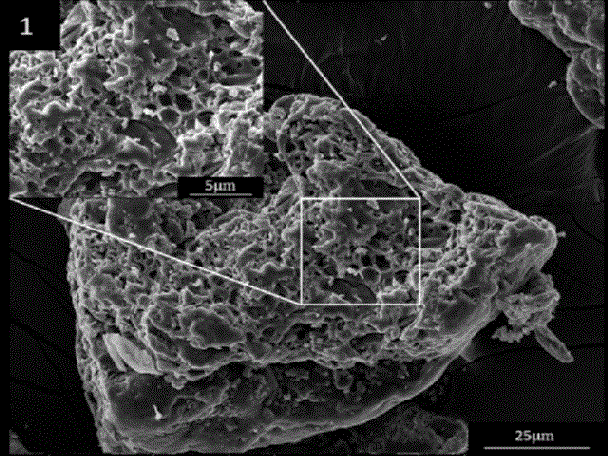
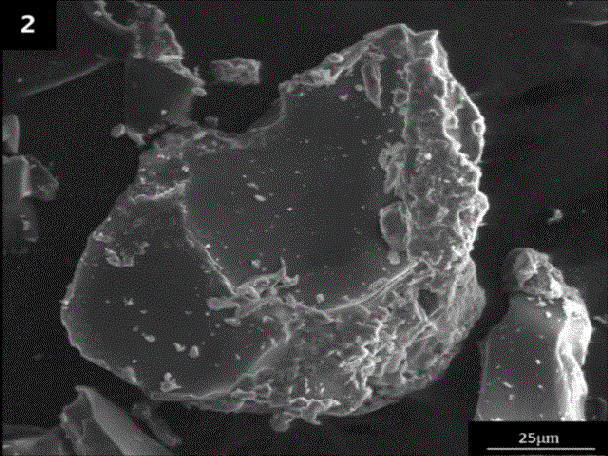
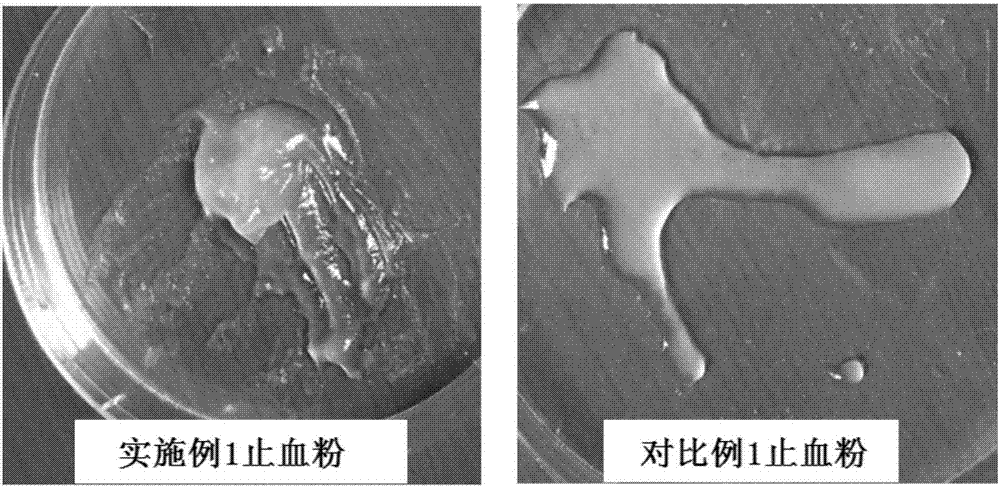
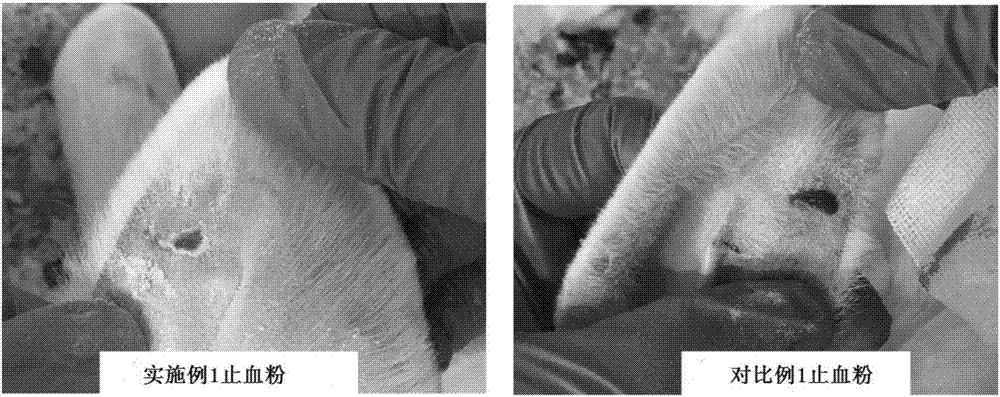
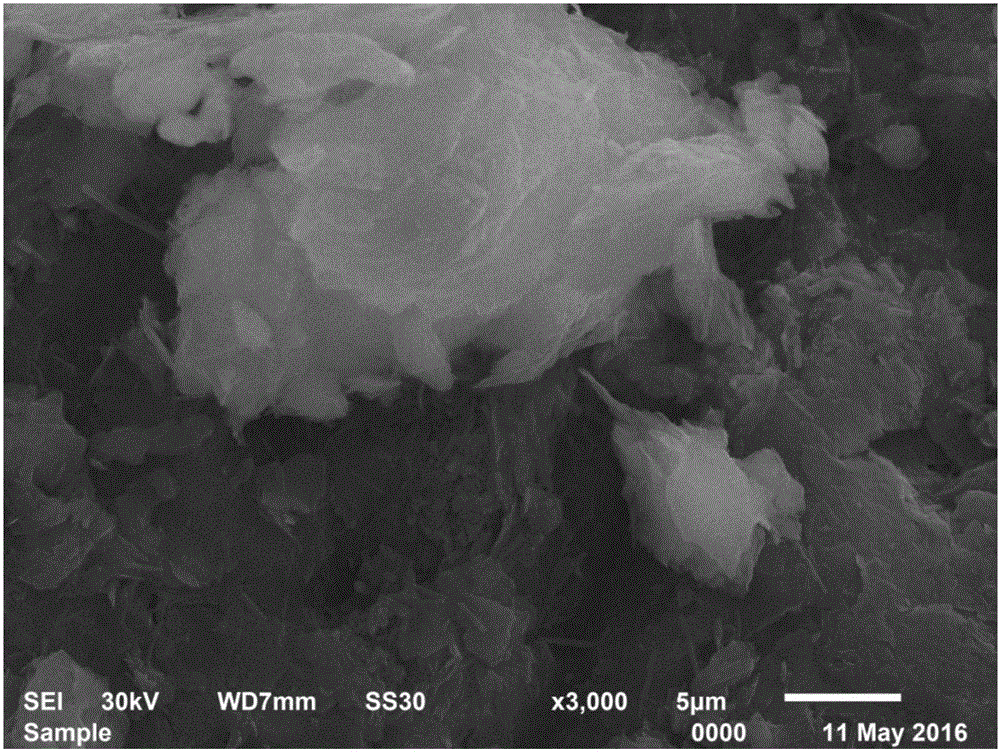
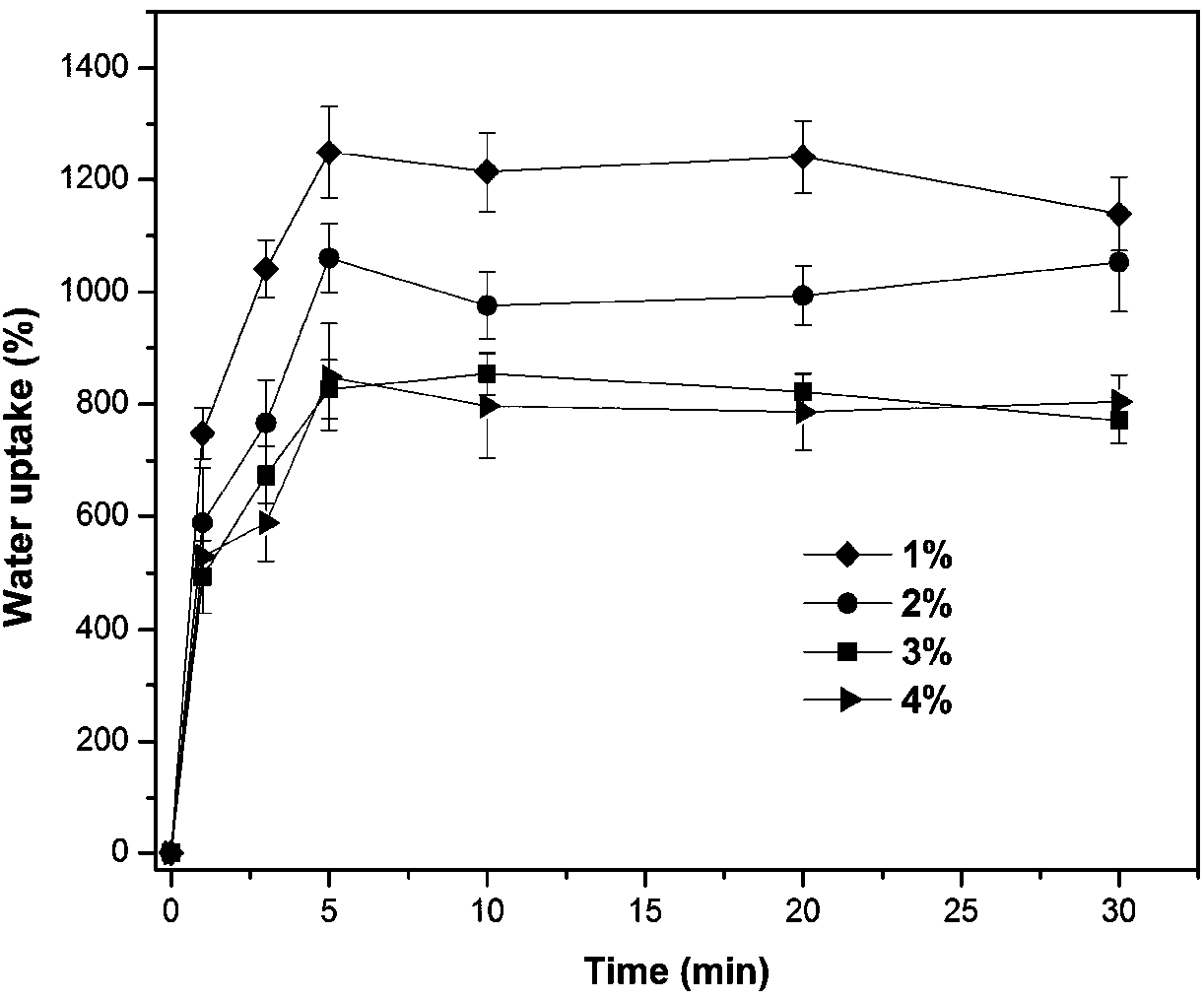
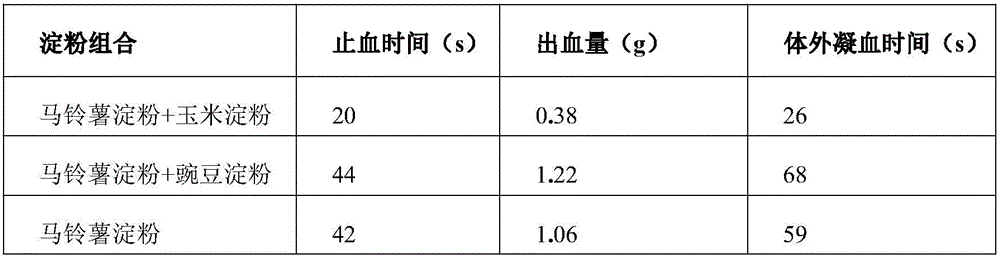
Patents
Literature
140 results about "HEMOSTATIC POWDER" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
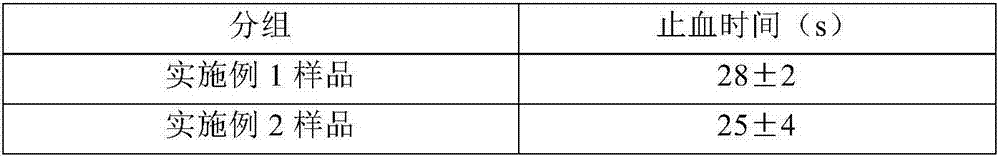
Arista (Arista™AH) is a synthetic hemostatic agent that includes an absorbable hemostatic powder consisting of Microporous Polysaccharide Hemospheres (MPH®).
