Patents
Literature
3812 results about "Lithium carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat manic-depressive disorder (bipolar disorder).
Method for preparing lithium cobaltate by directly using invalid lithium ion battery
InactiveCN102030375AReduce dispersionHigh purityCell electrodesCobalt compoundsElectrical batteryPotassium hydroxide
The invention provides a method for preparing lithium cobaltate by directly using an invalid lithium ion battery. The method comprises the following steps: crushing the invalid lithium ion battery or scraps generated when a lithium cobaltate battery is produced by a mechanical crusher at normal temperature; adding water and one or more of acetic acid, sulfuric acid, hydrochloric acid or nitric acid to produce mixed aqueous solution of the battery scraps and acid; filling the mixed aqueous solution into a hermetic pressure reactor, and controlling the temperature in the reactor to be between 50 and 150 DEG C; introducing or adding one leaching additive of sulfur dioxide or hydrogen, or adding hydrazine hydrate; stirring and leaching, cooling, and filtering; adding one precipitator of sodium carbonate, potassium carbonate and ammonium carbonate, or adding composite precipitator consisting of one of the sodium carbonate, the potassium carbonate and the ammonium carbonate and one of sodium hydroxide and potassium hydroxide to obtain mixture of lithium carbonate, cobalt carbonate and cobalt hydroxide; drying and calcining at high temperature to produce a lithium cobaltate product. The method is particularly suitable for the treatment scale of medium-sized and small enterprises, and is an effective method for directly materializing cobalt secondary resources.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Process for extracting lithium from salt lake brine by adsorptive method
InactiveCN1511964AEasy to makeNo pollutionProcess efficiency improvementLithium chlorideLithium carbonate
The present invention relates to adsorption process of extracting lithium from salt lake brine, and the process is suitable for producing lithium carbonate and lithium chloride with lithium-containing Qinghai saline lake brine, including concentrated lithium-containing Qinghai saline lake brine. The process includes sun shining saline lake brine to obtain concentrated lithium containing brine, adsorption of lithium ion with aluminum salt adsorbent, eluting adsorbed lithium ion with water, and refining and concentrating the elutriant to obtain material for preparing lithium carbonate and lithium chloride.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Solid composite electrolyte membrane and method of making
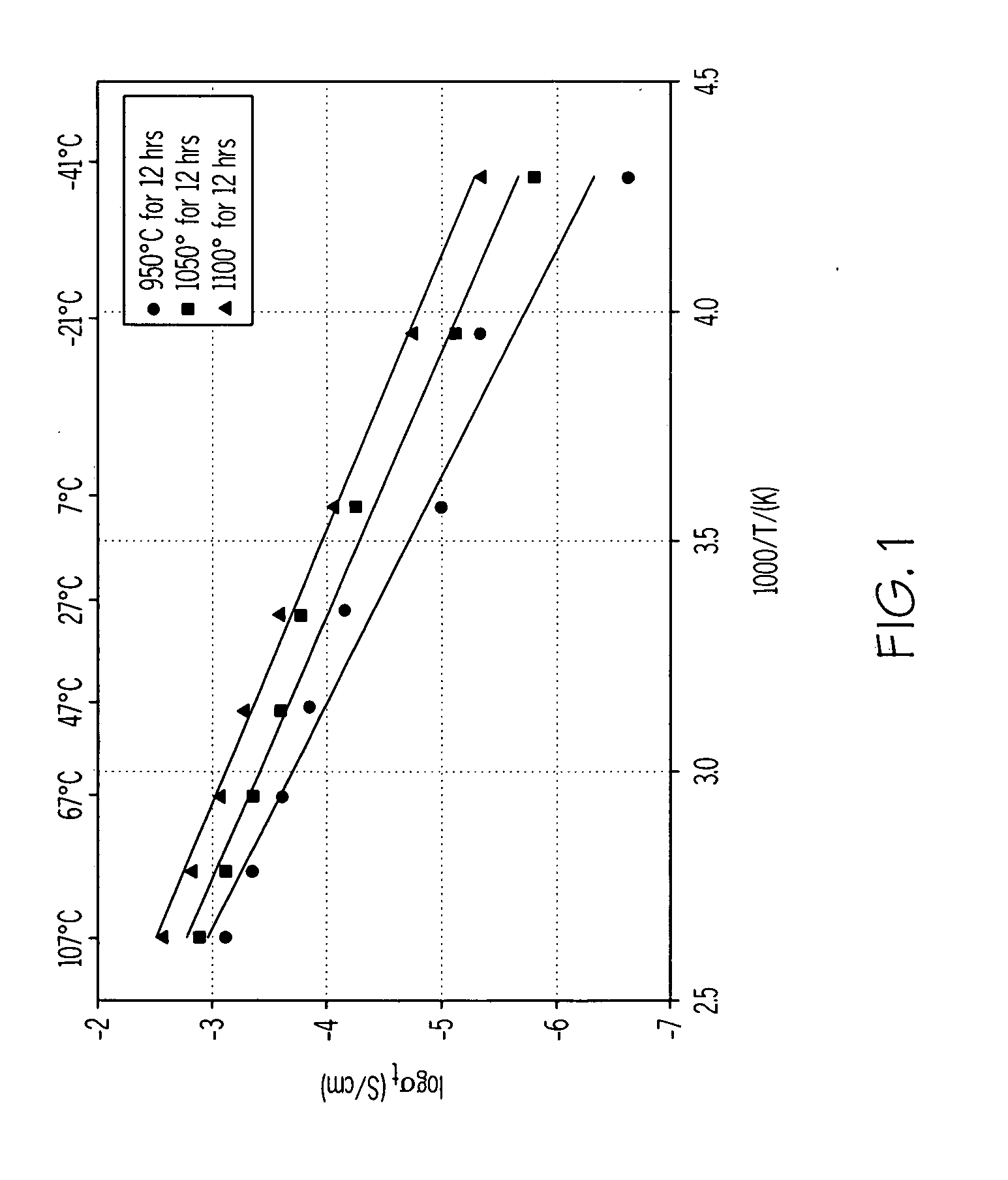
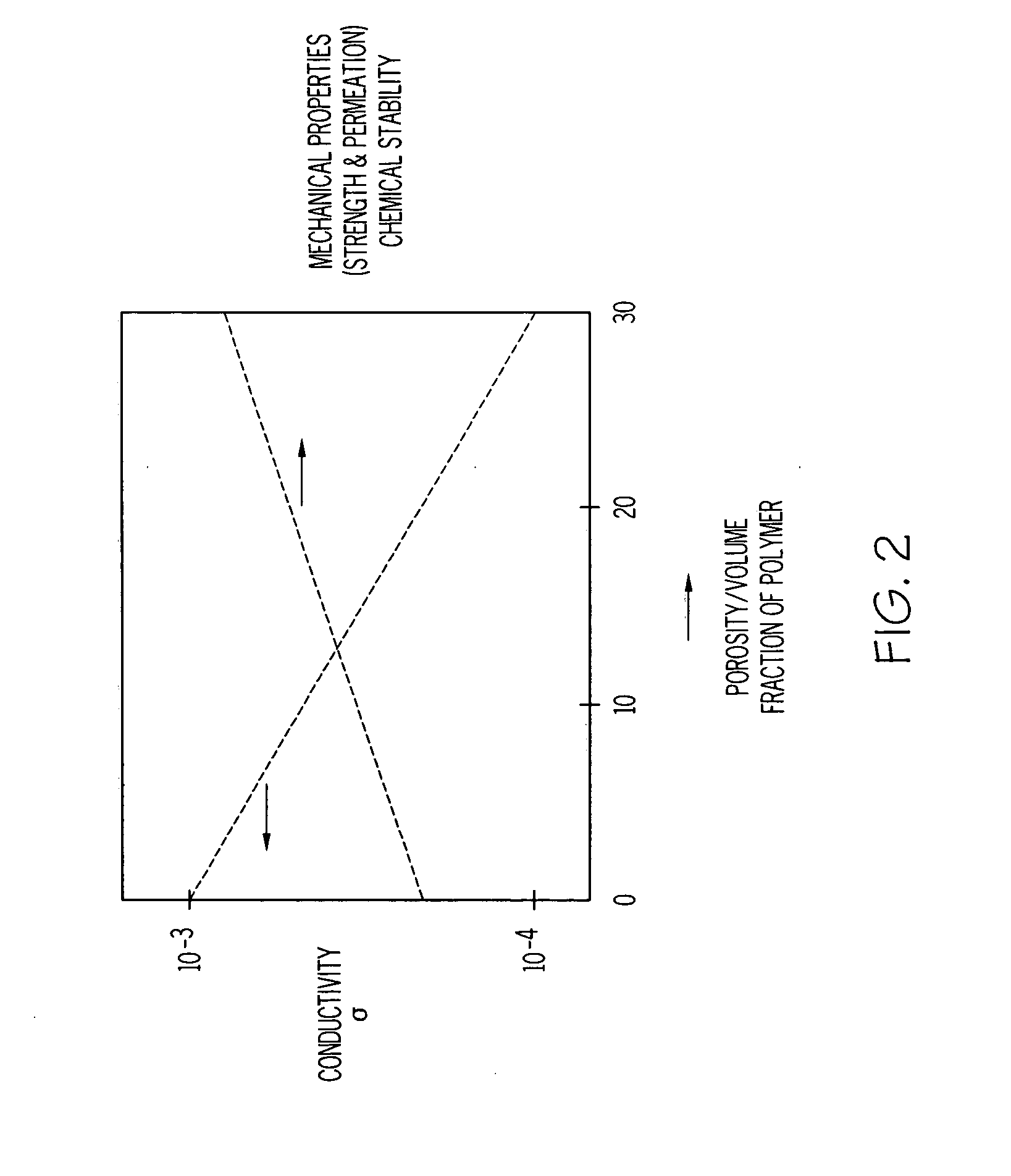
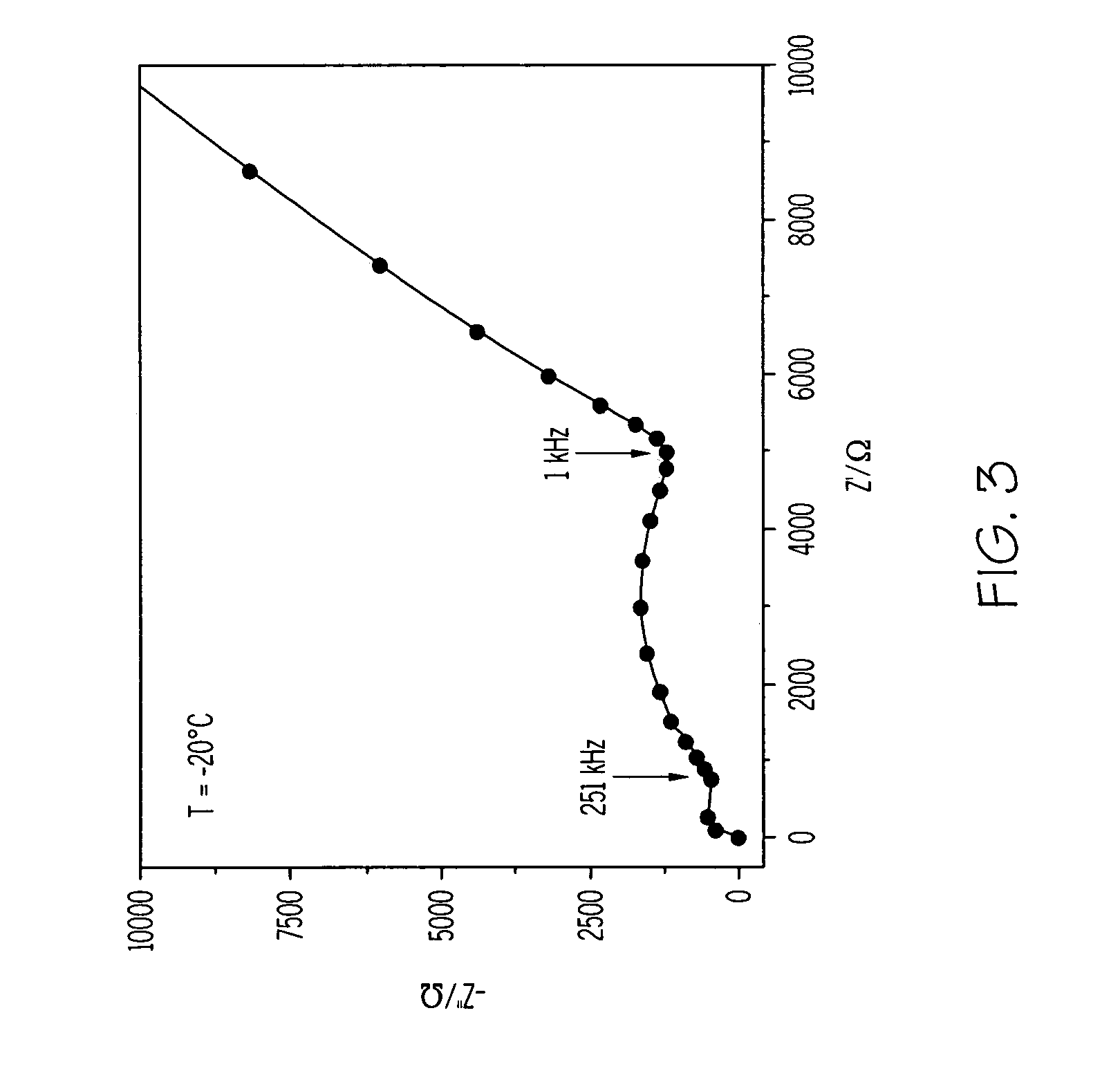
ActiveUS20070117026A1Improve conductivitySolid electrolytesSolid electrolyte cellsPorosityComposite electrolyte
A solid composite electrolyte membrane for use in a lithium battery is provided which exhibits a conductivity ranging from about 10−4 S cm−1 to about 10−3 S cm−1 at ambient temperature. The membrane is formed by providing a glass or glass-ceramic powder formed from a mixture of lithium carbonate, alumina, titanium dioxide, and ammonium dihydrogen phosphate. The powder is mixed with a conditioning agent and at least one solvent, followed by the addition of a binder and one or more plasticizers. The resulting slurry is cast into a tape which is then subjected to a binder burn-off and sintering process to form the membrane. The resulting membrane may be a glass-ceramic composite having a porosity ranging from 0 to 50%, or the membrane may be further infiltrated with a polymer to form a water-impermeable polymeric-ceramic composite membrane.
