
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Electrode wiping surgical device
Aspects of the present disclosure include a surgical device comprising electrodes on the sides of an end of an effector to aide in sealing during various surgical procedures, such as a liver resection. During a sealing procedure, the surgeon may wipe the surgical site with the end effector, causing the electrodes to touch the fractured area. Electrosurgical energy may be applied to the electrodes during the wiping, causing coagulation of smaller vessels, such as tiny blood vessels and bile ducts in the parenchyma of the liver. In some cases, due to the nature of some smaller vessels, electrosurgical energy should be delicately applied to cause sealing but to avoid overly damaging the remaining tissue. In some embodiments, the thin design of the electrodes allows for an appropriate amount of electrosurgical energy to be applied to the fractured area.
Owner:CILAG GMBH INTERNATIONAL
Abuse-deterrent drug formulations
ActiveUS20050281748A1Reduce the possibilityImprove lipophilicityTelevision system detailsPowder deliveryImmediate releaseActive agent
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, the drug is modified to increase its lipophilicity by forming a salt between the drug and one or more fatty acids wherein the concentration of the one or more fatty acids is one to 15 times the molar amount of the active agent, preferably two to ten times the molar amount of the active agent. In one embodiment the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble. The abuse-deterrent composition prevents the immediate release of a substantial portion of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Integrated body fluid collection and analysis device with sample transfer component
A single, integrated device in which a body fluid (e.g., blood) of a human or animal can be both collected and analyzed easily and without risk of contamination is disclosed. The collection portion and analysis portion of the device are permanently joined to permit movement of small quantities of body fluid under controlled conditions, to minimize any waste of the body fluid, to ensure that no contamination reaches the main body fluid volume, and to create a permanent physical record of the results of the analysis in association with the fluid sample itself: A wide variety of different body fluid components which may be indicative of various diseases, dysfunctions and abnormalities of the human or animal or the body fluid itself can be tested for. The device includes a container for collecting human or animal body fluid, one or more testing chambers containing one or more analysis units activated by body fluid; a transfer pump or vacuum assembly to transfer one or more samples into the analysis units; and one-way valves or the equivalent to prevent any portion of the withdrawn sample from being returned to the collection container. The body fluid acted upon may be blood, blood plasma, urine, bile, pleural fluid, ascites fluid, stomach or intestine fluid, colostrom, milk or lymph.
