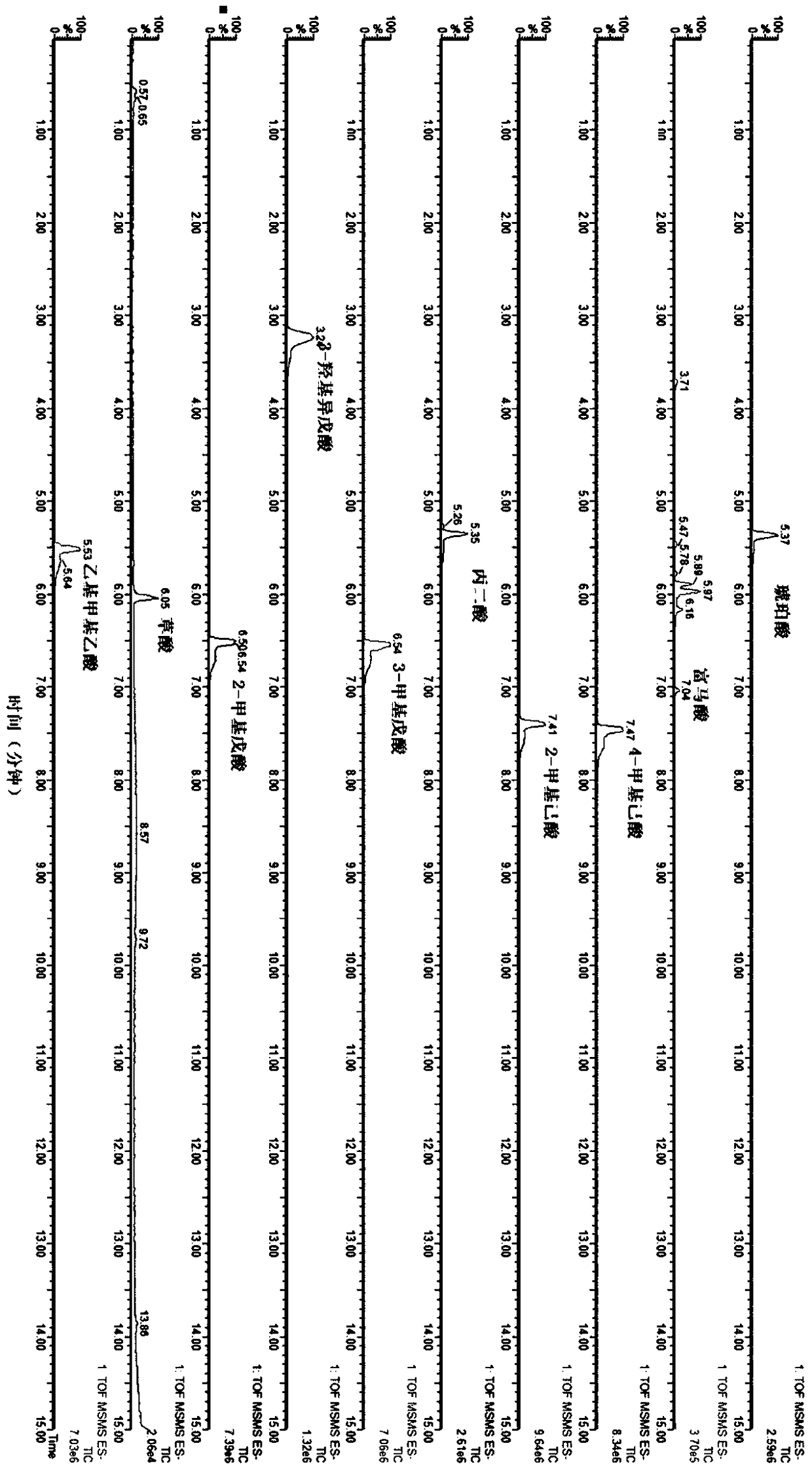
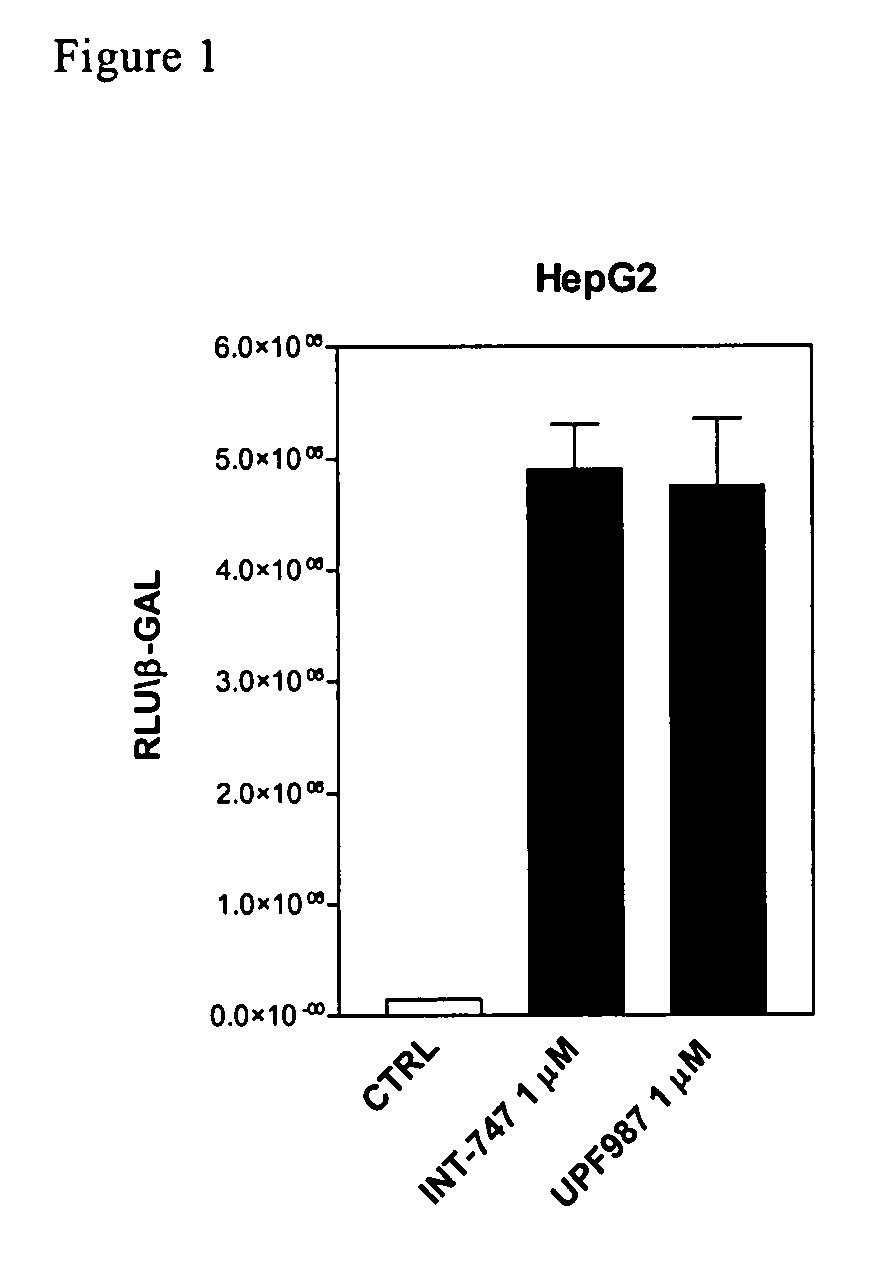
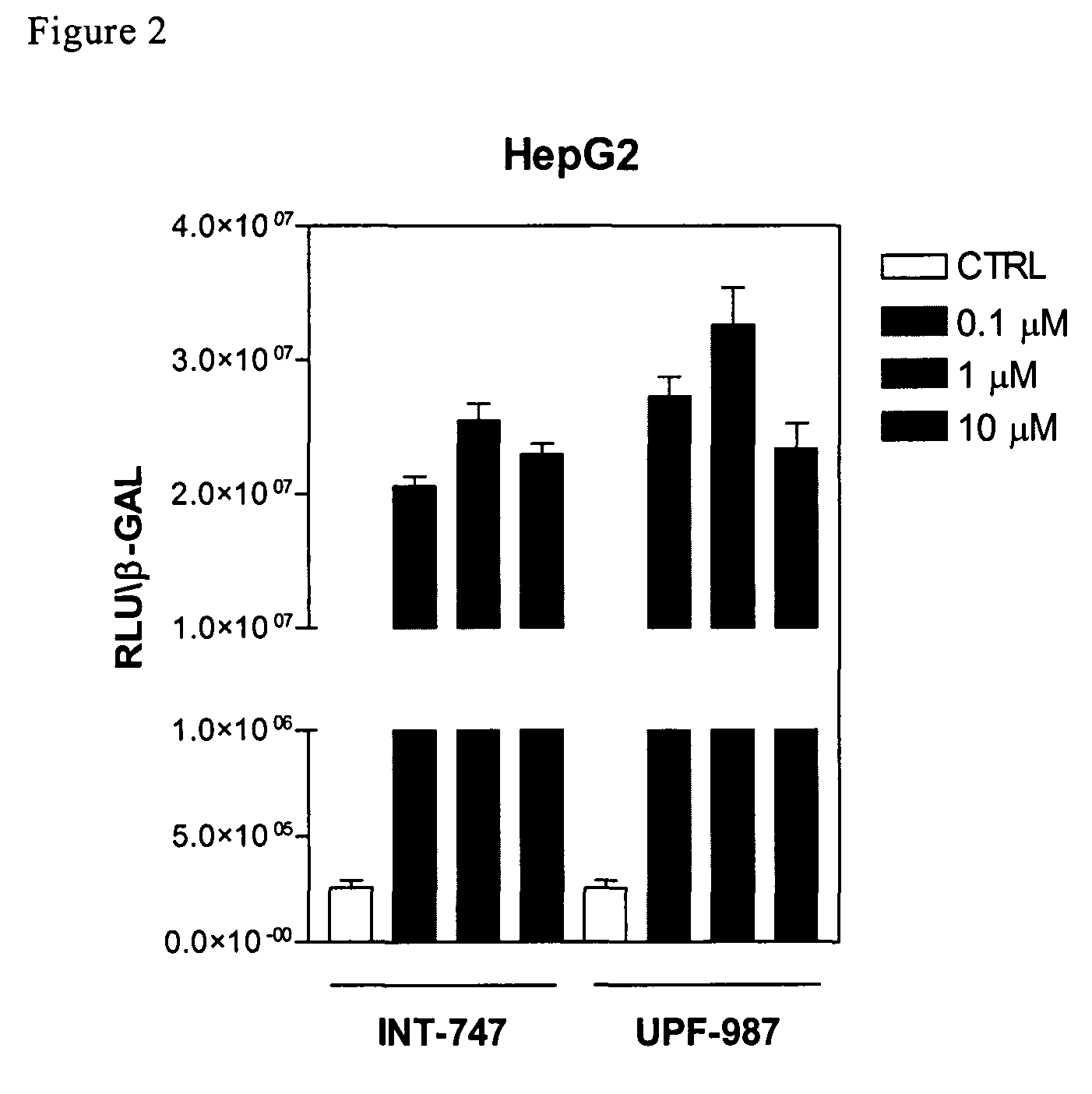
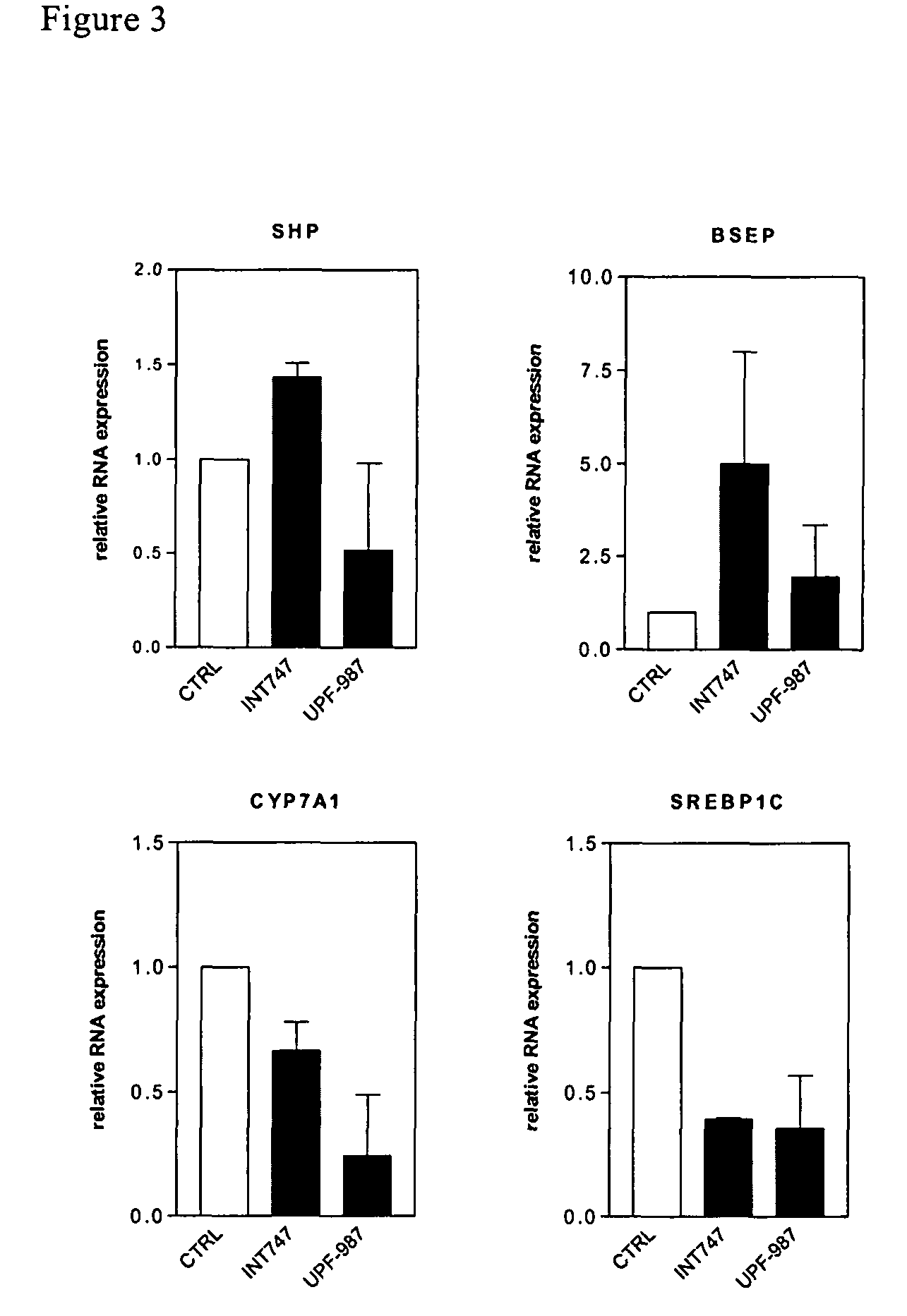
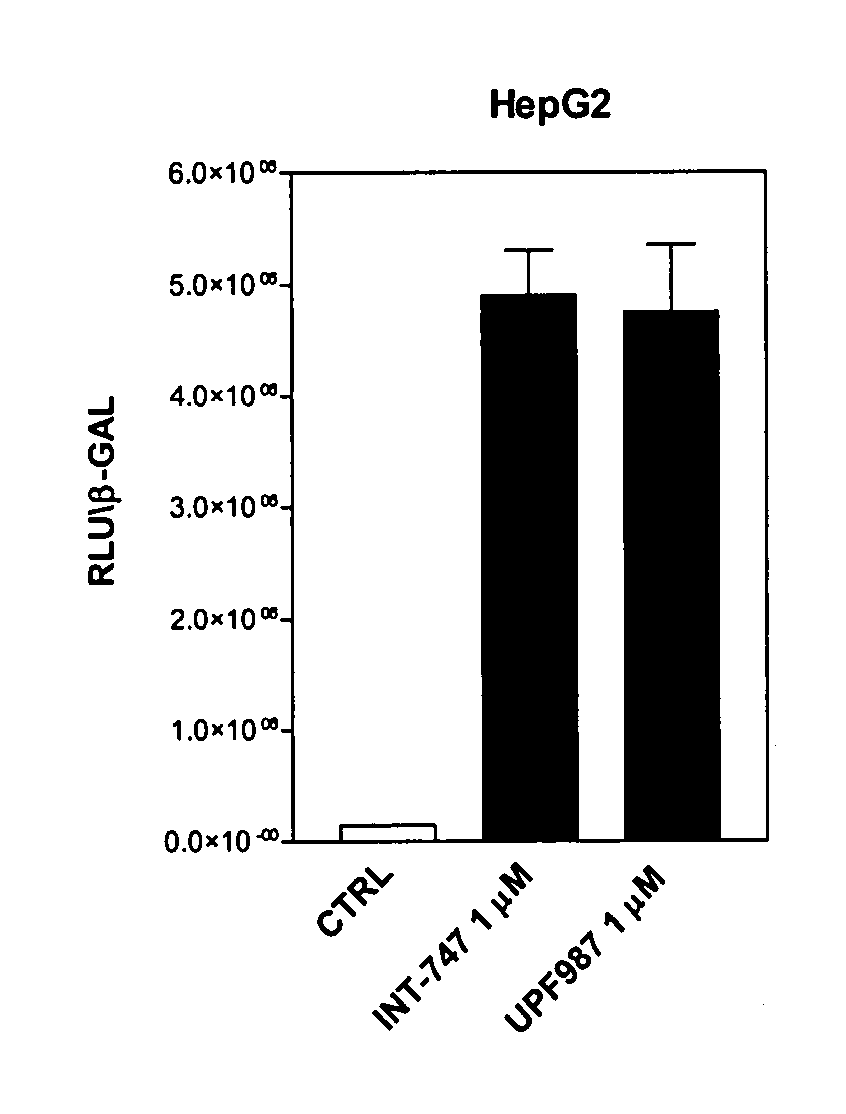
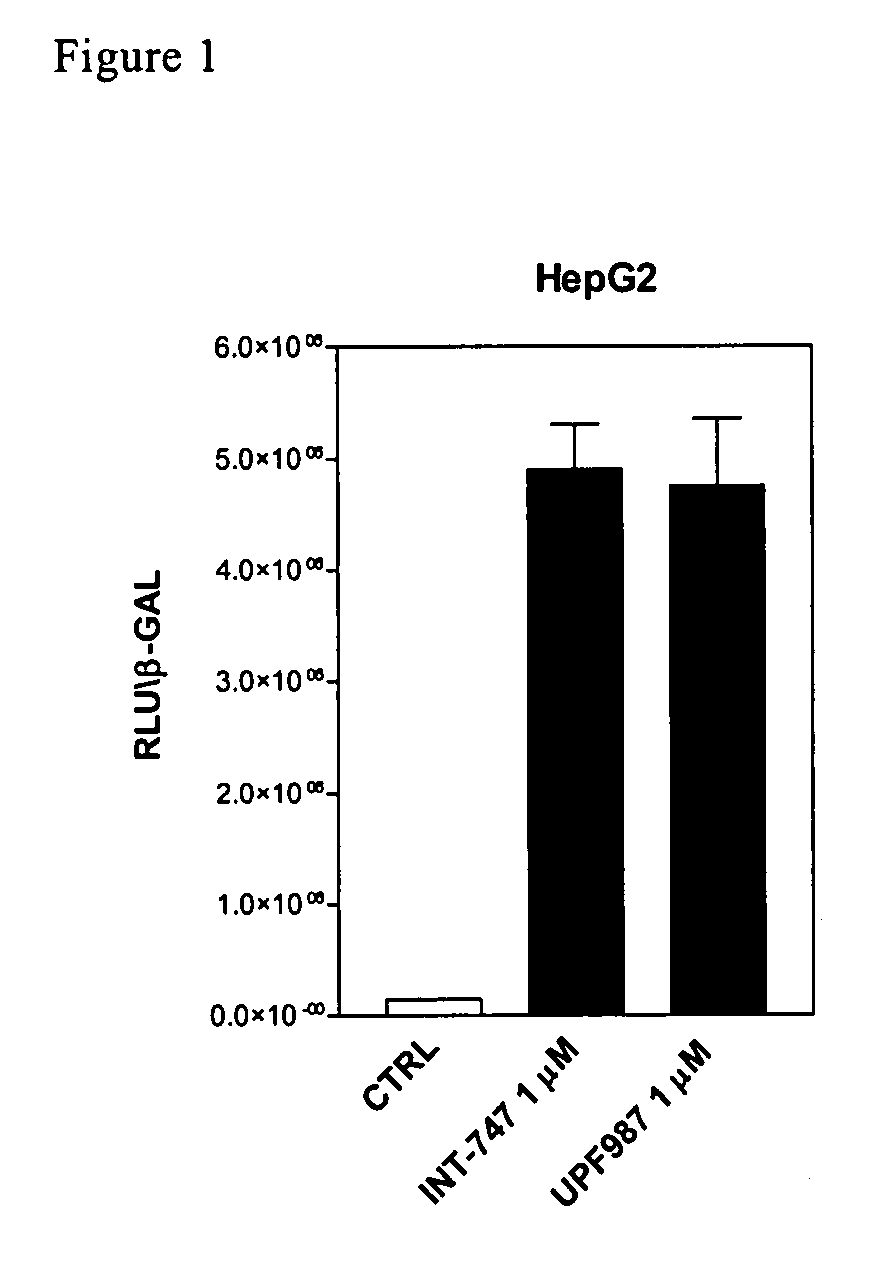
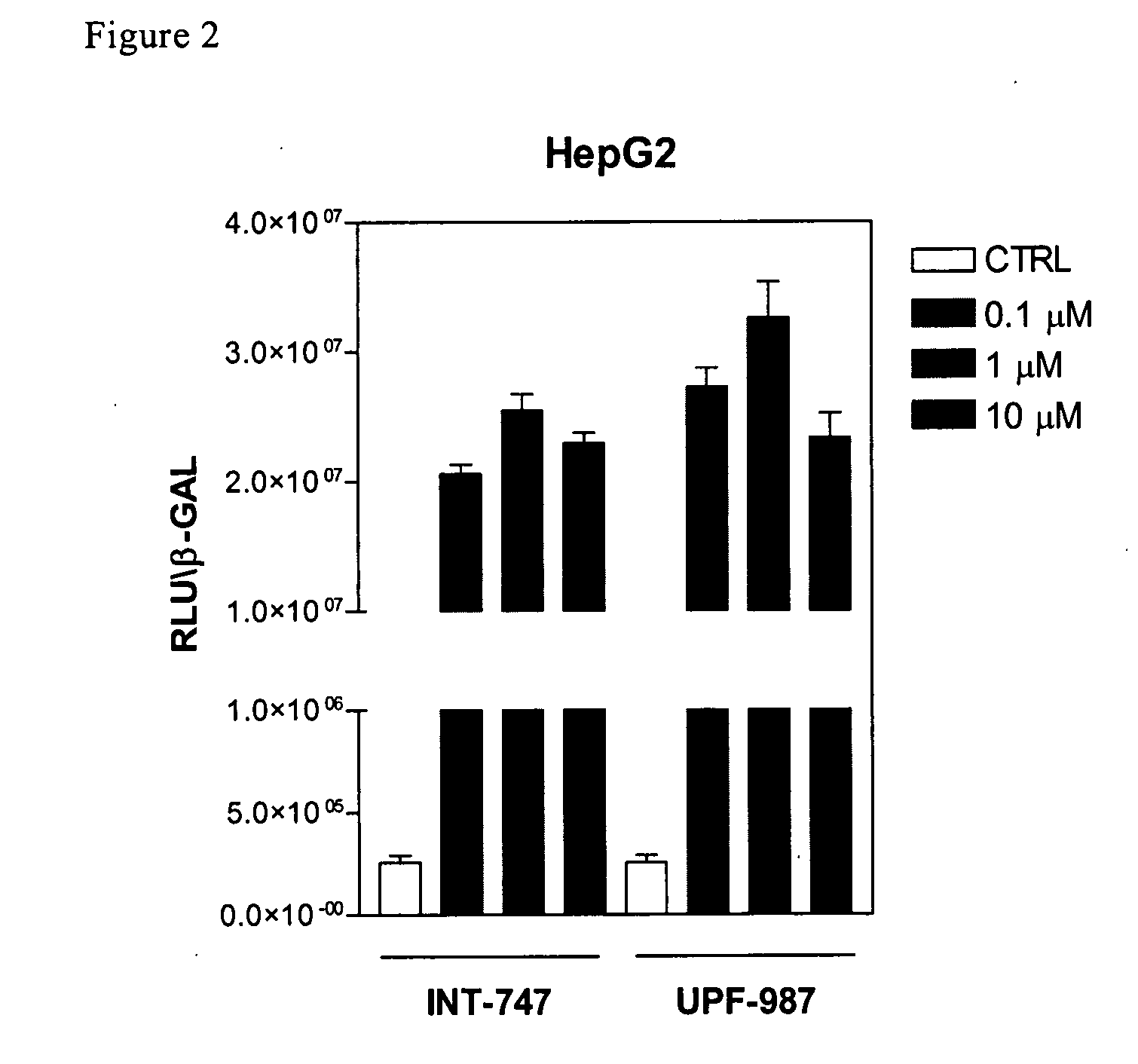
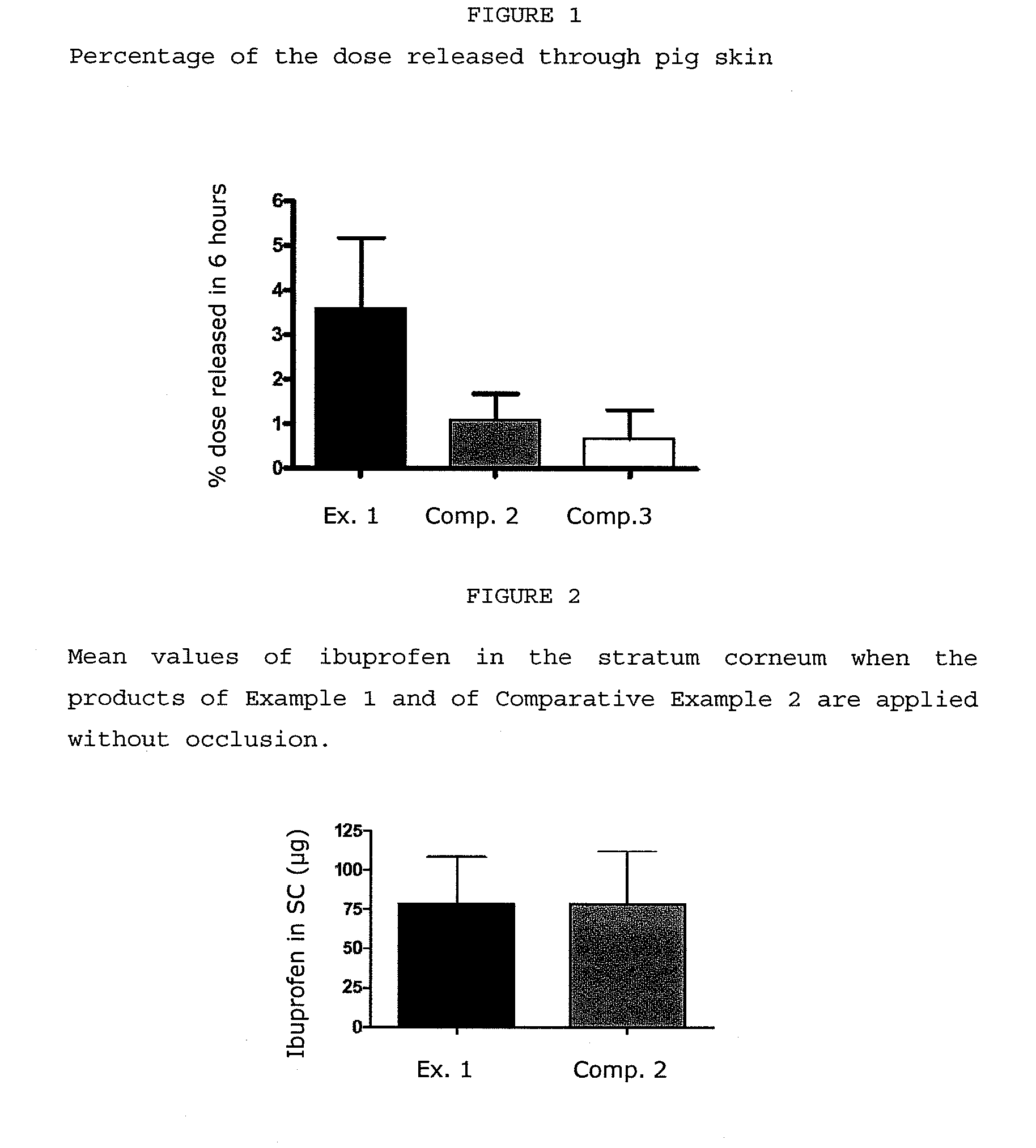
Patents
Literature
855 results about "Bile acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
