Patents
Literature
252 results about "Vasculogenesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
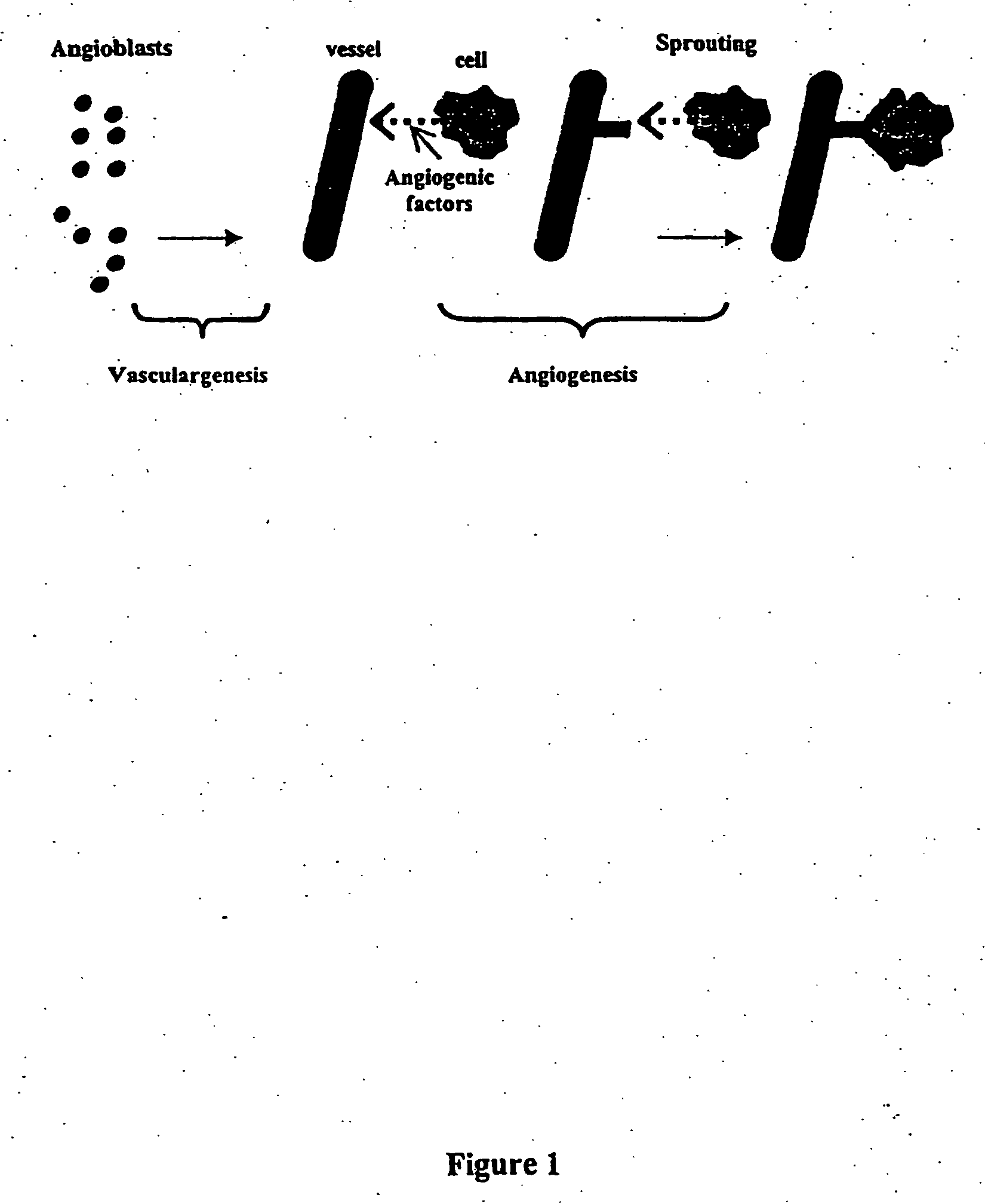
Vasculogenesis is the process of blood vessel formation in the embryo, occurring by a de novo production of endothelial cells. It is sometimes paired with angiogenesis, as the first stage of the formation of the vascular network, closely followed by angiogenesis.
Implantable device for penetrating and delivering agents to cardiac tissue
InactiveUSRE37463E1Avoid damageEliminate the effects ofTransvascular endocardial electrodesDiagnostic recording/measuringElectrical conductorCardiac wall
An implantable devices for the effective elimination of an arrhythmogenic site from the myocardium is presented. By inserting small biocompatible conductors and / or insulators into the heart tissue at the arrhythmogenic site, it is possible to effectively eliminate a portion of the tissue from the electric field and current paths within the heart. The device would act as an alternative to the standard techniques for the removal of tissue from the effective contribution to the hearts electrical action which require the destruction of tissue via energy transfer (RF, microwave, cryogenic, etc.). This device is a significant improvement in the state of the art in that it does not require tissue necrosis.In one preferred embodiment the device is a non conductive helix that is permanently implanted into the heart wall around the arrhythmogenic site. In variations on the embodiment, the structure is wholly or partially conductive, the structure is used as an implantable substrate for anti arrhythmic, inflammatory, or angiogenic pharmacological agents, and the structure is deliverable by a catheter with a disengaging stylet. In other preferred embodiments that may incorporate the same variations, the device is a straight or curved stake, or a group of such stakes that are inserted simultaneously.
Owner:BIOCARDIA
Treatment for heart disease
InactiveUS20070059288A1Poor cell survival and engraftmentImprove heart functionBiocideVirusesDiseaseCORONARY ARTERY DISEASE/MYOCARDIAL INFARCTION
The present invention provides a system for treating heart disease using a combination of pro-angiogenesis therapy and cellular cardiomyoplasty. The system is particularly useful in treating patients with damaged myocardium due coronary artery disease, myocardial infarction, congestive heart failure, and ischemia. A pro-angiogenic factor (e.g., VEGF) or a means of delivering a pro-angiogenic factor (e.g., a genetically engineered adenovirus, adeno-asssociated virus, or cells) is administered to the heart in order to promote new blood vessel growth in an ischemic or damaged area of the patient's heart. Cells such as skeletal myoblasts or stem cells (e.g., mesenchymal stem cells) with the potential to divide, differentiate, and integrate themselves into the injured myocardium are then administered into the affected area of the heart. By inducing new blood vessels growth in the injured myocardium, the cells are better able to grow and become an integral part of the heart. The invention also provides kits for use in treating a patient using the inventive method. Such kits may contain cells, catheters, syringes, needles, cell culture materials, polynucleotides, media, buffers, etc.
Owner:MYTOGEN
Stem cell-derived endothelial cells modified to disrupt tumor angiogenesis
InactiveUS20060024280A1Enhance the ability of the endothelial cells to disrupt and inhibit tumor angiogenesisHigh sensitivityBiocideGenetic material ingredientsAbnormal tissue growthTumor angiogenesis
The present invention provides cloned, genetically modified, endothelial cells, and the stem cells from which they are derived, which are produced by somatic cell nuclear transfer. The invention further provide novel therapeutic methods in which such cells are administered to a patient with tumors to inhibit and / or disrupt angiogenesis of the tumors, thereby inhibiting tumor growth and killing tumor cells.
Owner:ADVANCED CELL TECH INC
Multipotent Adult Stem Cells And Uses of Multipotent Adult Stem Cells To Treat Inflammation
InactiveUS20100172885A1Effective treatmentIncrease the number ofBiocideBone marrow stroma cellsDiseaseAutoimmune responses
Disclosed are cell preparations comprising multipotent adult stem cells and methods for using multipotent adult stem cells to treat autoimmune diseases, treat allergic responses, treat cancer, treat inflammatory diseases, treat fibrotic disorders, reduce inflammation and / or fibrosis, promote would healing, repair epithelial damage, and / or promote angiogenesis.
Owner:MESOBLAST INT
Treatment of Insulin Resistance and Diabetes
InactiveUS20080260703A1Increase perfusionImprove cell activityBiocidePeptide/protein ingredientsAngiogenesis growth factorSkeletal muscle
Disclosed are methods, compositions, and cells useful for increasing insulin sensitivity, as well as lack of insulin production in a host in need thereof. One aspect of the invention discloses methods of increasing skeletal muscle perfusion through administration of cells capable of directly and / or indirectly stimulatory of angiogenesis and / or vascular responsiveness. Another aspect provides means of increasing sensitivity to insulin through administration of a cell composition capable of integrating into host insulin responsive tissue and upregulating responsiveness either through mobilization of host cells capable of responding to insulin, mobilization of host cells capable of endowing insulin responsiveness on other host cells, exogenously administered cells taking the role of insulin responsiveness, or exogenously administered cells endowing insulin responsiveness on other host cells. Another aspect comprises modifying said host to allow for concurrent insulin sensitization and upregulated production of insulin.
Owner:CREATIVE MEDICAL HEALTH
Use of lipid conjugates in the treatment of diseases
InactiveUS7101859B2Reduce molecular weightIncrease rangeAntibacterial agentsBiocideLymphatic SpreadContact dermatitis
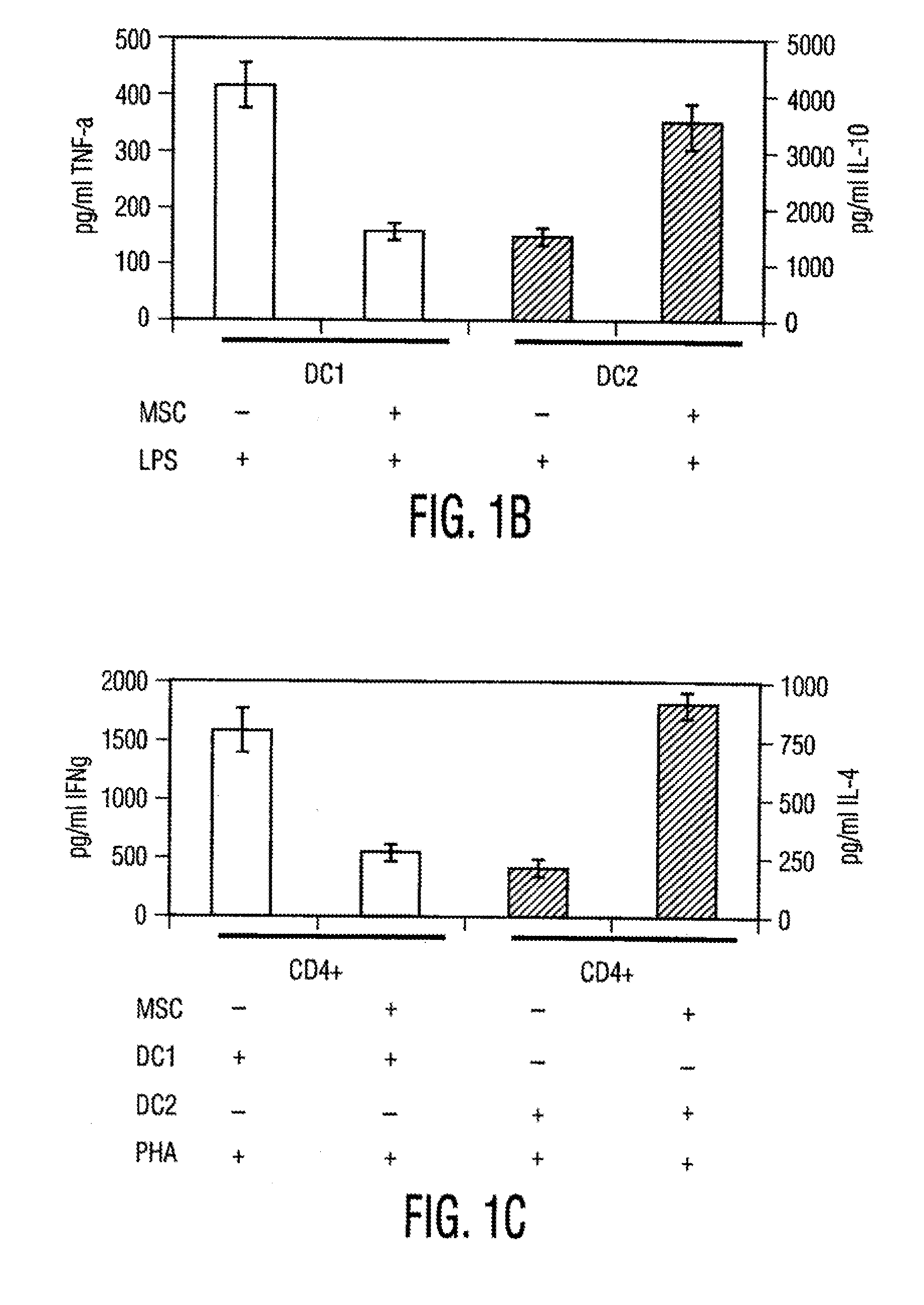
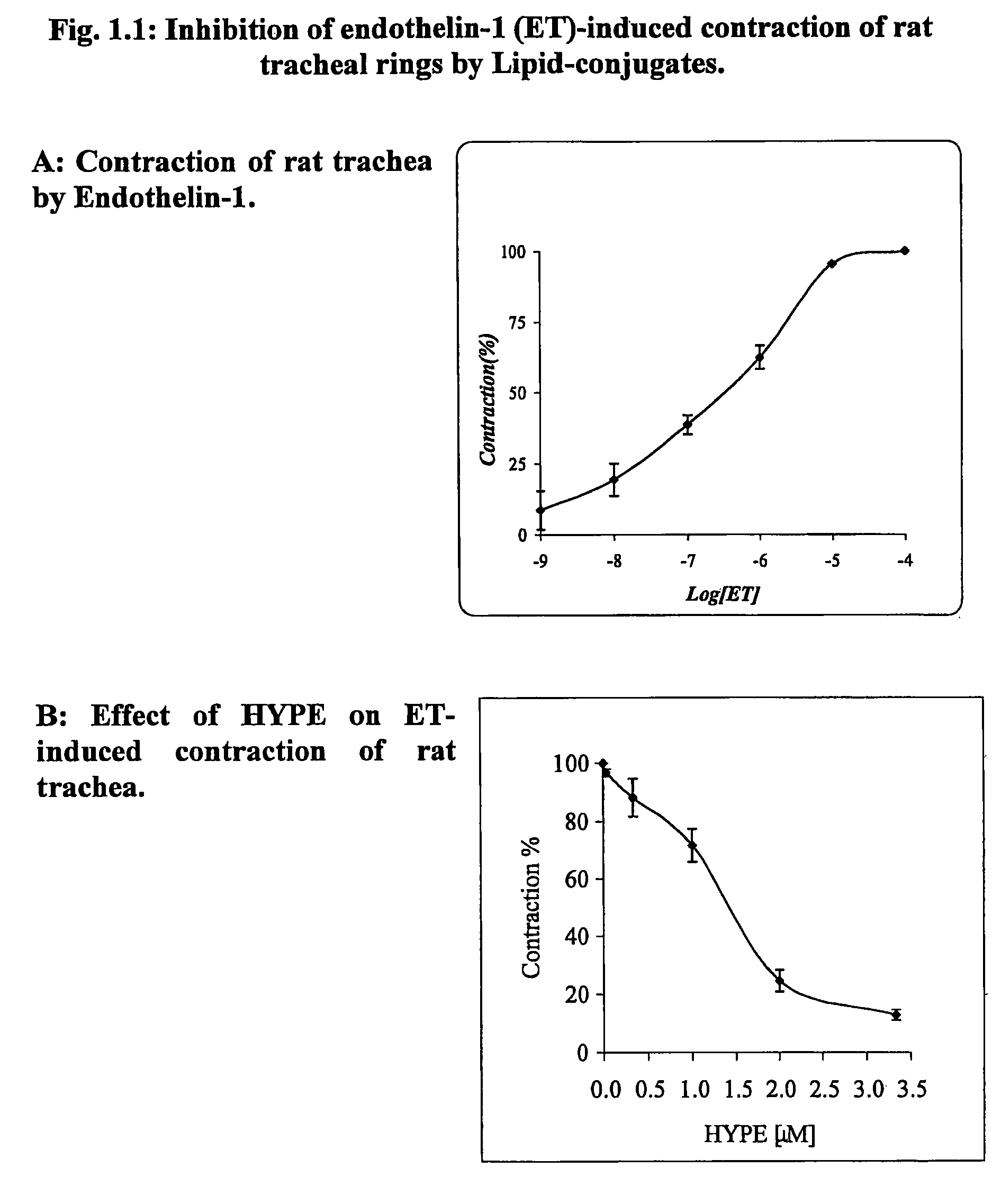
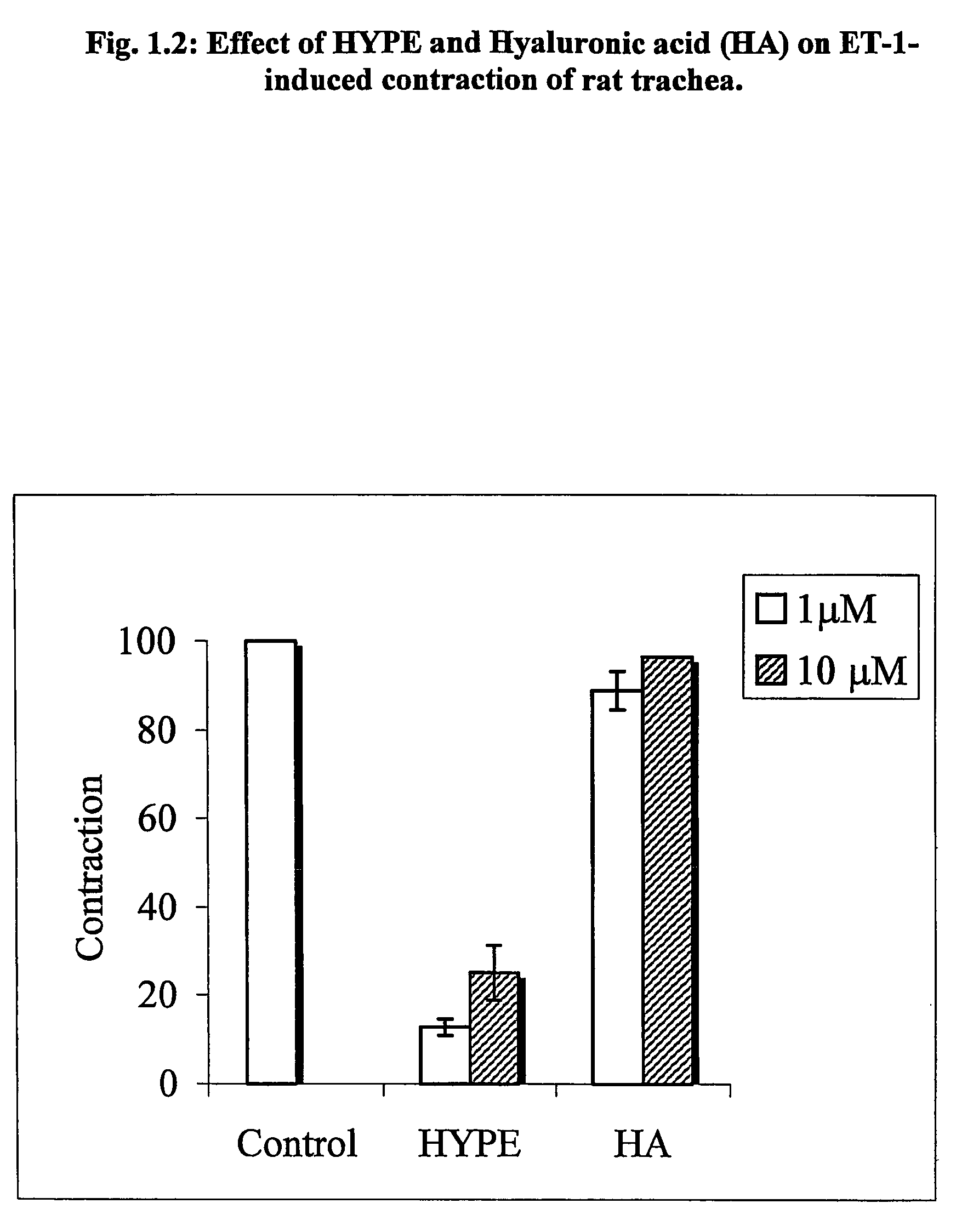
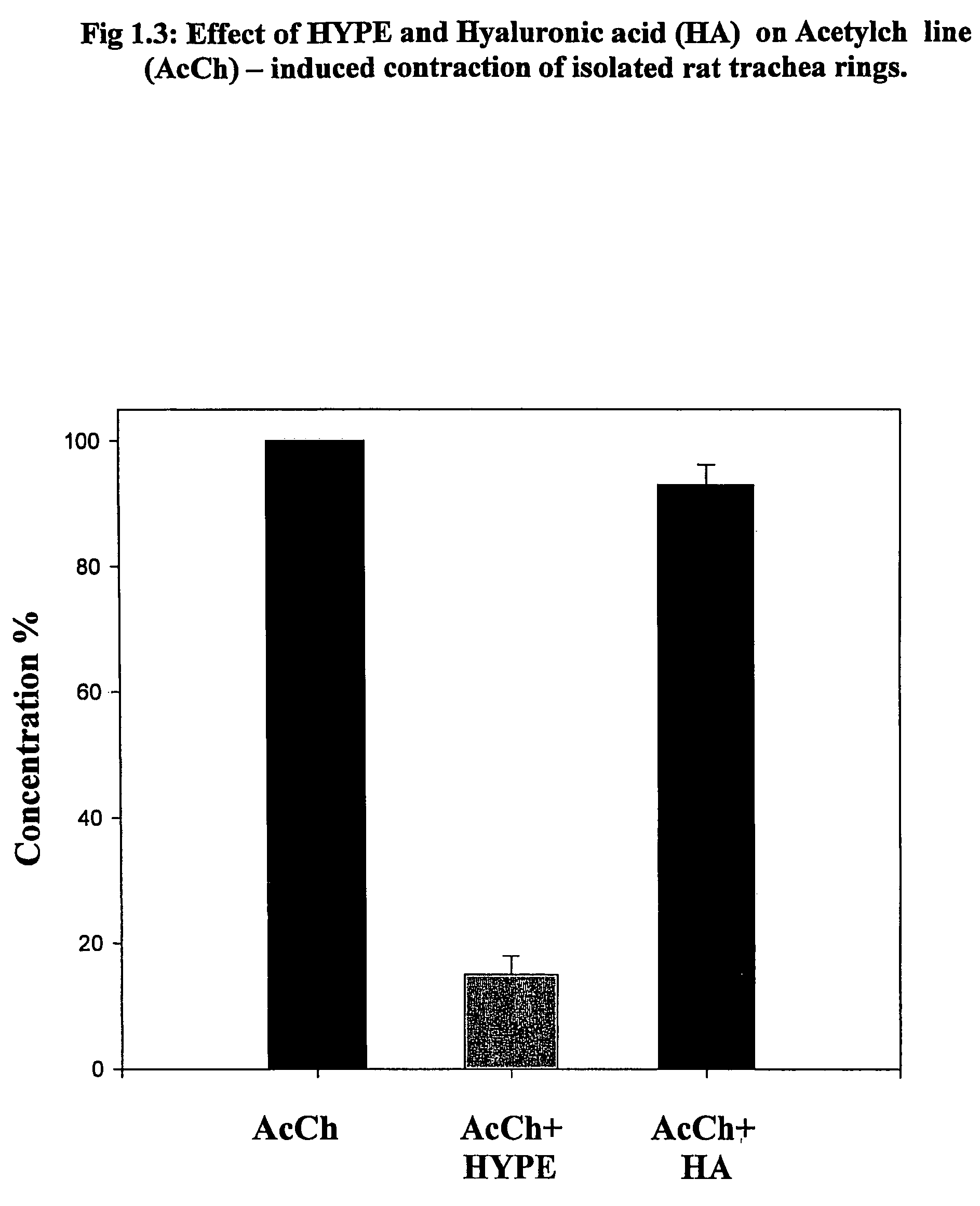
