Patents
Literature
614 results about "Thrombin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
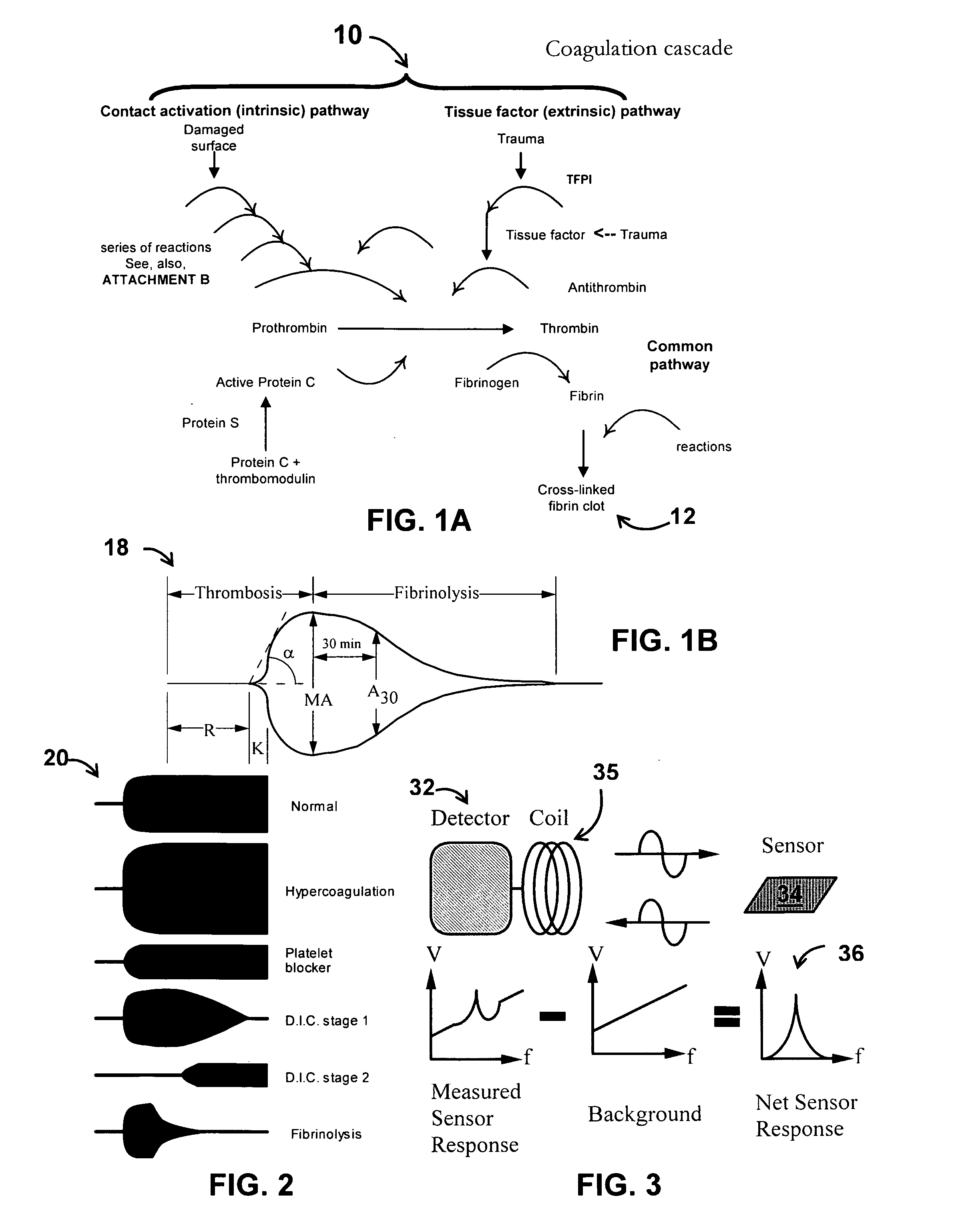
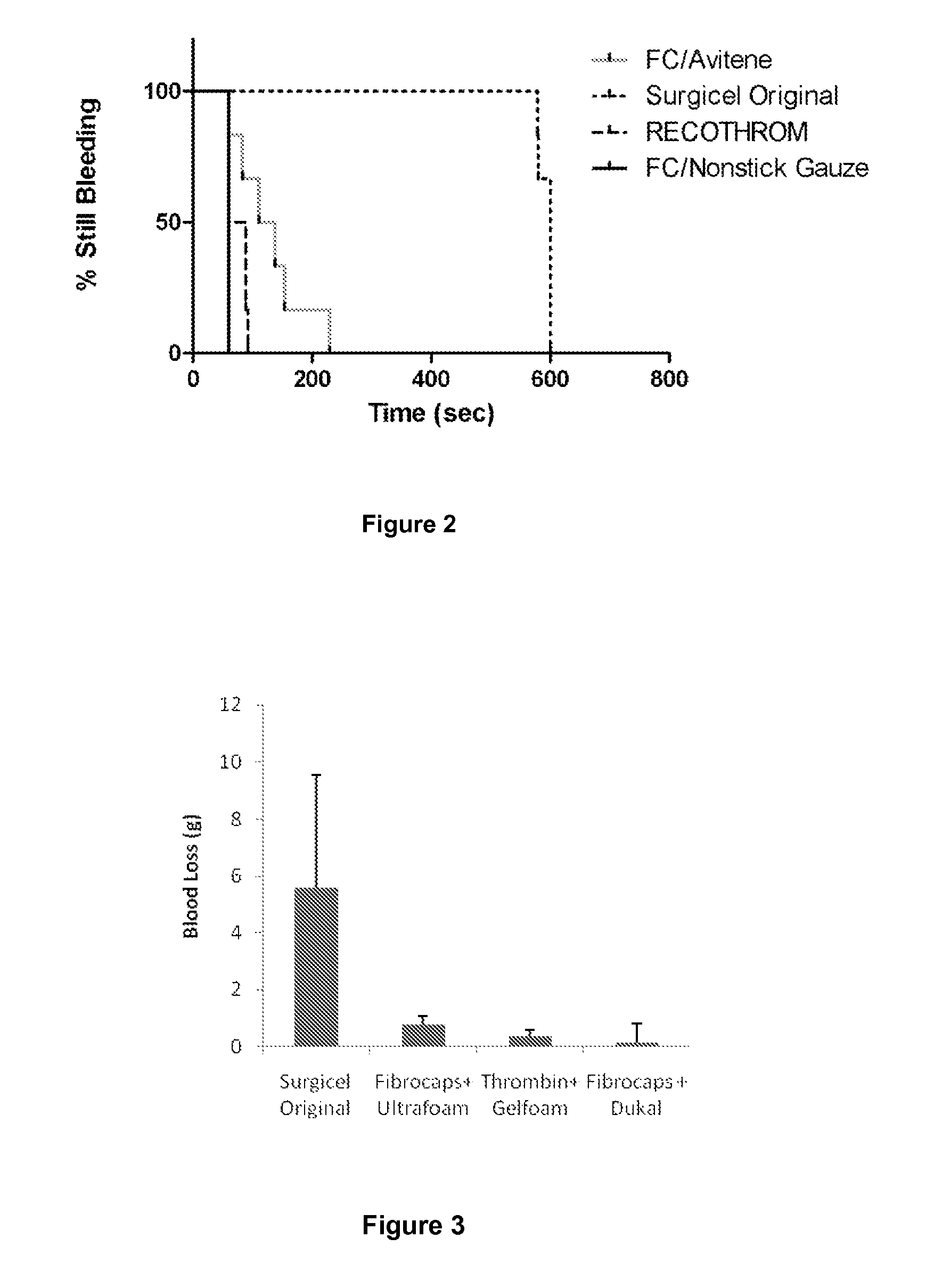
Thrombin (EC 3.4.21.5, fibrinogenase, thrombase, thrombofort, topical, thrombin-C, tropostasin, activated blood-coagulation factor II, blood-coagulation factor IIa, factor IIa, E thrombin, beta-thrombin, gamma-thrombin) is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. Prothrombin (coagulation factor II) is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.
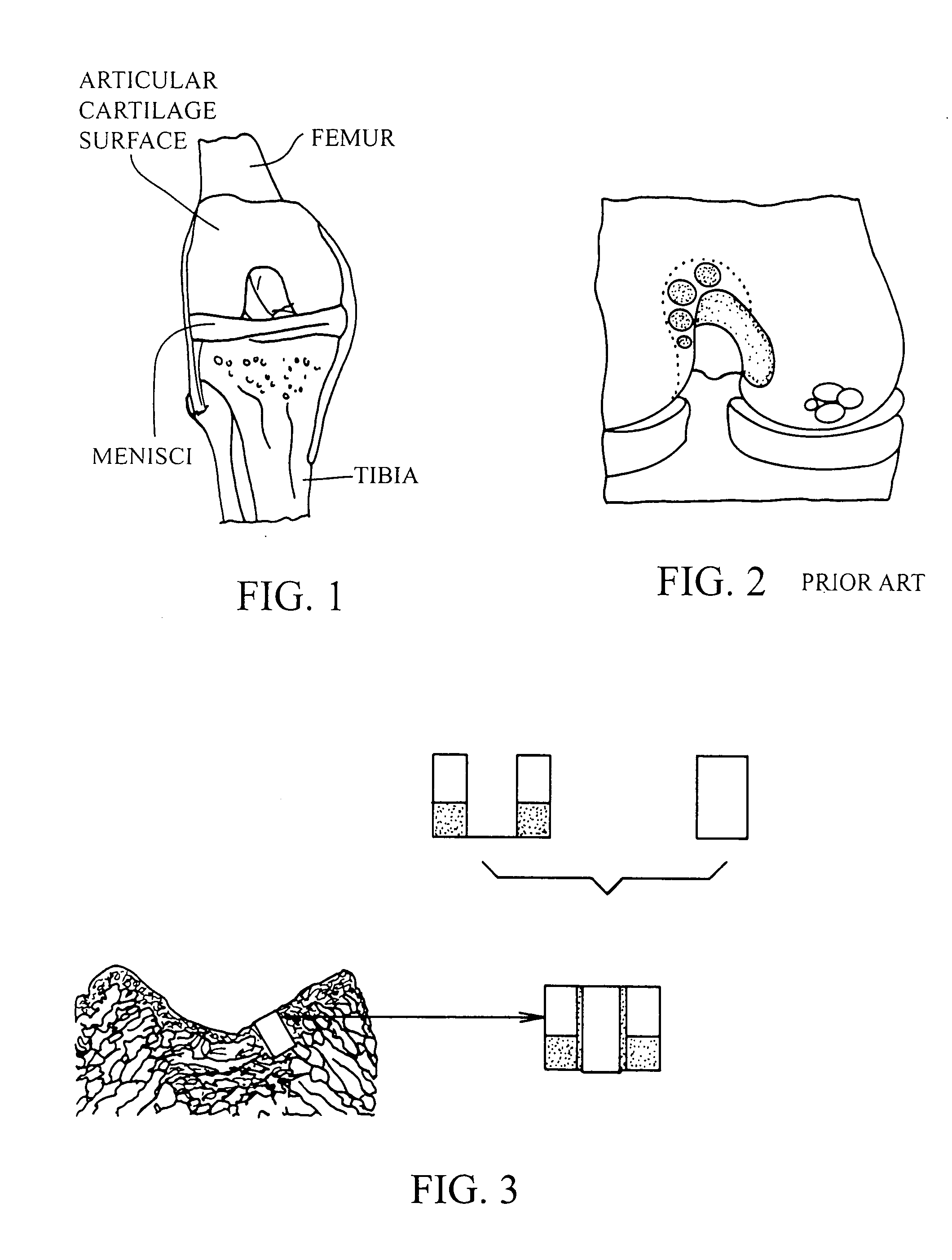
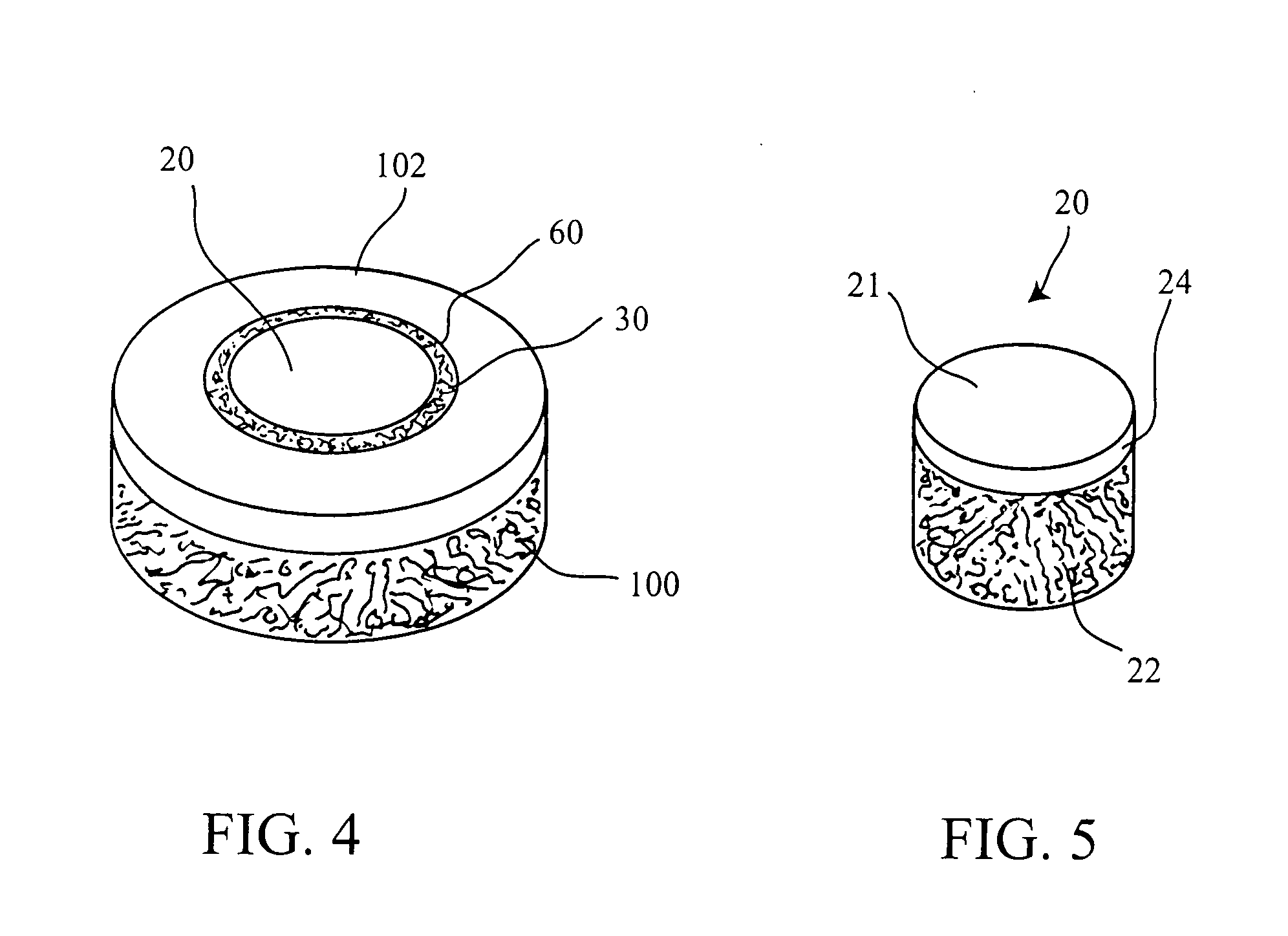
Cartilage implant plug with fibrin glue and method for implantation
InactiveUS20050064042A1Restores normal functionRelief painSurgical adhesivesPeptide/protein ingredientsThrombin activityChondral defect
The invention is directed toward a cartilage repair assembly comprising a shaped structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that said shaped bone and cartilage cap when centered in the bore does not engage the side wall of the bore and is positioned from the side wall of the bone a distance ranging from 10 microns to 1000 microns and is surrounded by milled cartilage and a fibrin thrombin glue. A method for inserting the assembly into a cartilage defect area is disclosed.
