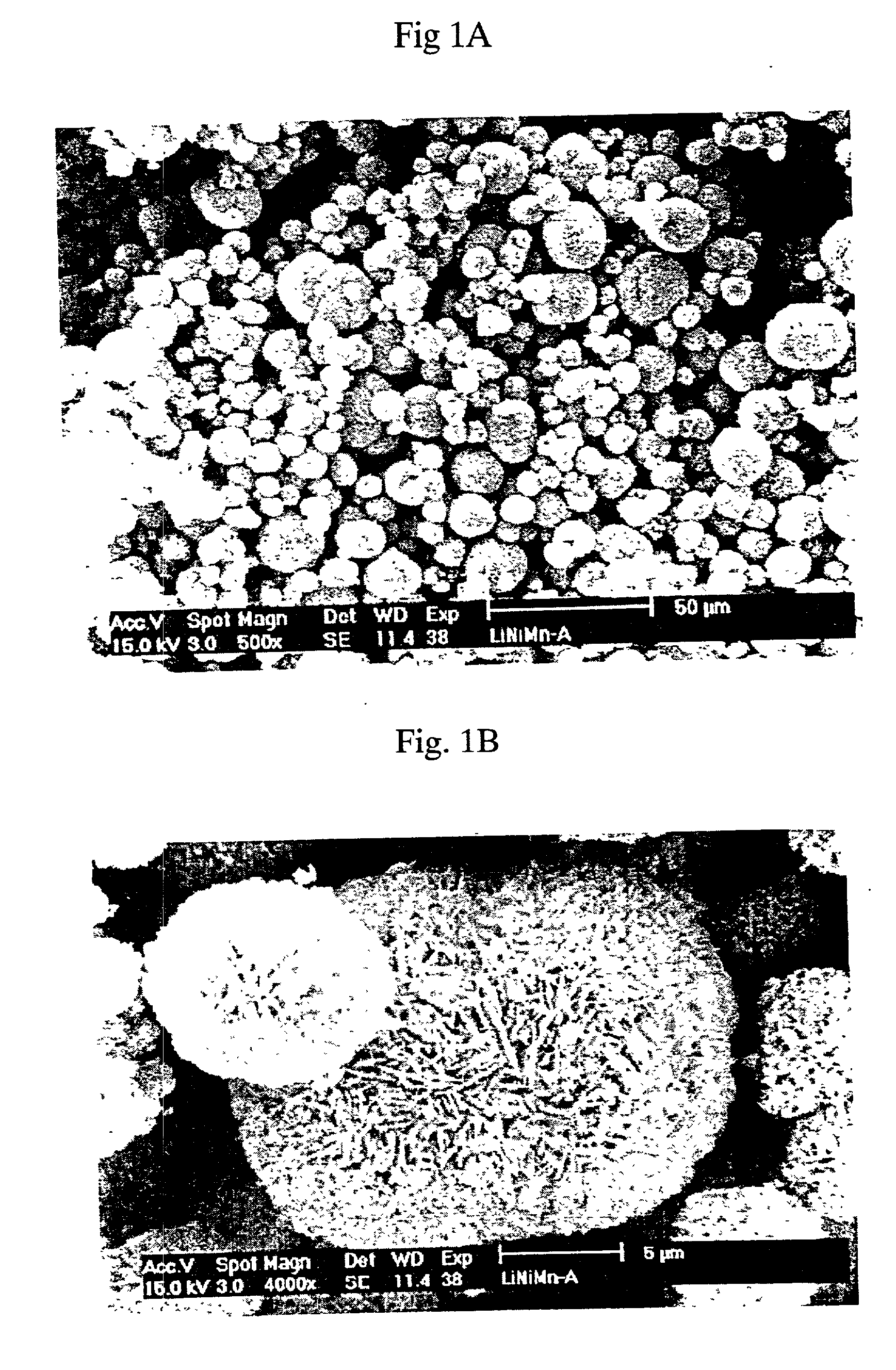
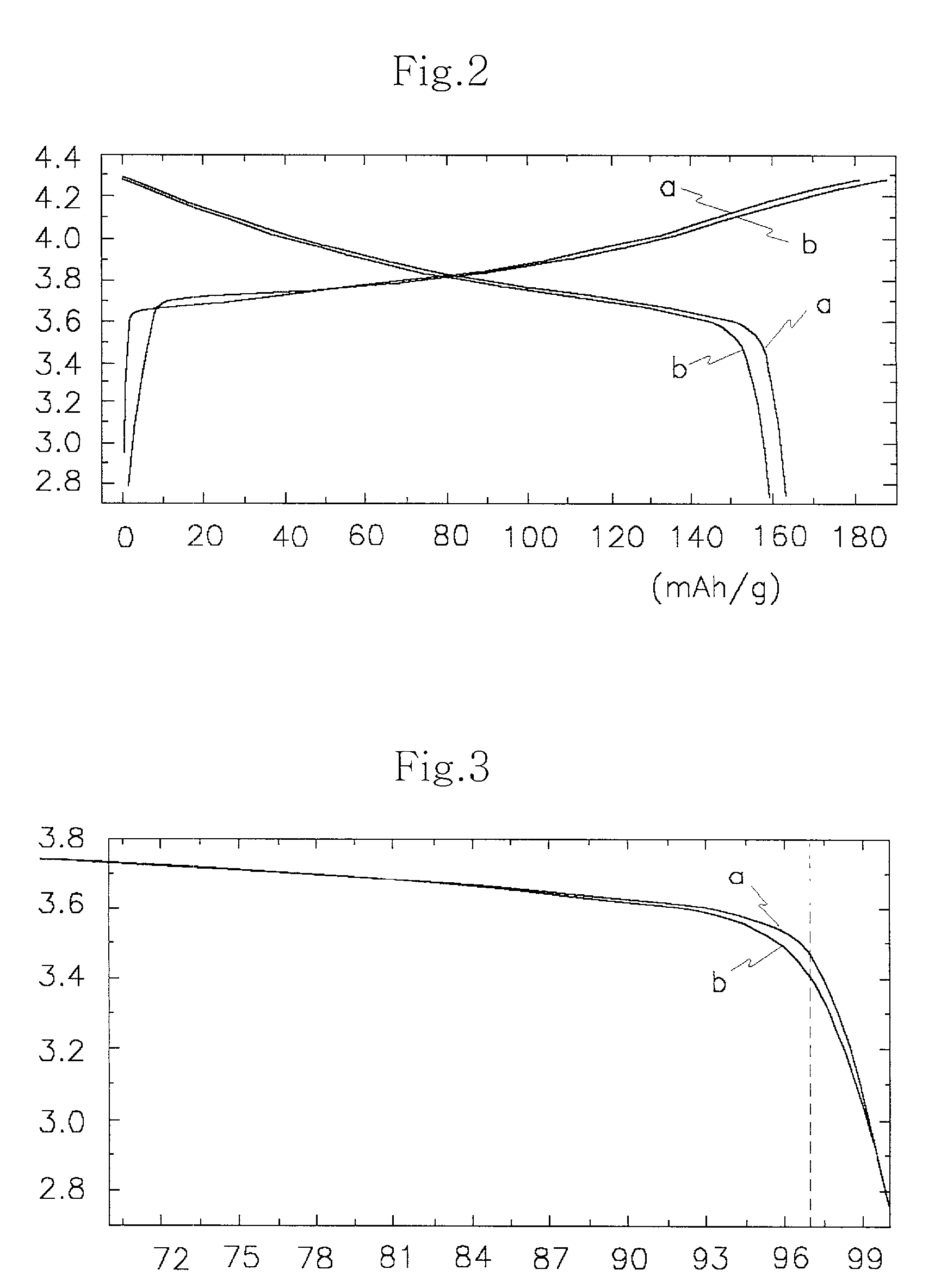
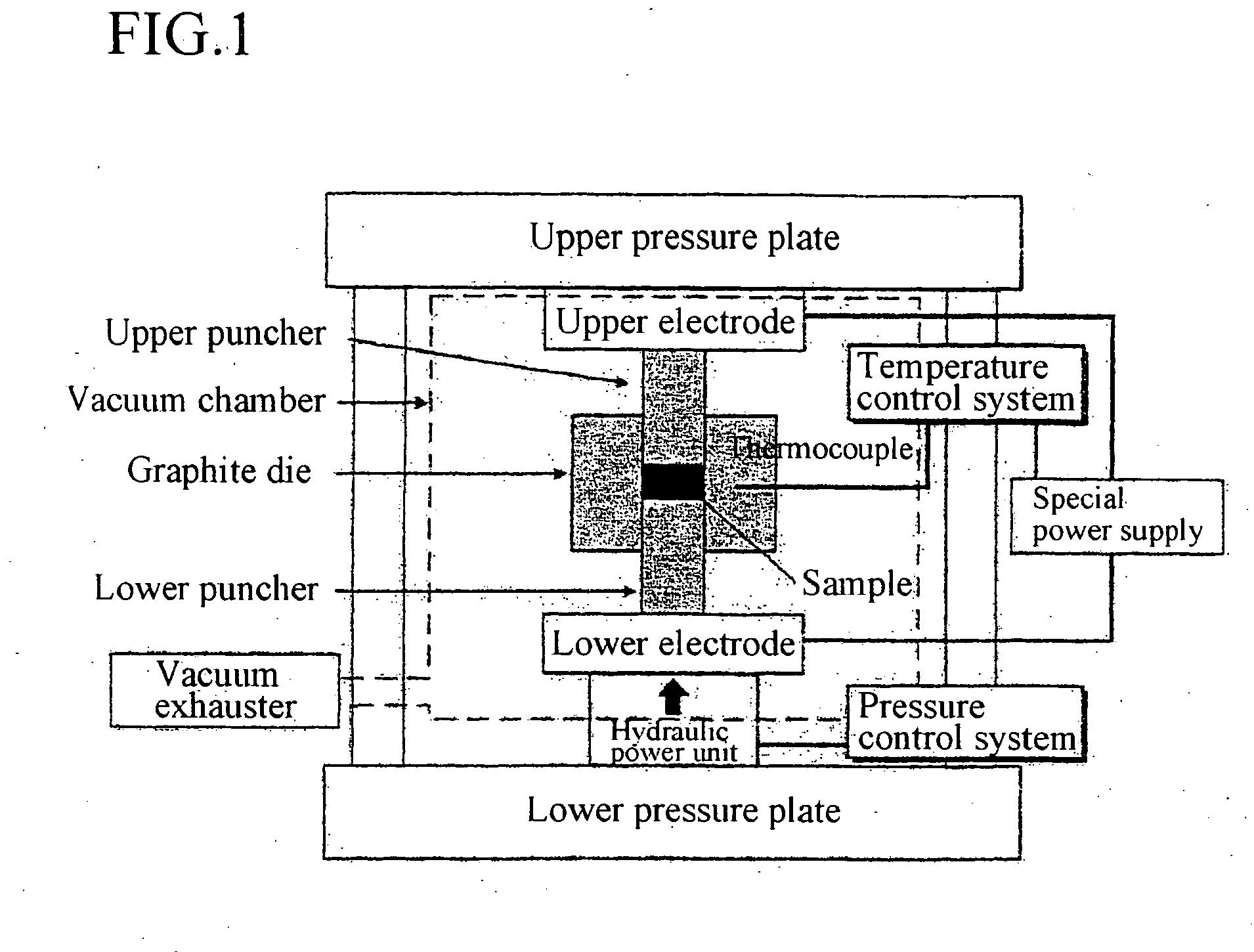
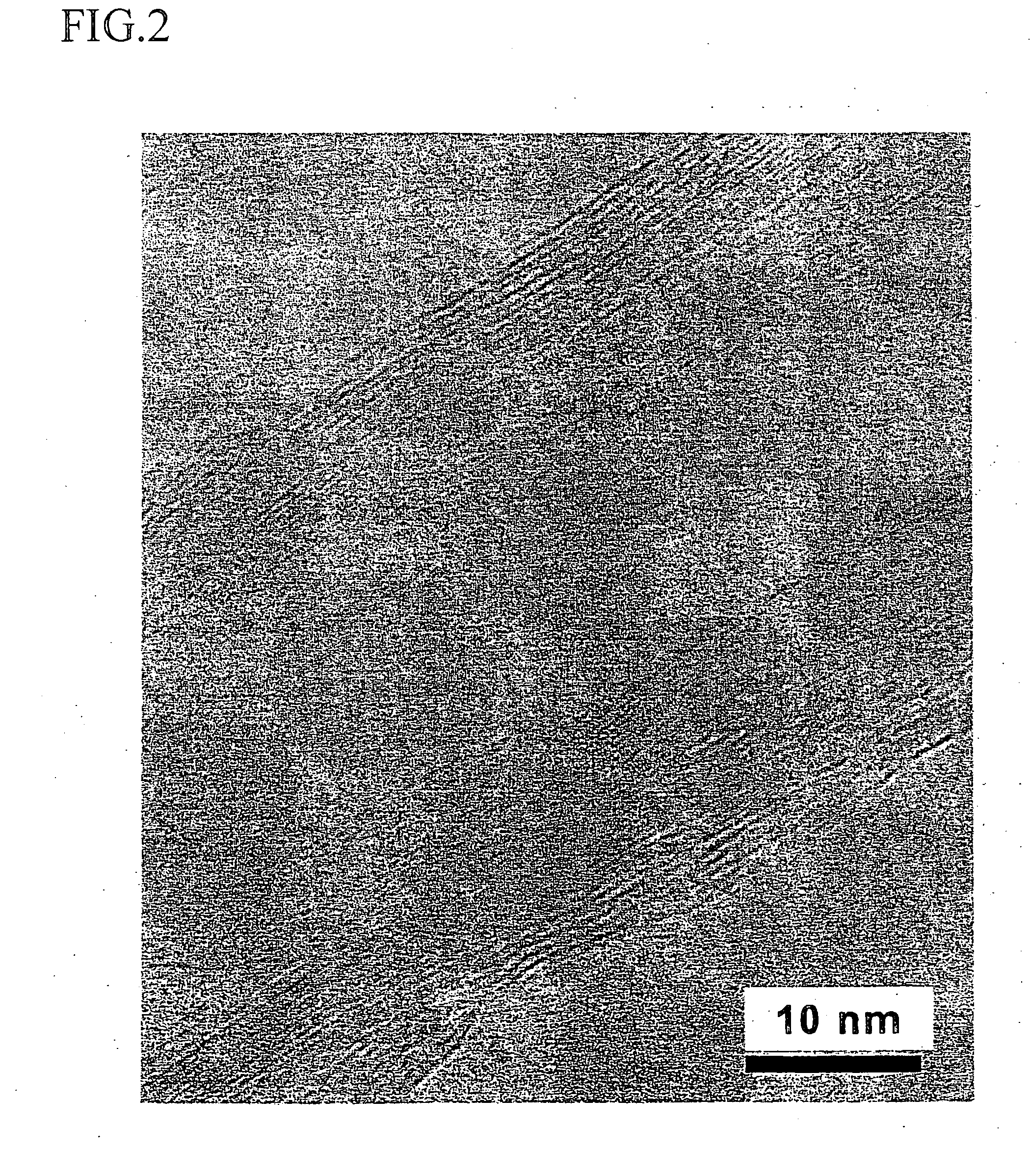
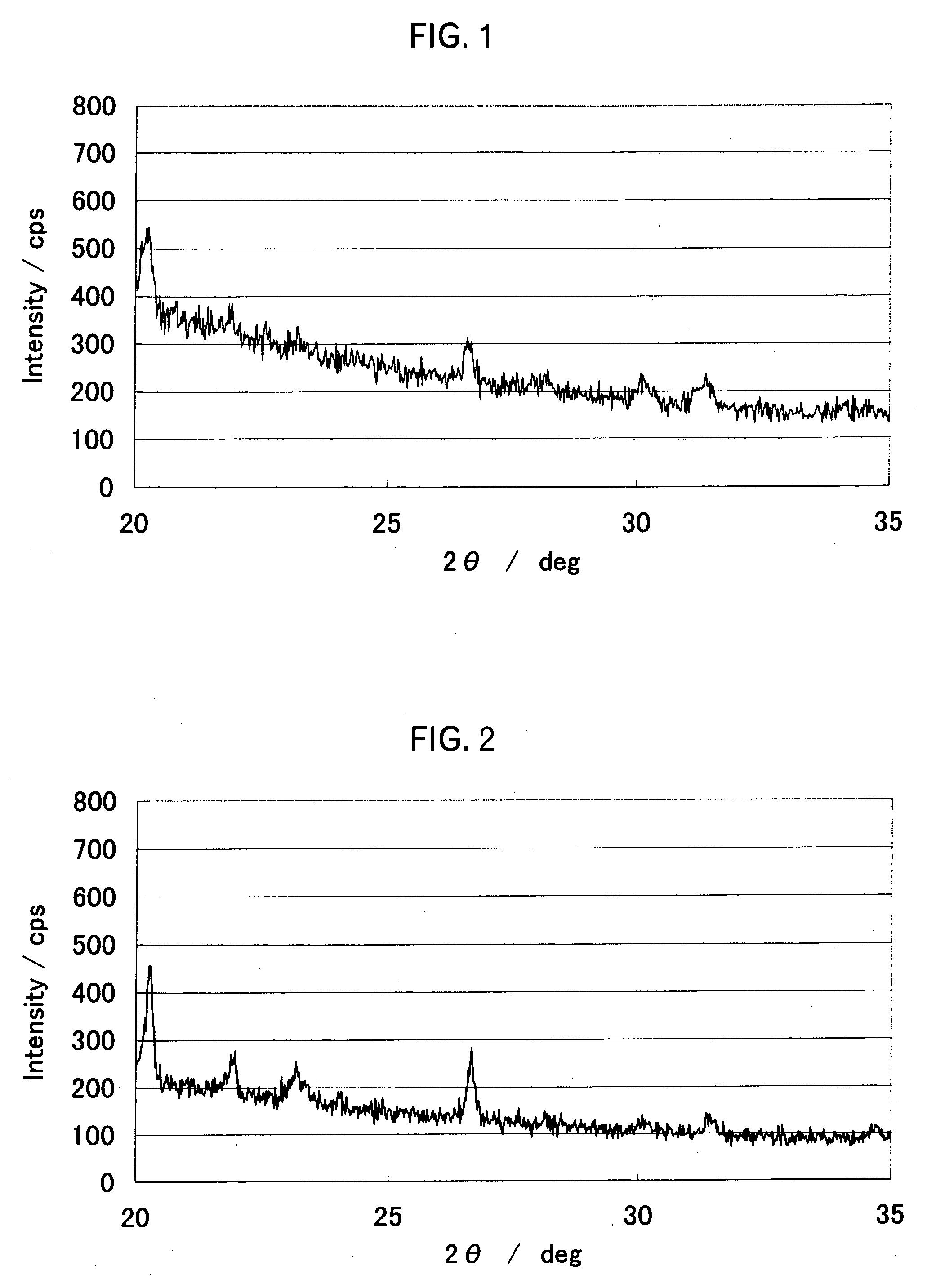
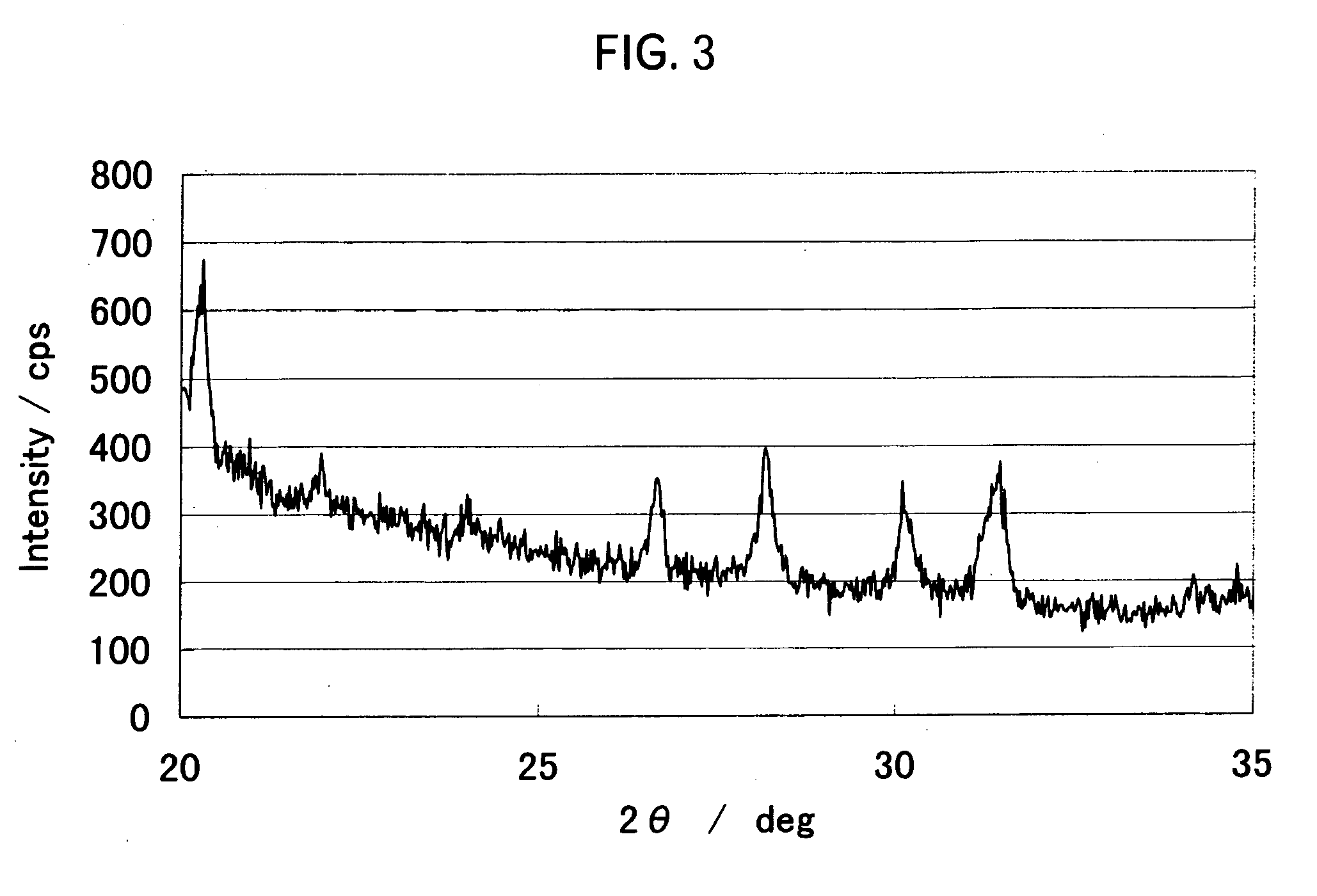
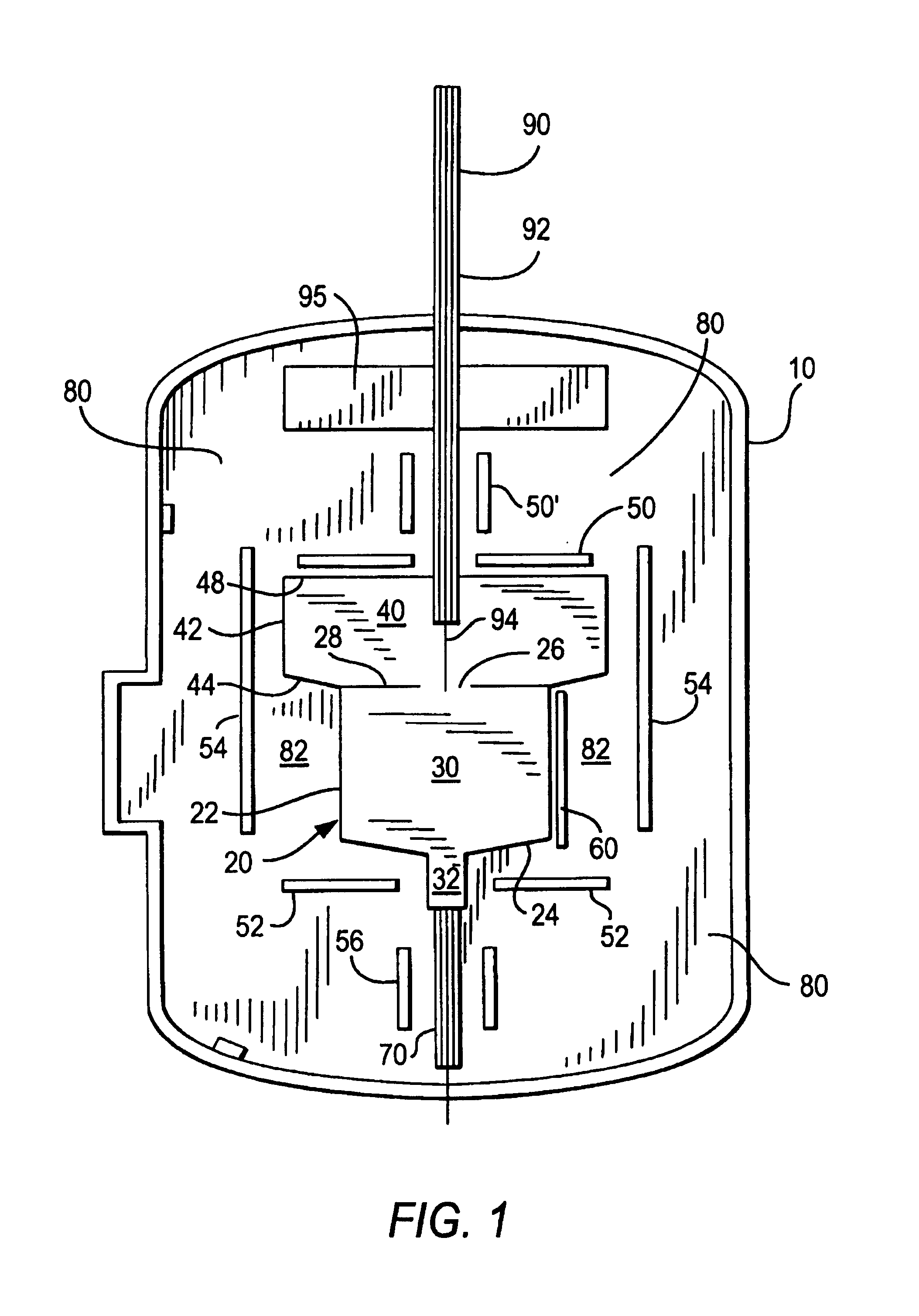
Patents
Literature
123results about "Halide preparation methods" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enhanced mercury control in coal-fired power plants
InactiveUS20030161771A1Enhanced capture of mercuryEliminating undesirable dischargeUsing liquid separation agentHalide preparation methodsPulverized fuel ashElemental mercury
A method of treating a coal combustion flue gas, which includes injecting a molecular halogen or thermolabile molecular halogen precursor able to decompose to form molecular halogen at flue gas temperature. The molecular halogen coverts elemental mercury to mercuric halide adsorbable by alkaline solids such as subbituminous or lignite coal ash, alkali fused bituminous coal ash capturable in whole or part by electrostatic precipitators (ESPs), baghouses (BHs), fabric filters (FFs), dry flue gas desulphurization solids, with or without subsequent adsorption by a liquid such as a flue gas desulphurization scrubbing liquor.
Owner:HAZELMERE RES
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
ActiveUS20110200508A1Electrolysis componentsLithium organic compoundsLithium carbonateLithium hydroxide
Owner:TERRALITHIUM LLC
Synthesis of bis(fluorosulfonyl)imide
The present invention provides methods for producing bis(fluorosulfonyl) compounds of the formula:F—S(O)2—Z—S(O)2—F Iby contacting a nonfluorohalide compound of the formula:X—S(O)2—Z—S(O)2—Xwith bismuth trifluoride under conditions sufficient to produce the bis(fluorosulfonyl) compound of Formula I, where Z and X are those defined herein.
Owner:SES HLDG PTE LTD
Positive active material for rechargeable lithium battery and method of preparing same
InactiveUS20020055042A1Improve thermal stabilityElectrode manufacturing processesZirconium compoundsPhysical chemistryLithium battery
Disclosed is a positive active material for a rechargeable lithium battery. The positive active material includes at least one compound represented by formulas 1 to 4 andl a metal oxide or composite metal oxide layer formed on the compound. <table-cwu id="TABLE-US-00001"> <number>1< / number> <tgroup align="left" colsep="0" rowsep="0" cols="3"> <colspec colname="OFFSET" colwidth="42PT" align="left" / > <colspec colname="1" colwidth="77PT" align="left" / > <colspec