Oxidized regenerated cellulose hemostatic powders and methods of making
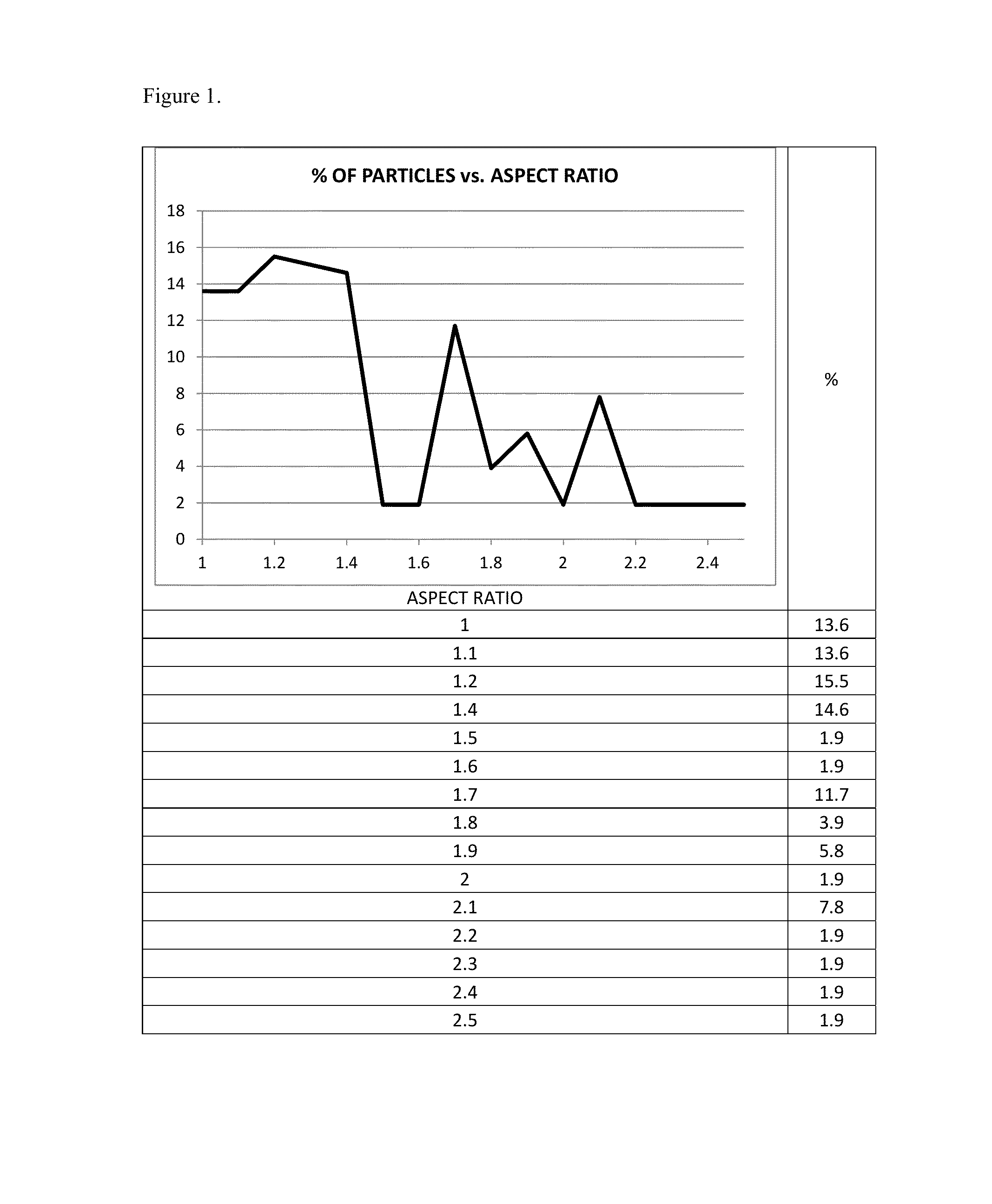
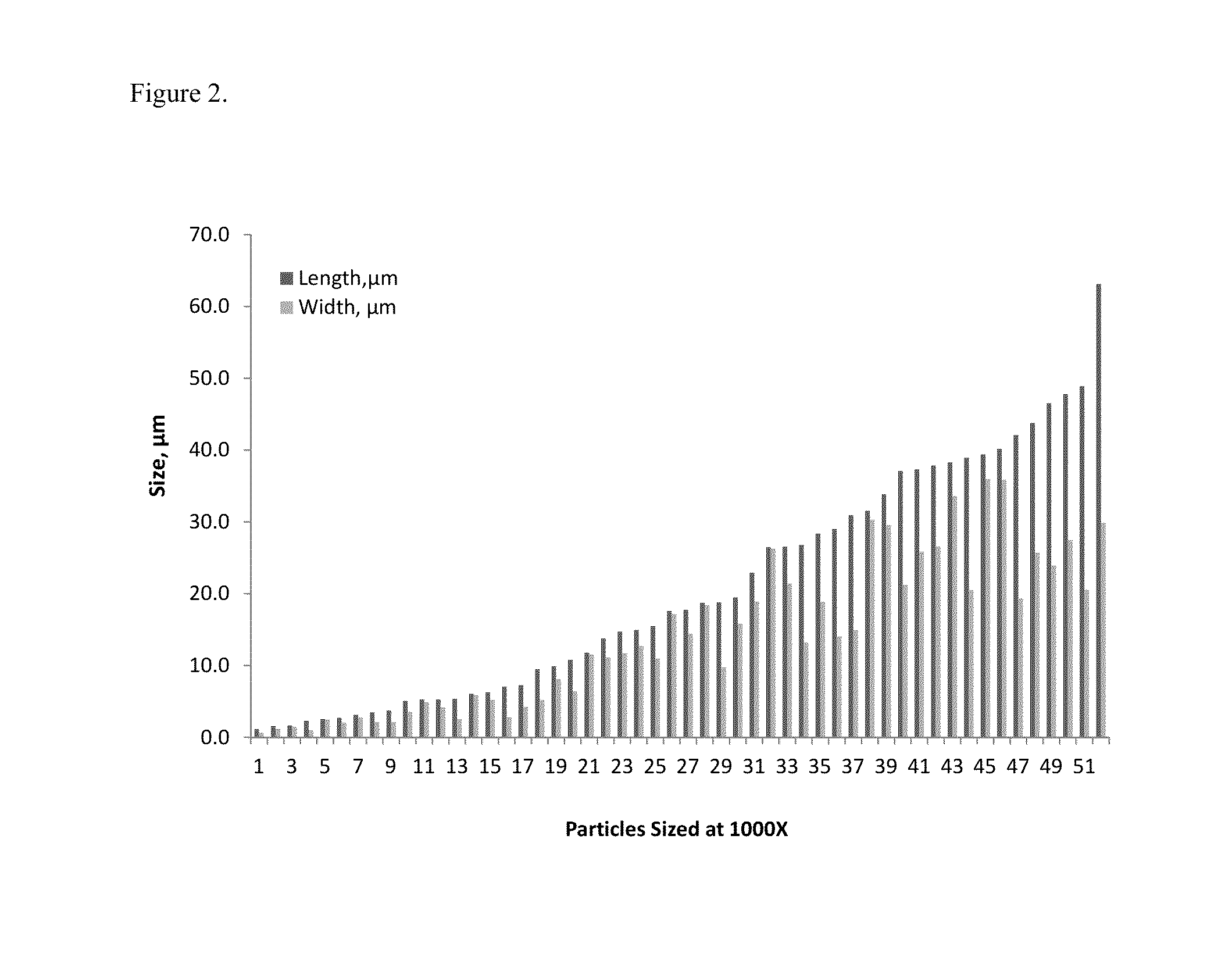
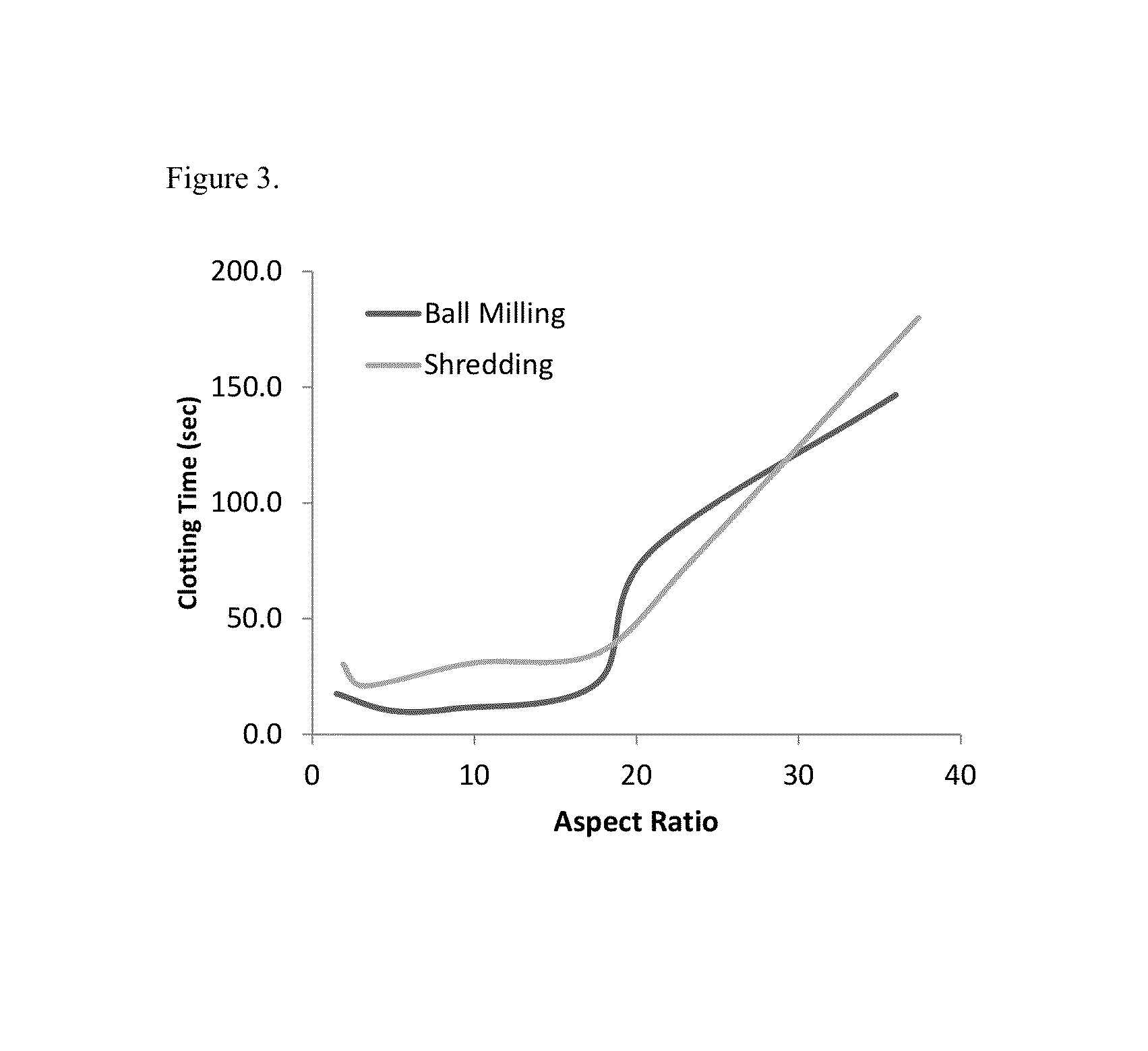
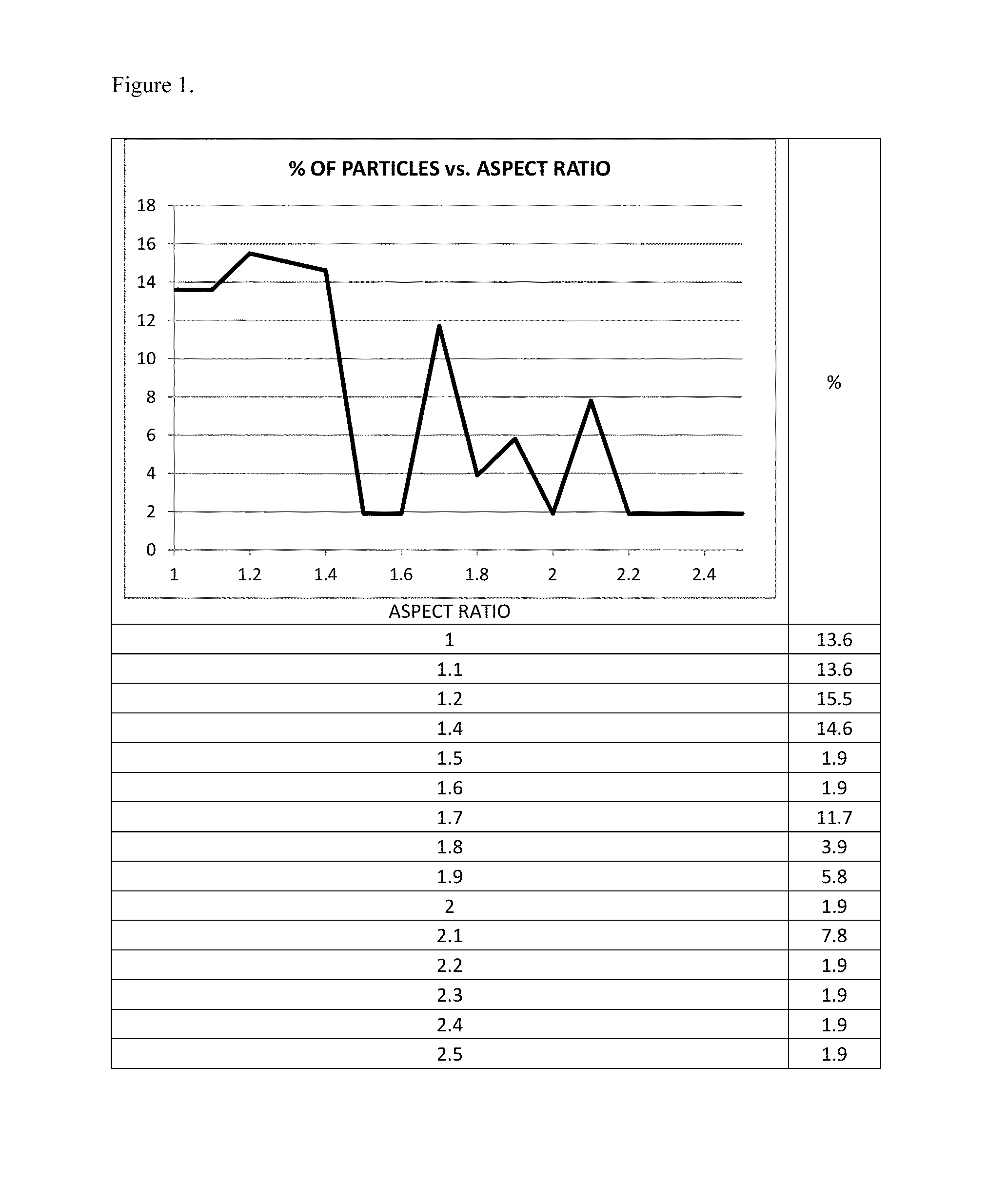
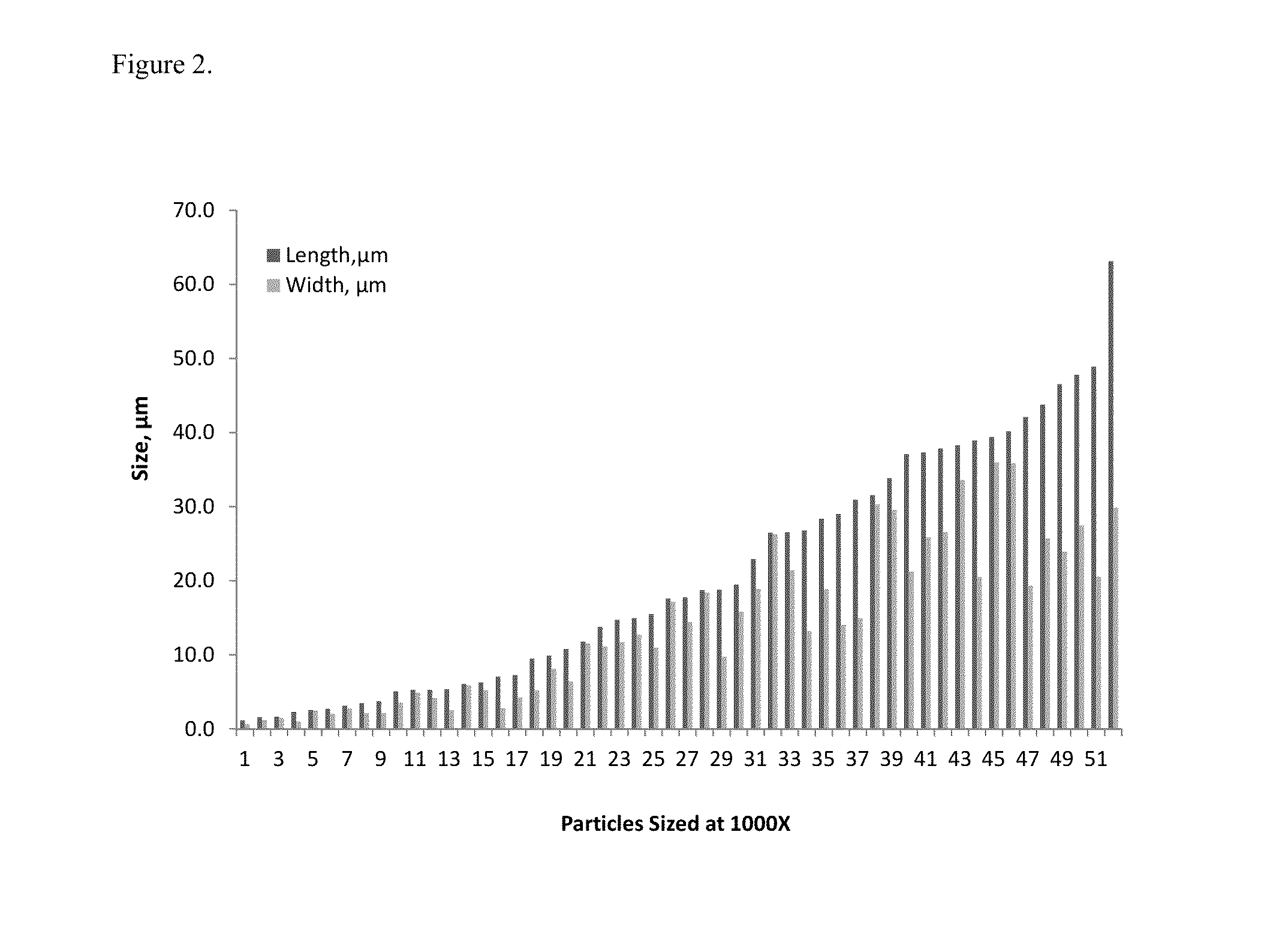
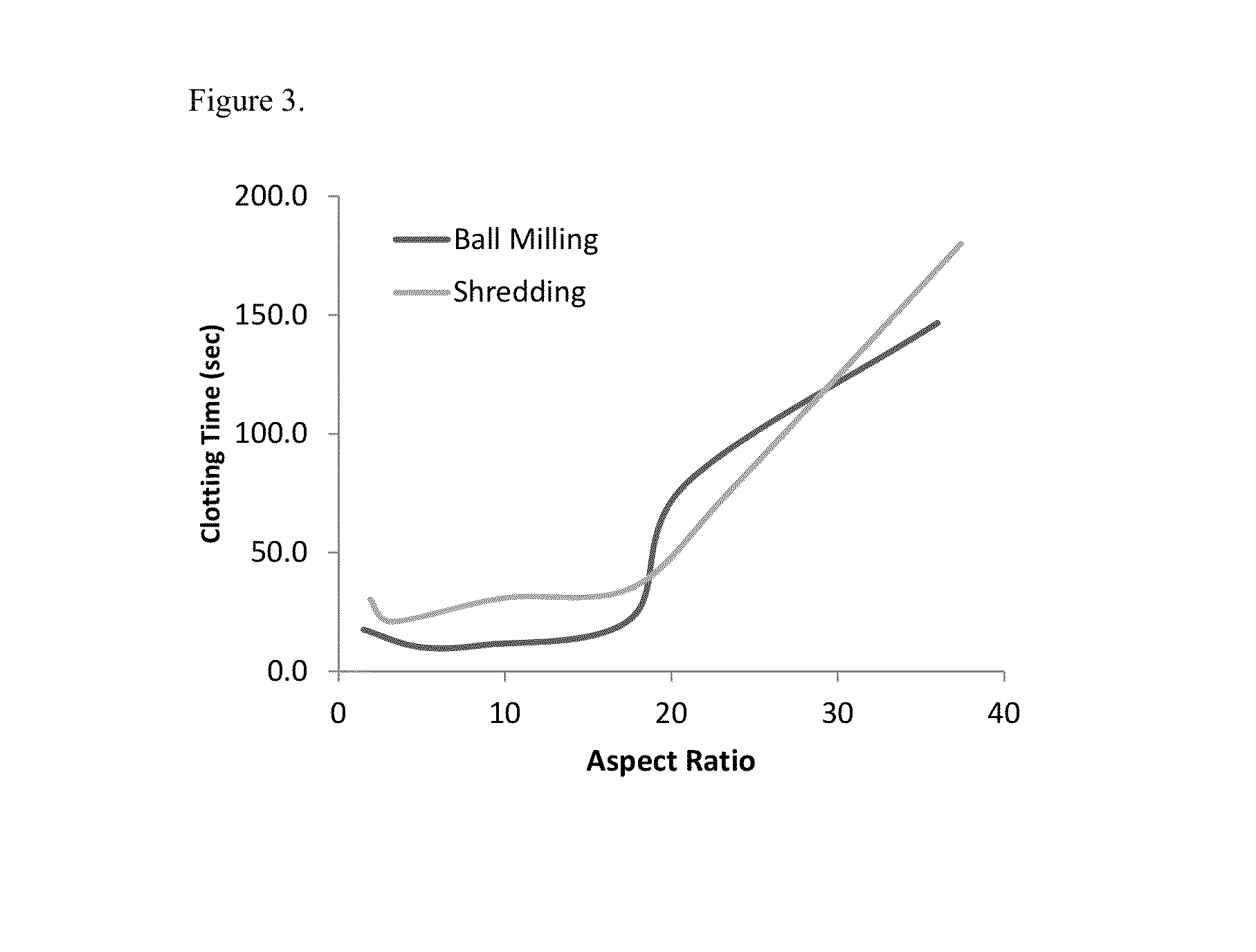
The present invention is directed to hemostatic material containing compacted ORC powder comprising particles having an average aspect ratio from about 1 to about 18, wherein said compacted ORC powder have preferably been processed in a compaction device, such as a ball milled ORC powder. The present invention further relates to methods of making the hemostatic material and a method of treating a wound by applying the hemostatic powder onto and / or into the wound of a patient.
Owner:ETHICON INC
Alkyl modified chitosan/mesoporous silicon dioxide composite quick hemostatic powder and preparation method thereof
InactiveCN102772820AFast hemostasisGood hemostasisAbsorbent padsBandagesHEMOSTATIC POWDERSide effect
The invention discloses an alkyl modified chitosan / mesoporous silicon dioxide composite quick hemostatic powder and a preparation method thereof. The method comprises the following steps: 1) preparation of alkyl modified chitosan; 2) preparation of mesoporous silicon dioxide particles; and 3) preparation of composite quick hemostatic powder: dissolving the alkyl modified chitosan in an acetic acid water solution, adding polyethyleneglycol 20000 to be dissolved, adding the mesoporous silicon dioxide particles prepared in the step 2), stirring at room temperature, freeze-drying the precipitate, suspending the precipitate in dimethylsulfoxide, regulating the pH value, centrifuging, washing with deionized water, and freeze-drying to obtain the alkyl modified chitosan / mesoporous silicon dioxide composite quick hemostatic powder. The hemostatic powder disclosed by the invention can quickly stop bleeding, and is suitable for pre-hospital emergency treatment of severe bleeding wounded persons. The material has the advantages of high hemostatic speed, excellent hemostatic effect, favorable biocompatibility and histocompatibility, and no side effect.
Owner:SANITARY EQUIP INST ACAD OF MILITARY MEDICAL SCI PLA
Multifunctional microporous styptic powder and preparation method thereof
ActiveCN105617449AImprove stabilitySimple methodSurgical adhesivesPharmaceutical delivery mechanismMicrowaveFiltration