Owner:MASSACHUSETTS INST OF TECH +1
Hemostatic sandwich bandage
The present invention relates to a haemostatic multilayer bandage that comprises preferably a thrombin layer between two fibrinogen layers. The dressing may contain other resorbable materials such as glycolic acid or lactic acid based polymers or copolymers. The inventive haemostatic bandage is useful for the treatment of wounded tissue.
Owner:AMERICAN NAT RED CROSS
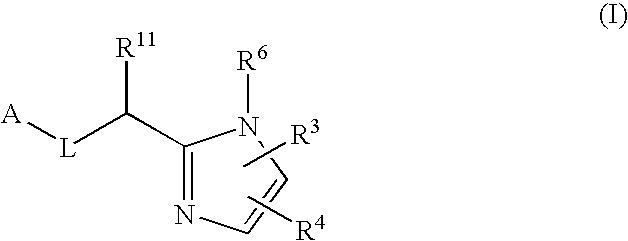
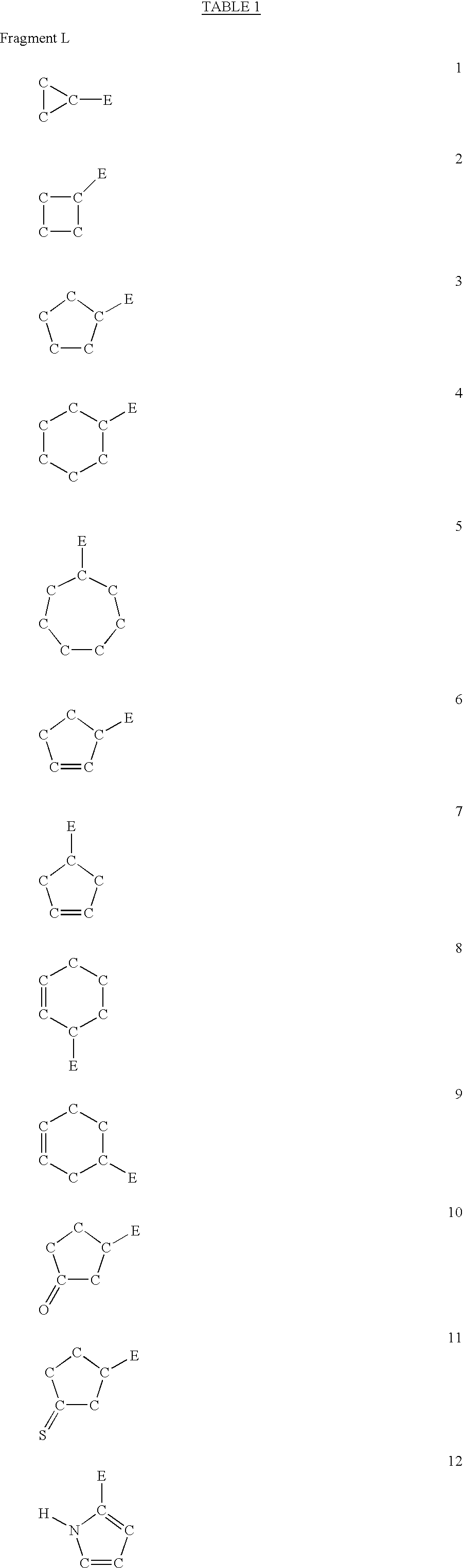
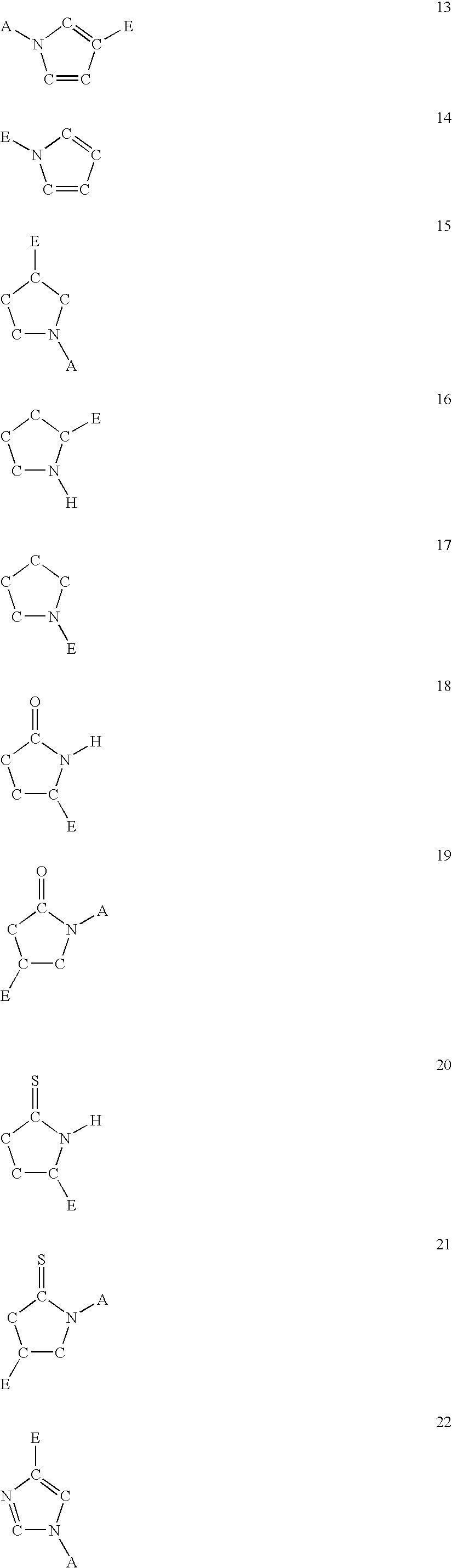
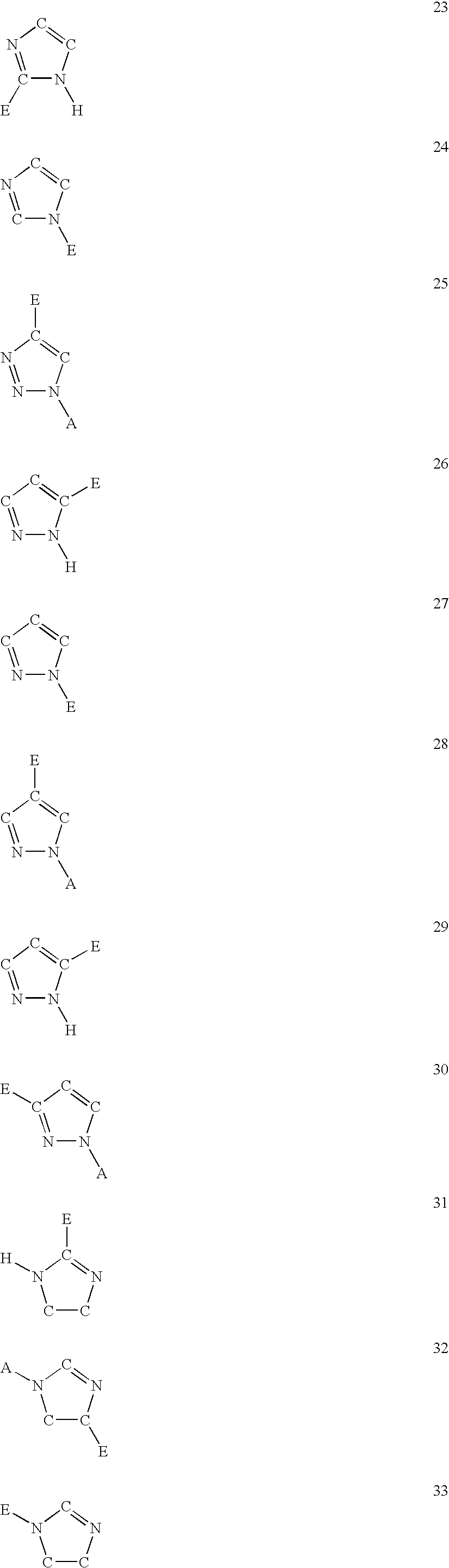
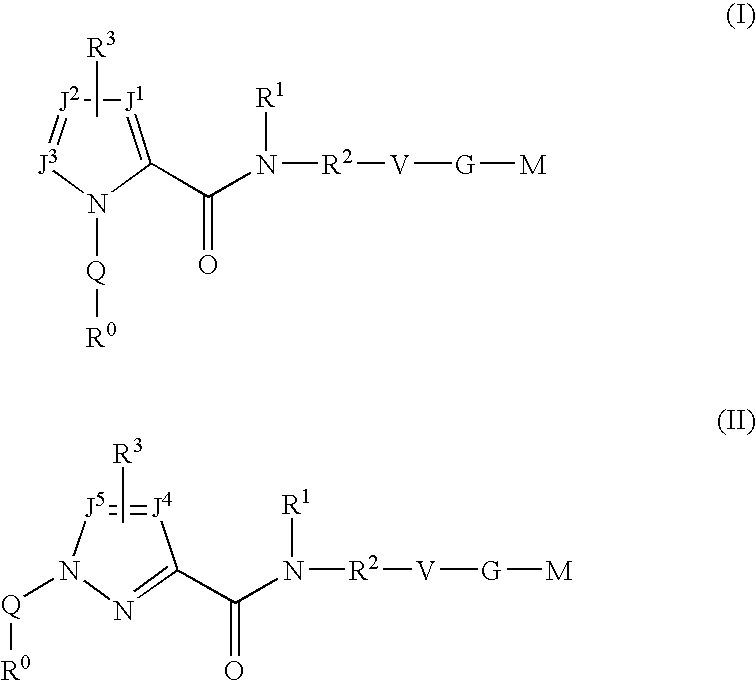
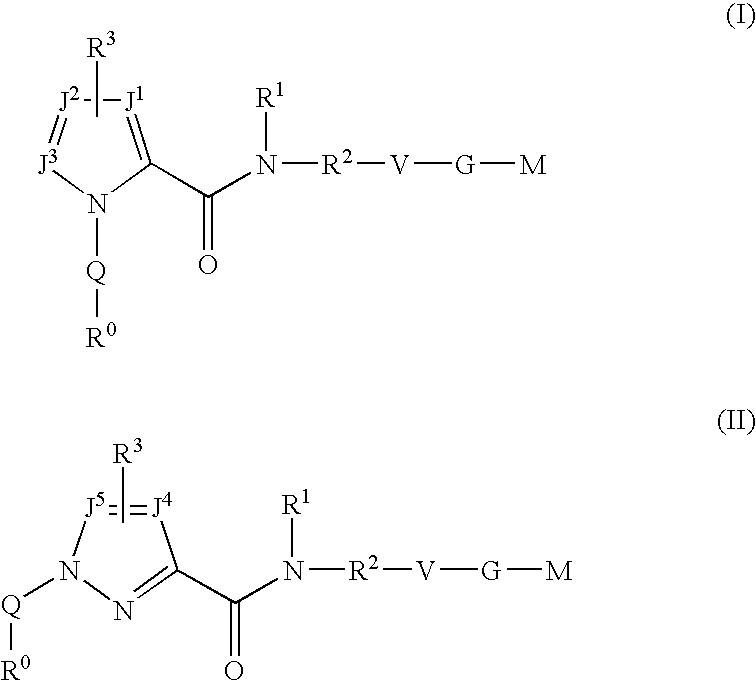
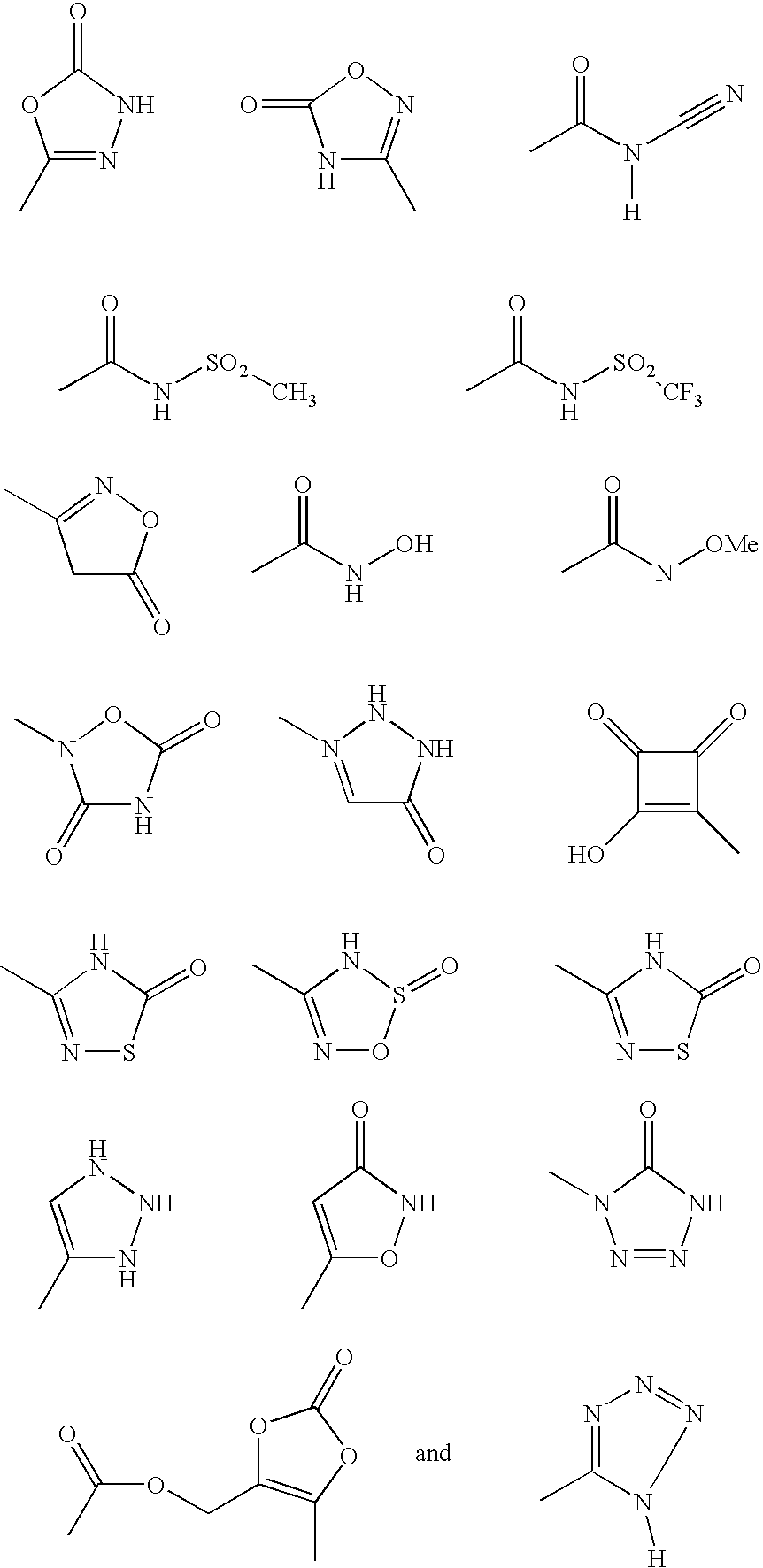
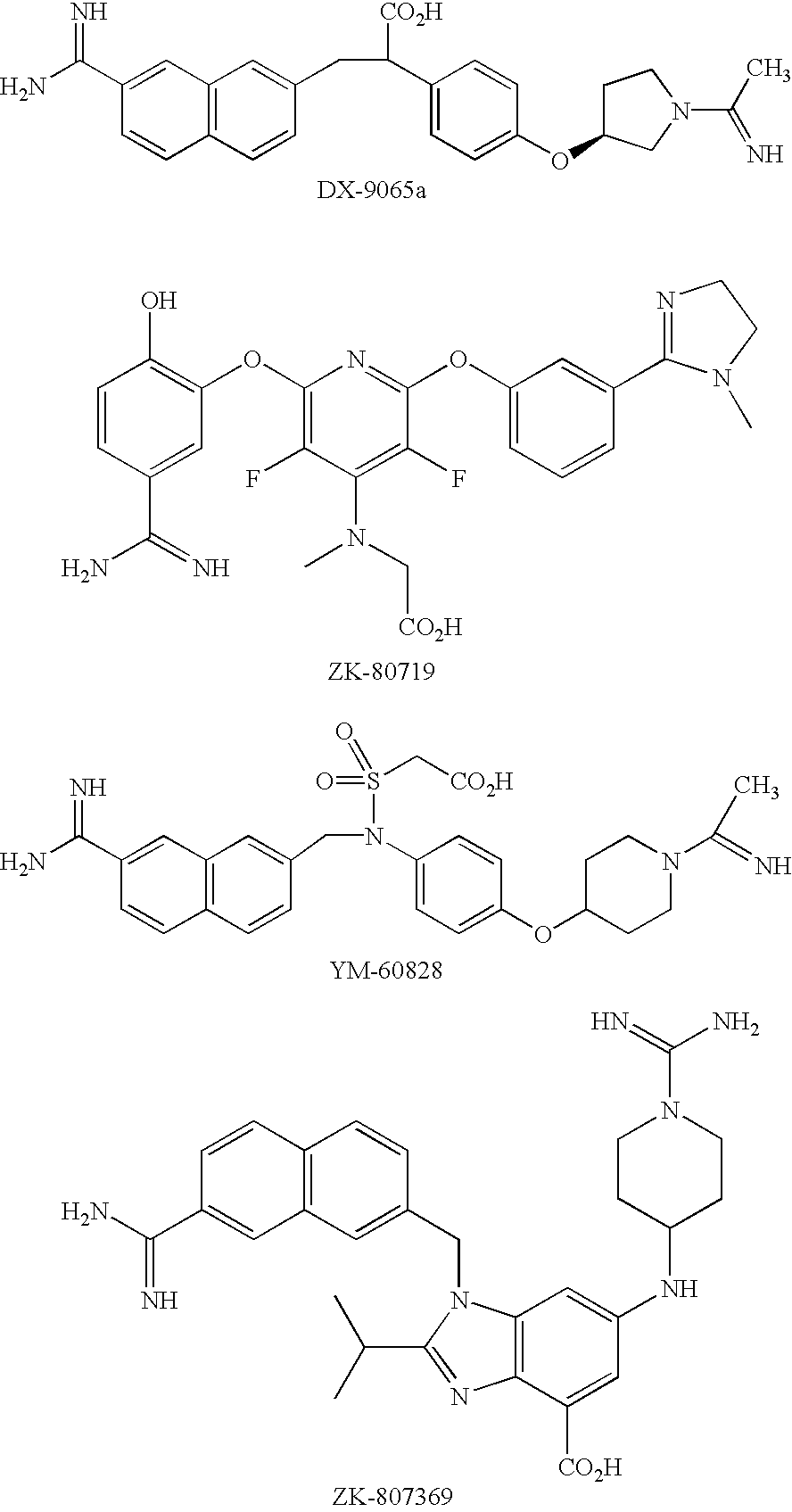
Five-membered heterocycles useful as serine protease inhibitors
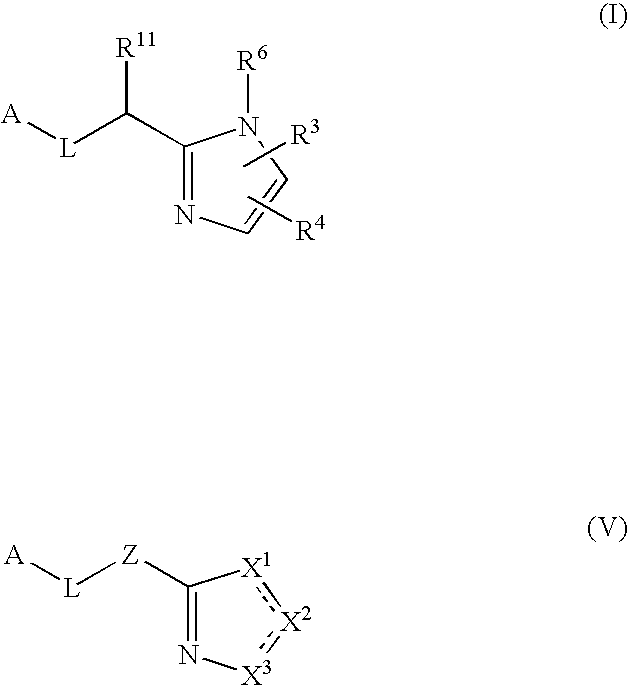
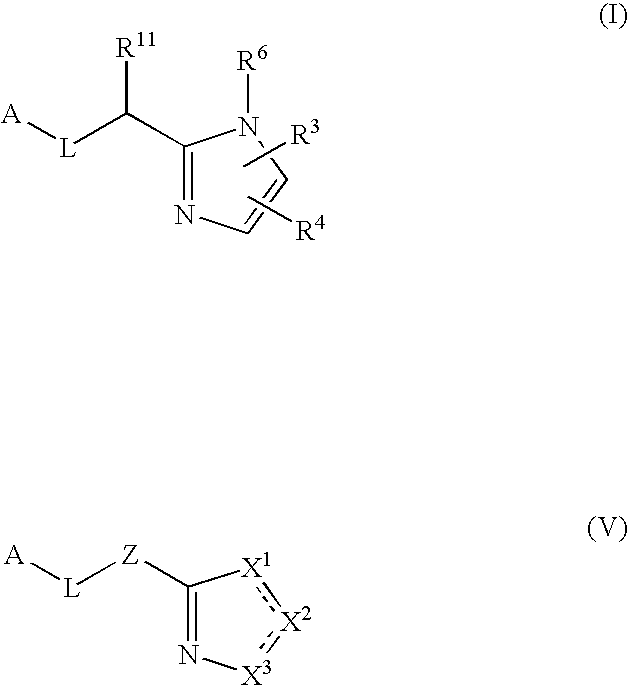
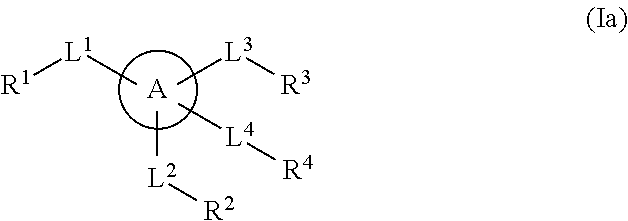
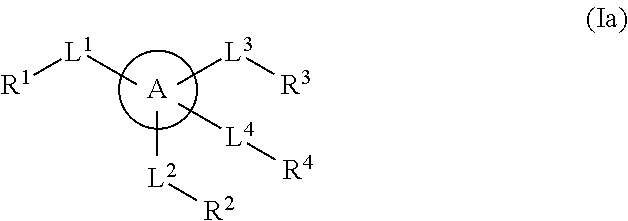
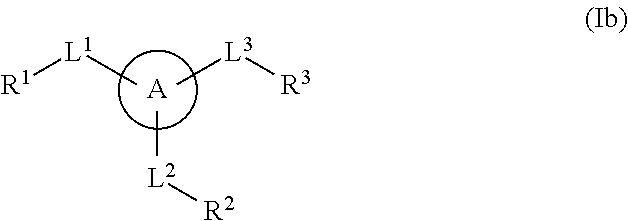
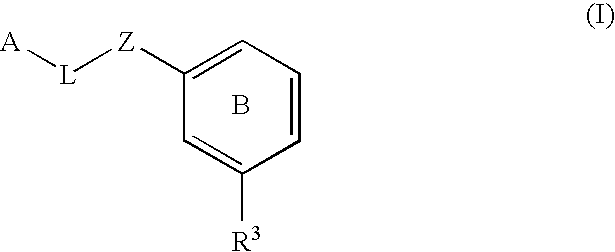
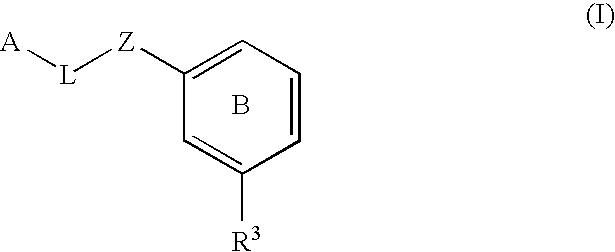
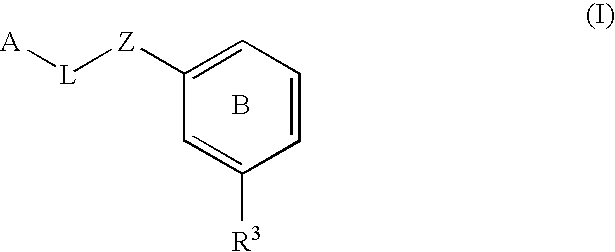
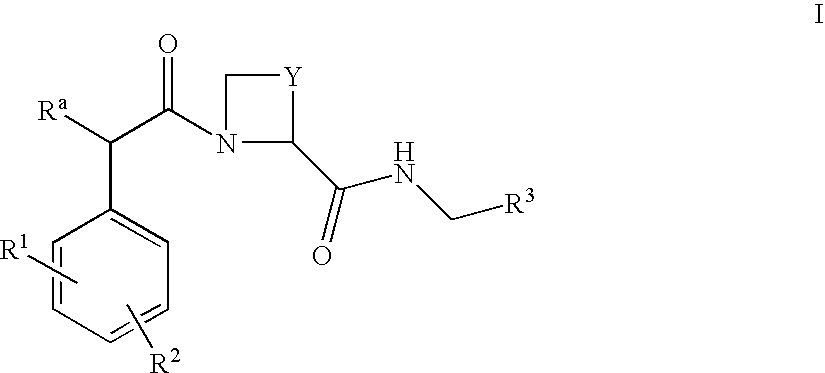
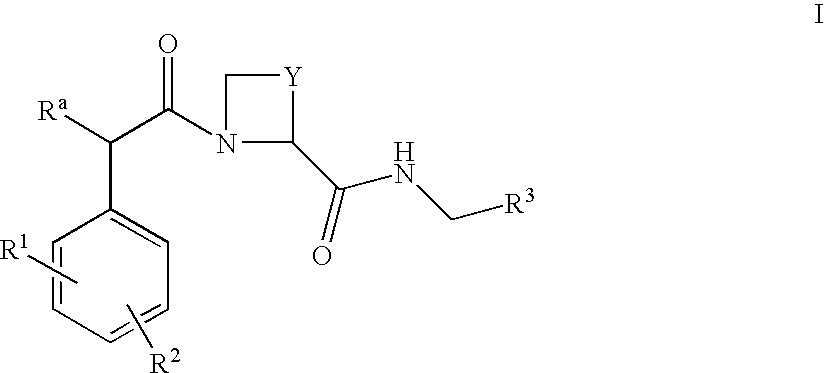
The present invention provides a method for treating a thrombotic or an inflammatory disorder administering to a patient in need thereof a therapeutically effective amount of at least one compound of Formula (I) or Formula (V): or a stereoisomer or pharmaceutically