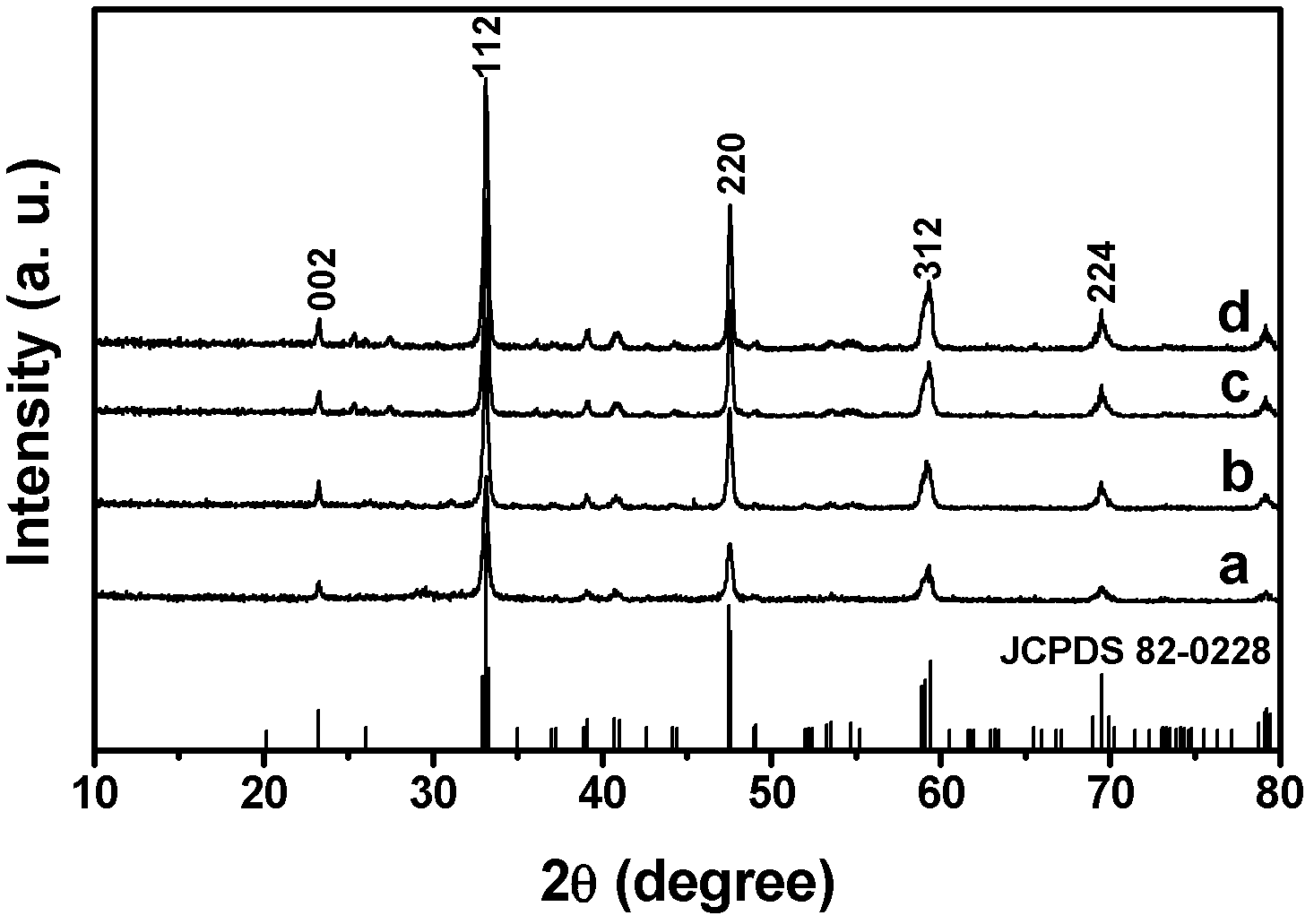
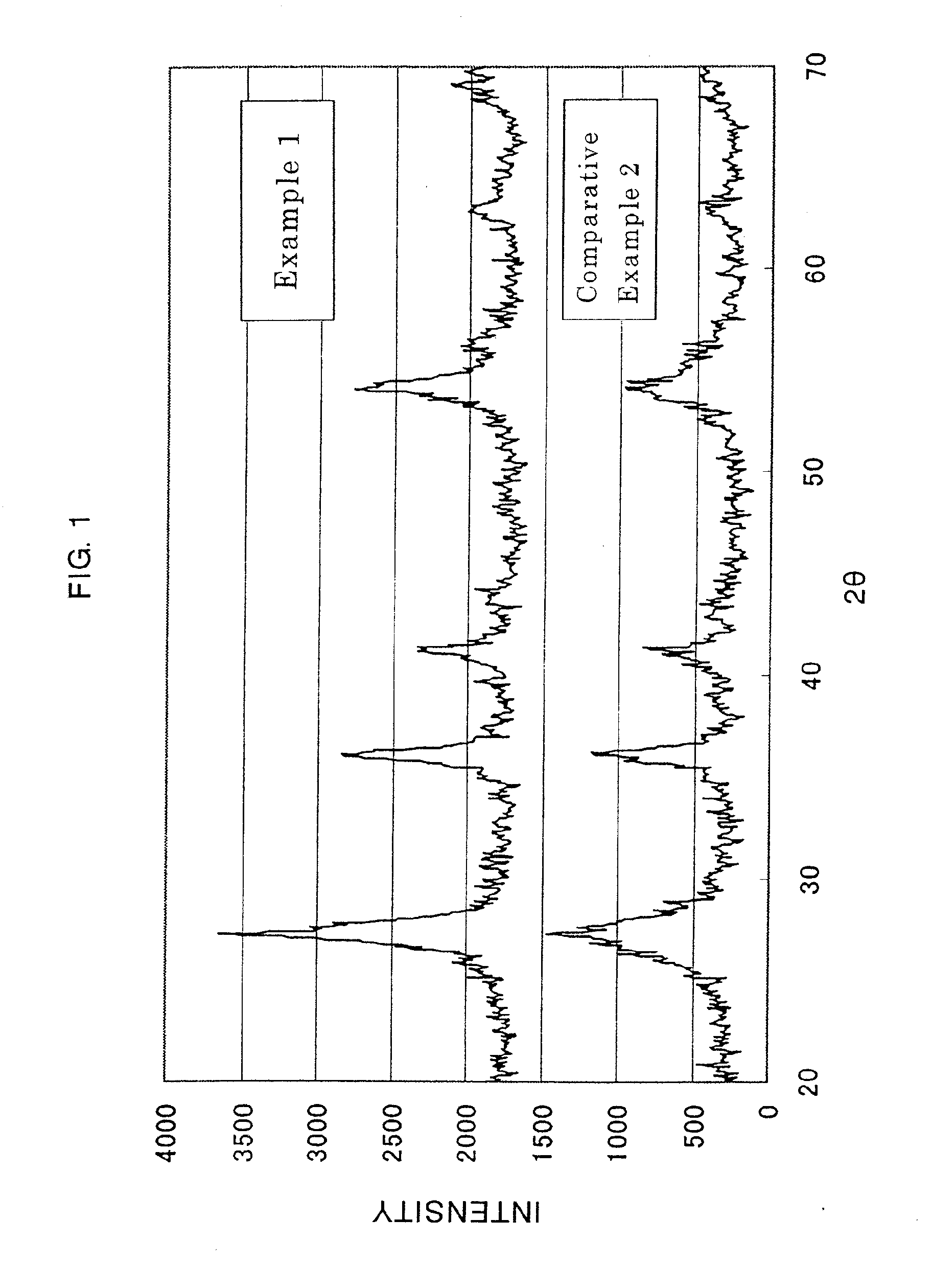
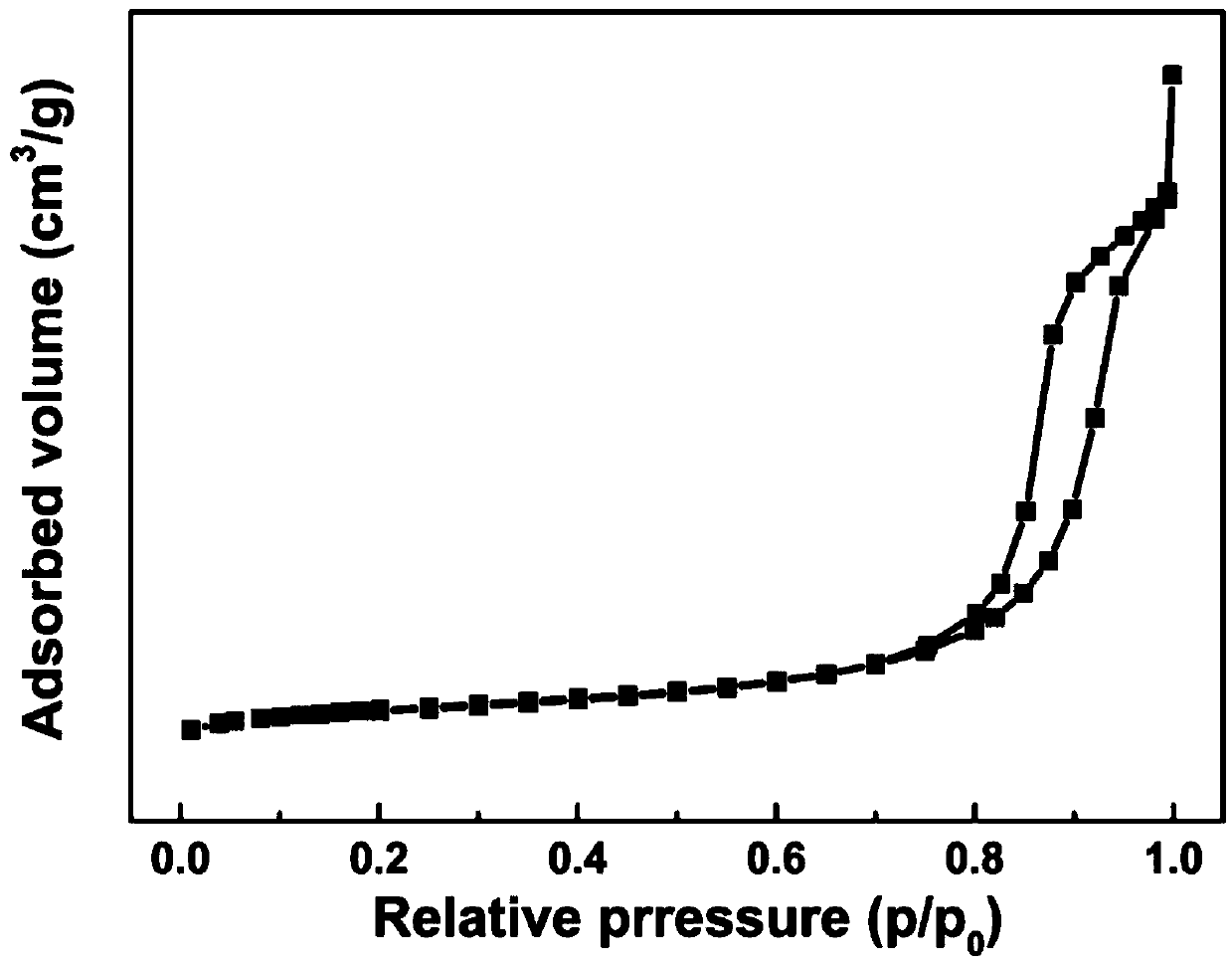
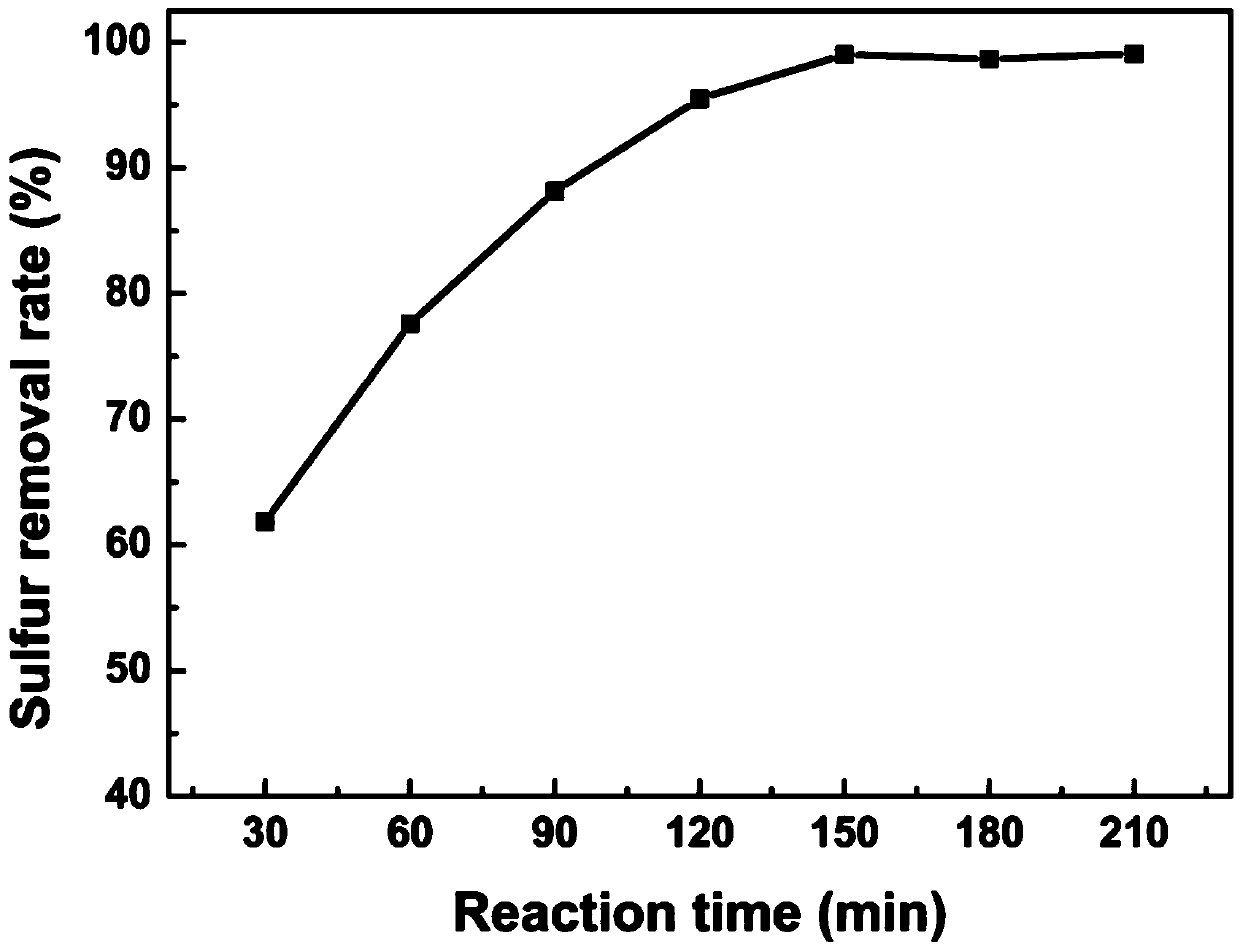
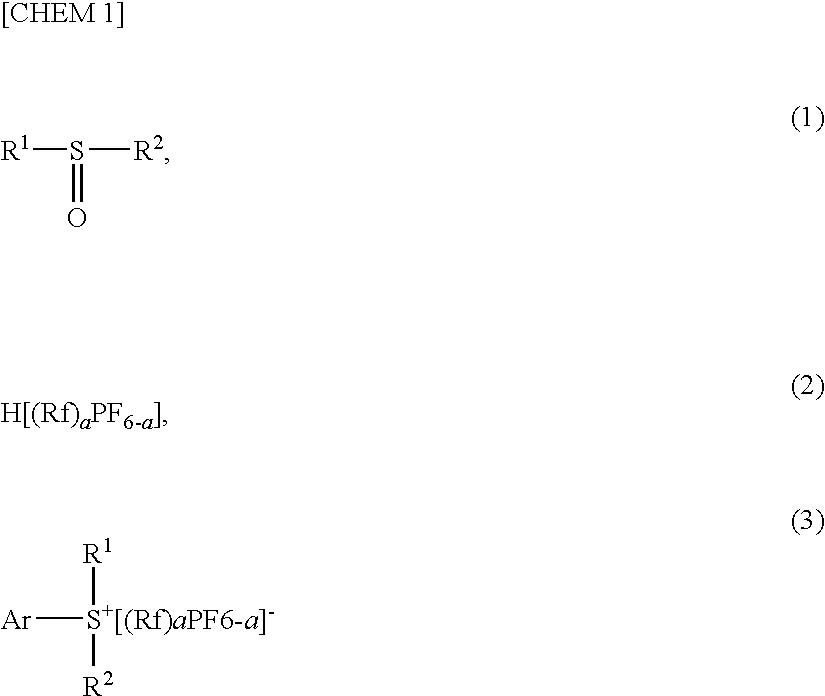Patents
Literature
82 results about "Excess acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polyester powder coating for solar water heater
InactiveCN101851460ASolve pollutionFix security issuesPowdery paintsPolyester coatingsAlcoholWeather resistance
The invention relates to the new material field of chemical engineering technique, in particular to a powder coating with integrated bottom and surface, i.e. a polyester powder coating for a solar water heater. The polyester powder coating is a thermosetting powder coating which is characterized by comprising the following components in percentage by weight: 61-74% of super-weather resistant terminal carboxyl polyester resin, 4.6-6% of curing agent, 15-30% of mixture of pigment and padding and 3-6% of assistant, wherein the super-weather resistant terminal carboxyl polyester resin is obtained by condensation polymerization of polyatomic alcohol and polyatomic acid or polyatomic anhydride under the condition of existence of excess acid, and the acid value range of the super-weather resistant terminal carboxyl polyester resin is 28-40mgKOH / g. The invention can solve the problem that the general powder coating can not simultaneously meet the requirements of super-weather resistance and rust protection.
Owner:JIANGSU LANLING POLYMER MATERIAL CO LTD
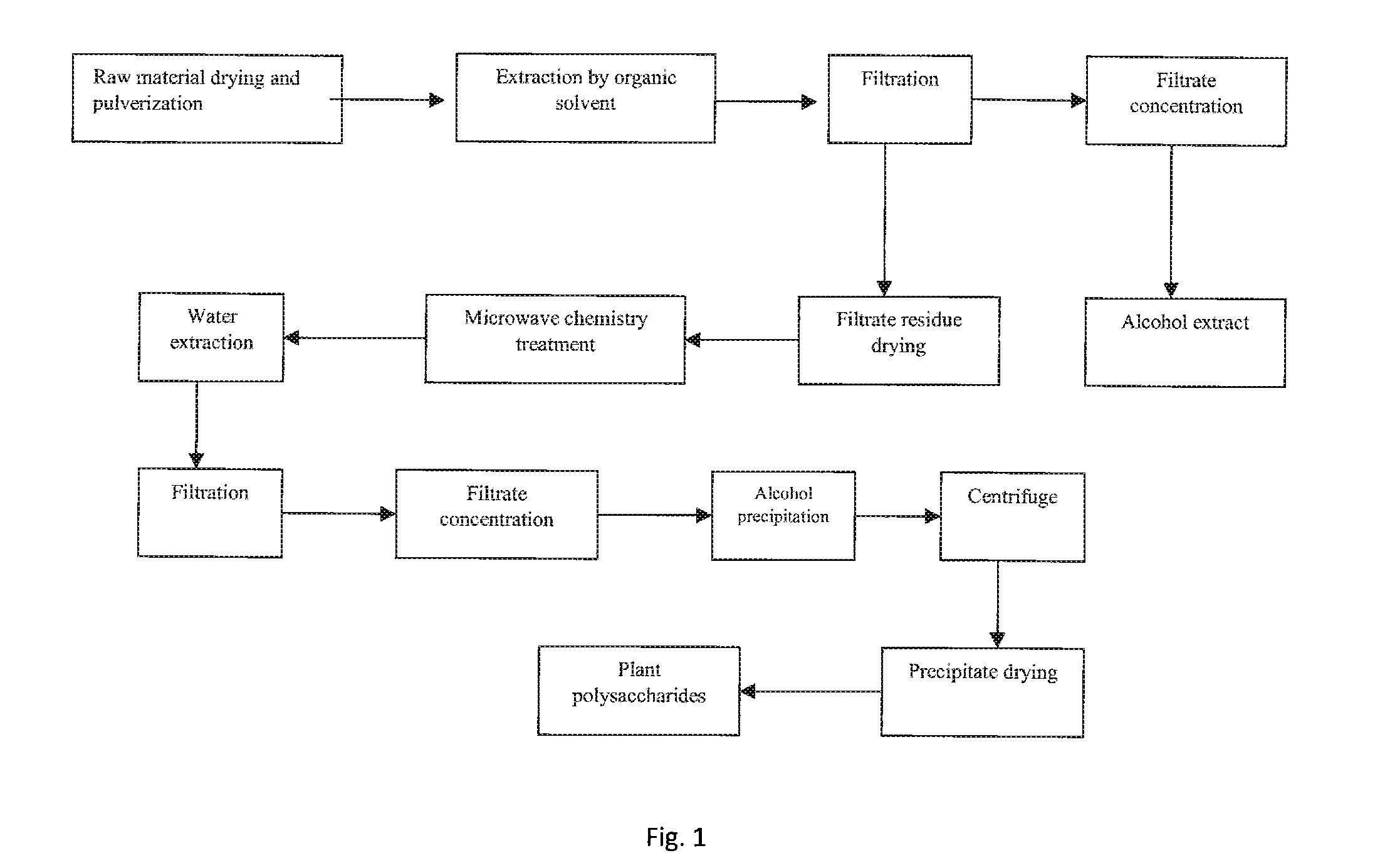
Method for extracting polysaccharides from higher plants and fungi through microwave chemical treatment
ActiveUS20140309414A1Evenly heatedHigh activitySugar derivativesSugar derivatives preparationChemical treatmentSolubility
This invention relates to a field of pharmaceutical chemistry, relates to a process of extracting high water soluble polysaccharides from higher plants or fungi. The present invention discloses a process of extracting higher plants and fungi polysaccharides based on a microwave chemistry method, comprising: putting the residue or pulverized higher plants and fungi after being degreased by an organic