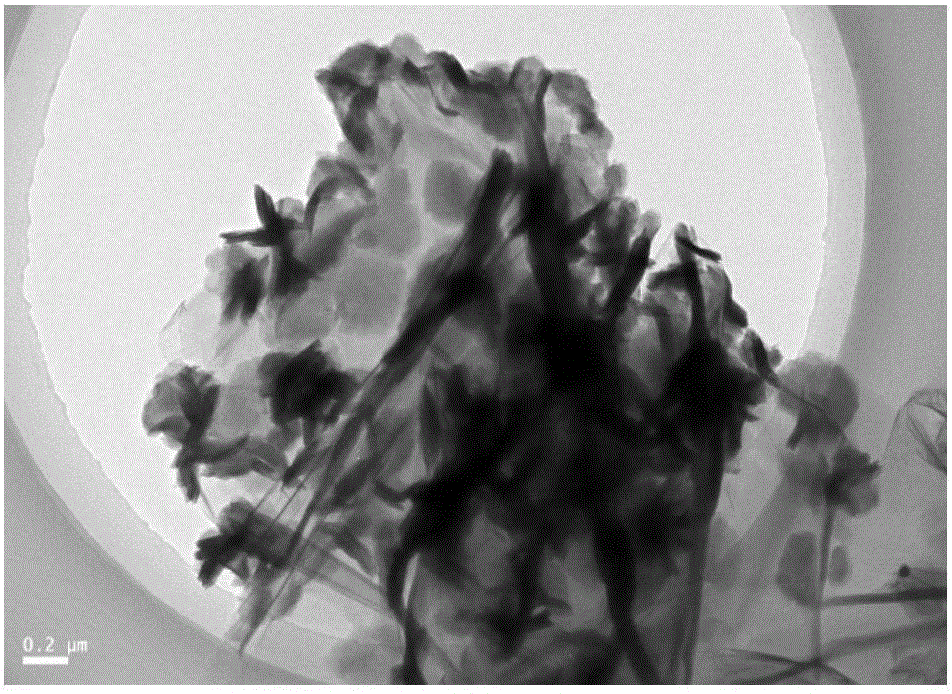
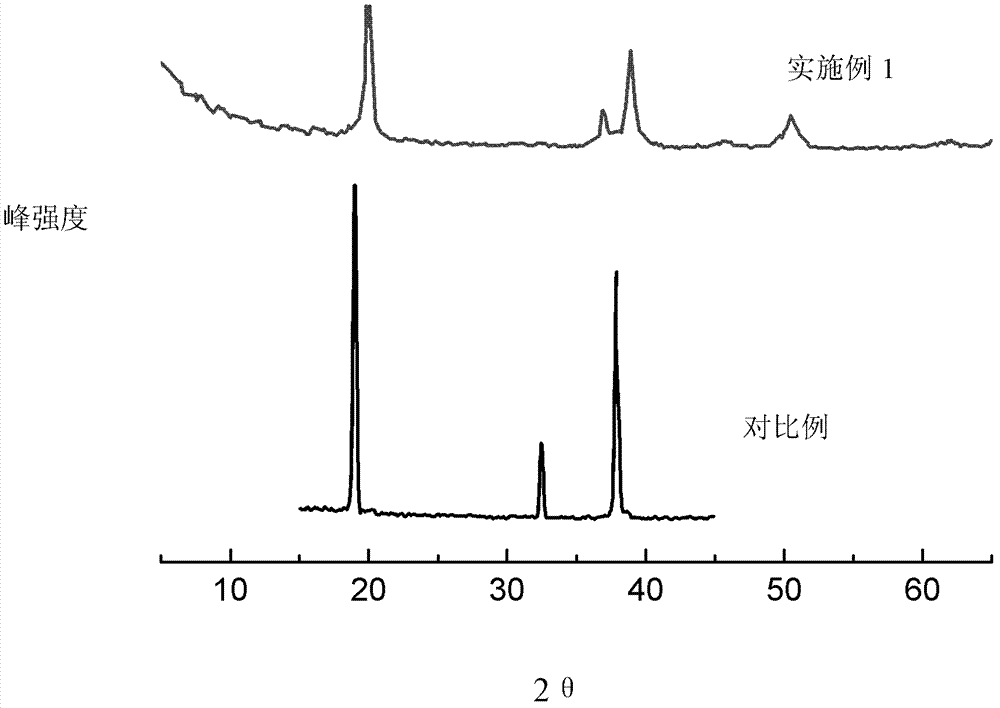
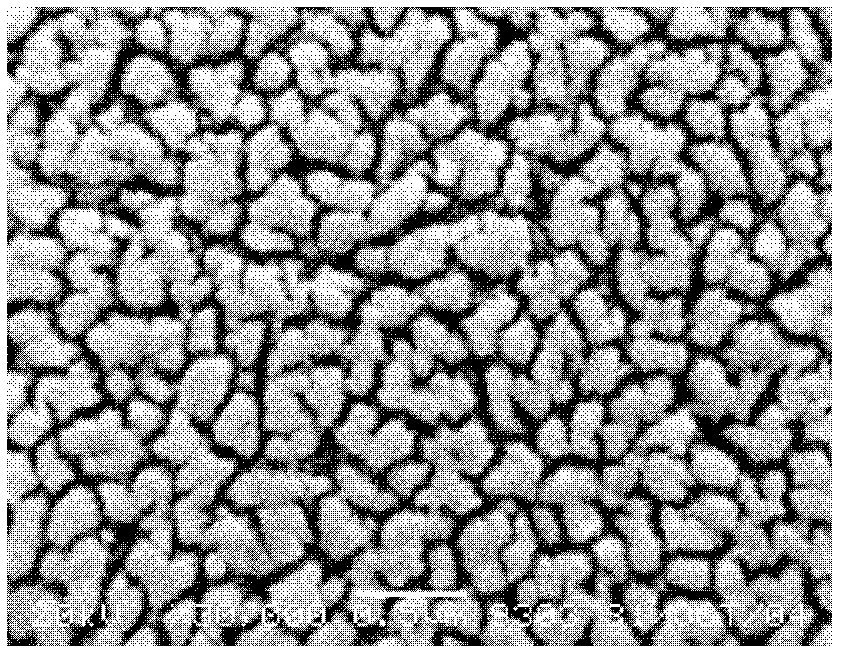
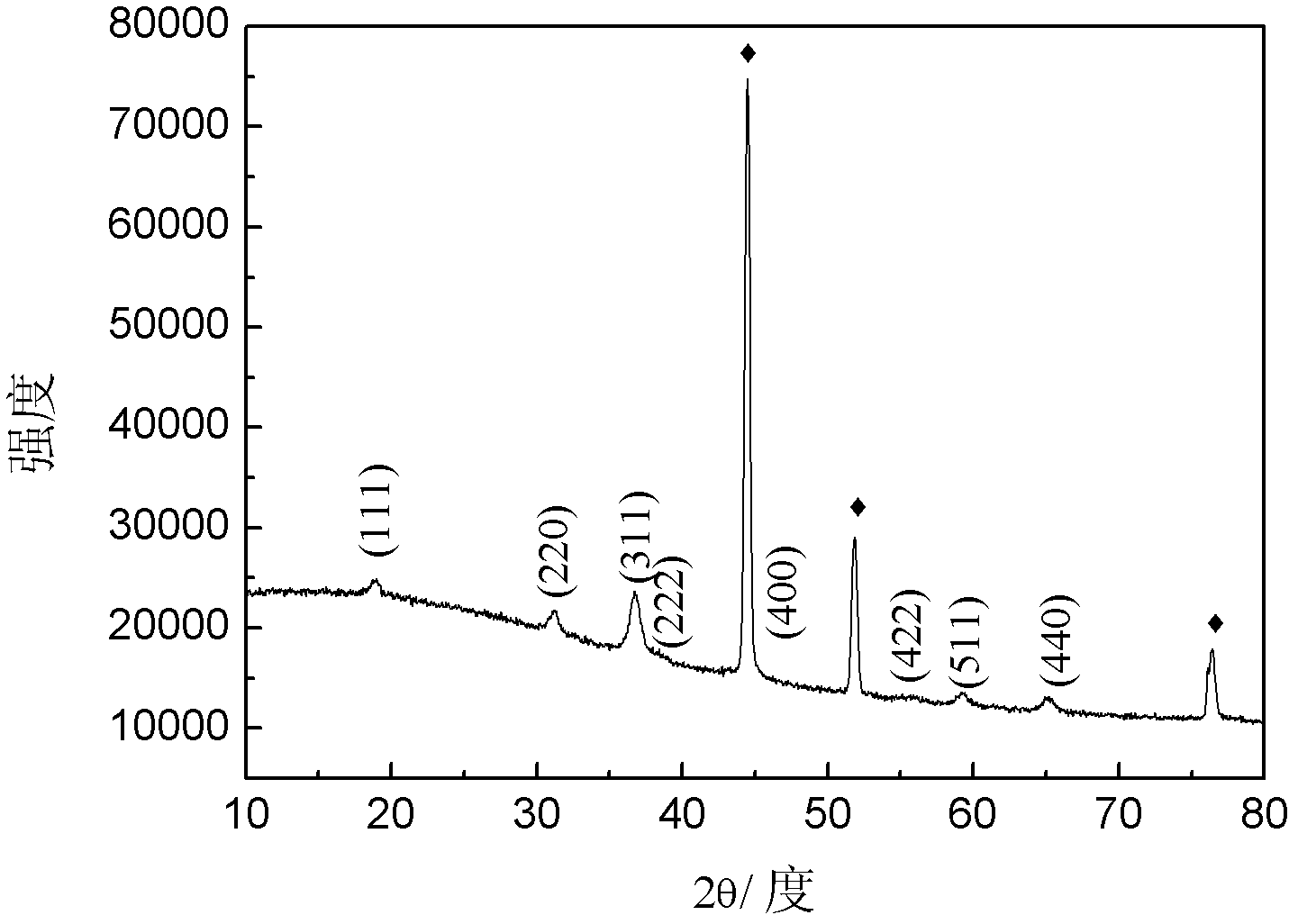
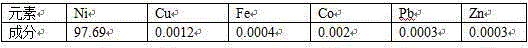Patents
Literature
728 results about "Cobalt hydroxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cobalt hydroxide may refer to: Cobalt hydroxide Cobalt hydroxide
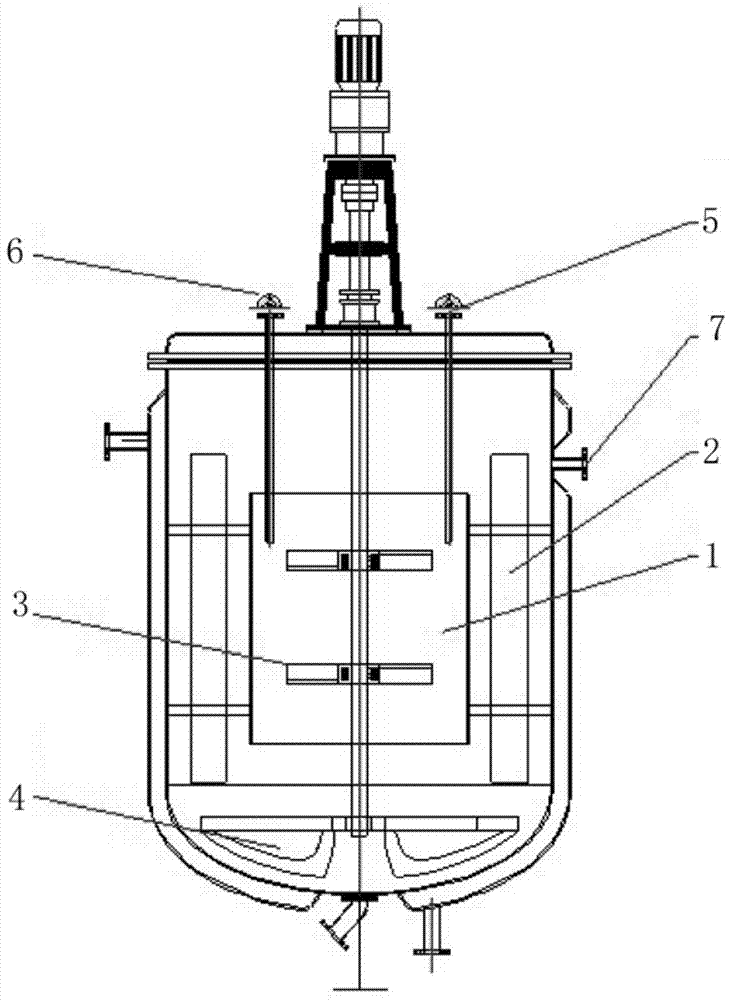
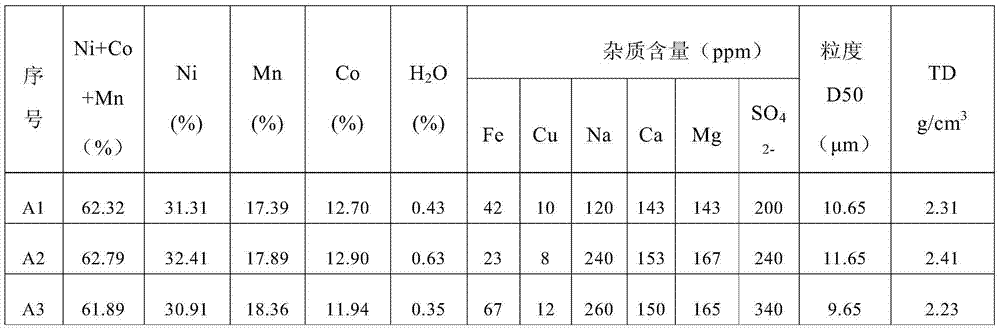
Method for preparing lithium cobaltate by directly using invalid lithium ion battery
InactiveCN102030375AReduce dispersionHigh purityCell electrodesCobalt compoundsElectrical batteryPotassium hydroxide
The invention provides a method for preparing lithium cobaltate by directly using an invalid lithium ion battery. The method comprises the following steps: crushing the invalid lithium ion battery or scraps generated when a lithium cobaltate battery is produced by a mechanical crusher at normal temperature; adding water and one or more of acetic acid, sulfuric acid, hydrochloric acid or nitric acid to produce mixed aqueous solution of the battery scraps and acid; filling the mixed aqueous solution into a hermetic pressure reactor, and controlling the temperature in the reactor to be between 50 and 150 DEG C; introducing or adding one leaching additive of sulfur dioxide or hydrogen, or adding hydrazine hydrate; stirring and leaching, cooling, and filtering; adding one precipitator of sodium carbonate, potassium carbonate and ammonium carbonate, or adding composite precipitator consisting of one of the sodium carbonate, the potassium carbonate and the ammonium carbonate and one of sodium hydroxide and potassium hydroxide to obtain mixture of lithium carbonate, cobalt carbonate and cobalt hydroxide; drying and calcining at high temperature to produce a lithium cobaltate product. The method is particularly suitable for the treatment scale of medium-sized and small enterprises, and is an effective method for directly materializing cobalt secondary resources.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Preparation method for nickel-manganese-cobalt anode material of lithium ion battery
InactiveCN102306765AImprove discharge capacityImprove cycle performanceCell electrodesManganeseLithium compound
The present invention relates to a preparation method for a nickel-manganese-cobalt anode material of a lithium ion battery. According to the present invention, in the presence of nitrogen atmosphere, a mixed solution containing nickel iron, manganese iron and cobalt ion reacts with a precipitating agent, then processes of aging, washing, drying and the like are performed to obtain a nickel-manganese-cobalt hydroxide precursor, the synthesized precursor material has spherical morphology, ideal particle size distribution and high tap density; the precursor, a lithium compound and a doped compound are mixed, then the sintering processing is performed for twice to prepare the nickel-manganese-cobalt three-element composite anode material. The method has characteristics of simple synthesis process, easy process controlling, low energy consumption, high efficiency and low cost, and is applicable for the industrial production; the prepared precursor material has characteristics of spherical morphology, uniform particle distribution and high tap density; the discharge capacity of the battery is improved through doping the metals; the cycle performance of the battery is stable.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Preparation of doped cobaltic-cobaltous oxide
InactiveCN101279771AImprove stabilityImprove electrochemical performanceCobalt oxides/hydroxidesSal ammoniacNitrate
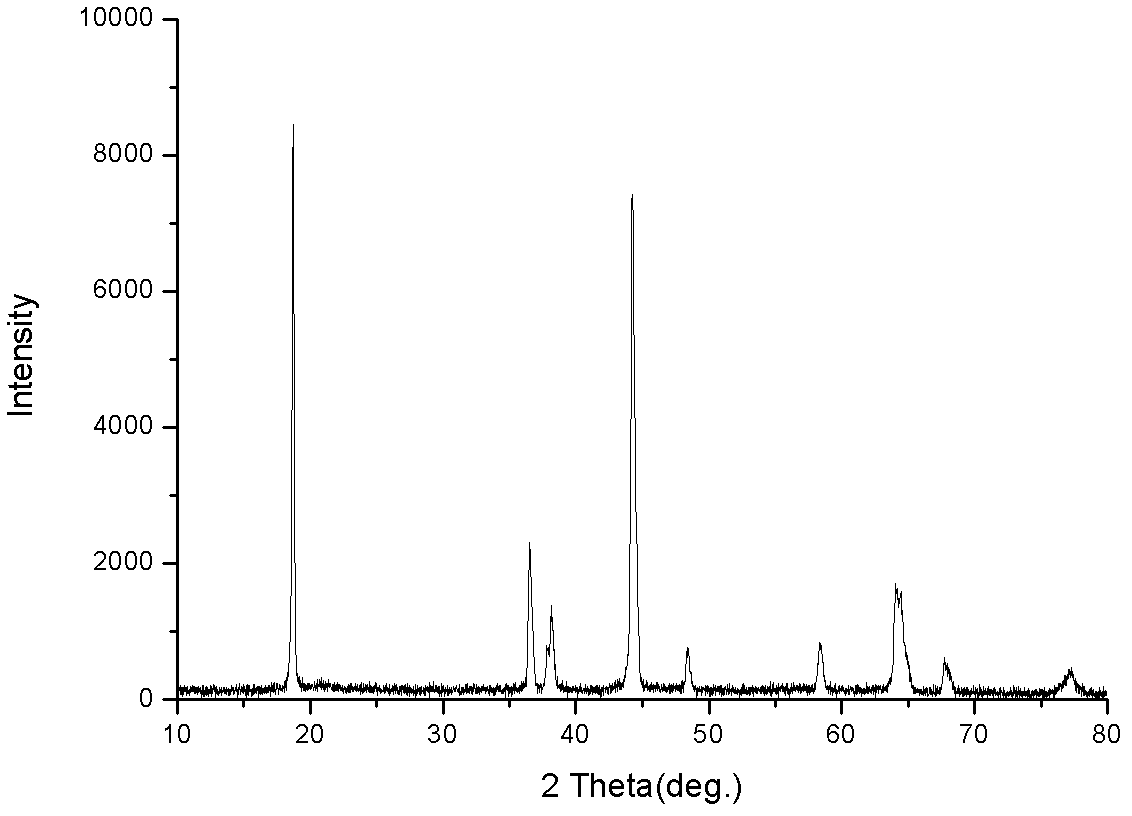
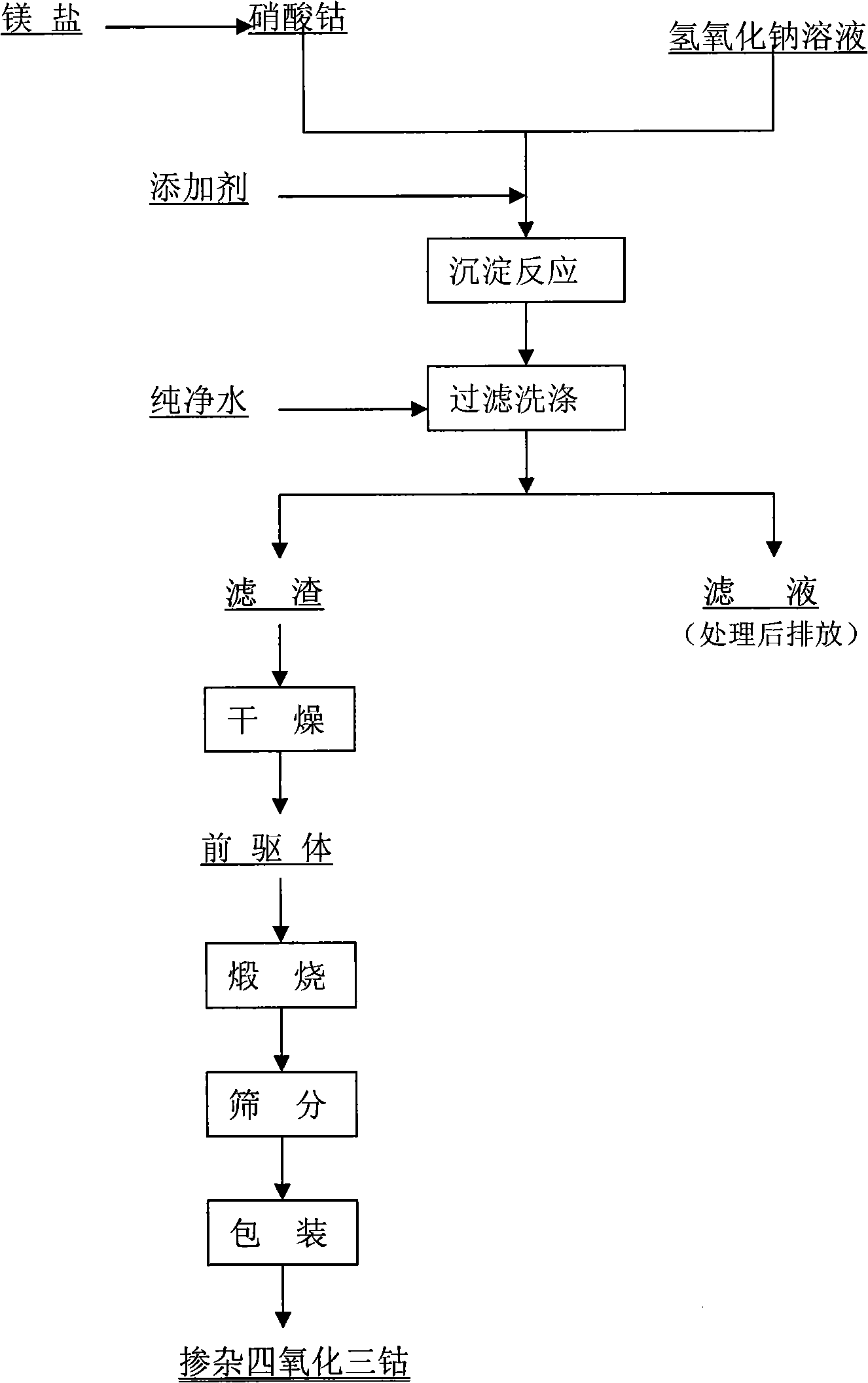
Disclosed is a process for preparing a doping cobaltosic oxide, which relates to a method for the production of a modified cobaltosic oxide used for a Lithium-ion battery anode material. The method is characterized in that the preparation process comprises: 1) mixing a cobalt nitrate solution containing doped chemical ions with a mixed precipitator solution containing ammonia and sodium hydroxide and making the mixture react for eight to twenty hours at a pH value of between 8.4 to 10 and a temperature of between 40 and 80 DEG C so as to prepare a cobalt hydroxide precipitation containing doped chemical; and 2) washing and drying the cobalt hydroxide precipitation containing the doped chemical and then burning the precipitation for two to six hours at a temperature of between 500 and 800 DEG C so as to obtain the doping cobaltosic oxide. The method of the invention can get even particles with regular shapes after the reaction, the particle sizes of the doping cobalt hydroxide are controllable in a certain range, and the doping cobaltosic oxide can be obtained by calcinations. The method of preparation is characterized in that a magnesium source, an aluminum source, a titanium source, etc. are induced to the cobalt nitrate solution directly, and the process and operation are simple and easy.