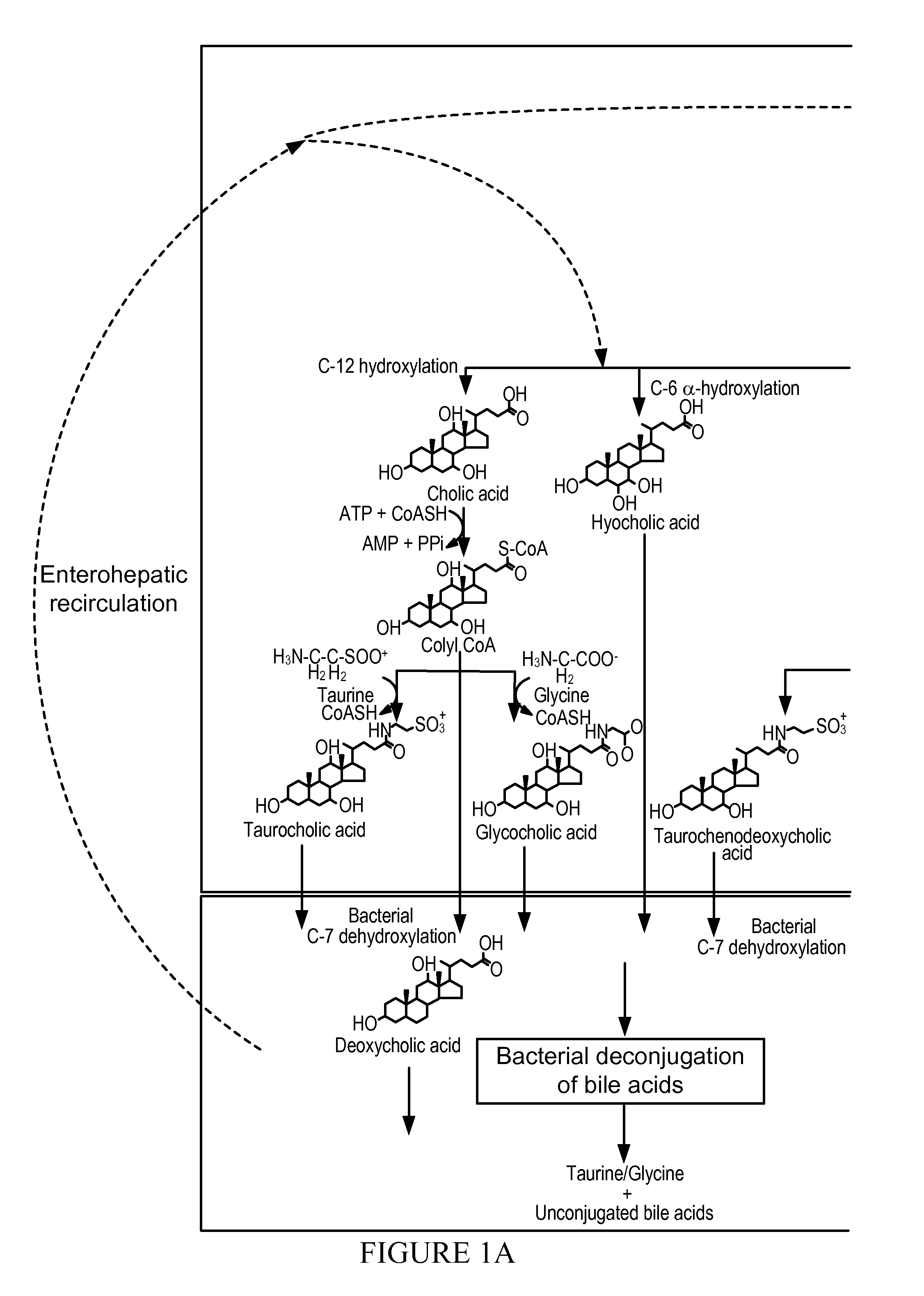
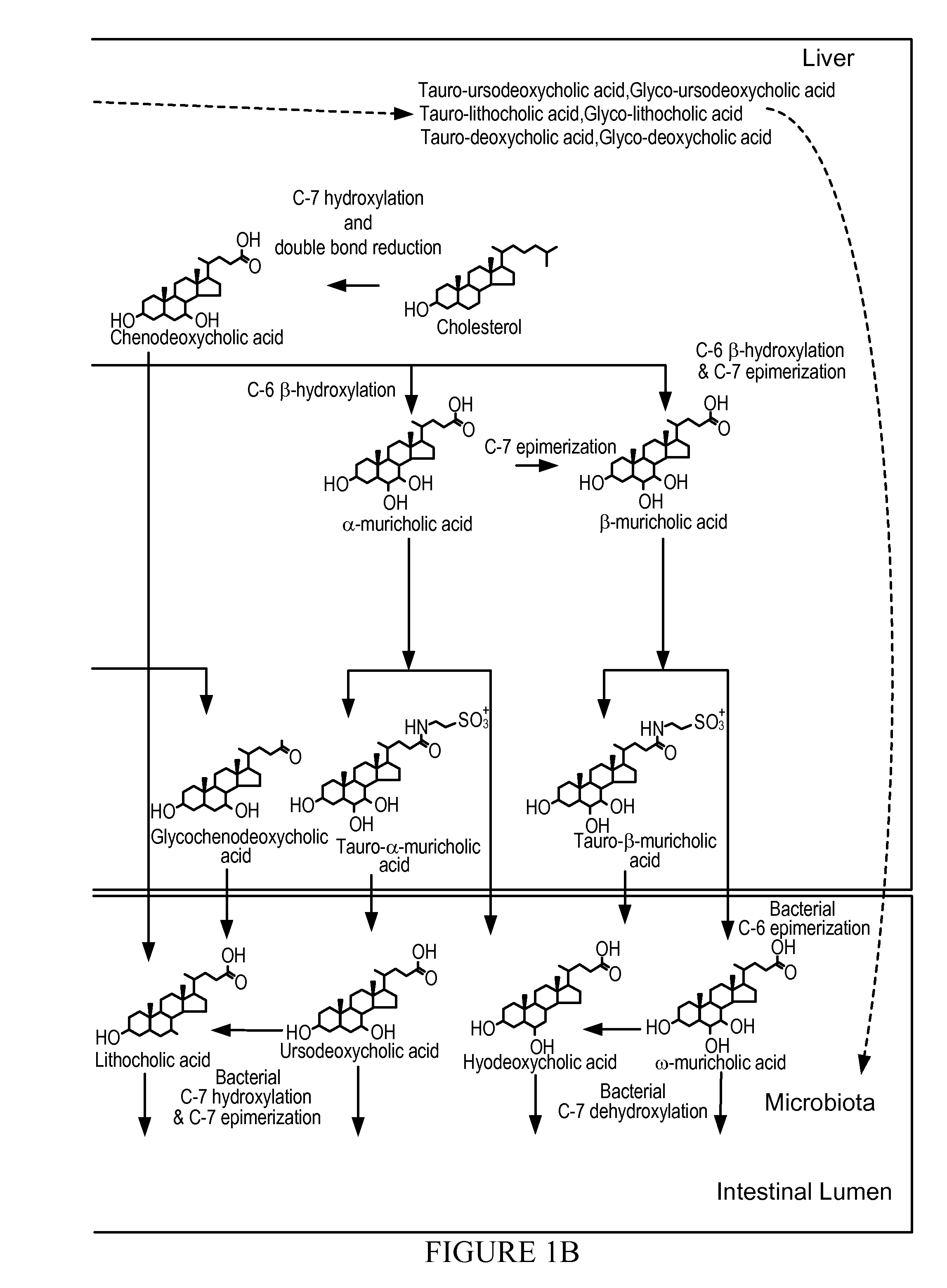
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Different molecular forms of bile acids can be synthesized in the liver by different species. Bile acids are conjugated with taurine or glycine in the liver, and the sodium and potassium salts of these conjugated bile acids are called bile salts.