colname="2" colwidth="98PT" align="center" / > <row> <entry>< / entry> <entry>< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyF2< / entry> <entry>(1)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyS2< / entry> <entry>(2)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aFa< / entry> <entry>(3)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aSa< / entry> <entry>(4)< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> < / tgroup> < / table-cwu> (where M is selected from the group consisting of Co, Mg, Fe, Sr, Ti, B, Si, Ga, Al, Sc, Y, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Ac, Th, Pa, U, Np, IPu, Am, Cm, Bk, Cf, Es, Fm, Md, No and Lr, 0.95<=x<=1.1, 0<=y<=0.99, 0<=,z<=0.5, and 0<=a<=0.5)
Owner:SAMSUNG SDI CO LTD
Carbon Nanotubes Aggregate, Method for Forming Same, and Biocompatible Material
InactiveUS20070209093A1Material nanotechnologyNanostructure manufactureCarbon nanotubeCompatibilization
Disclosed are a carbon nanotube aggregate and a method for forming a carbon nanotube aggregate. An aggregate can be obtained by fluorinating the surfaces of carbon nanotubes. The method for forming a carbon nanotube aggregate is characterized by comprising a step for firing a plurality of fluorinated carbon nanotubes.
Owner:STELLA CHEMIFA CORP
Lithium-containing composite oxide and its production method
ActiveUS20090148772A1Large volume capacity densityImprove securityCell electrodesLithium compoundsAlkaline earth metalDischarge rate
The present invention provides a lithium-containing composite oxide for a positive electrode for a lithium secondary battery, which has a large volume capacity density and high safety, and excellent durability for charge and discharge cycles and charge and discharge rate property, and its production method.The lithium-containing composite oxide is represented by the general formula LipNxMyOzFa (where N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, Sn, alkaline earth metal elements and transition metal elements other than Co, Mn and Ni, 0.9≦p≦1.2, 0.965≦x<2.00, 0<y≦0.035, 1.9≦z≦4.2, and 0≦a≦0.05), wherein when a powder of the lithium-containing composite oxide is classified into small particles with an average particle size of 2 μm≦Ds50≦8 μm and large particles with an average particle size of 10 μm≦Dl50≦25 μm, a content of the small particles is from 15 to 40% by weight and a content of the large particles is from 60 to 85% by weight, and 0.01≦ys≦0.06, 0≦yl≦0.02 and 0≦yl / ys<1, where (ys) is a ratio of the M element in the above general formula in the small particles and (yl) is a ratio of the M element in the general formula in the Large particles.
