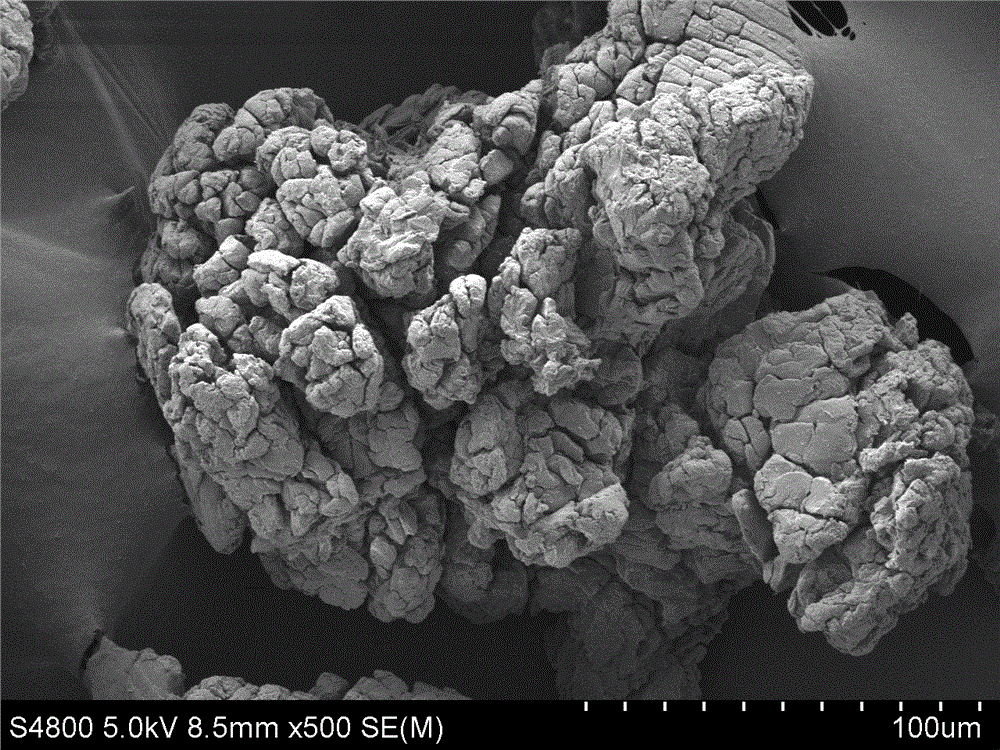
Potato starch-hyaluronic acid composite hemostatic powder and preparation method thereof
A potato starch and hyaluronic acid technology, applied in the field of medical devices, can solve the problems of poor water absorption of microporous polysaccharide particles, not firm enough gel, difficult strategic reserve, etc., and achieves easy degradation and discharge, good hemostatic effect, and low toxicity. residual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take 40g of high-purity potato starch granules and add them to deionized water, stir at a constant speed below 25°C to prepare a 40% starch suspension, adjust the pH of the suspension to 9.0 with 2% sodium hydroxide, and activate it 1 hour; slowly add 20ml of acetic anhydride / adipic acid mixture prepared at a ratio of 20:1 under uniform stirring conditions, and slowly add 2% sodium hydroxide to ensure that the pH of the reaction system is maintained at 9.0. Control the temperature of the process water bath to not be higher than 39°C; after the acetic anhydride / adipic acid mixture is added, continue to maintain the pH value at 9.0 with sodium hydroxide for 1 hour; slowly add succinic anhydride to the system until the pH value of the system is reached Until no change occurs, this process control is completed in about 2 hours. Neutralize the excess acid in the reaction system with 2% sodium hydroxide, wash with a large amount of deionized water several times, and filter. ...
Embodiment 2
[0033] Take 40g of high-purity potato starch granules and add them to deionized water, stir at a constant speed below 30°C to prepare a 40% starch suspension, adjust the pH of the suspension to 9.0 with 2% sodium hydroxide, and activate it 1 hour; slowly add 20ml of acetic anhydride / adipic acid mixture prepared at a ratio of 15:1 under uniform stirring conditions, and slowly add 2% sodium hydroxide to ensure that the pH of the reaction system is maintained at 9.0. Control the temperature of the process water bath to not be higher than 39°C; after the acetic anhydride / adipic acid mixture is added, continue to maintain the pH value at 9.0 with sodium hydroxide for 1 hour; slowly add succinic anhydride to the system until the pH value of the system is reached Until no change occurs, this process control is completed within 1.5 hours. Neutralize the excess acid in the reaction system with 2% sodium hydroxide, wash with a large amount of deionized water, filter and make a 40% suspe...
Embodiment 3
[0036] Take 40g of high-purity potato starch granules and add them to deionized water, stir at a constant speed below 30°C to prepare a 40% starch suspension, adjust the pH of the suspension to 9.5 with 2% sodium hydroxide, and activate it 1 hour; slowly add 20ml of acetic anhydride / adipic acid mixture prepared at a ratio of 20:1 under uniform stirring conditions, and slowly add 2% sodium hydroxide to ensure that the pH of the reaction system is maintained at 9.5. Control the temperature of the process water bath to not be higher than 35°C; after the acetic anhydride / adipic acid mixture is added, continue to maintain the pH at 9.0 with sodium hydroxide for 1 hour; slowly add succinic anhydride to the system until the pH of the system is reached Until no change occurs, this process control is completed within 2 hours. Neutralize the excess acid in the reaction system with 2% sodium hydroxide, wash with a large amount of deionized water several times, and filter.
[0037] The f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com