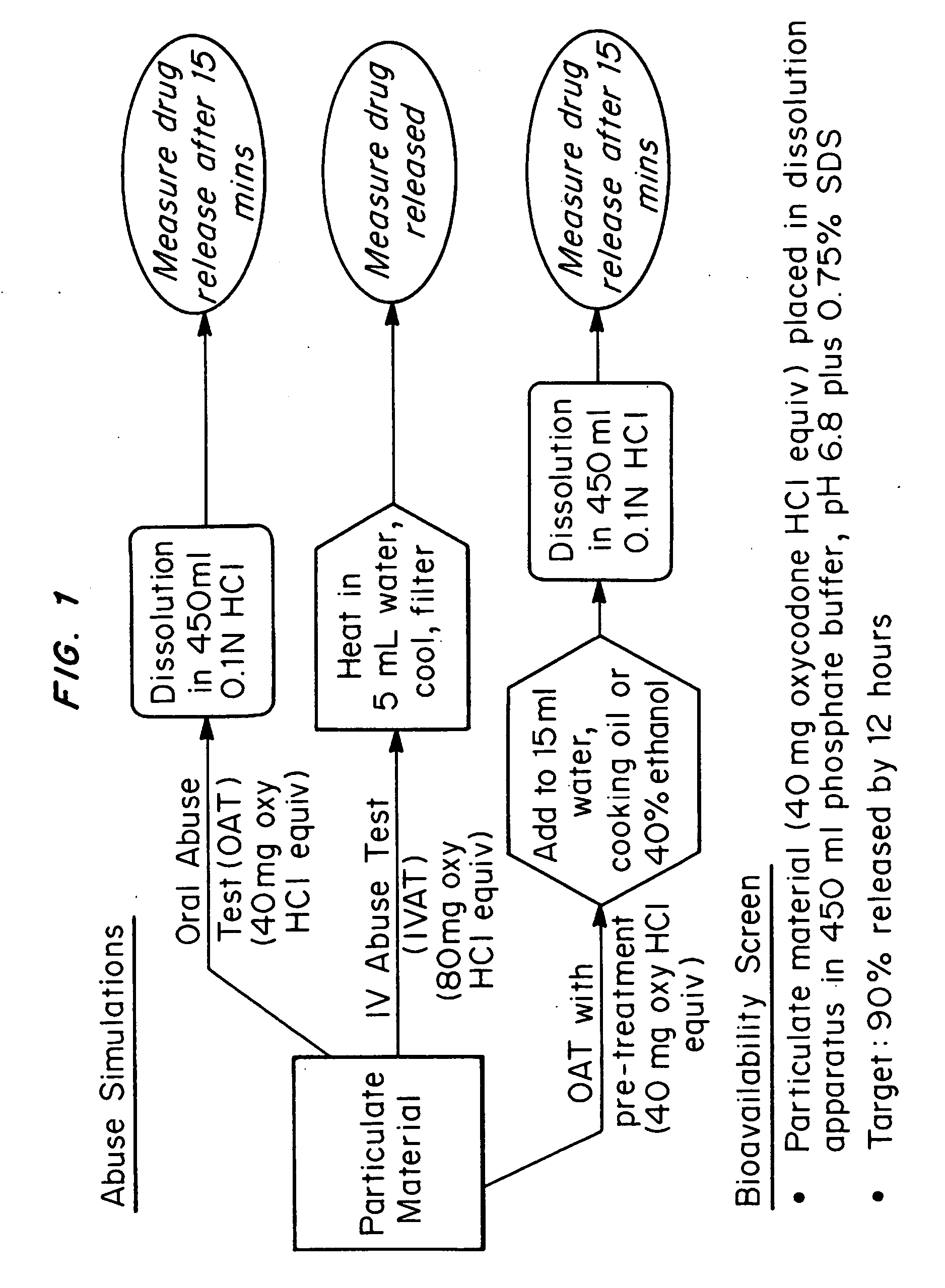
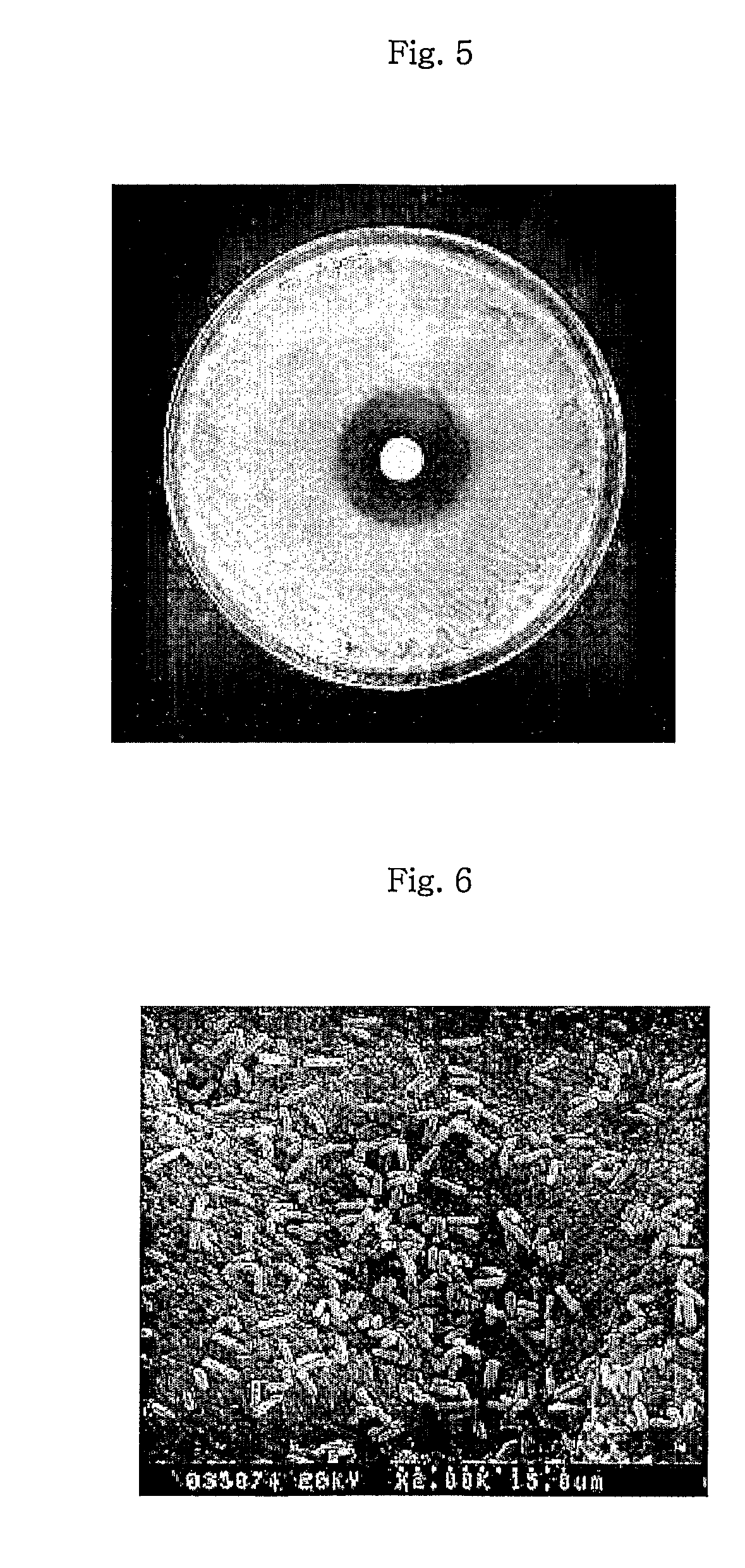
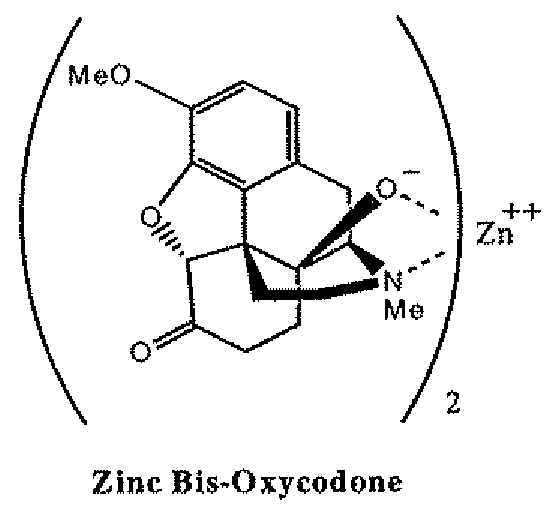
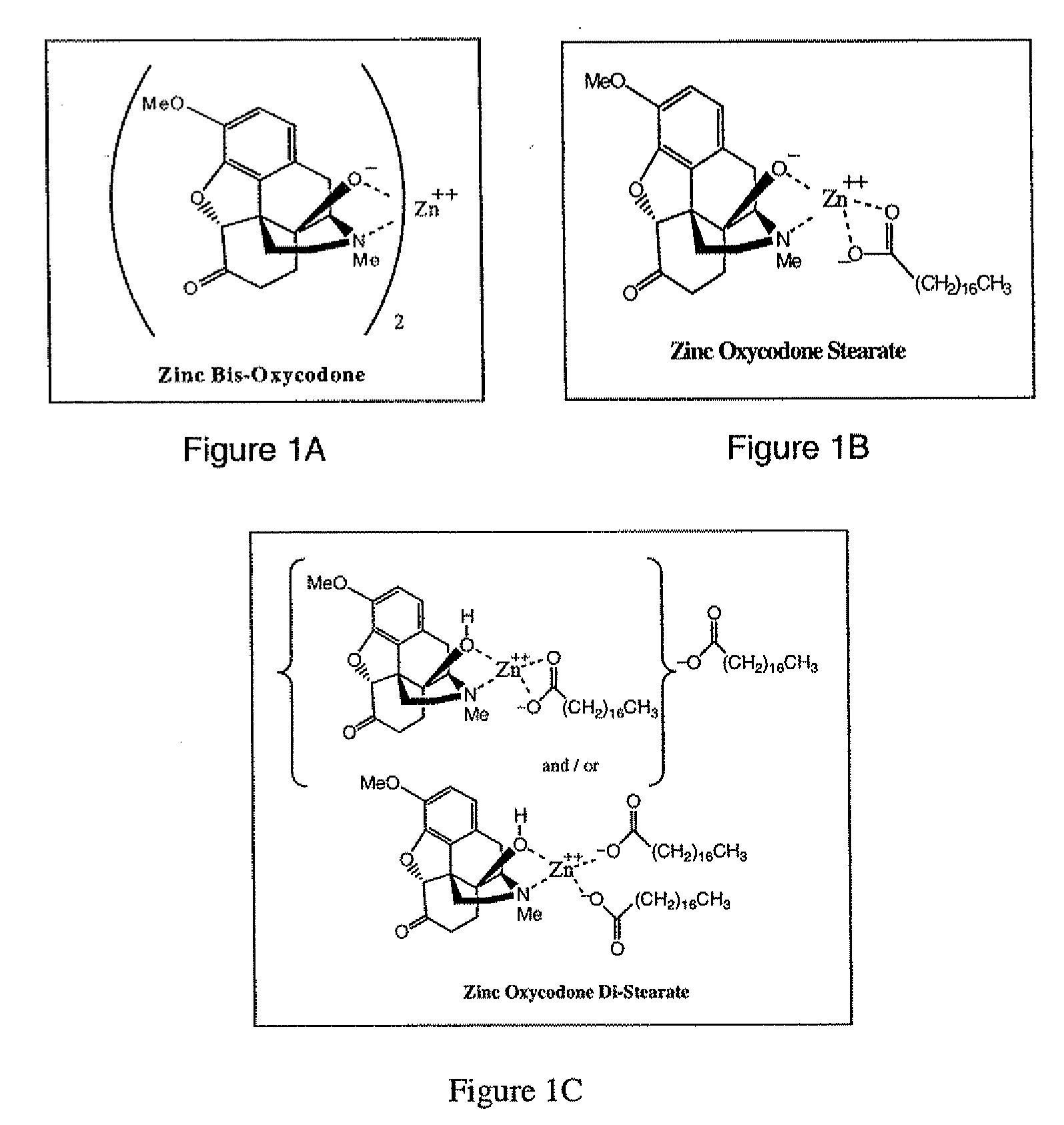
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
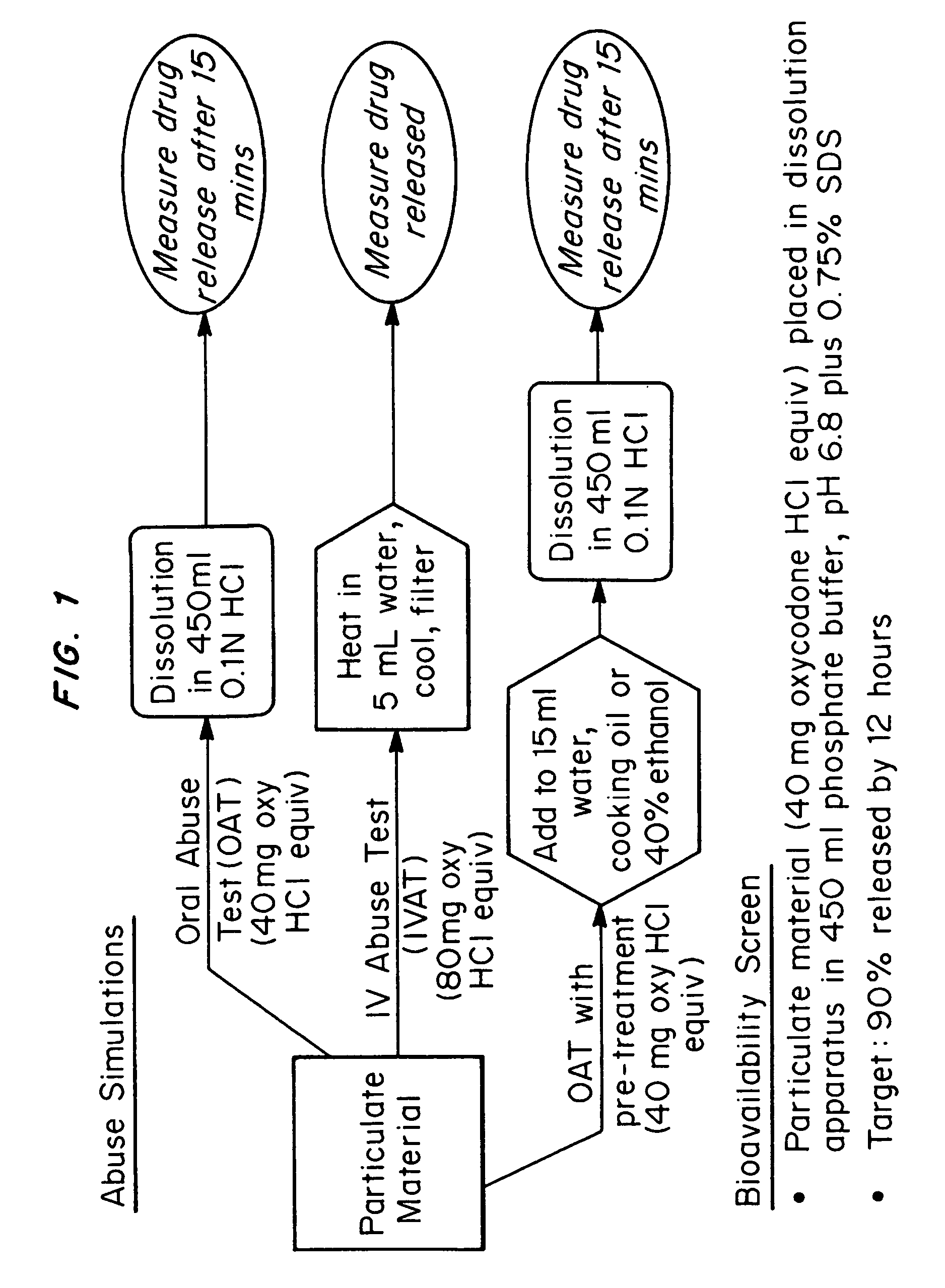
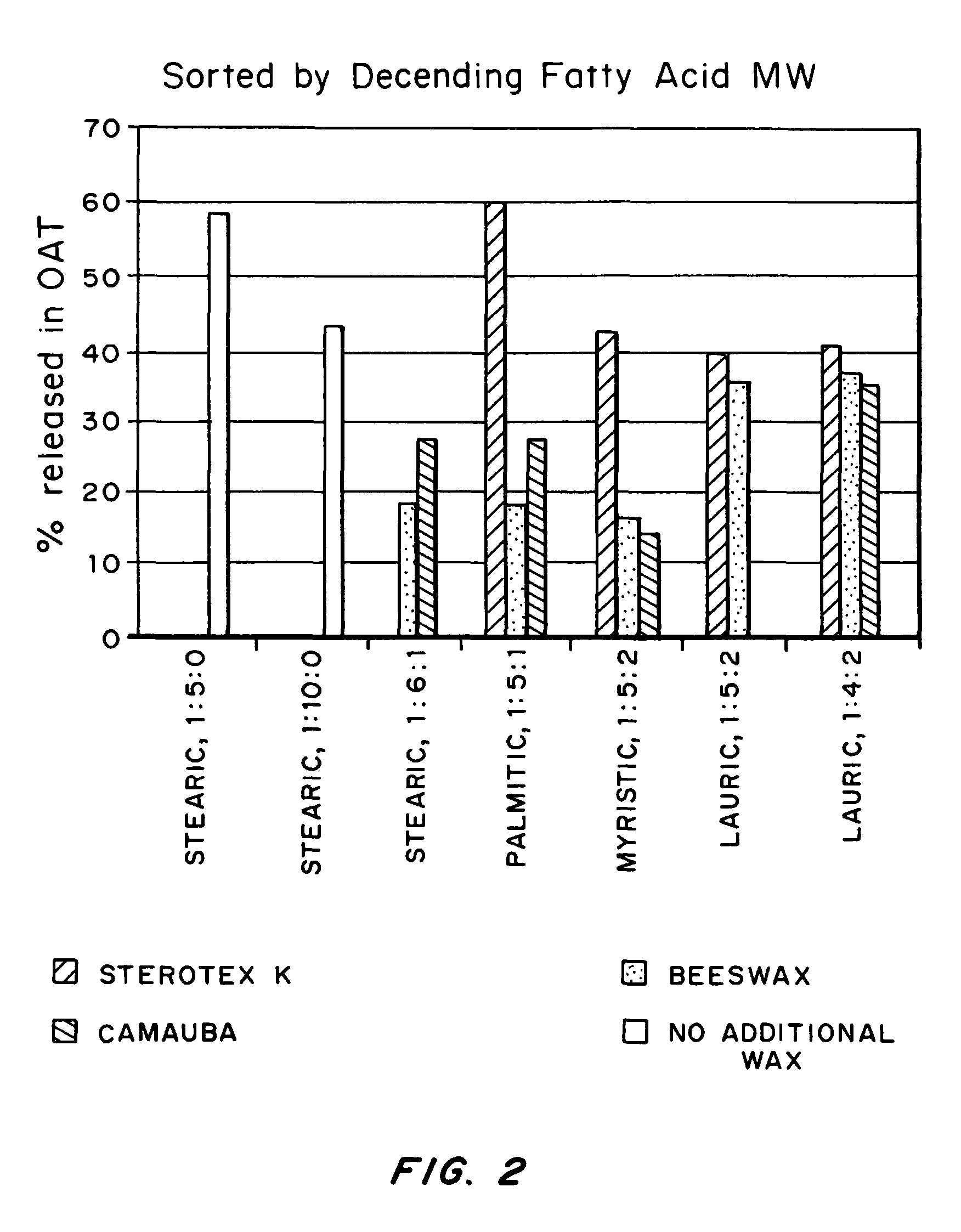
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Methods and compositions of bile acids
Methods and compositions are provided for treating metabolic disorders by modulating bile acid levels. Generally, the methods and compositions can modulate bile acid levels, such as serum bile acid levels, to treat a metabolic disorder. In one embodiment, a method of modulating a bile acid level includes measuring a bile acid level and delivering a composition effective to modulate the bile acid level. A method for modulating a bile acid profile includes comparing a bile acid profile to a target profile and delivering a bile acid cocktail to increase bile acid levels. In another embodiment, a pharmaceutical composition for increasing bile acid levels includes a bile acid cocktail effective to increase bile acid levels. The composition is further useful as part of an implantable system.