The invention provides novel methods for treating disease based upon the medicinal use of lipids and phospholipids covalently bound to physiologically acceptable monomers or polymers. Phosphatidylethanolamine moieties conjugated to physiologically acceptable monomers and polymers (PE conjugates) manifest an unexpectedly wide range of pharmacological effects, including stabilizing cell membranes; limiting oxidative damage to cell and blood components; limiting cell proliferation, cell extravasation and (tumor) cell migratory behavior; suppressing immune responses; and attenuating physiological reactions to stress, as expressed in elevated chemokine levels. The surprisingly manifold pharmacological properties of the PL-conjugates allow for the invention, disclosed herein, of novel methods for the treatment of a diverse range of disease states, including obstructive respiratory disease, including asthma; colitis and Crohn's disease; central nervous system insult, including blood brain barrier compromise, ischemic stroke, and multiple sclerosis; contact dermatitis; psoriasis; cardiovascular disease, including ischemic conditions and prophylaxis for invasive vascular procedures; cellular proliferative disorders, including anti-tumor vasculogenesis, invasiveness, and metastases; anti-oxidant therapy; hemolytic syndromes; sepsis; acute respiratory distress syndrome; tissue transplant rejection syndromes; autoimmune disease; viral infection; and hypersensitivity conjunctivitis. The therapeutic methods of the invention include administration of phosphatidylethanolamine bound to carboxymethylcellulose, heparin, hyaluronic acid, polyethylene glycol, and Polygeline (haemaccel). Disclosed herein are also new compounds comprised of phospholipid moieties bound to low molecular weight monomers and dimers, including mono- and disaccharides, carboxylated disaccharides, mono- and dicarboxylic acids, salicylates, bile acids, and fatty acids.
Owner:YEDGAR SAUL
Adipose derived stromal cells exhibiting characteristics of endothelial cells
The present invention encompasses an adipose-derived adult stromal (ADAS) cell exhibiting at least one characteristic of a pre-endothelial cell and / or an endothelial cell. The present invention also encompasses compositions and methods for generating an adipose-derived adult stromal to exhibit at least one characteristic of a pre-endothelial cell and / or an endothelial cell. Methods for using the cells in vascular transplantation, tissue engineering, regulation of angiogenesis, vasculogenesis, and the treatment of numerous disorders including heart disease are also included.
Owner:ARTECEL
Methods and therapeutic compositions comprising plant extracts for the treatment of cancer
A method of treating cancer by targeting two proteases, MMP-9 and cathepsin B is provided. Therapeutic compositions comprising one or more plant extracts that inhibit MMP-9 and / or cathepsin B, which are capable of inhibiting neoplastic and / or endothelial cell migration, tumor growth, tumor-induced angiogenesis and / or metastasis are also provided. The therapeutic compositions of the invention can be used in the treatment of cancer, and methods of inhibiting tumor growth, tumor metastasis, and / or tumor-induced angiogenesis using the therapeutic compositions alone or in combination with an anti-cancer agent are, therefore, also provided.
Owner:BIOPHARMACOPAE DESIGN INT
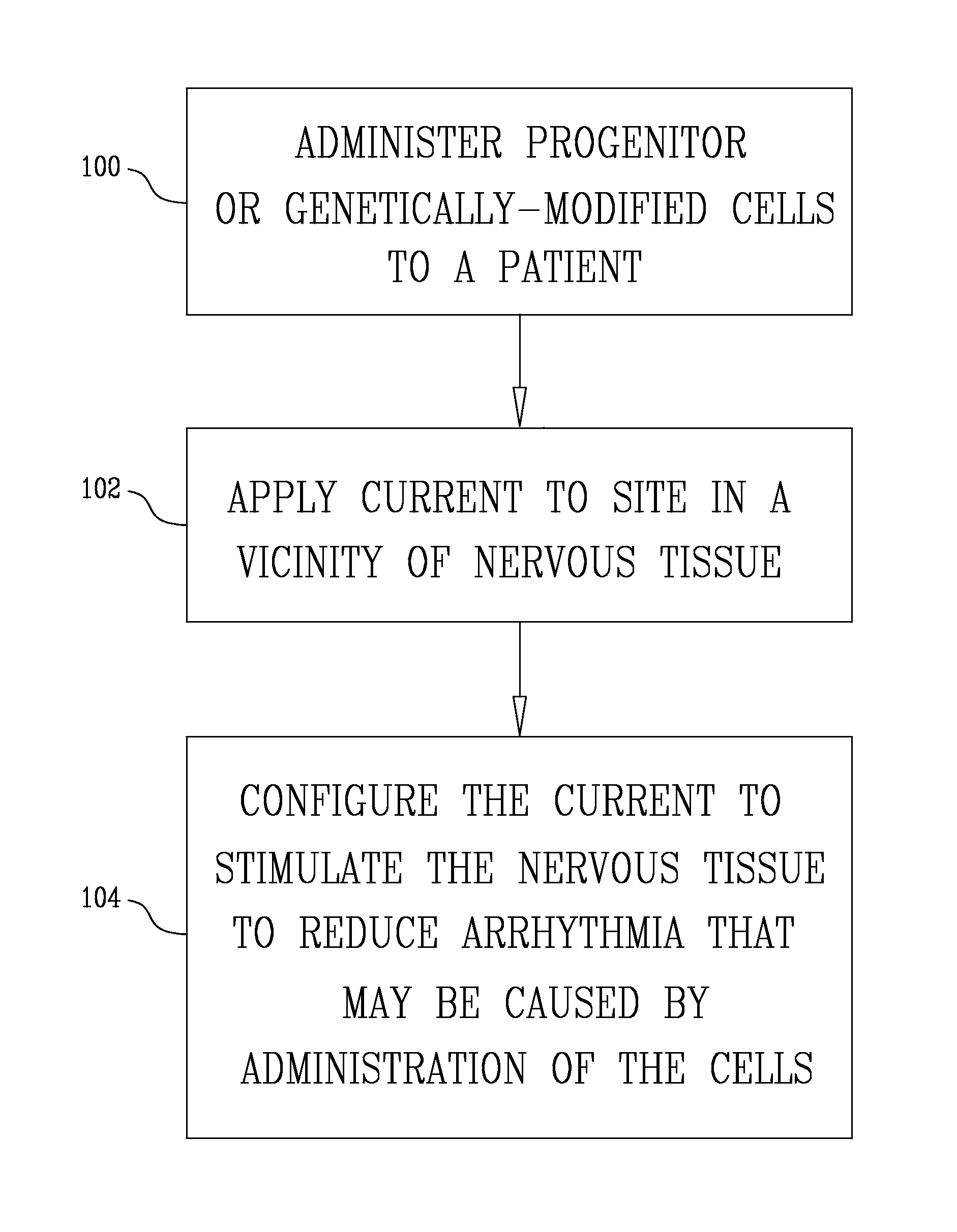
Administering bone marrow progenitor cells or myoblasts followed by application of an electrical current for cardiac repair, increasing blood supply or enhancing angiogenesis
InactiveUS8609082B2Improves the engraftment of the progenitorFunction increaseBiocideHeart stimulatorsProgenitorNeurulation
A method is provided including selecting a patient suffering from a condition, administering cells to the patient selected from the group consisting of: progenitor cells and genetically-modified cells, applying an electrical current to a site of the patient in a vicinity of nervous tissue, and configuring the current to stimulate the nervous tissue. Other embodiments are also described.
Owner:MEDTRONIC INC
VEGF variants
Applicants have defined the pro-inflammatory domain of the Vascular Endothelial Growth Factor VEGF164(165) protein molecule using VEGF164 protein mutants in which the heparin binding domain is inactivated through alanine scanning, site directed mutagenesis. The invention provides novel VEGF variants having a modified heparin binding domain. The VEGF variants modified heparin binding function compared to native VEGF while maintaining receptor binding function. The invention provides compositions and methods for treating disorders relating to angiogenesis and inflammation.
Owner:EYETECH
Biomarkers for cardiovascular disease
InactiveUS20110008346A1Reduce riskEarly detectionOrganic active ingredientsPeptide/protein ingredientsProgenitorVascular disease
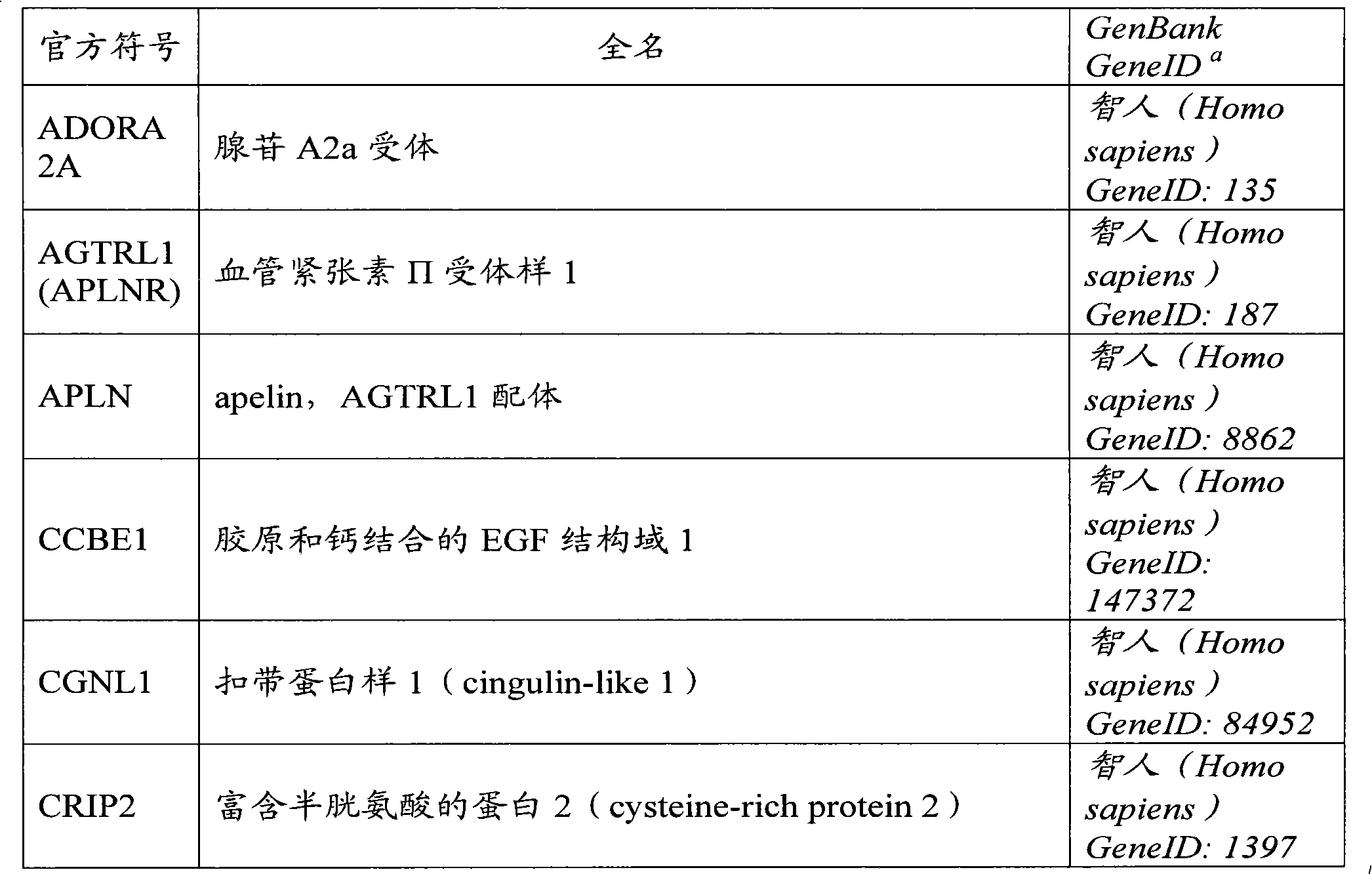
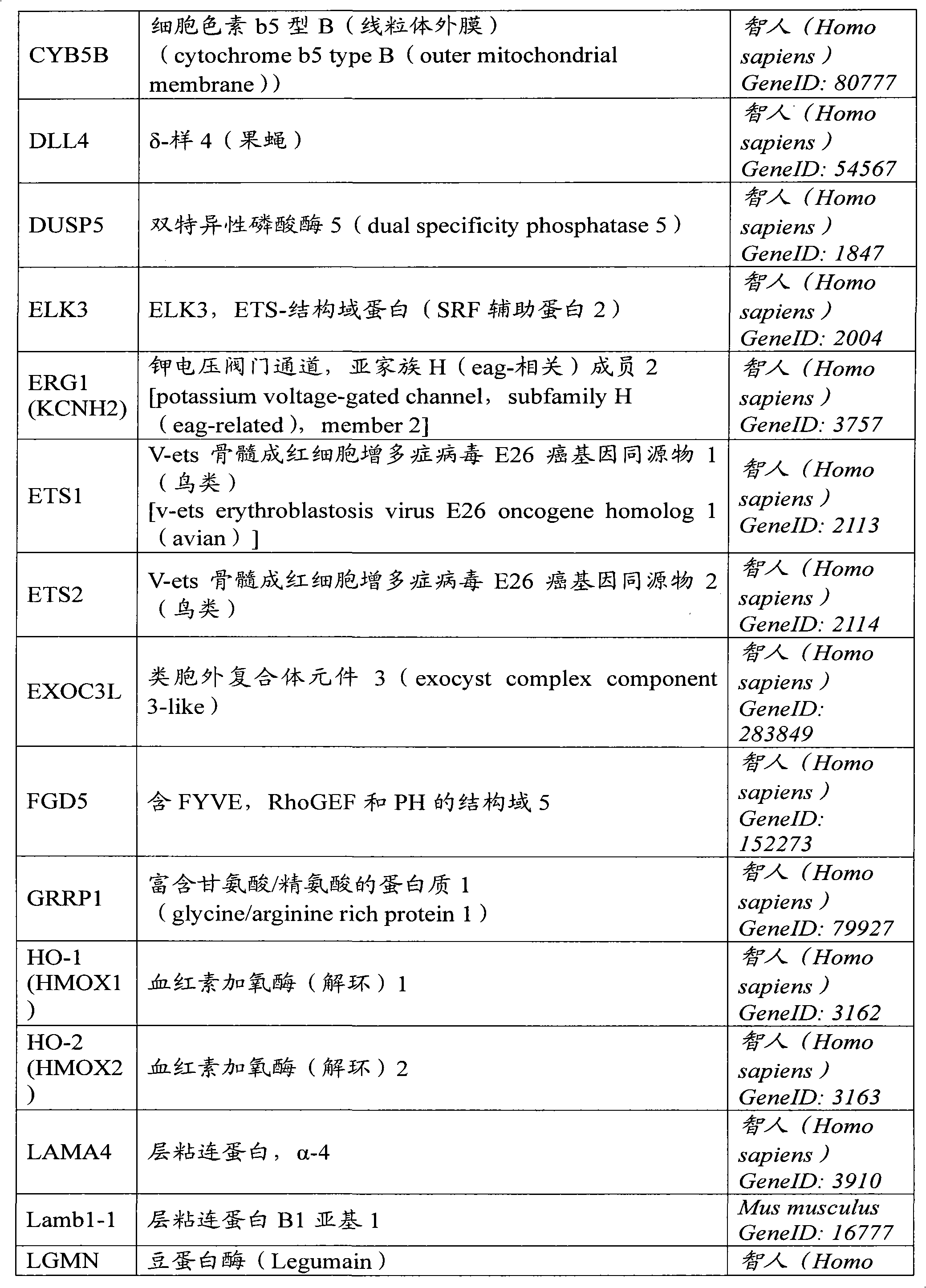
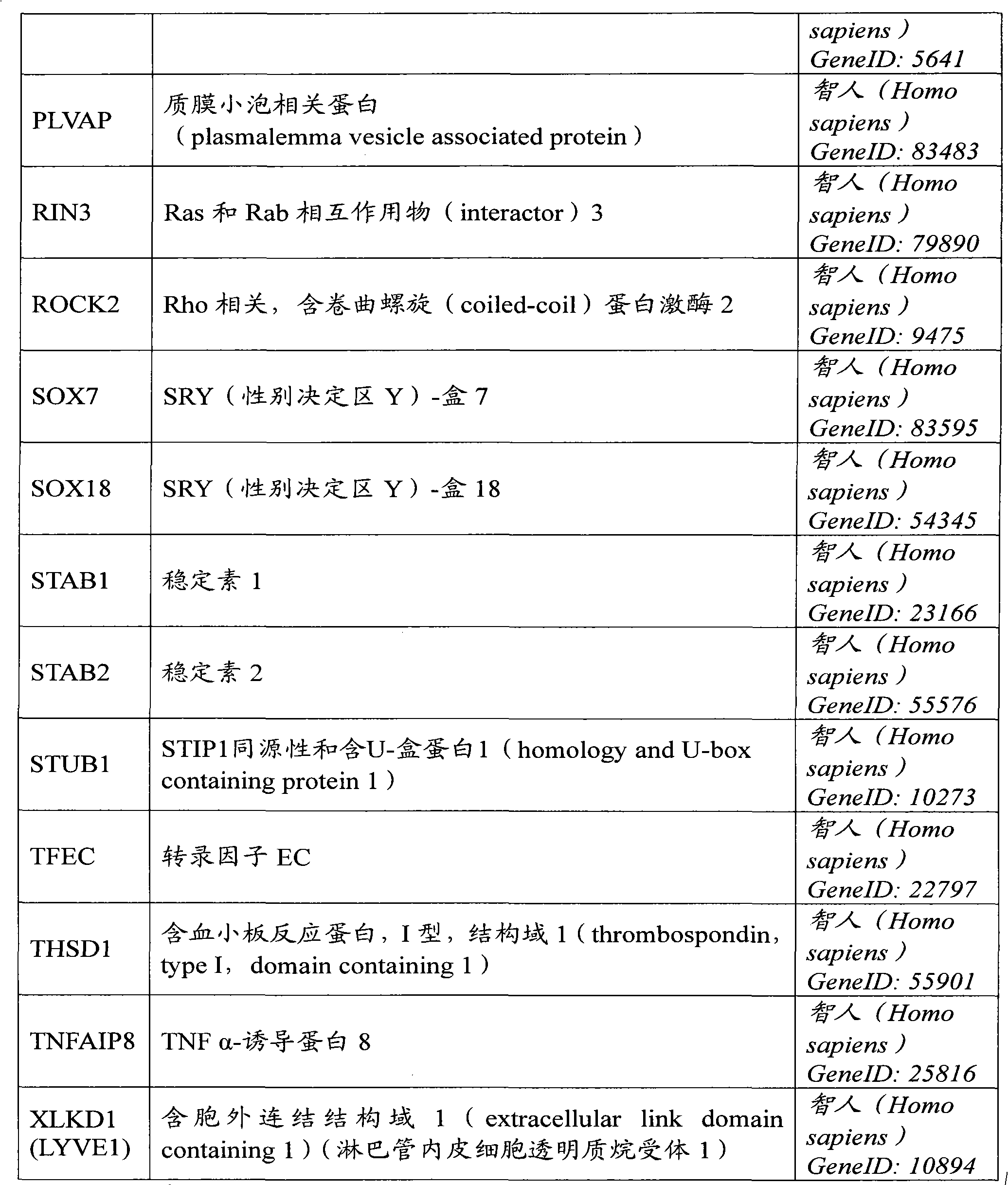
The present invention relates to a method of diagnosis or prognosis of cardiovascular disease in a subject, said method comprising the steps of detecting the presence of activated endothelial progenitor cells (EPCs) in a sample of a circulation fluid of said subject. The invention further relates to biomarkers for diagnosis or prognosis of cardiovascular disease in a patient, said biomarker comprising the expression product of a gene the expression of which is regulated during vasculogenesis.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Treatment of chronic post-traumatic encephalopathy
InactiveUS20140127171A1Inhibit host inflammatory reactionInhibit such inflammatory reactionCompounds screening/testingBiocidePost-Traumatic EncephalopathyNeural stem cell
Methods of treating chronic post-traumatic encephalopathy (PTE) using regenerative approaches is described. In one embodiment, molecules with capability of stimulating endogenous neural stem cells is provided. In another embodiment, cell therapeutics are provided capable of addressing angiogenic deficits in patients suffering from PTE. In another embodiment, cells are utilized to induce activation of endogenous progenitor cells in the central nervous system of PTE patients. Furthermore, low level laser irradiation is disclosed as a means of treatment of PTE either through direct administration to CNS tissue for stimulation of endogenous progenitor cells and reparative processes, or together with administration of exogenous stem cells, whether autologous or allogeneic. In a further embodiment exogenous stem cells are pretreated with laser prior to administration.