solvent into a microwave reaction chamber to react with an acid solution; and then distilling to remove excess acid or washing with organic solvent to remove the acid; adding water solution for extraction, subjecting the extracting solution after concentration to alcohol precipitation, separating precipitates aka polysaccharides therefrom.The present invention has significant advantages like fast processing rate, high polysaccharides yield, low organic acid consumption and efficient and easy to recycle, low water consumption, low power consumption, etc., and obtained polysaccharides have high yield and purity, good water solubility, and good biological activity.
Owner:SHENYANG KESI HIGH TECH
Lead-acid battery container formation technology
ActiveCN103943893AEasy dischargeReduce the impactFinal product manufactureSecondary cells charging/dischargingWater bathsWastewater
The invention relates to a container formation technology of a valve-closed charging type valve controlled sealed lead-acid battery. The technology comprises the following steps: adding acid into a valve controlled sealed battery, cooling off the battery in a water bath, allowing the battery to stand still for 2 to 4 hours, then covering the safety valve, binding the battery cover, and connecting the battery to a charging machine, wherein the battery container formation technology is divided into three phases. During the battery container formation process of the lead-acid battery container formation technology, acid is quantitatively added, so no acid is left after the container formation process, and thus the process of extracting acid from the lead-acid battery is saved. During the battery container formation process, a one-way valve is adopted to control the discharging of acid smog, and the acid extracting process after the battery container formation is also saved, so the environmental influence of the acid smog and processing of the extracted excess acid is reduced. The container formation technology simplifies the operation, reduces the production cost, does not generate wastewater due to electrode plate formation, reduces the environmental pollution, and improves the production efficiency.