acceptable salt or solvate form thereof, wherein the variables A, L, Z, R3, R4, R6, R11, X1, X2, and X3 are as defined herein. The compounds of Formula (I) are useful as selective inhibitors of serine protease enzymes of the coagulation cascade and / or contact activation system; for example thrombin, factor Xa, factor XIa, factor IXa, factor VIIa and / or plasma kallikrein. In particular, it relates to compounds that are selective factor XIa inhibitors. This invention also provides compounds within the scope of Formula I and relates to pharmaceutical compositions comprising these compounds.
Owner:BRISTOL MYERS SQUIBB CO
Compounds useful in the complement, coagulat and kallikrein pathways and method for their preparation
The present invention is concerned with new compounds, and particularly those having a fused bicyclic ring substituted with an amidine moiety. These compounds are each potent inhibitors of Factor D of the alternate pathway of complement, C1s of the classical pathway of complement, Factors Xa, XIIa, VIIa and thrombin of the coagulation pathway, plasmin in the fibrinolytic pathway, and kallikrein and high molecular weight kininogen in the inflammatory pathways. These proteases, which have serine in their active site, are called serine proteases and they are pivotal to most of the processes of inflammation and coagulation. In fact, these various systems are interactive with one another and it is difficult to activate one pathway without it influencing the others.