Owner:AALTO SCI
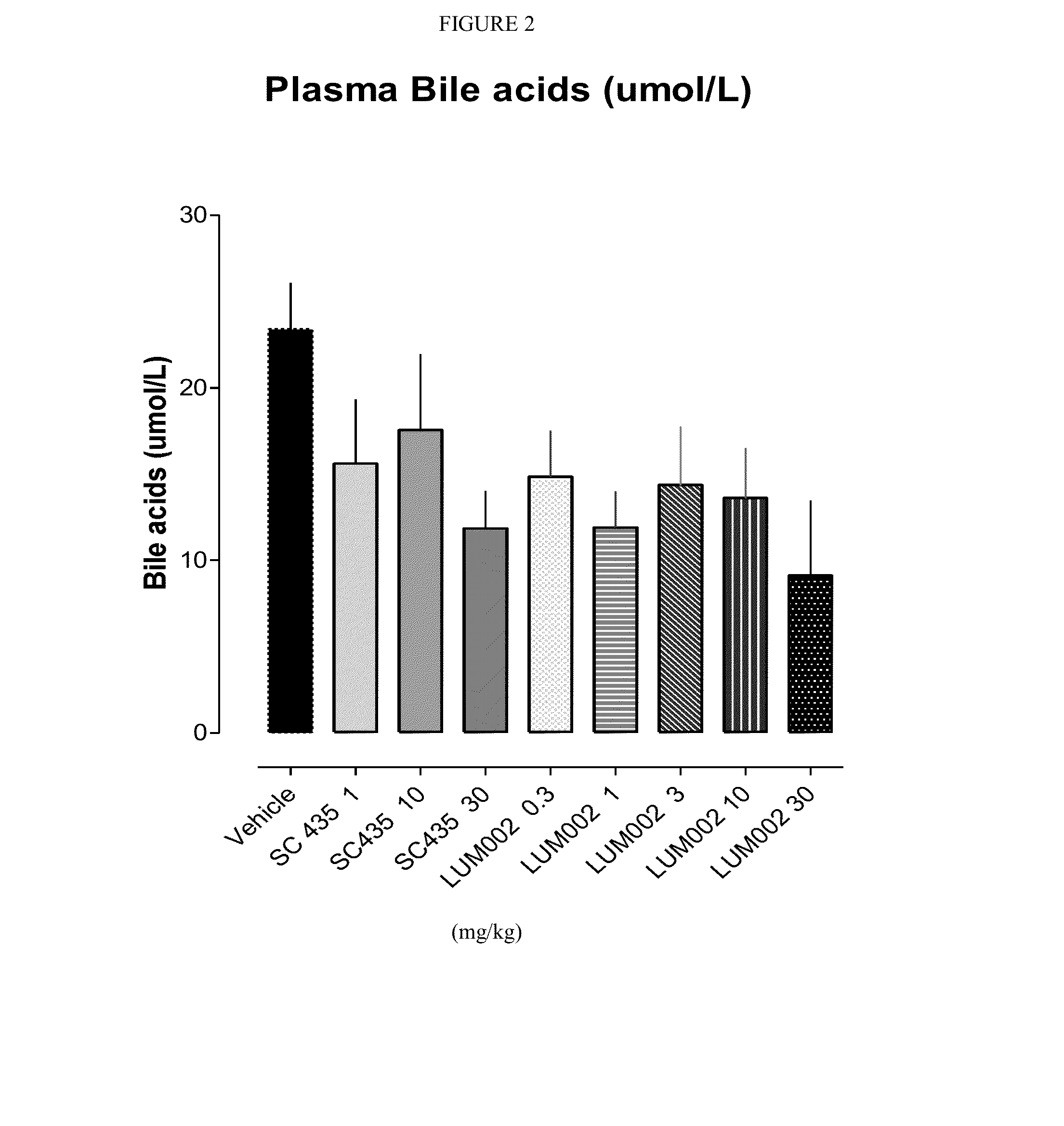
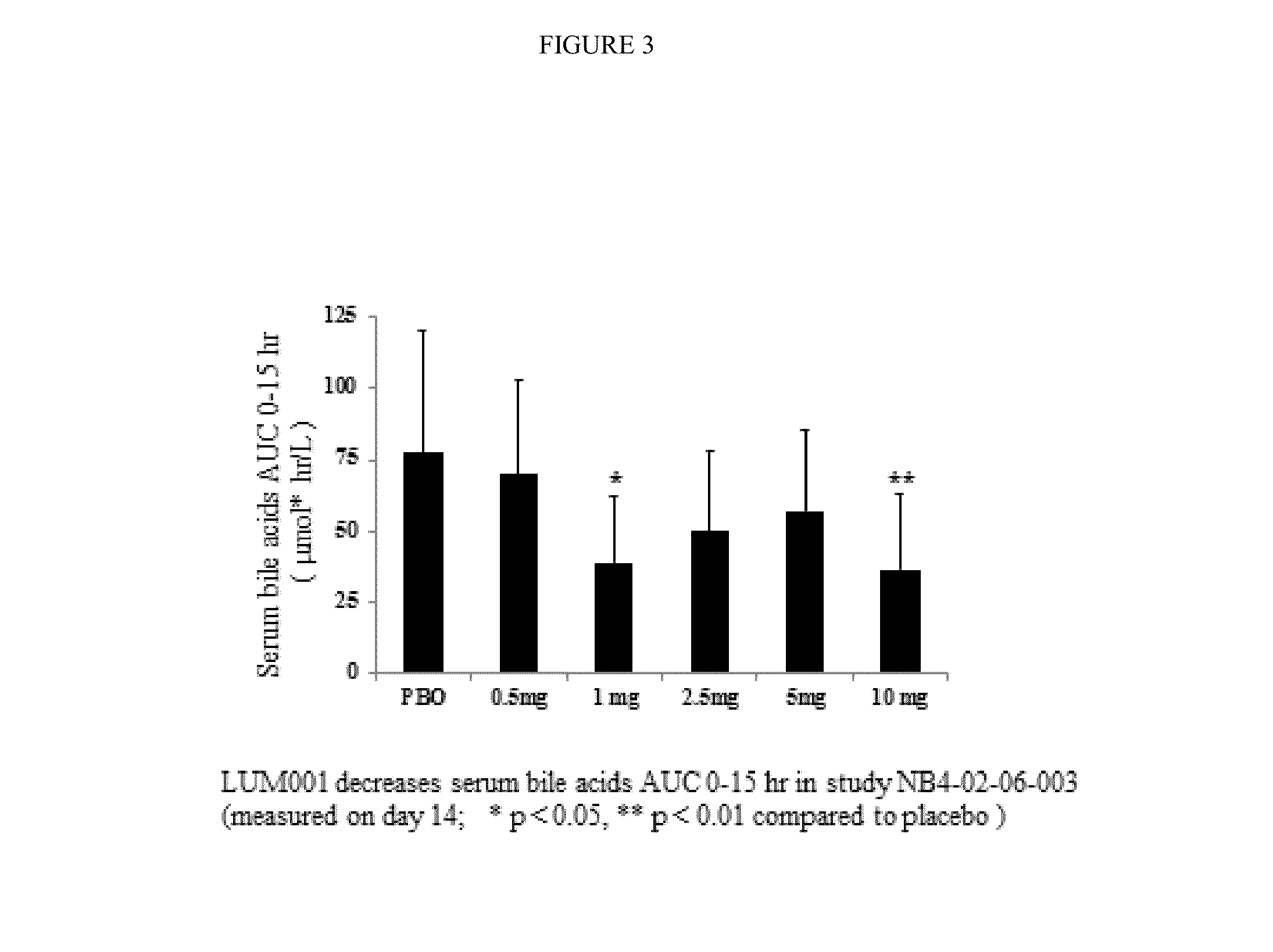
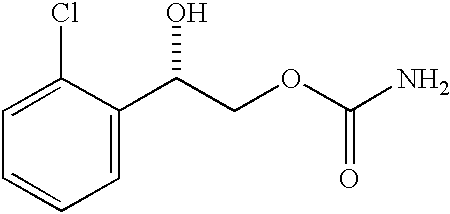
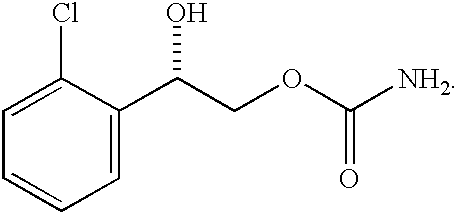
Bile Acid Recycling Inhibitors for Treatment of Hypercholemia and Cholestatic Liver Disease
InactiveUS20130108573A1Relieve symptomsReduce recurrenceBiocideCyclic peptide ingredientsDiseaseHepatic bile
Provided herein are methods of treating or ameliorating hypercholemia or a cholestatic liver disease by administering to an individual in need thereof a therapeutically effective amount of an Apical Sodium-dependent Bile Acid Transporter Inhibitor (ASBTI) or a pharmaceutically acceptable salt thereof. Also provided are methods for treating or ameliorating a liver disease, decreasing the levels of serum bile acids or hepatic bile acids, treating or ameliorating pruritis, reducing liver enzymes, or reducing bilirubin comprising administering to an individual in need thereof a therapeutically effective amount of ASBTI or a pharmaceutically acceptable salt thereof.
Owner:LUMENA PHARMA INC
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
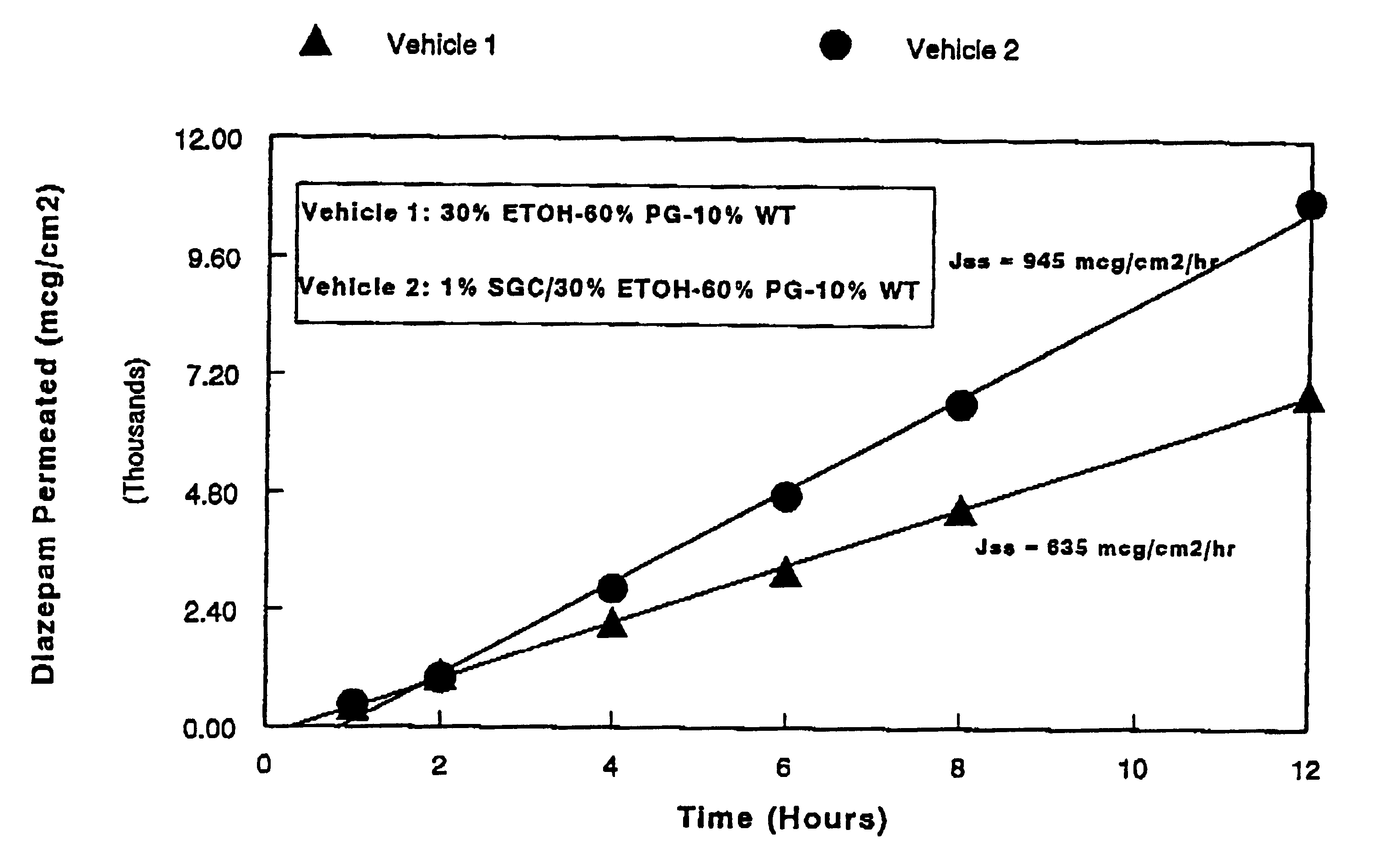
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
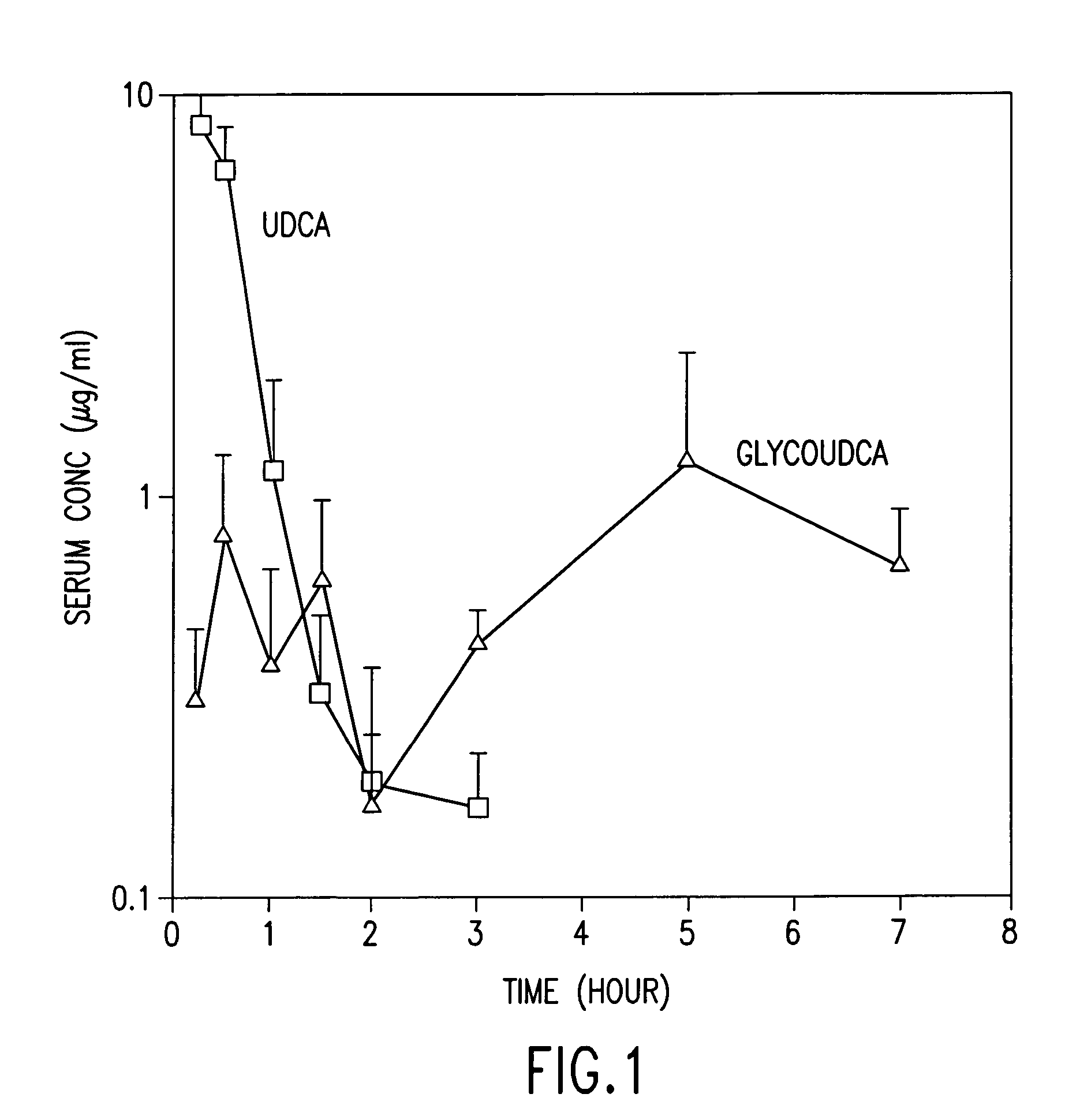
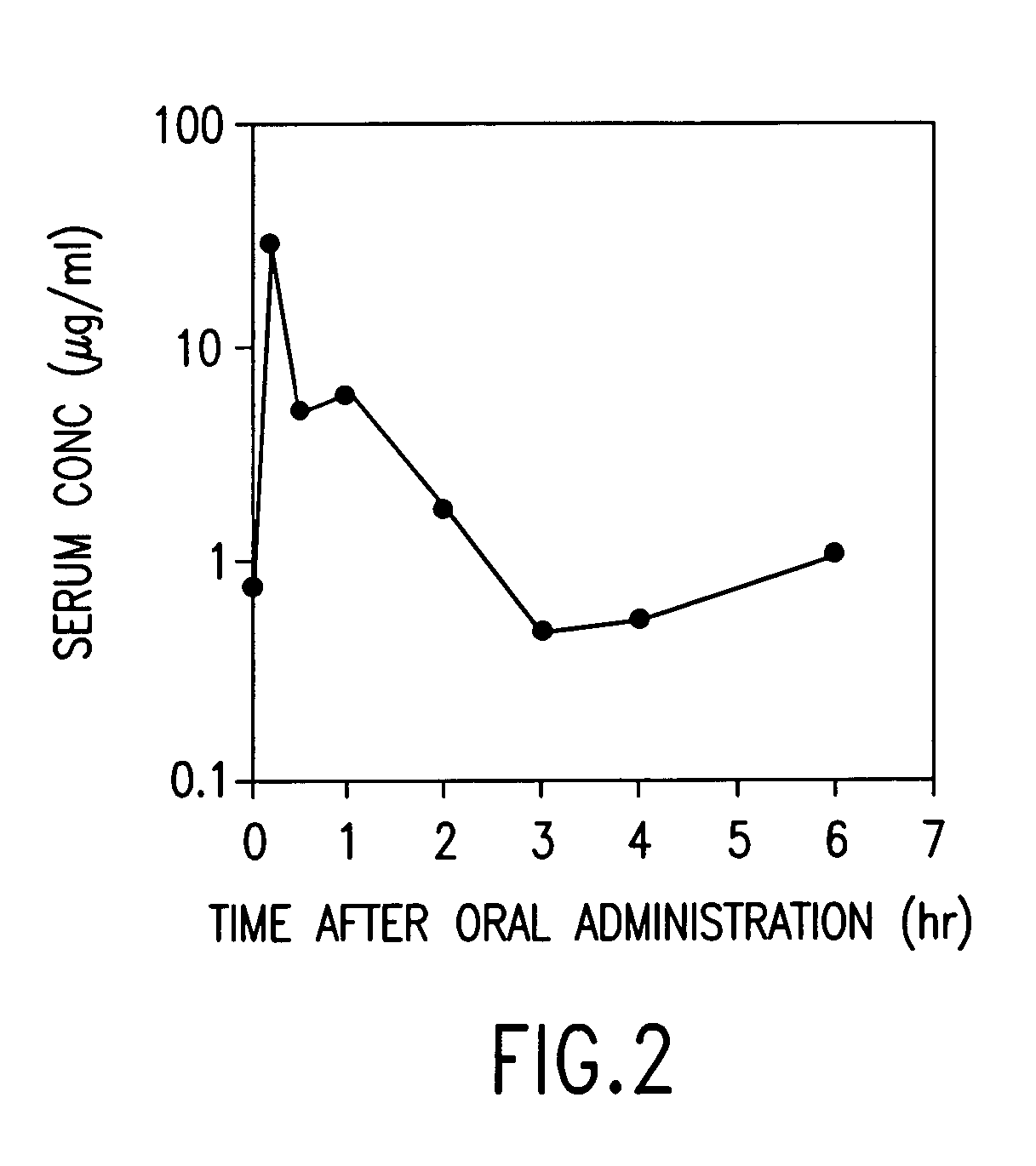
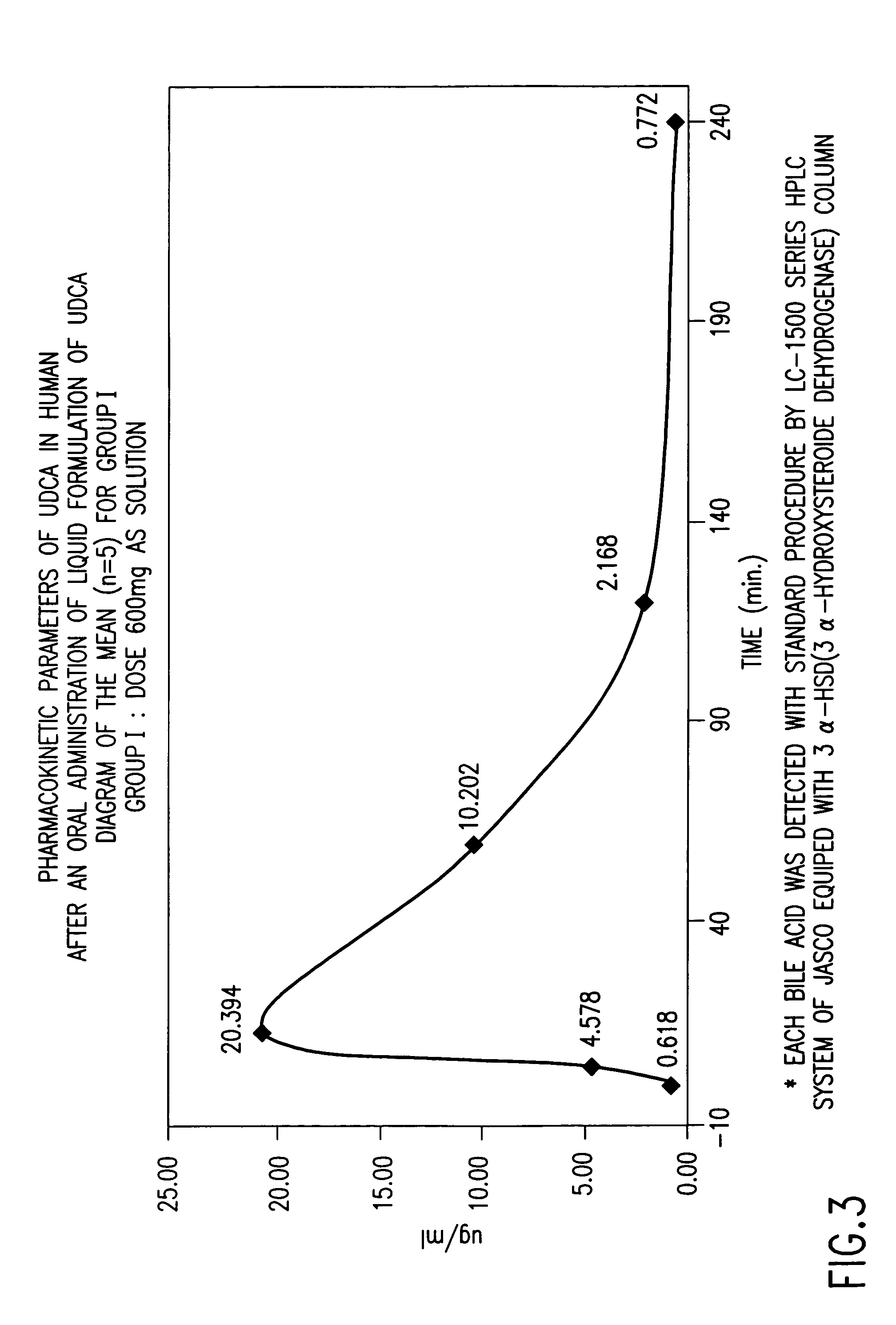
Dried forms of aqueous solubilized bile acid dosage formulation: preparation and uses thereof
InactiveUS20050158408A1Improve bioavailabilityPlasma bioavailabilityBiocideOrganic active ingredientsDrug compoundSoluble Non-Starch Polysaccharide
Compositions for pharmaceutical and other uses comprising clear aqueous solutions of bile acids which do not form any detectable precipitates over selected ranges of pH values of the aqueous solution and methods of making such solutions are disclosed. Compositions of the disclosure may comprise water; a bile acid in the form of a bile acid, bile acid salt, or a bile acid conjugated with an amine by an amide linkage; and either or both an aqueous soluble starch conversion product and an aqueous soluble non-starch polysaccharide. The composition remains in solution without forming a precipitate over a range of all pH values obtainable in an aqueous system. The composition, according to some embodiments, may further contain a pharmaceutical compound in a pharmaceutically effective amount. The disclosure further provides dried forms of primary aqueous solubilized bile acid formulations and methods of preparing such dried forms.
Owner:YOO SEO HONG
Blends of poly(ester amide) polymers
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
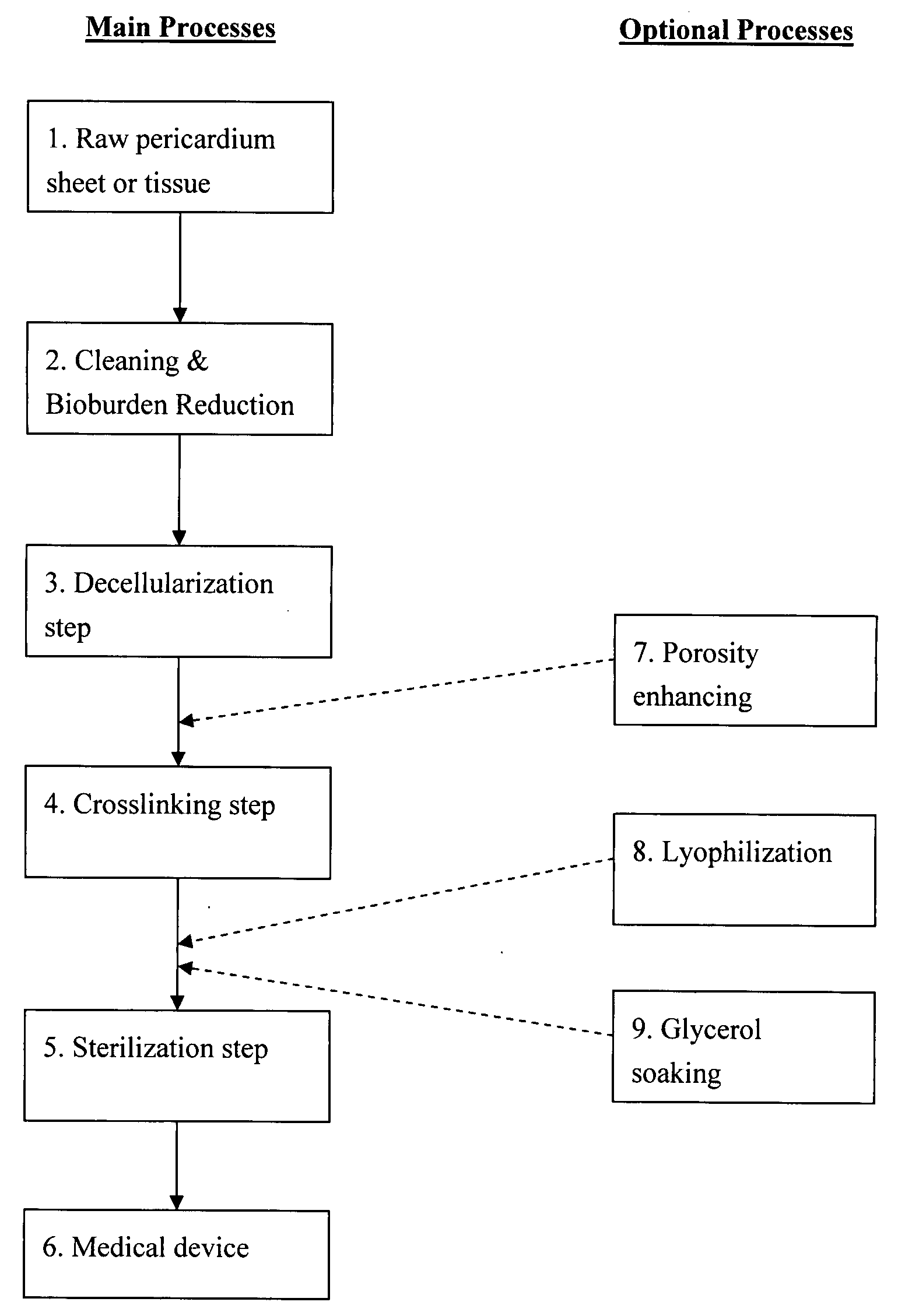
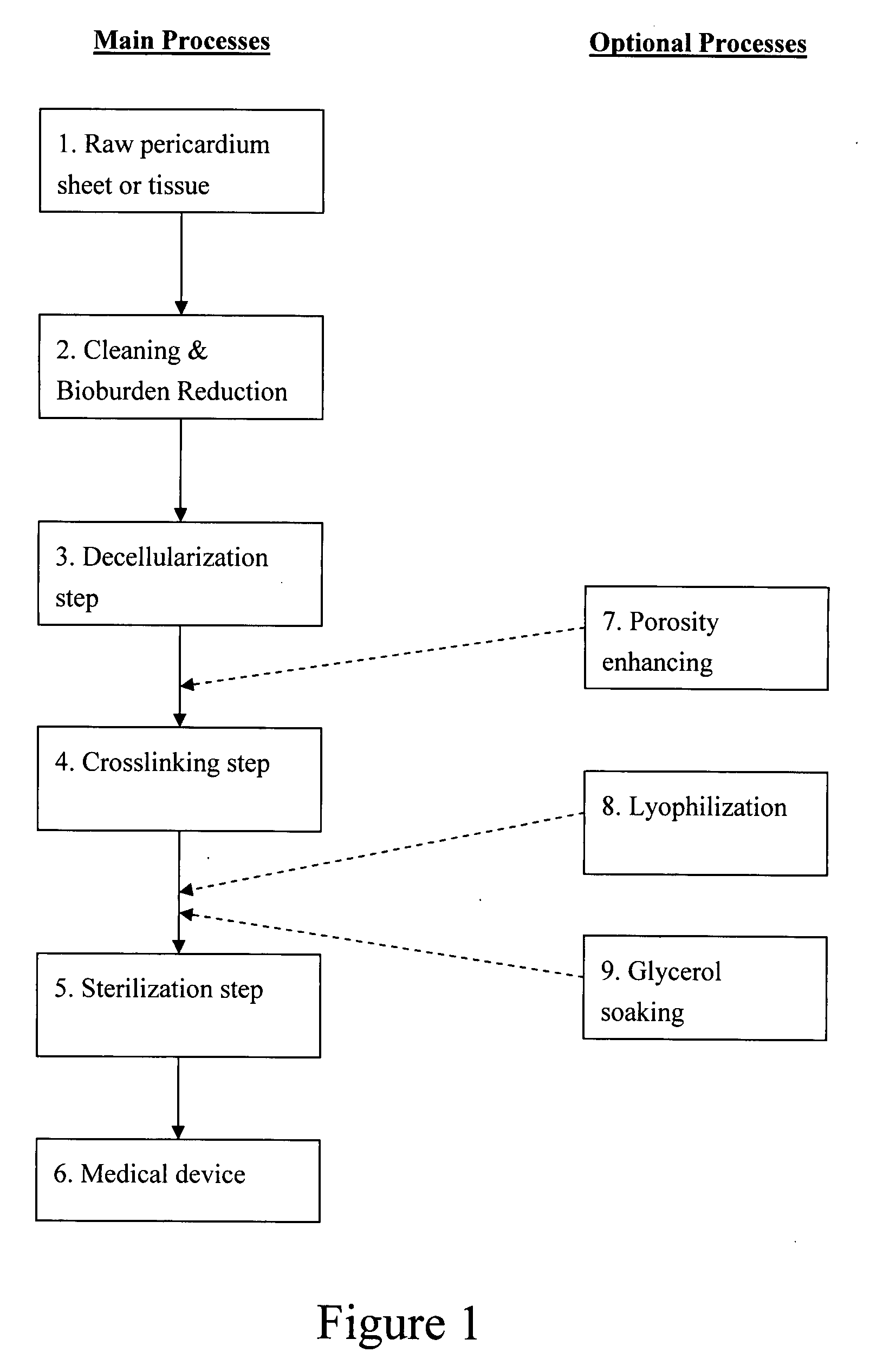
Decellularized pericardial tissue
InactiveUS20080195229A1Low antigenicityLow immunogenicityTissue regenerationProsthesisChemical treatmentPericardium
The invention discloses a decellularized pericardial tissue via chemical treatment with cholic acid or bile salts as a medical device and process of manufacture.