Owner:NOCERA ROGER +1
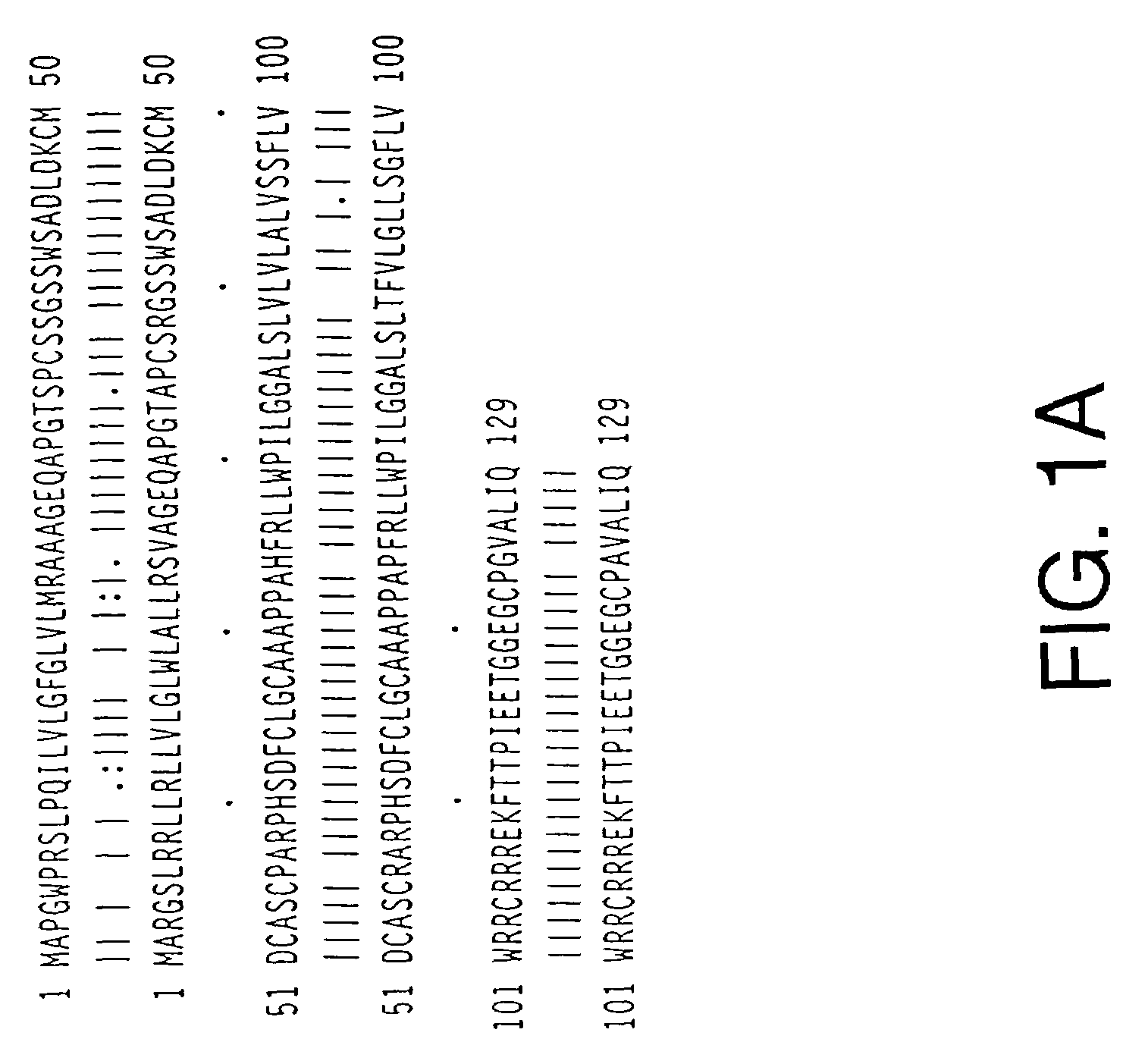
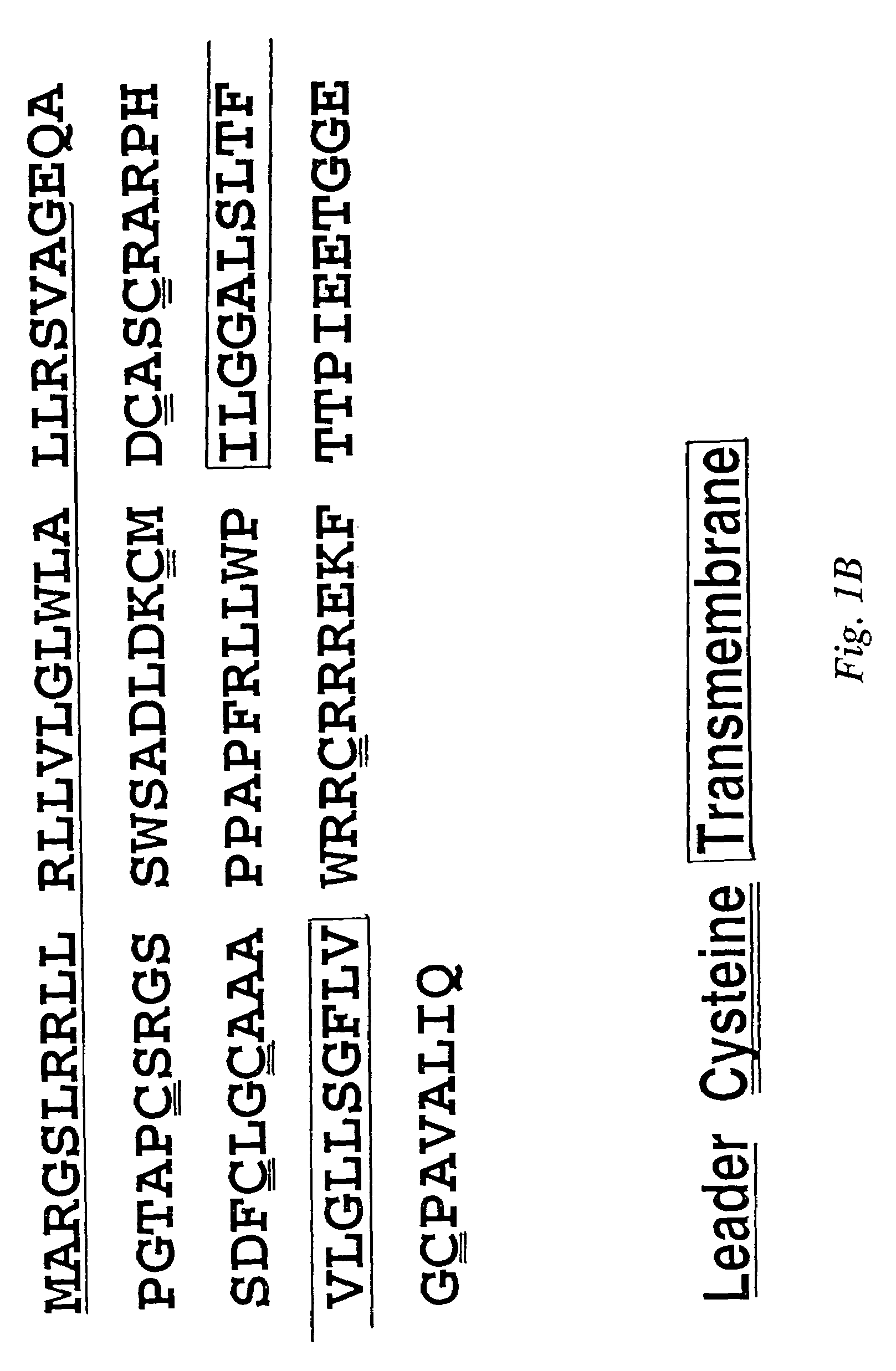
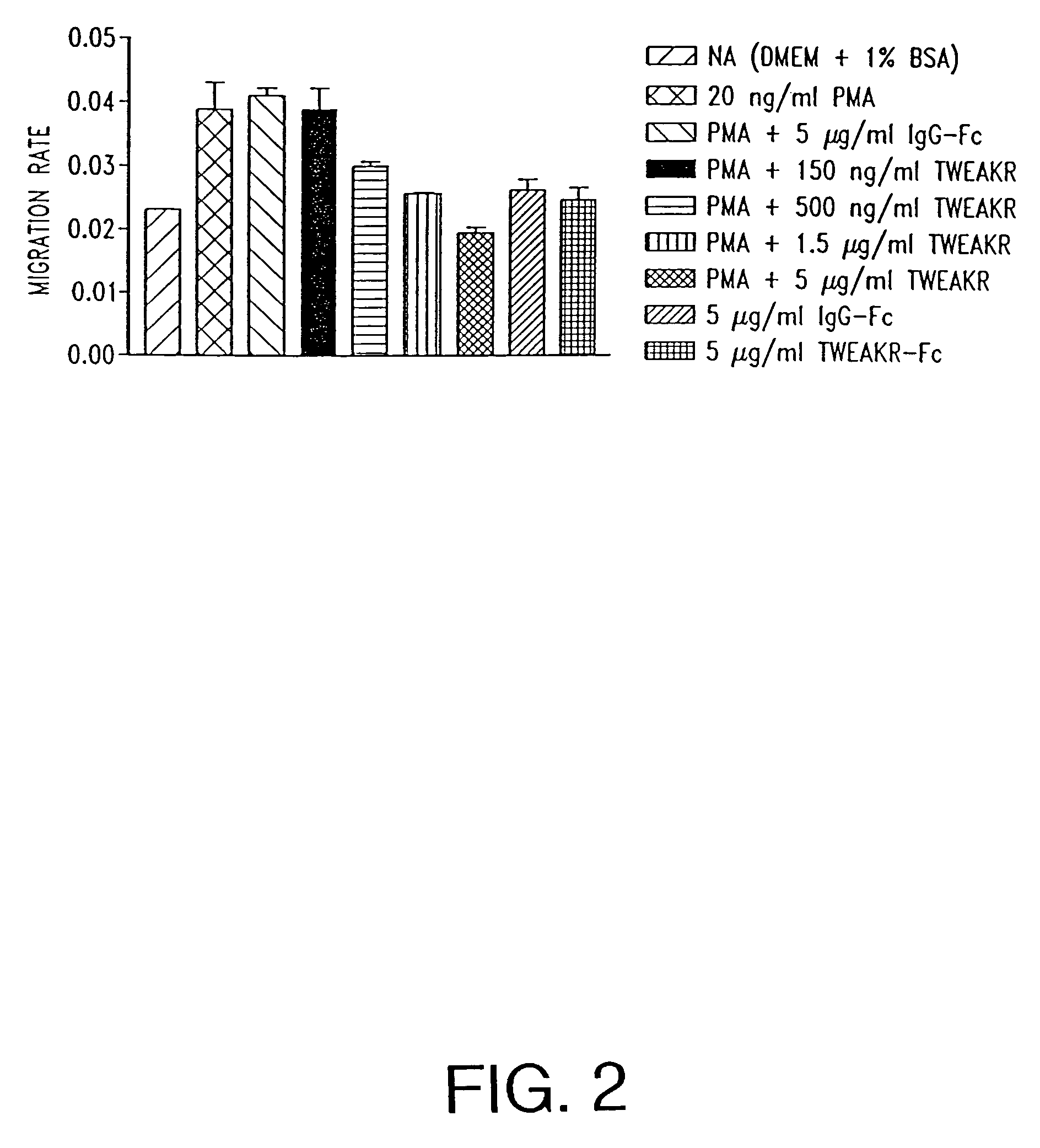
TWEAK receptor
The present invention provides the TWEAK receptor and methods for identifying and using agonists and antagonists of the TWEAK receptor. In particular, the invention provides methods of screening for agonists and antagonists and for treating diseases or conditions mediated by angiogenesis, such as solid tumors and vascular deficiencies of cardiac or peripheral tissue.
Owner:IMMUNEX CORP
Methods of screening agents for activity using teleosts
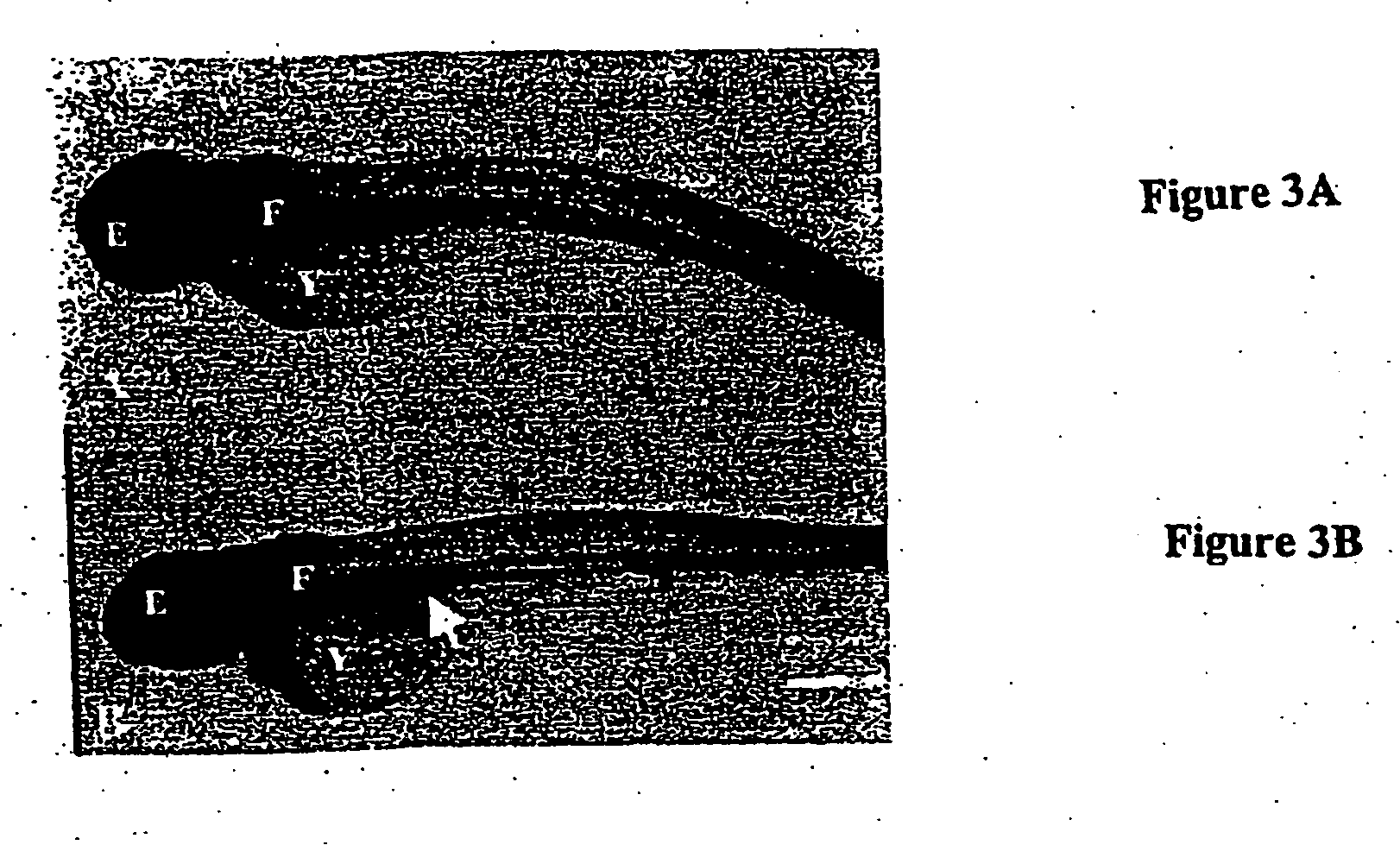
InactiveUS20060123487A1Compounds screening/testingLuminescence/biological staining preparationAngiogenesis growth factorBlood vessel
The present invention provides methods of screening an agent for activity using teleosts. Methods of screening an agent for angiogenesis activity, toxic activity and an effect cell death activity in teleosts are provided. The invention further provides high throughput methods of screening agents in multi-well plates.