Owner:SUMITOMO CHEM CO LTD
Nanocrystals in ligand boxes exhibiting enhanced chemical, photochemical, and thermal stability, and methods of making the same
ActiveUS7273904B2Improve stabilityEasy to detectMaterial nanotechnologyFrom normal temperature solutionsDendrimerCross-link
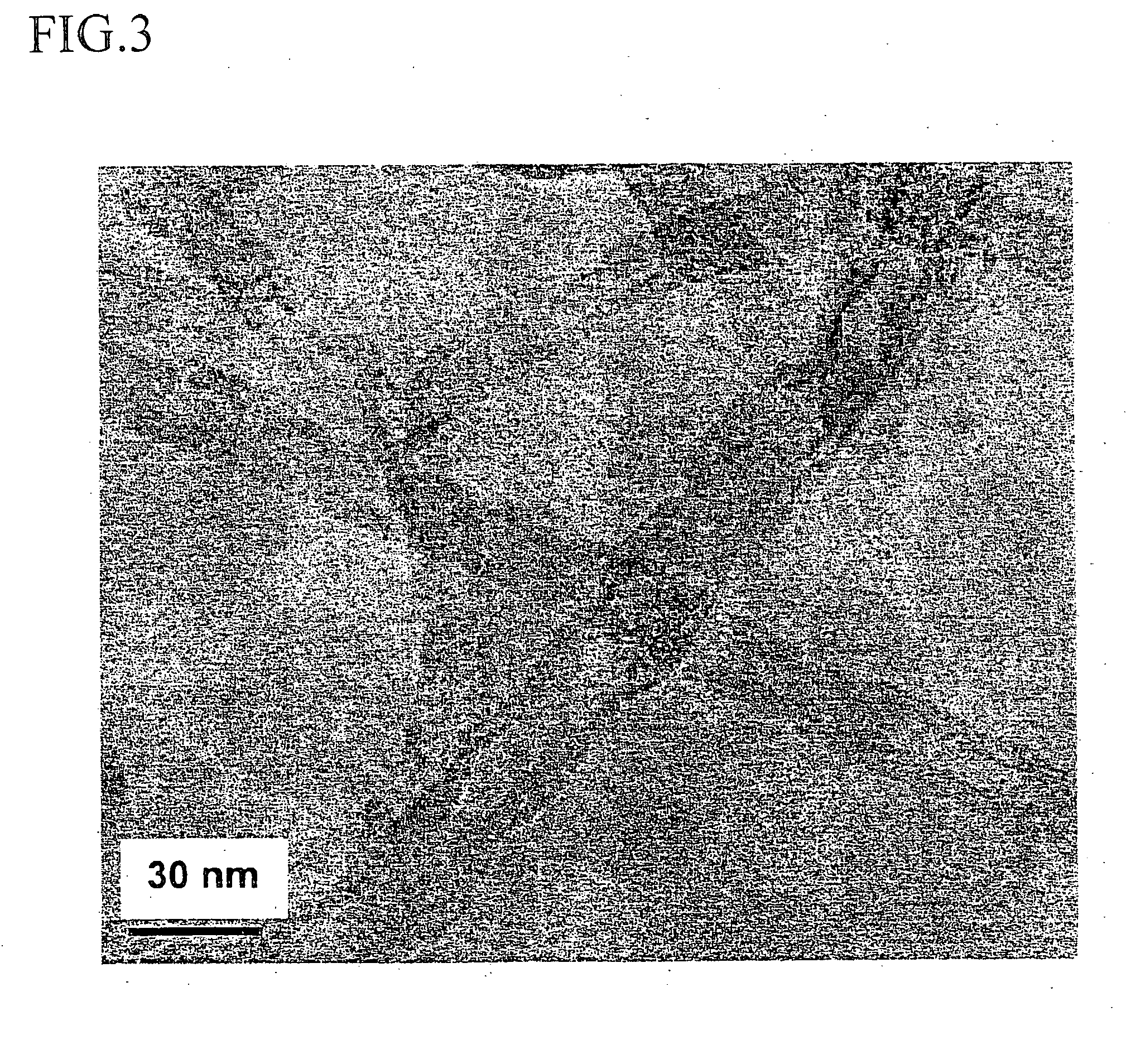
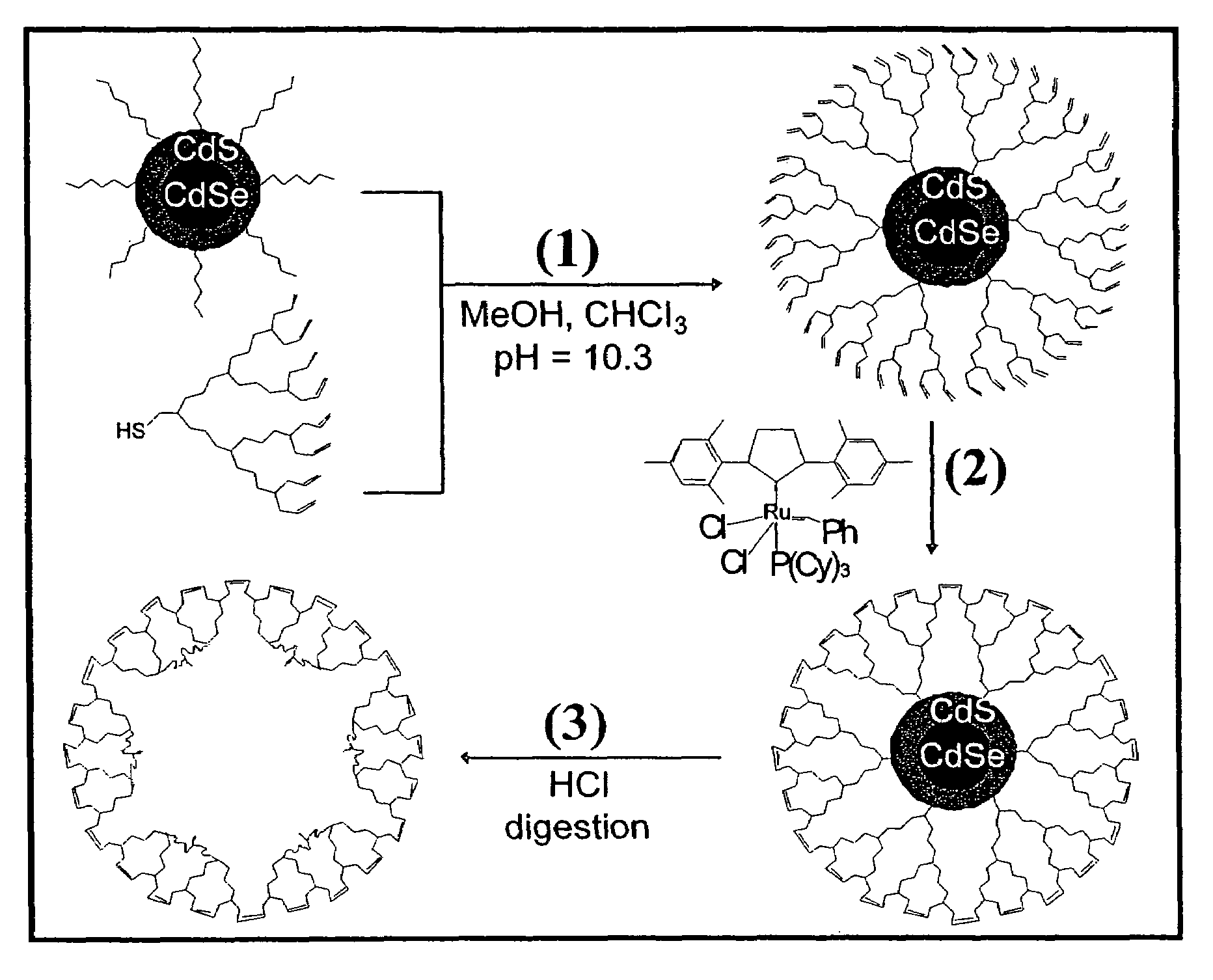
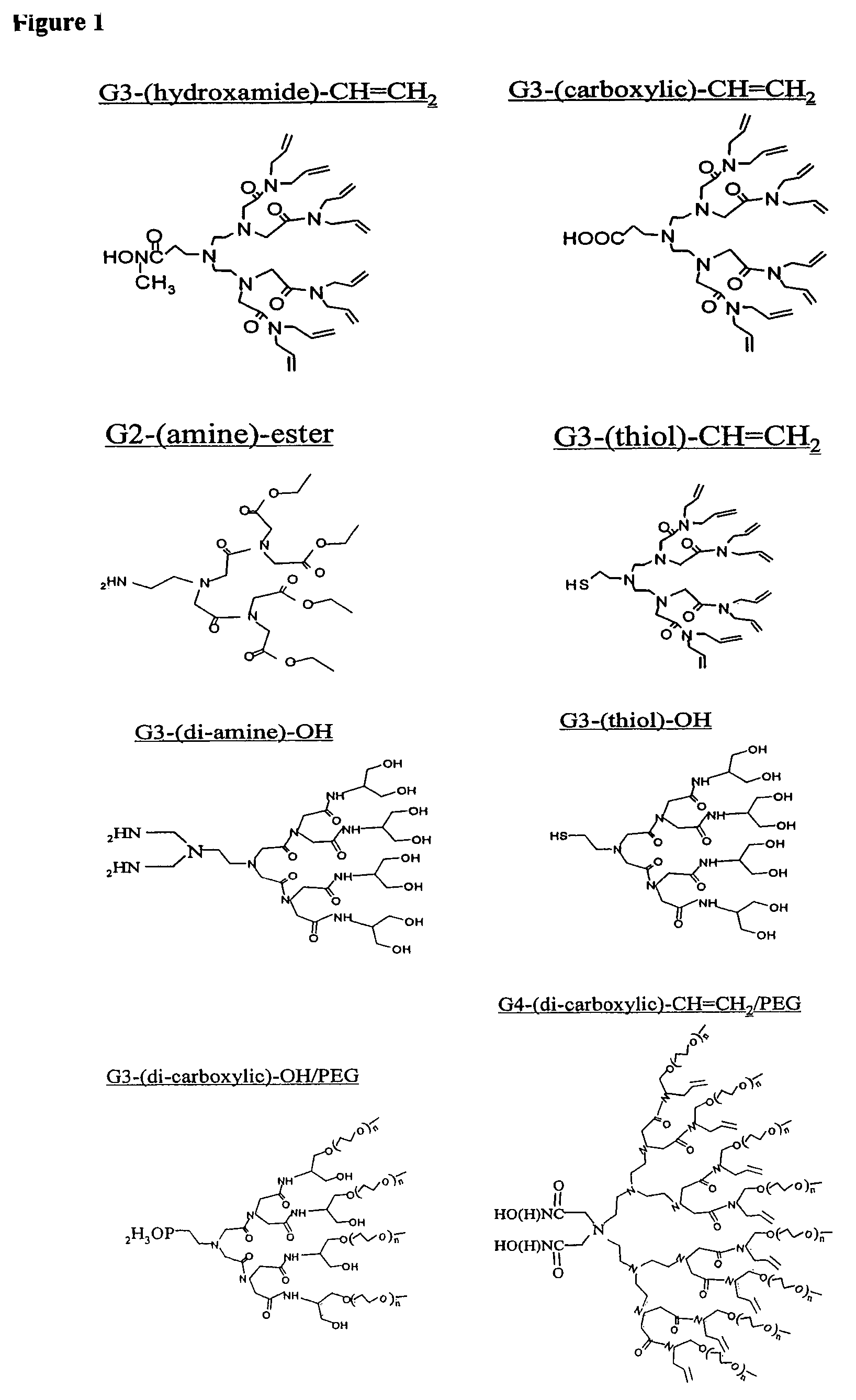
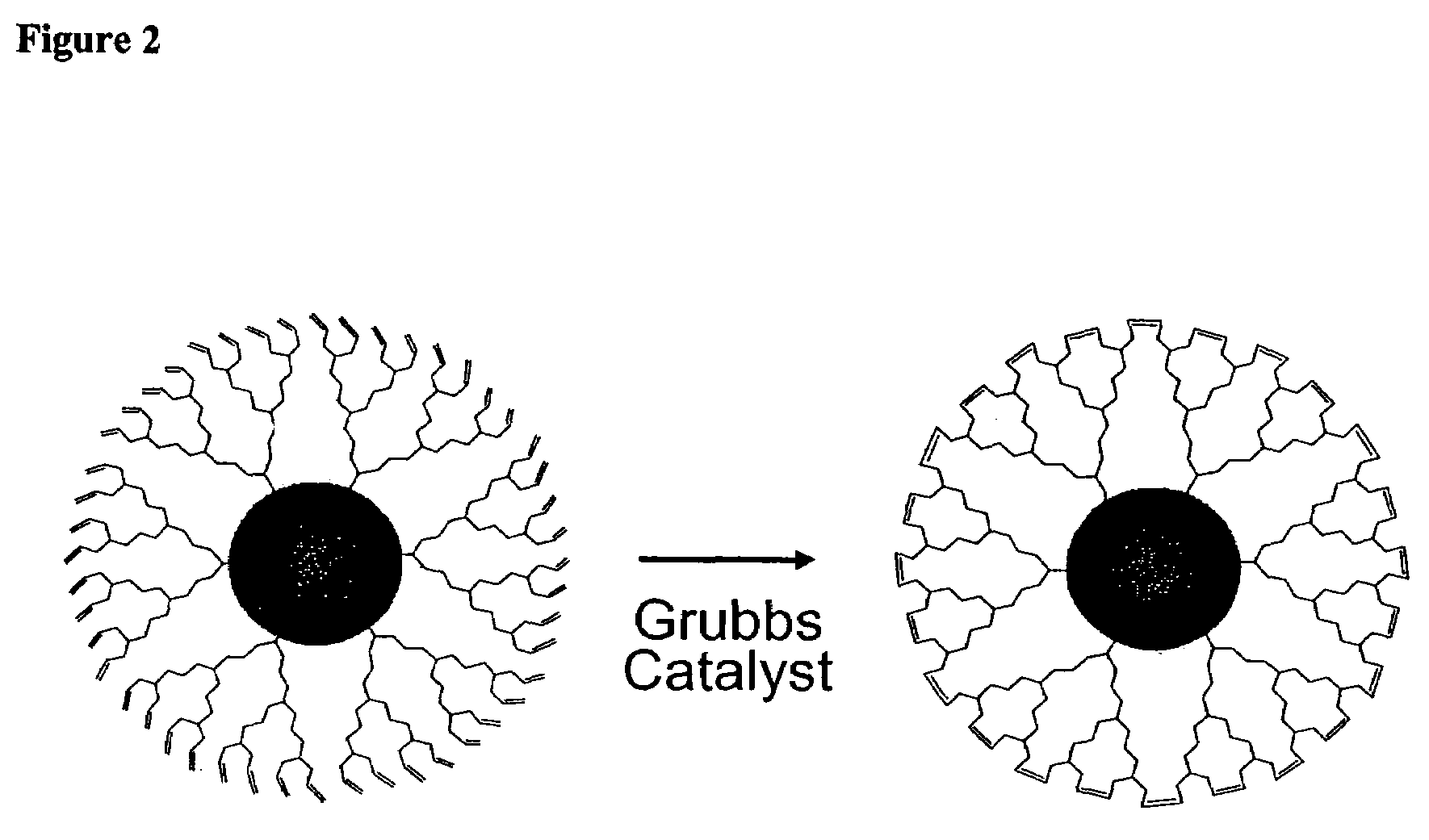
Dendron ligands or other branched ligands with cross-linkable groups were coordinated to colloidal inorganic nanoparticles, including nanocrystals, and substantially globally cross-linked through different strategies, such as ring-closing metathesis (RCM), dendrimer-bridging methods, and the like. This global cross-linking reaction sealed each nanocrystal within a dendron box to yield box-nanocrystals which showed dramatically enhanced stability against chemical, photochemical and thermal treatments in comparison to the non-cross-linked dendron-nanocrystals. Empty dendron boxes possessing a very narrow size distribution were formed by the dissolution of the inorganic nanocrystals contained therein upon acid or other etching treatments.