Owner:CHAOWEI POWER CO LTD
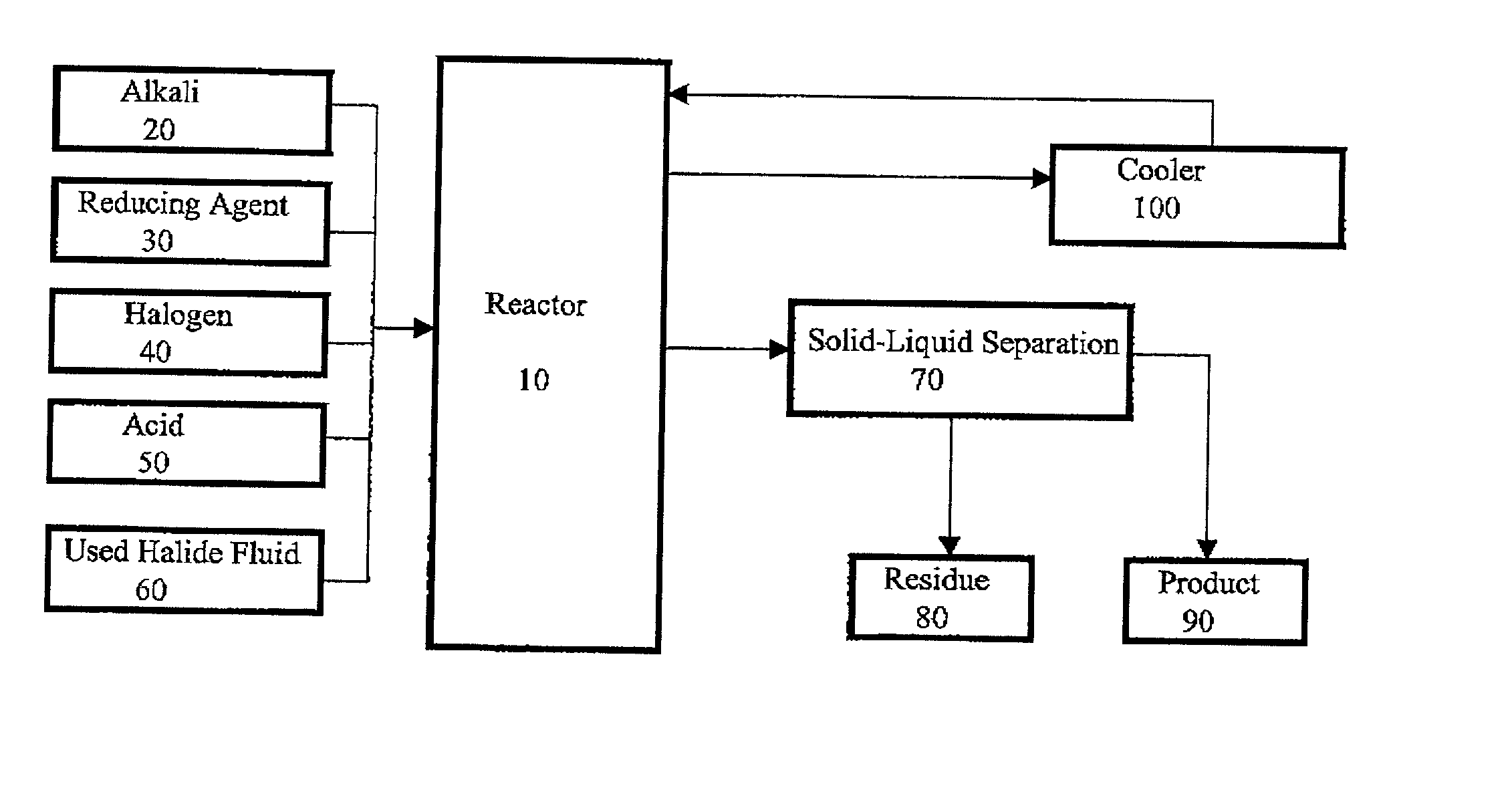
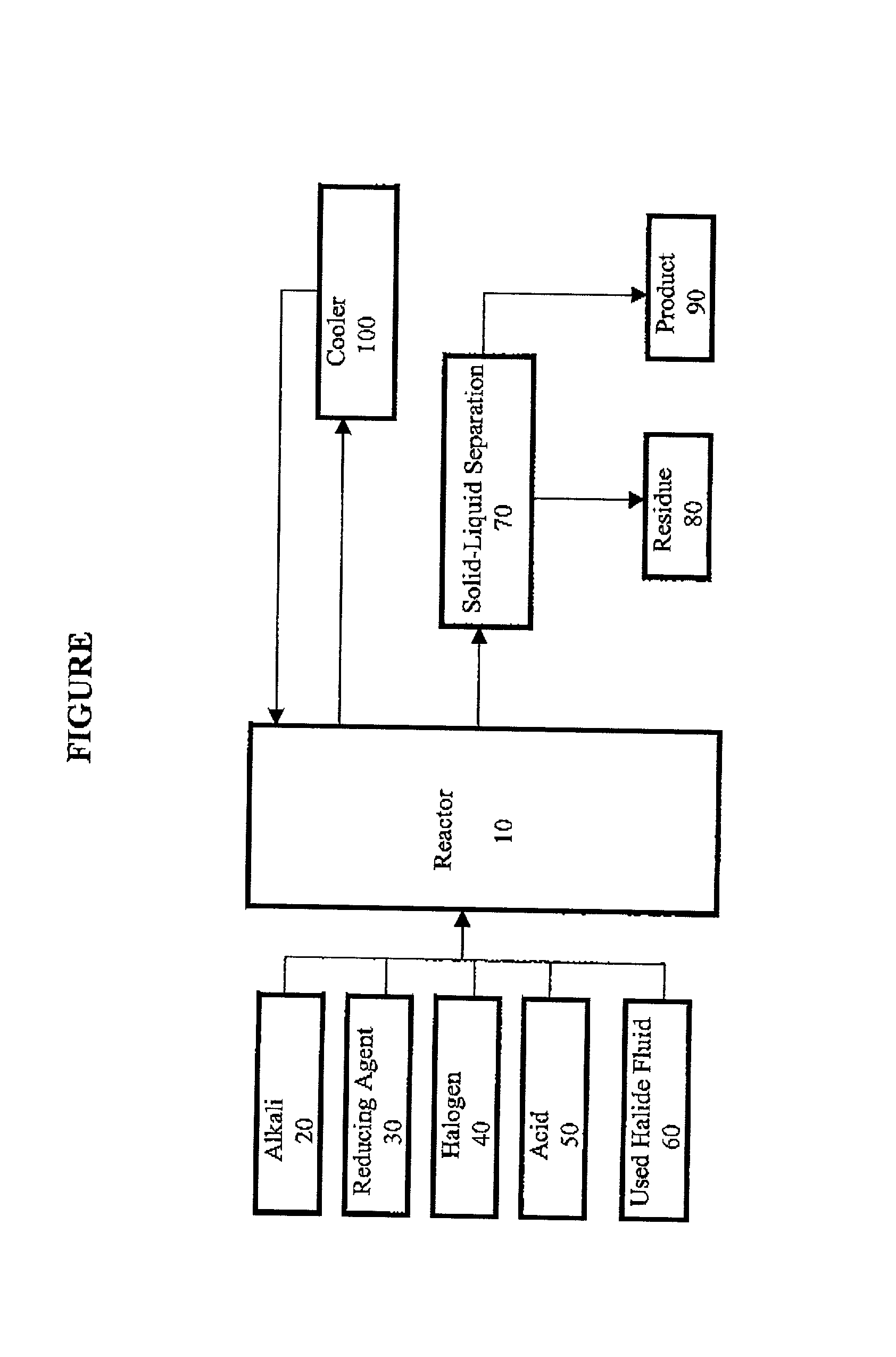
Method for regeneration of used halide fluids
InactiveUS20020130090A1Semi-permeable membranesHalide preparation methodsHalogenCrystallization temperature
A method for regenerating a used halide fluid comprising a density greater than 9.0 lbs / gal. and containing both soluble and insoluble impurities. This method comprises the steps of (1) adding acid to the used halide fluid so that the pH is within a range of approximately 0 to 10.0; (2) contacting the used halide fluid with halogen to increase the density to at least 10.0 lbs. / gal., adjust the desired true crystallization temperature of the fluid and oxidize soluble impurities; (3) adding a reducing agent while maintaining the temperature at a minimum of 10.degree. C.; (4) contacting the fluid with an alkali to neutralize excess acid; and (5) separating any suspended solid impurities from the fluid.
Owner:TETRA TECH INC
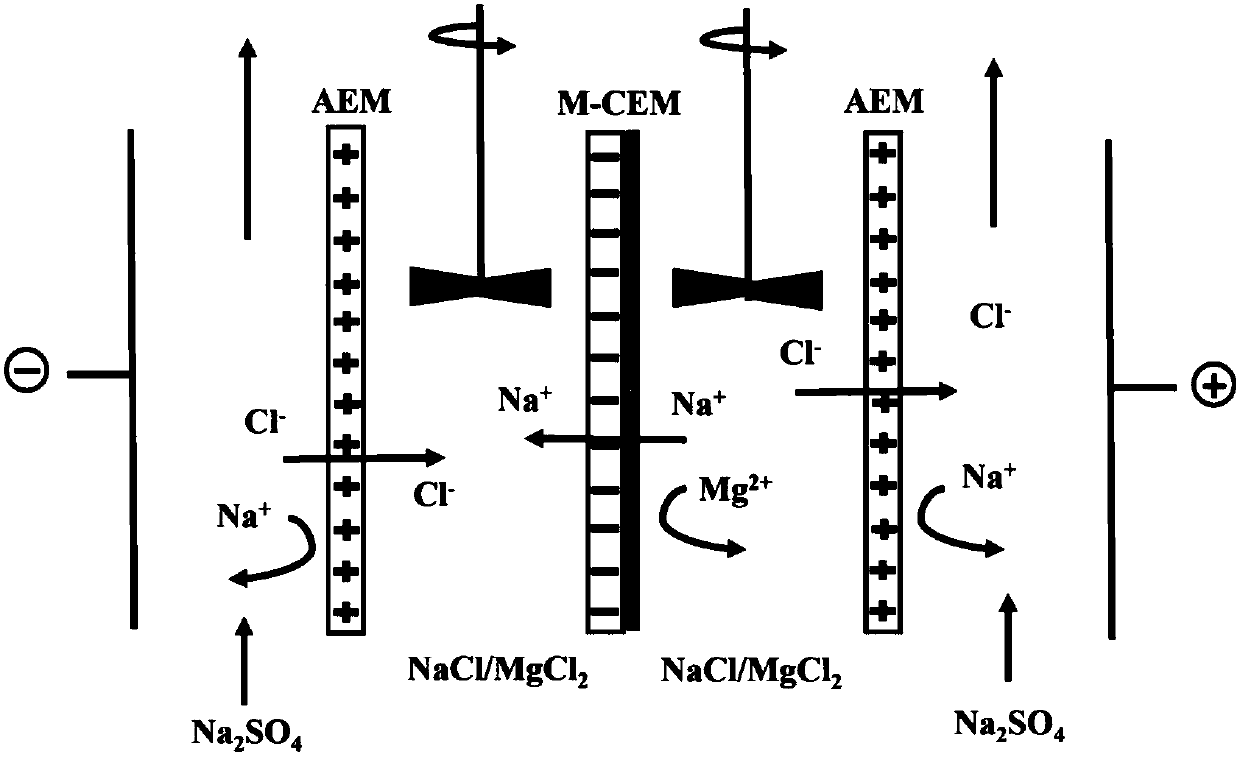
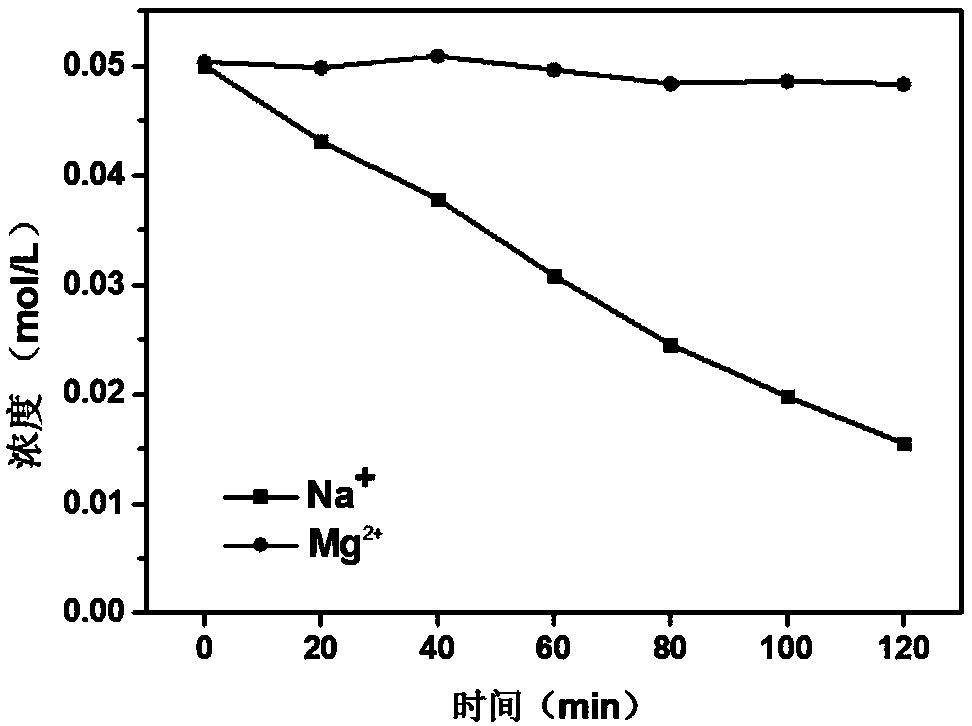
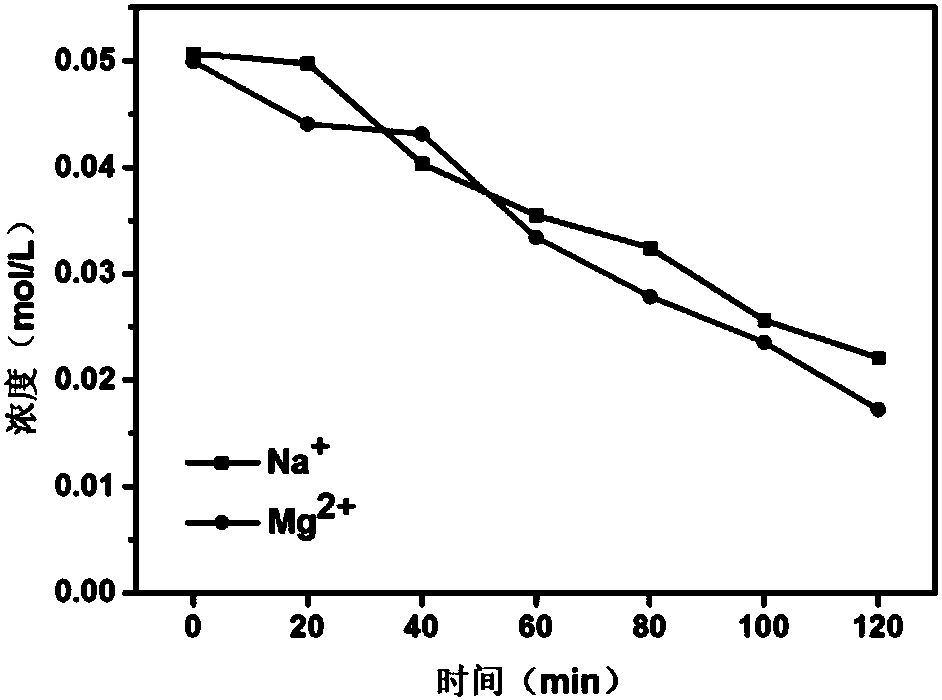
Method for preparing polypyrrole monovalent selective cation exchange membrane in situ
ActiveCN108097069AThe method steps are simpleEasy to operateSemi-permeable membranesPolypyrrolePyrrole
The invention discloses a method for preparing a polypyrrole monovalent selective cation exchange membrane in situ. The method comprises the following steps: (1) after an ordinary cation exchange membrane to be modified (white, 50 cm<2>) is immersed into a hydrochloric acid solution with the concentration of 0.2 mol / L for a period of time, washing an excess acid on the surface by using deionized water; (2) immersing the hydrogen-typed cation exchange membrane into a 2-5wt% pyrrole ethanol solution (200 mL) for 1 min; taking out the membrane, placing the membrane horizontally for more than 2 min; and after ethanol on the surface is completely evaporated, immersing the membrane into an oxidant solution with a certain concentration (such as ferric chloride, ammonium persulfate and hydrogen peroxide, 200 mL) for a period of time, wherein the membrane is black at the moment, and the result proves that the membrane is supported with polypyrrole; and (3) repeating the step (2) for a pluralityof times so as to obtain the polypyrrole selective cation exchange membrane with a plurality of layers. According to the method provided by the invention, the polymerization method of pyrrole is simple and effective, and the modified membrane has uniformly-deposited surface matter and significantly-improved monovalent ion selectivity.
