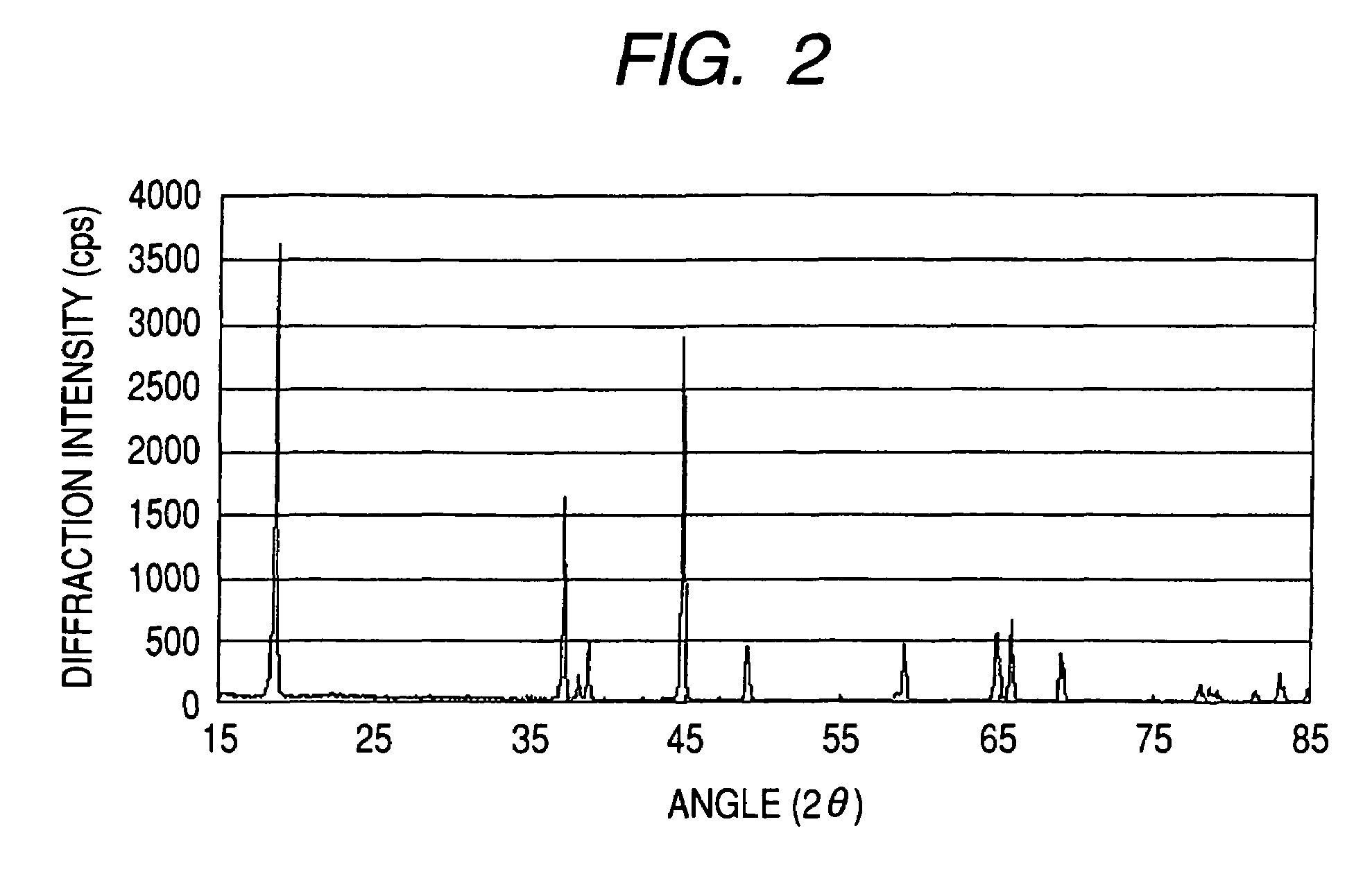
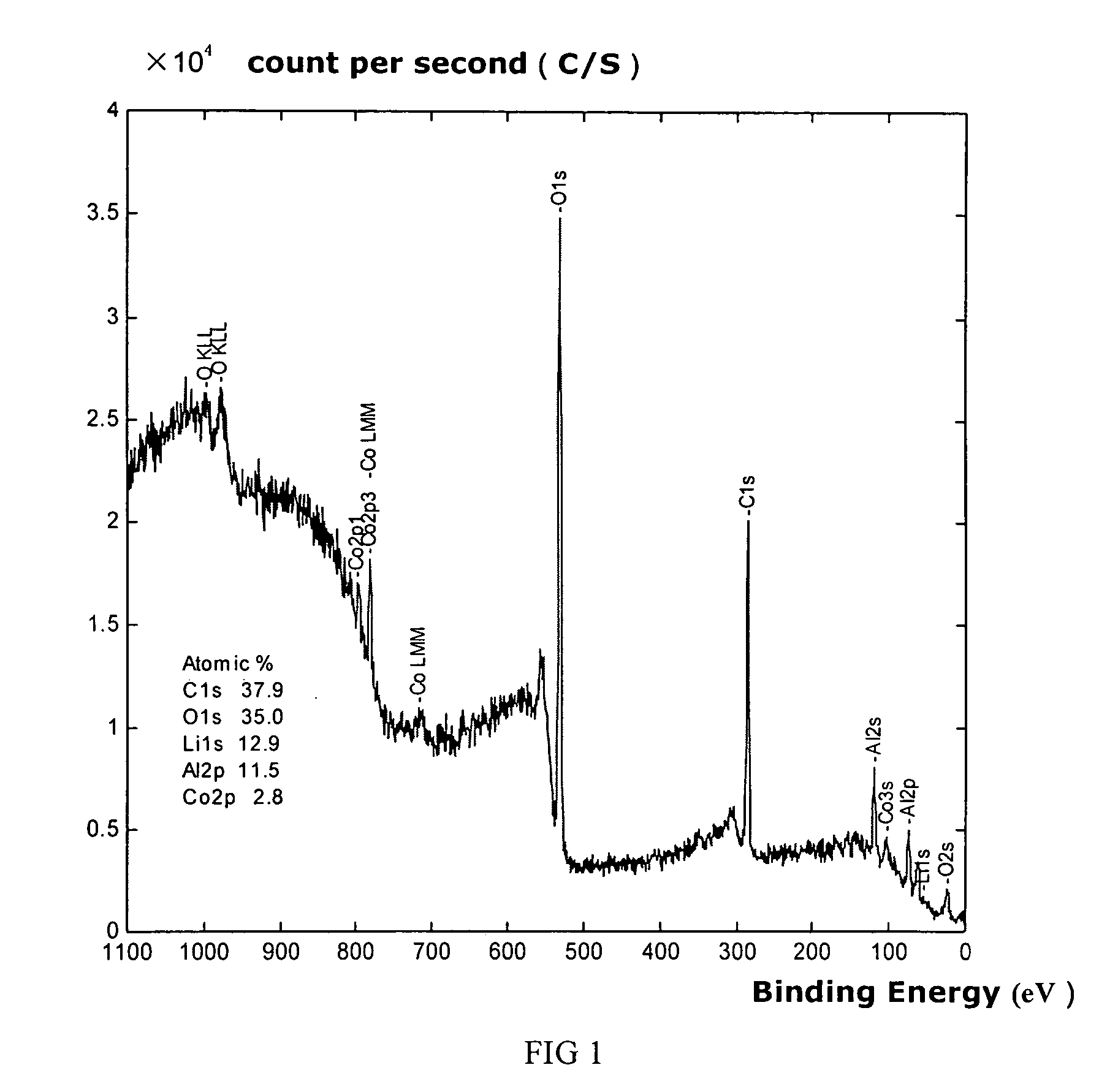
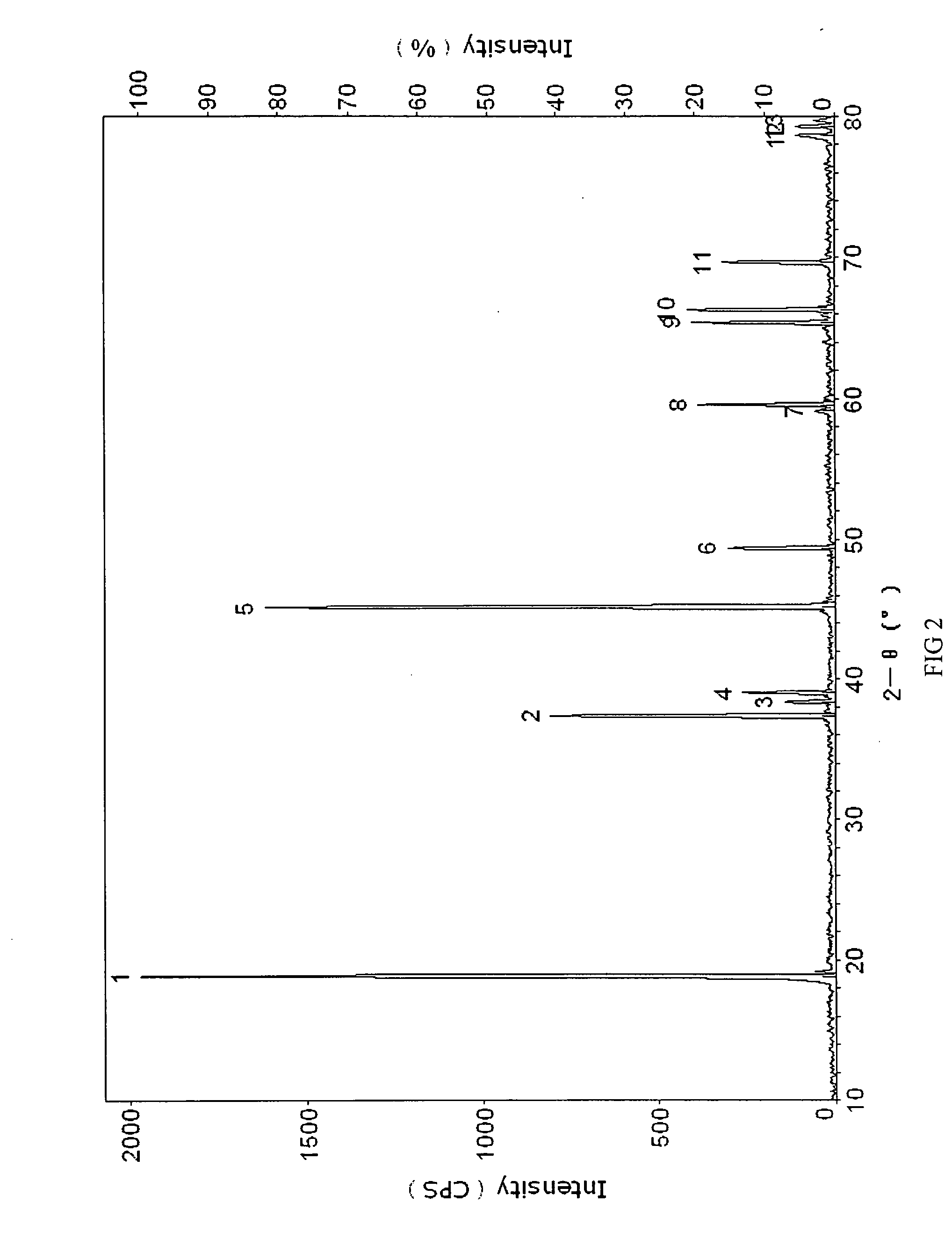
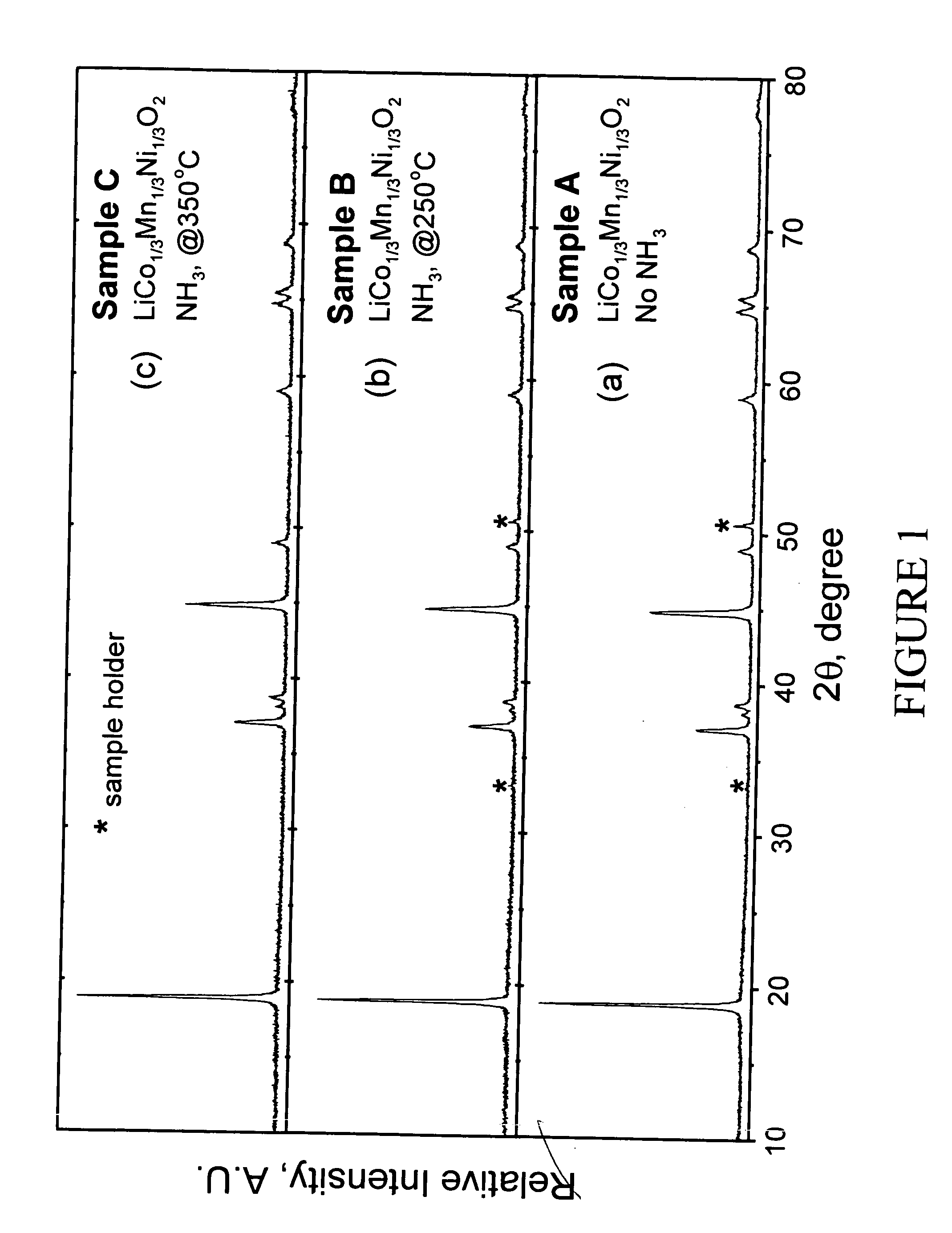
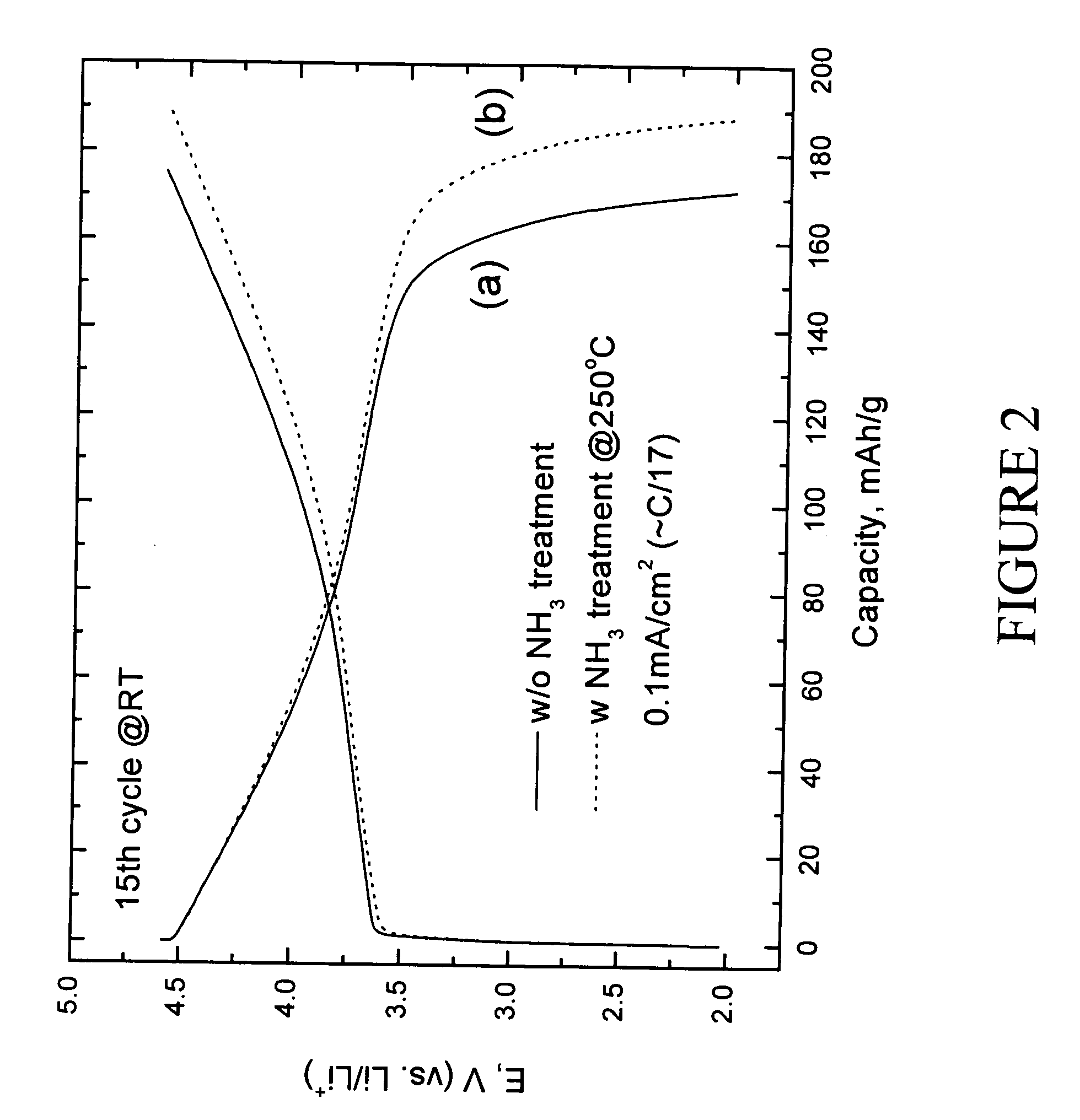
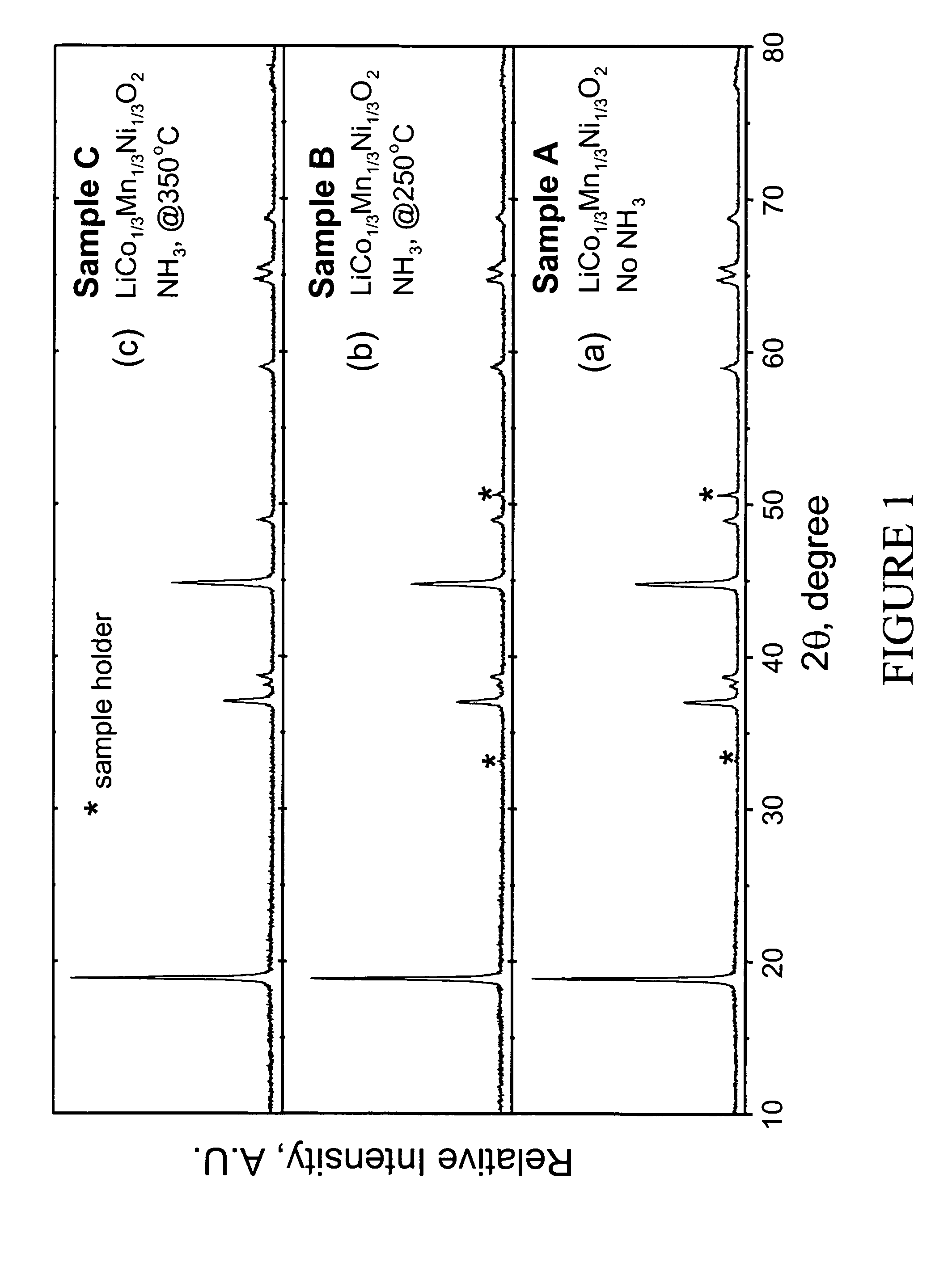
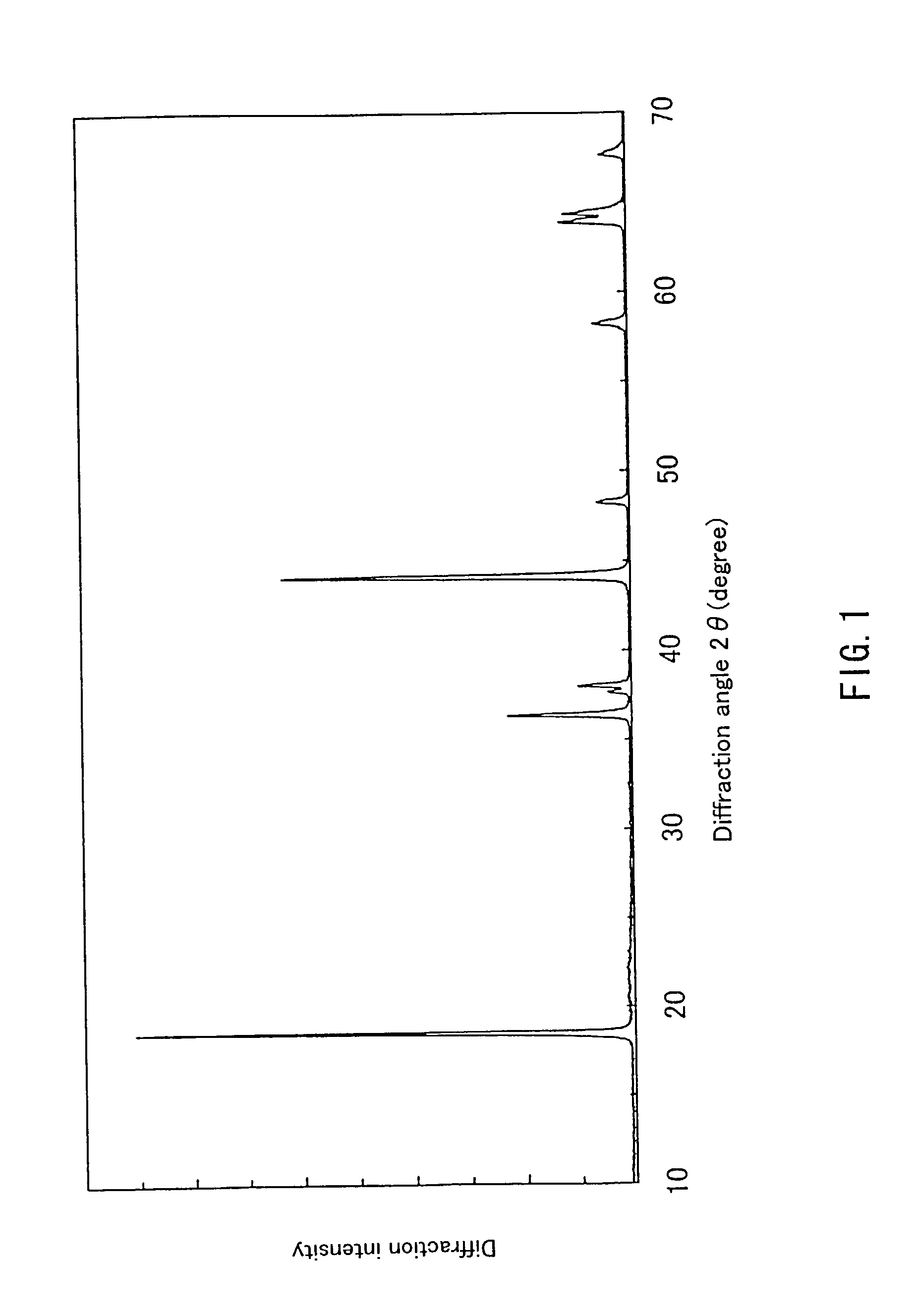
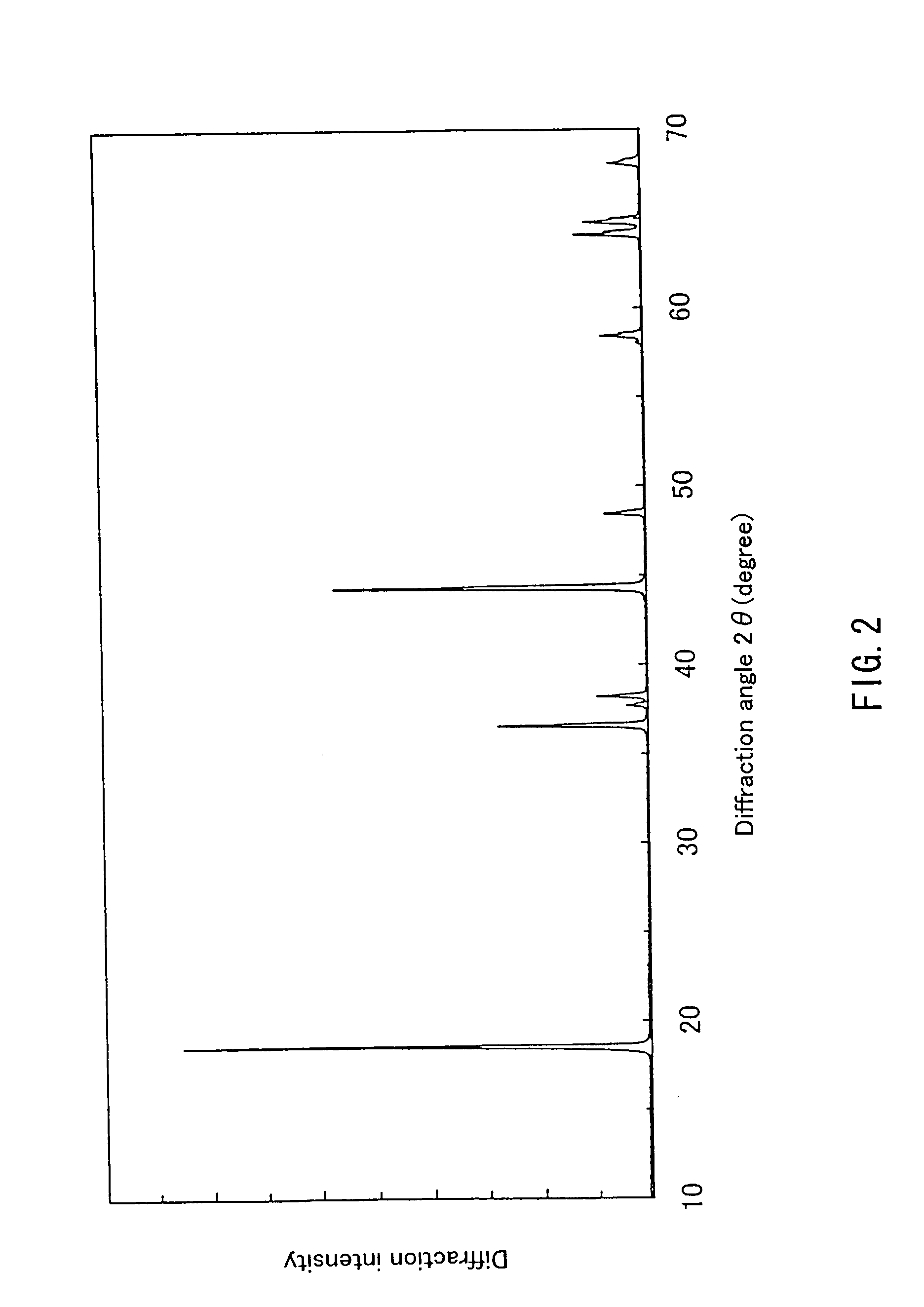
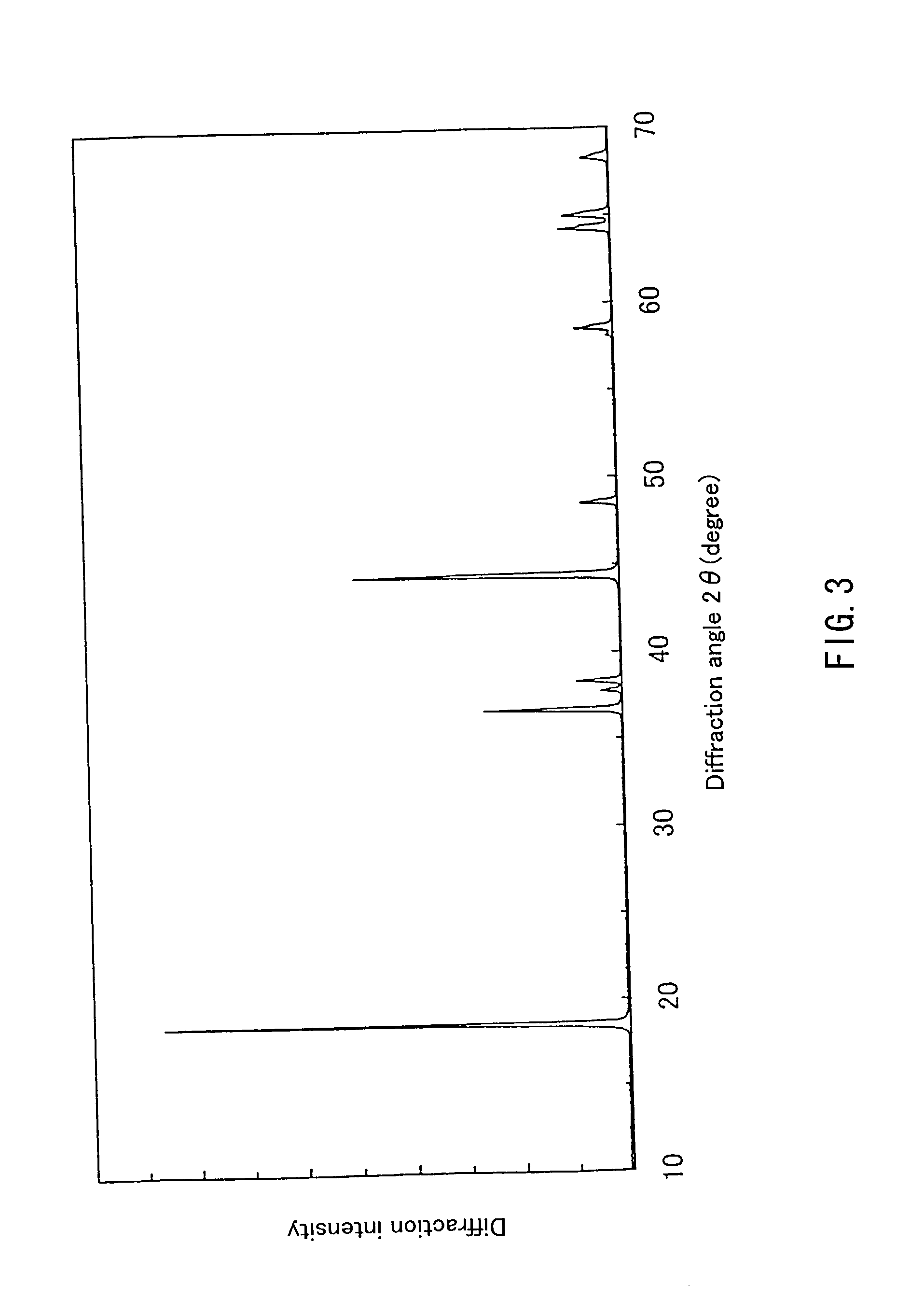
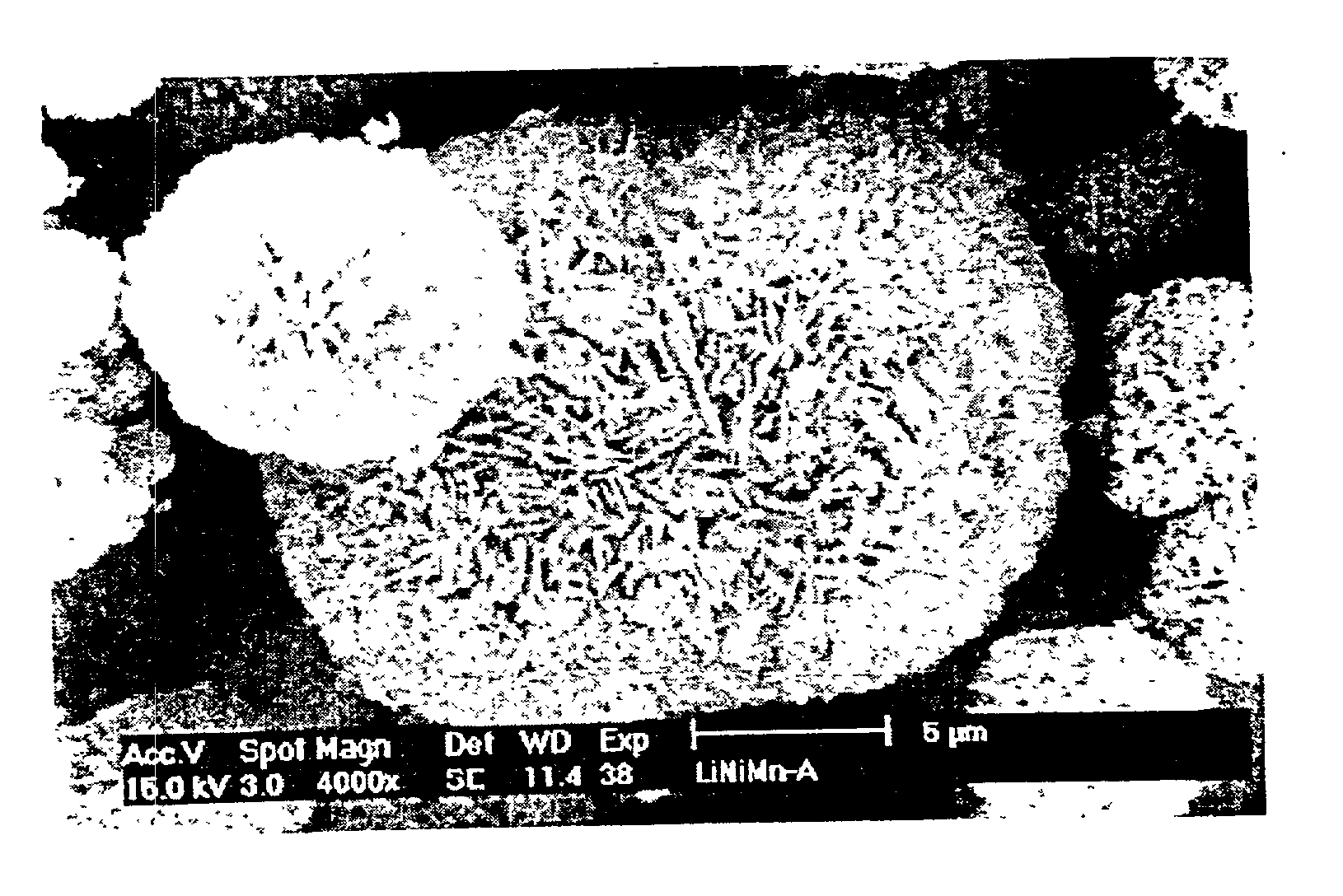
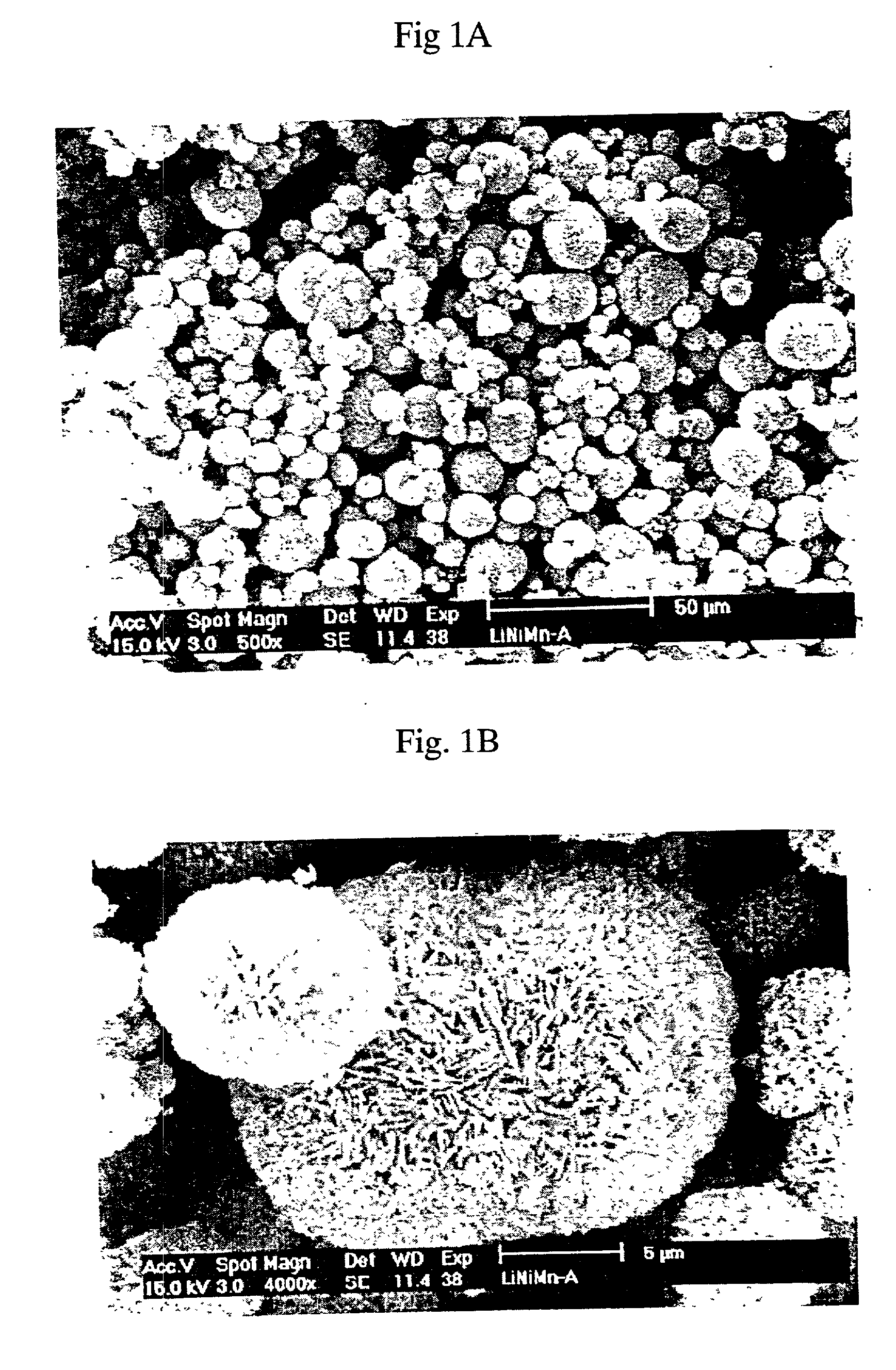
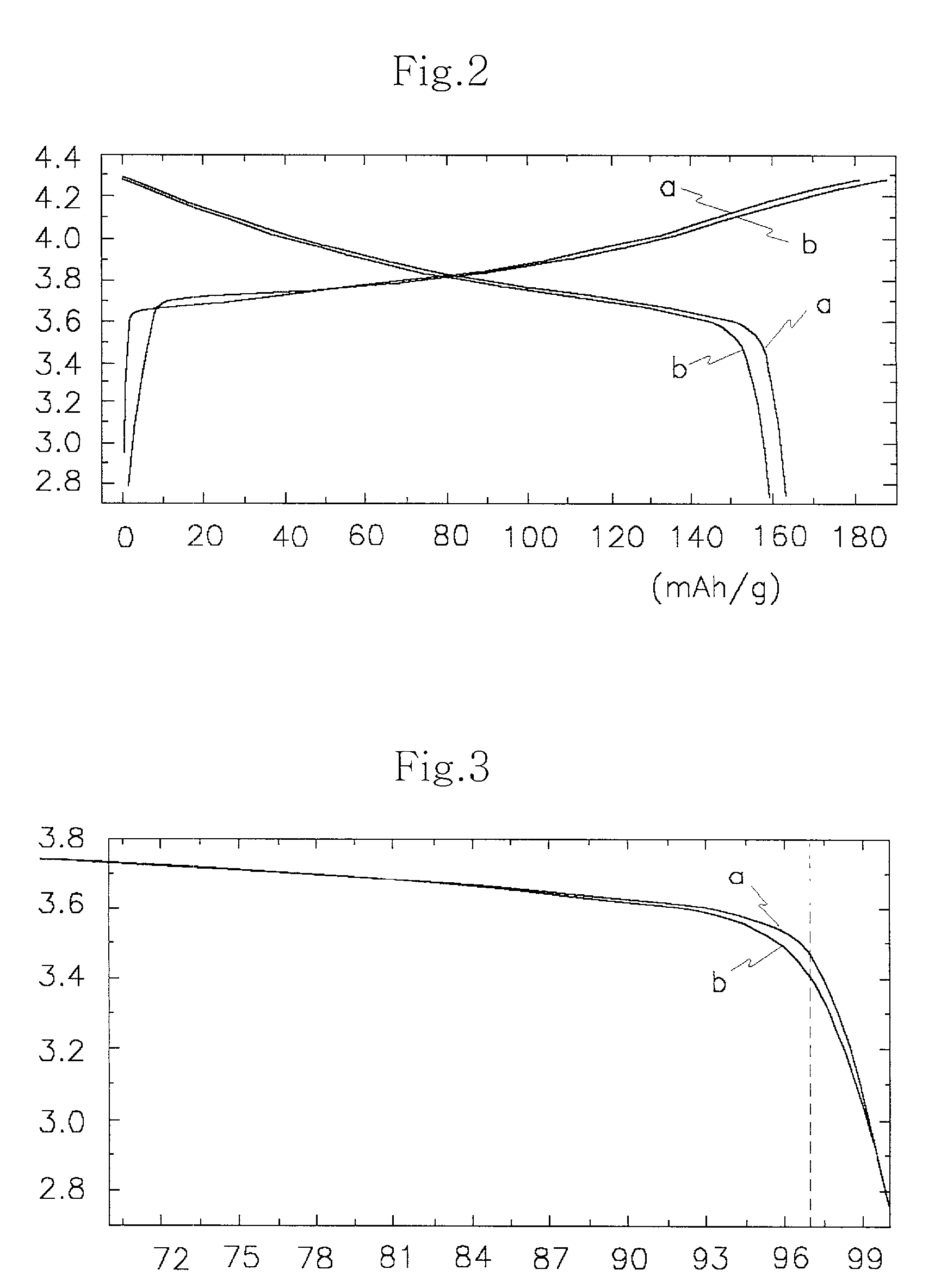
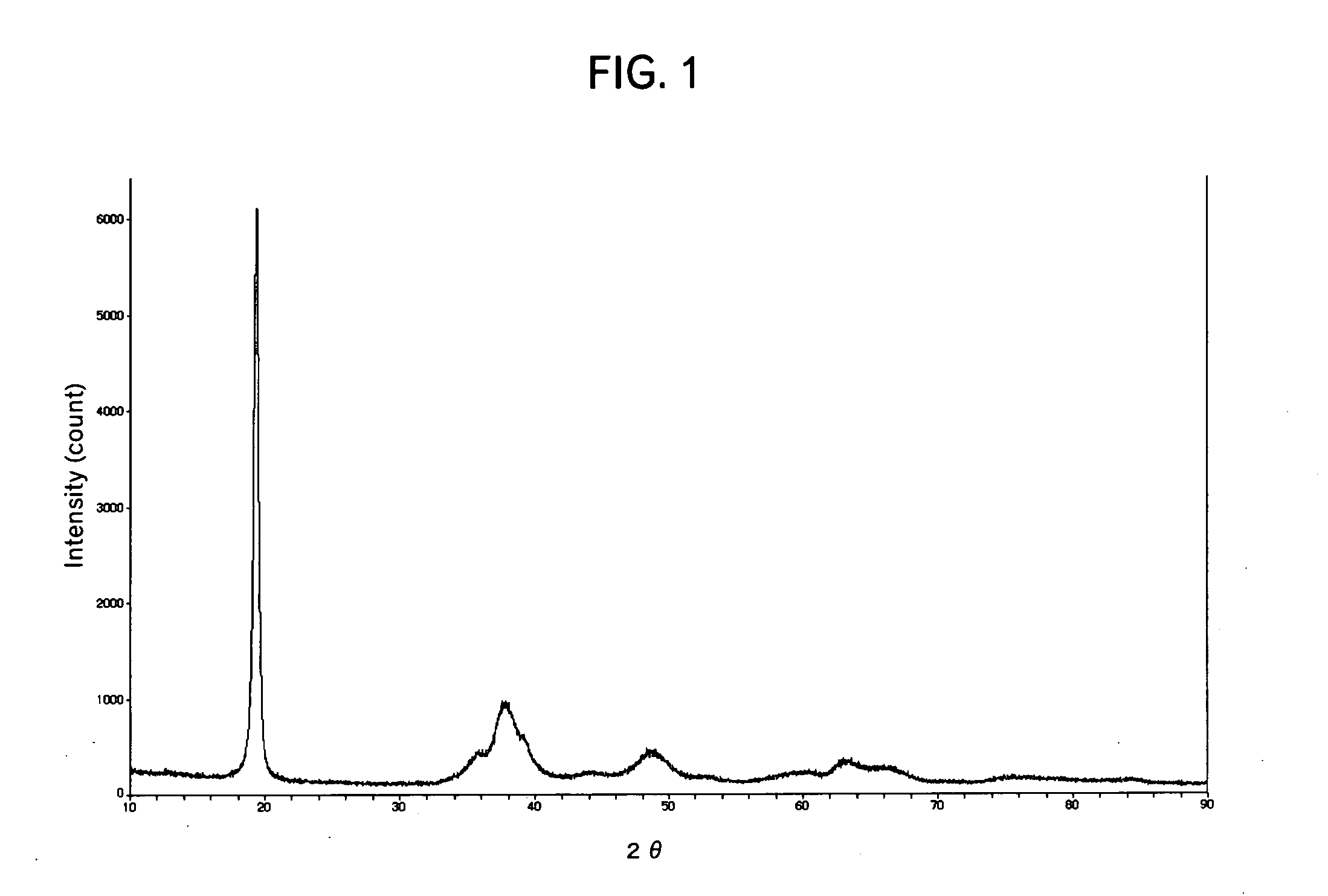
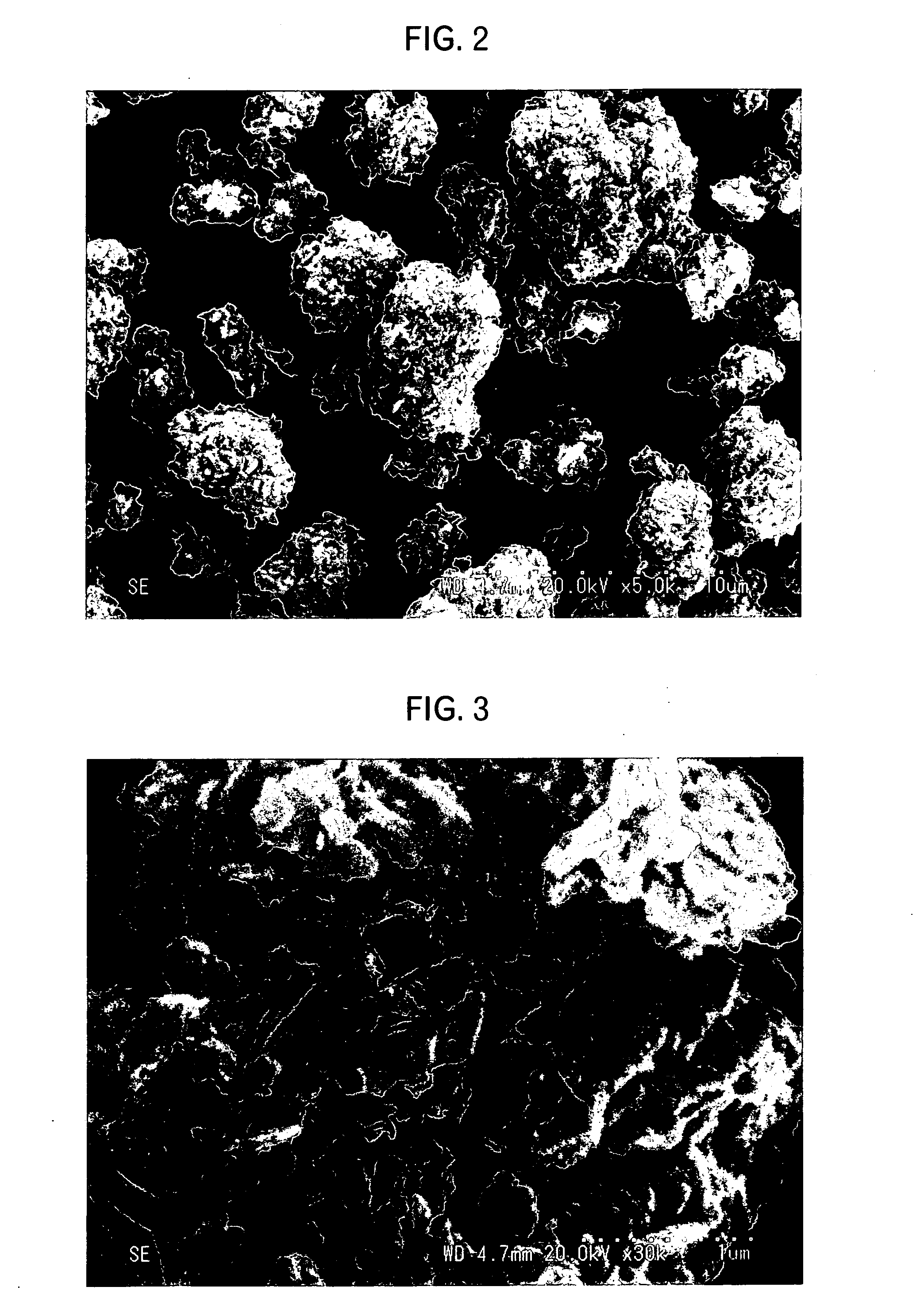
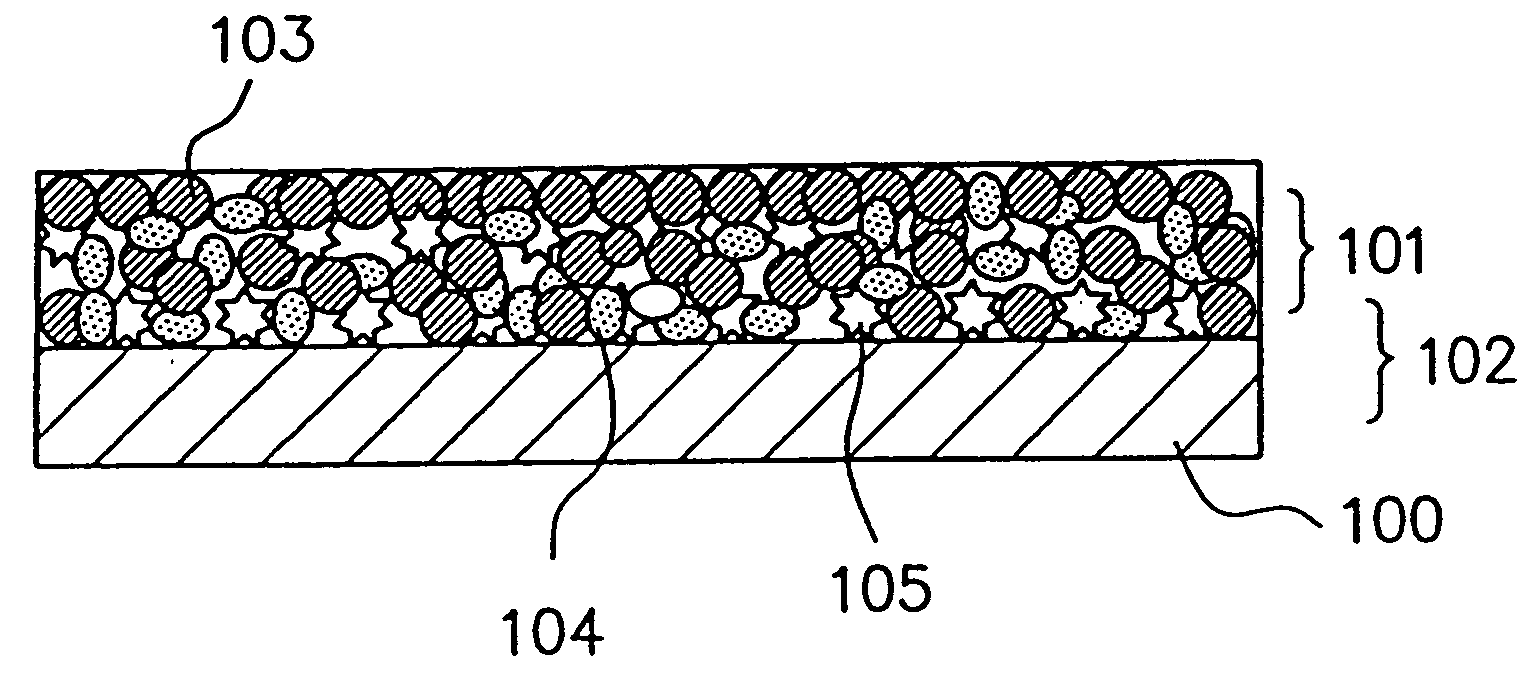
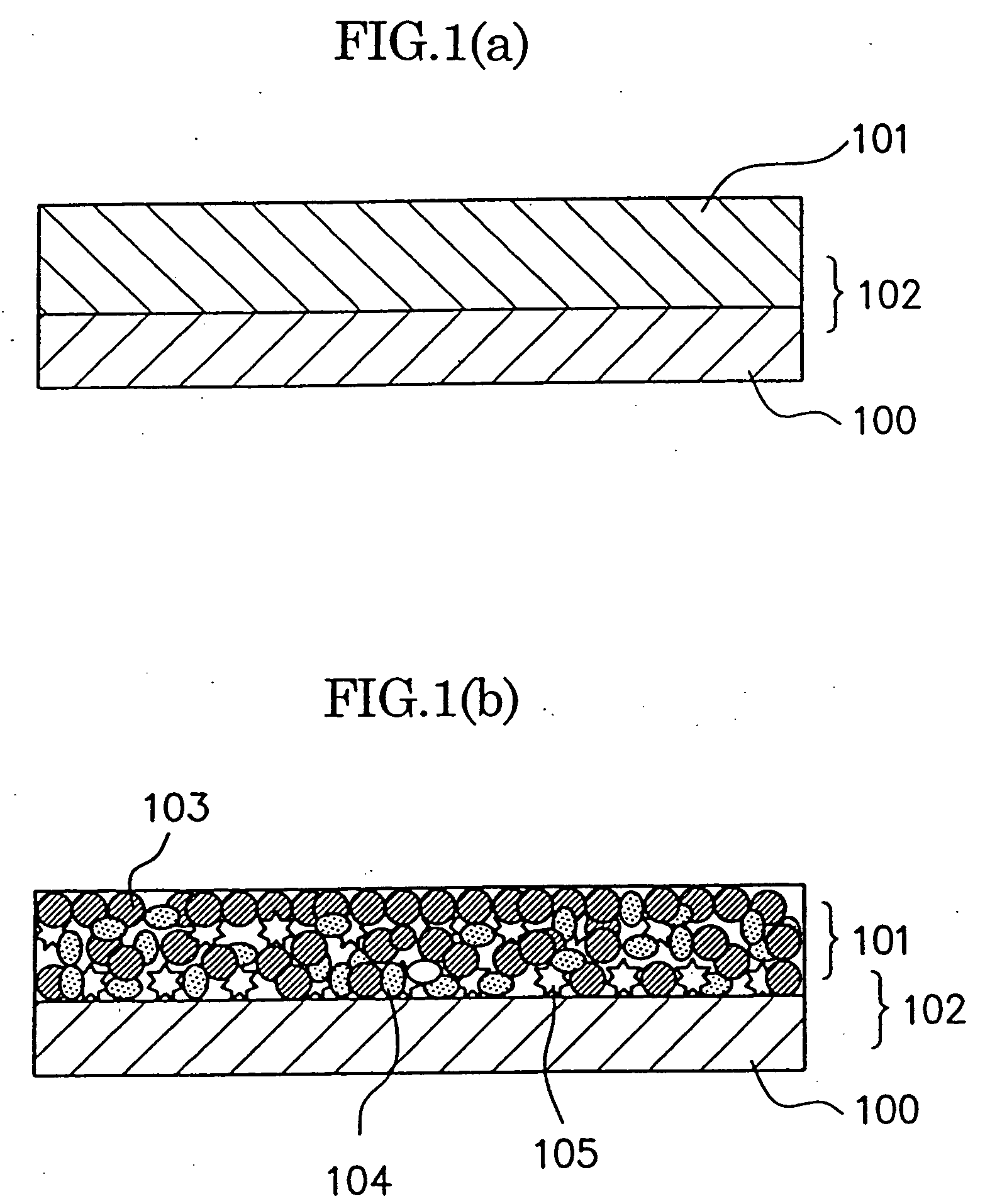
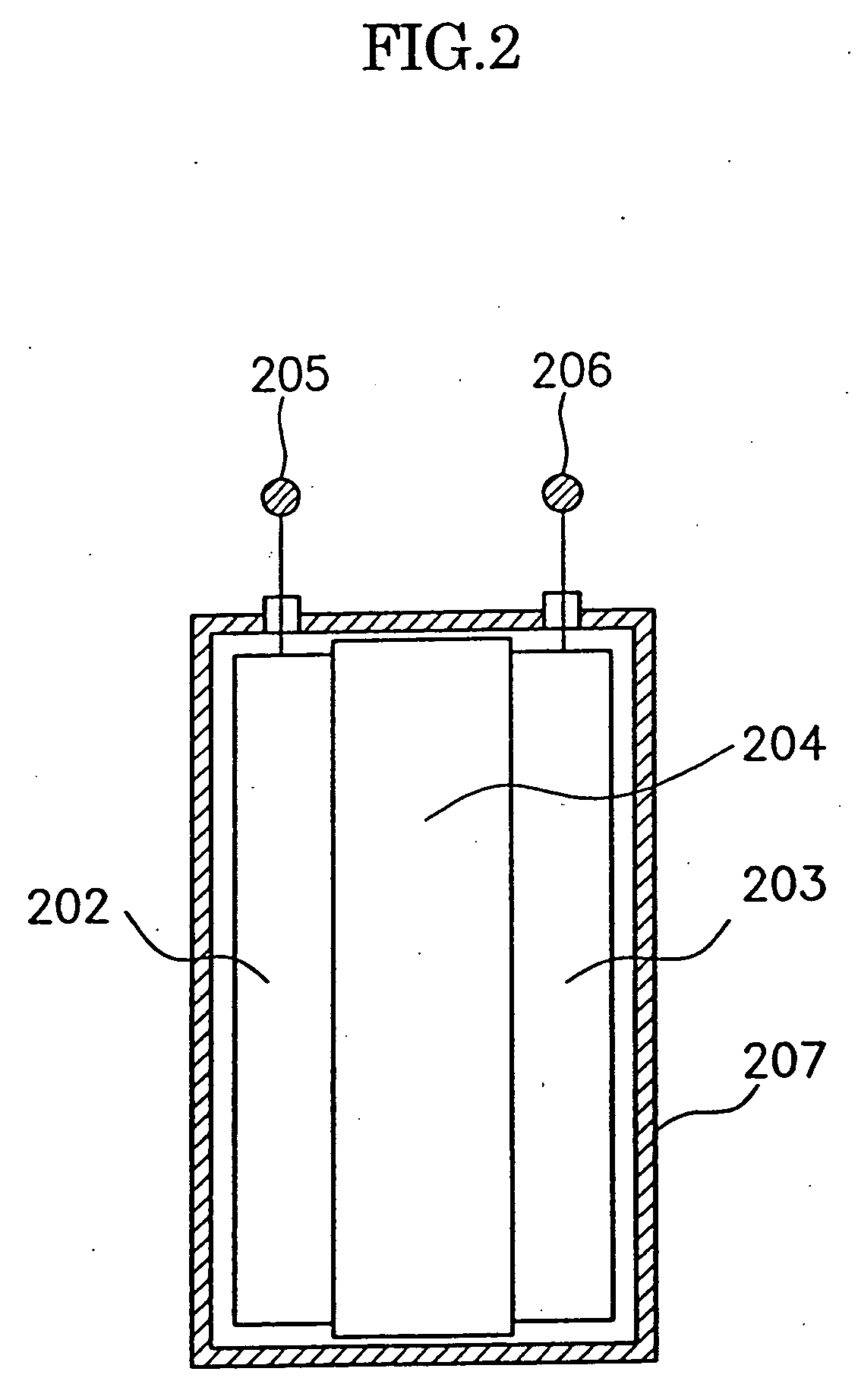
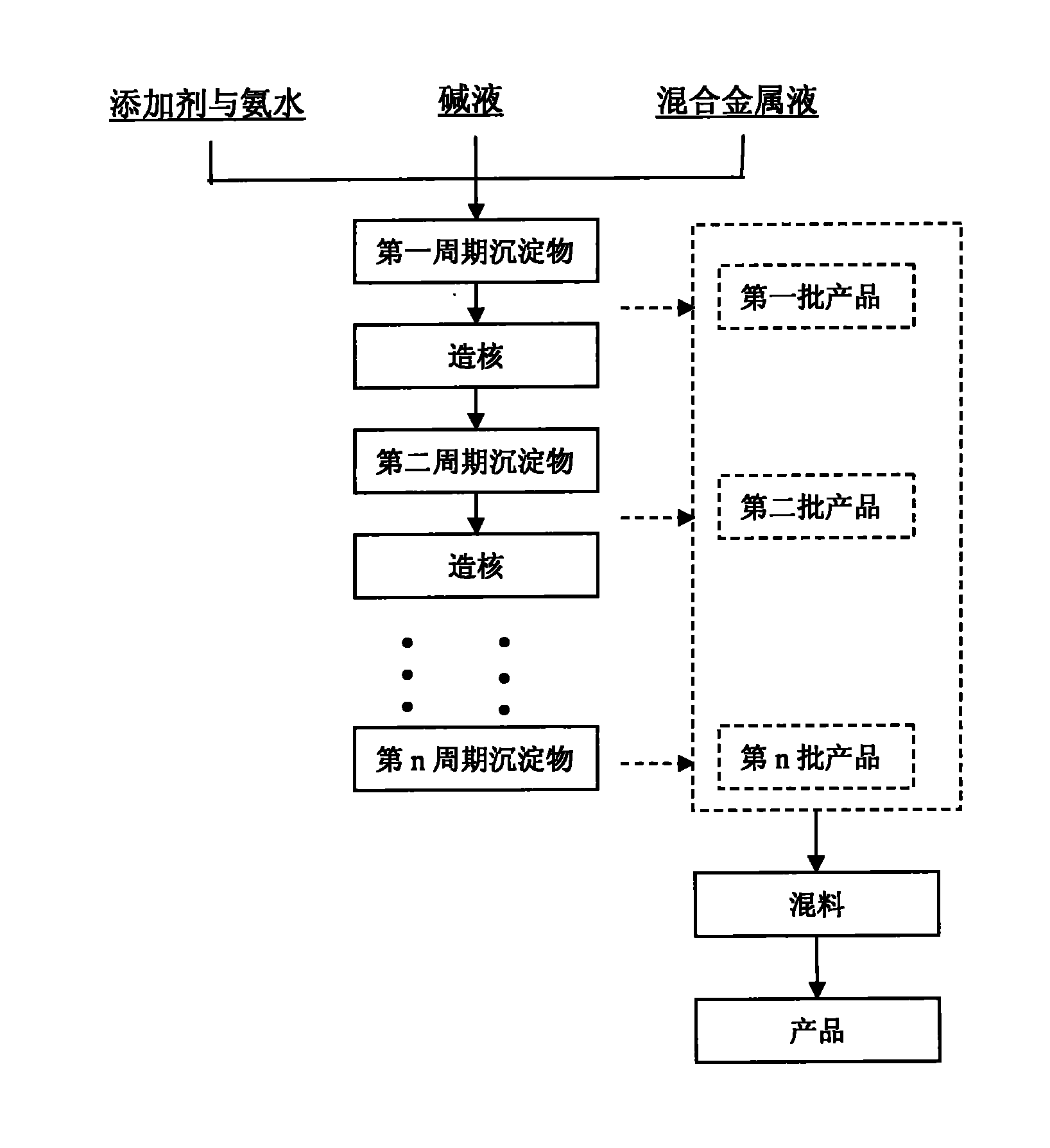
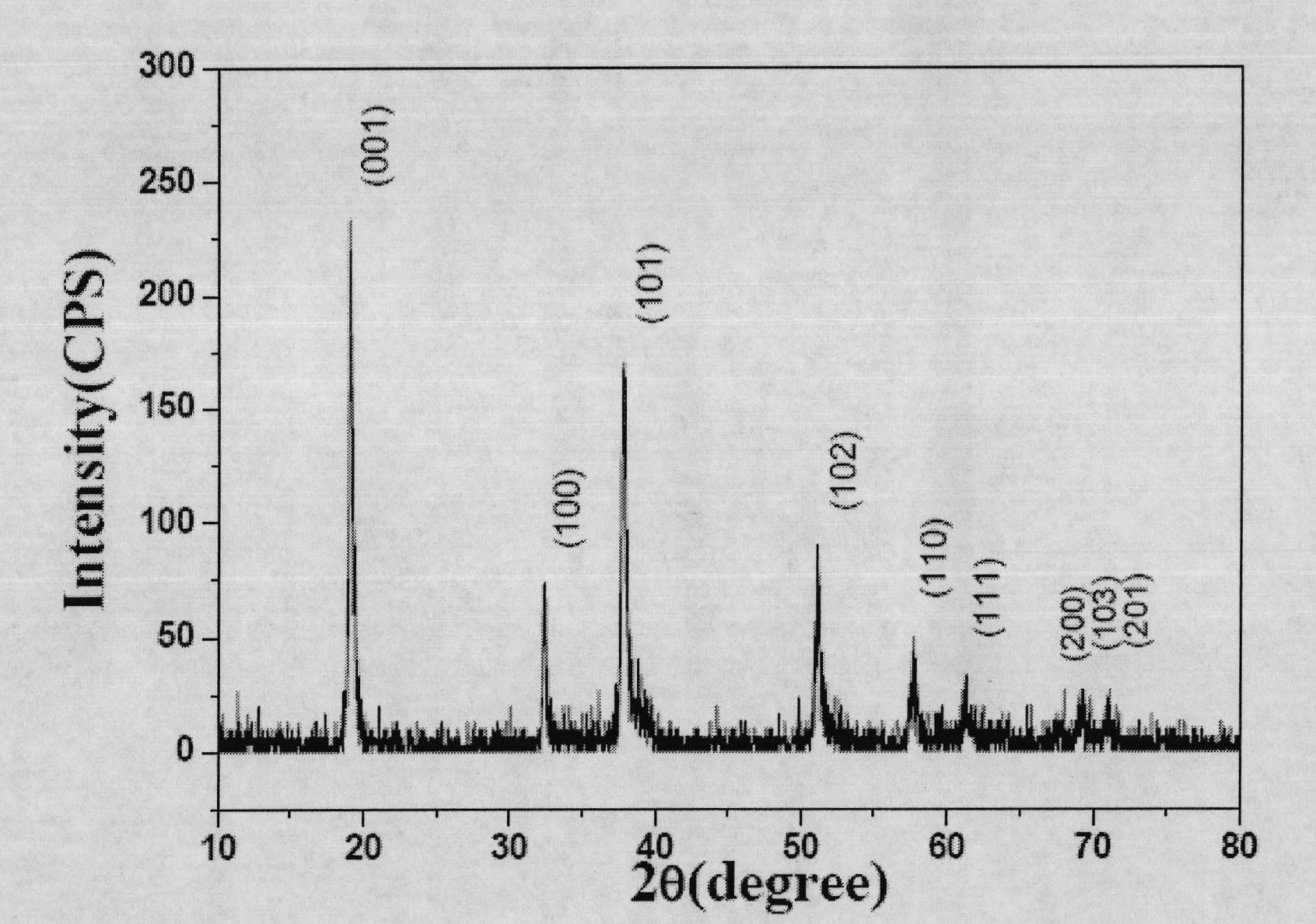
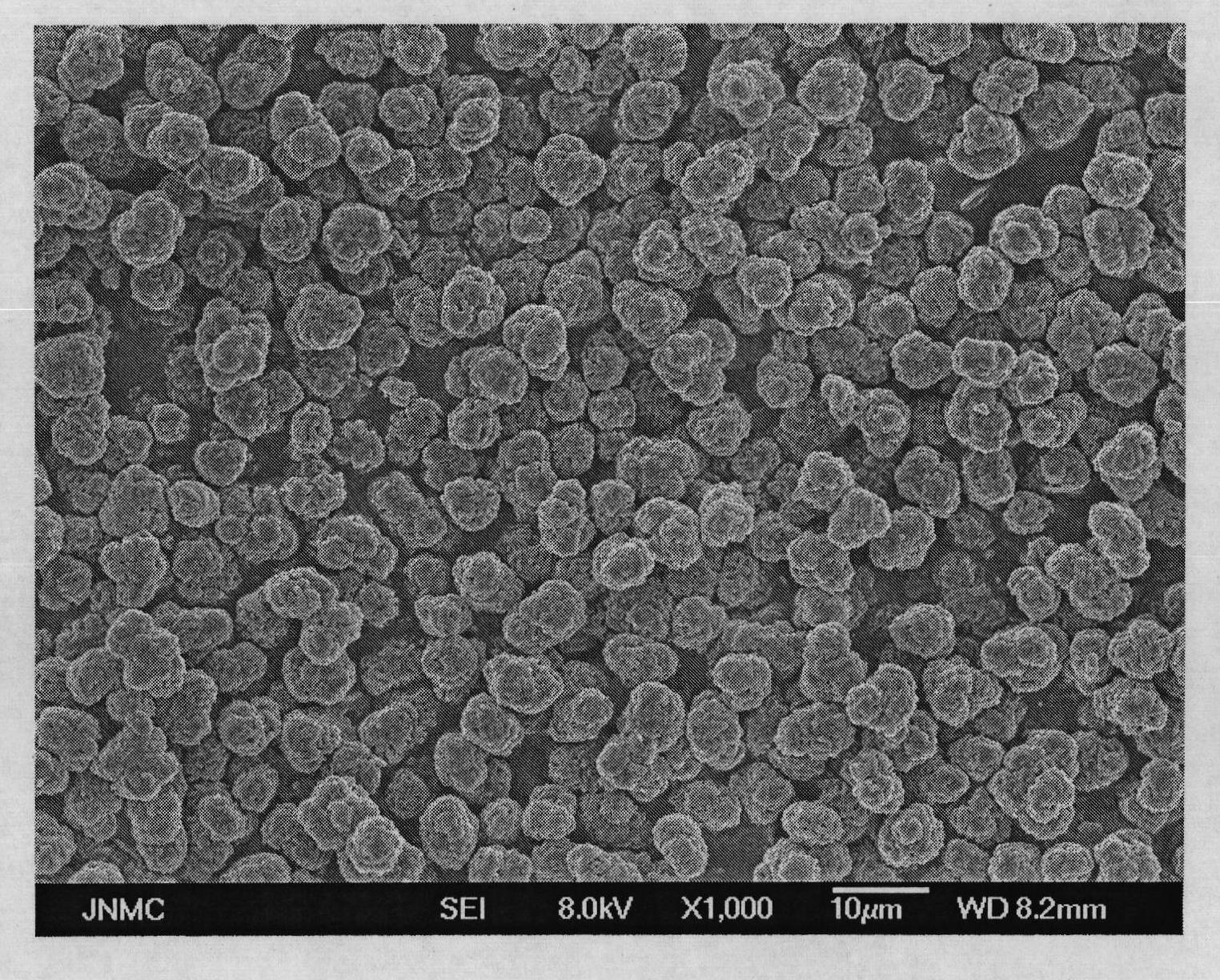
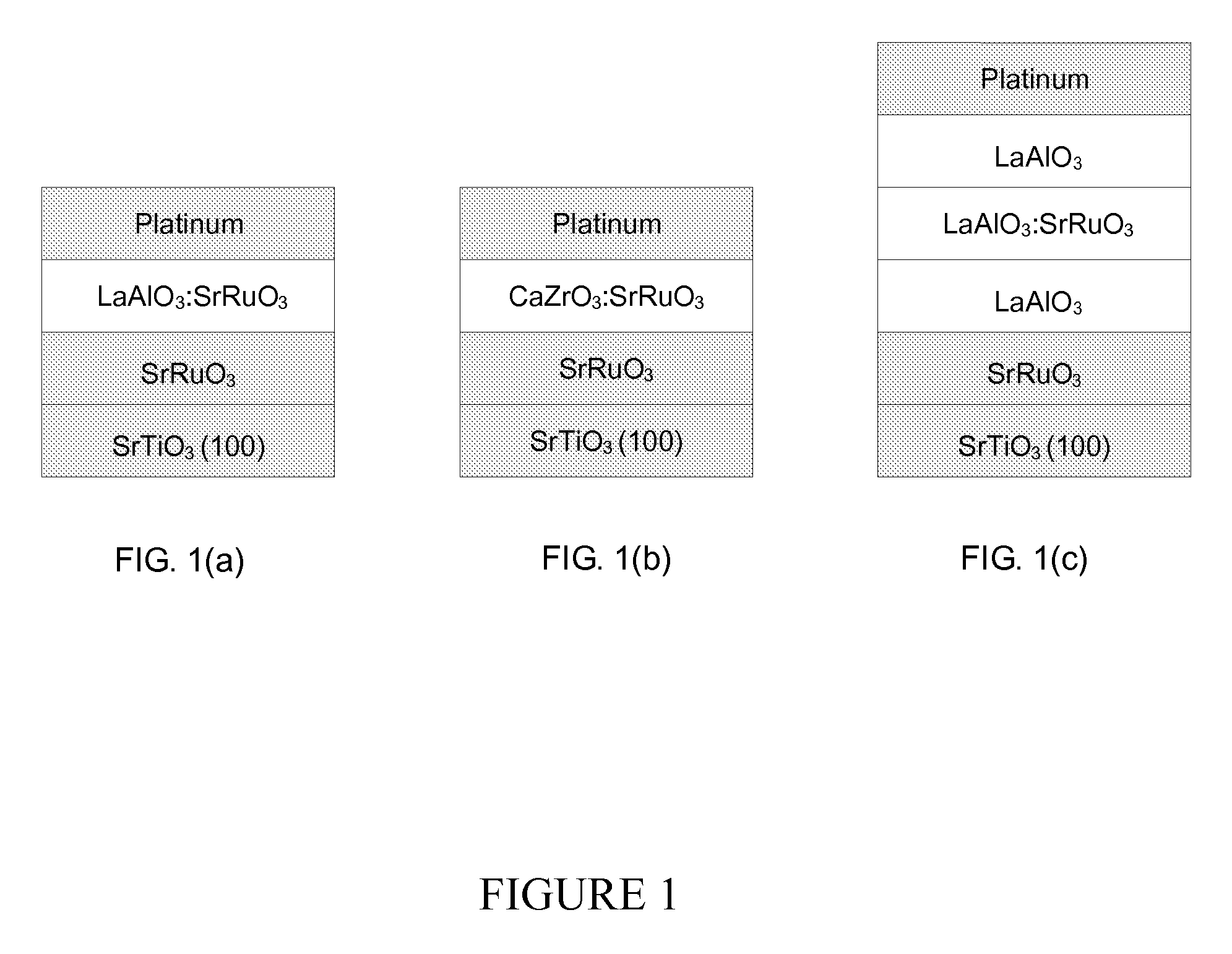
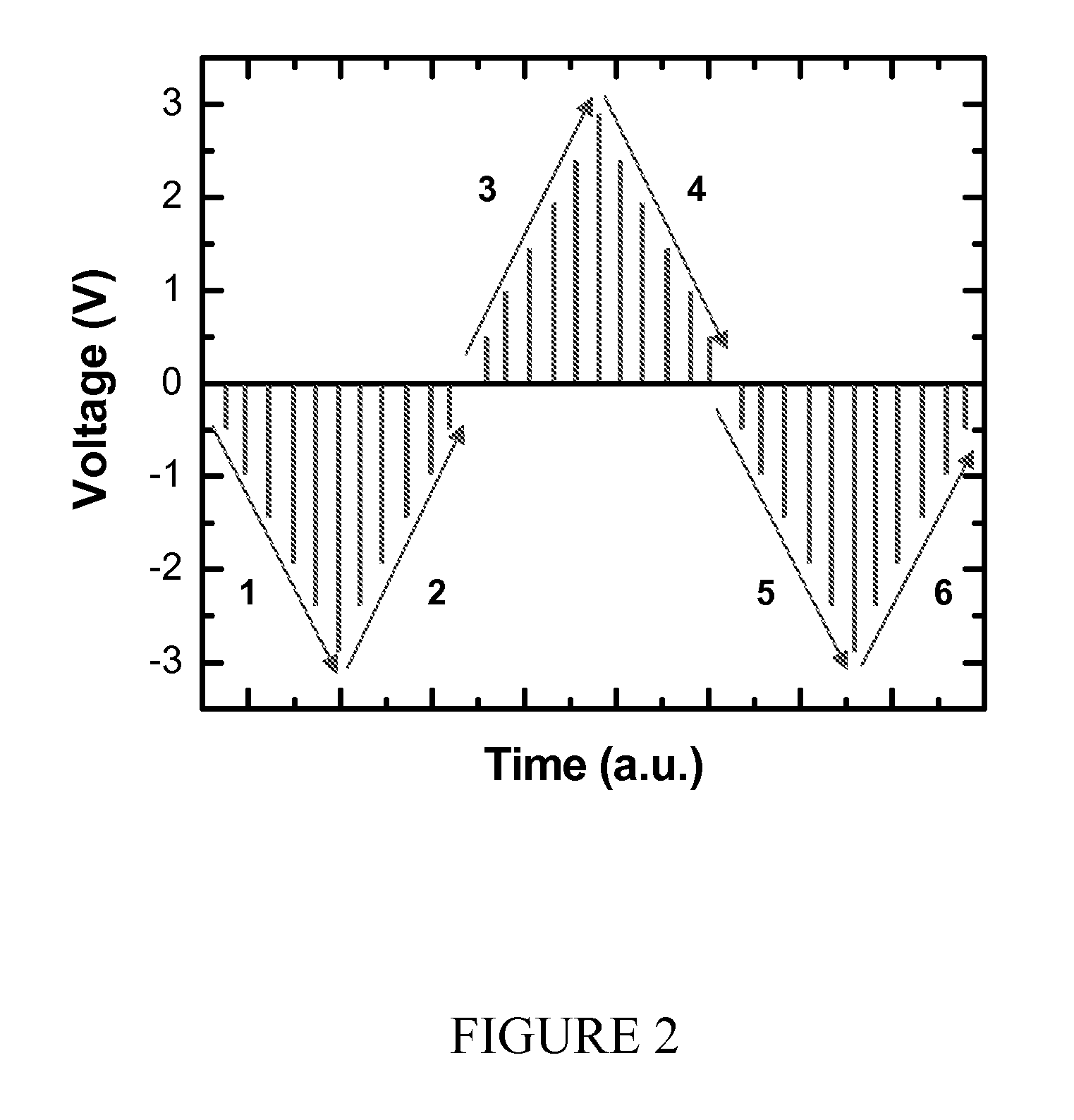
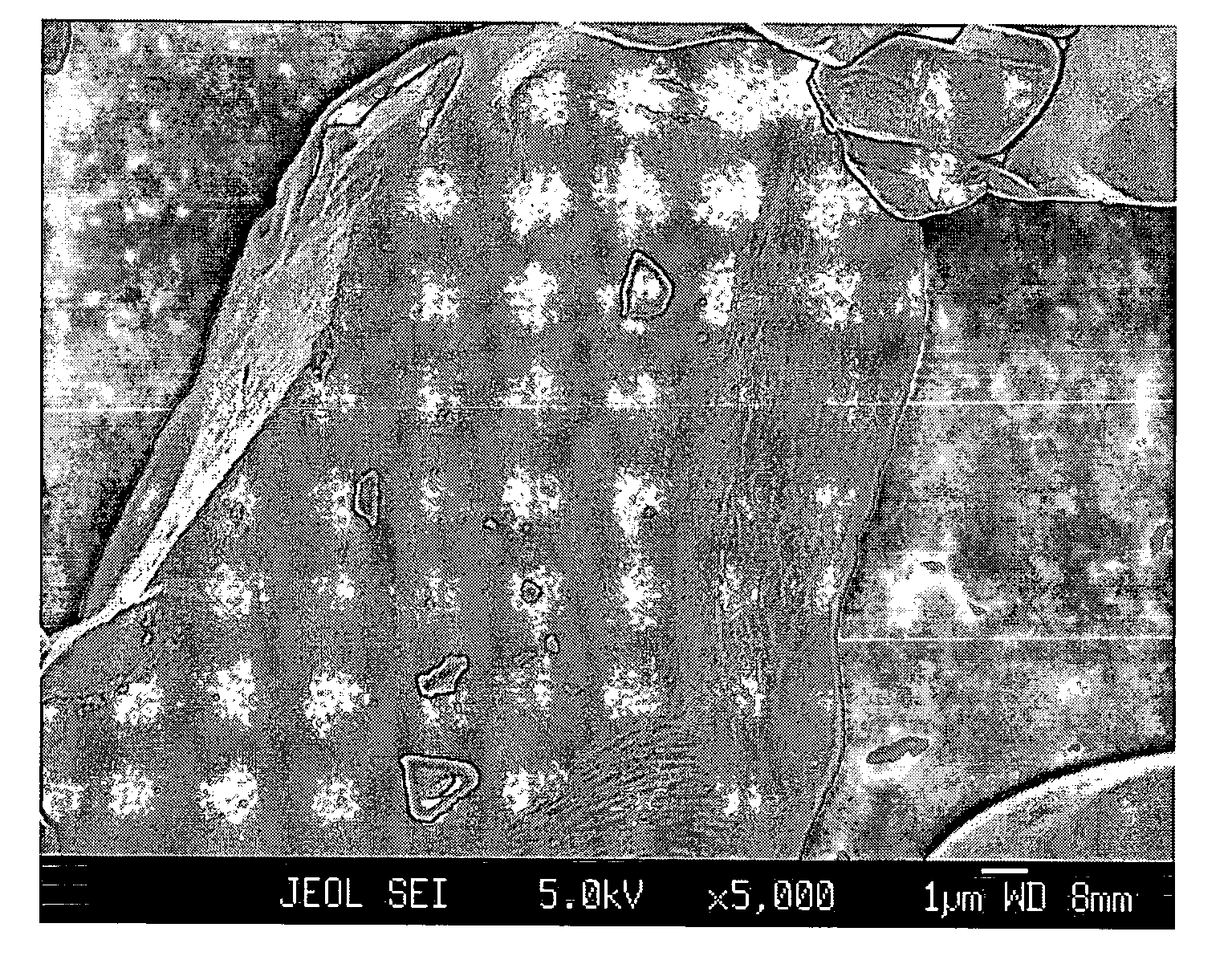
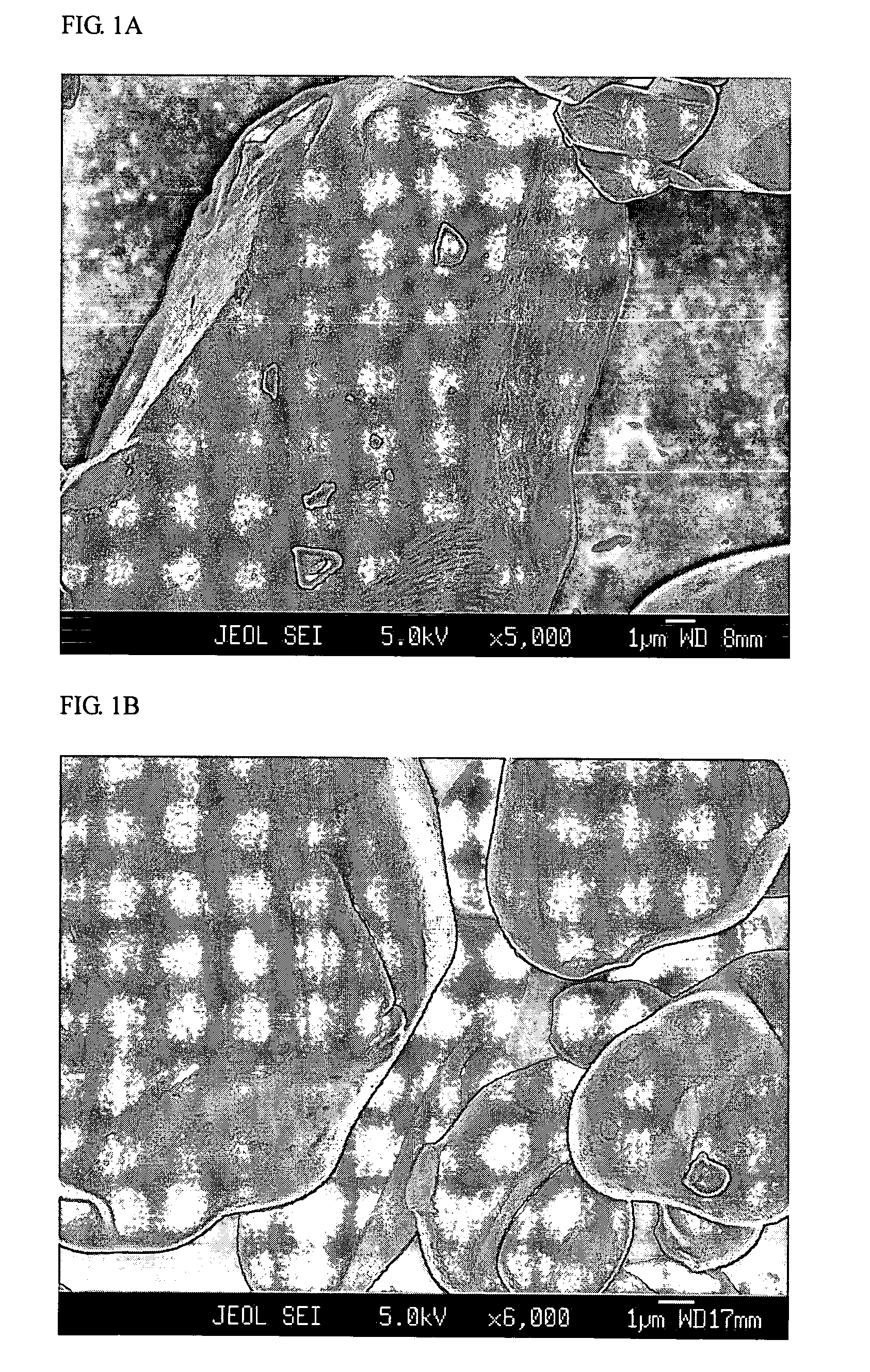
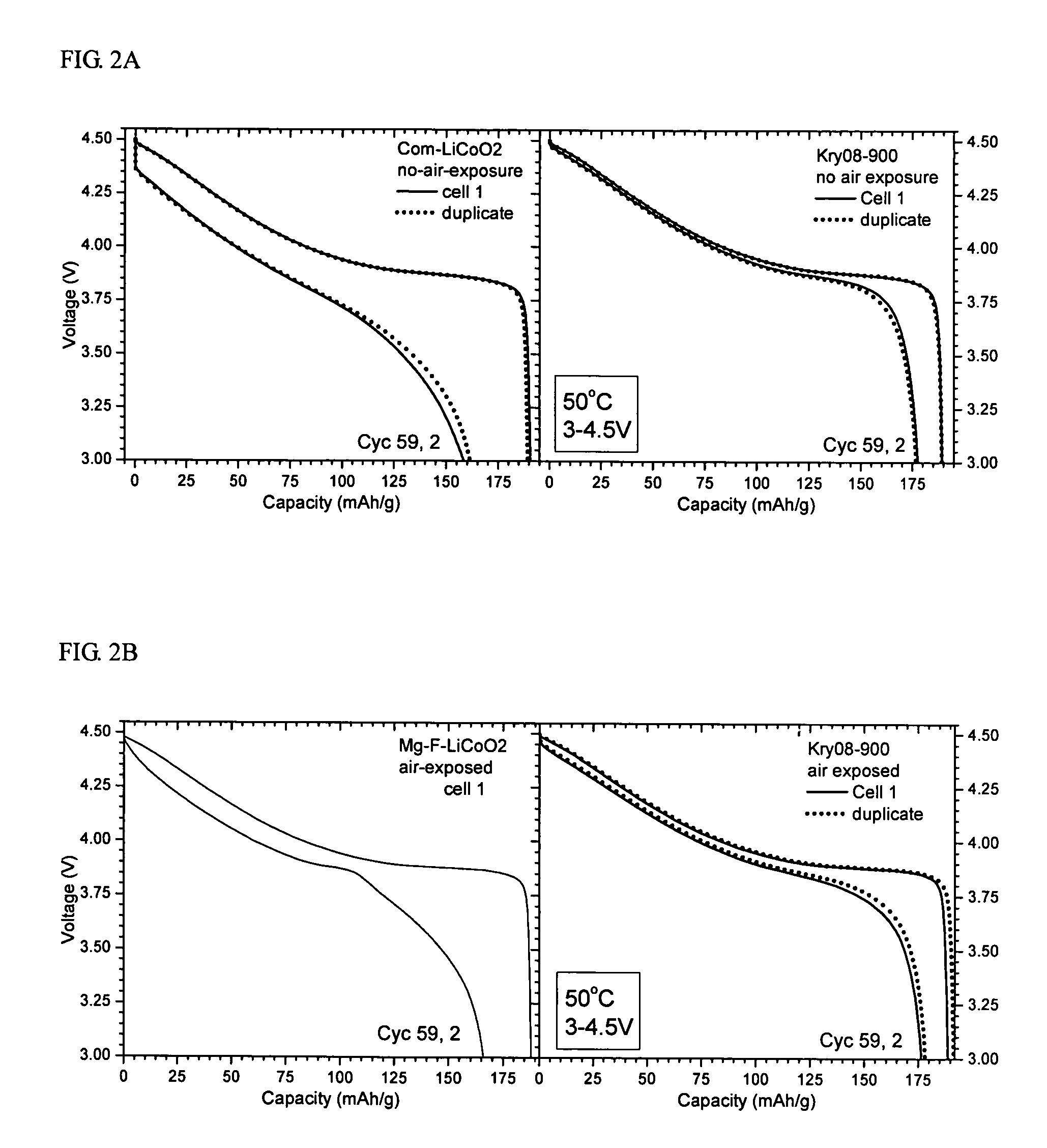
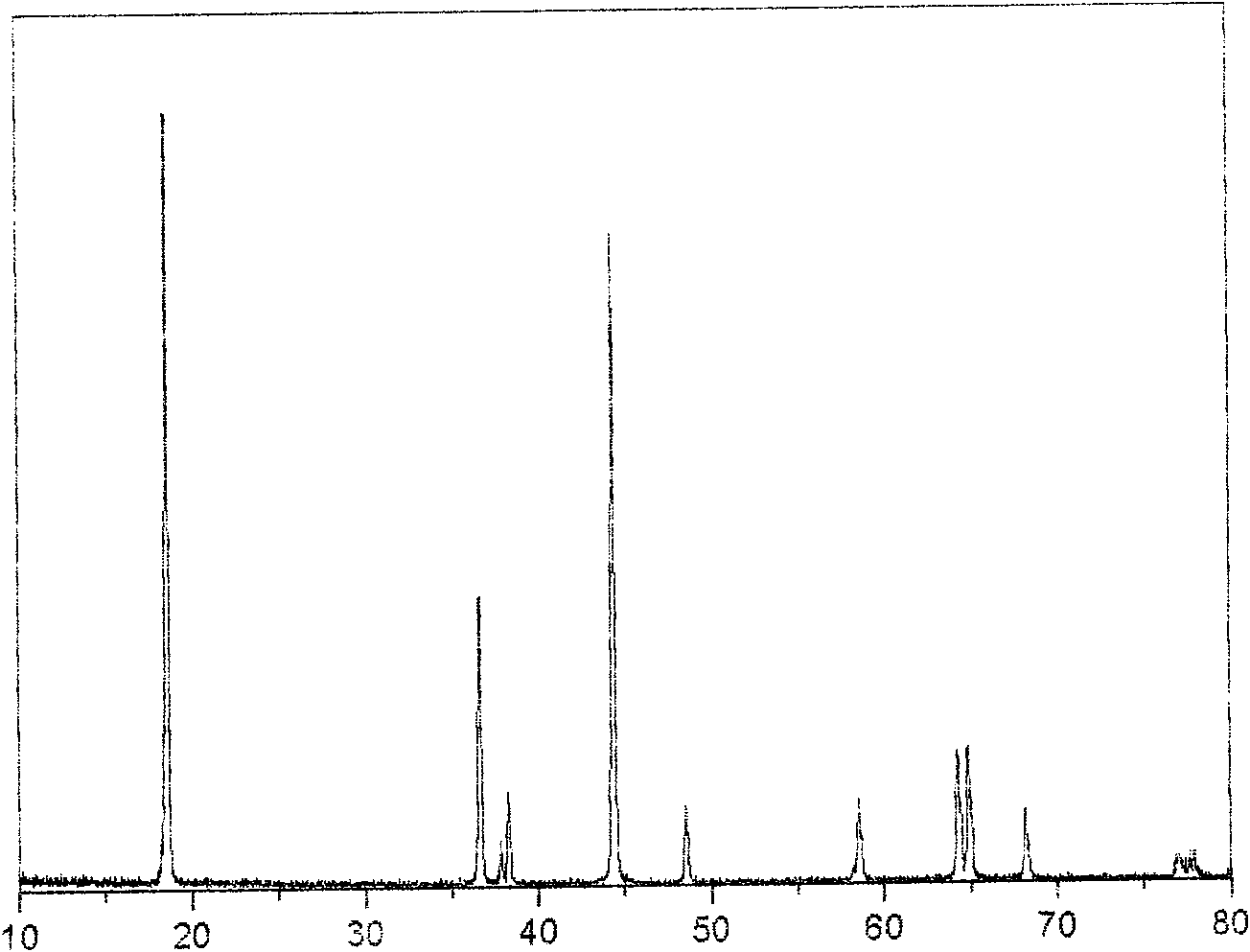
