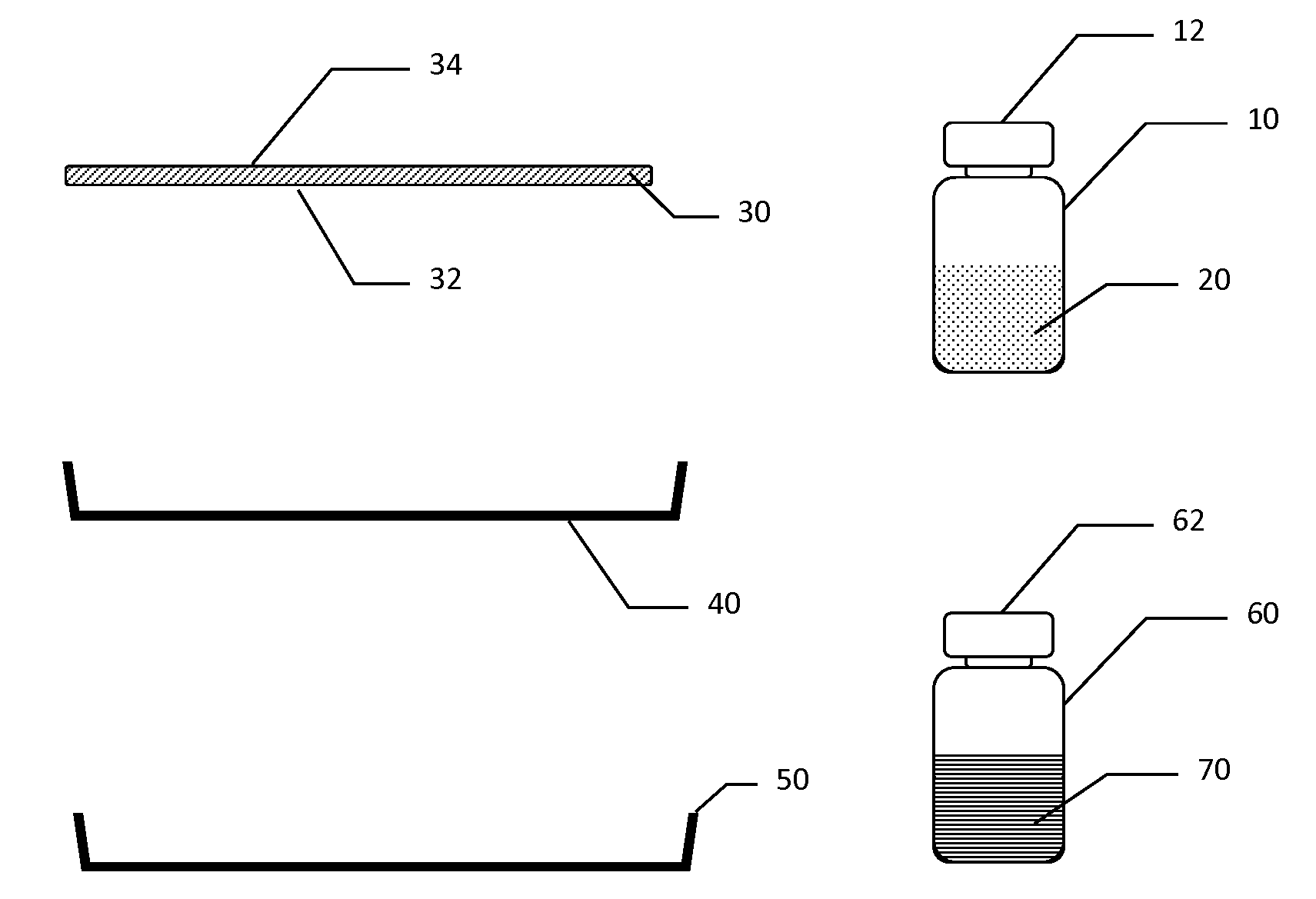
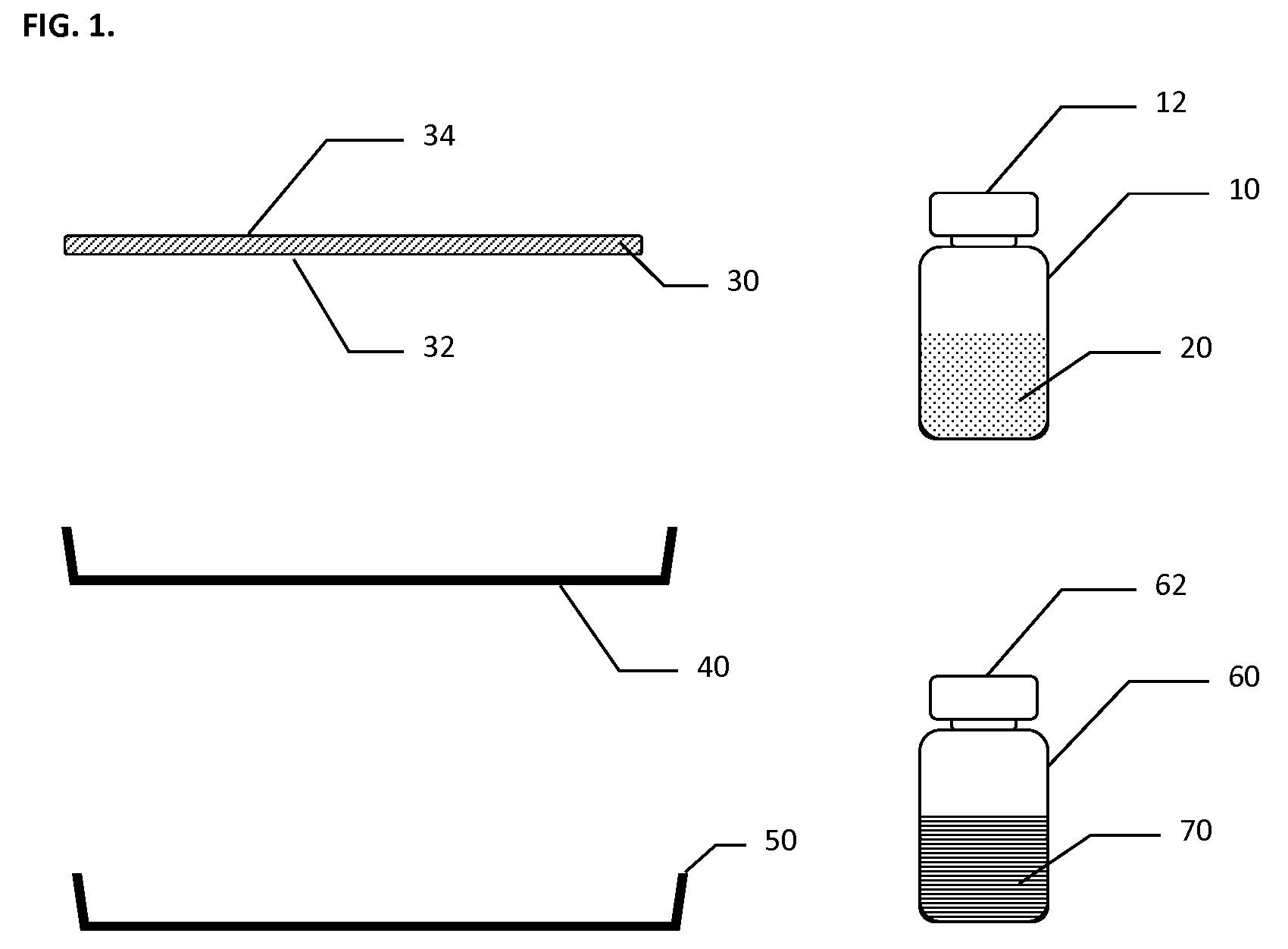
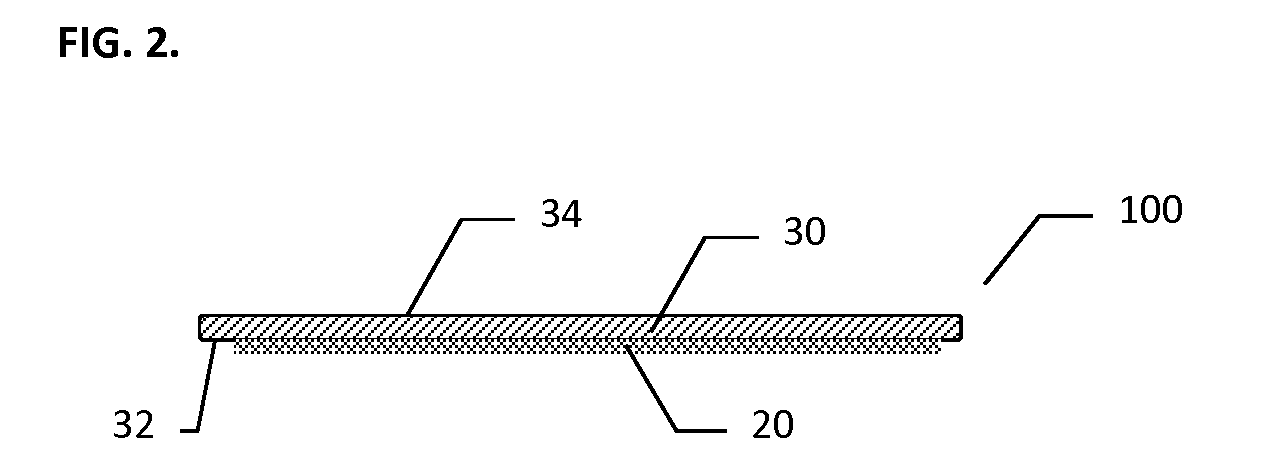
Hemostatic Pad Assembly Kit and Method
a technology of hemostasis and kit, which is applied in the field of hemostatic pads, can solve the problems of hydrolytic reaction and substantial bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hemostatic Test of Inventive Hemostatic Pad Preparation and Use
[0068]A scaffold made of composite matrix of a knitted oxidized regenerated cellulose (ORC) backing layer under a layer of Polyglactin 910 (PG910) non-woven fibers as described above was utilized. A dry hemostatic powder (lyophilized) derived from animal origin (porcine) was utilized. Approximately three hundred ninety (390) mg of thrombin powder (having about 20 IU / mg active thrombin) and about seventeen hundred (1700) mg of fibrinogen powder (containing about 0.4 mg / mg clottable fibrinogen) were available. The hemostatic powders were disaggregated from aggregated cake that formed during storage. The thrombin and fibrinogen powders were then transferred into a powder tray and thoroughly mixed and evenly