Owner:JINCHUAN GROUP LIMITED
Preparation method of Co3O4 with large grain size and uniformly doped with aluminum
ActiveCN108011101AEvenly distributedUniform particle size distributionCell electrodesCobalt oxides/hydroxidesCobalt(II,III) oxideMicrometer
The invention relates to a preparation method of Co3O4 with large grain size and uniformly doped with aluminum. The invention provides the preparation method of the Co3O4 which is uniformly doped withaluminum and is large in grain size and uniform in particle distribution, and the obtained Co3O4 with the large grain size and uniformly doped with aluminum can completely conform to the requirementof preparation of 4.45V high-voltage lithium cobalt oxide. According to the method, the large-grain size and aluminum-doped cobalt carbonate is synthesized by a wet method, and the problems of difficulty in enlargement of cobalt hydroxide (or hydroxyl cobalt) system grain size and non-uniform particle distribution are solved; with the regard to the problem of uniform aluminum doping of a cobalt carbonate system, a parameter is set from principle, an aluminum compound is prevented from being independently separated out and gathered, the doped Al element can be uniformly distributed in the Co3O4, the grain size reaches 15 micrometers or above, and the particle distribution is uniform; and the lithium cobalt oxide prepared from the aluminum-doped Co3O4 has high specific capacity and excellentcycle property under 4.45V.
Owner:취저우화여우코발트뉴머터리얼컴퍼니리미티드
Preparation method of hollow nickel cobaltate nano polyhedron
The invention discloses a preparation method of a hollow nickel cobaltate nano polyhedron. The method comprises the following steps: (1) mixing an organic metal framework complex ZIF-67 and an alcoholic solution of nickel nitrate uniformly, and reacting at the temperature condition of 80-100 DEG C; (2) cooling to room temperature after reaction, collecting precipitate, cleaning by ethyl alcohol or other non-polar solvents, centrifuging, removing the solvents, and carrying out vacuum drying so as to obtain a hollow nickel cobalt hydroxide polyhedron; and (3) annealing the hollow nickel cobalt hydroxide polyhedron in air heating so as to obtain the porous nickel cobaltate nano hollow polyhedron. The method is simple to operate, and is environmentally friendly, and the mold plate removing process is omitted; the hollow obtained nickel cobaltate nano polyhedron has the relatively high specific surface area, relatively low mass transfer resistance and relatively excellent structure stability; as an electrode material of the lithium ion battery, the hollow nickel cobaltate nano polyhedron is superior to an existing transition metal oxide electrode material in cost and performance.
Owner:NANJING UNIV OF TECH
Preparation method of lithium-ion battery cathode material coated aluminum
InactiveCN102299299ACoating process conditions are easy to controlImprove high temperature stabilityCell electrodesNickel saltElectrical battery
The invention relates to a method for preparing aluminum-coated anode materials of lithium-ion batteries, which is characterized in that: firstly, soluble nickel salts, cobalt salts, and manganese salts are formulated into salt solutions, and then mixed with ammonia-water-mixed sodium hydroxide or potassium hydroxide solutions React to form precursor particles, wash and dry; add water to the dried precursor particles to prepare a flowable slurry, stir, and add trivalent aluminum salt solution and sodium hydroxide or potassium hydroxide solution dropwise to the slurry at the same time , to obtain the precursor of nickel hydroxide cobalt manganese coated with aluminum; then mix the precursor with lithium source, and then sinter to obtain the positive electrode material of lithium ion battery coated with aluminum. The present invention has the following obvious advantages: raw materials are easy to obtain; coated The process conditions are easy to control, and it is easy to obtain a relatively uniform coating body; the prepared positive electrode material can make the lithium ion battery have superior high temperature stability and better cycle characteristics.