Owner:UNIV OF DAYTON THE
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
ActiveUS20110200508A1Electrolysis componentsLithium organic compoundsLithium carbonateLithium hydroxide
Owner:TERRALITHIUM LLC
Process for preparing high density spherical nickel-cobalt lithium manganate as anode material of lithium ion cell
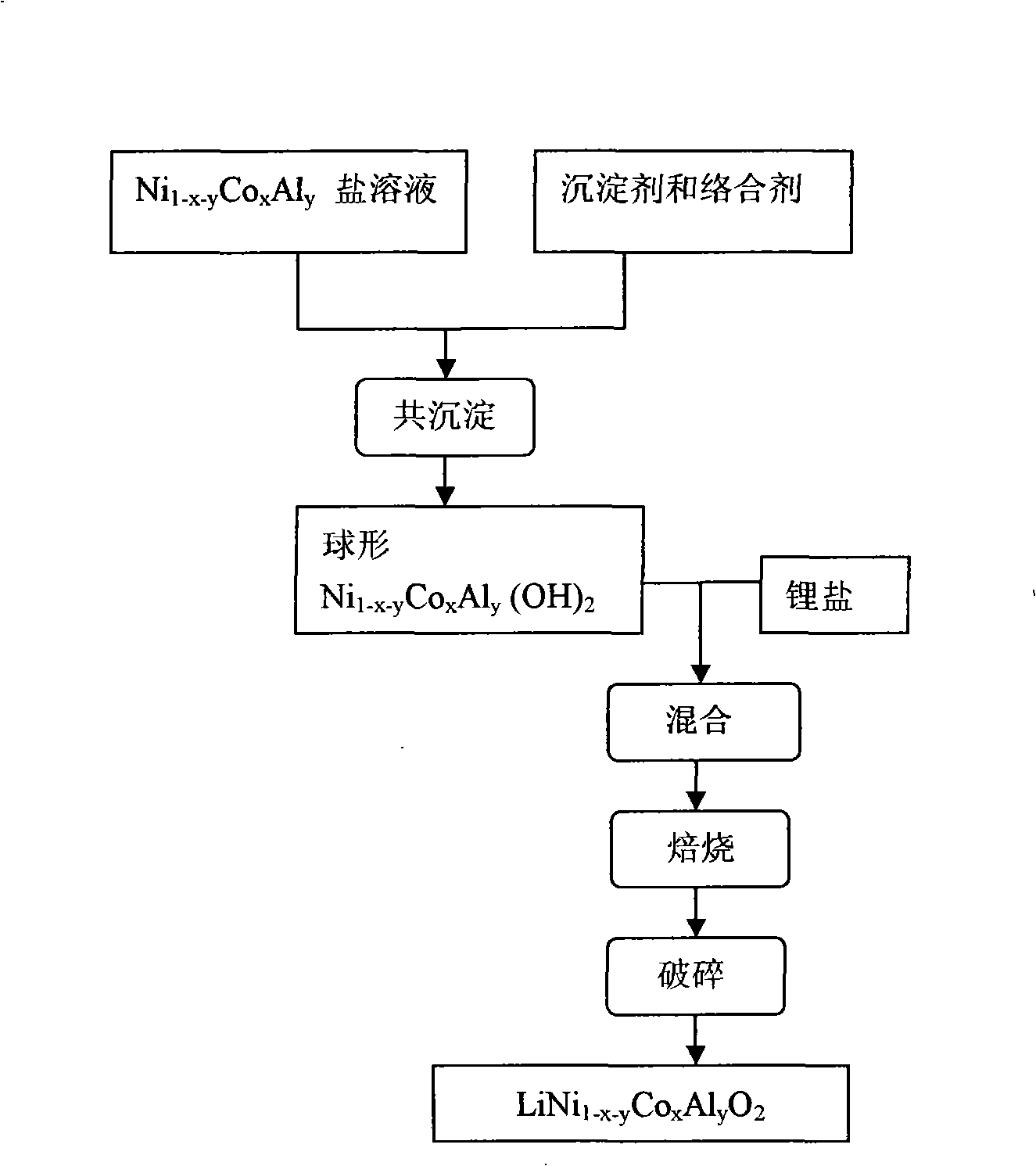
InactiveCN1622371AWell mixedImprove performanceElectrode manufacturing processesLithium compoundsNickel saltManganate
The present invention relates to energy source material technology, and is preparation process of high density spherical lithium nickel-cobalt-manganate as positive electrode material for lithium ion cell. The preparation process includes the reaction of nickel salt, cobalt salt, manganese salt, ammonium hydroxide and ammonian in water solution to synthesize spherical or spheroid precursor Ni1 / 3Co1 / 3Mn1 / 3 (OTHER)2, washing, drying and mixing with lithium carbonate; and high temperature treatment in the air at 750-950 deg.c for 8-48 hr to obtain spherical lithium nickel-cobalt-manganate. The spherical lithium nickel-cobalt-manganate has great bulk density reaching 2.25-2.50 g / cu cm after vibration densifying, average grain size of 3-7 microns, and reversible specific capacity up to 172-185 mA.hr / g.
Owner:TSINGHUA UNIV
Method for preparing nickel and cobalt doped lithium manganate by using waste and old lithium ionic cell as raw material
InactiveCN101450815ASimultaneous recyclingShort processManganates/permanganatesManganateManganese oxide
The invention discloses a method for preparing lithium nickel cobalt manganese oxide by taking a waste lithium ion battery as a raw material. The method is mainly characterized in that a waste lithium ion battery taking the lithium nickel cobalt manganese oxide, lithium nickel cobalt oxide and so on as a battery positive material is selected as the raw material and is pretreated through disassembly, separation, crushing, screening and so on, and then processes such as adhesive removal at high temperature and aluminum removal by sodium hydroxide are adopted to obtain an inactivated positive material containing nickel, cobalt and manganese; then a sulfuric acid and hydrogen peroxide system is adopted to leach, and P204 is adopted to remove impurities by extraction to obtain pure nickel, cobalt and manganese solution, and proper manganese sulfate, nickel sulfate or cobalt sulfate is blended to ensure that the mol ratio of nickel, cobalt and manganese elements in the solution is 1: 1: 1; and then ammonium carbonate is adopted to adjust the pH value to form a nickel cobalt manganese carbonate precursor, and then a proper amount of lithium carbonate is blended for high temperature sintering to synthesize a lithium nickel cobalt manganese oxide battery material. The first discharge capacity of the material is 150 mAh / g, the discharge capacity is still kept more than 130mAh / g after the circulation for 30 times, and the material has good electrochemical performance.
Owner:GUANGDONG BRUNP RECYCLING TECH +1
Full-component resource reclamation method for waste positive electrode materials of lithium ion batteries
ActiveCN102751549AAvoid secondary pollutionImprove separation efficiencyWaste accumulators reclaimingBattery recyclingRecovery methodDistillation
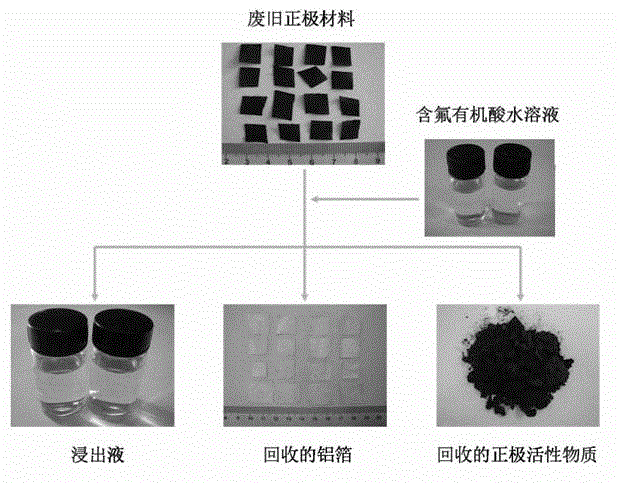
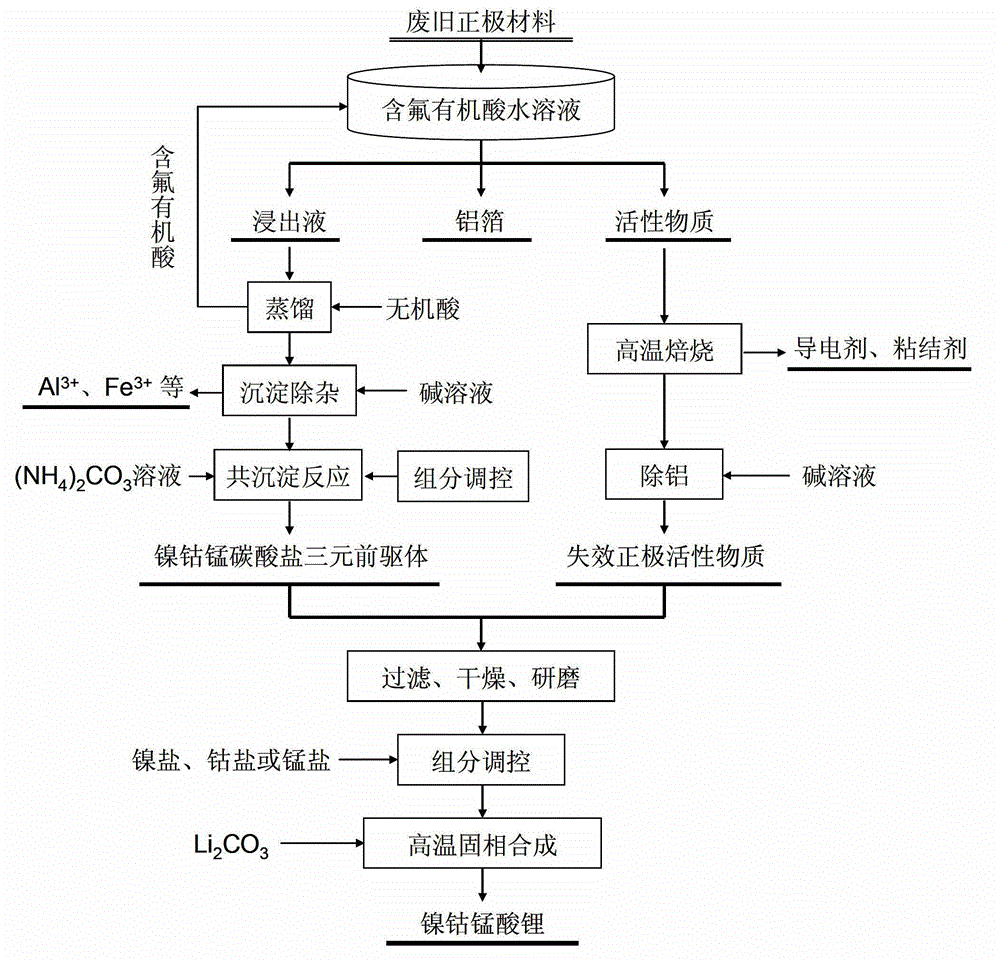
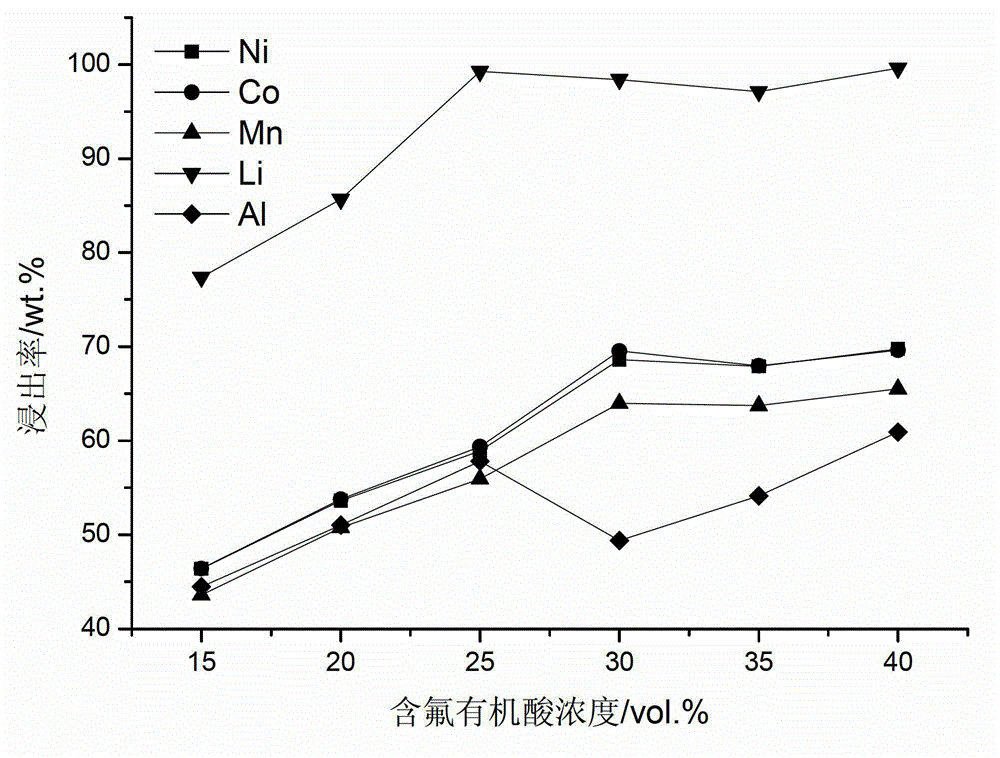
The invention provides a full-component resource reclamation method for waste positive electrode materials of lithium ion batteries. The method comprises the following steps: 1) separating active substances and aluminum foils in waste positive electrode materials of lithium ion batteries by using an aqueous solution of fluorine-containing organic acid and carrying out liquid-solid-solid separation so as to obtain leachate, the lithium-containing active substances and the aluminum foils; 2) respectively carrying out high temperature roasting and impurity removal with alkali liquor on the lithium-containing active substances; 3) respectively carrying out recovery of the fluorine-containing organic acid through addition of acid and distillation, deposition of impurity ions through addition of alkali and ammonium carbonate coprecipitation on the leachate so as to prepare nickel-cobalt-manganese carbonate ternary precursor; and 4) carrying out component regulation on a mixture of the treated active substances and the nickel-cobalt-manganese carbonate ternary precursor, adding lithium carbonate in a certain proportion and carrying out high temperature solid phase sintering so as to prepare a lithium nickel cobalt manganese oxide ternary positive electrode material. The method provided in the invention has the following advantages: the application scope of the method is wide; separation efficiency of the lithium-containing active substances and the aluminum foils is high; short-flow direct re-preparation of positive electrode materials in waste lithium ion batteries is realized; and the method is applicable to large-scale resource reclamation of waste lithium ion batteries.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Nano-filtration method for separating magnesium and enriching lithium from salt lake brine