Owner:BIOCRYST PHARM INC
System/unit and method employing a plurality of magnetoelastic sensor elements for automatically quantifying parameters of whole blood and platelet-rich plasma
InactiveUS20080261261A1Quantifying platelet-fibrin clot strengthBioreactor/fermenter combinationsBiological substance pretreatmentsClot formationBlood plasma
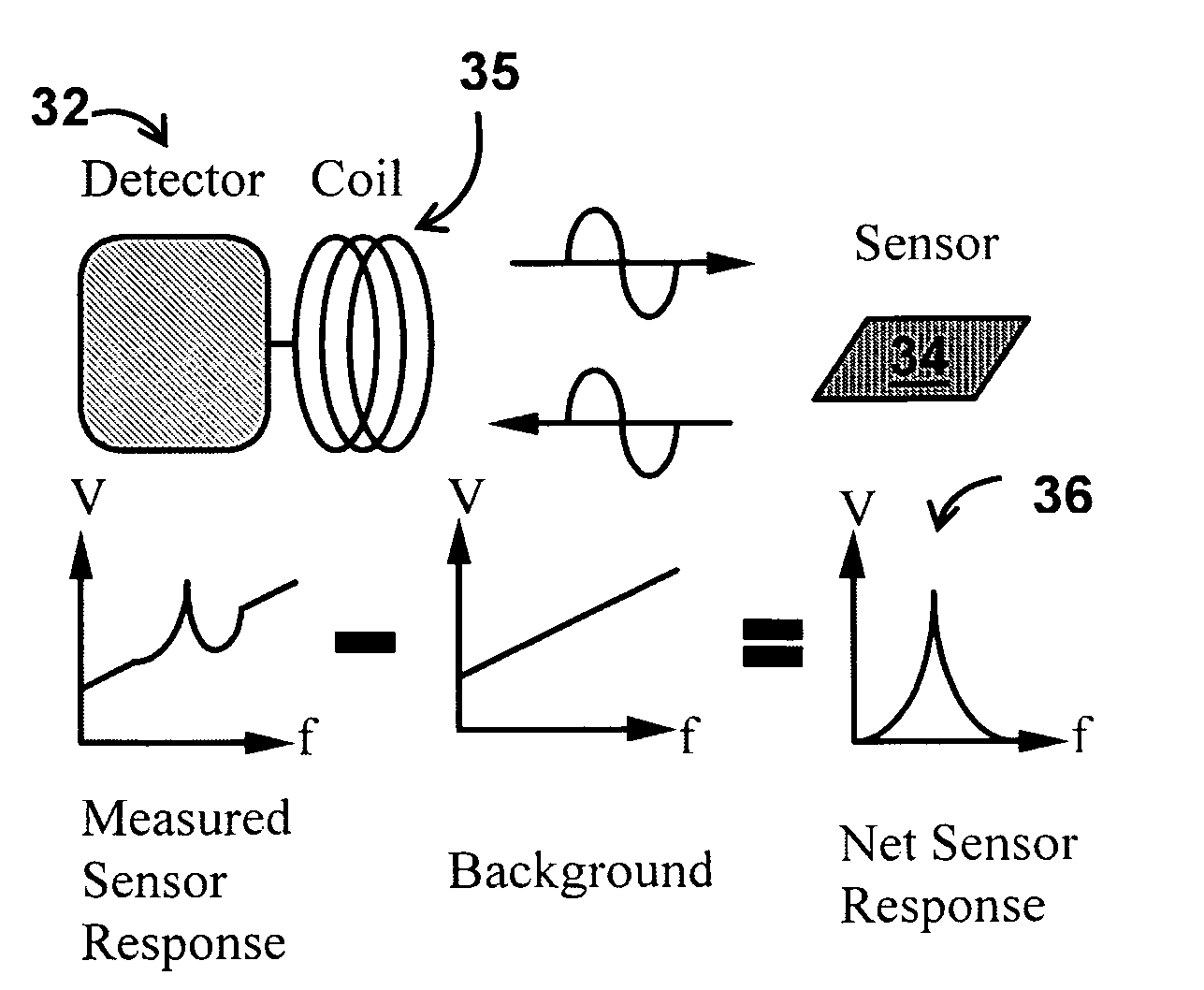
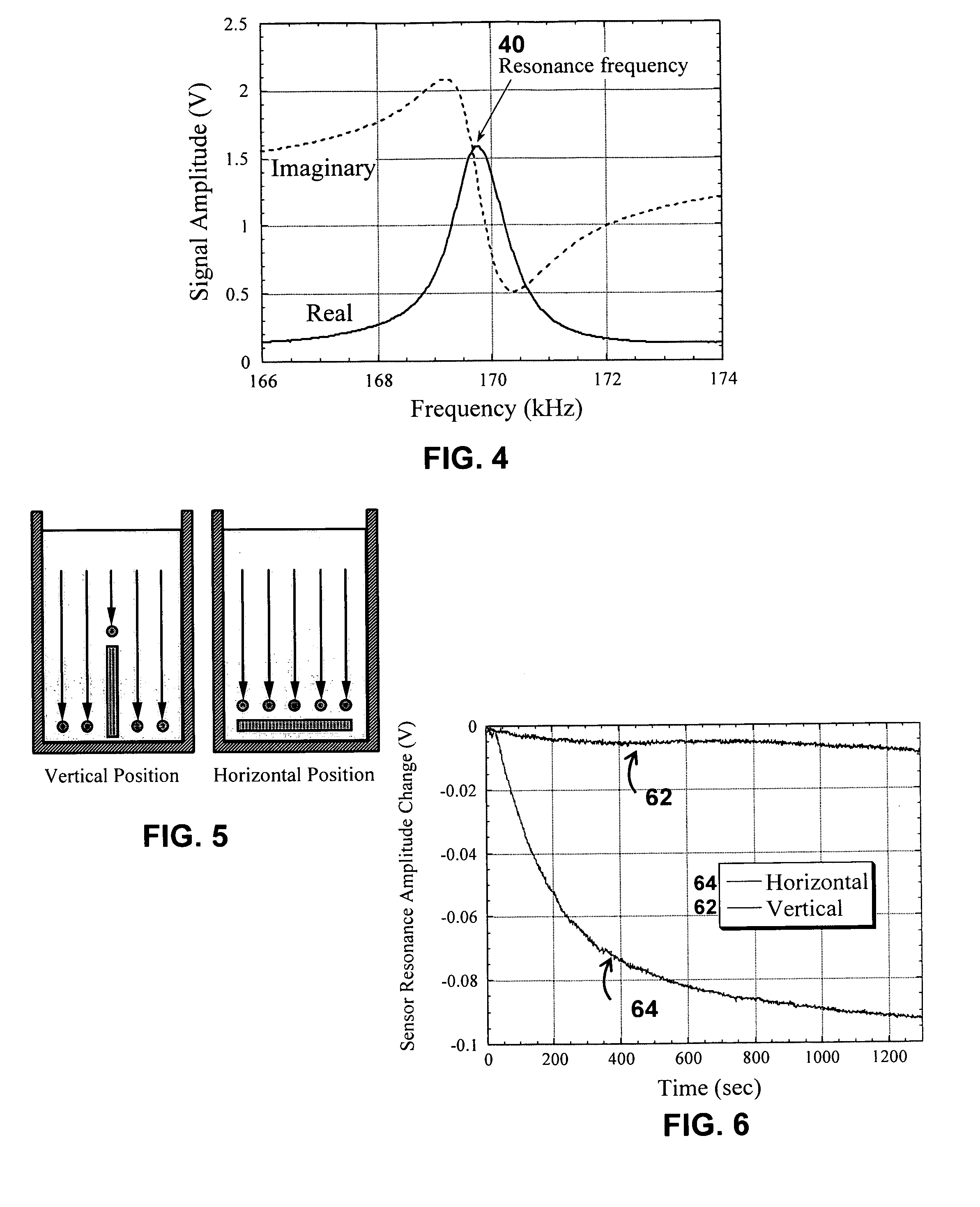
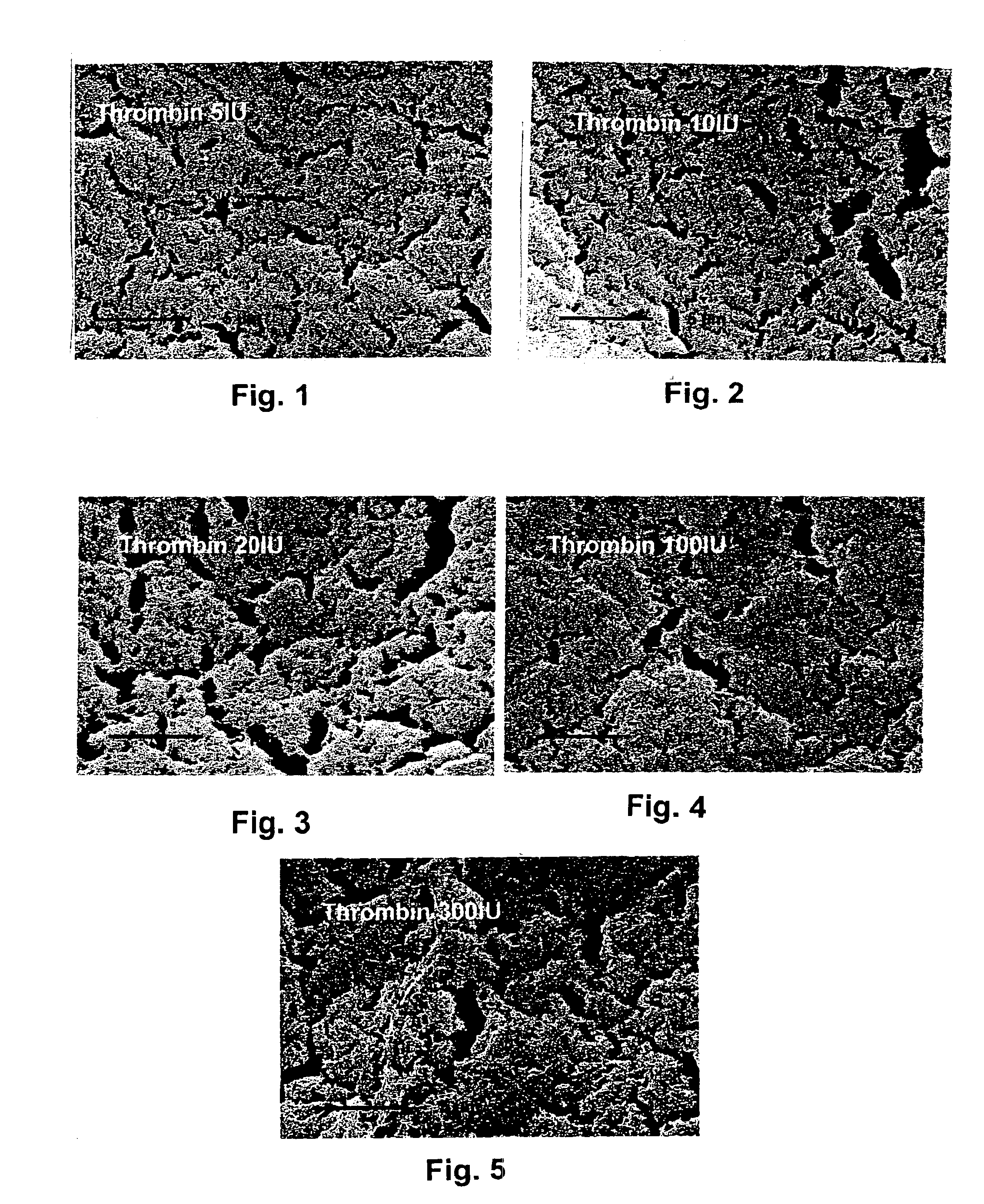
A system / analyzer-unit and method / platform—using information obtained from at least one, adapted for a plurality of, magnetoelastic sensor elements in contact with one or more samples comprising blood from a patient—for automatically quantifying one or more parameters of the patient's blood. Information obtained from emissions measured from each of the sensor elements is uniquely processed to determine a quantification about the patient's blood, such as, quantifying platelet aggregation to determine platelet contribution toward clot formation; quantifying fibrin network contribution toward clot formation; quantifying platelet-fibrin clot interactions; quantifying kinetics of thrombin clot generation; quantifying platelet-fibrin clot strength; and so on. Structural aspects of the analyzer-unit include: a cartridge having at least one bay within which a sensor element is positioned; each bay in fluid communication with both (a) an entry port for injecting a first blood sample composed of blood taken from the patient (human or other mammal), and (b) a gas vent through which air displaced by injecting the first blood sample into the bay.
Owner:KMG2 SENSORS CORP
Fibrin material and method for producing and using the same
This invention describes a bioerodible fibrin material which is obtained by mixing fibrinogen and thrombin reconstituted or diluted with a particular high tonic strength medium, free of calcium. Such a fibrin-based biomaterial develops a tight structure with thin fibers and small pore size suitable for use as an anti-adhesion barrier. In this invention, thrombin is no longer the variable which governs the tightness and the porosity of the fibrin material obtained, but still controls the clotting time. The mechanical behavior, high-water capacity, and releasable retention properties for therapeutic agents of this fibrin structure causes the fibrin material to be ideally suited for use as a drug delivery device, capable of delivering proteins, hormones, enzymes, antibiotics, antineoplastic agents and even cells for local and systemic treatment of human and non-human patients.
Owner:BAXTER INT INC
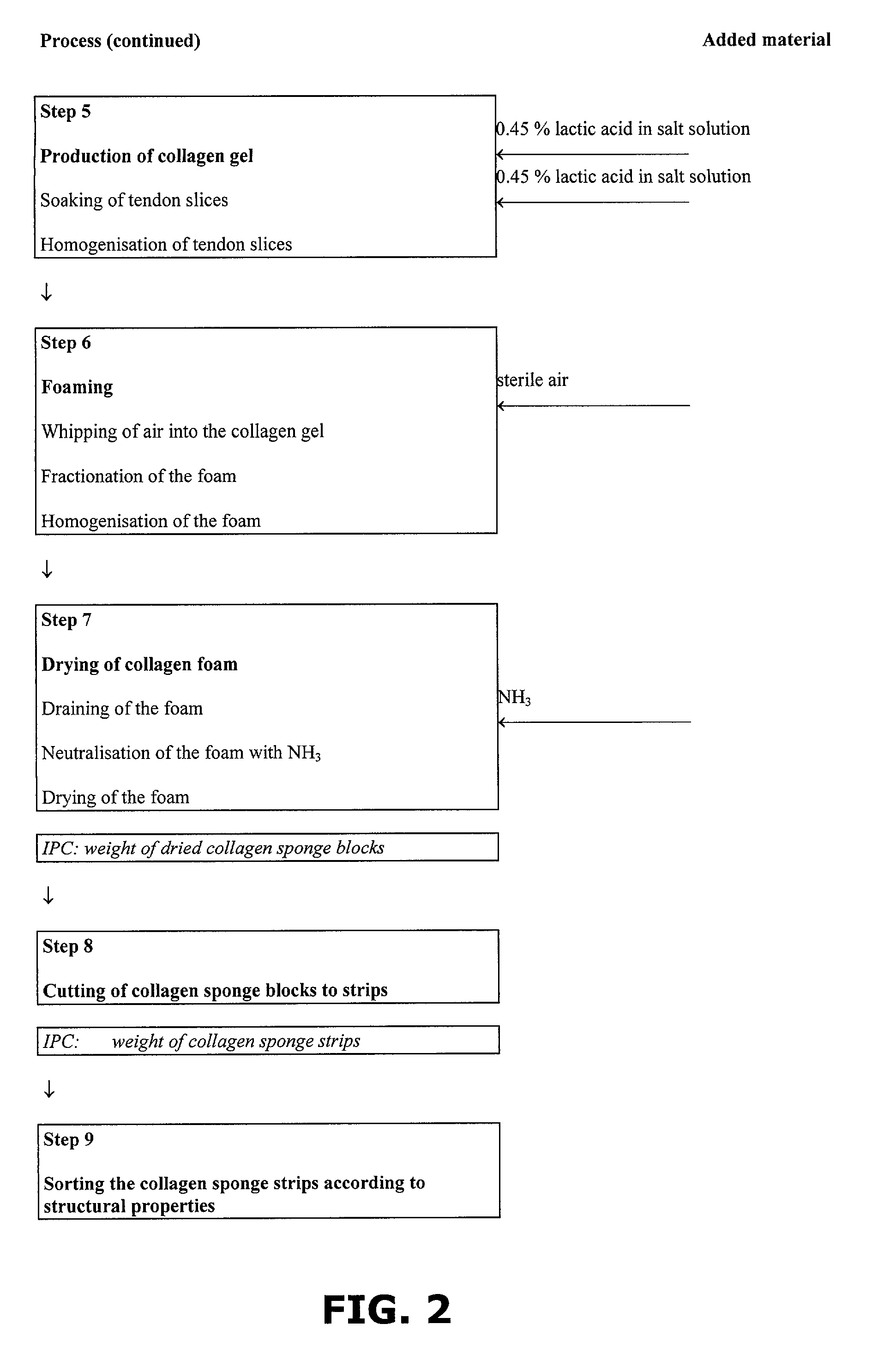
Method of preparing a collagen sponge, a device for extracting a part of a collagen foam, and an elongated collagen sponge
InactiveUS7098315B2Improve featuresHigh densitySurgical adhesivesPeptide/protein ingredientsAprotininFibrin glue
A method of preparing a collagen sponge comprises mixing air into a collagen gel, so as to obtain a collagen foam which is dried. From the dried product thereby obtained, collagen sponge is obtained by isolating parts of sponge with a chamber diameter of more than 0.75 mm and less than 4 mm, or parts with an average chamber diagonal dimension of 3 mm. The collagen sponge may be used as a material for sealing wounds, possibly with a coating comprising a fibrin glue, such as a combination of fibrinogen, thrombin and aprotinin. A device for extracting a part of a collagen foam and for degenerating another part of the collagen foam to a collagen gel is disclosed. An elongated collagen sponge having a through-going hole or bore and a flexible wall may be used for re-establishing walls in a mammalian gastrointestinal funnel or trachea system.