Owner:ZHEJIANG UNIV OF TECH
Method for measuring polyvinyl alcohol content in water
ActiveCN101303310ATest data is stableBoric acid-iodine spectrophotometry is stable and reliableMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsObservational errorWater quality
The invention discloses a method for measuring polyvinyl alcohol content in the water. An excessive sulfuric acid is added under neutral heating condition (60-90 DEG C.) to remove starch having interference to the measurement by hydrolysis, and the excessive acid reacts with the polyvinyl alcohol in the sample to generate an ester in the mean time. In presence of boracic acid, the polyvinyl alcohol and the ester generated by reaction of the polyvinyl alcohol and the sulfuric acid can both react with a boracic acid - iodine - potassium iodide solution to produce a stable blue-green complex compound, and an absorbency is measured by a spectrophotometer at a point of a special wavelength of 645nm. A standard work curve is drawn according to the linear relation between the absorbency and the concentration of the reactor, thus the concentration of the sample can be known by checking the drawn standard curve after the sample measurement. The invention controls measurement error within the range of 5%, and the measurement result is stable and accurate, which makes the invention a feasible method for measuring polyvinyl alcohol concentration in the printing waste water.
Owner:四川省环保科技工程有限责任公司
Recovery method for fuel cell membrane electrode key material
The invention provides a recovery method of key materials of membrane electrode assembly for fuel cells. The recovery method adopts concentrated sulfuric acid, concentrated nitric acid, or mixed acid of the concentrated sulfuric acid and the concentrated nitric acid in any proportion to treat waste membrane electrode assemblies, and is characterized in that acting force between proton exchange resin molecular chains in proton exchange membranes and catalyst layers is reduced by acid treatment, thereby separating the proton exchange resin molecular chains to scatter in acid to form a solution, catalyst noble metals can be sufficiently isolated by carbon carriers of oxidization supported catalysts, and additionally, gas diffusion layer materials (carbon paper or carbon fabric) can be isolated. The recovery method includes steps of (1) after cleaning, drying and shredding, immersing used membrane electrode assembly in the acid for further stirring and heating, (2) using NaOH solution to neutralize excess acid after reaction, and (3) isolating proton exchange resin solution, noble metal catalyst or gas diffusion layer materials. The recovery method has the advantages of simple operation, high recovery rate, low cost and the like.
Owner:WUHAN UNIV OF TECH
Method of tissue preservation
PendingUS20200128816A1PliablePractical applicationDead plant preservationFibre treatment to obtain bast fibreBiotechnologyAlcohol sugars
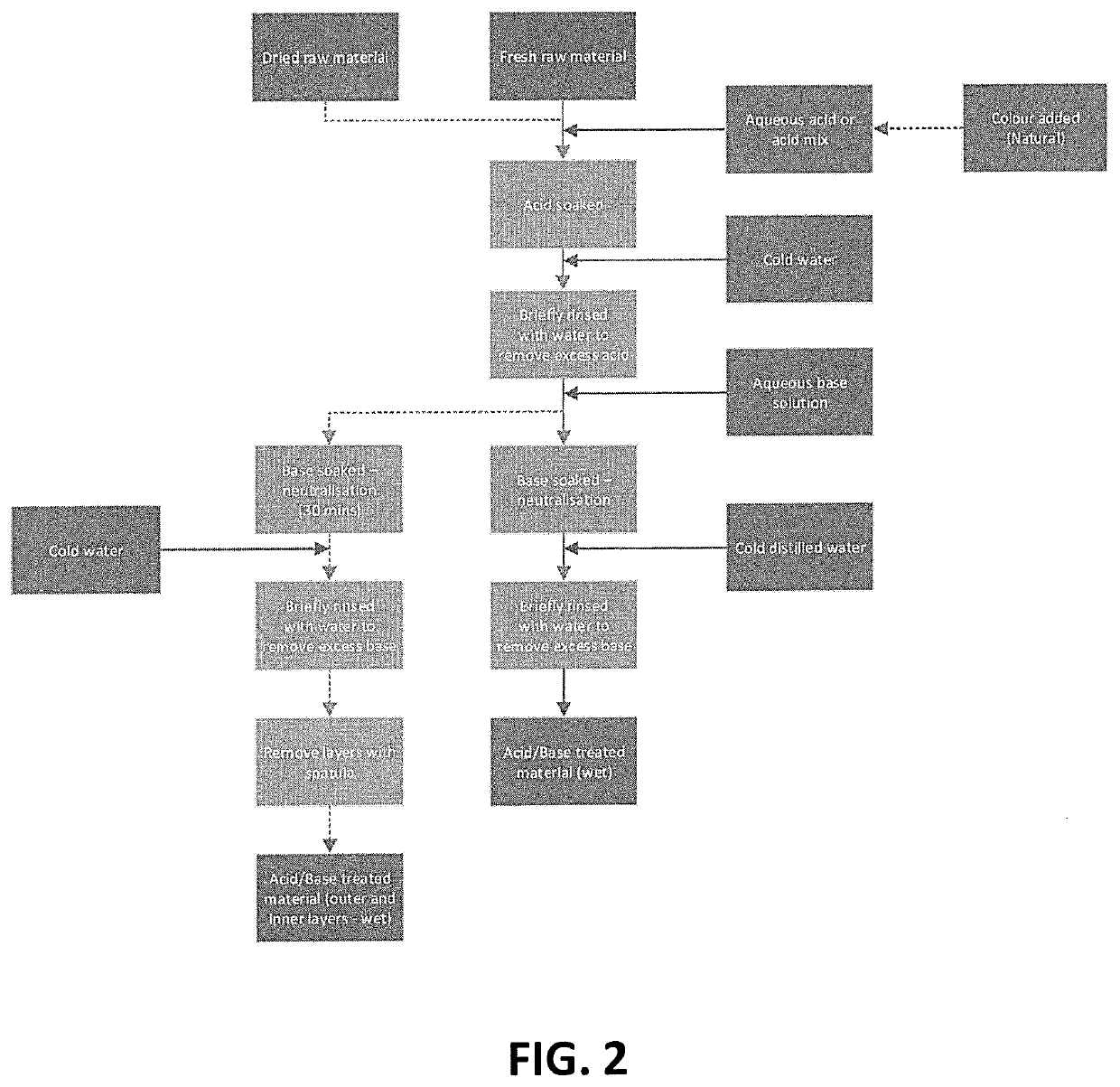
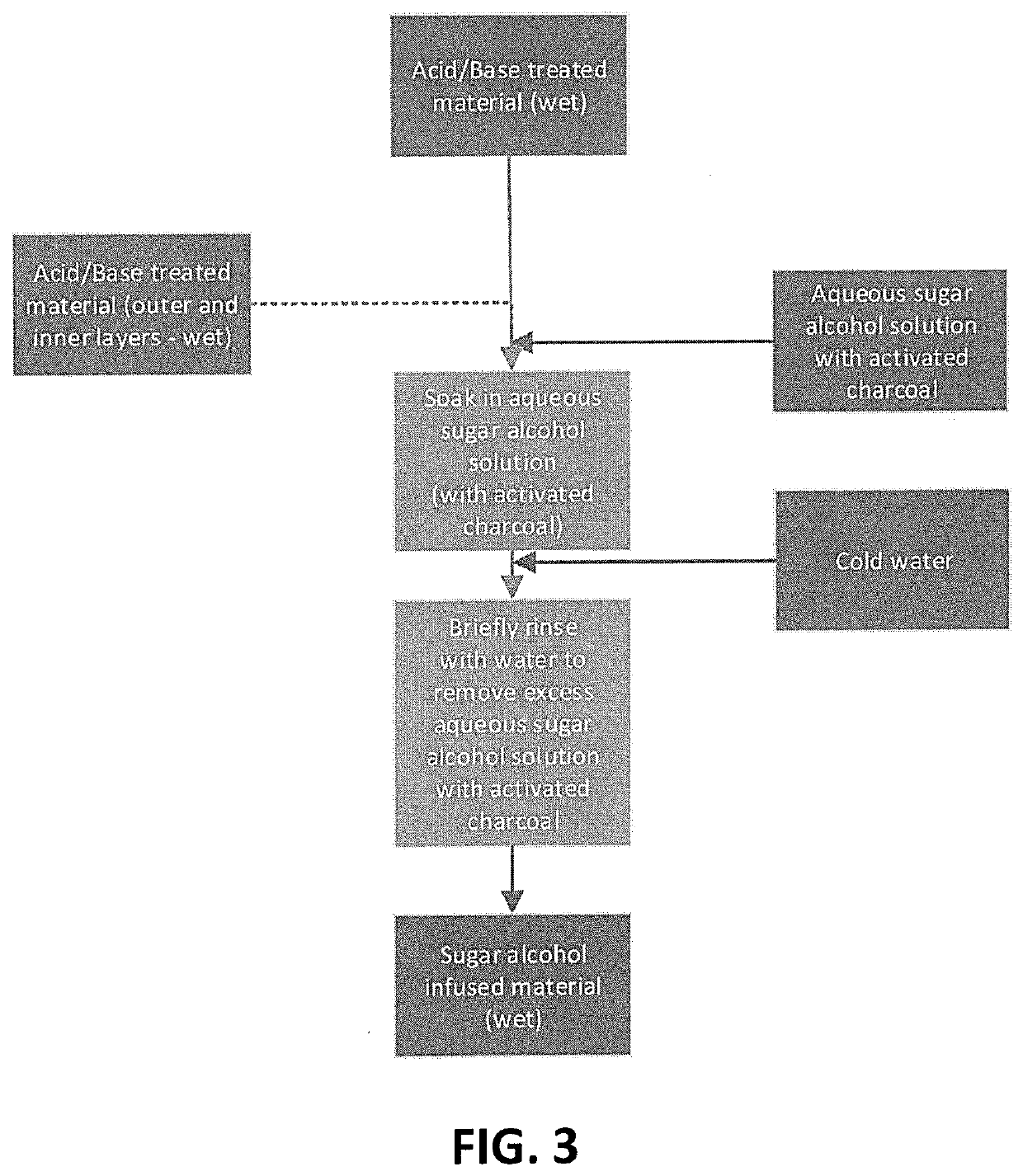
A process of preserving tissue from one or more plant, fungus or algae species is provided. The process comprises treating such tissue by subsequent steps of (i) incubation with a weak acid solution; (ii) removal of excess acid; (iii) incubation with an aqueous sugar alcohol solution; (iv) removal of excess sugar alcohol; and (v) drying. Also provided is a textile material having a sheet like structure, and characterized in having an average thickness of about 0.2-5 mm and comprising about 2% to about 20% sugar alcohol.