Owner:PHYLONIX PHARMA
Prominin-1 peptide fragments and uses thereof
ActiveUS20110190210A1Improve angiogenesisPromote migrationNervous disorderSkeletal disorderMedicineVasodilation
Described herein are peptide compositions of a prominin-1, which have regenerative activity. As such the peptides are useful when regeneration is needed, for example, to enhance angiogenesis, increase VEGF binding to endothelial cells, promote vasodilation, enhance cell migration, enhance cell proliferation, stimulate neuronal growth, prevent neurodegeneration, and / or promote neuroregeneration.
Owner:CHILDRENS MEDICAL CENT CORP
Gene Promoting Vascular Endothelial Cell Growth
ActiveUS20070281888A1Promoting transcriptionAntibacterial agentsOrganic active ingredientsVascular endotheliumAngina
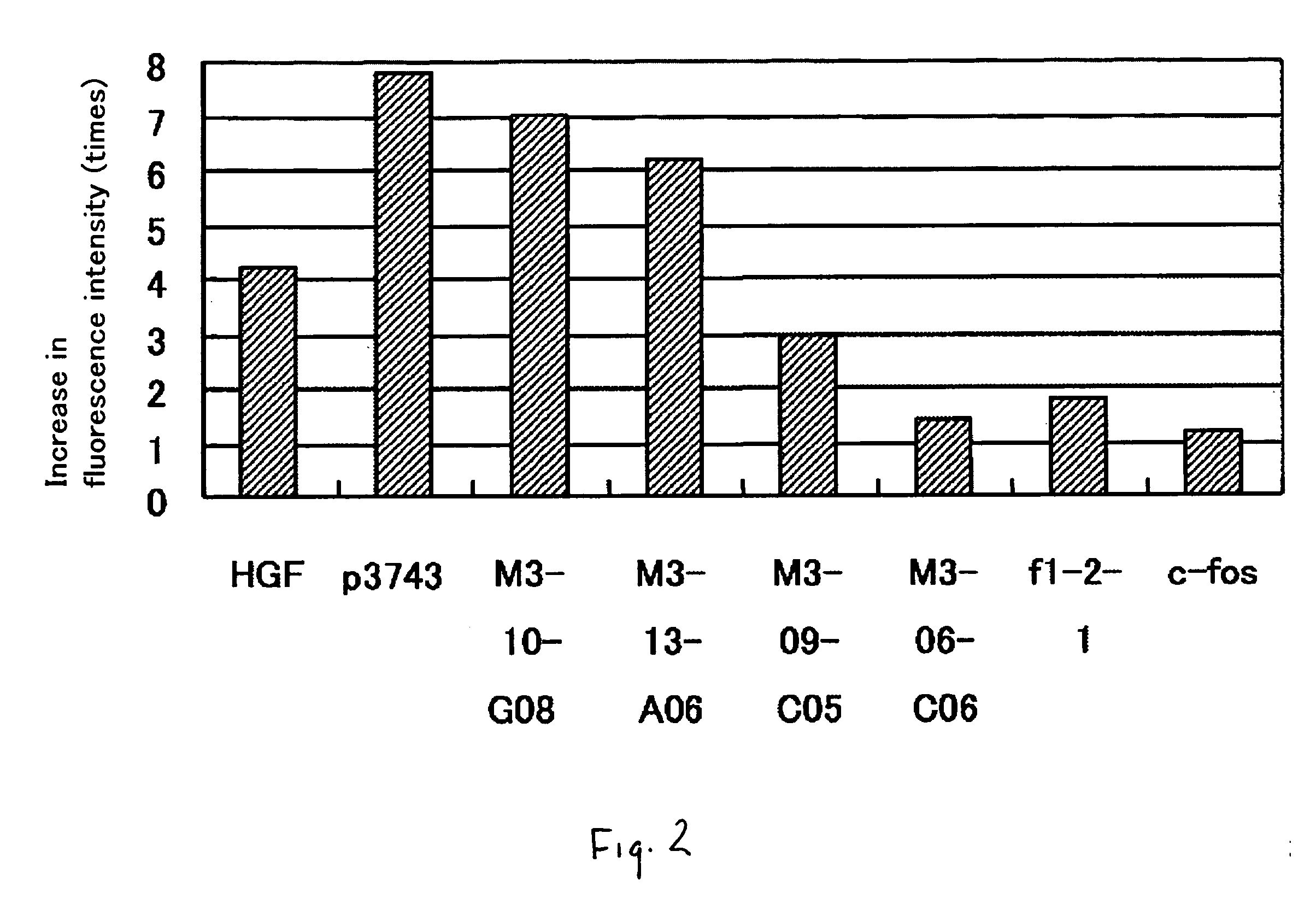
It is intended to provide a novel polypeptide having an activity of growing vascular endothelial cells, an activity of promoting transcription form c-fos promoter, an activity of promoting transciption from VEGF promoter and / or an angiogenic activity; a polynucleotide encoding this polypeptide; the above polypeptide and / or a pharmaceutical composition containing the polypeptide for treating a disease selected from the group consisting of obstructive arteriosclerosis, Buerger's disease, peripheral vascular disorder, angina, myocardial infraction, brain infarction, ischemic heart disease and ischemic brain disease; a method of treating these diseases; and an antibacterial composition. The above problems can be solved by isolating a novel peptide having the above-described activities and a nucleotide encoding this peptide.
Owner:FUNPEP CO LTD
Composition for skin regeneration, containing a secretion in the culture of an embryonic stem cell-derived endothelial progenitor cell or fractions thereof, and use thereof
InactiveUS20110300102A1Improve wrinklesPrevent skin agingCosmetic preparationsPeptide/protein ingredientsHigh concentrationCollagen VI
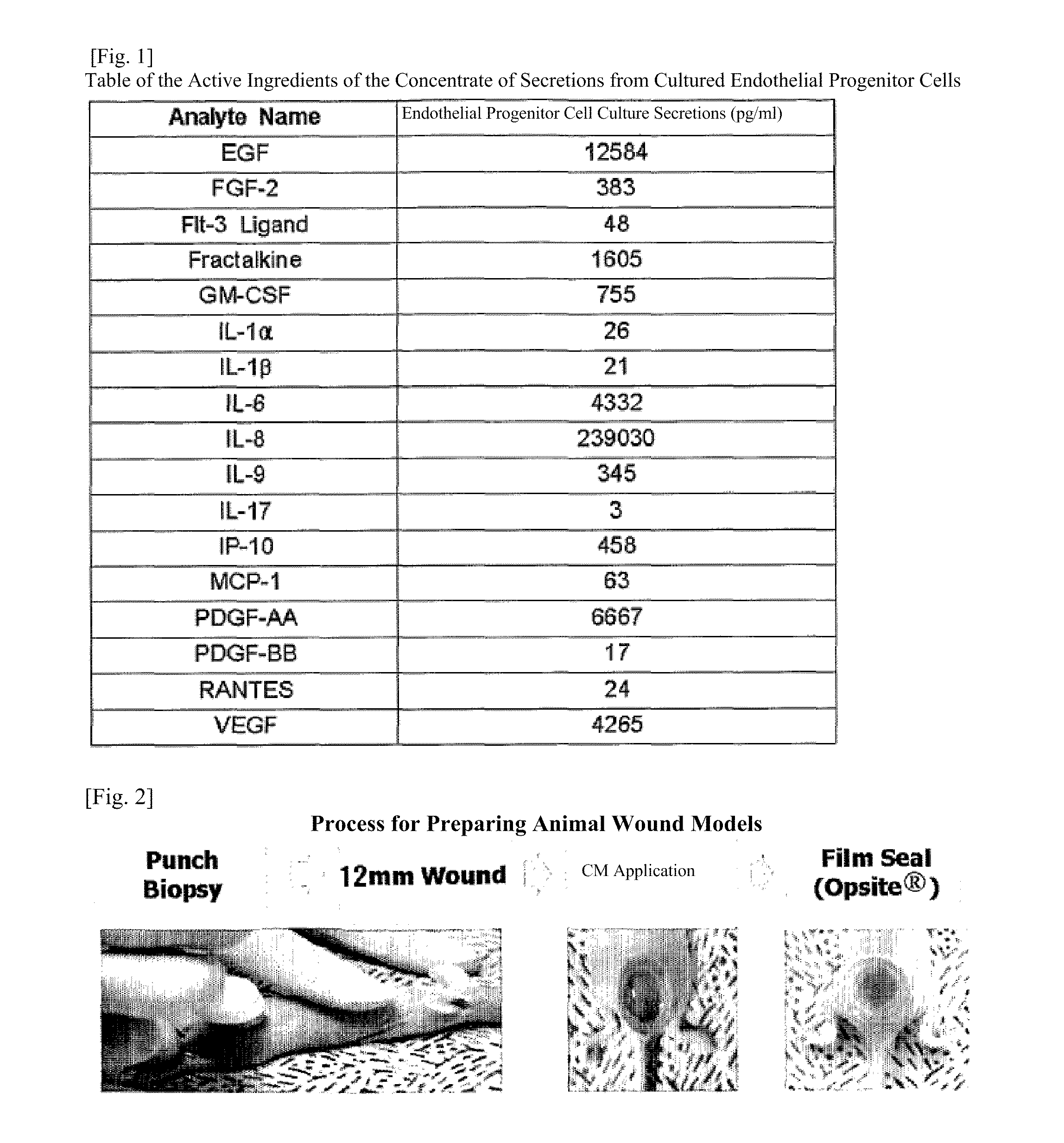
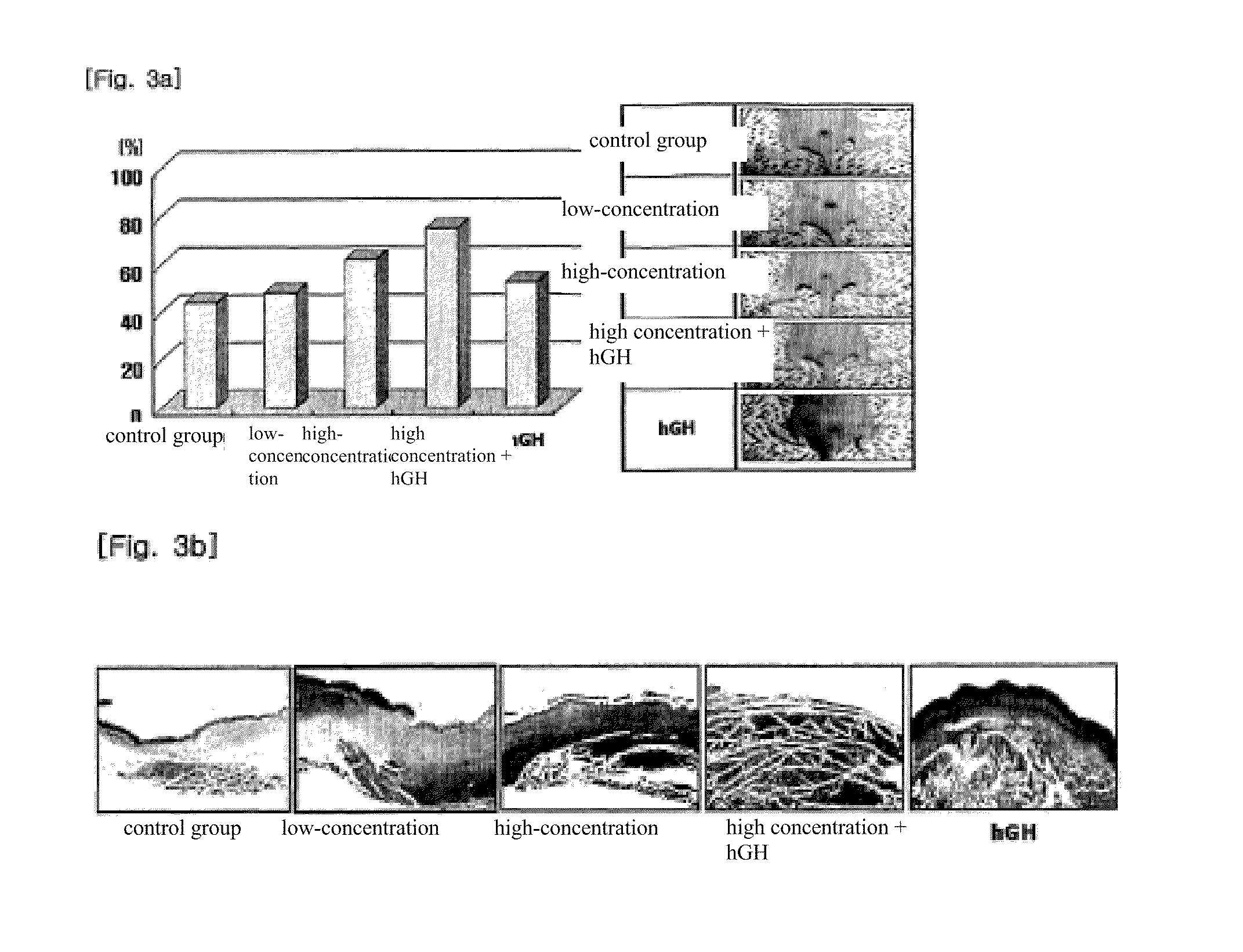
The present invention relates to a composition for skin regeneration, using a culture medium or a secretion in the culture of an embryonic stem cell-derived endothelial progenitor cell, and to the use thereof. As the present invention uses the secretion in the culture as a medicine and not the embryonic stem cell-derived endothelial progenitor cell itself, the risk of teratoma formation is prevented, and angiogenic activity is promoted to achieve improved effectiveness in healing wounds and burn wounds. The composition of the present invention, when used as a material in cosmetics, increases collagen synthesis and thus prevents skin aging. Particularly, the composition of the present invention in which the secretion in the culture is concentrated into a high concentration provides superior wound-healing effects as compared to a conventional human growth hormone (hGH).
Owner:CHABIO&DIOSTECH
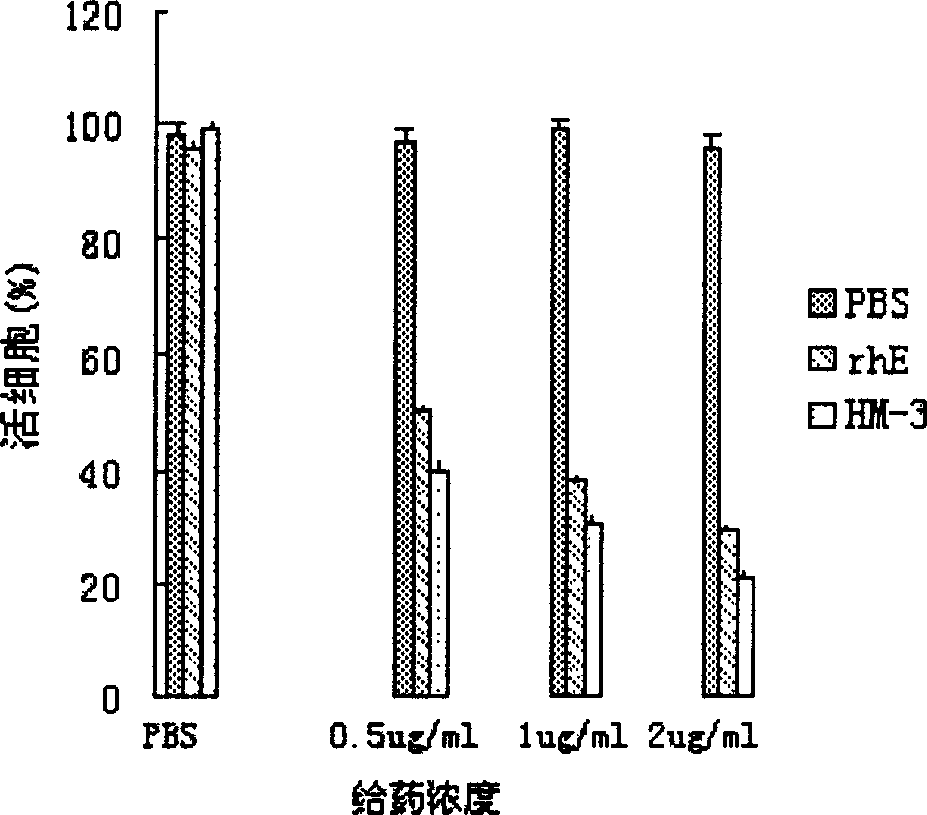
Blood vessel formation inhibitor IIM3 and its preparation method and application
InactiveCN1830487AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsAntineoplastic agentsEscherichia coliInclusion bodies
An efficient angiogenesis depressant HM-3 for treating the solid tumors including stomach cancer, lung cancer and liver cancer is prepared through expressing in colibacillus by genetic engineering method, separating the protein of inclusion body, dissolving, re-naturalizing, and separating-purifying by ion change and chromatography.