Owner:QUIJANO RODOLFO C +1
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS20060093842A1Improve stabilityIncrease drug release rateOrganic active ingredientsNervous disorderAbnormal tissue growthPEA polymer
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Method for the treatment of gallstones
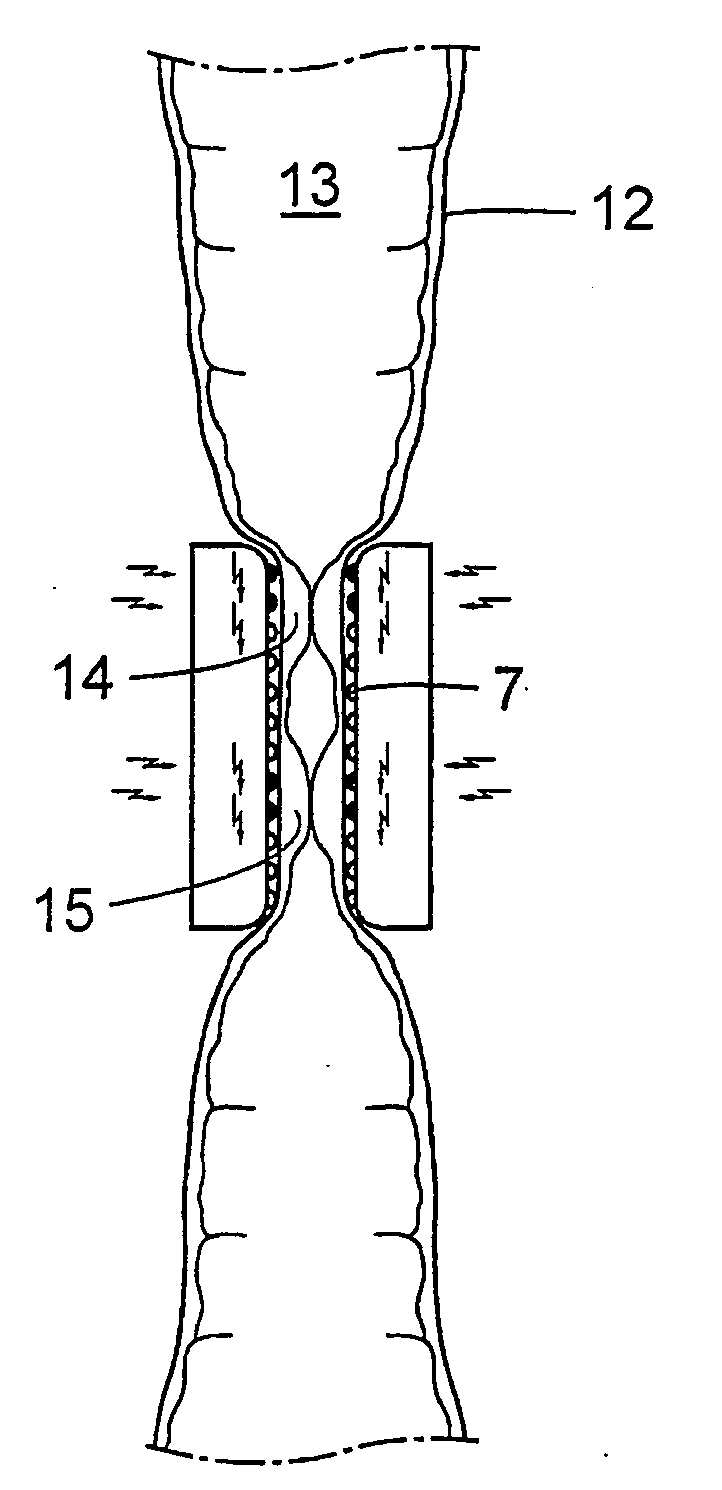
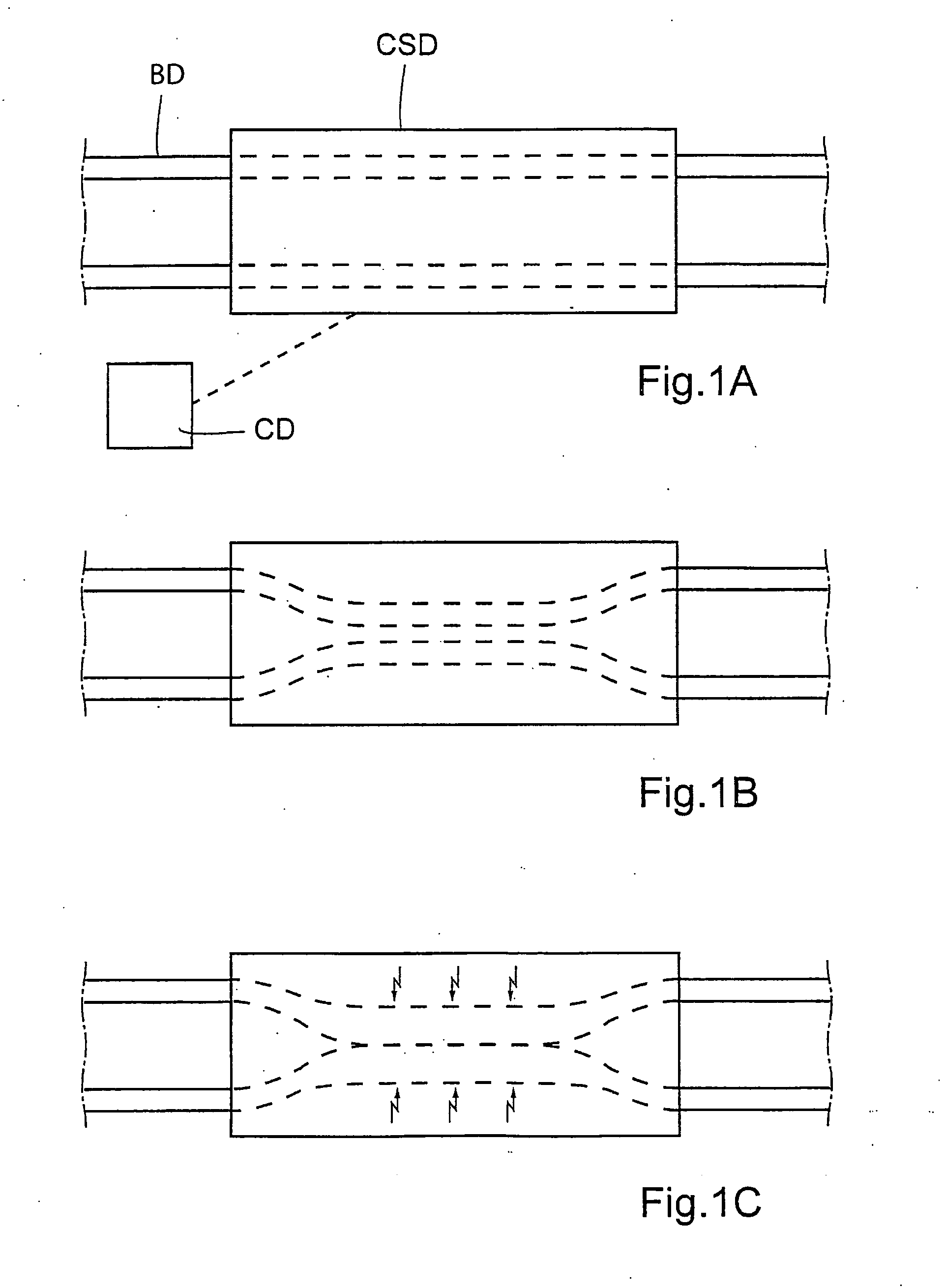
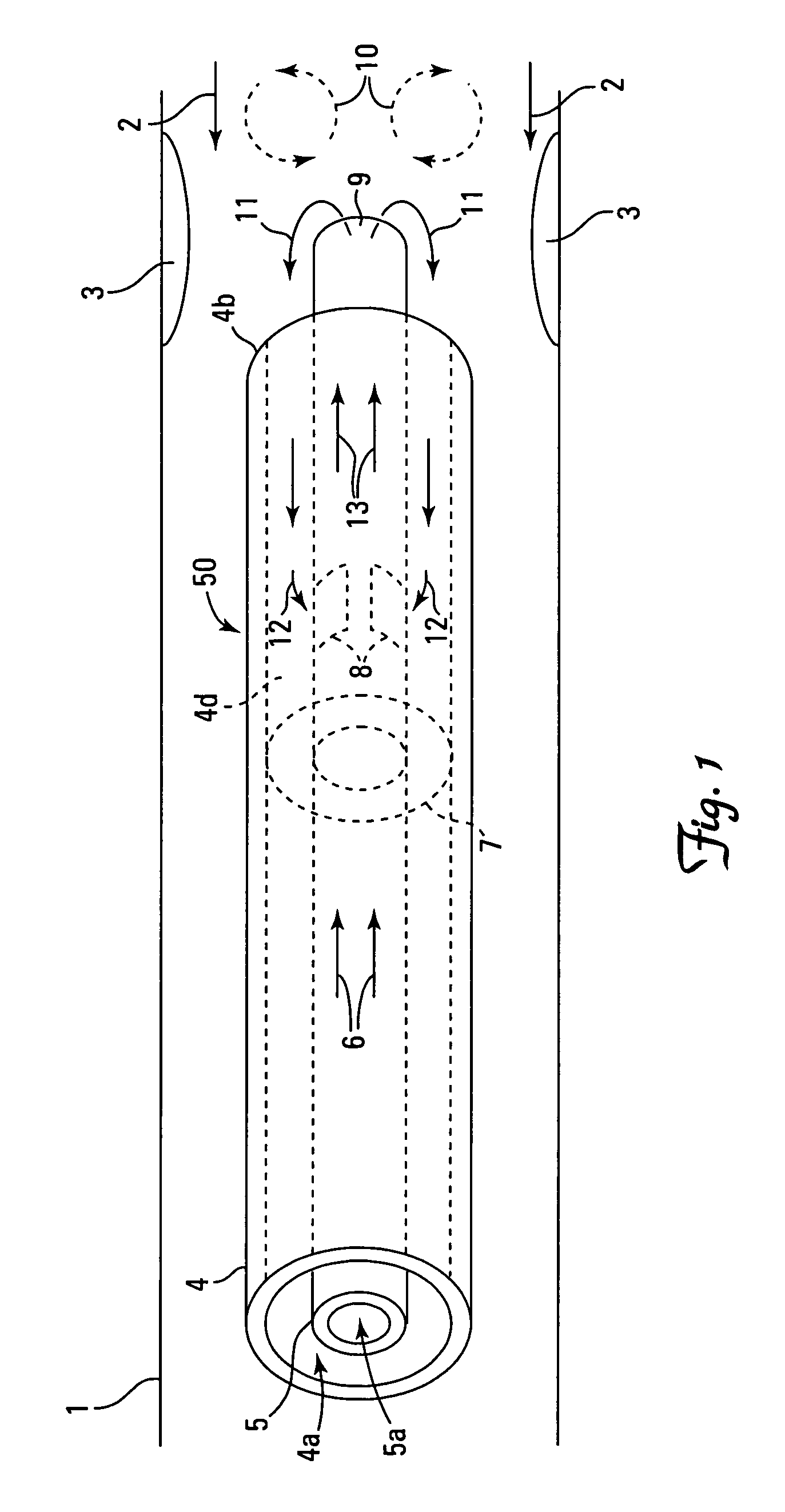
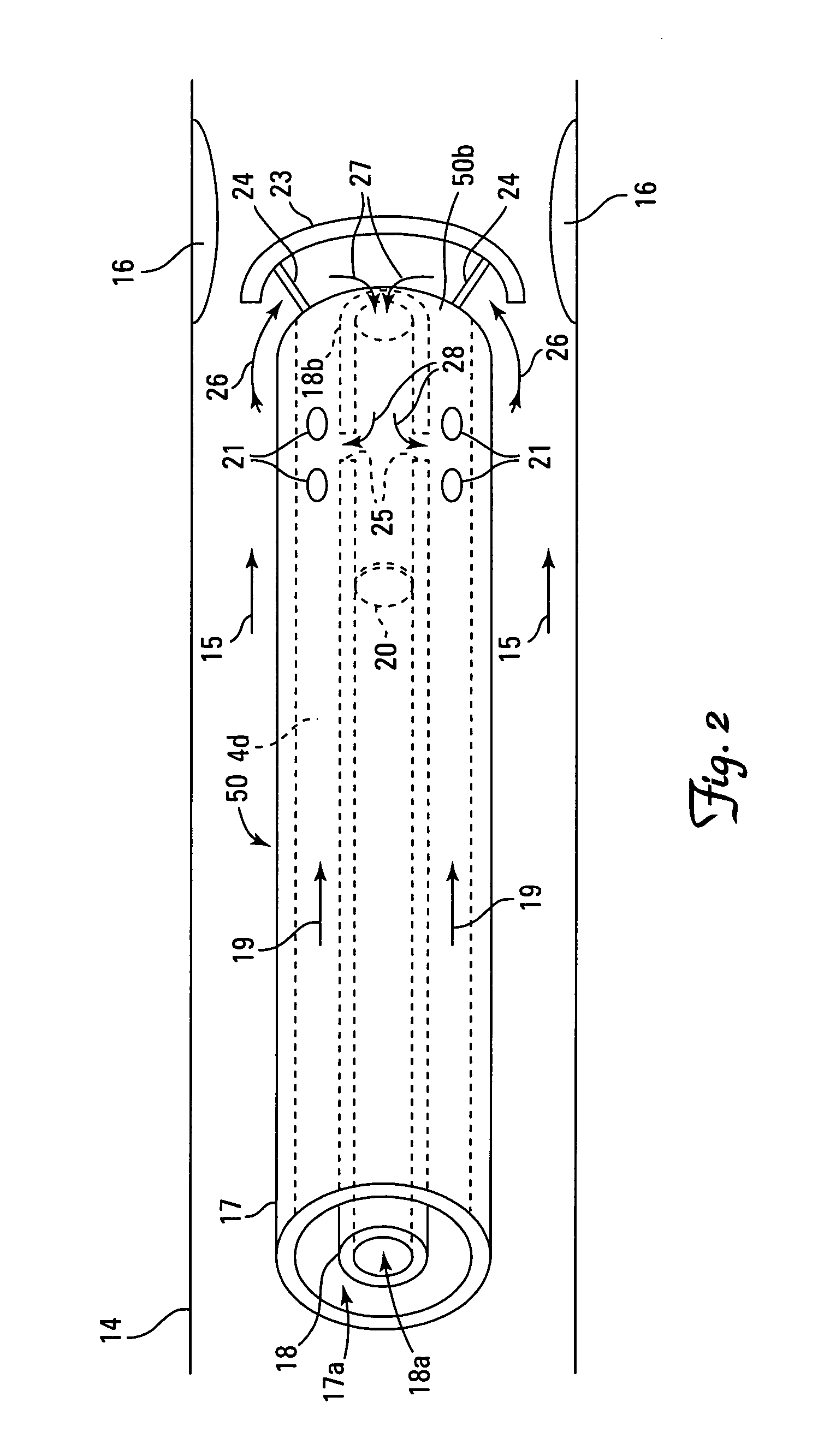
There is provided a method for controlling the movement of bile and / or gall stones in the biliary duct. The method comprises gently constricting (i.e., without substantially hampering the blood circulation in the tissue wall) at least one portion of the tissue wall to influence the movement of bile and / or gallstones in the biliary duct, and stimulating the constricted wall portion to cause contraction of the wall portion to further influence the movement of bile and / or gallstones in the biliary duct. The method can be used for restricting or stopping the movement of bile and / or gallstones in the biliary duct, or for actively moving the fluid in the biliary duct, with a low risk of injuring the biliary duct.
Owner:FORSELL PETER
Hair analysis method
InactiveUS6949344B1Reliable solubilizationAccurate methodPre-tanning chemical treatmentMicrobiological testing/measurementBetaineDigestion
A method the direct analysis of an analyte in keratinized structures, e.g., hair and fingernails, which comprises preparing a mixture containing a low redox potential activator compound such as dithiothreitol or dithioerythritol, an enzyme suitable for the digestion of the keratin structure, a sample of the keratin structure and a biological detergent that aids the digestion of the keratinized structure at a relatively low pH, e.g., between about 6.2 and 8; permitting the enzyme to at least substantially digest the sample of keratin structure, and subjecting the digest solution to analysis, preferably by radioimmunoassay, to determine the identity and amount of analyte in the keratin structure sample. To accelerate the method, cupric sulfate may be added to the mixture after degradation of the keratin sample. The enzyme may be a peptidase, endopeptidase or proteinase, with papain, chymopapain, and proteinase K being preferred for use in the invention. The preferred biological detergents include betaine, sulfo-betaine, alkylglucosides and bile acids.
Owner:PSYCHEMEDICS CORPORATION
Bifidobacterium possessing characteristic for anti pathogenesis bacterium in intestinal tract and antioxidation, and application
InactiveCN1796540AHave adhesive propertiesInhibitoryBacteriaBacteria material medical ingredientsEscherichia coliDisease
This invention describes a bifidobacterium, i.e., bifidobacterium longum BM358 that can resist intestinal pathogens as well as resist oxidation. The bifidobacterium longum has such properties as high security, outstanding acid and bile resistance, adhesion to intestinal epithelial cells, outstanding intestinal pathogen resistance and oxidation resistance. The bifidobacterium longum can be used to prevent and treat gastritis, gastrelcosis and duodenitis caused by pylorus helicobacteria, and enteritis, acute diarrhea and enterogastric disorder caused by colibacillus and other intestinal pathogens.
Owner:王敖喜 +1
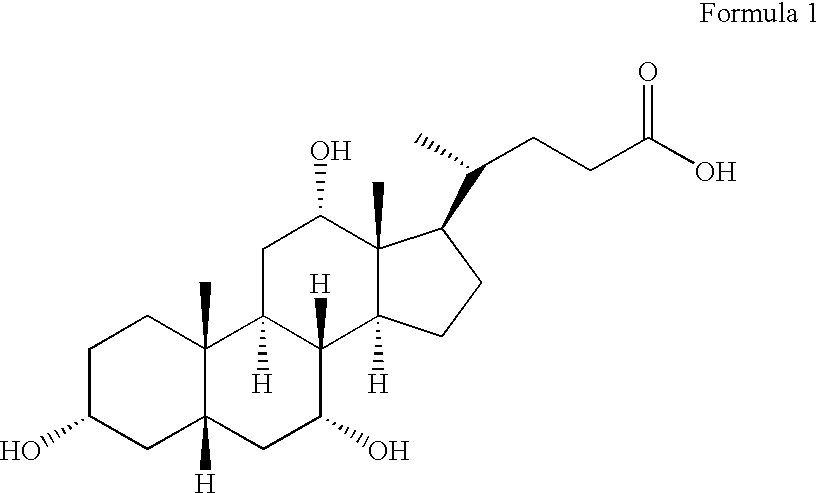
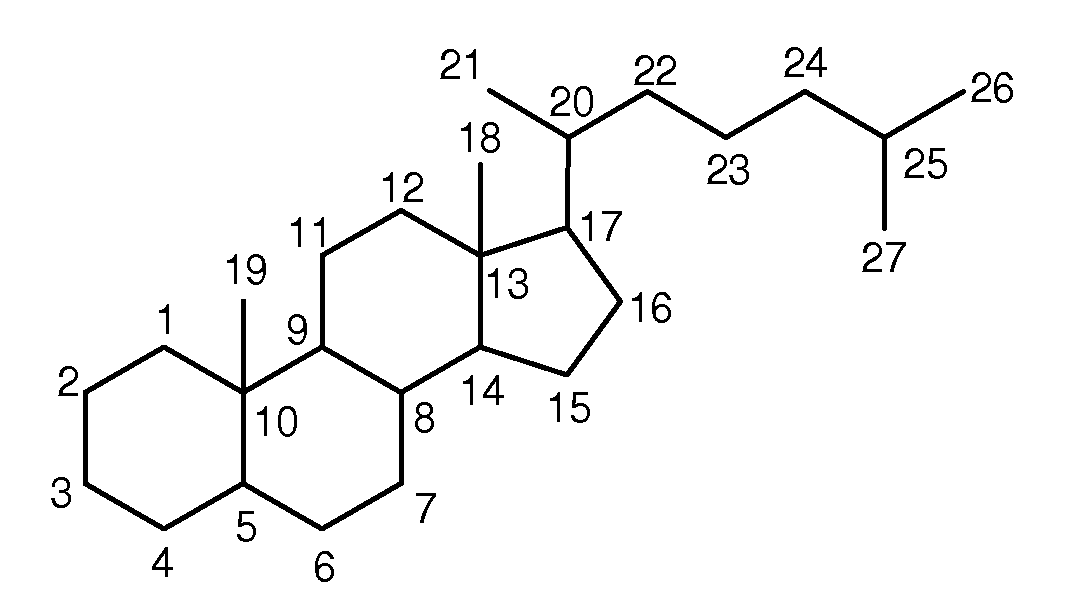
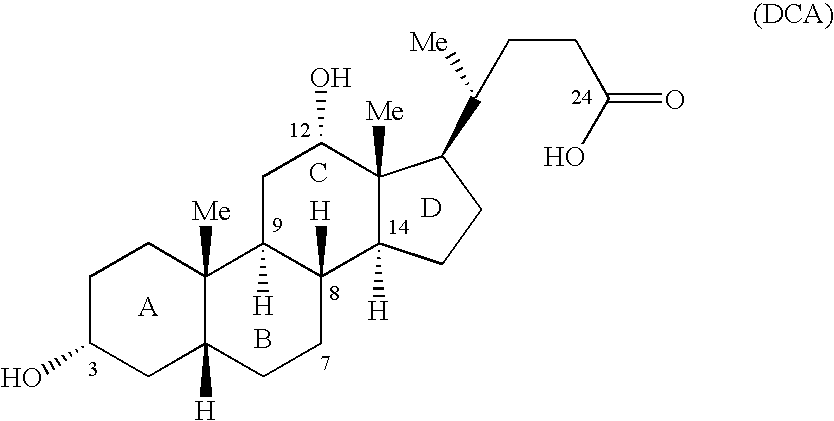
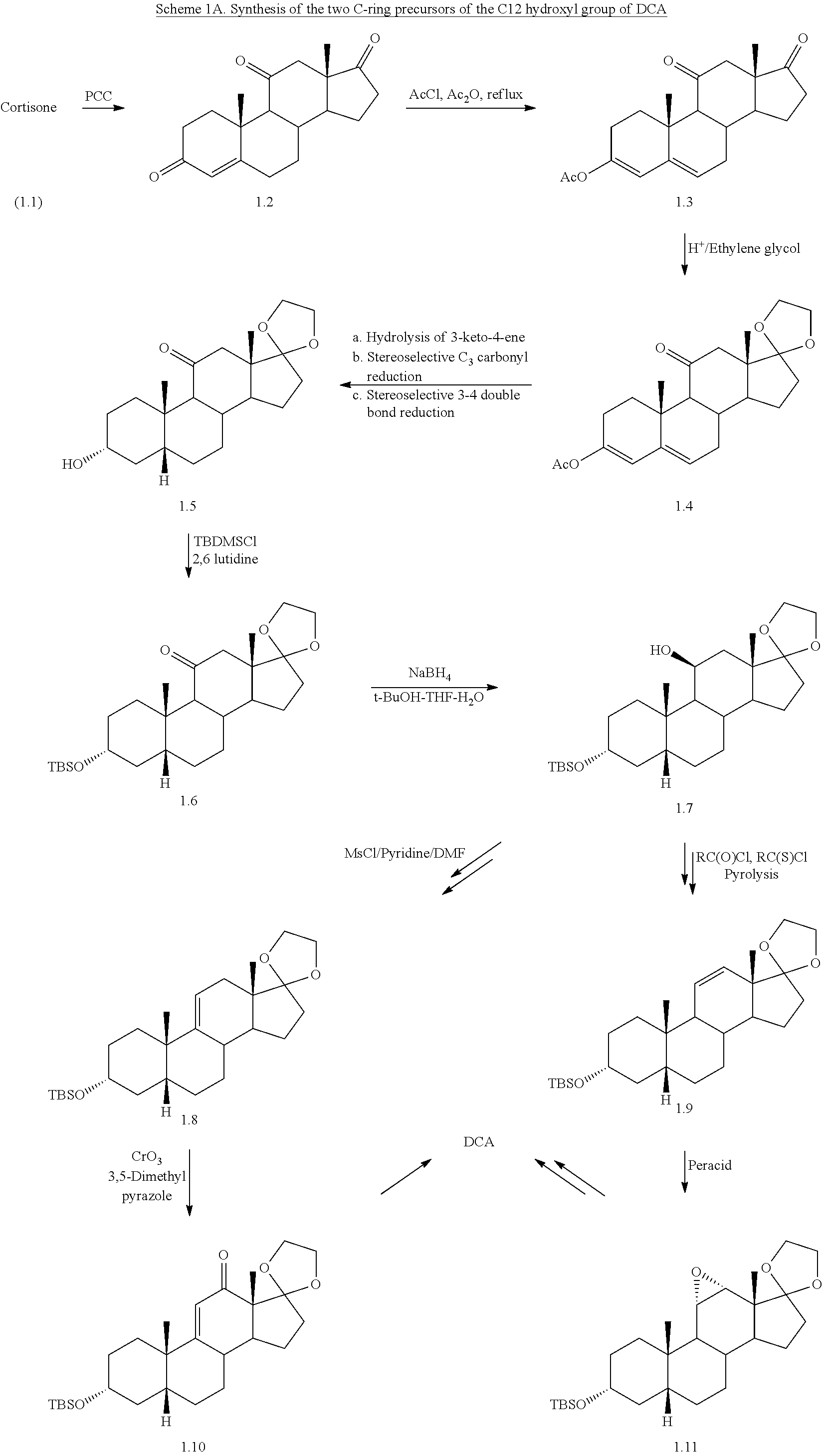
Synthetic bile acid compositions and methods
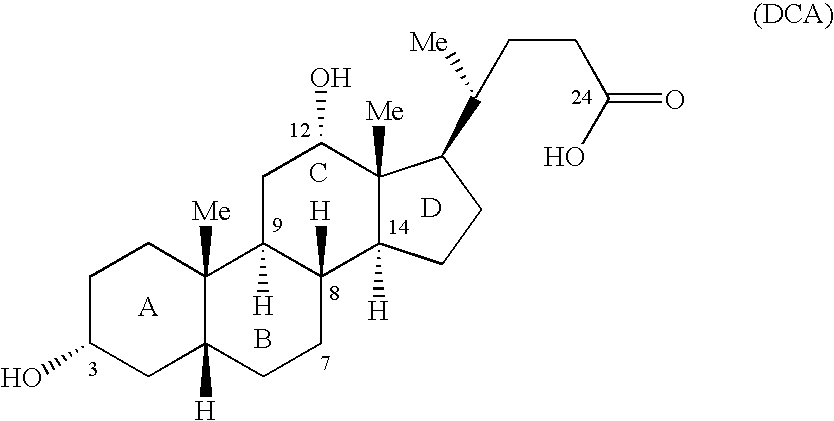
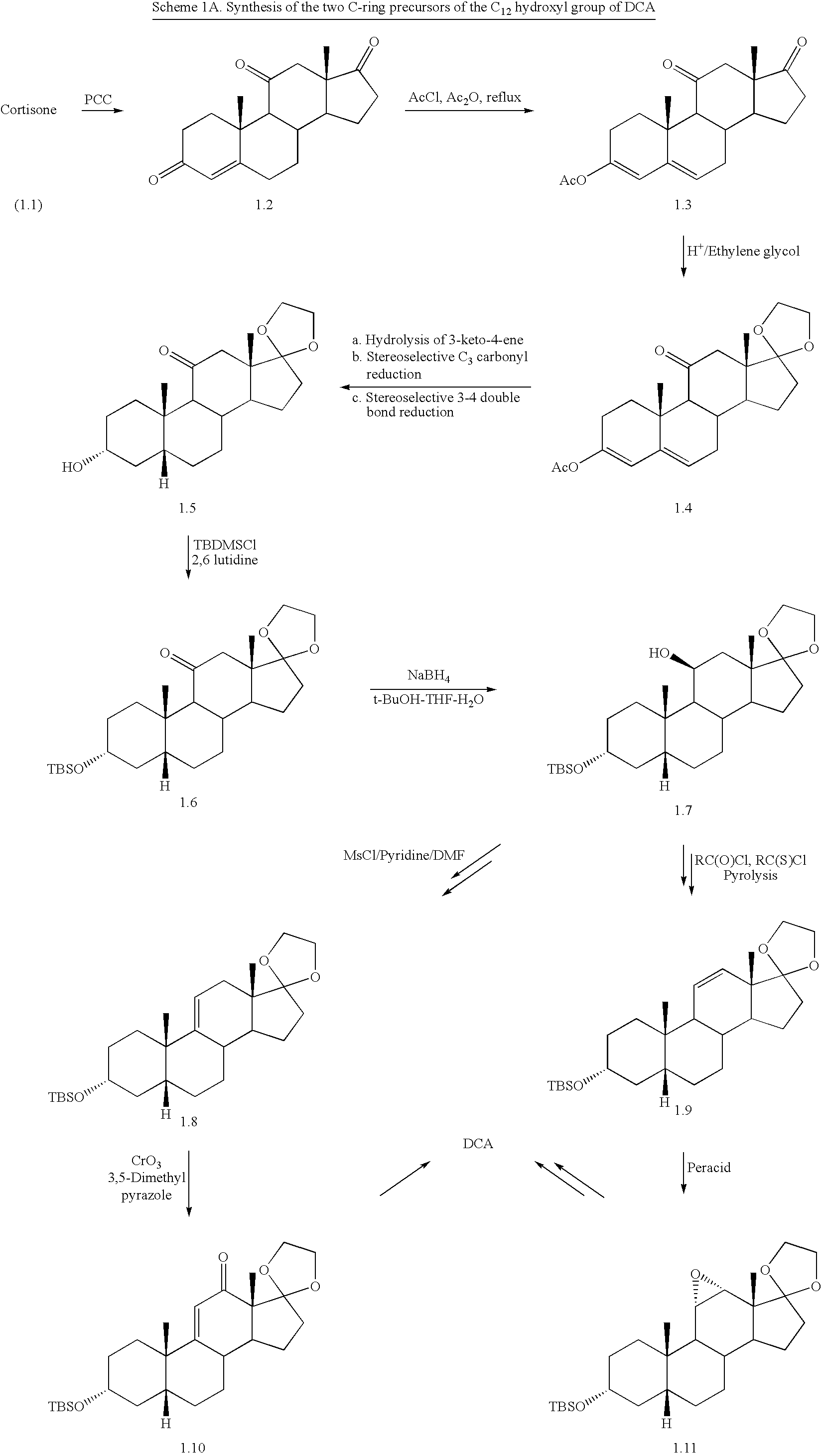
Bile acids and related compositions and methods of synthesis and use. More specifically, deoxycholic acid and related compositions, said compositions being free of all moieties of animal origin and free of pyrogenic moieties.