Owner:THE GENERAL HOSPITAL CORP +1
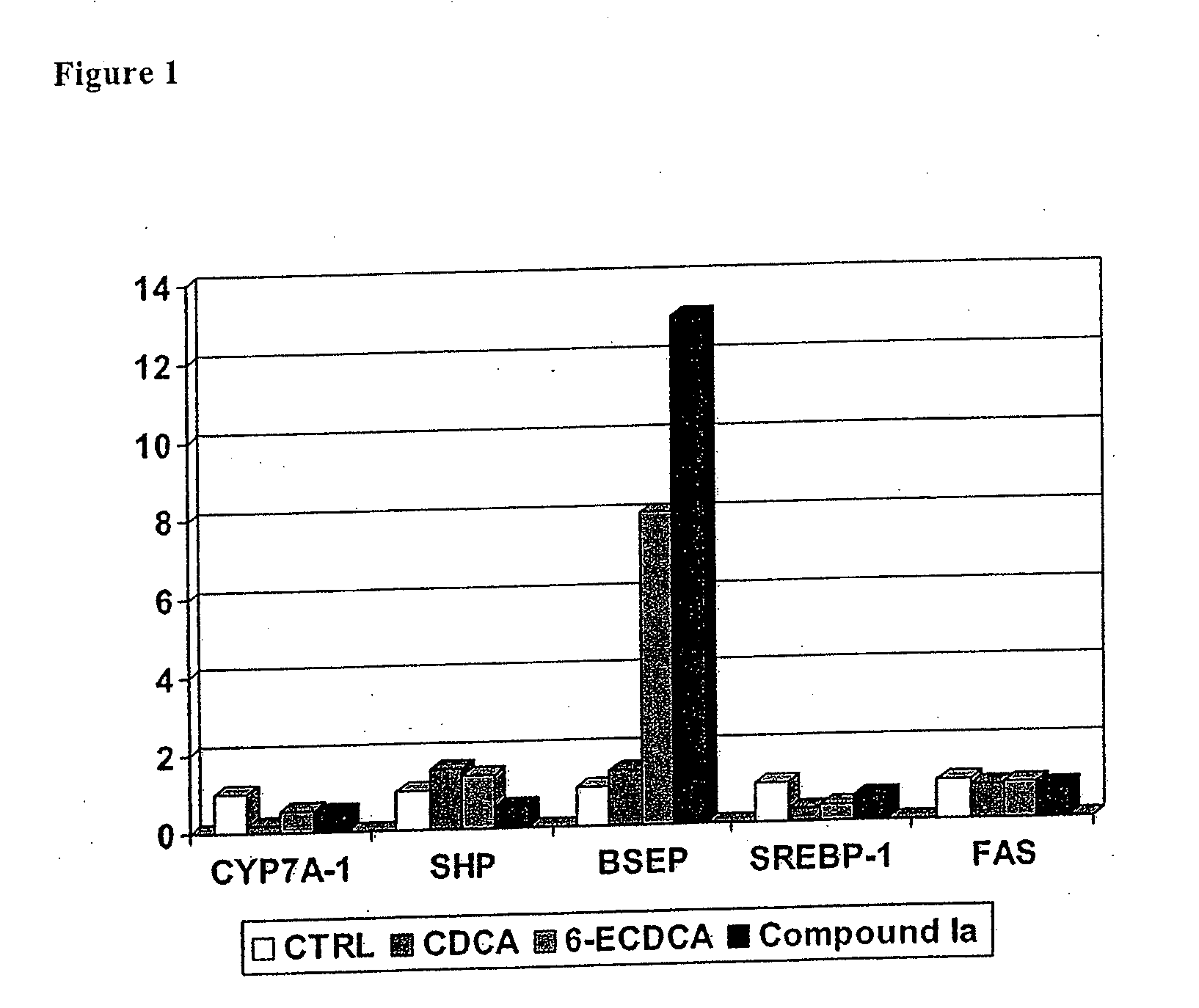
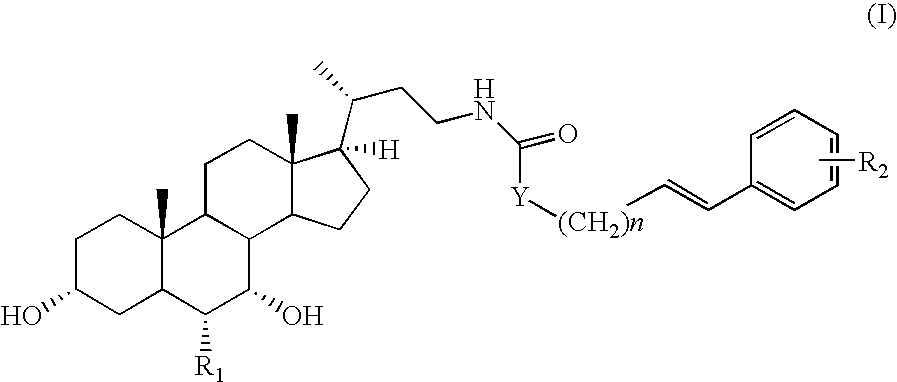
Bile acid derivatives as fxr ligands for the prevention or treatment of fxr-mediated diseases or conditions
The present invention relates to compounds of formula (I)wherein:R1 is hydrogen or an alkyl group;R2 is hydrogen or a halogen, nitro, alkyloxy, amino or carboxy group;Y is CH2, oxygen or sulfur;n is an integer from 1 to 4, and pharmaceutically acceptable salts, solvates or amino acid conjugates thereof for the treatment of FXR-mediated diseases or conditions.
Owner:INTERCEPT PHARMA INC
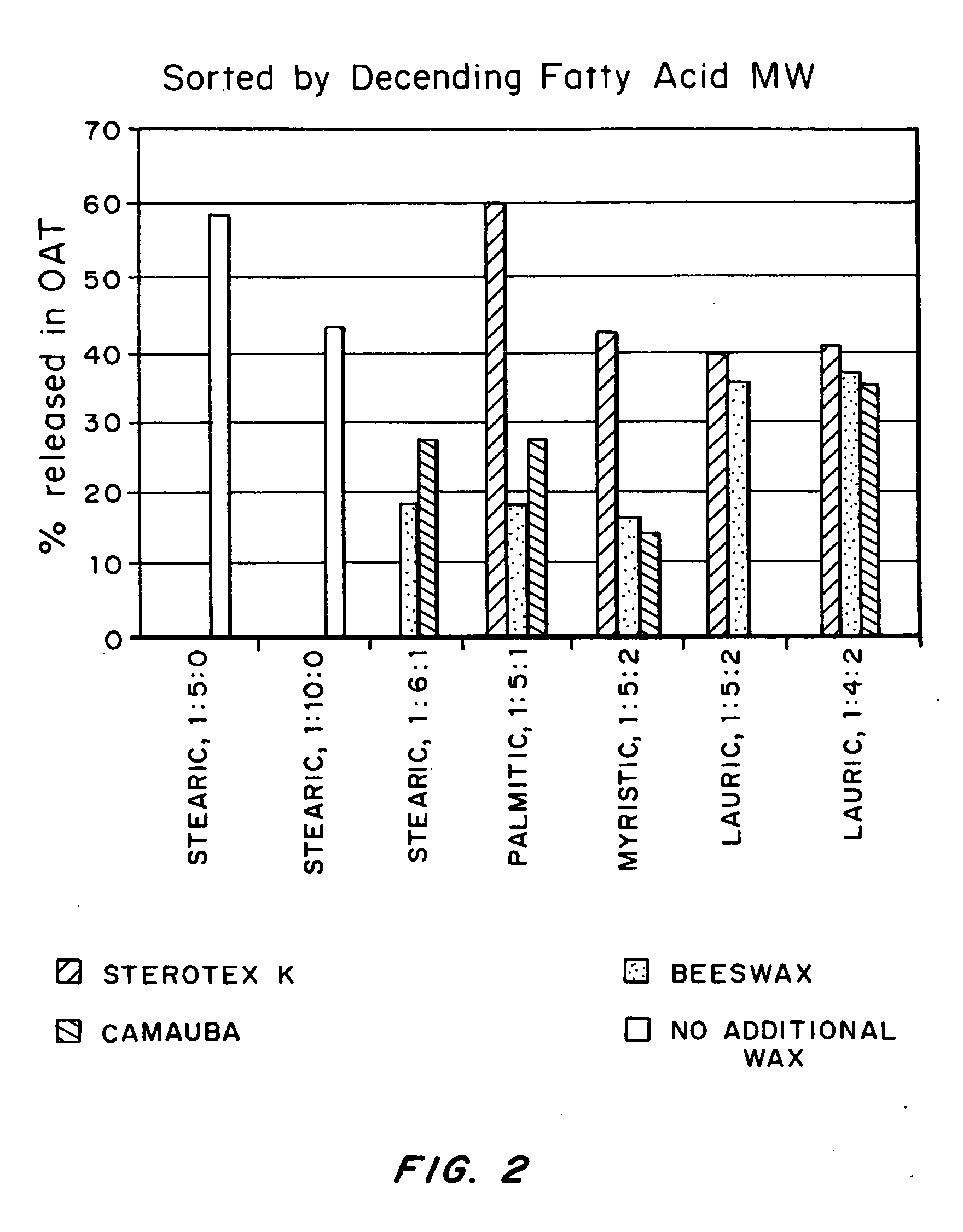
Abuse-deterrent drug formulations
ActiveUS20050281748A1Reduce the possibilityImprove lipophilicityTelevision system detailsPowder deliveryImmediate releaseActive agent
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, the drug is modified to increase its lipophilicity by forming a salt between the drug and one or more fatty acids wherein the concentration of the one or more fatty acids is one to 15 times the molar amount of the active agent, preferably two to ten times the molar amount of the active agent. In one embodiment the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble. The abuse-deterrent composition prevents the immediate release of a substantial portion of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
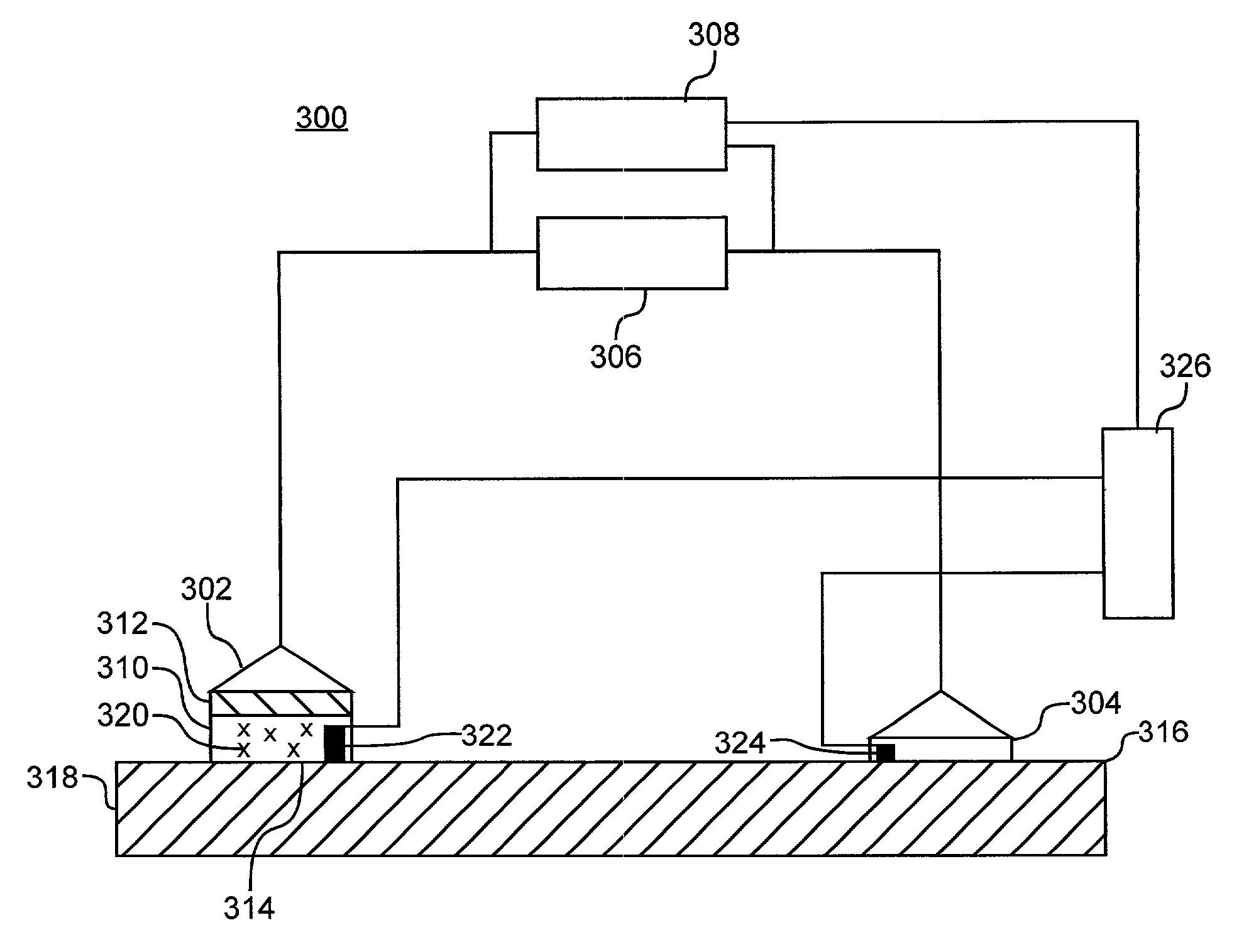
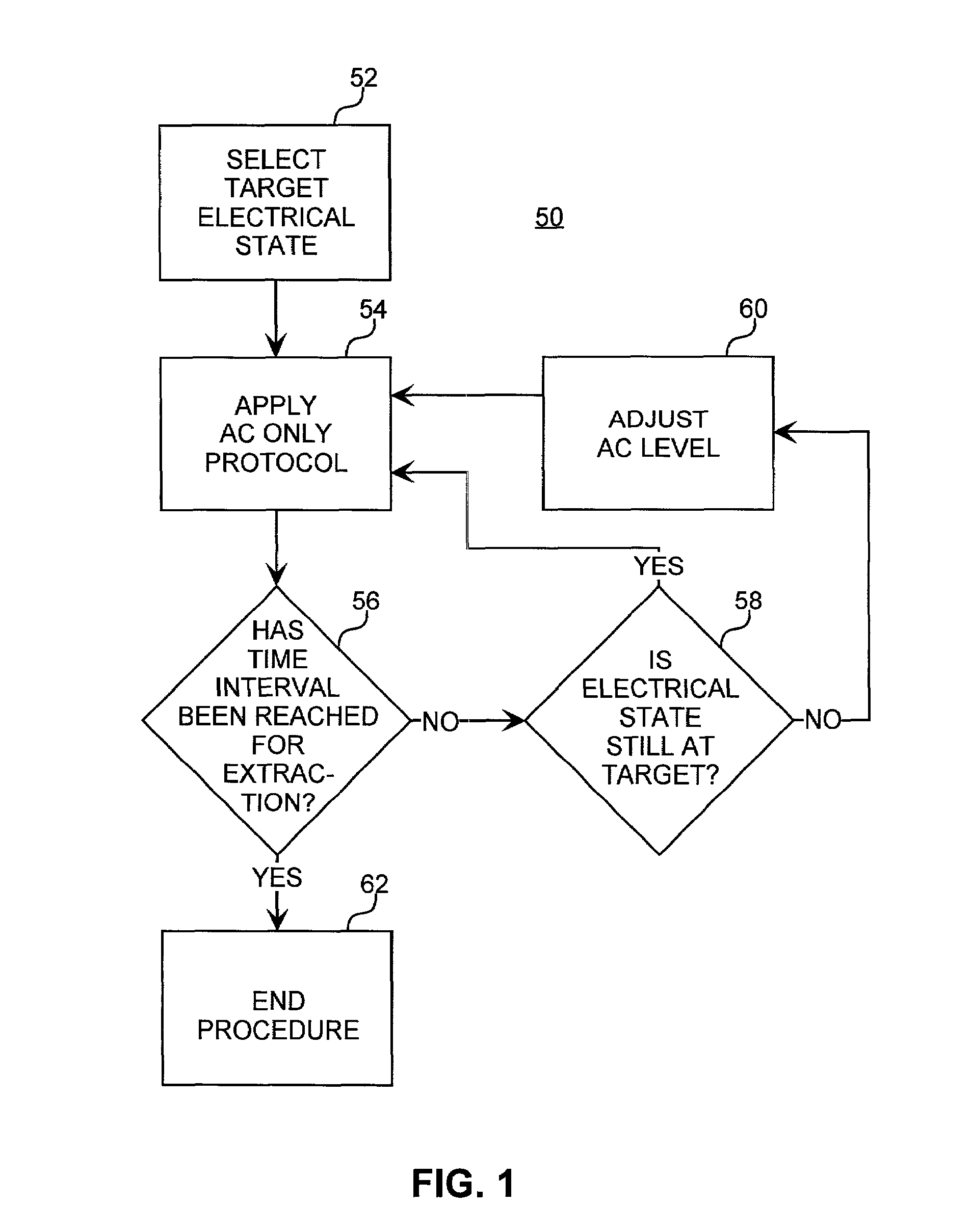
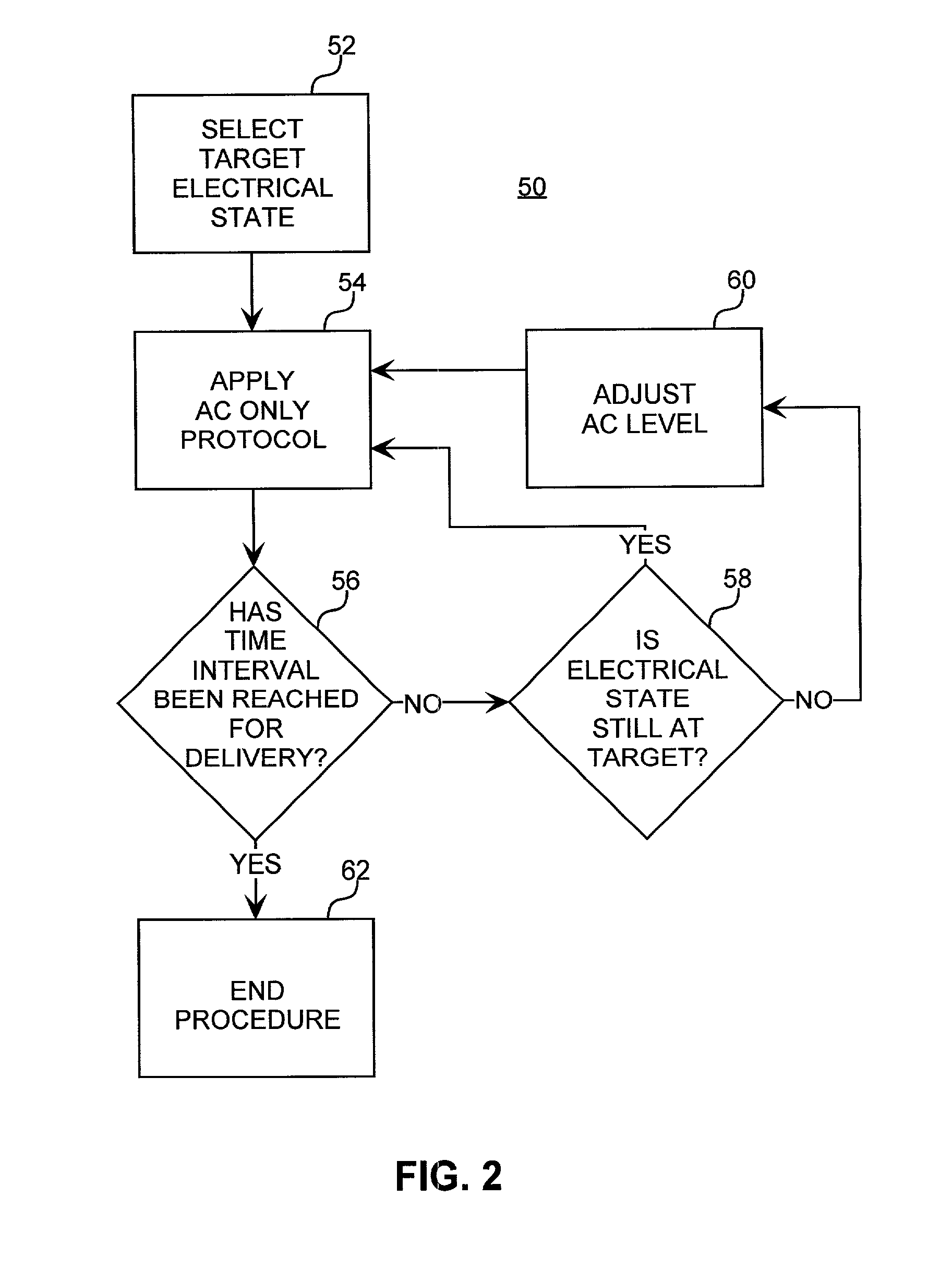
Method for increasing the battery life of an alternating current iontophoresis device using a barrier-modifying agent
InactiveUS7137975B2Less powerReduce voltageElectrotherapyMedical devicesElectricityElectrical resistance and conductance
An iontophoretic method for transporting compounds of interest across a body tissue is provided. The method utilizes an AC signal in conjunction with a barrier-modifying agent such as a fatty acid, fatty alcohol, bile acid, surfactant, or the like. The method enables the maintenance of a substantially constant electrical state in a localized region of the tissue through which transport occurs, thereby allowing a compound of interest to be transported across the tissue in a controlled and predictable manner. The barrier-modifying agent reduces the time as well as the voltage level required to achieve a target electrical resistance, thereby reducing patient discomfort and increasing the battery life of the iontophoresis device.