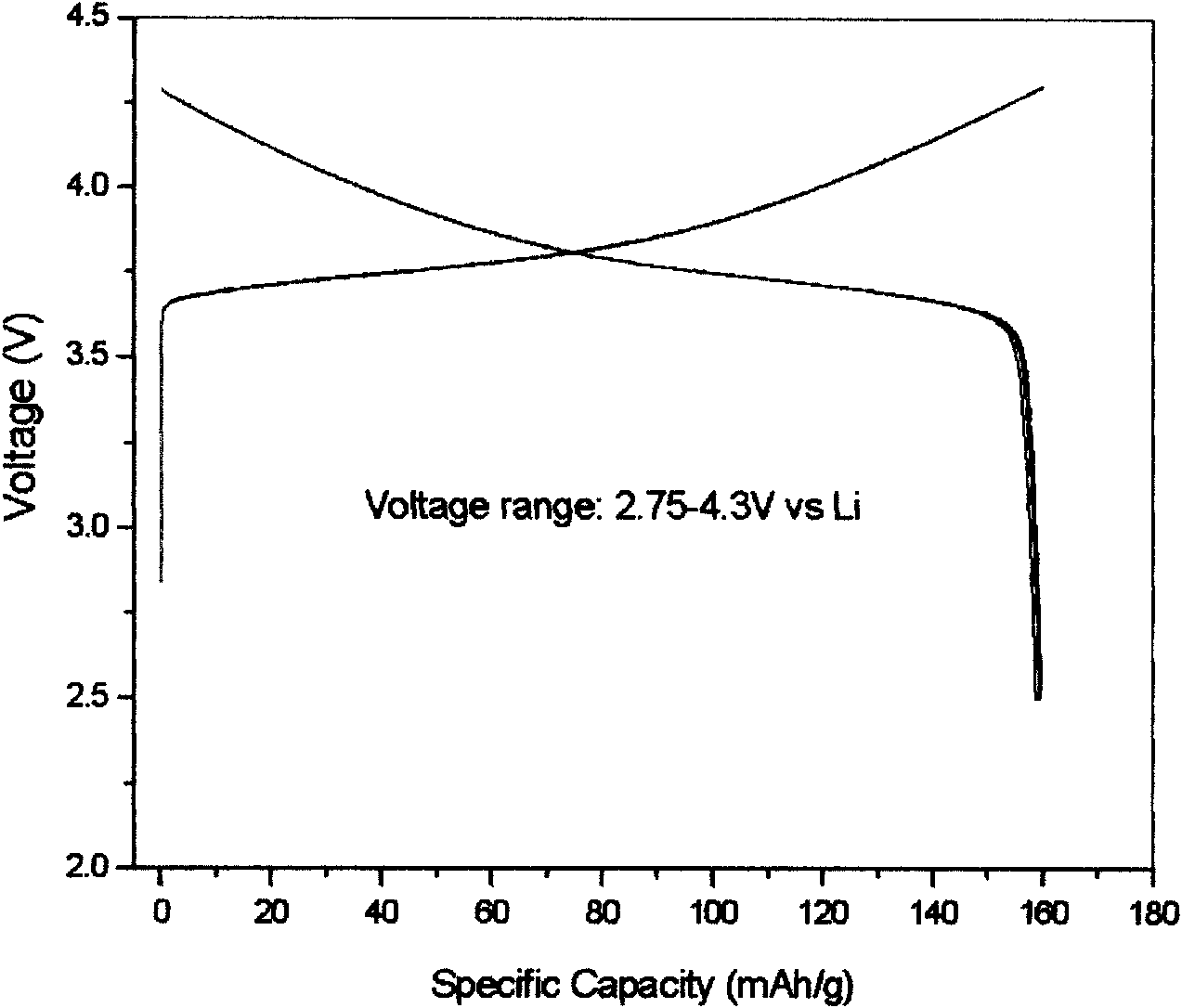
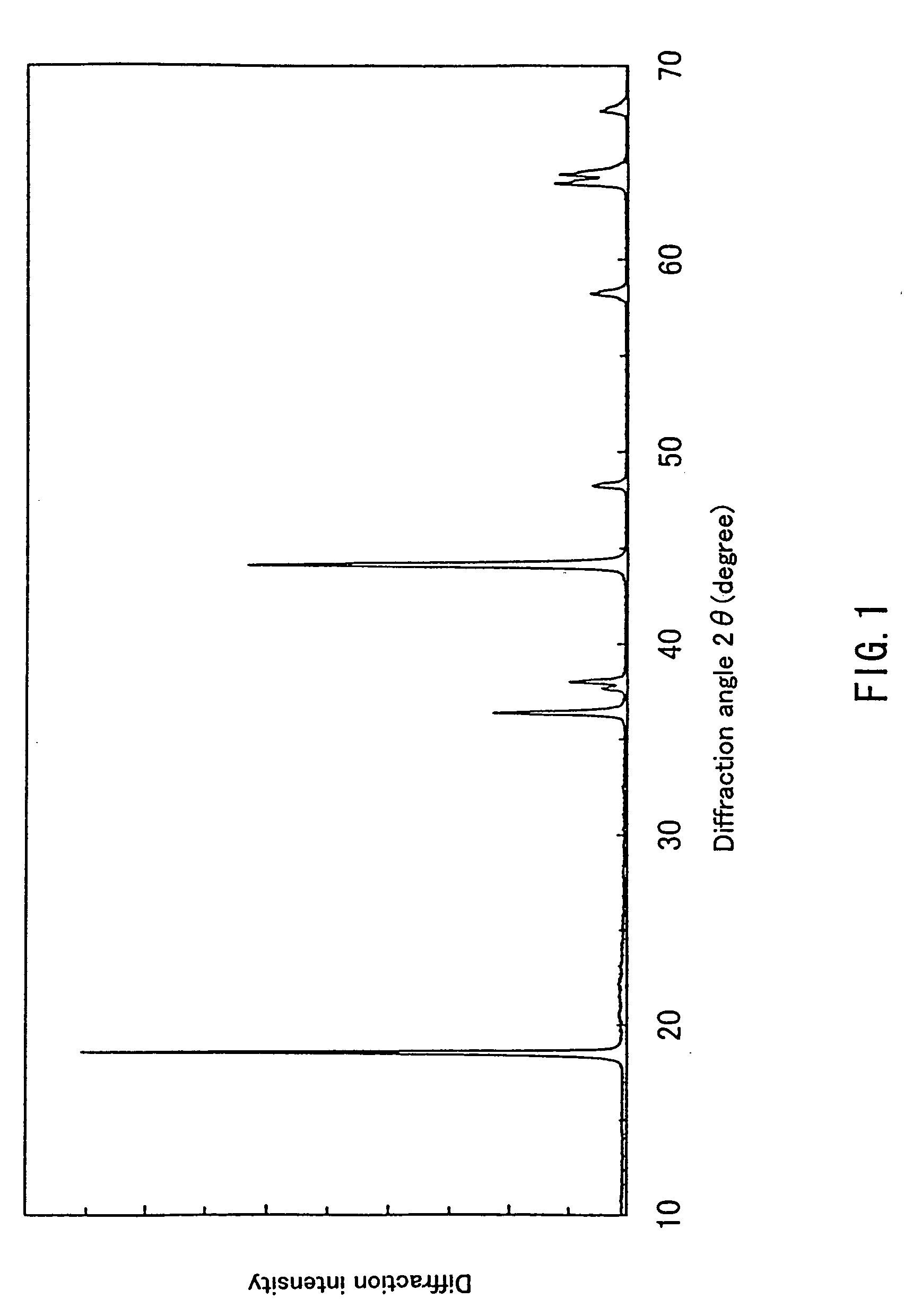
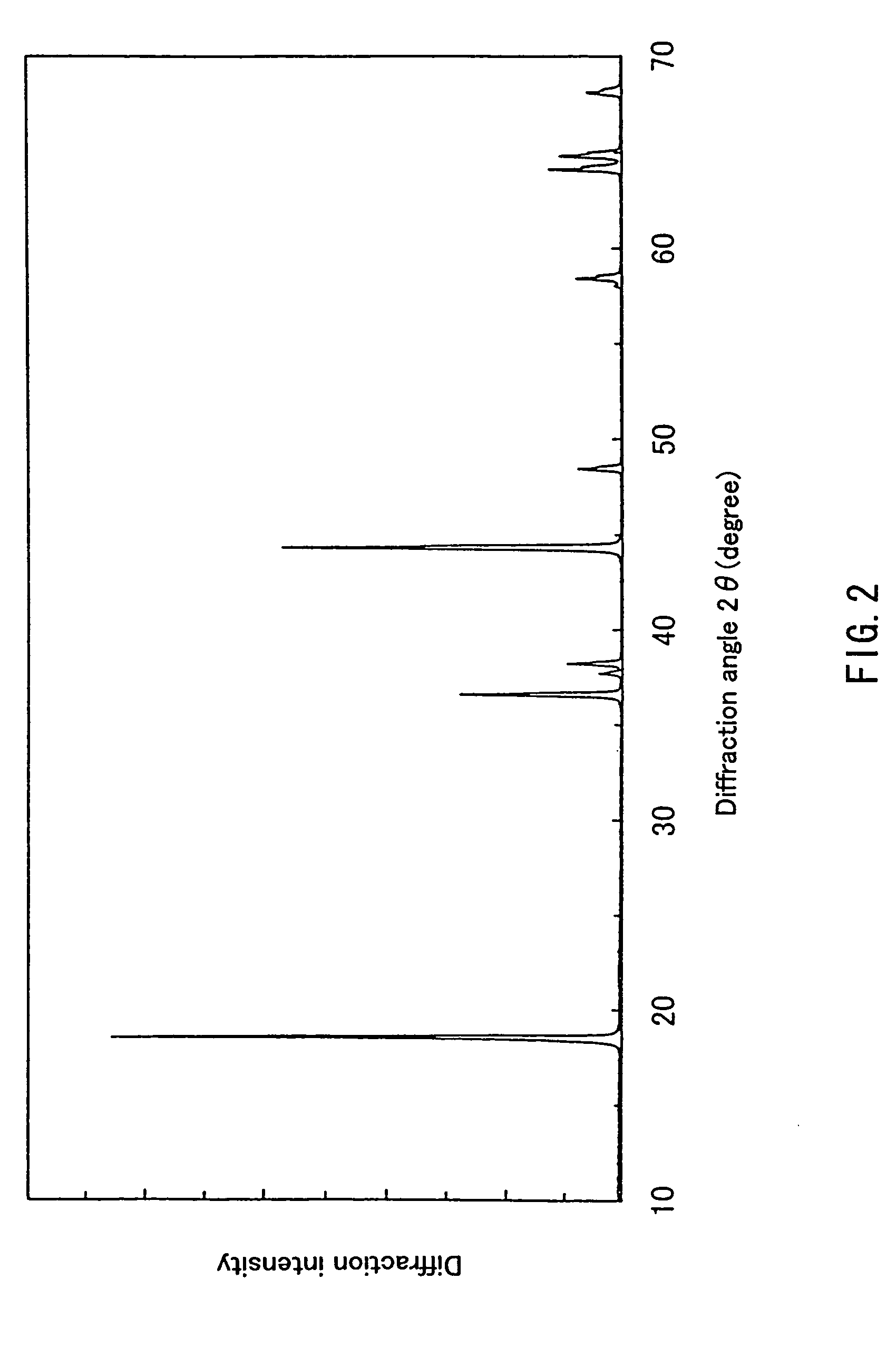
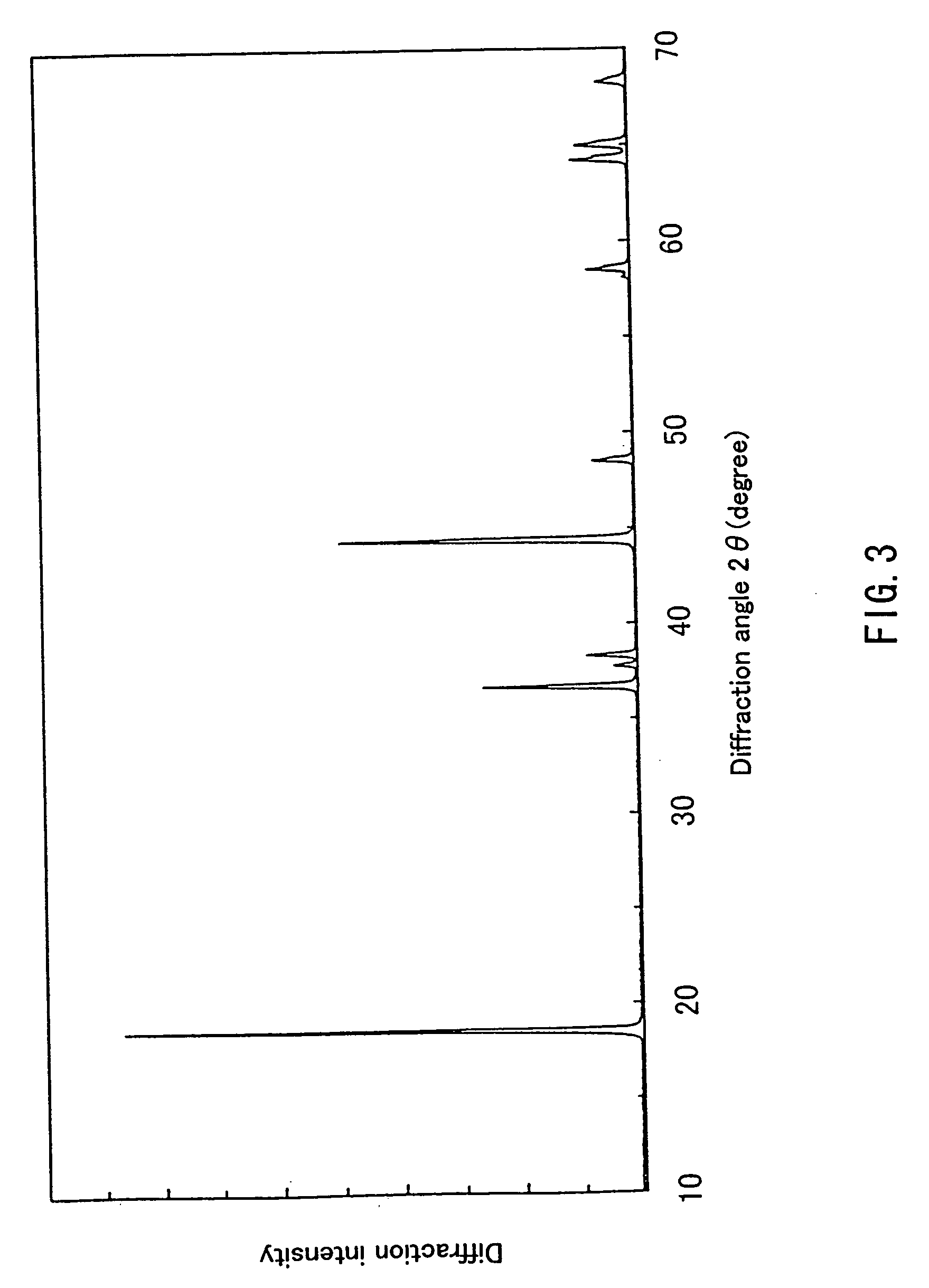
Patents
Literature
1829results about "Nickel oxides/hydroxides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS20080206124A1Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumPhysical chemistry
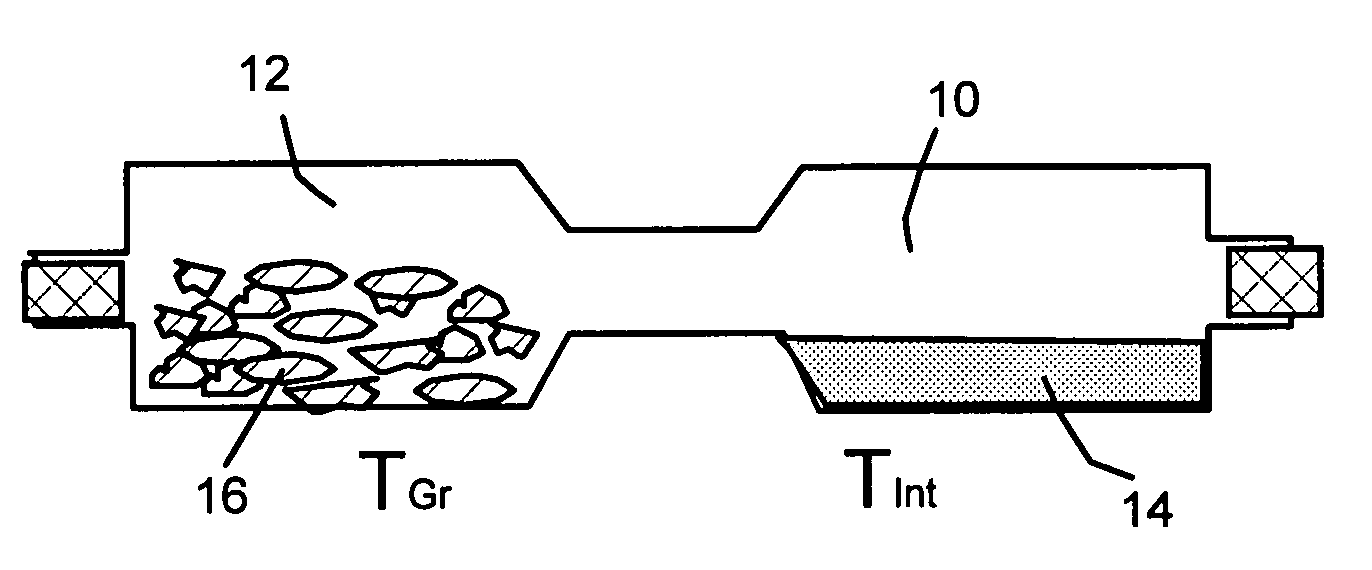
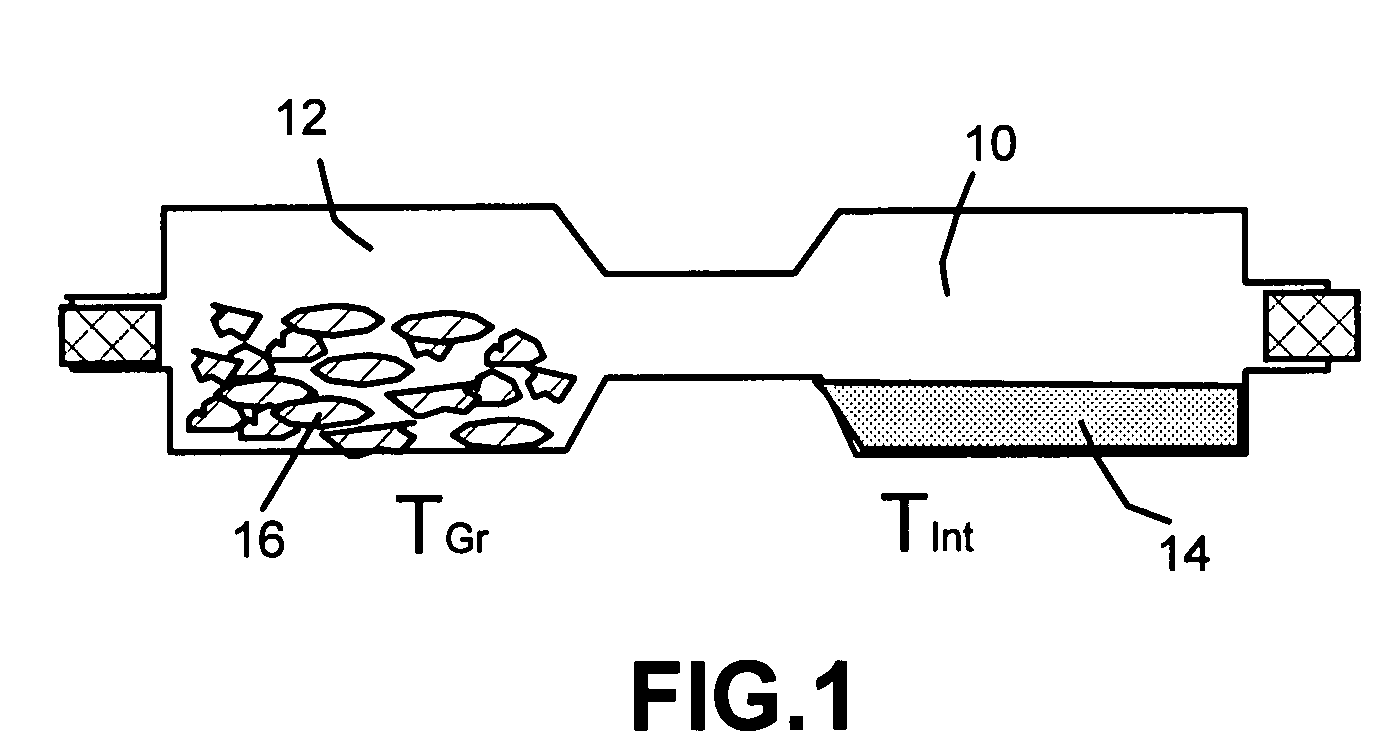
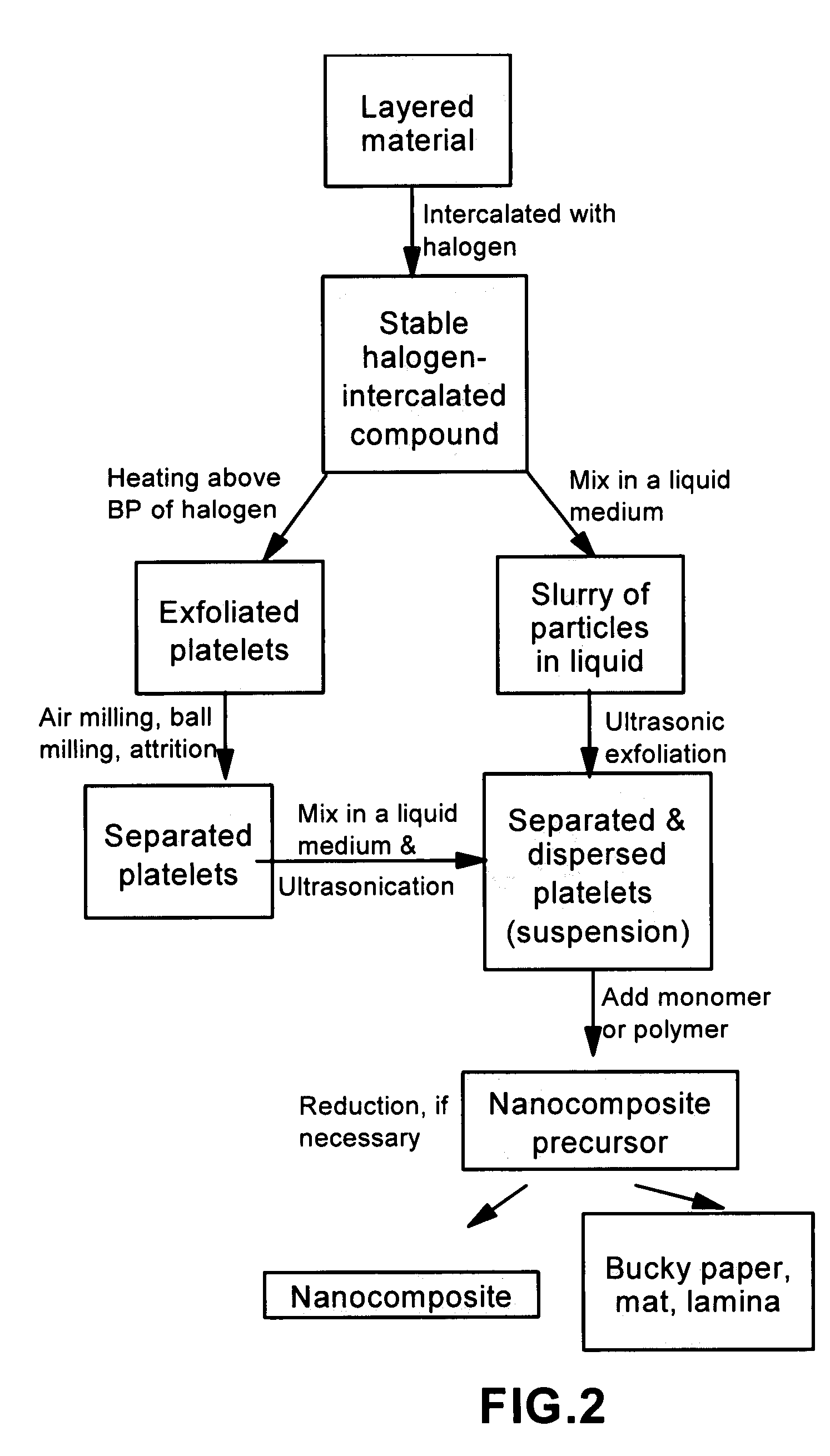
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
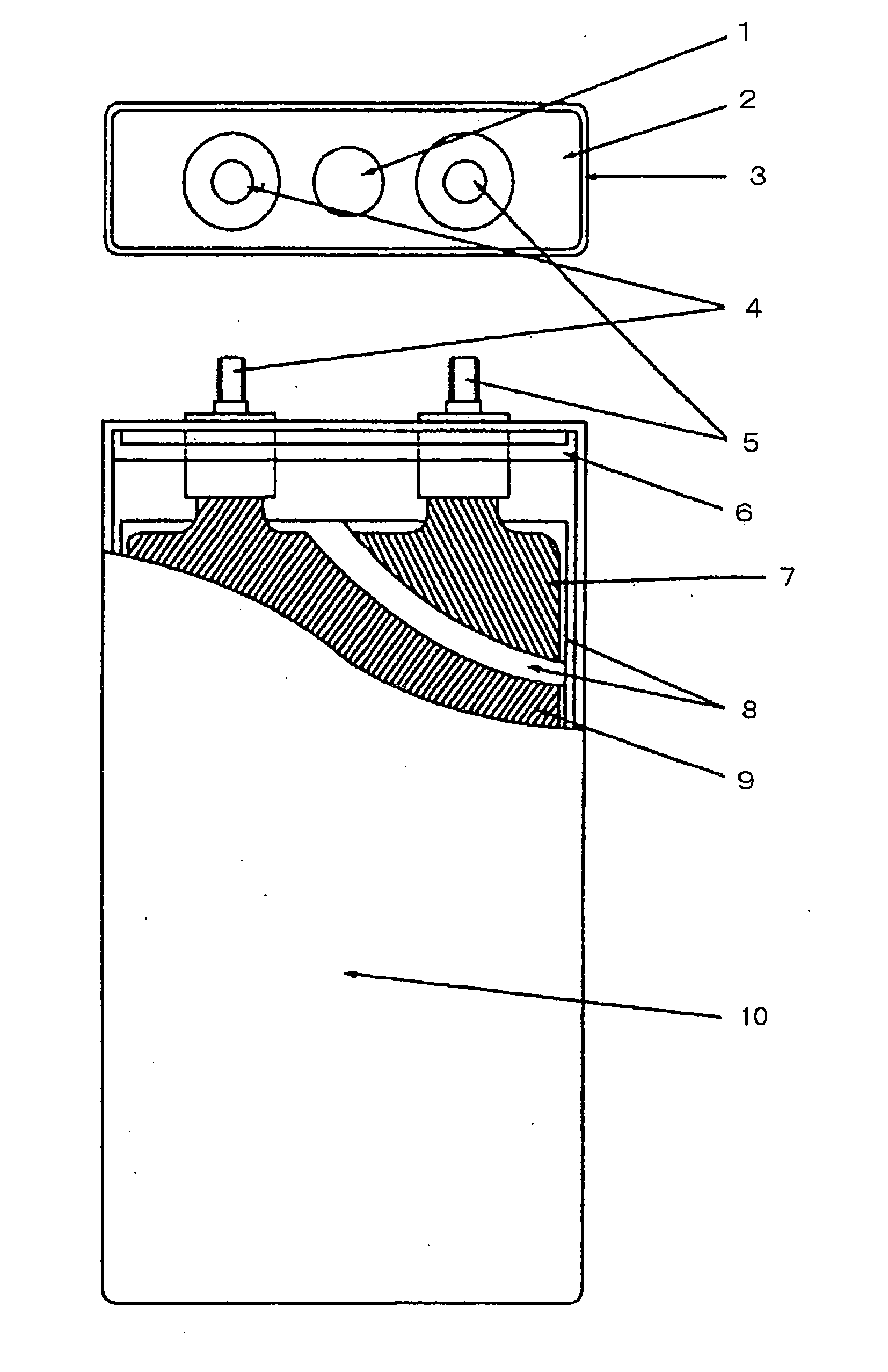
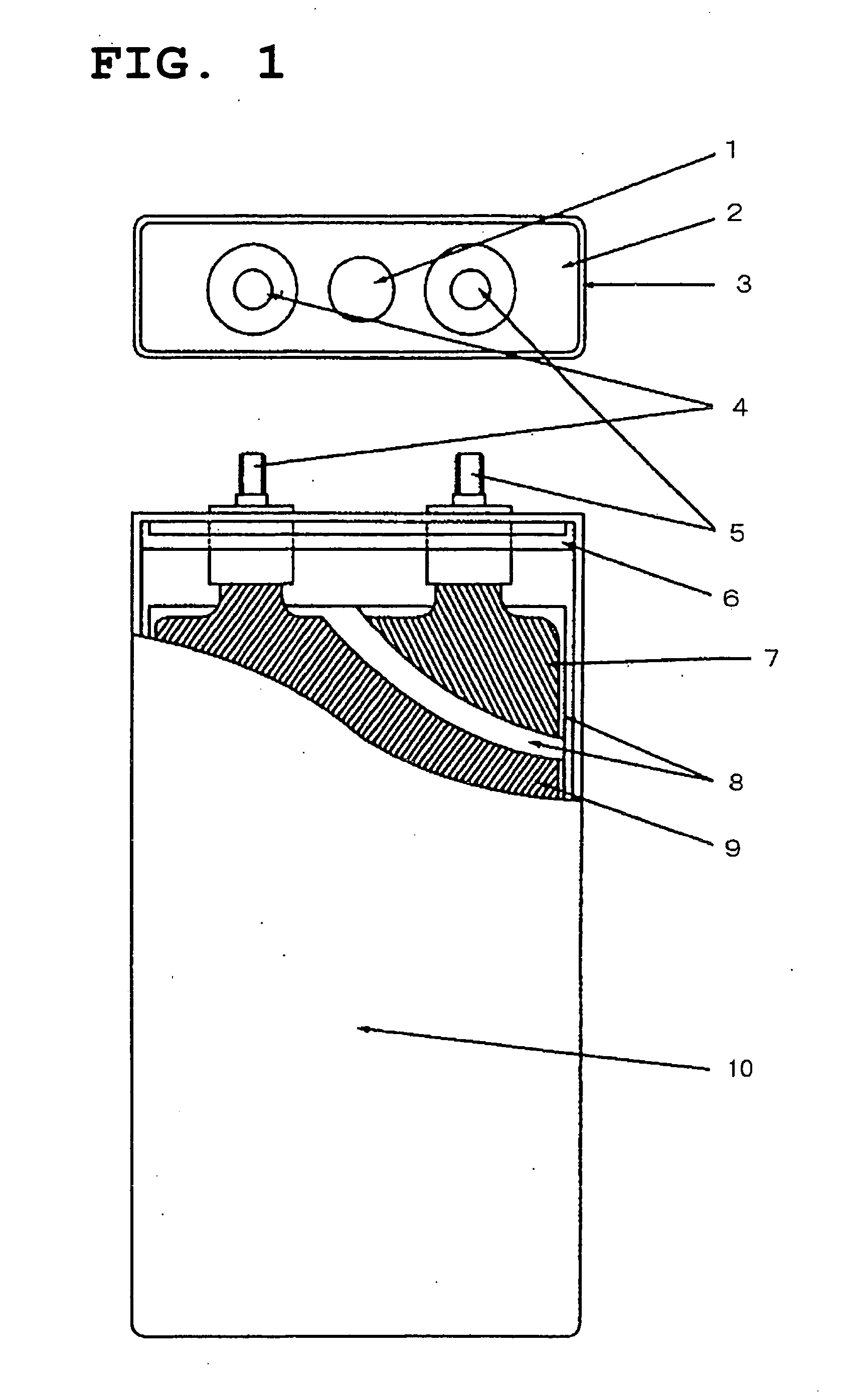
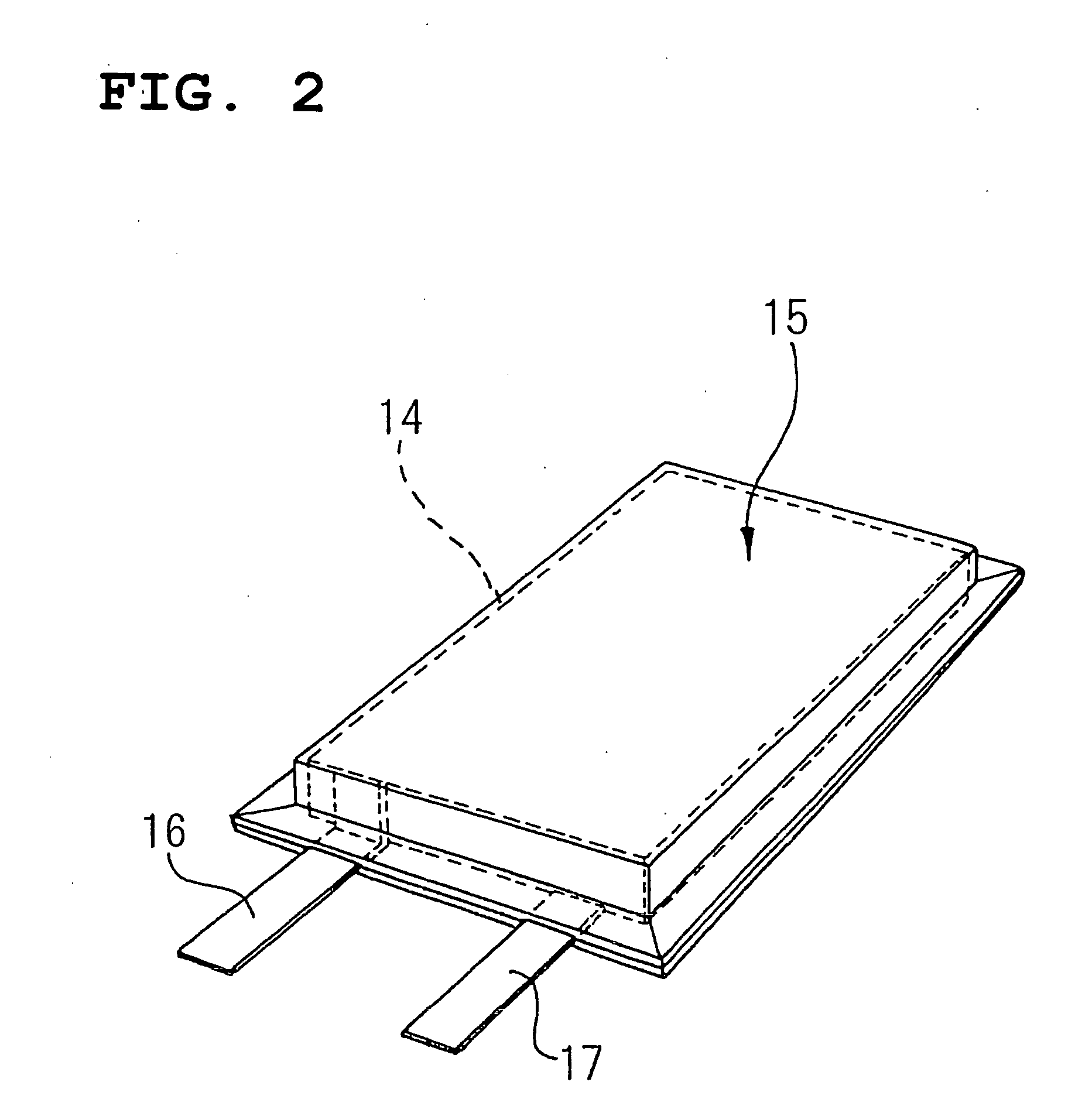
Positive-electrode active material and nonaqueous-electrolyte secondary battery containing the same
InactiveUS20030170540A1Improve the immunityLarge ion permeabilityIron oxides/hydroxidesElectrode thermal treatmentCrystal structureOxygen
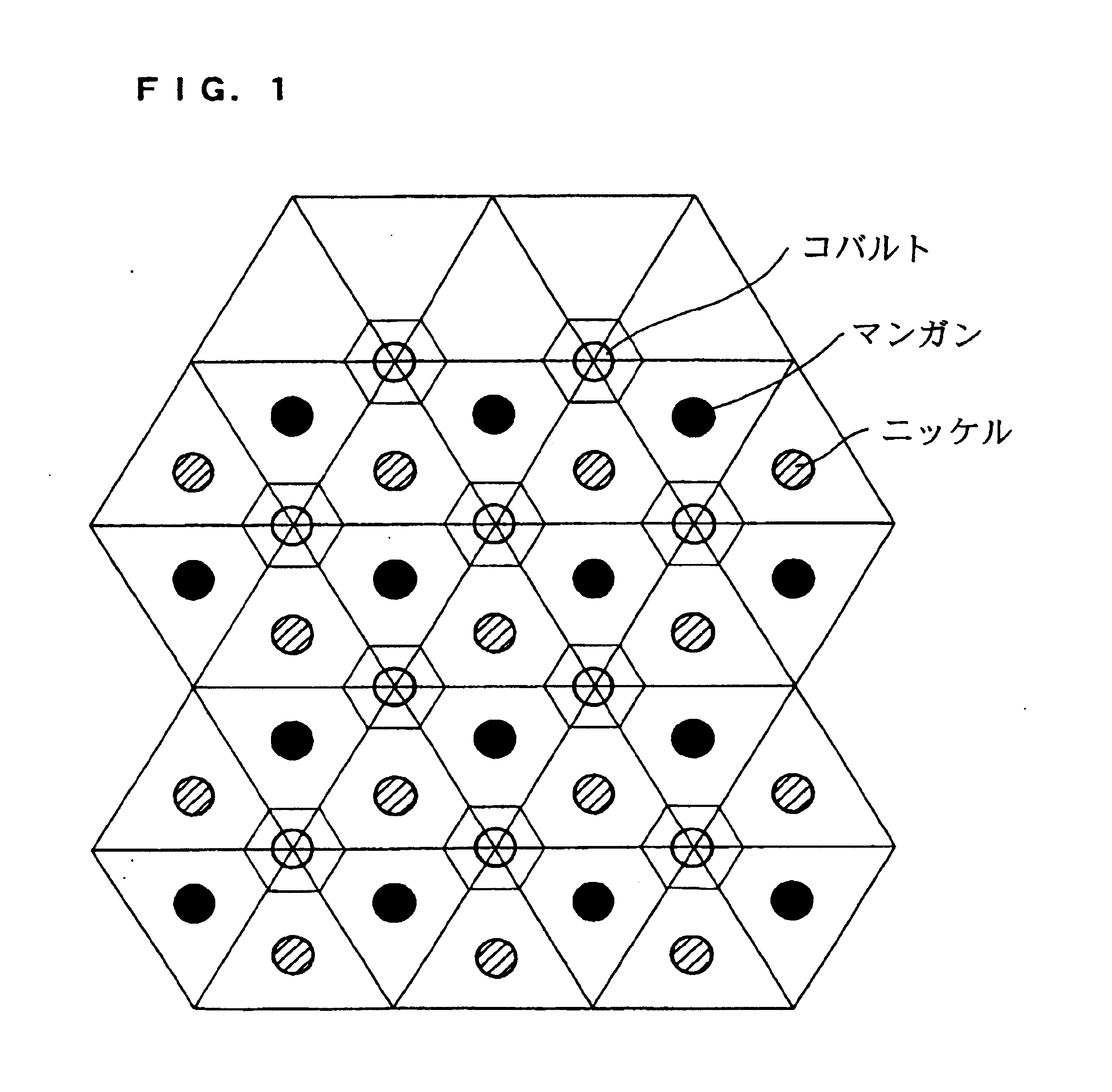