The invention discloses multifunctional microporous styptic powder and a preparation method and application thereof. The preparation method comprises the following steps: 1) mixing starch with water to obtain a starch solution or starch pasty liquid; 2) mixing carboxymethyl chitosan with water to obtain a carboxymethyl chitosan solution; 3) mixing the starch solution or the starch pasty liquid with the carboxymethyl chitosan solution to obtain a blended solution; 4) dispersing a pore-forming agent into the blended solution to obtain blended dispersion liquid; 5) dropwise adding an ionic crosslinker into the blended dispersion liquid under a stirring condition, mixing the obtained mixed system with a dispersing agent and an emulsifying agent, and performing crosslinking reaction and suction filtration; 6) removing the pore-forming agent from a product obtained in the step 5), and drying; 7) performing microwave treatment on a product obtained in the step 6) to obtain the styptic powder. The preparation method adopts a dual crosslinking method of ionic crosslinking and microwave treatment; used reagents are environmentally friendly, and free of residues; the biological activity of an amino group in the carboxymethyl chitosan can be retained easily.
Owner:YANTAI ZHENGHAI BIO TECH
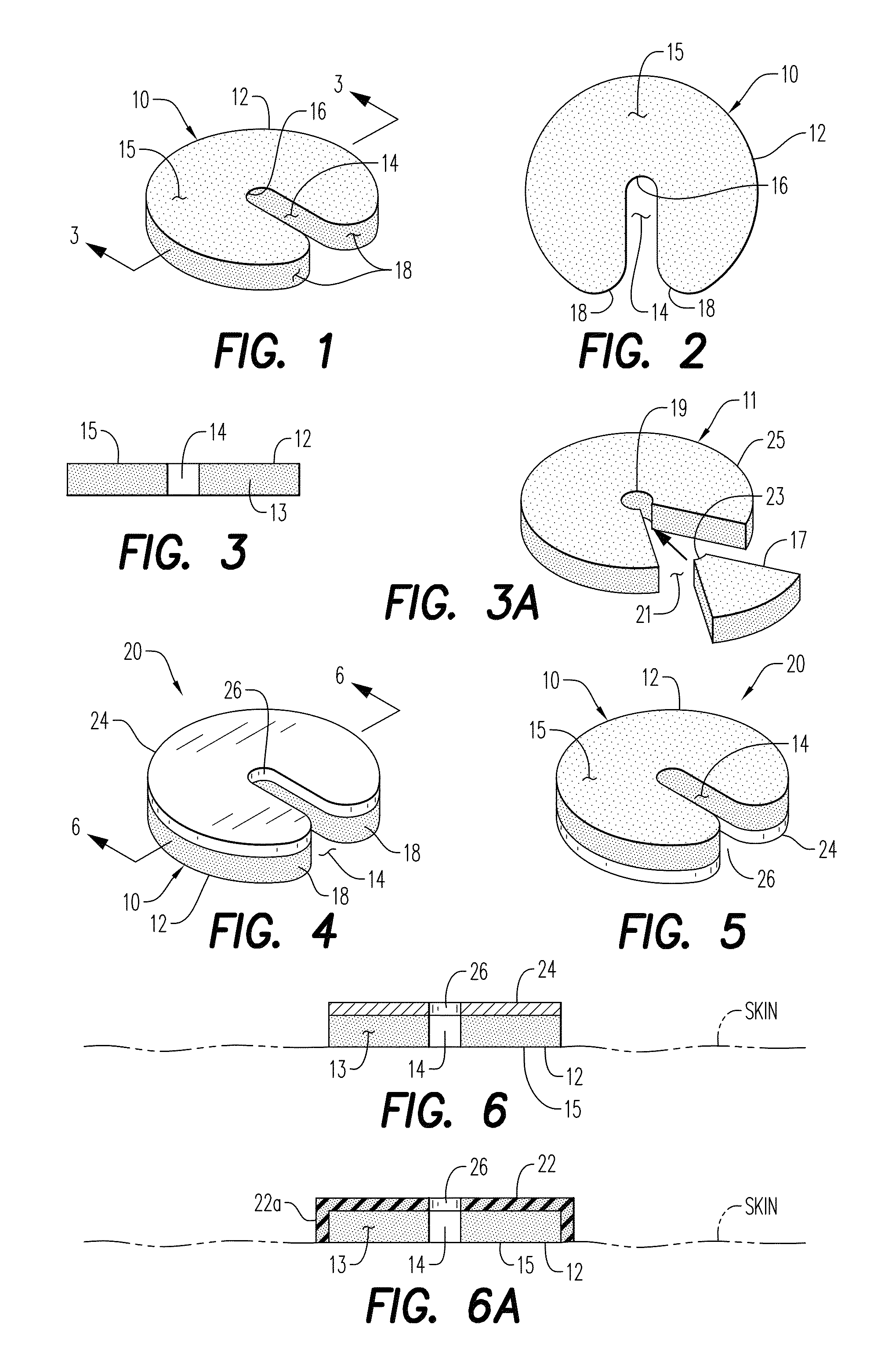
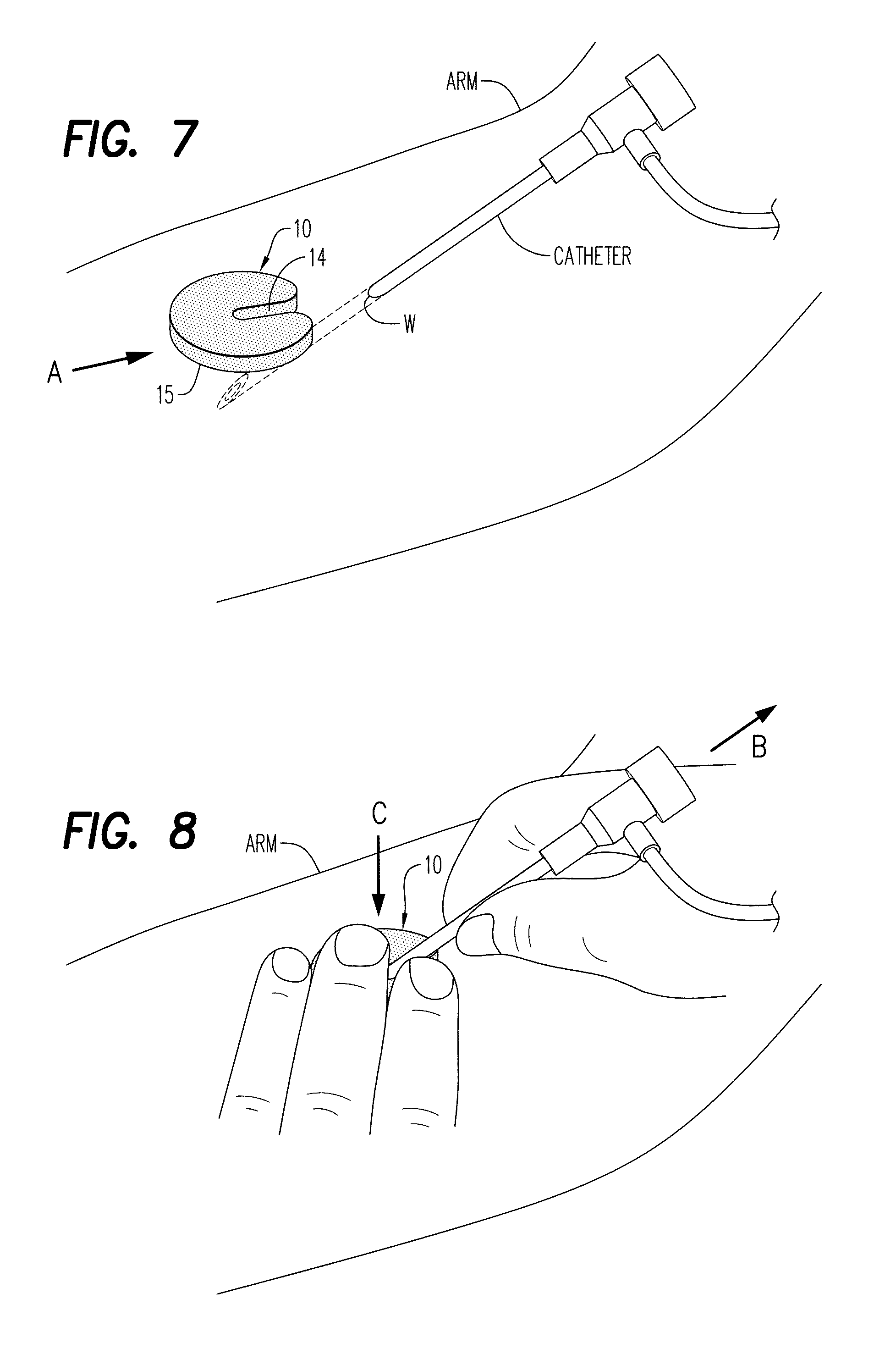
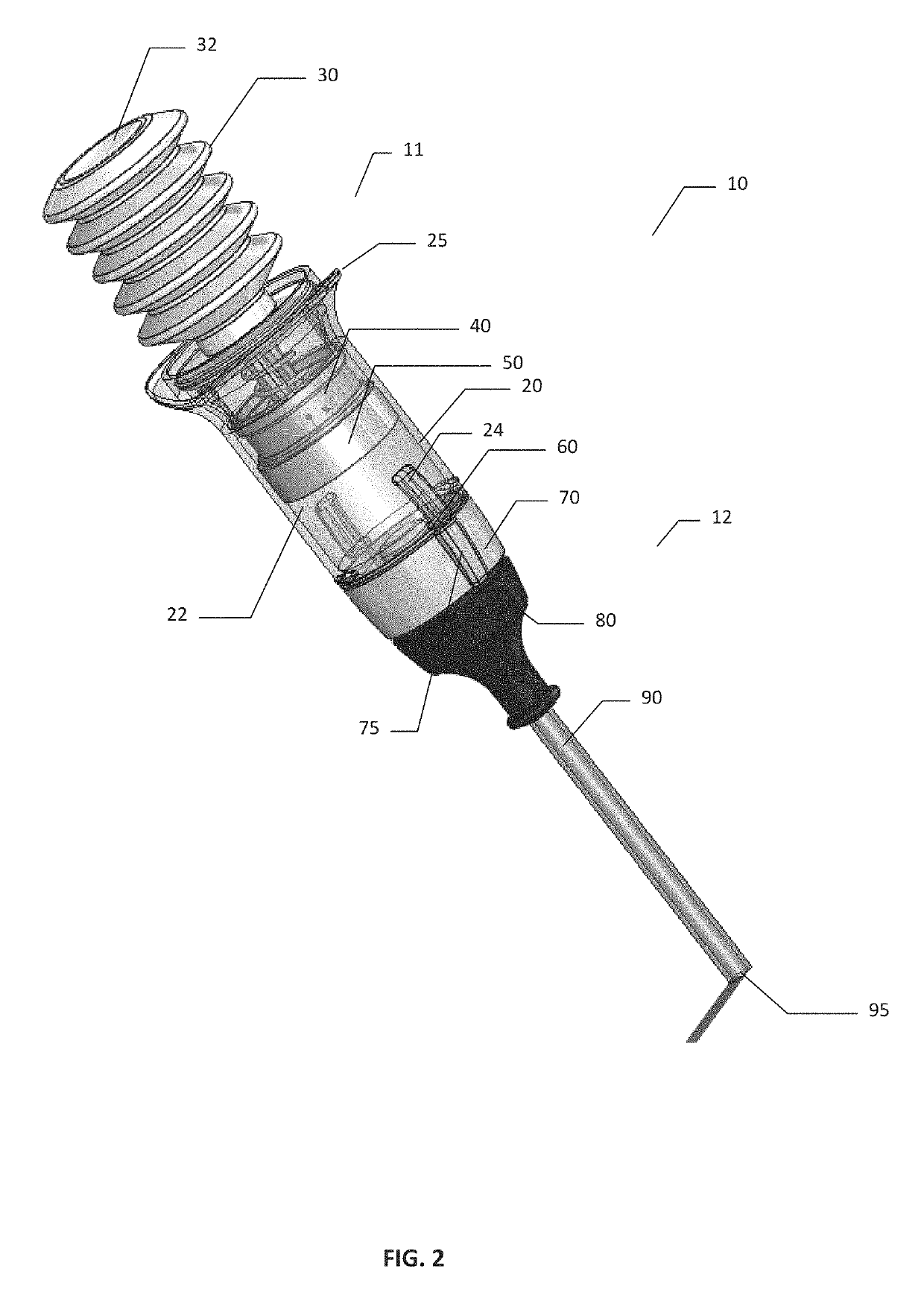
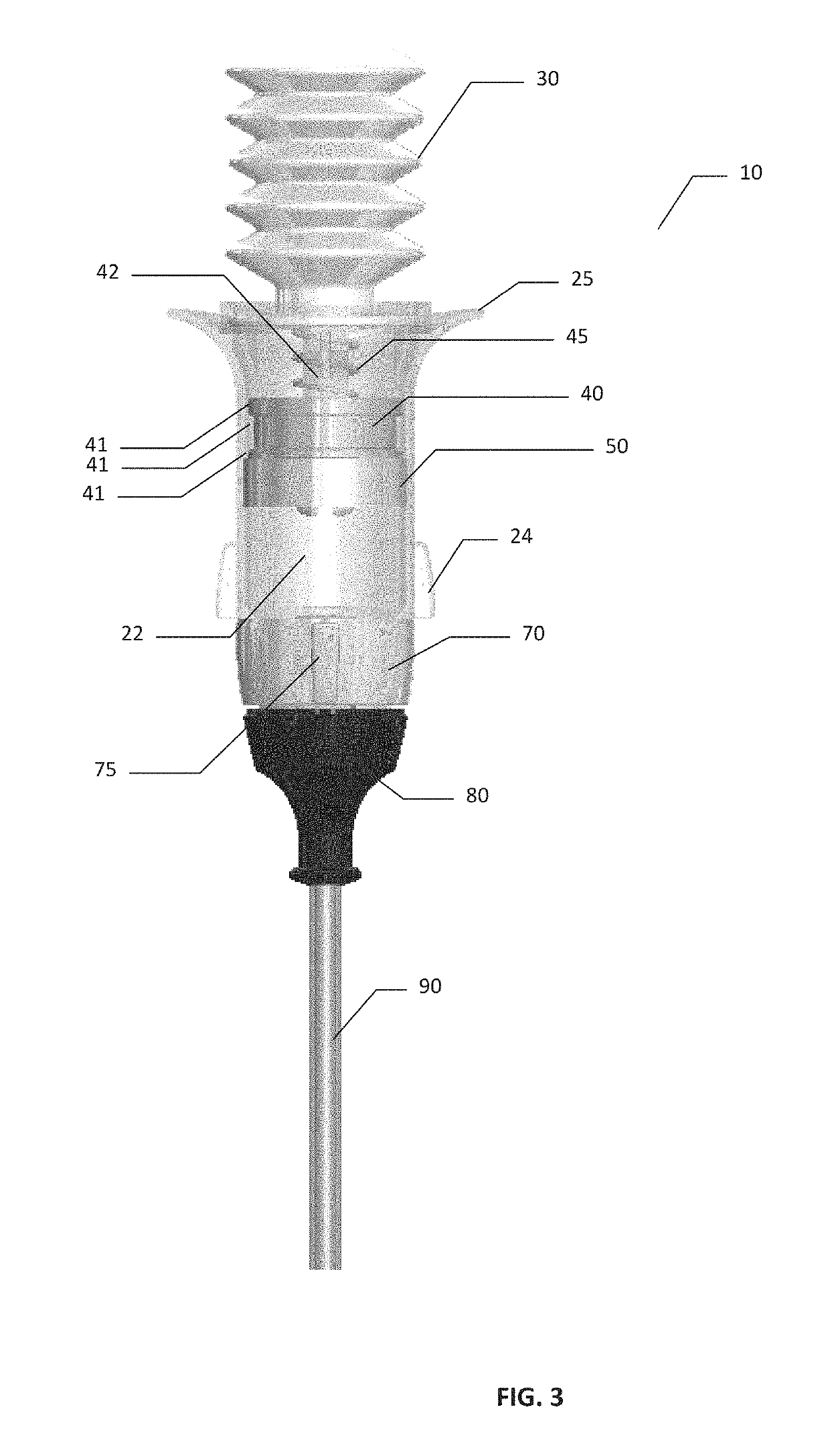
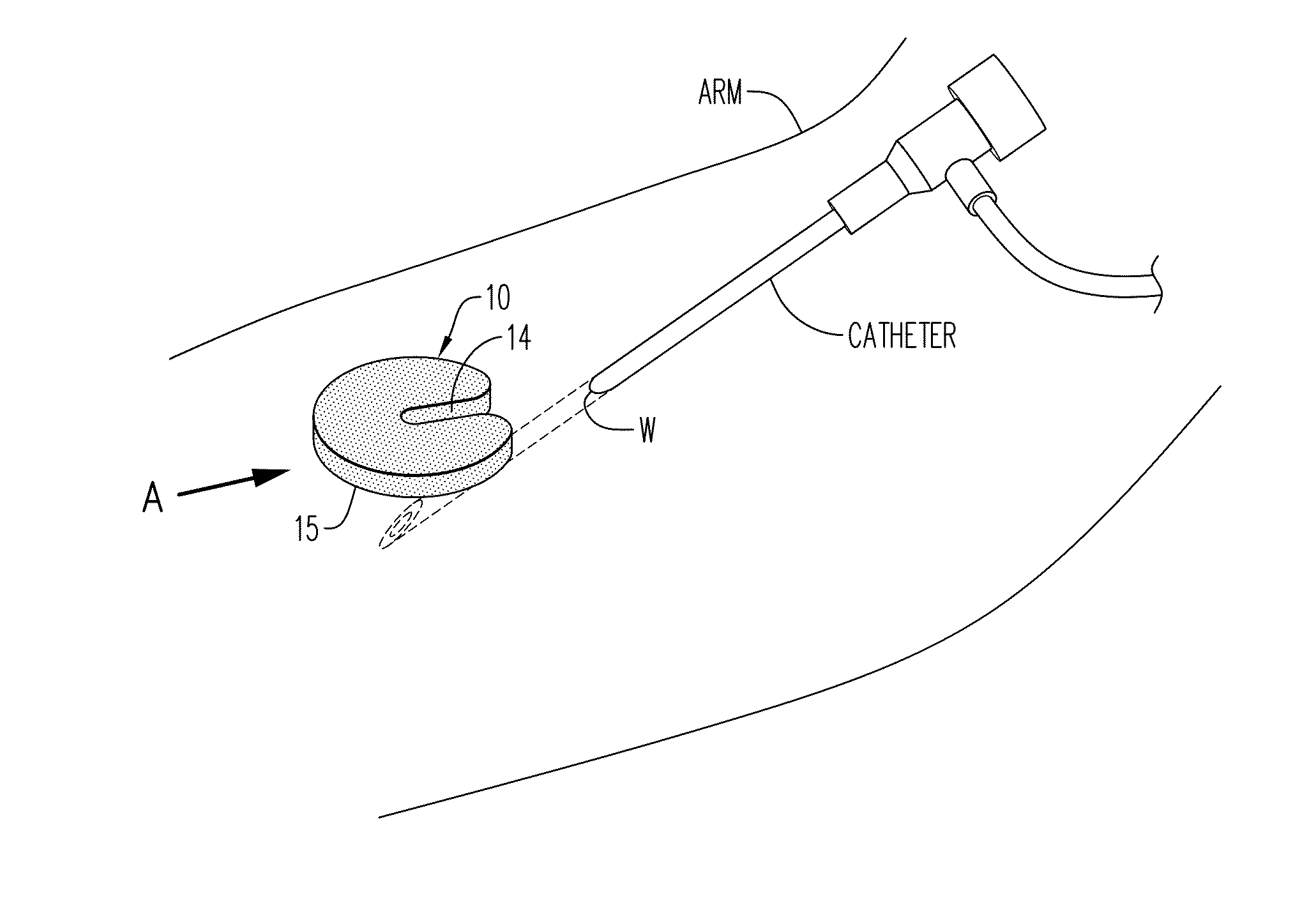
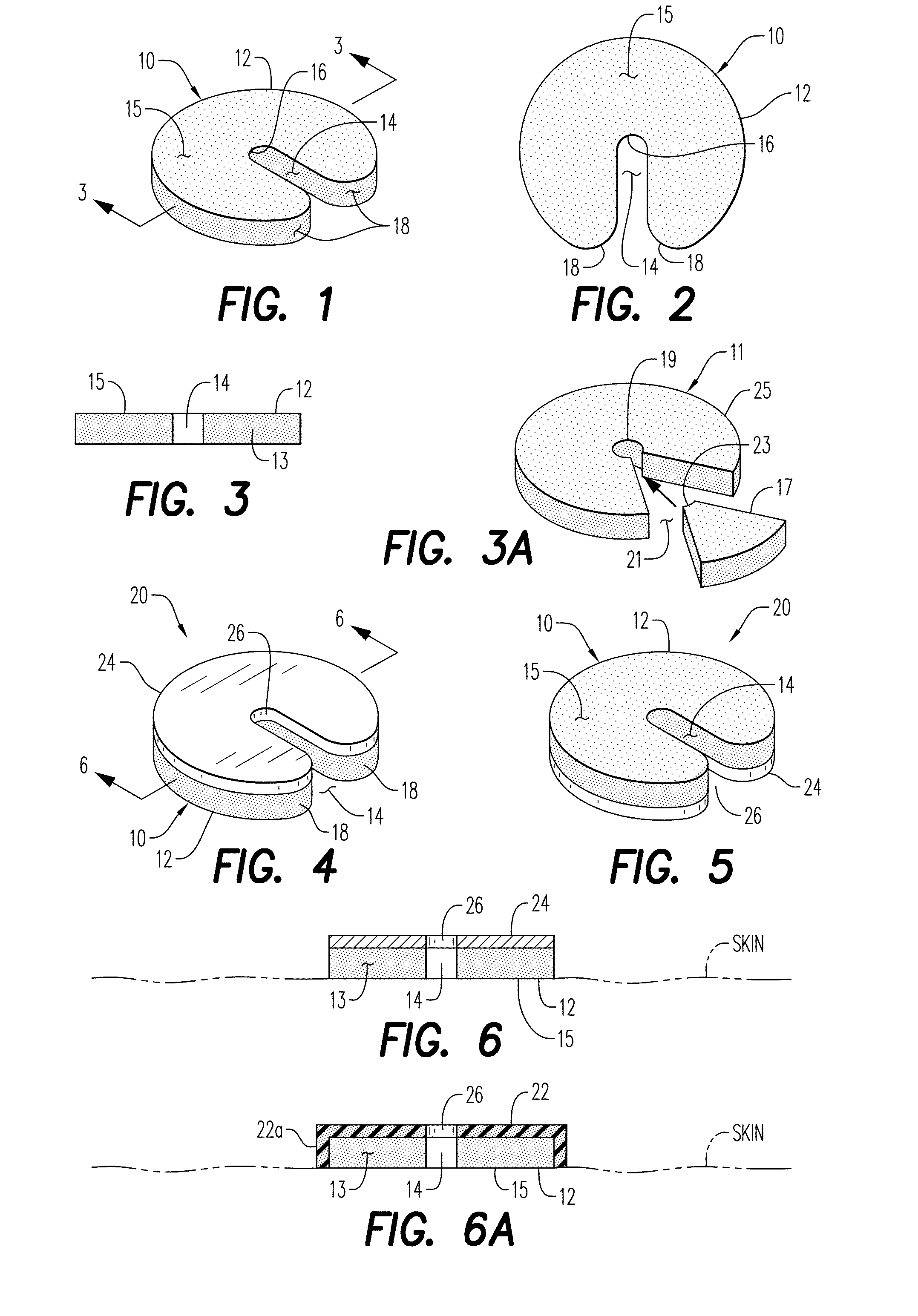
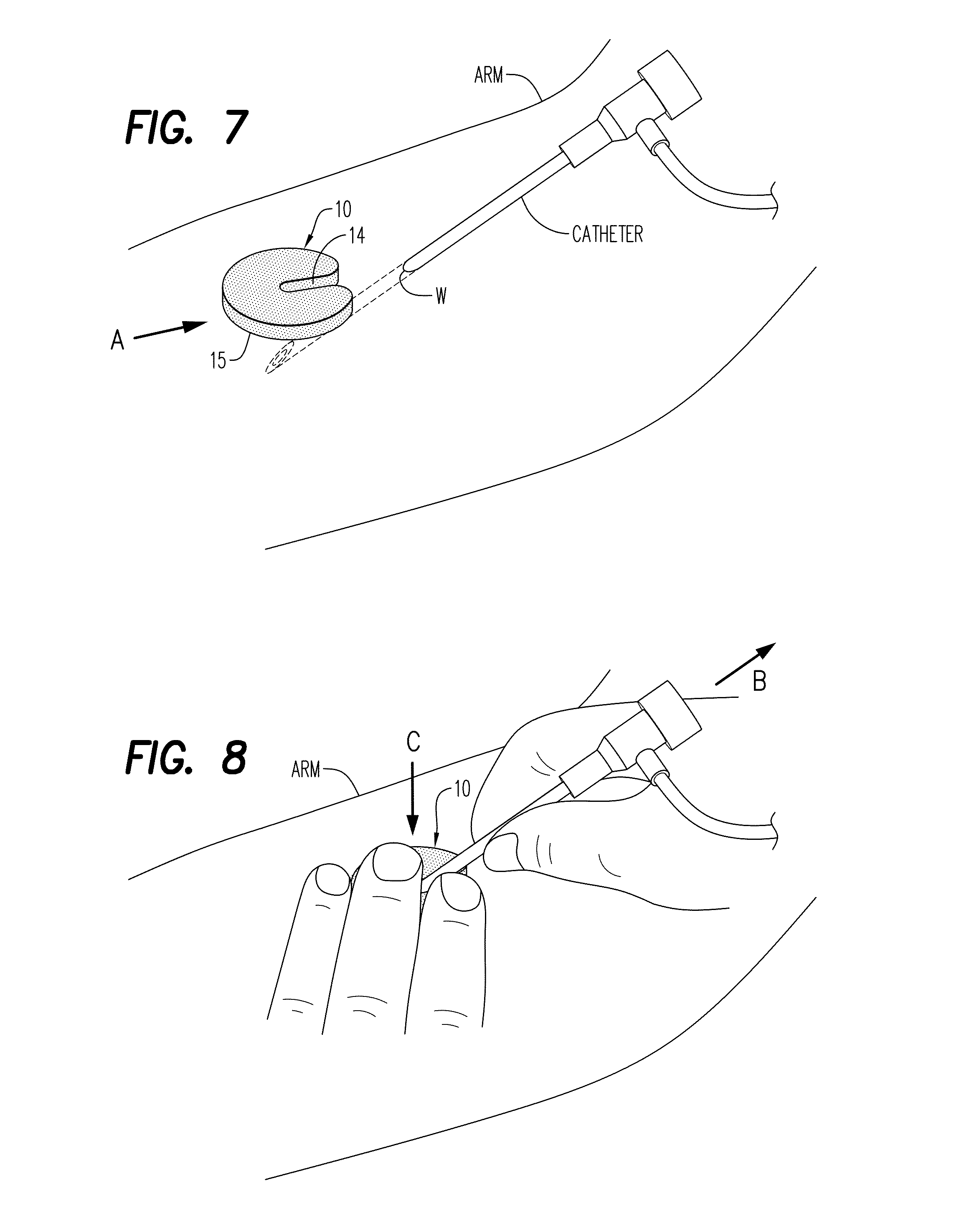
Hemostatic Powder Delivery Devices and Methods
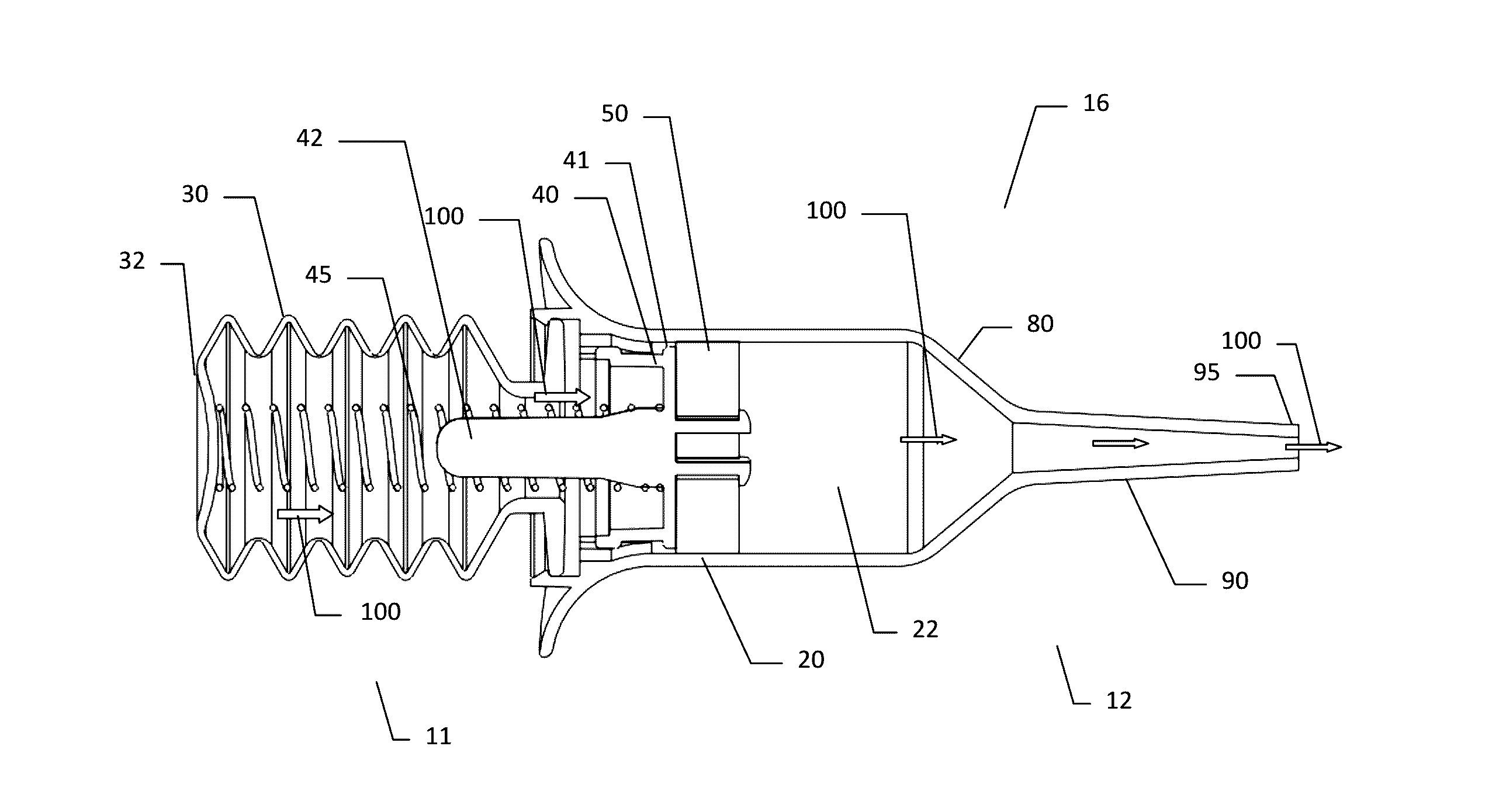
The present invention is directed to a device for the expression of a hemostatic powder having an elongated reservoir with a manual air pump, such as a bellows, at a proximal end and an expression port at a distal end. A porous filter is slidably disposed within the reservoir between the bellows and plunger and the expression port, and a spring is disposed within the reservoir between the air pump and the plunger. The powder is disposed within the reservoir between the porous filter and the expression port, and the pump is in a fluid communication with the expression port through the porous filter and through the powder.
Owner:ETHICON INC
Potato starch-hyaluronic acid composite hemostatic powder and preparation method thereof
ActiveCN103055343AImprove applicabilityThe effect is accurateOrganic active ingredientsPowder deliverySodium hyaluronateHEMOSTATIC POWDER
Belonging to the field of medical equipment, the invention particularly relates to a composite hemostatic powder and a preparation method thereof. The composite hemostatic powder provided in the invention is mainly prepared by: subjecting potato starch to modification crosslinking by acetic anhydride / adipic acid-succinic anhydride, then compounding the potato starch with a hyaluronate solution, and performing drying. The preparation method employs a relatively safe modification crosslinking pattern with low-toxic residue, and the security is significantly improved. Performance of the hemostatic powder can be adjusted by pregelatinization or