Owner:ACIONT
Dietary supplement for supressing appetite, enhancing and extending satiety, improving glycemic control, and stimulant free
This invention relates to a nutritional intervention composition for enhancing satiety prior to a meal and extending satiety after a meal. The nutritional intervention composition decreases food intake producing weight loss over time. The composition consists of Niacin, Vitamin B6, Calcium, Phosphorous, Magnesium, Chromium, Chitosan, Fenugreek, Ginseng, White willow bark, Garcinia cambogia, Aloe Vera gel powder, Momordica charantia, Griffonia simplicifolia, Lagerstroemia speciosa and Vanadyl sulfate. The invention does not require stimulants or anabolic ingredients. There are three phases of activity within the composition. One, enhanced satiety through elevated serotonin. Two, improved carbohydrate metabolism, reduced blood glucose and slowed gastric emptying. Three, enhanced fiber binding of lipids and excess bile acids.
Owner:NEEDLEMAN ALVIN +1
Bile Acid Recycling Inhibitors for Treatment of Pediatric Cholestatic Liver Diseases
InactiveUS20130109671A1Reduces and inhibits recyclingReduce harmBiocidePill deliveryHepatic DiseasesHepatic bile
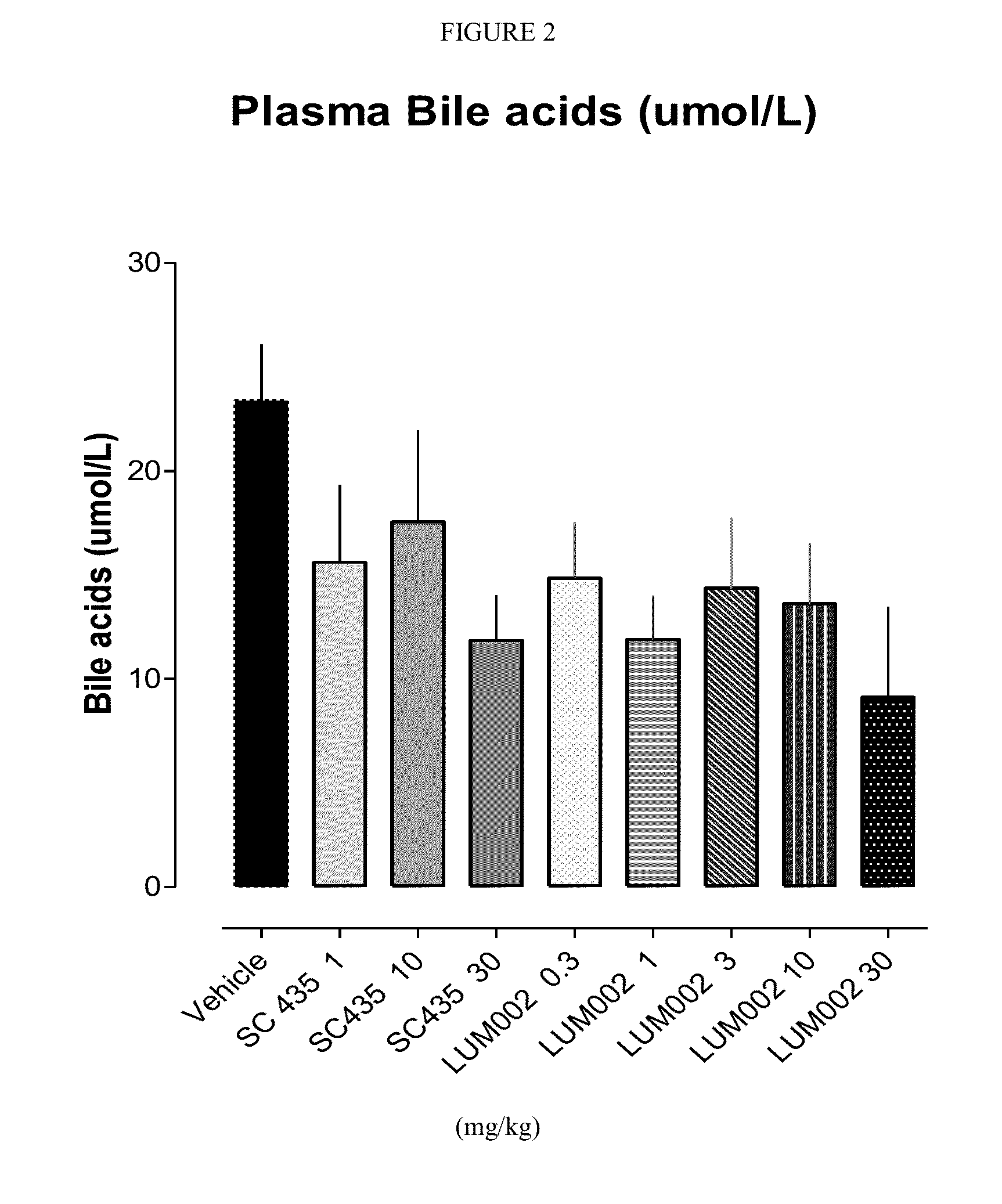
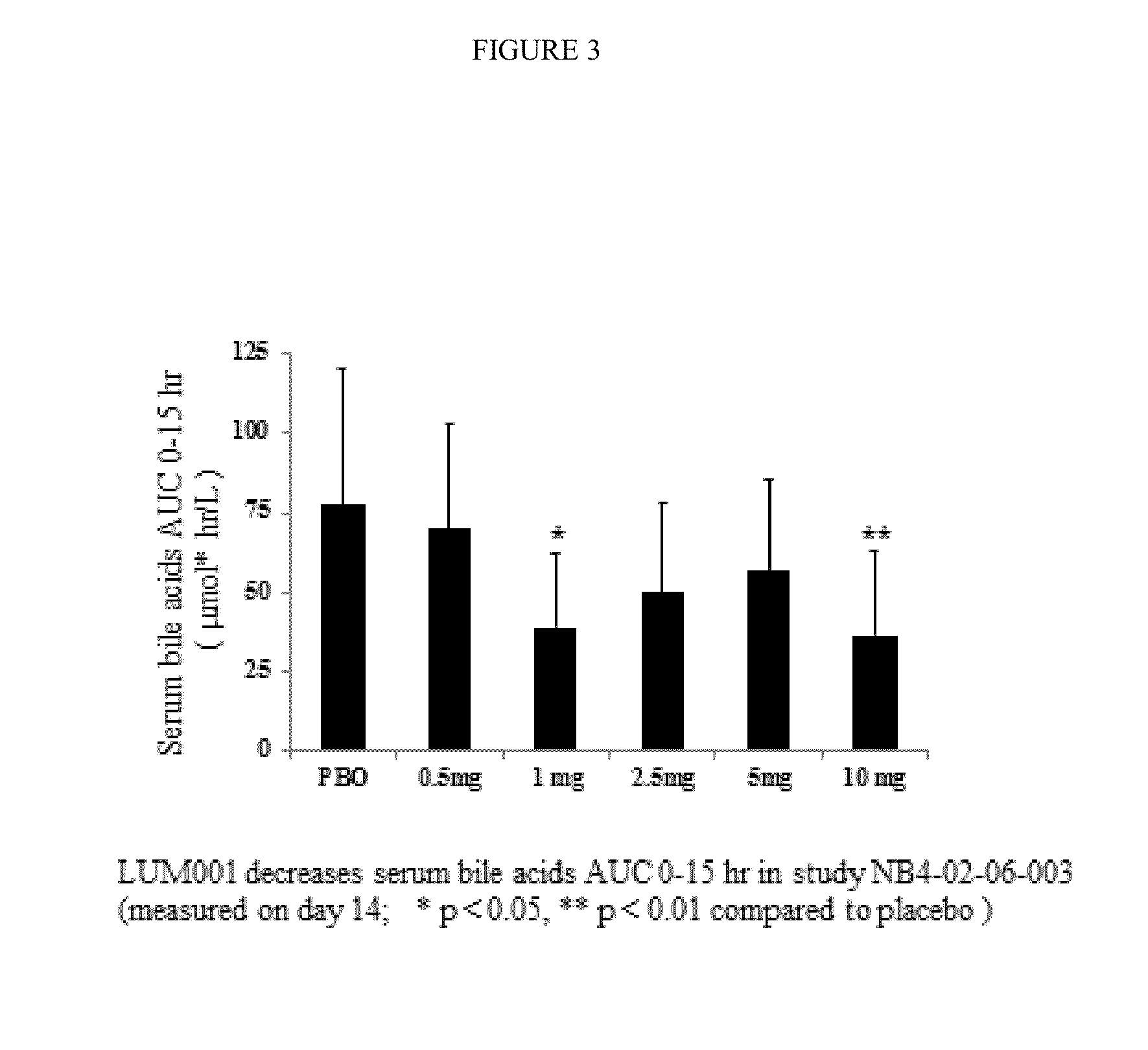
Provided herein are methods of treating or ameliorating a pediatric cholestatic liver disease by non-systemically administering to an individual in need thereof a therapeutically effective amount of a pediatric formulation comprising an Apical Sodium-dependent Bile Acid Transporter Inhibitor (ASBTI) or a pharmaceutically acceptable salt thereof. Also provided are methods for treating or ameliorating a pediatric liver disease, decreasing the levels of serum bile acids or hepatic bile acids, treating or ameliorating pruritis, reducing liver enzymes, or reducing bilirubin comprising non-systemically administering to an individual in need thereof a therapeutically effective amount of a pediatric formulation comprising an ASBTI or a pharmaceutically acceptable salt thereof.