Owner:深圳市天骄科技开发有限公司
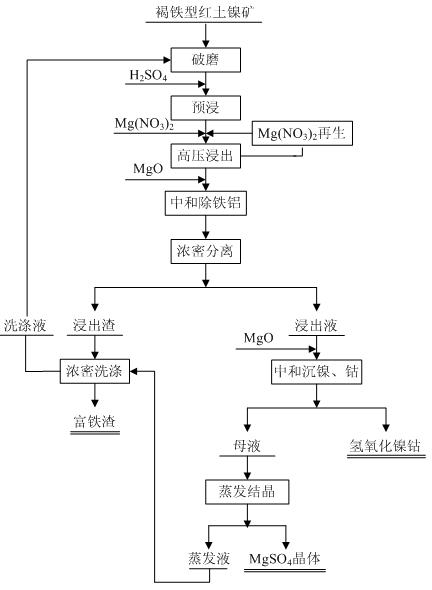
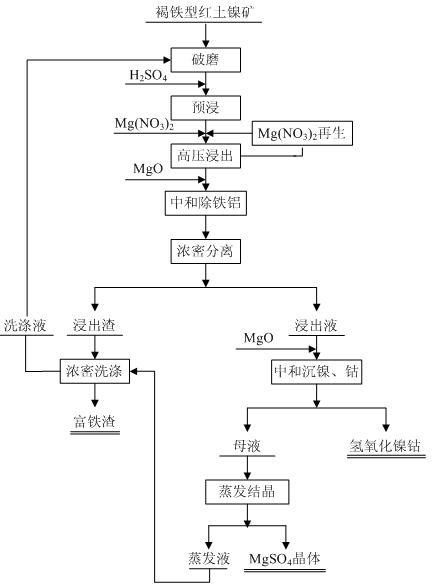
Method for leaching limonitic laterite nickel ore
InactiveCN102534206AReduce consumptionEasy to operate and controlProcess efficiency improvementSlagSlurry
The invention discloses a method for leaching limonitic laterite nickel ore and relates to a process method for recovering nickel, cobalt and iron through treatment of laterite nickel ore by wet process. The method is characterized in that the technical process comprises: (1) grinding raw limonitic laterite nickel ore into fine powder, making slurry, adding sulfuric acid, heating the slurry and leaching the slurry; (2) adding Mg(NO3)2 into the pre-leached slurry, heating with stirring, pressurizing the slurry and leaching the slurry; 3) at the end of leaching, neutralizing the slurry, removing iron and aluminum from the slurry, and separating to obtain a leaching solution and a leaching residue; and 4) washing the leaching residue to obtain washing liquid and iron-enriched slag, neutralizing the leaching solution, precipitating nickel and cobalt, obtaining nickel and cobalt hydroxides, evaporating a mother solution from which nickel and cobalt are separated to crystallize magnesium sulfate and comprehensively recovering magnesium sulfate. The method realizes the high-efficiency selective leaching of nickel and cobalt, the leaching rate reaches over 90 percent, the iron leaching rate is lower than 0.8 percent and iron-enriched slag with an iron content of over 55 percent is obtained.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Cobalt sulfide/carbon composite material with flower-like structure and preparation method thereof
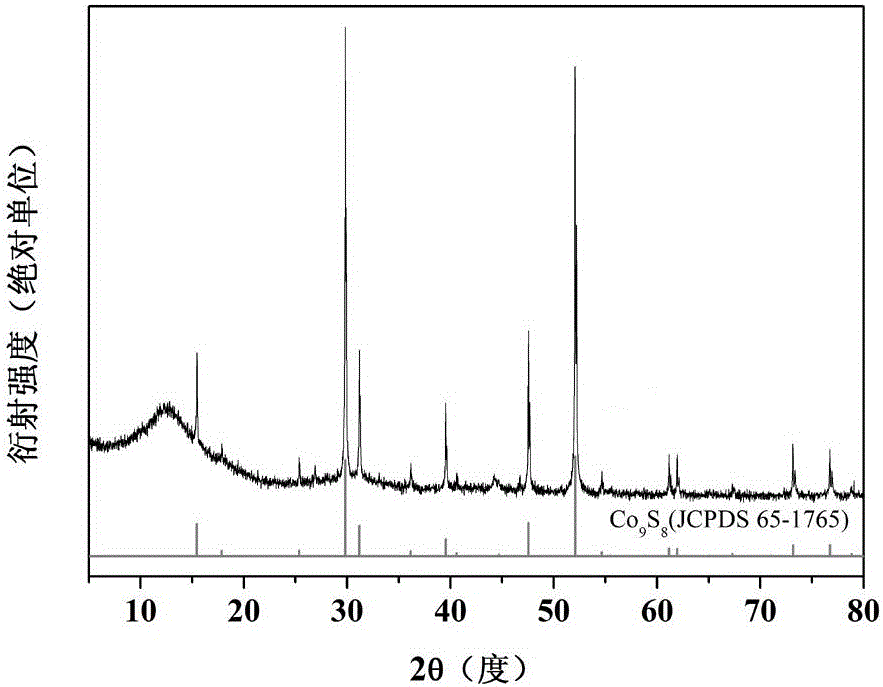
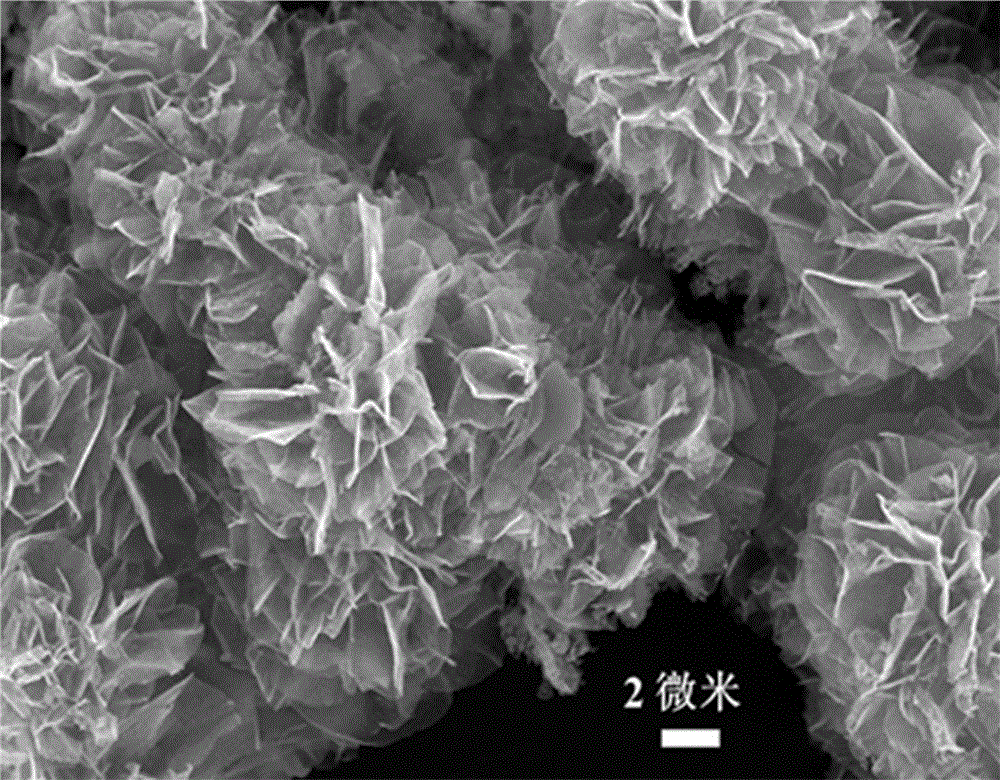
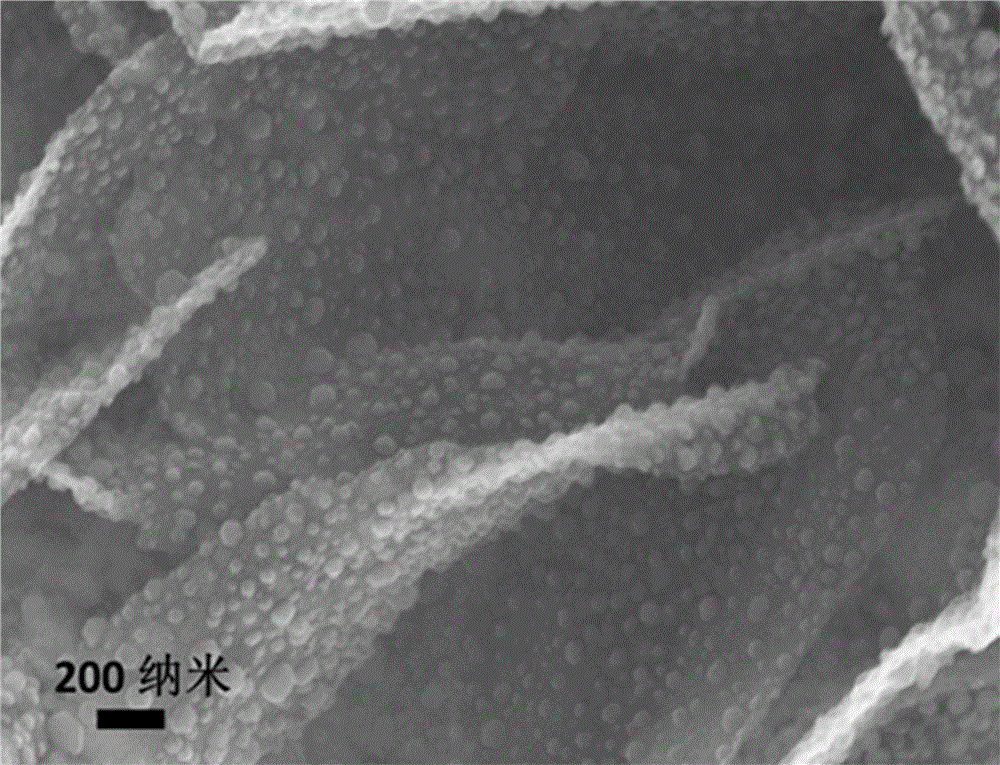
InactiveCN106099126ALarge specific surface areaExcellent electrocatalytic activity for oxygen reduction ORRMaterial nanotechnologyCell electrodesCarbon compositesFlower like
The invention provides a cobalt sulfide / carbon composite material with a flower-like structure and a preparation method thereof, and belongs to the technical field of fuel cell electrocatalysts and preparation thereof. The cobalt sulfide / carbon composite material with the flower-like structure is prepared from flower-like carbon with the diameter of 1-5 microns and Co9S8 with the particle sizes of 5-70nm; the mass percent content of the Co9S8 is 30%-80%; the flower-like carbon is prepared from interconnected carbon nanosheets of which the radial sizes are 0.5-3 microns and the thicknesses are 5-20nm; and Co9S8 particles are uniformly loaded on the carbon nanosheets. The preparation method of the cobalt sulfide / carbon composite material with the flower-like structure comprises the steps of intercalating anions of organic small molecules between layered cobalt hydroxide layers through hydrothermal reaction to obtain an intercalated structure precursor, mixing the intercalated structure precursor with powdered sulfur, and then carrying out high-temperature calcination to obtain the cobalt sulfide / carbon composite material with the flower-like structure. The method has the advantages that the method is environment-friendly and nontoxic, the technology is simple and the production cost is low.
Owner:BEIJING UNIV OF CHEM TECH
Preparation method of ternary anode material precursor
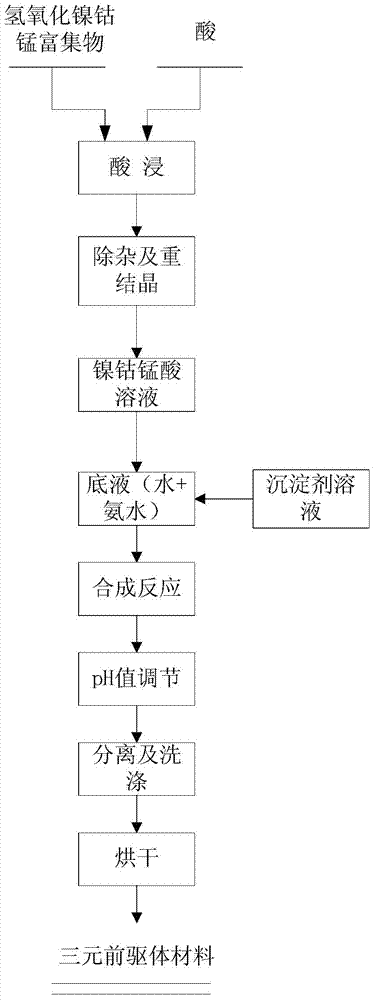
ActiveCN103545504AReduce manufacturing costLow costCell electrodesSecondary cellsNickel oxide hydroxideManganese
The invention discloses a preparation method of a ternary anode material precursor. According to the preparation method, an intermediate product namely nickel-manganese-cobalt hydroxide concentrate generated by producing electrolytic nickel from laterite, and the preparation process comprises the following process flows: A, acid leaching; B, impurity removal and recrystallization; C, preparation of a mixed salt; D, preparation of a precipitant; E, synthetic reaction; F, adjustment of the pH value; G, separation and washing; H, drying. The ternary anode material precursor prepared by the preparation method disclosed by the invention is uniform in particle size distribution, high in activity, high in density, high in specific capacity and low in cost.