surface pore-forming after starch modification crosslinking and by adopting different drying and sterilization ways. Also, blending of potato starch and sodium hyaluronate makes full use of the respective advantages of the two biological materials. Compared with existing products on the market, the composite hemostatic powder provided in the invention has excellent water-absorbing capacity, adhesion, gel forming property and anti-floating property, and shows better use performance. Being able to degrade completely in vivo, the hemostatic powder has no toxic or side effect and has definite effects, and also can be further compounded with other hemostatic materials, thus having broad market prospects.
Owner:SICHUAN SANTAI PHARM TECH CO LTD
Chinese medicine hemostatic powder
InactiveCN101537094AGood postoperative hemostasisReduce wound exudationPowder deliveryHeavy metal active ingredientsHEMOSTATIC POWDERMyrrh
The invention aims at providing a Chinese medicine hemostatic powder which is used for externally stanching and lowering the treatment cost of the prior hemostatic medicine. The Chinese medicine hemostatic powder is prepared by the following Chinese medicines according to the weight account: 1.5-3 portions of panax pseudo-ginseng var. notoginseng, 3-9 portions of marmor serpentinatum, 3-9 portions of frankincense, 3-9 portions of myrrh, 1-1.5 portions of dragon's blood, 3-10 portions of safflower, 10-18 portions of halloysitum rubrum and 3-9 portions of sapanwood. The hemostatic powder is the Chinese medicine for external use, which is prepared by combining the characteristics of wound external hemorrhage according to the medicine function effect, has good hemostatic action after the operation, and has remarkable effects of reducing the wound surface effusion, resisting infection and promoting the wound healing. Compared with the prior hemostatic medicine, the invention has equal hemostatic effect, but the cost is 20 percent of average price of the prior hemostatic medicine.
Owner:徐樊
Hemostatic device and method
ActiveUS8961479B2Improved delivery and controlSlow actingSurgical adhesivesSurgical needlesHEMOSTATIC POWDERThin layer
A hemostatic tablet preferably including potassium ferrate and a cation ion exchange resin pressure formed into a tablet for delivery to a bleeding wound. The tablet improves the rate of adhesion to a bleeding wound surface, and allows a significantly greater and more uniform pressure to be exerted by manual compression of the tablet on the wound site, as compared to that of a thin layer of scattered hemostatic powder. After the seal is formed from the interaction of blood or exudates with the immediate contacting surface of the tablet, the bulk of the unused tablet easily delaminates from the seal making clean up facile. If the unused portion of the tablet is not removed from the wound site, a reservoir of hemostatic dressing stops further bleeding and to provide antimicrobial protection and healing. The tablet may be applied to any surface orientation and take any shape and thickness possible.