Owner:LUMENA PHARMA INC
Abuse-deterrent drug formulations
ActiveUS7771707B2Reduce the possibilityImprove lipophilicityPowder deliveryTelevision system detailsActive agentWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, the drug is modified to increase its lipophilicity by forming a salt between the drug and one or more fatty acids wherein the concentration of the one or more fatty acids is one to 15 times the molar amount of the active agent, preferably two to ten times the molar amount of the active agent. In one embodiment the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble. The abuse-deterrent composition prevents the immediate release of a substantial portion of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Dried forms of aqueous solubilized bile acid dosage formulation: preparation and uses thereof
InactiveUS20050158408A1Improve bioavailabilityPlasma bioavailabilityBiocideOrganic active ingredientsDrug compoundSoluble Non-Starch Polysaccharide
Compositions for pharmaceutical and other uses comprising clear aqueous solutions of bile acids which do not form any detectable precipitates over selected ranges of pH values of the aqueous solution and methods of making such solutions are disclosed. Compositions of the disclosure may comprise water; a bile acid in the form of a bile acid, bile acid salt, or a bile acid conjugated with an amine by an amide linkage; and either or both an aqueous soluble starch conversion product and an aqueous soluble non-starch polysaccharide. The composition remains in solution without forming a precipitate over a range of all pH values obtainable in an aqueous system. The composition, according to some embodiments, may further contain a pharmaceutical compound in a pharmaceutically effective amount. The disclosure further provides dried forms of primary aqueous solubilized bile acid formulations and methods of preparing such dried forms.
Owner:YOO SEO HONG
Polyhydroxylated bile acids for treatment of biliary disorders
The invention provides, in part, polyhydroxylated bile acids for treating biliary disorders, for example, biliary disorders arising out of cholestasis of portal hypertension. The invention also provides, in part, polyhydroxylated bile acids for stimulating bile flow. New compounds 2α,3α,7α,12α-tetrahydroxy-5β-cholanoic acid and 3α.4α,7α,12α-tetrahydroxy-5β-cholanoic acid are disclosed, uses thereof and synthesis thereof.
Owner:QING BILE THERAPEUTICS
Quantitative detecting method for various metabolites in biological sample, and metabolic chip
ActiveCN109298115AFast contentContent quicklyComponent separationMass spectrometric analysisMetaboliteDerivatization
The invention discloses a quantitative detecting method for various metabolic components in a biological sample, and a metabolic chip used for the quantitative detecting method. The quantitative detecting method is characterized in that the biological sample is subjected to derivatization treatment, and the biological sample subjected to derivatization is detected through liquid chromatography andmass spectrometry combination; and during derivatization treatment, 3-nitrophenylhydrazine serves as a derivatization reagent, and 1-(3-dimethylaminopropyl)-3-ethly carbodiimide serves as a derivatization reaction catalyst. According to the quantitative detecting method, high-sensitivity detection can be achieved, and a plurality of level-crossing metabolic components can be covered during detection, and the quantitative detecting method is simple, fast and suitable for being applied to clinical and scientific research testing. The metabolic chip comprises a chip carrier microtitration plateand related reagents, and thus the multiple level-crossing metabolites such as amino acid, phenols, phenyl or benzyl derivatives, indole, organic acid, fatty acid, sugar and bile acid in the biological samples can be subjected to quantitative detection on the same microtitration plate.
Owner:麦特绘谱生物科技(上海)有限公司
Diet fiber fat reducing biscuit and its processing method
The slimming diet fiber biscuit is produced with flour and diet fiber as main material and supplementary material including salt, seasoning, etc. The diet fiber will expand after absorbing water and this makes the eater produce satiety to reduce the ingestion; and it will reduce the time for food to stay in intestinal tract to consume fat inside body and result in slimming effect. In addition, ingesting diet fiber can reduce the re-absorption amount and alter food digesting speed and digestion secretion amount to prevent gall stone, duodenal ulcer, peptic colonitis and other diseases.
Owner:LANZHOU YISHENG BIOCHEM TECH CO LTD
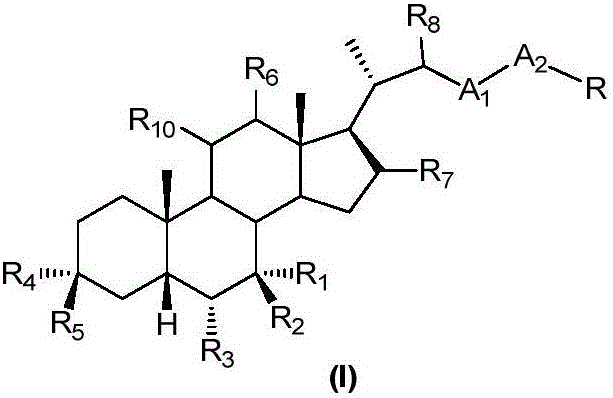
Bile acid derivatives as FXR ligands for the prevention or treatment of FXR-mediated diseases or conditions
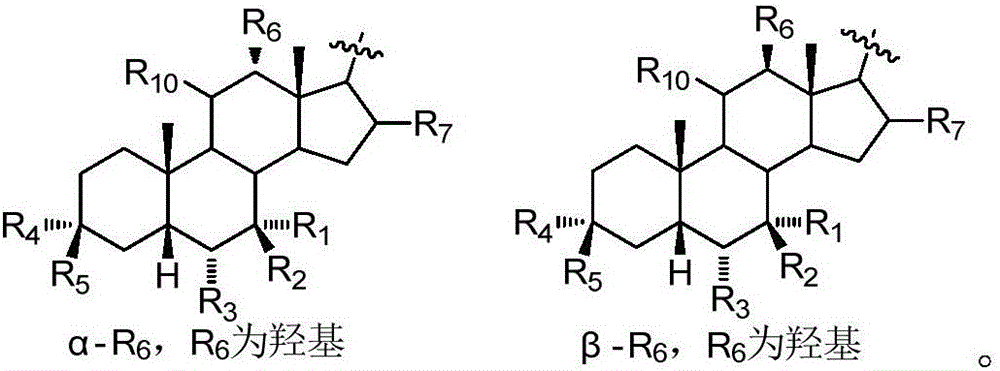
The present invention relates to compounds of formula (I):wherein R is hydrogen or alpha-hydroxy,the hydroxyl group in position 7 is in the alpha or beta position;and pharmaceutically acceptable salts, solvates or amino acid conjugates thereof.
Owner:INTERCEPT PHARMA INC
Bile acid derivatives as FXR ligands for the prevention or treatment of FXR-mediated diseases or conditions
The present invention relates to compounds of formula (I):wherein R is hydrogen or alpha-hydroxy,the hydroxyl group in position 7 is in the alpha or beta position;and pharmaceutically acceptable salts, solvates or amino acid conjugates thereof.
Owner:INTERCEPT PHARMA INC
Topical Ibuprofen Formulation
InactiveUS20090304782A1Improved transcutaneous absorption profileImprove permeabilityBiocideAntipyreticPharmacy medicinePhospholipid
The present invention relates to a liposomal liquid pharmaceutical composition comprising ibuprofen, at least one phospholipid, at least one C2-C6 alkanol and water, characterized in that the liposomes comprise, in addition to at least part of the phospholipid and at least part of the ibuprofen, at least one bile acid salt and to a method for preparing it. The invention also relates to the use of the composition for obtaining a medicinal product for the local relief of pain and inflammation and to a spray for the cutaneous application of the composition.
Owner:LAB ALCALA FARMA
Method of reducing C-reactive protein using growth hormone secretagogues
The present invention relates to a method of reducing C-reactive protein in a subject in need of treatment thereof, wherein the subject is at risk of having or the subject has already had a vascular event or suffering from an inflammatory disease or disorder. In one embodiment, the vascular event is a cardiovascular event (e.g., myocardial infarction). In another embodiment, the vascular event is a cerebrovascular event (e.g., stroke (such as transient ischemic attacks (TIAs)). In yet another embodiment the vascular event is a peripheral vascular event (e.g., intermittent claudication). The method comprises administering a therapeutically effective amount of at least one growth hormone secretagogue compound or a pharmaceutically acceptable salt, hydrate or solvate thereof. The growth hormone secretagogue can be coadministered with a second growth hormone secretagogue, HMG CoA reductase inhibitor, an ACAT inhibitor, a CETP inhibitor, an anti-inflammatory agent, an ACE inhibitor, a Beta blocker, a cholesterol absorption inhibitor, a nicotonic acid, a fibric acid derivative, a bile acid sequestering agent or a combination thereof.
Owner:HELSINN THERAPEUTICS (US) INC
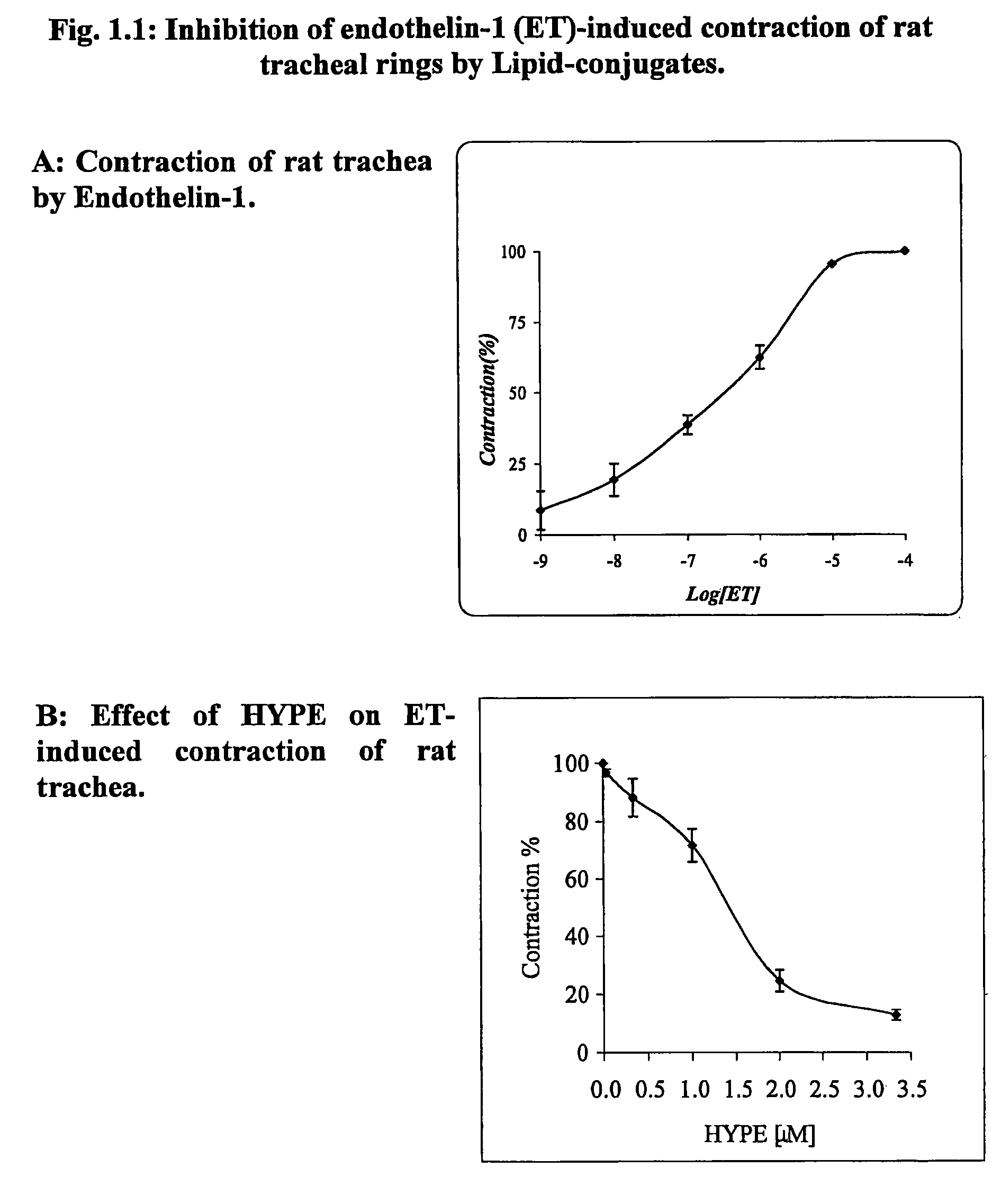
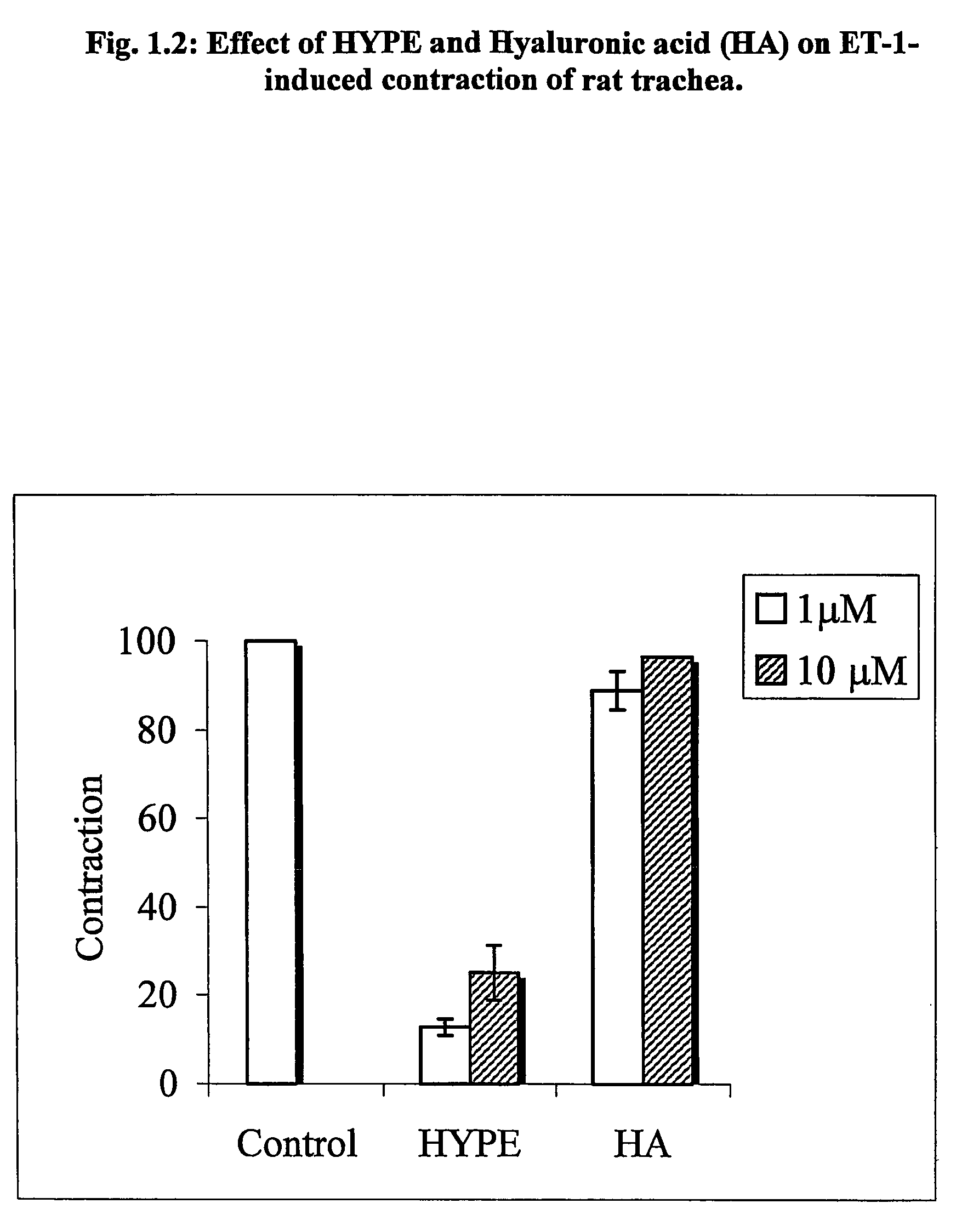
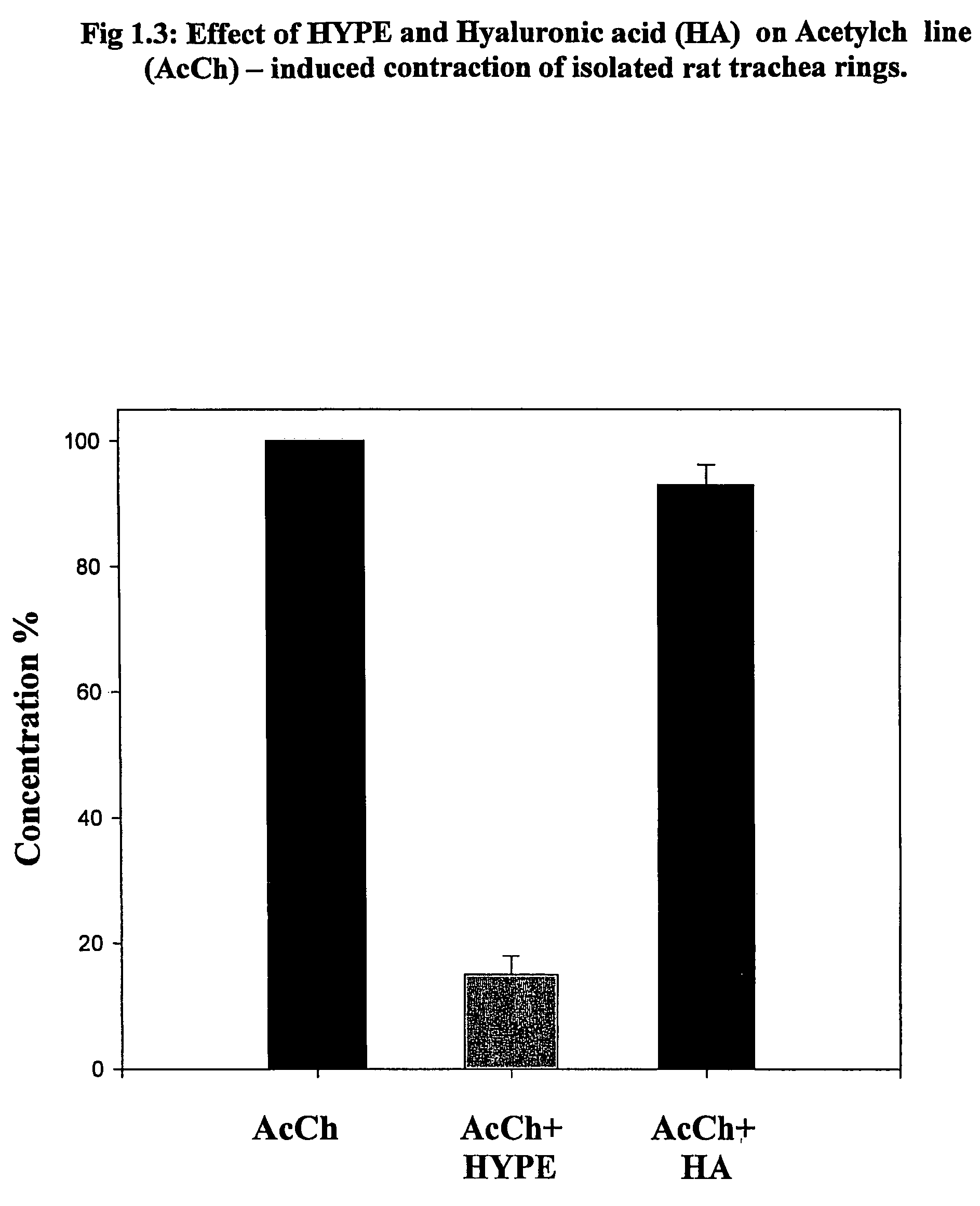
Use of lipid conjugates in the treatment of diseases
InactiveUS7101859B2Reduce molecular weightIncrease rangeAntibacterial agentsBiocideLymphatic SpreadContact dermatitis