Owner:江西赣锋循环科技有限公司
Making method of nickel hydroxide with coated gamma hydroxy cobalt oxide
ActiveCN101106193AAlkaline accumulator electrodesNickel oxides/hydroxidesHigh densityNickel oxide hydroxide
The invention relates to a preparation method for nickel hydroxide, which is used as a Ni-H storage battery positive material, coated with gamma hydroxy cobalt oxide. The preparation includes that the sphere surface of nickel hydroxide is coated with a layer of cobalt hydroxide firstly; then, under the conditions of high-density sodium hydroxide and oxygen, the cobalt hydroxide layer coated on the surface is oxidized the gamma hydroxy cobalt oxide, or the treatment of embedding doped metals is exerted on the gamma hydroxy cobalt oxide layer. By controlling the coating layer to be the cobalt hydroxide presenting a shape of flake, the method makes the resulted conducting network coated with the coating layer of gamma hydroxy cobalt oxide more uniform, more integrated and better conductive, and thus attributes the nickel hydroxide positive active material coated with gamma hydroxy cobalt oxide with some characteristics of high recovering rate of over-discharging capacity, good performance of big current discharging, low self-discharging, long lifetime of cycling use and so on.
Owner:JINCHI ENERGY MATERIALS CO LTD
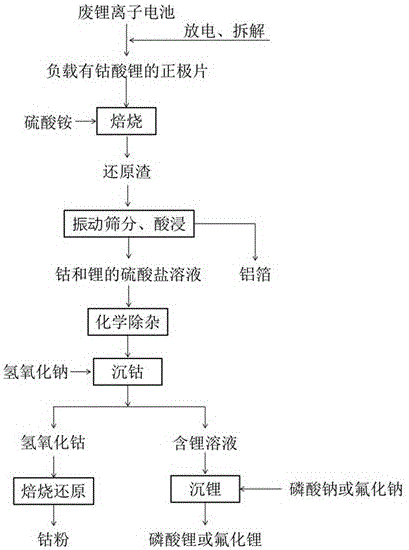
Method for recycling cobalt and lithium from positive plate of waste lithium ion battery
ActiveCN106505270AGuaranteed RecoveryHigh recovery rateWaste accumulators reclaimingProcess efficiency improvementSlagLithium-ion battery
The invention provides a simple and high-efficiency method for recycling cobalt and lithium from a positive plate of a waste lithium ion battery, and relates to a method for recycling cobalt and lithium from the positive plate of the waste lithium ion battery. The method is characterized by comprising the following processes of ammonium sulfate roasting, in which the positive plate of the waste lithium battery is mixed with ammonium sulfate, and high-temperature roasting is performed to obtain reduction roasting slag; screening, in which the reduction roasting slag is vibrated and is screened by a vibration screening machine, and an aluminum foil is separated and removed to obtain reduction slag containing cobalt and lithium; acid leaching, in which the aluminum foil is separated and removed from the reduction roasting slag, is leached in sulfuric acid and is filtered to obtain a sulfate solution containing cobalt and lithium; impurity removal, in which pH of the solution is adjusted by sodium carbonate, and a precipitant is filtered and removed to obtain a lithium-cobalt sulfate mixture solution; cobalt deposition, in which sodium hydroxide is added into the lithium-cobalt sulfate mixture solution, the pH of the solution is controlled to be 7.5-9.5, and filtering is performed to obtain the precipitant, the precipitant is washed with deionized water at 85-95 DEG C, cobalt hydroxide is dried, cobalt hydroxide after being dried is placed in a reduction furnace, and H2 is introduced for reduction to obtain cobalt powder; and lithium deposition, in which pH of a lithium-containing solution is adjusted to 7-8, and an excessive amount of a lithium deposition agent is added to a lithium salt product.
Owner:JINGMEN GEM NEW MATERIAL
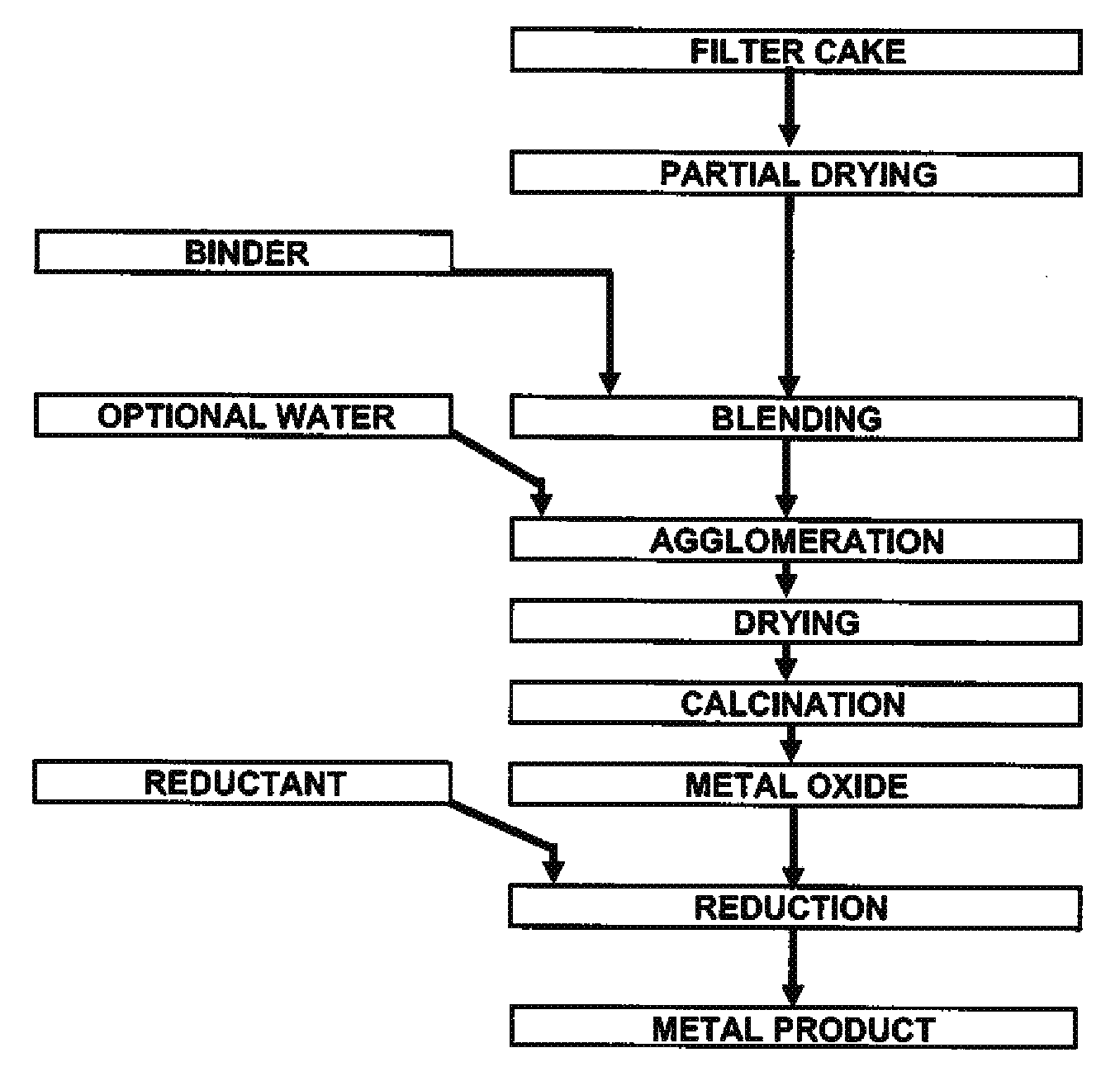
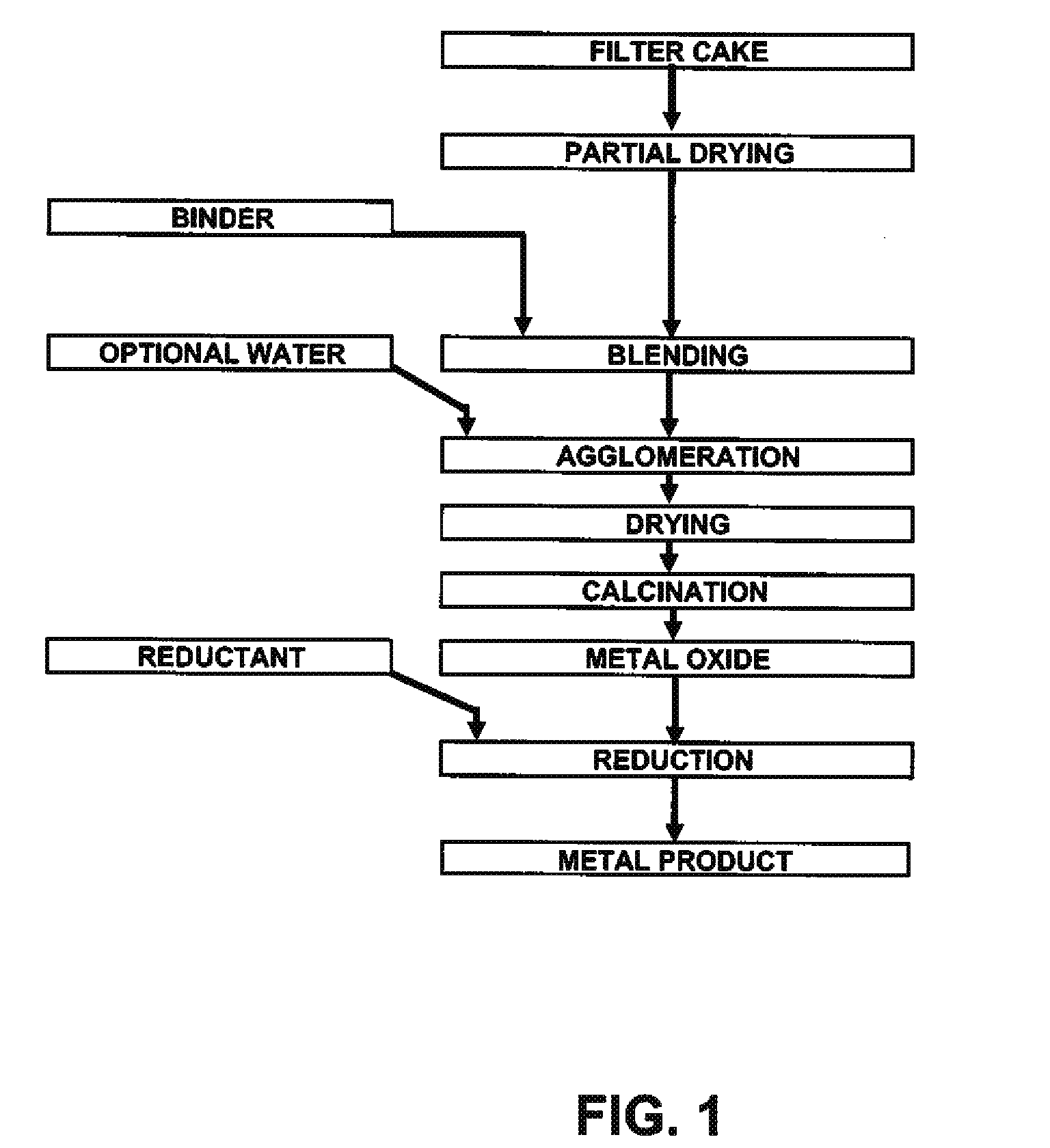
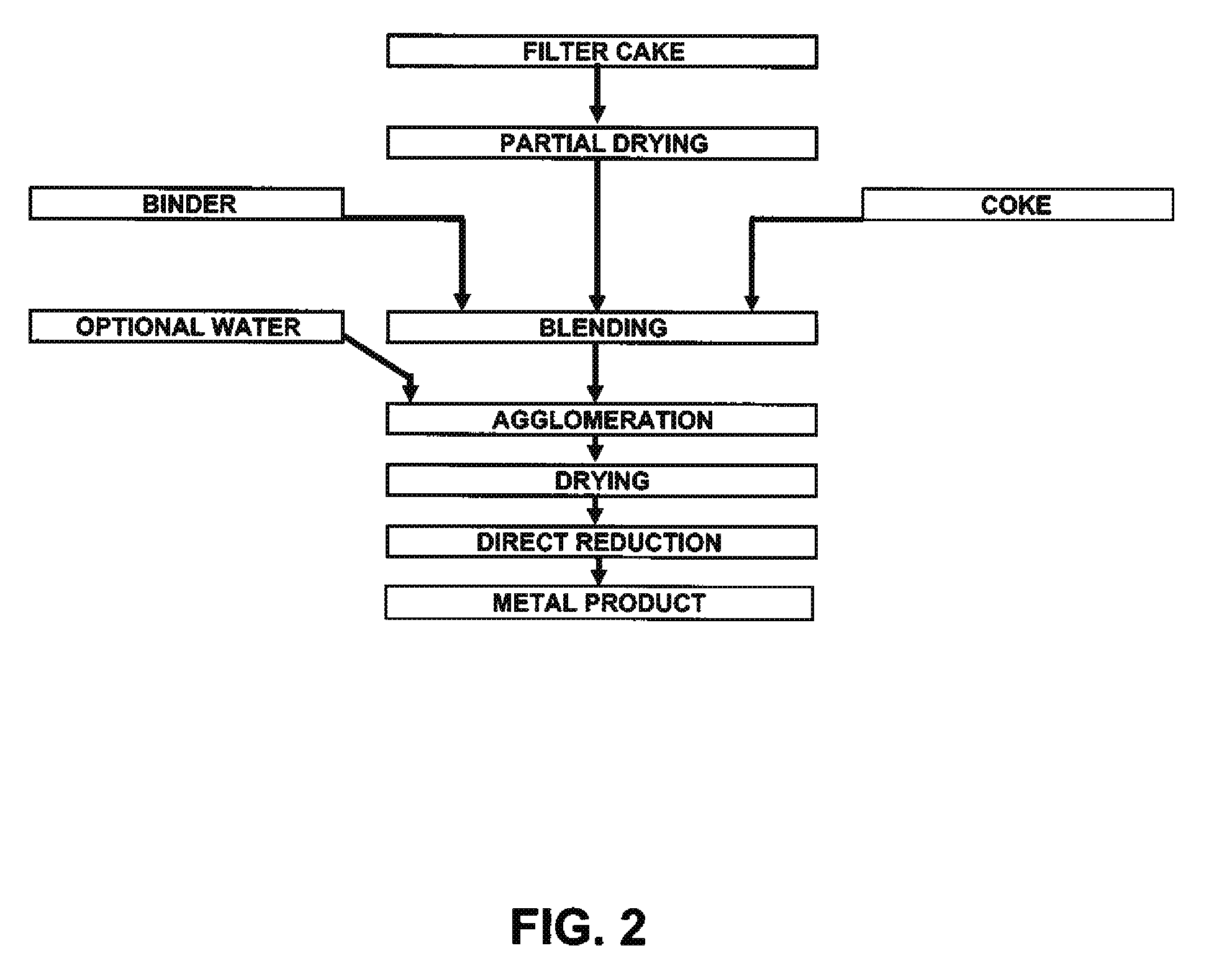
Process for production of nickel and cobalt using metal hydroxide, metal oxide and/or metal carbonate
A method for producing metal oxide from a metal salt selected from nickel hydroxide, cobalt hydroxide, mixed nickel-cobalt hydroxide, nickel carbonate, cobalt carbonate, mixed nickel-cobalt carbonate and combinations thereof includes providing a mixture of the metal salt, mixing the metal salt with a binder selected from the group consisting of inorganic binder, organic binder and combinations thereof, forming the mixture into agglomerates, and calcining the agglomerates to produce metal oxide. A method for making metallic nickel or cobalt includes providing a metal salt selected from the group consisting of nickel hydroxide, cobalt hydroxide, mixed nickel-cobalt hydroxide, nickel carbonate, cobalt carbonate and combinations thereof, mixing the metal salt with a binder selected from the group consisting of inorganic binder, organic binder and combinations thereof to form a mixture, optionally adding water, forming the mixture into agglomerates, drying the agglomerates, adding an effective reducing amount of coke and / or coal and directly reducing the dried agglomerates with an effective amount of heat to produce metallic nickel and / or cobalt. Coke particles may be added to the mixture prior to agglomeration. An agglomerate includes a metal salt selected from the group consisting of nickel hydroxide, cobalt hydroxide, mixed nickel-cobalt hydroxide, nickel carbonate, cobalt carbonate, mixed nickel-cobalt carbonate and combinations thereof; and a binder selected from the group consisting of inorganic binder, organic binder and combinations thereof.