InactiveCN1542147AEfficient recyclingSimple processSemi-permeable membranesLithium compoundsIon contentLithium chloride
The nanofiltration process is suitable for Mg-Li separation and Li enriching of Li containing bittern or Li containing solution from salt lake to prepare lithium carbonate or lithium chloride with the Li enriching bittern. Nanofiltration membrane is used in separating and enriching lithium from lithium containing bittern, which contains Mg, Ca and other cations and sulfate radical and other anions and has Li ion concentration of 0.1-11.5 g / L and Mg / Li ion weight ratio 1-200, to obtain lithium enriched bittern suitable for preparing lithium carbonate or lithium chloride. The said process is effective, and can obtain lithium enriched bittern with Mg / Li ion weight ratio 0.6-5 and Li ion content of 0.6-20 g / L.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Method for recovering waste lithium iron phosphate battery positive pieces
ActiveCN103280610AReduce pollutionWaste accumulators reclaimingProcess efficiency improvementPhosphoric acidImpurity
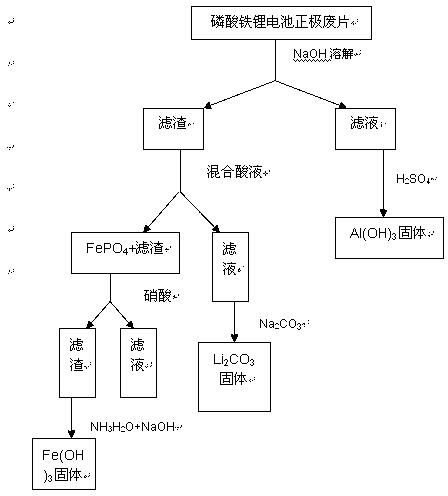
The invention discloses a method for recovering Al, Fe and Li in waste lithium iron phosphate battery positive pieces through an acid-alkali leaching process. The method comprises the following steps: dismounting the lithium iron phosphate battery positive pieces, dissolving the positive pieces by using an alkali, filtering, and dissolving obtained filter residues by a mixed acid solution for making Fe exist in an iron phosphate precipitate form and be separated from impurities comprising carbon black and the like and a lithium-containing solution; adding the lithium-containing solution to a 95DEG C saturated sodium carbonate solution for precipitation to obtain lithium carbonate; and adding an acid to obtain an iron-containing precipitate for leaching iron ions, and adding an alkaline solution to obtain Fe(OH)3. Al, Fe and Li in the waste lithium iron phosphate battery positive pieces are simply and effectively recovered through using low-concentration acids and alkalis and routine chemicals, and raw materials having economic benefits are recovered through using simple and effective equipment and the method.
Owner:STATE GRID JIANGXI ELECTRIC POWER CO LTD RES INST +1
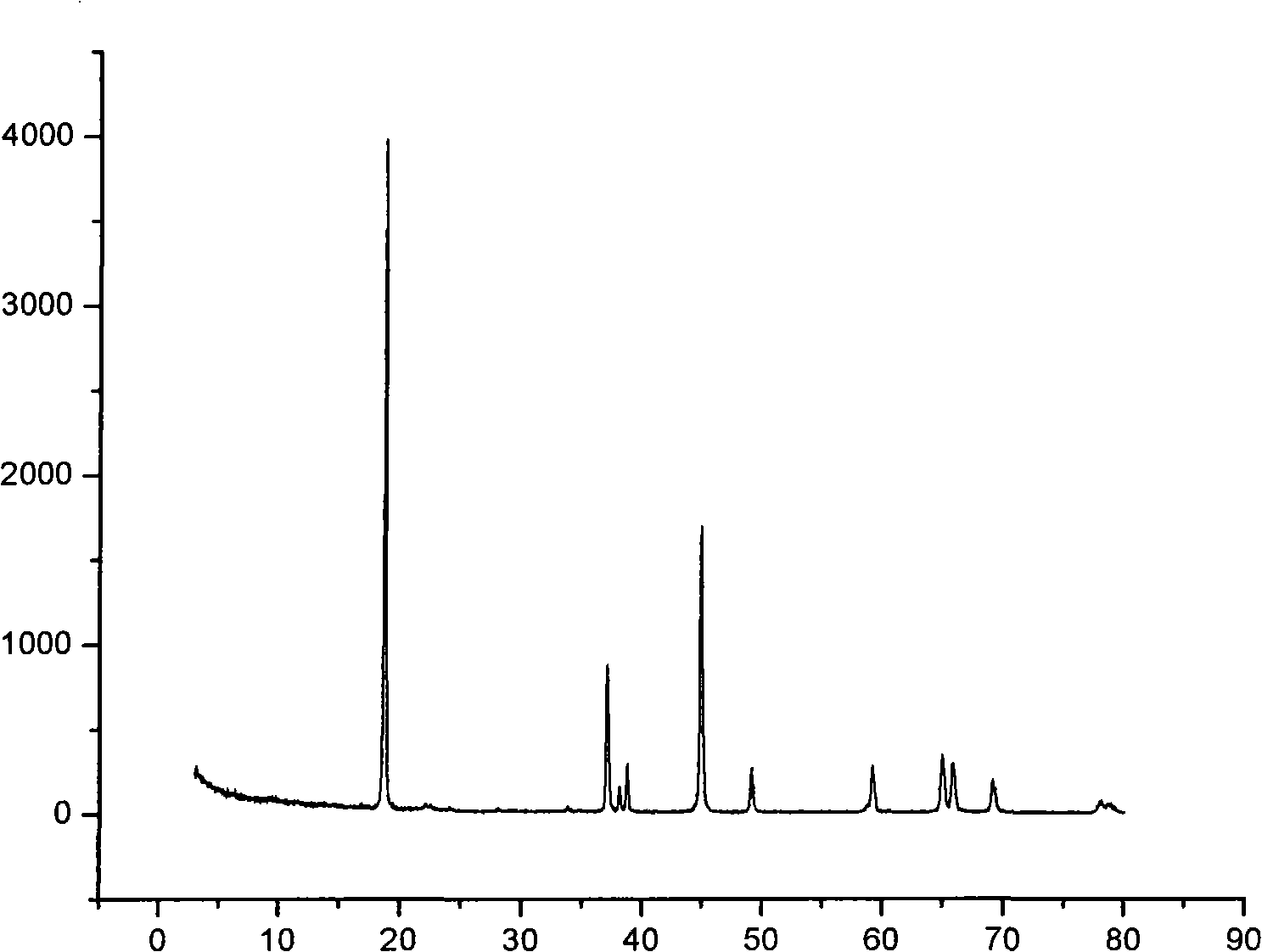
Spherical aluminum-doped nickel cobalt lithium for lithium ion battery and its making method
ActiveCN101262061AImprove liquidityImprove charge and discharge cycle stabilityElectrode manufacturing processesLithium compoundsDischarge efficiencyHigh rate
The invention discloses a preparation method of a spherical doped Al-Ni lithium cobalt oxide for lithium-ion battery. The preparation steps are that: first, sulfate, nitrate or chlorate of Al-Ni-Co react with strong alkali that is added with complex agent in liquid phase; the pH value, the temperature and the feeding speed of the reaction solution are controlled so as to produce a spherical precursor of Al-Ni-Co hydroxide; then the spherical precursor of Al-Ni-Co hydroxide is dried and evenly mixed with lithium hydroxide, lithium nitrate or lithium carbonate and dried; the obtained mixture is roasted into a spherical doped Al-Ni lithium cobalt oxide. The spherical doped Al-Ni lithium cobalt oxide has comparatively high tap density and remarkable cycle stability in the process of high-rate charge / discharge cycle, which improves over charge performance of Ni-Co substance and first obviously enhances charge / discharge efficiency; in addition, the preparation method of the spherical doped Al-Ni lithium cobalt oxide has the advantages of being simple, controllable and suitable for industrialized production with low energy consumption, high efficiency, short reaction time and low cost.
Owner:成都巴莫科技有限责任公司
Method for Producing Difluorophosphate, Nonaqueous Electrolyte Solution for Secondary Battery and Nonaqueous Electrolyte Secondary Battery
ActiveUS20080102376A1Phosphorus halides/oxyhalidesNon-aqueous electrolyte accumulatorsDifluorophosphateSource material
The present invention provides a simple method for producing a difluorophosphate from a source material, the difluorophosphate being useful as additives for nonaqueous electrolyte solutions for secondary batteries. In the method, a source material containing a carbonate and / or a borate is allowed to react with a source gas which contains P and F and which may further contain O as required. The source material may contain lithium carbonate. The source gas may be produced by decomposing LiPF6. The source gas may be produced in such a manner that LiPF6 and lithium carbonate are mixed and then subjected to reaction. The nonaqueous electrolyte solution contains the product obtained from the reaction.