The present invention provides a high-capacity and low-cost non-aqueous electrolyte secondary battery, comprising: a negative electrode containing, as a negative electrode active material, a ssubstance capable of absorbing / desorbing lithium ions and / or metal lithium; a separator; a positive electrode; and an electrolyte, wherein the positive electrode active material contained in the positive electrode is composed of crystalline particles of an oxide containing two kinds of transition metal elements, the crystalline particles having a layered crystal structure, and oxygen atoms constituting the oxide forming a cubic closest packing structure.
Owner:PANASONIC CORP +1
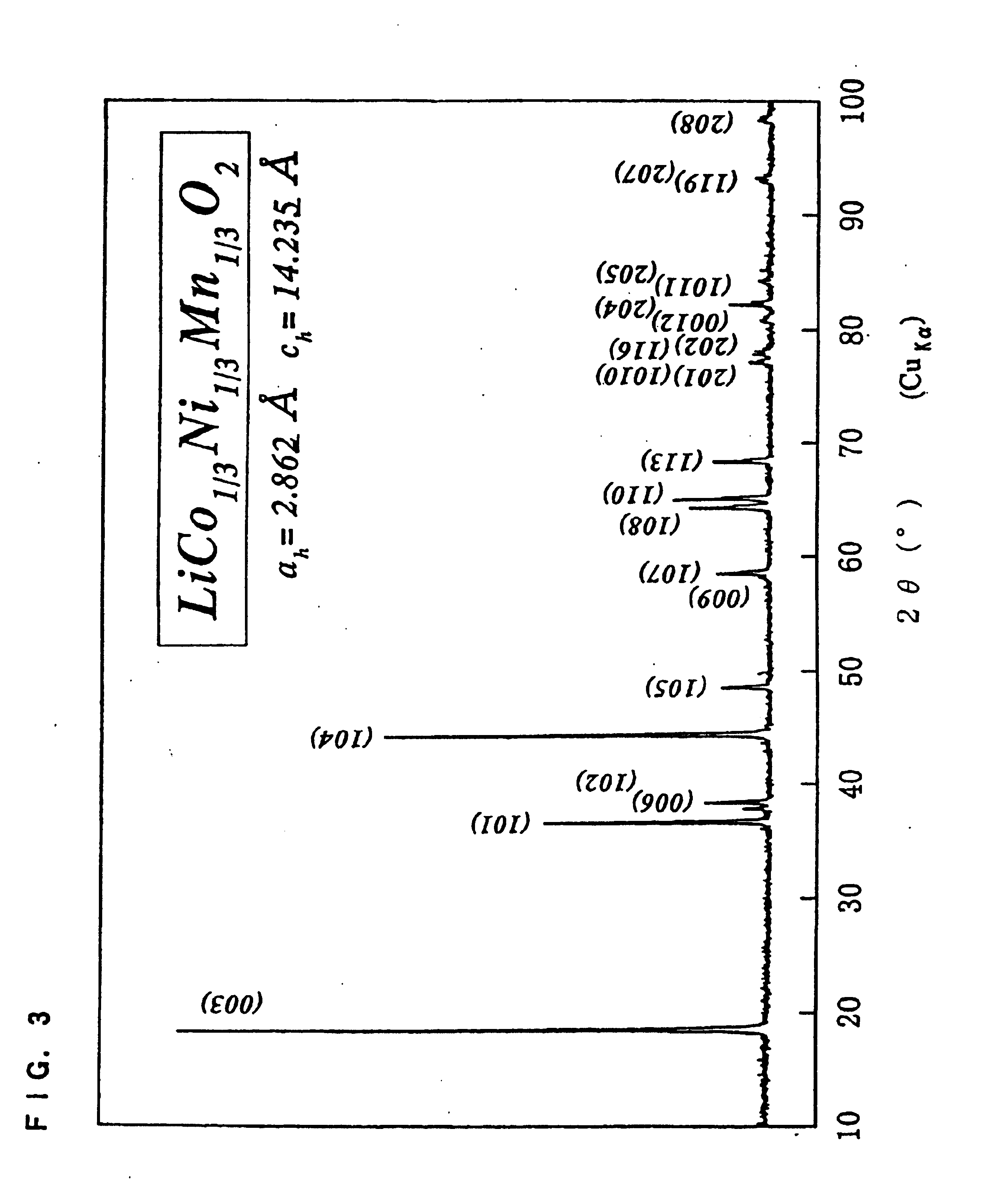
Layered Lithium Nickel Manganese Cobalt Composite Oxide Powder For Material Of Positive Electrode Of Lithium Secondary Battery, Process For Producing The Same, Positive Electrode Of Lithium Secondary Battery Therefrom, And Lithium Secondary Battery
ActiveUS20070202405A1Improve securityImprove battery performanceNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsManganeseCobalt
A powder of a layered lithium-nickel-manganese-cobalt composite oxide for use as a positive-electrode material for lithium secondary battery is provided which, when used as a positive-electrode material for lithium secondary battery, enables a cost reduction and higher safety to be reconciled with improved battery performances. The powder of a layered lithium-nickel-manganese-cobalt composite oxide for use as a positive-electrode material for lithium secondary battery is composed of secondary particles formed by the aggregation of primary particles. It has a composition represented by the following formula (I), has a volume resistivity of 5×105 Ω·cm or lower in the state of being compacted at a pressure of 40 MPa, and has a value of C / S, wherein C is the concentration of carbon contained therein (% by weight) and S is the BET specific surface area thereof (m2 / g), of 0.025 or smaller. The powder has been regulated so as to have a volume resistivity not higher than the specified value and a considerably reduced carbon content while having a composition in a limited range, whereby a cost reduction and higher safety can be reconciled with improved battery performances. Li1+zNixMnyCo1−x−yOδ (I) (0<z≦0.91, 0.1≦x≦0.55, 0.20≦y≦0.90, 0.50≦x+y≦1, 1.9≦δ≦3)
Owner:MITSUBISHI CHEM CORP
Positive electrode active material for lithium secondary cell and lithium secondary cell
ActiveUS7393476B2Improve cycle performanceIncrease energy densityMaterial nanotechnologyCell temperature controlCrystallographyComposite oxide
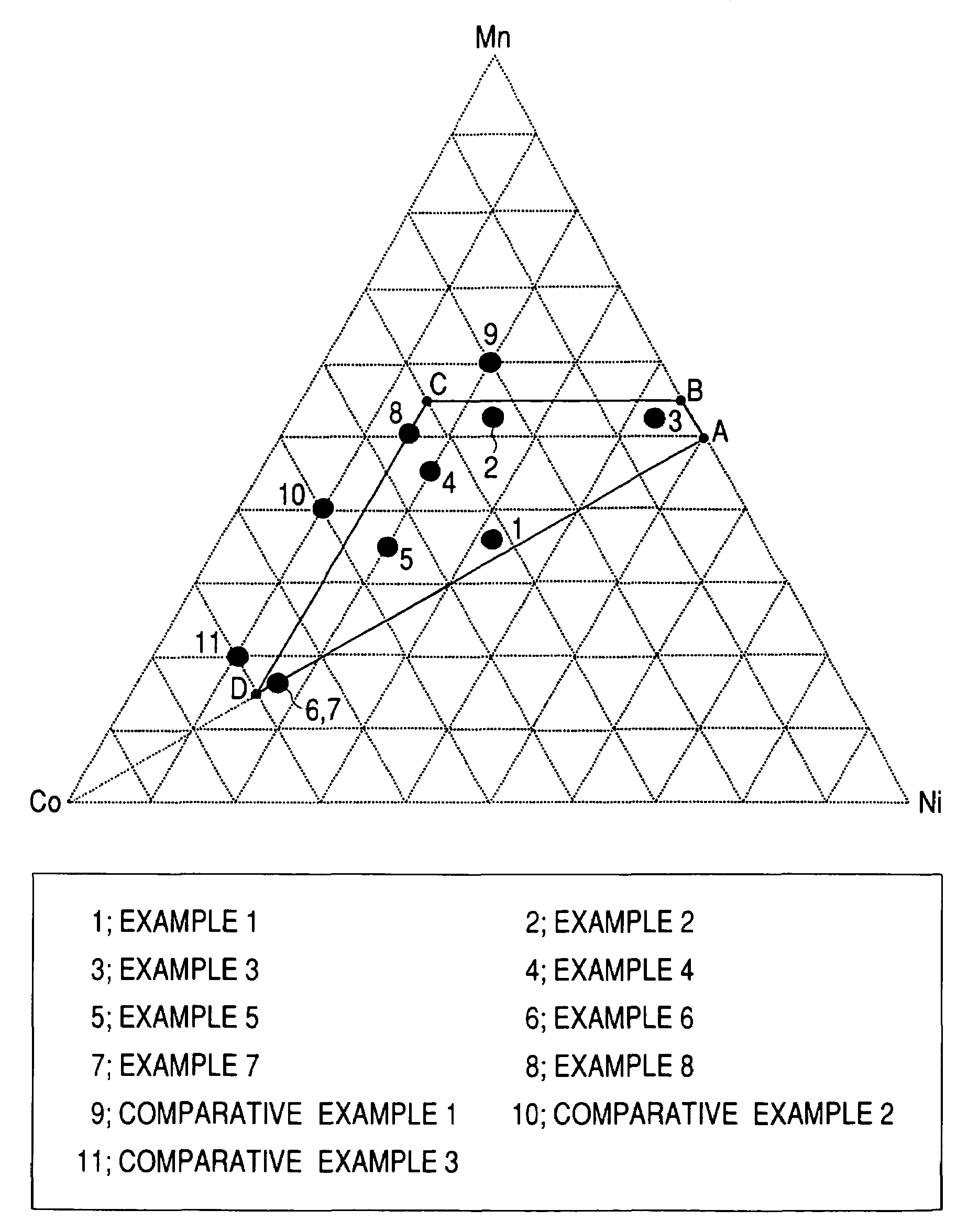
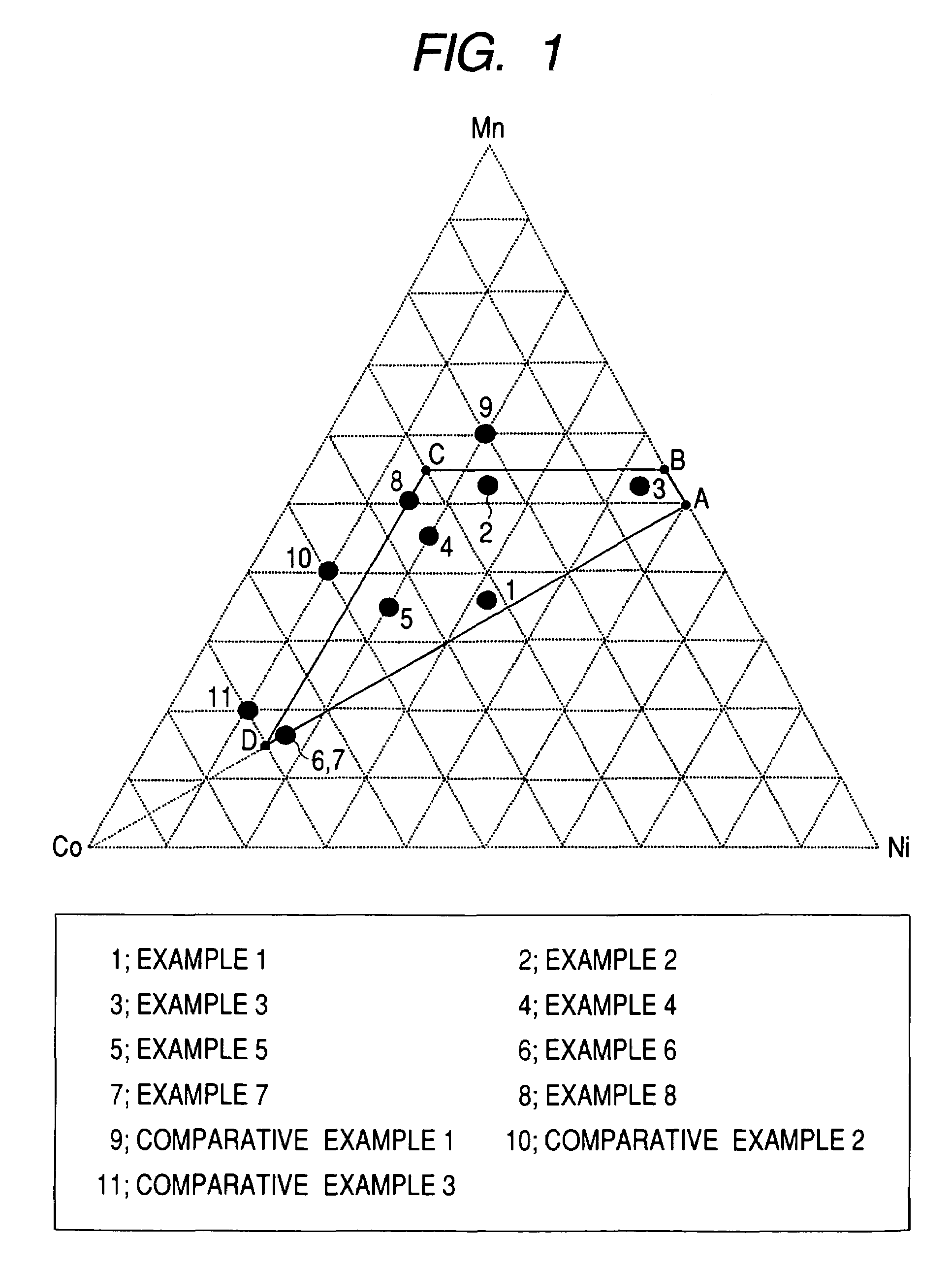
A positive active material for lithium secondary batteries, includes a composite oxide including an oxide which is represented by the composite formula LixMnaNibCocO2 and has an α-NaFeO2 structure, and an impurity phase including Li2MnO3. The values a, b, and c are within such a range that in a ternary phase diagram showing the relationship among these, (a, b, c) is present on the perimeter of or inside the quadrilateral ABCD defined by point A (0.5, 0.5, 0), point B (0.55, 0.45, 0), point C (0.55, 0.15, 0.30), and point D (0.15, 0.15, 0.7) as vertexes, and 0.95<x / (a+b+c)<1.35.