Owner:VALE CANADA
Foamed nickel composite material, and preparation method and application thereof
InactiveCN105070515AThe method route is simpleImprove cycle stabilityHybrid capacitor electrodesHybrid/EDL manufactureCapacitanceSolvent
The invention relates to a preparation method of a foamed nickel composite material. The method comprises the following steps that oxidized graphene and a surfactant are added to a water and methanol mixed solution, and ultrasonic processing is carried out to obtain a oxidized graphene solution; nickel nitrate and cobalt chloride are added into the oxidized graphene solution and stirred and mixed to form a uniform suspension; and the uniform suspension is poured into a hydro-thermal reactor kettle, foamed nickel is immersed into the suspension, solvothermal reaction is carried out at 100-200 DEG C, the oxidized graphene is changed to graphene by reduction while nickel and cobalt hydroxide is generated at the surface of the foamed nickel in situ, and thus, the foamed nickel composite material whose surface is covered with a graphene and nickel-cobalt hydrotalcite like layer is obtained. The invention also relates to the obtained foamed nickel composite material and application thereof. The preparation method is simple, easy to control and low in cost, and the obtained foamed nickel composite material which serves as electrode material of a super capacitor has higher specific capacitance and high cycle stability.
Owner:EAST CHINA UNIV OF SCI & TECH
Cobalt-covered lithium ion cell anode material precursor as well as preparation method and application
InactiveCN103359795AImprove performanceImproved magnification performanceCell electrodesManganese oxides/hydroxidesPower batteryLithium
The invention relates to a cobalt-covered compound polybasic lithium ion cell anode material precursor as well a as preparation method and application. The precursor has the following formula: NixCoy+zMn1-x-y(OH)2, wherein x is more than 0 and less than 0.8, y is more than 0 and less than 0.5 and z is more than 0 and less than 0.05; the precursor is formed by a core part and a nano cobaltosic oxide layer covering the surface of the core; the molecular formula of the core part is as follows: NikConMn1-k-n(OH)2, wherein k is more than 0 and less than 0.8 ad n is more than 0 and less than 0.5. According to the precursor disclosed by the invention, the outer surface of the core part is covered with one layer of nano cobalt hydroxide through a nano technology; and a covering layer is formed by uniformly growing in a liquid phase so that the very good and dense covering layer is formed on a spherical surface. Then, a strong oxidant is added under a strong alkali environment so that the cobalt hydroxide is oxidized into cobalt hydroxyl cobalt oxide and a cobaltosic oxide covering layer is formed on the surface of the material in a following sintering process. The cobaltosic oxide has the very good electronic conduction capability so that the heavy load discharge performance of the material is greatly improved and the material can be more suitable for the requirements of a power battery.
Owner:协鑫动力新材料(盐城)有限公司
Cobalt oxide anode material, amorphous carbon coated cobalt oxide anode material and preparation method and application of cobalt oxide anode material and amorphous carbon coated cobalt oxide anode material
InactiveCN102659192ASimple preparation processPromote circulationCell electrodesCobalt oxides/hydroxidesHigh rateRetention ratio
The invention discloses a preparation method of a cobalt oxide anode material or an amorphous carbon coated cobalt oxide anode material, which comprises the following steps of: placing a conductive metal substrate in aqueous solution containing soluble cobalt salt and hexamethylene tetramine and carrying out heat preservation for 3h to 12h at a temperature of 80 DEG C to 150 DEG C to obtain the conductive metal substrate on which a cobalt hydroxide thin film is deposited; calcining the conductive metal substrate for 1h to 3h at a temperature of 200 DEG C to 400 DEG C to obtain the cobalt oxide anode material; and soaking the cobalt oxide anode material into aqueous solution of glucose, drying and calcining for 1h to 8h at a temperature of 300 DEG C to 500 DEG C to obtain the amorphous carbon coated cobalt oxide anode material. The preparation method has a simple preparation process and good reproducibility, is easy to implement, has an environmental-friendly production process and low cost and is beneficial to industrial production. The invention also provides the cobalt oxide anode material and the amorphous carbon coated cobalt oxide anode material. The cobalt oxide anode material and the amorphous carbon coated cobalt oxide anode material have high capacity retention ratios and good high-rate capabilities and are particularly suitable to use as a cathode electrode of a lithium ion battery.
Owner:ZHEJIANG UNIV
Spherical tricobalt tetraoxide and method of preparing the same
ActiveUS20100135897A1Stable structureMaintain good propertiesIron oxides/hydroxidesCell electrodesCobalt(II,III) oxideHigh activity
A method of preparation of spherical tricobalt tetraoxide, including at least oxidizing a bivalent cobalt salt in a wet environment and in the presence of a precipitant, a complexing agent, and an oxidant to yield spherical cobalt oxyhydroxide.cobalt hydroxide according to the following equation Co2++3OH−+O→CoOOH.Co(OH)2; oxidizing the spherical hydroxy cobalt oxyhydroxide.cobalt hydroxide to yield spherical tricobalt tetraoxide according to the following equation 6 CoOOH.Co(OH)2+O→4 Co3O4+9 H2O; and roasting the spherical tricobalt tetraoxide at low or intermediate temperature to yield a black powder. The method is easily practiced and suitable for mass production, and the resultant spherical tricobalt tetraoxide has stable structure, reliable properties, and high activity.
Owner:NINGBO RONBAY LITHIUM BATTERY MATERIAL CO LTD
Isolation technology and process for extracting Ni and Co from nickel-containing high-cobalt hydroxide
InactiveCN105274332AImprove leaching rateEasy to separateProcess efficiency improvementPregnant leach solutionPhysical chemistry
The sulfuric acid total leaching process is adopted in an isolation technology and process for extracting nickel and cobalt from nickel-containing high-cobalt hydroxide, so that a high Ni and Co leaching rate is guaranteed. By means of the processes of sulfuric acid leaching, impurity removal, extraction, and Ni-Co isolation, leaching agents rich in metallic nickel and metallic cobalt are obtained, the leaching agents are directly fed into an electro-deposited nickel and electro-deposited cobalt production system, and accordingly metallic nickel and metallic cobalt are isolated and extracted. The obtained leaching agents rich in metallic nickel and metallic cobalt can directly enter the electro-deposited nickel and electro-deposited cobalt production system. In addition, the process for extracting nickel and cobalt from nickel-containing high-cobalt hydroxide is simple, efficient, environment-friendly and suitable for treating nickel-containing high-cobalt hydroxide.
Owner:JINCHUAN GROUP LIMITED
Ultra-fine hard alloy coated powder and method for preparing same
InactiveCN101186990AReduced tendency to aggregate and growWell mixedLiquid/solution decomposition chemical coatingCarbonizationTitanium carbide
The invention discloses a super-fine cemented carbide coating powder and process of preparation thereof. Super-fine hard-phase carbonization tungsten in the cemented carbide coating powder and other carbides such as titanium carbide, tantalum carbide, niobium carbide, vanadium carbide and / or chromium carbide are composed around by cobalt-phase ultra-fine powder particles. Karl Fischer's mean particle size of the super-fine cemented carbide coating powder is <=1 mu m. The super-fine tungsten carbide of the invention is put into the liquor of water-soluble metal cobalt-salt after being activated and dispersed with other hard-phase of carbide powder, the super-fine carbide powder and other hard-phase of carbide powder are taken as the core, chemical coprecipitation coating is employed in the reaction, and a uniform cobalt carbonate or cobalt hydroxide inhibitory coating is formed on the surface of the tungsten carbide powder and other hard-phase of carbide powder. The coprecipitation coating powder can be made into the super-fine cemented carbide coating powder by filtering, washing, and drying and low temperature reduction. The invention has the advantages of simple technique and low cost, which can take place the existing cemented carbide wet grinding mixture and the preparation method. High quality super-fine cemented carbide can be prepared by utilizing the powder of the invention.
Owner:CENT SOUTH UNIV +1
Production method of high stability CoSe2 / graphene composite electrode material
InactiveCN104971747AImprove stabilityStrong chemical resistancePhysical/chemical process catalystsCell electrodesPtru catalystCrystallinity
The invention discloses a production method of a high stability CoSe2 / graphene composite electrode material. The method comprises the steps: (1), synthesis of graphene oxide; (2) preparation of a cobalt hydroxide and graphene oxide mixed sol; (3) constant temperature vacuum aging; and (4) preparation of a CoSe2 / graphene composite electrode material. The present invention has the advantages of: (1), according to the above preparation method, CoSe2 is uniformly loaded on the graphene surface to achieve synthesis of a high stability CoSe2 / graphene composite electrode material; (2) the synthesis process is simple and easy to operate, and has mild conditions and low cost of raw materials; (3) compared with the existing non-noble metal electrode material, the CoSe2 / graphene composite electrode material prepared by the present invention has high efficiency electro-catalytic activity; and (4), the CoSe2 / graphene composite electrode material prepared by the present invention has good crystallinity, high stability and strong resistance to poison. The high stability CoSe2 / graphene composite electrode material can be used as a cathode catalyst for a polymer electrolyte membrane (PEMFC) fuel cell, has significantly increased catalytic activity in acid medium, reduces catalyst costs, and significantly enhances battery performance.