The invention provides novel methods for treating disease based upon the medicinal use of lipids and phospholipids covalently bound to physiologically acceptable monomers or polymers. Phosphatidylethanolamine moieties conjugated to physiologically acceptable monomers and polymers (PE conjugates) manifest an unexpectedly wide range of pharmacological effects, including stabilizing cell membranes; limiting oxidative damage to cell and blood components; limiting cell proliferation, cell extravasation and (tumor) cell migratory behavior; suppressing immune responses; and attenuating physiological reactions to stress, as expressed in elevated chemokine levels. The surprisingly manifold pharmacological properties of the PL-conjugates allow for the invention, disclosed herein, of novel methods for the treatment of a diverse range of disease states, including obstructive respiratory disease, including asthma; colitis and Crohn's disease; central nervous system insult, including blood brain barrier compromise, ischemic stroke, and multiple sclerosis; contact dermatitis; psoriasis; cardiovascular disease, including ischemic conditions and prophylaxis for invasive vascular procedures; cellular proliferative disorders, including anti-tumor vasculogenesis, invasiveness, and metastases; anti-oxidant therapy; hemolytic syndromes; sepsis; acute respiratory distress syndrome; tissue transplant rejection syndromes; autoimmune disease; viral infection; and hypersensitivity conjunctivitis. The therapeutic methods of the invention include administration of phosphatidylethanolamine bound to carboxymethylcellulose, heparin, hyaluronic acid, polyethylene glycol, and Polygeline (haemaccel). Disclosed herein are also new compounds comprised of phospholipid moieties bound to low molecular weight monomers and dimers, including mono- and disaccharides, carboxylated disaccharides, mono- and dicarboxylic acids, salicylates, bile acids, and fatty acids.
Owner:YEDGAR SAUL
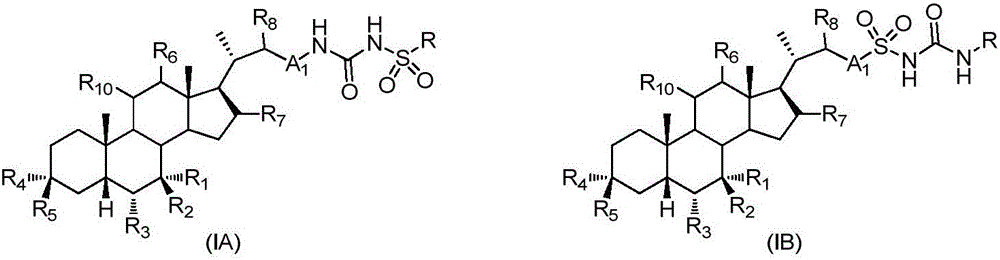
Sulfonylurea derivative and pharmaceutical composition and application thereof
The invention relates to a preparation method and application of a sulfonylurea compound and a composition containing the same component as FXR and / or TGR5 agonist, the FXR and / or TGR5 agonist is a compound shown as a formula (I), or a pharmaceutically acceptable salt, a solvate, a prodrug, an isomer and a stable isotope derivative thereof. The compounds can be used for treatment of FXR and / or TGR5 mediated diseases including primary biliary cirrhosis, nonalcoholic fatty liver, portal hypertension, bile acid diarrhea and cholestasis, type II diabetes and obesity and other field.
Owner:SHANGHAI DE NOVO PHARMA
Hair analysis method
InactiveUS6949344B1Reliable solubilizationAccurate methodPre-tanning chemical treatmentMicrobiological testing/measurementBetaineDigestion
A method the direct analysis of an analyte in keratinized structures, e.g., hair and fingernails, which comprises preparing a mixture containing a low redox potential activator compound such as dithiothreitol or dithioerythritol, an enzyme suitable for the digestion of the keratin structure, a sample of the keratin structure and a biological detergent that aids the digestion of the keratinized structure at a relatively low pH, e.g., between about 6.2 and 8; permitting the enzyme to at least substantially digest the sample of keratin structure, and subjecting the digest solution to analysis, preferably by radioimmunoassay, to determine the identity and amount of analyte in the keratin structure sample. To accelerate the method, cupric sulfate may be added to the mixture after degradation of the keratin sample. The enzyme may be a peptidase, endopeptidase or proteinase, with papain, chymopapain, and proteinase K being preferred for use in the invention. The preferred biological detergents include betaine, sulfo-betaine, alkylglucosides and bile acids.
Owner:PSYCHEMEDICS CORPORATION
Treatment of Obesity or Diabetes with Bile Acid Sequestrants
InactiveUS20110152204A1Enhances enteroendocrine peptide secretionReduction in blood levelBiocideMetabolism disorderLow affinityDiabetes mellitus
Provided herein are methods of treating obesity and diabetes with labile bile acid sequestrants. An effective amount of a labile bile acid sequestrant may be orally administered to an obese or diabetic individual. A labile bile acid sequestrant provided herein may have a low affinity in the colon or rectum of a human for at least one bile acid or bile acid mimic that stimulates L-cells. A labile bile acid sequestrant may be a non-systemic labile bile acid sequestrant.
Owner:SATIOGEN PHARMA
Cell and enzyme compositions for modulating bile acids, cholesterol and triglycerides
InactiveUS20070116671A1Reduce target compound levelReducing bile acid levelOrganic active ingredientsBiocideActive agentTriglyceride
The invention relates to immobilized or encapsulated enzyme and / or cells to lower bile acids and cholesterol. The invention also relates to methods of quantitatively measuring bile acids. The invention provides a composition for decreasing the amount of a target compound in the gastrointestinal tract of an animal, comprising: a) a biologically active agent which decreases the amount of the target compound; b) a retainer for retaining the biologically active agent by contacting the agent to limit movement of the agent; and c) a carrier.
Owner:CHR HANSEN AS
Dissolution of arterial plaque
InactiveUS20090035348A1Reducing plaque burdenLower cholesterol levelsBiocideHydrocarbon active ingredientsDissolutionAtheroma
Some embodiments of the present invention provide pharmaceutical formulations, for treating atherosclerosis in a mammal, including a bile acid and / or a terpene atherosclerotic plaque emulsifier. Some embodiments provide methods for administering such pharmaceutical formulations. In some embodiments, pharmaceutical formulations include a combination of a bile acid and a terpene in amounts effective to result in plaque regression, and the amount of each individual emulsifier in the combination can be lower than an amount that is effective to result in plaque regression when the emulsifier is administered alone. In some embodiments, a statin can be administered simultaneously or sequentially with the pharmaceutical formulation.
Owner:ATHERONOVA OPERATIONS
Acid tolerant Lactobacillus sakei probio-65 with the ability of growth suppression of pathogenic microorganisms and the anti-allergic effect
ActiveUS8034606B2Growth inhibitionEffectively inhibit harmful microorganismsBiocideCosmetic preparationsAcid-fastDisease
Disclosed are a novel tactic acid bacterium, Lactobacillus sakei Probio-65, and the use thereof. The L. sakei Probio-65 strain has acid tolerance, bile acid tolerance and antibiotic resistance, inhibits the growth of harmful pathogenic microorganisms in the body and the intestine of animals, and has immunuenhancing activity. In particular, the novel strain inhibits the growth of Staphylocccus aureus, which is known to be a factor aggravating atopic dermatitis. Thus, the novel strain is useful for preventing or treating atopic dermatitis and allergy-related disorders. Also, the novel strain stabilizes intestinal microflors by inhibiting the abnormal proliferation of harmful microorganisms in the intestine. The L. sakei Probio-65 strain or a culture thereof is useful in pharmaceutical, feed, food, and cosmetic compositions.
Owner:PROBIONIC CO LTD
Meat duck feed
InactiveCN102687817AReduce bile acid contentInhibit transformationAnimal feeding stuffSodium BentoniteAnimal science
The invention discloses a meat duck feed, belonging to the field of poultry feed. The meat duck feed contains the ingredients of 7 parts of corn, 46 parts of wheat, 11 parts of wheat middling, 10 parts of feed grade jujube powder, 2 parts of corn protein, 4 parts of cotton dregs, 4 parts of bean pulp, 2 parts of peanut meal, 4 parts of corn distillers, 2 parts of meat and bone meal, 2.9 parts of animal oil, 1.4 parts of mountain flour, 0.4 parts of calcium hydrophosphate, 0.7 parts of 70% lysine, 0.08 parts of methionine, 0.3 parts of chitosan, 0.05 parts of Chinese herbal medicine, 0.96 parts of bentonite, 0.25 parts of salt and 1 part of 1% premix, wherein the sum of the weight part percentages of the ingredients is 100%. The meat duck feed has the beneficial effects of reducing the in vivo bile acid of animals, preventing the cholesterol from being converted into cholesterol acid, increasing the feed intake quantity of the meat duck, improving the immunity and improving the carcassquality.