Owner:EMBLA PRODN HF
Preparation method of nitrogen-doped porous carbon material based on citric acid transition/alkali metal complex salt
InactiveCN108328599AFully contactedRich multi-level pore structureCell electrodesCarbon preparation/purificationPorous carbonAcid washing
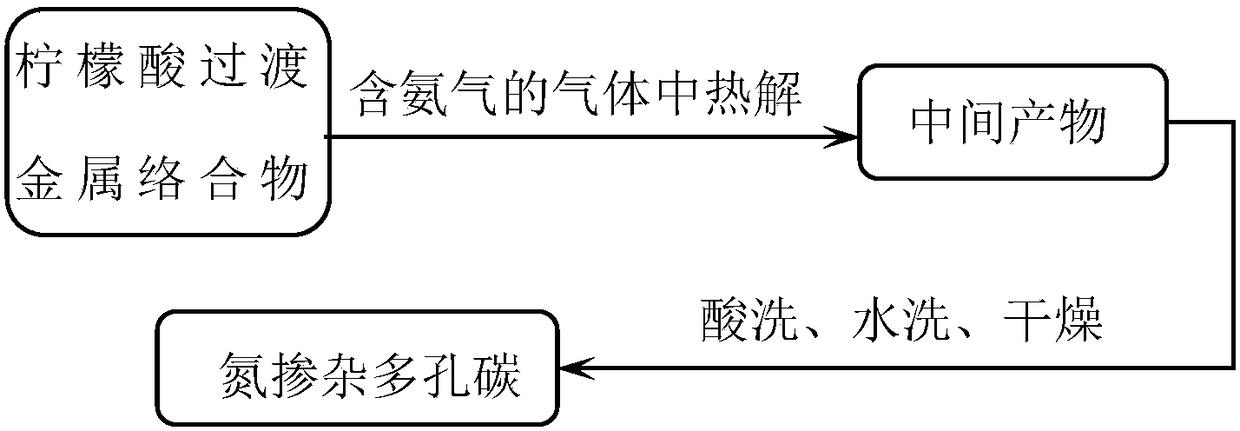
The invention provides a preparation method of a nitrogen-doped porous carbon material based on citric acid transition / alkali metal complex salt. The preparation method comprises the following steps:taking the citric acid transition / alkali metal complex salt as a carbon source, in the atmosphere containing ammonia gas, performing heat treatment for 1-24h under the temperature of 500-1,000 DEG C;after cooling, performing acid washing to remove impurities such as metal, and then washing with water to remove excess acid solution to neutral, and obtaining the nitrogen-doped porous carbon material after drying. The preparation method provided by the invention is simple and no pore-forming agent is needed, the preparation method is easy to operate, raw materials are wide in source, the methodis green and environmentally friendly, low in cost, is beneficial to realize larger-scale industrialized production, the prepared nitrogen-doped porous carbon material has the advantages that the nitrogen-doped porous carbon material has a multi-stage hole structure, a lamellar structure containing holes and has obvious graphite lattice fringes, the specific surface area is large, and the nitrogen-doped porous carbon material is rich in nitrogen heteroatoms and oxygen reduction activity point locations, has the good electrical catalysis oxygen reduction performance and stable performance, canbe used for preparing electricity catalysis oxygen reduction electrode and other catalyst carriers.
Owner:DONGGUAN UNIV OF TECH
Supercritical carbon dioxide method for extracting high purity rhubarb free anthraquinones
InactiveCN101053596AControl aciditySimple processAntibacterial agentsPlant ingredientsOrganic solventExcess acid
The invention discloses a method for extracting highly purified rhubarb dissociated anthraquinone by a hypercritical CO2 extraction method, including leaching the material with organic solvent to get leaching liquid, hydrolyzing the combined anthraquinone, balancing out the superfluous acid with inorganic alkali or acid after hydrolytic reaction, getting hydrolytic leaching liquid; injecting the hydrolytic leaching liquid into the extraction vessel for a hypercritical CO2 extraction, entering to the separation vessel after the extraction; recycling separated CO2, collecting discharging rhubarb dissociated anthraquinone and organic solvent, steaming out the organic solvent decompressly, obtaining the rhubarb dissociated anthraquinone powder. The invention adopts the liquid feed to effectively control the material liquid acidity in the extraction vessel and prevent the equipment corrosion and potential safety problems; meanwhile, it does not need install and detach the extraction vessel by entrainer pump liquid feed so that the extraction is processed continuously and greatly saves the extraction time and improve the output in unit time.
Owner:ZHEJIANG UNIV
Photoresist cleaning solutions and methods for pattern formation using the same
Disclosed are photoresist cleaning solutions, which are used to clean semiconductor substrates before or after an exposing step when photoresist patterns are formed. Methods for forming patterns using the same are also disclosed. The cleaning solutions include H2O and a nonionic surfactant compound represented by Formula 1. By spraying the disclosed cleaning solutions on a surface of the semiconductor substrate before or after exposing step to form a photoresist pattern, the desired pattern only is obtained and unnecessary patterns generated in undesired regions by ghost images are avoided as excess acid generated by the photoacid generator is neutralized and removed and damage to unexposed portions of the photoresist polymer is avoided. wherein R1 and R2 are independently H, C1-C20 alkyl, C5-C25 alkyl aryl or C1-C10 ester; m is 1 or 2; n is an integer ranging from 10 to 300; and o is 0 or 1.
Owner:SK HYNIX INC
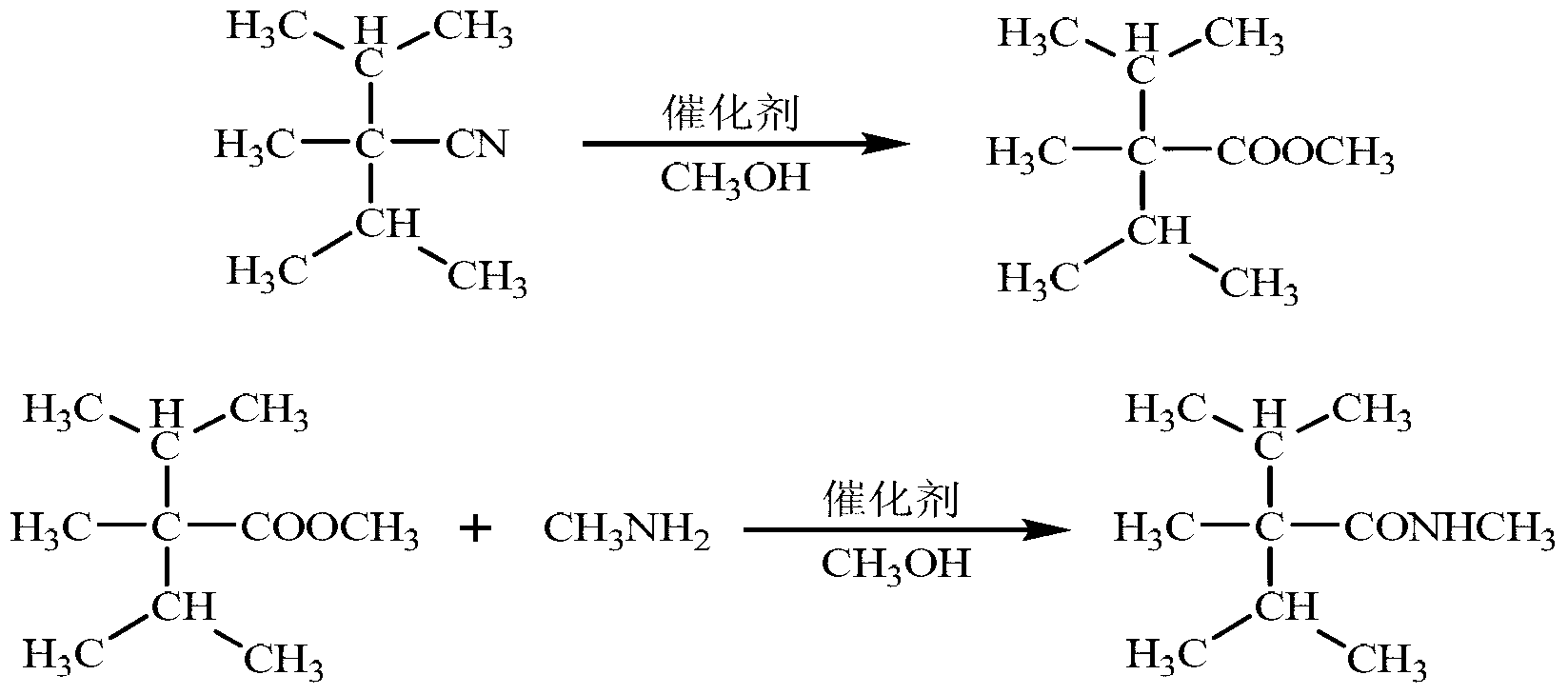
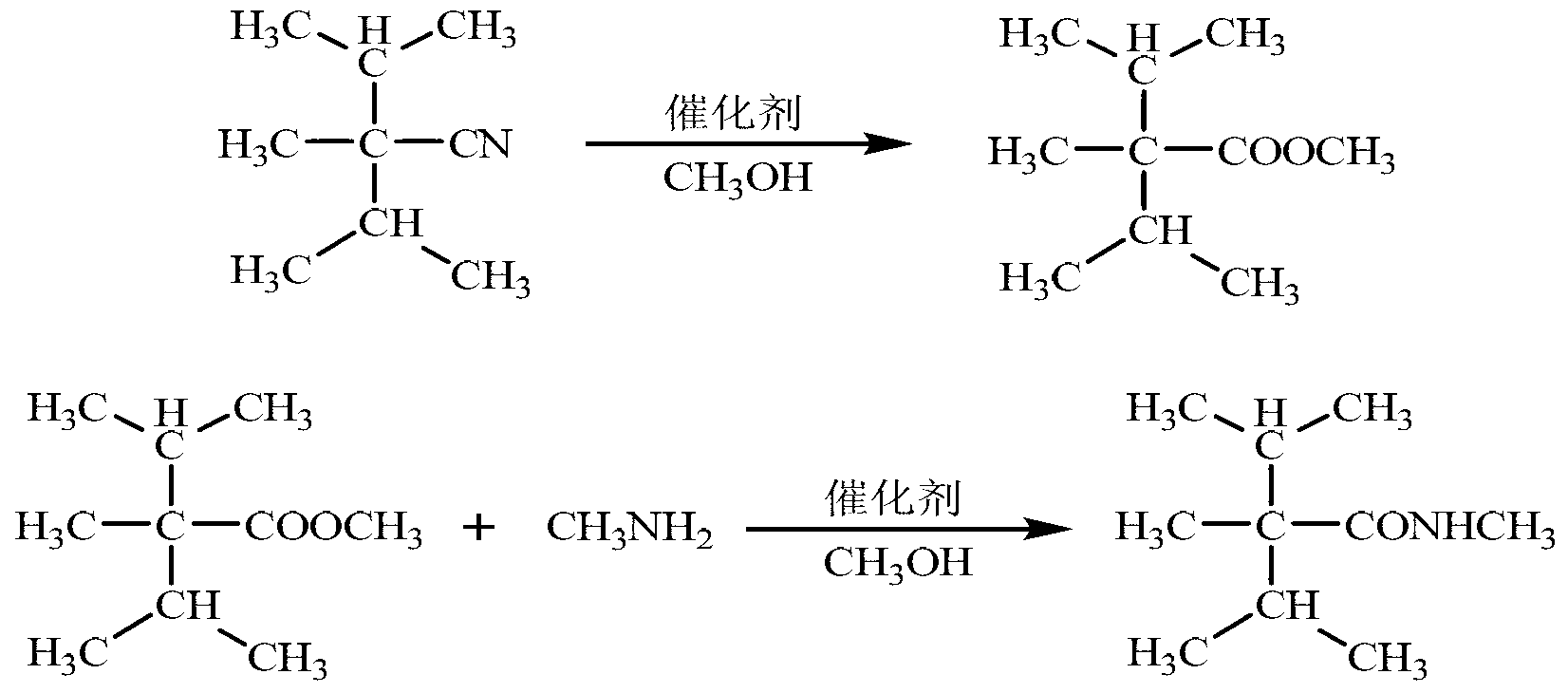
Synthetic method of cooling agent N-, 2, 3-trimethyl-2-isopropyl butyrylamide
InactiveCN103274959ALess simpleSimple processOrganic compound preparationCarboxylic acid amides preparationSodium bicarbonateAlcohol
The invention relates to a synthetic method of cooling agent N-, 2, 3-trimethyl-2-isopropyl butyrylamide. The method comprises the following steps: respectively adding 2, 2-isopropyl propionitrile, alcohol and acid into a 500 mL high-pressure reactor according to the mole ratio of 2, 2-isopropyl propionitrile, alcohol and acid gas of 1: (3-5): (3-5), performing backflow under stirring, reacting for 4 to 10 h, recycling excess acid gas and alcohol, cooling, washing sodium bicarbonate for two times, extracting saturated sodium chloride solution, standing, and performing liquid separation and drying, so as to obtain 2, 3-trimethyl-2-isopropyl butyric ester; respectively adding 2, 3-trimethyl-2-isopropyl butyric ester, amine and basic catalyst into a 500 mL high pressure reactor according to the mole ratio of 2, 3-trimethyl-2-isopropyl butyric ester, amine, basic catalyst of 1: (1.5-5): (0.04-0.3), washing, adding organic solvent to perform recrystallization, and then obtaining the cooling agent N-, 2, 3-trimethyl-2-isopropyl butyrylamide, wherein the content is 99.31 percent, and the yield is 87.32 percent. The method has the advantages that intermediate transformation rate is high, a small quantity of three wastes are produced, the method is safe to operate, the process is simple, amine can be circularly applied mechanically, the method is green and environmentally friendly, and the cold sense of products is lasting.