Owner:SUIHUA UNIV
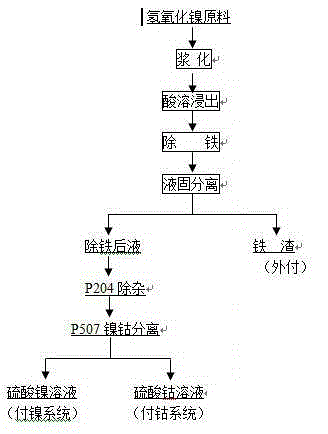
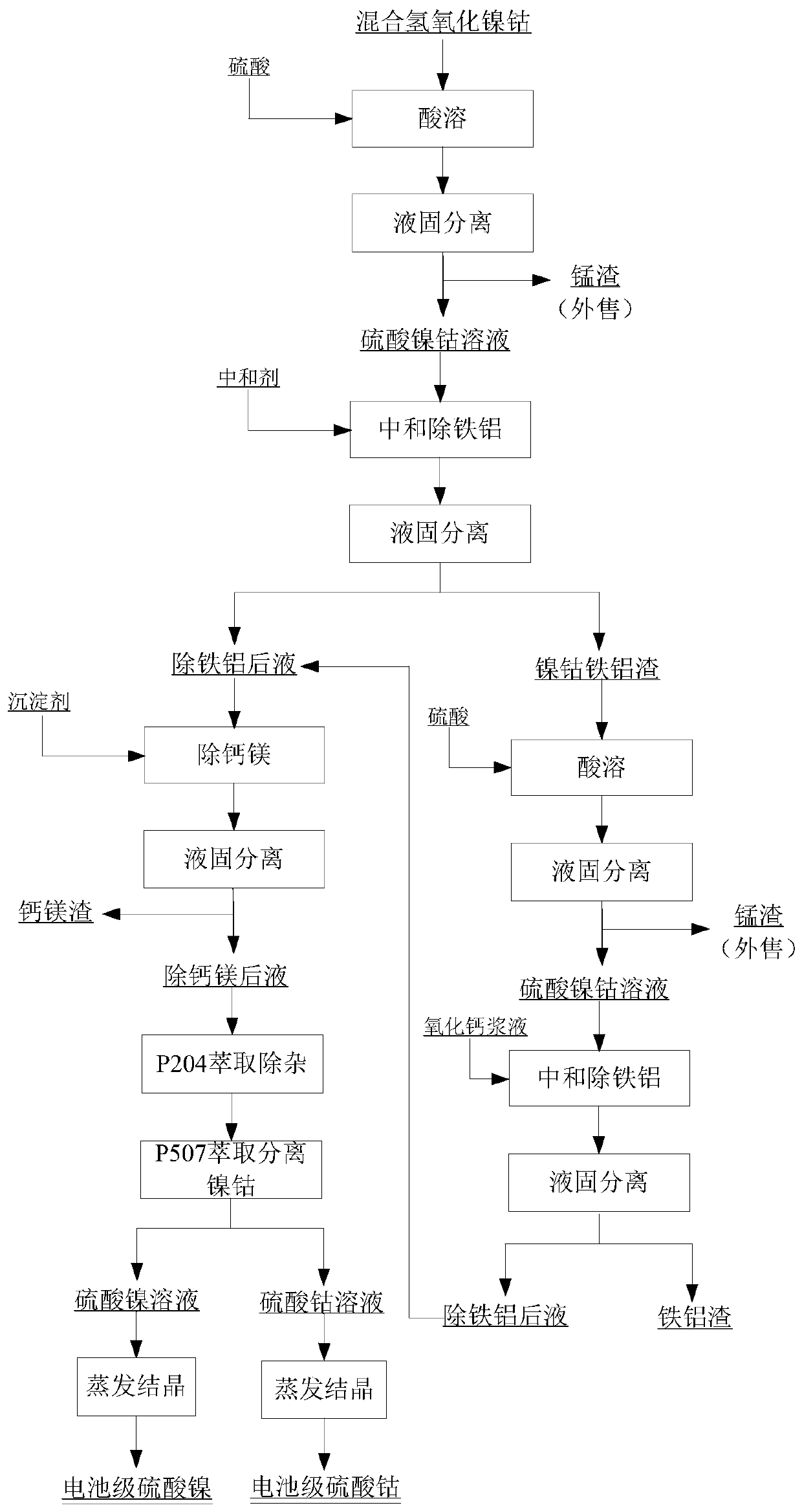
Method for preparing battery-grade nickel sulfate and cobalt sulfate from mixed nickel cobalt hydroxide
The invention discloses a method for preparing battery-grade nickel sulfate and cobalt sulfate from mixed nickel cobalt hydroxide, and belongs to the technical field of nickel cobalt hydrometallurgy.The method comprises the following steps that the mixed nickel cobalt hydroxide is leached by using sulfuric acid, iron and aluminum in the solution are removed by using a nickel / cobalt / manganese-based neutralizer, liquid-solid separation is carried out to obtain iron-removed slag, nickel and cobalt are recycled through acid dissolution, a precipitator (one or more of nickel fluoride, and cobalt fluoride and manganese fluoride) is added into iron-removed liquid to remove calcium and magnesium ions in the system. Impurities such as Mn, Cu and Zn are removed from the calcium and magnesium removed liquid by using a saponified P204 extractant, nickel and cobalt are separated from the P204 raffinate by using a saponified P507 extractant to obtain battery-grade nickel sulfate and a cobalt sulfate solution, and evaporative crystallization is carried out to obtain a product. According to the method, the use amount of calcium oxide is greatly reduced, the amount of calcium ions introduced intothe system and the corresponding nickel-cobalt loss are greatly reduced, the influence of calcium sulfate crystallization on extraction is avoided, the P507 extraction operation amount is reduced, andthe purification cost is reduced.
Owner:BEIJING MINING & METALLURGICAL TECH GRP CO LTD
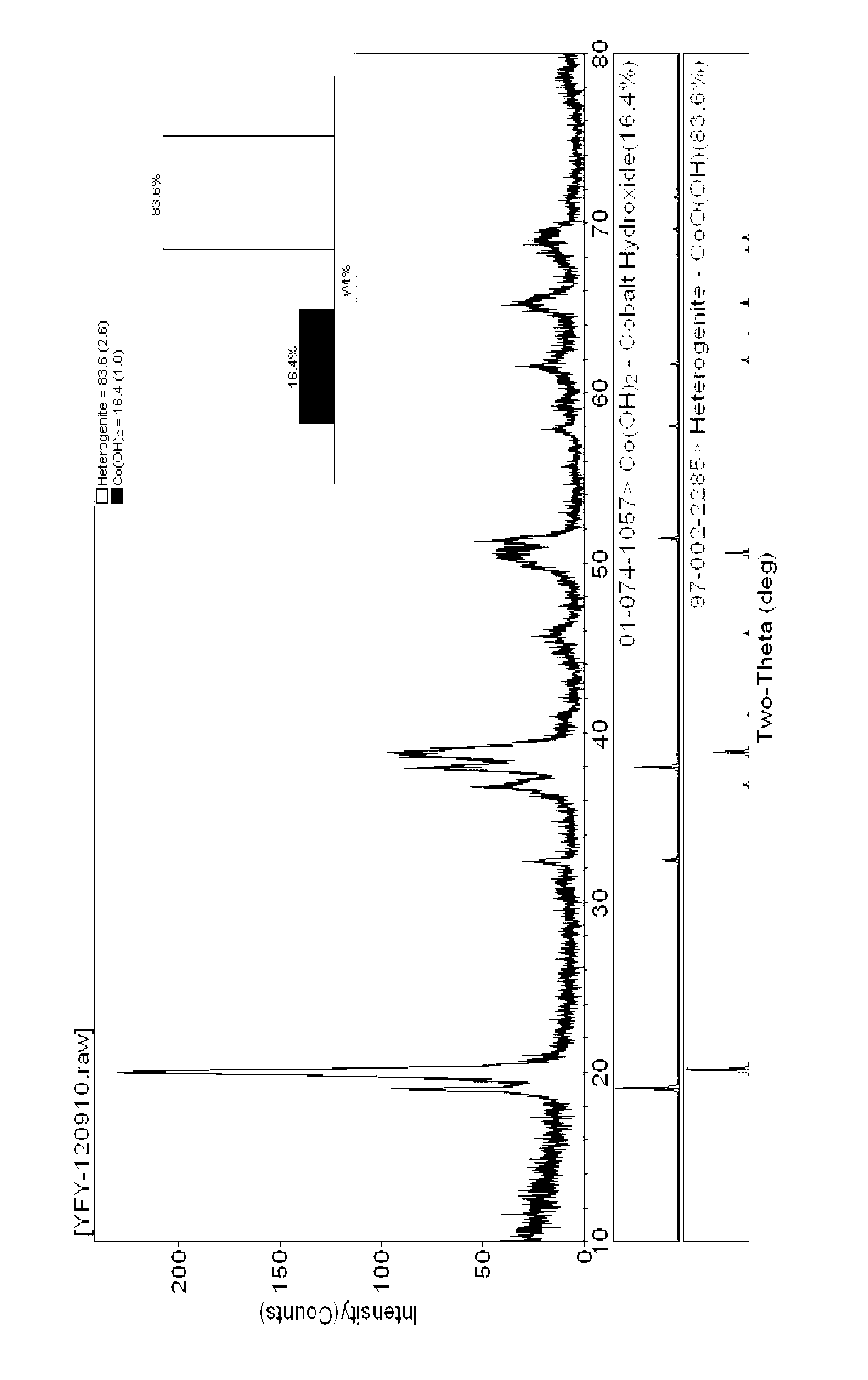
Preparation method for large-grained spherical cobalt oxyhydroxide
The present invention provides a preparation method for large-grained spherical cobalt oxyhydroxide. The specific method is as follows: (1) preparing a 0.5-2.0mol / L cobalt salt solution and a 5-10mol / L sodium hydroxide solution, and adding a complexing agent and a reducing agent in the sodium hydroxide solution; (2) under the protection of nitrogen, simultaneously pumping the cobalt salt solution and the sodium hydroxide solution prepared in the step (1) into a reaction vessel for a continuous reaction, wherein a pH value is controlled to be in a range of 10-12 by adjusting the flow rate of the sodium hydroxide solution, and a slurry particle size is controlled to be in a range of 15-20 microns; (3) obtaining cobalt hydroxide with good sphericity and crystallinity, washing and filtering, controlling a water content in a range of 10-30%, transferring into an oxidation reactor, and blowing air in for oxidation; and (4) placing the oxidized slurry into a blast oven for drying to obtain the large-grained spherical cobalt oxyhydroxide.
Owner:HUNAN YACHENG NEW MATERIAL CO LTD
Preparing method of anode material of lithium cobalt, nickel, manganese, oxygen lithium ion battery
InactiveCN1967911AReduced average particle size requirementsReduced settling timeElectrode manufacturing processesLithium compoundsMicrometerSpherical shaped
The invention relates to a method for preparing the anode material of lithium nickel manganese oxide lithium ion battery, wherein said method comprises that mixing nickel, manganese cobalt hydroxide and adhesive, preparing particles, sintering; mixing sintered product and lithium composite, sintering. The invention can prepare the ball anode material whose diameter is higher than 7 micrometer even when the diameter of forward element is lower than 1 micrometer, to reduce deposit time of forward element. The invention can increase contact area and improve capacity of battery.
Owner:BYD CO LTD
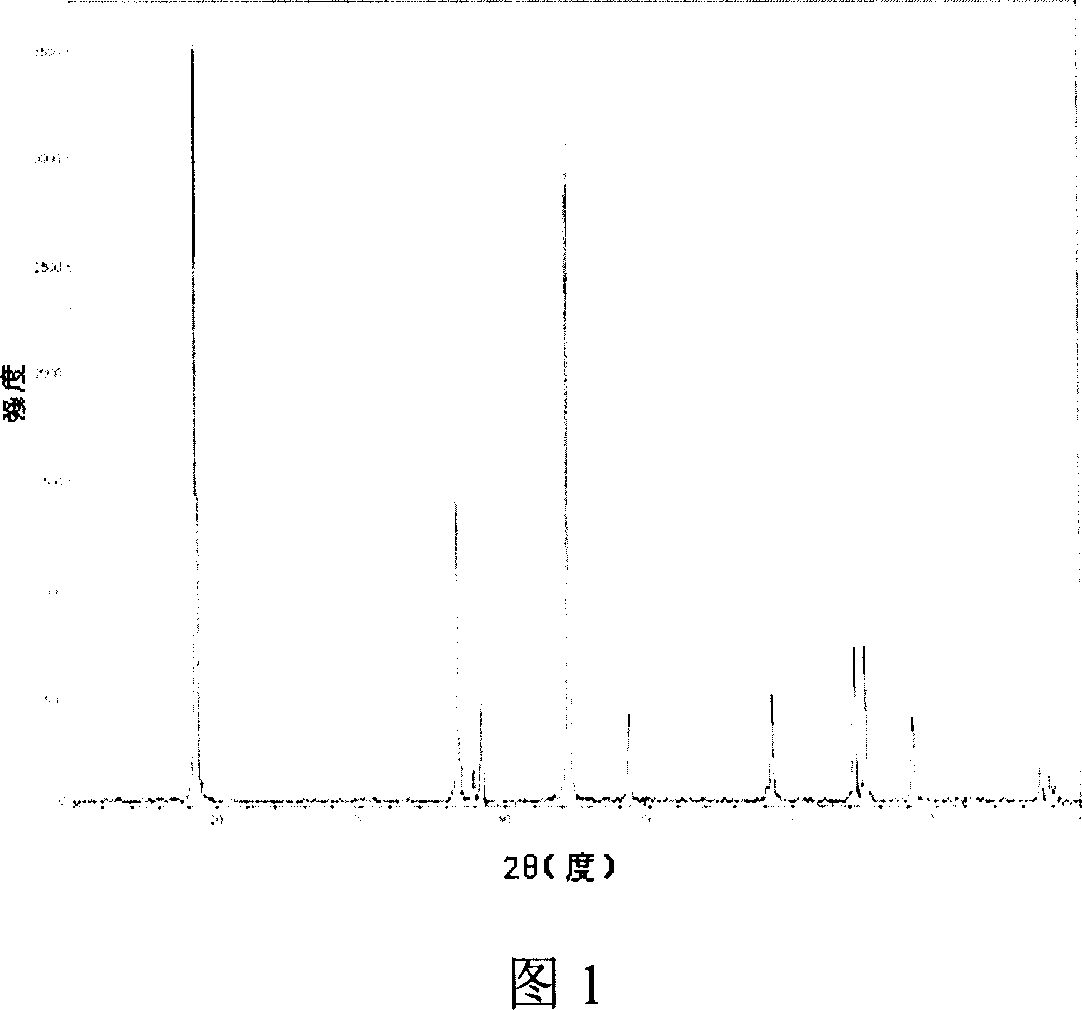
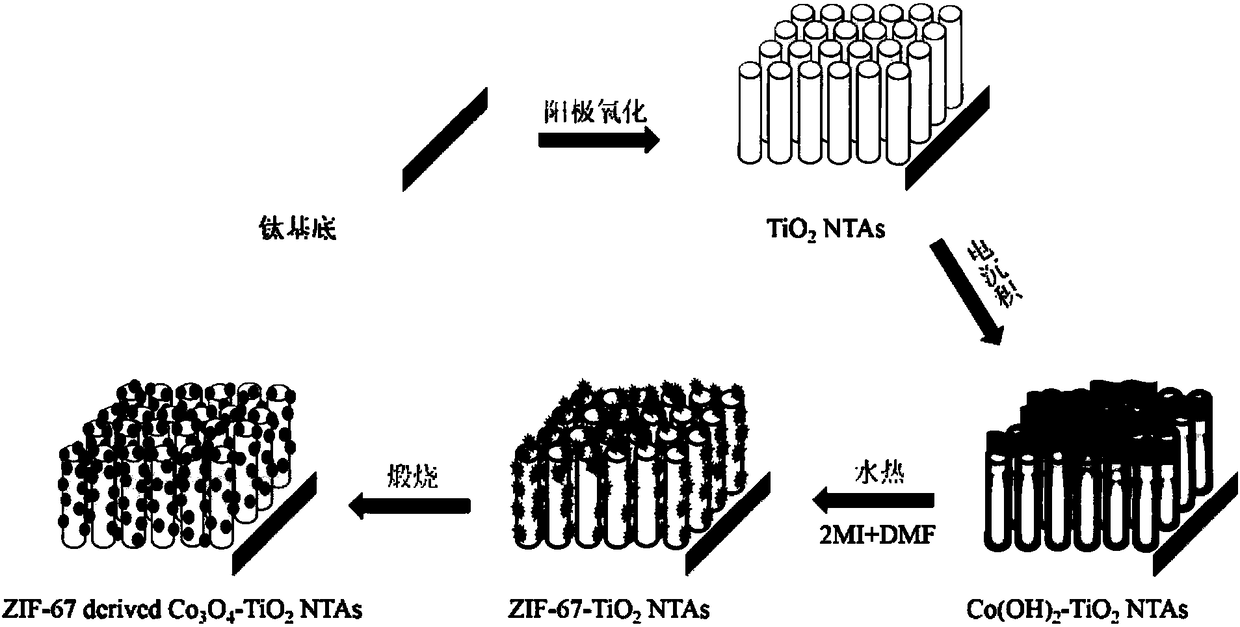
Preparation method for metal organic framework-derived tricobalt tetroxide-modified titanium dioxide nanotube array
InactiveCN108525667ASolve the repeatabilityFix stability issuesWater/sewage treatment by irradiationElectrolytic inorganic material coatingAbsorption capacityWorkstation
The invention discloses a preparation method for a metal organic framework-derived tricobalt tetroxide-modified titanium dioxide nanotube array. The method comprises the following steps: firstly performing pretreatment on a titanium sheet substrate material; performing electrochemical treatment on the treated titanium substrate material by using an ethylene glycol solution containing ammonium fluoride and water as an electrolyte, and performing calcination by using a muffle furnace to change a titanium dioxide crystal form; secondly, performing cobalt hydroxide electrodeposition by means of athree-electrode electrochemical workstation and by using cobalt nitrate hexahydrate as an electrolyte, a titanium dioxide nanotube array as a working electrode, a platinum sheet as a negative electrode and silver / silver chloride as a reference electrode; performing hydrothermal treatment on the titanium dioxide nanotube array to form ZIF-67 in situ; and finally, performing secondary calcination byusing a muffle furnace to obtain the ZIF-67-derived porous tricobalt tetroxide-modified titanium dioxide nanotube array. The method disclosed by the invention can effectively improve absorption capacity of TiO2 on visible light, promote separation of electron hole pairs, and improve photocatalytic degradation efficiency of organic pollutants.