Owner:ALLERGAN SALES LLC
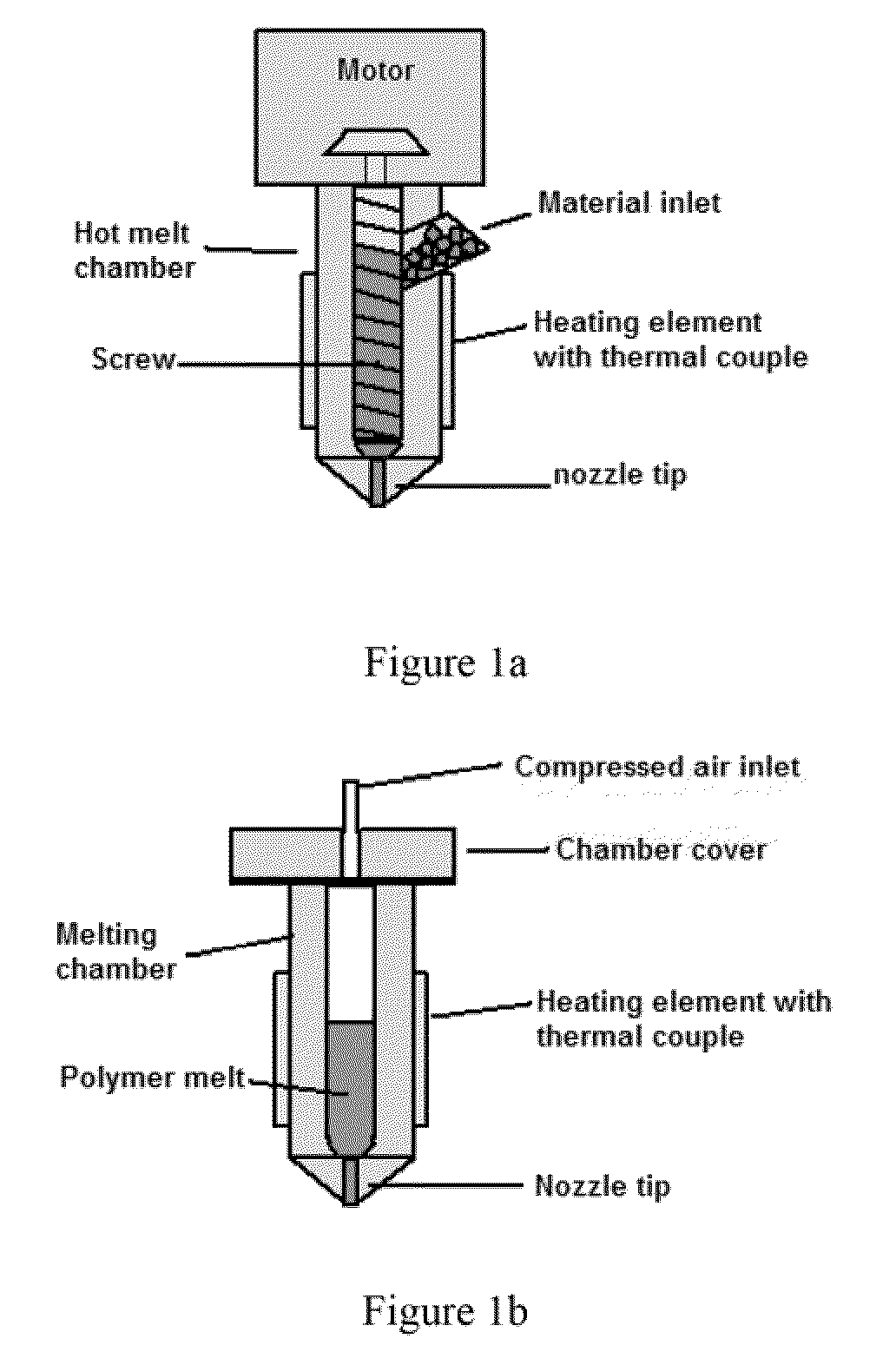
Methods and Apparatus for Fabricating Porous Three-Dimensional Tubular Scaffolds
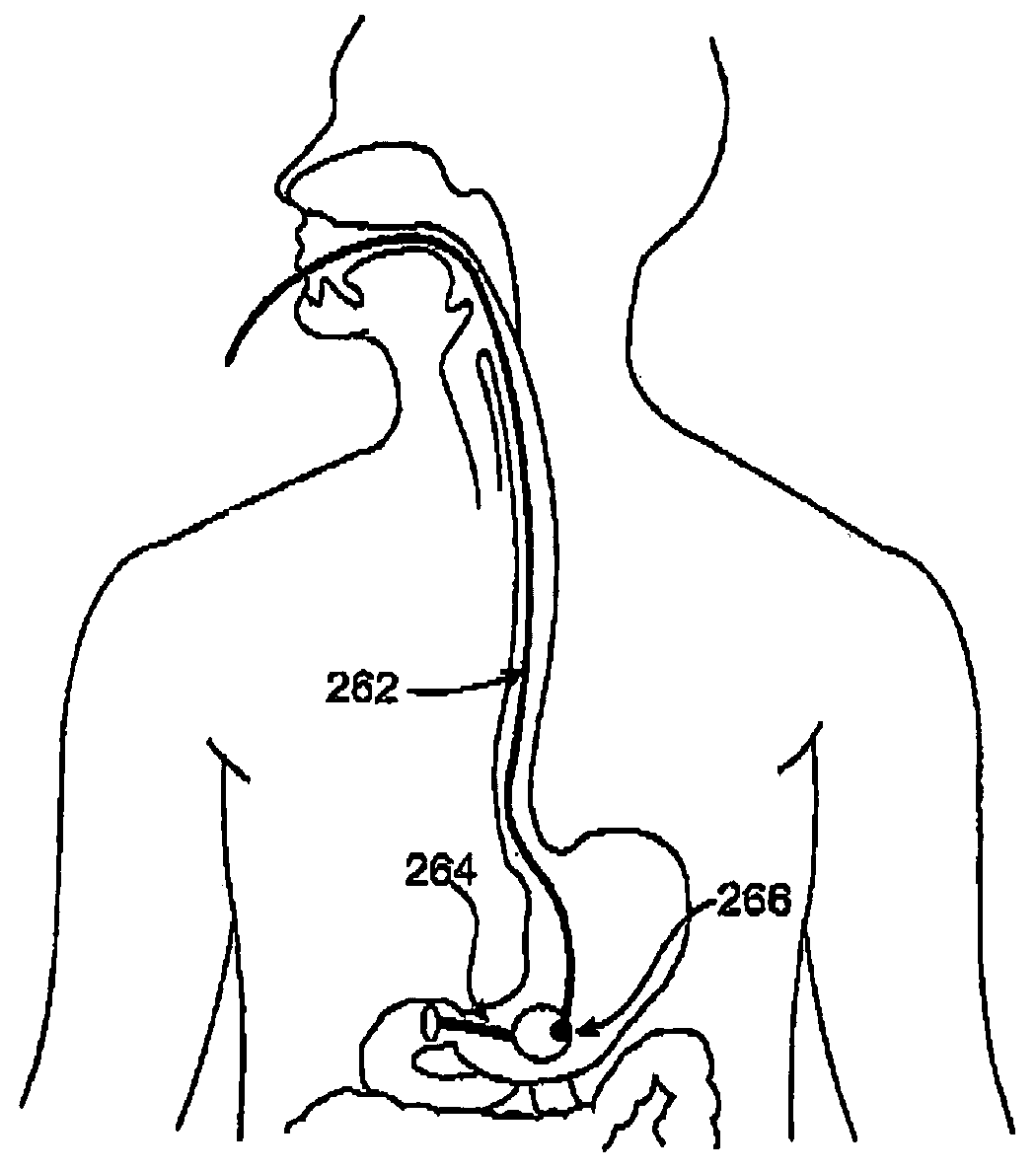
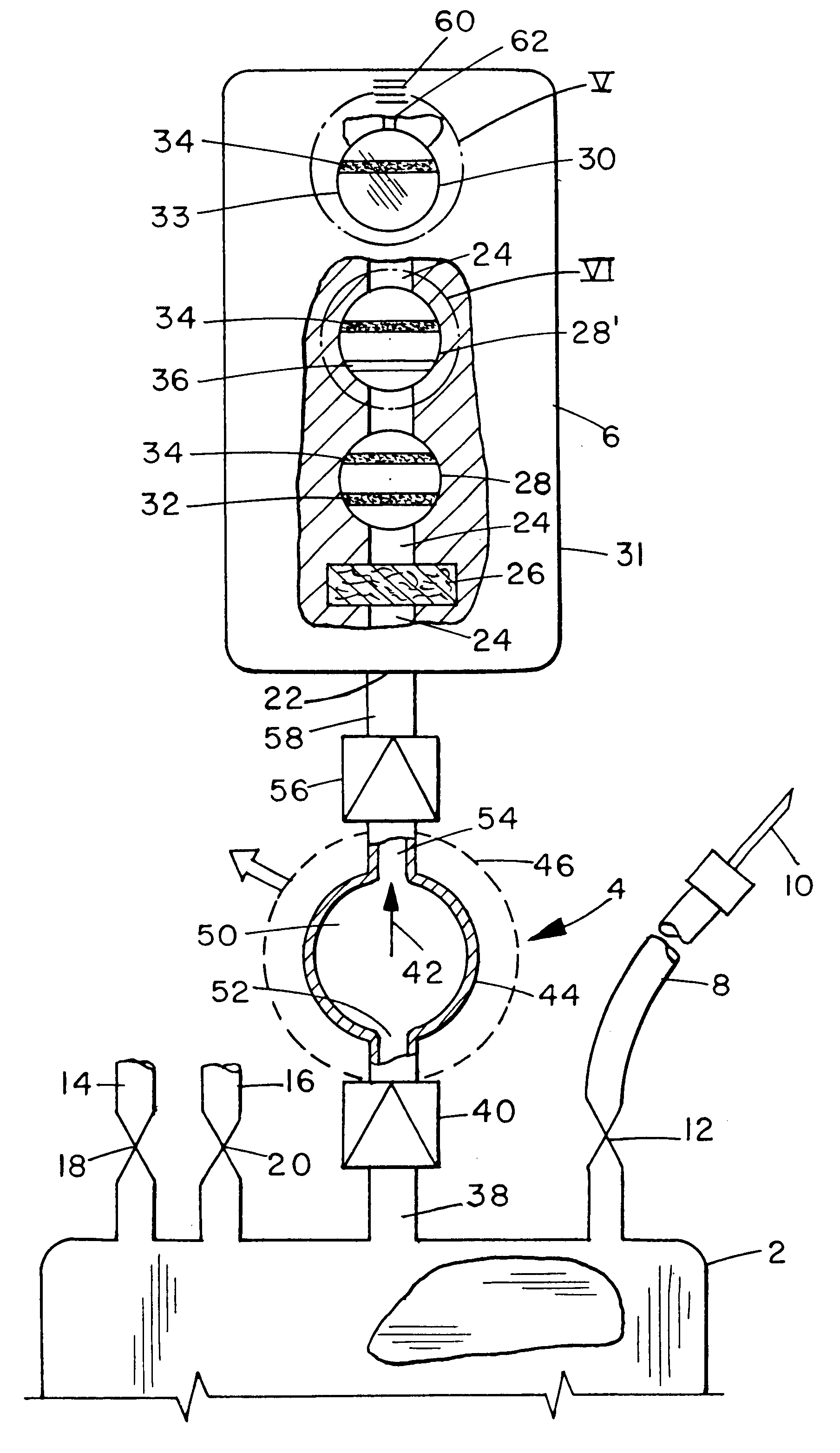
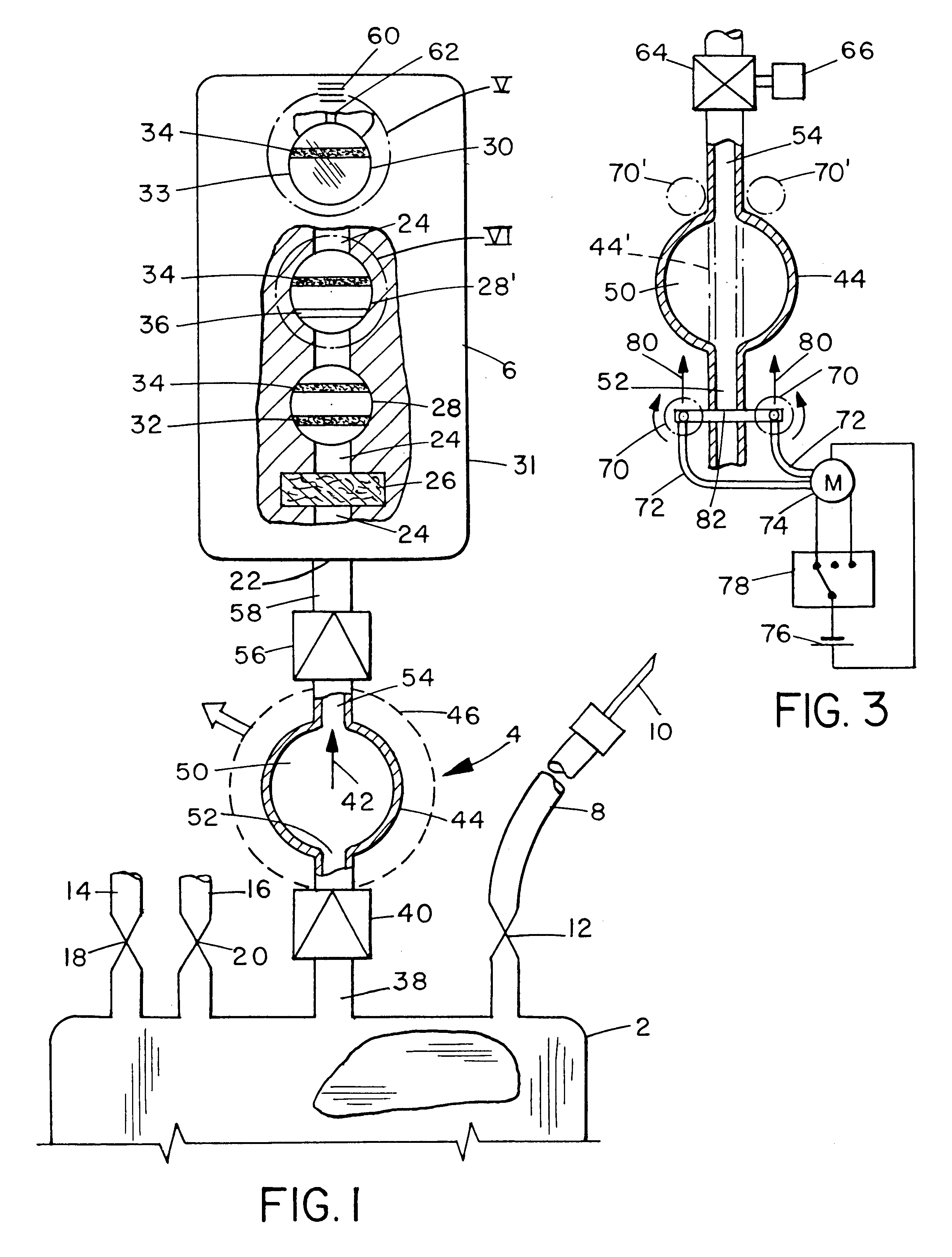
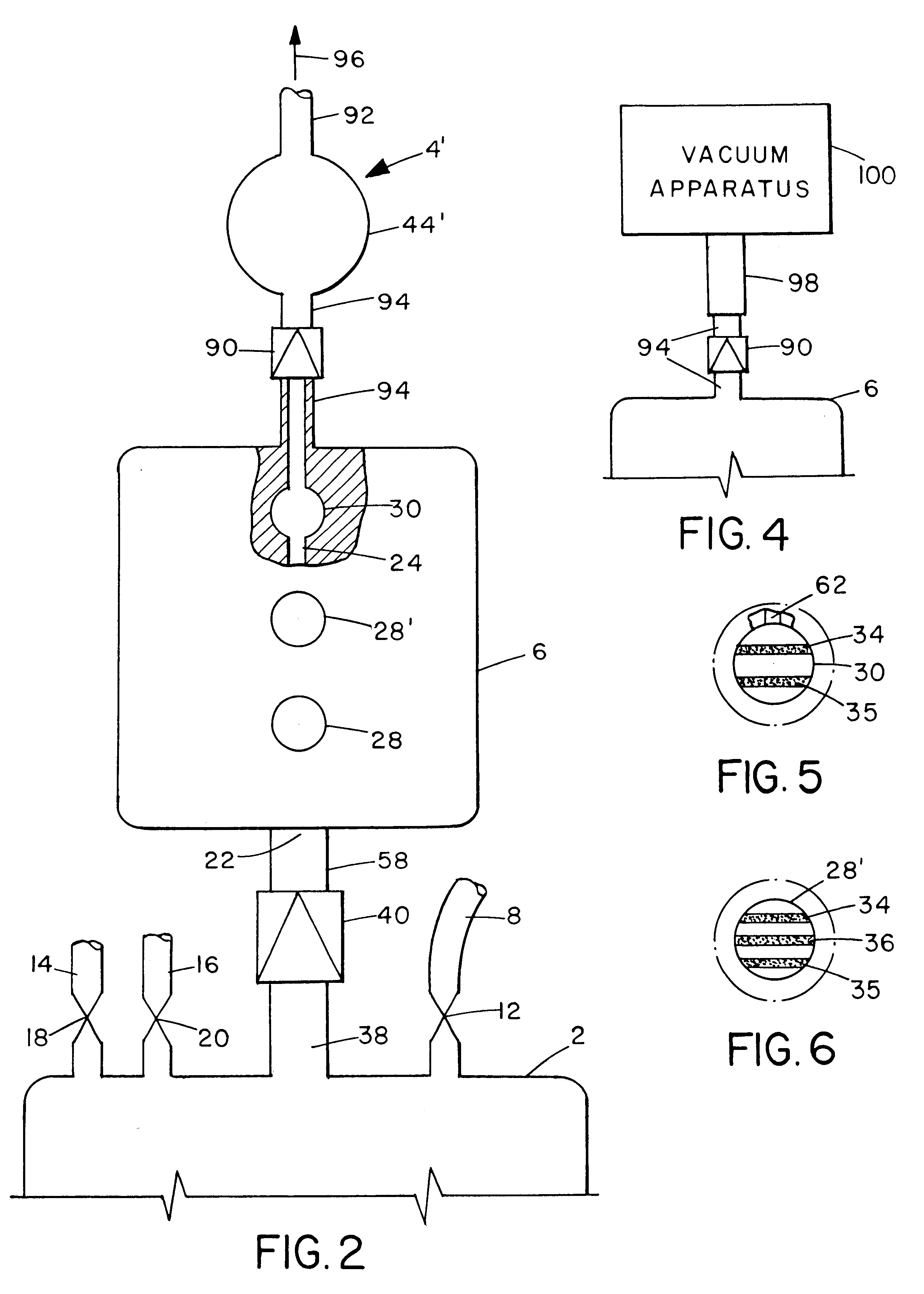
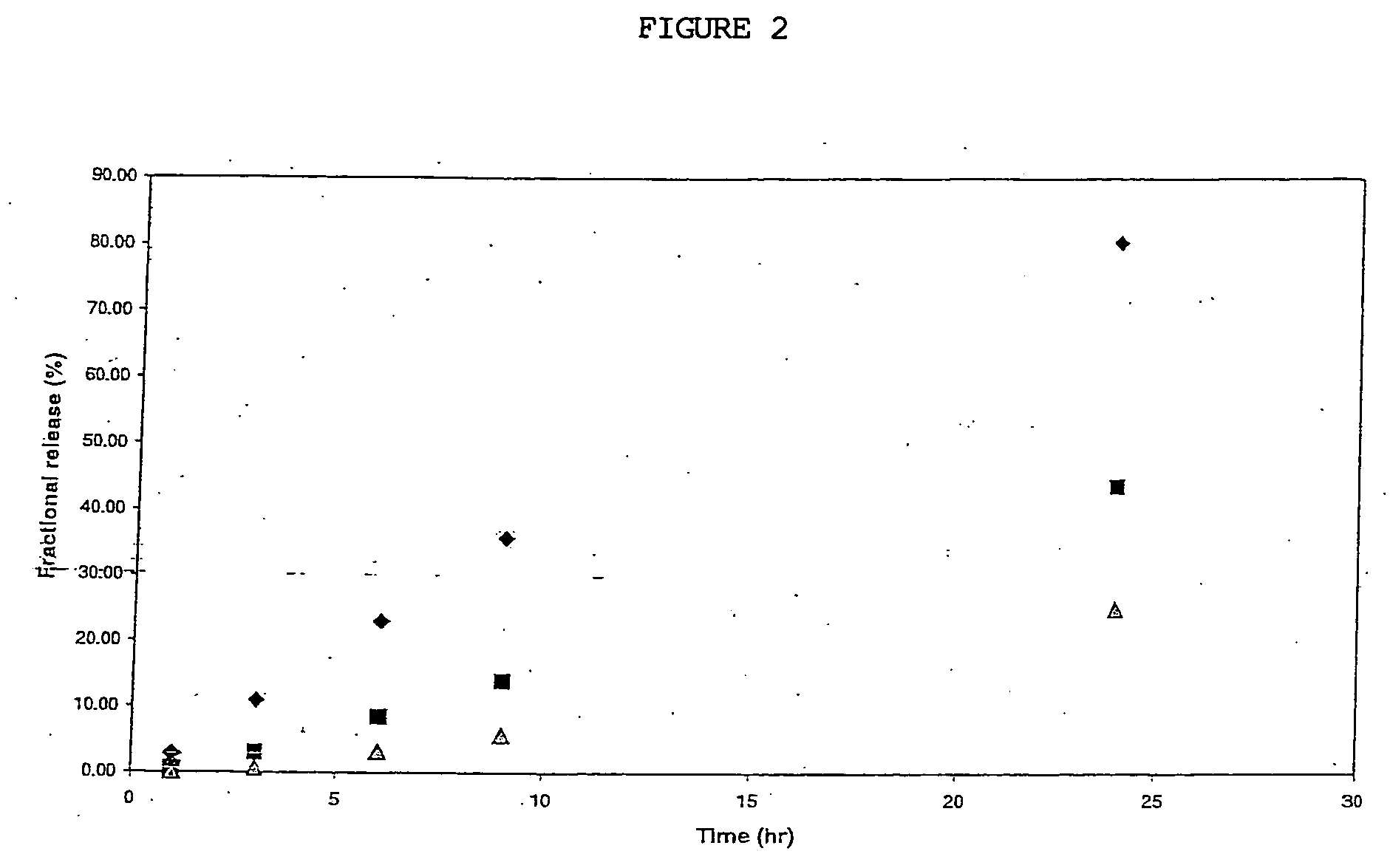
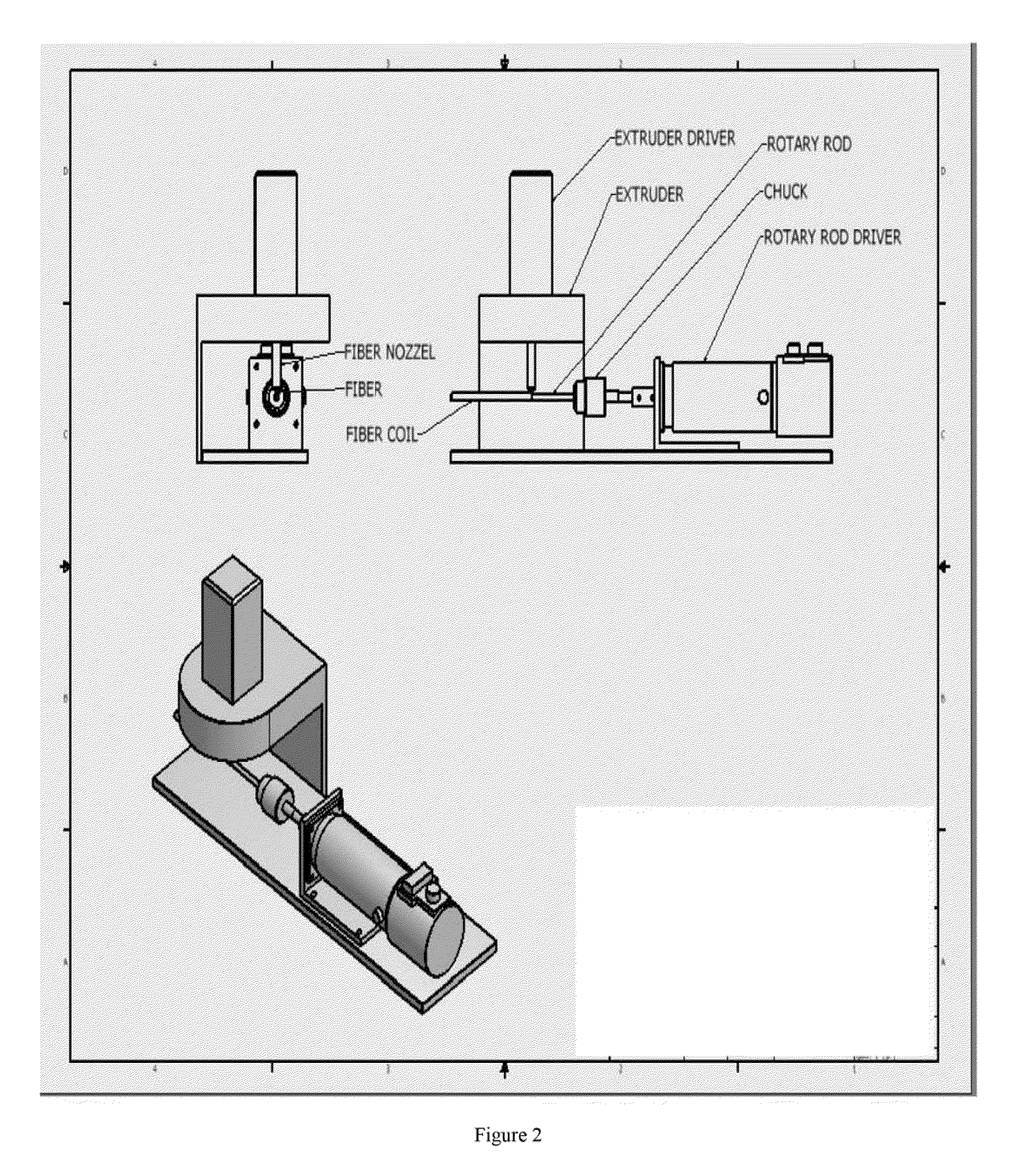
ActiveUS20100330144A1Reduce porosityProperties be controlledEnvelopes/bags making machineryLayered productsIntestinal structureBile fluid
Owner:BEIJING ADVANCED MEDICAL TECH
Cell and enzyme compositions for modulating bile acids, cholesterol and triglycerides
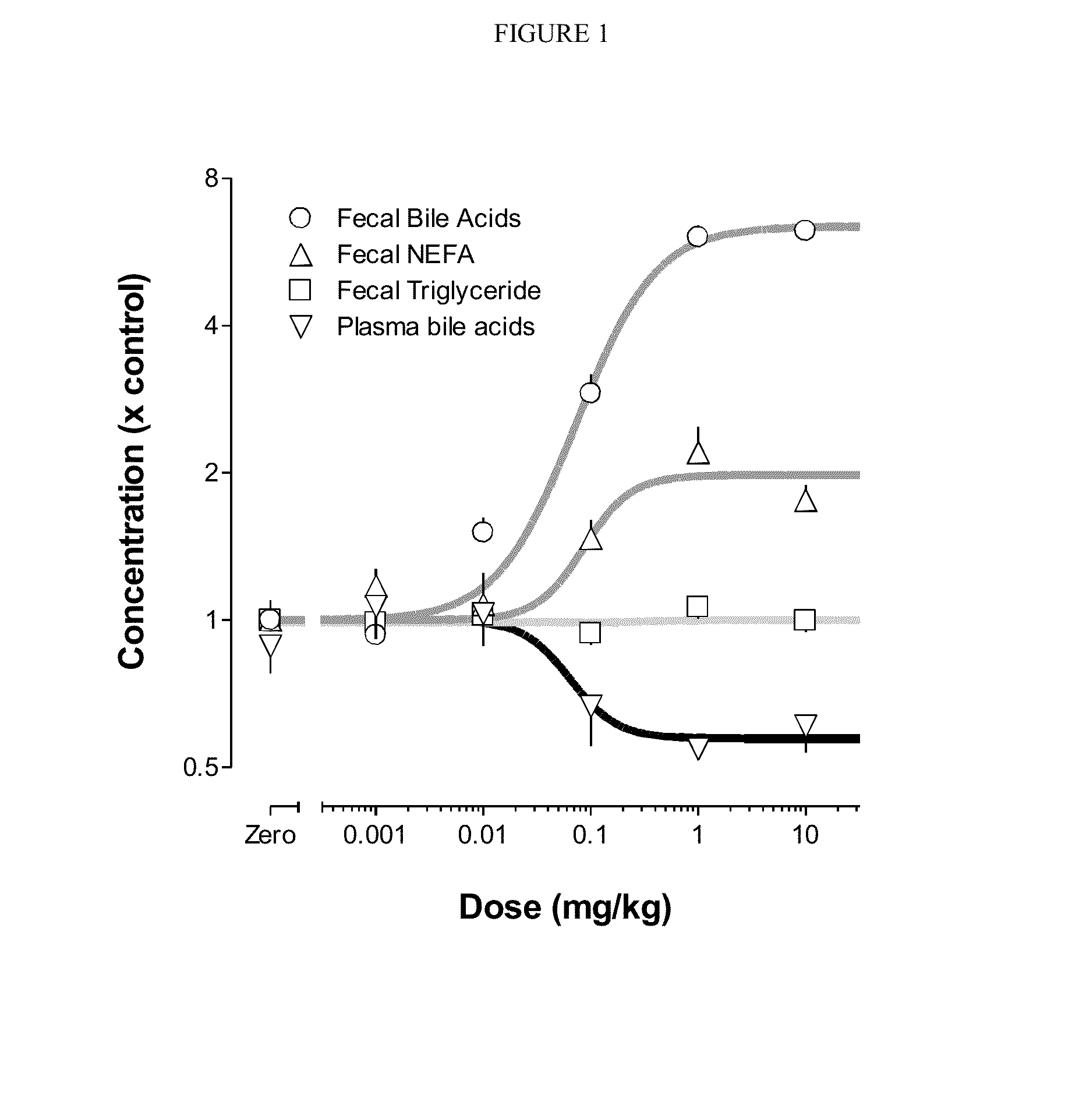
InactiveUS20070116671A1Reduce target compound levelReducing bile acid levelOrganic active ingredientsBiocideActive agentTriglyceride
The invention relates to immobilized or encapsulated enzyme and / or cells to lower bile acids and cholesterol. The invention also relates to methods of quantitatively measuring bile acids. The invention provides a composition for decreasing the amount of a target compound in the gastrointestinal tract of an animal, comprising: a) a biologically active agent which decreases the amount of the target compound; b) a retainer for retaining the biologically active agent by contacting the agent to limit movement of the agent; and c) a carrier.
Owner:CHR HANSEN AS
Valved stent for chronic venous insufficiency
InactiveUS20090254175A1Minimize turbulenceReduce molecular weightVenous valvesBlood vesselsChemical treatmentCoupling
The invention discloses a valved stent and process of manufacture for treating chronic venous insufficiency having the geometry of the supporting frame and its coupling to the membrane of a specific geometry that provides the valvular mechanism for optimal function. The membrane may comprise a decellularized pericardial tissue via chemical treatment with cholic acid or bile salts and crosslinked.
Owner:QUIJANO RODOLFO C +1
Catheter systems for delivery of agents and related method thereof
InactiveUS20050245896A1Minimize non-targeted and specious deliveryFocus agent deliveryMulti-lumen catheterEnemata/irrigatorsHuman bodyOuter Cannula