Owner:ANHUI SCI & TECH UNIV
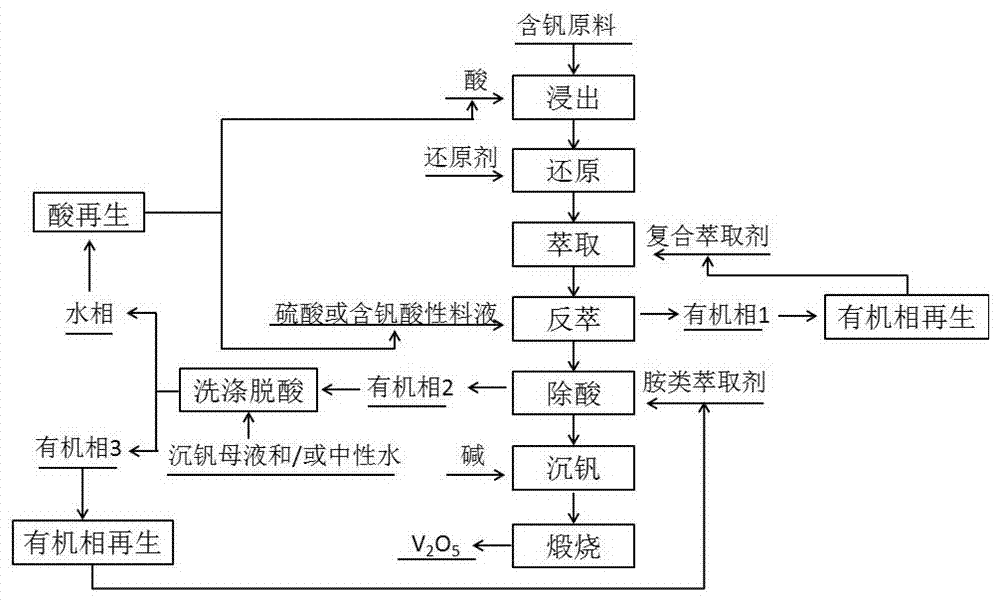
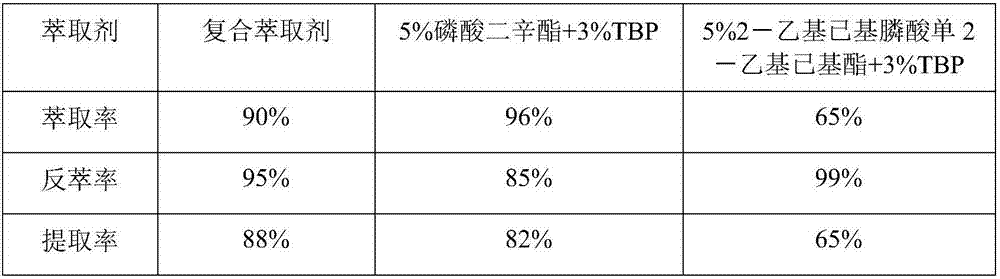
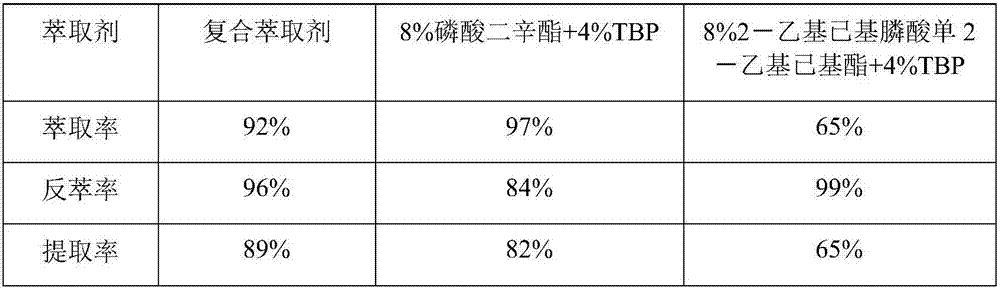
Method for preparing vanadium pentoxide through composite extraction
ActiveCN107226484AHigh extraction rateImprove extraction abilityVanadium compounds preparationVanadium oxidesPhysical chemistryPhosphoric acid
The present invention relates to a vanadium pentoxide preparation method, and provides a method for preparing vanadium pentoxide through composite extraction. The method comprises: (1) leaching; (2) reducing; (3) extracting, wherein a vanadium-containing material liquid is extracted with a composite extractant prepared from a phosphate organic matter and a modifier to prepare a vanadium-containing supported organic phase; (4) carrying out back extraction, wherein the supported organic phase is subjected to back extraction with sulfuric acid or a vanadium-containing sulfuric acid solution to prepare a vanadium-rich liquid; (5) removing acid, wherein the excess acid in the vanadium-rich liquid is extracted; (6) precipitating vanadium; and (7) calcining. According to the present invention, the vanadium is extracted with the composite extractant having strong extraction ability and strong back extraction ability, such that the vanadium extraction rate and the vanadium purity can be improved so as to improve the yield and the product of the vanadium pentoxide product, and the alkali consumption can be reduced so as to reduce the production cost.
Owner:CHONGQING KOOPPER CHEM IND
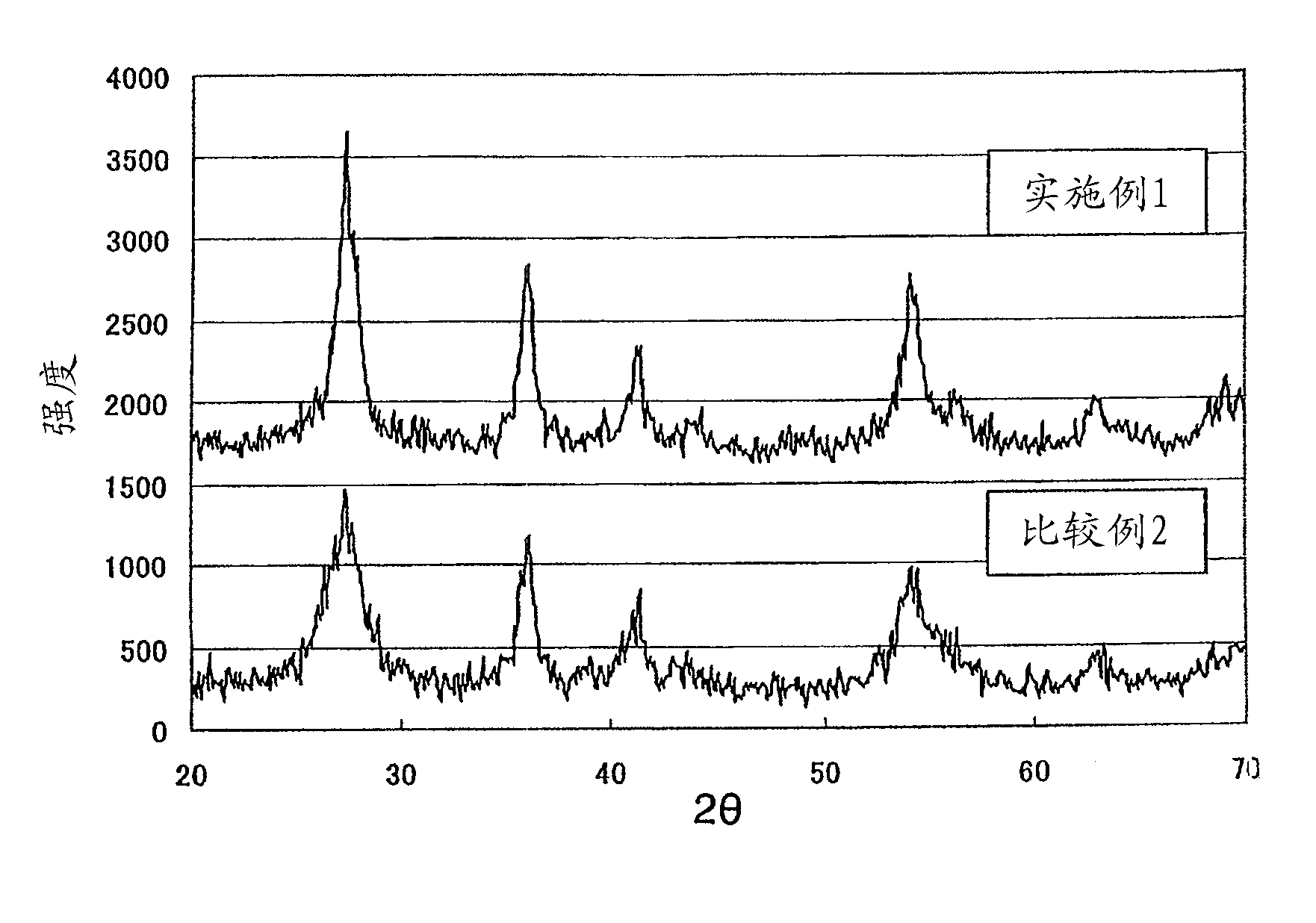
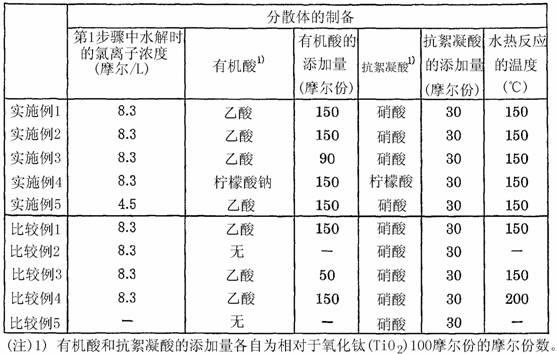
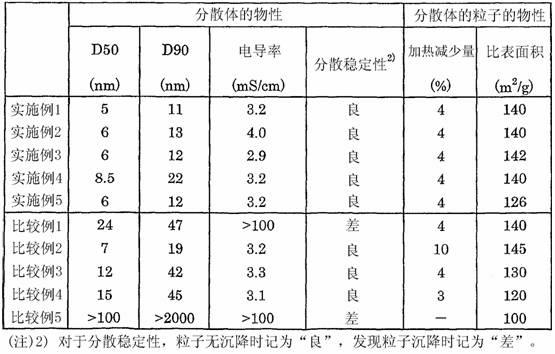
Dispersion of rutile titanium oxide particles, method for producing same and use of same
ActiveCN102325726AHigh crystallinityHigh refractive indexPigmenting treatmentMaterial nanotechnologyOrganic acidDynamic light scattering
Disclosed is a dispersion of rutile titanium oxide particles, wherein the D50 of the particle size distribution of the rutile titanium oxide particles is within the range of 1-15 nm and the D90 thereof is not more than 40 nm as measured by dynamic light scattering, the specific surface area of the rutile titanium oxide particles as determined by a BET method is within the range of 120-180 m2 / g, and the weight reduction rate is not more than 5% when the rutile titanium oxide particles are heated from 105 DEG C to 900 DEG C. The dispersion of rutile titanium oxide particles can be obtained by a method which comprises: a first step wherein an aqueous titanium tetrachloride solution is thermally hydrolyzed, thereby obtaining a slurry that contains deposited rutile titanium oxide particles; a second step wherein the slurry obtained in the first step is filtrated and washed with water, thereby removing water-soluble salts dissolved therein; a third step wherein the slurry obtained in the second step is subjected to a hydrothermal reaction in the presence of an organic acid; a fourth step wherein the slurry obtained in the third step is filtrated and washed with water; a fifth step wherein an acid is added to the slurry obtained in the fourth step and a wet dispersion process is performed, thereby obtaining a dispersion; and a sixth step wherein the excess acid and water-soluble salts are removed from the dispersion obtained in the fifth step.
Owner:SAKAI CHEM IND CO LTD
Simplified method to retrieve chitosan from acidic solutions thereof
InactiveUS20070265434A1Non-viscousPromote recoveryPharmaceutical non-active ingredientsFood supplementSalting out
Composition of salted out chitosan polymer containing mixture of certain salting out salts as well as methods for making and using same are disclosed. Chitosan polymer preparations produced by such methods are substantially free of chitosanase, undesirable salts and excess acid and retain their physiological as well as biological and physico-chemical properties. The chitosan preparations of the present invention are valuable for the dispensing of biologically active chitosan in forms of drugs or food supplement. Most-of these preparations easily dissolve in an aqueous acidic milieu such as the one of the stomach.