Owner:BEIJING JINGTAI MEIKANG BIOTECH CO LTC +1
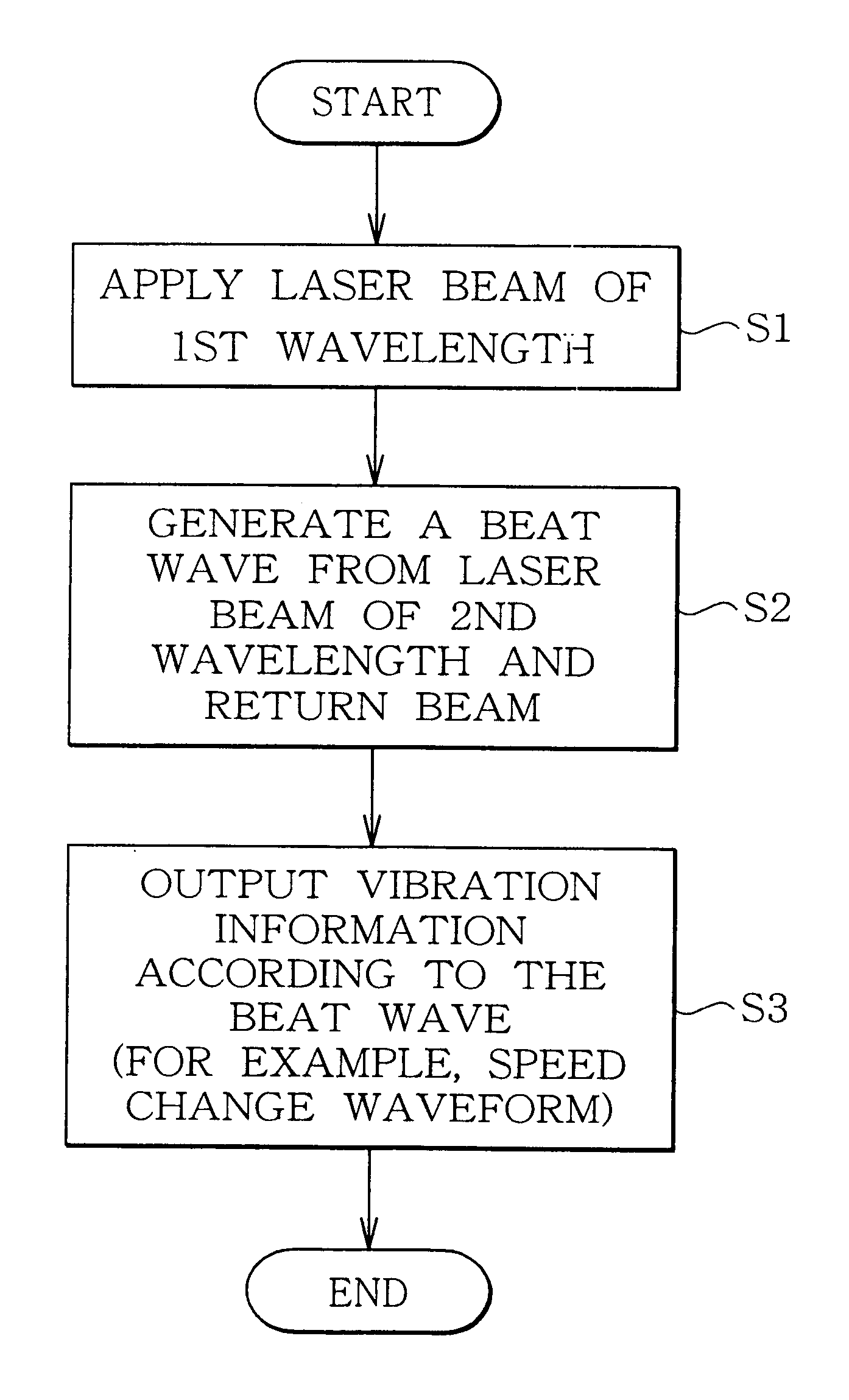
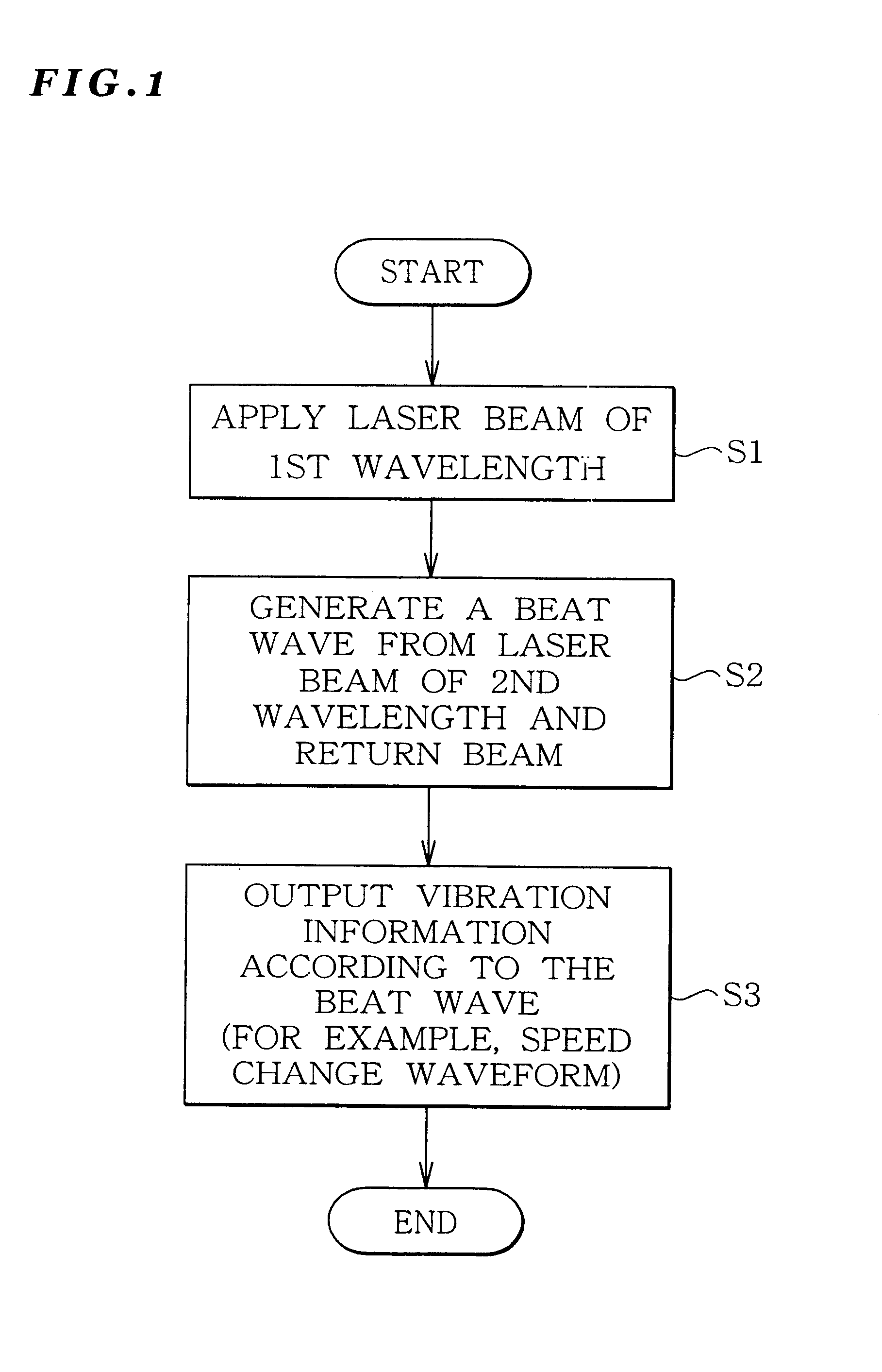
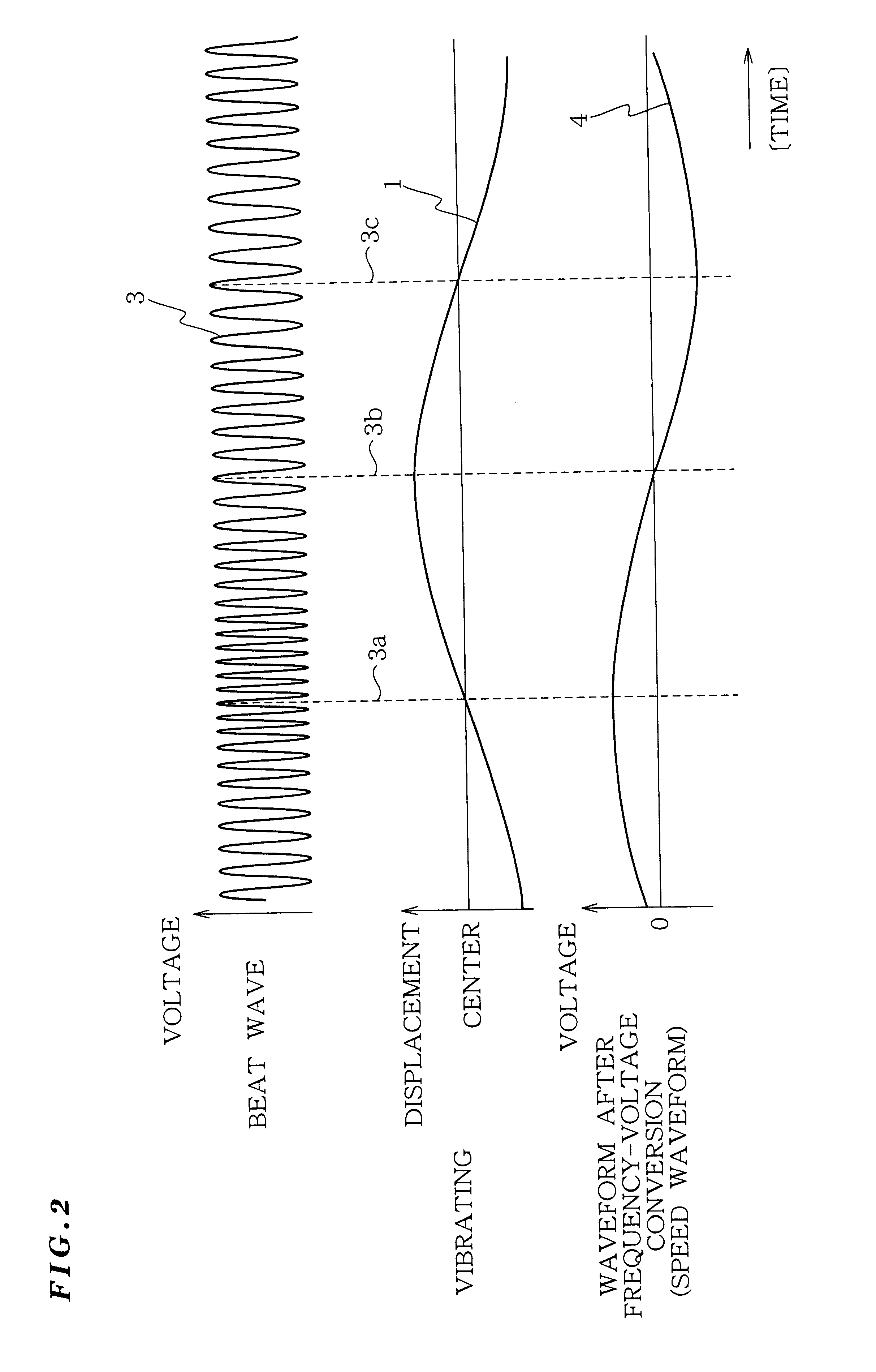
Vibration measurement method and apparatus
InactiveUS6301968B1Analysing solids using sonic/ultrasonic/infrasonic wavesSubsonic/sonic/ultrasonic wave measurementVibration measurementLight beam
The present invention provides a vibration measurement method and apparatus capable of accurately measuring displacement even if the displacement is very small. The vibration measurement method according to the present invention comprises: a laser beam application step (step S1) for applying a laser beam of a first wavelength to an object to be measured; a beat wave generation step (step S2) for mixing a laser beam of a second wavelength which is different from the first wavelength and the return beam reflected from the object to be measured; and a vibration information output step (step S3) for outputting the beat wave thus generated as a vibration information of the object to be measured.
Owner:SUZUKI MOTOR CORP
Process for producing nano-powders and powders of nano-particle loose aggregate
InactiveUS7238331B2Speed up the processSimple structureCalcium/strontium/barium carbonatesMaterial nanotechnologyPrillNanoparticle
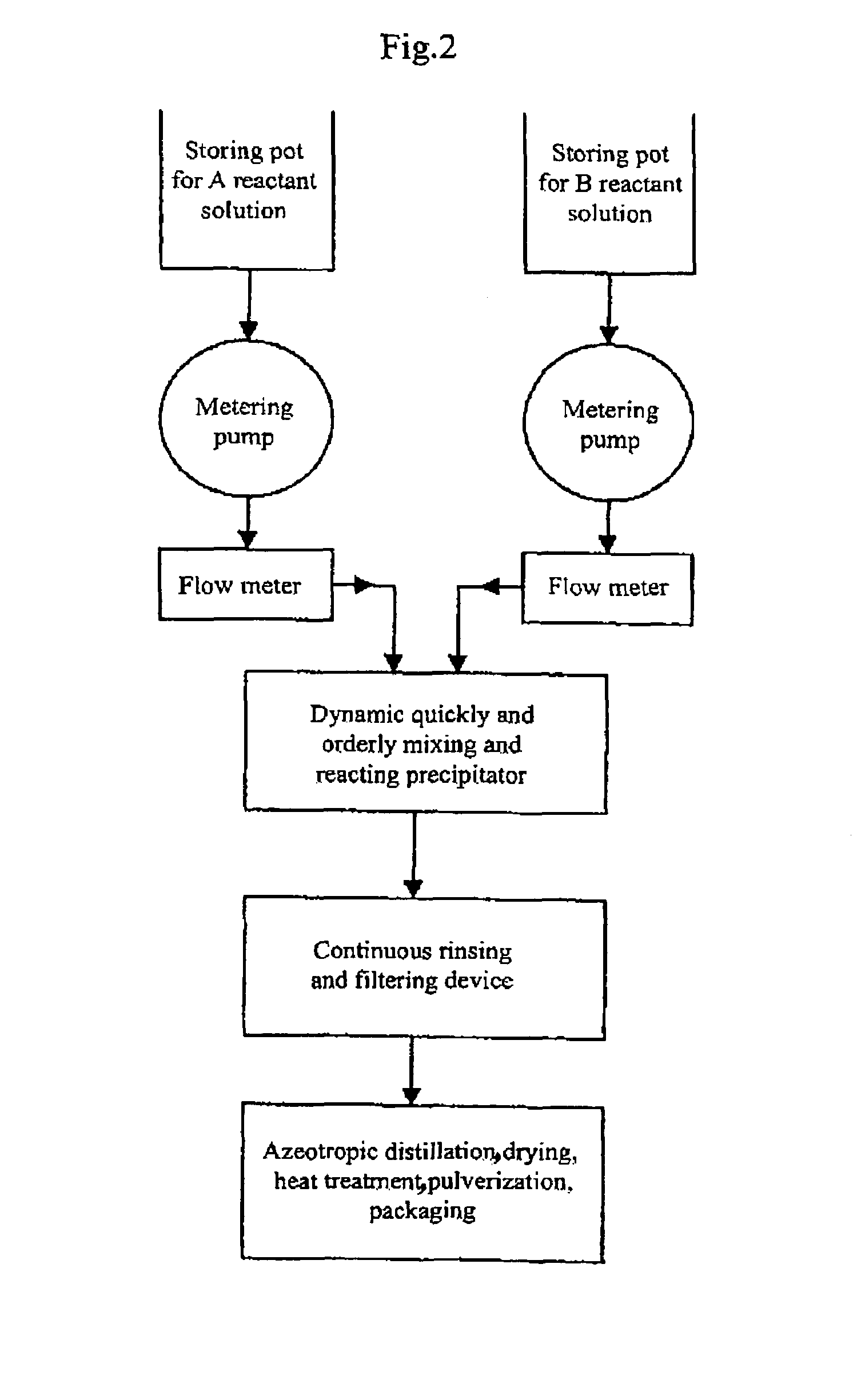
The present invention discloses a process for producing nano-powders and powders of nano-particle loose aggregate, which includes: (a) providing at least two reactant solutions A and B capable of rapidly reacting to form deposits; (b) supplying the at least two reactant solutions A and B at least at the reaction temperature into a mixing and reaction precipitator respectively, in which mixing reaction and precipitation are continuously carried out in sequence, the mixing and reaction precipitator being selected from at least one of a tubular ejection mixing reactor, a tubular static mixing reactor and an atomization mixing reactor; and (c) treating the deposit-containing slurry continuously discharged from the mixing reaction precipitator.