A method is disclosed for the delivery of therapeutic agents into tissues, blood vessels, and body ducts of the human body. A novel catheter enables controlled directing of emitted drug delivery to assist control of drug dwell time in targeted areas. One coaxial catheter embodiment provides capability for locating an outer lumen of the system into the target region, with localization of said outer lumen carried out by use of appropriate medical imaging modalities. In one embodiment, an inner lumen of the catheter means is primed with the agent to be delivered, and recirculated flow of the agent through pluralities of appropriately positioned port holes on the two lumens then occurs via one or more active and / or passive flow driving and guiding techniques intrinsic to the design of the coaxial catheter system. Another coaxial catheter embodiment carries and emits agent in an outer cannula and recaptures agent plus liquid or fluids in the body (such as blood, bile, serum, stable (non-flowing) liquid or kinetic liquid) in an inner cannula.
Owner:RGT UNIV OF MINNESOTA +1
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
InactiveUS20080199530A1Reduce the possibilityImprove lipophilicityPowder deliveryNervous disorderAdditive ingredientWater insoluble
Owner:COLLEGIUM PHARMA INC
Inactivated lactobacillus micro-ecological preparation and preparation method thereof
ActiveCN101612169AInhibition is effectiveEfficient killingBacteria material medical ingredientsAnimal feeding stuffMetaboliteLactase
The invention discloses an inactivated lactobacillus micro-ecological preparation and a preparation method thereof. The preparation comprises the following components in portion by mass: 10 to 60 portions of inactivated lactobacillus cell and lactobacillus fermentation product, and 30 to 50 portions of excipient, wherein the inactivated lactobacillus cell accounts for 10<8> to 10<9> cells per gram. The inactivated lactobacillus cell resists high temperature, gastric acid and bile, and has synergistic action with antibiotics; in addition, metabolic products produced in a lactobacillus fermentation process such as lactic acid, like bacteriocin and the like can effectively inhibit or kill enteric pathogenic bacteria of people or animals; and enzymes such as amylase, lactase and the like are helpful for digestion of the animals. The inactivated lactobacillus micro-ecological preparation effectively and economically solves the problem puzzling the prior viable bacteria micro-ecological preparation.