Owner:MU IONIC SOLUTIONS CORP +1
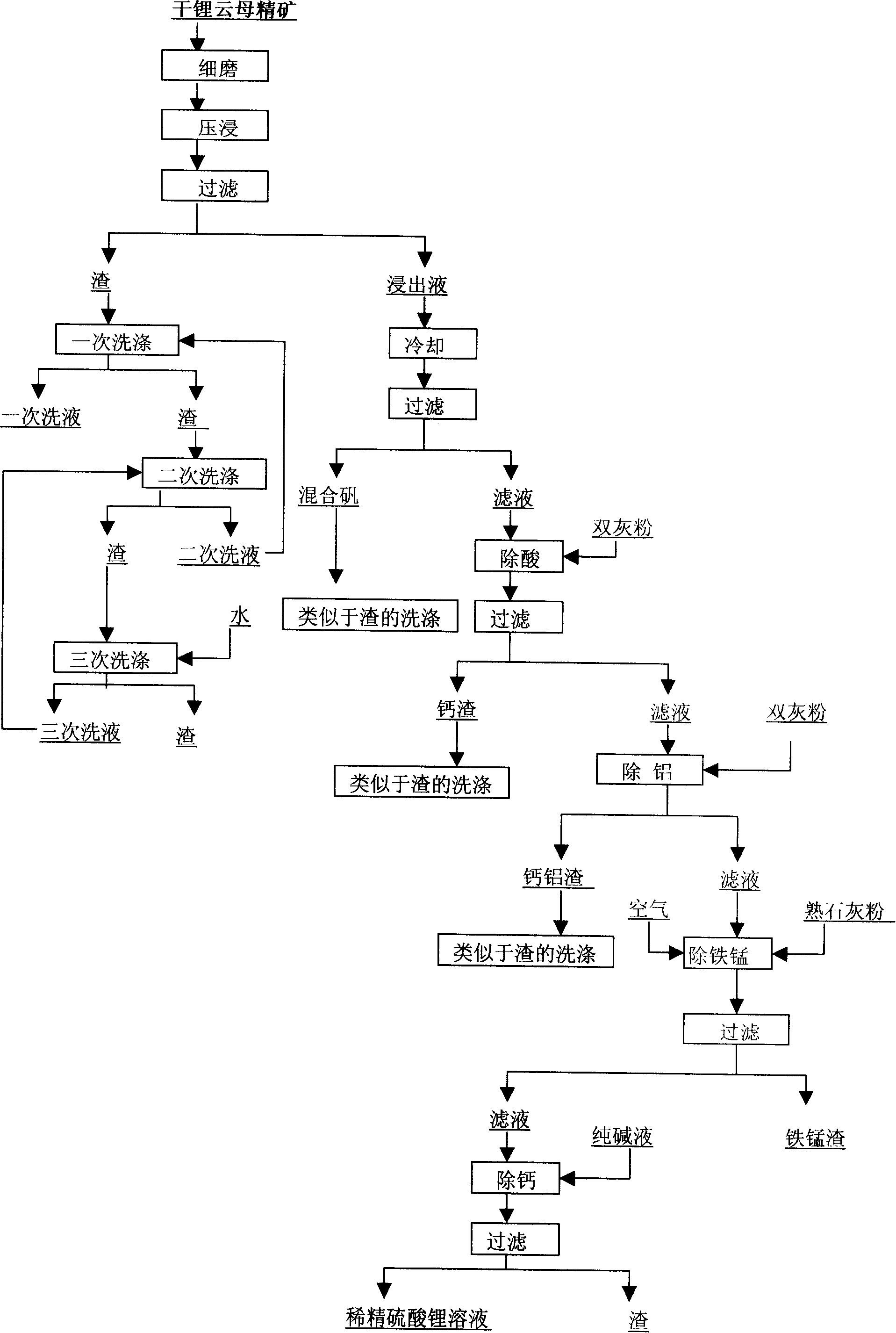
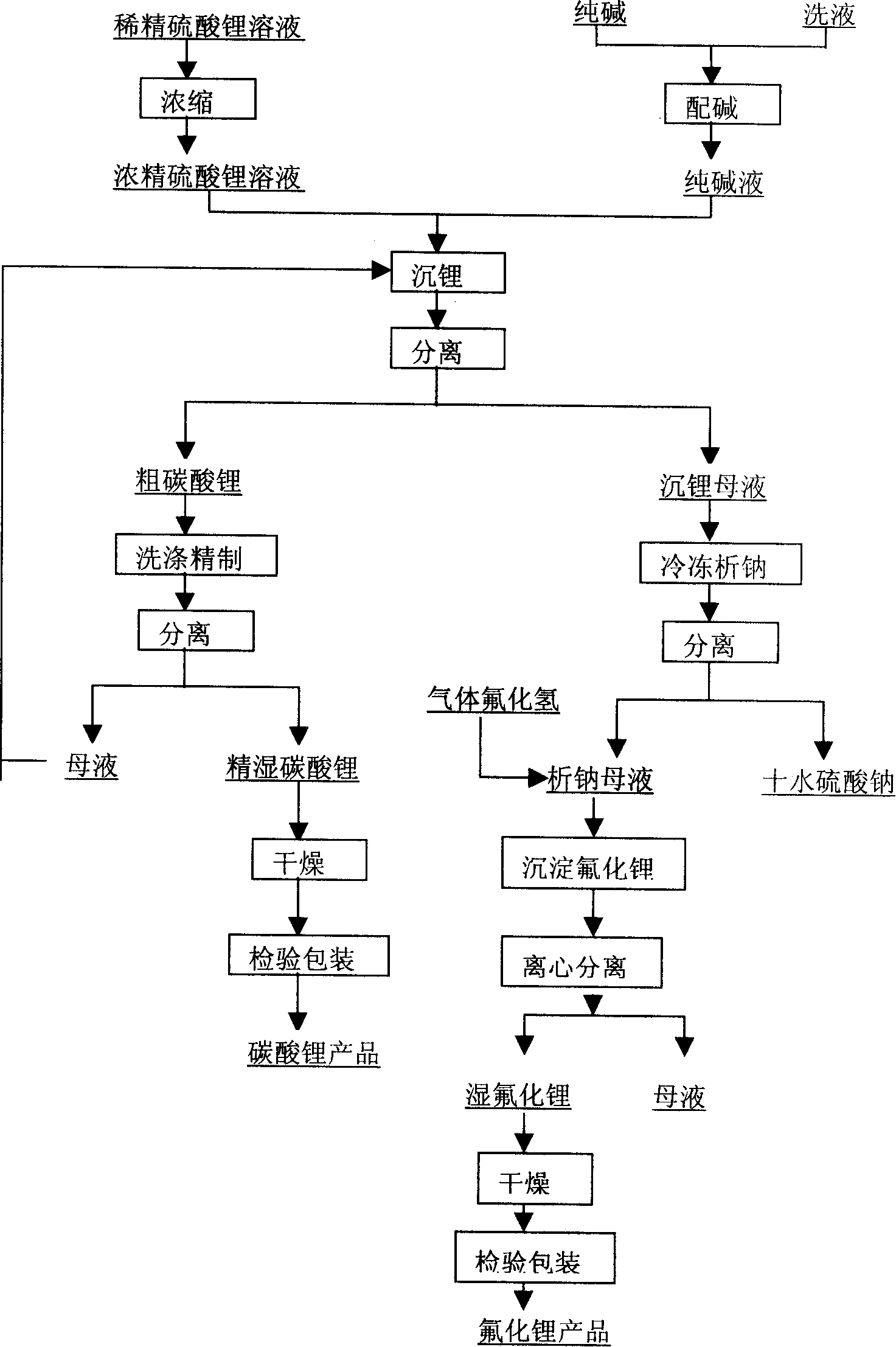
Method for producing refined lithium sulfate solution used in lepidolite lithium-extracting technique by sulfuric acid process
Provided is a process for producing refined lithium sulfate solution of lepidolite lithium extracting technology with sulfuric acid process, which takes lepidolite clean ore as raw material and sequentially includes the following steps, including leaching, alum cooling and decanting, acid removing, aluminum removing, decontaminating and deliming, thereby achieving refined lithium sulfate solution. The alum cooling and decanting process of the invention can precipitate kalium, rubidium and caesium in alum form, thereby the separation of lithium and kalium, rubidium and caesium is easily achieved, and the achieved alum dregs of kalium, rubidium and caesium are blend alum with high purity, which creates perfect condition for comprehensive utilization and simultaneously reduces the burdens of the separation of lithium and aluminum. The aluminum removing process can easily achieve the separation of lithium and aluminum. The process of the invention has the advantages that the energy consumption is relatively low, and the lithium yield is relatively high, most of the residues can be used and the process is favorable for comprehensive utilization. The invention further provides a process for producing lithium carbonate and lithium fluoride with the achieved refined lithium sulfate solution.
Owner:GANFENG LITHIUM CO LTD
Cement-base dual-liquid slip-casting material
The invention relates to a kind of pair-slurry affusing material prepared with cement as basic raw material. It is comprised of two kinds of dry powdery mixed materials A and B of affusing slurry pair-liquid formed after adding water. Their weight percents are respectively: mixed material A: sulfur aluminate cement 30% - 98.1%, retarder citric acid 0.1% - 0.5%, flocculant polyacrylamide 0% - 0.8%, water-reducing agent FDN 0.6% - 1.5% and inorganic padding pulverized coal ash 0% - 69.1%; mixed material B: Portland cement 20% - 88%, land plaster 6% - 15%, flocculant UWN 0% - 0.7%, accelerator lithium carbonate 0.4% - 1.0%, water-reducing agent FDN 0.5% - 1.3%, calcareousness 2.0% - 5.0% and inorganic padding pulverized coal ash 0% - 70.8%.
Owner:张振秋
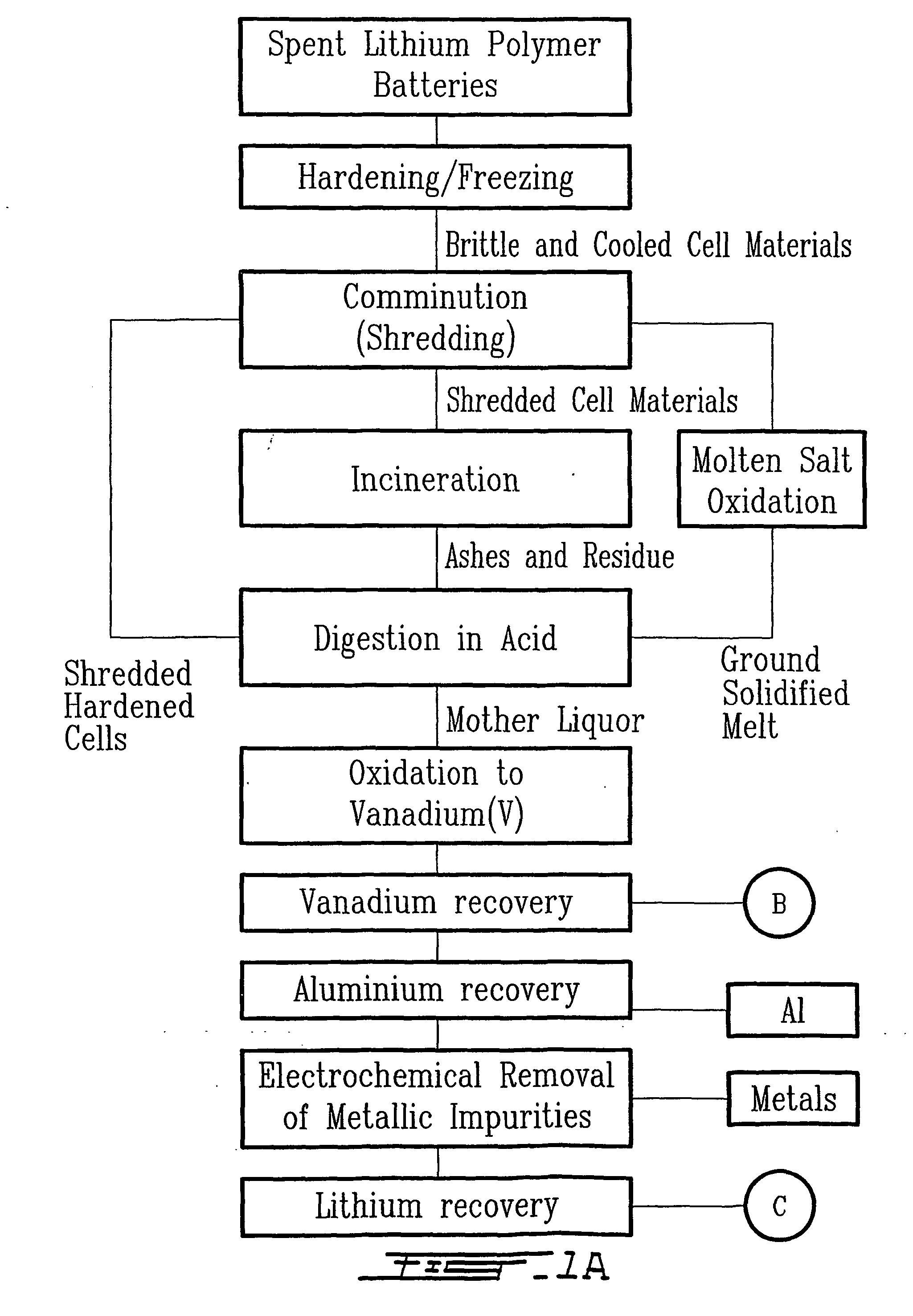
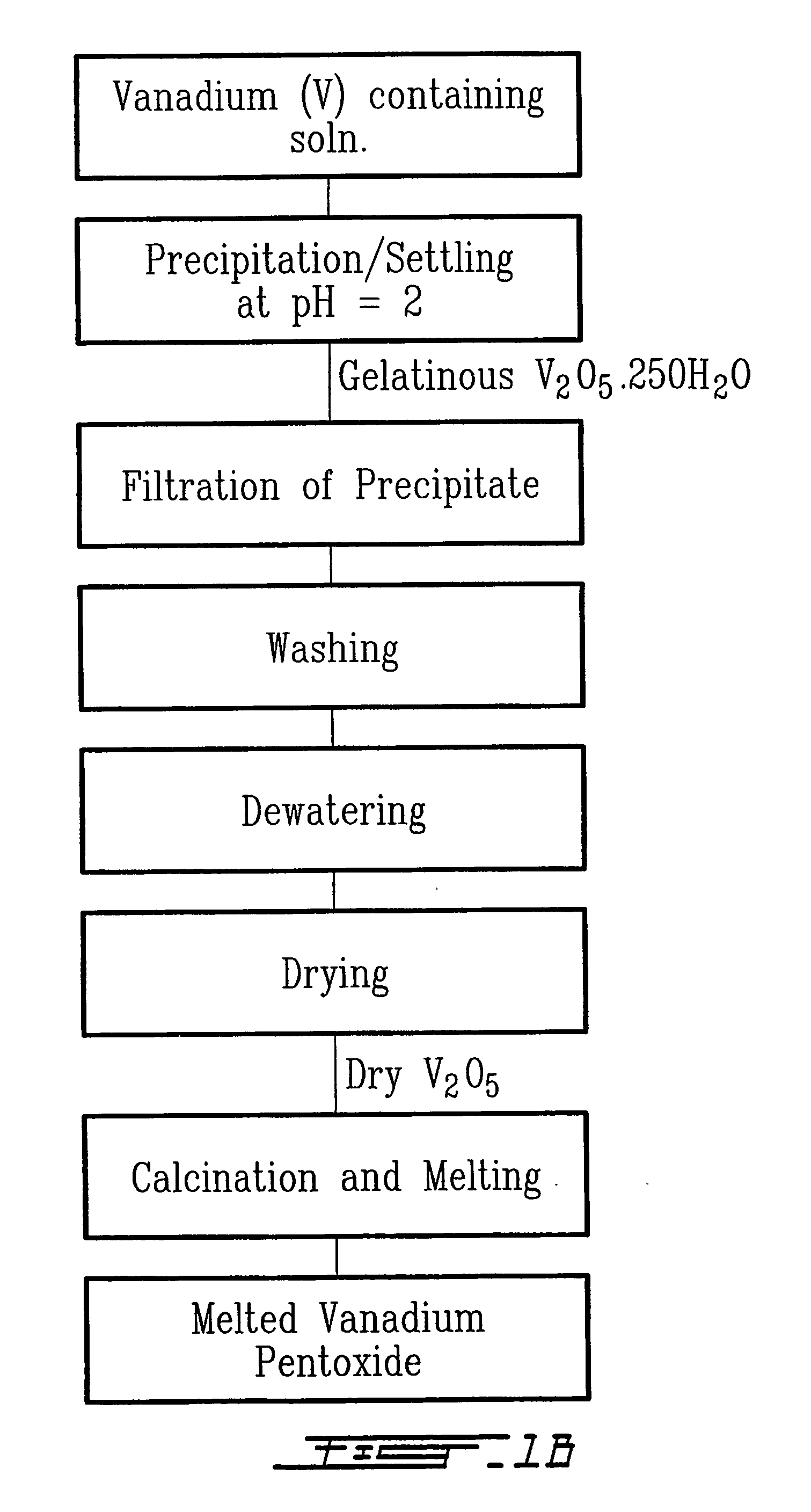
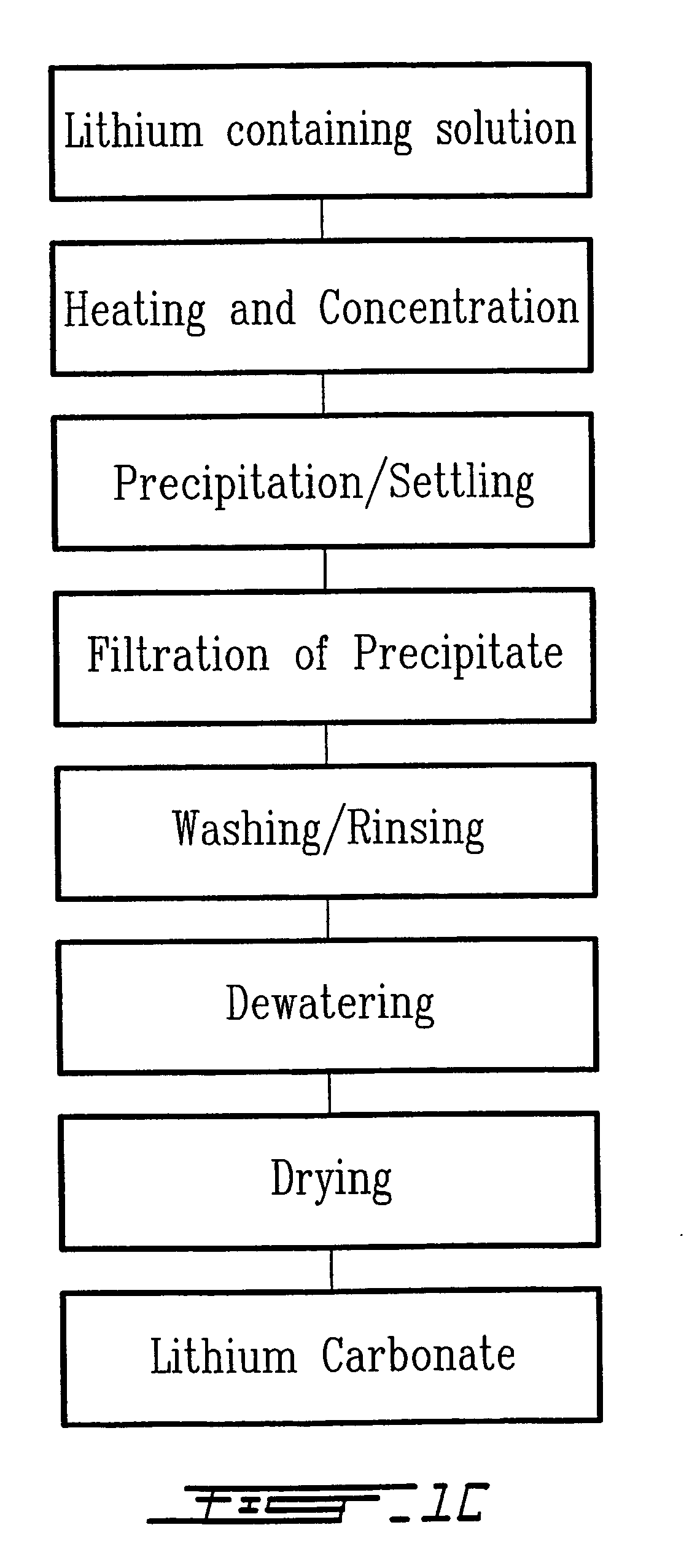
Method for recycling spent lithium metal polymer rechargeable batteries and related materials
The method relates to a pyrometallurgical and hydrometallurgical process for the recovery and recycling of lithium and vanadium compounds from a material comprising spent rechargeable lithium batteries, particularly lithium metal gel and solid polymer electrolyte rechargeable batteries. The method involves providing a mass of the material, hardening it by cooling at a temperature below room temperature, comminuting the mass of cooled and hardened material, digesting with an acid its ashes obtained by incineration, or its solidified salts obtained by molten salt oxidation, or the comminuted mass itself, to give a mother liquor, extracting vanadium compounds from the mother liquor, separating heavy metals and aluminium therefrom, and precipitating lithium carbonate from the remaining solution.
Owner:AVESTOR
Positive electrode active material for non-aqueous electrolyte secondary battery, production method thereof, and non-aqueous electrolyte secondary battery using the same