Owner:GS YUASA INT LTD
Materials for positive electrodes of lithium ion batteries and their methods of fabrication
InactiveUS20050130042A1Cycle wellEasy dischargeElectrode thermal treatmentSecondary cellsCeriumSolvent
This invention discloses materials for positive electrodes of secondary batteries and their methods of fabrication. Said materials comprise of granules of an active material for positive electrodes coated with an oxide layer. The active material is one or more of the following: oxides of lithium cobalt, oxides of lithium nickel cobalt, oxides of lithium nickel cobalt manganese, oxides of lithium manganese, LiCoO2, LiNi1-xCoxO2, LiNi1 / 3Co1 / 3Mn1 / 3O2, and LiMn2O4. The non-oxygen component in the oxide layer is one or more of the following: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B. Said non-oxygen component of the granules is between 0.01 wt. % to 10 wt. % of said granules of active material. The methods of fabrication for said materials includes the steps of mixing an additive and an active material for positive electrodes uniformly in water or solvent, evaporating said solvent or water, and heat treating the remaining mixture at 300° C. to 900° C. for between 1 hour to 20 hours. The additive is a compound of one or more of the following elements: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B where the element is between 0.01 wt. % to 10 wt. % of said active material. Using the materials of positive electrodes disclosed above or materials for positive electrodes fabricated in the methods disclosed above in batteries produces batteries with excellent cycling and high temperature properties.
Owner:BYD AMERICA CORP
Gradient cathode material for lithium rechargeable batteries
InactiveUS6921609B2Increase capacityImproved cyclabilityAluminium compoundsAlkaline accumulatorsManganesePotassium
A composition suitable for use as a cathode material of a lithium battery includes a core material having an empirical formula LixM′zNi1−yM″yO2. “x” is equal to or greater than about 0.1 and equal to or less than about 1.3. “y” is greater than about 0.0 and equal to or less than about 0.5. “z” is greater than about 0.0 and equal to or less than about 0.2. M′ is at least one member of the group consisting of sodium, potassium, nickel, calcium, magnesium and strontium. M″ is at least one member of the group consisting of cobalt, iron, manganese, chromium, vanadium, titanium, magnesium, silicon, boron, aluminum and gallium. A coating on the core has a greater ratio of cobalt to nickel than the core. The coating and, optionally, the core can be a material having an empirical formula Lix1Ax2Ni1−y1−z1Coy1Bz1Oa. “x1” is greater than about 0.1 a equal to or less than about 1.3. “x2,”“y1” and “z1” each is greater than about 0.0 and equal to or less than about 0.2. “a” is greater than 1.5 and less than about 2.1. “A” is at least one element selected from the group consisting of barium, magnesium, calcium and strontium. “B” is at least one element selected from the group consisting of boron, aluminum, gallium, manganese, titanium, vanadium and zirconium.
Owner:TIAX LLC
Lithium metal oxide electrodes for lithium batteries
InactiveUS20060188781A1Increase capacityImprove cycle stabilityActive material electrodesAlkali metal oxidesLithium metalOxidation state
An uncycled preconditioned electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2−2x) / (2+x)O2−δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn, and an uncycled electrode of the formula xLi2M′O3.(1−x)LiMO2, in which 0≦x<1, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more first-row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first- and second-row transition metal elements and Sn, the electrode being preconditioned in a proton-containing medium with a pH<7.0. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC +1
Lithium metal oxide electrodes for lithium batteries
InactiveUS7314682B2Electrode thermal treatmentActive material electrodesLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2−2x) / (2+x)O2−δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
Ink-jet printing ink compositions having magnetic properties and specific core/shell binder
InactiveUS6248805B1Improve propertiesImprove magnetic propertiesIron oxides/hydroxidesDuplicating/marking methodsCharge-transfer complexPrinting ink
Specific core-shell binders and magnetic additives for use in ink-jet printing ink compositions are provided. One class of specific core / shell binders has the general formula [AmBnC'p]x, where A and B are hydrophobic components in which A exhibits a glass transition temperature Tg between about -150° and +25° C. and B exhibits a glass transition temperature greater than 25° C., C' is a component that forms hydrophilic or water-soluble component in the polymer chain, and has an ionic or non-ionic structure, m<30 wt %, n>40 wt %, and p<30 wt %, with the total of m+n+p=100 wt %, and x=1 to 100,000. The molecular weight (weight average) of the polymer is between about 1,000 and 2,000,000. The polymers useful in the practice of the invention are prepared by emulsifying the monomers and then conducting a free-radical polymerization in water. The foregoing binder polymer is used in conjunction with magnetic additives comprising either (a) inorganic magnetic compound containing at least one of iron, cobalt, and nickel or (b) organic magnetic complexes containing at least one of iron, cobalt, and nickel or (c) organic charge transfer complexes that exhibit magnetic properties. The ratio of binder (I) to colorant (pigment) is greater that 1 to 10. The concentration of the magnetice additive is within the range of 1 to 30 wt %. The general ink formulation comprises: 5 to 50 wt % water-miscible solvent; 0.5 to 10 wt % colorant; 1 to 30 wt % magnetice additive; and water.
Owner:HEWLETT PACKARD DEV CO LP
Method of hydrothermal liquid phase sintering of ceramic materials and products derived therefrom
Provided here is a method of producing a monolithic body from a porous matrix, comprising: (i) providing a porous matrix having interstitial spaces and comprising at least a first reactant; (ii) contacting the porous matrix with an infiltrating medium that carries at least a second reactant; (iii) allowing the infiltrating medium to infiltrate at least a portion of the interstitial spaces of the porous matrix under conditions that promote a reaction between the at least first reactant and the at least second reactant to provide at least a first product; and (iv) allowing the at least first product to form and fill at least a portion of the interstitial spaces of the porous matrix, thereby producing a monolithic body, wherein the monolithic body does not comprise barium titanate.
Owner:RUTGERS THE STATE UNIV
Lithium transition metal oxide with gradient of metal composition
ActiveUS20060105239A1Improve electrochemical performanceIncrease energy densityCell electrodesManganese oxides/hydroxidesManganeseMaterials science
Disclosed are primary materials, precursor materials and final materials as well as methods to prepare these materials. The final materials are mixed lithium transition metal oxides, useful as performance optimized cathode materials for rechargeable lithium batteries. The transition metal is a solid solution mixture of manganese, nickel and cobalt, M=(Mn1-uNiu)1-u-yCoy, with 0.2
Owner:LG ENERGY SOLUTION LTD
Positive electrode active material and nonaqueous electrolyte secondary cell including the same
InactiveUS20050079416A1Large capacityImprove charge and discharge efficiencyOxygen/ozone/oxide/hydroxideIron oxides/hydroxidesMetallic lithiumManganese
A nonaqueous electrolytic secondary cell produced at low cost and having a large capacity comprises a negative electrode having an active material mainly composed of a material that at least absorbs and releases lithium ions or metallic lithium, a positive electrode, and an electrolyte. The active material of the positive electrode is an oxide containing nickel, manganese, and cobalt, and the contents of the elements are substantial the same.
Owner:PANASONIC CORP +1
Active substance of positive electrode and nonaqueous electrolyte battery containing the same
ActiveUS20050019659A1Increase energy densityImprove high rate discharge performanceSecondary cellsAlkali metal oxidesHigh rateHigh energy
A positive active material is provided which can give a battery having a high energy density and excellent high-rate discharge performance and inhibited from decreasing in battery, performance even in the case of high-temperature charge. Also provided is a non-aqueous electrolyte battery employing the positive active material. The positive active material contains a composite oxide which is constituted of at least lithium (Li), manganese (Mn), nickel (Ni), cobalt (Co), and oxygen (O) and is represented by the following chemical composition formula: LiaMnbNicCodOe (wherein 0<a≦1.3, |b−c|≦0.05, 0.6≦d<1, 1.7≦e≦2.3, and b+c+d=1). The non-aqueous electrolyte battery has a positive electrode containing the positive active material, a negative electrode, and a non-aqueous electrolyte.
Owner:GS YUASA INT LTD
Lithium-containing composite oxide and nonaqueous secondary cell using the same, and method for manufacturing the same
InactiveUS20030082452A1Stable structureHigh energy density per volumeElectrode thermal treatmentOrganic electrolyte cellsLithiumHigh density
Because of the composition represented by General Formula: Li1+x+alphaNi(1-x-y+delta) / 2Mn(1-x-y-delta) / 2MyO2 (where 0<=x<=0.05, -0.05<=x+alpha<=0.05, 0<=y<=0.4; -0.1<=delta<=0.1 (when 0<=y<=0.2) or -0.24<=delta<=0.24 (when 0.2<=y<=0.4); and M is at least one element selected from the group consisting of Ti, Cr, Fe, Co, Cu, Zn, Al, Ge and Sn), a high-density lithium-containing complex oxide with high stability of a layered crystal structure and excellent reversibility of charging / discharging can be provided, and a high-capacity non-aqueous secondary battery excellent in durability is realized by using such an oxide for a positive electrode.
Owner:MAXELL HLDG LTD
Positive active material for rechargeable lithium battery and method of preparing same
InactiveUS20020055042A1Improve thermal stabilityElectrode manufacturing processesZirconium compoundsPhysical chemistryLithium battery
Disclosed is a positive active material for a rechargeable lithium battery. The positive active material includes at least one compound represented by formulas 1 to 4 andl a metal oxide or composite metal oxide layer formed on the compound. <table-cwu id="TABLE-US-00001"> <number>1< / number> <tgroup align="left" colsep="0" rowsep="0" cols="3"> <colspec colname="OFFSET" colwidth="42PT" align="left" / > <colspec colname="1" colwidth="77PT" align="left" / > <colspec colname="2" colwidth="98PT" align="center" / > <row> <entry>< / entry> <entry>< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyF2< / entry> <entry>(1)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyS2< / entry> <entry>(2)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aFa< / entry> <entry>(3)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aSa< / entry> <entry>(4)< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> < / tgroup> < / table-cwu> (where M is selected from the group consisting of Co, Mg, Fe, Sr, Ti, B, Si, Ga, Al, Sc, Y, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Ac, Th, Pa, U, Np, IPu, Am, Cm, Bk, Cf, Es, Fm, Md, No and Lr, 0.95<=x<=1.1, 0<=y<=0.99, 0<=,z<=0.5, and 0<=a<=0.5)
Owner:SAMSUNG SDI CO LTD
Nanostructured fuel cell electrode
A fuel cell includes an electrolyte, a first electrode, and a second electrode. At least the first electrode comprises a nanostructured material.
Owner:BLOOM ENERGY CORP
Lithium composite oxide particle for positive electrode material of lithium secondary battery, and lithium secondary battery positive electrode and lithium secondary battery using the same
InactiveUS20060134521A1Improving low-temperature load characteristicGood paintabilityRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsAlkali metal oxidesElectrical batteryComposite oxide
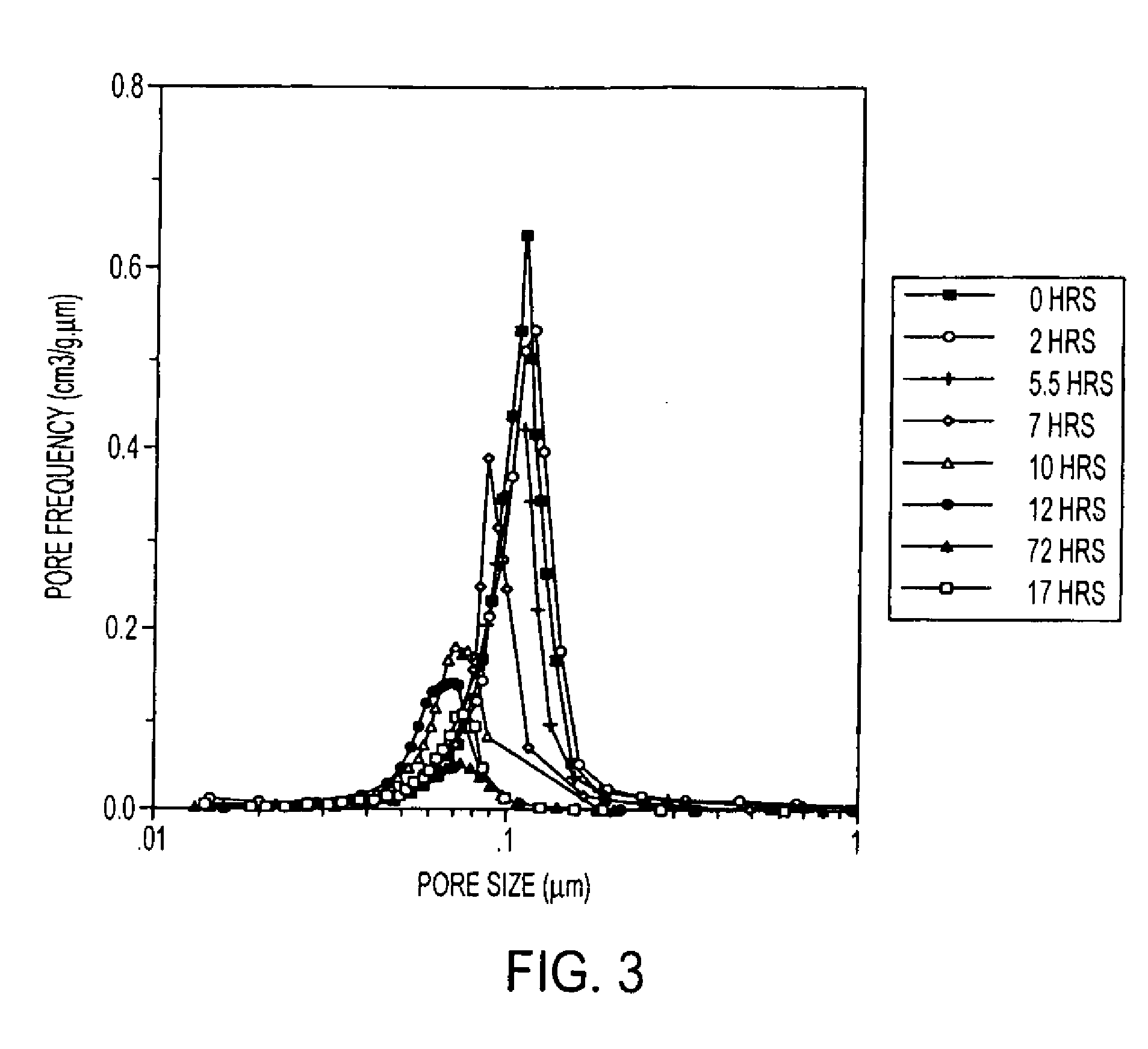
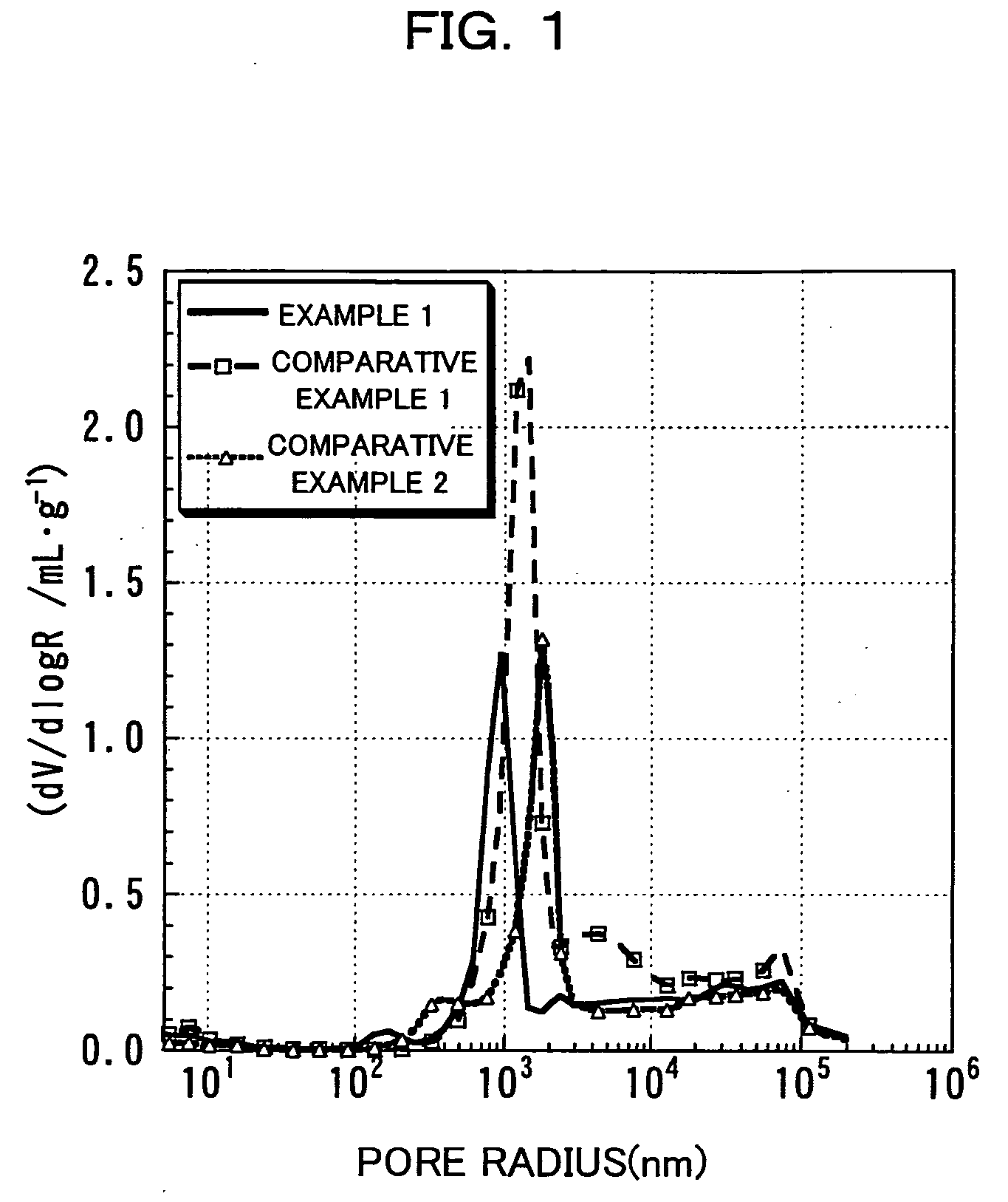
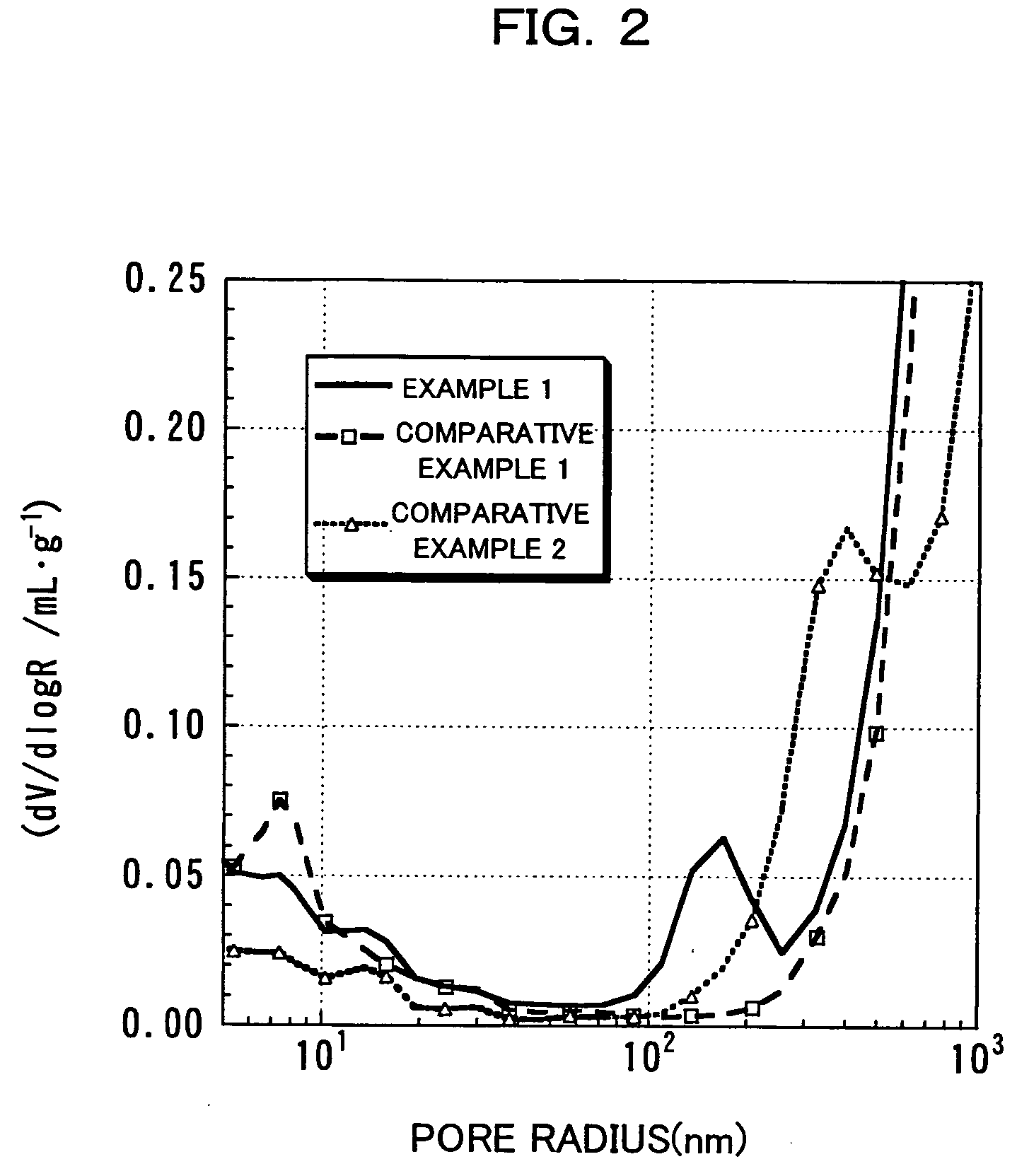
An excellent positive electrode material for a lithium secondary battery is provided that can increase low-temperature load characteristics of the battery as well as improving coatability. When measured by mercury intrusion porosimetry, the material meets Condition (A) and at least either Condition (B) or Condition (C). Condition (A) : on a mercury intrusion curve, the mercury intrusion volume from 50 MPa to 150 MPa is 0.02 cm3 / g or smaller. Condition (B): on the mercury intrusion curve, the mercury intrusion volume from 50 MPa to 150 MPa is 0.01 cm3 / g or larger. Condition (C): the average pore radius is within 10-100 nm, and the pore-size distribution curve has a main peak (with peak top at a pore radius of within 0.5-50 μm) and a sub peak (with peak top at a pore radius of within 80-300 nm).