Owner:TOPAZ INVESTMENT AS
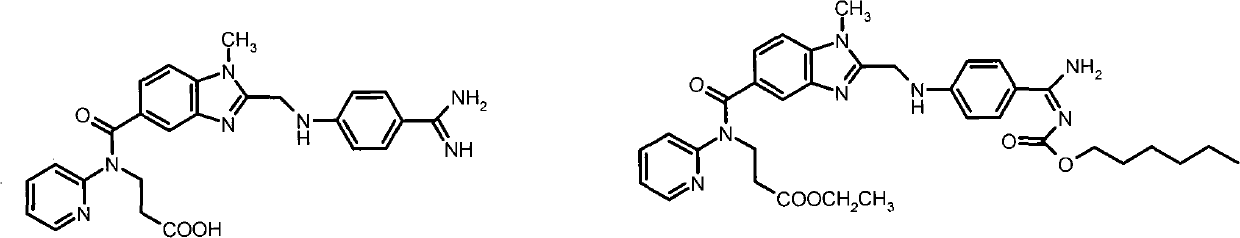
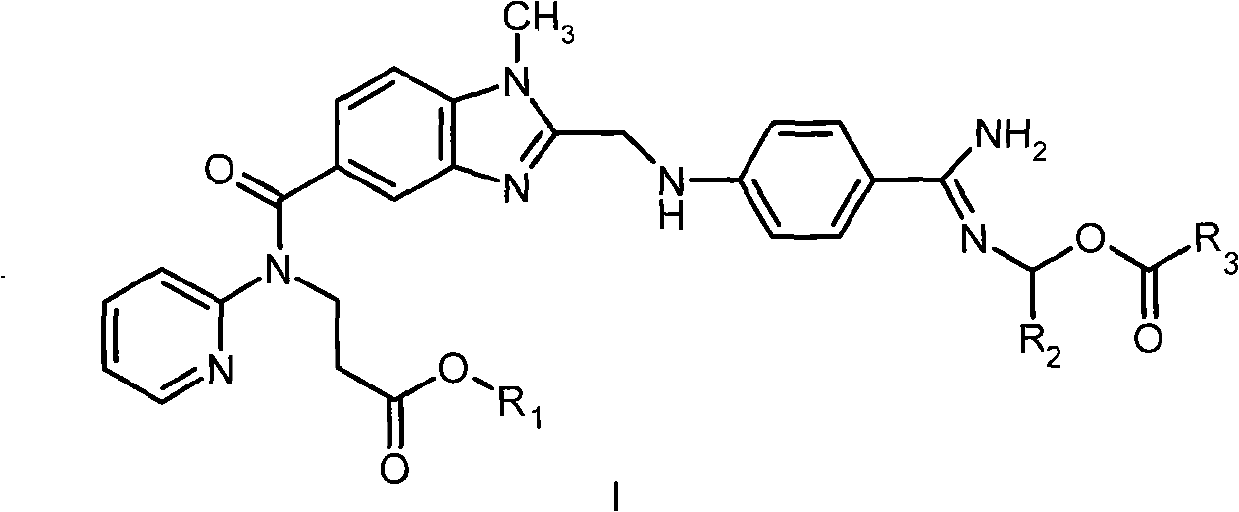
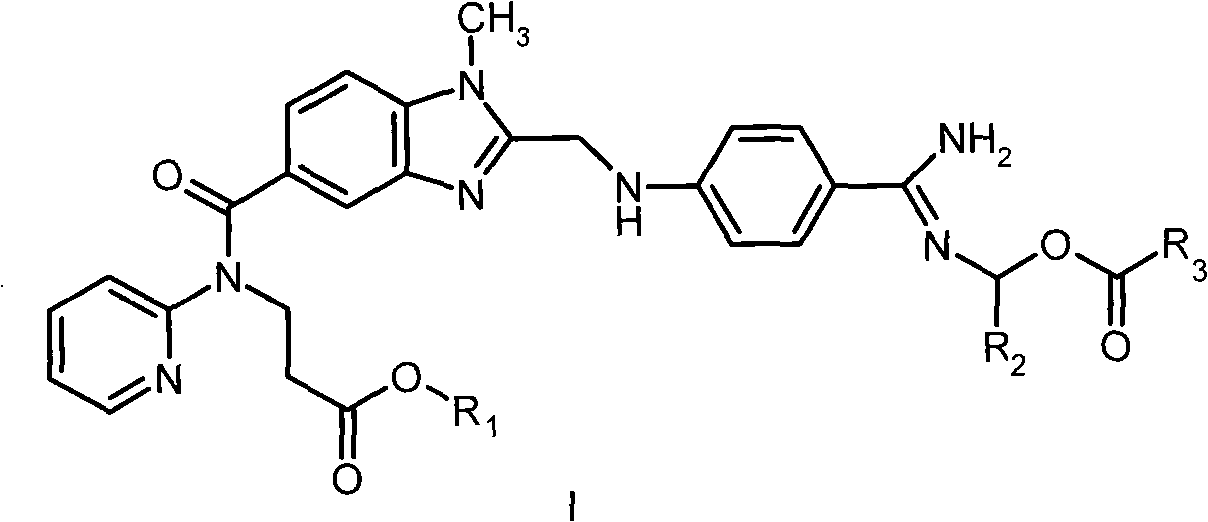
Dabigatran ester derivatives as prodrug
The invention relates to dabigatran ester derivatives as shown in a constitutional formula I, pharmaceutically acceptable nontoxic salts thereof, medical composite containing the compounds as active components, and application of thrombin inhibitor of the compounds and medical composites, wherein R1 represents H or C1 to C5 alkyl; R2 represents H, or C1 to C3 alkyl; and R3 represents C1 to C8 alkyl or substituted alkyl.
Owner:北京弘生医药科技有限公司
Multisubstituted aromatic compounds as inhibitors of thrombin
ActiveUS20130040950A1Effective treatmentPrevent disease and disorderBiocideNervous disorderDiseaseMedicine
There are provided inter alia multisubstituted aromatic compounds useful for the inhibition of thrombin, which compounds include substituted pyrazolyl or substituted triazolyl. There are additionally provided pharmaceutical compositions. There are additionally provided methods of treating and preventing a disease or disorder, which disease or disorder is amenable to treatment or prevention by the inhibition of thrombin.
Owner:VERSEON INT CORP
Antagonists of a non-selective cation channel in neural cells
InactiveUS20100092469A1Reduce mortalityReduces stroke sizeBiocideNervous disorderDiseaseNervous system
The present invention is directed to a combination of therapeutic compounds and treatment methods and kits using the combination. In particular, one of the combination affects the NCca-ATP channel of neural tissue, including neurons, glia and blood vessels within the nervous system. Exemplary SUR1 and / or TRPM4 antagonists that inhibit the NCca-ATP channel may be employed in the combination. The combination therapy also employs one or more of a non-selective cation channel blocker and / or an antagonist of VEFG, NOS, MMP, or thrombin. Exemplary indications for the combination therapy includes the prevention, diminution, and / or treatment of injured or diseased neural tissue, including astrocytes, neurons and capillary endothelial cells, that is due to ischemia, tissue trauma, brain swelling and increased tissue pressure, or other forms of brain or spinal cord disease or injury, for example. In other embodiments, there are methods and compositions directed to antagonists of TRPM4, including at least for therapeutic treatment of traumatic brain injury, cerebral ischemia, central nervous system (CNS) damage, peripheral nervous system (PNS) damage, cerebral hypoxia, or edema, for example.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
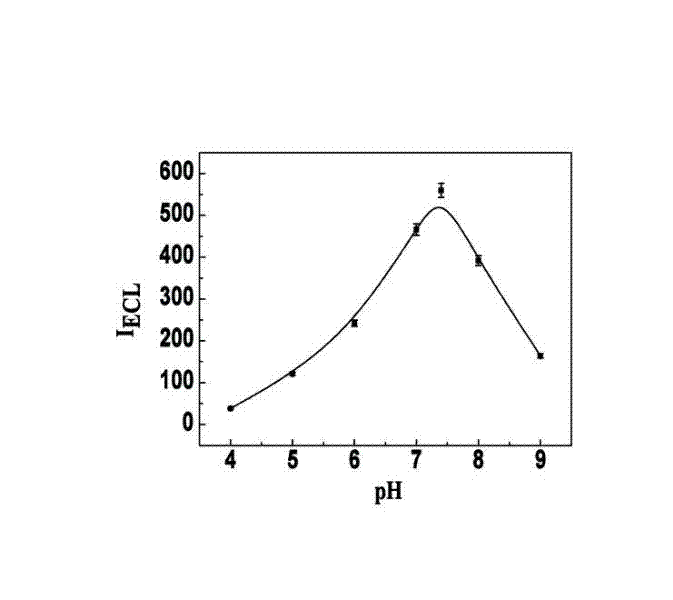
Manufacturing method and application of electrochemiluminescence sensor for detecting thrombin
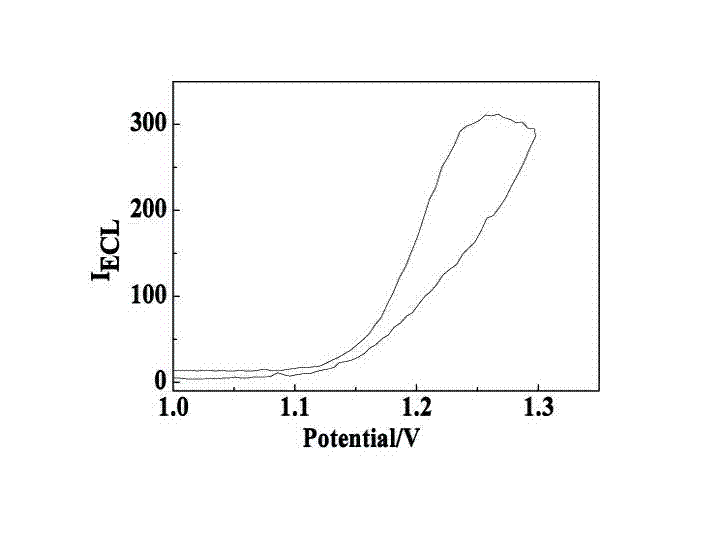
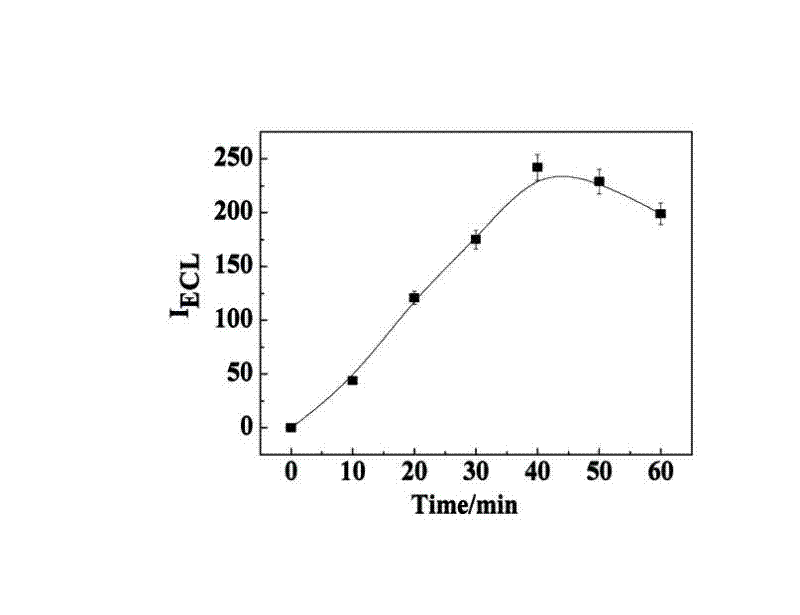
ActiveCN102507689AHigh sensitivityHigh luminous intensityChemiluminescene/bioluminescenceMaterial analysis by electric/magnetic meansSingle strandElectrochemiluminescence
The invention relates to a manufacturing method and application of an electrochemiluminescence sensor for detecting thrombin. Ruthenium bipyridine with active group is modified to the gold nanoparticle surface to form an electrochemiluminescence nanoparticle probe, which plays the role of amplifying electrochemiluminescence, and the upper part DNA single strand is modified on the working electrode surface to constitute an electrochemiluminescence sensor. The target analyte of thrombin is subjected to reaction with partial DNA double strands and then with DNA polymerase, Nb.BbvCI nicking enzyme and partial DNA single strand to obtain DNA hybrid solution which is added into the sensor, so as to increase electrochemiluminescence nanoparticle probes on the electrode surface and further lead to an enhanced electrochemiluminescence signal, and accordingly thrombin determination is realized. The inventive sensor has high selectivity and detection sensitivity.