Owner:SOCPRA SCI SANTE & HUMAINES S E C
Revaluation of aqueous waste streams in the propylene oxide/styrene co-production process
InactiveUS20110121228A1Raise the possibilityMaintain ecological balanceWater treatment parameter controlOther chemical processesWaste streamInorganic compound
The present disclosure describes a method for the revaluation of aqueous waste streams generated in the propylene oxide and styrene co-production processes. In particular, it discloses a method of reducing the organic contaminant load from a highly contaminated aqueous stream and recovery from said stream of organic compounds that may be recirculated or used as fuel in the co-generation of energy, and which comprises: acidification of said aqueous stream with inorganic acid at a pH less than 4.5; separation of the two resulting phases at a temperature greater than 40° C.; washing of the organic phase produced in the previous step with an aqueous solution of excess acid and separation of the two resulting phases.
Owner:REPSOL SA
Method for producing sulfonium fluorinated alkylfluorophosphate
InactiveUS20090163723A1Low costImprove performancePhotomechanical apparatusPhosphorus organic compoundsArylPolymer science
Disclosed is a low cost, efficient method for production of a salt composed of an arylsulfonium and a fluorinated alkylfluorophosphate which method does not required a large excess amount of acid. The method comprises reacting an aryl compound Ar—H and with a compound represented by the formula (I),wherein R1 and R2 denote a hydrocarbon group or a heterocycle group which may be substituted, or they are bonded with each other directly or via —O—, —S—, —SO—, —SO2—, —NH—, —NR′—, —CO—, —COO—, —CONH—, an alkylene group having 1 to 3 carbon atoms or a phenylene group to form a ring structure which may be substituted, wherein R′ denotes a C1-5 alkyl group or a C6-10 aryl group; in the presence of an acid represented by the formula (2), wherein Rf denotes an alkyl group 80% or more of whose hydrogen atoms are substituted by fluorine atoms, “a” is an integer of 1 to 5; and a dehydrating agent to produce the salt of sulfonium represented by the formula (3).
Owner:SAN APRO
Preparation method of praseodymium-doped calcium titanate luminescent powder
The invention provides a preparation method of praseodymium-doped calcium titanate luminescent powder. The method comprises: under room temperature, and according to Pr<3+> / Ca<2+> in a mole ratio of 0.005 / 1, weighing certain amounts of CaCO3 and Pr6O11 solids which are dissolved in dilute nitric acid, conducting heating to expel excess acid, and when the pH value of the solution reaches 2-3, reheating the solution to a melt state; leaving the solution to cool to room temperature, then respectively adding stoichiometric titanium tetrabutoxide and 20mL of a water-ethanol solution, then adding a certain amount of citric acid as a complexing agent, adding polyethylene glycol, and carrying out stirring for 2h, thus obtaining a transparent sol precursor; subjecting the obtained precursor solution to spray drying so as to obtain precursor powder; putting the precursor into a programmed heating furnace, and heating the precursor to a temperature of 600DEG C-1000DEG C, then maintaining the temperature constant for 3h, thus obtaining CaTiO3:Pr<3+> fluorescent powder. The praseodymium-doped calcium titanate fluorescent powder prepared by the method of the invention has good crystallization degree and high purity, and is a CaTiO3 pure phase in a perovskite structure.
Owner:HARBIN ENG UNIV
Alkaline Antioxidant Mineral Water
InactiveUS20130122150A1Beneficial to human healthEasy to operatePackagingBeer brewingSea saltAmino acid
An alkaline antioxidant mineral water comprises tripotassium phosphate and multiple healthful minerals in water, which is free of chlorine, fluoride, and heavy metals. The alkaline mineral water further includes organic potassium, calcium, zinc, magnesium, selenium and 79 trace minerals from ancient sea salt. The trace minerals are taken from the low-sodium technically-processed magnesium chloride marine deposits and chelated with amino acids. The amino acids are obtained from a non-animal source which is suitable for vegetarian and persons who are allergic to animal proteins. The alkaline antioxidant mineral water neutralizes and eliminates the excess acids in human body, thus improving blood circulation. In addition, it provides electrolytes and minerals, which are beneficial to human body.
Owner:KIM ROBERT
Dispersion of particles of rutile titanium oxide, process for producing the same, and use of the same
ActiveUS20110301270A1High crystallinityGood dispersionPigmenting treatmentMaterial nanotechnologySlurryRutile
The invention provides a dispersion of particles of rutile titanium oxide wherein the particles of rutile titanium oxide have a D50 in a range of 1 to 15 nm and a D90 of 40 nm or less in particle size distribution as determined by a dynamic light scattering method; a specific surface area in a range of 120 to 180 m2 / g as determined by a BET method; and a rate of weight loss of 5% or less as obtained by heating the particles of rutile titanium oxide from 105° C. to 900° C.Such a dispersion of particles of rutile titanium oxide is obtained by a process according to the invention, which comprises: a first step in which an aqueous titanium tetrachloride solution is heated and hydrolyzed to obtain a slurry containing the precipitated particles of rutile titanium oxide; a second step in which the slurry obtained in the first step is filtered and washed with water to remove water-soluble salts dissolved therein from the slurry; a third step in which the slurry obtained in the second step is subjected to a hydrothermal reaction in the presence of an organic acid; a fourth step in which the slurry obtained in the third step is filtered and washed with water; a fifth step in which an acid is added to the slurry obtained in the fourth step, and the resulting mixture is subjected to a wet dispersion treatment, thereby obtaining a dispersion; and a sixth step in which excess acid and water-soluble salts are removed from the dispersion obtained in the fifth step.
Owner:SAKAI CHEM IND CO LTD
Supported catalyst for catalytic oxidation desulfurization reaction and preparation method of supported catalyst
InactiveCN109675594AImprove catalytic performanceAchieve recyclabilityPhysical/chemical process catalystsHydrocarbon oils treatmentHeteropoly acidCatalytic oxidation
Owner:SHIHEZI UNIVERSITY
Method for preparing lignin anhydride cured epoxy resin by one-pot process
ActiveCN110358055AImproves tensile strength propertiesEfficient use of resourcesOrganic solventCarboxylic acid
The invention discloses a method for preparing a lignin anhydride cured epoxy resin by a one-pot process, and belongs to the field of biomass recycling and epoxy resin manufacturing. The method comprises the following steps: adding excess acid anhydride to perform carboxylating modification on an organic solvent lignin, and curing an epoxy resin with the remaining acid anhydride and the carboxylated lignin group to prepare the lignin anhydride cured epoxy resin. The whole preparation process is carried out in one pot, the preparation method has the characteristics of few process steps, low cost, greenness and environmental protection, and the obtained epoxy resin has the advantages of large lignin addition, uniform resin and good tensile properties, and has good industrial application prospects and values.
Owner:NANJING FORESTRY UNIV
High-ammonia-nitrogen wastewater reutilization zero-discharge treatment method and device of membrane aeration and membrane absorption coupling technology
ActiveCN105600995ASolve the hydrophilizationLow running costSpecific water treatment objectivesWater contaminantsHigh concentrationAeration system
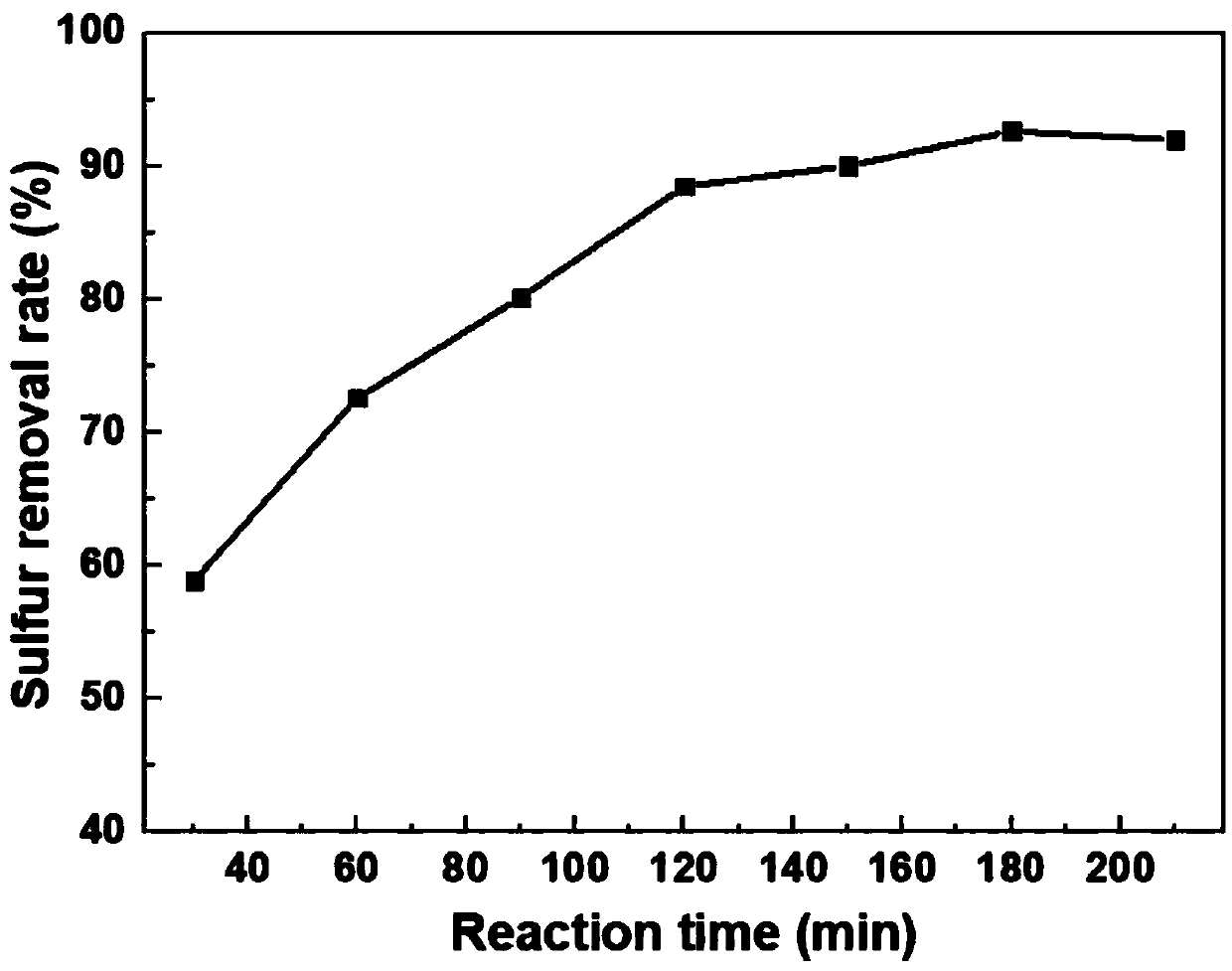
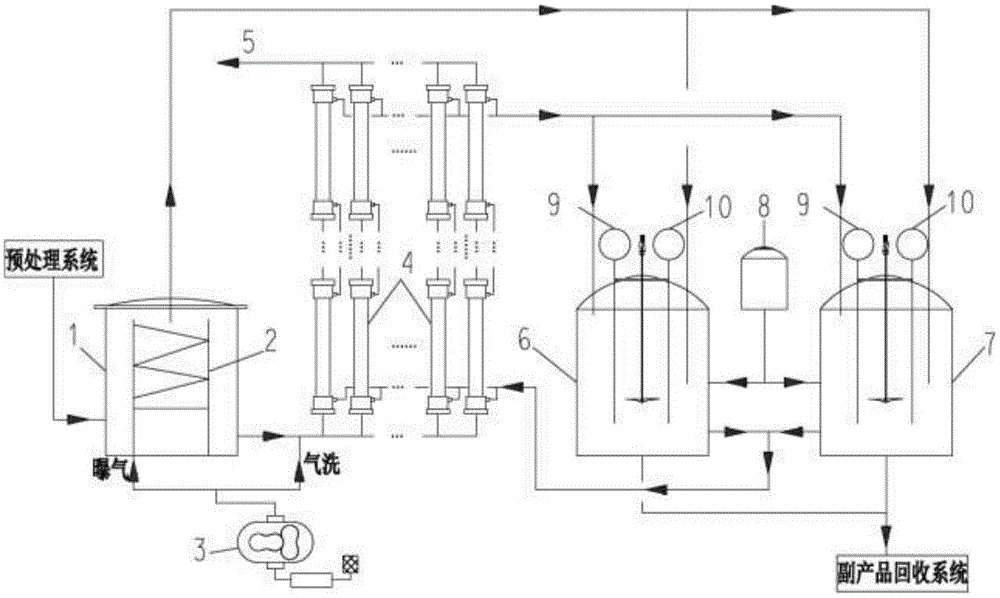
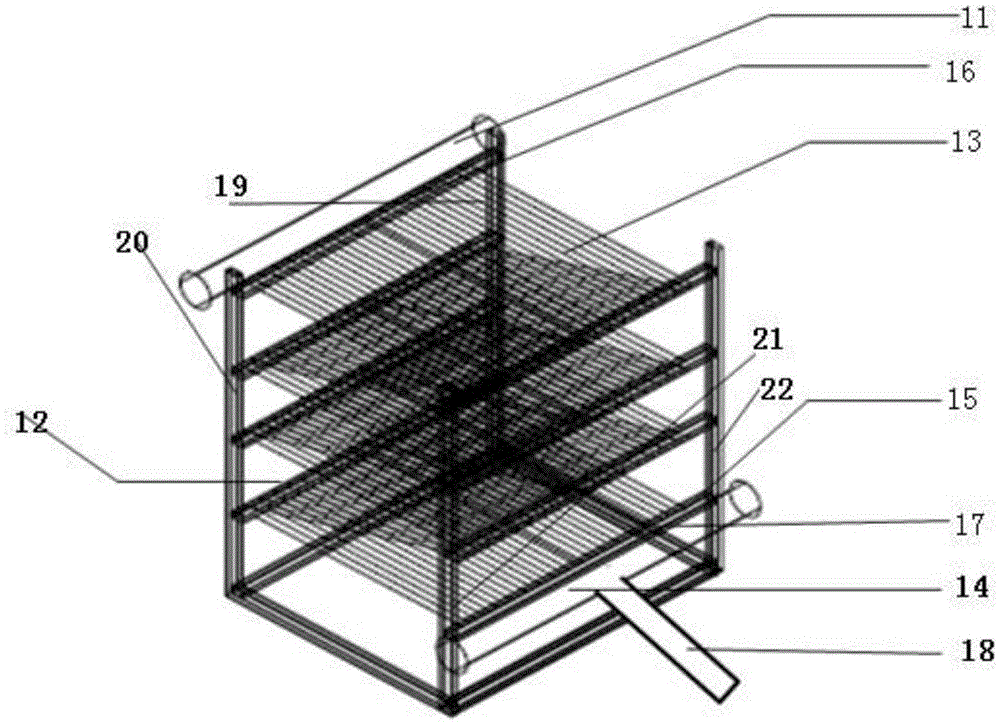
The invention discloses a high-ammonia-nitrogen wastewater reutilization zero-discharge treatment method and device of a membrane aeration and membrane absorption coupling technology and belongs to the field of environmental engineering. About 60% of ammonia nitrogen in ammonia-nitrogen wastewater is removed first in a membrane aeration mode, then about 99% of remaining ammonia nitrogen in the wastewater is removed in a membrane absorption multistage ammonia removal mode, and thus the high-ammonia-nitrogen raw water can be directly discharged after being treated; ammonia gas separated out from a membrane aeration system is directly introduced into an absorption liquid tank for the membrane absorption process to react with and remove excess acid in the absorption liquid tank; treated absorption liquid is introduced into a by-product recovery system to treat high-concentration absorption liquid. When the operating capability is reduced to 70% the original operating capability or long-time shutdown is needed, it is needed to start a cleaning-air purging system to maintain and clean the systems so that the systems can be kept stably operating for a long time.