A positive electrode active material for a non-aqueous electrolyte secondary battery of this invention includes: a lithium nickel composite oxide containing lithium, nickel, and at least one metal element other than lithium and nickel; and a layer containing lithium carbonate, aluminum hydroxide, and aluminum oxide, the layer being carried on the surface of the lithium nickel composite oxide. The lithium nickel composite oxide is composed such that the ratio of the nickel to the total of the nickel and the at least one metal element is 30 mol % or more. The layer is composed such that the amount of the lithium carbonate is 0.5 to 5 mol per 100 mol of the lithium nickel composite oxide. The total of aluminum atoms contained in the aluminum hydroxide and the aluminum oxide is 0.5 to 5 mol per 100 mol of the lithium nickel composite oxide.
Owner:PANASONIC CORP
Method for preparing equal dispersion ferric phosphate lithium nano crystal by hydrothermal synthetis method
InactiveCN101047242AExcellent inert environmentElectrode manufacturing processesPhosphateFerrous salts
A method for preparing uniformly scattered nanocrystal of iron-lithium phosphate by hydrothermal synthesis includes using ferrous salt and phosphoric acid as well as lithium hydroxide as raw materials to obtain reaction pioneer matter under temperature of 40-100deg.c first, then reacting on obtained pioneer matter in high pressure reactor with temperature of 150-200deg.c under hydrothermal condition and processing obtained product by high temperature under protection of inert gas to finally obtain said uniformly scattered nanocrystal with average particle diameter of 0.2-0.5micron.
Owner:胜利油田华鑫石油材料有限公司
Method for preparing high-density spherical lithium iron phosphate and lithium iron manganese phosphate
InactiveCN1632970AWide variety of sourcesImprove conductivityElectrode manufacturing processesLithium compoundsFerrous ammonium phosphatePersulfate
This invention discloses a high-density ball ferric phosphate lithium and manganous phosphate iron lithium process method used in lithium ion battery positive electrode material in energy material process technique field. The process method comprises the following steps: to use ferric persulfate, phosphor or complex builder or manganese sulfate to process the mixture liquid; to react with the hartshorn solution to generate ball ferrous phosphate ammonium or manganese phosphate ferrous front driver; to mix it with lithium carbonate with mole proportion of one to one after washing and drying; under protection of nitrogen gas and after 600 to 900 degrees for 8 to 48 hours to get the ferric phosphate lithium or manganese phosphate ferrous.
Owner:TSINGHUA UNIV
High temperature battery and electrolytes
A high temperature battery of one or more cells is disclosed in which each cell is made by holding an anode electrode and a cathode electrode, of different metallic substances, together through a fused flux wetted to an electrode, which fused flux is an electrolyte, to make an anode-to-cathode contact, and the anode-to-cathode contact is heated, by a heat source, to a high temperature above a threshold temperature to generate voltaic voltage, in excess of any thermoelectric voltage; such batteries with electrodes of various configurations are disclosed. The heat-activated flux and electrolyte, such as borax, may have, vegetable-growth ashes or chemical constituents of ashes, such as lithium carbonate, added to the heat-activated flux and electrolyte to catalyze or improve the current-generating capability of the battery. The preferred anode substance is aluminum, and the preferred cathode substance is copper. With the preferred cathode and anode substances and a heat-activated flux and electrolyte of fused borax between the cathode and anode, the open circuit voltage generated per cell when heated increases from 0.05 volts at 304° C. to 1.3 volts at 651° C.; the threshold temperature in this case is 279° C. Also disclosed is means to move the anode metal with respect to the cathode metal, when the heat-activated flux and electrolyte is fluid, for changing the battery characteristics and for replacing depleted heat-activated flux and electrolyte.