Owner:BIOLIFE
Sustained antibacterial hemostatic powder and preparation method thereof
InactiveCN106512076AAccelerate solidificationIncrease concentrationSurgical adhesivesMicrocapsulesHEMOSTATIC POWDERCarboxymethyl cellulose
The invention belongs to the field of medical equipment, particularly relates to the field of medical biopolymer materials and provides sustained antibacterial hemostatic powder and a preparation method of the sustained antibacterial hemostatic powder. The sustained antibacterial hemostatic powder is prepared from nanometer silver, sodium carboxymethyl cellulose and alginate, and by weight, 1-3 parts of the nanometer silver, 10-20 parts of the sodium carboxymethyl cellulose and 5-10 parts of the alginate are included. According to the sustained antibacterial hemostatic powder, the nanometer silver, the sodium carboxymethyl cellulose and the alginate are used as the raw materials, and the sustained antibacterial hemostatic powder is obtained through a spray drying method; and the hemostatic powder not only has the hemostatic function, but also has the excellent antibacterial function, as degradation of the sodium carboxymethyl cellulose and the alginate after the hemostatic powder is in contact with the blood or diffusate, the contained nanometer silver is released slowly, and thus the sustained antibacterial purpose is achieved.
Owner:GUANGDONG TAIBAO MEDICAL DEVICE TECH RES INST CO LTD
Polysaccharide hemostat and preparation method thereof
InactiveCN105169459AImprove the coagulation effectFaster gelation rateAbsorbent padsBandagesHEMOSTATIC POWDERMicrosphere
The invention relates to the field of bio-medicine techniques, and particularly relates to a polysaccharide hemostat and a preparation method thereof. The preparation method comprises the following steps: mixing high-viscosity high-deacetylation-degree chitosan with high-guluronic-acid-content (G) sodium alginate, then adding zinc chloride and calcium chloride into the obtained mixture, carrying out mixed coagulating bath on the obtained object, and stirring and crosslinking the obtained product, so that a polysaccharide hemostat is obtained. The invention also provides components of the polysaccharide hemostat and content thereof. According to the invention, high-viscosity high-deacetylation-degree chitosan and high-G-content (G) sodium alginate are taken as main components of the polysaccharide hemostat, so that obtained high-viscosity high-deacetylation-degree chitosan / zinc-calcium alginate composite microspheres are good in hemostatic effect.