Owner:武汉菲思特医学检验实验室有限公司
Method for producing a device applicable to biological tissues, particularly a patch for treating damaged tissues, and a device obtained by said method
ActiveUS20120070485A1Slow kineticsEasy to controlPeptide/protein ingredientsMetabolism disorderHaemostatic functionCross-link
The present invention relates to a device consisting of cross-linked nanofibrillary fibrin supported on and rooted to a microporous nonwoven fabric consisting of a biocompatible synthetic polymer material. An active ingredient is advantageously dispersed in the fibrin layer. The fibrin layer does not have a haemostatic function, but is suitable for retaining the active ingredient and releasing it with controlled kinetics. The device forming the object of the invention, preferably in the form of patches, is useful for in vitro cell cultures or for treating tissues damaged by wounds or necrosis, such as cardiac walls bearing the sequelae of infarction, or a tissue damaged by a diabetic ulcer. The patch according to the invention can be manufactured by inducing the polymerisation of the fibrin, under suitable conditions, directly on the support layer, which is suitably impregnated with thrombin (at least in a superficial portion of its thickness), and which has been conveniently prepared by means of a spray phase-inversion technique.
Owner:CONSIGLIO NAT DELLE RICERCHE +1
Hemostatic Pad Assembly Kit and Method
The present invention relates generally to agents and devices for promoting hemostasis and tissue sealing and, more particularly, to hemostatic pads comprising bioabsorbable scaffolds that can deliver lyophilized hemostasis promoting proteins, such as fibrinogen and thrombin, to a wound site or injured organ or tissue.
Owner:ETHICON INC
Substituted biaryl compounds as factor XIa inhibitors
The present invention provides compounds of Formula (I):or a stereoisomer, tautomer, pharmaceutically acceptable salt or solvate form thereof, wherein the variables A, L, Z, R3, and ring B are as defined herein. The compounds of Formula (I) are useful as selective inhibitors of serine protease enzymes of the coagulation cascade and / or contact activation system; for example thrombin, factor Xa, factor XIa, factor IXa, factor VIIa and / or plasma kallikrein. In particular, it relates to compounds that are selective factor XIa inhibitors. This invention also relates to pharmaceutical compositions comprising these compounds and methods of treating thromboembolic and / or inflammatory disorders using the same.
Owner:BRISTOL MYERS SQUIBB CO
Preparation method of hemostatic material
ActiveCN104721878AGood hemostatic effectSave rescue timeBiochemical fibre treatmentAbsorbent padsMedicineElectrospinning
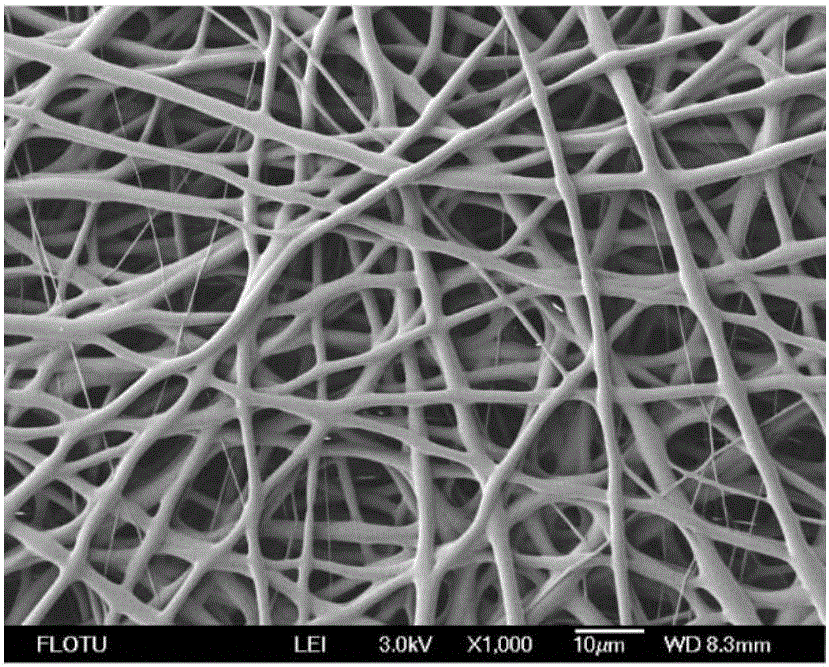
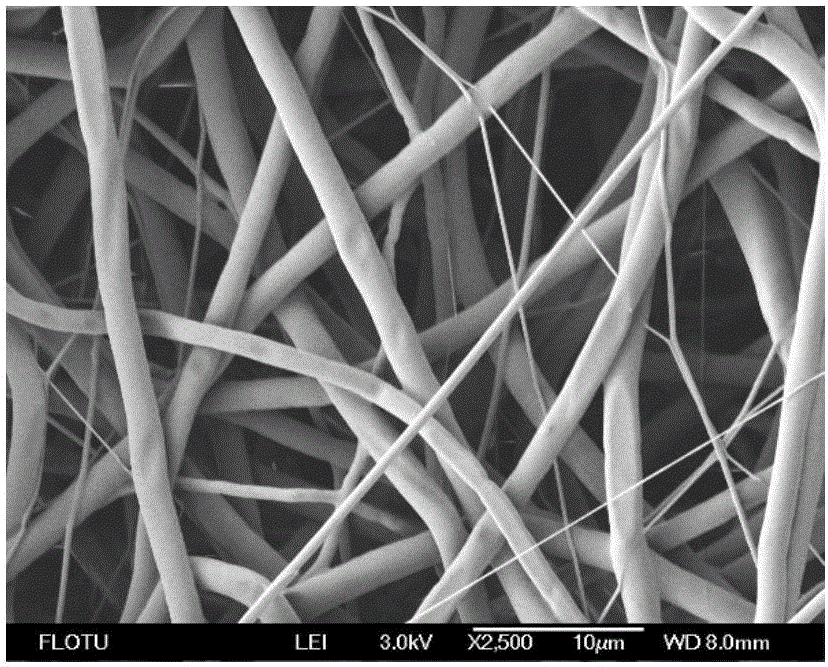
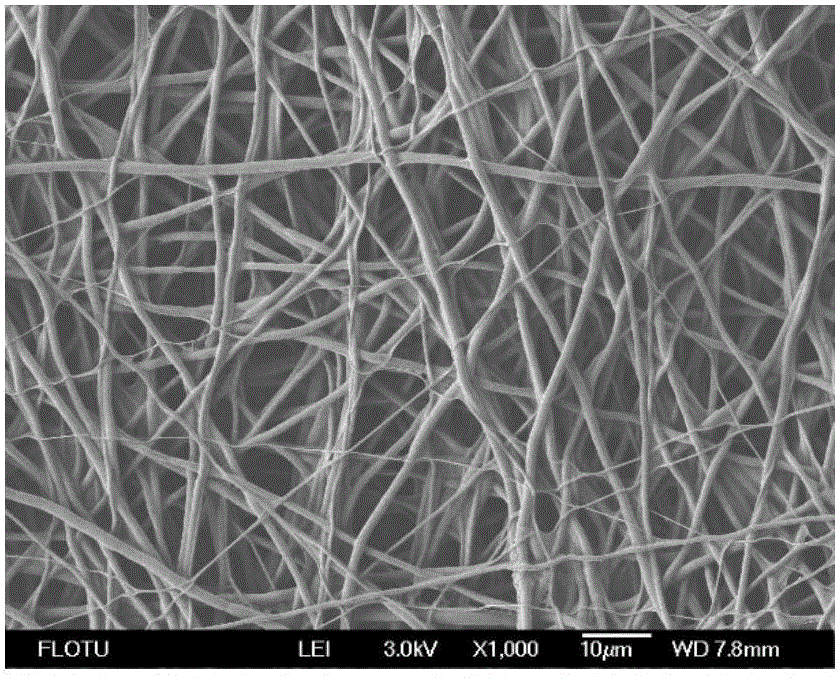
The invention discloses a preparation method for a hemostatic material, an absorbable carrier material prepared by an electrospinning technology or a combination of the absorbable carrier material and a blood coagulation factor film-shaped material prepared by the electrospinning technology, wherein blood coagulation factors are fibrinogen and thrombin from human or animal blood. The superfine fibre prepared by the electrospinning technology has the advantages that the specific surface area of the reticulate structure of the hemostatic material is increased, and the mutual fusion action between the reticulate structure and tissues is promoted. The hemostatic material is good in biocompatibility, more remarkable in hemostatic effect and convenient to use, and can be used for hemostasis in trauma treatment and clinical operations.
Owner:GUANGZHOU DIANFANG BIOTECH CO LTD
Dry powder fibrin sealant
ActiveUS8846105B2Increase absorbanceSpeed up the flowSurgical adhesivesPeptide/protein ingredientsFibrinogenFibrin adhesive
Owner:MALLINCKRODT PHARMA IP TRADING D A C
Haemostatic kit, a method of preparing a haemostatic agent and a method of promoting haemostatis
A haemostatic kit to be used as a medical device provides for a containment unit and a haemostatic agent in said containment unit, said haemostatic agent occupying less than 90% of the volume of the containment unit. This allows for facile and consequently sterile preparation of, for instance, a gelatin paste for use in haemostatis when combined with saline, thrombin or another agent to assist in haemostatis.
Owner:FERROSAN MEDICAL DEVICES
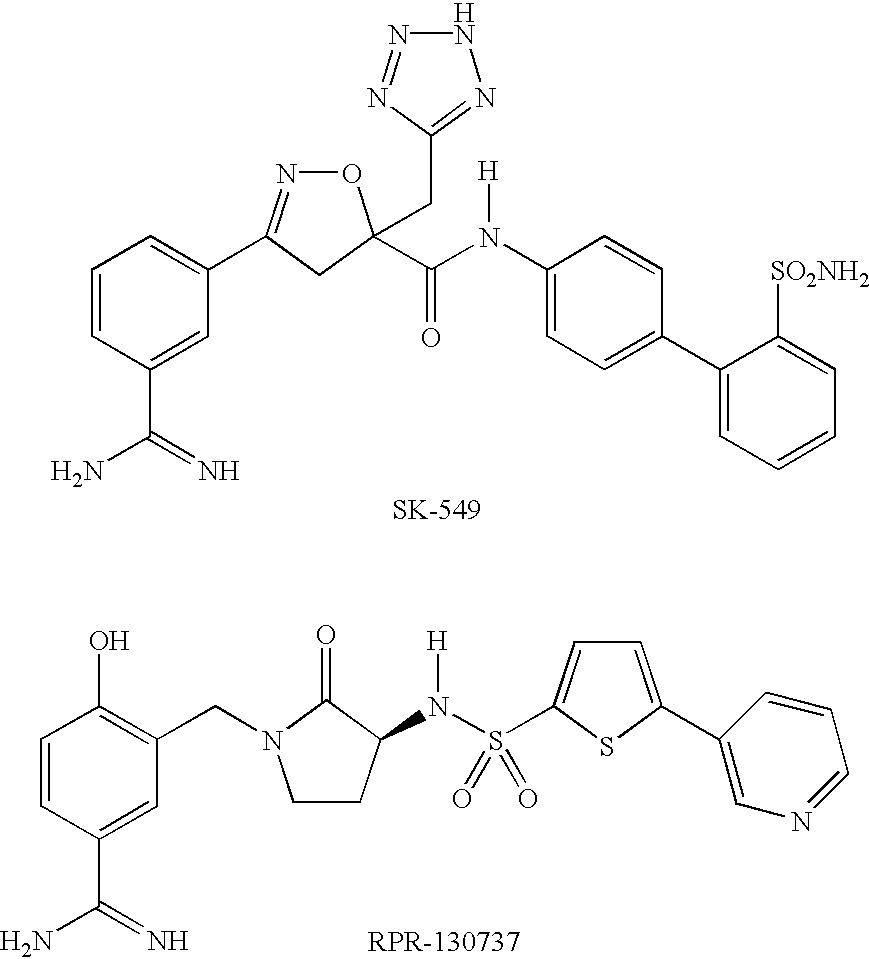
Thiochromane derivatives and their use as thrombin inhibitors
InactiveUS6716834B2Toxic reductionLonger actingOrganic active ingredientsBiocideThrombusPharmaceutical drug
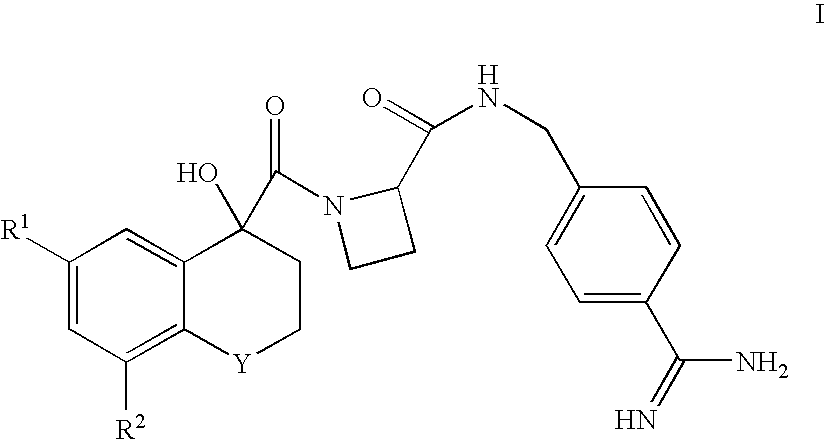
There is provided compounds of formulae I and IAwherein Y, R<1>, R<2>, R<3>, D<1 >and D<2 >have meanings given in the description which are useful as, or as prodrugs of, competitive inhibitors of trypsin-like proteases, such as thrombin, and in particular in the treatment of conditions where inhibition of thrombin is required (e.g. thrombosis) or as anticoagulants.
Owner:ASTRAZENECA AB
Injected bone repairing material and its prepn and application
InactiveCN1846789ABiologically activeClosed woundPeptide/protein ingredientsSkeletal disorderFiberBlood plasma
The present invention discloses one kind of injected bone repairing material and its preparation and application. The injected bone repairing material consists of solution I and solution II, the solution I is the mixture of platelet rich plasma (PRP) and 80-100 mg / ml concentration fibrinogen solution in the ratio of 1 to 3-6; and the solution II is solution mixture of calcium chloride in 40 mmol / ml concentration and thrombin in 400-1000 U / ml concentration. When the injected bone repairing material is used, solution I and solution II in the volume ratio of 1-9 are sucked with two syringes and injected into body to form gel with high adhesive force, with PRP accounting for 10-30 vol%. The injected bone repairing material is used in preparing implant for treating bone defect and bone nonunion and promoting fracture healing.
Owner:NAN FANG HOSPITAL
Formulations for Wound Therapy
InactiveUS20140369991A1Enhance powder flowImprove wettabilityPeptide/protein ingredientsPharmaceutical non-active ingredientsMedicineWound therapy
The present invention relates to novel formulations comprising a dry powder fibrin sealant comprised a mixture of fibrinogen and / or thrombin, for use in the treatment of wounds or injuries, in particular for use as a topical hemostatic composition or for surgical intervention.