Owner:BIOFORTE BIOTECHNOLOGY (SHENZHEN) CO LTD
Preparation of aqueous clear solution dosage forms with bile acids
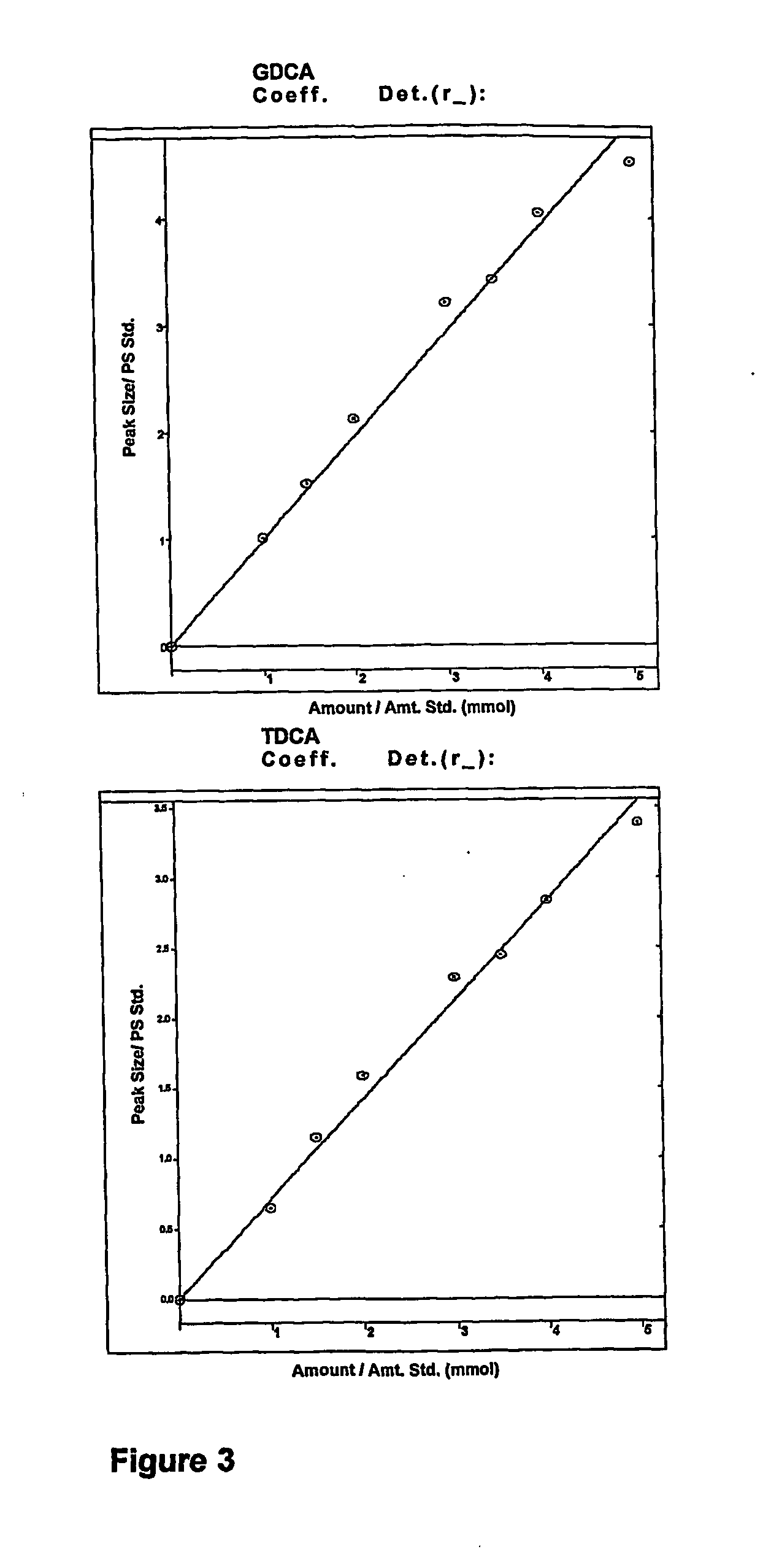
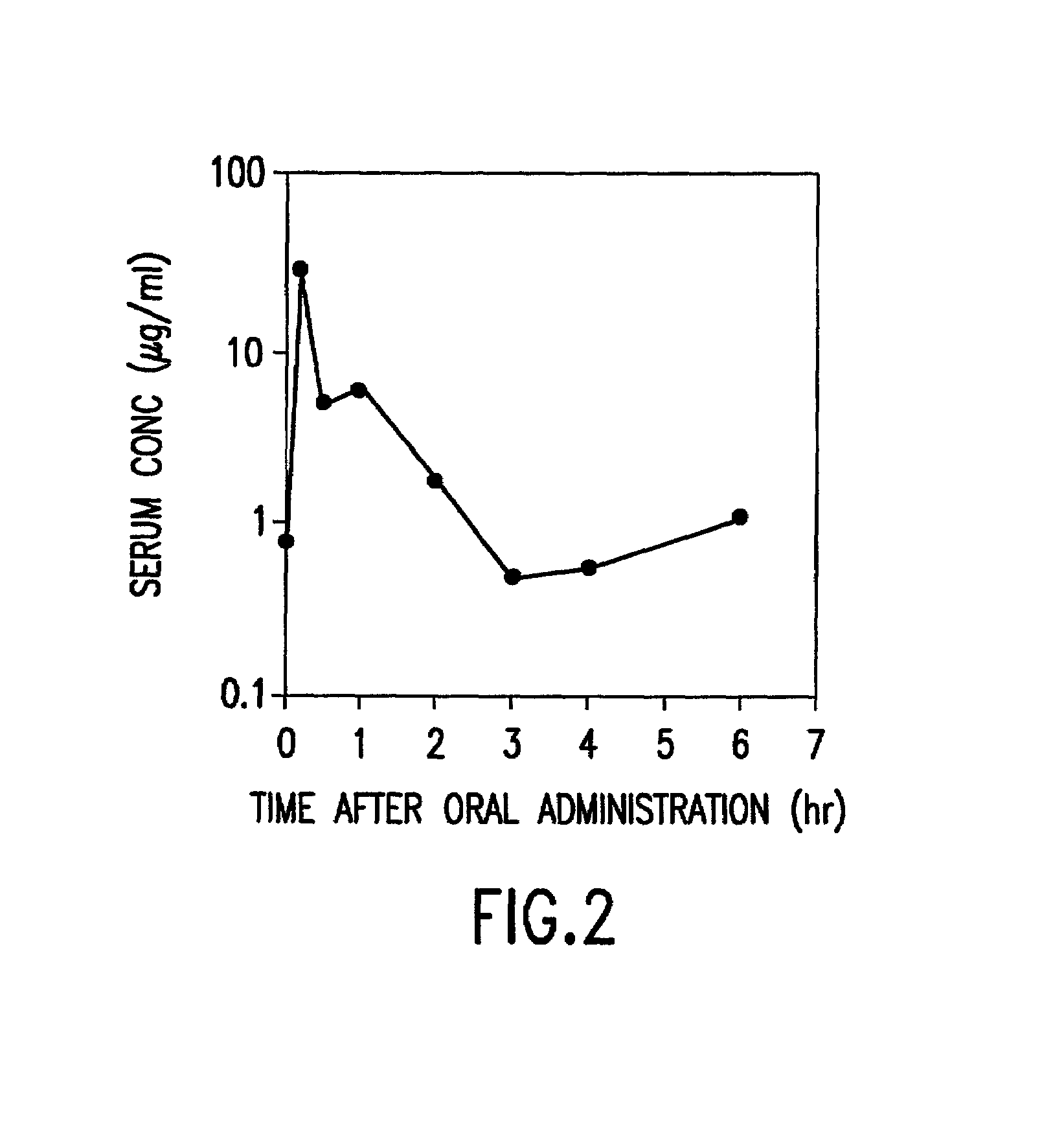
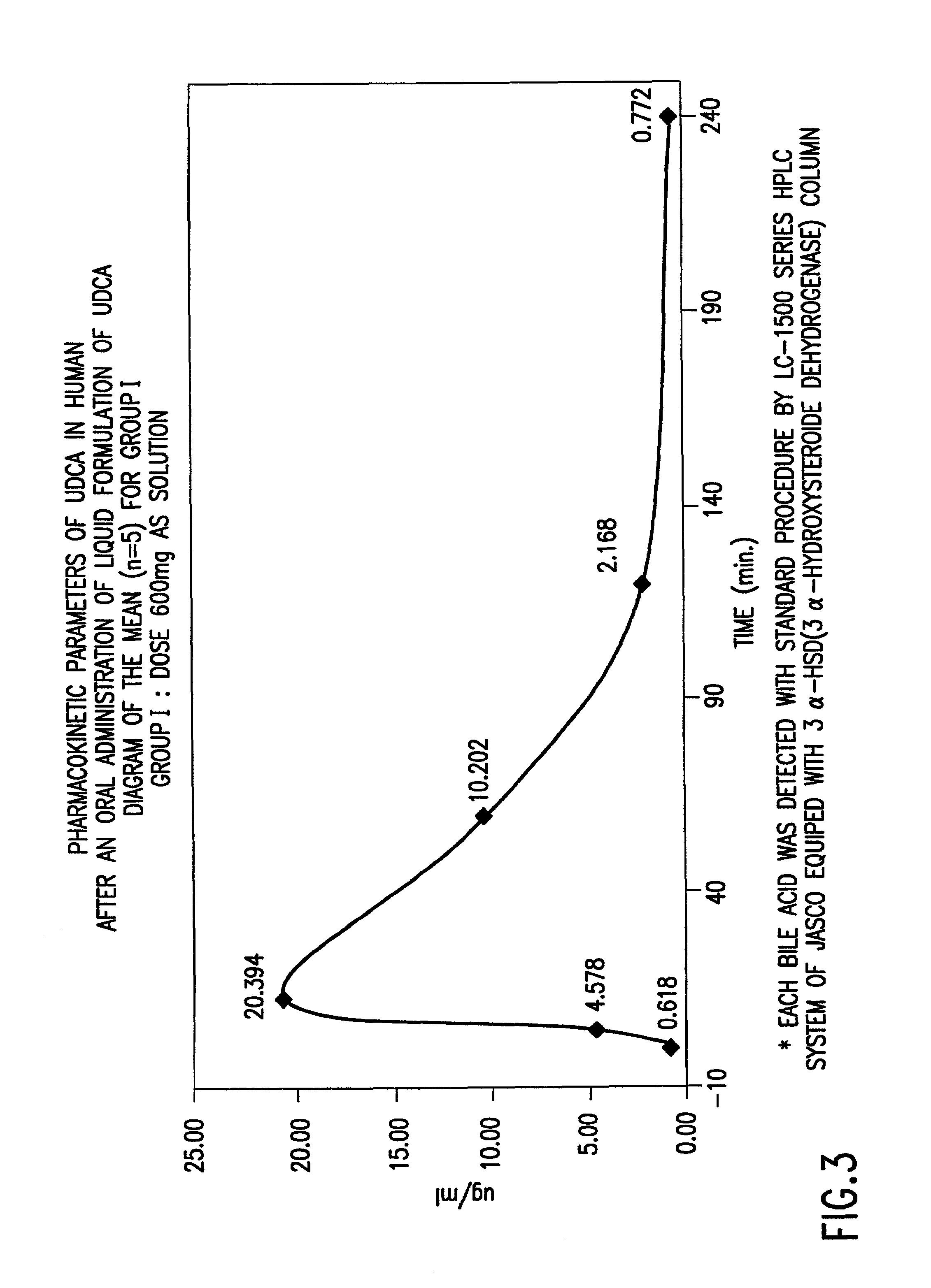
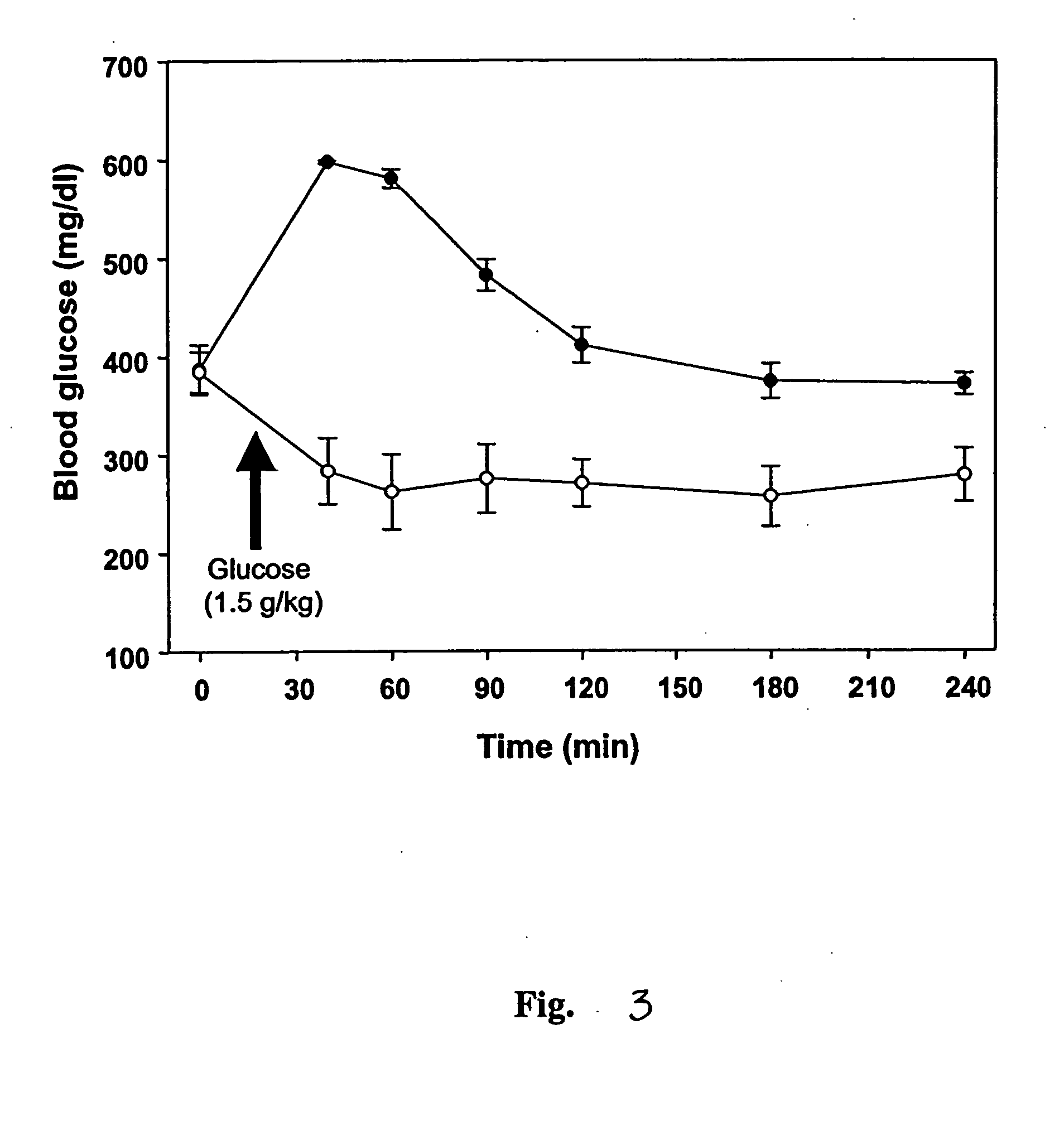
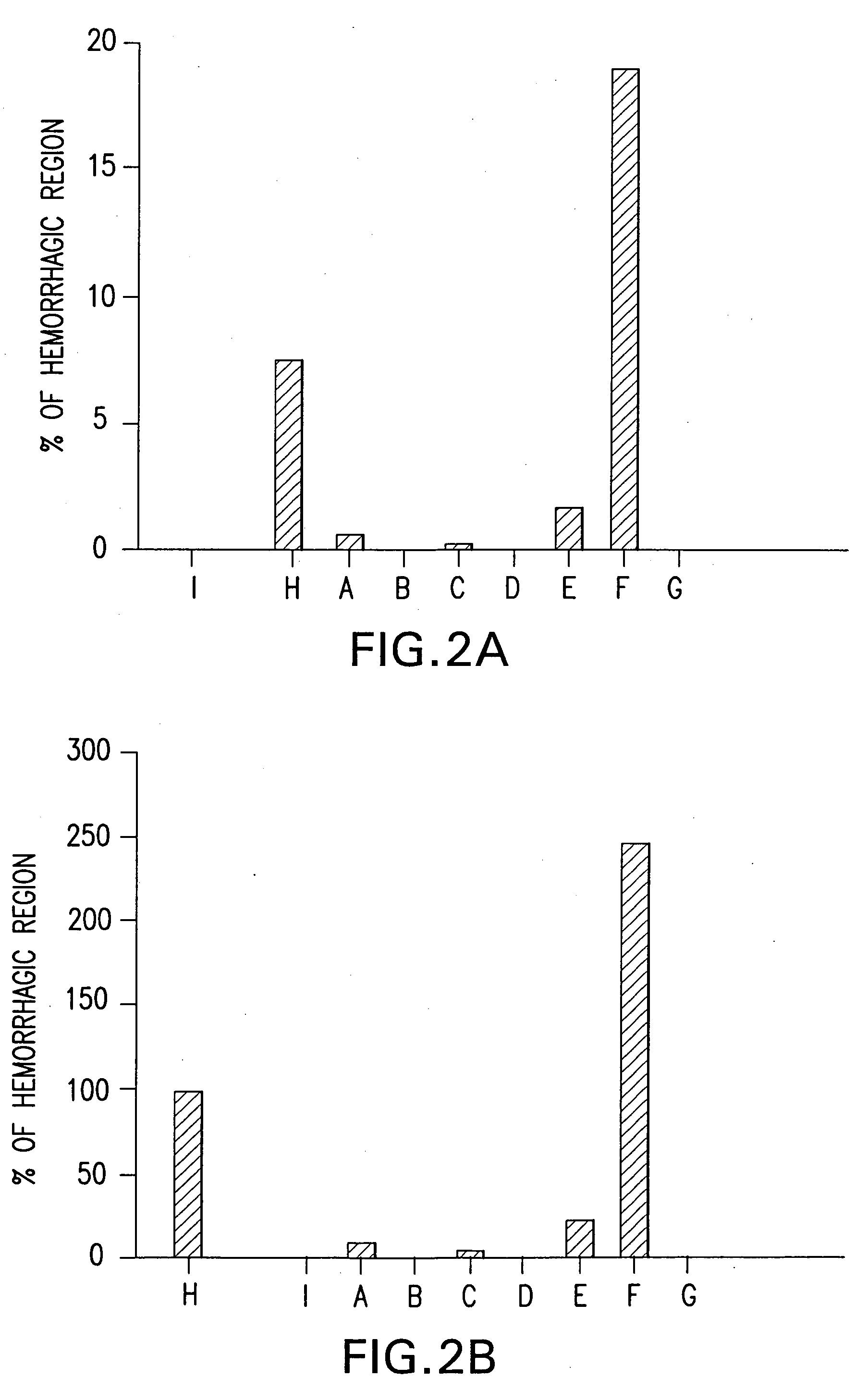
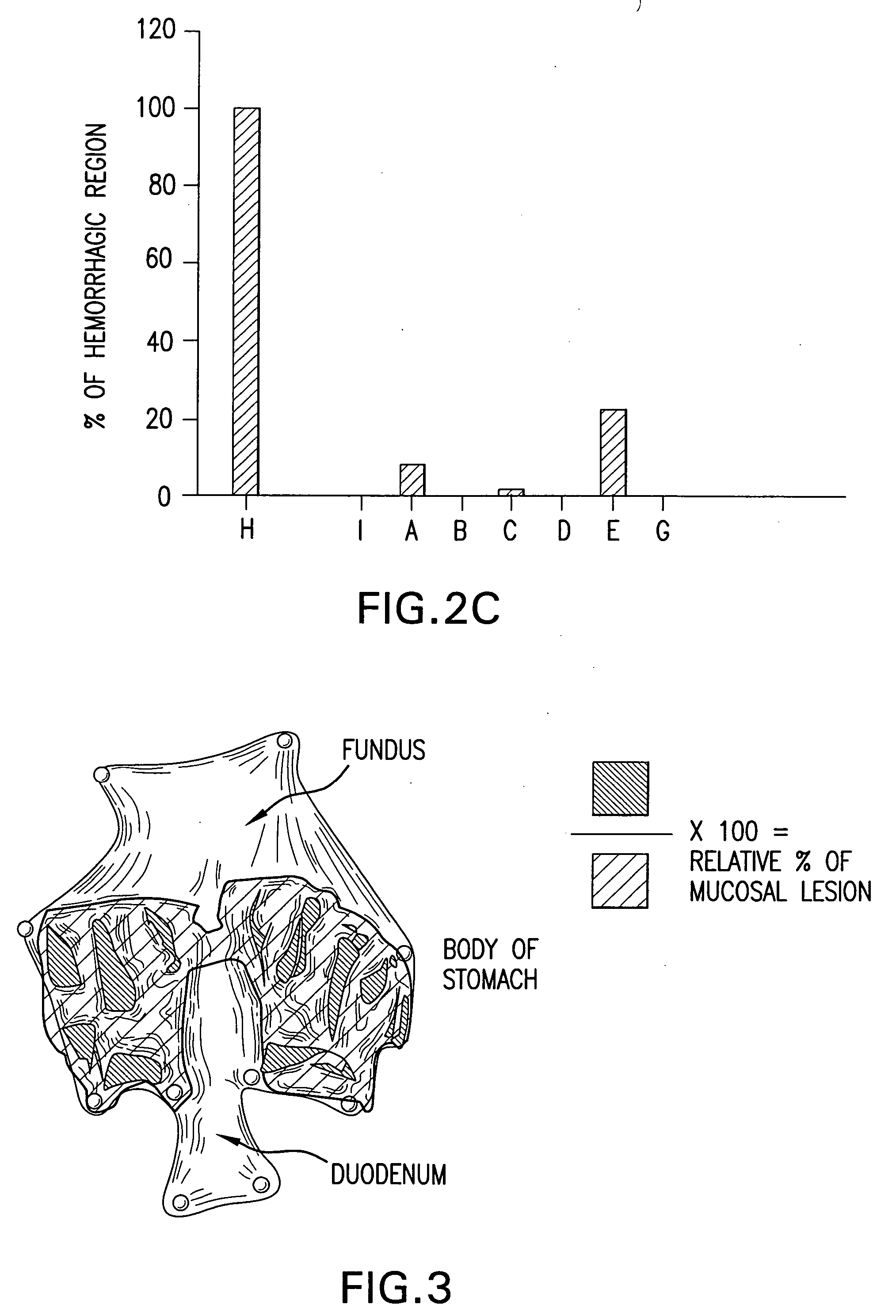
InactiveUS7303768B2Improve efficacyAlter absorptionOrganic active ingredientsHeavy metal active ingredientsBile fluidBile acid
Compositions for pharmaceutical and other uses comprising clear aqueous solutions of bile acids which do not form any detectable precipitates over selected ranges of pH values of the aqueous solution and methods of making such solutions. The compositions of the invention comprise water; a bile acid in the form of a bile acid, bile acid salt, or a bile acid conjugated with an amine by an amide linkage; and either or both an aqueous soluble starch conversion product and an aqueous soluble non-starch polysaccharide. The composition remains in solution without forming a precipitate over a range of pH values and, according to one embodiment, remains in solution for all pH values obtainable in an aqueous system. The composition, according to some embodiments, may further contain a pharmaceutical compound in a pharmaceutically effective amount. Non-limiting examples of pharmaceutical compounds include insulin, heparin, bismuth compounds, amantadine and rimantadine.