Owner:GUANGDONG TAIBAO MEDICAL SCI TECH
Porous hemostatic powder and its preparation method
InactiveCN103381189ASimple technologyEasy to operatePowder deliveryOrganic active ingredientsHEMOSTATIC POWDERHydroxyethyl starch
The invention provides a porous hemostatic powder which is prepared by carrying out physical drilling on a soluble material to form porous microspheres. A preparation method of the porous styptic powder comprises the following steps: putting the soluble material on a supersonic vibration bed device, starting the device, vibrating, and simultaneously carrying out laser radiation for 10-60 min. The soluble material undergoes laser drilling. The preparation method has advantages of simple technology, easy operation, low production cost and wide raw material sources. Soluble starch, carboxy methyl starch, hydroxyethyl starch, carboxymethylcellulose sodium, ethyl cellulose or sodium alginate and their derivatives are medically proved to be safe medical materials, are generally used as pharmaceutic adjuvants and sustained release agents of medicines, and are also used to prepare absorbable hemostatic gauze such as Tailing absorbable hemostatic gauze. These materials are all dissoluble; the size of molecular weight is controllable; preferably, the molecular weight is 500-12000Dalton; more preferably, the molecular weight is 1000-40000 Dalton; and the materials all can be absorbed by body.
Owner:QINGDAO ZHONGTENG BIOTECH
Hemostatic powder delivery devices and methods
The present invention is directed to a device for the expression of a hemostatic powder having an elongated reservoir with a manual air pump, such as a bellows, at a proximal end and an expression port at a distal end. A porous filter is slidably disposed within the reservoir between the bellows and plunger and the expression port, and a spring is disposed within the reservoir between the air pump and the plunger. The powder is disposed within the reservoir between the porous filter and the expression port, and the pump is in a fluid communication with the expression port through the porous filter and through the powder.
Owner:ETHICON INC
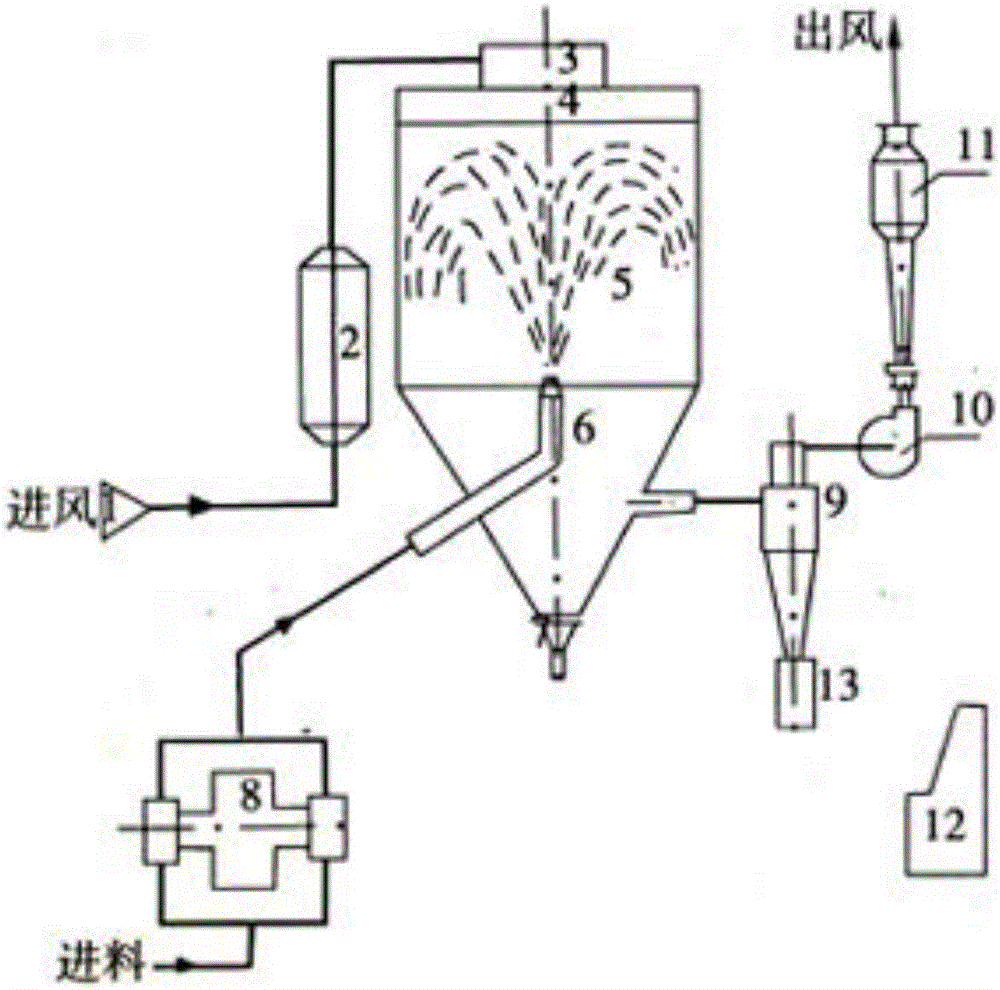
Method and application for preparing fully degradable chitosan hemostatic powder by spray drying process
InactiveCN106110378AReduced risk of concurrent inflammationShorten healing timeSurgical adhesivesPharmaceutical delivery mechanismHEMOSTATIC POWDERMicrosphere
Fully degradable chitosan hemostatic powder is disclosed. The hemostatic powder is chitosan, or composition of chitosan or / and other substance. The other component can be one or more of PVP, hyaluronic acid and oxidized starch. The particle diameter of the chitosan hemostatic powder is in the range of 20 micrometers to 2 millimeters. The chitosan hemostatic powder is porous microballs. The chitosan hemostatic powder is prepared with the spray drying process through chitosan or carboxymethyl chitosan solution of 5-10 g / L of mass fraction, and pH value of the solution is in the range of 4.0-7.4. The solvent used is distilled water or acetum, and the mass fraction of acetum is 10g / L. The prepared chitosan solution is subjected to spray drying, and powder is collected under the conditions that the caliber of the solution is 0.2-2 mm, inlet temperature is 160-180 DEG C, outlet temperature is 100-105 DEG C, and spray speed is 3-10 mL / min.
Owner:NANJING UNIVERSTIY SUZHOU HIGH TECH INST
Preparation method and applications of hemostatic material
InactiveCN111053943AHas antibacterial activityReduced antibacterial activitySurgical adhesivesPharmaceutical delivery mechanismHEMOSTATIC POWDERStarch Microspheres
The invention discloses a preparation method and applications of a hemostatic material. According to the invention, by using a microsphere surface modification method, the modified starch of a modified starch microsphere shell layer is activated and coupled with chitosan amino to obtain modified starch hemostatic microspheres with the surface modified with chitosan, wherein the modified starch microspheres have good water absorption performance and can rapidly absorb moisture in blood and concentrate blood coagulation factors so as to achieve the rapid hemostatic effect, the chitosan modification can enhance the hemostatic effect, activate blood coagulation factors and form blood coagulation blocks so as to promote blood coagulation, and chitosan has a certain antibacterial activity, so that the modification of the surface with chitosan can reduce the infection risk, the addition amount of chitosan can be reduced through surface modification, and the water absorption performance and the degradation and absorption performance of the modified starch microspheres are influenced to the minimum extent; and the hemostatic powder prepared by the invention can be used for quickly stoppingbleeding of bleeding parts such as body surfaces, in-vivo tissues, organs and the like, has a certain antibacterial function, and reduces the wound infection risk.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI
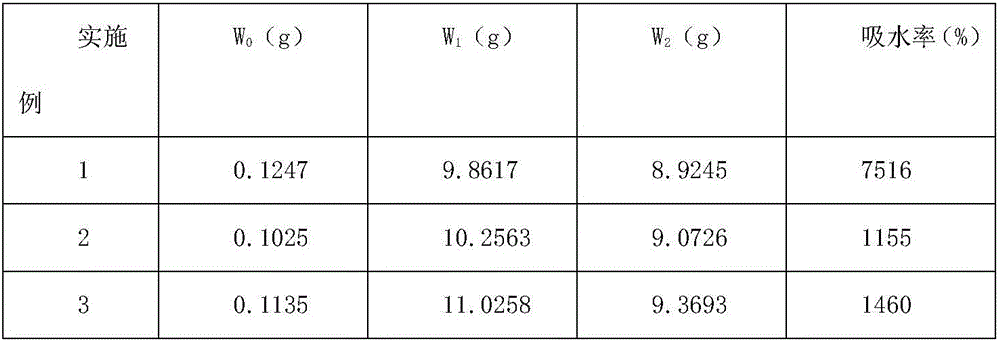
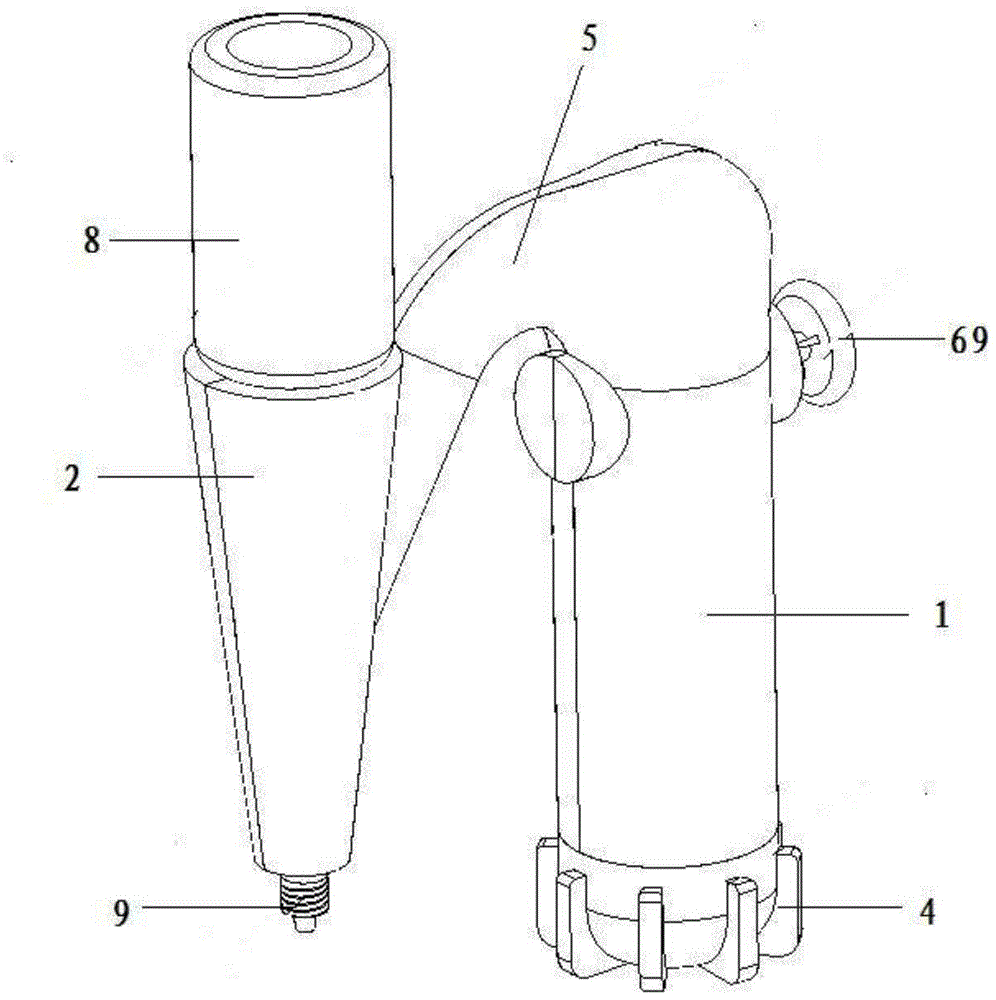
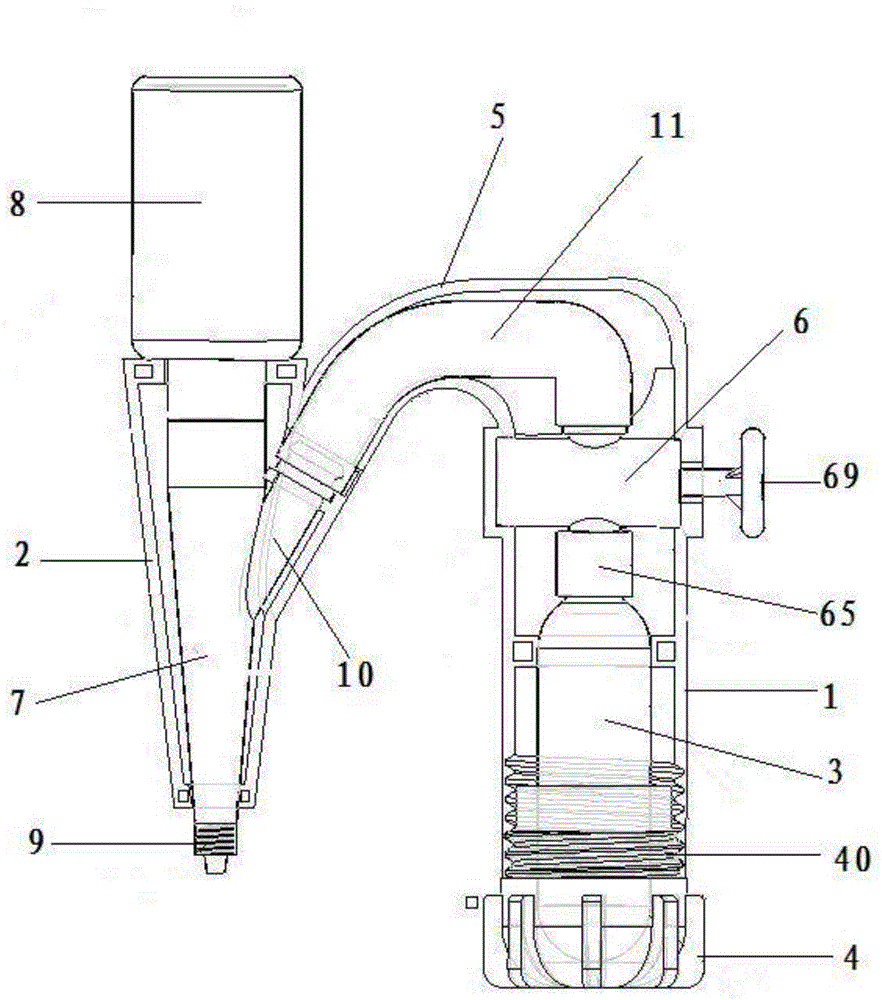
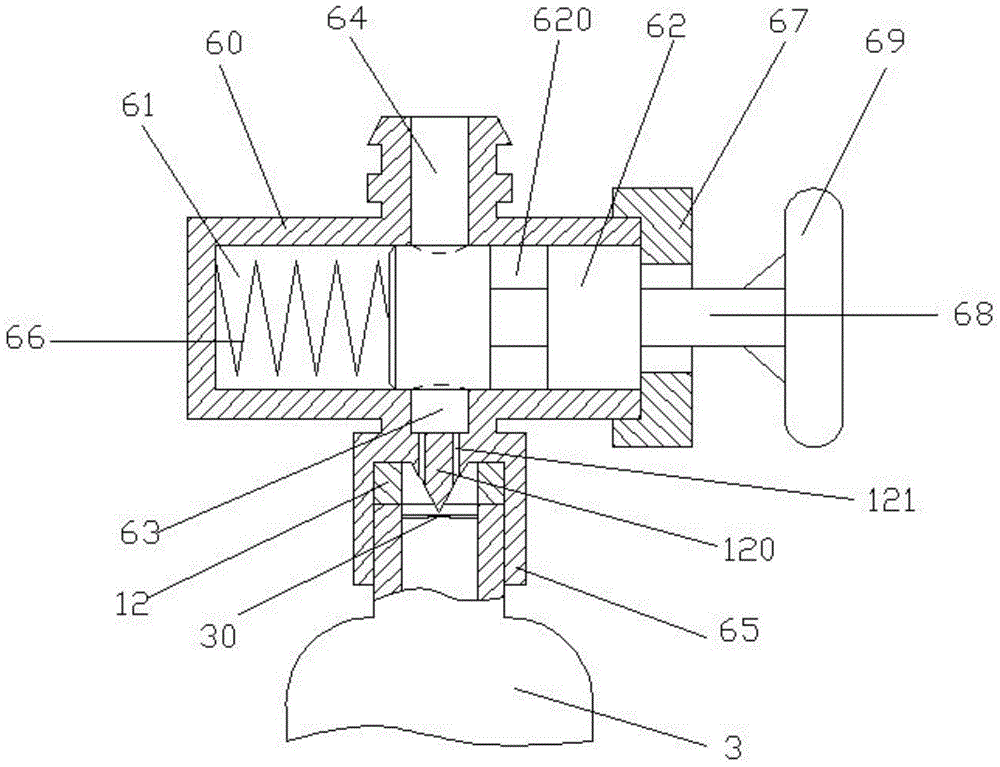
Hemostatic powder ejector
InactiveCN104958829APrecise deliveryConvey evenlyMedical devicesMedical insufflatorsComing outHEMOSTATIC POWDER
The invention relates to the technical field of medical instruments, and discloses a hemostatic powder ejector. The hemostatic powder ejector comprises an ejector main housing and an ejector sub-housing. A gas cylinder is disposed in the ejector main housing, the bottom of the ejector main housing is provided with an end cap, and an opening of the gas cylinder is provided with an air valve. A gas-powder mixing sleeve is disposed in the ejector sub-housing, a gas-powder mixing cavity is disposed in the gas-powder mixing sleeve, the upper end of the gas-powder mixing sleeve is provided with a medicine bottle, a medicine outlet of the medicine bottle communicates with the gas-powder mixing cavity, the lower end of the gas-powder mixing sleeve comes out of the ejector sub-housing and the lower end is provided with a nozzle. A gas tube is disposed in the connecting housing, one end of the gas tube is connected with the air valve, and the other end of the gas tube communicates with the gas-powder mixing cavity. The hemostatic powder ejector is convenient to operate, the hemostatic powder can be accurately transported to a focus and uniformly spayed on the focus to stop bleeding.