Owner:UNIV OF SCI & TECH LIAONING
Process for producing lithium-containing composite oxide for positive electrode for lithium secondary battery
ActiveUS20060154146A1Improve featuresSolve the small densityMagnesium halidesCell electrodesAlkaline earth metalNiobium
It is to provide a positive electrode active material for a lithium secondary battery, which has a large volume capacity density and high safety, is excellent in uniform coating properties and is excellent in the charge and discharge cyclic durability and low temperature characteristics even at a high charge voltage. A process for producing a lithium-containing composite oxide represented by the formula LipQqNxMyOzFa (wherein Q is at least one element selected from the group consisting of titanium, zirconium, niobium and tantalum, N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, alkaline earth metal elements and transition metal elements other than the Q element and the N element, 0.9≦p≦1.1, 0<q≦0.03, 0.97≦x<1.00, 0≦y<0.03, 1.9≦z≦2.1, q+x+y=1 and 0≦a≦0.02) from a lithium source, an Q element source and an N element source, and if necessary, at least one source selected from the group consisting of an M element source and a fluorine source, characterized by using as the Q element source an Q element compound aqueous solution having a pH of from 0.5 to 11.
Owner:SUMITOMO CHEM CO LTD
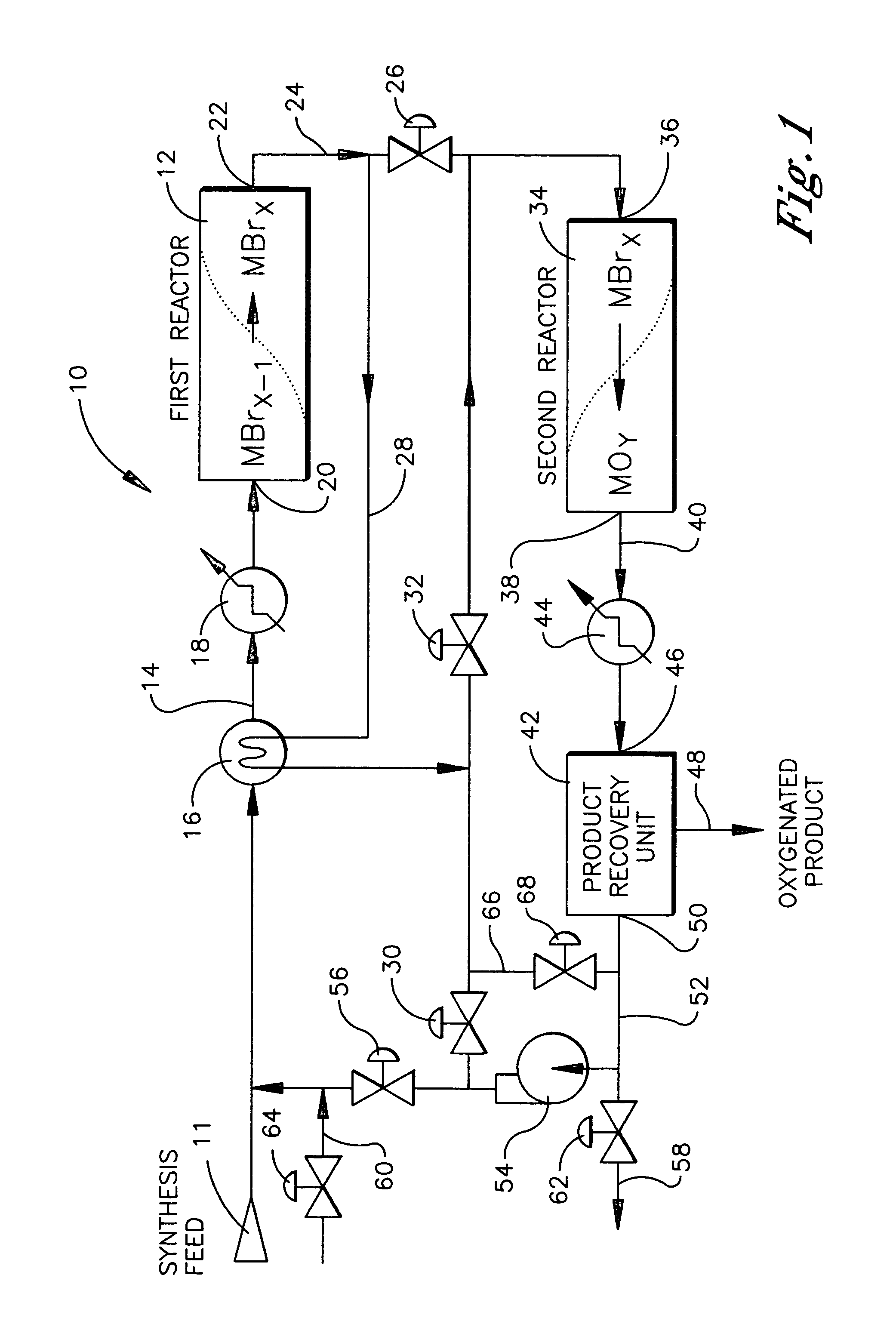
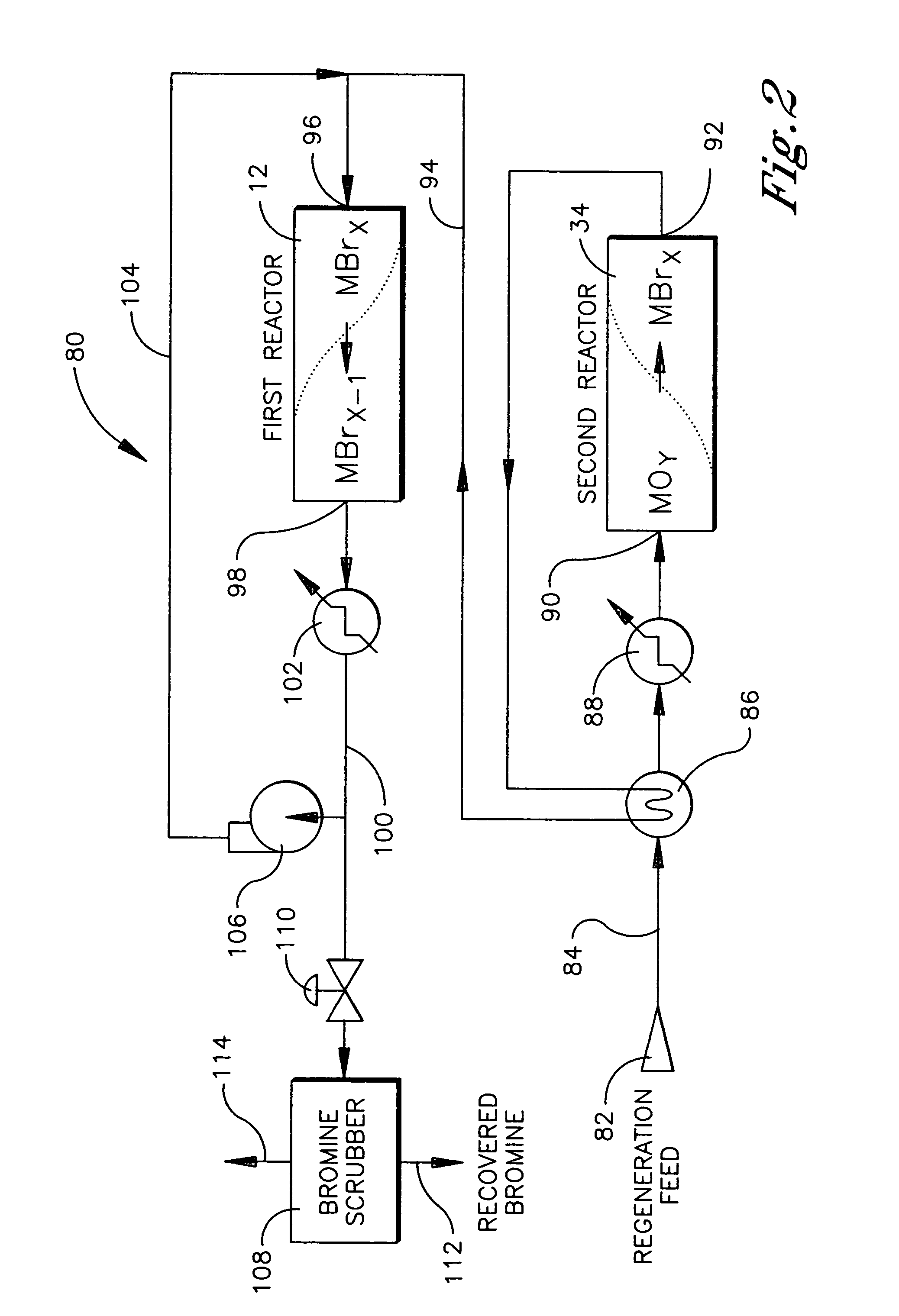
Conversion of alkanes to oxygenates
Owner:MARATHON OIL CO
Positive-electrode active material for non-aqueous electrolyte secondary battery and non-aqueous electrolyte secondary battery including the same
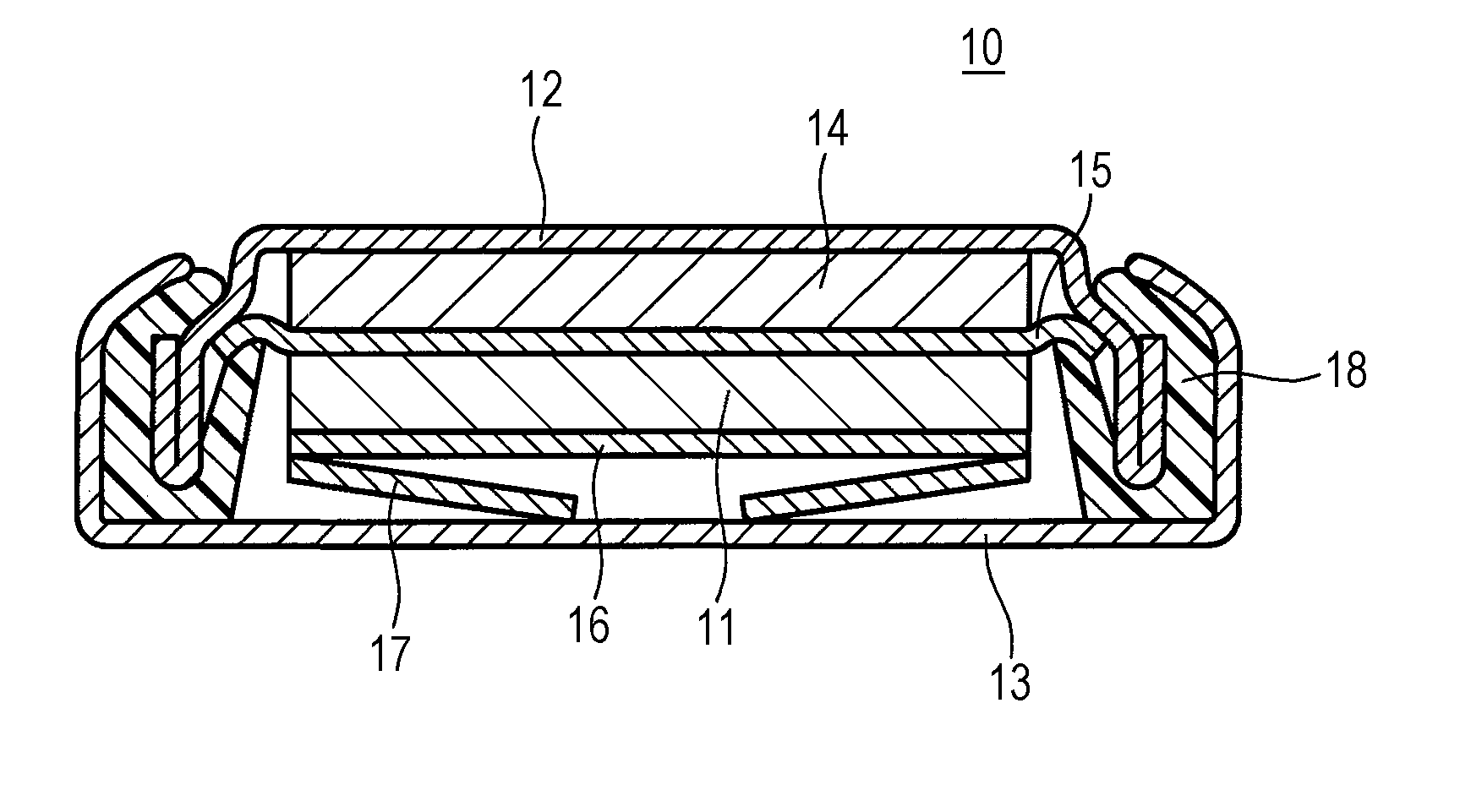
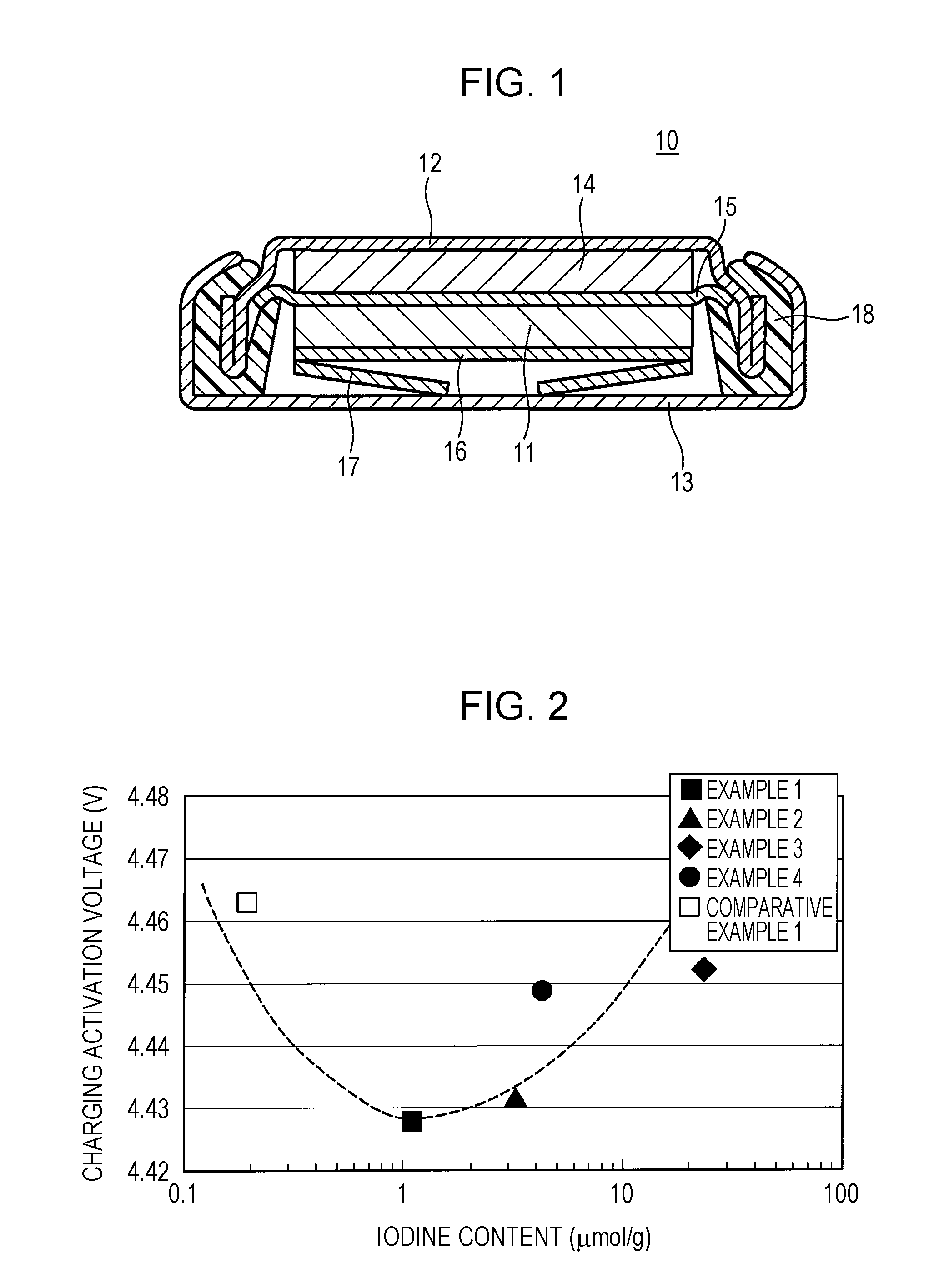
ActiveUS20150093646A1Improve battery performanceActivation voltageFinal product manufactureHalide preparation methodsBromineIodine
A positive-electrode active material for a non-aqueous electrolyte secondary battery according to the present disclosure contains a layered lithium(Li)-containing transition metal composite oxide that contains Li in the transition metal layer and more than 0.4 μmol / g and less than 25 μmol / g of iodine (I) or bromine (Br).