Owner:YOO SEO HONG
Traditional Chinese medicine for treating cardiac, cerebral and vascular diseases and preparation method thereof
InactiveCN101690764ARestore elasticityPrevent agglutinationHeavy metal active ingredientsAnthropod material medical ingredientsOysterCoronary heart disease
The invention relates to a traditional Chinese medicine for treating cardiac, cerebral and vascular diseases; the drug is composed of forty kinds of crude drugs by weight parts: milk veteh, salvia miltiorrhiza, root of common peony, ligusticum wallichii, lumbricus, sanguisuga, rhizoma acori graminei, polygala root, red ocher, oyster, angelica, the root of kudzu vine, arisaema cum bile and the like; the preparation method is that: (1) the crude drugs are prepared into water extract liquid; (2) the water extract liquid is prepared into oral liquid; (3)the water extract liquid obtained by the step (1) is dried, crushed, sieved, undersize material is extracted powder; (4) the extracted powder prepared by the step (3) is prepared into various dosage forms. The preparation method of crude powder is that: lime earth of the crude drugs is sieved, and then the crude drugs are crushed and sieved, the undersize material is taken out and is sterilized to be crude powder, and the crude powder is prepared into various dosage forms. The traditional Chinese medicine has the major functions of treating miocardial infarction and angina caused by coronary heart disease, high blood pressure and cerebral thrombosis, hemiplegia, cerebral infarction, cerebrovascular accident, sequel of apoplexy and other symptoms, and the medicine has obvious effect on headache, dizziness and heart cerebrovascular diseases caused by high blood pressure and cerebral circulation insufficiency.
Owner:卢速江
Delivery Agents for enhancing mucosal absorption of therapeutic agents
ActiveUS20050260237A1Reduce decreaseEnhanced interactionOrganic active ingredientsAntimycoticsSterolDipeptide
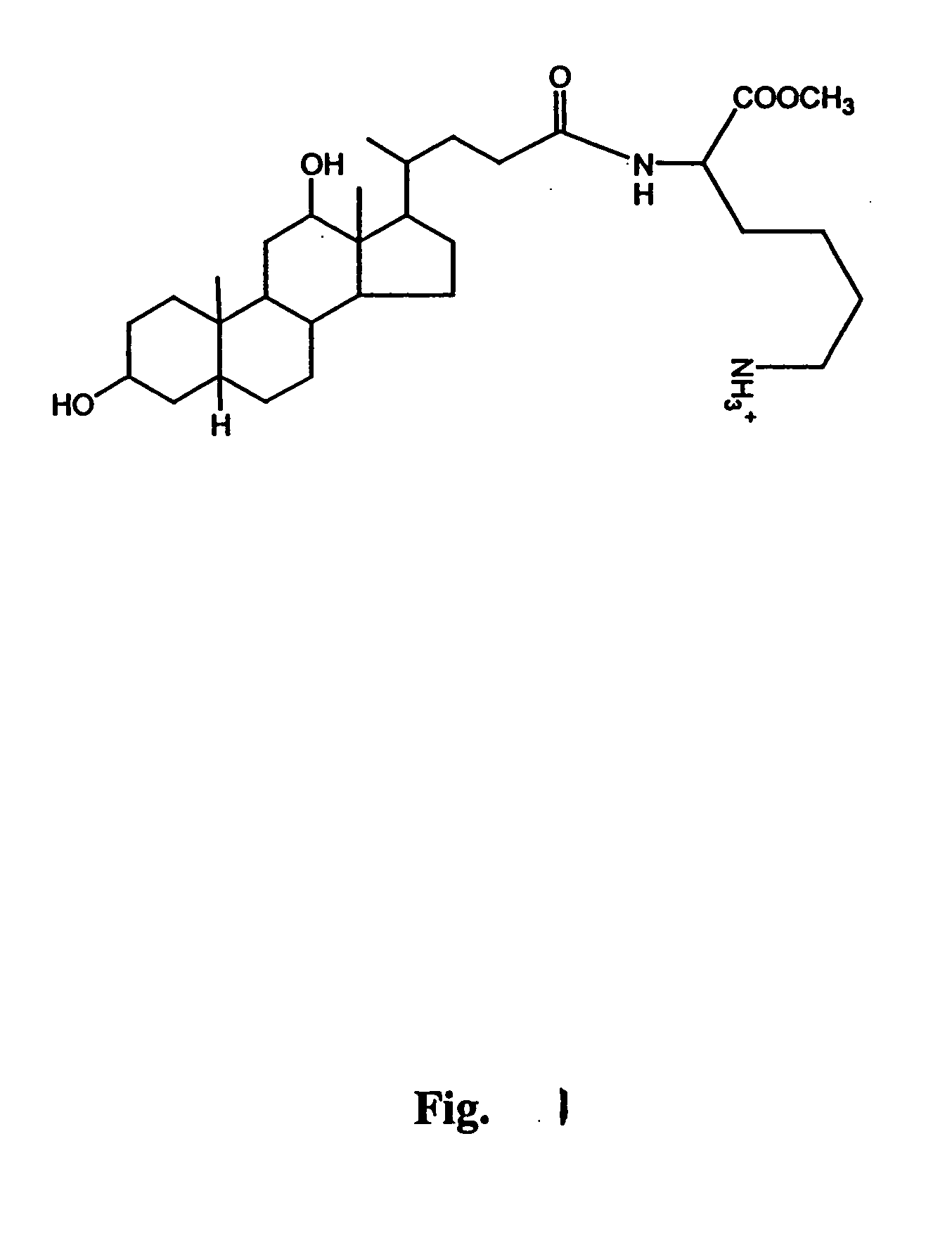
A delivery agent for delivering a biologically active agent to a warm-blooded animal includes a hydrophobic moiety covalently bonded to a hydrophilic moiety. The hydrophobic moiety can include bile acids, sterols, or hydrophobic small molecules. The hydrophilic moiety can include α-amino acids, dipeptides or tripeptides, or hydrophilic small molecules. An illustrative delivery agent is Nα-deoxycholyl-L-lysine-methylester. The delivery agent and the biologically active agent are mixed together to form a complex, which is then administered to the animal. These complexes are particularly useful for oral administration of biologically active agents, but other routes of administration may be used.
Owner:MEDIPLEX CORP
Acidproof and bile-salt-resisting rhamnose lactobacillus strain with anti-enterovirus and antioxidant functions
ActiveCN1982437AImprove adhesionResistantBacteriaBacteria material medical ingredientsEnterovirusMicroorganism
Lactobacillus rhamnosus and the use for it and its metabolin are disclosed. The strain is sieved and obtained from excrement of long-lived old. It's safe, has excellent antibacterial, acid-resisting and antioxidant performances. It can be used in dairy process and functional food production.
Owner:PRESIDENT ENTERPRISES (CHINA) INVESTMENT CO LTD +1
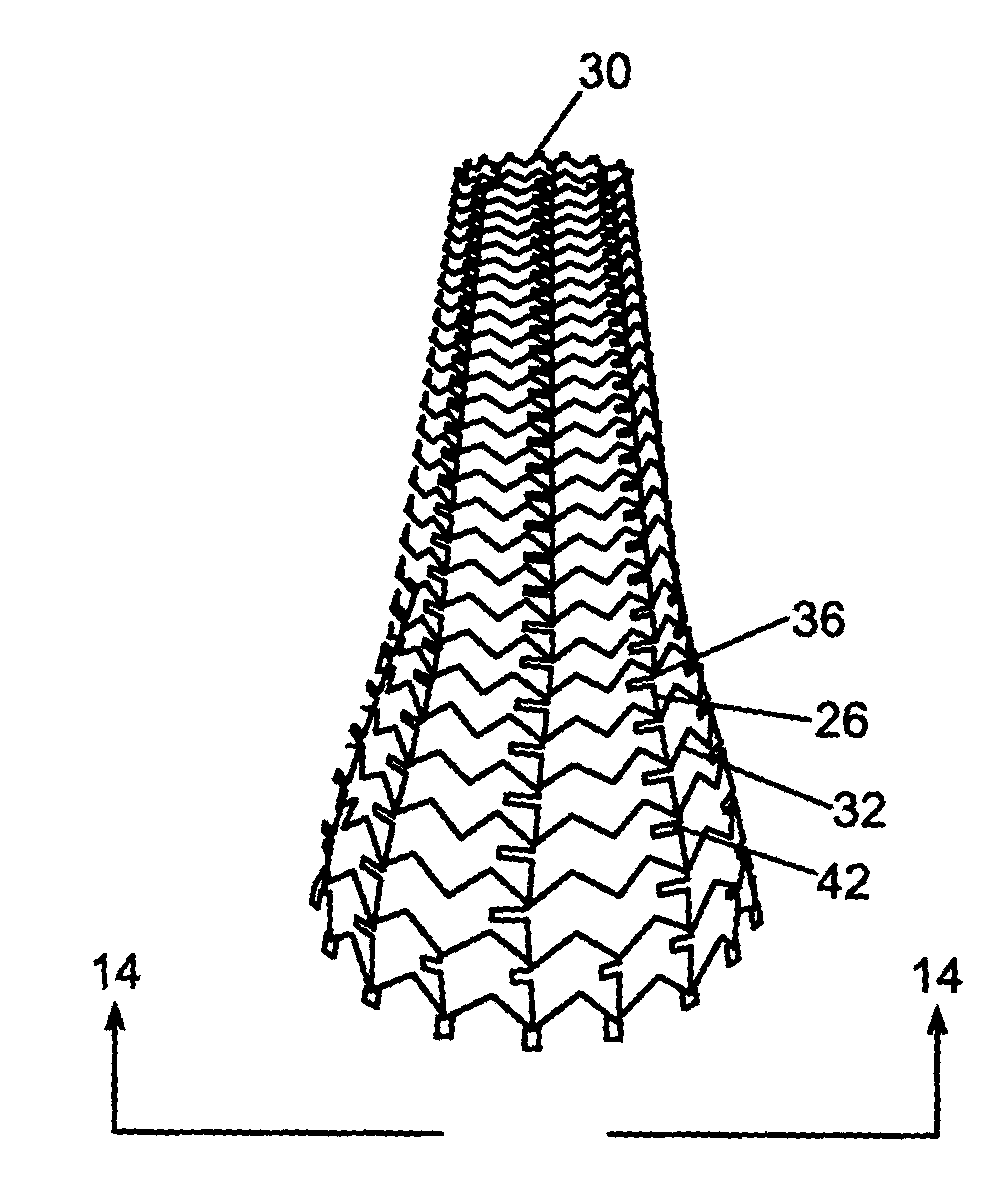
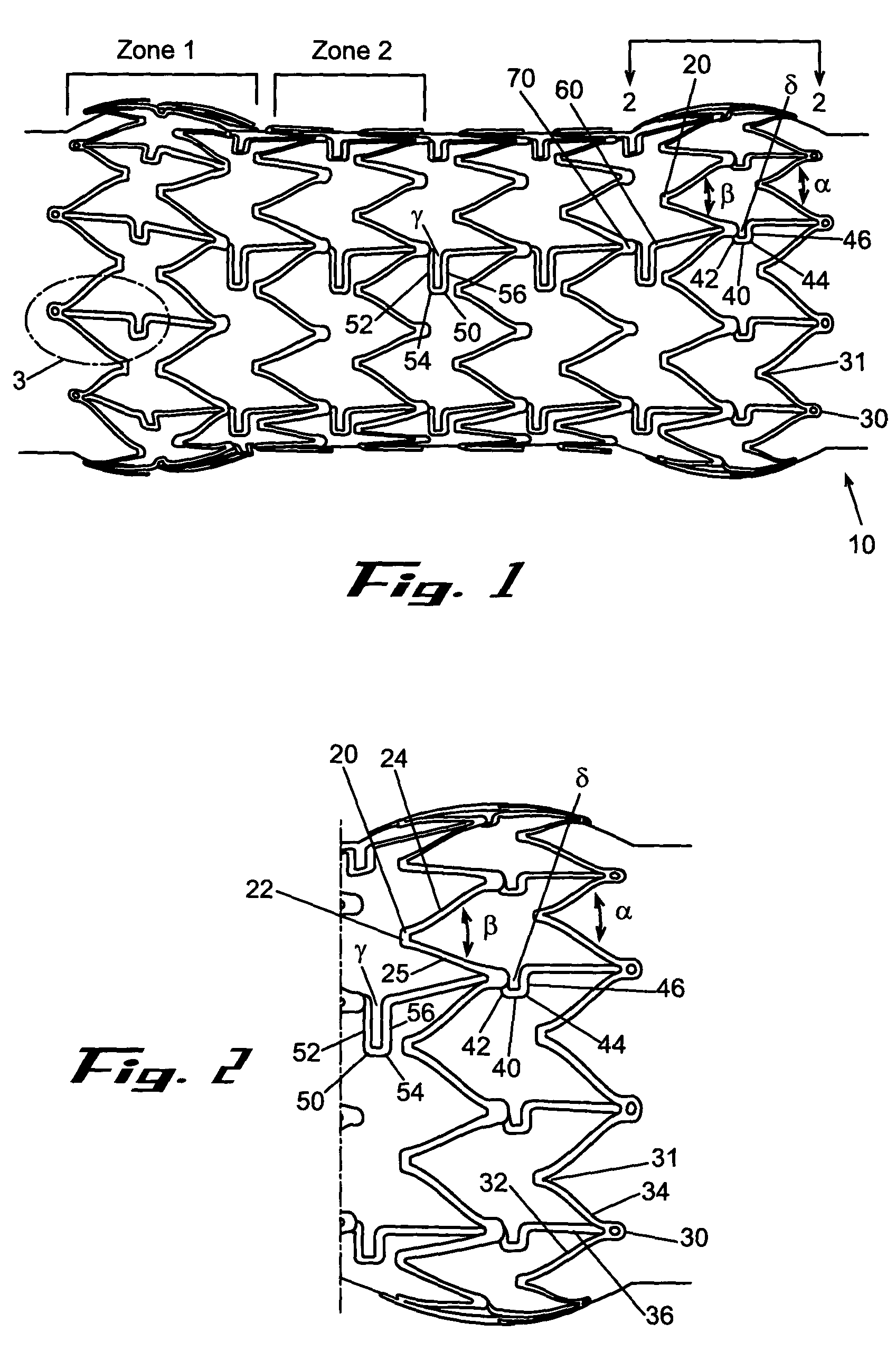
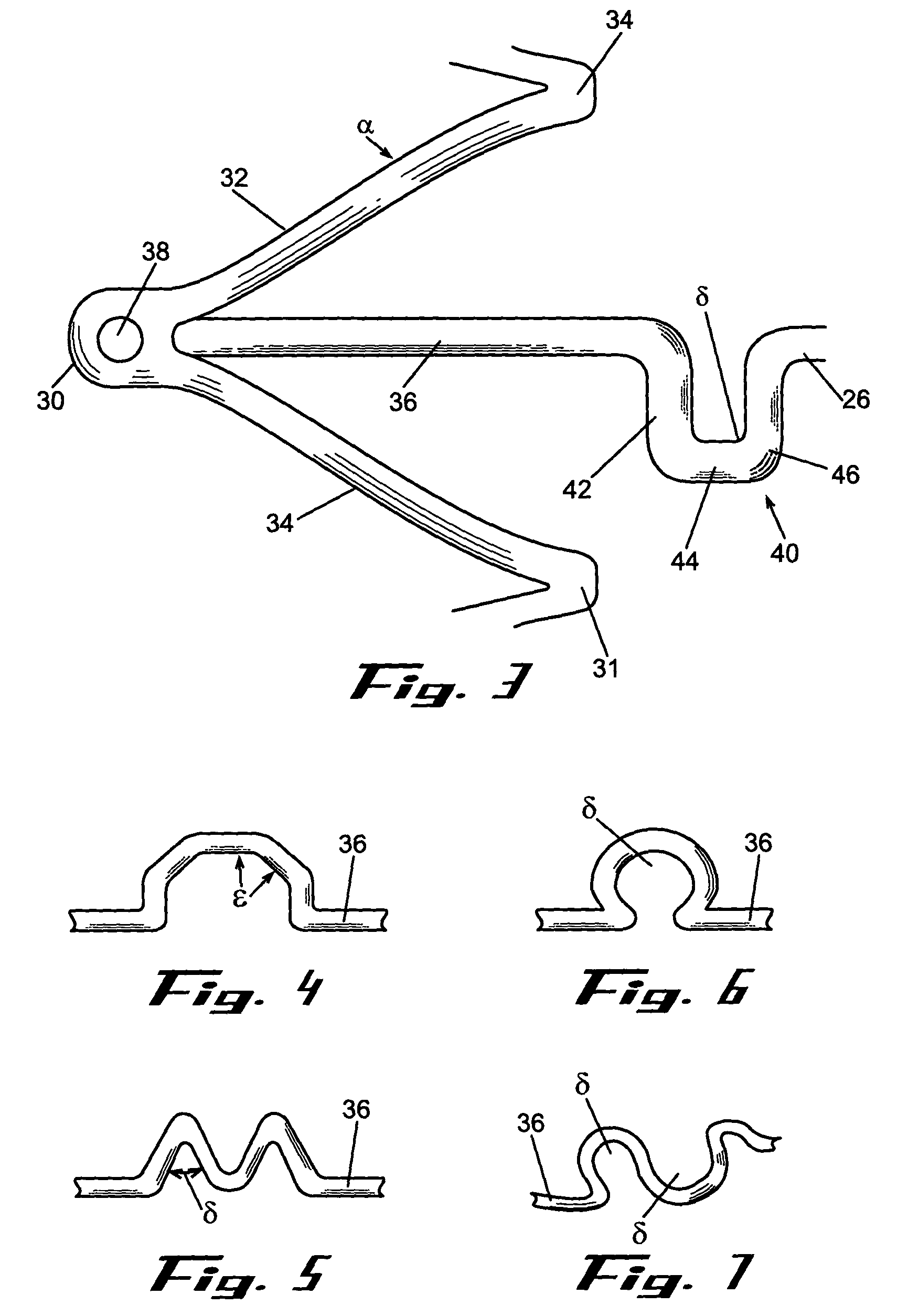
Removable biliary stent
The present invention, in an exemplary embodiment, provides a stent, which combines many of the excellent characteristics of both silicone and metal stents while eliminating the undesirable ones. In particular, a principal objective in accordance with the present invention is to provide a family of stents generally and a biliary stent in particular where the relative hardness / softness of regions of the stent can differ from other regions of the stent to provide additional patient comfort and resistance to radial forces. An exemplary embodiment also provides a family of stents with novel interstice configurations that facilitate flexibility, durability and / or proper installation. Additionally, the biliary stent in accordance with the present invention provides enhanced flow mechanics to ensure the adequate clearance of viscid fluids such as bile.