Owner:SHANGHAI YU XING MEDICAL DEVICE
Porous styptic powder and preparation method thereof
ActiveCN105920658AImprove water absorptionHigh strengthPharmaceutical delivery mechanismBandagesHEMOSTATIC POWDERFreeze-drying
The invention discloses porous styptic powder and a preparation method of the porous styptic powder. The preparation method comprises the following steps: rapidly cooling a solution containing a material needing to be crosslinked, carrying out ray irradiation crosslinking under low temperature, and carrying out freeze drying, thus obtaining the porous styptic powder. In the preparation method, superfine small ice crystals are obtained through rapid cooling, during the irradiation, the low temperature is guaranteed, so that the small ice crystals do not melt and are sized after the irradiation. With the adoption of the preparation method, the porous crosslinking material with small pore diameter, large porosity and large specific surface area is obtained; the ray irradiation crosslinking is adopted, in the preparation process, a crosslinking agent is not introduced, therefore, the prepared material has no biotoxicity caused by the crosslinking agent, and therefore, the preparation process is a safe, nontoxic and environmentally friendly production process; the prepared material has good prospects in the fields of hemostatic materials, drug carriers and the like.
Owner:厦门凝赋生物科技有限公司
Styptic powder and preparation method thereof
ActiveCN107213509AEasy to prepareEasy to operate industriallySurgical adhesivesPharmaceutical delivery mechanismRaw materialStarch
The invention discloses a styptic powder and a preparation method thereof. The method comprises the following steps: dissolving a starch raw material in water, emulsifying and dispersing, performing cross-linking reaction, drying under reduced pressure, washing, drying, and sterilizing. The styptic powder prepared by the preparation method has the characteristics of rapid adhesion to wound, rapid water absorption, arresting bleeding, and biodegradable property. The invention also discloses the styptic powder prepared by the method of the invention.
Owner:深圳市和福汇生物科技有限公司
Hemostatic device and method
ActiveUS20140330221A1Improved delivery and controlSlow actingBiocideHeavy metal active ingredientsHEMOSTATIC POWDERThin layer
A hemostatic tablet preferably including potassium ferrate and a cation ion exchange resin pressure formed into a tablet for delivery to a bleeding wound. The tablet improves the rate of adhesion to a bleeding wound surface, and allows a significantly greater and more uniform pressure to be exerted by manual compression of the tablet on the wound site, as compared to that of a thin layer of scattered hemostatic powder. After the seal is formed from the interaction of blood or exudates with the immediate contacting surface of the tablet, the bulk of the unused tablet easily delaminates from the seal making clean up facile. If the unused portion of the tablet is not removed from the wound site, a reservoir of hemostatic dressing stops further bleeding and to provide antimicrobial protection and healing. The tablet may be applied to any surface orientation and take any shape and thickness possible.
Owner:BIOLIFE
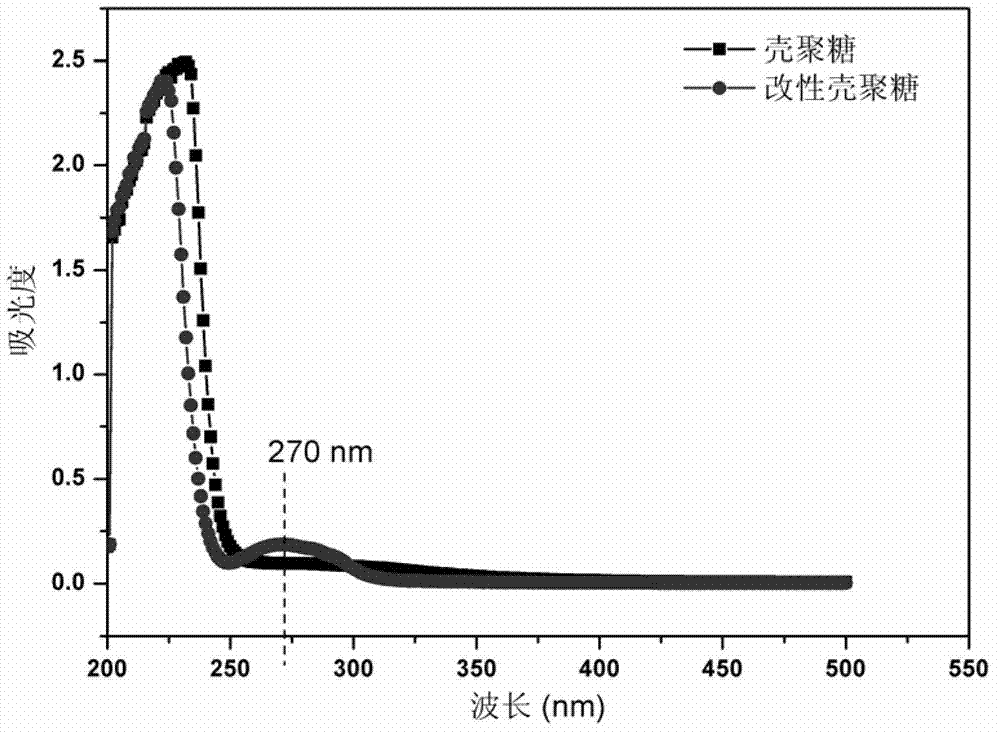
Preparation method of polyelectrolyte hemostatic powder based on carboxymethyl chitosan
InactiveCN106466492AImprove performanceSmall particlesAbsorbent padsBandagesPolyelectrolyteHEMOSTATIC POWDER
The invention belongs to the field of preparation of biomedical materials, and relates to a preparation method of a polyelectrolyte hemostatic powder based on carboxymethyl chitosan. The method includes the steps of: dissolving the carboxymethyl chitosan and an anionic polyelectrolyte or an amphoteric polyelectrolyte having biocompatibility in deionized water; forming a compound by reducing the pH value of the polyelectrolyte solution so as to protonize the amino groups in the carboxymethyl chitosan molecules, wherein the protonized amino groups are interacted with the anionic polyelectrolyte or the amphoteric polyelectrolyte through Coulomb force; and drying and crushing the polyelectrolyte compound to prepare the carboxymethyl chitosan polyelectrolyte hemostatic powder. The hemostatic powder has excellent biocompatibility and antibacterial property and has excellent hemostatic function.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Compound pseudo-ginseng, kaolin and bamboo fiber hemostatic gauze and preparation method thereof
InactiveCN106039383AAbsorbent padsAluminium/calcium/magnesium active ingredientsFiberHEMOSTATIC POWDER
The invention relates to the technical field of medical dressings and production processes of medical dressings, and in particular relates to a compound pseudo-ginseng, kaolin and bamboo fiber hemostatic gauze and a preparation method thereof. The hemostatic gauze comprises a base material, a hemostatic material and a binding agent, wherein the base material is made of a raw or regenerated bamboo fiber material; the hemostatic material is bonded to the base material; the binding agent is used for binding the hemostatic material to the base material; and the hemostatic material comprises a kaolin or kaolinite hemostatic clay material and pseudo-ginseng hemostatic powder. The base material of the hemostatic gauze provided by the invention comprises bamboo fiber, the hemostatic material comprises kaolin or kaolinite and pseudo-ginseng hemostatic powder, and the hemostatic clay material and the pseudo-ginseng hemostatic powder are bound to the bamboo fiber of the base material by using the binding agent, so that the hemostatic gauze is stable in property, the hemostatic clay material or the pseudo-ginseng hemostatic powder is not easy to drop off, and the hemostatic gauze is good in hemostatic effect.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
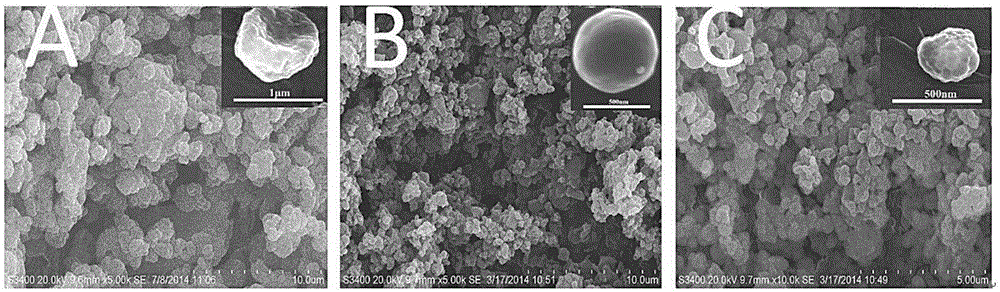
Absorbable starch microsphere hemostatic powder with antibacterial activity and application thereof
InactiveCN110115776AGood compatibilityStrong antibacterial activityAntibacterial agentsPowder deliveryHEMOSTATIC POWDERStarch Microspheres
The invention discloses starch microsphere hemostatic powder with antibacterial activity and application thereof. The preparation method of the hemostatic powder comprises the following steps of adopting soluble starch as a raw material and sodium trimetaphosphate as a crosslinking agent for preparing starch microspheres with a three-dimensional network structure by means of a reverse emulsification crosslinking approach; adding the prepared starch microspheres into a lysozyme solution to obtain the lysozyme-loaded antibacterial starch microsphere hemostatic powder. The preparation method is simple, and the prepared lysozyme-loaded antibacterial starch microsphere hemostatic powder has excellent hemostatic performance, high bacteriostatic activity and high biocompatibility and can be degraded and absorbed by the human body.
Owner:SHENYANG PHARMA UNIVERSITY
Rapid hemostatic powder for injury and preparation method of rapid hemostatic powder
The invention relates to the technical field of medical materials and particularly relates to rapid hemostatic powder for an injury and a preparation method of the rapid hemostatic powder. The rapid hemostatic powder for the injury comprises the following raw materials in parts by weight: 40-56 parts of chitosan quaternary ammonium salt, 28-36 parts of puffball extracts, 8-12 parts of sodium alginate, 1-5 parts of ramie root extracts, 1-5 parts of cirsium setosum extracts, 1-5 parts of madder extracts and 0.5-1.5 parts of carbonized hair extracts. The rapid hemostatic powder for the injury can take a hemostasis effect through various approaches and remarkably shorten the blood coagulation time, also has the effects of inhibiting and resisting bacteria, relieving pain and promoting wound healing and can be widely applied to rapid hemostasis, emergency treatment and treatment of skin scratch, injury and haemorrhoids and relief of scar formation.
Owner:DONGGUAN DAQING MEDICAL DEVICES CO LTD +2
Starch compound polysaccharide hemostatic powder and preparation method thereof
InactiveCN104474571AGood water swelling performanceFast absorptionAbsorbent padsBandagesHEMOSTATIC POWDERIon exchange
The invention relates to starch compound polysaccharide hemostatic powder and a preparation method thereof. The preparation method comprises the following steps: carrying out enzymatic denaturation on starch to obtain a porous starch solution, dissolving sodium hyaluronate in deionized water to obtain a sodium hyaluronate solution, mixing the two solutions according to a weight ratio of 50-95 parts of porous starch solution to 5-50 parts of sodium hyaluronate solution, stirring uniformly and drying, soaking the dried material in a calcium chloride solution with a certain concentration to carry out ion exchange, and grinding the material into powder after secondary drying. The material provided by the invention has efficient stypticity on wound surfaces, is biodegradable, has the functions of anti-adhesion and moisture retention, and is safe and non-toxic, simple in preparation process and low in cost.
Owner:CHONGQING LIANBAI BOCHAO MEDICAL EQUIP
Quick-acting hemostatic powder and hemostatic spray
InactiveCN105267875ARapid hemostasisEasy to processHeavy metal active ingredientsPowder deliveryHEMOSTATIC POWDERMyrrh
The invention discloses a quick-acting hemostatic powder and a hemostatic spray. The quick-acting hemostatic powder comprises following components by weight; the hemostatic spray is made from following components by weight: Radix Alangii 2-5 parts, myrrh 2-8 parts, peach twig 1-3 parts, Rhizoma Corydalis 3-5 parts, Herba Rabdosiae 6-9 parts, Trogopterus dung 1-2 parts, Semen Caesalpiniae 1-3 parts, Rhizoma Curcumae Longae 3-6 parts, frankincense 4-7 parts, Crinis Carbonisatus 8-10 parts, Herba Clinopodii 8-10 parts, Cera Chinensis 1-3 parts, Terra Flava Usta 2-5 parts, Radix Pteroxygoni Giraldii 1-3 parts, Herba Agrimoniae 5-7 parts, Flos Celosiae Cristatae 2-4 parts, Folium Callicarpae Formosanae 1-3 parts, Radix Melastomae 1-3 parts, Panax notoginseng 6-8 parts, Receptaculum Nelumbinis 5-7 parts, ophicalcite 1-2 parts, pollen Typhae 1-2 parts, Herba Achillis 1-3 parts, Folium Callicarpae Macrophyllae 2-5 parts, and Radix Toddaliae Asiaticae 5-8 parts. A preferred formula is obtained by matching various herbs effective in astringency, stasis removal and blood activation and through a plurality of tests and verifications, the purpose of quick hemostasis of a wound is achieved, and processing is facilitated; both the quick-acting hemostatic powder and the hemostatic spray are made from Chinese herbs, free of chemicals, safe and reliable, and free of side effect.