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
Method for forming a carbon nanotube aggregate
A carbon nanotube aggregate and a method for forming a carbon nanotube aggregate are provided. The carbon nanotube aggregate can be formed by treating carbon nanotubes with fluorine gas and sintering the resulting fluorinated carbon nanotubes. A carbon nanotube aggregate can be formed which does not contain a binder or resin matrix.
Owner:STELLA CHEMIFA CORP
Hydrogen sulfide abatement with scale control and/or well acidizing
InactiveUS6375907B1Avoid disadvantagesLow costHydrogen bromideLiquid degasificationHydrogen halidePower station
The emissions of hydrogen sulfide during the production of natural gas, oil or geothermal fluids from subterranean formations and the subsequent processing of these fluids is reduced by converting the hydrogen sulfide into a hydrogen halide or a halogen acid and then using the hydrogen halide or halogen acid for scale control and / or well acidizing. In a preferred embodiment, hydrogen sulfide produced with geothermal fluids is converted into hydrochloric acid, which is then used to reduce pH and control scale formation during the extraction of energy from geothermal fluids in a geothermal power plant.
Owner:UNION OIL OF CALIFORNIA
Quasi-One-Dimensional Polymers Based on the Metal-Chalcogen-Halogen System
InactiveUS20070274895A1Air stabilizationMaterial is straightforwardMaterial nanotechnologyPhysical/chemical process catalystsHalogenPhysical chemistry
The present Invention relates a quasi-one-dimensional material with sub-micron cross-section described by the formula M6CyHz, where the M=transition metal, C=chalcogen, H=halogen, and where y and z are integers such that 8.2<y+z<10, which materials are synthesized in a Single-step procedure at temperatures above 1000° C. The present invention also concerns the use of these materials in electronic, chemical, optical or mechanical applications.
Owner:INSTITUT JOZEF STEFAN
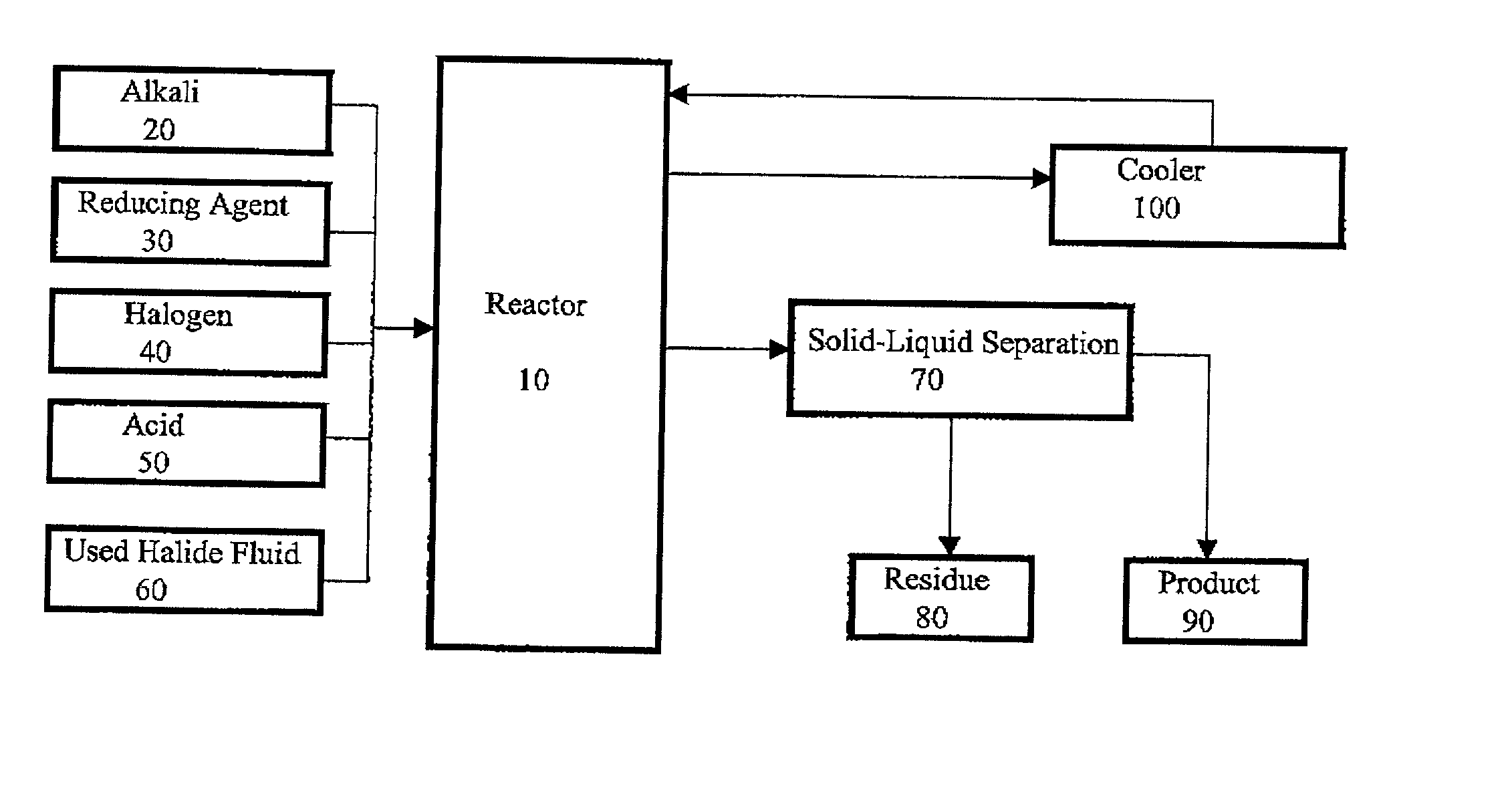
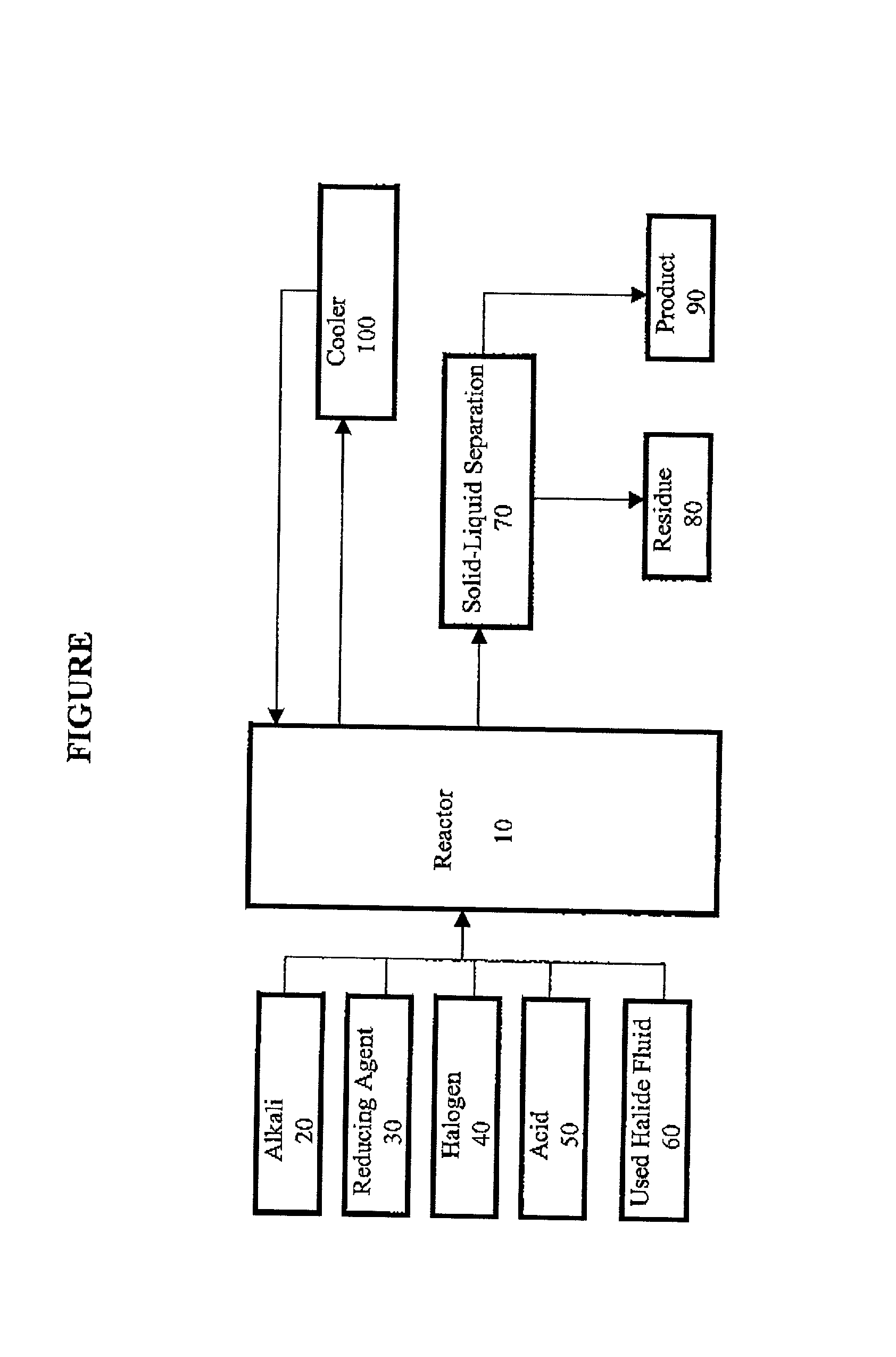
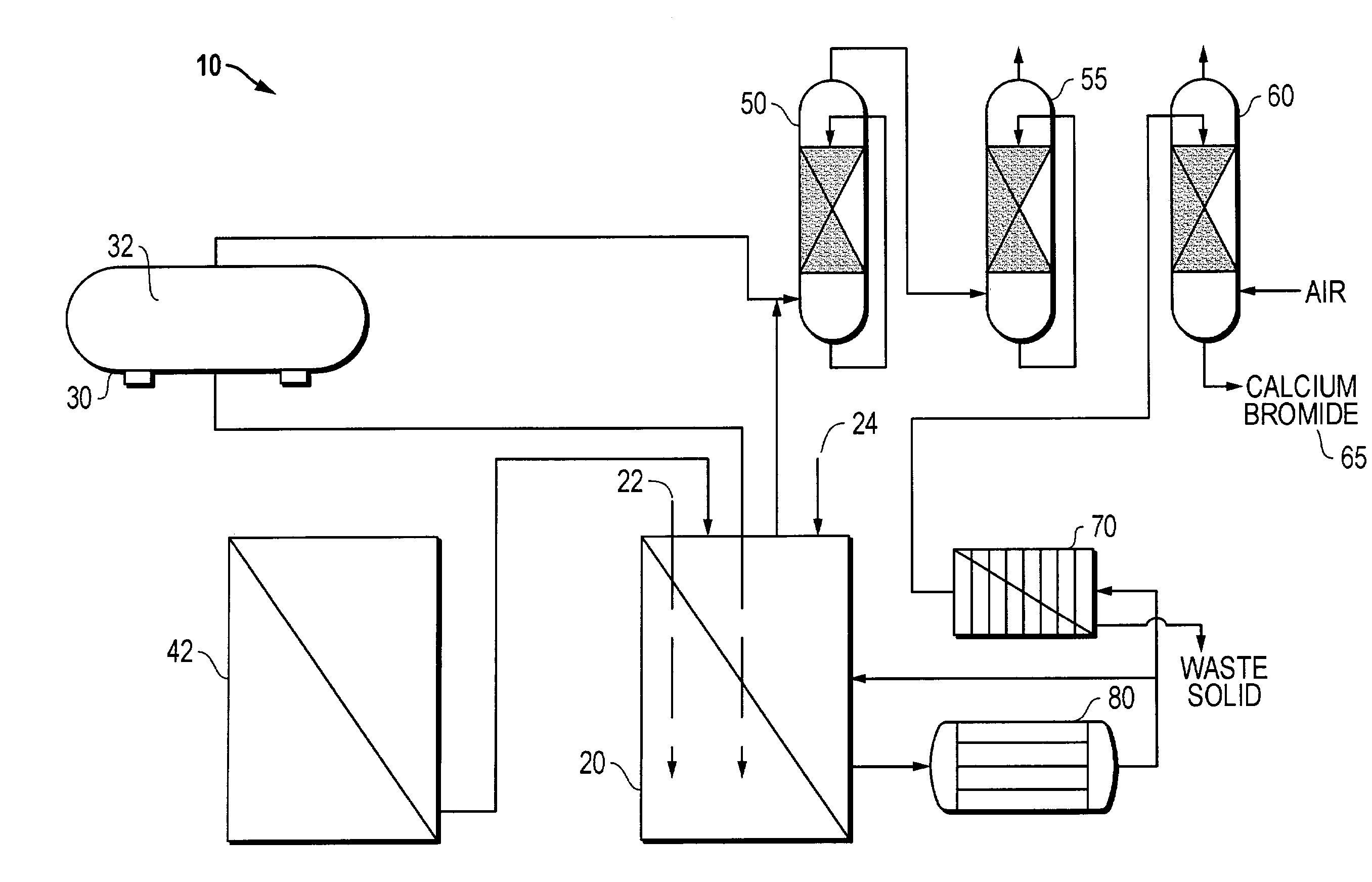
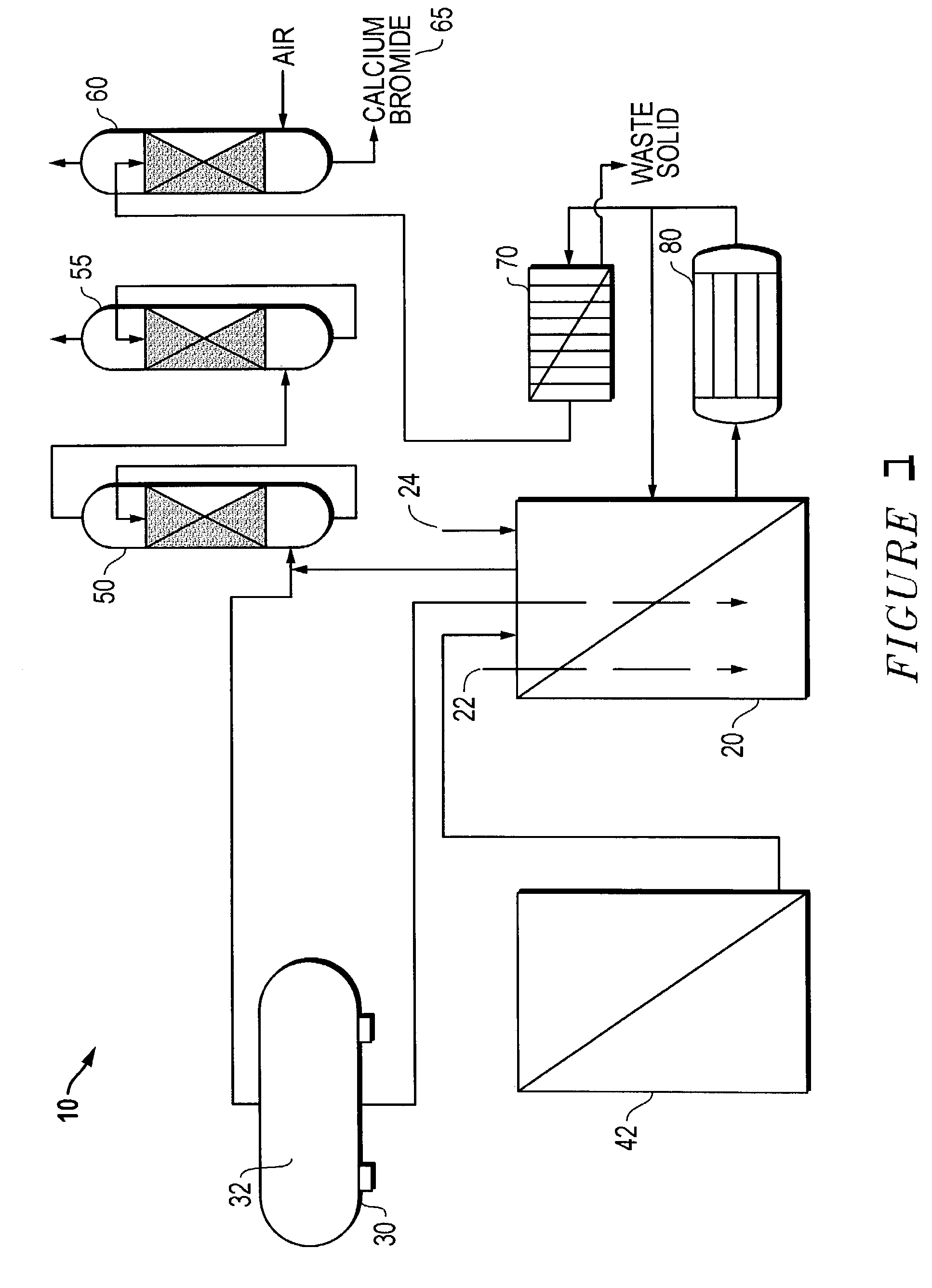
Method for regeneration of used halide fluids
InactiveUS20020130090A1Semi-permeable membranesHalide preparation methodsHalogenCrystallization temperature