Owner:WILSON JOHN T R
Method for recycling lithium, nickel, cobalt and manganese from waste ternary anode material
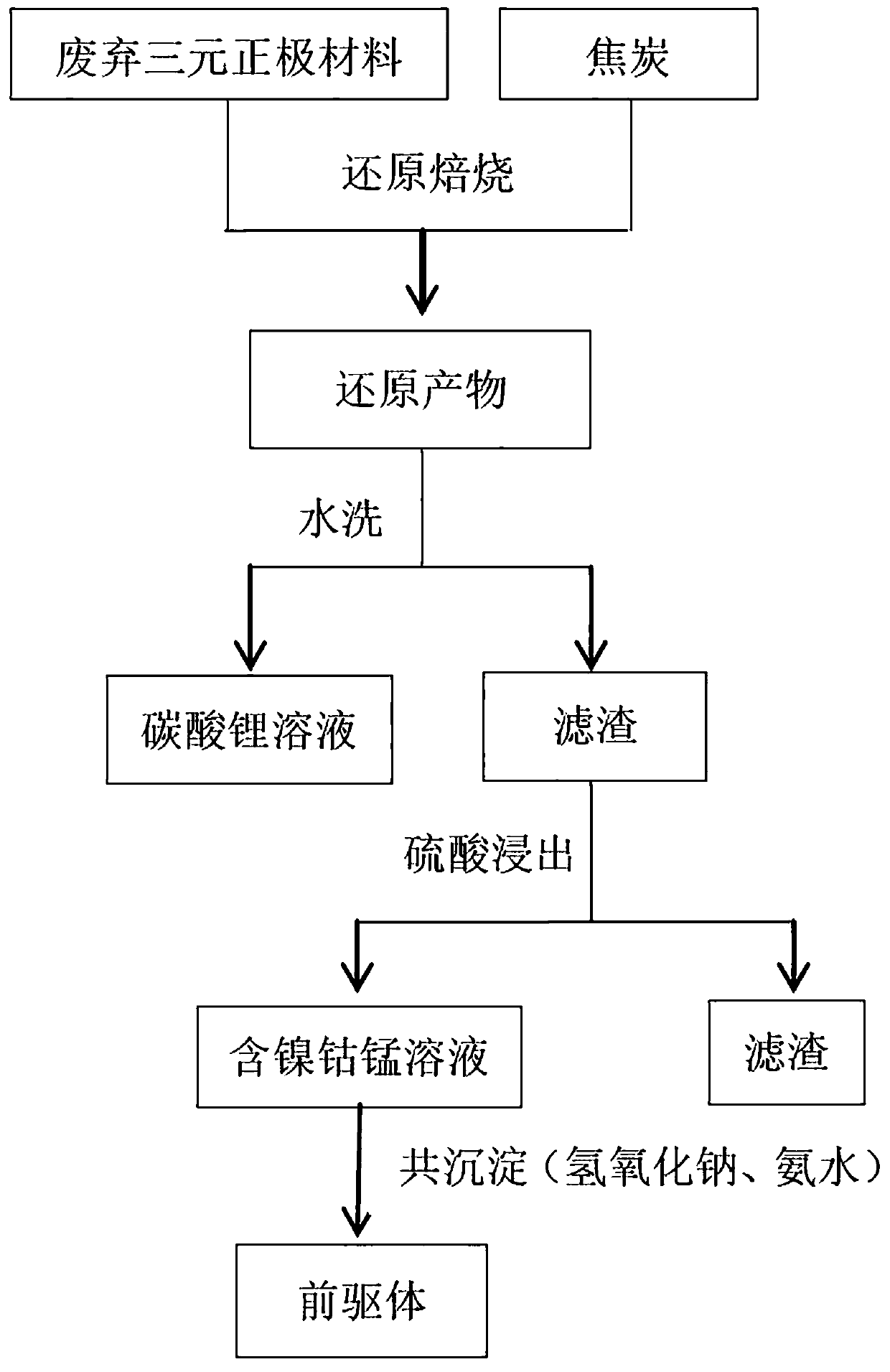
InactiveCN107666022AEfficient and harmless useShort processWaste accumulators reclaimingProcess efficiency improvementReaction temperatureManganese
The invention discloses a method for recycling lithium, nickel, cobalt and manganese from a waste ternary anode material. The method comprises the steps of with the waste ternary anode material as theraw material, adding a carbon reducing agent, carrying out mixing and dosing, carrying out roasting reduction at 500-700 DEG C in a protective atmosphere, adding the roasted product into water, carrying out water-soluble reaction, after the reaction is finished, filtering to obtain lithium carbonate filtrate and a filter residue I, carrying out sulfuric acid leaching on the filter residue I so asto obtain a filtrate containing nickel, cobalt and manganese and a filter residue II, adding sulfate so as to adjust the proportion of nickel, cobalt to manganese in the filtrate containing nickel, cobalt and manganese, carrying out precipitation reaction on the filtrate containing nickel, cobalt and manganese, a sodium hydroxide solution and an ammonia water solution in the protective atmosphere, controlling the reaction temperature at 50-70 DEG C and the pH value at 10-11, obtaining ternary precursor slurry after the precipitation reaction, filtering, washing, and drying, so as to obtain the ternary precursor. The method has the beneficial effects that the separation condition of lithium carbonate is simple, the costs of leaching processes of nickel, cobalt and manganese and the regeneration process of the ternary material precursor are low, and the recycling rate is high.
Owner:HUNAN UNIV OF TECH
Method for recycling waste lithium cobalt oxide lithium ion battery
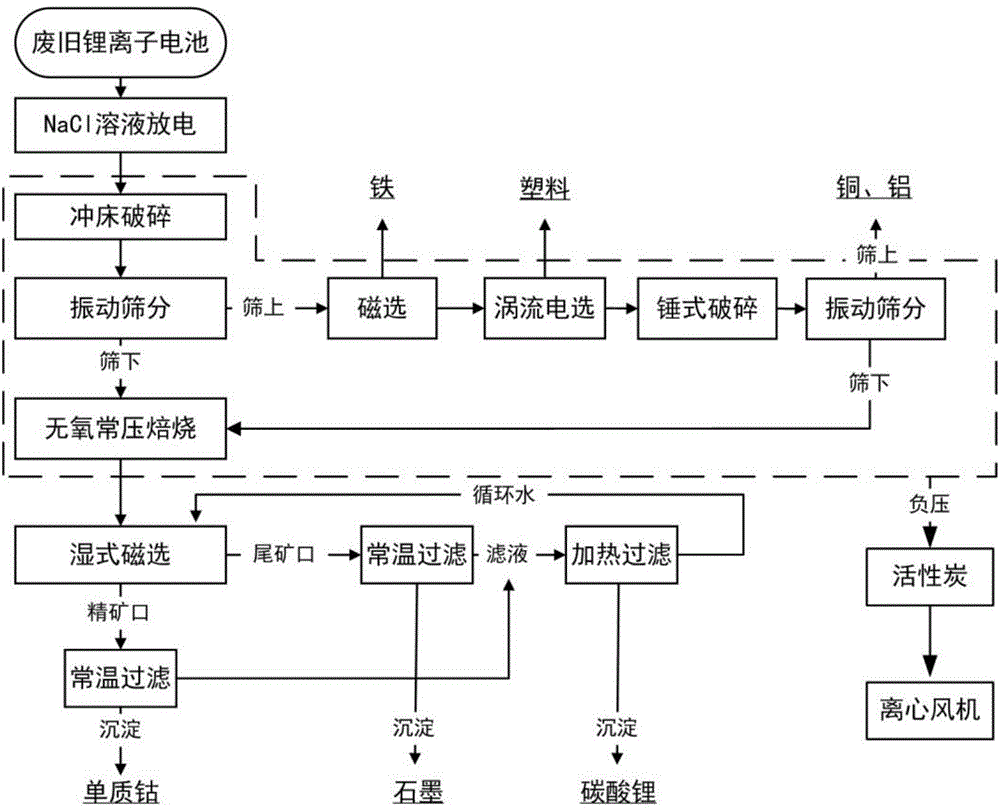
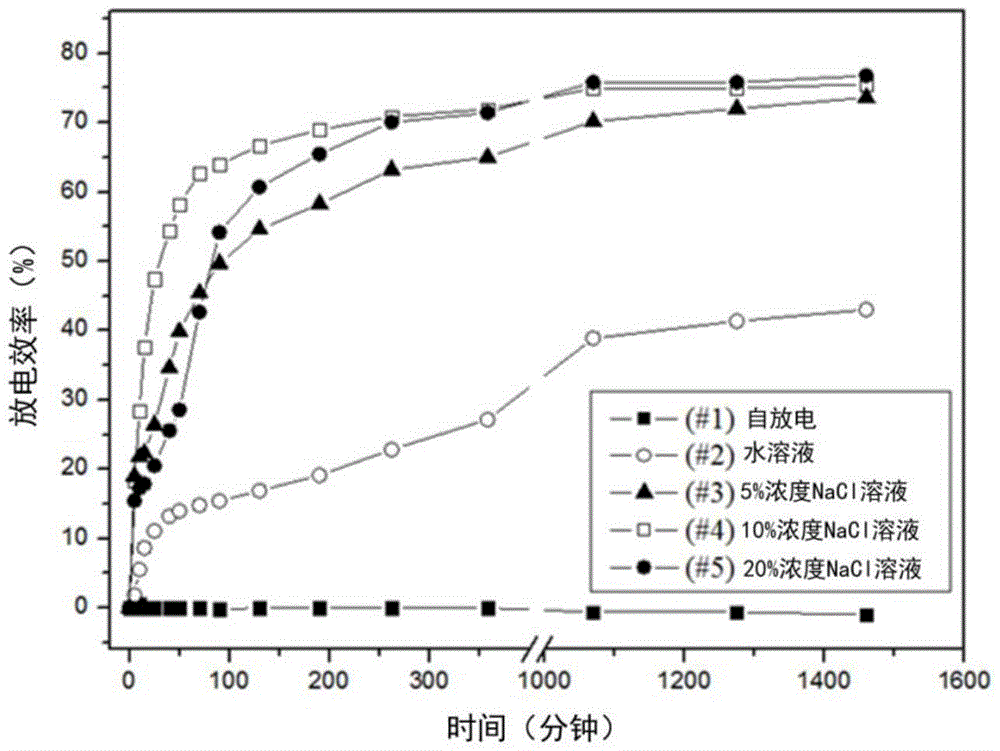
ActiveCN104577249AFully resourcedReduce lossesWaste accumulators reclaimingBattery recyclingPunch pressCobalt
The invention relates to a method for recycling waste lithium cobalt oxide lithium ion battery. The method is characterized in that valuable components in the waste lithium ion battery are completely recycled by integrating the processes of crushing by a punch press, magnetic separation, eddy current selection, anaerobic atmospheric roasting, temperature-variable filtering and the like, and products with the relatively high additional values, such as elemental crude cobalt, lithium carbonate, graphite, copper, aluminum, iron, and plastics are obtained. Manners of crushing by a punch press, magnetic separation, eddy current selection and the like are adopted for performing material separation, so that the original physical properties of materials are kept. Meanwhile, positive powder and negative powder of an electrode material are cooperatively treated, the negative graphite material is effectively utilized, the in-situ preparation of a resource can be realized, and the waste lithium ion battery can be relatively completely recycled. The anaerobic atmospheric roasting is adopted, so that the reaction condition is relatively loose, the loss of a graphite material is reduced, the cost is saved, the technological flow is simplified, and the industrial application practice can be facilitated.