Owner:CHENGDU ERJUE TECH
Hemostatic material and preparation method thereof
ActiveCN106729950AGood hemostasisStrong water absorptionSurgical adhesivesPharmaceutical delivery mechanismHEMOSTATIC POWDERMedicine
The invention belongs to the field of a biomedical material, and particularly relates to a hemostatic material and a preparation method thereof. The hemostatic material is prepared from one or more of starch raw materials through acidolysis, crosslinking, negative ionization and positive ionization; carboxymethyl chitosan is connected onto anion degradable starch, so that the hemostatic effect and the water absorptivity of the prepared hemostatic material are obviously improved; in addition, the hemostatic effect is superior to that of Arista fast hemostatic powder; the obvious progress is obtained.
Owner:成都迪康中科生物医学材料有限公司
Chinese medicine hemostatic powder
InactiveCN101537094BGood postoperative hemostasisReduce wound exudationHeavy metal active ingredientsPowder deliveryHEMOSTATIC POWDERPharmacology
The invention aims at providing a Chinese medicine hemostatic powder of anus postoperation hemostasis for external use which is used for externally stanching and lowering the treatment cost of the prior hemostatic medicine. The Chinese medicine hemostatic powder is prepared by the following Chinese medicines according to the weight account: 1.5-3 portions of panax pseudo-ginseng var. notoginseng, 3-9 portions of marmor serpentinatum, 3-9 portions of frankincense, 3-9 portions of myrrh, 1-1.5 portions of dragon's blood, 3-10 portions of safflower, 10-18 portions of halloysitum rubrum and 3-9 portions of sapanwood. The hemostatic powder is the Chinese medicine for external use, which is prepared by combining the characteristics of wound external hemorrhage according to the medicine function effect, has good hemostatic action after the operation, and has remarkable effects of reducing the wound surface effusion, resisting infection and promoting the wound healing. Compared with the prior hemostatic medicine, the invention has equal hemostatic effect, but the cost is 20 percent of average price of the prior hemostatic medicine.
Owner:徐樊
Novel absorbable hemostatic material
ActiveCN103446619AEasy to prepareReduce manufacturing costAbsorbent padsBandagesHEMOSTATIC POWDERPolyvinyl alcohol
The invention relates to a novel absorbable hemostatic material which is easy in preparation method, low in cost and good in hemostatic effect. At present most medical hemostatic materials in China are water-soluble hemostatic gauze, and the gauze does not have anti-inflammatory and anti-bacterial effects when being used for hemostasis, and has the defects that the hemostatic speed is low, the in vivo absorption time is long, an inflammatory reaction is easy to cause and the like, and therefore people turns to hemostatic powder, however, as existing hemostatic powder is complex to prepare, high in price, not ideal in hemostatic effect and the like, the hemostatic powder is not applied as a mainstream hemostatic product. The novel absorbable hemostatic material is a guluronic acid polymer generated by an complexation reaction or crosslinking reaction of a guluronic acid monomer, polyvinyl alcohol, lavender essential oil and clove essential oil. The novel absorbable hemostatic material is good in hemostatic effect, and has the advantages that the hemostatic speed is high, the in vivo absorption time is short, broad-spectrum sterilization and anti-inflammation effects are achieved, wound healing is promoted and the like, and in addition, the novel absorbable hemostatic material is not affected by the size and position of a wound surface, and widely applicable to the fast hemostasis for war wound, trauma and other conditions.
Owner:QINGDAO ZHONGTENG BIOTECH
Polysaccharide styptic powder, preparation method and applications thereof
InactiveCN105770963APromote absorptionSlow down the flowAbsorbent padsBandagesHEMOSTATIC POWDERBiocompatibility
The invention relates to polysaccharide styptic powder, a preparation method and applications thereof. Plant origin starch and etherified starch are taken as the raw materials, two starches are individually processed by ultrasonic waves, then two starches are emulsified and crosslinked, and finally the crosslinking product is subjected to extraction, liquid separation, washing, drying, and sterilization to obtain the styptic powder. The styptic powder has a good biocompatibility, can be directly applied to the common bleeding parts, deep bleeding parts, and bleeding parts that are hard to reach through surgery operation, and is simple and convenient to use. Starches are the raw materials of the styptic powder, thus the styptic powder is nontoxic and nonirritant, moreover, the sources of raw materials are wide, the cost is low, the technology is simple and feasible, and thus the styptic powder has a wide clinical application prospect.
Owner:CHONGQING LIANBAI BOCHAO MEDICAL EQUIP
Method for manufacturing specific hemostatic powder
InactiveCN101837097AStrong blood pressure resistanceEffective hemostasisPowder deliveryAnthropod material medical ingredientsHEMOSTATIC POWDERArterial hemorrhage
The invention provides a method for manufacturing specific hemostatic powder, which is a necessary medicament for rescuing body injury, i.e. hemorrhage caused by current war preparation, counter strike, accidents (knife injuries and traffic accidents) in life and natural disasters (earthquakes). The specific hemostatic powder has stronger blood pressure resistance (over 200mmHg) and effective hemostasis on medium-small arterial hemorrhage, besides basic requirement of effective hemostasis and medicament absorption in human tissues. According to the characters of the traditional Chinese medicines, the prepared hemostatic powder which is not studied domestically and overseas has the functions of antiphlogosis and tissue regeneration, and common wound is naturally healed in 4 to 5 days without any scar. Because of the advantages of tissue absorption and strong hemostatic efficacy, the hemostatic powder can effectively stop bleeding in only 2 minutes on parenchymatous organic rupture (liver and spleen rupture). The hemostatic powder has obvious hemostatic effect in a specific state (patients of hypertension, liver disease, cancer and thrombocytopenia, patients in blood transfusion, and patients lacking prothrombin and the like).
Owner:许雷
Cardiovascular clinical compression rapid hemostasis device
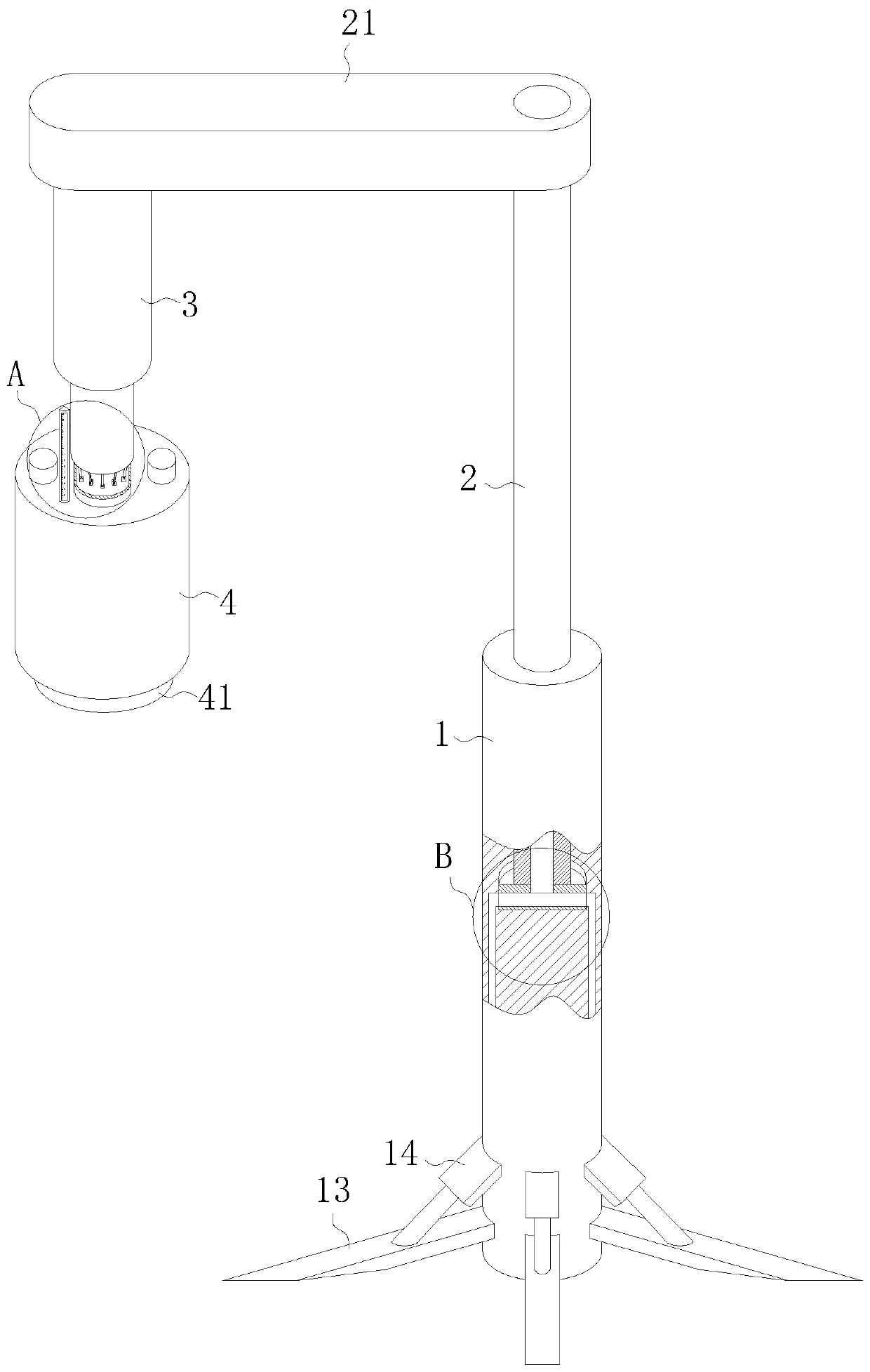
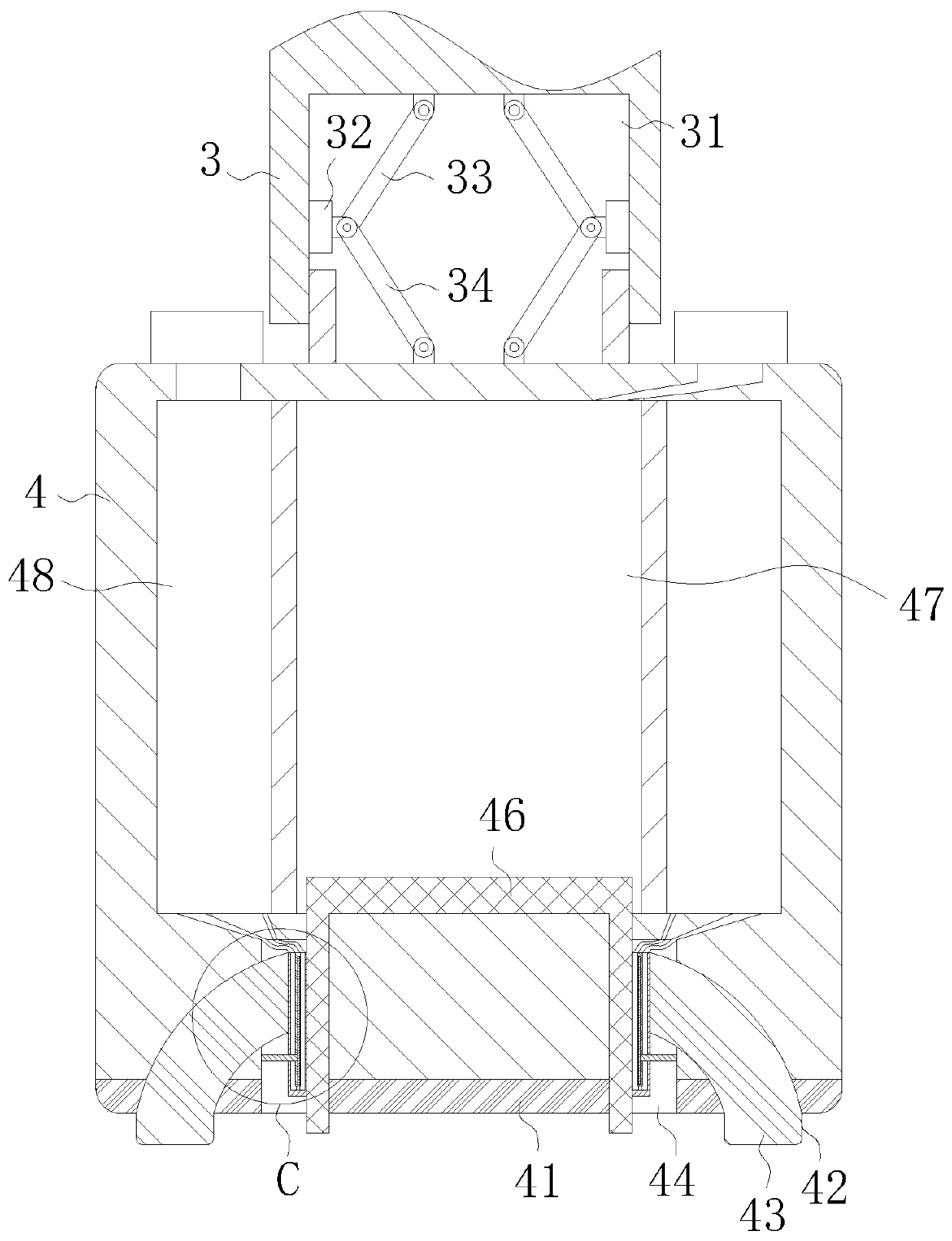
ActiveCN110151245AIncrease pressureIncrease and decrease pressureSurgeryMedical applicatorsBlood pressure increaseHEMOSTATIC POWDER
The invention belongs to the technical field of medical instruments, and particularly relates to a cardiovascular clinical compression rapid hemostasis device. The cardiovascular clinical compressionrapid hemostasis device comprises a vertical column, a rotating shaft, a fixed plate, a first air cylinder, a pressing head and a controller. One end of the rotating shaft is inserted into a first cavity formed in the upper end of the vertical column, and the rotating shaft is fixedly sleeved with a rotating ring in the first cavity; the side wall of a first groove is slidably connected to a circumferentially arranged electric push rod; the driving end of the electric push rod is hinged to a first hinge rod and a second hinge rod; a second groove is formed in the lower surface of the pressinghead; a third groove is formed in the lower surface of the pressing head; a third cavity is formed in the interior of the pressing head. The cardiovascular clinical compression rapid hemostasis device, while carrying out pressing hemostasis, can realize the intermittent intelligent adjustment of the pressing force and intermittent release of the buffer to ensure the necessary blood supply demand and to avoid the occurrence of blood pressure increase, and further can improve the hemostasis effect through circulating of ice water in combination with a hemostatic powder medical solution.
Owner:中挪(青岛)科技创新有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com