A method for regenerating a used halide fluid comprising a density greater than 9.0 lbs / gal. and containing both soluble and insoluble impurities. This method comprises the steps of (1) adding acid to the used halide fluid so that the pH is within a range of approximately 0 to 10.0; (2) contacting the used halide fluid with halogen to increase the density to at least 10.0 lbs. / gal., adjust the desired true crystallization temperature of the fluid and oxidize soluble impurities; (3) adding a reducing agent while maintaining the temperature at a minimum of 10.degree. C.; (4) contacting the fluid with an alkali to neutralize excess acid; and (5) separating any suspended solid impurities from the fluid.
Owner:TETRA TECH INC
Positive active material for rechargeable lithium battery and method of preparing same
InactiveUS6737195B2Improve thermal stabilityElectrode manufacturing processesZirconium compoundsMaterials scienceMetal
Disclosed is a positive active material for a rechargeable lithium battery. The positive active material includes at least one compound represented by formulas 1 to 4 and a metal oxide or composite metal oxide layer formed on the compound.(where M is selected from the group consisting of Co, Mg, Fe, Sr, Ti, B, Si, Ga, Al, Sc, Y, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No and Lr, 0.95<=x<=1.1, 0<y<=0.99, 0<=z<=0.5, and 0<=A≤0.5).
Owner:SAMSUNG SDI CO LTD
Methods and compositions for the treatment of cancer
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Clean method for preparing layered double hydroxides
ActiveUS20080170978A1Efficient responseProtect environmentLithium compoundsManganese oxides/hydroxidesFiltrationCleaning methods
Disclosed is a clean method for preparing layered double hydroxides (LDHs), in which hydroxides of different metals are used as starting materials for production of LDHs by atom-economical reactions. The atom efficiency of the reaction is 100% in each case because all the atoms of the reactants are converted into the target product since only M2+(OH)2, M3+(OH)3, and CO2 or HnAn− are used, without any NaOH or other materials. Since there is no by-product, filtration or washing process is unnecessary. The consequent reduction in water consumption is also beneficial to the environment.
Owner:BEIJING UNIV OF CHEM TECH
Process for producing high-purity silicon and apparatus
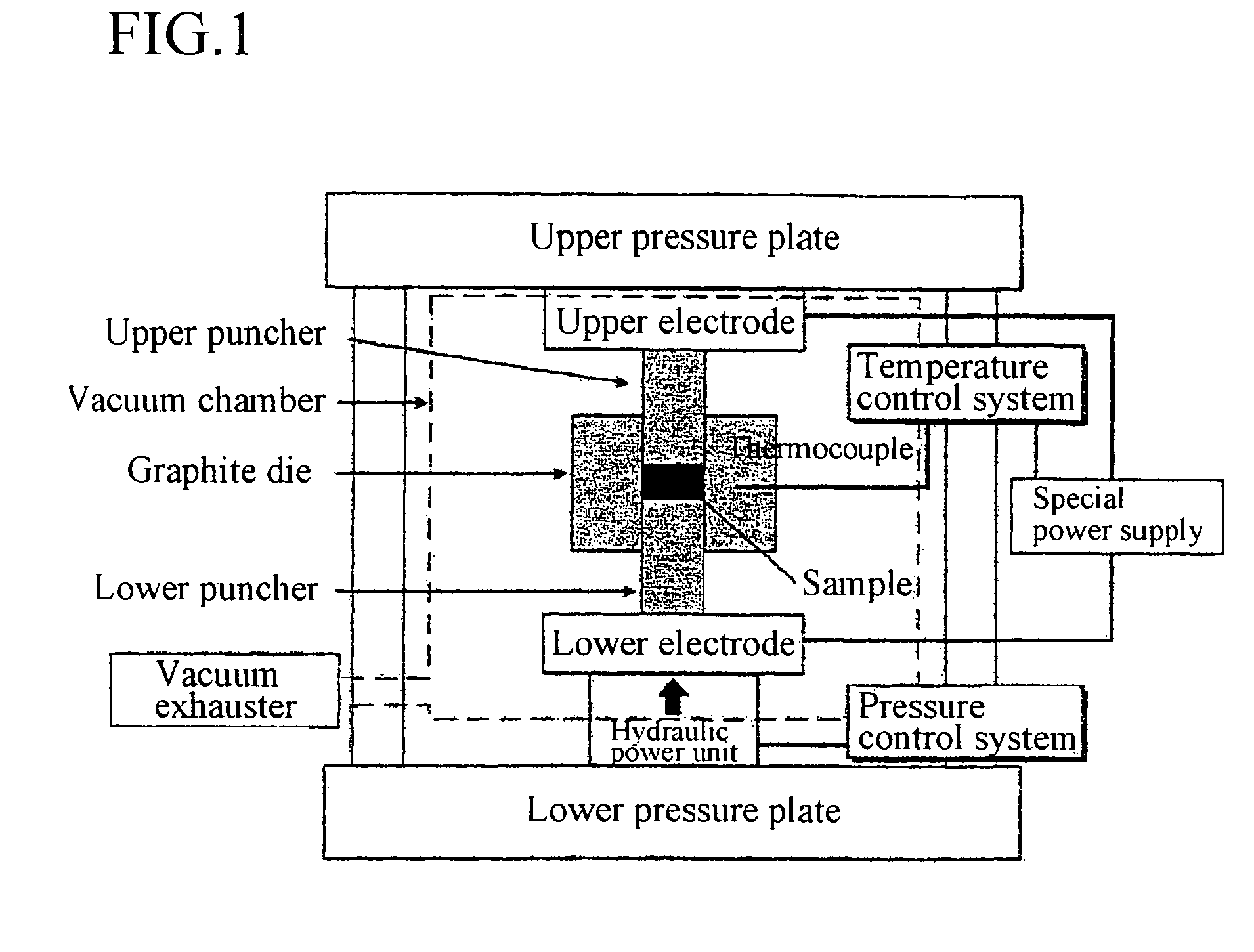
InactiveUS20060270199A1Crystal fineImprove reaction efficiencyPolycrystalline material growthSilicon halogen compoundsMolten stateGas phase
When high purity silicon is produced through a gas-phase reaction between silicon tetra-chloride and zinc in a reaction furnace, the produce silicon is obtained as block or molten state. after the reaction in which the silicon is not in contact with air and reaction temperature is maintained at melting point of the silicon or less.