Owner:MALLINCKRODT PHARMA IP TRADING D A C
Beta-Aminoacid-Derivatives As Factor Xa Inhibitors
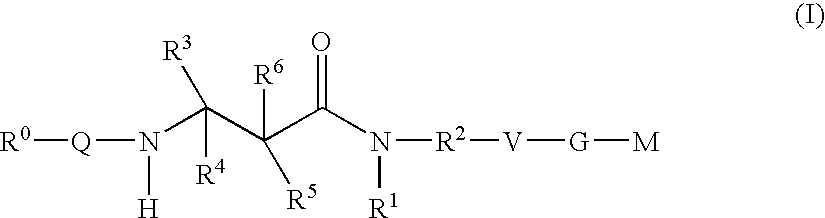
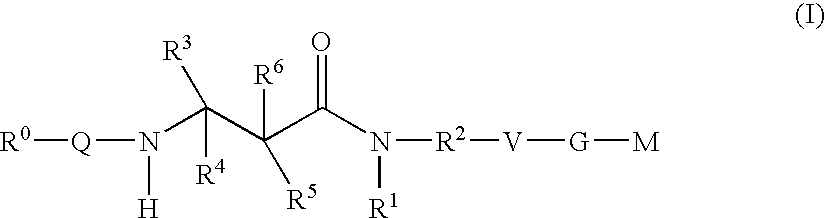
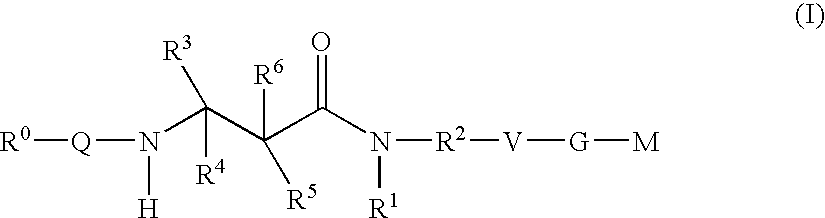
The present invention relates to compounds of the formula I, in which R0; R1; R2; R3; R4; R5, R, Q; V, G and M have the meanings indicated in the claims. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong antithrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardiovascular disorders like thromboemboic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and / or factor VIIa (FVIIa), and can in general be applied in conditions in which an undesired activity of factor Xa and / or factor VIIa is present or for the cure or prevention of which an inhibition of factor Xa and / or factor VIIa is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI AVENTIS DEUTSCHLAND GMBH
Method for prophylaxis and/or treatment of thromboembolism
InactiveUS6875446B2Reduce solubilityLimited food interactionPowder deliveryOrganic active ingredientsFiller ExcipientThrombus
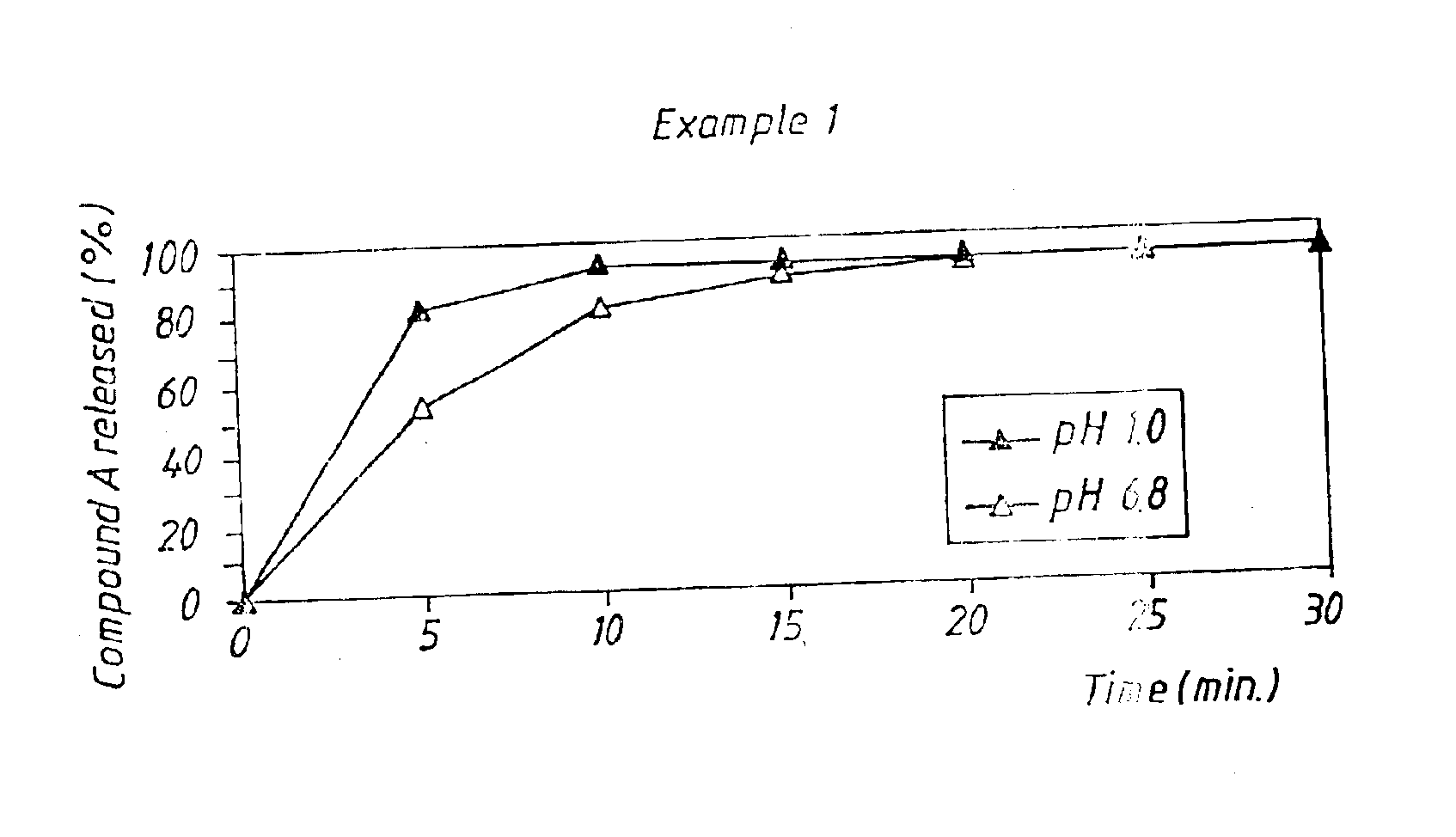
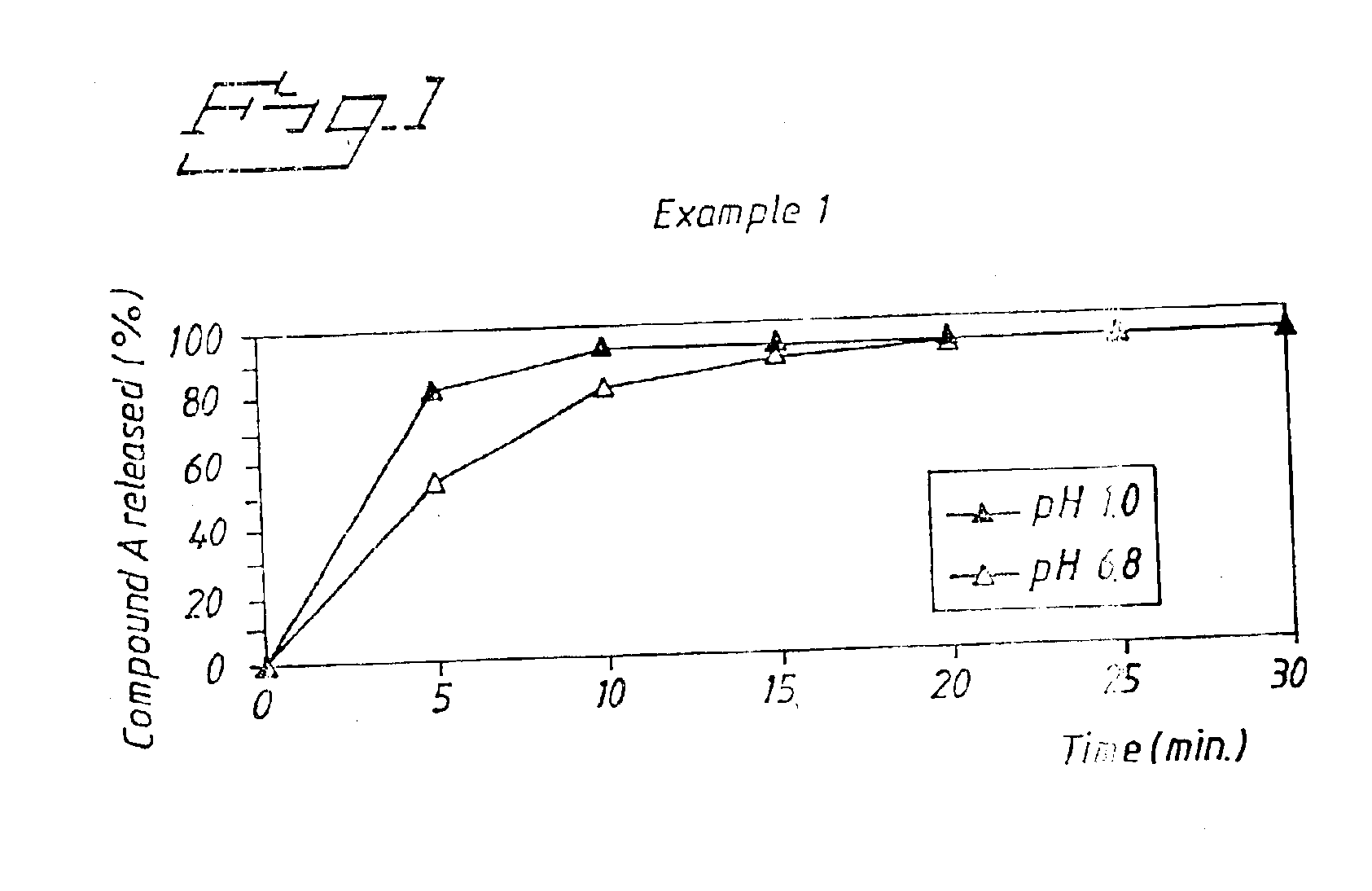
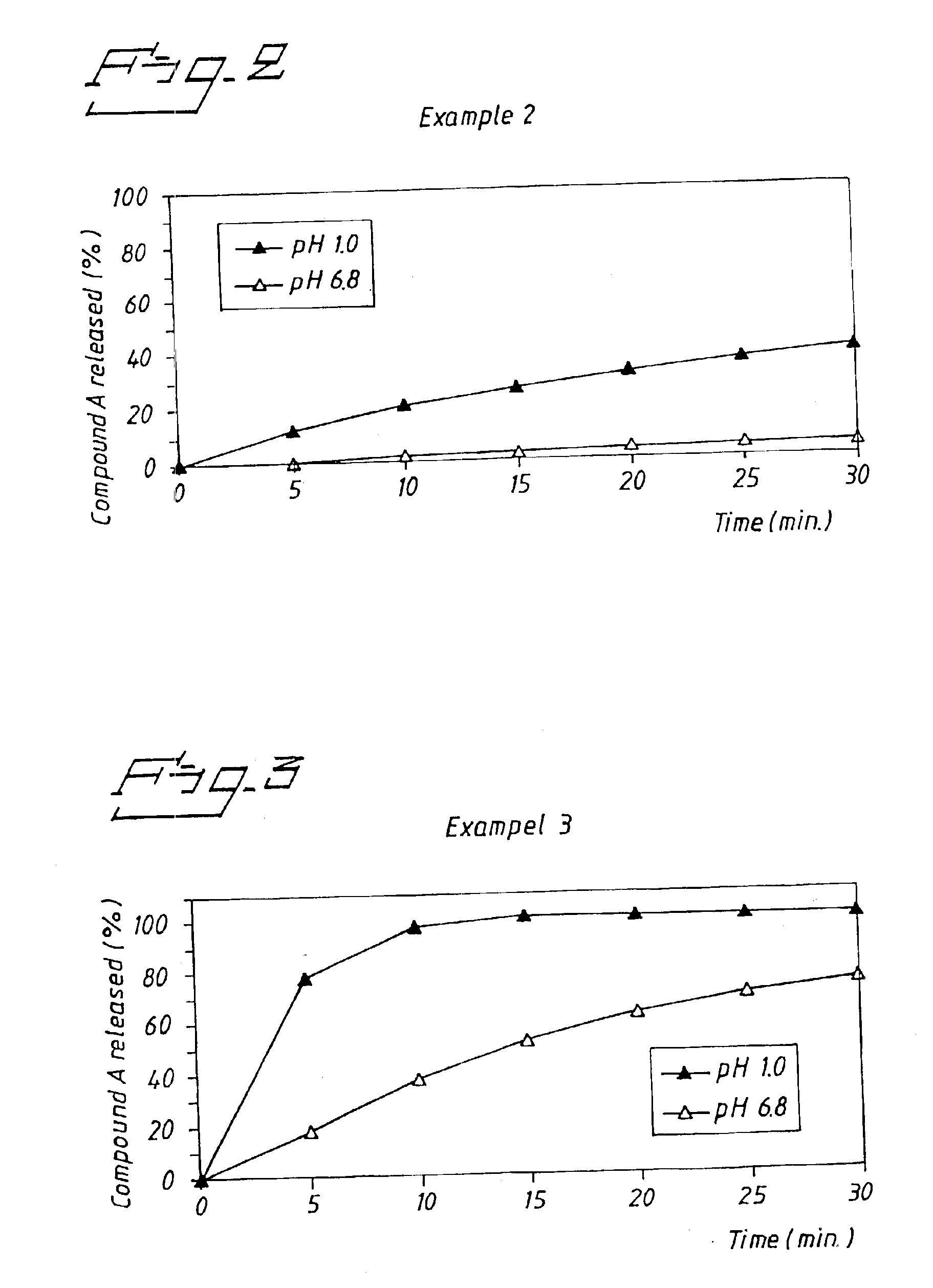
A new oral IR formulation in solid form for a low molecular weight thrombin inhibitor having pH dependant dissolution, characterized in that the formulation comprises a filler or a combination of fillers having disintegrant properties in an amount higher than 35% w / w of the formulation.