Owner:MITSUBISHI CHEM CORP
Multi-shell-layer metal oxide hollow ball and preparation method thereof
ActiveCN102464304AHigh specific surface areaSimple processOxide/hydroxide preparationZinc oxides/hydroxidesControllabilityNanoscopic scale
The invention provides a multi-shell-layer metal oxide hollow ball and a preparation method thereof. A hydrothermal method is used for preparing a carbon ball template; metal salts are dissolved in carbon ball suspension liquid, and the gradient distribution, the depth and the number of metal salts entering carbon balls are controlled through regulating adsorption conditions such as metal salt concentration, solution pH value, soaking temperature and time and the like; and the heat treatment is carried out on the carbon balls adsorbing metal ions, and the multi-shell-layer metal oxide hollow ball can be obtained. The shell-layer of the hollow ball prepared by the method is formed by accumulating nanometer crystal particles of metal oxides, the shell layer number can be regulated and changed from two to four, and both the size of the hollow ball and the thickness of the shell layers are controllable. The method provided by the invention is simple and is easy to implement, the controllability is high, the pollution is little, the cost is low, and in addition, the general applicability is realized. The prepared product has a hollow structure and the shell layers with the thickness inthe nanometer level, simultaneously, the internal space can be effectively utilized through the multilayer structure, and the multi-shell-layer metal oxide hollow ball is applied to gas sensitivity and photocatalysis and has the more excellent performance through being compared with the traditional nanometer material and a single-layer hollow ball.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
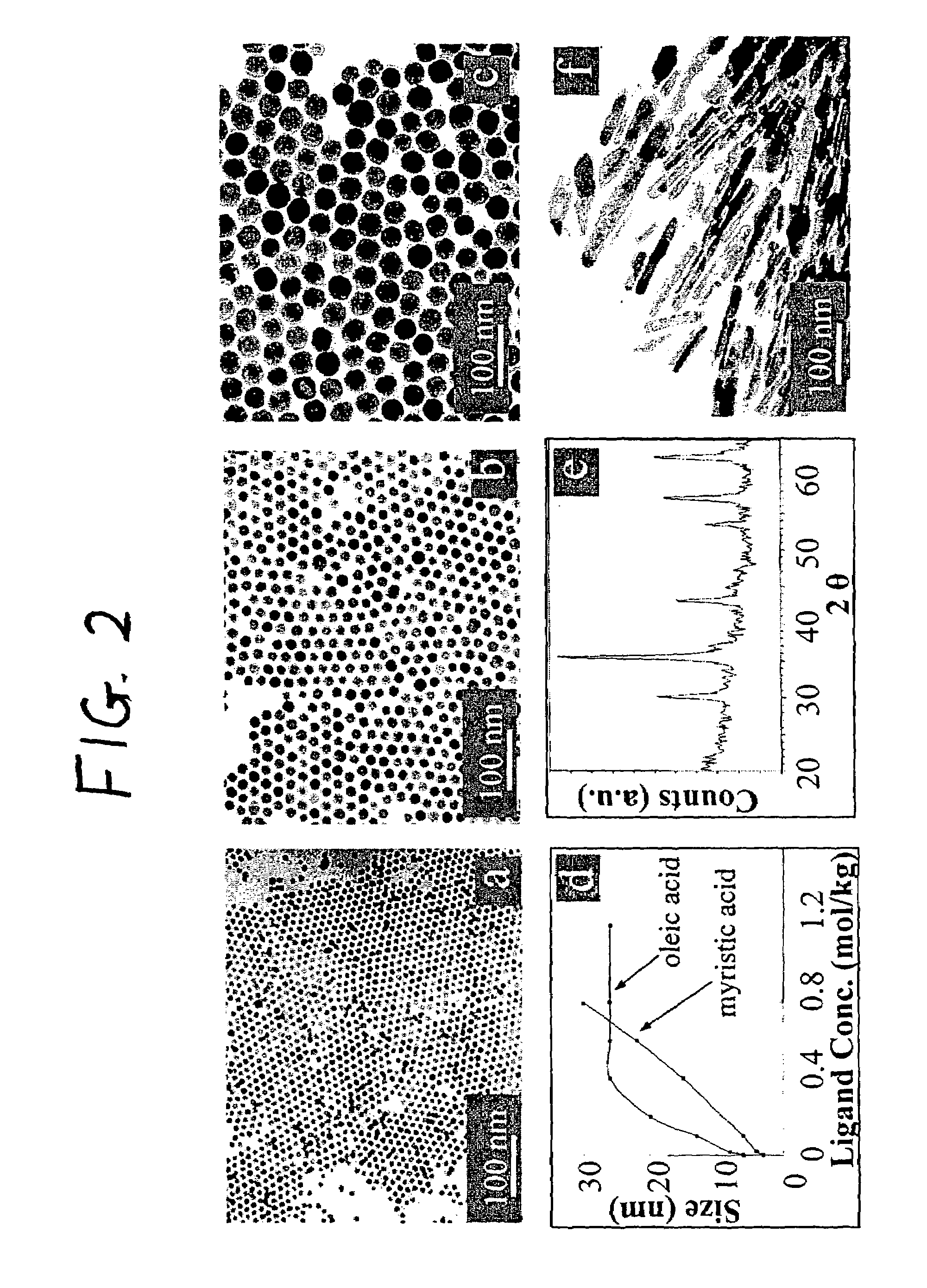
Synthetic control of metal oxide nanocrystal sizes and shapes
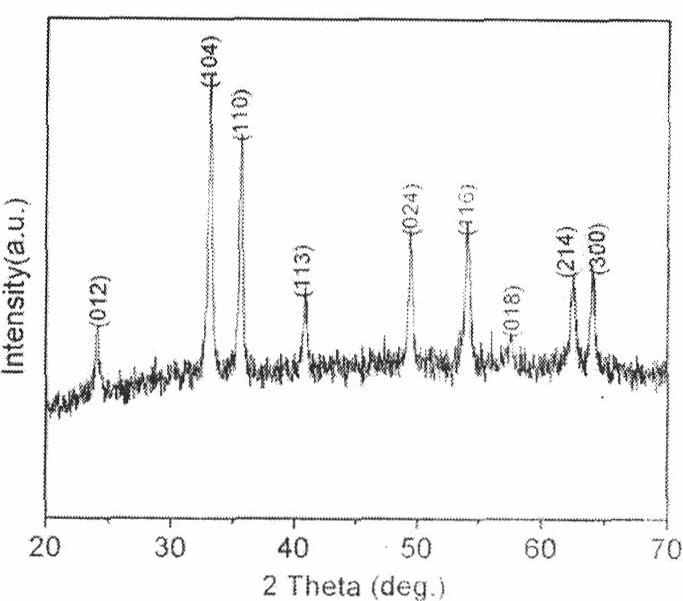
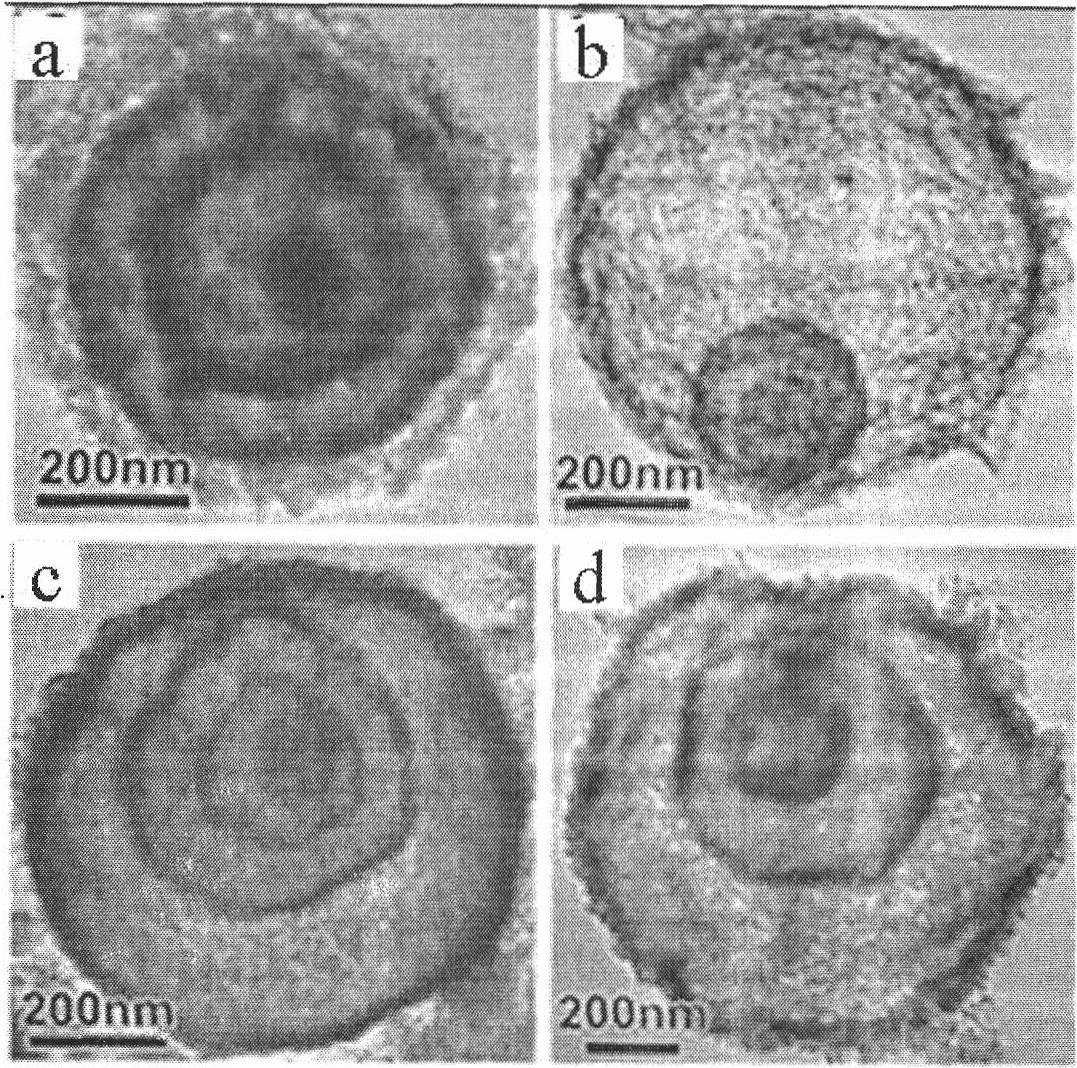
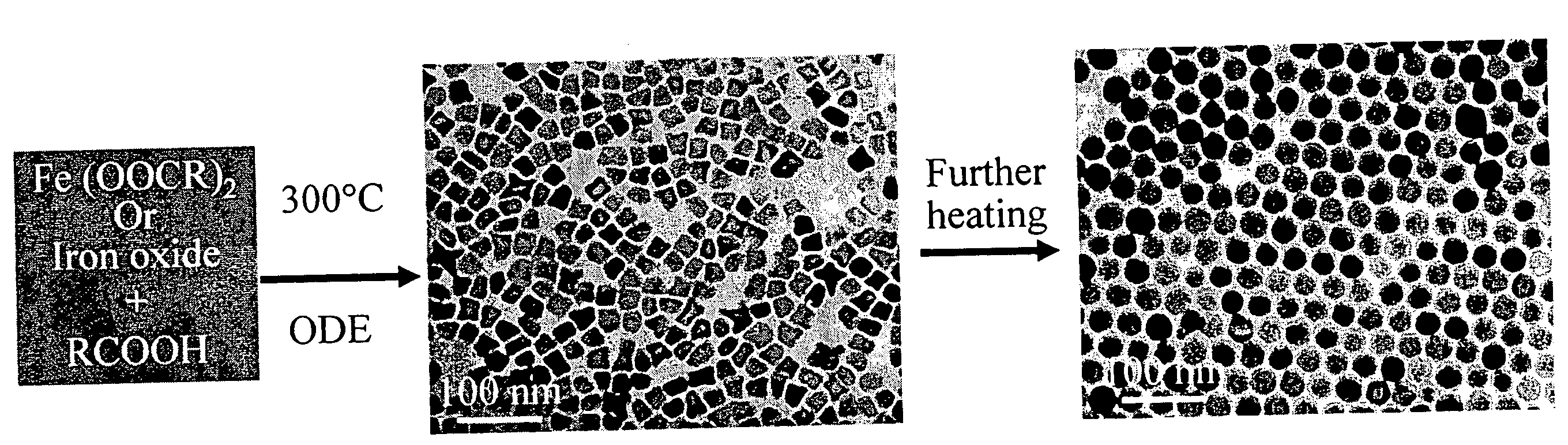
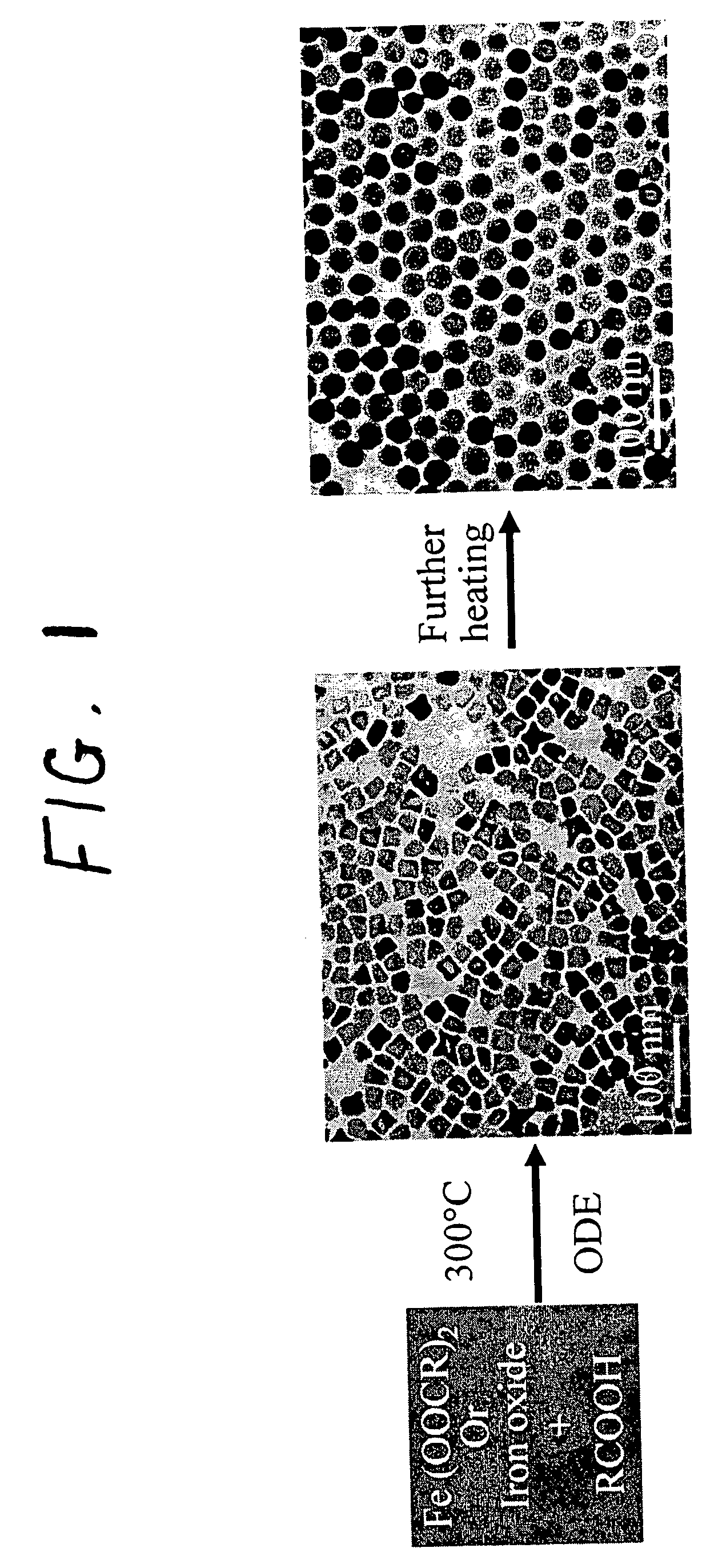
A general, reproducible, and simple synthetic method that employs readily available chemicals permits control of the size, shape, and size distribution of metal oxide nanocrystals. The synthesis entails reacting a metal fatty acid salt, the corresponding fatty acid, and a hydrocarbon solvent, with the reaction product being pyrolyzed to the metal oxide. Nearly monodisperse oxide nanocrystals of Fe3O4, Cr2O3, MnO, Co3O4, NiO, ZnO, SnO2, and In2O3, in a large size range (3-50 nm), are described. Size and shape control of the nanocrystals is achieved by varying the reactivity and concentration of the precursors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Electrode active material powder with size dependent composition and method to prepare the same
ActiveUS20070122705A1Increased gravimetric energy densityLow costElectrode thermal treatmentActive material electrodesLithiumPhysical chemistry
The present invention relates to a powderous electrode active material of lithium transition metal oxide LiaMbO2, wherein 0.9 < a < 1.1, 0.9 < b < 1.1 and M is dominantly transition metal chosen from Mn, Co and Nickel, having particles with a distribution of sizes, where the composition M varies with the size of the particles, and a preparation method thereof. The present invention also relates to an electrochemical cell, particularly rechargeable lithium battery, using the powderous electrode active material.
Owner:LG ENERGY SOLUTION LTD
Lithium-nickel-cobalt-maganese containing composite oxide, material for positive electrode active material for lithium secondary battery, and methods for producing these
ActiveUS20060083989A1Large capacityExcels in charge-discharge cycle durabilityElectrode rolling/calenderingFluoride preparationManganeseOxygen
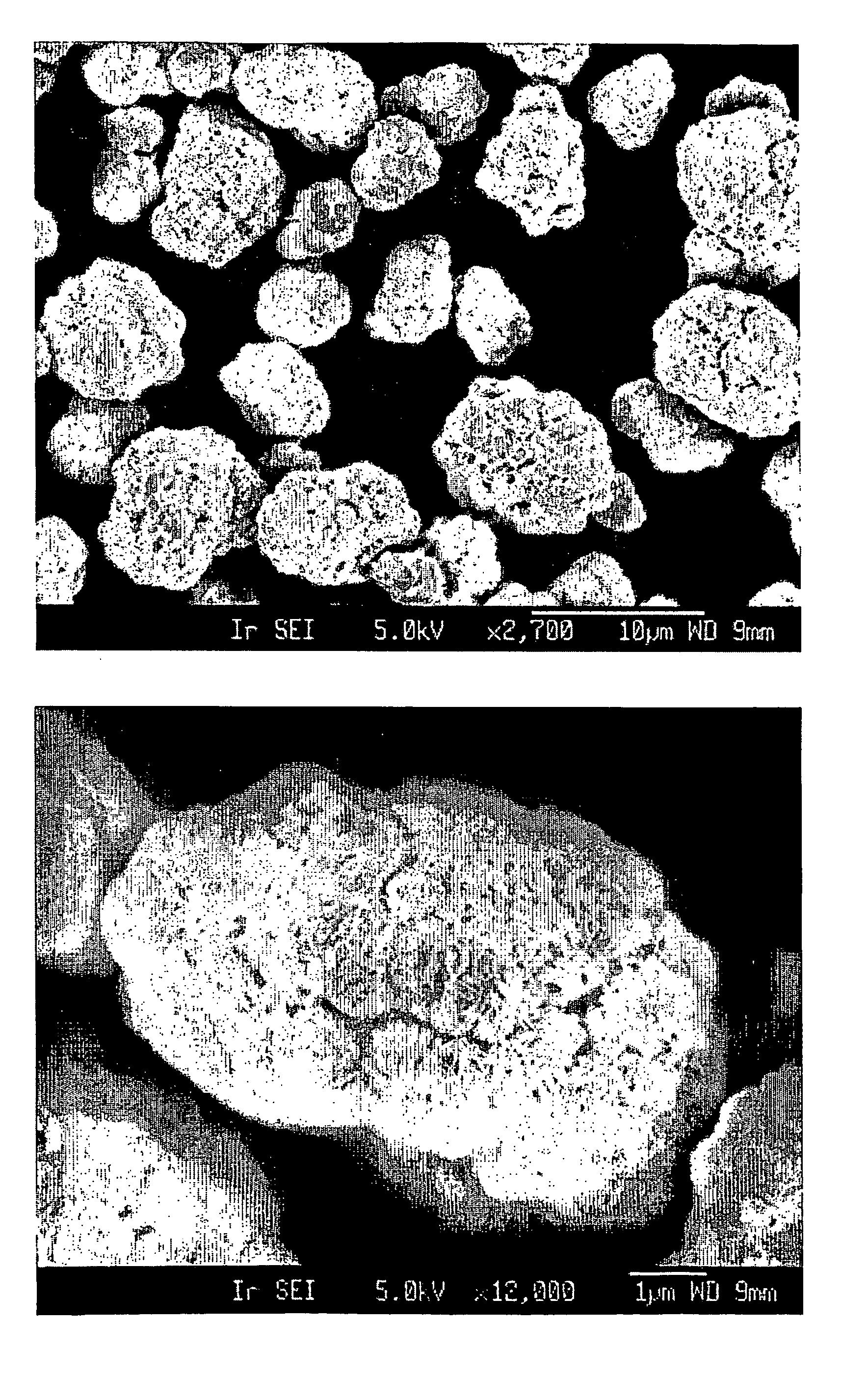
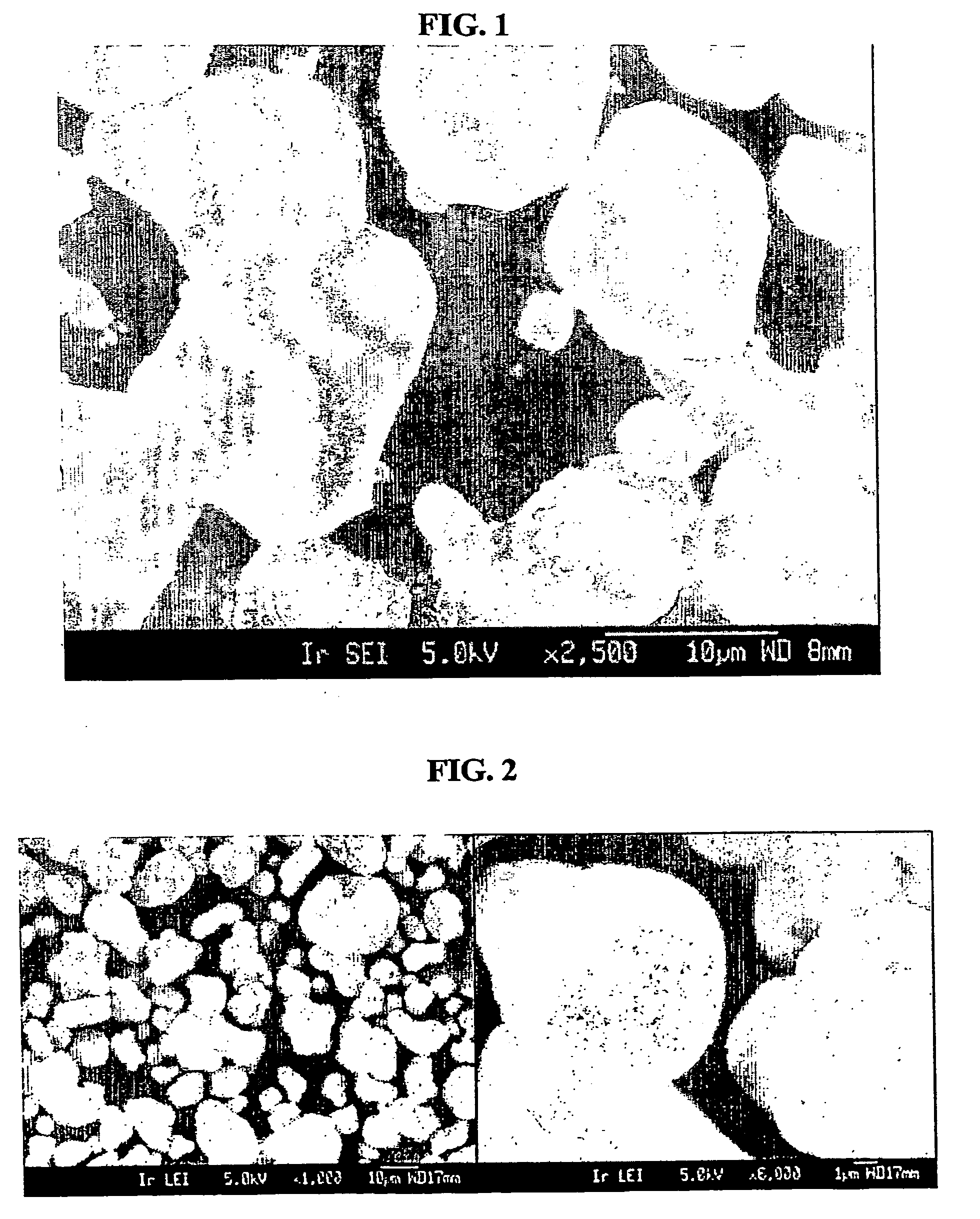
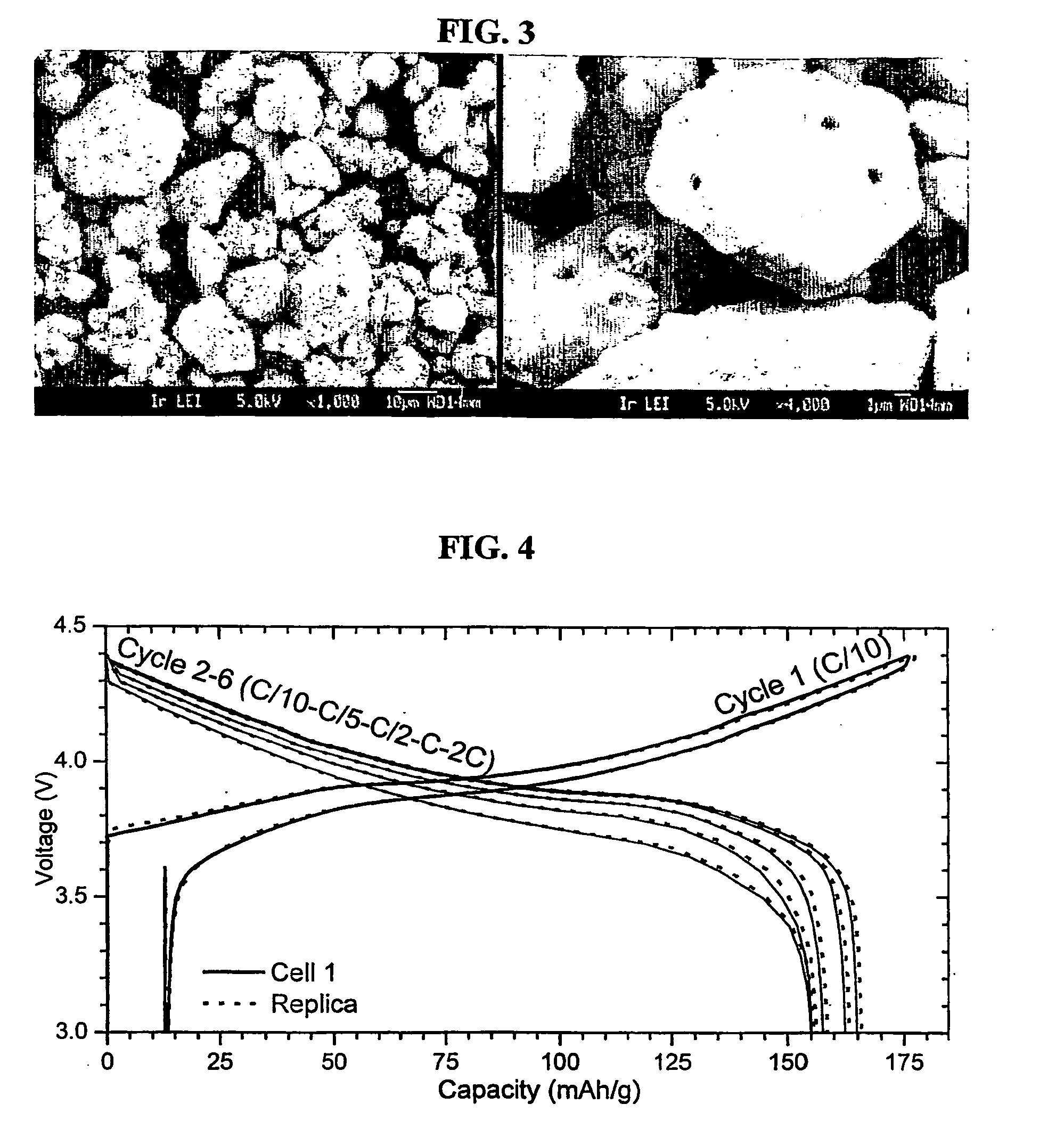
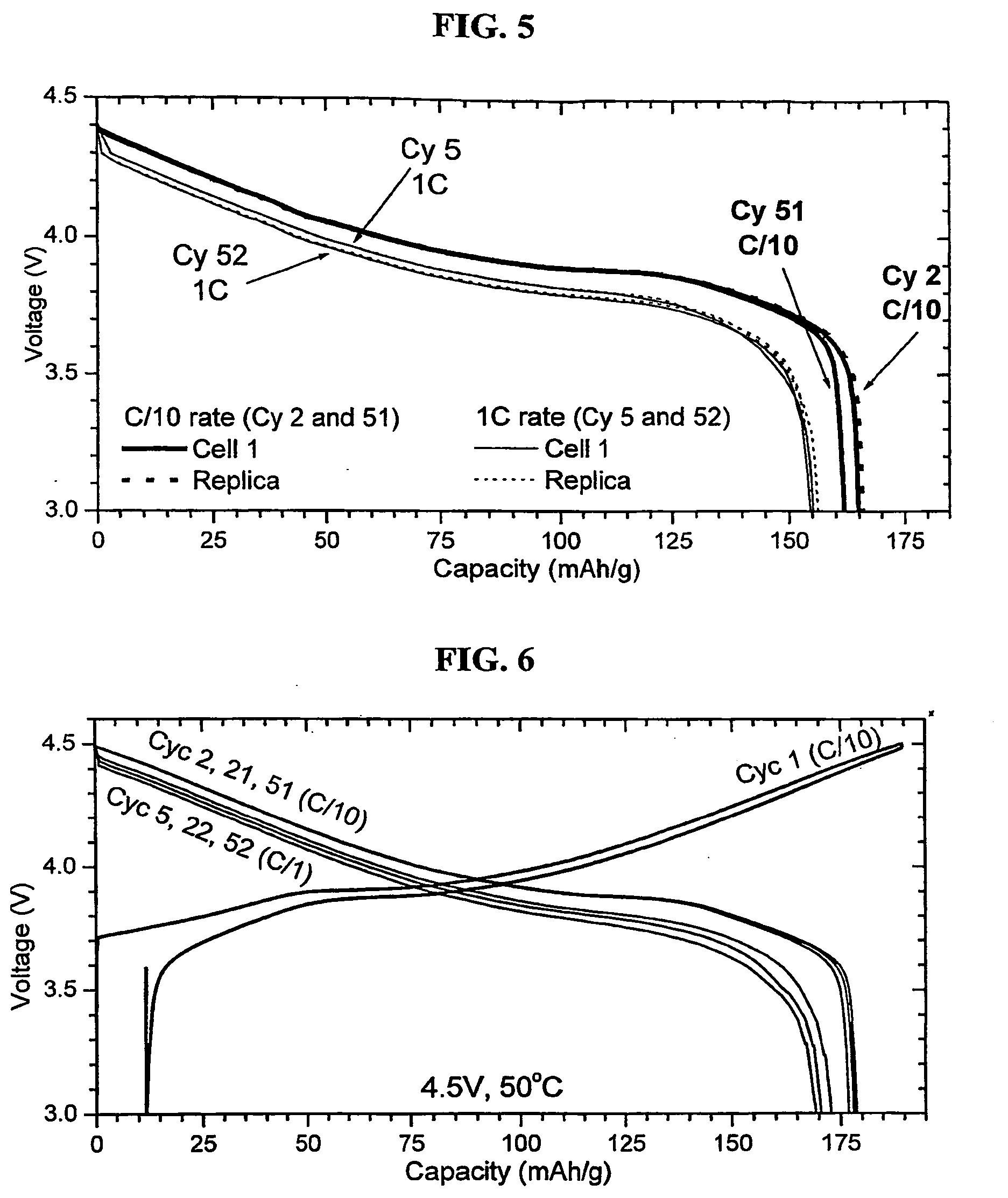
Coagulated particles of nickel-cobalt-manganese hydroxide wherein primary particles are coagulated to form secondary particles are synthesized by allowing an aqueous solution of a nickel-cobalt-manganese salt, an aqueous solution of an alkali-metal hydroxide, and an ammonium-ion donor to react under specific conditions; and a lithium-nickel-cobalt-manganese-containing composite oxide represented by a general formula, LipNixMn1-x-yCoyO2-qFq (where 0.98≦p≦1.07, 0.3≦x≦0.5, 0.1≦y≦0.38, and 0≦q≦0.05), which is a positive electrode active material for a lithium secondary cell having a wide usable voltage range, a charge-discharge cycle durability, a high capacity and high safety, is obtained by dry-blending coagulated particles of nickel-cobalt-manganese composite oxyhydroxide formed by making an oxidant to act on the coagulated particles with a lithium salt, and firing the mixture in an oxygen-containing atmosphere.