Owner:MERIT MEDICAL SYST INC
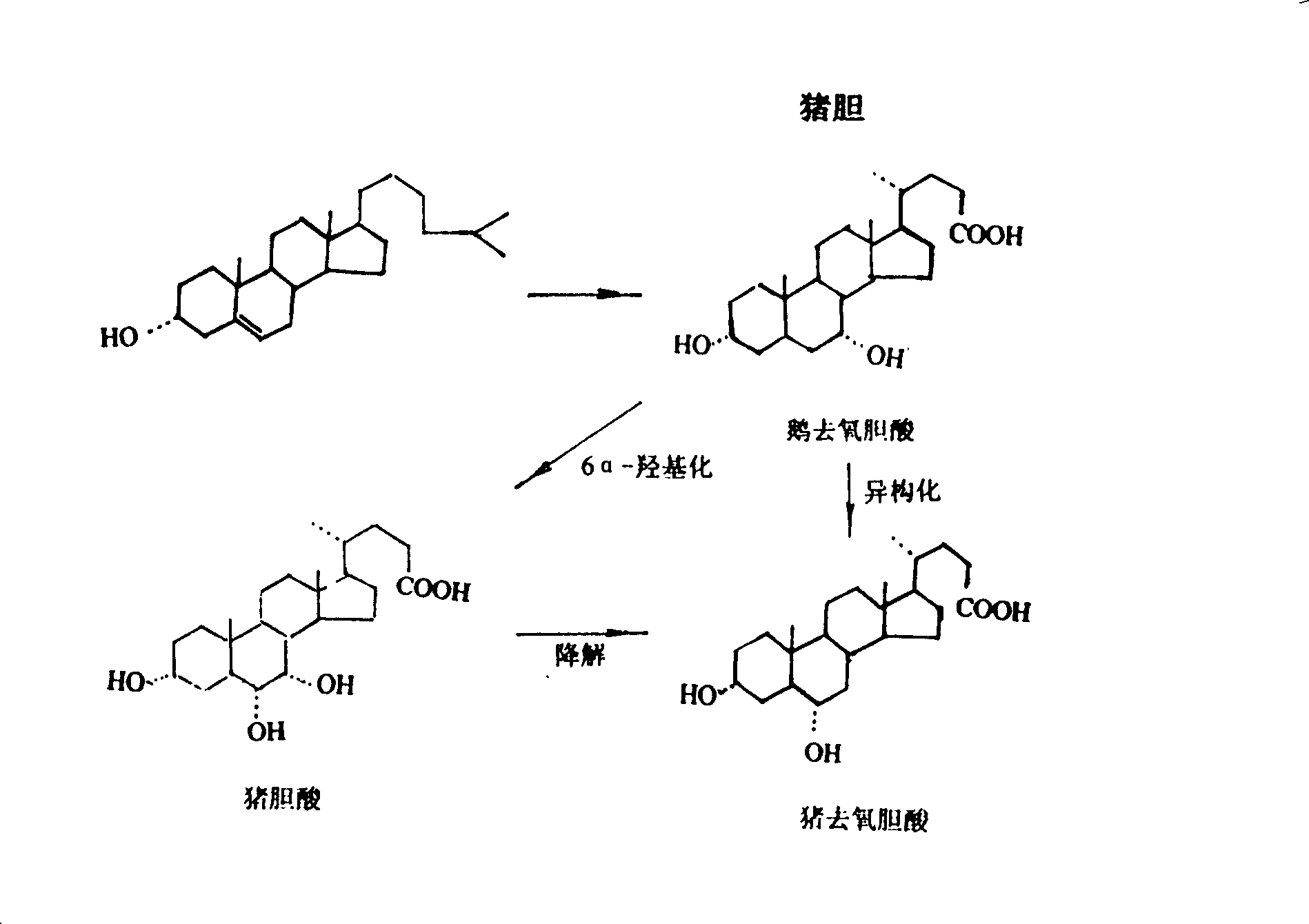
Method for producing ursodeoxycholic acid by using swine bladder as raw material
The invention provides a process of separating anthrodsoxycholic acid by using fresh swine bile as raw material, the invention further provides a process for preparing ursodeoxycholic acid by using fresh swine bile as raw material, which comprises extracting conjugated bile acid from swine bile after acidification, removing foreign materials such as aliphatic acid and protein and the like, preparing purified pig bile acid through hydrolysis (saponification) and hydroperoxide decolourization, extracting anthrodsoxycholic acid through solution immersion cleaning, preparing ursodeoxycholic acid through oxidization and reduction of anthrodsoxycholic acid which is obtained through separating. The process of the invention improves the preparation technology of ursodeoxycholic acid, and achieves positive effects on the aspects of technology simplification and output increase.
Owner:苏州天绿生物制药有限公司
Synthetic bile acid compositions and methods
Owner:ALLERGAN SALES LLC
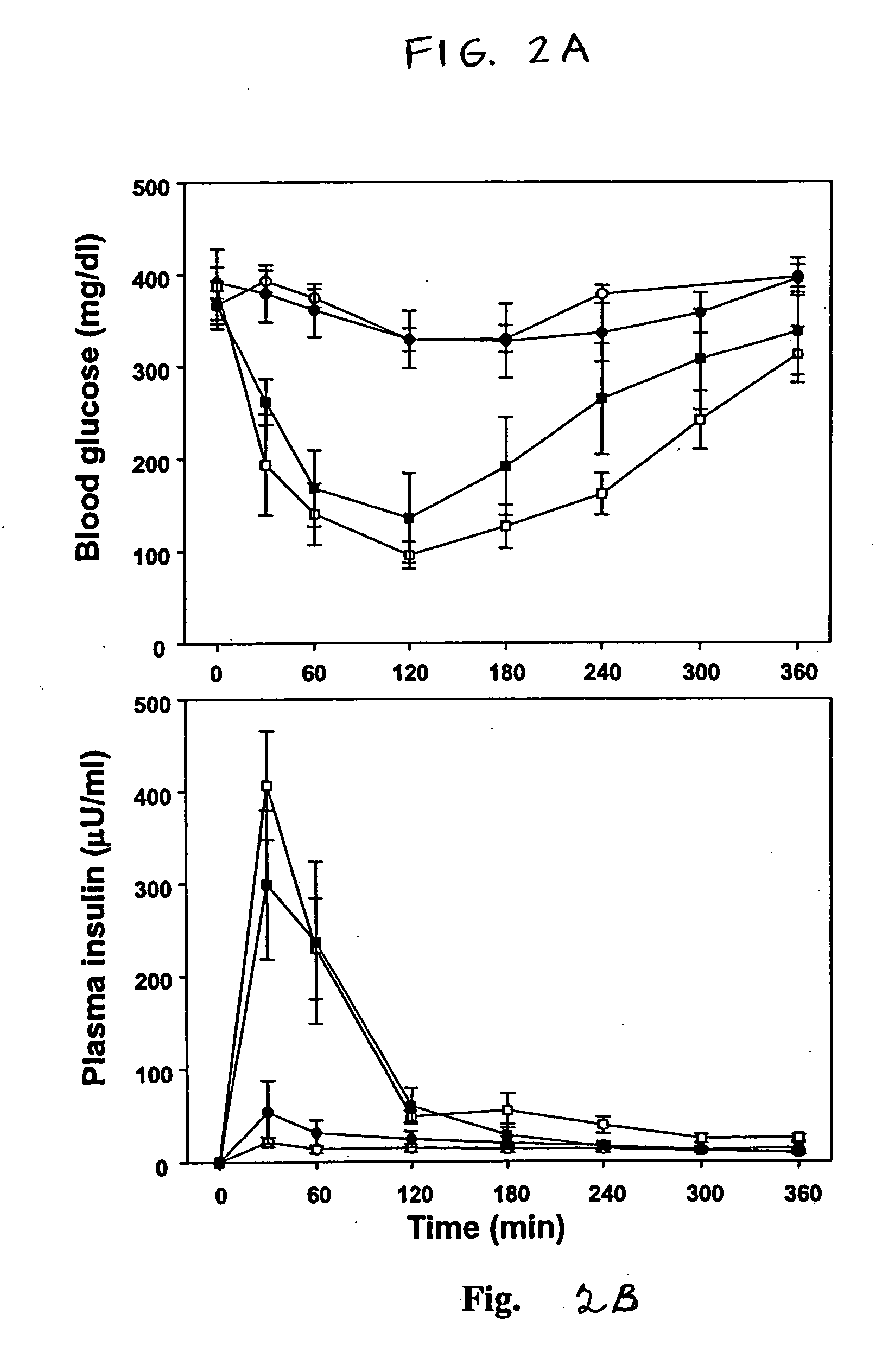
Induction of the formation of insulin-producing cells via gene transfer of pancreatic beta-cell-associated transcriptional factor
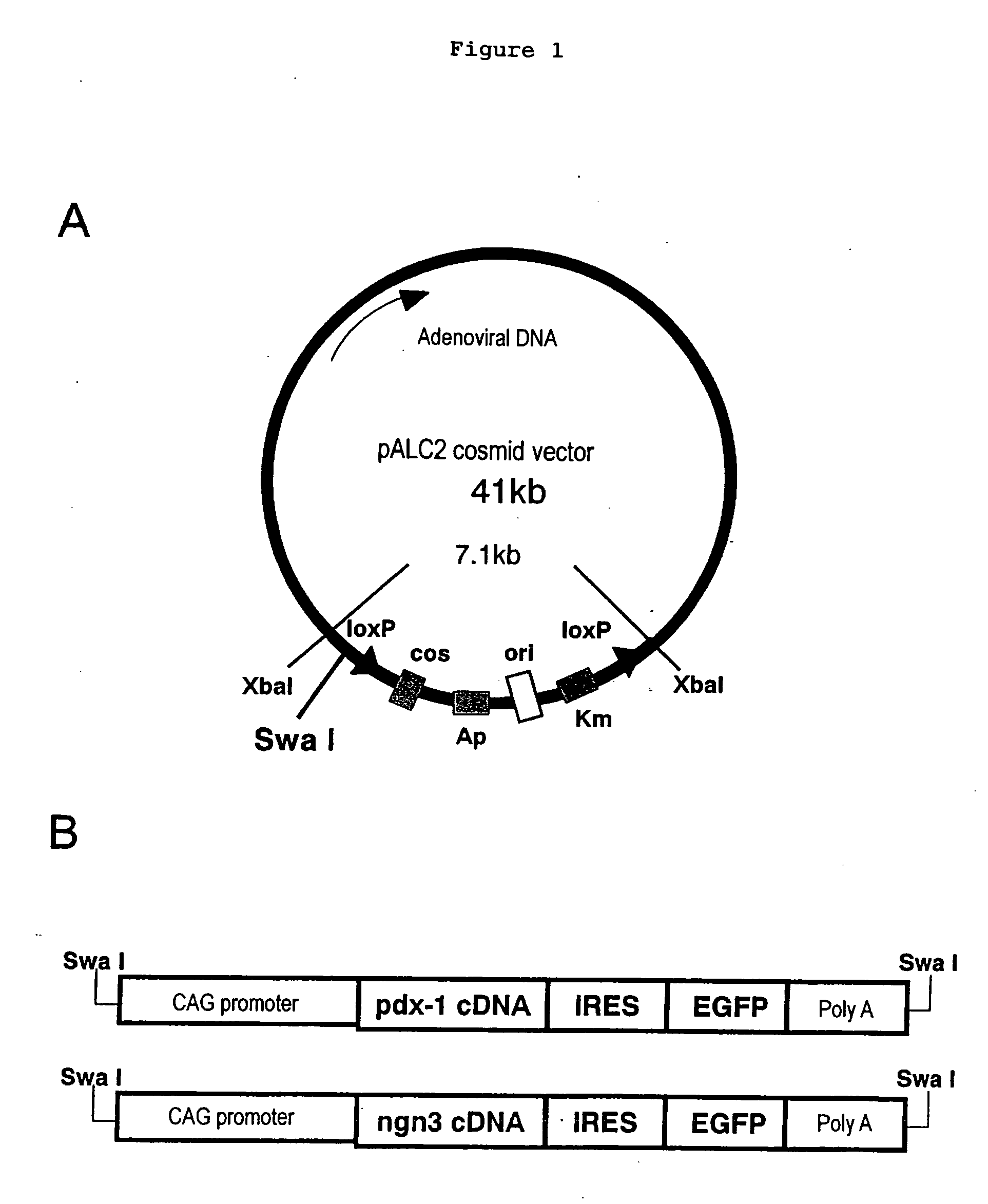
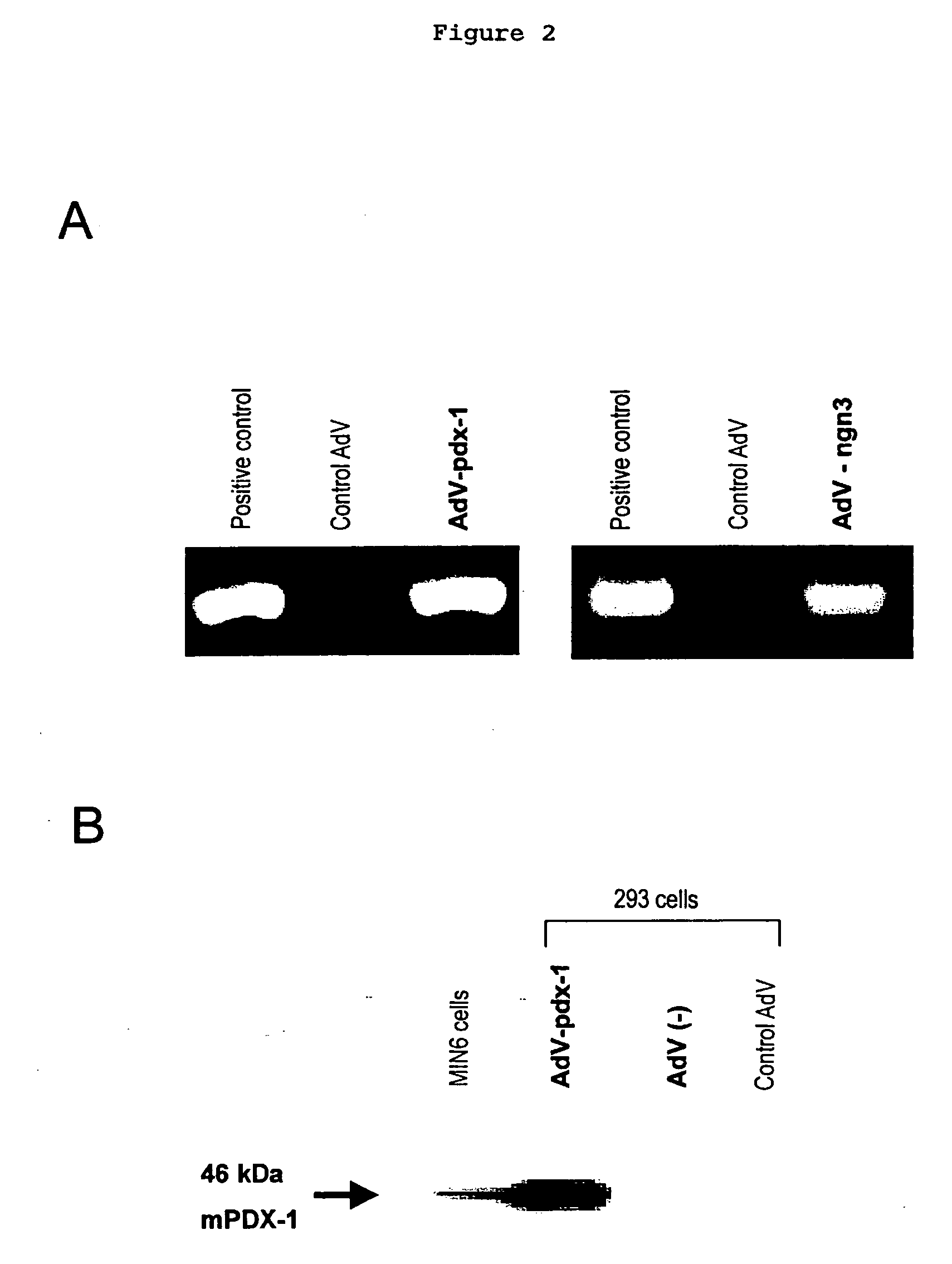
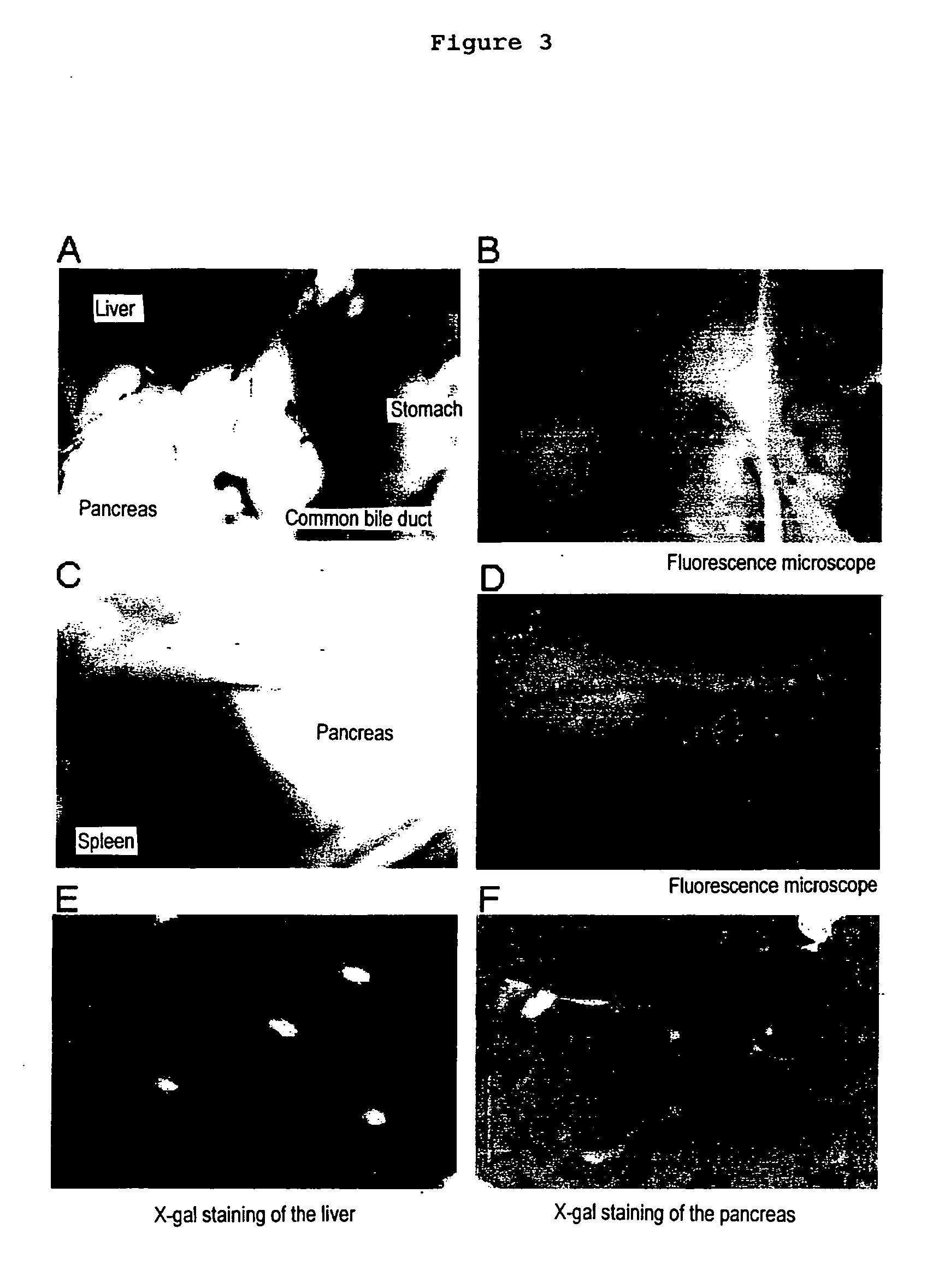
The present invention provides a method of inducing the formation of insulin-producing cells which comprises transferring a pancreatic β-cell associated transcriptional factor gene into the pancreas to induce the formation of insulin-producing cells. The pancreatic β-cell associated transcriptional factor gene is transferred into the pancreatic tissue stem cells by the ICBD injection without ligating the common bile ducts and thus the formation of insulin-producing cells is induced. In the present invention, pdx-1, neurogenin3, etc. are used as such pancreatic β-cell associated transcriptional factor gene and an adenoviral vector with the use of the Cre-loxP recombination system, etc. is used as a vector for transferring the pancreatic β-cell associated transcriptional factor gene into the pancreas. The method of the invention enables regeneration therapy for diabetes mellitus by inducing the formation of insulin-producing cells.
Owner:JAPAN SCI & TECH CORP
Bile preparations for gastrointestinal disorders
InactiveUS20060188530A1Impair its compositionHigh strengthBiocideDispersion deliveryDiseaseDrug compound
The present disclosure relates to methods and compositions to offset, ameliorate and / or alleviate one or more unwanted and / or adverse gastrointestinal effects. For example, in some embodiments, the present disclosure relates to compositions that include a bile acid, a carbohydrate and / or a pharmaceutical compound, wherein the pharmaceutical is associated with an adverse gastrointestinal effect in a subject (e.g., mammal or human). Non-limiting examples of pharmaceutical compounds may include a nonsteroidal anti-inflammatory drug, a gastric irritating drug (e.g., an antibiotic, an adrenal cortocoid steroid and an anti-cancer drug) and combinations thereof. The disclosure further relates to methods of ameliorating or eliminating at least one adverse gastrointestinal effects of a composition, comprising administering to a subject an aqueous solution comprising a bile acid and a carbohydrate.
Owner:YOO SEO HONG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com