Owner:徐寒梅
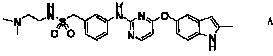
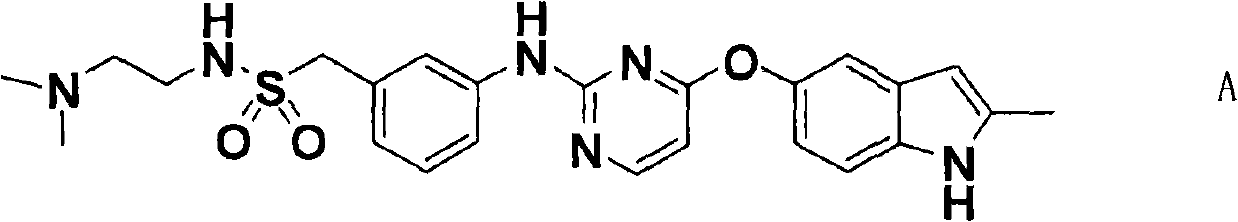
Compound and crystals thereof
ActiveCN102070618AImprove pharmaceutical propertiesOrganic active ingredientsSenses disorderDiseaseAutoimmune disease
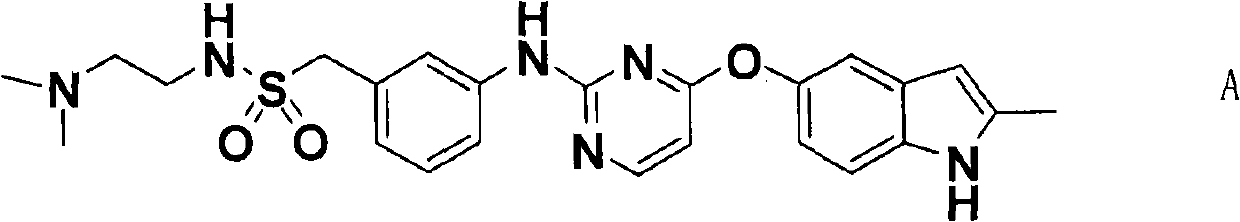
The invention discloses a pyrimidine compound of a structural formula A. The structural formula A is shown below. The pyrimidine compound can be used for preparing medicaments for treating diseases associated with vasculogenesis, in particular medicaments for treating tumor, age-related macular degeneration or autoimmune diseases.
Owner:HUTCHISON MEDIPHARMA LTD
New drug
InactiveUS20050054593A1Growth inhibitionKeep for a long timePeptide/protein ingredientsAntibody mimetics/scaffoldsLymphatic SpreadMurine fibrosarcoma
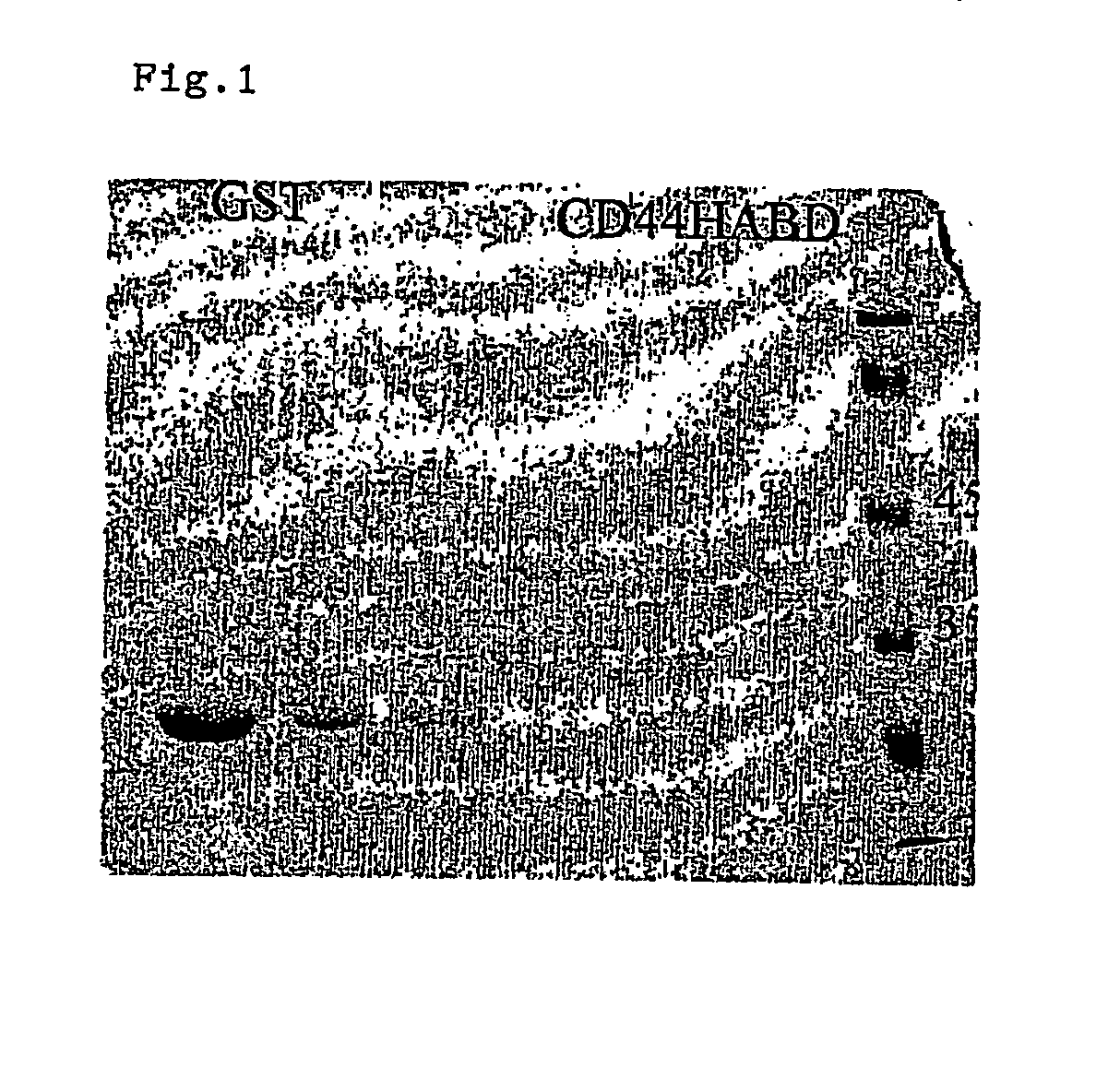
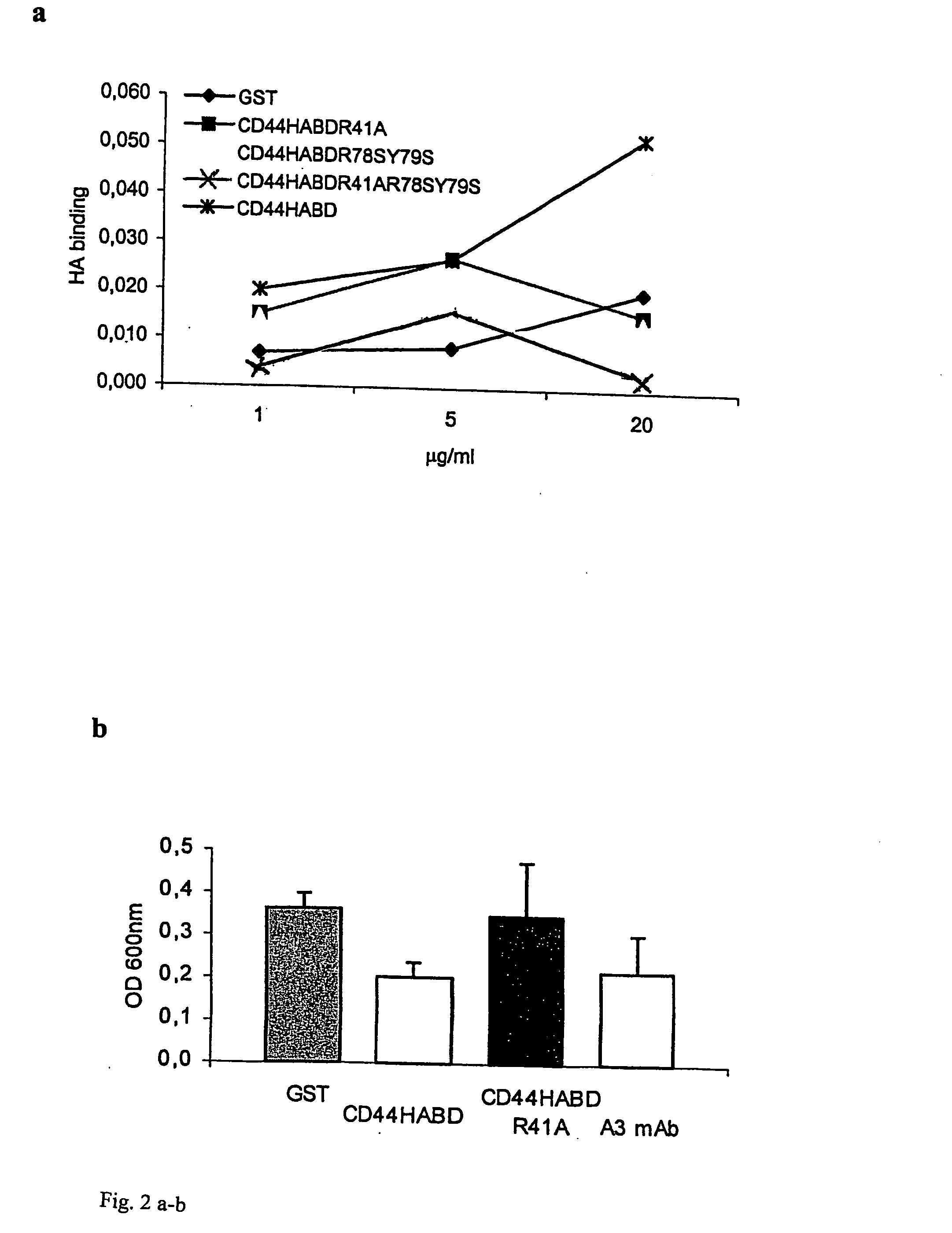
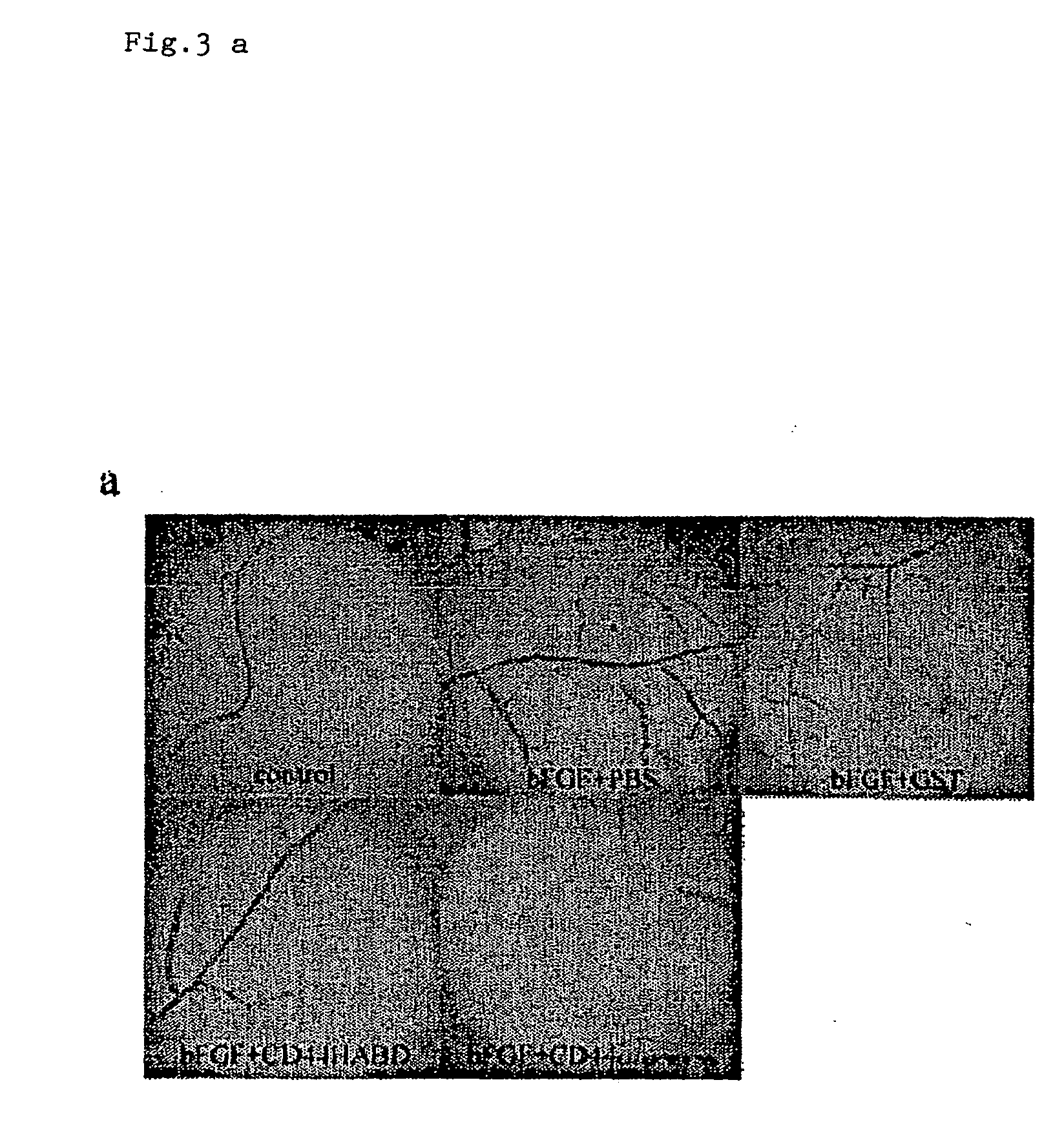
CD44, the receptor for hyaluronic acid, has complex functions in cellular physiology, cell migration and tumour metastasis. The inventors have previously found that human CD44 receptor overexpression in mouse fibrosarcoma cells inhibits subcutaneous tumour growth in mice [Kogerman et al., Oncogene 1997; 15:1407-16; Kogerman et al., Clin Exp Metastasis 1998; 16:83-93]. Here it is demonstrated that a tumour growth inhibitory effect of CD44 is caused by block of angiogenesis. Furthermore, the inventors have found that soluble recombinant CD44 hyaluronic acid binding domain (CD44HABD) inhibits angiogenesis in vivo in cLick and mouse and thereby inhibits human tumour growth of various origins. The anti-angiogenic effect of CD44-HABD is independent of hyaluronic acid (HA) binding, since non-HA-binding mutants of CD44HABD still maintain anti-angiogenic properties. The invention discloses soluble CD44 recombinant proteins as a novel class of angiogenesis inhibitors based on targeting of vascular cell surface receptor. A method of block of angiogenesis and treatment of human tumours using recombinant CD44 proteins as well as their analogues is disclosed. As a further embodiment of the invention, methods for screening for new drug targets using CD44 recombinant proteins and their analogues is presented.
Owner:CELECURE
Biomarkers for cardiovascular disease
InactiveCN101946009AMicrobiological testing/measurementCardiovascular disorderCardio vascular diseaseBlood vessel
The present invention relates to a method of diagnosis or prognosis of cardiovascular disease in a subject, said method comprising the steps of detecting the presence of activated endothelial progenitor cells (EPCs) in a sample of a circulation fluid of said subject. The invention further relates to biomarkers for diagnosis or prognosis of cardiovascular disease in a patient, said biomarker comprising the expression product of a gene the expression of which is regulated during vasculogenesis.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
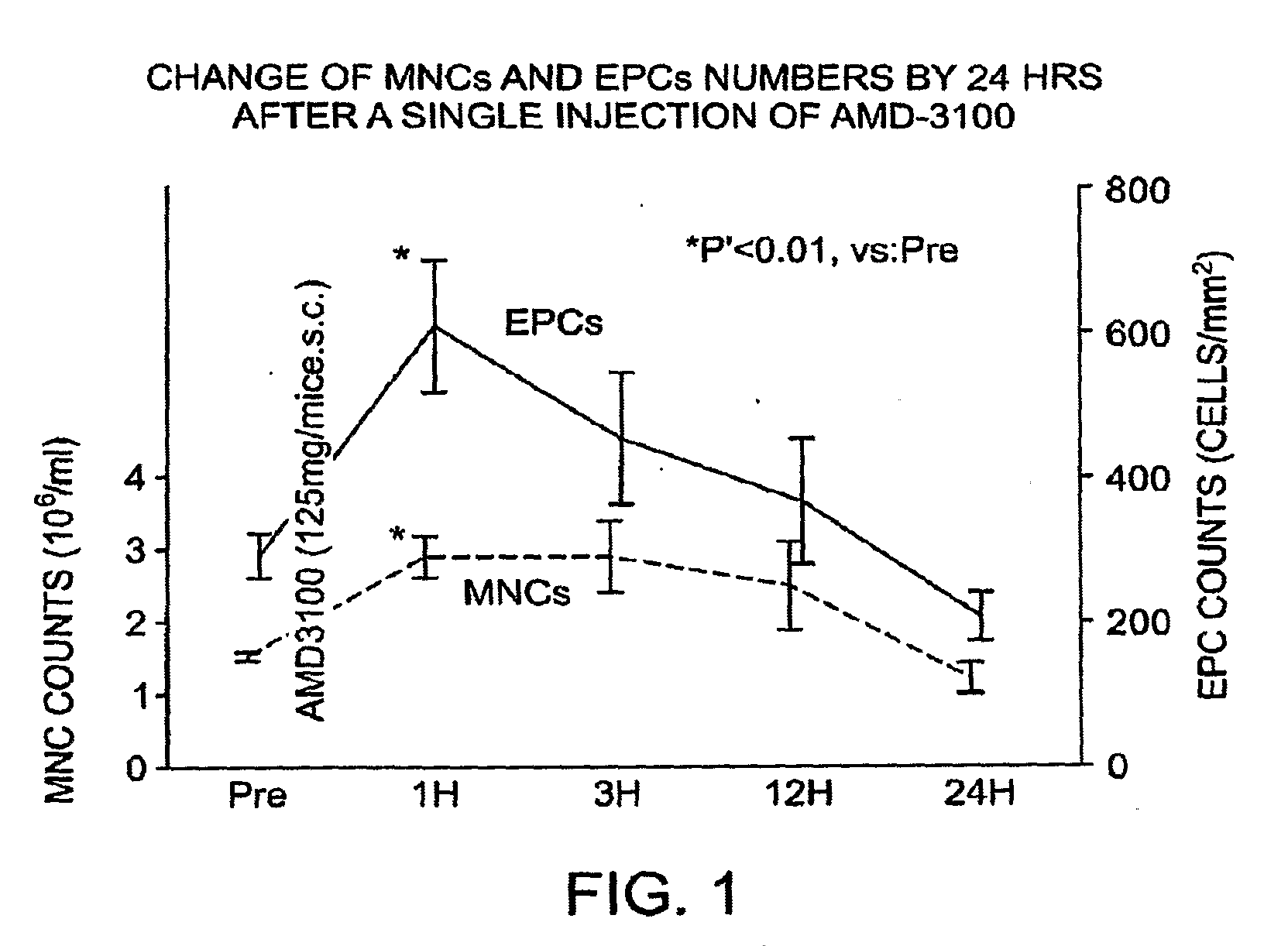
Combination of CXCR4 Antagonist and Morphogen to Increase Angiogenesis
InactiveUS20090221683A1Improve satisfactionHigh frequencyOrganic active ingredientsPeptide/protein ingredientsCXCR4 antagonistHeart disease
The present invention generally provides methods for preventing, treating or reducing the severity of symptoms associated with tissue ischemia by administering a CXCR4 antagonist in combination with at least one nucleic acid encoding a morphogen or effective fragment thereof. In one embodiment, the methods include elevating peripheral blood endothelial progenitor cells (EPCs), bone marrow-derived stem cells (BMSCs), or both cell types. The invention has a wide spectrum of applications including reducing or eliminating tissue ischemia, especially tissue ischemia associated with a myocardial infarct (heart attack).