Owner:SUMITOMO CHEM CO LTD
Electrode material for anode of rechargeable lithium battery, electrode structural body using said electrode material, rechargeable lithium battery using said electrode structural body, process for producing said electrode structural body, and process for producing said rechargeable lithium battery
InactiveUS20050175901A1Prolonged discharging cycle lifeLarge capacityNitrogen compoundsSelenium/tellurium compundsElectrochemical responseParticulates
An electrode material for an anode of a rechargeable lithium battery, containing a particulate comprising an amorphous Sn.A.X alloy with a substantially non-stoichiometric ratio composition. For said formula Sn.A.X, A indicates at least one kind of an element selected from a group consisting of transition metal elements, X indicates at least one kind of an element selected from a group consisting of O, F, N, Mg, Ba, Sr, Ca, La, Ce, Si, Ge, C, P, B, Pb, Bi, Sb, Al, Ga, In, Tl, Zn, Be, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, As, Se, Te, Li and S, where the element X is not always necessary to be contained. The content of the constituent element Sn of the amorphous Sn.A.X alloy is Sn / (Sn+A+X)=20 to 80 atomic %. An electrode structural body for a rechargeable lithium battery, comprising said electrode material for an anode and a collector comprising a material incapable of being alloyed with lithium in electrochemical reaction, and a rechargeable lithium battery having an anode comprising said electrode structural body.
Owner:CANON KK
Preparation method of high-temperature-resistant aerogel and aerogel-type porous ceramics
PendingCN107098352AImprove high temperature resistanceAvoiding Densification ProblemsSilicaZirconium compoundsThermal insulationWhiskers
The invention discloses a preparation method of high-temperature-resistant aerogel. The method comprises: adding high-temperature-resistant powder or crystal whiskers to sol, performing in-situ compounding of sol and high-temperature-resistant powder or crystal whiskers, and performing aging, modification and drying to obtain high-temperature-resistant aerogel. The invention further discloses a preparation method of aerogel-type porous ceramics. Porous ceramics having an aerogel hole structure kept is obtained by sintering the high-temperature-resistant aerogel. The prepared high-temperature-resistant aerogel and aerogel-type porous ceramics can resist high temperature of 1000-1800 DEC C or more, keep the nanometer hole structure from collapse, have high porosity and strength and have hole diameters which can be adjusted from micropores to large holes. The aerogel and aerogel-type porous ceramics can be used as a super thermal insulation material and can be widely applied to fields of purification separation, adsorption, chemical industrial catalytic carriers, sound absorption and damping, sensing elements and electrochemistry. The method is simple and easy to carry out and suitable for massive production, and allows high temperature resistance of aerogel to be improved and application fields of aerogel to be broadened.
Owner:浙江圣润纳米科技有限公司
Method for continuously synthesizing precursor of lithium ion battery positive material
InactiveCN102092798AInhibition formationSolve the problem of easy oxidationCell electrodesManganese oxides/hydroxidesSynthesis methodsManganese
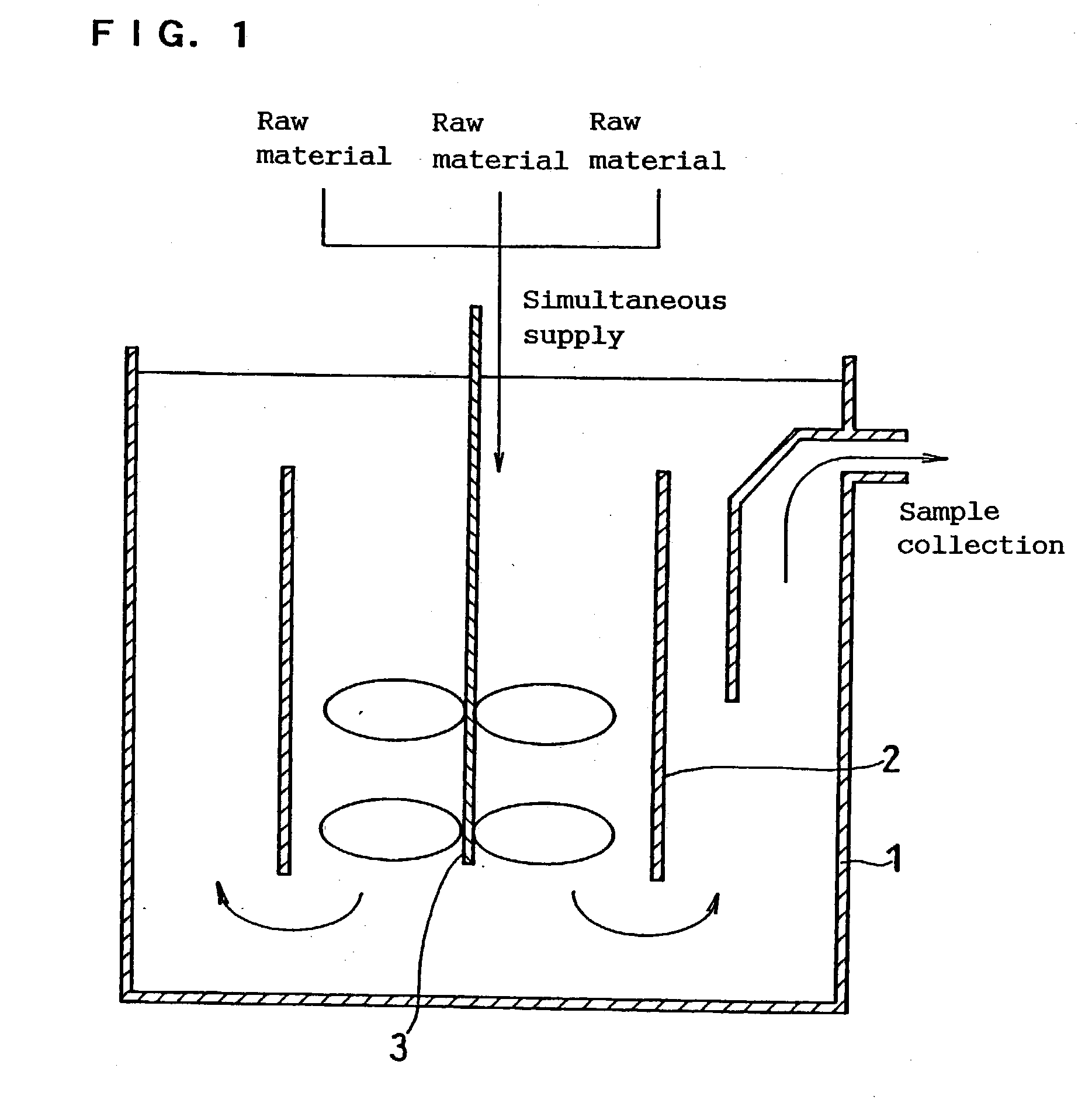
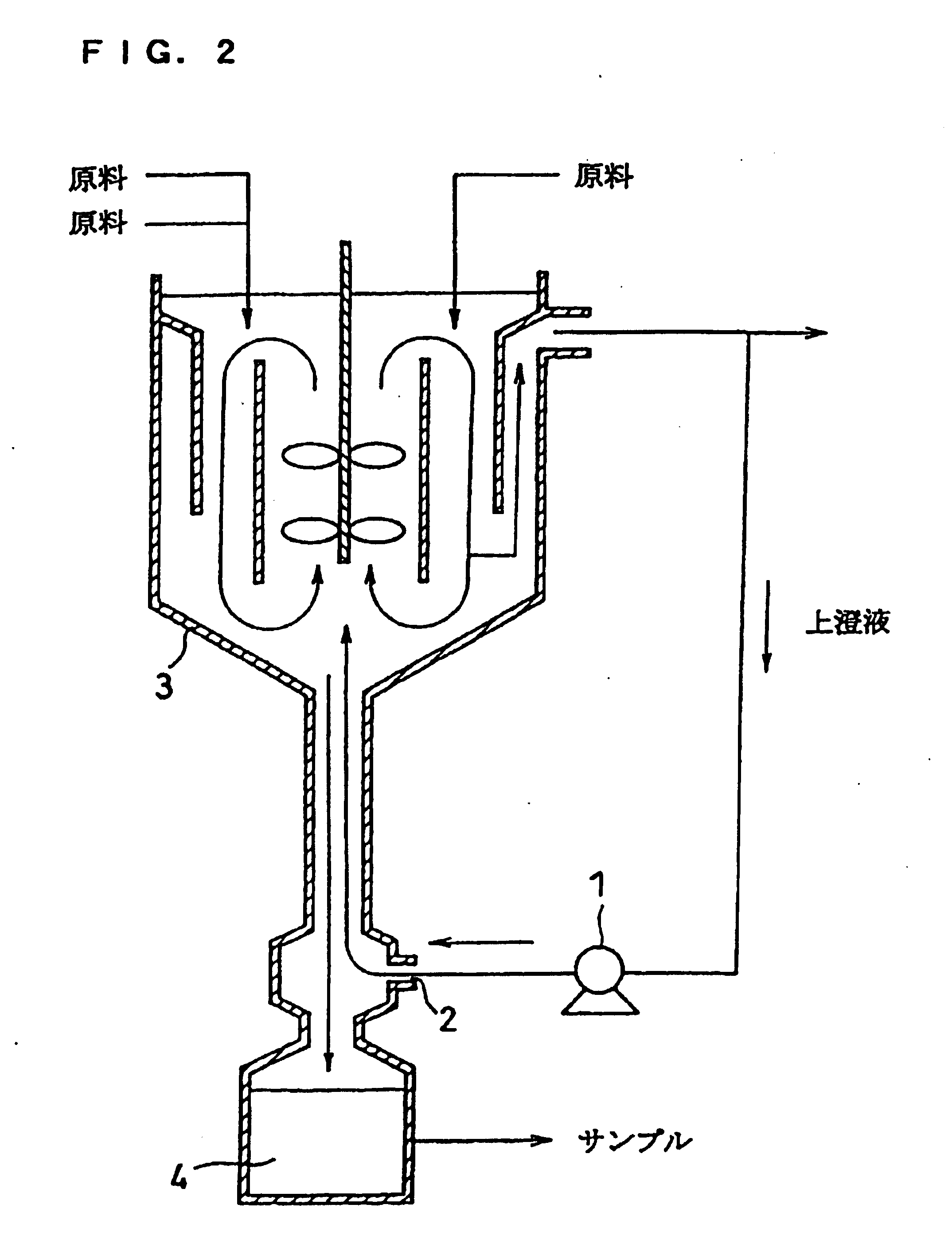
The invention provides a method for continuously synthesizing a precursor of a lithium ion battery positive material, relating to an improvement of a synthesis method of a nickle cobalt manganese termary positive material nickle cobalt lithium manganate of the lithium ion battery positive material. The method is characterized in that the synthesis process is as follows: merging a complexing agent ammonia, an aqueous solution of metal nickle cobalt manganese ions and a precipitator sodium hydroxide solution and then continuously adding the substances into a reaction kettle for a synthesis reaction under the strong stirring condition in the presence of protective gas; and aging, filtering and washing the effluent from the reaction kettle, and then drying to obtain the lithium ion battery positive material precursor spherical nickle cobalt manganese termary hydroxide. The method has the advantages that the preparation process is continuous, the particle size of the prepared nickle cobalt manganese compound hydroxide powder is controlled in a range of 5-20 microns, and the prepared nickle cobalt manganese compound hydroxide powder is even in distribution and excellent in electrochemistry property. The method has the advantages of high production efficiency, low production cost and significant economic and social benefits, and the energy is saved.
Owner:LANZHOU JINCHUAN NEW MATERIAL SCI & TECH +1
Methods of Making Binary Metal Oxide Nanostructures and Methods of Controlling Morphology of Same
ActiveUS20100278720A1Reduce crystallinityControl dimensionalityCopper oxides/halidesManganese oxides/hydroxidesPorous membraneNanostructure
The present invention includes a method of producing a crystalline metal oxide nanostructure. The method comprises providing a metal salt solution and providing a basic solution; placing a porous membrane between the metal salt solution and the basic solution, wherein metal cations of the metal salt solution and hydroxide ions of the basic solution react, thereby producing a crystalline metal oxide nanostructure.
Owner:WONG STANISLAUS S +1
Non-volatile resistance-switching oxide thin film devices
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Processing method for stainless steel acid cleaning waste water and liquid
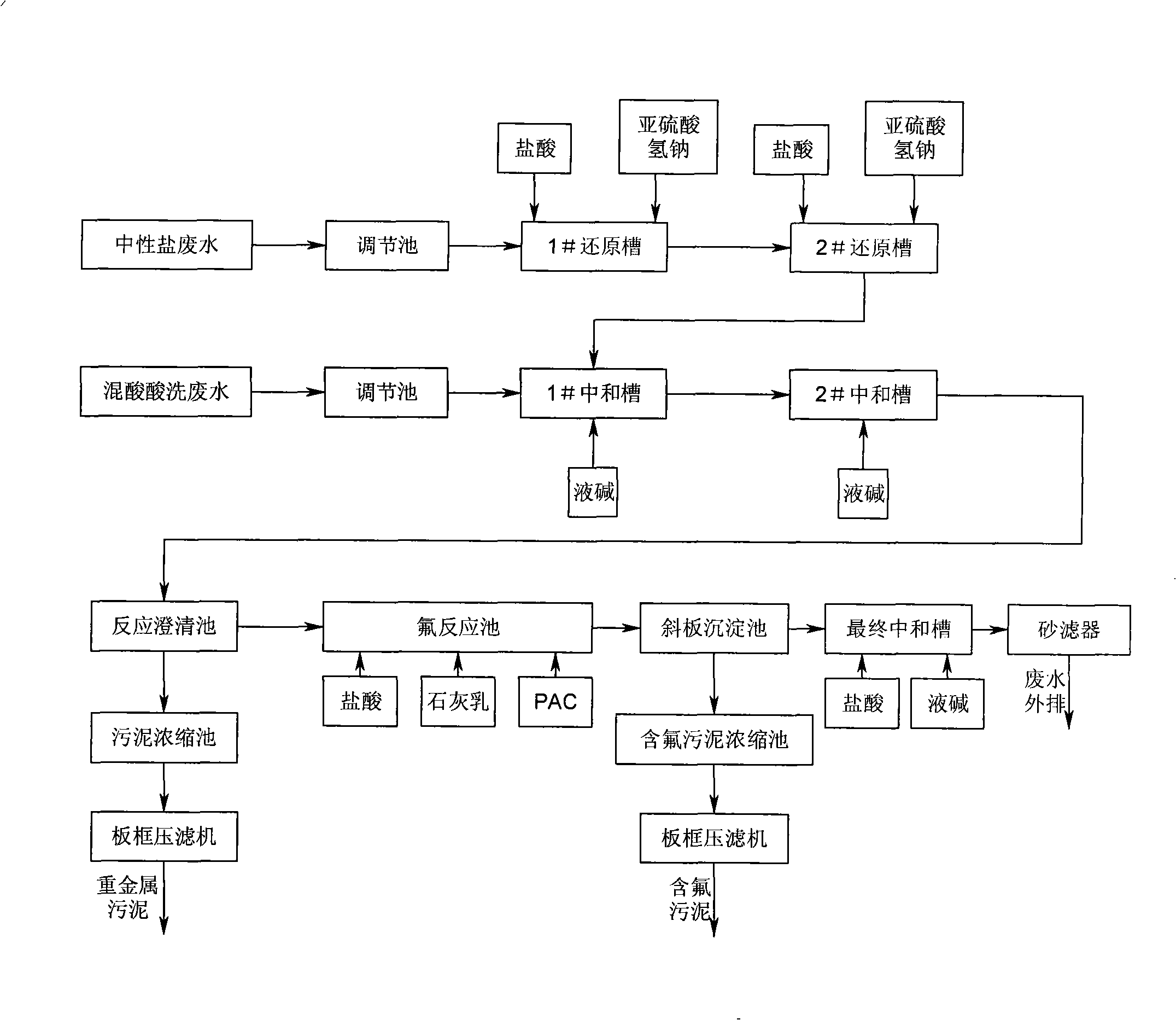
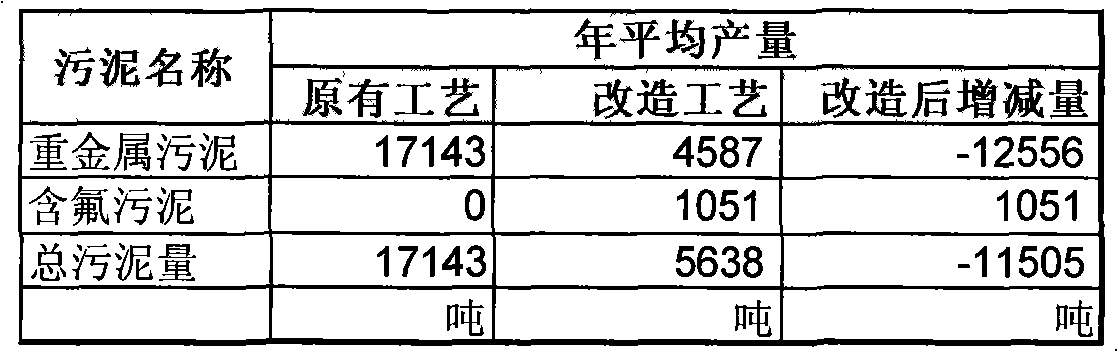
InactiveCN101269889AMeet emission compliance requirementsMeet compliance requirementsSludge treatment by de-watering/drying/thickeningIron oxides/hydroxidesLiquid wasteSludge
The invention relates to a processing method of stainless steel pickling waste liquid, which is characterized in that the processing method includes the steps that: neutral salt wastewater flows into a neutral reduction cell, and mixed acid pickling waste waters flow into a neutralizing tank; reducing agent is added into the neutral reduction cell, and then the reduced wastewater liquid is put into the neutralizing tank and is mixed with the adjusted mixed acid pickling waste waters, and liquid alkali is added into the neutralizing tank; the separated clear waste liquid removes fluorin ions in a settlement tank; neutralizing liquid is added into a final neutralizing tank to cause the pH value of treatment liquid to adjust to be neutralized wastewater liquid which passes through a sand filter to be discharged. The processing method has the advantages that metal ions and fluorin ions are performed the subsection treatment, and neutralizing agent is changed into the liquid alkali from lime cream, the requirement of reaching the standard of the waste liquid emission is not only achieved, but also the mud quantity produced by a mass of lime cream is greatly reduced, heavy metal mud of a retention pond only contains heavy metal compound, the salts of calcium fluoride and calcium sulphate, etc. are separated from the inclined plate settlement tank during the fluoridation stage, thereby the treatment cost is effectively lowered.
Owner:NINGBO BAOXIN STAINLESS STEEL
Powdered lithium transition metal oxide having doped interface layer and outer layer and method for preparation of the same
ActiveUS7364793B2Use of materialSimple processLiquid surface applicatorsElectrode thermal treatmentInterface layerMechanical stability
The present invention provides a powdered lithium transition metal oxide useful as a major component for cathode active material of rechargeable lithium batteries, comprising a lithium transition metal oxide particle, a doped interface layer formed near the surface of the particle, and a thermodynamically and mechanically stable outer layer, and a method of preparing the same.
Owner:LG ENERGY SOLUTION LTD
High-performance lithium ion battery cathode material and preparation method thereof
InactiveCN101847722AHigh tap densityImprove electrode processing performanceElectrode manufacturing processesNickel oxides/hydroxidesSingle crystalCobalt
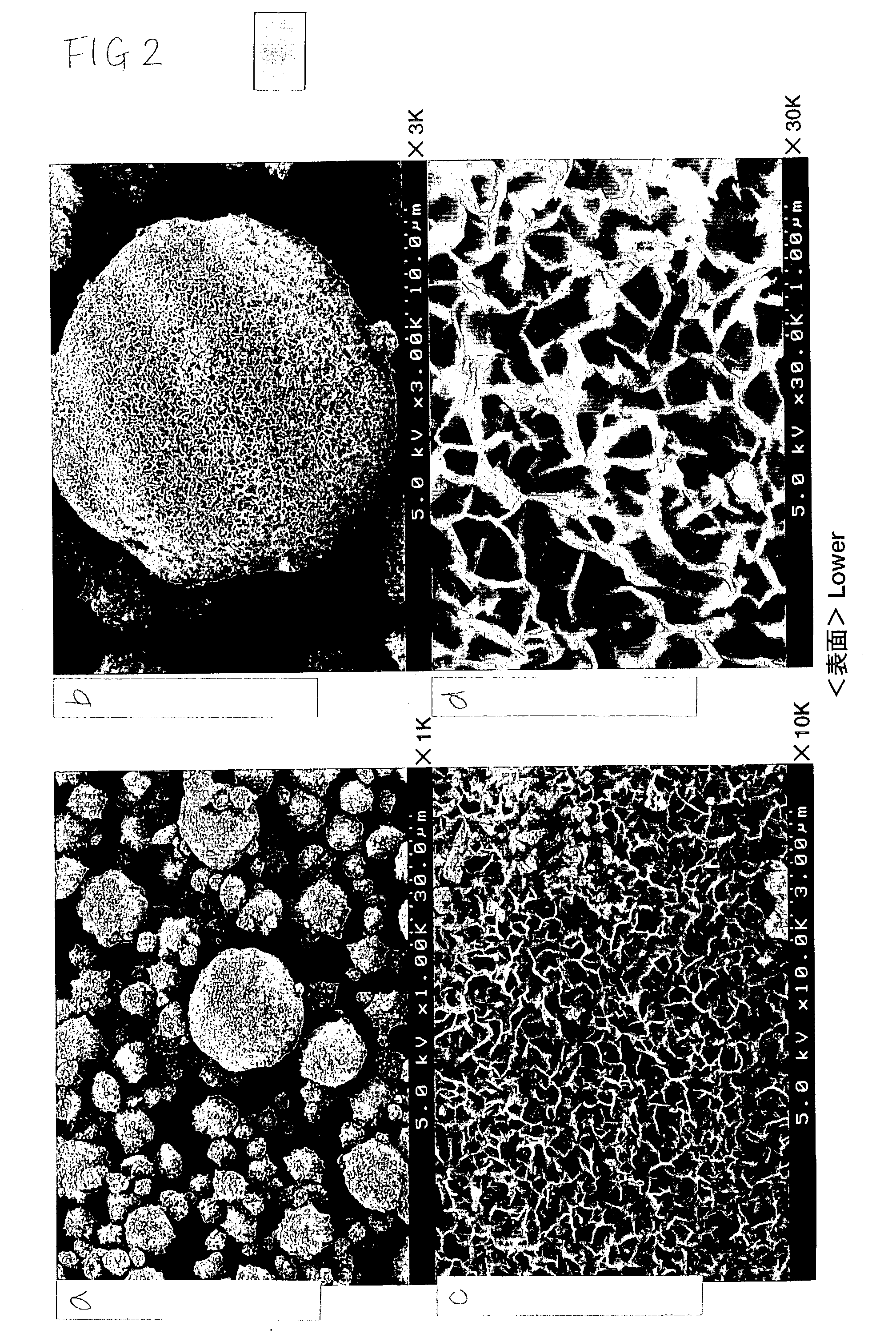
The invention relates to a lithium ion battery cathode material LiNixCoyM1-x-yO2 prepared from micron-sized single crystal particles and a preparation method thereof, wherein x is greater than 0 and is not more than 0.8, y is greater than 0 and is not more than 0.5, and M is one or two of Li, Mn, Al and Mg. The invention is characterized in that (1) composite oxide or hydroxide of transition metal nickel, transition metal cobalt and modified metal M is used as a raw material, the composite oxide or hydroxide is porous aggregate comprising nanocrystals, the average size of the aggregate is 2-50 micrometers, and the specific surface area of the aggregate is greater than 15m<2> / g (measured by BET method); (2) the composite metal oxide or hydroxide and lithium salts are milled in a ball mill, the micron-sized composite metal oxide or hydroxide is converted into nanocrystal particles to obtain a nano-sized mixed precursor of the composite metal oxide or hydroxide and the lithium salts, and the mixed precursor is sintered at uniform temperature to obtain the required lithium ion battery cathode material; and (3) the prepared lithium ion battery cathode material LiNixCoyM1-x-yO2 is basically prepared from micron-sized single crystal particles, and the average size of the single crystal particles is 2-20 micrometers. In addition, the product has excellent physical and electrochemical properties, such as ultra-low specific surface area, reasonable particle size distribution, good electrode processing properties, ultra-long cycle life, excellent rate capability, obvious high and low temperature cycling and storing properties and excellent safety; and the product can be widely used as a high-performance lithium ion battery cathode material. The invention provides the high-performance lithium ion battery cathode material and the preparation method thereof.
Owner:QINGDAO LNCM
Lithium-containing complex oxide, non-aqueous secondary battery using the lithium-containing complex oxide, and method for producing the lithium-containing complex oxide
InactiveUS20050260496A1Stable structureIncreased durabilityElectrode thermal treatmentOrganic electrolyte cellsLithiumHigh density
Because of the composition represented by General Formula: Li1+x+αNi(1−x−y+δ) / 2MyO2 (where 0≦x≦0.05, −0.05≦x+α≦0.05, 0≦y≦0.4; −0.1≦δ≦0.1 (when 0≦y≦0.2) or −0.24≦δ≦0.24 (when 0.2<y≦0.4); and M is at least one element selected from the group consisting of Ti, Cr, Fe, Co, Cu, Zn, Al, Ge and Sn), a high-density lithium-containing complex oxide with high stability of a layered crystal structure and excellent reversibility of charging / discharging can be provided, and a high-capacity non-aqueous secondary battery excellent in durability is realized by using such an oxide for a positive electrode.
Owner:MAXELL HLDG LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com