Owner:SUZHOU UNIV
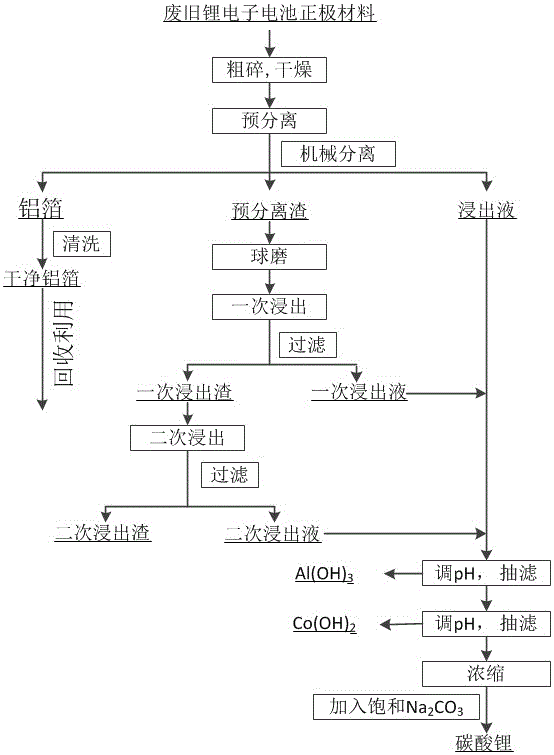
Method for mixed acid leaching and recovery of positive pole materials of waste and old lithium ion batteries
ActiveCN106848471AWide range of sourcesImprove leaching efficiencyWaste accumulators reclaimingBattery recyclingAluminium hydroxideFiltration
The invention provides a method for mixed acid leaching and recovery of metal components of positive pole materials of waste and old lithium ion batteries. The method comprises carrying out coarse crushing on wastes, drying the crushed wastes, pre-leaching the wastes through a mixed acid containing a reduction agent to obtain pre-separated residues, carrying out ball milling, carrying out primary and secondary leaching, mixing the primary and secondary leachates and the pre-leachate, adjusting pH of the mixture, carrying out suction filtration to obtain aluminum hydroxide and raffinate containing cobalt and lithium, adjusting pH of the raffinate containing cobalt and lithium at a high temperature, carrying out suction filtration to obtain cobalt hydroxide and raffinate containing lithium, carrying out concentration on the raffinate containing lithium at a high temperature, adding a saturated sodium carbonate solution into the concentrate to obtain high purity lithium carbonate, and recovering aluminum foil. The method utilizes a mixed acid leaching agent, has high leaching efficiency, can gradually acquire high purity aluminum, aluminum hydroxide, cobalt hydroxide and high purity lithium carbonate (having purity of 99.9%), realizes efficient recovery, overall recovery and collaborative recovery of high-value metals in the waste and old lithium-ion batteries and has a good application prospect.
Owner:BOTREE CYCLING SCI &TECH CO LTD
Three-element determination method of nickel-cobalt-manganese ternary material
ActiveCN104316643AImprove measurement accuracyHigh precisionMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationMurexideAcid dissolution
The invention relates to a three-element determination method of a nickel-cobalt-manganese ternary material. The method comprises the following steps: determining the total quantity of cobalt, nickel and manganese ions by EDTA titration and recording titration consumption volume; sampling to an alkaline medium of ammonia chloride and ammonium to form nickel amine, oxidizing cobalt by utilizing hydrogen peroxide, complexing with ammonium to form a trivalent cobalt ammonia complex to generate manganese dioxide precipitate, filtering out the precipitate to obtain the filter liquid, titrating the filter liquid by utilizing EDTA in the presence of murexide serving as an indicator to determine the content of nickel, and recording the titration consumption volume; enabling the ammonia gas to escape from the solution after the nickel is determined through titration under the alkaline and heating condition and generating cobalt hydroxide precipitate at the same time, dissolving the cobalt hydroxide precipitate by utilizing acid, measuring the content of cobalt through EDTA titration, and recording the titration consumption volume; and calculating the respective content of the three elements according to the EDTA consumption volume in each step, the concentration of EDTA and the sample mass. The method is accurate in detection, high in detection efficiency, safe, environment-friendly and applicable to the detection of content of three elements in an anode material.
Owner:JINGMEN GEM NEW MATERIAL +1
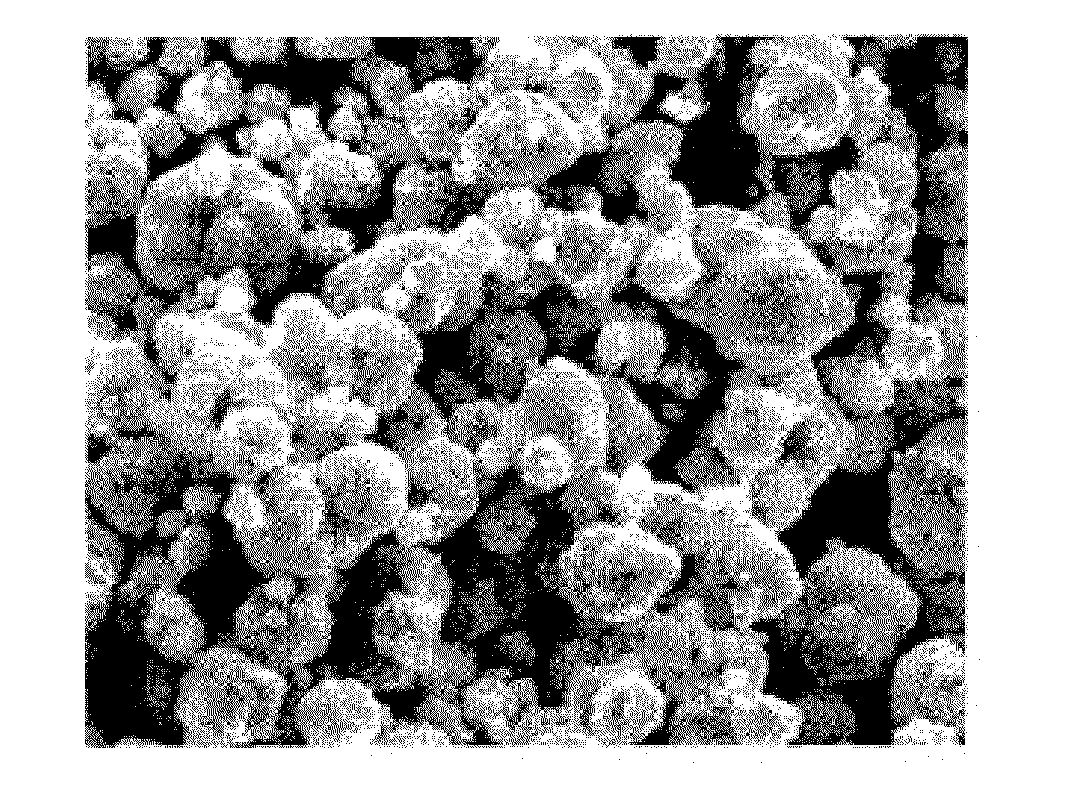
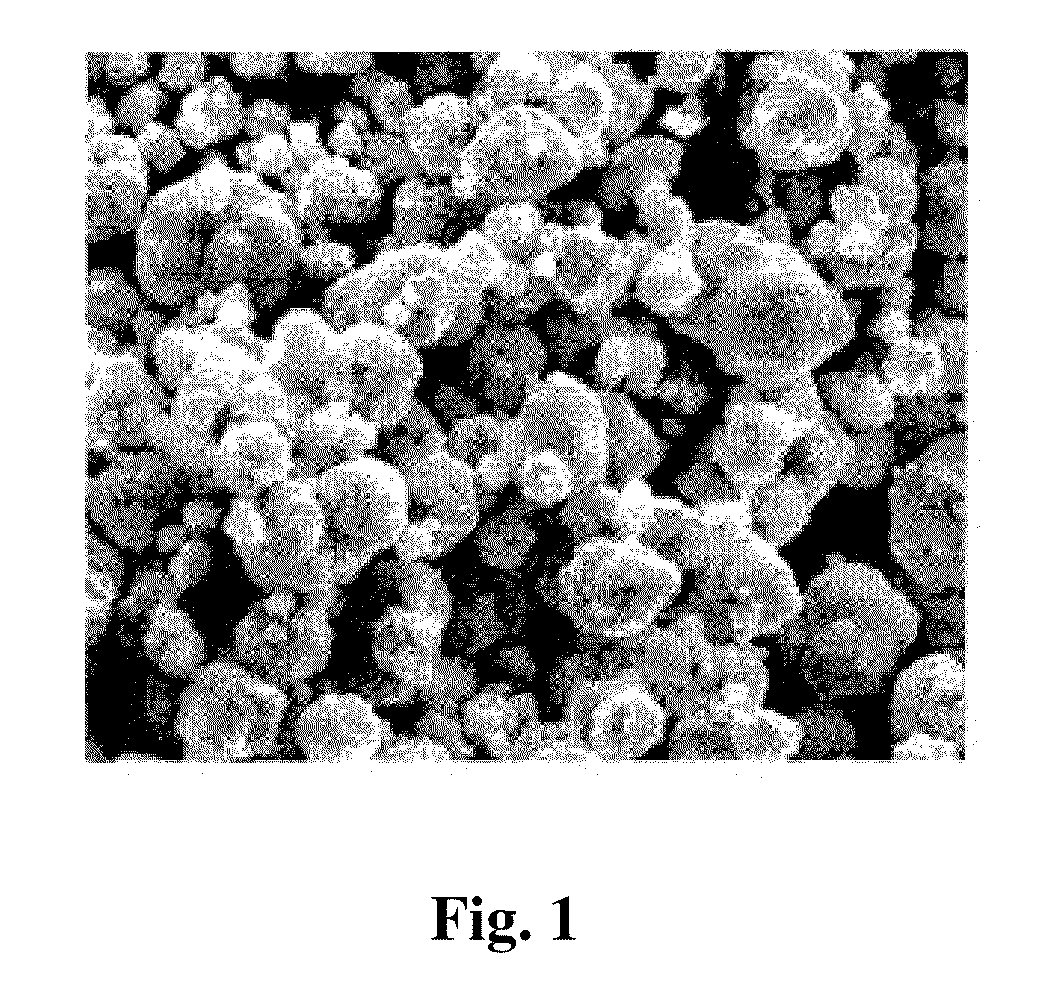
Method for preparing spherical cobalt hydroxide in the absence of complexing agent
The invention relates to a method for preparing a spherical cobalt hydroxide in the absence of a complexing agent. Though the shape of the cobalt hydroxide can be improved to a certain degree by complexing to control crystallization, the cobalt hydroxide is mostly particles in sheet shape or irregular block shape, and the addition of a complexing agent can cause negative effects. The technical scheme adopted by the invention comprises the following steps of: taking pure water as a base solution; adding a cobalt solution and a sodium hydroxide solution into the base solution with stirring at the same time to cause a precipitation reaction, wherein the pH value of the solution is kept between 5 and 7 in the process of adding the cobalt solution and the sodium hydroxide and is kept between 10.5 and 13.5 in the end of the process; adding an antioxidant into the solution to prevent the solution from being oxidized; and obtaining the cobalt hydroxide product after washing and drying the cobalt hydroxide slurry. The method adopts a forward feeding mode in the absence of complexing agent to form pink spherical cobalt hydroxide powder with very good crystallinity. The spherical cobalt hydroxide powder is easily filtered and washed, and the reaction condition is simple and easy to control. Therefore, the method is suitable for industrial mass production.