Owner:STEWARD RES & SPECIALTY PROJECTS
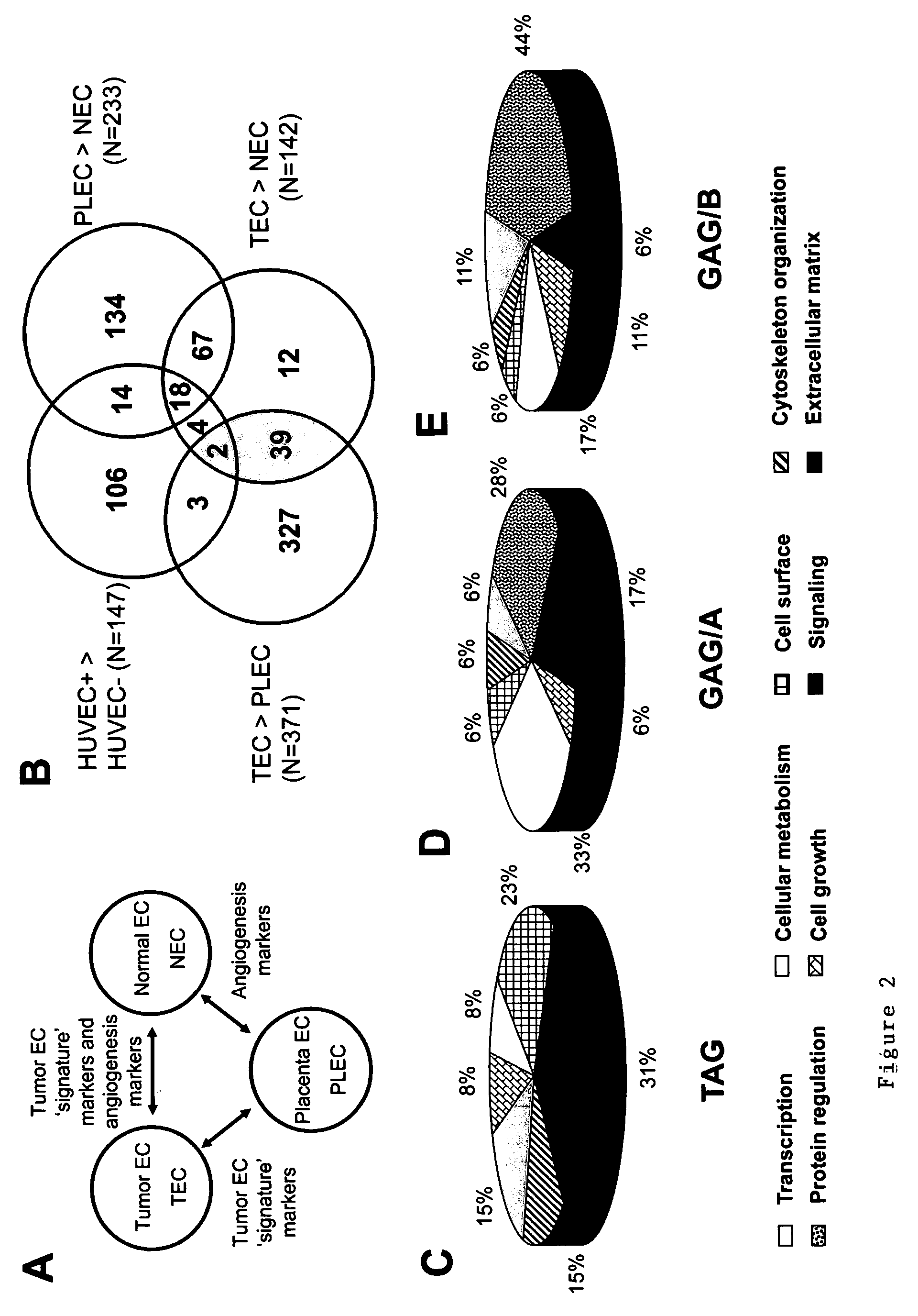
Tumor angiogenesis associated genes and a method for their identification
InactiveUS20090035312A1Inhibit angiogenesisReduced microvessel densityOrganic active ingredientsFungiAbnormal tissue growthMouse tumor
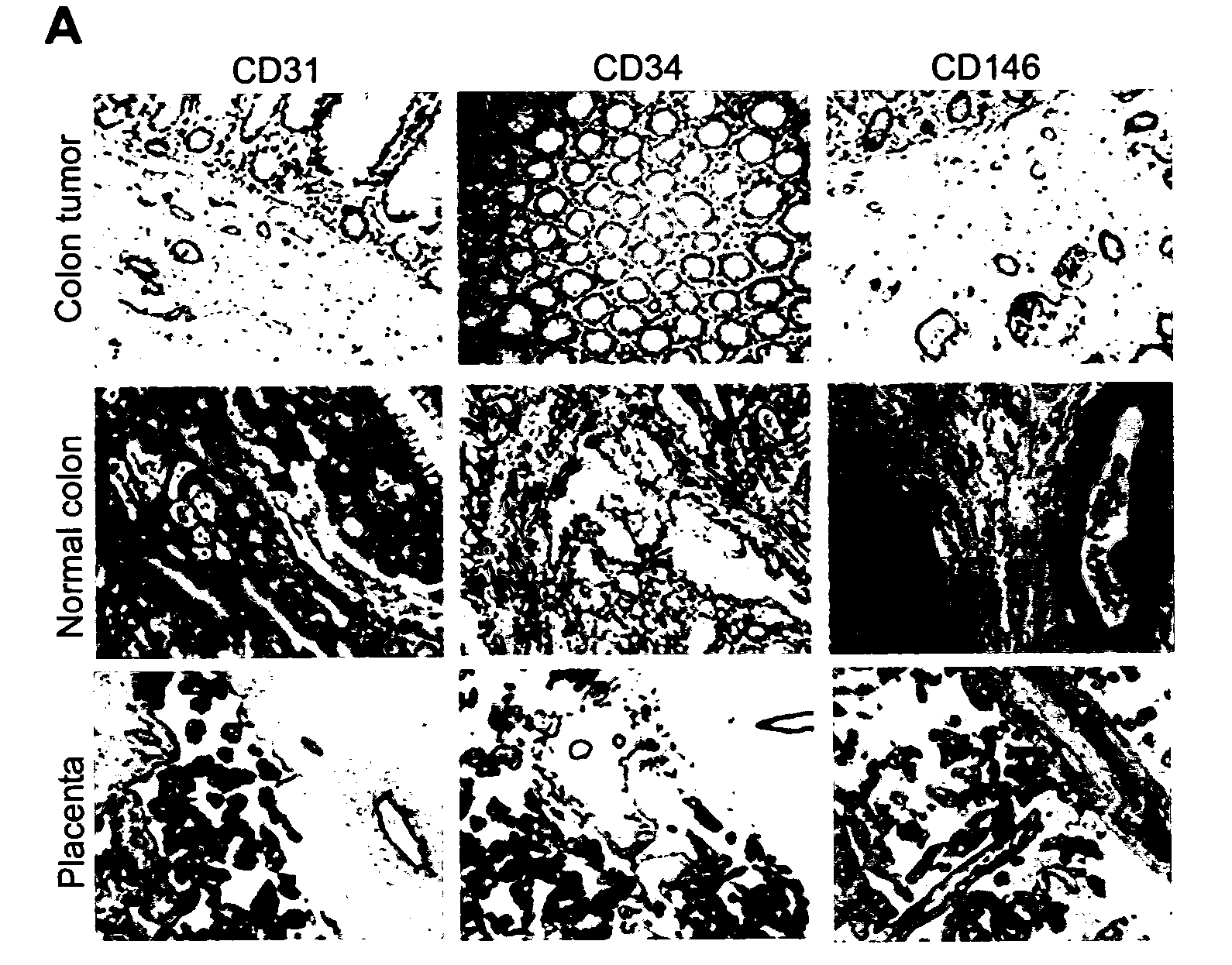
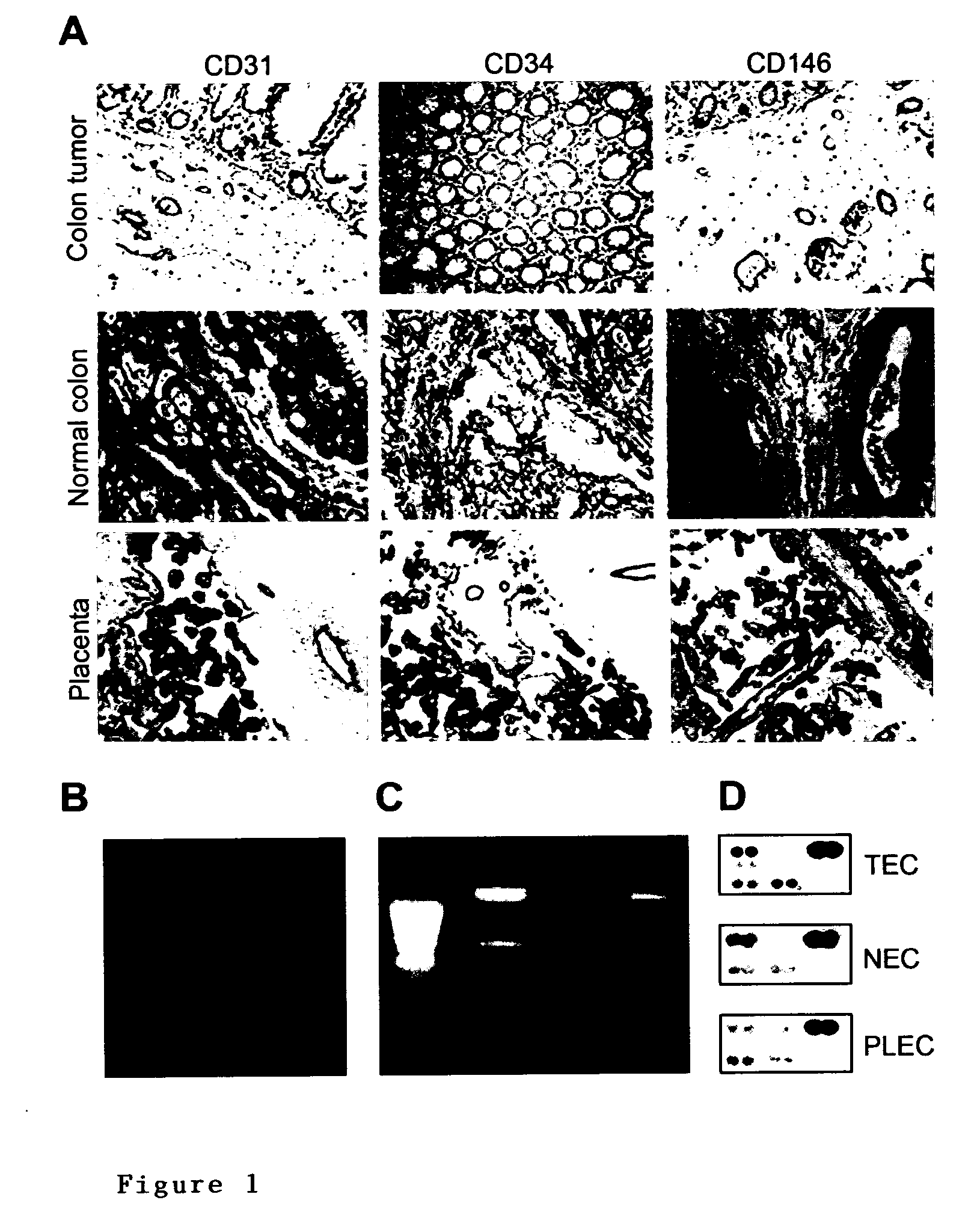
Methods of identifying specific target molecules for design of anti-angiogenic and vascular targeting approaches are disclosed. Transcriptional profiles of tumor endothelial cells were compared with that of normal resting endothelial cells, normal but angiogenically activated placental endothelial cells, and cultured endothelial cells. Although the majority of transcripts were classified as general angiogenesis markers, 17 genes were identified that show specific overexpression in tumor endothelium. Antibody targeting of four cell-surface expressed or secreted products (vimentin, CD59, HMGB1 and IGFBP7) inhibited angiogenesis in vitro and in vivo. Finally, targeting endothelial vimentin in a mouse tumor model significantly inhibited tumor growth and reduced microvessel density. The results demonstrate the utility of the identification and subsequent targeting of specific tumor endothelial markers for anticancer therapy.
Owner:MAASTRICHT UNIVERSITY
Liposome formulations
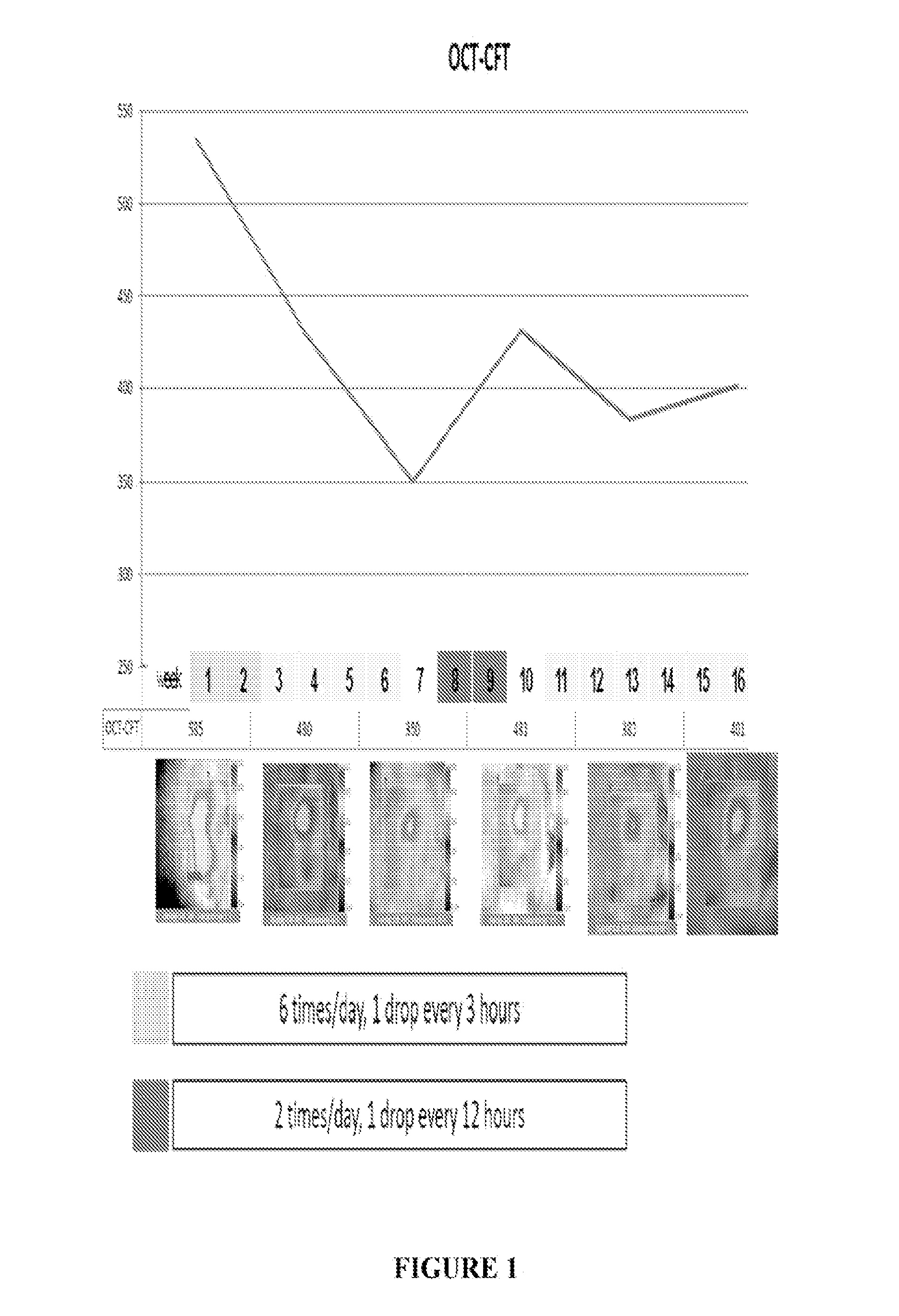
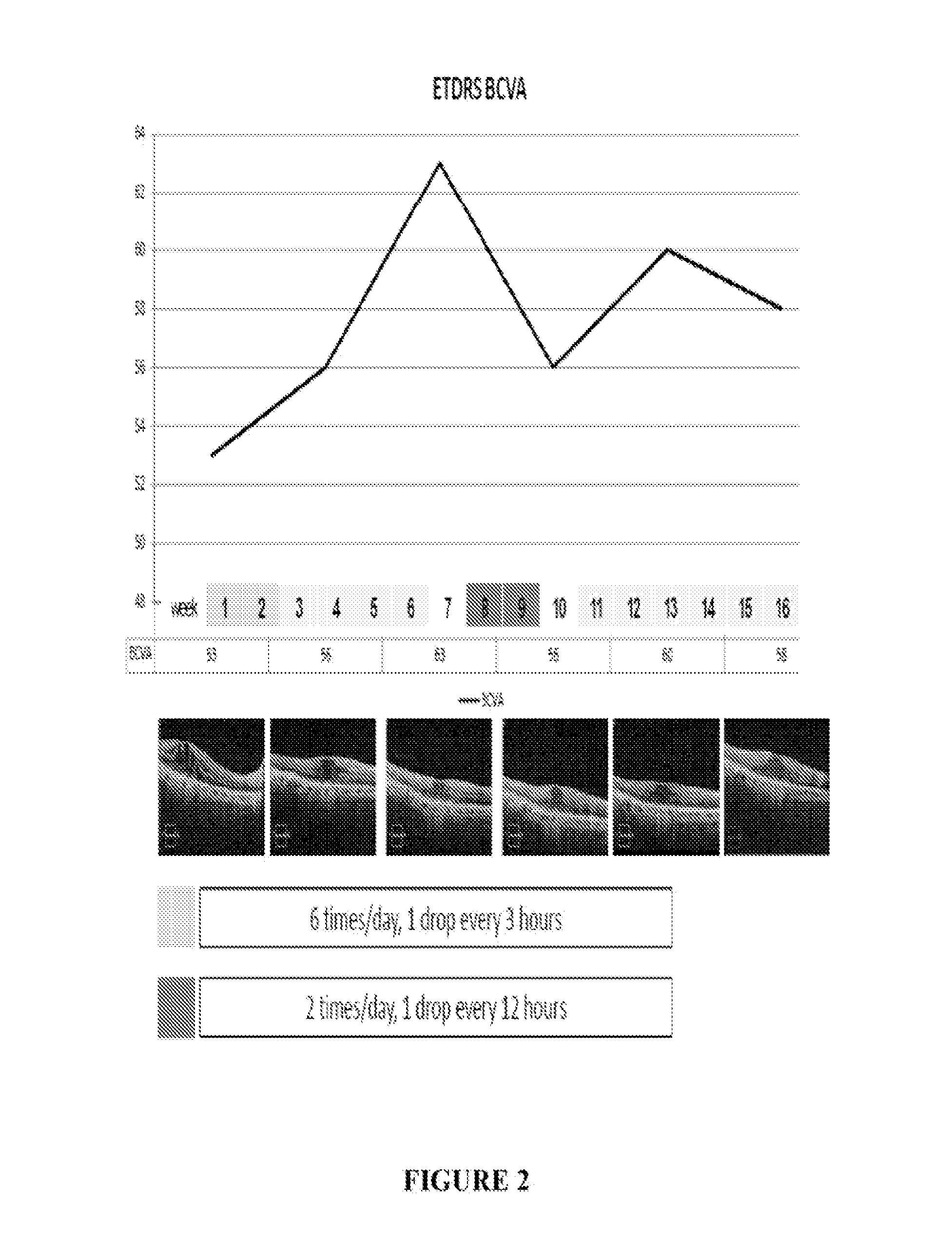
ActiveUS20150224055A1Promote penetrationImprove permeabilityOrganic active ingredientsSenses disorderRanibizumabPharmaceutical formulation
The present invention relates to pharmaceutical formulations comprising an anti angiogenic compound such as a monoclonal antibody or fragment thereof Selected from, for example, ranibizumab, which is a vascular endothelial growth factor binder which inhibits the action of VEGF, and a delivery agent selected from a pharmaceutically acceptable liposome. The formulations are useful in the treatment of a variety of angiogenic disorders and diseases in animals and people, and, preferably, in ophthalmic disorders selected from age-related macular degeneration, diabetic macular edema and corneal neovascularization.