Owner:ASAHI GLASS CO LTD
Apparatus and methods for the preparation of reaction vessels
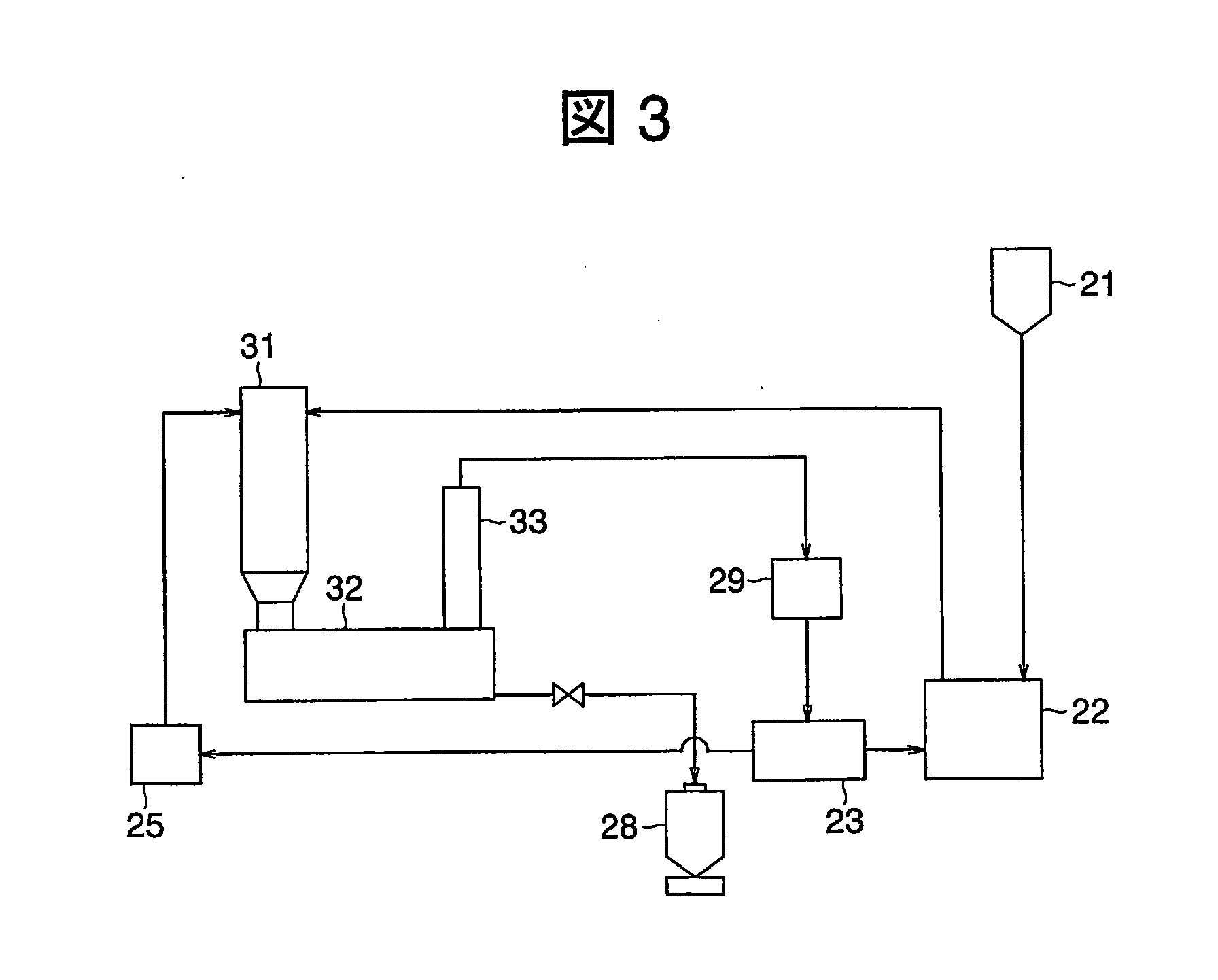
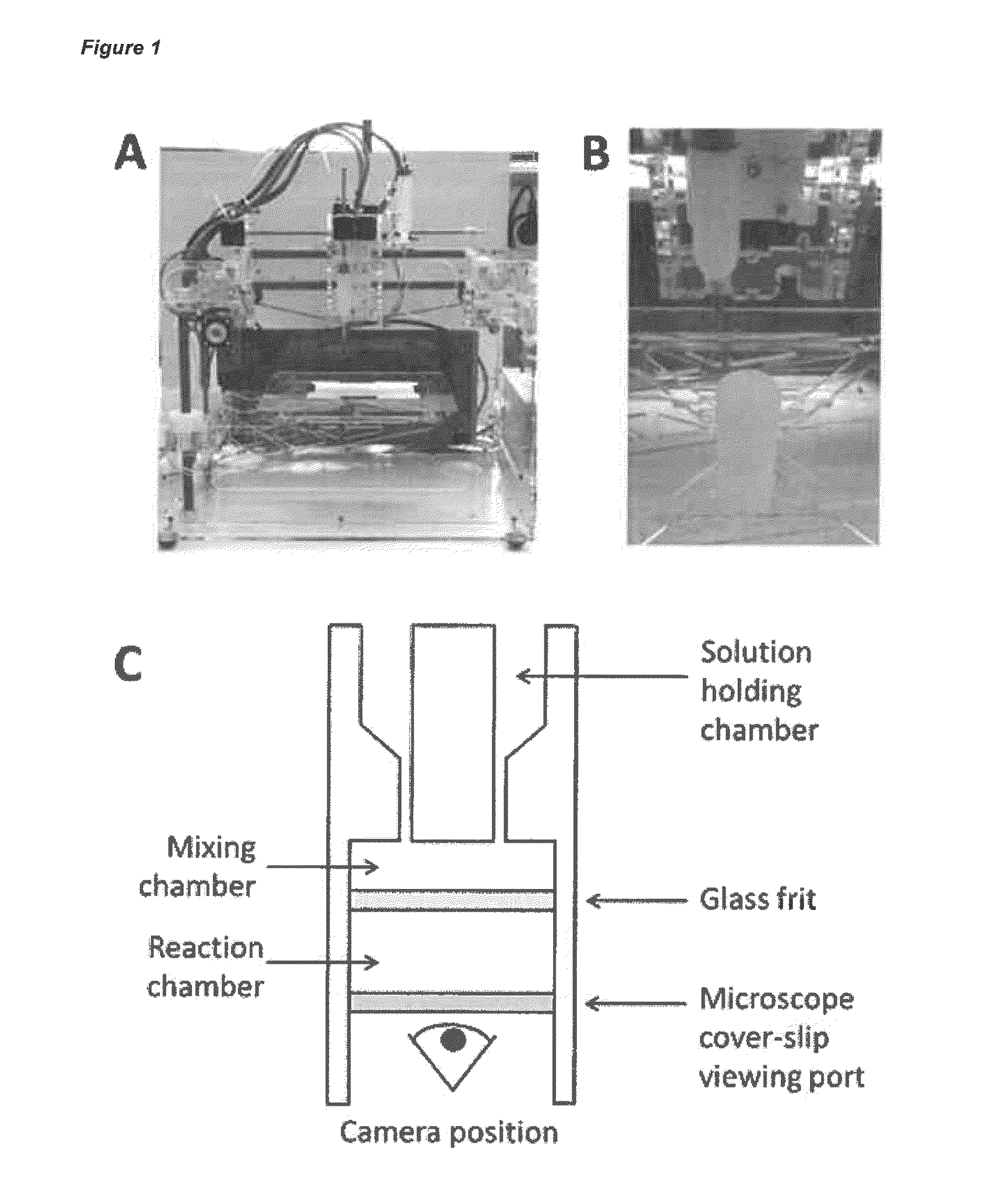
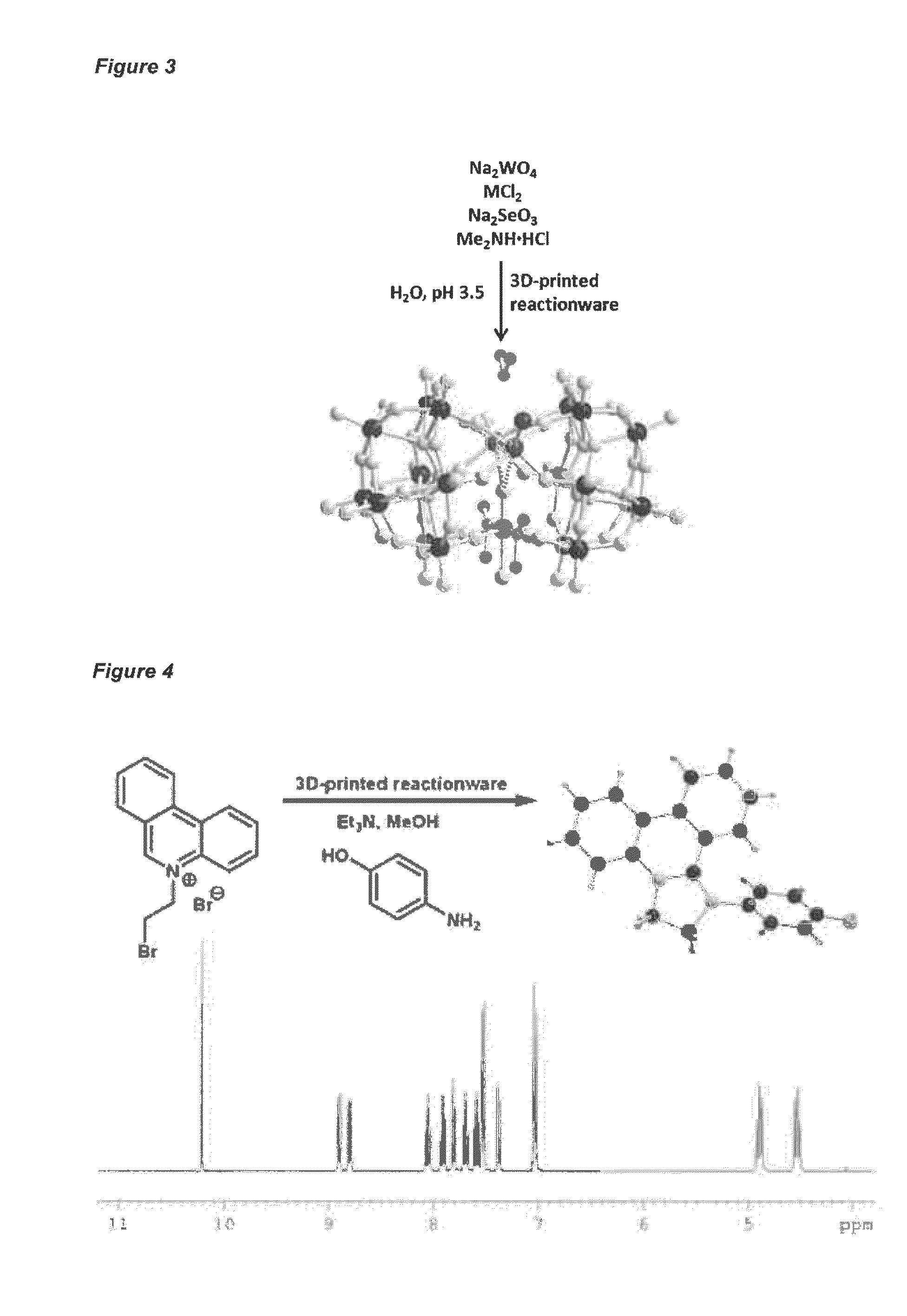
Provided are methods for preparing and using reaction vessels obtained or obtainable by 3D-printin methods, including a method for preparing a product compound, the method comprising the steps of: (i) providing a reaction vessel that is obtained by a 3-D printing method, wherein the reaction vessel has a reaction space; (ii) providing one or more reagents, optionally together with a catalyst or a solvent, for use in the synthesis of the product compound; and (iii) permitting the one or more reagents to react in the reaction space, optionally in the presence of the catalyst and the solvent, in the reaction vessel, thereby to form the product compound.
Owner:DEEPMATTER LTD
Positive electrode material for lithium secondary battery and process for producing the same
ActiveUS20050250013A1Improve battery performanceImproving charge-discharge cycle durabilitySolid electrolyte cellsActive material electrodesParticulatesLithium
There is obtained a material of a positive electrode for a secondary lithium-ion cell having high cycle durability and high safety in high-voltage and high-capacity applications, which is a particulate positive electrode active material for a secondary lithium-ion cell represented by a general formula, LiaCObAcBdOeFf (A is Al or Mg, B is a group-IV transition element, 0.90≦a≦1.10, 0.97≦b≦1.00, 0.0001≦c≦0.03, 0.0001≦d≦0.03, 1.98≦e≦2.02, 0≦f≦0.02, and 0.0001≦c+d≦0.03), where element A, element B and fluorine are evenly present in the vicinity of the particle surfaces.
Owner:SUMITOMO CHEM CO LTD
Method and device for growing large-volume oriented monocrystals
InactiveUS6969502B2Unusual sensitivityPrevent undesirable premature meltingAluminium silicatesBy zone-melting liquidsHeat flowMetallurgy
In the method for growing large-volume monocrystals crystal raw material is heated in a melting vessel with heating elements to a temperature above its melting point until a melt is formed. A monocrystal is then formed on the bottom of the melting vessel by lowering the temperature at least to the crystallization point. A solid / liquid phase boundary is formed between the monocrystal and the melt. The monocrystal grows towards the melt surface in a direction that is perpendicular to the phase boundary. A vertical axial temperature gradient is produced and maintained between the bottom of the melting vessel and its upper opening and heat inflow and / or heat outflow through side walls of the melting vessel is prevented, so that the solid / liquid phase boundary has a curvature radius of at least one meter. A crystal-growing device for performing this process is also described.
Owner:HELLMA MATERIALS
Alkali/Transition Metal Halo-And Hydroxy-Phosphates And Related Electrode Active Materials
InactiveUS20070190425A1Increase capacityImprove cycle performancePhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesHalogenPhosphate
The invention provides a novel polyanion-based electrode active material for use in a secondary or rechargeable electrochemical cell, wherein the electrode active material is represented by the general formula AaMb(XY4)2Zd.
Owner:BARKER JEREMY +2
Metal composite oxide with novel crystal structure and their use as ionic conductors
ActiveUS20060051278A1Predict ionic conductivityEasy to moveCell electrodesSulfur compoundsElectrical conductorCrystal structure
Disclosed is metal composite oxides having the new crystal structure. Also disclosed are ionic conductors including the metal composite oxides and electrochemical devices comprising the ionic conductors. The metal composite oxides have an ion channel formed for easy movement of ions due to crystallographic specificity resulting from the ordering of metal ion sites and metal ion defects within the unit cell. Therefore, the metal composite oxides according to the present invention are useful in an electrochemical device requiring an ionic conductor or ionic conductivity.
Owner:LG CHEM LTD
Method for producing a halide brine
ActiveUS7087209B2Simple and inexpensive methodLow costMagnesium halidesGallium/indium/thallium compoundsHalogenSalt water
A method for producing halide brine wherein an alkali and a reducing agent are added to an aqueous fluid having a density greater than 8.30 lb / gal., (0.996 kg / L) water, waste water or sea water for example. The resulting fluid is then contacted with a halogen to form a halide brine. The reaction occurs in a conventional reactor such as a mixing tank.
Owner:TETRA TECH INC
Process for recovery of ruthenium from a ruthenium-containing supported catalyst material
InactiveUS20080287282A1High purityHigh purity gradeCell electrodesFinal product manufactureHydrogen halideRuthenium Compounds
Process to recover ruthenium in the form of ruthenium halide, particularly ruthenium chloride, from a ruthenium-containing supported catalyst material comprising:a) chemically decomposing the ruthenium-containing supported catalyst material;b) producing a raw ruthenium salt solution;c) purifying the raw ruthenium salt solution and optionally stripping gaseous ruthenium tetroxide from the raw ruthenium salt solution; andd) treating the purified ruthenium compound obtained in c), particularly the ruthenium tetroxide, with hydrogen halide or hydrohalic acid to obtain ruthenium halide, particularly with hydrogen chloride or hydrochloric acid to obtain ruthenium chloride.
Owner:BAYER MATERIALSCIENCE AG
Clean method for preparing layered double hydroxides
ActiveUS8088349B2Prevent materialAvoid prolonged useLithium compoundsManganese oxides/hydroxidesCleaning methodsLayered double hydroxides
Disclosed is a clean method for preparing layered double hydroxides (LDHs), in which hydroxides of different metals are used as starting materials for production of LDHs by atom-economical reactions. The atom efficiency of the reaction is 100% in each case because all the atoms of the reactants are converted into the target product since only M2+(OH)2, M3+(OH)3, and CO2 or HnAn− are used, without any NaOH or other materials. Since there is no by-product, filtration or washing process is unnecessary. The consequent reduction in water consumption is also beneficial to the environment.
Owner:BEIJING UNIV OF CHEM TECH
Positive electrode active material for secondary cell, positive electrode for secondary cell using same, and secondary cell
InactiveUS7179566B2Increase working voltageReduce capacityHalide preparation methodsIron compoundsCrystal structureSpinel
A cathode active material for a secondary battery including a lithium-manganese composite oxide having a spinel structure and represented by the following general formula (I), Lia(MxMn2-x-y-zYyAz)(O4-wZw) (I), wherein 0.5≦x≦1.2, 0≦y, 0≦z, x+y+z<2, 0≦a≦1.2 and 0≦w≦1; M contains at least Co and may further contains at least one element selected from the group consisting of Ni, Fe, Cr and Cu; Y is at least one element selected lo from the group consisting of Li, Be, B, Na, Mg, Al, K and Ca; A is at least one of Ti and Si; and Z is at least one of F and Cl. When the cathode active material for the secondary battery is used as the cathode for the a secondary battery, a higher operating can be realized while suppressing the reliability reduction such as the capacity decrease after the cycles and the deterioration of the crystalline structure at a higher temperature.
Owner:NEC CORP
Lithium-containing composite oxide and its production method
ActiveUS8192715B2Solve the small densityImprove securityCell electrodesLithium compoundsAlkaline earth metalVolumetric Mass Density
The present invention provides a lithium-containing composite oxide for a positive electrode for a lithium secondary battery, which has a large volume capacity density and high safety, and excellent durability for charge and discharge cycles and charge and discharge rate property, and its production method. The lithium-containing composite oxide is represented by the general formula LipNxMyOzFa (where N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, Sn, alkaline earth metal elements and transition metal elements other than Co, Mn and Ni, 0.9≦̸p≦̸1.2, 0.965≦̸x<2.00, 0<y≦̸0.035, 1.9≦̸z≦̸4.2, and 0≦̸a≦̸0.05), wherein when a powder of the lithium-containing composite oxide is classified into small particles with an average particle size of 2 μm≦̸Ds50≦̸8 μm and large particles with an average particle size of 10 μm≦̸Dl50≦̸25 μm, a content of the small particles is from 15 to 40% by weight and a content of the large particles is from 60 to 85% by weight, and 0.01≦̸ys≦̸0.06, 0≦̸yl≦̸0.02 and 0≦̸yl / ys<1, where (ys) is a ratio of the M element in the above general formula in the small particles and (yl) is a ratio of the M element in the general formula in the large particles.
Owner:SUMITOMO CHEM CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com