Abuse-deterrent pharmaceutical compositions of opioids and other drugs
a technology of opioids and compositions, applied in the field of pharmaceutical compositions, can solve the problems of drug not being easily extracted from such a formulation, the formulation will require more than one step, and the potential for abus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Lipophilic Oxycodone Derivatives
[0071]A. Oxycodone Free Base
[0072]The free base of oxycodone was prepared from its hydrochloride salt by the following method: Oxycodone hydrochloride was dissolved in water and sodium carbonate was added in the amount required to neutralize hydrochloric acid. Methylene chloride was added in order to extract the formed oxycodone free base. The obtained organic layer was dried over sodium sulfate and methylene chloride was evaporated using rotary evaporator. The obtained oxycodone free base was purified by crystallization.
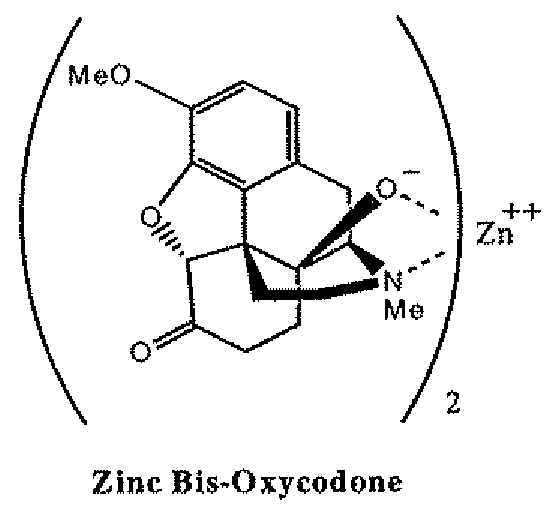
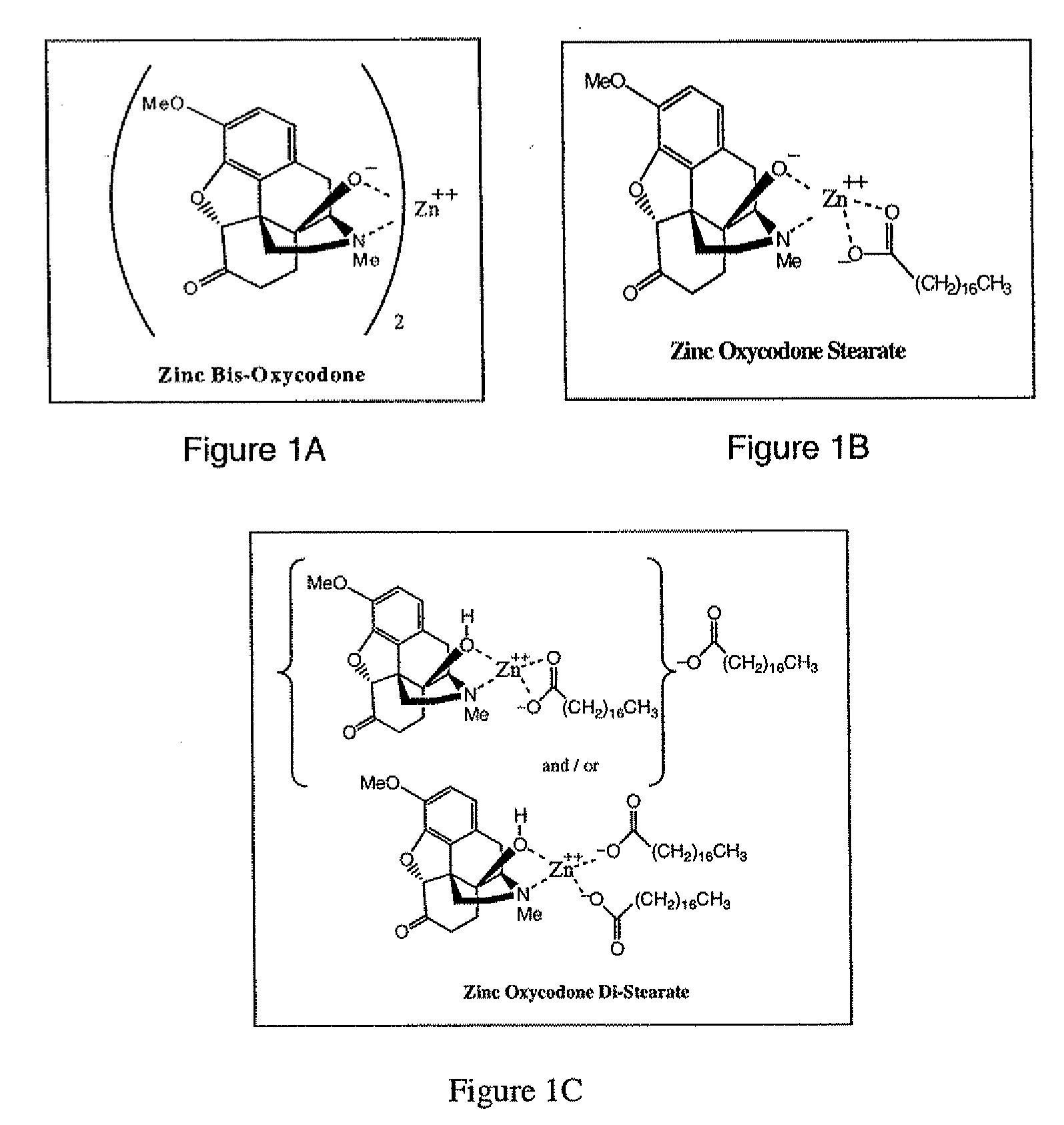
[0073]B. Zinc-Bis-Oxycodone
[0074]Zinc bis-oxycodone can be obtained in anhydrous media by reacting oxycodone free base with Zn(Et)2
[0075]C. Zinc Oxycodone Stearate
[0076]Zinc oxycodone stearate can be obtained in anhydrous media by reacting oxycodone free base with Zn(Et)(C18H35O2)
[0077]D. Zinc Oxycodone Di-Stearate
[0078]Zinc-oxycodone di-stearate can be obtained by co-melting Zn(C18H35O2)2 and oxycodone free base.
[0079...
example 2
Preparation of Drug Containing Microparticles
[0082]The free base, salts or complexes from Example 1 are added to molten hydrogenated vegetable oil, mixed, extruded and spheronized to form drug containing microparticles.
example 3
Preparation of Coated Drug Containing Microparticles
[0083]The drug-containing particles from Example 2 are spray coated with zein in a fluidized bed apparatus.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| lipophilic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com