Owner:OPKO PHARMA
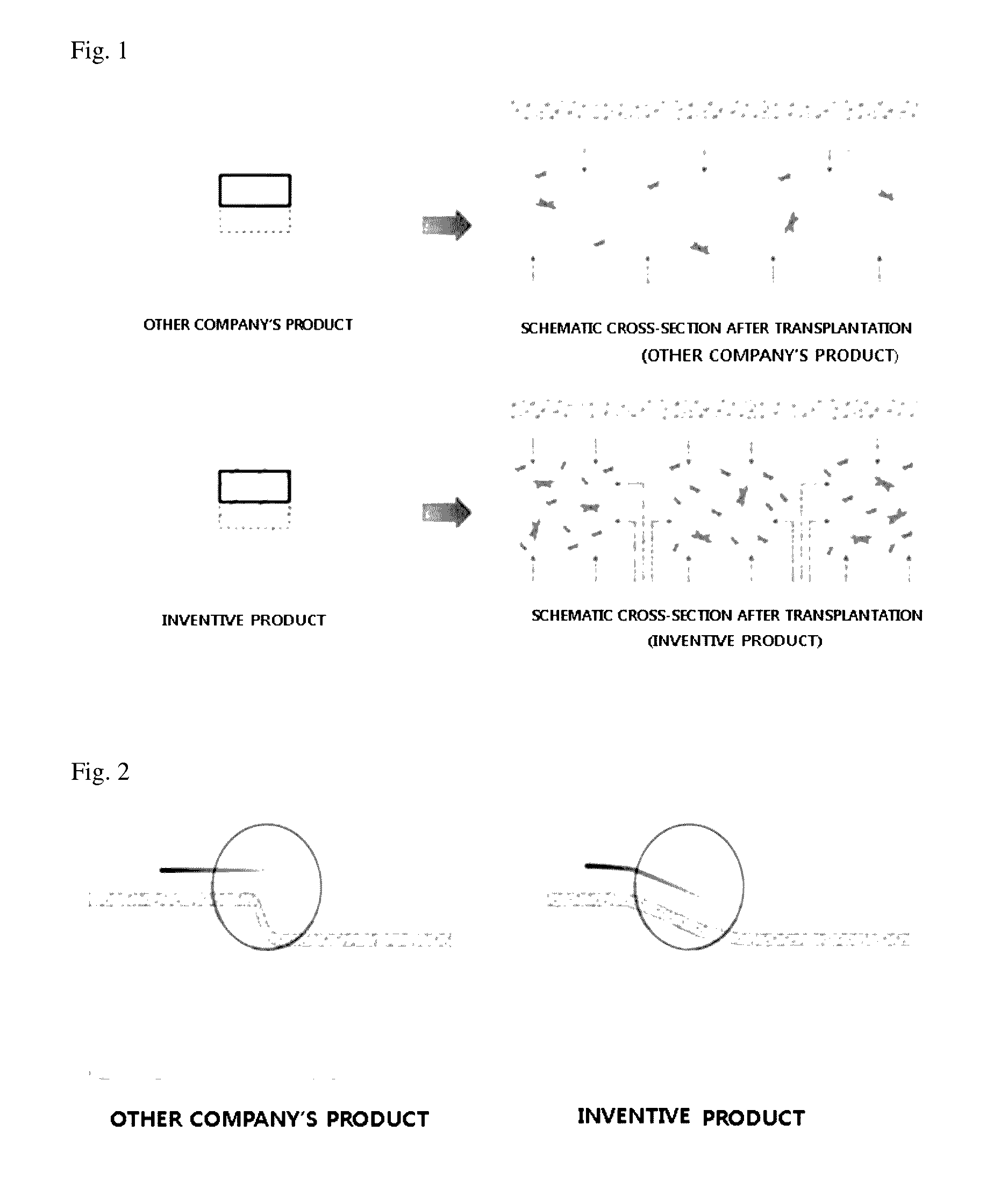
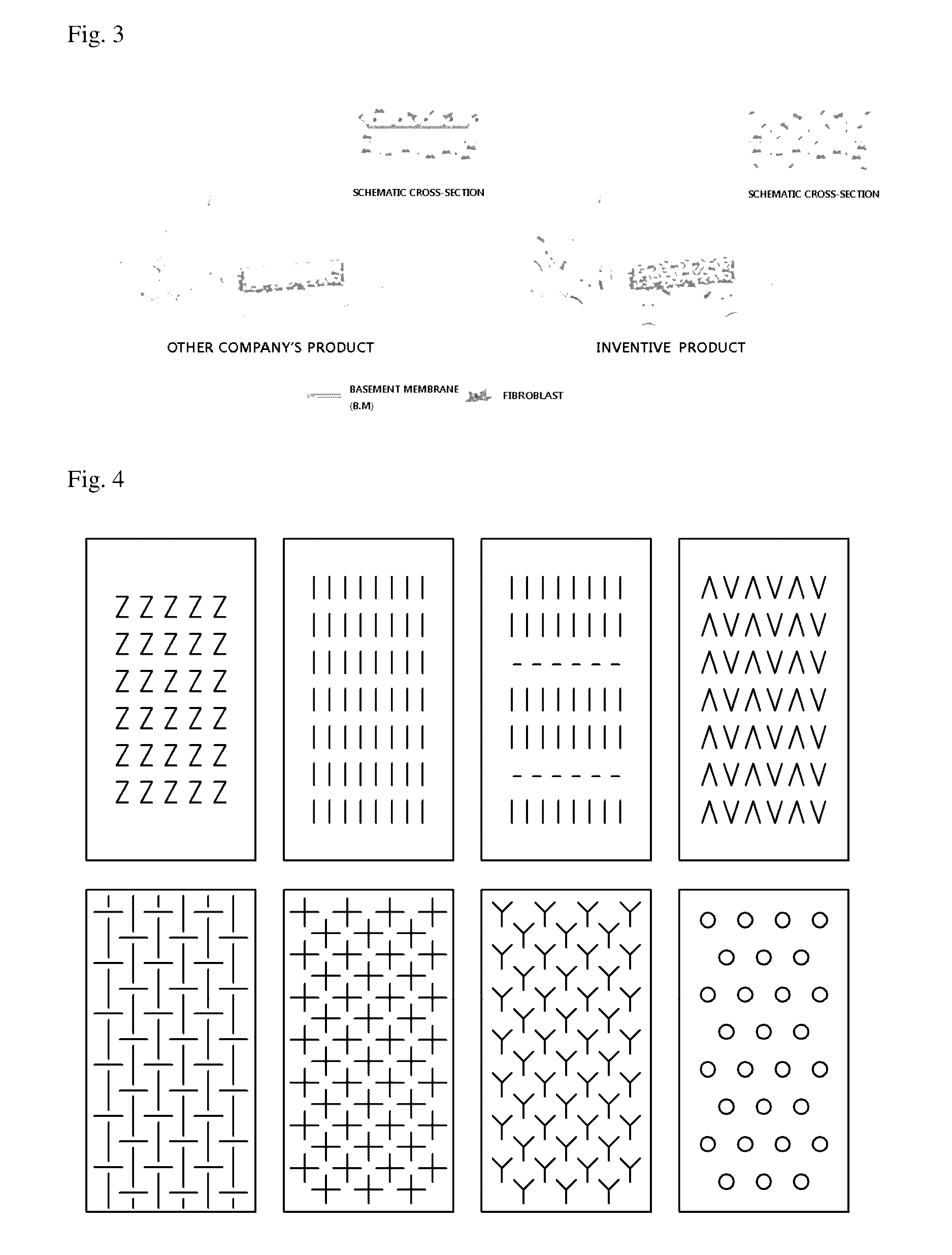
Acellular dermal graft
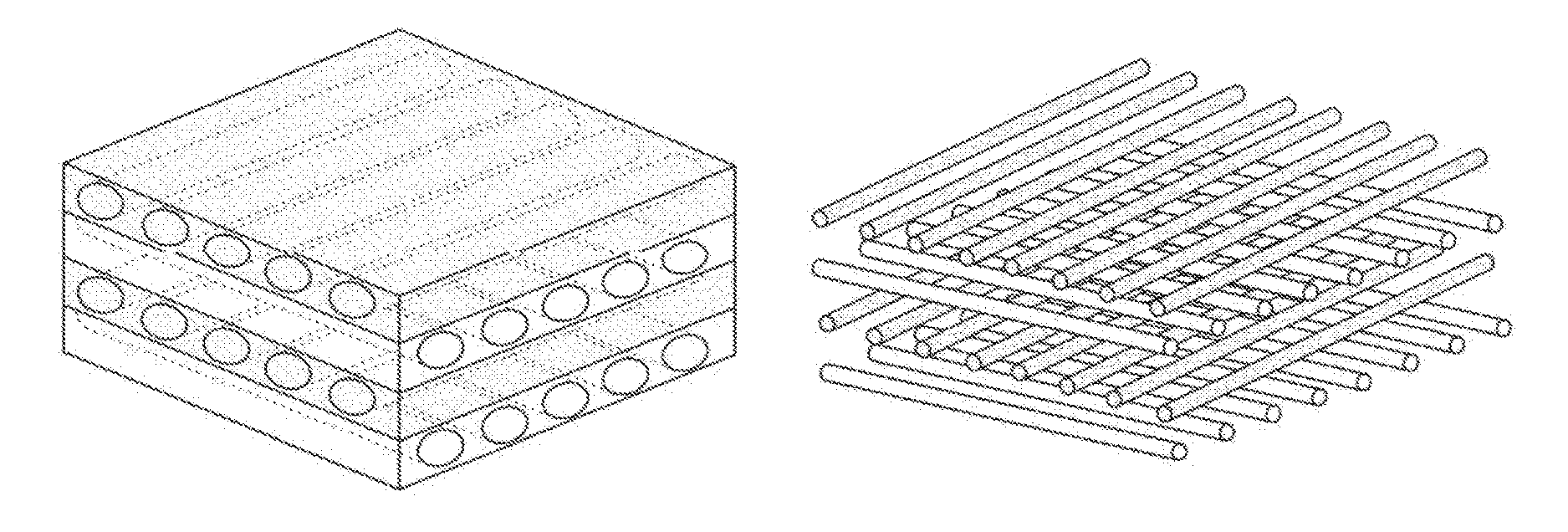
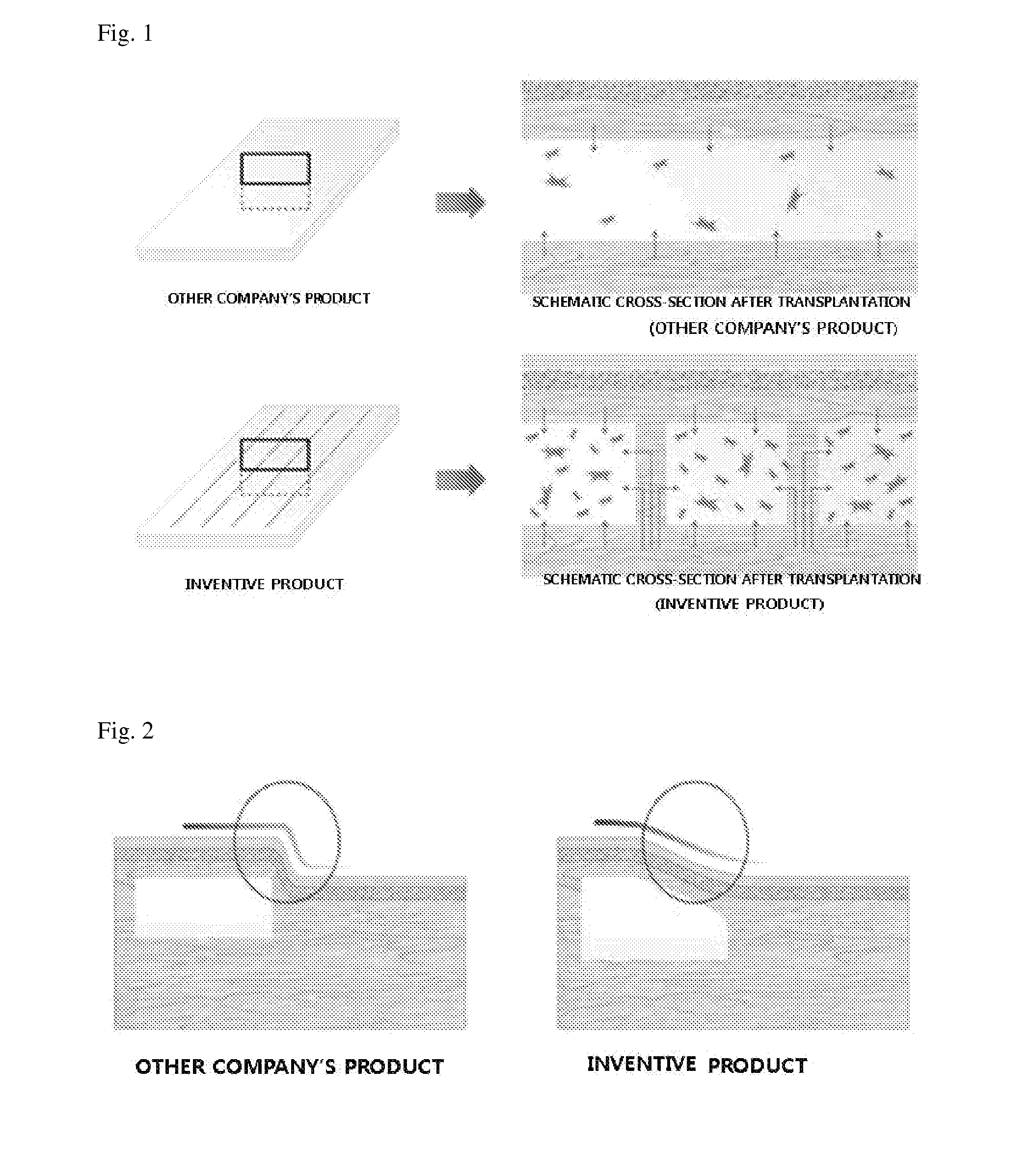
ActiveUS9532866B2Improves flexibility and extensibilityStablySkin implantsExtensibilityAutologous tissue
An acellular dermal graft is provided. The acellular dermal graft may be useful in minimizing side effects caused after transplantation since an environment favorable for formation of new blood vessels and proliferation of autologous tissues is provided by forming a multi-penetration structure in an acellular dermal tissue, removing a basement membrane layer and / or subjecting corners to slope cutting, and transplantation is stably performed within a short transplantation time due to improved extensibility and flexibility of tissues. The acellular dermal graft may be useful in reducing a time required to recover tissues after transplantation since the transplantation is stably performed due to improved grafting reaction with a host tissue by enhancing uptake of fibroblasts and promoting angiogenic activities. Also, the acellular dermal graft may be useful in maintaining smooth external appearance since hypodermic implantation is easily performed upon transplantation, and the skin at a graft site does not protrude after transplantation.
Owner:L&C BIO CO LTD
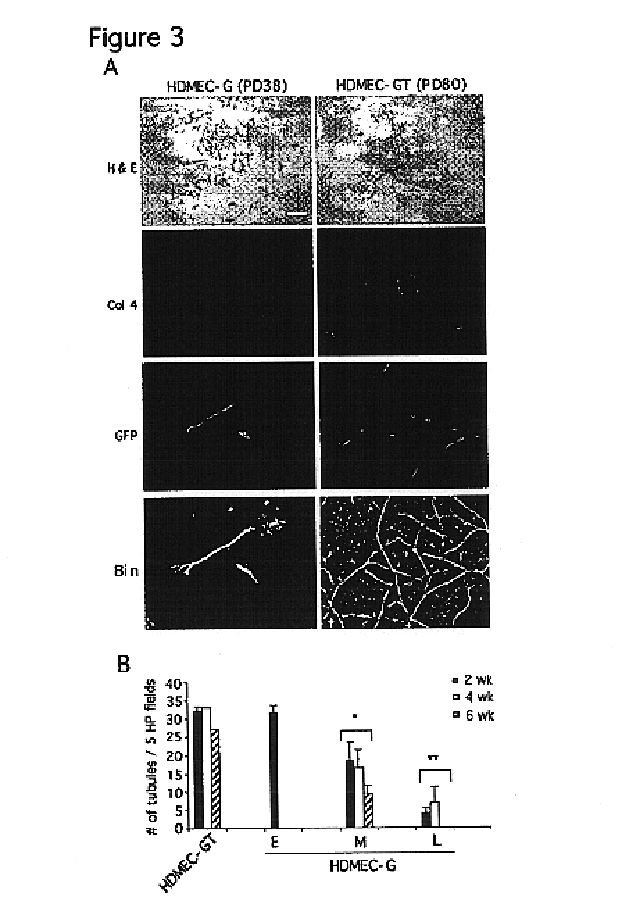
Vivo assay for anti angiogenic compounds
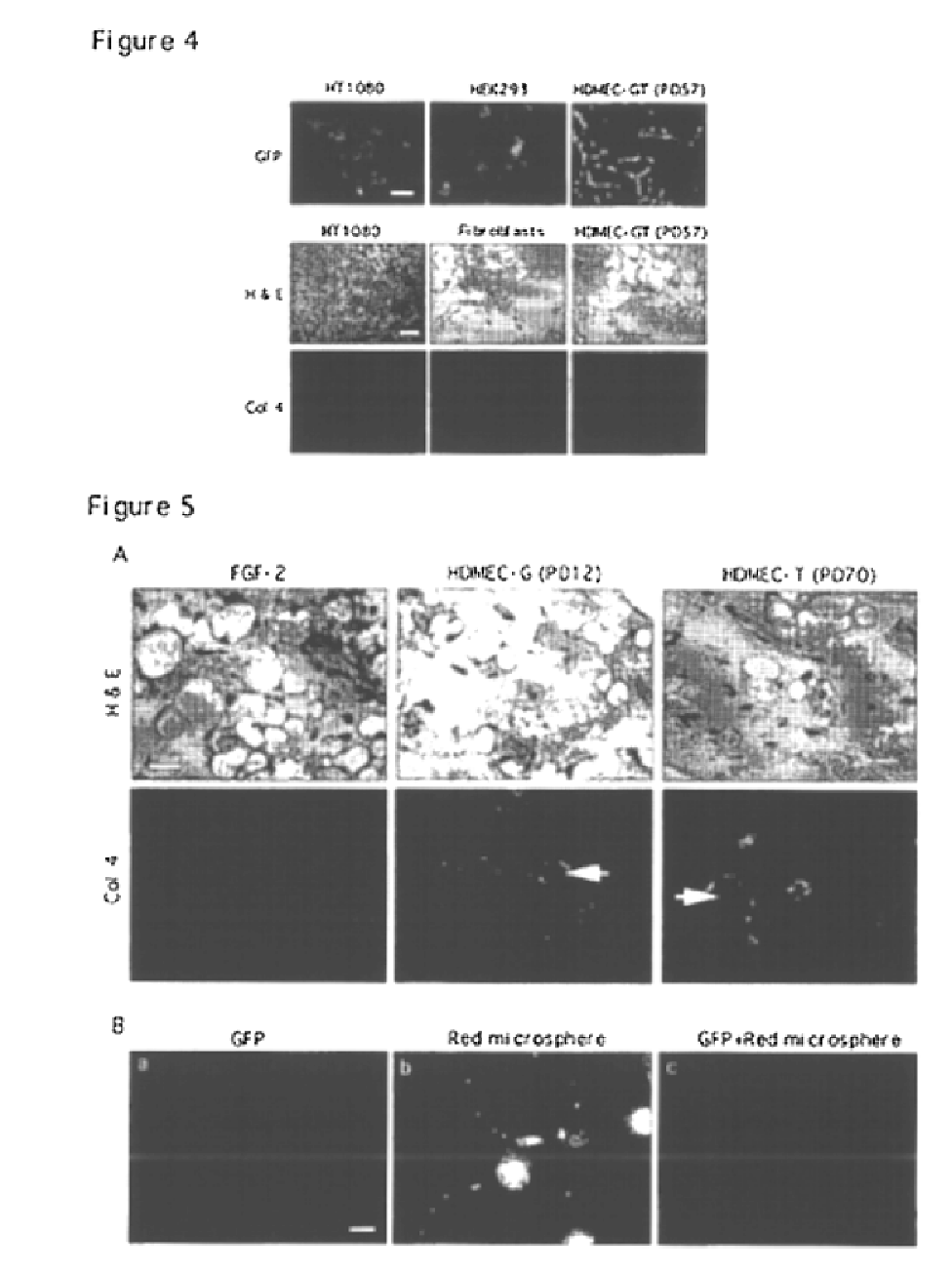
We report the use of telomerase-immortalized human microvascular endothelial cells in the formation of functional capillary blood vessels in vivo. Previously we showed the superior in vitro survival of human telomerase reverse transcriptase (hTERT)-transduced human endothelial cells. Here we show that retroviral-mediated transduction of hTERT in human dermal microvascular endothelial cells (HDMEC) results in cell lines that form microvascular structures when subcutaneously implanted in severe combined immunodeficiency (SCID) mice. The human origin of xenografted microvaculature was confirmed both by basement membrane immunoreactivity with anti-human type IV collagen staining and visualization of fluorescent vessels containing HDMEC that were co-transduced with hTERT and green fluorescent protein (eGFP). The lack of human vascular structures after implantation of HT1080 fibrosarcoma cells, 293 human embryonic kidney cells or human skin fibroblasts demonstrated the specificity of HDMEC at forming capillaries. Intravascular red fluorescent microspheres injected into the host circulation were found within green “telomerized” microvessels indicating functional murine-human vessel anastamoses. Whereas primary HDMEC-derived vessel density decreased steadily with time, telomerized HDMEC maintained durable vessels 6 weeks after xenografting. Modulation of implant vessel density by exposure to different angiogenic and angiostatic factors demonstrated the utility of this system for the study of human microvascular remodeling in vivo.