Owner:SHANGHAI JIAO TONG UNIV
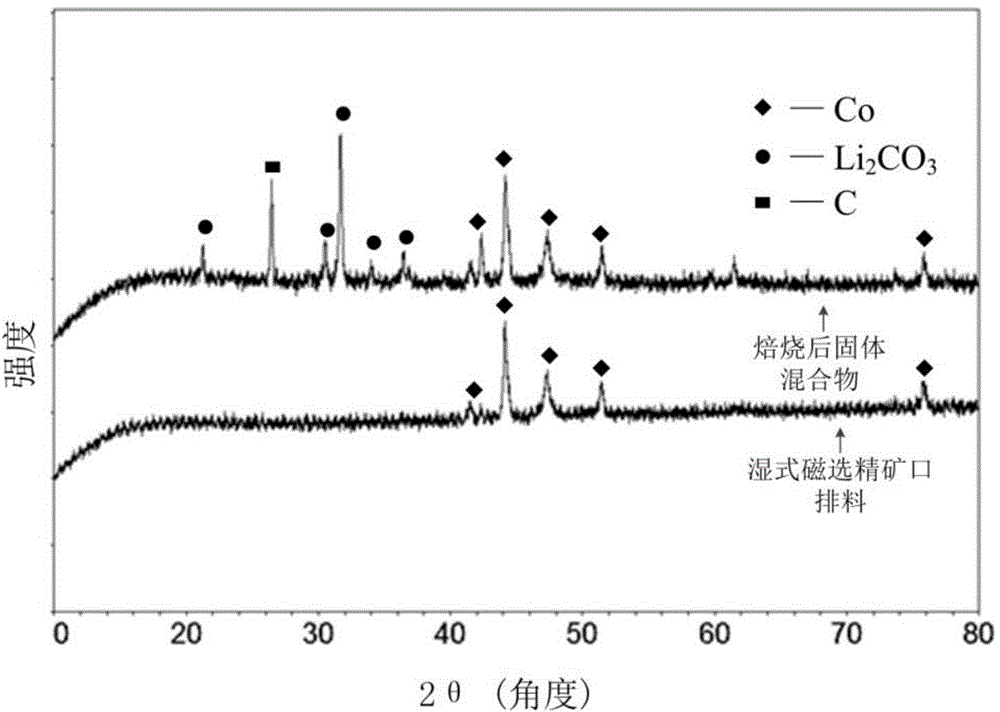
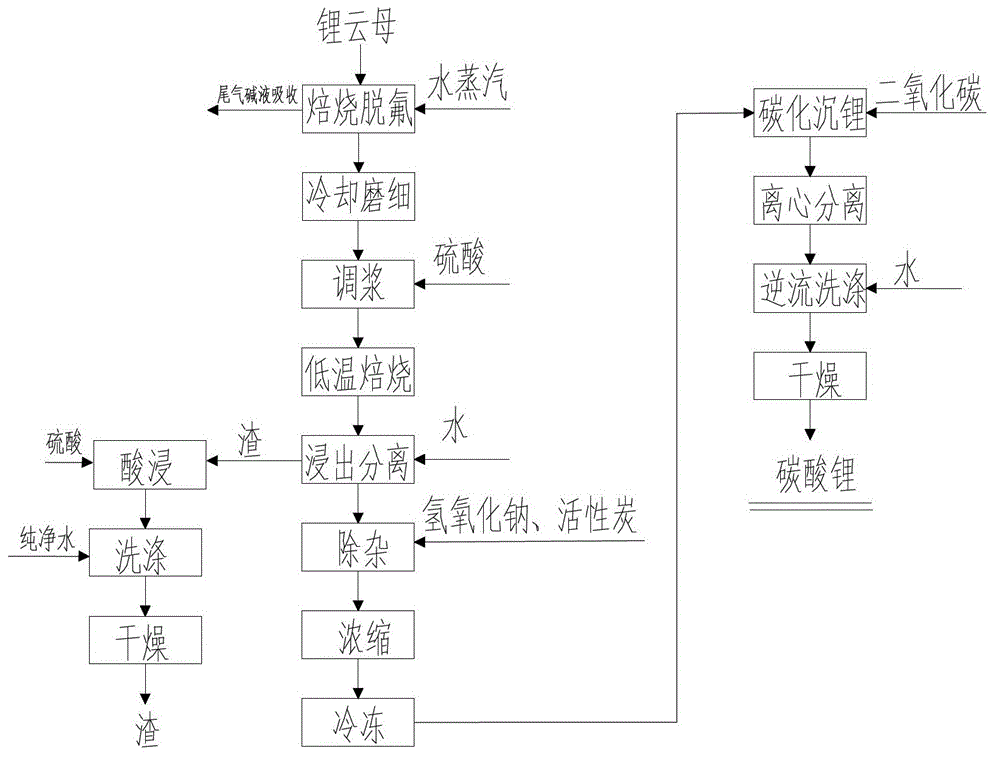
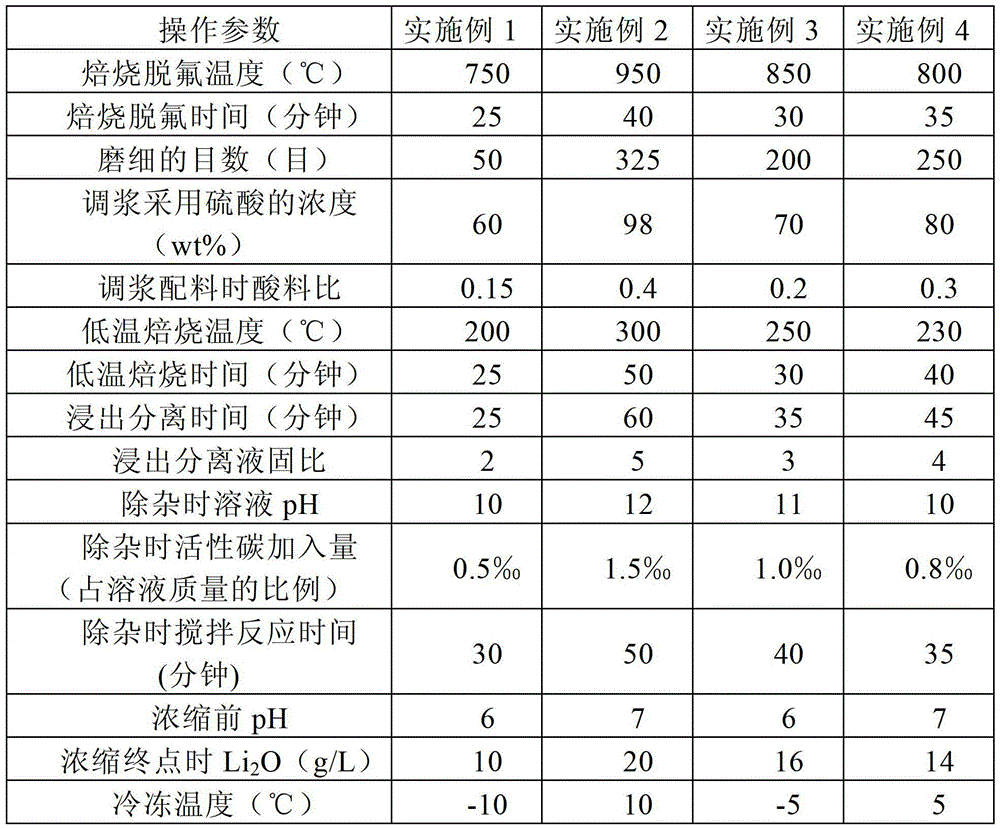
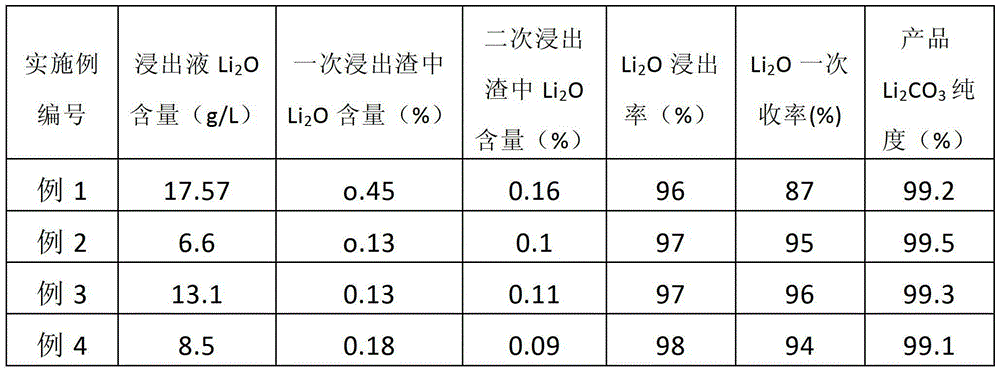
Method for preparing lithium carbonate from lepidolite through sulfuric acid roasting method
ActiveCN103145158AReduce dosageSimple cleaning processLithium carbonates/bicarbonatesResource utilizationLithium carbonate
The invention discloses a method for preparing lithium carbonate from lepidolite through a sulfuric acid roasting method, which comprises the following process steps: (1) roasting, and defluorinating; (2) cooling, and grinding; (3) pulping; (4) roasting at low temperature; (5) leaching, and separating; (6) removing impurities; (7) concentrating; (8) freezing; and (9) performing carbonization lithium precipitation to obtain the lithium carbonate product. The method for preparing lithium carbonate from lepidolite through a sulfuric acid roasting method is simple in process, low in sulfuric acid consumption, low in energy consumption and high in resource utilization rate, and causes less environment pollution.
Owner:GANFENG LITHIUM CO LTD
Method for recycling battery-grade iron phosphate in lithium iron phosphate battery and preparing lithium iron phosphate positive material by utilizing waste lithium ion phosphate battery
ActiveCN104953200AAchieve synthesisRealize the recovery of added valueWaste accumulators reclaimingBattery recyclingPhosphatePhosphoric acid
The invention relates to a method for recycling battery-grade iron phosphate in a lithium iron phosphate battery and preparing a lithium iron phosphate positive material by utilizing a waste lithium ion phosphate battery, and relates to a method for recycling a battery and preparing the battery positive material by utilizing the waste battery recycled material, solving the problems of the traditional method for recycling the LiFePO4 lithium ion battery positive electrode that the purity of the obtained element or substances is low and the obtained element or substances cannot be used for preparing the LiFePO4 lithium ion battery positive electrode. The method comprises the steps: I, crushing a positive pole piece, and carrying out heat treatment; II, dissolving the crushed positive pole piece in an acid solution; III, charging a surface active agent; IV, charging an alkaline solution, thereby obtaining a battery-grade iron phosphate; V, charging sodium carbonate to obtain a lithium carbonate; VI, mixing iron phosphate, lithium carbonate and a carbon source reduction agent; and VII, calcining. In the process for recycling the battery-grade iron phosphate in the lithium iron phosphate battery and preparing the lithium iron phosphate positive material by utilizing the waste lithium iron phosphate battery, no secondary pollution is produced, and the comprehensive and high-added-value recycling of the waste lithium iron phosphate battery can be realized.
Owner:HARBIN INST OF TECH
Method for producing positive plate material for lithium secondary cell
InactiveUS20050265909A1Improve battery performanceElectrode manufacturing processesActive material electrodesAlkaline earth metalLithium carbonate
A positive plate material for lithium secondary cells stably exhibiting excellent performance including the cell initial capacity, cycle characteristics, and the safety. The material is produced by dripping an aqueous solution of a salt (e.g., cobalt sulfate) of a doping element (e.g., a transition metal, an alkaline metal, an alkaline-earth metal, B, or Al) into an alkaline solution, a carbonate solution, or a hydrogencarbonate solution in any one of which a compound (e.g., manganese oxide) of a metal (Mn, Co, Ni, or the like) which is the major component of the positive plate material so as to precipitate the compound of the doping element on the major component compound and to cover the major component compound, mixing the major component compound covered with the doping element with a lithium compound (e.g., lithium carbonate), and firing the mixture.
Owner:NIKKO MATERIALS CO LTD
A method for recovering lithium and iron from electric vehicle lithium iron phosphate power battery
ActiveCN102285673ASimple processIron oxides/hydroxidesLithium carbonates/bicarbonatesPower batteryLithium iron phosphate
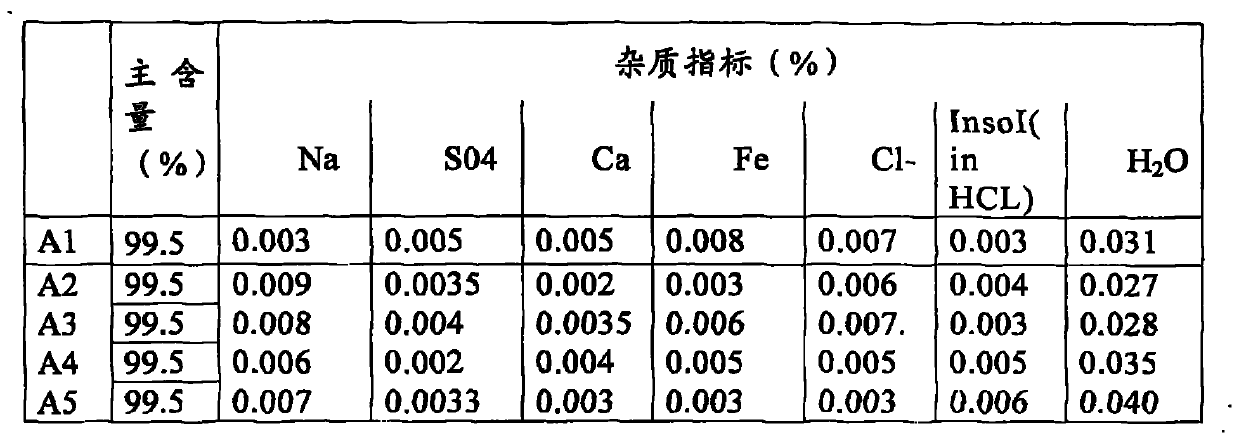
The invention discloses a method for recovering lithium and iron from a lithium iron phosphate power battery for an electromobile. The method comprises the following steps: 1) disassembling the lithium iron phosphate power battery so as to obtain a positive electrode material, smashing and screening so as to obtain a powder material; 2) adding an alkali solution in the powder material to dissolve aluminum and oxide of aluminum, and filtering so as to obtain filter mud; 3) lixiviating the filter mud with a mixed solution of an acid and a reducing agent so as to obtain lixivium; 4) adding an alkali to regulate the pH value of the lixivium to 1.5-3, precipitating to separate out iron hydroxide, and filtering so as to obtain filtrate; 5) firing iron hydroxide obtained in the step 4) so as to obtain iron oxide; 6) regulating the pH value of the lixivium to 5.0-8.0 with an alkali, precipitating impurities in the lixivium, and filtering so as to obtain filtrate; and 7) adding a solid sodium carbonate in the filtrate, and concentrating and crystallizing the obtained solution so as to obtain lithium carbonate. The recovering method disclosed by the invention has simple process, can be used for simultaneously recovering iron and lithium and can be directly used for production, and the purity of prepared lithium carbonate can reach above 98.5%.