distributed in the tray. The scaffold in this test was cut to size to cover end of kidney with approximately a one (1) cm margin, size of the scaffold was 3.5 cm×2.5 cm rectangular pieces.
[0069]A surgical practitioner pe...
example 2
Demonstration of Scaffold Wetting and Adherence
[0073]Exemplary scaffold materials that were used are as follows:[0074]Specimen SF: lightweight, layered ORC based absorbable hemostatic material;[0075]Specimen SS—structured non-woven fabric, needle punched with interlocking fibers, ORC based absorbable hemostatic material;[0076]Specimen SO—loose knit ORC based absorbable hemostatic material;[0077]Specimen CM or composite matrix, whereby the scaffold was made of composite multi-layer matrix of a knitted oxidized regenerated cellulose (ORC) reinforcing backing layer under a layer of Polyglactin 910 (PG910) non-woven fibers as a hemostatic powders carrier layer. Lyophilized biologic materials that were used as the hemostatic powders tested were: Bioseal Fibrinogen (˜850 mg per 5-ml vial); Bioseal Thrombin; (˜78 mg per 5 ml vial).
[0078]Soaking Method. One vial each of fibrinogen and thrombin was milled into powder in the respective vials, followed by combining both hemostatic powders and ...
example 3
Tissue Adhesion Evaluation
[0081]The composite matrix (Specimen CM) and Specimen SS, as described above, were further tested for tissue adhesion. The selected scaffolds were pre-cut into three quarter (¾) by four (4) inch strips and then coated with the blended, disagglomerated hemostatic powders directly before placing the sample strips onto the bovine corium to test for adhesion strength by evaluation of three different groups (number of tests in each group N=4) of hemostatic powder mixtures that each contain a different combination of hemostatic powders and scaffolds. The hemostatic powder dosages were prepared at the testing site and include the following: Specimen SS (Non-woven ORC) coated with blended disagglomerated hemostatic powders containing Fibrinogen: total of 130 mg or 6.7 mg / cm2; Thrombin: total of 1450 IU or 75 IU / cm2; Calcium Chloride total of 18 mg or 0.93 mg / cm2. Specimen CM (Composite Matrix) coated with blended disagglomerated hemostatic powders containing Fibrin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com