Owner:徐州金利饲料厂
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
InactiveUS20080199530A1Reduce the possibilityImprove lipophilicityPowder deliveryNervous disorderAdditive ingredientWater insoluble
Owner:COLLEGIUM PHARMA INC
Methods and compositions of gene delivery agents for systemic and local therapy
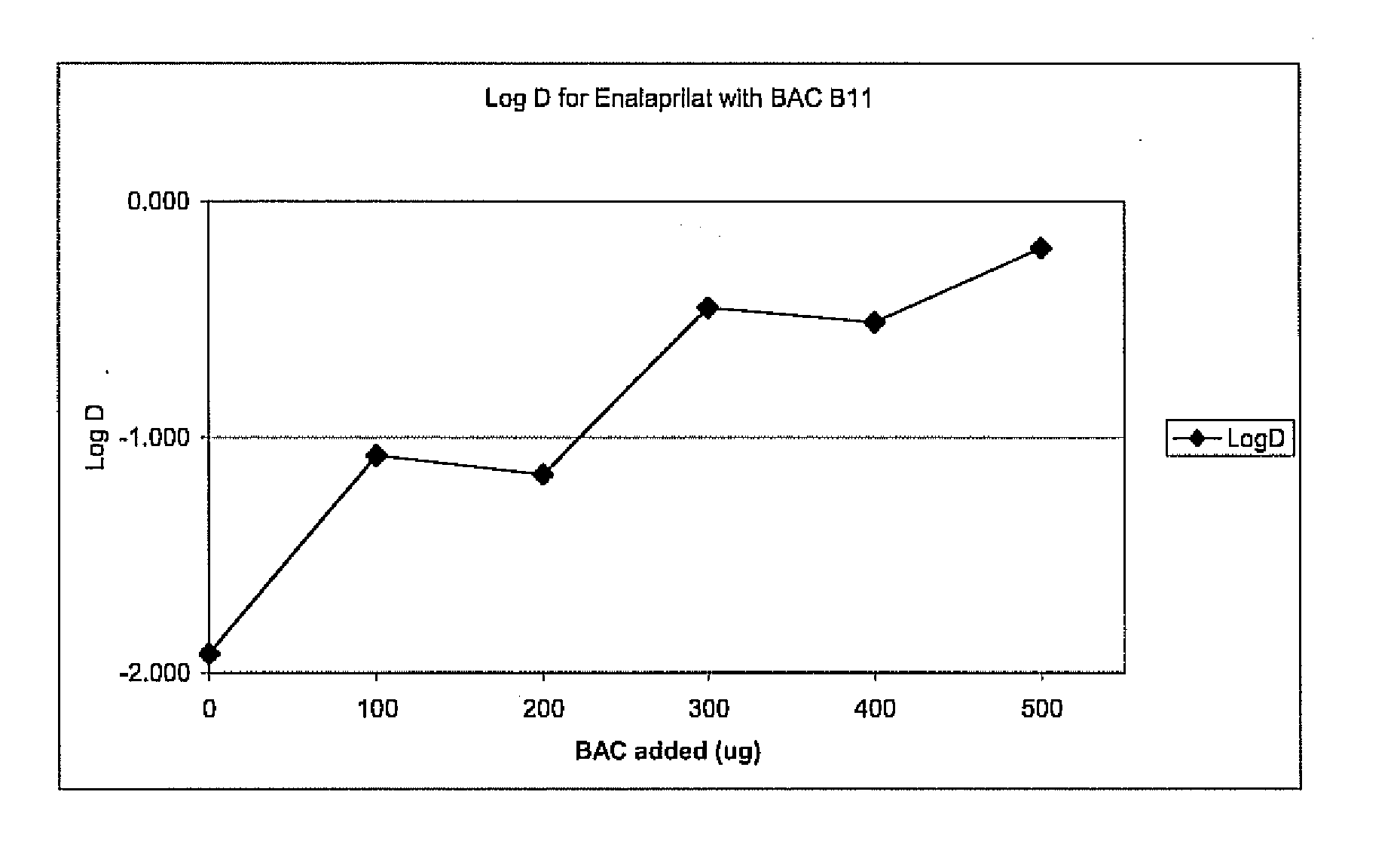
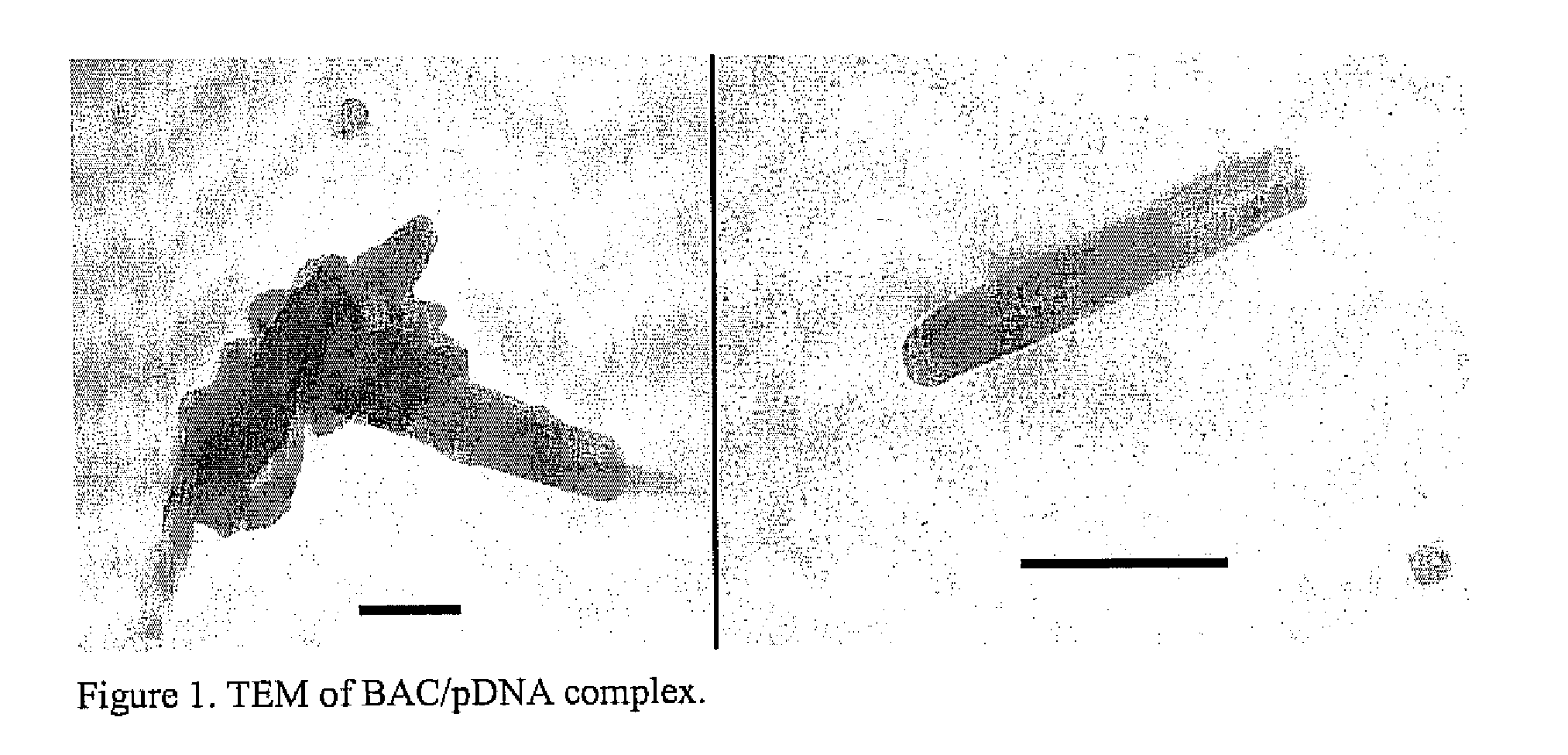
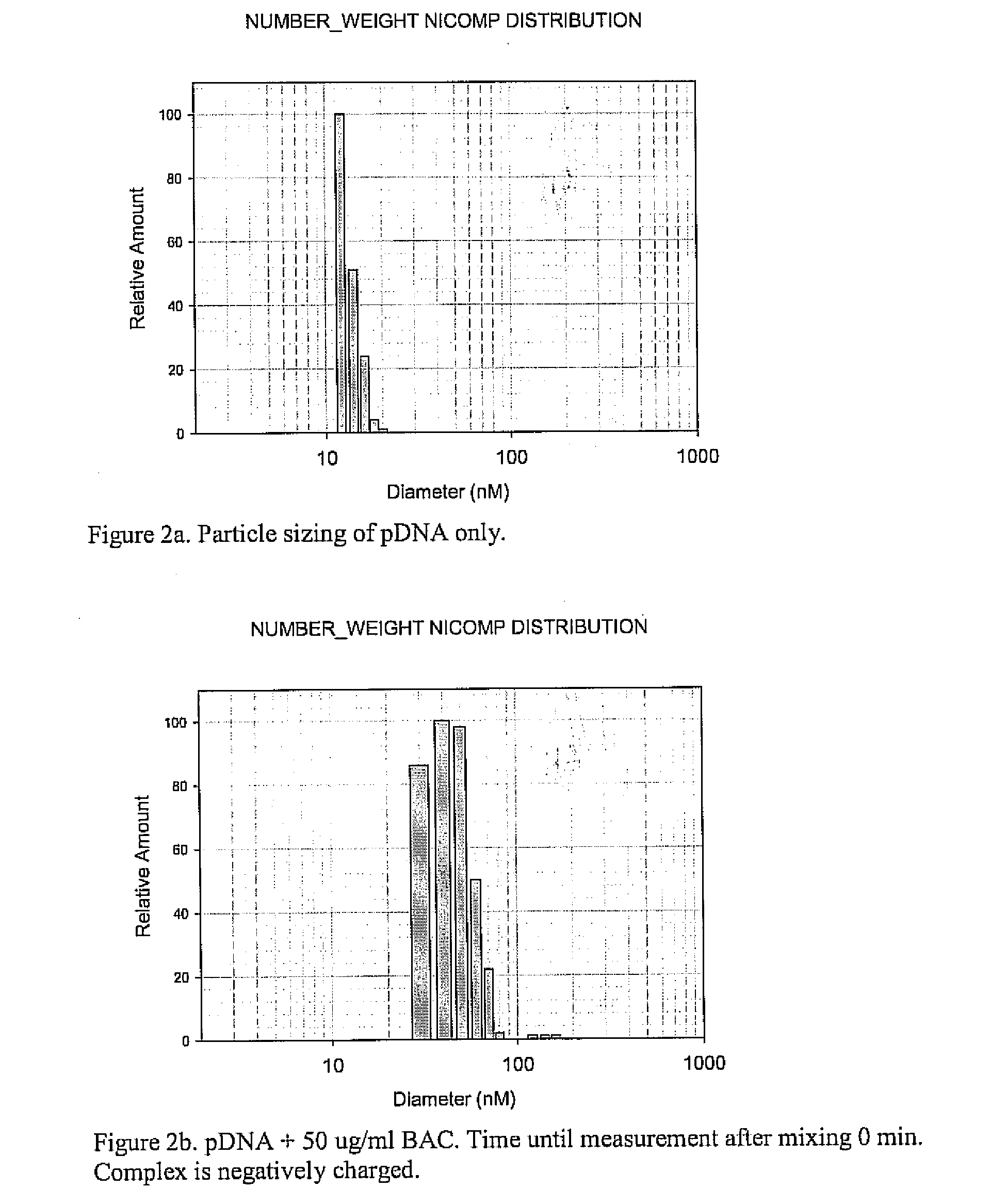
A method is provided for the delivery of a therapeutic to epithelial cells through the use of a bile acid conjugated to a peptide, the peptide being ionically charged at physiological pH. The complex is well suited for oral and other forms of therapeutic administration of therapeutic drugs known in the art in order to exact systemic and / or localized effect. Intestinal epithelial cells, as well as non-epithelial cells within the gastrointestinal tract and other target cells receive with greater efficiency a charged therapeutic when delivered with an oppositely charged bile acid conjugate (BAC) through oral administration, direct injection, or infusive administrations, thereby increasing bioavailability.
Owner:HILFINGER JOHN +2
Gastrin releasing peptide compounds
InactiveUS20060018830A1Minimizing interference signalHigh quantum yieldBiocideCarbamic acid derivatives preparationGastrin-releasing peptideImaging agent
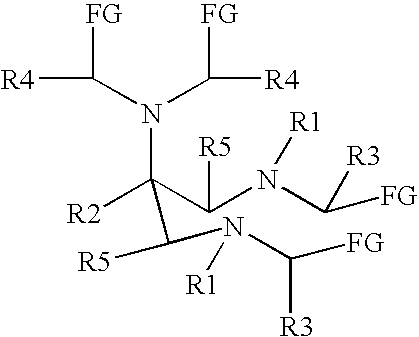
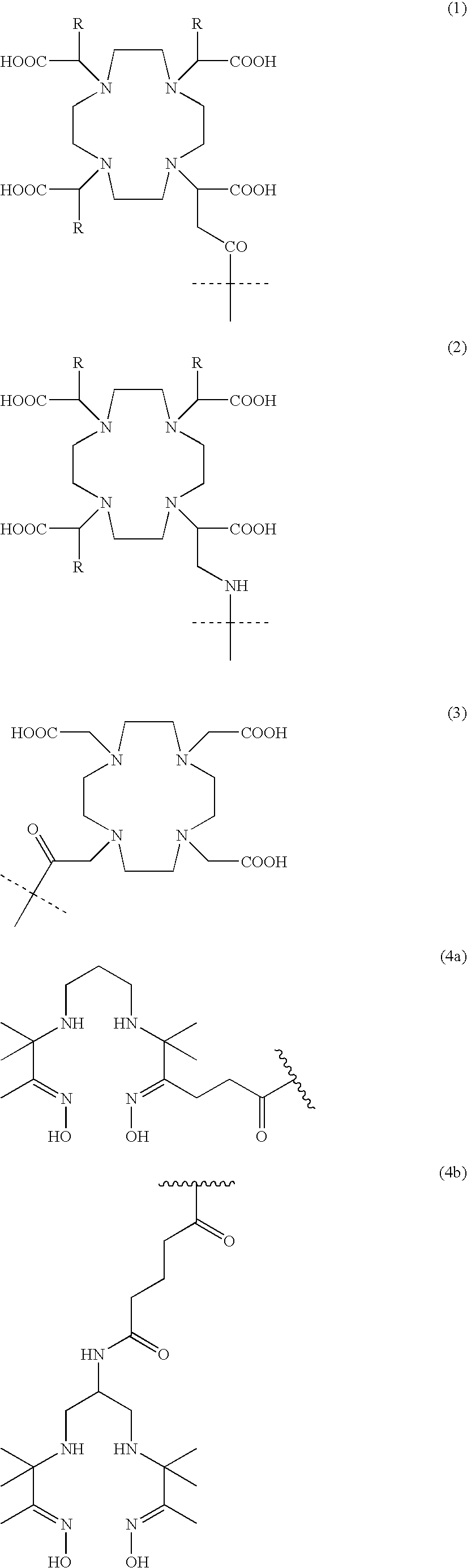
New and improved compounds for use in diagnostic imaging or therapy having the formula M-N-O-P-G, wherein M is a metal chelator having the structure: wherein R1-R5 and FG are as defined herein (in the form complexed with a metal radionuclide or not), N-O-P is the linker containing at least one non-alpha amino acid with a cyclic group, at least one substituted bile acid or at least one non-alpha amino acid, and G is the GRP receptor targeting peptide. In the preferred embodiment, M is an Aazta metal chelator or a derivative thereof. Methods for imaging a patient and / or providing radiotherapy or phototherapy to a patient using the compounds of the invention are also provided. Methods and kits for preparing a diagnostic imaging agent from the compound is further provided. Methods and kits for preparing a radiotherapeutic agent are further provided.
Owner:BRACCO IMAGINIG SPA
Feed additive, feed and preparation method thereof for preventing nutritional fatty liver of fish
The invention provides a feed additive, feed and preparation method thereof for preventing nutritional fatty liver of fish. Optimized compound of choline chloride, bile acid, carnitine, betaine and phospholipids is added into pellet feed for feeding fish, which can obviously reduce the settlement of fat in liver of fish caused by nutrient induction, avoid the generation of fatty liver diseases and promote the healthy growth of fish.
Owner:ZHEJIANG KESHENG FEED CO LTD
Preparation of aqueous clear solution dosage forms with bile acids
InactiveUS7303768B2Improve efficacyAlter absorptionOrganic active ingredientsHeavy metal active ingredientsBile fluidBile acid
Compositions for pharmaceutical and other uses comprising clear aqueous solutions of bile acids which do not form any detectable precipitates over selected ranges of pH values of the aqueous solution and methods of making such solutions. The compositions of the invention comprise water; a bile acid in the form of a bile acid, bile acid salt, or a bile acid conjugated with an amine by an amide linkage; and either or both an aqueous soluble starch conversion product and an aqueous soluble non-starch polysaccharide. The composition remains in solution without forming a precipitate over a range of pH values and, according to one embodiment, remains in solution for all pH values obtainable in an aqueous system. The composition, according to some embodiments, may further contain a pharmaceutical compound in a pharmaceutically effective amount. Non-limiting examples of pharmaceutical compounds include insulin, heparin, bismuth compounds, amantadine and rimantadine.
Owner:YOO SEO HONG
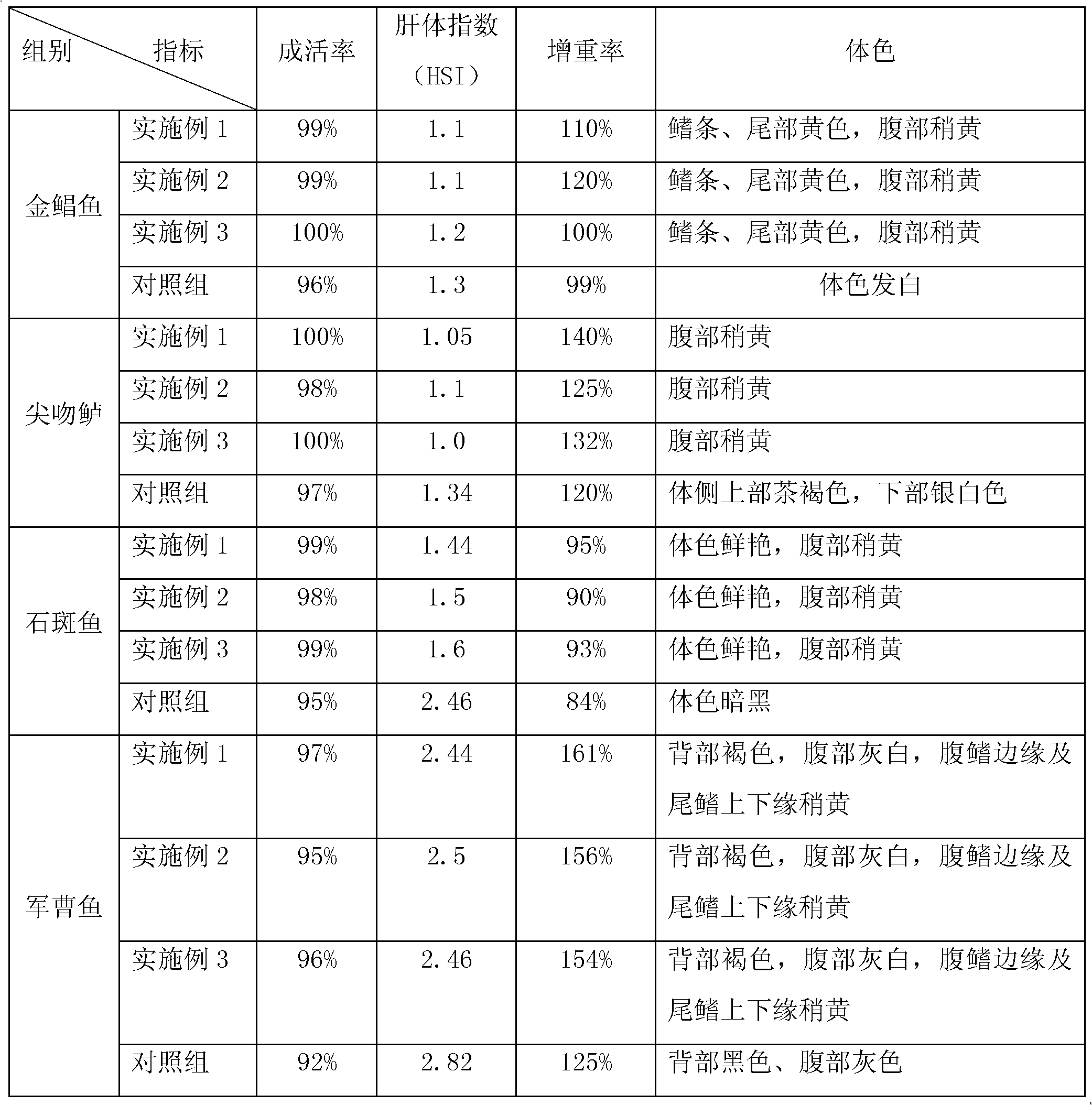
Compound feed capable of improving fatty liver and body colour of marine fish and preparation method thereof
ActiveCN102210411AImprove efficiencyAntioxidantFood processingClimate change adaptationBetaineBody colour
The invention provides a compound feed capable of improving the fatty liver and body colour of the marine fish. The compound feed comprises a premixed feed, a bile acid, curcumin and betaine, wherein the premixed feed comprises fish meal, soya bean meal, corn gluten meal, high protein flour, corn, shrimp shell meal, monocalcium phosphate, fish oil, liquid phosphatide, vitamin premixes, mineral premixes, and the like. Artificial marine-fish breeding experiments prove that the compound feed provided by the invention can meet the nutritional needs of marine-fish growth, effectively enhance the physique of the marine fish, improve the immunity of the marine fish, reduce the incidence of a fatty liver disease, and improve the body color of the marine fish, so that the body color of the marine fish is bright-colored and close to a wild natural color, thereby enhancing the quality of artificially cultured marine fish.
Owner:GUANGDONG EVERGREEN FEED INDAL +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com