Owner:GUANGDONG BRUNP RECYCLING TECH +1
Transparent lead-free fritted glaze with low expansion coefficient and preparation method thereof
The invention relates to a transparent lead-free fritted glaze with low expansion coefficient and a preparation method thereof. The fritted glaze comprises the following chemical ingredients of: 59 to 66 percent of SiO2, 10 to 15 percent of Al2O3, 5 to 10 percent of B2O3, 3 to 6 percent of CaO, 4 to 8 percent of MgO, 0 to 3 percent of K2O, 0 to 2 percent of Na2O, 0 to 1 percent of Li2O, 1 to 5 percent of ZnO and 0 to 2 percent of SrO; and the fritted glaze comprises the raw materials of: 10 to 25 percent of kaolin, 15 to 30 percent of quartz, 10 to 25 percent of potassium feldspar, 0 to 8 percent of dolomite, 2 to 6 percent of grammite, 10 to 20 percent of roasted talc, 1 to 6 percent of zinc oxide, 2 to 8 percent of boric acid, 5 to 15 percent of calcium borate, 1 to 5 percent of alumina, 0 to 2 percent of lithium carbonate and 0 to 3 percent of strontium carbonate. The expansion coefficient of the fritted glaze is 3.7-4.5*10-6 / DEG C (RT to 500 DEG C), the melted temperature is 1400 DEG C to 1500 DEG C, the glaze firing temperature is 1130 DEG C to 1230 DEG C; and the transparent lead-free fritted glaze has fine and bright glaze surface, high transparency, low expansion coefficient as well as no lead precipitation and is particularly suitable for double-fired hard porcelain body with lower expansion coefficient.
Owner:JINGDEZHEN CERAMIC INSTITUTE
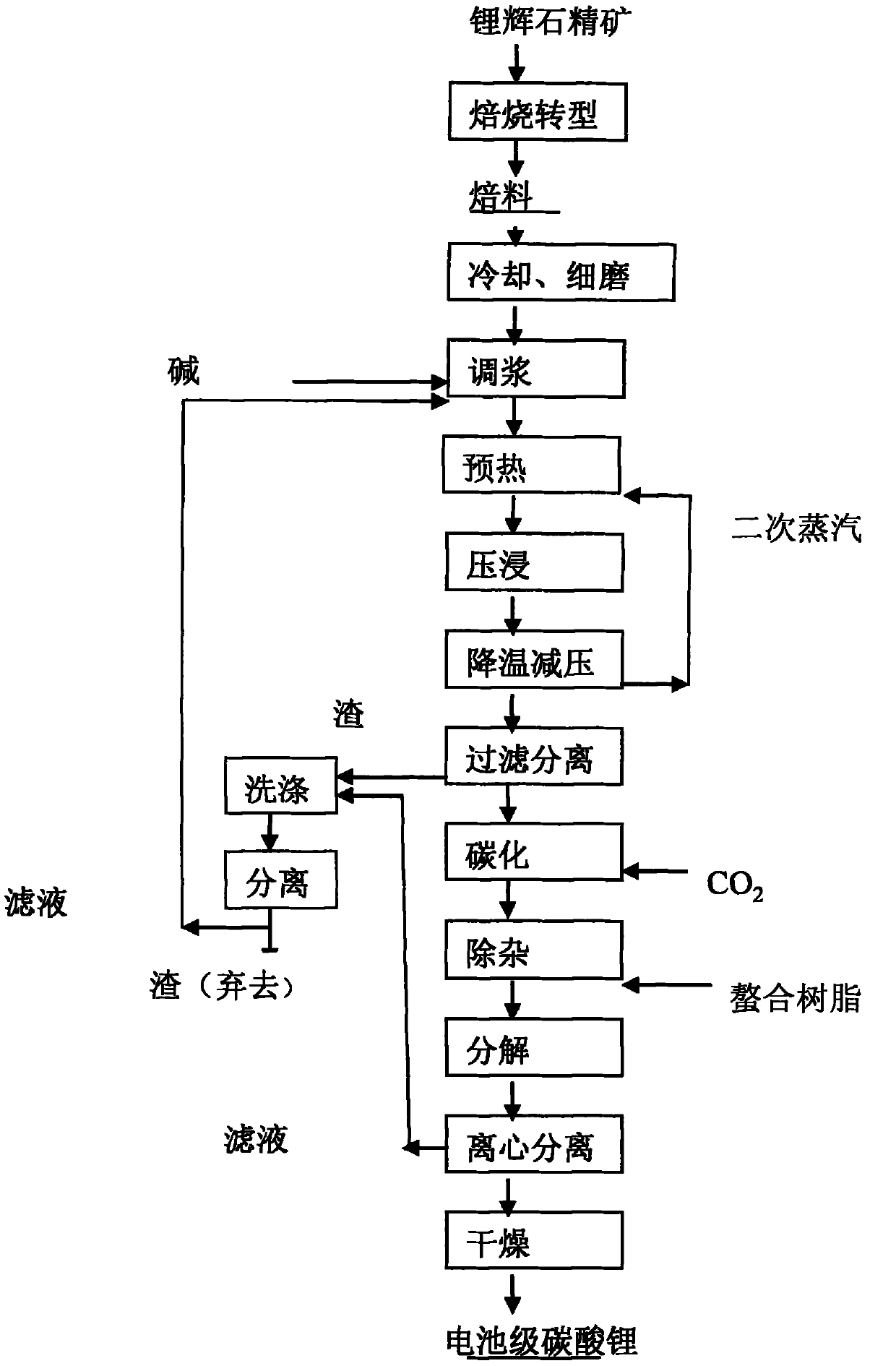
Method for preparing lithium carbonate by extracting lithium from lepidolite
ActiveCN101302018AImprove leaching rateIncrease concentrationLithium carbonates/bicarbonatesCarbonizationIon exchange
Owner:GANFENG LITHIUM CO LTD
Method for preparing spherical ferric lithium phosphate by oxidation control crystal-carbon thermal reduction method
InactiveCN101337666AWide variety of sourcesLow costCell electrodesPhosphorus compoundsElectric dischargePhosphoric acid
The invention discloses a method for preparing spherical lithium iron phosphate by adopting oxidation control crystallization-carbon thermal reduction. The preparation method comprises the steps of conducting reaction among a mixed water solution of bivalence malysite and phosphoric acid, an aqueous solution of ammonia and an oxidant; synthesizing a spherical precursor of a spherical hydration iron phosphate through the process of oxidation control crystallization; washing; drying; pre-burning for dewatering; mixing with lithium carbonate and a carbon source; and obtaining the lithium iron phosphate through high temperature carbon thermal reduction under the protection of inertial or reducing atmosphere. By using the preparation method, the spherical lithium iron phosphate, an anode material of a lithium ion battery, is prepared, and has high bulk density and high volume specific capacity. The average grain size of the spherical lithium iron phosphate is between 6 and 8 mum, the electric discharge specific capacity thereof for the first time can reach up to 145-150mAh / g at the room temperature of 0.5C.
Owner:TSINGHUA UNIV
Method for preparing lithium carbonate by using salt lake brine with high magnesium-to-lithium ratio
InactiveCN101698488AReduce energy consumptionNo way outLithium carbonates/bicarbonatesEvaporationCalcination
The invention provides a method for preparing industrial lithium carbonate by using salt lake brine with a high magnesium-to-lithium ratio. In the method, a TBP-CON-KS+FeCl3 is used as an extraction system to extract and back-extract impurity-free salt lake brine with a high magnesium-to-lithium ratio, the residual liquid obtained after back-extraction is converted by alkaline liquor for precipitation, the precipitate is washed to form an industrial lithium carbonate product and the lithium carbonate content is more than or equal to 99.0 percent and is in accordance with the requirements of GB / T 11 075-2003 standards. The method has the advantages that: liquid-liquid extraction with an organic solvent is adopted to realize the separation of lithium from magnesium, the lithium carbonate is precipitated by inorganic slats, the lithium carbonate is extracted from the salt lake brine with a high magnesium-to-lithium ratio, the process is simple, the control is easy, the operational reliability is high and the application range is wide; a process of calcination and diluted lithium solution evaporation and concentration is saved, the energy consumption is only 30 to 50 percent of that of the conventional process for producing lithium carbonate by using lithium-containing brine; initial raw material consumption comparison show that the production cost of the method is only about 8 percent of that of the prior art; and the raw material brine can return to a storage pool after the extraction of the lithium carbonate, so no by production disposal problem is involved, environmental pollution is relatively low and lithium yield in the whole process is more than or equal to 70 percent.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Comprehensive recycling method of lithium ion battery anode material
ActiveCN107267759AReduce sorting costsImprove recycling economicsWaste accumulators reclaimingProcess efficiency improvementSlagElectrical battery
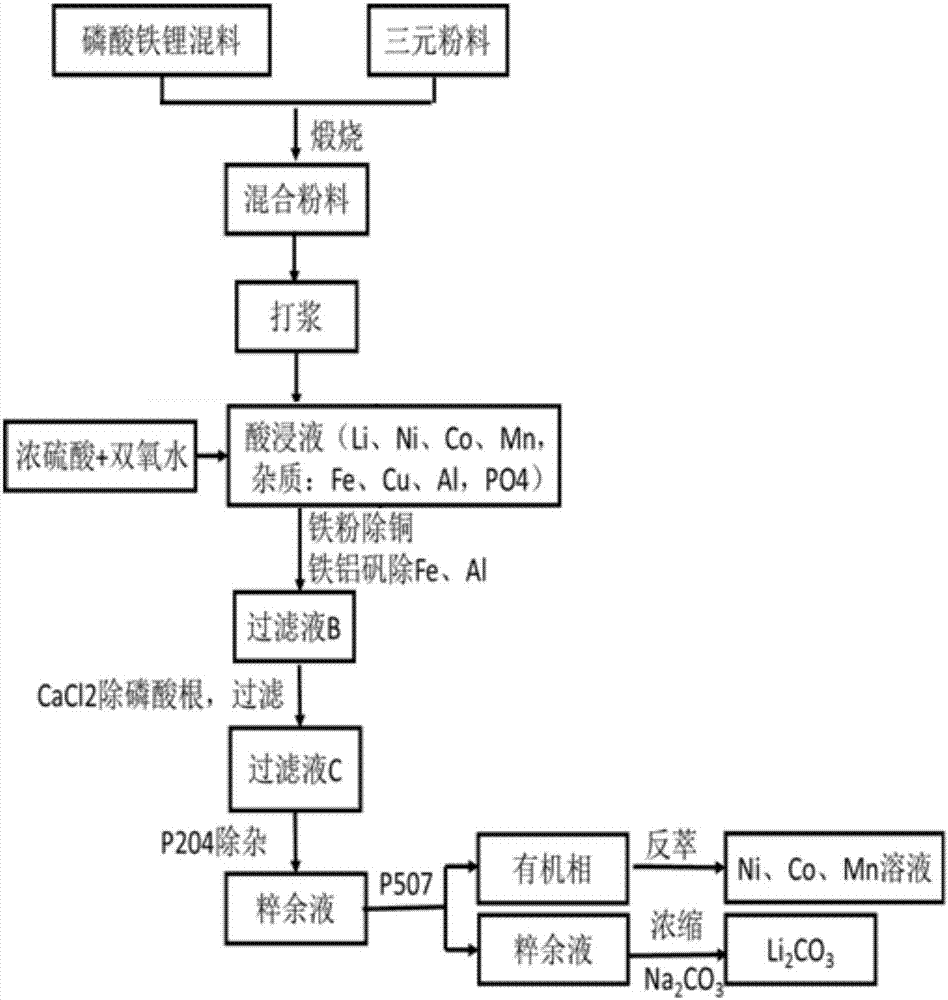
The invention provides a comprehensive recycling method of a lithium ion battery anode material. The method comprises the steps that the anode material of lithium iron phosphate and the anode material of a ternary battery are subjected to high-temperature pretreatment; a product is added into water for pulping processing; concentrated sulfuric acid and hydrogen peroxide are added, and filtering is performed to remove undissolved substances; iron powder is added, filtering is performed to remove copper elements, and heating is performed to generate iron aluminum vanadium slag; a calcium chloride solution is added, and filtering is performed to remove phosphate radicals; series-connection countercurrent extraction is performed through an extraction agent P204 to remove Fe and Ca impurities, and series-connection countercurrent extraction is performed through an extraction agent P507 to separate Ni, Co, Mn elements from Li elements; the organic phase is subjected to reverse extraction with sulfuric acid, a Ni, Ca and Mn solution is obtained, and recycling of nickel, cobalt and manganese is achieved; and the water phase is concentrated, and then a saturated sodium carbonate solution is added to generate lithium carbonate precipitation. By means of the comprehensive recycling method, the anode material of a lithium iron phosphate battery and the anode material of the ternary battery are recycled simultaneously, the battery separation cost is lowered, and the economic benefits of lithium battery recycling are increased.
Owner:南京国轩新能源有限公司
Method for extracting lithium salt from spodumene
ActiveCN101948124ASimple processReduce manufacturing costProcess efficiency improvementLithium carbonates/bicarbonatesSocial benefitsResource utilization
Owner:GANFENG LITHIUM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com