Owner:ASTRAZENECA AB
Antibacterial-hemostatic material with non-woven fabric fiber fabric structure and production method of antibacterial-hemostatic material
InactiveCN105012991AReduce dosageGood storage stabilityBiochemical fibre treatmentFibre typesPolyesterPorosity
The invention relates to antibacterial-hemostatic material with a non-woven fabric fiber fabric structure and a production method of the antibacterial-hemostatic material and belongs to the technical field of biomedical materials. The antibacterial-hemostatic material is characterized in that aliphatic polyester is dissolved into solvent, an electrospun fibrous membrane is produced through static spinning, chitosan is loaded on the surface of the electrospun fibrous membrane, thrombin is further loaded on the surface of the electrospun fibrous membrane, and the antibacterial-hemostatic material is produced. The material features of the aliphatic polyester and the chitosan are utilized, the structure features such as high porosity and high specific surface area of the formed electrospun fibrous membrane are utilized, stable loading of the thrombin is performed by the crosslinking action of the chitosan and glutaraldehyde, and the antibacterial-hemostatic material with the non-woven fabric fiber fabric structure is formed. The antibacterial-hemostatic material has the advantages that the material is good in antibacterial and hemostatic effects, the antibacterial and hemostatic effects of the material are obviously better than those of commercially available gelatin sponge, the material is good in mechanical performance, easy to store and convenient to remove after being used, the production method of the material is simple to operate and capable of reducing raw material use amount, and the material is applicable to fields such as surgery and trauma first-aid.
Owner:TSINGHUA UNIV
Mandelic acid derivatives and their use as throbin inhibitors
InactiveUS20040019033A1Preparation by organometalhalide reactionBiocideThrombin activityMethyl mandelic acid
There is provided a compound of formula I wherein R, R<1>, R<2>, Y and R<3 >have meanings given in the description and pharmaceutically acceptable derivatives (including prodrugs) thereof, which compounds and derivatives are useful as, or are useful as prodrugs of, competitive inhibitors of trypsin-like proteases, such as thrombin, and thus, in particular, in the treatment of conditions where inhibition of thrombin is required (e.g. thrombosis) or as anticoagulants.
Owner:ASTRAZENECA AB
Triazole-derivatives as blood clotting enzyme factor Xa inhibitors
The present invention is directed to the compound of formula I which is useful for inhibiting the activity of blood clotting enzyme Factor Xa. The present invention is also directed to compositions containing said compounds, processes for their preparation, their use, such as for inhibiting the formation of thrombin or for therapeutically treating a patient suffering from, or subject to, a disease state associated with a cardiovascular disorder.
Owner:SANOFI AVENTIS DEUT GMBH
Chitosan gel haemostatic material and preparation method thereof
ActiveCN104189941AGood biocompatibilityImprove mechanical propertiesAbsorbent padsBandagesWound healingCross-link
The invention relates to a chitosan gel haemostatic material and a preparation method thereof. The chitosan gel haemostatic material is compounded of chitosan, a cross-linking agent and thrombin. The chitosan gel haemostatic material disclosed by the invention is good in biocompatibility and biodegradability; hydrogel is excellent in mucous membrane viscosity and self-adaptability, and has functions of quickly stopping blooding in body, promoting wound healing and releasing thrombin slowly so as to prevent secondary blooding caused by postoperative motion; meanwhile, the haemostatic material has characteristics of bacteria resistance and bacteriostasis and is capable of effectively preventing wound infection.
Owner:BEIJING UNIV OF CHEM TECH
Factor xa inhibitors with aryl-amidines and derivatives, and prodrugs thereof
InactiveUS20030065176A1Good choicePrevent coagulationOrganic compound preparationOrganic chemistry methodsPharmaceutical medicinePyrrole
The present invention relates to a compound with aryl-amidines, particularly amidinoaryl-cyclopropanes, amidinoarylmethyl-pyrroles, amidinoaryl-benzenes, amidinoaryl-pyridines, or amindonoaryl-alanines, represented by formula (1), a pharmaceutically acceptable salt, a prodrug, a hydrate, a solvate or an isomer thereof, which are inhibitors of coagulation enzyme, factor Xa (FXa). The present invention also relates to a pharmaceutical composition containing the compound, and a method of using the same as an anticoagulant agent for treatment and prevention of thrombosis disorders.
Owner:LG CHEM INVESTMENT LTD
Formulations for wound therapy
InactiveUS9717821B2Increase absorbanceSpeed up the flowPeptide/protein ingredientsPharmaceutical non-active ingredientsWound therapyFibrinogen
The present invention relates to novel formulations comprising a dry powder fibrin sealant comprised of a mixture of fibrinogen and / or thrombin, for use in the treatment of wounds or injuries, in particular for use as a topical hemostatic composition or for surgical intervention.
Owner:MALLINCKRODT PHARMA IP TRADING D A C
Novel hemostatic compositions and dressings for bleeding
ActiveUS20150283286A1Activate blood clottingFacilitate rapid blood clottingPeptide/protein ingredientsPharmaceutical delivery mechanismArterial hemorrhageHemostatics
The present invention provides hemostatic compositions comprising components of the flowers of pharmaceutical chamomile (Chamomilla recutita), the leaves of dioecious nettle (Urtica dioica), kaolin, chitosan, fibrinogen and thrombin. Further inclusion of a biocompatible polymeric base, particularly an alignate, generates a composition with superior and broad spectrum hemostatic capabilities, including the ability to arrest arterial hemorrhage. The invention further provides methods of using the inventive compositions in to reduce or stop bleeding, as well as a variety of apparatuses useful in hemostatic contexts that incorporate the inventive compositions. In one particular embodiment, the invention provides hemostatic dressings in which a polymeric layer incorporating chamomile, nettle, kaolin, chitosan, fibrinogen and thrombin components is applied to a textile or fabric material, for example a non-woven viscose.
Owner:YES +1
Preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII
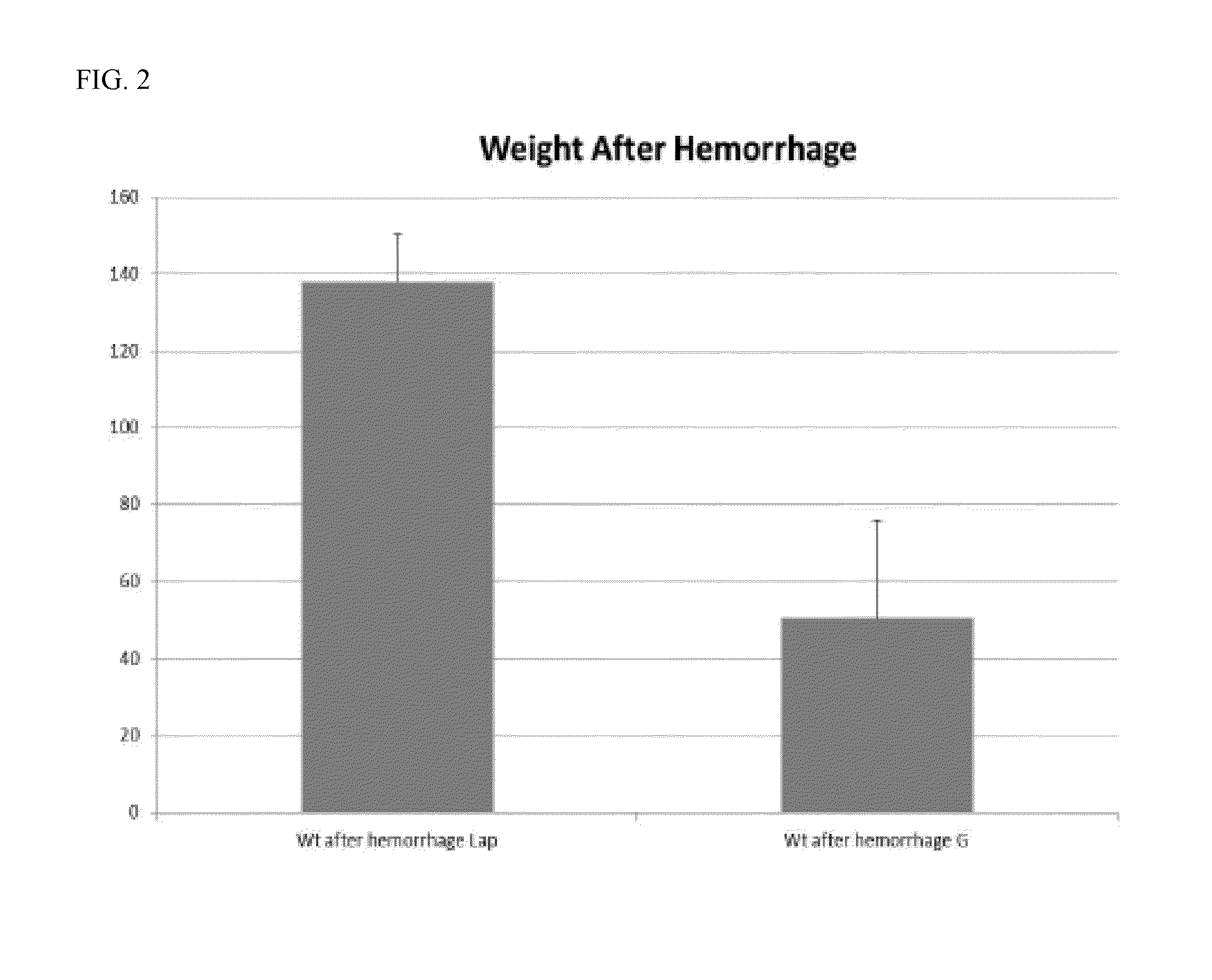
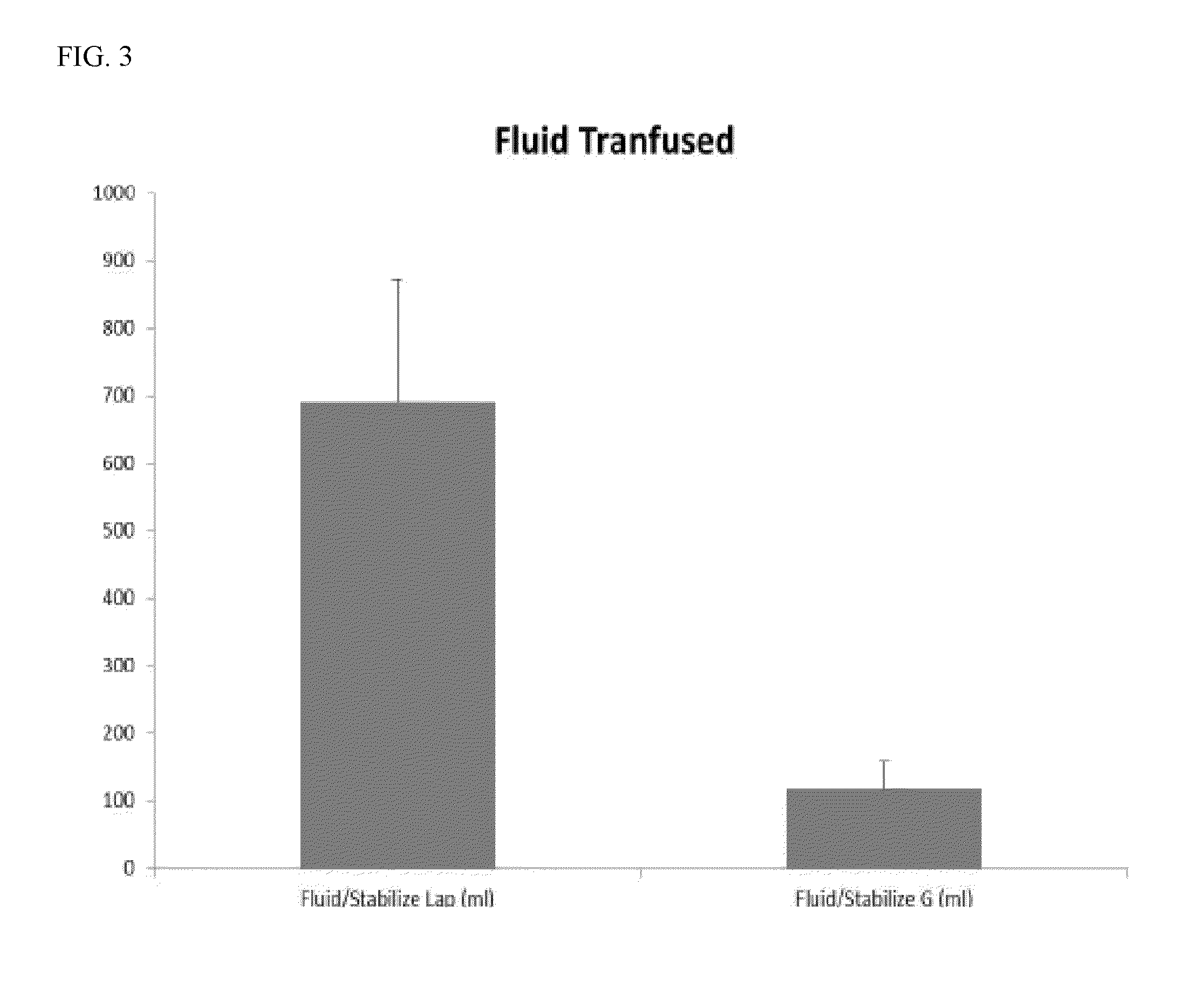
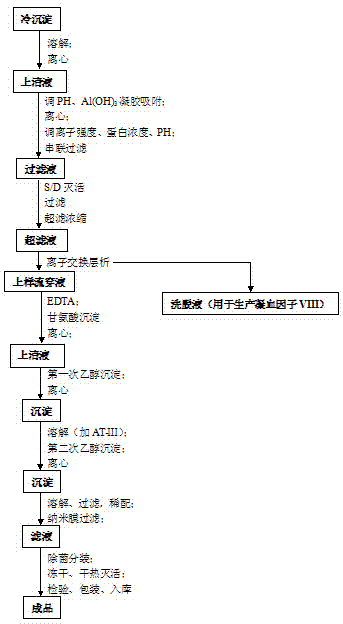
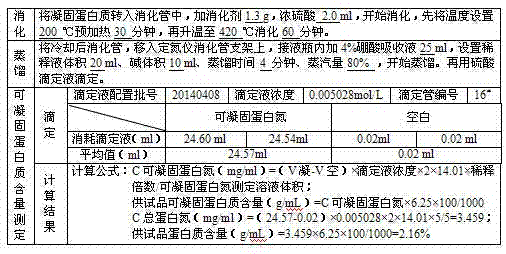
ActiveCN104231072AHigh purityShort reconstitution timeFactor VIIFibrinogenBlood coagulation factor VIIIEthanol precipitation
The invention discloses a preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII. The preparation process is characterized by comprising the steps of cryoprecipitation dissolution, 2% aluminium hydroxide gel absorption, ion strength adjustment, series connection filtering, S / D viral inactivation, ion-exchange chromatography, EDTA Ca2+ removal, glycine precipitation, primary low-temperature ethanol precipitation, AT-III thrombin inhibition, secondary low-temperature ethanol precipitation, nanofilm filtering and dry-heat inactivation. For ensuring the safety, a nanofilm ia added to filter virus except S / D and dry-heat inactivation. By means of an added AT-III inactivated thrombin and EDTA Ca2+ removal process, fibrinogen in the production process is effectively prevented from being activated into fibrous protein. The glycine precipitation is utilized to remove fibrous protein monomers and polymers in products so as obtain high-purity human fibrinogen. The preparation products are safe and reliable, redissolution time is short, the clinic first-aid demand is met, and meanwhile the preparation process has important significance on indirect saving of scarce plasma resources.
Owner:华润博雅生物制药集团股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com