Owner:HERRON G SCOTT
Acellular dermal graft
ActiveUS20150057751A1Good extensibilityIncrease flexibilitySkin implantsExtensibilityAutologous tissue
An acellular dermal graft is provided. The acellular dermal graft may be useful in minimizing side effects caused after transplantation since an environment favorable for formation of new blood vessels and proliferation of autologous tissues is provided by forming a multi-penetration structure in an acellular dermal tissue, removing a basement membrane layer and / or subjecting corners to slope cutting, and transplantation is stably performed within a short transplantation time due to improved extensibility and flexibility of tissues. The acellular dermal graft may be useful in reducing a time required to recover tissues after transplantation since the transplantation is stably performed due to improved grafting reaction with a host tissue by enhancing uptake of fibroblasts and promoting angiogenic activities. Also, the acellular dermal graft may be useful in maintaining smooth external appearance since hypodermic implantation is easily performed upon transplantation, and the skin at a graft site does not protrude after transplantation.
Owner:L&C BIO CO LTD
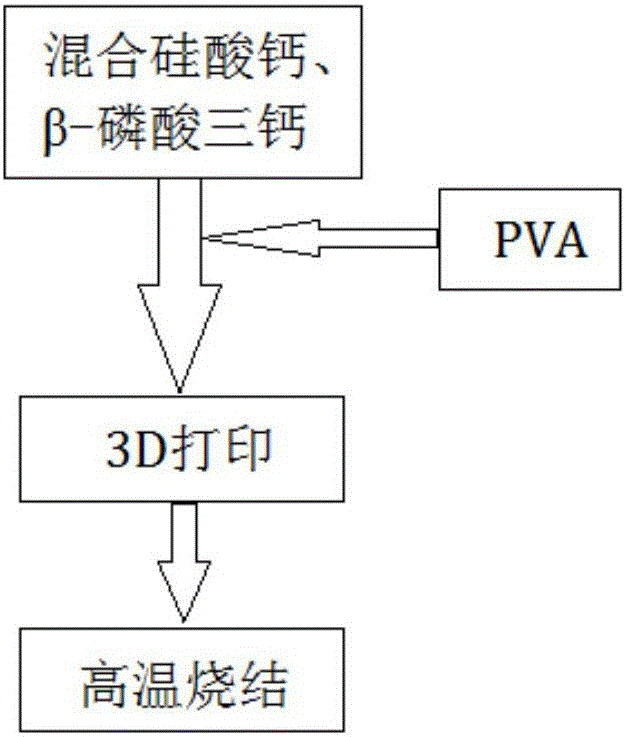
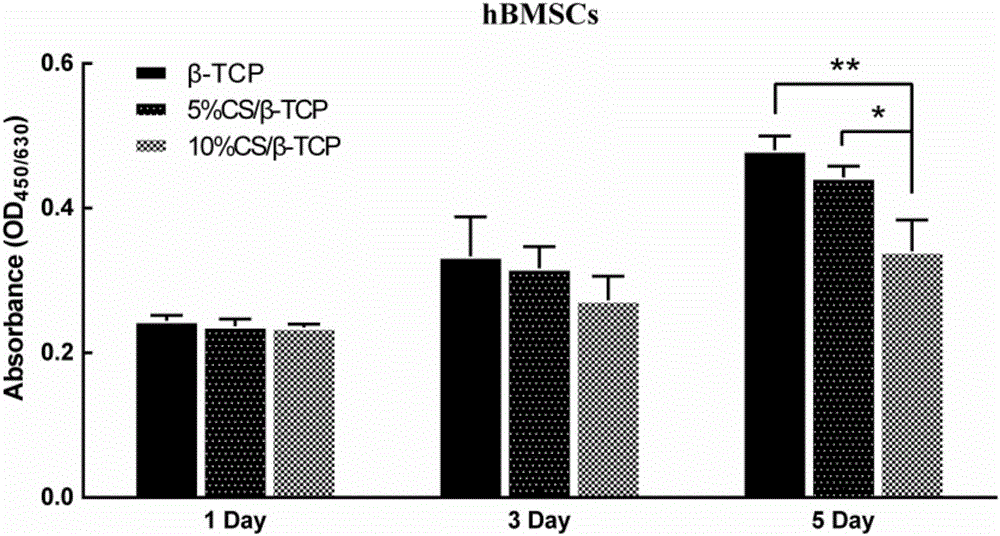
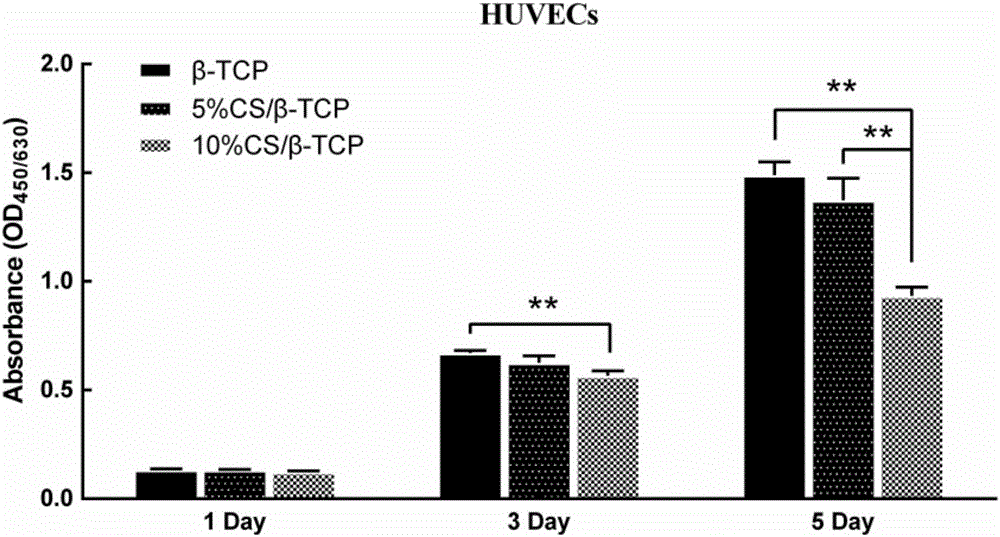
CS/beta-TCP (calcium silicate/beta-tricalcium phosphate) porous composite material for promoting osteogenesis and vasculogenesis and preparation method thereof
InactiveCN105999400AGood biocompatibilityConducive to adhesion and proliferationTissue regenerationProsthesisCalcium silicatePolyvinyl alcohol
A CS / β‐TCP porous composite material for promoting osteogenesis and its preparation method, by mixing calcium silicate (CS) and tricalcium phosphate (β‐TCP) powders according to the mass ratio, adding polyvinyl alcohol ( PVA) is uniformly mixed into a paste, and then 3D printed, and the printed three-dimensional model is dried at room temperature and then sintered at high temperature to obtain a CS / β-TCP porous composite material with a pore cross-section of a square of 350 μm×350 μm; the process of the present invention is simple , with better biocompatibility and uniform through-hole structure, which is beneficial to osteoinduction and angiogenesis.
Owner:SHANGHAI JIAO TONG UNIV
Methods and means for soft tissue engineering
ActiveUS9056084B2Tailoring is fastShort incubation timePowder deliveryPeptide/protein ingredientsCell freeAdipogenesis
Owner:LINIO BIOTECH OY
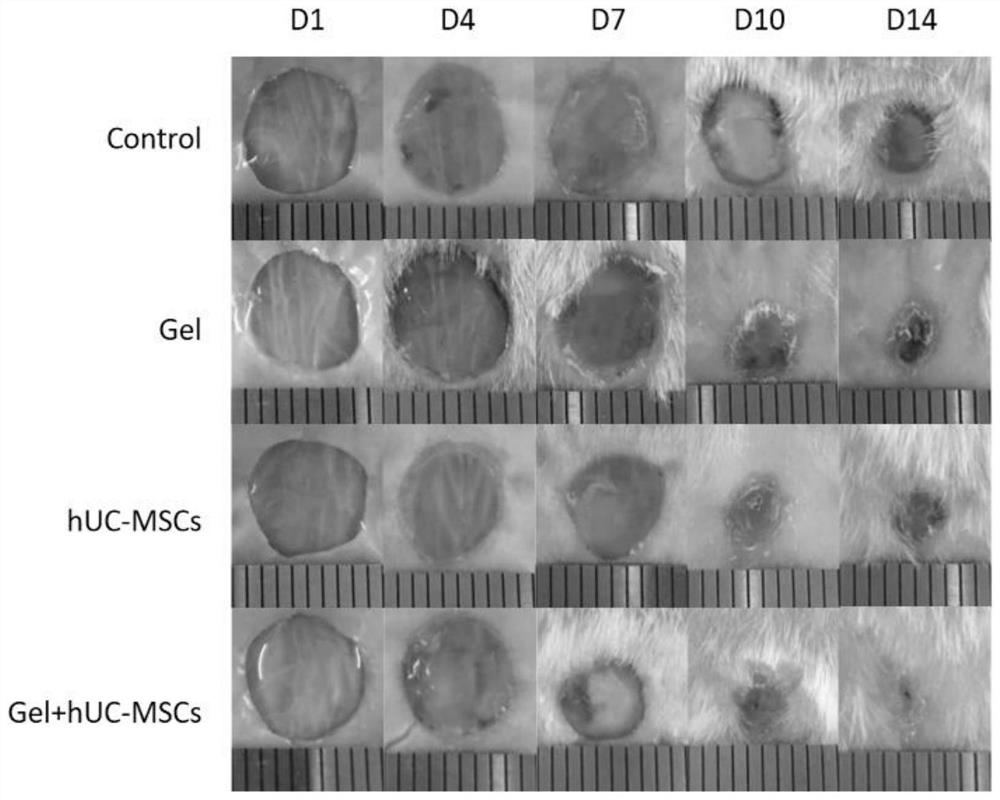
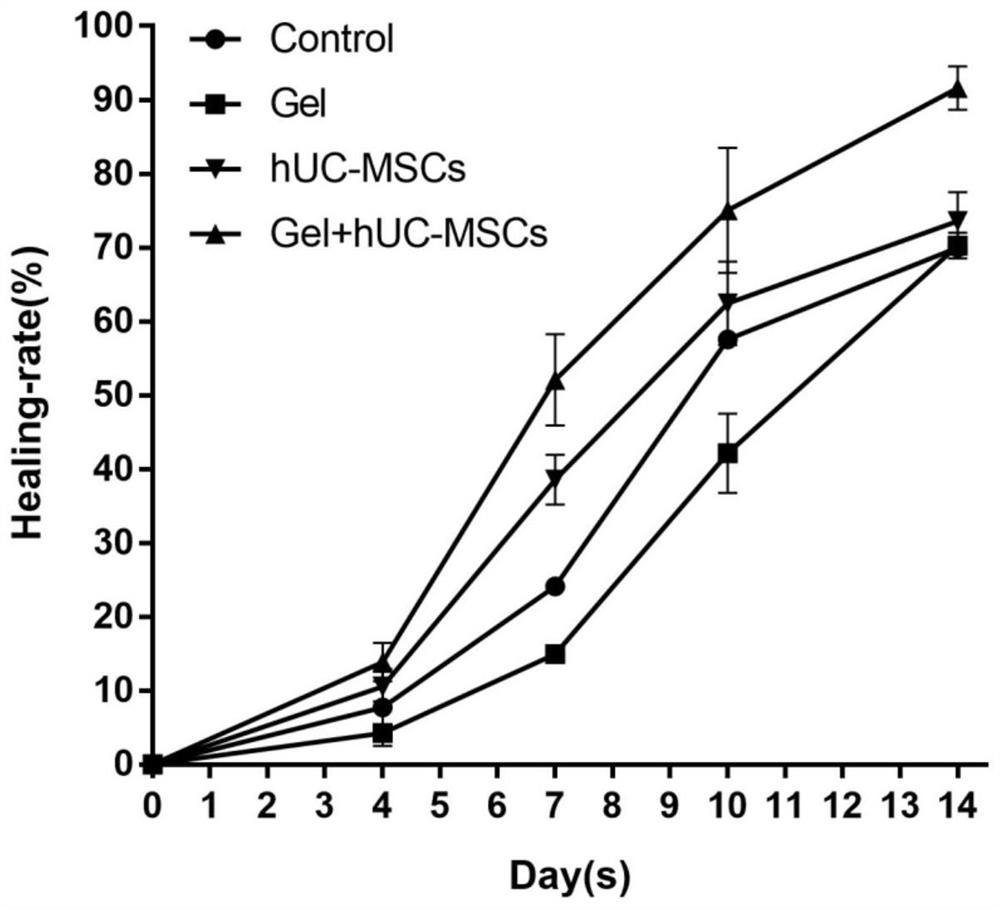
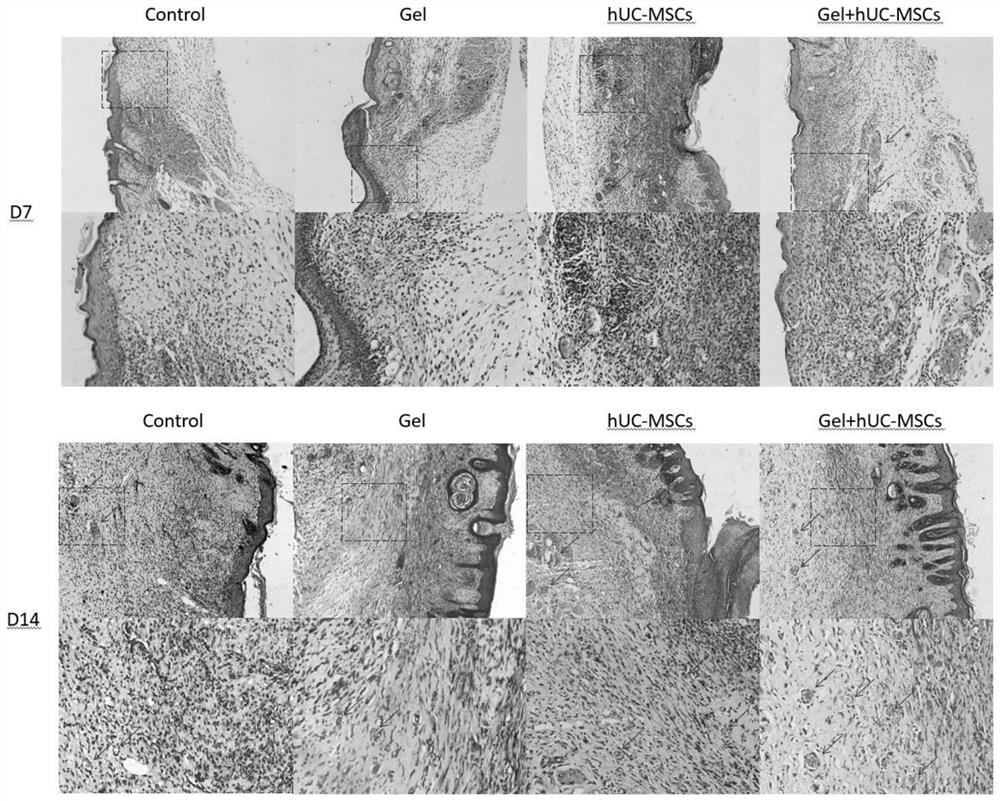
Fibrin hydrogel stent loaded with human umbilical cord mesenchymal stem cells and application of fibrin hydrogel stent
The invention discloses a fibrin hydrogel stent loaded with human umbilical cord mesenchymal stem cells and application of the fibrin hydrogel stent, and belongs to the technical field of hydrogel. The human umbilical cord mesenchymal stem cells are cultured until the density reaches 70 percent to 80 percent; treatment is performed by using a cell digestion solution, and the human umbilical cord mesenchymal stem cells are resuspended by using a fibrin raw solution to obtain a human umbilical cord mesenchymal stem cell-fibrin raw solution mixed solution; and a thrombin solution is added into the mixed solution to obtain the fibrin hydrogel stent loaded with the human umbilical cord mesenchymal stem cells. Through combined application of the fibrin hydrogel stent and the human umbilical cordmesenchymal stem cells, the healing speed of a wound is obviously accelerated; the re-epithelialization is promoted; the inflammatory response is inhibited; the angiogenesis is promoted; the scar formation is reduced; the wound is healed to be close to normal skin tissues to a greater extent; and the treatment requirement is met.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com