Owner:ZHEJIANG HUAYOU COBALT
Process for preparing a positive electrode material for lithium ion battery
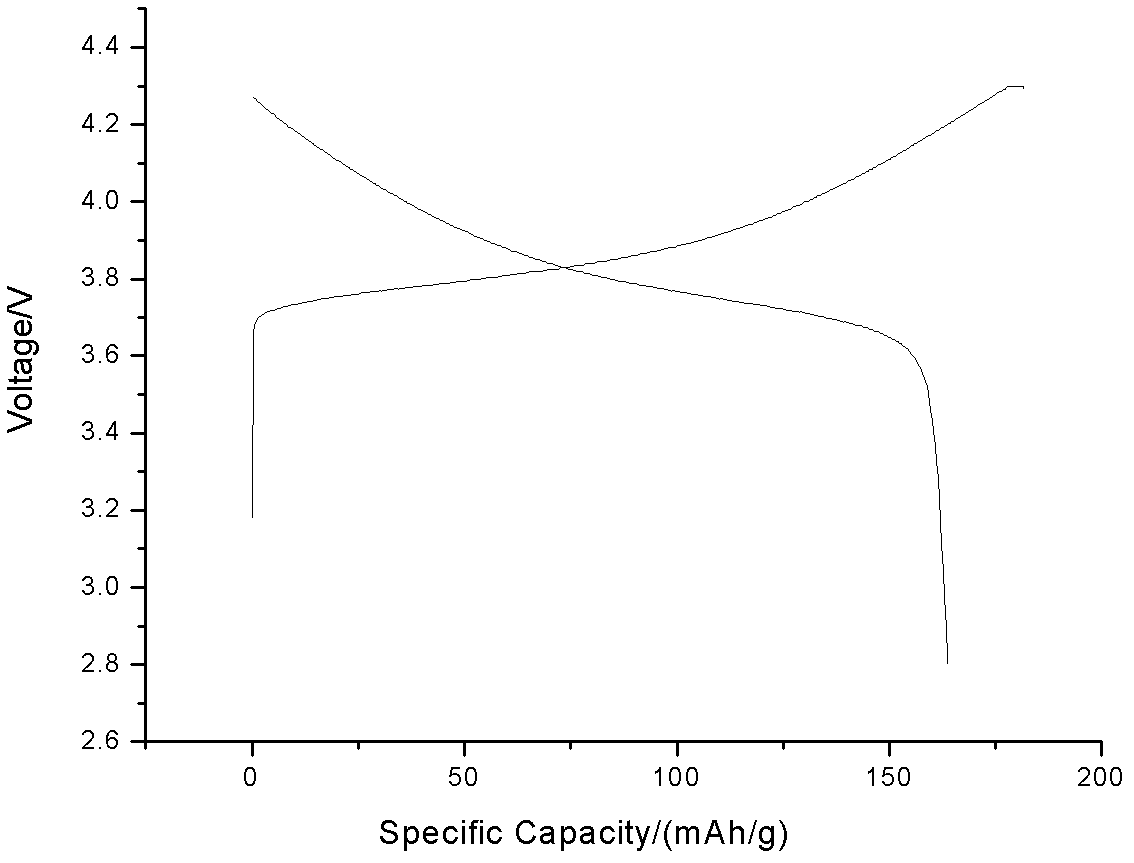
InactiveUS20090146115A1High tap densityLow tap densityElectrode thermal treatmentFinal product manufactureManganeseLithium compound
A process for preparing lithium-nickel-manganese-cobalt composite oxide used as a positive electrode material for the lithium ion battery, comprising subjecting a mixture containing a lithium compound and nickel-manganese-cobalt hydroxide to a first-stage sintering and a second-stage sintering, wherein said process further comprises adding a binder and / or binder solution after the first-stage sintering, and the mixture of the binder and / or binder solution and the product of first-stage sintering is sintered in said second-stage sintering. The tap density and volume specific capacity of the positive electrode material lithium-nickel-manganese-cobalt composite oxide prepared by the present process, come up to 2.4 g / cm3 and 416.4 mAh / cm3, respectively. Besides, the positive electrode material lithium-nickel-manganese-cobalt composite oxide prepared by the present process possesses the advantages of high specific capacity and good cycle stability.
Owner:BYD CO LTD
Lithium nickel and cobalt aluminate anode material and preparation method and lithium ion battery thereof
InactiveCN106532038ASurface low in alkalinityAlkaline compounds decreaseCell electrodesSecondary cellsNickel saltLithium compound
The invention provides a preparation method of a lithium nickel and cobalt aluminate anode material. The preparation method comprises the following steps that nickel salt, cobalt salt, aluminum salt, a first complexing agent and a first precipitant are mixed and are heated, precipitation reaction is performed to obtain aluminum nickel and cobalt hydroxide suspension liquid, the suspension liquid is mixed with manganese salt, a second complexing agent and a second precipitant, a first intermediate product is obtained after heating reaction, thermal treatment is performed to obtain a second intermediate product, the second intermediate product is mixed and sintered with a lithium compound to obtain a third intermediate product, the third intermediate product is mixed with a coating agent, and thermal treatment is performed to obtain the lithium nickel and cobalt aluminate anode material. Compared with the prior art, manganese hydroxide coating is conducted on the surface of aluminum nickel and cobalt hydroxide, and direct contact between surface nickel ions and air is effectively avoided in follow-up treatment, so that the alkali compound content of the lithium nickel and cobalt aluminate anode material is remarkably reduced. In addition, the material can react with residual lithium on the surface of a sintering product through the follow-up coated coating agent, and the alkalinity and moisture of the lithium nickel and cobalt aluminate anode material are reduced.
Owner:NINGBO RONBAY LITHIUM BATTERY MATERIAL CO LTD
Process for comprehensive recovery nickel, copper, cobalt, sulfur and magnesium from ore
ActiveCN101328536AAvoid pollutionDamage to life and healthSulfur preparation/purificationProcess efficiency improvementSlurryOxygen
The invention relates to a process for comprehensively reclaiming nickel, copper, cobalt, sulphur and magnesium from ore. The process comprises the following steps that: high magnesium-nickel concentrated ore is selected from the ore by floatation; the high magnesium-nickel concentrated ore is prepared into ore slurry; sulphuric acid and oxygen are added into the ore slurry and are subjected to pressurization and leaching to the ore slurry; the sulphuric acid in the pressurized and leached ore slurry is neutralized; the neutralized ore slurry is subjected to dense washing to produce a leached residue and a leached liquid; secondary concentrated ore is obtained from the leached residue by floatation; iron and copper in the leached liquid are removed; magnesium hydroxide is added into the leached liquid to remove copper so as to deposit and separate nickel hydroxide and cobalt hydroxide off; the leached liquid in which the nickel hydroxide and cobalt hydroxide are separated off is added with ammonia and carbon dioxide so as to deposit and separate magnesium carbonate off; and the deposited magnesium carbonate is subjected to roasting so as to produce magnesia. The method is utilized, discharges no sulfur dioxide, reclaims the non-ferrous metals of Ni, Cu, Co and magnesium in the ore simultaneously, improves the coefficient of recovery of valuable metal compositions in the ore and reduces energy consumption.
Owner:CHINA ENFI ENGINEERING CORPORATION
Penicillium, as well as preparation method and application
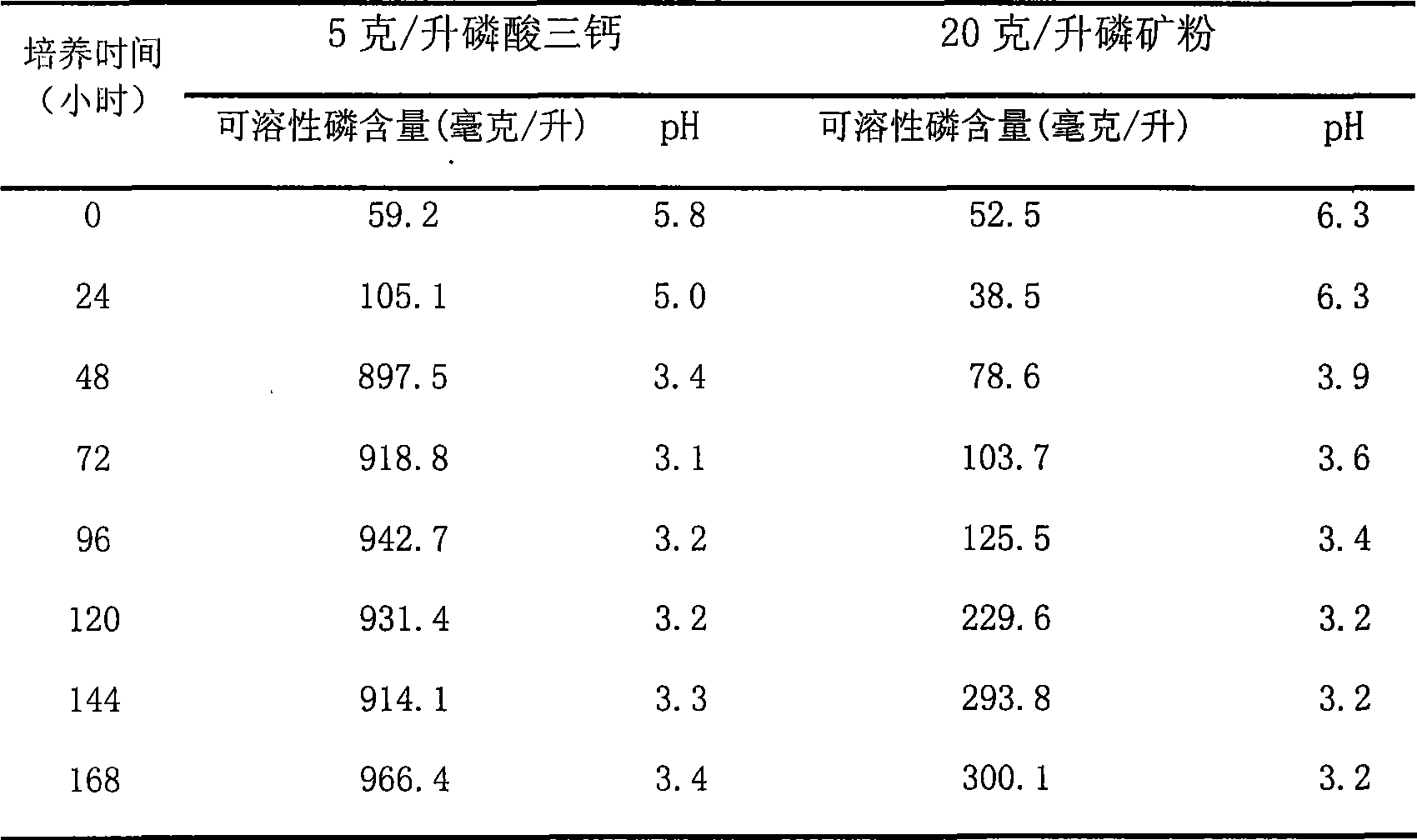
InactiveCN101434909AReach pollutionReduce pollutionFungiMicroorganism based processesEcological environmentPhosphate
The invention discloses a Penicillium, a preparation method and applications thereof, Penicillium fungus PSM11-5 is separated from a vanadium ore sample; insoluble tricalcium phosphate, sodium metavanadate, cobalt hydroxide and basic nickel carbonate are taken as indicating compounds; and a fungal strain is screened by testing the capability of decomposing the tricalcium phosphate, the sodium metavanadate, the cobalt hydroxide and the basic nickel carbonate. The Penicillium PSM11-5 is Penicillium sp.PSM11-5 CCTCCM208207. The strain is utilized for carrying out biological leaching of phosphorus and biological metallurgy, metals of phosphorus, vanadium, nickel, cobalt and the like are leached from lean ores, discarded ores, submarginal ores, difficult-to-mine ores, difficult dressing ores and refractory ores, thereby fully utilizing the mineral resources, reducing the metallurgical costs and protecting the ecological environment. The PSM11-5 is utilized for leaching the phosphorus from low-grade phosphate rock powder, a biological fertilizer is prepared to be applied to the soil, thereby leading the soil to contain higher content of soluble phosphorus which can be utilized by crops; the strain further leaches insoluble phosphorus which is deposited in the soil before, thereby reducing phosphorus fertilizer and reducing gas pollution caused by the phosphorus fertilizer and water pollution caused by the phosphorus fertilizer.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com