Owner:BEIJING E & E TECH
Method for Pattern Formation Using Photoresist Cleaning Solution
Photoresist cleaning solutions are used to clean semiconductor substrates before or after an exposing step when photoresist patterns are formed. The cleaning solutions include H2O and a nonionic surfactant compound represented by Formula 1. By spraying the disclosed cleaning solutions on a surface of the semiconductor substrate before or after exposing step to form a photoresist pattern, the desired pattern only is obtained and unnecessary patterns generated in undesired regions by ghost images are avoided as excess acid generated by the photoacid generator is neutralized and removed and damage to unexposed portions of the photoresist polymer is avoided. wherein R1 and R2 are independently H, C1-C20 alkyl, C5-C25 alkyl aryl or C1-C10 ester; m is 1 or 2; n is an integer ranging from 10 to 300; and o is 0 or 1.
Owner:SK HYNIX INC
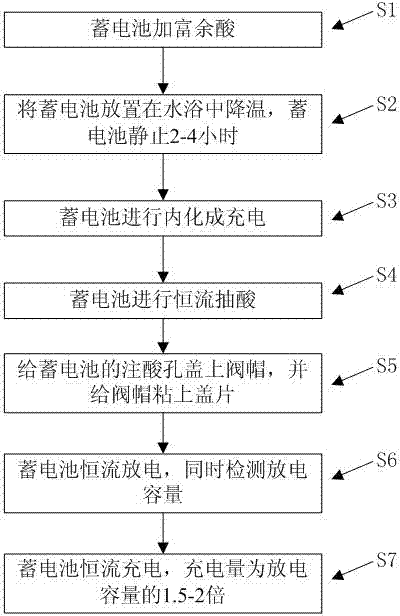
Valve-regulated lead acid battery inner formation technology capable of controlling battery separator saturation
ActiveCN103943888AEfficient formationFinal product manufactureSecondary cells charging/dischargingWater bathsEngineering
The invention discloses valve-regulated lead acid battery inner formation technology capable of controlling battery separator saturation. The valve-regulated lead acid battery inner formation technology comprises following steps: a storage battery is filled with excess acid, is subjected to cooling via water bath, is allowed to stand for 2 to 4h, is subjected to inner formation charging, and then is subjected to constant flow acid removing; an acid injection hole of the storage battery is covered with a valve bonnet, and the valve bonnet is glued with a cover plate; the storage battery is subjected to constant-current discharge, and discharge capacity is detected; and at last, the storage battery is subjected to constant current charge, wherein charging amount is 1.5 to 2 times of the discharge capacity. The valve-regulated lead acid battery inner formation technology is capable of controlling valve-regulated lead acid battery separator saturation to be optimal 95 to 98%, so that effective formation of gas compound channels is realized.
Owner:CHAOWEI POWER CO LTD
Method for manufacturing sulfonium salts
InactiveUS7060858B2Reduce yieldLow costOrganic chemistryOrganic compound preparationStrong acidsSulfonium
It is an object of the invention to provide a method which makes it possible to manufacture the desired sulfonium salts directly without using a metathesis process and without using acids in large excess amounts. An aryl compound (A) in which a hydrogen atom is bonded to at least one of the carbon atoms of the aryl group, and a sulfoxide compound (B) which can be expressed by the formula: R1 SO R2 (where R1 and R2 indicate hydrocarbon groups or heterocyclic groups which may be substituted, and which may be the same or different) are reacted in the presence of a strong acid (C) which can be expressed by the formula: HMXmYn (where M indicates an element of group IIIa or group Va of the periodic table, X indicates a halogen atom, Y indicates a hydroxyl group, m and n are integers which are such that m+n=4 and n=0 to 3 in cases where M is an element of group IIIa, and m and n are integers which are such that m+n=6 and n=0 to 2 in cases where M is an element of group Va).
Owner:SAN APRO
Preparation method of modified dextrin cigarette humectants
The invention relates to a preparation method of modified dextrin cigarette humectants. The preparation method includes steps of: (1) adding hydrochloric acid solution in 500g starch, mixing for 6 minutes, then reacting for 1-2 hours under 120-150 DEG C to obtain white solid, neutralizing excess acid to obtain dextrin, (2) blending the dextrin and deionized water into emulsion in 40%-70% concentration, regulating a pH value to 10.0-11.0, (3) heating up the emulsion to 40-60 DEG C, adding hydrogen peroxide dropwisely, and then reacting for 1-2 hours under constant temperature, (4) regulating the pH value to be neutral, (5) filtering, drying, and then obtaining the modified dextrin cigarette humectants. The starch can be starch of corn, potato, wheat and the like. The preparation method has the advantages that humidity preservation performance of tobacco is improved, and moreover the modified dextrin cigarette humectants are non-toxic.
Owner:SUZHOU GULI BIOTECH
Clean production method of bifunctional acrylic ester reactive diluent
ActiveCN105566113ANo outputMild reaction conditionsOrganic compound preparationCarboxylic acid esters separation/purificationNitrogen gasSolvent
The invention discloses a clean production method of a bifunctional acrylic ester reactive diluent. The clean production method is characterized by comprising the following specific steps: (1) performing esterification reaction: adding dihydric alcohol and acrylic acid at a molar ratio of dihydric alcohol to acrylic acid being 1:2 to 2.2 into a reactor, further adding an aqueous solvent, a catalyst, a polymerization inhibitor and an antioxidant subtractive agent, inletting air or a mixed gas of nitrogen and air from the bottom of the reactor, heating to 80-120 DEG C for refluxing, esterifying and separating water, detecting the acid value when substantially no water is separated out, and after a qualified standard is reached, cooling to 50 DEG C; (2) adding esterified water in the step 1, stirring and leaving to stand for layering, separating out an aqueous layer, as a raw material for next esterification reaction, containing the catalyst and a part of excess acid; (3) distilling the reaction solvent in vacuum, and detecting the acid value after finish of distillation; (4) removing the remaining acid through epoxide ring-opening reaction; (5) detecting product indexes. In the clean production method, neither the conventional processing method, comprising neutralizing and washing, nor the processing method, comprising neutralizing, adsorbing by using an adsorbent and filtering, is used. By the clean production method, both organic wastewater and solid chemical waste residues are not generated, so that the clean production method can completely meet clean production requirements in the industry; in the production process, no material loss is caused, and all processes are completed in the same reactor, so that the economic benefits are high.
Owner:HUNAN JINHAI SCI & TECH
System and Method for Managing All Cancers by Disabling the Cancer Cells Ability to Reproduce
PendingUS20190134228A1Avoid side effectsGood curative effectPeptide/protein ingredientsGeneral/multifunctional contrast agentsCancer cellOrganism
This invention provides tools and methods that prevent a cancer cell from growing and reproducing more cancer cells. When growth ceases the body's immune defenses are enabled to attack and destroy these cells if the cancer cell itself has not initiated its own natural apoptotic self-destruction processes. The tools and methods of the invention obstruct the metabolic adaptions required to support cancer growth. By addressing the increased rates of metabolism characteristic of all rapidly reproducing cancer cells using chemical and / or physical nanotechnology to identify, segregate, isolate these hypermetabolizing cells, the body's immune system and other natural defenses are empowered to further isolate and eliminate the diseased cells. The extreme growth rates required for their rapid reproduction involve massively increased rates of the biochemical reactions supporting the cancerous growth. Each excess reaction produces extra heat and raises the internal cell's temperature and the tissue space immediate to the rapidly growing cells. This heat signature is used as a primary biomarker that enables binding of a nanoviral particle engineered to migrate to at attach at the target site at the site and prevent the cell from continued metabolism. Preferably, the nanoparticle not only binds and blocks external membrane receptors on the target cell, but incorporates into the rapidly metabolizing cells additional metabolic blocking agents to stop their growth. When cell growth and proliferation are stopped, the body's natural defenses are able to segregate and eliminate these cells. The massively increased rates of metabolic reactions characteristic of cancer cells also produce excess acid. The decreased pH is useful as a secondary or confirmatory marker for identifying these cancer cells.
Owner:POSTREL RICHARD
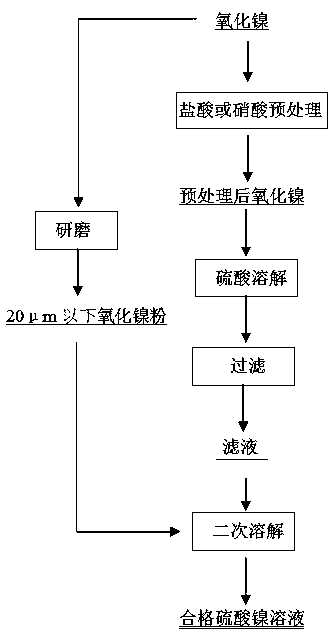
Method for producing nickel sulfate solution by taking nickel oxide as raw material,
The invention discloses a method for producing a nickel sulfate solution by taking nickel oxide as a raw material. The method comprises the following steps: (1) pretreating nickel oxide by adopting hydrochloric acid or nitric acid; (2) dissolving the nickel oxide obtained in the step (1) with sulfuric acid, and then carrying out solid-liquid separation to obtain a filtrate; and (3) adding nickel oxide powder into the filtrate obtained in the step (2) to obtain a nickel sulfate solution. According to the method disclosed by the invention, a surface dense oxidation film of the nickel oxide is pretreated by adopting hydrochloric acid or nitric acid, so that the time consumed by dissolving the nickel oxide by using sulfuric acid is shortened. The nickel oxide powder is adopted to neutralize excess acid, so that impurities are not introduced, meanwhile impurity components in the produced nickel sulfate solution are qualified, and the produced nickel sulfate solution can be used as an evaporation mother liquid needed by evaporation crystallization of nickel sulfate without the need of purification and impurity removal.
Owner:JINCHUAN GROUP LIMITED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com